An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Plants (Basel)


Current Progress in Nitrogen Fixing Plants and Microbiome Research
Kishan mahmud.
1 Center for Applied Genetic Technologies, University of Georgia, Athens, GA 30602, USA; [email protected]
Shiva Makaju
2 Department of Crop and Soil Sciences, University of Georgia, Athens, GA 30602, USA; ude.agu@ujakamhs
Razi Ibrahim
3 Institute of Plant Breeding, Genetics and Genomics, University of Georgia, Athens, GA 30602, USA; [email protected]
Ali Missaoui
In agroecosystems, nitrogen is one of the major nutrients limiting plant growth. To meet the increased nitrogen demand in agriculture, synthetic fertilizers have been used extensively in the latter part of the twentieth century, which have led to environmental challenges such as nitrate pollution. Biological nitrogen fixation (BNF) in plants is an essential mechanism for sustainable agricultural production and healthy ecosystem functioning. BNF by legumes and associative, endosymbiotic, and endophytic nitrogen fixation in non-legumes play major roles in reducing the use of synthetic nitrogen fertilizer in agriculture, increased plant nutrient content, and soil health reclamation. This review discusses the process of nitrogen-fixation in plants, nodule formation, the genes involved in plant-rhizobia interaction, and nitrogen-fixing legume and non-legume plants. This review also elaborates on current research efforts involved in transferring nitrogen-fixing mechanisms from legumes to non-legumes, especially to economically important crops such as rice, maize, and wheat at the molecular level and relevant other techniques involving the manipulation of soil microbiome for plant benefits in the non-legume root environment.
1. Introduction
A healthy, functioning soil ensures nutrient cycling for optimum plant growth for agricultural production [ 1 ]. However, agricultural productivity is often limited by available soil nutrients, especially nitrogen [ 2 ]. Nitrogen is not present in soil parent material despite the fact that nitrogen content in the atmosphere is highest among all the atmospheric gases [ 3 ]. Hence, soil nitrogen input for plant nutrition and crop productivity largely depends on organic matter degradation, synthetic fertilizer applications, and biological nitrogen fixation (BNF) via nitrogenase enzyme activity [ 4 , 5 ]. This limited bio-availability of N and the escalating reliance of crop growth on N have created a colossal N-based fertilizer industry worldwide [ 6 , 7 ]. Nitrogenous fertilizer production currently represents a significant expense for the efficient growth of various crops in the developed world. Synthetic N fertilizers are currently used in grain, grass, and fruit productions (about 60% for cereals and 10% with irrigated rice production) [ 8 ]. More than 50% of the applied N-based fertilizer is used by the plants and the remaining can be subjected to losses like surface runoff and leaching leading to nitrate contamination of soils and groundwater. In terms of energy efficiency, moreover, manufacturing nitrogen-based fertilizers requires six times more energy than that needed to produce either phosphorous or potassium based fertilizers [ 9 ]. Therefore, reducing dependence on nitrogenous fertilizers in agriculture in the developed world and developing countries may lead to potential gains in an agricultural setting. Biological nitrogen fixation (BNF) in economically important food and forage crops [ 10 ] has drawn attention to achieve sustainable agricultural goals in both both hemisphere of the world [ 11 ]. In livestock production systems in southeastern USA, strategically planting nitrogen-fixing legumes in cattle pastures has shown to increase the available soil nitrogen [ 12 ], thereby reducing the need to apply synthetic nitrogen sources. The diazotrophic microorganisms from bacteria or archaea domains are responsible for BNF and only some prokaryotes are able to use atmospheric nitrogen through BNF by encoding nitrogenase, an enzyme that catalyzes the conversion of N 2 gas to ammonia (NH 3 ) [ 8 , 13 , 14 ]. Despite the phylogenic and ecological diversity among diazotrophic bacteria and their hosts, a synchronized interaction is always a prerequisite between the microbial entities and the host plant to achieve a successful nitrogen fixation system. The importance of this process is enormous as it reduces the dependence on nitrogen fertilizers for plants and thus, for agriculture overall. It has been estimated that worldwide, biological nitrogen fixation produces roughly 200 million tons of nitrogen annually [ 15 , 16 ]. In fact, nearly 50% of the total nitrogen in crop fields is the contribution of BNF by diazotrophic bacteria of the total biosphere nitrogen [ 17 ]. Moreover, fixed nitrogen can also be transferred to intercropped non-legumes in the case of mixed cropping systems, such as the soybean–wheat system, or the next season crops in crop rotation [ 18 ]. In this review article, we explore current developments concerning the limitations and potential promises of nitrogen fixation in legumes and non-legumes.
2. Biological Nitrogen Fixation (BNF)
Nitrogen fixation is a dynamic and high energy demanding process [ 19 ]. The pathway for the biological reduction of inert N 2 into the reactive compound NH 3 (ammonia) under micro-aerobic conditions is as follows:
Free-living diazotrophs correspond to a small fraction of the plant rhizospheres ecosystem, and they belong to alphaproteobacteria ( Rhizobia , Bradyrhizobia , Rhodobacteria ), betaproteobacteria ( Burkholderia , Nitrosospira ), gammaproteobacteria ( Pseudomonas , Xanthomonus ), firmicutes, and cyanobacteria [ 20 ]. However, their presence, function, and importance can be explained by the “black queen” hypothesis which predicts that in free-living microbial communities, only a few “helpers” that carry the heaviest weight in terms of functions, such as high energy-requiring nitrogen fixation, support the rest of the flora and fauna population or the “beneficiaries” that rely on the “helpers” or the “beneficials” for nitrogen needs [ 21 ].
The symbiotic relationship between soil bacteria, collectively known as rhizobia (which includes the genera Rhizobium , Bradyrhizobium , Mesorhizobium , and Sinorhizobium ), and legume roots generates nodules (a new differentiated special organ) that fix atmospheric nitrogen through the action of the nitrogenase enzyme [ 22 ]. BNF by plants and its bacterial associations represent an important natural system for capturing atmospheric N and processing it into a reactive form of nitrogen through enzymatic reduction. BNF is considered an extremely sensitive process influenced by nutrient and environmental conditions and enables a plant to supply all or part of its requirements through interactions with endo-symbiotic, associative, and endophytic symbionts, thus offering a competitive advantage over any non-nitrogen-fixing plants [ 15 , 23 , 24 , 25 , 26 ]. The highly conserved nitrogenase complex in free-living and symbiotic diazotrophs enables them to participate in various types of associations/interactions with their host plants. BNF by plant–rhizobia symbiotic systems is mediated by endosymbiotic interaction when plants develop root nodules; in legumes and rhizobia, gram-negative alpha proteobacteria are the most common microbial species that associate (endo-symbiotic interaction) with legumes of the Fabaceae (Papilionaceae) family [ 27 , 28 , 29 ]. Actinomycetes such as the Parasponia species (family Cannabaceae) and Frankia sp. that associate with a broad spectrum of actinorhizal plants are well documented in nitrogen fixation as well [ 8 ]. Cyanobacteria (mainly Nostoc sp.) have also been found to colonize different plant organs, either intracellularly in the family Gunneraceae or extracellularly in Azolla , Cycadaceae , liverworts and hornworts. Associative nitrogen fixation (ANF) and/or endophytic symbioses are often observed among diazotrophs, such as Azospirillum spp., Azoarcus spp. and Herbaspirillum , with a wide variety of plant roots including cereals. The nitrogenase protein, as well as the associated proteins and non-proteins forming nitrogenase enzyme, are sensitive to the presence of oxygen [ 30 ]. For this extreme sensitivity to oxygen, obligate anaerobes such as Clostridium pasteurianum are ideal candidates for nitrogen fixation; however, facultative anaerobes such as Klebsiella oxytoca are also capable of fixing nitrogen but only when the oxygen is absent in the system [ 31 ]. Obligate aerobes, such as Azotobacter vinelandii can also shield nitrogenase from oxygen and perform nitrogen fixation by consuming oxygen via cytochrome oxidases [ 31 , 32 ].
2.1. The Nitrogenase Protein and Nodule Formation
As mentioned earlier, a protein complex called nitrogenase (composed of enzymes with metal co-factors) makes nitrogen fixation possible in plants. The first one is dinitrogenase and the second one is dinitrogenase reductase [ 33 ]. According to the active site co-factor binding metal, there exist three types of dinitrogenase in nature. (a) Molybdenum (Mo) nitrogenase; it is most abundant and carries the most significance in the nitrogen-fixing bacterial and archaeal niche and the alternative vanadium (V) and iron-only (Fe) nitrogenases [ 34 ]. The molybdenum dependent dinitrogenase is formed by nifD and nifK gene products and dinitrogenase reductase is a homodimer of the nifH gene product [ 30 , 35 ]. It is well documented that molybdenum nitrogenase is produced in all diazotrophs in nature, while some produce the vanadium or iron nitrogenase addition to Mo-nitrogenase [ 36 , 37 ]. The rhizobium bacteria residing in nodules fix atmospheric nitrogen gas to NH 3 , which plants can assimilate via glutamine synthase to form glutamine. In response, the bacteria derive plant carbohydrates, mainly as malate for food and an energy source for nitrogen fixation. Nodules are very complex structures, containing several processes which operate and interact at distinct levels. The process of nodule formation requires a coordinated exchange of signals between the two symbiotic partners [ 38 ]. Bacteria had their symbiotic genes first characterized by transposon mutagenesis; this achieved the definition of over 50 nodulation genes (Nod and Nol) in bacteria, and about the same number controlling nitrogen fixation; thus many nod- and fix-bacterial strains exist in many species of rhizobia. Legume–rhizobium symbiosis starts with molecular signaling between the two partners. Early nodulation gene cascades in legumes. Plants release signals such as flavonoids (e.g., the flavone 7,4 dihydroxyflavone and the isoflavone genistein) which are picked up by compatible bacteria in the rhizosphere [ 39 , 40 ] leading to the production of Nod factors (NF) which trigger early events in the nodulation process [ 41 , 42 ]. This triggers the downstream gene cascade including those involved in nucleoporin, cation channels, calcium spiking, early nodule expression, and cytokine signaling leading to cortical and pericyclic cell divisions, and concomitant bacterial infection. Rhizobia are entrapped by root hair curling after the Nod factor has been perceived, which results in initiating the formation of infection thread (a tubular structure). This infection thread facilitates the penetration of root hair cells and adjacent cortical cells [ 43 ]. Cell divisions in cortical and pericycle occur simultaneously resulting in the formation of the nodule primordium. Bacterial cell division facilitates the rhizobial traveling through the infection thread and is eventually freed into the induced nodule primordium cells [ 44 , 45 ]. As nodules mature with time, bacteria are enclosed within the symbiosome membrane, resultant from an inverted plasma membrane of plant origin. In this encapsulated chamber, the bacteria experience a micro-aerobic environment (lower oxygen concentration) and differentiate into bacteroids, fixing diffused nitrogen gas using their nitrogenase enzyme complex [ 46 , 47 ]. Depending on whether or not the meristem remains active for the life of the nodule, two main types of nodules are formed on the various legume species, (i) indeterminate or (ii) determinate. In the case of determinate nodules, nodular meristematic activity is terminated early and is usually initiated sub-epidermally in the outer cortex, thus giving rise to spherical nodules [ 48 ]. In indeterminate nodules, the inner cortex undergoes cell division (anticlinally) followed by periclinal divisions in the pericycle. Here, cylindrical nodules are formed due to more persistent meristems [ 49 , 50 ].
2.2. Genes Encoding Nitrogenase Enzyme
The understanding of the genetic basis of this relationship is of paramount importance and essential for the optimization of nitrogen acquisition rates in legumes themselves. Bacterial nif genes are well known to encode the components of the nitrogenase enzyme complex. nifH , nifD , and nifK genes encode the structural subunit of di-nitrogenase reductase and the 2 subunits of di-nitrogenase, respectively. Many rhizobial genes have been fully sequenced, for instance, Mesorhizobium loti , Sinorhizobium meliloti , and Bradyrhizobium japonicum [ 51 , 52 , 53 ]. These proteins have similar sequences and common structures and functions in many diazotrophs, for instance, Azotobacter vinelandii , Herbaspirillum seropedicae , Pseudomonas stutzeri , and Bradyrhizobium japonicum [ 54 , 55 , 56 , 57 ]. Furthermore, genetic and biochemical analyses revealed that many additional nif genes, including nifE , nifN , nifX , nifQ , nif W , nifV , nifA , nifB , nifZ , and nifS , play roles in the regulation of nif genes and maturation processes of electron transport and FeMo-cofactor biosynthesis and assembly [ 58 , 59 ]. In addition, the fixABCX genes first identified in Rhizobium meliloti [ 60 , 61 ] and subsequently in other diazotrophs were reported to encode a membrane complex participating in electron transfer to nitrogenase [ 62 ]. The degree of specificity between legumes and rhizobia varies. The Nod factors produced by Rhizobium etli and Rhizobium loti produce identical Nod factors; however, they have distinct host ranges ( Phaseolus spp. and Lotus spp., respectively) [ 63 ]. Moreover, different rhizobia nodulating the same plant may excrete completely different Nod factors. For instance, Rhizobium tropici and R. etli produce different Nod factors (sulfated and acetylfucosylated, respectively), but both are known to nodulate Proteus vulgaris [ 64 ]. More examples include Bradyrhizobium elkanii and Bradyrhizobium japonicum , which have a number of mutual hosts, but their Nod factors differ considerably [ 65 ].
2.3. Marker-Assisted Selection of Biological Nitrogen-Fixing Plants
Several studies have identified QTL associated with traits related to biological N fixation ( Table 1 [ 66 , 67 , 68 , 69 ]). The QTL markers can be used in marker-assisted selection for breeding plants with better nitrogen fixation attributes. A QTL for the total ureides (acyl derivatives of urea) was identified on chromosome 17 in soybean which explained 13.26% phenotypic variation [ 70 ]. Li and the team [ 71 ] cloned a candidate gene associated with a major QTL in soybean for increasing nodule size and named it INCREASING NODULE SIZE1 (GmINS1). The overexpression of GmINS1 increased the N content and the biomass of the soybean plant due to an increase in number, biomass, the abundance of infection cells, and nitrogenase activity of large nodules [ 71 ]. The result was the opposite when GmINS1 was suppressed by RNA interference [ 71 ].
Major genomic loci detected for BNF in different legume species [ 66 , 67 , 68 , 69 ].
| Species | Chromosome Number | QTL or Marker Interval | Plant Response | QTL-Effect, R (%) |
|---|---|---|---|---|
| Common bean ( L.) | 7 | N derived from atmosphere (Ndfa) | 14.9 | |
| Soybean [ (L.) Merr.] | 16 | Nodule size & number | 15.9–59 | |
| Soybean [ (L.) Merr.] | 17 | Nodule size & number | 12.6–18.6 | |
| 2 | TM0550–TM0324 | Acetylene reduction activity per plant (ARA/P) | 15.1 | |
| 2 | TM0550–TM0002 | ARA per nodule number (ARA/NN) | 11.1 | |
| 4 | TM0664 | ARA per nodule weight (ARA/NW) | 10.8 | |
| 5 | TM1417–TM0095 | ARA per nodule weight (ARA/NW) | 13 | |
| 3 | TM0083 | Nodule number (NN) | 21.6 | |
| 1 | TM0113–TM0805 | Stem length (SL) | 13.3 | |
| 1 | TM0027–TM0063 | Shoot length without inoculation (SL bac−) | 16.7 | |
| 1 | TM0113–TM0805 | Shoot length without inoculation (SL bac−) | 16 | |
| 5 | TM0095–TM0909 | Shoot dry weight without inoculation (SW bac−) | 10.7 | |
| Cowpea [ (L.) Walp.] | 4 (Likage group) | 2_12850/2_54418 | Nodule number | 48.4 |
| Cowpea [ (L.) Walp.] | 6 (Likage group) | 2_11936/2_49231 | Nodule fresh weight | 21.4 |
3. Host Plant
Plants are associated with a complex microbiome that contributes to plant nutrient assimilation, growth, and defense. Nitrogen-fixing microbial associations are efficient and well-characterized in legumes but are limited in cereals, including maize. Plants contribute substantially toward the organic carbon pool of soil in the form of lysed cells, mucilage, and root exudates [ 72 ]. Root exudates are a complex mixture of simple and complex sugars, amino and organic acids, fatty acids and vitamins [ 73 ]. Depending on the plant genotype and growth stage, soil texture, nutrient status of soil, water holding capacity of the soil and, most importantly, the rhizosphere microbial communities, the amount and types of exudates will vary. In turn, root exudate composition in the rhizosphere can influence the soil microbial community and availability of macro and micronutrients, especially nitrogen and phosphorus [ 74 ]. The root exudate composition also serves as a recruiting complex of unique prokaryotic and eukaryotic populations [ 75 ]. More interestingly, the build of specific microbiota by secreting particular carbon sources is often observed, for instance, dicarboxylates in tomato root exudates favor the growth of pseudomonas biocontrol strains and pea plants excrete homoserine to select Rhizobium leguminosarum [ 76 , 77 , 78 , 79 , 80 ]. Plants can also defend themselves through the secretion of phytochemicals that can inhibit the growth of certain microbial entities [ 80 ]. The ability to tolerate these chemicals can play an important role in the ability to colonize the plant. For example, the PGPR Pseudomonas putida is both tolerant of and attracted by the main antimicrobial benzoxazinoid produced by maize (a non-legume plant) [ 81 ]. In addition, transgenic plants expressing opine biosynthesis genes shown to redesign current rhizosphere populations to increase the densities of opine-catabolizing bacteria compared to wild-type [ 82 , 83 ].
3.1. Symbiotic Nitrogen Fixation in Legume Nodules
In both natural and cultivated ecosystems, legumes supply a significant amount of nitrogen [ 84 , 85 , 86 ], and the nitrogen fixed by perennial forage legumes can be as high as the amounts of nitrogen fertilizers used in conventional farming practices [ 87 , 88 ]. Moreover, rhizodeposition from legumes is another substantial source of available nitrogen and other essential plant nutrients in rhizosphere [ 89 , 90 , 91 ]. In temperate forests, a 28% increase in nitrogen availability was reported due to the direct effect of BNF and the indirect effect of rhizodeposition [ 92 ]. In North America, several leguminous plants such as pea ( Pisum sativum L.), faba bean ( Vicia faba ssp minor L.), and dry bean ( Phaseolus vulgaris L.) are mainly produced for animal and poultry feed [ 18 ]. The most common legumes for human consumption are dry bean, chickpea ( Cicer arietinum L.) and cowpea ( Vigna unguiculata L.), lentil ( Lens esculenta L.), pigeon pea ( Cajanus cajan L.), and peanut ( Arachis hypogea L.). All these legumes are capable of nitrogen-fixation and are often grown in intercropping or for crop rotation. Nitrogen fixed by symbiotic association of soybean root system with soil bacteria ( Rhizobia ) has a significant contribution to the growth, development, and maturity stages. The increase in nitrogen fixation capacity can be translated to the increase in plant parts including soybean pods. In the field-grown soybean [ Glycine max (L.) Merrill] cv. Chippewa in a Typic Eutrocrepts soil at physiological maturity (R7), the amounts of nitrogen derived from fixation ( Ndfs ), nitrogen from soil, and 15 N-labelled fertilizer ( Ndff ) were 47%, 50%, and 3%, respectively [ 93 ]. The contribution of nitrogen in soybean pods and seeds was higher from fixed nitrogen (55%) compared to the nitrogen from soil (43%) [ 93 ]. Muñoz, Qi [ 70 ] observed cultivated soybeans were more efficient in BNF compared to the wild soybeans.
Forage legumes are grown under a broad climatic spectrum, and they have the potential to give higher yield and provide essential nitrogen to the soil. The four major forage legumes, alfalfa ( Medicago sativa L.), red clover ( Trifolium pratense L.), subterranean clover ( T. subterraneum L.), and white clover ( T. repens L.) together comprise most of the hot and arid regions on earth’s grasslands [ 94 ]. Alfalfa ( Medicago saliva L.) is a cool-season perennial forage legume that obtains nitrogen from the soil and the BNF through symbiotic association of its root nodules with soil bacteria. For its ability to provide fixed nitrogen, alfalfa is increasingly gaining popularity as a companion forage in grass pastures [ 95 ]. The fixed nitrogen by alfalfa is not only used by itself but also is transferred to the subsequent crops, which is also termed as a “niche complementarity effect” [ 96 ]. For example, the transfer of nitrogen fixed by alfalfa to different grass species such as timothy ( Phleum pratense L.) and bromegrass ( Bromus inermis Leyss) was demonstrated using the 15 N dilution technique [ 97 ]. Heichel, Barnes [ 97 ] observed the contribution of N transfer from alfalfa to associated grasses in terms of absolute amounts at 5, 20, and 19 kg N ha −1 for the three test years, respectively. The direct excretion of N compounds from the root system and the decomposition of root and nodule debris were attributed to this contribution [ 97 ].
In addition to economically important crops and forages, considerable attention has been given to several plant species that can produce biofuel while fixing nitrogen. One important example is Pongamia pinnata , which in addition to being a medicinal and green manure plant, can nodulate with several strains of both Bradyrhizobium and Rhizobium ; however, best-selected inocula were B. japonicum strains CB1809 and USDA110 [ 98 , 99 , 100 ]. Pongamia resembles the general properties seen in annual legumes such as soybean and its nodules actively fix nitrogen where acetylene is reduced by bacterially encoded nitrogenase [ 101 ]. However, in addition, many current plant biofuel feedstocks such as oil palm, canola, willow, corn ( Zea mays ), sugarcane, jatropha, sorghum, and even algae may produce abundant fuels but are not nitrogen-fixing species [ 38 ].
3.2. Nitrogen Fixation in Non-Legumes
In a non-symbiotic system such as rhizosphere-associative nitrogen fixation, nitrogen-fixing bacteria fix the nitrogen by using carbon and energy sources supplied from the environment, and the bacteria release fixed N probably after lysis of the bacterial cells [ 102 , 103 ]. Symbiotic bacteria such as rhizobia and Frankia are located in nodules, whereas in rhizosphere-associative systems, the diazotrophic bacteria are essential in the free-living state and fix nitrogen using the supply of carbohydrates from the environment [ 104 , 105 ] in accord with the excretion of carbohydrates from the roots and the degradation of soil organic matter. In contrast to the legume-rhizobia symbiotic system in nodules, the associations of plants and microbes in rhizosphere soils and plant root interiors form adjusted or adapted nitrogen-fixing systems under physiologically nitrogen-deficient but energy-sufficient conditions. Over the last 50 years, nitrogen fixation in no-leguminous crops and bacterial associations have been investigated elaborately for their agronomic significance. For example, associative nitrogen fixation in sugarcane (Saccharum spp.), sweet potato ( Ipomoea batatas L.), and paddy rice ( Oryza sativa L.) are agronomically significant. Active expressions of the di-nitrogenase reductase-encoded gene ( nifH ) phylogenetically similar to those of Bradyrhizobium spp. and Azorhizobium sp. were abundantly found in the nitrogen-fixing sugarcane stems, sweet potato stems, and storage tubers. Setaria viridis , as well as Setaria italica (foxtail millet), is capable of securing a significant amount of fixed nitrogen from associations with Azospirillum brasilense [ 106 , 107 ]. Other promising associations include Azoarcus sp. strain BH72 and Kallar grass and Klebsiella pneumoniae and wheat [ 108 ]. A rhizosphere-associated nitrogen fixation can occur in three ways. First, rhizobia employ “crack entry” (a lack of Nod ABC genes, which results in a Nod factor-independent infection process [ 52 , 109 , 110 ] and invades xylem parenchyma tissues via cortical cells [ 111 ] in cut sugarcane stems [ 112 ] and sweet potato tuber [ 113 ]). Second, under low-oxygen level or micro-aerobic conditions, rhizobia may show free-living nitrogen fixation; for instance, Bradyrhizobium spp., nodulates Aeschynomene and Parasponia , Azorhizobium caulinodans , nodulates Sesbania rostrate , and Burkholderia , nodulates Mimosa , and all these rhizobia are capable of fixing nitrogen without a host plant under low-oxygen conditions [ 114 , 115 , 116 , 117 , 118 , 119 ]. Third, hormones of rhizobia origin that promote the growth of the host plant in accordance with fixed N acquisition; for instance, endophytic rhizobia promoting plant growth [ 64 ].
3.2.1. Bacterial Nitrogen Fixation in Sugarcane
Beijerinckia sp. from the rhizosphere of sugarcane was first isolated and observed in EMBRAPA Agrobiologia, Brazil [ 120 ]. The expression of nitrogenase nifH genes was examined by growing sugarcane cut-stems in Japan soils for 50 and 100 days by means of reverse transcription-polymerase chain reaction (RT-PCR) and the sequencing of nifH (encoding nitrogenase iron protein) nucleotides [ 112 ]. In that study, nifH sequences showed similarities with Bradyrhizobium spp. and Azorhizobium caulinodans , which suggested that the propagation of these nifH carrying Bradyrhizobium spp. may be a key factor in the endophytic nitrogen-fixation in the free-living state.
3.2.2. Bacterial Nitrogen Fixation in Sweet Potato
In growing sweet potato ( Ipomoea batatas L.), farmers use relatively infertile soil and apply a small dosage of chemical fertilizers [ 121 , 122 ]. Azospirillum sp. was first identified in fibrous roots and storage root peels of sweet potato, and in the same study, additional investigations also indicated a high input of total nitrogen in sweet potato stems and tubers by endophytic Bradyrhizobium spp. [ 121 ]. In recent years, an isolated endophytic diazotroph, Bradyrhizobium sp. strain AT1 [ 113 ] showed a nifH sequence similarity to Aeschynomene stem-nodulating Bradyrhizobium sp. ORS391 [ 123 ].
3.2.3. Bacterial Nitrogen Fixation in Paddy Field
Much higher nitrogenase activities in a paddy rice-soil system were detected compared to the paddy soil without rice plants and in an upland rice–soil system [ 124 ]. In flooded soil, the root–soil interface has been proposed as the nitrogen-fixing site and the bacteria sustaining such nitrogen fixation activity under dark in flooded conditions were thought to be heterotrophic diazotrophs such as Azotobacter and Clostridia [ 124 , 125 ]. Later, in long term repeated pot experiments at the IRRI (International Rice Research Institute) [ 126 ], nitrogen fixation by not only photosynthetic cyanobacteria but also consistently by the heterotrophic diazotrophs utilizing root secretions of carbonaceous origin in the rhizosphere was observed [ 127 ]. In addition, a positive nitrogen balance was calculated, suggesting significant atmospheric nitrogen input in paddy rice fields [ 128 ]. From nifD (a nitrogenase protein-encoded gene) segments from crude root DNA, cloned nifD genes similar to those of γ-proteobacteria ( Azotobacter vinelandii ) and α-proteobacteria ( Bradyrhizobium japonicum ) were detected [ 129 , 130 ].
