- The Bohr Model

Bohr Model Practice Problems
Why is the electron in a Bohr hydrogen atom bound less tightly when it has a quantum number of 3 than when it has a quantum number of 1?
What does it mean to say that the energy of the electrons in an atom is quantized?
Quantized energy means that the electrons can possess only certain discrete energy values; values between those quantized values are not permitted.
Using the Bohr model, determine the energy, in joules, necessary to ionize a ground-state hydrogen atom. Show your calculations.
The electron volt (eV) is a convenient unit of energy for expressing atomic-scale energies. It is the amount of energy that an electron gains when subjected to a potential of 1 volt; 1 eV = 1.602 × 10 –19 J. Using the Bohr model, determine the energy, in electron volts, of the photon produced when an electron in a hydrogen atom moves from the orbit with n = 5 to the orbit with n = 2. Show your calculations.
Using the Bohr model, determine the lowest possible energy, in joules, for the electron in the Li 2+ ion.
Using the Bohr model, determine the lowest possible energy for the electron in the He + ion.
−8.716 × 10 −18 J
Using the Bohr model, determine the energy of an electron with n = 6 in a hydrogen atom.
Using the Bohr model, determine the energy of an electron with n = 8 in a hydrogen atom.
−3.405 × 10 −20 J
How far from the nucleus in angstroms (1 angstrom = 1 × 10 –10 m) is the electron in a hydrogen atom if it has an energy of –8.72 × 10 –20 J?
What is the radius, in angstroms, of the orbital of an electron with n = 8 in a hydrogen atom?
Using the Bohr model, determine the energy in joules of the photon produced when an electron in a He + ion moves from the orbit with n = 5 to the orbit with n = 2.
Using the Bohr model, determine the energy in joules of the photon produced when an electron in a Li 2+ ion moves from the orbit with n = 2 to the orbit with n = 1.
1.471 × 10 −17 J
Consider a large number of hydrogen atoms with electrons randomly distributed in the n = 1, 2, 3, and 4 orbits.
(a) How many different wavelengths of light are emitted by these atoms as the electrons fall into lower-energy orbitals?
(b) Calculate the lowest and highest energies of light produced by the transitions described in part (a).
(c) Calculate the frequencies and wavelengths of the light produced by the transitions described in part (b).
How are the Bohr model and the Rutherford model of the atom similar? How are they different?
Both involve a relatively heavy nucleus with electrons moving around it, although strictly speaking, the Bohr model works only for one-electron atoms or ions. According to classical mechanics, the Rutherford model predicts a miniature “solar system” with electrons moving about the nucleus in circular or elliptical orbits that are confined to planes. If the requirements of classical electromagnetic theory that electrons in such orbits would emit electromagnetic radiation are ignored, such atoms would be stable, having constant energy and angular momentum, but would not emit any visible light (contrary to observation). If classical electromagnetic theory is applied, then the Rutherford atom would emit electromagnetic radiation of continually increasing frequency (contrary to the observed discrete spectra), thereby losing energy until the atom collapsed in an absurdly short time (contrary to the observed long-term stability of atoms). The Bohr model retains the classical mechanics view of circular orbits confined to planes having constant energy and angular momentum, but restricts these to quantized values dependent on a single quantum number, n . The orbiting electron in Bohr’s model is assumed not to emit any electromagnetic radiation while moving about the nucleus in its stationary orbits, but the atom can emit or absorb electromagnetic radiation when the electron changes from one orbit to another. Because of the quantized orbits, such “quantum jumps” will produce discrete spectra, in agreement with observations.
The spectra of hydrogen and of calcium are shown here.

