- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

U.S. Food and Drug Administration
- Search
- Menu
- Resources for You (Food)
- Agricultural Biotechnology
Science and History of GMOs and Other Food Modification Processes
Feed Your Mind Main Page
en Español (Spanish)
How has genetic engineering changed plant and animal breeding?
Did you know.
Genetic engineering is often used in combination with traditional breeding to produce the genetically engineered plant varieties on the market today.
For thousands of years, humans have been using traditional modification methods like selective breeding and cross-breeding to breed plants and animals with more desirable traits. For example, early farmers developed cross-breeding methods to grow corn with a range of colors, sizes, and uses. Today’s strawberries are a cross between a strawberry species native to North America and a strawberry species native to South America.
Most of the foods we eat today were created through traditional breeding methods. But changing plants and animals through traditional breeding can take a long time, and it is difficult to make very specific changes. After scientists developed genetic engineering in the 1970s, they were able to make similar changes in a more specific way and in a shorter amount of time.
A Timeline of Genetic Modification in Agriculture
A Timeline of Genetic Modification in Modern Agriculture

Circa 8000 BCE: Humans use traditional modification methods like selective breeding and cross-breeding to breed plants and animals with more desirable traits.
1866: Gregor Mendel, an Austrian monk, breeds two different types of peas and identifies the basic process of genetics.
1922: The first hybrid corn is produced and sold commercially.
1940: Plant breeders learn to use radiation or chemicals to randomly change an organism’s DNA.
1953: Building on the discoveries of chemist Rosalind Franklin, scientists James Watson and Francis Crick identify the structure of DNA.
1973: Biochemists Herbert Boyer and Stanley Cohen develop genetic engineering by inserting DNA from one bacteria into another.
1982: FDA approves the first consumer GMO product developed through genetic engineering: human insulin to treat diabetes.
1986: The federal government establishes the Coordinated Framework for the Regulation of Biotechnology. This policy describes how the U.S. Food and Drug Administration (FDA), U.S. Environmental Protection Agency (EPA), and U.S. Department of Agriculture (USDA) work together to regulate the safety of GMOs.
1992: FDA policy states that foods from GMO plants must meet the same requirements, including the same safety standards, as foods derived from traditionally bred plants.
1994: The first GMO produce created through genetic engineering—a GMO tomato—becomes available for sale after studies evaluated by federal agencies proved it to be as safe as traditionally bred tomatoes.
1990s: The first wave of GMO produce created through genetic engineering becomes available to consumers: summer squash, soybeans, cotton, corn, papayas, tomatoes, potatoes, and canola. Not all are still available for sale.
2003: The World Health Organization (WHO) and the Food and Agriculture Organization (FAO) of the United Nations develop international guidelines and standards to determine the safety of GMO foods.
2005: GMO alfalfa and sugar beets are available for sale in the United States.
2015: FDA approves an application for the first genetic modification in an animal for use as food, a genetically engineered salmon.
2016: Congress passes a law requiring labeling for some foods produced through genetic engineering and uses the term “bioengineered,” which will start to appear on some foods.

2017: GMO apples are available for sale in the U.S.
2019: FDA completes consultation on first food from a genome edited plant.
2020 : GMO pink pineapple is available to U.S. consumers.
2020 : Application for GalSafe pig was approved.
How are GMOs made?
“GMO” (genetically modified organism) has become the common term consumers and popular media use to describe foods that have been created through genetic engineering. Genetic engineering is a process that involves:
- Identifying the genetic information—or “gene”—that gives an organism (plant, animal, or microorganism) a desired trait
- Copying that information from the organism that has the trait
- Inserting that information into the DNA of another organism
- Then growing the new organism
How Are GMOs Made? Fact Sheet
Making a GMO Plant, Step by Step
The following example gives a general idea of the steps it takes to create a GMO plant. This example uses a type of insect-resistant corn called “Bt corn.” Keep in mind that the processes for creating a GMO plant, animal, or microorganism may be different.

To produce a GMO plant, scientists first identify what trait they want that plant to have, such as resistance to drought, herbicides, or insects. Then, they find an organism (plant, animal, or microorganism) that already has that trait within its genes. In this example, scientists wanted to create insect-resistant corn to reduce the need to spray pesticides. They identified a gene in a soil bacterium called Bacillus thuringiensis (Bt) , which produces a natural insecticide that has been in use for many years in traditional and organic agriculture.

After scientists find the gene with the desired trait, they copy that gene.
For Bt corn, they copied the gene in Bt that would provide the insect-resistance trait.

Next, scientists use tools to insert the gene into the DNA of the plant. By inserting the Bt gene into the DNA of the corn plant, scientists gave it the insect resistance trait.
This new trait does not change the other existing traits.

In the laboratory, scientists grow the new corn plant to ensure it has adopted the desired trait (insect resistance). If successful, scientists first grow and monitor the new corn plant (now called Bt corn because it contains a gene from Bacillus thuringiensis) in greenhouses and then in small field tests before moving it into larger field tests. GMO plants go through in-depth review and tests before they are ready to be sold to farmers.
The entire process of bringing a GMO plant to the marketplace takes several years.
Learn more about the process for creating genetically engineered microbes and genetically engineered animals .
What are the latest scientific advances in plant and animal breeding?
Scientists are developing new ways to create new varieties of crops and animals using a process called genome editing . These techniques can make changes more quickly and precisely than traditional breeding methods.
There are several genome editing tools, such as CRISPR . Scientists can use these newer genome editing tools to make crops more nutritious, drought tolerant, and resistant to insect pests and diseases.
Learn more about Genome Editing in Agricultural Biotechnology .
How GMOs Are Regulated in the United States
GMO Crops, Animal Food, and Beyond
How GMO Crops Impact Our World
www.fda.gov/feedyourmind
- Skip to main content
- Keyboard shortcuts for audio player
Intelligence Squared U.S.
Debate: should we genetically modify food.

Genomics researcher Alison Van Eenennaam, with Monsanto's Robert Fraley, argues that genetically modified foods have increased farmers' yields and profits around the world. Samuel LaHoz/Intelligence Squared U.S. hide caption
Genomics researcher Alison Van Eenennaam, with Monsanto's Robert Fraley, argues that genetically modified foods have increased farmers' yields and profits around the world.
Many plants we eat today are a result of genetic modifications that would never occur in nature. Scientists have long been altering the genes of food crops, to boost food production and to make crops more pest-, drought- and cold-resistant.
Proponents of genetically modified organisms, or GMOs, say that farmers who grow these crops are able to use fewer environmentally damaging pesticides. The increased yields of GMO crops, they also argue, are essential to feeding the world's growing population. And proponents say that numerous studies have shown that genetically modified foods are safe to eat.
Critics, however, say the claims of those benefits are overblown. They say farmers growing GMO crops have actually increased their use of herbicides. And widespread use of the crops, they say, have also led to an increase in herbicide- and pesticide-resistant weeds and insects. And, they argue, there is still no scientific consensus on the long-term safety of these foods.
Four scientists recently took on those questions in an Intelligence Squared U.S. debate, facing off two against two on the motion, "Genetically Modify Food." In these Oxford-style debates, the team that sways the most people to its side by the end is the winner.
Before the debate, the audience at the Kaufman Music Center in New York voted 32 percent in favor of the motion, with 30 percent against and 38 percent undecided. Afterward, 60 percent agreed with the motion, and 31 percent disagreed — making the side arguing in favor of the motion the winners of this debate.
More From The Debate
Those debating:
FOR THE MOTION
Robert Fraley is executive vice president and chief technology officer at Monsanto, where he has worked for more than 30 years. He currently oversees the company's global technology division which includes plant breeding, biotechnology and crop protection research facilities in dozens of countries. Fraley has authored more than 100 publications and patent applications. In 2013, he was honored as a World Food Prize Laureate and is the recipient of numerous awards, including the 2008 National Academy of Sciences Award for the Industrial Application of Science for his work on crop improvement and the National Medal of Technology from President Clinton in 1999.
Alison Van Eenennaam is a genomics and biotechnology researcher and cooperative extension specialist in the Department of Animal Science at University of California, Davis. The mission of her extension program is "to provide research and education on the use of animal genomics and biotechnology in livestock production systems." Her outreach program focuses on the development of science-based educational materials, including the controversial biotechnologies of genetic engineering and cloning. She has served on several national committees including the USDA National Advisory Committee on Biotechnology and 21st Century Agriculture, and as a temporary voting member of the 2010 FDA Veterinary Medicine Advisory Committee meeting on the AquAdvantage salmon, a genetically engineered Atlantic salmon. Van Eenennaam was the recipient of the 2014 Borlaug CAST Communication Award.

Science policy consultant Margaret Mellon argues that genetically modified crops have encouraged the evolution of resistant weeds and pests. Samuel LaHoz/Intelligence Squared U.S. hide caption
Science policy consultant Margaret Mellon argues that genetically modified crops have encouraged the evolution of resistant weeds and pests.
AGAINST THE MOTION
Charles Benbrook is a research professor at the Center for Sustaining Agriculture and Natural Resources at Washington State University, and leader of the center's program Measure to Manage: Farm and Food Diagnostics for Sustainability and Health. His career has focused on developing science-based systems for evaluating the public health, environmental and economic impacts of changes in agricultural systems, technology and policy. He spent the first 18 years of his career working in Washington, D.C., first for the Executive Office of the President, then as the staff director for a U.S. House of Representatives agricultural subcommittee. He was the executive director of the National Academy of Sciences Board on Agriculture, and has run a small consulting firm since 1991. He served as the chief scientist for The Organic Center, based in Washington, D.C., from 2004 to 2012, and has served as an appointed member on the USDA's Advisory Committee on 21st Century Agriculture since 2011. His 2012 peer-reviewed study documenting the big increase in herbicide use triggered by the planting of genetically engineered crops in the U.S. has been downloaded over 110,000 times.
Margaret Mellon is a science policy consultant in the areas of antibiotics, genetic engineering and sustainable agriculture. She holds a doctorate in molecular biology and a law degree from the University of Virginia. In 1993, Mellon founded the Food and Environment Program at the Union of Concerned Scientists to promote the adoption of science-based farming systems that are simultaneously productive, environmentally benign and resilient in the face of stress. The program critically evaluated products of genetic engineering for their contribution to sustainable agriculture and urged the reduction of unnecessary antibiotic use in animal agriculture. After almost 20 years, Mellon stepped down as head of the program in 2012 and, after two additional years as a senior scientist, left UCS in 2014. Mellon has published widely on the potential environmental impacts of biotechnology applications, and served three terms on USDA's Advisory Committee on Biotechnology and 21st Century Agriculture.
- food safety
- genetically modified food
- genetically engineered food
- genetically modified seeds
- Share full article

Learning to Love G.M.O.s
Overblown fears have turned the public against genetically modified food. But the potential benefits have never been greater.
Credit... Levon Biss for The New York Times
Supported by
By Jennifer Kahn
- July 20, 2021
Listen to This Article
To hear more audio stories from publications like The New York Times, download Audm for iPhone or Android .
On a cold December day in Norwich, England, Cathie Martin met me at a laboratory inside the John Innes Centre, where she works. A plant biologist, Martin has spent almost two decades studying tomatoes, and I had traveled to see her because of a particular one she created: a lustrous, dark purple variety that is unusually high in antioxidants, with twice the amount found in blueberries.
At 66, Martin has silver-white hair, a strong chin and sharp eyes that give her a slightly elfin look. Her office, a tiny cubby just off the lab, is so packed with binders and piles of paper that Martin has to stand when typing on her computer keyboard, which sits surrounded by a heap of papers like a rock that has sunk to the bottom of a snowdrift. “It’s an absolute disaster,” Martin said, looking around fondly. “I’m told that the security guards bring people round on the tour.” On the desk, there’s a drinks coaster with a picture of an attractive 1950s housewife that reads, “You say tomato, I say [expletive] you.”
Martin has long been interested in how plants produce beneficial nutrients. The purple tomato is the first she designed to have more anthocyanin, a naturally occurring anti-inflammatory compound. “All higher plants have a mechanism for making anthocyanins,” Martin explained when we met. “A tomato plant makes them as well, in the leaves. We just put in a switch that turns on anthocyanin production in the fruit.” Martin noted that while there are other tomato varieties that look purple, they have anthocyanins only in the skin, so the health benefits are slight. “People say, Oh, there are purple tomatoes already,” Martin said. “But they don’t have these kind of levels.”
The difference is significant. When cancer-prone mice were given Martin’s purple tomatoes as part of their diet, they lived 30 percent longer than mice fed the same quantity of ordinary tomatoes; they were also less susceptible to inflammatory bowel disease. After the publication of Martin’s first paper showing the anticancer benefit of her tomatoes, in the academic journal Nature Biotechnology in 2008, newspapers and television stations began calling. “The coverage!” she recalled. “Days and days and days and days of it! There was a lot of excitement.” She considered making the tomato available in stores or offering it online as a juice. But because the plant contained a pair of genes from a snapdragon — that’s what spurs the tomatoes to produce more anthocyanin — it would be classified as a genetically modified organism: a G.M.O.
That designation brings with it a host of obligations, not just in Britain but in the United States and many other countries. Martin had envisioned making the juice on a small scale, but just to go through the F.D.A. approval process would cost a million dollars. Adding U.S.D.A. approval could push that amount even higher. (Tomato juice is known as a “G.M. product” and is regulated by the F.D.A. Because a tomato has seeds that can germinate, it is regulated by both the F.D.A. and the U.S.D.A.) “I thought, This is ridiculous,” Martin told me.
Martin eventually did put together the required documentation, but the process, and subsequent revisions, took almost six years. “Our ‘business model’ is that we have this tiny company which has no employees,” Martin said with a laugh. “Of course, the F.D.A. is used to the bigger organizations” — global agricultural conglomerates like DowDuPont or Syngenta — “so this is where you get a bit of a problem. When they say, ‘Oh, we want a bit more data on this,’ it’s easy for a corporation. For me — it’s me that has to do it! And I can’t just throw money at it.”
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .
Advertisement
September 1, 2013
13 min read
The Truth about Genetically Modified Food
Proponents of genetically modified crops say the technology is the only way to feed a warming, increasingly populous world. Critics say we tamper with nature at our peril. Who is right?
By David H. Freedman
Robert Goldberg sags into his desk chair and gestures at the air. “Frankenstein monsters, things crawling out of the lab,” he says. “This the most depressing thing I've ever dealt with.”
Goldberg, a plant molecular biologist at the University of California, Los Angeles, is not battling psychosis. He is expressing despair at the relentless need to confront what he sees as bogus fears over the health risks of genetically modified (GM) crops. Particularly frustrating to him, he says, is that this debate should have ended decades ago, when researchers produced a stream of exonerating evidence: “Today we're facing the same objections we faced 40 years ago.”
Across campus, David Williams, a cellular biologist who specializes in vision, has the opposite complaint. “A lot of naive science has been involved in pushing this technology,” he says. “Thirty years ago we didn't know that when you throw any gene into a different genome, the genome reacts to it. But now anyone in this field knows the genome is not a static environment. Inserted genes can be transformed by several different means, and it can happen generations later.” The result, he insists, could very well be potentially toxic plants slipping through testing.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Williams concedes that he is among a tiny minority of biologists raising sharp questions about the safety of GM crops. But he says this is only because the field of plant molecular biology is protecting its interests. Funding, much of it from the companies that sell GM seeds, heavily favors researchers who are exploring ways to further the use of genetic modification in agriculture. He says that biologists who point out health or other risks associated with GM crops—who merely report or defend experimental findings that imply there may be risks—find themselves the focus of vicious attacks on their credibility, which leads scientists who see problems with GM foods to keep quiet.
Whether Williams is right or wrong, one thing is undeniable: despite overwhelming evidence that GM crops are safe to eat, the debate over their use continues to rage, and in some parts of the world, it is growing ever louder. Skeptics would argue that this contentiousness is a good thing—that we cannot be too cautious when tinkering with the genetic basis of the world's food supply. To researchers such as Goldberg, however, the persistence of fears about GM foods is nothing short of exasperating. “In spite of hundreds of millions of genetic experiments involving every type of organism on earth,” he says, “and people eating billions of meals without a problem, we've gone back to being ignorant.”
So who is right: advocates of GM or critics? When we look carefully at the evidence for both sides and weigh the risks and benefits, we find a surprisingly clear path out of this dilemma.
Benefits and worries
The bulk of the science on GM safety points in one direction. Take it from David Zilberman, a U.C. Berkeley agricultural and environmental economist and one of the few researchers considered credible by both agricultural chemical companies and their critics. He argues that the benefits of GM crops greatly outweigh the health risks, which so far remain theoretical. The use of GM crops “has lowered the price of food,” Zilberman says. “It has increased farmer safety by allowing them to use less pesticide. It has raised the output of corn, cotton and soy by 20 to 30 percent, allowing some people to survive who would not have without it. If it were more widely adopted around the world, the price [of food] would go lower, and fewer people would die of hunger.”
In the future, Zilberman says, those advantages will become all the more significant. The United Nations Food and Agriculture Organization estimates that the world will have to grow 70 percent more food by 2050 just to keep up with population growth. Climate change will make much of the world's arable land more difficult to farm. GM crops, Zilberman says, could produce higher yields, grow in dry and salty land, withstand high and low temperatures, and tolerate insects, disease and herbicides.
Credit: Jen Christiansen
Despite such promise, much of the world has been busy banning, restricting and otherwise shunning GM foods. Nearly all the corn and soybeans grown in the U.S. are genetically modified, but only two GM crops, Monsanto's MON810 maize and BASF's Amflora potato, are accepted in the European Union. Ten E.U. nations have banned MON810, and although BASF withdrew Amflora from the market in 2012, four E.U. nations have taken the trouble to ban that, too. Approval of a few new GM corn strains has been proposed there, but so far it has been repeatedly and soundly voted down. Throughout Asia, including in India and China, governments have yet to approve most GM crops, including an insect-resistant rice that produces higher yields with less pesticide. In Africa, where millions go hungry, several nations have refused to import GM foods in spite of their lower costs (the result of higher yields and a reduced need for water and pesticides). Kenya has banned them altogether amid widespread malnutrition. No country has definite plans to grow Golden Rice, a crop engineered to deliver more vitamin A than spinach (rice normally has no vitamin A), even though vitamin A deficiency causes more than one million deaths annually and half a million cases of irreversible blindness in the developing world.
Globally, only a tenth of the world's cropland includes GM plants. Four countries—the U.S., Canada, Brazil and Argentina—grow 90 percent of the planet's GM crops. Other Latin American countries are pushing away from the plants. And even in the U.S., voices decrying genetically modified foods are becoming louder. In 2016 the U.S. federal government passed a law requiring labeling of GM ingredients in food products, replacing GM-labeling laws in force or proposed in several dozen states.
The fear fueling all this activity has a long history. The public has been worried about the safety of GM foods since scientists at the University of Washington developed the first genetically modified tobacco plants in the 1970s. In the mid-1990s, when the first GM crops reached the market, Greenpeace, the Sierra Club, Ralph Nader, Prince Charles and a number of celebrity chefs took highly visible stands against them. Consumers in Europe became particularly alarmed: a survey conducted in 1997, for example, found that 69 percent of the Austrian public saw serious risks in GM foods, compared with only 14 percent of Americans.
In Europe, skepticism about GM foods has long been bundled with other concerns, such as a resentment of American agribusiness. Whatever it is based on, however, the European attitude reverberates across the world, influencing policy in countries where GM crops could have tremendous benefits. “In Africa, they don't care what us savages in America are doing,” Zilberman says. “They look to Europe and see countries there rejecting GM, so they don't use it.” Forces fighting genetic modification in Europe have rallied support for “the precautionary principle,” which holds that given the kind of catastrophe that would emerge from loosing a toxic, invasive GM crop on the world, GM efforts should be shut down until the technology is proved absolutely safe.
But as medical researchers know, nothing can really be “proved safe.” One can only fail to turn up significant risk after trying hard to find it—as is the case with GM crops.
A clean record
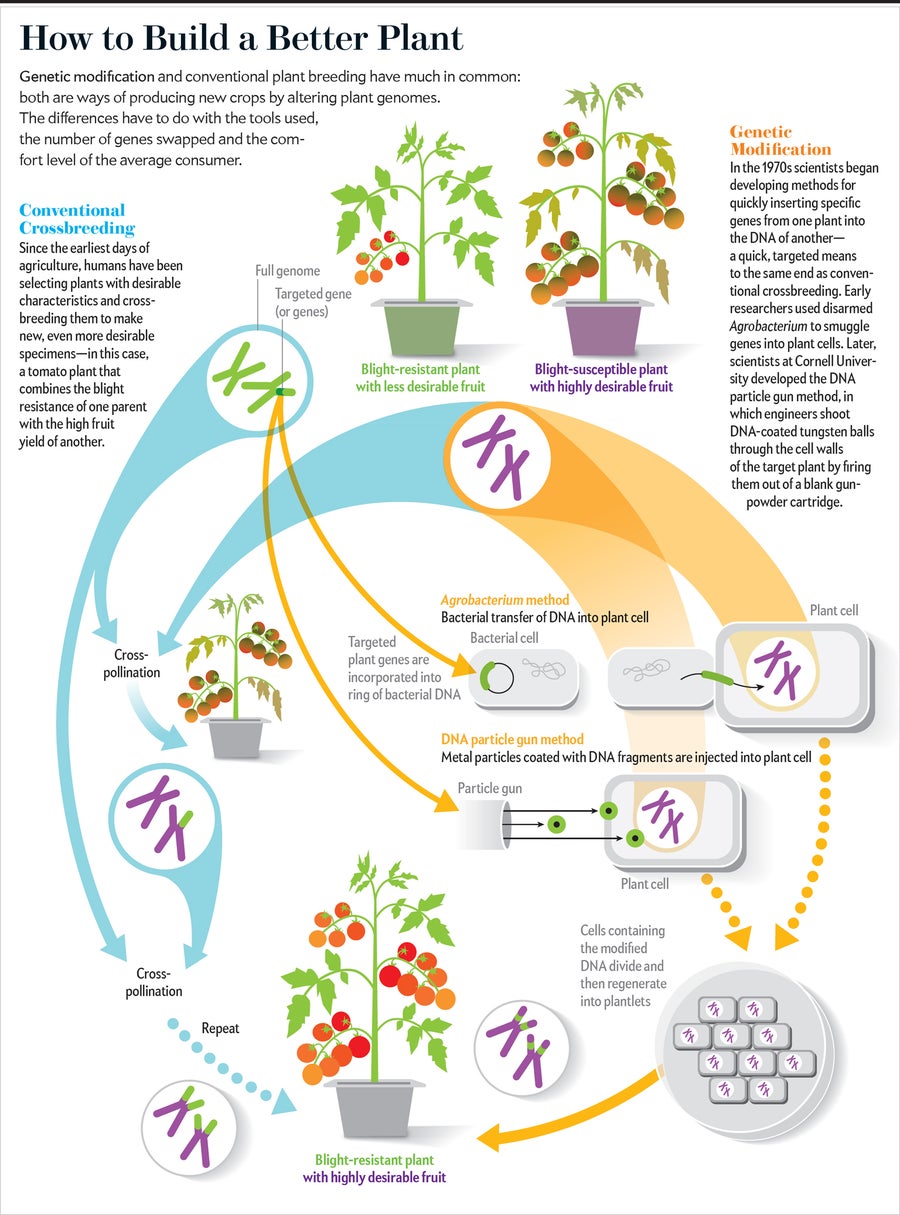
The human race has been selectively breeding crops, thus altering plants' genomes, for millennia. Ordinary wheat has long been strictly a human-engineered plant; it could not exist outside of farms, because its seeds do not scatter. For some 60 years scientists have been using “mutagenic” techniques to scramble the DNA of plants with radiation and chemicals, creating strains of wheat, rice, peanuts and pears that have become agricultural mainstays. The practice has inspired little objection from scientists or the public and has caused no known health problems.
The difference is that selective breeding or mutagenic techniques tend to result in large swaths of genes being swapped or altered. GM technology, in contrast, enables scientists to insert into a plant's genome a single gene (or a few of them) from another species of plant or even from a bacterium, virus or animal. Supporters argue that this precision makes the technology much less likely to produce surprises. Most plant molecular biologists also say that in the highly unlikely case that an unexpected health threat emerged from a new GM plant, scientists would quickly identify and eliminate it. “We know where the gene goes and can measure the activity of every single gene around it,” Goldberg says. “We can show exactly which changes occur and which don't.”
And although it might seem creepy to add virus DNA to a plant, doing so is, in fact, no big deal, proponents say. Viruses have been inserting their DNA into the genomes of crops, as well as humans and all other organisms, for millions of years. They often deliver the genes of other species while they are at it, which is why our own genome is loaded with genetic sequences that originated in viruses and nonhuman species. “When GM critics say that genes don't cross the species barrier in nature, that's just simple ignorance,” says Alan McHughen, a plant molecular geneticist at U.C. Riverside. Pea aphids contain fungi genes. Triticale is a century-plus-old hybrid of wheat and rye found in some flours and breakfast cereals. Wheat itself, for that matter, is a cross-species hybrid. “Mother Nature does it all the time, and so do conventional plant breeders,” McHughen says.
Could eating plants with altered genes allow new DNA to work its way into our own? It is possible but hugely improbable. Scientists have never found genetic material that could survive a trip through the human gut and make it into cells. Besides, we are routinely exposed to—and even consume—the viruses and bacteria whose genes end up in GM foods. The bacterium Bacillus thuringiensis , for example, which produces proteins fatal to insects, is sometimes enlisted as a natural pesticide in organic farming. “We've been eating this stuff for thousands of years,” Goldberg says.
In any case, proponents say, people have consumed as many as trillions of meals containing genetically modified ingredients over the past few decades. Not a single verified case of illness has ever been attributed to the genetic alterations. Mark Lynas, a prominent anti-GM activist who in 2013 publicly switched to strongly supporting the technology, has pointed out that every single news-making food disaster on record has been attributed to non-GM crops, such as the Escherichia coli –infected organic bean sprouts that killed 53 people in Europe in 2011.
Critics often disparage U.S. research on the safety of genetically modified foods, which is often funded or even conducted by GM companies, such as Monsanto. But much research on the subject comes from the European Commission, the administrative body of the E.U., which cannot be so easily dismissed as an industry tool. The European Commission has funded 130 research projects, carried out by more than 500 independent teams, on the safety of GM crops. None of those studies found any special risks from GM crops.
Plenty of other credible groups have arrived at the same conclusion. Gregory Jaffe, director of biotechnology at the Center for Science in the Public Interest, a science-based consumer-watchdog group in Washington, D.C., takes pains to note that the center has no official stance, pro or con, with regard to genetically modifying food plants. Yet Jaffe insists the scientific record is clear. “Current GM crops are safe to eat and can be grown safely in the environment,” he says. The American Association for the Advancement of Science, the American Medical Association and the National Academy of Sciences have all unreservedly backed GM crops. The U.S. Food and Drug Administration, along with its counterparts in several other countries, has repeatedly reviewed large bodies of research and concluded that GM crops pose no unique health threats. Dozens of review studies carried out by academic researchers have backed that view.
Opponents of genetically modified foods point to a handful of studies indicating possible safety problems. But reviewers have dismantled almost all of those reports. For example, a 1998 study by plant biochemist Árpád Pusztai, then at the Rowett Institute in Scotland, found that rats fed a GM potato suffered from stunted growth and immune system–related changes. But the potato was not intended for human consumption—it was, in fact, designed to be toxic for research purposes. The Rowett Institute later deemed the experiment so sloppy that it refuted the findings and charged Pusztai with misconduct.
Similar stories abound. Most recently, a team led by Gilles-Éric Séralini, a researcher at the University of Caen Lower Normandy in France, found that rats eating a common type of GM corn contracted cancer at an alarmingly high rate. But Séralini has long been an anti-GM campaigner, and critics charged that in his study, he relied on a strain of rat that too easily develops tumors, did not use enough rats, did not include proper control groups and failed to report many details of the experiment, including how the analysis was performed. After a review, the European Food Safety Authority dismissed the study's findings. Several other European agencies came to the same conclusion. “If GM corn were that toxic, someone would have noticed by now,” McHughen says. “Séralini has been refuted by everyone who has cared to comment.”
Some scientists say the objections to GM food stem from politics rather than science—that they are motivated by an objection to large multinational corporations having enormous influence over the food supply; invoking risks from genetic modification just provides a convenient way of whipping up the masses against industrial agriculture. “This has nothing to do with science,” Goldberg says. “It's about ideology.” Former anti-GM activist Lynas agrees. He has gone as far as labeling the anti-GM crowd “explicitly an antiscience movement.”
Persistent doubts
Not all objections to genetically modified foods are so easily dismissed, however. Long-term health effects can be subtle and nearly impossible to link to specific changes in the environment. Scientists have long believed that Alzheimer's disease and many cancers have environmental components, but few would argue we have identified all of them.
And opponents say that it is not true that the GM process is less likely to cause problems simply because fewer, more clearly identified genes are replaced. David Schubert, an Alzheimer's researcher who heads the Cellular Neurobiology Laboratory at the Salk Institute for Biological Studies in La Jolla, Calif., asserts that a single, well-characterized gene can still settle in the target plant's genome in many different ways. “It can go in forward, backward, at different locations, in multiple copies, and they all do different things,” he says. And as U.C.L.A.'s Williams notes, a genome often continues to change in the successive generations after the insertion, leaving it with a different arrangement than the one intended and initially tested. There is also the phenomenon of “insertional mutagenesis,” Williams adds, in which the insertion of a gene ends up quieting the activity of nearby genes.
True, the number of genes affected in a GM plant most likely will be far, far smaller than in conventional breeding techniques. Yet opponents maintain that because the wholesale swapping or alteration of entire packages of genes is a natural process that has been happening in plants for half a billion years, it tends to produce few scary surprises today. Changing a single gene, on the other hand, might turn out to be a more subversive action, with unexpected ripple effects, including the production of new proteins that might be toxins or allergens.
Opponents also point out that the kinds of alterations caused by the insertion of genes from other species might be more impactful, more complex or more subtle than those caused by the intraspecies gene swapping of conventional breeding. And just because there is no evidence to date that genetic material from an altered crop can make it into the genome of people who eat it does not mean such a transfer will never happen—or that it has not already happened and we have yet to spot it. These changes might be difficult to catch; their impact on the production of proteins might not even turn up in testing. “You'd certainly find out if the result is that the plant doesn't grow very well,” Williams says. “But will you find the change if it results in the production of proteins with long-term effects on the health of the people eating it?”
It is also true that many pro-GM scientists in the field are unduly harsh—even unscientific—in their treatment of critics. GM proponents sometimes lump every scientist who raises safety questions together with activists and discredited researchers. And even Séralini, the scientist behind the study that found high cancer rates for GM-fed rats, has his defenders. Most of them are nonscientists, or retired researchers from obscure institutions, or nonbiologist scientists, but the Salk Institute's Schubert also insists the study was unfairly dismissed. He says that as someone who runs drug-safety studies, he is well versed on what constitutes a good-quality animal toxicology study and that Séralini's makes the grade. He insists that the breed of rat in the study is commonly used in respected drug studies, typically in numbers no greater than in Séralini's study; that the methodology was standard; and that the details of the data analysis are irrelevant because the results were so striking.
Schubert joins Williams as one of a handful of biologists from respected institutions who are willing to sharply challenge the GM-foods-are-safe majority. Both charge that more scientists would speak up against genetic modification if doing so did not invariably lead to being excoriated in journals and the media. These attacks, they argue, are motivated by the fear that airing doubts could lead to less funding for the field. Says Williams: “Whether it's conscious or not, it's in their interest to promote this field, and they're not objective.”
Both scientists say that after publishing comments in respected journals questioning the safety of GM foods, they became the victims of coordinated attacks on their reputations. Schubert even charges that researchers who turn up results that might raise safety questions avoid publishing their findings out of fear of repercussions. “If it doesn't come out the right way,” he says, “you're going to get trashed.”
There is evidence to support that charge. In 2009 Nature detailed the backlash to a reasonably solid study published in the Proceedings of the National Academy of Sciences USA by researchers from Loyola University Chicago and the University of Notre Dame. The paper showed that GM corn seemed to be finding its way from farms into nearby streams and that it might pose a risk to some insects there because, according to the researchers' lab studies, caddis flies appeared to suffer on diets of pollen from GM corn. Many scientists immediately attacked the study, some of them suggesting the researchers were sloppy to the point of misconduct.
A way forward
There is a middle ground in this debate. Many moderate voices call for continuing the distribution of GM foods while maintaining or even stepping up safety testing on new GM crops. They advocate keeping a close eye on the health and environmental impact of existing ones. But they do not single out GM crops for special scrutiny, the Center for Science in the Public Interest's Jaffe notes: all crops could use more testing. “We should be doing a better job with food oversight altogether,” he says.
Even Schubert agrees. In spite of his concerns, he believes future GM crops can be introduced safely if testing is improved. “Ninety percent of the scientists I talk to assume that new GM plants are safety-tested the same way new drugs are by the FDA,” he says. “They absolutely aren't, and they absolutely should be.”
Stepped-up testing would pose a burden for GM researchers, and it could slow down the introduction of new crops. “Even under the current testing standards for GM crops, most conventionally bred crops wouldn't have made it to market,” McHughen says. “What's going to happen if we become even more strict?”
That is a fair question. But with governments and consumers increasingly coming down against GM crops altogether, additional testing may be the compromise that enables the human race to benefit from those crops' significant advantages.
David H. Freedman is a journalist who has been covering science, business and technology for more than 30 years.