3.2.4. Maize Mucilage and Microbiota Association for Nitrogen Fixation
A recent study carried out in nitrogen-depleted fields of Oaxaca, Mexico, demonstrated that the mucilage associated with the aerial roots of Sierra Mixe maize can aide a complex diazotrophic microbiome that can encode active nitrogenase, and the fixed nitrogen (29% to 82% of the plant nitrogen was derived from atmospheric nitrogen) can efficiently travel from the nitrogen-fixing microbiota to host plants [ 131 ]. In maize, aerial roots are known for enhancing nutrient and water uptake as well as an efficient gaseous exchange between plant tissue and the atmosphere [ 132 , 133 , 134 ].
3.2.5. Bacterial Nitrogen Fixation in Switchgrass
Switchgrass ( Panicum virgatum L.) is a warm-season C4 grass. It is native to the tallgrass prairies of North America, and it has been well-studied for its use as a forage grass and more recently for its potential as a cellulosic biofuel [ 135 , 136 ]. In the absence of substantial nitrogen deposition or soil organic nitrogen unavailability, rhizobia-associated nitrogen-fixing is solely responsible for nitrogen supply and switchgrass can incorporate recently fixed nitrogen into its tissues [ 137 , 138 ] and diverse communities of nitrogen-fixing bacteria are present in switchgrass rhizospheres such as Burkholderia spp., and Ralstonia taiwanensis of beta-proteobacteria have been found in the tissues of switchgrass which are known to form root nodules on host plants of Mimosa [ 139 ].
4. Current Strategies and Tools for Engineering Symbiotic Nitrogen Fixation in Non-Legumes
Although symbiotic nitrogen fixation is largely limited to legumes, there is an array of microorganisms, including some diazotrophs that inhabit the rhizosphere of other crop plants, which have been shown to enhance plant growth. The mechanisms involved in plants and microbes that lead to the formation and function of symbioses will help us in transferring these traits and processes to non-leguminous crops, especially in cereals. Advanced understanding of BNF, bacterial association with non-leguminous plants, and the microbial community composition of the rhizosphere population have led to several future research ideas for scientists; for instance, engineering non legume plants to nodulate and establish symbiotic nitrogen fixation, and the formulation of new associations between nitrogen-fixing microorganisms and crop plants [ 140 , 141 ]. Studies of evolutionary genomics suggest that relatively few genetic elements are needed to bestow nitrogen-fixation capabilities from legume to non-legume plants [ 142 ]. Transferring nitrogenase to plants requires the concatemerization of bacterial genetic units to create a minimum set of three genes [ 143 ]. The introduction of nitrogenase-encoding bacterial nif genes into non-legumes is challenging due to the complex nature of nitrogenase biosynthesis and the extreme sensitivity of nitrogenase to the presence of oxygen. Extensive genetic and biochemical studies have identified the common core set of genes/gene products required for functional nitrogenase biosynthesis [ 144 ].
In addition, potential subcellular (micro-pockets of air) low-oxygen environments offered via plastids and mitochondria to express active nitrogenase in plants making this engineering strategy feasible [ 145 ]. Although the nitrogen-fixing symbiosis is restricted to legumes, several components of the legume symbiotic signaling (SYM) pathway also play a role in the arbuscular mycorrhizal symbiosis. Many plants, including cereals, can form arbuscular mycorrhizal associations but lack the ability to form root nodules that can fix nitrogen. Like in legumes, the legume symbiotic signaling pathway (or SYM) also promotes the arbuscular mycorrhizal symbiosis. Since cereals contain the SYM pathway for arbuscular mycorrhizal associations, thus, this association can be engineered to perceive the rhizobial signaling molecules to activate this pathway, as well as by engineering its outputs of activation into an oxygen-limited nodule-like root organ for nitrogen fixation [ 64 ]. Recent phylogenomic studies suggest that a small set of genes could convert a species in association with arbuscular mycorrhizal fungi into a nitrogen-fixing symbiont [ 146 , 147 ].
In cereal crops, mitochondria and chloroplasts (“nitroplast”) [ 38 ] in plant cells are envisioned as suitable sites for performing the high energy-requiring nitrogenase enzyme production; however, a challenge for this approach would be the oxygen evolved by chloroplasts during photosynthesis that may be detrimental for the formation of the nitrogenase enzyme complex. A possible solution could be the temporal separation of photosynthesis and nitrogen fixation, which means the expression of nif gene only to dark periods (nights) or only in root systems (non-photosynthetic parts of plants) [ 19 ]. Also, a carbon secretion based approach in which a specialized carbon source encourages enhanced competition for carbon among nitrogen-fixing populations can also be utilized to establish appropriate signals between cereal crops and nitrogen-fixing microbes for effective colonization [ 64 ].
Previous studies have reported the influence of novel nutritional resources in the selection of microbial populations in the rhizosphere [ 83 , 148 ]. For instance, pea root mucilage is the sole source of carbon for some Rhizobium sp., Burkholderia sp., and Pseudomonas sp. [ 149 ]. Pursuing this “biased rhizosphere” approach to favor the growth of an introduced diazotroph which is able to use the novel rhizodeposition will involve the identification of appropriate plant and bacterial signals, receptors, and target genes [ 150 ]. Although transferring nitrogen fixation traits to crops beyond legumes has complex engineering problems, especially in the case of cereals, however, they might restructure the way cereal crops are grown. Even a small increase in available nitrogen in these self-supported nitrogen-fixing cereals will enable a substantial yield increase in the low-input farming systems of developing countries [ 151 ]. Moreover, in eukaryotes, some components of nitrogenase enzyme, for instance, active dinitrogenase reducatase can be expressed by mitochondrial targeting in yeast or plastid-targeting strategies in tobacco [ 69 , 152 , 153 ]. There is even an effort to synthesize an entire eukaryotic genome (Yeast 2.0). This expression of dinitrogenase in yeasts has important implications, especially for efficient nutrient uptake such as phosphate in cereal crop root habitats [ 131 , 154 ].
Opine molecules produced by transgenic plants are known to boost their rhizosphere with opine catabolizing bacteria; however, there is a risk of populating the rhizosphere with chemical compounds originating from pathogenic organisms [ 82 , 83 , 148 ]. Rhizopines are a rare group of compounds produced by a few species of rhizobia inside legume nodules and are exuded into the rhizosphere; namely, scyllo-inosamine 1 (SIA) and 3- O -methyl-scyllo-inosamine 2 (3- O -MSI) are believed to be suitable for ideal chemical signaling in the realm of trans-kingdom signaling between plants and rhizosphere bacteria, although engineering rhizopine-producing plants have not seen much success [ 155 , 156 , 157 , 158 ]. Rhizopines serves as the energy source (carbon and nitrogen) for Rhizobia and the gene responsible for rhizopine synthesis is (mosABC) and catabolism is (mocCABRDEF), which have been identified in the wild-type rhizobium Sinorhizobium meliloti L5-30 [ 159 , 160 ]. In the continued efforts in rhizosphere engineering of cereal crops, a recent study has successfully transferred the rhizopine biosynthesis genes into Hordeum vulgare (barley) [ 161 ]
5. Conclusions
Biological nitrogen fixation in plants can be a sustainable source of nitrogen and may divert our current dependence on industrial nitrogen production. This is especially true for food production in the developed world where agricultural production is still based on higher-yielding varieties and hybrids but with a simultaneous increase in inorganic nitrogen application. Not all the applied nitrogen in agricultural production is taken up by plants and the unused nitrogen has negative impacts on the environment, extending from eutrophication in nearby water bodies if the excess nitrogen is washed up by rainfall or surface runoff or nitrate poisoning in livestock. Future research should be focused on the efficient and strategic use of nitrogen-fixing legume plants, the use of higher legume plants such as Pongamia pinnata , in planned agroforestry, selecting highly competitive inoculants, and the use of non-legumes for nitrogen fixation. Artificial symbioses, associative nitrogen fixation in non-legume plants, especially in cereals such as rice, wheat, maize, targeted or biased rhizosphere, and understanding of endosymbiotic and endophytic nitrogen fixation with non-legume plants are some of the approaches that should be investigated to a greater extent.
Author Contributions
Conceptualization: K.M. and A.M.; Writing original draft preparation: K.M., S.M. and R.I.; Reviewing and Editing: K.M. and A.M.; Supervision: A.M.; Funding Acquisition: A.M. All authors have read and agreed to the published version of the manuscript.
Funding provided by The Center for Bioenergy Innovation a U.S. Department of Energy Research Center supported by the Office of Biological and Environmental Research in the DOE Office of Science.
Conflicts of Interest
The authors declare no conflict of interest.
- Open access
- Published: 16 October 2020
Fate of nitrogen in agriculture and environment: agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency
- Muhammad Anas 1 , 2 ,
- Fen Liao 2 ,
- Krishan K. Verma 2 ,
- Muhammad Aqeel Sarwar 3 ,
- Aamir Mahmood 1 ,
- Zhong-Liang Chen 2 ,
- Qiang Li 1 ,
- Xu-Peng Zeng 1 ,
- Yang Liu 4 &
- Yang-Rui Li 1 , 2
Biological Research volume 53 , Article number: 47 ( 2020 ) Cite this article
43k Accesses
277 Citations
68 Altmetric
Metrics details
Nitrogen is the main limiting nutrient after carbon, hydrogen and oxygen for photosynthetic process, phyto-hormonal, proteomic changes and growth-development of plants to complete its lifecycle. Excessive and inefficient use of N fertilizer results in enhanced crop production costs and atmospheric pollution. Atmospheric nitrogen (71%) in the molecular form is not available for the plants. For world’s sustainable food production and atmospheric benefits, there is an urgent need to up-grade nitrogen use efficiency in agricultural farming system. The nitrogen use efficiency is the product of nitrogen uptake efficiency and nitrogen utilization efficiency, it varies from 30.2 to 53.2%. Nitrogen losses are too high, due to excess amount, low plant population, poor application methods etc., which can go up to 70% of total available nitrogen. These losses can be minimized up to 15–30% by adopting improved agronomic approaches such as optimal dosage of nitrogen, application of N by using canopy sensors, maintaining plant population, drip fertigation and legume based intercropping. A few transgenic studies have shown improvement in nitrogen uptake and even increase in biomass. Nitrate reductase, nitrite reductase, glutamine synthetase, glutamine oxoglutarate aminotransferase and asparagine synthetase enzyme have a great role in nitrogen metabolism. However, further studies on carbon–nitrogen metabolism and molecular changes at omic levels are required by using “whole genome sequencing technology” to improve nitrogen use efficiency. This review focus on nitrogen use efficiency that is the major concern of modern days to save economic resources without sacrificing farm yield as well as safety of global environment, i.e. greenhouse gas emissions, ammonium volatilization and nitrate leaching.
Introduction
Nitrogen (N) plays an important role in crop plants. It is involved in various critical processes, such as growth, leaf area-expansion and biomass-yield production. Excess NUE can support good plant performance and better crop out-put. Various plant molecules such as amino acids, chlorophyll, nucleic acids, ATP and phyto-hormones, that contains nitrogen as a structural part, are necessary to complete the biological processes, involving carbon and nitrogen metabolisms, photosynthesis and protein production [ 1 , 2 ]. Insufficient amount of N available to plants can hinder the growth and development. Nitrogen can also improve root growth, increase the volume, area, diameter, total and main root length, dry mass and subsequently increase nutrient uptake and enhance nutrient balance and dry mass production [ 3 , 4 , 5 , 6 ].
Application of nitrogen increases greenness of plants, CO 2 assimilation rate, crop quality-yield and improve resistance to environmental stresses such as limited water availability and saline soil conditions [ 7 , 8 ]. Hou et al. [ 9 ] found that nitrogen application more important than the other major essential fertilizers/nutrient for successful crop production. Consequently, N requirement is the most central feature for plant production [ 10 ]. Slow development of plant and early leaf senescence due to deficient N can cause decreased both crop production and quality [ 11 ]. Excessive N fertilizer application is common practice by farmers of cotton regions in the northwest [ 12 ] which is not cost effective for crop production, and excess N prolongs the vegetative growth period, delays maturity [ 13 ], decrease sugar content, and also attracts insect pest and causes disease epidemics.
China has only 7% of global farm land with 20% world population that depends on it for feed [ 14 , 15 , 16 ]. It boosts up average yield of grain from 1.09 to 6.51 tonnes ha −1 in last 7 decades [ 17 ]. In China, chemical nitrogen (N) fertilizer input is the major element for the continuous increase of food production to mitigate the problem of food security [ 18 ]. Therefore, the low NUE all over the world especially in agriculture sector is not only wastage of resources (Fig. 1 a, b) and also problematic for environmental pollution (Fig. 1 c, d) and conflicting to sustainable agricultural productivity [ 19 , 20 , 21 ].
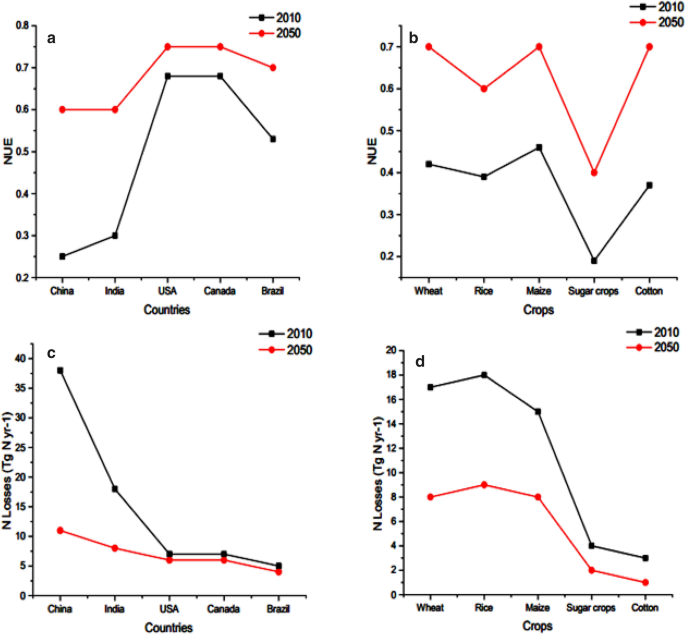
This diagram depicts country wise ( a ) and crop wise ( b ) NUE for 2010 and 2050 (proposed), while c , d shows nitrogen losses in teragram for 2010 and 2050 (proposed)
NUE and its status
NUE is an exploiting issue for discussion and research which depends on the physiological and metabolic changes, such as soil nitrogen uptake, assimilation from roots to other parts (Fig. 2 ), source-sink tissues interaction for transportation, signaling and regulatory pathways which are responsible for N status within plant and growth as well [ 22 ]. Normally, the ratio of yield and total N supplied is termed into NUE [ 23 ]. Several techniques have been adopted to observe NUE that can be separated into N uptake efficiency and N utilization efficiency. N uptake efficiency (NUpE) describes the nitrogen amount that a plant can take from sources of nitrogen while N utilization efficiency (NUtE) termed as the plant capability to assimilate plus remobilize N within the plant [ 4 , 22 , 24 ]. However, NUE is the resultant of NUpE and NUtE product. Numerous demarcations for NUE have been suggested over the years, which have showed a few differences in normal ways [ 4 , 25 , 26 ].
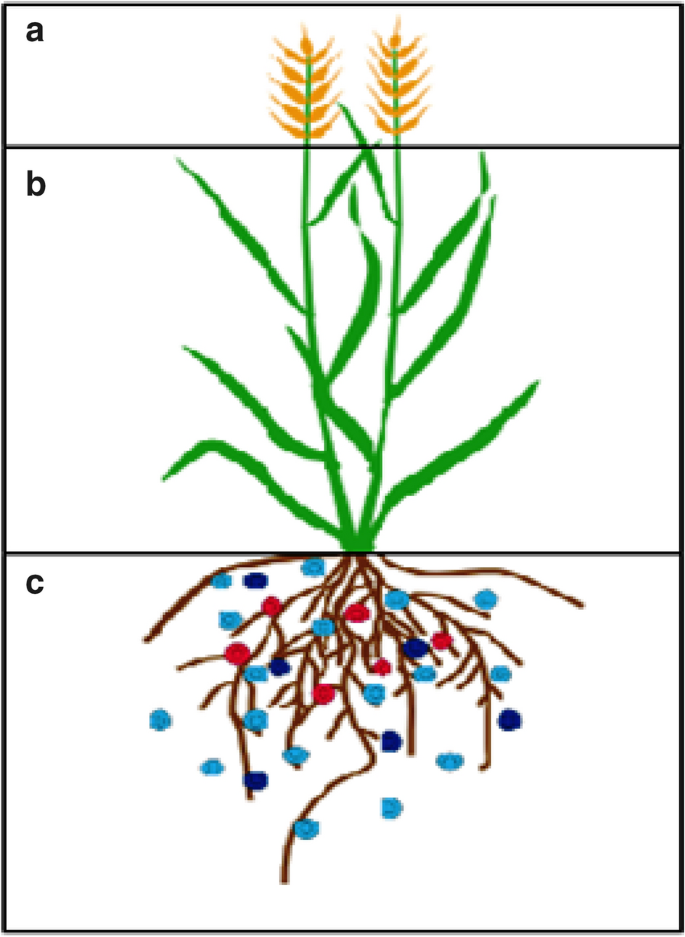
The major plant pats which have their own role for NUE. a Grain: responsive to fertilizers and nutrient storage component, b Shoot: nutrient redistribution, assimilation and transportation (source and sink), c Roots: Efficient nutrients uptake by transporters and channels
NUE, NUpE and NUtE can be measured by adopting the Eqs. 1 , 2 and 3 [ 4 , 24 ].
Nitrogen recovery and agronomic nitrogen efficiency (NRE) are the other common approaches used to observe NUE. NRE is termed as the percentage of pragmatic nitrogen fertilizer taken up by crop. It is an indicator for a crop to use the N fertilizer that has been supplied [ 27 ]. The yield increment per unit of N fertilizer given to the crop is denoted as agronomic nitrogen use efficiency (aNUE). It is an important index to measure gain or loss for excess amount of fertilizer [ 28 ]. Best aNUE is the surety of highest benefit–cost–ratio, which is a key economic relationship between input and output that relate both by linear curve [ 29 ].
The Eqs. 4 and 5 can be used to measure agronomic and recovery efficiencies like aNUE and NRE:
Y fertilized and Y not fertilized are yields (kg ha −1 ) when quantity of N fertilizer applied was F and zero; F applied is the total N (kg ha −1 ) applied [ 28 ].
Total NU fertilized and Total NU not fertilized showed N uptake for F and no fertilizer, respectively [ 30 ].
The variation in NUE can be understood by nitrogen doses, application methods and other agronomic factors which help to manage nitrogen has crucial effect for both profitable crop production and environment [ 31 ]. According to field demonstrations, Lou et al., [ 32 ] measured NRE and aNUE for different nitrogen rates, application methods and plant population in northwest, China, and found that the 70% and 80% of nitrogen loss can be minimized when nitrogen applied through drip fertigation and high plant population, respectively. Drip fertigation and high plant density can increase nitrogen recover efficiency for comparable yield. In contrast conventional method of nitrogen application and low plant population, more nitrogen losses, which leads to decrease yield in crops due to low amount of N available. The midseason rice NUE is less than 30% in China, which indicates that 70% nitrogen is going into the ecosystem as loss [ 33 ]. As comparison of USA and China from 1980 to 2010 for NUE in case of maize crop, the NUE declined from 30.2 to 29.9 in China but up-graded from 39.4 to 53.2 in USA [ 34 ]. Hajari et al. [ 35 ] demonstrated few varieties of sugarcane for nitrate and ammonium as a source of N fertilizer in their study and concluded that NO 3 − -N resulted in higher NUEs as compared to NH 4 + -N. Wheat and maize grown in a hydroponic culture containing NH 4 + -N showed that the photosynthetic and carbon assimilation rates decreased in the plants [ 35 , 36 ].
Available sources and forms nitrogen
The conversion of nitrogen from one form to others greatly influences the nitrogen use efficiency.In early growth stage NO 3 − form of nitrogen is important but it has not been commonly used as fertilizers alone, the other forms go the atmosphere by nitrification [ 37 ]. However, most widely used nitrogen fertilizer urea is abruptly nitrified (Fig. 4 ) after conversion to ammonium [ 37 ]. Although urea after application in soil can convert into nitrate and ammonium form, it is not still clear about urea uptake process and metabolic changes in plants [ 38 ]. Urea is also preferred and predominant source of N due to more nitrogen contents and low cost to produce it in South Africa [ 39 ].
The soil N (Fig. 3 ) is most important to observe the efficiency of N in the agricultural field conditions [ 40 , 41 , 42 , 43 , 44 ]. There are a lot of evidence from various field trials using 15 N-labeled fertilizer, N uptake is principally derived from soil (Fig. 3 ) rather than fertilizer [ 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 ]. However, many studies have been conducted and found that unfertilized N responses often give more yield than that of N fertilized [ 43 , 54 , 55 , 56 ], except those in which soil N availability is captured by accumulation of carbonaceous residues. Total soil nitrogen and organic carbon vary in soil profile, both decreases with the soil depth, however the ionic forms of N (NH 4 + , NO 2 − , and NO 3 − ) shape the mineral nitrogen dynamics because discrepant increments of mineral nitrogen stock in each soil layer takes place [ 57 , 58 ].
Sources of organic nitrogen available for mineralization in soil [ 59 ]
According to Neto et al. [ 60 ] when nitrogen concentration increases even though it is earlier applied, mineralization of nitrogen in soil is boosted and a part of N shares from the mineralized nitrogen. Nitrogen within the plants at anthesis stage also enhanced due to the transformation of nitrogenous compounds, which have stored nitrogen in earlier growth period [ 61 , 62 ]. Crop growth, development, biomass and yield have directly linked to nitrogen assimilation [ 61 , 63 ]. Mazzafera and Goncalves [ 64 ] analyzed xylem sap to study nitrogen transformation in coffee plants and found 52% of the total nitrogen is nitrate. But nitrate reductase reduces it into nitrite [ 65 ].
Sugarcane accumulates nitrogen 100–150 kg ha −1 in leaves and stalks, only about 55% is removed from stalks up to maturity [ 66 ]. The plant residues after harvesting are put into the field which gradually mineralized and release N in available forms [ 67 ]. Nitrogen consumption by enhanced N fertilization to the crop may lead to high N uptake but it is not necessary to increase biomass production [ 68 ]. Thus, over use of nitrogen fertilizer down-regulates the nitrogen use efficiency and increases production cost and environmental pollution.
Plants have the ability to acquire excessive NO 3 − nitrogen than the requirement for assimilation and store it in unassimilated pools like vacuoles of leaves [ 69 ], become available for utilization under low N [ 70 , 71 ]. Hajari et al. [ 35 ] and Robinson et al. [ 37 ] found, the NO 3 − -N per gram was higher in dry roots than the shoot on all growing media. Hajari et al. [ 35 ] claimed that the sugarcane plant is not able to translocate NO 3 − -N from root to shoot efficiently due to which limited N uptake and transport occur rather than assimilation which may affect the NUE in sugarcane. The application of a nutrient may increase (synergism) or decrease (antagonism) the contribution of the other nutrients in crop yield. The concentration of phosphorus and nitrogen varies over the growing period in the soil and create interaction either synergistic or antagonistic. The response of crop yield might be affected directly or indirectly [ 72 , 73 ]. Therefore, the supply of both N and P creat changes in chemical, physical, and biological properties of soil [ 74 , 75 ]. The nitrogen fertilizer has synergetic effect to phosphorus. The results indicated, addition of nitrogen along with phosphorus fertilizer produced better positive interaction than separately [ 76 ]. In the sugarcane field which has previously wild vegetation and low available phosphorus response nutrient limitations, it involves phosphorus as limiting source in high demand periods, and also microbial biomass [ 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 ].
Losses of nitrogen in the ecosystem
Worldwide high nitrogen fertilizer application results in economic loss and ecological hazardous due to extra consumption of resources, water eutrophication, and high rate of greenhouse gas emissions along with potential leaching. The inefficient N utilization with poor transformation of provided N results in unintentional fertilizer loss in soil, atmosphere and promoting contamination of groundwater, distort connecting biological communities and cause dangerous atmospheric deviation, through the emission of the poisonous ozone depleting substance nitrous oxide [ 82 ], eutrophication, air pollution, N leaching, water pollution, soil acidification and soil degradation [ 14 , 18 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 ] which is not suitable for environment friendly crop production and human life (Fig. 4 ).
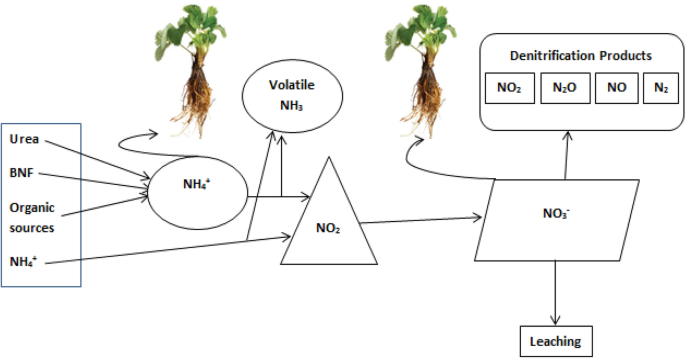
Summary of nitrogen sources and, their conversion, availability to plants and losses within/outside of soil
In agriculture, crop production requires plentiful N which is the most widely recognized limiting factor for crop growth, development and yield. A lot of synthetic N fertilizer is applied to arable land by growers to fulfill the demand for crop production. An abrupt increment in fertilizer applications in China was noted, and it consumed 30% of total N fertilizers synthesized around the world in 2002, in spite of the facts, its arable land accounts only 10% of the world aggregate. However, the use of vast amounts of synthetic N fertilizer to expand crop yield are not financially sustainable and put a substantial burden on farmers, and furthermore result in environmental pollution. Every crop cannot use about 50% nitrogen fertilizer during its growing season due to over fertilization [ 90 ].Moreover, plants grown under excessive nitrogen applications are more susceptible to lodging because of shoot overgrowth and tender, and pest damage and disease, and also degrade quality of the grains [ 91 ].
The N losses thru lixiviation, direct escape to the air, denitrification and/or percolation is higher due to over use of N fertilizer [ 92 ]. The synchronized application as the demand of plant at its critical stage can decrease losses of applied N fertilizer [ 93 , 94 , 95 ]. Over the last decade, crop response to N fertilization [ 96 , 97 ] was detected in sugarcane fields all over the Brazil for green cane trash blanketing systems (GCTBS) and also in situ quantify NH 3 volatilization [ 98 ], NO 3 leaching [ 99 , 100 , 101 ], and N 2 O emissions [ 102 , 103 ], N use efficiency [ 104 , 105 ]. About 60–80% synthetic N fertilizer is not taken up by sugarcane crop under GCTBS, and losses due to volatilization, denitrification and leaching has been observed, but most of the mineral N is not available for micro biota, while the remaining part available to the crop [ 96 ]. In spite of the fact that the mechanism of commercial fertilizers is relatively well familiar [ 106 ]. However, many researchers claim the impact of organic and organomineral is not understood on chemical and microbial properties of soil for successful crop cultivation in temperate areas [ 107 , 108 , 109 ].
Biotic factors like size and diversity of microbial community and abiotic factors temperature, soil moisture content, temperature have direct relation to regulate organic compounds mineralization in the soil (Fig. 3 ), however, seasonal climatic change during cropping season fluctuate the mineral N availability [ 110 ]. Rapid availability of mineral N in soil solution has been noted as a result of synthetic N fertilizer application [ 96 , 111 , 112 ], but there is a powerful race between crop plants and micro flora for existing mineral N (especially NH 4 + ), and cause a large variations over time [ 77 , 78 ].
Urea is the major N fertilizer that is applied to the field and also the main source of NH 3 gas emission (Fig. 4 ) from agronomic practices [ 113 ] contributing for about 20% of the emissions in Germany [ 114 ] and is highly important in many other countries like China. Nitrogen loss as NO 3 − leaching (Fig. 4 ) from sugarcane field has significant contribution to pollute environment in Australia [ 115 ]. Many researchers in Brazil also find out leaching losses of nitrogen in planted sugarcane throughout its growth [ 116 ]. However, during ratoon season, NO 3 − leaching is more important than the planted cane [ 100 ]. The skips within ratoon sugarcane field increased across the growth period, and decreased the crop N response. The unique response to applied N fertilizer can be attained by well-established ratoon crop similar to planted crop density.
