Study Documentation Templates and Tools
During planning and operationalization stages, before enrollment starts, research teams should discuss what forms will be needed for their study. These could be data collection forms, source documentation, even forms or logs for study management like visit checklists and progress notes.
In general, templates are an efficient way to build study documents but care needs to be taken that edits are made that are relevant and appropriate for each study. Study teams can use these templated tools and edit for each new study or can build their own templates based on their usual needs to use for all future studies.
The CRRO templated tools are not meant to be static, unchanging documents, but must be edited for each study to align with IRB-approved procedures that are in the INSPIR application, protocol, or other study document. However, not all templated tools that are available will be required or should be used on every study. Study teams should review all available tools and what will be needed for compliant and complete documentation.
CRRO templated tools are built upon a mix of institutional policies, federal regulations, ICH Good Clinical Practice, and current best practices. The CRRO cannot make any guarantee that these templated tools follow current policy or regulations as those may change without warning or announcement. These templated tools are not meant to be used as a policy or guidance document.
It is strongly recommended that study teams review the Overview and Instructions prior to using any of the templated tools. General questions on how to use these forms or implement them for a specific study can be answered by contacting the CRRO . Specific guidance on building these tools within Word, REDCap, or other systems are available from the CRRO .
Suggestions for additional tools or changes to current tools are welcome and can be submitted on our Tool Recommendation Form .
Adverse Event Form Adverse Event Log Biospecimen Storage and Tracking Log Delegation of Authority and Responsibilities Eligibility Criteria Checklist Essential Documents Location Cover Page Informed Consent Documentation IRB Submissions Log Note to File Template Participant Completion Form Participant Identification Log Phone Call Summary Report Pregnancy Testing Documentation Protocol Deviation Form Protocol Deviation Log Regulatory Binder Cover Page Schedule of Events Screening and Enrollment Log Site Visit Log SOP or MOP Template Staff License Log Training Log – Group Training Log – Individual Visit Checklist
FAQs on Regulatory Documentation Regulatory Binder Tabs REDCap e-Reg Lite AdobeSign Guidance

Not a member?
Find out what The Global Health Network can do for you. Register now.
Member Sites A network of members around the world. Join now.
- 1000 Challenge
- ODIN Wastewater Surveillance Project
- CEPI Technical Resources
- Global Health Research Management
- UK Overseas Territories Public Health Network
- Global Malaria Research
- Global Outbreaks Research
- Sub-Saharan Congenital Anomalies Network
- Global Pathogen Variants
- Global Health Data Science
- AI for Global Health Research
- MRC Clinical Trials Unit at UCL
- Virtual Biorepository
- Epidemic Preparedness Innovations
- Rapid Support Team
- The Global Health Network Africa
- The Global Health Network Asia
- The Global Health Network LAC
- Global Health Bioethics
- Global Pandemic Planning
- EPIDEMIC ETHICS
- Global Vector Hub
- Global Health Economics
- LactaHub – Breastfeeding Knowledge
- Global Birth Defects
- Antimicrobial Resistance (AMR)
- Human Infection Studies
- EDCTP Knowledge Hub
- CHAIN Network
- Brain Infections Global
- Research Capacity Network
- Global Research Nurses
- ZIKAlliance
- TDR Fellows
- Global Health Coordinators
- Global Health Laboratories
- Global Health Methodology Research
- Global Health Social Science
- Global Health Trials
- Zika Infection
- Global Musculoskeletal
- Global Pharmacovigilance
- Global Pregnancy CoLab
- INTERGROWTH-21ˢᵗ
- East African Consortium for Clinical Research
- Women in Global Health Research
- Coronavirus
Research Tools Resources designed to help you.
- Site Finder
- Process Map
- Global Health Training Centre
- Resources Gateway
- Global Health Research Process Map
- About This Site
Downloadable Templates and Tools for Clinical Research
Welcome to global health trials' tools and templates library. please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones have been added. please click on the orange text to download each template., the templates below have been shared by other groups, and are free to use and adapt for your researchstudies. please ensure that you read and adapt them carefully for your own setting, and that you reference global health trials and the global health network when you use them. to share your own templates and sops, or comment on these, please email [email protected]. we look forward to hearing from you.
These templates and tools are ordered by category, so please scroll down to find what you need.
|
|
| |
|
|
| |
|
|
| |
|
| ||
|
| ||
|
|
| |
|
|
| |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
|
| |
|
| ||
|
| ||
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
| ||
|
| ||
|
| ||
|
| ||
|
|
| |
|
|
| |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
|
| |
|
|
| |
|
| ||
|
| ||
|
| ||
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
|
| |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
|
| |
|
|
| |
|
| ||
|
|
| |
|
|
| |
|
| ||
|
| ||
|
|
| |
|
| ||
|
|
| |
|
| ||
|
| ||
|
|
| |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
|
|
|
|
|
| |
|
| ||
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
|
| |
|
|
| |
|
|
| |
| /td>< |
| |
|
| ||
|
|
| |
|
|
| |
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
|
| |
|
|
| |
|
|
| |
|
| ||
|
| ||
|
| ||
|
|
| |
|
|
| |
|
|
To share your own templates and SOPs, or comment on these, please email [email protected]. We look forward to hearing from you!
- Webinar on community engagement in clinical research involving pregnant women
- Free Webinar: Science, technology and innovation for upskilling knowledge-based economies in Africa
- Open Public Consultation on “Strengthened cooperation against vaccine preventable diseases”
Trial Operations Trial Management Ethics and Informed Consent Resources Trial Design Data Management and Statistics
training
This is Degena Bahrey Tadesse from Tigray, Ethiopia. I am new for this web I am assistant professor in Adult Health Nursing Could you share me the sample/templet research proposal for Global Research Nurses Pump-priming Grants 2023: Research Project Award
I have learned lot..Thanks..
i was wondering why there is no SOP on laboratory procedures ?
Hi, Can you provide me the SOP for electronic signatures in Clinical trial
Do you have an "SOP for Telephonic site selection visit". Kindly Share on my registered mail ID
Thank you for sharing the resources. It is very kind of you.
Hi These tolls are very useful! Thank you
Do you have a task and responsability matrix template for clinical trial managment ? Best
I am very much happy to find myself here as a clinician
Dear Getrude
We have a free 14-module course on research ethics on our training centre; you'll receive a certificate if you complete all the modules and quizzes. You can take it in your own time. Just visit 'Training centre' in the tabs above, then 'short courses'.
Kind regards The Editorial Team
need modules on free online gcp course on research ethics
Estimados: me parece excelente el aporte que han hecho dado que aporta. por un lado a mejorar la transparencia del trabajo como a facilitar el seguimiento y supervisión de los mismos. Muchas gracias por ello
We also have an up to date list of global health events available here: https://globalhealthtrials.tghn.org/community/training-events/
Dear Nazish
Thank you, I am glad you found the seminars and the training courses useful. We list many training events (all relevant to Global Health, and as many of them as possible are either free or subsidised) on the 'community' web pages above. Keep an eye on those for events and activities which you can get involved with. Also, if you post an 'introduction' on the introduction group stating where you are from and your research interests, we can keep you updated of relevant local events.
Thanks so much. These are very helpful seminars. Please let me know any other websites/links that provide free or inexpensive lectures on clinical Research. Appreciate your help.
Hi Nazish, and welcome to the Network. The items here are downloadable templates for you to use; it sounds like you may be seeking lectures and eLearning courses? If so - no problem! You can find free seminars with sound and slides here: https://globalhealthtrainingcentre.tghn.org/webinars/ , and you can find free, certified eLearning courses here: https://globalhealthtrials.tghn.org/elearning . Certificates are awarded for the eLearning courses for those scoring over 80% in the quiz at the end of each course. If you need anything else, do ask! Kind regards The Editorial Team
Hi, I am new to this website and also to the Clinical Research Industry for that matter I only am able to see the PDF of these courses, just wanted to know are these audio lectures and also happen to have audio clips that go with the pdf?
This site is impeccable and very useful for my job!!!!
Thank you for your kind comments.
Fantastic resources
I am delighted you found this website. I earlier introduced it to you because of your prolific interest in health care information and resource sharing....
Please Sign in (or Register ) to view further.
Useful Resources
Related articles.
- PRISMA for Abstracts: Reporting Systematic Reviews in Journal and Conference Abstracts BY Jai K Das
- 5 ways statistics can fool you—Tips for practicing clinicians BY Jai K Das
- How to prepare for a job interview and predict the questions you’ll be asked BY The Editorial Team
- Preparing for and Executing a Randomised Controlled Trial of Podoconiosis Treatment in Northern Ethiopia BY Henok Negussie, Thomas Addissie, Adamu Addissie, Gail Davey
- Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control BY WHO/ TDR
Most popular tags
- Archive (303)
- archive (104)
- data sharing (70)
- sharing (63)
- training (49)
- malaria (30)
- ACT consortium (25)
- informed consent (7)
- data management (6)
- trial management (6)
- careers (5)
- guidelines (5)
- monitoring (5)
- workshop (5)
- administration (4)
- clinical research (4)

An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Clinical Research Study Investigator’s Toolbox

Supporting Clinical Research
The purpose of the NIA Clinical Research Toolbox is to provide a Web-based informational repository for investigators and staff involved in clinical research. The Toolbox contains templates, sample forms, guidelines, regulations and informational materials to assist investigators in the development and conduct of high quality clinical research studies.
Study Startup
Recruitment and retention resources.
- NIA Guidance on Clinical Trials
Forms and Templates
- Glossary of Terms
Data Safety and Monitoring
As depicted in the NIA Guidance on Clinical Trials , NIA is responsible for overseeing the data and safety monitoring of the clinical research it supports. Data and safety monitoring of a clinical trial is commensurate with the risks posed to the study participants and with the size and complexity of the study.
Applicants requesting support for any intervention study must complete "PHS Human Subjects and Clinical Trials Information" form of the SF424 (R&R), describe a data and safety monitoring plan (DSMP), which discusses the need for an independent data and safety monitoring body or justifies why such a body is not needed to monitor the study and proposes an alternative safety monitoring mechanism. For example, for a single-site, low risk study, the PI may propose a local safety monitor, while a multi-site, higher risk study might propose a Data and Safety Monitoring Board (DSMB).
- For behavioral and social clinical trials, consider using the adapted DSMP Template (MS Word, 62K) .
- Guideline for Budgeting for Data and Safety Monitoring Activities (MS Word, 25K) aids investigators in budgeting for an independent DSMB or a Safety Officer when preparing the budget section of a grant application.
Data Sharing
The National Institutes of Health (NIH) advocates making available to the public the results and accomplishments of the activities that it funds. NIH assures that research resources developed with public funds become readily available to the broader research community in a timely manner for further research, development, application, and secondary data analysis. The expectation is that this will lead to products and knowledge of benefit to public health. To ensure that future research can build on previous efforts and discoveries, the National Institutes of Health (NIH) has developed a data sharing policy effective October 1, 2003, for applicants seeking NIH funding of $500,000 or more in direct costs in any one year. The policy expects final research data, especially unique data, from NIH-supported research efforts be made available to the investigators. The NIH policy on data sharing applies to:
- Basic research, clinical studies, surveys, and other types of research supported by the NIH.
- Human subjects and laboratory research.
- Data not produced with NIH funding but used in an NIH-supported activity in some instances.
Investigators are expected to include in their grant application a brief description of how final research data will be shared, or explain why data-sharing is not possible (for example: human subject protection concerns). Please see NIH’s Example Plan (MS Word, 55K) for a template you may modify to fit the data you plan to share.
Initial Proposal Concept Form (MS Word, 39K) - This form should be used to advocate for an initiative by the Division of Geriatrics and Clinical Gerontology (DGCG) for a clinical trial or trials that exceed $2 million in direct costs in any year of funding. DGCG Clinical Trials Advisory Panel, a task force of the National Advisory Council on Aging (NACA), will evaluate the concept proposals in October – November of each Fiscal Year and will provide its recommendations to DGCG, NACA, and to the NIA Director on initiatives for large clinical trials.
Back to top
The clinical protocol is a document that describes how a clinical study will be conducted by detailing the objective(s), design, methodology, statistical considerations and organization of a clinical study, and describes methods used to ensure the safety of the study participants and integrity of the data collected.
Protocol (MS Word, 93K) - The Clinical Intervention Study Protocol Template outlines a clinical study protocol and provides guidance on important content to include in each section. The template can be downloaded as an MS Word file for adaptation by the study investigator.
Manual of Procedures
A Manual of Procedures (MOP) is a handbook that details a study’s conduct and operations as well as facilitates consistency in protocol implementation and data collection across study participants and sites. It operationalizes the study protocol and describes each step of the study and how it is to be executed. A copy of the MOP should be provided to each member of the Study Team. Ideally, the MOP would contain an adequate amount of detail that any individual(s) at any site(s) could run the study consistently with only the information contained in the MOP and its appendices.
The NIA recognizes the importance of a MOP and has developed documents to assist principal investigators in writing their study MOP. Investigators with a multi-site study are required to submit a MOP, while single-site study investigators are strongly encouraged to review the MOP and determine which sections are necessary in order to ensure the study procedures are performed as intended. The Guidelines below provide details on each section of the MOP, while the MOP Outlines are an overview listing the sections that are most relevant in those types of studies.
- Manual of Procedures (MOP) Outline – Multi-Site (MS Word, 30K)
- Manual of Procedures (MOP) Guidelines – Multi-Site (MS Word, 2.9M)
- Manual of Procedures (MOP) Outline – Single-Site (MS Word, 27K)
- Manual of Procedures (MOP) Guidelines - Single-Site (MS Word, 170K)
The following documents can also be found within the MOP template:
- Schedule of Events presents the activities that take place at each contact with the participant.
- Protocol Deviation Log provides participant-specific documentation of missed visits and other actions that deviate from the protocol.
Informed Consent
The consent process provides individuals with sufficient information for making informed decisions about participation in a clinical research study. The following documents are provided as a tool to assist NIA investigators for developing a comprehensive informed consent:
- Informed Consent Checklist (MS Word, 54K) presents required and additional elements of the consent forms as set forth in Code of Federal Regulations.
- Informed Consent Version Tracker (MS Excel, 20K) provides a template with two examples of tools that sites may use to track informed consent versions; this helps minimize the use of expired versions and the occurrence of consent deviations.
- Informed Consent for Secondary Research with Data and Specimens (PDF, 736K) , from NIH's Office of Science Policy, provides points to consider and sample language for informed consent documents for research studies that plan to store and share data and/or biospecimens for future use.
Data Safety and Monitoring Boards
The Data and Safety Monitoring Board (DSMB) is an independent group of experts that advises the NIA Director and the study investigators. The members of the DSMB serve in an individual capacity and provide their expertise and recommendations. The need for DSMB oversight is based on assessment of the study’s overall risk. Investigators may propose a DSMB in their grant application, or NIA may require that a DSMB be established following consideration of review panel’s comments, NIA’s National Advisory Council on Aging (NACA) advice, and/or input from NIA staff.
- Sample Data and Safety Monitoring Board Charter (MS Word, 25.8K) The DSMB Charter describes the responsibilities of the DSMB to ensure ongoing, independent study review and assure the study is conducted according to the highest scientific and ethical standards.
- DSMB Conflict of Interest and Confidentiality Statement (MS Word, 22K) and DSMB Conflict of Interest and Confidentiality Statement (PDF, 130K) - All members of the DSMB are required to be independent of the studies being reviewed and need to certify this by signing a DSMB Conflict of Interest and Confidentiality statement.
- DSMB Report - Single Site Open (MS Word, 323K)
- DSMB Report - Single Site Closed (MS Word, 342K)
- DSMB Report - Multi Site Open (MS Word, 449K)
- DSMB Report - Multi Site Closed (MS Word, 348K)
Additional Startup Tools
- Data Management Tips (MS Word, 30K) help to ensure adequate data management processes and procedures in a clinical study. Investigators are encouraged to use Data Management Tips to describe how data will be handled in the study.
- Best Practices for Data Coordinating Centers – This Compendium, developed by the National Heart Lung and Blood Institute (NHLBI) provides helpful tips for clinical researchers and other stakeholders for developing large, multisite clinical trial programs.
The NIA Clinical Research Toolbox includes a broad array of resources to support communications, training, recruitment, engagement and more. Learn about research and retention resources below.
A collection of outreach, recruitment, and engagement resources providing tips and strategies for communicating with potential clinical trial participants.
ADORE (Alzheimer’s & Dementia Outreach, Recruitment & Engagement Resources) An NIA repository of resources to support the recruitment and retention of participants into clinical trials and studies on Alzheimer’s disease and related dementias.
Alzheimer’s and Dementia Information to share with Participants NIA resources for caregivers and people living with dementia.
Engagement and Access for Research-Active Institutions (EARA) Resource from the Office of the Director is a communication tool focused on outreach to research-active institutions and NIH institutes, centers, and offices.
Guidance regarding social media tools Developed by the NIH Office of Intramural Research and provides best practices when using social media tools and new technologies to recruit for clinical trials.
Person-first and Destigmatizing Language From the NIH Style Guide, this resource offers guidance on the principles for inclusive communication and defines person-first language and identity-first language.
Providing Care to a Diverse Older Adult Population An NIH resource on communicating with a diverse older patient population with tips for culturally sensitive care.
Recruiting and Communicating with Participants NCCIH presents strategies about how to be thoughtful about participants, staff, and community partners with emphasis on good communication skills and habits.
Talking to Your Patient About a Clinical Trial NIH provides communication guidance in the form of a modified checklist for talking to patients about clinical trials.
Talking With Older Patients NIH shares tips for communicating with older patients, including families and caregivers as part of the health care team, obtaining a thorough history from participants, discussing medical conditions and treatments, confusion and cognitive problems, and sensitive topics.
A set of resources related to developing and training a diverse clinical research workforce.
Chief Officer for Scientific Workforce Diversity (COSWD) An NIH resource dedicated to the diversity of the scientific workforce and use of evidence-based approaches to catalyze cultures of inclusive excellence.
Building a Diverse Scientific Workforce A NIDCD resource focused on enhancing the diversity of the biomedical, behavioral, and clinical research workforce.
Scientific Workforce Diversity Programs at NIA An NIA resource with opportunities for career development and training for researchers.
Scientific Workforce Diversity Seminar Series: How Do Research-Active Institutions ( e.g. HBCUs, TCUs, and MSIs) Impact the Diversity of the Scientific Workforce? This seminar, hosted by the NIH Chief Officer for Scientific Workforce Diversity (COSWD), featured a panel sharing data and perspectives on the critical role of Research-Active Institutions (RAIs) in enhancing the diversity of the scientific workforce. Panelists also discussed how NIH and other funders might better partner with and support these institutions in enhancing their impact.
Resources related to clinical trials planning, recruitment, and engagement.
AD/ADRD Clinical Studies Recruitment Planning Guide A resource developed by the NIA that outlines strategies for clinical research recruitment.
Accrual Stages An NCCIH resource on the five stages of study development including: Developing a Study; Selecting & Preparing to Open a Study; Recruiting and Communicating with Participants; Implementing the Study; and Evaluating Accrual and Reporting Lessons Learned.
National Strategy for Recruitment and Participation in Alzheimer's and Related Dementias Clinical Research Published by the NIH and NIA, the National Strategy is designed to engage broad segments of the public in Alzheimer’s and related dementias research.
OutreachPro NIA developed a free online recruitment materials generator for researchers and research teams to create customizable outreach materials. OutreachPro has a library of content designed specifically for African American, Hispanic/Latino, Asian American and Pacific Islander populations and available in English, Spanish, Simplified Chinese, Tagalog and Hindi. Explore the OutreachPro video playlist for videos on clinical trial participants, caregivers, and the benefits of participating in clinical research.
Recruiting & Communicating with Participants An NCCIH resource to engage intermediaries to aid in accrual to clinical research:
Recruitment & Retention A resource from the Diversity in Extramural Programs focused on recruitment planning.
Recruitment & Retention Planning: Getting Started Resources and tips from NINDS to create a recruitment and retention plan while you are writing the grant proposal and study protocol.
- Identify potentially eligible participants
- Engage participants in the Informed Consent Process
- Consider participant financial issues
- Maintain the morale and interest of staff, participants and their families
- Update participants regarding study related events and results
A collection of recruitment and retention resources focused on health disparities and inclusion of underrepresented populations.
CEAL (Community Engagement Alliance) CEAL is an NIH research network designed to work with communities and community-based organizations to identify promising engagement and outreach practices that communicate trustworthy, science-based information to communities experiencing health disparities.
Collection of Race and Ethnicity Data in Clinical Trials and Clinical Studies for FDA-Regulated Medical Products This guidance provides FDA’s expectations for, and recommendations on, use of a standardized approach for collecting and reporting race and ethnicity data in submissions including information collected and reported from clinical studies.
Community Health Disparities Recruitment From NHLBI, this Community Health Toolkit was designed to assist in planning, running, and evaluating programs and includes recruiting scenarios and informational handouts.
Cultural Competence Resources from across NIH are presented for researchers to consider regarding importance of cultural competence to promote effective and culturally informed recruitment and retention strategies.
Enhancing the Diversity of Clinical Trial Populations This FDA guidance recommends approaches that sponsors of clinical trials can take to increase enrollment of underrepresented populations in their clinical trials.
Health Disparities Framework This NIA page is designed to serve as a resource for scientists interested in investigating health disparities related to aging. It lists the priorities in aging research.
Health Equity Guiding Principles for Inclusive Communication The CDC provides guidance for health communicators to ensure their communication products and strategies adapt to the specific cultural, linguistic, environmental, and historical situation of each population or audience of focus.
NIH Inclusion Policies for Research Involving Human Subjects NIH is committed to supporting clinical research that benefits individuals of all sexes/genders, races, ethnicities, and ages. The information provided on this website is designed to assist the extramural community in addressing inclusion, including the Inclusion of Women and Minorities policy and the Inclusion Across the Lifespan policy, in NIH grant applications and progress reports.
Primary Barriers and Facilitators to Participation in Clinical Research The Office of Research on Women's Health at NIH provides a summary of the literature on the barriers and facilitators to recruiting from diverse backgrounds to clinical trials.
Recruitment and Retention From NIH, this website offers strategic considerations for outreach by population group and includes other recruitment resources.
Recruitment and Retention of Women in Clinical Research NIH Inclusion Outreach Toolkit on how to engage, recruit, and retain women in clinical research with strategies that are relevant to women and translatable across many subgroups of the U.S. population. This toolkit also includes case studies that highlight effective recruitment practices.
A collection of resources to help individuals learn more about clinical research, and find available clinical trials by location, condition, and intervention.
NIA Clinical Trials and Studies An NIA resource providing a variety of articles for potential clinical trial participants.
NIH Clinical Research Trials and You An NIH resource for people who want to learn more about clinical trials. It includes commonly asked questions and responses about participating in a clinical trial.
ClinicalTrials.gov An online database of clinical research studies that provides information about clinical research studies to the public, researchers, and health care professionals. Visit the Learn About Studies page to learn more.
Find Clinical Trials Tool for the public to find Alzheimer’s and related dementias clinical trials by location.
Personal Stories About Alzheimer’s and Related Dementias Research Watch English videos from clinical trials participants or Spanish videos from clinical trials participants .
ResearchMatch An NIH funded nonprofit program that helps to connect people interested in research studies with researchers from top medical centers across the US.
“Why I Participate” Videos From the NIA YouTube channel, this playlist contains videos of caregivers and those who are at risk for Alzheimer’s disease.
Plainlanguage.gov A resources design to help teams communicate in a way that their audience can understand the first time they read or hear it.
OutreachPro NIA developed a free online recruitment materials generator for researchers and research teams to create customizable outreach materials. OutreachPro has a library of content designed specifically for African American, Hispanic/Latino, Asian American and Pacific Islander populations and available in English, Spanish, Simplified Chinese, Tagalog, and Hindi. Explore the OutreachPro video playlist for videos on clinical trial participants, caregivers, and the benefits of participating in clinical research.
Recruitment Innovation Center (RIC) Advice, guidance and recommendations about community engagement, recruitment planning and feasibility assessment, recruitment materials, EHR-based cohort assessments, and expression of interest.
Community Informed Recruitment and Retention Template A tool to assist research teams in achieving recruitment and retention goals of historically underrepresented racial and ethnic populations.
Investigators must include in their application proposed adverse event (AE) and serious adverse event (SAE) definitions and discuss their monitoring and reporting. All clinical trials of drugs and biological products conducted under an Investigational New Drug Application (IND) must use definitions of adverse events and adverse reactions and follow the reporting requirements established by 21 Code of Federal Regulations (CFR) Part 312.32. Trials of medical devices conducted under an Investigational Device Exemption (IDE) must use the definitions and reporting requirements established by 21 CFR 812. All other interventional studies must propose their definitions of adverse events and their reporting procedures. See the NIA Guidance on Clinical Trials for additional information .
- Adverse Event Form ( MS Word , 38K or screen-readable PDF , 69K) provides a template for a study form for collecting information about adverse events that is reviewed by safety monitoring bodies.
- Serious Adverse Event Form ( MS Word , 31K or screen-readable PDF , 769K) provides a template for a study form for collecting information about serious adverse events. The form includes major components of the Food and Drug Administration (FDA) Form 3500.
- AE/SAE Process Flow (PDF, 119K) illustrates how adverse events and serious adverse events are handled within a study.
The NIA Safety Training Course (available below), an online training venue, provides an overview of human subject safety surveillance and reporting requirements in clinical research studies. The intent of the course is to help clinical study investigators and staff understand and implement NIA and regulatory requirements for safe, high quality clinical research. The topics covered include Good Clinical Practice (GCP), Human Subject Protections, Adverse Events and Unanticipated Problems, Safety Monitoring and Reporting Requirements, Safety Monitoring and Oversight: Data and Safety Monitoring Boards (DSMBs) and Safety Officers, Regulatory Requirements and Responsibilities of Principal Investigators, and Data and Safety Monitoring Plans. The course requires about 40 minutes to complete.
Administrative Forms
Screening Log (MS Excel, 47K) Provides documentation of all individuals who were evaluated for participation in a research study. The log typically contains a unique identification number for each person screened along with individuals’ date of birth, gender, race and ethnicity, screening date, and eligibility status.
Site Signature Log - Delegation of Authority Log ( MS Excel, 47K or screen-readable PDF, 294K ) A record of all study personnel and their specific responsibilities, signatures, and dates of involvement during the conduct of a clinical research study.
Note to File Template (MS Word, 20K) – Used by clinical site staff to document protocol deviations or other discrepancies identified during the conduct of the clinical research study and plans for resolution/prevention.
Sample Visit Flow and Schedule (MS Word, 25K) – The visit schedule tracks an individual participant’s progress through the study and helps to ensure that visits take place during the protocol-specified timeframe. The visit flow provides an overview of the activities that take place at each study visit, and may be customized for each study site.
Study Drug/Investigational Product Tracker (MS Excel, 12K) – Used to track study drug/investigational product disposition and accountability by the clinical research site. For multi-site studies under an investigational new drug (IND) application, this tracker could be used by coordinating centers to track the overall distribution of investigational product.
Study Drug/Investigational Product Compliance Log (MS Word, 30K) – Used to track study drug/investigational product disposition and accountability for each individual participant. This form may be used to track protocol adherence via amount dispensed and returned and is designed to be used in conjunction with the Study Drug/Investigational Product Tracker. May also be used to track study drug/investigational return or destruction.
Study-wide Forms
Adverse Events Form ( MS Word, 38K or screen-readable PDF, 68K )
Prior and Concomitant Medications ( MS Word, 34K or screen-readable PDF, 58K )
Protocol Deviations Form ( MS Word, 46K or screen-readable PDF, 80K )
Serious Adverse Events Form ( MS Word, 31K or screen-readable PDF, 769K )
Study Disposition Form ( MS Word, 32K or screen-readable PDF, 56K )
Baseline Visit Forms
Visit Checklist ( MS Word, 34K or screen-readable PDF, 53K )
Eligibility Form ( MS Word, 29K or screen-readable PDF, 184K )
Demographics Form ( MS Word, 32K or screen-readable PDF, 661K )
Medical History Form ( MS Word, 50K or screen-readable PDF, 87K )
Medical History Conventional ( MS Word, 54K or screen-readable PDF,184 K )
Vital Signs Form ( MS Word, 33K or screen-readable PDF, 101K )
Physical Exam Form ( MS Word, 73K or screen-readable PDF, 193K )
Randomization and Enrollment Form ( MS Word, 32K or screen-readable PDF, 806K )
Sign up for NIA training and career development updates
Last updated: July 25, 2024
nia.nih.gov
An official website of the National Institutes of Health
Clinical Trial Templates to Start Your Clinical Research
By Kate Eby | May 13, 2019
- Share on Facebook
- Share on LinkedIn
Link copied
In this article, you will find everything you need to start your clinical research trials, with easy-to-understand guidance and terminology, 26 adaptable templates, and project plans in Microsoft Word, Excel, Project, and SharePoint formats.
Included on this page, you'll find details on what a research protocol is, project management for clinical trials , research compliance templates , and post-clinical study research documentation and templates
What Is the Research Protocol?
All clinical research starts with the research protocol , a document that details all aspects of the trial: its background, rationale, objectives, design, methodology, statistical analysis plan, and organization. With the protocol, you can make sure you protect the participants and collect the data. Using protocol templates, you can start thinking through what you need to meet compliance standards with the Food and Drug Administration (FDA) and clinical study best practices.

