- U.S. Department of Health & Human Services

Encouraging First-in-Human Results for a Promising HIV Vaccine
Posted on June 6th, 2023 by Lawrence Tabak, D.D.S., Ph.D.

In recent years, we’ve witnessed some truly inspiring progress in vaccine development. That includes the mRNA vaccines that were so critical during the COVID-19 pandemic, the first approved vaccine for respiratory syncytial virus (RSV) , and a “universal flu vaccine” candidate that could one day help to thwart future outbreaks of more novel influenza viruses .
Inspiring progress also continues to be made toward a safe and effective vaccine for HIV, which still infects about 1.5 million people around the world each year [1]. A prime example is the recent first-in-human trial of an HIV vaccine made in the lab from a unique protein nanoparticle, a molecular construct measuring just a few billionths of a meter .
The results of this early phase clinical study, published recently in the journal Science Translational Medicine [2] and earlier in Science [3], showed that the experimental HIV nanoparticle vaccine is safe in people. While this vaccine alone will not offer HIV protection and is intended to be part of an eventual broader, multistep vaccination regimen, the researchers also determined that it elicited a robust immune response in nearly all 36 healthy adult volunteers.
How robust? The results show that the nanoparticle vaccine, known by the lab name eOD-GT8 60-mer , successfully expanded production of a rare type of antibody-producing immune B cell in nearly all recipients.
What makes this rare type of B cell so critical is that it is the cellular precursor of other B cells capable of producing broadly neutralizing antibodies (bnAbs) to protect against diverse HIV variants. Also very good news, the vaccine elicited broad responses from helper T cells. They play a critical supportive role for those essential B cells and their development of the needed broadly neutralizing antibodies.
For decades, researchers have brought a wealth of ideas to bear on developing a safe and effective HIV vaccine. However, crossing the finish line—an FDA-approved vaccine—has proved profoundly difficult.
A major reason is the human immune system is ill equipped to recognize HIV and produce the needed infection-fighting antibodies. And yet the medical literature includes reports of people with HIV who have produced the needed antibodies, showing that our immune system can do it.
But these people remain relatively rare, and the needed robust immunity clocks in only after many years of infection. On top of that, HIV has a habit of mutating rapidly to produce a wide range of identity-altering variants. For a vaccine to work, it most likely will need to induce the production of bnAbs that recognize and defend against not one, but the many different faces of HIV.
To make the uncommon more common became the quest of a research team that includes scientists William Schief, Scripps Research and IAVI Neutralizing Antibody Center, La Jolla, CA; M. Juliana McElrath, Fred Hutchinson Cancer Center, Seattle; and Kristen Cohen, a former member of the McElrath lab now at Moderna, Cambridge, MA. The team, with NIH collaborators and support, has been plotting out a stepwise approach to train the immune system into making the needed bnAbs that recognize many HIV variants.
The critical first step is to prime the immune system to make more of those coveted bnAb-precursor B cells. That’s where the protein nanoparticle known as eOD-GT8 60-mer enters the picture.
This nanoparticle, administered by injection, is designed to mimic a small, highly conserved segment of an HIV protein that allows the virus to bind and infect human cells. In the body, those nanoparticles launch an immune response and then quickly vanish. But because this important protein target for HIV vaccines is so tiny, its signal needed amplification for immune system detection.
To boost the signal, the researchers started with a bacterial protein called lumazine synthase (LumSyn). It forms the scaffold, or structural support, of the self-assembling nanoparticle. Then, they added to the LumSyn scaffold 60 copies of the key HIV protein. This louder HIV signal is tailored to draw out and engage those very specific B cells with the potential to produce bnAbs.
As the first-in-human study showed, the nanoparticle vaccine was safe when administered twice to each participant eight weeks apart. People reported only mild to moderate side effects that went away in a day or two. The vaccine also boosted production of the desired B cells in all but one vaccine recipient (35 of 36). The idea is that this increase in essential B cells sets the stage for the needed additional steps—booster shots that can further coax these cells along toward making HIV protective bnAbs.
The latest finding in Science Translational Medicine looked deeper into the response of helper T cells in the same trial volunteers. Again, the results appear very encouraging. The researchers observed CD4 T cells specific to the HIV protein and to the LumSyn in 84 percent and 93 percent of vaccine recipients. Their analyses also identified key hotspots that the T cells recognized, which is important information for refining future vaccines to elicit helper T cells.
The team reports that they’re now collaborating with Moderna, the developer of one of the two successful mRNA-based COVID-19 vaccines, on an mRNA version of eOD-GT8 60-mer. That’s exciting because mRNA vaccines are much faster and easier to produce and modify, which should now help to move this line of research along at a faster clip.
Indeed, two International AIDS Vaccine Initiative (IAVI)-sponsored clinical trials of the mRNA version are already underway, one in the U.S. and the other in Rwanda and South Africa [4]. It looks like this team and others are now on a promising track toward following the basic science and developing a multistep HIV vaccination regimen that guides the immune response and its stepwise phases in the right directions.
As we look back on more than 40 years of HIV research, it’s heartening to witness the progress that continues toward ending the HIV epidemic. This includes the recent FDA approval of the drug Apretude , the first injectable treatment option for pre-exposure prevention of HIV, and the continued global commitment to produce a safe and effective vaccine.
References:
[1] Global HIV & AIDS statistics fact sheet . UNAIDS.
[2] A first-in-human germline-targeting HIV nanoparticle vaccine induced broad and publicly targeted helper T cell responses . Cohen KW, De Rosa SC, Fulp WJ, deCamp AC, Fiore-Gartland A, Laufer DS, Koup RA, McDermott AB, Schief WR, McElrath MJ. Sci Transl Med. 2023 May 24;15(697):eadf3309.
[3] Vaccination induces HIV broadly neutralizing antibody precursors in humans . Leggat DJ, Cohen KW, Willis JR, Fulp WJ, deCamp AC, Koup RA, Laufer DS, McElrath MJ, McDermott AB, Schief WR. Science. 2022 Dec 2;378(6623):eadd6502.
[4] IAVI and Moderna launch first-in-Africa clinical trial of mRNA HIV vaccine development program . IAVI. May 18, 2022.
Progress Toward an Eventual HIV Vaccine , NIH Research Matters, Dec. 13, 2022.
NIH Statement on HIV Vaccine Awareness Day 2023 , Auchincloss H, Kapogiannis, B. May, 18, 2023.
HIV Vaccine Development (National Institute of Allergy and Infectious Diseases/NIH)
International AIDS Vaccine Initiative (IAVI) (New York, NY)
William Schief (Scripps Research, La Jolla, CA)
Julie McElrath (Fred Hutchinson Cancer Center, Seattle, WA)
McElrath Lab (Fred Hutchinson Cancer Center, Seattle, WA)
NIH Support: National Institute of Allergy and Infectious Diseases
Share this:
- Click to share on Facebook (Opens in new window)
- Click to share on X (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Tumblr (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to print (Opens in new window)
Posted In: Health , News , Science
Tags: AIDS , antibodies , B cells , bnAbs , broadly neutralizing antibodies , CD4 T cells , eOD-GT8 60mer , HIV , HIV vaccine , immune system , infectious disease , mRNA vaccine , nanoparticle , T cells
Good news . ¡Congratulation!
“As we look back on more than 40 years of HIV research, it’s heartening to witness the progress that continues toward ending the HIV epidemic.” Congratulations! So many astonishing discoveries and hopeful progress!!
And yet, as I approach nearly 40 years of Enduring ME/CFS (formerly Icelandic Disease; CFIDS; ‘Yuppie Flu’; Epstein-Barr; and now ‘Long-COVID’), my heart breaks when I think how little hope there is for a cure for me within my lifetime.
My anger rages when I consider how a similar investment in ME/CFS research 40 years ago could have prevented the latest ME/CFS Outbreak (which we are calling Long-COVID) from currently devastating another batch of people who have no idea how long ‘LONG’ is going to be……
And remember, the latest outbreak, exactly like previous outbreaks, COINCIDED WITH other viral illness outbreaks. THEY ARE NOT PROOF OF CAUSATION!!!! In fact, NONE of the previous viruses that have been implicated in ME/CFS have proved to be the cause. How is this obvious fact continually being missed?????
PLEASE, PLEASE, PLEASE LOOK FOR THE REAL CAUSATIVE VIRUS!!!!!
It must be a stealth virus (or fungus??) that requires an additional virus to allow it to access the body and (in my opinion) very quickly cross the blood-brain barrier causing the immediate ANS chaos that has a DISTINCTIVE HALLMARK PATTERN!!!!
Then the desperate victim spirals downward from there, constantly blocked from recovery by THE DEFINITIVE HALLMARK SYMPTOM of ‘Post-Exertion Malaise’ (which is neither a helpful nor accurate description of what it actually is and does to body and soul…..) until they reach a constant state of ENDURANCE, existence, survival, acceptance of what is NOT ACCEPTABLE, NOT NORMAL; NOT CRAZY, NOT LAZY; and NOT fully LIVING.
Alive but not living. Abandoned. Repeatedly Forgotten. Harshly Dismissed. A Fate that often feels worse than Death.
AND, as the above article clearly demonstrates, there are many scientists who are not daunted by a difficult challenge. THIS MYSTERY COULD BE SOLVED!!!!!! But such talent requires funding and focus. Both of which have been egregiously lacking in the case of ME/CFS.
Again, I will say it until I breathe my last breath, LOOK FOR THE VIRUS!!!!!!! It is there. Find it. Test for it. Cure it. Why should we have anything less than what HIV has gotten????? There is no good or defensible reason.
Does the 60mer structurally bind to any receptors? Is auto-immune priming something to consider? Safety studies for that may require a longer period of assessment.
Leave a Comment Cancel reply
@nihdirector on x, nih on social media.
Kendall Morgan, Ph.D.
Comments and Questions
If you have comments or questions not related to the current discussions, please direct them to Ask NIH .
You are encouraged to share your thoughts and ideas. Please review the NIH Comments Policy

- Visitor Information
- Privacy Notice
- Accessibility
- No Fear Act
- HHS Vulnerability Disclosure
- U.S. Department of Health and Human Services
- USA.gov – Government Made Easy
Discover more from NIH Director's Blog
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- News & Views
- Published: 09 December 2021
INFECTIOUS DISEASE
mRNA vaccines offer hope for HIV
- Lynn Morris ORCID: orcid.org/0000-0003-3961-7828 1 , 2
Nature Medicine volume 27 , pages 2082–2084 ( 2021 ) Cite this article
23k Accesses
9 Citations
120 Altmetric
Metrics details
- HIV infections
- Preclinical research
- RNA vaccines
mRNA technology may be uniquely positioned to tackle a major hurdle for HIV vaccines: the elicitation of broadly cross-reactive neutralizing antibodies. A preclinical study takes the first step toward this goal.
The remarkable success of mRNA vaccines against COVID-19 has been nothing short of miraculous. Whether this unique technology platform can be used to tackle the more complex task of developing a vaccine against human immunodeficiency virus (HIV) is now under intense scrutiny. In this issue of Nature Medicine , a preclinical study by Zhang et al. suggests that the mRNA platform may be up to the challenge 1 . The authors encapsulated mRNA encoding the HIV envelope glycoprotein (Env) (the equivalent of the SARS-CoV-2 spike protein), together with the structural HIV group-specific antigen protein (Gag), in a lipid nanoparticle, to produce virus-like particles (VLPs) in vivo. These Env-expressing VLPs elicited broadly neutralizing antibodies (bNAbs) and other immune responses that were protective against viral challenge in a macaque model. Although the VLPs were not nearly as immunogenic or as efficacious as mRNA vaccines against COVID-19, these results are encouraging and illuminate a pathway toward inducing the higher and more-durable antibody responses needed to prevent infection with HIV.
This is no small feat; Env is a formidable target that fails to induce the right kind of antibodies even in the majority of people who are infected with HIV. Unlike spike protein, which is relatively stable and contains just a few immunodominant epitopes (mostly restricted to the receptor-binding domain), Env is a complex trimeric protein with multiple dispersed antibody epitopes — many of which are conformational and heavily coated in sugars that shield them from antibody attack (Fig. 1 ). The ability to generate soluble trimeric Env proteins through the introduction of key mutations was a major advance in immunogen design, although these so-called ‘SOSIP proteins’ induced only autologous strain–specific neutralizing antibodies 2 . In the present study by Zhang et al., antibodies elicited by the Env–Gag mRNA were able to neutralize almost all members of a global panel of HIV isolates, classified as having a tier 2 phenotype 1 . This represents a major step forward for a vaccine against HIV, as this phenotype is typical of most circulating strains, which are difficult to neutralize due to a closed Env conformation.
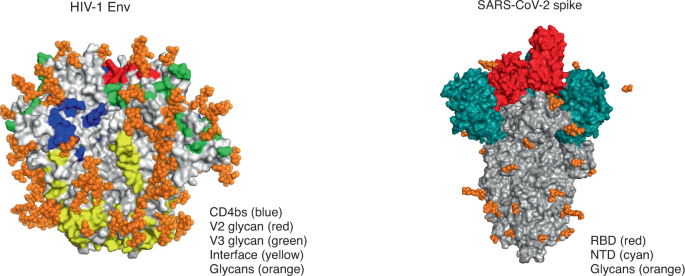
HIV Env (left) has multiple conformational bNAb epitopes (four of the six epitopes are shown here), is densely covered in glycans, and elicits a polyclonal neutralizing antibody response. Genes encoding antibodies to Env are heavily mutated, and bNAbs are rarely elicited by infection or vaccination. In contrast, the SARS-CoV-2 spike protein (right) is lightly covered in glycans, and the receptor-binding domain (RBD) is the main immunodominant epitope, with the N-terminal domain (NTD) targeted to a lesser extent. It generates a focused neutralizing antibody response; genes encoding antibodies to spike protein have limited or no mutations, and bNAbs are commonly elicited after infection and vaccination. CD4bs, CD4-binding site. Images from RCSB Protein Data Bank ( https://www.rcsb.org/ ) accession codes 4ZMJ (glycans transposed from 5FUU; HIV Env) and 6VSB (glycans transposed from 7CN9; SARS-CoV-2 spike protein). We thank T. Moyo-Gwete for preparation of this figure.
It is likely that a number of factors contributed to the greater immunogenicity of the mRNA vaccine designed by Zhang et al. 1 . The endogenous expression of the native Env on the surface of a VLP would preserve conformational epitopes, and VLPs were also shown to contain double the number of Env molecules present in an HIV viral particle. Furthermore, mRNA continues to be expressed for several days after administration, providing ongoing immunostimulation 3 . Another potential advantage is the ability of VLPs to bind to antigen-presenting cells, which guarantees delivery of mRNA into the appropriate cells and the induction of follicular helper T cells that are crucial for B cell development in germinal centers 4 . Zhang et al. also chose an HIV Env sequence that lacks a glycan at position 276; this enabled better access to the B cell precursors of bNAbs that target the CD4-binding site on the viral envelope 1 . Indeed, Env–Gag mRNA elicited antibodies to that site and, interestingly, to many other HIV epitopes — a considerable improvement on the strain-specific ‘glycan-hole’ responses seen with SOSIP proteins. It will be important in future studies to isolate B cells from mRNA-immunized animals to ascertain whether they are capable of broad neutralization and if they carry the genetic features associated with known bNAb lineages. Although the results from Zhang et al. 1 are encouraging for the HIV vaccine field, this is a complex and impractical protocol that requires multiple immunizations with high doses of mRNA. Moreover, the levels of bNAbs elicited in this study were extremely low and took a year to develop, and their role in protection from infection remains unclear.
mRNA technology has only recently come of age; it was originally hampered by instability and unfavorable immunogenicity, but decades of research have solved these problems, and the advantages of mRNA as a vaccine platform continue to emerge 5 . These include rapid development, ease of manufacture and scalability, which offer advantages over the traditional vector-based or protein-based vaccines being pursued in the HIV field. For example, mRNA would enable testing of sequential immunization and lineage-based approaches that require multiple immunogens with minor but important sequence changes 6 . mRNA is also considerably cheaper to produce and can be modified as needed, an important consideration for rapidly mutating viruses like HIV. Among the vaccines against COVID-19, those based on the mRNA platform have superior immunogenicity and stimulate both B cell responses and T cell responses 7 , 8 . The precise mechanisms underlying mRNA immunogenicity, however, are still unknown, and much remains to be learned if they are to be optimized and applied to HIV prevention. Nonetheless, the vast amount of safety data available from mRNA vaccines against COVID-19 will probably contribute to streamlined regulatory approval processes for vaccines against HIV and other diseases.
In contrast to the swift success of vaccines against COVID-19, the story of the development of vaccines against HIV has been long and troubled. Of the eight vaccine efficacy trials conducted so far, only one (with a viral-vector and protein-based vaccine prime–boost regimen) has shown moderate efficacy, although the follow-up trial failed to replicate this result 9 . This has shifted the field back to the pursuit of a vaccine able to induce bNAbs. Recently, the Antibody-Mediated Prevention clinical trials demonstrated that a monoclonal bNAb against the CD4-binding site can prevent infection of humans with HIV 10 , although it came with the sobering realization that high levels of bNAbs will probably be required (P. Gilbert, personal communication). Another approach to generating bNAbs is to trigger the B cell precursors of specific bNAb lineages. One such germline-targeting immunogen is eOD-GT8, a nanoparticle coated with HIV Env gp120 proteins, which binds rare B cells specific for the CD4-binding site in monkeys and humans 11 . eOD-GT8 has now been converted into an mRNA vaccine through the same platform as the successful Moderna vaccine against COVID-19, with human clinical trials due to start soon.
There is no doubt that HIV presents a much greater challenge for vaccine developers than COVID-19 does. The vast genetic diversity and the ability of HIV to integrate into the human genome necessities that a vaccine elicits antibodies able to block every viral particle. Whether further optimization of Env immunogens, together with the strong priming effect of an mRNA vaccine platform, is able to achieve this will require further investigation. The hope is that the lessons learned from the development of vaccines against COVID-19 will be used to solve the HIV problem and that this will be tackled with the same sense of urgency, given that HIV remains a major global health challenge.
Zhang. P. et al. Nat. Med . https://doi.org/10.1038/s41591-021-01574-5 (2021).
Sanders, R. W. et al. Science 349 , aac4223 (2015).
Article Google Scholar
Pardi, N. et al. J. Control. Release 217 , 345–351 (2015).
Article CAS Google Scholar
Pardi, N. et al. J. Exp. Med. 215 , 1571–1588 (2018).
Chaudhary, N., Weissman, D. & Whitehead, K. A. Nat. Rev. Drug Discov. 20 , 817–838 (2021).
Mu, Z., Haynes, B. F. & Cain, D. W. Vaccines 9 , 134 (2021).
Khoury, D. S. et al. Nat. Med. 27 , 1205–1211 (2021).
Lederer, K. et al. Immunity 53 , 1281–1295.e5 (2020).
Gray, G. E. et al. N. Engl. J. Med. 384 , 1089–1100 (2021).
Corey, L. et al. N. Engl. J. Med. 384 , 1003–1014 (2021).
Jardine, J. G. et al. Science 351 , 1458–1463 (2016).
Download references
Author information
Authors and affiliations.
Antibody Immunity Research Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
Lynn Morris
Center for the AIDS Program of Research in South Africa, University of KwaZulu-Natal, Durban, South Africa
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Lynn Morris .
Ethics declarations
Competing interests.
The author declares no competing interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Morris, L. mRNA vaccines offer hope for HIV. Nat Med 27 , 2082–2084 (2021). https://doi.org/10.1038/s41591-021-01602-4
Download citation
Published : 09 December 2021
Issue Date : December 2021
DOI : https://doi.org/10.1038/s41591-021-01602-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

