The Clinical Presentation
- First Online: 01 January 2013

Cite this chapter

- Sergio V. Delgado 3 &
- Jeffrey R. Strawn 4
993 Accesses
Presenting case material to colleagues requires preparation, whether the presentation is to be made casually during bedside rounds or in the formal environment of a national meeting. It is rewarding when a presentation is well received, particularly because it may prove helpful to other clinicians, allied health professionals, and researchers. Regardless of the setting, the presenter’s goal is to share their knowledge based on observations they have made and lessons they have learned from the case or cases. The most time-consuming aspect of the patient-oriented presentation is collecting and organizing as much information as possible about the patients, their families, and others who were involved in the patients’ care. Once these tasks are complete, the presenter must summarize the information and place it within the context of treatment data and consensus approaches. Tailoring the talk to the audience is also of paramount importance. Different groups will invariably come from different disciplines, and the presentation will need to be tailored to accommodate each audience’s background, interests and goals.
Make everything as simple as possible, but not simpler —Albert Einstein (1879–1955)
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Altman LK (2006) Socratic dialogue gives way to powerpoint®. New York Times, 12 Dec 2006
Google Scholar
Blechner MJ (2012) Confidentiality: against disguise, for consent. Psychotherapy (Chic) 49(1):16–18
Article Google Scholar
Clifft MA (1986) Writing about psychiatric patients. Guidelines for disguising case material. Bull Menninger Clin 50(6):511–524
PubMed CAS Google Scholar
Copeland HL, Hewson MG, Stoller JK et al (1998) Making the continuing medical education lecture effective. J Contin Educ Health Prof 18:227–234
Gabbard GO (2000) Disguise or consent: problems and recommendations concerning the publication and presentation of clinical material. Int J Psychoanal 81:1071–1086
Article PubMed Google Scholar
Gabbard GO, Williams P (2001) Preserving confidentiality in writing of case reports. Int J Psychoanal 82:1067–1068
Article PubMed CAS Google Scholar
Hull AL, Cullen RJ, Hekelman FP (1989) A retrospective analysis of grand rounds in continuing medical education. J Contin Educ Health Prof 9(4):257–266
Lorin MI, Palazzi DL, Turner TL et al (2008) What is a clinical pearl and what is its role in medical education? Med Teach 30(9–10):870–874
Sackett DL, Rosenberg WM, Gray JA et al (1996) Evidence based medicine: what it is and what it isn’t. Br Med J 312:71–72
Article CAS Google Scholar
Tuckett D (2000) Reporting clinical events in the journal: towards the constructing of a special case. Int J Psychoanal 81:1065–1069
Download references
Author information
Authors and affiliations.
Department of Psychiatry and Child Psychiatry, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA
Sergio V. Delgado
Department of Psychiatry and Behavioral Neuroscience, University of Cincinnati, Cincinnati, Ohio, USA
Jeffrey R. Strawn
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Delgado, S.V., Strawn, J.R. (2014). The Clinical Presentation. In: Difficult Psychiatric Consultations. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-39552-9_8
Download citation
DOI : https://doi.org/10.1007/978-3-642-39552-9_8
Published : 16 September 2013
Publisher Name : Springer, Berlin, Heidelberg
Print ISBN : 978-3-642-39551-2
Online ISBN : 978-3-642-39552-9
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Oral Presentations: Tips, Significance, Design, Guidelines & Presentation
1) Know your audience
It is always a good idea to structure your talk so that anyone in the audience can understand what you are presenting. A good scientist should be able to present complex, scientific ideas, no matter how technical, in a simple, easy to follow manner. Complexity is not a necessity, it is an annoyance.
Understand your purpose. This way you can get the point of your talk across appropriately and affectively by catering to your specific audience.
2) Be organized
- Whether you are giving a 15 minute talk or a 45 minute talk, make sure you give yourself enough time to deliver all the information you want in a calm manner. Allocate time for questions/answers.
- Be able to summarize your presentation in five minutes.
- Be concise. Use your space wisely. Use illustrations. Check grammar, spelling, and lay out of each slide.
- Keep an outline with you during the presentation; it will help you stay on track.
- Prepare back up slides. These will come in handy if a question comes up about a topic that needs further explanation.
3) Presentation
Practice your talk enough so that you have flow, but no so much that you have the entire talk memorized. Memorizing your talk will bore you and your audience, as it will be monotonous.
4) Be professional
- Know what you are presenting and be ready to answer question during and after the presentation. Do not answer questions vaguely. A knowledgeable scientist is specific and accurate with his/her information.
- Dress up to present with confidence and respect for the audience and the science involved.
- Be enthusiastic. Scientific talks can be boring, as often they are full of technical jargon. Be clear and talk simplistically.
- Make sure the presentation is visually pleasing. Add pertinent graphics and use fewer words.
5) Be aware of technical problems.
Make sure the format you choose for your presentation is compatible with your style of speech. Also, be prepared for technical disasters just before your talk. Be able to give your talk in another format just in case your first choice (ex: PowerPoint presentation) fails to load.
Significance
Oral presentations are an excellent means of communicating basic science or clinical research. Unlike a poster presentation or a written manuscript, the audience during an oral presentation is more attentive as they are focused on the presenter. For the researcher, this is a rare opportunity to shine! In as few as five minutes, the researcher can convey scientific information and give a years worth work some meaning that can be useful to thousands of people. Of course, this also means that in as little as five minutes, the researcher can cause a great deal of confusion by giving a bad presentation.
Just as is the case with written manuscripts and poster presentations, oral presentations must also communicate research to include all aspects of the scientific method. There are, however, no rules as to what order and which format this should be done in. In order to deliver a successful talk, the presenter should be organized, prepared, and enthusiastic about the research being presented.
Design: A General Guideline
Regardless of whether you choose a PowerPoint presentation or transparencies to deliver your talk, here are some general guidelines to keep in mind when designing your presentation.
1) Title (include authors and affiliations)
2) Introduction (Background, Purpose, Hypothesis)
3) Method (A brief introduction to the methodology without too much technical Jargon)
4) Results (Use graphs/charts/table, Provide an extra slide/transparency with a summary of the results, Explain the results)
5) Conclusions/Discussion (Clear explanation of the results, Clinical implications)
6) Future work (Provide information on where the project is headed)
7) Acknowledgment
Presentation
There some people for whom public speaking is as natural as having a conversation with their friends. Conveniently, however, public speaking is an art that can be perfected with enough practice. Here some things to consider before and during the presentation:
- Do not go over the time limit.
- Speak clearly and concisely. Be coherent. Do not ramble, play with the pointer, or move around in circles.
- Dress appropriately.
- Make eye contact.
- Make sure that each slide/transparency is not cluttered with too many points and ideas. Graphs, tables, and charts should be clearly labeled and easy to interpret.
- Practice your talk, but do not memorize a script.
- Be visually and orally interesting.
- Answer questions in a calm, non-condescending manner; do not argue with or interrupt the questioner.
- Be polite and graceful.
- Give a presentation that is focused with one underlying message.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 13 November 2020
Clinical presentations, laboratory and radiological findings, and treatments for 11,028 COVID-19 patients: a systematic review and meta-analysis
- Carlos K. H. Wong 1 , 2 na1 ,
- Janet Y. H. Wong 3 na1 ,
- Eric H. M. Tang 1 ,
- C. H. Au 1 &
- Abraham K. C. Wai 4
Scientific Reports volume 10 , Article number: 19765 ( 2020 ) Cite this article
6191 Accesses
55 Citations
3 Altmetric
Metrics details
- Health care
- Medical research
- Microbiology
- Risk factors
This systematic review and meta-analysis investigated the comorbidities, symptoms, clinical characteristics and treatment of COVID-19 patients. Epidemiological studies published in 2020 (from January–March) on the clinical presentation, laboratory findings and treatments of COVID-19 patients were identified from PubMed/MEDLINE and Embase databases. Studies published in English by 27th March, 2020 with original data were included. Primary outcomes included comorbidities of COVID-19 patients, their symptoms presented on hospital admission, laboratory results, radiological outcomes, and pharmacological and in-patient treatments. 76 studies were included in this meta-analysis, accounting for a total of 11,028 COVID-19 patients in multiple countries. A random-effects model was used to aggregate estimates across eligible studies and produce meta-analytic estimates. The most common comorbidities were hypertension (18.1%, 95% CI 15.4–20.8%). The most frequently identified symptoms were fever (72.4%, 95% CI 67.2–77.7%) and cough (55.5%, 95% CI 50.7–60.3%). For pharmacological treatment, 63.9% (95% CI 52.5–75.3%), 62.4% (95% CI 47.9–76.8%) and 29.7% (95% CI 21.8–37.6%) of patients were given antibiotics, antiviral, and corticosteroid, respectively. Notably, 62.6% (95% CI 39.9–85.4%) and 20.2% (95% CI 14.6–25.9%) of in-patients received oxygen therapy and non-invasive mechanical ventilation, respectively. This meta-analysis informed healthcare providers about the timely status of characteristics and treatments of COVID-19 patients across different countries.
PROSPERO Registration Number: CRD42020176589
Similar content being viewed by others
Global prevalence and effect of comorbidities and smoking status on severity and mortality of COVID-19 in association with age and gender: a systematic review, meta-analysis and meta-regression
Frequency, risk factors, and outcomes of hospital readmissions of COVID-19 patients
Risk factors for severe COVID-19 differ by age for hospitalized adults
Introduction.
Following the possible patient zero of coronavirus infection identified in early December 2019 1 , the Coronavirus Disease 2019 (COVID-19) has been recognized as a pandemic in mid-March 2020 2 , after the increasing global attention to the exponential growth of confirmed cases 3 . As on 29th March, 2020, around 690 thousand persons were confirmed infected, affecting 199 countries and territories around the world, in addition to 2 international conveyances: the Diamond Princess cruise ship harbored in Yokohama, Japan, and the Holland America's MS Zaandam cruise ship. Overall, more than 32 thousand died and about 146 thousand have recovered 4 .
A novel bat-origin virus, 2019 novel coronavirus, was identified by means of deep sequencing analysis. SARS-CoV-2 was closely related (with 88% identity) to two bat-derived severe acute respiratory syndrome (SARS)-like coronaviruses, bat-SL-CoVZC45 and bat-SL-CoVZXC21, but were more distant from SARS-CoV (about 79%) and MERS-CoV (about 50%) 5 , both of which were respectively responsible for two zoonotic human coronavirus epidemics in the early twenty-first century. Following a few initial human infections 6 , the disease could easily be transmitted to a substantial number of individuals with increased social gathering 7 and population mobility during holidays in December and January 8 . An early report has described its high infectivity 9 even before the infected becomes symptomatic 10 . These natural and social factors have potentially influenced the general progression and trajectory of the COVID-19 epidemiology.
By the end of March 2020, there have been approximately 3000 reports about COVID-19 11 . The number of COVID-19-related reports keeps growing everyday, yet it is still far from a clear picture on the spectrum of clinical conditions, transmissibility and mortality, alongside the limitation of medical reports associated with reporting in real time the evolution of an emerging pathogen in its early phase. Previous reports covered mostly the COVID-19 patients in China. With the spread of the virus to other continents, there is an imminent need to review the current knowledge on the clinical features and outcomes of the early patients, so that further research and measures on epidemic control could be developed in this epoch of the pandemic.