Download Research Protocol Template - Word
The full research protocol includes the following sections and topics:
- Title Pages: These pages provide general information about the protocol, including name, number, version number and date, trial phase, investigational product name, investigational new drug (IND) number, sponsor (or principal investigator in academia), funding organization, medical monitor, and coordinating center. The pages include the principal investigator’s signature (or sponsor), as well as site-specific information, such as the agreement, and protocol details. They also detail the study team and site, particularly in the case of multiple teams and sites.
- Objectives: List the study’s primary and secondary objectives.
- Background Information: Describe the problem under study and priority. Include the medical and scientific rationale that justifies researching the problem. Include data from other studies relevant to this proposed research. Include the name and description of the proposed intervention, including the dosage, route of administration, period, and frequency of intervention.
- Study Design: Describe the methodology and how it will answer the study question. This should include the type of study, primary and secondary outcome(s), population, sample size, study location, period of enrollment and follow-up, intervention and route of administration, randomization (as necessary), and any other relevant protocol information.
- Selection and Exclusion of Subjects: Provide statements describing how the participants must meet all the inclusion and exclusion criteria, and list the criteria. Clearly define the study population. For example, list the demographic criteria, required laboratory data, any prior therapies allowed or disallowed, ability to understand and meet all study requirements, if contraception is necessary, exclusion criteria such as specific health status, use of excluded drugs, cancer status, and chemical dependency status.
- Study Enrollment Procedures: Describe the methods and procedures for identifying and enrolling subjects, how they are documented, how consent is obtained, and any randomization procedures.
- Study Intervention, Duration, and Route of Administration: This section should describe each intervention and duration, as well as how each is administered. List expected adverse effects and dose escalation, if applicable. Discuss how the intervention is acquired, stored, and disposed of, as well as documentation for intervention accountability. In addition, note the medications restricted, allowed, and required, along with the extent to which these medications are tracked and documented.
- Study Procedures: This section includes a study evaluation schedule (presented as a chart) and explanations of the required assessments, what each period is, and any special considerations or instructions necessary. These should match what is available in the column headers of the chart above, and they should include information on the screening or baseline assessments, randomization, blinding, follow-up visits, and final assessments.
- Safety Assessment: List any expected adverse events, and how these could be managed. Mention any toxicities seen in earlier IND studies here. Also, include safety measures as identified in laboratory findings, methods and timing for safety parameters based on the risk profile, definitions for adverse events (AE) and serious adverse events (SAE) and laboratory values used to identify their possibility, timeframes for reporting and collecting information on AEs and SAEs, the reporting system, how you will follow up on AEs, and the specific guidelines for independent monitoring.
- Intervention Discontinuation: List criteria for intervention discontinuation and how you could meet them. Also list possible reasons for discontinuation, any modifications to the schedule should it be discontinued, duration of follow-up, any temporary discontinuation criteria, or any evaluations should participants be temporarily or permanently discontinued from the study.
- Statistical and Analytical Considerations: Include primary and secondary statistical hypotheses, why you chose the study design, the primary and secondary outcome measures, and the validity and reliability of these measures. Also discuss sample size and randomization, treatment assignment procedures, how you define the population, any interim analyses, primary and secondary outcome analyses, the statistical methods you use to consider any necessary intervention effect between groups, and if necessary, the expected positive within group correlations among different study arms.
- Data Collection: Detail how you will gather the data, the required forms, how to keep these forms confidential, and what source data to expect. Note site responsibility for data collection and management, and (if necessary) the responsibilities of the coordinating center.
- Quality Assurance: Describe training for study staff, whether there is a control committee and their required practices, any quality control metrics, how you will identify and document protocol deviations, how you will assure protocol compliance, and the schedule for reviews. If you have a manual of procedures (MOP), reference it here.
- Participants Rights: Include references to the Institutional Review Board (IRB) requirements, informed consent documents, procedures for participant confidentiality, and study discontinuation requirements.
- Committees: List any committees associated with the study, along with their roles.
- Publication: Outline the requirements and procedures for publication.
- References: List any citations referenced in this protocol.
- Supplements/Appendices: Include any additional documentation.
To track every aspect of the proposed research for each participant, create a case report form (CRF) that you can use in both paper and electronic formats. With CRFs, you can collect and analyze data for analysis, and then generate a conclusion for your study. For more information on the distinct phases of clinical trials, see “ Understanding the Phases of Clinical Trials .”
Concept Protocol Template
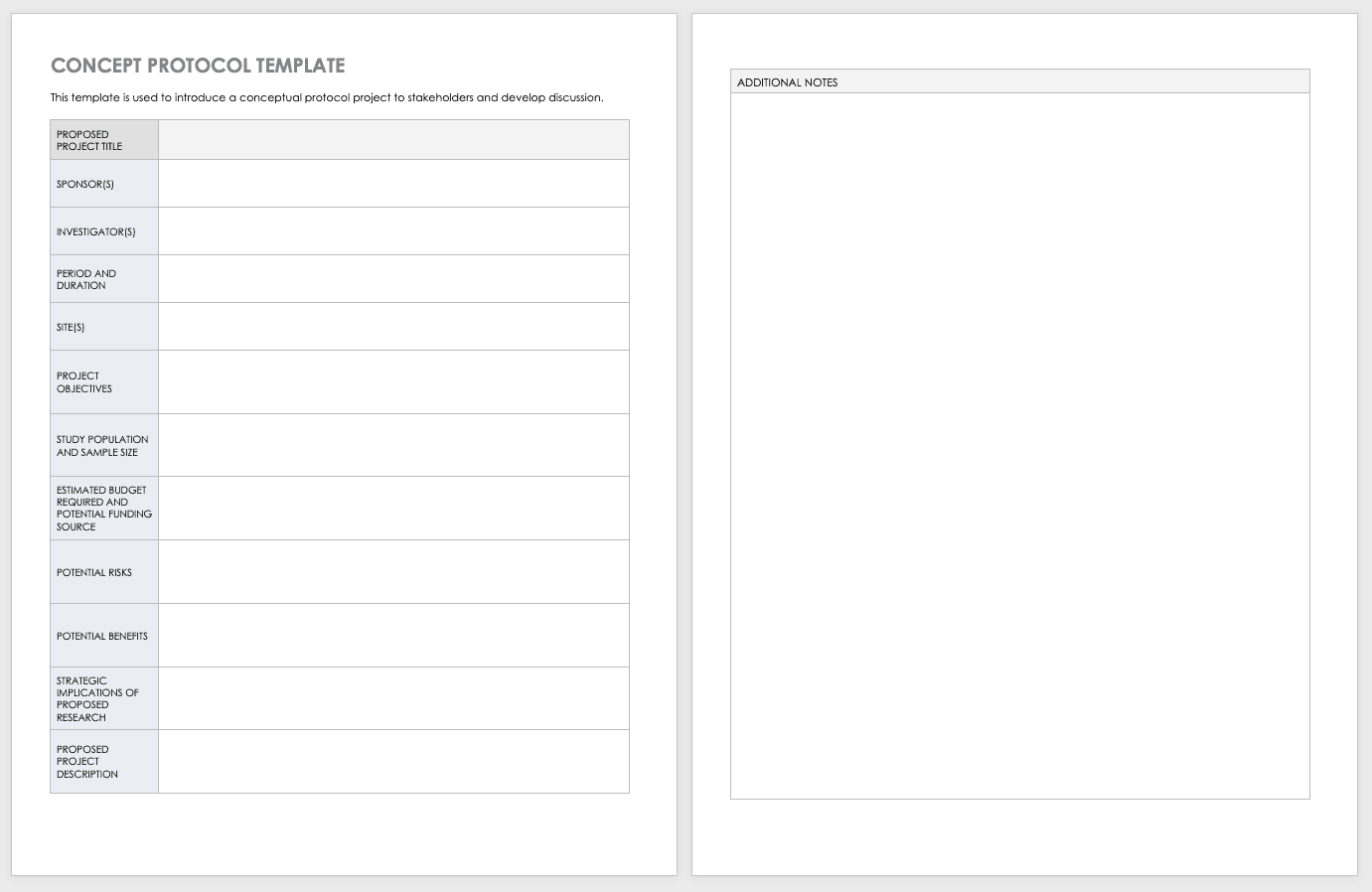
Before you start your full protocol, consider putting together a concept protocol. A concept protocol helps you introduce an abstract project to stakeholders and encourage discussion around the proposed project.
Download Concept Protocol Template for Clinical Research
Phase 1 Clinical Trial Protocol Template

For nonclinical research or clinical trials that are Phase 0 or Phase 1, use this free template. Phase 1 or nonclinical trials do not require the same amount of detail as a full study protocol.
Download Phase 1 Clinical Trial Protocol Template - Word
Research Compliance Templates
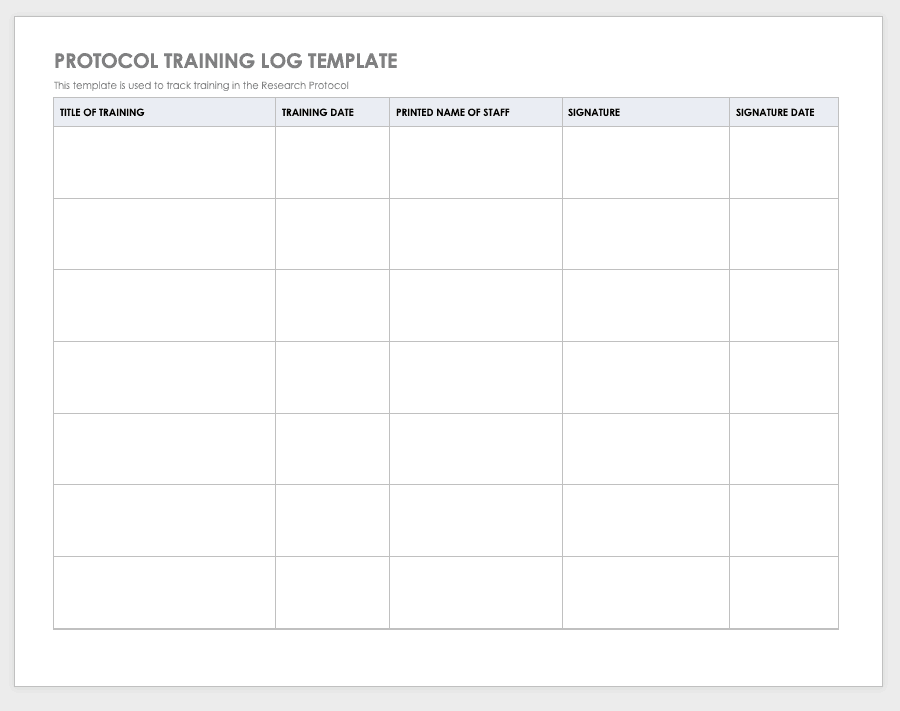
By training staff members on the research protocol, you’ll help them meet compliance standards and understand the purpose and details of the study. Use a training log to record all training that the site study staff completes, signing the log entry for verification.
Download Protocol Training Log Template
Excel | Word | PDF | Smartsheet
Protocol Deviation Template
Protocol deviations are inadvertent or unplanned changes or noncompliance with the research protocol. These events do not increase risk or decrease benefit, nor do they impinge on participants’ safety or rights. They do not compromise study data, but you should capture the deviation for reference.
Download Protocol Deviation Log Template
Excel | Word | PDF
Delegation of Authority Log Template
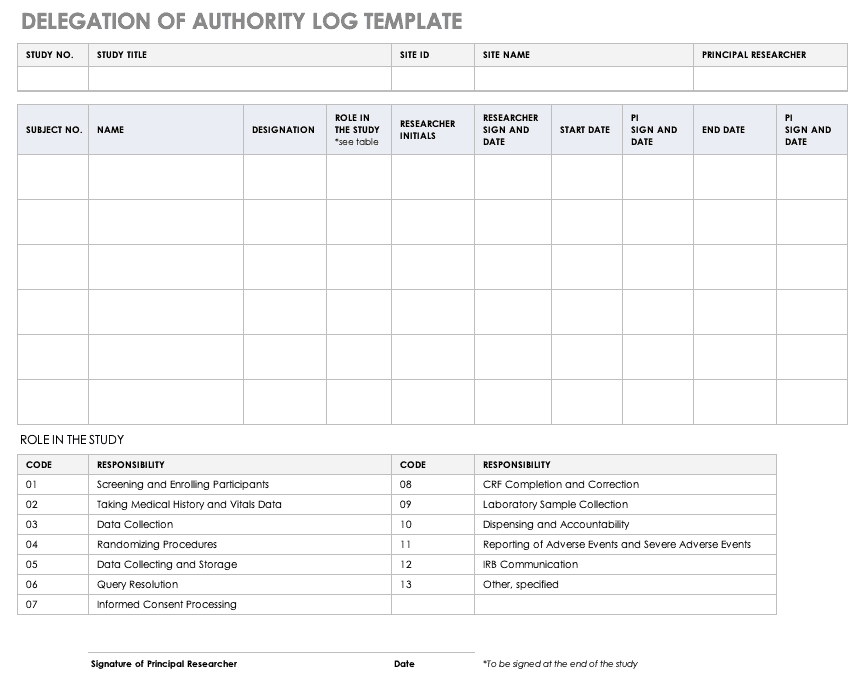
Once you’ve trained your staff and figured out their roles and responsibilities, the principal investigator must delegate authority. The delegation of authority log should be filled out and signed prior to the study’s start.
Download Delegation of Authority Log Template
Site Selection Visit Form Template
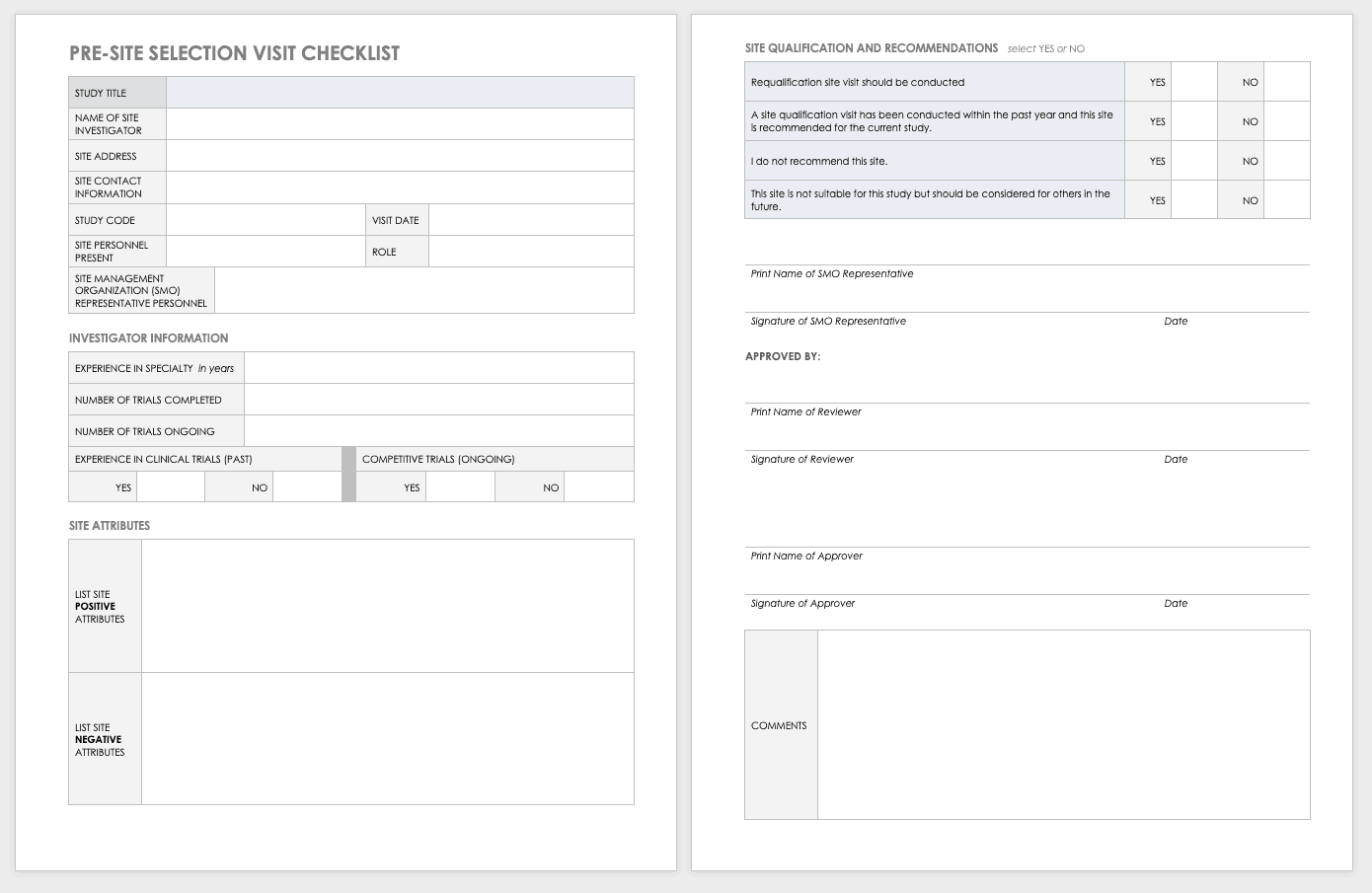
The sponsor must perform a site visit to determine its suitability as part of a multisite study. This means taking a tour to determine whether the site has the capabilities to meet the sponsor’s goals.
Download Site Selection Visit Form Template
Word | PDF | Smartsheet
Study Site Initiation Checklist
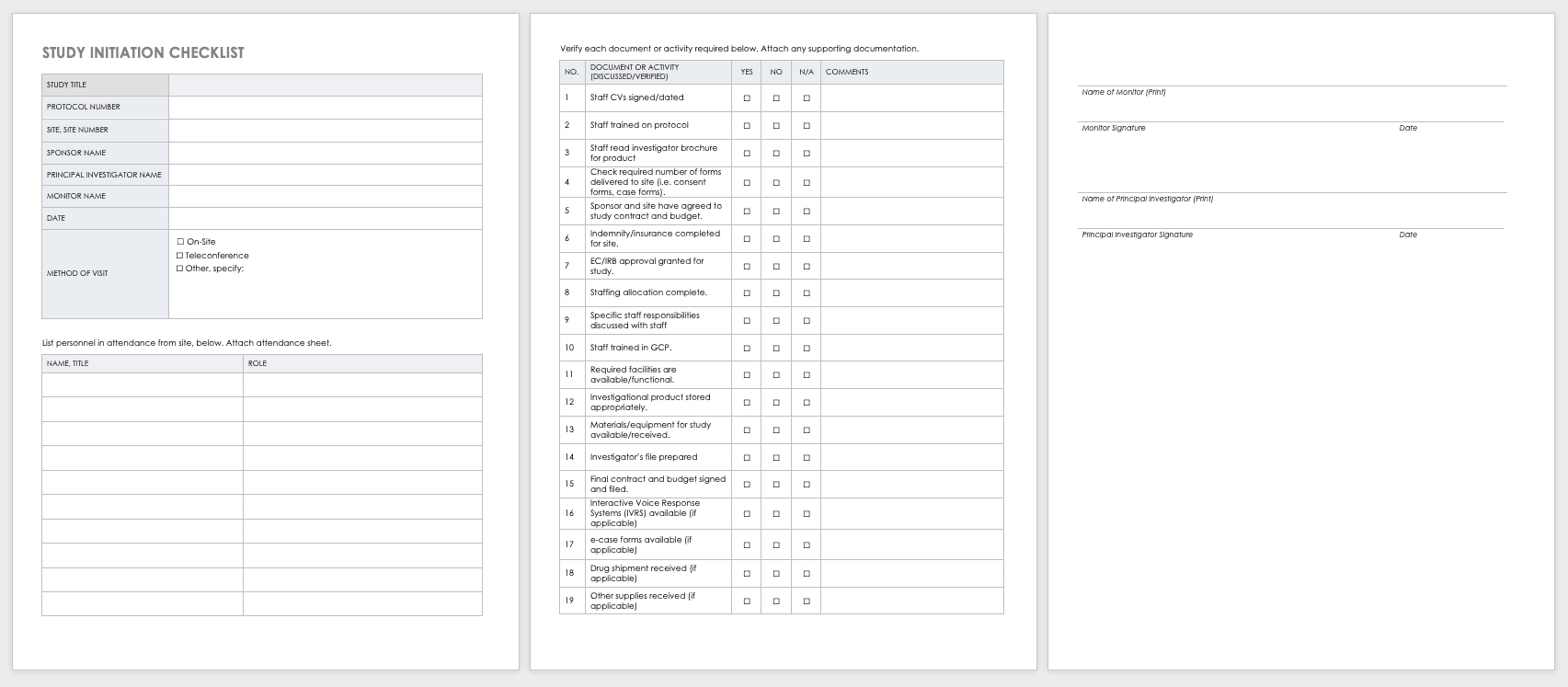
Teams must also perform an inspection to determine if a site has the appropriate staff, training, equipment, and supplies to be part of a multisite trial.
Download Study Site Initiation Checklist
Project Management for Clinical Trials, Practices, Templates, and Documents
Clinical trials are big projects. If the organization is not used to planning and wants to conduct clinical research, it must hire a project manager and work with senior leadership to introduce planning into the organization.
Together, they should develop the main goals and define their limits and the terms of success. They should set out a strategy for which tasks and sets of tasks to perform and in what manner. Test any planning tools or software before the trials start. When possible, use templates to ensure consistency and best practices.
Once the trial starts, evaluate your systems with standardized metrics. The project manager can track study deviations and apply corrective actions. Use the lessons learned from past and current projects to help guide future projects. Employing consistent tools gives you the opportunity to draw from a reservoir of data.
Clinical research can cost billions of dollars and years of time, resources, and effort. As
such, project management best practices and methodologies are critical to the success of a clinical trial, according to experts .
Many software systems are available to manage clinical trials. When very specialized, these are referred to as clinical trial management systems (CTMSs). However, other platforms can also manage clinical trials and may already be embedded with your information technology. Regardless of the platform you use, you should have full project management functionality, such as planning and reporting modules, as well as the ability to track participant contact information, deadlines, and milestones.
You may want to consider the following project management documents for your clinical research.
Project Management Plan (PMP) for Clinical Trials
A PMP delineates and acts as an agreed-upon document of scope, responsibilities, and guidance. You can use it throughout the project to help stay on track. Every clinical trial has difficult milestones, but a good project management plan can help you sidestep some of the regular issues.
You have many PMP software platforms to choose from, but regardless of your ultimate decision, your PMP must focus on protocol adherence, subject care, and service quality, along with how to achieve each standard. Here are the sections you should include in your PMP for a clinical trial:
- Project Objectives: This is an outline of the research objectives for the study, your quantifying standards, and your goals.
- Background and Strategic Context: By documenting background and context, you establish a foundation for decisions and discussion to follow.
- Study Governance: The governance covers the roles and responsibilities in the project, encouraging open communication, sharing, and accountability.
- Stakeholder Management Plan: This plan details how the staff and investigators will collaborate and effectively communication with stakeholders. This could include (as per the roles and responsibilities) regular emails, newsletters, consultation, oversight, training, and documentation.
- Scope: This document delineates assumptions, constraints, and deliverables (and their expected dates).
- Project Risk Assessment: This document helps you prepare for risks and decide on the risk profile.
Clinical Research Project Activity List
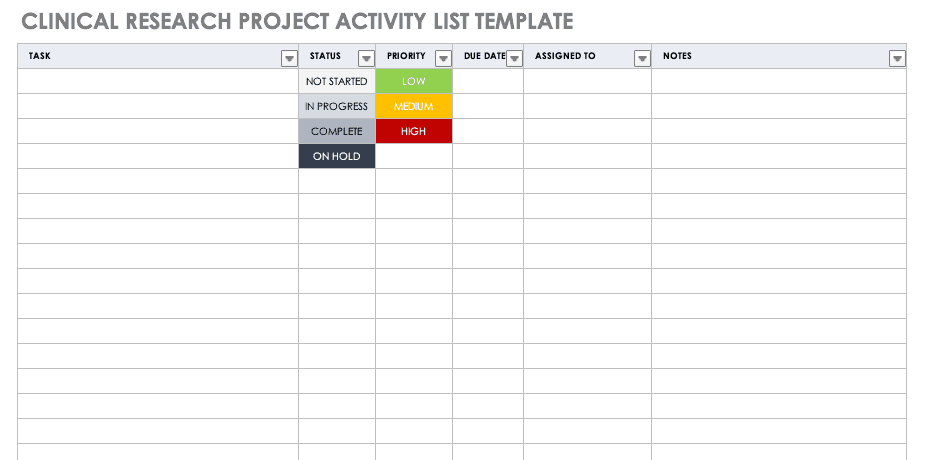
A project activity list is an itemized documentation of all the activities scheduled as part of the project. This list should be very detailed, including the status and priority of the task, when it is due, and to whom it is assigned.
Download Clinical Research Project Activity List Template
Excel | Smartsheet
Clinical Trial Timeline Template
A timeline enables you and your staff to track each major portion or milestone of your clinical trial. Your timeline should include these steps:
- Choose Research Questions and Study Design: Research always begins with questions. Your research question will determine how you design your study.
- Choose Outcomes: The outcomes for any trial are dependent on many factors, including scope, health conditions under study, target population, type of intervention. One resource to help develop outcomes is Core Outcome Measures in Effectiveness Trials (COMET) . This database details core outcome sets for comparison in clinical trials.
- Prospectively Register the Trial: Whether you are working through the FDA, World Health Organization (WHO), or another national agency, study transparency is critical. Prospective registration of trials is recommended. One resource for registration is the ISRCTN registry .
- Obtain Ethics Approval: Any trial involving human participants must go through an ethics review to safeguard the subjects’ rights, safety, well-being, and dignity. There are many options for institutional review, including through a university or a private or governmental organization. Without this step, research cannot commence.
- Prospectively Publish Protocol and Analysis Plan: Before a clinical trial, you must complete some pilot research. When you publish the research leading up to a clinical trial, along with the protocol and analysis for the trial itself, you increase transparency and accountability of the research.
- Planning for the Trial and Data Management: Many clinical research professionals recommend including patients in the planning phase of clinical trials, at least as stakeholders to review the plan. By completing the plan early and allowing potential participants to review it, you help improve recruitment and retention during the trial.
- Recruitment and Retention: Recruitment is getting the right people to take part in your trial, and retention is about keeping their interest and trust. A source of unending frustration for researchers, recruitment and retention can make or break a trial.
- Identify and Manage Trial Sites and Staff: This process is not as straightforward as it is often thought to be. Study coordinators must use feasibility checklists to choose sites and figure out how to get bring on staff who have the bandwidth to recruit for the study.
- Data Collection: The methods for collecting data are critical to any study. Advance planning and structure help you stay organized, comprehensive, and transparent so that your study can have a seamless analysis and solid conclusions.
- Data analysis: Flaws in analysis can generate poor, biased, or erroneous outcomes. In advance, researchers should consider patient blinding, randomization procedures, and sequence generation.
- Findings dissemination: Some researchers recommend threading all research on a trial topic. One resource for this is CrossRef , a database that links similar research. Regardless, the point of research is to capitalize on scientific progress and move it along. By having a plan to disseminate your results, you ensure that others capitalize on your research and move the knowledge forward.
Use this free template to develop your own clinical trial timeline. Add your own steps, milestones, and dates for a comprehensive, expansive view.

