myCBSEguide
- Class 9 Science Case...

Class 9 Science Case Study Questions
Table of Contents
myCBSEguide App
Download the app to get CBSE Sample Papers 2023-24, NCERT Solutions (Revised), Most Important Questions, Previous Year Question Bank, Mock Tests, and Detailed Notes.
If you are wondering how to solve class 9 science case study questions, then myCBSEguide is the best platform to choose. With the help of our well-trained and experienced faculty, we provide solved examples and detailed explanations for the recently added Class 9 Science case study questions.
You can find a wide range of solved case studies on myCBSEguide, covering various topics and concepts. Class 9 Science case studies are designed to help you understand the application of various concepts in real-life situations.
The rationale behind Science
Science is crucial for Class 9 students’ cognitive, emotional, and psychomotor development. It encourages curiosity, inventiveness, objectivity, and aesthetic sense.
In the upper primary stage, students should be given a variety of opportunities to engage with scientific processes such as observing, recording observations, drawing, tabulating, plotting graphs, and so on, whereas in the secondary stage, abstraction and quantitative reasoning should take a more prominent role in science teaching and learning. As a result, the concept of atoms and molecules as matter’s building units, as well as Newton’s law of gravitation, emerges.
Science is important because it allows Class 9 Science students to understand the world around us. It helps to find out how things work and to find solutions to problems at the Class 9 Science level. Science is also a source of enjoyment for many people. It can be a hobby, a career, or a source of intellectual stimulation.
Case study questions in Class 9 Science
The inclusion of case study questions in Class 9 science CBSE is a great way to engage students in critical thinking and problem-solving. By working through real-world scenarios, Class 9 Science students will be better prepared to tackle challenges they may face in their future studies and careers. Class 9 Science Case study questions also promote higher-order thinking skills, such as analysis and synthesis. In addition, case study questions can help to foster creativity and innovation in students. As per the recent pattern of the Class 9 Science examination, a few questions based on case studies/passages will be included in the CBSE Class 9 Science Paper. There will be a paragraph presented, followed by questions based on it.
Examples of Class 9 science class case study questions
Class 9 science case study questions have been prepared by myCBSEguide’s qualified teachers. Class 9 case study questions are meant to evaluate students’ knowledge and comprehension of the material. They are not intended to be difficult, but they will require you to think critically about the material. We hope you find Class 9 science case study questions beneficial and that they assist you in your exam preparation.
The following are a few examples of Class 9 science case study questions.
Class 9 science case study question 1
- due to its high compressibility
- large volumes of a gas can be compressed into a small cylinder
- transported easily
- all of these
- shape, volume
- volume, shape
- shape, size
- size, shape
- the presence of dissolved carbon dioxide in water
- the presence of dissolved oxygen in the water
- the presence of dissolved Nitrogen in the water
- liquid particles move freely
- liquid have greater space between each other
- both (a) and (b)
- none of these
- Only gases behave like fluids
- Gases and solids behave like fluids
- Gases and liquids behave like fluids
- Only liquids are fluids
Answer Key:
- (d) all of these
- (a) shape, volume
- (b) the presence of dissolved oxygen in the water
- (c) both (a) and (b)
- (c) Gases and liquids behave like fluids
Class 9 science case study question 2
- 12/32 times
- 18 g of O 2
- 18 g of CO 2
- 18 g of CH 4
- 1 g of CO 2
- 1 g of CH 4 CH 4
- 2 moles of H2O
- 20 moles of water
- 6.022 × 1023 molecules of water
- 1.2044 × 1025 molecules of water
- (I) and (IV)
- (II) and (III)
- (II) and (IV)
- Sulphate molecule
- Ozone molecule
- Phosphorus molecule
- Methane molecule
- (c) 8/3 times
- (d) 18g of CH 4
- (c) 1g of H 2
- (d) (II) and (IV)
- (c) phosphorus molecule
Class 9 science case study question 3
- collenchyma
- chlorenchyma
- It performs photosynthesis
- It helps the aquatic plant to float
- It provides mechanical support
- Sclerenchyma
- Collenchyma
- Epithelial tissue
- Parenchyma tissues have intercellular spaces.
- Collenchymatous tissues are irregularly thickened at corners.
- Apical and intercalary meristems are permanent tissues.
- Meristematic tissues, in its early stage, lack vacuoles, muscles
- (I) and (II)
- (III) and (I)
- Transpiration
- Provides mechanical support
- Provides strength to the plant parts
- None of these
- (a) Collenchyma
- (b) help aquatic plant to float
- (b) Sclerenchyma
- (d) Only (III)
- (c) provide strength to plant parts
Cracking Class 9 Science Case Study Questions
There is no one definitive answer to Class 9 Science case study questions. Every case study is unique and will necessitate a unique strategy. There are, nevertheless, certain general guidelines to follow while answering case study questions.
- To begin, double-check that you understand the Class 9 science case study questions. Make sure you understand what is being asked by reading it carefully. If you’re unclear, seek clarification from your teacher or tutor.
- It’s critical to read the Class 9 Science case study material thoroughly once you’ve grasped the question. This will provide you with a thorough understanding of the problem as well as the various potential solutions.
- Brainstorming potential solutions with classmates or other students might also be beneficial. This might provide you with multiple viewpoints on the situation and assist you in determining the best solution.
- Finally, make sure your answer is presented simply and concisely. Make sure you clarify your rationale and back up your claim with evidence.
A look at the Class 9 Science Syllabus
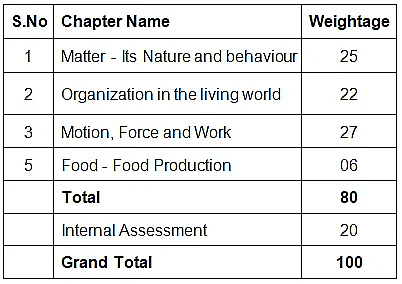
The CBSE class 9 science syllabus provides a strong foundation for students who want to pursue a career in science. The topics are chosen in such a way that they build on the concepts learned in the previous classes and provide a strong foundation for further studies in science. The table below lists the topics covered in the Class 9 Science syllabus of the Central Board of Secondary Education (CBSE). As can be seen, the Class 9 science syllabus is divided into three sections: Physics, Chemistry and Biology. Each section contains a number of topics that Class 9 science students must study during the course.
CBSE Class 9 Science (Code No. 086)
| I | Matter- Its Nature and Behaviour | 25 |
| II | Organization in the Living World | 22 |
| III | Motion, Force and Work | 27 |
| IV | Food; Food Production | 06 |
| 80 | ||
| 20 | ||
| 100 |
Theme: Materials Unit I: Matter-Nature and Behaviour Definition of matter; solid, liquid and gas; characteristics – shape, volume, density; change of state-melting (absorption of heat), freezing, evaporation (cooling by evaporation), condensation, sublimation. Nature of matter: Elements, compounds and mixtures. Heterogeneous and homogenous mixtures, colloids and suspensions. Particle nature and their basic units: Atoms and molecules, Law of constant proportions, Atomic and molecular masses. Mole concept: Relationship of mole to mass of the particles and numbers. Structure of atoms: Electrons, protons and neutrons, valency, the chemical formula of common compounds. Isotopes and Isobars.
Theme: The World of the Living Unit II: Organization in the Living World Cell – Basic Unit of life: Cell as a basic unit of life; prokaryotic and eukaryotic cells, multicellular organisms; cell membrane and cell wall, cell organelles and cell inclusions; chloroplast, mitochondria, vacuoles, endoplasmic reticulum, Golgi apparatus; nucleus, chromosomes – basic structure, number. Tissues, Organs, Organ System, Organism: Structure and functions of animal and plant tissues (only four types of tissues in animals; Meristematic and Permanent tissues in plants).
Theme: Moving Things, People and Ideas Unit III: Motion, Force and Work Motion: Distance and displacement, velocity; uniform and non-uniform motion along a straight line; acceleration, distance-time and velocity-time graphs for uniform motion and uniformly accelerated motion, derivation of equations of motion by graphical method; elementary idea of uniform circular motion. Force and Newton’s laws: Force and Motion, Newton’s Laws of Motion, Action and Reaction forces, Inertia of a body, Inertia and mass, Momentum, Force and Acceleration. Elementary idea of conservation of Momentum. Gravitation: Gravitation; Universal Law of Gravitation, Force of Gravitation of the earth (gravity), Acceleration due to Gravity; Mass and Weight; Free fall. Floatation: Thrust and Pressure. Archimedes’ Principle; Buoyancy. Work, energy and power: Work done by a Force, Energy, power; Kinetic and Potential energy; Law of conservation of energy. Sound: Nature of sound and its propagation in various media, speed of sound, range of hearing in humans; ultrasound; reflection of sound; echo.
Theme: Food Unit IV: Food Production Plant and animal breeding and selection for quality improvement and management; Use of fertilizers and manures; Protection from pests and diseases; Organic farming.
PRESCRIBED BOOKS:
- Science-Textbook for class IX-NCERT Publication
- Assessment of Practical Skills in Science-Class IX – CBSE Publication
- Laboratory Manual-Science-Class IX, NCERT Publication
- Exemplar Problems Class IX – NCERT Publication
myCBSEguide: A true helper
There are numerous advantages to using myCBSEguide to achieve the highest results in Class 9 Science.
- myCBSEguide offers high-quality study materials that cover all of the topics in the Class 9 Science curriculum.
- myCBSEguide provides practice questions and mock examinations to assist students in the best possible preparation for their exams.
- On our myCBSEguide app, you’ll find a variety of solved Class 9 Science case study questions covering a variety of topics and concepts. These case studies are intended to help you understand how certain principles are applied in real-world settings
- myCBSEguide is that the study material and practice problems are developed by a team of specialists who are always accessible to assist students with any questions they may have. As a result, students may be confident that they will receive the finest possible assistance and support when studying for their exams.
So, if you’re seeking the most effective strategy to study for your Class 9 Science examinations, myCBSEguide is the place to go!
Test Generator
Create question paper PDF and online tests with your own name & logo in minutes.
Question Bank, Mock Tests, Exam Papers, NCERT Solutions, Sample Papers, Notes
Related Posts
- Competency Based Learning in CBSE Schools
- Class 11 Physical Education Case Study Questions
- Class 11 Sociology Case Study Questions
- Class 12 Applied Mathematics Case Study Questions
- Class 11 Applied Mathematics Case Study Questions
- Class 11 Mathematics Case Study Questions
- Class 11 Biology Case Study Questions
- Class 12 Physical Education Case Study Questions
Leave a Comment
Save my name, email, and website in this browser for the next time I comment.
- CBSE Class 9 Study Material
CBSE Class 9 Science Important Case Study Questions with Answers for Term 2 Exam 2022 (PDF)
Check important case study questions of cbse class 9 science to prepare for the cbse term 2 exam 2022. all these questions have been put together by subject experts..

