
- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science

Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books

Use chemistry problems as a tool for mastering chemistry concepts. Some of these examples show using formulas while others include lists of examples.
Acids, Bases, and pH Chemistry Problems
Learn about acids and bases. See how to calculate pH, pOH, K a , K b , pK a , and pK b .
- Practice calculating pH.
- Get example pH, pK a , pK b , K a , and K b calculations.
- Get examples of amphoterism.
Atomic Structure Problems
Learn about atomic mass, the Bohr model, and the part of the atom.
- Practice identifying atomic number, mass number, and atomic mass.
- Get examples showing ways to find atomic mass.
- Use Avogadro’s number and find the mass of a single atom .
- Review the Bohr model of the atom.
- Find the number of valence electrons of an element’s atom.
Chemical Bonds
Learn how to use electronegativity to determine whether atoms form ionic or covalent bonds. See chemistry problems drawing Lewis structures.
- Identify ionic and covalent bonds.
- Learn about ionic compounds and get examples.
- Practice identifying ionic compounds.
- Get examples of binary compounds.
- Learn about covalent compounds and their properties.
- See how to assign oxidation numbers.
- Practice drawing Lewis structures.
- Practice calculating bond energy.
Chemical Equations
Practice writing and balancing chemical equations.
- Learn the steps of balancing equations.
- Practice balancing chemical equations (practice quiz).
- Get examples finding theoretical yield.
- Practice calculating percent yield.
- Learn to recognize decomposition reactions.
- Practice recognizing synthesis reactions.
- Practice recognizing single replacement reactions.
- Recognize double replacement reactions.
- Find the mole ratio between chemical species in an equation.
Concentration and Solutions
Learn how to calculate concentration and explore chemistry problems that affect chemical concentration, including freezing point depression, boiling point elevation, and vapor pressure elevation.
- Get example concentration calculations in several units.
- Practice calculating normality (N).
- Practice calculating molality (m).
- Explore example molarity (M) calculations.
- Get examples of colligative properties of solutions.
- See the definition and examples of saturated solutions.
- See the definition and examples of unsaturated solutions.
- Get examples of miscible and immiscible liquids.
Error Calculations
Learn about the types of error and see worked chemistry example problems.
- See how to calculate percent.
- Practice absolute and relative error calculations.
- See how to calculate percent error.
- See how to find standard deviation.
- Calculate mean, median, and mode.
- Review the difference between accuracy and precision.
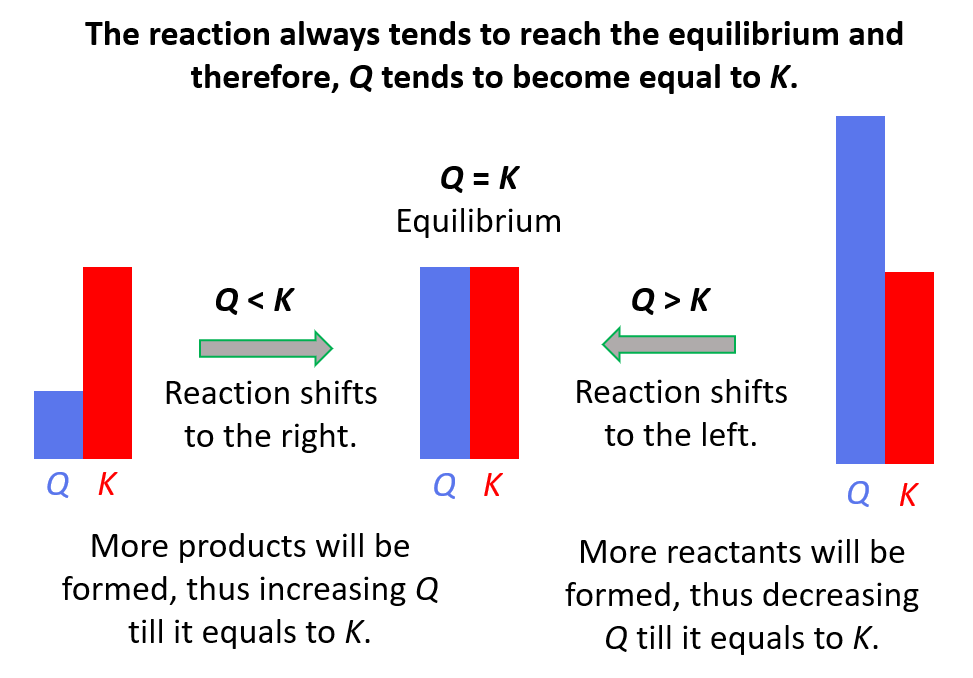
Equilibrium Chemistry Problems
Learn about Le Chatelier’s principle, reaction rates, and equilibrium.
- Solve activation energy chemistry problems.
- Review factors that affect reaction rate.
- Practice calculating the van’t Hoff factor.
Practice chemistry problems using the gas laws, including Raoult’s law, Graham’s law, Boyle’s law, Charles’ law, and Dalton’s law of partial pressures.
- Calculate vapor pressure.
- Solve Avogadro’s law problems.
- Practice Boyle’s law problems.
- See Charles’ law example problems.
- Solve combined gas law problems.
- Solve Gay-Lussac’s law problems.
Some chemistry problems ask you identify examples of states of matter and types of mixtures. While there are any chemical formulas to know, it’s still nice to have lists of examples.
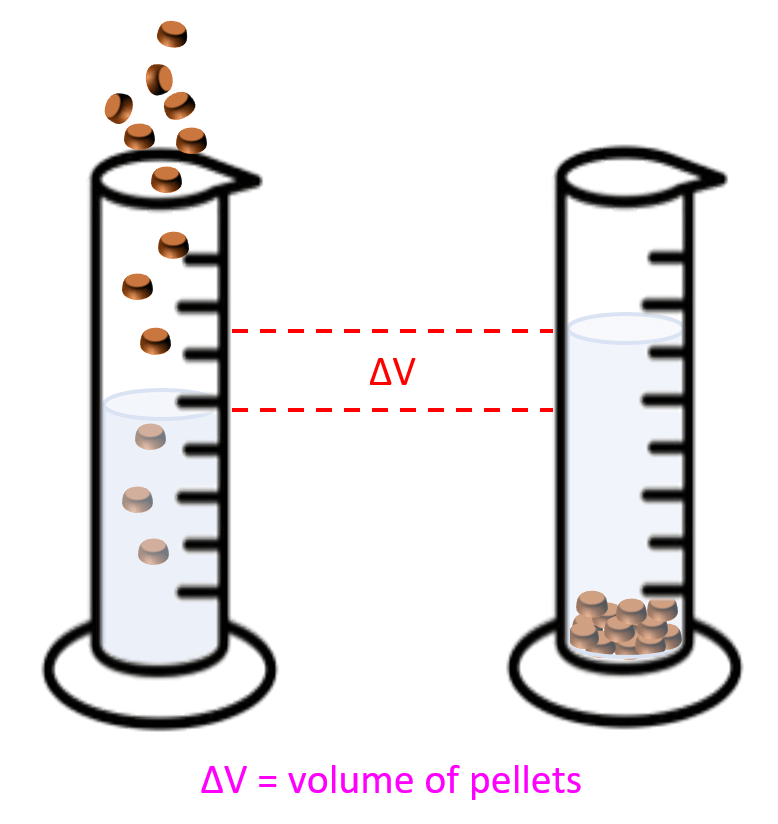
- Practice density calculations.
- Identify intensive and extensive properties of matter.
- See examples of intrinsic and extrinsic properties of matter.
- Get the definition and examples of solids.
- Get the definition and examples of gases.
- See the definition and examples of liquids.
- Learn what melting point is and get a list of values for different substances.
- Get the azeotrope definition and see examples.
- See how to calculate specific volume of a gas.
- Get examples of physical properties of matter.
- Get examples of chemical properties of matter.
- Review the states of matter.
Molecular Structure Chemistry Problems
See chemistry problems writing chemical formulas. See examples of monatomic and diatomic elements.
- Practice empirical and molecular formula problems.
- Practice simplest formula problems.
- See how to calculate molecular mass.
- Get examples of the monatomic elements.
- See examples of binary compounds.
- Calculate the number of atoms and molecules in a drop of water.
Nomenclature
Practice chemistry problems naming ionic compounds, hydrocarbons, and covalent compounds.
- Practice naming covalent compounds.
- Learn hydrocarbon prefixes in organic chemistry.
Nuclear Chemistry
These chemistry problems involve isotopes, nuclear symbols, half-life, radioactive decay, fission, fusion.
- Review the types of radioactive decay.
Periodic Table
Learn how to use a periodic table and explore periodic table trends.
- Know the trends in the periodic table.
- Review how to use a periodic table.
- Explore the difference between atomic and ionic radius and see their trends on the periodic table.
Physical Chemistry
Explore thermochemistry and physical chemistry, including enthalpy, entropy, heat of fusion, and heat of vaporization.
- Practice heat of vaporization chemistry problems.
- Practice heat of fusion chemistry problems.
- Calculate heat required to turn ice into steam.
- Practice calculating specific heat.
- Get examples of potential energy.
- Get examples of kinetic energy.
- See example activation energy calculations.
Spectroscopy and Quantum Chemistry Problems
See chemistry problems involving the interaction between light and matter.
- Calculate wavelength from frequency or frequency from wavelength.
Stoichiometry Chemistry Problems
Practice chemistry problems balancing formulas for mass and charge. Learn about reactants and products.
- Get example mole ratio problems.
- Calculate percent yield.
- Learn how to assign oxidation numbers.
- Get the definition and examples of reactants in chemistry.
- Get the definition and examples of products in chemical reactions.
Unit Conversions
There are some many examples of unit conversions that they have their own separate page!