- How We Eat and Drink Now
Are GMOs Safe? Breaking Down the Science of Science-ified Foods

T hirty years after tomatoes became the first genetically modified produce sold in the U.S., lots of people remain skeptical of science-ified foods. In a 2020 Pew Research Center survey , just 27% of Americans said they felt genetically modified foods are safe to eat, while 38% said they’re unsafe and 33% weren’t sure.
That’s not only a U.S. phenomenon. In the Philippines, for example, activists have been protesting the production of Golden Rice , a type of genetically modified rice harvested at scale for the first time last year . Unlike regular rice, Golden Rice is engineered to contain beta carotene, an addition meant to counter vitamin A deficiency and resulting vision loss. But opponents argue the rice has not been through adequate testing and that there are safer and healthier ways for people to consume vitamin A. “Golden Rice is simply not the solution to the wide, gaping wound of hunger and poverty,” a representative from MASIPAG, a Philippines-based, farmer-led group that opposes Golden Rice, told TIME in a statement.
Golden Rice is only the latest example in a long history of anti-genetically modified organism (GMO) sentiment. Over the years, protesters have torn up fields where genetically modified crops were planted and marched in the streets to criticize companies that produce GMOs. Much of the public’s concern seems to stem from fears that gene editing could introduce new toxicity into old foods; make foods more allergenic; or lead to disease-causing genetic mutations in the humans who eat these altered plants or animals. Since-debunked animal research from the 1990s also caused some people to believe that eating genetically modified food leads to organ damage.
Even though the U.S. Food and Drug Administration (FDA), U.S. Department of Agriculture , and U.S. Environmental Protection Agency —which work together to regulate GMOs and make sure they meet food-safety standards—say they are safe, many people remain wary of these science-enhanced foods. “Technophobia is a very common problem,” says Trey Malone, an agricultural economist at the University of Arkansas. “It’s this rosy retrospection that assumes that things used to be better back when. That leads to this belief system that creates pushback against gene-edited and GMO foods.”
What many people don’t realize, Malone says, is that humans have tinkered with their food for a very long time. Even thousands of years ago, farmers would save the best seeds from their harvests and use them to optimize future yields, sometimes breeding them with other plants to create even more desirable crops in years to come. Modern corn wouldn’t exist without this kind of selective breeding; nor would bananas, apples, and broccoli as we know them today. Many of the produce varieties currently available in grocery stores, like pluots and broccolini, are also a result of cross-breeding two species to create a new one.
More From TIME
Genetic modification is a related but more scientifically advanced process that involves making targeted tweaks to a plant or animal’s DNA to change or create specific traits. This process can be used to alter a food’s flavor, nutritional content, appearance, or defenses against pests like crop-killing insects, and has given rise to foods including Fresh Del Monte’s pink pineapples and non-browning Arctic apples . But while these flashy products grab lots of headlines, the truth is they make up only a fraction of the GMOs sold in the U.S.
Want to learn more about how we eat and drink now? Get guidance from experts:
- 9 Food Trends to Ditch in 2024
- How to Reduce Food Waste and Save Money
- How to Be a Healthier Drinker
- The Food Trends to Get Excited About in 2024
- How Food Can Improve Your Mood
Fred Gould, a professor of agriculture at North Carolina State University who chaired a 2016 National Academies of Sciences, Engineering, and Medicine report on genetically engineered crops, often leads educational sessions on GMOs. He likes to show a photograph of a supermarket produce section and ask how many of the vegetables in the picture are genetically modified. He gets lots of guesses as high as 90%—but the right answer is zero.
There are a handful of genetically modified fruits and veggies on the market, including summer squash, papayas, and the aforementioned pineapples and apples. And within the past decade, the FDA has approved genetically modified salmon (which grows faster than regular fish) and pork free of a specific allergen. But in the U.S., GMOs are much more likely to show up in processed foods like cooking oils, soy products, sweeteners, and snack foods. Almost all of the soybeans, corn, sugar beets, and canola planted in the U.S. are genetically modified, mainly for resistance against insects or pesticides. These crops are then used to make many of the packaged foods most Americans eat every day .
By eating these foods, the average American has for decades been part of a “natural experiment,” Gould says. People in the U.S. and Canada have been eating GMOs for decades, whereas they’re consumed less frequently overseas. If GMOs were linked to serious health problems, researchers would expect to see them reflected in comparisons of the health of North Americans relative to Europeans. But “when we look at the data,” Gould says, “we don’t see any signs.” Indeed, researchers have found no evidence of GMO-related increases in cancer, obesity, kidney disease, gastrointestinal issues, autism, or food allergies in the U.S. and Canada versus Europe. Research in animals has also shown no evidence that consuming GMOs causes genetic mutations, organ damage, or fertility problems.
“We’re very careful about saying there are no effects. We haven’t found any effects,” Gould says. There’s always a chance new risks could come to light with time, he says, but he feels that’s unlikely based on what the science has shown so far.
Malone agrees that, based on the available research, there’s no clear reason to fear genetically modified foods and plenty of reasons to embrace them. Gene-editing can not only make foods more nutritious, but also streamline their production processes to improve sustainability, he says. Planting genetically modified crops, research suggests , may increase yields and allow farmers to produce more food on less land, while simultaneously cutting down on chemical pesticide use. Meanwhile, fast-growing genetically modified salmon theoretically requires fewer resources to raise compared to conventional fish.
As Malone sees it, innovations like these are the strongest reason for people to embrace GMOs, particularly as it becomes clear that the status quo isn’t serving the planet or its people. “Production systems across the planet are realizing that we are going to have to confront climate change. We are going to have to adapt,” Malone says. “Agriculture can be part of the solution.”
More Must-Reads from TIME
- Heman Bekele Is TIME’s 2024 Kid of the Year
- The Reintroduction of Kamala Harris
- The 7 States That Will Decide the Election
- Why China Won’t Allow Single Women to Freeze Their Eggs
- Is the U.S. Ready for Psychedelics?
- The Rise of a New Kind of Parenting Guru
- The 50 Best Romance Novels to Read Right Now
- Can Food Really Change Your Hormones?
Write to Jamie Ducharme at [email protected]
Fact sheets
- Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- Endometriosis
- Excessive heat
- Mental disorders
- Polycystic ovary syndrome
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Questions and answers /
Food, genetically modified
These questions and answers have been prepared by WHO in response to questions and concerns from WHO Member State Governments with regard to the nature and safety of genetically modified food.
Genetically modified organisms (GMOs) can be defined as organisms (i.e. plants, animals or microorganisms) in which the genetic material (DNA) has been altered in a way that does not occur naturally by mating and/or natural recombination. The technology is often called “modern biotechnology” or “gene technology”, sometimes also “recombinant DNA technology” or “genetic engineering”. It allows selected individual genes to be transferred from one organism into another, also between nonrelated species. Foods produced from or using GM organisms are often referred to as GM foods.
GM foods are developed – and marketed – because there is some perceived advantage either to the producer or consumer of these foods. This is meant to translate into a product with a lower price, greater benefit (in terms of durability or nutritional value) or both. Initially GM seed developers wanted their products to be accepted by producers and have concentrated on innovations that bring direct benefit to farmers (and the food industry generally).
One of the objectives for developing plants based on GM organisms is to improve crop protection. The GM crops currently on the market are mainly aimed at an increased level of crop protection through the introduction of resistance against plant diseases caused by insects or viruses or through increased tolerance towards herbicides.
Resistance against insects is achieved by incorporating into the food plant the gene for toxin production from the bacterium Bacillus thuringiensis (Bt). This toxin is currently used as a conventional insecticide in agriculture and is safe for human consumption. GM crops that inherently produce this toxin have been shown to require lower quantities of insecticides in specific situations, e.g. where pest pressure is high. Virus resistance is achieved through the introduction of a gene from certain viruses which cause disease in plants. Virus resistance makes plants less susceptible to diseases caused by such viruses, resulting in higher crop yields.
Herbicide tolerance is achieved through the introduction of a gene from a bacterium conveying resistance to some herbicides. In situations where weed pressure is high, the use of such crops has resulted in a reduction in the quantity of the herbicides used.
Generally consumers consider that conventional foods (that have an established record of safe consumption over the history) are safe. Whenever novel varieties of organisms for food use are developed using the traditional breeding methods that had existed before the introduction of gene technology, some of the characteristics of organisms may be altered, either in a positive or a negative way. National food authorities may be called upon to examine the safety of such conventional foods obtained from novel varieties of organisms, but this is not always the case.
In contrast, most national authorities consider that specific assessments are necessary for GM foods. Specific systems have been set up for the rigorous evaluation of GM organisms and GM foods relative to both human health and the environment. Similar evaluations are generally not performed for conventional foods. Hence there currently exists a significant difference in the evaluation process prior to marketing for these two groups of food.
The WHO Department of Food Safety and Zoonoses aims at assisting national authorities in the identification of foods that should be subject to risk assessment and to recommend appropriate approaches to safety assessment. Should national authorities decide to conduct safety assessment of GM organisms, WHO recommends the use of Codex Alimentarius guidelines (See the answer to Question 11 below).
The safety assessment of GM foods generally focuses on: (a) direct health effects (toxicity), (b) potential to provoke allergic reaction (allergenicity); (c) specific components thought to have nutritional or toxic properties; (d) the stability of the inserted gene; (e) nutritional effects associated with genetic modification; and (f) any unintended effects which could result from the gene insertion.
While theoretical discussions have covered a broad range of aspects, the three main issues debated are the potentials to provoke allergic reaction (allergenicity), gene transfer and outcrossing.
Allergenicity
As a matter of principle, the transfer of genes from commonly allergenic organisms to non-allergic organisms is discouraged unless it can be demonstrated that the protein product of the transferred gene is not allergenic. While foods developed using traditional breeding methods are not generally tested for allergenicity, protocols for the testing of GM foods have been evaluated by the Food and Agriculture Organization of the United Nations (FAO) and WHO. No allergic effects have been found relative to GM foods currently on the market.
Gene transfer
Gene transfer from GM foods to cells of the body or to bacteria in the gastrointestinal tract would cause concern if the transferred genetic material adversely affects human health. This would be particularly relevant if antibiotic resistance genes, used as markers when creating GMOs, were to be transferred. Although the probability of transfer is low, the use of gene transfer technology that does not involve antibiotic resistance genes is encouraged.
Outcrossing
The migration of genes from GM plants into conventional crops or related species in the wild (referred to as “outcrossing”), as well as the mixing of crops derived from conventional seeds with GM crops, may have an indirect effect on food safety and food security. Cases have been reported where GM crops approved for animal feed or industrial use were detected at low levels in the products intended for human consumption. Several countries have adopted strategies to reduce mixing, including a clear separation of the fields within which GM crops and conventional crops are grown.
Environmental risk assessments cover both the GMO concerned and the potential receiving environment. The assessment process includes evaluation of the characteristics of the GMO and its effect and stability in the environment, combined with ecological characteristics of the environment in which the introduction will take place. The assessment also includes unintended effects which could result from the insertion of the new gene.
Issues of concern include: the capability of the GMO to escape and potentially introduce the engineered genes into wild populations; the persistence of the gene after the GMO has been harvested; the susceptibility of non-target organisms (e.g. insects which are not pests) to the gene product; the stability of the gene; the reduction in the spectrum of other plants including loss of biodiversity; and increased use of chemicals in agriculture. The environmental safety aspects of GM crops vary considerably according to local conditions.
Different GM organisms include different genes inserted in different ways. This means that individual GM foods and their safety should be assessed on a case-by-case basis and that it is not possible to make general statements on the safety of all GM foods.
GM foods currently available on the international market have passed safety assessments and are not likely to present risks for human health. In addition, no effects on human health have been shown as a result of the consumption of such foods by the general population in the countries where they have been approved. Continuous application of safety assessments based on the Codex Alimentarius principles and, where appropriate, adequate post market monitoring, should form the basis for ensuring the safety of GM foods.
The way governments have regulated GM foods varies. In some countries GM foods are not yet regulated. Countries which have legislation in place focus primarily on assessment of risks for consumer health. Countries which have regulatory provisions for GM foods usually also regulate GMOs in general, taking into account health and environmental risks, as well as control- and trade-related issues (such as potential testing and labelling regimes). In view of the dynamics of the debate on GM foods, legislation is likely to continue to evolve.
GM crops available on the international market today have been designed using one of three basic traits: resistance to insect damage; resistance to viral infections; and tolerance towards certain herbicides. GM crops with higher nutrient content (e.g. soybeans increased oleic acid) have been also studied recently.
The Codex Alimentarius Commission (Codex) is the joint FAO/WHO intergovernmental body responsible for developing the standards, codes of practice, guidelines and recommendations that constitute the Codex Alimentarius, meaning the international food code. Codex developed principles for the human health risk analysis of GM foods in 2003.
Principles for the risk analysis of foods derived from modern biotechnology
The premise of these principles sets out a premarket assessment, performed on a caseby- case basis and including an evaluation of both direct effects (from the inserted gene) and unintended effects (that may arise as a consequence of insertion of the new gene) Codex also developed three Guidelines:
Guideline for the conduct of food safety assessment of foods derived from recombinant-DNA plants
Guideline for the conduct of food safety assessment of foods produced using recombinant-DNA microorganisms
Guideline for the conduct of food safety assessment of foods derived from recombinant-DNA animals
Codex principles do not have a binding effect on national legislation, but are referred to specifically in the Agreement on the Application of Sanitary and Phytosanitary Measures of the World Trade Organization (SPS Agreement), and WTO Members are encouraged to harmonize national standards with Codex standards. If trading partners have the same or similar mechanisms for the safety assessment of GM foods, the possibility that one product is approved in one country but rejected in another becomes smaller.
The Cartagena Protocol on Biosafety, an environmental treaty legally binding for its Parties which took effect in 2003, regulates transboundary movements of Living Modified Organisms (LMOs). GM foods are within the scope of the Protocol only if they contain LMOs that are capable of transferring or replicating genetic material. The cornerstone of the Protocol is a requirement that exporters seek consent from importers before the first shipment of LMOs intended for release into the environment.
The GM products that are currently on the international market have all passed safety assessments conducted by national authorities. These different assessments in general follow the same basic principles, including an assessment of environmental and human health risk. The food safety assessment is usually based on Codex documents.
Since the first introduction on the market in the mid-1990s of a major GM food (herbicide-resistant soybeans), there has been concern about such food among politicians, activists and consumers, especially in Europe. Several factors are involved. In the late 1980s – early 1990s, the results of decades of molecular research reached the public domain. Until that time, consumers were generally not very aware of the potential of this research. In the case of food, consumers started to wonder about safety because they perceive that modern biotechnology is leading to the creation of new species.
Consumers frequently ask, “what is in it for me?”. Where medicines are concerned, many consumers more readily accept biotechnology as beneficial for their health (e.g. vaccines, medicines with improved treatment potential or increased safety). In the case of the first GM foods introduced onto the European market, the products were of no apparent direct benefit to consumers (not significantly cheaper, no increased shelflife, no better taste). The potential for GM seeds to result in bigger yields per cultivated area should lead to lower prices. However, public attention has focused on the risk side of the risk-benefit equation, often without distinguishing between potential environmental impacts and public health effects of GMOs.
Consumer confidence in the safety of food supplies in Europe has decreased significantly as a result of a number of food scares that took place in the second half of the 1990s that are unrelated to GM foods. This has also had an impact on discussions about the acceptability of GM foods. Consumers have questioned the validity of risk assessments, both with regard to consumer health and environmental risks, focusing in particular on long-term effects. Other topics debated by consumer organizations have included allergenicity and antimicrobial resistance. Consumer concerns have triggered a discussion on the desirability of labelling GM foods, allowing for an informed choice of consumers.
The release of GMOs into the environment and the marketing of GM foods have resulted in a public debate in many parts of the world. This debate is likely to continue, probably in the broader context of other uses of biotechnology (e.g. in human medicine) and their consequences for human societies. Even though the issues under debate are usually very similar (costs and benefits, safety issues), the outcome of the debate differs from country to country. On issues such as labelling and traceability of GM foods as a way to address consumer preferences, there is no worldwide consensus to date. Despite the lack of consensus on these topics, the Codex Alimentarius Commission has made significant progress and developed Codex texts relevant to labelling of foods derived from modern biotechnology in 2011 to ensure consistency on any approach on labelling implemented by Codex members with already adopted Codex provisions.
Depending on the region of the world, people often have different attitudes to food. In addition to nutritional value, food often has societal and historical connotations, and in some instances may have religious importance. Technological modification of food and food production may evoke a negative response among consumers, especially in the absence of sound risk communication on risk assessment efforts and cost/benefit evaluations.
Yes, intellectual property rights are likely to be an element in the debate on GM foods, with an impact on the rights of farmers. In the FAO/WHO expert consultation in 2003 , WHO and FAO have considered potential problems of the technological divide and the unbalanced distribution of benefits and risks between developed and developing countries and the problem often becomes even more acute through the existence of intellectual property rights and patenting that places an advantage on the strongholds of scientific and technological expertise. Such considerations are likely to also affect the debate on GM foods.
Certain groups are concerned about what they consider to be an undesirable level of control of seed markets by a few chemical companies. Sustainable agriculture and biodiversity benefit most from the use of a rich variety of crops, both in terms of good crop protection practices as well as from the perspective of society at large and the values attached to food. These groups fear that as a result of the interest of the chemical industry in seed markets, the range of varieties used by farmers may be reduced mainly to GM crops. This would impact on the food basket of a society as well as in the long run on crop protection (for example, with the development of resistance against insect pests and tolerance of certain herbicides). The exclusive use of herbicide-tolerant GM crops would also make the farmer dependent on these chemicals. These groups fear a dominant position of the chemical industry in agricultural development, a trend which they do not consider to be sustainable.
Future GM organisms are likely to include plants with improved resistance against plant disease or drought, crops with increased nutrient levels, fish species with enhanced growth characteristics. For non-food use, they may include plants or animals producing pharmaceutically important proteins such as new vaccines.
WHO has been taking an active role in relation to GM foods, primarily for two reasons:
on the grounds that public health could benefit from the potential of biotechnology, for example, from an increase in the nutrient content of foods, decreased allergenicity and more efficient and/or sustainable food production; and
based on the need to examine the potential negative effects on human health of the consumption of food produced through genetic modification in order to protect public health. Modern technologies should be thoroughly evaluated if they are to constitute a true improvement in the way food is produced.
WHO, together with FAO, has convened several expert consultations on the evaluation of GM foods and provided technical advice for the Codex Alimentarius Commission which was fed into the Codex Guidelines on safety assessment of GM foods. WHO will keep paying due attention to the safety of GM foods from the view of public health protection, in close collaboration with FAO and other international bodies.
Food, Genetically modified
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Food Sci Technol
- v.50(6); 2013 Dec

Genetically modified foods: safety, risks and public concerns—a review
Defence Food Research Laboratory, Siddarthanagar, Mysore, 570011 India
K. R. Anilakumar
Genetic modification is a special set of gene technology that alters the genetic machinery of such living organisms as animals, plants or microorganisms. Combining genes from different organisms is known as recombinant DNA technology and the resulting organism is said to be ‘Genetically modified (GM)’, ‘Genetically engineered’ or ‘Transgenic’. The principal transgenic crops grown commercially in field are herbicide and insecticide resistant soybeans, corn, cotton and canola. Other crops grown commercially and/or field-tested are sweet potato resistant to a virus that could destroy most of the African harvest, rice with increased iron and vitamins that may alleviate chronic malnutrition in Asian countries and a variety of plants that are able to survive weather extremes. There are bananas that produce human vaccines against infectious diseases such as hepatitis B, fish that mature more quickly, fruit and nut trees that yield years earlier and plants that produce new plastics with unique properties. Technologies for genetically modifying foods offer dramatic promise for meeting some areas of greatest challenge for the 21st century. Like all new technologies, they also pose some risks, both known and unknown. Controversies and public concern surrounding GM foods and crops commonly focus on human and environmental safety, labelling and consumer choice, intellectual property rights, ethics, food security, poverty reduction and environmental conservation. With this new technology on gene manipulation what are the risks of “tampering with Mother Nature”?, what effects will this have on the environment?, what are the health concerns that consumers should be aware of? and is recombinant technology really beneficial? This review will also address some major concerns about the safety, environmental and ecological risks and health hazards involved with GM foods and recombinant technology.
Introduction
Scientists first discovered in 1946 that DNA can be transferred between organisms (Clive 2011 ). It is now known that there are several mechanisms for DNA transfer and that these occur in nature on a large scale, for example, it is a major mechanism for antibiotic resistance in pathogenic bacteria. The first genetically modified (GM) plant was produced in 1983, using an antibiotic-resistant tobacco plant. China was the first country to commercialize a transgenic crop in the early 1990s with the introduction of virus resistant tobacco. In 1994, the transgenic ‘Flavour Saver tomato’ was approved by the Food and Drug Administration (FDA) for marketing in the USA. The modification allowed the tomato to delay ripening after picking. In 1995, few transgenic crops received marketing approval. This include canola with modified oil composition (Calgene), Bacillus thuringiensis (Bt) corn/maize (Ciba-Geigy), cotton resistant to the herbicide bromoxynil (Calgene), Bt cotton (Monsanto), Bt potatoes (Monsanto), soybeans resistant to the herbicide glyphosate (Monsanto), virus-resistant squash (Asgrow) and additional delayed ripening tomatoes (DNAP, Zeneca/Peto, and Monsanto) (Clive 2011 ). A total of 35 approvals had been granted to commercially grow 8 transgenic crops and one flower crop of carnations with 8 different traits in 6 countries plus the EU till 1996 (Clive 1996 ). As of 2011, the USA leads a list of multiple countries in the production of GM crops. Currently, there are a number of food species in which a genetically modified version exists (Johnson 2008 ). Some of the foods that are available in the market include cotton, soybean, canola, potatoes, eggplant, strawberries, corn, tomatoes, lettuce, cantaloupe, carrots etc. GM products which are currently in the pipeline include medicines and vaccines, foods and food ingredients, feeds and fibres. Locating genes for important traits, such as those conferring insect resistance or desired nutrients-is one of the most limiting steps in the process.
Foods derived from GM crops
At present there are several GM crops used as food sources. As of now there are no GM animals approved for use as food, but a GM salmon has been proposed for FDA approval. In instances, the product is directly consumed as food, but in most of the cases, crops that have been genetically modified are sold as commodities, which are further processed into food ingredients.
Fruits and vegetables
Papaya has been developed by genetic engineering which is ring spot virus resistant and thus enhancing the productivity. This was very much in need as in the early 1990s the Hawaii’s papaya industry was facing disaster because of the deadly papaya ring spot virus. Its single-handed savior was a breed engineered to be resistant to the virus. Without it, the state’s papaya industry would have collapsed. Today 80 % of Hawaiian papaya is genetically engineered, and till now no conventional or organic method is available to control ring spot virus.
The NewLeaf™ potato, a GM food developed using naturally-occurring bacteria found in the soil known as Bacillus thuringiensis (Bt), was made to provide in-plant protection from the yield-robbing Colorado potato beetle. This was brought to market by Monsanto in the late 1990s, developed for the fast food market. This was forced to withdraw from the market in 2001as the fast food retailers did not pick it up and thereby the food processors ran into export problems. Reports say that currently no transgenic potatoes are marketed for the purpose of human consumption. However, BASF, one of the leading suppliers of plant biotechnology solutions for agriculture requested for the approval for cultivation and marketing as a food and feed for its ‘Fortuna potato’. This GM potato was made resistant to late blight by adding two resistance genes, blb1 and blb2, which was originated from the Mexican wild potato Solanum bulbocastanum . As of 2005, about 13 % of the zucchini grown in the USA is genetically modified to resist three viruses; the zucchini is also grown in Canada (Johnson 2008 ).
Vegetable oil
It is reported that there is no or a significantly small amount of protein or DNA remaining in vegetable oil extracted from the original GM crops in USA. Vegetable oil is sold to consumers as cooking oil, margarine and shortening, and is used in prepared foods. Vegetable oil is made of triglycerides extracted from plants or seeds and then refined, and may be further processed via hydrogenation to turn liquid oils into solids. The refining process removes nearly all non-triglyceride ingredients (Crevel et al. 2000 ). Cooking oil, margarine and shortening may also be made from several crops. A large percentage of Canola produced in USA is GM and is mainly used to produce vegetable oil. Canola oil is the third most widely consumed vegetable oil in the world. The genetic modifications are made for providing resistance to herbicides viz. glyphosate or glufosinate and also for improving the oil composition. After removing oil from canola seed, which is ∼43 %, the meal has been used as high quality animal feed. Canola oil is a key ingredient in many foods and is sold directly to consumers as margarine or cooking oil. The oil has many non-food uses, which includes making lipsticks.
Maize, also called corn in the USA and cornmeal, which is ground and dried maize constitute a staple food in many regions of the world. Grown since 1997 in the USA and Canada, 86 % of the USA maize crop was genetically modified in 2010 (Hamer and Scuse 2010 ) and 32 % of the worldwide maize crop was GM in 2011 (Clive 2011 ). A good amount of the total maize harvested go for livestock feed including the distillers grains. The remaining has been used for ethanol and high fructose corn syrup production, export, and also used for other sweeteners, cornstarch, alcohol, human food or drink. Corn oil is sold directly as cooking oil and to make shortening and margarine, in addition to make vitamin carriers, as a source of lecithin, as an ingredient in prepared foods like mayonnaise, sauces and soups, and also to fry potato chips and French fries. Cottonseed oil is used as a salad and cooking oil, both domestically and industrially. Nearly 93 % of the cotton crop in USA is GM.
The USA imports 10 % of its sugar from other countries, while the remaining 90 % is extracted from domestically grown sugar beet and sugarcane. Out of the domestically grown sugar crops, half of the extracted sugar is derived from sugar beet, and the other half is from sugarcane. After deregulation in 2005, glyphosate-resistant sugar beet was extensively adopted in the USA. In USA 95 % of sugar beet acres were planted with glyphosate-resistant seed (Clive 2011 ). Sugar beets that are herbicide-tolerant have been approved in Australia, Canada, Colombia, EU, Japan, Korea, Mexico, New Zealand, Philippines, Russian Federation, Singapore and USA. The food products of sugar beets are refined sugar and molasses. Pulp remaining from the refining process is used as animal feed. The sugar produced from GM sugar beets is highly refined and contains no DNA or protein—it is just sucrose, the same as sugar produced from non-GM sugar beets (Joana et al. 2010 ).
Quantification of genetically modified organisms (GMOs) in foods
Testing on GMOs in food and feed is routinely done using molecular techniques like DNA microarrays or qPCR. These tests are based on screening genetic elements like p35S, tNos, pat, or bar or event specific markers for the official GMOs like Mon810, Bt11, or GT73. The array based method combines multiplex PCR and array technology to screen samples for different potential GMO combining different approaches viz. screening elements, plant-specific markers, and event-specific markers. The qPCR is used to detect specific GMO events by usage of specific primers for screening elements or event specific markers. Controls are necessary to avoid false positive or false negative results. For example, a test for CaMV is used to avoid a false positive in the event of a virus contaminated sample.
Joana et al. ( 2010 ) reported the extraction and detection of DNA along with a complete industrial soybean oil processing chain to monitor the presence of Roundup Ready (RR) soybean. The amplification of soybean lectin gene by end-point polymerase chain reaction (PCR) was achieved in all the steps of extraction and refining processes. The amplification of RR soybean by PCR assays using event specific primers was also achieved for all the extraction and refining steps. This excluded the intermediate steps of refining viz. neutralization, washing and bleaching possibly due to sample instability. The real-time PCR assays using specific probes confirmed all the results and proved that it is possible to detect and quantify GMOs in the fully refined soybean oil.
Figure 1 gives the overall protocol for the testing of GMOs. This is based on a PCR detection system specific for 35S promoter region originating from cauliflower mosaic virus (Deisingh and Badrie 2005 ). The 35S-PCR technique permits detection of GMO contents of foods and raw materials in the range of 0.01–0.1 %. The development of quantitative detection systems such as quantitative competitive PCR (QC-PCR), real-time PCR and ELISA systems resulted in the advantage of survival of DNA in most manufacturing processes. Otherwise with ELISA, there can be protein denaturing during food processing. Inter-laboratory differences were found to be less with the QC-PCR than with quantitative PCR probably due to insufficient homogenisation of the sample. However, there are disadvantages, the major one being the amount of DNA, which could be amplified, is affected by food processing techniques and can vary up to 5-fold. Thus, results need to be normalised by using plant-specific QC-PCR system. Further, DNA, which cannot be amplified, will affect all quantitative PCR detection systems.