Search strategy and selection criteria
The systematic review was conducted according to the protocol registered in the PROSPERO database (CRD42020176589). Following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline throughout this review, data were identified by searches of MEDLINE, Embase and references from relevant articles using the search terms "COVID", “SARS-CoV-2”, and “novel coronavirus” (Supplementary material 1 ). Articles published in English up to 27th March, 2020 were included. National containment measures have been implemented at many countries, irrespective of lockdown, curfew, or stay-at-home orders, since the mid of March 2020 12 , except for China where imposed Hubei province lockdown at 23th January 2020, Studies with original data including original articles, short and brief communication, letters, correspondences were included. Editorials, viewpoints, infographics, commentaries, reviews, or studies without original data were excluded. Studies were also excluded if they were animal studies, modelling studies, or did not measure symptoms presentation, laboratory findings, treatment and therapeutics during hospitalization.
After the removal of duplicate records, two reviewers (CW and CHA) independently screened the eligibility criteria of study titles, abstracts and full-texts, and reference lists of the studies retrieved by the literature search. Disagreements regarding the procedures of database search, study selection and eligibility were resolved by discussion. The second and the last authors (JW and AW) verified the eligibility of included studies.
Outcomes definitions
Signs and symptoms were defined as the presentation of fever, cough, sore throat, headache, dyspnea, muscle pain, diarrhea, rhinorrhea, anosmia, and ageusia at the hospital admission 13 .
Laboratory findings included a complete blood count (white blood count, neutrophil, lymphocyte, platelet count), procalcitonin, prothrombin time, urea, and serum biochemical measurements (including electrolytes, renal-function and liver-function values, creatine kinase, lactate dehydrogenase, C-reactive protein, Erythrocyte sedimentation rate), and treatment measures (i.e. antiviral therapy, antibiotics, corticosteroid therapy, mechanical ventilation, intubation, respiratory support, and renal replacement therapy). Radiological outcomes included bilateral involvement identified and pneumonia identified by chest radiograph.
Comorbidities of patients evaluated in this study were hypertension, diabetes, chronic obstructive pulmonary disease (COPD), cardiovascular disease, chronic kidney disease, liver disease and cancer.
In-patient treatment included intensive care unit admission, oxygen therapy, non-invasive ventilation, mechanical ventilation, Extracorporeal membrane oxygenation (ECMO), renal replacement therapy, and pharmacological treatment. Use of antiviral and interferon drugs (Lopinavir/ritonavir, Ribavirin, Umifenovir, Interferon-alpha, or Interferon-beta), antibiotic drugs, corticosteroid, and inotropes (Nor-adrenaline, Adrenaline, Vasopressin, Phenylephrine, Dopamine, or Dobutamine) were considered.
Data analysis
Three authors (CW, EHMT and CHA) extracted data using a standardized spreadsheet to record the article type, country of origin, surname of first author, year of publications, sample size, demographics, comorbidities, symptoms, laboratory and radiology results, pharmacological and non-pharmacological treatments.
We aggregated estimates across 90 eligible studies to produce meta-analytic estimates using a random-effects model. For dichotomous outcomes, we estimated the proportion and its respective 95% confidence interval. For laboratory parameters as continuous outcomes, we estimated the mean and standard deviation from the median and interquartile range if the mean and standard deviation were not available from the study 14 , and calculated the mean and its respective 95% confidence intervals. Random-effect models on DerSimonian and Laird method were adopted due to the significant heterogeneity, checked by the I 2 statistics and the p values. I 2 statistic of < 25%, 25–75% and ≥ 75% is considered as low, moderate, high likelihood of heterogeneity. Pooled estimates were calculated and presented by using forest plots. Publication bias was estimated by Egger’s regression test. Funnel plots of outcomes were also presented to assess publication bias.
All statistical analyses were conducted using the STATA Version 13.0 (Statacorp, College Station, TX). The random effects model was generated by the Stata packages ‘Metaprop’ for proportions 15 and ‘Metan’ for continuous variables 16 .
The selection and screen process are presented in Fig. 1 . A total of 241 studies were found by our searching strategy (71 in PubMed and 170 in Embase). 46 records were excluded due to duplication. After screening the abstracts and titles, 100 English studies were with original data and included in full-text screening. By further excluding 10 studies with not reporting symptoms presentation, laboratory findings, treatment and therapeutics, 90 studies 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 and 76 studies with more than one COVID-19 case 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 34 , 35 , 36 , 37 , 38 , 39 , 42 , 43 , 44 , 45 , 49 , 50 , 51 , 53 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 67 , 69 , 70 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 98 , 100 , 101 , 102 , 103 , 104 , 105 were included in the current systematic review and meta-analysis respectively. 73.3% 66 studies were conducted in China. Newcastle–Ottawa Quality Assessment Scale has been used to assess study quality of each included cohort study 107 . 30% (27/90) of included studies had satisfactory or good quality. The summary of the included study is shown in Table 1 .
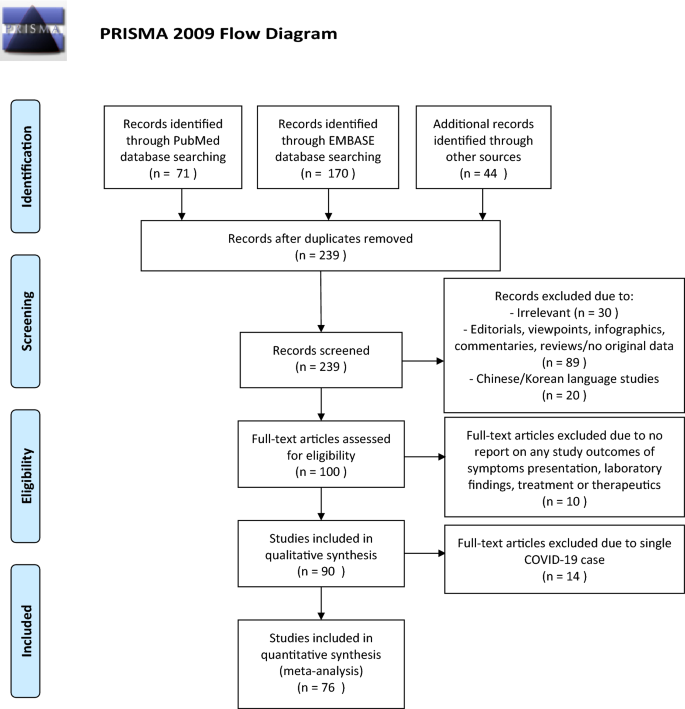
PRISMA flowchart reporting identification, searching and selection processes.
Of those 90 eligible studies, 11,028 COVID-19 patients were identified and included in the systematic review. More than half of patients (6336, 57.5%) were from mainland China. The pooled mean age was 45.8 (95% CI 38.6–52.5) years and 49.3% (pooled 95% CI 45.6–53.0%) of them were male.
For specific comorbidity status, the most prevalent comorbidity was hypertension (18.1%, 95% CI 15.4–20.8%), followed by cardiovascular disease (11.8%, 95% CI 9.4–14.2%) and diabetes (10.4%, 95% CI 8.7–12.1%). The pooled prevalence (95% CI) of COPD, chronic kidney disease, liver disease and cancer were 2.0% (1.3–2.7%), 5.2% (1.7–8.8%), 2.5% (1.7–3.4%) and 2.1% (1.3–2.8%) respectively. Moderate to substantial heterogeneity between reviewed studies were found, with I 2 statistics ranging from 39.4 to 95.9% ( p values between < 0.001–0.041), except for liver disease (I 2 statistics: 1.7%, p = 0.433). Detailed results for comorbidity status are displayed in Fig. 2 .
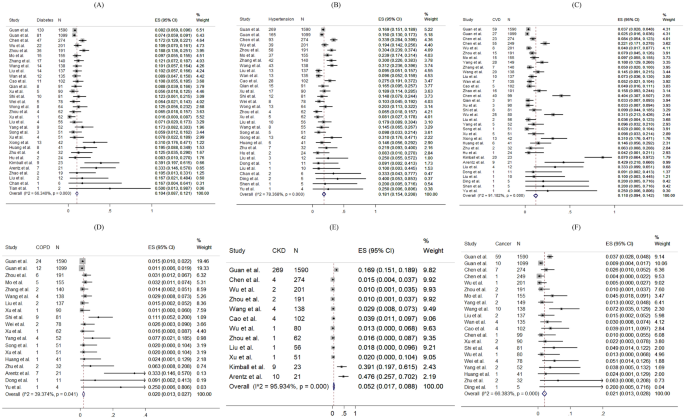
Random-effects meta-analytic estimates for comorbidities. ( A ) Diabetes mellitus, ( B ) Hypertension, ( C ) Cardiovascular disease, ( D ) Chronic obstructive pulmonary disease, ( E ) Chronic kidney disease, ( F ) Cancer.
Regarding the symptoms presented at hospital admission, the most frequent symptoms were fever (pooled prevalence: 72.4%, 95% CI 67.2–77.7%) and cough (pooled prevalence: 55.5%, 95% CI 50.7–60.3%). Sore throat (pooled prevalence: 16.2%, 95% CI 12.7–19.7%), dyspnoea (pooled prevalence: 18.8%, 95% CI 14.7–22.8%) and muscle pain (pooled prevalence: 22.1%, 95% CI 18.6–25.5%) were also common symptoms found in COVID-19 patients, but headache (pooled prevalence: 10.5%, 95% CI 8.7–12.4%), diarrhoea (pooled prevalence: 7.9%, 95% CI 6.3–9.6%), rhinorrhoea (pooled prevalence: 9.2%, 95% CI 5.6–12.8%) were less common. However, none of the included papers reported prevalence of anosmia and ageusia. The I 2 statistics varied from 68.5 to 97.1% (all p values < 0.001), indicating a high heterogeneity exists across studies. Figure 3 shows the pooled proportion of symptoms of patients presented at hospital.

Random-effects meta-analytic estimates for presenting symptoms. ( A ) Fever, ( B ) Cough, ( C ) Dyspnoea, ( D ) Sore throat, ( E ) Muscle pain, ( F ) Headache.
For laboratory parameters, white blood cell (pooled mean: 5.31 × 10 9 /L, 95% CI 5.03–5.58 × 10 9 /L), neutrophil (pooled mean: 3.60 × 10 9 /L, 95% CI 3.31–3.89 × 10 9 /L), lymphocyte (pooled mean: 1.11 × 10 9 /L, 95% CI 1.04–1.17 × 10 9 /L), platelet count (pooled mean: 179.5 U/L, 95% CI 172.6–186.3 U/L), aspartate aminotransferase (pooled mean: 30.3 U/L, 95% CI 27.9–32.7 U/L), alanine aminotransferase (pooled mean: 27.0 U/L, 95% CI 24.4–29.6 U/L) and C-reactive protein (CRP) (pooled mean: 22.0 mg/L, 95% CI 18.3–25.8 mg/L) and D-dimer (0.93 mg/L, 95% CI 0.68–1.18 mg/L) were the common laboratory test taken for COVID-19 patients. Above results and other clinical factors are depicted in Fig. 4 . Same with the comorbidity status and symptoms, high likelihood of heterogeneity was detected by I 2 statistics for a majority of clinical parameters.
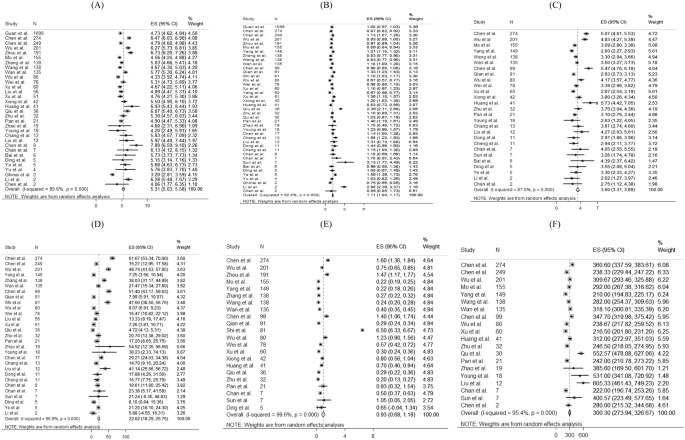
Random-effects meta-analytic estimates for laboratory parameters. ( A ) White blood cell, ( B ) Lymphocyte, ( C ) Neutrophil, ( D ) C-creative protein, ( E ) D-dimer, ( F ) Lactate dehydrogenase.