Chemistry Steps
General Chemistry Practice Problems
Chemistry Steps offers thousands of practice problems on topics of general chemistry such as atoms, molecules, isotopes, mole, molar mass, the stoichiometry of chemical reactions, and related molar calculations including limiting reactant and percent yield.
Over 1000 Practice Questions
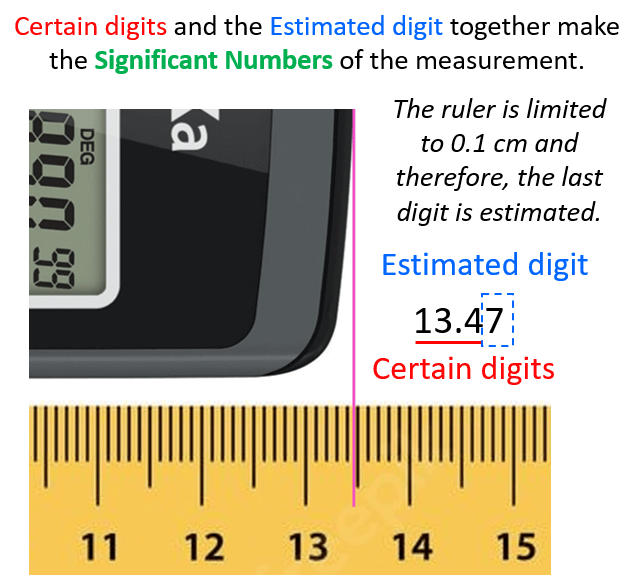
Significant Figures Practice Problems
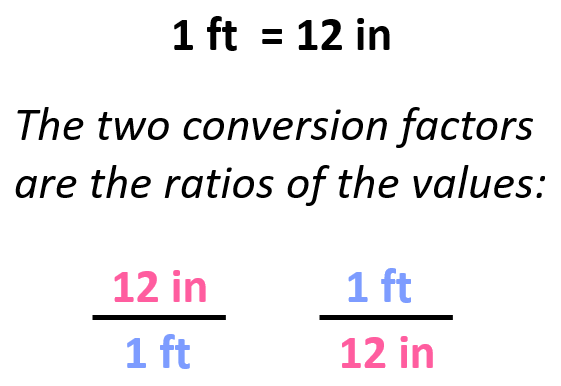
Dimensional Analysis Practice Problems
Naming Ionic and Covalent Compounds
Lewis Structures Practice Problems
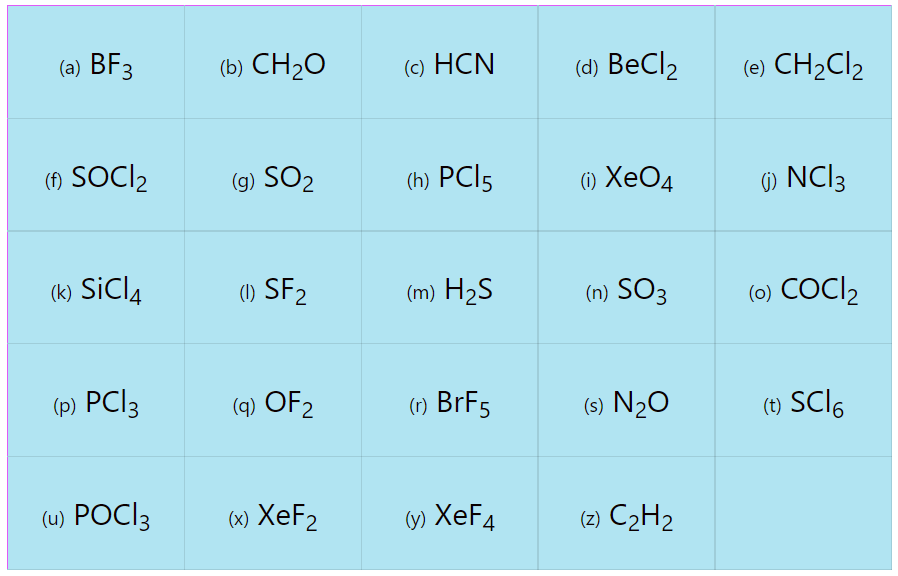
VSEPR Theory Practice Problems

