
- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center

- Why is biology important?
- What are the basic functional systems of animals?


good genes hypothesis
Our editors will review what you’ve submitted and determine whether to revise the article.
- Auburn University - College of Sciences and Mathematics - Choosing Mates: Good Genes Versus Genes that are a Good Fit
good genes hypothesis , in biology , an explanation which suggests that the traits females choose when selecting a mate are honest indicators of the male’s ability to pass on genes that will increase the survival or reproductive success of her offspring. Although no completely unambiguous examples are known, evidence supporting the good genes hypothesis is accumulating, primarily through the discovery of male traits that are simultaneously preferred by females and correlated with increased offspring survival. For example, female North American house finches ( Carpodacus mexicanus ) prefer to mate with bright, colourful males. Such male finches also have high overwinter survivorship. This preference suggests that mating with such males will increase offspring survival. British evolutionary biologist W.D. Hamilton and American behavioral ecologist Marlene Zuk first proposed this hypothesis in the early 1980s.
All Subjects
study guides for every class
That actually explain what's on your next test, good genes hypothesis, from class:, general biology i.
The good genes hypothesis suggests that certain traits are favored in mate selection because they indicate superior genetic quality. These traits increase the likelihood of offspring survival and reproduction.
congrats on reading the definition of good genes hypothesis . now let's actually learn it.
5 Must Know Facts For Your Next Test
- The hypothesis is often studied in the context of sexual selection and mate choice.
- Traits indicating 'good genes' can include physical characteristics, behaviors, or other phenotypic expressions.
- Good genes can confer advantages such as disease resistance, better metabolism, or more efficient resource use.
- Females may prefer males with these traits because their offspring are likely to inherit these advantageous qualities.
- Empirical evidence for the good genes hypothesis has been observed in various species, including birds, fish, and mammals.
Review Questions
- What does the good genes hypothesis propose about mate selection?
- How do 'good genes' benefit offspring according to this hypothesis?
- Can you name a type of trait that might be considered an indicator of good genes?
Related terms
Sexual Selection : The process by which certain traits increase an individual's chances of mating and reproducing based on attractiveness to potential mates
Mate Choice : The criteria individuals use to select their partners for reproduction
Phenotype : The observable characteristics or traits of an organism resulting from the interaction of its genotype with the environment
" Good genes hypothesis " also found in:
Subjects ( 1 ).
- Animal Behavior
© 2024 Fiveable Inc. All rights reserved.
Ap® and sat® are trademarks registered by the college board, which is not affiliated with, and does not endorse this website..
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.5(5); 2021 Oct

Should females prefer old males?
Julia carolina segami.
1 Department of Ecology and Genetics, Animal Ecology, Uppsala University, Uppsala SE‐75236 Sweden
Martin I. Lind
Anna qvarnström, associated data.
Figure S2. Age structure of the population per years since (A) 2008 until (I) 2016.
Table S1. A priori structure of the main models.
Table S2. Age structure total known age individuals monitored between 2002 and 2018.
Table S3. Linear mixed‐effect model (Gaussian) with fledgling number as response variable (cross‐sectional dataset).
Table S4. Generalized mixed‐effect model (genpois) with fledgling number as response variable (cross‐sectional dataset).
Table S5. Linear mixed‐effect model (Gaussian) with number of recruits as response variable (cross‐sectional dataset).
Table S6. Generalized mixed‐effect model (genpois) with number of recruits as response variable (cross‐sectional dataset).
Table S7. Linear mixed‐effect model with fledgling number as response variable (longitudinal dataset).
Table S8. Generalized mixed‐effect model (genpois) with fledgling number as response variable (longitudinal dataset).
Table S9. Linear mixed‐effect model (Gaussian) with number of recruits as response variable of longitudinal dataset (not including birds that died in year 1).
Table S10. Generalized mixed‐effect model (genpois) with number of recruits as response variable of longitudinal dataset (not including birds that died in year 1).
Table S11. Generalized mixed‐effect model (binomial) with recruitment as a response variable.
Table S 12. Generalized mixed‐effect model with binomial distribution for the response variable Recruitment (yes, no) with male's age and male's survival as explanatory variables.
Table S 13. Generalized mixed‐effect model with binomial distribution for the response variable Recruitment (yes, no) with male's age and male's survival as explanatory variables.
The data supporting the results are available in the public repository Dryad. DOI: https://doi.org/10.5061/dryad.f1vhhmgxg .
Whether females should prefer to mate with old males is controversial. Old males may sire offspring of low quality because of an aging germline, but their proven ability to reach an old age can also be an excellent indicator of superior genetic quality, especially in natural populations. These genetic effects are, however, hard to study in nature, because they are often confounded with direct benefits offered by old males to the female, such as experience and high territory quality. We, therefore, used naturally occurring extra‐pair young to disentangle different aspects of male age on female fitness in a natural population of collared flycatchers because any difference between within‐ and extra‐pair young within a nest should be caused by paternal genetic effects only. Based on 18 years of long‐term data, we found that females paired with older males as social partners experienced an overall reproductive advantage. However, offspring sired by old males were of lower quality as compared to their extra‐pair half‐siblings, whereas the opposite was found in nests attended by young males. These results imply a negative genetic effect of old paternal age, given that extra‐pair males are competitive middle‐age males. Thus, offspring may benefit from being sired by young males but raised by old males, to maximize both genetic and direct effects. Our results show that direct and genetic benefits from pairing with old males may act in opposing directions and that the quality of the germline may deteriorate before other signs of senescence become obvious.
Impact summary
Male age may influence the number and quality of the offspring he produces. This is due to senescence, the gradual deterioration of organism function with age, which can affect the germline cells quality (i.e., genetic or indirect parental effects) and the ability to provide resources (i.e., direct parental effects). However, proven ability to survive until old age may mean that older fathers instead will produce high‐genetic‐quality offspring, able to survive, and get old themselves. Additionally, old males may also be able to provide their offspring with better or more resources due to gained experience. These reproductive pros and cons of old male partners impose a dilemma for mate choosing females. Should they avoid or prefer old males to ensure direct and indirect benefits? This question has not yet been fully answered because direct and indirect effects of paternal age are hard to separate in the wild. We circumvented this problem by comparing nestlings sired by different males of different ages within the same brood. Therefore, we were able to distinguish the indirect (genetic) from direct (environmental) effects of biological fathers, as the environment and maternal genes were constant. We used 18 years of long‐term data on a collared flycatcher population of which 812 individuals were genotyped to assign paternity. We found that direct benefits increase with male age, but at the same time the germline quality decreases. Moreover, this deterioration happens before other signs of senescence become evident. These findings have profound consequences for the general understanding of the evolution of mate choice, which is rarely considered in the context of senescence. Our study is one of very few exploring the effects of male senescence in the wild and it is the first one to disentangle genetic from direct benefits in the context of mate choice.
Ageing is the physiological deterioration of organism functionwith age and is widespreadin nature (Shefferson et al. 2017 ). Increasing age can affect fitness of the individual (Lemaître and Gaillard 2017 ) and also its offspring through negative parental age affects (Monaghan et al. 2020 ), either because of reduced general phenotypic performance or because of accumulation of deleterious germline mutations. Therefore, age could influence partner choice and females may avoid mating with old males to minimize these negative effects. However, there are also numerous positive effects associated with increased age. In fact, females are often assumed to prefer to mate with older males to gain access to an experienced mate with superior resources and proved ability to survive (Grant and Grant 1987 ; Conner 1989 ; Côté and Hunte 1993 ; Takagi 2003 ; Dupont et al. 2018 ). How these negative and positive aspects of male age translate into number and quality of the offspring they sire and/or raise and hence influence the fitness of females that have chosen to breed with them remain open questions. Whether females should prefer to mate and pair with old males has therefore become a controversial subject (Brooks and Kemp 2001 ; Griffith et al. 2002 ; Beck and Promislow 2007 ; Dean et al. 2010 ; Lifjeld et al. 2011 ; Dupont et al. 2018 ; Rodríguez‐Muñoz et al. 2019 ). Accordingly, there is a need to investigate whether detrimental effects of old age are common in males and also to examine to what extent such effects are strong enough to compromise the reproductive success of females in the wild (Lemaître and Gaillard 2017 ).
Traditionally, natural selection was thought to favor females that prefer older males because such males should be more experienced and thereby also more likely to have access to high‐quality resources and to provide better parental care, which would provide direct benefits to the female (Grant and Grant 1987 ; Conner 1989 ; Côté and Hunte 1993 ; Takagi 2003 ; Dupont et al. 2018 ). Moreover, mating with older males can also give indirect (genetic) benefits to the female in terms of more fit offspring. The “good genes” hypothesis predicts a preference for older individuals based on the logic that survival until older ages requires high‐quality genes, which in turn will be passed on to the offspring (Trivers 1972 ; Kokko and Lindstrom 1996 ; Kokko 1998 ; Bouwman et al. 2007 ). In accordance with these expectations, there are several studies demonstrating that older males are more likely to attract females for mating (Conner 1989 ; Côté and Hunte 1993 ), to become socially paired (Weatherhead 1984 ; Alatalo et al. 1986 ), to be less likely to lose paternity in the broods they attend (Michálková et al. 2019 ), and to be more likely to gain extra pair paternity (Dickinson 2001 ; Bouwman et al. 2007 ; Cleasby and Nakagawa 2012 ; Michálková et al. 2019 ).
Possible negative aspects of pairing with relatively old males can be both in terms of reduced phenotypic skills (i.e., reduced resource holding potential or ability to provide parental care) as a consequence of senescence of the soma, but also genetically in terms of mutation accumulation in the male germline. Physiological senescence with increasing age is widespread across organisms (Shefferson et al. 2017 ), and examples from birds that could result in direct costs are reduced to foraging efficiency and nest defense (Newton and Rothery 2002 ; Bouwhuis and Vedder 2017 ). In contrast, purely genetic costs to the offspring of old fathers stayed for a long time undetected in wild animals. This contrasts to laboratory studies, where offspring from old male mice were found to have decreased reproduction and longevity (García‐Palomares et al. 2009 ), and negative paternal age effects on offspring life span have also been found in Drosophila (Priest et al. 2002 ) and captive populations of zebra finch (Noguera et al. 2018 ). Moreover, in humans, offspring life span decreases with increasing age of the father (Gavrilov and Gavrilova 1997 ; Kemkes‐Grottenthaler 2004 ). Such negative effects of male age were for a long time considered irrelevant in the context of evolution of mate choice in nature. Predation and other sources of mortality were thought to remove individuals from the population before the onset of senescence (Medawar 1951 ; Comfort 2011 ). Moreover, even if a few males would survive until the onset of senescence the likelihood of mating with them would be minimal meaning that selection on females to avoid mating with too old males should be negligible (Finch 1998 ). The few males that do show senescence may also compensate by being more experienced at acquiring resources or providing parental care further lowering the potential gain of avoiding these males as mates. However, these arguments have recently become questioned.
Because the reproductive success of males is very much determined by the ability to secure a mate and sire as many offspring as possible, males are expected to invest in secondary sexual characters, expensive behaviors (such as male‐male interactions, courtship, territory defense), and/or sperm competition, perhaps even at the expense of their germline maintenance (Lemaître and Gaillard 2017 ). Germline cells deteriorate with advanced age (Kong et al. 2012 ) and mutation accumulation in the germline cells can have negative effects on the quality of the offspring in natural populations (Pizzari et al. 2008 ; Velando et al. 2011 ). Mutation rates have also been shown to be higher in males than in females (Kong et al. 2012 ; Smeds et al. 2016 ) probably due to the large number of cell divisions during spermatogenesis. Moreover, recent findings in Drosophila suggest that the fitness effects of negative mutations often increase nonlinearly with age (Brengdahl et al. 2020 ). Thus, it is possible that effects of male senescence on offspring number and quality, especially in the form of mutation accumulation in the male germline, have been overlooked in natural populations. There is now growing evidence that various effects of senescence may be observed also in natural populations (Bonduriansky and Brassil 2002 ; Nussey et al. 2013 ; Bouwhuis and Vedder 2017 ; Froy et al. 2019 ; Gaillard and Lemaître 2020 ), which suggests that negative effects of advanced age can be important for mating decisions, at least if senescent males are present in the population or if the germline deteriorates before other visible signs of ageing occur. Still, relatively few studies have investigated possible effects of male reproductive senescence in the wild (Lemaître and Gaillard 2017 ), with a study on house sparrows being a rare exception. This study focused solely on possible genetic effects by using a cross‐fostering design and found that offspring of old parents had lower reproductive success, but with a sex‐specific effect such that old paternal age only influenced the fitness of sons and old maternal age only affected the fitness of daughters (Schroeder et al. 2015 ).
Taken together, the decision to mate with an old male reflects a balance between positive direct (Grant and Grant 1987 ; Conner 1989 ; Côté and Hunte 1993 ; Takagi 2003 ; Dupont et al. 2018 ) and genetic (Trivers 1972 ; Kokko and Lindstrom 1996 ; Bouwman et al. 2007 ) benefits of choosing an old mate, but also potential costs associated with mating with too old males that experience senescence of the soma (Bouwhuis and Vedder 2017 ) or germline (Pizzari et al. 2008 ; Velando et al. 2011 ; Kong et al. 2012 ), which are outlined in Figure 1 . How these different aspects of male age translate into number and quality of the offspring they sire and/or raise and hence influence the fitness of females that have chosen to breed with them remain open questions. The relative importance of these different effects has to our knowledge not previously been disentangled in studies on natural populations. Our study aims to fill this knowledge gap using two approaches: (1) using long‐term breeding data of collared flycatchers ( Ficedula albicollis ) to assess the effect of paternal age on offspring fledge number and offspring recruitment and (2) by using naturally occurring extra‐pair offspring to disentangle direct and genetic effects of paternal age on offspring quality while keeping maternal genetic effects constant. Collared flycatchers are small passerine birds that preferably breed in nest boxes which allows long term monitoring. The flycatcher population of Öland has been monitored over 18 years and pedigrees as well as age records are available since 2002 (Qvarnström et al. 2010 ). We used these long‐term breeding data to test whether females benefit from breeding with older males. In addition, we also genotyped a large number of offspring to specifically single out possible genetic effects associated with paternal age such as increased genetic quality of offspring with male age due to the male's proven ability to survive and/or decreased genetic quality of offspring with male age due to germline deterioration. In this case, we only have information of the age of the social male as the extra‐pair male is unknown. Nevertheless, we can infer the relative age of the extra‐pair male based on two facts. First, most extra‐pair young (EPY) are known to be sired by competitive middle‐aged males as revelated by other studies (Dickinson 2001 ; Bouwman et al. 2007 ; Cleasby and Nakagawa 2012 ; Michálková et al. 2019 ). Second, even if females would randomly mate with extra‐pair males the age structure of the studied population indicates that young males breeding for their first time would be cuckolded by equally old or older males (i.e., on average older males). Similarly, males belonging to the oldest age classes should be cuckolded by younger males.