Childhood HIV Vaccination Strategy Shows Promise in Study
- Share to Facebook
- Share to Twitter
- Share to LinkedIn
- Share on Email

Weill Cornell Medicine researchers have developed an experimental vaccine to protect against human immunodeficiency virus (HIV) infection based on " spike" proteins (shown in purple) on the surface of the virus. Credit: Shutterstock
Research at Weill Cornell Medicine suggests that childhood immunization against HIV could one day provide protection before risk of contracting this potentially fatal infection dramatically increases in adolescence.
The study , published Aug. 30 in Science Immunology, demonstrated that a series of six vaccinations containing a modified protein from the surface of HIV particles stimulated initial steps of a potent immune response in young non-human primates. This difficult-to-achieve response represents an important step toward providing full and potentially life-long protection against the virus, the researchers say.
Immunizing young children, rather than adults, makes sense because risk factors for HIV infection rise steeply when adolescents become sexually active, according to senior author Dr. Sallie Permar , the Nancy C. Paduano Professor in Pediatrics and chair of the Department of Pediatrics at Weill Cornell Medicine.
What’s more, evidence suggests that the immune systems of infants and children generally mount more effective responses to the virus than those of adults, said Dr. Permar. “One of the advancements we’ve made is to demonstrate that an HIV vaccine could be delivered on a schedule similar to routine vaccines already given to babies and children.”
Prepping the Immune System Early
HIV predominantly infects immune cells called CD4 T cells, leaving individuals vulnerable to opportunistic diseases. Without lifelong treatment, infection is fatal. In 2022, an estimated 140,000 adolescents between 10 and 19 years old worldwide became infected with the virus—a group that is overrepresented in the number of new infections.

Dr. Sallie Permar
Vaccine researchers are seeking ways to stimulate the immune system to make “broadly neutralizing antibodies” against the virus before a person is exposed to it. These antibodies attack a crucial part of the HIV virus—the protein on its surface that binds to CD4 T cells. In doing so, broadly neutralizing antibodies prevent many strains of HIV from entering the cell and infecting it.
In this study, the researchers started with an experimental vaccine developed previously from spike proteins on the envelope of HIV particles. Study authors Dr. John Moore , a professor of microbiology and immunology, and Dr. Rogier Sanders , an adjunct associate professor of research in microbiology and immunology at Weill Cornell Medicine and a professor at Amsterdam UMC , sought to improve this vaccine by altering the viral protein. They designed these changes to stimulate a specific set of antibody-producing B cells that protect CD4 T cells.
“An effective HIV vaccine needs to engage the right set of B cells in order to generate a broadly protective response,” said first author Dr. Ashley Nelson , an assistant professor of immunology research in pediatrics at Weill Cornell Medicine. “We discovered that introducing certain mutations into the envelope protein could accomplish that in the setting of a naïve immune system.”
Activating the Right B Cells for Protection
The researchers administered the modified vaccine to five young primates in three priming doses, starting less than a week after birth. They followed up with three doses of the vaccine matching the original HIV envelope protein, with the last dose given when the animals reached 78 weeks old, roughly equivalent to four or five years old for a human. As a control, five animals received all six doses of the original envelope protein vaccine.

Dr. Ashley Nelson
“While exposure to the modified protein got the immune response started off in the right direction, booster shots containing the original version of the viral protein were necessary to reach full potential,” Dr. Nelson said.
Three of the five animals who received the modified version of the priming vaccine developed antibodies that appeared to be precursors to the sought-after broadly neutralizing response. Tests suggested these antibodies attacked the site the virus uses to invade CD4 T cells. However, they were not yet fully effective against the same breadth of HIV strains as mature broadly neutralizing antibodies. One of the three animals also showed signs of developing the mature, broadly neutralizing response.
The next step is figuring out how to reliably elicit a full-on broadly neutralizing response, Dr. Nelson said. “We still need to identify the right combination of viral proteins to get us further down that path, starting from the earliest stages in life when multi-dose vaccines are commonly given.”
Many Weill Cornell Medicine physicians and scientists maintain relationships and collaborate with external organizations to foster scientific innovation and provide expert guidance. The institution makes these disclosures public to ensure transparency. For this information, please see the profile for Dr. Sallie Permar .
This work was supported in part by National Institutes of Health (NIH) grant P01 AI117915, Office of Research Infrastructure Program/OD grant P51 OD011107 and by the National Institute of Allergy and Infectious Diseases of the NIH grant P01 AI110657.
Related News
- Computational Approach Yields Novel Cancer Targets
- Analysis Could Guide the Future of Telehealth Policies
- Dr. Margaret McNairy Named NAM Emerging Leader in Health and Medicine
Back to News
Weill Cornell Medicine Office of External Affairs New York, NY --> Phone: (646) 962-9476
After decades of failures, researchers have renewed hopes for an effective HIV vaccine
The world needs an HIV vaccine if it ever hopes to beat a virus that still infects over 1 million people a year and contributes to hundreds of thousands of deaths.
Despite 20 years of failures in major HIV vaccine trials — four this decade alone — researchers say recent scientific advances have likely, hopefully, put them on the right track to develop a highly effective vaccine against the insidious virus.
But probably not until the 2030s.
“An effective vaccine is really the only way to provide long-term immunity against HIV, and that’s what we need,” Dr. Julie McElrath, the director of the vaccine and infectious disease division at the Fred Hutchinson Cancer Center in Seattle, said Monday at the Conference on Retroviruses and Opportunistic Infections in Denver.
All current HIV vaccine action is in the laboratory, animal studies or very early human trials.
Researchers at the retrovirus conference presented favorable results from two HIV vaccine studies. One found that a modification to the simian version of HIV spurred production of what are known as broadly neutralizing antibodies against the virus in monkeys. Another showed promise in the effort to coax the immune system’s B cells to make the powerful antibodies in humans.
“These trials illustrate as a proof of concept that we can train the immune system. But we need to further optimize it and test it in clinical trials,” Karlijn van der Straten, a Ph.D. student at the Academic Medical Center at Amsterdam University, who presented the human study, said at a news conference Monday.
Still, the scrappy scientists in this field face a towering challenge. HIV is perhaps the most complex pathogen ever known.
“The whole field has learned from the past,” said William Schief, who leads Moderna’s HIV vaccine efforts. “We’ve learned strategies that don’t work.”
The cost has already been immense. Nearly $17 billion was spent worldwide on HIV -vaccine research from 2000 to 2021. Nearly $1 billion more is spent annually, according to the Joint United Nations Program on HIV/AIDS and the nonprofit HIV group AVAC.
“Maintaining the funding for HIV vaccines right now is really important,” said Dr. Nina Russell, who directs HIV research at the Bill & Melinda Gates Foundation. She pointed to the field’s own “progress and the excitement” and to how “HIV vaccine science and scientists continue to drive innovation and science that benefits other infectious diseases and global health in general.”
Case in point: Covid. Thanks to HIV research, the mRNA vaccine technology was already available in 2020 to speed a coronavirus vaccine to market.
Why the HIV vaccine efficacy trials failed
In strong contrast to Covid, the HIV vaccine endeavor has spanned four decades. Only one of the nine HIV vaccine trials have shown efficacy: a trial conducted in Thailand and published in 2009 that reported a modest 31% reduction in HIV risk.
HIV vaccine researchers subsequently spent years seeking to retool and improve that vaccine strategy, leading to a series of trials that launched in the late 2010s — only to fail.
Researchers have concluded those latest trials were doomed because, aside from prompting an anti-HIV response based in immune cells, they only drove the immune system to produce what are known as non-neutralizing antibodies. Those weapons just weren’t strong enough for such a fearsome foe.
Preventing HIV through vaccination remains a daunting challenge because the immune system doesn’t naturally mount an effective defense against the virus, as it does with so many other vaccine-preventable infections, including Covid. An HIV vaccine must coax from the body a supercharged immune response with no natural equivalent.
That path to victory is based on a crucial caveat: A small proportion of people with HIV do produce what are known as broadly neutralizing antibodies against the virus. They attack HIV in multiple ways and can neutralize a swath of variants of the virus.
Those antibodies don’t do much apparent good for people who develop them naturally, because they typically don’t arise until years into infection. HIV establishes a permanent reservoir in the body within about a week after infection, one that their immune response can’t eliminate. So HIV-positive people with such antibodies still require antiretroviral treatment to remain healthy.
Researchers believe that broadly neutralizing antibodies could prevent HIV from ever seeding an infection, provided the defense was ready in advance of exposure. A pair of major efficacy trials, published in 2021 , demonstrated that infusions of cloned versions of one such antibody did, indeed, protect people who were exposed to certain HIV strains that are susceptible to that antibody.
However, globally, those particular strains of the virus comprise only a small subset of all circulating HIV. That means researchers can’t simply prompt a vaccine to produce that one antibody and expect it to be effective. Importantly, from this study they got a sense of what antibody level would be required to prevent infection.
It’s a high benchmark, but at least investigators now have a clearer sense of the challenge before them.
Also frustrating the HIV vaccine quest is that the virus mutates like mad. Whatever spot on the surface of the virus that antibodies target might be prone to change through mutation, thus allowing the virus to evade their attack. Consequently, researchers search for targets on the virus’ surface that aren’t highly subject to mutation.
Experts also believe warding off the mutation threat will require targeting multiple sites on the virus. So researchers are seeking to develop a portfolio of immune system prompts that would spur production of an array of broadly neutralizing antibodies.
Prompting the development of such antibodies requires a complex, step-by step process of coaxing the infection-fighting B cells, getting them to multiply and then guiding their maturation into potent broadly neutralizing antibody-producing factories.
HIV vaccine development ‘in a better place’
Dr. Carl Dieffenbach, the head of the AIDS division at the National Institute of Allergy and Infectious Diseases, said numerous recent technological advances — including mRNA, better animal models of HIV infection and high-tech imaging technology — have improved researchers’ precision in designing, and speed in producing, new proteins to spur anti-HIV immune responses.
Global collaboration among major players is also flourishing, researchers said. There are several early-stage human clinical trials of HIV-vaccine components underway.
Three mRNA- based early human trials of such components have been launched since 2022. Among them, they have been led or otherwise funded by the global vaccine research nonprofit group IAVI, Fred Hutch, Moderna, Scripps Research, the Gates Foundation, the National Institutes of Health, the U.S. Agency for International Development, and university teams. More such trials are in the works.
On Friday, Science magazine reported concerning recent findings that among the three mRNA trials, a substantial proportion of participants — 7% to 18%, IAVI said in a statement — experienced skin-related symptoms following injections, including hives, itching and welts.
IAVI said in its statement that it and partners are investigating the HIV trials’ skin-related outcomes, most of which were “mild or moderate and managed with simple allergy medications.”
Researchers have shown success in one of those mRNA trials in executing a particular step in the B-cell cultivation process.
That vaccine component also generated “helper” CD4 cells primed to combat HIV. The immune cells are expected to operate like an orchestra conductor for the immune system, coordinating a response by sending instructions to B cells and scaling up other facets of an assault on HIV.
A complementary strategy under investigation seeks to promote the development of “killer” CD8 cells that might be primed to kill off any immune cells that the antibodies failed to save from infection.
Crucially, investigators believe they are now much better able to discern top vaccine component candidates from the duds. They plan to spend the coming years developing such components so that when they do assemble the most promising among them into a multi-pronged vaccine, they can be much more confident of ultimate success in a trial.
“An HIV vaccine could end HIV,” McElrath said at the Denver conference. “So I say, ‘Let’s just get on with it.”
Dr. Mark Feinberg, president and CEO of IAVI, suggested that the first trial to test effectiveness of the vaccine might not launch until 2030 or later.
Even so, he was bullish.
“The field of HIV vaccine development is in a better place now than it’s ever been,” he said.
Benjamin Ryan is independent journalist specializing in science and LGBTQ coverage. He contributes to NBC News, The New York Times, The Guardian and Thomson Reuters Foundation and has also written for The Washington Post, The Nation, The Atlantic and New York.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Vaccines (Basel)