Protocol for the testing of genetically modified foods
In a recent work La Mura et al. ( 2011 ) applied QUIZ (quantization using informative zeros) to estimate the contents of RoundUp Ready™ soya and MON810 in processed food containing one or both GMs. They reported that the quantification of GM in samples can be performed without the need for certified reference materials using QUIZ. Results showed good agreement between derived values and known input of GM material and compare favourably with quantitative real-time PCR. Detection of Roundup Ready soybean by loop-mediated isothermal amplification combined with a lateral-flow dipstick has been reported recently (Xiumin et al. 2012 ).
GM foods-merits and demerits
Before we think of having GM foods it is very important to know about is advantages and disadvantages especially with respect to its safety. These foods are made by inserting genes of other species into their DNA. Though this kind of genetic modification is used both in plants and animals, it is found more commonly in the former than in the latter. Experts are working on developing foods that have the ability to alleviate certain disorders and diseases. Though researchers and the manufacturers make sure that there are various advantages of consuming these foods, a fair bit of the population is entirely against them.
GM foods are useful in controlling the occurrence of certain diseases. By modifying the DNA system of these foods, the properties causing allergies are eliminated successfully. These foods grow faster than the foods that are grown traditionally. Probably because of this, the increased productivity provides the population with more food. Moreover these foods are a boon in places which experience frequent droughts, or where the soil is incompetent for agriculture. At times, genetically engineered food crops can be grown at places with unfavourable climatic conditions too. A normal crop can grow only in specific season or under some favourable climatic conditions. Though the seeds for such foods are quite expensive, their cost of production is reported to be less than that of the traditional crops due to the natural resistance towards pests and insects. This reduces the necessity of exposing GM crops to harmful pesticides and insecticides, making these foods free from chemicals and environment friendly as well. Genetically engineered foods are reported to be high in nutrients and contain more minerals and vitamins than those found in traditionally grown foods. Other than this, these foods are known to taste better. Another reason for people opting for genetically engineered foods is that they have an increased shelf life and hence there is less fear of foods getting spoiled quickly.
The biggest threat caused by GM foods is that they can have harmful effects on the human body. It is believed that consumption of these genetically engineered foods can cause the development of diseases which are immune to antibiotics. Besides, as these foods are new inventions, not much is known about their long term effects on human beings. As the health effects are unknown, many people prefer to stay away from these foods. Manufacturers do not mention on the label that foods are developed by genetic manipulation because they think that this would affect their business, which is not a good practice. Many religious and cultural communities are against such foods because they see it as an unnatural way of producing foods. Many people are also not comfortable with the idea of transferring animal genes into plants and vice versa. Also, this cross-pollination method can cause damage to other organisms that thrive in the environment. Experts are also of the opinion that with the increase of such foods, developing countries would start depending more on industrial countries because it is likely that the food production would be controlled by them in the time to come.
Safety tests on commercial GM crops
The GM tomatoes were produced by inserting kanr genes into a tomato by an ‘antisense’ GM method (IRDC 1998 ). The results show that there were no significant alterations in total protein, vitamins and mineral contents and in toxic glycoalkaloids (Redenbaugh et al. 1992 ). Therefore, the GM and parent tomatoes were deemed to be “substantially equivalent”. In acute toxicity studies with male/female rats, which were tube-fed with homogenized GM tomatoes, toxic effects were reported to be absent. A study with a GM tomato expressing B. thuringiensis toxin CRYIA (b) was underlined by the immunocytochemical demonstration of in vitro binding of Bt toxin to the caecum/colon from humans and rhesus monkeys (Noteborn et al. 1995 ).
Two lines of Chardon LL herbicide-resistant GM maize expressing the gene of phosphinothricin acetyltransferase before and after ensiling showed significant differences in fat and carbohydrate contents compared with non-GM maize and were therefore substantially different come. Toxicity tests were only performed with the maize even though with this the unpredictable effects of the gene transfer or the vector or gene insertion could not be demonstrated or excluded. The design of these experiments was also flawed because of poor digestibility and reduction in feed conversion efficiency of GM corn. One broiler chicken feeding study with rations containing transgenic Event 176 derived Bt corn (Novartis) has been published (Brake and Vlachos 1998 ). However, the results of this trial are more relevant to commercial than academic scientific studies.
GM soybeans
To make soybeans herbicide resistant, the gene of 5-enolpyruvylshikimate-3-phosphate synthase from Agrobacterium was used. Safety tests claim the GM variety to be “substantially equivalent” to conventional soybeans (Padgette et al. 1996 ). The same was claimed for GTS (glyphosate-resistant soybeans) sprayed with this herbicide (Taylor et al. 1999 ). However, several significant differences between the GM and control lines were recorded (Padgette et al. 1996 ) and the study showed statistically significant changes in the contents of genistein (isoflavone) with significant importance for health (Lappe et al. 1999 ) and increased content in trypsin inhibitor.
Studies have been conducted on the feeding value (Hammond et al. 1996 ) and possible toxicity (Harrison et al. 1996 ) for rats, broiler chickens, catfish and dairy cows of two GM lines of glyphosate-resistant soybean (GTS). The growth, feed conversion efficiency, catfish fillet composition, broiler breast muscle and fat pad weights and milk production, rumen fermentation and digestibilities in cows were found to be similar for GTS and non-GTS. These studies had the following lacunae: (a) No individual feed intakes, body or organ weights were given and histology studies were qualitative microscopy on the pancreas, (b) The feeding value of the two GTS lines was not substantially equivalent either because the rats/catfish grew significantly better on one of the GTS lines than on the other, (c) The design of study with broiler chicken was not much convincing, (d) Milk production and performance of lactating cows also showed significant differences between cows fed GM and non-GM feeds and (e) Testing of the safety of 5-enolpyruvylshikimate-3-phosphate synthase, which renders soybeans glyphosate-resistant (Harrison et al. 1996 ), was irrelevant because in the gavage studies an E. coli recombinant and not the GTS product were used. In a separate study (Teshima et al. 2000 ), it was claimed that rats and mice which were fed 30 % toasted GTS or non-GTS in their diet had no significant differences in nutritional performance, organ weights, histopathology and production of IgE and IgG antibodies.
GM potatoes
There were no improvements in the protein content or amino acid profile of GM potatoes (Hashimoto et al. 1999a ). In a short feeding study to establish the safety of GM potatoes expressing the soybean glycinin gene, rats were daily force-fed with 2 g of GM or control potatoes/kg body weight (Hashimoto et al 1999b ). No differences in growth, feed intake, blood cell count and composition and organ weights between the groups were found. In this study, the intake of potato by animals was reported to be too low (Pusztai 2001 ).
Feeding mice with potatoes transformed with a Bacillus thuringiensis var. kurstaki Cry1 toxin gene or the toxin itself was shown to have caused villus epithelial cell hypertrophy and multinucleation, disrupted microvilli, mitochondrial degeneration, increased numbers of lysosomes and autophagic vacuoles and activation of crypt Paneth cells (Fares and El-Sayed 1998 ). The results showed CryI toxin which was stable in the mouse gut. Growing rats pair-fed on iso -proteinic and iso -caloric balanced diets containing raw or boiled non-GM potatoes and GM potatoes with the snowdrop ( Galanthus nivalis ) bulb lectin (GNA) gene (Ewen and Pusztai 1999 ) showed significant increase in the mucosal thickness of the stomach and the crypt length of the intestines of rats fed GM potatoes. Most of these effects were due to the insertion of the construct used for the transformation or the genetic transformation itself and not to GNA which had been pre-selected as a non-mitotic lectin unable to induce hyperplastic intestinal growth (Pusztai et al. 1990 ) and epithelial T lymphocyte infiltration.
The kind that expresses soybean glycinin gene (40–50 mg glycinin/g protein) was developed (Momma et al. 1999 ) and was claimed to contain 20 % more protein. However, the increased protein content was found probably due to a decrease in moisture rather than true increase in protein.
Several lines of GM cotton plants have been developed using a gene from Bacillus thuringiensis subsp. kurstaki providing increased protection against major lepidopteran pests. The lines were claimed to be “substantially equivalent” to parent lines (Berberich et al. 1996 ) in levels of macronutrients and gossypol. Cyclopropenoid fatty acids and aflatoxin levels were less than those in conventional seeds. However, because of the use of inappropriate statistics it was questionable whether the GM and non-GM lines were equivalent, particularly as environmental stresses could have unpredictable effects on anti-nutrient/toxin levels (Novak and Haslberger 2000 ).
The nutritional value of diets containing GM peas expressing bean alpha-amylase inhibitor when fed to rats for 10 days at two different doses viz. 30 % and 65 % was shown to be similar to that of parent-line peas (Pusztai et al. 1999 ). At the same time in order to establish its safety for humans a more rigorous specific risk assessment will have to be carried out with several GM lines. Nutritional/toxicological testing on laboratory animals should follow the clinical, double-blind, placebo-type tests with human volunteers.
Allergenicity studies
When the gene is from a crop of known allergenicity, it is easy to establish whether the GM food is allergenic using in vitro tests, such as RAST or immunoblotting, with sera from individuals sensitised to the original crop. This was demonstrated in GM soybeans expressing the brasil nut 2S proteins (Nordlee et al. 1996 ) or in GM potatoes expressing cod protein genes (Noteborn et al. 1995 ). It is also relatively easy to assess whether genetic engineering affected the potency of endogenous allergens (Burks and Fuchs 1995 ). Farm workers exposed to B. thuringiensis pesticide were shown to have developed skin sensitization and IgE antibodies to the Bt spore extract. With their sera it may now therefore be possible to test for the allergenic potential of GM crops expressing Bt toxin (Bernstein et al. 1999 ). It is all the more important because Bt toxin Cry1Ac has been shown to be a potent oral/nasal antigen and adjuvant (Vazquez-Padron et al. 2000 ).
The decision-tree type of indirect approach based on factors such as size and stability of the transgenically expressed protein (O’Neil et al. 1998 ) is even more unsound, particularly as its stability to gut proteolysis is assessed by an in vitro (simulated) testing (Metcalf et al. 1996 ) instead of in vivo (human/animal) testing and this is fundamentally wrong. The concept that most allergens are abundant proteins may be misleading because, for example, Gad c 1, the major allergen in codfish, is not a predominant protein (Vazquez-Padron et al. 2000 ). However, when the gene responsible for the allergenicity is known, such as the gene of the alpha-amylase/trypsin inhibitors/allergens in rice, cloning and sequencing opens the way for reducing their level by antisense RNA strategy (Nakamura and Matsuda 1996 ).
It is known that the main concerns about adverse effects of GM foods on health are the transfer of antibiotic resistance, toxicity and allergenicity. There are two issues from an allergic standpoint. These are the transfer of a known allergen that may occur from a crop into a non-allergenic target crop and the creation of a neo-allergen where de novo sensitisation occurs in the population. Patients allergic to Brazil nuts and not to soy bean then showed an IgE mediated response towards GM soy bean. Lack ( 2002 ) argued that it is possible to prevent such occurrences by doing IgE-binding studies and taking into account physico-chemical characteristics of proteins and referring to known allergen databases. The second possible scenario of de novo sensitisation does not easily lend itself to risk assessment. He reports that evidence that the technology used for the production of GM foods poses an allergic threat per se is lacking very much compared to other methodologies widely accepted in the food industry.
Risks and controversy
There are controversies around GM food on several levels, including whether food produced with it is safe, whether it should be labelled and if so how, whether agricultural biotechnology and it is needed to address world hunger now or in the future, and more specifically with respect to intellectual property and market dynamics, environmental effects of GM crops and GM crops’ role in industrial agricultural more generally.
Many problems, viz. the risks of “tampering with Mother Nature”, the health concerns that consumers should be aware of and the benefits of recombinant technology, also arise with pest-resistant and herbicide-resistant plants. The evolution of resistant pests and weeds termed superbugs and super weeds is another problem. Resistance can evolve whenever selective pressure is strong enough. If these cultivars are planted on a commercial scale, there will be strong selective pressure in that habitat, which could cause the evolution of resistant insects in a few years and nullify the effects of the transgenic. Likewise, if spraying of herbicides becomes more regular due to new cultivars, surrounding weeds could develop a resistance to the herbicide tolerant by the crop. This would cause an increase in herbicide dose or change in herbicide, as well as an increase in the amount and types of herbicides on crop plants. Ironically, chemical companies that sell weed killers are a driving force behind this research (Steinbrecher 1996 ).
Another issue is the uncertainty in whether the pest-resistant characteristic of these crops can escape to their weedy relatives causing resistant and increased weeds (Louda 1999 ). It is also possible that if insect-resistant plants cause increased death in one particular pest, it may decrease competition and invite minor pests to become a major problem. In addition, it could cause the pest population to shift to another plant population that was once unthreatened. These effects can branch out much further. A study of Bt crops showed that “beneficial insects, so named because they prey on crop pests, were also exposed to harmful quantities of Bt.” It was stated that it is possible for the effects to reach further up the food web to effect plants and animals consumed by humans (Brian 1999 ). Also, from a toxicological standpoint, further investigation is required to determine if residues from herbicide or pest resistant plants could harm key groups of organisms found in surrounding soil, such as bacteria, fungi, nematodes, and other microorganisms (Allison and Palma 1997 ).
The potential risks accompanied by disease resistant plants deal mostly with viral resistance. It is possible that viral resistance can lead to the formation of new viruses and therefore new diseases. It has been reported that naturally occurring viruses can recombine with viral fragments that are introduced to create transgenic plants, forming new viruses. Additionally, there can be many variations of this newly formed virus (Steinbrecher 1996 ).
Health risks associated with GM foods are concerned with toxins, allergens, or genetic hazards. The mechanisms of food hazards fall into three main categories (Conner and Jacobs 1999 ). They are inserted genes and their expression products, secondary and pleiotropic effects of gene expression and the insertional mutagenesis resulting from gene integration. With regards to the first category, it is not the transferred gene itself that would pose a health risk. It should be the expression of the gene and the affects of the gene product that are considered. New proteins can be synthesized that can produce unpredictable allergenic effects. For example, bean plants that were genetically modified to increase cysteine and methionine content were discarded after the discovery that the expressed protein of the transgene was highly allergenic (Butler and Reichhardt 1999 ). Due attention should be taken for foods engineered with genes from foods that commonly cause allergies, such as milk, eggs, nuts, wheat, legumes, fish, molluscs and crustacean (Maryanski 1997 ). However, since the products of the transgenic are usually previously identified, the amount and effects of the product can be assessed before public consumption. Also, any potential risk, immunological, allergenic, toxic or genetically hazardous, could be recognized and evaluated if health concerns arise. The available allergen data bases with details are shown in Table 1 .
Allergen databases (Kleter and Peijnenburg 2002 )
| Name | Website | Type of allergen | Details |
|---|---|---|---|
| AgMoBiol | Food, Pollen | The Agricultural Molecular Biology Laboratory of the Peking University Protein Engg. & Plant Genetic Engg. | |
| Central Science Lab | Proteins | Food and Drug Administration Centre for Food Safety and Applied Nutrition, Sand Hutton, York, UK | |
| FARRP | Proteins | 658 allergens, The Food Allergy Research & Resource Program, University of Nebraska-Lincoln | |
| NCFST | Gluten | National Centre for Safety & Technology, Illinois Institute of Technology | |
| PROTALL | Plant | Biochemical and clinical data- The PROTALL project, FAIR- CT98-4356, The Institute of Food Research, UK | |
| SDAP | Proteins | Allergenic Proteins (Ivanciuc et al. ) | |
| SwissPort | Proteins | SIB Swiss Institute of Bioinformatics, Geneva) | |
| WHO/International Union of Immunological Societies | Proteins | Nomenclature (Chapman ) | |
| Allergome | Proteins | Mari and Riccioli ( ) | |
| Internet Symposium on Food Allergens-2002 | – | Food Allergen data collections |
More concern comes with secondary and pleiotropic effects. For example, many transgenes encode an enzyme that alters biochemical pathways. This could cause an increase or decrease in certain biochemicals. Also, the presence of a new enzyme could cause depletion in the enzymatic substrate and subsequent build up of the enzymatic product. In addition, newly expressed enzymes may cause metabolites to diverge from one secondary metabolic pathway to another (Conner and Jacobs 1999 ). These changes in metabolism can lead to an increase in toxin concentrations. Assessing toxins is a more difficult task due to limitations of animal models. Animals have high variation between experimental groups and it is challenging to attain relevant doses of transgenic foods in animals that would provide results comparable to humans (Butler and Reichhardt 1999 ). Consequently, biochemical and regulatory pathways in plants are poorly understood.
Insertional mutagenesis can disrupt or change the expression of existing genes in a host plant. Random insertion can cause inactivation of endogenous genes, producing mutant plants. Moreover, fusion proteins can be made from plant DNA and inserted DNA. Many of these genes create nonsense products or are eliminated in crop selection due to incorrect appearance. However, of most concern is the activation or up regulation of silent or low expressed genes. This is due to the fact that it is possible to activate “genes that encode enzymes in biochemical pathways toward the production of toxic secondary compounds” (Conner and Jacobs 1999 ). This becomes a greater issue when the new protein or toxic compound is expressed in the edible portion of the plant, so that the food is no longer substantially equal to its traditional counterpart.
There is a great deal of unknowns when it comes to the risks of GM foods. One critic declared “foreign proteins that have never been in the human food chain will soon be consumed in large amounts”. It took us many years to realize that DDT might have oestrogenic activities and affect humans, “but we are now being asked to believe that everything is OK with GM foods because we haven’t seen any dead bodies yet” (Butler and Reichhardt 1999 ). As a result of the growing public concerns over GM foods, national governments have been working to regulate production and trade of GM foods.
Reports say that GM crops are grown over 160 million hectares in 29 countries, and imported by countries (including European ones) that don’t grow them. Nearly 300 million Americans, 1350 million Chinese, 280 million Brazilians and millions elsewhere regularly eat GM foods, directly and indirectly. Though Europeans voice major fears about GM foods, they permit GM maize cultivation. It imports GM soy meal and maize as animal feed. Millions of Europeans visit the US and South America and eat GM food.
Around three million Indians have become US citizens, and millions more go to the US for tourism and business and they will be eating GM foods in the USA. Indian activists claim that GM foods are inherently dangerous and must not be cultivated in India. Activists strongly opposed Bt cotton in India, and published reports claiming that the crop had failed in the field. At the same time farmers soon learned from experience that Bt cotton was very profitable, and 30 million rushed to adopt it. In consequence, India’s cotton production doubled and exports zoomed, even while using much less pesticide. Punjab farmers lease land at Rs 30,000 per acre to grow Bt cotton.
Public concerns-global scenario
In the late 1980s, there was a major controversy associated with GM foods even when the GMOs were not in the market. But the industrial applications of gene technology were developed to the production and marketing status. After words, the European Commission harmonized the national regulations across Europe. Concerns from the community side on GMOs in particular about its authorization have taken place since 1990s and the regulatory frame work on the marketing aspects underwent refining. Issues specifically on the use of GMOs for human consumption were introduced in 1997, in the Regulation on Novel Foods Ingredients (258/97/EC of 27 January 1997). This Regulations deals with rules for authorization and labelling of novel foods including food products made from GMOs, recognizing for the first time the consumer’s right to information and labelling as a tool for making an informed choice. The labelling of GM maize varieties and GM soy varieties that did not fall under this Regulation are covered by Regulation (EC 1139/98). Further legislative initiatives concern the traceability and labelling of GMOs and the authorization of GMOs in food and feed.
The initial outcome of the implementation of the first European directive seemed to be a settlement of the conflicts over technologies related to gene applications. By 1996, the second international level controversy over gene technology came up and triggered the arrival of GM soybeans at European harbours (Lassen et al. 2002 ). The GM soy beans by Monsanto to resist the herbicide represented the first large scale marketing of GM foods in Europe. Events such as commercialisation of GM maize and other GM modified commodities focused the public attention on the emerging biosciences, as did other gene technology applications such as animal and human cloning. The public debate on the issues associated with the GM foods resulted in the formation of many non-governmental organizations with explicit interest. At the same time there is a great demand for public participation in the issues about regulation and scientific strategy who expresses acceptance or rejection of GM products through purchase decisions or consumer boycotts (Frewer and Salter 2002 ).
Most research effort has been devoted to assessing people’s attitudes towards GM foods as a technology. Numerous “opinion poll”—type surveys have been conducted on national and cross-national levels (Hamstra 1998 ). Ethical concerns are also important, that a particular technology is in some way “tampering with nature”, or that unintended effects are unpredictable and thus unknown to science (Miles and Frewer 2001 ).
Consumer’s attitude towards GM foods
Consumer acceptance is conditioned by the risk that they perceive from introducing food into their consumption habits processed through technology that they hardly understand. In a study conducted in Spain, the main conclusion was that the introduction of GM food into agro-food markets should be accompanied by adequate policies to guarantee consumer safety. These actions would allow a decrease in consumer-perceived risk by taking special care of the information provided, concretely relating to health. For, the most influential factor in consumer-perceived risk from these foods is concern about health (Martinez-Poveda et al. 2009 ).
Tsourgiannis et al. ( 2011 ) conducted a study aimed to identify the factors that affect consumers purchasing behaviour towards food products that are free from GMO (GM Free) in a European region and more precisely in the Prefecture of Drama-Kavala-Xanthi. Field interviews conducted in a random selected sample consisted of 337 consumers in the cities of Drama, Kavala, Xanthi in 2009. Principal components analysis (PCA) was conducted in order to identify the factors that affect people in preferring consuming products that are GM Free. The factors that influence people in the study area to buy GM Free products are: (a) products’ certification as GM Free or organic products, (b) interest about the protection of the environment and nutrition value, (c) marketing issues and (d) price and quality. Furthermore, cluster and discriminant analysis identified two groups of consumers: (a) those influenced by the product price, quality and marketing aspects and (b) those interested in product’s certification and environmental protection (Tsourgiannis et al. 2011 ).
Snell et al. ( 2012 ) examined 12 long-term studies (of more than 90 days, up to 2 years in duration) and 12 multigenerational studies (from 2 to 5 generations) on the effects of diets containing GM maize, potato, soybean, rice, or triticale on animal health. They referenced the 90-day studies on GM feed for which long-term or multigenerational study data were available. Many parameters have been examined using biochemical analyses, histological examination of specific organs, hematology and the detection of transgenic DNA. Results from all the 24 studies do not suggest any health hazards and, in general, there were no statistically significant differences within parameters observed. They observed some small differences, though these fell within the normal variation range of the considered parameter and thus had no biological or toxicological significance. The studies reviewed present evidence to show that GM plants are nutritionally equivalent to their non-GM counterparts and can be safely used in food and feed.
GM foods: issues with respect to India
In a major setback to the proponents of GM technology in farm crops, the Parliamentary Committee on Agriculture in 2012 asked Indian government to stop all field trials and sought a bar on GM food crops such as Bt. brinjal. Raising the “ethical dimensions” of transgenics in agricultural crops, as well as studies of a long-term environmental and chronic toxicology impact, the panel noted that there were no significant socio-economic benefits to farmers.
Countries like India have great security concerns at the same time specific problems exist for small and marginal farmers. India could use a toxin free variety of the Lathyrus sativus grown on marginal lands and consumed by the very poor. GM mustard is a variety using the barnase-barstar-bar gene complex, an unstable gene construct with possible undesirable effects, to achieve male sterile lines that are used to make hybrid mustard varieties. In India we have good non-GM alternatives for making male sterile lines for hybrid production so the Proagro variety is of little use. Being a food crop, GM mustard will have to be examined very carefully. Even if there were to be benefits, they have to be weighed against the risks posed to human health and the environment. Apart from this, mustard is a cross-pollinating crop and pollen with their foreign genes is bound to reach non-GM mustard and wild relatives. We do not know what impact this will have. If GM technology is to be used in India, it should be directed at the real needs of Indian farmers, on crops like legumes, oilseeds and fodder and traits like drought tolerance and salinity tolerance.
Basmati rice and Darjeeling tea are perhaps India’s most easily identifiable premium products in the area of food. Basmati is highly prized rice, its markets are growing and it is a high end, expensive product in the international market. Like Champagne wine and truffles from France, international consumers treat it as a special, luxury food. Since rice is nutritionally a poor cereal, it is thought that addition of iron and vitamin A by genetic modification would increase the nutritional quality. So does it make any sense at all to breed a GM Basmati, along the lines of Bt Cotton? However, premium wine makers have outright rejected the notion of GM doctored wines that were designed to cut out the hangover and were supposed to be ‘healthier’. Premium products like special wines, truffles and Basmati rice need to be handled in a special, premium way (Sahai 2003 ).
Traceability of GMOs in the food production chain
Traceability systems document the history of a product and may serve the purpose of both marketing and health protection. In this framework, segregation and identity preservation systems allow for the separation of GM and non-GM products from “farm to fork”. Implementation of these systems comes with specific technical requirements for each particular step of the food processing chain. In addition, the feasibility of traceability systems depends on a number of factors, including unique identifiers for each GM product, detection methods, permissible levels of contamination, and financial costs. Progress has been achieved in the field of sampling, detection, and traceability of GM products, while some issues remain to be solved. For success, much will depend on the threshold level for adventitious contamination set by legislation (Miraglia et al. 2004 ).
Issues related to detection and traceability of GMOs is gaining interest worldwide due to the global diffusion and the related socio-economical implications. The interest of the scientific community into traceability aspects has also been increased simultaneously. Crucial factors in sampling and detection methodologies are the number of the GMOs involved and international agreement on traceability. The availability of reliable traceability strategies is very important and this may increase public trust in transparency in GMO related issues.
Heat processing methods like autoclaving and microwave heating can damage the DNA and reduce the level to detectable DNA. The PCR based methods have been standardised to detect such DNA in GM soybean and maize (Vijayakumar et al. 2009 ). Molecular methods such as multiplex and real time PCR methods have been developed to detect even 20 pg of genomic DNA in genetically modified EE-1 brinjal (Ballari et al. 2012 ).
DNA and protein based methods have been adopted for the detection and identification of GMOs which is relatively a new area of diagnostics. New diagnostic methodologies are also being developed, viz. the microarray-based methods that allow for the simultaneous identification of the increasing number of GMOs on the global market in a single sample. Some of these techniques have also been discussed for the detection of unintended effects of genetic modification by Cellini et al. ( 2004 ). The implementation of adequate traceability systems requires more than technical tools alone and is strictly linked to labelling constraints. The more stringent the labelling requirements, the more expensive and difficult the associated traceability strategies are to meet these requirements.
Both labelling and traceability of GMOs are current issues that are considered in trade and regulation. Currently, labelling of GM foods containing detectable transgenic material is required by EU legislation. A proposed package of legislation would extend this labelling to foods without any traces of transgenics. These new legislations would also impose labelling and a traceability system based on documentation throughout the food and feed manufacture system. The regulatory issues of risk analysis and labelling are currently harmonised by Codex Alimentarius. The implementation and maintenance of the regulations necessitates sampling protocols and analytical methodologies that allow for accurate determination of the content of GM organisms within a food and feed sample. Current methodologies for the analysis of GMOs are focused on either one of two targets, the transgenic DNA inserted- or the novel protein(s) expressed- in a GM product. For most DNA-based detection methods, the polymerase chain reaction is employed. Items that need consideration in the use of DNA-based detection methods include the specificity, sensitivity, matrix effects, internal reference DNA, availability of external reference materials, hemizygosity versus homozygosity, extra chromosomal DNA and international harmonisation.
For most protein-based methods, enzyme-linked immunosorbent assays with antibodies binding the novel protein are employed. Consideration should be given to the selection of the antigen bound by the antibody, accuracy, validation and matrix effects. Currently, validation of detection methods for analysis of GMOs is taking place. New methodologies are developed, in addition to the use of microarrays, mass spectrometry and surface plasmon resonance. Challenges for GMO detection include the detection of transgenic material in materials with varying chromosome numbers. The existing and proposed regulatory EU requirements for traceability of GM products fit within a broader tendency towards traceability of foods in general and, commercially, towards products that can be distinguished from one another.
Gene transfer studies in human volunteers
As of January 2009, there has only been one human feeding study conducted on the effects of GM foods. The study involved seven human volunteers who previously had their large intestines removed for medical reasons. These volunteers were provided with GM soy to eat to see if the DNA of the GM soy transferred to the bacteria that naturally lives in the human gut. Researchers identified that three of the seven volunteers had transgenes from GM soya transferred into the bacteria living in their gut before the start of the feeding experiment. As this low-frequency transfer did not increase after the consumption of GM soy, the researchers concluded that gene transfer did not occur during the experiment. In volunteers with complete digestive tracts, the transgene did not survive passage through intact gastrointestinal tract (Netherwood 2004 ). Other studies have found DNA from M13 virus, GFP and even ribulose-1, 5-bisphosphate carboxylase (Rubisco) genes in the blood and tissue of ingesting animals (Guertler et al. 2009 ; Brigulla and Wackernagel 2010 ).
Two studies on the possible effects of giving GM feed to animals found that there were no significant differences in the safety and nutritional value of feedstuffs containing material derived from GM plants (Gerhard et al. 2005 ; Beagle et al. 2006 ). Specifically, the studies noted that no residues of recombinant DNA or novel proteins have been found in any organ or tissue samples obtained from animals fed with GM plants (Nordlee 1996 ; Streit 2001 ).
Future developments
The GM foods have the potential to solve many of the world’s hunger and malnutrition problems, and to help protect and preserve the environment by increasing yield and reducing reliance upon synthetic pesticides and herbicides. Challenges ahead lie in many areas viz. safety testing, regulation, policies and food labelling. Many people feel that genetic engineering is the inevitable wave of the future and that we cannot afford to ignore a technology that has such enormous potential benefits.
Future also envisages that applications of GMOs are diverse and include drugs in food, bananas that produce human vaccines against infectious diseases such as Hepatitis B (Kumar et al. 2005 ), metabolically engineered fish that mature more quickly, fruit and nut trees that yield years earlier, foods no longer containing properties associated with common intolerances, and plants that produce new biodegradable plastics with unique properties (van Beilen and Yves 2008 ). While their practicality or efficacy in commercial production has yet to be fully tested, the next decade may see exponential increases in GM product development as researchers gain increasing access to genomic resources that are applicable to organisms beyond the scope of individual projects.
One has to agree that there are many opinions (Domingo 2000 ) about scarce data on the potential health risks of GM food crops, even though these should have been tested for and eliminated before their introduction. Although it is argued that small differences between GM and non-GM crops have little biological meaning, it is opined that most GM and parental line crops fall short of the definition of substantial equivalence. In any case, we need novel methods and concepts to probe into the compositional, nutritional, toxicological and metabolic differences between GM and conventional crops and into the safety of the genetic techniques used in developing GM crops if we want to put this technology on a proper scientific foundation and allay the fears of the general public. Considerable effort need to be directed towards understanding people’s attitudes towards this gene technology. At the same time it is imperative to note the lack of trust in institutions and institutional activities regarding GMOs and the public perceive that institutions have failed to take account of the actual concerns of the public as part of their risk management activities.
Contributor Information
A. S. Bawa, Email: ni.oc.oohay@awabrednirama .
K. R. Anilakumar, Email: moc.liamg@rkramukalina .
- Allison S, Palma PM. Commercialization of transgenic plants: potential ecological risks. BioScience. 1997; 47 :86–96. doi: 10.2307/1313019. [ CrossRef ] [ Google Scholar ]
- Ballari VR, Martin A, Gowda LR (2012) Detection and identification of genetically modified EE-1 brinjal ( Solanum melongena ) by single, multiplex and SYBR® real-time PCR. J Sci Food Agric. doi:10.1002/jsfa.5764, Published online 22 June 2012 [ PubMed ]
- Beagle JM, Apgar GA, Jones KL, Griswold KE, Radcliffe JS, Qiu X, Lightfoot DA, Iqbal MJ. The digestive fate of Escherichia coli glutamate dehydrogenase deoxyribonucleic acid from transgenic corn in diets fed to weanling pigs. J Anim Sci. 2006; 84 (3):597–607. [ PubMed ] [ Google Scholar ]
- Berberich SA, Ream JE, Jackson TL, Wood R, Stipanovic R, Harvey P, Patzer S, Fuchs RL. The composition of insect-protected cottonseed is equivalent to that of conventional cottonseed. J Agric Food Chem. 1996; 44 :365–371. doi: 10.1021/jf950304i. [ CrossRef ] [ Google Scholar ]
- Bernstein IL, Bernstein JA, Miller M, Tierzieva S, Bernstein DI, Lummus Z, Selgrade MK, Doerfler DL, Seligy VL. Immune responses in farm workers after exposure to Bacillus thuringiensis pesticides . Environ Health Perspect. 1999; 107 :575–582. doi: 10.1289/ehp.99107575. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brake J, Vlachos D. Evaluation of transgenic Event 176 “Bt” corn in broiler chicken. Poult Sci. 1998; 77 :648–653. [ PubMed ] [ Google Scholar ]
- Brian H. Unintended effects of Bt crops. World Watch. 1999; 12 :9–10. [ Google Scholar ]
- Brigulla M, Wackernagel W. Molecular aspects of gene transfer and foreign DNA acquisition in prokaryotes with regard to safety issues. Appl Microbiol Biotechnol. 2010; 86 (4):1027–1041. doi: 10.1007/s00253-010-2489-3. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Burks AW, Fuchs RL. Assessment of the endogenous allergens in glyphosate-tolerant and commercial soybean varieties. J Allergy Clin Immunol. 1995; 96 :1008–1010. doi: 10.1016/S0091-6749(95)70243-1. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Butler T, Reichhardt T. Long-term effect of GM crops serves up food for thought. Nature. 1999; 398 (6729):651–653. doi: 10.1038/19348. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cellini F, Chesson A, Colquhoun I, Constable A, Davies HV, Engel KH, Gatehouse AMR, Karenlampi S, Kok EJ, Leguay JJ, Lehasranta S, Noteborn HPJM, Pedersen J, Smith M. Unintended effects and their detection in genetically modified crops. Food Chem Toxicol. 2004; 42 :1089–1125. doi: 10.1016/j.fct.2004.02.003. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chapman MD. Allergen nomenclature. In: Lockey RF, Dennis Ledford K, editors. Allergens and allergen immunotherapy. 4. New York: Informa Healthcare; 2008. pp. 47–58. [ Google Scholar ]
- Clive J (1996) Global review of the field testing and commercialization of transgenic plants: 1986 to 1995. The International Service for the Acquisition of Agri-biotech Applications. http://www.isaaa.org/kc/Publications/pdfs/isaaabriefs/Briefs%201.pdf . Retrieved on 17 July 2010
- Clive J. Global status of commercialized Biotech/GM crops. ISAAA Briefs 43 . Ithaca: International Service for the Acquisition of Agri-biotech Applications; 2011. [ Google Scholar ]
- Conner AJ, Jacobs JME. Genetic engineering of crops as potential source of genetic hazard in the human diet. Mutat Res Genet Toxicol Environ Mutagen. 1999; 443 :223–234. doi: 10.1016/S1383-5742(99)00020-4. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Crevel RWR, Lerkhof MAT, Koning MMG. Allergenicity of refined vegetable oils. Food Chem Toxicol. 2000; 38 (4):385–393. doi: 10.1016/S0278-6915(99)00158-1. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Deisingh AK, Badrie N. Detection approaches for genetically modified organisms in foods. Food Res Int. 2005; 38 :639–649. doi: 10.1016/j.foodres.2005.01.003. [ CrossRef ] [ Google Scholar ]
- Domingo JL. Health risks of genetically modified foods: many opinions but few data. Science. 2000; 288 :1748–1749. doi: 10.1126/science.288.5472.1748. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ewen SWB, Pusztai A. Effects of diets containing genetically modified potatoes expressing Galanthus nivalis lectin on rat small intestine. Lancet. 1999; 354 :1353–1354. doi: 10.1016/S0140-6736(98)05860-7. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fares NH, El-Sayed AK. Fine structural changes in the ileum of mice fed on delta-endotoxin-treated potatoes and transgenic potatoes. Nat Toxins. 1998; 6 :219–233. doi: 10.1002/(SICI)1522-7189(199811/12)6:6<219::AID-NT30>3.0.CO;2-K. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Frewer LI, Salter B. Public attitudes, scientific advice and the politics of regulatory policy the case of BSE. Sci Public Policy. 2002; 29 :137–145. doi: 10.3152/147154302781781092. [ CrossRef ] [ Google Scholar ]
- Gerhard F, Andrew C, Karen A. Animal nutrition with feeds from genetically modified plants. Arch Anim Nutr. 2005; 59 :1–40. doi: 10.1080/17450390512331342368. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Guertler P, Paul V, Albrech C, Meyer HH. Sensitive and highly specific quantitative real-time PCR and ELISA for recording a potential transfer of novel DNA and Cry1Ab protein from feed into bovine milk. Anal Bioanal Chem. 2009; 393 :1629–1638. doi: 10.1007/s00216-009-2667-2. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hamer H, Scuse T (2010) National Agricultural Statistics Service (NASS), Agricultural Statistics Board, US Department of Agriculture. Acreage report, NY
- Hammond BG, Vicini JL, Hartnell GF, Naylor MW, Knight CD, Robinson EH, Fuchs RL, Padgette SR. The feeding value of soybeans fed to rats, chickens, catfish and dairy cattle is not altered by genetic incorporation of glyphosate tolerance. J Nutr. 1996; 126 :717–727. [ PubMed ] [ Google Scholar ]
- Hamstra A (1998) Public opinion about Biotechnology. A survey of surveys. European Federation of Biotechnology, The Hague
- Harrison LA, Bailey MR, Naylor MW, Ream JE, Hammond BG, Nida DL, Burnette BL, Nickson TE, Mitsky TA, Taylor ML, Fuchs RL, Padgette SR. The expressed protein in glyphosate-tolerant soybean, 5-enolpyruvylshikimate-3-phosphate synthase from Agrobacterium sp. strain CP4, is rapidly digested in vitro and is not toxic to acutely gavaged mice. J Nutr. 1996; 126 :728–740. [ PubMed ] [ Google Scholar ]
- Hashimoto W, Momma K, Katsube T, Ohkawa Y, Ishige T, Kito M, Utsumi S, Murata K. Safety assessment of genetically engineered potatoes with designed soybean glycinin: compositional analyses of the potato tubers and digestibility of the newly expressed protein in transgenic potatoes. J Sci Food Agric. 1999; 79 :1607–1612. doi: 10.1002/(SICI)1097-0010(199909)79:12<1607::AID-JSFA408>3.0.CO;2-T. [ CrossRef ] [ Google Scholar ]
- Hashimoto W, Momma K, Yoon HJ, Ozawa S, Ohkawa Y, Ishige T, Kito M, Utsumi S, Murata K. Safety assessment of transgenic potatoes with soybean glycinin by feeding studies in rats. Biosci Biotechnol Biochem. 1999; 63 :1942–1946. doi: 10.1271/bbb.63.1942. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- IRDC (1998) Alliance for biointegrity. http://www.biointegrity.org including Calgene FLAVR SAVR™ tomato report, pp 1–604; International Research and Development Corp. first test report, pp 1736–1738; Conclusions of the expert panel regarding the safety of the FLAVR SAVR™ tomato, ENVIRON, Arlington VA, USA pp 2355–2382; Four week oral (intubation) toxicity study in rats by IRDC, pp 2895–3000
- Ivanciuc O, Schein CH, Braun W. SDAP: database and computational tools for allergenic proteins. Nucleic Acids Res. 2003; 31 :359–362. doi: 10.1093/nar/gkg010. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Joana C, Isabel M, Joana SA, Oliveira MBPP. Monitoring genetically modified soybean along the industrial soybean oil extraction and refining processes by polymerase chain reaction techniques. Food Res Int. 2010; 43 :301–306. doi: 10.1016/j.foodres.2009.10.003. [ CrossRef ] [ Google Scholar ]
- Johnson SR. Quantification of the impacts on US Agriculture of Biotechnology-Derived Crops Planted in 2006. Washington DC: National Centre for Food and Agricultural Policy; 2008. [ Google Scholar ]
- Kleter GA, Peijnenburg AACM. Screening of transgenic proteins expressed in transgenic food crops for the presence of short amino acid sequences identical to potential, IgE-binding linear epitopes of allergens. BMC Struct Biol. 2002; 2 :8–19. doi: 10.1186/1472-6807-2-8. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kumar GBS, Ganapathi TR, Revathi CJ, Srinivas L, Bapat VA. Expression of hepatitis B surface antigen in transgenic banana plants. Planta. 2005; 222 :484–493. doi: 10.1007/s00425-005-1556-y. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lack G. Clinical risk assessment of GM foods. Toxicol Lett. 2002; 127 :337–340. doi: 10.1016/S0378-4274(01)00517-3. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- La Mura M, Allnutt TR, Greenland A, Mackay LD. Application of QUIZ for GM quantification in food. Food Chem. 2011; 125 :1340–1344. doi: 10.1016/j.foodchem.2010.10.002. [ CrossRef ] [ Google Scholar ]
- Lappe MA, Bailey EB, Childress C, Setchell KDR. Alterations in clinically important phytoestrogens in genetically modified, herbicide-tolerant soybeans. J Med Food. 1999; 1 :241–245. doi: 10.1089/jmf.1998.1.241. [ CrossRef ] [ Google Scholar ]
- Lassen J, Allansdottir A, Liakoupulos M, Olsson A, Mortensen AT. Testing times: the reception of round-up ready soya in Europe. In: Bauer M, Gaskell G, editors. Biotechnology—the making of a global controversy. Cambridge: Cambridge University Press; 2002. pp. 279–312. [ Google Scholar ]
- Louda SM (1999) Insect Limitation of weedy plants and its ecological implications. In: Traynor PL, Westwood J H (eds) Proceedings of a workshop on: ecological effects of pest resistance genes in managed ecosystems. Information Systems for Biotechnology. Blacksburg, Virginia, pp 43–48, http://www.isb.vt.edu
- Mari A, Riccioli D. The allergome web site—a database of allergenic molecules. Aim, structure, and data of a web-based resource. J Allergy Clin Immunol. 2004; 113 :S301. [ Google Scholar ]
- Martinez-Poveda A, Molla-Bauza MB, Gomis FJC, Martinez LMC. Consumer-perceived risk model for the introduction of genetically modified food in Spain. Food Policy. 2009; 34 :519–528. doi: 10.1016/j.foodpol.2009.08.001. [ CrossRef ] [ Google Scholar ]
- Maryanski JH. Bioengineered foods: will they cause allergic reactions? NY: U.S. Food and Drug Administration (FDA)/Centre for Food Safety and Applied Nutrition (CFSAN); 1997. [ Google Scholar ]
- Metcalf DD, Astwood JD, Townsend R, Sampson HA, Taylor SL, Fuchs RL (1996) Assessment of the allergenic potential of foods derived from genetically engineered crop plants. In: Crit Rev Food Sci Nutr 36(S):S165–S186. CRC, Boca Raton [ PubMed ]
- Miles S, Frewer LI. Investigating specific concerns about different food hazards—higher and lower order attributes. Food Qual Prefer. 2001; 12 :47–61. doi: 10.1016/S0950-3293(00)00029-X. [ CrossRef ] [ Google Scholar ]
- Miraglia M, Berdal K, Brera C, Corbisier P, Holst-jensen A, Kok E, Marvin H, Schimmel H, Rentsch J, van Rie J, Zagon J. Detection and traceability of genetically modified organisms in the food production chain. Food Chem Toxicol. 2004; 42 :1157–1180. doi: 10.1016/j.fct.2004.02.018. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Momma K, Hashimoto W, Ozawa S, Kawai S, Katsube T, Takaiwa F, Kito M, Utsumi S, Murata K. Quality and safety evaluation of genetically engineered rice with soybean glycinin: analyses of the grain composition and digestibility of glycinin in transgenic rice. Biosci Biotechnol Biochem. 1999; 63 :314–318. doi: 10.1271/bbb.63.314. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nakamura R, Matsuda T. Rice allergenic protein and molecular-genetic approach for hypoallergenic rice. Biosci Biotechnol Biochem. 1996; 60 :1215–1221. doi: 10.1271/bbb.60.1215. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Netherwood T. Assessing the survival of transgenic plant DNA in the human gastrointestinal tract. Nat Biotechnol. 2004; 22 :204–209. doi: 10.1038/nbt934. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nordlee JA. Identification of Brazil-Nut allergen in transgenic soybeans. New Engl J Med. 1996; 334 :688–692. doi: 10.1056/NEJM199603143341103. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nordlee JA, Taylor SL, Townsend JA, Thomas LA. Identification of a Brazil nut allergen in transgenic soybean. New Engl J Med. 1996; 334 :688–692. doi: 10.1056/NEJM199603143341103. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Noteborn HPJM, Bienenmann-Ploum ME, van den Berg JHJ, Alink GM, Zolla L, Raynaerts A, Pensa M, Kuiper HA. Safety assessment of the Bacillus thuringiensis insecticidal crystal protein CRYIA(b) expressed in transgenic tomatoes. In: Engel KH, Takeoka GR, Teranishi R, editors. ACS Symp series 605 Genetically modified foods—safety issues. Washington, D.C: American Chemical Society; 1995. pp. 135–147. [ Google Scholar ]
- Novak WK, Haslberger AG. Substantial equivalence of antinutrients and inherent plant toxins in genetically modified novel foods. Food Chem Toxicol. 2000; 38 :473–483. doi: 10.1016/S0278-6915(00)00040-5. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- O’Neil C, Reese G, Lehrer SB. Allergenic potential of recombinant food proteins. Allergy Clin Immunol Int. 1998; 10 :5–9. [ Google Scholar ]
- Padgette SR, Taylor NB, Nida DL, Bailey MR, MacDonald J, Holden LR, Fuchs RL. The composition of glyphosate-tolerant soybean seeds is equivalent to that of conventional soybeans. J Nutr. 1996; 126 :702–716. [ PubMed ] [ Google Scholar ]
- Pusztai A (2001) Safety tests on commercial crops. American Institute of Biological Sciences. actionbioscience.org, http://www.actionbioscience.org/biotech/pusztai.html viewed 2 March 2010
- Pusztai A, Ewen SWB, Grant G, Peumans WJ, van Damme EJM, Rubio L, Bardocz S. Relationship between survival and binding of plant lectins during small intestinal passage and their effectiveness as growth factors. Digestion. 1990; 46 (suppl 2):308–316. doi: 10.1159/000200402. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pusztai A, Grant G, Bardocz S, Alonso R, Chrispeels MJ, Schroeder HE, Tabe LM, Higgins TJV. Expression of the insecticidal bean alpha-amylase inhibitor transgene has minimal detrimental effect on the nutritional value of peas fed to rats at 30 % of the diet. J Nutr. 1999; 129 :1597–1603. [ PubMed ] [ Google Scholar ]
- Redenbaugh K, Hiatt W, Martineau B, Kramer M, Sheehy R, Sanders R, Houck C, Emlay D (1992) Safety assessment of genetically engineered fruits and vegetables: a case study of the Flavr Savr Tomato. CRC Press, Boca Raton
- Sahai S. Genetically modified crops: issues for India. Fin Agric. 2003; 35 :7–11. [ Google Scholar ]
- Snell C, Bernheim A, Bergé J-B, Kuntz M, Pascal G, Paris A, Agnès ER. Assessment of the health impact of GM plant diets in long-term and multigenerational animal feeding trials: a literature review. Food Chem Toxicol. 2012; 50 :1134–1148. doi: 10.1016/j.fct.2011.11.048. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Steinbrecher RA. From green to gene evolution: the environmental risks of genetically engineered crops. Ecologist. 1996; 26 :273–281. [ Google Scholar ]
- Streit L. Association of the Brazil nut protein gene and Kunitz trypsin inhibitor alleles with soybean protease inhibitor activity and agronomic traits. Crop Sci. 2001; 41 :1757–1760. doi: 10.2135/cropsci2001.1757. [ CrossRef ] [ Google Scholar ]
- Taylor NB, Fuchs RL, MacDonald J, Shariff AB, Padgette SR. Compositional analysis of glyphosate-tolerant soybeans treated with glyphosate. J Agric Food Chem. 1999; 47 :4469–4473. doi: 10.1021/jf990056g. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Teshima R, Akiyama H, Okunuki H, Sakushima J-i, Goda Y, Onodera H, Sawada J-i, Toyoda M. Effect of GM and non-GM soybeans on the immune system of BN rats and B10A mice. J Food Hyg Soc Jpn. 2000; 41 :188–193. doi: 10.3358/shokueishi.41.188. [ CrossRef ] [ Google Scholar ]
- Tsourgiannis L, Karasavvoglou A, Florou G. Consumers’ attitudes towards GM free products in a European region. The case of the Prefecture of Drama-Kavala-Xanthi in Greece. Appetite. 2011; 57 :448–458. doi: 10.1016/j.appet.2011.06.010. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- van Beilen JB, Yves P. Harnessing plant biomass for biofuels and biomaterials: production of renewable polymers from crop plants. Plant J. 2008; 54 (4):684–701. doi: 10.1111/j.1365-313X.2008.03431.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vazquez-Padron RI, Moreno-Fierros L, Neri-Bazan L, Martinez-Gil AF, de la Riva GA, Lopez-Revilla R. Characterization of the mucosal and sytemic immune response induced by Cry1Ac protein from Bacillus thuringiensis HD 73 in mice. Braz J Med Biol Res. 2000; 33 :147–155. doi: 10.1590/S0100-879X2000000200002. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vijayakumar KR, Martin A, Gowda LR, Prakash V. Detection of genetically modified soya and maize: impact of heat processing. Food Chem. 2009; 117 :514–521. doi: 10.1016/j.foodchem.2009.04.028. [ CrossRef ] [ Google Scholar ]
- Xiumin W, Da T, Qingfeng G, Fang T, Jianhua W. Detection of Roundup Ready soybean by loop-mediated isothermal amplification combined with a lateral-flow dipstick. Food Control. 2012; 29 :213–220. [ Google Scholar ]
Home — Essay Samples — Nursing & Health — Nutrition & Dieting — Genetically Modified Food
Essays on Genetically Modified Food
Embark on a deep dive into the complex and contentious world of genetically modified organisms (GMOs) with our curated collection of genetically modified food essay samples. These essays are designed to provide students with a multifaceted view of the GMO debate, offering insights into the scientific, ethical, environmental, and socio-economic dimensions of genetically modified foods. Whether you are seeking inspiration, looking to bolster your argument, or searching for comprehensive analyses, our collection is an invaluable resource for anyone writing a genetically modified food essay.
The GMO Debate
Genetically modified foods have been at the center of global debates due to their potential impact on food security, health, environment, and biodiversity. Essays on this topic explore various angles, including the benefits of GMOs in addressing world hunger , the concerns over their health and environmental effects, and the ethical considerations of manipulating genetic material. By engaging with these essays, students can cultivate a balanced perspective, appreciating the complexities of the GMO debate.
Highlights from Our Essay Collection
Our genetically modified food essay samples encompass a wide range of perspectives, ensuring that students can find material that resonates with their specific research interests and viewpoints. From critical essays examining the risks associated with GMO consumption to persuasive essays advocating for the role of GMOs in sustainable agriculture, our collection offers a rich tapestry of arguments and discussions. Each essay not only serves as a model for academic writing but also as a springboard for generating unique ideas and approaches to the topic.
Utilizing Our Essays to Your Advantage
- Idea Generation: Let our essays inspire your topic selection and approach, offering new perspectives on the genetically modified food debate.
- Research Starting Point: Use the essays as a foundation for further research, helping you to identify key points, studies, and statistics relevant to your argument.
- Structural Blueprint: Analyze the organization and flow of our essays to guide the structuring of your own argument, ensuring clarity and persuasiveness.
- Citation Guidance: Learn from the referencing styles used in our essays to enhance the credibility and academic integrity of your work.
The debate over genetically modified foods is both vital and vast, touching on issues that affect our health, environment, and global food supply. Our collection of genetically modified food essay samples is here to guide students through the intricacies of this debate, providing a solid foundation for informed and compelling writing. Dive into our essays to enrich your understanding and articulate your position on genetically modified foods with confidence and clarity.
Explore our genetically modified food essay samples today and embark on a journey of discovery and debate. Let these essays empower you to craft a thoughtful and persuasive genetically modified food essay that contributes meaningfully to the ongoing conversation.
The Advantages of Genetic Engineering in Food Production
Genetically modified technology in the agricultural industry, made-to-order essay as fast as you need it.
Each essay is customized to cater to your unique preferences
+ experts online
Genetically Modified Food: is It Beneficial Or Harmful
Why labelling of genetically modified foods should be mandatory, should genetically modified foods be banned, genetically modified food: benefits and risks, let us write you an essay from scratch.
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
The Ethics of Meat Consumption
Kfc's triumph and turmoil in china, thesis statement on genetically modified foods, relevant topics.
- Healthy Food
- Energy Drink
- Eating Habits
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
Genetically Modified Foods and Environment Research Paper
- To find inspiration for your paper and overcome writer’s block
- As a source of information (ensure proper referencing)
- As a template for you assignment
Introduction
Concept of genetic modification, benefits of genetic modification, human and environment harm associated with genetic modification, lack of validity to support gm crops bio-safety, controversy in countries over use of gm foods.
Cultivation of crops for food has been as old as man himself. As time goes by farmers have been trying to improve characteristics of plants used for food such as taste and resistance to diseases. Crops which have been grown in a healthy manner seem to have better yields and even good taste. However, this technique does not work well because it has natural limitations. Plants which are from different species can not be bred together to bring out desired characteristics the farmer may want.
It is on this background researchers that are in the field of genetic engineering and biotechnology have come up with a concept of genetic modification in attempt to address this limitation to farmers. Genetic modification is a biotechnology that involves use of gene from one organism into another. This technology is very different from traditional breeding techniques that farmers used to employ to improve their crop yields several centuries ago (Gura, 2000 p 412).
Increased production of genetically modified foods to many has been considered as a breakthrough in addressing issue of food security. Currently, various regions of the world particularly developing countries have been faced with both acute and chronic food shortages that have led to loss of many lives due to malnutrition.
Nevertheless, bio-safety of genetically modified foods has raised a lot of concern in public health domain. Adverse effects of gene transfer in genetically modified foods have been found to be a threat to human health and in ecological system.
Therefore, the main purpose of this paper is to present evidence based argument in support that, despite achieved breakthroughs in genetic engineering to provide us with genetically modified foods, there are overwhelming drawbacks that outweigh good intentions of using genetically modified foods (Gura, 2000 p 414).
Before proceeding further it would be relevant to highlight how genetic modification works. Genetic modification shortened as GM is a process which involves altering with the how genes in the plants, animals or micro-organisms function. It also involves getting a gene form organism A. to organism B. All these need to be living organism for the process to work.
Essentially, GM is possible by altering deoxyribonucleic acid (DNA) of an organism in some aspects. This may be in form of inserting a genetic material into an organism from another organism which is of the same species or unrelated species. It is possible to introduce a genetic material from one plan to another or from plant to an animal or vice versa (Ibid).
Deoxyribonucleic acid (DNA) is a genetic material that is found in the cells of living things that carries coded information which allows survival of any living thing in terms of how an organism’s cells functions, reproduce and get repaired. It has been found that in a single cell of an organism, there are thousand of different types of genes.
These genes make up DNA and determine genetic make up or characteristics of an organism. Advanced understanding of genetic materials has facilitated emergence of genetically modified foods in the world. Genetic modification involves number of steps (Gura, 2000 p412).
For instance, to genetically modify a plant, a researcher will have to look for a plant which has characteristics that are needed. This follows location of the specific gene in the DNA that gives the desired attributes of the plant. After specific gene providing desired characteristics in the plant is identified, it is inserted to the plant which needs to be modified.
At this point, there is integration of identified specific gene with a piece of DNA from bacteria that acts as a carrier. Also, something called ‘promoter’ which acts as a switch is inserted to this gene to ensure that this gene works appropriately to the plant it is transferred. Interestingly, it is only few number of cells in the plant being modified accept new gene introduced (Gura, 2000 p412).
In order to find out which cells in the plant have taken up new genetic material, a carrier package gene is also introduced to the plant. Carrier packaged are introduced in the plant by use of soil bacterium or attaching very minute particles of tungsten and firing them into plant tissue at a very high speed. It is worth noting that genetic modification does not only involve gene transfer. It can involve altering specific gene in a plant or organism by changing or switching off certain gene in order to achieve desired characteristic (Gura, 2000 p413).
Research findings indicates that out of the trials which have been carried out to determine the success of GM, plants and animals with specific qualities desired can be produced with high level of accuracy and in a more efficient way compared to traditional techniques. GM has made it possible to transfer genes from one species to another which would not be possible using traditional methods.
There are number of alleged advantages as a result of genetic modification. The first advantage cited is that of pest control. It has been said that huge crop losses do occur due to insect pest infestations that can cost farmers in terms of financial investments as well as lead to country’s food shortage.
It is argue that annually, famers use several tons of pesticides to control pests during crop growing process which is a health threat to consumers. As such production of genetically modified crops such as B.t. corn can help farmers eliminate need for using chemical pesticides and significantly cut down the cost of buying these chemicals (Hurley,Babcock & Hellmich, 2000 p2).
Another benefit of using genetic modification in agriculture is that it helps come up with crops which have tolerance in herbicide used during weeding. It is argued that weeding by physical means for some crops may be costly and therefore, many farmers opt to use herbicide sprays.
This process is also time-consuming as farmers try to take caution not to spray to food crops. By using of plants that are genetically-engineered to have resistant to particular herbicides, it is said that this can save huge amounts of herbicide sprays used and prevent environmental pollution (Hurley,Babcock & Hellmich, 2000 p6).
A good example is that of a certain soy-bean strain which has been genetically modified by Mansanto Company that is able to resist herbicides. It is argued that if farmers grow this type of soy-bean, they do not need multiple applications of herbicides but just one weed-killer application that significantly reduce the cost and minimize dangers associated.
Crops that are tolerance to cold have been developed using antifreeze gene obtained from cold water fish. Such plants include tobacco and potatoes. It is argued that with antifreeze gene, such crops are able to withstand cold temperatures.
In addition to above mentioned benefits, genetic modification has been embraced due to creating plants which are able to resist drought or high saline content salt. This is seen as a great hallmark especially in current world where land for cultivation is reducing.
People are now able to grow crops for food in region that could earlier not support growth of normal crops. There being high prevalence rates of malnutrition in developing world, genetic modification has been providing some sort of relieve. For example, there are some parts of the world where population depends on one type of crop, like rice, as main staple in diet.
In attempt to address vitamin A deficiency, one research was conducted by the Swiss Federal Institute of Technology Institute for Plant Sciences came up with a strain of rice which has increased content level of beta-carotene, a precursor of vitamin A.
This project was funded by Rockfeller Foundation to help address malnutrition in the world. It is speculated that researches are still going on to produce this particular strain of rice with relatively higher content of iron to help address anemia which is also a high prevalent form of micro-nutrient deficiency especially in young children (Rockfellor Foundation, 1999 p1470).
Globally supporters of genetically modified food argue that its is necessary to use GM foods in order to meet food requirement of world’s ever increasing population and address setbacks brought by crop diseases.
But I would pose one question: Do we really need genetically modified foods to address this problem? What are the risks associated with the use of genetically modified food to human being and our environment? Malnutrition still continues to remain a major public health concern particularly in developing countries. But is it that people are having malnutrition and hunger due to the fact that there is no sufficient food to feed them?
It should be noted that issues of food security do not revolve only around on inadequate or availability of food. There are about three dimensions of food security namely, availability, accessibility and utilization. Food may be available but individuals lack economic means to have access to food however plenty it might be in the market. In addition, complex issues ranging from social, political to economic are determinant to accessibility of land and other resources.
For that reason, concern of increased level of production through introduction of genetically modified foods is just one part of the whole picture in addressing food insecurity. It is realized that most of genetically modified foods grown, they are for market purposes.
Countries that produce maize and soy-bean are developed countries and these crops are used as animal fodders and some of it being added in processed food commodities. This does not seem sufficiently enough to address malnutrition and hunger in the world. Many countries which have been faced with food crisis have always raised concerns in regard to food aid given as genetically modified crops due to their health and environmental effects (Clark & Lehman, 2001 p27).
Great controversy has ensued and will continue between those who support the use of genetically modified crops and those who do not support use of it. But who is right between the two groups? Over the recent past there has GMO controversies being reported in popular media and in scientific papers.
Those who support GM crops maintain there are potential benefits associated with the use of such crops which include high yields rate, increased tolerance of crops in new crop growing zones as well as reduced dependence on use of biocides. Notwithstanding this they argue that these crops have no associated risk to human being or environment. However, these claims have been criticized through practical ways.
Several potential harms have been identified such as damage to insects and other soil organism which are useful, enhancing antibiotic resistant diseases and involuntary exposure to not only human beings but also to livestock and wildlife to toxins and allergens which have not been detected (FAO/WHO, 2000 p26).
A search conducted on GM food safety from Medline database indicated that on issue of toxicity of transgenic foods 44 citations were identified, with only one citation reporting experimentation while seven citations were from opinions. A search on adverse effects of transgenic foods provided 67 citations, two reporting experimentation while sixteen were citations from opinions.
Finally, a search on modified foods yielded 101 citations with six citations reporting experimentation while 37 were citations based on opinion. In total sixty authors were identified offering their opinions without support of concrete data. Most of these were those who supported idea of transgenic food safety (Clark & Lehman, 2001 p7-8).
On the same note several studies conducted by generic modified companies lacked citations and this questions the evidence presented to us besides lacking valid reason to explain why evidence presented by those support GM foods that are safe has not been subjected to peer review. For this reason, claims for GM crops are safe and posses no environmental harm for sure have not gone through peer-review for validation this lack sufficient ground to be trusted (Clark & Lehman, 2001 p27).
There has been a recent global controversy over genetically modified foods where United States of America has been seen taking different stand for genetically modified food as compared with other countries particularly from European region.
For instance, a study conducted in these regions indicated that United State respondents were supportive to genetically modified foods as compared to those who were from Europe.
However, biotechnology did not seem to receive strong support in United State but European respondents were more supportive to genetic testing. Though no solid reason was found as to existence of resistance of genetically modified food in Europe media coverage seemed to be the most probable cause that influenced of public perceptions (Gaskell et al, 2000 p1).
From the above presentation, I find that there is not concrete evidence to support that there is no harm in consuming genetically modified foods. Bio-safety assessment is supposed to be undertaken taking into consideration of the harm associated with use of GM crops both to human and environment.
Nevertheless, such studies prove to be expensive in terms of cost and should be continuous. There seems no commercial organization promoting use of GM crops or governmental body willing to undertake such studies to provide public with evidence-based research.
Much of what we are currently hearing is commentaries and opinions which do not have any single data to support claims that it is safe to use genetically modified food. We can not get blinded that increasing food production to feed ever growing population is a sound reason to embrace genetically modified foods.
Neither should we need to go for it because of reduced farming cost in saving insecticides and herbicides for crop production. There may be potentially high risks that are associated with use of genetic modified food that are not uncovered which may prove so costly and endangering life in this planet earth that managing malnutrition and hunger caused by poor policies which can be effectively addressed.
Clark, E. Ann & Lehman, Hugh. (2001). Assessment of GM crops in commercial agriculture. Journal of Agriculture and Environmental Ethics , 14, pp. 7-27
FAO/WHO (2000). Safety aspects of genetically modified foods of plants origin.
Report of a joint FAO/WHO Expert consultation on food derived from biotechnology, World Health Organization, Geneva, p. 26
Gaskell George, Bauer,W. Martin, Durant,J ohn & Allum, C. Nicholas. (2000) World apart? The reception of genetically modified foods in Europe and the US. Science Journal. Vol. 285, p. 1
Gura, Tylor. Reaping the plant gene harvest. Science journal, 289, p. 413
Hurley, M. Terrance, Babcock, A. Bruce & Hellmich, L. Richard. (2000). Bt corn and insect resistance: an economic assessment of refugees. Journal of Agricultural and Resources Economic. Vol, 26, p. 2
Rockfellor Foundation. (1999). Rice biotechnology: Rockfeller to end network after 15 years of success. Science Journal, Volume, 286, p. 1470
- Business Ethics-Labeling Genetically Modified Food
- Should All Genetically Modified Foods Be Labeled?
- Genetically Modified Corn in the United States of America
- Genetically Modified Foods Projects
- Analyzing the Prospects of Genetically Modified Foods
- American Food Over the Decades
- Are Americans Killing Themselves
- Should We Begin to Think About What We Eat?
- Food Culture and Obesity
- Influence of Television Advertising on Children’s Eating Behavior
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2018, November 6). Genetically Modified Foods and Environment. https://ivypanda.com/essays/genetically-modified-foods-and-environment/
"Genetically Modified Foods and Environment." IvyPanda , 6 Nov. 2018, ivypanda.com/essays/genetically-modified-foods-and-environment/.
IvyPanda . (2018) 'Genetically Modified Foods and Environment'. 6 November.
IvyPanda . 2018. "Genetically Modified Foods and Environment." November 6, 2018. https://ivypanda.com/essays/genetically-modified-foods-and-environment/.
1. IvyPanda . "Genetically Modified Foods and Environment." November 6, 2018. https://ivypanda.com/essays/genetically-modified-foods-and-environment/.
Bibliography
IvyPanda . "Genetically Modified Foods and Environment." November 6, 2018. https://ivypanda.com/essays/genetically-modified-foods-and-environment/.