What causes the lines in these spectra? Why are the colors of the lines different? Suggest a reason for the observation that the spectrum of calcium is more complicated than the spectrum of hydrogen.
Browse Course Material
Course info.
- Prof. Donald Sadoway
Departments
- Materials Science and Engineering
As Taught In
- Chemical Engineering
Learning Resource Types
Introduction to solid state chemistry, 3. atomic models: rutherford & bohr.
« Previous | Next »
Session Overview
| Structure of the Atom | |
| Thomson’s plum pudding model, Rutherford’s model of the nucleus, Bohr’s model of the hydrogen atom, Rutherford-Geiger-Marsden experiment, Planck-Einstein relationship, isotopes of hydrogen | |
| lanthanides, actinides, electron, mass, J. J. Thomson, proton, electrical charge, amber, alpha particle, beta particle, ionization, conservation of mass, Johannes Geiger, Ernest Marsden, coulomb, Niels Bohr, Bohr model of hydrogen, energy quantization, orbital angular momentum, Planck-Einstein relationship, joule, Newtonian force, Coulombic force, Max Planck, photon, energy, frequency, Planck’s constant, isotope, Henry Cavendish, Harold Urey, Ernest Rutherford, blackbody radiation | |
| lanthanum (La), magnesium (Mg), chlorine (Cl), titanium (Ti), helium (He), hydrogen (H) | |
| nuclear fission, nanotechnology |
Prerequisites
Before starting this session, you should be familiar with:
- Session 2: The Periodic Table
Looking Ahead
Prof. Sadoway discusses the atomic spectra of hydrogen ( Session 4 ).
Learning Objectives
After completing this session, you should be able to:
- Understand Thomson’s “plum pudding” model .
- Understand Rutherford’s “nuclear” model .
- Explain the Bohr model of hydrogen .
- Understand Bohr’s quantization condition.
Archived Lecture Notes #1 (PDF) , Sections 1-3
| Book Chapters | Topics |
|---|---|
| 1.5, “The Atom.” | The electron; radioactivity; the atomic model |
| 6.2, “The Quantization of Energy.” | Blackbody radiation; the photoelectric effect |
| 6.3, “Atomic Spectra and Models of the Atom.” | Line spectra; the Bohr model; uses of emission and absorption spectra |
Lecture Video
- Download video
- Download transcript
Lecture Slides (PDF - 9.3MB)
Periodic Table and Table of Constants
Lecture Summary
Prof. Sadoway talks about the principles of modern chemistry and how that led to the understanding of the structure of the atom . He details Bohr’s postulates for the hydrogen atom and discusses how the Planck-Einstein relationship applies to electron transitions. He defines the different isotopes of hydrogen.
This lecture includes the following:
- Electrons are distributed uniformly throughout the atom
- Conclusions from the gold foil experiment
- Majority of the mass is found in the nucleus
- Electrons orbit around the nucleus
- Explanation of blackbody radiation and atomic spectra
- Electrons follow circular orbits around a nucleus
- Orbital angular momentum is quantized hence only certain orbits are possible
- Electrons in stable orbits do not radiate
- Electrons change orbits by radiating or absorbing photons
Problems (PDF)
Solutions (PDF)
Textbook Problems
| [Saylor] Sections | Conceptual | Numerical |
|---|---|---|
| 1.5, “The Atom.” | none | 4 |
| 1.6, “Isotopes and Atomic Masses.” | none | 10 |
| 6.1, “Waves and Electromagnetic Energy.” | none | 8 |
| 6.2, “The Quantization of Energy.” | none | 3, 6 |
For Further Study
Supplemental readings.
Ottaviani, J. Suspended in Language: Niels Bohr’s Life, Discoveries, and the Century He Shaped . GT Labs: Ann Arbor, MI, 2004. ISBN: 9780978803728.
Rozental, S. Niels Bohr: His Life and Work as Seen by His Friends and Colleagues . New York, NY: Wiley, 1967.
Bohr, Niels H. D. On the Constitution of Atoms and Molecules . New York, NY: W.A. Benjamin, 1963.
Bohr, Niels H. D. Atomic Physics and Human Knowledge . New York, NY: Wiley, 1958.
Bohr, Niels. “ On the Constitution of Atoms and Molecules. ” Philosophical Magazine Series 6 26 (July 1913): 1-15.
Cathcart, B. The Fly in the Cathedral: How a Small Group of Cambridge Scientists Won the Race to Split the Atom . New York, NY: Penguin, 2005. ISBN: 9780670883219.
Andrade, E. N. Rutherford and the Nature of the Atom . Garden City, NY: Doubleday, 1964.
Frayn, M. Copenhagen: A Play in Two Acts . New York, NY: S. French, 2000.
Miller, D. P. Discovering Water: James Watt, Henry Cavendish and the Nineteenth Century Water Controversy . Burlington, VT: Ashgate, 2004. ISBN: 9780754631774.
Cavendish Laboratory
How Atoms Work
Joseph Thompson - 1906 Nobel Prize in Physics
Ernest Rutherford - 1908 Nobel Prize in Chemistry
Johannes Geiger
Ernest Marsden
Max Planck - 1918 Nobel Prize in Physics
Albert Einstein - 1921 Nobel Prize in Physics
Niels Bohr - 1922 Nobel Prize in Physics
Robert Millikan - 1923 Nobel Prize in Physics
Henry Cavendish
Werner Heisenberg - 1932 Nobel Prize in Physics
Harold Urey - 1934 Nobel Prize in Chemistry
Charles-Augustin de Coulomb
James Prescott Joule
Other OCW and OER Content
| Content | Provider | Level | Notes |
|---|---|---|---|
| MIT OpenCourseWare | Undergraduate (first-year) |
| |
| HyperPhysics | High school |

You are leaving MIT OpenCourseWare
- Structure of Atom
- Bohrs Model

Bohr's Model Of An Atom
What is bohr’s model of an atom.
The Bohr model of the atom was proposed by Neil Bohr in 1915. It came into existence with the modification of Rutherford’s model of an atom. Rutherford’s model introduced the nuclear model of an atom, in which he explained that a nucleus (positively charged) is surrounded by negatively charged electrons.
Introduction to the Bohr Model
Bohr theory modified the atomic structure model by explaining that electrons move in fixed orbitals (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy. Rutherford explained the nucleus of an atom and Bohr modified that model into electrons and their energy levels.