Current Status of HIV-1 Vaccines
Anna hargrave.
1 Department of Biomedical Sciences, Kentucky College of Osteopathic Medicine, University of Pikeville, Pikeville, KY 41501, USA; ude.ekipu@evargrahanna
Abu Salim Mustafa
2 Department of Microbiology, College of Medicine, Kuwait University, Kuwait City 12037, Kuwait; [email protected]
3 Department of Restorative Sciences, College of Dentistry, Kuwait University, Kuwait City 12037, Kuwait; [email protected]
Javed H. Tunio
4 Department of Internal Medicine, Carver College of Medicine, University of Iowa, Iowa City, IA 52242, USA; ude.awoiu@oinut-devaJ
Shumaila Nida M. Hanif
Associated data.
Not applicable.
HIV-1 infection and its progression to AIDS remains a significant global health challenge, particularly for low-income countries. Developing a vaccine to prevent HIV-1 infections has proven to be immensely challenging with complex biological acquisition and infection, unforeseen clinical trial disappointments, and funding issues. This paper discusses important landmarks of progress in HIV-1 vaccine development, various vaccine strategies, and clinical trials.
1. Introduction
In 2020, 37.6 million people were living with HIV infections, and 1.5 million people acquired HIV infections within the year. Since the HIV epidemic began, 34.7 million people have died due to an AIDS-related illness. In 2020, 690,000 people died due to an AIDS-related illness [ 1 ]. It has been estimated that 85% of all HIV cases are transmitted sexually. In contrast, the other 15% of cases are transmitted from shared injection needles, infected blood transfusions, or from mother to child [ 2 ].
Treatment of HIV-1 infections drastically changed as antiretroviral drugs evolved. This started with azidothymidine, an inhibitor of viral reverse transcriptase, in 1987, and it decreased the amount of HIV RNA in the bloodstream. This treatment plan was altered from a single-drug regimen for a more effective two-drug regimen. Then, clinicians tested a three-drug regimen, including the newly developed protease inhibitors, and this was referred to as the highly effective combination antiretroviral therapy (ART) in 1996. Provided that patients with HIV-1 infections consistently adhere to ART, they have close to normal life expectancies and do not transmit the virus to an uninfected sexual partner. The virus is not transmitted in this circumstance because ART suppresses the level of the virus to incredibly low levels [ 3 ].
Fourteen years later, in 2012, clinicians started using ART to prevent HIV-1 infections and referred to this as preexposure prophylaxis or PrEP. A single pill taken once daily has been shown to be 99% effective in preventing HIV-1 infection via sexual acquisition [ 3 ]. Currently, all people at high risk for HIV infection should be offered PrEP according to the US Preventive Services Task Force Grade A [ 3 ].
While the development of effective antiretroviral drugs for patients with HIV-1 infections and their application as PrEP to help prevent infection to at-risk people is an important landmark in scientific history, it does not replace the need for an effective vaccine [ 3 , 4 , 5 , 6 ]. Given that developing countries have approximately 90% of the people with HIV-1 infections and antiretroviral drugs are inaccessible, it is clear that a vaccine is required to end this epidemic [ 4 ].
To end this epidemic, two possible immunization strategies must be considered as possible solutions, therapeutic and prophylactic vaccines. The aim of prophylactic vaccines is to prevent the infection or disease while therapeutic vaccines are aimed at treating the individual already infected with HIV. This review is focused on the prophylactic vaccines, and as such, it considers clinical trials that assess the risk of contracting HIV-1 infections after receiving a prophylactic vaccine instead of reduction in viral load.
2. Challenges of the HIV-1 Vaccine
2.1. biological perspective.
The biological challenges of HIV vaccine development include a high rate of mutation and recombination during viral replication, four main groups of HIV with nine subtypes/clades across the world, no appropriate animal models, and limited information regarding the correlates of immune protection.
The high rate of mutation of HIV is due to the error-prone viral reverse transcriptase and has been estimated to lead to 1–10 mutations per genome per replication cycle. This mutation rate mostly leads to changes within the Env glycoprotein, allowing glycan shielding so the virus can evade the immune system. Though there is considerable genetic diversity present in the Env glycoprotein, this structure is the main target of neutralizing antibodies [ 6 ].
HIV-1 infections are classified into a group (M, N, O, P) and subtype or clade (A, B, C, D, F, G, H, J, K). Within a clade, genetic variation can be as high as 30%. Additionally, 10–20% of people infected with HIV in certain regions of Africa have two or more viral subtypes [ 6 ]. These viral subtypes lead to recombinant strains such as A/Ga recombinant in West Africa and B/C recombinant in China [ 2 ].
The lack of appropriate animal models posed a unique challenge to researchers before the early 1990s. Chimpanzees, an endangered species classified as a nonhuman primate (NHP), contracted HIV, but it did not follow the course of the disease in humans. This led to the US and Japan separately developing SHIV, a chimeric virus with gag and pol genes from SIV and env gene from HIV, because it is a more pathogenically relevant model for the generation of an HIV vaccine. The standard animal model widely accepted today is macaque monkeys infected with SHIV administered with low doses and intravaginally [ 7 ]. It is worth noting that this standard accepted animal model is only a model and may not reflect the disease pathology and immune response in humans.
Researchers are also challenged by the lack of information regarding the correlates of immune protection. This is due to the complex progression of HIV-1 infection since the infection is never able to be cleared by the immune system because of the reservoir of latently infected memory CD4+ T-cells [ 4 ]. During the preliminary stage of infection for the majority of patients, T-cells attack immunodominant highly variable regions of HIV-1, leading to escaped HIV-1 variants with decoy epitopes and ineffective protection against them [ 8 ].
An interesting characteristic that may provide insight into immune correlates is the three unique subsets of the population with HIV-1 infections. There are viremic controllers who suppress the viral load from 50 to 2000 RNA copies per mL, elite controllers who suppress the viral load to <50 RNA copies per mL, and long-term nonprogressors. Long-term nonprogressors maintain stable CD4+ T-cells above 500 cells per microliter for a decade without ART. The mechanisms behind these subsets’ response to HIV-1 are not fully understood. Some genetic studies suggest the difference in amino acids in proteins that code for HLA class I alleles are correlated to controllers or progressors. HLA-B*57, HLA-B*27, HLA-B*52, and HLA-B*14 are more frequently found in controllers, while HLA-B*07, HLA-B*08, and HLA-B*25 are linked to an increased risk of progression. Though genetic variation is an important aspect of understanding the biology behind viremic and elite controllers, no HLA Class I allele is able to suppress the virus. Researchers believe that the mechanism likely involves HLA association with CD8+ T-cells and other nongenetic factors [ 9 ].
Another characteristic that may provide insight into immune correlates is the neutralizing antibodies present in approximately 10–30% of people with HIV-1 infections. These antibodies are developed from heavy chain mutation and are not produced until years into a chronic natural infection [ 10 ]. While they do not provide any protection, they may offer information regarding what is needed for the immune system to prevent HIV-1 acquisition.
2.2. Funding
There is a gap between the resources and funding needed to develop an effective vaccine and the countries that have large populations with HIV-1 infections. Unfortunately, the financial backing behind HIV vaccine research and development has decreased since 2010. Eighty-five percent of the funding has been contributed by the US government and the Bill & Melinda Gates Foundation. In 2018, the combined contribution from these two groups amounted to approximately 680 million US dollars [ 11 ].
2.3. A Brief History of HIV Vaccine Development
From 1987 to 2021, there have been three major approaches driving HIV-1 vaccine development. Each of these approaches involved one or more clinical trials and is summarized in Table 1 . Though the first two approaches have mostly been concluded, it is worth noting that each approach has been continually reexplored as researchers learn more [ 7 , 12 ].
Historic phase 2b and 3 HIV-1 vaccine clinical trials.
| Name | Year Started and Country/Continent | Phase | Molecular Basis of Vaccine | Efficacy/Response | Relevant Information | NCT Number or Author |
|---|---|---|---|---|---|---|
| Vax 003 | 1999, Thailand | 3 | AIDSVAX B/E | No efficacy | Bivalent subunit vaccine, 2 Gp120 from clades B and E were combined and alum adjuvant added | NCT00006327 |
| Vax 004 | 1998, North America and The Netherlands | 3 | AIDSVAX B/B | No efficacy | Bivalent subunit vaccine, 2 Gp120 from clade B were combined and alum adjuvant added | NCT00002441 |
| RV 144 | 2003, Thailand | 3 | ALVAC-HIV and AIDSVAX B/E | 31.2% efficacy against HIV-1 acquisition | NA | NCT00223080 |
| HVTN 502/Step and HVTN 503/Phambili | 2004, North and South America, Australia, Caribbean, and South Africa | 2b | MRKAd5 HIV-1 gag/pol/nef B | No efficacy | Both studies prematurely terminated. People with high titer to adenovirus were more likely to contract HIV. Uncircumcised men had a higher risk of contracting HIV [ , , ]. | NCT00095576 and NCT00413725 |
In the late 1980s, the first approach focused on generating a vaccine that would induce neutralizing antibodies because neutralizing antibodies and their associated subsequent cytotoxic T lymphocyte responses were believed to provide enough protection against HIV-1. Vaccines designed and tested targeted gp120 or gp160 HIV-1 envelope proteins [ 13 ]. This was based on the observation that neutralizing antibodies could be produced in response to envelope glycoproteins present on the virus because this had occurred with the recombinant hepatitis B vaccine. This approach mostly ended in 2003, after the VaxGen trials testing gp120 vaccines produced poor results [ 12 ].
The second approach was based on administering a viral vector to induce a CD8+ T cell response [ 13 ]. In the early 2000s, researchers started focusing on how CD8+ T-cells controlled HIV infection [ 12 ] because when CD8+ T-cells decreased significantly during acute infection, the immune system was no longer able to control the virus, as observed in animal and human studies [ 6 ]. The goal in inducing a CD8+ T cell reaction was to control post-infection viremia and potentially prevent HIV acquisition. Using a recombinant vector with HIV genes as a vaccine, the virus would produce HIV proteins that would be presented to the immune system via the Class I antigen-presenting pathway [ 14 ]. This approach ended approximately after the STEP trial was terminated [ 12 ].
Briefly, the STEP trial starting in 2004 and its associated counterpart, “Phambili”, starting in 2007 were the first T-cell-based vaccine candidates. Both tested recombinant Ad5 vector with HIV-1 clade B gag/pol/nef inserts. It is worth noting that no envelope genes were present [ 14 ] because this would allow the immune system to attack the proteins and DNA within the core of the virus. Both clinical trials were ended prematurely because the STEP trial provided no efficacy and did not decrease viral load in participants who contracted HIV. The results also indicated that some participants who had Ad5-neutralizing antibodies and/or were uncircumcised were more likely to contract HIV as compared to the placebo group [ 7 , 14 , 15 ]. Hence, it was an example of product failure.
The third and current approach is to utilize a heterologous prime-boost to elicit humoral and cell-mediated immune responses [ 13 ]. The prime-boost strategy is based on priming with a virus and boosting with a recombinant protein. A homologous prime-boost is utilized for diphtheria, tetanus, and pertussis (DTP) and involves administering the same vaccine at intervals to boost the previous responses. The heterologous prime-boost utilizes the same antigens in different types of vaccines and has been proven to be more immunogenic than the homologous series [ 16 ]. This has been employed in numerous clinical trials, and it has been able to significantly improve the humoral and cellular immune response while simultaneously inducing neutralizing antibodies [ 6 ]. Prime-boost strategies’ outcome change is based on the selection of antigen, vector type, delivery route, dose, adjuvant, schedule, and sequence of immunizations [ 17 ]. Theoretically, this approach provides a heightened immune response (in terms of breadth and depth) that is focused on the inserts, not the vectors, and produces unique populations of effector-like memory T-cells that gather at the nonlymphoid organs [ 17 ].
This approach was employed in the only modestly successful trial to date, RV 144, and its results were made public in 2009, only two years after STEP’s disappointing results [ 7 ]. This trial is discussed in detail under “Milestone Event”.
Though the prime-boost approach has proven to be somewhat successful, there has yet to be an HIV vaccine, so more research into the understanding of HIV’s mechanism and its immune correlates, development of an effective and safe vaccine, and clinical trials are necessary.
2.4. Important Advances in Scientific Technology Impacting HIV Vaccine Development
Since researchers have been developing an HIV vaccine for two and a half decades, the fields of molecular biology, bioinformatics, and “omics” based technology have all developed from inception or significantly expanded [ 2 , 12 ].
Molecular biology and bioinformatics techniques rapidly evolved and led to the HIV genome sequencing and cloning and identification of structural proteins of the virus [ 12 ]. “Omics” based technology ranging from “vaccinomics,” genomics, and reverse immunology allowed researchers to design highly specific DNA vaccines [ 2 ].
Since the beginning of HIV vaccine development, it is worth noting the significant changes from the initial recombinant vectors to more effective and safer vectors. This transition to improve recombinant vectors was specifically between the first and second approaches discussed in the history of HIV development section. Initially, a recombinant vaccinia virus was utilized in 1987, and it posed serious potential concerns [ 17 ]. Individuals who had already received the smallpox vaccine would not mount an appropriate immune response if vaccinated against HIV-1 in the same vector, so receiving this vaccine would be a futile effort. Immunocompromised individuals likely would be severely ill because of the replicating virus [ 17 ].
In 1975, researchers developed MVA, a nonreplicating highly attenuated vaccinia virus with over 200 poxvirus proteins [ 18 ]. It was tested and proven to be safe, inducing a cell-mediated immune response [ 17 ]. In the early 1990s, scientists developed two other nonreplicating poxviruses, NYVAC (highly attenuated vaccinia virus) and ALVAC (an avian poxvirus, canarypox). Both of these poxviruses are considered to be safe [ 6 ]. Currently, the most common recombinant vectors utilized in clinical trials since 2015 are ALVAC, Ad26, and/or MVA, as given in Table 2 . Though recombinant vector is an obvious aspect of immunization, it determines immunogenicity and can drastically change the clinical trial results. An example of this is that plasmid vectors containing Env or Gag in the full-length form have poor immunogenicity and are ineffective. To circumvent this, several clinical trials rely on administering the plasmid with the HIV gene followed by a highly immunogenic recombinant vector [ 2 ].
Recent and ongoing HIV-1 clinical trials.
| Name | Year Started and Country/Continent | Phase | Molecular Basis of Vaccine | Efficacy/Response or Completion Date | Relevant Comments | NCT Number or Author |
|---|---|---|---|---|---|---|
| HVTN 702 | 2016, South Africa | 2b/3 | ALVAC-HIV and subtype C gp120/MF59 | No efficacy | NCT02968849 | |
| Antibody-Mediated Protection, HVTN 703 | 2016, sub-Saharan Africa | 2b | VRC01 broadly neutralizing monoclonal antibody infusion | Did not prevent HIV-1 acquisition | Similar to HVTN 704 but clinical participants were women | NCT02568215 |
| Antibody-Mediated Protection, HVTN 704 | 2016, Brazil, Peru, Switzerland, USA | 2b | VRC01 broadly neutralizing monoclonal antibody infusion | Did not prevent HIV-1 acquisition | Similar to HVTN 703, but clinical participants were men who have sex with men and transgender women | NCT02716675 |
| IAVI A003/CHOP HVDDT 001 | 2014, United Kingdom | 1 | rAAV1-PG9DP | Safe and tolerable, but antibody levels not detected in all participants | NCT01937455 | |
| APPROACH | 2015, east Africa, South Africa, Thailand, and USA | ½ | Ad26.Mos.HIV and Clade C gp140 or MVA mosaic vaccine with gp140 | Safe and tolerable, efficacy will be assessed with HVTN 705 | NCT02315703 | |
| Imbokodo, HVTN 705 | 2017, sub-Saharan Affrica | 2b | Ad26.Mos4.HIV and adjuvanted clade C gp140 and Mosaic gp140 protein | Completion date in 2022 | NCT03060629 | |
| HVTN 706 | 2019, Europe, North, and South America | 3 | Ad26.Mos4.HIV and adjuvanted clade C gp140 and Mosaic gp140 protein | Completion date in 2022 | ||
| CR108152 | 2016, USA and Rwanda | ½ | Ad26.Mos.HIV or Ad26.Mos4.HIV and clade C gp140 plus adjuvant | Completion date in 2023 | NCT02788045 | |
| HIV-CORE 004 | 2014, Kenya | ½ | pSG2.HIVconsv DNA, MVA.HIVconsv and Ad35-GRIN | Safe and tolerable, all participants had HIVcons specific T cell responses | NCT02099994 | |
| HIV-CORE 0052 | 2021, United Kingdom | 1 | ChAdOx1.tHIVconsv1, MVA.tHIVconsv3 (M3), or MVA.tHIVconsv4 (M4) | Completion date in 2022 | NCT04586673 | |
| PrepVacc | 2020, Mozambique; South Africa; Tanzania; and Uganda | 2b | DNA-HIV-PT123 and AIDSVAX or 2 injections of CN54gp140 + MPLAL and MVA | Completion date in 2023 | All participants on PREP | NCT04066881 |
| IAVI G001 | 2018, USA | 1 | eOD-GT8 60mer + AS01B/DPBS sucrose/IM | Results have not been published in peer reviewed journal | NCT03547245 | |
| IAVI G002 | Estimated start date 2021, USA | 1 | Core-g28v2 60mer mRNA and eOD-GT8 60mer mRNA Vaccine | Completion date in 2023 | NCT05001373 |
2.5. Milestone Event
Given the numerous challenges in HIV-1 vaccine development, scientists doubted whether a vaccine could be generated to provide immunity against this virus. One significant breakthrough that illustrated that a preventative HIV-1 vaccine is possible was the result of the RV144 trial, obtained in 2009 [ 14 ].
Briefly, a summary of the RV144 trial protocol is as follows. This phase 3 efficacy trial conducted in Thailand tested ALVAC-HIV, a recombinant canarypox vector. This vector included Env (clade E), group-specific antigen (gag) (clade B), and protease (pro) (clade B) and is classified as a prime [ 6 ]. Following the priming events, study participants also received AIDSVAX, a protein boost with alum as an adjuvant. This protein boost was a combination of gp120 clade B (Note that this protein was modified. Eleven amino acids from N-terminal were deleted, and protein was tagged with herpes simplex virus gD), strain MN, and strain A244 (from CRF01_AE). The ALVAC priming events occurred at weeks 0 and 4, while the protein boost injections were given alongside the ALVAC at weeks 12 and 24 [ 19 ].
It is worth noting that both of these components had previously been tested in other trials. ALVAC-HIV, the vector prime, was not as immunogenic as some of the other vectors. AIDSVAX with a bivalent clade B gp120, the protein boost, had also been tested separately with no vector prime, and this was unsuccessful in preventing HIV-1 infection [ 14 ].
RV144 trial had a vaccine efficacy of 60% at 12 months and 31% at 3.5 years [ 17 ]. Though researchers expected the correlate of reduced risk to be CD8+ T cell response or neutralizing antibodies, the trial results indicated that the strongest correlate of reduced risk was nonneutralizing antibody response to the V1-V2 loop of gp120 [ 12 ]. The results also found that high levels of Env-specific IgA antibodies were correlated to infection risk in the vaccinated participants of the RV144 trial. Haynes et al. hypothesized that the high levels of Env-specific IgA antibodies weaken the effects of protective antibodies [ 20 ].
Given the unexpected results of RV144, there is renewed interest in the role of antibodies outside the classic role of neutralization, and this is particular focused on antibody-dependent cell-mediated cytotoxicity (ADCC) [ 12 ].
2.6. Setbacks Following RV144’s Modest Success
Based on the RV144 trial’s modest efficacy, a similar trial in South Africa, HVTN 702, was launched. The vaccine regimen was designed to increase the efficacy and immune response duration of RV144. These modifications from RV144 included changing the clade present in the vaccine due to regional differences, changing the adjuvant in the protein boost from alum to MF49, and changing the timing of the vaccinations from four injections in six months to five injections over twelve months [ 6 ].
HVTN 702 was terminated prematurely because the independent data and safety monitoring board found that the vaccine was not effective, with approximately the same number of HIV infections in the participants who received the vaccine as the participants who received the placebo [ 6 ].
The rationale behind the study design of HVTN 702 may have led to this product failure in clinical trial. The HVTN 702 clinical trial differed from the RV144 clinical trial in terms of vaccination schedule, clades, subtypes of proteins, lack of tagging of proteins, genes in immunogens, and/or adjuvants. Based on nonhuman primate studies, the alum to MF49 adjuvant change may have contributed to low efficacy in HVTN 702 [ 19 ]. MF49 adjuvant was previously utilized to increase neutralizing antibodies and T-cell responses [ 6 ] and was tested in another phase I/II clinical trial (HVTN 100). This trial found the regimen to be safe and tolerable, so HVTN 702 proceeded to test its efficacy [ 19 ]. More research is needed to understand why HVTN 702 was projected to be more effective than RV144 but showed no efficacy [ 6 ].
2.7. Broadly Neutralizing Antibodies and the Subsequent Antibody-Mediated Prevention (AMP) Trials
Researchers have attempted to produce immunogens that induce the immune system to synthesize broadly neutralizing antibodies (bNAbs) for several years. BNAbs inhibit the virions from entering the host cells, preventing HIV integration into the genome. Their role is particularly important because bNAbs are able to protect against the strain that the patient has been infected with as well as multiple different immunological strains [ 21 ]. Though Env-specific bNAbs are produced in patients with chronic HIV-1 infections, an antibody must undergo extensive somatic mutation with possible insertions or deletions in the immunoglobin heavy and light chains in the germinal center. BNAbs also typically have a third heavy-chain complementarity-determining region (HCDR3) loop, and this feature allows the antibody to combat the Env glycan shield. Some researchers tracked the evolution of the antibody to its development into a bNAb in an effort to understand the generation of bNAb [ 21 ]. However, despite all the different versions of HIV envelope glycoproteins studied and synthesized, these glycoproteins or fragments have been unable to elicit a neutralizing response to primary isolates of HIV-1 [ 7 ].
The breakthrough that synthesized bNAbs was high throughput single-cell BCR-amplification assays [ 10 ]. This was completed by separating HIV-1 Env-reactive memory B cells from antigen-specific B cells, from plasma cells, and from clonal memory B cell cultures [ 20 ]. Dozens of new antibodies, including PG6, PG16, and VRC01, have been isolated and characterized based on which target of four highly conserved regions of HIV-1 Env they bind. Scheid classified a set of potent antibodies that mimic CD4 binding as “highly active agonist CD4bs antibodies [ 10 , 22 ]”. This set includes 3BNC117 and VRC01 [ 10 ].
To investigate whether these bNAbs could induce a protective immune response in human subjects with HIV-1 infections, two early phase clinical trials were completed. Caskey et al. studied how a passive infusion of 3BNC117 affected the viral load in participants with HIV-1 infections and without HAART treatment [ 23 ]. Lynch et al. ran a similar trial with a VRC01 infusion on HAART-treated or HAART-untreated individuals with HIV-1 infections [ 24 ]. Both trials testing 3BNC117 and VRC01 indicated that infusion of a bNAb decreased the viral load in participants with HIV-1 infections not on HAART medication [ 14 , 23 , 24 ]. Since these trials utilized passive immunization, further research is necessary to adapt the bNAb to an active immunization strategy.
Researchers assessed VRC01 further and found that it protected against HIV-1 clades B and C in vitro. Subsequently, they tested VRC01 in two simultaneous proof-of-concept trials, HVTN 703 and 704, starting in 2016. These trials were completed to learn whether this bnAb could prevent HIV-1 acquisition [ 25 ].
The results of the antibody-mediated protection (AMP) trials published in 2021, shown in Table 2 , indicate that VRC01 was unable to prevent HIV-1 acquisition as compared to the placebo. However, the HIV isolates that were sensitive to VRC01 proved that bnAb does have the potential to prevent HIV-1 acquisition. Corey et al. suggested that multiple potent antibodies could be combined to result in a preventative HIV treatment [ 25 ].
2.8. Gene Therapy Application to Induce Broadly Neutralizing Antibodies
BNAb as passive immunotherapy would be challenging to administer to a small population with the health infrastructure in place and the cost of biological production. Given the obstacles to administering this therapy, it is clear that an active vaccination eliciting bNAbs would be more effective as a prophylactic vaccine. A concept to circumvent this issue is vectored immunoprophylaxis, where an adeno-associated vector and the bnAb genes are injected into the muscle. Researchers used this gene therapy concept and conducted a phase 1 trial (IAVI A003/CHOP HVDDT 001) with rAAV vector coding for PG9 antibody, shown in Table 2 . The results indicated that more research needs to be completed to increase the antibody expression because the PG9 antibody level was detected indirectly. Overall, it was safe and well-tolerated in the 16 participants tested [ 26 ].
2.9. Mosaic Vaccine Design, APPROACH, and HVTN 705
Given HIV-1’s vast genetic diversity and several strains, some researchers reconstructed global HIV-1 sequences in silico to generate mosaic immunogens [ 10 ]. These immunogens have the maximum of potential T cell epitopes and, if administered as a vaccine, induce broader cellular and humoral immune responses as compared to wild-type of consensus HIV-1 antigens [ 27 ]. In theory, mosaic antigens could generate a global HIV-1 vaccine [ 28 ]. A phase I clinical trial testing mosaic Env and Gag-pol antigen set in adenovirus serotype 26 proved that the vaccine induced strong Env-specific immune responses in the bloodstream and colorectal mucosa [ 28 ].
APPROACH, a phase I/II clinical trial, investigated the safety and effects of two different mosaic vaccines in Ad26 or MVA vectors followed by a protein boost of Env gp140 ( Table 2 ). At the end of the study, Env-specific binding antibody responses were measured for each experimental group. Though both vectors were safe and well-tolerated, Ad26 had the strongest immunogenicity. Barouch et al. found that Env-specific binding antibody responses, antibody-dependent cellular phagocytosis responses, and T-cell responses were present in 100%, 80%, and 83% of participants. This research group also administered a similar vaccine regimen in rhesus monkeys and found that it provided a 66% protection against SHIV-SF16P3 infection in six virus challenges [ 28 ]. While this is an exciting development, it is important to note that the virus challenges consisted of one strain and the mosaic vaccine may not be able to protect against different strains [ 27 ].
Building on the positive phase I/II results of the Ad26 mosaic vaccine, a phase II clinical trial, HVTN 705/Imbokodo, evaluated the efficacy and was expected to be completed in 2022 [ 28 ]. Unfortunately, this study was terminated in 2021 after the primary endpoint results showed that the vaccine did not confer any statistically significant efficacy. This study proved that the necessary immune response to confer protection against HIV is greater than the immune response to confer protection against other viruses such as COVID-19 [ 29 ]. The Ad26 vector was successfully utilized to manufacture Ad26.COV2.S, a recombinant vaccine to protect against COVID-19 [ 30 ].
2.10. HIVconsv Vaccine
Some research groups believe that a rationally designed HIVconsv immunogen is the key to an effective HIV vaccine. In theory, this immunogen causes T-cells to bind conserved regions of HIV-1 proteins and is designed with alternating clade consensus sequences between each conserved sequence. In humans, a vaccine of HIVconsv inserted into DNA, simian adenovirus, and poxvirus MVA vectors induce broadly specific T cell responses [ 8 ].
A phase I clinical trial, known as HIV-CORE 004, evaluated pSG2.HIVcons plasmid DNA, MVA.HIVconsv and Ad35-GRIN. Ad35-GRIN contained HIV-1 clade A Gag, reverse transcriptase, integrase, and Nef fusion protein (GRIN). All participants had HIVconsv-specific T cell responses. In vitro, vaccine-induced T-cells inhibited replication of the majority of viruses tested from HIV-1 clades A, B, C, and D [ 8 ].
A second generation tHIVconsvX, based on this concept, will be studied in HIV-CORE 0052, a phase I clinical trial. This is designed to test the safety and immunogenicity of the conserved mosaic HIV-1 vaccine [ 31 ].
2.11. mRNA-Based Vaccine Technology for HIV
In an attempt to produce an effective HIV-1 vaccine, researchers are developing messenger ribonucleic-acid (mRNA)-based vaccines. mRNA vaccines have been developed for Zika, influenza [ 32 ], and 2 vaccines for COVID-19 virus, BNT162b2 (Pfizer-BioNTech, New York, NY, USA) [ 33 ] and mRNA-1273 (Moderna, Cambridge, MA, USA) [ 34 ], and this is in part due to the lack of infectious risk, ease of manufacturing, and flexibility of immunogens associated with this type of vaccine.
Given the biological challenges of HIV-1, as discussed previously, this strategy to vaccination will likely be more complicated than other strategies. Mu et al. hypothesized that this scenario for a possible effective HIV-1 mRNA vaccine involves a sequential immunization of mRNAs encoding Env-based immunogens that will produce germline precursors that develop into bNAb in the germinal centers [ 21 ].
In phase 1 clinical trial of an mRNA-based HIV vaccine, IAVI G001, researchers administered participants with two doses of eOD-GT8 60mer vaccine or placebo [ 35 ]. The eOD aspect of this nanoparticle-mRNA vaccine is an engineered outer domain of the Env gp120 binding CD4. This eOD design of the vaccine targets germline B cells and helps mature them into bNAB [ 32 ]. The initial results presented at the HIV Research and Prevention virtual conference indicated that 97% of participants who received the eOD-GT8 60mer vaccine developed VRC01-class IgG B cells. These B cells are considered to be the progenitor to the VRC01 class of bNAbs. While this is an exciting development, it is essential to consider that the results have not been peer reviewed and that VRC01 antibodies must mutate to become bNAbs [ 35 ]. A similar phase 1 clinical trial, IAVI G002, is slated to begin in September 2021 [ 36 ].
2.12. Future Directions
Though there have been significant achievements in preventing HIV-1 infections in at-risk populations, it does not replace the necessity for the efficacious prophylactic vaccine. This has become apparent now more than ever because of the pandemic of COVID-19. This pandemic illustrated the significant gaps in healthcare and its accessibility to populations at risk for developing HIV-1 infections. People at risk for developing HIV-1 infections and people with HIV-1 infections have been negatively impacted in terms of HIV testing, treatment access, availability of preexposure prophylaxis, and treatment of opportunistic infections [ 37 ]. The success of developing a vaccine to protect against COVID-19 in a relatively short time frame involved collaborations, and it has renewed interest in the development of a prophylactic HIV-1 vaccine [ 38 ].
3. Conclusions
More research, funding, and clinical trials are necessary to eradicate the HIV-1 epidemic. Though researchers have been developing a vaccine for thirty years, the mosaic antigens, broadly neutralizing antibodies, and gene therapy application to induce broadly neutralizing antibodies show significant advancement and may potentially provide us with an HIV-1 vaccine in the future.
Author Contributions
Conceptualization and mentoring, S.N.M.H.; A.H. (Anna Hargrave), writing—original draft preparation; A.S.M., editing and writing; A.H. (Asma Hanif), review and editing; J.H.T., review and editing. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
Institutional Review Board Statement
Informed consent statement, data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih research matters.
December 13, 2022

