- Search Menu
- Sign in through your institution
- Advance Articles
- Supplements
- Early Career Investigators
- Author Guidelines
- Detailed inclusive language guidelines
- Submission Site
- Why Publish with Us?
- Open Access
- Author Resource Centre
- Early Career Investigator Research Section
- Editorial Board
- Diversity, Equity, and Inclusion
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Data availability, author contributions, conflicts of interest, reducing ovarian cancer mortality through screening: an impossible dream.
- Article contents
- Figures & tables
- Supplementary Data
Evan R Myers, Reducing ovarian cancer mortality through screening: an impossible dream?, JNCI: Journal of the National Cancer Institute , 2024;, djae175, https://doi.org/10.1093/jnci/djae175
- Permissions Icon Permissions
Although ovarian cancer is “only” the 10th most common cancer in women, it is the fifth-leading cause of cancer death ( 1 ). Sixty-five percent of ovarian cancers are diagnosed after the disease has spread within the peritoneal cavity (stage III) or distant organs (stage IV) ( 2 ). Because 5-year survival for localized disease is over 90% compared with 30% for distant disease ( 2 ), efforts at developing effective early-detection strategies for reducing ovarian cancer mortality ( 3-5 ) have been ongoing since the 1980s. Unfortunately, large randomized trials have repeatedly failed to show a significant reduction in mortality in screened patients ( 6 , 7 ). In this issue of the Journal, Ishizawa and colleagues ( 8 ), using data from the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial ( 6 ) and UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) ( 7 ), use an innovative approach to gain insight into factors contributing to these disappointing results.
Using an approach initially validated using lung cancer screening data ( 9 ), the authors modeled cancer progression as a 3-state (no clinical disease, preclinical detectable disease, and clinical disease) continuous-time Markov chain. In the absence of screening, there are transition rates between no disease and preclinical disease (λ 1 ) and between preclinical disease and symptomatic disease leading to diagnosis (λ 2 ); in a screening setting, the preclinical state can be detected based on the sensitivity of the tests. Varying the values of λ 1, λ 2 , and sensitivity results in different estimates of the number of screen-detected cases within a specified interval. By calibrating the value of the 3 parameters so that model estimates approximate reported cases from the trials, it is possible to derive an estimate for the mean sojourn time—the “window of opportunity” for effective early detection. The authors further extended this approach to separately estimate sojourn time between early-stage and advanced-stage cancers. Individual-level data from PLCO and published summary data from UKCTOCS were used; in addition, stage-specific and histology-specific incidence and survival data from Surveillance, Epidemiology, and End Results were used to estimate histology-specific survival hazard ratios relative to high-grade serous carcinoma, the most common subtype with the poorest survival, and subsequently estimate histology-specific sojourn times.
Using the PLCO data, estimated overall mean sojourn time was 2.1 years (95% confidence interval [CI] = 1.9 to 2.4 years), with a sensitivity over the 6 years of screening of 65.7% (95% CI = 60.2% to 71.2%). Sojourn time estimates did not differ when analyzed on the basis of screening modality (cancer antigen 125 plus ultrasound vs cancer antigen 125 alone), although sensitivity with multimodal screening (80.8%, 95% CI = 74.3% to 87.4%) was statistically significantly higher than cancer antigen 125 alone (36.2%, 95% CI = 29.6% to 43.2%). Estimated sensitivity for early-stage cancers (39.1%, 95% CI = 34.9% to 43.3%) was statistically signficantly lower than for advanced cancers (82.9%, 95% CI = 78.0% to 87.8%). Critically, estimated time in the early-stage preclinical state was 1.1 years (95% CI = 1.0 to 1.1 years).
Results using the published UKCTOCS data were similar: estimated mean sojourn times in the multimodal screening arm of 2.0 years (95% CI = 1.8 to 2.1 years) and 2.4 years (95% CI = 2.2 to 2.5 years) in the transvaginal ultrasound arm. Estimated sojourn times for aggressive histologic subtypes (including high-grade serous carcinoma) were significantly shorter (range = 0.9-1.8 years) than for histologic types with better prognosis (range = 2.9-6.6 years).
The population-level time course of cancer progression from an initial mutation until diagnosis with symptoms is an inherently “unknowable unknown,” but estimates of the unobserved rate of progression are necessary for developing models to compare the potential impact of different cancer-control strategies. A variety of approaches exist for inferring these rates based on population-level data; Ishizawa and colleagues ( 8 ) have demonstrated an innovative approach to deriving estimates from empiric data, for example. Although the authors note that the lack of individual-level data from UKCTOCS is a limitation, the fact that estimates of mean sojourn time on the basis of the individual-level data from PLCO are similar to those on the basis of UKCTOCS summary data provides additional confidence in the validity of their approach.
Neither trial demonstrated a significant reduction in ovarian cancer mortality among screened women (despite a stage shift in UKCTOCS). The analysis by Ishizawa and colleagues ( 8 ) provides some explanation for these findings. First, the mean sojourn time for ovarian cancer is short; as the authors point out, it is substantially shorter than estimates for other screen-detectable cancers, such as prostate, lung, breast, and colorectal cancers. Second, the sojourn time for early-stage preclinical disease was only 1 year, and sensitivity of all screening modalities was lower for early-stage disease. Third, the sojourn time for high-grade serous carcinoma, the most frequent cause of ovarian cancer death, was substantially shorter than for histologic subtypes with better prognoses. As the authors point out, these findings are consistent with our current understanding of the biology of high-grade serous carcinoma. Most of these cancers arise in the fallopian tube epithelium—there are no physical barriers to cancer cell migration to the surface of the ovary, and from the surface of the ovary to the surfaces of other organs within the peritoneum. Given that the biology of high-grade serous carcinoma is so different from that of cervical cancer, the paradigm for successful cancer mortality reduction through screening, it is not surprising that we do not yet have an “ovarian Pap test.”
The authors discuss some of the implications of these findings for future efforts to develop effective ovarian cancer screening tests. A short sojourn time implies that screening intervals may need to be less than 1 year, and new tests should ideally have a higher sensitivity for early-stage disease than current modalities. Because a higher sensitivity would almost necessarily be associated with lower specificity, however, the combination of more frequent screening and lower specificity would inevitably lead to a high number of false-positive results—a particular problem for ovarian cancer, where definitive diagnosis requires surgery. Further mathematical modeling, informed by analyses such as this one, can be used to explore combinations of sensitivity, specificity, and screening frequency that could result in acceptable trade-offs of benefits (especially mortality reduction) and harms (such as false positives and unnecessary surgeries) for screening, either in the general population or in specific high-risk populations. As new screening tests and strategies are developed, those whose preliminary characteristics resulted in acceptable benefit/harm trade-offs could be considered for clinical trials, with mortality as the endpoint. As the UKCTOCS investigators point out, to date, findings of benefit for surrogate outcomes in ovarian cancer screening have not translated into mortality reduction ( 10 ).
Even if a strategy with characteristics that could potentially be acceptable were identified, however, another issue must be considered, both in study design and in implementation: Ovarian cancer incidence and incidence-based mortality have been declining significantly since the mid-1990s, and these declines are greatest for high-grade serous carcinoma ( 11 ). This decline in incidence may partially be attributable to several factors, including the increased use of contraceptive methods that are associated with lower risk and opportunistic salpingectomy, but age-specific data suggest a decline even in women born in the 1920s, who would not have benefited from oral contraceptives. Although the declining incidence in high-grade serous carcinoma is indisputably good news, it creates a potential problem for study design: Not accounting for potential cohort trends in cancer incidence could lower a study’s power. For example, Figure 1 shows the expected 5-year cancer incidence in women aged 55, 60, 65, and 70 years based on Surveillance, Epidemiology, and End Results cross-sectional data in 1993-1995, when the PLCO started enrollment, compared with observed estimates for each age cohort over 20 years of follow-up. Especially for the younger age groups, observed cancer incidence was lower than predicted based on cross-sectional data before beginning enrollment. If these trends continue, there are major feasibility issues for screening trials. Declining incidence will also increase the harm/benefit ratio (and decrease the cost-effectiveness) of any screening strategy.

Difference between expected 5-year probability of first diagnosis of ovarian cancer, by age cohort, based on Surveillance, Epidemiology, and End Results cross-sectional estimates, 1993-1995, compared with observed age-specific incidence in subsequent years. Expected probabilities for the cumulative 5-year incidence starting at ages 55, 60, 65, and 70 years are based on cross-sectional age-specific incidence in 1993-1995, derived from DevCan (referent) (solid line). “Observed” lines plot 5-year cumulative probabilities for each age group for the years 2000-2002, 2009-2011, and 2015-2017. For example, “observed” incidence for women aged 55 years in 1993-1995 would be those of 60-year-old women in 1999-2001, 65-year-old women in 2003-2005, and 70-year-old women in 2009-2011. Unanticipated cohort effects could affect the precision of estimates of screening test effectiveness.
Given the inherent challenges to ovarian cancer screening and the continuing decline in incidence and mortality, the relative value of additional efforts to develop screening modalities compared with more optimal utilization of primary prevention strategies must be considered. In addition, better understanding of the causes of the ongoing decline in incidence at the population level is needed. First, to the extent that the decline is attributable to interventions such as contraception and salpingectomy, estimating the potential impact of optimizing access is crucial. Second, because there may be age-period-cohort trends in other exposures (both risk reducing and risk increasing) that have contributed to the declining incidence, estimates of those effects are needed to help with predictions of future trends.
Randomized trials with mortality as the primary endpoint will always be needed to prove screening effectiveness, but mathematical models, such as those developed and used by the National Cancer Institute–sponsored Cancer Intervention and Surveillance Modeling Network, are also necessary for estimating the population-level impact of implementing screening. The work by Ishizawa and colleagues ( 8 ) illustrates how modeling can provide insight into trial results. Moving forward, more modeling work will be critical for helping us understand the feasibility of future screening strategies for ovarian cancer, from the perspective of pragmatic study design and population-level implementation, and inform decisions about the optimal balance of resources devoted to primary prevention, screening, and improved treatment to continue and perhaps expedite the ongoing decline in mortality.
The data used to generate the figure in this editorial are available from Surveillance, Epidemiology, and End Results (seer.cancer.gov) and were accessed using DevCan, version 6.7.5, software (DevCan: Probability of Developing or Dying of Cancer Software; Statistical Research and Applications Branch, National Cancer Institute, 2007 [ http://srab.cancer.gov/devcan/ ]).
Evan R. Myers, MD, MPH (Conceptualization; Data curation; Software; Validation; Visualization; Writing—original draft; Writing—review & editing).
No funding was used for this editorial.
Dr Myers has no disclosures.
Siegel RL , Giaquinto AN , Jemal A. Cancer statistics, 2024 . CA Cancer J Clin . 2024 ; 74 ( 1 ): 12 - 49 .
Google Scholar
SEERExplorer: an interactive website for SEER cancer statistics. Surveillance Research Program, National Cancer Institute; April 17, 2024 . [updated: June 27, 2024; cited July 17, 2024]. https://seer.cancer.gov/statistics-network/explorer/ . Accessed July 22, 2024. Data source(s): SEER Incidence Data, November 2023 Submission (1975-2021), SEER 22 registries (excluding Illinois and Massachusetts).
Campbell S , Goswamy R , Goessens L , et al. Real-time ultrasonography for determination of ovarian morphology and volume: a possible early screening test for ovarian cancer? Lancet . 1982 ; 1 ( 8269 ): 425 - 426 .
Zurawski VR , Broderick SF , Pickens P , et al. Serum CA 125 levels in a group of nonhospitalized women: Relevance for the early detection of ovarian cancer . Obstet Gynecol . 1987 ; 69 : 606 - 611 .
Jacobs I , Bridges J , Reynolds C , et al. Multimodal approach to screening for ovarian cancer . Lancet . 1988 ; 1 ( 8580 ): 268 - 271 .
Buys SS , Partridge E , Black A , et al. ; PLCO Project Team . Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening randomized controlled trial . JAMA . 2011 ; 305 ( 22 ): 2295 - 2303 .
Menon U , Gentry-Mahraj A , Burnell M , et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial . Lancet . 2021 ; 397 ( 10290 ): 2182 - 2193 .
Ishizawa S , Niu J , Tammemagi MC , et al. Estimating sojourn time and sensitivity of screening for ovarian cancer using a Bayesian framework [published online ahead of print July 23, 2024] . J Natl Cancer Inst . 2024 :djae145. doi: 10.1093/jnci/djae145 .
Shen S , Han SX , Petousis P , et al. A Bayesian model for estimating multi-state disease progression . Comput Biol Med . 2017 ; 81 : 111 - 120 .
Menon U , Gentry-Maharaj A , Burnell M , et al. Tumour stage, treatment, and survival of women with high-grade serous tubo-ovarian cancer in UKCTOCS: An exploratory analysis of a randomised controlled trial . Lancet Oncol . 2023 ; 24 ( 9 ): 1018 - 1028 .
Somasegar S , Reddy RA , Chow S , et al. Trends in ovarian, fallopian tube, and primary peritoneal cancer incidence, mortality, and survival: a 15-year population-based analysis . Gynecol Oncol . 2024 ; 184 : 190 - 197 .
| Month: | Total Views: |
|---|---|
| August 2024 | 481 |
| September 2024 | 29 |
Email alerts
- Estimating sojourn time and sensitivity of screening for ovarian cancer using a Bayesian framework
Citing articles via
Looking for your next opportunity.
- Recommend to your Library
Affiliations
- Online ISSN 1460-2105
- Print ISSN 0027-8874
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Open access
- Published: 31 August 2020
A real-world study on characteristics, treatments and outcomes in US patients with advanced stage ovarian cancer
- Daniel C. Beachler ORCID: orcid.org/0000-0003-2788-3061 1 ,
- Francois-Xavier Lamy 2 ,
- Leo Russo 3 ,
- Devon H. Taylor 1 ,
- Jade Dinh 1 ,
- Ruihua Yin 4 ,
- Aziza Jamal-Allial 1 ,
- Samuel Dychter 5 ,
- Stephan Lanes 1 &
- Patrice Verpillat 2
Journal of Ovarian Research volume 13 , Article number: 101 ( 2020 ) Cite this article
8228 Accesses
11 Citations
1 Altmetric
Metrics details
Detailed epidemiologic descriptions of large populations of advanced stage ovarian cancer patients have been lacking to date. This study aimed to describe the patient characteristics, treatment patterns, survival, and incidence rates of health outcomes of interest (HOI) in a large cohort of advanced stage ovarian cancer patients in the United States (US).
This cohort study identified incident advanced stage (III/IV) ovarian cancer patients in the US diagnosed from 2010 to 2018 in the HealthCore Integrated Research Database (HIRD) using a validated predictive model algorithm. Descriptive characteristics were presented overall and by treatment line. The incidence rates and 95% confidence intervals for pre-specified HOIs were evaluated after advanced stage diagnosis. Overall survival, time to treatment discontinuation or death (TTD), and time to next treatment or death (TTNT) were defined using treatment information in claims and linkage with the National Death Index.
We identified 12,659 patients with incident advanced stage ovarian cancer during the study period. Most patients undergoing treatment received platinum agents (75%) and/or taxanes (70%). The most common HOIs (> 24 per 100 person-years) included abdominal pain, nausea and vomiting, anemia, and serious infections. The median overall survival from diagnosis was 4.5 years, while approximately half of the treated cohort had a first-line time to treatment discontinuation or death (TTD) within the first 4 months, and a time to next treatment or death (TTNT) from first to second-line of about 6 months.
Conclusions
This study describes commercially insured US patients with advanced stage ovarian cancer from 2010 to 2018, and observed diverse treatment patterns, incidence of numerous HOIs, and limited survival in this population.
Ovarian cancer is the most lethal gynecologic malignancy [ 1 ] and the fifth most common cause of cancer death for women in the United States (US) [ 1 ]. Epithelial ovarian cancer is primarily treated with surgery and platinum-based chemotherapy, and can also be treated with radiation, hormone, or targeted therapy. Many new treatments, including poly ADP-ribose polymerase (PARP) inhibitors, are indicated specifically for advanced stage ovarian cancer, [ 2 ] while potential new therapies, such as immunotherapies, are being investigated [ 3 ].
Randomized trials have suggested that adverse events including hypertension, neutropenia, liver-related toxicity, fatigue, anemia and diarrhea can occur commonly after initiation of certain ovarian cancer therapies, [ 4 , 5 , 6 ] but less is known about the incidence and types of health outcomes of interest (HOIs) occurring in the general ovarian cancer population. Randomized trials are tightly controlled studies that commonly use small and narrowly defined populations. Recent publications have suggested that trial populations are significantly younger, have higher income, and have fewer co-morbidities than the general cancer population [ 7 , 8 , 9 ].
Real world evidence on the characteristics, treatment patterns, incidence of HOIs, and outcomes (including survival) of advanced stage ovarian cancer patients has been limited [ 10 ], partially due to the lack of specific cancer information, such as the stage of disease, in large administrative claims databases. Recently, we developed a validated algorithm to define advanced stage ovarian cancer using supervised machine learning techniques [ 11 ]. In this study, we applied this algorithm to an administrative claims database to identify a large cohort of advanced stage ovarian cancer patients and described their characteristics, treatment patterns, survival, and incidence rates of HOIs that could be utilized as comparator incidence rates for new and future ovarian cancer therapies indicated for advanced stage ovarian cancer.
Population and design
This study included incident advanced stage ovarian cancer patients in the US using the HealthCore Integrated Research Database (HIRD). The HIRD is a longitudinal medical and pharmacy claims database from health plan members across each region of the US. Member enrollment, medical care, outpatient prescription drug use, outpatient laboratory test result data, and health care utilization are tracked for health plan members.
Claims databases lack certain types of clinical information not needed for billing purposes, such as cancer stage. To overcome this limitation, we linked claims data with three state cancer registries (Ohio, Kentucky, and New York) and the HealthCore Integrated Research Environment (HIRE) Oncology data. HIRE Oncology is a pre-authorization program in which clinical data is obtained through physicians’ submissions of intentions to use certain cancer treatments, and has shown good agreement with medical records with regard to cancer stage [ 12 ]. Advanced stage was defined in the registries and HIRE Oncology as epithelial ovarian cancer, either locally advanced (Stage IIIa, IIIb or IIIc) or metastatic (Stage IV). Subsequently, we developed a claims-based predictive model algorithm for advanced stage ovarian cancer among the subset of patients with clinical data using least absolute shrinkage and selection operator (lasso) regression and 20-fold cross validation [ 11 ]. The predictive model for advanced stage (III or IV) had a high PPV (95%), specificity (90%), and sensitivity (70%) when validated using data from the state cancer registries and HIRE Oncology, using an 80% probability threshold for defining a case [ 11 ].
To identify patients with confirmed incident advanced stage ovarian cancer, patients needed to meet the following inclusion criteria: at least one diagnosis code in any claims position for ovarian cancer (codes starting with International Classification of Diseases [ICD]-9: 1830 or ICD-10: C56; Supplemental Table 1 ) in the HIRD between January 1, 2010 and January 31, 2018, continuously enrolled in a health plan captured by the HIRD for at least 6 months prior to the first ovarian cancer diagnosis (to restrict to newly diagnosed (incident) cases), and identified as an advanced cancer patient by either matching to a cancer registry, HIRE Oncology, [ 11 , 12 ] or meeting the predictive algorithm for advanced disease [ 11 ].
Follow-up for this cohort of advanced stage ovarian cancer was identical to the inclusion period (January 2010 to January 2018). For each patient, the predictive probability of advanced stage ovarian cancer was computed each time a patient had a claim in the predictive model (hypothetical example of a patient results in Supplemental Fig. 1 , Supplemental Table 2 ). The date of incident advanced cancer (i.e. the index date for the start of follow-up) was defined as the first date the patient met the advanced stage predictive model’s probability threshold of 80% or higher (Supplemental Fig. 1 , Supplemental Table 2 ). The date of incident advanced cancer defined by the predictive model was within 1 month of cancer registry date for 84% of the patients and the median difference between the registry and model was 1 day apart. For patients with confirmed advanced disease who did not meet the predictive model algorithm, we used the cancer registry or HIRE Oncology date as the date of incident advanced cancer. Cases are defined as “advanced stage at diagnosis” if their advanced stage date (from cancer registry, HIRE Oncology, or predictive model) was within 1 month of their first cancer diagnosis in claims, otherwise they are defined as “Diagnosed as early stage and progressed to advanced stage”.
Follow-up started for an individual at the advanced stage index date and continued until they were censored (either by death, end of health plan enrollment, or end of study period (January 2018)). We did not require a set amount of person-time after the advance stage index date, thus a subset of patients in this cohort died or lost to follow-up soon after the advanced stage index date.
Patients were described in terms of demographic and clinical characteristics, prior and concomitant treatments, key incident HOIs, lines of treatment, and mortality. Selected characteristics were presented stratified by treatment line, which was inferred based on observed patterns of medication use which included assumptions such as 28-day cycles and a new line occurring when there were more than 60 days between two cycles or if there were treatment switches or a treatment added. We also identified the 25 most frequently dispensed medication classes during the 12 months before the advanced stage ovarian cancer index date and separately for the 12 months after the advanced stage ovarian cancer index date. The medication classes were defined at the four-digit Generic Product Identifier (GPI) level. Diagnoses are not linked to a specific prescription, and thus some of the record treatments may have been specified for other cancers such as breast cancer, if a patient had multiple malignancies.
