- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

U.S. Food and Drug Administration
- Search
- Menu
- News & Events for Human Drugs
FDA approves first treatment for Fibrodysplasia Ossificans Progressiva
The U.S. Food and Drug Administration (FDA) has approved Sohonos (palovarotene) capsules for reduction in the volume of new heterotopic ossification (extra-skeletal bone formation) in adults and children aged 8 years and older for females, and 10 years and older for males with fibrodysplasia ossificans progressiva. Sohonos is the first drug approved for patients with fibrodysplasia ossificans progressiva.
Adults and pediatric patients 14 years and older should take 5 mg of Sohonos once daily, with an increase in dose at the time of a flare-up to 20 mg once daily for 4 weeks, followed by 10 mg once daily for 8 weeks for a total of 12 weeks (20/10 mg flare-up treatment). Patients under 14 years, based on their body weight, should take from 2.5 to 5 mg of Sohonos.
Disease or Condition
Fibrodysplasia ossificans progressiva is a rare, autosomal dominant disease where connective tissue such as muscle, tendons and ligaments gradually turn into bone tissue, causing limited movement, deformities and severe disability.
Effectiveness
The safety and effectiveness of Sohonos was evaluated in clinical studies that enrolled a total of 164 subjects with fibrodysplasia ossificans progressiva, including 139 subjects in the indicated population of females, aged 8 years and above and males, aged 10 years and above (8/10 years and older). Most of the subjects received open label treatment with the chronic daily/flare-up regimen, consisting of 5 mg daily dosage of oral Sohonos with a 20/10 mg dosage as needed for 12 weeks at the time of flare-up (4 weeks of 20 mg once daily followed by 10 mg once daily for 8 weeks), with all doses reduced by weight in subjects who were less than 90% skeletally mature. The mean age of these subjects was 19 years (range 8 to 61 years); 51% were male.
Safety Information
Sohonos contains a boxed warning for embryo-fetal toxicity and premature epiphyseal closure (early closure of bone growth) in growing pediatric patients. Healthcare providers should verify that people who can become pregnant are not pregnant prior to beginning treatment and periodically during therapy. Monitoring linear growth in growing pediatric patients is also recommended. Before taking Sohonos, all growing pediatric patients should undergo skeletal maturity baseline assessments. Continued monitoring is recommended every 6 to 12 months until patients reach skeletal maturity or final adult height.
Sohonos comes with warnings and precautions: Sohonos is associated with dry skin, lip dry, pruritis, rash, alopecia, erythema, skin exfoliation, and dry eye; therefore prevention or treatment with skin emollients, sunscreen, artificial tears and dosage reduction may be required in some patients. Sohonos is associated with metabolic bone disorders, and decreased vertebral bone mineral content and bone density may occur; therefore, spinal fracture should be assessed periodically using radiologic methods. Sohonos is associated with depression, anxiety, mood alterations and suicidal thoughts and behaviors; therefore, patients should contact their healthcare provider if new or worsening symptoms develop. Sohonos is also associated with night-blindness and can make driving at night hazardous.
The most common adverse reactions include dry skin, lip dry, arthralgia, pruritis, pain in extremity, rash, alopecia, erythema, headache, back pain, skin exfoliation, nausea, musculoskeletal pain, myalgia, dry eye, hypersensitivity, peripheral edema, and fatigue. See the full prescribing information for all risks associated with Sohonos.
Designations
Sohonos received a priority review , fast track designation and breakthrough therapy designation for this indication.
FDA granted this approval to Ipsen Biopharmaceuticals, Inc.
PERSPECTIVE article
Fibrodysplasia ossificans progressiva: what have we achieved and where are we now follow-up to the 2015 lorentz workshop.

- 1 Department of Internal Medicine, Section Endocrinology, Amsterdam University Medical Center (Amsterdam UMC), Vrije Universiteit Amsterdam, Amsterdam Movement Sciences, Amsterdam, Netherlands
- 2 Department of Clinical Genetics and Bone Histomorphology, Amsterdam University Medical Center (Amsterdam UMC), Vrije Universiteit Amsterdam, Amsterdam, Netherlands
- 3 Department of Clinical Chemistry, Amsterdam University Medical Center (Amsterdam UMC), Vrije Universiteit Amsterdam, Amsterdam Movement Sciences, Amsterdam, Netherlands
- 4 Freie Universität Berlin, Institute for Chemistry and Biochemistry, Berlin, Germany
- 5 Department of Periodontology, Academic Centre for Dentistry Amsterdam (ACTA), University of Amsterdam and Vrije Universiteit, Amsterdam, Netherlands
- 6 Department of Cell and Chemical Biology, Leiden University Medical Center, Leiden, Netherlands
- 7 Translational Research Program in Pediatric Orthopaedics, Abramson Research Center, Division of Orthopaedic Surgery, The Children’s Hospital of Philadelphia, Philadelphia, PA, United States
- 8 Department of Medicine, Mayo Clinic, Rochester, MN, United States
- 9 Department of Orthopaedic Surgery and Genetics, and the Center for Research in FOP and Related Disorders, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, United States
- 10 Department of Molecular Cell Biology and Immunology, Cancer Center Amsterdam, Amsterdam University Medical Center (Amsterdam UMC), Vrije Universiteit Amsterdam, Amsterdam, Netherlands
- 11 Division of Biomechanics, Department of Mechanical Engineering, Katholieke Universiteit (KU) Leuven, Leuven, Belgium
- 12 Prometheus division of skeletal tissue engineering, Katholieke Universiteit (KU) Leuven, Leuven, Belgium
- 13 Department of Orthopaedic Surgery and Medicine, Center for Research in FOP and Related Disorders, The Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, United States
- 14 Department of Endocrinology and Metabolism, and the Institute for Human Genetics, Department of Medicine, University of California, San Francisco, CA, United States
- 15 Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States
- 16 Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health (DINOGMI), Università degli Studi di Genova, Medical Genetics Unit, IRCCS Istituto Giannina Gaslini, Genova, Italy
- 17 Rheumatology Section, Department of Pediatrics, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina
- 18 Teaching and Research Institute of the Hospital Israelita Albert Einstein, Sao Paulo, Brazil
- 19 Berlin-Brandenburg Center for Regenerative Therapies, Charité Medical University of Berlin, Berlin, Germany
- 20 Laboratory for Myology, Faculty of Behavioural and Movement Sciences, Vrije Universiteit Amsterdam, Amsterdam Movement Sciences, Amsterdam, Netherlands
- 21 Centre for Metabolic Bone Disease, Royal National Orthopaedic Hospital, Stanmore, United Kingdom
- 22 Department of Physiology, Amsterdam University Medical Center (Amsterdam UMC), Vrije Universiteit Amsterdam, Amsterdam, Netherlands
- 23 Department of Pediatrics, Garmisch-Partenkichen Medical Center, Garmisch-Partenkirchen, Germany
- 24 Department of Dermatology, Amsterdam University Medical Center (AmsterdamUMC), Vrije Universiteit Amsterdam, Amsterdam, Netherlands
- 25 Division of Paediatric Rheumatology, Departmet of Paediatrics and Child Heath, Red Cross War Memorial Children’s Hospital, University of Cape Town, Cape Town, South Africa
- 26 Botnar Research Centre, University of Oxford, Oxford, United Kingdom
- 27 Departamento de Cièncias Fisiológicas, Facultad de Medicina y Ciencias de la Salud, Universitat de Barcelona, Barcelona, Spain
Fibrodysplasia ossificans progressiva (FOP) is an ultra-rare progressive genetic disease effecting one in a million individuals. During their life, patients with FOP progressively develop bone in the soft tissues resulting in increasing immobility and early death. A mutation in the ACVR1 gene was identified as the causative mutation of FOP in 2006. After this, the pathophysiology of FOP has been further elucidated through the efforts of research groups worldwide. In 2015, a workshop was held to gather these groups and discuss the new challenges in FOP research. Here we present an overview and update on these topics.
Introduction
Fibrodysplasia ossificans progressiva (FOP) is an ultra-rare progressive genetic disease characterized by heterotopic ossification (HO) of muscles, tendons and ligaments, often preceded by periodic painful soft tissue swellings called flare-ups. During their lives, patients develop a “second” skeleton, resulting in increasing immobility and early death often due to thoracic insufficiency, infectious diseases, and traumatic falls ( 1 ).
Progress of FOP research ( Figure 1 ) has been slow due to three main factors. Firstly, obtaining tissue samples to examine the pathophysiology is difficult. Biopsies are contraindicated because of the increased risk for flare-ups in FOP. Secondly, FOP is frequently misdiagnosed, and so systematic data on early pathophysiology has been difficult to obtain. Finally, for a long time there were no cell or animal models for FOP as the causative genetic mutation was unknown. In 2006, the genetic cause of FOP was identified to be a missense mutation (R206H) in the ACVR1 gene encoding the activin receptor-like kinase (ALK2) ( 2 ). The mutation induces hyperactivity of the ALK2 in response to bone morphogenetic protein (BMP) ligands as well as constitutive activity in the absence of ligands ( 3 , 4 ). Also, while activing A induces ALK4-mediated canonical SMAD 2/3 signaling, the mutated ALK2 causes activin A to induce SMAD 1/5/9 signaling too, resulting in a skeletogenic signal instead of the usual response to activin A ( 5 ).
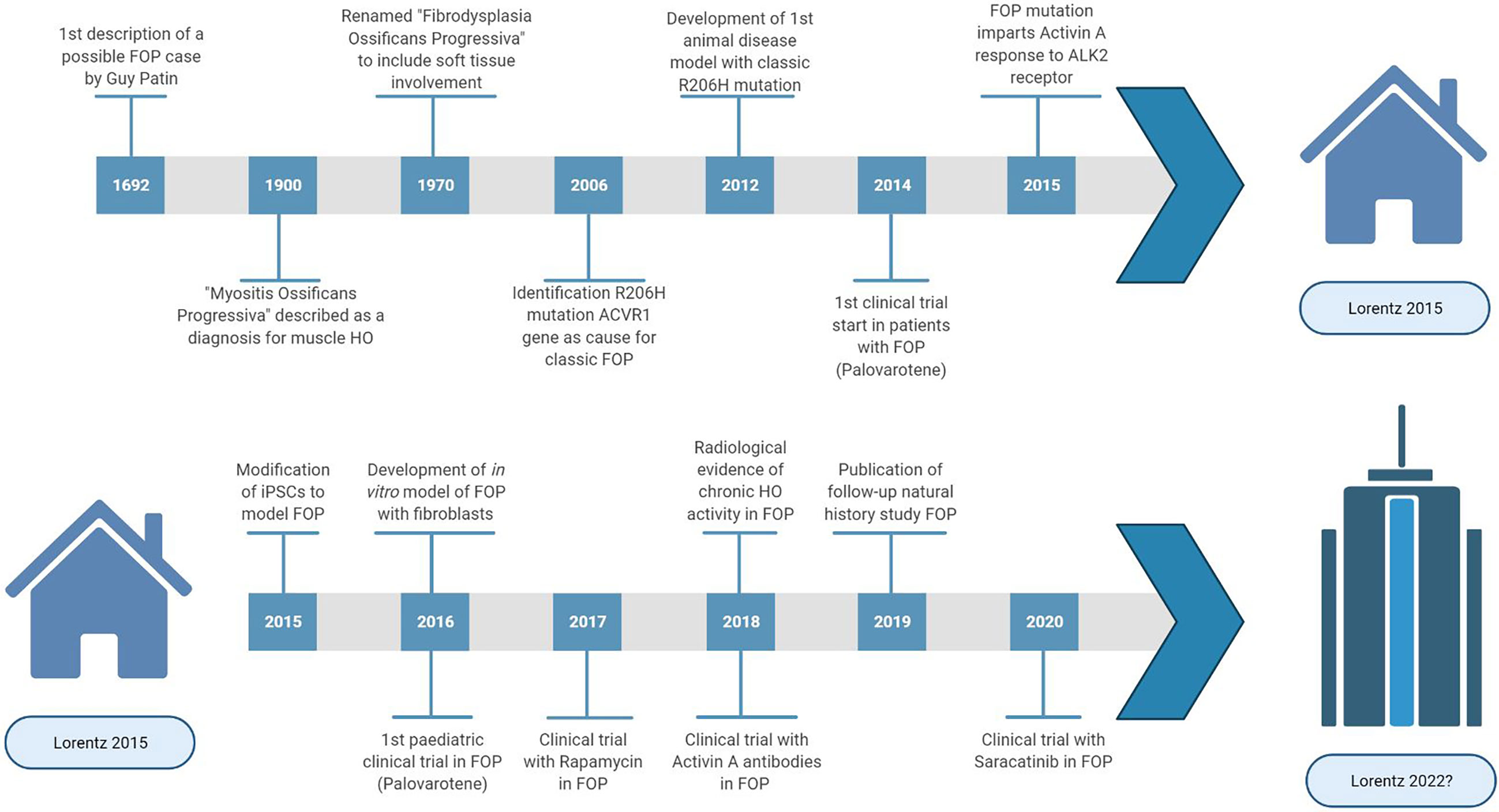
Figure 1 Highlights and key discoveries in FOP research leading up to the 2015 Lorentz meeting and after.
To date, there are no approved treatments to stop or reverse this disease, no biomarker to quantify FOP activity and many unanswered questions regarding pathophysiology.
In 2015, a Lorentz workshop was held, bringing international experts with a range of scientific backgrounds relevant to FOP research together for a week of scientific workshops discussing complex research problems and stimulating new initiatives for FOP treatment. Here we provide a comprehensive survey about the recent developments of basic and translational research on FOP.
Identification of Heterotopic Ossification Progenitor Cells
HO is a complex, multi-stage process involving various cell types ( 6 ), but the exact progenitor cells that form the heterotopic bone are yet to be identified. Multiple populations of progenitor cells associated with muscle tissue have demonstrated osteogenic differentiation. Muscle stem cells (MuSCs) are muscle-resident stem cells essential for muscle growth and regeneration ( 7 ) and were initially a leading candidate for the HO progenitor cell. However, in vivo lineage tracing studies have shown that these cells do not significantly contribute to BMP-induced HO ( 8 , 9 ), strongly arguing against MuSCs inducing HO in FOP ( 10 ).
Endothelial cells (EC) have also been proposed as a progenitor cell candidate. The endothelial marker Tie2 has been found in chondrocytes and osteoblasts in histological examination of HO tissues from individuals with FOP ( 11 ) and lineage tracing studies have identified Tie2 expression in roughly half the chondrocytes and osteocytes in heterotopic bone ( 11 ). However, Tie2 is not specific to ECs and more than 90% of the Tie2+ cells found in heterotopic bone are also platelet-derived growth factor receptor (PDGFR)α + Sca1 + indicating a mesenchymal rather than an endothelial origin ( 12 ).
In fact, these markers are also present in fibro/adipogenic progenitors (FAPs), another muscle tissue-resident progenitor. Cre/lox lineage tracing showed that FAPs can cause injury-induced and spontaneous HO in a FOP mouse model, greatly dependent on activin A signaling ( 13 ). Given the complexity of bone formation, perhaps cells from multiple origins are present and involved in ultimately forming the heterotopic bone.
Inflammatory Triggers of HO
The contribution of the immune system in FOP is a keen focus of research. HO lesions harbor many cells of the immune system, such as lymphocytes, macrophages and mast cells ( 14 , 15 ). Depletion of mast cells and macrophages have been reported to reduce HO volume in a FOP mouse model ( 16 ). The role of macrophages in HO has been investigated in different in vivo models with differing results ( 17 , 18 ), leading to the idea that macrophage populations in FOP lesions are more heterogeneous than often presumed and may be responding to different types of injury signals.
The ALK2 mutation is also present in other cell types. Thus, the mutated ALK2 likely also affects immune responses. ECSIT (Evolutionarily Conserved Signal Intermediate in the Toll pathway) has been reported as a possible mechanism linking toll-like receptor activation in the innate immune response to aberrant SMAD signaling in FOP ( 19 ).
Blood samples taken from patients with FOP without symptoms of a flare-up have shown significantly elevated levels of pro-inflammatory interleukins indicating that patients with FOP may be in a constant pro-inflammatory state. Monocytes derived from patients with FOP, when stimulated with lipopolysaccharide, showed prolonged and increased cytokine and chemokine secretion, and prolonged activation of nuclear factor (NF)-κB ( 20 , 21 ). A study of peripheral blood mononuclear cells from patients with FOP showed increased expression levels of DNAX accessory molecule-1 (DNAM-1) in monocytes, suggesting a functional effect in monocyte migration, and could represent a biomarker for the inflammatory state in FOP ( 22 ). Monocytes are also precursors for circulating osteogenic cells found in FOP lesions ( 23 ).
The hypoxic condition in inflamed tissues is another factor contributing to FOP pathogenesis, possibly through hypoxia inducible factor-1-α (HIF-1-α) which has been reported to promote amplification of BMP signaling through retention of the mutated ALK2 receptor in signaling endosomes ( 24 ). The fibroproliferative stage with extracellular matrix production that normally occurs after injury also appears to be overactive in FOP, leading to tissue stiffening and increased mechano-sensitivity in favor of osteogenic processes ( 25 ).
Vascularization in FOP
Angiogenesis is an important process involved in the development of FOP lesions. The inflammation, soft tissue destruction, and subsequent infiltration of immune cells all depend on vascularization. In the fibroproliferative phase the inflamed tissue is infiltrated by chondrocytes promoting a proteoglycan-enriched environment, which becomes progressively hypoxic. Hypoxic conditions favor chondrocyte differentiation partially by sustaining BMP signaling activation ( 24 ), and induce expression of vascular endothelial growth factor (VEGF), promoting the infiltration of blood vessels, which in turn drives endochondral bone formation. Interestingly, monocytes isolated from FOP patients showed increased VEGF secretion upon an inflammatory trigger compared to controls ( 18 ).
BMP and VEGF signaling play key roles in regulating blood vessel homeostasis; gene mutations in components of the BMP signaling pathway are associated with cardiovascular conditions ( 26 ), and disturbances in the angiogenesis-osteogenesis axis can cause bone disorders ( 27 ). Whether the mutant FOP ALK2 also disturbs EC function through aberrant BMP signaling is currently under investigation.
Angiogenesis is initiated by the formation of tip cells supported by proliferating stalk cells to forming new sprouts from pre-existing vessels. This process is coordinated by VEGF-, BMP2- and BMP6 signaling. During angiogenesis, BMP2 primarily signals through ALK3, whereas BMP6 signals through ALK2. Upon ALK2 knockdown, hypersprouting was observed in in vitro EC models, whereas ALK3 knockdown appeared to have the opposite effect ( 28 ). Recent data showed that EC’s derived from human induced pluripotent stem cells (hiPSC) follow the same principle and hiPSCs derived from patients with FOP show activin A induced SMAD 1/5 signaling ( 29 ).
Vascular leakage and edema have also been described in HO lesions in FOP ( 30 ). BMP6 stimulation in ECs causes internalization of VE-cadherin changing the endothelial architecture. VE-cadherin in turn appears to interact with ALK2 in a ligand-dependent manner by stabilizing the BMP receptors in the EC junctions ( 31 ). ECs from patients with FOP appear to have decreased expression of vascular endothelial (VE)-cadherin under inflammatory conditions ( 32 ), possibly due to an altered interaction of VE-cadherin signaling with the mutated ALK2 receptor complex.
Suitability of FOP Disease Models
Since the discovery of the mutation ( 2 ), several cellular and animal models have been developed to examine the effects of FOP ALK2 mutations on BMP signaling and chondro/osteogenesis.
Availability of human cell models is limited due to restrictions on obtaining patient material and our incomplete knowledge of the progenitor cell types relevant to FOP HO. Dermal fibroblasts derived from patients with FOP have been successfully transdifferentiated to cells of an osteogenic lineage ( 33 ). Periodontal ligament fibroblasts have also been isolated and induced to osteogenesis and osteoclastogenesis ( 34 ). hiPSCs obtained from patients with FOP are able to differentiate to ECs ( 29 , 35 , 36 ) and pericytes with increased mineralization, but did not develop into mature osteoblasts ( 36 ). Connective tissue progenitor cells from discarded primary teeth have been used to examine the effects of FOP mutations on BMP signaling and chondrogenic/osteogenic differentiation ( 19 , 24 , 37 , 38 ). C2C12 myoblasts have been altered to express ALK2 R206H with doxycycline dependent promoter to simulate FOP ( 39 ).
A fruit fly model carrying the classical R206H mutation demonstrated over activation of BMP signaling by the ALK2 R206H receptor but also ligand independent signaling of the receptor ( 40 ), consistent with earlier in vitro analyses ( 41 – 43 ). An embryonic chicken model was used to study the role of several ALK2 mutations and demonstrated that the FOP ALK2 Q207E and ALK2 R206H mutation, along with the engineered constitutively active ALK2 Q207D mutation, caused FOP-like phenotypes with skeletal malformations and HO ( 44 ).
In mice, activating mutations in ALK2 are lethal during embryonic development ( 45 ), therefore investigations of the in vivo effects of ALK2 activating mutations have required either chimeric/mosaic expression of mutant cells or a conditional gene expression model. The first such mouse model, using a Cre-Lox inducible ALK Q207D transgene was developed prior to the identification of ALK2 as relevant to FOP ( 45 ). Later, this model was used with adenovirus expressing Cre and tamoxifen-responsive Cre alleles to induce postnatal activation of the ALK2 Q207D transgene ( 46 ). Although the ALK2 Q207D substitution is not a naturally occurring FOP mutation in humans, these mouse models provided the first mammalian systems to study the effects of excessive BMP signaling by ALK2, importantly demonstrating a requirement for tissue injury and inflammation in addition to mutant ALK2 expression for the development of heterotopic bone ( 47 ).
Subsequently, researchers have developed mouse models harboring the common FOP ALK2 R206H mutation. A chimeric model with a variable proportion of cells expressing a heterozygous ALK2 R206H allele yielded intermittent live births, mimicking classic FOP features such as HO development in response to muscle injury, hind limb digit malformation, and joint fusions ( 48 ). A Cre-dependent knock-in model with inducible ALK2 R206H expression has been used to mimic HO formation in response to various injuries, highlighting the importance of activin A in ALK R206H signaling function ( 49 – 52 ). The progression of HO formation in ALK2 R206H mouse models appears to closely reproduce the events of HO formation from an early-stage immune cell response to a robust fibroproliferative stage that transitions to endochondral ossification ( 15 , 16 , 48 ). These models also feature the distinct patterns of HO within the axial and extra-axial skeleton and exhibit both injury-dependent and spontaneous progression of HO ( 50 , 52 ).
A zebrafish FOP model has also been developed and embryonic development assays have been used to investigate the mechanism through which mutant ALK2 receptors enhance BMP-phosphorylated (p)SMAD 1/5 signaling ( 53 – 55 ).
A novel approach is a computational disease model. Computational models of endochondral ossification have previously been developed ( 56 , 57 ). In these models the interplay between growth factors, angiogenesis, oxygen, recruitment, proliferation and differentiation of osteoprogenitor cells can be considered. These models could be adapted to simulate endochondral ossification in FOP and provide an additional way to evaluate the effect of therapeutic interventions in FOP.
In summary, there are numerous in vitro and in vivo models available with the potential to further investigate and understand FOP. It will also be important to establish how closely these model systems reflect the pathophysiology of FOP in humans and how well they address the various complexities of the FOP phenotype. Acknowledging the advantages and disadvantages of each of these models can allow them to complement each other, maximizing the information gained in preclinical FOP research.
Possible Targets for Therapy in FOP
Despite many efforts, still there is no effective and specific treatment approved for FOP. Therapy is focused on treating flare-ups with glucocorticoids and nonsteroidal anti-inflammatory drugs upon presentation ( 58 ). Taking the different stages of HO in FOP into consideration it is possible to identify different processes which can be considered as targets to develop therapies by different approaches ( Figure 2 ).
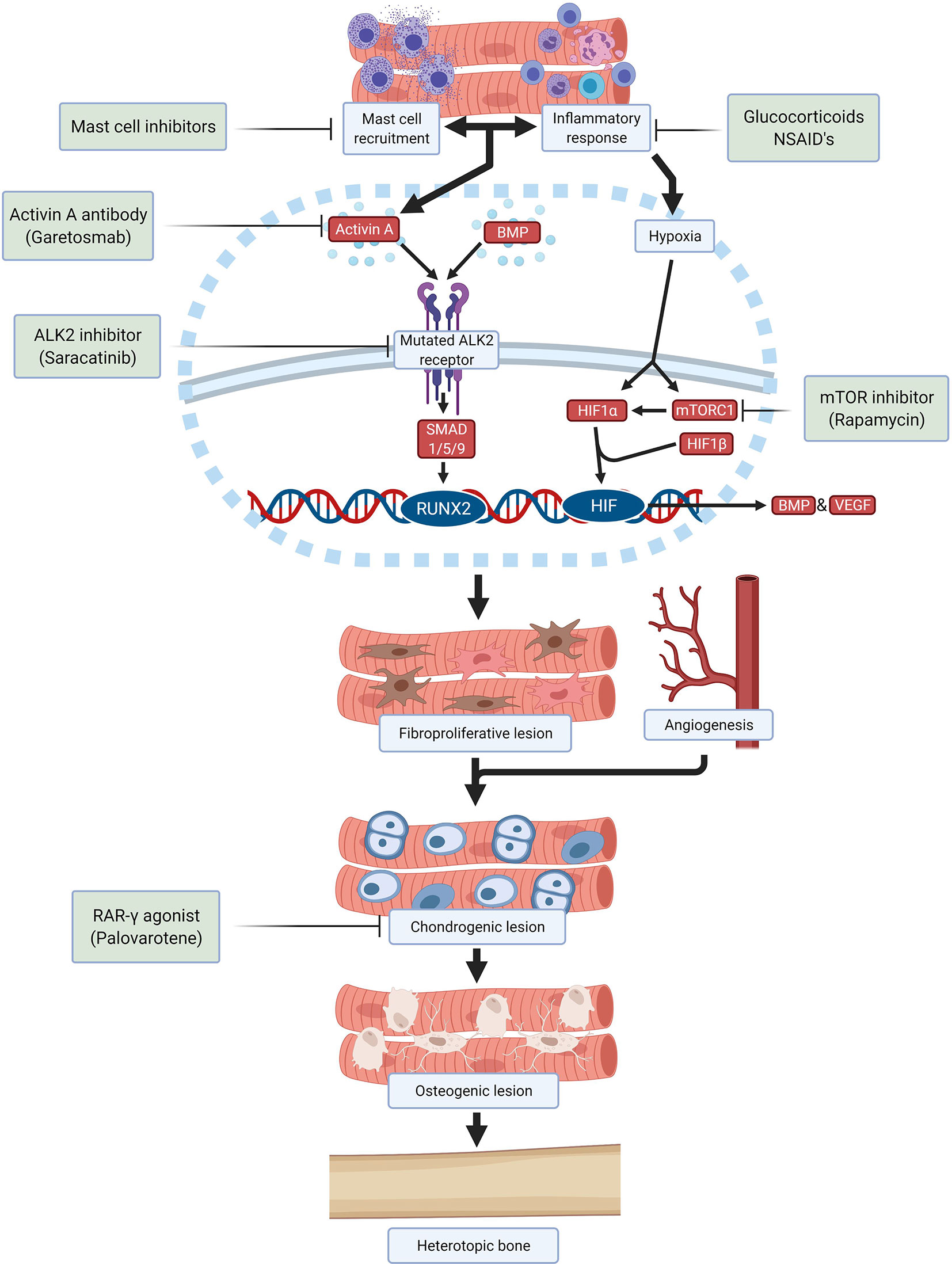
Figure 2 Schematic overview of drugs and investigational compounds currently used and/or evaluated in FOP treatment and their respective targets.