Progress toward an eventual HIV vaccine
At a glance.
- An experimental HIV vaccine elicited broadly neutralizing antibody precursors in people.
- With further development, the approach could lead to an effective vaccine strategy for HIV and AIDS.

More than 1 million new HIV infections occur each year. Ending the global HIV/AIDS pandemic will require an effective HIV vaccine. Vaccines work by inducing the immune system to make antibodies that can neutralize a particular pathogen. But doing so for HIV has been challenging because there are countless variants worldwide, and the immune system doesn’t normally make antibodies that can protect against a wide range of them.
More than a decade ago , researchers at the Vaccine Research Center of NIH’s National Institute of Allergy and Infectious Diseases discovered a class of rare antibodies called broadly neutralizing antibodies (bnAbs) against HIV. These could neutralize many HIV strains at once. The bnAbs have been shown to prevent HIV infection in animals and humans. But inducing bnAbs with a vaccine has proven difficult. This is because bnAb-precursor B cells—the immune cells that develop into bnAb-producing B cells—are only rarely activated by the envelope proteins that form a protective coating for HIV.
One strategy to produce bnAbs involves specifically stimulating these rare precursor B cells. To do this, researchers led by Dr. William Schief at the Scripps Research Institute engineered a molecule based on a region of the HIV envelope protein called the CD4 binding site. They developed a modified protein to prime the precursor B cells to react. The protein, called eOD-GT8, was designed to incorporate into a self-assembling nanoparticle with 60 copies. The nanoparticle vaccine was previously shown to induce production of bnAb precursors in mice.
Based on this evidence, a research team led by Drs. Juliana McElrath at the Fred Hutchinson Cancer Center, Adrian McDermott at NIH’s Vaccine Research Center, and Schief conducted a phase 1 clinical trial of the eOD-GT8 vaccine to begin assessing its safety and efficacy in people. Results appeared in Science on December 2, 2022.
Forty-eight participants were immunized with either a low or high dose of the vaccine or a placebo. Immunization consisted of two doses, given eight weeks apart. The vaccine had a favorable safety profile, with no serious adverse events reported.
Before and after vaccination, the researchers measured the presence in participants’ blood and lymph nodes of bnAb-precursor B cells targeting eOD-GT8. They found that these cells increased in 97% of vaccine recipients after at least one of the two doses. Frequencies of these B cells increased by more than 500-fold compared to before vaccination.
The team also examined the receptors that B cells use to recognize pathogens. These receptors resemble antibodies and recognize their targets in a similar way. Receptors on the eOD-GT8-targeting bnAb-precursor B cells shared several molecular features with bnAbs. The team also saw early steps in the development of bnAbs. These include an increase in mutations in the receptor genes after the second vaccine dose and an increase in the affinity of the receptors for the vaccine.
These findings establish proof of concept and a crucial first step for the strategy of eliciting bnAbs against HIV. But this priming vaccine alone cannot induce production of mature bnAbs. Booster vaccines will be needed to elicit bnAb production and protection against HIV. The results support further development of such boosters.
“This trial and additional analyses will help inform design of the remaining stages of a candidate HIV vaccine regimen—while also enabling others in the field to develop vaccine strategies for additional viruses,” McElrath says.
—by Brian Doctrow, Ph.D.
Related Links
- “Slow” Vaccine Delivery Improves Immune Response To HIV
- Combination Antibody Treatment For HIV
- Experimental mRNA HIV Vaccine Shows Promise in Animals
- Antibody Combination Suppresses HIV
- Test Vaccine Active Against Many HIV Strains
- Engineered Antibody Protects Monkeys From HIV-Like Virus
- HIV Vaccine Progress in Animal Studies
- Antibodies Protect Human Cells from Most HIV Strains
- HIV and AIDS: Know the Facts
References: Vaccination induces HIV broadly neutralizing antibody precursors in humans. Leggat DJ, Cohen KW, Willis JR, Fulp WJ, deCamp AC, Kalyuzhniy O, Cottrell CA, Menis S, Finak G, Ballweber-Fleming L, Srikanth A, Plyler JR, Schiffner T, Liguori A, Rahaman F, Lombardo A, Philiponis V, Whaley RE, Seese A, Brand J, Ruppel AM, Hoyland W, Yates NL, Williams LD, Greene K, Gao H, Mahoney CR, Corcoran MM, Cagigi A, Taylor A, Brown DM, Ambrozak DR, Sincomb T, Hu X, Tingle R, Georgeson E, Eskandarzadeh S, Alavi N, Lu D, Mullen TM, Kubitz M, Groschel B, Maenza J, Kolokythas O, Khati N, Bethony J, Crotty S, Roederer M, Karlsson Hedestam GB, Tomaras GD, Montefiori D, Diemert D, Koup RA, Laufer DS, McElrath MJ, McDermott AB, Schief WR. Science . 2022 Dec 2;378(6623):eadd6502. doi: 10.1126/science.add6502. Epub 2022 Dec 2. PMID: 36454825.
Funding: NIH’s National Institute of Allergy and Infectious Diseases (NIAID); International AIDS Vaccine Initiative (IAVI); Bill and Melinda Gates Foundation Collaboration for AIDS Vaccine Discovery; Swedish Research Council; Ragon Institute of MGH, MIT, and Harvard.
Connect with Us
- More Social Media from NIH
To revisit this article, visit My Profile, then View saved stories .
- The Big Story
- Newsletters
- Steven Levy's Plaintext Column
- WIRED Classics from the Archive
- WIRED Insider
- WIRED Consulting
There’s New Hope for an HIV Vaccine
Since it was first identified in 1983, HIV has infected more than 85 million people and caused some 40 million deaths worldwide.
While medication known as pre-exposure prophylaxis , or PrEP, can significantly reduce the risk of getting HIV, it has to be taken every day to be effective. A vaccine to provide lasting protection has eluded researchers for decades. Now, there may finally be a viable strategy for making one.
An experimental vaccine developed at Duke University triggered an elusive type of broadly neutralizing antibody in a small group of people enrolled in a 2019 clinical trial. The findings were published today in the scientific journal Cell .
“This is one of the most pivotal studies in the HIV vaccine field to date,” says Glenda Gray, an HIV expert and the president and CEO of the South African Medical Research Council, who was not involved in the study.
A few years ago, a team from Scripps Research and the International AIDS Vaccine Initiative (IAVI) showed that it was possible to stimulate the precursor cells needed to make these rare antibodies in people. The Duke study goes a step further to generate these antibodies, albeit at low levels.
“This is a scientific feat and gives the field great hope that one can construct an HIV vaccine regimen that directs the immune response along a path that is required for protection,” Gray says.
Vaccines work by training the immune system to recognize a virus or other pathogen. They introduce something that looks like the virus—a piece of it, for example, or a weakened version of it—and by doing so, spur the body’s B cells into producing protective antibodies against it. Those antibodies stick around so that when a person later encounters the real virus, the immune system remembers and is poised to attack.
While researchers were able to produce Covid-19 vaccines in a matter of months, creating a vaccine against HIV has proven much more challenging. The problem is the unique nature of the virus. HIV mutates rapidly, meaning it can quickly outmaneuver immune defenses. It also integrates into the human genome within a few days of exposure, hiding out from the immune system.
“Parts of the virus look like our own cells, and we don’t like to make antibodies against our own selves,” says Barton Haynes, director of the Duke Human Vaccine Institute and one of the authors on the paper.
The particular antibodies that researchers are interested in are known as broadly neutralizing antibodies, which can recognize and block different versions of the virus. Because of HIV’s shape-shifting nature, there are two main types of HIV and each has several strains. An effective vaccine will need to target many of them.
Some HIV-infected individuals generate broadly neutralizing antibodies, although it often takes years of living with HIV to do so, Haynes says. Even then, people don’t make enough of them to fight off the virus. These special antibodies are made by unusual B cells that are loaded with mutations they’ve acquired over time in reaction to the virus changing inside the body. “These are weird antibodies,” Haynes says. “The body doesn’t make them easily.”

