
Animal Research

Animal research case studies

UCL is a leading centre for biomedical research in the UK. Scientific research is conducted not by shadowy figures in ivory towers, but by human beings working earnestly to address major issues facing society today.

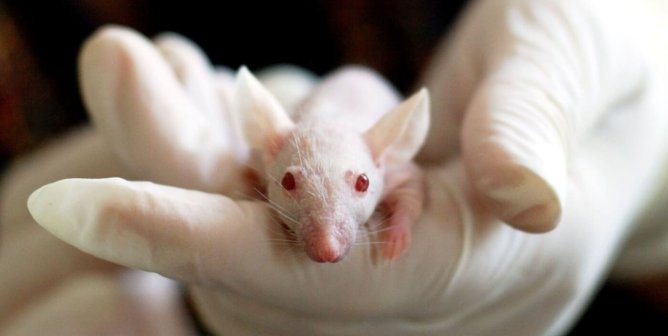
Dr Clare Stanford: using mice to find treatments for ADHD
Dr Clare Stanford is a Reader in Experimental Psychopharmacology at UCL. Despite the intimidating title, Clare is a down-to-earth, compassionate researcher with a real commitment to animal welfare. She is chair of the Bloomsbury AWERB and does not hold back from questioning the ethics of research objectives , as well as the way it is carried out.
Clare is currently working on a mouse model for Attention Deficit Hyperactivity Disorder (ADHD). This is a strongly inherited psychiatric disorder, which causes problems for patients by making them hyperactive, excessively impulsive and inattentive. ADHD is often regarded as a childhood issue, but about 65% of people carry it through to adulthood where the associated problems are far worse. It has been associated with alcohol and drug misuse in later life, and an estimated 25% of the prison population have ADHD . There is also an increased risk of other health complications, including asthma and epilepsy.
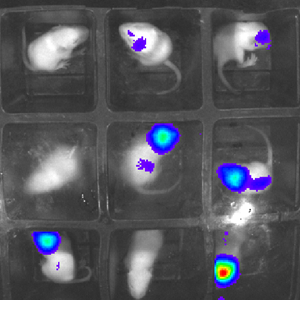
Dr Simon Waddington and Rajvinder Karda: reducing mouse use with glowing firefly genes
Although animal research remains a necessary part of modern research, current methods are far from perfect. By injecting the genes that fireflies use to emit light into newborn mice, UCL scientists have developed a way to drastically reduce the numbers of mice needed for research into disease and development.
At the moment, researchers often need to cull and perform autopsies on animals to see how diseases develop on a molecular level. This means that an animal needs to be killed for every data point recorded, so some studies might use dozens of mice to get reliable data on disease progression.
The new technique could allow researchers to get molecular-level data by simply taking a picture with specialist equipment rather than killing an animal, allowing them to get data more regularly and ethically. An experiment that previously need 60 mice can be done with around 15, and the results are more reliable.

Dr Karin Tuschl: Using zebrafish to treat a rare form of childhood Parkinsonism
Using genetically modified zebrafish, UCL scientists have identified a novel gene affected in a devastating disorder with childhood-onset Parkinsonism. Indeed, when a drug that worked in the fish was given to one of the children, she regained the ability to walk.
The research studied a group of nine children who suffered from severely disabling neurological symptoms including difficulties in walking and talking. Dr Karin Tuschl and her team at the UCL Great Ormond Street Institute of Child Health and UCL Department of Cell and Developmental Biology used state of the art genome editing in zebrafish to validate the identity of the gene affected in these children.
The scientists disrupted a gene known as slc39a14 in the fish, which is important for transporting metals in the body. Disrupting the transporter in fish led to a build up of manganese in the brain and impaired motor behaviour. As similar symptoms were seen in the patients, this confirmed that slc39A14 is required to clear manganese from the body and protect it from manganese toxicity. It also confirmed that the scientists had found the gene causing the disease in the patients.
Undercover investigation exposes the horrors of animal testing—and more than 80 dogs who need our help
Kitty Block and Sara Amundson
Today we are releasing the results of our seven-month undercover investigation at one of America’s largest animal testing laboratories. We’re asking you to join us in changing an outdated industry— animal testing—and, more immediately, in urging the release of more than 80 dogs still suffering at the lab.
Our undercover investigator worked on studies with more than 6,000 animals—including 250 dogs, 500 primates, 62 “minipigs” and more than 5,100 mice and rats—at Inotiv, a contract testing laboratory in Indiana. Inotiv has laboratories and breeding facilities in multiple states, including Colorado, Maryland, North Carolina, Texas and Virginia. What we saw was heartbreaking. Our investigator documented animals being force-fed high doses of drugs via tubes or intravenously, sometimes several times a day. Some animals were unable to move because of the drugs’ toxic effects; others died during procedures. The studies conducted at Inotiv were intended to test drug toxicity and were funded by dozens of pharmaceutical companies.
Most of the animals our investigator came to know didn’t make it out alive, including one beagle we call Riley, because we believe he deserves a name and not just a number. He was used to test a substance so toxic that it brought him near death after only two days of dosing. The investigator captured video showing Riley hypersalivating, trembling, vomiting and moaning on the floor, unable to stand. Our investigator tried to comfort him while he was dying, but Riley was left to suffer overnight because the veterinarian was unavailable on a weekend evening. Riley was euthanized the following day.
Other animals who died horrible deaths during our investigation were two young cynomolgus macaques who were accidentally hanged in their restraint chairs.
We documented numerous potential violations of the Animal Welfare Act, including failure to provide adequate veterinary care, failure to minimize animal pain and distress, and failure to ensure proper staffing. We urgently reached out to the U.S. Department of Agriculture and asked the agency to investigate these findings, but the devastating truth is that this suffering—and Riley’s fate—are simply reflections of the realities of contemporary animal testing.
Pharmaceutical companies and the Food and Drug Administration have a responsibility to ensure drugs are safe for humans, but our continuing reliance on animal testing creates a false sense of security. Nearly 90% of drugs tested in animals ultimately fail in human trials , and a large proportion of these failures is attributable to unexpected toxicity in humans … even after animal tests. In what business model is such a high failure rate acceptable?
We uncovered an example of this failure. A company contracting with Inotiv ended its pursuit of a drug after encountering unexpected liver toxicity in human patients. At the same time the company decided to end the human trials, mice and primates were being dosed with the same drug at Inotiv while our investigator was there. We are hoping these tests have by now come to an end since the pharmaceutical company has stopped pursuit of this compound due to the serious adverse effects in humans.
Today, combinations of modern, non-animal methods based on human biology—such as human organs on chips and next-generation computer modeling—are increasingly providing better real-world predictions of human reactions to drugs and chemicals than some animal tests. For example, a recent study found that organ chips detected toxicity in almost seven out of eight drugs that proved toxic in patients but had cleared animal testing—an 87% success rate. But because animal testing remains the surest path to regulatory approval, it continues.
We ask that you join us in bringing the science of safety testing into the 21 st century—bringing the system from low-tech and cruel to high-tech and effective. Even more urgently, we’re asking for your help in securing the release of 82 dogs still alive in the lab today.
Eighty beagle puppies are being dosed every day and may suffer the same fate as Riley if we don’t act quickly. Two additional adult beagles have been used to practice harmful procedures for years. Please join us in urging Inotiv (the testing laboratory) and Crinetics (the pharmaceutical company that hired Inotiv) to immediately end the tests and release these 82 beagles to us so that we can find them loving homes.
With your help, we’ll continue our work toward the day when invasive experiments on dogs and other animals are a thing of the past—and saving these dogs is just the start.
Help us secure the release of the 82 dogs still alive in the lab
Sara Amundson is president of the Humane Society Legislative Fund.
Taking Suffering Out of Science
About the author
Kitty Block is President and CEO of the Humane Society of the United States and CEO of Humane Society International, the international affiliate of the HSUS
Subscribe to the blog
Get all of Kitty's latest updates delivered to your inbox.
Also of interest

Our undercover investigation reveals why hippos desperately need federal protections under the Endangered Species Act

South Korea shuts down major dog meat market; More than 80 dogs rescued from vendors

Undercover investigation exposes Petland's treatment of sick puppies
Watch CBS News
Harvard study on monkeys reignites ethical debate over animal testing
Updated on: November 21, 2022 / 12:54 PM EST / CBS/AFP
Mother monkeys permanently separated from their newborns sometimes find comfort in plush toys; this recent finding from Harvard experiments has set off intense controversy among scientists and reignited the ethical debate over animal testing.
The paper, "Triggers for mother love," was authored by neuroscientist Margaret Livingstone and appeared in the Proceedings of the National Academy of Sciences (PNAS) in September to little fanfare or media coverage.
But once news of the study began spreading on social media, it provoked a firestorm of criticism and eventually a letter to PNAS signed by over 250 scientists calling for a retraction.
Animal rights groups meanwhile recalled Livingstone's past work, which included temporarily suturing shut the eyelids of infant monkeys in order to study the impact on their cognition.

"We cannot ask monkeys for consent, but we can stop using, publishing, and in this case actively promoting cruel methods that knowingly cause extreme distress," wrote Catherine Hobaiter, a primatologist at the University of St. Andrews, who co-authored the retraction letter.
Hobaiter told AFP she was awaiting a response from the journal before further comment, but expected news soon.
Harvard and Livingstone, for their part, have strongly defended the research.
Livingstone's observations "can help scientists understand maternal bonding in humans and can inform comforting interventions to help women cope with loss in the immediate aftermath of suffering a miscarriage or experiencing a still birth," said Harvard Medical School in a statement .
The school added it was "deeply concerned about the personal attacks directed at scientists who conduct critically important research for the benefit of humanity."
Livingstone, in a separate statement , said: "I have joined the ranks of scientists targeted and demonized by opponents of animal research, who seek to abolish lifesaving research in all animals."
Such work routinely attracts the ire of groups such as People for the Ethical Treatment of Animals (PETA), which opposes all forms of animal testing.
In its statement, Harvard Medical School said PETA had published content regarding the study on its website that was "misleading and contains factual inaccuracies."
This controversy has notably provoked strong responses in the scientific community, particularly from animal behavior researchers and primatologists, said Alan McElligot of the City University of Hong Kong's Centre for Animal Health and a co-signer of the PNAS letter.
He told AFP that Livingstone appears to have replicated research performed by Harry Harlow, a notorious American psychologist, from the mid-20th century.
Harlow's experiments on maternal deprivation in rhesus macaques were considered groundbreaking, but may have also helped catalyze the early animal liberation movement.
"It just ignored all of the literature that we already have on attachment theory," added Holly Root-Gutteridge, an animal behavior scientist at the University of Lincoln in Britain.
McElligot and Root-Gutteridge argue the case was emblematic of a wider problem in animal research, in which questionable studies and papers continue to pass institutional reviews and are published in high impact journals.
McElligot pointed to a much-critiqued 2020 paper extolling the efficiency of foot snares to capture jaguars and cougars for scientific study in Brazil.
More recently, experiments on marmosets that included invasive surgeries have attracted controversy.
The University of Massachusetts Amherst team behind the work says studying the tiny monkeys, which have 10-year lifespans and experience cognitive decline in their old age, are essential to better understand Alzheimer's in people.
Opponents argue results rarely translate across species.
When it comes to testing drugs, there is evidence the tide is turning against animal trials.
In September, the Senate passed the bipartisan FDA Modernization Act, which would end a requirement that experimental medicines first be tested on animals before any human trials.
The vast majority of drugs that pass animal tests fail in human trials, while new technologies such as tissue cultures, mini organs and AI models are also reducing the need for live animals.
Opponents also say the vast sums of money that flow from government grants to universities and other institutes — $15 billion annually, according to watchdog group White Coat Waste — perpetuate a system in which animals are viewed as lab resources.
"The animal experimenters are the rainmaker within the institutions, because they're bringing in more money," said primatologist Lisa Engel-Jones, who worked as a lab researcher for three decades but now opposes the practice and is a science adviser for PETA.
"There's financial incentive to keep doing what you've been doing and just look for any way you can to get more papers published, because that means more funding and more job security," added Emily Trunnel, a neuroscientist who experimented on rodents and also now works for PETA.
Most scientists do not share PETA's absolutist stance, but instead say they adhere to the "three Rs" framework — refine, replace and reduce animal use.
On Livingstone's experiment, Root-Gutteridge said the underlying questions might have been studied on wild macaques who naturally lost their young, and urged neuroscientists to team up with animal behaviorists to find ways to minimize harm.
"Do I wish we lived in a world where generating this important knowledge were possible without the use of lab animals? Of course!" Livingstone said in her statement . "Alas, we are not there yet."
- Harvard Medical School
More from CBS News

JD Vance defends amplifying false claims about immigrants

Transcript: David Becker and Chris Krebs on "Face the Nation with Margaret Brennan," Sept. 15, 2024

Idaho murders suspect jailed 300 miles from crime after trial moved

How dogs wearing backpacks are helping to rewild an English woodland
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Ethical and Scientific Considerations Regarding Animal Testing and Research
* E-mail: [email protected]
Affiliations Physicians Committee for Responsible Medicine, Washington, D.C., United States of America, Department of Medicine, The George Washington University, Washington, D.C., United States of America
Affiliation Physicians Committee for Responsible Medicine, Washington, D.C., United States of America
- Hope R. Ferdowsian,

