
- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons

Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

Phase Diagrams for Pure Substances
- Last updated
- Save as PDF
- Page ID 3864

- Truro School in Cornwall
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
This page explains how to interpret the phase diagrams for simple pure substances - including a look at the special cases of the phase diagrams of water and carbon dioxide.
The Basic Phase Diagram
At its simplest, a phase can be just another term for solid, liquid or gas. If you have some ice floating in water, you have a solid phase present and a liquid phase. If there is air above the mixture, then that is another phase. But the term can be used more generally than this. For example, oil floating on water also consists of two phases - in this case, two liquid phases. If the oil and water are contained in a bucket, then the solid bucket is yet another phase. In fact, there might be more than one solid phase if the handle is attached separately to the bucket rather than molded as a part of the bucket.
You can recognize the presence of the different phases because there is an obvious boundary between them - a boundary between the solid ice and the liquid water, for example, or the boundary between the two liquids. A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapor (gas) states of a pure substance. This is the phase diagram for a typical pure substance.
These diagrams (including this one) are nearly always drawn highly distorted in order to see what is going on more easily. There are usually two major distortions. We'll discuss these when they become relevant. If you look at the diagram, you will see that there are three lines, three areas marked "solid", "liquid" and "vapor", and two special points marked "C" and "T".
The Three Areas
These are easy! Suppose you have a pure substance at three different sets of conditions of temperature and pressure corresponding to 1, 2 and 3 in the next diagram.
Under the set of conditions at 1 in the diagram, the substance would be a solid because it falls into that area of the phase diagram. At 2, it would be a liquid; and at 3, it would be a vapor (a gas).
Phase Transitions
Moving from solid to liquid by changing the temperature.
Suppose you had a solid and increased the temperature while keeping the pressure constant - as shown in the next diagram. As the temperature increases to the point where it crosses the line, the solid will turn to liquid. In other words, it melts.
If you repeated this at a higher fixed pressure, the melting temperature would be higher because the line between the solid and liquid areas slopes slightly forward.
So what actually is this line separating the solid and liquid areas of the diagram?
It simply shows the effect of pressure on melting point. Anywhere on this line, there is an equilibrium between solid and liquid. You can apply Le Chatelier's Principle to this equilibrium just as if it was a chemical equilibrium. If you increase the pressure, the equilibrium will move in such a way as to counter the change you have just made.
If it converted from liquid to solid, the pressure would tend to decrease again because the solid takes up slightly less space for most substances. That means that increasing the pressure on the equilibrium mixture of solid and liquid at its original melting point will convert the mixture back into the solid again. In other words, it will no longer melt at this temperature.
To make it melt at this higher pressure, you will have to increase the temperature a bit. Raising the pressure raises the melting point of most solids. That's why the melting point line slopes forward for most substances.
Moving from solid to liquid by changing the pressure
You can also play around with this by looking at what happens if you decrease the pressure on a solid at constant temperature.
Moving from liquid to vapor
In the same sort of way, you can do this either by changing the temperature or the pressure.
The liquid will change to a vapor - it boils - when it crosses the boundary line between the two areas. If it is temperature that you are varying, you can easily read off the boiling temperature from the phase diagram. In the diagram above, it is the temperature where the red arrow crosses the boundary line.
So, again, what is the significance of this line separating the two areas? Anywhere along this line, there will be an equilibrium between the liquid and the vapor. The line is most easily seen as the effect of pressure on the boiling point of the liquid. As the pressure increases, so the boiling point increases.
The critical point
You will have noticed that this liquid-vapor equilibrium curve has a top limit (labeled as C in the phase diagram), which is known as the critical point . The temperature and pressure corresponding to this are known as the critical temperature and critical pressure. If you increase the pressure on a gas (vapor) at a temperature lower than the critical temperature, you will eventually cross the liquid-vapor equilibrium line and the vapor will condense to give a liquid.