Haynes and his colleagues aimed to speed up that process in healthy, HIV-negative people. Their vaccine uses synthetic molecules that mimic a part of HIV’s outer coat, or envelope, called the membrane proximal external region. This area remains stable even as the virus mutates. Antibodies against this region can block many circulating strains of HIV.
The trial enrolled 20 healthy participants who were HIV-negative. Of those, 15 people received two of four planned doses of the investigational vaccine, and five received three doses. The trial was halted when one participant experienced an allergic reaction that was not life-threatening. The team found that the reaction was likely due to an additive in the vaccine, which they plan to remove in future testing.
Still, they found that two doses of the vaccine were enough to induce low levels of broadly neutralizing antibodies within a few weeks. Notably, B cells seemed to remain in a state of development to allow them to continue acquiring mutations, so they could evolve along with the virus. Researchers tested the antibodies on HIV samples in the lab and found that they were able to neutralize between 15 and 35 percent of them.
Jeffrey Laurence, a scientific consultant at the Foundation for AIDS Research (amfAR) and a professor of medicine at Weill Cornell Medical College, says the findings represent a step forward, but that challenges remain. “It outlines a path for vaccine development, but there’s a lot of work that needs to be done,” he says.
For one, he says, a vaccine would need to generate antibody levels that are significantly higher and able to neutralize with greater efficacy. He also says a one-dose vaccine would be ideal. “If you’re ever going to have a vaccine that’s helpful to the world, you’re going to need one dose,” he says.
Targeting more regions of the virus envelope could produce a more robust response. Haynes says the next step is designing a vaccine with at least three components, all aimed at distinct regions of the virus. The goal is to guide the B cells to become much stronger neutralizers, Haynes says. “We’re going to move forward and build on what we have learned.”
You Might Also Like …
In your inbox: The best and weirdest stories from WIRED’s archive
How the brain decides what to remember
The Big Story: Meet Priscila, queen of the rideshare mafia
Silicon Valley's soulless plutocrats flip for Donald Trump
Event: Join us for The Big Interview on December 3 in San Francisco

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Clinical trials and recent progress in HIV vaccine development
Affiliations.
- 1 Department of Biotechnology Quaid-i, Azam University Islamabad Pakistan, Islamabad Capital Territory, Pakistan. [email protected].
- 2 Department of Biotechnology Quaid-i, Azam University Islamabad Pakistan, Islamabad Capital Territory, Pakistan.
- 3 Department of Biotechnology Quaid-i, Azam University Islamabad Pakistan, Islamabad Capital Territory, Pakistan. [email protected].
- PMID: 39192058
- DOI: 10.1007/s10142-024-01425-9
The greatest obstacle for scientists is to develop an effective HIV vaccine. An effective vaccine represents the last hope for halting the unstoppable global spread of HIV and its catastrophic clinical consequences. Creating this vaccine has been challenging due to the virus's extensive genetic variability and the unique role of cytotoxic T lymphocytes (CTL) in containing it. Innovative methods to stimulate CTL have demonstrated significant therapeutic advantages in nonhuman primate model systems, unlike traditional vaccination techniques that are not expected to provide safe and efficient protection against HIV. Human clinical trials are currently evaluating these vaccination strategies, which involve plasmid DNA and live recombinant vectors. This review article covers the existing vaccines and ongoing trial vaccines. It also explores the different approaches used in developing HIV vaccines, including their molecular mechanisms, target site effectiveness, and potential side effects.
Keywords: Enveloped-based vaccines; HIV; Recombinant vaccines; Viral-based vaccines non-structural based vaccines.
© 2024. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
PubMed Disclaimer
- Ajbani S (2016a) HIV vaccine development: current scenario and future prospects. J AIDS Clin Res, 7(11), 626. DOI: https://doi.org/610.4172/2155-6113.1000626
- Ajbani S (2016b) HIV vaccine development: current scenario and future prospects. J AIDS Clin Res, 7(11), 626.DOI: https://doi.org/610.4172/2155-6113.1000626
- Andersen-Nissen E, Fiore-Gartland A, Ballweber Fleming L, Carpp LN, Naidoo AF, Harper MS, Voillet V, Grunenberg N, Laher F, Innes C (2021) Innate immune signatures to a partially-efficacious HIV vaccine predict correlates of HIV-1 infection risk. PLoS pathogens, 17(3), e1009363. https://doi.org/1009310.1001371/journal.ppat.1009363
- Andrabi R, Bhiman JN, Burton DR (2018) Strategies for a multi-stage neutralizing antibody-based HIV vaccine. Curr Opin Immunol 53:143–151. https://doi.org/110.1016/j.coi.2018.1004.1025
- Åsjö B, Stavang H, Sørensen B, Baksaas I, Nyhus J, Langeland N (2002) Phase I trial of a therapeutic HIV type 1 vaccine, Vacc-4x, in HIV type 1-infected individuals with or without antiretroviral therapy. AIDS Res Hum Retroviruses 18(18):1357–1365. https://doi.org/1310.1089/088922202320935438 - DOI - PubMed
Publication types
- Search in MeSH
Related information
Linkout - more resources.
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
New study highlights potential of childhood immunization against HIV
- Download PDF Copy
Research at Weill Cornell Medicine suggests that childhood immunization against HIV could one day provide protection before risk of contracting this potentially fatal infection dramatically increases in adolescence.
The study, published Aug. 30 in Science Immunology, demonstrated that a series of six vaccinations containing a modified protein from the surface of HIV particles stimulated initial steps of a potent immune response in young non-human primates. This difficult-to-achieve response represents an important step toward providing full and potentially life-long protection against the virus, the researchers say.
Immunizing young children, rather than adults, makes sense because risk factors for HIV infection rise steeply when adolescents become sexually active, according to senior author Dr. Sallie Permar, the Nancy C. Paduano Professor in Pediatrics and chair of the Department of Pediatrics at Weill Cornell Medicine.
What's more, evidence suggests that the immune systems of infants and children generally mount more effective responses to the virus than those of adults. One of the advancements we've made is to demonstrate that an HIV vaccine could be delivered on a schedule similar to routine vaccines already given to babies and children." Dr. Sallie Permar, the Nancy C. Paduano Professor in Pediatrics and chair of the Department of Pediatrics at Weill Cornell Medicine
Prepping the immune system early
HIV predominantly infects immune cells called CD4 T cells , leaving individuals vulnerable to opportunistic diseases. Without lifelong treatment, infection is fatal. In 2022, an estimated 140,000 adolescents between 10 and 19 years old worldwide became infected with the virus—a group that is overrepresented in the number of new infections.
Vaccine researchers are seeking ways to stimulate the immune system to make "broadly neutralizing antibodies " against the virus before a person is exposed to it. These antibodies attack a crucial part of the HIV virus—the protein on its surface that binds to CD4 T cells. In doing so, broadly neutralizing antibodies prevent many strains of HIV from entering the cell and infecting it.
In this study, the researchers started with an experimental vaccine developed previously from spike proteins on the envelope of HIV particles. Study authors Dr. John Moore, a professor of microbiology and immunology, and Dr. Rogier Sanders, an adjunct associate professor of research in microbiology and immunology at Weill Cornell Medicine and a professor at Amsterdam UMC, sought to improve this vaccine by altering the viral protein. They designed these changes to stimulate a specific set of antibody-producing B cells that protect CD4 T cells.
Related Stories
- Clarification urgently needed for detected signal of semaglutide-linked suicidal ideation
- Research identifies 31 additional cancers potentially linked to obesity
- Untreated hypertension increases Alzheimer’s risk, research shows
"An effective HIV vaccine needs to engage the right set of B cells in order to generate a broadly protective response," said first author Dr. Ashley Nelson, an assistant professor of immunology research in pediatrics at Weill Cornell Medicine. "We discovered that introducing certain mutations into the envelope protein could accomplish that in the setting of a naïve immune system."
Activating the right B cells for protection
The researchers administered the modified vaccine to five young primates in three priming doses, starting less than a week after birth. They followed up with three doses of the vaccine matching the original HIV envelope protein, with the last dose given when the animals reached 78 weeks old, roughly equivalent to four or five years old for a human. As a control, five animals received all six doses of the original envelope protein vaccine.
"While exposure to the modified protein got the immune response started off in the right direction, booster shots containing the original version of the viral protein were necessary to reach full potential," Dr. Nelson said.
Three of the five animals who received the modified version of the priming vaccine developed antibodies that appeared to be precursors to the sought-after broadly neutralizing response. Tests suggested these antibodies attacked the site the virus uses to invade CD4 T cells. However, they were not yet fully effective against the same breadth of HIV strains as mature broadly neutralizing antibodies. One of the three animals also showed signs of developing the mature, broadly neutralizing response.
The next step is figuring out how to reliably elicit a full-on broadly neutralizing response, Dr. Nelson said. "We still need to identify the right combination of viral proteins to get us further down that path, starting from the earliest stages in life when multi-dose vaccines are commonly given."
Weill Cornell Medicine
Nelson, A. N., et al. (2024) Immunization with germ line–targeting SOSIP trimers elicits broadly neutralizing antibody precursors in infant macaques . Science Immunology . doi.org/10.1126/sciimmunol.adm7097 .
Posted in: Child Health News | Medical Research News | Disease/Infection News
Tags: Adolescents , Allergy , Antibodies , Antibody , CD4 , Cell , Children , HIV , Immune Response , Immune System , Immunization , Immunology , Infectious Diseases , Medicine , Microbiology , Pediatrics , Protein , Research , Vaccine , Virus
Suggested Reading

Cancel reply to comment
- Trending Stories
- Latest Interviews
- Top Health Articles

How can microdialysis benefit drug development
Ilona Vuist
In this interview, discover how Charles River uses the power of microdialysis for drug development as well as CNS therapeutics.

Global and Local Efforts to Take Action Against Hepatitis
Lindsey Hiebert and James Amugsi
In this interview, we explore global and local efforts to combat viral hepatitis with Lindsey Hiebert, Deputy Director of the Coalition for Global Hepatitis Elimination (CGHE), and James Amugsi, a Mandela Washington Fellow and Physician Assistant at Sandema Hospital in Ghana. Together, they provide valuable insights into the challenges, successes, and the importance of partnerships in the fight against hepatitis.

Addressing Important Cardiac Biology Questions with Shotgun Top-Down Proteomics
In this interview conducted at Pittcon 2024, we spoke to Professor John Yates about capturing cardiomyocyte cell-to-cell heterogeneity via shotgun top-down proteomics.

Latest News

Newsletters you may be interested in
Your AI Powered Scientific Assistant
Hi, I'm Azthena, you can trust me to find commercial scientific answers from News-Medical.net.
A few things you need to know before we start. Please read and accept to continue.
- Use of “Azthena” is subject to the terms and conditions of use as set out by OpenAI .
- Content provided on any AZoNetwork sites are subject to the site Terms & Conditions and Privacy Policy .
- Large Language Models can make mistakes. Consider checking important information.
Great. Ask your question.
Azthena may occasionally provide inaccurate responses. Read the full terms .
While we only use edited and approved content for Azthena answers, it may on occasions provide incorrect responses. Please confirm any data provided with the related suppliers or authors. We do not provide medical advice, if you search for medical information you must always consult a medical professional before acting on any information provided.
Your questions, but not your email details will be shared with OpenAI and retained for 30 days in accordance with their privacy principles.
Please do not ask questions that use sensitive or confidential information.
Read the full Terms & Conditions .
Provide Feedback

Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Identification of a clade-specific hla-c*03:02 ctl epitope gy9 derived from the hiv-1 p17 matrix protein.