Figure 5 presents the distribution of the pharmacological treatments received for COVID-19 patients. 10.6% of patients admitted to intensive care units (pooled 95% CI 8.1–13.2%). For drug treatment, 63.9% (pooled 95% CI 52.5–75.3%), 62.4% (pooled 95% CI 47.9–76.8%) and 29.7% (pooled 95% CI 21.8–37.6%) patients used antibiotics, antiviral, and corticosteroid, respectively. 41.3% (pooled 95% CI 14.3–68.3%) and 50.7% (pooled 95% CI 9.2–92.3%) reported using Lopinavir/Ritonavir and interferon-alpha as antiviral drug treatment, respectively. Among 14 studies reporting proportion of corticosteroid used, 7 studies (50%) specified the formulation of corticosteroid as systemic corticosteroid. The remaining one specified the use of methylprednisolone. No reviewed studies reported the proportion of patients receiving Ribavirin, Interferon-beta, or inotropes.
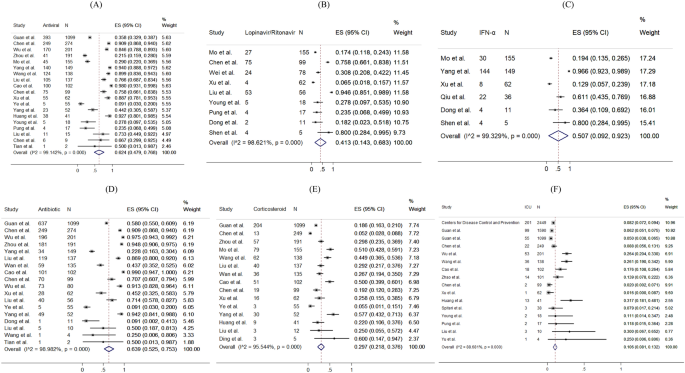
Random-effects meta-analytic estimates for pharmacological treatments and intensive unit care at hospital. ( A ) Antiviral or interferon drugs, ( B ) Lopinavir/Ritonavir, ( C ) Interferon alpha (IFN-α), ( D ) Antibiotic drugs, ( E ) Corticosteroid, ( F ) Admission to Intensive care unit.
The prevalence of radiological outcomes and non-pharmacological treatments were presented in Fig. 6 . Radiology findings detected chest X-ray abnormalities, with 74.4% (95% CI 67.6–81.1%) of patients with bilateral involvement and 74.9% (95% CI 68.0–81.8%) of patients with viral pneumonia. 62.6% (pooled 95% CI 39.9–85.4%), 20.2% (pooled 95% CI 14.6–25.9%), 15.3% (pooled 95% CI 11.0–19.7%), 1.1% (pooled 95% CI 0.4–1.8%) and 4.7% (pooled 95% CI 2.1–7.4%) took oxygen therapy, non-invasive ventilation, mechanical ventilation, ECMO and dialysis respectively.
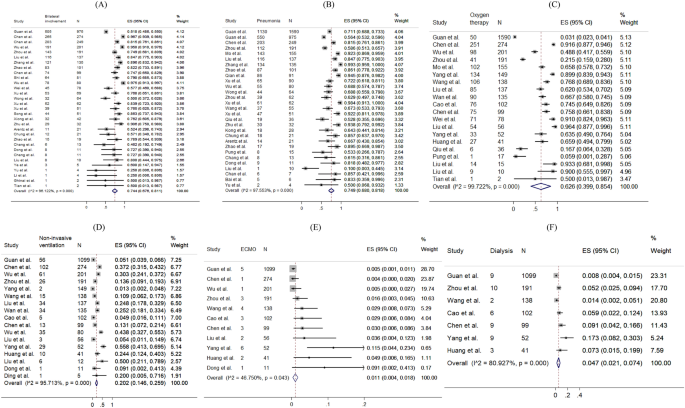
Random-effects meta-analytic estimates for radiological findings and non-pharmacological treatments at hospital. ( A ) Bilateral involvement, ( B ) Pneumonia, ( C ) Oxygen therapy, ( D ) Non-invasive ventilation, ( E ) Extracorporeal membrane oxygenation (ECMO), ( F ) Dialysis.
The funnel plots and results Egger’s test of comorbidity status, symptoms presented, laboratory test and treatment were presented in eFigure 1 – S5 in the Supplement. 63% (19/30) of the funnel plots (eFigure 1 – S5 ) showed significance in the Egger’s test for asymmetry, suggesting the possibility of publication bias or small-study effects caused by clinical heterogeneity.
This meta-analysis reveals the condition of global medical community responding to COVID-19 in the early phase. During the past 4 months, a new major epidemic focus of COVID-19, some without traceable origin, has been identified. Following its first identification in Wuhan, China, the virus has been rapidly spreading to Europe, North America, Asia, and the Middle East, in addition to African and Latin American countries. Three months since Wuhan CDC admitted that there was a cluster of unknown pneumonia cases related to Huanan Seafood Market and a new coronavirus was identified as the cause of the pneumonia 108 , as on 1 April, 2020, there have been 858,371 persons confirmed infected with COVID-19, affecting 202 countries and territories around the world. Although this rapid review is limited by the domination of reports from patients in China, and the patient population is of relative male dominance reflecting the gender imbalance of the Chinese population 109 , it provides essential information.
In this review, the pooled mean age was 45.8 years. Similar to the MERS-CoV pandemic 110 , middle-aged adults were the at-risk group for COVID-19 infections in the initial phase, which was different from the H1N1 influenza pandemic where children and adolescents were more frequently affected 111 . Biological differences may affect the clinical presentations of infections; however, in this review, studies examining the asymptomatic COVID-19 infections or reporting any previous infections were not included. It is suggested that another systematic review should be conducted to compare the age-specific incidence rates between the pre-pandemic and post-pandemic periods, so as to understand the pattern and spread of the disease, and tailor specific strategies in infection control.
Both sexes exhibited clinical presentations similar in symptomatology and frequency to those noted in other severe acute respiratory infections, namely influenza A H1N1 112 and SARS 113 , 114 . These generally included fever, new onset or exacerbation of cough, breathing difficulty, sore throat and muscle pain. Among critically ill patients usually presented with dyspnoea and chest tightness 22 , 29 , 39 , 72 , 141 (4.6%) of them with persistent or progressive hypoxia resulted in the requirement of intubation and mechanical ventilation 115 , while 194 (6.4%) of them required non-invasive ventilation, yielding a total of 11% of patients requiring ventilatory support, which was similar to SARS 116 .
The major comorbidities identified in this review included hypertension, cardiovascular diseases and diabetes mellitus. Meanwhile, the percentages of patients with chronic renal diseases and cancer were relatively low. These chronic conditions influencing the severity of COVID-19 had also been noted to have similar effects in other respiratory illnesses such as SARS, MERS-CoV and influenza 117 , 118 . Higher mortality had been observed among older patients and those with comorbidities.
Early diagnosis of COVID-19 was based on recognition of epidemiological linkages; the presence of typical clinical, laboratory, and radiographic features; and the exclusion of other respiratory pathogens. The case definition had initially been narrow, but was gradually broadened to allow for the detection of more cases, as milder cases and those without epidemiological links to Wuhan or other known cases had been identified 119 , 120 . Laboratory investigations among COVID-19 patients did not reveal specific characteristics—lymphopenia and elevated inflammatory markers such as CRP are some of the most common haematological and biochemical abnormalities, which had also been noticed in SARS 121 . None of these features were specific to COVID-19. Therefore, diagnosis should be confirmed by SARS-CoV–2 specific microbiological and serological studies, although initial management will continue to be based on a clinical and epidemiological assessment of the likelihood of a COVID-19 infection.
Radiology imaging often plays an important role in evaluating patients with acute respiratory distress; however, in this review, radiological findings of SARS-CoV-2 pneumonia were non-specific. Despite chest radiograph usually revealed bilateral involvement and Computed Tomography usually showed bilateral multiple ground-glass opacities or consolidation, there were also patients with normal chest radiograph, implying that chest radiograph might not have high specificity to rule out pneumonia in COVID-19.
Limited clinical data were available for asymptomatic COVID-19 infected persons. Nevertheless, asymptomatic infection could be unknowingly contagious 122 . From some of the official figures, 6.4% of 150 non-travel-related COVID-19 infections in Singapore 123 , 39.9% of cases from the Diamond Princess cruise ship in Japan 124 , and up to 78% of cases in China as extracted on April 1st, 2020, were found to be asymptomatic 122 . 76% (68/90) studies based on hospital setting which provided care and disease management to symptomatic patients had limited number of asymptomatic cases of COVID-19 infection. This review calls for further studies about clinical data of asymptomatic cases. Asymptomatic infection intensifies the challenges of isolation measures. More global reports are crucially needed to give a better picture of the spectrum of presentations among all COVID-19 infected persons. Also, public health policies including social and physical distancing, monitoring and surveillance, as well as contact tracing, are necessary to reduce the spread of COVID-19.
Concerning potential treatment regime, 62.4% of patients received antivirals or interferons (including oseltamivir, lopinavir-ritonavir, interferon alfa), while 63.9% received antibiotics (such as moxifloxacin, and ceftriaxone). In this review, around one-third of patients were given steroid, suggestive as an adjunct to IFN, or sepsis management. Interferon and antiviral agents such as ribavirin, and lopinavir-ritonavir were used during SARS, and the initial uncontrolled reports then noted resolution of fever and improvement in oxygenation and radiographic appearance 113 , 125 , 126 , without further evidence on its effectiveness. At the time of manuscript preparation, there has been no clear evidence guiding the use of antivirals 127 . Further research is needed to inform clinicians of the appropriate use of antivirals for specific groups of infected patients.
Limitations of this meta-analysis should be considered. First, a high statistical heterogeneity was found, which could be related to the highly varied sample sizes (9 to 4226 patients) and study designs. Second, variations of follow-up period may miss the event leading to heterogeneity. In fact, some patients were still hospitalized in the included studies. Third, since only a few studies had compared the comorbidities of severe and non-severe patients, sensitivity analysis and subgroup analysis were not conducted. Fourthly, the frequency and severity of signs and symptoms reported in included studies, primarily based on hospitalized COVID-19 patients were over-estimated. Moreover, different cutoffs for abnormal laboratory findings were applied across countries, and counties within the same countries. Lastly, this meta-analysis reviewed only a limited number of reports written in English, with a predominant patient population from China. This review is expected to inform clinicians of the epidemiology of COVID-19 at this early stage. A recent report estimated the number of confirmed cases in China could reach as high as 232,000 (95% CI 161,000, 359,000) with the case definition adopted in 5th Edition. In this connection, further evidence on the epidemiology is in imminent need.
Oliveira N. Shrimp vendor identified as possible coronavirus ‘patient zero,’ leaked document says. 27 March 2020. New York Daily News. 2020.
World Health Organization. Basic protective measures against the new coronavirus (2020). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public . Accessed 7 Oct 2020.
