CBSE NCERT Solutions
NCERT and CBSE Solutions for free

Class 11 Chemistry Assignments
We have provided below free printable Class 11 Chemistry Assignments for Download in PDF. The Assignments have been designed based on the latest NCERT Book for Class 11 Chemistry . These Assignments for Grade 11 Chemistry cover all important topics which can come in your standard 11 tests and examinations. Free printable Assignments for CBSE Class 11 Chemistry , school and class assignments, and practice test papers have been designed by our highly experienced class 11 faculty. You can free download CBSE NCERT printable Assignments for Chemistry Class 11 with solutions and answers. All Assignments and test sheets have been prepared by expert teachers as per the latest Syllabus in Chemistry Class 11. Students can click on the links below and download all Pdf Assignments for Chemistry class 11 for free. All latest Kendriya Vidyalaya Class 11 Chemistry Assignments with Answers and test papers are given below.
Chemistry Class 11 Assignments Pdf Download
We have provided below the biggest collection of free CBSE NCERT KVS Assignments for Class 11 Chemistry . Students and teachers can download and save all free Chemistry assignments in Pdf for grade 11th. Our expert faculty have covered Class 11 important questions and answers for Chemistry as per the latest syllabus for the current academic year. All test papers and question banks for Class 11 Chemistry and CBSE Assignments for Chemistry Class 11 will be really helpful for standard 11th students to prepare for the class tests and school examinations. Class 11th students can easily free download in Pdf all printable practice worksheets given below.
Topicwise Assignments for Class 11 Chemistry Download in Pdf
Advantages of Class 11 Chemistry Assignments
- As we have the best and largest collection of Chemistry assignments for Grade 11, you will be able to easily get full list of solved important questions which can come in your examinations.
- Students will be able to go through all important and critical topics given in your CBSE Chemistry textbooks for Class 11 .
- All Chemistry assignments for Class 11 have been designed with answers. Students should solve them yourself and then compare with the solutions provided by us.
- Class 11 Students studying in per CBSE, NCERT and KVS schools will be able to free download all Chemistry chapter wise worksheets and assignments for free in Pdf
- Class 11 Chemistry question bank will help to improve subject understanding which will help to get better rank in exams
Frequently Asked Questions by Class 11 Chemistry students
At https://www.cbsencertsolutions.com, we have provided the biggest database of free assignments for Chemistry Class 11 which you can download in Pdf
We provide here Standard 11 Chemistry chapter-wise assignments which can be easily downloaded in Pdf format for free.
You can click on the links above and get assignments for Chemistry in Grade 11, all topic-wise question banks with solutions have been provided here. You can click on the links to download in Pdf.
We have provided here topic-wise Chemistry Grade 11 question banks, revision notes and questions for all difficult topics, and other study material.
We have provided the best collection of question bank and practice tests for Class 11 for all subjects. You can download them all and use them offline without the internet.
Related Posts

Class 11 Business Studies Assignments

Class 11 Sociology Assignments

Class 11 Psychology Assignments
- Sample Paper
- Question Paper
- NCERT Solutions
- NCERT Books
- NCERT Audio Books
- NCERT Exempler
- Model Papers
- Past Year Question Paper
- Writing Skill Format
- RD Sharma Solutions
- HC Verma Solutions
- CG Board Solutions
- UP Board Solutions
- Careers Opportunities
- Courses & Career
- Courses after 12th
Home » 11th Class » NCERT Solutions for Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry | Question Answer PDF
NCERT Solutions for Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry | Question Answer PDF
NCERT Solutions for Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry has been published by Aglasem. You can now download the Class 11 Chemistry Ch 1 Questions and Answers PDF here. This NCERT Solutions for Class 11 Chemistry contains answers of all questions asked in Chapter 1 in textbook, Chemistry Part I . Therefore you can refer it to solve Some Basic Concepts of Chemistry exercise questions and learn more about the topic.
NCERT Solutions for Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry
Class – Class 11 Subject – Chemistry Chapter – Ch 1 Chapter Name – Some Basic Concepts of Chemistry Book – Chemistry Part I Study Material – NCERT Solutions

NCERT Solutions for Class 11 Chemistry Chapter 1 PDF
While you can read NCERT Solutions for Class 11 Chemistry Ch 1 for all exercises here on aglasem. You can also download this NCERT Solutions PDF to refer ncer question answer at anytime when you study Some Basic Concepts of Chemistry. Here it is.
NCERT Solutions for Class 11 Chemistry Chapter 1 PDF Download Link – Click Here to Download Solutions PDF
How to download NCERT Solutions for Class 11 Chemistry Chapter 1 PDF?
You can download the complete NCERT solutions for chapter 1 of this NCERT Book i.e. Chemistry Part I with following steps.
- First search NCERT Solutions for Class 11 Chemistry Ch 1 PDF aglasem and come to this page.
- Now you will see the exercise questions answers of Some Basic Concepts of Chemistry and download pdf link on it.
- Click the Download PDF link to obtain the Some Basic Concepts of Chemistry questions with answers document.
- NCERT Solutions for Class 11 Chemistry
There are more chapters to study besides Some Basic Concepts of Chemistry in this subject. So here are NCERT solutions for all topics of Chemistry taught in 11th class here at aglasem.
- Chapter 1 Some Basic Concepts of Chemistry
- Chapter 2 Structure of Atom
- Chapter 3 Classification of Elements and Periodicity in Properties
- Chapter 4 Chemical Bonding and Molecular Structure
- Chapter 5 Thermodynamics
- Chapter 6 Equilibrium
- Chapter 7 Redox Reactions
- Chapter 8 Organic Chemistry – Some Basic Principles and Techniques
- Chapter 9 Hydrocarbons
NCERT Solutions for Class 11
Just like you got Chemistry Ch 1 solutions here. You can see exercise questions answers of other subjects and their topics too on aglasem. Here are NCERT solutions for all subjects of 11th standard NCERT books.
- NCERT Solutions for Class 11 Accountancy
- NCERT Solutions for Class 11 Biology
- NCERT Solutions for Class 11 Business Studies
- NCERT Solutions for Class 11 Economics
- NCERT Solutions for Class 11 English
- NCERT Solutions for Class 11 Geography
- NCERT Solutions for Class 11 Hindi
- NCERT Solutions for Class 11 History
- NCERT Solutions for Class 11 Maths
- NCERT Solutions for Class 11 Physics
- NCERT Solutions for Class 11 Political Science
- NCERT Solutions for Class 11 Psychology
- NCERT Solutions for Class 11 Sociology
NCERT Solutions for Class 11 Chemistry Chapter 1 – An Overview
The key highlights of this study material are as follows.
| Aspects | Details |
|---|---|
| Class | |
| Subject | Chemistry |
| Chapter Number | Ch 1 |
| Chapter Name | Some Basic Concepts of Chemistry |
| Book Name | Chemistry Part I |
| Book By | NCERT (National Council of Educational Research and Training) |
| Educational Resource Here | NCERT Solutions of Class 11 Chemistry Ch 1 for All Exercise |
| More Questions Answers of This Subject | |
| Download Book Chapter PDF | |
| All Questions Answers For This Class | |
| Complete Solutions |
If you have any queries on NCERT Solutions for Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry, then please ask in comments below.
To get study material, exam alerts and news, join our Whatsapp Channel .
NCERT Solutions for Class 11 Biology Chapter 11 Photosynthesis in Higher Plants | Question Answer PDF
Ncert solutions for class 11 economics chapter 4 human capital formation in india | question answer pdf, related posts.

AP Inter 1st Year Previous Year Question Papers – Download PDF Andhra Pradesh Board PYQP
Ap inter 1st year sanskrit question paper | ap pyqp pdf download, ap inter 1st year surveying theory question paper | ap pyqp pdf download, ap inter 1st year tamil question paper | ap pyqp pdf download, leave a reply cancel reply, cbse board quick links.
- CBSE Date Sheet
- CBSE Result
- CBSE Syllabus
- CBSE Sample Papers
- CBSE Question Papers
- CBSE Practice Papers
CISCE Board Quick Links
- CISCE Time Table
- CISCE Results
- CISCE Specimen Papers
- CISCE Syllabus
- CISCE Question Papers
Class Wise Study Material
Board exams 2023.
- Solved Sample Papers
- Revision Notes
- State Board
Study Material
- Class Notes
- Courses After Class 12th
- JEE Main 2024
- Fashion & Design
- Terms of Use
- Privacy Policy
© 2019 aglasem.com
Discover more from AglaSem Schools
Subscribe now to keep reading and get access to the full archive.
Continue reading
Talk to our experts
1800-120-456-456
Important Questions for CBSE Class 11 Chemistry Chapter 1 - Some Basic Concepts of Chemistry
- Class 11 Important Question
- Chapter 1: Some Basic Concepts Of Chemistry