Published: September 7, 2011
- https://doi.org/10.1371/journal.pone.0024059
- Reader Comments
Citation: Ferdowsian HR, Beck N (2011) Ethical and Scientific Considerations Regarding Animal Testing and Research. PLoS ONE 6(9): e24059. https://doi.org/10.1371/journal.pone.0024059
Editor: Catriona J. MacCallum, Public Library of Science, United Kingdom
Copyright: © 2011 Ferdowsian, Beck. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: The authors are grateful to the National Science Foundation (grant SES-0957163) and the Arcus Foundation (grant 0902-34) for the financial support for the corresponding conference, Animals, Research, and Alternatives: Measuring Progress 50 Years Later. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: HRF and NB are employed by Physicians Committee for Responsible Medicine, which is a non-governmental organization which promotes higher ethical standards in research and alternatives to the use of animals in research, education, and training. Physicians Committee for Responsible Medicine is a nonprofit organization, and the authors adhered to PLoS ONE policies on sharing data and materials.
In 1959, William Russell and Rex Burch published the seminal book, The Principles of Humane Experimental Technique, which emphasized r eduction, r efinement, and r eplacement of animal use, principles which have since been referred to as the “3 Rs”. These principles encouraged researchers to work to reduce the number of animals used in experiments to the minimum considered necessary, refine or limit the pain and distress to which animals are exposed, and replace the use of animals with non-animal alternatives when possible. Despite the attention brought to this issue by Russell and Burch and since, the number of animals used in research and testing has continued to increase, raising serious ethical and scientific issues. Further, while the “3 Rs” capture crucially important concepts, they do not adequately reflect the substantial developments in our new knowledge about the cognitive and emotional capabilities of animals, the individual interests of animals, or an updated understanding of potential harms associated with animal research. This Overview provides a brief summary of the ethical and scientific considerations regarding the use of animals in research and testing, and accompanies a Collection entitled Animals, Research, and Alternatives: Measuring Progress 50 Years Later , which aims to spur ethical and scientific advancement.
Introduction
One of the most influential attempts to examine and affect the use of animals in research can be traced back to1959, with the publication of The Principles of Humane Experimental Technique [1] . William Russell and Rex Burch published this seminal book in response to marked growth in medical and veterinary research and the concomitant increase in the numbers of animals used. Russell and Burch's text emphasized r eduction, r efinement, and r eplacement of animal use, principles which have since been referred to as the “3 Rs”. These principles encouraged researchers to work to reduce the number of animals used in experiments to the minimum considered necessary, refine or limit the pain and distress to which animals are exposed, and replace the use of animals with non-animal alternatives when possible.
Despite the attention brought to this issue by Russell and Burch, the number of animals used in research and testing has continued to increase. Recent estimates suggest that at least 100 million animals are used each year worldwide [2] . However, this is likely an underestimate, and it is impossible to accurately quantify the number of animals used in or for experimentation. Full reporting of all animal use is not required or made public in most countries. Nevertheless, based on available information, it is clear that the number of animals used in research has not significantly declined over the past several decades.
The “3 Rs” serve as the cornerstone for current animal research guidelines, but questions remain about the adequacy of existing guidelines and whether researchers, review boards, and funders have fully and adequately implemented the “3 Rs”. Further, while the “3 Rs” capture crucially important concepts, they do not adequately reflect the substantial developments in our new knowledge about the cognitive and emotional capabilities of animals; an updated understanding of the harms inherent in animal research; and the changing cultural perspectives about the place of animals in society [3] , [4] . In addition, serious questions have been raised about the effectiveness of animal testing and research in predicting anticipated outcomes [5] – [13] .
In August 2010, the Georgetown University Kennedy Institute of Ethics, the Johns Hopkins University Center for Alternatives to Animal Testing, the Institute for In Vitro Sciences, The George Washington University, and the Physicians Committee for Responsible Medicine jointly held a two day multi-disciplinary, international conference in Washington, DC, to address the scientific, legal, and political opportunities and challenges to implementing alternatives to animal research. This two-day symposium aimed to advance the study of the ethical and scientific issues surrounding the use of animals in testing and research, with particular emphasis on the adequacy of current protections and the promise and challenges of developing alternatives to the use of animals in basic research, pharmaceutical research and development, and regulatory toxicology. Speakers who contributed to the conference reviewed and contributed new knowledge regarding the cognitive and affective capabilities of animals, revealed through ethology, cognitive psychology, neuroscience, and related disciplines. Speakers also explored the dimensions of harm associated with animal research, touching on the ethical implications regarding the use of animals in research. Finally, several contributors presented the latest scientific advances in developing alternatives to the use of animals in pharmaceutical research and development and regulatory toxicity testing.
This Collection combines some papers that were written following this conference with an aim to highlight relevant progress and research. This Overview provides a brief summary of the ethical and scientific considerations regarding the use of animals in research and testing, some of which are highlighted in the accompanying Collection.
Analysis and Discussion
Ethical considerations and advances in the understanding of animal cognition.
Apprehension around burgeoning medical research in the late 1800s and the first half of the 20 th century sparked concerns over the use of humans and animals in research [14] , [15] . Suspicions around the use of humans were deepened with the revelation of several exploitive research projects, including a series of medical experiments on large numbers of prisoners by the Nazi German regime during World War II and the Tuskegee syphilis study. These abuses served as the impetus for the establishment of the Nuremberg Code, Declaration of Helsinki, and the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (1974) and the resulting Belmont Report [16] – [18] . Today, these guidelines provide a platform for the protection of human research subjects, including the principles of respect, beneficence, and justice, as well as special protections for vulnerable populations.
Laws to protect animals in research have also been established. The British Parliament passed the first set of protections for animals in 1876, with the Cruelty to Animals Act [19] . Approximately ninety years later, the U.S. adopted regulations for animals used in research, with the passage of the Laboratory Animal Welfare Act of 1966 [20] . Subsequent national and international laws and guidelines have provided basic protections, but there are some significant inconsistencies among current regulations [21] . For example, the U.S. Animal Welfare Act excludes purpose-bred birds, rats, or mice, which comprise more than 90% of animals used in research [20] . In contrast, certain dogs and cats have received special attention and protections. Whereas the U.S. Animal Welfare Act excludes birds, rats and mice, the U.S. guidelines overseeing research conducted with federal funding includes protections for all vertebrates [22] , [23] . The lack of consistency is further illustrated by the “U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training” which stress compliance with the U.S. Animal Welfare Act and “other applicable Federal laws, guidelines, and policies” [24] .
While strides have been made in the protection of both human and animal research subjects, the nature of these protections is markedly different. Human research protections emphasize specific principles aimed at protecting the interests of individuals and populations, sometimes to the detriment of the scientific question. This differs significantly from animal research guidelines, where the importance of the scientific question being researched commonly takes precedence over the interests of individual animals. Although scientists and ethicists have published numerous articles relevant to the ethics of animal research, current animal research guidelines do not articulate the rationale for the central differences between human and animal research guidelines. Currently, the majority of guidelines operate on the presumption that animal research should proceed based on broad, perceived benefits to humans. These guidelines are generally permissive of animal research independent of the costs to the individual animal as long as benefits seem achievable.
The concept of costs to individual animals can be further examined through the growing body of research on animal emotion and cognition. Studies published in the last few decades have dramatically increased our understanding of animal sentience, suggesting that animals' potential for experiencing harm is greater than has been appreciated and that current protections need to be reconsidered. It is now widely acknowledged by scientists and ethicists that animals can experience pain and distress [25] – [29] . Potential causes of harm include invasive procedures, disease, and deprivation of basic physiological needs. Other sources of harm for many animals include social deprivation and loss of the ability to fulfill natural behaviors, among other factors. Numerous studies have demonstrated that, even in response to gentle handling, animals can show marked changes in physiological and hormonal markers of stress [30] .
Although pain and suffering are subjective experiences, studies from multiple disciplines provide objective evidence of animals' abilities to experience pain. Animals demonstrate coordinated responses to pain and many emotional states that are similar to those exhibited by humans [25] , [26] . Animals share genetic, neuroanatomical, and physiological similarities with humans, and many animals express pain in ways similar to humans. Animals also share similarities with humans in genetic, developmental, and environmental risk factors for psychopathology [25] , [26] . For example, fear operates in a less organized subcortical neural circuit than pain, and it has been described in a wide variety of species [31] . More complex markers of psychological distress have also been described in animals. Varying forms of depression have been repeatedly reported in animals, including nonhuman primates, dogs, pigs, cats, birds and rodents, among others [32] – [34] . Anxiety disorders, such as post-traumatic stress disorder, have been described in animals including chimpanzees and elephants [35] , [36] , [37] .
In addition to the capacity to experience physical and psychological pain or distress, animals also display many language-like abilities, complex problem-solving skills, tool related cognition and pleasure-seeking, with empathy and self-awareness also suggested by some research. [38] – [44] . Play behavior, an indicator of pleasure, is widespread in mammals, and has also been described in birds [45] , [46] . Behavior suggestive of play has been observed in other taxa, including reptiles, fishes and cephalopods [43] . Self-awareness, assessed through mirror self-recognition, has been reported for chimpanzees and other great apes, magpies, and some cetaceans. More recent studies have shown that crows are capable of creating and using tools that require access to episodic-like memory formation and retrieval [47] . These findings suggest that crows and related species display evidence of causal reasoning, flexible learning strategies, imagination and prospection, similar to findings in great apes. These findings also challenge our assumptions about species similarities and differences and their relevance in solving ethical dilemmas regarding the use of animals in research.
Predictive Value of Animal Data and the Impact of Technical Innovations on Animal Use
In the last decade, concerns have mounted about how relevant animal experiments are to human health outcomes. Several papers have examined the concordance between animal and human data, demonstrating that findings in animals were not reliably replicated in human clinical research [5] – [13] . Recent systematic reviews of treatments for various clinical conditions demonstrated that animal studies have been poorly predictive of human outcomes in the fields of neurology and vascular disease, among others [7] , [48] . These reviews have raised questions about whether human diseases inflicted upon animals sufficiently mimic the disease processes and treatment responses seen in humans.