Google Trend. When will coronavirus end (2020). https://trends.google.com/trends/explore?date=today%203-m&q=when%20will%20coronavirus%20end,%2Fm%2F01cpyy . Accessed 10 Oct 2020.
Worldometer. COVID-19 Coronavirus Pandemic (2020). https://www.worldometers.info/coronavirus/ . Accessed 13 Oct 2020.
Lu, R. et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395 (10224), 565–574 (2020).
Article CAS PubMed PubMed Central Google Scholar
Ralph, R. et al. 2019-nCoV (Wuhan virus), a novel Coronavirus: human-to-human transmission, travel-related cases, and vaccine readiness. J. Infect. Dev. Ctries. 14 (1), 3–17 (2020).
Article CAS PubMed Google Scholar
Sun, Z., Thilakavathy, K., Kumar, S. S., He, G. & Liu, S. V. Potential factors influencing repeated SARS outbreaks in China. Int. J. Environ. Res. Public Health 17 (5), 1633 (2020).
Article CAS PubMed Central Google Scholar
Zhao, S. et al. The association between domestic train transportation and novel coronavirus (2019-nCoV) outbreak in China from 2019 to 2020: a data-driven correlational report. Travel Med. Infect. Dis. 33 , 101568 (2020).
Article PubMed PubMed Central Google Scholar
Li, Q. et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 382 (13), 1199–1207 (2020).
Chen, J. Pathogenicity and transmissibility of 2019-nCoV-A quick overview and comparison with other emerging viruses. Microbes Infect. 22 (2), 69–71 (2020).
World Health Organization. Database of publications on coronavirus disease (COVID-19) (2020). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov . Accessed 30 Mar 2020.
Wong, C. K. H. et al. Impact of national containment measures on decelerating the increase in daily new cases of COVID-19 in 54 countries and 4 epicenters of the pandemic: comparative observational study. J. Med. Internet Res. 22 (7), e19904 (2020).
Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Symptoms of Coronavirus (2020).
Wan, X., Wang, W., Liu, J. & Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14 (1), 135 (2014).
Nyaga, V. N., Arbyn, M. & Aerts, M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch. Public Health 72 (1), 39 (2014).
Harris, R. J. et al. metan: fixed- and random-effects meta-analysis. Stata J. 8 (1), 3–28 (2008).
Article Google Scholar
Xu, X. et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur. J. Nucl. Med. Mol. Imaging 47 (5), 1275–1280 (2020).
Cao, J. et al. Clinical features and short-term outcomes of 18 patients with corona virus disease 2019 in intensive care unit. Intensive Care Med. 46 (5), 851–853 (2020).
Xiong, Y. et al. Clinical and high-resolution CT features of the COVID-19 infection: comparison of the initial and follow-up changes. Invest. Radiol. 55 (6), 332–339 (2020).
Arentz, M. et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA 323 (16), 1612–1614 (2020).
Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395 (10223), 497–506 (2020).
Guan, W. J. et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382 (18), 1708–1720 (2020).
Zhao, D. et al. A comparative study on the clinical features of coronavirus 2019 (COVID-19) pneumonia with other pneumonias. Clin. Infect. Dis. 71 (15), 756–761 (2020).
Xu, X. W. et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 19 (368), m606 (2020).
Chan, J. F. et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395 (10223), 514–523 (2020).
Chen, N. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395 (10223), 507–513 (2020).
Pung, R. et al. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet 395 (10229), 1039–1046 (2020).
Wang, D. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323 (11), 1061–1069 (2020).
Young, B. E. et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 323 (15), 1488–1494 (2020).
Chen, H. et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 395 (10226), 809–815 (2020).
Huang, W. H. et al. 2019 novel coronavirus disease (COVID-19) in Taiwan: reports of two cases from Wuhan, China. J. Microbiol. Immunol. Infect. 53 (3), 481–484 (2020).
Cheng, S. C. et al. First case of coronavirus disease 2019 (COVID-19) pneumonia in Taiwan. J. Formos. Med. Assoc. 119 (3), 747–751 (2020).
Holshue, M. L. et al. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 382 (10), 929–936 (2020).
Wei, M. et al. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA 323 (13), 1313–1314 (2020).
Bernard Stoecklin, S. et al. First cases of coronavirus disease 2019 (COVID-19) in France: surveillance, investigations and control measures, January 2020. Euro Surveill. 25 (6), 20–26 (2020).
Shi, H. et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 20 (4), 425–434 (2020).
Zhu, N. et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 382 (8), 727–733 (2020).
Ghinai, I. et al. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet 395 (10230), 1137–1144 (2020).
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395 (10229), 1054–1062 (2020).
Yang, X. et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 8 (5), 475–481 (2020).
Kim, J. Y. et al. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: implication for infection prevention and control measures. J. Korean Med. Sci. 35 (5), e61 (2020).
Okada, P. et al . Early transmission patterns of coronavirus disease 2019 (COVID-19) in travellers from Wuhan to Thailand, January 2020. Euro Surveill . 25 (8), 6–10 (2020).
Arashiro, T., Furukawa, K. & Nakamura, A. COVID-19 in 2 persons with mild upper respiratory tract symptoms on a cruise ship, Japan. Emerg. Infect. Dis. 26 (6), 1345–1348 (2020).
Lillie, P. J. et al. Novel coronavirus disease (Covid-19): the first two patients in the UK with person to person transmission. J. Infect. 80 (5), 578–606 (2020).
Tian, S. et al. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 15 (5), 700–704 (2020).
Haveri, A. et al . Serological and molecular findings during SARS-CoV-2 infection: the first case study in Finland, January to February 2020. Euro Surveill . 25 (11), 16–21 (2020).
Nicastri, E. et al . Coronavirus disease (COVID-19) in a paucisymptomatic patient: epidemiological and clinical challenge in settings with limited community transmission, Italy, February 2020. Euro Surveill . 25 (11) (2020).
Van Cuong, L. et al. The first Vietnamese case of COVID-19 acquired from China. Lancet Infect Dis. 20 (4), 408–409 (2020).
Spiteri, G. et al . First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Euro Surveill . 25 (9), 2–7 (2020).
Rothe, C. et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 382 (10), 970–971 (2020).
Tong, Z. D. et al. Potential presymptomatic transmission of SARS-CoV-2, Zhejiang Province, China, 2020. Emerg. Infect. Dis. 26 (5), 1052–1054 (2020).
Bai, Y. et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA 323 (14), 1406–1407 (2020).
Yu, P., Zhu, J., Zhang, Z. & Han, Y. A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period. J. Infect. Dis. 221 (11), 1757–1761 (2020).
Li, P. et al. Transmission of COVID-19 in the terminal stages of the incubation period: a familial cluster. Int. J. Infect. Dis. 96 , 452–453 (2020).
Tang, A. et al. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg. Infect. Dis. 26 (6), 1337–1339 (2020).
Kam, K. Q. et al. A well infant with coronavirus disease 2019 with high viral load. Clin. Infect. Dis. 71 (15), 847–849 (2020).
Zhou, S., Wang, Y., Zhu, T. & Xia, L. CT Features of Coronavirus Disease 2019 (COVID-19) Pneumonia in 62 Patients in Wuhan. China. AJR Am J Roentgenol. 214 (6), 1287–1294 (2020).
Article PubMed Google Scholar
Zhao, W., Zhong, Z., Xie, X., Yu, Q. & Liu, J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am. J. Roentgenol. 214 (5), 1072–1077 (2020).
Cheng, Z. et al. Clinical features and chest CT manifestations of coronavirus disease 2019 (COVID-19) in a single-center study in Shanghai, China. AJR Am. J. Roentgenol. 215 (1), 121–126 (2020).
Chung, M. et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology 295 (1), 202–207 (2020).
Liu, K. et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 133 (9), 1025–1031 (2020).
Chang, L. M. et al. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA 323 (11), 1092–1093 (2020).
Team C-NIRS. COVID-19, Australia: Epidemiology Report 7 (Reporting week ending 19:00 AEDT 14 March 2020). Commun. Dis. Intell. 44 (2018).
Pan, F. et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology 295 (3), 715–721 (2020).
Wang, S. et al. A case report of neonatal 2019 coronavirus disease in China. Clin. Infect. Dis. 71 (15), 853–857 (2020).
Bastola, A. et al. The first 2019 novel coronavirus case in Nepal. Lancet Infect. Dis. 20 (3), 279–280 (2020).
Qiu, H. et al. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect. Dis. 20 (6), 689–696 (2020).
Zhang, J. J. et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 75 (7), 1730–1741 (2020).
Ye, G. et al. Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. J. Infect. 80 (5), e14–e17 (2020).
Liu, Y. et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 63 (3), 364–374 (2020).
Chen, T. et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 26 (368), m1091 (2020).
Guan, W. J. et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 55 (5), 2000547 (2020).
Wong, H. Y. F. et al. Frequency and Distribution of Chest Radiographic Findings in Patients Positive for COVID-19. Radiology 296 (2), E72–E78 (2020).
Xu, T. et al. Clinical features and dynamics of viral load in imported and non-imported patients with COVID-19. Int J Infect Dis. 94 , 68–71 (2020).
Shen, C. et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 323 (16), 1582–1589 (2020).
Kimball, A. et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility—King County, Washington, March 2020. Morb. Mortal. Wkly. Rep. 69 (13), 377–381 (2020).
Article CAS Google Scholar
Team CC-R. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12-March 16, 2020. Morb. Mortal. Wkly. Rep. 69 (12), 343–346 (2020).
Wu, J. et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu Province: a multicenter descriptive study. Clin. Infect. Dis. 71 (15), 706–712 (2020).
Yang, W. et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J. Infect. 80 (4), 388–393 (2020).
Zhu, L. et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am. J. Transplant. 20 (7), 1859–1863 (2020).
Zhu, W. et al. Initial clinical features of suspected coronavirus disease in two emergency departments outside of Hubei, China. J. Med. Virol. 92 , 1525–1532 (2019).
Wu, C. et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 180 (7), 934–943 (2020).
Wang, Z., Chen, X., Lu, Y., Chen, F. & Zhang, W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci. Trends 14 (1), 64–68 (2020).
Wang, Y. et al. Clinical outcomes in 55 patients with severe acute respiratory syndrome coronavirus 2 who were asymptomatic at hospital admission in Shenzhen, China. J. Infect. Dis. 221 (11), 1770–1774 (2020).
Wan, S. et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med.. Virol. 92 (7), 797–806 (2020).
Tian, S. et al. Characteristics of COVID-19 infection in Beijing. J. Infect. 80 (4), 401–406 (2020).
Sun, D. et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J. Pediatr. 16 (3), 251–259 (2020).
Song, F. et al. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 295 (1), 210–217 (2020).
Hu, Z. et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci. China Life Sci. 63 (5), 706–711 (2020).
Qu, R. et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J. Med. Virol. 92 , 1533–1541 (2020).
Qian, G. Q. et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM 113 (7), 474–481 (2020).
Mo, P. et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin. Infect. Dis . (2020).
Liu, W. et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med. J. (Engl) 133 (9), 1032–1038 (2020).
Liu, K., Chen, Y., Lin, R. & Han, K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J. Infect. 80 (6), e14–e18 (2020).
Liu, F. et al. Patients of COVID-19 may benefit from sustained Lopinavir-combined regimen and the increase of Eosinophil may predict the outcome of COVID-19 progression. Int. J. Infect. Dis. 95 , 183–191 (2020).
Liu, D. et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am. J. Roentgenol. 215 (1), 127–132 (2020).
Guillen, E. et al. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation?. Am. J. Transplant. 20 (7), 1875–1878 (2020).