Download Clinical Trial Timeline Template
For a different perspective, add your project details to this free template so you can view your timeline visually.
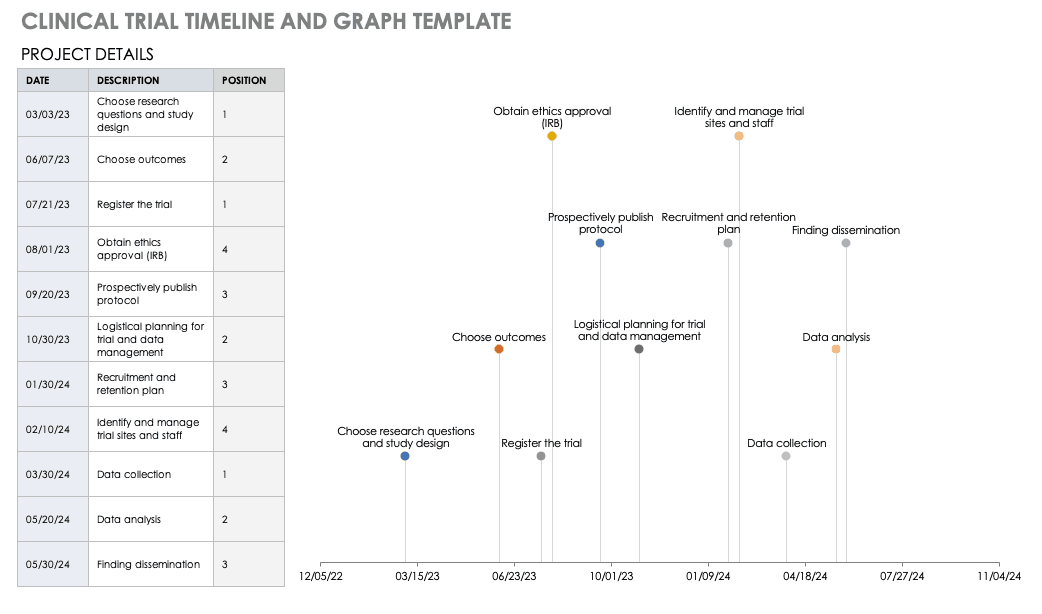
Download Trial Timeline and Graph Template
Microsoft Project Management for Clinical Trials
First released in 1985, Project is a well-respected Microsoft product for project management. Microsoft Project was not traditionally available as a part of Office Suites, a package of programs for professionals and professional organizations. However, Microsoft recently included it as a part of the Windows 2016 suite.
Microsoft Project Management has the following features:
- Built-in templates
- Project portfolio management
- IT management
- Presentations
- Out-of-the-box reports
- Multiple timelines
- Real-time reporting
- Dependency management
- Priority assignment
- Lean management
- Gantt charts/project mapping
- Calendar views
- Setting baselines/KPIs
- Project budgeting
- Issue tracking
- Task creation
- Resource management
- Cloud access
Microsoft Project has built-in templates that you can apply to clinical trial management.
Microsoft SharePoint for Clinical Trials
SharePoint is a collaboration platform that is integrated with Microsoft Office. SharePoint manages and stores documents , and it enables multiple users to access the documents via their own site or a standardized Microsoft site. A subscription to Microsoft Office 365’s SharePoint does not require a server, but customization options are limited; the flexible authentication and authorization systems are built in.
SharePoint Server, available in Standard or Enterprise versions, can be developed as either
virtual or hosted services in a business’s IT department. SharePoint Server enables the organization to control the SharePoint features available to staff, and you can scale it to meet different numbers of users.
Windows SharePoint Services 3.0 is a Microsoft-hosted version that comes with Microsoft Office. Microsoft provides a template in SharePoint for Clinical Trials: Clinical Trial Initiation and Management application template for Windows SharePoint Services 3.0 . You can download and add this template to your SharePoint Services, which enables you to create the following:
- Clinical Trial Protocols: This includes the objectives, study design, project plan, subject selection, and budget.
- Protocol Documents: This includes additional documents relative to your study.
- Calendar: Track milestones in the project.
- Threaded Document Discussions: Team members can start and track discussions within documents.
- Task Creation and Assignment: You can create and assign tasks to users, who receive email notifications.
- Archiving: You can move documents or groups of documents to archive status, keeping them but not making them visible.
The clinical trial template has site lists of libraries for clinical trial protocols, protocol documents, announcements, calendars, issues, tasks, and document discussions. These can be further customized with different versions of SharePoint. To download this template, you will need access to SharePoint Server 3.0.
Clinical Research Budget Plan Template
In many instances, you set the clinical trial budget after much negotiation with a sponsor. Other times, you need to build a budget before the sponsor is even on board, as a way to convince them of the project’s feasibility. The key cost drivers for any clinical research project are the following:
- Patient Grants: These include the costs for screening failures, baseline patient measurements, and procedural costs.
- Site Costs: This covers any expenses associated with the site, such as start-up fees, IRB fees, storage fees, and site management costs.
- Non-Patient Costs: This includes consultation fees, monitoring board fees, and any medical device costs.
- Labor Costs: You must account for all the staff required for the project and their full-time equivalency (FTE).
- Site Management: These costs include pre-study visits, initiation fees, monitoring, and close-out fees.
- Miscellaneous: These include investigator meetings, any technology needs, and ad hoc travel.
- Unexpected Costs: These are costs resulting from protocol amendments, value added tax (VAT), delays, and inflation.
Before you start putting together your research budget, you must gather the following:
- Schedule of assessments from the protocol
- Standard institutional fees from your institution, if applicable
- Evaluation and procedural costs
- Staff allocation and their hourly rates
- Indirect cost rate
- Subject compensation costs
- Data storage fee estimate
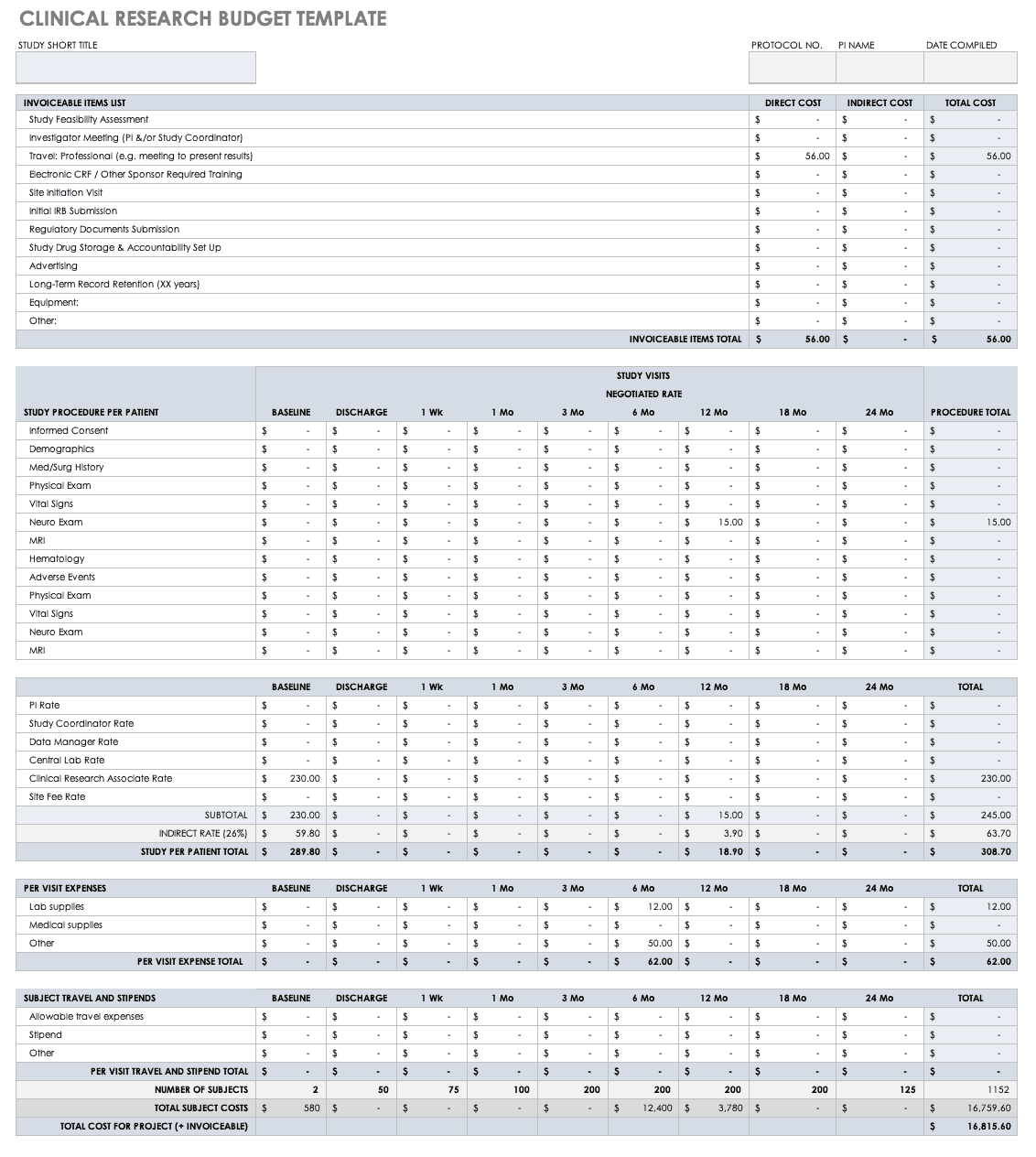
Put together your own clinical trial budget with this free clinical research budget template.
Download Clinical Research Budget Template - Excel
Clinical Research Tracking Log Templates
Clinical research requires scrupulous planning, a well-developed team, regulatory adherence, and above all, excellent documentation. It is therefore critical for clinical trial project managers to have a completed scope of work and to develop all the forms and templates before the trial begins. Some of these documents are for planning, and some, like those included below, are for operational purposes.
Regulatory Binder Checklist

Strong clinical practice thrives with a regulatory binder checklist. This checklist keeps track of all paper versions of essential regulatory study documents. Each document should also include any electronic locations. This document should be regularly updated, customized for unique studies, and stored in reverse chronological order.
Download Regulatory Binder Checklist
Clinical Study Document Tracking Log
It is important to not only track all paperwork related to a clinical trial, but also be able to locate it easily between various staff and sites. A clinical trial document tracking log can help you keep a written trail of the documents and when they were submitted and approved. You should also keep copies of the documents with the log. Use this free template to develop your own clinical study document tracking log. You can also adapt the log for specific correspondence, such as documents relating to FDA or IRB submissions, but it should not be mixed with regulatory documentation.
Download Clinical Study Document Tracking Log
Data and Safety Monitoring Plan (DSMP) Template
Before you can undertake a study, you must develop a DSMP for how to keep participants safe and how to secure data and ensure accuracy. The DSMP has several sections:
- The study purpose
- An adherence statement
- Any protocol amendments
- Multisite agreements
- A plan for subject privacy
- Confidentiality during adverse event reporting
- Expected risks
- Adverse events, unanticipated problems, and serious adverse events: how they are defined, their relation to the study, expectations, severity grading, and reporting procedures in single-site and multisite trials, and whether they are IND or non-IND studies
- Events of special interest
- Pregnancy reporting
- Rules to halt the study for participants
- Quality control and quality assurance
- Subject accrual and compliance
- Sample size justification
- Stoppage rules
- Monitoring committee designation
- Safety review plan
- Study report plan for independent monitors
- Plan to submit reports from onsite monitoring and audits
- Data handling and record keeping
- Informed consent
- Reporting changes in study status

Create your own data and safety monitoring plan using this free template. It lays out each section so you can specify them for your research. The principal investigator should sign and date this document once it is complete so that it may be filed.
Download Data and Safety Monitoring Plan Template - Word
Research Communication Plan Template
A communication plan should describe how you will converse with internal and external stakeholders during your project. Your communication plan should include a brief overview of your project and a breakdown of the messages you need to get out. You should adapt the messages for different audiences and define who will deliver these messages. The messages should include the following:
- The purpose and benefits of the research
- The known effectiveness of the intervention, or (if the intervention is under study) the disclosure that the effectiveness is unknown
- How participants will be protected
- The risks and benefits of participating

Develop your own communication plan using this free clinical trial communication plan template. This template also includes a section for situation analysis and risk analysis that asks for inputs on strengths, weaknesses, opportunities, and threats.
Download Clinical Trial Communication Plan Template - Word
Participant Management in Clinical Trials Using Templates
A few main documents help ensure that your participants are tracked and well-cared for before and during your research study.
Enrollment Log for Clinical Trials Template
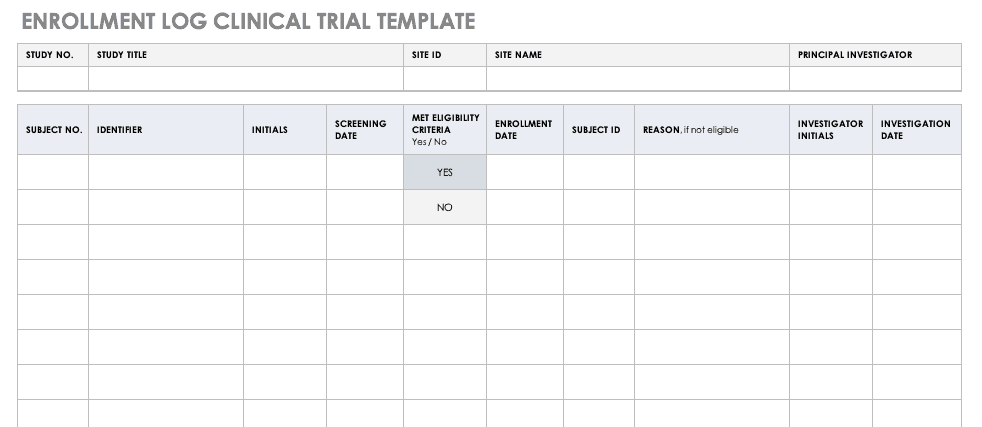
This log keeps track of everyone that has been enrolled for participation in your study. This does not mean that they have met the eligibility requirements or have been otherwise screened, but it is a record that they have signed up to be admitted.
Download Enrollment Log for Clinical Trials Template
Informed Consent Form Templates
Informed consent is the central tenet of ethical research with human subjects. The consent process typically involves a researcher delineating what is involved in the study, its risks and benefits, what a participant’s duties entail, and answering any questions they have. Before you perform any research, make sure the informed consent document is signed and the participant receives a copy, unless the informed consent document has been waived by an institutional review board (IRB). Federal regulations 45 CFR 46.116 govern what you must provide in the informed consent process in the United States.
To prepare informed consent documentation, researchers must do the following:
- Use plain, easily understandable language no higher than an 8th-grade reading level.
- Tailor documents to the potential population.
- Avoid technical jargon.
- Use the second or third person (you/he/she) to present study details.
- Include a statement of agreement.
- Ensure that the consent document is consistent with information in the IRB application.
These templates assist the principal investigator in the design of their informed consent forms (ICFs). You can adapt them to accommodate the details of any study and include both the information sheet and the consent form. Modify each section with the appropriate description described in italics. Use the general template for any type of research.
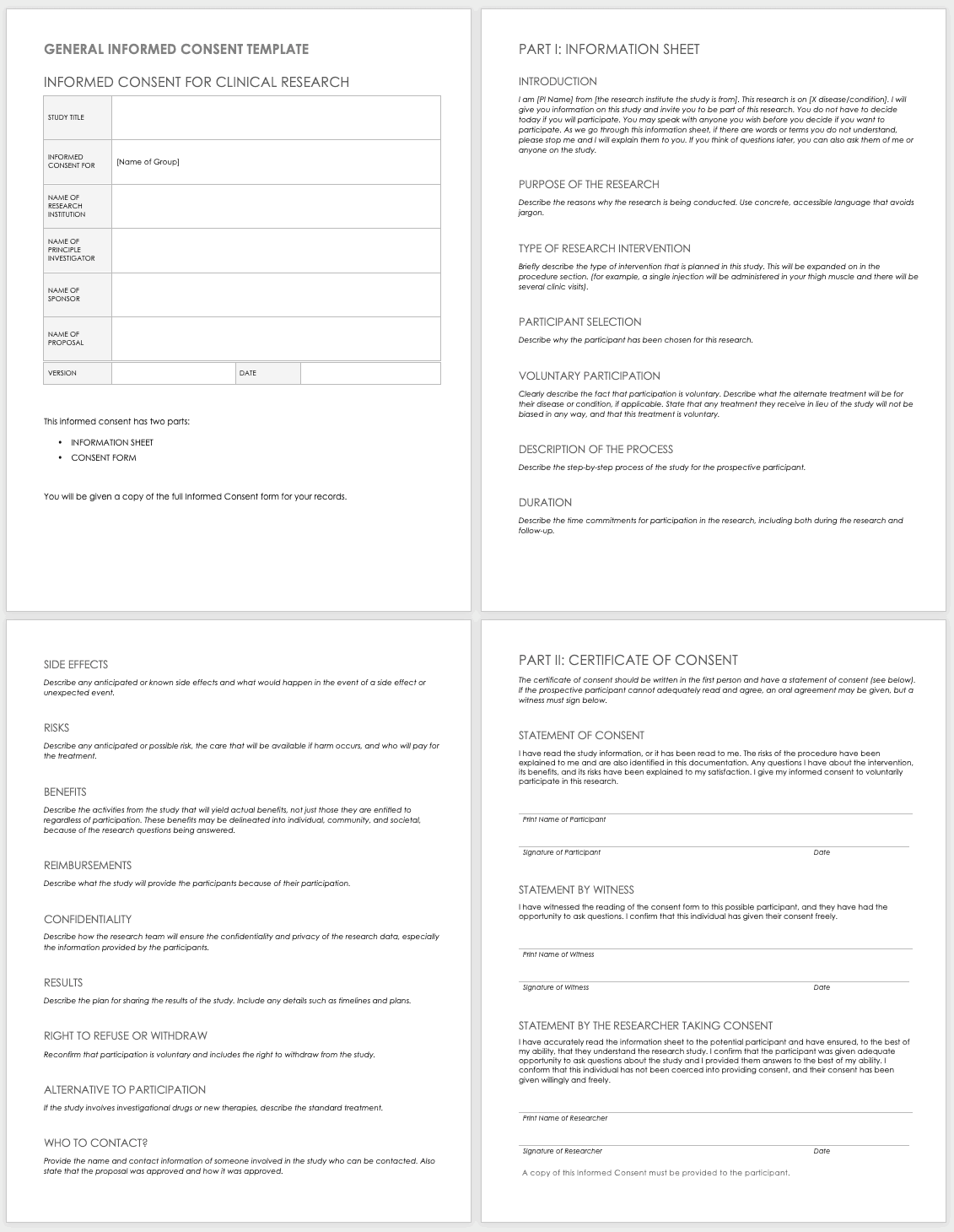
Download General Informed Consent Template - Word
Use the clinical trial template for medical research.