Saracatinib, a kinase inhibitor targeting src-family kinases originally developed as a treatment for various solid tumors, is a potent ALK2 inhibitor with efficacy against HO in preclinical models and is now being repositioned as a potential treatment for FOP in an ongoing phase 2 clinical trial (NCT04307953) ( 59 ). Several other ALK2 inhibitors have been developed with the goal of improving potency and selectivity for ALK2 receptor inhibition, with promising safety in phase 1 studies and are anticipated to advance to phase 2 efficacy studies in the near future.
Alternatively, the stimulation of the ALK2 receptor by ALK2 ligands can be prevented. A neutralizing antibody specific for activin A (garetosmab) has been evaluated in a phase 2 clinical trial (NCT03188666) after promising preclinical results ( 49 ). Recently, mTOR (mammalian target of rapamycin) has been identified as a key factor in the early hypoxic and inflammatory stages of HO ( 21 ). Besides its important immunoregulatory function, mTOR signaling is required for chondrogenesis and osteogenesis induction. Crosstalk between mTOR signaling and BMP signaling may amplify HO in FOP ( 60 ). In preclinical studies, rapamycin successfully inhibited HO in a mouse model and a clinical trial is being performed to evaluate its efficacy and safety in patients with FOP (UMIN000028429) ( 60 , 61 ).
Downstream signaling initiated by activation of ALK2 also offers opportunities to prevent HO. Palovarotene, a retinoic acid receptor-gamma (RAR-γ) agonist, inhibits HO in FOP mouse models by blocking chondrogenic differentiation of the progenitor cells and is currently being investigated in multiple phase 2 and phase 3 trials (NCT02279095, NCT02190747, NCT03312634) ( 51 , 62 , 63 ). Other therapies being investigated are VEGF inhibitors, ligand traps, phosphoinositide 3-kinases (PI3K)-inhibitors, siRNAs, HIF1-α blockers and transforming growth factor-β activated kinase (TAK)1 inhibitors. Once a successful therapeutic strategy for preventing HO in FOP is available, surgical intervention may become feasible for excising heterotopic bone and restoring function.
Clinical Trials in Ultra-Rare Diseases
Therapeutic development in FOP shares many challenges faced by other ultra-rare diseases such as a limited understanding of natural history to inform trial design, dearth of validated and surrogate outcome measures to quantify the disease during the limited time span of a clinical trial, and small numbers of patients available for clinical trials ( 64 , 65 ).
The randomized controlled trial (RCT) is the gold standard for determining drug efficacy in a clinical trial setting. Randomization minimizes selection bias and distributes potential confounders between study groups. RCT power decreases rapidly with diminished smaller cohorts as inter-individual differences become more pronounced, increasing the risk of known and unknown covariates affecting the trial results.
An uncontrolled trial may be feasible when the natural history of a disease is well-established. In this design, the effect of the intervention can be compared against the natural history of the disease. In FOP however, the natural history of the disease is still being investigated and it is known that disease progression varies between individuals ( 66 ). Additionally, subject may report less adverse events in a non-interventional natural study than in an interventional clinical trial, creating bias against the drug. However, studies have been performed to mitigate this potential bias ( 67 ).
Both trial designs have their drawbacks but remain important options for determining drug efficacy in FOP. Future trials in FOP should acknowledge these disadvantages, implementing smart trial designs and statistical methods to address inherent limitations of a small and heterogeneous population, thus maximizing the information obtained while supporting patient safety ( 65 , 68 ). There is an urgent need to establish an imaginative and equitable approach towards clinical trials in FOP given the multitude of drugs being developed and the limited number of patients.
Determination of Disease Activity in FOP
Another problem that FOP faces is the difficulty to evaluate individual disease activity. Clinical symptoms of a flare-up such as pain, swelling, erythema and warmth are non-specific, and it is not possible to predict whether the acute phase will end up with HO or will resolve ( 66 ). A multitude of inflammatory, chondrogenic and osteogenic bone markers have been investigated, and although some were markedly elevated in patients with FOP, none have shown an association with disease activity or been able to predict HO formation adequately ( 69 – 71 ).
Conventional imaging techniques are only able to detect HO after formation of bone tissue. MRI (magnetic resonance imaging) and ultrasonography are suitable to detect soft tissue edema associated with the inflammatory stage of HO but are non-specific and unable to reveal bone formation ( 72 , 73 ). Nucleotide imaging such as the [ 18 F]-sodium fluoride (NaF) PET (positron emission tomography) scan can detect bone formation before it is visible on conventional CT (computed tomography). Interestingly, PET/CT and MRI scanning revealed that not every flare-up resulted in HO and showed continuous FOP activity not related to a flare-up ( 74 ).
Determination of disease activity with a suitable biomarker and imaging techniques is necessary for evaluation of potential therapies in FOP. A combination of markers may be needed to reflect the multiple stages of HO in FOP; ongoing efforts exist on FOP biomarker development ( 20 ).
Discussion and Future Research
Looking back at the topics discussed in 2015, the meeting identified key issues in which progress has been made through collaborative approaches ( Figure 1 ). However, it is also clear that FOP research and treatment still face many challenges. Big questions remain regarding the pathophysiology of FOP such as the identity of the HO progenitor cell and the effect of the ALK2 mutation on the immune response and angiogenesis. Also, with the advent of clinical trials for FOP, it has become clear that we still need to obtain as much information as possible in the preclinical phase including cell and molecular mechanisms. This requires further use and development of in vitro and in vivo disease models, and perhaps exploring options such as computational modelling. During clinical trials, the information gained must be maximized through means of careful trial design and proper evaluation of disease activity. To achieve this in FOP, international collaboration is paramount and has to be fostered. Maybe the time is ripe to make a point and gather the FOP research community in a new meeting to share and discuss the most recent research strategies again.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author Contributions
RdR wrote the manuscript and created the figures with input from all authors. EE, GP, NB, PKn, DM, and RR organised the original workshop in which topics were discussed. GP, NB, PK, TS, GSD, MP, RP, ES, ME, HO, PY, RR, DM, EE, RB, CC, TV, SH, RJ, RK, PKo, RM, CN, PD, JT, and FV all attended the 2015 workshop, provided valuable contributions to the discussions and comments on the manuscript. RdR, BS, EB, TR, ES, FK, CSc, CSt, PD, and EH all provided valuable updates to the discussion of the research done since the 2015 meeting. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the Lorentz organization for their help and facilities making this workshop possible. The workshop was made possible with grants by the Lorentz Center, the Dutch FOP foundation and ZonMw. Images were created with Biorender.com .
1. Kaplan FS, Zasloff MA, Kitterman JA, Shore EM, Hong CC, Rocke DM. Early Mortality and Cardiorespiratory Failure in Patients With Fibrodysplasia Ossificans Progressiva. J Bone Joint Surg Am Vol (2010) 92(3):686–91. doi: 10.2106/JBJS.I.00705
CrossRef Full Text | Google Scholar
2. Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, et al. A Recurrent Mutation in the BMP Type I Receptor ACVR1 Causes Inherited and Sporadic Fibrodysplasia Ossificans Progressiva. Nat Genet (2006) 38(5):525–7. doi: 10.1038/ng1783
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Hino K, Ikeya M, Horigome K, Matsumoto Y, Ebise H, Nishio M, et al. Neofunction of ACVR1 in Fibrodysplasia Ossificans Progressiva. Proc Natl Acad Sci United States America (2015) 112(50):15438–43. doi: 10.1073/pnas.1510540112
4. Song GA, Kim HJ, Woo KM, Baek JH, Kim GS, Choi JY, et al. Molecular Consequences of the ACVR1(R206H) Mutation of Fibrodysplasia Ossificans Progressiva. J Biol Chem (2010) 285(29):22542–53. doi: 10.1074/jbc.M109.094557
5. Lin H, Shi F, Gao J, Hua P. The Role of Activin A in Fibrodysplasia Ossificans Progressiva: A Prominent Mediator. Biosci Rep (2019) 39(8). doi: 10.1042/BSR20190377
6. Kaplan FS, Lounev VY, Wang H, Pignolo RJ, Shore EM. Fibrodysplasia Ossificans Progressiva: A Blueprint for Metamorphosis. Ann N Y Acad Sci (2011) 1237:5–10. doi: 10.1111/j.1749-6632.2011.06195.x
7. Forcina L, Miano C, Pelosi L, Musarò A. An Overview About the Biology of Skeletal Muscle Satellite Cells. Curr Genomics (2019) 20(1):24–37. doi: 10.2174/1389202920666190116094736
8. Asakura A, Komaki M, Rudnicki M. Muscle Satellite Cells are Multipotential Stem Cells That Exhibit Myogenic, Osteogenic, and Adipogenic Differentiation. Differentiation (2001) 68(4-5):245–53. doi: 10.1046/j.1432-0436.2001.680412.x
9. Lounev VY, Ramachandran R, Wosczyna MN, Yamamoto M, Maidment AD, Shore EM, et al. Identification of Progenitor Cells That Contribute to Heterotopic Skeletogenesis. J Bone Joint Surg Am Vol (2009) 91(3):652–63. doi: 10.2106/JBJS.H.01177
10. Lees-Shepard JB, Goldhamer DJ. Stem Cells and Heterotopic Ossification: Lessons From Animal Models. Bone (2018) 109:178–86. doi: 10.1016/j.bone.2018.01.029
11. Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of Vascular Endothelial Cells Into Multipotent Stem-Like Cells. Nat Med (2010) 16(12):1400–6. doi: 10.1038/nm.2252
12. Wosczyna MN, Biswas AA, Cogswell CA, Goldhamer DJ. Multipotent Progenitors Resident in the Skeletal Muscle Interstitium Exhibit Robust BMP-Dependent Osteogenic Activity and Mediate Heterotopic Ossification. J Bone Mineral Res (2012) 27(5):1004–17. doi: 10.1002/jbmr.1562
13. Lees-Shepard JB, Yamamoto M, Biswas AA, Stoessel SJ, Nicholas SE, Cogswell CA, et al. Activin-Dependent Signaling in Fibro/Adipogenic Progenitors Causes Fibrodysplasia Ossificans Progressiva. Nat Commun (2018) 9(1):471. doi: 10.1038/s41467-018-02872-2
14. Gannon FH, Valentine BA, Shore EM, Zasloff MA, Kaplan FS. Acute Lymphocytic Infiltration in an Extremely Early Lesion of Fibrodysplasia Ossificans Progressiva. Clin Orthopaedics Rel Res (1998) 346):19–25. doi: 10.1097/00003086-199801000-00005
15. Gannon FH, Glaser D, Caron R, Thompson LD, Shore EM, Kaplan FS. Mast Cell Involvement in Fibrodysplasia Ossificans Progressiva. Hum Pathol (2001) 32(8):842–8. doi: 10.1053/hupa.2001.26464
16. Convente MR, Chakkalakal SA, Yang E, Caron RJ, Zhang D, Kambayashi T, et al. Depletion of Mast Cells and Macrophages Impairs Heterotopic Ossification in an Acvr1(R206H) Mouse Model of Fibrodysplasia Ossificans Progressiva. J Bone Mineral Res (2018) 33(2):269–82. doi: 10.1002/jbmr.3304
17. Cho SW, Soki FN, Koh AJ, Eber MR, Entezami P, Park SI, et al. Osteal Macrophages Support Physiologic Skeletal Remodeling and Anabolic Actions of Parathyroid Hormone in Bone. Proc Natl Acad Sci United States America (2014) 111(4):1545–50. doi: 10.1073/pnas.1315153111
18. Tirone M, Giovenzana A, Vallone A, Zordan P, Sormani M, Nicolosi PA, et al. Severe Heterotopic Ossification in the Skeletal Muscle and Endothelial Cells Recruitment to Chondrogenesis Are Enhanced by Monocyte/Macrophage Depletion. Front Immunol (2019) 10:1640. doi: 10.3389/fimmu.2019.01640
19. Wang H, Behrens EM, Pignolo RJ, Kaplan FS. ECSIT Links TLR and BMP Signaling in FOP Connective Tissue Progenitor Cells. Bone (2018) 109:201–9. doi: 10.1016/j.bone.2017.12.024
20. Barruet E, Morales BM, Cain CJ, Ton AN, Wentworth KL, Chan TV, et al. NF-Kappab/MAPK Activation Underlies ACVR1-Mediated Inflammation in Human Heterotopic Ossification. JCI Insight (2018) 3(22):122958. doi: 10.1172/jci.insight.122958
21. Matsuo K, Chavez RD, Barruet E, Hsiao EC. Inflammation in Fibrodysplasia Ossificans Progressiva and Other Forms of Heterotopic Ossification. Curr Osteoporosis Rep (2019) 17(6):387–94. doi: 10.1007/s11914-019-00541-x
22. Del Zotto G, Antonini F, Azzari I, Ortolani C, Tripodi G, Giacopelli F, et al. Peripheral Blood Mononuclear Cell Immunophenotyping in Fibrodysplasia Ossificans Progressiva Patients: Evidence for Monocyte DNAM1 Up-Regulation. Cytomet Part B Clin Cytomet (2018) 94(4):613–22. doi: 10.1002/cyto.b.21594
23. Suda RK, Billings PC, Egan KP, Kim JH, McCarrick-Walmsley R, Glaser DL, et al. Circulating Osteogenic Precursor Cells in Heterotopic Bone Formation. Stem Cells (Dayton Ohio) (2009) 27(9):2209–19. doi: 10.1002/stem.150
24. Wang H, Lindborg C, Lounev V, Kim JH, McCarrick-Walmsley R, Xu M, et al. Cellular Hypoxia Promotes Heterotopic Ossification by Amplifying BMP Signaling. J Bone Mineral Res (2016) 31(9):1652–65. doi: 10.1002/jbmr.2848
25. Haupt J, Stanley A, McLeod CM, Cosgrove BD, Culbert AL, Wang L, et al. ACVR1(R206H) FOP Mutation Alters Mechanosensing and Tissue Stiffness During Heterotopic Ossification. Mol Biol Cell (2019) 30(1):17–29. doi: 10.1091/mbc.E18-05-0311
26. Cai J, Pardali E, Sánchez-Duffhues G, ten Dijke P. BMP Signaling in Vascular Diseases. FEBS Lett (2012) 586(14):1993–2002. doi: 10.1016/j.febslet.2012.04.030
27. Saran U, Gemini Piperni S, Chatterjee S. Role of Angiogenesis in Bone Repair. Arch Biochem Biophys (2014) 561:109–17. doi: 10.1016/j.abb.2014.07.006
28. Benn A, Hiepen C, Osterland M, Schutte C, Zwijsen A, Knaus P. Role of Bone Morphogenetic Proteins in Sprouting Angiogenesis: Differential BMP Receptor-Dependent Signaling Pathways Balance Stalk vs. Tip Cell Competence. FASEB J (2017) 31(11):4720–33. doi: 10.1096/fj.201700193RR
29. Hildebrandt S, Kampfrath B, Fischer K, Hildebrand L, Haupt J, Stachelscheid H, et al. ActivinA Induced SMAD1/5 Signaling in an iPSC Derived EC Model of Fibrodysplasia Ossificans Progressiva (FOP) Can Be Rescued by the Drug Candidate Saracatinib. Stem Cell Rev Rep (2021) 17(3):1039–52. doi: 10.1007/s12015-020-10103-9
30. el-Labban NG, Hopper C, Barber P. Ultrastructural Finding of Vascular Degeneration in Fibrodysplasia Ossificans Progressiva (FOP). J Oral Pathol Med (1995) 24(3):125–9. doi: 10.1111/j.1600-0714.1995.tb01152.x
31. Benn A, Bredow C, Casanova I, Vukicevic S, Knaus P. VE-Cadherin Facilitates BMP-Induced Endothelial Cell Permeability and Signaling. J Cell Sci (2016) 129(1):206–18. doi: 10.1242/jcs.179960
32. Sánchez-Duffhues G, Williams E, Benderitter P, Orlova V, van Wijhe M, Garcia de Vinuesa A, et al. Development of Macrocycle Kinase Inhibitors for ALK2 Using Fibrodysplasia Ossificans Progressiva-Derived Endothelial Cells. JBMR Plus (2019) 3(11):e10230. doi: 10.1002/jbm4.10230
33. Micha D, Voermans E, Eekhoff MEW, van Essen HW, Zandieh-Doulabi B, Netelenbos C, et al. Inhibition of Tgfβ Signaling Decreases Osteogenic Differentiation of Fibrodysplasia Ossificans Progressiva Fibroblasts in a Novel In Vitro Model of the Disease. Bone (2016) 84:169–80. doi: 10.1016/j.bone.2016.01.004
34. de Vries TJ, Schoenmaker T, Micha D, Hogervorst J, Bouskla S, Forouzanfar T, et al. Periodontal Ligament Fibroblasts as a Cell Model to Study Osteogenesis and Osteoclastogenesis in Fibrodysplasia Ossificans Progressiva. Bone (2018) 109:168–77. doi: 10.1016/j.bone.2017.07.007
35. Barruet E, Morales BM, Lwin W, White MP, Theodoris CV, Kim H, et al. The ACVR1 R206H Mutation Found in Fibrodysplasia Ossificans Progressiva Increases Human Induced Pluripotent Stem Cell-Derived Endothelial Cell Formation and Collagen Production Through BMP-Mediated SMAD1/5/8 Signaling. Stem Cell Res Ther (2016) 7(1):115. doi: 10.1186/s13287-016-0372-6
36. Cai J, Orlova VV, Cai X, Eekhoff EMW, Zhang K, Pei D, et al. Induced Pluripotent Stem Cells to Model Human Fibrodysplasia Ossificans Progressiva. Stem Cell Rep (2015) 5(6):963–70. doi: 10.1016/j.stemcr.2015.10.020
37. Billings PC, Fiori JL, Bentwood JL, O’Connell MP, Jiao X, Nussbaum B, et al. Dysregulated BMP Signaling and Enhanced Osteogenic Differentiation of Connective Tissue Progenitor Cells From Patients With Fibrodysplasia Ossificans Progressiva (FOP). J Bone Mineral Res (2008) 23(3):305–13. doi: 10.1359/jbmr.071030
38. Kaplan J, Kaplan FS, Shore EM. Restoration of Normal BMP Signaling Levels and Osteogenic Differentiation in FOP Mesenchymal Progenitor Cells by Mutant Allele-Specific Targeting. Gene Ther (2012) 19(7):786–90. doi: 10.1038/gt.2011.152
39. Ebner JK, König GM, Kostenis E, Siegert P, Aktories K, Orth JHC. Activation of G(q) Signaling by Pasteurella Multocida Toxin Inhibits the Osteoblastogenic-Like Actions of Activin A in C2C12 Myoblasts, a Cell Model of Fibrodysplasia Ossificans Progressiva. Bone (2019) 127:592–601. doi: 10.1016/j.bone.2019.07.031
40. Le VQ, Wharton KA. Hyperactive BMP Signaling Induced by ALK2(R206H) Requires Type II Receptor Function in a Drosophila Model for Classic Fibrodysplasia Ossificans Progressiva. Dev Dynamics (2012) 241(1):200–14. doi: 10.1002/dvdy.22779
41. Shen Q, Little SC, Xu M, Haupt J, Ast C, Katagiri T, et al. The Fibrodysplasia Ossificans Progressiva R206H ACVR1 Mutation Activates BMP-Independent Chondrogenesis and Zebrafish Embryo Ventralization. J Clin Invest (2009) 119(11):3462–72. doi: 10.1172/JCI37412
42. Fukuda T, Kohda M, Kanomata K, Nojima J, Nakamura A, Kamizono J, et al. Constitutively Activated ALK2 and Increased SMAD1/5 Cooperatively Induce Bone Morphogenetic Protein Signaling in Fibrodysplasia Ossificans Progressiva. J Biol Chem (2009) 284(11):7149–56. doi: 10.1074/jbc.M801681200
43. van Dinther M, Visser N, de Gorter DJ, Doorn J, Goumans MJ, de Boer J, et al. ALK2 R206H Mutation Linked to Fibrodysplasia Ossificans Progressiva Confers Constitutive Activity to the BMP Type I Receptor and Sensitizes Mesenchymal Cells to BMP-Induced Osteoblast Differentiation and Bone Formation. J Bone Mineral Res (2010) 25(6):1208–15. doi: 10.1359/jbmr.091110
44. Haupt J, Deichsel A, Stange K, Ast C, Bocciardi R, Ravazzolo R, et al. ACVR1 P.Q207E Causes Classic Fibrodysplasia Ossificans Progressiva and is Functionally Distinct From the Engineered Constitutively Active ACVR1 P.Q207D Variant. Hum Mol Genet (2014) 23(20):5364–77. doi: 10.1093/hmg/ddu255
45. Fukuda T, Scott G, Komatsu Y, Araya R, Kawano M, Ray MK, et al. Generation of a Mouse With Conditionally Activated Signaling Through the BMP Receptor, ALK2. Genesis (2006) 44(4):159–67. doi: 10.1002/dvg.20201
46. Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, et al. BMP Type I Receptor Inhibition Reduces Heterotopic [Corrected] Ossification. Nat Med (2008) 14(12):1363–9. doi: 10.1038/nm.1888
47. Mohedas AH, Xing X, Armstrong KA, Bullock AN, Cuny GD, Yu PB. Development of an ALK2-Biased BMP Type I Receptor Kinase Inhibitor. ACS Chem Biol (2013) 8(6):1291–302. doi: 10.1021/cb300655w
48. Chakkalakal SA, Zhang D, Culbert AL, Convente MR, Caron RJ, Wright AC, et al. An Acvr1 R206H Knock-in Mouse has Fibrodysplasia Ossificans Progressiva. J Bone Mineral Res (2012) 27(8):1746–56. doi: 10.1002/jbmr.1637
49. Upadhyay J, Xie L, Huang L, Das N, Stewart RC, Lyon MC, et al. The Expansion of Heterotopic Bone in Fibrodysplasia Ossificans Progressiva Is Activin A-Dependent. J Bone Mineral Res (2017) 32(12):2489–99. doi: 10.1002/jbmr.3235
50. Hatsell SJ, Idone V, Wolken DM, Huang L, Kim HJ, Wang L, et al. ACVR1R206H Receptor Mutation Causes Fibrodysplasia Ossificans Progressiva by Imparting Responsiveness to Activin A. Sci Trans Med (2015) 7(303):303ra137. doi: 10.1126/scitranslmed.aac4358
51. Chakkalakal SA, Uchibe K, Convente MR, Zhang D, Economides AN, Kaplan FS, et al. Palovarotene Inhibits Heterotopic Ossification and Maintains Limb Mobility and Growth in Mice With the Human ACVR1(R206H) Fibrodysplasia Ossificans Progressiva (FOP) Mutation. J Bone Mineral Res (2016) 31(9):1666–75. doi: 10.1002/jbmr.2820
52. Dey D, Bagarova J, Hatsell SJ, Armstrong KA, Huang L, Ermann J, et al. Two Tissue-Resident Progenitor Lineages Drive Distinct Phenotypes of Heterotopic Ossification. Sci Trans Med (2016) 8(366):366ra163. doi: 10.1126/scitranslmed.aaf1090
53. LaBonty M, Pray N, Yelick PC. A Zebrafish Model of Human Fibrodysplasia Ossificans Progressiva. Zebrafish (2017) 14(4):293–304. doi: 10.1089/zeb.2016.1398
54. LaBonty M, Yelick PC. Animal Models of Fibrodysplasia Ossificans Progressiva. Dev Dynamics (2018) 247(2):279–88. doi: 10.1002/dvdy.24606
55. Allen RS, Tajer B, Shore EM, Mullins MC. Fibrodysplasia Ossificans Progressiva Mutant ACVR1 Signals by Multiple Modalities in the Developing Zebrafish. eLife (2020) 9:e53761. doi: 10.7554/eLife.53761
56. Carlier A, van Gastel N, Geris L, Carmeliet G, Van Oosterwyck H. Size Does Matter: An Integrative In Vivo -in Silico Approach for the Treatment of Critical Size Bone Defects. PloS Comput Biol (2014) 10(11):e1003888. doi: 10.1371/journal.pcbi.1003888
57. Carlier A, Geris L, van Gastel N, Carmeliet G, Van Oosterwyck H. Oxygen as a Critical Determinant of Bone Fracture Healing-a Multiscale Model. J Theor Biol (2015) 365:247–64. doi: 10.1016/j.jtbi.2014.10.012
58. Kaplan FS, Al Mukaddam M, Baujat G, Brown M, Cali A, Cho T-J, et al. The Medical Management of Fibrodysplasia Ossificans Progressiva: Current Treatment Considerations. Proc Intl Clin Council FOP (2021) 2:1–128.