Dong, X. et al. Eleven faces of coronavirus disease 2019. Allergy 75 (7), 1699–1709 (2020).
Fan, C. et al . Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry? Clin. Infect. Dis. (2020).
Chen, R. et al. Safety and efficacy of different anesthetic regimens for parturients with COVID-19 undergoing Cesarean delivery: a case series of 17 patients. Can. J. Anaesth. 67 (6), 655–663 (2020).
Chen, L. et al. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg. Microbes Infect. 9 (1), 313–319 (2020).
Chen, J. et al. Clinical progression of patients with COVID-19 in Shanghai, China. J. Infect. 80 (5), e1–e6 (2020).
Ding, Q., Lu, P., Fan, Y., Xia, Y. & Liu, M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J. Med. Virol. 92 , 1549–1555 (2020).
Covid-19 National Emergency Response Center E & Case Management Team KCfDC, Prevention. Early epidemiological and clinical characteristics of 28 cases of coronavirus disease in South Korea. Osong Public Health Res. Perspect. 11 (1), 8–14 (2020).
Li, Y., Guo, F., Cao, Y., Li, L. & Guo, Y. Insight into COVID-2019 for pediatricians. Pediatr. Pulmonol. 55 (5), E1–E4 (2020).
Ai, J. W., Zhang, Y., Zhang, H. C., Xu, T. & Zhang, W. H. Era of molecular diagnosis for pathogen identification of unexplained pneumonia, lessons to be learned. Emerg Microbes Infect. 9 (1), 597–600 (2020).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25 (9), 603–605 (2010).
Khan N. New virus discovered by Chinese scientists investigating pneumonia outbreak. Wall Street J . (2020).
国家统计局 (National Bureau of Statistics). 2019 年国民经济运行总体平稳 发展主要预期目标较好实现 (In 2019, the overall stable development of the national economic operation is expected to achieve the main goals (2020). http://www.stats.gov.cn/tjsj/zxfb/202001/t20200117_1723383.html . Accessed 30 Mar 2020.
Park, J. E., Jung, S., Kim, A. & Park, J. E. MERS transmission and risk factors: a systematic review. BMC Public Health 18 (1), 574 (2018).
Van Kerkhove, M. D. et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 8 (7), e1001053 (2011).
Wang, C. et al. Epidemiological and clinical characteristics of the outbreak of 2009 pandemic influenza A (H1N1) at a middle school in Luoyang, China. Public Health 126 (4), 289–294 (2012).
Lee, N. et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 348 (20), 1986–1994 (2003).
Booth, C. M. et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 289 (21), 2801–2809 (2003).
Fowler, R. A. et al. Critically ill patients with severe acute respiratory syndrome. JAMA 290 (3), 367–373 (2003).
Christian, M. D., Poutanen, S. M., Loutfy, M. R., Muller, M. P. & Low, D. E. Severe acute respiratory syndrome. Clin Infect Dis. 38 (10), 1420–1427 (2004).
Mertz, D. et al. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ 23 (347), f5061 (2013).
Badawi, A. & Ryoo, S. G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int. J. Infect. Dis. 49 , 129–133 (2016).
Tsang, T. K. et al. Effect of changing case definitions for COVID-19 on the epidemic curve and transmission parameters in mainland China: a modelling study. Lancet Public Health. 5 (5), e289–e296 (2020).
国家卫生健康委办公厅 (Office of National Health Comission). 新型冠状病毒肺炎诊疗方案 (试行第七版) (Clinical Guideline for Novel Coronavirus Pneumonia—Interim 7th Edition) (2020).
File, T. M. Jr. & Tsang, K. W. Severe acute respiratory syndrome: pertinent clinical characteristics and therapy. Treat. Respir. Med. 4 (2), 95–106 (2005).
Day, M. Covid-19: four fifths of cases are asymptomatic, China figures indicate. BMJ 2 (369), m1375 (2020).
Wei, W. E. et al. Presymptomatic transmission of SARS-CoV-2—Singapore, January 23–March 16, 2020. Morb. Mortal. Wkly. Rep. 69 (14), 411–415 (2020).
Mizumoto, K., Kagaya, K., Zarebski, A. & Chowell, G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 25 (10), 2000180 (2020).
Article PubMed Central Google Scholar
Poutanen, S. M. et al. Identification of severe acute respiratory syndrome in Canada. N. Engl. J .Med. 348 (20), 1995–2005 (2003).
Tsang, K. W. et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 348 (20), 1977–1985 (2003).
Cao, B. et al. A trial of Lopinavir–Ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 382 (19), 1787–1799 (2020).
Download references
Acknowledgements
There was no funding source for this study.
Author information
These authors contributed equally: Carlos K. H. Wong and Janet Y. H. Wong.
Authors and Affiliations
Department of Family Medicine and Primary Care, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China
Carlos K. H. Wong, Eric H. M. Tang & C. H. Au
Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China
Carlos K. H. Wong
School of Nursing, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China
Janet Y. H. Wong
Emergency Medicine Unit, Li Ka Shing, Faculty of Medicine, The University of Hong Kong, Hong Kong, China
Abraham K. C. Wai
You can also search for this author in PubMed Google Scholar
Contributions
C.W., J.W. and A.W. contributed equally to all aspects of study design, conduct, data interpretation, and the writing of the manuscript. C.W., E.T. and C.H.A. contributed to eligibility screening, data extraction from eligible studies, and data analysis and interpretation.
Corresponding author
Correspondence to Abraham K. C. Wai .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary figure 1., supplementary figure 2., supplementary figure 3., supplementary figure 4., supplementary figure 5., supplementary material 6., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Wong, C.K.H., Wong, J.Y., Tang, E.H.M. et al. Clinical presentations, laboratory and radiological findings, and treatments for 11,028 COVID-19 patients: a systematic review and meta-analysis. Sci Rep 10 , 19765 (2020). https://doi.org/10.1038/s41598-020-74988-9
Download citation
Received : 04 May 2020
Accepted : 25 September 2020
Published : 13 November 2020
DOI : https://doi.org/10.1038/s41598-020-74988-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Comorbidity genetic risk and pathways impact sars-cov-2 infection outcomes.
- Rachel K. Jaros
- Tayaza Fadason
- Justin M. O’Sullivan
Scientific Reports (2023)
Adrenal function in relation to cytokines and outcome in non-critically ill patients with COVID-19
- N. Athanasiou
- A. Diamantopoulos
- D. A. Vassiliadi
Journal of Endocrinological Investigation (2023)

The Role of Multidimensional Prognostic Index to Identify Hospitalized Older Adults with COVID-19 Who Can Benefit from Remdesivir Treatment: An Observational, Prospective, Multicenter Study
- Carlo Custodero
- Nicola Veronese
- Julia Schlotmann
Drugs & Aging (2023)
Prevalence of hypertension and associated risks in hospitalized patients with COVID-19: a meta-analysis of meta-analyses with 1468 studies and 1,281,510 patients
- Yousof Khairy
- Deniz Naghibi
- Saber Azami-Aghdash
Systematic Reviews (2022)
An alternative approach to determination of Covid-19 personal risk index by using fuzzy logic
- Hakan Şimşek
- Elifnaz Yangın
Health and Technology (2022)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Presentation skills:...
Presentation skills: plan, prepare, phrase, and project
- Related content
- Peer review
- Laura Brammar , careers adviser, C2 Careers
- laura.brammar{at}careers.lon.ac.uk
In the third of her series on getting the dream job, Laura Brammar looks at giving an interview presentation
Many doctors have extensive experience of delivering presentations at conferences, during research projects, or to medical students during their training. Nevertheless, for many medical professionals having to deliver a presentation is still something they dread rather than relish. Equally, candidate presentations are becoming an established feature of selection and assessment for many roles within medicine.
Applicants may be asked to prepare and deliver a presentation as part of the interview process for anything from a salaried general practitioner post to a senior consultant post. For that reason alone, it’s vital to grasp the nettle and strengthen those presentation skills, which you can draw on throughout your medical career.
Break it down to just four P’s
To prepare most effectively for your presentation, you might find considering four main areas particularly useful: planning, preparation, phrasing, and projection.
Planning —A good presentation begins with the early stage of planning. Common complaints about ineffectual and dull presentations revolve around the apparent lack of structure shown by the presenter.
You will generally be given the topic of your presentation in advance. Topics vary, but they usually relate to your specialty—for example, “What do you see as the main current issues/future direction of this specialty?”—or link to contextual factors related to the role—“How, in your opinion, could the current system of X work more effectively?”
Think of the title as your research question or hypothesis and structure your presentation so that you answer that question directly. A simple but effective framework for any presentation is: tell them what you’re going to say, say it, and tell them what you’ve just said. Whether your presentation relates to the latest National Institute for Health and Clinical Excellence (NICE) guidelines for your specialty or a business plan in response to a proposed polyclinic, this structure will help keep your audience engaged and your presentation within the time limit.
Indeed, timing is crucial when giving a presentation. Most candidates are overambitious about what can be squeezed into just five to ten minutes. Be realistic about what you can achieve in the time limit and plan your presentation accordingly. As a rule of thumb, less is almost always more and remember to build in time for questions at the end.
Preparation —Having a clear structure can give you a useful framework that underpins your presentation. In a similar way, using particular resources to support your point can be a good method to employ during a presentation.
While the use of PowerPoint is becoming increasingly popular, in these circumstances you need to check before slaving over your slides. Remember that you are the focus of the presentation, not the screen; avoid distracting animations and excessive detail. Even if PowerPoint is an option you still need to plan for technological meltdowns; bring hard copies and overhead slides as a back-up.
You may consider it worth while to produce a brief summarising handout of the main points. Aim to distribute this before you begin so that you can create a clear and confident start, rather than compete with the rustle of paper as you try to introduce yourself.
Phrasing —Many candidates get anxious about the fact that they may “um” and “er” during a presentation. The vast majority of people feel nervous when they are presenting. Accept that and remember that, to an extent, it is what your audience will expect; from the selectors’ perspective, a completely laidback candidate might appear unmotivated and flippant. So while you want to aim for a fluid and articulate delivery, it’s not the end of the world if you occasionally need to pause between sentences. Indeed pauses can be an excellent way of emphasising your points and retaining your audience’s attention.
Essentially, use your structure to help you—for example, “First, I’d like to talk about . . .; next, let’s look at . . . ; and, finally, in summary . . ..”Also, be aware of your pace and volume.
Projection —Many people associate the term “presentation skills” with aspects of non-verbal communication, such as gestures and facial expression. Even when you feel nervous there are ways successfully to convey confidence to your audience. The following suggestions will help you to show a positive and calm attitude, which in turn will help you to maintain control over your presentation.
Breathe—If you are particularly nervous before you start, take a few moments to slow down your breathing; it may help to think about balancing the length of your inhalation and exhalation and breathe deeply and evenly.
Share your eye contact—If feasible, make eye contact with all your audience throughout your presentation; if you are presenting to a large group, make sure you address both sides of the room during your session.
Take time to pause—Use pauses to illustrate the structure of your session. Brief pauses can also help you to slow down your delivery and maintain the focus of your audience.
Project your voice—Check that those at the back can hear you before you start. Maintain your volume throughout and aim at projecting your voice to the back of the room.
Own the space—If possible, try not to stay stuck to one spot for the duration of the presentation. Clearly, now isn’t the time to try out gestures that feel unnatural or forced. However, convey your confidence through the way you stand and emphasise your message through your body language.
Smile—Despite feeling anxious, displaying a smile can make you feel more relaxed. Even better, it also gives your audience confidence in you and in your message.