Download Informed Consent for Clinical Trials Template - Word
Eligibility Criteria (Inclusion/Exclusion) Checklist
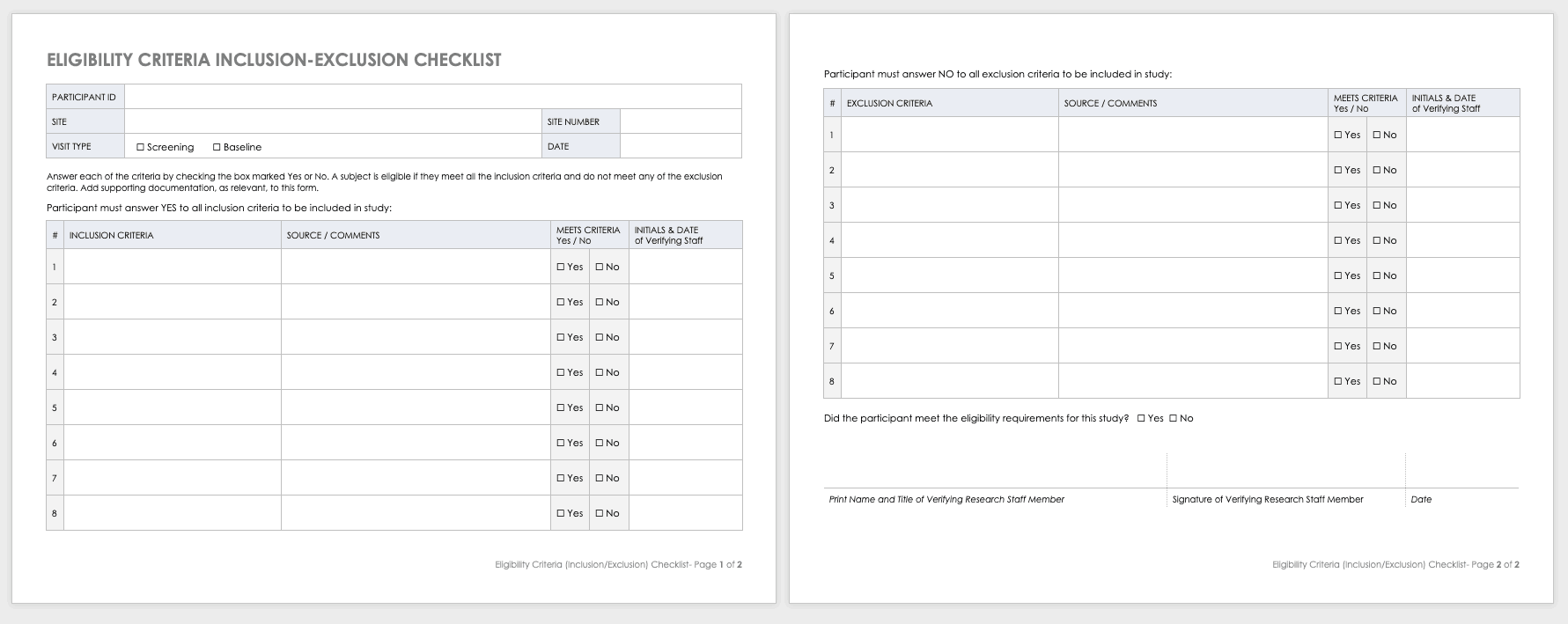
Eligibility criteria are an essential part of clinical trials. They define the population under investigation.
Inclusion criteria are the standards that participants must meet to enroll in the study. For example, in a study on a new diabetes medication, you would likely want participants who have already been diagnosed with diabetes.
Exclusion criteria specify the characteristics that disqualify participants from taking part in the research. For example, in the diabetes study above, the proposed diabetes drug may target a specific age demographic. One exclusion criterion could be a participant whose age falls outside of the range.
Download Eligibility Checklist Inclusion-Exclusion Template
Concomitant Medication Log Template
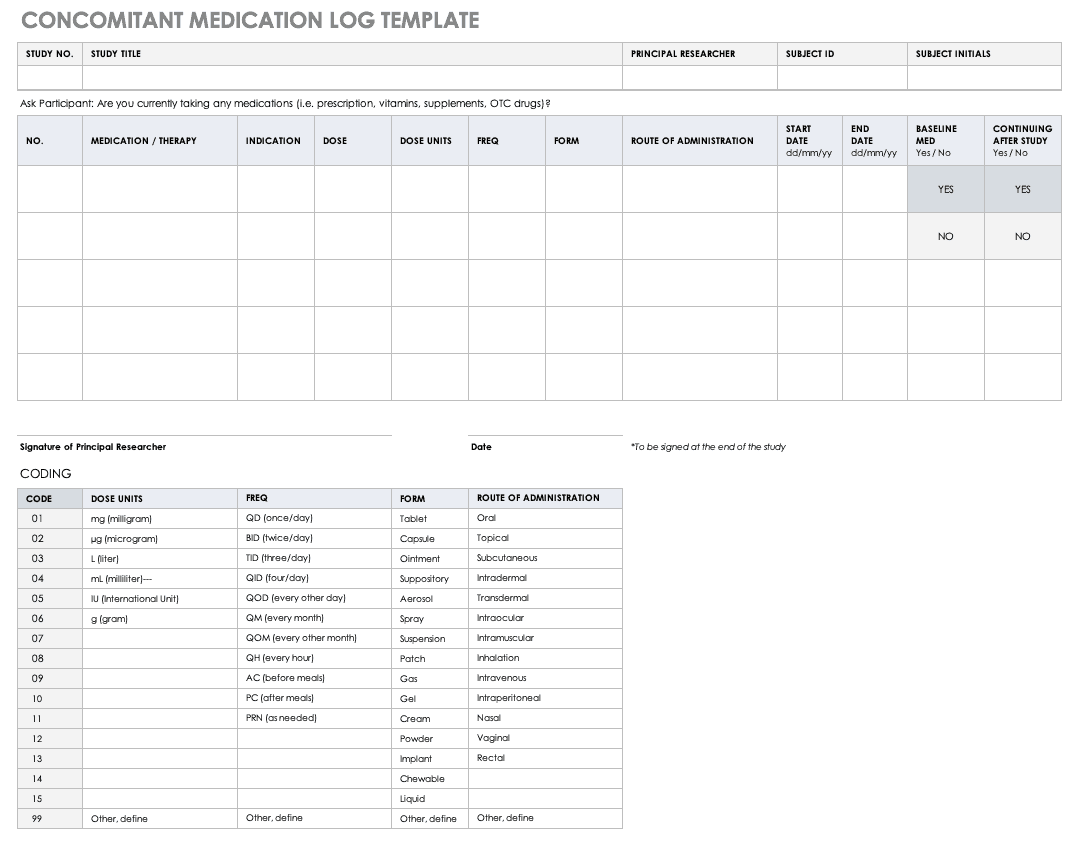
Properly documenting any medications that participants are taking is imperative to understanding the reactions occurring in their bodies, as well as what could spur adverse and severe adverse events during the study. Fill out a concomitant medication log for every participant and account for everything participants take, even seemingly innocuous items like multivitamins.
Download Concomitant Medication Log Template
Excel | Word | PDF
Adverse Event Form
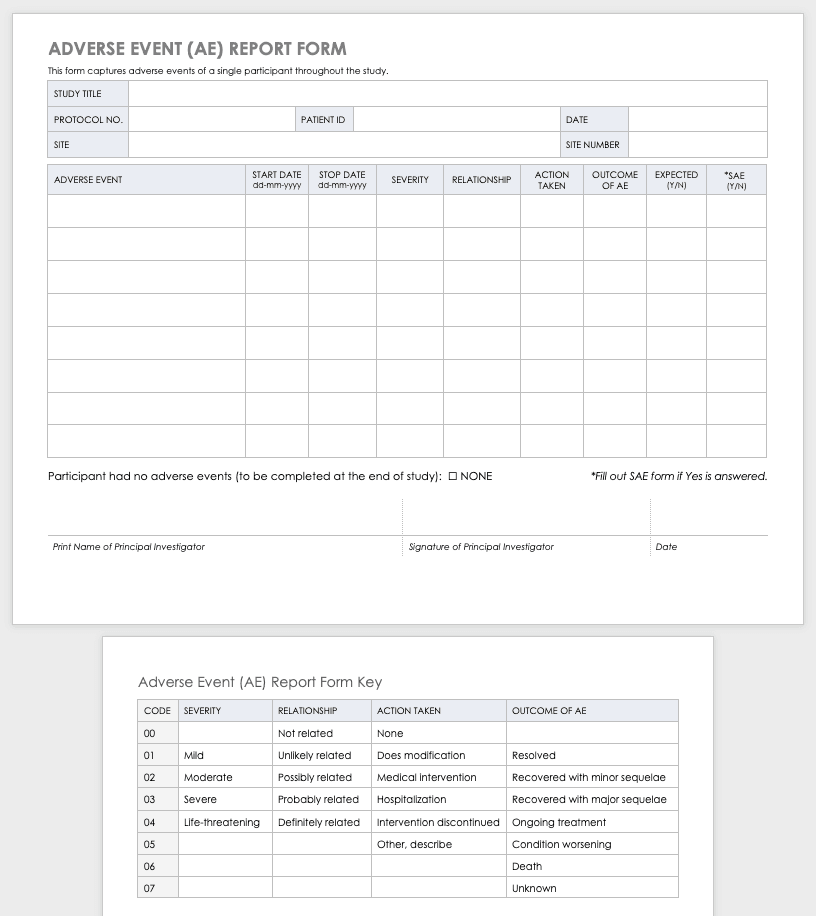
Clinical research can result in complications for the participants and trigger an adverse or severe adverse event. An adverse or severe adverse event is when participants in a clinical trial have negative medical symptoms that can be shown in laboratory or physical testing. Each participant in a clinical trial should have an adverse event log that tracks any adverse events through the duration of the study.
Download Adverse Event Form Template
Severe Adverse Event Form

A severe adverse event (SAE) is a special case of an adverse event in which the outcomes are acute. Examples of SAEs include death, life-threatening complications, or anything leading to immediate hospitalization, physical disability, or congenital abnormalities. Log SAEs in the AE form, but fill out an additional SAE form.
Download Severe Adverse Event Form Template
Word | PDF | Smartsheet
Post-Clinical Study Research Documentation and Templates
After you complete or terminate a clinical trial, you should prepare several additional documents. Here are some examples of this documentation:
- Investigational Product Accountability Log: You generally provide an accountability log to the authorities that tracks drug products to show product disposition and accountability per participant. It also helps you track the drug product stock and any imbalance at the end of the study.
- Investigational Product Destruction: Due to regulations governing the proper disposition of investigational products in clinical research, you must properly dispose of products left at the end of a study (as evidenced by the product accountability log). This form describes and ensures that you have properly handled any leftover products.
- Close-out Checklist/Report: A study close-out checklist and report helps ensure that you complete all closing procedures, archive the paperwork, and resolve electronic data.
Clinical Study Summary Report Template

Assemble the summary report at the end of a study to get results into the sponsor’s or public’s hands while you complete the full report. A summary report is typically about 2-3 page-long document that encompasses the highlights from the trial.
Download Study Summary Report Template - Word
Clinical Study Report (Full) Template

The full clinical study report (CSR) encompasses all aspects and details of the research you’ve conducted. It is not a sales or marketing tool; instead, it is a scientific report details the methodology and shows scientific rigor.
Download Clinical Study Report Template - Word
Public Links and Resources for Clinical Trials
The following are publicly available resources, tools, and links for clinical trial practitioners and principal investigators:
- PROMIS : Patient-Reported Outcomes Measurement Information System (PROMIS) software gives clinicians health status patient measures that are physical, mental, and social patient-reported metrics. Funded by the National Institutes of Health (NIH), PROMIS can be used in clinical trials as measures of conditions and disease and as a comparison to the general population. The measures in PROMIS are free to administer on paper, by computer (computer adaptive tests), or with an app. The computer adaptive tests may be conducted on REDCap , Assessment Center , or Epic .
- REDCap: REDCap (Research Electronic Data Capture) is an electronic data capture system that works on browsers to develop research databases. It was developed at Vanderbilt University to support clinical research data collection and is a free resource to nonprofit organizations. It is limited to organizations joining the REDCap consortium and is not open-source or available for commercial use.
- Good Clinical Practice (GCP) Training: GCP is an international quality standard designed for use by staff involved in clinical trials. The guidelines for this are from the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). These regulate the ethical guidelines, documentation, record keeping, training, facilities, technology, and inspections. The purpose of these guidelines is to keep clinical trials scientifically rigorous and to delineate the roles and responsibilities of research staff. The National Institutes of Health administers training for GCP.
- Quality Management Study-wide Review Tool: Developed by the NIH, this review tool is for PIs and study teams to manage their quality reviews, and may be customized for unique studies.
- Quality Management Subject Review Tool: Also developed by the NIH, this review tool provides study teams the structure for review of participant data, and may be customized for the unique study. This should be developed in concert with the DSMP.
- AccrualNet: AccrualNet is sponsored by the National Cancer Institute (NCI), and offers advice and training to staff on how to recruit study participants.
- Regulatory Education for Industry (REdI): The FDA offers a Clinical Investigator Training Course for researchers conducting investigational new drug (IND) or device exemption (IDE) studies.
- ResearchMatch: Available to volunteers and researchers affiliated with the NIH Clinical and Translational Science Award (CTSA) program, this site helps match prospective participants with specific studies.
- Grant Policies and Guidance: The NIH and National Center for Complementary and Integrative Health (NCCIH) offer links to many resources that are policy- and grant-specific to the NIH and NCCIH, updated regularly.
- Protocol Amendments: The NIH and NCCIH offer regularly updated guidance for NIH policy and protocol changes.
- Clinical Terms of Award for Human Subjects Research: The NIH and NCCIH offer guidance for clinical trial grant awardees for compliance.
- NIH Single IRB (sIRB) Policy for Multisite Research: The NIH offers a FAQ page for multisite research that includes policy, contract and application information, responsibilities, exceptions, and costs.
- Dictionary of Cancer Terms: The National Cancer Institute (NCI) offers a dictionary of cancer terms for researchers and laypersons. You can add this dictionary to your website as a widget.
- Informed Consent FAQs: The U.S. Department of Health and Human Services (HHS) and the Office for Human Research Protections (OHRP) offer a FAQ page about informed consent for researchers and lay persons.\Informed Consent Language (ICL) Database: The National Comprehensive Cancer Network (NCCN) offers a database to help write informed consents. This database is specific to medical conditions and different risk language.
Improve Clinical Trial Research with Smartsheet for Healthcare
Empower your people to go above and beyond with a flexible platform designed to match the needs of your team — and adapt as those needs change.
The Smartsheet platform makes it easy to plan, capture, manage, and report on work from anywhere, helping your team be more effective and get more done. Report on key metrics and get real-time visibility into work as it happens with roll-up reports, dashboards, and automated workflows built to keep your team connected and informed.
When teams have clarity into the work getting done, there’s no telling how much more they can accomplish in the same amount of time. Try Smartsheet for free, today.
Discover why over 90% of Fortune 100 companies trust Smartsheet to get work done.