Google Scholar
59. Williams E, Bagarova J, Kerr G, Xia DD, Place ES, Dey D, et al. Saracatinib is an Efficacious Clinical Candidate for Fibrodysplasia Ossificans Progressiva. JCI Insight (2021) 6(8):e95042. doi: 10.1172/jci.insight.95042
60. Wu J, Ren B, Shi F, Hua P, Lin H. BMP and mTOR Signaling in Heterotopic Ossification: Does Their Crosstalk Provide Therapeutic Opportunities? J Cell Biochem (2019) 120(8):12108–22. doi: 10.1002/jcb.28710
61. Hino K, Zhao C, Horigome K, Nishio M, Okanishi Y, Nagata S, et al. An mTOR Signaling Modulator Suppressed Heterotopic Ossification of Fibrodysplasia Ossificans Progressiva. Stem Cell Rep (2018) 11(5):1106–19. doi: 10.1016/j.stemcr.2018.10.007
62. Shimono K, Tung WE, Macolino C, Chi AH, Didizian JH, Mundy C, et al. Potent Inhibition of Heterotopic Ossification by Nuclear Retinoic Acid Receptor-γ Agonists. Nat Med (2011) 17(4):454–60. doi: 10.1038/nm.2334
63. Pacifici M. Retinoid Roles and Action in Skeletal Development and Growth Provide the Rationale for an Ongoing Heterotopic Ossification Prevention Trial. Bone (2018) 109:267–75. doi: 10.1016/j.bone.2017.08.010
64. Augustine EF, Adams HR, Mink JW. Clinical Trials in Rare Disease: Challenges and Opportunities. J Child Neurol (2013) 28(9):1142–50. doi: 10.1177/0883073813495959
65. Hsiao EC, Di Rocco M, Cali A, Zasloff M, Al Mukaddam M, Pignolo RJ, et al. Special Considerations for Clinical Trials in Fibrodysplasia Ossificans Progressiva (FOP). Br J Clin Pharmacol (2019) 85(6):1199–207. doi: 10.1111/bcp.13777
66. Pignolo RJ, Bedford-Gay C, Liljesthrom M, Durbin-Johnson BP, Shore EM, Rocke DM, et al. The Natural History of Flare-Ups in Fibrodysplasia Ossificans Progressiva (FOP): A Comprehensive Global Assessment. J Bone Mineral Res (2016) 31(3):650–6. doi: 10.1002/jbmr.2728
67. Pignolo RJ, Cheung K, Kile S, Fitzpatrick MA, De Cunto C, Al Mukaddam M, et al. Self-Reported Baseline Phenotypes From the International Fibrodysplasia Ossificans Progressiva (FOP) Association Global Registry. Bone (2020) 134:115274. doi: 10.1016/j.bone.2020.115274
68. Chow SC, Chang M. Adaptive Design Methods in Clinical Trials - A Review. Orphanet J Rare Dis (2008) 3:11. doi: 10.1186/1750-1172-3-11
69. Lindborg CM, Brennan TA, Wang H, Kaplan FS, Pignolo RJ. Cartilage-Derived Retinoic Acid-Sensitive Protein (CD-RAP): A Stage-Specific Biomarker of Heterotopic Endochondral Ossification (HEO) in Fibrodysplasia Ossificans Progressiva (FOP). Bone (2018) 109:153–7. doi: 10.1016/j.bone.2017.09.016
70. Hildebrand L, Gaber T, Kühnen P, Morhart R, Unterbörsch H, Schomburg L, et al. Trace Element and Cytokine Concentrations in Patients With Fibrodysplasia Ossificans Progressiva (FOP): A Case Control Study. J Trace Elements Med Biol (2017) 39:186–92. doi: 10.1016/j.jtemb.2016.10.001
71. Al Kaissi A, Kenis V, Ben Ghachem M, Hofstaetter J, Grill F, Ganger R, et al. The Diversity of the Clinical Phenotypes in Patients With Fibrodysplasia Ossificans Progressiva. J Clin Med Res (2016) 8(3):246–53. doi: 10.14740/jocmr2465w
72. Botman E, Raijmakers P, Yaqub M, Teunissen B, Netelenbos C, Lubbers W, et al. Evolution of Heterotopic Bone in Fibrodysplasia Ossificans Progressiva: An [(18)F]NaF PET/CT Study. Bone (2019) 124:1–6. doi: 10.1016/j.bone.2019.03.009
73. Al Mukaddam M, Rajapakse CS, Pignolo RJ, Kaplan FS, Smith SE. Imaging Assessment of Fibrodysplasia Ossificans Progressiva: Qualitative, Quantitative and Questionable. Bone (2018) 109:147–52. doi: 10.1016/j.bone.2017.08.011
74. Eekhoff EMW, Botman E, Coen Netelenbos J, de Graaf P, Bravenboer N, Micha D, et al. [18f]NaF PET/CT Scan as an Early Marker of Heterotopic Ossification in Fibrodysplasia Ossificans Progressiva. Bone (2018) 109:143–6. doi: 10.1016/j.bone.2017.08.012
Keywords: fibrodysplasia ossificans progessiva (FOP), trials, therapy, disease models, inflammation, angiogenesis
Citation: de Ruiter RD, Smilde BJ, Pals G, Bravenboer N, Knaus P, Schoenmaker T, Botman E, Sánchez-Duffhues G, Pacifici M, Pignolo RJ, Shore EM, van Egmond M, Van Oosterwyck H, Kaplan FS, Hsiao EC, Yu PB, Bocciardi R, De Cunto CL, Longo Ribeiro Delai P, de Vries TJ, Hilderbrandt S, Jaspers RT, Keen R, Koolwijk P, Morhart R, Netelenbos JC, Rustemeyer T, Scott C, Stockklausner C, ten Dijke P, Triffit J, Ventura F, Ravazzolo R, Micha D and Eekhoff EMW (2021) Fibrodysplasia Ossificans Progressiva: What Have We Achieved and Where Are We Now? Follow-up to the 2015 Lorentz Workshop. Front. Endocrinol. 12:732728. doi: 10.3389/fendo.2021.732728
Received: 29 June 2021; Accepted: 22 September 2021; Published: 10 November 2021.
Reviewed by:
Copyright © 2021 de Ruiter, Smilde, Pals, Bravenboer, Knaus, Schoenmaker, Botman, Sánchez-Duffhues, Pacifici, Pignolo, Shore, van Egmond, Van Oosterwyck, Kaplan, Hsiao, Yu, Bocciardi, De Cunto, Longo Ribeiro Delai, de Vries, Hilderbrandt, Jaspers, Keen, Koolwijk, Morhart, Netelenbos, Rustemeyer, Scott, Stockklausner, ten Dijke, Triffit, Ventura, Ravazzolo, Micha and Eekhoff. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruben D. de Ruiter, [email protected] ; Elisabeth M. W. Eekhoff, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Recent progress in drug development for fibrodysplasia ossificans progressiva
- Open access
- Published: 10 May 2022
- Volume 477 , pages 2327–2334, ( 2022 )
Cite this article
You have full access to this open access article

- Xinmiao Meng 1 na1 ,
- Haotian Wang 2 na1 &
- Jijun Hao ORCID: orcid.org/0000-0002-6769-9069 3
4660 Accesses
12 Citations
6 Altmetric
Explore all metrics
Fibrodysplasia Ossificans Progressiva (FOP) is a rare genetic disease caused by heterozygous missense mutations in Activin A receptor type I which is also known as Activin-like kinase 2 (ALK2), a type I receptor of Bone Morphogenetic Proteins(BMP). Patients with FOP usually undergo episodic flare-ups and the heterotopic ossification in soft and connective tissues. Molecular mechanism study indicates that Activin A, the ligand which normally transduces Transforming Growth Factor Beta signaling, abnormally activates BMP signaling through ALK2 mutants in FOP, leading to heterotopic bone formation. To date, effective therapies to FOP are unavailable. However, significant advances have recently been made in the development of FOP drugs. In this article, we review the recent advances in understanding the FOP mechanism and drug development, with a focus on the small-molecular and antibody drugs currently in the clinical trials for FOP treatment.
Similar content being viewed by others

Genetics and future therapy prospects of fibrodysplasia ossificans progressiva

Successful experience of tofacitinib treatment in patients with Fibrodysplasia Ossificans Progressiva

Study methodology and insights from the palovarotene clinical development program in fibrodysplasia ossificans progressiva
Avoid common mistakes on your manuscript.
Introduction
FOP is a rare human genetic disorder in which ectopic bone formation occurs in connective tissue such as tendons, ligaments, and skeletal muscles throughout the body, leading to progressive loss of mobility, chronic pain, and eventual premature death mainly due to cardiorespiratory failure [ 1 ]. A worldwide prevalence of FOP is approximately one in two million population without ethnic, racial, or geographic predisposition [ 2 ]. One main symptom of FOP is a malformation of big toes at birth which also serves as an early diagnostic hallmark for FOP [ 2 , 3 ]. In 2006, the first heterozygous missense causative mutation of FOP (617G>A; R206H) was reported in the gene-encoding ACVR1 [ 4 ]. Since then, additional new heterozygous missense causative mutations in ACVR1 have been reported, and further studies indicated that ACVR1 R206H mutation occurs in approximately 97% of FOP patients [ 5 , 6 ] (Fig. 1 ). ACVR1, also known as ALK2, is a type I receptor of BMP signaling essential for normal skeleton formation and embryonic patterning [ 7 , 8 ]. For a more complete view of FOP etiology, clinical characteristics, diagnosis, and management, we refer the readers to the excellent reviews in these topics [ 2 , 3 , 9 ].
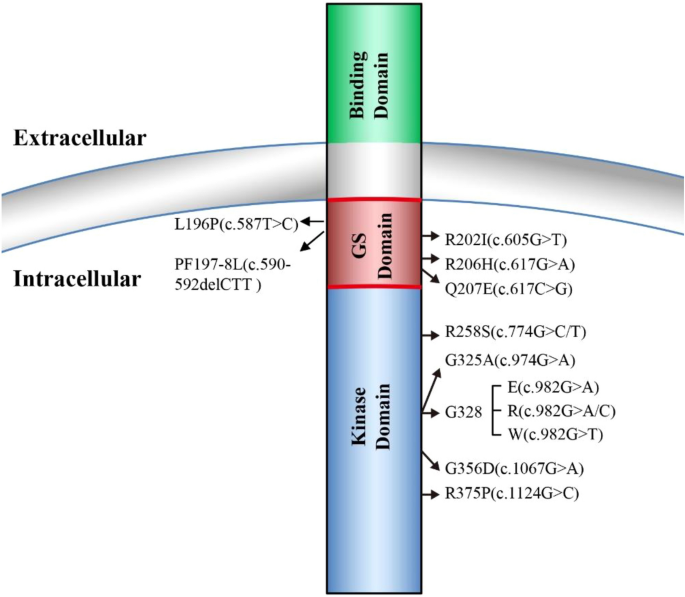
FOP causative mutations in ALK2 (ACVR1). ALK2 consists of ligand-binding domain, transmembrane domain, GS-rich domain, and serine/threonine kinase domain. All the identified FOP causative mutations are located either in either GS-rich domain or the serine/threonine kinase domain
Early mechanistic studies showed that FOP ALK2 mutants result in leaky BMP signaling in a basal condition and hyper-responsiveness upon BMP ligand stimulation [ 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 ]. However, recent findings have confirmed that activin A, the ligand which normally transduces TGF-β signaling, abnormally activates BMP signaling through FOP-mutated ALK2 [ 18 , 19 , 20 , 21 ]. This abnormal activin A-induced BMP signaling is thought to trigger heterotopic ossification of connective tissues [ 22 ]. To date, although effective therapies for FOP are unavailable, significant advances have been achieved in the development of potential FOP drugs, resulting in several promising therapies currently in clinical trials [ 23 ]. In this article, we review the recent progress in FOP mechanism studies and drug development, with a focus on the small-molecular and antibody drugs in the clinical trials for FOP treatment.
BMP signaling and FOP
BMPs are secreted multi-functional growth factors, and they belong to the TGF-β super family. BMPs consist of more than 20 family members which play central roles in regulating cellular morphogenesis, differentiation, proliferation, and apoptosis during embryogenesis and adult homeostasis [ 24 ]. The BMPs signal transduction is mainly mediated through the canonic Smads-dependent pathway in which BMPs first bind to a heterotetrametric complex consisting of a type II receptor homodimer and a type I receptor homodimer (Fig. 2 ). Then the type II receptors phosphorylate and activate the type I receptors, which in turn phosphorate Smad1/5/9 (also known as Smad1/5/8). The phosphorylated Smad1/5/9 subsequently form a complex with Smad4, which then translocates into the nucleus where it binds to BMP response elements and activates transcription of BMPs target genes [ 24 , 25 ].
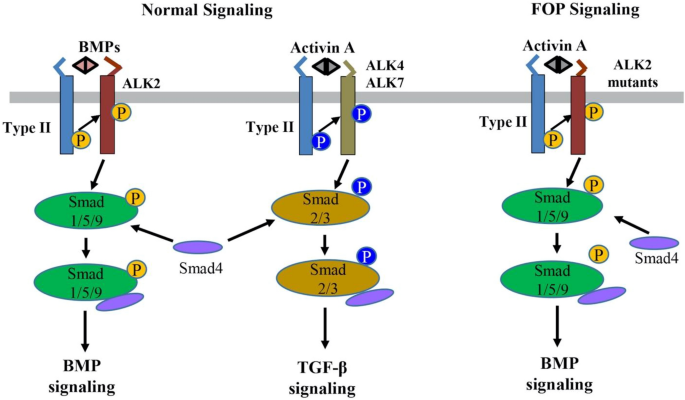
The normal BMP/TGF-β signaling pathways and abnormal activin A-induced BMP signaling through the ALK2 mutants in FOP. BMP or activin A ligands assemble and bind to a heterotetramer complex consisting of a type II receptor homodimer and a type I receptor homodimer (e.g., ALK2 for BMP and ALK4/7 for activin A). The type II receptor phosphorylates the type I receptor, which subsequently phosphorylates Smads (Smad1/5/9 for BMPs and Smad2/3 for activin A) to transduce normal BMP and TGF-β signaling, respectively. In contrast, in FOP, activin A can abnormally cross-signal BMP signaling through the ALK2 mutants
Four type I receptors, ALK1, ALK2, ALK3, and ALK6, are able to mediate BMP signaling and malfunctions of these four types I receptors are involved in many diseases including cancer [ 26 , 27 ]. In FOP, the most common mutation R206H is located at the intracellular glycine-serine-rich (GS) domain of ALK2, where FKBP12 protein (also known as FKBP1A) binds to ALK2 to prevent ALK2 activation in the absence of BMP ligands [ 12 , 15 , 16 ]. ALK2 R206H has been shown to induce basal leaky BMP signaling in the absence of BMP ligands and hyper-responsiveness upon BMP ligand stimulation that was initially thought to result in the ectopic endochondral ossification in FOP [ 15 , 16 , 17 , 28 ]. Later, additional FOP mutations have been identified in both GS domain and kinase domain of ALK2, which are associated with the disease onset ages and the extent of heterotopic ossification [ 5 , 10 , 29 , 30 , 31 , 32 ].
Nevertheless, recent findings have proved that activin A, a ligand which normally transduces TGF-β signaling, abnormally activates BMP signaling in FOP [ 18 , 19 , 20 , 21 ]. In normal physiological conditions, BMPs utilize ALK1/ALK2/ALK3/ALK6 as the type I receptors to activate Smad1/5/9-dependent BMP signaling, while activin A signals through ALK4/ALK7 as the type I receptors for Smad2/3-dependent TGF-β signaling and activin A does not transduce Smad1/5/9-dependent BMP signaling [ 33 ] (Fig. 2 ). However, recent multiple studies have demonstrated that activin A can activate Smad1/5/9-dependent BMP signaling in cells expressing ALK2 R206H in vitro and induced heterotopic ossification in a conditional knock-in mouse model of FOP in vivo [ 18 , 19 , 20 , 21 , 34 , 35 ]. In addition, this heterotopic ossification in the FOP mouse model can be blocked by the activin A-specific antibodies supporting that activin A cross-signal BMP pathway via mutated FOP ALK2 receptors [ 18 , 19 , 20 , 21 ]. Advances in understanding of the FOP molecular mechanism have led to significant progress in FOP drug development.
Recent drug development for FOP
Based on the molecular mechanism underlying FOP, multiple potential therapeutic targets have been selected for drug development to treat the disease.
Targeting ALK2
Since FOP is caused by the missense mutations of ALK2, ALK2 has been long thought as a potential therapeutic target for FOP and significant efforts have been made to develop ALK2 inhibitors.
Dorsomorphin, the first ALK2 chemical inhibitor, was identified from an in vivo screening of BMP inhibitors using zebrafish embryos [ 36 ] (Fig. 3 ). Unfortunately, Dorsomorphin displays notable off-targets against serval other kinases including Vascular Endothelial Growth Factor Receptor 2 (VEGFR2), ALK5, AMP-activated kinase (AMPK) and platelet-derived growth factor receptor β (PDGFRβ) [ 37 ], raising concerns about its clinical safety [ 37 , 38 ]. To develop more selective ALK2 inhibitors, we and colleagues have synthesized 63 Dorsomorphin analogs and identified DMH1 from those analogs by using zebrafish embryo screening [ 37 ]. In contrast to Dorsomorphin, DMH1 is more selective to ALK2, and it does not exhibit detectable activities against the closely related kinases such as VEGFR2, ALK5, AMPK, and PDGFRβ [ 37 ]. Meanwhile, another ALK2 inhibitor, LDN-193189, was developed, and it shows better potency and selectivity than Dorsomorphin [ 39 ] (Fig. 3 ). Nevertheless, both DMH1 and LDN-193189 cannot well distinguish ALK2 from other BMP type I receptors (ALK1/3/6) which are essential for development and homeostasis [ 40 , 41 , 42 , 43 ]. Therefore, developing better ALK2 inhibitor is critical for FOP treatment with minimum side effects. Further investigations discovered more selective ALK2 inhibitors, ML347 and LDN-212854 with negligible inhibitory activities for all other kinases except ALK1 [ 44 , 45 ] (Fig. 3 ). Very recently, Ullrich et al. reported a new potent and selective ALK2 inhibitor, compound 23, which displays excellent biochemical and cellular potency, selectivity, and a favorable in vitro profiles for absorption, distribution, metabolism, and excretion [ 46 ]. However, none of the above selective ALK2 inhibitors have moved into clinical trials.
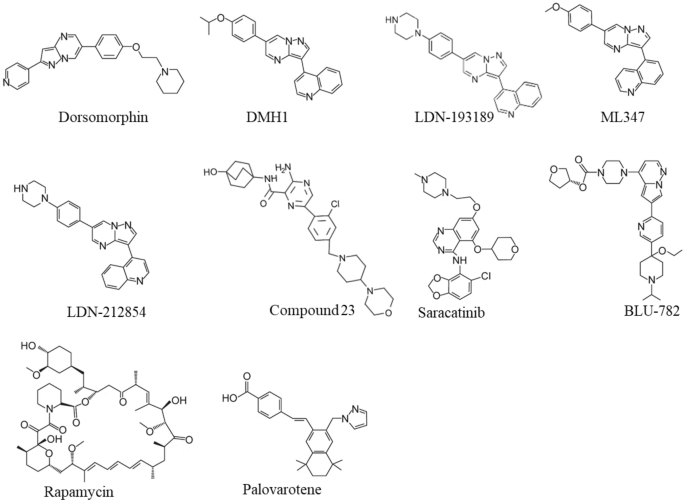
Chemical Structures of Small-Molecular Inhibitors of ALK2, Rapamycin, and Palovarotene
Recently, Williams et al. screened over 220 small-molecular kinase inhibitors which have either been approved previously by FDA or in clinical trials [ 47 ]. They identified a potent and selective ALK2 inhibitor, Saracatinib (also known as AZD0530), an orally bioavailable drug developed by AstraZeneca for the treatment of ovarian adenocarcinoma [ 47 , 48 ] (Fig. 3 ). Since Saracatinib effectively blocks heterotopic ossification in preclinical FOP models and displays excellent pharmacokinetic parameters and safety, Phase II clinical trial of Saracatinib for FOP was recently initiated in August 2020 (NCT04307953) [ 49 , 50 ] (Table 1 ). Another selective ALK2 inhibitor, INCB000928 that was originally developed to treat anemia as an iron homeostasis modulator, is now being evaluated for the efficacy and tolerability in the treatment of FOP in the phase II clinical trial (NCT05090891) [ 51 , 52 ] (Table 1 ). Other than small-molecular ALK2 inhibitors, an anti-ALK2 monoclonal antibody, DS-6016a, was developed as well by Daiichi Sankyo and Saitama Medical University in Japan. The Phase I clinical trial of DS-6016a to assess its safety, tolerability, and pharmacokinetics in healthy participants is ongoing, and the study results have not been released to date (NCT04818398) [ 53 ] (Table 1 ).
Nevertheless, these ALK2-targeting potential drugs indiscriminately target both wild-type ALK2 and FOP-mutated ALK2, leading to inhibition of important physiologic BMP signaling essential for normal cellular and tissue function. To overcome this challenge, Blueprint Medicines, Inc. developed a small molecule called BLU-782 (also known as IPN60130), which selectively targets the FOP-mutated ALK2 with minimal interference to the wild-type ALK2 [ 54 ] (Fig. 3 ). The Phase I clinical trial BLU-782 in healthy volunteers to establish its safety of the investigational drug was recently completed (NCT03858075), and the result showed that BLU-782 is well tolerated with approximately 24 h of half-life and displays excellent properties of pharmacokinetics and pharmacodynamics [ 55 , 56 ] (Table 1 ).
Targeting activin A
Activin A normally mediates TGF-β signaling by using Activin Receptors type IIA or IIB (ActR-IIA/ActR-IIB) as type II receptors and ALK4/7 as type I receptors followed by the downstream-phosphorylated Smad2/3 as intracellular signal transducers (Fig. 2 ). However, recent studies have confirmed that activin A abnormally activates BMP-Smad1/5/9 signaling through mutant ALK2 in FOP [ 18 , 19 , 20 , 21 , 34 , 35 ]. Given this interesting discovery, activin A has become a promising therapeutic target for FOP treatment. REGN2477 (also known as Garetosmab), a human anti-activin A-neutralizing antibody, was examined in the FOP mouse model, and the result showed that REGN2477 effectively inhibited heterotopic ossification [ 19 ]. The Phase I clinical trial of REGN2477 was completed, and the result demonstrated that REGN2477 displays great safety, tolerability, and pharmacokinetics [ 57 ]. Recently its Phase II clinical trial was initiated with a plan to administer 10 mg/kg REGN2477 intravenously every 4 weeks to FOP patients (NCT03188666) [ 58 ]. As activin A also plays important roles in multiple biological functions such as ovarian follicle maturation, spermatogenesis, steroidogenesis, muscle growth, immunity, inflammation, neuronal differentiation, and bone remodeling [ 59 , 60 , 61 , 62 , 63 , 64 ], the potential side effects of REGN2477 for activin A inhibition must be carefully monitored in FOP patients (Table 1 ).
Targeting other associated transcriptional effectors
It is believed that activin A induces chondrogenesis via BMP signaling in FOP by differentiating connective tissue progenitor cells into chondrocytes and osteoblasts prior to eventual formation of heterotopic bones in soft tissues [ 34 , 65 ]. Thus, inhibition of chronogenesis may be a good strategy to prevent heterotopic ossification in FOP.
Rapamycin (also known as Sirolimus) is an immunosuppressive drug used to prevent transplant rejection and lymphangioleiomyomatosis, and it has been recently identified as a potential drug for the treatment of FOP (Fig. 3 ). In a high-throughput screening by using FOP patient-derived induced pluripotent stem cells (FOP-iPSCs) to identify signaling pathways involved in activin A-induced chondrogenesis, Hino et al. found that the mammalian target of rapamycin (mTOR) signaling is critical in enhanced chondrogenesis initiated by activin A and heterotopic ossification in FOP [ 66 ]. They further showed that Rapamycin attenuated heterotopic ossification in both FOP-ALK2 R206H conditional transgenic mice and the mice with activin A-triggered heterotopic ossification derived from FOP-iPSCs [ 66 ]. Given the promising preclinical studies and its proved safety profile, Phase II/III clinical trials of Rapamycin for randomized, placebo-controlled studies and subsequent open-label extension studies were initiated at Kyoto University Hospital in Japan (UMIN000028429), and the outcomes of this trial has not been publicly released (Table 1 ). Nevertheless, a case report recently showed that Rapamycin did not show clear benefits to heterotopic ossification reduction in two young patients with classic FOP-ALK2 R206H mutation at the administrated dose [ 67 ].
Palovarotene
Retinoid signaling mediated by nuclear retinoic acid receptors (RAR) plays a critical biological role in chondrogenesis and normal skeleton formation and retinoic acid signaling agonists could effectively block chondrogenesis and subsequent heterotopic ossification in FOP [ 68 , 69 , 70 , 71 ]. In 2011, Shimono et al. showed that palovarotene (also known as R667), a specific agonist of the retinoic acid signaling by targeting nuclear retinoic acid receptor-γ (RARγ) with well characterized safety profile, inhibited heterotopic ossification in a transgenic mouse model expressing ALK2 Q207D mutation [ 72 ] (Fig. 3 ). Later, Chakkalakal et al. examined palovarotene in a knock-in mouse model carrying the classic FOP-ALK2 R206H mutation and demonstrated that palovarotene effectively blocks trauma-induced and spontaneous heterotopic ossification without comprising limb mobility and growth [ 73 ]. Importantly, palovarotene maintained joint, limb, and body motion, providing clear evidence for its encompassing therapeutic potential as a treatment for FOP [ 73 ]. In 2014, Clementia Pharmaceuticals initiated a double-blinded, placebo-controlled Phase II clinical trial to evaluate whether palovarotene prevents heterotopic ossification during and following a flare-up in FOP patients (NCT02190747). The trial was completed in 2016, and the result shows that palovarotene reduces the percentage of FOP patients developing heterotopic ossification, the time to remission and patient-reported pain associated with the flare-up area [ 74 ]. Currently, the Phase III clinical trial of palovarotene in FOP patients is in progress (NCT03312634). In addition, the rollover Phase III study was launched in November 2021 to further evaluate the safety and efficacy of palovarotene in adult and pediatric participants with FOP who have previously received palovarotene treatment (NCT05027802) [ 75 ] (Table 1 ).
In recent years, significant progresses have been made in understanding the molecular mechanism underlying FOP and developing FOP therapies. The discovery of causative mutations in ALK2 has made it a promising druggable target for FOP. Numerous small-molecular inhibitors and antibodies targeting ALK2 have been developed. Among them, Saracatinib, DS-6016a, and BLU-782 are currently in FOP clinical trials. In addition, as activin A abnormally transduces BMP signaling in FOP, REGN2477 antibody-targeting activin A has been studied for the treatment of FOP, and its efficacy is currently under evaluation in a Phase II clinical trial. Moreover, potential drugs targeting transcriptional effectors associated with the early heterotopic ossification have also shown promise in the treatment of FOP, and their efficacies are being evaluated in clinical trials. For instance, a Phase II clinical trial has showed that RARγ agonist Palovarotene effectively reduces the percentage of FOP patients developing heterotopic ossification and the time to remission (NCT02190747) [ 74 ]. Additionally, Rapamycin was shown to attenuate heterotopic ossification in FOP mouse models [ 66 ], and a Phase II clinical trial for Rapamycin is currently ongoing. In summary, rapid, and exciting advances have been made in our understating of FOP mechanism and drug development. Several potential drugs are currently under clinical trials to treat FOP at multiple targets, which allows more effective combinatorial pharmacological management for FOP. Nevertheless, as physiological BMP signaling is critical to homeostasis and indiscriminately blocking BMP signaling to treat FOP may raise some concerns, therapeutic agents like BLU-782 that selectively targets only the mutant ALK2 with minimal interference to the wild-type ALK2 may represent an excellent strategy for FOP treatment in the future.
Data availability
Not applicable.