Duan et al. [ 117 ] discuss their findings about N application to long and short vines of sweet potato, the both long-vine and short-vine cultivars have the peak yield for nitrogen applied as 30 and 90 kg ha −1 respectively. The cultivars of same production potential have reduced their yields, and the root yield of long vine is significantly lower than that of short vine for nitrogen 120 kg ha −1 . Wu et al. [ 118 ] also claim the cultivar Zijing No. 2 decrease in the root yield for N application (75 kg ha −1 ) in fertile soil. Thus, the genotypic differences in sweet potato have a great influence on the partitioning of dry matter as well as uptake of nitrogen [ 119 ]. Wilson [ 120 ] classified cultivars of sweet potato for N-responsiveness, nonresponsive and depressive natures. Nitrogen buildup and distribution for short stature tuber roots are greater, and similarly exhibit more yield in response to high N conditions [ 121 ]. Besides, the cultivars that require higher N, give higher root yield in fertile soils [ 118 ].
Total nitrogen fertilizer can be reduced up to 360 kg ha −1 with respect to 430 kg ha −1 for cropping system based on the wheat–maize rotations, along with improved agronomic practices. It was resulted in increase in maize yield by 7–14%, but reduction in wheat yield, N 2 O and NO emissions by 1–2%, 7% and 29%, respectively [ 122 ]. In addition, best fertilization practices are an option to improve NUE and also seasonal collective N 2 O emission decrease [ 123 ]. Leaching process can be minimized by adopting legume crops in cropping system up to 50% than the conservative systems [ 124 ]. Soybean reduces 50–60% of N demand by biological nitrogen fixation [ 125 ]. Graham et al. [ 126 ] and Resende et al. [ 127 ] observed that addition of synthetic fertilizers decreased soil N stocks, while Ladha et al. [ 108 ] reported an increase in soil C pool and N stocks for long–term organic fertilizer application.
Agronomic and physiological approaches
Application rates.
Irrational application of nitrogen is a major problem of low nitrogen use efficiency [ 128 , 129 , 130 ]. Therefore, agronomic principles and practices should utilized in modern techniques to enhance nitrogen use efficiency, so as the reduced application rate of fertilizer inputs without yield reduction is key factor [ 32 ]. Soil characteristics and agro-climatic conditions highly force the application level of fertilizer [ 131 ]. Crops can use only up to 35% of the supplied N during its complete life cycle [ 39 ] and the remaining is escaped to the environment by various mechanisms and functions (Fig. 4 ) [ 132 , 133 ].
Improvements in NUE by decreasing nitrogen dose may delay leaf senescence which results in no yield loss. Late-season leaf senescence due to low nitrogen application rate provides relatively higher photosynthetic capacity to crop and ultimately increase yield production. Mulvaney et al. [ 109 ] proposed N mineralization in soil is positively regulated by synthetic nitrogen fertilizer. These findings indicate that N may exceeds the demand of sugarcane crop (200 kg ha −1 year −1 ) and affect C:N ratio in soil for long time continuous applications.
Srivastava and Suarez [ 134 ] confirmed N recommendation rate for sugarcane varies worldwide for 45 to 300 kg ha −1 but 60 to 140 kg ha −1 is recommended for Brazil. Dametie and Fantaye [ 135 ] summarised the results of sugarcane N uptake studies by various researchers in the globe, and indicated that the usual need of ratoon crop for nitrogen is 1.5 kg Mg −1 cane yield. N uptake varied from 0.88 to 1.47 kg Mg −1 in Hawaii, and stubble cane production required 1.3 kg Mg −1 [ 136 , 137 ]. By the compilation of numerous results for nitrogen dosage and technically recommendations in Brazil, the usual rate is 1.0–1.4 kg Mg −1 cane [ 138 ].
Nitrogen fertilizer application dose can be minimized by 20% without yield loss in Australia [ 139 ]. The N fertilizer in China has possibility to use moderately at low rate by integration management practices [ 140 ]. The reports from different regions/countries suggest that N use efficiency can increased by decreasing N application rate [ 141 , 142 , 143 , 144 ]. However, it also depends on agronomic traits, fertility of soil, management and yield potential [ 141 , 142 , 143 , 144 ].
The N application rate can also be determined by vegetative growth and productivity index, for example, coffee plants showed high rates for it between 2400 and 3600 kg ha −1 per year [ 60 , 145 ] and N as urea applied 600 to 800 kg ha −1 to maintain this productivity in Brazil. Official recommendations for nitrogen fertilizer are 400 kg ha −1 year −1 [ 61 ] and apply in tow or four splits. But the coffee growers applied urea between 600 and 800 kg ha −1 in 26 splits during coffee cycle. In fact, they attempted this practice to stop N deficiency, but causing low nitrogen use efficiency [ 146 ]. Luo et al. [ 32 ] suggests that 20% N can be reduced, when plant density is high, without yield loss and also can reduce for drip fertigation.
Application methods
The international plant nutrition institute is convincing the best agronomic practices, 4R nutrient application principles, i.e. source of fertilizer, rate, time and site/place [ 147 ]. Soil fertility varies with in the field abruptly which has strong impact on yield and nutrient uptake by cultivated crops, and this major problem can be handled by adopting site-specific nitrogen fertilization. Site-specific N fertilization provides significant impacts in terms of economy and ecology in heterogeneous fields [ 148 , 149 , 150 ] which results in enhanced yield, quality and ultimately high nitrogen use efficiency.
Spectral measurement is a suitable approach to know the nitrogen requirements of crops and site-specific application for precise farming [ 151 ]. The principle behind laser-induced chlorophyll fluorescence (LICF) is used to the measure the N situation of the crop stand by close distance [ 152 ] as well as 3–4 m [ 153 ]. The plant nitrogen is measured indirectly by chlorophyll content via fluorescence signals ratio at 690 and 730 nm [ 154 , 155 ]. It indicates that high amount of chlorophyll resulted in lower fluorescence radiation ratio F690/F730 because reabsorbed radiations have more strength at 690 nm. Rubisco acts as the sink of N and has close relation to chlorophyll content, thus the ratio F690/F730 describes the N content of the plant [ 156 ].
Crop canopy sensor calibration is too sensitive to field variability like the ramp calibration strip [ 157 ] or the calibration plot methods [ 158 ]. The reference area for canopy sensor within a field should be given according to field and soil variability [ 159 ] that also relates to the sugarcane plant density variation. The calibration should be done for every crop and season, separately [ 160 ]. Yong et al. [ 161 ] applied nitrogen fertilizer at various concentrations among the rows of maize-soybean relay intercropped field at three different distances (15 cm, 30 cm and 45 cm) and concluded that crop performed better for 15 cm and 30 cm treatments. The NUE and total grain yield of the maize-soybean relay intercropping system were significantly higher in 15 cm and 30 cm. So, lower N application at 15–30 cm from fertilizer application location to the maize row was optimal.
Productivity of low land rice has a great dependence on the selection of varieties and their nutrient utilization capacity. Under dose of N fertilizer may happen, especially when N is subject to immobilization following ratoon crop fertilization for unburned sites [ 56 ]. Crop response to inputs is also influenced by climate, for example, high altitude of Andhra Pradesh is endowed with the special soil and climate where varietal responses to inputs vary relatively to coastal plains. Different nitrogen sources should be jointly applied to fulfill the requirement of nitrogen to improve crop productivity [ 162 ].
The supply of N fertilizer to sugarcane is affected by soil profiles that are hard to measure inside the agricultural land [ 53 ]. Indeed, even the selection of reference regions, that get satisfactory measures for nitrogen, according to Raun et al. [ 163 ], can be risky with regards to evaluating sugarcane N feedback; depending upon where reference zones were set up, the harvest N reaction can differ altogether. For instance, producers may realize that a yield did or did not respond to N application,and such conflicting results found in various experiments were demonstrated by Duan et al. [ 117 ]. Hence, use of canopy sensors to quantify the N response is troublesome because of variable plant density inside the fields. In that capacity, different elements can veil the N impacts, like soil compaction, pest attack and diseases. Zillmann et al. [ 164 ] announced a comparative issue when they conducted a test for N connected to maize. For all the experimental area, the crop response for N was not similar as proposed.
The canopy sensor has to be utilized when the sugarcane tallness is between 40 and 70 cm to get estimation affectability to sugarcane vigor fluctuation [ 165 , 166 ]. At this stage, sugarcane has attained around 10–30% of total biomass with 27–68% N, which is dependent on genotype, soil fertility, climate and developmental stage [ 167 ]. N requirement of crop prior to treatment can achieved by various sources, i.e. mineralization of organic sources and endophytic nitrogen fixation by bacteria related to plant roots [ 53 , 168 , 169 , 170 ], and also other inputs to the field like vinasse, poultry manure and farmyard manure etc.
Drip fertigation
Northwestern China has an arid climate, cotton production in this region is not possible without irrigation and N fertilization [ 171 ]. Drip fertigation is a good option to supply water and fertilizers in precise quantities [ 172 , 173 ]. Drip fertigation with mulching is going to be extensively used in recent years [ 174 ]. It is well documented that the nutrient and water use efficiency both can be enhanced through drip fertigation that improves crop production for each unit of nutrients and water [ 172 , 175 ]. It has more advantage of the soluble fertilizers that can be put in specific quantity alongside the good crop health and potential yield because of maintained fertigation in the root zone [ 173 ]. Many studies pointed out fertigation can improve fertilizer use efficiency by decreasing application rates without losing crop yield [ 176 , 177 ] and especially drip fertigation of cotton field with reduced nitrogen, improved its efficiency [ 175 , 178 ]. It improved cotton yield, yield components, and leaf area index (LAI) by 20 to 30% as compared to furrow irrigation [ 179 ]. However, maximum nitrogen recovery was obtained by sacrificing cotton yield at lower N level under drip fertigation [ 180 ]. So, an optimum N level for drip fertigation has important role to achieve highest cotton yield.
Traditional high nitrogen application without considering method of application and plant population gives more seed cotton yield. Anyhow, N can be reduced up to 15–30% when drip fertigation is employed and 20% in case of high plant population without sacrificing seed cotton yield. The findings of Luo et al. [ 32 ] are that N reduction up to 30% has non-significant seedcotton yield reduction for drip fertigation. However, drip fertigation shows increase by 5 and 20.7% in seedcotton yield for 15 and 30% nitrogen reduction.
In other words, drip fertigation with high plant population is an important attribute to save nitrogen with sustainable yield for arid culture. Many experiments have conducted to find agronomic practices, high planting density, diversified planting geometry [ 181 ] organic fertilizers and improvement of application method of nutrients are helpful to regulate cotton yield for reduced nitrogen conditions in the Yellow River valley, China [ 11 , 12 , 140 ].
N and plant density
The plant density is an important tool to testify N rate without sacrifice of yield either by increase or decrease in number of plants per unit area [ 12 , 140 , 182 ]. It varies active crop canopy reflectance on the base of ground for sensors [ 183 ]. This idea has been proficiently utilized to control N application for rice [ 121 ], maize [ 184 , 185 , 186 , 187 , 188 ], cotton [ 189 ] and wheat [ 188 , 190 , 191 ]. The application of nitrogen based on canopy sensor depends on chlorophyll of crop canopy which describes nitrogen status [ 192 ], but it is not as valid for sugarcane. The field-scale sensor observations at the leaf level poorly show a relationship with nitrogen and chlorophyll status [ 166 ]. It is due to irregular sugarcane canopy which may show ground soil to the sensor. Dynamic and manually monitored canopy reflectance sensors are available, which consider all the parameters for sugarcane biomass variation, principally effected by plant population, as described by Amaral et al. [ 138 ].
Amaral et al. [ 138 ] conducted strip experiments for different nitrogen rates and validated that the uniform distribution of canopy has no trouble for canopy sensor. Variation in the canopy is mainly affected by plant population and vigor rather than the nitrogen supply. Six trials with differing nitrogen supply were conducted at different locations, five out of six trials has non-significant response to variable nitrogen supply and the sixth trial may have variation in soil characters, deeper root zone and more water holding capacity, therefore increases soil nutrient utilization and crop vigor.
Intercropping
Intercropped crops are significantly influenced by fertilization methods and show better growth for diverse nitrogen supply for interspecific rows instead of intraspecific [ 193 ]. Interspecific applications accelerate resource use efficiency, soil productivity and also have positive impacts on the environment [ 194 , 195 , 196 , 197 ]. This system involves more than one crop in a season, and can be observed in the Huang Huai Hai, China [ 198 ], and relay intercropping system is common in the Southwest China where one crop or three crops in 2 years are grown [ 199 ]. So, better nitrogen fertilization methods and relay or intercropping systems based on soybean (legume crop) greatly influenced on soybean yield with decreasing environmental cost. But environmental features like rainfall, light intensity and heat can be limiting factors for cropping systems. Maize-soybean relay intercropping occupies largest planting area in Southwest China that is helpful to improve nitrogen, light use efficiencies and soil nutrient availability [ 20 , 199 , 200 , 201 , 202 , 203 , 204 ].
There are many previous studies indicating that high N input has undesirable outcome for biological nitrogen fixation [ 205 ]. When nitrogen availability studied for legume-nonlegume mixtures, high content of mineral nitrogen in soil triggers the microbial nitrogen fixation and hence availability of nitrogen decrease for nonlegume crop [ 206 ]. However, low input of nitrogen increased significantly fixation and stimulated the translocation of fixed N to nonlegume [ 203 , 207 ].
NUE regulating enzymes and genes
The major sources of nitrogen, taken up by higher plants, are nitrate and ammonium as synthetic fertilizers, organic compounds and amino acids etc. It depends upon the availability of nitrogen, and within the plants it is controlled by many metabolic pathways and genes expression levels [ 208 ]. Nitrogen use efficiency is dependent of soil nitrogen conditions, photo synthetically fixed carbon dioxide to provide precursor for biosynthesis of many amino acids and vice versa [ 209 , 210 ]. It has been also claimed that all the inorganic nitrogenous fertilizers first converted to ammonium before uptake by higher plants [ 211 ]. Nitrate reduction occurs in roots as well as shoots but nitrate reduced directly in cytoplasm while in plastids/chloroplast via nitrite [ 208 ]. Reduction of nitrate to nitrite occurs in cytosol by nitrate reductase enzyme (Table 1 ) [ 212 ]. Nitrite is transported into chloroplasts in leaves where nitrite is converted to ammonium ions due to nitrite reductase (Table 1 ) [ 213 ]. The products of ammonia, glutamine and glutamate, act as donor of the nitrogen during biosynthesis for nucleic acid, chlorophyll and amino acids. The isoenzymes of glutamine synthetase, glutamate synthase, and glutamate dehydrogenase (Table 1 ) have been proposed for three major ammonium assimilation processes: primary nitrogen assimilation, reassimilation of photorespiratory ammonia, and “recycled” nitrogen [ 213 ]. Organic nitrogen in the form of amino acids transferred from source organs to sink (Fig. 2 ), for example, glutamine and glutamate can be used to form aspartate and asparagine [ 211 , 214 ]. The ammonium nitrogen is transferred into amino acids by the enzymes e.g. glutamine synthetase, glutamate synthase, asparagine synthetase and aspartate amino transferase (Table 1 ). The coherent situation existed for glutamate dehydrogenase either it is involved in assimilation of ammonium nitrogen or carbon cycling [ 215 , 216 ].
The ammonium assimilating enzymes are important during grain filling stage due to its remobilization. The biosynthesis of amino acids from ammonia is occurred by the GS and GOGAT pathways (Fig. 5 ) [ 217 ]. Nitrogen reutilization is an important phenomenon involving NADH-GOGAT enzyme, rice grain weight increased up to 80% due to over production of NADH-GOGAT [ 218 ]. Glutamine dehydrogenase involves for senescing of leaves and also controversy as deaminating (Fig. 5 ) [ 219 , 220 ] and aminating directions [ 23 ]. Young leaves recycle nitrogen from chloroplast by GS2 and Fd-GOGAT. In GOGAT catalyzed proteolysis, GS2 and de facto NiR are responsible for breakdown of chloroplast during senescence. Production of glutamine during leaf senescence is basically dependent on GS1 isoform. Substrates for GDH are produced from chloroplast proteins proteolysis, and deaminating activity provides 2-oxoglutarate and ammonia. Glutamine for new sink organ is produced by GS1 reassimilation of ammonia [ 221 ].
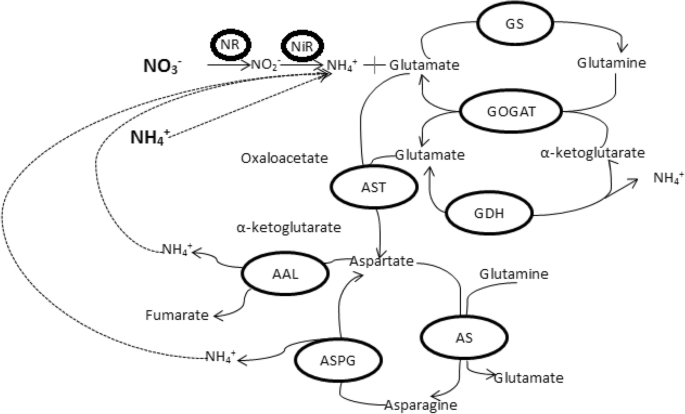
Schematic diagram to show the fate of nitrogen within the plant Bolded NO − 3 and NH + 4 are nitrogen uptake forms by roots through different transporters
Each monomer of homodimer nitrate reductase associated with three prosthetic groups: flavin adenine dinucleotide (FAD), a molybdenum cofactor (MoCo) and a haem. NR reduces chlorate into toxic chlorite, responsible gene for that in mutant has been identified, the Nia genes encoding the NR apoenzyme and the Cnx genes encoding the MoCo cofactor. [ 208 , 222 ]. The Nii genes have one to two copies encoding the NiR enzyme [ 208 ]. GS having decameric structure is controlled by two classes of genes, GLN1 and GLN2 , [ 223 ]. GLN2 (single nuclear gene) encodes chloroplastic GS2, involved in ammonium assimilation or re-assimilation either from nitrate reduction in C 3 and C 4 plants or photorespiratory product of C 3 plants [ 224 ]. On the other hand, GS1 isoform is encoded by GLN1 gene family which recycles ammonium during leaf senescing and transport in the phloem sap [ 225 ]. Vanoni et al. [ 226 ] reported that GOGAT (mechanistic structure) has two forms Fd GOGAT (in leaf chloroplast) and NADH GOGAT (in plastids of non-photosynthetic tissues). Three genes ( ASN1, ASN2 and ASN3 ) encode asparagine synthase, and substrate ammonia is utilized by asparagine synthase to form asparagine [ 227 ]. Storage compounds, long-range transporter and glutamine has lower N/C ratio than asparagine [ 228 , 229 ]. In plastids; bicarbonate, adenosine tri-phosphate and amide/ammonium from glutamine act as substrate for carbamoylphosphate synthase (CPSase) to form precursor (carbamoylphosphate) of citrulline and arginine. The subunits (small and large) of carbamoylphosphate synthase (CPSase) encoded by car A and B genes, respectively [ 230 ]. Finally, glutamate is produced by mitochondrial NADH-glutamate dehydrogenase for higher levels of ammonium [ 23 ].
NUE responsive genes manipulation
Crop varieties that are highly N efficient, high yields with reduced N input is the main solution for improving NUE [ 231 , 232 , 233 ]. Recent studies documented that shoot-to-root signaling pathways, feedback mechanisms and amino acids transportation in roots and shoots influence the nitrogen uptake and its metabolism [ 234 , 235 , 236 , 237 , 238 ]. With the aim of improving NUE, approaches have been adopted on the basis of genetic changes for nitrogen uptake [ 239 , 240 , 241 ], nitrate allocation [ 242 ], nitrogen metabolism [ 218 , 243 , 244 , 245 , 246 , 247 , 248 , 249 ] and the regulation [ 250 ].
Many critical candidate genes also have been over-expressed and knocked out in order to test for biomass and plant nitrogen status. Nitrate influx increased due to over-expression of HATS-like NRT2.1 but at the same time NUE and its utilization phenotypically remains unchanged [ 46 ]. Overexpression of genes encoding for NR/NiR in transgenic plants to improve NUE has no surety for its utility. Nitrate reductase related gene overexpression in tobacco plants showed delayed NR-activity for drought conditions and quick recovery for re-watering after short time drought [ 251 ]. It has been observed that nitrate level decreased in transgenic Arabidopsis, tobacco and potato plants without improving in biomass, number of tubers and seeds respectively. Regardless of the nitrogen available sources, Nia or Nii genes overexpression improved mRNA levels besides N uptake affect without any change in the yield and growth, indicating the composite post-transcriptional regulation of NR [ 252 ].
When we talk about GS1 and GS2 genes expression, the overexpressed GS2 has been testified along with Rubisco promoter in Nicotiana tabaccum and CaMV 35S promoter in Oryza sativa [ 4 , 217 ]. It enhanced growth rate in Nicotiana tabaccum and photorespiration and drought tolerance in Oryza sativa . Overexpression of GS1 genes with promoters having different combinations, RolD, CaMV 35S and Rubisco subunit (rbcS) have been reported with positive results for plant biomass and grain yield. For example, grain yield and roots are significantly higher with more N content in nitrogen efficient wheat lines under the control of the rbcS promoter observed [ 248 ]. Similarly, biomass and leaf protein in Nicotiana tabacum (over expressed GS1 ) increased under the control of CaMV 35S promoter [ 253 ]. Another overexpression of GS1 gene depicted 30% increase in yield of maize due to more kernel number and size [ 231 ]. In conclusion, GS activity has direct relation with biomass or yield in transgenic plants [ 254 ]. Over-expression of NADH-GOGAT increased in grain yield for transgenic rice plant [ 231 ]. So, it is important to know the alleles of genes and promoters to improve yield by overexpressing GS or GOGAT genes. Overexpressed ASN1 in Arabidopsis increased soluble protein content in seed, total protein and plants ability to grow for limited nitrogen supply [ 229 ]. These results suggested that NUE can be improved by manipulating downstream steps in N-remobilization. Further studies of carbon metabolism pathways also have potential to improve NUE [ 255 , 256 , 257 ].
Several external and endogenous factors influenced the expression of genes which are highly regulated at the transcriptional as well as post-translational levels [ 208 ]. Lea et al. [ 218 ] demonstrated that post-translational regulation affects the amino acids, ammonium, and nitrate levels, whereas transcriptional regulation has only minor influence. Plants unregulated for NR accumulate high concentrations of asparagine and glutamine in leaves. Thus further characterization can provide the useful properties for crops.
Asparagine synthetase (AS) encoded by a small gene family, catalyzes the formation of asparagine (Asn) (Fig. 5 ) and glutamate from glutamine (Gln) and aspartate [ 258 ]. The role of AS and GS interaction in primary N metabolism is very crucial [ 259 , 260 ]. GS negatively correlates with the AS transcript levels and polypeptides in the transgenic plants suggesting that AS showed compensation for GS ammonium assimilatory activity [ 260 , 261 ]. It is hypothized that AS might be important in regulation of the reduced N flux into plants due to decreased GS activity. However, the GS is essential to synthesize Gln for biosynthesis of Asp via NADH-GOGAT and AspAT [ 260 ]. Lam et al. [ 229 ] demonstrated the results of overexpressed the ASN1 gene in Arabidopsis as enhanced soluble seed protein content, total protein content with better growth on N-limiting medium. However, in case of ASN2 gene endogenous ammonium accumulation was less compared to wild-type plants as growing on 50-mM ammonium medium [ 22 ]. Signaling processes are attractive clues for metabolic engineering. Physiological activity of glutamate dehydrogenase (GDH) is still unclear as compared to GS/GOGAT enzymes [ 215 ]. Ameziane et al. [ 241 ] investigated GDH activity in transgenic tobacco plant, and the biomass production increased in gdhA transgenic plants without considering growing conditions either controlled conditions or field.
Microarray and whole genome sequencing
It has been observed that N uptake remains constant throughout domestication of extraordinary maize varieties but utilization of N enhanced, which support the hypothesis of conventional breeding programs improving NRE capacity [ 262 ]. Interestingly, inconsistency of overexpressed key enzymes (NR, NiR, GS, and GOGAT) for an improvement of NUE or phenotypic change is also a challenge [ 218 , 231 , 254 , 262 ]. Due to these reasons, new molecular techniques like microarray and transcriptome (Fig. 6 ) are consider as emerging tools to study the response of plants whole genome.
Work flow chart for transcriptomic profiling for crops
The arrangement of known and unknown DNA samples on a solid support is known as microarray. Every microarray contains thousands of spots, each has less than 200 µM diameter and called probe [ 263 ]. These arrays may be in different formats and also probes can be smaller as oligonucleotides, cDNA or genomic sequences. Different techniques (photolithographic, nib, pin or inkjet) are employed to format. The probes are labelled radioactively or fluorescently and hybridization controlled electronically [ 264 ].
Whole genome sequencing is a modern approach to understand the changes at genomic level, expression level of genes and specific genes related to the desired traits. Good quality genome sequence information of ideotype rice and Arabidopsis plants are available for microarray analysis, but the transcriptomic profiling (Fig. 6 ) for whole genome sequencing of RNA is an excellent emerging technology for all plants [ 265 , 266 ]. Molecular and physiological techniques have been employed in last two decades to know the differentially expressed genes (DEGs) in Oryza sativa [ 267 , 268 ], Sorghom bicolor [ 269 ], Glycin max [ 270 ] and Camilia sinensis [ 271 ] for low nitrogen levels. Past studies mostly relied on single genotype for genes expression all over the world for low and normal nitrogen conditions either for nitrate or ammonium [ 267 , 268 , 269 , 270 , 271 ]. However, two genotypes of Camilia sinensis were studied and compared for both levels of nitrogen in ammonium form. Genotypic contrast for global genes expression and comparative analysis helped to compact the knowledge of candidate genes for NUE. A lot of information in literature regarding quantitative trait loci (QTLs) responding NUE are also available [ 272 , 273 , 274 ]. The combination of DEGs and QTLs datasets has great importance to develop new nitrogen use efficient genotypes in future [ 275 ].
Recent next generation sequencing technologies for transcriptomic profiling are helpful to understand the genes transcription and regulation of transcripts at all levels [ 276 ]. Illumina’s RNA-sequencing platform was used for transcriptomic exploration of genes expression to investigate the response of nitrogen nutritional stress in plants. It has been reported that the amino acid transporters in wheat plants play important role to transport nitrogen for development and a biotic stress conditions [ 277 ]. Based on the transcriptomic profiling Dai et al. studied the regulatory mechanism for storage protein in wheat grain in response to nitrogen supply during grain development [ 278 ]. Asparagine has crucial importance for nitrogen uptake in roots and considered as ideal nitrogen transporting molecule [ 258 , 279 , 280 ]. According to Curci et al. genes encoding asparagine were down regulated in leaves and roots of durum wheat under limited nitrogen [ 276 ]. It has been clearly observed that genes were down regulated in roots and leaves which were involved in carbon, nitrogen, amino acid metabolisms, and photosynthetic activity for plants grown under nitrogen free conditions [ 268 ].