Transforming the understanding and treatment of mental illnesses.
Información en español
Celebrating 75 Years! Learn More >>
- Opportunities & Announcements
- Funding Strategy for Grants
- Grant Writing & Approval Process
- Managing Grants
- Clinical Research
- Small Business Research
NIMH Clinical Research Toolbox
The NIMH Clinical Research Toolbox serves as an information repository for NIMH staff and the clinical research community, particularly those receiving NIMH funding. The Toolbox contains resources such as NIH and NIMH policy and guidance documents, templates, sample forms, links to additional resources, and other materials to assist clinical investigators in the development and conduct of high-quality clinical research studies.
Use of these templates and forms is optional; the resources can be used as-is or customized to serve study team needs. In cases where institutions provide research teams with institution-specific templates and forms for clinical research documentation, NIMH expects researchers to follow their institutional policies for document use. Nevertheless, the materials on this page can be consulted to assure that study teams are meeting NIMH expectations.
Protocol Templates
Protocol associated documents, regulatory documents and associated case report forms, clinical research education, support, and training (crest) program overview.
- Human Subject Risk
Data and Safety Monitoring for Clinical Trials
Reportable events, recruitment, suicide prevention research, good clinical practice training, data sharing, educational presentations, clinical research start up.
NIMH encourages investigators to consider using one of the protocol templates below when developing a clinical research protocol. In cases where an institutional review board (IRB) has a recommended or required protocol template, reviewing the documents included below is still suggested as there may be sections that a study team may opt to include in an effort to develop a comprehensive research protocol.
NIH has developed a Clinical e-Protocol Writing Tool to support the collaborative writing and review of protocols for behavioral and social sciences research involving humans, and of phase 2 and 3 clinical trial protocols that require a Food and Drug Administration (FDA) Investigational New Drug (IND) or Investigational Device Exemption (IDE) Application.
NIH-FDA Phase 2 and 3 IND/IDE Clinical Trial Protocol Template
This clinical trial protocol template is a suggested format for Phase 2 and 3 clinical trials funded by NIH that are being conducted under a FDA IND or IDE Application.
Investigators for such trials are encouraged to use this template when developing protocols for NIH-funded clinical trial(s). This template may also be useful to others developing phase 2 and 3 IND/IDE clinical trials.
NIH Behavioral and Social Clinical Trials Template
This clinical trial protocol template is a suggested format for behavioral or psychosocial clinical trials funded by NIH. Investigators for such studies are encouraged to use this template when developing protocols for NIH-funded clinical trial(s). This template may also be useful to others developing behavioral of psychosocial research studies.
Back to Table of Contents
NIMH Clinical Manual of Procedures (MOP) Template [Word]
This template provides a recommended structure for developing consistent instructions on study procedure implementation and data collection across participant and clinical site activities. It details the study’s organization, operations, procedures, data management, and quality control.
NIMH Clinical Monitoring Plan Template [Word]
This template provides a recommended structure for a plan to conduct internal or independent review of Good Clinical Practices (GCP), human subject safety, and data integrity throughout the lifecycle of a study.
Informed Consent Materials
Often study teams will be provided with informed consent form templates and guidance on requirements for the informed consent process by their institutions. Below is additional guidance and materials to support a thorough informed consent process.
Sample NDA Informed Consent Language
The NIMH Data Archive (NDA) receives de-identified human subjects data collected from hundreds of research projects across many scientific domains, and makes these data available to enable collaborative science. This NDA sample informed consent language for data sharing can be adapted when using one of the NDA platforms.
Regulatory Document Checklists by Study Type The following checklists are intended to help the investigator community identify a set of core documents to be organized within a single study specific folder, either electronically, hard copy, or a mixture of both formats. NIMH encourages study teams to verify what additional documents, or alternative formats of the documents in the checklists, their institution and IRB require.
NIMH Regulatory Document Checklist for non-Clinical Trial Human Subjects Research [Word]
Study teams can use this checklist to compile essential documents for the conduct of a NIMH-funded study that does not meet the NIH definition of a clinical trial and is research on human subjects.
NIMH Regulatory Document Checklist for Clinical Trials without Investigational Product [Word]
Study teams can use this checklist to compile essential documents for the conduct of a NIMH-funded NIH defined clinical trial that does not involve an investigational drug or device.
NIMH Regulatory Document Checklist for Human Subjects Research Clinical Trials with Investigational Product not under a FDA IND/IDE [Word]
Study teams can use this checklist to compile essential documents for the conduct of a NIMH-funded NIH defined clinical trial with an investigational drug or device that is not under a FDA IND or IDE.
NIMH Regulatory Document Checklist for a Study under a FDA IND or IDE [Word]
Study teams can use this checklist to compile essential documents for the conduct of a NIMH-funded NIH defined clinical trial or non-clinical trial with an investigational drug or device under a FDA IND or IDE.
Necessary Documents for Reportable Events
NIMH Reportable Events Log Template [Word]
This document provides a log template for documenting reportable events. The types of events that require reporting may vary by institution, IRB, sponsor, state, and other factors.
NIMH Study-Wide Protocol Deviation Log Template [Word]
This document provides a log template for tracking all protocol deviations/violations across a study.
NIMH Subject-Specific Protocol Deviation Log Template [Word]
This document provides a log template for tracking subject-specific protocol deviations/violations. If captured electronically, subject-specific deviation logs can be exported into a study-wide deviation log.
NIMH Study-Wide Adverse Events (AE) Log Template [Word]
This document provides a log template for tracking all adverse events (AEs), including serious adverse events (SAEs), across a study.
NIMH Subject-Specific Adverse Event (AE) Log Template [Word]
This document provides a log template for tracking adverse events (AEs), including serious adverse events (SAEs), for each subject. If captured electronically, subject-specific AE logs can be exported into an electronic study-wide AE log.
Necessary Documents for Studies with Pharmacy/Investigational Product
FDA Form 1572 Statement of Investigator
This FDA form should be signed by the investigator prior to study initiation to provide certain information to the sponsor, and assure that he/she will comply with FDA regulations related to the conduct of a clinical investigation of an investigational drug or biologic.
NIMH Investigational Product (IP) Management Standard Operating Procedure (SOP) Template [Word]
This document provides a sample standard operating procedures (SOP) template to document how investigational product (IP) will be received, stored, monitored, labeled, dispensed, and destroyed.
NIMH Investigational Product Storage Temperature Log Template [Word]
This document provides a log template for recording the daily temperatures for investigational product (IP).
NIMH Master Investigational Product Dispensing and Accountability Log Template [Word]
This document provides a log template for capturing all investigational product (IP) dispensed to and returned by participants for the duration of the study.
NIMH Subject-Specific Investigational Product Dispensation and Accountability Log Template [Word]
This document provides a log template for capturing all investigational product (IP) dispensed to an individual participant and returned by that participant. This log is typically placed in each subject’s study binder (study blind is maintained, if applicable).
Screening and Enrollment Logs and Materials
NIMH Participant Pre-Screening Log Template [Word]
This document provides a log template for all potential participants who have completed initial screening procedures (i.e. phone screens or internet screening surveys; typically, prior to signing written informed consent). This log should capture the number of participants eligible for an official screening visit, as well as the number ineligible with the reasons for ineligibility listed.
NIMH Participant Enrollment Log Template [Word]
This document provides a log template for chronologically documenting the participants who have been enrolled in the study.
NIMH Inclusion/Exclusion Checklist Template [Word]
This document provides a sample checklist to customize according to protocol-specific eligibility criteria. A qualified and appropriately-delegated study team member should sign and date to confirm eligibility once all criteria have been assessed. If criteria are assessed on different visit dates, this checklist should be reformatted to reflect which criteria are assessed on which visit dates, and who is responsible for assessing them.
NIMH Documentation of Informed Consent Template [Word]
This document provides a sample form template for documenting the informed consent process.
Additional Participant Tracking Logs and Materials
NIMH Concomitant Medication Log Template [Word]
This document provides a log template for recording each participant’s medications throughout the study. This log is typically reviewed at all subject study visits and is located in each participant’s study binder.
NIMH Research Sample Inventory/Tracking Log [Word]
This document provides a log template for tracking the collection and storage of research samples.
Staff Training and Administrative Tracking Logs and Materials
NIMH Good Clinical Practice (GCP) Training Log Template [Word]
This document provides a log template for documenting completion of Good Clinical Practice (GCP) training requirements. Note: all NIH-funded investigators and staff who are involved in the conduct, oversight, or management of clinical trials should be trained in Good Clinical Practice (GCP), consistent with principles of the International Conference on Harmonisation (ICH) E6 (R2). Individual institutions may require GCP training regardless of funding source or clinical trial status.
NIMH Study Training Log Template [Word]
This document provides a log template for documenting staff trainings for study-specific procedures (i.e., trainings for diagnostic interview administration, study protocol adherence, phlebotomy, outcomes measures, OSHA Bloodborne Pathogens, etc.).
NIMH Delegation of Authority Log Template [Word]
This document can be used to record all study staff members’ significant study-related duties, as delegated by the Principal Investigator (PI). Most studies opt to use a log format, such as the Delegation of Authority log, because it captures study staff on one page and includes space to document the addition or removal of specific study tasks for individual staff members.
NIMH Monitoring Visit Log Template [Word]
This document is typically completed by the clinical site monitor to document dates and purpose of clinical site monitoring visits.
NIMH Note to File (NTF) Template [Word]
This document provides a sample template for generating notes-to-file, which are written to acknowledge a discrepancy or problem with the study’s conduct, or for other administrative purposes (such as to document where study materials are stored).
On-Site Monitoring
Even though it is the NIMH’s expectation that grantees will provide adequate oversight of their clinical research, NIMH Program Officials may require additional levels of on-site monitoring conducted by NIMH staff. Clinical monitoring helps ensure the rights and well-being of human subjects are protected; the reported clinical research study data are accurate, complete, and verifiable; and the conduct of the study is in compliance with the study protocol, Good Clinical Practice (GCP), and the regulations of applicable agencies.
The NIMH Clinical Research Education, Support, and Training (CREST) Program provides ongoing educational and technical support from NIMH staff for clinical research project grants selected for consultation and/or site visit(s). The CREST Program aims to ensure that the reported clinical research study data are accurate, complete, and verifiable, the conduct of the study is in compliance with the study protocol, Good Clinical Practice (GCP) and the regulations of applicable agencies, and the rights and well-being of human subjects are protected, in accordance with 45 CFR 46 (Protection of Human Subjects) and, as applicable, 21 CFR part 50 (Protection of Human Subjects).
To promote clinical research that is compliant with GCP and human subject regulations, the CREST Program includes phone conversations, email consultation, and/or site visit(s) from NIMH staff, as needed, to assess and provide written feedback and recommendations on planned or ongoing clinical research protocols. Documents relating to the conduct of the clinical research, such as current IRB approved protocols, informed consent documents, source documents, and drug accountability records, as applicable, may be reviewed for compliance with applicable Federal regulations, and institutional and IRB policies.
Research project grants selected for inclusion in the CREST Program might include clinical research studies with “significantly-greater-than-minimal risk” to subjects (e.g., an intervention or invasive procedure with high potential for serious adverse events; see NIMH Risk-Based Monitoring Guidance ); a study intervention under a FDA Investigational New Drug or Investigational Device Exemption; or other studies identified by NIMH staff that may benefit from inclusion in CREST. CREST is separate and distinct from “for cause” audits of clinical research. Research grants may be included in CREST at any time during the study lifecycle, although projects are generally identified and selected for the program at the initiation of the grant.
NIMH Clinical Research Education Support and Training (CREST) Program Overview
This page provides a description of the NIMH CREST Program’s purpose, process for inclusion, and operating procedures.
Site Visits
NIMH Clinical Research Education, Support, and Training Program (CREST): Comprehensive Visit Report Template [Word]
This template provides a recommended structure for a CREST site visit report, as well as a sample matrix of regulatory criteria that CREST monitors look at while at site initiation visits (SIVs), interim monitoring visits (IMVs) and close out visits (COVs). It is to be used as a starting point for preparing for a CREST site visit or for writing a site visit report.
NIMH CREST Site Initiation Visit (SIV) Sample Agenda [Word]
This document provides a sample site initiation visit agenda to be customized by the Principal Investigator (PI) and site monitor prior to the visit.
Human Subjects Research
This section provides resources, including policy and guidance documents related to the conduct of human subject research. The resources included below represent those frequently of interest to NIMH investigators, specifically: overviews of human subject research, data and safety monitoring, human subject risk, reportable events, and recruitment. There are numerous other NIH webpages devoted to human subjects research; see Research Involving Human Subjects , NIH Human Subjects Policies and Guidance , and New Human Subjects and Clinical Trial Information Form .
Human Subject Regulations Decision Charts
The Office for Human Research Protections (OHRP) has developed graphic aids to help guide investigators in deciding if an activity is research involving human subjects that must be reviewed by an IRB under the requirements of the U.S. Department of Health and Human Services (HHS) regulations ( 45 CFR 46 ).
Human Subjects in Research: Things to Consider
This NIMH webpage presents items which investigators should pay particular attention to when proposing to use human subjects in NIMH-funded studies.
Human Subjects Risk
NIMH Guidance on Risk-Based Monitoring
This NIMH guidance aims to clarify risk level definitions and the NIMH’s monitoring expectations to mitigate these risks. This guidance will assist study teams in determining the level of data and safety monitoring that should be established for a study based on the probability and magnitude of anticipated harm and discomfort.
The policies, guidance and documentation in this section outline NIMH expectations for data and safety monitoring of clinical trials . For human subject research that does not meet criteria for NIH clinical trial designation, investigators still have an option of including a data and safety monitoring plan (DSMP; i.e., in studies that may have significant risk to participants). The initial links below apply to all NIMH-funded clinical trials, while the second section provides documentation for clinical trials under the oversight of a NIMH-constituted data and safety monitoring board (DSMB).
All Clinical Trials
NIMH Policy Governing the Monitoring of Clinical Trials
This NIMH policy outlines NIH and NIMH expectations for data and safety monitoring of clinical trials. This policy also assures that the NIMH is notified by NIMH-funded researchers in a timely manner of all directives emanating from monitoring activities.
Guidance for Developing a Data and Safety Monitoring Plan for Clinical Trials Sponsored by NIMH
This guidance was created to aid investigators developing a data and safety monitoring plan (DSMP) to ensure the safety of research participants and to protect the validity and integrity of study data in clinical trials supported by NIMH. This guidance applies to data and safety monitoring for all NIMH-supported clinical trials (including grants, cooperative agreements, and contracts).
NIMH Policy Governing Independent Safety Monitors and Independent Data and Safety Monitoring Boards
This policy establishes expectations for the monitoring of NIMH-supported clinical trials by Independent Safety Monitors (ISMs) and/or independent data and safety monitoring boards (DSMBs) to assure the safety of research participants, regulatory compliance, and data integrity.
Trials Reviewed by a NIMH-Constituted DSMB
The materials below are for studies designated for review by a NIMH-constituted DSMB. Study teams developing materials for a study-constituted independent DSMB may benefit from reviewing the data report template and the protocol amendment memo.
NIMH Clinical Trials Operations Branch Liaison Orientation Letter [Word]
This letter provides an orientation to working with the NIMH Clinical Trials Operations Branch which supports study teams reporting to the NIMH DSMB.
NIMH DSMB Reporting Guide Full Report Template [PDF]
This template provides a recommended structure for data reports used for DSMB review and oversight. The report template includes standard data tables. Study teams are encouraged to utilize this template as a starting point, and use, remove, and/or modify the existing tables as appropriate for the study under review.
NIMH DSMB Amendment Memo Template [Word]
This template may be used when submitting a study protocol or consent document amendment to the NIMH DSMB.
NIMH Reportable Events Policy
This policy outlines the expectations of NIMH-funded researchers relating to the submission of reportable events (i.e., Adverse Events (AEs); Serious Adverse Events (SAEs); Unanticipated Problems Involving Risks to Subjects or Others ; protocol violations; non-compliance (serious or continuing); suspensions or terminations by monitoring entities (i.e., Institutional Review Board (IRB), Independent Safety Monitor (ISM)); and suspensions or terminations by regulatory agencies (i.e., Office for Human Research Protections (OHRP) or the Food and Drug Administration (FDA)).
( For associated documentation, see: Guidance on Regulatory Documents and Associated Case Report Forms )
NIMH Policy for the Recruitment of Participants in Clinical Research
This policy is intended to support effective and efficient recruitment of participants into all NIMH extramural-funded clinical research studies proposing to enroll 150 or more subjects per study, and all clinical trials, regardless of size.
NIMH Recruitment of Participants in Clinical Research Policy
This policy outlines NIMH expectations regarding the establishment of recruitment plans and milestones for overall study enrollment, and as appropriate, recruitment plans for females and males, members of racial and ethnic minority groups, and children, as well as recruitment reporting.
Frequently Asked Questions (FAQ) about Recruitment Milestone Reporting (RMR)
This NIMH FAQ document provides responses to several of the most common questions surrounding RMR.
Points to Consider about Recruitment and Retention While Preparing a Clinical Research Study
These “points to consider” are meant to serve as a resource as investigators plan a clinical research study and a NIMH grant application. It also outlines common barriers that can impact clinical recruitment and retention.
Additional Resources and Trainings
Conducting Research with Participants at Elevated Risk for Suicide: Considerations for Researchers
This web document is intended to support the development of NIMH research grant applications in suicide research, including those related to clinical course, risk and detection, and interventions and implementation, as well as to support research conduct that is safe, ethical and feasible.
Based on the NIH Good Clinical Practice (GCP) policy , all NIH-funded clinical investigators and clinical trial staff who are involved in the design, conduct, oversight, or management of clinical trials are requirement to be trained in GCP. Below are links to some GCP courses that meet NIH GCP training expectations.
Good Clinical Practice for Social and Behavioral Research – E-Learning Course
The NIH Office of Behavioral and Social Sciences Research (OBSSR) offers a self-paced Good Clinical Practice (GCP) training course with nine video modules. Learners complete knowledge checks and exercises throughout the course.
National Institute of Allergies and Infectious Diseases (NIAID) GCP Learning Center
NIAID has created a self-paced Good Clinical Practice (GCP) training course that includes four modules. These modules educate the learner on the history of human subject research, the regulatory framework, planning human subject research, and conducting human subject research.
National Drug Abuse Treatment (NDAT) Clinical Trials Network
This NDAT course includes 12 modules based on International Council for Harmonisation (ICH) Good Clinical Practice (GCP) and the Code of Federal Regulations (CFR) for clinical research studies in the U.S. The course is self-paced and takes approximately six hours to complete.
The following notices and links present NIMH expectations and tools for data sharing.
Data Sharing Expectations for NIMH-Funded Clinical Trials
This notice establishes NIMH’s data sharing expectations, including the request to include a detailed data sharing plan as part of grant applications.
Data Harmonization
This notice encourages investigators in the mental health research community to utilize data collection protocols using a common set of tools and resources to facilitate sharing, comparing, and integration of data from multiple sources.
NIMH Data Archive
The NIMH Data Archive is an informatics platform for the sharing of de-identified human subject data from all clinical research funded by the NIMH.
Educational Materials
The following educational materials are provided to support the training of NIMH-funded clinical research investigators and staff.
Good Clinical Practices (GCP) for NIMH-Sponsored Studies [PowerPoint]
This training presentation defines Good Clinical Practice (GCP) and describes its application in NIMH-funded research. Topics include: investigator responsibilities, training and qualifications, resources and staffing, delegation of responsibilities, informed consent, documentation and storage of data, assessment and reporting, protocol adherence, drug accountability, adverse events/unanticipated problems and noncompliance. Note that this presentation does not replace the Good Clinical Practice (GCP) training required for NIH funded investigators.
Good Documentation Practices for NIMH-Sponsored Studies [PowerPoint]
This training presentation provides an overview of good documentation practices to follow throughout the duration of NIMH-funded research. The presentation defines and gives examples of good documentation practices.
Introduction to Site-Level Quality Management for NIMH-Sponsored Studies [PowerPoint]
This training presentation provides an overview of the process of establishing and ensuring the quality of processes, data, and documentation associated with clinical research activities. Quality Management (QM) is defined in relationship to site-level documentation, processes, and activities. Tools that are available to support site-level QM are also described.
NIMH Clinical Monitoring and Clinical Research Education, Support, and Training Program (CREST) Overview [PowerPoint]
This training presentation provides an overview of Clinical Monitoring, types of site monitoring visits and what takes place during these visits as well as an overview of follow-up activities. The presentation specifically describes the NIMH Clinical Research Education Support and Training (CREST) Program, its goals, study portfolio selection process, and standard procedures.
Additional NIMH Links and Contacts:
- Office of Clinical Research
- Clinical Trials Operations Branch (CTOB)
- NIMH Clinical Research Policies, Guidance, and Resources
- Human Research Protection Branch (HRPB)
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
- Research paper
- Research Paper Format | APA, MLA, & Chicago Templates

Research Paper Format | APA, MLA, & Chicago Templates
Published on November 19, 2022 by Jack Caulfield . Revised on January 20, 2023.
The formatting of a research paper is different depending on which style guide you’re following. In addition to citations , APA, MLA, and Chicago provide format guidelines for things like font choices, page layout, format of headings and the format of the reference page.
Scribbr offers free Microsoft Word templates for the most common formats. Simply download and get started on your paper.
APA | MLA | Chicago author-date | Chicago notes & bibliography
- Generate an automatic table of contents
- Generate a list of tables and figures
- Ensure consistent paragraph formatting
- Insert page numbering
Instantly correct all language mistakes in your text
Upload your document to correct all your mistakes in minutes