We described characteristics for patients who were platinum therapy sensitive, platinum resistant, or platinum refractory, which were defined similarly to previously published studies [ 13 , 14 ]. The categorization was defined using medication dispensing data for platinum sensitive agents (cisplatin, carboplatin, or oxaliplatin) and other chemotherapies, and time until use of a second-line therapy.
We linked claims to the US National Death Index (NDI) to identify mortality outcomes and cause of death, following NDI standards for identification of death [ 15 ]. We also evaluated two real-world surrogates of cancer progression in this cohort, time to treatment discontinuation or death (TTD), and time to next treatment or death (TTNT) [ 16 ]. We defined TTD as the time from the date of initiation of a first-line systemic anti-cancer therapy after the advanced stage index date to the earliest of discontinuation (> 60 days without first-line treatment; event), death (event) or loss to follow-up in the HIRD (administrative censor, not an event). TTNT was defined as the time from the date of the first-line treatment after the advanced stage index date to the earliest of a second-line treatment (event), death (event), or loss to follow-up in the HIRD (administrative censor, not an event). We restricted mortality, TTD, and TTNT analyses to the patients available for linkage to the NDI, as a subset of the cohort was unable to be linked due to privacy restrictions. This study was approved by the New England Institutional Review Board (Work Order Number 1–9472-1).
Statistical analysis
Patient characteristics and treatments received were described by counts and percentages for categorical variables and statistics such as mean, standard deviation (SD), and median for continuous variables. Person-time incidence rates and Poisson 95% confidence intervals (CIs) were calculated for pre-specified HOIs. These pre-specified HOIs were identified with attention to the Medical Dictionary for Regulatory Activities (MedDRA) classification system and FDA approved standardized case definitions, when possible. MedDRA is not always directly translatable to use in administrative claims data but can sometimes be approximated with ICD codes. These HOIs required two or more ICD-9/ICD-10 diagnosis codes in any setting or at least one ICD-9/ICD-10 diagnosis code in the inpatient setting (codes available upon request). For the main analysis, the incidence rate of each HOI was determined from the case definition date for advanced ovarian cancer (index date) through the first HOI of a given type, or the end of the patient’s follow-up due to a censoring event, whichever is sooner. Incidence rates of HOIs after systemic anticancer therapy (while with advanced stage disease) were also conducted. We also assessed severe HOIs as those requiring hospitalization or ER visit as defined by the primary diagnosis on the facility claim.
Administrative claims-based assessments of disease incidence can be inaccurate for repeated events, as it is not always possible to distinguish between a patient who has a past medical history of a condition and one who has been newly diagnosed or experienced an acute event. For this reason, for most HOIs, patients were followed from cohort entry (or treatment initiation from some analyses) until their first recorded event of a given type, and then censored from follow-up for that event type. Unless otherwise specified, we excluded patients who presented the HOI prior to start of study follow-up (i.e. prevalent cases during the baseline period) from these HOI analyses.
The product-limit estimator was used to describe median values and rates of mortality, TTD, and TTNT at one, three, and 5 years and the corresponding Kaplan-Meier curves [ 17 ]. In a sensitivity analysis, we also evaluated the rates of mortality when excluding the last 6 months of data provided from the NDI (July 1, 2017 to December 31, 2017) given prior evidence of lower sensitivity of newly released data [ 15 ].
Descriptive characteristics
We identified 12,659 advanced ovarian cancer patients that met the eligibility criteria for this cohort. Most patients were classified as incident advanced stage at diagnosis (96.7%) rather than incident early stage cancers that progressed to an advanced stage (3.3%) which may often represent recurrent cases. At the time of advanced stage, these patients had a mean (±SD) age of 62 ± 14 years, and 50% were followed after their advanced cancer date for over 17.3 months (Table 1 ). The comorbidity burden was elevated with a median Deyo-Charlson Comorbidity Index (DCI) score of 6 [ 18 ]. The most frequently dispensed medication class in the 12 months before and after the advanced stage index date was opioid combinations (pre: 41.6%; post: 46.3%; Supplemental Table 3 ). Medication use appeared to increase after the advanced stage index date particular for 5-HT3 receptor agonists (pre: 19.7%; post: 37.1%) and phenothiazines (pre: 13.8%; post: 27.0%) which are both often used to treat nausea (Supplemental Table 3 ).
Regarding the treatment for ovarian cancer, close to half of advanced ovarian cancer patients had at least one ovarian cancer-related surgery during follow-up (i.e. after the advanced stage index date) (40.5%), primarily palliative surgery for relief of small bowel obstruction (34.9%; Supplemental Table 3 ). More than two-thirds received radiotherapy or systemic anti-cancer therapy (68.5%) after the advanced stage index date, the most common being platinum agents (75.3%; carboplatin = 66.3%, cisplatin = 14.1%, and oxaliplatin = 4.4% of treated patients) and taxanes (70.0%; paclitaxel = 64.2% and docetaxel = 12.8% of treated patients). Common specific agents used were carboplatin (66.3%) and paclitaxel (64.2%). There were 68.5% of patients for whom we observed a first line of treatment (including systemic therapy and radiotherapy), 43.9% had a second line, 30.5% had a third line, and 20.5% had four or more lines (Supplemental Table 4 ). Following first line therapy, there were 12.1% categorized as platinum sensitive, 15.3% as platinum resistant, and 41.7% as platinum refractory (Supplemental Table 4 ). The age, DCI, and treatment use were largely similar between platinum sensitive and platinum refractory/resistant patients (results available upon request.
Systemic anti-cancer medication class use differed by treatment line (Table 2 ). The majority of patients were taking platinum and taxane agents in the first treatment line, while the use of angiogenesis inhibitors, hormonal and related agents, antineoplastic antibodies, and antineoplastic antibiotics all became more widely used in later treatment lines (> 25% in the fourth line or higher; Table 2 ). The most commonly used agents, carboplatin and paclitaxel, were most frequently used in the first treatment line, and the proportion of patients using them were lower in the subsequent treatment lines (~ 50% in first line vs. < 37% in all subsequent treatment lines; Table 2 ). There were 12% of patients who had a breast cancer diagnosis (in addition to their ovarian cancer diagnosis) noted during their first treatment line therapy, suggesting a small subset of first line therapies may have been for breast cancer.
Health outcomes of interest (HOIs)
The most common pre-defined HOIs among advanced stage ovarian cancer patients included abdominal pain, nausea and vomiting, anemia, and serious infections (each > 24 per 100 person-years; Table 3 ). Advanced stage ovarian cancer patients also frequently developed malaise/fatigue, hypertension, constipation, pain in joints or limbs, and renal failure (each > 10 per 100-person years; Table 3 ). Endocrinopathies and immune/autoimmune related event rates were less frequent (e.g., colitis: 3.1 per 100 person-years, type 1 diabetes: 0.5 per 100 person-years; Table 3 ).
Of the 25,868 person-years of follow-up in the advanced ovarian cancer cohort, 15,938 person-years (62%) were after a systemic anti-cancer therapy. When restricting to time after anti-cancer therapy, rates of many HOIs were similar compared to rates after the advanced stage index date, which included pre and post anti-cancer treatment time (e.g., any rash - after advanced stage: 3.0 per 100 person-years, after anti-cancer therapy: 3.1 person-years; renal failure – after advanced stage: 9.6 per 100 person-years, after anti-cancer therapy: 10.2 per 100 person-years; Table 3 ). However, incidence rates of some HOIs, such as serious infections, nausea and vomiting, malaise and fatigue, and thrombocytopenia, were higher after treatment (Table 3 ).
When restricting to severe HOIs occurring as the primary discharge diagnosis in inpatient or emergency room facilities, the incidence rates of all events were lower than overall HOI event rates, especially events such as nausea and vomiting, anemia, malaise/fatigue, and constipation, which declined over three-fold compared to the overall incidence rate (Table 3 , Supplemental Table 4 ). Serious infections, abdominal pain, and renal failure were some of the most common hospitalized events noted as the primary discharge diagnosis (each > 4 per 100 person-years; Supplemental Table 5 ).
Mortality, TTD, and TTNT analyses
In this cohort of 12,659 incident advanced stage ovarian cancer patients, 8374 patients were eligible to be linked to the NDI and thus available for the mortality analyses (66.2% of incident ovarian cancer cases). Characteristics between these patients and those who could not be linked to the NDI were largely similar except patients eligible for NDI linkage were older (median age 64 vs. 58) and had a less recent advanced stage index date; Supplemental Table 6 ).
The median overall survival in this cohort was 4.5 years (95%CI = 4.17, 4.86; Fig. 1 , Table 4 ). Approximately 25% of the cohort had died within 1.28 years (95%CI = 1.20, 1.37; Fig. 1 ), and the five-year survival was 47.7% (95%CI = 0.462–0.493; Table 4 ). Survival results were similar when excluding data after June 30, 2019 (five-year survival = 46.8% (95%CI = 0.451–0.484; Supplemental Table 7 ).
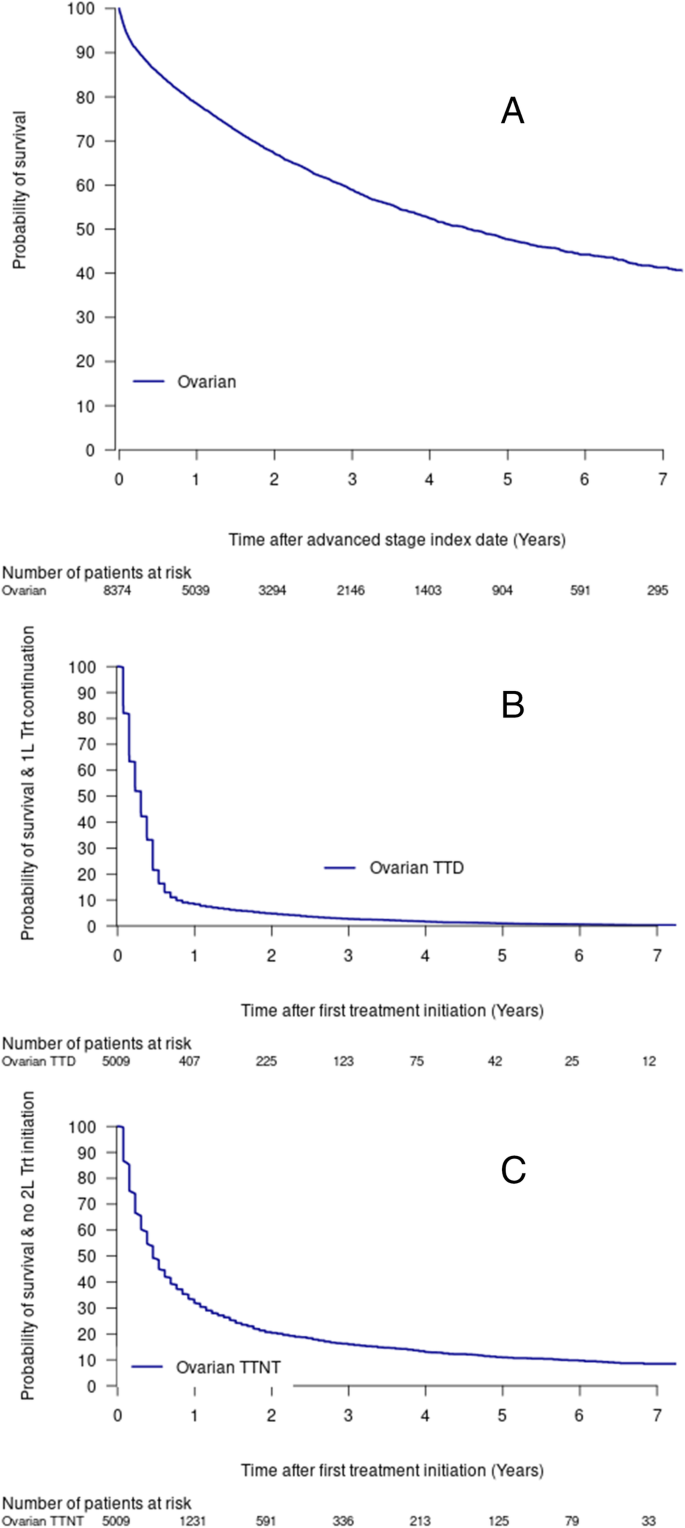
Advanced stage ovarian cancer, overall survival ( a ), time to treatment discontinuation or death (TTD) ( b ), time to next treatment or death (TTNT) ( c ). Abbreviations: 1 L, 1st Line; 2 L: 2nd Line; Trt, Treatment; TTD, treatment discontinuation or death; TTNT, time to next treatment or death
The TTD and TTNT estimates among treated patients were lower than overall survival estimates with approximately half of the treated cohort having a treatment discontinuation or death within the first 4 months (Fig. 1 , Table 4 ), or a second line treatment or death by about 6 months (0.46 years, 95%CI = 0.46, 0.53; Fig. 1 , Table 4 ).
After NDI linkage, few fatal HOI events were identified, with hypertension, serious infections, and renal failure being the most common (data available upon request).
This study identified a large cohort of incident advanced stage ovarian cancer patients in US administrative claims and examined descriptive data on demographics, treatment patterns, safety events, and mortality rates. Incidence rates of serious infections, and symptoms such as abdominal pain, malaise and fatigue, and nausea and vomiting were high. Incidence rates of HOIs could be used as comparator rates for safety signals to help inform and contextualize the safety of new or future therapies for advanced stage ovarian cancer, especially for uncontrolled clinical trials. Our study, which used our previously validated predictive model for advanced stage ovarian cancer, [ 11 ] provides detailed information on the routine care of advanced stage ovarian cancer. In this population, over one-third of individuals received an ovarian-related surgery and over two-thirds of individuals received radiotherapy or systemic anti-cancer therapy during follow-up (i.e. after their advanced stage index date). Surgeries and treatments may have occurred prior to this advanced stage date (e.g. when they had early stage ovarian cancer or just before the index date), or after they have dropped out of the study (e.g. due to health plan discontinuation) as no minimal follow-up time was required.
The most commonly used treatments were chemotherapies such as alkylating agents and mitotic inhibitors, particularly in the first and second line of therapy. Other treatments such as antimetabolites and hormonal agents were more common in later lines of therapy. This cohort included some patients who had multiple malignances, and as diagnoses are not linked to a specific prescription, some of the included treatments may represent treatment for diseases outside of ovarian cancer. In addition, the treatment line algorithm may have some level of misclassification, as the results represent the treatment lines since the model estimated date of advanced cancer. Thus, some of the treatments noted in the first line could have been used in an adjuvant setting.
This study observed high incidence rates of certain HOIs during follow-up such as anemia, diarrhea, hypertension and fatigue that have been noted as adverse events in trials [ 4 , 5 , 6 ] and other smaller observational studies [ 19 , 20 ]. This study also provides incidence rates of less common immune and endocrine-related events that have been unable to be robustly evaluated in previous studies given their limited sample size. While each of the 61 pre-specified HOI events did occur in at least one patient in this cohort, most of the immune and endocrine events were rare in advanced stage ovarian cancer patients, but events such as colitis and hypothyroidism were more common with incidence rates over three per 100 person-years of observation. The incidence of colitis and hypothyroidism in these women was not significantly higher after systemic therapy (Table 3 ). While, it is known that treatments such as platinum chemotherapy are associated with adverse events that impact quality of life, few studies have examined the occurrence of adverse events occurring among advanced stage ovarian cancer patients in a large real-world population. This is partially due to the lack of clinical stage information readily available in administrative claims. This study tried to provide proxies for such data through the incidence of HOIs among an advanced stage cancer population.
The HOIs in our study were not validated and it is expected that accuracy varies by safety event. In claims research, diagnosis, procedure, and prescription dispensing codes are used to reconstruct patients’ medical histories. As such, claims diagnoses are subject to misclassification and incidence estimates can vary widely based on the case definition used – a rate based on a definition that is very sensitive but not specific may be an overestimate, while a rate based on a definition that is specific but poorly sensitive may be an underestimate [ 21 ]. This is particularly relevant given that some of the outcomes used in the current study are based on clinical characteristics that are less likely to be assigned a diagnosis code (e.g., nausea, fatigue), and therefore would be captured in a claims database with poor sensitivity. These HOI algorithms would not capture fatal safety events if they occurred outside the healthcare system, although our linkage to the NDI could detect fatal HOIs, suggesting that HOIs were rarely noted on death certificates.
Survival of advanced stage ovarian cancer patients, while still relatively low, has been improving over time potentially due to the increasing number of therapeutic options. This study is also the first to our knowledge to provide estimates of TTD and TTNT (previously used as surrogates of disease progression during treatment) [ 22 , 23 , 24 ] for advanced stage ovarian cancer patients, in addition to overall survival. These proxies have been examined in other cancers and are correlated with progression free survival [ 16 , 22 ]. In our study, we observe near ubiquitous treatment discontinuation (TTD) and transfer to second line (or later) therapies (TTNT) within a few months of initiation of the first line therapy for advanced disease, and while overall survival was longer than the TTD and TTNT measures, it was still poor with approximately half the patients dying within 5 years. We found that almost all patients with advanced stage ovarian cancer (> 95%) were diagnosed at an advanced stage, rather than progressing from an earlier stage. This may be an indication of a lack of screening for this disease suggesting that symptoms may be initially mistaken for other diseases or are not present until later in the disease progression, which could contribute to accelerated mortality. Recent trials suggest that the use of PARP inhibitors (e.g., veliparib and olaparib) alone or in combination with chemotherapy or VEGF inhibitors significantly improves progression-free survival in first-line, as maintenance therapy and after first-line platinum exposure in ovarian cancer [ 25 , 26 , 27 ]. If these findings are confirmed through a benefit in overall survival, these new treatment strategies will likely reshape the treatment landscape of the disease in the coming years with widespread use and likely improve the outcomes currently observed in this patient population.
Our cohort included both stage III and IV tumors among commercial insured US patients. This population is likely younger and with a higher social economic status than the general US ovarian cancer population, given our limited data on Medicare ( > 65 year old) population and the lack of Medicaid data. The median overall survival, which was evaluated in a subset of population that was older than our overall population, was 4.5 years. In contrast, the 5-year survival rates based on Surveillance, Epidemiology, and End Results (SEER) data (US cancer registry) were 74% for regional tumors (spread to regional lymph nodes) at diagnosis and 29% for distant tumors (i.e. metastasized) (46% at 3 years) [ 28 ] suggesting overall survival may be modestly higher in our sample compared to SEER data assuming our sample largely is composed of distant stage cancers. While this difference could be related to age and higher income of our sample, there are also other explanations. For example, the start of follow-up time for SEER is the date of cancer diagnosis while in this study it is the date a patient has met the threshold of advanced stage cancer. Additionally, there could be imperfect sensitivity of NDI linkage for mortality, which would bias mortality rates downward. Published literature suggests NDI has a high sensitivity (97%) [ 29 ]. However, the sensitivity could be lower in patients with incomplete identifying information (e.g., missing social security number) which is present on at least a small subset of the HIRD.
In our main survival analyses, we censored a patient’s follow-up at the time they lost healthcare coverage eligibility in the HIRD (e.g., changed insurance plans). Some patients may leave their workplace and their related health plan as the disease progresses and deaths could occur at a differential rate – relatively soon after discontinuation of the health plan. To examine this possibility, we conducted an additional analysis where we did not censor at the discontinuation of the health plan. When using all available NDI mortality data, we found that the survival for ovarian cancer was similar to when censoring at health plan discontinuation (data available upon request) – suggesting that informed censoring was not a major source of bias.
This study of over ten thousand advanced stage ovarian cancer patients in the US from 2010 to 2018 provides a description of the diverse treatment patterns, numerous HOIs, and relatively short survival time for these women. These data on incidence rates of HOIs could be utilized as comparator rates of safety events for new and future ovarian cancer therapies indicated for advanced stage ovarian cancer, which will be of particularly importance given the numerous new treatment options, such as PARP inhibitors, and increasing survival of this population.
Availability of data and materials
Data and further materials for this manuscript cannot be shared given privacy regulations.
Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Reuer EJ, Cronin KA. SEER Cancer statistics review, 1975–2016 Bethesda. MD: National Cancer Institute; 2019. [Based on November 2018 SEER data submission, posted to the SEER web site, April 9]. Available from: https://seer.cancer.gov/csr/1975_2016/ .
Google Scholar
Kim JY, Cho CH, Song HS. Targeted therapy of ovarian cancer including immune check point inhibitor. Korean J Intern Med. 2017;32(5):798–804.
Article CAS Google Scholar
Cortez AJ, Tudrej P, Kujawa KA, Lisowska KM. Advances in ovarian cancer therapy. Cancer Chemother Pharmacol. 2018;81(1):17–38.
du Bois A, Floquet A, Kim JW, Rau J, del Campo JM, Friedlander M, et al. Incorporation of pazopanib in maintenance therapy of ovarian cancer. J Clin Oncol. 2014;32(30):3374–82.