The agronomic and molecular approaches altogether have potential to improve nitrogen use efficiency. Nitrogen losses can be minimized by precision agriculture, cut off nitrogen dose, intercropping of legume and non-legume crops, improving plant populations and introducing nitrogen efficient genotypes. Although the studies have been conducted to improve nitrogen use efficiency of many crops by manipulating single or more genes but now the advanced technologies like whole genome sequencing are more important for future studies. Molecular breeding instead of conventional breeding is going to be more popular as of advancement in technologies. Wild genotypes are another option to improve NUE due to their more resistance against diseases, insect pest and have yield potential.
Availability of data and materials
Not applicable.
Abbreviations
- Nitrogen use efficiency
Nitrogen utilization efficiency
Nitrogen uptake efficiency
Agronomic nitrogen use efficiency
Adenosine triphosphate
Polymerase chain reaction
Nitrogen recovery efficiency
Nitrate reductase
Nitrite reductase
Glutamine synthetase
Frink CR, Waggoner PE, Ausubel JH. Nitrogen fertilizer: retrospect and prospect. Proc Natl Acad Sci. 1999;96:1175–80.
Article CAS PubMed PubMed Central Google Scholar
Crawford NM, Forde BG. Molecular and developmental biology of inorganic nitrogen nutrition. Arabidopsis Book. 2002;1:e1.
Article Google Scholar
Stitt M, Krapp A. The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant Cell Environ. 1999;22:583–621.
Article CAS Google Scholar
Good AG, Shrawat AK, Muench DG. Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production? Trends Plant Sci. 2004;9:597–605.
Article CAS PubMed Google Scholar
Ding L, Wang K, Jiang G, Biswas D, Xu H, Li L, Li Y. Effects of nitrogen deficiency on photosynthetic traits of maize hybrids released in different years. Ann Bot. 2005;96:925–30.
Diaz C, Saliba-Colombani V, Loudet O, Belluomo P, Moreau L, Daniel-Vedele F, Morot-Gaudry J-F, Masclaux-Daubresse C. Leaf yellowing and anthocyanin accumulation are two genetically independent strategies in response to nitrogen limitation in Arabidopsis thaliana . Plant Cell Physiol. 2006;47:74–83.
Bondada B, Oosterhuis D, Norman R, Baker W. Canopy photosynthesis, growth, yield, and boll 15 N accumulation under nitrogen stress in cotton. Crop Sci. 1996;36:127–33.
Chen W, Hou Z, Wu L, Liang Y, Wei C. Effects of salinity and nitrogen on cotton growth in arid environment. Plant Soil. 2010;326:61–73.
Hou Z, Li P, Li B, Gong J, Wang Y. Effects of fertigation scheme on N uptake and N use efficiency in cotton. Plant Soil. 2007;290:115–26.
Bondada BR, Oosterhuis DM. Canopy photosynthesis, specific leaf weight, and yield components of cotton under varying nitrogen supply. J Plant Nutr. 2001;24:469–77.
Dong H, Li W, Eneji AE, Zhang D. Nitrogen rate and plant density effects on yield and late-season leaf senescence of cotton raised on a saline field. Field Crops Res. 2012;126:137–44.
Mao S. Cotton Farming in China Shanghai. Shanghai: Shanghai Scientific and Technical Press; 2013.
Google Scholar
S.C. Hodges, Fertilization, in: K.L.E. Edmisten (ed.) Cotton Information Publ.AG- 417, North Carolina Cooperative Extension Service, Raleigh, NC, (2002) 40-54.
Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C. Food security: the challenge of feeding 9 billion people. Science. 2010;327:812–8.
CAS PubMed Google Scholar
Tilman D, Balzer C, Hill J, Befort BL. Global food demand and the sustainable intensification of agriculture. Proc Natl Acad Sci USA. 2011;108:20260–4.
Larson C. Losing arable land, China faces stark choice: adapt or go hungry. Am Assoc Adv Sci. 2013;339(6120):644.
CAS Google Scholar
Zhou L. Grain production and food security in China. In: Zhou L, editor. Reform and development of agriculture in China. Singapore: Springer; 2017. p. 141–69.
Zhu Z, Chen D. Nitrogen fertilizer use in China-Contributions to food production, impacts on the environment and best management strategies. Nutr Cycl Agroecosyst. 2002;63:117–27.
Kwong KNK, Bholah A, Volcy L, Pynee K. Nitrogen and phosphorus transport by surface runoff from a silty clay loam soil under sugarcane in the humid tropical environment of Mauritius. Agr Ecosyst Environ. 2002;91:147–57.
Wang X, Deng X, Pu T, Song C, Yong T, Yang F, Sun X, Liu W, Yan Y, Du J. Contribution of interspecific interactions and phosphorus application to increasing soil phosphorus availability in relay intercropping systems. Field Crops Research. 2017;204:12–22.
Xing G, Zhu Z. Regional nitrogen budgets for China and its major watersheds. Biogeochemistry. 2002;57:405–27.
Moose S, Below FE. Biotechnology approaches to improving maize nitrogen use efficiency. In: Kriz AL, Larkins A, editors. Molecular genetic approaches to maize improvement. Berlin: Springer; 2009. p. 65–77.
Chapter Google Scholar
Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A. Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot. 2010;105:1141–57.
Article PubMed PubMed Central Google Scholar
Moll R, Kamprath E, Jackson W. Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization 1. Agron J. 1982;74:562–4.
Brauer EK, Shelp BJ. Nitrogen use efficiency: re-consideration of the bioengineering approach. Botany. 2010;88:103–9.
A.G. Good, P.H. Beatty, Biotechnological approaches to improving nitrogen use efficiency in plants: alanine aminotransferase as a case study, The molecular and physiological basis of nutrient use efficiency in crops, (2011) 165-191.
Rochester IJ, O’Halloran J, Maas S, Sands D, Brotherton E. Monitoring nitrogen use efficiency in your region. Aust Cottongrower. 2007;28:24–7.
Novoa R, Loomis RS. Green plants play a unique role among living organisms through their ability to reduce carbon in photosynthesis. Plant Soil. 1981;58:177–204.
Vanlauwe B, Kihara J, Chivenge P, Pypers P, Coe R, Six J. Agronomic use efficiency of N fertilizer in maize-based systems in sub-Saharan Africa within the context of integrated soil fertility management. Plant Soil. 2011;339:35–50.
Dilz K. Efficiency of uptake and utilization of fertilizer nitrogen by plants. In: Jenkinson DS, Smith KA, editors. Nitrogen efficiency in agricultural soils. London and New York: Elsevier; 1988. p. 1–26.
Rochester IJ. Assessing internal crop nitrogen use efficiency in high-yielding irrigated cotton. Nutr Cycl Agroecosyst. 2011;90:147–56.
Luo Z, Liu H, Li W, Zhao Q, Dai J, Tian L, Dong H. Effects of reduced nitrogen rate on cotton yield and nitrogen use efficiency as mediated by application mode or plant density. Field Crops Research. 2018;218:150–7.
Zhang F, Cui Z, Fan M, Zhang W, Chen X, Jiang R. Integrated soil–crop system management: reducing environmental risk while increasing crop productivity and improving nutrient use efficiency in China. J Environ Qual. 2011;40:1051–7.
Yu P, Li X, White PJ, Li C. A large and deep root system underlies high nitrogen-use efficiency in maize production. PLoS ONE. 2015;10:0126293.
Hajari E, Snyman SJ, Watt MP. Nitrogen use efficiency of sugarcane (Saccharum spp.) varieties under in vitro conditions with varied N supply, Plant Cell. Tissue and Organ Culture. 2015;122:21–9.
Cramer M, Lewis O. The influence of nitrate and ammonium nutrition on the growth of wheat ( Triticum aestivum ) and maize ( Zea mays ) plants. Ann Bot. 1993;72:359–65.
Robinson N, Brackin R, Vinall K, Soper F, Holst J, Gamage H, Paungfoo-Lonhienne C, Rennenberg H, Lakshmanan P, Schmidt S. Nitrate paradigm does not hold up for sugarcane. PLoS ONE. 2011;6:e19045.
Witte CP. Urea metabolism in plants. Plant Sci. 2011;180:431–8.
Meyer J, Schumann A, Wood R, Nixon D, Van Den Berg M, Recent advances to improve nitrogen use efficiency of sugarcane in the South African sugar industry, In: Proceedings of the International Society of Sugar Cane Technologists, (2007), 238–246.
Cassman KG, Peng S, Olk D, Ladha J, Reichardt W, Dobermann A, Singh U. Opportunities for increased nitrogen-use efficiency from improved resource management in irrigated rice systems. Field Crops Research. 1998;56:7–39.
Liu L, Xu W, Tang C, Wang Z, Yang J. Effect of indigenous nitrogen supply of soil on the grain yield and fertilizer-N use efficiency in rice. Rice Sci. 2005;12:267–74.
Mengel K, Hütsch B, Kane Y. Nitrogen fertilizer application rates on cereal crops according to available mineral and organic soil nitrogen. Eur J Agron. 2006;24:343–8.
Mulvaney RL, Khan S, Ellsworth T. Need for a soil-based approach in managing nitrogen fertilizers for profitable corn production. Soil Sci Soc Am J. 2006;70:172–82.
Cui Z, Zhang F, Chen X, Miao Y, Li J, Shi L, Xu J, Ye Y, Liu C, Yang Z. On-farm evaluation of an in-season nitrogen management strategy based on soil Nmin test. Field Crops Res. 2008;105:48–55.
Takahashi DT. 15 N nitrogen field studies with sugarcane. Hawaii Plant Rec. 1964;57:198–222.
Olson R, Murphy L, Moser H, Swallow C. Fate of tagged fertilizer Nitrogen applied to winter wheat 1. Soil Sci Soc Am J. 1979;43:973–5.
Wilson C, Wells B, Norman R. Seasonal uptake patterns of fertilizer nitrogen applied in split applications to rice. Soil Sci Soc Am J. 1989;53:1884–7.
Omay A, Rice C, Maddux L, Gordon W. Corn yield and nitrogen uptake in monoculture and in rotation with soybean. Soil Sci Soc Am J. 1998;62:1596–603.
Schindler F, Knighton R. Fate of fertilizer nitrogen applied to corn as estimated by the isotopic and difference methods. Soil Sci Soc Am J. 1999;63:1734–40.
Stevens W, Hoeft R, Mulvaney RL. Fate of nitrogen-15 in a long-term nitrogen rate study. Agron J. 2005;97:1046–53.
Lopez-Bellido L, Lopez-Bellido RJ, Lopez-Bellido FJ. Fertilizer nitrogen efficiency in durum wheat under rainfed Mediterranean conditions: Effect of split application. Agron J. 2006;98:55–62.
Dourado-Neto D, Powlson D, Bakar RA, Bacchi OOS, Basanta M, Keerthisinghe G, Ismaili M, Rahman S, Reichardt K, Safwat M. Multiseason recoveries of organic and inorganic nitrogen-15 in tropical cropping systems. Soil Sci Soc Am J. 2010;74:139–52.
Franco HCJ, Otto R, Faroni CE, Vitti AC, de Oliveira ECA, Trivelin PCO. Nitrogen in sugarcane derived from fertilizer under Brazilian field conditions. Field Crops Research. 2011;121:29–41.
Lory J, Scharf P. Yield goal versus delta yield for predicting fertilizer nitrogen need in corn. Agron J. 2003;95:994–9.
Franco HC, Trivelin PC, Eduardo FC, Vitti AC, Otto R. Stalk yield and technological attributes of planted cane as related to nitrogen fertilization. Scientia Agricola. 2010;67:579–90.
Rossetto R, Dias F, Landell M, Cantarella H, Tavares S, Vitti A, Perecin D. N and K fertilisation of sugarcane ratoons harvested without burning. Proceeding of the International Society of Sugar Cane Technologists. 2010;27:1–8.
Meier EA, Thorburn PJ, Wegener MK, Basford KE. The availability of nitrogen from sugarcane trash on contrasting soils in the wet tropics of North Queensland. Nutr Cycl Agroecosyst. 2006;75:101–14.
Thorburn PJ, Dart IK, Biggs IM, Baillie CP, Smith MA, Keating BA. The fate of nitrogen applied to sugarcane by trickle irrigation. Irrig Sci. 2003;22:201–9.
Leghari SJ, Wahocho NA, Laghari GM, Hafeez Laghari A, Mustafa Bhabhan G, Hussain Talpur K, Bhutto TA, Wahocho SA, Lashari AA. Role of nitrogen for plant growth and development: a review. Adv Environ Biol. 2016;10:209–18.
Neto AP, Favarin JL, de Almeida REM, dos Santos Dias CT, Tezotto T, Alves ALG, Moraes MF. Changes of nutritional status during a phenological cycle of coffee under high nitrogen supply by fertigation. Commun Soil Sci Plant Anal. 2011;42:2414–25.
dos Reis AR, Favarin JL, Gallo LA, Moraes MF, Tezotto T, Lavres Junior J. Influence of nitrogen fertilization on nickel accumulation and chemical composition of coffee plants during fruit development. J Plant Nutr. 2011;34:1853–66.
Reis AR, Favarin JL, Gallo LA, Malavolta E, Moraes MF, Lavres J. Nitrate reductase and glutamine synthetase activity in coffee leaves during fruit development. Revista Brasileira de Ciência do Solo. 2009;33:315–24.
Fenilli TAB, Reichardt K, Dourado-Neto D, Trivelin PCO, Favarin JL, Costa FMP, Bacchi OOS. Growth, development, and fertilizer- 15 N recovery by the coffee plant. Scientia Agricola. 2007;64:541–7.
Mazzafera P, Gonçalves KV. Nitrogen compounds in the xylem sap of coffee. Phytochemistry. 1999;50:383–6.
Wray JL, Fido RJ. Nitrate reductase and nitrite reductase. In: Dey PM, Harborne JB, editors. Methods in plant biochemistry. Waltham: Academic Press; 1990.
Franco AL, Cherubin MR, Pavinato PS, Cerri CE, Six J, Davies CA, Cerri CC. Soil carbon, nitrogen and phosphorus changes under sugarcane expansion in Brazil. Sci Total Environ. 2015;515:30–8.
Article PubMed CAS Google Scholar
Ferreira DA, Franco HC, Otto R, Vitti AC, Fortes C, Faroni CE, Garside AL, Trivelin PC. Contribution of N from green harvest residues for sugarcane nutrition in B razil. GCB Bioenergy. 2016;8:859–66.
Muchow R, Robertson M. Relating crop nitrogen uptake to sugarcane yield. Proceeding of Australian Society of Sugar Cane Technologists. 1994;16:122–30.
Granstedt RC, Huffaker RC. Identification of the leaf vacuole as a major nitrate storage pool. Plant Physiol. 1982;70:410–3.
Burns IG, Walker RL, Moorby J. How do nutrients drive growth? Plant Soil. 1997;196:321–5.
Crawford NM, Glass AD. Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci. 1998;3:389–95.
Duncan EG, Sulivan CA, Roper MM, Biggs JS, Peoples MB. Influence of co-application of nitrogen with phosphorus, potassium and sulphur on the apparent efficiency of nitrogen fertiliser use, grain yield and protein content of wheat: review. Field Crops Research. 2018;226:56–65.
Groot CC, Marcelis LFM, van Den Boogard R, Kaiser WN, Lambers H. Interaction of nitrogen and phosphorus nutrition in determining growth. Plant Soil. 2003;248:257–68.
Chen H, Zhang W, Gurmesa GA, Zhu X, Li D, Mo J. Phosphorus addition affects soil nitrogen dynamics in a nitrogen-saturated and two nitrogen-limited forests. Eur J Soil Sci. 2017;68:472–9.
Yokoyama D, Imai N, Kitayama K. Effects of nitrogen and phosphorus fertilization on the activities of four different classes of fine-root and soil phosphatases in Bornean tropical rain forests. Plant Soil. 2017;416:463–76.
Alzubaidi A, Aljanabi AS, Al-Rawi A. Interaction between nitrogen and phosphorus fertilizers and soil salinity and its effect on growth and ionic composition of corn ( Zea mays L.). In: El Bassam N, Dambroth M, Loughman BC, editors. Genetic aspects of plant mineral nutrition developments in plant and soil sciences. Dordrecht: Springer; 2018. p. 42.
Kuzyakov Y, Xu X. Competition between roots and microorganisms for nitrogen: mechanisms and ecological relevance. New Phytol. 2013;198:656–69.
Schimel JP, Bennett J. Nitrogen mineralization: challenges of a changing paradigm. Ecology. 2004;85:591–602.
Vitousek PM, Howarth RW. Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry. 1991;13:87–115.
Vitousek PM, Sanford RL Jr. Nutrient cycling in moist tropical forest. Annu Rev Ecol Syst. 1986;17:137–67.
Xu X, Thornton PE, Post WM. A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Glob Ecol Biogeogr. 2013;22:737–49.
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science. 2008;320:889–92.
Tilman D, Fargione J, Wolff B, Dantonio C, Dobson A, Howarth R, Schindler D, Schlesinger WH, Simberloff D, Swackhamer D. Forecasting agriculturally driven global environmental change. Science. 2001;292:281–4.
Vitousek PM, Naylor R, Crews T, David MB, Drinkwater L, Holland E, Johnes P, Katzenberger J, Martinelli L, Matson P. Nutrient imbalances in agricultural development. Science. 2009;324:1519–20.
Liu J, You L, Amini M, Obersteiner M, Herrero M, Zehnder AJ, Yang H. A high-resolution assessment on global nitrogen flows in cropland, Proc Natl Acad Sci USA. 2010;107:8035–40.
Guo JH, Liu XJ, Zhang Y, Shen J, Han W, Zhang W, Christie P, Goulding K, Vitousek P, Zhang F. Significant acidification in major Chinese croplands. Science. 2010;327:1008–10.
Chen X-P, Cui Z-L, Vitousek PM, Cassman KG, Matson PA, Bai J-S, Meng Q-F, Hou P, Yue S-C, Römheld V. Integrated soil–crop system management for food security. Proc Natl Acad Sci USA. 2011;108:6399–404.
Zhao X, Zhou Y, Min J, Wang S, Shi W, Xing G. Nitrogen runoff dominates water nitrogen pollution from rice-wheat rotation in the Taihu Lake region of China. Agr Ecosyst Environ. 2012;156:1–11.
Zhang WF, Dou ZX, He P, Ju XT, Powlson D, Chadwick D, Norse D, Lu YL, Zhang Y, Wu L. New technologies reduce greenhouse gas emissions from nitrogenous fertilizer in China. Proc Natl Acad Sci USA. 2013;110:8375–80.
Socolow RH. Nitrogen management and the future of food: lessons from the management of energy and carbon. Proc Natl Acad Sci. 1999;96:6001–8.
Zhang Q. Strategies for developing green super rice. Proc Natl Acad Sci USA. 2007;104:16402–9.
Peoples M, Herridge D, Ladha J. Biological nitrogen fixation: an efficient source of nitrogen for sustainable agricultural production. In: Ladha JK, Peoples MB, editors. Management of biological nitrogen fixation for the development of more productive and sustainable agricultural systems. Berlin: Springer; 1995. p. 3–28.
Matson PA, Naylor R, Ortiz-Monasterio I. Integration of environmental, agronomic, and economic aspects of fertilizer management. Science. 1998;280:112–5.
Panek J, Matson P, Ortiz-Monasterio I, Brooks P. Distinguishing nitrification and denitrification sources of N2O in a Mexican wheat system using 15 N. Ecol Appl. 2000;10:506–14.
Matějková Š, Kumhálová J, Lipavský J. Evaluation of crop yield under different nitrogen doses of mineral fertilization. Plant, Soil and Environment. 2010;56:163–7.
Mariano E, Leite JM, Megda MX, Torres-Dorante L, Trivelin PC. Influence of nitrogen form supply on soil mineral nitrogen dynamics, nitrogen uptake, and productivity of sugarcane. Agron J. 2015;107:641–50.
Otto R, Mulvaney RL, Khan S, Trivelin P. Quantifying soil nitrogen mineralization to improve fertilizer nitrogen management of sugarcane. Biol Fertil Soils. 2013;49:893–904.
Cantarella H, Trivelin PCO, Contin TLM, Dias FLF, Rossetto R, Marcelino R, Coimbra RB, Quaggio JA. Ammonia volatilisation from urease inhibitor-treated urea applied to sugarcane trash blankets. Scientia Agricola. 2008;65:397–401.
Ghiberto P, Libardi P, Brito A, Trivelin PCO. Leaching of nutrients from a sugarcane crop growing on an Ultisol in Brazil. Agric Water Manag. 2009;96:1443–8.
Ghiberto P, Libardi P, Trivelin P. Nutrient leaching in an Ultisol cultivated with sugarcane. Agric Water Manag. 2015;148:141–9.
Oliveira JCM, Reichardt K, Bacchi OO, Timm LC, Dourado-Neto D, Trivelin PCO, Tominaga TT, Navarro RC, Piccolo MC, Cássaro FAM. Nitrogen dynamics in a soil-sugar cane system. Scientia Agricola. 2000;57:467–72.
Signor D, Cerri CEP, Conant R. N 2 O emissions due to nitrogen fertilizer applications in two regions of sugarcane cultivation in Brazil. Environ Res Lett. 2013;8:015013.
Carmo JB, Filoso S, Zotelli LC, de Sousa Neto ER, Pitombo LM, Duarte-Neto PJ, Vargas VP, Andrade CA, Gava GJ, Rossetto R. Infield greenhouse gas emissions from sugarcane soils in Brazil: effects from synthetic and organic fertilizer application and crop trash accumulation. Gcb Bioenergy. 2013;5:267–80.
Fortes C, Trivelin PCO, Vitti AC. Long-term decomposition of sugarcane harvest residues in Sao Paulo state, Brazil. Biomass Bioenergy. 2012;42:189–98.
Vieira-Megda MX, Mariano E, Leite JM, Franco HCJ, Vitti AC, Megda MM, Khan SA, Mulvaney RL, Trivelin PCO. Contribution of fertilizer nitrogen to the total nitrogen extracted by sugarcane under Brazilian field conditions. Nutr Cycl Agroecosyst. 2015;101:241–57.
Otto R, Castro S, Mariano E, Castro S, Franco H, Trivelin PCO. Nitrogen use efficiency for sugarcane-biofuel production: what is next? Bioenergy Res. 2016;9:1272–89.
Khan S, Mulvaney RL, Ellsworth T, Boast C. The myth of nitrogen fertilization for soil carbon sequestration. J Environ Qual. 2007;36:1821–32.
Ladha JK, Reddy CK, Padre AT, Van Kessel C. Role of nitrogen fertilization in sustaining organic matter in cultivated soils. J Environ Qual. 2011;40:1756–66.
Mulvaney RL, Khan S, Ellsworth T. Synthetic nitrogen fertilizers deplete soil nitrogen: a global dilemma for sustainable cereal production. J Environ Qual. 2009;38:2295–314.
Darrouzet-Nardi A, Weintraub MN. Evidence for spatially inaccessible labile N from a comparison of soil core extractions and soil pore water lysimetry. Soil Biol Biochem. 2014;73:22–32.
Inselsbacher E. Recovery of individual soil nitrogen forms after sieving and extraction. Soil Biol Biochem. 2014;71:76–86.
Inselsbacher E, Oyewole OA, Näsholm T. Early season dynamics of soil nitrogen fluxes in fertilized and unfertilized boreal forests. Soil Biol Biochem. 2014;74:167–76.
Sommer SG, Schjoerring JK, Denmead O. Ammonia emission from mineral fertilizers and fertilized crops. Adv Agron. 2004;82:82008.
Haenel HD, Rosemann C, Dammgen U, Freibauer A, Doring U, Wulf S, Eurich-Menden B, Dohler H, Schreiner C, Osterburg B. Calculations of gaseous and particulate emissions from German agriculture 1990–2014: Report on methods and data (RMD) submission 2016. Johann Heinrich von Thuenen-Institut, Braunschweig, Report. 2016;39:412.
Skocaj DM, Everingham YL, Schroeder BL. Nitrogen management guidelines for sugarcane production in Australia: can these be modified for wet tropical conditions using seasonal climate forecasting? Springer Sci Rev. 2013;1:51–71.
Ghiberto PJ, Libardi PL, Brito AS, Trivelin PCO. Nitrogen fertilizer leaching in an Oxisol cultivated with sugarcane. Scientia Agricola. 2011;68:86–93.
Duan W, Wang Q, Zhang H, Xie B, Li A, Hou F, Dong S, Wang B, Qin Z, Zhang L. Differences between nitrogen-tolerant and nitrogen-susceptible sweetpotato cultivars in photosynthate distribution and transport under different nitrogen conditions. PLoS ONE. 2018;13:e0194570.
Article PubMed PubMed Central CAS Google Scholar
Wu C, Liu Q, Kong F, Li H, Shi Y. Effects of nitrogen application rates on root yield and nitrogen utilization in different purple sweetpotato varieties. Aata Agronomica Sinica. 2016;42:113–22.
Walker D, Woodson W. Nitrogen rate and cultivar effects on nitrogen and nitrate concentration of sweet potato leaf tissue. Commun Soil Sci Plant Anal. 1987;18:529–41.
W. L.A., Eco-physiology of tropical crops, in: KT Alvim PDT (ed.) Root crops, Academic Press, New York, 1977, p. 187–236.
Xue L, Li G, Qin X, Yang L, Zhang H. Topdressing nitrogen recommendation for early rice with an active sensor in South China. Precis Agric. 2014;15:95–110.
Liu C, Wang K, Meng S, Zheng X, Zhou Z, Han S, Chen D, Yang Z. Effects of irrigation, fertilization and crop straw management on nitrous oxide and nitric oxide emissions from a wheat–maize rotation field in northern China. Agr Ecosyst Environ. 2011;140:226–33.
Yan G, Zheng X, Cui F, Yao Z, Zhou Z, Deng J, Xu Y. Two-year simultaneous records of N2O and NO fluxes from a farmed cropland in the northern China plain with a reduced nitrogen addition rate by one-third. Agr Ecosyst Environ. 2013;178:39–50.
Drinkwater LE, Wagoner P, Sarrantonio M. Legume-based cropping systems have reduced carbon and nitrogen losses. Nature. 1998;396:262.
Salvagiotti F, Cassman KG, Specht JE, Walters DT, Weiss A, Dobermann A. Nitrogen uptake, fixation and response to fertilizer N in soybeans: a review. Field Crops Res. 2008;108:1–13.
Graham M, Haynes R, Meyer J. Changes in soil chemistry and aggregate stability induced by fertilizer applications, burning and trash retention on a long-term sugarcane experiment in South Africa. Eur J Soil Sci. 2002;53:589–98.
de Resende AS, Xavier RP, de Oliveira OC, Urquiaga S, Alves BJ, Boddey RM. Long-term effects of pre-harvest burning and nitrogen and vinasse applications on yield of sugar cane and soil carbon and nitrogen stocks on a plantation in Pernambuco, NE Brazil. Plant and Soil. 2006;281:339–51.
Ju X-T, Xing G-X, Chen X-P, Zhang S-L, Zhang L-J, Liu X-J, Cui Z-L, Yin B, Christie P, Zhu Z-L. Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc Natl Acad Sci USA. 2009;106:3041–6.
Zhang Y, Li S, Fu W, Wen H. Effects of nitrogen application on yield, photosynthetic characteristics and water use efficiency of hybrid millet. J Plant Nutr Fertil. 2014;20:1119–26.
Pei-ling LI, Fu-cang ZH. Coupling effects of partitioning alternative drip irrigation with plastic mulch and nitrogen fertilization on cotton dry matter accumulation and nitrogen use. Chin J Appl Ecol. 2013;24:416–22.
Schumann A. Prospects for improving nitrogen fertiliser use efficiency with a new soil test and ammonia volatilisation model. Pro Ann Congr S Afr Sugar Technol Assoc. 2000;73:70–8.
Weigel A, Miles N, Nyandeni B, Naidoo G, Wettergreen T. Ammonia volatilisation losses from nitrogen fertilisers: laboratory studies. Proc Ann Congr S Afr Sugar Technol Assoc. 2014;87:353–7.
Weigel A, Naidoo G, Eustice T. Atmospheric nitrogen deposition: what does it tell you. Proc Ann Congr S Afr Sugar Technol Assoc. 2009;82:639–41.
Srivastava S. Sugarcane. In: Wichmann W, editor. IAF world fertilizer use manual. Paris: International Fertilizer Industry Association; 1992. p. 257–66.
A.A.A.F. Dametie, Determination of optimum nitrogen rate for sugarcane at Wonji-Shoa sugarcane plantation, in: 1st Ethiopian Sugar Industries Biennial Conference, Adama, Ethiopia, (2009) 23–24.
van DlLLEWIJN C. Botany of sugarcane, Botany of sugarcane. Waltham: The Chronica Botanica Co. Book Department; 1952.
Rakkiyappan P, Thangavelu S, Bhagyalakshmi K, Radhamani R. Uptake of nitrogen, phosphorus and potassium by some promising mid late maturing sugarcane clones. Sugar Tech. 2007;9:23–7.
Amaral LR, Molin JP, Portz G, Finazzi FB, Cortinove L. Comparison of crop canopy reflectance sensors used to identify sugarcane biomass and nitrogen status. Precis Agric. 2015;16:15–28.
Rochester I, Ceeney S, Maas S, Gordon R, Hanna L, Hill J. Monitoring nitrogen use efficiency in cotton crops. Austral Cottongrow. 2009;30:42.
Dong H, Kong X, Li W, Tang W, Zhang D. Effects of plant density and nitrogen and potassium fertilization on cotton yield and uptake of major nutrients in two fields with varying fertility. Field Crops Res. 2010;119:106–13.
Boquet DJ. Cotton in ultra-narrow row spacing. Agron J. 2005;97:279–87.
Clawson EL, Cothren JT, Blouin DC, Satterwhite JL. Timing of maturity in ultra-narrow and conventional row cotton as affected by nitrogen fertilizer rate. Agron J. 2008;100:421–31.
Janat M. Response of cotton to irrigation methods and nitrogen fertilization: yield components, water-use efficiency, nitrogen uptake, and recovery. Commun Soil Sci Plant Anal. 2008;39:2282–302.
Kumbhar A, Buriro U, Junejo S, Oad F, Jamro G, Kumbhar B, Kumbhar S. Impact of different nitrogen levels on cotton growth, yield and N-uptake planted in legume rotation. Pak J Bot. 2008;40:767–78.
CONAB, Monitoring of the Brazilian coffee production of 2009, in, Companhia Nacional de Abastecimento, Brasilia, (2010).
Neto AP, Favarin JL, dos Reis AR, Tezotto T, de Almeida REM, Junior JL, Gallo LA. Nitrogen metabolism in coffee plants in response to nitrogen supply by fertigation. Theor Exp Plant Physiol. 2015;27:41–50.
Bruulsema T, Lemunyon J, Herz B. Know your fertilizer rights. Crops Soils. 2009;42:13–8.
Ancev T, Whelan B, McBratney A, Stafford J. Evaluating the benefits from precision agriculture: the economics of meeting traceability requirements and environmental targets. Precis Agric. 2005;5:985–92.
U. Schmidhalter, F.-X. Maidl, H. Heuwinkel, M. Demmel, H. Auernhammer, P. Noack, M. Rothmund, Precision farming–adaptation of land use management to small scale heterogeneity, in: Perspectives for Agroecosystem Management, Elsevier, (2008) 121–199.