Awkward audience moments
While you may have organised thoroughly your planning, preparation, phrasing, and projection, the one area you cannot control or necessarily predict is your audience’s reactions. Many people find the thought of their audience’s responses, especially during the question and answer session, far more terrifying than the presentation itself.
Here are a few suggestions for how to deal with some common difficult situations.
Random interruptions —If someone asks a question in the middle of your presentation, make a decision whether it would be appropriate to deal with it now or later. Don’t be forced to change your structure unless you believe it is really necessary. Acknowledge the question and reassure the person that there will be opportunities to discuss that later. Equally, if it is an unrelated or irrelevant question remember to acknowledge it but make it clear that such a topic isn’t going to be dealt with explicitly on this occasion. You can always offer to research that question for them at a later opportunity.
Audience looks bored —Many people feel they are poor presenters because their audiences can look distracted or even bored. The key thing here is to ask yourself if they are actually bored or whether they are just presenting you with a professional and impartial expression. In your clinical work you need to be able to focus on a task and not be distracted by personal emotional considerations or anxieties; this is no different. Treat the presentation as a professional exercise and move on.
Someone isn’t listening and is talking to someone nearby —Depending on your audience (senior consultants or medical students, for example) you may want to vary your specific response to this. However, a good technique with any audience is to pause in your delivery, look at the culprits while smiling, and wait for their attention before you start again. This is an effective (and non-aggressive) way of acknowledging that they are distracting both you and the rest of the group. That is usually all it takes to get their full attention. However, if they are persistent offenders maintain your professionalism and carry on regardless.
Questions you can’t answer —Sometimes the dread of the questions at the end of a presentation can overshadow the whole experience. Avoid this by framing your question and answer session with a reassurance that you’ll do your best to deal with any questions now and will guarantee to follow up any additional questions after the session. If you are asked a reasonable question which you genuinely can’t answer you may want to try the following:
Acknowledge that it’s a valid question
Invite any suggestions from the audience first
Admit that you can’t give a full answer at this moment; don’t bluff an answer
Offer to follow up a response and email the person later.
Remember that part of good medical practice is to know your limits and work within the parameters of your knowledge; it sounds far more confident and impressive to admit you can’t answer a question fully at this moment, rather than try to cobble together a poor answer and pretend you know.
Want to practise in a supportive environment?
The BMA Careers Service works with many individual medics who wish to improve their presentation skills through a tailormade practice presentation service. A bespoke practice presentation session, based on your actual material, can be excellent preparation for the real thing. During the session you can rehearse your presentation fully, practise answering focused questions, and gain immediate and constructive one to one feedback on your overall performance ( www.bma.org.uk/ap.nsf/Content/Hubcareersadvicefordoctors ).
Competing interests : None declared.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Wiley - PMC COVID-19 Collection

Clinical presentation and management of COVID ‐19
Irani thevarajan.
1 Victorian Infectious Diseases Service, Royal Melbourne Hospital, Melbourne VIC
2 University of Melbourne, Melbourne VIC
Kirsty L Buising
Benjamin c cowie.
3 WHO Collaborating Centre for Viral Hepatitis, Doherty Institute, Melbourne VIC
- The rapid spread of severe acute respiratory syndrome coronavirus 2 led to the declaration of a global pandemic within 3 months of its emergence.
- The majority of patients presenting with coronavirus disease 2019 ( COVID ‐19) experience a mild illness that can usually be managed in the community. Patients require careful monitoring and early referral to hospital if any signs of clinical deterioration occur.
- Increased age and the presence of comorbidities are associated with more severe disease and poorer outcomes.
- Treatment for COVID ‐19 is currently predominantly supportive care, focused on appropriate management of respiratory dysfunction.
- Clinical evidence is emerging for some specific therapies (including antiviral and immune‐modulating agents). Investigational therapies for COVID ‐19 should be used in the context of approved randomised controlled trials.
- Australian clinicians need to be able to recognise, diagnose, manage and appropriately refer patients affected by COVID ‐19, with thousands of cases likely to present over the coming years.
In December 2019, a novel coronavirus emerged in Wuhan, Hubei Province, China, leading to a global pandemic. The virus, named severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), causes a clinical syndrome termed coronavirus disease 2019 (COVID‐19).
The first reports of an undiagnosed pneumonia in Wuhan on 8 December 2019 were followed by an alert from China to the World Health Organization (WHO) about a cluster of pneumonia cases on 30 December. Isolation of a novel coronavirus occurred on 3 January 2020. On 30 January, the WHO declared a public health emergency of international concern, and a pandemic was declared on 12 March 2020.
Clinical presentation
Similar to other coronaviruses, SARS‐CoV‐2 is predominantly spread by respiratory droplets, although spread by contact with contaminated fomites also occurs, as does transmission by aerosols in certain circumstances. 1
Based on the experience in China, the typical incubation period of COVID‐19 infection has been estimated to be a median of 5.1 days (95% CI, 4.5–5.8 days), with 97.5% of those who develop symptoms doing so within 11 days of exposure (95% CI, 8.2–15.6 days). This has informed the use of a 14‐day time period for quarantining potentially exposed individuals in an effort to limit onward spread. 2
The recognition of asymptomatic infection has been an area of intense interest in understanding the epidemiology of COVID‐19. The ratio of asymptomatic to symptomatic infection is currently uncertain. Cross‐sectional studies have reported asymptomatic infection in women attending a maternity service in New York (33 of 215 infected, 88% asymptomatic) 3 and in general population testing in Iceland (87 of 10 797 infected, 41% asymptomatic). 4 In such cross‐sectional studies, a proportion of those who were asymptomatic at the time of testing may in fact have been in the pre‐symptomatic phase of infection. In a study conducted in a nursing home in the United States, 48 of 76 residents tested positive, with 27 (56%) being asymptomatic at the time of testing. However, 24 (89%) of these individuals went on to develop symptoms at a median of 4 days (interquartile range [IQR], 3–5 days) after the positive test result. 5
Symptomatic COVID‐19 infection usually presents as a respiratory syndrome, most commonly with fever and cough. 6 , 7
Fever has been reported in up to 99% of people at some time during the course of their illness, but importantly in one cohort, it was reported to be present at the time of hospital presentation in only 44% of patients, and at some time during the hospital admission in 89%. 8 Other common symptoms are cough, dyspnoea, fatigue, anorexia, anosmia, myalgia and sometimes confusion. Diarrhoea may occur in up to 10% of patients. 9 Symptoms reported less frequently (< 5% of cases) include sore throat, rhinorrhoea, headache, chest pain, dizziness, abdominal pain and nausea. 6 , 7
Around 80% of COVID‐19 infections present as a mild respiratory illness in a patient who is ambulatory and can generally be managed outside the hospital. Around 15% typically need hospital care (usually for moderate to severe pneumonia), and another 5% have critical illness requiring more intensive supports. 10
Of those who require hospitalisation, the median time from first symptoms to onset of dyspnoea is 5 days (IQR, 1–10 days), the median time to hospital admission is 7 days (IQR, 4–8 days), and in those who develop more severe manifestations, the median time to acute respiratory distress syndrome is 8 days (IQR, 6–12 days). 6 About a quarter of patients who are hospitalised may need transfer to the intensive care unit (ICU) for the management of complications such as hypoxaemic respiratory failure or hypotension requiring vasopressor support. 11
At presentation to hospital, the most common laboratory feature of COVID‐19 infection is lymphopenia (reported in 70.3% of cases). 6 Radiological imaging may reveal a clear chest, unilateral or bilateral consolidation, or ground glass opacity.
Nasopharyngeal specimens, deep nasal swabs, throat swabs or lower respiratory samples (eg, sputum) sent for molecular detection of SARS‐CoV‐2 by polymerase chain reaction (PCR) are currently the best means of specific diagnosis of COVID‐19 in Australia. Faecal samples may also be PCR positive for COVID‐19 but the role of the oral–faecal route for transmission remains unclear. 12 Patients with more severe disease tend to have higher viral loads in respiratory samples. Mild cases have been shown to clear the virus earlier, with over 90% returning negative PCR test results by day 10 compared with severe cases who more often remain positive beyond day 10. 13 Viral loads appear to be highest early in the illness. Prolonged viral shedding after the onset of symptoms has been described. 14 The virus has also been detected by PCR in asymptomatic patients with comparable viral loads to those still symptomatic. 15
Patients with suspected or confirmed COVID‐19 should be assessed for features of severe disease and risk factors for progression to severe disease. This assists in determining whether a patient can safely be managed in the community or requires referral and admission to a health care facility able to provide acute inpatient care. Current data suggest that older patients and those with comorbidities have increased risk of progression to severe disease and mortality. In a large surveillance report from China including over 44 000 confirmed cases of COVID‐19, the case fatality rate was < 0.5% for patients aged < 50 years, but rose to 8.0% for those in their 70s, and 14.8% in those aged > 80 years. 16 While these surveillance‐based case fatality rates are possibly overestimates, being influenced by under‐recognition of lower severity cases, the impact of increasing age and the presence of comorbidities on risk of severe and fatal illness should be recognised, 8 and such patients should generally be offered more careful monitoring.
Clinical features that have been identified more often in COVID‐19 infected patients who have had a fatal outcome compared with those who survive are: dyspnoea at presentation (70.6% v 24.7%; P < 0.001); lower initial oxygen saturation (median oxygen saturation, 85% [IQR, 75–91%] v 97% [IQR, 95–98%]; P < 0.001); and higher total white blood cell count but lower lymphocyte count at presentation accompanied by a lower lymphocyte count, expressed as a lower lymphocyte percentage (median, 7.1% [IQR, 4.5–12.7%] v 23.5% [IQR, 15.3–31.3%]; P < 0.001). 17 In developing a predictive model, Chinese researchers found four factors independently associated with disease progression during hospitalisation in 208 consecutive patients: presence of comorbidity, age > 60 years, lymphocyte count < 1.0 × 10 9 /L, and elevated lactate dehydrogenase levels. 18
A propensity for deterioration in the second week of illness has been recognised in some cohorts of patients, typically 5–10 days after the onset of symptoms. 19 All patients should be warned about symptoms of concern (such as increasing breathlessness), and early referral for hospital admission should be suggested for any patient with signs of clinical deterioration. Individual circumstances need to be considered when determining the ideal monitoring strategy and site of care for each patient ( Box ).
Assessing disease severity and consideration for setting of care for patients diagnosed with COVID‐19
| Disease severity | Clinical features | Setting of care |
|---|---|---|
| Mild illness/lower risk of progression to severe disease | Mild upper respiratory symptoms (eg, cough, sore throat, myalgia, fatigue) AND Age < 60 years AND No major comorbidities | Ideally manage out of hospital (eg, at home or in a step‐down facility), unless symptoms progress to lower tract symptoms such as dyspnoea (see below) |
| Moderate illness/intermediate risk of progression to severe disease | Stable patient presenting with respiratory and systemic symptoms or signs: AND No major comorbidities | If patient amenable to community level management, careful monitoring into second week of illness is recommended AND Early referral for hospital admission if any evidence of clinical deterioration |
| Severe illness | Patient meeting any of the following criteria: on room air < 92% | Assessment for hospital admission |
| Clinical deterioration and at risk for critical illness | Worsening respiratory state as determined by any of the following criteria: > 92% | Early referral to intensive care unit if goals of care include intensive care unit management |
Adapted from World Health Organization interim guidance, 21 Australasian Society for Infectious Diseases interim guidelines, 20 and National COVID‐19 Clinical Evidence Taskforce living guidelines. 19
General management
It is critically important to ensure optimal infection prevention from the time a patient with suspected COVID‐19 is first assessed until their infection is resolved, irrespective of the site of care. This can present particular challenges for health care staff, who must learn to use personal protective equipment safely, and for patients and their loved ones who must manage the difficulties associated with isolation.