Behold the future of the apple: If the gene inserted into this apple plantlet makes it resistant to the fire blight bacterium, it could help save apple growers tens of millions of dollars a year. Researchers are also working on an apple that could vaccinate children against a virus that is the leading cause of pneumonia.
Food: How Altered?
Here's what you need to know about the warming planet, how it's affecting us, and what's at stake.
Scientists continue to find new ways to insert genes for specific traits into plant and animal DNA. A field of promise—and a subject of debate—genetic engineering is changing the food we eat and the world we live in.
In the brave new world of genetic engineering, Dean DellaPenna envisions this cornucopia: tomatoes and broccoli bursting with cancer-fighting chemicals and vitamin-enhanced crops of rice, sweet potatoes, and cassava to help nourish the poor. He sees wheat, soy, and peanuts free of allergens; bananas that deliver vaccines; and vegetable oils so loaded with therapeutic ingredients that doctors "prescribe" them for patients at risk for cancer and heart disease. A plant biochemist at Michigan State University, DellaPenna believes that genetically engineered foods are the key to the next wave of advances in agriculture and health.
While DellaPenna and many others see great potential in the products of this new biotechnology, some see uncertainty, even danger. Critics fear that genetically engineered products are being rushed to market before their effects are fully understood. Anxiety has been fueled by reports of taco shells contaminated with genetically engineered corn not approved for human consumption; the potential spread of noxious "superweeds" spawned by genes picked up from engineered crops; and possible harmful effects of biotech corn pollen on monarch butterflies.
In North America and Europe the value and impact of genetically engineered food crops have become subjects of intense debate, provoking reactions from unbridled optimism to fervent political opposition.
Just what are genetically engineered foods, and who is eating them? What do we know about their benefits—and their risks? What effect might engineered plants have on the environment and on agricultural practices around the world? Can they help feed and preserve the health of the Earth's burgeoning population?
Q: Who's eating biotech foods? A: In all likelihood, you are.
Most people in the United States don't realize that they've been eating genetically engineered foods since the mid-1990s. More than 60 percent of all processed foods on U.S. supermarket shelves—including pizza, chips, cookies, ice cream, salad dressing, corn syrup, and baking powder—contain ingredients from engineered soybeans, corn, or canola.
In the past decade or so, the biotech plants that go into these processed foods have leaped from hothouse oddities to crops planted on a massive scale—on 130 million acres (52.6 million hectares) in 13 countries, among them Argentina, Canada, China, South Africa, Australia, Germany, and Spain. On U.S. farmland, acreage planted with genetically engineered crops jumped nearly 25-fold from 3.6 million acres (1.5 million hectares) in 1996 to 88.2 million acres (35.7 million hectares) in 2001. More than 50 different "designer" crops have passed through a federal review process, and about a hundred more are undergoing field trials.
Q: How long have we been genetically altering our food? A: Longer than you think.
Genetic modification is not novel. Humans have been altering the genetic makeup of plants for millennia, keeping seeds from the best crops and planting them in following years, breeding and crossbreeding varieties to make them taste sweeter, grow bigger, last longer. In this way we've transformed the wild tomato, Lycopersicon , from a fruit the size of a marble to today's giant, juicy beefsteaks. From a weedy plant called teosinte with an "ear" barely an inch long has come our foot-long (0.3-meter-long) ears of sweet white and yellow corn. In just the past few decades plant breeders have used traditional techniques to produce varieties of wheat and rice plants with higher grain yields. They have also created hundreds of new crop variants using irradiation and mutagenic chemicals.
But the technique of genetic engineering is new, and quite different from conventional breeding. Traditional breeders cross related organisms whose genetic makeups are similar. In so doing, they transfer tens of thousands of genes. By contrast, today's genetic engineers can transfer just a few genes at a time between species that are distantly related or not related at all.
Genetic engineers can pull a desired gene from virtually any living organism and insert it into virtually any other organism. They can put a rat gene into lettuce to make a plant that produces vitamin C or splice genes from the cecropia moth into apple plants, offering protection from fire blight, a bacterial disease that damages apples and pears. The purpose is the same: to insert a gene or genes from a donor organism carrying a desired trait into an organism that does not have the trait.
The engineered organisms scientists produce by transferring genes between species are called transgenic. Several dozen transgenic food crops are currently on the market, among them varieties of corn, squash, canola, soybeans, and cotton, from which cottonseed oil is produced. Most of these crops are engineered to help farmers deal with age-old agriculture problems: weeds, insects, and disease.
Farmers spray herbicides to kill weeds. Biotech crops can carry special "tolerance" genes that help them withstand the spraying of chemicals that kill nearly every other kind of plant. Some biotech varieties make their own insecticide, thanks to a gene borrowed from a common soil bacterium, Bacillus thuringiensis , or Bt for short.
Bt genes code for toxins considered to be harmless to humans but lethal to certain insects, including the European corn borer, an insect that tunnels into cornstalks and ears, making it a bane of corn farmers. So effective is Bt that organic farmers have used it as a natural insecticide for decades, albeit sparingly. Corn borer caterpillars bite into the leaves, stems, or kernels of a Bt corn plant, the toxin attacks their digestive tracts, and they die within a few days.
Other food plants—squash and papaya, for instance—have been genetically engineered to resist diseases. Lately scientists have been experimenting with potatoes, modifying them with genes of bees and moths to protect the crops from potato blight fungus, and grapevines with silkworm genes to make the vines resistant to Pierce's disease, spread by insects.
With the new tools of genetic engineering, scientists have also created transgenic animals. Atlantic salmon grow more slowly during the winter, but engineered salmon, "souped-up" with modified growth-hormone genes from other fish, reach market size in about half the normal time. Scientists are also using biotechnology to insert genes into cows and sheep so that the animals will produce pharmaceuticals in their milk. None of these transgenic animals have yet entered the market.
Q: Are biotech foods safe for humans? A: Yes, as far as we know.
"Risks exist everywhere in our food supply," points out Dean DellaPenna. "About a hundred people die each year from peanut allergies. With genetically engineered foods we minimize risks by doing rigorous testing."
According to Eric Sachs, a spokesperson for Monsanto, a leading developer of biotech products: "Transgenic products go through more testing than any of the other foods we eat. We screen for potential toxins and allergens. We monitor the levels of nutrients, proteins, and other components to see that the transgenic plants are substantially equivalent to traditional plants."
Three federal agencies regulate genetically engineered crops and foods—the U.S. Department of Agriculture (USDA), the Environmental Protection Agency (EPA), and the Food and Drug Administration (FDA). The FDA reviews data on allergens, toxicity, and nutrient levels voluntarily submitted by companies. If that information shows that the new foods are not substantially equivalent to conventional ones, the foods must undergo further testing. Last year the agency proposed tightening its scrutiny of engineered foods, making the safety assessments mandatory rather than voluntary.
"When it comes to addressing concerns about health issues, industry is being held to very high standards" says DellaPenna, "and it's doing its best to meet them in reasonable and rigorous fashion."
In the mid-1990s a biotech company launched a project to insert a gene from the Brazil nut into a soybean. The Brazil nut gene selected makes a protein rich in one essential amino acid. The aim was to create a more nutritious soybean for use in animal feed. Because the Brazil nut is known to contain an allergen, the company also tested the product for human reaction, with the thought that the transgenic soybean might accidentally enter the human food supply. When tests showed that humans would react to the modified soybeans, the project was abandoned.
For some people this was good evidence that the system of testing genetically engineered foods works. But for some scientists and consumer groups, it raised the specter of allergens or other hazards that might slip through the safety net. Scientists know that some proteins, such as the one in the Brazil nut, can cause allergic reactions in humans, and they know how to test for these allergenic proteins. But the possibility exists that a novel protein with allergenic properties might turn up in an engineered food—just as it might in a new food produced by conventional means—and go undetected. Furthermore, critics say, the technique of moving genes across dramatically different species increases the likelihood of something going awry—either in the function of the inserted gene or in the function of the host DNA—raising the possibility of unanticipated health effects.
An allergy scare in 2000 centered around StarLink, a variety of genetically engineered corn approved by the U.S. government only for animal use because it showed some suspicious qualities, among them a tendency to break down slowly during digestion, a known characteristic of allergens. When StarLink found its way into taco shells, corn chips, and other foods, massive and costly recalls were launched to try to remove the corn from the food supply.
No cases of allergic response have been pinned to StarLink. In fact, according to Steve L. Taylor, chair of the Department of Food Science and Technology at the University of Nebraska, "None of the current biotech products have been implicated in allergic reactions or any other healthcare problem in people." Nevertheless, all new foods may present new risks. Only rigorous testing can minimize those risks.
You May Also Like