Article Google Scholar
Matulonis UA, Berlin S, Ivy P, Tyburski K, Krasner C, Zarwan C, et al. Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J Clin Oncol. 2009;27(33):5601–6.
Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374(9698):1331–8.
Unger JM, Hershman DL, Fleury ME, Vaidya R. Association of Patient Comorbid Conditions with Cancer Clinical Trial Participation. JAMA Oncol. 2019;5(3):326–33.
Unger JM, Hershman DL, Albain KS, Moinpour CM, Petersen JA, Burg K, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31(5):536–42.
Ludmir EB, Mainwaring W, Lin TA, Miller AB, Jethanandani A, Espinoza AF, et al. Factors Associated With Age Disparities Among Cancer Clinical Trial Participants. JAMA Oncol. 2019;5(12). https://doi.org/10.1001/jamaoncol.2019.2055 .
Eisenhauer EA. Real-world evidence in the treatment of ovarian cancer. Ann Oncol. 2017;28(suppl_8):viii61–viii5.
Esposito DB, Russo L, Oksen D, Yin R, Desai VCA, Lyons JG, et al. Development of predictive models to identify advanced-stage cancer patients in a US healthcare claims database. Cancer Epidemiol. 2019;61:30–7.
Kern DM, Barron JJ, Wu B, Ganetsky A, Willey VJ, Quimbo RA, et al. A validation of clinical data captured from a novel Cancer care quality program directly integrated with administrative claims data. Pragmat Obs Res. 2017;8:149–55.
Johnson SJ, Sorg RA, Borker RD, Duh MS. Chemotherapy Treatment Patterns in Elderly Patients Initially Diagnosed With Advanced Ovarian Cancer. Clin Ovarian Gynecol Cancer. 2012;5(2):67–77.e1.
Cooke SL, Brenton JD. Evolution of platinum resistance in high-grade serous ovarian cancer. Lancet Oncol. 2011;12(12):1169–74.
Statistics CNCfH. NDI early release pilot program 2019 updated February 8 ,2016. Available from: https://www.cdc.gov/nchs/ndi/ndi_early_release.htm .
Vago E, Liwing J, Pan F, Mehra M, Zheng Y, Nahi H. Estimates of PFS, TTNT PER Treatment Line and their Relationship for Multiple Myeloma Patients. Glasgow: ISPOR EU; 2017.
Book Google Scholar
Pocock SJ, Clayton TC, Altman DG. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet. 2002;359(9318):1686–9.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9.
Le Saux O, Taylor A, Chia V, Pillas D, Kaur M, Freyer G. Cross-sectional study on comorbidities and adverse events in patients with advanced and recurrent ovarian cancer in France. Clin Epidemiol. 2015;7:431–40.
PubMed PubMed Central Google Scholar
Dunton CJ. Management of treatment-related toxicity in advanced ovarian cancer. Oncologist. 2002;7(Suppl 5):11–9.
Esposito D, Titievsky L, Beachler DC, Hawes JCL, Isturiz R, Scott DA, et al. Incidence of outcomes relevant to vaccine safety monitoring in a US commercially-insured population. Vaccine. 2018;36(52):8084–93.
Hari P, Romanus D, Palumbo A, Luptakova K, Rifkin RM, Tran LM, et al. Prolonged duration of therapy is associated with improved survival in patients treated for relapsed/refractory multiple myeloma in routine clinical Care in the United States. Clin Lymphoma Myeloma Leuk. 2018;18(2):152–60.
Blumenthal GM, Gong Y, Kehl K, Mishra-Kalyani P, Goldberg KB, Khozin S, et al. Analysis of time-to-treatment discontinuation of targeted therapy, immunotherapy, and chemotherapy in clinical trials of patients with non-small-cell lung cancer. Ann Oncol. 2019;30(5):830–8.
Gong Y, Kehl KL, Oxnard GR, Khozin S, Mishra-Kalyani PS, Blumenthal GM. Time to treatment discontinuation (TTD) as a pragmatic endpoint in metastatic non-small cell lung cancer (mNSCLC): A pooled analysis of 8 trials. JCO. 2018;20(15_suppl):9064 (36).
Gonzalez-Martin A, Pothuri B, Vergote I, DePont CR, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian Cancer. N Engl J Med. 2019;381(25):2391–402.
Ray-Coquard I, Pautier P, Pignata S, Perol D, Martin-Gonzalez A, Berger R, et al. Olaparib plus Bevacizumab as first-line maintenance in ovarian Cancer. N Engl J Med. 2019;381(25):2416–28.
Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian Cancer. N Engl J Med. 2019;381:2403–15.
SEER Explorer - Ovary. Suvival by time since diagnosis, vol. 5; 2019. Available from: https://seer.cancer.gov/explorer/application.php .
Boyle CA, Decoufle P. National sources of vital status information: extent of coverage and possible selectivity in reporting. Am J Epidemiol. 1990;131(1):160–8.
Download references
Acknowledgments
Doreen-allen Kahangire (Merck Healthcare KGaA (Darmstadt, Germany), operational), Nianya Liu (HealthCore, Inc., programming), Shiva Krishna Vojjala (HealthCore, Inc., programming).
Cancer incidence data used in certain analyses were obtained from the Ohio Cancer Incidence Surveillance System (OCISS), Ohio Department of Health (ODH), a cancer registry partially supported by the National Program of Cancer Registries at the Centers for Disease Control and Prevention (CDC) through Cooperative Agreement Number NU58DP006284. Use of these data does not imply that ODH or CDC agrees or disagrees with the analyses, interpretations or conclusions in this report.
This study was funded by Pfizer Inc. and Merck HealthCare KGaA.
Author information
Authors and affiliations.
Safety and Epidemiology, HealthCore, Inc, 123 Justison Street, Suite 200, Wilmington, DE, 19801, USA
Daniel C. Beachler, Devon H. Taylor, Jade Dinh, Aziza Jamal-Allial & Stephan Lanes
Global Epidemiology, Merck KGaA, Darmstadt, Germany
Francois-Xavier Lamy & Patrice Verpillat
Global Medical Epidemiology, Pfizer Inc, Collegeville, PA, USA
Ingenio Rx, Anthem Inc, Andover, MA, USA
Global Product Development, Pfizer Inc, La Jolla, CA, USA
Samuel Dychter
You can also search for this author in PubMed Google Scholar
Contributions
Beachler, Daniel : Conceptualization, Data Curation, Formal Analysis, Methodology, Investigation, Project Administration, software, validation, writing – original draft, Lamy, Francois Xavier : Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review and editing; Russo, Leo: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review and editing; Taylor, Devon: Project administration, writing – review and editing; Dinh, Jade: Formal Analysis, Software, Writing – review & editing; Yin, Ruihua : Formal Analysis, Software, Writing – review & editing; Jamal-Allial, Aziza - Formal Analysis, Software, Visualization, Writing – review & editing; Dychter, Samuel : Conceptualization, Supervision, Writing – review and editing; Lanes, Stephan: Conceptualization, Methodology, Supervision, Writing – review and editing; Verpillat, Patrice : Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review and editing. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Daniel C. Beachler .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the New England Institutional Review Board (Work Order Number 1–9472-1). The current study was designed as an analysis based on claims data from a large insured population in the US. There was no active enrollment or active follow-up of study subjects, and no data was collected directly from individuals. The HIPAA Privacy Rule permits PHI in a limited data set to be used or disclosed for research, without individual authorization, if certain criteria are met (further described 45 CFR Part 160 and Subparts A and E of Part 164). Thus informed consent was not required.
Consent for publication
Not applicable, as all results presented in this manuscript were aggregated.
Competing interests
DB, DT, JD, AJA, and SL are employees of HealthCore, Inc. (a subsidiary of Anthem Inc.), which received funding from Pfizer Inc. and Merck Healthcare KGaA (Darmstadt, Germany) for this study. FXL and PV are employees of Merck Healthcare KGaA (Darmstadt, Germany) and LR and SD are employees of Pfizer Inc. RY was an employee of HealthCore, Inc. at the time of this study, and is a current employee of Anthem Inc.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: figure s1..
Example of a hypothetical patient “A” progressing from early to advanced stage ovarian cancer and definition of index date. Most patients (96.7%) in our cohort were classed as advanced stage at diagnosis. This hypothetical example would have been classified in those who “progressed from early to advanced stage ovarian cancer”, which represented 3.3% of patients in the cohort.
Additional file 2: Table S1.
Codes used to define ovarian cancer. Table S2. Probability of advanced stage ovarian cancer for hypothetical patient “A” over time in the HIRD used to identify their index date. Table S3. Top 25 most common prescribed medication among 12,659 advanced stage ovarian cancer patients, 12 months before and after their advanced stage ovarian cancer date. Table S4. Advanced stage ovarian cancer cohort, cancer treatment received on or after the advanced stage date ( N = 12,659). Table S5. Advanced stage ovarian cancer cohort, hospital or emergency room incidence rates of selected health outcomes of interest. Table S6. Characteristics by National Death Index (NDI) linkable status. Table S7 . Ovarian cancer overall survival, excluding last 6 months of follow-up (July–December 2017).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Beachler, D.C., Lamy, FX., Russo, L. et al. A real-world study on characteristics, treatments and outcomes in US patients with advanced stage ovarian cancer. J Ovarian Res 13 , 101 (2020). https://doi.org/10.1186/s13048-020-00691-y
Download citation
Received : 17 April 2020
Accepted : 27 July 2020
Published : 31 August 2020
DOI : https://doi.org/10.1186/s13048-020-00691-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Epidemiology
- Ovarian Cancer
- Advanced stage
- Treatment patterns
- Health outcome of interest
Journal of Ovarian Research
ISSN: 1757-2215
- General enquiries: [email protected]
Ovarian Cancer
Explore the latest in ovarian cancer, including recent advances in epidemiology, screening, genetic testing, and management of the disease.
Publication
Article type.
This economic evaluation estimates the incremental lifetime health outcomes, costs, and cost-effectiveness associated with population-based BRCA testing compared with family history–based testing in a simulated cohort of women in Canada.
- Symptom-Triggered Testing Speeds Up Diagnosis of Aggressive Ovarian Cancer JAMA News September 6, 2024 Reproductive Health Oncology Gynecologic Cancer Gynecology Women's Health Full Text | pdf link PDF free
- The Intersection of Endometriosis and Ovarian Cancer Prevention JAMA Surgery Opinion August 14, 2024 Reproductive Health Gynecology Women's Health Obstetrics and Gynecology Oncology Full Text | pdf link PDF
- Surgeon Type and Anastomotic Leaks in Ovarian Cancer JAMA Surgery Opinion August 7, 2024 Reproductive Health Oncology Gynecologic Cancer Gynecology Women's Health Full Text | pdf link PDF
This study compares surgical outcomes between gynecologic oncologist, general surgeons, and a 2-surgeon team approach of patients with advanced epithelial ovarian cancer who underwent bowel surgery during cytoreductive debulking.
This narrative review describes the identification of male BRCA1/2 pathogenic variant carriers, associated cancer risks, and management recommendations.
This population-based cohort study investigates ovarian cancer risk in women with vs without endometriosis and the association between ovarian cancer histotypes and endometriosis subtypes.
- New Insights in Endometriosis Subtypes and Ovarian Cancer Risk JAMA Opinion July 17, 2024 Reproductive Health Oncology Gynecologic Cancer Gynecology Women's Health Full Text | pdf link PDF has multimedia
This cohort study examines the association between primary cytoreduction status and survival for patients with less-common, advanced-stage epithelial ovarian carcinoma.
This cohort study investigates nail abnormality prevalence among patients with ( BRCA1 -associated protein) BAP1 tumor predisposition syndrome.
This cohort study characterizes the clinical and tumor characteristics of germline RAD51C/D PV carriers, including the evaluation of homologous recombination deficiency status.
This cohort study assesses whether time between most recent childbirth and breast cancer diagnosis is associated with mortality among patients with young-onset breast cancer and germline BRCA1/2 pathogenic variants.
This cohort study examines overall and progression-free survival, using a BRCA -like genomic copy number aberration profile classifier, in patients with ovarian cancer receiving olaparib plus bevacizumab or placebo plus bevacizumab.
This Viewpoint highlights the need for recognition that ovarian cancer affects women from racial and ethnic minority groups worldwide and that the rates of ovarian cancer are increasing in those populations while decreasing among White women.
- Increasing the Uptake of Cancer Risk Management Strategies for Women With BRCA1/2 Sequence Variations JAMA Oncology Opinion February 29, 2024 Genetics and Genomics Oncology Women's Health Breast Cancer Obstetrics and Gynecology Full Text | pdf link PDF
This cohort study examines the association between magnetic resonance imaging (MRI) surveillance and the risk of breast cancer mortality in women with BRCA1 or BRCA2 sequence variations.
This cohort study evaluates the association between bilateral oophorectomy and all-cause mortality among women with BRCA1 or BRCA2 sequence variations.
This prognostic study examines the utility of tumor-stroma proportion as a predictive biomarker for chemoresistance among patients with ovarian cancer.
This economic evaluation examines the cost-effectiveness of population-based multigene testing compared with family history–based testing in simulated patients with breast and ovarian cancer.
This economic evaluation estimates the cost-effectiveness of prevention strategies for ovarian and breast cancer among individuals with pathogenic variants in cancer susceptibility genes BRCA1 , BRCA2 , PALB2 , RAD51C , RAD51D , and BRIP1 .
Select Your Interests
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Advances in ovarian cancer therapy
Affiliations.
- 1 Maria Skłodowska-Curie Institute - Oncology Center, Gliwice Branch, Wybrzeże Armii Krajowej 15, Gliwice, 44-100, Poland.
- 2 Maria Skłodowska-Curie Institute - Oncology Center, Gliwice Branch, Wybrzeże Armii Krajowej 15, Gliwice, 44-100, Poland. [email protected].
- PMID: 29249039
- PMCID: PMC5754410
- DOI: 10.1007/s00280-017-3501-8
Epithelial ovarian cancer is typically diagnosed at an advanced stage. Current state-of-the-art surgery and chemotherapy result in the high incidence of complete remissions; however, the recurrence rate is also high. For most patients, the disease eventually becomes a continuum of symptom-free periods and recurrence episodes. Different targeted treatment approaches and biological drugs, currently under development, bring the promise of turning ovarian cancer into a manageable chronic disease. In this review, we discuss the current standard in the therapy for ovarian cancer, major recent studies on the new variants of conventional therapies, and new therapeutic approaches, recently approved and/or in clinical trials. The latter include anti-angiogenic therapies, polyADP-ribose polymerase (PARP) inhibitors, inhibitors of growth factor signaling, or folate receptor inhibitors, as well as several immunotherapeutic approaches. We also discuss cost-effectiveness of some novel therapies and the issue of better selection of patients for personalized treatment.
Keywords: Biological drugs; Clinical trials; Ovarian cancer; Targeted therapy.
PubMed Disclaimer
Similar articles
- Updates and New Options in Advanced Epithelial Ovarian Cancer Treatment. Kurnit KC, Fleming GF, Lengyel E. Kurnit KC, et al. Obstet Gynecol. 2021 Jan 1;137(1):108-121. doi: 10.1097/AOG.0000000000004173. Obstet Gynecol. 2021. PMID: 33278287 Free PMC article. Review.
- Targeted agents and combinations in ovarian cancer: where are we now? McLachlan J, Lima JP, Dumas L, Banerjee S. McLachlan J, et al. Expert Rev Anticancer Ther. 2016;16(4):441-54. doi: 10.1586/14737140.2016.1162101. Expert Rev Anticancer Ther. 2016. PMID: 26942837 Review.
- Clinical Trials of Novel Targeted Therapies in Ovarian Cancer: Moving Beyond Poly ADP Ribose Polymerase (PARP) Inhibitors. Guo Q, Yang Q, Li J, Liu G, Nikoulin I, Jia S. Guo Q, et al. Curr Pharm Biotechnol. 2018;19(14):1114-1121. doi: 10.2174/1389201020666181226123054. Curr Pharm Biotechnol. 2018. PMID: 30585545 Review.
- Management and Treatment of Recurrent Epithelial Ovarian Cancer. Armbruster S, Coleman RL, Rauh-Hain JA. Armbruster S, et al. Hematol Oncol Clin North Am. 2018 Dec;32(6):965-982. doi: 10.1016/j.hoc.2018.07.005. Hematol Oncol Clin North Am. 2018. PMID: 30390768 Review.
- An update on current and emerging therapies for epithelial ovarian cancer: Focus on poly(adenosine diphosphate-ribose) polymerase inhibition and antiangiogenesis. Chung C, Lee R. Chung C, et al. J Oncol Pharm Pract. 2017 Sep;23(6):454-469. doi: 10.1177/1078155216657165. Epub 2016 Jun 29. J Oncol Pharm Pract. 2017. PMID: 27357817 Review.
- Multiparametric MRI-based radiomics nomogram for differentiation of primary mucinous ovarian cancer from metastatic ovarian cancer. Shi SY, Li YA, Qiang JW. Shi SY, et al. Abdom Radiol (NY). 2024 Aug 31. doi: 10.1007/s00261-024-04542-y. Online ahead of print. Abdom Radiol (NY). 2024. PMID: 39215773
- Stanniocalcin Protein Expression in Female Reproductive Organs: Literature Review and Public Cancer Database Analysis. Khatun M, Modhukur V, Piltonen TT, Tapanainen JS, Salumets A. Khatun M, et al. Endocrinology. 2024 Aug 27;165(10):bqae110. doi: 10.1210/endocr/bqae110. Endocrinology. 2024. PMID: 39186548 Free PMC article. Review.
- MicroRNA‑mediated approaches in ovarian cancer therapy: A comprehensive systematic review. Putri HMAR, Novianti PW, Pradjatmo H, Haryana SM. Putri HMAR, et al. Oncol Lett. 2024 Aug 12;28(4):491. doi: 10.3892/ol.2024.14624. eCollection 2024 Oct. Oncol Lett. 2024. PMID: 39185494 Free PMC article.
- Constraint-based modelling predicts metabolic signatures of low and high-grade serous ovarian cancer. Meeson KE, Schwartz JM. Meeson KE, et al. NPJ Syst Biol Appl. 2024 Aug 24;10(1):96. doi: 10.1038/s41540-024-00418-5. NPJ Syst Biol Appl. 2024. PMID: 39181893 Free PMC article.
- Targeted Nanocarrier-Based Drug Delivery Strategies for Improving the Therapeutic Efficacy of PARP Inhibitors against Ovarian Cancer. Gralewska P, Gajek A, Marczak A, Rogalska A. Gralewska P, et al. Int J Mol Sci. 2024 Jul 30;25(15):8304. doi: 10.3390/ijms25158304. Int J Mol Sci. 2024. PMID: 39125873 Free PMC article. Review.
- Kurman RJ, Shih IM. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer-shifting the paradigm. Hum Pathol. 2011;42(7):918–931. doi: 10.1016/j.humpath.2011.03.003. - DOI - PMC - PubMed
- Kujawa KA, Lisowska KM. Ovarian cancer—from biology to clinic. Postep Hig Med Dosw. 2015;69:1275–1290. doi: 10.5604/17322693.1184451. - DOI - PubMed
- Bignotti E, Tassi RA, Calza S, Ravaggi A, Bandiera E, Rossi E, Donzelli C, Pasinetti B, Pecorelli S, Santin AD. Gene expression profile of ovarian serous papillary carcinomas: identification of metastasis-associated genes. Am J Obstet Gynecol. 2007;196(3):245–246. doi: 10.1016/j.ajog.2006.10.874. - DOI - PubMed
- Lisowska KM, Olbryt M, Student S, Kujawa KA, Cortez AJ, Simek K, Dansonka-Mieszkowska A, Rzepecka IK, Tudrej P, Kupryjanczyk J. Unsupervised analysis reveals two molecular subgroups of serous ovarian cancer with distinct gene expression profiles and survival. J Cancer Res Clin. 2016;142(6):1239–1252. doi: 10.1007/s00432-016-2147-y. - DOI - PMC - PubMed
- Lisowska KM, Olbryt M, Dudaladava V, Pamula-Pilat J, Kujawa K, Grzybowska E, Jarzab M, Student S, Rzepecka IK, Jarzab B, Kupryjanczyk J. Gene expression analysis in ovarian cancer—faults and hints from DNA microarray study. Front Oncol. 2014;4:6. doi: 10.3389/fonc.2014.00006. - DOI - PMC - PubMed
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- Europe PubMed Central
- PubMed Central
Other Literature Sources
- The Lens - Patent Citations
- scite Smart Citations
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Advances in Ovarian Cancer Research
An ovarian tumor grown in a mouse using human cells. Special techniques were used to create the high-resolution, 3-D view of the cancer’s cell structure and inner workings.
The most common ovarian cancers are those that begin in the epithelial cells that line the fallopian tubes or ovaries . These, along with cancers that form in the peritoneum , are called epithelial ovarian cancers . Other types of ovarian cancer arise in other cells, including germ cell tumors , which start in the cells that make eggs, and stromal cell tumors , which start in supporting tissues.
NCI-funded researchers are working to advance our understanding of how to prevent, detect early, and treat ovarian cancer.