The first two panels show expected relationships between female fitness in terms of number and quality of offspring produced and the age of her mate through several possible processes. The second two panels illustrate how naturally occurring extra pair young can be used to disentangle effects mediated by resources or genes provided by the males. This is because these offspring share the same nest environment, including resources provided by the social male, and maternal genes but are sired by males of (on average) different ages. (A) Positive effects of male age on female fitness. Increased male experience and/or selective removal of males of low quality may lead to higher quality of the resources such as territories and paternal care that are provided by the older males. Selective removal of low‐quality males may also ensure genetic benefits to females selecting older males that have proven their ability to survive. The expected net benefits to females with increasing male age will level off as selection will have removed the males of lowest quality and due to an expected less sharp learning curve late in life. (B) Negative effects of male age on female fitness. Reproductive senescence may negatively affect the phenotypic quality of males toward the end or their lives, leading to middle‐aged males being of highest phenotypic quality and therefore providing the best direct benefits to their females. Old males may also experience decreased germline quality as deleterious mutations accumulate with male age leading to a decline in genetic benefits obtained by females selecting older males as mates. These negative fitness effects are expected to accelerate in association with “tipping points” when a decline in male general phenotypic quality, for example, makes them unable to defend high‐quality territories. (C) Direct material benefits from socially pairing with males of different ages will have similar effects on the number and phenotypic quality of within‐ (WPY) and extra‐pair young (EPY). (D) Assuming that extra‐pair young are mainly sired by middle‐aged competitive males, we expect EPY to be of superior genetic quality as compared to WPY when the social male is very young or very old. This is because very young social males have not yet proven their ability to survive to older ages (i.e., selection has not yet removed low‐quality males) and very old social males may experience germline senescence.
DATA COLLECTION
The collared flycatcher ( Ficedula albicollis ) is a small migratory passerine of the family Muscicapidae ; they overwinter in sub‐Saharan Africa and breed in Europe (Qvarnström, Rice, and Ellegren 2010 ). At the beginning of the reproductive season in early May, males arrive to the breeding grounds and establish territories that they advertise to females. Once a female starts building the nest, the male defends its territory from other males and predators. After laying eggs, the female starts incubating and the male provides her food. When the eggs hatch, both the male and the female share the task of feeding the nestlings and guarding the nest. They are insectivorous with a preference for caterpillar larvae as food for their offspring (Shirihai and Svensson 2018 ). The population breeding on Öland (57°100N, 16°580E) has been monitored in deciduous and mixed forests with over 2000 nest boxes across the island during the reproductive season between May and July since 2002 (Qvarnström, Rice, and Ellegren 2010 ). Metal rings with a unique identity code are placed on every bird and morphological measurements as well as a blood samples are taken every year. Additionally, the offspring are weighted (±0.1g) at day 6 and day 12 before fledgling with a spring Pesola of 30 g. This long‐term data collection allowed us to determine social pedigrees, age, and fitness measurements. In addition, we used a subset of breeding events to specifically test for possible effects of paternal age on the genetic quality of the offspring. We genotyped 812 offspring from 154 nests. These nests are from the following years: 2002, 2004–2005, 2008, and 2010–2016.
To assess extra‐pair and within‐pair paternity of social couples offspring, we compared 12 microsatellite loci (FhU1, FhU2, FhU3, FhU4, Fhy223, Fhy301, Fhy304, Fh401, Fhy403, Fhy407, Fhy454, and PdoU5) of the offspring with their known mother and their social father using the software Cervus 3.0.3 (Marshall et al. 1998 ; Kalinowski et al. 2007 ) as previously described in similar studies (Alund et al. 2013 , 2018 ). For this analysis, data were simulated for 10,000 offspring with five candidate fathers assuming the sampling of 70% of the population (Jones et al. 2010 ). Only individuals where at least six microsatellite loci were compared were included and the confidence level used to establish extra pair paternity in the pairwise comparison between offspring and social father was >95%. It was not possible to identify the extra‐pair sires.
STATISTICAL ANALYSIS
Our overall strategy for statistical analyses was to construct a limited number of biologically relevant models to test the hypotheses outlined in Figure 1 . These models are presented in Table S1 . In general, these models include both a linear and quadratic effect of male age, because nonlinear fitness effects of age may be present for both direct (Forslund and Pärt 1995 ) and genetic effects (Fig. 1D ) (Pizzari et al. 2008 ). These linear and nonlinear fitness effects are also expected to interact with predictors of interests. For offspring recruitment probability, we expect that the effect of male survival to next year can interact with age and age 2 due to expected age‐dependent solutions to life‐history trade‐offs (Clutton‐Brock 1984 ). We also expect that the effect of EPY status on offspring mass can differ depending upon parental age, in both a linear and nonlinear fashion. Moreover, although reproductive data are often underdispersed and arguments have been raised to analyze this type of data using flexible generalized linear models (Brooks et al. 2019 ), other arguments favor the robustness and interpretability of Gaussian models for this type of data (Knief and Forstmeier 2020 ). We therefore analyzed reproductive data using both methods, and because our results are robust to the method used, we present the Gaussian models in the Results section and the corresponding generalized linear models in the Supporting Information.
Long‐term breeding dataset
To understand the age structure of the population, data of individuals of known age (i.e., ringed the year of birth or as 1‐year old when plumage reveals exact age of the male) between 2002 and 2018 were visually explored with a population pyramid plot and age percentages of different age classes were calculated.
We analyzed the effect of the social fathers age on the number of fledged offspring (i.e., offspring who left the nest alive) and number of recruits (i.e., offspring of the given clutch that returned after migration to breed in the population on subsequent years), using both cross‐sectional data and longitudinal data of 1‐year‐old individuals to test for selection between age class 1 and 2. These males ( n = 1094) were monitored between 2002 and 2018, and the total number of broods is 1527 of which 117 had 0 fledglings. All experimental (manipulated) broods in the study area were excluded for these analyses. We constructed separate linear mixed‐effect models with fledgling number or number of recruits as response variables. We fitted the age of the social father as explanatory variable and also age 2 , to investigate any nonlinear effect of age. For that purpose, age was mean centered before calculating age 2 . Male ID and year were included in the models as random effects on the intercept. Because both number of fledgling and number of recruits are count data that are generally underdispersed, we used both Gaussian distribution of the package lme4 (Bates et al. 2015 ) and the “genpois” family distribution available in the glmmTMB package (Brooks et al. 2017 ). Because we got consistent results with both distributions, the Gaussian distribution models are presented in the Results section and the correspondent models fitted with glmmTMB can be found in the Supporting Information.
To test whether recruitment does not only depend on age but also on the survival of the parent to the next age category, we constructed a generalized mixed‐effect model with binomial distribution. Offspring recruitment (fail, success) was fit as response variable and age, age 2 , and male survival to the next year as explanatory variables, including all interactions. Male ID and year were included as random effects.
To determine whether mass at time of fledge is a good predictor for recruitment in our population, we constructed a generalized linear mixed‐effect model with binomial distribution having successful or unsuccessful recruitment as a response variable. Mass at day 12 (briefly before fledgling) and lay date were fitted as explanatory variables, whereas nest ID and year were fitted as random effects.
Extra‐pair offspring dataset
To determine the effect of the age of the social father on the offspring's weight at fledgling, we constructed a linear mixed‐effect model with the weight of the offspring at day 12 as the response variable. As explanatory variables we included the paternity status (EPY or within‐pair young [WPY]) and the number of nestlings in the nest, because there is evidence that this is an important factor influencing nestling weight (Källander and Smith 1990 ). We also fitted father's age and age 2 . Year and male ID were included as random effects on the intercept, male ID was then removed as it did not explain any variation in the model and caused singularity problems to the Gaussian model (male ID was retained in the Generalized model [Table S14 ], where it did not cause singularity issues). To avoid pseudo replication because individuals from the same brood can share the same father, we included a random slope of brood ID over paternity status. All statistical analyses were conducted in R 3.5.3 (R Core Team 2019 ), mixed‐effect Gaussian and binomial models were implemented using the package lme4 (Bates et al. 2015 ), and Poisson models were implemented using the package glmmTMB (Brooks et al. 2017 ). All plots were created with the package ggplot2 (Wickham 2016 ) and show the last age category grouped when and additional category would yield less than 5% of all the data points. Significance was assessed using confidence intervals that were obtained using the confint function with Wald method.
The population shows a pyramidal age structure, where females and males show a similar age distribution with the majority of the population being composed by birds of 1 (45.8%) and 2 (25.5%) years old (Fig. S1 ; Table S2 ). Every age category experiences an approximate 50% reduction and thus, we find very few individuals of age 4 (8.5%) or older (5.3%). These older individuals are approximately 6.5% of the male population and 4% of the female population with the oldest individual registered of 9 years old for males and 8 years old for females.
PATERNAL AGE AND OVERALL REPRODUCTIVE PERFORMANCE
Direct effects of the age of the social partner influence the number and quality of offspring that a female produces in the same way regardless of whether these offspring are WPY or EPY. Based on gained experience and selective removal of males with poor phenotypic quality (Fig. 1A ), we expected females to experience higher reproductive performance when breeding together with older males. We found that females paired with older males as their social partners produced more (0.25 ± 0.06, t = 4.51, P < 0.001; Table S3 ; Fig. 2A ) fledged offspring and more recruits that returned to the breeding population as adults themselves (0.06 ± 0.02, t = 3.53, P < 0.001; Table S5 ; Fig. 2B ). In addition to the linear effect of the social fathers age on the number of fledglings, a significant quadratic effect of age captures the curvature of the response, where the positive effect of increased age of the social father weakens after age 2 (Table S3 ; Fig. 2 ). The increased benefit from breeding with older males was hence mainly driven by a lower performance of females paired with 1‐year‐old males that were breeding for the first time (Fig. 2 ). This increased performance observed between the first and second year of breeding in the cross‐sectional data can be driven either by increased performance of males due to gained experience or by selective removal of males of relatively poor quality. To disentangle these two possibilities, we therefore re‐analyzed the dataset using only 1‐year‐old individuals that were known to survive until their second year of breeding or after. Both fledglings and recruitment models lose the significant effect of age 2 , the linear effect of age is only borderline significant (depending upon model), and importantly the parameter estimate of age is much closer to zero in the longitudinal dataset ( Tables S7 and S9 ). These results imply that selective removal of low‐quality individuals between the first and second breeding attempts plays an important role for explaining the benefits associated with selecting older males as breeding partners (Fig. 2 ).

The number of fledglings (A) and recruits back into the breeding population (B) as a function of the social male's age in collared flycatchers. Symbols represent mean ± SE of the number of fledged offspring depending on the social male's age. Red symbol indicates data on males of age 1 that also survived to year 2, therefore controlling for selective disappearance. The white symbol indicates data on the males of age 1 who did not survive to year 4. There is a significant positive relationship between male age and reproductive performance that levels off with increasing age, but there is no apparent evidence for negative effects of male senescence on reproductive output when genetic and material effects are entangled.
In addition, we also analyzed the probability that a fledged offspring would recruit into the breeding population. For our a priori model, which included a linear as well as quadratic effect of age and all interactions, we found no relationship between male survival to next year and offspring recruitment probability after fledgling (Table S13 ). However, if we allow for model simplification using AIC, the best model (which excludes interactions) showed that offspring in the nest of older males (−0.13 ± 0.05, Z = –2.49, P = 0.013; Table S12 ; Fig. 3 ) and males who survive to the next year (−0.21 ± 0.1, Z = −2.18, P = 0.03; Table S12 ; Fig. 3 ) have a higher recruitment probability.

Probability of recruitment given successful fledgling depending on social male's age and survival to the next year. White symbols represent the mean probability ± SE of recruitment of offspring raised by males that died, magenta dots represent the mean probability ± SE of recruitment of offspring raised by males that survived, and black dots represent the mean ± SE of both. Offspring are more likely to recruit to the breeding population when they have fledged from nests attended by older males and males that survived to the following year themselves.
PATERNAL AGE AND GENETIC CONTRIBUTION TO OFFSPRING CONDITION
To estimate offspring quality, we used mass at day 12, which significantly predicts recruitment back to the breeding population (0.268 ± 0.026, Z = 10.116, P < 0.001, Table S11 ). We used 812 offspring from 154 social nests for which both the social male's age and paternity in the brood were known to disentangle possible effects of paternal age on the genetic quality of the offspring from direct effects of paternal age. We found a significant interaction between EPY status and age 2 (Table 1 ) on offspring mass (condition). The interaction between EPY status and age should be interpreted as a more or less negligible linear effect on age for both EPY (nonsignificant weakly negative) and WPY (estimated to be flat), whereas the interaction between EPY status and age 2 highlights fundamental differences in the nonlinear response, where EPY has a strong convex relationship between age and offspring mass with a minimum in young age and increased mass both for age 1 and especially age 4. In contrast, the quadratic relationship is convex and closer to zero for WPY. These interactions are illustrated in Figure 4 , showing that WPY have a relatively higher mass than EPY in nests attended by 1‐ and especially 2‐year‐old males. In nests attended by very old males (4 year or older), we find that WPY have a relatively lower mass than EPY (Table 1 ; Fig. 4 ). Because competitive middle‐aged males mainly sire the EPY, both these results imply a negative genetic effect of paternal age on offspring mass as predicted based on age‐dependent germline deterioration (Fig. 1D ).
Linear mixed‐effect model with offspring mass at day 12 as a responsevariable
| Estimate | SE | CI 2.5%–97.5% | |||
|---|---|---|---|---|---|
| Intercept | 14.217 | 0.548 | 25.926 | ||
| Age | −0.168 | 0.177 | −0.951 | 0.344 | −0.52–0.18 |
| Paternity status (WPY) | 0.671 | 0.210 | 3.194 | ||
| (Age) | 0.347 | 0.157 | 2.212 | ||
| Total offspring in nest | −0.046 | 0.074 | −0.626 | 0.532 | −0.19–0.10 |
| Age: Paternity status (WPY) | 0.188 | 0.129 | 1.450 | 0.152 | −0.08–0.44 |
| Paternity status (WPY): (Age) | −0.40 | 0.136 | −2.921 |
| Random effects | σ | CI 2.5% –97.5% | groups |
|---|---|---|---|
| Nest ID (intercept) | 1.168 | 0.96–1.42 | 154 |
| Paternity status | 0.687 corr −0.54 | –0.74 to −0.22 | – |
| Year (intercept) | 0.597 | 0.39 to 0.99 | 11 |
| Residual | 0.824 | 0.78 to 0.87 | – |
The explanatory variables are Age of the social father (Age), Paternity status (either within‐pair offspring [WPY] or extra‐pair offspring [EPY]), and total number of offspring in the nest. As random effects, we have Year and a random slope for Paternity status on Nest ID. Number of observations: 812. Number of broods: 154. A confidence interval that does not overlap with 0 indicates significance for the value in this case the Estimate. And in the P column all values of p <0.05 are considered significant and hence they are in bold. P <0.05 in all cases matches the significance given by the calculation of confidence intervals.