Reetz FR, Fixen PE. Economic analysis of site-specific nutrient management systems. In: Robert PC, Rust RH, Larson WE, editors. Proceeding of site-specific management for agricultural systems: second international conference, March 27-30, 1994. Minnesota: Minneapolis; 1995. p. 734–52.
Mistele B, Schmidhalter U. Estimating the nitrogen nutrition index using spectral canopy reflectance measurements. Eur J Agron. 2008;29:184–90.
Schächtl J, Huber G, Maidl F-X, Sticksel E, Schulz J, Haschberger P. Laser-induced chlorophyll fluorescence measurements for detecting the nitrogen status of wheat ( Triticum aestivum L.) canopies. Precis Agric. 2005;6:143–56.
Bredemeier C, Schmidhalter U. Laser-induced chlorophyll fluorescence sensing to determine biomass and nitrogen uptake of winter wheat under controlled environment and field conditions. Precis Agric. 2005;6:273–80.
Chappelle EW, Wood FM, McMurtrey JE, Newcomb WW. Laser-induced fluorescence of green plants. 1: A technique for the remote detection of plant stress and species differentiation. Appl Opt. 1984;23:134–8.
Lichtenthaler HK, Rinderle U. The role of chlorophyll fluorescence in the detection of stress conditions in plants. CRC Crit Rev Anal Chem. 1988;19:S29–85.
Chappelle EW, McMurtrey JE, Wood FM, Newcomb WW. Laser-induced fluorescence of green plants. 2: LIF caused by nutrient deficiencies in corn. Appl Opt. 1984;23:139–42.
Raun W, Solie J, Taylor R, Arnall D, Mack C, Edmonds D. Ramp calibration strip technology for determining midseason nitrogen rates in corn and wheat. Agron J. 2008;100:1088–93.
Shaver T, Khosla R, Westfall D. Evaluation of two crop canopy sensors for nitrogen variability determination in irrigated maize. Precis Agric. 2011;12:892–904.
Bausch WC, Brodahl MK. Strategies to evaluate goodness of reference strips for in-season, field scale, irrigated corn nitrogen sufficiency. Precis Agric. 2012;13:104–22.
P. Magalhães, G. Sanches, H. Franco, C. Driemeier, O. Kölln, O. Braunbeck, Precision agriculture in sugarcane production: A key tool to understand its variability, in: 12th International Conference on Precision Agriculture, Sacramento, CA, 2014, pp. 20-23.
Yong TW, Ping C, Qian D, Qing D, Feng Y, Wang XC, Liu WG, Yang WY. Optimized nitrogen application methods to improve nitrogen use efficiency and nodule nitrogen fixation in a maize-soybean relay intercropping system. J Integr Agric. 2018;17:664–76.
Kaupa P, Rao BR. Nitrogen mineralization and efficiency from co-applied animal manures and mineral fertilizer in sweetpotato under humid tropical conditions. Field Crops Res. 2014;168:48–56.
Raun WR, Solie JB, Johnson GV, Stone ML, Mullen RW, Freeman KW, Thomason WE, Lukina EV. Improving nitrogen use efficiency in cereal grain production with optical sensing and variable rate application. Agron J. 2002;94:815–20.
Zillmann E, Graeff S, Link J, Batchelor WD, Claupein W. Assessment of cereal nitrogen requirements derived by optical on-the-go sensors on heterogeneous soils. Agron J. 2006;98:682–90.
G. Portz, L. Amaral, J.P. Molin, J. Jasper, Optimum sugarcane growth stage for canopy reflectance sensor to predict biomass and nitrogen uptake, in: Internation Conference on Precision Agriculture, (2012).
Rios do Amaral L, Molin JP. The effectiveness of three vegetation indices obtained from a canopy sensor in identifying sugarcane response to nitrogen. Agron J. 2014;106:273–80.
E. Oliveira, Sugarcane nutritional balance related to nitrogen fertilization, Portuguese with English abstract.) Ph. D. Dissertation, University of São Paulo, Piracicaba, SP, Brazil. 2011. http://www.teses.usp.br/teses/disponiveis/11/11140/tde-20042011–094249/en.php . Accessed 30 Apr 2015.
Boddey RM, Urquiaga S, Alves BJ, Reis V. Endophytic nitrogen fixation in sugarcane: present knowledge and future applications. Plant Soil. 2003;252:139–49.
Gava GJC, Trivelin PCO, Vitti AC, Oliveira MW. Urea and sugarcane straw nitrogen balance in a soil-sugarcane crop system. Pesquisa Agropecuária Brasileira. 2005;40:689–95.
Schultz N, Morais RF, Silva JA, Baptista RB, Oliveira RP, Leite JM, Pereira W, Júnior C, de Barros J, Alves BJR. Agronomic evaluation of varieties of sugar cane inoculated with diazotrophic bacteria and fertilized with nitrogen. Pesquisa Agropecuária Brasileira. 2012;47:261–8.
Li Y, Wang F, Sun J, Liu H, Yang J, Xian F, Su H. Coupling effect of water and nitrogen on mechanically harvested cotton with drip irrigation under plastic film in arid area of western Inner Mongolia, China. J Appl Ecol. 2016;27:845–54.
Neelam P, Rajput T. Simulation and modeling of water movement in potato ( Solanum tuberosum ) under subsurface drip system. Indian J Agric Sci. 2011;81:25–32.
Ayyadurai P, Manickasundaram P. Growth, nutrient uptake and seed cotton yield as influenced by foliar nutrition and drip fertigation in cotton hybrid. International Journal of Agricultural Sciences. 2014;10:276–9.
Dai J, Dong H. Intensive cotton farming technologies in China: achievements, challenges and countermeasures. Field Crops Res. 2014;155:99–110.
B. Bar-Yosef, Advances in fertigation. In: Advances in Agronomy, Elsevier, (1999) 1–77.
Yan KF, Peng FT, Qi YJ, Dang ZQ, Zhang JH, Jiang XM. Effects of different irrigation and fertilizer methods on space variation of nitrate nitrogen and nitrogen fertilization absorption and utilization of plants. Water Saving Irrigation. 2015;9:33–8.
Ma HY, Shi WQ, Liu YA, Xian AM, Wang JG. Effect of different irrigation and fertilization methods on yield and water and fertilizer use efficiency of pineapple. Chin J Trop Crops. 2016;37:1882–8.
Jayakumar M, Surendran U, Manickasundaram P. Drip fertigation effects on yield, nutrient uptake and soil fertility of Bt cotton in semi arid tropics. Int J Plant Prod. 2014;8:375–90.
Li J, Zhang J, Rao M. Wetting patterns and nitrogen distributions as affected by fertigation strategies from a surface point source. Agric Water Manag. 2004;67:89–104.
Li Z, Wang H, Zhang F, Wu L, Wang Z, Zhou J. Effects of water-fertilizer coupling on field cotton growth and yield under fertigation in Xinjiang. J Drain Irrig Mach Eng. 2015;33:1069–77.
Anas M, Jabbar A, Sarwar MA, Ullah R, Abuzar MK, Ijaz A, Latif S. Intercropping sunflower with mungbean for improved productivity and net economic return under irrigated conditions. Pak J Agric Res. 2017;30:338.
Wang SL, Du HY, Qi H, Zhang Q, Lin YZ, Wang ZZ, Li ZF, Feng GY. Effects of density, fertilizer and chemical control on cotton development traits and yield. J Hebei Agric Sci. 2012;16:22–5.
Singh I, Srivastava AK, Chandna P, Gupta RK. Crop sensors for efficient nitrogen management in sugarcane: potential and constraints. Sugar Tech. 2006;8:299–302.
Scharf PC, Lory JA. Calibrating reflectance measurements to predict optimal sidedress nitrogen rate for corn. Agron J. 2009;101:615–25.
Holland K, Schepers J. Derivation of a variable rate nitrogen application model for in-season fertilization of corn. Agron J. 2010;102:1415–24.
Kitchen NR, Sudduth KA, Drummond ST, Scharf PC, Palm HL, Roberts DF, Vories ED. Ground-based canopy reflectance sensing for variable-rate nitrogen corn fertilization. Agron J. 2010;102:71–84.
Schmidt J, Beegle D, Zhu Q, Sripada R. Improving in-season nitrogen recommendations for maize using an active sensor. Field crops Res. 2011;120:94–101.
Solie JB, Monroe AD, Raun WR, Stone ML. Generalized algorithm for variable-rate nitrogen application in cereal grains. Agron J. 2012;104:378–87.
G. Vellidis, H. Savelle, R. Ritchie, G. Harris, R. Hill, H. Henry, NDVI response of cotton to nitrogen application rates in Georgia, USA, In: Precision Agriculture 2011–Proceedings of the 8th European Conference on Precision Agriculture (8ECPA), Prague, Czech Republic, (2011) 358–368.
Mullen RW, Freeman KW, Raun WR, Johnson GV, Stone ML, Solie JB. Identifying an in-season response index and the potential to increase wheat yield with nitrogen. Agron J. 2003;95:347–51.
Raun W, Solie J, Stone M, Martin K, Freeman K, Mullen R, Zhang H, Schepers J, Johnson G. Optical sensor-based algorithm for crop nitrogen fertilization. Commun Soil Sci Plant Anal. 2005;36:2759–81.
Schepers J, Francis D, Vigil M, Below FE. Comparison of corn leaf nitrogen concentration and chlorophyll meter readings. Commun Soil Sci Plant Anal. 1992;23:2173–87.
Wu B, Fullen MA, Li J, An T, Fan Z, Zhou F, Zi S, Yang Y, Xue G, Liu Z. Integrated response of intercropped maize and potatoes to heterogeneous nutrients and crop neighbours. Plant Soil. 2014;374:185–96.
Oljaca S, Cvetkovic R, Kovacevic D, Vasic G, Momirovic N. Effect of plant arrangement pattern and irrigation on efficiency of maize (Zea mays) and bean ( Phaseolus vulgaris ) intercropping system. J Agric Sci. 2000;135:261–70.
Li L, Zhang F, Li X, Christie P, Sun J, Yang S, Tang C. Interspecific facilitation of nutrient uptake by intercropped maize and faba bean. Nutr Cycl Agroecosyst. 2003;65:61–71.
Li L, Sun J, Zhang F, Li X, Rengel Z, Yang S. Wheat/maize or wheat/soybean strip intercropping: II. Recovery or compensation of maize and soybean after wheat harvesting. Field Crops Research. 2001;71:173–81.
Hochman Z, Carberry P, Robertson M, Gaydon D, Bell L, McIntosh P. Prospects for ecological intensification of Australian agriculture. Eur J Agron. 2013;44:109–23.
Liu X, Rahman T, Yang F, Song C, Yong T, Liu J, Zhang C, Yang W. PAR interception and utilization in different maize and soybean intercropping patterns. PLoS ONE. 2017;12:e0169218.
Yang F, Liao D, Wu X, Gao R, Fan Y, Raza MA, Wang X, Yong T, Liu W, Liu J. Effect of aboveground and belowground interactions on the intercrop yields in maize-soybean relay intercropping systems. Field Crops Res. 2017;203:16–23.
Gao Y, Duan A, Qiu X, Sun J, Zhang J, Liu H, Wang H. Distribution and use efficiency of photosynthetically active radiation in strip intercropping of maize and soybean. Agron J. 2010;102:1149–57.
Xiang D, Yong T, Yang W, Gong W, Cui L, Lei T. Effect of phosphorus and potassium nutrition on growth and yield of soybean in relay strip intercropping system. Sci Res Essays. 2012;7:342–51.
Yang F, Huang S, Gao R, Liu W, Yong T, Wang X, Wu X, Yang W. Growth of soybean seedlings in relay strip intercropping systems in relation to light quantity and red: far-red ratio. Field Crops Res. 2014;155:245–53.
Yong T, Liu X, Yang F, Song C, Wang X, Liu W, Su B, Zhou L, Yang W. Characteristics of nitrogen uptake, use and transfer in a wheat-maize-soybean relay intercropping system. Plant Prod Sci. 2015;18:388–97.
Yan Y, Gong W, Yang W, Wan Y, Chen X, Chen Z, Wang L. Seed treatment with uniconazole powder improves soybean seedling growth under shading by corn in relay strip intercropping system. Plant Prod Sci. 2010;13:367–74.
Gan Y, Stulen I, Posthumus F, van Keulen H, Kuiper P. Effects of N management on growth, N 2 fixation and yield of soybean. Nutr Cycl Agroecosyst. 2002;62:163–74.
Thilakarathna MS, McElroy MS, Chapagain T, Papadopoulos YA, Raizada MN. Belowground nitrogen transfer from legumes to non-legumes under managed herbaceous cropping systems. A review. Agron Sustain Dev. 2016;36:58.
Stern W. Nitrogen fixation and transfer in intercrop systems. Field Crops Res. 1993;34:335–56.
Meyer C, Stitt M. Nitrate reductase and signalling. In: Lea PJ, Morot-Gaudry J-F, editors. Plant Nitrogen. New York: Springer; 2001. p. 37–59.
Frink CR, Waggoner PE, Ausubel JH. Nitrogen fertilizer: retrospect and prospect. Proc Natl Acad Sci USA. 1999;96:1175–80.
Lea PJ, Morot-Gaudry JF. Plant Nitrogen. Berlin: Springer; 2001. p. 11.
Book Google Scholar
Lea PJ, Miflin BJ. Transport and metabolism of asparagines and other nitrogen compounds within the plant. In: Miflin BJ, editor. The biochemistry of plants: amino acids and derivatives. New York: Academic Press; 1980. p. 569–607.
Crawford NM, Arst HN. The molecular genetics of nitrate assimilation in fungi and plants. Annu Rev Genet. 1993;27:115–46.
Lea PJ. Nitrogen metabolism. In: Lea PJ, Leegood RC, editors. Plant biochemistry and molecular biology. New York: Wiley; 1993. p. 155–80.
Peoples MB, Gifford RM. Long-distance transport of carbon and nitrogen from sources to sinks in higher plants. In: Dennis DT, Turpin DH, editors. Plant physiology, biochemistry and molecular biology. New York: Wiley; 1993. p. 434–47.
Dubois F, Terce-Laforgue T, Gonzalez-Moro BM, Estavillo MB, Sangwan R, Gallais A, Hirel B. Glutamate dehydrogenase in plants: is there a new story for an old enzyme? Plant Physiol Biochem. 2003;41:565–76.
Hirel B, Andrieu B, Valadier MH, Renard S, Quillere I, Chelle M, Pommel B, Fournier C, Drouet JL. Physiology of maize II: identification of physiological markers representative of the nitrogen status of maize ( Zea mays ) leaves during grain filling. Physiol Plant. 2005;124:178–88.
Oliveira I, Brears T, Knight T, Clark A, Coruzzi G. Overexpression of cytosolic glutamine synthetase Relation to nitrogen, light, and photorespiration. Plant Physiol. 2002;129:1170–80.
Yamaya T, Obara M, Nakajima H, Sasaki S, Hayakawa T, Sato T. Genetic manipulation 979 and quantitative-trait loci mapping for nitrogen recycling in rice. J Exp Bot. 2002;53:917–25.
Miflin BJ, Habash DZ. The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J Exp Bot. 2002;53:979–87.
Aubert S, Bligny R, Douce R, Gout E, Ratcliffe RG, Roberts JKM. Contribution of glutamate dehydrogenase to mitochondrial glutamate metabolism studied by 13 C and 31 P nuclear magnetic resonance. J Exp Bot. 2001;52:37–45.
Ward M, Grimes H, Huffaker R. Latent nitrate reductase activity is associated with the plasma membrane of corn roots. Planta. 1989;177:470–5.
Unno H, Uchida T, Sugawara H, Kurisu G, Sugiyama T, Yamaya T, et al. Atomic structure of plant glutamine synthetase: a key enzyme for plant productivity. J Biol Chem. 2006;281:29287–96.
Keys A, Bird I, Cornelius M, Lea P, Wallsgrove R, Miflin B. Photorespiratory nitrogen cycle. Nature. 1978;275:741–3.
Bernard SM, Habash DZ. The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytol. 2009;182:608–20.
Vanoni M, Dossena L, van den Heuvel R, Curti B. Structure–function studies on the complex iron-sulfur flavoprotein glutamate synthase: the key enzyme of ammonia assimilation. Photosynth Res. 2005;83:219–38.
Masclaux-Daubresse C, Reisdorf-Cren M, Pageau K, Lelandais M, Grandjean O, Kronenberger J, Valadier MH, Feraud M, Jouglet T, Suzuki A. Glutamine synthetase-glutamate synthase pathway and glutamate dehydrogenase play distinct roles in the sink-source nitrogen cycle in tobacco. Plant Physiol. 2006;140:444–56.
Rochat C, Boutin JP. Metabolism of phloem-borne amino acids in maternal tissues of fruit of nodulated or nitrate-fed pea plants ( Pisum sativum L.). J Exp Bot. 1991;42:207–14.
Lam HM, Wong P, Chan HK, Yam KM, Chen L, Chow CM, Coruzzi GM. Overexpression of the ASN1 gene enhances nitrogen status in seeds of Arabidopsis. Plant Physiol. 2003;132:926–35.
Potel F, Valadier MH, Ferrario-Mery S, Grandjean O, Morin H, Gaufichon L, Boutet-Mercey S, Lothier J, Rothstein SJ, Hirose N, Suzuki A. Assimilation of excess ammonium into amino acids and nitrogen translocation in Arabidopsis thaliana —roles of glutamate synthases and carbamoylphosphate synthetase in leaves. FEBS J. 2009;276:4061–76.
Skopelitis D, Paranychianakis N, Paschalidis K, Pliakonis E, Delis I, Yakoumakis D, Kouvarakis A, Papadakis A, Stephanou E, Roubelakis-Anfelakis K. Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell. 2006;18:2767–81.
Garnett T, Plett D, Heuer S, Okamoto M. Genetic approaches to enhancing nitrogen-use efficiency (NUE) in cereals:challenges and future directions. Funct Plant Biolohy. 2015;42:921–94.
Miller AJ, Fan X, Shen Q, Smith SJ. Amino acids and nitrate as signals for the regulation of nitrogen acquisition. J Exp Bot. 2008;59:111–9.
Sanders A, Collier R, Trethewy A, Gould G, Sieker R, Tegeder M. AAP1 regulates import of amino acids into developing Arabidopsis embryos. Plant J. 2009;59:540–52.
Tan Q, Zhang L, Grant J, Cooper P, Tegeder M. Increased phloem transport of S-methylmethionine positively affects sulfur and nitrogen metabolism and seed development in pea plants. Plant Physiolol. 2010;154:1886–96.
Forde B. Glutamate signalling in roots. J Exp Bot. 2014;65:779–87.
Santiago J, Tegeder M. Connecting source with sink: the role of Arabidopsis AAP8 in phloem loading of amino acids. Plant Physiol. 2016;171:508–21.
Fang Z, Xia K, Yang X, Grotemeyer MS, Meier S, Rentsch D, Xu X, Zhang M. Altered expression of the PTR/NRT1 homologue OsPTR9 affects nitrogen utilization efficiency, growth and grain yield in rice. Plant Biotechnol J. 2013;11:446–58.
Araus V, Vidal EA, Puelma T, Alamos S, Mieulet D, Guiderdoni E, Gutiérrez RA. Members of BTB gene family of scaffold proteins suppress nitrate uptake and nitrogen use efficiency. Plant Physiol. 2016;171:1523–32.
CAS PubMed PubMed Central Google Scholar
Chen J, Fan X, Qian K, Zhang Y, Song M, Liu Y, Xu G, Fan X. pOsNAR2.1:OsNAR2.1 expression enhances nitrogen uptake efficiency and grain yield in transgenic rice plants. Plant Biotechnol J. 2017. https://doi.org/10.1111/pbi.12714 .
Y.F. Tsay, S.C. Fan, H.Y. Chen. Method for changing nitrogen utilization efficiency in plants. U.S. Patent Application (2011) 12/832, 2347.
Ameziane R, Bernhard K, Lightfoot D. Expression of the bacterial gdhA gene encoding a NADHP glutamate dehydrogenase in tobacco affects plant growth and development. Plant Soil. 2000;22:147–57.
Chichkova S, Arellano J, Vance CP, Hernández G. Transgenic tobacco plants that overexpress alfalfa NADH-glutamate synthase have higher carbon and nitrogen content. J Exp Bot. 2001;52:2079–87.
Habash DZ, Massiah AJ, Rong HL, Wallsgrove RM, Leigh RA. The role of cytosolic glutamine synthetase in wheat. Ann Appl Biol. 2001;138:83–9.
Seiffert B, Zhou Z, Wallbraun M, Lohaus G, Möllers C. Expression of a bacterial asparagine synthetase gene in oilseed rape (Brassica napus) and its effect on traits related to nitrogen efficiency. Physiol Plant. 2004;121:656–65.
Good A, Johnson S, de Pauw M, Carroll R, Savidov N, Vidmar J, Lu Z, Taylor G, Stroeher V. Engineering nitrogen use efficiency with alanine aminotransferase. Can J Bot. 2007;85:252–62.
Shrawat A, Carroll R, de Pauw M, Taylor G. Good A Genetic engineering of improved nitrogen use efficiency in rice by the tissue-specific expression of alanine aminotransferase. Plant Biotechnol J. 2008;6:722–32.
Peña PA, Quach T, Sato S, Ge Z, Nersesian N, Changa T, Dweikat I, Soundararajan M, Clemente TE. Expression of the maize Dof1 transcription factor in wheat and sorghum. Front Plant Sci. 2017;8:434.
Yanagisawa S, Akiyama A, Kisaka H, Uchimiya H, Miwa T. Metabolic engineering with Dof1 transcription factor in plants: improved nitrogen assimilation and growth under low-nitrogen conditions. Proc Natl Acad Sci USA. 2004;101:7833–8.
Ferrario-Mery S, Besin E, Pichon O, Meyer C, Hodges M. The regulatory PII protein controls arginine biosynthesis in Arabidopsis. FEBS Lett. 2006;580:2015–20.
Ferrario-Mery S, Valadier M, Foyer C. Overexpression of nitrate reductase in tobacco delays drought-induced decreases in nitrate reductase activity and mRNA. Plant Physiol. 1998;117:293–302.
Hoshida H, Tanaka Y, Hibino T, Hayashi Y, Anaka TA, Takabe T. Enhanced tolerance to salt stress in transgenic rice that overexpresses chloroplast glutamine synthetase. Plant Mol Biol. 2000;43:103–11.
Migge A, Carrayol E, Hirel B, Becker T. Leaf-specific overexpression of plastidic glutamine synthetase stimulates the growth of transgenic tobacco seedlings. Planta. 2000;210:252–60.
Deprost D, Yao L, Sormani R, Moreau M, Leterreux G, et al. The Arabidopsis TOR kinase links plant growth, yield, stress resistance and mRNA translation. EMBO Rep. 2007;8:864–70.
Castaings L, Camargo A, Pocholle D, Gaudon V, Texier Y, Boutet-Mercey S, Taconnat L, Renou J-P, Daniel-Vedele F, Fernandez E, Meyer C, Krapp A. The nodule inception-like protein 7 modulates nitrate sensing and metabolism in Arabidopsis. Plant J. 2009;57:426–35.
Pathak RR, Ahmad A, Lochab S, Raghuram N. Molecular physiology of plant nitrogen use efficiency and biotechnological options for its enhancement. Curr Sci. 2009;94:1394–403.
Lea US, Leydecker MT, Quillere I, Meyer C, Lillo C. Posttranslational regulation of nitrate reductase strongly affects the levels of free amino acids and nitrate, whereas transcriptional regulation has only minor influence. Plant Physiolology. 2006;140:1085–94.
Lam HM, Hsieh MH, Coruzzi G. Reciprocal regulation of distinct asparagine synthetase genes by light and metabolites in Arabidopsis thaliana. Plant J. 1998;16:345–53.
Harrison J, Brugiere N, Phillipson B, Ferrario-Mery S, Becker T, Limami A, Hirel B. Manipulating the pathway of ammonia assimilation through genetic engineering and breeding: consequences on plant physiology and plant development. Plant Soil. 2000;221:81–93.
LopesCardoso MI, Carvalho H, Lima L, Melo P, Cullimore J. Nodule specific modulation of glutamine synthetase in transgenic Medicago truncatula . Plant Physiol. 2003;133:243–52.
Harrison J, Pou de Crescenzo MA, Sene O, Hirel B. Does lowering glutamine synthetase activity in nodules modify nitrogen metabolism and growth of Lotus japonicus ? Plant Physiol. 2003;133:253–62.
Wong HK, Chan HK, Coruzzi GM, Lam HM. Correlation of ASN2 gene expression with ammonium metabolism in Arabidopsis. Plant Physiol. 2004;134:332–8.
Hirel B, Le Gouis J, Ney B, Gallais A. The challenge of improving nitrogen use efficiency in crop plants: towards a more central role for genetic variability and quantitative genetics within integrated approaches. J Exp Bot. 2007;58:2369–87.
Radtkey R, Feng L, Muralhidar M, Duhon M, Canter D, DiPierro D, Fallon S, Tu E, McElfresh K, Nerenberg M, Sosnowski R. Rapid, high fidelity analysis of simple sequence repeats on an electronically active DNA microchip. Nucleic Acids Res. 2000;28:E17.
Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63.
Rawal HC, Kumar S, Mithra SVA, Solanke AU. High quality unigenes and microsatellite markers from tissue specific transcriptome and development of a database in Clusterbean ( Cyamopsis tetragonoloba , L. Taub). Genes. 2017;8:313.
Article PubMed Central CAS Google Scholar
Lian X, Wang S, Zhang J, Feng Q, Zhang L, Fan D, Li X, Yuan D, Han B, Zhang Q. Expression profiles of 10,422 genes at early stage of low nitrogen stress in rice assayed using a cDNA microarray. Plant Mol Biol. 2006;60:617–31.
Yang SY, Hao DL, Song ZZ, Yang GZ, Wang L, Su YH. RNA-Seq analysis of differentially expressed genes in rice under varied nitrogen supplies. Gene. 2015;555:305–17.
Gelli M, Duo Y, Konda AR, Zhang C, Holding D, Dweikat I. Identification of differentially expressed genes between sorghum genotypes with contrasting nitrogen stress tolerance by genome-wide transcriptional profiling. BMC Genomics. 2014;15:179.
Hao QN, Zhou XA, Ai HS, Wang C, Zhou R, Chen SL. Identification of genes associated with nitrogen-use efficiency by genome-wide transcriptional analysis of two soybean genotypes. BMC Genomics. 2011;12:525.
Li W, Xiang F, Zhong M, Zhou L, Liu H, Li S, Wang X. Transcriptome and metabolite analysis identifies nitrogen utilization genes in tea plant ( Camellia sinensis ). Sci Rep. 2017;7:1693.
Cho YI, Jiang WZ, Chin JH, Piao ZZ, Cho YG, McCouch SR, Koh HJ. Identification of QTLs associated with physiological nitrogen use efficiency in rice. Mol Cell. 2007;23:72–9.
Wei D, Cui KH, Ye GY, Pan JF, Xiang J, Huang JL, Nie L. QTL mapping for nitrogen-use efficiency and nitrogen-deficiency tolerance traits in rice. Plant Soil. 2012;359:281–95.
Zhou Y, Tao Y, Tang D, Wang J, Zhong J, Wang Y, Liang G. Identification of QTLs associated with nitrogen uptake and nitrogen use efficiency using high throughput genotyped CSSLs in rice ( Oryza sativa L.). Front Plant Sci. 2017;8:1166.
Pandit A, Rai V, Bal S, Sinha S, Kumar V, Chauhan M, Gautam RK, Singh R, Sharma PC, Singh AK, et al. Combining QTL mapping and transcriptome profiling of bulked RILs for identification of functional polymorphism for salt tolerance genes in rice ( Oryza sativa L.). Mol Genet Genomics. 2013;284:121–36.
Curci PL, Bergès H, Marande W, Maccaferri M, Tuberosa R, Sonnante G. Asparagine synthetase genes ( AsnS1 , and AsnS2 ) in durum wheat: structural analysis and expression under nitrogen stress. Euphytica. 2018;214:36.
Wan Y, King R, Mitchell RAC, Hassani-Pak K, Hawkesford MJ. Spatiotemporal expression patterns of wheat amino acid transporters reveal their putative roles in nitrogen transport and responses to abiotic stress. Sci Rep. 2017;7:5461–74.
Dai ZW, Plessis A, Vincent J, Duchateau N, Besson A, Dardevet M, Prodhomme D, Gibon Y, Hilbert G, Pailloux M, et al. Transcriptional and metabolic alternations rebalance wheat grain storage protein accumulation under variable nitrogen and sulfur supply. Plant J. 2015;83:326–43.
Kirkman MA, Miflin BJ. The nitrate content and amino acid composition of the xylem fluid of spring wheat throughout the growing season. J Sci Food Agric. 1979;30:653–60.
Todd J, Screen S, Crowley J. Identification and characterization of four distinct asparagine synthetase (AsnS) genes in maize ( Zea mays L.). Plant Sci. 2008;175:799–808.
Curci PL, Cigliano RA, Zuluaga DL, Janni M, Sanseverino W, Sonnante G. Transcriptomic response of durum wheat to nitrogen starvation. Sci Rep. 2017;7:1176–90.
Download references
Acknowledgements
The authors would like to thank the Mr. Babar Usman and Wajid Saeed for their help and support.
The present study was supported by National Key R&D Program of China (2019YFD1000503) and Guangxi Special Fund for Scientific Base and Talent (GKAD17195100), Guangxi Sugarcane Innovation Team of National Agricultural Industry Technology System (gjnytxgxcxtd-03-01), and Fund for Construction of Guangxi Key Laboratory of Sugarcane Genetic Improvement (2019), Guangxi Ctop Genetic Improvement and Biotechnology Laboratory (2020) and Fund of Guangxi Academy of Agricultural Sciences (2015YT02). The funding bodies played no role in the design, execution, interpretation of the data, or writing of the manuscript.
Author information
Authors and affiliations.
College of Agriculture, Guangxi University, Nanning, 530005, China
Muhammad Anas, Aamir Mahmood, Qiang Li, Xu-Peng Zeng & Yang-Rui Li
Key Laboratory of Sugarcane Biotechnology and Genetic Improvement (Guangxi), Ministry of Agriculture/Guangxi Key Laboratory of Sugarcane Genetic Improvement, Sugarcane Research Institute, Guangxi Academy of Agricultural Sciences, Nanning, 530007, Guangxi, China
Muhammad Anas, Fen Liao, Krishan K. Verma, Zhong-Liang Chen & Yang-Rui Li
Crop Sciences Institute, National Agricultural Research Centre, Islamabad, Pakistan
Muhammad Aqeel Sarwar
Guangxi Crop Genetic Improvement and Biotechnology Laboratory, Nanning, 530007, China
You can also search for this author in PubMed Google Scholar
Contributions
MA wrote the abstract, introduction and get ideas, QL and XPZ designed the figures, KKV and MAS did proof reading, FL, Z-LC and AM give their ideas to adjust the contents, LY and YRL gave main idea. Y-RL revised and finalized the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Yang Liu or Yang-Rui Li .
Ethics declarations
Ethics approval and consent to participate.
All applicable international, national, and/or institutional guidelines were followed.
Consent for publication
All authors of this paper consent for publishing manuscript and figures in this journal.
Competing interests
All authors declared no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Anas, M., Liao, F., Verma, K.K. et al. Fate of nitrogen in agriculture and environment: agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol Res 53 , 47 (2020). https://doi.org/10.1186/s40659-020-00312-4
Download citation
Received : 17 December 2019
Accepted : 20 September 2020
Published : 16 October 2020
DOI : https://doi.org/10.1186/s40659-020-00312-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Assimilation
Biological Research
ISSN: 0717-6287
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Nitrogen and the future of agriculture: 20 years on
This article belongs to Ambio’s 50th Anniversary Collection. Theme: Solutions-oriented research
- Behind the paper
- Published: 14 March 2021
- Volume 51 , pages 17–24, ( 2022 )