Which leafy greens are healthiest—and which might make you sick?

This 'Gate to Hell' has burned for decades. Will we ever shut it?

In 1969, the U.S. turned off Niagara Falls. Here’s what happened next.
Often overlooked in the debate about the health effects of these foods is one possible health benefit: Under some conditions corn genetically engineered for insect resistance may enhance safety for human and animal consumption. Corn damaged by insects often contains high levels of fumonisins, toxins made by fungi that are carried on the backs of insects and that grow in the wounds of the damaged corn. Lab tests have linked fumonisins with cancer in animals, and they may be potentially cancer-causing to humans. Among people who consume a lot of corn—in certain parts of South Africa, China, and Italy, for instance—there are high rates of esophageal cancer, which scientists associate with fumonisins. Studies show that most Bt corn has lower levels of fumonisins than conventional corn damaged by insects.
Should genetically engineered foods be labeled? Surveys suggest that most Americans would say yes (although they wouldn't want to pay more for the labeling). Professor Marion Nestle, chair of the Department of Nutrition and Food Studies at New York University, favors labeling because she believes consumers want to know and have the right to choose. However, no engineered foods currently carry labels in the U.S. because the FDA has not found any of them to be substantially different from their conventional counterparts. Industry representatives argue that labeling engineered foods that are not substantially different would arouse unwarranted suspicion.
Q: Can biotech foods harm the environment? A: It depends on whom you ask.
Most scientists agree: The main safety issues of genetically engineered crops involve not people but the environment. "We've let the cat out of the bag before we have real data, and there's no calling it back," says Allison Snow, a plant ecologist at Ohio State University.
Snow is known for her research on "gene flow," the movement of genes via pollen and seeds from one population of plants to another, and she and some other environmental scientists worry that genetically engineered crops are being developed too quickly and released on millions of acres of farmland before they've been adequately tested for their possible long-term ecological impact.
Advocates of genetically engineered crops argue that the plants offer an environmentally friendly alternative to pesticides, which tend to pollute surface and groundwater and harm wildlife. The use of Bt varieties has dramatically reduced the amount of pesticide applied to cotton crops. But the effects of genetic engineering on pesticide use with more widely grown crops are less clear-cut.
What might be the effect of these engineered plants on so-called nontarget organisms, the creatures that visit them? Concerns that crops with built-in insecticides might damage wildlife were inflamed in 1999 by the report of a study suggesting that Bt corn pollen harmed monarch butterfly caterpillars.
Monarch caterpillars don't feed on corn pollen, but they do feed on the leaves of milkweed plants, which often grow in and around cornfields. Entomologists at Cornell University showed that in the laboratory Bt corn pollen dusted onto milkweed leaves stunted or killed some of the monarch caterpillars that ate the leaves. For some environmental activists this was confirmation that genetically engineered crops were dangerous to wildlife. But follow-up studies in the field, reported last fall, indicate that pollen densities from Bt corn rarely reach damaging levels on milkweed, even when monarchs are feeding on plants within a cornfield.
"The chances of a caterpillar finding Bt pollen doses as high as those in the Cornell study are negligible," says Rick Hellmich, an entomologist with the Agricultural Research Service and one author of the follow-up report. "Butterflies are safer in a Bt cornfield than they are in a conventional cornfield, when they're subjected to chemical pesticides that kill not just caterpillars but most insects in the field."
Perhaps a bigger concern has to do with insect evolution. Crops that continuously make Bt may hasten the evolution of insects impervious to the pesticide. Such a breed of insect, by becoming resistant to Bt, would rob many farmers of one of their safest, most environmentally friendly tools for fighting the pests.
To delay the evolution of resistant insects, U.S. government regulators, working with biotech companies, have devised special measures for farmers who grow Bt crops. Farmers must plant a moat or "refuge" of conventional crops near their engineered crops. The idea is to prevent two resistant bugs from mating. The few insects that emerge from Bt fields resistant to the insecticide would mate with their nonresistant neighbors living on conventional crops nearby; the result could be offspring susceptible to Bt. The theory is that if growers follow requirements, it will take longer for insects to develop resistance.
It was difficult initially to convince farmers who had struggled to keep European corn borers off their crops to let the insects live and eat part of their acreage to combat resistance. But a 2001 survey by major agricultural biotech companies found that almost 90 percent of U.S. farmers complied with the requirements.
Many ecologists believe that the most damaging environmental impact of biotech crops may be gene flow. Could transgenes that confer resistance to insects, disease, or harsh growing conditions give weeds a competitive advantage, allowing them to grow rampantly?
"Genes flow from crops to weeds all the time when pollen is transported by wind, bees, and other pollinators," says Allison Snow. "There's no doubt that transgenes will jump from engineered crops into nearby relatives." But since gene flow usually takes place only between closely related species, and since most major U.S. crops don't have close relatives growing nearby, it's extremely unlikely that gene flow will occur to create problem weeds.
Still, Snow says, "even a very low probability event could occur when you're talking about thousands of acres planted with food crops." And in developing countries, where staple crops are more frequently planted near wild relatives, the risk of transgenes escaping is higher. While no known superweeds have yet emerged, Snow thinks it may just be a matter of time.
Given the risks, many ecologists believe that industry should step up the extent and rigor of its testing and governments should strengthen their regulatory regimes to more fully address environmental effects. "Every transgenic organism brings with it a different set of potential risks and benefits," says Snow. "Each needs to be evaluated on a case-by-case basis. But right now only one percent of USDA biotech research money goes to risk assessment."
Q: Can biotech foods help feed the world? A: There are obstacles to overcome.
"Eight hundred million people on this planet are malnourished," says Channapatna Prakash, a native of India and an agricultural scientist at the Center for Plant Biotechnology Research at Tuskegee University, "and the number continues to grow."
Genetic engineering can help address the urgent problems of food shortage and hunger, say Prakash and many other scientists. It can increase crop yields, offer crop varieties that resist pests and disease, and provide ways to grow crops on land that would otherwise not support farming because of drought conditions, depleted soils, or soils plagued by excess salt or high levels of aluminum and iron. "This technology is extremely versatile," Prakash explains, "and it's easy for farmers to use because it's built into the seed. The farmers just plant the seeds, and the seeds bring new features in the plants."
Some critics of genetic engineering argue that the solution to hunger and malnutrition lies in redistributing existing food supplies. Others believe that the ownership by big multinational companies of key biotechnology methods and genetic information is crippling public-sector efforts to use this technology to address the needs of subsistence farmers. The large companies that dominate the industry, critics also note, are not devoting significant resources to developing seed technology for subsistence farmers because the investment offers minimal returns. And by patenting key methods and materials, these companies are stifling the free exchange of seeds and techniques vital to public agricultural research programs, which are already under severe financial constraints. All of this bodes ill, say critics, for farmers in the developing world.
Prakash agrees that there's enough food in the world. "But redistribution is just not going to happen," he says. "The protest against biotech on political grounds is a straw man for a larger frustration with globalization, a fear of the power of large multinational corporations. People say that this technology is just earning profit for big companies. This is true to some extent, but the knowledge that companies have developed in the production of profitable crops can easily be transferred and applied to help developing nations."
"Biotechnology is no panacea for world hunger," says Prakash, "but it's a vital tool in a toolbox, one that includes soil and water conservation, pest management, and other methods of sustainable agriculture, as well as new technologies."
The debate over the use of biotechnology in developing countries recently went from simmer to boil about rice, which is eaten by three billion people and grown on hundreds of millions of small farms.
"White rice," explains Dean DellaPenna, "is low in protein. It has very little iron, and virtually no vitamin A."However, in 1999 a team of scientists led by Ingo Potrykus, of the Swiss Federal Institute of Technology, and Peter Beyer, of the University of Freiburg, Germany, announced a new breakthrough: They had introduced into rice plants two daffodil genes and one bacterial gene that enable the rice to produce in its grains beta-carotene, a building block of vitamin A. According to the World Health Organization, between 100 million and 140 million children in the world suffer from vitamin A deficiency, some 500,000 go blind every year because of that deficiency, and half of those children die within a year of losing their sight. "Golden rice," so named for the yellow color furnished by the beta-carotene, was hailed by some as a potential solution to the suffering and illness caused by vitamin A deficiency.
Skeptics consider golden rice little more than a public relations ploy by the biotechnology industry, which they say exaggerated its benefits. "Golden rice alone won't greatly diminish vitamin A deficiency," says Marion Nestle. "Beta-carotene, which is already widely available in fruit and vegetables, isn't converted to vitamin A when people are malnourished. Golden rice does not contain much beta-carotene, and whether it will improve vitamin A levels remains to be seen."
Potrykus and Beyer are now developing new versions of the rice that may be more effective in delivering beta-carotene for the body to convert to vitamin A. Their plan is to put the improved rices free of charge into the hands of poor farmers. According to Beyer, golden rice is still at least four years away from distribution. It could take much longer if opposing groups delay plans for field trials and safety studies.
Q: What next? A: Proceed with caution.
Whether biotech foods will deliver on their promise of eliminating world hunger and bettering the lives of all remains to be seen. Their potential is enormous, yet they carry risks—and we may pay for accidents or errors in judgment in ways we cannot yet imagine. But the biggest mistake of all would be to blindly reject or endorse this new technology. If we analyze carefully how, where, and why we introduce genetically altered products, and if we test them thoroughly and judge them wisely, we can weigh their risks against their benefits to those who need them most.
Related Topics
- ENVIRONMENT AND CONSERVATION
- AGRICULTURE
- ENGINEERING

How to take larger-than-life photos of the world’s tiny creatures

How do fireflies get their glow? We finally have some answers.

When does old age begin? Science says later than you might think


Death cap mushrooms are extremely deadly—and they’re spreading

Is Ehlers-Danlos Syndrome really so rare—or is it misdiagnosed?
- Interactive Graphic
- Environment
- Paid Content
History & Culture
- History & Culture
- History Magazine
- The Big Idea
- Mind, Body, Wonder
- Terms of Use
- Privacy Policy
- Your US State Privacy Rights
- Children's Online Privacy Policy
- Interest-Based Ads
- About Nielsen Measurement
- Do Not Sell or Share My Personal Information
- Nat Geo Home
- Attend a Live Event
- Book a Trip
- Inspire Your Kids
- Shop Nat Geo
- Visit the D.C. Museum
- Learn About Our Impact
- Support Our Mission
- Advertise With Us
- Customer Service
- Renew Subscription
- Manage Your Subscription
- Work at Nat Geo
- Sign Up for Our Newsletters
- Contribute to Protect the Planet
Copyright © 1996-2015 National Geographic Society Copyright © 2015-2024 National Geographic Partners, LLC. All rights reserved
Pardon Our Interruption
As you were browsing something about your browser made us think you were a bot. There are a few reasons this might happen:
- You've disabled JavaScript in your web browser.
- You're a power user moving through this website with super-human speed.
- You've disabled cookies in your web browser.
- A third-party browser plugin, such as Ghostery or NoScript, is preventing JavaScript from running. Additional information is available in this support article .
To regain access, please make sure that cookies and JavaScript are enabled before reloading the page.

IELTS Essay: Genetically Modified Foods
by Dave | EBooks | 18 Comments
This is an IELTS writing task 2 sample answer essay on the topic of genetically modified foods from the real IELTS exam.
It is only available as a full Ebook on my Patreon.com/howtodoielts .
One of the most important issues facing the world today is a shortage of food and some think genetically modified foods are a possible solution.
To what extent do you agree or disagree?
You can sign up and get access for as little as $1 a month!
Contributing helps me to publish these essays for students every week, so please consider signing up for my Patreon !

Recommended For You

Latest IELTS Writing Task 1 2024 (Graphs, Charts, Maps, Processes)
by Dave | Sample Answers | 147 Comments
These are the most recent/latest IELTS Writing Task 1 Task topics and questions starting in 2019, 2020, 2021, 2022, 2023, and continuing into 2024. ...

Recent IELTS Writing Topics and Questions 2024
by Dave | Sample Answers | 342 Comments
Read here all the newest IELTS questions and topics from 2024 and previous years with sample answers/essays. Be sure to check out my ...

Find my Newest IELTS Post Here – Updated Daily!
by Dave | IELTS FAQ | 18 Comments

IELTS Essay: Fast Food
by Dave | Real Past Tests | 5 Comments
This is an IELTS writing task 2 sample answer essay on the topic of fast food becoming cheaper and more available and the advantages/disadvantages. ...

IELTS Essay: Activities for Leisure Time
by Dave | Real Past Tests | 3 Comments
This is an IELTS writing task 2 sample answer essay from the general training exam on the topic of planning your activities for your leisure time. ...