CBSE Class 11 Chemistry Chapter-1 Important Questions - Free PDF Download
Class 11 is a crucial and significant year for high school students because it is during this year that you set the pedestal for all the essential topics and concepts you will cover in the Class 12 board exams .
A brief comprehension of the chapter - some basic concepts of chemistry can help you understand and appreciate the role of chemistry in different spheres of life. The Important Questions of Some Basic Concepts of Chemistry Class 11 is based majorly upon the topics and concepts that will help you secure a good average.
It is of prime importance that students remain aware of all the crucial questions about Class 11 Chemistry so that you can prepare better for their final exams.
A student who finds it challenging to comprehend the topics and concepts that fall under this chapter must practice all the important questions for class 11 chemistry chapter 1 . These essential questions can aid the preparation of the students through the concepts of this chapter.
Download CBSE Class 11 Chemistry Important Questions 2024-25 PDF
Also, check CBSE Class 11 Chemistry Important Questions for other chapters:
CBSE Class 11 Chemistry Important Questions | ||
Sl.No | Chapter No | Chapter Name |
1 | Chapter 1 | Some Basic Concepts of Chemistry |
2 | Chapter 2 |
|
3 | Chapter 3 |
|
4 | Chapter 4 |
|
5 | Chapter 5 |
|
6 | Chapter 6 |
|
7 | Chapter 7 |
|
8 | Chapter 8 |
|
9 | Chapter 9 |
|
10 | Chapter 10 |
|
11 | Chapter 11 |
|
12 | Chapter 12 |
|
13 | Chapter 13 |
|
14 | Chapter 14 |
|
Study Important Question for Class 11 Chemistry Chapter 1 – Some Basic Concepts of Chemistry
1 MARK QUESTIONS
1. What is chemistry?
Ans: Chemistry is the scientific study of the composition, characteristics, and interactions of matter.
2. How has chemistry contributed to the nation's development?
Ans: Weather patterns, brain function, computer operation, chemical industries, manufacturing, fertilizers, alkalis, acids, salts, dyes, polymers, medicines, soaps, detergents, metals, alloys, and other fields of chemistry have all contributed to the national economy.
3. Differentiate solids, liquids & gases in terms of volume & shapes.
Ans: The differences between solids, liquids, and gases are:
Property | Solids | Liquids | Gases |
Definite | Definite | Not definite | |
Fixed | Not fixed, take the shape of the container | Not fixed, takes the shape of the container. |
4. Name the different methods that can be used for the separation of components of a mixture.
Ans: Physical procedures such as handpicking, filtrations, crystallization, distillation, and others can be used to separate the components of a mixture.
5. Classify the following as pure substances and mixtures – Air, glucose, gold, sodium and milk.
Ans: From the substances given in the question Glucose, Gold, and Sodium are the pure substances while air milk is the mixtures.
6. What is the difference between molecules and compounds? Give examples of each.
Ans: The difference is tabulated below:
Molecules | Compound |
Molecules are made up of either distinct atoms or the same atoms. | When two or more distinct atoms join in a simple proportion, a compound is created. |
For example, a hydrogen molecule has two hydrogen atoms, but a water molecule has two hydrogen atoms and one oxygen atom. | For example, water (${{\text{H}}_{\text{2}}}\text{O}$), Carbon dioxide ($\text{C}{{\text{O}}_{\text{2}}}$), etc. |
7. How can we separate the components of a compound?
Ans: The constituents of a compound cannot be separated by physical methods. They can only be separated by chemical methods.
8. How are physical properties different from chemical properties?
Ans: Color, odor, and other physical properties can be measured or observed without changing the substance's identity or composition, whereas chemical properties require a chemical change to be measured.
9. What are the two different systems of measurement?
Ans: The different systems of measurement are the English system and the metric system.
10. What is the SI unit of density?
Ans: $\text{Kg }{{\text{m}}^{\text{-3}}}$ or $\text{Kg/}{{\text{m}}^{\text{3}}}$ is the SI unit of density.
11.What are the reference points in a thermometer with the Celsius scale?
Ans: The thermometers with Celsius scale are calibrated form ${{\text{0}}^{\text{o}}}\text{C}$ to $\text{10}{{\text{0}}^{\text{o}}}\text{C}$ where there two temperatures are the freezing and boiling of water.
12. What is the SI unit of volume? What is the other common unit which is not an SI unit of volume?
Ans: The SI unit of volume is ${{\text{m}}^{\text{3}}}$ while litre (L) is the common unit which is not an SI unit.
13. What is the difference between precision and accuracy?
Ans: The difference between precision and accuracy are:
Precision | Accuracy |
Precision refers to how near different measurements for the same amount are to each other. | When comparing the observed value to the real value of the outcome, accuracy informs us how close they are. |
14. What do you understand by significant figures?
Ans: Significant figures are used to define those numbers which have some uncertainty in the form of digits. Considering the following example, if we have a 5.756 value, then it has 4 significant figures.
15. State law of definite proportions.
Ans: Law of definite proportions also known by the name of the law of constant proportions which states that a given element always contains exactly the same proportion of elements by weight.
16. State Avogadro’s law.
Ans: Avogadro’s law states that at the same temperature and pressure, gases that have equal volume will contain an equal number of molecules.
17. Define one atomic mass unit (amu).
Ans: One atomic mass unit is defined as the mass that is exactly equivalent to 1/12 th of the mass of a carbon atom, whereas the mass of a carbon atom is 12.0107 u.
18. What is formula mass?
Ans: When a material has a three-dimensional structure and does not include discrete molecules as component particles, the molecular mass is calculated by summing the atomic masses of all the individual atoms present in that composition.
19. What is the value of one mole?
Ans: A mole of a material or particle is defined as having exactly $\text{6}\text{.022 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{23}}}$ particles, which can be atoms, molecules, or ions, with $\text{6}\text{.022 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{23}}}$ being Avogadro's number.
20. At NTP, what will be the volume of molecules of $\text{6}\text{.022 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{23}}}\text{ }{{\text{H}}_{\text{2}}}$?
Ans: Under NTP circumstances, $\text{6}\text{.022 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{23}}}$ hydrogen molecules will contain precisely 22.4 litres of hydrogen.
21. Calculate the number of molecules present in 0.5 moles of $\text{C}{{\text{O}}_{\text{2}}}$?
Ans: 1 mole of $\text{C}{{\text{O}}_{\text{2}}}$ contains exactly $\text{6}\text{.022 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{23}}}$ molecules, then 0.5 moles will contain: $\text{6}\text{.022 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{23}}}\text{ }\!\!\times\!\!\text{ 0}\text{.5 = 3}\text{.011 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{23}}}$
So, 0.5 moles of $\text{C}{{\text{O}}_{\text{2}}}$ contains $\text{3}\text{.011 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{23}}}$ molecules.
22. 1L of a gas at STP weighs 1.97g. What is molecular mass?
Ans: Molecular mass can be calculated by multiplying the weight by 22.4, so the 22.4 L of gas will weigh:
$\text{1}\text{.97 }\!\!\times\!\!\text{ 22}\text{.4 = 44}\text{.1 g}$
Hence, the molecular mass is 44.1 g.
23. What is stoichiometry?
Ans: Stoichiometry is formed by combining two Greek words: stoikhein, which means element, and metron, which means measurement. As a result, we may say that stoichiometry is concerned with calculating the masses of reactants and products in chemical processes.
24. The substance which gets used up in any reaction is called _________ .
Ans: Limiting reagent
25. What is 1 molal solution?
Ans: One molal solution is defined as a solution containing one mole of a solute per kilogram or 1000 g of solvent.
2 MARKS QUESTION
1. How can we say that sugar is solid and water is liquid?
Ans: Sugar's constituent particles are densely packed, and it also has its own volume and form, making it a solid, whereas water's constituent particles are not as densely packed. It has a defined volume but no defined form, therefore it is classified as a liquid.
2. How is matter classified at macroscopic level?
Ans: Macroscopic classification of matter is given as follows:
3. Classify the following substances as elements, compounds and mixtures – water, tea, silver, steel, carbon dioxide and platinum.
Ans: From the substances given in the question water and carbon dioxide are compounds, silver and platinum are elements while tea and steel are mixture.
4. Write seven fundamental quantities and their units.
Ans: The seven fundamental quantities and their SI units are listed as follows:
Physical Quantity | SI unit |
1. Length (l) | Metre (m) |
2. Mass (m) | Kilogram (kg) |
3. Time (t) | Second (s) |
4. Electric Current (I) | Ampere (A) |
5. Thermodynamic Temperature (T) | Kelvin (K) |
6. Amount of substance (n) | Mole (mol) |
7. Luminous Intensity (I) | Candela (cd) |
5. What is the difference between mass & weight? How is mass measured in the laboratory?
Ans: The difference between mass and weight is:
Mass | Weight |
The quantity of matter in a material is its mass. | The force of gravity exerted by the earth on an item or a body is its weight. |
Mass is a scalar quantity as it only has a magnitude. | Weight is a vector quantity as it has magnitude and is directed towards the center of the Earth. |
The mass of a material is generally determined in the laboratory using an analytical balance.
6. How is volume measured in the laboratory? Convert 0.5L into mL and $\text{30 c}{{\text{m}}^{\text{3}}}$ to $\text{d}{{\text{m}}^{\text{3}}}$ .
Ans: In laboratories, volume of a liquid is generally measured by using burette, graduated cylinder, pipette etc.
$\text{1 L = 1000 mL}$
so, 0.5 L will be equal to:-
$\text{0}\text{.5 L = 0}\text{.5 }\!\!\times\!\!\text{ 1000 mL}$
$\text{0}\text{.5 L = 500 mL}$
Now, $\text{1000 c}{{\text{m}}^{\text{3}}}\text{ = 1 d}{{\text{m}}^{\text{3}}}$
So, $\text{30 c}{{\text{m}}^{\text{3}}}$ will be equal to:-
$\text{30 c}{{\text{m}}^{\text{3}}}\text{ = }\dfrac{\text{1}}{\text{1000}}\text{ }\!\!\times\!\!\text{ 30d}{{\text{m}}^{\text{3}}}$
$\text{30 c}{{\text{m}}^{\text{3}}}\text{ = 0}\text{.03 d}{{\text{m}}^{\text{3}}}$
7. Convert $\text{3}{{\text{5}}^{\text{o}}}\text{C}$ to $^{\text{o}}\text{F}$ and K.
Ans: To convert $\text{3}{{\text{5}}^{\text{o}}}\text{C}$to $^{\text{o}}\text{F}$
We will use the following formula,
$^{\text{o}}\text{F=}\dfrac{\text{9}}{\text{5}}{{\text{(}}^{\text{o}}}\text{C)+32}$
Putting the value of 35 in $^{\text{o}}\text{C}$, we get,
$^{\text{o}}\text{F = }\dfrac{\text{9}}{\text{5}}\text{ (35) + 32}$
$\text{63 + 32 = 9}{{\text{5}}^{\text{o}}}\text{F}$
Now, to convert $\text{3}{{\text{5}}^{\text{o}}}\text{C}$ to K,
We will us the following relationship,
$\text{K =}{{\text{ }}^{\text{o}}}\text{C + 273}\text{.15}$
Putting the values, we get:
K = 35 + 273.15
8. What does the following prefixes stand for:
Ans: $\text{Pico = 1}{{\text{0}}^{\text{-12}}}$
Ans: $\text{Nano = 1}{{\text{0}}^{\text{-9}}}$
Centi
Ans: $\text{Centi = 1}{{\text{0}}^{\text{-2}}}$
Ans: $\text{Deci = 1}{{\text{0}}^{\text{-1}}}$
9. Explain the law of multiple proportions with an example.
Ans: The law of multiple proportions states that if two elements can combine to form more than one compound, the masses of one element which combine with a fixed mass of another element are in a ratio of small whole numbers.
For example:
Hydrogen and oxygen can combine to form water (whose chemical formula is ${{\text{H}}_{\text{2}}}\text{O}$) as well as hydrogen peroxide (whose chemical formula is ${{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}$).
Here, the masses of oxygen (16g and 32 g) combines with a fixed mass of hydrogen (2g) element bear a simple ratio which is 16:32 = 1:2
10. Write Postulates of Dalton’s atomic theory.
Ans: Postulates of Dalton’s atomic theory are as follows–
1. Matter consists of indivisible atoms.
2. All atoms of an element have a similar atomic mass. But atoms of different elements have different atomic masses.
3. Compounds are formed when atoms of different elements combine in a fixed ratio.
4. Chemical reaction involves the reorganization of atoms. These are neither created nor destroyed.
11. Calculate the molecular mass of-
${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\text{, }{{\text{C}}_{\text{12}}}{{\text{H}}_{\text{22}}}{{\text{O}}_{\text{11}}}\text{, }{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{, }{{\text{H}}_{\text{3}}}\text{P}{{\text{O}}_{\text{4}}}$
Ans: The molecular mass is the sum of the atomic masses of the individual elements present in a molecule. The molecular masses of the given compounds are calculated as follows with the help of the molar masses of the elements.
The molar mass of C= 12
The molar mass of H= 1
The molar mass of O= 16
The molar mass of S= 32
The molar mass of P= 31
\[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\text{ = (2 }\!\!\times\!\!\text{ 12) + (6 }\!\!\times\!\!\text{ 1) = 30 g/ mol}\]
\[{{\text{C}}_{\text{12}}}{{\text{H}}_{\text{22}}}{{\text{O}}_{\text{11}}}\text{ = (12 }\!\!\times\!\!\text{ 12) + (22 }\!\!\times\!\!\text{ 1) + (11 }\!\!\times\!\!\text{ 16) = 342 g/ mol}\]
\[{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{ = (2 }\!\!\times\!\!\text{ 1) + (32) + (16 }\!\!\times\!\!\text{ 4) = 98 g/ mol}\]
\[{{\text{H}}_{\text{3}}}\text{P}{{\text{O}}_{\text{4}}}\text{ = (3 }\!\!\times\!\!\text{ 1) + (31) + (16 }\!\!\times\!\!\text{ 4) = 98 g/ mol}\]
12. Give one example each of molecule in which empirical formula and molecular formula are
Ans: Molecule having same molecular formula and the empirical formula is Carbon dioxide, that is $\text{C}{{\text{O}}_{\text{2}}}$.
Ans: When molecular formula and empirical formula are different, the example of such molecule is,
Hydrogen peroxide: the molecular formula is ${{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}$ and empirical formula is HO.
13. Calculate the number of moles in the following masses:
7.85g of Fe
Ans: Given 7.85g of Fe
56g of Fe contains $\text{6}\text{.022 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{23}}}$ atoms = 1 mole
56g of Fe = 1 mole
So, $\text{7}\text{.85 g of Fe = }\dfrac{\text{1}}{\text{56}}\text{ }\!\!\times\!\!\text{ 7}\text{.85 = 0}\text{.14 moles}$
7.9 mg of Ca
Ans: As, 40g of Ca = $\text{40 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{3}}}\text{ mg}$ of Ca
40g of Ca contain 1 mole of Ca
Or we can write $\text{4 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{4}}}\text{ mg Ca = 1 mole}$
Therefore, $\text{7}\text{.9 mg of Ca = }\dfrac{\text{7}\text{.9}}{\text{4 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{4}}}}$
$\text{= 1}\text{.97 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-4}}}\text{ moles}$
14. How much potassium chlorate should be heated to produce 2.24 L of oxygen at NTP?
Ans: The reaction for heating of potassium chlorate is:
\[\text{2 KCl}{{\text{O}}_{\text{3}}}\xrightarrow{\text{ }\!\!\Delta\!\!\text{ }}\text{2 KCl + 3}{{\text{O}}_{\text{2}}}\]
From the reaction, it is evident that 2 moles of potassium chlorate liberate 3 moles of oxygen.
Therefore, we have:
67.2 L of oxygen is produced from 245g of $\text{KCl}{{\text{O}}_{\text{3}}}$
Then, 2.24L of oxygen will be produced from = $\dfrac{\text{245}}{\text{67}\text{.2}}\text{ }\!\!\times\!\!\text{ 2}\text{.24}$
$\text{=8}\text{.17 g of KCl}{{\text{O}}_{\text{3}}}$
15. Write an expression for molarity and molality of a solution.
Ans: Molarity is the number of moles of solute per litre of a solution, that is,
$\text{Molarity = }\dfrac{\text{number of moles of solutes}}{\text{Volume of solution in Litres}}$
While molality is the number of moles od solute per kilogram of a solvent, that is,
$\text{Molality = }\dfrac{\text{number of moles of solutes}}{\text{Mass of solvent in kg}}$
16. Calculate the weight of lime CaO obtained by heating 200kg of 95% pure limestone $\text{CaC}{{\text{O}}_{\text{3}}}$ .
Ans: 100 kg impure sample has pure $\text{CaC}{{\text{O}}_{\text{3}}}$ = 95% = 95 kg
Therefore, 200kg impure sample has pure $\text{CaC}{{\text{O}}_{\text{3}}}$ = $\dfrac{\text{95 }\!\!\times\!\!\text{ 200}}{\text{100}}\text{ = 190 kg}$
From the below reaction:
\[\text{CaC}{{\text{O}}_{\text{3}}}\xrightarrow{\text{ }\!\!\Delta\!\!\text{ }}\text{ CaO + C}{{\text{O}}_{\text{2}}}\]
We can observe that 100kg $\text{CaC}{{\text{O}}_{\text{3}}}$ will give CaO = 56 kg
Therefore, 190 kg $\text{CaC}{{\text{O}}_{\text{3}}}$ will give CaO = $\dfrac{\text{56 }\!\!\times\!\!\text{ 190}}{\text{100}}\text{ = 106}\text{.4 kg}$
17. 4 litres of water added to 2L of 6 molar HCl solution. What is the molarity of the resulting solution?
Ans: Let the initial volume ${{\text{V}}_{\text{1}}}\text{ = 2 L}$
The final volume, ${{\text{V}}_{\text{2}}}\text{ = 4 L + 2 L = 6 L}$
Given, Initial Molarity, ${{\text{M}}_{\text{1}}}\text{ = 6 M}$
Let, Final molarity = ${{\text{M}}_{\text{2}}}$
Using the following relationship, ${{\text{M}}_{\text{1}}}{{\text{V}}_{\text{1}}}\text{=}{{\text{M}}_{\text{2}}}{{\text{V}}_{\text{2}}}$
$\text{6 M }\!\!\times\!\!\text{ 2 L = }{{\text{M}}_{\text{2}}}\text{ }\!\!\times\!\!\text{ 6 L}$
We have, ${{\text{M}}_{\text{2}}}\text{=}\dfrac{\text{6 M }\!\!\times\!\!\text{ 2 L}}{\text{6 L}}\text{ = 2 M}$
18. What volume of 10M HCl and 3M HCl should be mixed to obtain 1L of 6M HCl solution?
Ans: Let the required volume og 10M HCl be V liters.
The, the required volume of 3M HCl be (1 – V) liters.
Using the resultant Molarity formula,
${{\text{M}}_{\text{1}}}{{\text{V}}_{\text{1}}}\text{ + }{{\text{M}}_{\text{2}}}{{\text{V}}_{\text{2}}}\text{ = }{{\text{M}}_{\text{3}}}{{\text{V}}_{\text{3}}}$ .
$\text{10 }\!\!\times\!\!\text{ V + 3 }\!\!\times\!\!\text{ (1-V) = 6 }\!\!\times\!\!\text{ 1}$
10V + 3 – 3V = 6
$\text{V = }\dfrac{\text{3}}{\text{7}}\text{ = 0}\text{.428 L = 428 ml}$
Then the volume of 10M HCl required = 428 ml and volume of 3M HCl required will be:
1000ml – 428ml = 572ml
3 MARKS QUESTION
1. How many significant figures are present in
$\text{4}\text{.01 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{2}}}$
Ans: There are rules that must be followed for counting the number of significant figures in a given number.
If a number terminates in zeros, but these zeros do not reach the right side of the decimal point, these zeros might be significant or not important.
So, the given number is $\text{4}\text{.01 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{2}}}$, therefore, there are three significant figures in this.
8.256
Ans: All the non-zero digits and the zeros are important between the non-zero digits.
So, the given number is 8.256, therefore, there are four significant figures in this.
Ans: If a number terminates in zeros, but these zeros do not reach the right side of the decimal point, these zeros might be significant or not important.
So, the given number is 100, therefore, there is only one significant figure in this.
2. Vitamin C is essential for the prevention of scurvy. Combustion of
0.2000g of vitamin C gives 0.2998g of $\text{C}{{\text{O}}_{\text{2}}}$ and 0.819g of ${{\text{H}}_{\text{2}}}\text{O}$ . What is the empirical formula of Vitamin C?
Ans: The empirical formula is the simplest form of any molecular formula, and it can be also the same as the molecular formula.
First, we have to find the percentage of carbon, hydrogen, and oxygen in the given amount of compounds. These are given below:
Percentage of carbon = $\dfrac{\text{2}}{\text{18}}\text{ }\!\!\times\!\!\text{ 0}\text{.2998 }\!\!\times\!\!\text{ }\dfrac{\text{100}}{\text{0}\text{.2}}\text{= 40}\text{.88}$
Percentage of Hydrogen = $\dfrac{\text{2}}{\text{18}}\text{ }\!\!\times\!\!\text{ 0}\text{.819 }\!\!\times\!\!\text{ }\dfrac{\text{100}}{\text{0}\text{.2}}\text{ = 4}\text{.55}$
Percentage of Oxygen = $100\text{ - (40}\text{.88 + 4}\text{.55) = 54}\text{.57}$
So, we have the percentage of all the elements, now we can find the empirical formula as in the table given below:
Element | % | Atomic Mass | Relative no. of atoms | Simplest Molar Ratio | Simplest Whole Number |
C | 40.88 | 12 | $\dfrac{40.88}{12}=3.40$ | $\dfrac{3.40}{3.40}=1$ | $\text{1 }\!\!\times\!\!\text{ 3 = 3}$ |
H | 4.55 | 1 | $\dfrac{4.55}{1}=4.55$ | $\dfrac{4.55}{3.40}=1.33$ | $\text{1}\text{.33 }\!\!\times\!\!\text{ 3 = 4}$ |
O | 54.57 | 16 | $\dfrac{54.57}{16}=3.41$ | $\dfrac{3.41}{3.40}=1$ | $\text{1 }\!\!\times\!\!\text{ 3 = 3}$ |
So, the empirical formula of Vitamin C = ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{4}}}{{\text{O}}_{\text{3}}}$.
Important Questions For Class 11 Chemistry Chapter 1 - Free PDF Download
Some Basic Concepts of Chemistry is a chapter that deals with the theoretical and practical aspects and the role of chemistry in each student's life. This chapter presents the definition, balanced equations, empirical formula and molecular formula for a compound, and numerical, which may be difficult for students to understand. Lack of practice and a solid course plan and curriculum may act as a barrier preventing you from securing good scores.
To get a thorough comprehension of all the essential concepts and topics under this chapter and break the barriers, students must indulge in continuous practice. Regular practice of Chapter 1 Chemistry Class 11 Important Questions can help students improve, become through the concepts and topics, and be efficient during preparation or revision.
Students must implement the practice of the crucial questions in their schedule to enhance and better their preparation process. Students can download the Important Questions of Chemistry Class 11, Chapter 1, for free and achieve their study goals.
Chemistry Class 11 Chapter 1 Important Questions
Some Basic Concepts of Chemistry Class 11 Important Questions will help students gain comprehensive knowledge and understanding of the concepts and theories under this chapter. Continuous practice of the essential questions for class 12 chemistry chapter 1 will help you implement the plan and tactics to enhance preparation. A comprehensive brief of the chapter for students to learn are as follows:
Chemistry is often referred to as the Central Science because it plays a significant role in connecting the physical sciences, such as Chemistry with Applied Science and Life Sciences such as Engineering and Medicine. Chemistry is defined as the study of interaction, composition, and properties of matter.
The matter is defined as anything that possesses mass, occupies space, and the presence that can be felt by the five senses. Matter exists in three forms, namely, as a solid, liquid, and gas. Solids are substances that possess a definite structure and a definite shape like sugar, iron, etc. Liquids are substances that have a definite volume but lack a definite form and take the shape of the vessel in which they are put - for example, mercury, milk, water, etc. Gases are substances that can neither possess definite shape or definite volume like hydrogen, oxygen, nitrogen, etc.
Macroscopic Classification of Matter
The matter is classified as:
Mixtures: Mixtures consist of two or more substances in any ratio that are present in it which are called its components. A mixture can be heterogeneous or homogeneous.
Pure Substances: It is a material that contains only one substance. A pure substance is classified into elements and compounds.
Properties of Matter
A substance holds unique characteristics or attributed properties. The properties of matter can be classified into two primary categories- physical properties and Chemical properties. Physical properties can be observed or measured without changing the substance's composition and without changing the identity of the substances such as melting point, boiling point, colour, odour, etc. Chemical properties are the chemical changes that produce a characteristic reaction such as combustibility, basicity or acidity, etc.
Measurements
Physical Quantities: Physical Quantities are those that come across during our scientific studies. A physical quantity consists of two parts- The number and The Unit. A unit is referred to as the standard of reference chosen to measure any physical quantity such as Unit of length, Unit of density, Unit of time, Unit of electric current, etc.
Law of Chemical Combination
Law of Conservation of Life.
Law of Multiple Proportion.
Gay Lussac's Law of gaseous Volumes.
Law of Definite or Constant Proportions.
Avogadro's Law.
Law of Reciprocal Proportions.
Chemical Reactions
Chemical reactions are the rearrangement of atoms. Atoms from different molecules rearranged to form other molecules.
Stoichiometry
The study of chemical reactions and the calculations related to the reactions is known as Stoichiometry. The coefficients that are used to balance the chemical reactions are known as the Stoichiometric Coefficients.
Dilution Law
When a solution is diluted, more solutions are added, and the solute's moles remain unchanged. If the volume of a solution with a Molarity of M is changed from V 1 to V 2 , it can be stated that M 1 V 1 = M 2 V 2 can solve the concentration or volume of the concentrated or dilute solution.
Introduction to Equivalent Concept
Equivalent Concept helps understand the processes in chemistry and the chemical reactions derived into simple format through the Equivalent Concept.
Valency Factor (Z): The number of 'H' ions supplied by one molecule or a mole of an acid or the number of -OH ions provided by one mole of a base of one molecule.
Equivalent Mass: The mass of an acid or base that furnishes one mol H+ or OH- is Equivalent Mass.
Equivalents: The equivalents are equal to the weight of acids or bases taken by the Equivalent weight.
Law of Chemical Equivalence
The Law of Chemical Equivalence states that the number of gram equivalents of reactant and the number of gram equivalents of product in a reaction is equal. For example in a reaction, aA + bB = cC + dD irrespective of the stoichiometric coefficients, one eq. of A with one eq. of B gives one eq. of C and one eq. of D.
Important Questions of Chemistry Class 11 Chapter 1
To get a better overview of the Important Questions of Some Basic Concepts of Chemistry Class 11, we have enlisted a few important and potential questions that are most likely to appear in the examination:
Draw a comparison between solids, liquids, and gases regarding their shape and volume.
State how chemical properties differ in their characteristics from the physical properties.
Define S.I and state the S.I unit of density.
What do you mean by significant figures?
Solve the numerical by finding the volume needed for making 2.5 litres of its 0.25 M solution, given that the density of methanol is 0.793 kg L –1 .
State the difference between weight and mass. Explain how mass is measured in a laboratory.
State the Law of Multiple proportions along with a suitable example.
Determine the mass of one 12C atom in grams?
Solve the numerical by calculating the distance covered by light in 2.00 ns if the light speed is 3.0 × 10 8 m s –1 .
Explain how many water vapour volumes would be produced if ten volumes of dihydrogen gas react with five volumes of dioxygen gas?
Benefits of Important Questions For Class 11 Chemistry Chapter 1
Some Basic Concepts of Chemistry Important Questions help students secure a good average with a detailed understanding of the chapter's fundamentals. With the basic knowledge of the concepts, theories, and formulas, you can score better percentages in your annual or board examinations with regular practice. A few benefits of Chemistry Chapter 1, Class 11 Important Questions are as follows:
Chemistry chapter 1 class 11, is a fundamental chapter that will help students get a brief comprehension of the subject matter and clear their examinations of all sorts.
The Chemistry Class 11 Chapter 1 Important Questions are curated by Vedantu experts with thorough research and keeping in mind the highest probability of its chance in the examinations.
Chapter 1 Chemistry Class 11 Important Questions help students comprehend all the topics and concepts in a simple, easy, and understandable manner.
The crucial questions help students manage your time efficiently, boost their confidence during examinations, plan your preparation accordingly, and continuous practice will make your self-assessment better.
Some Basic Concepts of Chemistry Important Questions is a valuable and credible source of study material written in well structured and easily understandable language for students. The Important Questions of Some Basic Concepts of Chemistry Class 11 will let students know the important topics, develop a comprehensive understanding, create a well-structured plan and study schedule while preparing for Class 11 Chemistry Chapter 1 . Regular practice of essential questions will help students achieve a high score in their exam and boost their overall attributes.
Important Related Links for CBSE Class 11 Chemistry
CBSE Class 11 Study Materials |
|
|
|
|
|
|