Calculating Different Concentrations

Colligative Properties Practice Problems
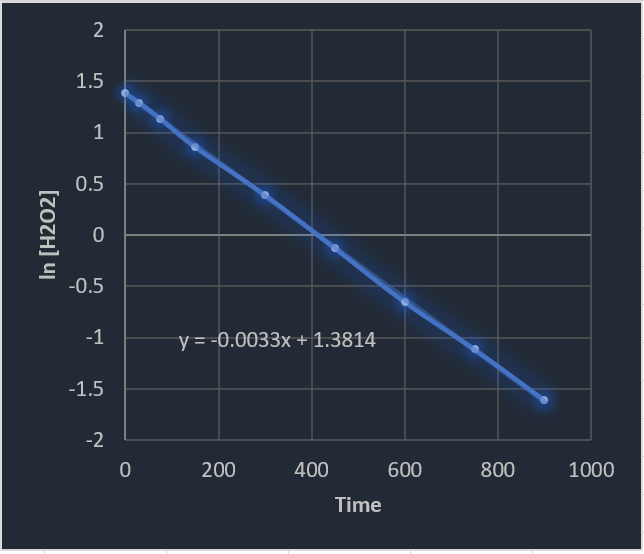
Kinetics Practice Problems

Chemical Equilibrium Practice Problems
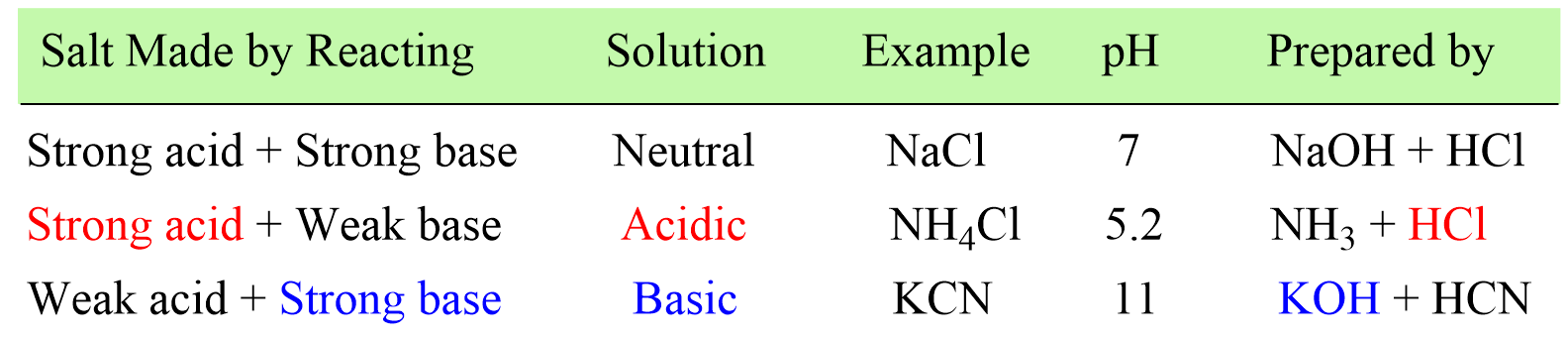
Acids and Bases Practice Problems
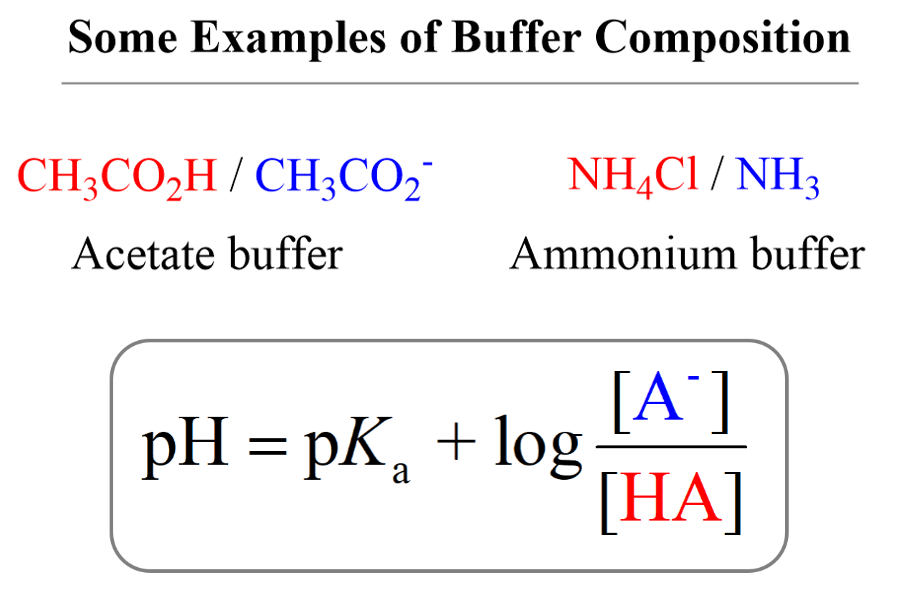
Buffer Solutions Practice Problems
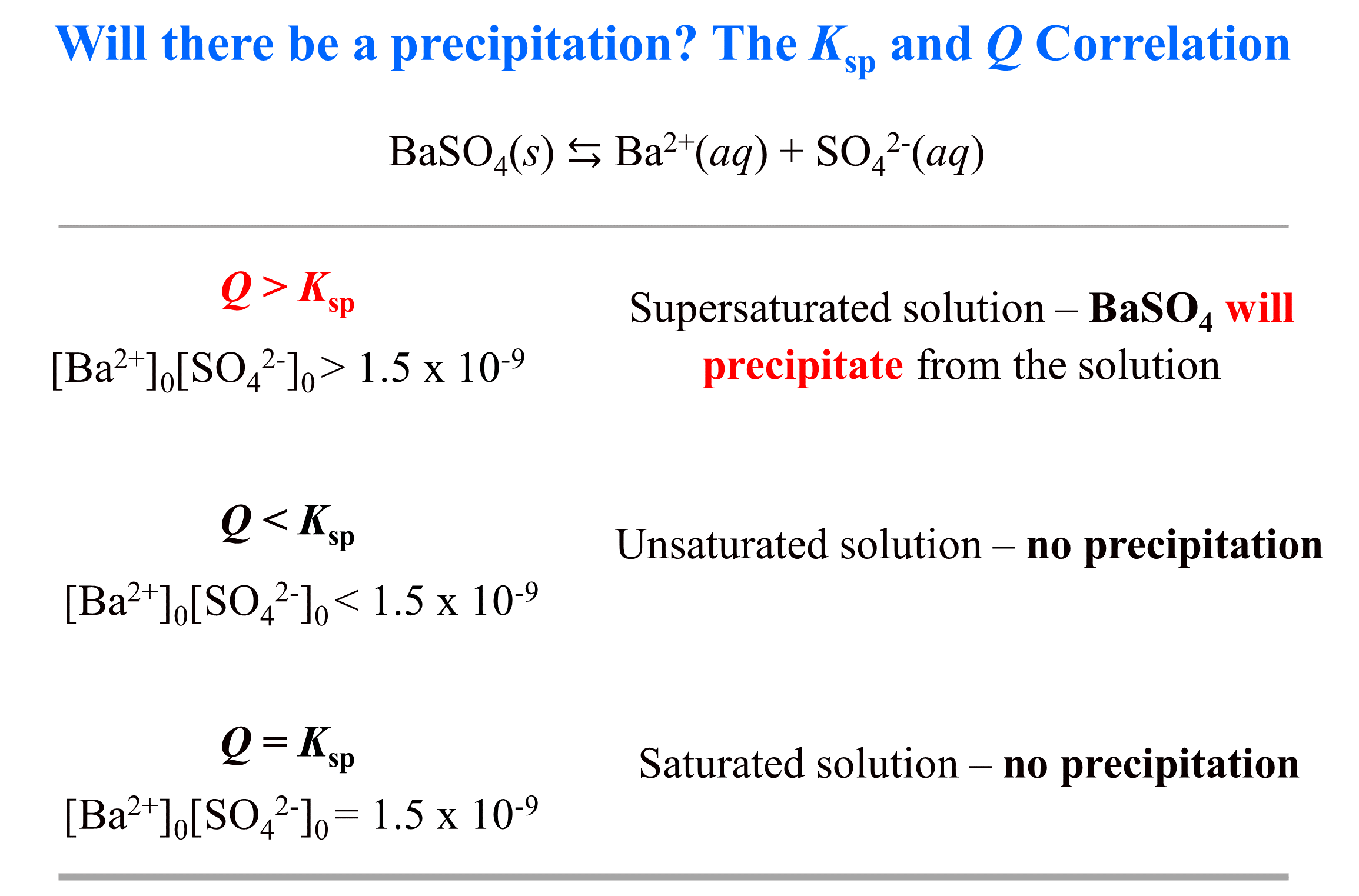
K sp and Molar Solubility Practice Problems
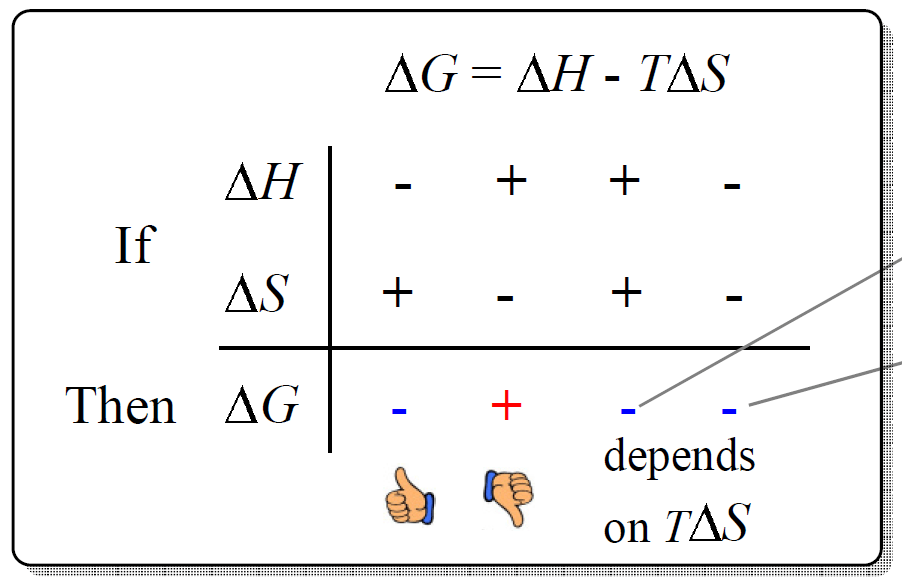