Mass at day 12 of extra‐pair (EPY, red color) and within‐pair (WPY, blue color) offspring depending on the social male's age. Within‐pair offspring are relatively heavier when the social male is young, but the opposite pattern with relatively heavier extra‐pair young is found when the social male is old. Offspring of males of age 5 or older are grouped in the plot but not on the analysis. (A) Mean ± SE of each age group. (B) Visualization of raw data points (with mean ± SE superimposed on top).
We found that female collared flycatchers benefit from breeding with old males, and especially should avoid 1‐year‐old males as social partners, to ensure overall high reproductive output. There was no evidence of reproductive male senescence lowering the direct, material benefits for females pairing with very old males. However, a comparison between WPY and EPY within a clutch revealed evidence consistent with deterioration of the germline of old males. Extra‐pair offspring had higher weight than within‐pair offspring in nest attended by old social males. Because old males are rare in the population, we assume that the EPY in these nests were sired by on average younger males. This result therefore demonstrates a decoupling of direct (material) and indirect (genetic) benefits of pairing with old males. Thus, female collared flycatchers could maximize their fitness by having their offspring sired by young males but raised by old males, where the direct benefits have an overall stronger effect on female fitness. Below we discuss these findings in more detail together with possible constraints on optimal female mating strategies.
The decision to mate with a male of a particular age reflects a balance of both direct and indirect benefits and costs associated with male age. Direct (or material) benefits are associated with resource provisioning and nest defense, and older males can often provide more resources due to increased experience and access to higher quality territories (Grant and Grant 1987 ; Conner 1989 ; Forslund and Pärt 1995 ; Takagi 2003 ). Indirect effects are instead related to the genetic effects of choosing a particular male, in terms of increased genetic quality of the offspring (Kempenaers and Dhondt 1993 ; Jennions and Petrie 2000 ; Griffith et al. 2002 ). Direct and genetic benefits are hard to disentangle in the wild, because both effects contribute to reproductive success of the social male, but we achieved this goal by using naturally occurring extra‐pair offspring. Any difference in performance between half‐sib WPY and EPY in a nest should be caused by genetic effects only, because males are not able to recognize its genetic offspring and therefore do not provide differential paternal care (Kempenaers and Sheldon 1996 ).
Firstly, we investigated the direct effect of paternal age. We found that females should prefer older males as social partners, and specifically avoid choosing young males as social partners. Both the number of fledglings and recruits (offspring returning to the breeding populations) increased with male age, which agree with a number of studies finding improved reproductive performance with age in birds (reviewed in Forslund and Pärt 1995 ). A significant nonlinear decline in the positive effect of male age suggested that the result was, as expected, mainly driven by low performance of 1‐year‐old males. Several mechanisms can underlie such patterns, such as increased experience (a direct effect), but also selective disappearance of low‐condition individuals, as well as age‐assortative mating (Zhang et al. 2015 ; Bouwhuis and Vedder 2017 ). By analyzing a longitudinal dataset with only individuals that survived at least 2 years, we found the effect of paternal age on the number of hatchlings and recruits almost disappeared, therefore selective disappearance of year‐1 males rather than increased experience is causing the increased reproductive performance of females having older males as their social partners. This result also excludes age‐assortative mating (where males and females of the same age preferentially breed together) as a possible explanation, because males surviving more than 1 year had a relatively high reproductive performance already as young. Moreover, we also found that the probability that a fledgling would recruit back into the breeding population increased not only with social male age but also with its survival to the following year. This suggests a good‐genes effect may be present for overwintering survival, further arguing for the importance of breeding with old males with proven ability to survive. Thus, the overall reproductive performance of females is clearly boosted by avoiding first‐year breeding males as social mates because selective disappearance ensures a generally higher quality of older males.
Although reproductive performance often increases with paternal age in birds (Forslund and Pärt 1995 ; Rebke et al. 2010 ; Torres et al. 2011 ; Auld et al. 2013 ; Zhang et al. 2015 ), few studies have separated the role of increased experience from selective disappearance of low‐performing males, and only in long‐lived birds. Selective disappearance contributed to the increased reproductive performance with age in blue‐footed booby (Torres et al. 2011 ) and mute swans (Auld et al. 2013 ), but had only a small effect in the common tern (Rebke et al. 2010 ; Zhang et al. 2015 ). For short‐lived birds, the role of selective disappearance has not been investigated in relation to male performance, but selective disappearance of low‐quality females is important in great tits (Bouwhuis et al. 2009 ) and collared flycatchers (Evans et al. 2011 ). There is thus a need for further studies aimed at separating the role of selective disappearance and increased experience for male reproductive performance, especially in short‐lived species.
We did not observe a decline in the reproductive performance of very old males (Figs. 2 and 3 ) as would have been expected based on reproductive senescence and a general decline in phenotypic performance and the ability to provide resources at very old ages (Fig. 1 ). This result contrasts against the observed strong decline in the reproductive performance of female collared flycatchers late in life (Gustafsson and Pärt 1990 ) and with evidence for senescence from other natural populations (Bonduriansky and Brassil 2002 ; Nussey et al. 2013 ; Bouwhuis and Vedder 2017 ; Froy et al. 2019 ; Gaillard and Lemaître 2020 ). Most of the previous scientific works have focused on females making it premature to make the conclusion that actuarial senescence is generally more evident in females even if this indeed appears to be the case in collared flycatchers. However, it has been suggested that sex‐specific reproductive strategies may result in sexual selection favoring males that invest heavily in keeping a competitive phenotype in shape also at old ages and perhaps even at the expense of their germline maintenance (Lemaître and Gaillard 2017 ). Effects of germline senescence are difficult to assess, because genetic effects generally are masked by direct effects of the breeding environment in natural populations (but see below).
Secondly, we assessed the indirect effect of paternal age. Because selective removal of poor‐quality 1‐year‐old individuals resulted in increased reproductive success, we expected that the females would also get genetic benefits from choosing old males. However, there may also be a genetic cost associated with choosing old males due to germline senescence (Fig. 1B ). To separate direct and genetic effects, we used naturally occurring extra‐pair paternity to investigate the relative difference in mass between WPY and EPY within a clutch. This approach has previously been used to investigate if females gain genetic benefits from extra‐pair mating (e.g., Kempenaers et al. 1992 ; Foerster et al. 2003 ; Charmantier et al. 2004 ), but has to our knowledge never been used to investigate effects of male germline senescence. The shared nest environment means that both EPY and WPY benefit from being raised by old males. Because most males in the population are young (Fig. S1 ), but middle‐aged males are most likely to get extra‐pair paternity (Dickinson 2001 ; Bouwman et al. 2007 ; Cleasby and Nakagawa 2012 ; Michálková et al. 2019 ), we expect that EPY in nest with young males as social partners are sired by older males, whereas EPY in nests with very old males as social partners are sired by relatively younger, competitive middle‐aged males, because very old males are rare in the population. The half‐siblings (i.e., within‐ and between‐pair young) sharing the same nest are exposed to the same direct effects of paternal age (Fig. 1C ), which in the flycatcher case lack evidence for a negative effect of actuarial male senescence in broods raised by very old males (Figs. 2 and 3 ). If females gain genetic benefits by mating with old males that have proven their ability to survive (Fig. 1A ), we expect that EPY in nests attended by young males should be of better condition (have higher mass) than WPY (Kempenaers et al. 1992 ; Fig. 1D ). For nests attended by very old males, we also expected EPY to be of better condition (have higher mass), but in this case due to germline deterioration among these very old social males (Fig. 1D ). As expected, EPY indeed outperformed WPY in nests attended by very old males. Surprisingly, however, we found evidence for negative performance effects associated with paternal age also in nests attended by young males where within‐pair offspring outperformed EPY that probably had been sired by “middle‐aged” competitive males. These results imply that the germline of these competitive males may deteriorate before other signs of senescence become obvious. This finding is consistent with the idea that sexual selection has led to the evolution of males that prioritize somatic maintenance at the expense of their germline maintenance (Lemaître and Gaillard 2017 ). One should, however, note that the negative effect was stronger for nests attended by 2‐year‐old rather than 1‐year‐old males. A possible explanation to this finding is that that the two sources of genetic benefits (i.e., avoidance of the effects of germline deterioration by having offspring sired by young males vs. ensuring the effects of good genes by having offspring sired by old males with proven ability to survive) even each other out in nests attended by 1‐year‐old males.
Because mating outside the social pair bond is associated with obvious costs to females, such as exposure to sexually transmitted disease (Sheldon 1993 ) or reduced parental care by the social mate (Arnqvist and Kirkpatrick 2005 ; Griffin et al. 2013 ), much previous scientific work has been allocated toward finding possible adaptive explanations to the wide spread occurrence of active female extra‐pair mating in birds. The most commonly proposed adaptive explanation is that females, who generally are constraint in their choice of social male, can obtain genetic benefits from extra‐pair mating (e.g., Jennions and Petrie 2000 ; Westneat and Stewart 2003 ; Akçay and Roughgarden 2007 ). That old males, with proven ability to survive and more attractive traits, often gain more extra‐pair matings as compared to other males is generally interpreted as evidence in support of this assumption (Akçay and Roughgarden 2007 ; Cleasby and Nakagawa 2012 ). However, this interpretation has recently become questioned (Brooks and Kemp 2001 ; Griffith et al. 2002 ; Beck and Promislow 2007 ; Dean et al. 2010 ; Lifjeld et al. 2011 ; Dupont et al. 2018 ; Rodríguez‐Muñoz et al. 2019 ) and some studies have even found evidence suggesting that EPY may be of lower genetic quality as compared to WPY (Sardell et al. 2011 ; Hsu et al. 2014 ). Thus, the overall evidence for large genetic benefits of extra‐pair mating is limited (Forstmeier et al. 2014 ). Our study implies that there may be negative genetic consequences of extra‐pair mating with old males. Specifically, our results are consistent with a deteriorating germline in old males. There are several mechanisms that can underlie deteriorating of the germline. Mutation accumulation is thought to be a major cause (Kong et al. 2012 ), but possibly also telomere shortening (Noguera et al. 2018 ) as well as passive epigenetic effects (Schroeder et al. 2015 ). We did not investigate the mechanism, but note that they all are evolutionary very similar, resulting from accumulation of deleterious effects with age because of weakened selection (Hamilton 1966 ).
Negative parental age effects are seen as a hallmark of ageing, and several laboratory studies have found that offspring of old parents have shorter life span (Lansing 1947 ; Rockstein 1957 ; Tracey 1958 ; O'Brian 1961 ; Kiritani and Kimura 1967 ; Gavrilov and Gavrilova 1997 ; Priest et al. 2002 ; García‐Palomares et al. 2009 ; Lind et al. 2015 ) and/or reduced fecundity (Priest et al. 2002 ; García‐Palomares et al. 2009 ). Although most often studied in females, negative paternal age effects have also been found in males of captive zebra finch (Noguera et al. 2018 ), Drosophila (Priest et al. 2002 ), and mice (García‐Palomares et al. 2009 ). However, although negative paternal age effects are found in the lab, they are much harder to detect in nature, where they can be masked by positive direct effects of increasing paternal age. Moreover, even negative effects on reproduction with age could reflect somatic ageing and diminished direct effects (Newton and Rothery 2002 ) and hide indirect (genetic) effects. To our knowledge, only one previous study has investigated genetic paternal age effect in wild birds, and found reduced lifetime reproduction of male offspring from old fathers in the house sparrow (Schroeder et al. 2015 ). The study on house sparrows did not consider the relative effects of direct (material) and indirect (genetic) parental age effects on offspring performance, which is the aim of our study. Instead, it focused on paternal genetic effects by using a cross‐foster design and did not investigate the effect of paternal genetic contribution to offspring performance against the same maternal genetic background. By using naturally occurring extra‐pair offspring, we can place our findings in relation to direct benefits from mating with older males and we are testing paternal genetic effects against the same maternal genetic background (i.e., by comparing halfsiblings). Although we found that within‐pair offspring of old social males are smaller than extra‐pair offspring in the same brood, WPY produced by these old males actually have a similar fledging weight as compared to WPY produced by younger males. This finding suggests that old males compensate the disadvantages of the deteriorated germline with increased parental investment. Because the reproductive success of males is very much determined by the ability to attract a mate (Andersson 1994 ), males are expected to invest heavily in secondary sexual characters, perhaps even at the expense of their germline maintenance (Lemaître and Gaillard 2017 ). Thus, males should prioritize keeping their soma in shape. In line with this, male ornamentation in collared flycatchers shows no signs of senescence, but is rather increasing with age (Evans et al. 2011 ), and Houbara bustards that invest considerably in sexual display also show rapid senescence of spermatogenic function (Preston et al. 2011 ). If males prioritize keeping their soma in shape, females may prefer old males to ensure direct benefits in terms of high‐quality territories and parental care.
Our findings also reveal that the ideal situation for a nestling is to be sired by a young male but to be raised in the nest attended by an old male. As a result, despite evident germline ageing, females should prefer old males as social partners because of the direct benefits but obtain extra‐pair mating with younger males. However, older males are generally dominant in aggressive interactions with other males (Andersson 1994 ) suggesting that young males may be prevented from successfully courting and mating with females socially paired with older males. Moreover, young males often arrive later in the season at the breeding grounds as compared to old males reducing the window when they may seek extra‐pair mating before the females lay all their eggs (Canal et al. 2012 ; Edme et al. 2016 ). Finally, males may respond to perceived lost paternity by depressed paternal investment, for example, in terms of reduced paternal care of offspring by their social mate, resulting in selection against EPC behavior in females (Arnqvist and Kirkpatrick 2005 ). Thus, both male‐male interactions and social males’ responses to perceived lost paternity are likely to constrain optimal female mating strategies in relation to male age.
To conclude, we used a combination of long‐term breeding data and naturally occurring EPY to disentangle the different aspects of male age on female fitness in a natural population of collared flycatchers. We found that females paired with older males experienced an overall reproductive advantage due to increased phenotypic quality of males belonging to the older age categories. Selective disappearance of low‐quality males was found to be a major driver behind the advantage of choosing old males as breeding partners. There was moreover evidence for a good gene benefit of choosing old males because males who survived were also more likely to have offspring recruiting back to the breeding population. However, we also found evidence for a negative genetic effect of mating with old males. Within the same nest, offspring sired by old males were of relatively lower quality as compared to their EPY half‐siblings, whereas the opposite was found in nests attended by young males. Direct and indirect benefits from pairing with old males hence act in opposing directions. Female flycatchers gain direct benefits from pairing with old males but genetic benefits from having young males siring their offspring.
Supporting information
Figure S1. Age structure of the population since 2002 until 2018.
Acknowledgments
We thank all the people involved in the fieldwork of the Qvarnström lab flycatcher project for helping with the data collection, C. Cunha for help with Figure 1, and R. Dufva for performing the molecular lab work. We especially thank the editor, associate editor, and two anonymous reviewers for constructive and insightful comments, which greatly improved the manuscript. This work was supported by the Swedish Research Council (Grant number 2016–05138 and 2012–03722 to AQ, grant number 2016–05195 to MIL) and the Stiftelsen för Zoologisk Forskning (to JCS).
Author Contributions
AQ conceived the study. AQ and JCS collected data. JCS performed paternal and all statistical analysis. All authors contributed to the analytical approach and interpretation of the data. All authors wrote the manuscript and approved the final version.
Data Archiving
All animal monitoring and handling were in accordance with Swedish laws, following the ethical standards of Linköping's committee of ethical animal research (Linköpings djurförsöketiska nämnd).
LITERATURE CITED
- Akçay, E., and Roughgarden J.. 2007. Extra‐pair paternity in birds: review of the genetic benefits . Evol. Ecol. Res. 9 :855–868. https://repository.upenn.edu/biology_papers/12 [ Google Scholar ]
- Alatalo, R. V., Gustafsson L., and Lundberg A.. 1986. Do females prefer older males in polygynous bird species? Am. Nat. 127 :241–245. [ Google Scholar ]
- Alund, M., Immler S., Rice A. M., and Qvarnstrom A.. 2013. Low fertility of wild hybrid male flycatchers despite recent divergence . Biol. Lett. 9 20130169. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Alund, M., Schmiterlöw S. P., McFarlane S. E., and Qvarnström A.. 2018. Optimal sperm length for high siring success depends on forehead patch size in collared flycatchers . Behav. Ecol. 29 :1436–1443. [ Google Scholar ]
- Andersson, M. B.1994. Sexual selection . Princeton Univ. Press, Princeton, NJ. [ Google Scholar ]
- Arnqvist, G., and Kirkpatrick M.. 2005. The evolution of infidelity in socially monogamous passerines: the strength of direct and indirect selection on extrapair copulation behavior in females . Am. Nat. 165 :S26–S37. [ PubMed ] [ Google Scholar ]
- Auld, J. R., Perrins C. M., and Charmantier A.. 2013. Who wears the pants in a mute swan pair? Deciphering the effects of male and female age and identity on breeding success . J. Anim. Ecol. 82 :826–835. [ PubMed ] [ Google Scholar ]
- Bates, D., Mächler M., Bolker B. M., and Walker S. C.. 2015. Fitting linear mixed‐effects models using lme4 . J. Stat. Softw. 67 :1–48. [ Google Scholar ]
- Beck, C. W., and Promislow D. E. L.. 2007. Evolution of female preference for younger males . PLoS ONE 2 e939. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bonduriansky, R., and Brassil C. E.. 2002. Rapid and costly ageing in wild male flies . Nature 420 :377. [ PubMed ] [ Google Scholar ]
- Bouwhuis, S., and Vedder O.. 2017. Avian escape artists? Patterns, processes and costs of senescence in wild birds. In Shefferson R. P., Jones O. R., & Salguero‐Gomez R. (Eds.), The evolution of senescence in the tree of life (pp. 156–174). Cambridge Univ. Press, Cambridge, U.K. [ Google Scholar ]
- Bouwhuis, S., Sheldon B. C., Verhulst S., and Charmantier A.. 2009. Great tits growing old: selective disappearance and the partitioning of senescence to stages within the breeding cycle . Proc. R. Soc. B Biol. Sci. 276 :2769–2777. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bouwman, K. M., van Dijk R. E., Wijmenga J. J., and Komdeur J.. 2007. Older male reed buntings are more successful at gaining extrapair fertilizations . Anim. Behav. 73 :15–27. [ Google Scholar ]
- Brengdahl, M. I., Kimber C. M., Elias P., Thompson J., and Friberg U.. 2020. Deleterious mutations show increasing negative effects with age in Drosophila melanogaster . BMC Biol. 18 :128. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Brooks, M. E., Kristensen K., van Benthem K. J., Magnusson A., Berg C. W., Nielsen A., et al. 2017. glmmTMB balances speed and flexibility among packages for zero‐inflated generalized linear mixed modeling . R J. 9 :378–400. [ Google Scholar ]
- Brooks, M. E., Kristensen K., Darrigo M. R., Rubim P., Uriarte M., Bruna E., et al. 2019. Statistical modeling of patterns in annual reproductive rates . Ecology 100 e02706. [ PubMed ] [ Google Scholar ]
- Brooks, R., and Kemp D. J.. 2001. Can older males deliver the good genes? Trends Ecol. Evol. 16 :308–313. [ PubMed ] [ Google Scholar ]
- Canal, D., Jovani R., and Potti J.. 2012. Male decisions or female accessibility? Spatiotemporal patterns of extra pair paternity in a songbird . Behav. Ecol. 23 :1146–1153. [ Google Scholar ]
- Charmantier, A., Blondel J., Perret P., and Lambrechts M. M.. 2004. Do extra‐pair paternities provide genetic benefits for female blue tits Parus caeruleus ? J. Avian Biol. 35 :524–532. [ Google Scholar ]
- Cleasby, I. R., and Nakagawa S.. 2012. The influence of male age on within‐pair and extra‐pair paternity in passerines . Ibis 154 :318–324. [ Google Scholar ]
- Clutton‐Brock, T. H.1984. Reproductive effort and terminal investment in iteroparous animals . Am. Nat. 123 :212–229. [ Google Scholar ]
- Comfort, A.2011. The biology of senescence . Rinehart, New York. [ Google Scholar ]
- Conner, J.1989. Older males have higher insemination success in a beetle . Anim. Behav. 38 :503–509. [ Google Scholar ]
- Côté, I. M., and Hunte W.. 1993. Female redlip blennies prefer older males . Anim. Behav. 46 :203–205. [ Google Scholar ]
- Dean, R., Cornwallis C. K., Løvlie H., Worley K., Richardson D. S., and Pizzari T.. 2010. Male reproductive senescence causes potential for sexual conflict over mating . Curr. Biol. 20 :1192–1196. [ PubMed ] [ Google Scholar ]
- Dickinson, J.2001. Extrapair copulations in western bluebirds ( Sialia mexicana ): female receptivity favors older males . Behav. Ecol. Sociobiol. 50 :423–429. [ Google Scholar ]
- Dupont, S. M., Barbraud C., Chastel O., Delord K., Ruault S., Weimerskirch H., et al. 2018. Young parents produce offspring with short telomeres: a study in a long‐lived bird, the Black‐browed Albatross ( Thalassarche melanophrys ) . PLoS ONE 13 e0193526. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Edme, A., Munclinger P., and Krist M.. 2016. Female collared flycatchers choose neighbouring and older extra‐pair partners from the pool of males around their nests . J. Avian Biol. 47 :552–562. [ Google Scholar ]
- Evans, S. R., Gustafsson L., and Sheldon B. C.. 2011. Divergent patterns of age‐dependence in ornamental and reproductive traits in the collared flycatcher . Evolution 65 :1623–1636. [ PubMed ] [ Google Scholar ]
- Finch, C. E.1998. Variations in senescence and longevity include the possibility of negligible senescence . J. Gerontol. A Biol. Sci. Med. Sci. 53 :B235–B239. [ PubMed ] [ Google Scholar ]
- Foerster, K., Delhey K., Johnsen A., Lifjeld J. T., and Kempenaers B.. 2003. Females increase offspring heterozygosity and fitness through extra‐pair matings . Nature 425 :714–717. [ PubMed ] [ Google Scholar ]
- Forslund, P., and Pärt T.. 1995. Age and reproduction in birds — hypotheses and tests . Trends Ecol. Evol. 10 :374–378. [ PubMed ] [ Google Scholar ]
- Forstmeier, W., Nakagawa S., Griffith S. C., and Kempenaers B.. 2014. Female extra‐pair mating: adaptation or genetic constraint? Trends Ecol. Evol. 29 :456–464. [ PubMed ] [ Google Scholar ]
- Froy, H., Sparks A. M., Watt K., Sinclair R., Bach F., Pilkington J. G., et al. 2019. Senescence in immunity against helminth parasites predicts adult mortality in a wild mammal . Science 365 :1296–1298. [ PubMed ] [ Google Scholar ]
- Gaillard, J. M., and Lemaître J. F.. 2020. An integrative view of senescence in nature . Funct. Ecol. 34 :4–16. [ Google Scholar ]
- García‐Palomares, S., Navarro S., Pertusa J. F., Hermenegildo C., García‐Pérez M. A., Rausell F., et al. 2009. Delayed fatherhood in mice decreases reproductive fitness and longevity of offspring . Biol. Reprod. 80 :343–349. [ PubMed ] [ Google Scholar ]
- Gavrilov, L. A., and Gavrilova N. S.. 1997. Parental age at conception and offspring longevity . Rev. Clin. Gerontol. 7 :5–12. [ Google Scholar ]
- Grant, B. R., and Grant P. R.. 1987. Mate choice in Darwin's finches . Biol. J. Linn. Soc. 32 :247–270. [ Google Scholar ]
- Griffin, A. S., Alonzo S. H., and Cornwallis C. K.. 2013. Why do cuckolded males provide paternal care? PLoS Biol. 11 e1001520. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Griffith, S. C., Owens I. P. F., and Thuman K. A.. 2002. Extra pair paternity in birds: a review of interspecific variation and adaptive function . Mol. Ecol. 11 :2195–2212. [ PubMed ] [ Google Scholar ]
- Gustafsson, L., and Pärt T.. 1990. Acceleration of senescence in the collared flycatcher Ficedula albicollis by reproductive costs . Nature 347 ( 6290 ):279–281. 10.1038/347279a0 [ CrossRef ] [ Google Scholar ]
- Hamilton, W. D.1966. The moulding of senescence by natural selection . J. Theor. Biol. 12 :12–45. [ PubMed ] [ Google Scholar ]
- Hsu, Y. H., Schroeder J., Winney I., Burke T., and Nakagawa S.. 2014. Costly infidelity: low lifetime fitness of extra‐pair offspring in a passerine bird . Evolution 68 :2873–2884. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jennions, M. D., and Petrie M.. 2000. Why do females mate multiply? A review of the genetic benefits . Biol. Rev. 75 :21–64. [ PubMed ] [ Google Scholar ]
- Jones, A. G., Small C. M., Paczolt K. A., and Ratterman N. L.. 2010. A practical guide to methods of parentage analysis . In Molecular Ecology Resources 10 ( 1 ):6–30. 10.1111/j.1755-0998.2009.02778.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kalinowski, S. T., Taper M. L., and Marshall T. C.. 2007. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment . Mol. Ecol. 16 :1099–1106. [ PubMed ] [ Google Scholar ]
- Källander, H., and Smith H. G.. 1990. Manipulation of the brood size of pied flycatchers. Pp. 257–268 in Blondel J., Gosler A., Lebreton J. D., and McCleery R., eds. Population biology of passerine birds . Springer, Berlin. [ Google Scholar ]
- Kemkes‐Grottenthaler, A.2004. Parental effects on offspring longevity ‐ evidence from 17th to 19th century reproductive histories . Ann. Hum. Biol. 31 :139–158. [ PubMed ] [ Google Scholar ]
- Kempenaers, B., and Dhondt A.. 1993. Why do females engage in extra‐pair copulations? A review of hypotheses and their predictions . Belgian J. Zool. 123 :93–103. [ Google Scholar ]
- Kempenaers, B., and Sheldon B. C.. 1996. Why do male birds not discriminate between their own and extra‐pair offspring? Anim. Behav. 51 :1165–1173. [ Google Scholar ]
- Kempenaers, B., Verheyen G. R., Van Den Broeck M., Burke T., Van Broeckhoven C., and Dhondt A.. 1992. Extra‐pair paternity results from female preference for high‐quality males in the blue tit . Nature 357 :494–496. [ Google Scholar ]
- Kiritani, K., and Kimura K.. 1967. Effects of parental age on the life cycle of the southern green stink bug, Nezara viridula L. (Heteroptera: Pentatomidae) . Appl. Entomol. Zool. 2 :69–78. [ Google Scholar ]
- Knief, U., and Forstmeier W.. 2020. Violating the normality assumption may be the lesser of two evils . bioRxiv . 10.1101/498931 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kokko, H.1998. Good genes, old age and life‐history trade‐offs . Evol. Ecol. 12 :739–750. [ Google Scholar ]
- Kokko, H., and Lindstrom J.. 1996. Evolution of female preference for old mates . Proc. R. Soc. B Biol. Sci. 263 :1533–1538. [ Google Scholar ]
- Kong, A., Frigge M. L., Masson G., Besenbacher S., Sulem P., Magnusson G., et al. 2012. Rate of de novo mutations and the importance of father's age to disease risk . Nature 488 :471–475. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lansing, A. I.1947. A transmissible, cumulative, and reversible factor in aging . J. Gerontol. 2 :228–239. [ PubMed ] [ Google Scholar ]
- Lemaître, J. F., and Gaillard J. M.. 2017. Reproductive senescence: new perspectives in the wild . Biol. Rev. 92 :2182–2199. [ PubMed ] [ Google Scholar ]
- Lifjeld, J. T., Kleven O., Jacobsen F., McGraw K. J., Safran R. J., and Robertson R. J.. 2011. Age before beauty? Relationships between fertilization success and age‐dependent ornaments in barn swallows . Behav. Ecol. Sociobiol. 65 :1687–1697. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lind, M. I., Berg E. C., Alavioon G., and Maklakov A. A.. 2015. Evolution of differential maternal age effects on male and female offspring development and longevity . Funct. Ecol. 29 :104–110. [ Google Scholar ]
- Marshall, T. C., Slate J., Kruuk L. E. B., and Pemberton J. M.. 1998. Statistical confidence for likelihood‐based paternity inference in natural populations . Mol. Ecol. 7 :639–655. [ PubMed ] [ Google Scholar ]
- Medawar, P. B.1951. An unsolved problem of biology . H. K. Lewis & Co Ltd, Lond. [ Google Scholar ]
- Michálková, R., Tomášek O., Adámková M., Kreisinger J., and Albrecht T.. 2019. Extra‐pair paternity patterns in European barn swallows Hirundo rustica are best explained by male and female age rather than male ornamentation . Behav. Ecol. Sociobiol. 73 :119. [ Google Scholar ]
- Monaghan, P., Maklakov A. A., and Metcalfe N. B.. 2020. Intergenerational transfer of ageing: parental age and offspring lifespan . Trends Ecol. Evol. 35 :927–937. [ PubMed ] [ Google Scholar ]
- Newton, I., and Rothery P.. 2002. Age‐related trends in different aspects of the breeding performance of individual female Eurasian sparrowhawks ( Accipiter nisus ) . Auk 119 :735–748. [ Google Scholar ]
- Noguera, J. C., Metcalfe N. B., and Monaghan P.. 2018. Experimental demonstration that offspring fathered by old males have shorter telomeres and reduced lifespans . Proc. R. Soc. B Biol. Sci. 285 20180268. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nussey, D. H., Froy H., Lemaitre J. F., Gaillard J. M., and Austad S. N.. 2013. Senescence in natural populations of animals: widespread evidence and its implications for bio‐gerontology . Ageing Res. Rev. 12 :214–225. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- O'Brian, D. M.1961. Effects of parental age on the life cycle of Drosophila melanogaster . Ann. Entomol. Soc. Am. 54 :412–416. [ Google Scholar ]
- Pizzari, T., Dean R., Pacey A., Moore H., and Bonsall M. B.. 2008. The evolutionary ecology of pre‐ and post‐meiotic sperm senescence . Trends Ecol. Evol. 23 :131–140. [ PubMed ] [ Google Scholar ]
- Preston, B. T., Jalme M. S., Hingrat Y., Lacroix F., and Sorci G.. 2011. Sexually extravagant males age more rapidly . Ecol. Lett. 14 :1017–1024. [ PubMed ] [ Google Scholar ]
- Priest, N. K., Mackowiak B., and Promislow D. E. L.. 2002. The role of parental age effects on the evolution of aging . Evolution 56 :927–935. [ PubMed ] [ Google Scholar ]
- Qvarnström, A., Rice A. M., and Ellegren H.. 2010. Speciation in Ficedula flycatchers . Philos. Trans. R. Soc. B Biol. Sci. 365 :1841–1852. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- R Core Team . 2019. A language and environment for statistical computing . Available via https://www.r‐project.org/ .
- Rebke, M., Coulson T., Becker P. H., and Vaupel J. W.. 2010. Reproductive improvement and senescence in a long‐lived bird . Proc. Natl. Acad. Sci. USA 107 :7841–7846. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rockstein, M.1957. Longevity of male and female house flies . J. Gerontol. 12 :253–256. [ PubMed ] [ Google Scholar ]
- Rodríguez‐Muñoz, R., Hopwood P., Fisher D., Skicko I., Tucker R., Woodcock K., et al. 2019. Older males attract more females but get fewer matings in a wild field cricket . Anim. Behav. 153 :1–14. [ Google Scholar ]
- Sardell, R. J., Arcese P., Keller L. F., and Reid J. M.. 2011. Sex‐specific differential survival of extra‐pair and within‐pair offspring in song sparrows, Melospiza melodia . Proc. R. Soc. B Biol. Sci. 278 :3251–3259. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Schroeder, J., Nakagawa S., Rees M., Mannarelli M. E., and Burke T.. 2015. Reduced fitness in progeny from old parents in a natural population . Proc. Natl. Acad. Sci. USA 112 :4021–4025. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shefferson, R. P., Jones O. R., and Salguero‐Gómez R.. 2017. The evolution of senescence in the tree of life . Cambridge Univ. Press, Cambridge, U.K. [ Google Scholar ]
- Sheldon, B. C.1993. Sexually transmitted disease in birds: occurrence and evolutionary significance . Philos. Trans. R. Soc. London, B 339 :491–497. [ PubMed ] [ Google Scholar ]
- Shirihai, H., and Svensson L.. 2018. Handbook of Western palearctic birds, Volume 2 . Passerines: flycatchers to buntings . 1st ed.Bloomsbury Publishing, Lond. [ Google Scholar ]
- Smeds, L., Qvarnström A., and Ellegren H.. 2016. Direct estimate of the rate of germline mutation in a bird . Genome Res. 26 :1211–1218. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Takagi, M.2003. Different effects of age on reproductive performance in relation to breeding stage in Bull‐headed Shrikes . J. Ethol. 21 :9–14. [ Google Scholar ]
- Torres, R., Drummond H., and Velando A.. 2011. Parental age and lifespan influence offspring recruitment: a long‐term study in a seabird . PLoS ONE 6 e27245. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tracey, K. M.1958. Effects of parental age on the life cycle of the mealworm, Tenebrio molitor Linnaeus . Ann. Entomol. Soc. Am. 51 :429–432. [ Google Scholar ]
- Trivers, R. L.1972. Parental investment and sexual selection. Pp. 136–179 in Campbell B., ed. Sexual selection and the descent of man, 1871–1971 . Aldine, Chicago. [ Google Scholar ]
- Velando, A., Noguera J. C., Drummond H., and Torres R.. 2011. Senescent males carry premutagenic lesions in sperm . J. Evol. Biol. 24 :693–697. [ PubMed ] [ Google Scholar ]
- Weatherhead, P. J.1984. Mate choice in avian polygyny: why do females prefer older males? Am. Nat. 123 :873–875. [ Google Scholar ]
- Westneat, D. F., and Stewart I. R. K.. 2003. Extra‐pair paternity in birds: causes, correlates, and conflict . Annu. Rev. Ecol. Evol. Syst. 34 :365–396. [ Google Scholar ]
- Wickham, H.2016. ggplot2: elegant graphics for data analysis . Springer‐Verlag, New York. Available via https://ggplot2.tidyverse.org . [ Google Scholar ]
- Zhang, H., Vedder O., Becker P. H., and Bouwhuis S.. 2015. Age‐dependent trait variation: the relative contribution of within‐individual change, selective appearance and disappearance in a long‐lived seabird . J. Anim. Ecol. 84 :797–807. [ PubMed ] [ Google Scholar ]