FAQs on Important Questions for CBSE Class 11 Chemistry Chapter 1 - Some Basic Concepts of Chemistry
1. How can I download the important questions of Chapter 1 of Class 11 Chemistry?
To download the important questions of Chapter 1 of Class 11 Chemistry follow the given method:
Visit the official website of Vedantu(vedantu.com), hit on the link- Important Questions for Class 11 Chemistry.
On clicking this link, you will find the page of Class 11 Chemistry.
Now, choose the chapter of your choice.
At the peak of this PDF file, students will be able to see the "Download PDF" option.
Click this option.
You will be able to study offline after the file gets downloaded free of cost.
Students can also download the PDF of Important Questions from the Vedantu app.
2. What are the differences between mixtures and compounds?
The differences between mixtures and compounds are as follows:
Two or more elements are chemically combined to form compounds.
The ratio of elements is fixed in the compounds.
The composition of compounds is the same throughout which means they are homogeneous.
Two or more compounds are simply mixed and not chemically combined to form mixtures.
The constituents of mixtures are not available in a fixed ratio.
The nature of the mixtures is either homogeneous or heterogeneous.
3. What do you understand about atomic mass and average atomic mass?
(a) Atomic Mass - It is defined as the number of times an atom is heavier than the atom of carbon taken as 12. It can be seen that the obtained atomic masses are the relative atomic masses and not the actual masses of the atoms. This unified mass is equal to 1/12th of the mass of an atom of the carbon-12 isotope.
(b) Average Atomic Mass - The average relative mass of an atom of an element as compared to the mass of carbon atoms.
4. What is the importance of Chemistry in the following fields:
In food and agriculture
In Sanitation and health
(a) In Agriculture and Food -
Chemicals such as urea, sodium nitrate, ammonium phosphate, etc are used in making fertilizers.
Pesticides and insecticides are used to protect crops from harmful insects.
Preservatives are made from chemicals that are used to preserve food items for longer periods.
(b) In Health and Sanitation -
Many life-saving drugs like penicillin and sulpha drugs have been discovered. For AIDS victims, AZT is used.
Phenols are used to kill harmful microorganisms.
Chlorine is used for the sterilization of water.
5. How to get good results in Chapter 1 of Class 11 Chemistry?
Class 11 is that phase of a student's life in which they build their basics. Therefore, they have to concentrate on their studies to make their concepts strong and to attain good marks. Chapter 1 is a little bit difficult so students have to study hard to get decent marks. For this, students must focus on the NCERT book and the questions given in this book. Also, important questions of this chapter will enable students to comprehend the concepts. By solving sample papers, students can clear their doubts.
CBSE Class 11 Chemsitry Important Questions
Cbse study materials.