CBSE Class 9 Term 2 Exam 2022: Important case based questions for CBSE Class 9 Science are provided here students to prepare for the upcoming Term 2 Exam 2022. All the questions provided below are curated by the subject experts. These questions are really helpful to revise important concepts and prepare the case study questions for the exam. Answers to all questions have been provided for reference. So, students should practice the chapter-wise questions to clearly understand the right way to attempt the case based questions. Download the chapter-wise questions in PDF.
Check some of the important case study questions below:
Q. Read the following and answer the questions :
A student was asked by his teacher to verify the law of conservation of mass in the laboratory. He prepared 5% aqueous solutions of NaCl and Na 2 SO 4 . He mixed 10 mL of both these solutions in a conical flask. He weighed the flask on a balance. He then stirred the flask with a rod and weighed it after sometime. There was no change in mass.
- Was the student able to verify the law of conservation of mass?
- If not, what was the mistake committed by him?
- In your opinion, what he should have done?
- What is the molar mass of Na 2 SO 4 ?
- No, he could not verify the law of conservation of mass in-spite of the fact that there was no change in mass.
- No chemical reaction takes place between NaCl and Na 2 SO 4 . This means that no reaction actually took place in the flask.
- He should have performed the experiment by using aqueous solutions of BaCl 2 and Na 2 SO 4 . A chemical reaction takes place in this case and a white precipitate of BaSO 4 is formed.
- Will the weight of the precipitate be the same as that of the reactants before mixing?
- If not, what she should have done?
- Which law of chemical combination does this support?
- State the law of conservation of mass.
- No, it will not be the same.
- She should have weighed the total contents of the beaker after the reaction and not the precipitate alone.
- It supports the law of conservation of mass.
- Mass can neither be created nor destroyed during a chemical reaction.
Get here latest School , CBSE and Govt Jobs notification and articles in English and Hindi for Sarkari Naukari , Sarkari Result and Exam Preparation . Download the Jagran Josh Sarkari Naukri App .
- SSC MTS Exam Date 2024
- UGC NET Admit Card 2024
- Har Ghar Tiranga Campaign UPSC
- UGC NET City Intimation Slip 2024
- UP Police Constable Mock Test
- Independence Day Poems
- Independence Day Speech in Hindi
- Independence Day Drawing
- Independence Day Speech
- India Post GDS Cut Off
- Education News
- CBSE Study Material
- CBSE Class 9
Latest Education News
UP Police Exam City Slip 2024: Download UPPRPB Constable Pre Admit Card at uppbpb.gov.in
Bihar Sakshamta Pariksha Phase 2 Admit Card 2024: आज जारी होगा बिहार सक्षमता परीक्षा फेज 2 एडमिट कार्ड, 23 अगस्त को होगा एग्जाम
MJPRU Result 2024 OUT at mjpruiums.in; Download UG and PG Marksheet PDF
CCSU Result 2024 OUT at ccsuniversity.ac.in; Direct Link to Download Even Semester UG and PG Marksheet
eShram Card: क्या है ई-श्रम कार्ड? लाभ, पात्रता और ऑनलाइन अप्लाई की सभी डिटेल्स यहां देखें, e-shram Card Download का तरीका
Olympic Games 2026-2034: अगला ओलंपिक गेम कब और किस शहर में किया जायेगा आयोजित? जानें यहां
CRPF Full Form With All Details: Roles and Responsibilities, History, Symbol
SBI का ATM लगवाने के नियम, प्रक्रिया और जरुरी डाक्यूमेंट्स के बारें में जानें यहां
Highlights of PM Modi’s Speech: महिलाओं के खिलाफ अपराध के लिए दी गई सजा का प्रचार-प्रसार किया जाना चाहिए- पीएम मोदी
ISC Biotechnology Specimen Paper 2024-25: CISCE Class 12 Biotechnology Sample Paper, Download PDF
JSSC Stenographer Recruitment 2024 Notification Released for 455 Vacancies, Check Details
BPSC TRE 3.0 OMR Sheet 2024 at bpsc.bih.nic.in: Raise Objection till Aug 28
India Post GDS Selection Process 2024 for 44228 Vacancies: Know the Selection Criteria for Gramin Dak Sevak Posts
MP Board Class 10 Social Science Syllabus 2024-25: Download Free Syllabus PDF!
BPSC TRE 3.0 OMR Sheet 2024 OUT: बिहार शिक्षक भर्ती परीक्षा 3.0 की OMR Sheet जारी, यहां से डाउनलोड करें PDF
NEET UG Counselling 2024 Choice Filling Begin at mcc.nic.in, Get Direct Link Here
रक्षाबंधन पर निबंध हिंदी में - Raksha Bandhan Essay in Hindi
TNPSC CTS Recruitment 2024: Apply For 861 Diploma / ITI Level Posts, Check Application Process And Others Details
MAT 2024 CBT Mode Admit Card Out at mat.aima.in, Get Direct Link Here to Download
MP Board Class 10 Latest Syllabus 2024-25: Download Subject-Wise Pdfs For Free
CBSE Case Study Questions for Class 9 Science - Pdf PDF Download
| 1 Crore+ students have signed up on EduRev. Have you? |
CBSE Case Study Questions for Class 9 Science
Case based questions for Class 9 Science involve exploring a real-world situation through scientific analysis and inquiry. These questions allow students to make connections between science concepts and the world around them, as well as develop critical thinking skills. For example, a case study may involve challenging a student to determine the cause of an illness in a local population by researching the disease, its symptoms, and the local environment. Through this exercise, students learn how to identify a problem, break it down into parts, and come up with a solution that is supported by evidence. This type of question helps students to understand how science is at the centre of solving real-world problems.
Chapter Wise Case Based Questions for Class 9 Science
Chapter-wise case-based questions for Class 9 Science are a set of questions based on specific chapters or topics covered in the science textbook. These questions are designed to help students apply their understanding of scientific concepts to real-world situations and events.
The CBSE Class 9 Case Based Questions can be accessed from Chapetrwise Links provided below:
Chapter 1: Matter In Our Surroundings
Chapter 2: is matter around us pure.