Kaplan FS, Shen Q, Lounev V, Seemann P, Groppe J, Katagiri T et al (2008) Skeletal metamorphosis in fibrodysplasia ossificans progressiva (FOP). J Bone Miner Metab 26(6):521–530
Article PubMed PubMed Central Google Scholar
Pignolo RJ, Shore EM, Kaplan FS (2013) Fibrodysplasia ossificans progressiva: diagnosis, management, and therapeutic horizons. Pediatr Endocrinol Rev 10(Suppl 2):437–448
PubMed PubMed Central Google Scholar
Kaplan FS, Xu M, Glaser DL, Collins F, Connor M, Kitterman J et al (2008) Early diagnosis of fibrodysplasia ossificans progressiva. Pediatrics 121(5):e1295–e1300
Article PubMed Google Scholar
Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH et al (2006) A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet 38(5):525–527
Article CAS PubMed Google Scholar
Furuya H, Ikezoe K, Wang L, Ohyagi Y, Motomura K, Fujii N et al (2008) A unique case of fibrodysplasia ossificans progressiva with an ACVR1 mutation, G356D, other than the common mutation (R206H). Am J Med Genet A 146A(4):459–463
Kaplan FS, Groppe JC, Xu M, Towler OW, Grunvald E, Kalunian K et al (2022) An ACVR1(R375P) pathogenic variant in two families with mild fibrodysplasia ossificans progressiva. Am J Med Genet Part A 188(3):806–817
Huse M, Muir TW, Xu L, Chen YG, Kuriyan J, Massague J (2001) The TGF beta receptor activation process: an inhibitor- to substrate-binding switch. Mol Cell 8(3):671–682
Huse M, Chen YG, Massague J, Kuriyan J (1999) Crystal structure of the cytoplasmic domain of the type I TGF beta receptor in complex with FKBP12. Cell 96(3):425–436
Haga N, Nakashima Y, Kitoh H, Kamizono J, Katagiri T, Saijo H et al (2020) Fibrodysplasia ossificans progressiva: review and research activities in Japan. Pediatr Int 62(1):3–13
Kaplan FS, Xu M, Seemann P, Connor JM, Glaser DL, Carroll L et al (2009) Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat 30(3):379–390
Article CAS PubMed PubMed Central Google Scholar
Groppe JC, Shore EM, Kaplan FS (2007) Functional Modeling of the ACVR1 (R206H) mutation in FOP. Clin Orthop Relat R 462:87–92
Article Google Scholar
Groppe JC, Wu J, Shore EM, Kaplan FS (2011) In vitro analyses of the dysregulated R206H ALK2 kinase-FKBP12 interaction associated with heterotopic ossification in FOP. Cells Tissues Organs 194(2–4):291–295
Chaikuad A, Alfano I, Kerr G, Sanvitale CE, Boergermann JH, Triffitt JT et al (2012) Structure of the bone morphogenetic protein receptor ALK2 and implications for fibrodysplasia ossificans progressiva. J Biol Chem 287(44):36990–36998
Bagarova J, Vonner AJ, Armstrong KA, Borgermann J, Lai CS, Deng DY et al (2013) Constitutively active ALK2 receptor mutants require type II receptor cooperation. Mol Cell Biol 33(12):2413–2424
Shen Q, Little SC, Xu MQ, Haupt J, Ast C, Katagiri T et al (2009) The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J Clin Invest 119(11):3462–3472
CAS PubMed PubMed Central Google Scholar
Song GA, Kim HJ, Woo KM, Baek JH, Kim GS, Choi JY et al (2010) Molecular consequences of the ACVR1(R206H) mutation of fibrodysplasia ossificans progressiva. J Biol Chem 285(29):22542–22553
van Dinther M, Visser N, de Gorter DJ, Doorn J, Goumans MJ, de Boer J et al (2010) ALK2 R206H mutation linked to fibrodysplasia ossificans progressiva confers constitutive activity to the BMP type I receptor and sensitizes mesenchymal cells to BMP-induced osteoblast differentiation and bone formation. J Bone Min Res 25(6):1208–1215
Google Scholar
Hino K, Ikeya M, Horigome K, Matsumoto Y, Ebise H, Nishio M et al (2015) Neofunction of ACVR1 in fibrodysplasia ossificans progressiva. Proc Natl Acad Sci USA 112(50):15438–15443
Hatsell SJ, Idone V, Wolken DM, Huang L, Kim HJ, Wang L et al (2015) ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aac4358
Olsen OE, Wader KF, Hella H, Mylin AK, Turesson I, Nesthus I et al (2015) Activin A inhibits BMP-signaling by binding ACVR2A and ACVR2B. Cell Commun Signal 13:27
Article PubMed PubMed Central CAS Google Scholar
Upadhyay J, Xie L, Huang L, Das N, Stewart RC, Lyon MC et al (2017) The expansion of heterotopic bone in fibrodysplasia ossificans progressiva is activin A-dependent. J Bone Min Res. https://doi.org/10.1002/jbmr.3235
Alessi Wolken DM, Idone V, Hatsell SJ, Yu PB, Economides AN (2018) The obligatory role of Activin A in the formation of heterotopic bone in fibrodysplasia ossificans progressiva. Bone 109:210–217
Connor JM, Woodrow JC, Evans DAP (1982) Histocompatibility antigens in patients with ectopic ossification due to fibrodysplasia ossificans progressiva. Ann Rheum Dis 41(6):646–647
Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A (2011) Bone morphogenetic proteins: a critical review. Cell Signal 23(4):609–620
Sanchez-Duffhues G, Williams E, Goumans MJ, Heldin CH, Ten Dijke P (2020) Bone morphogenetic protein receptors: structure, function and targeting by selective small molecule kinase inhibitors. Bone 138:115472
Loomans HA, Andl CD (2016) Activin receptor-like kinases: a diverse family playing an important role in cancer. Am J Cancer Res 6(11):2431–2447
Lin S, Svoboda KK, Feng JQ, Jiang X (2016) The biological function of type I receptors of bone morphogenetic protein in bone. Bone Res 4:16005
Botello-Smith WM, Alsamarah A, Chatterjee P, Xie C, Lacroix JJ, Hao J et al (2017) Polymodal allosteric regulation of type 1 serine/threonine kinase receptors via a conserved electrostatic lock. PLoS Comput Biol 13(8):e1005711
Whyte MP, Wenkert D, Demertzis JL, DiCarlo EF, Westenberg E, Mumm S (2012) Fibrodysplasia ossificans progressiva: middle-age onset of heterotopic ossification from a unique missense mutation (c.974G>C, p.G325A) in ACVR1. J Bone Min Res 27(3):729–737
Article CAS Google Scholar
Bocciardi R, Bordo D, Di Duca M, Di Rocco M, Ravazzolo R (2009) Mutational analysis of the ACVR1 gene in Italian patients affected with fibrodysplasia ossificans progressiva: confirmations and advancements. Eur J Hum Genet 17(3):311–318
Petrie KA, Lee WH, Bullock AN, Pointon JJ, Smith R, Russell RG et al (2009) Novel mutations in ACVR1 result in atypical features in two fibrodysplasia ossificans progressiva patients. PLoS ONE 4(3):e5005
Cappato S, Traberg R, Gintautiene J, Zara F, Bocciardi RA (2021) case of fibrodysplasia ossificans progressiva associated with a novel variant of the ACVR1 gene. Mol Genet Genomic 9(10):e1774
CAS Google Scholar
Massague J (2012) TGF beta signalling in context. Nat Rev Mol Cell Biol 13(10):616–630
Wang H, Shore EM, Pignolo RJ, Kaplan FS (2018) Activin A amplifies dysregulated BMP signaling and induces chondro-osseous differentiation of primary connective tissue progenitor cells in patients with fibrodysplasia ossificans progressiva (FOP). Bone 109:218–224
Xie C, Jiang WJ, Lacroix JJ, Luo Y, Hao JJ (2020) Insight into molecular mechanism for activin A-induced bone morphogenetic protein signaling. Int J Mol Sci 21(18):6498
Article CAS PubMed Central Google Scholar
Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA et al (2008) Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol 4(1):33–41
Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR et al (2010) In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol 5(2):245–253
Luo Y, Alsamarah A, Zhang K, Hao J (2016) Development of new therapeutic agents for fibrodysplasia ossificans progressiva. Curr Mol Med 16(1):4–11
Cuny GD, Yu PB, Laha JK, Xing X, Liu JF, Lai CS et al (2008) Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg Med Chem Lett 18(15):4388–4392
Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P (2002) Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J 21(7):1743–1753
Hu-Lowe DD, Chen EH, Zhang LL, Watson KD, Mancuso P, Lappin P et al (2011) Targeting activin receptor-like kinase 1 inhibits angiogenesis and tumorigenesis through a mechanism of action complementary to anti-VEGF therapies. Can Res 71(4):1362–1373
Chida A, Shintani M, Nakayama T, Furutani Y, Hayama E, Inai K et al (2012) Missense mutations of the BMPR1B (ALK6) gene in childhood idiopathic pulmonary arterial hypertension. Circ J 76(7):1501–1804
Cunha SI, Pietras K (2011) ALK1 as an emerging target for antiangiogenic therapy of cancer. Blood 117(26):6999–7006
Engers DW, Frist AY, Lindsley CW, Hong CC, Hopkins CR (2013) Synthesis and structure-activity relationships of a novel and selective bone morphogenetic protein receptor (BMP) inhibitor derived from the pyrazolo[1.5-a] pyrimidine scaffold of Dorsomorphin: the discovery of ML347 as an ALK2 versus ALK3 selective MLPCN probe. Bioorganic Med Chem Lett 23(11):3248
Mohedas AH, Xing XC, Armstrong KA, Bullock AN, Cuny GD, Yu PB (2013) Development of an ALK2-biased BMP type I receptor kinase inhibitor. ACS Chem Biol 8(6):1291–1302
Ullrich T, Arista L, Weiler S, Teixeira-Fouchard S, Broennimann V, Stiefl N et al (2022) Discovery of a novel 2-aminopyrazine-3-carboxamide as a potent and selective inhibitor of activin receptor-like kinase-2 (ALK2) for the treatment of fibrodysplasia ossificans progressiva. Bioorg Med Chem Lett. https://doi.org/10.1016/j.bmcl.2022.128667
Williams E, Bagarova J, Kerr G, Xia DD, Place ES, Dey D et al (2021) Saracatinib is an efficacious clinical candidate for fibrodysplasia ossificans progressiva. JCI Insight. https://doi.org/10.1172/jci.insight.95042
Hennequin LF, Allen J, Breed J, Curwen J, Fennell M, Green TP et al (2006) N-(5-chloro-1,3-benzodioxol-4-yl)-7-[2-(4-methylpiperazin-1-yl)ethoxy]-5- (tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine, a novel, highly selective, orally available, dual-specific c-Src/Abl kinase inhibitor. J Med Chem 49(22):6465–6488
Connor JM (1996) Fibrodysplasia ossificans progressive: lessons from rare maladies. N Engl J Med 335(8):591–593
Saracatinib Trial TO Prevent FOP (2020). https://clinicaltrials.gov/ct2/show/NCT04307953 . Accessed 12 Dec 2021
Chen YY, Stubbs MC, Pusey M, Wen XM, Collins RJ, Kapilashrami K et al (2020) Characterization of INCB00928, a potent and selective ALK2 inhibitor for the treatment of anemia. Blood 136:52–52
To assess the efficacy, safety, and tolerability of INCB000928 in participants With Fibrodysplasia Ossificans Progressiva (2021). https://clinicaltrials.gov/ct2/show/NCT05090891 . Accessed 12 Dec 2021
Study of single-ascending doses of DS-6016a in healthy Japanese subjects (2021). https://clinicaltrials.gov/ct2/show/NCT04818398 . Accessed 12 Dec 2021
Blueprint medicines presents foundational preclinical data supporting the development of BLU-782, a highly selective ALK2 inhibitor, for the treatment of patients with fibrodysplasia ossificans progressive (2018)
Safety, tolerability, pharmacokinetics, and food effect of BLU-782 in healthy adults (2019). https://clinicaltrials.gov/ct2/show/NCT03858075 . Accessed 14 Dec 2021
Alison DFA, Riadh L, Michael P, Cori AS, Sara G, Faith S, Sean K, Gordon W, Mark H, Robert S, Rachel S, Morgan L, Pauplis R, Vivek K, Andy B, Timothy L (2019) A clinical update on BLU-782, an investigational ALK2 inhibitor in development for fibrodysplasia ossificans progressiva (FOP). https://www.blueprintmedicines.com/wp-content/uploads/2019/09/Blueprint-Medicines-ASBMR-2019-BLU-782-Poster1.pdf .
Vanhoutte F, Liang S, Ruddy M, Zhao A, Drewery T, Wang Y et al (2020) Pharmacokinetics and pharmacodynamics of garetosmab (Anti-activin A): results from a first-in-human phase 1 study. J Clin Pharmacol 60(11):1424–1431
A study to examine the safety, tolerability and effects on abnormal bone formation of REGN2477 in patients with fibrodysplasia ossificansprogressiva (2017). https://clinicaltrials.gov/ct2/show/NCT03188666 . Accessed 16 Dec 2021
Ries A, Schelch K, Falch D, Pany L, Hoda MA, Grusch M (2020) Activin A: an emerging target for improving cancer treatment? Expert Opin Ther Targets 24(10):985–996
Knight PG, Satchell L, Glister C (2012) Intra-ovarian roles of activins and inhibins. Mol Cell Endocrinol 359(1–2):53–65
Hedger MP, Winnall WR (2012) Regulation of activin and inhibin in the adult testis and the evidence for functional roles in spermatogenesis and immunoregulation. Mol Cell Endocrinol 359(1–2):30–42
Petrakou E, Fotopoulos S, Anagnostakou M, Anatolitou F, Samitas K, Semitekolou M et al (2013) Activin-A exerts a crucial anti-inflammatory role in neonatal infections. Pediatr Res 74(6):675–681
Mayer K, Buchbinder A, Morty RE (2012) Activin A: a mediator governing inflammation, immunity, and repair. Am J Respir Crit Care Med 185(4):350–352
Rodriguez-Martinez G, Molina-Hernandez A, Velasco I (2012) Activin A promotes neuronal differentiation of cerebrocortical neural progenitor cells. PLoS ONE 7(8):e43797
Lees-Shepard JB, Yamamoto M, Biswas AA, Stoessel SJ, Nicholas SE, Cogswell CA et al (2018) Activin-dependent signaling in fibro/adipogenic progenitors causes fibrodysplasia ossificans progressiva. Nat Commun 9(1):471
Hino K, Horigome K, Nishio M, Komura S, Nagata S, Zhao C et al (2017) Activin-A enhances mTOR signaling to promote aberrant chondrogenesis in fibrodysplasia ossificans progressiva. J Clin Invest 127(9):3339–3352
Kaplan FS, Zeitlin L, Dunn SP, Benor S, Hagin D, Al Mukaddam M et al (2018) Acute and chronic rapamycin use in patients with fibrodysplasia ossificans progressiva: a report of two cases. Bone 109:281–284
Pacifici M (2018) Retinoid roles and action in skeletal development and growth provide the rationale for an ongoing heterotopic ossification prevention trial. Bone 109:267–275
Underhill TM, Cash DE, Linney E (1994) Constitutively active retinoid receptors exhibit interfamily and intrafamily promoter specificity. Mol Endocrinol 8(3):274–285
CAS PubMed Google Scholar
Weston AD, Rosen V, Chandraratna RA, Underhill TM (2000) Regulation of skeletal progenitor differentiation by the BMP and retinoid signaling pathways. J Cell Biol 148(4):679–690
Weston AD, Hoffman LM, Underhill TM (2003) Revisiting the role of retinoid signaling in skeletal development. Birth Defects Res C Embryo Today 69(2):156–173
Shimono K, Tung WE, Macolino C, Chi AH, Didizian JH, Mundy C et al (2011) Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-gamma agonists. Nat Med 17(4):454–460
Chakkalakal SA, Uchibe K, Convente MR, Zhang D, Economides AN, Kaplan FS et al (2016) palovarotene inhibits heterotopic ossification and maintains limb mobility and growth in mice with the human ACVR1(R206H) fibrodysplasia ossificans progressiva (FOP) Mutation. J Bone Min Res 31(9):1666–1675
Kaplan FS, Hsiao EC, Baujat G, Keen R, Grogan DR, Pignolo RJ (2017) Efficacy and safety of palovarotene in fibrodysplasia ossificans progressiva (FOP): a randomized, placebo-controlled, double-blind study. J Bone Min Res 32:S114–S114
An efficacy and safety study of palovarotene for the treatment of fibrodysplasia ossificans progressiva (2017). https://clinicaltrials.gov/ct2/show/NCT03312634 . Accessed 15 Dec 2021
Download references
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Xinmiao Meng and Haotian Wang have contributed equally.
Authors and Affiliations
College of Arts and Sciences, Cornell University, Ithaca, NY, 14850, USA
Xinmiao Meng
College of Arts and Sciences, University of Pennsylvania, Philadelphia, PA, 191041, USA
Haotian Wang
College of Veterinary Medicine, Western University of Health Sciences, Pomona, CA, 91766, USA
You can also search for this author in PubMed Google Scholar
Contributions
XM and HW reviewed articles, prepared figures, and wrote original draft. JH supervised the review, organized the figures, wrote, and edited the manuscript. All authors contributed to the critical reading and writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Correspondence to Jijun Hao .
Ethics declarations
Conflict of interest.
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This is a review article, and no ethical approval is required.
Consent for publication
Its publication has been approved by all co-authors.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Meng, X., Wang, H. & Hao, J. Recent progress in drug development for fibrodysplasia ossificans progressiva. Mol Cell Biochem 477 , 2327–2334 (2022). https://doi.org/10.1007/s11010-022-04446-9
Download citation
Received : 13 January 2022
Accepted : 08 April 2022
Published : 10 May 2022
Issue Date : October 2022
DOI : https://doi.org/10.1007/s11010-022-04446-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Fibrodysplasia ossificans progressiva
- Heterotopic ossification
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 18 April 2022
Current challenges and opportunities in the care of patients with fibrodysplasia ossificans progressiva (FOP): an international, multi-stakeholder perspective
- Robert J. Pignolo ORCID: orcid.org/0000-0002-8533-9438 1 ,
- Christopher Bedford-Gay 2 , 3 ,
- Amanda Cali 4 , 5 , 6 ,
- Michelle Davis 2 ,
- Patricia L. R. Delai 7 ,
- Kristi Gonzales 2 ,
- Candace Hixson 2 ,
- Alastair Kent 8 ,
- Hope Newport 2 ,
- Manuel Robert 9 , 10 ,
- Christiaan Scott 11 &
- Frederick S. Kaplan 12
Orphanet Journal of Rare Diseases volume 17 , Article number: 168 ( 2022 ) Cite this article
4216 Accesses
6 Citations
8 Altmetric
Metrics details
Fibrodysplasia ossificans progressiva (FOP) is an ultra-rare, disabling genetic disorder characterized by congenital malformations of the great toes and progressive heterotopic ossification of soft and connective tissues. Assiduous attention to the unmet needs of this patient community is crucial to prevent potential iatrogenic harm and optimize care for individuals with FOP.
To gather international expert opinion and real-world experience on the key challenges for individuals with FOP and their families, highlight critical gaps in care, communication, and research, and provide recommendations for improvement.
An international group of expert clinicians, patients and patient advocates, caregivers and representatives from the international FOP community participated in a virtual, half-day meeting on 22 March 2021 to discuss the key unmet needs of individuals with FOP.
Individuals with FOP often face the frustration of long diagnostic journeys, the burden of self-advocacy and the navigation of novel care pathways. Globally, patients with FOP are also confronted with inequities in access to diagnosis and specialist care, and consequently, unequal access to registries, clinical trials, and essential support from patient associations. Organizations such as the International FOP Association, the International Clinical Council on FOP, and national FOP organizations work to provide information, facilitate access to expert clinical guidance, nurture patient empowerment, fund FOP research and/or foster meaningful collaborations with the research community. The non-profit Tin Soldiers Global FOP Patient Search program aims to identify and provide a pathway to diagnosis and care for individuals with FOP, particularly in underserved communities. Such global initiatives and the increasingly widespread use of telemedicine and digital platforms offer opportunities to improve vital access to care and research.
Conclusions
This multi-stakeholder perspective highlights some of the unmet needs of individuals with FOP and their families. Regional and international organizations play an important role in improving the quality of life of those they reach in the global FOP community. However, globally, fundamental issues remain around raising awareness of FOP among healthcare professionals, identifying individuals with FOP, reducing time to diagnosis, and ensuring access to best practice in care, support, and clinical research. Medical writing support was industry-sponsored.
Introduction
Fibrodysplasia ossificans progressiva (FOP; OMIM #135100) is an ultra-rare, disabling genetic disorder characterized by congenital malformations of the great toes and progressive heterotopic ossification (HO) of soft and connective tissues [ 1 ]. Approximately 97% of individuals with FOP carry the same spontaneous missense mutation in the ALK2/ACVR1 gene, involved in the bone morphogenetic protein (BMP) signaling pathway [ 2 , 3 ]. The estimated prevalence of FOP (diagnosed cases) has been reported as up to 1.43 per million individuals [ 4 ]; however, regional variability is high [ 4 , 5 ].
Episodes of soft tissue swelling, pain, reduced movement, stiffness and warmth, referred to as ‘flare‐ups’, often emerge in young children with FOP [ 6 ]. Although FOP flare‐ups can occur spontaneously, they are frequently triggered by intra-muscular injections, unnecessary biopsies, muscle fatigue, dental work, minor trauma or influenza‐like viral illnesses [ 7 ]. Flare-ups often lead to HO [ 8 ], but FOP progression can also occur in the absence of flare-ups [ 6 ]. HO is cumulative and permanent, leading to progressive disability and severe functional limitations in joint mobility. As individuals age, their need for assistance in performing activities of daily living increases and, by the third decade of life, most individuals with FOP are confined to a wheelchair [ 9 ]. Many clinical aspects of FOP resemble a premature aging phenotype [ 10 ].
Individuals with FOP have a median life expectancy of 56 years [ 11 ]. However, a wide variation in lifespan has been observed, with some patients reaching their seventies [ 11 ]. In underserved regions, Footnote 1 the effect of limited access to diagnosis and care on the life expectancy of individuals living with FOP remains unknown. Death is often a result of cardiorespiratory failure, due to thoracic insufficiency syndrome, or pneumonia [ 11 ]. There is no definitive medical treatment for FOP; current clinical management is supportive and aims to mitigate the risks that could cause further disease progression and complications [ 12 ]. Attempts to treat individuals with FOP by physicians who lack the necessary experience or knowledge of FOP can inadvertently result in flare-ups and/or irreversible disease progression.
Due to the rarity and disabling nature of FOP, patients and their caregivers require specialist, multi-disciplinary care, and support with many aspects of life. The FOP community faces numerous challenges including low awareness of FOP among healthcare professionals (HCPs) and policymakers, high rates of misdiagnosis worldwide [ 13 ], poor access to specialist FOP care, and limited support and information for individuals who are isolated from the global FOP community [ 4 ]. These factors are exacerbated in developed or developing regions where access to healthcare is limited due to resource constraints and/or competing healthcare challenges, such as high rates of communicable diseases. This includes highly populated regions such as Asia and Africa, where rates of FOP diagnosis are largely unknown but most likely very low. The recent increase in targeted clinical trials offers hope that an effective disease-modifying therapy, or therapies, may be identified [ 14 ]. However, such developments also present new medical and logistical challenges for individuals with FOP, and it is vital to implement provisions to ensure equitable access to care, support, and research. Identifying the most important challenges and raising awareness of these among policymakers is crucial for improving quality of life for individuals with FOP and their families worldwide.
A multi-stakeholder meeting was convened to give a voice to the FOP community, represented by an international group of clinical experts/researchers from the International Clinical Council on FOP (ICC) [ 15 ], patients, patient advocates, caregivers, and members of FOP organizations such as the International FOP Association (IFOPA) [ 16 ], FOP Friends [ 17 ], Tin Soldiers [ 18 ] and Fundación FOP [ 19 ]. Drawing on their collective expertise and/or lived experience, the multi-stakeholder group highlighted the main challenges for patients with FOP and their caregivers, priority areas for change, and opportunities to further improve the care of people living with FOP worldwide. This paper presents the outcomes of these discussions along with recommendations for the achievement of best practice and global equity in FOP care.
A targeted literature review was conducted to identify recent evidence on awareness of FOP, unmet needs and best practices in FOP care, and patient engagement with research. The information gathered was used to develop a proposed agenda of discussion topics for the multi-stakeholder meeting.
An international group of expert clinicians (representing the ICC), patient representatives/advocates, caregivers, and representatives from the IFOPA, Tin Soldiers, and other FOP national organizations, were invited to participate in a virtual, half-day meeting to provide a broad range of perspectives on the key unmet needs of individuals with FOP and their families. A project charter outlining the roles and responsibilities of all parties involved in the project was shared with prospective attendees ahead of the meeting, for full transparency. Consistent with best-practice reporting of patient involvement in research, we report patient involvement in this project using the standardized Guidance for Reporting Involvement of Patients and the Public (GRIPP) checklist (Additional file 1 : Table S1) [ 20 ].
A total of 12 participants attended the virtual meeting, held on 22 March 2021. The multi-stakeholder group included individuals from 5 countries (Argentina, Brazil, South Africa, United Kingdom and the United States of America). An expert in rare disease policy development and patient engagement was selected to chair the meeting. The format of the meeting was organized as a virtual roundtable discussion, with a participant introducing each discussion topic with a short talk, followed by open discussion. Topics were developed around pathways of care, patient involvement in research, and future opportunities made possible by advancements in technology. Discussions held both during the meeting and the development of the manuscript were analyzed thematically and resulted in the identification of key challenges and opportunities for improving the care of individuals with FOP worldwide (Fig. 1 ).
Overview of the multi-stakeholder meeting and key challenges and opportunities identified. A multi-stakeholder meeting was convened to give a voice to the FOP community, represented by an international group of clinical experts/researchers from the ICC, patients, patient advocates, and caregivers, representing FOP Friends, Tin Soldiers and Fundación FOP, and members of the IFOPA. Drawing on their collective expertise and/or lived experience, the multi-stakeholder group highlighted the main challenges for patients with FOP and their caregivers, priority areas for change, and opportunities to further improve the care of people living with FOP worldwide. FOP: fibrodysplasia ossificans progressiva; HCP: healthcare professional; ICC: International Clinical Council on FOP; IFOPA: International FOP Association
Pathways to care
Diagnostic journey for patients and families.
Delayed diagnosis is a common phenomenon across rare diseases [ 21 ]. Although time to diagnosis for individuals with FOP has decreased in recent years [ 13 , 22 ], patients and their caregivers still face a prolonged diagnostic journey. Data from the FOP Registry (as of December 2020) show that on average, individuals with FOP receive a correct diagnosis around 1.5 years following symptom onset, with over 50% of individuals initially misdiagnosed [ 13 , 22 ]. Common incorrect diagnoses include cancer (e.g. osteosarcoma), juvenile fibromatosis and non-hereditary myositis ossificans [ 13 , 23 ]. Prolonged diagnostic journeys and misdiagnosis may be more common for individuals with an atypical FOP mutation [ 22 ] and/or for those who live in an underserved region. As a result of misdiagnosis, it is common for patients with FOP to undergo unnecessary surgical procedures or medical interventions. Such interventions can exacerbate disease progression and contribute to disability [ 23 ]. Therefore, the timely and accurate diagnosis of FOP is crucial to prevent premature loss of mobility and preserve quality of life for individuals with FOP [ 23 ].
According to recent FOP Registry data, individuals with FOP receive a correct diagnosis after seeing an average of 3.3 HCPs [ 13 ]. These results highlight that there is a disconnect between the HCPs first consulted and those who are most likely to accurately diagnose patients [ 9 , 13 ]. Geneticists and orthopedic physicians were identified as the specialists most likely to provide a definitive diagnosis of FOP [ 9 , 13 , 22 ]. Although newborn screening for FOP has recently been proposed in Brazil, the implementation of these programs more widely is, in part, limited by country-specific differences in newborn screening policies. However, there are also important ethical considerations over screening for a disease with no approved treatment, concerns over the risk of misdiagnosis based on clinical observation in newborns, and regulatory/cost-effectiveness barriers related to genetic testing. Therefore, the routine screening of newborns to diagnose FOP, either clinically or genetically, is unlikely to become more widespread in the near future. Efforts should focus on raising awareness of the characteristic features of FOP among the HCPs who are most likely to see patients early in life or when they first present with symptoms.
Diagnosis of FOP can be made clinically. Individuals will often present in their first decade of life with great toe malformations and lumps/swellings on the scalp, head, neck and/or back [ 6 ]; FOP is the only condition with this combination of symptoms. Increased communication of this unique symptom combination among pediatricians and other HCPs may help to reduce the frequency of misdiagnosis and shorten the time to a correct diagnosis and care. Consideration of these symptoms in isolation, particularly the lumps and swellings characteristic of FOP, can lead to misdiagnosis and unnecessary medical procedures, potentially causing irreparable harm. The ability to provide a clinical diagnosis of FOP is particularly valuable for individuals living in regions where genetic testing is either not available or cost prohibitive. However, where available, genetic testing can also be beneficial, particularly for individuals with an atypical genetic mutation or for those who have great toe malformations without HO [ 24 ].
There has been a recent drive to optimize rare disease diagnosis through the use of web search, social media and medical data repositories in recognition that many parents, caregivers, and HCPs increasingly use these resources to search for a diagnosis [ 25 ]. Although such approaches can be helpful, it is important that individuals are steered towards trustworthy sources of information and advice. The IFOPA is currently optimizing search results to ensure that individuals who search for FOP-related symptoms, such as malformations of the great toes, are directed to the IFOPA website and the wide variety of resources provided (Table 1 ) [ 16 ]. Increasing the accessibility of reliable information available online for both patients and HCPs could help to reduce patient/caregiver anxiety and the time to diagnosis.