NCERT Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry
NCERT Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry Solutions to each chapter is provided in the list so that you can easily browse through different chapters NCERT Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry and select need one. NCERT Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry Question Answers Download PDF . NCERT Chemistry Class 11 Solutions .
Also, you can read the NCERT book online in these sections Solutions by Expert Teachers as per Central Board of Secondary Education ( CBSE ) Book guidelines. NCERT Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry Solutions are part of All Subject Solutions . Here we have given NCERT Class 11 Chemistry Part: I, Part: II Notes . NCERT Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry Notes, NCERT Class 11 Chemistry Textbook Solutions for All Chapters, You can practice these here.
Some Basic Concepts of Chemistry
1. Calculate the molar mass of the following:
Ans: Molecular mass of H 2 O
= 2(1.008 amu) + 16.00 amu
= 2.016 amu + 16.00 amu
= 16.016 amu.
Ans: Molecular mass of CO 2
= 12.01 amu + 2 × 16.00 amu
= 12.01 amu + 32 amu
= 44.01 amu.
(iii) CH 4
Ans: Molecular mass of CH 4
= 12.1 amu + 4 × (1.008 amu)
= 12.1 amu + 4.032 amu
= 16.132 amu.
2. Calculate the mass per cent of different elements present in sodium sulphate (Na2SO4).
Ans: Mass % of an element
= Mass of that element in the compound / Molar mass of compound × 100
Now, molar mass of Na 2 SO = 23 × 2 + 32 + 4 × 16
= 46 +32 + 64 =142
Na = 23 × 2 / 142 × 100
= 46 / 142 × 100
= 0.323 100
S = 32/142 × 100
= 0.225 × 100
O = 64/142 × 100
= 0.4507 × 100
3. Determine the empirical formula of an oxide of iron, which has 69.9% iron and 30.1% dioxygen by mass.
| Iron | Fe | 69.9 | 55.85 | 69.9/55.85 = 1.25 | 1.25/1.25 = 1 | 2 |
| Oxygen | O | 30.1 | 16.00 | 30.1 /16.00 =1.88 | 1.88/ 1.25 = 1.5 | 3 |
∴ Empirical formula – Fe 2 O 3 .
4. Calculate the amount of carbon dioxide that could be produced when:
(i) 1 mole of carbon is burnt in air.
(ii) 1 mole of carbon is burnt in 16 g of dioxygen.
(iii) 2 moles of carbon are burnt in 16 g of dioxygen.
Ans: The balanced equation for the combustion of carbon in dioxygen/air is
(i) C + O 2 = CO 2
1 mole of carbon burnt in air will produce 44 grams of CO₂.
(ii) 1 mole of carbon is burnt in 16g of dioxygen.
16g of dioxygen corresponds to 16/32 = 0.5 moles.
i.e., dioxygen is the limiting reactant.
Hence, CO 2 produced= 0.5 × 44 = 22 g.
(iii) 2 mole of carbon are burnt 16g of dioxygen.
16g of of dioxygen corresponds to 16/32 = 0.5 mole.
Here,dioxygen is the limiting reagent.
Hence, CO 2 corresponds = 0.5 × 44 = 22 g.
5. Calculate the mass of sodium acetate (CH 3 COONa) required to make 500 mL of 0.375 molar aqueous solution. Molar mass of sodium acetate is 82.0245 g mol– 1 .
Ans: Molarity (M) = 0.375 M
Volume (V) = 500 mL = 0.500 L
Molar mass of sodium acetate (CH 3 COONa) = 82.0245 g/mol
Sodium acetate = 0.375/2 mole.
Moral mass of sodium acetate required = 0.375/2
Mole × 82.0245 g mol -1 = 15.380g.
6. Calculate the concentration of nitric acid in moles per litre in a sample which has a density, 1.41 g mL–1 and the mass per cent of nitric acid in it being 69%.
Ans: Mass percent of HNO 3 = 69%
Molar mass of nitric acid (HNO 3 )
= 1 + 14 + 48= 63 g mol -1
Moles of HNO3,n = 69/ HNO 3
= 69/63 = 1.095
7. How much copper can be obtained from 100 g of copper sulphate (CuSO 4 )?
Ans: 1 mole of CuSO 4 contains 1 mole (1 g atom) of Cu
Copper (Cu): 63.55 g/mol
Sulphur (S): 32.07 g/mol
Oxygen (O): 16.00 g/mol × 4 = 64.00 g/mol
Molar mass of CuSO 4
= 63.5 + 32 + 4 × 16
= 159.5 g mol-1
Thus, Cu that can be obtained from 159.5 g of CuSO 4 = 63.5g
Therefore Cu that can be obtained from 100g of CuSO 4
= 63.5/ 159.5 × 100g
8. Determine the molecular formula of an oxide of iron, in which the mass per cent of iron and oxygen are 69.9 and 30.1, respectively.
Ans: Calculation of empirical Formula:
| Fe | 69.9 | 55.8 | 69.9/55.8 = 1.25 | 1 | 2 |
| o | 30.1 | 16.00 | 30.1/16.00 = 1.88 | 1.5 | 3 |
So, empirical formula of iron oxide is Fe 2 O 3.
Empirical formula mass of Fe2O3 = 2 x 55.85 + 3 × 16.00
= 111.7 + 48.00
= 159.7g mol-1
N = Molar mass/empirical formula mass
= 159.8 / 159.7 = 1
Hence, Thus, the molecular formula of the oxide of iron is Fe 2 O 3 .
9. Calculate the atomic mass (average) of chlorine using the following data:
| Cl | 75.77 | 34.9689 |
| Cl | 24.23 | 36.9659 |
Ans: Fractional abundance of 35 Cl = 75.77% = 0.7577
Molar mass of 35 Cl = 34.9689g/ mol
Fractional abundance of 37 Cl = 24.23% = 0.2423
Molar mass of 37 Cl = 36.9659
∴ Average atomic mass of chlorine
= (0.7577) (34.9689) + (0.2423) (36.9659)
= 0.7577 × 34.9689 = 26.4959
= 0.2423 × 36.9659 = 8.9566
= 26.4959 + 8.9566 = 35.4527.
10. In three moles of ethane (C 2 H 6 ), calculate the following:
(i) Number of moles of carbon atoms.
Ans: 1 mole of C 2 H 6 contains 2 moles of carbon atoms.
Number of moles of carbon atoms = 3 × 2 = 6 mol
(ii) Number of moles of hydrogen atoms.
Ans: 1 mole of C 2 H 6 contains 6 moles of hydrogen atoms.
Number of moles of hydrogen atoms = 3 × 6 = 18 mol
(iii) Number of molecules of ethane.
Ans: 1 mole of C 2 H 6 contains Avogadro,s no. i.e. 6.02 × 10 23 molecules.
Number of molecules of ethane = 3 × 6.023 × 10 23
= 18.069 × 10 23 molecules.
11. What is the concentration of sugar (C 12 H 22 O 11 ) in mol L–1 if its 20 g are dissolved in enough water to make a final volume up to 2L?
Ans: Molar mass of sugar (C 12 H 22 O 11 )
= (12 × 12) + (22 × 1) + (11 ×16.00)
= 144 + 22+ 176
No. of moles in 20g of sugar = Mass/molars
= 20/342 g mol -1
= 0.0585 mol
Volume of solution = mole of solute/ volume of sol in L
= 0.0585 mol/ 2l
= 0.0293 mol L -1.
12. If the density of methanol is 0.793 kg L–1, what is its volume needed for making 2.5 L of its 0.25 M solution?
Ans: Molar mass of methanol = Molar × Molar mass of methanol
= 0.625 × 32.042 g /mol
Calculation of mass methanol to volume using its density:
Volume = mass/ density = 20.00g / 0.793 kg × 1000 g/kg
= 0.025 × 1000 g/kg
= 25.22 ml.
13. Pressure is determined as force per unit area of the surface. The SI unit of pressure, pascal is as shown below: 1Pa = 1N m–2 If mass of air at sea level is 1034 g cm–2, calculate the pressure in pascal.
Ans: Force (F) = Mass (m) × Gravity (g)
Assuming = 9.81 m/s2
Mass per unit are, m/a = 1034 gm
Convert mass from g to kg: 1034g = 1.034 kg
Force = 1.034 × 9.81 m/ s 2 = 10.13734 N/m 2
Convert the mass of air from g/cm² to kg/m
1034g/cm2 = 1034g/ cm 2 × 1kg / 1000 g × (100 cm/m) 2
= 1034g × 0.001 2 1
Pressure is the force (i.e, weight) acting per unit area
Weight = mg
Pressure = weight per unit area
= 1034g × 9.81 m/ s / cm 2 × 1kg /1000g × 100cm /1m × 1N / kg ms-2 × 1Pa / 1N m-2
= 1.01332 × 10 5 pa.
14. What is the SI unit of mass? How is it defined?
Ans: Kilogram is used as SI unit of mass. It is represented by the symbol kg. It is defined as the mass of platinum-iridium block stored at international bureau of weight and measures in france. The system of units, including unit definitions, keeps on changing with time. Whenever the accuracy of measurement of a particular unit was enhanced substantially by adopting new principles, member nations of metre treaty (signed in 1875), agreed to change the formal definition of that unit.
15. Match the following prefixes with their multiples:
| (i) | micro | 10 |
| (ii) | deca | 10 |
| (iii) | mega | 10 |
| (iv) | giga | 10 |
| (v) | femto | 10 |
Ans:
| (i) | micro | 10 |
| (ii) | deca | 10 |
| (iii) | mega | 10 |
| (iv) | giga | 10 |
| (v) | femto | 10 |
16. What do you mean by significant figures?
Ans: The uncertainty in the experimental or the calculated values is indicated by mentioning the number of significant figures. Significant figures are meaningful digits which are known with certainty plus one which is estimated or uncertain. The uncertainty is indicated by writing the certain digits and the last uncertain digit.
17. A sample of drinking water was found to be severely contaminated with chloroform, CHCl3, supposed to be carcinogenic in nature. The level of contamination was 15 ppm (by mass).
(i) Express this in per cent by mass.
Ans: 15 ppm means 15 parts in million (10 6 ) parts
Percent by mass = Mass of chloroform/ mass of solution × 100
= 15/ 10 6 × 100
= 1.5 × 10 -3 %
(ii) Determine the molality of chloroform in the water sample.
Ans: Molar mass of chloroform (CHCL 3 )
= Mass of chloroform/ Molar mass of chloroform × (mass of solution – mass of chloroform) × 100
= 12 + 1 3 × 35.5
= 118.5 g mol-1
100g of the sample contain chloroform
= 1.5 × 10-3g
1000g 91 kg of the sample will contain chloroform Molality = 15 × 10-2 / 118.65 mole
= 1.266 × 10 -4 mole
18. Express the following in the scientific notation:
Ans: 0.0048 × 10 -3
(ii) 234,000
Ans: 234,000 × 10 5
(iii) 8008
Ans: 8008 × 10 3
(iv) 500.0
Ans: 500.0 × 10 2
(v) 6.0012
Ans: 6.0012 × 10 0
19. How many significant figures are present in the following?
Ans: Significant figure: 2
(ii) 208
Ans: Significant figure: 3
(iii) 5005
Ans: Significant figure: 4
(iv) 126,000
(v) 500.0
(vi) 2.0034
Ans: Significant figure:5
20. Round up the following upto three significant figures:
Ans: Three significant figures: 34.2
(ii) 10.4107
Ans: Three significant figures: 10.4
(iii) 0.04597
Ans: Three significant figures: 0.045
(iv) 2808
Ans: Three significant figures: 280
21. The following data are obtained when dinitrogen and dioxygen react together to form different compounds:
| (i) | 14g | 16g |
| (ii) | 14g | 32g |
| (iii) | 28g | 32g |
| (iv) | 28g | 80g |
(a) Which law of chemical combination is obeyed by the above experimental data? Give its statement.
Ans: When the mass of dinitrogen is 28g, mass of dioxygen combined is 32, 64, 32, and 80g. The corresponding ratio is 1:2:1:5. It is a simple whole number ratio. Hence the given data obey the low of multiple proportions.
Low of multiple proportions: When two elements combine to form two or more chemical compounds, then masses of one of the elements which combine with a fixed mass of the other, bear a simple ratio to one another.
(b) Fill in the blanks in the following conversions:
(i) 1 km = ____________ mm = ________ pm.
Ans: 1 km = 1 km × 1000m/1km × 100m/1m × 10mm /1cm
= 1000 × 100 × 10
= 100000mm
= 10 6 mm
1 km = 1 km × 1000m/1km × 1pm/ 10 -12 m
= 10 15 pm
(ii) 1 mg = ___________ kg = _____________ ng.
Ans: 1 mg = 1mg × 1g/1000g × 1mg/1000g
1 mg = 1 mg × 1 /1000 mg × 1ng 10 -9 g
mL = 1 cm 3
(iii) 1 mL = ____________ L = _____________ dm3
Ans: 1 ml = 1 ml × 1g/1000 mg
= 1 cm 3 × 1 dm × 1 dm × 1 dm / 10 cm × 10 cm × 10 cm
= 10 -3 dm 3 .
22. If the speed of light is 3.0 × 10 8 m s–1, calculate the distance covered by light in 2.00 ns.
Ans: Convert time from nanoseconds to seconds:
2.00 ns = 2.00 × 10 −9 s
Distance = Speed × Time
= 3.0 × 10 8 m s–1 × 2.00 ns
= 3.0 × 10 8 m s–1 × 2.00 ns × 10 −9 s/ 1ns
= 6.00 × 10 -1 m s -1 = 0.600 m.
23. In a reaction
A + B2 AB2
Identify the limiting reagent, if any, in the following reaction mixtures.
(i) 300 atoms of A + 200 molecules of B.
Ans: According to the given reaction, 1 atom of A reacts with 1 atom of B.
∴ 200 molecules of B will react with 200 atoms of A 100 atoms of A will remain unreacted Hence, B 2 is the limiting reagent.
(ii) 2 mol A + 3 mol B.
Ans: According to the given reaction, 1 mol of A reacts with 1 mol of B.
∴ 2 mol of A will react with only 2 mol of B. As a result, 1 mol of B will not be consumed. Hence, Ais the limiting reagent.
(iii) 100 atoms of A + 100 molecules of B.
Ans: There is no limiting reagent.
(iv) 5 mol A + 2.5 mol B.
Ans: 2.5 mol of B will combine with only 2.5 mol of A. As a result, 2.5 mol of A will be left as such. Hence, B is the limiting reagent.
(v) 2.5 mol A + 5 mol B.
Ans: 2.5 mol of A will combine with only 2.5 mol of B and the remaining 2.5 mol of B will be left as such. Hence, A is the limiting reagent.
24. Dinitrogen and dihydrogen react with each other to produce ammonia according to the following chemical equation: N 2 (g) + H 2 (g) 2NH 3 (g)
(i) Calculate the mass of ammonia produced if 2.00 × 103 g dinitrogen reacts with 1.00 × 103 g of hydrogen.
Ans: Mass of ammonia produced
28g of N 2 produces 34g of NH 3
1g of N 2 will produce = 34/28 g NH 3
2000 g of N 2 will produce NH 3 = 34/28 × 2000
= 2428.57 g of NH 3 .
(ii) Will any of the two reactants remain unreacted?
Ans: Yes,H 2 will remain unreacted.
(iii) If yes, which one and what would be its mass?
Ans: Mass left unreacted
= 1000 g – 428.6 g
= 571.4 g.
25. How are 0.50 mol Na 2 CO 3 and 0.50 M Na 2 CO 3 different?
Ans: 0.50 mol HCl means there is a total of 0.50 moles of HCl present, while 0.50 M HCl (M stands for molarity) indicates that there is a concentration of 0.50 moles of HCl per litre of solution.
Molar mass of Na 2 CO 3
= 2 × 23 + 3 × 16
= 106 g mol -1
0.5 M Na 2 CO 3 means 0.05 × 106 g
26. If 10 volumes of dihydrogen gas reacts with five volumes of dioxygen gas, how many volumes of water vapour would be produced?
Ans: H 2 and O 2 react according to the equation:
2H 2 (g) + O 2 (g) = 2H 2 O(g)
The volume of a gas is directly proportional to the number of moles.
Thus, 2 volumes of dihydrogen react with 1 volume of oxygen to form 2 volumes of water.
Therefore, 10 volumes of H₂ will produce 10 volumes of H₂O (water vapour). So, the reaction will produce 10 volumes of water vapour.
27. Convert the following into basic units:
(i) 28.7 pm.
Ans: 1 picometer (pm) = 10 −12 metres
28.7pm=28.7 × 10 −12 m / 1pm
=2.87 × 10 −11 m
(ii) 15.15 pm
15.15 us = 15.15 × 10 -12
= 1.515 × 10 -11 m
(iii) 25365 m
Ans: 25365 mg = 2.5365 × 1g /1000 mg × 1kg 1000g
2.5365 × 10-2 kg. Since.
28. Which one of the following will have the largest number of atoms?
(i) 1 g Au (s)
Ans: 1 g Au (s) =1/197 mol
= 1/197 × 6.02 × 10 23 atoms
= 6.02 × 10 23 / 197 atoms.
(ii) 1 g Na (s)
Ans: 1 g Na (s) = 1/23 mol
= 1/ 23 × 6.02 × 10 23 atoms
= 6.02 × 10 23 / 23 atoms.
(iii) 1 g Li (s)
Ans: 1 g Li (s) = 1/7 mol
= 1/ 7 × 6.02 × 10 23 atoms
= 6.02 × 10 23 / 7 atoms.
(iv) 1 g of Cl2(g)
Ans: 1 g of Cl2(g) = 1/71 mol
= 1/71 × 6.02 × 10 23 atoms
= 6.02 × 10 23 / 71 atoms.
29. Calculate the molarity of a solution of ethanol in water, in which the mole fraction of ethanol is 0.040 (assume the density of water to be one).
Ans: Mole fraction of component in solution = moles of a particular component /Total number of moles of solution.
Xc 2 H 5 OH = n(C 2 H 5 OH) / n(C 2 H 5 OH) + n(H 2 O)
0.0040 = n(C 2 H 5 OH) / n(C 2 H 5 OH) + n(H 2 O)
No of moles in 1 L of water
= 1000 g / 18g mol-1
= 55.55 moles
Let n be the number of moles of ethanol. The mole fraction of ethanol is 0.040.
n(C 2 H 5 OH) / n(C 2 H 5 OH) + 55.55 = 0.040
Cross-multiply to solve for n:
n = 0.040 (n + 55.55)
n = 0.040n + 2.222
n = 2.222 / 1- 0.040n
n = 2.31 moles.
30. What will be the mass of one 12 C atom in g?
Ans: The molar mass 12 C of is 12 g/mol.
= 12 grams/ 6.022 × 10 23 atoms
= 1.993 × 10 -23 grams.
31. How many significant figures should be present in the answer of the following calculations?
(i) 0.02856 × 298.15 × 0.112 0.5785 / 0.5785
Ans: The answer of the calculation 0.02856 × 298.15 × 0.112 / 0.5785 the number with the least number of significant figures is 0.1120, which has 3 significant figures.
The least precise number of answer = 0.112
(ii) 5 × 5.364
Ans: Leaving the exact number (5), the second term has 4 significant figures. Hence, the answer should have 4 significant figures.
(iii) 0.0125 + 0.7864 + 0.0215
Ans: In the given addition, the least number of decimal places in the term is 4. Hence, the answer should have 4 significant.
32. Use the data given in the following table to calculate the molar mass of naturally occurring argon isotopes:
| 36Ar | 35.96755 g mol | 0.337% |
| 38Ar | 37.96272 g mol | 0.063% |
| 40Ar | 39.9624 g mol | 99.600% |
Ans: Molar mass of Ar
= ΣfiAi = 0.00337 × 35.96755 + 0.00063 × 37.96272 + 0.996 × 39.9624
= 0.1212 + 0.002393 + 39.8025
= 39.926 g mol -1
∴ Molar mass of naturally occurring argon isotopes is 39.926 g mol -1
33. Calculate the number of atoms in each of the following:
(i) 52 moles of Ar.
Ans: 1 mole of Ar = 6.022 × 10 23 atoms of Ar
= 52 mol of he = 6.022 × 10 23 atoms
= 3.131 × 10 25
(ii) 52 u of He.
Ans: Atomic mass of He = 4 amu
∴ 52 u of he = 1/4 × 52 atoms
= 13 atoms.
(iii) 52 g of He.
Ans: Gram atomic mass of He = 4 g
Or 4 g of He contains = 6.022 × 10 23 atoms
∴ 52 g of he = 16.022* 10 23 /4 × 52 atoms
= 7.8286 × 10 24
34. A welding fuel gas contains carbon and hydrogen only. Burning a small sample of it in oxygen gives 3.38 g carbon dioxide, 0.690 g of water and no other products. A volume of 10.0 L (measured at STP) of this welding gas is found to weigh 11.6 g. Calculate:
(i) Empirical formula.
(ii) Molar mass of the gas. and
(iii) Molecular formula.
Ans: Moles of Carbon: Given 92.2 g of carbon, moles of carbon = 92.212 = 7.7 mol.
Moles of Hydrogen: Given 7.7 g of hydrogen, moles of hydrogen = 7.71 = 7.7 mol
Mole Ratio: The ratio of C is 7.77.7=1:1.
Calculation Of Empirical Formula:
| 92.32 | 12 | 92.32/12=7.69 | 1 | 1 | |
| 7.68 | 1 | 7.68/1=7.68 | 1 | 1 |
Empirical formula = CH
10.0L of the gas at STP weigh = 11.6
∴ 22.4 L of the gas at S.T.P. will weight
= 11.6/ 10.0 × 22.4
∴ Molar mass = 26 g mol -1
Empirical formula mass of CH
∴ n = Molecular mass / Empirical formula mass = 26/13
The molecular formula = 2 × CH = C 2 H 2
35. Calcium carbonate reacts with aqueous HCl to give CaCl2 and CO2 according to the reaction, CaCO 3 (s) + 2 HCl (aq) → CaCl 2 (aq) + CO 2 (g) + H2O(l) What mass of CaCO 3 is required to react completely with 25 mL of 0.75 M HCl?
Ans: Calculation of mass of HCl in 25 ml of 0.75 M HCl
1000 mL of 0.75 mol
= 0.75 × 36.5 g
= 24.375 g
∴ 25 mL of 0.75 HCl will contain HCl
= 24.375 g / 1000× 25g
= 0.6844 g
CaCO 3 + 2HCl = CaCl 2 + H 2 O + CO 2
According to the above equation, 100g of CaCO 3 is required to react completely with CaCO 3 . That is
73g of HCl = 100g of CaCO 3
1g of HCl = (100 / 73) g
= (100 / 73) × 0.684 g
= 1.369 × 0.684
36. Chlorine is prepared in the laboratory by treating manganese dioxide (MnO 2 ) with aqueous hydrochloric acid according to the reaction 4 HCl (aq) + MnO 2 (s) → 2H2O (l) + MnCl 2 (aq) + Cl 2 (g) How many grams of HCl react with 5.0 g of manganese dioxide?
Ans: 1 mole of MnO 2 i.e
Molar mass of MnO 2 = 55 + 2 × 16
= 55 + 32 = 87g
Molar mass of HCl = 1.01 (H) + 35.45 (Cl) = 36.5 g/mol
React with 4 mole of HCl, i.e., 4 × 36.5
= 146 of HCI.
Therefore, 5.0g of MnO 2 will react with HCl
= 146/87 × 5.0
= 8.40 g.
Related Posts