- Case Based Questions: Is Matter Around Us Pure?
Chapter 3: Atoms And Molecules
- Case Based Questions: Atoms And Molecules
Chapter 4: Structure Of The Atom
- Case Based Questions: Structure Of The Atom
Chapter 5: The Fundamental Unit Of Life
- Case Based Questions: The Fundamental Unit Of Life- 1
- Case Based Questions: The Fundamental Unit Of Life- 2
Chapter 6: Tissues
- Case Based Questions: Tissues- 1
- Case Based Questions: Tissues- 2
Chapter 7: Motion
- Case Based Questions: Motion-1
- Case Based Questions: Motion- 2
Chapter 8: Force And Laws Of Motion
- Case Based Questions: Force And Laws Of Motion
Chapter 9: Gravitation
- Case Based Questions: Gravitation
Chapter 10: Work And Energy
- Case Based Questions: Work And Energy- 1
- Case Based Questions: Work And Energy- 2
Chapter 11: Diversity In Living Organisms
Chapter 12: sound, chapter 13: natural resources, chapter 14: improvement in food resource, chapter 15: why do we fall ill.
- Case Based Questions: Why Do We Fall Ill?
Weightage of Case Based Questions in Class 9 Science
Why are Case Study Questions important in Science Class 9?
- Enhance critical thinking: Case study questions require students to analyze a real-life scenario and think critically to identify the problem and come up with possible solutions. This enhances their critical thinking and problem-solving skills.
- Apply theoretical concepts: Case study questions allow students to apply theoretical concepts that they have learned in the classroom to real-life situations. This helps them to understand the practical application of the concepts and reinforces their learning.
- Develop decision-making skills: Case study questions challenge students to make decisions based on the information provided in the scenario. This helps them to develop their decision-making skills and learn how to make informed decisions.
- Improve communication skills: Case study questions often require students to present their findings and recommendations in written or oral form. This helps them to improve their communication skills and learn how to present their ideas effectively.
- Enhance teamwork skills: Case study questions can also be done in groups, which helps students to develop teamwork skills and learn how to work collaboratively to solve problems.
In summary, case study questions are important in Class 9 because they enhance critical thinking, apply theoretical concepts, develop decision-making skills, improve communication skills, and enhance teamwork skills. They provide a practical and engaging way for students to learn and apply their knowledge and skills to real-life situations.
Class 9 Science Curriculum at Glance
The Class 9 Science curriculum in India covers a wide range of topics and concepts. Here is a brief overview of the Science curriculum at a glance:
- Physics: The Physics section includes topics such as motion, force, work and energy, sound, and light.
- Chemistry: The Chemistry section includes topics such as matter, atoms and molecules, structure of the atom, and chemical reactions.
- Biology: The Biology section includes topics such as cell structure and functions, tissues, diversity in living organisms, natural resources, and environmental management.
- Practical Work: The Science curriculum also includes practical work, where students perform experiments to observe and understand scientific phenomena.
The Class 9 Science curriculum is designed to provide a strong foundation in science and prepare students for higher education in the field. The curriculum is structured to develop critical thinking, problem-solving, and analytical skills, and to promote the application of scientific concepts in real-life situations. The curriculum is also designed to help students prepare for competitive exams and develop a strong scientific base for future academic and professional pursuits.
Students can also access Case Based Questions of all subjects of CBSE Class 9
- Case Based Questions for Class 9 Maths
- Case Based Questions for Class 9 Social Science
- Case Based Questions for Class 9 English
- Case Based Questions for Class 9 Hindi
- Case Based Questions for Class 9 Sanskrit
Frequently Asked Questions (FAQs) on Case Based Questions for Class 9 Science
Are case-based questions on the class 9 science exam.
Yes, case-based questions are often included in science exams at the class 9 level as they test students' ability to apply their scientific knowledge and skills to real-world situations.
How are case-based questions different from traditional science questions?
Traditional science questions typically focus on testing students' knowledge of specific facts, concepts, and theories. Case-based questions, on the other hand, require students to use their knowledge and understanding to analyze and interpret real-world situations and make informed decisions.
How can students prepare for case-based questions in science?
To prepare for case-based questions in science, students should practice analyzing data and interpreting scientific experiments. They should also work on developing their critical thinking and problem-solving skills.
Top Courses for Class 9
FAQs on CBSE Case Study Questions for Class 9 Science - Pdf
| 1. What are case study questions in Class 9 Science? |
| 2. How are case study questions helpful in Class 9 Science? |
| 3. What is the weightage of case study questions in Class 9 Science? |
| 4. How can students prepare for case study questions in Class 9 Science? |
| 5. Can you provide an example of a case study question in Class 9 Science? |
| Views | |
| Last updated |
Extra Questions
Practice quizzes, sample paper, cbse case study questions for class 9 science - pdf, viva questions, mock tests for examination, study material, shortcuts and tricks, semester notes, important questions, video lectures, past year papers, previous year questions with solutions, objective type questions.