Table of contents
Formatting an apa paper, formatting an mla paper, formatting a chicago paper, frequently asked questions about research paper formatting.
The main guidelines for formatting a paper in APA Style are as follows:
- Use a standard font like 12 pt Times New Roman or 11 pt Arial.
- Set 1 inch page margins.
- Apply double line spacing.
- If submitting for publication, insert a APA running head on every page.
- Indent every new paragraph ½ inch.
Watch the video below for a quick guide to setting up the format in Google Docs.
The image below shows how to format an APA Style title page for a student paper.
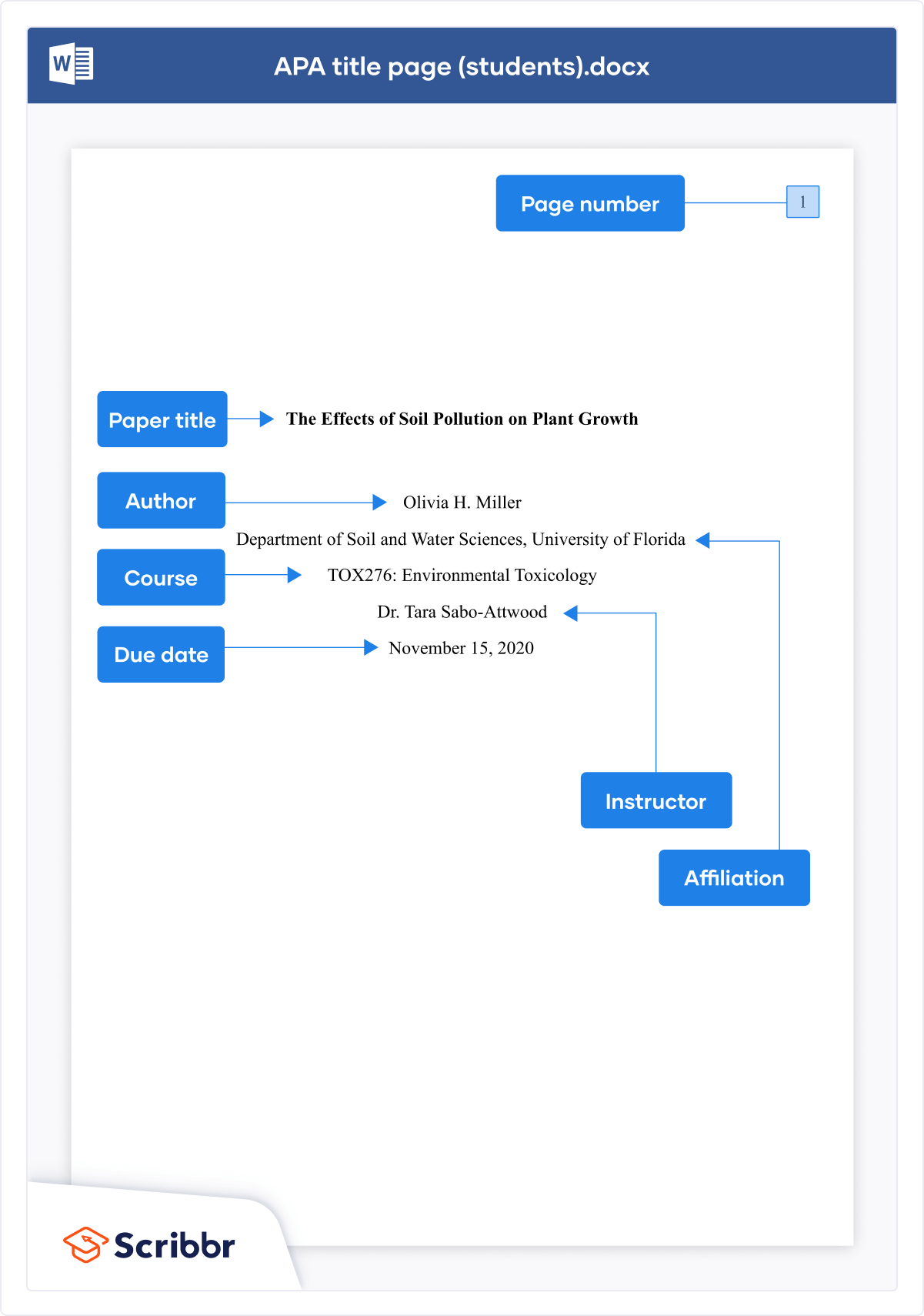
Running head
If you are submitting a paper for publication, APA requires you to include a running head on each page. The image below shows you how this should be formatted.
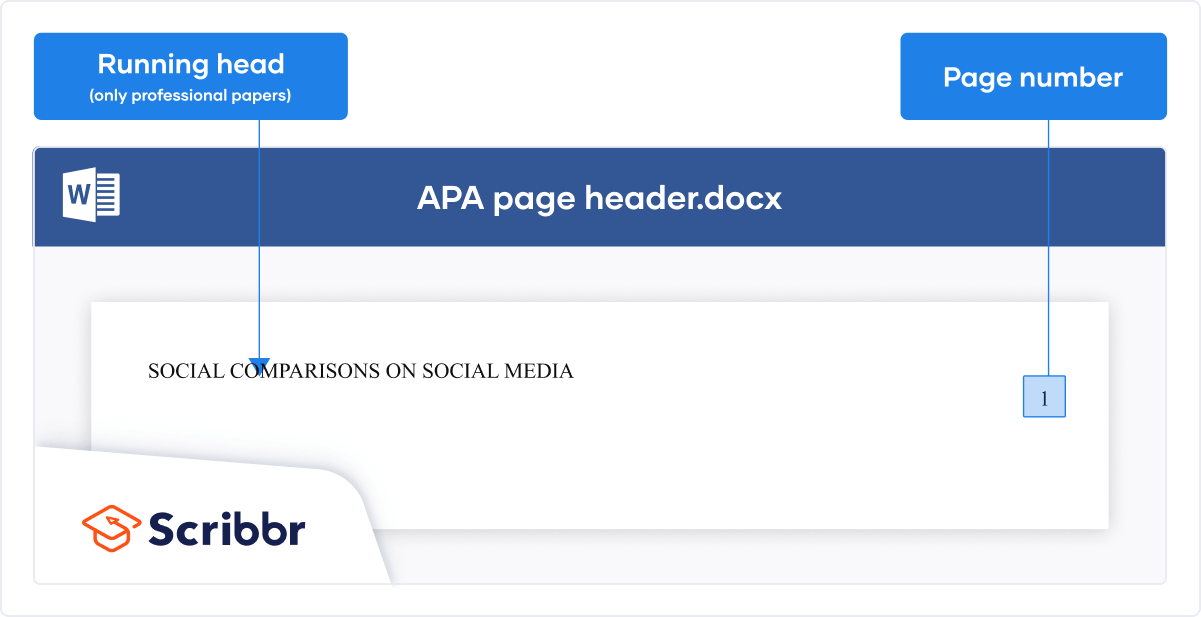
For student papers, no running head is required unless you have been instructed to include one.
APA provides guidelines for formatting up to five levels of heading within your paper. Level 1 headings are the most general, level 5 the most specific.
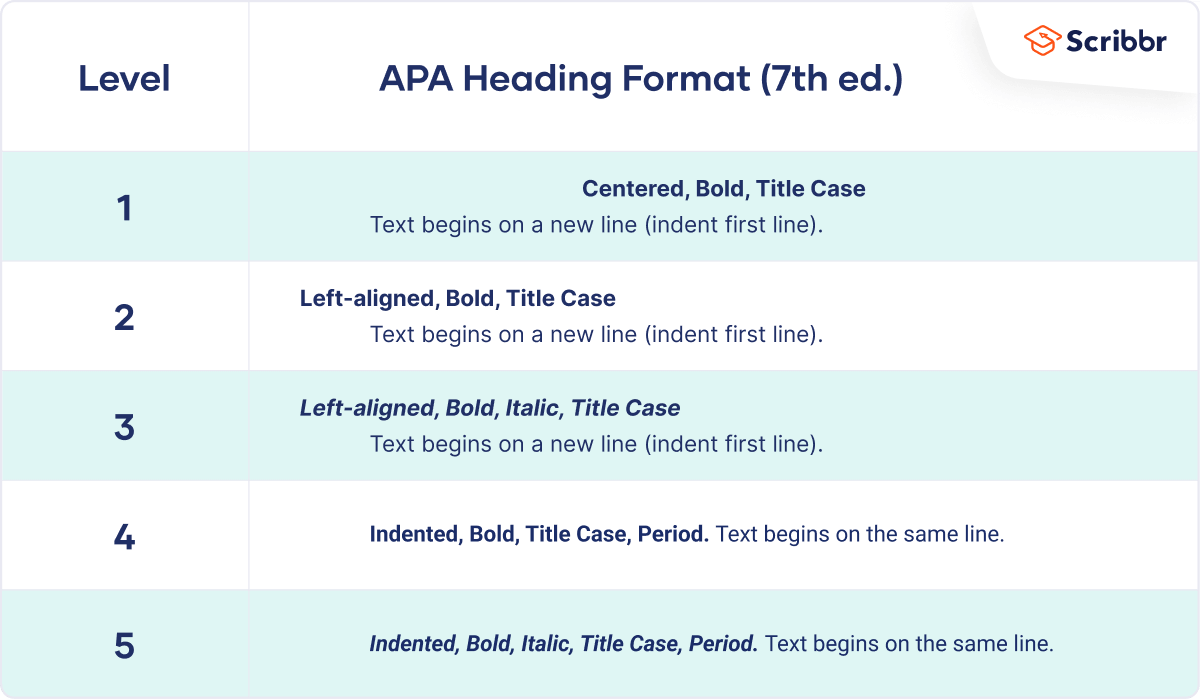
Reference page
APA Style citation requires (author-date) APA in-text citations throughout the text and an APA Style reference page at the end. The image below shows how the reference page should be formatted.
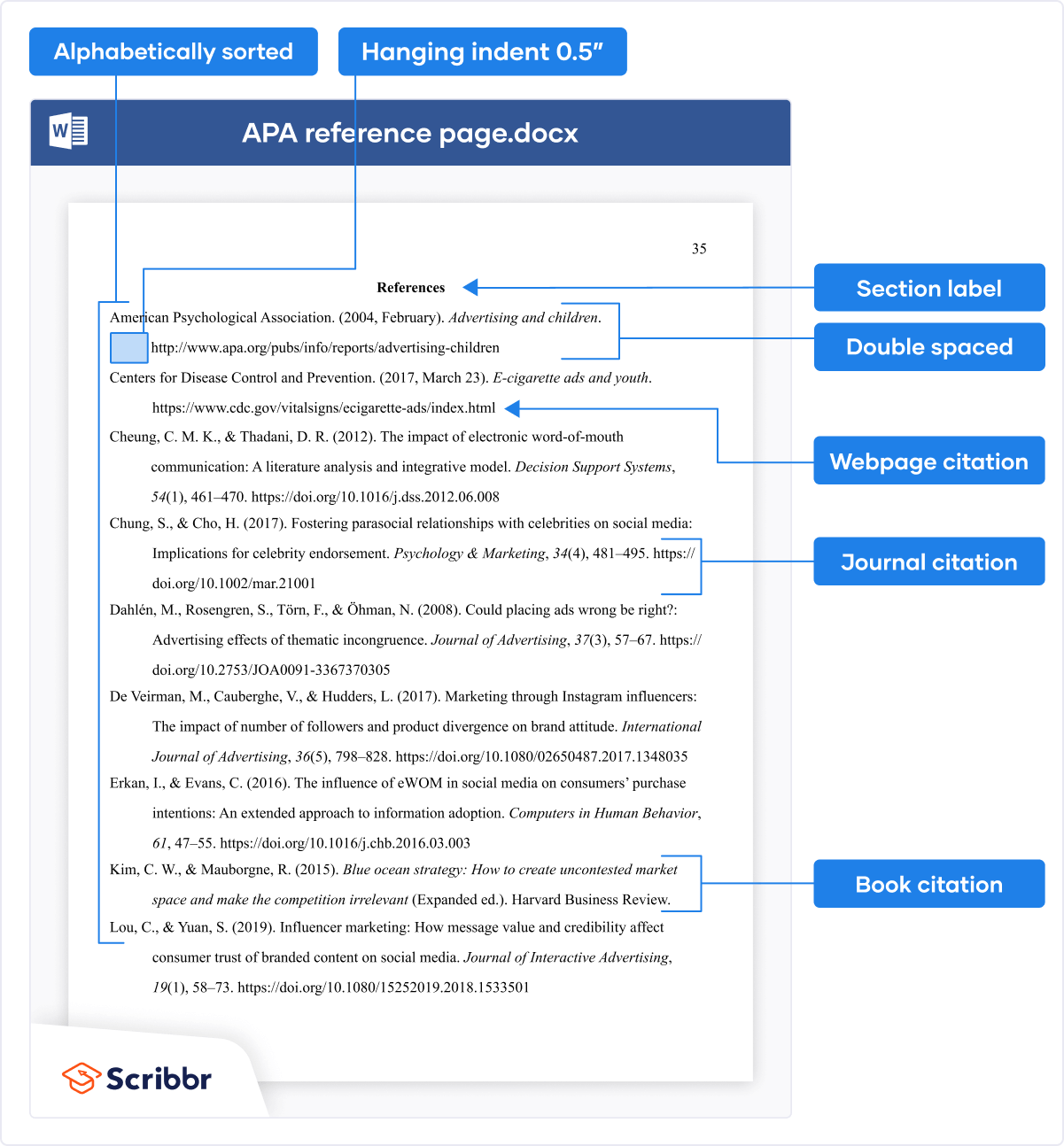
Note that the format of reference entries is different depending on the source type. You can easily create your citations and reference list using the free APA Citation Generator.
Generate APA citations for free
Don't submit your assignments before you do this
The academic proofreading tool has been trained on 1000s of academic texts. Making it the most accurate and reliable proofreading tool for students. Free citation check included.
Try for free
The main guidelines for writing an MLA style paper are as follows:
- Use an easily readable font like 12 pt Times New Roman.
- Use title case capitalization for headings .
Check out the video below to see how to set up the format in Google Docs.
On the first page of an MLA paper, a heading appears above your title, featuring some key information:
- Your full name
- Your instructor’s or supervisor’s name
- The course name or number
- The due date of the assignment
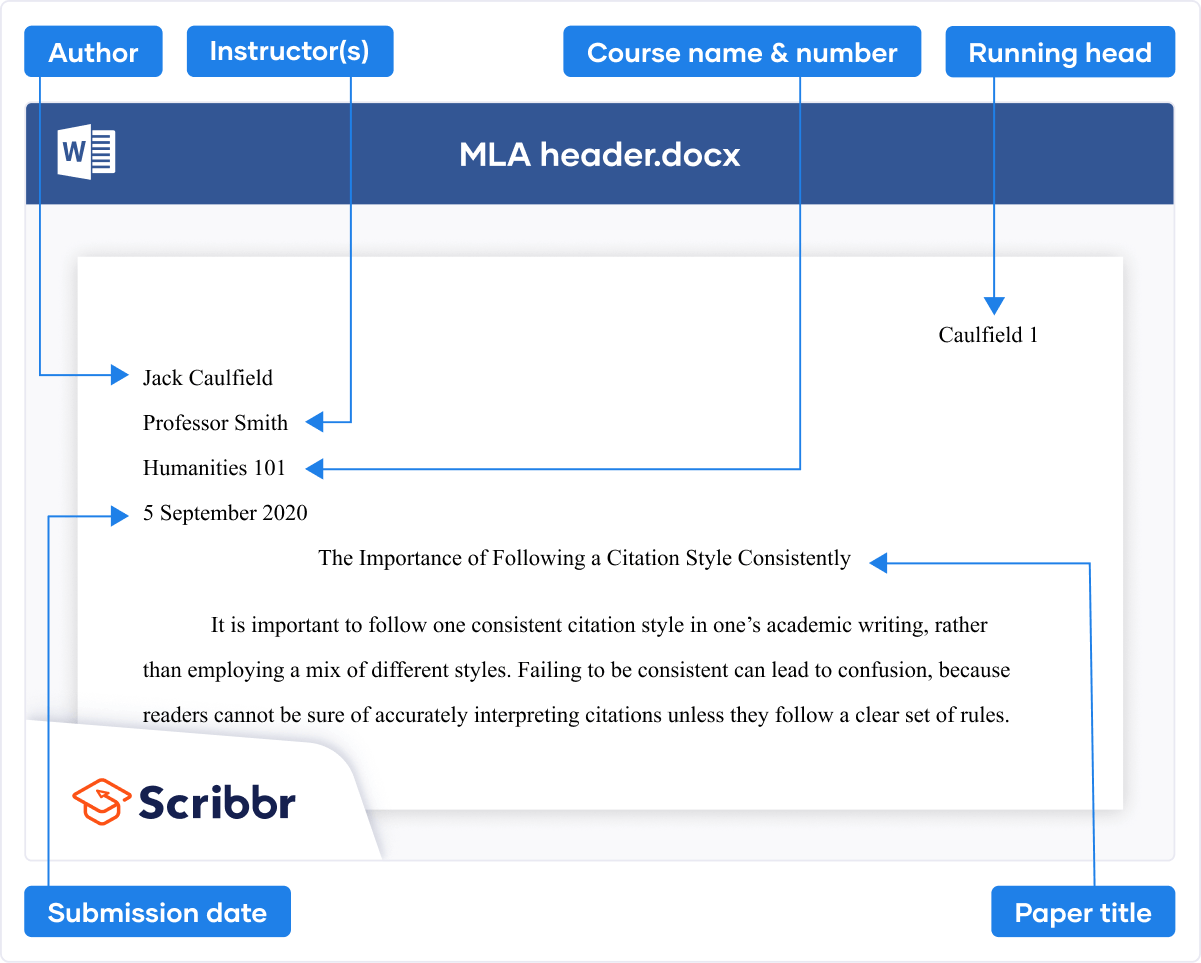
Page header
A header appears at the top of each page in your paper, including your surname and the page number.
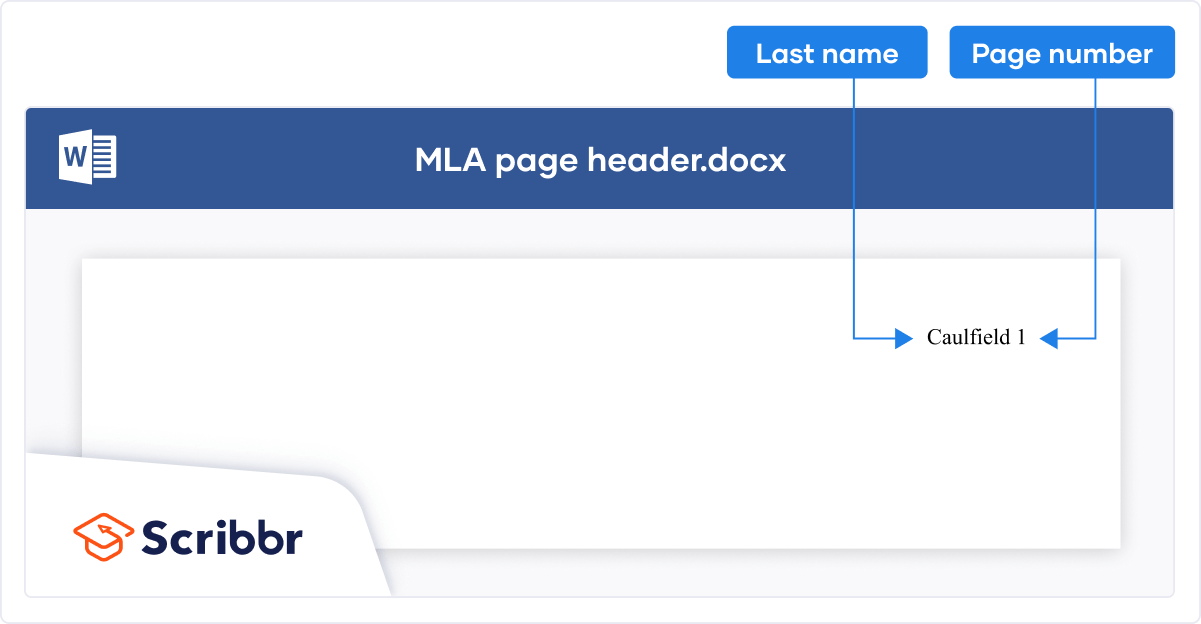
Works Cited page
MLA in-text citations appear wherever you refer to a source in your text. The MLA Works Cited page appears at the end of your text, listing all the sources used. It is formatted as shown below.
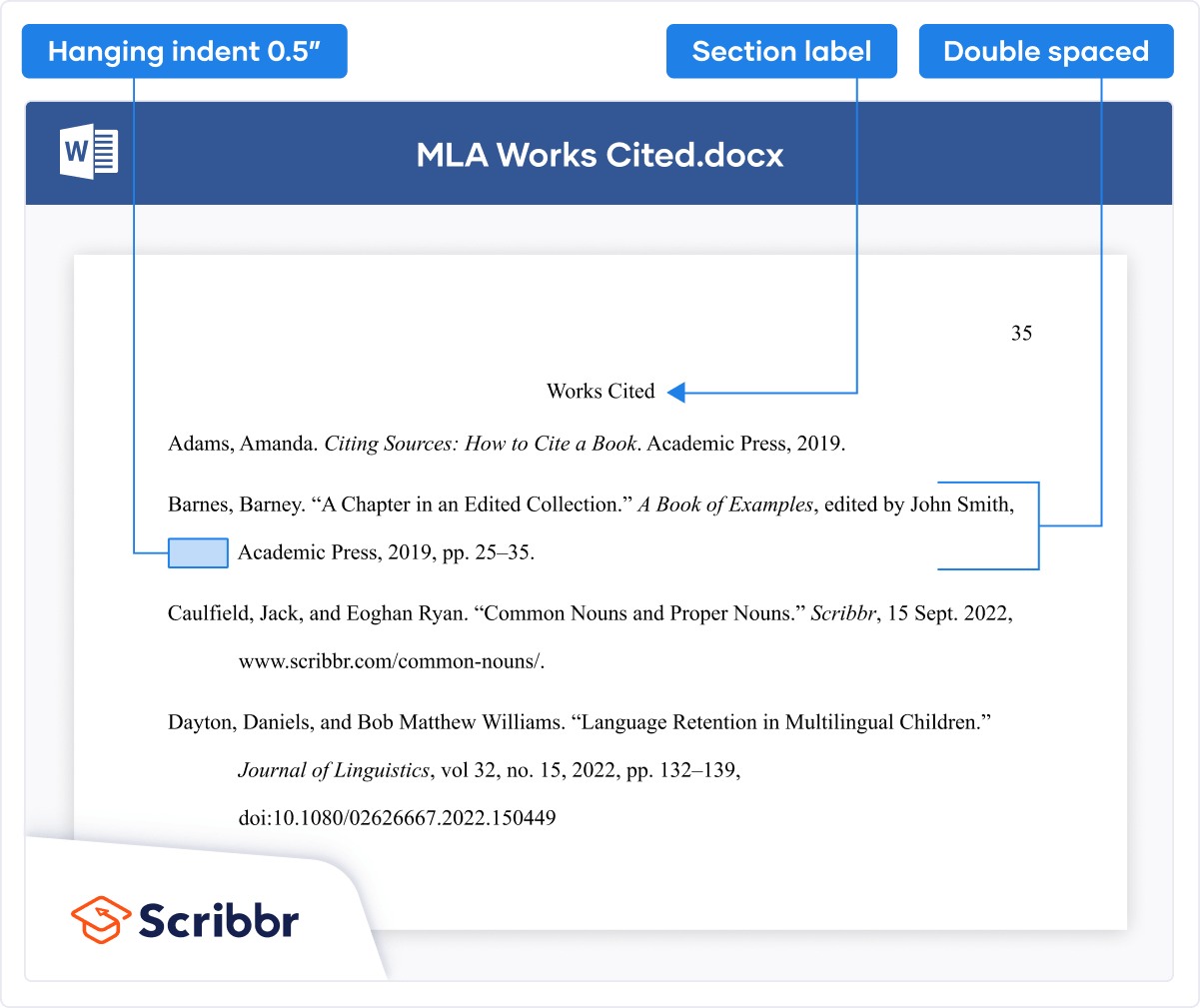
You can easily create your MLA citations and save your Works Cited list with the free MLA Citation Generator.
Generate MLA citations for free
The main guidelines for writing a paper in Chicago style (also known as Turabian style) are:
- Use a standard font like 12 pt Times New Roman.
- Use 1 inch margins or larger.
- Place page numbers in the top right or bottom center.
Chicago doesn’t require a title page , but if you want to include one, Turabian (based on Chicago) presents some guidelines. Lay out the title page as shown below.
Bibliography or reference list
Chicago offers two citation styles : author-date citations plus a reference list, or footnote citations plus a bibliography. Choose one style or the other and use it consistently.
The reference list or bibliography appears at the end of the paper. Both styles present this page similarly in terms of formatting, as shown below.
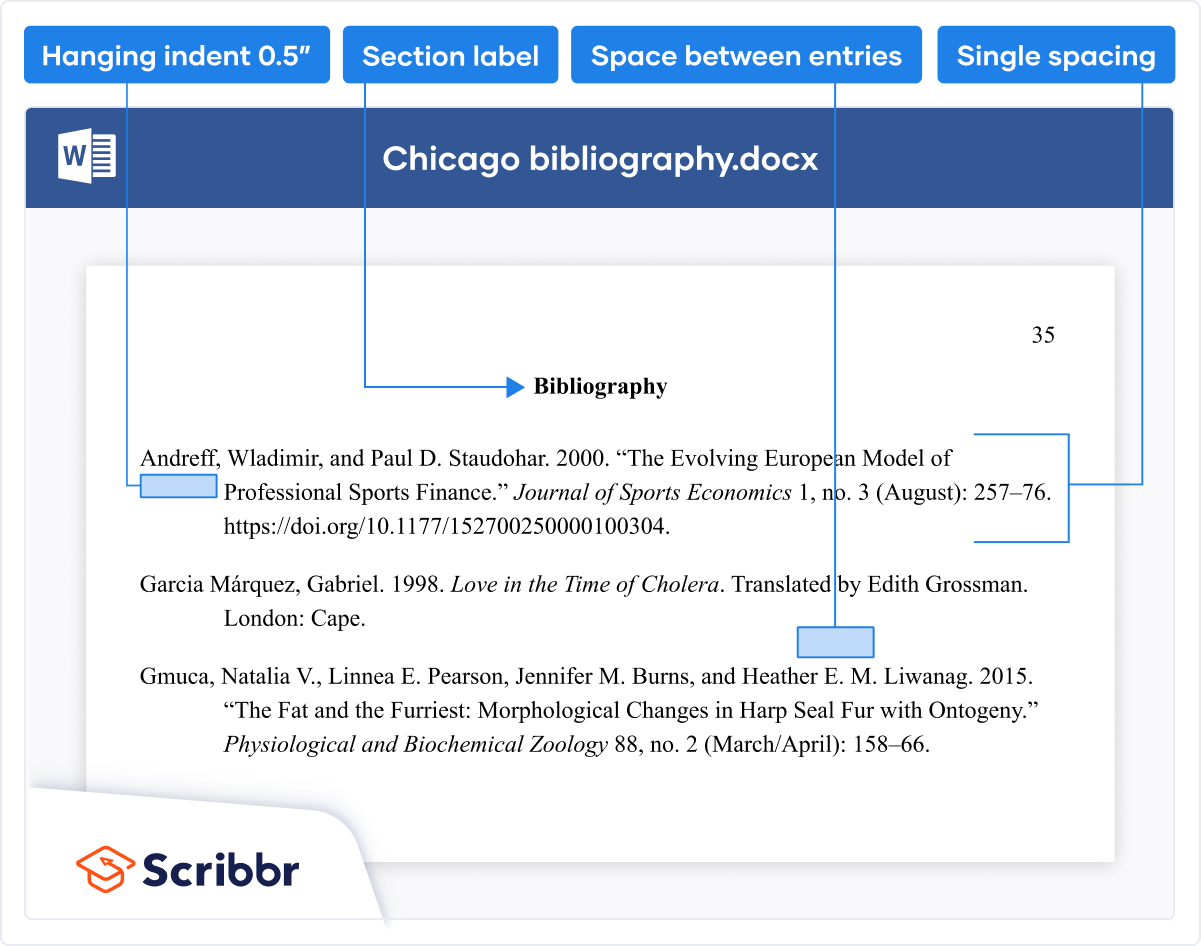
To format a paper in APA Style , follow these guidelines:
- Use a standard font like 12 pt Times New Roman or 11 pt Arial
- Set 1 inch page margins
- Apply double line spacing
- Include a title page
- If submitting for publication, insert a running head on every page
- Indent every new paragraph ½ inch
- Apply APA heading styles
- Cite your sources with APA in-text citations
- List all sources cited on a reference page at the end
The main guidelines for formatting a paper in MLA style are as follows:
- Use an easily readable font like 12 pt Times New Roman
- Include a four-line MLA heading on the first page
- Center the paper’s title
- Use title case capitalization for headings
- Cite your sources with MLA in-text citations
- List all sources cited on a Works Cited page at the end
The main guidelines for formatting a paper in Chicago style are to:
- Use a standard font like 12 pt Times New Roman
- Use 1 inch margins or larger
- Place page numbers in the top right or bottom center
- Cite your sources with author-date citations or Chicago footnotes
- Include a bibliography or reference list
To automatically generate accurate Chicago references, you can use Scribbr’s free Chicago reference generator .
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
Caulfield, J. (2023, January 20). Research Paper Format | APA, MLA, & Chicago Templates. Scribbr. Retrieved August 23, 2024, from https://www.scribbr.com/research-paper/research-paper-format/
Is this article helpful?

Jack Caulfield
Other students also liked, apa format for academic papers and essays, mla format for academic papers and essays, chicago style format for papers | requirements & examples, get unlimited documents corrected.
✔ Free APA citation check included ✔ Unlimited document corrections ✔ Specialized in correcting academic texts

Free Download
Research Paper Template
The fastest (and smartest) way to craft a research paper that showcases your project and earns you marks.
Available in Google Doc, Word & PDF format 4.9 star rating, 5000 + downloads
Step-by-step instructions
Tried & tested academic format
Fill-in-the-blanks simplicity
Pro tips, tricks and resources

What It Covers
This template’s structure is based on the tried and trusted best-practice format for academic research papers. Its structure reflects the overall research process, ensuring your paper has a smooth, logical flow from chapter to chapter. Here’s what’s included:
- The title page/cover page
- Abstract (or executive summary)
- Section 1: Introduction
- Section 2: Literature review
- Section 3: Methodology
- Section 4: Findings /results
- Section 5: Discussion
- Section 6: Conclusion
- Reference list
Each section is explained in plain, straightforward language , followed by an overview of the key elements that you need to cover within each section.
You can download a fully editable MS Word File (DOCX format), copy it to your Google Drive or paste the content to any other word processor.
download your copy
100% Free to use. Instant access.
I agree to receive the free template and other useful resources.
Download Now (Instant Access)

FAQs: Research Paper Template
What format is the template (doc, pdf, ppt, etc.).
The research paper template is provided as a Google Doc. You can download it in MS Word format or make a copy to your Google Drive. You’re also welcome to convert it to whatever format works best for you, such as LaTeX or PDF.
What types of research papers can this template be used for?
The template follows the standard best-practice structure for formal academic research papers, so it is suitable for the vast majority of degrees, particularly those within the sciences.
Some universities may have some additional requirements, but these are typically minor, with the core structure remaining the same. Therefore, it’s always a good idea to double-check your university’s requirements before you finalise your structure.
Is this template for an undergrad, Masters or PhD-level research paper?
This template can be used for a research paper at any level of study. It may be slight overkill for an undergraduate-level study, but it certainly won’t be missing anything.
How long should my research paper be?
This depends entirely on your university’s specific requirements, so it’s best to check with them. We include generic word count ranges for each section within the template, but these are purely indicative.
What about the research proposal?
If you’re still working on your research proposal, we’ve got a template for that here .
We’ve also got loads of proposal-related guides and videos over on the Grad Coach blog .
How do I write a literature review?
We have a wealth of free resources on the Grad Coach Blog that unpack how to write a literature review from scratch. You can check out the literature review section of the blog here.
How do I create a research methodology?
We have a wealth of free resources on the Grad Coach Blog that unpack research methodology, both qualitative and quantitative. You can check out the methodology section of the blog here.
Can I share this research paper template with my friends/colleagues?
Yes, you’re welcome to share this template. If you want to post about it on your blog or social media, all we ask is that you reference this page as your source.
Can Grad Coach help me with my research paper?
Within the template, you’ll find plain-language explanations of each section, which should give you a fair amount of guidance. However, you’re also welcome to consider our private coaching services .
Additional Resources
If you’re working on a research paper or report, be sure to also check these resources out…
1-On-1 Private Coaching
The Grad Coach Resource Center
The Grad Coach YouTube Channel
The Grad Coach Podcast
Sign in to your site’s geographic location:
3 tips to create outstanding clinical trial source documents.

Creating exceptional clinical research source documents is crucial for successful clinical trials. The best way to ensure that your source documents are ready is to know and understand the Clinical Study Protocol (CSP), which serves as the basis for your source documents. Use the CSP as your primary reference when creating source documents. This is because it explains exactly what happens in each step of the clinical trial.
First, read the CSP. Once you have a general idea of what each source document should contain, dig a little deeper. Use these three targeted sections in the CSP to gather crucial information needed to write an outstanding clinical trial source document.
1. Clinical Trial Participant Inclusion and Exclusion Criteria in your Source Documents

The Inclusion & Exclusion Criteria section has some of the most valuable information needed to reach study endpoints. It’s also included in every CSP. That said, write your source documents to collect information about a patient. You can ask them directly, or collect information through observation from clinical trial staff. To use this section of the CSP to your advantage, go through each of the inclusion and exclusion criteria and make sure that there is a question in your source documents that confirms each criterion.
For example, if an exclusion criterion in a particular study omits a patient who has a BMI that is >=31, include a BMI question in the demographic source document(s). Note that not all criterion are explicitly stated in the study activities portion of the CSP. So, if you want to make an extremely valuable source document, try writing a note in italics. Include the note under the question that states the exclusionary value and include the criteria number.
2. Use Study Endpoints in Your Source Documents

Study endpoints explain why the clinical trial is being conducted. Thus, during source document create, you’ll answer most of your questions in the study endpoints section of the CSP.
To create great source documents, use a similar process for study endpoints that you did for the inclusion and exclusion criterion. Find the endpoints in the CSP and look for specific values, queues, or milestones that need to be monitored throughout the study.
One way to include information about a desired outcome is to write a note about the endpoint in a source question. Or, you can include it in the “details and directions” section of a source document.
Here’s an example. One study endpoint measures a Six Minute Walk Test in patients at 30 days post treatment, and again at 1 year. Within the source, create a question that asks for the difference between the walk tests at the two dates. This allows the investigator or staff to assess if there is a change from baseline.
3. Don’t Forget Appendices & Tables in Your Clinical Trial Source
At this point, almost all of the source documents for the clinical trial have been written and polished. However, if you want to create a truly outstanding source document, don’t forget the appendices and tables section of the CSP.
The appendices and tables section may also include information to further explain the study, its purposes, and detailed information about specific procedures.
Here’s an example. An appendix in a study called Appendix B: Definitions offers all definitions for terms used throughout the study. Include these specific definitions within the relevant source document.
From the example above, in the Six Minute Walk Test (6MWT), include a definition from the appendices and table section, links to resources, and CSP reference information.
While there are many tricks to the trade, make sure you know what is being written, why, and for whom. Get to know the clinical study protocol to create great source documents.
Here at Team CRIO, our study designers create well-written digitized documents that include alerts for protocol and regulatory deviations. These handy features take the guesswork out of deviations and help to keep you organized. We strive to get the most out of a clinical study protocol in our eSource and CTMS digital documents.