NCERT Class 11 Chemistry Chapter 2…
Read More »
NCERT Class 11 Chemistry Chapter 3…
NCERT Class 11 Chemistry Chapter 4…
NCERT Class 11 Chemistry Chapter 5…
NCERT Class 11 Chemistry Chapter 6…
NCERT Class 11 Chemistry Chapter 7…
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *
NCERT Solutions for Class 11 Chemistry Free PDF Download
Ncert solutions for class 11 chemistry.
NCERT Solutions for Class 11 Chemistry cover all the important concepts in detail. The NCERT Solution for Class 11 Chemistry presents the answers in the simplest way possible, to ensure the understanding of the subject which are prepared by our experts.
We have compiled NCERT Solutions for Class 11 Chemistry chapter-wise and exercise-wise so that students can easily find the answers to the questions. We have ensured that students get a good knowledge of the subject and score better marks in the exams. Our NCERT Solutions for Class 11 Chemistry are free to download.
Download NCERT Solutions for the other subjects here .
Download NCERT Solutions for Class 11 of all subjects here .
Toppr provides the last 10 years of question papers, free study materials, 1000+ hours of video lectures, mock tests, and live doubts solving. Download Toppr app for Android and iOS or signup for free.
NCERT Solutions for Class 11 Chemistry Chapterwise
Chapter 1 – Some Basic Concepts of Chemistry
Chapter 2 – Structure Of The Atom
Chapter 3 – Classification of Elements and Periodicity in Properties
Chapter 4 – Chemical Bonding and Molecular Structure
Chapter 5 – States of Matter
Chapter 6 – Thermodynamics
Chapter 7 – Equilibrium
Chapter 8 – Redox Reactions
Chapter 9 – Hydrogen
Chapter 10 – The s-Block Elements
Chapter 11 – The p-Block Elements
Chapter 12 – Organic Chemistry: Some Basic Principles and Techniques
Chapter 13 – Hydrocarbons
Chapter 14 – Environmental Chemistry

Chapterwise NCERT Solutions for Class 11 Chemistry
Ncert solutions for class 11 chemistry chapter 1 – some basic concepts of chemistry.
In this chapter, laws of chemical combination, Dalton’s atomic theory, mole concept, empirical and molecular formula, stoichiometry and its calculations are discussed. Download NCERT Solutions for basic concepts of chemistry here .
NCERT Solutions for Class 11 Chemistry Chapter 2 – Structure Of the Atom
In this chapter you are going to study about Thomson’s model, Rutherford’s model, Bohr’s model, and their limitations, de Broglie’s relationship, Heisenberg uncertainty principle, orbitals and their shapes, Aufbau principle, Pauli’s exclusion principle, and Hund’s rule, electronic configuration of atoms and their stability in orbitals. Download NCERT Solutions for the structure of an atom here .
NCERT Solutions for Class 11 Chemistry Chapter 3 – Classification of Elements and Periodicity in Properties
This chapter tells you about the significance of classification and history of the periodic table, modern periodic law, periodic trends in properties of elements, ionization and electron gain enthalpy, electronegativity and the nomenclature of the elements with atomic number greater than 100. Download NCERT Solutions for this chapter here .
NCERT Solutions for Class 11 Chemistry Chapter 4 – Chemical Bonding and Molecular Structure
This chapter discusses the valence electrons, different types of bond, Lewis structure, polar character, valence bond theory, the theory of VSEPR, concept of hybridization, molecular orbital and theory of the homonuclear diatomic molecules and hydrogen bond. Download NCERT Solutions for Chemical bonding and molecular structure here .
NCERT Solutions for Class 11 Chemistry Chapter 5 – States of Matter
In this chapter, you will understand about the states of matter, intermolecular interactions, types of bonding, Boyle’s law, Charles law, Gay Lussac’s law, Avogadro’s law, Avogadro’s number, ideal gas equation and empirical derivation of gas equation, and its deviation. Download NCERT solutions for states of the matter here .
NCERT Solutions for Class 11 Chemistry Chapter 6 – Thermodynamics
In this chapter, you study about the Laws of thermodynamics (I, II, III), Hess’s law, enthalpy of bond dissociation, atomization, phase transition, entropy as a state function, Gibb’s energy change. Download NCERT Solutions for Thermodynamics here .
NCERT Solutions for Class 11 Chemistry Chapter 7 – Equilibrium
This chapter deals with the concept of equilibrium, dynamic nature and constant of equilibrium, factors affecting equilibrium, the Le Chatelier’s principle, also study about the concept of ionic equilibrium, electrolytes, ionization and pH, Henderson Equation, hydrolysis, common ion effect, etc. Download NCERT Solutions for Equilibrium here .
NCERT Solutions for Class 11 Chemistry Chapter 8 – Redox Reactions
In this chapter, you will learn about the concept of oxidation and reduction, redox reactions and balancing them, its applications. Download NCERT Solutions for Redox Reactions here .
NCERT Solutions for Class 11 Chemistry Chapter 9 – Hydrogen
This chapter tells you about the position of hydrogen in the periodic table, its occurrence, isotopes, properties, uses of hydrogen, hydrides, properties of water, heavy water, hydrogen peroxide, and hydrogen as a fuel. Download NCERT Solutions for Hydrogen here .
NCERT Solutions for Class 11 Chemistry Chapter 10 – The s -Block Elements
In this chapter, you are going to study the electronic configuration of the element in s block, its occurrence, and the diagonal relationship of elements, trends in the variation of properties, chemical reactivity, and their uses. Download NCERT Solutions for the s-Block Elements here .
NCERT Solutions for Class 11 Chemistry Chapter 11 – The p -Block Elements
In this chapter, you are going to study the electronic configuration of the element in p block, its occurrence, the variation of the properties, oxidation states, trends in the chemical reactivity, and anomalous properties of the first element of each group. Download NCERT Solutions for The p-Block Elements here .
NCERT Solutions for Class 11 Chemistry Chapter 12 – Organic Chemistry – Some Basic Principles and Technique
The chapter talks about methods of purification, qualitative and quantitative analysis, classification and IUPAC nomenclature of organic compounds, the electronic displacements in a covalent bond, the inductive effect, electromeric effect, homolytic and heterolytic fission, the concept of free radicals, electrophiles, and nucleophiles, types of organic reactions. Download NCERT Solutions for Organic Chemistry here .

NCERT Solutions for Class 11 Chemistry Chapter 13 – Hydrocarbons
In this chapter, you will get to know about the classification of Hydrocarbons and Aliphatic Hydrocarbons and their nomenclature, IUPAC names, physical properties, chemical reactions including pyrolysis, etc. Download NCERT Solutions for Hydrocarbons here .
NCERT Solutions for Class 11 Chemistry Chapter 14 – Environmental Chemistry
In this chapter, you will study environmental pollution – air, water and soil pollution, smog, ozone and the effects of depletion of the ozone layer, greenhouse effect, and global warming. Also study about various pollutants and their means, strategies for controlling pollution. Download NCERT Solutions for Environmental Chemistry here .
Why choose Toppr?
With Toppr App, you get free classes with conceptual videos. You can access the free pdf downloads of solutions along with free online tests. Download the Toppr App for Android and iOS or sign up for free.
Solved Questions For You:
Question 1. Calculate the mass and charge of one mole of electrons.
Mass of one mole of electrons is 9 . 1 1 × 1 0 −31 × 6 . 0 2 3 × 1 0 23 = 5 . 4 8 6 × 1 0 −7 kg.
Question 2. What do you mean by significant figures?
In a given number, all the certain digits plus one doubtful digit correspond to significant figures. They depend on the precision of the scale or the instrument used for the measurement.
Question 3. What is the SI unit of mass? How is it defined?
The kilogram is used as an SI unit of mass. It is represented by the symbol kg.
Question 4. What is the lowest value of n that allows g orbitals to exist?
| n | |
| 1 | s |
| 2 | s,p |
| 3 | s,p,d |
| 4 | s,p,d,f |
| 5 | s,p,d,f,g |
The lowest value of n that allows g orbitals to exist is 5.
Customize your course in 30 seconds
Which class are you in.

NCERT Solutions for Class 11
- NCERT Solutions for Class 11 Chemistry Chapter 7 Free PDF Download
- NCERT Solutions for Class 11 Physics Chapter 6
- NCERT Solutions for Class 11 Biology Chapter 6 Free PDF Download
- NCERT Solutions for Class 11 Biology Chapter 2 Free PDF Download
- NCERT Solutions for Class 11 Biology Chapter 18 Free PDF Download
- NCERT Solutions for Class 11 Biology Chapter 10 Free PDF Download
- NCERT Solutions for Class 11 Biology Chapter 22 Free PDF Download
- NCERT Solutions for Class 11 Biology Chapter 16 Free PDF Download
- NCERT Solutions for Class 11 Biology Chapter 20 Free PDF Download
- NCERT Solutions for Class 11 Biology Chapter 19 Free PDF Download
One response to “NCERT Solutions for Class 11 Biology Chapter 19 Free PDF Download”
Iam student pls help me
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Download the App