Thermodynamics, Δ H , Δ S , and Δ G Practice Problems
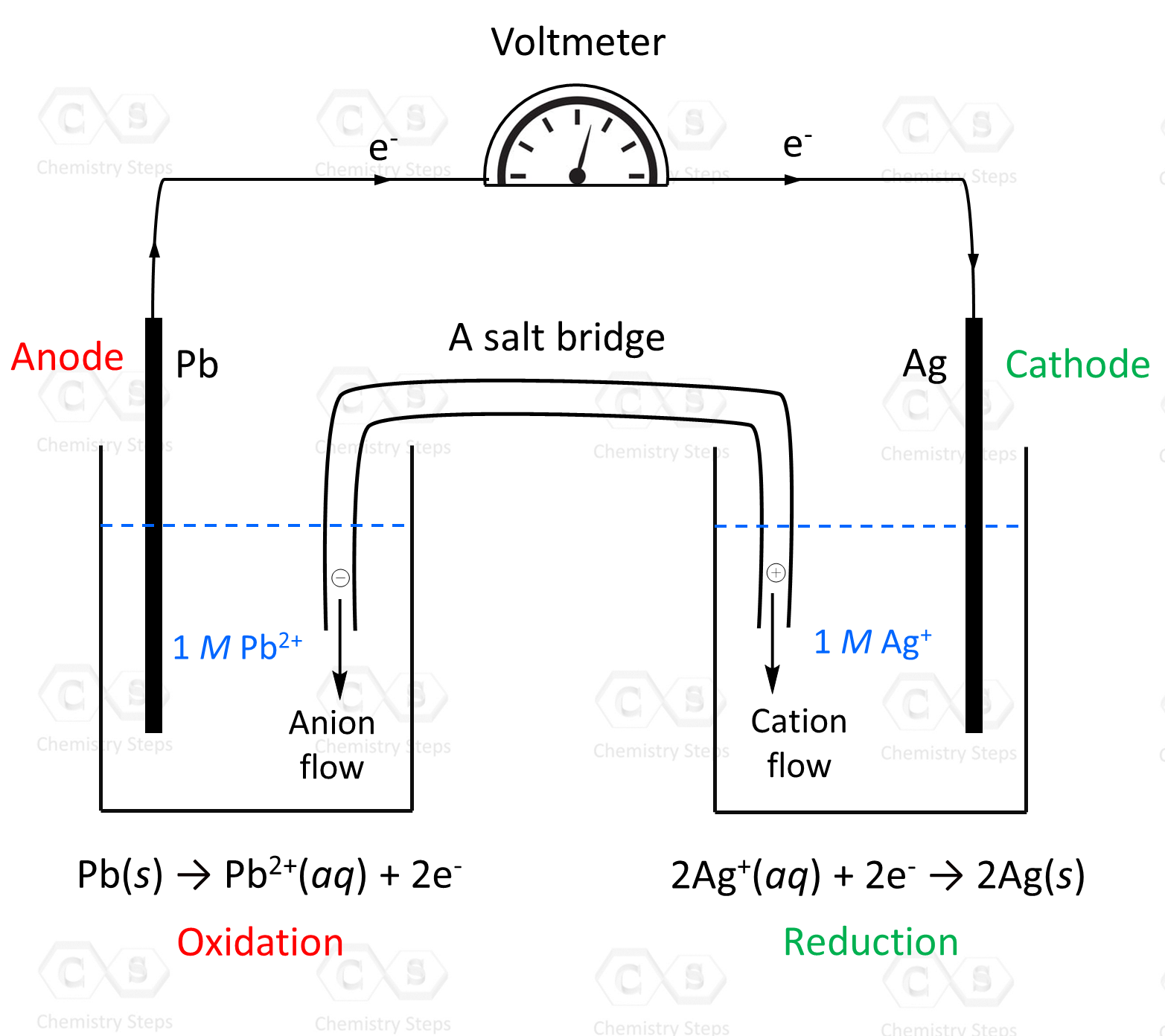
General Chemistry Quizzes
You read all the articles of the chapter and watched lots of videos, but need to assess your skills before the general chemistry exam?
Multiple choice quizzes might be the “easy” way of glancing through the key concepts and getting feedback on what you need to work on more.
Here are the general chemistry quizzes available to practice the following topics:

Quiz: Matter, Chemical and Physical Properties
Nomenclature and formulas quiz, atomic structure, mass, and isotopes quiz, significant figures quiz, dimensional analysis in chemistry quiz, mass, moles, and number of particles quiz, the stoichiometry of chemical reactions quiz, reactions in aqueous solutions quiz, oxidation state and redox reactions quiz, a multiple-choice quiz on gases, thermochemistry quiz, electronic structure of atoms quiz, periodic table and periodic trends, chemical bonding and lewis structures quiz, geometry and hybridization quiz, chemical kinetics quiz, chemical equilibrium quiz, acids and bases quiz, chemical thermodynamics quiz, electrochemistry quiz.
Example Questions
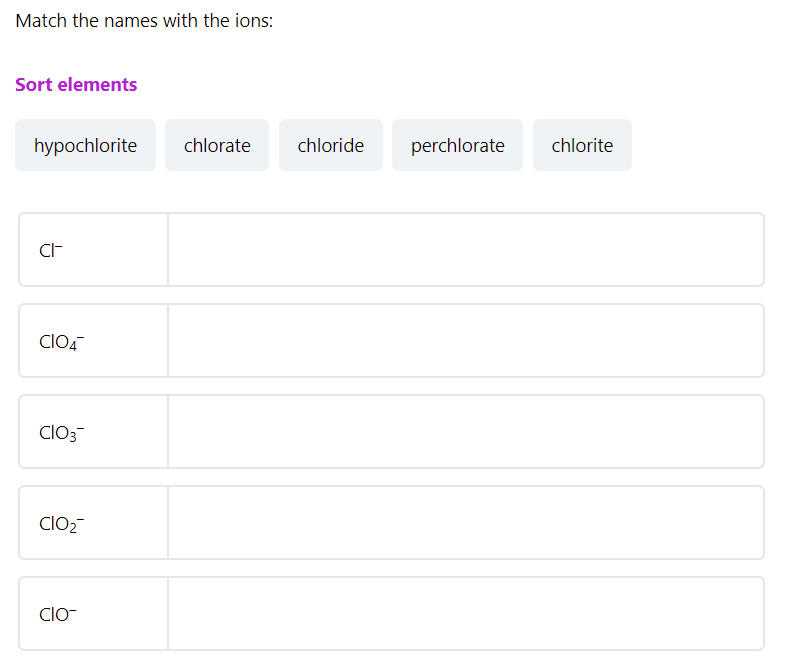
Each question has a hint including the related articles and the Study guide for the given topic!
How do I access the quizzes?
The quizzes are available to Chemistry Steps General and Semester subscribers .
What else is included as part of the membership?
- General Chemistry 1 and 2 Summary Study Guides. These are comprehensive and beautiful study guides summarizing the key concepts of Gen Chem 1 and 2. Once you register, go to your account and download them under the CS Benefits tab.
Detailed Beautiful Powerful