The value of animal use for predicting human outcomes has also been questioned in the regulatory toxicology field, which relies on a codified set of highly standardized animal experiments for assessing various types of toxicity. Despite serious shortcomings for many of these assays, most of which are 50 to 60 years old, the field has been slow to adopt newer methods. The year 2007 marked a turning point in the toxicology field, with publication of a landmark report by the U.S. National Research Council (NRC), highlighting the need to embrace in vitro and computational methods in order to obtain data that more accurately predicts toxic effects in humans. The report, “Toxicity Testing in the 21 st Century: A Vision and a Strategy,” was commissioned by the U.S. Environmental Protection Agency, partially due to the recognition of weaknesses in existing approaches to toxicity testing [49] . The NRC vision calls for a shift away from animal use in chemical testing toward computational models and high-throughput and high-content in vitro methods. The report emphasized that these methods can provide more predictive data, more quickly and affordably than traditional in vivo methods. Subsequently published articles address the implementation of this vision for improving the current system of chemical testing and assessment [50] , [51] .
While a sea change is underway in regulatory toxicology, there has been much less dialogue surrounding the replacement of animals in research, despite the fact that far more animals are used in basic and applied research than in regulatory toxicology. The use of animals in research is inherently more difficult to approach systematically because research questions are much more diverse and less proscribed than in regulatory toxicology [52] . Because researchers often use very specialized assays and systems to address their hypotheses, replacement of animals in this area is a more individualized endeavour. Researchers and oversight boards have to evaluate the relevance of the research question and whether the tools of modern molecular and cell biology, genetics, biochemistry, and computational biology can be used in lieu of animals. While none of these tools on their own are capable of replicating a whole organism, they do provide a mechanistic understanding of molecular events. It is important for researchers and reviewers to assess differences in the clinical presentation and manifestation of diseases among species, as well as anatomical, physiological, and genetic differences that could impact the transferability of findings. Another relevant consideration is how well animal data can mirror relevant epigenetic effects and human genetic variability.
Examples of existing and promising non-animal methods have been reviewed recently by Langley and colleagues, who highlighted advances in fields including orthodontics, neurology, immunology, infectious diseases, pulmonology, endocrine and metabolism, cardiology, and obstetrics [52] .
Many researchers have also begun to rely solely on human data and cell and tissue assays to address large areas of therapeutic research and development. In the area of vaccine testing and development, a surrogate in-vitro human immune system has been developed to help predict an individual's immune response to a particular drug or vaccine [53] , [54] . This system includes a blood-donor base of hundreds of individuals from diverse populations and offers many benefits, including predictive high-throughput in vitro immunology to assess novel drug and vaccine candidates, measurement of immune responses in diverse human populations, faster cycle time for discovery, better selection of drug candidates for clinical evaluation, and reductions in the time and costs to bring drugs and vaccines to the market. In the case of vaccines, this system can be used at every stage, including in vitro disease models, antigen selection and adjuvant effects, safety testing, clinical trials, manufacturing, and potency assays. When compared with data from animal experiments, this system has produced more accurate pre-clinical data.
The examples above illustrate how innovative applications of technology can generate data more meaningful to humans, and reduce or replace animal use, but advances in medicine may also require novel approaches to setting research priorities. The Dr. Susan Love Research Foundation, which focuses on eradicating breast cancer, has challenged research scientists to move from animal research to breast cancer prevention research involving women. If researchers could better understand the factors that increase the risk for breast cancer, as well as methods for effective prevention, fewer women would require treatment for breast cancer. Whereas animal research is largely investigator-initiated, this model tries to address the questions that are central to the care of women at risk for or affected by breast cancer. This approach has facilitated the recruitment of women for studies including a national project funded by the National Institutes of Health and the National Institute of Environmental Health to examine how environment and genes affect breast cancer risk. This study, which began in 2002, could not have been accomplished with animal research [55] .
Similarly, any approach that emphasizes evidence-based prevention would provide benefits to both animals and humans. Resource limitations might require a strategic approach that emphasizes diseases with the greatest public health threats, which increasingly fall within the scope of preventable diseases.
It is clear that there have been many scientific and ethical advances since the first publication of Russell and Burch's book. However, some in the scientific community are beginning to question how well data from animals translates into germane knowledge and treatment of human conditions. Efforts to objectively evaluate the value of animal research for understanding and treating human disease are particularly relevant in the modern era, considering the availability of increasingly sophisticated technologies to address research questions [9] . Ethical objections to the use of animals have been publically voiced for more than a century, well before there was a firm scientific understanding of animal emotion and cognition [15] . Now, a better understanding of animals' capacity for pain and suffering is prompting many to take a closer look at the human use of animals [56] .
Articles in the accompanying Collection only briefly touch on the many scientific and ethical issues surrounding the use of animals in testing and research. While it is important to acknowledge limitations to non-animal methods remain, recent developments demonstrate that these limitations should be viewed as rousing challenges rather than insurmountable obstacles. Although discussion of these issues can be difficult, progress is most likely to occur through an ethically consistent, evidence-based approach. This collection aims to spur further steps forward toward a more coherent ethical framework for scientific advancement.
Acknowledgments
The authors thank the conference speakers and participants for their participation.
Author Contributions
Conceived and designed the experiments: HRF NB. Contributed reagents/materials/analysis tools: HRF NB. Wrote the paper: HRF NB.
- 1. Russell WMS, Burch RL (1959) The principles of humane experimental technique. London: Methuen. 238 p.
- View Article
- Google Scholar
- 3. Ibrahim DM (2006) Reduce, refine, replace: the failure of the three R's and the future of animal experimentation. University of Chicago Legal Forum, 2006; Arizona Legal Studies Discussion Paper No. 06-17. Available: http://ssrn.com/abstract=888206 . Accessed 2011 Jan 7.
- 15. Lederer SE (1995) Subjected to science. Baltimore: The Johns Hopkins University Press. 192 p.
- 16. United States (1947) Nuremberg code. Trials of War Criminals before the Nuremberg Military Tribunals under Control Council Law No. 10. Washington, D.C.: U.S. Government Printing Office. Available: http://ohsr.od.nih.gov/guidelines/nuremberg.html . Accessed 2011 Jan 7.
- 17. World Medical Association (1964) Declaration of Helsinki. 18 th WMA General Assembly. Helsinki, Finland: Available: http://history.nih.gov/research/downloads/helsinki.pdf . Accessed 2011 Jan 7.
- 18. National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (1979) The Belmont Report. Washington, D.C.: US Department of Health, Education, and Welfare. Available: http://ohsr.od.nih.gov/guidelines/belmont.html . Accessed 2011 Jan 7.
- 19. Parliament of the United Kingdom (1876) Cruelty to Animals Act 1876. Available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1872363/ . Accessed 2011 Jan 7.
- 20. Animal Welfare Act. 7 U.S.C. §§ 2131–2159.
- 22. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council (1996) Guide for the care and use of laboratory animals. Washington, D.C.: National Academy Press. 140 p.
- 23. Office of Laboratory Animal Welfare (2002) Public Health Service policy on humane care and use of laboratory animals. Available: http://grants.nih.gov/grants/olaw/references/phspol.htm#PublicHealthServicePolicyonHumaneCareandUseofLaboratory . Accessed 2011 Jan 18.
- 24. Office of Laboratory Animal Welfare (2002) U.S. Government principles for the utilization and care of vertebrate animals used in testing, research and training. Available: http://grants.nih.gov/grants/olaw/references/phspol.htm . Accessed 2011 Jan 7.
- 25. Gregory NG (2004) Physiology and behavior of animal suffering. Oxford, U.K.: Blackwell Science. 280 p.
- 26. McMillan FD, editor. (2005) Mental Health and Well-Being in Animals. Oxford, U.K.: Blackwell Publishing Professional. 301 p.
- 27. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council (2009) Recognition and alleviation of pain and distress in laboratory animals. Washington, D.C.: National Academy Press. 196 p.
- 31. Panksepp J (2004) Affective neuroscience: the foundations of human and animal emotions. Oxford: Oxford University Press. 480 p.
- 32. Koob GF, Ehlers CL, Kupfers DJ, editors. (1989) Animal Models of Depression. Boston, MA: Birkhäuser. 295 p.
- 39. Shettleworth SJ (1998) Cognition, evolution, and behavior. Oxford, U.K.: Oxford University Press. 704 p.
- 40. deWaal F (2009) The age of empathy: nature's lessons for a kinder society. New York, NY: Random House, Inc. 304 p.
- 44. Burghardt GM (2005) The genesis of animal play: testing the limits. Cambridge, U.K.: MIT Press. 501 p.
- 49. Committee on Toxicity Testing and Assessment of Environmental Agents, National Research Council (2007) Toxicity testing in the 21st century: a vision and a strategy. Washington, DC: National Academy Press. 216 p.
- 55. Dr. Susan Love Research Foundation, National Cancer Institute Cancer Biomedical Informatics Grid (2009) Health of Women Study. Available: http://cabig.cancer.gov/action/collaborations/howstudy/ . Accessed 2011 Jan 10.
- 56. Beauchamp TL, Orlans FB, Dresser R, Morton DB, Gluck JP (2008) The Human Use of Animals: Case Studies in Ethical Choice, 2 nd ed. New York, NY: Oxford University Press. 287 p.
Ethical care for research animals
WHY ANIMAL RESEARCH?
The use of animals in some forms of biomedical research remains essential to the discovery of the causes, diagnoses, and treatment of disease and suffering in humans and in animals., stanford shares the public's concern for laboratory research animals..
Many people have questions about animal testing ethics and the animal testing debate. We take our responsibility for the ethical treatment of animals in medical research very seriously. At Stanford, we emphasize that the humane care of laboratory animals is essential, both ethically and scientifically. Poor animal care is not good science. If animals are not well-treated, the science and knowledge they produce is not trustworthy and cannot be replicated, an important hallmark of the scientific method .
There are several reasons why the use of animals is critical for biomedical research:
• Animals are biologically very similar to humans. In fact, mice share more than 98% DNA with us!
• Animals are susceptible to many of the same health problems as humans – cancer, diabetes, heart disease, etc.
• With a shorter life cycle than humans, animal models can be studied throughout their whole life span and across several generations, a critical element in understanding how a disease processes and how it interacts with a whole, living biological system.
The ethics of animal experimentation
Nothing so far has been discovered that can be a substitute for the complex functions of a living, breathing, whole-organ system with pulmonary and circulatory structures like those in humans. Until such a discovery, animals must continue to play a critical role in helping researchers test potential new drugs and medical treatments for effectiveness and safety, and in identifying any undesired or dangerous side effects, such as infertility, birth defects, liver damage, toxicity, or cancer-causing potential.
U.S. federal laws require that non-human animal research occur to show the safety and efficacy of new treatments before any human research will be allowed to be conducted. Not only do we humans benefit from this research and testing, but hundreds of drugs and treatments developed for human use are now routinely used in veterinary clinics as well, helping animals live longer, healthier lives.
It is important to stress that 95% of all animals necessary for biomedical research in the United States are rodents – rats and mice especially bred for laboratory use – and that animals are only one part of the larger process of biomedical research.
Our researchers are strong supporters of animal welfare and view their work with animals in biomedical research as a privilege.
Stanford researchers are obligated to ensure the well-being of all animals in their care..
Stanford researchers are obligated to ensure the well-being of animals in their care, in strict adherence to the highest standards, and in accordance with federal and state laws, regulatory guidelines, and humane principles. They are also obligated to continuously update their animal-care practices based on the newest information and findings in the fields of laboratory animal care and husbandry.
Researchers requesting use of animal models at Stanford must have their research proposals reviewed by a federally mandated committee that includes two independent community members. It is only with this committee’s approval that research can begin. We at Stanford are dedicated to refining, reducing, and replacing animals in research whenever possible, and to using alternative methods (cell and tissue cultures, computer simulations, etc.) instead of or before animal studies are ever conducted.