1. Introduction
2.1. hiv-1 clades c and a have private and shared hla-c*03:02-epitopes and preferentially accommodate hydrophobic residues in the distal pocket, 2.2. c*03:02-restricted stable epitopes are mainly derived from structural proteins of hiv, 2.3. positions 62, 142, and 151 in hla-c*03:02 and p6 in the gy9 epitope provide the structural basis for the preferential binding of gy9, 2.4. the gy9 epitope elicits a clade-specific hla-c*03:02 ifn-γ response, 3. discussion, limitations of the study, 4. materials and methods, 4.1. patient recruitment, 4.2. hla-c*03:02 homology modeling and validation, 4.3. hiv-1 ligand prediction and preparation for docking, 4.4. molecular docking protocol and analysis, 4.5. molecular dynamics simulation protocol and analysis, 4.6. hiv-1 epitope conservancy analysis, 4.7. hiv-1 genotyping, 4.8. hiv-1-specific ifn-γ and il-2 dual elispot assay, 5. conclusions, supplementary materials, author contributions, institutional review board statement, informed consent statement, data availability statement, acknowledgments, conflicts of interest.
- McLaren, P.J.; Carrington, M. The Impact of Host Genetic Variation on Infection with HIV-1. Nat. Immunol. 2015 , 16 , 577–583. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cohen, O.J.; Paolucci, S.; Bende, S.M.; Daucher, M.; Moriuchi, H.; Moriuchi, M.; Cicala, C.; Davey, R.T.; Baird, B.; Fauci, A.S. CXCR4 and CCR5 Genetic Polymorphisms in Long-Term Nonprogressive Human Immunodeficiency Virus Infection: Lack of Association with Mutations Other than CCR5-Δ32. J. Virol. 1998 , 72 , 6215–6217. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Walker, B.D.; Pereyra, F.; Jia, X.; McLaren, P.J.; Telenti, A.; de Bakker, P.I.W.; De Bakker, P.I.W.; Walker, B.D. The Major Genetic Determinants of HIV-1 Control Affect HLA Class I Peptide Presentation. Science 2010 , 330 , 1551–1557. [ Google Scholar ] [ CrossRef ]
- Boudreau, J.E.; Hsu, K.C. Natural Killer Cell Education in Human Health and Disease. Curr. Opin. Immunol. 2018 , 50 , 102–111. [ Google Scholar ] [ CrossRef ]
- Kaseke, C.; Tano-Menka, R.; Senjobe, F.; Gaiha, G.D. The Emerging Role for CTL Epitope Specificity in HIV Cure Efforts. J. Infect. Dis. 2021 , 223 , S32–S37. [ Google Scholar ] [ CrossRef ]
- Pymm, P.; Tenzer, S.; Wee, E.; Weimershaus, M.; Burgevin, A.; Kollnberger, S.; Gerstoft, J.; Josephs, T.M.; Ladell, K.; McLaren, J.E.; et al. Epitope Length Variants Balance Protective Immune Responses and Viral Escape in HIV-1 Infection. Cell Rep. 2022 , 38 , 110449. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kutsch, O.; Vey, T.; Kerkau, T.; Hünig, T.; Schimpl, A. HIV Type 1 Abrogates TAP-Mediated Transport of Antigenic Peptides Presented by MHC Class I. AIDS Res. Hum. Retroviruses 2002 , 18 , 1319–1325. [ Google Scholar ] [ CrossRef ]
- Li, L.; Bouvier, M. Structures of HLA-A*1101 Complexed with Immunodominant Nonamer and Decamer HIV-1 Epitopes Clearly Reveal the Presence of a Middle, Secondary Anchor Residue. J. Immunol. 2004 , 172 , 6175–6184. [ Google Scholar ] [ CrossRef ]
- Carlson, J.M.; Brumme, C.J.; Martin, E.; Listgarten, J.; Brockman, M.A.; Le, A.Q.; Chui, C.K.S.; Cotton, L.A.; Knapp, D.J.H.F.; Riddler, S.A.; et al. Correlates of Protective Cellular Immunity Revealed by Analysis of Population-Level Immune Escape Pathways in HIV-1. J. Virol. 2012 , 86 , 13202–13216. [ Google Scholar ] [ CrossRef ]
- Stewart-Jones, G.B.E.; Gillespie, G.; Overton, I.M.; Kaul, R.; Roche, P.; McMichael, A.J.; Rowland-Jones, S.; Jones, E.Y. Structures of Three HIV-1 HLA-B*5703-Peptide Complexes and Identification of Related HLAs Potentially Associated with Long-Term Nonprogression. J. Immunol. 2005 , 175 , 2459–2468. [ Google Scholar ] [ CrossRef ]
- Kyobe, S.; Mwesigwa, S.; Kisitu, G.P.; Farirai, J.; Katagirya, E.; Mirembe, A.N.; Ketumile, L.; Wayengera, M.; Katabazi, F.A.; Kigozi, E.; et al. Exome Sequencing Reveals a Putative Role for HLA-C*03:02 in Control of HIV-1 in African Pediatric Populations. Front. Genet. 2021 , 12 , 720213. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Leslie, A.; Matthews, P.C.; Listgarten, J.; Carlson, J.M.; Kadie, C.; Ndung’U, T.; Brander, C.; Coovadia, H.; Walker, B.D.; Heckerman, D.; et al. Additive Contribution of HLA Class I Alleles in the Immune Control of HIV-1 Infection. J. Virol. 2010 , 84 , 9879–9888. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gonzalez-Galarza, F.F.; McCabe, A.; dos Santos, E.J.M.; Jones, J.; Takeshita, L.; Ortega-Rivera, N.D.; Del Cid-Pavon, G.M.; Ramsbottom, K.; Ghattaoraya, G.; Alfirevic, A.; et al. Allele Frequency Net Database (AFND) 2020 Update: Gold-Standard Data Classification, Open Access Genotype Data and New Query Tools. Nucleic Acids Res. 2020 , 48 , D783–D788. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gijsbers, E.F.; Anton Feenstra, K.; van Nuenen, A.C.; Navis, M.; Heringa, J.; Schuitemaker, H.; Kootstra, N.A. HIV-1 Replication Fitness of HLA-B*57/58:01 CTL Escape Variants Is Restored by the Accumulation of Compensatory Mutations in Gag. PLoS ONE 2013 , 8 , e81235. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Borghans, J.A.M.; Mølgaard, A.; de Boer, R.J.; Keşmir, C. HLA Alleles Associated with Slow Progression to AIDS Truly Prefer to Present HIV-1 p24. PLoS ONE 2007 , 2 , e920. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chen, H.; Piechocka-Trocha, A.; Miura, T.; Brockman, M.A.; Julg, B.D.; Baker, B.M.; Rothchild, A.C.; Block, B.L.; Schneidewind, A.; Koibuchi, T.; et al. Differential Neutralization of Human Immunodeficiency Virus (HIV) Replication in Autologous CD4 T Cells by HIV-Specific Cytotoxic T Lymphocytes. J. Virol. 2009 , 83 , 3138–3149. [ Google Scholar ] [ CrossRef ]
- Murakoshi, H.; Zou, C.; Kuse, N.; Akahoshi, T.; Chikata, T.; Gatanaga, H.; Oka, S.; Hanke, T.; Takiguchi, M. CD8+ T Cells Specific for Conserved, Cross-Reactive Gag Epitopes with Strong Ability to Suppress HIV-1 Replication. Retrovirology 2018 , 15 , 46. [ Google Scholar ] [ CrossRef ]
- Adland, E.; Carlson, J.M.; Paioni, P.; Kløverpris, H.; Shapiro, R.; Ogwu, A.; Riddell, L.; Luzzi, G.; Chen, F.; Balachandran, T.; et al. Nef-Specific CD8+ T Cell Responses Contribute to HIV-1 Immune Control. PLoS ONE 2013 , 8 , e73117. [ Google Scholar ] [ CrossRef ]
- Altfeld, M.; Addo, M.M.; Eldridge, R.L.; Yu, X.G.; Thomas, S.; Khatri, A.; Strick, D.; Phillips, M.N.; Cohen, G.B.; Islam, S.A.; et al. Vpr Is Preferentially Targeted by CTL During HIV-1 Infection. J. Immunol. 2001 , 167 , 2743–2752. [ Google Scholar ] [ CrossRef ]
- Ferrando-Martínez, S.; Casazza, J.P.; Leal, M.; Machmach, K.; Muñoz-Fernández, M.; Viciana, P.; Koup, R.A.; Ruiz-Mateos, E. Differential Gag-Specific Polyfunctional T Cell Maturation Patterns in HIV-1 Elite Controllers. J. Virol. 2012 , 86 , 3667–3674. [ Google Scholar ] [ CrossRef ]
- Falivene, J.; Ghiglione, Y.; Laufer, N.; Socías, M.E.; Holgado, M.P.; Ruiz, M.J.; Maeto, C.; Figueroa, M.I.; Giavedoni, L.D.; Cahn, P.; et al. Th17 and Th17/Treg Ratio at Early HIV Infection Associate with Protective HIV-Specific CD8+ T-Cell Responses and Disease Progression. Sci. Rep. 2015 , 5 , 11511. [ Google Scholar ] [ CrossRef ]
- Boulet, S.; Song, R.; Kamya, P.; Bruneau, J.; Shoukry, N.H.; Tsoukas, C.M.; Bernard, N.F. HIV Protective KIR3DL1 and HLA-B Genotypes Influence NK Cell Function Following Stimulation with HLA-Devoid Cells. J. Immunol. 2010 , 184 , 2057–2064. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zappacosta, F.; Borrego, F.; Brooks, A.G.; Parker, K.C.; Coligan, J.E. Peptides Isolated from HLA-Cw*0304 Confer Different Degrees of Protection from Natural Killer Cell-Mediated Lysis. Proc. Natl. Acad. Sci. USA 1997 , 94 , 6313–6318. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- del Moral-Sánchez, I.; Sliepen, K. Strategies for Inducing Effective Neutralizing Antibody Responses against HIV-1. Expert Rev. Vaccines 2019 , 18 , 1127–1143. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Arnold, G.F.; Velasco, P.K.; Holmes, A.K.; Wrin, T.; Geisler, S.C.; Phung, P.; Tian, Y.; Resnick, D.A.; Ma, X.; Mariano, T.M.; et al. Broad Neutralization of Human Immunodeficiency Virus Type 1 (HIV-1) Elicited from Human Rhinoviruses That Display the HIV-1 gp41 ELDKWA Epitope. J. Virol. 2009 , 83 , 5087–5100. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Payne, R.P.; Branch, S.; Kløverpris, H.; Matthews, P.C.; Koofhethile, C.K.; Strong, T.; Adland, E.; Leitman, E.; Frater, J.; Ndung’U, T.; et al. Differential Escape Patterns within the Dominant HLA-B*57:03-Restricted HIV Gag Epitope Reflect Distinct Clade-Specific Functional Constraints. J. Virol. 2014 , 88 , 4668–4678. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Brackenridge, S.; Evans, E.J.; Toebes, M.; Goonetilleke, N.; Liu, M.K.P.; di Gleria, K.; Schumacher, T.N.; Davis, S.J.; McMichael, A.J.; Gillespie, G.M. An Early HIV Mutation within an HLA-B*57-Restricted T Cell Epitope Abrogates Binding to the Killer Inhibitory Receptor 3DL1. J. Virol. 2011 , 85 , 5415–5422. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yang, Y.; Sun, W.; Guo, J.; Zhao, G.; Sun, S.; Yu, H.; Guo, Y.; Li, J.; Jin, X.; Du, L.; et al. In Silico Design of a DNA-Based HIV-1 Multi-Epitope Vaccine for Chinese Populations. Hum. Vaccines Immunother. 2015 , 11 , 795–805. [ Google Scholar ] [ CrossRef ]
- Haynes, B.F.; McElrath, M.J. Progress in HIV-1 Vaccine Development. Curr. Opin. HIV AIDS 2013 , 8 , 326–332. [ Google Scholar ] [ CrossRef ]
- Song, H.; Giorgi, E.E.; Ganusov, V.V.; Cai, F.; Athreya, G.; Yoon, H.; Carja, O.; Hora, B.; Hraber, P.; Romero-Severson, E.; et al. Tracking HIV-1 Recombination to Resolve Its Contribution to HIV-1 Evolution in Natural Infection. Nat. Commun. 2018 , 9 , 1928. [ Google Scholar ] [ CrossRef ]
- Hraber, P.; Korber, B.T.; Lapedes, A.S.; Bailer, R.T.; Seaman, M.S.; Gao, H.; Greene, K.M.; McCutchan, F.; Williamson, C.; Kim, J.H.; et al. Impact of Clade, Geography, and Age of the Epidemic on HIV-1 Neutralization by Antibodies. J. Virol. 2014 , 88 , 12623–12643. [ Google Scholar ] [ CrossRef ]
- Chikata, T.; Paes, W.; Akahoshi, T.; Partridge, T.; Murakoshi, H.; Gatanaga, H.; Ternette, N.; Oka, S.; Borrow, P.; Takiguchi, M. Identification of Immunodominant HIV-1 Epitopes Presented by HLA-C*12:02, a Protective Allele, Using an Immunopeptidomics Approach. J. Virol. 2019 , 93 , e00634-19. [ Google Scholar ] [ CrossRef ]
- Bugembe, D.L.; Ekii, A.O.; Ndembi, N.; Serwanga, J.; Kaleebu, P.; Pala, P. Computational MHC-I Epitope Predictor Identifies 95% of Experimentally Mapped HIV-1 Clade A and D Epitopes in a Ugandan Cohort. BMC Infect. Dis. 2020 , 20 , 172. [ Google Scholar ] [ CrossRef ]
- Hsin, J.; Arkhipov, A.; Yin, Y.; Stone, J.E.; Schulten, K. Using VMD: An Introductory Tutorial. Curr. Protoc. Bioinform. 2008 , 24 , 5.7.1–5.7.48. [ Google Scholar ] [ CrossRef ]
- Knapp, B.; van der Merwe, P.A.; Dushek, O.; Deane, C.M. MHC Binding Affects the Dynamics of Different T-Cell Receptors in Different Ways. PLoS Comput. Biol. 2019 , 15 , e1007338. [ Google Scholar ] [ CrossRef ]
- Li, X.; Singh, N.K.; Collins, D.R.; Ng, R.; Zhang, A.; Lamothe-Molina, P.A.; Shahinian, P.; Xu, S.; Tan, K.; Piechocka-Trocha, A.; et al. Molecular Basis of Differential HLA Class I-Restricted T Cell Recognition of a Highly Networked HIV Peptide. Nat. Commun. 2023 , 14 , 2929. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. Gmx_MMPBSA: A New Tool to Perform End-State Free Energy Calculations with GROMACS. J. Chem. Theory Comput. 2021 , 17 , 6281–6291. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ndongala, M.L.; Kamya, P.; Boulet, S.; Peretz, Y.; Rouleau, D.; Tremblay, C.; Leblanc, R.; Côté, P.; Baril, J.; Thomas, R.; et al. Changes in Function of HIV-Specific T-Cell Responses with Increasing Time from Infection. Viral Immunol. 2010 , 23 , 159–168. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Excler, J.-L.; Robb, M.L.; Kim, J.H. Prospects for a Globally Effective HIV-1 Vaccine. Vaccine 2015 , 33 , D4–D12. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cohen, G.B.; Gandhi, R.T.; Davis, D.M.; Mandelboim, O.; Chen, B.K.; Strominger, J.L.; Baltimore, D. The Selective Downregulation of Class I Major Histocompatibility Complex Proteins by HIV-1 Protects HIV-Infected Cells from NK Cells. Immunity 1999 , 10 , 661–671. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mann, J.K.; Byakwaga, H.; Kuang, X.T.; Le, A.Q.; Brumme, C.J.; Mwimanzi, P.; Omarjee, S.; Martin, E.; Lee, G.Q.; Baraki, B.; et al. Ability of HIV-1 Nef to Downregulate CD4 and HLA Class I Differs among Viral Subtypes. Retrovirology 2013 , 10 , 100. [ Google Scholar ] [ CrossRef ]
- Fellay, J.; Shianna, K.V.; Ge, D.; Colombo, S.; Ledergerber, B.; Weale, M.; Zhang, K.; Gumbs, C.; Castagna, A.; Cossarizza, A.; et al. A Whole-Genome Association Study of Major Determinants for Host Control of HIV-1. Science 2007 , 317 , 944–947. [ Google Scholar ] [ CrossRef ]
- Kulkarni, S.; Savan, R.; Qi, Y.; Gao, X.; Yuki, Y.; Bass, S.E.; Martin, M.P.; Hunt, P.; Deeks, S.G.; Telenti, A.; et al. Differential microRNA Regulation of HLA-C Expression and Its Association with HIV Control. Nature 2011 , 472 , 495–498. [ Google Scholar ] [ CrossRef ]
- Thomas, R.; Apps, R.; Qi, Y.; Gao, X.; Male, V.; O’Huigin, C.; O’Connor, G.; Ge, D.; Fellay, J.; Martin, J.N.; et al. HLA-C cell Surface Expression and Control of HIV/AIDS Correlate with a Variant Upstream of HLA-C. Nat. Genet. 2009 , 41 , 1290–1294. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Korber, B.; Fischer, W. T Cell-Based Strategies for HIV-1 Vaccines. Hum. Vaccines Immunother. 2020 , 16 , 713–722. [ Google Scholar ] [ CrossRef ]
- Shin, S.Y. Recent Update in HIV Vaccine Development. Clin. Exp. Vaccine Res. 2016 , 5 , 6–11. [ Google Scholar ] [ CrossRef ]
- Zipeto, D.; Beretta, A. HLA-C and HIV-1: Friends or Foes? Retrovirology 2012 , 9 , 39. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Buranapraditkun, S.; Hempel, U.; Pitakpolrat, P.; Allgaier, R.L.; Thantivorasit, P.; Lorenzen, S.-I.; Sirivichayakul, S.; Hildebrand, W.H.; Altfeld, M.; Brander, C.; et al. A Novel Immunodominant CD8+ T Cell Response Restricted by a Common HLA-C Allele Targets a Conserved Region of Gag HIV-1 Clade CRF01_AE Infected Thais. PLoS ONE 2011 , 6 , e23603. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hashimoto, M.; Akahoshi, T.; Murakoshi, H.; Ishizuka, N.; Oka, S.; Takiguchi, M. CTL Recognition of HIV-1-Infected Cells Via Cross-Recognition of Multiple Overlapping Peptides from a Single 11-Mer Pol Sequence. Eur. J. Immunol. 2012 , 42 , 2621–2631. [ Google Scholar ] [ CrossRef ]
- Perdomo-Celis, F.; Taborda, N.A.; Rugeles, M.T. CD8+ T-Cell Response to HIV Infection in the Era of Antiretroviral Therapy. Front. Immunol. 2019 , 10 , 1896. [ Google Scholar ] [ CrossRef ]
- Addo, M.M.; Yu, X.G.; Rathod, A.; Cohen, D.; Eldridge, R.L.; Strick, D.; Johnston, M.N.; Corcoran, C.; Wurcel, A.G.; Fitzpatrick, C.A.; et al. Comprehensive Epitope Analysis of Human Immunodeficiency Virus Type 1 (HIV-1)-Specific T-Cell Responses Directed against the Entire Expressed HIV-1 Genome Demonstrate Broadly Directed Responses, but No Correlation to Viral Load. J. Virol. 2003 , 77 , 2081–2092. [ Google Scholar ] [ CrossRef ]
- Rosás-Umbert, M.; Gunst, J.D.; Pahus, M.H.; Olesen, R.; Schleimann, M.; Denton, P.W.; Ramos, V.; Ward, A.; Kinloch, N.N.; Copertino, D.C.; et al. Administration of Broadly Neutralizing Anti-HIV-1 Antibodies at ART Initiation Maintains Long-Term CD8+ T Cell Immunity. Nat. Commun. 2022 , 13 , 6473. [ Google Scholar ] [ CrossRef ]
- Joglekar, A.V.; Liu, Z.; Weber, J.K.; Ouyang, Y.; Jeppson, J.D.; Noh, W.J.; Lamothe-Molina, P.A.; Chen, H.; Kang, S.-G.; Bethune, M.T.; et al. T Cell Receptors for the HIV KK10 Epitope from Patients with Differential Immunologic Control Are Functionally Indistinguishable. Proc. Natl. Acad. Sci. USA 2018 , 115 , 1877–1882. [ Google Scholar ] [ CrossRef ]
- Tassiopoulos, K.; Landay, A.; Collier, A.C.; Connick, E.; Deeks, S.G.; Hunt, P.; Lewis, D.E.; Wilson, C.; Bosch, R. CD28-Negative CD4+ and CD8+ T Cells in Antiretroviral Therapy–Naive HIV-Infected Adults Enrolled in Adult Clinical Trials Group Studies. J. Infect. Dis. 2012 , 205 , 1730–1738. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ziegler, M.C.; Nelde, A.; Weber, J.K.; Schreitmüller, C.M.; Martrus, G.; Huynh, T.; Bunders, M.J.; Lunemann, S.; Stevanovic, S.; Zhou, R.; et al. HIV-1 Induced Changes in HLA-C∗03: 04-Presented Peptide Repertoires Lead to Reduced Engagement of Inhibitory Natural Killer Cell Receptors. AIDS 2020 , 34 , 1713–1723. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Knapp, B.; Ospina, L.; Deane, C.M. Avoiding False Positive Conclusions in Molecular Simulation: The Importance of Replicas. J. Chem. Theory Comput. 2018 , 14 , 6127–6138. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wan, S.; Knapp, B.; Wright, D.W.; Deane, C.M.; Coveney, P.V. Rapid, Precise, and Reproducible Prediction of Peptide–MHC Binding Affinities from Molecular Dynamics That Correlate Well with Experiment. J. Chem. Theory Comput. 2015 , 11 , 3346–3356. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yusuf, M.; Destiarani, W.; Widayat, W.; Yosua, Y.; Gumilar, G.; Tanudireja, A.S.; Rohmatulloh, F.G.; Maulana, F.A.; Baroroh, U.; Hardianto, A.; et al. Coarse-Grained Molecular Dynamics-Guided Immunoinformatics to Explain the Binder and Non-Binder Classification of Cytotoxic T-Cell Epitope for SARS-CoV-2 Peptide-Based Vaccine Discovery. PLoS ONE 2023 , 18 , e0292156. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Koita, O.A.; Dabitao, D.; Mahamadou, I.; Tall, M.; Dao, S.; Tounkara, A.; Guiteye, H.; Noumsi, C.; Thiero, O.; Kone, M.; et al. Confirmation of Immunogenic Consensus Sequence HIV-1 T-cell Epitopes in Bamako, Mali and Providence, Rhode Island. Hum. Vaccines 2006 , 2 , 119–128. [ Google Scholar ] [ CrossRef ]
- Goepfert, P.A.; Bansal, A.; Edwards, B.H.; Ritter, G.D.; Tellez, I.; McPherson, S.A.; Sabbaj, S.; Mulligan, M.J. A Significant Number of Human Immunodeficiency Virus Epitope-Specific Cytotoxic T Lymphocytes Detected by Tetramer Binding Do Not Produce Gamma Interferon. J. Virol. 2000 , 74 , 10249–10255. [ Google Scholar ] [ CrossRef ]
- Retshabile, G.; Mlotshwa, B.C.; Williams, L.; Mwesigwa, S.; Mboowa, G.; Huang, Z.; Rustagi, N.; Swaminathan, S.; Katagirya, E.; Kyobe, S.; et al. Whole-Exome Sequencing Reveals Uncaptured Variation and Distinct Ancestry in the Southern African Population of Botswana. Am. J. Hum. Genet. 2018 , 102 , 731–743. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bbosa, N.; Kaleebu, P.; Ssemwanga, D. HIV Subtype Diversity Worldwide. Curr. Opin. HIV AIDS 2019 , 14 , 153–160. [ Google Scholar ] [ CrossRef ]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera? A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004 , 25 , 1605–1612. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Antunes, D.A.; Moll, M.; Devaurs, D.; Jackson, K.R.; Lizée, G.; Kavraki, L.E. DINC 2.0: A New Protein–Peptide Docking Webserver Using an Incremental Approach. Cancer Res 2017 , 77 , e55–e57. [ Google Scholar ] [ CrossRef ]
- El-Hachem, N.; Haibe-Kains, B.; Khalil, A.; Kobeissy, F.H.; Nemer, G. AutoDock and AutoDockTools for Protein-Ligand Docking: Beta-Site Amyloid Precursor Protein Cleaving Enzyme 1(BACE1) as a Case Study. In Methods in Molecular Biology ; Springer: Berlin/Heidelberg, Germany, 2017; pp. 391–403. [ Google Scholar ]
- Kadukova, M.; Grudinin, S. Convex-PL: A Novel Knowledge-Based Potential for Protein-Ligand Interactions Deduced from Structural Databases Using Convex Optimization. J. Comput. Aided. Mol. Des. 2017 , 31 , 943–958. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure Visualization for Researchers, Educators, and Developers. Protein Sci. 2021 , 30 , 70–82. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015 , 1–2 , 19–25. [ Google Scholar ] [ CrossRef ]
- Best, R.B.; Zhu, X.; Shim, J.; Lopes, P.E.M.; Mittal, J.; Feig, M.; MacKerell, A.D. Optimization of the Additive CHARMM All-Atom Protein Force Field Targeting Improved Sampling of the Backbone ϕ, ψ and Side-Chain χ 1 and χ 2 Dihedral Angles. J. Chem. Theory Comput. 2012 , 8 , 3257–3273. [ Google Scholar ] [ CrossRef ]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM General Force Field: A Force Field for Drug-like Molecules Compatible with the CHARMM All-Atom Additive Biological Force Fields. J. Comput. Chem. 2010 , 31 , 671–690. [ Google Scholar ] [ CrossRef ]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012 , 4 , 17. [ Google Scholar ] [ CrossRef ]
- Miah, M.M.; Tabassum, N.; Afroj Zinnia, M.; Islam, A.B.M.M.K. Drug and Anti-Viral Peptide Design to Inhibit the Monkeypox Virus by Restricting A36R Protein. Bioinform. Biol. Insights 2022 , 16 , 11779322221141164. [ Google Scholar ] [ CrossRef ]