NCERT Solutions for Class 11 Chemistry
NCERT Solutions for Class 11 Chemistry in PDF format English Medium MCQ, Extra Questions for CBSE and State Board. As per the new textbook published for academic year 2024-25, there are only 9 chapters in class 11 chemistry Syllabus. Chapter wise NCERT Solutions for Class 11 Chemistry Chapter 1. Some Basic Concepts of Chemistry Chapter 2. Structure of Atom Chapter 3. Classification of Elements and Periodicity in Properties Chapter 4. Chemical Bonding and Molecular Structure Chapter 5. Thermodynamics Chapter 6. Equilibrium Chapter 7. Redox Reactions Chapter 8. Organic Chemistry – Some Basic Principles and Techniques Chapter 9. Hydrocarbons
| Class: 11 | Chemistry |
| Content: | NCERT Solutions and Extra Questions |
| Mode of Content: | Text, Images, PDF and Videos |
| Academic Year: | Session 2024-25 |
| Medium: | Hindi and English Medium |
NCERT books Solutions, notes and assignments according to current CBSE syllabus, are also available to download along with the answers given at the end of the book. If you are having any suggestion for the improvement, your are welcome. The improvement of the website and its contents are based on your suggestion and feedback.
The branch of science under which the structure of matter, its properties, uses and interaction with other types of a matter and various forms of energy are studied is called chemistry.
1. Since times immortal. The man come on earth his curious nature urge him. Due to this he made new discoveries. In Indus Valley cultures making of metals techniques were found. They were make copper and bronze articles. In Egyptian culture the methods to preserve dead bodies as ‘mummies. Chemicals used for it remain mystery. Even today we do not know about the chemicals. Egyptians had the knowledge of making soap, dyes, glass, etc. Pyramids of Egypt are the examples of their progress. Chemist’ of Egypt discovered touch stone, universal solvent. They did not succeed in their attempts. They learnt about process like distillation and sublimation. These are help us even today.
2. For treatment, different systems of medicine were found. Allopathy in Europe, Unani in Greece and Chinese in China. In India, many expert in Ayurveda contributed notably. Sage Kanad termed the smallest part of as particle matter/elements. On this basis, John Dalton gave his Atomic Theory in 1808 AD. For example:. This body made from Earth, Water, Air, Fire and sky. The concept of five elements is the Research of Indian scientist only.
3. Ancient Indians had the knowledge of metallurgy, fermentation. They can prepare bases, extracts and distillates. The polish done on the ‘Iron Pillar’ of Mehrauli in Delhi. This is a subject of research. There is no rusting has occurred on it till date.
Provide us feedback to improve the quality of the content. Class 11 Chemistry NCERT Solutions are in the form of PDF and Videos. Download NCERT Books and Offline Apps 2024-25 based on new CBSE Syllabus. Ask your doubts related to NIOS or UP Board, MP Board or other Boards and share your knowledge with your friends and other users through Discussion Forum.
Copyright 2024 by Tiwari Academy | A step towards Free Education