A recent study of the epidemiology of FOP, based on information from the patient registration databases of the IFOPA and 16 other regional/national FOP organizations, found that the apparent prevalence of individuals with FOP ranged from approximately 0.65 per million in North America to 0.05 and 0.04 per million in Africa and the Asia Pacific region, respectively [ 4 ]. The authors concluded that this variability is likely associated with limited awareness of FOP, delays in diagnosis, lack of supporting regional infrastructure, and poor access to a local FOP organization or the international FOP community in underserved areas [ 4 ]. To address global inequalities in access to diagnosis, the Tin Soldiers Global FOP Patient Search program aims to increase global awareness of FOP, to identify undiagnosed individuals and connect patients with the FOP community [ 18 ]. By employing a novel approach that used a mixed-media campaign and community-based programs, the Tin Soldiers initiative increased the number of known cases in Africa from 25 to 32 over a five-month period from December 2020 to April 2021 [ 26 ]. These newly diagnosed individuals were subsequently connected to national and international support structures, patient organizations and medical care. This positive outcome demonstrates the potential of a dedicated global patient identification program to increase rates of diagnosis and access to care pathways for individuals living in underserved regions.
Education for healthcare professionals
There is a need for more unified, global initiatives to educate HCPs on caring for patients with rare bone diseases. Many scientific societies are expanding the educational programming available at congresses and using special journal issues to raise awareness, highlight recent research, and present new perspectives on rare bone diseases [ 27 ]. To address the need to educate HCPs on caring for patients with FOP specifically, the ICC created the Global Health & Education Task Force with the following goals: (1) identify FOP global health providers; (2) develop a global database of treating FOP physicians, across all relevant specialties; (3) formulate a pathway for new ICC membership; and (4) develop education platforms for new members.
Many countries are also becoming increasingly aware of the importance of providing educational programs for HCPs to develop their expertise in rare diseases. These programs can be used to encourage collaboration and the sharing of information among HCPs (locally and globally) and also to foster communication between HCPs and patient organizations [ 28 ]. For example, the Rare Bone Disease TeleECHO, a monthly video teleconference, promotes active learning and peer-to-peer knowledge sharing of rare bone diseases for HCPs around the world [ 29 ]. Similarly, the Tin Soldiers Continuing Medical Education (CME) Master series provides virtual and international educational opportunities for HCPs to interact with and learn about FOP from their peers [ 30 ]. However, in areas of digital poverty other initiatives may be needed, such as enlisting the help of local faith-based health initiatives and/or humanitarian medical non-governmental organizations to reach physicians and nurses [ 18 ].
Although it may not be feasible to educate all HCPs on FOP-specific symptoms, simplifying the key messaging for clinicians around rare diseases may expedite the diagnosis process for all individuals with a rare disease. For example, a drive to raise awareness among clinicians that the presence of a bilateral physical malformation is possibly indicative of a genetic disease, may increase the likelihood of timely referral to a geneticist. Messaging specifically targeted to the pediatric healthcare community (e.g. pediatricians and pediatric nurses, nurse practitioners and physician assistants, and the neonatal care team) and/or the most common frontline HCPs in a given country (i.e. community pharmacists in Egypt, Jordan, Lebanon and Somalia [ 31 ]) is also needed to improve the likelihood of an individual receiving a correct diagnosis prior to receiving any unnecessary and/or potentially harmful medical procedures.
Care coordination
As FOP does not fall under one medical specialty, patients with FOP interact with a number of HCPs and specialists during the management of their care. A coordinated, multidisciplinary approach to providing care for patients with FOP is critical to ensure high-quality care and patient safety [ 24 , 32 ]. However, expertise in rare diseases is often concentrated in a limited number of medical centers, typically where clinicians have taken a special interest in the rare disease [ 21 ]. This is also the case for FOP, where the standard of care and access to specialists for individuals with FOP can vary considerably by geographic region.
With variable access to specialists, patients should have a primary physician who can coordinate a local care team and who is willing to consult with FOP experts [ 12 ] (Fig. 2 ). With the help of the patient community and national organizations, the ICC is actively recruiting and continually expanding its global network of FOP specialists through the Global Health & Education Task Force [ 15 ]. In addition, the Tin Soldiers formed the ‘African Clinicians Collective’ that includes ten African clinicians, nine of whom currently treat individuals with FOP [ 18 ]. Nevertheless, despite best efforts, it is not feasible for FOP experts to be involved in every care decision. To assist local HCPs with the day-to-day management of patients with FOP, the ICC maintains up-to-date FOP Treatment Guidelines, last updated in April 2021 [ 12 ]. These guidelines provide an important resource for HCPs, but are also invaluable for patients and/or their caregivers to advocate for themselves in a medical setting.

Care coordination pathway (current and future). The journey to diagnosis for patients with FOP can involve a number of different HCPs. The IFOPA and other national/global FOP organizations and initiatives also play an important role in the identification and referral of patients. Following diagnosis, patients with FOP should be supported by a primary physician who is willing to consult with FOP experts and can coordinate a local care team of specialists without expertise in FOP [ 12 ]. FOP experts from the ICC can provide guidance and education for the local physician as required. There are also a variety of CME opportunities available for all HCPs. As the global network of FOP specialists continues to expand, it is the long-term goal of the ICC to create multiple national/regional centers of FOP care. These centers would combine medical, surgical, anesthesia, physical and occupational therapy, and dental expertise in FOP in physical locations to improve care and minimize risks for patients. These centers could also function as key sites for clinical research. CME: Continuing Medical Education; FOP: fibrodysplasia ossificans progressiva; HCP: healthcare professional; ICC: International Clinical Council on FOP; IFOPA: International FOP Association
There is an increased awareness that specialized treatment, knowledge, and resources for the care management of rare and complex diseases need to be centralized. For example, European Reference Networks (ERNs) were launched in 2017 as virtual networks of HCPs across Europe to facilitate discussion and improve the management of rare diseases [ 33 ]. Specifically, the RarERN Path has been developed as a reference organizational model for care pathways that can be adapted to best fit the specific disease and geographical context [ 33 ]. The long-term goal of the ICC’s global network of FOP specialists is the development of national/regional centers of FOP care; combining medical, surgical, anesthesia, physical and occupational therapy, and dental expertise in FOP in physical locations to improve care and minimize risks for patients [ 24 ]. In addition to focusing on care, these centers could also function as key sites for clinical research. Many existing centers currently serve as clinical trial sites for ongoing interventional studies.
Transition from pediatric to adult care
The transition from pediatric to adult care, also known as transitional care, has been characterized as “the purposeful, planned movement of adolescents and young adults with chronic physical and medical conditions from child-centered to adult-oriented health care systems” [ 34 ]. For chronic health conditions, the transition from pediatric to adult care can be challenging for the patient and caregiver [ 35 ], and this is especially true for rare diseases [ 36 ]. For the patient, assuming responsibility for managing insurance, prescriptions, diet, dental care, and other key needs can be overwhelming, regardless of the specific underlying disease. The importance of a successful transition to adult care for health outcomes has been established [ 35 , 36 ]. Individuals who encounter difficulties navigating the transition to adult care can often experience disruptions in care that increase the likelihood of future medical complications, higher emergency department/hospital use, lower treatment adherence and reduced quality of life [ 37 ]. Similar to other rare diseases [ 36 ], individuals with FOP often experience a delayed and disrupted transition to adult care.
The transition from familiar physicians, family-centered systems, and multidisciplinary pediatric care to a less supportive and more fragmented adult healthcare system can be a particularly challenging time for patients and their caregivers [ 36 ]. One cause of anxiety for patients with a rare disease is that adult physicians often lack the necessary expertise and experience of treating patients with rare diseases, especially those that are first encountered in childhood [ 36 ]. There is often a related fear among patients and their caregivers that adult physicians may not consider or value the patient’s own expertise and lived experience [ 37 , 38 ]. As an ultra-rare disease that does not fall under one medical specialty, it is particularly challenging to manage adult care for individuals with FOP. It can be difficult to find a primary physician willing to assume responsibility for the coordination of a patient’s care into adulthood. Identifying an adult-care physician early on in the transition process and including them in care management discussions with the patient, caregivers and pediatrician can help to mitigate the anxiety experienced by patients and caregivers, and ensure the continuity of high-quality, specialized care [ 36 , 37 , 38 ].
Active involvement of the patient and caregivers in care decision-making has been shown to increase the likelihood of a successful transition to adult care [ 36 , 39 ]. To facilitate anticipatory care, and to account for variability in FOP disease progression, it is beneficial to start preparing an individual for the transition to adult care from an early age [ 37 ]. Although learning self-advocacy skills can be overwhelming at first, with the support of a guardian, a young patient can gradually gain experience and confidence in assuming an active role in their own care management. It may also be beneficial for individuals who share an FOP diagnosis to help support and mentor younger individuals and their families during the transition [ 40 ]. The goal should be to ensure connectivity and continuity of care, in an age- and disease progression-appropriate context. The IFOPA provides many resources and programs to help patients emotionally and practically negotiate this transition, including the Transition of Care webinars and the Advocacy Series and Resilient Living Program (Table 1 ). In addition, national FOP organizations play a key role in providing targeted support and guidance for families within local communities.
Patient empowerment and support
At present, patients or caregivers typically assume primary responsibility for informing and educating new clinicians, dentists, or other care providers about FOP and how to manage care for individuals with FOP. However, developing advocacy skills can be time-consuming and challenging, and not every patient or caregiver has the ability to act as an advocate. As the life expectancy for individuals with FOP increases, it may become difficult for aging caregivers to provide the level of physical care needed (e.g. lifting) over time. Therefore, it is important to educate and empower a patient’s wider support network, such as their adult siblings, and not just their primary caregiver.
The IFOPA and other national organizations specifically aim to educate and nurture advocacy skills to empower patients and their support network by providing many online resources for managing day-to-day and emergency care (Table 1 ; Additional file 1 : Table S2). An important aspect of managing day-to-day care, particularly for children with FOP, is within an educational setting. Therefore, organizations such as the IFOPA and FOP Friends have created resources in a child-friendly format to help raise awareness of FOP among educators and school-age children to ensure the safety of children with FOP among their peers (Table 1 ). In addition to physical care, providing emotional support is a fundamental but often overlooked and underprioritized aspect of care [ 24 ], and the IFOPA has created resources to address the social and mental health needs of the FOP community. This support can be particularly important for individuals who did not develop symptoms nor receive a diagnosis of FOP until their teenage years, after experiencing a relatively normal childhood.
Social media and mobile phone messaging apps are very important to the FOP community and serve as a platform to ask questions, share resources, feel connected to peers, and problem-solve in real time. In addition to providing practical information and guidance for parents of children with a rare disease, access to support groups and communication with other parents is an important social need [ 41 ]. Communities created through online and mobile phone platforms and supported by patient organizations can encourage a network of collaborative self-management among patients and caregivers that can enhance care and lead to better clinical outcomes [ 21 ]. Social media can also be an important tool for children with FOP to interact with their peers and mitigate isolation.
However, it is important to recognize the limitations of these tools. Social media can be difficult to navigate for individuals with underlying mental health issues, such as anxiety or depression. Furthermore, richer interactions may be required to offer optimal support. Although efforts have been made to expand reach across various digital/mobile phone platforms and languages, maintaining clear messaging across platforms can be challenging. Additional barriers can be caused by limited access to the internet and enabled devices in some parts of the world [ 42 ]. Nevertheless, these tools offer the opportunity to increase access and vital connectivity to regional or international FOP communities for individuals worldwide.
Patient involvement and access
The identification of the classic FOP gene mutation ( ALK2/ACVR1 R206H ) and the dysregulated BMP signaling pathway was a key milestone in the history of FOP [ 2 ]. The discovery identified druggable targets for treatments and fueled international interest in research and development (R&D) in FOP [ 2 , 14 ]. This recent drive in R&D has resulted in several promising treatments for FOP reaching clinical trial stage [ 43 ]. Patients played a pivotal role in the discovery of the FOP gene and continue to be instrumental in advancing FOP research by being actively involved in online surveys and participating in advisory panels for industry, clinical trials, non-interventional studies, the “Tooth Ferry” Program, and the IFOPA’s FOP Registry and FOP Biobank [ 44 , 45 , 46 ].
Patients are often keen to participate in clinical trials, but opportunities can be limited (particularly in the Global South). Pharmaceutical companies often rely on existing patient networks, created and maintained through a patient organization or specialist clinicians, to increase awareness of an upcoming trial and to identify participants. The lack of an established, local patient network can, therefore, be a major barrier to initiating a clinical trial in a certain geographical region. Other barriers to establishing clinical trials in underserved areas include cumbersome country-specific legal regulations that cause delays or roadblocks, a very low number of known diagnosed patients, lack of local supportive infrastructure for clinical trials and inadequate research training, and prioritization of local sites for trials of treatments for more prevalent infectious diseases.
As there is an increased drive to expand clinical trials into new regions and increase participation for underserved patient groups, there are important ethical concerns to be considered. It is crucial that the relevant risks and benefits of a clinical trial are clearly communicated, taking into account cultural context; the message should convey that clinical trials are experiments, not proven treatments [ 14 , 47 ]. There are also ethical considerations around access to post-trial care and/or treatments for participants following a clinical trial. This is especially important if the treatment provided during the trial resulted in a meaningful clinical benefit for the participant [ 48 ].
Guidance for clinical trials in FOP have recently been developed by the Clinical Trials Committee of the ICC [ 47 ]. These guidelines emphasize the responsibility of pharmaceutical companies to engage with patients and their families during all stages of clinical trial development. The patient perspective should be reflected in the practicalities of the trial (e.g. need for onsite visits, imaging, etc.), but also to provide insight into the FOP-specific safety considerations that are necessary to include in the trial design, and the assessment of measurable outcomes that are meaningful to patients [ 47 ]. Ensuring that the voice of the patient is heard and incorporated into research studies and clinical trial design is essential to maintaining a positive relationship between the FOP community and the pharmaceutical industry.
Maintaining patient-friendly communication with the FOP community throughout a clinical trial or research study is important, even when there is no new information to provide. The IFOPA provides educational resources to explain the drug development process and how clinical studies and trials are conducted to enable individuals to accurately interpret research results and understand how these findings may be relevant to themselves or their family members. Although there are many possible avenues to communicate research outcomes, some of the most accessible and well-known to the FOP community are the Annual Reports of the FOP Collaborative Research Project at the University of Pennsylvania [ 46 ], lay summaries provided by IFOPA for research projects that they fund, and the IFOPA’s FOP Family Gathering. In addition, the FOP Drug Development Forum organized by the IFOPA brings together academic researchers, clinical care specialists and patients to discuss and plan future research with the patient voice central to these discussions. However, there is a need for all stakeholders to go beyond the proactive communication of research to actively sharing non-proprietary research information with the FOP community to build knowledge and limit the duplication of research. As such, the IFOPA, in collaboration with the ICC, has developed guidelines for data-sharing specific to various research activities. This “Open Science” approach to data sharing and collaboration has been identified as particularly important within the rare disease community to reduce the time to diagnosis and treatment [ 49 ].
National patient organizations and networks also play an important role in communicating research updates and information to individuals within a given country or region (Additional file 1 : Table S2). For example, the national FOP organization for Argentina (Fundación FOP) connects the Spanish-speaking patients and families throughout Latin America with the international FOP community. However, there are still regions that are not directly supported by a patient organization, which can be very isolating for patients and their families [ 4 ].
To improve access to care, reliable information, and clinical studies and trials for all individuals with FOP, there have been several recent initiatives to increase regional representation and expand the reach of the international FOP community. For example, the FOP Registry, launched in 2015 by the IFOPA, is a centralized, international registry for patients with FOP that captures demographic data and has facilitated longitudinal studies of patient health and quality of life [ 50 ]. As of December 2020, the Registry had 323 enrolled patients from 69 countries, approximately 36% of the world’s known FOP population. In addition to providing valuable research information, it is hoped that the Registry will increase the known patient base for this ultra-rare disease and improve clinical care by increasing the speed of development of novel treatments [ 44 ]. It is important to note that language (the Registry is currently available in seven languages), educational level and/or limited access to the internet/enabled devices may be barriers to participation in the FOP Registry for some individuals. Addressing these issues that limit regional representation and ethnic diversity are crucial to ensure that patients, regardless of their location, receive appropriate care and treatment, access to clinical trials and up-to-date information and support.
Gaps in research
Despite many crucial advancements arising from FOP research from over 60 institutions worldwide, such as the discovery of the genetic mutation underlying FOP [ 2 ], there is still much to understand about disease progression. The natural course and co-morbidities of FOP in poorly resourced areas remain particularly understudied. Future research should also prioritize the identification of biomarkers that can be used to predict flare-up and HO status, and evaluate treatment efficacy. In addition, as FOP shares similarities with other inflammatory diseases, understanding the role of inflammation in the context of FOP could provide valuable insight and new avenues to treatments. Finally, although FOP is often categorized as a “new bone” disease, it is also a disease of the joints [ 51 , 52 ]. As such, further research on the joint manifestations of FOP is warranted.
Natural history studies of FOP are important to understand disease progression and to identify clinically meaningful outcome measures to assess in short-term (typically 1–2 years) clinical trials [ 6 ]. However, conducting these studies in ultra-rare diseases can be fraught with challenges, including small numbers of geographically-dispersed patients and a lack of established biomarkers, validated measurement tools, and clinically-meaningful disease progression endpoints. A recent 3-year, global natural history study of FOP (NCT02322255; sponsored by Ipsen) provided valuable information on the baseline, cross-sectional disease phenotypes of 114 individuals with FOP [ 52 , 53 , 54 ] and additional longitudinal data are forthcoming. These studies will be crucial to better understand the natural history of FOP and identify common secondary health issues. In addition, the increased use of FOP-specific assessments, such as the Cumulative Analogue Joint Involvement Scale (CAIJS), will help to better understand the disease progression of FOP [ 55 ]. An initial clinical staging system for FOP has also been proposed [ 56 ], which is currently being validated using data from a longitudinal natural history study on FOP and the FOP Registry. Further expansion of the FOP Registry will continue to yield valuable data on the longitudinal natural history of FOP for patients globally, and can also be used in the future to facilitate post-marketing surveillance of new therapies/treatments [ 50 ].
Future directions
Changes to ‘routine’ medical practice and the rise of telemedicine during the coronavirus disease (COVID-19) pandemic could provide a framework to improve access to care and clinical studies/trials for individuals with FOP around the world. Telemedicine can facilitate access to specialists for the management of care in FOP, particularly for patients who are not able to travel or have limited access to local medical specialists.
By necessity, remote medical assessments became the norm during the COVID-19 pandemic. As the feasibility and benefits of remote medical consultations are recognized and understood, this may lead to positive changes regarding how data are collected for patient registries, disease diagnosis, natural history studies and clinical trials, ultimately improving accessibility [ 49 ]. Wearable medical devices transmitting digital health data may also be a way to increase access to research and clinical trials for individuals [ 49 ]. For example, FOP-PROMPT is a patient-reported outcomes (PRO) tool that can be used to capture the signs, symptoms and impacts most important to people living with FOP via a mobile phone app [ 57 ]. It may be possible to incorporate this daily symptom tracker into future clinical trials to enhance the timespan of data collected. However, not all medical information can be collected remotely (e.g. whole-body computed-tomography scans) and the importance of in-person interaction between a patient and clinician cannot be minimized [ 49 ].
Although the COVID-19 pandemic has been an isolating time for patients and their families, the circumstances necessitated by the pandemic have forced a new level of comfort with video-conferencing and online webinars. The IFOPA has seen increased engagement with online meetings of the international FOP community, such as the annual FOP Family Gathering. This event is an opportunity for families and local HCPs to learn about the latest developments in treatments and care from FOP experts, and tips and strategies for disease management and daily living from other people living with FOP. Traditionally an in-person event, the necessitated virtual format increased accessibility to individuals who would have been unable to travel and participate in person. Digital platforms can provide an opportunity for individuals to connect with the international FOP community while efforts are ongoing to establish dedicated patient organizations in underserved countries and regions. The events organized by national and regional patient organizations, whether in person or virtual, are crucial to build the FOP community and provide support and information for individuals (Table 1 ) [ 4 ].
Although there are many opportunities for digital platforms and telemedicine to have a positive impact on the global FOP community in the future, it is also important to be aware of limitations posed by cost, digital poverty, and language barriers. For example, although the 2020 IFOPA Virtual FOP Family Gathering was translated into 17 languages, it was not possible to provide translation for every language due to the associated costs. Individuals also have differential access to the internet and data based on their location and means, including rural communities in developed countries [ 58 ]. To reach patients who do not have access to the internet, national telephone helplines can be used to reach a higher number of individuals in their local language at limited cost [ 59 ]. Similarly, it may be possible to make key information available on a mobile phone app that can be referred to during medical appointments.
As an ultra-rare, disabling disease with no definitive treatment at present, FOP poses significant challenges for patients and their caregivers. Individuals with FOP often face a long diagnostic journey and a constant need to advocate for themselves in new medical circumstances. Continued education of HCPs on the characteristics of FOP, particularly targeting those HCPs most likely to see a patient when they first develop symptoms, will help to reduce the rate of misdiagnosis and shorten the diagnostic journey for patients. Once a diagnosis is received, a local primary physician should be appointed to coordinate care between local non-specialists and FOP experts. Furthermore, as the transition from pediatric to adult care can be challenging for individuals and often result in disruptions to care, it is important to identify a primary adult physician early to encourage knowledge-sharing with the pediatrician and ensure a positive transition process.
With ongoing and anticipated clinical trials, there is hope that an effective disease-modifying therapy, or therapies, will be identified. However, there are safety and ethical concerns to consider during clinical trial development that relate to the unique needs of the FOP community, post-trial care and/or access to new treatments. The patient perspective should be reflected at all stages of clinical trial development to ensure that FOP-specific safety considerations are addressed and that the measurable outcomes are meaningful to patients. Communications on FOP research should be frequent and conveyed in plain language that is easily translatable, to ensure the timely sharing of information globally and reduce the spread of misinformation or misunderstanding due to poor translation. Although additional research is needed to fully understand the disease progression of FOP, the sharing of non-proprietary research information between stakeholders will help to further build knowledge and limit duplication.
In many regions of the world, individuals have poor access to diagnosis and expert care, face barriers to participating in registries and trials, and may be isolated from the wider FOP community. Innovations in telemedicine and increased communication via digital platforms have the potential to improve accessibility of care and information worldwide. It is critical to ensure that all individuals with FOP are afforded equitable access to care and treatment. Along with the continuing efforts from the IFOPA, the ICC, national FOP organizations, the Tin Soldiers Global FOP Patient Search, and Industry, increasing awareness of the unmet needs facing the FOP community among key audiences will be vital to address these challenges and improve the care of patients globally.
Availability of data and materials
Not applicable.
In the context of this paper, the term ‘underserved’ is used to indicate any community, whether in a developed or developing region, that faces inequities in access to information, diagnosis, specialist care and/or support from patient organizations.
Kaplan FS, Tabas JA, Gannon FH, Finkel G, Hahn GV, Zasloff MA. The histopathology of fibrodysplasia ossificans progressiva. An endochondral process. J Bone Joint Surg Am. 1993;75(2):220–30.
Article CAS PubMed Google Scholar
Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38(5):525–7.
Zhang W, Zhang K, Song L, Pang J, Ma H, Shore EM, et al. The phenotype and genotype of fibrodysplasia ossificans progressiva in China: a report of 72 cases. Bone. 2013;57(2):386–91.
Article PubMed PubMed Central Google Scholar
Liljesthröm M, Pignolo R, Kaplan F. Epidemiology of the global fibrodysplasia ossificans progressiva (FOP) community. J Rare Dis Res Treat. 2020;5(2):31–6.
Article Google Scholar
Baujat G, Choquet R, Bouée S, Jeanbat V, Courouve L, Ruel A, et al. Prevalence of fibrodysplasia ossificans progressiva (FOP) in France: an estimate based on a record linkage of two national databases. Orphanet J Rare Dis. 2017;12(1):123.
Pignolo RJ, Bedford-Gay C, Liljesthröm M, Durbin-Johnson BP, Shore EM, Rocke DM, et al. The natural history of flare-ups in fibrodysplasia ossificans progressiva (FOP): a comprehensive global assessment. J Bone Miner Res. 2016;31(3):650–6.
Kaplan FS, Pignolo RJ, Al Mukaddam M, Shore EM. Genetic Disorders of Heterotopic Ossification: Fibrodysplasia Ossificans Progressiva and Progressive Osseous Heteroplasia. In: Bilezikian J, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 9th ed. Washington DC: The American Society for Bone and Mineral Research; 2019. p. 865–70.
Google Scholar
Pignolo RJ, Shore EM, Kaplan FS. Fibrodysplasia ossificans progressiva: clinical and genetic aspects. Orphanet J Rare Dis. 2011;6(1):80.
Pignolo RJ, Cheung K, Kile S, Fitzpatrick MA, De Cunto C, Al Mukaddam M, et al. Self-reported baseline phenotypes from the International Fibrodysplasia Ossificans Progressiva (FOP) Association Global Registry. Bone. 2020;134:115274.
Article PubMed Google Scholar
Pignolo RJ, Wang H, Kaplan FS. Fibrodysplasia ossificans progressiva (FOP): a segmental progeroid syndrome. Front Endocrinol. 2020;10(908).
Kaplan FS, Zasloff MA, Kitterman JA, Shore EM, Hong CC, Rocke DM. Early mortality and cardiorespiratory failure in patients with fibrodysplasia ossificans progressiva. J Bone Joint Surg Am. 2010;92(3):686–91.
Kaplan FS, Al Mukaddam M, Baujat G, Brown M, Cali A, Cho T-J, et al. The medical management of fibrodysplasia ossificans progressiva: current treatment considerations. Proc Int Clin Council FOP. 2021;2:1–127.
Sherman LA, Cheung K, De Cunto C, Kile S, Pignolo RJ, Kaplan FS. The diagnostic journey in fibrodysplasia ossificans progressiva: insights from the FOP registry. Annual Meeting of the American Society for Bone and Mineral Research. 2020.
Kaplan F, Al Mukaddam M, Baujat G, Cali A. The Twilight Zone: Benefit, Risk & Hope in Clinical Trials for Fibrodysplasia Ossificans Progressiva (FOP). The ICC. 2020.
International Clinical Council on Fibrodysplasia Ossificans Progressiva. https://www.iccfop.org/ . Accessed 29 April 2021.
International Fibrodysplasia Ossificans Progressiva Association. https://www.ifopa.org/ . Accessed 29 April 2021.
FOP Friends. https://fopfriends.com/ . Accessed 19 May 2021.
Tin Soldiers Global FOP Patient Search. https://tinsoldiers.org/ . Accessed 19 May 2021.
Fundación FOP. http://fundacionfop.org.ar/ . Accessed 14 July 2021.
Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ. 2017;358:j3453.
Article CAS PubMed PubMed Central Google Scholar
Stoller JK. The challenge of rare diseases. Chest. 2018;153(6):1309–14.
International Fibrodysplasia Ossificans Progressiva Association (IFOPA). 2020 FOP Registry Annual Report. 2021. https://www.ifopa.org/2020_fop_registry_annual_report . Accessed 18 May 2021.
Kitterman JA, Kantanie S, Rocke DM, Kaplan FS. Iatrogenic harm caused by diagnostic errors in fibrodysplasia ossificans progressiva. Pediatrics. 2005;116(5):e654.
Di Rocco M, Baujat G, Bertamino M, Brown M, De Cunto CL, Delai PLR, et al. International physician survey on management of FOP: a modified Delphi study. Orphanet J Rare Dis. 2017;12(1):110.
Svenstrup D, Jørgensen HL, Winther O. Rare disease diagnosis: a review of web search, social media and large-scale data-mining approaches. Rare Diseases. 2015;3(1):e1083145.