This page highlights some of what’s new in the latest research in ovarian cancer, including clinical advances that may soon translate into improved care, NCI-supported programs that are fueling progress, and research findings from recent studies.
Prevention of Ovarian Cancer
Women who carry harmful or potentially harmful mutations in the BRCA1 or BRCA2 genes are at increased risk of developing ovarian cancer. Surgery to remove the ovaries and fallopian tubes in these women is the recommended treatment method and can reduce their lifetime risk of getting ovarian cancer by 95%. However, having this surgery causes immediate menopause. This may cause health problems if it is much earlier than naturally occurring menopause.
Research has shown that the most common type of ovarian cancer begins in the fallopian tubes , not in the ovaries. This discovery has led doctors to reconsider ways of preventing ovarian cancer.
- Removing fallopian tubes only. An ongoing NCI-sponsored clinical trial is testing whether removing the fallopian tubes but delaying removal of the ovaries will be as safe and effective to reduce the risk of ovarian cancer in women with BRCA1 mutations as removing both the ovaries and fallopian tubes at the same time. This would allow women to maintain premenopausal levels of hormones produced by the ovaries and delay many of the complications associated with menopause.
- Removal of fallopian tubes in people seeking to prevent pregnancy. The discovery that epithelial ovarian cancers most often start in the fallopian tubes has also led to changes in the way some gynecologists approach surgery to prevent pregnancy. Women seeking tubal ligation to prevent pregnancy (often called having your tubes tied) may be offered the option of having their tubes removed instead. Doing so might reduce the possibility of ovarian cancer in the future.
- Removal of fallopian tubes in people undergoing a hysterectomy. Similarly, some gynecologists recommend that their patients who are undergoing a hysterectomy also have their fallopian tubes removed.
- Testing relatives for gene mutations. NCI is funding efforts to test the relatives of women who have been diagnosed with ovarian cancer in the past. Researchers are locating women diagnosed with ovarian cancer with the hope to test them and/or their family members for ovarian cancer-related gene mutations. As a result, family members who learn they carry a mutation can take steps to reduce their risk. The overall goal is not only to prevent ovarian cancer, but also to find the best ways to communicate sensitive genetic information to ovarian cancer patients and their family members.
Ovarian Cancer Treatment
Surgery and chemotherapy are the main treatments for ovarian cancer. The location and type of cells where the cancer begins, and whether the cancer is high-grade or low-grade , influences how the cancer is treated. Surgery can cure most people with early-stage ovarian cancer that has not spread beyond the ovaries. For advanced ovarian cancer, the goal of surgery is to remove as much of the cancer as possible, called surgical debulking .
Platinum-based chemotherapy drugs, such as cisplatin or carboplatin , given in combination with other drugs, such as the targeted therapy bevacizumab (Avastin) , are usually effective in treating epithelial ovarian cancer at any stage. However, in most people with advanced ovarian cancer, the cancer comes back. Treating the cancer again with platinum drugs may work, but eventually the tumors become resistant to these drugs.
Targeted Therapy
Targeted therapy uses drugs or other agents to attack specific types of cancer cells. PARP inhibitors are a type of targeted therapy that can stop a cancer cell from repairing its damaged DNA , causing the cell to die. Cancers in people who have certain mutations in the BRCA genes are considered particularly susceptible to PARP inhibitors. That’s because BRCA genes are involved in the repair of some types of DNA damage, so cancers with alterations in these genes already have defects in DNA repair.
The use of PARP inhibitors has transformed treatment for people with advanced epithelial ovarian cancer who have harmful mutations in a BRCA gene. Since the 2014 approval of olaparib (Lynparza) , the first PARP inhibitor to be approved, the number of PARP inhibitors has grown and their uses for people with ovarian cancer have expanded. Now researchers are studying the benefits of combining PARP inhibitors with other drugs.
Clinical trials have shown that using PARP inhibitors as long-term therapy in women with advanced epithelial ovarian cancer delayed progression of the cancer.
A different targeted therapy, mirvetuximab soravtansine (Elahere) , is now available to treat women with ovarian cancer that is no longer responding to platinum drugs. FDA recently approved the drug to treat people with platinum-resistant ovarian tumors that produce an excess of a protein called FR-α. Results from a large clinical trial showed that people with this type of ovarian cancer treated with mirvetuximab lived longer overall than people treated with standard chemotherapy .
Treatment after Cancer Progression
Typically, chemotherapy and targeted therapies are stopped once ovarian cancer begins to come back. Clinical trials have shown that where there was more than a 6 month delay between stopping treatment and cancer being found again, resuming the drug bevacizumab (Avastin) in combination with platinum-based chemotherapy for patients previously treated with bevacizumab slowed the growth of platinum-sensitive disease . And in women who no longer benefited from platinum-based chemotherapy, non–platinum-based chemotherapy combined with bevacizumab kept the cancer in check longer than chemotherapy alone.
Targeted therapies may also be helpful for people with low-grade ovarian cancer. A trial of the drug trametinib in women with low-grade serous ovarian cancer that had come back showed that it delayed the cancer’s growth compared with treating the cancer with chemotherapy again.
Secondary Surgery
For women with advanced epithelial ovarian cancer that has come back after being in remission, clinical trials have studied the use of secondary surgery or surgery to remove more tumor after the initial surgery with varying results.
- An NCI-funded phase 3 clinical trial, in patients whose doctor felt that a second surgery could be helpful for treating the cancer, found that secondary surgery followed by chemotherapy did not increase overall survival compared with chemotherapy alone. Of the studies listed, this one reflected the most likely scenario in real-world practice.
- A trial done in China studied a group of patients more likely to benefit from the intervention. The trial tested secondary surgery followed by chemotherapy and did show improvements in how long women with recurrent epithelial ovarian cancer lived without their cancer growing .
- In a third trial, conducted in Europe, researchers identified people with recurrent ovarian cancer who were most likely to benefit from surgery. They found that women who had secondary surgery followed by chemotherapy lived an average of nearly 8 months longer than women who only received chemotherapy.
In the Chinese and European trials, and in an analysis of 64 clinical trials and other studies , the benefits of secondary surgery were observed only in women who had all of their visible cancer removed.
NCI-Supported Research Programs
Many NCI-funded researchers at the National Institutes of Health campus, and across the United States and the world, are seeking ways to address ovarian cancer more effectively. Some research is basic, exploring questions as diverse as the biological underpinnings of ovarian cancer and the social factors that affect cancer risk. And some is more clinical, seeking to translate this basic information into improving patient outcomes.
The Women’s Malignancies Branch in NCI’s Center for Cancer Research conducts basic and clinical research in breast and gynecologic cancers, including early-phase clinical trials at the NIH Clinical Center in Bethesda, Maryland.
The Ovarian Specialized Programs of Research Excellence (SPOREs) promote collaborative translational cancer research. This group works to improve prevention and treatment approaches, along with molecular diagnostics , in the clinical setting to help people with ovarian cancer.
The Ovarian Cancer Cohort Consortium , part of the NCI Cohort Consortium, is an international consortium of more than 20 cohort studies that follow people with ovarian cancer to improve understanding of ovarian cancer risk, early detection, tumor differences, and prognosis.
NCI’s clinical trials programs, the National Clinical Trials Network , Experimental Therapeutics Clinical Trials Network , and NCI Community Oncology Research Program , all conduct or sponsor clinical studies of ovarian cancer.
Clinical Trials for Ovarian Cancer
NCI funds and oversees both early- and late-phase clinical trials to develop new treatments and improve patient care. Trials are available for the treatment of ovarian cancer.
Ovarian Cancer Research Results
The following are some of our latest news articles on ovarian cancer research:
Approval of Elahere Expands Treatment Options for Some Advanced Ovarian Cancers
Implanted “Drug Factories” Deliver Cancer Treatment Directly to Tumors
Trametinib Is a New Treatment Option for Rare Form of Ovarian Cancer
When Ovarian Cancer Returns, Surgery May Be a Good Choice for Selected Patients
How Does Ovarian Cancer Form? A New Study Points to MicroRNA
Ovarian Cancer Studies Aim to Reduce Racial Disparities, Improve Outcomes
View the full list of Ovarian Cancer Research Results and Study Updates .

New insight into links between menopause timing and cancer risk

13 September 2024
New research has found four genes with some of the largest known effects on the timing of menopause discovered to date.
New research has found four genes with some of the largest known effects on the timing of menopause discovered to date, providing new insight into links between menopause timing and cancer risk.
Genes come in pairs, and when women only have one working copy of the four new genes identified, they have menopause between two and five and a half years earlier than average.
Published in ‘Nature’, the large-scale analysis was funded by the Medical Research Council (MRC) and Wellcome.
The team first looked at variation in data from genetic sequencing of 106,973 post-menopausal female participants in the UK Biobank study, an MRC-funded biomedical resource.
Rare genetic changes
Researchers focused on rare types of genetic changes which cause a loss of the protein and investigated their effect on the timing of menopause.
The genetic changes studied are all rare in the population, however their influence on menopause is five times greater than the impact of any previously identified common genetic variant.
The strongest effect was found from gene variants in ZNF518A, only found in one in 4,000 women.
These variants shortened reproductive lifespan more than most previously identified genes.
Effect of genes
Discovering the effect of the genes gives scientists a better understanding of the biological mechanisms underpinning menopause and links to other diseases.
When unrepaired DNA damage occurs in eggs, they can die. The rate at which eggs are lost determines when women experience menopause.
The team’s previous work has shown that many genes that influence the timing of menopause are likely to do this by affecting the genetic integrity of eggs.
Links with cancer
The same factors affect other cells and tissue types in parallel, and in this new study, the team found that many of the genes linked to menopause timing are also risk factors for cancer.
These include changes in the BRCA1 and BRCA2 genes, which result in earlier menopause and also in an increased risk of cancer.
This is thought to be the process at play in a fifth new gene linked to menopause timing (SAMHD1).
The team discovered that changes in this gene can cause women to go through menopause over a year later than average.
The researchers also found for the first time that changes in this gene cause predisposition to various cancers in men and women.
Professor John Perry, co-lead from the MRC Epidemiology Unit at the University of Cambridge, said:
Past research suggests the female ovary ages at a faster rate than other organ in the body, and this is a model system for understanding the biology of broader ageing. Our latest research builds on this concept, demonstrating that studying ovarian ageing will not only lead to a better understanding of the biology behind infertility and other reproductive disorders, but will enhance our understanding of fundamental processes that regulate DNA damage and cancer risk in the general population.
Study co-lead Professor Anna Murray, of the University of Exeter Medical School, added:
For decades, menopause has been under-researched, yet now this is a rapidly evolving area of science. The timing of menopause has a huge impact on women as they plan their careers and lives, and understanding the genetic changes is of particular interest in terms of potential treatments that could prolong reproductive life in future.

Further information
The paper is entitled ‘ Genetic links between ovarian ageing, cancer risk and de novo mutation rates ’, and is published in ‘Nature’.
Top image: Credit: insta_photos, iStock, Getty Images Plus via Getty Images
Share this page
- Share this page on Twitter
- Share this page on LinkedIn
- Share this page on Facebook
This is the website for UKRI: our seven research councils, Research England and Innovate UK. Let us know if you have feedback or would like to help improve our online products and services .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 05 October 2021
Next steps in the early detection of ovarian cancer
- Robert C. Bast 1 ,
- Chae Young Han 1 ,
- Zhen Lu 1 &
- Karen H. Lu 2
Communications Medicine volume 1 , Article number: 36 ( 2021 ) Cite this article
6606 Accesses
17 Citations
13 Altmetric
Metrics details
- Diagnostic markers
- Ovarian cancer
A recent ovarian cancer screening trial found no reduction in mortality, despite increased detection of early stage disease. Here, we discuss these findings and examine next steps to develop more effective approaches for the early detection of ovarian cancer.
Ovarian cancer afflicts more than 300,000 women each year worldwide. Despite improved care with cytoreductive surgery and combination chemotherapy, the majority of patients will die from their disease. When cancer is limited to the ovaries in stage I, up to 90% of patients can be cured with currently available treatment 1 . Even when disease has spread to pelvic organs in stage II, up to 70% survive for more than 10 years. With further spread over the surface of the abdominal cavity (stage III) or outside the abdomen (stage IV), long term survival is reduced to 20% or lower. Approximately 25–30% of patients are currently diagnosed in stage I or II. It has long been assumed that increasing the fraction of women with ovarian cancer detected at an early stage could improve survival and decrease mortality.
The negative outcome of the UKCTOCS calls into question where we should go next to find an effective strategy for early detection of ovarian cancer.
The United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS)
The UKCTOCS, the largest ovarian cancer screening trial conducted to date, randomized postmenopausal women at average risk for developing ovarian cancer to a control group (101,314), annual transvaginal sonography (TVS) for 7 years (50,623), or “multi-modal screening” for 7 years (50,625) involving a “two-step process”, where changes in annual CA125 ovarian tumor biomarker blood tests were analyzed with a Bayesian Risk of Ovarian Cancer Algorithm (ROCA) prompting TVS in a small fraction of patients with a significant increase in CA125 2 . Executing a screening study of this magnitude constitutes a remarkable achievement. In the initial report in 2016, there was no significant reduction in mortality overall, but a 20% reduction was found in a pre-specified subset of women with incident disease who had been diagnosed after 7 years of screening on the multi-modal arm ( p = 0.021). Given wide statistical bounds around the estimate, re-analysis was planned after 5 years of additional follow-up. The update confirmed a stage shift with an increase in early stage disease and a decrease in late stage disease in the screened population, but failed to confirm a reduction in mortality 1 .
Failure to sustain a mortality advantage despite an increase in early stage disease could relate to inadequate therapy. As collaborating sites were chosen for expertize in gynecologic oncology, surgery is likely to have been state-of-the-art. It would be important to know that all early stage patients received six cycles of carboplatin and paclitaxel. If the choice and duration of chemotherapy were at the discretion of collaborating oncologists, some might have chosen single agent carboplatin or used only three cycles of combination chemotherapy for early stage disease.
Early stage (I/II) disease detected in the multimodal arm of the UKCTOCS was associated with increased mortality, consistent with the possibility that rising CA125 detected additional micro-metastatic disease or identified visible tumors that resisted conventional chemotherapy. While PARP-inhibitor maintenance therapy is generally prescribed for advanced stage (III/IV) disease, patients with screen-detected homologous repair deficient early stage (I/II) ovarian cancer might also benefit. Novel agents, including SIK2 inhibitors 3 , are being developed to enhance primary treatment with carboplatin and paclitaxel potentially improving care for both early and late stage disease.
Furthermore, the magnitude of the stage shift observed in the UKCTOCS may not have been sufficient to reduce mortality. The fraction of stage I/II patients in the UKCTOCS increased from 28.4% with no screening to 38.1% with CA125 followed by TVS. The fraction of patients with stage IV disease decreased from 20.7 to 15.1%. A much greater stage shift was observed in the single arm Normal Risk Ovarian Screening Study (NROSS) that has been conducted, in parallel, over the last 19 years in post-menopausal women at average risk in the United States, using the identical two-step multi-modal screening plan with the CA125 based ROCA followed by TVS. Among the 7597 women screened, 16 epithelial ovarian cancers have been detected—2 were borderline and 14 invasive—with 11 (69%) in early stage (I or II) (updated from ref. 4 ). One of 16 cases (6%) was detected in stage IV. Both trials confirmed that adequate specificity could be attained with the two-step strategy, requiring no more than 2–4 operations to detect each case of ovarian cancer. The reason for a greater stage shift in the smaller trial is not clear. This could reflect statistical variation with the smaller size of the NROSS. Difficulties were, however, encountered with TVS imaging in the UKCTOCS. In a retrospective review of 1000 archived cases, ovaries and fallopian tubes could be identified in only 50% of cases 5 . TVS imaging could have been more reliable in the NROSS. Another difference between the trials relates to processing of blood for measurement of CA125. In the NROSS, blood was drawn in glass tubes without gel, serum was separated and frozen on the same day, while in the UKCTOCS blood was drawn in gel separation tubes, shipped at ambient temperature and separated after up to 56 h. A modest systematic reduction in CA125 levels in the UKCTOCS could have decreased the ability to detect early stage disease. In addition, particular care was taken in the NROSS to follow elevations of CA125 with repeated TVS and to minimize time to surgical intervention.
The negative outcome of the UKCTOCS calls into question, where we should go next to find an effective strategy for early detection of ovarian cancer. While some might suspend attempts to detect early stage ovarian cancer awaiting a novel and disruptive technology, the two-step screening strategy has already achieved adequate specificity and a clear stage shift, although sensitivity is not yet adequate. There are opportunities for improvement both in serum biomarkers and in imaging. Only 80% of ovarian cancers express CA125 and serum levels of CA125 are elevated in only 70% of stage I/II cancers. A recent review identified 35 biomarkers that complement CA125 and could potentially improve sensitivity of the initial step in screening 6 . A combination of CA125, HE4, and CA72.4 detects 16% of cases missed by CA125 7 . Through a collaboration sponsored by the NCI Early Detection Research Network (EDRN), CA125 detected 72% of early stage cases at 98% specificity, whereas a combination of CA125, HE4 antigen-autoantibody complexes 8 and osteopontin 9 detected 89% at 94% specificity 10 . A second-generation ROCA algorithm is being developed and can be tested prospectively for specificity in the NROSS cohort.
In addition to detecting a greater fraction of early stage patients, panels of biomarkers could improve lead time with detection of cancers at longer intervals before clinical presentation. Autoantibodies could arise in response to very small volumes of early disease, which would be particularly important for high grade serous lesions arising from the fallopian tube. Anti-p53 autoantibodies have been detected in more than 20% of patients with early and late stage ovarian cancer 11 . Assaying serum samples from the UKCTOCS, titers of anti-p53 autoantibodies increased 8 months before elevation of CA125 and 22 months prior to clinical presentation in patients who did not exhibit increases in serum CA125 12 . This is the first of >120 biomarkers tested by our group that increased lead time over CA125. Among 19 promising autoantibodies tested, anti-p53, anti-CTAG1, and anti-IL-8 detected the greatest fraction of early stage ovarian cancer patients 11 .
A variety of additional biomarkers are being developed to detect ovarian cancer including ctDNA, methylated DNA, and miRNAs. Alterations in cervical and peripheral blood ctDNA can complement CA125 in detecting early stage disease 12 . While ovarian cancer has been included in DNA-based pan-cancer screening strategies 13 , 14 , detecting stage I/II disease has proven challenging. Future research should optimize the integration of DNA and protein biomarkers.
Whatever the biomarker panel chosen, screening could be performed more frequently. In patients at high risk, largely related to germ-line BRCA1/2 mutations, screening with the ROCA every 3 months proved more effective than annual screening 15 , 16 , While it is difficult to imagine more frequent screening for ovarian cancer alone in patients at conventional risk, blood might be drawn every six months to screen for multiple cancers in women over 50. Ovarian cancer screening could be paired with DNA-based pan-cancer screening strategies or combined with site-specific blood tests that are being developed to detect colorectal adenomas, and breast and pancreatic cancers 17 .
Imaging, the second step in two-stage screening, poses perhaps the greatest unmet need. As a single modality, TVS lacks adequate sensitivity and specificity for early detection of ovarian cancer. The majority of high-grade serous cancers probably arise in the fallopian tubes. Even in expert hands, fallopian tubes could not be imaged in 23% of 549 healthy women 18 . CT, PET-CT, and MRI also have problems with sensitivity, specificity, exposure to radiation and cost for screening 7 .
One possible solution in patients with rising serum biomarkers and negative TVS is falloposcopy, where a fiberoptic scope is threaded through the uterus and fallopian tube to visualize the fimbriae and ovary. This would be particularly relevant for women with BRCA1/2 mutations who are delaying risk reducing surgery. The EDRN is currently evaluating the feasibility of this approach. Another technology that is being developed is superconducting quantum interference detection (SQUID), which is a sensitive method for detecting faint magnetic fields. Anti-CA125 antibodies have been conjugated with ferritin nanospheres. Only antibody conjugated nanospheres bound to cells are detected by magnetic relaxation. Ex vivo, 10 6 ovarian cancer cells (0.1 mm) can be detected 7 . If uptake of antibody-coated nanospheres can be optimized in xenografts, this approach might be utilized to detect recurrent ovarian cancer and then tested in healthy women with rising biomarkers and negative TVS.
Conclusions
Given the specificity of the two-step screening strategy, opportunities to improve both phases and the impressive stage shift in the CA125-based NROSS trial, further development of this approach appears worthy of pursuit. The potential benefit for ovarian cancer patients is substantial. Computer simulations suggest that an effective strategy for early detection could reduce mortality by 10–30%, a dramatic improvement over our current attempts to improve therapy 7 .
Menon, U. et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet 397 , 2182–2193 (2021).
Article Google Scholar
Jacobs, I. J. et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet 387 , 945–956 (2016).
Fan, D. et al. A novel salt inducible kinase 2 inhibitor, ARN-3261, sensitizes ovarian cancer cell lines and xenografts to carboplatin. Cancers 13 , 446 (2021).