Organizations and Resources
There are many outreach and advocacy organizations in the field of biomedical research.
- Learn more about outreach and advocacy organizations

Stanford Discoveries
What are the benefits of using animals in research? Stanford researchers have made many important human and animal life-saving discoveries through their work.
- Learn more about research discoveries at Stanford

Ethics of Medical Research with Animals
The Case for Phasing Out Experiments on Primates
Whether they realize it or not, most stakeholders in the debate about using animals for research agree on the common goal of seeking an end to research that causes animals harm. [1] The central issues in the controversy are about how much effort should be devoted to that goal and when we might reasonably expect to achieve it. Some progress has already been made: The number of animals used for research is about half what it was in the 1970s, and biomedical research has reached the point where we can reasonably begin to envision a time when it could advance without causing harm to animals. With some effort and aggressive development of new biomedical research technologies, full replacement of animals in harmful research is within our grasp. The goal will not be reached all at once, however, and phasing out invasive research on all nonhuman primates should be the priority.
Approximately 70,000 nonhuman primates are used for research in the United States each year, according to the U.S. Department of Agriculture, and another 45,000 are held or bred for research. They include macaques, baboons, marmosets, and other monkeys, as well as some chimpanzees. Moreover, these numbers are increasing in the United States and Canada. The rise is driven in part by the “high-fidelity” notion (supported by very little careful scientific justification) that primates are likely to be better models than mice and rats for studying human diseases, and partly by the sheer availability of primates.
The availability factor is a result of historical accident. In the 1960s, the United States invested in a significant infrastructure for primate research through creation of the National Primate Research Centers. The primate center program was the result of two unrelated occurrences. First, in the 1950s, hundreds of thousands of wild primates were captured and imported to support the race to develop a poliomyelitis vaccine. By 1960, with polio vaccines in use, this “race” was essentially over, but laboratories still had tens of thousands of primates. Then, they became swept up in another kind of race. The Russians had beaten the United States into space by launching the first satellite, creating panic that Russian science was outpacing U.S. science. American scientists made the argument that, because the Russians had a big primate research center, the United States should also have one or more primate centers. Seven facilities, formally recognized as government-supported institutions, were set up to provide support for and opportunities to do research in nonhuman primates.
The centers did not produce the hoped-for results. Three federal assessments found that the research conducted by the centers fell far short of expectations in terms of quality, and many deficiencies were also noted. [2] In the early 1980s, these centers were “rescued,” in a sense, by the discovery that primates at the California Regional Primate Research Center were suffering from a simian version of AIDS. Suddenly, there was renewed focus on research in nonhuman primates. There are now eight National Primate Research Centers, the objective of which continues to be “to provide support for scientists who use NHPs in their research.” [3]
Primates are used for a wide variety of research purposes. An analysis of one thousand federally funded studies that involved nonhuman primates found that research on HIV accounted for about 27 percent of the funding, followed by colony maintenance (likely because caring for primates is costly) at 15 percent, neurological research at 14 percent, and developmental research at 10 percent. [4]
Arguments for Phasing Out Primate Research
Phasing out primate use should be a priority for ethical, scientific, and economic reasons. The ethical concerns fall into two categories. One of them is the nature of the primates themselves. They are well known for their cognitive and emotional abilities. Studies demonstrate that they have mathematical, memory, and problem-solving skills and that they experience emotions similar to those of humans—for example, depression, anxiety, and joy. Chimpanzees can learn human languages, such as American Sign Language. Primates also have very long lifespans, which is an ethical issue because they are typically held in laboratories for decades and experimented on repeatedly. The other category of ethical concern is how primates are treated. Each year, thousands are captured from the wild, mostly in Asia and Mauritius, and transported to other countries. For example, China sets up breeding colonies, and the infants are sold to various countries, including the United States and European countries. The animals experience considerable stress, such as days of transport in small crates and restrictions on food and water intake. Studies show that it takes months for their physiological systems to return to baseline levels, [5] and then they face the trauma of research, including infection with virulent diseases, social isolation, food and water deprivation, withdrawal from drugs, and repeated surgeries.
Providing for the welfare of primates in a laboratory setting is very challenging. According to the Animal Welfare Act, each facility must develop and follow a plan for environmental enhancement to promote the psychological well-being of nonhuman primates. The plan must address social grouping; enriching the environment, with special consideration for great apes; caring for infants, young juveniles, and those primates showing signs of psychological distress; and ensuring the well-being of those primates who are used in a protocol requiring restricted activity.
Social companionship is the most important psychological factor for most primates. Federal law requires institutions to house primates in groups unless there is justification, such as debilitation as a result of age or other conditions, for housing them alone. But a recent analysis of documents from two large facilities obtained by The Humane Society of the United States demonstrates that primates spent an average of 53 percent of their lives housed alone. In many instances, a metal shape hung for a month on the bars of a metal cage was deemed to constitute adequate “enrichment.” [6]
As we have done with chimpanzees, we need to critically analyze uses of other nonhuman primates. A good starting point would be the formation of a working group of diverse stakeholders who agree that ending primate research is a worthwhile goal.
The Bateson report recommended that all proposed primate studies be assessed using the following parameters: scientific value, probability of medical or other benefit, availability of alternatives, and likelihood and extent of animal suffering. [9] The report indicated that if a proposed use would cause severe suffering, it should be allowed only if there is a high likelihood of benefit. The report considered approximately 9 percent of the studies it examined to be of low importance and to inflict high levels of suffering. [10] The report was critical of some of the neuroscience research, which represented nearly half of the research surveyed. It found that half of the thirty-one neuroscience studies took a high toll on animal welfare, although most were also considered to be of high scientific value. Two of the studies were of concern because they posed a “high welfare impact,” but moderate-quality science and little medical benefit. [11] The report recommended that more consideration be given to alternatives to nonhuman primates, including brain imaging, noninvasive electrophysiological technologies, in vitro and in silico techniques, and even research on human subjects. [12] The report recommended other ways of reducing the number of primates needed for research, including data sharing, publication of all results, and periodic review of outcomes, benefits, and impact of the research. “Researchers using NHPs have a moral obligation to publish results—even if negative—in order to prevent work from being repeated unnecessarily,” the report states. [13]
In addition to the ethical and scientific arguments for ending research involving primates, there are economic reasons. Primates are very expensive to maintain. The eight National Primate Research Centers alone receive $1 billion of the National Institutes of Health’s total $32 billion budget. The care and upkeep of primates other than chimpanzees is twenty to twenty-five dollars per day, compared with twenty cents to about $1.60 per day for small rodents. We argue that much of the research with nonhuman primates is either of questionable value or has not been carefully evaluated and justified. Therefore, these funds might be better spent on other research models, including several technologies that could replace nonhuman primates and other animals. Francis Collins, director of the NIH, argued in 2011 that new high-throughput approaches could overcome the drawbacks of animal models—they are slow, expensive, and not sufficiently relevant to human biology and pharmacology. [14]
Several such technologies are available. The U.S. Army recently announced that it would end the use of monkeys for chemical casualty training courses and replace them with alternatives such as simulators that mimic the effects of nerve gas on victims. [15]
Following Chimpanzees
The process that culminated in the phasing out of invasive research on chimpanzees in the United States in 2011 can and should be applied to all other nonhuman primates. Public opinion and ethical challenges drove that process. Even before the 2011 IOM report, scientists in the United States were having difficulty justifying why they should perform experiments on chimpanzees when their colleagues in other countries had stopped doing so. Unlike nonhuman primates in general, the number of chimpanzees in U.S. labs has been declining since reaching its peak in the late 1990s.
The main drivers for efforts to phase out research on chimpanzees are their genetic, biological, and behavioral similarities with humans. [16] Chimpanzees are humans’ closest relative. Chimpanzee cognition has been studied extensively, and their capabilities are considerable. As with other primates, the impact of laboratory life—including barren housing and social isolation—on chimpanzees can last decades due to their long lifespan and thus raises significant welfare concerns. There is evidence that some chimpanzees used in research suffer from a form of posttraumatic stress disorder similar to that of humans. In their 2008 article, Gay Bradshaw and colleagues described the plight of a chimpanzee named Jeannie who endured invasive research and social isolation for over a decade. She exhibited abnormal behavior, including self-injury, bouts of aggression, and, according to laboratory documentation, a “nervous breakdown.” When retired to a sanctuary, she recovered partially, but was ultimately diagnosed with complex PTSD. The paper concluded: “The costs of laboratory-caused trauma are immeasurable in their life-long psychological impact on, and consequent suffering of, chimpanzees.” [17]
As we have done with chimpanzees, we need to critically analyze current uses of other nonhuman primates, the viability of alternative models, and the economic issues involved to forge the best way forward. A good starting point would be the formation of a working group of diverse stakeholders who agree that ending primate research is a worthwhile goal. Such a working group—possibly organized by the NIH and the National Academies—would analyze the necessity of primate use and identify existing and potential alternatives.
The stakeholder group could develop a concrete plan to work on common-ground issues. This would involve developing priorities, short-term outcomes, and related activities. The ongoing Human Toxicology Project Consortium’s work to ultimately replace all animals for toxicity testing is a good example of this approach. (See “No Animals Harmed: Toward a Paradigm Shift in Toxicity Testing,” in this volume.) The mission of the consortium is to “serve as a catalyst for the prompt, global, and coordinated implementation of ‘21 st Century’ toxicology, which will better safeguard human health and hasten the replacement of animal use in toxicology.” [18] Because science is ever-changing, there must be an ongoing analysis of new technologies and challenges, and regulatory authorities must adjust regulations accordingly. In the United States, many stakeholders express frustration with the fact that the Food and Drug Administration, for example, favors data from outdated tests, including those that involve primates and other animals.
Phasing out invasive research on all nonhuman primates would take courage on the part of leaders in science and policy. It is a formidable task, but similarly transformative changes in how we conduct biomedical research have been achieved. At various points in the past century and a quarter, restrictions have been placed on particular kinds of human and animal research because of ethical issues, despite objections that such restrictions would slow scientific progress; think, for instance, of the Helsinki Declaration to protect human subjects in research and the animal welfare laws in the United States and the European Union. However, these laws have not slowed the pace of discovery about biology and disease processes. If anything, there has been an acceleration of such discovery in the half-century since these restrictions went into effect.
In the early 1950s, Sir Peter Medawar pressed the Universities Federation for Animal Welfare to develop a report on how laboratory animal welfare could be improved and how distress and suffering in the research laboratory might be reduced. That initiative led to publication of a volume on humane experimental approach that is now regarded as the foundation for the concept of the Three Rs of replacement, reduction, and refinement of animal studies. [19] Ten years later, in 1969, Medawar correctly predicted that laboratory animal use would peak within ten years and then start to decline. He argued that animal research would allow researchers to develop the knowledge and understanding that would lead, eventually, to the replacement of animal use in laboratories. In 2010, forty years after Medawar’s prediction, laboratory animal use is approximately 50 percent of what it was in 1970. Francis Collins has pointed to the down sides of animal-based research—that is “time-consuming, costly, and may not accurately predict efficacy in humans.” [20] He has also suggested that nonanimal technologies might be quicker and more effective in new drug discovery programs. Given the trends and political will, we believe that we could reach Medawar’s prediction of complete replacement by 2050.
Now is the time for an internationally coordinated effort to define a strategy to replace all invasive research on primates. At the very least, we need to move quickly to reverse the increase in laboratory primate use in the United States and Canada. Until replacement is a realistic option, we must reduce the number of primates used and refine studies to reduce their suffering, for the sake of both animal welfare and science.
Kathleen M. Conlee is vice president for animal research issues with The Humane Society of the United States. She worked for several years at a primate breeding and research facility and also worked with great apes in a sanctuary setting. Her current work focuses on the long-term goal of replacing the use of animals in harmful research and testing, the ongoing development of nonanimal alternatives, and the short-term goals of ending invasive chimpanzee research and retiring chimpanzees from laboratories to sanctuaries.
Andrew N. Rowan is chief scientific officer at The Humane Society of the United States and chief executive officer of The Humane Society International. He has written numerous books and peer-reviewed publications regarding animal research, including a book titled The Animal Research Controversy: Protest, Process and Public Policy (Center for Animals and Public Policy, Tufts University School of Veterinary Medicine, 1995). He is a biochemist in training, and a focus of his career has been promotion of the three Rs in animal research: replacement of nonhuman animals, reduction in number of animals used, and refinement to decrease animal suffering.
[[12]]12. Ibid., 4, 5, 16.[[12]
- 1. C. Blakemore, “Should We Experiment on Animals? Yes,” Telegraph, October 28, 2008. ↵
- 2. A.N. Rowan, Of Mice, Models and Men (Albany: State University of New York Press, 1984). ↵
- 3. Department of Health and Human Services, Funding Opportunity for the National Primate Research Centers, http://grants.nih.gov/grants/guide/pa-files/PAR-11-136.html, accessed July 7, 2011. ↵
- 4. K.M. Conlee, E.H. Hoffeld, and M.L. Stephens, “A Demographic Analysis of Primate Research in the United States,” Alternatives to Laboratory Animals 32, suppl. 1A (2004): 315-22. ↵
- 5. P.E. Honess, P.J. Johnson, and S.E. Wolfensohn, “A Study of Behavioural Responses of Non-Human Primates to Air Transport and Re-Housing,” Laboratory Animals 38, no. 2 (2004): 119-32; J.M. Kagira et al., “Hematological Changes in Vervet Monkeys ( Chlorocedub aethiops ) during Eight Months’ Adaptation to Captivity,” American Journal of Primatology 69, no. 9 (2007): 1053-63. ↵
- 6. J. Balcombe and K.M. Conlee, “Primate Life in Two American Laboratories,” presentation to the Eighth World Congress on Alternatives and Animal Use in the Life Sciences, held in Montreal, Quebec, Canada, on August 21-25, 2011. ↵
- 7. P. Bateson, A Review of Research Using Nonhuman Primates: A Report of a Panel Chaired by Professor Sir Patrick Bateson, FRS (London and Wiltshire, U.K.: Biotechnology and Biological Sciences Research Council, Medical Research Council, and Wellcome Trust, 2011) http://www.mrc.ac.uk/Utilities/Documentrecord/index.htm?d =MRC008083; J.A. Smith and K.M. Boyd, eds., The Use of Non-Human Primates in Research and Testing (Leicester, U.K.: British Psychological Society, 2002); D. Weatherall, The Use of Non-Human Primates in Research: A Working Group Report Chaired by Sir David Weatherall FRS FmedSci (London: Academy of Medical Sciences, 2006), http://www.acmedsci.ac.uk/images/project/nhpdownl.pdf. ↵
- 8. Institute of Medicine, Committee on the Use of Chimpanzees in Biomedical and Behavioral Research, Chimpanzees in Biomedical and Behavioral Research: Assessing the Necessity (Washington, D.C.: National Academies Press, 2011), 4. ↵
- 9. Bateson, A Review of Research Using Nonhuman Primates , 2. ↵
- 10. Ibid., 1. ↵
- 11. Ibid., 12-13. ↵
- 13. Ibid., 3. ↵
- 14. F.S. Collins, “Reengineering Translational Science: The Time Is Right,” Science Translational Medicine 3, no. (2011): 1-6. ↵
- 15. B. Vastag, “Army to Phase Out Animal Nerve-Agent Testing,” Washington Post, October 13, 2011. ↵
- 16. G.W. Bradshaw et al., “Building Inner Sanctuary: Complex PTSD in Chimpanzees,” Journal of Trauma and Dissociation 9, no. 1 (2008): 9-34; J.A. Smith and K.M. Boyd, eds., The Boyd Group Papers on the Use of Non-Human Primates in Research and Testing (Leicester, U.K.: British Psychological Society, 2002). ↵
- 17. Bradshaw et al., “Building Inner Sanctuary,” 31. ↵
- 18. Human Toxicology Project Consortium Web site, http://htpconsortium.wordpress.com/about-2, accessed February 13, 2012. ↵
- 19. W.M.S. Russell and R.L. Burch, The Principles of Humane Experimental Technique (London: Methuen, 1959). ↵
- 20. F.S. Collins, “Reengineering Translational Science,” 3. ↵
- Share full article
Advertisement
Supported by
student opinion
Is Animal Testing Ever Justified?
The E.P.A. recently said it would move away from requiring the testing of potentially harmful chemicals on animals. Do you support the decision?