Scott C, Kaplan F, Friedman C, Delai P, Al Mukaddam M, Cali A, et al. While looking for one, you may find another: Tin Soldiers and the search for undiagnosed individuals with fibrodysplasia ossificans progressiva (FOP). Pediatr Rheumatol. 2021:19(Suppl 1):O6.
Shore EM, Pacifici M. JBMRPlus: Special Issue on Rare Bone Diseases 2019. JBMR plus. 2019;3(8):e10218.
Dharssi S, Wong-Rieger D, Harold M, Terry S. Review of 11 national policies for rare diseases in the context of key patient needs. Orphanet J Rare Dis. 2017;12(1):63.
Tosi LL, Rajah EN, Stewart MH, Gillies AP, Hart TS, Lewiecki EM. The rare bone disease TeleECHO program: leveraging telehealth to improve rare bone disease care. Curr Osteoporos Rep. 2020;18:344–9.
Tin Soldiers Fibrodysplasia Ossificans Progressiva (FOP) Global CME Master Series. https://tinsoldiers.org/master_series/ . Accessed 19 May 2021.
El Bizri L, Jarrar LG, Ali WKA, Omar AH. The role of community pharmacists in increasing access and use of self-care interventions for sexual and reproductive health in the Eastern Mediterranean Region: examples from Egypt, Jordan, Lebanon and Somalia. Health Res Pol Syst. 2021;19(1):49.
Kilmartin E, Grunwald Z, Kaplan FS, Nussbaum BL. General anesthesia for dental procedures in patients with fibrodysplasia ossificans progressiva: a review of 42 cases in 30 patients. Anesth Analg. 2014;118(2):298–301.
Talarico R, Cannizzo S, Lorenzoni V, Marinello D, Palla I, Pirri S, et al. RarERN Path: a methodology towards the optimisation of patients’ care pathways in rare and complex diseases developed within the European Reference Networks. Research Square; 2020.
Blum RWM, Garell D, Hodgman CH, Jorissen TW, Okinow NA, Orr DP, et al. Transition from child-centered to adult health-care systems for adolescents with chronic conditions: a position paper of the Society for Adolescent Medicine. J Adolesc Health. 1993;14(7):570–6.
Schraeder K, Dimitropoulos G, McBrien K, Li JY, Samuel S. Perspectives from primary health care providers on their roles for supporting adolescents and young adults transitioning from pediatric services. BMC Fam Pract. 2020;21(1):140.
Stepien KM, Kieć-Wilk B, Lampe C, Tangeraas T, Cefalo G, Belmatoug N, et al. Challenges in transition from childhood to adulthood care in rare metabolic diseases: results from the first multi-center European survey. Front Med (Lausanne). 2021;8:652358.
White PH, Cooley WC. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2018;142(5):e20182587.
Rutishauser C, Akré C, Surìs JC. Transition from pediatric to adult health care: expectations of adolescents with chronic disorders and their parents. Eur J Pediatr. 2011;170(7):865–71.
Coyne I, Sheehan A, Heery E, While AE. Healthcare transition for adolescents and young adults with long-term conditions: qualitative study of patients, parents and healthcare professionals’ experiences. J Clin Nurs. 2019;28(21–22):4062–76.
Doyle M. Peer support and mentorship in a US rare disease community: findings from the cystinosis in emerging adulthood study. Patient. 2015;8(1):65–73.
Pelentsov LJ, Laws TA, Esterman AJ. The supportive care needs of parents caring for a child with a rare disease: a scoping review. Disabil Health J. 2015;8(4):475–91.
Hootsuite & We Are Social. Digital 2021 April Global Statshot Report. 2021. https://blog.hootsuite.com/simon-kemp-social-media/ . Accessed 17 June 2021.
Pignolo RJ, Kaplan FS. Druggable targets, clinical trial design and proposed pharmacological management in fibrodysplasia ossificans progressiva. Expert Opin Orphan Drugs. 2020;8(4):101–9.
FOP Registry. Available from: https://www.ifopa.org/fopregistry . Accessed 5 May 2021.
IFOPA FOP Biobank. Available from: https://www.ifopa.org/biobank . Accessed 5 May 2021.
Kaplan FS, Al Mukaddam MM, Shore EM. 27th Annual Report of the Fibrodysplasia Ossificans Progressiva (FOP) Collaborative Research Project. Perelman School of Medicine at the University of Pennsylvania; 2019.
Hsiao EC, Di Rocco M, Cali A, Zasloff M, Al Mukaddam M, Pignolo RJ, et al. Special considerations for clinical trials in fibrodysplasia ossificans progressiva (FOP). Br J Clin Pharmacol. 2019;85(6):1199–207.
Cho HL, Danis M, Grady C. Post-trial responsibilities beyond post-trial access. Lancet. 2018;391(10129):1478–9.
Rubinstein YR, Robinson PN, Gahl WA, Avillach P, Baynam G, Cederroth H, et al. The case for open science: rare diseases. JAMIA Open. 2020;3(3):472–86.
Mantick N, Bachman E, Baujat G, Brown M, Collins O, De Cunto C, et al. The FOP Connection Registry: design of an international patient-sponsored registry for fibrodysplasia ossificans progressiva. Bone. 2018;109:285–90.
Towler OW, Peck SH, Kaplan FS, Shore EM. Dysregulated BMP signaling through ACVR1 impairs digit joint development in fibrodysplasia ossificans progressiva (FOP). Dev Biol. 2021;470:136–46.
Towler OW, Shore EM, Kaplan FS. Skeletal malformations and developmental arthropathy in individuals who have fibrodysplasia ossificans progressiva. Bone. 2020;130:115116.
Pignolo RJ, Baujat G, Brown MA, De Cunto C, Di Rocco M, Hsiao EC, et al. Natural history of fibrodysplasia ossificans progressiva: cross-sectional analysis of annotated baseline phenotypes. Orphanet J Rare Dis. 2019;14(1):98.
Kou S, De Cunto C, Baujat G, Wentworth KL, Grogan DR, Brown MA, et al. Patients with ACVR1R206H mutations have an increased prevalence of cardiac conduction abnormalities on electrocardiogram in a natural history study of Fibrodysplasia Ossificans Progressiva. Orphanet J Rare Dis. 2020;15(1):193.
Kaplan FS, Al Mukaddam M, Pignolo RJ. A cumulative analogue joint involvement scale (CAJIS) for fibrodysplasia ossificans progressiva (FOP). Bone. 2017;101:123–8.
Pignolo RJ, Kaplan FS. Clinical staging of fibrodysplasia ossificans progressiva (FOP). Bone. 2018;109:111–4.
International Fibrodysplasia Ossificans Progressiva Association (IFOPA). FOP-PROMPT Development Study Underway. https://www.ifopa.org/fop_prompt_development_study_underway . Accessed 13 July 2021.
Crowe AL, McKnight AJ, McAneney H. Communication needs for individuals with rare diseases within and around the healthcare system of Northern Ireland. Front Public Health. 2019;7:236.
Mazzucato M, Houyez F, Facchin P. The importance of helplines in National Plans. Orphanet J Rare Dis. 2014;9(1):O12.
Article PubMed Central Google Scholar
International Clinical Council on Fibrodysplasia Ossificans Progressiva (ICC). Coronavirus (COVID-19) Precautions for FOP Patients & Families. 2020. http://www.iccfop.org/dvlp/wp-content/uploads/2020/12/Coronavirus_Precautions_for_FOP_Families_123020.pdf . Accessed 19 May 2021.
International Clinical Council on Fibrodysplasia Ossificans Progressiva (ICC). Coronavirus (COVID-19) Vaccine recommendations. 2021. http://www.iccfop.org/dvlp/wp-content/uploads/2021/03/Coronavirus-vaccination-FOP-031821-1.pdf . Accessed 19 May 2021.
Download references
Acknowledgements
The authors thank Odette Schwegler, Executive Director of Tin Soldiers Global FOP Patient Search, for her valuable review of the manuscript. The authors acknowledge Debbie Nixon, DPhil, from Costello Medical, UK for publication coordination and Marielle Brown, PhD, and Arianna Psichas, PhD, from Costello Medical, UK, for medical writing and editorial assistance based on the authors’ input and direction. Medical writing was funded by Ipsen.
This project was initiated by Ipsen. Ipsen provided funding to Costello Medical to independently organize the roundtable, invite participants and identify a chair with expertise in rare disease policy development. Ipsen had no influence on the planning and execution of the roundtable or the development of the resulting manuscript, other than a courtesy review of the final draft for accuracy. The authors did not receive honoraria for their participation in the roundtable or subsequent manuscript development. Medical writing assistance for this article, provided by Marielle Brown, PhD, and Arianna Psichas, PhD, Costello Medical, UK, was funded by Ipsen in accordance with Good Publication Practice (GPP3) guidelines ( http://www.ismpp.org/gpp3 ). Frederick S. Kaplan’s contribution was supported in part by The Isaac & Rose Nassau Professorship of Orthopaedic Molecular Medicine.
Author information
Authors and affiliations.
Department of Medicine, Mayo Clinic, Rochester, MN, USA
Robert J. Pignolo
International FOP Association, Kansas City, MO, USA
Christopher Bedford-Gay, Michelle Davis, Kristi Gonzales, Candace Hixson & Hope Newport
FOP Friends, Manchester, UK
Christopher Bedford-Gay
The Radiant Hope Foundation, Mountain Lakes, NJ, USA
Amanda Cali
The Ian Cali FOP Research Fund, PENN Medicine, Center for Research in FOP & Related Disorders, Philadelphia, PA, USA
Tin Soldiers: Global Patient Identification Program, Johannesburg, South Africa
Hospital Israelita Albert Einstein, Instituto de Ensino E Pesquisa, Sao Paolo, Brazil
Patricia L. R. Delai
Independent Patient Advocacy Consultant, Downham Market, UK
Alastair Kent
Fundación FOP, Buenos Aires, Argentina
Manuel Robert
Patient Author, Buenos Aires, Argentina
Red Cross Children’s Hospital, University of Cape Town, Cape Town, South Africa
Christiaan Scott
Departments of Orthopaedic Surgery and Medicine, The Center for Research in FOP and Related Disorders, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
Frederick S. Kaplan
You can also search for this author in PubMed Google Scholar
Contributions
Substantial contributions to study conception and design: RJP, CBG, AC, MD, PLRD, KG, CH, AK, HN, MR, CS, FSK; substantial contributions to analysis and interpretation of the data: RJP, CBG, AC, MD, PLRD, KG, CH, AK, HN, MR, CS, FSK; drafting the article or revising it critically for important intellectual content: RJP, CBG, AC, MD, PLRD, KG, CH, AK, HN, MR, CS, FSK; final approval of the version of the article to be published: RJP, CBG, AC, MD, PLRD, KG, CH, AK, HN, MR, CS, FSK. All authors read and approved the final manuscript.

Corresponding authors
Correspondence to Robert J. Pignolo or Frederick S. Kaplan .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
RJP: Research investigator: Clementia/Ipsen, Regeneron; Advisory board: President of the International Clinical Council (ICC) on FOP; CBG: IFOPA Board Member, IFOPA Executive Committee Member, FOP Friends Chair and Trustee, International President’s Council Chair; AC: Trustee of the Radiant Hope Foundation, Trustee of the Ian Cali FOP Research Fund/Penn Medicine, Co-founder and Advisory Board member of the Tin Soldiers Patient Identification Program, Executive Producer of the Tin Soldiers documentary, Past IFOPA Chairman of the Board, Executive Associate of the International Clinical Council (ICC) on FOP; MD: Member of the Rare Bone Disease Alliance Steering Committee and the Global Genes RARE Global Advocacy Leadership Council; PLRD: Research investigator: Clementia/Ipsen; Member of the International Clinical Council on FOP; MR: Fundación FOP Board Member, IFOPA member; CS: Vice Chairman of the Board, Board member of Tin Soldiers Patient Identification Program, Member of the International Clinical Council (ICC) on FOP, Member of the IFOPA Medical Advisory Board, Tin Soldiers Board Member, Member of the International President’s Council; KG, CH, AK, HN: No competing interests to declare; FSK: Research investigator: Clementia/Ipsen, Regeneron; Advisory Board: IFOPA Medical Advisory Board; Founder and Past-President of the International Clinical Council (ICC) on FOP; Chair of the Publications Committee of the ICC. In April 2019, Ipsen acquired Clementia Pharmaceuticals.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: table s1..
GRIPP2 patient and public involvement. Table S2. National FOP patient organizations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Pignolo, R.J., Bedford-Gay, C., Cali, A. et al. Current challenges and opportunities in the care of patients with fibrodysplasia ossificans progressiva (FOP): an international, multi-stakeholder perspective. Orphanet J Rare Dis 17 , 168 (2022). https://doi.org/10.1186/s13023-022-02224-w
Download citation
Received : 05 August 2021
Accepted : 06 February 2022
Published : 18 April 2022
DOI : https://doi.org/10.1186/s13023-022-02224-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Fibrodysplasia ossificans progressiva
- Unmet needs
- Patient care
- Health policy
- Rare disease
Orphanet Journal of Rare Diseases
ISSN: 1750-1172
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

- Rare Disease News
- Resource Library
- Rare Disease Facts and Statistics
- NORD’s Rare Disease Database
- Rare Disease Video Library
- What It Means To Be Undiagnosed
- Find A Rare Disease Organization
- Stories That Inspire
- RareEdu ® – Online Learning Platform
- Rare Disease Day
- State Resource Center – Find Local Resources
- Publications On Rare Disease
- Getting Help & Support
- Managing Your Disease
- How NORD Can Help
- Speak To Someone at NORD
- Rare Disease Center Of Excellence
- Patient Assistance Programs
- Explore Clinical Trials
- Find A Patient Organization
- Caregiver Resources
- State Resource Center – Discover Local Resources
- Rare Diseases Defined
- Financial & Medical Assistance
- Call Center & Information Services
- Bringing Together Your Community
- NORD Member List
- Start a Rare Disease Organization
- Membership Program
- Becoming Research Ready
- Launching Registries & Natural History Studies
- Patient-Focused Drug Development
- Rare Disease Centers of Excellence
- Continuing Medical Education (CME)
- Corporate Council
- National Partnerships
- Global Parnerships
- Diversity, Equity & Inclusion
- List of Rare Diseases
- Gene Therapy for Rare Disease
- Find Clinical Trials & Research Studies
- Request for Proposals
- Research Grant Programs
- Data Standards for Rare Diseases
- Resources for Patients
- Find a Rare Disease Care Center
- IAMRARE ® Program Powered by NORD
- Rare Disease Cures Accelerator (RDCA-DAP)
- Add Your Expertise
- Today’s Policy Issues
- NORD’s Policy Statements
- Rare Disease Advisory Councils
- NORD State Report Card
- Join the Rare Action Network®
- Policy & Advocacy Taskforce
- Contact your Representative
- Take Action on Key Issues
- Learn about our current policy goals
- Do-It-Yourself NORD Fundraiser
- Students for Rare
- Sports & Fitness Fundraisers
- Media Inquiries
- Attend An Upcoming Event
- Find a Rare Disease Patient Organization
- Stay Informed With NORD’s Email Newsletter
- Rare Disease Day®
- Share Your Story
- Careers At NORD
- Intern At NORD
- Jobs At Patient Disease Organizations
- Donate to NORD
- Volunteer with NORD
- Visit the NORD Store
- For Clinicians & Researchers
- For Patient Organizations
Rare Disease Database
Disease overview, signs & symptoms.
- Affected Populations
Disorders with Similar Symptoms
Standard therapies, clinical trials and studies, programs & resources.
- Complete Report

Fibrodysplasia Ossificans Progressiva
Last updated: 06/26/24 Years published: 1987, 1990, 1996, 2002, 2006, 2008, 2011, 2014, 2017, 2020, 2024
Acknowledgment
NORD gratefully acknowledges Frederick S. Kaplan, MD, Isaac & Rose Nassau Professor of Orthopaedic Molecular Medicine; Chief, Division of Orthopaedic Molecular Medicine and Co-Director, Center for Research in FOP & Related Disorders, The Perelman School of Medicine at The University of Pennsylvania, and Eileen M. Shore, PhD, Cali-Weldon Professor of Orthopaedic Molecular Medicine and Genetics and Co-Director of The Center for Research in FOP & Related Disorders, The Perelman School of Medicine at The University of Pennsylvania, for assistance in the preparation of this report.
Fibrodysplasia ossificans progressiva (FOP) is an extremely rare genetic connective tissue disorder characterized by the abnormal development of bone in areas of the body where bone is not normally present (heterotopic ossification), such as the ligaments, tendons and skeletal muscles. Specifically, this disorder causes the body’s skeletal muscles and soft connective tissues to undergo a metamorphosis, essentially a transformation into bone, progressively locking joints in place and making movement difficult or impossible. Patients with FOP have malformed big toes that are present at birth (congenital). Other skeletal malformations may occur. The abnormal episodic development of bone at multiple soft tissue sites leads to stiffness in affected areas, limited movement and eventual fusion (ankylosis) of affected joints (neck, back, shoulders, elbows, hips knees, wrists, ankles, jaw – often in that order).
Episodic flare-ups (inflammatory soft tissue swellings) of FOP usually begin during early childhood and progress throughout life. Most cases of FOP occur as the result of a sporadic new variant in the ACVR1 gene in the bone morphogenetic protein (BMP) signaling pathway, which is important during the formation of the skeleton in the embryo and the repair of the skeleton following birth.
- Print / Download as PDF
- Next section >
- < Previous section
- myositis ossificans progressiva
All individuals with classic FOP have malformations of the great toes and, in approximately 50% of patients, the thumbs. These changes in the skeleton are present at birth (congenital) and are the first clinical signs of this disorder. The most common skeletal malformation associated with FOP is a shortened great toe with a malformed distal first metatarsal and a missing or abnormal first phalanx and/or interphalangeal joint. Other malformations of the toes and fingers may include inward turning of the great toe toward the other toes (hallux valgus), abnormally short fingers and toes (microdactyly) and/or permanent fixation of the fifth finger in a bent position (clinodactyly). Other congenital signs of FOP include proximal medial tibial osteochondromas, malformation of the upper part of the spinal column (cervical vertebrae) and an abnormally short broad neck of the bone in the thigh that extends from the knee to the pelvis (femur).
Progressive endochondral bone formation in connective tissues (heterotopic ossification) usually occurs during early childhood and progresses throughout life. Abnormal development of bone may occur spontaneously but often occurs following an episode of soft tissue injury or a viral illness. The first sign of heterotopic ossification is the appearance of firm tender swellings (often referred to as flare-ups) on certain parts of the body, especially the back, neck and/or shoulders. These soft tissue swellings mature through a cartilage-to-bone (endochondral) pathway to form mature heterotopic bone. The ectopic bone growth usually involves tendons, ligaments, skeletal muscle tissue and connective tissues such as fascia and aponeuroses. In many patients, pain and stiffness occurs in these areas. On some occasions, a low-grade fever may herald the development of these swellings. Although the swellings eventually regress, they usually harden into mature bone as they decrease in size.
In the affected areas, bone slowly replaces connective tissue. The neck, back, chest, arms and legs are usually the first areas affected. The disease eventually affects the hips, ankles, wrists, elbows, shoulders and/or jaw as well as the abdominal wall. In some affected individuals, the progression of bone development may be rapid; in others, the process may be gradual. Even among identical twins, the disease progression may vary greatly, reflecting different environmental impacts such as traumatic episodes.
Chronic swelling in various parts of the body is a common physical characteristic of individuals with FOP. Swelling may occur coordinately with the abnormal bone formation that characterizes FOP, or it may occur when recently formed bone presses on lymphatic vessels, obstructing the flow of tissue fluid. In addition, swelling may also be caused by a lack of pumping action within the hardened (ossified) muscle and can cause blood and tissue fluids to pool in a limb (e.g., arms and/or legs).
Abnormal development of bone eventually leads to stiffness and limited movement of affected joints. If the jaw is involved, the person may have trouble eating and/or speaking. In addition, abnormal development of bone may lead to progressive deformity of the spine including side-to-side (scoliosis) and, in some people, front-to-back curvature of the spine (kyphosis). As is the case for skeletal bone, the bone that develops in abnormal areas may fracture and then undergo fracture repair. As the disease progresses, individuals with FOP experience increasingly limited mobility that causes problems with balance, difficulty walking and/or sitting and/or severely restricted movement.
FOP may eventually result in complete immobilization. Affected individuals may have progressive pain and stiffness in affected areas, complete fusion of the spine and/or pain in affected areas of the body caused by abnormal bony growths that compress the nerves in these areas (entrapment neuropathies). As mobility begins to deteriorate, affected individuals may have an increased susceptibility to respiratory infection or right sided congestive heart failure due to thoracic insufficiency. Hearing impairment is seen in approximately 50% of affected individuals. Some people with more severe forms of FOP may have hair loss or mild cognitive delay.
Most cases of FOP occur sporadically, and there is a single affected individual within a family. When a familial pattern has been identified, FOP is inherited in an autosomal dominant pattern.
Dominant genetic disorders occur when only a single copy of a disease-causing gene variant is necessary to cause the disease. The gene variant can be inherited from either parent or can be the result of a new ( de novo ) changed gene in the affected individual that is not inherited. The risk of passing the gene variant from an affected parent to a child is 50% for each pregnancy. The risk is the same for males and females.
In 2006, an international team of researchers led by Eileen M. Shore, PhD and Frederick Kaplan MD at the University of Pennsylvania, published results of research identifying the genetic cause of FOP. The team found that FOP is caused by a change (variant) in the ACVR1 gene in the bone morphogenetic protein (BMP) signaling pathway.
Bone morphogenetic proteins are regulatory proteins important in embryonic skeletal formation and in post-natal repair of the skeleton. The ACVR1 encodes a BMP receptor called activin receptor type IA, or ACVR1, one of four known BMP type I receptors. BMP receptors, located at the cell surface, help determine the fate of the stem cells in which they are expressed by transmitting signals into the cell. The classic clinical FOP presentation is caused by the specific substitution of a particular amino acid (arginine, at position 206) in the ACVR1 protein for another amino acid (histidine). This amino acid substitution induces activation of signaling by the ACVR1 receptor.
Affected populations
FOP is a very rare inherited connective tissue disorder that was first identified in the 18th century. The prevalence of FOP is estimated to be 1/1,000,000. FOP affects males and females equally, and people from all ethnicities.
Symptoms of the following disorders may be similar to those of fibrodysplasia ossificans progressiva. Comparisons may be useful for differential diagnosis:
Aggressive juvenile fibromatosis is a condition in which cells called fibroblasts increase in number in tendons, ligaments and other connective tissues. This overgrowth of cells may invade adjacent tissues, causing pain and disability. The resulting lesions may resemble the tissue swelling associated with FOP. However, individuals with aggressive juvenile fibromatosis do not have the toe malformation that is associated with FOP, nor do they develop heterotopic ossification.
Progressive osseous heteroplasia (POH) is an extremely rare inherited disorder characterized by the abnormal development of bone in areas of the body where bone is not normally present (heterotopic ossification). Unlike FOP, the initial bone growth may develop on the surface of the skin (osseous plaques). These areas may become progressively widespread and come together (coalesce) to form even larger areas of hardened and thickened skin (dermal ossification). POH spreads to deeper levels of the skin and to various muscle, fatty and connective tissues of the body. As the disorder continues to progress, the abnormal development of bone may lead to stiffening and limited movement of affected joints. In severe cases, affected joints may become permanently fixed (ankylosed). In addition, arms and legs may become malformed and not grow to full length. POH is caused by a variant in the GNAS gene. GNAS variants have been identified in approximately 70% of affected individuals. POH is a distinct developmental disorder and belongs to a spectrum of clinical conditions that have the common feature of ossification of the skin (osteoma cutis). Although skeletal malformations have been noted, individuals with POH do not have the toe malformation that is characteristic of FOP. (For more information on this disorder, choose “Progressive Osseous Heteroplasia” as your search term in the Rare Disease Database.)
Misdiagnosis of FOP is common but can be avoided by examining the individual’s toes for the characteristic feature, short great toes. The diagnosis may be confirmed by a thorough clinical evaluation, characteristic physical findings, and sequencing of the ACVR1 gene.
Treatment Biopsies should be avoided when FOP is suspected because these tests may result in rapid bone formation in those areas where tissue is removed. Intramuscular injections (e.g., immunizations) must be avoided. Injections of local anesthetics and stretching of the jaw for dental therapy should be avoided. People with FOP should avoid any situations, such as falls, that may cause blunt trauma, since trauma may cause abnormal bone development. Various viral illnesses including influenza and influenza-like illnesses may provoke flare-ups of the condition.
In 2023, palovarotene (Sohonos), a retinoic acid receptor γ (RARγ) agonist, was approved by the U.S. Food and Drug Administration (FDA) as the first treatment for FOP to reduce extra-skeletal bone formation in adults and children aged 8 years and older for females, and 10 years and older for males.
Preventative (prophylactic) antibiotic therapy may be appropriate to prevent infection in affected individuals with an increased susceptibility to respiratory infections due to progressive mobility impairment.
Certain types of drugs have been used to relieve pain and swelling associated with FOP during acute flare-ups (most notably corticosteroids) and non-steroidal anti-inflammatory medication between flare-ups.
Affected individuals may benefit from occupational therapy. Special shoes, braces and other devices that assist in walking and weight-bearing have been used to help people with FOP. An occupational therapist can help obtain special devices or tools to assist in daily activities.
A team approach for infants diagnosed with FOP is also recommended and may include special social, educational and medical services.
Genetic counseling is recommended for individuals with FOP and their families. Other treatment is symptomatic and supportive.
Information on current clinical trials is posted on the Internet at www.clinicaltrials.gov . All studies receiving U.S. government funding, and some supported by private industry, are posted on this government website.
For information about clinical trials being conducted at the National Institutes of Health (NIH) Clinical Center in Bethesda, MD, contact the NIH Patient Recruitment Office:
Tollfree: (800) 411-1222 TTY: (866) 411-1010 Email: [email protected]
Some current clinical trials also are posted on the following page on the NORD website: https://rarediseases.org/living-with-a-rare-disease/find-clinical-trials/
For information about clinical trials sponsored by private sources, contact: www.centerwatch.com
For information about clinical trials conducted in Europe, contact: https://www.clinicaltrialsregister.eu/
Contacts for additional information about fibrodysplasia ossificans progressiva:
For basic research questions:
Eileen M. Shore, PhD Professor, Departments of Orthopaedic Surgery and Genetics Perelman School of Medicine University of Pennsylvania 309A Stemmler Hall 3450 Hamilton Walk Philadelphia, PA 19104-6081 phone: 215-898-2330 fax: 215-573-2133 email: [email protected]
For clinical questions:
Frederick S. Kaplan, M.D. Isaac & Rose Nassau Professor of Orthopaedic Molecular Medicine Chief, Division of Orthopaedic Molecular Medicine Perelman School of Medicine The University of Pennsylvania c/o Department of Orthopaedic Surgery Penn Musculoskeletal Center – Suite 600 3737 Market Street Philadelphia, PA 19104 Tel: 215-294-9145 Fax: 215-222-8854 Email: [email protected]
REVIEW ARTICLES
Pignolo RJ and Kaplan FS. Druggable targets, clinical trial design and proposed pharmacological management in fibrodysplasia ossificans progressiva. Expert Opinion on Orphan Drugs 2020; 8:4, 101-109.
Shore EM, Kaplan FS. Role of altered signal transduction in heterotopic ossification and fibrodysplasia ossificans progressiva. Curr Osteoporosis Rep. 2011;9: 83-88.
Shore EM, Kaplan FS. Inherited human diseases of heterotopic bone formation. Nat Rev Rheumatol. 2010;6: 518-527.