- Vishnoi, S.; Bhattacharya, S.; Walsh, E.M.; Okoh, G.I.; Thompson, D. Computational Peptide Design Cotargeting Glucagon and Glucagon-like Peptide-1 Receptors. J. Chem. Inf. Model. 2023 , 63 , 4934–4947. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lapointe, H.R.; Dong, W.; Lee, G.Q.; Bangsberg, D.R.; Martin, J.N.; Mocello, A.R.; Boum, Y.; Karakas, A.; Kirkby, D.; Poon, A.F.Y.; et al. HIV Drug Resistance Testing by High-Multiplex “Wide” Sequencing on the MiSeq Instrument. Antimicrob. Agents Chemother. 2015 , 59 , 6824–6833. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tachbele, E.; Kyobe, S.; Katabazi, F.A.; Kigozi, E.; Mwesigwa, S.; Joloba, M.; Messele, A.; Amogne, W.; Legesse, M.; Pieper, R.; et al. Genetic Diversity and Acquired Drug Resistance Mutations Detected by Deep Sequencing in Virologic Failures among Antiretroviral Treatment Experienced Human Immunodeficiency Virus-1 Patients in a Pastoralist Region of Ethiopia. Infect. Drug Resist. 2021 , 14 , 4833–4847. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pineda-Peña, A.-C.; Faria, N.R.; Imbrechts, S.; Libin, P.; Abecasis, A.B.; Deforche, K.; Gómez-López, A.; Camacho, R.J.; de Oliveira, T.; Vandamme, A.-M. Automated Subtyping of HIV-1 Genetic Sequences for Clinical and Surveillance Purposes: Performance Evaluation of the New REGA Version 3 and Seven Other Tools. Infect. Genet. Evol. 2013 , 19 , 337–348. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| HIV-1 Clade | Protein and Position | Peptide Sequence | No. of H Bonds | RMSD | Binding Energy (kcal/mol) | Convex-PL Score | Amino Acids Involved in H Bond Interaction | |
|---|---|---|---|---|---|---|---|---|
| C/A1 | Gag * | GTEELRSLY | GY9 | 7 | 0.811 | –8.6 | 7.23 | Lys65, Tyr83, Tyr98, Thr142, Trp146 and Tyr158 |
| A1 | Env | KAYETEMHN | KN9 | 11 | 0.202 | –7.4 | 7.36 | Tyr6, Arg61, Glu62, Lys65, Tyr66, Ser76, Glu151 and Tyr158 |
| C | Pol * | GAERQGTLNF | GF10 | 7 | 1.165 | –8.7 | 7.22 | Tyr6, Glu62, Lys65, Tyr98, Thr142, Lys145 and Gln154 |
| C | Pol * | AQNPEIVIY | AY9 | 6 | 1.547 | –7.7 | 7.16 | Glu62, Lys65, Lys145, Tyr158 and Tyr170 |
| C/A1 | Nef | GAFDLSFFL | GL9 | 7 | 1.030 | –9.1 | 7.24 | Tyr8, Glu62, Lys65, Thr72, Tyr98, Lys145 and Tyr158 |
| C/A1 | Nef | WVYNTQGYF | WF9 | 7 | 0.873 | –9.7 | 7.53 | Tyr6, Arg68, Tyr98, Thr142, Lys145, Trp146 and Glu151 |
| A1 | Vif | VVSPRCEY | VY8 | 8 | 1.473 | –8.3 | 7.10 | Gln69, Thr72, Ser76, Tyr83, Tyr98, Thr142, Ly145 and Glu151 |
| A1 | Rev * | VSVESPVIL | VL9 | 7 | 1.638 | –8.0 | 7.27 | Tyr8, Arg61, Thr72, Asn79, Tyr83 and Tyr98 |
| HIV-1 Subtype | Peptide Sequence | Energy Components (kcal/mol) | ||||
|---|---|---|---|---|---|---|
| Van der Waals | Electrostatics | Polar Solvation | ΔG Binding Energy | |||
| C/A1 | GTEELRSLY | GY9 | −53.01 | −547.44 | 521.66 | −88.41 |
| C | GAERQGTLNF | GF10 | −53.45 | −335.96 | 347.44 | −50.59 |
| C | AQNPEIVIY | AY9 | −68.75 | −258.85 | 285.79 | −51.24 |
| A1 | VSVESPVIL | VL9 | −67.29 | −367.42 | 394.36 | −49.73 |
| HIV1 Clade | Amino Acid Residue and Position | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| G1 | T2 | E3 | E4 | L5 | R6 | S7 | L8 | Y9 | |
| A1 | . | . | . | . | . | R/K | . | . | Y/F |
| C | . | . | . | . | . | K | . | . | Y/F/H |
| D | . | . | . | . | I | K | . | . | Y/F |
| K | . | . | . | . | I | K | . | . | Y/F |
| ΔΔG GY9 | NA | −8.18 | −10.3 | −4.82 | −2.83 | −27.00 | −5.85 | −2.82 | −7.33 |
| Conservancy score | 5 | 4 | 2 | 3 | 3 | 2 | 5 | 5 | 2 |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Kyobe, S.; Mwesigwa, S.; Nkurunungi, G.; Retshabile, G.; Egesa, M.; Katagirya, E.; Amujal, M.; Mlotshwa, B.C.; Williams, L.; Sendagire, H.; et al. Identification of a Clade-Specific HLA-C*03:02 CTL Epitope GY9 Derived from the HIV-1 p17 Matrix Protein. Int. J. Mol. Sci. 2024 , 25 , 9683. https://doi.org/10.3390/ijms25179683
Kyobe S, Mwesigwa S, Nkurunungi G, Retshabile G, Egesa M, Katagirya E, Amujal M, Mlotshwa BC, Williams L, Sendagire H, et al. Identification of a Clade-Specific HLA-C*03:02 CTL Epitope GY9 Derived from the HIV-1 p17 Matrix Protein. International Journal of Molecular Sciences . 2024; 25(17):9683. https://doi.org/10.3390/ijms25179683
Kyobe, Samuel, Savannah Mwesigwa, Gyaviira Nkurunungi, Gaone Retshabile, Moses Egesa, Eric Katagirya, Marion Amujal, Busisiwe C. Mlotshwa, Lesedi Williams, Hakim Sendagire, and et al. 2024. "Identification of a Clade-Specific HLA-C*03:02 CTL Epitope GY9 Derived from the HIV-1 p17 Matrix Protein" International Journal of Molecular Sciences 25, no. 17: 9683. https://doi.org/10.3390/ijms25179683
Article Metrics
Article access statistics, supplementary material.
ZIP-Document (ZIP, 1604 KiB)
Further Information
Mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
HIV research grants for students underrepresented in medicine
Medical Student Education Sep 06, 2024
Seeking medical students from communities that are underrepresented in medicine in the US who are considering careers in HIV prevention and/or vaccine research. Application Deadline: Monday, December 2 Want to learn more? Join us for a RAMP Informational Webinar
Wednesday, September 18 2:00 pm – 3:00 pm PT (5:00 pm – 6:00 pm ET)
The HIV Vaccine Trials Network, in collaboration with the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, is investing in a young generation of HIV prevention researchers by providing medical students from communities that are underrepresented in medicine in the US with opportunities to conduct independent research while receiving mentoring, project and salary funding, training, and professional development opportunities.
The Request for Applications can be found on October 1.
Grant recipients will be mentored by HVTN-affiliated investigators while conducting research projects in areas of basic, clinical, behavioral, and social science. Projects will be:
Short-Term Projects: 8-10 weeks
Timed with a summer break or fourth-year research elective Travel to an HIV Vaccine Clinical Research Site in the US or abroad Attend and present at an HVTN Conference Maximum award of $20,000
Project Options
During the application process, scholars will have the opportunity to rank all of the research projects according to their interests. Projects for the 2025-2026 cycle will be posted on October 1.
To see the projects of our current and former scholars , check out their profiles.
Eligibility Criteria
Medical students who self-identify as being from communities that are underrepresented in medicine in the US Attending US MD, DO programs, or international medical schools that are US certification eligible (ECFMG) In good academic standing US citizen or US permanent resident or DACA status Interested in pursuing a future career in HIV research
Application Materials
To apply, visit http://www.hvtn.org/ramp . The Request For Applications (RFA) and application materials will be available on October 1. Carefully read the Request for Applications before applying. Applications are due online by 5:00 PM PT on Monday, December 2.
For More Information All correspondence concerning the program should be addressed to:
Linda Oseso, MPH RAMP Program Manager HIV Vaccine Trials Network
Fred Hutchinson Cancer Center 1100 Fairview Ave. N., Mail Stop M2-B500 Seattle, WA 98109 206-667-2175 [email protected]
Medical Student Education
The Medical Student Education team includes student affairs, curricular affairs and student support professionals across the state who support medical students at every step of their journey.
Suggested for you
History of HIV Vaccine Research
HIV was identified as the cause of AIDS. U.S. HHS Secretary Margaret Heckler declared that an AIDS vaccine will be ready for testing within two years.
The first HIV vaccine clinical trial opened at the National Institutes of Health (NIH) Clinical Center in Bethesda, Maryland. This Phase 1 trial enrolled 138 healthy, HIV-negative volunteers. The gp160 subunit vaccine showed no serious adverse effects.
The NIAID AIDS Vaccine Evaluation Group (AVEG), the first U.S. cooperative HIV vaccine clinical trials group, began enrolling volunteers in its first trial.
NIAID launched the first Phase 2 HIV vaccine clinical trial. This trial included HIV-negative volunteers with a history of high-risk behavior -- injection drug use, multiple sex partners, or sexually transmitted infections. Participants were counseled repeatedly to avoid any behaviors that put them at risk of HIV infection.
The first annual HIV Vaccine Awareness Day to honor vaccine study volunteers was observed.
The first large-scale HIV vaccine trial began. VaxGen initiated a Phase 3 trial of AIDSVAX (VAX004) in North America and the Netherlands involving more than 5,400 volunteers.
NIAID began the first African preventive HIV vaccine trial in Uganda.
The first large-scale HIV vaccine trial in a developing country began. VaxGen initiated a Phase 3 trial of AIDSVAX (VAX003) involving over 2,500 volunteers in Thailand.
The newly established Vaccine Research Center (VRC) was dedicated to immunization advocates Dale and Betty Bumpers.
NIAID formed the HIV Vaccine Trials Network (HVTN), a network of clinical sites in the United States and abroad dedicated to developing a preventive HIV vaccine by testing and evaluating candidate vaccines in all phases of clinical trials. The network included more than 25 sites in the United States, Africa, Asia, South America, and the Caribbean.
The first African HIV vaccine trial was completed in Uganda.
The U.S. and Royal Thai governments jointly initiated RV144, a Phase 3 trial to evaluate a novel HIV vaccine strategy commonly referred to as "prime-boost."
Formation of the Global HIV Vaccine Enterprise was proposed in the journal Science .
Both VaxGen candidates failed to confer protection against HIV in Phase 3 trials.
NIAID halted the Phase 2 Step and Phambili studies due to safety concerns.
The Phase 2 HVTN 505 study was initiated to evaluate a “prime-boost” vaccine regimen developed by the VRC.
Results of the Phase 3 Thai Trial (RV144) revealed that the vaccine combination demonstrated a modest preventive effect in humans. The trial, which enrolled more than 16,000 volunteers, was the first, and to date only, large clinical study to demonstrate efficacy for an investigational HIV vaccine.
VRC scientists identified two potent antibodies that neutralize most strains of HIV in the laboratory (VRC01 and VRC02).
The Pox-Protein Public-Private Partnership (P5), an international collaborative team committed to building on the modest success of RV144, was formed.
HVTN 505 was expanded to include protection from HIV as primary endpoint.
Additional analyses of samples from RV144 provided insight into what types of immune responses may be needed for an effective vaccine.
HVTN 505 immunizations were stopped due to lack of efficacy.
The Phase 1/2 HVTN 100 study, part of the P5 research endeavor, launched to test the safety of an experimental HIV vaccine regimen based upon the RV144 findings, as well as its ability to generate an immune response.
NIAID launched the AMP Studies to test whether intravenous infusions of the antibody VRC01 are safe, tolerable and effective at preventing HIV infection. The trials were also designed to answer fundamental scientific questions for HIV prevention and vaccine research.
HVTN 702, part of the P5 research endeavor, launched to test whether a new version of the RV144 HIV vaccine candidate safely prevents HIV infection among adults in South Africa.
NIAID and partners launched Imbokodo or HVTN 705/HPX2008, a Phase 2b proof-of-concept study evaluating the safety and efficacy of an experimental regimen based on a “mosaic” vaccine designed to induce immune responses against a wide variety of global HIV strains.
- What Are HIV and AIDS?
- How Is HIV Transmitted?
- Who Is at Risk for HIV?
- Symptoms of HIV
- U.S. Statistics
- Impact on Racial and Ethnic Minorities
- Global Statistics
- HIV and AIDS Timeline
- In Memoriam
- Supporting Someone Living with HIV
- Standing Up to Stigma
- Getting Involved
- HIV Treatment as Prevention
- Pre-exposure Prophylaxis (PrEP)
- Post-exposure Prophylaxis (PEP)
- Preventing Sexual Transmission of HIV
- Alcohol and HIV Risk
- Substance Use and HIV Risk
- Preventing Perinatal Transmission of HIV
HIV Vaccines
- Long-acting HIV Prevention Tools
- Microbicides
- Who Should Get Tested?
- HIV Testing Locations
- HIV Testing Overview
- Understanding Your HIV Test Results
- Living with HIV
- Talking About Your HIV Status
- Locate an HIV Care Provider
- Types of Providers
- Take Charge of Your Care
- What to Expect at Your First HIV Care Visit
- Making Care Work for You
- Seeing Your Health Care Provider
- HIV Lab Tests and Results
- Returning to Care
- HIV Treatment Overview
- Viral Suppression and Undetectable Viral Load
- Taking Your HIV Medicine as Prescribed
- Tips on Taking Your HIV Medicine as Prescribed
- Paying for HIV Care and Treatment
- Other Health Issues of Special Concern for People Living with HIV
- Alcohol and Drug Use
- Coronavirus (COVID-19) and People with HIV
- Hepatitis B & C
- Vaccines and People with HIV
- Flu and People with HIV
- Mental Health
- Mpox and People with HIV
- Opportunistic Infections
- Sexually Transmitted Infections
- Syphilis and People with HIV
- HIV and Women's Health Issues
- Aging with HIV
- Emergencies and Disasters and HIV
- Employment and Health
- Exercise and Physical Activity
- Nutrition and People with HIV
- Housing and Health
- Traveling Outside the U.S.
- Civil Rights
- Workplace Rights
- Limits on Confidentiality
- National HIV/AIDS Strategy (2022-2025)
- Implementing the National HIV/AIDS Strategy
- Prior National HIV/AIDS Strategies (2010-2021)
- Key Strategies
- Priority Jurisdictions
- HHS Agencies Involved
- Learn More About EHE
- Ready, Set, PrEP
- Ready, Set, PrEP Pharmacies
- AHEAD: America’s HIV Epidemic Analysis Dashboard
- HIV Prevention Activities
- HIV Testing Activities
- HIV Care and Treatment Activities
- HIV Research Activities
- Activities Combating HIV Stigma and Discrimination
- The Affordable Care Act and HIV/AIDS
- HIV Care Continuum
- Syringe Services Programs
- Finding Federal Funding for HIV Programs
- Fund Activities
- The Fund in Action
- About PACHA
- Members & Staff
- Subcommittees
- Prior PACHA Meetings and Recommendations
- I Am a Work of Art Campaign
- Awareness Campaigns
- Global HIV/AIDS Overview
- U.S. Government Global HIV/AIDS Activities
- U.S. Government Global-Domestic Bidirectional HIV Work
- Global HIV/AIDS Organizations
- National Black HIV/AIDS Awareness Day February 7
- HIV Is Not A Crime Awareness Day February 28
- National Women and Girls HIV/AIDS Awareness Day March 10
- National Native HIV/AIDS Awareness Day March 20
- National Youth HIV & AIDS Awareness Day April 10
- HIV Vaccine Awareness Day May 18
- National Asian & Pacific Islander HIV/AIDS Awareness Day May 19
- HIV Long-Term Survivors Awareness Day June 5
- National HIV Testing Day June 27
- Zero HIV Stigma July 21
- Southern HIV/AIDS Awareness Day August 20
- National Faith HIV/AIDS Awareness Day August 25
- National African Immigrants and Refugee HIV/AIDS and Hepatitis Awareness Day September 9
- National HIV/AIDS and Aging Awareness Day September 18
- National Gay Men's HIV/AIDS Awareness Day September 27
- National Latinx AIDS Awareness Day October 15
- World AIDS Day December 1
- Event Planning Guide
- U.S. Conference on HIV/AIDS (USCHA)
- National Ryan White Conference on HIV Care & Treatment
- AIDS 2020 (23rd International AIDS Conference Virtual)
Want to stay abreast of changes in prevention, care, treatment or research or other public health arenas that affect our collective response to the HIV epidemic? Or are you new to this field?
HIV.gov curates learning opportunities for you, and the people you serve and collaborate with.
Stay up to date with the webinars, Twitter chats, conferences and more in this section.
- Long-Acting HIV Prevention Tools
- Share on Facebook
- Share on Twitter
- Share on LinkedIn
- Share on Email
Is There a Vaccine to Prevent HIV?
No. There is currently no vaccine available that will prevent HIV infection.
However, scientists around the world, with support from the National Institutes of Health (NIH), are working to develop one. Some of the areas being studied include:
- Whether a preventive vaccine protects people from getting HIV.
- Whether preventive vaccines are safe.
- Whether a preventive vaccine controls HIV if a person gets HIV while enrolled in a study. (It is possible for someone to get HIV through sexual contact or from sharing drug injection equipment while they are participating in a clinical trial. But a person cannot get HIV from the HIV vaccine being tested.)
- What immune responses occur in people who receive a preventive vaccine.
- Different ways of giving preventive vaccines, such as using a needle and syringe versus a needle-free device.
WATCH : Louis Shackelford, MPH, of the HIV Vaccine Trials Network and NIAID’s Dr. Carl Dieffenbach discuss the latest in HIV vaccine research.
READ NIAID's 2024 HIV Vaccine Awareness Day blog post.
What Are Vaccines and What Do They Do?
Vaccines play an important role in keeping us healthy. They protect us from serious and sometimes deadly diseases.
Vaccines are products made from very small parts of weak or dead germs (such as viruses, bacteria, or toxins) that can cause diseases. They help your immune system fight infections faster and more effectively.
When you get a vaccine, it teaches your immune system to recognize and clear those germs, helping your body remember and fight them off if you get exposed to them in the future. And since vaccines are not made of infectious germs, they won’t make you sick.
Vaccines are usually administered by a shot, but sometimes can be administered by mouth or nasal spray. They are widely used to prevent diseases like polio, chicken pox, measles, mumps, rubella, influenza (flu), hepatitis A and B, and human papillomavirus (HPV).
Learn more about how vaccines protect you and others .
Why Do We Need a Vaccine to Prevent HIV?
Developing a safe, effective, and affordable preventive HIV vaccine has game-changing potential for controlling and ultimately ending the HIV/AIDS pandemic.
Today, more people with HIV than ever before have access to life-saving treatment with HIV medicines (called antiretroviral therapy or ART), which is good for their health. When people with HIV take HIV medicine as prescribed and get and keep an undetectable viral load , they can live long and healthy lives and will not transmit HIV to their HIV-negative partners through sex. In addition, people without HIV and who are likely to benefit can take pre-exposure prophylaxis (PrEP), HIV medicine used to prevent HIV. Yet, unfortunately, in 2022, an estimated 31,800 new HIV infections occurred in the United States, and in 2022 approximately 1.3 million people newly acquired HIV worldwide. To control and ultimately end HIV globally, we need a powerful array of HIV prevention tools that are widely accessible to all who would benefit from them.
Vaccines historically have been the most effective means to prevent and even eradicate infectious diseases. They safely and cost-effectively prevent illness, disability, and death. Like smallpox and polio vaccines, a preventive HIV vaccine could help save millions of lives. That’s why developing a preventive HIV vaccine has been a pillar of NIH’s scientific mission since the start of the HIV pandemic.
The long-term goal is to develop a safe and effective vaccine that protects people worldwide from acquiring HIV. Even if a vaccine only protects some people who get vaccinated, or even if it provides less than total protection by reducing the risk of getting HIV, it could still have a major impact on the rates of transmission and help control the pandemic, particularly for populations most affected by HIV. A partially effective vaccine could decrease the number of people who acquire HIV, further reducing the number of people who can pass the virus on to others. By substantially reducing the number of people who acquire and transmit HIV, we can stop the epidemic.
Can You Participate in HIV Preventive Vaccine Clinical Trials?
A list of clinical trials on preventive HIV vaccines is available from the database of ClinicalTrials.gov study summaries. Click on the title of any trial in the list to see more information about the study.
If you are interested in participating in a vaccine study, you can also contact the NIH Vaccine Research Center by calling 866-833-LIFE (5433) or by emailing [email protected] .