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
4 Chapter 4: Sexual Selection
Mason Tedeschi; Lisa Limeri; and Anastasia Chouvalova
Learning Objectives
By the end of this section, students will be able to:
4.1 Compare and contrast artificial, natural, and sexual selection.
4.2 Describe the relationship between sexual selection and sexual dimorphism
4.3 Contrast intrasexual selection and intersexual selection.
4.4 Describe the hypothesized mechanisms of intersexual selection (sexy sons, good genes, handicap, and direct benefits hypotheses).
Sexual Selection
When discussing natural selection and adaptations, we often focus on traits that help an organism survive – surviving cold winters, avoiding predators, obtaining food, etc. But what about traits that do not directly aid in survival? Or better yet, what about traits that actually hinder survival? Consider the elaborate train feathers of a peacock (Fig 4.1). These feathers, while visually stunning, make it nearly impossible for an individual to fly. Not only that, but these feathers also make it easier for a predator to spot a peacock from a distance and they can also be used by a predator to apprehend a peacock so it cannot escape. Rather than helping a peacock survive, their tail feathers actually make it more difficult to escape predation. So does such a trait evolve?
This question can be answered by viewing this trait not as aiding in survival, but as a trait that aids in reproduction. Exaggerated traits, like the tail feathers of a peacock, are used to attract females for mating, ensuring that a male passes his genes on to the next generation.
Elaborate peacock trains (Fig 4.1) represent an example of sexual selection. Sexual selection is a “special case” of natural selection in which individuals compete for mates in order to pass on their genes to future generations. The peacock’s train is used to attract females for mating, ensuring that a male passes his genes on to the next generation. In essence, sexual selection acts on an individual’s ability to successfully reproduce- even if that ability comes at a cost to survival.