CBSE Case Study Questions for Class 9 Science - Pdf Free PDF Download
Importance of cbse case study questions for class 9 science - pdf, cbse case study questions for class 9 science - pdf notes, cbse case study questions for class 9 science - pdf class 9, study cbse case study questions for class 9 science - pdf on the app.
| cation olution |
| Join the 10M+ students on EduRev |
Welcome Back
Create your account for free.

Forgot Password
Unattempted tests, change country, practice & revise.
CBSE Expert

Case Study Questions of Chapter 2 Is Matter Around Us Pure? PDF Download
Case study Questions on Class 9 Science Chapter 2 are very important to solve for your exam. Class 9 Science Chapter 2 Case Study Questions have been prepared for the latest exam pattern. You can check your knowledge by solving case study-based questions for Class 9 Science Chapter 2 Is Matter Around Us Pure?

In CBSE Class 9 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage-based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked.
Is Matter Around Us Pure? Case Study Questions With answers
Here, we have provided case-based/passage-based questions for Class 9 Science Chapter 2 Is Matter Around Us Pure?
Case Study/Passage-Based Questions
Question 1:
Akshita wants to separate the mixture of dyes constituting a sample of ink. She marked a line by the ink on the filter paper and placed the filter paper in a glass containing water as shown in the figure. The filter paper was removed when the water moved near the top of the filter paper.

(i) Identify the technique used by the Akshita. (a) Sedimentation (b) Filtration (c) Chromatography (d) Distillation
Answer: (c) Chromatography.
(ii) What would you expect to see, if the ink contains three different coloured components? (a) We will not see any band on the filter paper. (b) We would see three bands on the filter paper at various lengths. (c) We would see infinite bands on the filter paper. (d) We would see the single band on the filter paper.
Answer: (b) The components of the ink will travel with water and we would see three bands on the filter paper at various lengths.
(iii) An application where you can use this technique is: (a) To separate salt from sand (b) To separate the wheat from the husk (c) To separate oil from water (d) To separate drugs from the blood.
Answer: (d) To separate drugs from blood.
(iv) The above process is used for the separation of : (a) insoluble substances (b) single solute that dissolves in the soluble solvent. (c) solutes that dissolve in the same solvent. (d) solutes that dissolve in the different solvents.
Answer: (c) For the separation of those solutes that dissolve in the same solvent.
(v) What is chromatography? (a) It is an agricultural method to separate grains (b) A method to separate magnetic impurities from non-magnetic impurities
(c) The process of separating the suspended particles of an insoluble substance (d) Method of separating and identifying various components in a mixture, which are present in small trace quantities.
Answer: (d) Method of separating and identifying various components in a mixture, which are present in small trace quantities.
Question 2:
A homogeneous mixture of two or more substances is called a true solution. it consists of solute and solvent. The particle size of the true solution is less than 1 nanometer. A suspension is a heterogeneous mixture in which the solute particle does not dissolve but remains suspended throughout the bulk of the medium. A colloid is a mixture that is actually heterogeneous but appears to be homogeneous as the particles are uniformly spread throughout the solution.
(i) which one of the following is most stable?
A)True solution
B)Suspensions
D) both A and B
Answer: A)True solution
ii) which type of mixture can be separated by filtration?
D)All of these
Answer: B)Suspensions
iii) which statement is incorrect about the Tyndall effect. *
A)True solution shows Tyndall effect
B)Suspensions show the Tyndall effect
C)Colloid show Tyndall effect
D)Both B and C show the Tyndall effect
Answer: A)True solution shows Tyndall effect
iv) Which is the correct order of stability of solution *
A) True < Colloid<Suspension
B)Colloid<Suspension<True
C)Colloid<True<Suspension
D)Suspension<Colloid<True
Answer: D)Suspension Hope the information shed above regarding Case Study and Passage Based Questions for Class 9 Science Chapter 2 Is Matter Around Us Pure? with Answers Pdf free download has been useful to an extent. If you have any other queries about CBSE Class 9 Science Is Matter Around Us Pure? Case Study and Passage Based Questions with Answers, feel free to comment below so that we can revert back to us at the earliest possible Save my name, email, and website in this browser for the next time I comment. Key Features No thanks, I’m not interested!Leave a Comment Cancel reply
Download India's best Exam Preparation App Now.

Class 9 Science Case Study Questions Chapter 3 Atoms and Molecules
- Post author: studyrate
- Post published:
- Post category: class 9th
- Post comments: 0 Comments
Case study Questions in Class 9 Science Chapter 3 are very important to solve for your exam. Class 9 Science Chapter 3 Class 9 Science Case Study Questions have been prepared for the latest exam pattern. You can check your knowledge by solving case study-based questions for Class 9 Science Chapter 3 Atoms and Molecules
Join our Telegram Channel, there you will get various e-books for CBSE 2024 Boards exams for Class 9th, 10th, 11th, and 12th.