Zimmer C. The mystery of the second skeleton. Atlantic Monthly. 2013;311(5): 72-82.
JOURNAL ARTICLES
Adegbite NS, Xu M, Kaplan FS, Shore EM, Pignolo RJ. Diagnostic and mutational spectrum of progressive osseous heteroplasia (POH) and other forms of GNAS-based heterotopic ossification. Am J Med Genet A. 2008;146A:1788-1796.
Chakkalakal SA, Zhang D, Culbert AL, Convente MR, Caron RJ, Wright AC, Maidment AD, Kaplan FS, Shore EM. An Acvr1 Knock-in mouse has fibrodysplasia ossificans progressiva. J Bone Miner Res. 2012; 27:1746-1756.
Convente MR, Chakkalakal SA, Yang E, Caron RJ, Zhang D, Kambayashi T, Kaplan FS, Shore EM. Depletion of mast cells and macrophages impairs heterotopic ossification in an ACVR1 (R206H) mouse model of fibrodysplasia ossificans progressiva. J Bone Miner Res. 2018; 33: 269-282.
Deirmengian GK, Hebela NM, O’Connell M, Glaser DL, Shore EM, Kaplan FS. Proximal tibial osteochondromas in patients with fibrodysplasia ossificans progressiva. J Bone Joint Surg Am. 2008;90:366-374.
Di Rocco M, Forleo-Neto E, Pignolo RJ, et al. Garetosmab in fibrodysplasia ossificans progressiva: a randomized, double-blind, placebo-controlled phase 2 trial. Nat Med. 2023;29(10):2615-2624. doi:10.1038/s41591-023-02561-8
Gupta RR, Delai PLR, Glaser DL, Rocke DM, Al Mukaddam M, Pignolo RJ, Kaplan FS. Prevalence and risk factors for kidney stones in fibrodysplasia ossificans progressiva. Bone 2018;109: 120-123.
Hatsell SJ, Idone V, Wolken DM, et al. ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci Transl Med. 2015;7(303):303ra137. doi:10.1126/scitranslmed.aac4358
Hino K, Ikeya M, Horigome K, Matsumoto Y, Ebise H, Nishio M, Sekiguchi K, Shibata M, Nagata S, Matsuda S, Toguchida J. Neofunction of ACVR1 in fibrodysplasia ossificans progressiva. PNAS 2015; doi/10.1073/pnas.1510540112.
Hsiao EC, DiRocco M, Cali A. Zasloff M, Al Mukaddam M, Pignolo R, Grunwald Z, Netelenbos C, Keen R, Baujat G, Brown MA, Cho T-J De Cunto C, Delai P, Haga N, Morhart R, Scott C, Zhang K, Diecidue RJ, Friedman CS, Kaplan FS, Eekhoff EMW. Special considerations for clinical trials in fibrodysplasia ossificans progressiva. Br J Clin Pharmacol 2019; 85(6): 1199-1207.
Kaplan FS, Al Mukaddam M, Pignolo RJ. Longitudinal patient-reported mobility assessment in fibrodysplasia ossificans progressiva (FOP). Bone 2018;109:150-161.
Kaplan FS, Glaser DL, Shore EM, Pignolo RJ, Xu M, Zhang Y, Senitzer D, Forman SJ, Emerson SG. Hematopoietic stem cell contribution to ectopic skeletogenesis. J Bone Joint Surg Am. 2007;89:347-357.
Kaplan FS, Xu M, Seemann P, et al. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat. 2009;30(3):379-390. doi:10.1002/humu.20868
Kaplan FS, Xu M, Glaser DL Collins F, Connor M, Kitterman J, Sillence D, Zackai E, Ravitsky V, Zasloff M, Ganguly A, Shore EM. Early diagnosis of fibrodysplasia ossificans progressiva. Pediatrics. 2008;121:e1295-e1300.
Kaplan FS, Zasloff MA, Kitterman JA, Shore EM, Hong CC, Rocke DM. Early mortality and cardiorespiratory failure in patients with fibrodysplasia ossificans progressiva. J Bone Joint Surg Am. 2010;92:686-691.
Kitterman JA, Kantanie S, Rocke DM, Kaplan FS. Iatrogenic harm caused by diagnostic errors in fibrodysplasia ossificans progressiva. Pediatrics. 2005;116:e654-61.
Lees-Shepard JB, Yamamoto M, Biswas AA, Stoessel SJ, Nicholas SE, Cogswell CA, Devarakonda PM, Schneider MJ Jr, Cummins SM, Legendre NP, Yamamoto S, Kaartinen V, Hunter JW, Goldhamer DJ. Activin-dependent signaling in fibro/adipogenic progenitors causes fibrodysplasia ossificans progressiva. Nat Commun. 2018 Feb 2;9(1):471. doi: 10.1038/s41467-018-02872-2.
Lindborg CM, Al Mukaddam M, Baujat G, Cho TJ, DeCunto CL, Delai PLR, Eekhoff EMW, Haga N, Hsiao EC, Morhart R, de Ruiter R, Scott C, Seemann P, Szczepanek M, Tabarkiewicz J, Pignolo RJ, Kaplan FS. Most fractures treated non-operatively in individuals with fibrodysplasia ossificans progressiva heal with a paucity of flareups, heterotopic ossification, and loss of mobility. Clin Orthop Relat Res. 2023; 481: 2447-2458.
Lounev V, Groppe JC, Brewer N, Wentworth KL, Smith V, Xu M, Schomburg L, Bhargava P, Al Mukaddam M, Hsiao EC, Shore EM, Pignolo RJ, Kaplan FS. MMP-9 deficiency confers resilience in Fibrodysplasia Ossificans Progressiva in a man and mice. J Bone Miner Res. 2024; 39(4): 382-398.
Pignolo R.J., Baujat G, Brown MA, DeCunto C, DiRocco M, Hsiao EC, Keen R, Al Mukaddam M, LeQuan Sang K-H, Wilson A, White B, Grogan DR, Kaplan FS. Natural history of fibrodysplasia ossificans progressiva: cross-sectional analysis of annotated baseline phenotypes. Orphanet J. Rare Diseases 2019; 14: 98. https://doi.org/10.1186/s13023-019-1068-7
Pignolo RJ, Baujat G, Brown MA, De Cunto C, Hsiao EC, Keen R, Al Mukaddam M, Le Quan Sang K-H, Wilson A, Marino R, Strahs A, Kaplan FS. The natural history of fibrodysplasia ossificans progressiva: A prospective, global 36-month study. Genetics in Medicine. https://doi.org/10.1016/j.gim. 2022.08.013
Pignolo RJ, Durbin-Johnson BP, Rocke DM, Kaplan FS. Joint -specific risk of impaired function in fibrodysplasia ossificans progressiva (FOP). Bone 2018; 109:124-133.
Pignolo RJ, Bedford-Gay C Liljesthröm M Durbin-Johnson BP, Shore EM , Rocke DM , Kaplan FS. The natural history of flare-ups in fibrodysplasia ossificans progressiva (FOP): a comprehensive global assessment. J Bone Miner Res. 2016;31(3):650-6.
Pignolo RJ, Hsiao EC, Al Mukaddam M, Baujat G, Berglund SK, Brown MA, Cheung AM, DeCunto C, Delai P, Haga N, Kannu P, Keen R, Le Quan Sang, Mancilla EE, Marino R, Strahs A, Kaplan FS. Reduction of new heterotopic ossification (HO) in the open-label phase 3 MOVE trial of palovarotene for fibrodysplasia ossificans progressiva (FOP). J Bone Miner Res. 2023; 38(3): 381-394.
Shen Q, Little SC, Xu M, Haupt J, Ast C, Katagiri T, Mundlos S, Seemann P. Kaplan FS, Mullins MC, Shore EM. The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J Clin Invest. 2009;119(11):3462-3472.
Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho T-J, Choi IH, Connor JM, Delai P, Glaser DL, Le Merrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nature Genetics. 2006;38:525-527.
Towler OW, Shore EM, Kaplan FS. Skeletal malformations and developmental arthropathy in individuals who have fibrodysplasia ossificans progressiva. Bone 2020 Jan;130:115116. doi: 10.1016/j.bone.2019.115116. Epub 2019 Oct 23.
Wang H, Lindborg C, Lounev V, Kim J, McCarrick-Walmsley R, Xu M, Mangiavini L, Groppe J, Shore E, Schipani E, Kaplan F, Pignolo R. Cellular Hypoxia Promotes Heterotopic Ossification by Amplifying BMP Signaling. J Bone Miner Res. 2016; 31(9):1652-65.
Wang H, Shore EM, Pignolo RJ, Kaplan FS. Activin A amplifies dysregulated BMP signaling and induced chondro-osseous differentiation of primary connective tissue progenitor cells in patients with fibrodysplasia ossificans progressiva (FOP). Bone 2018;109: 218-224.
INTERNET Kaplan FS, Al Mukaddam M, Baujat G, Brown M, Cali A, Cho T-J, Crowe C, DeCunto C, Delai P, Diecidue, R, Di Rocco M, Eekhoff EMW, Friedman C, Grunwald Z, Haga N. Hsiao E, Keen R, Kitterman J, Levy C, Morhart R, Netelenbos C, Scott C, Shore EM, Zasloff M, Zhang K, Pignolo RJ. Proc Intl. Clin. Council The medical management of fibrodysplasia ossificans progressiva: current treatment considerations. FOP 2021 ; 1:1-128. https://www.iccfop.org/dvlp/wp-content/uploads/2021/08/guidelines-aug-2021.pdf Accessed May 16, 2024.
Fibrodysplasia Ossificans Progressiva; FOP. Online Mendelian Inheritance in Man (OMIM). Entry No:135100. Last Updated 11/07/2022. Available at: http://omim.org/entry/135100 Accessed May 16, 2024.
Akesson LS, Savarirayan R. Fibrodysplasia Ossificans Progressiva. 2020 Jun 11 [Updated 2023 May 11]. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK558090/ Accessed May 16, 2024.
Orphanet,the European database for rare diseases and contains a unique, multi-lingual nomenclature of rare diseases, along with several relevant resources.
Online Mendelian Inheritance in Man (OMIM) is a compendium of human genes and genetic phenotypes that is freely available, containing information on all known mendelian disorders and over 16,000 genes. Because OMIM is designed to be used primarily by physicians and other health professionals, although it is open to the public, the information is complex and users seeking information about a personal medical or genetic condition are advised to consult with a qualified physician for diagnosis and for answers to personal questions.
The information provided on this page is for informational purposes only. The National Organization for Rare Disorders (NORD) does not endorse the information presented. The content has been gathered in partnership with the MONDO Ontology. Please consult with a healthcare professional for medical advice and treatment.
- Assistance Programs
Patient Organizations
More information, rarecare ® assistance programs.
NORD strives to open new assistance programs as funding allows. If we don’t have a program for you now, please continue to check back with us.
Additional Assistance Programs
Medicalert assistance program.
NORD and MedicAlert Foundation have teamed up on a new program to provide protection to rare disease patients in emergency situations.
Rare Disease Educational Support Program
Ensuring that patients and caregivers are armed with the tools they need to live their best lives while managing their rare condition is a vital part of NORD’s mission.
Rare Caregiver Respite Program
This first-of-its-kind assistance program is designed for caregivers of a child or adult diagnosed with a rare disorder.
International FOP Association (IFOPA)
Nih/national institute of arthritis and musculoskeletal and skin diseases, progressive osseous heteroplasia association.
The information provided on this page is for informational purposes only. The National Organization for Rare Disorders (NORD) does not endorse the information presented. The content has been gathered in partnership with the MONDO Disease Ontology. Please consult with a healthcare professional for medical advice and treatment.
GARD Disease Summary
The Genetic and Rare Diseases Information Center (GARD) has information and resources for patients, caregivers, and families that may be helpful before and after diagnosis of this condition. GARD is a program of the National Center for Advancing Translational Sciences (NCATS), part of the National Institutes of Health (NIH).
Orphanet has a summary about this condition that may include information on the diagnosis, care, and treatment as well as other resources. Some of the information and resources are available in languages other than English. The summary may include medical terms, so we encourage you to share and discuss this information with your doctor. Orphanet is the French National Institute for Health and Medical Research and the Health Programme of the European Union.
Online Mendelian Inheritance In Man (OMIM) has a summary of published research about this condition and includes references from the medical literature. The summary contains medical and scientific terms, so we encourage you to share and discuss this information with your doctor. OMIM is authored and edited at the McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University School of Medicine.
- For Patients & Caregivers
- For Organizations

Every donation matters.
Your support helps to ensure everyone’s free access to NORD’s rare disease reports.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Fibrodysplasia ossificans progressiva
Frederick s. kaplan.
Departments of Orthopedic Surgery & Medicine, The University of Pennsylvania School of Medicine, c/o Hospital of The University of Pennsylvania, Philadelphia, PA, USA
Martine Le Merrer
U781 INSERM, Hopital Necker-Enfants Malades, Paris, France
David L. Glaser
Department of Orthopedic Surgery, The University of Pennsylvania School of Medicine, c/o Hospital of the University of Pennsylvania, Philadelphia, PA, USA
Robert J. Pignolo
Department of Medicine, The University of Pennsylvania School of Medicine, Philadelphia, PA, USA
Robert Goldsby
Department of Pediatrics and Cardiovascular Research Institute, The University of California San Francisco, San Francisco, CA, USA
Joseph A. Kitterman
Department of Biochemistry, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA
Eileen M. Shore
Departments of Orthopedic Surgery and Genetics, The University of Pennsylvania School of Medicine, Philadelphia, PA, USA
Fibrodysplasia ossificans progressiva (FOP), a rare and disabling genetic condition of congenital skeletal malformations and progressive heterotopic ossification (HO), is the most catastrophic disorder of HO in humans. Episodic disease flare-ups are precipitated by soft tissue injury, and immobility is cumulative. Recently, a recurrent mutation in activin receptor IA/activin-like kinase 2 (ACVR1/ALK2), a bone morphogenetic protein (BMP) type I receptor, was reported in all sporadic and familial cases of classic FOP, making this one of the most highly specific disease-causing mutations in the human genome. The discovery of the FOP gene establishes a critical milestone in understanding FOP, reveals a highly conserved target for drug development in the TGF-β/BMP signalling pathway, and compels therapeutic approaches for the development of small molecule signal transduction inhibitors for ACVR1/ALK2. Present management involves early diagnosis, assiduous avoidance of iatrogenic harm, and symptomatic amelioration of painful flare-ups. Effective therapies for FOP, and possibly for other common conditions of HO, may potentially be based on future interventions that block ACVR1/ALK2 signalling.
Fibrodysplasia ossificans progressiva (FOP), a rare and catastrophic genetic disorder of progressive heterotopic ossification (HO), is the most disabling condition of extraskeletal ossification known in humans. FOP causes immobility through progressive metamorphosis of skeletal muscle and soft connective tissue into a second skeleton of heterotopic bone. At the present time, there is no effective treatment. 1 – 6
HISTORICAL DESCRIPTIONS OF FOP
Possible cases of FOP date back to antiquity. FOP, known by many names throughout history, was first described in detail more than 250 years ago by a London physician. In a letter to The Royal Society of Medicine, dated 14 April 1736 (published in 1740), John Freke of Saint Bartholomew’s Hospital, London wrote: ‘There came a boy of healthy look, and about 14 years of age, to ask of us at the hospital, what should be done to cure him of many large swellings on his back, which began about 3 years since, and have continued to grow as large on many parts as a penny loaf particularly on the left side. They arise from all the vertebrae of the neck and reach down to the os sacrum; they likewise arise from every rib of his body, and joining together in all parts of his back, as the ramifications of coral do, they make as it were, a fixed bony pair of bodice’. 7
Nearly 200 years later in 1918, Jules Rosenstirn from Mount Zion Hospital in San Francisco, USA wrote: ‘One does not wonder that a disease, so baffling in its course from the first causes to its ultimate state, should invite the speculative as well as the patiently investigating observer to lift the obscuring veil and solve this embarrassing puzzle’. 8
FOP was, until recently, one of medicine’s most elusive mysteries. To patients who suffer from FOP, it is a painful metamorphosis into progressive immobility and a lifelong obstacle to physical freedom. While definitive treatments and cures are not yet available, the goals of FOP research are well articulated: to establish the genetic, molecular and cellular basis of FOP; and to use that knowledge to establish effective prevention, treatment and eventually cure.
CLASSIC CLINICAL FEATURES OF FOP
Two clinical features define classic FOP: malformation of the great toes; and progressive HO in specific spatial patterns ( Figure 1 ). Individuals with FOP appear normal at birth except for the characteristic malformations of the great toes which are present in all classically affected individuals. 9 During the first decade of life, children with FOP develop painful and highly inflammatory soft tissue swellings (or flare-ups) that transform soft connective tissues, including aponeuroses, fascia, ligaments, tendons and skeletal muscles, into an armament-like encasement of bone. 10 , 11 Ribbons, sheets and plates of heterotopic bone replace skeletal muscles and connective tissues through a process of endochondral ossification that leads to permanent immobility. 12 – 15 Minor trauma such as intramuscular immunizations, mandibular blocks for dental work, muscle fatigue and blunt muscle trauma from bumps, bruises, falls or influenza-like illnesses can trigger painful new flare-ups of FOP leading to progressive HO. 16 – 20 Surgical attempts to remove heterotopic bone commonly lead to episodes of explosive and painful new bone growth. 1 , 3 , 5 , 6 HO in FOP progresses in characteristic anatomical and temporal patterns that mimic the patterns of normal embryonic skeletal formation. FOP involvement is typically seen first in the dorsal, axial, cranial and proximal regions of the body and later in the ventral, appendicular, caudal and distal regions. 1 , 3 , 5 , 10 , 11 Several skeletal muscles including the diaphragm, tongue and extra-ocular muscles are enigmatically spared from FOP. Cardiac muscle and smooth muscle are not involved in the FOP process. 1 , 3 , 5

Characteristic clinical features of fibrodysplasia ossificans progressive (FOP). (a) Extensive heterotopic bone formation typical of FOP is seen by three-dimensional reconstructed computed tomography scan of the back of a 12-year-old child. (b) Anteroposterior radiograph of the feet of a 3-year-old child shows symmetrical great toe malformations. Source: Shore et al. Nature Genet 2006; 38 : 525–527. Copyright held by the authors.
The clinical features of early lesional involvement in the axial regions are often different from those seen in the appendicular regions. 21 Axial lesions may appear very rapidly, more rapidly than almost any neoplasm. In the axial regions, swelling is often mistaken for tumours, as large bulbous lesions may appear on the neck and back, whereas in the limbs, the swelling is often diffuse and may be mistaken for acute thrombophlebitis; a complication that can occur in patients with FOP due to generalized immobility and associated venous stasis. 21 The qualitative differences in swelling in the axial versus the appendicular regions in patients with FOP may reflect regional differences in the anatomy of the subaponeurotic spaces, as well as differences in the anatomy of the fascial compartments.
Bone formation in FOP is episodic, but disability is cumulative. Most patients with FOP are confined to a wheelchair by the third decade of life, and require lifelong assistance in performing activities of daily living. 1 – 6 Severe weight loss may result following ankylosis of the jaw, and pneumonia or right-sided heart failure may complicate rigid fixation of the chest wall. 22 The severe disability of FOP results in low reproductive fitness, and fewer than 10 multigenerational families are known worldwide. 23 The median age of survival is approximately 45 years, and death often results from complications of thoracic insufficiency syndrome (TIS). 22
MISDIAGNOSIS OF FOP
FOP is commonly misdiagnosed, as clinicians often fail to associate the rapidly developing soft tissue swellings that appear on the head, neck and upper back with the malformed great toes. 24 The correct diagnosis of FOP can be made clinically even before radiographic evidence of HO is seen if rapidly waxing and waning soft tissue lesions are associated with symmetrical malformations of the great toes. When such associations are not made, FOP is commonly misdiagnosed as aggressive juvenile fibromatosis (extra-abdominal desmoid tumours), lymphoedema or soft tissue sarcomas. Children often undergo unnecessary and harmful diagnostic biopsies that exacerbate progression of the condition. 24 This can be particularly dangerous at any anatomical site, but especially so in the neck or back where asymmetric HO can lead to rapidly progressive spinal deformity and exacerbation of TIS.
CERVICAL SPINE ANOMALIES IN FOP
In addition to malformations of great toes and thumbs, early developmental anomalies are frequently observed in the cervical spine. 25 Stiffness of the neck is an early finding in most patients and can precede the appearance of HO at that site. Characteristic anomalies of the cervical spine include large posterior elements, tall narrow vertebral bodies, and fusion of the facet joints between C2 and C7; findings that are strikingly similar to those seen in mice with homozygous deletions of the gene encoding noggin, a secreted bone morphogenetic protein antagonist. 25
OTHER SKELETAL ANOMALIES IN FOP
Other skeletal anomalies often associated with FOP include short malformed thumbs, clinodactyly, short broad femoral neck and proximal medial tibial osteochondromas. The latter two findings are reminiscent of patients who have multiple hereditary exostoses, although the genes associated with multiple hereditary exostoses are not mutated in patients who have FOP. Nevertheless, these shared clinical findings may illuminate common pathway anomalies. 1 – 3 , 5
THE TEMPOROMANDIBULAR JOINT IN FOP
Patients with FOP may have developmental anomalies of the temporomandibular joints (TMJs), although a comprehensive study of TMJ anatomy has not yet been undertaken in the FOP community. Spontaneous or post-traumatic extra-articular ankylosis of the TMJs is common, and leads to severe disability with resultant difficulties in eating and poor oral hygiene. 1 – 3 , 5
SUBMANDIBULAR SWELLING IN FOP
Submandibular swelling can be a life-threatening complication, especially when associated with massive anterior neck swelling and difficulty in swallowing. 17 Special measures to decrease swelling, including a course of glucocorticoids and respiratory support, may be warranted. 17
HEARING IMPAIRMENT IN FOP
Hearing impairment is a common feature of FOP and occurs in approximately 50% of patients. The onset is usually in childhood or adolescence, and is generally slowly progressive. Hearing loss is usually conductive in nature and may be due to middle ear ossification; however, in some patients, the hearing impairment is neurological in nature. 26
CARDIOPULMONARY FUNCTION IN FOP
Patients with FOP develop TIS that can lead to life-threatening complications. Features contributing to TIS in patients with FOP include: costovertebral malformations with orthotopic ankylosis of the costovertebral joints; ossification of intercostal muscles, paravertebral muscles and aponeuroses; and progressive spinal deformity including kyphoscoliosis or thoracic lordosis. Pneumonia and right-sided heart failure are the major life-threatening hazards that result from TIS in patients with FOP. Prophylactic measures to maximize pulmonary function, minimize respiratory compromise, and prevent influenza and pneumonia are helpful in decreasing the morbidity and mortality from TIS in patients with FOP. 22 , 27 , 28
Assiduous attention should be directed towards the prevention and therapy of intercurrent chest infections. Such measures should include prophylactic pneumococcal pneumonia and influenza vaccinations (given subcutaneously), chest physiotherapy and prompt antibiotic treatment of early chest infection. Upper abdominal surgery should be avoided if possible, as it interferes with diaphragmatic respiration. Sleep studies to assess sleep apnoea may be helpful, and positive pressure assisted breathing devices such as bipap masks without the use of supplemental oxygen may also be helpful. 22
Patients with FOP who have advanced TIS and who use unmonitored oxygen have a high risk of sudden death. Sudden correction of oxygen tension in the presence of chronic carbon dioxide retention suppresses respiratory drive. Patients who have FOP and severe TIS should not use supplemental oxygen in an unmonitored setting. 22
Additional understanding of the complex chest wall dynamics in a true genetic model of FOP should greatly enhance understanding of the pathophysiology of these dreaded complications.
RADIOGRAPHIC FEATURES OF FOP
Joint malformations and soft tissue ossification are the characteristic radiographic features of FOP. Malformation of the great toes, thumbs, cervical spine and proximal femurs, along with the presence of proximal medial tibial osteochondromas, can make the diagnosis more certain. 1 – 3 , 5
Radiographic and bone scan findings suggest normal modelling and remodelling of heterotopic bone. 29 , 30 The incidence of fractures is not increased in patients with FOP, although fracture healing is characteristically accelerated in heterotopic bone. 31 Bone scans are abnormal before HO can be detected by conventional radiographs. Computerized tomography and magnetic resonance imaging of early lesions have been described, but are superfluous. 5 , 6 The definitive diagnosis of FOP can be made by simple clinical evaluation that associates progressively ossifying soft tissue lesions with malformations of the great toes. 5 , 24 Clinical diagnosis of FOP can be confirmed by DNA diagnostic testing of the ACVR1 gene (see below).
LABORATORY FINDINGS IN FOP
Routine biochemical evaluations of bone mineral metabolism are usually normal, although serum alkaline phosphatase activity may be increased, especially during disease flare-ups. 3 , 5 , 6 Urinary basic fibroblast growth factor levels may be elevated during disease flare-ups coinciding with the pre-osseous angiogenic phase of fibroproliferative lesions. 32 Nephrolithiasis is more common in older patients with FOP, and may be due to increased immobilization and dehydration in the setting of generalized increased bone remodelling and mineral turnover. 5
HISTOPATHOLOGY OF FOP LESIONS
The histological stages of FOP lesions have been well described. 12 – 15 Early FOP lesions contain an intense perivascular B-cell and T-cell lymphocytic infiltrate. Subsequent migration of mononuclear inflammatory cells into affected muscle precedes widespread myonecrosis. 13
Following a brief inflammatory stage, an intense fibroproliferative reaction associated with robust angiogenesis and neovascularity is noted. 13 , 14 These early- to intermediate-stage lesions are microscopically indistinguishable from aggressive juvenile fibromatosis. As the lesion matures, fibroproliferative tissue undergoes an avascular condensation into cartilage followed by a revascularization stage and osteogenesis in a characteristic process of endochondral ossification. The resultant HO is normal, histologically mature lamellar bone with marrow elements. 12 – 15
Mast cells have been identified at every histological stage, and are found in much greater abundance compared with normal skeletal muscle and non-lesional FOP muscle. In fact, during the intense fibroproliferative stage of the lesion, mast cells are found at a density much higher than in any other inflammatory myopathy. 33
All stages of histological development are present in an active FOP lesion, indicating that different regions within the lesion mature at different rates. Although heterotopic bone formation in FOP is similar in some respects to bone formation in embryonic skeletal development and postnatal fracture healing, important differences are the lack of inflammation in embryonic skeletal induction and the relative absence of lymphocytic inflammatory cells in early fracture healing. 15 , 34
EPIDEMIOLOGIC, GENETIC AND ENVIRONMENTAL FACTORS IN FOP
FOP is extremely rare with a worldwide prevalence of approximately one in two million. There appears to be no ethnic, racial, gender or geographic predisposition. 3 , 6 Most cases arise as a result of a spontaneous new mutation. When observed, genetic transmission is autosomal dominant and can be inherited from either mothers or fathers. 23 , 35
Both genetic and environmental factors affect the phenotype of FOP. A study of three pairs of monozygotic twins with FOP found that within each pair, congenital toe malformations were identical. However, postnatal HO varied greatly depending on life history and environmental exposure. This study indicated that genetic determinants strongly influence disease phenotype during prenatal development, and that environmental factors strongly influence postnatal progression of HO 36
FOP AND THE BMP SIGNALLING PATHWAY
The classic and invariable FOP phenotype of great toe malformations and progressive heterotopic endochondral ossification suggested that the primary molecular pathology involves the bone morphogenetic protein (BMP) signalling pathway. 37 A number of seminal discoveries provided evidence of profound dysregulation of the BMP signalling pathway in cells from patients who had FOP. 38 – 47
DISCOVERY OF THE FOP GENE
In order to identify the chromosomal locus for the FOP gene, a conservative genome-wide linkage analysis was conducted using a subset of five families with the most stringent and unambiguous features of FOP. This approach identified linkage of FOP to 2q23–24. 9 The gene encoding activin receptor IA (ACVR1) [also known as activin-like kinase 2 (ALK2)], a BMP type I receptor, was identified in the linkage interval. DNA sequencing of the ACVR1 gene determined that the same heterozygous mis-sense mutation in the glycine–serine (GS) activation domain (c.617G>A;R206H) occurs in all classically affected individuals examined. 9 The discovery of the FOP gene was the culmination of a monumental 15-year search.