Article CAS Google Scholar
Lu, K. H. et al. A 2-stage ovarian cancer screening strategy using the risk of ovarian cancer algorithm (ROCA) identifies early-stage incident cancers and demonstrates high positive predictive value. Cancer 119 , 3454–3461 (2013).
Stott, W. et al. Sonographers’ self‐reported visualization of normal postmenopausal ovaries on transvaginal ultrasound is not reliable: results of expert review of archived images in UKCTOCS. Ultrasound Obstet. Gynecol. 51 , 401–408 (2018).
Bast, R. C. Jr et al. Biomarkers and strategies for early detection of ovarian cancer. Cancer Epidemiol. Biomark. Prev. 29 , 2504–2512 (2020).
Simmons, A. R. et al. Complementary longitudinal serum biomarkers to CA125 for early detection of ovarian cancer. Cancer Prev. Res. 12 , 391–400 (2019).
Yang, W. L. et al. Human epididymis protein 4 antigen-autoantibody complexes complement cancer antigen 125 for detecting early-stage ovarian cancer. Cancer 126 , 725–736 (2019).
Guo, J. et al. Osteopontin, macrophage migration inhibitory factor and anti-interleukin-8 autoantibodies complement CA125 for detection of early stage ovarian cancer. Cancers 11 , 596 (2019).
Han, C. Y. et al. Multi-biomarker panel assessment of serological assays in early detection of ovarian cancer. In 12th EDRN Scientific Workshop (2021).
Yang, W. L. et al. Elevation of TP53 autoantibody before CA125 in preclinical invasive epithelial ovarian cancer. Clin. Cancer Res. 23 , 5912–5922 (2017).
Bast, R. C. Jr, et al. Critical questions in ovarian cancer research and treatment: report of an AACR Special Conference. Cancer 125 , 1963–1972 (2019)
Cohen, J. D. et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359 , 926–930 (2018).
Lennon, A. M. et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science https://doi.org/10.1126/science.abb9601 (2020).
Rosenthal, A. N. et al. Evidence of stage shift in women diagnosed with ovarian cancer during phase II of the United Kingdom Familial Ovarian Cancer Screening Study. J. Clin. Oncol. 35 , 1411–1420 (2017).
Skates, S. J. et al. Early detection of ovarian cancer using the risk of ovarian cancer algorithm with frequent CA125 testing in women at increased familial risk—combined results from two screening trials. Clin. Cancer Res. 23 , 3628–3637 (2017).
Bast, R. C. & Srivastava, S. The National Cancer Institute Early Detection Research Network: two decades of progress in cancer biomarkers. Cancer Epidemiol. Biomark. Prev. 29 , 2396–2400 (2020).
Lefringhaus, J. R. et al. Probability of fallopian tube and ovarian detection with transvaginal ultrasonography in normal women. Women’s Health 12 , 303–311 (2016).
Google Scholar
Download references
Acknowledgements
This work was supported by funds from the NCI Early Detection Research Network (5 U01 CA200462-02, RCB), the MD Anderson Ovarian SPOREs (P50 CA83639 and P50CA217685, R.C.B.), National Cancer Institute, Department of Health and Human Services; the Cancer Prevention Research Institute of Texas (RP160145, R.C.B.); Golfer’s Against Cancer; the Tracey Joe Wilson Foundation; and generous donations from the Ann and Henry Zarrow Foundation, the Mossy Foundation, the Roberson Endowment, Stuart and Gaye Lynn Zarrow, Barry and Karen Elson, and Arthur and Sandra Williams.
Author information
Authors and affiliations.
Department of Experimental Therapeutics, University of Texas M.D. Anderson Cancer Center, Houston, TX, 77030, USA
Robert C. Bast, Chae Young Han & Zhen Lu
Department of Gynecologic Oncology and Reproductive Medicine, University of Texas M.D. Anderson Cancer Center, Houston, TX, 77030, USA
Karen H. Lu
You can also search for this author in PubMed Google Scholar
Contributions
R.C.B. wrote the commentary. C.Y.H., Z.L., and K.H.L. contributed data and reviewed the manuscript.
Corresponding author
Correspondence to Robert C. Bast .
Ethics declarations
Competing interests.
Dr. Bast receives royalties for the discovery of CA125 from Fujirebio Diagnostics Inc. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Bast, R.C., Han, C.Y., Lu, Z. et al. Next steps in the early detection of ovarian cancer. Commun Med 1 , 36 (2021). https://doi.org/10.1038/s43856-021-00037-9
Download citation
Received : 26 July 2021
Accepted : 15 September 2021
Published : 05 October 2021
DOI : https://doi.org/10.1038/s43856-021-00037-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Large-scale proteomics reveals precise biomarkers for detection of ovarian cancer in symptomatic women.
- Emma Ivansson
- Julia Hedlund Lindberg
- Stefan Enroth
Scientific Reports (2024)
Single-cell RNA sequencing reveals a pro-metastatic subpopulation and the driver transcription factor NFE2L1 in ovarian cancer cells
- Junseong Park
- Yoon-Seob Kim
- Yeun-Jun Chung
Genes & Genomics (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

- Entertainment
- Life & Style

To enjoy additional benefits
CONNECT WITH US

Understanding ovarian cancer: its causes, symptoms, and screening methods Premium
This september, during ovarian cancer awareness month, understanding the cancer’s lesser-known facts — from subtle symptoms to genetic and lifestyle risks — can lead to earlier detection, personalised treatment, and preventive strategies.
Updated - September 13, 2024 12:09 pm IST

For representative purposes. | Photo Credit: Getty Images
Note: The information in this article is provided as such and is not intended as medical advice. If you have any concerns, please consult your physician.
Ovarian cancer is the most lethal gynecological malignancy. It is often called a “silent killer” because its symptoms are non-specific and mimic less serious conditions that lead to late diagnosis and a poor prognosis. In India, ovarian cancer ranks among the top three cancers, contributing to 6.6% of all women’s cancers .
In 2022, India reported 47,333 new ovarian cancer cases and 32,978 deaths. These alarming figures highlight the gravity of the disease. Understanding lesser-known aspects of ovarian cancer can enhance prevention, early detection, and treatment efforts, offering hope to patients and healthcare providers.
Unlike other cancers, ovarian cancer presents vague symptoms such as bloating, pelvic or abdominal pain, loss of appetite, feeling full quickly, and an urgent or frequent need to urinate. Other signs include indigestion, constipation, back pain, persistent fatigue, weight loss, and postmenopausal vaginal bleeding. These symptoms are often mistaken for common ailments, leading to late diagnosis. A 2004 study reported women with (malignant) ovarian cancer typically experience these symptoms 20 to 30 times a month, and which are more severe than those without the disease. Unfortunately, because of the overlapping nature of these symptoms with other common ailments, they can be dismissed until the cancer has advanced. Healthcare providers must be vigilant when women report persistent symptoms.
Cancer subtypes and screening
Ovarian cancer is not a uniform disease. It has two main subtypes: type I and type II. Type I tumours are less common, typically diagnosed at an early stage, and have a better prognosis. Type II tumours are more common, more aggressive, usually diagnosed at an advanced stage, and are responsible for most deaths due to ovarian cancer.
The survival rate for patients with ovarian cancer depends on the stage of detection and access to appropriate treatment. Researchers reported in September 2022 that around 20% of patients with advanced ovarian cancer who receive optimal surgery and platinum-based chemotherapy could be disease-free at 10 years and might be considered potentially cured.
Unlike breast or cervical cancer, there are no effective screening tests for ovarian cancer. The CA125 blood test, often included in cancer screening packages, is not recommended for routine screening in women at average risk due to its limited specificity. While CA125 is useful to monitor ovarian cancer after diagnosis, it is less effective at screening asymptomatic women as it can lead to false positives and unnecessary further testing, anxiety, and potentially over-treatment.
Due to the absence of a reliable screening tool, awareness of risk factors and symptoms becomes crucial. Regular consultations with healthcare providers and discussions about family history can lead to earlier detection and better management of ovarian cancer.
Genes and endometriosis
While it’s possible to develop ovarian cancer even without a family history of breast or ovarian cancer, it’s the most heritable of all cancers: 65-85% of hereditary ovarian cancer cases involve mutations in the BRCA1 or the BRCA2 genes. Women with these mutations have a significantly higher risk of developing ovarian cancer — up to 50% for BRCA1 and around 15% for BRCA2. Some other genes have also been associated with hereditary ovarian cancer.
Genetic testing allows for personalised risk management, including tailored clinical surveillance, chemoprevention, and prophylactic surgeries, which can reduce the risk of developing ovarian cancer in high-risk women. Endometriosis, a condition where uterine-like tissue grows outside the uterus, has been linked to an increased risk of certain types of ovarian cancer, particularly endometrioid and clear-cell cancers. This said, endometriosis-linked ovarian cancer risk is low and radical measures, such as a bilateral salpingo-oophorectomy, to prevent ovarian cancer alone are rarely justified.
Lifestyle factors
Certain lifestyle factors are linked to ovarian cancer risk. For example, the use of talcum powder in the genital area has long been debated, with mixed evidence about its potential link to ovarian cancer. In the past, some talc products contained asbestos, a known carcinogen, but the evidence regarding modern, asbestos-free talc is still inconclusive. Experts including the American Cancer Society have called for more research to settle these questions. Another emerging area of concern is the use of chemical hair products. Some studies have shown a possible link between the prolonged use of hair dyes and an increased risk of ovarian cancer.
Additionally, frequent use of hair straighteners, relaxers or pressing products that release formaldehyde gas — a known carcinogen — may elevate the risk of developing ovarian cancer. Again, more research is required.
HRT, menopause, and genetic counselling
Hormone replacement therapy (HRT), commonly used to alleviate menopausal symptoms, has been linked to a higher risk of ovarian cancer even when used for less than five years. Postmenopausal women considering HRT should weigh this risk against the benefits and explore alternatives with their healthcare provider to make informed decisions.
For women with a family history of ovarian or breast cancer, genetic counselling is a valuable tool. This process helps identify individuals at risk for hereditary cancers and provides tailored guidance on preventive measures and potential treatments. Through personalised risk management strategies, genetic counselling can improve outcomes and help reduce the likelihood of developing ovarian cancer.
Ovarian cancer may be elusive, but knowledge is power. This September, during Ovarian Cancer Awareness Month, understanding its lesser-known facts — from subtle symptoms to genetic and lifestyle risks — can lead to earlier detection, personalised treatment, and preventive strategies. Empower yourself and others with this knowledge — it can save lives.
Anup Rawool is a Clinical and Cancer Geneticist and the founder of Sahaj Genetics Clinic, Comprehensive Medical Genetics & Counseling Center. Vid Karmarkar is the founder and CEO of Canseva Foundation.
Published - September 11, 2024 08:30 am IST
Related Topics
Text and Context / cancer
Top News Today
- Access 10 free stories every month
- Save stories to read later
- Access to comment on every story
- Sign-up/manage your newsletter subscriptions with a single click
- Get notified by email for early access to discounts & offers on our products
Terms & conditions | Institutional Subscriber
Comments have to be in English, and in full sentences. They cannot be abusive or personal. Please abide by our community guidelines for posting your comments.
We have migrated to a new commenting platform. If you are already a registered user of The Hindu and logged in, you may continue to engage with our articles. If you do not have an account please register and login to post comments. Users can access their older comments by logging into their accounts on Vuukle.
Civilsdaily
No. 1 UPSC IAS Platform for preparation
Mother and Child Health – Immunization Program, BPBB, PMJSY, PMMSY, etc.
Ovarian cancer: understanding the silent killer.
From UPSC perspective, the following things are important :
Prelims level: Ovarian Cancer
Why in the News?
- In India, Ovarian Cancer ranks among top three cancers affecting women , accounting for 6.6% of all female cancer cases.
- In 2022 , India alone reported 47,333 new ovarian cancer cases and 32,978 deaths , emphasizing the critical need for awareness, early detection, and effective treatment.
What is Ovarian Cancer?
| Referred to as the due to its subtle symptoms Late diagnosis is common. | |
| • Bloating • Pelvic/abdominal pain • Loss of appetite • Frequent urination • Indigestion, back pain, fatigue, and weight loss | |
| • : Less common, early diagnosis, better prognosis • : More common, aggressive, diagnosed at advanced stages | |
| • No effective screening tests available • used post-diagnosis but not recommended for routine screening due to limited specificity | |
| • Linked to mutations in , increasing risk by up to 50% • aids in personalized risk management | |
| • Associated with a higher risk of certain ovarian cancers, though overall risk remains low | |
| • regarding usage and its potential link to ovarian cancer • (dyes, straighteners) may increase risk, though further research is required | |
| • is linked to a higher risk of ovarian cancer • helps at-risk individuals with preventive measures |
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024

JOIN THE COMMUNITY
Join us across social media platforms., your better version awaits you.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Ovarian Res

Investigation on factors associated with ovarian cancer: an umbrella review of systematic review and meta-analyses
Kiarash tanha.
1 Department of Biostatistics, School of Public Health, Iran University of Medical Sciences, Tehran, Iran
Azadeh Mottaghi
2 Research Center for Prevention of Cardiovascular Diseases, Institute of Endocrinology and Metabolism, Iran University of Medical Sciences, Tehran, Iran
Marzieh Nojomi
3 Preventive Medicine and Public Health Research Center, Psychosocial Health Research Institute, Community and Family Medicine Department, School of Medicine,Iran University of Medical Sciences, Tehran, Iran
4 Department of Sociology & Anthropology, Nipissing University, Ontario North Bay, Canada
Marzieh Moradi
5 Department of Epidemiology, School of Public Health, Iran University of Medical Sciences, Tehran, Iran
Rezvan Rajabzadeh
6 School of Health, North Khorasan University of Medical Sciences, Bojnurd, Iran
Samaneh Lotfi
Leila janani.
7 Imperial Clinical Trials Unit, School of Public Health, Faculty of Medicine, Imperial College London, London, UK
Associated Data
The data for supporting the research findings are available from the corresponding author upon reasonable request.
Following cervical and uterine cancer, ovarian cancer (OC) has the third rank in gynecologic cancers. It often remains non-diagnosed until it spreads throughout the pelvis and abdomen. Identification of the most effective risk factors can help take prevention measures concerning OC. Therefore, the presented review aims to summarize the available studies on OC risk factors. A comprehensive systematic literature search was performed to identify all published systematic reviews and meta-analysis on associated factors with ovarian cancer. Web of Science, Cochrane Library databases, and Google Scholar were searched up to 17th January 2020. This study was performed according to Smith et al. methodology for conducting a systematic review of systematic reviews. Twenty-eight thousand sixty-two papers were initially retrieved from the electronic databases, among which 20,104 studies were screened. Two hundred seventy-seven articles met our inclusion criteria, 226 of which included in the meta-analysis. Most commonly reported genetic factors were MTHFR C677T (OR=1.077; 95 % CI (1.032, 1.124); P-value<0.001), BSML rs1544410 (OR=1.078; 95 %CI (1.024, 1.153); P-value=0.004), and Fokl rs2228570 (OR=1.123; 95 % CI (1.089, 1.157); P-value<0.001), which were significantly associated with increasing risk of ovarian cancer. Among the other factors, coffee intake (OR=1.106; 95 % CI (1.009, 1.211); P-value=0.030), hormone therapy (RR=1.057; 95 % CI (1.030, 1.400); P-value<0.001), hysterectomy (OR=0.863; 95 % CI (0.745, 0.999); P-value=0.049), and breast feeding (OR=0.719, 95 % CI (0.679, 0.762) and P-value<0.001) were mostly reported in studies. Among nutritional factors, coffee, egg, and fat intake significantly increase the risk of ovarian cancer. Estrogen, estrogen-progesterone, and overall hormone therapies also are related to the higher incidence of ovarian cancer. Some diseases, such as diabetes, endometriosis, and polycystic ovarian syndrome, as well as several genetic polymorphisms, cause a significant increase in ovarian cancer occurrence. Moreover, other factors, for instance, obesity, overweight, smoking, and perineal talc use, significantly increase the risk of ovarian cancer.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13048-021-00911-z.
Following cervical and uterine cancer, ovarian cancer (OC) has the third rank in gynecologic cancers. A woman’s risk of getting ovarian cancer during her lifetime is about 1 in 78. Mortality rate of ovarian cancer is about 1 in 108. (These statistics don’t count low malignant potential ovarian tumors.) It often remains non-diagnosed until it spreads throughout the pelvis and abdomen, making its treatment even more difficult. At its early stages, when it is limited to the ovary, the treatment success has a higher rate. The silent tumor growth in OC increases its mortality rate and deteriorates its prognosis [ 1 ]. OC has a 46 % five-year survival rate. Early detection is important. Most women with Stage 1 ovarian cancer have an excellent prognosis. Stage 1 patients with grade 1 tumors have a 5-year survival of over 90 %, as do patients in stages 1 A and 1B [ 2 ].
Besides the undetectable progress of this type of cancer, improper screening methods further delay its diagnosis [ 3 ]. Due to the low prevalence of ovarian cancer even amongst postmenopausal women (1:2500), an efficient screening tool requires high sensitivity (>75 %) and extremely high specificity (99.7 %) [ 4 ].
A significant increase is estimated in its mortality rate by 2040. Nonetheless, identification of the most effective risk factors can be helpful in prevention measures concerning OC [ 5 ]. Conflicting results can be found in the literature describing the role of several factors (e.g., nutritional, environmental, and genetic factors, as well as lifestyle, drug use, and medical history). Genetic predisposition is related to a higher risk of ovarian cancer that also tends to occur at a younger age. BRCA1 and 2 mutation carriers harbor significantly increased ovarian cancer risk (40–45 % resp. 15–20 %) by the age of 70. Risk of OC in the high risk women under 40 years old is low [ 6 ]. Several studies on ovarian cancer have been published that have examined various factors influencing the incidence, prevalence and mortality rate. Some of these studies were purely observational and some were meta-analyzes. So far, no study has been published that has summarized and re-analyzed the results of various meta-analyzes in this field, and this issue shows the importance of this study. The present study examined up to 50 factors (nutritional and genetic factors, drugs use, some diseases, breast feeding, smoking and physical activity) that other studies had examined and sometimes presented conflicting results.
The presented umbrella meta-analysis and systematic review is focused on any kind of risk factors on ovarian cancer among all women and aimed to summarize the available reviews and find the most important OC risk factors.
This study is focused on any kind of risk factors on ovarian cancer among all women.
A systematic review of systematic reviews was conducted to identify the associated factors with OC. This study was performed according to Smith et al. methodology for conducting a systematic review of systematic reviews [ 7 ].
Study question
What are the most important factors associated with ovarian cancer found in systematic reviews?
Literature search
A comprehensive systematic literature search was performed to identify all published systematic reviews and meta-analysis on associated factors with OC. Medline through PubMed, Scopus, Embase, Web of Science, Cochrane Library databases, and Google Scholar all were searched up to 17th January 2020 without time limitation. The search strategy included the use of Mesh terms and keywords related to subject and study design (ovarian; ovary; cancer; carcinoma; neoplasm; tumor; Malignancy; review; systematic review; systematic literature review; meta-analysis). The detailed search strategy for the Medline can be found in the supplementary, Table 1 S. The reference lists of selected articles were also manually searched to identify any additional related documents.
Study selection
This overview only included systematic reviews of factors associated with OC.
The articles which met the following criteria were included in our study: (1) systematic reviews or meta-analysis; (2) have evaluated risk factors of Ovarian cancer; (3) have at least abstracts in English. The articles that were narrative reviews or had assessed prognostic factors of OC or did not provide at least abstract in English were excluded. Characteristic of included studies are illustrate in Table 1 .