In CBSE Class 9 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage-based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked.
Atoms and Molecules Case Study Questions With Answers
Here, we have provided case-based/passage-based questions for Class 9 Science Chapter 3 Atoms and Molecules
Case Study/Passage-Based Questions
Case Study 1: The knowledge of the valencies of various radicals helps us to write the formulae of chemical compounds. The total positive charge on positive ions (cations) is equal to the total negative charge on negative ions (anions) in a molecule. Therefore, in writing the formula of a compound, the positive and negative ions are adjusted in such a way that the total number of positive charges of positive ions (cations) becomes equal to the total number of negative charges of negative ions (anions). There is another simple method for writing the formulae of ionic compounds. In this method, the valencies (or positive or negative charges) of the ions can be ‘crossed over’ to give subscripts. The purpose of crossing over of charges is to find the number of ions required to equalise the number of positive and negative charges.
Element X has two valencies 5 and 3 and Y has valency 2. The elements X and Y are most likely to be respectively (a) copper and sulphur (b) sulphur and iron (c) phosphorus and fluorine (d) nitrogen and iron.
Answer: (d) nitrogen and iron.
The formula of the sulphate of an element X is X 2 (SO 4 ) 3 . The formula of nitride of element X will be (a) X 2 N (b) XN 2 (c) XN (d) X 2 N 3
Answer: (c) XN
The formula of a compound is X 3 Y. The valencies of elements X and Y will be respectively (a) 1 and 3 (b) 3 and 1 (c) 2 and 3 (d) 3 and 2
Answer: (a) 1 and 3
Case Study/Passage Based Questions
Case Study 2: A mole of an atom is a collection of atoms whose total mass is the number of grams equal to the atomic mass. Since an equal number of moles of different elements contain an equal number of atoms it becomes convenient to express the amounts of the elements in terms of moles. A mole represents a definite number of particles viz, atoms, molecules, ions or electrons. This definite number is called the Avogadro number or Avogadro constant which is equal to 6.022 × 1023. Hence a mole represents 6.022 × 1023 particles of the substance. One mole of a substance represents one gram-formula of the substance. One mole of a gas at standard temperature and pressure occupies 22.4 litres.
How many grams of sodium must be taken to get 1 mole of the element? (a) 23 g (b) 35.5 g (c) 63.5 g (d) 46 g
Answer: (a) 23 g
What is the mass in grams of a single atom of chlorine? (Atomic mass of chlorine = 35.5) (a) 6.54 × 10 23 g (b) 5.9 × 10 –23 g (c) 0.0025 g (d) 35.5 g
Answer: (b) 5.9 × 10–23 g
How many number of moles are there in 5.75 g of sodium ? (Atomic mass of sodium = 23) (a) 0.25 (b) 0.5 (c) 1 (d) 2.5
Answer: (a) 0.25
What is the mass in grams of 2.42 mol of zinc? (Atomic mass of Zn = 65.41) (a) 200 g (b) 25 g (c) 85 g (d) 158 g
Answer: (d) 158 g
Case Study 3: According to Dalton’s atomic theory, all matter whether an element, a compound, or a mixture is composed of small particles called atoms which can neither be created nor destroyed during a chemical reaction. Dalton’s theory provides a simple explanation for the laws of chemical combination. He used his theory to explain the law of conservation of masses, the law of constant proportions, and the law of multiple proportions, based on various postulates of the theory. Dalton was the first scientist to use the symbols for the elements in a very specific sense. When he used a symbol for an element he also meant a definite quantity of that element, that is one atom of that element.
Which postulate of Dalton’s atomic theory is the result of the law of conservation of mass? (a) Atoms can neither be created nor destroyed. (b) Each element is composed of extremely small particles called atoms. (c) All the atoms of a given element are identical. (d) During chemical combination, atoms of different elements combine in simple ratios.
Answer: (a) Atoms can neither be created nor destroyed.
Which postulate of Dalton’s atomic theory explains law of definite proportions? (a) Atoms of an element do not change during a chemical reaction. (b) An element consists of atoms having fixed mass and the number and kind of atoms in a given compound is fixed. (c) Different elements have different kind of atoms. (d) Atoms are of various kinds
Answer: (b) An element consists of atoms having fixed mass and the number and kind of atoms in a given compound is fixed.
“If 100 g of calcium carbonate (whether in the form of marble or chalk) is decomposed, 56 g of calcium oxide and 44 g of carbon dioxide are formed.” Which law of chemical combination is illustrated by this statement? (a) Law of constant proportions (b) Law of conservation of mass (c) Law of multiple proportions (d) Law of conservation of energy
Answer: (b) Law of conservation of mass
When 5 g calcium is burnt in 2 g oxygen, 7 g of calcium oxide is produced. When 5 g of calcium is burnt in 20 g of oxygen, then also 7 g of calcium oxide is produced. Which law of chemical combination is being followed? (a) Law of conservation of mass (b) Law of multiple proportions (c) Law of constant proportions (d) No law is being followed.
Answer: (c) Law of constant proportions
Case Study 4: Atoms and molecules are the building blocks of matter. An atom is the smallest unit of an element that retains its chemical properties, while a molecule is a group of two or more atoms held together by chemical bonds. Atoms consist of a positively charged nucleus, which contains protons and neutrons, surrounded by negatively charged electrons in energy levels or shells. The number of protons in an atom determines its atomic number and defines its unique identity as an element. The electrons in an atom occupy specific energy levels, and the outermost shell is known as the valence shell. Atoms gain, lose, or share electrons to achieve a stable electron configuration, forming chemical bonds and giving rise to molecules. Understanding the concept of atoms and molecules is crucial for comprehending various chemical reactions and the composition of substances.
What is the smallest unit of an element that retains its chemical properties? a) Proton b) Electron c) Nucleus d) Atom Answer: d) Atom
What is a group of two or more atoms held together by chemical bonds called? a) Element b) Compound c) Molecule d) Nucleus Answer: c) Molecule
What are the positively charged particles present in the nucleus of an atom called? a) Electrons b) Protons c) Neutrons d) Valence electrons Answer: b) Protons
Which part of an atom contains electrons in energy levels or shells? a) Protons b) Neutrons c) Nucleus d) Valence shell Answer: d) Valence shell
What do atoms do to achieve a stable electron configuration? a) Gain, lose, or share electrons b) Absorb protons c) Increase their atomic number d) Create chemical bonds Answer: a) Gain, lose, or share electrons
Hope the information shed above regarding Case Study and Passage Based Questions for Class 9 Science Chapter 3 Atoms and Molecules with Answers Pdf free download has been useful to an extent. If you have any other queries about the CBSE Class 9 Science Atoms and Molecules Case Study and Passage-Based Questions with Answers, feel free to comment below so that we can revert back to us at the earliest possible By Team Study Rate
You Might Also Like
Class 9 maths case study questions of chapter 2 polynomials pdf download, mcq questions of class 9 maths chapter 13 surface areas and volumes with answers, class 9 geography case study questions chapter 5 natural vegetation and wildlife, leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.
- Work and Energy Class 9 Case Study Questions Science Chapter 10