Darwin identified a special case of natural selection that he called sexual selection. Sexual selection acts on traits that affect an individual’s ability to attract mates and thus produce offspring. Sexual selection often leads to the evolution of dramatic traits that often appear maladaptive in terms of survival but persist because they give their owners greater reproductive success. Sexual selection can be so strong that it selects traits that are actually detrimental to the individual’s survival.
Sexual selection occurs through two mechanisms: intrasexual selection of mates, also known as mate competition and [latex]null[/latex] and intersexual selection , also known as mate selection/choice.
Mechanisms of Sexual Selection
Sexual selection, the process through which individuals compete for mates, primarily takes two forms: intersexual selection and intrasexual selection. Intersexual selection, often referred to as mate choice, involves individuals of one sex choosing among members of the opposite sex based on the attractiveness of certain traits that those individuals possess. Intrasexual selection, also called mate competition, involves one sex competing with members of the same sex for access to mates.
Sexual selection results from competition over mates. Which sex competes for mates and which sex is choosy? In general, the sex that invests more resources in offspring is the one that will be more choosy, because they have more to lose by making a bad choice about a mate. This investment difference begins with gametes. Females produce eggs, which are much larger in size (and thus more costly to produce) and males produce sperm, which are small and energetically cheap (Fig 4.2). This difference in gamete investment is known as anisogamy .
This early investment difference resulting from anisogamy causes the general trend that in most species, females are the choosy sex and males are the sex competing for female to mate with. Another reason why females are typically the choosy sex has to do with the level of investment in offspring care, known as parental care. For example, following sexual reproduction and fertilization , most mammals develop within the body of their mothers. The developing offspring of most mammals then get their food and oxygen from the blood of their mothers through a spongy organ called the placenta. Even marsupial offspring, though not fully developed when born, are usually carried by their mothers in a pouch until they are able to walk on their own.
However, it is important to note that not all species follow this trend. In some animals, males provide a great deal of parental care to their offspring. For example, in emperor penguins each female produces a single egg. She then transfers the egg to her male mate and leaves to spend the winter in the open ocean in search of food and other resources. During the Antarctic winter, which lasts about four months, male emperor penguins huddle in groups, guarding their eggs and keeping them warm. Another example is seahorses, where males incubate eggs and care for young in a pouch. When they mate, a female deposits eggs into the male’s pouch and leaves, providing no further parental care. Thus, male seahorses invest far more resources into offspring than females do, and it’s the males who are the choosy sex and females compete for male mates.
Question #1
Which sex is typically the choosy sex? The sex that…
A. invests more resources into offspring. B. produces a larger number of gametes. C. is larger in size. D. has more elaborate coloration and ornamentation.
Intrasexual Selection: Competition
Intrasexual (within sex) competition takes the form of conflicts between members of the same sex competing for mates. These intrasexual competitions are often ritualized, but may also pose significant threats to the competitors’ survival. Intrasexual selection involves mating displays and aggressive mating rituals such as rams butting heads—the winner of these battles is the one that is able to mate. Many of these rituals use up considerable energy but result in the selection of the healthiest, strongest, and/or most dominant individuals for mating. Sometimes the competition is for territory, with prospective mates more likely to mate with individuals with higher quality territories.
Intersexual Selection: Mate Choice
Intersexual (between sexes) selection occurs when members of the choosy sex select a mate based on a trait or suite of traits, such as feather colors, the performance of a mating dance, or the building of an elaborate structure. There are several, non-exclusive models of how and why mating preferences evolve. Broadly, there are two types of fitness benefits that drive mate choice: direct benefits and indirect benefits.
Direct Benefits
Direct benefits increase the fitness of choosy individuals through material resources. Members of the competing sex will sometimes provide members of the choosy sex with a food gift before mating. These resources, called nuptial gifts , provide nourishment to prospective mates that they may not otherwise get. For example, male great grey shrikes- a predatory bird- will present prey items (e.g., rodents, other birds, lizards) to females immediately before mating. A female great grey shrike will choose a mate according to the size of the prey item presented to her. Nuptial gifts are observed in many insects and spiders where males present nuptial food gifts to females in the hopes that she will choose to mate with him.
In extreme cases, the competing sex will even sacrifice parts or all of themselves to members of the choosy sex. For example, in some species of ground crickets, females receive a nuptial gift by chewing on a specialized spur structure on the male hind tibia (i.e. leg) while mating. Most predatory species of preying mantids practice a type of extreme nuptial feeding known as sexual cannibalism, in which a female will eat her mate prior to, during, or after copulation. Most often, a female mantid will begin feeding by biting off the head of a male, as they would with regular prey. Because copulatory movement in males is controlled by nerves in the abdomen, not the head, removal of a male’s head does not affect mating, sperm transfer, or proper fertilization. The reason for sexual cannibalism has been heavily debated. Experiments show that females on low quality diets are more likely to cannibalize her mates, compared with females given high quality diets. Thus, nuptial gifts are typically considered a direct benefit, because they enhance a female’s survival and reproduction. Some suggest that males that submit to females and are cannibalized gain a selective advantage by producing higher quality offspring. In any event, this type of sexual behavior is quite rare because the costs are often assumed to out-weigh the benefits, particularly for males.
Question #2
What are nuptial gifts?
A. A form of indirect benefits to the choosy sex. B. Resources typically provided by the competitive sex to the choosy sex. C. Resources typically provided by the choosy sex to the competitive sex. D. A form of intrasexual competition.
Indirect Benefits
Indirect benefits do not directly benefit the choosing individual, but rather, indirectly benefit them by increasing the fitness of their offspring. There are multiple hypothesized mechanisms of indirect benefits. Three of the most common and well-supported are the Sexy Sons, Good Genes and Handicap Hypotheses.
The Sexy Sons Hypothesis: The sexy sons hypothesis postulates that members of the choosy sex who select mates with attractive traits will benefit by producing offspring who also possess the attractive traits and thus will be reproductively successful. As such, the attractive (sexy) sons will be more likely to attract females, and thus the choosy female’s genes will continue to spread.
The Good Genes Hypothesis. The good genes hypothesis posits that males develop impressive ornaments to show off their efficient metabolism or effectiveness at obtaining food, or their ability to fight disease. Females then choose males with the most impressive traits because it signals their genetic superiority, which they will then pass on to their offspring.
The Handicap Hypothesis. Exaggerated traits, such as the Peacock’s train, that exist to attract mates can reduce the owner’s survival. Why would females prefer to mate with males that have traits that reduce survival? The Handicap hypothesis poses that a male with a large, elaborate train must be especially strong and fast to escape predators while having a handicap, and thus makes an ideal mate.
In both the handicap principle and the good genes hypothesis, the trait is an honest signal of the males’ quality, thus giving females a way to find the fittest mates— males that will pass the best genes to their offspring.
Question #3
Individuals with a large, elaborate traits are ideal mates because they must be especially strong and fast to escape predators despite having such an unwieldy trait. This describes which hypothesized mechanism of sexual selection?
A. Sexy Sons hypothesis B. Good genes hypothesis C. Handicap hypothesis D. Direct benefits hypothesis
Post-Copulatory Sexual Selection
Sexual selection does not come to a halt after animals have mated. If a female mates with multiple males, such that sperm from several individuals remains in her body for an extended period of time, sexual selection can continue long after a male and female have mated. Just like with sexual selection before mating, post-copulatory sexual selection occurs in two forms: cryptic female choice – an extension of mate choice; and sperm competition – an extension of mate competition.
Cryptic female choice
Using physical or chemical mechanisms, females can bias paternity and affect male reproductive success by choosing whether certain sperm are successful in fertilizing their eggs. The term “cryptic” is used to describe an internal, and thereby hidden, process that females employ to choose the sperm from males that they prefer. The research suggests that cryptic female choice is likely a consequence of sexual conflict regarding the frequency and mode of mating. While males increase their fitness by successfully mating with as many females as possible, and thereby fertilizing as many eggs as possible from different females, females can incur fitness costs associated with mating with many males. Cryptic female choice reduces these costs by allowing females to mate multiply (as males wish to do), but then only select sperm from the favorable males afterwards. Here, females benefit by influencing paternity in favor of the males they prefer- possibly because they provide some direct or indirect benefit to her.
Sperm competition
Sperm competition, an extension of intrasexual competition, is the process by which sperm from two or more males compete for fertilization of a female’s eggs. Sperm competition is often compared to having tickets in a raffle: a male has a better chance of having their ticket drawn (i.e. fathering offspring) if he has more tickets in the raffle (i.e. he releases more sperm per ejaculate into a female’s reproductive tract). Alternatively, males may not release more sperm, but instead they evolve faster, more motile sperm that allow an individual’s sperm to reach a female’s eggs first. Among the best evidence we have for sperm competition is the evolution of longer sperm tails in animals that have multiple partners (Fig 4.3).
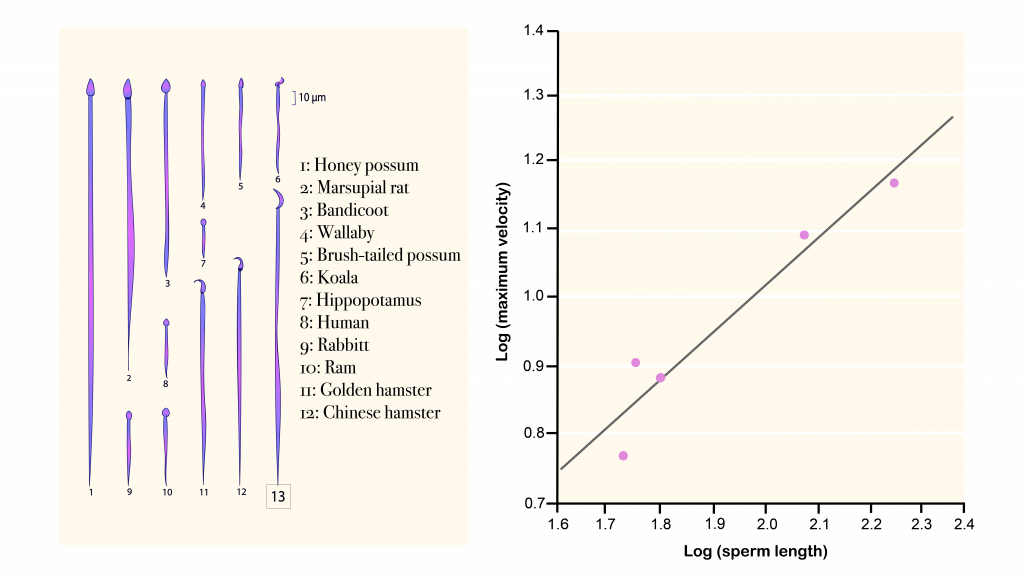
Sexual Dimorphism
Just as with natural selection, sexual selection can lead to changes in the genetic composition of a population that can be seen through physical changes to the way an organism looks. Both mate choice and mate competition can lead to the evolution of elaborate traits, termed secondary sexual traits, (secondary because they are not the primary traits involved in sexual reproduction or sperm transfer). Secondary sexual traits aid in sexual reproduction by improving an individual’s ability to obtain mates. Typically, one sex possesses an elaborate secondary sexual trait or traits, but the other sex does not, a condition called sexual dimorphism (Fig 4.4). Both mate choice and mate competition can involve the evolution of secondary traits that are sexually dimorphic.