Bohr’s Model of an Atom
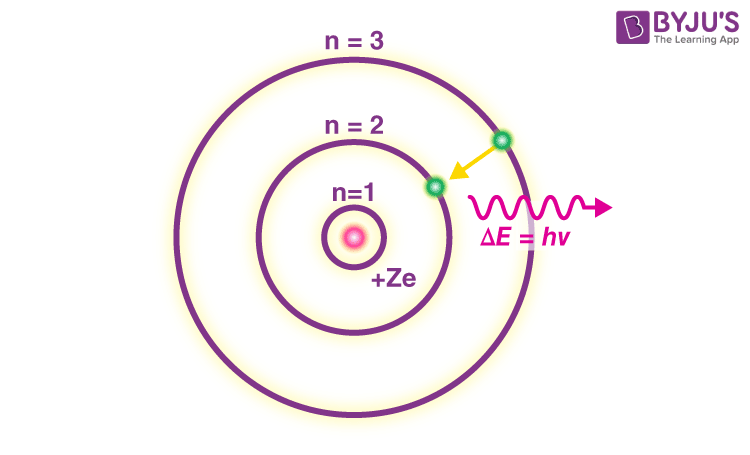
Bohr’s model consists of a small nucleus (positively charged) surrounded by negative electrons moving around the nucleus in orbits. Bohr found that an electron located away from the nucleus has more energy, and the electron which is closer to nucleus has less energy
Postulates of Bohr’s Model of an Atom
- In an atom, electrons (negatively charged) revolve around the positively charged nucleus in a definite circular path called orbits or shells.
- Each orbit or shell has a fixed energy and these circular orbits are known as orbital shells.
- The energy levels are represented by an integer (n=1, 2, 3…) known as the quantum number. This range of quantum number starts from nucleus side with n=1 having the lowest energy level. The orbits n=1, 2, 3, 4… are assigned as K, L, M, N…. shells and when an electron attains the lowest energy level, it is said to be in the ground state.
- The electrons in an atom move from a lower energy level to a higher energy level by gaining the required energy and an electron moves from a higher energy level to lower energy level by losing energy.
Recommended Video
Bohr’s model of atom – structure of atom.

Limitations of Bohr’s Model of an Atom
- Bohr’s model of an atom failed to explain the Zeeman Effect (effect of magnetic field on the spectra of atoms).
- It also failed to explain the Stark effect (effect of electric field on the spectra of atoms).
- It violates the Heisenberg Uncertainty Principle .
- It could not explain the spectra obtained from larger atoms.
Related Videos
Atomic structure – important questions.

Bohr’s Model of Atom – Numerical Problems

Bohr’s Model of Atom – Atomic Structure Concepts
Bohr theory is applicable to
Frequently asked questions – faqs, how do electrons move according to bohr’s model.
The theory notes that electrons in atoms travel around a central nucleus in circular orbits and can only orbit stably at a distinct set of distances from the nucleus in certain fixed circular orbits. Such orbits are related to certain energies and are also referred to as energy shells or energy levels.
How did Bohr discover electrons?
Bohr was the first to discover that electrons move around the nucleus in different orbits and that an element’s properties are determined by the number of electrons in the outer orbit.
Did Bohr’s model have neutrons?
The nucleus in the atom’s Bohr model holds most of the atom’s mass in its protons and neutrons. The negatively charged electrons, which contribute little in terms of mass, but are electrically equivalent to the protons in the nucleus, orbit the positively charged core.
How did Sommerfeld modify Bohr’s theory?
Many modifications have been introduced to the Bohr model, most notably the Sommerfeld model or Bohr – Sommerfeld model, which suggested that electrons move around a nucleus in elliptical orbits rather than circular orbits of the Bohr model. The Bohr – Sommerfeld system was essentially incoherent, contributing to many paradoxes.
Who discovered electrons?
J. J. Thomson in 1897 discovered Electron when he was studying the properties of the cathode ray.
To follow more about Bohr’s model of an atom, download BYJU’S-the learning app.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
| CHEMISTRY Related Links | |
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Byju’s is the best learning app
Byjus is easy to learn
Byjus is so great & interesting.
byjus is the best learning app
Byjus is the best learning app ,,, soo much interesting 👍👍👍👍
it is easy to learn through byjus
Byjus.com is best learning app
Best app of learning
Has been helping me for a pretty long time.
Byju’s is really helpful and so intresting and also easily understandable . Thank you so much for sharing this small part .
I Need bohr’s atomic theory and it’s limitations
Check here Niels Bohr Atomic Model And Limitations
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