Table 1
Characteristic of included studies
| No. | Author | Year | No. of Articles | No. of Patient (total) | No. of Cases | No. of Control | Evaluated Factors |
|---|---|---|---|---|---|---|---|
| 1 | Yan Qiao | 2018 | 21 | 309 | - | - | Aspirin |
| 2 | Hongmei Chen | 2017 | 14 | 11,690 | 4448 | 7242 | VDR rs2228570 |
| 3 | Li-Hui Yan | 2018 | 46 | 84,772 | 36,298 | 48,474 | BRCA2 N372H |
| 4 | Jie Ruan | 2018 | 24 | 1217 | - | - | P16INK4a |
| 5 | liang Tang | 2018 | 13 | 13,064 | 5461 | 7603 | HER2 and ESR2 polymorphisms |
| 6 | Ross Penninkilampi | 2018 | 27 | - | 14,311 | - | Talc Use |
| 7 | Chao-Huan Xu | 2017 | 7 | 3016 | 1,345 | 1,671 | Genetic polymorphisms |
| 8 | Xu-Ming Zhu | 2017 | 10 | 4621 | 1930 | 2464 | Genetic polymorphisms |
| 9 | JieNa Li | 2017 | 9 | 4024 | 1333 | 2691 | ERCC2 rs13181 |
| 10 | Jing Li | 2017 | 7 | - | 1898 | - | C-reactive protein |
| 11 | Dongyu Zhang | 2017 | 14 | 2,342,245 | 4184 | | Diabetes mellitus |
| 12 | Xingxing Song | 2017 | 15 | 493,415 | 7453 | 485,962 | Calcium Intake |
| 13 | Wera Berge | 2016 | 27 | 34,176 | 15,154 | 19,022 | Talc Use |
| 14 | Xin Zhan | 2017 | 18 | 701,857 | 8,683 | 693,174 | Tea consumption |
| 15 | A Darelius | 2017 | 11 | - | - | - | Hysterectomy |
| 16 | Zhiyi Zhou | 2017 | 13 | 2,951,539 | 13,616 | 2,937,923 | Pelvic inflammatory disease |
| 17 | Yang Deng | 2017 | 8 | 14,014 | 6613 | 7401 | Androgen receptor gene |
| 18 | Bamia Christina | 2016 | 32 | - | 11,411 | - | Coffee Intake |
| 19 | Lihua Wang | 2017 | 13 | 3,708,313 | 5534 | 3,702,779 | Diabetes mellitus |
| 20 | lilin he | 2017 | 8 | 45,624 | 19,260 | 26,364 | MTHFR C677T |
| 21 | Chunpeng Wang | 2016 | 38 | 409,061 | 40,609 | 368,452 | Endometriosis, Tubal Ligation, Hysterectomy |
| 22 | Chunyan Shen | 2016 | 12 | 1235 | 806 | 429 | Adenomatous polyposis coli (APC) gene |
| 23 | Xiyue Xiao | 2016 | 12 | 901 | 612 | 289 | P16INK4a |
| 24 | Fangfang Zeng | 2016 | 7 | 33,456 | 2011 | 31,445 | Inflammatory markers |
| 25 | Dongyu Zhang | 2016 | 23 | 499,950 | 15,163 | 484,787 | Aspirin |
| 26 | Wenlong Qiu | 2016 | 25 | 900,000 | 6612 | 893,388 | Dietary fat intake |
| 27 | Qiang Wang | 2016 | 9 | 740 | 485 | 255 | CDH1 promoter |
| 28 | Xiaoli Hua | 2016 | 12 | 2,361,494 | 6,275 | 2,355,219 | Dietary Flavonoids |
| 29 | Li-feng Shi | 2015 | 12 | 2,353,945 | 8896 | 2,345,049 | Hormone therapy |
| 30 | Christos Iavazzo | 2016 | 4 | 725 | 385 | 340 | Hypodontia |
| 31 | Sang-Hee Yoon | 2016 | 3 | 5,659,211 | 3509 | 5,655,702 | salpingectomy |
| 32 | Wei Liu | 2016 | 35 | 42,650 | 19,527 | 23,123 | A1298C POLYMORPHISM |
| 33 | Vida Mohammadi | 2019 | 7 | 381,810 | 3653 | 378,157 | flavonoids |
| 34 | Lifeng Li | 2016 | 9 | - | - | - | Metformin |
| 35 | Arefe Parvaresh | 2019 | 13 | - | - | - | Quercetin |
| 36 | Xiaowei Yu | 2016 | 14 | 11,471 | 3796 | 7675 | ERCC2 rs13181 - XRCC2 rs3218536 |
| 37 | Rui Hou | 2015 | 20 | 1,117,992 | 12,046 | 1,105,946 | Dietary fat |
| 38 | Zhen Liu | 2015 | 26 | 34,817 | 12,963 | 21 854 | overweight, obesity |
| 39 | N. Keum | 2015 | 18 | - | 2636 | - | Egg intake |
| 40 | Liangxiang Su | 2015 | 4 | 12,016 | 2344 | 9672 | BRCA2 N372H |
| 41 | Sai-tian Zeng | 2014 | 12 | 629,453 | 3728 | 625,725 | Egg intake |
| 42 | Xiaolian Zhang | 2015 | 5 | 4233 | 1791 | 2,196 | Vascular Endothelial Polymorphisms |
| 43 | Li-Ping Feng | 2014 | 19 | 469,095 | 9438 | 459,657 | Breastfeeding |
| 44 | collaborative Group | 2015 | 52 | 12,110 | - | - | Menopausal hormone use |
| 45 | Huang Yan-Hong | 2015 | 13 | 1,996,841 | 5857 | 1,990,984 | alcohol consumption |
| 46 | Jiyi Hu | 2015 | 8 | 305,338 | 3555 | 301,783 | cruciferous vegetables |
| 47 | Jing Liao | 2014 | 21 | 3117 | 2842 | 4305 | progesterone receptor Polymorphisms |
| 48 | Xingzhong Hu | 2015 | 5 | 5884 | 2336 | 3548 | RAD51 Gene 135G/C |
| 49 | Jing Liu | 2014 | 19 | - | - | - | Milk, Yogurt, and Lactose Intake |
| 50 | Jun Qin | 2014 | 62 | 92,857 | 42,315 | 50,542 | STK15 polymorphisms |
| 51 | Luliang Liu | 2015 | 15 | 14,798 | 7,450 | 7,348 | MMP-12-82 A/G polymorphism |
| 52 | X.Y. Shi | 2015 | 3 | 7026 | - | - | MTHFR A1298C polymorphism |
| 53 | M. Zhai | 2015 | 4 | 10,169 | 3565 | 6604 | Arg188His polymorphism |
| 54 | Yue-Dong Wang | 2014 | 15 | 1653 | 822 | 831 | serum levels of osteopontin |
| 55 | John A. Barry | 2014 | 3 | 72,973 | 919 | 72,054 | polycystic ovary syndrome |
| 56 | Xinli Li | 2014 | 10 | 72,054 | 6127 | 65,927 | dietary lycopene intake |
| 57 | Xue Qin | 2014 | 4 | 1133 | 474 | 659 | Asn680Ser polymorphism |
| 58 | Shujing Shi | 2014 | 13 | 16,230 | 5,927 | 10,303 | RAD51 135 G>C and XRCC2 G>A (rs3218536) |
| 59 | M. A. Alqumber | 2014 | 12 | 2257 | 993 | 1264 | 72 Arg.Pro Polymorphism |
| 60 | Pei-yue Jiang | 2014 | 15 | 889,033 | 6,087 | 882,946 | Fish Intake |
| 61 | Danhua Pu | 2014 | 7 | 7356 | 3493 | 3863 | MTHFR Polymorphism |
| 62 | Xinwei Pan | 2013 | 8 | 7724 | 3,723 | 4,001 | Ala222Val |
| 63 | Yulan Yan | 2013 | 4 | 9108 | 3,635 | 5,473 | XRCC3 Thr241Met polymorphism |
| 64 | Tracy E. Crane | 2013 | 24 | 519,431 | 2091 | 517,340 | Dietary Intake |
| 65 | Su Li | 2014 | 14 | 10,964 | - | - | VDR rs2228570 |
| 66 | Dan Cheng | 2014 | 22 | 15,343 | 6836 | 8507 | RAD51 Gene 135G/C polymorphism |
| 67 | Bo Han | 2014 | 11 | 379,868 | 4,306 | 375,562 | Cruciferous vegetables |
| 68 | Xin-Lan Qu | 2014 | 10 | 297,892 | 4392 | 293,500 | Phytoestrogen Intake |
| 69 | Jin-Ze Du | 2014 | 8 | 3940 | 1,293 | 2,647 | COMT rs4680 Polymorphism |
| 70 | Li-Yuan Han | 2014 | 10 | 6001 | 2578 | 3423 | GST Genetic Polymorphisms |
| 71 | Da-Peng Li | 2014 | 40 | 415,949 | 17,139 | 398,810 | Breastfeeding |
| 72 | Yong-Jun Ma | 2014 | 6 | 3839 | 1,766 | 2,073 | Rs11615 (C>T) |
| 73 | Jalal Poorolajal | 2014 | 19 | - | - | - | BMI |
| 74 | Li-Min Zhou | 2014 | 6 | 435,398 | 2983 | 432,415 | Recreational Physical Activity |
| 75 | Piyemeth Dilokthornsakul | 2013 | 4 | - | - | - | Metformin |
| 76 | Chenglin Li | 2013 | 18 | 227,859 | 5677 | 222,182 | Folate intake and MTHFR polymorphism C677T |
| 77 | Susan J. Jordan | 2013 | 22 | - | - | - | hysterectomy |
| 78 | Nan-Nan Luan | 2013 | 35 | 720,617 | 14,465 | 706,152 | Breastfeeding |
| 79 | Xue Qin | 2013 | 7 | 4,809 | 1977 | 2832 | VDR |
| 80 | Laura J. Havrilesky | 2013 | 55 | 31,056 | 10,031 | 21,025 | Oral Contraceptive |
| 81 | Ting-Ting Gong | 2012 | 27 | 1,020,516 | 9859 | 1,010,657 | Age at menarche |
| 82 | Yanling Liu | 2013 | 6 | 10,768 | 4,107 | 6,661 | VDR |
| 83 | Louise Baandrup | 2012 | 21 | 563,976 | 11,759 | 552,217 | NSAIDs |
| 84 | Jung-Yun Lee | 2012 | 19 | - | - | - | Diabetes Mellitus |
| 85 | Chengbin Ma | 2013 | 10 | 18, 628 | 5, 932 | 12,696 | MTHFR C677T polymorphism |
| 86 | Ying-Yu Ma | 2013 | 6 | 3745 | 1534 | 2211 | MDM2 309T.G Polymorphism |
| 87 | Gwan Gyu Song | 2013 | 12 | 8775 | 3716 | 5059 | VDR |
| 88 | Ketan Gajjar | 2012 | 5 | 3795 | 1199 | 2596 | Cytochrome P1B1 (CYP1B1) |
| 89 | Xiaojian Ni | 2012 | 17 | 193,424 | 10 373 | 183,051 | NSAIDs |
| 90 | Lu Liu | 2012 | 4 | 7127 | 3,496 | 3,631 | C677T and A1298C polymorphism |
| 91 | T.N. Sergentanis | 2012 | 11 | 5025 | 1,680 | 3345 | MspI and Ile462-Val and Thr461Asn |
| 92 | 2012 | 51 | 123,056 | 28 114 | 94,942 | Smoking | |
| 93 | Megan S Rice | 2012 | 30 | 18,929 | - | - | Tubal ligation and Hysterectomy |
| 94 | Matteo Rota | 2012 | 27 | 15,762,134 | 16,554 | 15,745,580 | Alcohol drinking |
| 95 | Collaborative Group | 2012 | 47 | 106,468 | 25,157 | 81,313 | Body Size |
| 96 | Su-Qin Shen | 2012 | 18 | 7368 | 2,193 | 5,175 | TP53 Arg72Pro |
| 97 | Xiao-Ping Ding | 2012 | 8 | 7457 | 3,379 | 4,078 | MTHFR C677T Polymorphism |
| 98 | M.G.M. Braem | 2011 | 150 | - | - | - | Genetic variants |
| 99 | 2011 | 18 | 21,973 | 117 | 22,090 | Asbestos | |
| 100 | David Cibula | 2011 | 3 | - | - | - | Oral contraceptives |
| 101 | Sarah J. Oppeneer | 2011 | 16 | - | 7234 | - | Tea Consumption |
| 102 | Lu Yin | 2011 | 10 | 157,292 | - | - | Circulating vitamin D |
| 103 | A Wallin | 2011 | 8 | 754 836 | 2349 | 752,487 | Red and processed meat consumption |
| 104 | D. Cibula | 2011 | 13 | - | - | - | Tubal ligation |
| 105 | Ru-Yan Liao | 2010 | 4 | 15,104 | 5532 | 9572 | TGFBR1*6A/9A polymorphism |
| 106 | Linda S. Cook | 2010 | 20 | - | - | - | vitamin D |
| 107 | K. P. Economopoulos (2010) | 2010 | 2 | 4240 | 2049 | 2191 | Meat, fish |
| 108 | Hee Seung Kim | 2010 | 10 | 135,871 | 65,578 | 70,293 | Wine |
| 109 | S-K Myung | 2009 | 7 | 169 051 | 3516 | 165 535 | Soy intake |
| 110 | BG Chittenden | 2009 | 1 | 4547 | 476 | 4071 | Polycystic ovary syndrome |
| 111 | Bo Zhou | 2008 | 27 | 1,584,610 | 12,955 | 1,571,655 | Hormone replacement therapy |
| 112 | HG Mulholland | 2008 | 2 | - | - | - | Dietary glycemic index |
| 113 | Catherine M. Olsen | 2007 | 12 | 2778 | 1269 | 1509 | Recreational Physical Activity |
| 114 | J Steevens | 2007 | 21 | - | 280 | - | Tea and coffee drinking |
| 115 | C. M. Greiser | 2007 | 42 | 48,153 | 12 238 | | Menopausal hormone therapy |
| 116 | Catherine M. Olsen | 2007 | 28 | 1,640,615 | 53,182 | 1,587,433 | Obesity |
| 117 | S. J. Jordan | 2006 | 9 | 6474 | 910 | 5564 | smoking |
| 118 | Stefanos Bonovas | 2005 | 8 | 746,293 | | 741,888 | Paracetamol |
| 119 | Susanna C. Larsson | 2006 | 21 | - | - | - | Milk, milk products and lactose intake |
| 120 | Grimes DA | 2009 | 3 | 500 | - | - | Oral contraceptives |
| 121 | Stefanos Bonovas | 2005 | 10 | 320,544 | 3803 | 316,741 | Nonsteroidal anti-inflammatory drugs |
| 122 | L-Q Qin | 2005 | 22 | 134,406 | 8372 | 126,034 | Milk/dairy products consumption |
| 123 | Sonya Kashyap | 2004 | 10 | 13,480 | 3624 | 9856 | Assisted Reproductive Technology |
| 124 | M. Huncharek | 2003 | 16 | 11,933 | - | - | Cosmetic talc |
| 125 | V Bagnardi | 2001 | 235 | 117 471 | 235 | | Alcohol drinking |
| 126 | Michael Huncharek | 2009 | 8 | 6,689 | 2529 | 4160 | Dietary Fat Intake |
| 127 | S. S. Coughlin | 2000 | 15 | - | - | - | Estrogen replacement therapy |
| 128 | Pushkal P. Garg | 1998 | 9 | 259,794 | 4392 | 255,402 | Hormone replacement therapy |
| 129 | John F. Stratton | 1998 | 15 | - | 6077 | - | Family history |
| 130 | Bowen Zheng | 2018 | 13 | 142,189 | 5777 | 136,412 | Dietary fiber intake |
| 131 | Hai-Fang Wang | 2017 | 22 | 1,485,988 | - | - | Empirically derived dietary patterns |
| 132 | Hui Xu | 2018 | 19 | 567,742 | - | - | Dietary fiber intake |
| 133 | Dongyu Zhang | 2018 | 14 | 180,833 | 7500 | | Non-herbal tea consumption |
| 134 | Yun-Long Huo | 2018 | 6 | 81,791 | 7878 | 73,913 | antidepressant medication |
| 135 | Massimiliano Berretta | 2018 | 9 | 787,076 | 3,541 | | Coffee consumption |
| 136 | Jiaqi Li | 2018 | 7 | 65,754 | - | - | vitamin D receptor |
| 137 | Xianling Zeng | 2018 | 11 | 9987 | 4097 | 5890 | RAD51 135 G/C polymorphism |
| 138 | Marieke GM Braem | 2012 | 3 | 330,849 | 1244 | 329,605 | Coffee and tea consumption |
| 139 | Shanliang Zhong | 2014 | 19 | 730,703 | 9,459 | | Nonoccupational physical activity |
| 140 | Xiumin Huang | 2018 | 17 | 149,177 | 7609 | 73,168 | dietary fiber intake |
| 141 | Ting Liu | 2013 | 17 | 16,363 | 6,365 | 9,998 | Progesterone receptor PROGINS |
| 142 | Yanyang Pang | 2018 | 10 | 2354 | - | - | Dietary protein intake |
| 143 | Ke Wei Foong | 2017 | 43 | 3,491,943 | - | - | Obesity |
| 144 | Lingling Zhou | 2015 | 2 | 774 | 389 | 385 | SNP rs763110 |
| 145 | Rizzuto I | 2013 | 25 | 182,972 | - | - | ovarian stimulating drugs for infertility |
| 146 | Yanqiong Liu | 2014 | 5 | 624 | - | - | Statin |
| 147 | Ahmad Sayasneh | 2011 | 8 | - | 653 | - | Endometriosis |
| 148 | Jia li | 2018 | 25 | 957,152 | - | - | Endometriosis |
| 149 | Ho Kyung Sung | 2016 | 32 | 530,950 | 7639 | 523,311 | Breastfeeding |
| 150 | Mahdieh Kamali | 2017 | 17 | 10,817 | 4464 | 6353 | XRCC2 rs3218536 |
| 151 | Menelaos Zafrakas | 2014 | 16 | - | 17,445 | - | Endometriosis |
| 152 | Dagfinn Aune | 2015 | 28 | - | - | - | Anthropometric factors |
| 153 | QIAO WANG | 2015 | 4 | 1985 | 627 | 1358 | circulating insulin |
| 154 | Yihua Yin | 2013 | 11 | 6192 | 2,673 | 3519 | glutathione S-transferase |
| 155 | Ximena Gianuzzi | 2016 | 14 | 8130 | 1,149 | 6981 | Insulin growth factor (IGF) |
| 156 | Li-Ling Liu | 2014 | 4 | 2675 | 1073 | 1602 | transforming growth factor b receptor |
| 157 | Yong-qiang Wang | 2012 | 4 | 580,581 | 2444 | 578,137 | TGFBR1 Polymorphisms |
| 158 | Dongyang Li | 2018 | 44 | 1,082,092 | 48,345 | 1,033,747 | Dietary inflammatory index |
| 159 | Si Huang | 2018 | 10 | 4605 | 2394 | 2211 | miR-502-binding site |
| 160 | Eileen Deuster | 2017 | 200 | - | - | - | VDR |
| 161 | Ru Chen | 2017 | 28 | 3362 | 2,171 | 1191 | MGMT Promoter |
| 162 | Joanna Kruk | 2017 | 26 | - | - | - | Dietary alkylresorcinols |
| 163 | Xue-Feng Li | 2017 | 11 | 33,209 | 14,030 | 19,179 | lncRNA H19 polymorphisms |
| 164 | Yan Jiang | 2017 | 1 | 285 | 165 | 120 | ARLTS1 polymorphism |
| 165 | Qiuyan Li | 2017 | 7 | - | - | - | BRCA2 rs144848 polymorphism |
| 166 | Mohamed Hosny Osman | 2017 | 1 | 2,116,029 | 7124 | 2,108,905 | Cardiac glycosides |
| 167 | Erjiang Zhao | 2017 | 4 | - | - | - | Glutathione S-transferase |
| 168 | Giuseppe Grosso | 2017 | 4 | - | - | - | Diet |
| 169 | Limin Miao | 2017 | 6 | 6027 | 2156 | 3871 | BRCA1 P871L polymorphism |
| 170 | Na-Na Yang | 2017 | 4 | 2110 | 944 | 1166 | XRCC1 polymorphism |
| 171 | Giuseppe Grosso | 2016 | 53 | - | - | - | Dietary flavonoid |
| 172 | Juan Enrique Schwarze | 2017 | 4 | - | - | - | Reproduction technologies |
| 173 | Rosanne M. Kho | 2016 | 10 | - | - | - | Hysterectomy |
| 174 | K Robinson | 2016 | 11 | - | - | - | Bisexual |
| 175 | Hong-Bae Kim | 2016 | 6 | 1937 | - | - | Benzodiazepine |
| 176 | Chuanjie Zhang | 2017 | 3 | 2628 | 1276 | 1352 | NFκB1-94ins/del ATTG |
| 177 | Minjie Chu | 2016 | 2 | 18,540 | 6,857 | 11,683 | H19 lncRNA |
| 178 | Duan Wang | 2016 | 4 | 3036 | 1463 | 1573 | NFKB1 −94 ins/del ATTG |
| 179 | Jun Wang | 2016 | 19 | 3,87,71,388 | 13,116 | 38,758,272 | BMI |
| 180 | Yun-Feng Zhang | 2015 | 1 | 549 | 229 | 320 | IL-27 Genes |
| 181 | Ping Wang | 2016 | 2 | - | - | - | MDM2 SNP285 |
| 182 | Wenkai Xia | 2015 | 4 | 1248 | 497 | 751 | ESR2 |
| 183 | Lei Chen | 2016 | 2 | - | - | - | L55M polymorphism |
| 184 | Davide Serrano | 2015 | 3 | 5456 | 2313 | 3143 | VDR |
| 185 | Ranadip Chowdhury | 2015 | 41 | - | - | - | Breastfeeding |
| 186 | Zhi-Ming Dai | 2015 | 3 | 3530 | 1475 | 2055 | VDR |
| 187 | Claudio Pelucchi | 2014 | 4 | - | 2,010 | - | Dietary acrylamide |
| 188 | Yu-Fei Zhang | 2015 | 6 | 619 714 | 2933 | | Tea consumption |
| 189 | Jin-Lin Cao | 2015 | 2 | 9245 | 3102 | 6143 | TERT Genetic Polymorphism |
| 190 | Myung-Jin Muna | 2015 | 6 | | 4107 | 6661 | VDR |
| 191 | NaNa Keum | 2015 | 6 | - | - | - | Weight Gain |
| 192 | Sheng-Song Chen | 2015 | 2 | 1185 | 556 | 629 | MMP-12 82 A/G polymorphism |
| 193 | Bei-bei Zhang | 2014 | 45 | 57,328 | 28,956 | 28,372 | Genetic 135G/C polymorphism |
| 194 | Sara Raimondi | 2014 | 5 | 97,275 | 45,218 | 52,057 | BsmI polymorphism |
| 195 | Shang Xie | 2014 | 15 | 11,644 | 5873 | 5771 | LIG4 gene polymorphisms |
| 196 | Wen-Qiong Xue | 2014 | 4 | | 36,299 | 48,483 | BRCA2 N372H |
| 197 | Patrizia Gnagnarella | 2014 | 6 | 10,588 | 4051 | 6537 | VDR |
| 198 | Peter Boyle | 2014 | 2 | - | - | - | Sweetened carbonated beverage consumption |
| 199 | Tara M. Friebel | 2014 | 5 | - | - | - | BRCA1 and BRCA2 |
| 200 | Xin Wang | 2014 | 41 | 42,121 | 17,814 | 24,307 | FAS rs2234767G/A Polymorphism |