- NCERT Solutions
- NCERT Class 11
- NCERT 11 Chemistry
NCERT Solutions for Class 11 Chemistry
Ncert solutions for class 11 chemistry download chapter-wise pdf for 2023-24.
NCERT Solutions for Class 11 Chemistry is a study material which is developed by the faculty at BYJU’S by keeping in mind the grasping power of Class 11 students. NCERT Solutions for Class 11 are drafted in a simple and understandable manner to help students ace the exam without fear. Chemistry is a subject which contains a lot of chemical reactions and symbols. It might be difficult for the students to remember all these concepts effectively if not revised on a regular basis. So, making use of proper study material is very important.
At BYJU’S, students can access chapter-wise NCERT Solutions for Class 11 to get their doubts clarified instantly. The faculty has provided both online and offline modes of solutions which can be used free of cost. The chapter-wise links of NCERT Solutions for Class 11 Chemistry are as follows:
Chapter-Wise NCERT Solutions for Class 11 Chemistry
Chapter 1: Some Basic Concepts of Chemistry
Chapter 2: structure of atom, chapter 3: classification of elements and periodicity in properties, chapter 4: chemical bonding and molecular structure.
- Chapter 5: Thermodynamics
- Chapter 6: Equilibrium
- Chapter 7: Redox Reactions
- Chapter 8: Organic Chemistry – Some Basic Principles & Techniques
- Chapter 9: Hydrocarbons
The following chapters have been removed from the NCERT Class 11 Chemistry textbook 2023-24.
- States of Matter
- The s-Block Elements
- The p-Block Elements
- Environmental Chemistry
From the NCERT Solutions at BYJU’S, students will clearly learn about all the chemical reactions which occur in our day-to-day activities. The solutions explain each and every minute concept in the best way possible so that students do not face any problems in the exam. It not only boosts their exam preparation, but also provides a strong foundation of fundamental concepts, which frequently appear in various competitive exams. Using these solutions, students will understand how to approach complex questions that would appear in the exam and answer them with confidence.
Download NCERT Solutions for Class 11 Chemistry PDF to revise all the concepts.
NCERT Solutions Class 11 Chemistry
NCERT Solutions for Class 11 Chemistry at BYJU’S is designed in such a way that students will be able to grasp all the concepts instantly. Each and every chemical reaction is explained with suitable examples and explanations in order to provide a quality learning experience for the students. For a student of Class 11, learning all the concepts from the NCERT Textbook would be a difficult task if they do not have a proper understanding of the concepts. The main objective of creating these solutions is to help students face the annual exam without fear. It not only enables logical and analytical thinking approach among students, but also helps them to attain good grades.
NCERT Solutions Class 11 Chemistry Chapter Details and Exercises
This chapter explains the importance of chemistry, and concepts like molecular mass and atomic mass. A few basic theories and laws, like Avogadro’s law , Dalton’s atomic theory and the law of conservation of mass, are explained in brief in this chapter. You will also solve problems based on determining the molecular weight of compounds, mass percent and concentration. You will get a clear idea about the empirical and molecular formulae, molarity, molality and mole concept.
Topics Covered in Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry :
General Introduction: Importance and scope of chemistry. Historical approach to particulate nature of matter, laws of chemical combination, Dalton’s atomic theory: the concept of elements, atoms and molecules. Atomic and molecular masses. Mole concept and molar mass; percentage composition and empirical and molecular formula; chemical reactions, stoichiometry and calculations based on stoichiometry.
Also, access the following resources for Class 11 Chapter 1 Some Basic Concepts of Chemistry at BYJU’S:
- CBSE Class 11 Notes Chapter 1 – Some Basic Concepts of Chemistry
- Chemistry Revision Notes for Class 11 Chapter 1 Some Basic Concepts of Chemistry
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 1 – Some Basic Concepts Of Chemistry Solutions
- Important Questions for Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry
Students will learn about Thomson’s atomic model, subatomic particles, Bohr’s model , Rutherford’s atomic model and quantum mechanical model of atom. Problems on relationship between frequency and wavelength, energy associated with electromagnetic radiation and subatomic particles are present in this chapter. You will get to know how to write the electron configurations and the electron transition in different shells which is important for the exam.
Topics Covered in Class 11 Chemistry Chapter 2 Structure of Atom :
Discovery of electron, proton and neutron; atomic number, isotopes and isobars. Thomson’s model and its limitations, Rutherford’s model and its limitations, Bohr’s model and its limitations, concept of shells and subshells, dual nature of matter and light, de Broglie’s relationship, Heisenberg uncertainty principle, concept of orbitals, quantum numbers, shapes of s, p, and d orbitals, rules for filling electrons in orbitals – Aufbau principle, Pauli exclusion principle and Hund’s rule, electronic configuration of atoms, stability of half filled and completely filled orbitals.
Also, access the following resources for Class 11 Chapter 2 Structure of Atom at BYJU’S:
- Structure of Atom Class 11 Notes
- Chemistry Revision Notes for Class 11 Chapter 2 Structure of Atom
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 2 – Structure of Atom
From the exam point of view, classification of elements is very important. Most of the questions in the final exam and various competitive exams appear from this chapter. So learning all the concepts from this chapter is important. From this chapter, students will also learn about the s-block, p-block, d-block and f-block elements from the periodic table , trends in physical and chemical properties and chemical reactivity. By learning this chapter thoroughly, students will obtain good score in the exam.
Topics Covered in Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties :
Significance of classification, brief history of the development of periodic table, modern periodic law and the present form of periodic table, periodic trends in properties of elements – atomic radii, ionic radii, inert gas radii, ionization enthalpy, electron gain enthalpy, electronegativity, valence.
Also, access the following resources for Class 11 Chapter 3 Classification of Elements and Periodicity in Properties at BYJU’S:
- Classification of Elements and Periodicity in Properties Class 11 Notes – Chapter 3
- Chemistry Revision Notes for Class 11 Chapter 3 Classification of Elements and Periodicity in Properties
- Important Questions Chemistry Class 11 Chapter 3 Classification of Elements and Periodicity in Properties
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 3 – Classification of Elements and Periodicity in Properties
Chemistry is based on the things which we observe in our surroundings and is called the central science. The topics discussed in this chapter are – VSEPR Theory, Lewis structures, Valence Bond Theory , polar character of covalent bonds and hydrogen bonding. Students will learn how to draw Lewis dot symbols for molecules, atoms and polyatomic ions. The solutions at BYJU’S contain diagrams for each concept to provide visual learning experience for the students.
Topics Covered in Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure :
Valence electrons, ionic bond, covalent bond, bond parameters, Lewis structure, polar character of covalent bond, covalent character of ionic bond, valence bond theory, resonance, geometry of covalent molecules, VSEPR theory, concept of hybridization involving s, p and d orbitals and shapes of some simple molecules, molecular orbital theory of homonuclear diatomic molecules (qualitative idea only), hydrogen bond.
Also, access the following resources for Class 11 Chapter 4 Chemical Bonding and Molecular Structure at BYJU’S:
- Chemical Bonding Class 11 Notes
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 4 – Chemical Bonding and Molecular Structure
Chapter 5: States of Matter
This chapter explains the intermolecular forces and how they affect the physical state of a substance. It deals with other concepts associated with gaseous and liquid states of matter. This is the main reason why this chapter is important for the exam. Students will be able to understand the Boyle’s Law , Charle’s Law, Gay Lussac’s Law and Avogadro Law which carry more marks as per the exam pattern. Problems on finding partial pressure, critical temperature and pressure, Van der Waals force and other intermolecular forces are present in this chapter.
Topics Covered in Class 11 Chemistry Chapter 5 States of Matter :
Three states of matter, intermolecular interactions, type of bonding, melting and boiling points, role of gas laws in elucidating the concept of the molecule, Boyle’s law, Charles’ law, Gay Lussac’s law, Avogadro’s law, ideal behaviour, empirical derivation of gas equation, Avogadro’s number, ideal gas equation, deviation from ideal behaviour, liquefaction of gases, critical temperature. Liquid State – Vapour pressure, viscosity and surface tension (qualitative idea only, no mathematical derivations).
Also, access the following resources for Class 11 Chapter 5 States of Matter at BYJU’S:
- States Of Matter Class 11 Notes – Chapter 5
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 5 – States of Matter
Chapter 6: Thermodynamics
It is a branch of science which explains the relationship between heat and the other energy forms. On the basis of exchange of matter and energy, thermodynamic systems are divided into three types – open, closed and isolated systems. Students will learn more about the concepts like terms in thermodynamics, applications, calorimetry, enthalpies, spontaneity and Gibbs energy change and equilibrium. The problems in the NCERT Solutions are answered in a systematic manner completely based on the latest syllabus of the CBSE board.
Topics Covered in Class 11 Chemistry Chapter 6 Thermodynamics :
Concepts of system, types of systems, surroundings, work, heat, energy, extensive and intensive properties, state functions. First law of thermodynamics – internal energy and enthalpy, heat capacity and specific heat, measurement of ΔU and ΔH, Hess’s law of constant heat summation, enthalpy of bond dissociation, combustion, formation, atomization, sublimation, phase transition, ionization, and dilution. Introduction of entropy as a state function, free energy change for spontaneous and nonspontaneous process, equilibrium.
Also, access the following resources for Class 11 Chapter 6 Thermodynamics at BYJU’S:
- Thermodynamics Class 11 Notes – Chapter 6
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 6 – Thermodynamics
Chapter 7: Equilibrium
From this chapter, students will learn buffer solutions, equilibrium constant and the common ion effect . As this chapter contains a lot of concepts, a thorough understanding of each of them is important to perform well in the annual exam. The solutions contain clear cut explanations for each and every concept so that all the queries of the students are clarified. The concepts discussed in this chapter are – solid liquid equilibrium, applications of equilibrium constant, factors affecting equilibria, acids, bases and salts, buffer solutions etc.
Topics Covered in Class 11 Chemistry Chapter 7 Equilibrium:
Equilibrium in physical and chemical processes, dynamic nature of equilibrium, law of mass action, equilibrium constant, factors affecting equilibrium – Le Chatelier’s principle; ionic equilibrium – ionization of acids and bases, strong and weak electrolytes, degree of ionization, concept of pH. Hydrolysis of salts (elementary idea), buffer solutions, solubility product, common ion effect (with illustrative examples).
Also, access the following resources for Class 11 Chapter 7 Equilibrium at BYJU’S:
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 7 – Equilibrium
- Equilibrium Class 11 Notes – Chapter 7
Chapter 8: Redox Reactions
The fact here is that the field of electrochemistry deals with the redox reactions . It is important for the students to learn this chapter in order to score good marks in the final exam. Students will get questions based on the classical idea of redox reaction, redox reactions in terms of electron transfer reactions, oxidation number and electrode processes. Students are recommended to first complete the entire chapter using the NCERT textbook prescribed by the CBSE board.
Topics Covered in Class 11 Chemistry Chapter 8 Redox Reaction :
Concept of oxidation and reduction, redox reactions, oxidation number, balancing redox reactions, applications of redox reactions.
Also, access the following resources for Class 11 Chapter 8 Redox Reactions at BYJU’S:
- Redox Reactions Class 11 Notes – Chapter 8
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 8 – Redox Reactions
Chapter 9: Hydrogen
Hydrogen is the lightest element with one electron and proton. The most abundant element in the entire universe is dihydrogen . Hydrogen is an important element which makes up 80% of the entire mass of the universe. It is very important for the students to know about this element in order to secure good marks in the final exam. Students will also learn about the preparation and properties of dihydrogen and its applications in our daily life. It might be difficult for the students to grasp all these concepts at a stretch, so making use of a perfect study material will help you through it.
Topics Covered in Class 11 Chemistry Chapter 9 Hydrogen :
Position of hydrogen in periodic table, occurrence, isotopes, preparation, properties and uses of hydrogen; hydrides – ionic, covalent and interstitial; physical and chemical properties of water, heavy water; hydrogen peroxide – preparation, reactions and structure; hydrogen as a fuel.
Also, access the following resources for Class 11 Chapter 9 Hydrogen at BYJU’S:
- Hydrogen Class 11 Notes – Chapter 9
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 9 – Hydrogen
- Chemistry Revision Notes for Class 11 Chapter 9 Hydrogen
Chapter 10: The s-Block Elements
Students will learn about the Alkali metals and Alkaline earth metals which are the group 1 and group 2 in the periodic table. Some of the concepts like physical and chemical properties of s-block elements, general characteristics of compounds and about important compounds like calcium. The biological importance of calcium and magnesium is also explained clearly in this chapter. Periodic table and the elements present in them is a hot topic in the exam perspective. For this reason, students are highly recommended to learn all these concepts thoroughly to score well.
Topics Covered in Class 11 Chemistry Chapter 10 The s-Block Elements :
Group 1 and Group 2 elements: General introduction, electronic configuration, occurrence, anomalous properties of the first element of each group, diagonal relationship, trends in the variation of properties (such as ionization enthalpy, atomic and ionic radii), trends in chemical reactivity with oxygen, water, hydrogen and halogens; uses.
Preparation and properties of some important compounds: Sodium carbonate, sodium chloride, sodium hydroxide and sodium hydrogen carbonate, biological importance of sodium and potassium. CaO, CaCO 3 , and industrial use of lime and limestone, biological importance of Mg and Ca.
Also, access the following resources for Class 11 Chapter 10 The s-Block Elements at BYJU’S:
- The s-Block Elements Class 11 Notes – Chapter 10
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 10 – The S Block Elements
Chapter 11: The p-Block Elements
The elements present between the 13th and 18th group are called the p-block elements. As this chapter has a lots of concepts, it is very important for the students to revise it regularly. Students will get a gist of the p-block elements, where the last electron enters or is found on the p-subshell. Some of the concepts which you will learn in this chapter are – boron family , important compounds of boron, uses of aluminium and boron, carbon family, allotropes of carbon and important compounds of silicon and carbon.
Topics Covered in Class 11 Chemistry Chapter 11 The p-Block Elements :
General Introduction to p-Block Elements Group 13 elements: General introduction, electronic configuration, occurrence, variation of properties, oxidation states, trends in chemical reactivity, anomalous properties of first element of the group; Boron – physical and chemical properties, some important compounds: borax, boric acids, boron hydrides. Aluminium: uses, reactions with acids and alkalies. Group 14 elements: General introduction, electronic configuration, occurrence, variation of properties, oxidation states, trends in chemical reactivity, anomalous behaviour of first element. Carbon – catenation, allotropic forms, physical and chemical properties; uses of some important compounds: oxides. Important compounds of silicon and a few uses: silicon tetrachloride , silicones, silicates and zeolites.
Also, access the following resources for Class 11 Chapter 11 The p-Block Elements at BYJU’S:
- What Are P-Block Elements?
- The p-Block Elements Class 11 Notes – Chapter 11
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 11 – The P Block Elements
Chapter 12: Organic Chemistry – Some Basic Principles and Techniques
When it comes to exam preparation, both organic and inorganic chemistry plays an important role in attaining good marks. This chapter will introduce you to concepts such as organic compounds, structural representation, classification, nomenclature, isomerism , purification of organic compounds and quantitative analysis. If students are not able to learn these concepts using the NCERT textbook they can download the PDF of solutions which are available online for absolutely free of cost.
Topics Covered in Class 11 Chemistry Chapter 12 Organic Chemistry – Some Basic Principles :
General introduction, methods of purification, qualitative and quantitative analysis, classification and IUPAC nomenclature of organic compounds. Electronic displacements in a covalent bond: inductive effect, electromeric effect, resonance and hyper conjugation. Homolytic and heterolytic fission of a covalent bond: free radicals, carbocations, carbanions; electrophiles and nucleophiles, types of organic reactions
Also access the following resources for Class 11 Chapter 12 Organic Chemistry – Some Basic Principles and Techniques at BYJU’S:
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 12 – Organic Chemistry Solutions Some Basic Principles and Techniques
- Chemistry Revision Notes for Class 11 Chapter 12 Organic Chemistry – Some Basic Principles and Techniques
Chapter 13: Hydrocarbons
The scientific study of properties, structure, reactions, composition and synthesis of organic compounds is called organic chemistry. It teaches the students about concepts like alkanes and its preparation from alkyl halides, unsaturated hydrocarbons and carboxylic acids. Students will also learn about the physical and chemical properties of alkanes as per the prescribed CBSE syllabus. As this chapter is important from the exam point of view, students must highly concentrate on all the concepts and memorise it thoroughly to perform well in the exam.
Topics Covered in Class 11 Chemistry Chapter 13 Hydrocarbons :
Classification of hydrocarbons Alkanes: Nomenclature, isomerism, conformations (ethane only), physical properties, chemical reactions including free radical mechanism of halogenation, combustion and pyrolysis. Alkenes: Nomenclature, structure of double bond (ethene), geometrical isomerism, physical properties, methods of preparation; chemical reactions: addition of hydrogen, halogen, water, hydrogen halides (Markovnikov’s addition and peroxide effect), ozonolysis, oxidation, mechanism of electrophilic addition. Alkynes: Nomenclature, structure of triple bond (ethyne), physical properties, methods of preparation, chemical reactions: acidic character of alkynes, addition reaction of – hydrogen, halogens, hydrogen halides and water. Aromatic hydrocarbons: Introduction, IUPAC nomenclature; Benzene: resonance, aromaticity; chemical properties: mechanism of electrophilic substitution – nitration sulphonation, halogenation, Friedel Craft’s alkylation and acylation; directive influence of functional group in mono-substituted benzene; carcinogenicity and toxicity
Also access the following resources for Class 11 Chapter 13 Hydrocarbons at BYJU’S:
- Hydrocarbons Class 11 Notes – Chapter 13
- Chapter 13 – Hydrocarbons
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 13 – Hydrocarbons
Chapter 14: Environmental Chemistry
Various concepts which are related to environment like water pollution , atmospheric pollution, soil pollution and its reasons are explained briefly in this chapter. Along with this students will also learn about the use of pesticides to reduce soil pollution and strategies to control pollution like waste disposal. By learning this chapter thoroughly, students will get to know about the pollution occurring in nature by our activities.
Topics Covered in Class 11 Chemistry Chapter 14 Environmental Chemistry :
Environmental pollution : Air, water and soil pollution, chemical reactions in atmosphere, smogs, major atmospheric pollutants; acid rain, ozone and its reactions, effects of depletion of ozone layer, greenhouse effect and global warming – pollution due to industrial wastes; green chemistry as an alternative tool for reducing pollution, strategy for control of environmental pollution.
Also access the following resources for Class 11 Chapter 14 Environmental Chemistry at BYJU’S:
- Environmental Chemistry Class 11 Notes – Chapter 14
- NCERT Exemplar Class 11 Chemistry Solutions for Chapter 14 – Environmental Chemistry Solutions
In the NCERT Textbook of Chemistry, there are about 14 chapters which contains various concepts which are crucial for the exam. It might be difficult for the students to remember all the chemical formulas and reactions during the final exam so regular practise is necessary. To understand the syllabus students can make use of the NCERT Class 11 Books from BYJU’S.
Features of NCERT Solutions for Class 11 Chemistry
The NCERT Solutions for Class 11 Chemistry provided by BYJU’S features:
- Brief explanations are provided for each question.
- The numerical equations are solved in a step wise manner.
- The answers are clear cut and precise completely based on the CBSE syllabus.
- Subject matter experts design the solutions after conducting a vast research.
- PDFs are available with a free download option.
Note: CBSE Class 11 Chemistry students can bookmark this page in their browser to easily access NCERT Solutions for Class 11 Chemistry in the future.
CBSE Marking Scheme 2023-24
The entire syllabus as per CBSE board is divided portions containing all the concepts divided equally. By doing this, the faculty explain the relationship between the various topics which are of higher importance. At the end of each academic year, the CBSE conducts exams in accordance to the syllabus allotted for each year. This mainly aims to provide students the ability to learn the various concepts that are crucial from the exam perspective.
| 1 | Some Basic Concepts of Chemistry | 7 |
| 2 | Structure of Atom | 9 |
| 3 | Classification of Elements and Periodicity in Properties | 6 |
| 4 | Chemical Bonding and Molecular Structure | 7 |
| 5 | Chemical Thermodynamics | 9 |
| 6 | Equilibrium | 7 |
| 7 | Redox Reactions | 4 |
| 8 | Organic Chemistry: Some basic Principles and Techniques | 11 |
| 9 | Hydrocarbons | 10 |
| Total | 70 |
Toppers Prefer BYJU’S NCERT Solutions. Here’s Why…
The highly experienced subject experts at BYJU’S design the solutions with the aim of helping students ace the exams. The solutions contain explanations in simple language so that students can grasp the concepts effectively. Students can rely on these concepts as they strictly follow the NCERT Class 11 Chemistry Syllabus . The solutions offered by BYJU’S give students a competitive edge by meeting the following criteria:
1. The solutions are created by the best faculty in the respective field
The highly qualified academic tutors design the solutions with the main objective of providing the best educational content to the students across the country. The solutions are very elaborate so that students get all their doubts cleared instantly.
2. Helps students answer complex questions
The explanatory solutions provided by the experts at BYJU’S are student friendly. The complex questions and numericals are solved in a step wise manner in order to make students grasp the method of solving them. The solutions are concept focused, which in turn, provides a strong foundation of fundamental concepts which would help students in their future studies.
3. Best study material for revision purpose
Among the various study materials available online, students can refer to the NCERT Solutions at BYJU’S to speed up their exam preparation. All the concepts are covered in the solutions so that students do not miss any concept which is important for the exam. From theory questions to numerical problems, NCERT Solutions provides the best knowledge to the students.
After going through our NCERT Solutions for Class 11 Chemistry, Also Check Out:
- NCERT Exemplar Problems for Class 11 Chemistry
- CBSE Revision Notes for Class 11 Chemistry
Have any questions/issues regarding our NCERT Solutions for Class 11 Chemistry? Our support team is always available to resolve your queries. Register with BYJU’S and get in touch with them NOW!
Frequently Asked Questions on NCERT Solutions for Class 11 Chemistry
Is the ncert solutions for class 11 chemistry free of cost, can i get my doubts clarified using the ncert solutions for class 11 chemistry at byju’s, how many chapters are present in the ncert solutions for class 11 chemistry , is the ncert solutions for class 11 chemistry the best study material for chapter revision.
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
NCERT Solutions for Class 6, 7, 8, 9, 10, 11 and 12
NCERT Solutions for Class 11 Chemistry
NCERT Solutions for Class 11 Chemistry : For students who want to go the extra mile and understand more and more concepts, NCERT solutions are perfect for you. NCERT solutions for class 11 chemistry helps students cover all the topics in detail. Students have the liberty and can choose any topic of their own from Class 11 Chemistry . They can download the Class 11 Chemistry NCERT Solutions , which they want to study with the comfort of their house.
- Chapter 1 Some Basic Concepts of Chemistry
- Chapter 2 Structure of The Atom
- Chapter 3 Classification of Elements and Periodicity in Properties
Chapter 4 Chemical Bonding and Molecular Structure
- Chapter 5 States of Matter
- Chapter 6 Thermodynamics
Chapter 7 Equilibrium
- Chapter 8 Redox Reactions
Chapter 9 Hydrogen
- Chapter 10 The sBlock Elements
- Chapter 11 The pBlock Elements
- Chapter 12 Organic Chemistry: Some Basic Principles and Techniques
Chapter 13 Hydrocarbons
Chapter 14 environmental chemistry.
NCERT Solutions for Class 11 Chemistry PDF
NCERT Exemplar Class 11 Chemistry Solutions
- Chapter 2 Structure of Atom
- Chapter 10 The s-Block Elements
- Chapter 11 The p-Block Elements
CBSE Class 11 Chemistry Lab Manual
- Introduction
- Viva Questions With Answers
- Viva Questions with Answers on Determination of Melting point
- Viva Questions with Answers on Determination of Boiling point
- To purify impure sample of benzoic acid by the process of crystallisation
- Viva Questions with Answers on Purification of Chemical Substances by Crystallisation
- Determine the pH values of hydrochloric acid at different strengths using; (i) pH papers and (ii) universal indicator solution
- To determine the pH of various samples of NaOH solution using: (i) pH paper, and (ii) universal indicator solution
- Determine the pH of solutions of some salts using pH paper or universal indicator
- Determine the pH of vegetable and fruit juices using pH paper and universal indicator
- Compare the pH of solutions of hydrochloric acid and acetic acid having same concentration
- (a) To study the change in pH of acetic acid (a weak acid) solution by addition of sodium acetate (b) To study the change in pH of ammonium hydroxide (a weak base) solution by the addition of ammonium chloride
- Viva Questions with Answers on Experiments Based On pH Change
- To study the shift of equilibrium between ferric ions and thiocyanate ions by increasing the concentration of either of them
- Viva Questions with Answers on Effect of Change of Concentration on Chemical Equilibrium
- You are provided with the solution of a hydrogen carbonate of a monovalent alkali metal (M) with strength equal to 10.0 gms per litre. Find out the atomic mass of the metal (M).
- Viva Questions with Answers on Quantitative Estimation (Volumetric Analysis)
- To analyse the given salt for acidic and basic radicals
- Viva Questions with Answers on Qualitative Analysis
- Viva Questions with Answers on Detection of Elements in Organic Compounds
Move to Top of the page.
Class 11 Chemistry NCERT Solutions
NCERT solutions for class 11 chemistry has all the solutions to the questions given in the textbook. The solutions available are in depth and in the simplest way possible. Thus, this chemistry class 11 NCERT solutions ensures that the students have the utmost understanding of the topic. This will help the students beyond examination marks. This will help them develop a core understanding of the subject. Because this subject demands to understand rather than just memorizing solutions of class 11 chemistry . Here below we are providing you with the overview of all the chemistry class 11 chapters that are there in the NCERT textbook.
Chapter 1 Some Basics Concepts of Chemistry
This class 11 Chemistry NCERT chapter 1 explains the role that is played in everyday life by chemistry. Furthermore, this chapter will explain the laws of chemical combinations and the nature of the matter. In this chemistry class 11 chapter 1, students will go in details about Dalton’s atomic theory where the concepts of molecules and atoms are explained. Also, class 11 chemistry chapter 1 deals with the molecular masses and concepts of atomic masses.
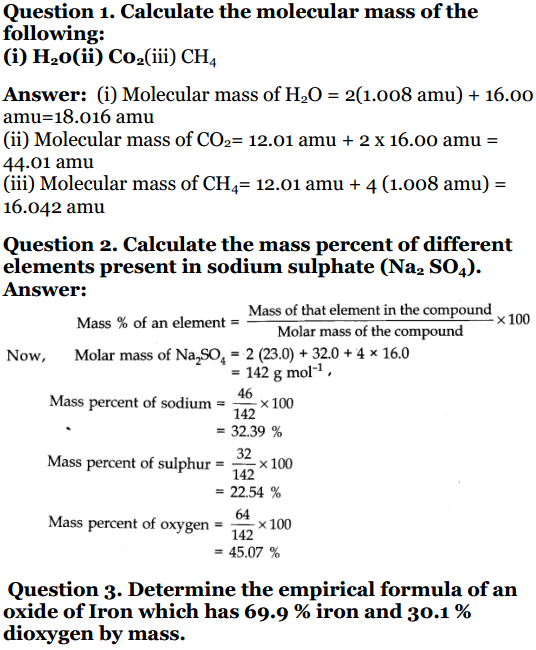
Chapter 2 Structure of Atoms
This Chemistry ncert class 11 chapter 2 will be fundamental for atoms and thus students will get to know about the discovery of proton, electron, and neutrons. They will also study what isotopes, isobars, atomic numbers, etc is. This chapter also describes details of Thomson’s model along with its limitations. Besides this, it talks about Bohr’s model and Rutherford’s model and its limitations. There is some detail being thrown into the dual nature of light and matter, Heisenberg’s uncertainty principle, de Broglie’s principle, the shapes of s, d, and p orbitals, quantum numbers, etc. There are also topics like Hund’s rule and Pauli’s exclusion principle which are also discussed in this chapter.
Chapter 3 Periodicity in Properties and Classification of Elements
In this chapter, you will learn about a brief history of the periodic table and its development, the significance of classifying periodic table, how the present form of the periodic table was formed, etc. Furthermore, the information about the trends in the periodic table for atoms like ionic radii, radii, inert gas radii, electronegativity, electron gain, valency, etc. is more discussed in this chapter. A total of 40 questions are there in CBSE Class 11 Chemistry NCERT Solutions for students to practice.