PROTEIN MODELLING OF THE FOP MUTATION
ACVR1/ALK2 is a BMP type I receptor, and protein structure homology modelling of the recurrent mutation predicts destabilization of the GS domain, consistent with an overactive BMP signalling pathway as the underlying cause of the ectopic chondrogenesis, osteogenesis and joint fusion seen in FOP. This mutation is consistent with a wealth of previous findings of an overactive BMP signalling pathway in FOP cells, and provides a rational basis for understanding both the postnatal HO and the congenital skeletal malformations that are ignominious signatures of this devastating disease. 9
Hypothetical protein structure models are being developed to understand both inter-and intramolecular interactions of the mutant receptor. The GS domain of all TGF-β/BMP type I receptors is a critical site for binding and activation of pathway-specific Smad signalling proteins, and is a binding site of FKBP12, an inhibitory protein that prevents leaky activation of the type I receptor in the absence of ligand. 48 , 49 FKBP12 also recruits a Smad7-Smurf1 ubiquitin ligase that functions normally to regulate the abundance of the receptor at the membrane. 50 Both leaky activation of BMP signalling and accumulation of BMP type I receptors at the cell membrane are seen in FOP cells, suggesting possible aberrant association with FKBP12 in FOP. The most likely possibility is that FKBP12 interactions with the GS domain become altered, leading to promiscuous ACVR1/ALK2 activity ( Figure 2 ). However, exactly how the R206H mutation in ACVR1/ALK2 specifically perturbs BMP signalling in FOP remains undetermined but could involve dysregulation of BMP receptor oligomerization, internalization, degradation and/or activation of downstream signalling. This is presentlythe subject of intense investigation.

Hypothetical schema of bone morphogenetic protein (BMP) signalling in fibrodysplasia ossificans progressiva (FOP) cells. In control cells (A), in the absence of ligand, the Smad/Smurf-FKBP12 (SM-FKBP12) complex binds activin receptor IA (ACVR1; a BMP type I receptor) and prevents its promiscuous phosphorylation by the constitutively active type II BMP receptor (not shown). SM-FKBP12 also promotes ubiquitin-associated degradation of ACVR1 in the absence of ligand, thus maintaining low steady-state levels of ACVR1 at the cell membrane. Following ligand binding in control cells (B), SM-FKBP12 dissociates from ACVR1, thus allowing the constitutively active BMP type II receptor (not shown) to phosphorylate ACVR1, and promote Smad 1, 5 and8 phosphorylation and downstream BMP signalling. In FOP cells, SM-FKBP12 does not appear to bind appropriately to the mutant receptor [ACVR1 (R206H)]. Thus, inhibition of BMP signalling is impaired in the absence of ligand, and basal leakiness of BMP signalling occurs (C). Additionally, it is suspected that since the SM-FKBP12 complex cannot properly target the mutant ACVR1 (R206H) receptor for ubiquitin-associated degradation, ACVR1 may be expected to accumulate at the cell surface. Thus, in the presence of ligand (D), hyper-responsive BMP signalling may be predicted to occur. Arrows, signalling promoted; blunt-end lines, signalling inhibited; lock, SM-FKBP12 binding to ACVR1; dashed lines, SM-FKBP12 binding to ACVR1 impaired; open cups, extracellular ligand-binding domain of ACVR1; filled-in circles, BMP ligand; filled-in circles inside open cups, BMP ligand binding to ACVR1.
ACVR1/ALK2: A DRUGGABLE TARGET FOR THE SECOND SKELETON
The ultimate goal of FOP research is the development of treatments that will prevent, halt or even reverse progression of the condition. The prevention and treatment of HO in FOP, as for any of the more common forms of HO, will be based on at least one of four principles: disrupting the relevant inductive signalling pathways; suppressing the immunological and/or inflammatory triggers; altering the relevant osteoprogenitor cells in the target tissues; and/or modifying the tissue environment conducive to heterotopic osteogenesis.
The discovery of the FOP gene identifies ACVR1/ALK2 as a specific druggable target for FOP. 51 The identification of the recurrent heterozygous mis-sense point mutation that causes FOP in all classically affected individuals provides a specific druggable target and a rational point of intervention in a critical signalling pathway. Plausible therapeutic approaches to inhibiting BMP signalling in FOP include inhibitory RNA technology, monoclonal antibodies directed against ACVR1/ALK2, and (most plausibly) orally available small molecule selective signal transduction inhibitors (STIs) of ACVR1/ALK2. 51
Small molecule STIs have proven to be invaluable for investigating signal transduction pathways. Such molecules also have the potential for development into powerful therapeutic agents. The development of specific STIs for promiscuous ACVR1/ALK2 signalling in FOP have the potential to modify the natural history of the disease. Residues close to the ATP-binding site of ACVR1/ALK2 could be exploited to achieve selectivity, even among closely related receptor serine threonine kinases such as ALK3 (BMPRIA) and ALK6 (BMPRIB). Small soluble molecule inhibitors designed to specifically block ACVR1/ALK2 signalling intracellularly will need to be designed, screened and tested in cell and animal models of FOP. ACVR1/ALK2 STIs will need to have sufficient efficacy, tolerance to resistance, and acceptable safety profiles. 51
Selective inhibitors have been developed for the ALKs that signal through Smads 2 and 3 [ALK4, 5 (TβRI) and 7]. At the present time, there are no known selective inhibitors of ACVR1/ALK2 or the other three BMP pathway type I receptors (ALK 1, 3 and 6) that signal through the BMP-pathway-specific Smads 1, 5 and 8. Such selective inhibitors are desperately needed. 51
ANIMAL MODELS OF FOP
Animal models of FOP will be important for understanding the pathophysiology of FOP and for testing possible therapies. 52 Laboratory-generated animal models with some features of FOP have provided the opportunity to better understand the biology of HO and to study the effectiveness and safety of currently available and emerging therapies. Development of a knock-in mouse model carrying the specific FOP-disease-causing mutation in ACVR1/ALK2 will be necessary to establish specificity of treatment in FOP. Such a genetically engineered knock-in mouse is presently being developed.
CURRENT MANAGEMENT OF FOP
The rarity, variable severity and episodic clinical course of FOP pose substantial uncertainties when evaluating experimental therapies. 53 Accordingly, medical intervention is currently supportive. Surgical release of joint contractures is generally unsuccessful and risks new, trauma-induced HO. Osteotomy of heterotopic bone or surgical removal of heterotopic bone to mobilize joints is generally counterproductive because additional HO develops at the operative site. Rarely, a joint may be repositioned surgically to improve the patient’s overall functional status. Spinal bracing is ineffective and surgical intervention is associated with numerous complications. 27
Guidelines for symptomatic management of disease flare-ups have been published, and highlight the anecdotal utility of glucocorticoids in managing new flare-ups affecting the function of major joints in the appendicular skeleton. 53 Non-steroidal anti-inflammatory medications, cyclo-oxygenase-2 inhibitors, leukotriene inhibitors and mast cell stabilizers are useful anecdotally in managing chronic discomfort and ongoing flare-ups, but to date there is no proven efficacy with any therapy in altering the natural history of the disease. 53 A recent report documented the failure of bone marrow transplantation to cure the condition, but suggested that chronic immunosuppression may have some utility, although its general use is not recommended. 34
PROPHYLACTIC ISSUES IN FOP
Dental therapy must involve assiduous attention to prophylaxis of caries and must avoid intramuscular injection of local anesthetics, especially mandibular blocks and stretching of the jaw. 54 All intramuscular injections must be avoided. 16 Prevention of falls is crucial. 19 Prophylaxis against influenza and pneumonia, as well as measures to prevent respiratory infection and cardiopulmonary complications of restrictive chest well disease, are vitally important. 20
ANAESTHESIA IN PATIENTS WITH FOP
General anaesthesia is particularly dangerous in patients with FOP. Guidelines for general anaesthesia have been reported. 54 Overstretching of the jaw for intubation may cause additional trauma to the TMJs, and lead to disease flare-ups. In older patients whose TMJs are ankylosed, oral access for intubation may not be possible. General anaesthesia in FOP patients should be accomplished through an awake fibre-optic nasal intubation under light sedation so that the patient can control secretions. This should be performed by well-trained anaesthesia teams who are familiar and experienced with this type of procedure. 54
REHABILITATION ISSUES IN FOP
As heterotopic bone accumulates in FOP, range of motion is progressively lost leading to near-complete immobility. Present and future rehabilitation approaches should be focused on enhancing activities of daily living. Occupational therapy and vocational education consultations may be useful. Despite the widespread HO and progressive disability, most patients lead productive and fulfilling lives. 55
THE INTERNATIONAL FOP ASSOCIATION
The International FOP Association (IFOPA) was founded in June 1988 to educate patients, doctors and the public about FOP; to support medical research into FOP; and to support patients with FOP and their families by providing a network of communication to help end the isolation that accompanies this rare and severely disabling condition. Additional information can be found on the IFOPA website ( www.ifopa.org ). In recent years, many regional FOP organizations have arisen worldwide to support patient-related activities.
RESEARCH AGENDA AND SUMMARY
While the mutation that causes classic FOP has been discovered, much work remains to elucidate the molecular mechanism by which this mutation leads to the complex disease phenotype of skeletal malformations and episodic progression of HO.
It will be essential to fully understand the role of the inflammatory pathways in triggering flare-ups of the disease, and to better understand the interaction of the immune system with the as-yet-unidentified connective tissue progenitor cells that are mobilized by disease flare-ups. 56 Additionally, the molecular micro-environment in which HO develops needs to be more fully understood in the context of the disease-causing mutation that underlies the pathophysiology of the episodic flare-ups. The critical relationships between the mutant receptor, the environmental triggers, the responsive stem cells and the micro-environmental niches in which this renegade skeletal metamorphosis takes place will be vitally important to understand in order to design and develop the most effective treatment and prevention strategies. Accurate and clinically available premonitory markers of FOP flare-ups are desperately needed to assess potential therapies.
All of these important goals, and of course the ultimate goal of using this knowledge to develop better treatments and eventually a cure, will require the development of relevant cell and animal models.
A complete understanding of the genetic, molecular and cellular basis of HO in FOP will likely have broad therapeutic implications for patients with more common forms of HO, such as non-genetic forms of HO that may occur following total hip replacement, head injuries, spinal cord injuries, athletic injuries, blast injuries from war, and end-stage valvular heart disease. 57 , 58
It may even be possible some day to harness the gene mutation that causes the renegade bone formation in FOP to create bone and new skeletal elements in a controlled way for patients who have osteoporosis, for those with severe bone loss from trauma or neoplasms, for those with fractures that fail to heal or spinal fusions that are slow to heal, or for those with congenital malformations of the spine and limbs. With the recent identification of the mutation responsible for FOP, 9 we have reached a monumental milestone on our epic journey to understand FOP; knowledge which is needed to help the children with FOP and that has the potential to help many others. For the moment, the clinical management of FOP is focused in the prevention of flare-ups, the symptomatic management of disease symptoms and the optimization of function. The pathway to more effective management of FOP is through the research laboratory.
Practice points
This very brief guide will summarize the current symptomatic management of FOP.
Activities: avoid soft tissue injuries, contact sports, overstretching of soft tissues and muscle fatigue. Avoid biopsies, surgical removal of heterotopic bone and all non-emergent surgical procedures
Anaesthesia: if general anaesthesia is required, perform awake intubation by nasotracheal fibre-optic technique
Falls: locked upper limbs may accentuate head trauma from falls. Epidural haematomas are common (surgical emergency). Use protective headgear in children who have upper limb involvement
Flare-up (back/chest) : use non-steroidal anti-inflammatory medications with gastrointestinal precautions. Use analgesics and/or muscle relaxants, as needed
Flare-up (limbs/throat): prednisone – 2 mg/kg PO once daily for 4 days; begin within first 24 h of flare-up. Keep medication on-hand for emergencies. Use analgesias and/or muscle relaxants, as needed, with gastrointestinal precautions
Flare-ups (protection): most flare-ups result from over-use and soft tissue injuries. Prednisone 2 mg/kg PO once daily for 3 days to prevent flare-up after severe soft tissue injury. Do not use after minor bumps or bruises
Hearing: conductive hearing impairment is common. Perform periodic audiology evaluations. Hearing aids may improve conductive hearing loss
Immunizations: avoid all intramuscular immunizations. Subcutaneous immunizations are acceptable when FOP is quiescent. Avoid any immunizations during flare-ups
Influenza: administer influenza vaccines subcutaneously, but never during flare-ups. Avoid live attenuated flu vaccine; it may cause flu-like symptoms and exacerbate FOP. Household contacts of FOP patients should be immunized annually.
IVs: superficial IV access and venepuncture is acceptable. Traumatic IVs and arterial punctures may cause HO
Limb swelling: lymphoedema and transient neuropathy may occur with flare-ups of limbs. Elevate legs while sleeping and recumbent. Use support stockings. Take one baby aspirin daily with food. Rule-out deep vein phlebitis with Doppler ultrasound
Occupational therapy: perform periodic occupational therapy evaluations as activities of daily living change
Physiotherapy: avoid passive range of motion. Warm water hydrotherapy may be helpful
Pulmonary function: perform baseline pulmonary function tests and echocardiogram. Repeat periodically. Supplemental oxygen should not be used in an unmonitored setting
School: use school aides to protect and assist children. Request medical letter and preschool evaluation
Surgery: avoid surgery, except in emergencies
Teeth: avoid mandibular blocks, overstretching of the jaw and muscle fatigue
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Frederick S. Kaplan, Departments of Orthopedic Surgery & Medicine, The University of Pennsylvania School of Medicine, c/o Hospital of The University of Pennsylvania, Philadelphia, PA, USA.
Martine Le Merrer, U781 INSERM, Hopital Necker-Enfants Malades, Paris, France.
David L. Glaser, Department of Orthopedic Surgery, The University of Pennsylvania School of Medicine, c/o Hospital of the University of Pennsylvania, Philadelphia, PA, USA.
Robert J. Pignolo, Department of Medicine, The University of Pennsylvania School of Medicine, Philadelphia, PA, USA.
Robert Goldsby, Department of Pediatrics and Cardiovascular Research Institute, The University of California San Francisco, San Francisco, CA, USA.
Joseph A. Kitterman, Department of Pediatrics and Cardiovascular Research Institute, The University of California San Francisco, San Francisco, CA, USA.
Jay Groppe, Department of Biochemistry, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA.
Eileen M. Shore, Departments of Orthopedic Surgery and Genetics, The University of Pennsylvania School of Medicine, Philadelphia, PA, USA.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
A new era for fibrodysplasia ossificans progressiva: a druggable target for the second skeleton
Affiliation.
- 1 University of Pennsylvania School of Medicine, Department of Orthopaedic Surgery, Hospital of the University of Pennsylvania, Silverstein Two, 34th & Spruce Street, Philadelphia, PA 19104, USA. [email protected]
- PMID: 17477807
- DOI: 10.1517/14712598.7.5.705
Fibrodysplasia ossificans progressiva (FOP) is a disabling genetic condition that leads to the formation of a second (heterotopic) skeleton, and is the most catastrophic disorder of heterotopic ossification in humans. Throughout childhood and early adult life, FOP progressively immobilizes all of the joints of the normotopic skeleton, rendering movement impossible. At present, there is no effective prevention or treatment. Recently, a recurrent mutation in the glycine-serine activation domain of the activin receptor IA/activin-like kinase-2, a bone morphogenetic protein type I receptor, was reported in all sporadic and familial cases of classic FOP, making this one of the most highly specific disease-causing mutations in the human genome. The discovery of the FOP gene establishes a critical milestone in understanding FOP, reveals a highly conserved druggable target in the TGF-beta/bone morphogenetic protein signaling pathway and compels therapeutic approaches for the development of small molecule signal transduction inhibitors for activin-like kinase-2. Effective therapies for FOP, and possibly for a vast array of more common conditions of heterotopic ossification, will be based on blocking activin-like kinase-2, a critical node in the BMP signaling pathway.
PubMed Disclaimer
Similar articles
- Fibrodysplasia ossificans progressiva. Kaplan FS, Le Merrer M, Glaser DL, Pignolo RJ, Goldsby RE, Kitterman JA, Groppe J, Shore EM. Kaplan FS, et al. Best Pract Res Clin Rheumatol. 2008 Mar;22(1):191-205. doi: 10.1016/j.berh.2007.11.007. Best Pract Res Clin Rheumatol. 2008. PMID: 18328989 Free PMC article. Review.
- Recent progress in drug development for fibrodysplasia ossificans progressiva. Meng X, Wang H, Hao J. Meng X, et al. Mol Cell Biochem. 2022 Oct;477(10):2327-2334. doi: 10.1007/s11010-022-04446-9. Epub 2022 May 10. Mol Cell Biochem. 2022. PMID: 35536530 Free PMC article. Review.
- Hints on transcriptional control of essential players in heterotopic ossification of Fibrodysplasia Ossificans Progressiva. Ravazzolo R, Cappato S, Bocciardi R. Ravazzolo R, et al. Bone. 2018 Apr;109:187-191. doi: 10.1016/j.bone.2017.10.028. Epub 2017 Oct 31. Bone. 2018. PMID: 29100956 Review.
- Deregulated bone morphogenetic protein receptor signaling underlies fibrodysplasia ossificans progressiva. de Gorter DJ, Jankipersadsing V, Ten Dijke P. de Gorter DJ, et al. Curr Pharm Des. 2012;18(27):4087-92. doi: 10.2174/138161212802430495. Curr Pharm Des. 2012. PMID: 22630080
- Acute and chronic rapamycin use in patients with Fibrodysplasia Ossificans Progressiva: A report of two cases. Kaplan FS, Zeitlin L, Dunn SP, Benor S, Hagin D, Al Mukaddam M, Pignolo RJ. Kaplan FS, et al. Bone. 2018 Apr;109:281-284. doi: 10.1016/j.bone.2017.12.011. Epub 2017 Dec 11. Bone. 2018. PMID: 29241828
- Palovarotene for Fibrodysplasia Ossificans Progressiva (FOP): Results of a Randomized, Placebo-Controlled, Double-Blind Phase 2 Trial. Pignolo RJ, Baujat G, Hsiao EC, Keen R, Wilson A, Packman J, Strahs AL, Grogan DR, Kaplan FS. Pignolo RJ, et al. J Bone Miner Res. 2022 Oct;37(10):1891-1902. doi: 10.1002/jbmr.4655. Epub 2022 Aug 17. J Bone Miner Res. 2022. PMID: 35854638 Free PMC article. Clinical Trial.
- Saracatinib is an efficacious clinical candidate for fibrodysplasia ossificans progressiva. Williams E, Bagarova J, Kerr G, Xia DD, Place ES, Dey D, Shen Y, Bocobo GA, Mohedas AH, Huang X, Sanderson PE, Lee A, Zheng W, Economides AN, Smith JC, Yu PB, Bullock AN. Williams E, et al. JCI Insight. 2021 Apr 22;6(8):e95042. doi: 10.1172/jci.insight.95042. JCI Insight. 2021. PMID: 33705358 Free PMC article.
- Propranolol and ascorbic acid in control of fibrodysplasia ossificans progressiva flare-ups due to accidental falls. Palhares DB, Nascimento DR, Palhares MG, Lopes S, de L, Cristhina P, Mauro J, Alves F, Vieira RO, Souza-Fagundes EM, Underwood A, Milsted A, Augusto R, Martins AS. Palhares DB, et al. Intractable Rare Dis Res. 2019 Feb;8(1):24-28. doi: 10.5582/irdr.2018.01095. Intractable Rare Dis Res. 2019. PMID: 30881854 Free PMC article.
- Discovery of 3-(4-sulfamoylnaphthyl)pyrazolo[1,5-a]pyrimidines as potent and selective ALK2 inhibitors. Jiang JK, Huang X, Shamim K, Patel PR, Lee A, Wang AQ, Nguyen K, Tawa G, Cuny GD, Yu PB, Zheng W, Xu X, Sanderson P, Huang W. Jiang JK, et al. Bioorg Med Chem Lett. 2018 Nov 1;28(20):3356-3362. doi: 10.1016/j.bmcl.2018.09.006. Epub 2018 Sep 6. Bioorg Med Chem Lett. 2018. PMID: 30227946 Free PMC article.
- Cartilage-derived retinoic acid-sensitive protein (CD-RAP): A stage-specific biomarker of heterotopic endochondral ossification (HEO) in fibrodysplasia ossificans progressiva (FOP). Lindborg CM, Brennan TA, Wang H, Kaplan FS, Pignolo RJ. Lindborg CM, et al. Bone. 2018 Apr;109:153-157. doi: 10.1016/j.bone.2017.09.016. Epub 2017 Sep 28. Bone. 2018. PMID: 28963080 Free PMC article.
Publication types
- Search in MeSH
Related information
- PubChem Compound
- PubChem Compound (MeSH Keyword)
- PubChem Substance
Grants and funding
- R01-AR41916/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full text sources.
- Taylor & Francis
Other Literature Sources
- The Lens - Patent Citations
- Genetic Alliance
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

IMAGES
VIDEO
COMMENTS
Fibrodysplasia ossificans progressiva (FOP) is an ultra-rare progressive genetic disease effecting one in a million individuals. During their life, patients with FOP progressively develop bone in the soft tissues resulting in increasing immobility and ...
Fibrodysplasia ossificans progressiva is a rare, autosomal dominant disease where connective tissue such as muscle, tendons and ligaments gradually turn into bone tissue, causing limited movement ...
Summary Fibrodysplasia ossificans progressiva (FOP) is a rare genetic condition with soft tissue progressive ossification, leading to severe disability. We describe a 27-years-old female affected by FOP who died after a fall. An autopsy was performed. Upper and lower extremities resulted in fixed flexion, with kyphoscoliosis of the spine and chest wall deformity. Moreover, a cranial fracture ...
Drug trial shows reduced abnormal bone formation in those with fibrodysplasia ossificans progressiva Date: September 28, 2023 Source: Vanderbilt University Medical Center Summary:
Fibrodysplasia ossificans progressiva (FOP) is an ultra-rare progressive genetic disease effecting one in a million individuals. During their life, patients with FOP progressively develop bone in the soft tissues resulting in increasing immobility and early death.
Fibrodysplasia ossificans progressiva (FOP) is rare genetic disease featuring progressive heterotopic ossification of soft tissues of the musculoskeletal system which leads to severe disability and premature death. Recognition of this disease is important ...
Fibrodysplasia ossificans progressiva (FOP) is an ultra-rare progressive genetic disease characterized by heterotopic ossification (HO) of muscles, tendons and ligaments, often preceded by periodic painful soft tissue swellings called flare-ups.
Fibrodysplasia ossificans progressiva (FOP) is an enigmatic, ultra-rare genetic disorder characterized by progressive heterotopic ossification, wherein soft connective tissues undergo pathological transformation into bone structures. This incapacitating process severely limits patient mobility and p …
Fibrodysplasia ossificans progressiva (FOP) is a rare genetic skeletal disorder manifesting progressive heterotopic ossification (HO) and congenital malformation of the great toes. Since 2007, we have conducted research on FOP. Here, we review the findings on FOP published to date, including the res …
Summary: Fibrodysplasia ossificans progressiva is a rare disease characterised by progressive heterotopic bone formation. Here, we present a comprehensive summary of the recent literature on this debilitating condition and discuss approaches to solving this clinical puzzle.
Fibrodysplasia Ossificans Progressiva (FOP) is a rare genetic disease caused by heterozygous missense mutations in Activin A receptor type I which is also known as Activin-like kinase 2 (ALK2), a type I receptor of Bone Morphogenetic Proteins(BMP). Patients with FOP usually undergo episodic flare-ups and the heterotopic ossification in soft and connective tissues. Molecular mechanism study ...
Background Fibrodysplasia ossificans progressiva (FOP) is an ultra-rare, disabling genetic disorder characterized by congenital malformations of the great toes and progressive heterotopic ossification of soft and connective tissues. Assiduous attention to the unmet needs of this patient community is crucial to prevent potential iatrogenic harm and optimize care for individuals with FOP ...
A mutation in the gene that causes fibrodysplasia ossificans progressiva (FOP) doesn't just cause extra bone growth but is tied to a problem in generating new muscle tissue after injury, according ...
Results from individuals receiving standard care for up to 3 years in this natural history study show the debilitating effect and progressive nature of FOP cross-sectionally and longitudinally, with greatest progression during childhood and early adulthood.
Fibrodysplasia ossificans progressiva is described as a rare genetic disorder characterized by the organization of heterotopic hard tissues within the soft tissues, such as ligaments, tendons, and skeletal muscle.[1][2][3] It comes under the category of an autosomal dominant disorder. The tissue formed in such patients is not just the mineralized calcium phosphate, but it resembles the new ...
Fibrodysplasia Ossificans Progressiva. NORD gratefully acknowledges Frederick S. Kaplan, MD, Isaac & Rose Nassau Professor of Orthopaedic Molecular Medicine; Chief, Division of Orthopaedic Molecular Medicine and Co-Director, Center for Research in FOP & Related Disorders, The Perelman School of Medicine at The University of Pennsylvania, and ...
Abstract. Fibrodysplasia ossificans progressiva (FOP) is an ultra-rare genetic disorder of extraskeletal bone formation, but could appropriately be viewed as a seminal disorder of osteochondrogenesis. Many, if not most, of the musculoskeletal features of FOP are related to dysregulated chondrogenesis including abnormal articular cartilage ...
Fibrodysplasia ossificans progressiva ( / ˌfaɪbroʊdɪˈspleɪʒ ( i) ə ɒˈsɪfɪkænz prəˈɡrɛsɪvə /; [ 1] abbr. FOP ), also called Münchmeyer disease or formerly myositis ossificans progressiva, is an extremely rare connective tissue disease in which fibrous connective tissue such as muscle, tendons, and ligaments turn into bone tissue ( ossification ). It is the only known medical ...
Fibrodysplasia ossificans progressiva (FOP), a rare and disabling genetic condition characterized by congenital malformations of the great toes and progressive heterotopic endochondral ossification (HEO) which is the most catastrophic of HEO disorders in humans. Flare-ups of FOP are episodic; immobility is cumulative.
Fibrodysplasia ossificans progressiva (FOP) is characterized by congenital bilateral hallux valgus malformations and early-onset heterotopic ossification, which may be spontaneous or precipitated by trauma including intramuscular vaccinations. Painful, recurrent soft-tissue swellings (flare-ups) may precede localized heterotopic ossification. Heterotopic ossification can occur at any location ...
Go to: Fibrodysplasia ossificans progressiva (FOP), a rare and disabling genetic condition of congenital skeletal malformations and progressive heterotopic ossification (HO), is the most catastrophic disorder of HO in humans. Episodic disease flare-ups are precipitated by soft tissue injury, and immobility is cumulative.
Fibrodysplasia ossificans progressiva (FOP) is a disabling genetic condition that leads to the formation of a second (heterotopic) skeleton, and is the most catastrophic disorder of heterotopic ossification in humans. Throughout childhood and early adult life, FOP progressively immobilizes all of the joints of the normotopic skeleton, rendering ...