Last Updated on August 13, 2024 by XAM CONTENT
Hello students, we are providing case study questions for class 9 science. Case study questions are the new question format that is introduced in CBSE board. The resources for case study questions are very less. So, to help students we have created chapterwise case study questions for class 9 science. In this article, you will find case study questions for cbse class 9 science chapter 10 Work and Energy.
| Work and Energy | |
| Case Study Questions | |
| Competency Based Questions | |
| CBSE | |
| 9 | |
| Science | |
| Theme | Moving Things, People and Ideas |
| Class 9 Studying Students | |
| Yes | |
| Mentioned | |
Table of Contents
Case Study Questions on Work and Energy
Question 1:
Work is said to be done when the force applied on an object produces a displacement of the object in the direction of force applied. For example, when we push or pull a heavy load or lift it above the floor then we are doing work, but a man carrying heavy load and standing still is not doing any work. Work, which is the product of force and displacement, has only magnitude and no direction. So, it is a scalar quantity.
Read the given passage carefully and give the answer of the following questions:
Q 1. A man raises a box of mass 50 kg to a height of 2 m in 10 s, while another man raises the same box to the same height in 50 s. What is the ratio of work done by them?
Difficulty Level: Medium
Q 2. If force and displacement of the particle (in direction of force) are doubled, what should be the amount of work?
Difficulty Level: Easy
Q 3. A coolie lifts a luggage of 10 kg from the ground and put it on his head 1.8 m above the ground. What would be the work done by him on the luggage?
Q 4. A student carries a bag weighing 5 kg from the ground floor to his class on the first floor that is 2 m high. What is the work done by the boy?
Q 5. Calculate the value of work done in holding a suitcase of 15 kg while waiting for a bus for 40 minutes.
1. We know that, $W=F \times s \Rightarrow$ Work done is independent of time taken. Hence, in both the cases, $W=$ $50 \times 10 \times 2=1000 \mathrm{~J}$ Thus, ratio of work done $=1000: 1000=1: 1$
2. The work should be 4 times.
3. Mass of luggage, $m=10 \mathrm{~kg}$ and displacement, $s=1.8 \mathrm{~m}$ Work done, $W=F \times s=m g \times s$ $$ =10 \times 10 \times 1.8=180 \mathrm{~J} $$
4. Here, mass of bag, $m=5 \mathrm{~kg}$ and displacement, $s=2 \mathrm{~m}$ Work done, $W=F \times s=m g \times s$ $$ =5 \mathrm{~kg} \times 10 \mathrm{~m} \mathrm{~s}^{-2} \times 2 \mathrm{~m}=100 \mathrm{~J} $$
5. Displacement in holding a suitcase while waiting for a bus, i.e., stationary position $=0$ $$ \begin{aligned} & \therefore \quad \text { Work done }=\text { force } \times \text { zero } \\ & \quad \Rightarrow \text { Work done }=\text { zero. } \end{aligned} $$
Case study questions for other chapters of class 9 science is given below.
- Gravitation Class 9 Case Study Questions Science Chapter 9
- Force and Laws of Motion Class 9 Case Study Questions Science Chapter 8
- Motion Class 9 Case Study Questions Science Chapter 7
- Tissues Class 9 Case Study Questions Science Chapter 6
- The Fundamental Unit of Life Class 9 Case Study Questions Science Chapter 5
Is Matter Around Us Pure Class 9 Case Study Questions Science Chapter 2
Matter in our surroundings class 9 case study questions science chapter 1.
We hope the given case study questions for Work and Energy Class 9 helps you in your learning.
Helpful Links for CBSE Class 9 Science Preparation
- Chapter Tests for CBSE Class 9 Science
- Worksheets for CBSE Class 9 Science
- 100 Important Numerical Problems for CBSE Class 9 Physics
- 65 Important Case Study Questions for CBSE Class 9 Science
Topics from which case study questions may be asked
- Work done by a Force
- Kinetic and Potential energy
- Law of conservation of energy (excluding commercial unit of Energy).
Work is said to be done when the force applied on the body displaces its position in the direction of the applied force.
Conditions for Work to be Done:
- A force should act on the body.
- The body must be displaced from its position.
Work Done by a Constant Force: It is the product of the force and the distance moved by the body in the direction of the applied force, i.e., W = F x s. It is a scalar quantity.
SI Unit of Work: The SI unit of work is Newton-metre (N-m) or Joule (J).
1 Joule: It is the amount of work done on a body when a force of 1 N displaces it by 1 m along the line of action of the force.
Frictional force acts in the direction opposite to the direction of displacement, so work done by friction will be negative.
Energy is the capacity or the ability of the body to do work. Its SI unit is Joule (J) and is a scalar quantity.
Forms of Energy: There are various forms of energy such as kinetic energy, potential energy, etc.
Heavy objects which are moving with a high speed possess more kinetic energy as compared to smaller objects moving with less speed.
For further practice on case study questions related to Gravitation Class 9 Science, we recommend exploring the link given below.
| Work and Energy | |
| CBSE Class 9 Students | |
| MCQs | |
| 30 | |
| Instant Solutions after Completion of Quiz | |
| Free |
How to take quiz or test using the given link
It’s quite simple!
Step 1: Click on the given link. You will see the below screen.
Step 2: Fill in the necessary details. There is no need to register. Just fill your email and name and click on the button “Take Assessment”. The below screen will appear.
Step 3: Click on start assessment. Now you are ready to take test.
Frequently Asked Questions (FAQs) on Work and Energy Case Study Questions
Q1: what are case study questions for cbse examinations.
A1: Case study questions in CBSE examinations typically involve scenarios or real-life examples, requiring students to apply their understanding of concepts to solve problems or analyze situations.
Q2: Why are case study questions important for understanding class 9 science chapters?
A2: Case study questions provide a practical context for students to apply theoretical knowledge to real-world situations, fostering deeper understanding and critical thinking skills.
Q3: How should students approach answering case study questions for CBSE?
A3: Students should carefully read the case study, identify the key issues or problems presented, analyze the information provided, apply relevant concepts and principles of chemical reactions and equations, and formulate well-supported solutions or responses.
Q4: Are there any resources available online for students to practice case study questions on class 9 science chapters for CBSE exams?
A4: Yes, several educational websites offer case study questions for CBSE students preparing for science examinations. We also offer a collection of case study questions for all classes and subject on our website. Visit our website to access these questions and enhance your learning experience. If you need more case study questions for your preparation, then you visit Physics Gurukul website.
Q5: How can students effectively prepare for case study questions on “Gravitation” for CBSE exams?
A5: Effective preparation strategies include regular revision of concepts, solving practice questions, analyzing case studies from previous exams, seeking clarification on doubts, and consulting with teachers or peers for guidance and support.
Q6: How can teachers incorporate case study questions on “Gravitation” class 9 science into classroom teaching?
A6: Teachers can integrate case studies into lesson plans, group discussions, or interactive activities to engage students in active learning, promote problem-solving skills, and facilitate a deeper understanding of “Force and Laws of Motion”.
Q7: When is work done by a force zero?
A7: Work done by a force is zero when the direction of force and displacement of an object are perpendicular to each other or when the displacement is zero.
Q8: A student sitting in a class does his examination paper in three hours. How much work is done by the student?
A8: No work is done by the student as there is no displacement.
Q9: What kind of energy transformations take place at a thermal power station?
A9: At a thermal power station, the chemical energy of coal is changed into heat energy which is further changed into electrical energy.
Q10: When a body is thrown upwards, its velocity becomes zero at the highest point. What will be its acceleration at this point?
A10: The acceleration at this point is equal to the value of g in the downward direction
Q11: A bullet is fired from a gun. Which will have a greater kinetic energy; the bullet or the gun?
A11: The bullet will possess greater kinetic energy.
Q12: Two identical objects, one of iron and the other of wood, are dropped from the same height on sand. Which will penetrate more and why?
A12: Iron object will penetrate more as it has more potential energy because of its greater mass.
Download Customised White Label Study Materials in MS Word Format
We are providing teaching resources to teachers and coaching institute looking for customised study materials in MS word format. Our High-quality editable study material which is prepared by the expert faculties are Highly useful for Teachers, Mentors, Tutors, Faculties, Coaching Institutes, Coaching Experts, Tuition Centers.