Patients with mild disease (about 80%) 10 can often be managed in the community if they are able to self‐isolate. They must also be capable of monitoring their own condition, be aware of which symptoms should prompt medical review, and be able to escalate any concerns. 19 , 20 , 21 For some patients, a more proactive program of monitoring by phone or telehealth or in‐person monitoring (eg, hospital in the home, regular review by general practitioner, or hospital admission) may be required. Strategies for care should be individualised to suit patient circumstances. Patients whose home environment is not conducive to safe management, or which is unacceptable from an infection prevention perspective, may require admission either to hospital or to alternative safe accommodation. Discussion with public health authorities is essential to ensure that appropriate isolation and follow‐up mechanisms are in place. In the face of high health care demand during the peak of a pandemic, safe management of low risk patients in the community will likely be essential to preserve hospital capacity for the more severely ill.
Patients with moderate or severe illness will generally require admission to hospital. This includes those who are dyspnoeic on minor exertion, tachypnoeic at rest (respiratory rate > 22 breaths/min), hypoxaemic (pulse oximetry [SpO 2 ] < 94% on room air), hypotensive (systolic blood pressure < 100 mmHg), have an acutely altered mental state, or who have extensive pulmonary infiltrates evident on chest imaging. 19 , 20 , 21
Severe illness, indicated by, among other features, a respiratory rate > 30 breaths/min, SpO 2 < 92% on room air 19 , 21 or sustained hypotension, warrants urgent hospitalisation and consideration of the need for intensive care if suitable for a given patient.
Respiratory management
Supplemental oxygen should be administered for patients with SpO 2 < 92%. 19 , 20 Once stabilised, the target SpO 2 range is usually 92–96%. The target will be lower in those with chronic hypercapnoeic respiratory failure (eg, 88–92%). 19 , 20 , 21
Manoeuvres to improve gas exchange should be implemented, such as positioning patients appropriately in bed (on either side with regular turning), elevating the bed head to 30 degrees, encouraging deep breathing every hour while awake, sitting patients out of bed every day when possible, and mobilising when able. For mechanically ventilated patients with persistent hypoxaemia, prone positioning may be effective. 19 , 22
In the setting of progressive hypoxaemia despite low or moderate flow oxygen (via nasal prongs or Hudson mask), high flow oxygen can be considered. Whether high flow oxygen devices (> 10 mL/min) are potentially aerosol‐generating is being studied, but current guidelines 1 , 23 advise that airborne precautions be taken by staff (personal protective equipment including N95/P2 masks) and single rooms where possible.
There are emerging views that the respiratory dysfunction observed in COVID‐19 infections is not uniform. 22 Initial recommendations have focused on consideration of early intubation and mechanical ventilation for patients with acute respiratory distress syndrome due to COVID‐19. 1 , 19 , 20 , 21 Experience from a multicentre Italian COVID‐19 patient cohort suggests that non‐invasive ventilation such as continuous positive airways pressure and bilevel positive airways pressure may also have a role both within and outside ICUs. 24 These non‐invasive ventilation devices are clearly aerosol‐generating and as such should only be used with appropriate precautions in place. 1 , 23 Advice from experts in respiratory medicine or critical care should be sought.
Other management considerations
Empirical antibiotic therapy for bacterial pneumonia should be considered in patients whose illness is severe, where there is evidence of sepsis or septic shock, or where the patient is clinically deteriorating. 19 , 20 Empirical treatment for influenza with a neuraminidase inhibitor should be considered for patients with severe pneumonia (guided by local epidemiology) until influenza PCR results are available. 20 , 21 Empirical antibiotics are not recommended for patients with mild or moderate pneumonia unless there is additional clinical evidence to suggest bacterial infection. De‐escalation of empirical antimicrobial therapy should be undertaken as appropriate, guided by microbiology results (where available) and clinical judgement. 21
Hypovolaemia may be contributed to by reduced oral intake and increased losses, but management requires cautious administration of intravenous fluids with regular assessments given the risk of exacerbating pulmonary oedema in the setting of acute respiratory distress syndrome 19 , 22 and given the possibility of underlying cardiac injury. 25
A range of possible complications related to SARS‐CoV‐2 infection have been reported and their incidence is being monitored. These include thromboembolic events in the lungs 22 and cerebrovascular system, 26 Prophylaxis with anticoagulants for adults with moderate, severe or critical COVID‐19 infection is generally recommended, unless there are contraindications. 19 , 21 Acute cardiac injury presenting with electrocardiogram changes, arrhythmias, left ventricular dysfunction, cardiomyopathy and congestive cardiac failure have also been described, and assessment of baseline electrocardiogram is suggested for patients with moderate or severe COVID‐19 illness. 25 , 27
There is considerable interest in monitoring large patient cohorts and conducting analysis of linked datasets at a population level to establish whether there are any rare or longer term complications or associations of COVID‐19 with other medical conditions. Given the very recent emergence of SARS‐CoV‐2, data are currently limited but it is likely that information will emerge in coming months from populations that have experienced a high attack rate. An example of a rare condition with potential association is paediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2, presenting as hyperinflammatory shock with features similar to atypical Kawasaki disease. 28 Similarly, there is interest in monitoring long term incidence of cardiovascular complications, thromboembolic disease, chronic respiratory dysfunction, renal or neurological disorders, and post‐infectious inflammatory syndromes after COVID‐19, in addition to inspection of large datasets for complications that are as yet unsuspected.
Specific therapies
A range of pharmacotherapies have been proposed as possible treatments for COVID‐19. Early evidence of clinical benefit for some agents has emerged. The WHO interim guidance on the clinical management of COVID‐19 21 asserts that investigational therapeutics should be used only in approved randomised controlled trials. This position is endorsed by the Australasian Society for Infectious Diseases interim guidelines for the clinical management of COVID‐19 in adults, 20 and the Australian guidelines for the clinical care of people with COVID‐19, 19 which state that even where conditional recommendations for use of disease modifying agents are made, whenever possible these should be administered in the context of randomised trials with appropriate ethical approval.
The understandable interest in evaluating potential treatments has led to a large number of clinical trials being registered globally; by late April 2020, over 1100 clinical studies were registered, including over 500 randomised controlled trials. 29
Antimicrobials
Lopinavir–ritonavir.
Lopinavir–ritonavir, a combined antiretroviral agent, was proposed as a potential treatment for severe acute respiratory syndrome in 2003, based on apparent reductions in mortality in preliminary research in Hong Kong. 30 Given its hypothesised role, five of the first 18 patients diagnosed with COVID‐19 in Singapore were administered this agent. 31
On 18 March 2020, a randomised controlled open label trial of lopinavir–ritonavir in 199 hospitalised adults with COVID‐19 in China was published. 32 No benefit was observed in participants treated with the antiviral compared with controls. Nearly 14% of those receiving lopinavir–ritonavir were unable to complete 14 days of treatments owing to adverse events.
Chloroquine and hydroxychloroquine
Chloroquine and hydroxychloroquine are antimalarial agents which also have immunomodulatory properties that led to established indications for use in the treatment of rheumatological conditions. Potential adverse effects include retinal toxicity, QT interval prolongation and other cardiological and dermatological effects.
In early February 2020, chloroquine was reported to inhibit SARS‐CoV‐2 replication in vitro. 33 By mid‐February, treatment of COVID‐19 with chloroquine was being described as a “breakthrough”: a published letter stated that the results of treatment in over 100 patients in China had demonstrated that chloroquine was “superior to the control treatment”, but no data were provided. 34 A small French open label non‐randomised clinical trial examining hydroxychloroquine with or without azithromycin suggested a significant viral load reduction in those receiving therapy; 35 however, concerns have been raised about the design and analysis of the study. 36
Despite the lack of clinical evidence from randomised clinical trials, several institutional and local guidelines, and notable public figures, have supported the potential use of chloroquine or hydroxychloroquine for the treatment of COVID‐19. 37 , 38
However, given the current lack of evidence of clinical benefit and reports of significant limitations of supply of hydroxychloroquine for patients with rheumatological conditions, in March 2020, the Pharmaceutical Society of Australia and the Australasian Society for Infectious Diseases called for immediate cessation of prescribing and dispensing of hydroxychloroquine for indications relating to COVID‐19, outside use in approved clinical trials. 39 , 40
On 5 June 2020, the chief investigators on the RECOVERY trial (comprising over 11 500 patients enrolled from hospitals across the United Kingdom) issued a press release stating that no beneficial effect of hydroxychloroquine had been observed. 41 No difference in 28‐day mortality, duration of admission, or other outcomes were observed between the 1542 patients randomised to hydroxychloroquine and the 3132 patients randomised to usual care. Further details regarding this analysis are awaited.
In January 2020, the first patient diagnosed with COVID‐19 in the US received the investigational nucleotide prodrug remdesivir, supplied on a compassionate basis. 42 Developed as a potential therapy for Ebola, there is in vitro evidence that remdesivir inhibits replication of coronaviruses, including Middle East respiratory syndrome coronavirus and SARS‐CoV‐2. 33 , 43 By late March 2020, four clinical trials to assess the efficacy of remdesivir against COVID‐19 had commenced in the US and two were registered in China. 44 On 29 April, results of the first randomised clinical trial conducted in China were published; 45 while this found no clinical benefit of remdesivir, the trial was underpowered (237 participants) owing to the success of public health measures in controlling COVID‐19 in China. The authors noted a non‐significant numerical reduction in time to clinical improvement in patients commencing treatment earlier in the course of illness.
On 27 May 2020, the first positive results of a randomised double‐blind controlled trial of a treatment for COVID‐19 were published. 46 This international multicentre study reported the preliminary results of 1059 hospitalised patients who received up to 10 days of remdesivir or placebo. Those receiving remdesivir had a significantly shorter median recovery time of 11 days compared with 15 days for those receiving placebo (rate ratio for recovery, 1.32; 95% CI, 1.12–1.55; P < 0.001); no significant difference in mortality was found. Benefit was reported for the group requiring oxygen but not yet requiring invasive or non‐invasive ventilatory support. This new evidence has led Australian national guidelines to adopt a conditional recommendation for use of remdesivir outside of a trial setting where necessary. 19
Combination therapy with interferon beta‐1b, lopinavir–ritonavir and ribavirin
In May 2020, a randomised trial in Hong Kong reported results of a comparison of lopinavir–ritonavir alone ( n = 24) with a combination of lopinavir–ritonavir, ribavirin and subcutaneous interferon beta‐1b ( n = 52). 47 The combination group experienced a faster median time to viral clearance (7 days v 13 days; P < 0.0001) and shorter median length of hospital stay (8 days v 15 days; P = 0.0030) if the combination was commenced in the first 7 days from symptom onset. Importantly, the cohort of patients studied was not particularly unwell, with very few requiring ICU support and no deaths in the group.
Immunomodulatory treatments
Corticosteroids.
Interim guidance from the WHO states that corticosteroids should not be used in routine treatment of COVID‐19. 21 This is based on systematic reviews in the context of severe acute respiratory syndrome and Middle East respiratory syndrome which showed lack of effectiveness, and possible harm. 48
In a study of 138 hospitalised patients with COVID‐19 in Wuhan, 49 72.2% of ICU patients and 35.3% of non‐ICU patients received glucocorticoid therapy. The authors commented that while the dose of methylprednisolone varied depending on disease severity, no effective outcomes were observed.