Cite this article

- Kenneth G. Cassman 1 &
- Achim Dobermann 2
Avoid common mistakes on your manuscript.
Global view
Adequate crop yields to feed 7.8 billion people today, and nearly 10 billion by mid-century (Vollset et al. 2020 ), depend on large amounts of nitrogen (N) input, which in turn represent an inherent weakness in our global food production system. The challenge has nothing to do with N supply as that is virtually unlimited. Nearly 80% of the atmosphere is composed of N 2 , a relatively inert gas, that can be converted to reactive N forms (mostly nitrate, NO 3 − and ammonium, NH 4 + ) via biological and industrial processes and used to satisfy crop N requirements. Instead, the main problem is one of excess: it is difficult to precisely supply enough N to meet crop physiological requirements while also controlling the fate of reactive N to avoid losses to the environment. As a result, cropping systems responsible for the bulk of humanity’s food supply leak too much NO 3 − , causing severe degradation of water quality and riparian and aquatic ecosystems. Gaseous losses as ammonia, nitrogen oxides (NO x ), and nitrous oxide (N 2 O) reduce air quality and account for much of agriculture’s contribution to climate change. To make up for these losses, the global annual input of fertilizer N to cropland is double the N input from the natural processes of biological N fixation (BNF) (Fowler et al. 2013 ). At the same time, crops in large parts of the world, and particularly in sub-Saharan Africa (SSA), suffer chronic N deficiency and low yields due to lack of sufficient input of N and other nutrients (Berge et al. 2019 ; Senthilkumar et al. 2020 ).
These concerns were well documented and recognized within the scientific community when our 2002 Ambio paper (Cassman et al. 2002 ) was presented at the 2nd International Nitrogen Conference in Washington DC (October 2001). Achieving better synchrony between N supply and demand across the wide range of cropping systems and environments in which crops are grown was identified as the solution to both meet crop N needs and better protect environmental quality. But it was difficult to find robust and relevant field data on N fertilizer efficiency (NFE, also called recovery efficiency of fertilizer N) of major food crops as quantified by the proportion of applied N taken up the crop. Such data are essential for monitoring and mapping N losses to identify crops and regions with greatest potential for improvement, or areas where progress has been made.
To that end, our paper brought together the best available data on NFE for the major grain crops rice, wheat, and maize to establish benchmarks based on direct measurements taken as much as possible in farmer-managed production fields. Although there is a copious literature on NFE measured in “small-plot” experiments, estimates from those studies are not representative of NFE in larger, non-uniform production fields as managed by farmers. Our results documented relatively low NFE of 18–49% occurring at production scale in several major cereal systems depending on crop species, cropping system, and country. While not good news for the environment because such low levels of NFE equate to more than 50 Tg of applied fertilizer N not taken up by crops Footnote 1 and at risk of loss, it documents enormous room for improvement.
In recent years, approaches to estimate N use efficiency Footnote 2 have focused on constructing N budgets and N use efficiency indicators based on estimates of N inputs and outputs at farm (Quemada et al. 2020 ), national (Zhang et al. 2015 ), and global scales (Ladha et al. 2016 ). General conclusions from this work are consistent with our 2002 Ambio paper: N use efficiency varies widely but is often well below achievable levels. Using input–output budgeting approaches, global N use efficiency from crop production is currently in the 40–50% range. Footnote 3 Few studies since 2002 have added new data on NFE from farmer-managed production fields, or on critical nutrient budget components such as N inputs from biological fixation and the amount of N released from mineralization of soil organic matter (SOM). Hence, large uncertainties remain with data and coefficients used to construct such nutrient budgets (Zhang et al. 2020 ).
At a global scale, average N use efficiency has increased slowly during the past 20 years. A general proxy for NFE trajectories can be seen in time-trends for N fertilizer use versus the amount of N removed with harvested crop materials. In high-yield cropping systems of North America, for example, N removal was considerably less than applied N fertilizer from 1960 to 1970 (an indication of N excess), and considerably more than applied in recent decades (Fig. 1 a). This crossover reflects improved NFE due to rising crop yields, modest improvements in N fertilizer management, and increased soybean production, the latter a legume crop with large capacity to meet its N demand through BNF, thus requiring little N fertilizer (Salvagiotti et al. 2008 ). Greater N removal than N fertilizer inputs in Latin America also reflects both increasing crop yields and large expansion of soybean-based cropping systems in Argentina, Brazil, Paraguay, and Uruguay (Fig. 1 g).
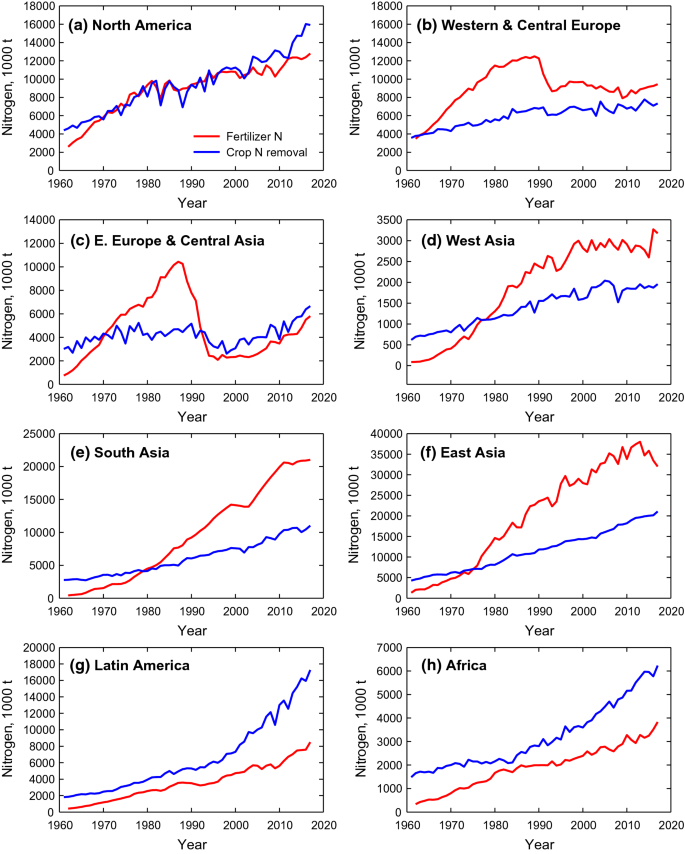
Trends in fertilizer N use and crop N removal by region, 1961–2017. Data source IFA Nutrient Use Efficiency database, IFASTAT, 2020. The dataset contains national scale estimates for N inputs to cropland from fertilizer, manure and biological N fixation, as well as N removal by harvested crops. Grasslands and forage crops are not included
A narrowing gap between N fertilizer inputs and N removal also indicates a trend towards improved NFE as seen in the high-yield systems of Western and Central Europe (Fig. 1 b) and more recently in East Asia, which mostly reflects trends in China (Fig. 1 f). Improved NFE in these regions largely results from more judicious use of N fertilizer in response to policies and regulations promoting higher NFE rather than the benefits of increasing crop yields. In Eastern Europe and Central Asia, the decline in N fertilizer use that resulted from collapse of the former U.S.S.R. has been reversed along with rising crop yields, with indications of a trend towards higher NFE (Fig. 1 c). Offsetting these positive trends is the widening gap between N fertilizer inputs and N removal in cropping systems of West and South Asia, which suggest decreasing NFE (Fig. 1 d, 1e ).
After years of stagnation, fertilizer use in sub-Saharan Africa has started to increase, from about 8 kg nutrients ha −1 in 2000 to about 20 kg ha −1 today, Footnote 4 although the gap between crop N removal and fertilizer N use has widened in recent years (Fig. 1 h). As a result, current farm yields are less than 33% of attainable yield with currently available technologies (van Ittersum et al. 2016 ) and soil nutrient stocks are being depleted. Closing the African yield gap will require reducing the gap between physiological nutrient requirements and the amount of applied nutrients on a massive scale (Berge et al. 2019 ).
Increased public concern about N pollution in many high- and middle-income countries has led to regulatory action and a range of policies related to N pollution (Kanter et al. 2020 ). Limits on the amount of N fertilizer have been implemented in some countries, while other jurisdictions rely on voluntary measures or use a relatively straightforward “N balance” approach (the difference between N inputs and N removal in harvested materials) as the means to track progress towards environmental goals (McLellan et al. 2018 ). A key challenge in all such programs is the capacity to link NFE at the level of a farmer’s field or farm to environmental outcomes at the watershed or groundwater resource scale because of large deficiencies in ability to accurately model crop N uptake, N cycling through soil organic matter, and losses of reactive N via leaching and gaseous emissions. While farmers can easily monitor N balance, it is much more difficult to estimate NFE because it is confounded by uptake of indigenous soil N from mineralization of SOM and residual N from applications to the previous crop. Although ability to predict N supply from soil N mineralization across a wide range of soil types and climates remains a difficult scientific challenge, it would give farmers a powerful tool to improve synchronization of crop demand and N supply throughout the growing season through improved timing and amount of N topdressings. Despite widespread concerns about N pollution from agriculture and regulations to reduce it, we believe that public- and private-sector investment explicitly focused on raising crop yields while also decreasing N losses is woefully inadequate relative to the magnitude of the challenge and associated societal benefits.
Nitrogen innovations
In our 2002 paper, we proposed an “N synchrony framework” for evaluating promising technologies to achieve “just-in-time N supply,” without excess or deficiency, throughout the crop growth cycle as the means to achieve meaningful increases in NFE at scale. The need to concomitantly increase crop yields and NFE was the primary justification for this approach (Cassman 1999 ) because crops that absorb more of the available N and transform it into economic yield offer the best path to achieve both goals, thereby decreasing the amount of N at risk of loss to the environment. A number of promising technology options were identified. Here, we evaluate progress over the subsequent two decades.
Trends in Fig. 1 suggest that in some regions, either motivated by voluntary incentive schemes or regulatory policies (van Grinsven et al. 2015 ), changes in management practices and/or adoption of new technologies have contributed to improved N efficiency. It is difficult, however, to attribute improvements to adoption of specific technologies because NFE improvement can occur from technologies other than fertilizer management. Hence, widespread adoption of practices that contribute to more uniform plant stands, higher plant densities, more vigorous early season growth rates, and higher yields have indirectly increased NFE when there is excess N supply. In mechanized systems, these practices include use of integrated pest management, GMO crops that reduce yield loses from weeds and insect pests, and precision-farming technologies such as auto-steer tractors guided by global positioning systems that avoid strips without N fertilization and N fertilizer overlaps. A general movement away from a single large N fertilizer application before planting or at sowing towards smaller, split applications during the growing season has been promoted to farmers in high-yield cereal production systems worldwide, although it is difficult to obtain good data on the degree of adoption by farmers.
In contrast, compared to its theoretical promise (Cassman and Plant 1992 ), adoption of variable-rate fertilization technology has been disappointing. Lack of adoption by large-scale mechanized farmers appears to reflect an unproven value proposition for the required investments in equipment, software, data, and labor to implement variable-rate fertilizer application, while low levels of motorized mechanization represents the biggest adoption barrier for medium and small farms in developing countries (Lowenberg-DeBoer and Erickson 2019 ). To overcome these barriers for small farms, a considerable body of research has produced a number of “low-tech” site-specific nutrient management (SSNM) tools that give consistent increases in crop yields and profits (typically by 10–20% relative to current farmers’ practice) and N use efficiency (typically by 30-40%) in many crops, including rice, wheat, maize, and other crops in Asia and Africa (Dobermann et al. 2002 ; Khurana et al. 2008 ; Chen et al. 2014 ; Pasuquin et al. 2014 ; Saito et al. 2015 ; Rurinda et al. 2020 ; Wang et al. 2020 ). These substantial improvements have been achieved across a wide range of environments and are typically associated with a modest reduction in N fertilizer rates and a shift towards more split N applications to improve congruence between N supply and crop N demand. It is likely that adoption of these tools also reduces NO 3 leaching and N 2 O emission losses (Pampolino et al. 2007 ; An et al. 2015 ). We know of no other intervention that has demonstrated such robust, win–win performance across large regions.
Yet despite robust validation of the SSSM approach, it has been a challenge to achieve widespread adoption by millions of smallholder farmers, for which a combination of conventional and digital extension education tools have been tested (Pampolino et al. 2012 ; Cui et al. 2018 ; Sharma et al. 2019 ; Zossou et al. 2020 ). Experience thus far suggests multiple reasons for limited impact at larger scale, including: weak capacity in government extension systems, insufficient private-sector uptake, decision tools that are still too complex to use and do not have sustainable business models, lack of integration with financial and input supply services, insufficient integration of other agronomically relevant and geospatial information, and lack of policy-driven incentives to promote farmer adoption.
“Smart” fertilizer formulations and specialty products designed to regulate specific N transformation pathways in soil to better synchronize N supply and demand have been around for decades. Yet progress towards defining the conditions under which these products work has been slow, and inconsistency of expected returns from their use have limited widespread adoption. Enhanced-efficiency fertilizers Footnote 5 currently account for less than 5% of the global fertilizer N market. Nitrogen transformation inhibitors and other N fertilizer “stabilizers” applied to broadacre crops account for the bulk of that market, whereas controlled- and slow-release fertilizers are mostly applied to high-value crops such as turf, ornamentals, nurseries and plantations. Biological nitrification inhibition—the ability of plant roots to suppress soil-nitrifier activity through production and release of nitrification inhibitors—has been proposed as an innovative genetic modification pathway to reduce N 2 O emissions (Subbarao et al. 2017 ). Although scientifically interesting, we doubt it has potential for real-world impact due to the complexity of regulating expression of this trait in response to both N supply and crop N demand as they change throughout the crop growth cycle.
Crop genetic improvement is often emphasized for its promise to improve N use efficiency, as mentioned in our Ambio paper (Cassman et al. 2002 ). However, we are not aware of new crop varieties with proven improvement of specific traits governing N efficiency or BNF despite many millions of dollars of public- and private-sector investment towards these goals. Breeding for N use efficiency must coordinate genetic control of physiological and metabolic factors influencing N uptake from soil and N utilization efficiency and allocation within the plant. And both processes involve numerous finely tuned biochemical pathways and feedback mechanisms that are highly sensitive to environmental conditions (Cormier et al. 2016 ; van Lammerts Bueren and Struik 2017 ), making “trade-off” free genetic solutions unlikely (Denison 2015 ).
For similar reasons, there has been little progress towards increased contributions from BNF by legume crops, or developing cereal crops with N fixation capability (Ladha and Reddy 1995 ; Beatty and Good 2011 ; Rogers and Oldroyd 2014 ). Impressive basic research has been conducted on different approaches, including improvement of root-associated endophytic bacteria to develop nodule-independent N 2 -fixing systems, engineering root nodule symbiosis in cereals, and transferring nitrogenase genes into cereal crops. Despite progress in expanding scientific knowledge, none of these approaches—if they work—are likely to reach commercialization within the next 20 or 30 years because of the number of genes involved. In recent years, commercial activity has focused on identification and/or improvement of endophytic bacteria for associative N fixation. Several companies aim to commercialize endophytic products in the near future, but more rigorous, independent field testing is still required. At best, such microbial formulations have potential to contribute relatively small amounts of about 20–30 kg N ha −1 during a cropping season, compared to typical N uptake requirements of about 200 kg N/ha for high-yielding cereal crops, and similar large amounts from legume BNF inputs.
Two notable trends have emerged in the global N balance since we wrote our paper. First, industrial N is becoming a key ingredient in global sustainability chains, including uses to reduce emissions in transportation and power stations, construction, extraction and processing of mineral resources, feed additives, wastewater treatment, and pharmaceuticals. Industrial uses of inorganic N (mainly as ammonia, urea, and ammonium nitrate) now represent around 20% (ca. 33 Mt) of total industrial N production. Annual growth of that sector has averaged 4–5% during the past 20 years and is likely to continue or accelerate. Efficiencies and losses from these multiple end-uses have not been well quantified, but this is also a rapidly emerging area to support development of new green technologies.
Second, there has been massive growth in livestock production and associated nutrient pollution as demand for meat and dairy products has soared with rapid economic and income growth, particularly in countries such as China and India. Nitrogen losses from livestock systems and virtual trade in N are now estimated at 65 Tg N year −1 , or one-third of all human-induced N losses to the environment (Uwizeye et al. 2020 ). Continued growth in livestock production is anticipated as economic growth spreads to other populous, low-income countries in Asia, Latin America, and Sub-Sahara Africa where farm animals can play a pivotal role in creating a new, circular food system for meeting both dietary requirements and environmental protection (van Zanten et al. 2019 ). This megatrend calls for innovation in developing improved livestock production systems and business models that include opportunities for nutrient recovery and recycling. Several major companies and venture capital startups are investing in such technologies.
It is frequently suggested that food system solutions must include substantial shifts in diets to reduce consumption of livestock products and large reductions in food losses or waste (Foley et al. 2011 ; Springmann et al. 2018 ; Willett et al. 2019 ). In countries like China, such transformations could contribute significantly to future cropland, grassland and nutrient needs (Ma et al. 2019 ). While technically feasible, however, we see little scope for either option to substantially reduce N input requirements in crop production within a relevant time frame for impact. To our knowledge there is scarce evidence that diets can be substantially modified in developed countries with high meat and dairy consumption, or in low-income developing countries where current levels of meat and dairy consumption are so low that nutrition is greatly improved by increased consumption as economic development proceeds. We are also uncertain about the potential for alternative protein sources and production systems from industrial scale cell culture, insects, or plant-based meat mimics. If these technologies can displace meat production from livestock, they could achieve a large reduction in N use and losses from conventional agriculture. But it is not at all clear that diet change of any kind will achieve widespread consumer acceptance simply because dietary preferences are so tightly interwoven with culture and heritage.
We are left with the synchronization framework proposed 20 years ago, and an urgent call for innovation and open thinking to redesign agriculture in terms of crops, cropping systems, integrated crop-livestock systems with better nutrient recycling and recovery, and the agricultural landscape itself in terms of hydraulic flows and conservation features such as riparian buffers and constructed wetlands. Capture and recycling of N (and P) from multiple waste streams will likely become increasingly important.
We remain highly skeptical of the potential for single-factor genetic improvements in N use efficiency, or other “single-factor” genetic technologies to pay off. Instead, we see most promise in systematic agronomic approaches that harness Big Data and geospatial extrapolation frameworks to accelerate the process of optimizing crop and soil management practices governing both yields and resource use efficiencies at production scale (Cassman and Grassini 2020 ). Traditional, replicated field studies with two or three treatment variables are not up to the task of optimizing the 10–20 management factors (including selection of the optimal hybrid or cultivar) that affect NFE, crop yield, profit, and other key performance indicators. Machine learning and other artificial intelligence approaches can play an important role in developing optimized, tailored, and site-specific management solutions (Saikai et al. 2020 ), particularly once it becomes possible to move seamlessly from data to prescriptive analytics and automated decision making (Smith 2020 ). The rapid spread of GPS-enabled smartphones presents a unique opportunity for directly reaching 2 billion people in smallholder farming households, provided the smartphone apps and messages are scientifically sound, give actionable advice, and utilize feedback mechanisms to enable rigorous testing and continuous improvement (Fabregas et al. 2019 ). Public access to high-quality, high spatial resolution data on soil properties, long-term weather records, current weather conditions, and robust weather forecasts represent essential and relatively low-cost “public goods” to support the needed innovation and should be given a high priority by policy-makers.
We also note that while substantial improvement in NFE is necessary, it is not sufficient to meet water quality standards in major crop producing regions like the US Corn Belt. Cover crops, conservation tillage, and modified cropping systems may also be required (Castellano and David 2014 ), as well as retirement of the most sensitive land from crop production. For example, strategic conversion of < 3% of cropland in the Upper Mississippi, Ohio, and Missouri River watersheds is estimated to achieve a 45% reduction in nitrate losses and reduce extent of the hypoxia zone within regulatory targets (McLellan et al. 2015 ).
The current global food system relies on a handful of staple crops, Footnote 6 many of which are grown in a few “breadbasket” regions with good soils and favorable climates, to produce consistently high yields in sufficient quantities to meet global demand for calories, protein, and vegetable oil. While many other crops contribute importantly to human food supply and nutrition at a national scale, none are widely traded on global markets to supply the majority of countries that rely on food imports for a substantial portion of total food requirements. Hence, our current food system depends on globalized production and trade in crop and livestock commodities, and modern science and technology to produce them. We see continuation of this globalized food system in a post-pandemic world because it fosters crop production where soils and climate give comparative advantages in capture of sunlight and conversion into human food with greatest efficiency in use of inputs such as labor, nutrients, water, and energy. While some nations may have comparative advantage for production of rice, others have advantages for maize, soybean, wheat, potatoes, or oil palm. Global trade, economic development, social and economic equality provide the means to support balanced diets in a world without hunger and without further environmental degradation. And while recent studies have estimated N balance limits within the planetary boundary concept (Steffen et al. 2015 ; Zhang et al. 2015 ), the range is too large to be an effective target at the field or farm level. Emphasis should be given to better estimating acceptable N loss limits at the field level so that farmers and supporting agricultural industries can innovate to reach them across the wide range of soils, climates, and crops that comprise our major food production systems. It is notable that the knowledge and tools to better estimate environmental performance limits at a field scale, as influenced by soil, weather, cropping system, and management practices, would also strongly contribute to developing technologies that improve NFE by achieving greater synchrony in N supply and demand.
Despite the enormity of the challenge, we are confident that environmental damage and human health problems caused by N losses from modern, high-yield agriculture can be eliminated within 30 years. But our confidence assumes that policy-makers have the vision to make adequate R&D investments with a ruthless focus on accelerating yield growth of major food crops on existing farmland while concomitantly increasing NFE, as well as transparent environmental performance standards, and robust, low-cost metrics that allow farmers to monitor progress towards those standards. Policies to meet this grand challenge include adequate public investment in education and human resource development, as well as free public access to high-quality data on soil properties, historical and real-time weather data, and water resources at a spatial resolution sufficient to drive innovation towards increasingly precise crop and soil management in farmers’ fields to optimize productivity and avoid negative environmental consequences.
Current annual global fertilizer N use on cropland is about 100 Tg (excluding grasslands and forage crops).
N use efficiency is a broader measure than NFE because, in addition to applied N fertilizer, it also includes N supply from mineralization of soil organic matter, and N inputs from manure, BNF and atmospheric deposition, all of which contribute to the N supply for crop uptake.
Most published estimates are for time series until 2010, with global cropland N use efficiency estimates ranging from about 42 to 47%, e.g., Lassaletta et al. ( 2014 ); Zhang et al. ( 2015 ).
Based on IFASTAT Fertilizer Consumption and Fertilizer Use By Crops databases, IFA, Paris.
This includes controlled-release fertilizers, slow-release fertilizers, sulfur-coated fertilizers, stabilized nitrogen fertilizers (urease inhibitors, nitrification inhibitors, etc.).
The key crops include rice, wheat, maize, potatoes and sweet potatoes, cassava, soybean, oil palm, sugarcane, and sugarbeets.
An, N., M. Fan, F. Zhang, P. Christie, J. Yang, J. Huang, S. Guo, X. Shi, et al. 2015. Exploiting co-benefits of increased rice production and reduced greenhouse gas emission through optimized crop and soil management. PLoS ONE 10: e0140023.
Article Google Scholar
Beatty, P.H., and A.G. Good. 2011. Future prospects for cereals that fix nitrogen. Science 333: 416–417. https://doi.org/10.1126/science.1209467 .
Article CAS Google Scholar
Cassman, K.G., and R.E. Plant. 1992. A model to predict crop response to applied fertilizer nutrients in heterogenous fields. Fertilizer Research 31: 151–163.
Cassman, K.G. 1999. Ecological intensification of cereal production systems: Yield potential, soil quality, and precision agriculture. Proceedings of the National Academy of Sciences 96: 5952–5959.
Cassman, K.G., A. Dobermann, and D.T. Walters. 2002. Agroecosystems, nitrogen-use efficiency, and nitrogen management. Ambio 31: 132–140. https://doi.org/10.1579/0044-7447-31.2.132 .
Cassman, K.G., and P. Grassini. 2020. A global perspective on sustainable intensification research. Nature Sustainability 3: 262–268. https://doi.org/10.1038/s41893-020-0507-8 .
Castellano, M.J., and M.B. David. 2014. Long-term fate of nitrate fertilizer in agricultural soils is not necessarily related to nitrate leaching from agricultural soils. Proceedings of the National academy of Sciences of the United States of America 111: E766. https://doi.org/10.1073/pnas.1321350111 .
Chen, X., Z. Cui, M. Fan, P. Vitousek, M. Zhao, W. Ma, Z. Wang, W. Zhang, et al. 2014. Producing more grain with lower environmental costs. Nature 514: 486–489. https://doi.org/10.1038/nature13609 .
Cormier, F., J. Foulkes, B. Hirel, D. Gouache, Y. Moënne-Loccoz, and J. Le Gouis. 2016. Breeding for increased nitrogen-use efficiency: A review for wheat ( T. aestivum L.). Plant Breeding 135: 255–278. https://doi.org/10.1111/pbr.12371 .
Cui, Z., H. Zhang, X. Chen, C. Zhang, W. Ma, C. Huang, W. Zhang, G. Mi, et al. 2018. Pursuing sustainable productivity with millions of smallholder farmers. Nature 555: 363–366. https://doi.org/10.1038/nature25785 .
Denison, R.F. 2015. Evolutionary tradeoffs as opportunities to improve yield potential. Field Crops Research 182: 3–8.
Dobermann, A., C. Witt, D. Dawe, S. Abdulrachman, G.C. Gines, R. Nagarajan, S. Satawathananont, T.T. Son, et al. 2002. Site-specific nutrient management for intensive rice cropping systems in Asia. Field Crops Research 74: 37–66.
Fabregas, R., M. Kremer, and F. Schilbach. 2019. Realizing the potential of digital development: The case of agricultural advice. Science . https://doi.org/10.1126/science.aay3038 .
Foley, J.A., N. Ramankutty, K.A. Brauman, E.S. Cassidy, J.S. Gerber, M. Johnston, N.D. Mueller, C. Connell, et al. 2011. Solutions for a cultivated planet. Nature 478: 337–342. https://doi.org/10.1038/nature10452 .
Fowler, D., M. Coyle, U. Skiba, M.A. Sutton, J.N. Cape, S. Reis, L.J. Sheppard, A. Jenkins, et al. 2013. The global nitrogen cycle in the twenty-first century. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences 368: 20130164. https://doi.org/10.1098/rstb.2013.0164 .
Kanter, D.R., O. Chodos, O. Nordland, M. Rutigliano, and W. Winiwarter. 2020. Gaps and opportunities in nitrogen pollution policies around the world. Nature Sustainability . https://doi.org/10.1038/s41893-020-0577-7 .
Khurana, H.S., S.B. Phillips, M.M. Bijay-Singh, A. Alley, A.S. Sidhu, Yadvinder-Singh, and S.B. Peng. 2008. Agronomic and economic evaluation of site-specific nutrient management for irrigated wheat in northwest India. Nutrient Cycling in Agroecosystems 82: 15–31.
Ladha, J.K., and P.M. Reddy. 1995. Extension of nitrogen fixation to rice? Necessity and possibilities. GeoJournal 35: 363–372. https://doi.org/10.1007/BF00989144 .
Ladha, J.K., A. Tirol-Padre, C.K. Reddy, K.G. Cassman, S. Verma, D.S. Powlson, C. van Kessel, D. de Richter, et al. 2016. Global nitrogen budgets in cereals: A 50-year assessment for maize, rice, and wheat production systems. Scientific Reports 6: 19355. https://doi.org/10.1038/srep19355 .
Lassaletta, L., G. Billen, B. Grizetti, J. Anglade, and J. Garnier. 2014. 50 year trends in nitrogen use efficiency of world cropping systems: The relationship between yield and nitrogen input to cropland. Environmental Research Letters 9: 105011.
Lowenberg-DeBoer, J., and B. Erickson. 2019. Setting the record straight on precision agriculture adoption. Agronomy Journal 111: 1552–1569. https://doi.org/10.2134/agronj2018.12.0779 .
Ma, L., Z. Bai, W. Ma, M. Guo, R. Jiang, J. Liu, O. Oenema, G.L. Velthof, et al. 2019. Exploring future food provision scenarios for China. Environmental Science and Technology 53: 1385–1393. https://doi.org/10.1021/acs.est.8b04375 .
McLellan, E., D. Robertson, K. Schilling, M. Tomer, J. Kostel, D. Smith, and K. King. 2015. Reducing nitrogen export from the Corn Belt to the Gulf of Mexico: Agricultural strategies for remediating hypoxia. JAWRA Journal of the American Water Resources Association 51: 263–289. https://doi.org/10.1111/jawr.12246 .
McLellan, E.L., K.G. Cassman, A.J. Eagle, P.B. Woodbury, S. Sela, C. Tonitto, R.D. Marjerison, and H.M. van Es. 2018. The nitrogen balancing act: Tracking the environmental performance of food production. BioScience 68: 194–203. https://doi.org/10.1093/biosci/bix164 .
Pampolino, M.F., I.J. Manguiat, S. Ramanathan, H.C. Gines, P.S. Tan, T.T.N. Chi, R. Rajendran, and R.J. Buresh. 2007. Environmental impact and economic benefits of site-specific nutrient management (SSNM) in irrigated rice systems. Agricultural Systems 93: 1–24.
Pampolino, M.F., C. Witt, J.M. Pasuquin, A. Johnston, and M.J. Fisher. 2012. Development approach and evaluation of the Nutrient Expert software for nutrient management in cereal crops. Computers and Electronics in Agriculture 88: 103–110. https://doi.org/10.1016/j.compag.2012.07.007 .
Pasuquin, J.M., M.F. Pampolino, C. Witt, A. Dobermann, T. Oberthur, M.J. Fisher, and K. Inubushi. 2014. Closing yield gaps in maize production in Southeast Asia through site-specific nutrient management. Field Crops Research 156: 219–230.
Quemada, M., L. Lassaletta, L.S. Jensen, O. Godinot, F. Brentrup, C. Buckley, S. Foray, S.K. Hvid, et al. 2020. Exploring nitrogen indicators of farm performance among farm types across several European case studies. Agricultural Systems 177: 102689. https://doi.org/10.1016/j.agsy.2019.102689 .
Rogers, C., and G.E.D. Oldroyd. 2014. Synthetic biology approaches to engineering the nitrogen symbiosis in cereals. Journal of Experimental Botany 65: 1939–1946. https://doi.org/10.1093/jxb/eru098 .
Rurinda, J., S. Zingore, J.M. Jibrin, T. Balemi, K. Masuki, J.A. Andersson, M.F. Pampolino, I. Mohammed, et al. 2020. Science-based decision support for formulating crop fertilizer recommendations in sub-Saharan Africa. Agricultural Systems 180: 102790.
Saikai, Y., V. Patel, and P.D. Mitchell. 2020. Machine learning for optimizing complex site-specific management. Computers and Electronics in Agriculture 174: 105381. https://doi.org/10.1016/j.compag.2020.105381 .
Saito, K., S. Diack, I. Dieng, and M.K. N’Diaye. 2015. On-farm testing of a nutrient management decision-support tool for rice in the Senegal River valley. Computers and Electronics in Agriculture 116: 36–44. https://doi.org/10.1016/j.compag.2015.06.008 .
Salvagiotti, F., K.G. Cassman, J.E. Specht, D.T. Walters, A. Weiss, and A. Dobermann. 2008. Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crops Research 108: 1–13.
Senthilkumar, K., J. Rodenburg, I. Dieng, E. Vandamme, F.S. Sillo, J.-M. Johnson, A. Rajaona, J.A. Ramarolahy, et al. 2020. Quantifying rice yield gaps and their causes in Eastern and Southern Africa. Journal of Agronomy and Crop Science 206: 478–490. https://doi.org/10.1111/jac.12417 .
Sharma, S., P. Panneerselvam, R. Castillo, S. Manohar, R. Raj, V. Ravi, and R.J. Buresh. 2019. Web-based tool for calculating field-specific nutrient management for rice in India. Nutrient Cycling in Agroecosystems 113: 21–33. https://doi.org/10.1007/s10705-018-9959-x .
Smith, M.J. 2020. Getting value from artificial intelligence in agriculture. Animal Production Science 60: 46. https://doi.org/10.1071/AN18522 .
Springmann, M., M. Clark, D. Mason-D’Croz, K. Wiebe, B.L. Bodirsky, L. Lassaletta, W. de Vries, S.J. Vermeulen, et al. 2018. Options for keeping the food system within environmental limits. Nature 562: 519–525. https://doi.org/10.1038/s41586-018-0594-0 .
Steffen, W., K. Richardson, J. Rockström, S.E. Cornell, I. Fetzer, E.M. Bennett, R. Biggs, S.R. Carpenter, et al. 2015. Sustainability. Planetary boundaries: Guiding human development on a changing planet. Science 347: 1259855. https://doi.org/10.1126/science.1259855 .
Subbarao, G.V., J. Arango, K. Masahiro, A.M. Hooper, T. Yoshihashi, Y. Ando, K. Nakahara, S. Deshpande, et al. 2017. Genetic mitigation strategies to tackle agricultural GHG emissions: The case for biological nitrification inhibition technology. Plant Science 262: 165–168.
ten Berge, H.F.M., R. Hijbeek, M.P. van Loon, J. Rurinda, K. Tesfaye, S. Zingore, P. Craufurd, J. van Heerwaarden, et al. 2019. Maize crop nutrient input requirements for food security in sub-Saharan. Africa 23: 9–21.
Google Scholar
Uwizeye, A., I.J.M. de Boer, C.I. Opio, R.P.O. Schulte, A. Falcucci, G. Tempio, F. Teillard, F. Casu, et al. 2020. Nitrogen emissions along global livestock supply chains. Nature Food . https://doi.org/10.1038/s43016-020-0113-y .
van Grinsven, H.J.M., L. Bouwman, K.G. Cassman, H.M. van Es, M.L. McCrackin, and A.H.W. Beusen. 2015. Losses of ammonia and nitrate from agriculture and their effect on nitrogen recovery in the European Union and the United States between 1900 and 2050. Journal of Environmental Quality 44: 356–367. https://doi.org/10.2134/jeq2014.03.0102 .
van Ittersum, M.K., L.G.J. van Bussel, J. Wolf, P. Grassini, J. van Wart, N. Guilpart, L. Claessens, H. de Groot, et al. 2016. Can sub-Saharan Africa feed itself? Proceedings of the National Academy of Sciences 113: 14964–14969. https://doi.org/10.1073/pnas.1610359113 .
van Lammerts Bueren, E.T., and P.C. Struik. 2017. Diverse concepts of breeding for nitrogen use efficiency. A review. Agronomy for Sustainable Development . https://doi.org/10.1007/s13593-017-0457-3 .
van Zanten, H.H.E., M.K. van Ittersum, and I.J.M. de Boer. 2019. The role of farm animals in a circular food system 21: 18–22. https://doi.org/10.1016/j.gfs.2019.06.003 .
Vollset, S.E., E. Goren, C.-W. Yuan, J. Cao, A.E. Smith, T. Hsiao, C. Bisignano, G.S. Azhar, et al. 2020. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: A forecasting analysis for the Global Burden of Disease Study. The Lancet . https://doi.org/10.1016/S0140-6736(20)30677-2 .
Wang, Y., C. Li, Y. Li, L. Zhu, S. Liu, L. Yan, G. Feng, and Q. Gao. 2020. Agronomic and environmental benefits of nutrient expert on maize and rice in Northeast China. Environmental Science and Pollution Research . https://doi.org/10.1007/s11356-020-09153-w .
Willett, W., J. Rockström, B. Loken, M. Springmann, T. Lang, S. Vermeulen, T. Garnett, D. Tilman, et al. 2019. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. The Lancet 393: 447–492. https://doi.org/10.1016/S0140-6736(18)31788-4 .
Zhang, X., E.A. Davidson, D.L. Mauzerall, T.D. Searchinger, P. Dumas, and Y. Shen. 2015. Managing nitrogen for sustainable development. Nature 528: 51–59. https://doi.org/10.1038/nature15743 .
Zhang, X., E.A. Davidson, T. Zou, L. Lassaletta, Z. Quan, T. Li, and W. Zhang. 2020. Quantifying nutrient budgets for sustainable nutrient management. Global Biogeochemical Cycles . https://doi.org/10.1029/2018GB006060 .
Zossou, E., K. Saito, A. Assouma-Imorou, K. Ahouanton, and B.D. Tarfa. 2020. Participatory diagnostic for scaling a decision support tool for rice crop management in northern Nigeria. Development in Practice . https://doi.org/10.1080/09614524.2020.1770699 .
Download references
Acknowledgements
The authors wish to recognize the contributions of Daniel T. Walters, who was a co-author and dear colleague on our original 2002 Ambio paper, but who passed away in 2010.
Author information
Authors and affiliations.
University of Nebraska, 202 Keim Hall, Lincoln, NE, 68583, USA
Kenneth G. Cassman
International Fertilizer Association, 49 Avenue d’Iena, 75116, Paris, France
Achim Dobermann
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Kenneth G. Cassman .
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cassman, K.G., Dobermann, A. Nitrogen and the future of agriculture: 20 years on. Ambio 51 , 17–24 (2022). https://doi.org/10.1007/s13280-021-01526-w
Download citation
Published : 14 March 2021
Issue Date : January 2022
DOI : https://doi.org/10.1007/s13280-021-01526-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research
An Overview of the Kjeldahl Method of Nitrogen Determination. Part II. Sample Preparation, Working Scale, Instrumental Finish, and Quality Control
- This person is not on ResearchGate, or hasn't claimed this research yet.