Sign Up Today to Access All of This
CS General $29
* Answers and Solutions to
Practice Problems
* Gen Chem 1 and 2 Study Guides
No Recurring Payments
CS Semester $49
* Answers and Solutions to
Practice Problems
4 Months
Reset password New user? Sign up
Existing user? Log in
- Number Theory
- Probability
- Everyday Math
- Classical Mechanics
- Electricity and Magnetism
- Computer Science
- Quantitative Finance
Take a guided, problem-solving based approach to learning Chemistry. These compilations provide unique perspectives and applications you won't find anywhere else.
The Chemical Reaction
What's inside.
- Introduction
- Chemical Kinetics
- Reaction Principles
- Elements of Reactivity
Physics of the Everyday
- In the House
- On the Field
- Fuel the World
- Out in Nature
- Infrastructure
Community Wiki
Browse through thousands of Chemistry wikis written by our community of experts.
Fundamentals
- Chemistry in action
- Atoms, Molecules, Elements, Compounds
- Dalton's Atomic Model
- Bohr's Model
- Periodic Table of the Elements
- Atomic and Molecular Weights
- Mole Concept
- Concentration Terminology
- Nuclear Decay
- Balancing Chemical Reactions
- Limiting Reagents
- Laws of Chemical Combination
- Try It At Home Chemistry Experiments
- Chemistry Of Drugs
Chemical Bonding
- Ionic Compounds
- Lewis Structure
- Hybridisation
- Valence Shell Electron Pair Repulsion (VSPER) Theory
- Sigma and Pi Bonds
- Molecular Orbital Theory
- Coordinate Bonds
- Polarity of a Molecule
- Van der Waals Force
- Dipole Interactions
- Hydrogen Bonds
- Surface Tension
- Strength of Intermolecular Forces
- Chemistry of Diamonds
Reaction Mechanics
- Chemical Equilibrium
- Basics of Statistical Mechanics
- Le Chatelier's Principle
- Acids and Bases
- Acid/Base Equilibrium
- Thermal Expansion
- Rate of Chemical Reactions
- Order of Chemical Reactions
- Activation Energy
- Chemistry of Fireworks
- Thermometry
- Electrolytic Cells and Electrolysis
- Galvanic Cells
Organic Chemistry
- Structural Representations of Organic Compounds
- IUPAC Nomenclature - Branched Chain Alkanes, Cyclic Compounds Unsaturated Compounds
- Structural Isomerism
- Geometric Isomerism
- Optical Isomerism
- Electrophiles and Nucleophiles
- Inductive Effect, Electromeric Effect, Resonance Effects, and Hyperconjugation
- Reactive Intermediates
- Common Types of Organic Reactions
- Grignard Reagent
- Alkanes, Alkenes, Alkynes
- Do objects gain protons to become positive?
- Chemistry Of Poisons
- Chemistry Of Nutrition
- Reverse Transcription
- Mutation and DNA repair
- Viral Replication
Problem Loading...
Note Loading...
Set Loading...
What are the answers to Holt Chemistry page thirty-three Problem Solving Additional Problems work sheet?
I tell my students that the answers are unimportant. Working through to a solution becomes a skill that helps a student in life, not just chemistry. That is, a lists of 1 a, 2 b, 3 c, 4 d, etc. helps only the idiot who thinks a list of correct answers means learning.
For example, the first question, "Why are only a few metals found in nature in their pure form, whereas most exist only as ores, which are metal containing compounds? a. Most metals have many valence electrons. b. Metals tend to lose valence electrons easily. c. Metal atoms tend to form bonds with other metal atoms easily. d. Most metal are located on the higher periods of the Periodic Table .
The first choice 'many valence electrons' applies well to group VII, the halogens, all non metals, so 'a.' is not a good choice. The second choice, 'metals lose electrons easily,' is a property of most metals but the noble metals like gold cause doubt, so lets see if there is a better answer. The third choice, 'metals bond with metals easily,' also true but metallic bonds are not the substance of bonds in compounds. The fourth choice, 'metals are located on the higher periods,' is true enough but gold is right there and so this choice is weak. 'B' is the best choice as the question gives us an out for the rule not having to hold for all metals, just most metals.
This type of analysis models the type of learning your teacher wishes for you. If you don't trust this discussion, just ask your teacher.
Nitrogen monoxide and oxygen, both colorless gases, form a red-brown gas when mixed. Nitrogen monoxide and oxygen are called the? a-products b-reactants c-synthetics d-equilibria red brown?
The answers to the Holt Chemistry page thirty-three will not be located via the internet . Students will need to review the textbook information to know the answers.
The answers to the Hold chemistry chapter 12 section 1 review can not be located online. Students will have to obtain assistance from the teacher if they need help with the answers.
How can I know the acceleration if I hit a speed of 50 m/s after 5 seconds
Add your answer:
What are the two main practices that aid in solving chemistry problems?
The two main practices that aid in solving chemistry problems are understanding the underlying concepts and principles involved in the problem, and practicing problem-solving techniques consistently. By mastering the fundamental concepts and regularly applying problem-solving strategies, you can effectively tackle a wide range of chemistry problems.

Why does the study of chemistry depend so much on problem solving?
Chemistry relies on problem solving because it involves understanding and predicting the behavior of matter at the molecular level. By solving problems, chemists can make sense of experimental data, design experiments, and explain complex phenomena. Problem solving also helps chemists to develop critical thinking skills and apply fundamental concepts to real-world situations.
What is Zumdahl chemistry?
Zumdahl chemistry refers to the chemistry textbooks authored by Steven S. Zumdahl. These textbooks are widely used in high school and college chemistry courses due to their clear explanations and comprehensive coverage of key concepts in chemistry. Zumdahl's approach emphasizes problem-solving skills and practical applications of chemistry principles.
What are the answers to Holt chemistry's stoichiometry problem solving packet?
Na2CO3+2H(C2H3O2) >2Na(C2H3O2) + CO2+H20
What is the best definition for context chemistry apex?
Context chemistry in the APEX program refers to understanding chemical concepts in relation to real-world applications and problem-solving scenarios. It emphasizes the relevance of chemistry in everyday life and industry, providing a practical framework for learning and applying chemical principles.
Top Categories

Please ensure that your password is at least 8 characters and contains each of the following:
- a special character: @$#!%*?&
Homework Q&A
Drag image here or click to upload
- Anatomy & Physiology
- Astrophysics
- Earth Science
- Environmental Science
- Organic Chemistry
- Precalculus
- Trigonometry
- English Grammar
- U.S. History
- World History

Chemistry AI Solver
HIX Tutor's AI chemistry homework helper is your go-to companion to master chemistry problem-solving.