IELTS Essay: Choosing a Job
by Dave | General Training | 0 Comment
This is an IELTS writing task 2 sample answer from the general training exam on the topic of choosing a job. Jobs come ...
Submit a Comment Cancel reply
You must be logged in to post a comment.
18 Comments
Many people are starving all over the world due to food scarcity, and some are suggesting that one way of combatting this global phenomenon is through modifying the genetics of food. While scientifically enhancing food elements might be an effective way to expand food resources, I disagree that it would do much in terms of curbing worldwide hunger because this scientific innovation will most likely only benefit countries with financial capabilities and ultimately still leave the poorest of the poor lacking food.
Modifying the DNA structure of food offers endless possibilities to humans. Unbeknownst to some people, this innovation is not new and has been used for a long time to grow fruits without seeds as an example. If we move forward with this innovation, we can increase the number of plants we grow, or decrease the harvesting time needed for fruits to ripen. As a result, there would be more food for people to consume.
However, despite its potential, the cost is one reason why this practice is not implemented worldwide. Genetically modifying food is an expensive process so countries that are already struggling financially to feed their citizens, will not be able to afford this kind of technology. Poverty-stricken societies, mostly living in third-world countries with little to no access to sophisticated innovations, will remain hungry, thereby, not solving this global dilemma. A similar example is vertical farming. This agricultural innovation allows fruits and vegetables to be grown in tall buildings, but despite its tangible benefits to the environment, most countries fail to adopt this technology due to budget. The same can be said in terms of food genetics.
In conclusion, although the modification of food genetics can increase food resources, this does not mean that the benefit will extend to poor countries, and the poor with no budget to keep up with this technology will continue to starve. Therefore, governments all over the world should get involved and look into a more practical way of dealing with famine.
By the way, for those who are correcting their own essays, one way to check grammar is via Grammarly. I downloaded it as a chrome extension, and I would paste my work on gmail so it detects my error. BUT of course, do this only after you’re done writing an essay, otherwise it’s cheating 🙂
well done mate.
Agreed! Try to balance your body paragraphs a bit better, Chris!
World population and dependent rate is increasing daily so, the biggest challenge is to produce adequate foods. Some people think that genetically modify foods is a good option. I agree with this because, genetically improved foods can produce more harvest, adhere to different weather condition and also produce it in short duration. In the past, usually people grow two or three seasons in every year. So that was enough for their consumption throughout the year. Due to higher population and different food choices, it has more demand for foods nowadays. As a result, many countries enhanced their testing and invest more money for genetic improvements. It has been shown great success so far and, we can see those foods are now in the supermarkets. If I take my country as an example, Sri Lankan has been working two seasons each year those days. But nowadays they produce some foods all over the year because of the genetic improvements. Genetically modified foods can adhere to the different weather conditions and produce more harvest than traditional foods. Some people discuss the bad effects of the heretical improvements. Even though we all know it has bad effects, we have no choice to reject it. Still we have the choice to purchase organic foods. But those are more expensive, and everyone cannot afford those in daily basis. If I take chicken meat as an example, it has higher demand, and we cannot fulfill the demand without changing DNA. In These days chicken meat can produce within 45 days. Finally, even though it has some bad effects genetically modified food is a good option unless we find another good way to feed hungry people.
Love your examples – keep doing that.
Lots of little mistakes with grammar and vocabulary thought, so keep working on those!
Food scarcity is one of the greatest challenges of the 21st century. Some people advocate that genetically modified foods can be a viable solution to this problem. From my perspective, this might pose some risk, however, I am largely in agreement with this suggestion. To begin with, why I believe that modified organisms might be the key component of this issue, modified organisms are basically made with altering DNA sequences by genetic engineers, therefore this approach offers endless possibilities to humans. To illustrate this, by changing the DNA structure of food, an upward trend can be maintained in the nutrition levels which food contains, as a result, people who have access to an only limited amount of food might not be suffered from malnutrition a problem which even leads to death. What is more, these changes in genetic material might improve the yield of food, thus with less labour, more people can obtain these valuable nourishments. Moving onto the reason why I claim this might not be an effective solution to hunger, even today most people are not dying from the shortage of food, they are dying on account of the inequity in access to food. Even genetic engineering solved this lack of food problem, this solution will be only available to people who can afford it, hence poverty-stricken people will keep dying from undernourishment.
All in all, what can be concluded from the aforementioned remarks that although genetic engineering might solve this lack of food problem, there will be people who cannot afford it.
Nice essay, Nilüfer!
Really accurate and nice ideas.
The 3rd paragraph could be longer and could use a more specific example. Try rewriting that one.
Over the decades, the advancement of technology increased giving rise for many innovations such as foods which are genetically engineered. Nowadays one of the most important problem faced by the world is food shortage. Few people think that a good solution for this would be genetically modified foods. In my opinion, I believe with the increase of food shortage, the new type of agriculture will be much beneficial.
All around the world, especially many countries in Africa is going though food shortage, where people are starving of hunger each day with no way to getting access to healthy foods. With the introduction of genetically engineered food, lives of these peoples will change massively. Modified food types are been altered to contain all the nutrients such as vitamins and minerals which the body is required in-order to stay healthy making the population healthier than ever before.
With regards to governments, it would be easier to cultivate these types of foods due to reasons for which to grow they require controlled environments which may also result in less labour. The prices may drop with less labour going into production and less resources needed than normal agriculture. Furthermore, extensive number of researches and tests have been conducted with some still going on, the data of these have proven that the newly innovated agriculture food sources show no side effects and risks to the human body.
In conclusion, with the help of genetically engineered foods the shortage of foods around world may drop and populations will be healthier than ever before and governments should promote and fund these types of new innovations.
please can anyone give an estimated band score for this essay and would highly appreciate if tips were given to improve.❤️
Excellent work, Upendra!
While one of the major challenges faced in the 21st century is that of food availability, some believe that genetically engineered crops are a potential solution for the same. I completely back this idea as hybrid crops can be grown in odd weather and are resistant to some diseases.
The primary reason why hybrid plants contributes to food security is that they can be grown in unfavorable environments. This is to say that crops are seasonal and its yield depends on weather conditions, thus, some crops can be grown under controlled climatic conditions such as in poly houses. As a result, not only the yield of the crops is enhanced but also food is available throughout the year. To cite an example, Dubai has reduced its food dependency on imports by 30 percent as they are able to cultivate crops in a desert using hybrid crops grown in a maintained environment. Thus, countries are becoming self-sufficient in the terms of food security and are reducing malnutrition problems in their nations.
Another reason is that genetically modified crops are resistant to some natural diseases such as fungal attacks and their regeneration time is reduced. As the new plants can withstand natural attacks and can reproduce in minimal amount of time, farmers prefer sowing of hybrid plants. Subsequently, the requirement of pesticide use has taken a backseat while the income of farmers has increased, thereby, hybrid plantation is encouraged by farmers. Therefore, getting a sight of winter vegetables in summers is not a big surprise nowadays like carrots in tropical countries like India. For instance, a report published in the editorial section of the Washington post by editor-in-chief Martin Baron in 2015 revealed that genetically enhanced crops has aided the USA to achieve its targets for food safety and security by 50 percent.
To encapsulate, it can be concluded that as the demand of food is rising across the globe, use of hybrid crops is a good solution. I believe they are harvested in all the seasons and their yield is increased over the years since they are prone to natural diseases. Hence, all the nations must encourage farmers to adopt new crops for cultivation in order to resolve food crisis in their country.
Hi, Can someone check whether the content is appropriate or not. also are the examples relevant to the arguments?
Thanks in advance.
One of the most important issues facing the world today is a shortage of food and some think genetically modified foods are a possible solution. To what extent do you agree or disagree?
A highly controversial issue today in the air is apropos with paucity of foods. Some people assert that genetically engineered checkbox is a valid way to cover it as well as has sparkling debate on other side too. This essay looks on the both side. However, I am in side with those decrying propagation of an advancement.
Many people in argue with agricultural development with alternation of old food considerably outweigh its merits.There are numerous countries are suffering from poverty and starving of grains such as Africa and NZ. Reproductive method can bring active change and prosperity. This factor can reduce the labour costs which alternatively government can use for production and cultivation process. Due to climate change and environmental issues farmers are unable to feed their pockets ; This remarkable solution leads in their favour too. Every people today are dimly aware of dangerous future with trade depression and dearness where refashioning process can become beneficial for government and community as well.
On contrary point , in the such cases if the results won’t meet with the expectations then situations become more cruel. It is also possible to say that modified process can loose such vitamins and minerals from the original food which is unhealthy. Going with an advancement of moderate lifestyle it raising the scope of side effects and even leads to death also.
To sum up , Both side have major influences on their advantages and disadvantages. It should be countered by concerned with government and citizen’s support possible. Although this thought is an unlikely to-be entirely eliminated in a short term period with better development of technology. In my opinion It is a valuable suggestion of modify the genetic foods.
Good work, Hemangi!
In recent decades, the lack of foods is becoming a massive problem worldwide. Using genetically modified foods (GMO) can be treated as one of the best methods to tackle this issue.
The primary reason for using GMO foods is that they provide an alternative option for farmers to produce at a lower cost. In fact, the production of GMO foods should require less freshwater, fewer pesticides compare to conventional crops. As a result, the farmers may produce the same number of foods at much less price. Furthermore, the price of modified foods also can be attracted to buyers as it can provide cheaper options from them. It can be seen in many developing countries such as Ethiopia or Madagascar, where locals can purchase GMO foods at a reasonable price as the price is 15% less than traditional foods.
Another reason is that genetically modified foods can provide better nutrients to consumers. Due to the research from New York times, thanks to the combination among various kind of foods, it has created the new types of African crops have much more vitamins than traditional crops. It has brought a huge opportunity to farmers in regions where people suffer from nutritional deficiencies. Chad Republic, for example, is one of the poorest countries in Africa, where it does not have much soil with enough nutrients for cultivation. Based on new types of GMO foods, Chad’s national economy has grown massively for the last decade.
In conclusion, the growth of GMO foods is provided the new opportunity for farmers in business world. At the same time, its foods can also bring a higher quality of nutrients to customers
Nice Jimmy and sorry for the late reply – hope your studies are going well!
Overpopulation is one of the most challenging issues among others like globalization, climate change, and deforestation. Around 40 years ago the experts implemented a new project to solve the problem, they initiated to grow GM foods. In the last couple of years, GMO veggies and fruits were spread all over the planet. Many people speak about the negative and positive sides of GM foods, but no one knows what is best for human health. The primary reason why GM food was created was hunger in undeveloped countries. A couple of decades have passed the problem still exists. In fact, the poor countries are getting poorer and developed countries are refusing consumption of such kinds of food. For instance, in Europe, it is forbidden to plant GM vegetables and fruits due to protect the ecosystem. Furthermore, bumblebees and other insects do not eat GM food eventually, do not pollinate the environment. This became a serious question for scientists putting the entire continent in danger. In addition, GM foods are new to human DNA, meaning we do not know how they affect our bodies in long term. 30 years of observation seems to be not enough to answer the solid questions. Food is fuel and people are extremely careful what they put inside, even though GM foods cost noticeably affordable some people still prefer to buy organic food. In conclusion, nowadays GM foods are presented in many countries as alternatives. However not every country is considering GM foods healthy nutrition. In addition, hundreds of doctors believe that GM food was not researched enough. Only time can tell what are consequences. I personally believe that GM foods are not the best solution, it is significant that businesses and government work together and carry on studying these types of foods.
Nice ideas but work on the balance of your paragraphs, Sally.
The conclusion is way too long!
Some people claim that genetically modified foods are the viable solution to curbing the hunger issue throughout the world. Although I agree with this viewpoint, I believe some measures in changing people’s perspective toward these foods should be done in advance.
There is an assumption that scientifically enhanced foods will reduce the problem of food scarcity since one commonly cited advantage of these foods is their mass production as well as their longer shelf life that grossly contributes to alleviating hunger worldwide, especially in poorer countries. In other words, these crops are more abundant, cheaper, and last longer than conventional agricultural products, so they do not decay before reaching the consumer. Moreover, they are more resistant to harmful insects, parasites, and fungi, making them more appealing in terms of mentioned issues and particularly for nations with outdated farming methods.
Nevertheless, there are a number of significant negative points to these nascent types of food, the first of which is their unknown potential dire repercussions to our health. Some studies have declared that these foods will eventually affect our organisms adversely since they have not been used for so long, so their consequences will be flagged up in upcoming decades. Furthermore, not inclined are most people to eat these foods because they prefer those that have not been tampered with in laboratories. Thus, they do not currently help reduce famine throughout the globe, which is a drawback. Should they assist in decreasing starvation rates, the public has to be psychologically persuaded to consume them beforehand by informing them about their benefits.
In conclusion, I support modifying the genetics of food as one of the feasible helpful steps that can be taken to combat the global concern of food shortage, however, more research as well as notifying about their positive side to the society is needed.
Good work, Zeinab!
Some really good adjectives and collocations in your writing that other students can learn a lot from!
Careful of some informal ones like ‘flagged up’ – keep working hard!
Exclusive Ebooks, PDFs and more from me!
Sign up for patreon.
Don't miss out!
"The highest quality materials anywhere on the internet! Dave improved my writing and vocabulary so much. Really affordable options you don't want to miss out on!"
Minh, Vietnam
Hi, I’m Dave! Welcome to my IELTS exclusive resources! Before you commit I want to explain very clearly why there’s no one better to help you learn about IELTS and improve your English at the same time... Read more
Patreon Exclusive Ebooks Available Now!
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 13 August 2024
Reducing climate change impacts from the global food system through diet shifts
- Yanxian Li ORCID: orcid.org/0000-0002-1947-7541 1 ,
- Pan He ORCID: orcid.org/0000-0003-1088-6290 2 , 3 ,
- Yuli Shan ORCID: orcid.org/0000-0002-5215-8657 4 ,
- Ye Hang ORCID: orcid.org/0000-0002-1368-905X 4 ,
- Shuai Shao ORCID: orcid.org/0000-0002-9525-6310 6 ,
- Franco Ruzzenenti 1 &
- Klaus Hubacek ORCID: orcid.org/0000-0003-2561-6090 1
Nature Climate Change ( 2024 ) Cite this article
2087 Accesses
142 Altmetric
Metrics details
- Climate-change impacts
- Climate-change mitigation
How much and what we eat and where it is produced can create huge differences in GHG emissions. On the basis of detailed household-expenditure data, we evaluate the unequal distribution of dietary emissions from 140 food products in 139 countries or areas and further model changes in emissions of global diet shifts. Within countries, consumer groups with higher expenditures generally cause more dietary emissions due to higher red meat and dairy intake. Such inequality is more pronounced in low-income countries. The present global annual dietary emissions would fall by 17% with the worldwide adoption of the EAT-Lancet planetary health diet, primarily attributed to shifts from red meat to legumes and nuts as principal protein sources. More than half (56.9%) of the global population, which is presently overconsuming, would save 32.4% of global emissions through diet shifts, offsetting the 15.4% increase in global emissions from presently underconsuming populations moving towards healthier diets.
Similar content being viewed by others
Simple dietary substitutions can reduce carbon footprints and improve dietary quality across diverse segments of the US population

The ongoing nutrition transition thwarts long-term targets for food security, public health and environmental protection