By Natalie Proulx
Find all our Student Opinion questions here.
On Sept. 10, the Environmental Protection Agency said it would move away from requiring the testing of potentially harmful chemicals on animals, a decision that was hailed by animal rights groups but criticized by environmentalists and researchers who said the practice was necessary to rigorously safeguard human health.
What are your thoughts on animal testing? Do you think it is ever justified? Why or why not?
In “ E.P.A. Says It Will Drastically Reduce Animal Testing ,” Mihir Zaveri, Mariel Padilla and Jaclyn Peiser write about the decision:
The E.P.A. Administrator Andrew Wheeler said the agency plans to reduce the amount of studies that involve mammal testing by 30 percent by 2025, and to eliminate the studies entirely by 2035, though some may still be approved on a case-by-case basis. The agency said it would also invest $4.25 million in projects at four universities and a medical center that are developing alternate ways of testing chemicals that do not involve animals. “We can protect human health and the environment by using cutting-edge, ethically sound science in our decision-making that efficiently and cost-effectively evaluates potential effects without animal testing,” Mr. Wheeler said in a memo announcing the changes. The E.P.A. has for decades required testing on a variety of animals — including rats, dogs, birds and fish — to gauge their toxicity before the chemicals can be bought, sold or used in the environment.
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .
- Help Animals
- Investigations
- Animal Rights Issues
- Students Opposing Speciesism (SOS)
- Join the Action Team
- Monthly Giving
- Your Legacy For Animals
- PETA Vanguard Society
- ‘In Honor of’ and Memorial Gifts
- Membership Services
- More Ways to Support PETA
- Report Cruelty to Animals
- Urgent Alerts
- Our Campaigns
- Action Team
- Activist Guide
- Get Active Online
- Leaflets & Stickers
- Adoptable Animals
- Rescue Stories
- Recent Investigations
- Investigations & Rescue Fund
- Animals Used for Experimentation
- Entertainment
- Companion Animals
- Personal Care & Fashion
- Food & Health
- Humane Home
- Animal Companions
- Cruelty-Free Database
- PETA Shopping Guide
- PETA Approved Vegan
- PETA Presents
- Work at PETA
- PETA Global
- Vanguard Society
- More Ways to Support
- Gifts in Wills
A Century of Suffering: 10 Gruesome Experiments on Animals From the Last 100 Years
Mice, rats, dogs, monkeys, rabbits, and other animals have been suffering in experiments for more than a century. During the last 100 years, sensitive animals trapped in laboratories have been burned, shocked, poisoned, forcibly impregnated, decapitated, locked away in isolation from other members of their species, and made to endure countless other atrocities. Below, you can learn more about the sordid history of animal testing — including the landmark investigation that launched PETA more than 40 years ago.

The History of Animal Testing — 10 Shocking Experiments on Animals From the Last Century
1. maryland psychologist severed spinal cords, repeatedly shocked monkeys (1958–1981).
At the Institute for Behavioral Research in Silver Spring, Maryland, psychologist and animal experimenter Edward Taub—a man with no medical or veterinary training—kept 17 monkeys in cramped wire cages that were caked with feces. The animals were subjected to debilitating surgeries in which their spinal nerves were severed, rendering one or more of their limbs useless. They were then forced to try to regain function in their impaired limbs through cruel methods such as electric shocks and pinches with pliers.
PETA’s groundbreaking investigation into this hellhole led to the nation’s first arrest and criminal conviction of an animal experimenter for cruelty to animals, the first confiscation of abused animals from a laboratory, and the first U.S. Supreme Court victory for animals used in experiments.

2. Pfizer Injects Horses With Snake Venom (1961–Present)
In Pfizer laboratories, snake venom has been repeatedly injected into 111 horses and large quantities of their blood have been drawn. These painful procedures can cause horses to fall ill, lose weight, and become anemic, and no pain relief is provided.
3. Government Experimenter Inflicts Permanent Brain Damage on Monkeys (1983–Present)
The National Institutes of Health’s Elisabeth Murray carves out a section of monkeys’ skulls and then injects toxins into the brain or suctions out portions of it, causing permanent and traumatic damage. The monkeys are then held alone in a small metal cage. A guillotine-like door at the front of the cage is suddenly raised, revealing realistic-looking fake spiders or snakes, which are inherently frightening to monkeys. The animals endure this same torture repeatedly. When Murray has finished with them, they may be killed or recycled into other experiments to be further tormented.
4. Sensory-Deprivation Experiments on Baby Monkeys (1983–Present)
Margaret Livingstone, a Harvard University experimenter, has spent her entire 40-year career tormenting animals, including by tearing baby monkeys away from their mothers and sewing their eyes shut—or making sure they never see a human or monkey face in other ways—just to see how badly it damages their brain and visual development. Livingstone calls it science. We call it psychosis. Harvard must shut down her lab permanently and have her head examined.
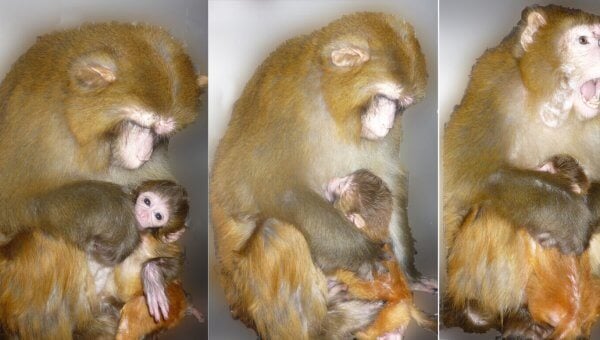
Figure 1 in Triggers for Mother Love | Margaret S. Livingstone | CC BY-NC-ND
5. Oregon Experimenter Killed and Cut Open Pregnant Monkeys (1997–2017)
Kevin Grove at the Oregon National Primate Research Center confined female monkeys to cramped cages and fed them unhealthy, high-fat diets until they became obese. He then artificially inseminated them. Some of these pregnant monkeys were killed and cut open, and their brains and fetuses were removed and examined. Those who weren’t killed gave birth, and their newborns were taken away from them almost immediately, traumatizing both mother and baby.
6. Columbia Experimenters Cut Baboons’ Eyes Out, Induced Strokes (2001–2011)
In experiments conducted at Columbia University, baboons’ eyes were cut out, sometimes while they were conscious, and the arteries to their brains were clamped in a crude procedure intended to induce strokes.
7. Animals Beheaded With Kitchen Scissors in UNC Experiments (2001–2003)
At the University of North Carolina, animals were used in alcohol, dopamine, and nicotine experiments. Mice and rats who had been inadequately gassed or undergone improper cervical dislocation (neck-breaking) were still alive in a cooler used to store dead animals. An experimenter admitted that he was not numbing young rats with ice before cutting their heads off with scissors and removing their brains.
8. Johns Hopkins Experimenter Cuts Into Owls’ Skulls, Implants Electrodes in Brains (2005–Present)
Shreesh Mysore, an experimenter at Johns Hopkins University, cuts into owls’ skulls to expose their brains and then screws and glues metal devices to their heads. These birds—nocturnal hunters who would fly great distances in their natural habitat—are forced into plastic tubes so small that they can’t even move their wings. Then, Mysore bombards them with lights and sounds. He pokes electrodes around in their brains while they’re conscious, mutilating the tissue so severely that they become “unusable” to him—at which point he kills them.

9. University Experimenter Traps Birds, Wounds Them Without Painkillers (2008–Present)
At Tufts and Yale universities, experimenter Christine Lattin injected sparrows and other birds with chemicals to destroy their adrenal glands, used a biopsy punch to inflict painful wounds on birds’ legs, and fed sparrows food that was laced with crude oil.
Now at Louisiana State University (LSU), she’s begun a new round of pointless experiments on birds. House sparrows breeding in nest boxes at the LSU College of Agriculture will be captured, banded, and fitted with digital ID transmitters. At the end of the breeding season, Lattin will recapture and then kill all the birds— and their chicks —before removing their brains for analysis.

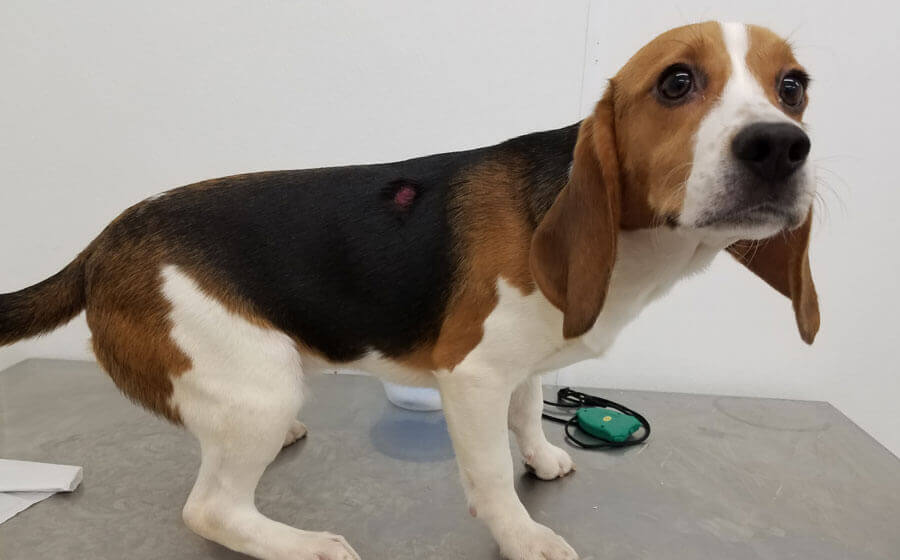
10. Liberty Research Workers Drilled Holes Into Young Beagles’ Skulls (2016–2017)
A 2017 PETA eyewitness investigation showed that workers at Liberty Research, Inc.—a laboratory in New York—drilled holes into the skulls of young beagles so that distemper virus could be injected directly into their brains. Some dogs blinked and even whimpered during the painful procedure—indicating that they were not adequately anesthetized—and woke up moaning. Likely in severe pain, some banged their heads on cage walls, causing blood to spurt from their wounds. Some foamed at the mouth, and others had seizures. They were left to suffer without any apparent treatment and were killed at the end of the study.
Want to Learn More About the History of Animal Testing?
Be sure to check out PETA’s interactive online timeline, “Without Consent.” It explores the troubled history of animal testing and challenges what we’ve been told for decades is normal.

It’s speciesist to believe that animals in laboratories don’t feel emotions or experience pain. Animals used in experiments are no different from the cats and dogs with whom many people lovingly share their homes, yet they are afforded few—if any—of the same protections or considerations. As a result, more than 100 million animals suffer and die in the U.S. every year in cruel chemical, drug, food, and cosmetics tests as well as in medical training exercises and curiosity-driven medical experiments at universities .
What You Can Do for These Animals
Now that you’ve learned more about the history of animal testing, discover what you can do for animals in the present day. PETA makes it easy to take action for animals suffering in cruel and useless experiments like the ones described above. It only takes a minute using your phone or computer, so what are you waiting for?

From PETA and executive producer Bill Maher, the new docuseries ‘The Failed Experiment’ exposes what most people don’t know about experiments on animals.
Related Posts

The work of the PETA Foundation’s crack legal team goes far beyond what’s shown in HBO’s “Chimp Crazy.” Here are some of our other historic cases!
Are animal experimenters who work with dangerous pathogens in laboratories capable of protecting public health from an outbreak? Spoiler alert: They’re not.
Personal trauma over experimenting on mice and other animals is creating a brain drain in scientific fields. PETA knows that institutions can recover and evolve.
By submitting this form, you’re acknowledging that you have read and agree to our privacy policy and agree to receive e-mails from us.

“Almost all of us grew up eating meat, wearing leather, and going to circuses and zoos. We never considered the impact of these actions on the animals involved. For whatever reason, you are now asking the question: Why should animals have rights? ”
— Ingrid E. Newkirk, PETA President and co-author of Animalkind
Text CRAZY to 73822 to take action for chimpanzees suffering in human homes & roadside zoos! Then watch Chimp Crazy, HBO’s new docuseries to learn more.
Terms for automated texts/calls from PETA: http://peta.vg/txt . Text STOP to end, HELP for more info. Msg/data rates may apply. U.S. only.
share this!
November 3, 2022
Controversial monkey study reignites animal testing debate
by Issam AHMED

Mother monkeys permanently separated from their newborns sometimes find comfort in plush toys: this recent finding from Harvard experiments has set off intense controversy among scientists and reignited the ethical debate over animal testing.
The paper, "Triggers for mother love" was authored by neuroscientist Margaret Livingstone and appeared in the Proceedings of the National Academy of Sciences ( PNAS ) in September to little fanfare or media coverage .
But once news of the study began spreading on social media, it provoked a firestorm of criticism and eventually a letter to PNAS signed by over 250 scientists calling for a retraction.
Animal rights groups meanwhile recalled Livingstone's past work, that included temporarily suturing shut the eyelids of infant monkeys in order to study the impact on their cognition.
"We cannot ask monkeys for consent, but we can stop using, publishing, and in this case actively promoting cruel methods that knowingly cause extreme distress," wrote Catherine Hobaiter, a primatologist at the University of St Andrews, who co-authored the retraction letter.
Hobaiter told AFP she was awaiting a response from the journal before further comment, but expected news soon.
Harvard and Livingstone, for their part, have strongly defended the research.
Livingstone's observations "can help scientists understand maternal bonding in humans and can inform comforting interventions to help women cope with loss in the immediate aftermath of suffering a miscarriage or experiencing a still birth," said Harvard Medical School in a statement.
Livingstone, in a separate statement, said: "I have joined the ranks of scientists targeted and demonized by opponents of animal research, who seek to abolish lifesaving research in all animals."
Such work routinely attracts the ire of groups such as People for the Ethical Treatment of Animals (PETA), which opposes all forms of animal testing .
This controversy has notably provoked strong responses in the scientific community , particularly from animal behavior researchers and primatologists, said Alan McElligot of the City University of Hong Kong's Centre for Animal Health and a co-signer of the PNAS letter.
He told AFP that Livingstone appears to have replicated research performed by Harry Harlow, a notorious American psychologist, from the mid-20th century.
Harlow's experiments on maternal deprivation in rhesus macaques were considered groundbreaking, but may have also helped catalyze the early animal liberation movement.
"It just ignored all of the literature that we already have on attachment theory," added Holly Root-Gutteridge, an animal behavior scientist at the University of Lincoln in Britain.
Harm reduction
McElligot and Root-Gutteridge argue the case was emblematic of a wider problem in animal research , in which questionable studies and papers continue to pass institutional reviews and are published in high impact journals.
McElligot pointed to a much-critiqued 2020 paper extolling the efficiency of foot snares to capture jaguars and cougars for scientific study in Brazil.
More recently, experiments on marmosets that included invasive surgeries have attracted controversy.
The University of Massachusetts Amherst team behind the work says studying the tiny monkeys, which have 10-year-lifespans and experience cognitive decline in their old age, are essential to better understand Alzheimers in people.
Opponents argue results rarely translate across species.
When it comes to testing drugs, there is evidence the tide is turning against animal trials.
In September, the US Senate passed the bipartisan FDA Modernization Act, which would end a requirement that experimental medicines first be tested on animals before any human trials .
The vast majority of drugs that pass animal tests fail in human trials, while new technologies such as tissue cultures, mini organs and AI models are also reducing the need for live animals.
Opponents also say the vast sums of money that flow from government grants to universities and other institutes—$15 billion annually, according to watchdog group White Coat Waste—perpetuate a system in which animals are viewed as lab resources.
"The animal experimenters are the rainmaker within the institutions, because they're bringing in more money," said primatologist Lisa Engel-Jones, who worked as a lab researcher for three decades but now opposes the practice and is a science advisor for PETA.
"There's financial incentive to keep doing what you've been doing and just look for any way you can to get more papers published, because that means more funding and more job security," added Emily Trunnel, a neuroscientist who experimented on rodents and also now works for PETA.
Most scientists do not share PETA's absolutist stance, but instead say they adhere to the "three Rs" framework—refine, replace and reduce animal use.
On Livingstone's experiment, Root-Gutteridge said the underlying questions might have been studied on wild macaques who naturally lost their young, and urged neuroscientists to team up with animal behaviorists to find ways to minimize harm.
Journal information: Proceedings of the National Academy of Sciences
Explore further
Feedback to editors