21 CFR Part 11 Regulation Compliance Update
Compliance Update: The 21 CFR Part 11 Regulation is a cornerstone of conducting clinical trials in today’s world. The release of the regulation 1997 established guidelines for the use of electronic records and electronic signatures in FDA-regulated industries and had a significant impact on the pharmaceutical and medical device industries. The FDA began working on...

The Current State of Clinical Trials in Ukraine—A Conversation with Dr. Roman Fishchuk
Meet Dr. Roman Fishchuk, an esteemed otorhinolaryngologist (ENT) whose journey in the healthcare field has been marked by a commitment to providing medical care and innovative treatment options through clinical trials in his native Ukraine. Having graduated from Ivano-Frankivsk National Medical University, Dr. Fishchuk specialized in otolaryngology and completed a Master’s degree at the University...

Transitioning to CRIO? How to Get Your Data Ready for Migration
You have just signed on to use CRIO and you are excited to get started. Even if you are moving primarily from paper, you may have data that you want to migrate into CRIO rather than having to re-enter it. For many of our new clients, they have a wealth of information available in spreadsheets...
Get articles delivered to your inbox, every week
Forms, Tools, & Templates
Filter by Category(ies) Expand/Collapse Category Filter
- Budget/ Finance
- Communication
- Data Management
- OCR Applications
- Project Management
- Protocol Templates
- Recruitment
- Regulatory File/ Investigator Site File
- Source Documentation
- Study Start-Up
- Third Party/ Vendors
- Trial Documents
- Trial Master File
- Regulatory File
0">Matching of items
0 && show_pagination">Page of
There are no matching items
- Forms, Tools, & Templates Description Category(ies) Keyword(s)
- 02.04.02 Investigator's Brochure Addendum Log Track versions of the Investigator’s Brochure Trial Documents TrialDocuments
- 01.01.01 Work Instructions TMF - PennBox Instructions explaining training requirements, user roles, access, and use of the TMF in the Veeva Vault system Trial Master File TrialMasterFile Penn Box
- 01.01.01 Work Instructions TMF - Veeva Instructions explaining training requirements, user roles, access, and use of the TMF in the PennBox system. Trial Master File TrialMasterFile Veeva
- 01.02.03 Notice of Disclosure of FCOI Template for written disclosure of FCOI IND; IDE; Sponsor INDIDESponsor
- 01.02.03 Statement of Financial Interest - Sponsor Team Member Form to be used by Penn Faculty or staff of the Sponsor team to state presence/absence of financial interests for a particular study. Sponsor Sponsor
- 01.03.01 DSMB Charter Used to clearly describe how the DSMB will function, including frequency of meetings, data to be reviewed, how safety items will be communicated, etc. DSMB DSMB
- 01.03.02 DSMB Contact List Used to compile contact information for the DSMB members and their administrative support staff. May be useful as a reference during trial progress. DSMB DSMB
- 01.03.03 DSMB Meeting Minutes Used to document discussions during DSMB meetings. Includes documentation of attendance and disclosure of financial conflicts. DSMB DSMB
- 01.03.03 DSMB Outcome Email Letter Used to document the outcome of the DSMB meeting to provide to the PI(s). The PI may need this confirmation to provide to their IRB or other local regulatory review committee. DSMB DSMB
- 01.03.03_DSMB_Report Used to provide a summary of trial data to the DSMB. Specific section will differ by study, may be revised based on DSMB request. DSMB DSMB
- 01.03.05 DSMB Qualification Form Used to document potential DSMB member's agreement to serve as part of the DSMB. Includes description of confidentiality and disclosure of financial conflict of interest provisions. DSMB DSMB
- 01.05.01 Correspondence Log Template Log for documenting correspondence including the date, time, participants, and summary of discussion Project Management ProjectManagement
- 02.01.01 Investigator Brochure Template Guide for developing an Investigator's Brochure Trial Documents TrialDocuments Trial Document; Investigator; brochure
- 03.01.01 Expanded Access eIND Instructions for submitting an emergency IND request IND IND
- 03.01.01 Expanded Access sIND Instructions for submitting a (compassionate use) single patient IND request IND IND
- 03.01.01 IDE Application Template Original Investigational Device Exemption (IDE) template in accordance with 21CFR812.20 IDE IDE
- 03.01.01 IND Application Template Template for an application to the FDA for an Investigational New Drug (IND) IND IND
- 03.01.01 Submitting to the FDA This document describes the different way a submission can be sent to the FDA. It applies to clinical research (IND/IDE) and also to Expanded Access of drugs and devices. Sponsor Sponsor
- 03.03.02 IDE Progress Report Template Template for completing an IDE Progress Report IDE IDE
- 03.03.02 IND Annual Report Template Template for completing an IND Annual Report IND IND Investigator Annual Report
- 03.03.03 Closing an IND Guide to Sponsor How to discontinue an IND with the FDA. Definitions of the various IND statuses are included below for reference purposes. IND IND IND, Sponsor, FDA
- 03.04.01 Regulatory History Log Template for tracking all regulatory submissions of an IND/IDE IND; IDE; Sponsor INDIDESponsor
- 05.02.01 IB Signature Page This document tracks the Principal Investigator's acknowledgement of an Investigator Brochure for their IND study. IND IND Investigator Brochure, Principal Investigator, signature page, IND
- 05.02.02 PI Signature Page The document to be signed by the Principal Investigator of a study at each revision of the study's protocol. Protocol Templates ProtocolTemplates
- 05.02.07 PI Qualification Form Template for documenting the qualifications of a Principal Investigator Sponsor Sponsor
- 05.02.10 Site FCOI This work instruction assists the Regulatory Specialists with understanding what documentation is required to ensure the qualification of the sponsor team members and individuals acting as vendors and to determine how qualifications should be filed in the Trial Master File (TMF).The sponsor must ensure qualification of its team members and vendors. IND; IDE INDIDE
- 05.02.19 IDE Investigator Agreements The investigator agreement of compliance to all requirements of the investigational plan, IDE regulations, and other applicable regulations of the FDA for investigational devices. IDE IDE Statement,Investigator
- 07.01.01 Work Instructions - Pharmacovigilance Work instructions on sponsor pharmacovigilance (safety) management. This includes a discussion of the sponsor responsibilities related to pharmacovigilance, as well as instructions on conducting the activities, and additional reference resources. Sponsor Sponsor
- 07.02.02 SAE Form Form used to document an SAE that occurs in a trial and to report the relevant information to the sponsor. Trial Documents TrialDocuments
- 09.01.01 GLP Qualification Guide Guide to ensuring vendors providing investigational product are qualified and follow Good Laboratory Practices Third Party/ Vendors ThirdPartyVendors
- 09.02.03 Transfer of Obligations Guide to transferring IND/IDE sponsor obligations to another party IND; IDE INDIDE
- 21 CFR Part 11 for EMR Memo- Penn Medicine Electronic Health Records in Support of Clinical Research Communication; Study Start-Up CommunicationStudyStartUp part 11, 21 cfr part 11, EMR, compliance, pennchart
- CLIA, CAP, Lab References and Lab Director’s CV_HUP CLIA, CAP, Lab References and Lab Director’s CV. Documents obtained annually and posted with permission from Dept. of Pathology and Laboratory Medicine, Penn Medicine-HUP Regulatory File/ Investigator Site File; Source Documentation RegulatoryFileInvestigatorSiteFileSourceDocumentation CLIA, CAP, Lab References
- CLIA, CAP, Lab References and Lab Director’s CV_PRESBY CLIA, CAP, Lab References and Lab Director’s CV. Documents obtained annually and posted with permission from Dept. of Pathology and Laboratory Medicine, Penn Medicine-Presbyterian Regulatory File/ Investigator Site File; Source Documentation RegulatoryFileInvestigatorSiteFileSourceDocumentation CLIA, CAP, Lab References, Presby
- CRF Testing Script - Blank Template CRF Testing Script - Blank Template Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, sponsor, crf, crms, data management
- CRF Testing Tracker CRF Testing Tracker Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, sponsor, crf, crms, data management
- CRF build and Schedule of Events tracker CRF build and Schedule of Events tracker Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, sponsor, crf, crms, data management
- CRSPR Email Listserve Clinical Research Staff Portal and Registry (CRSPR) is an email listserve to communicate important, timely information to Penn Medicine's Clinical Research Professionals. Needs PennKey login. Sign up by creating a profile, to receive emails from OCR, IRB, CHPS, etc. OCR Applications OCRApplications CRSPR, listserve, application, email, portal, registry
- Case Report Form Amendments Log Track Case Report Form (CRF) versions and changes Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile CRF
- Clinical Research Resource Feasibility Assessment Budget/ Finance BudgetFinance
- Clinical Trial Protocol Template This protocol template is designed to help research teams develop a clinical trial protocol that includes an investigational intervention (drug, biologic, vaccine or device). Protocol Templates ProtocolTemplates
- Close Out Visit Checklist - Investigator Checklist for closing out a protocol Monitoring Monitoring
- Close Out Visit Checklist- Sponsor Investigator Checklist for closing out a protocol by a Sponsor or Sponsor Investigator Monitoring Monitoring
- Closing IDE Guide to Sponsor How to discontinue an IDE with the FDA. Definitions of the various IDE statuses are included below for reference purposes. IDE IDE
- Cosmos Access Form Complete this form to request access to EPIC/PennChart's COSMOS network for patient data. PennChart access is a pre-requirement. Data Management; Recruitment; Study Start-Up DataManagementRecruitmentStudyStartUp cosmos, patient data, pennchart, data
- Cost Finder Tool with research rates for both Hospital Billing (HB) and Professional Billing (PB), components of an item/service/procedure. Select at least one entity (HUP, PPMC, PAH OR CCH) prior to searching.Pricing may vary by entity. Ability to search by CPT code (preferred) or procedure name. OCR Applications; Study Start-Up OCRApplicationsStudyStartUp Cost Finder, finance, start up
- Data Safety Monitoring Plan (DSMP) With Guidance This template is intended to be used to develop a data safety monitoring plan. Monitoring Monitoring DSMP, monitoring, Data Safety Monitoring Plan
- Database Activation Authorization Database Activation Authorization Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, sponsor, crf, crms, data management
- Database Lockdown Checklist and CRMS Examples Database Lockdown Checklist and CRMS Examples Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, sponsor, crms, source
- Delegation of Responsibility and Signature Log Document study personnel and their role on the study, as delegated by the Principal Investigator. Also serves as a signature log to identify signatures on research forms. Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Device Investigational Plan Template Guide to developing an Investigational Plan for medical device research IDE IDE
- Device Product Information This guidance document assists US IDE, IND, and foreign clinical research device applicant holders in determining what product information is needed and where to place device product information. IDE IDE
- Electronic Data Management Tool This tool is used to assist the determination of what data management tool would be most beneficial for your research study. Budget/ Finance BudgetFinance
- Electronic Database Build and Activation Summary Plan Electronic Database Build and Activation Summary Plan Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation data management , sponsor, source documentation
- GLP Vendor Qualification Form Template to qualify non-clinical vendors Third Party/ Vendors ThirdPartyVendors
- Good Laboratory Practice (GLP) Qualification Guide Guide to ensuring vendors providing investigational product are qualified and follow Good Laboratory Practices IND; IDE; Sponsor INDIDESponsor Third Party/Vendors; Sponsor; Investigational Product
- ICH GCP Essential Documents Table of regulatory files that should be maintained by the PI and Sponsor throughout a clinical research study Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- IND Enabling Nonclinical Safety Study Work Instructions These work instructions are intended as guidance for personnel who (i) conduct or assist in the conduct of Nonclinical Safety Studies (NSS) to be submitted in support of an IND Application or (ii) have oversight of such activities. Although such studies usually occur prior to the submission of an IND, these work instructions also apply to NSS performed after IND approval and in support of clinical development. If an individual is subject to a FINANCIAL CONFLICT OF INTEREST (FCOI) management plan issued by the Vice Provost for Research, the conditions of the management plan will apply to the conduct of NSS. IND IND
- IND IDE Exemption Amendment Risk Assessment This form provides instructions, and may be used to document, the Sponsor Investigator’s risk assessment for minor changes to a protocol that has previously received an IND or IDE exemption determination. Protocol Templates; Sponsor ProtocolTemplatesSponsor
- IND-IDE Sponsor Responsibilities Guide Responsibilities of an IND/IDE Sponsor IND; IDE INDIDE
- Informed Consent Form (ICF) Version Log Tracking log of approvals for Informed Consent Form Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Investigational Product Accountability Study Log Study-level investigational product accountability log (all subjects on one log) Sponsor Sponsor
- Investigational Product Accountability Subject Log Subject specific investigational product accountability log Sponsor Sponsor
- Memo from Penn IRB Memo from Penn IRB regarding the IRB not disclosing names of its members. This is a necessary component of the IDE application. IDE IDE
- Monitoring Analysis Template This tool is intended to assist in the planning for monitoring a clinical trial. Monitoring Monitoring
- Monitoring Assessment Guidance Document This document is designed to assist study teams with completing an appropriate monitoring summary for the Penn IRB at the time of continuing review. Monitoring Monitoring
- Monitoring Close Out Visit Template Monitoring visit report template for the conduct of a close out monitoring visit at the end of a clinical trial. Monitoring; Sponsor; Trial Master File MonitoringSponsorTrialMasterFile
- Monitoring Findings Template Monitoring Findings Template Monitoring Monitoring findings, monitoring, site
- Monitoring Visit Report - Sponsor Summary Template for reporting monitoring findings to Sponsor Monitoring Monitoring
- Monitoring Visit Tracking Log Log used to document monitoring visits over the course of a trial Monitoring Monitoring
- Note to File Template To be used to create a Note to File which are written to identify a discrepancy or problem in the conduct of the clinical research study. Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- On-Site Query Report Form Worksheet for documenting the identification and resolution of queries Monitoring Monitoring
- Participant Visit Schedule Tool for tracking the proposed and actual dates for subject study visits Study Start-Up StudyStartUp
- PennCRMS CRF Guidelines and Tips PennCRMS CRF Guidelines and Tips Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, sponsor, crf, crms, data management
- PennChart - Patient Registration Form EMPI If you need your patient/subject setup in PennChart within 24 hours Budget/ Finance BudgetFinance
- Pre-Screen Phone Script (Incoming Call) Pre-screen phone script template for incoming calls from potential research participants IND; Recruitment; Regulatory File/ Investigator Site File INDRecruitmentRegulatoryFileInvestigatorSiteFile
- Pre-Screen Phone Script (Outgoing Call) Pre-screen phone script template for outgoing calls to potential research participants Recruitment; Regulatory File/ Investigator Site File RecruitmentRegulatoryFileInvestigatorSiteFile
- Principal Investigator (PI) Qualification Form Template for documenting the qualifications of a Principal Investigator Third Party/ Vendors ThirdPartyVendors PI; Qualification, principal investigator
- Principal Investigator Compliance Assessment (PICA) The Principal Investigator Compliance Assessment (PICA) is a tool which can be used to monitor or assess the overall conduct of a study. This document is required for all active, high risk studies conducted at Penn. Monitoring Monitoring
- Prospective Reimbursement Analysis/ Medicare Coverage Analysis - Budget Template Template for completing the prospective reimbursement analysis/ medicare coverage analysis and industry sponsored clinical research budget. Budget/ Finance; Regulatory File/ Investigator Site File BudgetFinanceRegulatoryFileInvestigatorSiteFile
- Prospective Study Design with no Investigational Product (IP) Template Use this template to develop a clinical research protocol that does not involve an investigational product. E.g. Comparative effectiveness study, a cohort design, case control study, etc. Protocol Templates ProtocolTemplates
- Protocol Adverse Event Log A Log for recording adverse events Regulatory File RegulatoryFile Adverse Event, Adverse, Event, Log
- Protocol Amendments Log Track versions of the IRB approved Protocol Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Protocol Deviation Log To track all protocol deviations. Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Protocol Training Log Log for documenting the training of research personnel on the research protocol Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- RBA Business Admin Approval Tipsheet Tipsheet for the RBA. Shows how to route request for RBN to your BA and OCR Finance for RBN creation Budget/ Finance; OCR Applications; Project Management; Study Start-Up BudgetFinanceOCRApplicationsProjectManagementStudyStartUp RBA, RBN, Research Billing application, Research Billing number, finance, BA
- RBA New Request Tipsheet Tipsheet with steps on how to submit a new request for a Research Billing Number in the Research Billing Application Budget/ Finance; OCR Applications; Project Management; Study Start-Up BudgetFinanceOCRApplicationsProjectManagementStudyStartUp RBA, Research Billing Application, Finance, RBN, billing number
- Recording, Assessment, and Reporting of Deviations This document aims to help teams with the recording of deviations and exceptions of an approved protocol, and the reporting requirements to the Penn IRB and Sponsor IND; IDE; Regulatory File/ Investigator Site File; Sponsor INDIDERegulatoryFileInvestigatorSiteFileSponsor
- Recruitment Letter - Physician to Established Patient Template for recruitment letter from an external, referring physician to his/her patient about a research study Recruitment Recruitment
- Recruitment Letter - Physician to Physician Letter Template Template for recruitment letter from one physician to another about a research study Recruitment Recruitment
- Recruitment Letter – PI to Patient Template Template for recruitment letter from the Principal Investigator to his/her patient about a research study Recruitment Recruitment
- Recruitment Letter- Physician to Unknown Potential Subject Template for recruitment letter from a physician to a potential research subject about a research study Recruitment Recruitment
- Reportable Event Form (Electronic) Form for capturing the details of a Reportable Event for informing a sponsor, manufacturer, or other reporting entity Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Reportable Event Form (Paper) Form for capturing the details of a Reportable Event for informing a sponsor, manufacturer, or other reporting entity Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Research Billing Application (RBA) All studies utilizing UPHS services/procedures and/or Imaging Core/Service Center(s) will need to be registered in the Research Billing Application (RBA), regardless of the payor (insurance or research grant). A Research Billing Number (RBN) will be generated for those studies where all or a portion of visits/tests/procedures associated with the research protocol are being billed to the research grant. OCR Applications OCRApplications RBA, billing, research billing application, hospital, services, grant, payor, insurance
- Residual Balance Transfer Request Form Form used to request the residual balance to be transferred from appropriate contracts Budget/ Finance BudgetFinance
- Retrospective Study Protocol Template This protocol template is designed to facilitate the creation of a retrospective clinical research protocol. Protocol Templates ProtocolTemplates
- Risk Assessment Template for performing a clinical trial risk assessment for monitoring Monitoring Monitoring
- Screening and Enrollment Log Document subjects who have been screened and/or enrolled Recruitment; Regulatory File/ Investigator Site File RecruitmentRegulatoryFileInvestigatorSiteFile
- Single Patient Emergency Use of an Investigational Product Guide to requesting and managing a single patient emergency use of an investigational product. IND; IDE; Sponsor INDIDESponsor
- Site Guidance for Source Documentation This document contains information about Source Documentation for clinical trials. Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Site Initiation Visit Checklist Checklist for conducting a Site Initiation Visit IND; IDE; Sponsor INDIDESponsor
- Site Qualification Report Tool for documenting the review and qualification assessment of a site and principal investigator Monitoring; Regulatory File/ Investigator Site File MonitoringRegulatoryFileInvestigatorSiteFile
- Site Visit Log Document the dates of Monitoring Visits Monitoring Monitoring
- Specimen Preparation Checklist Instructions for collecting, labeling and processing specimens for shipment Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Specimen Shipping Log Log the collection and shipment of specimens Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Sponsor - IDE Investigator List Template for the submission to the FDA-CDHR of the semi-annual list of investigators required by 21CFR 812.150 Monitoring; Regulatory File/ Investigator Site File MonitoringRegulatoryFileInvestigatorSiteFile
- Sponsor Monitoring Plan Guide Guide for developing an IND/IDE sponsor's monitoring plan IND; IDE; Sponsor INDIDESponsor
- Sponsor Welcome Letter Template for use in negotiating with an industry sponsor. Budget/ Finance BudgetFinance
- Study Admin File_Device Template for developing a study administration file or regulatory binder for a device trial Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Study Admin File_Drug Template for developing a study administration file or regulatory binder for a drug trial Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Study Admin File_Social Behavioral Template for developing a study administration file or regulatory binder for a social behavioral study Regulatory File/ Investigator Site File RegulatoryFileInvestigatorSiteFile
- Study Feasibility Assessment Tool for assessing the resources, recruitment potential, and logistical considerations of a particular study Study Start-Up StudyStartUp
- Subject Contact Information Sheet Collect contact information for research subjects Source Documentation SourceDocumentation
- Subject Eligibility Checklist Template for documenting review of Inclusion and Exclusion criteria Source Documentation SourceDocumentation
- Testing CRFs Overview in PennCRMS Testing CRFs Overview in PennCRMS Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, sponsor, crf, crms, data management
- Trial Master File Template for a Trial Master File Trial Master File TrialMasterFile
- Understanding Using an EDC - PennCRMS W Understanding Using an EDC - PennCRMS Workflows Data Management; Sponsor; Source Documentation DataManagementSponsorSourceDocumentation edc, crms, sponsor, source, crf
- W-9 IRS Form (No Form) Federal form used to report income paid to an individual Budget/ Finance BudgetFinance
- Zone 2 Core Document Change Control Sponsor checklist to document steps taken for an operationally compliant revision of core documents Sponsor Sponsor
- What is Translational Science?
- News & Events
- Michigan Institute for Clinical and Health Research at the University of Michigan
- Letters of Support
- All MICHR Support
Study Management Templates and Guidance
A guide to regulatory documentation and maintaining high standards in human participant research
What is this resource?
The study management templates are adaptable and downloadable templates that can be used to organize and maintain research study documentation as you would include in a regulatory binder.
The templates can also help you adhere to high standards of practice in the conduct of studies involving human participants. The study management templates are a University of Michigan resource available to all study team members.
These templates are designed to help meet requirements for FDA-regulated clinical trials. They may be useful, but not required, to organize study documentation for other studies as well. Please customize the templates to match your study-specific requirements. Guidance documents are also provided to assist you with study management.
How this advances translational science
Implementing operations that facilitate and support the quality and impact of research.
Translational Science Categories:
Designing a Study / Implementing a Study /
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Perspect Clin Res
- v.2(2); Apr-Jun 2011
Good documentation practice in clinical research
Chitra bargaje.
Department of Clinical Trials and Safety, Global Quality and Regulatory Compliance, Bristol Myers Squibb, Mumbai, India
One of the most common inspection findings in investigator site inspections is lack of reliable, accurate and adequate source documentation. This also happens to be the most common pitfall identified during sponsor audits. The importance of good documentation practice needs to be emphasized to investigator sites to ensure that the study results are built on the foundation of credible and valid data. This article focuses on the key principles of good documentation practice and offers suggestions for improvement.
INTRODUCTION
Inadequate/inaccurate case histories form the second most commonly cited deficiency in US-FDA inspections of clinical investigator sites.
Similarly, source documentation issues ranked 5th among the top 10 findings from European Medicines Agency (EMA) inspections of investigator sites in 2009[ 1 ] and in some instances the findings were classified ‘critical’. Not surprisingly, clinical trial monitors and auditors also report documentation issues as a frequent area of GCP concern.
I would like to share an experience at a recent investigator site audit.
During the audit opening meeting we were informed that all the source data is on paper and no electronic documentation is used. The site was actually using MS word to document the data collected during the study. In normal practice the site did not use MS word to generate medical records. This method was adopted only for clinical trial subjects. For the trial subjects there were no other hand-written progress notes which the site would normally use for routine patients.
There were two underlying potential issues here:
- First, the site was following a different practice for documenting progress for clinical research subjects. Were the subjects’ records missing any elements of standard care because of the deviation from routine practice?
- Second, the site thought they had no electronic documentation, although MS word was used to record all subject data.
This example, illustrates a common occurrence in clinical trial research where a lack of understanding of basic GCP principles may have a negative impact on the quality of the study.
WHAT IS THE PURPOSE OF SOURCE DOCUMENTATION?
To understand the importance of good source documentation we should first review the purpose of source documentation. The most important purpose of source documentation in a clinical trial is to reconstruct the trial as it happened. It should enable an independent observer to reconfirm the data. Documentation should be such that it is able to provide audit trail to permit investigation if and when required.
Source documentation is the medical record of the subject before, during and after the trial.
It is the tool which confirms the eligibility criteria of the subject in the given trial.
It documents the progress of the subject from consenting till the subject completes the study. It records the accountability of the investigational product dispensed, consumed and returned by the subject. It serves as the complete medical record of the subject as the reference to the treating physician at any point of time.
Finally it forms a strong foundation for the data that gets transcribed into a CRF which ultimately gets translated into a clinical study report.
Irrespective of clinical trial, accurate documentation supports the fundamental principle of protecting subject’s rights, safety and well-being.
There can not be two thoughts to emphasize the need for reliable and quality documentation.
PRINCIPLES OF GOOD DOCUMENTATION PRACTICE
So, what does it mean when we say ‘Good Documentation’ and how do we practice it?
Any basic training in clinical research will definitely include these phrases:
‘What is not documented is not done!’
‘Document what is done as well as what is not done!’
Roots of good documentation principles are in the ICH-GCP where source data and source document is first defined.
ICH E6 1.51 source data
All information in original records and certified copies of original records of clinical findings, observations, or other activities in a clinical trial necessary for the reconstruction and evaluation of the trial. Source data are contained in source documents (original records or certified copies).
The words in italics describe some inherent qualities of source data.
ICH E6 1.52 source documents
Original documents, data and records (e.g., hospital records, clinical and office charts, laboratory notes, memoranda, subjects’ diaries or evaluation checklists, pharmacy dispensing records, recorded data from automated instruments, copies or transcriptions certified after verification as being accurate copies, microfiches, photographic negatives, microfilm or magnetic media, X-rays, subject files, and records kept at the pharmacy, at the laboratories and at medico-technical departments involved in the clinical trial).
This definition describes the various types of documents which collectively form the source document.
Key attributes for good documentation were first described by US-FDA in the form of ALCOA -attributable, legible, contemporaneous, original and accurate. These are also adapted by World Health Organization (WHO). These criteria evolved with time. EMA has added some more ‘letters’ to describe qualities of good source documentation particularly for electronic documentation.[ 2 – 4 ]
Let‘s look at these attributes described by different authorities collectively.
Attributable
It should be clear who has documented the data.
Readable and signatures identifiable.
Contemporaneous
The information should be documented in the correct time frame along with the flow of events. If a clinical observation cannot be entered when made, chronology should be recorded. Acceptable amount of delay should be defined and justified.[ 4 ]
Original, if not original should be exact copy; the first record made by the appropriate person. The investigator should have the original source document.
Accurate, consistent and real representation of facts.
Long-lasting and durable.
Available and accessible
Easily available for review of treating physicians and during audits/inspections. The documents should be retrievable in reasonable time.
Complete till that point in time.
Demonstrate the required attributes consistently.
Based on real and reliable facts.
Corroborated
The data should be backed up by evidence.
Interestingly, it should be noted that the Drug Controller General India (DCGI) would emphasize on the condition in addition to the completeness, legibility and accessibility of investigator source data file as noted in DCGI’s guidance document for inspections.[ 5 ] My understanding of ‘condition’ is the state of the source documents, in terms of filing, storing and readability.
The degree to which the data fulfills the data quality criteria establishes acceptability of the data. It also determines the degree of excellence of the data quality. Qualities like consistency, credibility and corroboration help establish data integrity along with the data quality.
These are the expectations from clinical trial documentation however in reality many issues are observed in terms of quality of source documentation.
COMMON FINDINGS WITH RESPECT TO SOURCE DOCUMENTATION
‘Failure to maintain adequate and accurate case histories that record all observations and other data pertinent to the investigation on each individual administered the investigational drug or employed as a control in the investigation’ is cited in 6 out of the 10 warning letters issued by US-FDA to clinical investigators in 2010.[ 6 ]
At one investigator site source documents were not available because the computer ‘crashed’ . So in the absence of availability, adequacy of the records could not be evaluated. The investigator was warned for ‘failure to retain records required to be maintained for the required timeframe per regulations’ .
I would like to highlight some of the findings from the warning letters in detail here. These findings give an idea of regulatory expectations and lacunae in documentation noted during inspections. I am sure readers would be able to relate to some of these findings with their personal experience.
- Eligibility criteria could not be confirmed. For e.g., (a)IVRS user manual states “Complete call worksheets prior to contacting the IVRS; then file completed worksheets with each subject’s source documentation.” The IVRS worksheets were not kept in the subjects’ files or maintained at the site and as such it could not be confirmed that patients were stratified in the right arm and received the medication they were assigned to. (b) All the items in the exclusion criteria checklist are checked except for the exclusion criterion related to the history of thrombocytopenia, including heparin-induced thrombocytopenia, or a platelet count <100,000 cells/microliter. In the absence of lab report this exclusion criteria could not be confirmed on the basis of the incomplete checklists.
- Multiple records for same data points making it unable to determine which served as the accurate source record, for e.g., multiple versions of visual analog scales completed for same visit with different values.
- Discrepancies in records to confirm primary efficacy endpoint of the study, for e.g., the total administered dose of morphine, as reflected in hospital records was different from the Case Report Form. The primary efficacy endpoint of the protocol was to measure the reduction in the requirement for morphine use in the 24 hours following surgery measured by total morphine usage compared to placebo.
- Clinical significance for out of range lab values not documented on the lab reports or conflicting information found in the source documentation-e.g., significant high glucose value marked as clinically nonsignificant on the lab report although the subject was referred to for primary physician for further follow-up.
- Missing pages from subject interview scales, numerous unexplained corrections months after the initial entries and conflicting information; incorrect subject identifiers, incorrect date e.g., same date on screening visit, visit week 1 and week 4.
- Numerous AEs not reported in CRFs, delays in transcribing data in CRFs, discrepancies between source and the CRF. Lack of timely reporting of AEs in eCRFs jeopardizes subject safety and reliability and integrity of data captured at the site.
- Incorrect/incomplete documentation regarding the disposition of drugs-dates, quantity and use by subjects.
Although some of these issues may appear minor prima facie such as some checkboxes not checked, a lab report not marked for significance for out of range value, some discrepancies in source and CRF, unexplained corrections, these issues point toward lack of understanding of good documentation requirements. For an independent observer such data would fail to provide confidence and assurance of data quality and safety of the subjects enrolled. The data may be deemed unfit for use. All exposure of patients to new drugs and the efforts and time spent by the investigator team would be wasted.
Systematic deficiencies in documentation can lead to questions about the integrity of the data, potentially resulting in health authority decisions to exclude the data from analysis.
In essence, we can definitely say that the quality of documentation can make or break the study at a given site.
WHAT ARE THE POSSIBLE ROOT CAUSES FOR REPEATED DEFICIENCIES IN SOURCE DOCUMENTATION?
Clinical research documentation involves a variety of documents from various sources and is often completed by several people. Thus rendering this process to be complicated and posing challenges to meet requirements. Moreover clinical research happens over a long period of time which adds to the challenge of maintaining continuity in the documentation practice.
Inadequacies in documentation could be the result of lack of training and experience in good understanding of clinical research and documentation requirements. As a result the principal investigator (PI) and staff may continue documentation per the routine medical practice. In India, the documentation in routine medical practice may not be as extensive as what would be expected for clinical research.
Additional unmonitored medical records are discovered at the time of audits/inspections. Such as: Diaries of coordinator, inpatient records of the hospital, electronic records, etc., for the simple reason that the staff does not realize that these form a part of source record. These unmonitored records may have important data which do not find its way to the CRF. This would have an impact on the availability of important information in CRFs. Reliability and integrity of data might me affected as a result.
In many FDA warning letters one can observe that inadequate case histories, consenting or drug disposal records are often attributed to the lack of investigator’s supervision in ensuring compliance. The PI delegates responsibilities to the study team and may not provide adequate time to review the source data due to lack of time or commitment. The study documentation is completely left on the shoulders of study coordinator’s.
Various tools are used for data collection. At times sponsor provides source document worksheets to ensure complete documentation. If these worksheets are not designed accurately to align with the protocol and CRF source data quality is directly impacted. These worksheets are often completed as checkboxes without any additional notes, comments or supporting documents. Source document worksheets sometimes also result in multiple records. The sites continue to maintain the clinical practice routine documentation and worksheets are completed in addition for the study. As such, these worksheets are no longer a primary source and thus no source document.
Workload of the existing staff can be another important reason leading to poor documentation. This may cause errors like source data for one subject entered in another subject record, pages misfiled, use of incorrect consent forms and similar issues.
Study coordinator/PI work with various sponsors/CROs at a time. These different sponsors/CROs communicate different level of expectations regarding source documentation. If the site is not experienced enough and they do not have a standard procedure to follow they may get confused with variations in guidance they receive. This may negatively impact the quality of data.
Certain technical inadequacies may also lead to poor source documentation. For e.g., the ECG machine is old and does not print the date, time and subject identifiers, printer or fax machine does not work. If the fax is not working it may result in not receiving important data i.e., lab reports, data queries, investigational product allocation confirmations, SAE transmission confirmations, etc. Important email correspondence with sponsor/CROs if not printed and archived may get lost.
HOW CAN THE DOCUMENTATION BE IMPROVED?
Based on the various causes noted above, I would like to offer some suggestions to improve the quality of source documentation at sites.
- PI should delegate responsibilities to staff adequately trained in protocol and GCP. Particular training should be provided on ALCOA and other good documentation practice requirements. Medical decisions should be delegated to medically qualified staff. Training of site staff should be repeated at defined frequency. New hires should be adequately trained before trial participation.
- PI should commit for involvement, and supervision throughout the entire duration of the study. There should be an agreed and documented procedure for PI to ensure supervision of the study by meetings with site staff, monitors; review of documentation, timely resolution of medical, ethical or GCP issues. The PI or designated subinvestigators should validate the medical data. The PI should also supervise the work of SMO staff and external facilities if used. In case there are performance issues with SMO staff or external facility PI should immediately inform the supervisor as well as sponsor.
- Site should develop a SOP for good documentation. This SOP should be shared with the sponsor/CRO and agreed upon before the start of the trial. This SOP should address aspects including but not limited to consenting process, verifying eligibility, use of right tools such as diaries, source document worksheets, OPD papers, copies of prescriptions, etc; ways to avoid multiple records and in case of multiple records should define the source for the study, method of corrections, review of safety labs and other reports. Documented procedure at site level should encompass management, maintenance, archival and retrieval of source documentation. Sites should have measures for continuous improvement and maintaining high-quality data. Sites should develop process for quality control.
- Before the trial commences all technical aspects such as for e-CRFs, fax, printers, etc. should be clarified and issues resolved. In case of any difficulties during the trial, sponsor should be informed and back-up plans agreed upon till the issue is resolved. In case when original lab records or investigational records are sent to central location for assessment, process should be in place to ensure a duplicate copy or certified copy is available in the site source records.
- Sponsor/CRO also plays an important role in ensuring quality of source documentation. Sponsor/CRO should ensure PI’s commitment and involvement throughout the study. Sponsor/CRO should assess the site’s documentation practice during pre-study visit and during the study; provide training to the site staff to reinforce expectations. Time spent effectively during pre-study evaluation on source documentation would help a great deal to minimize documentation issues later. The source data and their respective capture methods should be clearly defined prior to trial recruitment i.e. in the protocol or study specific source data agreement.
CONCLUSIONS
Source documentation should demonstrate the ALCOA and other attributes as described by regulatory authorities and GCP. Source documentation related findings are the most commonly cited during inspections and audits. PI’s commitment and involvement in the trial makes a huge difference. Efforts to train the sites, understand the sites practices right from the pre-study visit and continuous monitoring and training would definitely help in improving and maintaining the quality of site source documentation practices.
Ultimately the source document should speak for itself. It should narrate the medical journey of the patient as it happened to an independent observer-an auditor or inspector and thus form a strong foundation for a good clinical research.
Acknowledgments
I would like to thank Jessica Parchman (Group Director) and Kristel Van De Voorde (Director) from Global Quality and Regulatory Compliance, Bristol Myers Squibb for reviewing the article and providing valuable suggestions in shaping this article.
Research Administration
Clinical research subject/source documentation templates, source document templates, regulatory documentation templates, other documentation.
Right-Side Nav