Get Instant Chemistry Homework Help At HIX Tutor
HIX Tutor's AI-powered chemistry homework solver is the ultimate companion for completing your chemistry assignment. Effortlessly solving complex reaction calculations and offering detailed problem solutions, this is an indispensable tool for students seeking clear, instant, and accurate assistance in chemistry studies.
Cutting-Edge Features of Our Chemistry AI
All-round chemistry support.
From basic concepts to complex chemistry problems, our AI chemistry equation solver provides thorough assistance for all levels.
Step-by-Step Solutions
The AI chemistry homework solver breaks down complex equations into understandable steps, offering detailed guidance to enhance learning.
Efficient and Economical
Streamline your study sessions with our efficient AI chemistry problem solver, saving valuable time and resources while achieving better academic results.
Multilingual Support
HIX Tutor’s AI chemistry homework helper is available in multiple languages, ensuring no student is left behind due to language barriers.
Available 24/7
Day or night, our AI chemistry problem solver is always ready to assist, providing reliable and instant chemistry help whenever you need it.
Worldwide Accessibility
Our chemistry AI problem solver is universally accessible, offering consistent and effective chemistry solutions to students worldwide.
How to Get Quick Chemistry Homework Help From HIX Tutor?
Experience the transformative power of HIX Tutor's AI chemistry homework solver with the following easy steps:
Initiate Your Chemistry Inquiry
Enter or upload your chemistry assignment questions into HIX Tutor's Chemistry AI homework solver to get started.
Experience AI-Driven Problem Solving
Once you input your query, our chemistry equation solver will use AI algorithms to dissect and process your question.
Obtain Comprehensive Solutions
Our chemistry helper will generate step-by-step explanations, making complex concepts and reactions understandable.
Other AI Homework Helpers
From basic arithmetic to advanced calculus, get understandable steps for complex math problems.
Demystify topics ranging from basic mechanics to advanced electromagnetism with detailed solutions
Clarify doubts, and get insights on complex processes and terminologies. Get accurate answers to hard.
The Dynamic Capabilities of Our Chemistry AI
| 🧪 Chemistry expertise | Accurate solutions instantly |
|---|---|
| ⏰ Always ready to assist | Reliable problem-solving 24/7 |
| 🌍 Global educational access | Worldwide reach and use |
| 🔍 Detailed solution breakdown | Easy-to-understand explanations |
| 💡 Time-saving study aid | Quick, efficient homework help |
1. What is a Chemistry AI?
A Chemistry AI applies artificial intelligence techniques to solve complex chemistry problems, such as predicting reaction outcomes, designing novel compounds, or optimizing synthesis pathways. By using AI algorithms, Chemistry AI can streamline research and development processes in drug discovery, materials science, and other areas of chemical research.
2. Can this chemistry problem solver tackle diverse homework queries?
Yes, it's equipped to handle a range of chemistry homework questions, from basic concepts to advanced topics like stoichiometry and thermodynamics. While highly specific or research-level queries might have limitations, it effectively covers most educational levels.
3. How accurate is the HIX Tutor chemistry homework helper?
Empowered by state-of-the-art AI, HIX Tutor's chemistry homework helper boasts a wide-ranging chemical knowledge base, and it is able to provide accurate and reliable solutions across a vast array of chemistry topics.
4. Is HIX Tutor’s chemistry problem solver suitable for various academic levels?
Absolutely. HIX Tutor's chemistry AI homework solver is versatile enough to aid students across different academic levels. From high school chemistry to college-level assignments, it provides valuable support, enhancing understanding for students of all academic levels.
5. Is this chemistry AI free?
Yes, our Chemistry AI Solver is indeed available for free. You can solve chemistry problems without incurring any costs.
6. What's the best Chemistry AI?
HIX Tutor is the best chemistry solver designed to assist students and researchers in solving complex chemistry problems. It offers a wide range of features, including step-by-step solutions and detailed explanations of key concepts. With HIX Tutor, users can better understand chemical reactions, improve problem-solving skills, and gain a deeper understanding of the subject matter.
Discover Frequently Asked Chemistry Questions and Their Answers
- Formula for Cd2+ and PO43- ? also whats its name
- What is the Lewis structure for a perchlorate ion?
- Which of these structures has dipole-dipole interactions? Water (H2O), ethyl alcohol (CH3CH2OH), and hexane (C6H14).
- Is a single molecule of oxygen held together by two nonpolar covalent bonds?
- Why do the alkali metals have to be stored under oil?
- How do you know the number of electrons in the outermost energy level of an atom?
- How do you define a valence electron? How do you determine the valence number of an atom?
- Do ionic bonds occur between atoms from adjacent groups?
- Why are the melting point and boiling point of graphite so remarkably high?
- Which statement is INCONSISTENT with Dalton's atomic theory?
- What is Ka for #HClO_4#?
- How do you rank Bronsted acids?
- Which is the stronger acid in each of the following pair #H_2SeO_3# or #H_2SeO_4#?
- Which is the stronger acid in each of the following pair #HBrO_2# or #HBrO#?
- How do you draw the cis and trans isomers for 1-ethyl-3-methylcyclobutane?
- How does a hydrolysis reaction separate a polymer into its monomers?
- Why are there cis and trans isomers?
- What does it mean for a molecule to be optically active?
- What is the most stable conformer for 3,3-dimethylhexane, viewed along the #C_3-C_4# bond using Newman projections?
- Which group contains a chiral carbon? #A.# #"1-bromopropane"#. #B.# #"2-bromopropane"#. #C.# #H_3C−CH_2CHBrCH_2CH_3#. #D.# #"cyclohexyl bromide"#

Ease Your Chemistry Learning Journey With Our Chemistry AI Problem Solver
HIX Tutor’s AI chemistry homework helper is your reliable partner for assignment help and exam preparation. Try it now and experience a new standard in AI-powered education.