Researchers test ChatGPT, other AI models against real-world students
5 hours ago

Study finds mine-drainage treatment cost effective, but far more costs lay ahead

Researchers develop new method for delivering RNA and drugs into cells


Scientists discover how TGF-Beta sends its message even while tethered to the cell membrane

New algorithm rights wrongs of precipitation-type classification over Tibetan Plateau

An AI tool for scanning sand grains opens windows into recent time and the deep past
6 hours ago

Scientists discover nonstomatal control of water loss in critical crops

Researchers find golden eagles improve their flight skills with age
7 hours ago

Optogenetic control reveals collective cell behavior

Designing a better water filter: A fabric-like filter to remove tiny plastics and lead from drinking water
8 hours ago
Relevant PhysicsForums posts
Why does a series of pulses generate a pitch.
Sep 15, 2024
Epothilone B study connected to 'Hard Problem of Consciousness' Model
Sep 9, 2024
Any stereo audio learning resources for other languages?
Sep 8, 2024
Too much fluoride might lower IQ in kids?
Sep 6, 2024
The predictive brain (Stimulus-Specific Error Prediction Neurons)
Sep 1, 2024
Any suggestions to dampen the sounds of a colostomy bag?
Aug 31, 2024
More from Biology and Medical
Related Stories

Observations in macaques provide new insights into how mothers form attachments to their newborns
Sep 19, 2022

FDA takes steps away from animal testing requirement
Aug 10, 2022

New testing methods can spare laboratory animals
Feb 4, 2022
Scientists successfully transition cell line to be completely animal-free
Jul 26, 2022

University can continue barn owl testing after permit battle with PETA
Jul 12, 2022
Cell-based tests promise respite for lab animals: study
Jan 26, 2016
Recommended for you

New 'grumpy' fish species discovered in the Red Sea
10 hours ago

Researchers find evidence that bumblebees make the same memory errors as humans
11 hours ago

Genomics reveals sled dogs' Siberian lineage

Flowers use adjustable 'paint by numbers' petal designs to attract pollinators, researchers discover
Sep 13, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- NATURE INDEX
- 04 November 2022
US agency seeks to phase out animal testing
- Rachel Nuwer 0
Rachel Nuwer is a freelance writer based in New York City.
You can also search for this author in PubMed Google Scholar

Biomedical advances mean there might be sound alternatives to using animals such as mice for testing. Credit: Paul Chinn/The San Francisco Chronicle/Getty
The future of drug development might be animal-free — or, at least, involve far fewer animals than is currently the norm. Last June, the US Food and Drug Administration (FDA) set out proposals for the New Alternative Methods Program that will focus on replacing, reducing and refining the use of laboratory animals through the adoption of cutting-edge alternative methods. The aim is to produce findings that are more relevant to humans, streamline product development and reduce costs.
The shift, which has been years in the making, would be undertaken across all of the FDA’s centres, including ones that oversee the approval of new pharmaceuticals, medical devices, veterinary medicines, cosmetics and more. FDA scientists are conducting their own research in service of this goal and are collaborating extensively with colleagues in industry, academia and other sectors of the US government. Any methods eventually adopted in place of research involving animals would be rigorously vetted and “fully validated and based on the best science”, says Namandjé Bumpus, chief scientist at the FDA. Bumpus and her colleagues have not received any pushback from researchers about making this shift, she adds, or heard any concerns from the scientific community about cutting back on the use of animals.
Although there is no set timeline, FDA officials say the programme is a priority. It would be funded from US$5 million it has requested as part of its 2023 budget to develop a ‘comprehensive strategy’ on alternative testing methods. According to Paul Locke, an environmental-health scientist and lawyer at Johns Hopkins University in Baltimore, Maryland, who specializes in alternatives to animal testing, the FDA is taking a necessary step towards ensuring that the US government stays up to speed with the latest science. “I’m really excited about what the FDA’s doing here,” he says. “They’ve put a stake in the ground and said, ‘Hey, we want to be there using these tools, they’re consistent with our mission and they’re consistent with what twenty-first-century science looks like.’”
Biomedical advances fuel alternatives
Animal-based testing has been the gold standard for research for decades, and it remains an important requirement for establishing the safety and efficacy of products being brought to market today. But key differences exist between humans and the rodents, rabbits, non-human primates and other animals that researchers depend on for testing, and as biomedical understanding has advanced, scientists have begun to come up against the limitations of using other species as proxies for humans. “A mouse or a rat doesn’t always handle or process medicines and chemicals in the same way humans do,” Bumpus says. “Developing more in vitro systems that are based on human cells, human tissues and human models could, in some instances, be more predictive.”
The FDA’s interest in moving towards new approaches also reflects the current thinking of the biomedical community at large. In 2014, the United Kingdom, for example, announced plans to reduce the use of animal tests in scientific research, aiming to replace those tests with ‘scientifically valid alternatives’ where possible. In 2021, the European Parliament voted in favour of plans to phase out animal testing in research.
“A key for bringing about change is to do so among the multiple major regulatory agencies,” says David Strauss, director of the FDA’s Division of Applied Regulatory Science. “Drug-development programmes are global, and companies want to market their products in many countries around the world.”
That sentiment is echoed by Bumpus. “There’s a lot of energy around this across the globe,” she adds.
Animal-rights advocates have been calling for an end to animal testing for years; now, methods being developed in labs around the world have made this a realistic possibility for the future. “We think we’re at a potential tipping point,” says Strauss. The technologies include, for example, induced pluripotent stem cells — cells that scientists program to have the potential to turn into any cell type found in the body — and ‘organs-on-a-chip’, which are small devices containing living human tissues that mimic an organ, organ system or even an entire body 1 . Developments in artificial intelligence and machine learning are also allowing scientists to harness existing data to build computer models that can make predictions about a new drug’s safety and efficacy.
More funding needed
In addition to being more relevant to humans, says Locke, once these types of technology are qualified and validated for specific uses, they will probably be faster and cheaper than using animals, allowing products to be brought to market more rapidly and efficiently. These are still early days, however, and so far, the FDA has only a handful of successful animal-testing-replacement stories it can point to.
Funding for developing and validating alternative methods is also an issue, Locke adds — both internally at the FDA, and externally for scientists whose labs depend on federal funds to pioneer new approaches. The FDA is not primarily a funding agency, and the US National Institutes of Health, which is the largest public funder of biomedical research in the world, currently has no programme dedicated to developing alternatives to animal testing. “If we had a legitimate funding programme to push these technologies forward, it would accelerate their progress greatly,” Locke points out.
For now, despite the promising alternatives to animal testing, federal regulators have approved only a few cutting-edge methods, and such techniques will not completely replace animal testing any time soon. But they do hold great promise, Locke says, especially if other government agencies and countries join the FDA in its effort.
“There’s a lot of moving pieces here,” he says. “The FDA has started the ball rolling, but we need more work to make sure we can use these new methodologies appropriately.”
doi: https://doi.org/10.1038/d41586-022-03569-9
Sung, J. H. et al. Anal. Chem. 91 , 330–351 (2019).
Article PubMed Google Scholar
Download references
Related Articles
- Drug discovery
- Health care
- Medical research

Agonist antibody to guanylate cyclase receptor NPR1 regulates vascular tone
Article 11 SEP 24
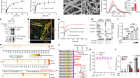
Fibrin drives thromboinflammation and neuropathology in COVID-19
Article 28 AUG 24
Debate rages over Alzheimer’s drug lecanemab as UK limits approval
News 22 AUG 24

Mosquito-borne diseases are surging in Europe — how worried are scientists?
News 16 SEP 24

First private spacewalk a success! What the SpaceX mission means for science
News 12 SEP 24