Traits that are subject to selection via mate choice are referred to as ornaments or sexual signals . Ornaments can involve different signal modalities, including visual signals like the bright colors of many birds and butterflies; olfactory (i.e. chemical) signals like the scent patches that many mammal species use to attract mates; auditory signals used by chorusing frogs and some insects like crickets; or even tactile signals like the vibratory signals used by some spiders when they tap their legs on the surface of a leaf to attract mates. Sexual signals can also involve multiple signal modalities. For example, male jumping spiders will often use both visual and vibratory signals when trying to attract females for mating.
Question #4
Which of the following correctly describes sexual dimorphism?
A. An evolutionary force that improves reproductive success in both males and females. B. Traits related to sexual reproduction that are present in both males and females. C. Traits that improve an individual’s ability to survive. D. Differences between males and females within a species resulting from sexual selection.
Question #5
What is the primary difference between natural and sexual selection?
A. Natural selection promotes traits enhancing the likelihood of reproduction whereas sexual selection promotes traits enhancing fitness. B. Natural selection promotes traits enhancing survival whereas sexual selection promotes traits enhancing the likelihood of reproduction. C. While natural selection occurs in individuals, sexual selection occurs on a population level. D. There is no difference between them.
Adapted from Various Authors, Introductory Biology: Evolutionary and Ecological Perspectives. University of Minnesota. Retrieved from https://pressbooks.umn.edu/introbio/
reproduction of individuals with favorable genetic traits that survive environmental change because of those traits, leading to evolutionary change
competition between members of the same sex for a mate
selection of a desirable mate of the opposite sex, contrast to intrasexual selection.
a type of sexual reproduction where male and female organisms produce gametes of unequal size
the union of two haploid cells from two individual organisms of two different sexes.
the act of a bird sitting on their eggs to keep the egg warm and to eventually incite hatching
in sexual reproduction, these are valuable nutritional resources provided by one of the partners to the other partner
in mate selection, this is the act of the female mating with multiple males but latently selecting which mate will fertilize her eggs, without the male knowing
in sexual reproduction, this occurs when sperm from multiple males rival for the fertilization of one female egg
phenotypic difference between a population's males and females
characteristics of organisms that decorate the organism, rather than provide a useful functionality
synonymous with ornaments, that is, characteristics of organisms that decorate the organism, rather than provide a useful functionality
Introductory Biology 2 Copyright © 2023 by Mason Tedeschi; Lisa Limeri; and Anastasia Chouvalova is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.
Share This Book
The Good Genes Hypothesis
- Living reference work entry
- First Online: 13 January 2023
- Cite this living reference work entry

- Urszula M. Marcinkowska 2
408 Accesses
23 Altmetric
Honest signalling
The good genes hypothesis proposes that the characteristics preferred by females are a signal of males’ ability to pass on genes which will increase the survival and reproductive success of the offspring sired with a male possessing them.