- Cracow University of Technology

Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
- Marwa Abou-Taleb
- Hosam El-Sayed
- Zobia Waheed

- Lutviana Tiaswuni
- Ahmad Ni'matullah Al-Baarri

- Hayatun Nupus
- Syaifuddin Syaifuddin
- Akhmad Rizalli Saidy

- Abdellatif Janati-Idrissi

- Xingrong Sun

- Felipe Neri Hernádez Soto
- Jacqueline Emeterio Moreno
- María Karina Colín Velázquez
- Ahlam A. Alfazairy
- Deena A. Elsakhawy
- Fatma A. El-Meniawi
- Ibrahem A. Rawash
- Ashfak Mahmud

- Papa Niokhor Diouf

- Shirley Anderson
- J Assoc Offic Anal Chem
- Walter M Gantenbein

- Gregory Möller
- Brink Marcia King
- Sebranek Joseph G.
- Cindy G Price
- Neil B Webb
- Wertice J Smith
- Aron M Yoffe

- Stuart M Barlow
- John Van Camp

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 09 December 2023
Nitrogen fertilization application strategies improve yield of the rice cultivars with different yield types by regulating phytohormones
- Yue Zou 1 , 2 ,
- Yuchen Zhang 1 ,
- Jiehao Cui 1 ,
- Jiacong Gao 1 ,
- Liying Guo 1 &
- Qiang Zhang 1 , 2
Scientific Reports volume 13 , Article number: 21803 ( 2023 ) Cite this article
- Plant hormones
- Plant physiology
Rice ( Oryza sativa L. ) is the most important food crop worldwide, and its sustainable development is essential to ensure global food security. Panicle morphological and physiological characteristics plays an important role in rice yield formation. However, under different nitrogen (N) fertilization strategies, it is not clear whether the morphological and physiological state of panicles at panicle development stage affects the formation of yield. To understand how the panicle differentiation and development, and grain yield are affected by the N fertilization strategies, and clarify the relationship between related traits and yield in the process of panicle development in different cultivars. In this study consisted of no N fertilizer and four N fertilization strategies, a panicle weight type (PWT) rice cultivar, Dongfu 114 (DF114) and a panicle number type (PNT) rice cultivar, Longdao 11 (LD11) were grown in the field. The results showed that N fertilization strategies could improve the nitrogen use efficiency and yield of rice, but the response of different rice varieties to N fertilizer strategies was different. Different from the DF114, the further increase of panicle N fertilizer ratio could not further improve the yield of LD11, and the highest grain yield of DF114 and LD11 was obtained under N4 and N3 conditions, respectively. In addition, this study found that N fertilizer strategies can affect the content of phytohormones in rice at the panicle differentiation stage, and then regulate the differentiation and development of rice panicles to affect yield. It is of great significance to optimize the application mode of N fertilizer according to the characteristics of varieties to improve rice yield and ensure food security.
Similar content being viewed by others
Scheduling of nitrogen fertilizer topdressing during panicle differentiation to improve grain yield of rice with a long growth duration

The nitrogen topdressing mode of indica-japonica and indica hybrid rice are different after side - deep fertilization with machine transplanting

Effects of low nitrogen on seedling growth, photosynthetic characteristics and antioxidant system of rice varieties with different nitrogen efficiencies
Introduction.
Rice ( Oryza sativa L. ) is one of the most important food crops in the world, about half of the world's people use rice as their staple food. Its sustainable and healthy development is an important support to ensure world's food security. Under the severe situation of increasing population and decreasing land, people have steadily improved the yield of rice by improving rice cultivars, advancing cultivation techniques and increasing production inputs, among them, increasing nitrogen (N) input is one of the effective measures 1 , 2 , 3 . N is one of the most important nutrient elements, which can greatly affect the yield of rice. Since the last century, due to the increase of N application, rice yield has also increased significantly. However, rice plants can only use part of the applied N fertilizer, accounting for about 20–30% of the total amount of fertilizer, excessive and unreasonable N fertilizer input has also brought a series of problems such as unstable grain production, decreased nitrogen use efficiency (NUE), increased production costs, exacerbating environmental pollution, which has seriously affected the sustainable development of rice production 4 , 5 , 6 , 7 , 8 .
In view of the above problems, many scholars have carried out a lot of research on how to improve the NUE in paddy fields, and have made great progress in the comparison, screening, evaluation and utilization of NUE of rice germplasm, the physiological and biochemical characteristics, root morphology, dry matter production and accumulation characteristics of different nitrogen use efficiency genotypes, and the effects of field N management on rice yield, quality and NUE 9 , 10 , 11 , 12 , 13 , 14 , 15 . In addition, under the context of sustainable agricultural production, appropriate N management measures are needed to protect the environment and increase rice productivity. Over the past few decades, many optimized nitrogen fertilization strategies have been proposed and tested in field trials, such as top dressing, precise quantitative fertilization, site-specific nutrient management, etc. 1 , 16 , 17 , 18 , 19 . In these strategies, the improvement of rice yield is mainly through increasing the number of fertilization, or regulating the use of panicle fertilizer at the stage of panicle development. However, different types of rice cultivars have different responses to N fertilizer application strategies 5 , 20 .
Phytohormones are a class of trace organic matter produced by plants themselves and have obvious physiological effects at very low concentrations 21 , 22 . At present, phytohormones include auxins (IAA), gibberellins (GAs), cytokinins (CTK), abscisic acid (ABA) and ethylene (ETH), and new phytohormones such as brassinosteroids (BRs), polyamines (PAs), jasmonates (JA), salicylic acid (SA) and strigolactone (SL) 23 . A large number of studies have shown that phytohormones play an important regulatory role in rice panicle development, grain filling, grain quality and quality, and different phytohormones play different roles and mechanisms. However, the biological mechanism of how phytohormones respond to N fertilization strategies to regulate rice N uptake and utilization efficiency, panicle morphogenesis and yield formation in different rice cultivars is still unclear. In the present study, a panicle weight type (PWT) rice cultivar, Dongfu 114 (DF114) and a panicle number type (PNT) rice cultivar, Longdao 11 (LD11) were used to compare the effects of N fertilization strategies on panicle development characteristics and yield of rice cultivars with contrasting yield types. This study will provide a theoretical basis for the rational and effective application of N fertilizer in rice production.
Materials and methods
Plant materials and growth conditions.
The field experiments was conducted at the Acheng rice experiment base belonging to Northeast Agricultural University (45° 39′ N, 127° 34′ E) during the rice growing season in 2021, and repeated in 2022. The region is a cold temperate semi humid continental climate. The meteorological data such as temperature, rainfall, humidity and sunshine hours during the rice growing season in the experimental year were from the National Meteorological Data Center (Table 1 ). The soil texture of the test field is brown soil, and the soil nutrient content is shown in Table 2 . Two rice cultivars with different yield types, DF114 and LD11 were used. The two rice cultivars have similar growth periods. The experimental materials were provided by the rice research group of Northeast Agricultural University.
Experimental design
The experiments were laid out in a complete randomized block design with three replicates. The N fertilizer strategies was the main plot treatment, and the rice cultivars formed the sub-plot treatment. Each plot was 8-m in length and 6-m in width with 30 cm row spacing and 13.3 cm terrace spacing, and two seedlings per hill. The main plots were separated by a berm. The seeds were sown on 16 April 2021, and 16 April 2022. At the four-leaf hpaniclet stage, seedlings with similar growth were selected and transplanted on May 18, 2021, and May 17, 2022. The experimental treatments included N0 (no N fertilizer), N1 (farmers routinely N fertilizer strategies, base fertilizer:tiller fertilizer of 6:4), N2 (base fertilizer : tiller fertilizer : panicle fertilizer of 6:2:2), N3 (base fertilizer:tiller fertilizer:panicle fertilizer of 5:3:2), N4 (base fertilizer:tiller fertilizer:panicle fertilizer of 4:3:3), a total of 5 treatments. The N was applied as urea(46%), at a rates were 150 kg ha −1 . Phosphate fertilizer (P 2 O 5 ) was applied once as a basal fertilizer at a rate of 90 kg ha −1 . Potash fertilizer (K 2 O) was applied as a basal and panicle fertilizer at a ratio of 5:5, at a rate of 90 kg ha −1 . With the exception of the different nitrogen fertilizer strategies, the other cultivation requirements were identical for all plots in both ypanicles. Chemicals were used to control weeds, diseases, and insects to prevent yield loss.
Sampling and measurements
Nitrogen uptake and utilization.
At the maturity stage, five representative hills were taken from each plot in 2021 and 2022. Aboveground plants sampled were separated into stems, leaves and grains. Dry weight of each part was determined by oven-drying at 80 °C to constant weight and weighed separately, and recorded the dry weight of each part. Tissue N content was measured with an elemental analyzer to calculate N uptake of each part (EA1110, Thermo Electon SPA., Italy). The calculation formula of N absorption and utilization related indicators is as follows:
Grain yield and its components
In 2021 and 2022, grain yield (GY) and its components were measured at maturity. GY was measured from a harvest area of 1 m 2 area for the three replicates in each plot, adjusted to 14% moisture. The yield components were measured for nine replicates in each plot. The panicle number from each samples were recorded to measure the effective panicles number (EP). The filled grains andunfilled grains per panicle were recorded to calculate the number of grains per panicle (GNP) and seed setting rate (SSR). The 1000-grain weight (TGW) was weighed and recorded.
Tillering dynamic survey
In 2021 and 2022, after 14 days of transplanting, 10 randomly selected plants were investigated every 7 days, and tillers were recorded until the number of tillers did not change. Calculate maximum tiller number (MTN) and productive tiller percentage (PTP) ccording to tillering dynamic survey.
Floret and grain development
At spikelet differentiation stage, pikelets differentiated number (SDN) were measured in 2021 and 2022. The differentiation of florets was observed using a planing microscope, and the SDN was recorded. The grain per panicle was used as the spikelets surviving number to calculate the spikelets degenerated number (SRN) and degraded percentage (RP).
The panicle biomass and nitrogen concentration at panicle development stage
According to the heading stage data of the tested cultivars, this study sampled at five time points: 14 days before heading stage, 7 days before heading stage, heading stage, 7 days after heading stage and 14 days after heading stage, which were named S1, S2, S3, S4 and S5, respectively. The dry weight of each panicle was determined by oven-drying at 80 °C to constant weight and weighed separately, and recorded the panicle biomass. The panicle N concentration was measured with an elemental analyzer (EA1110, Thermo Electon SPA., Italy).
Panicle and root phytohormones content at panicle development stage
The panicles and roots per plot were sampled to determine phytohormones content were obtained at S1, S2, S3, S4 and S5 stages. The contents of IAA, ABA, GA and ZR were determined with enzyme-linked immunosorbent assay (ELIAS) method, according to the operation guide of ELIAS kit of China Agricultural University 24 , 25 .
Statistical analysis
In this study, for the experimental variables, one-way analysis of variance (ANOVA) of SPSS 22.0 (SPSS Inc., Chicago, IL, USA ) was used to evaluate the differences between different N fertilizer application strategies. According to Fisher's LSD, there were significant differences between different N fertilizer application strategies at the p < 0.05 level. Graphs were drawn using edgeR software ( http://www.r-project.org/ ) and Origin 2023b software (OriginLab, Northampton, MA, USA).
Ethical statement
We ensure that all rice seeds used in this study originated from northeast agricultural university in Heilongjiang Province, China. The legality of these seeds complies with the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora. The rice seeds collected in the study are all cultivated rice in China rather than endangered and wild species. These varieties have passed the legal variety certification procedures in China and are licensed for production, planting, and market operations. The authors declare that the cultivation of plants and carrying out study in the Acheng rice experiment base of northeast agricultural university complies with all relevant institutional, national and international guidelines and treaties.
As shown in Table 3 , the indexes of N uptake and utilization in both cultivars were significantly different among different N treatments. Compared with farmers routinely N fertilizer strategies, the N fertilization strategies were significantly increased NRE, PFPN, NAE and NPE of two rice cultivars. The NRE, PFPN, NAE and NPE of DF114 were the highest under N4 treatment, and the NRE, PFPN, NAE and NPE of LD11 were the highest under N3 treatment. This result indicates that the N fertilization strategies could improve the N uptake and utilization of rice, and there were some differences among different yield types of rice cultivars.
Grain yield and yield components
As shown in Table 4 , the GY and yield components in two yield types of rice were significantly different among different N treatments. Compared with farmers routinely N fertilizer strategies, the N fertilization strategies were significantly increased GY and GPN of two rice cultivars. The GY and GPN of DF114 was the highest under N4 treatment, and the GY and GPN of LD11 was the highest under N3 treatment. Compared with farmers routinely N fertilizer strategies, the N fertilization strategies were significantly increased EP and SSR (except LD11 in 2022) of two rice varieties. The EP of two rice cultivars were the highest under N3 treatment. Compared with farmers routinely N fertilizer strategies, the N fertilization strategies were decreased TGW of two rice cultivars (except LD11 in 2022). Interestingly, increase the application ratio of panicle N fertilizer can guarantee the GPN and SSR of rice, but excessive N fertilizer postponing reduces the EP of PNT rice cultivar LD11.
Dynamic changes of tillers
As shown in Fig. 1 , the N fertilization strategies significantly affected the tillering of rice, and the tillering dynamic trends of DF114 and LD11 were basically the same under among N fertilization strategies. At 20–30 days after transplanting, the number of tillers increased rapidly and reached the highest number of tillers at about 35 days (LD11 reached the highest number of tillers at about 42 days in 2022). After 50 days of transplanting, the growth center of the plant was transferred to the stem and panicle, and the nutrients transported to the tillers were greatly reduced. The new tillers did not occur, and some of the small tillers that had been born began to die in succession until the number of tillers in the population tended to be stable after heading. In this study, compared with the farmers routinely N fertilizer strategies, the N fertilization strategies were significantly reduced the MTN of rice, but also significantly increased the PTP of rice to ensure a higher EP of rice.
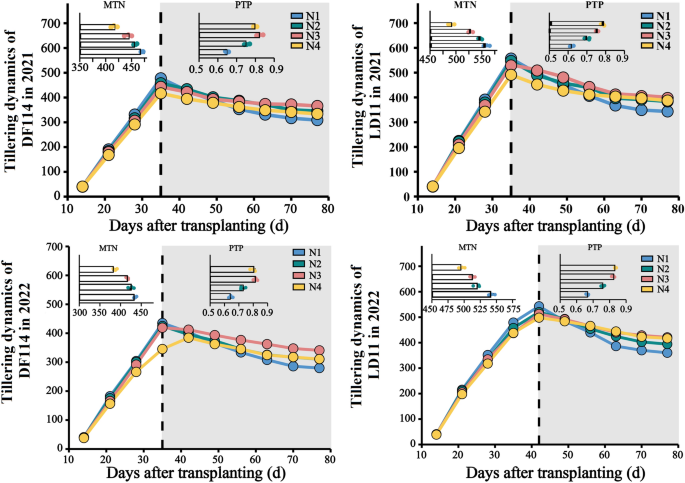
Tillering dynamics of rice under different treatments in 2021 and 2022. MTN maximum tiller number, PTP productive tiller percentage.
The spikelets differentiation and degeneration, and grain development in two yield types of rice were significantly different among different N treatments (Table 5 ). Compared with the farmers routinely N fertilizer strategies, the N fertilization strategies were significantly increased SDN of two rice cultivars (except DF114 in 2022). Compared with the farmers routinely N fertilizer strategies, the N fertilization strategies were significantly decreased SRN and RP of two rice cultivars. The SRN and RP of DF114 were the lowest under N4 treatment, and the GPN of LD11 was the lowest under N3 treatment.
The relationships among grain yield and the floret and grain development
The formation of rice GY mainly depends on the number of EP, GPN, SSR and TGW. As shown in Fig. 2 , the EP and GPN were significant positively, and TGW was significant negatively correlated with GY in both rice cultivars. The SSR were significant positively with GY of DF114. The farmers routinely N fertilizer strategies can produce more tillers, but there are more ineffective tillers. Correlation analysis showed that PTP was an important basis for higher EP to ensure higher yield. The differentiation and degeneration of spikelets determine the GPN of rice. Correlation analysis showed that the SRN was significant negatively correlated with GPN of DF114, and the SDN was significant positively correlated with GPN of LD11. These results indicated that panicle and grain development was the main factor responsible for the difference in yield among two rice cultivars under difference N treatments.
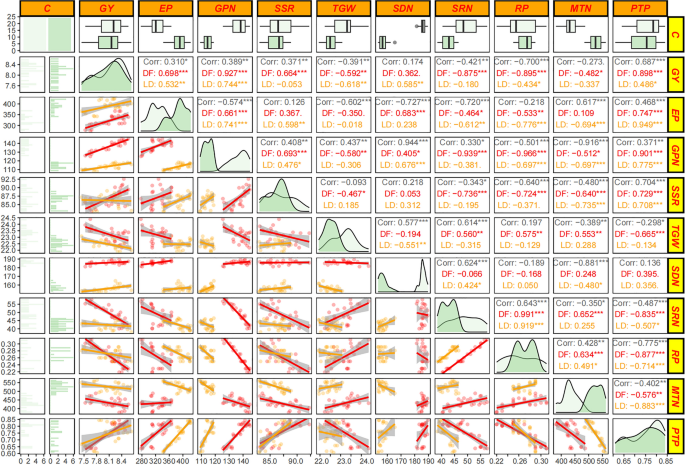
The relationships among grain yield and the floret and grain development. The *, ** and *** indicate that at the level of 0.05, 0.01 and 0.001, respectively. GN grain yield, EP effective panicles, GPN grain per panicle, SSR seed setting rate, TGW 1000-grain weight, SDN spikelets differentiated number, SRN spikelets degraded number, RP degraded percentage, MTN maximum tiller number, PTP productive tiller percentage.
At the panicle development stage, the responses of different yield type rice cultivars to N fertilizer treatments were coincident (Fig. 3 ). With the development of panicle, the N concentration of young panicle decreased and the biomass increased. Compared with the farmers routinely N fertilizer strategies, the N fertilization strategies were significantly increased panicle biomass (PB) of two rice varieties during the period from S3 to S5, the panicle biomass of DF114 was the highest under N4 treatment, and the panicle biomass of LD11 was the highest under N3 treatment. Compared with the farmers routinely N fertilizer strategies, the N fertilization strategies were significantly impacted panicle N concentration (PN) of two rice varieties during the period from S1 to S5.
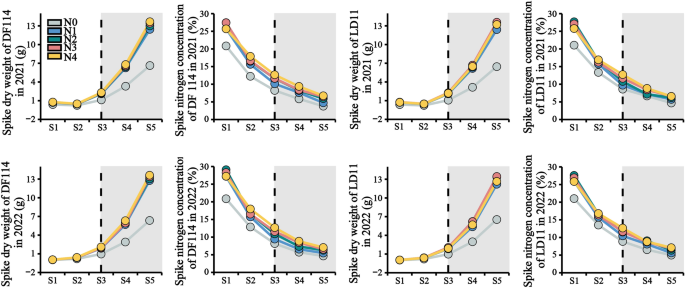
The N concentration and panicle dry weight of the rice under different treatment during critical period.
The phytohormone content in panicle and root at panicle development stage
The IAA content in the panicle of tow rice cultivars gradually decreased with the developmental stage of the young panicle under different N treatments. The ABA content in the panicle of tow rice cultivars were decreased first and then increased with the developmental stage of the young panicle under different N treatments. Compared with the farmers routinely N fertilizer strategies, the N fertilization strategies were significantly increased the ABA content of DF114 panicle at S3-S5 stages, and increased the ABA content of LD11 panicle at S1-S5 stages. The GA content in the panicle of tow rice cultivars were decreased first and then increased with the developmental stage of the young panicle under different N treatments. Compared with the farmers routinely N fertilizer strategies, the N fertilization strategies were significantly increased the GA content of two rice cultivars panicle at S3–S5 stages. The ZR content in the panicle of tow rice cultivars were decreased first and then increased with the developmental stage of the young panicle under different N treatments. Compared with the farmers routinely N fertilizer strategies, the N fertilization strategies were significantly increased the ZR content of DF114 panicle at S2-S5 stages, and increased the ZR content of LD11 panicle at S1, S2 and S5 (Fig. 4 ).
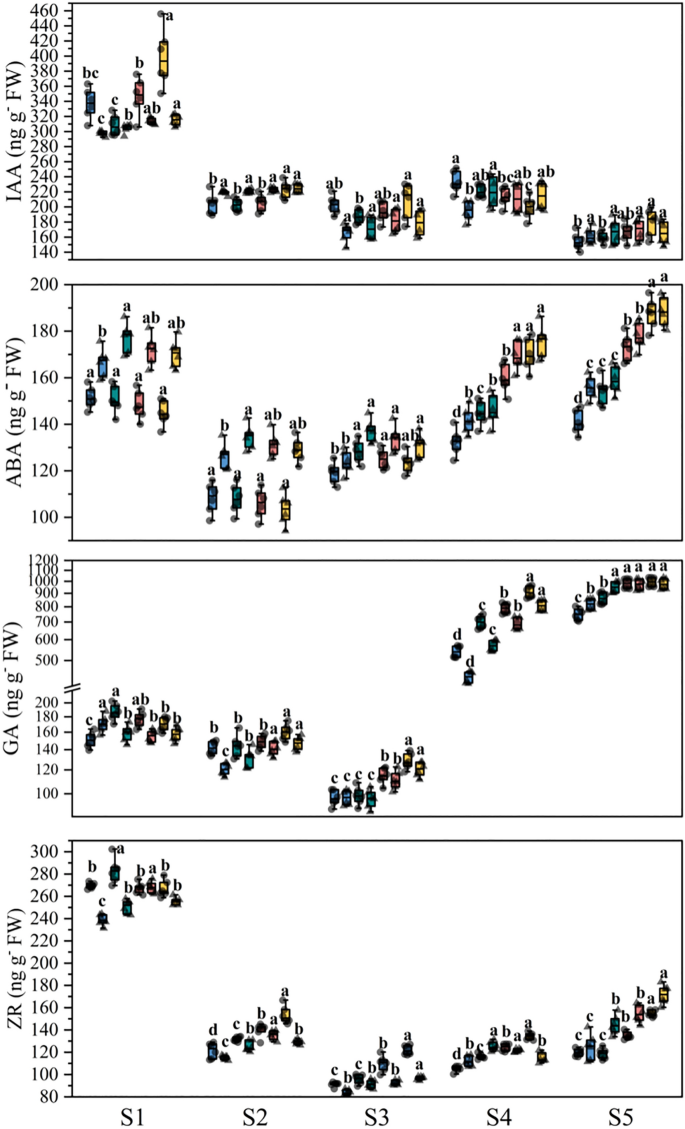
The phytohormones content in panicle at panicle development stage. Result is the average from 2021 to 2022. The different small letters above the box indicate significant difference in the same panicle differentiation stage among the same cultivars at P < 0.05. From left to right represent N1, N2, N3 and N4, respectively.
The IAA content in the root of tow rice cultivars gradually decreased with the developmental stage of the young panicle under different N treatments. Compared with the farmers routinely N fertilizer strategies, the N fertilization strategies were significantly increased the IAA content of DF114 root at S1 stages, and increased the IAA content of LD11 root at S1–S5 stages. The ABA content in the root of tow rice cultivars were increased with the developmental stage of the young panicle under different N treatments. Compared with the farmers routinely N fertilizer strategies, the N fertilization strategies were significantly increased the ABA content of DF114 root at S4 and S5 stages, and increased the IAA content of LD11 root at S3-S5 stages. The GA content in the root of tow rice cultivars were increased first and then decreased with the developmental stage of the young panicle under different N treatments. The ZR content in the root of tow rice cultivars were increased with the developmental stage of the young panicle under different N treatments. Compared with the farmers routinely N fertilizer strategies, the N fertilization strategies were significantly increased the ZR content of DF114 root at S2 and S5 stages (Fig. 5 ).
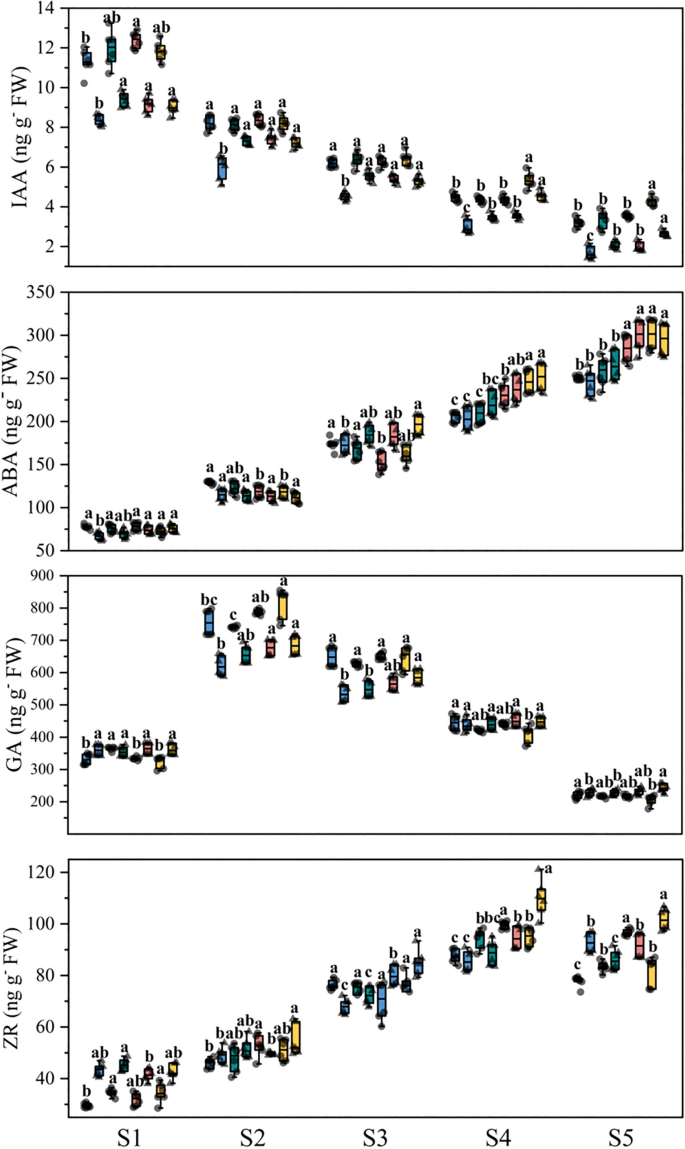
The phytohormone content in root at panicle development stage. Result is the average from 2021 to 2022. The different small letters above the box indicate significant difference in the same panicle differentiation stage among the same cultivars at P < 0.05. From left to right represent N1, N2, N3 and N4, respectively.
Correlations between panicle and grain development, and panicle and root phytohormones
The results of correlations analysis (Figs. 2 , 6 ) showed that the spikelet differentiation and grain filling were the main reasons for the different responses of the two rice cultivars to N treatments. The results of principal component analysis show that the SRN of DF114 were significantly negatively correlated with P-ZR, P-GA and PN at panicle differentiation stage. The SSR of DF114 were significantly positively correlated with P-ZR, P-ABA and R-ABA at after flowering stage. The SDN of LD11 were significantly positively correlated with P-IAA at panicle differentiation stage. These results indicate that phytohormones play an important role in rice yield formation, and reasonable optimization of N fertilizer application strategies can regulate rice phytohormone levels to improve NUE and yield.
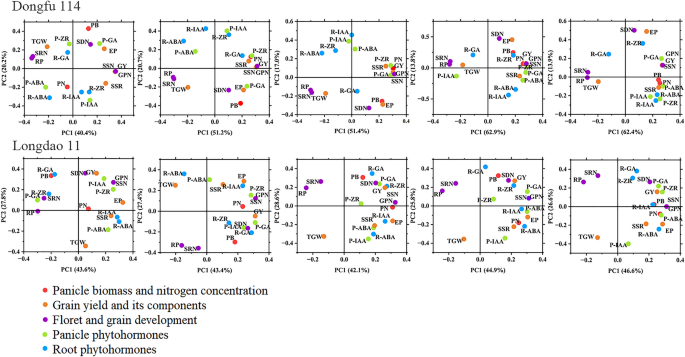
Principal component analysis (PCA) of panicle differentiation and development traits, panicle and root phytohormones determined on rice under different treatment during critical panicle differentiation stages.
The N is one of the most important nutrient elements in the process of rice growth, and the amount of N fertilizer significantly affects the yield of rice 17 . Farmers usually apply most of the N fertilizer at the early stage of nutrition. At this time, the absorption capacity of rice roots to N is limited, and a large amount of N is retained in soil and irrigation water. The results of 15 N tracing shown that the NUE of N fertilizer applied to rice before regreening was lower, and a large amount of N fertilizer was lost and volatilized 26 , 27 . In this study area, farmers applied 150 kg hm −2 of N fertilizer. Although the amount of fertilizer applied is less than some areas in southern China, there is still a large amount of the N fertilizer applied at the initial stage of tillering. Some studies have shown that an appropriate increase in the proportion of panicle fertilizer can improve the NUE and yield of rice 28 , 29 . In this study, higher panicle fertilizer ratio can effectively improve the NUE and yield of both rice cultivars. However, there were some differences among different cultivars, DF114 had the highest NUE and yield under N4 treatment, and LD11 had the highest NUE and yield under N3 treatment. This result was in agreement with previous observations that rice cultivars with different productivity and NUE can achieve maximum yield under precise quantitative cultivation 19 , 30 , 31 . It can be seen that although the optimized N fertilizer application strategies can improve the NUE of rice, the response of rice varieties with different yield types to the optimized N fertilizer application strategies is different. The increase of panicle N fertilizer proportion is more conducive to improving the NUE and yield of PWT rice cultivars.
The effects of N application strategies on rice NUE and yield were related to the response characteristics of multiple yield components to N application strategies 32 . Tillers are composed of effective tillers and ineffective tillers. Excessive ineffective tillers not only consume nutrients, but also deteriorate population permeability and aggravate the occurrence of pests and diseases 33 . Previous studies have found that the PTP of rice is closely related to a number of population quality indicators, and it is an intuitive and comprehensive indicator for diagnosing population quality 17 , 18 , 19 . Under normal circumstances, rice plants have a stable law of leaf and tiller extension. However, excessive N application in the early stage of rice growth will shorten the time interval of leaf emergence, accelerate the occurrence of tillers, and increase the number of ineffective tillers. On the contrary, insufficient N fertilizer will also lead to a longer time interval for leaf emergence, a slower rate of tillering, and insufficient panicles 34 . In this study, postponing N fertilizer significantly reduced the MTN of rice and significantly increased the spike rate. However, the further increase of panicle fertilizer ratio also significantly reduced the number of effective panicles of LD11. Therefore, under the premise of ensuring the number of panicles, it is an important way to improve rice NUE and yield an by reducing the seedling peak, reducing the ineffective tillers and increasing the PTP.
The reproductive development process of crops is closely related to the formation of yield. Many studies have suggested that the key to further increase the yield of major grain crops such as rice is to increase the GPN 35 . The development stage of young panicle is the key period to determine the number of spikelets per panicle of rice, including rachis differentiation stage, primary and secondary branch differentiation stage and spikelet differentiation stage 36 . The number of spikelets is the basis of the number of filled grains, which together with the SSR determines the GPN of rice 37 . According to previous studies, the application of N fertilizer at the panicle differentiation stage of rice can promote floret differentiation 38 , 39 . In this study, the response of floret differentiation of the two varieties to N fertilizer management patterns was quite different. The SDN of PWT varieties was significantly increased with the increase of panicle N fertilizer ratio. Compared with farmers routinely N fertilizer strategies,, the increase of panicle fertilizer ratio significantly reduced the SRN and RP, and then increased the GPN and expanded the sink capacity, which was conducive to improving N absorption and utilization efficiency and yield.
In order to illustrate the biological basis of the response of panicle traits to N fertilization strategies, this study measured the panicle N and phytohormines content at the main stage of panicle development. Phytohormines play a essential role in regulating the development of plant organs, nutrient absorption and transport, and defensive adaptation to stress 40 , 41 . IAA is the most bizarre phytohormines discovered so far. Its content is very low in plants, but it plays an important role in crop organogenesis and morphogenesis, tissue differentiation tendency and apical dominance 42 , 43 , 44 . High N concentration in differentiated organs is conducive to promoting biosynthesis of IAA and differentiation of tissues and organs 45 . ABA is called senescence hormone, which can induce the occurrence of plant senescence, but the role of ABA in the senescence process is contradictory. Some studies have shown that ABA can coordinate the senescence process and ensure food production in the stage of crop yield formation 46 , 47 , 48 . ZR is a plant endogenous hormone belonging to cytokinin. It can accelerate cell division and plays an important role in regulating the size and activity of meristem, and directly affects the initiation and development of reproductive organs 49 . The rice LONELY GUY (LOG) gene encodes a cytokinin-activating enzyme that regulates the biosynthesis of cytokinin in rice. It is an important regulatory factor necessary for maintaining the activity of meristem. The meristem development of the log mutant is terminated in advance, the number of branches and spikelets is significantly reduced, and the floral organ cannot start normally 50 . Correlation analysis showed that SDN and SSR were the main factors affecting yield of DF114. PCA results showed that ZR content in panicle of DF114 was significantly correlated with N concentration in panicle, and was significantly negatively correlated with SRN and RP in floret differentiation stage. ABA content in panicle was significantly positively correlated with SSR in grain filling stage. The floret differentiation of LD11 was the main factor affecting the yield. PCA results showed that IAA in the panicle of LD11 was significantly positively correlated with SDN in the floret differentiation stage. These results suggest that N fertilizer strategies can affect the content of phytohormones in rice at the panicle differentiation stage, and then regulate the differentiation and development of rice panicles to affect yield. It is of great significance to optimize the application mode of N fertilizer according to the characteristics of varieties to improve rice yield and ensure food security.
Conclusions
Compared with farmer fertilization, N fertilizer application strategy significantly improved the NUE and yield of rice, but the response of rice varieties with different yield types to N fertilizer application strategy was different. Correlation analysis showed that panicle development related indexes SDN, SRN, RP and SSR were the main factors affecting NUE and yield of PNT and PWT rice varieties.The results showed that in the early stage of panicle development, the higher IAA content in the panicle of PNT rice varieties was beneficial to promote the differentiation of spikelets to ensure the yield.. While the indexes of SRN, RP and SSR in the middle and late stages of panicle development of PWT rice varieties were significantly correlated with yield. These results will lay a theoretical foundation for guiding the construction of high-yield and high-efficiency cultivation models for different yield types of rice varieties.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request (Q.Z., [email protected]).
Peng, S. B. et al. Strategies for overcoming low agronomic nitrogen use efficiency in irrigated rice systems in China. Field Crops Res. 96 (1), 37–47 (2006).
Article Google Scholar
Peng, S. B. et al. Improving nitrogen fertilization in rice by site-specific N management: A review. Agron. Sustain. Dev. 30 (3), 649–656 (2010).
Article CAS Google Scholar
Liu, X. et al. Enhanced nitrogen deposition over China. Nature 494 , 459–462 (2013).
Article ADS PubMed CAS Google Scholar
Shen, J. L. et al. Atmospheric dry and wet nitrogen deposition on three contrasting land use types of an agricultural catchment in subtropical central China. Atmos. Environ. 67 (1), 415–424 (2013).
Article ADS CAS Google Scholar
Yi, J. et al. Uptake and utilization of different nitrogen forms in erect panicle japonica rice cultivar. J. Plant Interact. 14 (1), 397–406 (2019).
Li, H., Hu, B. & Chu, C. Nitrogen use efficiency in crops: Lessons from Arabidopsis and rice. J. Exp. Bot. 68 , 2477–2488 (2017).
Article PubMed CAS Google Scholar
Zhou, W. et al. Effects of postponing nitrogen applications on leaf senescence of indica hybrid rice. Res. Crops 14 (2), 357–366 (2013).
Google Scholar
Zhang, A. P., Liu, R. L., Gao, J., Yang, S. Q. & Chen, Z. Regulating N application for rice yield and sustainable eco-agro development in the upper reaches of Yellow River rasin. China. Sci. World J. 1 , 1–11 (2014).
CAS Google Scholar
Mi, G. et al. Ideotype root architecture for efficient nitrogen acquisition by maize in intensive cropping systems. China-Life Sci. 53 , 1369–1373 (2010).
Ju, C. et al. Root and shoot traits for rice varieties with higher grain yield and higher nitrogen use efficiency at lower nitrogen rates application. Field Crops Res. 175 , 47–55 (2015).
Xin, W. et al. An integrated analysis of the rice transcriptome and metabolome reveals root growth regulation mechanisms in response to nitrogen availability. Int. J. Mol. Sci. 20 , 5893 (2019).
Article PubMed PubMed Central CAS Google Scholar
Zhang, H., Xue, Y., Wang, Z., Yang, J. & Zhang, J. Morphological and physiological traits of roots and their relationships with shoot growth in ‘super’ rice. Field Crops Res. 113 , 31–40 (2009).
Uga, Y. et al. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 45 , 1097–1102 (2013).
Sun, L., Lu, Y., Yu, F., Kronzucker, H. J. & Shi, W. Biological nitrification inhibition by rice root exudates and its relationship with nitrogen-use efficiency. New Phytol. 212 , 646–656 (2016).
Gu, J. et al. Roles of nitrogen and cytokinin signals in root and shoot communications in maximizing of plant productivity and their agronomic applications. Plant Sci. 274 , 320–331 (2018).
Dobermann, A. et al. Site-specific nutrient management for intensive rice cropping systems in Asia. Field Crops Res. 74 , 37–66 (2002).
Ju, X. T. et al. Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc. Natl. Acad. Sci. USA 106 (9), 3041–3046 (2009).
Article ADS PubMed PubMed Central CAS Google Scholar
Kamiji, Y., Yoshida, H., Palta, J. A., Sakuratani, T. & Shiraiwa, T. N applications that increase plant N during panicle development are highly effective in increasing spikelet number in rice. Field Crops Res. 122 (3), 242–247 (2011).
Li, M. et al. Accumulation and utilization of nitrogen, phosphorus and potassium of irrigated rice cultivars with high productivities and high N use efficiencies. Field Crops Res. 161 (1), 55–63 (2014).
Sun, H. Y. et al. Heterotrimeric G proteins regulate nitrogen-use efficiency in rice. Nat. Genet. 46 (2), 652–656 (2014).
Cernaro, V., Donato, V., Romeo, A., Lacquaniti, A. & Buemi, M. Regeneration and the plant we have inside. J. Mol. Genet. Med. Int. J. Biomed. Res. 8 (137), 1 (2014).
Fahad, S., Hussain, S., Matloob, A., Khan, F. A. & Huang, J. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regul. 75 (2), 391–404 (2014).
Macgowan, M. J. Leader strategies in managing maladaptive group behavior: An empirical report from school-based groups in a rct. Physiol. Mol. Biol. Plants 14 (1), 23–38 (2008).
Liu, Y., Ding, Y., Wang, Q., Meng, D. & Wang, S. Effects of nitrogen and 6-benzylaminopurine on rice tiller bud growth and changes in endogenous hormones and nitrogen. Crop Sci. 51 , 786–792 (2011).
Yu, N., Zhang, J., Liu, P., Zhao, B. & Ren, B. Integrated agronomic practices management improved grain formation and regulated endogenous hormone balance in summer maize ( Zea May L.). J. Integr. Agric. 19 (7), 1768–1776 (2020).
Qian, D. et al. Effect of nitrogen application methods on crop yield and grain filling characteristics of maize in maize-soybean relay strip intercropping system. Acta Agron. Sin. 40 (11), 2028 (2014).
Wang, D. Y. et al. 15 N tracer-based analysis of genotypic differences in the uptake and partitioning of N applied at different growth stages in transplanted rice. Field Crop Res. 211 , 27–36 (2017).
Ju, X. T., Liu, X. J., Zhang, F. S., & Roelcke, M. Nitrogen fertilization, soil nitrate accumulation, and policy recommendations in several agricultural regions of China. AMBIO J. Hum. Environ. 33 (6), 300–305 (2004).
Bhattacharyya, P. et al. Effects of rice straw and nitrogen fertilization on greenhouse gas emissions and carbon storage in tropical flooded soil planted with rice. Soil Tillage Res. 124 (4), 119–130 (2012).
Sui, B. et al. Optimizing nitrogen supply increases rice yield and nitrogen use efficiency by regulating yield formation factors. Field Crops Res. 150 (1), 99–107 (2013).
Jiang, L. G. et al. Characterizing physiological N-use efficiency as influenced by nitrogen management in three rice cultivars. Field Crops Res. 88 (2–3), 239–250 (2004).
Zhao, L. M. et al. Effects of irrigation methods and rice planting densities on yield and photosynthetic characteristics of matter production in cold area. Trans CSAE 31 (06), 159–169 (2015).
Peng, S. B. et al. Research strategy in improving fertilizer-nitrogen use efficiency of irrigated rice in China. Sci. Agric. Sin 09 , 1095–1103 (2002).
Lin, J. J. et al. Subdivision of nitrogen use efficiency of rice based on 15 N tracer. Acta Agron Sin. 40 (08), 1424–1434 (2014).
Wang, J. et al. Optimizing N management to balance rice yield and environmental risk in the Yangtze River’s middle reaches. Environ. Sci. Pollut. Res. Int. 26 (5), 4901–4912 (2018).
Article PubMed Google Scholar
Li, G., Zhang, H., Li, J., Zhang, Z. & Li, Z. Genetic control of panicle architecture in rice. Crop J. 9 (3), 590–597 (2021).
Liu, K. et al. Spikelet differentiation and degeneration in rice varieties with different panicle sizes. Food Energy Secur. 11 , e320 (2022).
Article ADS Google Scholar
Yoshida, H., Horle, T. & Shiraiwa, T. A model explaining genotypic and environmental variation of rice spikelet number per unit area measured by cross-locational experiments in Asia. Field Crops Res. 97 (2–3), 337–343 (2006).
Ding, Y. F. & Maruyama, S. Proteins and carbohydrates in developing rice panicles with different numbers of spikelets. Plant Prod. Sci. 7 (1), 16–21 (2004).
Zhang, H. et al. Hormones in the grains and roots in relation to post-anthesis development of inferior and superior spikelets in japonica/indica hybrid rice. Plant Physiol. Biochem. 47 (3), 195–204 (2009).
Zhang, H., Tan, G., Wang, Z., Yang, J. & Zhang, J. Ethylene and ACC levels in developing grains are related to the poor appearance and milling quality of rice. Plant Growth Regul. 58 (1), 85–96 (2009).
Barazesh, S. & McSteen, P. Hormonal control of grass infl orescence development. Trends Plant Sci. 13 , 656–662 (2008).
Pagnussat, G. C., Alandete-Saez, M., Bowman, J. L. & Sundaresan, V. Auxin-dependent patterning and gamete specifi cation in the Arabidopsis female gametophyte. Science 324 , 1684–1689 (2009).
Sundberg, E. & Østergaard, L. Distinct and dynamic auxin activities during reproducti ve development. Cold Spring Harb. Perspect. Biol. 1 , a001628 (2009).
Article PubMed PubMed Central Google Scholar
Mcsteen, P. Auxin and monocot development. Cold Spring Harb. Perspect. Biol. 2 , a001479 (2010).
Kato, T. Effect of spikelet removal on the grain filling of Akenohoshi, a rice cultivar with numerous spikelets in a panicle. J. Agric. Sci. 142 , 177–181 (2004).
Peng, S. et al. Strategies for overcoming low agronomic nitrogen use efficiency in irrigated rice systems in China. Field Crops Res. 96 , 37–47 (2006).
Dong, M. et al. Comparative proteomics analysis of superior and inferior spikelets in hybrid rice during grain filling and response of inferior spikelets to drought stress using isobaric tags for relative and absolute quantification. J. Proteom. 1 , 51–54 (2014).
Kiba, T., Kudo, T., Kojima, M. & Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 62 , 1399–1409 (2011).
Kurakawa, T. et al. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 445 , 652–655 (2007).
Download references
This work was supported by grants from the Science and Technology Development Plan Project of Jilin Province, China (20230202031NC) and the Open Project of the Key Laboratory of Germplasm Innovation and Physiological Ecology of Cold and Grain Crops, Ministry of Education (CXSTOP202202).
Author information
Authors and affiliations.
Agronomy College Jilin Agricultural University, Changchun, 130118, China
Yue Zou, Yuchen Zhang, Jiehao Cui, Jiacong Gao, Liying Guo & Qiang Zhang
Key Laboratory of Germplasm Innovation and Physiological Ecology of Grain Crops in Cold Region, Ministry of Education, Harbin, 150030, China
Yue Zou & Qiang Zhang
You can also search for this author in PubMed Google Scholar
Contributions
Y.Z. and L.G. designed the study and provided experimental materials. Y.Z. and J.C. performed the measurement of architecture and physiological characteristics. J.G. and Q.Z. prepared the figures and tables. Y.Z. and L.G. wrote the paper.
Corresponding authors
Correspondence to Liying Guo or Qiang Zhang .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Zou, Y., Zhang, Y., Cui, J. et al. Nitrogen fertilization application strategies improve yield of the rice cultivars with different yield types by regulating phytohormones. Sci Rep 13 , 21803 (2023). https://doi.org/10.1038/s41598-023-48491-w
Download citation
Received : 11 July 2023
Accepted : 27 November 2023
Published : 09 December 2023
DOI : https://doi.org/10.1038/s41598-023-48491-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