Chemistry Assistant
Ai-powered chemistry problem solver.
- Homework Help: Students can use the Chemistry Assistant to help understand and work through chemistry problems in their homework.
- Teaching Aid: Teachers can use this tool to generate solutions to chemistry problems, aiding in lesson planning and student instruction.
- Exam Preparation: Use the Chemistry Assistant to prepare for chemistry exams by solving practice problems and getting explanations of chemistry terms and principles.
- Research Assistance: Researchers can use this tool to help work through chemistry problems in their work.
Yes, the Chemistry Assistant is designed to handle a wide range of chemistry problems, from basic to advanced. However, it's always important to cross-verify the solutions provided by the AI with trusted resources or professionals in the field to ensure accuracy and understanding, especially with more complex problems and principles.
While the Chemistry Assistant is specifically designed for chemistry problems, HyperWrite offers other AI tools for different subjects and needs. You can explore more tools at app.hyperwriteai.com/tools .
New & Trending Tools
Get webpage text from url, ai web scraper, ai resume updater.

IMAGES
VIDEO
COMMENTS
The mass of a molecule of water is the sum of the masses of two hydrogen atoms and one oxygen atom, and is equal to 18.02 amu. Therefore, 1 mol of water has a mass of 18.02 g. In the same way, you can relate amount, mass, and number of formula units for ionic compounds, such as NaCl, CaBr2, and Al2(SO4)3.
Now, with expert-verified solutions from Holt Chemistry 6th Edition, you'll learn how to solve your toughest homework problems. Our resource for Holt Chemistry includes answers to chapter exercises, as well as detailed information to walk you through the process step by step. With Expert Solutions for thousands of practice problems, you can ...
Exercise 13. At Quizlet, we're giving you the tools you need to take on any subject without having to carry around solutions manuals or printing out PDFs! Now, with expert-verified solutions from Holt Chemistry 4th Edition, you'll learn how to solve your toughest homework problems. Our resource for Holt Chemistry includes answers to chapter ...
Holt ChemFile: Problem-Solving Workbook 130 Percentage Yield Name Class Date Problem Solving continued Additional Problems 1. Ethyl acetate is a sweet-smelling solvent used in varnishes and fingernail-polish remover. It is produced industrially by heating acetic acid and ethanol together in the presence of sulfuric acid, which is added to speed ...
Now, with expert-verified solutions from Chemistry 5th Edition, you'll learn how to solve your toughest homework problems. Our resource for Chemistry includes answers to chapter exercises, as well as detailed information to walk you through the process step by step. With Expert Solutions for thousands of practice problems, you can take the ...
Holt ChemFile: Problem-Solving Workbook 119 Limiting Reactants Name Class Date Problem Solving continued Additional Problems 1. Heating zinc sulfide in the presence of oxygen yields the following: ZnS O 2 → ZnO SO 2 If 1.72 mol of ZnS is heated in the presence of 3.04 mol of O 2, which reactant will be used up? Balance the equation first. 2 ...
Some chemistry problems ask you identify examples of states of matter and types of mixtures. While there are any chemical formulas to know, it's still nice to have lists of examples. Practice density calculations. Identify intensive and extensive properties of matter. See examples of intrinsic and extrinsic properties of matter.
These pages present some common chemistry problems and strategies for solving them. The pages recommend a problem solving strategy then show you how to work through each step of the problem. As you work through the problems, you will notice that: The current step is displayed in bold. Information used in the current step is highlighted in red.
Chemistry Steps offers thousands of practice problems on topics of general chemistry such as atoms, molecules, isotopes, mole, molar mass, the stoichiometry of chemical reactions, and related molar calculations including limiting reactant and percent yield. Over 1000 Practice Questions. Sign Up and Get Now.
Find step-by-step solutions and answers to Pearson Chemistry - 9780132525763, as well as thousands of textbooks so you can move forward with confidence. ... Problem Solving in Chemistry. Page 28: Assessment. Page 31: Standardized Test Prep. Exercise 1. Exercise 2. Exercise 3. ... Solving Conversion Problems. Page 95: Assessment. Page 99 ...
Organic Chemistry. Structural Representations of Organic Compounds. IUPAC Nomenclature - Branched Chain Alkanes, Cyclic Compounds Unsaturated Compounds. Structural Isomerism. Geometric Isomerism. Optical Isomerism. Electrophiles and Nucleophiles. Inductive Effect, Electromeric Effect, Resonance Effects, and Hyperconjugation. Reactive Intermediates.
The two main practices that aid in solving chemistry problems are understanding the underlying concepts and principles involved in the problem, and practicing problem-solving techniques consistently.
Practicing chemistry problems is essential for mastering the subject and developing a solid understanding of its concepts. Chemistry is a complex science that deals with the structure, properties, composition, and reactions of matter. It requires critical thinking, problem-solving skills, and a deep knowledge of various theories and principles.
Problem solving is a complex set of activities, processes, and behaviors for which various models have been used at various times. Specifically, "problem solving is a process by which the learner discovers a combination of previously learned rules that they can apply to achieve a solution to a new situation (that is, the problem)". 2 Zoller identifies problem solving, along with critical ...
Free math problem solver answers your chemistry homework questions with step-by-step explanations. Mathway. Visit Mathway on the web. Start 7-day free trial on the app. Start 7-day free trial on the app. Download free on Amazon. Download free in Windows Store. Take a photo of your math problem on the app. get Go. Chemistry. Basic Math.
Now, with expert-verified solutions from Basic Chemistry 6th Edition, you'll learn how to solve your toughest homework problems. Our resource for Basic Chemistry includes answers to chapter exercises, as well as detailed information to walk you through the process step by step. With Expert Solutions for thousands of practice problems, you can ...
HIX Tutor is the best chemistry solver designed to assist students and researchers in solving complex chemistry problems. It offers a wide range of features, including step-by-step solutions and detailed explanations of key concepts. With HIX Tutor, users can better understand chemical reactions, improve problem-solving skills, and gain a ...
This AI tool helps answer chemistry questions and solve chemistry problems. HyperWrite's Chemistry Assistant is an AI-powered tool designed to answer chemistry questions and think through solving chemistry problems. By leveraging advanced AI models, this tool simplifies complex chemistry problems and provides detailed, understandable solutions.