You can also learn about current HIV vaccine studies and opportunities to participate from the NIH-supported HIV Vaccine Trials Network Exit Disclaimer .
Related HIV.gov Blogs
Icymi: research roundup from aids 2024, putting people first: ias closing day, day 4 aids 2024: long-acting injectables, bi-directional learning, pacha & more.
- Vaccine HIV Vaccine
- HIV Vaccine Awareness Day
- HIVinfo.NIH.gov – What Is an HIV Preventive Vaccine?
- NIAID – HIV Vaccine Development
- NIAID – NIAID Marks HIV Vaccine Awareness Day, 2024
- NIAID - Proof-of-Concept Study Shows an HIV Vaccine Can Generate Key Antibody Response in People, 2024
- NIAID – Progress Toward an HIV Vaccine (2018)
- NIAID – History of HIV Vaccine Research
- HIV Vaccine Trials Network

IMAGES
VIDEO
COMMENTS
Many technologic advances and research capabilities of the HIV vaccine field have been leveraged rapidly to develop highly successful SARS‐CoV‐2 vaccines; however, among HIV vaccine efficacy trials completed to date, the RV144 Thai trial remains the only study to demonstrate a positive signal with an estimated efficacy of 31% at 3.5 years ...
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has launched a Phase 1 clinical trial evaluating three experimental HIV vaccines based on a messenger RNA (mRNA) platform—a technology used in several approved COVID-19 vaccines. NIAID is sponsoring the study, called HVTN 302, and the ...
NEW YORK and LA JOLLA, CA—A phase 1 clinical trial testing a novel vaccine approach to prevent HIV has produced promising results, IAVI and Scripps Research announced today. The vaccine showed success in stimulating production of rare immune cells needed to start the process of generating antibodies against the fast-mutating virus; the targeted response was detected in 97 percent of ...
Encouraging First-in-Human Results for a Promising HIV ...
The field has made steady progress towards a vaccine since the first HIV infection was reported in 1981 — but there's still a long way to go, says Rama Rao Amara, an immunologist at Emory ...
This louder HIV signal is tailored to draw out and engage those very specific B cells with the potential to produce bnAbs. As the first-in-human study showed, the nanoparticle vaccine was safe when administered twice to each participant eight weeks apart. People reported only mild to moderate side effects that went away in a day or two.
Although several preventive HIV vaccine candidates have been designed to stimulate CD8+ T-cell activity, they did not prevent HIV acquisition or control viral replication in clinical trials. Understanding and addressing this lack of effect is a scientific priority of HIV vaccine research.
An effective HIV vaccine may need to prompt strong responses from immune cells called CD8+ T cells to protect people from acquiring HIV, according to a new study from researchers at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, and colleagues. The study findings, appearing in Science, draw comparisons between the immune system ...
120 Altmetric. Metrics. mRNA technology may be uniquely positioned to tackle a major hurdle for HIV vaccines: the elicitation of broadly cross-reactive neutralizing antibodies. A preclinical study ...
The researchers administered the modified vaccine to five young primates in three priming doses, starting less than a week after birth. They followed up with three doses of the vaccine matching the original HIV envelope protein, with the last dose given when the animals reached 78 weeks old, roughly equivalent to four or five years old for a human.
HIV is a virus that remains a major health concern and results in an infection that has no cure even after over 30 years since its discovery. As such, HIV vaccine discovery continues to be an area of intensive research. In this review, we summarize the most recent HIV vaccine efficacy trials, clinical trials initiated within the last 3 years ...
Introduction. Much has happened in the field of human immunodeficiency virus-1 (HIV) vaccine research in recent years. The field of vaccinology has advanced considerably in the last decade, giving rise to novel vaccines for human pathogens. HIV is a pathogen that has challenged vaccine researchers for over 30 years.
After decades of failures, researchers have renewed ...
This paper discusses important landmarks of progress in HIV-1 vaccine development, various vaccine strategies, and clinical trials. 1. Introduction. In 2020, 37.6 million people were living with HIV infections, and 1.5 million people acquired HIV infections within the year.
HIV Vaccine Research Update with Dr. Dieffenbach from CROI 2023. 02-22-2023 The ongoing search for an HIV vaccine was among the topics discussed during the 2023 Conference on Retroviruses and Opportunistic Infections (CROI). HIV.gov spo….
During a CROI special session on HIV vaccine research, Dr. Susan Buchbinder, the co-chair of the Mosaico Study, provided a review of the study, which began in 2019. It involved an investigational HIV vaccine regimen tested among 3,900 men who have sex with men and transgender people in Europe, North America, and South America. ...
During a CROI special session on HIV vaccine research, Dr. Susan Buchbinder, the co-chair of the Mosaico Study, provided a review of the study, which began in 2019. It involved an investigational HIV vaccine regimen tested among 3,900 men who have sex with men and transgender people in Europe, North America, and South America.
An experimental HIV vaccine elicited broadly neutralizing antibody precursors in people. With further development, the approach could lead to an effective vaccine strategy for HIV and AIDS. Colorized transmission electron micrograph of numerous HIV virus particles (blue) replicating from a T cell (red). NIAID Integrated Research Facility.
The Duke study goes a step further to generate these antibodies, albeit at low levels. "This is a scientific feat and gives the field great hope that one can construct an HIV vaccine regimen ...
Joseph Prezioso/AFP/Getty Images. CNN —. The first participants have been vaccinated in a Phase 1 clinical trial of an experimental HIV vaccine that utilizes Moderna's mRNA technology, the ...
An experimental HIV vaccine has been found to induce broadly neutralizing antibody precursors among a small group of volunteers in a Phase 1 study. The findings suggest that a two-dose regimen of ...
Eliciting potent and broadly neutralizing antibodies (bnAbs) is a major goal in HIV-1 vaccine development. Here, we describe how germline-targeting immunogen BG505 SOSIP germline trimer 1.1 (GT1.1), generated through structure-based design, engages a diverse range of VRC01-class bnAb precursors.
The greatest obstacle for scientists is to develop an effective HIV vaccine. An effective vaccine represents the last hope for halting the unstoppable global spread of HIV and its catastrophic clinical consequences. Creating this vaccine has been challenging due to the virus's extensive genetic variability and the unique role of cytotoxic T ...
An empirical approach to HIV vaccine development relies on observation and experimentation to quickly move vaccine candidates into human clinical trials. The quest to develop a preventive HIV vaccine was reinvigorated in 2009 when results from the large RV144 trial showed for the first time that an investigational vaccine regimen could confer a ...
Noting it is an exciting time in HIV vaccine research, Carl explained that scientists are exploring how to take what we have learned about bNAbs, which prevented acquisition of some HIV strains, and turn that into an HIV vaccine. In addition, Carl and Louis discussed how bNAbs are being studied for use in HIV treatment and even, possibly, a ...
Research at Weill Cornell Medicine suggests that childhood immunization against HIV could one day provide protection before risk of contracting this potentially fatal infection dramatically ...
While previous research on HIV-1 vaccine candidates has predominantly focused on the protective HLA-B alleles, it is noteworthy that HIV-nef attachment selectively downregulates the cell surface expression of both HLA-A and B molecules [40,41]. This downregulation phenomenon facilitates immune evasion through CTL escape by virally infected cells.
Timed with a summer break or fourth-year research elective Travel to an HIV Vaccine Clinical Research Site in the US or abroad Attend and present at an HVTN Conference Maximum award of $20,000. Project Options. During the application process, scholars will have the opportunity to rank all of the research projects according to their interests.
NIAID began the first African preventive HIV vaccine trial in Uganda. The first large-scale HIV vaccine trial in a developing country began. VaxGen initiated a Phase 3 trial of AIDSVAX (VAX003) involving over 2,500 volunteers in Thailand. The newly established Vaccine Research Center (VRC) was dedicated to immunization advocates Dale and Betty ...
HIV Vaccines ... HIV Vaccines