Introduction
The good genes hypothesis (GGH) was formulated by evolutionary biologist W.D. Hamilton and behavioral ecologist M. Zuk ( 1982 ). It proposes that the characteristics preferred by females are a signal of males’ ability to pass on genes (coding that certain characteristic) which will increase the survival and reproductive success of the offspring sired with a male possessing them. In such case, sexual selection would lead to the increase of the proportion of genes (coding the attractive trait) that are beneficial under prevailing conditions. In other words, good genes increase fitness of the offspring. In an evolutionary and ecological understanding individual’s fitness depends on how many copies of own...
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Institutional subscriptions
Achorn, A. M., & Rosenthal, G. G. (2019). It’s not about him: Mismeasuring ‘good genes’ in sexual selection. Trends in Ecology & Evolution, 35 (3), 206–219. https://doi.org/10.1016/j.tree.2019.11.007
Boothroyd, L. G., Scott, I., Gray, A. W., Coombes, C. I., & Pound, N. (2013). Male facial masculinity as a Cue to health outcomes. Evolutionary Psychology, 11 (5), 1044–1058. https://doi.org/10.1177/147470491301100508
Article Google Scholar
Foo, Y. Z., Simmons, L. W., & Rhodes, G. (2017). Predictors of facial attractiveness and health in humans. Scientific Reports, 7 . https://doi.org/10.1038/srep39731
Hakkarainen, T. J., Krams, I., Coetzee, V., Skrinda, I., Kecko, S., Krama, T., … & Rantala, M. J. (2021). MHC class II heterozygosity associated with attractiveness of men and women. Evolutionary Psychology , 19 (1). https://doi.org/10.1177/1474704921991994 .
Hamilton, W. D., & Zuk, M. (1982). Heritable true fitness and bright birds: A role for parasites? Science, 218 (4570), 384–387. https://doi.org/10.1126/science.7123238
Marcinkowska, U. M., Ziomkiewicz, A., Kleisner, K., Galbarczyk, A., Klimek, M., Sancilio, A., … & Bribiescas, R. G. (2020). Oxidative stress as a hidden cost of attractiveness in postmenopausal women. Scientific Reports, 10 (1). https://doi.org/10.1038/s41598-020-76627-9 .
Prum, R. O. (2010). The Lande-Kirkpatrick mechanism is the null model of evolution by intersexual selection: Implications for meaning, honesty, and design in intersexual signals. Evolution, 64 (11), 3085–3100.
Rantala, M. J., Coetzee, V., Moore, F. R., Skrinda, I., Kecko, S., Krama, T., … & Krams, I. (2013). Adiposity, compared with masculinity, serves as a more valid Cue to immunocompetence in human mate choice. Proceedings of the Royal Society B-Biological Sciences, 280 (1751). https://doi.org/10.1098/rspb.2012.2495 .
Scott, I. M. L., Clark, A. P., Boothroyd, L. G., & Penton-Voak, I. S. (2013). Do Men’s faces really signal heritable immunocompetence? Behavioral Ecology, 24 (3), 579–589. https://doi.org/10.1093/beheco/ars092
Stephen, I. D., Hiew, V., Coetzee, V., Tiddeman, B. P., & Perrett, D. I. (2017). Facial shape analysis identifies valid Cues to aspects of physiological health in Caucasian, Asian, and African populations. Frontiers in Psychology, 8 . https://doi.org/10.3389/fpsyg.2017.01883
Weatherhead, P., & Robertson, R. (1979). Offspring quality and the polygyny threshold: “The sexy son hypothesis”. The American Naturalist, 113 (2), 201–208. https://doi.org/10.1086/283379
Zahavi, A. (1975). Mate selection—A selection for a handicap. Journal of Theoretical Biology, 53 (1), 205–214. https://doi.org/10.1016/0022-5193(75)90111-3
Zaidi, A. A., White, J. D., Mattern, B. C., Liebowitz, C. R., Puts, D. A., Claes, P., & Shriver, M. D. (2019). Facial masculinity does not appear to be a condition-dependent male ornament and does not reflect MHC heterozygosity in humans. Proceedings of the National Academy of Sciences of the United States of America, 116 (5), 1633–1638. https://doi.org/10.1073/pnas.1808659116
Download references
Author information
Authors and affiliations.
Institute of Public Health, Jagiellonian University Medical College, Cracow, Poland
Urszula M. Marcinkowska
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Department of Psychology, Oakland University, ROCHESTER, MN, USA
Todd K. Shackelford
Section Editor information
Department of Psychology, Oakland University, Rochester, MI, USA
Oakland University, Rochester, MI, USA
Gavin Vance
Department of Psychology, Oakland University, Rochester Hills, MI, USA
Madeleine K. Meehan
Rights and permissions
Reprints and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this entry
Cite this entry.
Marcinkowska, U.M. (2023). The Good Genes Hypothesis. In: Shackelford, T.K. (eds) Encyclopedia of Sexual Psychology and Behavior. Springer, Cham. https://doi.org/10.1007/978-3-031-08956-5_1081-1
Download citation
DOI : https://doi.org/10.1007/978-3-031-08956-5_1081-1
Received : 29 August 2022
Accepted : 29 August 2022
Published : 13 January 2023
Publisher Name : Springer, Cham
Print ISBN : 978-3-031-08956-5
Online ISBN : 978-3-031-08956-5
eBook Packages : Springer Reference Behavioral Science and Psychology Reference Module Humanities and Social Sciences Reference Module Business, Economics and Social Sciences
- Publish with us
Policies and ethics
- Find a journal
- Track your research
What are signs of good genes? A closer look at the genetics behind desirable traits
Hey there! As a genetics geek, I wanted to provide a more in-depth look at what scientists mean when they talk about "good genes." There‘s fascinating research on the genetic influences behind traits like athleticism, attractiveness, and intelligence. However, it‘s also a sensitive topic, as focusing too much on "good genes" promotes some unhealthy attitudes about human value. Still, understanding the complex interplay between genetics and personal traits offers insight into human nature. Let‘s explore further!
What physical traits reflect good genes?
Certain physical features and abilities signal reproductive fitness and can be influenced partly by genetics. But what specific genes might be behind these desirable traits? Let‘s break it down:
Athleticism and strength
Variants of a few key genes seem linked to elite athletic performance and strength potential:
- ACTN3 – This impacts fast twitch muscle fibers. The RR variant boosts power and sprint speed [1].
- ACE – Linked to endurance and cardiovascular performance. Carrying the I allele enhances stamina [2].
- MYLK – Regulates muscle contraction. Certain variants help with strength and speed [3].
- NOS3 – Boosts blood and oxygen flow to muscles. The T allele aids high intensity exercise [4].
Other muscular genes include PPARGC1A, a metabolism regulator, and NRF2 which enhances muscle repair [5]. Genetic differences underlie ~50% of variance in athletic ability [1].
Physical attractiveness
Features like facial symmetry, smooth skin, feminine/masculine proportions are tied to hotness, but what genes influence these traits?
- MC1R – Key pigment gene affecting hair color, skin tone, UV sensitivity [6].
- OCA2, SLC24A5 – Also influence melanin production and eye/skin color [7].
- EDA2R – Associated with facial masculinity/femininity and attractiveness ratings [8].
- PAX3 – Linked to facial symmetry according to twin studies [9].
But many criticize attractiveness genetic research as subjective and flawed. Perceptions of beauty clearly also involve cultural preferences too!
Intelligence and cognitive abilities
While IQ has genetic factors, teasing out exactly which genes influence intelligence has proven tricky:
- Twin studies estimate IQ is about 50-80% heritable [10].
- But no single "smart gene" exists; 700+ genes likely involved [11].
- APOE, CHRM2, COMT, BDNF, KIAA0319 may impact IQ and cognition [12].
- Education still plays a huge role in cognitive development!
Longevity and health
Robust immune function and overall vitality also indicate genetic quality. Relevant genes include:
- MHC gene complex – Controls immune fitness. MHC diversity boosts disease resistance [13].
- FOXO3 – Linked to longer lifespans according to centenarian studies [14].
- APOE – Influences heart health and cholesterol. The ε2 variant is protective [15].
But lifestyle habits clearly affect disease risk and life expectancy regardless of genetic variations. Genes merely influence probabilities.
How are genetic traits inherited?
Clearly genetics affect the expression of certain physical characteristics and abilities. But how exactly are these trait-influencing genes passed on?
Autosomal inheritance
Most genes are located on the 22 chromosome pairs that both males and females inherit from both parents. Different versions of these genes are called alleles, and some are dominant while others are recessive. Without getting too technical, the key point is that these non-sex-linked autosomal genes are passed down equally from mom and dad.
X-linked inheritance
The X and Y sex chromosomes determine gender. Females inherit two X chromosomes (one from each parent) while males get an X from mom and Y from dad. Genes on the X but not Y chromosome are X-linked and passed from fathers to daughters, or from mothers to both sons and daughters.
Mitochondrial inheritance
Mitochondria have their own distinct DNA passed solely down the maternal line. Since mitochondria provide energy for cells, their DNA can influence metabolism, aging, and other traits.
Y-linked inheritance
A small number of genes on the Y chromosome are passed directly from fathers to sons. These mainly influence male sexual characteristics.
So in summary, while some genes come just from mom or just from dad, the majority are inherited equally from both parents.
Can you judge genes just by looks?
This is a tricky question. While statistical associations exist between genotype, phenotype, and reproductive success, there‘s no foolproof way to assess multi-gene traits like attractiveness, health, and intelligence just by eyeballing someone‘s physical appearance.
Some people clearly win the genetic lottery in certain areas, but caution is needed to avoid reinforcing unhealthy biases around human worth being tied to genetic differences. Attractiveness is also very subjective and culturally-influenced.
In the end, we‘re all complex genetic mosaics. While studies can correlate single gene variants with specific traits, most characteristics result from a symphony of thousands of interacting genes. We should be wary of putting too much weight on singular genetic differences.
The takeaway
Genetics undeniably shape key aspects of our physical selves. However, genes merely predispose, while environment determines how tendencies manifest. No one gene makes us athletic, smart, or sexy! The expression of genetic potential requires the proper environment, opportunities, nutrition, training, and support systems.
Understanding the genetics behind human diversity and achievement is endlessly fascinating. But it‘s equally important we don‘t lose appreciation for each person‘s inherent worth and limitless capacity to learn, grow, and excel regardless of genetic variations. Hope this gives you food for thought! Let me know if you want to discuss more!
References:
How useful was this post?
Click on a star to rate it!
Average rating 0 / 5. Vote count: 0
No votes so far! Be the first to rate this post.
Share this:
You may like to read,.
- What does dice mean slang? An in-depth look at dice terminology and idioms
- What does ❤ 💜 mean from a girl?
- What is Theatrical Version DVD? A Film Buff‘s Perspective
- What is 13 BTS? An In-Depth Look at the Meaning Behind This Symbolic Number
- What is the Real Name for RPG? An In-Depth Look at This Iconic Weapon
- Why do gamers say bot?
- Why is it Called Scuderia? – The Meaning and History Behind the Iconic Term
- Is 20/50 Vision Bad? An In-Depth Look

How our Genes Lie: Honest and Dishonest Genes in Sexual Selection
Samuel Gascoigne Lake Forest College Lake Forest, Illinois 60045
Natural selection has been understood for over a hundred years, but the mechanisms by which it works have not been identified. One of the forms it takes is sexual selection. Sexual selection is an evolutionary pressure conferred by the opposite sex of the same species. The good genes hypothesis, posed in the 1930s, attempted to reconcile mate choice and the selection for certain traits. The selfish gene hypothesis, first declared in 1976, attempted to explain mate choice as well as our behaviors. With our modern understanding of genetics and DNA that holds the information, these two hypotheses can be applied to identify the honest and dishonest genes that are passed down generation after generation.
Introduction
While the molecular basis is unknown, the role of genes in heredity has been common knowledge since the 1930s. The good genes hypothesis proposed that individuals choose mates on certain phenotypes that pose a genetic advantage for the next generation. To apply this to humans, the attractiveness we prescribe to an individual reflects that individual’s genetic superiority. This is an incomplete model given that different people find different individuals attractive. A possible supplement to the model is the selfish gene hypothesis. The selfish gene hypothesis proposes that our mate choice is a result of our interest to pass our genetic code on to the next generation. A human application of this would be that we choose our mates based on that individual’s similarity to our own genome, thereby probabilistically increasing the longevity of our genes. Both hypotheses have merit but fail to independently explain the presence of honest and dishonest genes; but, together, honest and dishonest genes are made inevitable.
When discussing honest and dishonest genes, it is important to clarify that sexual selection works via the selection of phenotypes, not genotypes. Phenotypes are observable characteristics of an organism and these traits are influenced by the organism’s genes. Since genotypes cannot be seen, phenotypes are used for selection as they are an indirect manifestation of the organism’s genes and experiences. An example of this is if a male peacock has a mutation in a gene important in feather development. A result of this mutation is an upregulation of a hormone responsible for feather growth, thereby increasing the relative size of the peacock’s plumage. Since plumage size is a sexually selected trait in peacocks, the mutated peacock would be selected to a greater degree by hens than a wild-type peacock. The disparity in the selection of males with varying secondary-sexual traits, affected by variation in genotype, is the basis of sexual selection contributing to the evolution of the organism. Yet, while advantageous mutations account for an evolutionary change over the course of multiple generations, genes do not independently explain why a trait is sexually selected. For that, genes must manifest into phenotypes that suggest an evolutionary fitness of the organism. Unfortunately, the path from gene to trait is not without its own set of variation.
Environment plays a key role in phenotype and the development of a sexually selected trait. Genotype does not determine phenotype. Genes code for proteins. Phenotypes can be anything from horn allometry, as in Onthophagus beetles, all the way to call syllables, as in bush crickets. What links genes to corresponding proteins are, most often, a suite of developmental and cellular mechanisms. It is this developmental and cellular link between genes and phenotypes that explain the plasticity of phenotypes. Phenotypic plasticity is the phenomenon that multiple phenotypes can arise from a single genotype; one example is the case of monozygotic twins. Imagine a pair of monozygotic twins, Jim and Jeff. Jim frequents a gym regularly and ensures he maintains a balanced diet. Jeff, on the other hand, frequents a buffet regularly and ensures his freezer is filled with his favorite midnight snacks. It would not be a surprise to find out that Jim has a lower body mass index (BMI) than Jeff despite having the exact same genotype. There was nothing that predisposed Jeff to a higher BMI than Jim. What ensured his increased insulation was the environment he experienced. In summary, genes lead to phenotype, but the phenotype is also moderated by the environment.
Honest and Dishonest Genes
What determines the honesty of a gene is how accurately it depicts, via a phenotype, the fitness of the organism. From a sexual selection standpoint, the evolution of honest genes would be favorable. In addition, over the course of multiple generations, the scruples of sexually selective pressures would refine the accuracy of the honest genes as it would lead to a sensitive and more prosperous method of selection. This is a case of resolution. Imagine a doe is searching for a buck for mating. Two bucks, Skip and Skippy, appear with similar size and muscle proportion. The only way they differ are their coats and horns. Skip has a relatively dull coat and small horns relative to body size while Skippy has a full shiny coat with a large ornament rack relative to body size. Skippy is favorably selected by the doe for mating. In this situation, genes that synthesize androgenic hormones and genes involved in insulin/insulin-like growth factor signaling (IIS) are honest genes; androgens are positively correlated with hair development and IIS is positively correlated with rack size. This situation is favorable for the doe and Skippy as they both have an increased probability of passing their genes on to the next generation. Skip, on the other hand, draws the short straw in the field of honest genes. He, therefore, favors a dishonest set of cards.
Imagine the doe and two bucks scenario once again with Skippy still being the more sexually favored. Now include a mutant Skip. This Skip has a mutation in genes involved in IIS that increase IIS and, further downstream, upregulate androgenic hormones. Mutant Skip has a glossy coat and large rack relative to body size which catches the doe’s eyes to a greater extent than Skippy’s features. In turn, mutant Skip is selected instead of Skippy. While the genes involved were originally honest, the mutation in Skip’s genome made the environment insubstantial in affecting the final phenotype and thus lead to dishonest phenotypes. In this scenario, the doe and Skip win. However, the doe wins at a probability of smaller magnitude as the offspring may be less fit than the offspring of an honest mate. The disparity in winning magnitude offers logic toward a selective pressure in does to increase their resolution for sexual selection; the better the does are at discerning honest genes, the more likely their genes will survive to the following generations. However, the presence of dishonest genes in species either supports the idea that dishonest genes are inevitable with random mutation or, more poignantly, the disparity in winning magnitude due to potential filial unfitness is not enough to select against dishonest genes.
The Two Theories
The presence of honest and dishonest genes highlights a sexually divergent initiative in sexual selection. The sexual selector prefers honest genes, while the sexual selectee prefers either honest or dishonest genes – whatever offers an advantage in increasing gene longevity. In turn, a theory of sexual selection must reconcile both initiatives.
Together, the good genes hypothesis and the selfish gene hypothesis explain the honest-dishonest genes phenomenon. The good genes hypothesis explains honest genes. In the good genes hypothesis, genes that accurately illustrate the fitness of the organism are preferably selected above inaccurate genes. This theory explains the disparate winning advantage in dishonest selection and offers a selective pressure against dishonest genes. Evidence for this theory can be found in IIS-dependent traits. Almost all animals use IIS for cellular and physiological development. One of the reasons IIS is so conserved is that IIS is upregulated in high nutrition. Therefore, an organism in high nutrition has full or increased development due in part to high IIS. In turn, it makes sense that sexual selection would work on traits that are insulin sensitive, allowing greater selection accuracy of well fed mates. However, the presence of dishonest genes indicates a second manner of sexual selection at work.
The selfish gene hypothesis explains the presence and longevity of dishonest genes despite the selective pressure against them offered by the good genes hypothesis. In the selfish gene hypothesis, animal behavior, including mate choice, is explained to increase the longevity of an individual organism’s genes. An example of this would be the mutant described above, Skip. The mutant Skip illustrates the presence of a dishonest gene via a mutation. According to the selfish gene hypothesis, what offers a dishonest gene its longevity, in addition to the phenotypic advantage, is the fact that organisms with the same gene tend to mate with one another, thus increasing the probable lifetime of the dishonest gene.
Moller, A., & Alatalo, R. (1999). Good-genes effects in sexual selection. Proceedings of The Royal Society B: Biological Sciences, 266(1414), 85-91. http://dx.doi.org/10.1098/rspb.1999.0607
Neff, B., & Pitcher, T. (2004). Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Molecular Ecology, 14(1), 19-38. http://dx.doi.org/10.1111/ j.1365-294x.2004.02395.x
Chandler, C., Ofria, C., & Dworkin, I. (2012). RUNAWAY SEXUAL SELECTION LEADS TO GOOD GENES. Evolution, 67(1), 110- 119. http://dx.doi.org/10.1111/j.1558-5646.2012.01750.x
Dawkins, R. (2016). The extended selfish gene.
Emlen, D., Warren, I., Johns, A., Dworkin, I., & Lavine, L. (2012). A Mechanism of Extreme Growth and Reliable Signaling in Sexually Selected Ornaments and Weapons. Science, 337(6096), 860- 864. http://dx.doi.org/10.1126/science.1224286
Loyau, A., Jalme, M., & Sorci, G. (2005). Intra- and Intersexual Selection for Multiple Traits in the Peacock (Pavo cristatus). Ethology, 111(9), 810-820. http://dx.doi.org/10.1111/j.1439- 0310.2005.01091.x
Kodric-Brown, A., & Brown, J. (1984). Truth in Advertising: The Kinds of Traits Favored by Sexual Selection. The American Naturalist, 124(3), 309-323. http://dx.doi.org/10.1086/284275
Related Links:
- 036-037_Gascoigne_NewsViews (PDF)
- Request new password
You are here
Good gene hypothesis.
Good Gene Hypothesis : Courtship displays are ancient reproductive strategies that have been tightly associated with elaborate plumage. This elaboration is meant to convey superior fitness to females, however, the mechanism of this is unknown. The prevailing theory detailing this correlation is the “Good Gene Hypothesis”. This theory suggests that exotic or more elaborate plumage serves as a handicap by making them less optimal for flight or running as well as more easily identifiable by predators. However, if a male is able to survive with these additional stressors then a female can infer that they are superior in terms of stamina or survival skills. Furthermore, this theory suggests that plumage can reveal parasite load and hormonal levels as they have both been shown to affect brilliance or color scheme. Due to the high associated cost associated with developing, maintaining and surviving with such elaborate feather schemes, many species have developed post-breeding molting cycles to eliminate these handicaps during the non-breeding seasons.
- drosen's blog
Perfect Paragraphs
Each week, post your own Perfect Paragraph and comment on three Perfect Paragraphs . Suggest improvements. Don't just say "Looks good."
Recent comments
- a vs an 6 years 4 months ago
- introductory phrases 6 years 4 months ago
- what are SNP's 6 years 4 months ago
- proves 6 years 4 months ago
- broad term 6 years 4 months ago
- "As to be able to join this 6 years 4 months ago
- Who is 'they'? Try not to use 6 years 4 months ago
- Morrill should have two r's. 6 years 4 months ago
- explain humanities 6 years 4 months ago
- explanation 6 years 4 months ago
Blogs Updated This Week
- benjaminburk
- brettconnoll
- dfainkichen
- jonathanrubi