Adoption of the ‘planetary health diet’ has different impacts on countries’ greenhouse gas emissions
Food choices impact both our health and the environment 1 , 2 . The food system is responsible for about one-third of global anthropogenic GHG emissions 3 , 4 and climate goals become unattainable without efforts to reduce food-related emissions 5 , 6 . However, not everyone contributes the same way to food-related emissions because of disparities in lifestyle, food preferences and affordability within and across countries 7 , 8 , 9 . High levels of food consumption (especially animal-based diets), one of the leading causes of obesity and non-communicable diseases 10 , 11 , lead to substantial emissions 9 , 12 . Simultaneously, >800 million people still suffer from hunger and almost 3.1 billion people cannot afford a healthy diet 13 . Ending hunger and malnutrition while feeding the growing population by extending food production will further exacerbate climate change 14 , 15 . Given the notable increase in emissions driven by food consumption despite efficiency gains 16 , changing consumer lifestyles and choices are needed to mitigate climate change 17 .
Research shows that widespread shifts towards healthier diets, aligned with the sustainable development goals (SDGs) of the United Nations 18 , offer solutions to this complex problem by eradicating hunger (SDG 2), ensuring health (SDG 3) and mitigating emissions (SDG 13) 19 , 20 , 21 , 22 . Numerous dietary options have been proposed as guidelines for diet shifts 1 , 23 , 24 . The planetary health diet 12 , proposed by the EAT-Lancet Commission, stands out as a prominent option. It aims to improve health while limiting the impacts of the food system within planetary boundaries by providing reference intake levels for different food categories 9 , 25 . It is flexibly compatible with diversities and preferences of regional and local diets 12 . Previous research has estimated changes in country-specific environmental impacts, including GHG emissions 26 , 27 , 28 and water consumption 25 , resulting from adopting the planetary health diet. However, there is limited evidence on how different population groups will contribute differently in this process 7 .
Food consumption and associated emissions differ as a result of disparities in consumer choices guided by social and cultural preferences, wealth and income 29 . Quantifying food-related emissions along the entire supply chain for different products and population groups provides information for emission mitigation through changing consumer choices 17 . With the improved availability of household consumption data, recent studies have revealed inequality in energy consumption 30 , 31 and carbon emissions 17 , 32 , 33 , 34 . Although there are several studies on income- or expenditure-specific food-related emissions within individual countries based on survey-based data 35 , 36 , 37 , 38 , previous studies have not assessed global food-related emissions with a detailed breakdown into specific products and population groups. Furthermore, reducing the overconsumption of wealthy or otherwise overconsuming groups can increase the availability of resources for reducing hunger and malnutrition 7 . However, it remains unclear how emissions from different population groups would change in response to global diet shifts.
To fill these gaps, this study evaluates GHG emissions (CO 2 , CH 4 and N 2 O) throughout the global food supply chains (including agricultural land use and land-use change, agricultural production and beyond-farm processes) 16 induced by diets, termed ‘dietary emissions’, in 2019 and the potential emission changes of global diet shifts. Food loss and waste during household consumption 25 , 39 , 40 have been subtracted from the national food supply to obtain dietary intake. We quantify dietary emissions of 140 products 16 (classified into 13 food categories 12 ) on the basis of the global consumption-based emissions inventory of detailed food products 16 . By linking detailed food intake amounts to the food consumption patterns of 201 global expenditure groups (grouped according to the per capita total expenditure of each group) from the household-expenditure dataset 41 based on the World Bank Global Consumption Database (WBGCD) 42 , we analyse the unequal distribution of dietary emissions in 139 countries or areas, covering 95% of the global population. Despite limitations, the total expenditure of consumers, which effectively reflects patterns in household income, consumption and asset accumulation, is a useful approximation to represent levels of income and wealth 31 , 43 . Additionally, we build a scenario of shifting from diets in 2019 to the global planetary health diet to estimate emission changes ( Methods ). This study investigates differences in dietary emissions among regions, countries and population groups, identifying areas where efforts are needed to mitigate emissions during the global transition towards a healthier and more planet-friendly diet.
Present dietary emissions across countries
In this study, dietary emissions account for emissions along the entire global food production supply chains, which are allocated to final consumers of diets. We use the term ‘GHG footprints’ to specifically refer to the dietary emissions of an individual over 1 year 17 , 34 . The total dietary emissions and country-average per capita GHG footprints show different distributions across countries in 2019 (Fig. 1a ; for detailed food categories see Supplementary Figs. 1 – 9 ). The present total global dietary emissions reach 11.4 GtCO 2 e (95% confidence interval 8.2–14.7 Gt) (details of uncertainty ranges in Supplementary Tables 1 and 2 ). China (contributing 13.5% of emissions) and India (8.9%), the world’s most populous countries (Supplementary Table 3 ), are the largest contributors to global dietary emissions. Alongside Indonesia, Brazil, the United States, the Democratic Republic of Congo, Pakistan, Russia, Japan and Mexico, the top ten contributors represent 57.3% of global dietary emissions but with very unequal per capita emissions within and between countries. We find the highest country-average per capita footprints in Bolivia, with 6.1 tCO 2 e, followed by Luxembourg, Slovakia, Mongolia, the Netherlands and Namibia, with >5.0 tCO 2 e (Supplementary Discussion 2.1 ). Haiti (0.36 tCO 2 e) and Yemen (0.38 tCO 2 e) have the lowest country-average footprints, followed by Burundi, Ghana and Togo. Insufficient food intake of residents due to limited food affordability 44 , 45 is the root cause of low footprints in these low- and lower-middle-income countries 46 .
a , Total and per capita dietary emissions for 139 countries/areas. b , Regional dietary emissions from different food categories and populations. The bar chart (left primary axis) shows the regional emission amounts and the line chart (right secondary axis) shows the number of regional populations. Columns are ordered by the descending per capita GDP of regions (Supplementary Tables 5 and 6 ). USA, United States; AUS, Australia; WE, Western Europe; CAN, Canada; JPN, Japan; RUS, Russia; ROEA, Rest of East Asia; EE, East Europe; CHN, China; ROO, Rest of Oceania; NENA, Near East and North Africa; BRA, Brazil; ROLAC, Rest of Latin America and the Caribbean; ROSEA, Rest of Southeast Asia; IDN, Indonesia; IND, India; ROSA, Rest of South Asia; and SSA, Sub-Saharan Africa. Details for the division and scope of regions are shown in Supplementary Fig. 10 and Supplementary Tables 7 and 8 . Country classification by income levels is based on the World Bank 46 . Credit: World Countries basemap, Esri ( https://hub.arcgis.com/datasets/esri::world-countries/about ).
Source data
While animal-based (52%) and plant-based (48%) products contribute nearly equally to global dietary emissions 4 , 16 , the latter accounts for 87% of calories in global diets (Supplementary Table 4 ). The three main sources of emissions, namely red meat (beef, lamb and pork) (5% of calories), grains (51%) and dairy products (5%), contribute to 29%, 21% and 19% of global emissions, respectively. The substantial emissions from red meat and dairy products are attributed to their considerably higher emissions per unit of calories compared to other categories (Supplementary Table 4 ).
To highlight emission differences at a regional level, we further group the country-level results into 18 regions according to geographical locations and development levels (Fig. 1b and Supplementary Fig. 10 ). In most regions, animal-based products contribute fewer calories (less than a quarter) (Supplementary Data 21 ) but yield more emissions than plant-based products, especially in Australia (84% from animal-based products), the United States (71%) and the region Rest of East Asia (71%) where residents excessively consume both red meat and dairy products. However, the consumption of plant-based products in Indonesia (83% of total calories), Rest of Southeast Asia (92%) and Sub-Saharan Africa (77%) accounts for the most emissions, at 92%, 73% and 64%, respectively. Southeast Asia including Indonesia has a high-emission proportion from grains (42%) due to the prevalent meals dominated by rice. The typical food basket in Sub-Saharan Africa is broadly made up of grains, tubers, legumes and nuts 25 , 47 , representing over half of the regional emissions.
Unequal distribution of dietary emissions within countries
We find substantial differences in per capita GHG footprints within countries and regions. To clearly present the distribution of footprints within each country and region, individuals are sorted in ascending order of their total expenditure levels and then sequentially allocated to ten expenditure deciles with equal population size (Supplementary Fig. 11 and Fig. 2a ). As expenditures increase, individuals tend to have higher levels of footprints, with the largest increase attributed to red meat and dairy products. Richer populations usually have higher per capita footprints related to animal-based products than the poorer in most regions (Fig. 2b ). However, there are differences in per capita footprints within expenditure deciles. For example, even in high-income countries such as Australia and Japan, the dietary intake of red meat for some people in the poorest deciles falls below the recommended levels (Supplementary Data 15 ). Rest of East Asia is one exception, with the poorest decile having high footprints due to a substantial intake of red meat, as seen in Mongolia where beef and mutton are the most common dish 48 .
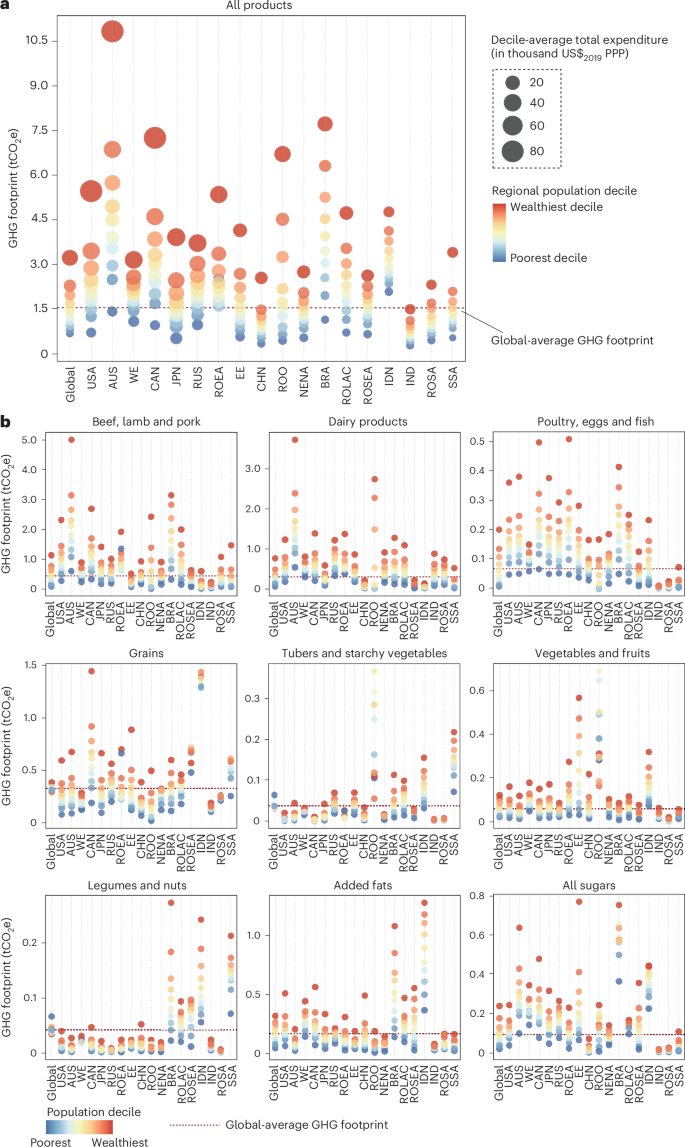
a , GHG footprints from all types of food categories. The size of the bubble refers to the average total expenditure represented by the decile. b , GHG footprints from different food categories. The colours of bubbles in a and b indicate expenditure deciles ranging from the poorest in blue to the wealthiest in red and are comparable only within each region.
Footprints related to plant-based products in specific regions show a different trend from animal-based products as expenditures increase. The middle expenditure groups are responsible for the highest footprints associated with grains in Sub-Saharan Africa and Southeast Asia and the highest footprints of tubers, vegetables and fruits (mainly starchy tropical fruits 49 ) in the Rest of Oceania. These locally produced, high-carbohydrate products are traditional staple foods. In poor countries, agricultural policy primarily targets improving the productivity of staple food, with little investment in the market and facilities for nutrient-rich products 50 , 51 . Consequently, the need for dietary diversity for middle- and low-income people is not adequately addressed 50 , leading to increased consumption of these lower-cost products. However, wealthier consumers can afford more expensive products, such as red meat, reducing their reliance on these staple products.
We use the GHG footprint Gini (GF-Gini) coefficient, calculated on the basis of data from 201 expenditure groups, to measure the dietary emission inequality within a country (Fig. 3 ), with 0 indicating perfect equality and 1 indicating perfect inequality. The inequality of dietary emissions tends to decline with the increase of the per capita GDP of a country, especially for animal-based products. We find the highest inequality of dietary emissions of food products generally in low-income countries, most of which are located in Sub-Saharan Africa. In Sub-Saharan Africa, the highest spending 10% of the population contributes 40% of the regional emissions from red meat, 39% from poultry and 35% from dairy products. In contrast, high-income countries generally have relatively low inequality with high levels of emissions despite country-to-country variations. The GF-Gini coefficients for all types of products of most Western European countries are <0.20 (Supplementary Tables 9 and 10 ), which is lower than for other high-income countries such as the United States, Australia, Canada and Japan.
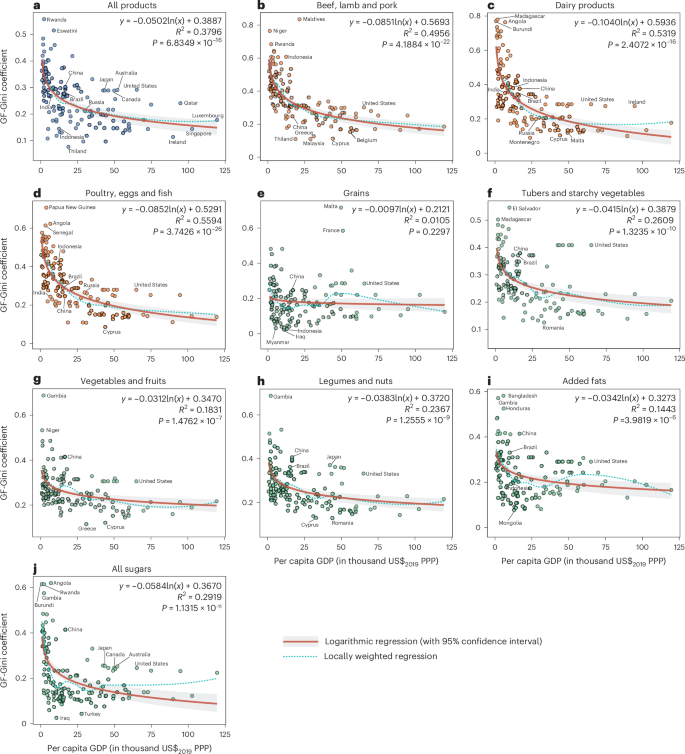
a – j , The x axis represents the country-average per capita GDP, and the y axis represents the national GF-Gini coefficients of all types of ( a ) and different ( b – j ) food categories. b , Beef, lamb and pork. c , Dairy products. d , Poultry, eggs and fish. e , Grains. f , Tubers and starchy vegetables. g , Vegetables and fruits. h , Legumes and nuts. i , Added fats. j , All sugars. Logarithmic regression (red solid line) and locally weighted regression analysis (blue dotted line) are used to determine the relationship between the national GF-Gini coefficient (dependent variable) and the country-average per capita GDP (independent variable). The coefficients of determination ( R 2 ) and the exact P values from the two-sided Student’s t -test for the logarithmic regression are indicated in each subgraph. The error bands (grey shaded areas) represent 95% confidence intervals around the fitted logarithmic regression lines. Blue, orange and green dots represent all types of products, animal-based products and plant-based products, respectively.
Dietary emission shares across consumer groups
There are notable differences in dietary emission shares associated with food categories across expenditure deciles between regions (Fig. 4 ). In high-income countries, expenditure groups have relatively similar patterns of dietary emissions, with large shares of red meat and dairy products contributing the largest amount of emissions. Even poor consumer groups in high-income countries tend to be more likely to be able to afford animal-based products as a result of relatively lower prices for dairy products, eggs, white meat and processed red meat. This contrasts with the high prices of animal-based products due to supply constraints in most low- and lower-middle-income countries 52 , 53 . Except in high-income countries, starchy staple foods (including grains and tubers), with low prices but high-carbohydrate content 44 , 54 , constitute a large proportion of dietary emissions because of the high level of consumption, especially in Southeast Asia and Sub-Saharan Africa. As individuals’ expenditures increase in these countries, emission shares from starchy staple foods in total emissions decrease substantially. These changes demonstrate that as the affordability of food increases, populations tend to adopt instead more diverse diets composed of fewer starchy staple foods and more meat, dairy products, vegetables and fruits. This trend generally aligns with Bennett’s Law 25 , 55 , 56 . For example, research shows that with rapid economic growth, China’s urban or high-income groups increase their intake of non-starchy foods to fulfil their requirements of dietary diversity 35 , while poorer groups, often engaging in strenuous physical jobs, predominantly consume inexpensive starchy staple foods. One exception is Rest of Oceania, where poorer groups have higher percentages of emissions from not only tubers but also vegetables and fruits. Owing to relatively low expenditure on food, poor populations in this island region usually choose locally cultivated tubers and fruits (such as cassava, taro and bananas) 57 , 58 with high intensities of land-use emissions 59 .
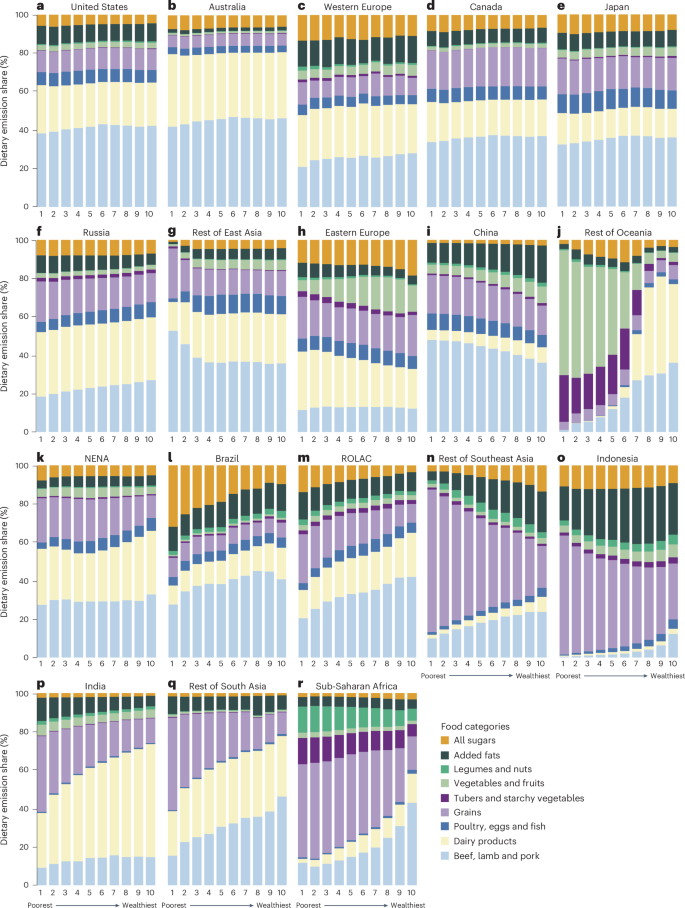
The numbers at the bottom of each bar represent the expenditure levels of regional expenditure deciles, ranging from the poorest (1) to the wealthiest (10). Food categories are shown in the colour legend. a , United States. b , Australia. c , Western Europe. d , Canada. e , Japan. f , Russia. g , Rest of East Asia. h , Eastern Europe. i , China. j , Rest of Oceania. k , NENA. l , Brazil. m , ROLAC. n , Rest of Southeast Asia. o , Indonesia. p , India. q , Rest of South Asia. r , Sub-Saharan Africa.
Emission changes from adopting the planetary health diet
To estimate the emission changes from a global diet shift, we build a hypothetical scenario by assuming that everyone in all countries adopts the planetary health diet ( Methods ). Results indicate that the global dietary emissions would decrease by 17% (1.94 (1.51–2.39) GtCO 2 e) compared with the 2019 level (details of the uncertainty ranges can be found in Supplementary Tables 11 and 12 ). The presently overconsuming groups (56.9% of the global population) would save 32.4% of global emissions through diet shifts, more than offsetting the 15.4% increase in global emissions from the presently underconsuming groups (43.1% of the global population) as a result of adopting healthier diets (Supplementary Table 13 ). National dietary emissions in 100 countries would decline by 2.88 GtCO 2 e, whereas the other 39 countries (mainly low- and lower-middle-income countries 46 in Sub-Saharan Africa and South Asia) would have an increase in emissions by 938 MtCO 2 e (Fig. 5a ; for detailed food categories see Supplementary Figs. 12 – 20 ).
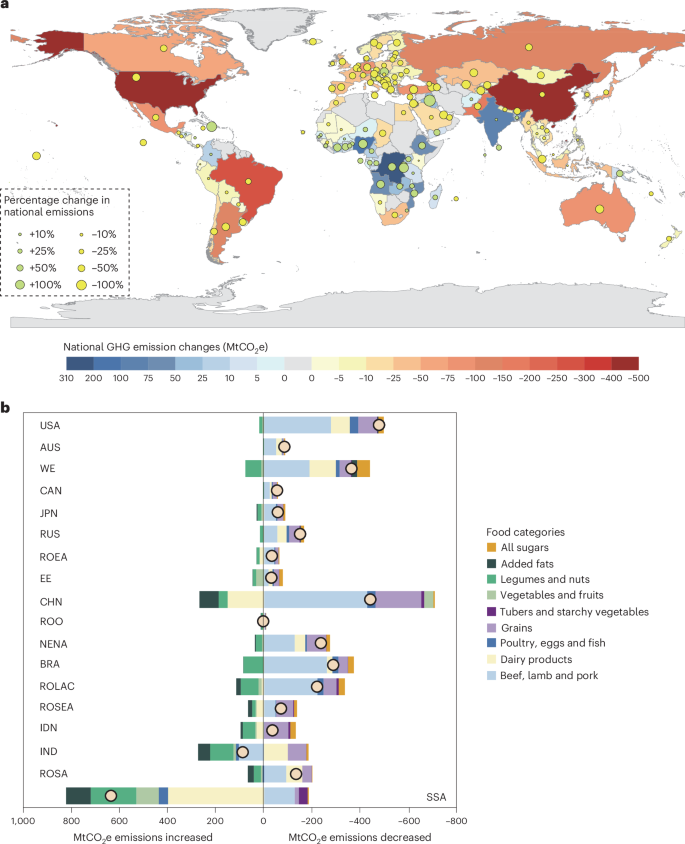
a , Volume changes and percentage changes of national emissions for 139 countries/areas. b , Regional emission changes from different food categories. Abbreviations of 18 regions and the source of the base map are listed in Fig. 1 caption.
Countries would be affected differently regarding emission changes by adopting the planetary health diet, reflected in the percentage change in national emissions (Fig. 5a ). Uzbekistan (−74%), Australia (−70%), Qatar (−67%), Turkey (−65%) and Tajikistan (−64%) would see the largest percentage decrease. In comparison, most of the countries with an estimated considerable percentage increase are located in Sub-Saharan Africa and the Middle East, with the largest percentage increase from Iraq (+155%). Notably, with the increase in per capita GDP, the percentage change in overall dietary emissions of countries shows a shift from a positive to a negative trend, primarily led by changes in animal-based emissions (Supplementary Fig. 21 ).
Global emission reduction would be dominantly driven by red meat and grains (Fig. 5b ). The reduction in meat, eggs and fish would lead to 2.04 GtCO 2 e of emission reduction, of which 94% is driven by the decrease in red meat. China (22%), the United States (15%) and Brazil (14%) would be the largest contributors to emission reduction associated with a decrease in red meat consumption. A decline in grains would result in 914 MtCO 2 e of emission reduction, of which 56% would happen in Asia. A further 240 and 89 MtCO 2 e reduction in emissions would come from reduced sugars and tubers, respectively. However, increased proteins (legumes and nuts and dairy products), added fats and vegetables and fruits would partly offset the above-reduced emissions by 41%. Intake of legumes and nuts would increase in all regions, leading to a further 757 MtCO 2 e of emissions, whereas most of the emission increase related to added fats (largely vegetable oils) (279 Mt) and dairy products (143 Mt) would take place in Sub-Saharan Africa, China and other Asian countries. Global dietary emissions associated with vegetables and fruits would increase by 163 Mt, despite declines in China and Rest of Oceania.
The decline in per capita GHG footprints would be achieved primarily in wealthy consumer groups in high- and upper-middle-income countries, while increased footprints would occur mainly in poor groups in most countries (Fig. 6a ). Results show that the shifts of chief protein sources from animal-based to plant-based proteins according to the planetary health diet 12 would contribute the most to changes in footprints globally (Fig. 6b ). For example, in Australia, Brazil, Canada and the United States where diets are dominated by red meat and dairy products, the top and upper-middle expenditure groups would have notable reductions in footprints. However, most populations in South and Southeast Asia and Sub-Saharan Africa would have a considerable increase in footprints because of the present low levels of red meat intake. Meanwhile, the present intake of plant-based proteins in all countries is below the recommended level 25 . Footprints related to legumes and nuts would increase for most expenditure groups in all regions to meet nutrient demands. This increase is particularly substantial in Rest of Oceania, Brazil, Indonesia and Sub-Saharan Africa, where most of the consumed legumes and nuts are domestically produced with high land-use emission intensities 59 , 60 , assuming the present production and trade patterns remain unchanged.
a , Changes in GHG footprints from all types of food categories. The size of the bubble refers to the average total expenditure represented by the decile. b , Changes in GHG footprints from different food categories. The colours of bubbles in a and b indicate expenditure deciles ranging from the poorest in blue to the wealthiest in red and are comparable only within each region.
Discussion and conclusions
This study uncovers the extent of inequality of dietary emissions within countries based on detailed expenditure data 17 , 34 and underlines the dependence of dietary emissions on expenditure and income levels. Emissions aggregated at expenditure deciles may lose some fine-grained information from the 201 expenditure groups. For example, people from the lowest expenditure groups in affluent countries may experience malnutrition or even hunger, which is not adequately captured at a decile level. Nevertheless, the GF-Gini coefficient calculated from 201 groups provides an accurate reflection of emission inequality. Results show that affluent countries consume high-emission diets but show relatively lower levels of inequality, whereas many poor countries tend to have diets with lower emissions but higher levels of inequality.
The objective of the diet shift scenario is to assess the potential implications of emission mitigation of the food system resulting from changing consumer choices. Widespread diet shifts offer dual benefits by moving 43.1% of the global population out of underconsumption and mitigating 17% of global dietary emissions. The simulated changes in the volume of global emissions under the planetary health diet approximate the findings by ref. 26 (Supplementary Discussion 1 ). However, worldwide diet shifts require tailored policies targeted at regions, countries, expenditure groups and products instead of ‘one-size-fits-all’ policies.
We find that, compared to plant-based products, animal-based products, particularly red meat and dairy products, exhibit greater potential for reducing both emission volumes and emission disparities among different expenditure groups. Priorities lie in reducing the overconsumption of specific emission-intensive products in affluent countries (particularly the high-expenditure groups), such as beef in Australia and the United States, to achieve health 9 , 12 and climate benefits 25 , 26 , 28 . Incentives, such as implementing subsidies or taxation on environmental externalities through food or carbon pricing 61 , ecolabelling 62 and expanding the availability of less emission-intensive products (for instance, menu design for diverse vegetarian foods 63 ), can encourage consumers to make dietary changes. Moreover, a well-designed (primarily urban) food environment can reshape residents’ dietary patterns 35 and the parallel development of urban planning and infrastructure can alleviate the time and financial burdens of shifts to healthier diets 64 . However, in countries such as Mongolia, where diets heavily rely on red meat and dairy products because of their traditional nomadic lifestyle and limited accessibility of diverse foods, especially in rural areas 48 , diet shifts may not be feasible but there is a need to improve national nutritional education 48 .
Low-income countries face more severe challenges in reaching healthier diets. On the one hand, diet shifts require increased food consumption in these countries. For example, in Sub-Saharan Africa, the planetary health diet requires a 3.4-fold increase in dairy consumption for the entire population and a 69-fold increase for the poorest decile (Supplementary Fig. 22 ). However, Sub-Saharan Africa and South and Southeast Asia, which have experienced stagnating agriculture production efficiency for decades 8 , cannot produce domestically nor afford to import the food required for diet shifts 65 . It is crucial to enhance the production efficiency of feed and food crops through various measures such as crop and soil management techniques 8 , 66 and the introduction of high-yielding crop varieties and hybrids 67 , 68 . Moreover, increasing the proportions of nutrient-rich products in food imports 65 and reducing restrictive trade policies which tend to raise food prices 25 , 69 help to address this challenge. On the other hand, poor populations often opt for lower-cost, calorie-dense but less nutritionally beneficial foods. High cost and low affordability remain the largest barriers for these individuals to select healthier diets 44 , 54 , 70 , 71 . Others 44 found that >1.58 billion low-income populations worldwide cannot afford the cost of the planetary health diet. Therefore, policy efforts (for instance, pricing interventions 72 , technical assistance to reduce food production costs 73 and so on) should focus on making food more affordable and accessible, especially for lower expenditure groups 37 , 74 . However, studies indicate that lower food prices may decrease the income of agricultural households 75 , 76 , widen wealth gaps between individuals employed in food- and non-food sectors, especially in low-income agrarian countries and exacerbate rural poverty 1 , 77 . In this sense, policies aimed at promoting diet shifts should be deliberately and cautiously designed with vulnerable groups in mind to reduce inequality 37 , 61 .
Lastly, altered food demand due to diet shifts can induce notable structural adjustments within the global agri-food system. Although this study does not assess the feasibility of countries supplying sufficient food if the planetary health diet was adopted, results indicate that the composition of global food production would change considerably to adapt to the substantial changes in demand 8 , 25 , 77 . The diet shifts would necessitate the global supply (in calorie content) of red meat decrease by 81%, all sugars by 72%, tubers by 76% and grains by 50%, while that of legumes and nuts increase by 438%, added fats by 62% and vegetables and fruits by 28% (Supplementary Data 16 ). Research 77 , 78 confirms that changed food demand could cause fluctuating prices of agricultural products and land in global markets, triggering spillover effects between different food categories or to other non-food sectors (for example, stimulating biofuel production) and partly offsetting the benefits of diet shifts. Therefore, policy-making should focus on alleviating these effects. Incentives such as increased subsidies or tax breaks can generate new economic opportunities and motivations for industries that need to scale up production to meet the heightened demand for products (for example, plant-based proteins). By contrast, for emission-intensive food industries that need to downsize, measures such as gradual crop substitution 25 , 79 could be adopted to optimize production and reduce the costs of production transformations while safeguarding the interests of producers.
In this study, we first assess the GHG emissions from diets comprising 140 products 16 (Supplementary Table 14 ) in 139 countries or areas (we collectively use the term ‘country’ because most of them are individual countries) (Supplementary Data 1 ) in 2019 based on the global consumption-based emission inventory of detailed food products from ref. 16 . The inventory 16 provides data (in mass units) of GHG emissions (including CO 2 , CH 4 and N 2 O) generated during supply chain processes, including agricultural land use and land-use change (LULUC), agricultural activities and beyond-farm processes (excluding emissions from household and end of life) 4 . All emissions are allocated to final consumers of food products. The year 2019 (the latest year before the COVID-19 pandemic) is selected as a baseline year, which can reflect the level of present dietary intake without the interference of the pandemic 80 , 81 . Subsequently, dietary emissions from different expenditure groups are quantified by matching diets with the household-expenditure dataset 42 to reflect the differences and potential inequality of dietary emissions. Finally, to measure the magnitude of the emission impact of the global diet shift, we model the transition from diets in 2019 to the widespread adoption of the planetary health diet. The research framework of this study is shown in Supplementary Fig. 23 .
The following data sources are mainly used in this study. The consumption-based food emissions inventory 16 is based on data derived from the FAOSTAT 82 , comprising national emission accounts of supply chain processes and data on food trade and production. Data on food loss and waste throughout the global supply chain and at the household level as well as food supply data, all used for linking emissions with diets, are obtained from FAOSTAT 83 and previous research 25 , 39 . The household-expenditure data 41 are built on the basis of the WBGCD 42 and further refined and supplemented by consumer expenditure surveys from high-income countries 17 , 41 to bridge the dietary emissions with different expenditure groups. Detailed data sources used for calculation are provided in Supplementary Table 15 . Data processing, assumptions and uncertainties for all calculations are also given.
Dietary energy intake and emissions
Accounting of food consumption and supply chain emissions.
The estimation of the present dietary emissions and the emission changes for adopting the EAT-Lancet planetary health diet 12 is based on the accounting framework designed by ref. 16 . They assess global GHG emissions induced by the consumption of food products in 181 countries based on the physical trade flow approach 84 , 85 . Consumption-based GHG emissions along global supply chains, including local production and international trade, are calculated as follows 16 , 84 :
where E i,r refers to the consumption-based GHG emission of product i in country r . G i / P i represents the vector of direct emission intensity of product i from entire food supply chain processes, of which G i denotes total emissions generated from entire supply chain process of product i , P i is the production vector of product i . \({(I-{A}^{i})}^{-1}\) is the trade structure of product i , of which A i is the matrix of export shares and I is the identity matrix with the same dimension as matrix A i . DMI i refers to the vector of direct material input of product i and DMC i,r is the vector of domestic material consumption of product i in country r with values set to zero for other countries. The DMI of a country is defined as the total inputs of products and the DMC is defined as the amount of products consumed domestically. DMI equals DMC plus exports of products (or production plus imports). F i refers to the vector of total (or consumption-based) emission intensity of product i from food supply chain processes, that is, total emissions induced by per unit of domestic consumption of product i . All variables in equation ( 1 ) are in units of mass (metric tonnes).
Feed products are excluded from diets because emissions from feed crops have been allocated to livestock products that consume feed during production 16 . Food loss and waste (FLW) along supply chains and households are subtracted to quantify the net intake amount of food products from the household stage.
Dietary calorie conversions
We use the annual per capita food supply (FS) quantity of 140 food products from the supply utilization accounts of FAOSTAT 83 and population from the United Nations 86 to calculate the total supply amount of product i in country r (FS i,r , in the unit of mass):
where \({{\rm{FS}}}_{{\rm{per}}}^{i}\) denotes the per capita supply of product i per year and p r refers to the population in country r .
To be consistently matched with the DMC , the FS values should be limited within the coverage of the DMC and values that exceed this range are removed. At the same time, to aggregate food products into food categories and compare their nutritional contents with the reference level from the planetary health diet, we convert the quantity of food consumption or supply into calorie content using product-specific nutritive factors (calories per unit weight of product) 87 , 88 from FAO (Supplementary Table 14 ).
Subtracting food loss and waste at the household level
The food supply derived from FAOSTAT datasets does not exclude FLW that happens during household consumption 25 . FLW before dietary intake can be divided into two parts: the FLW during supply chain processes (including agricultural production, postharvest handling and storage, processing and packaging and distribution) as well as the FLW during the food preparation and supply for household consumption 39 , 40 . The food supply value provided by FAOSTAT only excludes FLW during supply chain processes. Therefore, we exclude household FLW using the method by ref. 25 to calculate the annual dietary intake for each product as follows:
where DI i,r and \({{\rm{DI}}}_{{\rm{per}}}^{i,r}\) refer to the national and per capita caloric intake amount of product i in country r each year, respectively. \({{\rm{FS}}}_{{\rm{energy}}}^{i,r}\) and \({{\rm{FS}}}_{{\rm{energy}\_per}}^{i,r}\) are the national and per capita supply quantity (in calorie content) of product i annually, respectively. Parameter \({f}_{{\rm{FLW}}}^{\;i,r}\) is the FLW factor in the household consumption stage 39 of food product i in country r . Others 39 provide regional FLW factors, expressed as the weight percentage of food that is lost or wasted at different stages of food production and consumption, for different food categories. As a result, household food waste is subtracted from the FS to obtain the dietary intake amount of each product. Detailed household FLW factors are shown in Supplementary Table 16 .
Quantifying dietary GHG emissions
Our equation ( 1 ) can be transformed into the following equation to calculate the total emission intensity of food calorie consumption:
where \({F}_{{\rm{energy}}}^{\,i,r}\) represents total emissions per unit of calorie content of product i in country r , \({{\rm{DMC}}}_{{\rm{energy}}}^{i,r}\) refers to total calorie content of product i consumed domestically in country r . Then, emissions from the dietary intake (without FLW) of product i in country r ( \({E}_{{\rm{intake}}}^{\,i,r}\) ) are calculated as follows:
Classification of food categories
The EAT-Lancet Commission report provides coverage of different food categories in the planetary health diet and their recommended caloric intake levels at 2,500 kcal for adults each day 12 (Supplementary Table 17 ). In this study, we classify 140 products into 13 aggregated food categories according to the planetary health diet 12 , including grains, tubers or starchy vegetables, vegetables, fruits, dairy products, red meat (beef, lamb and pork), chicken and other poultry, eggs, fish, legumes, nuts, added fats (both unsaturated and saturated oils) and all sugars. On the basis of the data availability of the FAOSTAT 4 , 82 , the food products in this study include both primary and processed products (primary and secondary food processing) which can be classified into specific food categories 16 . Ultraprocessed products that combine ingredients from several food categories, such as ice creams made from both dairy and sugar, are not considered. Detailed coverages of each food category and their mapping relationship with specific products are shown in Supplementary Table 18 .
Matching diets with the household-expenditure dataset
We explore the dietary emissions from consumers with different expenditure levels (defined as expenditure groups) using the household-expenditure dataset 41 for the year 2011. The dataset, containing 116 countries and almost 90% of the global population (Supplementary Table 19 ), is primarily based on the household survey microdata from the WBGCD 42 , supplemented by consumer expenditure surveys of national statistical offices from high-income countries such as the United States and European countries 17 , 41 . For every country in the dataset, 201 expenditure groups (grouped according to the per capita total expenditure of each group) and the corresponding population share are listed. The annual per capita expenditure of people in different expenditure groups ranges from <US$50 to ~US$1 million per year (expressed in 2011 Purchasing Power Parities, PPP) 31 , 34 . For each expenditure group, the expenditure for 33 different sectors of goods and services (including 11 food items) and the corresponding expenditure share in national consumption of each sector are provided 31 , 34 , 41 . For some affluent (or poor) countries that do not have a sufficient representative number of people at the bottom (or top) end of the expenditure spectrum, the population in the corresponding expenditure groups is empty. Expenditure shares of 11 food items are matched with the 140 products in this study (Supplementary Table 20 ). We calculate the dietary intake of different food products for each expenditure group in each country by multiplying the food expenditure share of groups with the total dietary intake amounts of food products of each country.
This study assumes that the amount of food consumption is proportionate to food expenditures and the purchasing price for the same product is unchanged across 201 groups ignoring higher prices for high-quality or luxury food items within the same food category. Although the assumption of an unchanged purchasing price is an unsolved limitation shared by similar studies using monetary expenditure data 31 , 34 , 41 , household expenditures on food can still effectively highlight the differences in food consumption and emissions across consumer groups with different affordability of, and spending on, food. We also assume that the proportion of food sources from local production and trade for the same food category remains constant across the 201 groups. In other words, the magnitude of dietary emissions is solely determined by the size and pattern of food expenditure of each group and the associated supply chains for each food consumption item.
For countries that are major food consumers (and emitters) but without data in WBGCD, expenditure shares from countries with similar development levels and eating habits and neighbouring geographical locations are used to calculate the distribution of their food expenditure. We finally select 201 expenditure groups in 139 countries/areas, covering 95% of the global population in 2019 (Supplementary Table 3 and Supplementary Data 3 ). Details for dealing with missing data are provided in Supplementary Table 7 . Countries or areas are then classified into 18 regions for comparison according to geographical locations (Supplementary Table 8 ). The WBGCD expenditure data from the year 2011 are adjusted to PPP in 2019 to represent the expenditure level of populations in figures. Results of emissions from 13 types of food categories of 201 expenditure groups at the national and regional levels are shown in Supplementary Data 8 , 10 and 11 .
Analysis of GF-Gini coefficients
Calculation of gf-gini coefficients.
This study uses the GF-Gini coefficient 33 , 89 , which is based on the well-known Gini coefficient 90 , to measure the inequality of GHG footprints from 201 expenditure groups within countries, regions and globally. The GF-Gini coefficient ranges from 0 to 1, indicating the emission distribution across expenditure groups changes from perfect equality to perfect inequality. The GF-Gini coefficient of each food category is calculated as 33 :
where Gini j indicate the GF-Gini coefficient of food category j (including product i , i = 1, 2, 3, …, n ). Expenditure groups and their population are reordered in ascending order of per capita GHG footprint of food category j and m refers to the reordered number of groups ( m = 1, 2, 3, …, 201). \({D}_{m}^{j}\) and \({Y}_{m}^{j}\) represent the proportions of population and GHG footprints (of food category j ) for each expenditure group, respectively. \({T}_{m}^{j}\) is the cumulative proportion of GHG footprints of each expenditure group. The results of national, regional and global GF-Gini coefficients are shown in Supplementary Tables 9 and 10 .
Regression analysis
We use the regression approach to examine the relationship between the national GF-Gini coefficients and the per capita GDP 91 , 92 of 139 countries/areas. The GF-Gini coefficient of each country is regarded as the dependent variable ( y ) and the national per capita GDP acts as the independent variable ( x ). Initially, locally weighted regression is applied to illustrate the trend lines within the scatterplot. Subsequently, we test different regression methods for validation based on the general trend. Ultimately, we found that logarithmic regression is the most fitting for dietary emissions of most food categories, particularly in the case of animal-based products. Thus, the logarithmic regression is applied.
Scenario of the planetary health diet
Scenario setting and assumptions.
To estimate the emission changes resulting from the transition from the 2019 diet to the global planetary health diet, we build a hypothetical scenario by assuming that individuals belonging to 201 different expenditure groups in all countries will all reach the reference intake level of 13 types of food categories 12 . First, we assume that the proportion of food sources from local production and trade in each country is unchanged, that is, emission changes from dietary shifts would be calculated on the basis of emissions from local production and imports accounting for emissions along global food supply chains, similar to studies by refs. 25 , 26 . At the same time, emission changes induced by decreased food consumption in countries following the planetary health diet, such as carbon uptake from agriculture abandonment 59 or emission increase from non-food biomass production in saved agricultural land 77 , are not considered in this study. Second, we assume that agricultural and food-related production technology, trade patterns and emission intensities of food supply chain processes remain unchanged during the diet transition. Third, fluctuations in food prices induced by altered food demand or the affordability of the planetary health diet for different consumer groups are not considered in this study.
Diet gaps for different food categories
The diet gap (DG) reflects gaps between present dietary intake and the planetary health diet 12 , 25 , as follows:
where \({{\rm{DG}}}_{{\rm{per}}}^{j,r}\) is defined as the percentage ratio of the present per capita caloric intake of food category j in country r each year ( \({{\rm{DI}}}_{{\rm{per}}}^{\,j,r}\) ) to the annual reference level ( \({{\rm{DI}}}_{{\rm{EAT}}\_{\rm{per}}}^{i}\) ). \({{\rm{DI}}}_{{\rm{EAT}\_day\_per}}^{\,j}\) is the recommended per capita caloric intake of food category j each day 12 (Supplementary Table 17 ). We assume a uniform annual calorie reference level for each food category across all populations in all countries. We allow flexibility in local diets by keeping the composition of each food category unchanged, requiring only that the calorie content reaches the reference level. According to the definition, present food intake is considered insufficient compared with reference levels when DG is <100%, while it is deemed excessive and should be reduced when DG is >100%. Daily per capita caloric intake of food categories from 201 expenditure groups of countries or regions are shown in Supplementary Data 12 and 13 . We calculate the DG for food categories of 201 expenditure groups at national and regional levels (Supplementary Data 14 and 15 ).
According to equation ( 1 ), the total emissions per unit of calorie content of food category j in country r ( \({F}_{{\rm{energy}}}^{\;j,r}\) ) can be calculated as:
where E j,r refers to the national emissions due to consumption of food category j in country r . Thus, emission changes for adopting the planetary health diet are calculated as follows:
where \(\Delta {E}_{{\rm{intake}}}^{\;j,r}\) represents the national emission changes of food category j in country r , \({E}_{{\rm{intake}}}^{\;j,r}\) is the national emissions from intake of food category j in country r . Changes in dietary emissions of food categories from 201 groups are shown in Supplementary Data 9 . The number of people with increased/decreased emissions from 201 groups is shown in Supplementary Data 19 .
Uncertainty analysis
We assess the uncertainty range of dietary emissions from different food products using a Monte Carlo approach, which simulates the uncertainties caused by activity data, emission factors and parameters in each emission process 16 , 59 , 93 . More details can be found in Supplementary Methods 1 .
Limitations
This study has the following limitations regarding data analysis and scenario setting.
In terms of data analysis, this study is limited by the data availability. First, we use regional household food loss and waste factors of aggregated food categories without more detailed product division at the national level because of a lack of data. There might also be differences between calculated and actual food intake amounts that are unable to be removed, such as animal bones or fruit skins 25 . Second, we use the consumer household-expenditure dataset based on WBGCD for the year 2011, which provides the most precise and detailed differentiation of consumer groups and their consumption patterns within countries so far. We assume that the shares in food expenditure and population for each expenditure group are the same as in 2011. Third, we assume that the composition of different products aggregated in one category consumed by expenditure groups is the same as the national consumption composition and there is no difference in the price of food products purchased by people from different expenditure groups. In addition, data for some populous high- or upper-middle-income countries are missing from the household-expenditure dataset. However, the countries are the world’s major food consumers and emitters, their emission changes due to diet shifts are important for the global food system. We use the expenditure shares of similar countries in the household-expenditure dataset to allocate the distributions of food expenditure in these countries.
In terms of scenario setting, we focus on the impact induced by changes in consumer choices without changing the proportion of food supply sources (domestic production and imports). We do not consider altering the proportions of supply sources and associated emissions in this study. However, future studies may explore the impacts of the production side and supply chains for diet shifts. Moreover, as we focus on the present emission inequality and mitigation potentials within the food system, we assume that the income and expenditure levels of expenditure groups remain unchanged. However, a shift in food supply may affect household income and subsequently alter the household food budgets, especially for populations employed in, or countries reliant on, food-related sectors. Additionally, as a result of data and model limitations, this study does not consider price fluctuations induced by food demand and subsequent changes in household affordability or spillover effects (between food categories or to non-food sectors). Future studies may combine assessment models incorporating elasticities to project the long-term feasibilities and consequences of diet shifts.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data for LULUC, agricultural and beyond-farm emissions and data for physical food consumption are curated by the FAO and can be freely obtained from FAOSTAT 82 , available from ref. 16 . Data of food loss and waste rate are retrieved from FAOSTAT 82 and ref. 25 . The global household-expenditure data are obtained from the World Bank 42 and refs. 17 , 41 . Population data used in this study are obtained from World Population Prospects of the United Nations 86 . Data on per capita GDP in countries can be collected from the World Bank 91 and the International Monetary Fund 92 . Supplementary datasets are also available on Zenodo ( https://doi.org/10.5281/zenodo.11934909 ) 94 . Source data are provided with this paper.
Code availability
Data collection is performed in MATLAB and Microsoft Excel. Code developed for data processing in MATLAB and R in this study is available from Zenodo ( https://doi.org/10.5281/zenodo.11880402 ) 95 .
Springmann, M., Godfray, H. C. J., Rayner, M. & Scarborough, P. Analysis and valuation of the health and climate change cobenefits of dietary change. Proc. Natl Acad. Sci. USA 113 , 4146–4151 (2016).
Article CAS Google Scholar
Kesse-Guyot, E. et al. Sustainability analysis of French dietary guidelines using multiple criteria. Nat. Sustain. 3 , 377–385 (2020).
Article Google Scholar
Crippa, M. et al. Food systems are responsible for a third of global anthropogenic GHG emissions. Nat. Food 2 , 198–209 (2021).
Tubiello, F. N. et al. Pre-and post-production processes increasingly dominate greenhouse gas emissions from agri-food systems. Earth Syst. Sci. Data 14 , 1795–1809 (2022).
Clark, M. A. et al. Global food system emissions could preclude achieving the 1.5 °C and 2 °C climate change targets. Science 370 , 705–708 (2020).
Ivanovich, C. C., Sun, T., Gordon, D. R. & Ocko, I. B. Future warming from global food consumption. Nat. Clim. Change 13 , 297–302 (2023).
Béné, C. et al. Five priorities to operationalize the EAT-Lancet Commission report. Nat. Food 1 , 457–459 (2020).
Navarre, N., Schrama, M., de Vos, C. & Mogollón, J. M. Interventions for sourcing EAT-Lancet diets within national agricultural areas: a global analysis. One Earth 6 , 31–40 (2023).
Laine, J. E. et al. Co-benefits from sustainable dietary shifts for population and environmental health: an assessment from a large European cohort study. Lancet Planet. Health 5 , e786–e796 (2021).
Craig, W. J. Health effects of vegan diets. Am. J. Clin. Nutr. 89 , S1627–S1633 (2009).
Afshin, A. et al. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 393 , 1958–1972 (2019).
Willett, W. et al. Food in the Anthropocene: the EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 393 , 447–492 (2019).
The State of Food Security and Nutrition in the World 2022: Repurposing Food and Agricultural Policies to Make Healthy Diets More Affordable (FAO, 2022); https://www.fao.org/documents/card/en/c/cc0639en
Bajželj, B. et al. Importance of food-demand management for climate mitigation. Nat. Clim. Change 4 , 924–929 (2014).
Springmann, M. et al. Options for keeping the food system within environmental limits. Nature 562 , 519–525 (2018).
Li, Y. et al. Changes in global food consumption increase GHG emissions despite efficiency gains along global supply chains. Nat. Food 4 , 483–495 (2023).
Hubacek, K., Baiocchi, G., Feng, K. & Patwardhan, A. Poverty eradication in a carbon constrained world. Nat. Commun. 8 , 912 (2017).
Sustainable Development Goals: 17 Goals to Transform Our World (United Nations, 2017); https://www.un.org/sustainabledevelopment/sustainable-development-goals/
Humpenöder, F. et al. Projected environmental benefits of replacing beef with microbial protein. Nature 605 , 90–96 (2022).
Hasegawa, T., Havlík, P., Frank, S., Palazzo, A. & Valin, H. Tackling food consumption inequality to fight hunger without pressuring the environment. Nat. Sustain. 2 , 826–833 (2019).
Kim, B. F. et al. Country-specific dietary shifts to mitigate climate and water crises. Glob. Environ. Change 62 , 101926 (2020).
Denton, F. et al. in Climate Change 2022: Mitigation of Climate Change (eds Shukla, P. R. et al.) 1727–1790 (Cambridge Univ. Press, 2022).
Tilman, D. & Clark, M. Global diets link environmental sustainability and human health. Nature 515 , 518–522 (2014).
Springmann, M. et al. Health and nutritional aspects of sustainable diet strategies and their association with environmental impacts: a global modelling analysis with country-level detail. Lancet Planet. Health 2 , e451–e461 (2018).
Tuninetti, M., Ridolfi, L. & Laio, F. Compliance with EAT-Lancet dietary guidelines would reduce global water footprint but increase it for 40% of the world population. Nat. Food 3 , 143–151 (2022).
Semba, R. D. et al. Adoption of the ‘planetary health diet’ has different impacts on countries’ greenhouse gas emissions. Nat. Food 1 , 481–484 (2020).
Guo, Y. et al. Environmental and human health trade-offs in potential Chinese dietary shifts. One Earth 5 , 268–282 (2022).
Sun, Z. et al. Dietary change in high-income nations alone can lead to substantial double climate dividend. Nat. Food 3 , 29–37 (2022).
Mbow, C. et al. in Climate Change and Land (eds Shukla, P. R. et al.) Ch. 5 (IPCC, 2019); https://www.ipcc.ch/site/assets/uploads/sites/4/2022/11/SRCCL_Chapter_5.pdf
Millward-Hopkins, J. & Oswald, Y. Reducing global inequality to secure human wellbeing and climate safety: a modelling study. Lancet Planet. Health 7 , e147–e154 (2023).
Guan, Y. et al. Burden of the global energy price crisis on households. Nat. Energy 8 , 304–316 (2023).
Hubacek, K. et al. Global carbon inequality. Energy Ecol. Environ. 2 , 361–369 (2017).
Mi, Z. et al. Economic development and converging household carbon footprints in China. Nat. Sustain. 3 , 529–537 (2020).
Bruckner, B., Hubacek, K., Shan, Y., Zhong, H. & Feng, K. Impacts of poverty alleviation on national and global carbon emissions. Nat. Sustain. 5 , 311–320 (2022).
He, P., Baiocchi, G., Hubacek, K., Feng, K. & Yu, Y. The environmental impacts of rapidly changing diets and their nutritional quality in China. Nat. Sustain. 1 , 122–127 (2018).
Rao, N. D. et al. Healthy, affordable and climate-friendly diets in India. Glob. Environ. Change 49 , 154–165 (2018).
He, P., Feng, K., Baiocchi, G., Sun, L. & Hubacek, K. Shifts towards healthy diets in the US can reduce environmental impacts but would be unaffordable for poorer minorities. Nat. Food 2 , 664–672 (2021).
Reynolds, C. J., Horgan, G. W., Whybrow, S. & Macdiarmid, J. I. Healthy and sustainable diets that meet greenhouse gas emission reduction targets and are affordable for different income groups in the UK. Public Health Nutr. 22 , 1503–1517 (2019).
Gustavsson, J., Cederberg, C., Sonesson, U., Van Otterdijk, R. & Meybeck, A. Global Food Losses and Food Waste-Extent, Causes and Prevention (FAO, 2011); https://www.fao.org/3/mb060e/mb060e00.htm
Kummu, M. et al. Lost food, wasted resources: global food supply chain losses and their impacts on freshwater, cropland and fertiliser use. Sci. Total Environ. 438 , 477–489 (2012).
Zhong, H., Feng, K., Sun, L., Cheng, L. & Hubacek, K. Household carbon and energy inequality in Latin American and Caribbean countries. J. Environ. Manag. 273 , 110979 (2020).
Global Consumption Database (World Bank, 2022); https://datatopics.worldbank.org/consumption/
Wier, M., Birr-Pedersen, K., Jacobsen, H. K. & Klok, J. Are CO 2 taxes regressive? Evidence from the Danish experience. Ecol. Econ. 52 , 239–251 (2005).
Hirvonen, K., Bai, Y., Headey, D. & Masters, W. A. Affordability of the EAT-Lancet reference diet: a global analysis. Lancet Glob. Health 8 , e59–e66 (2020).
The State of Food Security and Nutrition in the World 2023 (FAO, 2023); https://doi.org/10.4060/cc3017en
World Bank Country and Lending Groups (World Bank, 2021); https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
Okou, C., Spray, J. A. & Unsal, M. F. D. Staple Food Prices in Sub-Saharan Africa: An Empirical Assessment (International Monetary Fund, 2022); https://www.imf.org/en/Publications/WP/Issues/2022/07/08/Staple-Food-Prices-in-Sub-Saharan-Africa-An-Empirical-Assessment-520567
Delgermaa, D., Yamaguchi, M., Nomura, M. & Nishi, N. Assessment of Mongolian dietary intake for planetary and human health. PLoS Glob. Public Health 3 , e0001229 (2023).
Burkhart, S., Underhill, S. & Raneri, J. Realizing the potential of neglected and underutilized bananas in improving diets for nutrition and health outcomes in the Pacific Islands. Front. Sustain. Food Syst. 6 , 805776 (2022).
Pingali, P. Agricultural policy and nutrition outcomes—getting beyond the preoccupation with staple grains. Food Secur. 7 , 583–591 (2015).
Sibhatu, K. T. & Qaim, M. Rural food security, subsistence agriculture and seasonality. PloS ONE 12 , e0186406 (2017).
Headey, D. D. & Alderman, H. H. The relative caloric prices of healthy and unhealthy foods differ systematically across income levels and continents. J. Nutr. 149 , 2020–2033 (2019).
Bai, Y., Alemu, R., Block, S. A., Headey, D. & Masters, W. A. Cost and affordability of nutritious diets at retail prices: evidence from 177 countries. Food Policy 99 , 101983 (2021).
Batis, C. et al. Adoption of healthy and sustainable diets in Mexico does not imply higher expenditure on food. Nat. Food 2 , 792–801 (2021).
Bennett, M. K. International contrasts in food consumption. Geogr. Rev. 31 , 365–376 (1941).
D’Odorico, P. et al. The global food–energy–water nexus. Rev. Geophys. 56 , 456–531 (2018).
Traditional Pacific Island Crops (Univ. Hawaii, 2024); https://guides.library.manoa.hawaii.edu/paccrops
Fiji—Agricultural Commodities (International Trade Administration, 2022); https://www.trade.gov/country-commercial-guides/fiji-agricultural-commodities
Hong, C. et al. Global and regional drivers of land-use emissions in 1961–2017. Nature 589 , 554–561 (2021).
Hong, C. et al. Land-use emissions embodied in international trade. Science 376 , 597–603 (2022).
Darmon, N., Lacroix, A., Muller, L. & Ruffieux, B. Food price policies improve diet quality while increasing socioeconomic inequalities in nutrition. Int. J. Behav. Nutr. Phys. Act. 11 , 66 (2014).
Poore, J. & Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 360 , 987–992 (2018).
Bacon, L. & Krpan, D. (Not) Eating for the environment: the impact of restaurant menu design on vegetarian food choice. Appetite 125 , 190–200 (2018).
Swinburn, B. A. et al. The global syndemic of obesity, undernutrition and climate change: the Lancet Commission report. Lancet 393 , 791–846 (2019).
Geyik, O., Hadjikakou, M., Karapinar, B. & Bryan, B. A. Does global food trade close the dietary nutrient gap for the world’s poorest nations? Glob. Food Secur. 28 , 100490 (2021).
Pradhan, P., Fischer, G., Van Velthuizen, H., Reusser, D. E. & Kropp, J. P. Closing yield gaps: how sustainable can we be. PloS ONE 10 , e0129487 (2015).
Sánchez, P. A. Tripling crop yields in tropical Africa. Nat. Geosci. 3 , 299–300 (2010).
Huang, J., Pray, C. & Rozelle, S. Enhancing the crops to feed the poor. Nature 418 , 678–684 (2002).
The State of Food Security and Nutrition in the World 2020. Transforming Food Systems for Affordable Healthy Diets (FAO, 2020); https://www.fao.org/documents/card/en?details=ca9692en
Allcott, H. et al. Food deserts and the causes of nutritional inequality. Q. J. Econ. 134 , 1793–1844 (2019).
Springmann, M., Clark, M. A., Rayner, M., Scarborough, P. & Webb, P. The global and regional costs of healthy and sustainable dietary patterns: a modelling study. Lancet Planet. Health 5 , e797–e807 (2021).
Darmon, N. & Drewnowski, A. Contribution of food prices and diet cost to socioeconomic disparities in diet quality and health: a systematic review and analysis. Nutr. Rev. 73 , 643–660 (2015).
Baylis, K., Peplow, S., Rausser, G. & Simon, L. Agri-environmental policies in the EU and United States: a comparison. Ecol. Econ. 65 , 753–764 (2008).
Swinnen, J. The right price of food. Dev. Policy Rev. 29 , 667–688 (2011).
Headey, D. D. Food prices and poverty. World Bank Econ. Rev. 32 , 676–691 (2018).
Google Scholar
Headey, D. & Hirvonen, K. Higher food prices can reduce poverty and stimulate growth in food production. Nat. Food 4 , 699–706 (2023).
Gatto, A., Kuiper, M. & van Meijl, H. Economic, social and environmental spillovers decrease the benefits of a global dietary shift. Nat. Food 4 , 496–507 (2023).
Puma, M. J., Bose, S., Chon, S. Y. & Cook, B. I. Assessing the evolving fragility of the global food system. Environ. Res. Lett. 10 , 024007 (2015).
Davis, K. F. et al. Alternative cereals can improve water use and nutrient supply in India. Sci. Adv. 4 , eaao1108 (2018).
Le Quéré, C. et al. Temporary reduction in daily global CO 2 emissions during the COVID-19 forced confinement. Nat. Clim. Change 10 , 647–653 (2020).
Shan, Y. et al. Impacts of COVID-19 and fiscal stimuli on global emissions and the Paris Agreement. Nat. Clim. Change 11 , 200–206 (2021).
FAOSTAT Database (FAO, 2022); https://www.fao.org/faostat/en/
Supply Utilization Accounts, Food Blances, FAOSTAT Online Database (FAO, 2022); https://www.fao.org/faostat/en/#data/SCL
Kastner, T., Kastner, M. & Nonhebel, S. Tracing distant environmental impacts of agricultural products from a consumer perspective. Ecol. Econ. 70 , 1032–1040 (2011).
Kastner, T., Erb, K.-H. & Haberl, H. Rapid growth in agricultural trade: effects on global area efficiency and the role of management. Environ. Res. Lett. 9 , 034015 (2014).
World Population Prospects 2022 (United Nations, 2022); https://population.un.org/wpp/Download/Standard/Population/
Food Balance Sheets—A Handbook (FAO, 2001); https://www.fao.org/3/x9892e/X9892e05.htm#P8217_125315
Nutritive Factors (FAO, 2023); https://www.fao.org/economic/the-statistics-division-ess/publications-studies/publications/nutritive-factors/en/
Wiedenhofer, D. et al. Unequal household carbon footprints in China. Nat. Clim. Change 7 , 75–80 (2017).
Gini, C. Measurement of inequality of incomes. Econ. J. 31 , 124–125 (1921).
The World Bank Data: GDP per Capita (Current US$) (World Bank, 2023); https://data.worldbank.org/indicator/NY.GDP.PCAP.PP.CD
Datasets, World Economic Outlook (April 2023): GDP per Capita, Current Prices (IMF, 2023); https://www.imf.org/external/datamapper/NGDPDPC@WEO/OEMDC/ADVEC/WEOWORLD
Xu, X. et al. Global greenhouse gas emissions from animal-based foods are twice those of plant-based foods. Nat. Food 2 , 724–732 (2021).
Li, Y. et al. Supplementary Datasets for ‘Reducing climate change impacts from the global food system through diet shifts’. Zenodo https://doi.org/10.5281/zenodo.11934909 (2024).
Li, Y. et al. Code for ‘Reducing climate change impacts from the global food system through diet shifts’. Zenodo https://doi.org/10.5281/zenodo.11880402 (2024).
Download references
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant nos 72243004, 32101315, 71904098). Y.S. and S.S. acknowledge support from the National Natural Science Foundation of China (grant no. 72243004). Yu Li acknowledges support from the National Natural Science Foundation of China (grant no. 32101315). P.H. acknowledges support from the National Natural Science Foundation of China under a Young Scholar Programme Grant (grant no. 71904098). Yanxian Li and Y.H. acknowledge the funding support by the China Scholarship Council PhD programme. We thank Y. Zhou for supporting visualization and J. Yan for assisting in writing and revising. For the purpose of open access, a CC BY public copyright license is applied to any author accepted manuscript arising from this submission.
Author information
Authors and affiliations.
Integrated Research on Energy, Environment and Society (IREES), Energy and Sustainability Research Institute Groningen (ESRIG), University of Groningen, Groningen, the Netherlands
Yanxian Li, Franco Ruzzenenti & Klaus Hubacek
School of Earth and Environmental Sciences, Cardiff University, Cardiff, UK
Department of Earth System Science, Tsinghua University, Beijing, China
School of Geography, Earth and Environmental Sciences, University of Birmingham, Birmingham, UK
Yuli Shan & Ye Hang
School of Public Administration, Chongqing Technology and Business University, Chongqing, China
School of Business, East China University of Science and Technology, Shanghai, China
You can also search for this author in PubMed Google Scholar
Contributions
Yanxian Li, Y.S. and K.H. designed the research. Yanxian Li performed the analysis with support from P.H., Yu Li, Y.H. and S.S. on analytical approaches and visualization. Yanxian Li led the writing with efforts from P.H., Y.S., F.R. and K.H. Y.S. and K.H. supervised and coordinated the overall research. All co-authors reviewed and commented on the manuscript.
Corresponding authors
Correspondence to Yuli Shan or Klaus Hubacek .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Climate Change thanks Catharina Latka and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information.
Supplementary Methods, Discussion, Figs. 1–23, Tables 1–24 and references.
Reporting Summary
Supplementary data.
Detailed data for calculated results in this study.
Source Data Fig. 1
Source data for creating Fig. 1.
Source Data Fig. 2
Source data for creating Fig. 2.
Source Data Fig. 3
Source data for creating Fig. 3.
Source Data Fig. 4
Source data for creating Fig. 4.
Source data Fig. 5
Source data for creating Fig. 5.
Source Data Fig. 6
Source data for creating Fig. 6.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Li, Y., He, P., Shan, Y. et al. Reducing climate change impacts from the global food system through diet shifts. Nat. Clim. Chang. (2024). https://doi.org/10.1038/s41558-024-02084-1
Download citation
Received : 07 November 2023
Accepted : 05 July 2024
Published : 13 August 2024
DOI : https://doi.org/10.1038/s41558-024-02084-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Consumer Goods & FMCG ›
- Food & Nutrition
Industry-specific and extensively researched technical data (partially from exclusive partnerships). A paid subscription is required for full access.
Consumers concerned about genetically modified food in the UK 2024, by age group
As of 2024, UK consumers between the ages of 55 and 74 were most concerned about genetically modified food, with 66 percent stating they were either highly or somewhat concerned. Only 47 percent of consumers between the ages of 16 and 24 were concerned.
Share of consumers concerned about genetically modified food in the United Kingdom in 2024, by age group
| Characteristic | Share of respondents |
|---|---|
| - | - |
| - | - |
| - | - |
| - | - |
| - | - |
| - | - |
To access all Premium Statistics, you need a paid Statista Account
- Immediate access to all statistics
- Incl. source references
- Download as PDF, XLS, PNG and PPT
Additional Information
Show sources information Show publisher information Use Ask Statista Research Service
Europe, United Kingdom
May 31 to June 4, 2024
2,056 respondents
16 years and older
Online interviews were administered randomly to members of the YouGov Plc UK panel only.
Online interview
Other statistics on the topic
- Global genetically modified crops by countries 2019, based on acreage
- Percentage of genetically modified crops in the U.S. by type 1997, 2018, 2019 & 2020
- Acreage of genetically modified crops 2015-2019, by country
- Global adoption rate for major biotech crops worldwide 2019, by type
To download this statistic in XLS format you need a Statista Account
To download this statistic in PNG format you need a Statista Account
To download this statistic in PDF format you need a Statista Account
To download this statistic in PPT format you need a Statista Account
As a Premium user you get access to the detailed source references and background information about this statistic.
As a Premium user you get access to background information and details about the release of this statistic.
As soon as this statistic is updated, you will immediately be notified via e-mail.
… to incorporate the statistic into your presentation at any time.
You need at least a Starter Account to use this feature.
- Immediate access to statistics, forecasts & reports
- Usage and publication rights
- Download in various formats
* For commercial use only
Basic Account
- Free Statistics
Starter Account
- Premium Statistics
The statistic on this page is a Premium Statistic and is included in this account.
Professional Account
- Free + Premium Statistics
- Market Insights
1 All prices do not include sales tax. The account requires an annual contract and will renew after one year to the regular list price.
Statistics on " Genetically modified (GM) crops in Canada "
- Seeded area of genetically engineered crops in Canada 2012-2023
- Seeded area of genetically engineered canola in Canada 2012-2023
- Genetically engineered canola share of seeded canola area in Canada 2012-2023
- Canada's seeded area of genetically engineered soybeans 2012-2023
- Genetically engineered soybeans share of seeded soybeans area in Canada 2012-2023
- Seeded area of genetically engineered corn in Canada 2011-2023
- Genetically engineered corn share of seeded corn area in Canada 2012-2023
- Seeded area of genetically modified soybeans in Ontario 2012-2024
- Seeded area of genetically modified soybeans in Quebec 2012-2024
- Share of consumers who want GM food products to be labeled in Canada 2018
- Canadian perception regarding safety of GM foods 2018
- Canadian perception of the safety of GM foods for consumption by province 2017
- Canadians concerned about eating GM foods 2006-2019
- Share of Canadian consumers who refuse to eat genetically modified food 2018
Other statistics that may interest you Genetically modified (GM) crops in Canada
Market overview
- Premium Statistic Acreage of genetically modified crops 2015-2019, by country
- Premium Statistic Global genetically modified crops by countries 2019, based on acreage
- Basic Statistic Global adoption rate for major biotech crops worldwide 2019, by type
- Premium Statistic Percentage of genetically modified crops in the U.S. by type 1997, 2018, 2019 & 2020
- Premium Statistic Seeded area of genetically engineered crops in Canada 2012-2023
Genetically modified crops
- Premium Statistic Seeded area of genetically engineered canola in Canada 2012-2023
- Premium Statistic Genetically engineered canola share of seeded canola area in Canada 2012-2023
- Premium Statistic Canada's seeded area of genetically engineered soybeans 2012-2023
- Premium Statistic Genetically engineered soybeans share of seeded soybeans area in Canada 2012-2023
- Premium Statistic Seeded area of genetically engineered corn in Canada 2011-2023
- Premium Statistic Genetically engineered corn share of seeded corn area in Canada 2012-2023
- Premium Statistic Seeded area of genetically modified soybeans in Ontario 2012-2024
- Premium Statistic Seeded area of genetically modified soybeans in Quebec 2012-2024
Consumer attitudes
- Basic Statistic Share of consumers who want GM food products to be labeled in Canada 2018
- Premium Statistic Canadian perception regarding safety of GM foods 2018
- Premium Statistic Canadian perception of the safety of GM foods for consumption by province 2017
- Premium Statistic Canadians concerned about eating GM foods 2006-2019
- Premium Statistic Share of Canadian consumers who refuse to eat genetically modified food 2018
Further Content: You might find this interesting as well