Why does heart disease affect so many young South Asians?
News Feature 11 SEP 24
Long-lasting heart-failure treatment could be a game-changer
News & Views 11 SEP 24
Lipid recycling by macrophage cells drives the growth of brain cancer
Why some women enter menopause early — and how that could affect their cancer risk
News 11 SEP 24
Faculty Positions in Biology and Biological Engineering: Caltech, Pasadena, CA, United States
The Division of Biology and Biological Engineering (BBE) at Caltech is seeking new faculty in the area of Molecular Cell Biology.
Pasadena, California
California Institute of Technology (Caltech)
Assistant Professor of Molecular and Cellular Biology
We seek applications for a tenure-track faculty position in the Department of Molecular and Cellular Biology.
Cambridge, Massachusetts
Harvard University - Department of Molecular and Cellular Biology
Husbandry Technician I
Memphis, Tennessee
St. Jude Children's Research Hospital (St. Jude)
Lead Researcher - Developmental Biology of Medulloblastoma (Paul Northcott Lab)
Clinical research associate rn i.
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Ethical and Scientific Considerations Regarding Animal Testing and Research
Hope r. ferdowsian.
1 Physicians Committee for Responsible Medicine, Washington, D.C., United States of America
2 Department of Medicine, The George Washington University, Washington, D.C., United States of America
Conceived and designed the experiments: HRF NB. Contributed reagents/materials/analysis tools: HRF NB. Wrote the paper: HRF NB.
In 1959, William Russell and Rex Burch published the seminal book, The Principles of Humane Experimental Technique, which emphasized r eduction, r efinement, and r eplacement of animal use, principles which have since been referred to as the “3 Rs”. These principles encouraged researchers to work to reduce the number of animals used in experiments to the minimum considered necessary, refine or limit the pain and distress to which animals are exposed, and replace the use of animals with non-animal alternatives when possible. Despite the attention brought to this issue by Russell and Burch and since, the number of animals used in research and testing has continued to increase, raising serious ethical and scientific issues. Further, while the “3 Rs” capture crucially important concepts, they do not adequately reflect the substantial developments in our new knowledge about the cognitive and emotional capabilities of animals, the individual interests of animals, or an updated understanding of potential harms associated with animal research. This Overview provides a brief summary of the ethical and scientific considerations regarding the use of animals in research and testing, and accompanies a Collection entitled Animals, Research, and Alternatives: Measuring Progress 50 Years Later , which aims to spur ethical and scientific advancement.
Introduction
One of the most influential attempts to examine and affect the use of animals in research can be traced back to1959, with the publication of The Principles of Humane Experimental Technique [1] . William Russell and Rex Burch published this seminal book in response to marked growth in medical and veterinary research and the concomitant increase in the numbers of animals used. Russell and Burch's text emphasized r eduction, r efinement, and r eplacement of animal use, principles which have since been referred to as the “3 Rs”. These principles encouraged researchers to work to reduce the number of animals used in experiments to the minimum considered necessary, refine or limit the pain and distress to which animals are exposed, and replace the use of animals with non-animal alternatives when possible.
Despite the attention brought to this issue by Russell and Burch, the number of animals used in research and testing has continued to increase. Recent estimates suggest that at least 100 million animals are used each year worldwide [2] . However, this is likely an underestimate, and it is impossible to accurately quantify the number of animals used in or for experimentation. Full reporting of all animal use is not required or made public in most countries. Nevertheless, based on available information, it is clear that the number of animals used in research has not significantly declined over the past several decades.
The “3 Rs” serve as the cornerstone for current animal research guidelines, but questions remain about the adequacy of existing guidelines and whether researchers, review boards, and funders have fully and adequately implemented the “3 Rs”. Further, while the “3 Rs” capture crucially important concepts, they do not adequately reflect the substantial developments in our new knowledge about the cognitive and emotional capabilities of animals; an updated understanding of the harms inherent in animal research; and the changing cultural perspectives about the place of animals in society [3] , [4] . In addition, serious questions have been raised about the effectiveness of animal testing and research in predicting anticipated outcomes [5] – [13] .
In August 2010, the Georgetown University Kennedy Institute of Ethics, the Johns Hopkins University Center for Alternatives to Animal Testing, the Institute for In Vitro Sciences, The George Washington University, and the Physicians Committee for Responsible Medicine jointly held a two day multi-disciplinary, international conference in Washington, DC, to address the scientific, legal, and political opportunities and challenges to implementing alternatives to animal research. This two-day symposium aimed to advance the study of the ethical and scientific issues surrounding the use of animals in testing and research, with particular emphasis on the adequacy of current protections and the promise and challenges of developing alternatives to the use of animals in basic research, pharmaceutical research and development, and regulatory toxicology. Speakers who contributed to the conference reviewed and contributed new knowledge regarding the cognitive and affective capabilities of animals, revealed through ethology, cognitive psychology, neuroscience, and related disciplines. Speakers also explored the dimensions of harm associated with animal research, touching on the ethical implications regarding the use of animals in research. Finally, several contributors presented the latest scientific advances in developing alternatives to the use of animals in pharmaceutical research and development and regulatory toxicity testing.
This Collection combines some papers that were written following this conference with an aim to highlight relevant progress and research. This Overview provides a brief summary of the ethical and scientific considerations regarding the use of animals in research and testing, some of which are highlighted in the accompanying Collection.
Analysis and Discussion
Ethical considerations and advances in the understanding of animal cognition.
Apprehension around burgeoning medical research in the late 1800s and the first half of the 20 th century sparked concerns over the use of humans and animals in research [14] , [15] . Suspicions around the use of humans were deepened with the revelation of several exploitive research projects, including a series of medical experiments on large numbers of prisoners by the Nazi German regime during World War II and the Tuskegee syphilis study. These abuses served as the impetus for the establishment of the Nuremberg Code, Declaration of Helsinki, and the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (1974) and the resulting Belmont Report [16] – [18] . Today, these guidelines provide a platform for the protection of human research subjects, including the principles of respect, beneficence, and justice, as well as special protections for vulnerable populations.
Laws to protect animals in research have also been established. The British Parliament passed the first set of protections for animals in 1876, with the Cruelty to Animals Act [19] . Approximately ninety years later, the U.S. adopted regulations for animals used in research, with the passage of the Laboratory Animal Welfare Act of 1966 [20] . Subsequent national and international laws and guidelines have provided basic protections, but there are some significant inconsistencies among current regulations [21] . For example, the U.S. Animal Welfare Act excludes purpose-bred birds, rats, or mice, which comprise more than 90% of animals used in research [20] . In contrast, certain dogs and cats have received special attention and protections. Whereas the U.S. Animal Welfare Act excludes birds, rats and mice, the U.S. guidelines overseeing research conducted with federal funding includes protections for all vertebrates [22] , [23] . The lack of consistency is further illustrated by the “U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training” which stress compliance with the U.S. Animal Welfare Act and “other applicable Federal laws, guidelines, and policies” [24] .
While strides have been made in the protection of both human and animal research subjects, the nature of these protections is markedly different. Human research protections emphasize specific principles aimed at protecting the interests of individuals and populations, sometimes to the detriment of the scientific question. This differs significantly from animal research guidelines, where the importance of the scientific question being researched commonly takes precedence over the interests of individual animals. Although scientists and ethicists have published numerous articles relevant to the ethics of animal research, current animal research guidelines do not articulate the rationale for the central differences between human and animal research guidelines. Currently, the majority of guidelines operate on the presumption that animal research should proceed based on broad, perceived benefits to humans. These guidelines are generally permissive of animal research independent of the costs to the individual animal as long as benefits seem achievable.
The concept of costs to individual animals can be further examined through the growing body of research on animal emotion and cognition. Studies published in the last few decades have dramatically increased our understanding of animal sentience, suggesting that animals' potential for experiencing harm is greater than has been appreciated and that current protections need to be reconsidered. It is now widely acknowledged by scientists and ethicists that animals can experience pain and distress [25] – [29] . Potential causes of harm include invasive procedures, disease, and deprivation of basic physiological needs. Other sources of harm for many animals include social deprivation and loss of the ability to fulfill natural behaviors, among other factors. Numerous studies have demonstrated that, even in response to gentle handling, animals can show marked changes in physiological and hormonal markers of stress [30] .
Although pain and suffering are subjective experiences, studies from multiple disciplines provide objective evidence of animals' abilities to experience pain. Animals demonstrate coordinated responses to pain and many emotional states that are similar to those exhibited by humans [25] , [26] . Animals share genetic, neuroanatomical, and physiological similarities with humans, and many animals express pain in ways similar to humans. Animals also share similarities with humans in genetic, developmental, and environmental risk factors for psychopathology [25] , [26] . For example, fear operates in a less organized subcortical neural circuit than pain, and it has been described in a wide variety of species [31] . More complex markers of psychological distress have also been described in animals. Varying forms of depression have been repeatedly reported in animals, including nonhuman primates, dogs, pigs, cats, birds and rodents, among others [32] – [34] . Anxiety disorders, such as post-traumatic stress disorder, have been described in animals including chimpanzees and elephants [35] , [36] , [37] .
In addition to the capacity to experience physical and psychological pain or distress, animals also display many language-like abilities, complex problem-solving skills, tool related cognition and pleasure-seeking, with empathy and self-awareness also suggested by some research. [38] – [44] . Play behavior, an indicator of pleasure, is widespread in mammals, and has also been described in birds [45] , [46] . Behavior suggestive of play has been observed in other taxa, including reptiles, fishes and cephalopods [43] . Self-awareness, assessed through mirror self-recognition, has been reported for chimpanzees and other great apes, magpies, and some cetaceans. More recent studies have shown that crows are capable of creating and using tools that require access to episodic-like memory formation and retrieval [47] . These findings suggest that crows and related species display evidence of causal reasoning, flexible learning strategies, imagination and prospection, similar to findings in great apes. These findings also challenge our assumptions about species similarities and differences and their relevance in solving ethical dilemmas regarding the use of animals in research.
Predictive Value of Animal Data and the Impact of Technical Innovations on Animal Use
In the last decade, concerns have mounted about how relevant animal experiments are to human health outcomes. Several papers have examined the concordance between animal and human data, demonstrating that findings in animals were not reliably replicated in human clinical research [5] – [13] . Recent systematic reviews of treatments for various clinical conditions demonstrated that animal studies have been poorly predictive of human outcomes in the fields of neurology and vascular disease, among others [7] , [48] . These reviews have raised questions about whether human diseases inflicted upon animals sufficiently mimic the disease processes and treatment responses seen in humans.
The value of animal use for predicting human outcomes has also been questioned in the regulatory toxicology field, which relies on a codified set of highly standardized animal experiments for assessing various types of toxicity. Despite serious shortcomings for many of these assays, most of which are 50 to 60 years old, the field has been slow to adopt newer methods. The year 2007 marked a turning point in the toxicology field, with publication of a landmark report by the U.S. National Research Council (NRC), highlighting the need to embrace in vitro and computational methods in order to obtain data that more accurately predicts toxic effects in humans. The report, “Toxicity Testing in the 21 st Century: A Vision and a Strategy,” was commissioned by the U.S. Environmental Protection Agency, partially due to the recognition of weaknesses in existing approaches to toxicity testing [49] . The NRC vision calls for a shift away from animal use in chemical testing toward computational models and high-throughput and high-content in vitro methods. The report emphasized that these methods can provide more predictive data, more quickly and affordably than traditional in vivo methods. Subsequently published articles address the implementation of this vision for improving the current system of chemical testing and assessment [50] , [51] .
While a sea change is underway in regulatory toxicology, there has been much less dialogue surrounding the replacement of animals in research, despite the fact that far more animals are used in basic and applied research than in regulatory toxicology. The use of animals in research is inherently more difficult to approach systematically because research questions are much more diverse and less proscribed than in regulatory toxicology [52] . Because researchers often use very specialized assays and systems to address their hypotheses, replacement of animals in this area is a more individualized endeavour. Researchers and oversight boards have to evaluate the relevance of the research question and whether the tools of modern molecular and cell biology, genetics, biochemistry, and computational biology can be used in lieu of animals. While none of these tools on their own are capable of replicating a whole organism, they do provide a mechanistic understanding of molecular events. It is important for researchers and reviewers to assess differences in the clinical presentation and manifestation of diseases among species, as well as anatomical, physiological, and genetic differences that could impact the transferability of findings. Another relevant consideration is how well animal data can mirror relevant epigenetic effects and human genetic variability.
Examples of existing and promising non-animal methods have been reviewed recently by Langley and colleagues, who highlighted advances in fields including orthodontics, neurology, immunology, infectious diseases, pulmonology, endocrine and metabolism, cardiology, and obstetrics [52] .
Many researchers have also begun to rely solely on human data and cell and tissue assays to address large areas of therapeutic research and development. In the area of vaccine testing and development, a surrogate in-vitro human immune system has been developed to help predict an individual's immune response to a particular drug or vaccine [53] , [54] . This system includes a blood-donor base of hundreds of individuals from diverse populations and offers many benefits, including predictive high-throughput in vitro immunology to assess novel drug and vaccine candidates, measurement of immune responses in diverse human populations, faster cycle time for discovery, better selection of drug candidates for clinical evaluation, and reductions in the time and costs to bring drugs and vaccines to the market. In the case of vaccines, this system can be used at every stage, including in vitro disease models, antigen selection and adjuvant effects, safety testing, clinical trials, manufacturing, and potency assays. When compared with data from animal experiments, this system has produced more accurate pre-clinical data.
The examples above illustrate how innovative applications of technology can generate data more meaningful to humans, and reduce or replace animal use, but advances in medicine may also require novel approaches to setting research priorities. The Dr. Susan Love Research Foundation, which focuses on eradicating breast cancer, has challenged research scientists to move from animal research to breast cancer prevention research involving women. If researchers could better understand the factors that increase the risk for breast cancer, as well as methods for effective prevention, fewer women would require treatment for breast cancer. Whereas animal research is largely investigator-initiated, this model tries to address the questions that are central to the care of women at risk for or affected by breast cancer. This approach has facilitated the recruitment of women for studies including a national project funded by the National Institutes of Health and the National Institute of Environmental Health to examine how environment and genes affect breast cancer risk. This study, which began in 2002, could not have been accomplished with animal research [55] .
Similarly, any approach that emphasizes evidence-based prevention would provide benefits to both animals and humans. Resource limitations might require a strategic approach that emphasizes diseases with the greatest public health threats, which increasingly fall within the scope of preventable diseases.
It is clear that there have been many scientific and ethical advances since the first publication of Russell and Burch's book. However, some in the scientific community are beginning to question how well data from animals translates into germane knowledge and treatment of human conditions. Efforts to objectively evaluate the value of animal research for understanding and treating human disease are particularly relevant in the modern era, considering the availability of increasingly sophisticated technologies to address research questions [9] . Ethical objections to the use of animals have been publically voiced for more than a century, well before there was a firm scientific understanding of animal emotion and cognition [15] . Now, a better understanding of animals' capacity for pain and suffering is prompting many to take a closer look at the human use of animals [56] .
Articles in the accompanying Collection only briefly touch on the many scientific and ethical issues surrounding the use of animals in testing and research. While it is important to acknowledge limitations to non-animal methods remain, recent developments demonstrate that these limitations should be viewed as rousing challenges rather than insurmountable obstacles. Although discussion of these issues can be difficult, progress is most likely to occur through an ethically consistent, evidence-based approach. This collection aims to spur further steps forward toward a more coherent ethical framework for scientific advancement.
Acknowledgments
The authors thank the conference speakers and participants for their participation.
Competing Interests: HRF and NB are employed by Physicians Committee for Responsible Medicine, which is a non-governmental organization which promotes higher ethical standards in research and alternatives to the use of animals in research, education, and training. Physicians Committee for Responsible Medicine is a nonprofit organization, and the authors adhered to PLoS ONE policies on sharing data and materials.
Funding: The authors are grateful to the National Science Foundation (grant SES-0957163) and the Arcus Foundation (grant 0902-34) for the financial support for the corresponding conference, Animals, Research, and Alternatives: Measuring Progress 50 Years Later. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
JavaScript appears to be disabled on this computer. Please click here to see any active alerts .
EPA Leads International Case Study to Reduce Animal Testing for Chemical Safety
Published November 4, 2019
EPA is prioritizing efforts to reduce, refine, and replace animal testing in chemical safety research. In a new case study recently published in Toxicological Sciences , EPA identified an innovative approach to rapidly screen chemicals for biological impacts using new “ in vitro ,” or “in test-tube” methods. This work supports the September 2019 EPA Administrator Memo advocating the benefits of new approach methods for predicting potential hazards without the use of traditional methods that rely on animal testing.