NASA Discovers a Long-Sought Global Electric Field on Earth

- A rocket team reports the first successful detection of Earth’s ambipolar electric field: a weak, planet-wide electric field as fundamental as Earth’s gravity and magnetic fields.
- First hypothesized more than 60 years ago, the ambipolar electric field is a key driver of the “polar wind,” a steady outflow of charged particles into space that occurs above Earth’s poles.
- This electric field lifts charged particles in our upper atmosphere to greater heights than they would otherwise reach and may have shaped our planet’s evolution in ways yet to be explored.
Using observations from a NASA suborbital rocket, an international team of scientists has, for the first time, successfully measured a planet-wide electric field thought to be as fundamental to Earth as its gravity and magnetic fields. Known as the ambipolar electric field, scientists first hypothesized over 60 years ago that it drove how our planet’s atmosphere can escape above Earth’s North and South Poles. Measurements from the rocket, NASA’s Endurance mission , have confirmed the existence of the ambipolar field and quantified its strength, revealing its role in driving atmospheric escape and shaping our ionosphere — a layer of the upper atmosphere — more broadly.
Understanding the complex movements and evolution of our planet’s atmosphere provides clues not only to the history of Earth but also gives us insight into the mysteries of other planets and determining which ones might be hospitable to life. The paper was published Wednesday, Aug. 28, 2024, in the journal Nature .
An Electric Field Drawing Particles Out to Space
Since the late 1960s, spacecraft flying over Earth’s poles have detected a stream of particles flowing from our atmosphere into space. Theorists predicted this outflow, which they dubbed the “polar wind,” spurring research to understand its causes.
Some amount of outflow from our atmosphere was expected. Intense, unfiltered sunlight should cause some particles from our air to escape into space, like steam evaporating from a pot of water. But the observed polar wind was more mysterious. Many particles within it were cold, with no signs they had been heated — yet they were traveling at supersonic speeds.
“Something had to be drawing these particles out of the atmosphere,” said Glyn Collinson, principal investigator of Endurance at NASA’s Goddard Space Flight Center in Greenbelt, Maryland, and lead author of the paper. Scientists suspected a yet-to-be-discovered electric field could be at work.
The hypothesized electric field, generated at the subatomic scale, was expected to be incredibly weak, with its effects felt only over hundreds of miles. For decades, detecting it was beyond the limits of existing technology. In 2016, Collinson and his team got to work inventing a new instrument they thought was up to the task of measuring Earth’s ambipolar field.
How the Ambipolar Field Works
A weak electric field in the upper atmosphere may loft charged particles into space..
Scientists theorized this electric field should begin at around 150 miles (250 kilometers) altitude, where atoms in our atmosphere break apart into negatively charged electrons and positively charged ions. Electrons are incredibly light — the slightest kick of energy could send them shooting out to space. Ions are at least 1,836 times heavier and tend to sink toward the ground. If gravity alone were in play, the two populations, once separated, would drift apart over time. But given their opposite electric charges, an electric field forms to tether them together, preventing any separation of charges and counteracting some of the effects of gravity.
This electric field is bidirectional, or “ambipolar,” because it works in both directions. Ions pull the electrons down with them as they sink with gravity. At the same time, electrons lift ions to greater heights as they attempt to escape to space, like a tiny dog tugging on its sluggish owner’s leash. The net effect of the ambipolar field is to extend the height of the atmosphere, lifting some ions high enough to escape with the polar wind. Animation credits: NASA/Conceptual Image Lab/Wes Buchanan/Krystofer Kim
Launching a Rocket from the Arctic
The team’s instruments and ideas were best suited for a suborbital rocket flight launched from the Arctic. In a nod to the ship that carried Ernest Shackleton on his famous 1914 voyage to Antarctica, the team named their mission Endurance. The scientists set a course for Svalbard, a Norwegian archipelago just a few hundred miles from the North Pole and home to the northernmost rocket range in the world.
“Svalbard is the only rocket range in the world where you can fly through the polar wind and make the measurements we needed,” said Suzie Imber, a space physicist at the University of Leicester, UK, and co-author of the paper.
On May 11, 2022, Endurance launched and reached an altitude of 477.23 miles (768.03 kilometers), splashing down 19 minutes later in the Greenland Sea. Across the 322-mile altitude range where it collected data, Endurance measured a change in electric potential of only 0.55 volts.
“A half a volt is almost nothing — it’s only about as strong as a watch battery,” Collinson said. “But that’s just the right amount to explain the polar wind.”

Hydrogen ions, the most abundant type of particle in the polar wind, experience an outward force from this field 10.6 times stronger than gravity. “That’s more than enough to counter gravity — in fact, it’s enough to launch them upwards into space at supersonic speeds,” said Alex Glocer, Endurance project scientist at NASA Goddard and co-author of the paper.
Heavier particles also get a boost. Oxygen ions at that same altitude, immersed in this half-a-volt field, weigh half as much. In general, the team found that the ambipolar field increases what’s known as the “scale height” of the ionosphere by 271%, meaning the ionosphere remains denser to greater heights than it would be without it.
“It’s like this conveyor belt, lifting the atmosphere up into space,” Collinson added.
Endurance’s discovery has opened many new paths for exploration. The ambipolar field, as a fundamental energy field of our planet alongside gravity and magnetism, may have continuously shaped the evolution of our atmosphere in ways we can now begin to explore. Because it’s created by the internal dynamics of an atmosphere, similar electric fields are expected to exist on other planets, including Venus and Mars.
“Any planet with an atmosphere should have an ambipolar field,” Collinson said. “Now that we’ve finally measured it, we can begin learning how it’s shaped our planet as well as others over time.”
By Miles Hatfield and Rachel Lense NASA’s Goddard Space Flight Center, Greenbelt, Md. Media Contact: Sarah Frazier, [email protected]
Endurance was a NASA-funded mission conducted through the Sounding Rocket Program at NASA’s Wallops Flight Facility in Virginia. The Svalbard Rocket Range is owned and operated by Andøya Space. The European Incoherent Scatter Scientific Association (EISCAT) Svalbard radar, located in Longyearbyen, made ground-based measurements of the ionosphere critical to interpreting the rocket data. The United Kingdom Natural Environment Research Council (NERC) and the Research Council of Norway (RCN) funded the EISCAT radar for the Endurance mission. EISCAT is owned and operated by research institutes and research councils of Norway, Sweden, Finland, Japan, China, and the United Kingdom (the EISCAT Associates). The Endurance mission team encompasses affiliates of the Catholic University of America, Embry-Riddle Aeronautical University, the University of California, Berkeley, the University of Colorado at Boulder, the University of Leicester, U.K., the University of New Hampshire, and Penn State University.
Related Terms
- Goddard Space Flight Center
- Heliophysics
- Heliophysics Division
- Science & Research
- Sounding Rockets
- Sounding Rockets Program
What are nitrogen oxides?
What happens to nitrogen oxides when they enter the environment, how might i be exposed to nitrogen oxides, how can nitrogen oxides affect my health, how likely are nitrogen oxides to cause cancer, how does nitrogen oxides affect children, how can families reduce the risk of exposure to nitrogen oxides, is there a medical test to show whether i've been exposed to nitrogen oxides, has the federal government made recommendations to protect human health, where can i get more information, toxfaqs™ for nitrogen oxides.
Spanish: Óxidos de Nitrógeno
CAS#: 10102-43-9 (nitric oxide); #10102-44-0 (nitrogen dioxide)
This fact sheet answers the most frequently asked health questions about nitrogen oxides. For more information, call the ATSDR Information Center at 1-800-232-4636. This fact sheet is one in a series of summaries about hazardous substances and their health effects. It is important you understand this information because this substance may harm you. The effects of exposure to any hazardous substance depend on the dose, the duration, how you are exposed, personal traits and habits, and whether other chemicals are present.
Everybody is exposed to small amounts of nitrogen oxides in ambient air. Higher exposure may occur by burning wood or kerosene or near gas stoves or if you smoke. Exposure to high levels of nitrogen oxides can damage the respiratory airways. Contact with the skin or eyes can cause burns. Nitrogen dioxide and nitric oxide have been found in at least 9 and 6 of the 1,585 National Priorities List sites identified by the Environmental Protection Agency (EPA), respectively.
Nitrogen oxides are a mixture of gases that are composed of nitrogen and oxygen. Two of the most toxicologically significant nitrogen oxides are nitric oxide and nitrogen dioxide; both are nonflammable and colorless to brown at room temperature. Nitric oxide is a sharp sweet-smelling gas at room temperature, whereas nitrogen dioxide has a strong, harsh odor and is a liquid at room temperature, becoming a reddish-brown gas above 70°F.
Nitrogen oxides are released to the air from the exhaust of motor vehicles, the burning of coal, oil, or natural gas, and during processes such as arc welding, electroplating, engraving, and dynamite blasting. They are also produced commercially by reacting nitric acid with metals or cellulose.
Nitrogen oxides are used in the production of nitric acid, lacquers, dyes, and other chemicals. Nitrogen oxides are also used in rocket fuels, nitration of organic chemicals, and the manufacture of explosives.
- Nitrogen oxides are broken down rapidly in the atmosphere by reacting with other substances commonly found in the air. The reaction of nitrogen dioxide with chemicals produced by sunlight leads to the formation of nitric acid, which is a major constituent of acid rain. Nitrogen dioxide also reacts with sunlight, which leads to the formation of ozone and smog conditions in the air we breathe.
- Small amounts of nitrogen oxides may evaporate from water, but most of it will react with water and form nitric acid.
- When released to soil, small amounts of nitrogen oxides may evaporate into air. However, most of it will be converted to nitric acid or other compounds.
- Nitrogen oxides do not build up in the food chain.
- The general population is primarily exposed to nitrogen oxides by breathing in air. People who live near combustion sources such as coal burning power plants or areas with heavy motor vehicle use may be exposed to higher levels of nitrogen oxides.
- Households that burn a lot of wood or use kerosene heaters and gas stoves tend to have higher levels of nitrogen oxides in them when compared to houses without these appliances.
- Nitric oxide and nitrogen dioxide are found in tobacco smoke, so people who smoke or breathe in second-hand smoke may be exposed to nitrogen oxides.
- Workers employed in facilities that produce nitric acid or certain explosives like dynamite and trinitrotoluene (TNT), as well as workers involved in the welding of metals may breath in nitrogen oxides during their work.
Low levels of nitrogen oxides in the air can irritate your eyes, nose, throat, and lungs, possibly causing you to cough and experience shortness of breath, tiredness, and nausea. Exposure to low levels can also result in fluid build-up in the lungs 1 or 2 days after exposure. Breathing high levels of nitrogen oxides can cause rapid burning, spasms, and swelling of tissues in the throat and upper respiratory tract, reduced oxygenation of body tissues, a build-up of fluid in your lungs, and death.
If you were to come into skin or eye contact with high concentrations of nitrogen oxide gases or nitrogen dioxide liquid, you would likely experience serious burns.
We do not know if exposure to nitrogen oxides will result in reproductive effects in humans.
The Department of Health and Human Services (DHHS), the International Agency for Research on Cancer (IARC), and the EPA have not classified nitrogen oxides for potential carcinogenicity.
Children would probably be affected by exposure to nitrogen oxides in the same ways as adults. But we do not know whether children differ from adults in their susceptibility to nitrogen oxides.
Exposure of pregnant animals to nitrogen oxides has resulted in toxic effects in developing fetuses. Nitrogen oxides have also caused changes in the genetic material of animal cells. But we do not know if exposure to nitrogen oxides might cause developmental effects in humans.
Families with indoor gas stoves, space heaters, or indoor cigarette smoke can minimize indoor exposure to nitrogen oxides by periodically allowing fresh outdoor air into the home. Farm families should not allow children to play near silos that contain silage.
Specific tests for the presence of nitrogen oxides in blood or urine are not generally useful to the doctor. If a severe exposure has occurred, blood and urine analyses and other tests may show whether damage has been done to your respiratory airways. Some of these tests may be done at the doctor's office, others may require a clinic or hospital that have specialized equipment.
The EPA has established that the average concentration of nitrogen dioxide in ambient air in a calendar year should not exceed 0.053 parts of nitrogen dioxide per million parts of air (0.053 ppm).
The Occupational Safety and Health Administration (OSHA) has set a limit of 25 ppm of nitric oxide in workplace air during an 8-hour workday, 40-hour work week. OSHA has also set a 15-minute exposure limit of 5 ppm for nitrogen dioxide in workplace air.
Agency for Toxic Substances and Disease Registry (ATSDR). 2002. Managing Hazardous Materials Incidents. Volume III - Medical Management Guidelines for Acute Chemical Exposures: Nitrogen Oxides . Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service.
If you have questions or concerns, please contact your community or state health or environmental quality department or:
For more information, contact: Agency for Toxic Substances and Disease Registry Office of Innovation and Analytics, Toxicology Section 4770 Buford Highway Chamblee, GA 30341-3717 Phone: 1-800-CDC-INFO 888-232-6348 (TTY) Email: Contact CDC-INFO
ATSDR can also tell you the location of occupational and environmental health clinics. These clinics specialize in recognizing, evaluating, and treating illnesses resulting from exposure to hazardous substances.

COMMENTS
This may, at least in part, explain the great interest in as well as difficulties associated with environmental microbiology research on the nitrogen cycle, which has encouraged and motivated many scientists to study it and resulted in a greater abundance of research articles and reviews on the nitrogen cycle and metabolism than on energy ...
Plant nitrogen source in the soil is challenging to track. Compiling the most comprehensive global δ15N dataset, a new study shows the plant use of various available soil nitrogen forms (ammonium ...
Nitrogen-cycle research in the Stein lab is supported by the Natural Sciences and Engineering Research Council of Canada. Nitrogen-cycle research in the Klotz lab has been supported during the last two decades by the NSF, DOE, USDA, the Gordon and Betty Moore Foundation and institutional incentive funds from the University of Louisville and UNC ...
The process of converting N 2 into biologically available nitrogen is called nitrogen fixation. N 2 gas is a very stable compound due to the strength of the triple bond between the nitrogen atoms ...
Biological nitrogen fixation, which reduces atmospheric dinitrogen gas (N 2) into reactive ammonia (NH3), is central in the nitrogen biogeochemical cycle as the only path to incorporate the abundant dinitrogen gas into biomass.This process represents a main driver of fertilization for aquatic and terrestrial systems and is continuously studied to increase crop yields in agriculture ().
Nitrogen is an abundant element on Earth; it makes up 78.1% of Earth's atmosphere and is an essential nutrient for all forms of life. Much of this nitrogen is in the form of unreactive nitrogen (N 2) gas and is not available for use by most living organisms.But a portion of it, fixed by natural or anthropogenic processes, is in a reactive form [Nr, which includes nitrogen oxides (NO x ...
Nitrogen is one of the most important nutrient for plant growth and development; it is strongly associated with a variety of abiotic stress responses. As sessile organisms, plants have evolved to develop efficient strategies to manage N to support growth when exposed to a diverse range of stressors. This review summarizes the recent progress in the field of plant nitrate (NO3-) and ammonium ...
Abstract. Humans continue to transform the global nitrogen cycle at a record pace, reflecting an increased combustion of fossil fuels, growing demand for nitrogen in agriculture and industry, and pervasive inefficiencies in its use. Much anthropogenic nitrogen is lost to air, water, and land to cause a cascade of environmental and human health ...
Nitrogen (N) is used in many of life's fundamental biomolecules, and it is also a participant in environmental redox chemistry. Biogeochemical processes control the amount and form of N available to organisms ("fixed" N). These interacting processes result in N acting as the proximate limiting nutrient in most surface environments. Here, we review the global biogeochemical cycle of N and ...
The work was published in Science on 11 April 1. New cellular 'organelle' discovered inside fruit-fly intestines. "The textbooks say nitrogen fixation only occurs in bacteria and archaea ...
BNF by legumes and associative, endosymbiotic, and endophytic nitrogen fixation in non-legumes play major roles in reducing the use of synthetic nitrogen fertilizer in agriculture, increased plant nutrient content, and soil health reclamation. This review discusses the process of nitrogen-fixation in plants, nodule formation, the genes involved ...
Nitrogen is the main limiting nutrient after carbon, hydrogen and oxygen for photosynthetic process, phyto-hormonal, proteomic changes and growth-development of plants to complete its lifecycle. Excessive and inefficient use of N fertilizer results in enhanced crop production costs and atmospheric pollution. Atmospheric nitrogen (71%) in the molecular form is not available for the plants. For ...
Impacts on forest growth. Forest growth generally still increases at N input levels where adverse impacts on plant species diversity and soil quality already occur (Figure 1).At higher N deposition levels, however, above 10-15 kg N ha −1 yr −1, N leaching starts to increase as the forest approaches 'nitrogen saturation' [36], associated with soil acidification and elevated leaching ...
Introduction. Nitrogen (N) fuels life while creating a multitude of environmental threats. It is both an essential input for food production and a key contributor to issues ranging from climate change, stratospheric ozone depletion and biodiversity loss, to air and water pollution [1].Scientific research into N - its properties, dynamics and impacts - is at a watershed moment: the policy ...
Global view. Adequate crop yields to feed 7.8 billion people today, and nearly 10 billion by mid-century (Vollset et al. 2020), depend on large amounts of nitrogen (N) input, which in turn represent an inherent weakness in our global food production system. The challenge has nothing to do with N supply as that is virtually unlimited.
Biological nitrogen (N 2) fixation, the reduction of abundant inert atmospheric N 2 gas to ammonia, is a key metabolic innovation that maintains the fertility of terrestrial and aquatic environments. N 2 fixation in eukaryotes is only known to exist through diverse symbioses with Bacteria or Archaea, the microbes capable of N 2 fixation (5).
Nitrogen is an essential element for plant growth and development and is required for the formation of chlorophyll, amino acids, and nucleic acids. 34 Plants obtain nitrogen primarily from ...
The Kjeldahl method uses sulfuric acid, a variety of catalysts, and salts to convert organically bound nitrogen in samples to ammonium with its subsequent measurement (Sáez-Plaza et al., 2013).
Sui, B. et al. Optimizing nitrogen supply increases rice yield and nitrogen use efficiency by regulating yield formation factors. Field Crops Res. 150 (1), 99-107 (2013). Article Google Scholar
The nitrogen contamination management strategies for surface water in China should be targeted by region and period specifically. (2) The health risk assessment of nitrogen across China showed that there were 1%, 1%, 12%, and 46% probability exceeding the unacceptable risk level (HI>1) for children in the SL, PR, HR*, and YR, respectively.
Research Article. Effect of feeding time and time of harvest of fresh pasture on urinary and faecal nitrogen excretion patterns in sheep. ... This low nitrogen utilisation efficiency leads to higher urinary nitrogen excretion (Beltran et al. Citation 2019). In the present study, feed harvested in the afternoon had a higher dry matter and higher ...
An international team of scientists has successfully measured a planet-wide electric field thought to be as fundamental to Earth as its gravity and magnetic fields. Known as the ambipolar electric field, scientists first hypothesized over 60 years ago that it drove atmospheric escape above Earth's North and South Poles. Measurements from a suborbital rocket have confirmed the existence of ...
Everybody is exposed to small amounts of nitrogen oxides in ambient air. Higher exposure may occur by burning wood or kerosene or near gas stoves or if you smoke. Exposure to high levels of nitrogen oxides can damage the respiratory airways. Contact with the skin or eyes can cause burns. Nitrogen dioxide and nitric oxide have been found in at least 9 and 6 of the 1,585 National Priorities List ...
Gas and propane stoves emit nitrogen dioxide (NO 2) pollution indoors, but the exposures of different U.S. demographic groups are unknown.We estimate NO 2 exposure and health consequences using emissions and concentration measurements from >100 homes, a room-specific indoor air quality model, epidemiological risk parameters, and statistical sampling of housing characteristics and occupant ...