COMMENTS
It has been proposed that genetically modified foods are integral in the enhancement of safe food security, enhanced quality, and increased shelf-life, hence becoming cost-effective to consumers and farmers. Proponents of this technology also argue that genetically modified foods have many health benefits, in addition to being environmentally ...
A number of studies show the economic benefits of using genetically modified products. Between 1996 and 2011, farmers' income worldwide increased by $92 million from the use of genetically modified crops. Part of the revenue is due to the more efficient treatment of weeds and insects, while another part is due to lower overall production costs.
The term "genetic modified organisms (GMO)" has become a controversial topic as its benefits for both food producers and consumers are companied by potential biomedical risks and environmental side effects. Increasing concerns from the public about GMO, particularly in the form of genetic modified (GM) foods, are aimed at the short- and ...
2015: FDA approves an application for the first genetic modification in an animal for use as food, a genetically engineered salmon. 2016: ...
For instance, papers published recently argue about the basis for the EU's regulation on the GM crops ... The relevance of gene transfer to the safety of food and feed derived from genetically modified (GM) plants. Food and Chemical Toxicology, 42 (7), 1127-1156. 10.1016/j.fct.2004.02.001 ...
Many plants we eat today are a result of genetic modifications that would never occur in nature. Scientists have long been altering the genes of food crops, to boost food production and to make ...
Roughly 94 percent of soybeans grown in the United States are genetically modified, as is more than 90 percent of all corn, canola and sugar beets, together covering roughly 170 million acres of ...
Nearly all the corn and soybeans grown in the U.S. are genetically modified, but only two GM crops, Monsanto's MON810 maize and BASF's Amflora potato, are accepted in the European Union. Ten E.U ...
January 5, 2024 9:30 AM EST. T hirty years after tomatoes became the first genetically modified produce sold in the U.S., lots of people remain skeptical of science-ified foods. In a 2020 Pew ...
GMOs, or genetically modified organisms, can help farmers increase yields, but may also have potential negative effects. Learn the pros and cons and how to identify GMO foods.
In 1971, the first debate over the risks to humans of exposure to GMOs began when a common intestinal microorganism, E. coli, was infected with DNA from a tumor-inducing virus (Devos et al ., 2007 ...
Generally consumers consider that conventional foods (that have an established record of safe consumption over the history) are safe. Whenever novel varieties of organisms for food use are developed using the traditional breeding methods that had existed before the introduction of gene technology, some of the characteristics of organisms may be altered, either in a positive or a negative way.
Genetically modified (GM) technology is a highly controversial topic for today's global food consumer. The commercial development of GM crops began in 1996 with GM corn and has expanded every ...
Quantification of genetically modified organisms (GMOs) in foods Testing on GMOs in food and feed is routinely done using molecular techniques like DNA microarrays or qPCR. These tests are based on screening genetic elements like p35S, tNos, pat, or bar or event specific markers for the official GMOs like Mon810, Bt11, or GT73.
Originally, the genetically modified foods are derived from the organisms. The fact is that, genetic modification is the changing of the DNA code by the means of the genetic engineering, thus, the genes of the organisms are deviating from the normal genes of similar organisms, consequently, these organisms may be regarded as mutants.
The debate over genetically modified foods is both vital and vast, touching on issues that affect our health, environment, and global food supply. Our collection of genetically modified food essay samples is here to guide students through the intricacies of this debate, providing a solid foundation for informed and compelling writing.
These genes make up DNA and determine genetic make up or characteristics of an organism. Advanced understanding of genetic materials has facilitated emergence of genetically modified foods in the world. Genetic modification involves number of steps (Gura, 2000 p412). For instance, to genetically modify a plant, a researcher will have to look ...
On U.S. farmland, acreage planted with genetically engineered crops jumped nearly 25-fold from 3.6 million acres (1.5 million hectares) in 1996 to 88.2 million acres (35.7 million hectares) in ...
Regents Exam in ELA (Common Core) Rating Guide — Jan. '16. [33] The essay introduces a reasonable claim, as directed by the task (I believe that it is right to genetically alter foods). The essay demonstrates some analysis of the texts (The food can be modified to help poorer countries and can reduce some problems within the farms), but ...
Genetically Modified Food Essay Title: Genetically Modified Foods: Balancing Risks and Benefits Introduction: Genetically modified (GM) foods, also known as genetically engineered (GE) foods, have become a contentious issue in the global food system. These foods are produced from crops whose DNA has been altered using genetic engineering techniques to introduce desirable traits such as pest ...
This is an IELTS writing task 2 sample answer essay on the topic of genetically modified foods from the real IELTS exam. ... One of the most important issues facing the world today is a shortage of food and some think genetically modified foods are a possible solution.
Decisive factors influencing consumers behavior. Master Thesis. by Sina Brinkmeier & Jule Persson. Abstract. Genetically modified (GM) food could be a solution to secure the world's food supply, which is in jeopardy due to the ongoing climate crisis. Thus, it is of great importance that consumers would be willing to consume this type of food.
Food choices impact both our health and the environment 1,2.The food system is responsible for about one-third of global anthropogenic GHG emissions 3,4 and climate goals become unattainable ...
The food enzyme glucan 1,4-α-maltohydrolase (4-α-d-glucan α-maltohydrolase; EC 3.2.1.133) is produced with the genetically modified Saccharomyces cerevisiae strain LALL-MA+ by Danstar Ferment AG.The genetic modifications do not give rise to safety concerns. The food enzyme is free from viable cells of the production organism and its DNA.
Share of consumers concerned about genetically modified food in the United Kingdom in 2024, by age group [Graph], Food Standards Agency, July 30, 2024. [Online].