| 201 | Yeqiong Xu | 2013 | 7 | 11,009 | 4210 | 6799 | VDR |
| 202 | H S Kim | 2014 | 35 | 444 255 | - | - | Endometriosis |
| 203 | Yazhou He | 2014 | 7 | 69,524 | 30,868 | 38,656 | XRCC2 Arg188His Polymorphismc |
| 204 | Weifeng Tang | 2014 | 14 | 27,269 | 11,245 | 16,024 | Aurora-A V57I (rs1047972) Polymorphism |
| 205 | Yeqiong Xu | 2014 | 3 | 937 | 457 | 480 | Polymorphisms |
| 206 | Mengmeng Zhao | 2014 | 42 | 39,505 | 19,142 | 20,363 | Rad51 G135C |
| 207 | Xiao Yang | 2014 | 21 | | 6127 | 9238 | NFKB1 −94ins/del ATTG Promoter |
| 208 | Bai-Lin Zhang | 2014 | 7 | - | 9956 | - | Blood Groups |
| 209 | Ursula Schwab | 2014 | - | - | - | - | Dietary fat on cardiometabolic |
| 210 | Tie-Jun Liang | 2013 | 21 | 8720 | 3,498 | 5,222 | 137G>C polymorphism |
| 211 | Wei Wang | 2013 | 39 | 41,698 | 19,068 | 22,630 | RAD51 135 G.C Polymorphism |
| 212 | Lei Xu | 2013 | 47 | 43,295 | 19,810 | 23,485 | FASL rs763110 Polymorphism |
| 213 | Jingxiang Chen | 2013 | 19 | 48,670 | 14,814 | 33,856 | TCF7L2 Gene Polymorphism |
| 214 | Monica Franciosi | 2013 | 53 | 1,050,984 | - | - | Metformin |
| 215 | Zhou Zhong-Xing | 2013 | 41 | 42,169 | 17,858 | 24,311 | FAS-1377 G/A (rs2234767) Polymorphism |
| 216 | Zhibin Yu | 2013 | 73 | 38,278 | 15,942 | 22,336 | Interleukin 10 - 819 C/T Polymorphism |
| 217 | Shangqian Wang | 2013 | 2 | 1706 | 794 | 912 | PAI-1 4G/5G Polymorphism |
| 218 | Li Li Li | 2013 | 8 | 746,455 | - | - | Fertilization |
| 219 | XIN XU | 2012 | 21 | 17,623 | 8,415 | 9,208 | PAI-1 promoter |
| 220 | Dominique Trudel | 2012 | 22 | - | - | - | Green tea |
| 221 | Tian-Biao Zhou | 2012 | 6 | 2,658 | 1,461 | 1,197 | Gene Polymorphism |
| 222 | Xin-Min Pan | 2011 | 17 | 27,759 | 13 691 | 14 068 | MLH1 -93 G>A polymorphism |
| 223 | 2011 | - | - | 4830 | - | Height | |
| 224 | C. Pelucchi | 2011 | 3 | - | 1594 | - | Acrylamide |
| 225 | Bo Peng | 2010 | 4 | 1240 | 443 | 797 | Polymorphisms |
| 226 | Bahi Takkouche | 2009 | 10 | - | - | - | Hairdressers |
| 227 | Bahi Takkouche | 2005 | 2 | 556 | 238 | 318 | Hair Dyes |
| 228 | V. G. Kaklamani | 2003 | 1 | 907 | 659 | 248 | TGFBR1*6A |
| 229 | Song Mao | 2018 | 3 | - | - | - | klotho expression |
| 230 | Mukete Franklin Sona | 2018 | 15 | 1 915 179 | 31 893 | 1,911,045 | Type 1 diabetes mellitus |
| 231 | Christine Schwarz | 2018 | 4 | - | - | - | Night shift work |
| 232 | Xiaoqing Shi | 2018 | - | 1208 | 604 | 604 | NME1 polymorphisms |
| 233 | H.J. van der Rhee | 2006 | 2 | - | - | - | Sunlight |
| 234 | Nadin Younes | 2018 | 44 | - | 805 | - | Polymorphisms |
| 235 | Yue Xu | 2016 | 1 | - | - | - | BHMT gene rs3733890 |
| 236 | Zhong Tian | 2013 | 46 | 51,413 | 22,993 | 28,420 | CYP1A2*1F polymorphism |
| 237 | Yu Wang | 2018 | 1 | 79,988 | - | - | Renal transplants |
| 238 | T. O. Yang | 2014 | - | 453 023 | 2009 | 451,014 | Birth weight |
| 239 | Lanhua Tang | 2017 | - | - | - | - | Night work |
| 240 | Steven M. Koehler | 2012 | 8 | - | - | - | BMP-2 |
| 241 | Yan Zhang | 2013 | 9 | 5632 | 2,331 | 3,301 | VDR |
| 242 | Ivana Rizzuto | 2013 | 25 | 182,972 | - | - | Stimulating drugs for infertility |
| 243 | Xiao-san Zhang | 2018 | 7 | 105,507 | 6783 | 98,724 | Bisphosphonates use |
| 244 | Yun Ye | 2018 | 10 | 1045 | - | - | B7-H4 expression |
| 245 | Junga Lee | 2018 | 34 | - | - | - | Physical activity |
| 246 | Huijun Yang | 2019 | 26 | 1,174,527 | 11 410 | 1 163 117 | Age at menarche |
| 247 | M. Kadry Taher | 2019 | 27 | 214,447 | 15,303 | 199,144 | Perineal use of talc powder |
| 248 | Yanjun Wu | 2019 | 13 | 2,471,030 | 19,959 | 2,451,071 | Age at last birth |
| 249 | A. Moazeni-Roodi | 2019 | 19 | 37,036 | 13,562 | 23,474 | MDM2 40 bp indel polymorphism |
| 250 | Fateme Shafiei (2018) | 2019 | 22 | 40 140 | 8568 | 31,572 | Caffeine |
| 251 | Lindsay J. Wheeler | 2019 | 11 | 13,591 | 4,484 | 9,107 | Intrauterine Device Use |
| 252 | Yuhang Long | 2019 | 16 | 437,689 | 4,553 | 433,136 | vitamin C intake |
| 253 | M. Arjmand (2020) | 2019 | 16 | 4184 | 1106 | 3078 | Circulating omentin levels |
| 254 | Claudia Santucci | 2019 | 37 | - | 70,646 | - | smoking |
| 255 | A. Salari-Moghaddam | 2019 | 14 | - | 4434 | - | Caffeine |
| 256 | M. Karimi-Zarchi | 2019 | 11 | 12,720 | 4990 | 7730 | MTHFR 677 C>T Polymorphism |
| 257 | Fan Yang | 2019 | 2 | 445 | - | - | ERCC1 gene polymorphisms |
| 258 | Tingting Yang | 2019 | 3 | - | - | - | Work Stress |
| 259 | Youxu Leng | 2019 | 14 | - | 4597 | - | vitamin E |
| 260 | Jalal Choupani | 2019 | 4 | 9532 | 843 | 110 | mir-196a-2 rs11614913 |
| 261 | Xiaqin Huo | 2019 | 18 | - | 14,440 | - | Hysterectomy |
| 262 | A. Bodurtha Smith | 2019 | 58 | 292,730 | 528 | 292,202 | HIV |
| 263 | Alireza Sadeghia | 2019 | 21 | 900,000 | - | - | Dietary Fat Intake |
| 264 | Kui Zhang | 2019 | 13 | 40,404 | 6449 | 33,955 | Fermented dairy foods |
| 265 | Zohre Momenimovahed | 2019 | 20 | - | - | - | Fertility Drugs |
| 266 | Christina Bamia | 2019 | 31 | - | 13,111 | - | Coffee consumption |
| 267 | Boris Janssen | 2019 | 115 | - | - | - | predicted pathogenic PALB2 |
| 268 | Yang Liu | 2019 | 12 | 1,193,201 | - | - | Menopausal Hormone Replacement |
| 269 | Javaid Iqbal | 2018 | 2 | 5093 | 1114 | 3979 | Hormone Levels |
| 270 | Sen Li | 2019 | 12 | 12,933 | 5057 | 7876 | Genetic polymorphism of MTHFR C677T |
| 271 | Guisheng He | 2019 | 45 | 1,059,975 | 329,035 | 730,940 | TERT rs10069690 polymorphism |
| 272 | Yizi Wang | 2019 | 36 | 4, 229,061 | - | - | Statin use |
| 273 | Jun Yu | 2019 | 83 | 21,612 | - | - | SFRP promoter hypermethylation |
| 274 | Qiao Wen | 2019 | 7 | 1,710,080 | - | - | Metformin |
| 275 | Suszynska M1 | 2019 | 5 | 3748 | 1919 | 1829 | EPHX1 polymorphism rs1051740 |
| 276 | Tian Xu1 | 2019 | 21 | 29,981 | 13,675 | 16,306 | HOTAIR polymorphisms |
| 277 | Jinghua Shi | 2018 | 13 | 901,287 | - | - | Metformin |
Four authors (RR, MM, SL, and KT) independently screened the titles and abstracts of citations to identify potentially relevant studies. Then, the full texts of potentially eligible articles were obtained and reviewed for further assessment according to the inclusion and exclusion criteria. Controversies were resolved by consulting a third person (LJ).
Data extraction
Data were extracted from eligible studies using a prespecified form in Microsoft Excel by four authors (RR, MM, SL, and KT) independently. The following information was collected: first author, year of publication, number of included primary studies, number of participants, age of participants, factors associated with OC, besides the measure of association (e.g., RR, OR), and its confidence intervals. Any discrepancy was resolved through discussion with a third author (LJ). EndNote X9 was used to extracting the records and removing duplicates (The EndNote Team. EndNote. EndNote X9 ed. Philadelphia, PA: Clarivate; 2013.).
Risk of bias assessment
The SIGN checklist was used to assess the methodological quality of systematic reviews (2); it is composed of 12 items containing ‘yes,‘ ‘no,‘ ‘can’t,‘ or ‘not applicable’ options. Generally, the methodological quality of the studies in this checklist was categorized into low quality, acceptable, and high quality, (Fig. 1 ).

SIGN Checklist scoring
The quality assessment of the eligible studies was undertaken independently by four authors (RR, MM, SL, and KT). Any disagreements were resolved through discussion.
Data synthesis
All statistical analyses were performed using Stata version 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.).
Most of the studies reported measures of the association between each factor and OC using the odds ratio (OR) or risk ratio (RR) with their corresponding CIs. Only one study used a standardized incidence rate ratio (SIR) and standardized mean difference (SMD) as an effect size. Thus, OR or RR and 95 % confidence intervals (CIs) were used to present the association between the factors and OC. For conducting the meta-analysis, all related information about measures of association (e.g., Pooled OR, Pooled RR, Standard error, 95 % Confidence Interval) were extracted and converted to pooled effect size and its SE for every factor in each study.
Since the reported combined effects from systematic reviews were used in the analysis, so primary studies may have been included in different systematic reviews and meta-analyses in the different years which we were not able to exclude them in the analysis. Heterogeneity was evaluated among the primary studies using the forest plots, Cochran’s Q statistic, and I 2 statistic. A random-effects model using restricted maximum-likelihood was used if heterogeneity was high (I 2 > 50 %); otherwise, a fixed-effects model was applied.
Since the number of first reviews combined for the meta-analysis was less than 10, Egger’s regression asymmetry tests were used for assessing the publication bias instead of funnel plots (Egger et al., 1997), where p <0.10 was considered as evidence of bias. The characteristics of the included studies were descriptively summarized using a structured table.
Twenty-eight thousand sixty-two papers were initially retrieved from the electronic databases, among which 20,104 studies were screened. Two hundred seventy-seven articles met our inclusion criteria, 226 of which included in the meta-analysis (Fig. 2 ). The eligible articles were those published between 1998 (when meta-analyses in this field first became available) and 2020. All of the studies had utilized a healthy control group against women with OC.

PRISMA flow diagram
Overall, from the 277 eligible meta-analyses or systematic reviews, 216 putative risk/protective factors of OC were reported.
Due to the number of evaluated factors, all were categorized into 5 main groups: (1) Nutritional factors, (2) Drug use and Medical history, (3) Diseases, (4) Genetic factors, (5) Other factors.
Among all of the studied factors, 109 had one quantitative synthesis report, and 53 did not have any quantitative synthesis of individual findings but reported valuable data in systematic review articles (Table 2 S and Table 3 S).
Meta-analysis results of the outcomes of interest
Meta-analyses were conducted on the 53 associated factors with OC with sufficient data (two or more reports with the same measures). Most commonly reported genetic factors were MTHFR C677T (OR=1.077; 95 % CI (1.032, 1.124); P-value<0.001), BSML rs1544410 (OR=1.078; 95 %CI (1.024, 1.153); and P-value=0.004) and Fokl rs2228570 (OR=1.123; 95 % CI (1.089, 1.157); P-value<0.001), which were significantly associated with increasing risk of OC ( Fig. 3 ). The results of publication bias assessed using the Egger’s test indicate significant publication bias only for MTHFR C677T factor (P-value=0.017).

Meta-analysis of OR for MTHFR C677T, BSML rs1544410 and Fokl rs2228570
Among the other factors, coffee intake (OR=1.106; 95 % CI (1.009, 1.211); P-value=0.030), hormone therapy (RR=1.057; 95 % CI (1.030, 1.400); P-value<0.001), hysterectomy (OR=0.863; 95 % CI (0.745, 0.999); P-value=0.049), and breast feeding (OR=0.719; 95 % CI (0.679, 0.762); P-value<0.001) were mostly reported in studies. Final results of all conducted meta-analysis are presented in Table 2 .
Results of all conducted meta-analysis
| Variables | Measure of Association | Odds Ratio (95 % CI) | P-value | I % | No. of study in analysis | |
|---|---|---|---|---|---|---|
| Alcohol use | RR | 1.015 (0.974 – 1.052) | 0.485 | 0.01 | 3 | |
| Coffee intake | OR | 1.106 (1.009 – 1.211) | 0.030 | 0.00 | 4 | |
| RR | 1.036 (0.967 – 1.109) | 0.317 | 0.00 | 3 | ||
| Egg intake | RR | 1.147 (1.045 – 1.250) | <0.001 | 17.73 | 2 | |
| Fat intake | RR | 1.188 (1.090 – 1.296) | <0.001 | 0.00 | 3 | |
| Fiber intake | OR | 0.760 (0.714 – 0.810) | <0.001 | 0.00 | 3 | |
| Milk intake | RR | 1.016 (0.664 – 1.554) | 0.941 | 0.08 | 2 | |
| Tea intake | OR | 0.833 (0.741 – 0.936) | 0.002 | 0.00 | 3 | |
| RR | 0.856 (0.779 – 0.959) | 0.005 | 0.00 | 2 | ||
| Vegetables intake | RR | 0.896 (0.837 – 0.958) | <0.001 | 0.00 | 2 | |
| Aspirin | OR | 0.894 (0.854 – 0.935) | <0.001 | 0.00 | 3 | |
| Metformin | RR | 0.718 (0.602 – 0.855) | <0.001 | 0.00 | 3 | |
| NSAIDs | RR | 0.898 (0.819 – 0.984) | 0.020 | 0.00 | 3 | |
| Oral contraceptive | OR | 0.655 (0.515 – 0.833) | <0.001 | 78.23 | 2 | |
| Statin | RR | 0.849 (0.749 – 0.962) | 0.010 | 0.00 | 2 | |
| Hormone therapy (estrogen) | RR | 1.305 (1.210 – 1.407) | <0.001 | 0.00 | 2 | |
| Hormone therapy (Overall) | RR | 1.057 (1.030 – 1.400) | <0.001 | 94.44 | 4 | |
| Hormone therapy (estrogen-progestin) | OR | 1.190 (1.043 – 1.357) | 0.009 | 82.24 | 2 | |
| Hysterectomy | OR | 0.863 (0.745 – 0.999) | 0.049 | 67.12 | 4 | |
| Tubal ligation | OR | 0.693 (0.657 – 0.731) | <0.001 | 0.00 | ||
| Diabetes | RR | 1.24 (1.32 – 1.35) | <0.001 | 0.00 | 3 | |
| Endometriosis | OR | 1.433 (1.294 – 1.586) | <0.001 | 3.05 | 2 | |
| Poly cystic ovarian syndrome | OR | 1.580 (1.081 – 2.310) | 0.018 | 29.48 | 2 | |
| Asn680Ser | OR | 1.120 (0.594 – 2.110) | 0.726 | 86.32 | 2 | |
| BRCA2 N372H rs144848 | OR | 1.079 (1.018 – 1.143) | 0.010 | 44.61 | 4 | |
| BSML rs1544410 | OR | 1.078 (1.024 – 1.153) | 0.004 | 0.00 | 8 | |
| ESR2 rs3020450 | OR | 0.818 (0.719 – 1.040) | 0.151 | 61.20 | 2 | |
| Fokl rs2228570 | OR | 1.123 (1.089 – 1.157) | <0.001 | 0.00 | 8 | |
| GSTM1 | OR | 1.015 (0.928 – 1.111) | 0.741 | 0.00 | 2 | |
| MTHFR A1298C | OR | 0.997 (0.943 – 1.054) | 0.907 | 0.00 | 3 | |
| MTHFR C677T | OR | 1.077 (1.032 – 1.124) | <0.001 | 45.55 | 9 | |
| NFƙB1 | OR | 1.680 (1.08 – 2.62) | 0.020 | 69.07 | 2 | |
| P16INK4a | OR | 2.657 (1.173 – 6.014) | 0.019 | 51.28 | 2 | |
| RAD51 135G-C | OR | 0.996 (0.922 – 1.075) | 0.910 | 0.00 | 4 | |
| ERCC1 rs11615 | OR | 0.987 (0.756 – 1.287) | 0.920 | 0.00 | 2 | |
| ERCC2 rs13181 | OR | 1.42 (1.15 – 1.76) | 0.001 | 0.00 | 2 | |
| VGEGF rs699947 | OR | 0.983 (0.644 – 1.502) | 0.938 | 78.04 | 2 | |
| VDR rs731236 | OR | 0.996 (0.882 – 1.125) | 0.842 | 56.81 | 6 | |
| FASL rs763110 | OR | 0.640 (0.520 – 0.788) | <0.001 | <0.01 | 2 | |
| VEGFA rs833061 | OR | 0.834 (0.324 – 2.149) | 0.707 | 76.02 | 2 | |
| RAD51 rs1801320 | OR | 0.656 (0.349 – 1.232) | 0.189 | 41.43 | 3 | |
| FAS/APO-1 rs2234767 | OR | 1.001 (0.956 – 1.068) | 0.982 | 0.00 | 3 | |
| MMP-12 rs2276109 | OR | 1.588 (0.694 – 3.630) | 0.273 | 88.80 | 2 | |
| VEGF rs3025039 | OR | 0.869 (0.719 – 1.04) | 0.144 | 0.00 | 2 | |
| VDR rs7975232 | OR | 0.990 (0.901 – 1.088) | 0.842 | 0.00 | 5 | |
| VDR rs11568820 | OR | 1.164 (1.087 – 1.248) | <0.001 | 0.00 | 4 | |
| XRCC2r rs3218536 | OR | 0.887 (0.750 – 1.050) | 0.163 | 51.57 | 3 | |
| Acrylamide | RR | 0.994 (0.930 – 1.063) | 0.865 | 0.00 | 2 | |
| Obesity | RR | 1.274 (1.194 – 1.36) | <0.001 | 0.00 | 2 | |
| Overweight | OR | 1.079 (1.041 – 1.119) | <0.001 | 24.04 | 3 | |
| RR | 1.071 (1.041 – 1.102) | <0.001 | 0.00 | 3 | ||
| Height | RR | 1.128 (1.064 – 1.196) | <0.001 | 87.71 | 3 | |
| Weight | RR | 1.067 (0.977 – 1.165) | 0.149 | 74.99 | 2 | |
| Smoking | RR | 1.311 (0.847 – 2.029) | 0.225 | 98.13 | 3 | |
| Recreational physical activity | RR | 0.830 (0.745 – 0.925) | <0.001 | 0.00 | 3 | |
| Perineal talc | OR | 1.297 (1.242 – 1.355) | <0.001 | 0.00 | 2 | |
| RR | 1.250)1.177 – 1.327) | <0.001 | 38.11 | 2 | ||
| Breast feeding | OR | 0.719 (0.679 – 0.762) | <0.001 | 4.63 | 4 | |
The risk of bias was assessed using the SIGN checklist. Among 277 included studies, 24.19 %, 39.35 %, and 36.46 % had “low quality”, “acceptable” and “high quality,“ respectively.