COMMENTS
Directions. Draw the Bohr Models showing all the electrons in each energy level. Magnesium compounds are used in the production of uranium for nuclear reactors. Draw the Bohr model for magnesium. Sodium is found in salts that can be used to seed clouds to increase rainfall. Draw the Bohr model for sodium. Neon is often found in lasers.
Bohr Models 1. 1 1 H Hydrogen 1.01 2 3 4 Li Be Lithium Beryllium 6.94 9.01 11 12 Na Mg Sodium Magnesium 22.99 24.31 19 20 K Ca Potassium Calcium 39.10 40.08 37 38 Rb ... ASSIGNMENT #1: Getting to Know Subatomic Particles pg 9 +Atomic Structure Worksheet pages 10-11 This assignment is
ASSIGNMENT #1:Bohr Model Practice, Worksheet pages 6-8 This assignment is to be completed below in the space provided. Use the innermost circle as the nucleus, and fill the electron shells with the correct number of electrons for each of the first 20 elements in the Periodic Table. eg. Hydrogen has been completed for you as an example. 1.
Bohr Model Practice. For each element, write the total number of electrons on the line. Then color the correct number of electrons for each orbit. Remember, fill the orbit closest to the nucleus first, but never exceed the number each orbit can hold. Check the Periodic Table to find out how many electrons each element actually has. Sodium (Na
Bohr Model Practice Problems. In 1943, Niels Bohr described the atom as a planetary system with electrons orbiting around the nucleus. Bohr's model is incomplete, but it nonetheless is helpful in understanding why some atoms are very reactive and others are less so. Directions: complete each of the following models using what you know about ...
Bohr Model Diagrams. Draw a nucleus with the element symbol inside. Write the number of Protons and Neutrons the element has inside the nucleus. Put a + by the P and a little o by the N. Note: Round mass to nearest 1 when figuring neutrons. hold a maximum number of electrons. IMPORTANT: The outer-most shell of an atom (no matter what level) can ...
SCIENCE 9 BOHR MODEL ASSIGNMENT BACKGROUND: The Bohr model of the atom has a positive nucleus with protons and the electrons are in energy rings/levels around the nucleus. ... and the third level also holds 8 (18 in truth, but it splits to 8 and 10). ASSIGNMENT: In the table below draw the Bohr model for the first 18 elements on the periodic ...
Symbol Atomic Number_ Mass Number. Procedure: 1. Draw Bohr atomic models for each of the atoms using your Periodic Table 2. To represent the # of protons write a P- followed by the number of protons. Place in nucleus. 3. To represent the # of neutrons write a N- followed by the number of neutrons. Place in nucleus.
Chapter 1 The Bohr Atom1 IntroductionNiels Bohr was a Danish physicist who made a fundamental contribution to our understanding of. tomic structure and quantum mechanics. He made the first successful attempt at modeling the hydrogen atom by assuming that the electron executes orbital motio.
Name _____ Period_____ Date _____ The Structure of Atoms Complete the table Sub-atomic Particle Symbol Location in the atom Mass of particle
The Bohr model for the hydrogen atom is the prototype of the semi‐classical approach to atomic and molecular structure. Although it was superseded by quantum mechanics many decades ago, ... This assignment shows that, given its simplicity, the Bohr model achieves acceptable results. However, the students should note that the He result ...
The electron volt (eV) is a convenient unit of energy for expressing atomic-scale energies. It is the amount of energy that an electron gains when subjected to a potential of 1 volt; 1 eV = 1.602 × 10 -19 J. Using the Bohr model, determine the energy, in electron volts, of the photon produced when an electron in a hydrogen atom moves from the orbit with n = 5 to the orbit with n = 2.
Resources. Lecture Slides (PDF - 9.3MB) Periodic Table and Table of Constants. Lecture Summary. Prof. Sadoway talks about the principles of modern chemistry and how that led to the understanding of the structure of the atom.He details Bohr's postulates for the hydrogen atom and discusses how the Planck-Einstein relationship applies to electron transitions.
models are named Dalton, Thomson, Rutherford, Bohr and Schrödinger — the last names of five physicists. In class, your group will research your assigned model and begin preparing a 5- to 7 ...
(6) Failure of Bohr Model (i) Bohr theory was very successful in predicting and accounting the energies of line spectra of hydrogen i.e. one electron system. It could not explain the lin e spectra of atoms containing more than one electron. (ii) This theory could not explain the presence of multiple spectral lines.
What is Bohr's Model of an Atom? The Bohr model of the atom was proposed by Neil Bohr in 1915. It came into existence with the modification of Rutherford's model of an atom. Rutherford's model introduced the nuclear model of an atom, in which he explained that a nucleus (positively charged) is surrounded by negatively charged electrons.