Related Posts
Case Study Questions Class 9 Science Matter in our Surroundings
Case study questions class 9 science chapter 1 matter in our surroundings.
CBSE Class 9 Case Study Questions Science Matter in our Surroundings. Important Case Study Questions for Class 9 Exam. Here we have arranged some Important Case Base Questions for students who are searching for Paragraph Based Questions Matter in our Surroundings.
CBSE Case Study Questions Class 9 Science – Matter in our Surroundings
Case study 1:.
Answer the following questions by referring above paragraph.
ii.) Thoughts coming in our mind are example of matter. True or false
b.) Particles of matter are continuously moving
Case Study 2:
Solids have a definite shape, distinct boundaries and fixed volumes, that is, have negligible compressibility. Solids have a tendency to maintain their shape when subjected to outside force. Solids may break under force but it is difficult to change their shape, so they are rigid.
Gas has very low density hence are light. Gas can flow easily and hence are called fluid.
Case Study 3:
Particles start vibrating with greater speed. The energy supplied by heat overcomes the forces of attraction between the particles. The particles leave their fixed positions and start moving more freely. A stage is reached when the solid melts and is converted to a liquid. The minimum temperature at which a solid melts to become a liquid at the atmospheric pressure is called its melting point.
b.) Deposition
c.) Boiling point
iv.) Define melting point and boiling point
Case Study 4:
a.) Boiling point
d.) None of these
c.) They are light weight as compare to other particles
a.) From the surface
Case Study 5:
i.) Evaporation is surface phenomenon. True or false
c.) Does not have any effect from wind speed
Leave a Reply Cancel reply
We have a strong team of experienced teachers who are here to solve all your exam preparation doubts, rs aggarwal class 5 solutions chapter 17 – basic geometrical concepts, sikkim scert class 4 english chapter 4b no one plays with me, granny solution, maharashtra board class 4 english chapter 8 a great leader solution, maharashtra board class 4 english chapter 5 six honest serving-men solution.

Gurukul of Excellence
Classes for Physics, Chemistry and Mathematics by IITians
Join our Telegram Channel for Free PDF Download
Case Study and Passage Based Questions for Class 9 Science Chapter 2 Is Matter Around Us Pure?
- Last modified on: 2 years ago
- Reading Time: 4 Minutes
Case Study Questions:
Question 1:
Akshita wants to separate the mixture of dyes constituting a sample of ink. She marked a line by the ink on the filter paper and placed the filter paper in a glass containing water as shown in figure. The filter paper was removed when the water moved near the top of the filter paper.

(i) Identify the technique used by the Akshita. (a) Sedimentation (b) Filtration (c) Chromatography (d) Distillation
(ii) What would you expect to see, if the ink contains three different coloured components? (a) We will not see any band on the filter paper. (b) We would see three bands on the filter paper at various lengths. (c) We would see infinite bands on the filter paper. (d) We would see single band on the filter paper.
(iii) An application where you can use this technique is: (a) To separate salt from sand (b) To separate wheat from husk (c) To separate oil from water (d) To separate drugs from blood.
(iv) The above process is used for the separation of : (a) insoluble substances (b) single solute that dissolves in soluble solvent. (c) solutes that dissolve in the same solvent. (d) solutes that dissolve in the different solvents.
(v) What is chromatography ? (a) It is an agricultural method to separate grains (b) A method to separate magnetic impurities from non-magnetic impurities (c) The process of separating the suspended particles of an insoluble substance (d) Method of separating and identifying various components in a mixture, which are present in small trace quantities.
You may also like:
Case Study and Passage Based Questions for Class 9 Science Chapter 3 Atoms and Molecules
Last modified on:3 years agoReading Time:5MinutesCase Study Questions for Class 9 Science Chapter 3 Atoms and Molecules In CBSE Class 9 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage based as well. In that, a paragraph will be given, and then…
Case Study and Passage Based Questions for Class 9 Science Chapter 4 Structure of Atom
Last modified on:3 years agoReading Time:4MinutesCase Study Questions for Class 9 Science Chapter 4 Structure of Atom In CBSE Class 9 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage based as well. In that, a paragraph will be given, and then…
Case Study and Passage Based Questions for Class 9 Science Chapter 10 Gravitation
Last modified on:3 years agoReading Time:4MinutesCase Study Questions for Class 9 Science Chapter 10 Gravitation In CBSE Class 9 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage based as well. In that, a paragraph will be given, and then questions based…
Case Study and Passage Based Questions for Class 9 Science Chapter 11 Work and Energy
Last modified on:3 years agoReading Time:4MinutesCase Study Questions for Class 9 Science Chapter 11 Work and Energy In CBSE Class 9 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage based as well. In that, a paragraph will be given, and then…
Case Study and Passage Based Questions for Class 9 Science Chapter 13 Why Do We Fall Ill
Last modified on:3 years agoReading Time:4MinutesCase Study Questions for Class 9 Science Chapter 13 Why Do We Fall Ill In CBSE Class 9 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage based as well. In that, a paragraph will be given,…
Download CBSE Books
Exam Special Series:
- Sample Question Paper for CBSE Class 10 Science (for 2024)
- Sample Question Paper for CBSE Class 10 Maths (for 2024)
- CBSE Most Repeated Questions for Class 10 Science Board Exams
- CBSE Important Diagram Based Questions Class 10 Physics Board Exams
- CBSE Important Numericals Class 10 Physics Board Exams
- CBSE Practical Based Questions for Class 10 Science Board Exams
- CBSE Important “Differentiate Between” Based Questions Class 10 Social Science
- Sample Question Papers for CBSE Class 12 Physics (for 2024)
- Sample Question Papers for CBSE Class 12 Chemistry (for 2024)
- Sample Question Papers for CBSE Class 12 Maths (for 2024)
- Sample Question Papers for CBSE Class 12 Biology (for 2024)
- CBSE Important Diagrams & Graphs Asked in Board Exams Class 12 Physics
- Master Organic Conversions CBSE Class 12 Chemistry Board Exams
- CBSE Important Numericals Class 12 Physics Board Exams
- CBSE Important Definitions Class 12 Physics Board Exams
- CBSE Important Laws & Principles Class 12 Physics Board Exams
- 10 Years CBSE Class 12 Chemistry Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Physics Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Maths Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Biology Previous Year-Wise Solved Papers (2023-2024)
- ICSE Important Numericals Class 10 Physics BOARD Exams (215 Numericals)
- ICSE Important Figure Based Questions Class 10 Physics BOARD Exams (230 Questions)
- ICSE Mole Concept and Stoichiometry Numericals Class 10 Chemistry (65 Numericals)
- ICSE Reasoning Based Questions Class 10 Chemistry BOARD Exams (150 Qs)
- ICSE Important Functions and Locations Based Questions Class 10 Biology
- ICSE Reasoning Based Questions Class 10 Biology BOARD Exams (100 Qs)
✨ Join our Online NEET Test Series for 499/- Only for 1 Year
1 thought on “ Case Study and Passage Based Questions for Class 9 Science Chapter 2 Is Matter Around Us Pure? ”
- Pingback: Is Matter Around Us Pure Class 9 Case Study Questions Science Chapter 2 - XAM CONTENT
Leave a Reply Cancel reply