This study focuses on OC risk factors and protective measures. The factors can be classified into nutritional, drug use and medical history, diseases, and genetic. As regards nutritional factors, intake of coffee, egg, and fat can significantly enhance the risk of OC. Estrogen and estrogen-progesterone therapies (generally, hormone therapy) are also associated with the elevated risk of OC. Several diseases (e.g., diabetes, endometriosis, and polycystic ovarian syndrome), as well as some genetic polymorphisms (e.g., BRCA2 N372H rs144848, BSML rs1544410, Fokl rs2228570, MTHFR C677T, P16INK4a, ERCC2 rs13181, MMP-12 rs2276109, and VDR rs11568820), can significantly increase the incidence of OC. Other factors, like obesity, overweight, smoking, and the use of perineal talc, are also accompanied by an increased risk of OC.
Coffee is rich in several anti-oxidant and anti-carcinogenic bioactive compounds (e.g., phenolic acids, cafestol, and kahweol, respectively) [ 6 ]. This beverage has shown an inverse correlation with liver and endometrial cancer risk [ 4 ]. Furthermore, coffee and caffeine have an inverse relationship with sex hormones (testosterone and estradiol) [ 2 ]. High levels of these hormones have exhibited direct association with enhanced breast and ovarian cancer [ 8 , 9 ]. Coffee contains acrylamide, which has been shown to increase the risk of breast and ovarian cancer as well [ 10 ]. The meta-analysis in the present study indicates a positive correlation between coffee drinking and OC risk.
Eggs are rich in cholesterol and choline, thus providing quite high protein per energy content, all of which are linked to the risk of breast, ovarian, and prostate cancers. Nonetheless, the majority of these studies on the mentioned cancers have not explored egg consumption as a primary exposure of interest, restricting a robust assessment of the hypothesized correlations. Since eggs have been considered as a source of protein and fat, its intake association with the OC risk has been primarily explored to examine the impact of protein or fat [ 11 ]. In this meta-analysis, egg consumption has been shown to be significantly and positively correlated with OC.
As one of the most controversial nutritional factors, dietary fat can enhance the development of hormone-related cancers (e.g., breast, endometrial, and OCs). However, the reports on this field are discrepant. High-fat diets may stimulate over-secretion of ovarian estrogen, leading to tumor-promoting mechanisms through mitogenic impacts on ERα- positive or negative tumor cells [ 12 ].
Epidemiologic reports indicate an association between estrogen exposure duration and OC induction and biology [ 13 ]. Recent research has expressed that besides inhibiting estrogen-driven growth in the uterus, progesterone can protect the ovaries against neoplastic transformation [ 14 ]. Despite the available poor knowledge of the etiology of OC, the role of estrogen and progestin seems biologically plausible. Based on a theory, high levels of menopausal gonadotropins due to estradiol expression may elevate OC risk. In other words, HRT can decrease the risk of OC by reducing the levels of menopausal gonadotropins. However, due to small HRT-related decrease, the mentioned advantages could be overruled by the estrogen-induced proliferation of ovarian cells. Moreover, the epithelial surface of both normal and malignant ovaries expresses estrogen receptors [ 15 ]. Furthermore, progestin is responsible for the declined risk associated with oral contraceptive use. Pregnancy can also offer a biologic basis for weak correlations with HRT formulations, including progestins [ 16 ]. The current work indicates a significant positive association between hormone therapy (estrogen, estrogen-progestin, and overall) and OC.
Diabetes mellitus (DM) is also positively and significantly associated with the risk of OC. Although the carcinogenic influence of DM on the ovary has not been completely understood, some mechanisms have been introduced to describe it partially. Hyperinsulinemia (often associated with insulin resistance) is commonly observed in type 2 DM patients. Chronic hyperinsulinemia has an association with tumor promotion due to the oncogenic potentials of insulin by stimulating cellular signaling cascade or incrementing growth factor-related cell proliferation [ 17 ]. Moreover, increased levels of insulin are associated with high bioactivity of insulin growth factor-1 (IGF-1) [ 18 ]. Considering the anti-apoptosis and mitogenic influences of IGF-1 on normal and cancerous human cells, type 2 DM can promote tumor development [ 19 ]. Besides, hyperglycemia has been recognized as one of the major health consequences of DM. Based on numerous animal and clinical studies, hyperglycemia is related to oxidative stress [ 20 ]. Oxidative stress refers to an imbalance between the reactive oxygen species (ROS) production and antioxidant defense mechanisms. ROS can damage the biomolecules of the cells, including those involved in cell proliferation and repair [ 21 ].
Based on the results, the risk of developing OC is 43 % in women with endometriosis. The endometriosis mechanisms in epithelial OC can be divided into 3 types. The first one is estrogen-dependent. Ness et al. introduce endometriosis as a precursor for epithelial OC, which is easily developed in the low-progesterone and high-estrogen conditions [ 22 ]. The second involves the genetic mutation in endometriotic tissues, like hepatocyte nuclear factor-1β (HNF-1β) [ 23 ] and ARID1A [ 24 ]. Furthermore, chronic inflammations, heme, or free iron-induced oxidative stress in endometriotic tissues also exhibit an association with epithelial OC [ 25 ].
The risk of OC shows a 60 % increase in women suffering from polycystic ovary syndrome (PCOS). PCOS has various risk factors, including obesity, diabetes, inflammation, metabolic syndrome, and aging. However, it is not clear whether the elevated risk of endometrial cancer is due to separate risk factors (e.g., diabetes, obesity) or PCOS itself. PCOS has its own metabolic characteristics, including hyperinsulinism, hyperglycemia, insulin resistance, and hyperandrogenism, enhancing cancer risk. Moreover, such a relationship between PCOS and endometrial cancer could be due to common inherited genetic variants. Other factors, such as parity (nulliparous versus multi), age at first pregnancy, and use/length of hormone therapy (HRT, OCP), could confound the results.
Some genetic factors may enhance the risk of developing OC. In the present study, Asn680Ser, BRCA2 N372H rs144848, BSML rs1544410, Fokl rs2228570, GSTM1 , MTHFR C677T, NFƙB1 , P16 INK4a , ERCC2 rs13181, MMP-12 rs2276109, and VDR rs11568820 have been found to increase the risk of OC significantly. Among the mentioned polymorphisms, P16INK4a has the strongest impact on the risk of OC (2.6-fold increase), followed by NFƙB1 and MMP-12. rs2276109.
Some studies have mentioned the crucial role of p16 INK4a inactivation as the result of aberrant hypermethylation in the lung, liver, stomach, breast, and uterus carcinogeneses [ 26 , 27 ]. In a meta-analysis on 6 eligible research encompassing 261 patients, Hu et al. show a correlation between p16 INK4a promoter hypermethylation and elevated risk of endometrial carcinoma [ 27 ]. A meta-analysis by Xiao et al. also report the significant association of aberrant methylation of p16 INK4a promoter with OC [ 28 ]. This could be regarded as a potential molecular marker for monitoring the diseases and providing new insights into OC therapies.
NFκB1 can significantly inhibit cell apoptosis through regulation of the level of survival genes, such as BCL-2 homolog A1, PAI-2, and IAP family. Moreover, studies have indicated the role of the NFκB1 signaling pathway in cellular proliferation by IL-5 enhancement, MAPK phosphorylation, and cyclin D1 expression modulation [ 29 ].
Numerous meta-analyses have addressed the relationship between NFκB1 promoter -94ins/del ATTG polymorphism and cancer risk, although their findings are not entirely consistent. For instance, Yang et al. [ 30 ] and Duan et al. [ 31 ] express that the polymorphism in NFκB1 -94ins/del ATTG promoter can increase the overall cancer risk. These results do not agree with those reported by Zou et al. [ 32 ]. Such contradictions can be assigned to the bias as the result of a limited sample size.
MMP-12 is involved in the pro-tumorigenesis process through inhibiting cancer cell apoptosis and promoting cancer cell invasion and migration [ 33 ]. As SNP of MMP-12-82 A>G can influence the MMP-12 expression and enhance the cancer risk, the correlation between MMP-12 promoter gene polymorphism and the cancer risk has been extensively addressed in recent years.
Obesity, overweight, smoking, and the use of perineal talc could be mentioned as other factors associated with OC risk. The biological mechanisms underlying the relation of overweight and obesity with OC are not clarified and consistent. Based on a study by Kuper et al. [ 34 ], progesterone and leptin could be possible endocrine mediators of the weight effect on OC risk. Such an impact could be assigned to elevated insulin levels, androgens, and free IGF-I due to obesity [ 35 ]. Regarding disassociation of BMI with OC risk among postmenopausal women, Reeves et al. [ 36 ] express that association of BMI with OC risk is under the mediation of hormones, as its impact on OC risk remarkably differs in premenopausal and postmenopausal subjects. BMI shows an inverse association with sex hormone-binding globulin and progesterone, while it is positively correlated with free testosterone in premenopausal women [ 37 ]. The mentioned hormone factors seem to be independently or cooperatively involved in the carcinogenic process.
Concerning biological mechanisms, the direct correlation of smoking with mucinous tumors can be assigned to the similarity of this neoplasm with cervical adenocarcinoma and colorectal cancers [ 38 ], both of which have exhibited direct association with tobacco exposure. Similarly, endometriois and clear cell cancers have some biological similarities with endometrial cancer, which is inversely related to tobacco smoking due to the possible anti-estrogenic influence of smoking. The tobacco smoking could exert strong impacts in the early stages of (ovarian) carcinogenesis. Thus, the more powerful tobacco-associated risk for mucinous could be explained by the fact that for the mucinous histotype, there is a continuum from benign to borderline and invasive disease, while serous OCs are often high grade and not originated from the borderline tumors [ 39 ]. Furthermore, the smoking-induced mutation in the somatic KRAS gene is more common in mucinous rather than serous borderline ovarian tumors [ 40 ], and also in borderline tumors than invasive cancer [ 41 ].
The ovarian carcinogenesis mechanism of perineal talc use has remained unclear. Based on a hypothesis, however, as an external stimulus, talc can ascend from the vagina to the uterine tubes and trigger a chronic inflammatory response, further promoting the OC development. Cellular injuries, oxidative stresses, and local elevation of inflammatory mediators (e.g., cytokines and prostaglandins) could be mutagenic, thus encouraging carcinogenesis [ 42 ]. Supporting this hypothesis, hysterectomy or bilateral tubal ligation, which may dramatically decline the ovarian exposure to inflammatory mediators, is related to a decreased OC risk [ 43 – 45 ].
Conclusions
Numerous studies have addressed the effective factors of OC; however, these works have resulted in contradicting outcomes. The current study explores all previous meta-analyses and systematic reviews to provide a valuable summary of the OC protective and risk factors, among which nutritional and genetic factors play a more profound role. Although the genetic factors cannot be changed due to their inheritance, nutritional ones could be well regulated to prevent OC.
Acknowledgements
Not applicable.
Statement of significance
Nutritional and genetic factors play a more profound role in ovarian cancer risk. Coffee intake, hormone therapy are risk factors while hysterectomy and breast feeding have protective role.
Authors’ contributions
All authors have read and approved the manuscript. LJ and MN conceptualized and designed the study and critically revised the manuscript for important intellectual content. MM, RR, SL, and KT acquired data. LJ and KT analyzed data, interpreted the study results, and critically revised the manuscript for important intellectual content. AM drafted the manuscript and critically revised the manuscript for important intellectual content.
This project was supported by Vice Chancellor for Research & Technology, Iran University of Medical Sciences( No. 1084).
Availability of data and materials
Declarations.
This project was registered and approved by the Iran University of Medical Sciences Ethics committee ( Code: IR.IUMS.REC 1396.32585 ).
The authors have no conflicts of interest associated with the publication of this manuscript to declare. The authors report no financial disclosures related to the current work.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kiarash Tanha and Azadeh Mottaghi are co-first author
Contributor Information
Kiarash Tanha, Email: [email protected] .
Azadeh Mottaghi, Email: [email protected] .
Marzieh Nojomi, Email: ri.ca.smui@imojonm .
Marzieh Moradi, Email: moc.liamg@0002idaromheyizram .
Rezvan Rajabzadeh, Email: [email protected] .
Samaneh Lotfi, Email: moc.oohay@09ls_rhem .
Leila Janani, Email: [email protected] .

IMAGES
VIDEO
COMMENTS
2. Targeting Numerous Signaling Pathways of Ovarian Cancer. Surgery and chemoradiotherapy are the most frequently used treatment options for ovarian cancer (OC) [].However, severe side effects have been associated with chemo- and radiotherapy (RT), while the only minor therapeutic benefit from RT eventually leads to succumbing to the disease and poor survival outcomes [].
Introduction. Cancer is the most common cause of mortality in most parts of the world, 1 and currently is the most common impediment to achieving desirable life expectancy in most countries. 2 Ovarian cancer is one of the most common gynecologic cancers that rank third after cervical and uterine cancer. 2 It also has the worst prognosis and the highest mortality rate. 3 Although ovarian cancer ...
Epidemiology of Ovarian Cancer. Ovarian cancer ranks as the fifth leading cause of malignancy-associated mortality in females (10,11).In 2008, an estimated 225,500 women were diagnosed as having ovarian cancer worldwide, and in 2012 it was estimated that there were 238,700 new cases, and 151,900 women died of ovarian cancer ().In general, ovarian cancer is more common in developed countries ...
Although ovarian cancer is "only" the 10th most common cancer in women, it is the fifth-leading cause of cancer death ().Sixty-five percent of ovarian cancers are diagnosed after the disease has spread within the peritoneal cavity (stage III) or distant organs (stage IV) ().Because 5-year survival for localized disease is over 90% compared with 30% for distant disease (), efforts at ...
Cellular & Molecular Biology Letters (2021) Recent preclinical and clinical research has led to exciting advances related to high-grade serous ovarian cancer, from examining its cellular origins ...
Globally, ovarian cancer is the eighth most common cancer in women, accounting for an estimated 3.7% of cases and 4.7% of cancer deaths in 2020. Until the early 2000s, age-standardized incidence ...
Objective: To provide an overview of the risk factors, modifiable and non-modifiable, for ovarian cancer as well as prevention, diagnostic, treatment, and long-term survivorship concerns. This article will also examine current and future clinical trials surrounding ovarian cancer. Data sources: A review of articles dated 2006-2018 from CINAHL, UpToDate, and National Comprehensive Cancer ...
Ovarian cancer is the most lethal gynecological cancer worldwide, accounting for 207.252 deaths in 2020 1.Due to non-specific or absence of symptoms at an early stage, patients typically present ...
Despite documented evidence that ovarian cancer cells express immune-checkpoint molecules, such as PD-1 and PD-L1, and of a positive correlation between the presence of tumour-infiltrating lymphocytes and favourable overall survival outcomes in patients with this tumour type, the results of trials testing immune-checkpoint inhibitors (ICIs) in these patients thus far have been disappointing.
Aims and scope. Journal of Ovarian Research is an open access, peer reviewed, online journal that aims to provide a forum for high-quality basic and clinical research on ovarian function, abnormalities, and cancer. The journal focuses on research that provides new insights into ovarian functions as well as prevention and treatment of diseases ...
Ovarian cancer (OC) is the predominant primary tumor in the human reproductive system. Abnormal sialylation has a significant impact on tumor development, metastasis, immune evasion, angiogenesis, and treatmen... Di Wu, Li-yuan Sun, Xin-yu Chang and Guang-mei Zhang. Journal of Ovarian Research 2024 17:176.
Background Detailed epidemiologic descriptions of large populations of advanced stage ovarian cancer patients have been lacking to date. This study aimed to describe the patient characteristics, treatment patterns, survival, and incidence rates of health outcomes of interest (HOI) in a large cohort of advanced stage ovarian cancer patients in the United States (US). Methods This cohort study ...
Historically, ovarian cancer (OC) was thought to metastasize by surface-to-surface spread, but recent developments have yielded a new understanding of the paths of metastatic spread. Given the histologic and molecular heterogeneity of OC, we will focus on high-grade serous carcinoma (HGSC). Here, we provide a critical and more holistic view of the evidence supporting various routes of ...
Introduction. In 2018, there will be approximately 22,240 new cases of ovarian cancer diagnosed and 14,070 ovarian cancer deaths in the United States. 1 Ovarian cancer accounts for 2.5% of all malignancies among females but 5% of female cancer deaths because of low survival rates, largely driven by late stage diagnoses. 2 Improving prevention and early detection is a research priority because ...
Abstract. Although cure rates remain low and effective screening strategies are elusive, the recent advances in systemic therapies over the past year highlighted in this review have prolonged survival for women with ovarian cancer. In 2022, the first antibody-drug conjugate for platinum-resistant ovarian cancer received accelerated US Food ...
Ovarian cancer (OC) is the deadliest cancer among women placing it with 4th place for all the fatal disease among women. Cancer statistics from 2019 show that the estimated number of new cases is 22 240 with deaths around 14 170 cases. There are three histological types associated with the disease.
Ovarian cancer is the leading cause of death from gynaecological malignancies, with 21,410 estimated new diagnoses and 13,770 deaths in 2021 in the USA 1,2.Most ovarian cancers are epithelial ...
Mintu Pal et al. [32] CA-125, the gold standard tumor marker, exhibits high levels in ovarian cancer but lacks specificity for early-stage screening due to high levels in non-cancerous conditions.HE4, with FDA approval, is a prospective single serum biomarker but with low sensitivity and specificity. Combining CA-125 with other biomarkers like CA 19-9, EGFR, G-CSF, Eotaxin, IL-2R, cVCAM, and ...
Abstract. Ovarian cancer (OC) is the most lethal reproductive system cancer and a leading cause of cancer-related death. The high mortality rate and poor prognosis of OC are primarily due to its tendency for extensive abdominal metastasis, late diagnosis in advanced stages, an immunosuppressive tumor microenvironment, significant adverse reactions to first-line chemotherapy, and the ...
Explore the latest in ovarian cancer, including recent advances in epidemiology, screening, genetic testing, and management of the disease. ... and Policy Promoting EDI in Genetics Research PTSD and Cardiovascular Disease Red Blood Cell Transfusion: 2023 AABB International Guidelines Reimagining Children's Rights in the US Spirituality in ...
Abstract. Epithelial ovarian cancer is typically diagnosed at an advanced stage. Current state-of-the-art surgery and chemotherapy result in the high incidence of complete remissions; however, the recurrence rate is also high. For most patients, the disease eventually becomes a continuum of symptom-free periods and recurrence episodes.
High-grade serous ovarian cancer, the most common form of the disease, is often fatal. This study investigated the genomic and immune characteristics of tumors from women who survived more than 10 ...
Advances in Ovarian Cancer Research. An ovarian tumor grown in a mouse using human cells. Special techniques were used to create the high-resolution, 3-D view of the cancer's cell structure and inner workings. Credit: Chris Booth, Kyle Cowdrick, Frank C. Marini. National Cancer Institute \ Comprehensive Cancer Center of Wake Forest Univ.
New research has found four genes with some of the largest known effects on the timing of menopause discovered to date. ... The paper is entitled 'Genetic links between ovarian ageing, cancer risk and de novo mutation rates', and is published in 'Nature'. Top image: Credit: insta_photos, iStock, Getty Images Plus via Getty Images ...
Introduction. Ovarian cancer is the sixth most common cancer worldwide among women in developed countries and the most lethal of all gynecologic malignancies.() Currently, most women have advanced stage disease at the time of diagnosis.Despite aggressive surgery and chemotherapy, the prognosis for these women is poor, with a 5-year survival rate of less than 30%.
This work was supported by funds from the NCI Early Detection Research Network (5 U01 CA200462-02, RCB), the MD Anderson Ovarian SPOREs (P50 CA83639 and P50CA217685, R.C.B.), National Cancer ...
In India, ovarian cancer ranks among the top three cancers, contributing to 6.6% of all women's cancers. In 2022, India reported 47,333 new ovarian cancer cases and 32,978 deaths. These alarming ...
In India, Ovarian Cancer ranks among top three cancers affecting women, accounting for 6.6% of all female cancer cases. In 2022 , India alone reported 47,333 new ovarian cancer cases and 32,978 deaths , emphasizing the critical need for awareness, early detection, and effective treatment.
BRCA1 and 2 mutation carriers harbor significantly increased ovarian cancer risk (40-45 % resp. 15-20 %) by the age of 70. Risk of OC in the high risk women under 40 years old is low . Several studies on ovarian cancer have been published that have examined various factors influencing the incidence, prevalence and mortality rate.