Researches led by EPA’s Katie Paul Friedman used high throughput toxicological methods to explore the scale at which in vitro techniques could be used as an alternative to current animal-based techniques for calculating a chemical’s POD. High throughput in vitro experiments use automated technologies ( including robots! ) to expose living cells or proteins to chemicals. The cells or proteins are then screened for changes in biological activity that may suggest toxic effects. Based on the dose at which the potential toxic effects are observed, scientists calculate a POD value that is meant to model how different types of cells might respond to chemicals if they were in the human body.
Although modeling the interactions of chemicals in vitro is challenging and may not fully represent what occurs inside a living human body, in vitro methods present several advantages over traditional animal-based testing. First, in vitro studies require fewer resources, which saves time, cost, and animals.
“These savings enable the screening of many more chemicals, providing more information to decision makers about which chemicals may be more interesting for investment of additional resources,” says Katie.

The team found that for 90% of nearly 500 chemicals reviewed, the in vitro -based methods provide a POD value less than or equal to the animal-derived POD values. These results gave scientists confidence that the use of in vitro -based POD doses may provide an alternative that is equivalent to current techniques.
To support this research, EPA is working with international scientists from regulatory agencies in Canada, the European Union, and Singapore as part of a global workshop known as “Accelerating the Pace of Chemical Risk Assessment” (APCRA). EPA and APCRA will continue meeting annually to discuss the results of this and other case studies exploring in vitro risk assessment techniques. For more information about how EPA and its partners are changing the game in chemical safety, read about our computational toxicology and exposure research.
- Science Matters Home
- Researchers at Work Profiles
- All Stories
We need your support today
Independent journalism is more important than ever. Vox is here to explain this unprecedented election cycle and help you understand the larger stakes. We will break down where the candidates stand on major issues, from economic policy to immigration, foreign policy, criminal justice, and abortion. We’ll answer your biggest questions, and we’ll explain what matters — and why. This timely and essential task, however, is expensive to produce.
We rely on readers like you to fund our journalism. Will you support our work and become a Vox Member today?
- Future Perfect
What’s worse than a cruel animal experiment? A cruel and fake animal experiment.
Raising the consequences for animal testing experiments gone wrong.
by Marina Bolotnikova

Last December, in the wake of animal cruelty allegations against Elon Musk’s brain chip startup Neuralink, Vox’s Kenny Torrella wrote about a concept he called “the moral math of animal testing”: the view held by many people that trading some amount of animal suffering is worth it if it can save enough human lives by advancing medicine.
Experimentation on live animals is a divisive, morally charged subject. Slightly more than half of Americans say they oppose using animals in scientific research, according to a 2018 Pew survey, but it depends a lot on how you phrase the question and who is asking. When asked by the biomedical industry whether they support “the humane use of animals” to develop “lifesaving medicines,” many more people say they do, or aren’t sure. These gaps reflect the public’s lack of understanding of how vivisection works in general: Most people don’t know whether animal testing is humane, effective, or necessary, nor do they always know how to define those terms.
Not everyone will agree with my view of vivisection, which is that it’s unjustifiable in nearly all circumstances. But I would think most people will agree that animal experiments should have to clear an especially high bar — that they have to be truly necessary for saving human lives and irreplaceable with non-animal methods.
That is, unfortunately, not how animal testing in the US works at all. Scientists harm and kill animals for all sorts of studies that have nothing to do with saving human lives. Researchers at Oregon Health & Science University, for example, have forced prairie voles to drink alcohol to test whether it makes them cheat on their partners. A Harvard neuroscientist recently came under fire for separating caged mother monkeys from their babies and giving them surrogate stuffed animals to bond with, thus demonstrating, she wrote in a top scientific journal, that “infant/mother bonds may be triggered by soft touch.”
The worst kind of fraud
Animal experimentation is also not immune to outright fraud, a problem that’s “disturbingly common” in science, as Vox’s Kelsey Piper wrote in June. Last week, federal investigators found that William Armstead, a former professor at the University of Pennsylvania’s medical school, had faked the results of multiple federally funded studies that involved cutting open piglets’ skulls and inducing brain injuries. The studies were meant to test drugs for treating brain injuries in humans. (Armstead left the university while he was under investigation for this misconduct.)
Some of Armstead’s fabrications, which included relabeling results from past studies as new ones, appear designed to make a drug his team was studying look more effective, Ivan Oransky, co-founder of Retraction Watch — a blog that tracks retractions of scientific papers — told the Philadelphia Inquirer last week. Armstead’s team doctored 51 scientific figures across five published studies, three federal grant applications, and other documents. The faked data renders the research useless; it’s now been retracted from journals and can’t be incorporated into future work. “A bunch of pigs were subjected to some pretty terrible conditions for no reason,” Oransky told the Inquirer.
Armstead now faces a seven-year ban on conducting federally funded research, a penalty that’s relatively rare in its severity. But his case isn’t an isolated one.
Last year, a pivotal 2006 mouse study, which had been thought to shed light on the pathology of Alzheimer’s disease and shaped years of federally funded research, was credibly accused of being fraudulent and remains under investigation.
Also last year, federal officials found Deepak Kaushal, then-head of the federally funded Southwest National Primate Research Center in San Antonio, to have falsified results in a published study of a tuberculosis treatment tested on monkeys, and used those results in two NIH grant applications. Kaushal was placed under a one-year supervision period — the lightest imaginable slap on the wrist — with no lasting consequences for his ability to experiment on animals. He also, according to initial reports, got to keep his job as director of the lab. After criticism from some in the research community and animal advocates, he was later demoted from that position; it’s unclear whether he’ll be reinstated after his supervision period.
The price of suffering
All these revelations should raise alarms about how misconduct is handled in research involving animal testing. When a top primate researcher is allowed to keep experimenting on monkeys after falsifying data, it sends a message to everyone in the research community that recklessly handling animal experiments, while temporarily embarrassing, may not be that big a deal.
“The NIH tends to give anybody on their pay line the benefit of the doubt,” neuroscientist Katherine Roe, who worked at NIH for more than eight years and is now chief of PETA’s science advancement and outreach division, told me. (PETA, despite its reputation, has a top-notch team of scientists challenging unethical animal research). “The penalties for research fraud are not what they should be.”
To shift the incentive structure, we need better federal regulation that raises the cost of torturing animals for botched experiments. Right now, the consequences for misconduct in federally funded research don’t take into account whether the work involved animal testing, Roe said. Federal research regulations could be amended so that scientists found responsible for misconduct in work involving vulnerable populations, including non-human animals, be permanently barred from testing on them in future federally sponsored research, a change that’s been proposed by PETA, explained Emily Trunnell, a senior scientist for the organization.
That would be a good start. But it would require the authorities who oversee science to view the animal experiments themselves, and not just lying about their results, as morally implicated, something the research community has been loath to do because it threatens to undermine the whole endeavor of animal testing.
On a higher level, we have to start seeing it as the public’s right and duty to make democratic decisions about whether and how animals are used in scientific research, especially when our money is paying for it. Scientists are an exalted class, often allowed to self-regulate, but their expertise in a narrow subject matter shouldn’t let them overrule democratic governance of research ethics. Ethics belongs to us all. And the public expects a much higher bar than too many animal researchers currently set for themselves.
- Animal Welfare
Most Popular
- A thousand pigs just burned alive in a barn fire
- Who is Ryan Wesley Routh? The suspect in the Trump Florida assassination attempt, explained.
- Sign up for Vox’s daily newsletter
- Take a mental break with the newest Vox crossword
- The new followup to ChatGPT is scarily good at deception
Today, Explained
Understand the world with a daily explainer plus the most compelling stories of the day.
This is the title for the native ad
More in Future Perfect

The safety paradox at the heart of OpenAI’s “Strawberry” model.

Scientists are trapped in an endless loop of grant applications. How can we set them free?

Why the new luxury is flip phones and vinyl LPs

561 research papers in, the case for degrowth is still weak.

It’s not just the Princess of Wales. More and more young people are getting cancer.

We buy stuff. We throw it away. There’s a system to stop this toxic cycle.

IMAGES
VIDEO
COMMENTS
IBR was a federally funded laboratory in Silver Spring, Maryland, now closed down for reasons that will soon become apparent. It was run out of a warehouse by psychologist and animal experimenter Edward Taub, a man with no medical training. There, we found 17 monkeys living in tiny rusty wire cages caked with years of accumulated feces in a ...
Using genetically modified zebrafish, UCL scientists have identified a novel gene affected in a devastating disorder with childhood-onset Parkinsonism. Indeed, when a drug that worked in the fish was given to one of the children, she regained the ability to walk. The research studied a group of nine children who suffered from severely disabling ...
Numerous published studies have shown that animal experimentation wastes resources and lives, as more than 90% of basic science research—most of which involves animal experimentation—fails to lead to treatments for humans. And 95% of new medications that are found to be effective in animals fail in human clinical trials.
According to the UK Home Office (12), in the year 2016, 48.6% of the animal tests in medical research were conducted for genetically oriented studies. Moreover, 28.5% of the medical research involving animal testing was for basic biological research, 13.5% was for regulatory. testing, 8.6% was for translating research from animals to humans ...
Even in the case of thalidomide, animal testing in 10 strains of rats, 11 breeds of rabbits, two breeds of dogs, three strains of hamsters, eight species of primates, ... A recent case study that exemplifies some of the aforementioned mechanisms is the court case of the biopharmaceutical company Vanda against the FDA. Vanda was developing ...
Introduction. Annually, more than 115 million animals are used worldwide in experimentation or to supply the biomedical industry. 1 Nonhuman animal (hereafter "animal") experimentation falls under two categories: basic (i.e., investigation of basic biology and human disease) and applied (i.e., drug research and development and toxicity and safety testing).
We're asking you to join us in changing an outdated industry— animal testing—and, more immediately, in urging the release of more than 80 dogs still suffering at the lab. ... Our undercover investigator worked on studies with more than 6,000 animals—including 250 dogs, 500 primates, 62 "minipigs" and more than 5,100 mice and rats ...
McElligot and Root-Gutteridge argue the case was emblematic of a wider problem in animal research, in which questionable studies and papers continue to pass institutional reviews and are published ...
Introduction. Animal model-based research has been performed for a very long time. Ever since the 5 th century B.C., reports of experiments involving animals have been documented, but an increase in the frequency of their utilization has been observed since the 19 th century [].Most institutions for medical research around the world use non-human animals as experimental subjects [].
Technologically advanced non-animal research methods—such as those using human cells, computational models, or clinical studies—can be used in place of animal testing. These methods are more humane, have the potential to be faster, and are more relevant to humans. Scientists in PETA's Science Advancement & Outreach division, a part of the Laboratory Investigations Department, have ...
This two-day symposium aimed to advance the study of the ethical and scientific issues surrounding the use of animals in testing and research, with particular emphasis on the adequacy of current protections and the promise and challenges of developing alternatives to the use of animals in basic research, pharmaceutical research and development ...
There are several reasons why the use of animals is critical for biomedical research: • Animals are biologically very similar to humans. In fact, mice share more than 98% DNA with us! • Animals are susceptible to many of the same health problems as humans - cancer, diabetes, heart disease, etc. • With a shorter life cycle than humans ...
Some drug companies have chafed at FDA's animal testing requirement, arguing that animal studies cost them millions of dollars, slowing drug development and making the medicines that do reach the market far more expensive. In 2019, Vanda Pharmaceuticals sued the agency, charging that its requirement of additional toxicity testing of an ...
Animals in laboratories endure appalling suffering, such as being deliberately poisoned, brain-damaged and subjected to inescapable electric shocks. The pain and misery inflicted on the victims is enough, on its own, to make vivisection worthy of public condemnation.
The Case for Phasing Out Experiments on Primates. Whether they realize it or not, most stakeholders in the debate about using animals for research agree on the common goal of seeking an end to research that causes animals harm. [1] The central issues in the controversy are about how much effort should be devoted to that goal and when we might ...
The E.P.A. Administrator Andrew Wheeler said the agency plans to reduce the amount of studies that involve mammal testing by 30 percent by 2025, and to eliminate the studies entirely by 2035 ...
The History of Animal Testing — 10 Shocking Experiments on Animals From the Last Century. 1. Maryland Psychologist Severed Spinal Cords, Repeatedly Shocked Monkeys (1958-1981) At the Institute for Behavioral Research in Silver Spring, Maryland, psychologist and animal experimenter Edward Taub—a man with no medical or veterinary training ...
Animal research is not always king: researchers should explore the alternatives. Technological advances can reduce the numbers of laboratory animals used in studies — but they need to be ...
McElligot and Root-Gutteridge argue the case was emblematic of a wider problem in animal research, in which questionable studies and papers continue to pass institutional reviews and are published ...
US agency seeks to phase out animal testing. The Food and Drug Administration commits to exploring alternative methods to replace laboratory animals in developing new drugs and products. By ...
Ethical Considerations and Advances in the Understanding of Animal Cognition. Apprehension around burgeoning medical research in the late 1800s and the first half of the 20 th century sparked concerns over the use of humans and animals in research , .Suspicions around the use of humans were deepened with the revelation of several exploitive research projects, including a series of medical ...
Published November 4, 2019. EPA is prioritizing efforts to reduce, refine, and replace animal testing in chemical safety research. In a new case study recently published in Toxicological Sciences , EPA identified an innovative approach to rapidly screen chemicals for biological impacts using new "in vitro," or "in test-tube" methods.This work supports the September 2019 EPA ...
That is, unfortunately, not how animal testing in the US works at all. Scientists harm and kill animals for all sorts of studies that have nothing to do with saving human lives.