- Health Care
- UTMB Support Areas
Documentation of Study Visit Interventions/Observations
The purpose of source documentation is to reconstruct the research study as it happened. It should allow for data to be confirmed, validated, and support the fundamental principle of protecting the participant’s rights, safety and well-being.
These templates are intended to assist in documenting study procedures, interventions, observations, etc.
Below are several examples that can be customized to meet your specific study needs/requirements.
Best Practice Considerations
The research record should contain documentation of each participant’s study involvement from the time they sign consent through to whatever point they complete participation.
- Anything not completed should include record of why and be signed and dated by the person completing the note.
- Attaching a note to file or documenting in a running note missing items, dates, incomplete questions, missing assessments, etc., is another way to document why a record is incomplete, but the note to file entry must also be signed and dated.
- With electronic study documentation, consider creating a data field to capture any general notes about why study interventions were not completed or were partially complete
Hardcopy source documents/data collections forms should contain at the very least the HRPO#, participant ID, and study visit date on each loose page.
Research team members should document each task they perform.
Screening/Enrollment
- Demographics and Contact Form (DOC)
- Including Health History (DOC)
- Without Health History (DOC)
- Guide to Using the Screening & Enrollment Log (DOC)
- Screening & Enrollment Log Template (XLS)
- Subject Contact Information
Study Visits/Phone Visit Documentation
- Urine Pregnancy Test Record
- Vital Signs CRF Example 1
- Vital Signs CRF Example 2
- Vital Signs CRF Example 3
- Multiple Visits
- Single Visit
- End of Study/Early Termination Visit Source Document
- First Dose Documentation
- Follow-up Phone Call
- Telephone Communication Record
- Inclusion-Exclusion Checklist
- Inclusion-Exclusion Checklist with Interview Section
- Medical History
- Phone Screen Checklist
- Physical Exam Form Example 1
- Physical Exam Form Example 2
- Study Visit Source Document
- Study Specific Source Document Template
- Protocol Deviation Form
Memos/Notes to Files
- Action Form
- Note to File Example 1
- Note to File Example 2
- Note to File Example 3
- Running Note
- Correspondence (Research Team/Participant)
- COVID-19 Note to File
Adverse/Serious Adverse Event Documentation
- Adverse Event Example Template
- Adverse Event Tracking Log
Medication Logs
- Concurrent Medications CRF – Extended Version
- Concurrent Medications Sheet Example 1
- Concurrent Medications Sheet Example 2
- Daily Temperature Log
- Deviation Log
- Device Dispensing Log
- Drug Dispensing Log
- PI Delegation Log
- Receipt Log
- Specimen Collection Log
- Specimen Shipping Log
- Study Device Accountability Log
- Study Drug Accountability Log
- Study Personnel Log
- Study Team Contact Log
- Telephone Call Log
- Screening and Enrollment Log Guide and Template

- What is the UNC Scientific Review Committee (SRC)?
- The SRC Mission and Scope
- How Does the SRC Review Process Work?
- When is SRC Review Required?
- Protocol Development Tips and Resources
- Committee Members
- Required Costs
- Budget Development Tools
- Clinical Trial Registration Overview and UNC Policy Statement
- Registering an Investigator-Initiated Clinical Trial Overview
- Trial Registration Highlights
- ICMJE Data Sharing Statement Guidance
- ClinicalTrials.gov Protocol Registration and Results System
- Ongoing Record Maintenance Requirements
- Informed Consent Posting Requirements
- Additional Resources
- How Do I Request a Coverage Analysis?
- How Can I Find Research Costs For A Grant Proposal?
- Linking Subjects in Epic
- Quality Assurance Reviews
- FDA Inspections
- Sponsor Audits
- Monitoring Letter Review
- Clinical Research Updates April 2021
- CITI Training
- Conflict of Interest Training
- LMS: CRMS & BCA Training
- Network of Research Professionals 🡥
Forms & Templates
- Billing Coverage Analysis Resources
- Compliance Checks for PS Proj ID Setup
- Research Participant Reimbursement Information
- Protocol Builder
- Subject Injury Language for Informed Consent Forms
- Adverse Event Log
- Close-out Checklist
- Concomitant Medication/Therapies Worksheet
- Delegation of Responsibility Log
- Deviation Log
- Device Accountability Log
- Eligibility Checklist Template
- Enrollment Log
- FDA Part 11 Certification
- Feasibility Assessment
- Good Clinical Practices Checklist
- Guidance for Completing a Supervisory Plan for Clinical Research
- Informed Consent Process and HIPAA Authorization Documentation
- Inventory List for Study Storage Documents
- Master Subject ID Log
- Comprehensive Monitoring Plan Template
- Concise Monitoring Plan Template
- Monitoring Visit Log
- Participant Enrollment Documentation
- PI Supervisory Plan for Clinical Research
- Protocol/Amendment Tracking Log
- Data Managment
- Informed Consent Process
- Investigational Product (IP) Accountability Standard Operating Procedure and Work Instructions
- Management and Reporting Adverse Events
- Study Start-Up Checklist
- Training Log
- Transfer of Subject Checklist
Forms, Tools & Templates
Start-up forms.
- Intake Form for New Studies
- MTA/DUA Form
- Amendment Intake Form
- Conflict of Interest Form
- Epic Access for Study Monitors Request Form
- Cosmos Access Request Form
- SharePoint Form - If you are requesting data from Epic, please complete this form (Coming soon)
Source Document Templates
- Adverse Event (AE) Log
- Audit Follow-Up Report Template
- Deviation Log
- Device Accountability Log
- Delegation of Authority (DOA) Log
- Enrollment Log
- ICF Checklist
- Medical History
- Monitor Visit Log
- Note to File
- Phone Contact Form
- Regulatory Binder Checklist
- Serious Adverse Event (SAE) Log
- Sample Tracking Log
- Screening Log
- Subject Communication Log
- Training Log
Here’s how you know
- U.S. Department of Health and Human Services
- National Institutes of Health
Required Templates
Access study document templates required in the NCCIH clinical study review process.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Required Protocol Templates
| [80KB Word file] | Recommended Protocol Template for NCCIH-funded clinical studies |
| [377KB Word file] | Optional Clinical Trial Template for Behavioral and Social Sciences Interventions |
| [356KB Word file] | Optional IND/IDE Clinical Trial Protocol Template |
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Other Required Templates
| [123KB Word file] | Provides a recommended structure for a data and safety monitoring plan. Includes example text, instruction, and sample data tables, as well as a report template. |
| (60KB Word file) | Includes sample text and instructions. |

COMMENTS
Study Documentation Templates and Tools. During planning and operationalization stages, before enrollment starts, research teams should discuss what forms will be needed for their study. These could be data collection forms, source documentation, even forms or logs for study management like visit checklists and progress notes.
Find free and adaptable templates and tools for various aspects of clinical research, such as protocols, consent forms, logs, agreements, and more. Browse by category or search by keyword to access the resources shared by other groups and Global Health Trials.
The purpose of the NIA Clinical Research Toolbox is to provide a Web-based informational repository for investigators and staff involved in clinical research. The Toolbox contains templates, sample forms, guidelines, regulations and informational materials to assist investigators in the development and conduct of high quality clinical research ...
The NCCIH Clinical Research Toolbox provides a web-based information repository for investigators and staff involved in NCCIH-funded clinical research. The Toolbox is a one-stop shop for required templates, sample forms, and information materials to assist clinical investigators in the development and conduct of high-quality clinical research ...
All clinical research starts with the research protocol, a document that details all aspects of the trial: its background, rationale, objectives, design, methodology, statistical analysis plan, and organization.With the protocol, you can make sure you protect the participants and collect the data. Using protocol templates, you can start thinking through what you need to meet compliance ...
In cases where institutions provide research teams with institution-specific templates and forms for clinical research documentation, NIMH expects researchers to follow their institutional policies for document use. ... source documents, and drug accountability records, as applicable, may be reviewed for compliance with applicable Federal ...
Learn how to format a research paper in APA, MLA, or Chicago style with free templates and guidelines. See examples of title pages, headings, citations, and reference pages for each style.
Research Paper Template. The fastest (and smartest) way to craft a research paper that showcases your project and earns you marks. Available in Google Doc, Word & PDF format. 4.9 star rating, 5000+ downloads. Download Now (Instant access)
So, if you want to make an extremely valuable source document, try writing a note in italics. Include the note under the question that states the exclusionary value and include the criteria number. 2. Use Study Endpoints in Your Source Documents. Study endpoints explain why the clinical trial is being conducted.
The Toolbox is a web-based information repository for investigators and staff involved in NCCIH-funded clinical research. You can find it under our "Grants and Funding" section. This resource serves as a one-stop shop for required templates, sample forms, FAQs, and policies. The materials are available to assist clinical investigators in ...
Forms, Tools, & Templates Description Category(ies) Keyword(s); 02.04.02 Investigator's Brochure Addendum Log Track versions of the Investigator's Brochure Trial Documents ; 01.01.01 Work Instructions TMF - PennBox Instructions explaining training requirements, user roles, access, and use of the TMF in the Veeva Vault system Trial Master File Penn Box; 01.01.01 Work Instructions TMF - Veeva ...
Source Documents. Source documents must follow the requirements detailed in the Source Documentation section of the DAIDS SCORE Manual. The examples below describe acceptable clinical trial source documents. All source documentation may either be handwritten using dark (blue or black) ink, typed, or computer generated.
The study management templates are a University of Michigan resource available to all study team members. These templates are designed to help meet requirements for FDA-regulated clinical trials. They may be useful, but not required, to organize study documentation for other studies as well. Please customize the templates to match your study ...
Roots of good documentation principles are in the ICH-GCP where source data and source document is first defined. ICH E6 1.51 source data All information in original records and certified copies of original records of clinical findings, observations, or other activities in a clinical trial necessary for the reconstruction and evaluation of the ...
Clinical Research Subject/Source Documentation Templates. Source Document Templates. Regulatory Documentation Templates. Other Documentation. Resources. OCR master. Adverse Event (Grading) Log. ... Research Quick Find Tool. Right-Side Nav. Research Experts. Funding Institutional. ECRT. PeopleSoft. infoEd. OnCore. Close Off-Canvas Mobile Menu ...
HIPAA Consent Template: Authorization Language for Research Use HIPAA Highlights for Researchers ... Compliments of Mountainside MD Press and Conducting Clinical Research. 2 Informed Consent Form Requirements Checklist Informed Consent Form Template ... information from your medical records or source documents when you Þll out a CRF ...
Multi-site Appendix G-1: Demographics Form. Multi-site Appendix G-2: Medical History Form. Multi-site Appendix G-3: Prior and Concomitant Medications Form. Multi-site Appendix G-4: Vital Signs Form. Multi-site Appendix G-5: Study Disposition Form. Multi-site Appendix H: Sample Clinical Trial Closeout Procedures.
The purpose of source documentation is to reconstruct the research study as it happened. It should allow for data to be confirmed, validated, and support the fundamental principle of protecting the participant's rights, safety and well-being. These templates are intended to assist in documenting study procedures, interventions, observations, etc.
Device Accountability Log. Eligibility Checklist Template. Enrollment Log. FDA Part 11 Certification. Feasibility Assessment. Good Clinical Practices Checklist. Guidance for Completing a Supervisory Plan for Clinical Research. Informed Consent Process and HIPAA Authorization Documentation. Inventory List for Study Storage Documents.
Phone Contact Form. Regulatory Binder Checklist. Serious Adverse Event (SAE) Log. Sample Tracking Log. Screening Log. Subject Communication Log. Training Log. Start-Up Forms Intake Form for New Studies MTA/DUA Form Amendment Intake Form.
Creating templates for source documents, Using an electronic Case Report Form (eCRF) as a source document, ... Source documents at a clinical trial site may include the participant's research, clinical, hospital, institutional, and/or medical office records. These records may be maintained in paper or electronic format
Institute for Clinical and Translational Research. 4240 Health Sciences Learning Center. 750 Highland Avenue Madison, WI 53705. Email: [email protected]. Phone: (608) 263-1018. The Institute for Clinical and Translational Research is supported by the Clinical and Translational Science Award (CTSA) program, the National Center for Advancing ...
Required Protocol Templates. NCCIH Protocol Template [80KB Word file] Recommended Protocol Template for NCCIH-funded clinical studies. Protocol Template for Behavioral & Science Research [377KB Word file] Optional Clinical Trial Template for Behavioral and Social Sciences Interventions. NIH-FDA Phase 2 and 3 Protocol Template [356KB Word file]