Editable Study Materials for Your Institute - CBSE, ICSE, State Boards (Maharashtra & Karnataka), JEE, NEET, FOUNDATION, OLYMPIADS, PPTs
Discover more from Gurukul of Excellence
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading

COMMENTS
Find a comprehensive collection of case study questions for Class 9 Science, covering various topics and concepts. Learn the benefits, tips, and importance of practicing case studies for science education and exams.
Class 9 science case study question 3. A few layers of cells beneath the epidermis are generally simple permanent tissue. Parenchyma is the most common simple permanent tissue. It consists of relatively unspecialized cells with thin cell walls. They are living cells.
Download solved examples and explanations for Class 9 Science case study questions based on real or hypothetical scenarios. Learn how to apply scientific concepts, enhance analytical skills, and think critically with case study questions.
Find chapter-wise case based questions with answers for CBSE Class 9 Science Term 2 Exam 2022. These questions are curated by subject experts and help to revise important concepts and prepare for the exam.
Case Study/Passage-Based Questions. Case Study 1: Akshita wants to separate the mixture of dyes constituting a sample of ink. She marked a line by the ink on the filter paper and placed the filter paper in a glass containing water as shown in the figure. The filter paper was removed when the water moved near the top of the filter paper.
Case based questions for Class 9 Science involve exploring a real-world situation through scientific analysis and inquiry. These questions allow students to make connections between science concepts and the world around them, as well as develop critical thinking skills. For example, a case study may involve challenging a student to determine ...
Case Study and Passage Based Questions for Class 9 Science Chapter 7 Diversity in Living Organisms. March 29, 2022 Physics Gurukul 1 Comment.
Last modified on:3 years agoReading Time:4MinutesCase Study Questions for Class 9 Science Chapter 11 Work and Energy In CBSE Class 9 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage based as well. In that, a paragraph will be given, and then…
Case Study/Passage Based Questions. The given diagrams show the atomic structures of elements X and Y. Element X and Y could be _ and _ respectively. (a) Be and B (b) C and O. (c) F and N (d) C and N. Show Answer. Valency of elements X and Y are respectively, (a) 4 and 3 (b) 2 and 5. (c) 1 and 4 (d) 3 and 4.
Maths Case-Study Qs. Maths Case-Study Qs. VIEW ALL. TopperLearning offers an online platform to access case studies for CBSE Class 9 students. Explore your analytical and problem-solving skills by solving case studies with our expert guidance. Get started today!
Case study Questions on Class 9 Science Chapter 9 are very important to solve for your exam. Class 9 Science Chapter 9 Case Study Questions have been prepared for the latest exam pattern. You can check your knowledge by solving case study-based questions for Class 9 Science Chapter 9 Force and Laws of Motion
CBSE Case Study Questions Class 9 Science - The Fundamental Unit of Life. CASE 1. All living Organisms are made up of cells and these cells perform all the functions essential for the survival of the Organism eg. Respiration, digestion, excretion etc. In Unicellular organisms, a single cell carries out all these functions and in multicellular ...
Case study Questions on Class 9 Science Chapter 2 are very important to solve for your exam. Class 9 Science Chapter 2 Case Study Questions have been prepared for the latest exam pattern. You can check your knowledge by solving case study-based questions for Class 9 Science Chapter 2 Is Matter Around Us Pure?
b) Dissolution, combustion, sublimation, and oxidation. c) Fermentation, photosynthesis, respiration, and digestion. d) Oxidation, reduction, precipitation, and ionization. Answer: a) Evaporation, condensation, melting, and freezing. Hope the information shed above regarding Case Study and Passage Based Questions for Class 9 Science Chapter 1 ...
CBSE Case Study Questions Class 9 Science - Force and Laws of Motion. Case 1: (1) Newton's first law of motion states that a body at rest will remain at rest position only and a body which is in motion continues to be in motion unless otherwise they are acted upon by an external force. In other words, all objects resist a changein their ...
CBSE Case Study Questions Class 9 Science - Tissues. CASE 1. The growth of plants occurs only in certain specific regions. This is because the dividing tissue, also known as meristematic tissue, is located only at these points. Depending on the region where they are present, meristematic tissues are classified as apical, lateral and intercalary.
Last modified on:3 years agoReading Time:4MinutesCase Study Questions for Class 9 Science Chapter 11 Work and Energy In CBSE Class 9 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage based as well. In that, a paragraph will be given, and then…
Q14: List all the factors which affects the rate of evaporation. A14: The factors affecting the rate of evaporation are: (a) an increase in surface area. (b) an increase in temperature. (c) an increase in wind speed. (d) a decrease in humidity. Hello students, we are providing case study questions for class 9 science.
Here we are providing case study questions for class 9 science chapter 12 sound. Students are suggested to go through each and every case study questions for better understanding of the chapter. Case Study/Passage Based Questions: Question 1:
Here, we have provided case-based/passage-based questions for Class 9 Science Chapter 3 Atoms and Molecules. Case Study/Passage-Based Questions. Case Study 1: The knowledge of the valencies of various radicals helps us to write the formulae of chemical compounds. The total positive charge on positive ions (cations) is equal to the total ...
Reading Time: 10 minutes Last Updated on August 13, 2024 by XAM CONTENT. Hello students, we are providing case study questions for class 9 science. Case study questions are the new question format that is introduced in CBSE board.
At Case Study Questions there will given a Paragraph. In where some Questions will made on that respective Case Based Study. There will various types of marks will given 1 marks, 2 marks, 3 marks or 4 marks. CBSE Case Study Questions Class 9 Science - Matter in our Surroundings Case Study 1: 1.) A matter is anything that has mass and occupies ...
Last modified on:3 years agoReading Time:4MinutesCase Study Questions for Class 9 Science Chapter 11 Work and Energy In CBSE Class 9 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage based as well. In that, a paragraph will be given, and then…
CASE STUDY QUESTIONS CLASS IX SCIENCE CHAPTER 2 IS MATTER AROUND US PURE. S. UDY QUESTIONS CLASS IXSCIENCECHAPTER 2 - IS MATTER AROUND US PURE1. A solution which can dissolve. ore of the solute at a given temperature is called unsaturated solution. However, a solution w. ich cannot dissolve any more of the solute is called saturated solution ...