Good Genes for Mental Health
Recent research connects mental health to our dna..
Posted November 28, 2019 | Reviewed by Abigail Fagan

When we think of “good genes,” we tend to focus on beauty. But genetics are increasingly important in the study of mental health, including the origin of disorders like schizophrenia.
Schizophrenia is widely misunderstood; it derives from a combination of biological factors, as well as one’s immediate environment. Malnutrition, exposure to certain illnesses and things like childhood abuse all contribute to this chronic disease.
Schizophrenia is marked by both delusions and hallucinations. A hallucination means seeing things that aren’t there—like a ghost or spirit, usually in the form of a close relative who has passed. Hearing voices is part of having hallucinations, too. Delusions are essentially a fixed set of false beliefs, despite a plethora of evidence to the contrary.
In obsessive-compulsive disorder (OCD), delusions—not hallucinations—are connected to behaviors like excessive hand-washing or what may be viewed as superstitions, such as avoiding cracks in the sidewalk. Part of the difficulty with diagnosing schizophrenia (or any mental health concern) is the high comorbidity rate with other clinical disorders.
The schizophrenic spectrum includes depression , mania , impaired social function, and neglect of personal hygiene. Schizoaffective disorder is part of the schizophrenic spectrum and often involves aspects of bipolar disorder , like mood swings. With schizoaffective disorder, symptoms may last anywhere from two weeks to a year or longer.
Any illness within the schizophrenic spectrum affects how a person thinks. If a family member or friend suddenly begins seeing you as a threat or feels as though others are out to get them, it may be a sign that elements of the schizophrenic spectrum are at play. The sad part is that you can't force people to get help. Unlike a physical wound that bleeds, perforations in our psychology can be more easily masked as eccentricities.
But new research published in Nature Communications (2019) led by a team at Cardiff University shows that behavioral inflexibility is connected to our genes, specifically something called cytoplasmic FMRP interacting protein 1 (CYFIP1).[i] The deletion of the gene within chromosome 15 can increase a person’s risk up to four-fold for psychiatric issues like schizophrenia.
How It Works
Chromosome 15 (known as 15q11.2) is one of 23 pairs of chromosomes. Humans have duplicate copies of chromosomes—one from each parent. Think of it as a back-up system. Like using an external hard-drive with a computer.
Chromosome 15 has over 100-million base pairs. What does that mean? A base pair is made up of two nucleobases (nitrogen-based compounds that contribute to the formation of nucleotides, or the building blocks of DNA). Nucleobases are held together by hydrogen bonds, an elemental bond via electrostatic force that includes hydrogen. With all those millions of base pairs, you might be surprised to learn that Chromosome 15 only contributes to about 3% of the DNA in your cells, yet one small change can make a world of difference when it comes to human programming.
People throw around the term “DNA” like it’s a frisbee. DNA is actually an acronym for deoxyribonucleic acid—it’s essentially the double-helix, or the two chains that coil around each other and carry genetic instructions for the growth and development of all organisms, including certain viruses.
The researchers at Cardiff showed that when one copy of CYFIP1 was missing, it created abnormalities in the insulating sheath that forms around nerves in the brain called myelin . Oligodendrocytes, or glial cells in the central nervous system that do not produce electrical impulses, are important to brain function because they help maintain balance in the body by protecting neurons through the production of myelin.
Neurons are nerve cells in the brain that communicate with each other through synapses—the conductors of messages from cell to cell. Without myelin to protect the nerves, cellular damage can happen, causing miscommunications within the brain itself. That’s schizophrenia, just at the cellular level: A misfiring in the brain that causes a lack of flexibility in one’s thinking. Having fixed ideas that are not based in reality contributes to avoidant behavior, translating to anything from the discard of family members to suicidal thoughts. Being overwhelmed by social obligations or devaluing necessary social connections is part of the inflexibility that presents when there’s an interruption in myelin production in the brain.

The Bottom Line
Part of raising awareness about mental health means helping others to understand how our psychology is influenced by our biology, which is entirely dependent on our genetics. We tend to perceive these scientific disciplines as separate from one another. But through emerging research on the gut-brain axis , it’s clear that we are what we eat in more ways than one. It’s no surprise then that malnutrition is one of the contributing factors in schizophrenia. As research continues to uncover the connections between our genes and our biochemical functions, there is greater hope of finding a cure for chronic diseases like schizophrenia.
As we approach the end of 2019, it’s important to be mindful of the context behind our mental health. Part of the stigma of psychiatric disease is that it’s somehow an individual’s fault when, in fact, there are often biological factors stemming from our genes that contribute to the development of mental health disorders—a good thing to keep in mind as we come together with family this holiday season.
Choosing compassion over judgment and kindness over cruelty is the best gift we can give to ourselves and others. The real key to surviving anything is having the wherewithal to use patience in negative social situations, sometimes caused by mental health disorders in otherwise high-functioning individuals.
Compassion and kindness both derive from increased mindfulness . Mindfulness means being self-aware. Meditation can help there. If you have difficulty concentrating, try incorporating simple mind-games that will help improve your focus, like simplifying numbers on license plates (1+1=2, etc.)—that’s something that requires no extra effort, time or money. You just have to either walk or drive outside on a regular basis, and you can begin to improve your brain function by keeping it active. This also increases mindfulness because you automatically become more aware of your surroundings—a healthy addition as we all begin a new chapter in the new year.
Ana L. Silva, Josephine E. Haddon, Yasir Ahmed Syed, Simon Trent, Tzu-Ching E. Lin, Yateen Patel, Jenny Carter, Niels Haan, Robert C. Honey, Trevor Gumby, Yaniv Assaf, Michael J. Owen, David E. J. Linden, Jeremy Hall, Lawrence S. Wilinson. "Cy-fip 1 haploinsufficient rats show white matter changes, myelin thinning, abnormal oligodendrocytes and behavioural inflexibility." Nature Communications , 2019; 10 (1) DOI: 10.1038/s41467-019-11119-7

Rebecca Housel, Ph.D. , is a 28-year survivor of high-grade brain cancer, an internationally-sold author, and a former award-winning professor.
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- United States
- Brooklyn, NY
- Chicago, IL
- Houston, TX
- Los Angeles, CA
- New York, NY
- Portland, OR
- San Diego, CA
- San Francisco, CA
- Seattle, WA
- Washington, DC
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Self Tests NEW
- Therapy Center
- Diagnosis Dictionary
- Types of Therapy

It’s increasingly common for someone to be diagnosed with a condition such as ADHD or autism as an adult. A diagnosis often brings relief, but it can also come with as many questions as answers.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience
Search This Blog
- How do organisms develop and organised themselves?
- Why is finding "patient zero" in an pandemic so im...
- What is the 'Good Genes Hypothesis' and is it true?
GeneticsPerspective
What is the 'good genes hypothesis' and is it true, post a comment, popular posts from this blog, knock-out, knock-down and knock-sideways, nkx2-5 gene overview.

CRISPR in the clinic

COMMENTS
good genes hypothesis, in biology, an explanation which suggests that the traits females choose when selecting a mate are honest indicators of the male's ability to pass on genes that will increase the survival or reproductive success of her offspring. Although no completely unambiguous examples are known, evidence supporting the good genes hypothesis is accumulating, primarily through the ...
The good genes hypothesis (GGH) was formulated by evolutionary biologist W.D. Hamilton and behavioral ecologist M. Zuk ().It proposes that the characteristics preferred by females are a signal of males' ability to pass on genes (coding that certain characteristic) which will increase the survival and reproductive success of the offspring sired with a male possessing them.
The good genes hypothesis is a theory in evolutionary biology suggesting that individuals choose mates based on certain traits that indicate genetic quality. These traits are thought to be reliable signals of an individual's fitness and health, which can be passed on to offspring, thereby enhancing their chances of survival and reproductive success.
The hypothesis is often studied in the context of sexual selection and mate choice. Traits indicating 'good genes' can include physical characteristics, behaviors, or other phenotypic expressions. Good genes can confer advantages such as disease resistance, better metabolism, or more efficient resource use. Females may prefer males with these ...
The "good genes" hypothesis predicts a preference for older individuals based on the logic that survival until older ages requires high ... White symbols represent the mean probability ± SE of recruitment of offspring raised by males that died, magenta dots represent the mean probability ± SE of recruitment of offspring raised by males ...
The good genes hypothesis posits that males develop impressive ornaments to show off their efficient metabolism or effectiveness at obtaining food, or their ability to fight disease. Females then choose males with the most impressive traits because it signals their genetic superiority, which they will then pass on to their offspring. The ...
Introduction. Sexual selection is a complex process and involves many variables still not completely clear to researchers. Among these variables, there is one called "good genes," an indirect benefit that cannot be observed directly but has hypothetical indicators of masculinity, symmetry, health, and physical attractiveness.
The good genes hypothesis proposes that the characteristics preferred by females are a signal of males' ability to pass on genes which will increase the survival and reproductive success of the offspring sired with a male possessing them. Introduction The good genes hypothesis (GGH) was formu-lated by evolutionary biologist W.D. Hamilton
ACE - Linked to endurance and cardiovascular performance. Carrying the I allele enhances stamina [2]. MYLK - Regulates muscle contraction. Certain variants help with strength and speed [3]. NOS3 - Boosts blood and oxygen flow to muscles. The T allele aids high intensity exercise [4]. Other muscular genes include PPARGC1A, a metabolism ...
The good genes hypothesis, posed in the 1930s, attempted to reconcile mate choice and the selection for certain traits. The selfish gene hypothesis, first declared in 1976, attempted to explain mate choice as well as our behaviors. With our modern understanding of genetics and DNA that holds the information, these two hypotheses can be applied ...
The good genes hypothesis predicts that females benefit from choosing attractive males because these males will produce offspring with superior viability. The critical problem for this hypothesis is that attractive males need to have higher viability. ... meaning that sexual selection can increase population fitness if there is a positive ...
The good genes hypothesis states that the choosy sex will mate with individuals who possess traits that signify overall genetic quality. In doing so, they gain an evolutionary advantage for their offspring through indirect benefit. ... This means that the males must incubate the eggs and defend the nest for an extended period of time. Since ...
Good Gene Hypothesis: Courtship displays are ancient reproductive strategies that have been tightly associated with elaborate plumage. This elaboration is meant to convey superior fitness to females, however, the mechanism of this is unknown. The prevailing theory detailing this correlation is the "Good Gene Hypothesis". This theory ...
The good genes hypothesis posits that sexual selection on condition-dependent traits indirectly increases mean condition and therefore population health. Here, using mathematical models, we show that this effect should rarely be expected when sexual traits cause harm: Instead, good genes selection leads to larger harming traits, reduced female ...
coined by Galton (1883), Greek for 'good genes' or 'true genes'; controlled selective breeding of humans to improve viability. breeding value for total fitness. models of sexual selection in which the preference for a trait increases the fitness of the average chooser with respect to offspring viability.
There is allure to the idea that mating preferences can increase population mean fitness and local adaptation. A loose construction of 'good genes' and 'genetic quality' remains the default explanation for mating preferences and sexual dimorphisms outside the immediate field of sexual selection and in the popular literature.
The magnitude of the effect of good genes as a viability benefit accruing to choosy females remains a controversial theoretical and empirical issue. We collected all available data from the literature to estimate the magnitude of good-genes viability effects, while adjusting for sample size.
When we think of "good genes," we tend to focus on beauty. But genetics are increasingly important in the study of mental health, including the origin of disorders like schizophrenia ...
Good genes can, however, cause substantial exaggeration if preference genes are nearly selectively neutral. Alternatively, direct selection on preference genes, acting on mating behavior itself or on the genes' pleiotropic effects, can cause mating preferences and male display traits to be exaggerated by any degree.
gene combinations in the offspring [8]. Only recently, however, has this genetic compatibility hypothesis gained empirical support and widespread consideration as an explanation for patterns of mate preference [9-14] (Boxes 1,2). Ornaments and good genes versus dissimilarity and compatibility Female mate preferences for ornamentation versus ...
The good genes hypothesis was originally proposed in the 1980s and propagates the idea that observed mates are selected by their ability to pass on genes that increase reproductive success. How might it work? An organisms phenotype is determined by its genotype. Meaning your body observable properties (organs, tissues and cells) are determined ...
The science is still in its infancy, but gene-edited foods are already on the shelves in Japan: tomatoes rich in a chemical that supposedly promotes calmness; red sea bream with extra edible flesh ...