However, on 22 June 2020, a preliminary report regarding interim findings from the UK RECOVERY trial suggested that low dose dexamethasone (6 mg daily orally or intravenous for 10 days) may substantially reduce mortality in hospitalised patients with COVID‐19 who received supplemental oxygen or mechanical ventilation. 50 In comparing 2104 patients randomised to receive dexamethasone with 4321 randomised to receive usual care, dexamethasone was found to reduce mortality by 35% (rate ratio, 0.65; 95% CI, 0.51–0.82; P < 0.001) among ventilated patients, and for those receiving oxygen without mechanical ventilation, mortality was reduced by 20% (rate ratio, 0.80; 95% CI, 0.70–0.92; P = 0.002). No benefit of dexamethasone was observed among hospitalised patients who did not require respiratory support. While peer review and formal publication of this analysis is awaited, it is likely that these findings will be reflected in national and international guidelines.
Interleukin 6 antagonists
Tocilizumab is a humanised monoclonal antibody which binds to interleukin 6 (IL‐6) receptors, resulting in reduced immune activation and inflammation. It is licensed in Australia for use in autoimmune conditions including rheumatoid arthritis and giant cell arteritis. In addition to complications of immunosuppression including serious infections, adverse effects include hepatotoxicity and gastrointestinal complications. The theory behind use of tocilizumab or other agents that target the IL‐6 pathway (eg, sarilumab) in the context of COVID‐19 is that part of the pathogenesis in some patients may be attributable to an acute inflammatory syndrome or cytokine storm, which is associated with elevated IL‐6 levels. Clinical trials of these agents are currently underway. 44
Other agents
Numerous immunomodulatory agents have been proposed as potential adjunctive treatments for COVID‐19, with a range of different immunological targets including other inflammatory cytokines. These include anakinra (an IL‐1 receptor antagonist), bevacizumab (an antivascular endothelial growth factor agent), and eculizumab (which inhibits terminal complement and prevents formation of the membrane attack complex). 44 , 51 While clinical trials are underway overseas for several proposed agents, no data exist to support their use at this time. 44
Passive immunotherapy
A preliminary, uncontrolled case series of five critically ill Chinese patients with COVID‐19 who received convalescent plasma containing high SARS‐CoV‐2‐specific antibody titres was published on 27 March 2020. 52 While improvement in clinical status was reported following this intervention, the small sample size and uncontrolled nature of the study precludes drawing any conclusions regarding the efficacy of this intervention. Once again, further research is needed.
Holistic care
A global pandemic causes understandable fear and anxiety for many people in the community. For those at particular risk of worse outcomes of infection — older people and those with significant pre‐existing illness or multiple comorbidities — COVID‐19 represents a particular threat. In addition, the health care workforce is under substantial strain and faces a potentially overwhelming challenge in delivering care to patients. Ensuring emotional care for the most vulnerable and those experiencing high levels of stress will be a fundamental determinant of the resilience of our society during this challenge.
For vulnerable and frail patients at particular risk of poor outcomes, it is important to provide personalised care and to develop an understanding of each individual's perspectives and preferences for health management. Involving caregivers and family members in decision making and establishing goals of care is necessary. 21 Discussing goals of care early and, where appropriate, assisting patients to make advance care directives or resuscitation plans early in illness (or before infection) may provide substantial peace of mind and allow families to face the pandemic openly and with unity as they support vulnerable loved ones.
It is essential to ensure that all patients receive the best standard of care irrespective of the setting in which the care is delivered, or of the existence of any proposed limitations to life‐extending interventions. Under no circumstances should the best possible symptom control and compassionate, individualised care be denied any patient affected by COVID‐19.
SARS‐CoV‐2 has caused a global pandemic with a profound public health impact, changing the daily lives of billions of people. It has exposed weaknesses in even strong and well resourced health systems internationally, and the economic impact alone will be staggering.
However, never before has the global community had the tools currently available to address a pandemic threat. A strong commitment to social and public health strategies and communicable disease control will ensure our health system retains the capacity to address COVID‐19, including sufficient hospital and intensive care resources to care for those with severe illness.
Biomedical innovations such as new and rapid point‐of‐care diagnostics, effective specific treatments and preventive vaccines are very high priorities which are rightly attracting substantial attention and funding. In the interim, high quality, evidence‐based clinical care — scaled up to face the pandemic challenge — together with robust public health interventions will save the lives of thousands in Australia, and millions globally.
Competing interests
No relevant disclosures.
Commissioned; externally peer reviewed.
Acknowledgements
We gratefully acknowledge the contributions of Anna Deng, Louis Irving, Ashleigh Qama and Lien Tran to this article.
The unedited version of this article was published as a preprint on mja.com.au on 8 April 2020
- Cambridge Dictionary +Plus
clinical presentation
Meanings of clinical and presentation.
Your browser doesn't support HTML5 audio
(Definition of clinical and presentation from the Cambridge English Dictionary © Cambridge University Press)
- Examples of clinical presentation

Word of the Day
a day that you spend somewhere that is not your home or usual place of work

It’s not really my thing (How to say you don’t like something)

Learn more with +Plus
- Recent and Recommended {{#preferredDictionaries}} {{name}} {{/preferredDictionaries}}
- Definitions Clear explanations of natural written and spoken English English Learner’s Dictionary Essential British English Essential American English
- Grammar and thesaurus Usage explanations of natural written and spoken English Grammar Thesaurus
- Pronunciation British and American pronunciations with audio English Pronunciation
- English–Chinese (Simplified) Chinese (Simplified)–English
- English–Chinese (Traditional) Chinese (Traditional)–English
- English–Dutch Dutch–English
- English–French French–English
- English–German German–English
- English–Indonesian Indonesian–English
- English–Italian Italian–English
- English–Japanese Japanese–English
- English–Norwegian Norwegian–English
- English–Polish Polish–English
- English–Portuguese Portuguese–English
- English–Spanish Spanish–English
- English–Swedish Swedish–English
- Dictionary +Plus Word Lists
{{message}}
There was a problem sending your report.
- Definition of clinical
- Definition of presentation
- Other collocations with presentation

IMAGES
VIDEO
COMMENTS
clinical presentation: The constellation of physical signs or symptoms associated with a particular morbid process, the interpretation of which leads to a specific diagnosis
Presenting patient cases is a key part of everyday clinical practice. A well delivered presentation has the potential to facilitate patient care and improve efficiency on ward rounds, as well as a means of teaching and assessing clinical competence.1 The purpose of a case presentation is to communicate your diagnostic reasoning to the listener, so that he or she has a clear picture of the ...
2 A well-organized comprehensive knowledge domain has practical implications in clinical problem solving, and appropriate teaching and learning methods play an important role in achieving the educational goals. 3. Clinical presentation (CP) is a relatively new and innovative approach to teaching medicine.
A clinical presentation is the mode by which a patient presents to a physician and represents the clinical problem a physician is expected to manage. Big picture: The curriculum begins and ends with a focus on the patient, specifically the patient's clinical presentation. Deconstruct and independent learning: Each clinical presentation is ...
Presenting a patient is an essential skill that is rarely taught Clinical presenting is the language that doctors use to communicate with each other every day of their working lives. Effective communication between doctors is crucial, considering the collaborative nature of medicine. As a medical student and later as a doctor you will be expected to present cases to peers and senior colleagues ...
This topic will review the clinical presentation, diagnosis, and initial evaluation of diabetes in nonpregnant adults. Screening for and prevention of diabetes, the etiologic classification of diabetes mellitus, the treatment of diabetes, as well as diabetes during pregnancy are discussed separately. (See "Screening for type 2 diabetes mellitus" .)
There are a multitude of presentation formats for sharing and discussing clinical cases, diagnostic formulations or dilemmas, treatment approaches, and ethical issues. These presentation formats vary in terms of the number and type of participants, the use of multimedia , the availability of continuing medical education credits, etc. (Hull et ...
just in case your first choice (ex: PowerPoint presentation) fails to load. Significance. Oral presentations are an excellent means of communicating basic science or clinical research. Unlike a poster presentation or a written manuscript, the audience during an oral presentation is more attentive as they are focused on the presenter.
Definition of a Clinical Presentation A clinical presentation must: Represent a common or important way in which a patient, group of patients, community or population actually presents to the physician and which a graduate would be expected to handle.
Clinical presentation is a skill that is needed for doctors to share the medical problem for a patient with other physicians. It is also used to evaluate resident in training and for other purposes.
2 meanings: of or relating to a clinic [...].... Click for more definitions.
Clinical presentation - Patients with CKD may present with symptoms and signs resulting directly from diminished kidney function, such as edema or hypertension. However, many have no clinical symptoms, and kidney disease is often detected in these patients when an elevated serum creatinine, reduced estimated GFR (eGFR), or an abnormal ...
Epidemiological studies published in 2020 (from January-March) on the clinical presentation, laboratory findings and treatments of COVID-19 patients were identified from PubMed/MEDLINE and ...
Whether your presentation relates to the latest National Institute for Health and Clinical Excellence (NICE) guidelines for your specialty or a business plan in response to a proposed polyclinic, this structure will help keep your audience engaged and your presentation within the time limit. Indeed, timing is crucial when giving a presentation.
Areas covered. Direct person-to-person respiratory transmission has rapidly amplified the spread of coronavirus. In the absence of any clinically proven treatment options, the current clinical management of COVID-19 includes symptom management, infection prevention and control measures, optimized supportive care, and intensive care support in severe or critical illness.
clinical. (klɪnɪkəl ) adjective [ADJECTIVE noun] Clinical means involving or relating to the direct medical treatment or testing of patients. [...] [medicine] clinically (klɪnɪkli ) adverb [usually ADVERB adjective/-ed] See full entry for 'clinical'. Collins COBUILD Advanced Learner's Dictionary.
The epidemiology, definitions, risk factors, clinical presentation, diagnosis, and outcomes of sepsis are reviewed here. The pathophysiology and treatment of sepsis are discussed separately. (See "Pathophysiology of sepsis" and "Evaluation and management of suspected sepsis and septic shock in adults".)
Clinical presentation. Similar to other coronaviruses, SARS‐CoV‐2 is predominantly spread by respiratory droplets, although spread by contact with contaminated fomites also occurs, as does transmission by aerosols in certain circumstances.1 Based on the experience in China, the typical incubation period of COVID‐19 infection has been estimated to be a median of 5.1 days (95% CI, 4.5-5. ...
23.6 Sleep apnoea: definition, prevalence, and role in cardiovascular diseases Notes. Notes. 23.7 ... 38.15 Carotid sinus syndrome: clinical presentation, diagnosis, and management Notes. Notes. 38.16 Bradycardia in athletes: clinical evaluation and management Notes. Notes. 38.17 ...
CLINICAL PRESENTATION. Clinical features — Most patients with VAP present with a gradual or sudden onset of the following more than 48 hours after intubation [ 5 ]: Symptoms - dyspnea (few patients have symptoms since most are nonverbal on mechanical ventilation) Signs - fever, tachypnea, increased or purulent secretions, hemoptysis ...
Presentation (medical) This definition of medical jargon . In medicine, a presentation is the appearance in a patient of illness or disease—or signs or symptoms thereof—before a medical professional. In practice, one usually speaks of a patient as presenting with this or that. Examples include:
The definition, classification, etiology, and pathophysiology of shock are discussed in this review. The clinical presentation and diagnostic evaluation of undifferentiated shock and the evaluation of patients with specific forms of shock are discussed separately.
Examples of CLINICAL PRESENTATION in a sentence, how to use it. 16 examples: This review describes the causative organisms, pathogenesis, clinical presentation, epidemiology…