This NCERT Chemistry class 11 chapter 4 will help you understand what a covalent bond and an ionic bond is. There are more details about the parameters of the bonds, covalent bond and it’s the polar character, the bond theory of valence, covalent bond and it’s geometry, resonance, etc. Furthermore, this chapter discusses the VSEPR theory, concepts of hybridization that involve s, d, and p orbitals, various shapes of some molecules, and many more. There are a total of 40 questions in this chapter that can help students to practice.
Chapter 5 States of Matter – Liquid and Gas
This chapter will make students understand about three states of matter along with the types of bonding and intermolecular interactions. There are also some insights about the boiling point and melting points given in the chapter. Furthermore, the roles of gas laws are discussed and how Gay lussac’s law, Boyle’s law, Avogadro’s law, etc are helping students understand their ideal behavior. Along with this, the Avogadro’s number, empirical deviation in the gas equation, and the ideal equation required for the numerical are illustrated.
Chapter 6 Chemical Thermodynamics
This chemistry class 11 chapter 6 helps with the concepts of various systems and their different types. There is also discussion provided about the surroundings in the form of heat, work, energy, intensive and extensive properties, and state functions. There is a discussion about the first law of thermodynamics in this chapter. It involves the enthalpy and internal energy, specific heat, heat capacity, measurement of heat, etc.
This chemistry NCERT Solutions class 11 chapter 7 talks about the concepts of equilibrium in chemical and physical processes and details related to the equilibrium’s dynamic nature. There are also some insights related to the law of mass action, the factors affecting equilibrium and the equilibrium constant as per Le Chatelier’s principle. Furthermore, the information about the acid strength, ionization of polybasic acids, Henderson equation, the concept of pH, etc are also discussed.
Chapter 8 Redox Reaction
This chapter will provide in-depth knowledge to students about the reduction and oxidation and various insights about the redox reactions. Furthermore, information about balancing the redox reactions, oxidation number, etc will also be provided. There are a total of thirty questions in the chapter that also discusses the loss and gains of electrons.
Through this chapter, you will learn about the occurrence of hydrogen and it’s position in the periodic table. Along with this, there will be some information about the isotopes, their properties and how they are prepared is also discussed in this chapter. Information related to interstitial and hydrogen ionic covalent bonds is also discussed in this chapter.
Chapter 10 S-block Elements
This chapter discusses the elements present in group 1 and 2. It discusses the electronic configuration along with their occurrence. Every first element in the group shows some anomalous behavior which is also discussed in this chapter. There are diagonal relationships like atomic radii, variation in terms of properties in ionization enthalpy, ionic radii, etc is also discussed. How some of the important compounds like sodium chloride, sodium carbonate, sodium hydrogen carbonate, and sodium hydroxide are prepared is also discussed in this chapter.
Chapter 11 Some P-block Elements
This chapter provides more of a general view of the p-block elements to the students. There is also in-depth and detailed information about the elements in group 13 being discussed in this chapter. Also, the variation of oxidation states and their properties is also discussed. The chemical and physical properties of boron along with its important compounds like boric acid, borax, boron hydrides, etc are discussed in this chapter.
Chapter 12 Organic Chemistry – Some Basic Techniques and Principles
This chapter talks more about various purification methods along with quantitative and qualitative analysis being used for it. Furthermore, information related to IUPAC nomenclature and classification of various organic compounds is also discussed in this chapter. Along with this, the electronic displacements occurring in a covalent bond in the form of electromeric effect, inductive effect, hyperconjugation, resonance are also discussed in-depth.
In this NCERT class 11 chemistry chapter 13, students will get to know in detail about the classification of hydrocarbons and their uses, properties, and related reactions. Furthermore, this chapter talks about alkanes, alkynes, and alkenes. It also talks about related nomenclature, physical properties, IUPAC names, chemical reactions, combustion, isomerism, etc.
This chapter 14 Environmental Chemistry will talk about the environmental part of chemistry like environmental pollution related to air, soil, and water. Furthermore, all the chemical reactions happening in the atmosphere due to smog, major atmospheric pollutants, etc is also discussed. This final chapter in NCERT solutions for class 11 chemistry further discusses about ozone, acid rains and it’s reactions. A total of 20 questions will help students understand various alternative tools required for reducing pollution.
NCERT solutions for class 11 chemistry is the right recipe for students who want to go beyond marks in the exams. We will keep you updated on all exam related stuff on this website.
More Resources for CBSE Class 11
NCERT Solutions
CBSE Chemistry Practical Class 11 Lab Manual
Free Resources
Quick Resources
NCERT Books

NCERT Books for Class 11 Chemistry PDF Download in English & Hindi Medium
NCERT Books Class 11 Chemistry: The National Council of Educational Research and Training (NCERT) publishes Chemistry textbooks for Class 11. The NCERT Class 11th Chemistry textbooks are well known for it’s updated and thoroughly revised syllabus. The NCERT Chemistry Books are based on the latest exam pattern and CBSE syllabus.
NCERT keeps on updating the Chemistry books with the help of the latest question papers of each year. The Class 11 Chemistry books of NCERT are very well known for its presentation. The use of NCERT Books Class 11 Chemistry is not only suitable for studying the regular syllabus of various boards but it can also be useful for the candidates appearing for various competitive exams, Engineering Entrance Exams, and Olympiads.
NCERT Class 11 Chemistry Books in English PDF Download
NCERT Class 11 Chemistry Books are provided in PDF form so that students can access it at any time anywhere. Class 11 NCERT Chemistry Books are created by the best professors who are experts in Chemistry and have good knowledge in the subject.
NCERT Books for Class 11 Chemistry – English Medium
- Chapter 1: Some Basic Concepts of Chemistry
- Chapter 2: Structure of Atom
- Chapter 3: Classification of Elements and Periodicity in Properties
- Chapter 4: Chemical Bonding and Molecular Structure
- Chapter 5: States of Matter & Solid State
- Chapter 6: Thermodynamics
- Chapter 7: Equilibrium
- Chapter 8: Redox Reactions
- Chapter 9: Hydrogen
- Chapter 10: The s – Block Elements
- Chapter 11: The p – Block Elements
- Chapter 12: Organic Chemistry – Some Basic Principles and Techniques
- Chapter 13: Hydrocarbons
- Chapter 14: Environmental Chemistry
- NCERT Answers Part 1
- NCERT Answers Part 2
- Class 11 Chemistry NCERT Book PDF Download Part I
- Class 11 Chemistry NCERT Book PDF Download Part II
NCERT Books for Class 11 Chemistry Hindi Medium
रसायन (भाग 1 तथा भाग 2)
- अध्याय 1: रसायन विज्ञान की कुछ मूल अवधारणाएँ
- अध्याय 2: परमाणु की संरचना
- अध्याय 3: तत्वों का वर्गीकरण एवं गुणधर्मों में आवर्तिता
- अध्याय 4: रासायनिक आबंधन तथा आण्विक संरचना
- अध्याय 5: द्रव्य के अवस्थाएँ तथा ठोस अवस्था
- अध्याय 6: उष्मागतिकी
- अध्याय 7: साम्यावस्था
- अध्याय 8: अपचयोपचय अभिक्रियाएँ
- अध्याय 9: हाइड्रोजन
- अध्याय 10: s – बलॉक तत्व
- अध्याय 11: p – बलॉक तत्व I & II
- अध्याय 12: कार्बनिक रसायन: कुछ आधारभूत सिद्धांत तथा तकनीकें
- अध्याय 13: हाइड्रोकार्बन
- अध्याय 14: पर्यावरणीय रसायन
- उत्तरमाला भाग 1
- उत्तरमाला भाग 2
- Class 11 Chemistry NCERT Book in Hindi PDF Download Part I
- Class 11 Chemistry NCERT Book in Hindi PDF Download Part II
The NCERT syllabus mainly focuses on this book to make it student-friendly to make it useful for both the students and the competitive exam aspirants. The book covers a detailed Chemistry based on the syllabuses of various boards. NCERT Chemistry Books for Class 11 is perfectly compatible with almost every Indian education state and central boards.
We hope that this detailed article on NCERT Books Class 11 Chemistry helps you in your preparation and you crack the Class 11 exams or competitive exams with excellent scores.
Leave a Comment Cancel reply
You must be logged in to post a comment.

IMAGES
VIDEO
COMMENTS
NCERT Solutions Class 11 Computer Science. Question 3. Determine the empirical formula of an oxide of Iron which has 69.9 % iron and 30.1 % dioxygen by mass. Answer: Question 4. Calculate the amount of carbon dioxide that could be produced when. (i) 1 mole of carbon is burnt in air. (ii) 1 mole of carbon is burnt in 16 g of dioxygen. (iii) 2 ...
Class 11 Assignments. We have provided below free printable Class 11 Chemistry Assignments for Download in PDF. The Assignments have been designed based on the latest NCERT Book for Class 11 Chemistry. These Assignments for Grade 11 Chemistry cover all important topics which can come in your standard 11 tests and examinations.
Solution: Molecular mass of Na 2 SO 4 = 2 × Atomic mass of Na + Atomic mass of S + 4 × Atomic mass of O. = 2 × 23 + 32 + 4 × 16 = 46 + 32 + 64 = 142 u. Question 3. Determine the empirical formula of an oxide of iron which has 69.9% iron and 30.1% oxygen by mass. Solution: Hence, the empirical formula is Fe 2 CO 3.
NCERT Solutions Class 11 Chemistry Chapter 1 - Free PDF Download. NCERT Solutions for Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry are provided on this page for Class 11 Chemistry students. These solutions can also be downloaded in a PDF format for free by clicking the download button provided below.
This NCERT Solutions for Class 11 Chemistry contains answers of all questions asked in Chapter 1 in textbook, Chemistry Part I. Therefore you can refer it to solve Some Basic Concepts of Chemistry exercise questions and learn more about the topic. NCERT Solutions for Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry
The CBSE Chemistry Chapter 1, Some Basic Concepts of Chemistry Class 11 notes introduces fundamental concepts crucial for understanding the subject. It covers essentials like matter, its properties, and measurement units. Focus on comprehending the mole concept, which plays a pivotal role in chemical calculations.
NCERT Solutions Class 11 Chemistry Chapter 1: This chapter introduces students to the classification of matter based on the nature of their substances which include pure substances and mixtures and the properties of these matters and their measurement. This chapter recalls the International System of Units (SI). It also deals with other topics like Mass, Wright, Density, and Temperature of ...
With a focus on clarity and depth, these resources serve as indispensable tools for students navigating through chemistry class 11, chapter 1 of their Chemistry syllabus. 1. Class 11 Chemistry Chapter 1 NCERT Solutions FREE PDF Download. 2. Quick Insights of "Some Basic Concepts of Chemistry" Class 11 NCERT Solutions.
We will help you to get complete NCERT Solutions for Class 11 Chemistry and other subjects. We are providing you the free PDF download links of the class 11 Chemistry Chapter 1. Toppr provides free study materials, previous 10 years of question papers, 1000+ hours of video lectures. Download Toppr for Android and iOS or signup for free.
The Chemistry Class 11 Chapter 1 Important Questions are curated by Vedantu experts with thorough research and keeping in mind the highest probability of its chance in the examinations. Chapter 1 Chemistry Class 11 Important Questions help students comprehend all the topics and concepts in a simple, easy, and understandable manner.
Also, you can read the NCERT book online in these sections Solutions by Expert Teachers as per Central Board of Secondary Education Book guidelines. NCERT Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry Solutions are part of All Subject Solutions.Here we have given NCERT Class 11 Chemistry Part: I, Part: II Notes.NCERT Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry ...
Download Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry Important Questions with Answers PDF by clicking on the button below. Download PDF. Recommended Video Some Basic Concepts of Chemistry Class 11 - 100 Days Crash Course
NCERT Solutions for Class 11 Chemistry -Chapterwise. Chapter-1 Some basic concepts of Chemistry. Chapter-2 Structure of Atom. Chapter-3 Classification of Elements and Periodicity in Properties. Chapter-4 Chemical Bonding and Molecular Structure. Chapter-5 States of Matter Gases and Liquid. Chapter-6 Thermodynamics.
Chapterwise NCERT Solutions for Class 11 Chemistry NCERT Solutions for Class 11 Chemistry Chapter 1 - Some Basic Concepts of Chemistry . In this chapter, laws of chemical combination, Dalton's atomic theory, mole concept, empirical and molecular formula, stoichiometry and its calculations are discussed.
11). Calculate: (i) the molality of the solution. (ii) the mole fraction of sugar. 11. Calculate the molarity of pure water if its density at room temperature is 0.997 g/cm 3. 12. Calculate the mass of carbon tetrachloride which can be produced by the reaction of 10.0 g of carbon with 100.0 g of chlorine. Determine the mass of excess reagent ...
Chemistry Worksheets for Class 11 Chemistry Chapter wise. Below is the most comprehensive collection of CBSE NCERT Worksheets for Class 11 Chemistry that you can download for free. These free worksheets are based on the latest CBSE NCERT books and syllabus and cover all relevant Questions and Answers in Class 11 Chemistry.
As per the new textbook published for academic year 2024-25, there are only 9 chapters in class 11 chemistry Syllabus. Chapter wise NCERT Solutions for Class 11 Chemistry Chapter 1. Some Basic Concepts of Chemistry Chapter 2. ... NCERT books Solutions, notes and assignments according to current CBSE syllabus, are also available to download ...
Topics Covered in Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry : General Introduction: Importance and scope of chemistry. Historical approach to particulate nature of matter, laws of chemical combination, Dalton's atomic theory: the concept of elements, atoms and molecules. Atomic and molecular masses.
NCERT Solutions for Class 11 Chemistry Free PDF Download (Chapter-wise) Chapter 1 Some Basic Concepts of Chemistry. Chapter 2 Structure of Atom. Chapter 3 Classification of Elements and Periodicity in Properties. Chapter 4 Chemical Bonding and Molecular Structure. Chapter 5 States of Matter. Chapter 6 Thermodynamics.
This chemistry NCERT Solutions class 11 chapter 7 talks about the concepts of equilibrium in chemical and physical processes and details related to the equilibrium's dynamic nature. There are also some insights related to the law of mass action, the factors affecting equilibrium and the equilibrium constant as per Le Chatelier's principle.
Chapter 9: Hydrogen. Chapter 10: The s - Block Elements. Chapter 11: The p - Block Elements. Chapter 12: Organic Chemistry - Some Basic Principles and Techniques. Chapter 13: Hydrocarbons. Chapter 14: Environmental Chemistry. NCERT Answers Part 1. NCERT Answers Part 2. Class 11 Chemistry NCERT Book PDF Download Part I.