This works fine as long as the gas is below the critical temperature. What, though, if your temperature was above the critical temperature? There wouldn't be any line to cross! That is because, above the critical temperature, it is impossible to condense a gas into a liquid just by increasing the pressure. All you get is a highly compressed gas. The particles have too much energy for the intermolecular attractions to hold them together as a liquid. The critical temperature obviously varies from substance to substance and depends on the strength of the attractions between the particles. The stronger the intermolecular attractions, the higher the critical temperature.
Moving from solid to vapor
There's just one more line to look at on the phase diagram. This is the line in the bottom left-hand corner between the solid and vapor areas. That line represents solid-vapor equilibrium. If the conditions of temperature and pressure fell exactly on that line, there would be solid and vapor in equilibrium with each other - the solid would be subliming. (Sublimation is the change directly from solid to vapor or vice versa without going through the liquid phase.)
Once again, you can cross that line by either increasing the temperature of the solid, or decreasing the pressure. The diagram shows the effect of increasing the temperature of a solid at a (probably very low) constant pressure. The pressure obviously has to be low enough that a liquid can't form - in other words, it has to happen below the point labelled as T .
You could read the sublimation temperature off the diagram. It will be the temperature at which the line is crossed.
The Triple Point
Point T on the diagram is called the triple point. If you think about the three lines which meet at that point, they represent conditions of:
- solid-liquid equilibrium
- liquid-vapor equilibrium
- solid-vapor equilibrium
Where all three lines meet, you must have a unique combination of temperature and pressure where all three phases are in equilibrium together. That's why it is called a triple point.
If you controlled the conditions of temperature and pressure in order to land on this point, you would see an equilibrium which involved the solid melting and subliming, and the liquid in contact with it boiling to produce a vapor - and all the reverse changes happening as well. If you held the temperature and pressure at those values, and kept the system closed so that nothing escaped, that's how it would stay.
Normal melting and boiling points
The normal melting and boiling points are those when the pressure is 1 atmosphere. These can be found from the phase diagram by drawing a line across at 1 atmosphere pressure.
Example \(\PageIndex{1}\): Phase Diagram for Water
There is only one difference between this and the phase diagram that we've looked at up to now. The solid-liquid equilibrium line (the melting point line) slopes backwards rather than forwards.
In the case of water, the melting point gets lower at higher pressures. Why?
If you have this equilibrium and increase the pressure on it, according to Le Chatelier's Principle the equilibrium will move to reduce the pressure again. That means that it will move to the side with the smaller volume. Liquid water is produced. To make the liquid water freeze again at this higher pressure, you will have to reduce the temperature. Higher pressures mean lower melting (freezing) points.
Now lets put some numbers on the diagram to show the exact positions of the critical point and triple point for water.
Notice that the triple point for water occurs at a very low pressure. Notice also that the critical temperature is 374°C. It would be impossible to convert water from a gas to a liquid by compressing it above this temperature. The normal melting and boiling points of water are found in exactly the same way as we have already discussed - by seeing where the 1 atmosphere pressure line crosses the solid-liquid and then the liquid-vapor equilibrium lines.
Just one final example of using this diagram. Imagine lowering the pressure on liquid water along the line in the diagram below.
The phase diagram shows that the water would first freeze to form ice as it crossed into the solid area. When the pressure fell low enough, the ice would then sublime to give water vapor. In other words, the change is from liquid to solid to vapor.
Example \(\PageIndex{2}\): Phase Diagram for Carbon Dioxide
The only thing special about this phase diagram is the position of the triple point which is well above atmospheric pressure. It is impossible to get any liquid carbon dioxide at pressures less than 5.11 atmospheres.
That means that at 1 atmosphere pressure, carbon dioxide will sublime at a temperature of -78°C. This is the reason that solid carbon dioxide is often known as "dry ice". You can't get liquid carbon dioxide under normal conditions - only the solid or the vapor.
Contributors and Attributions
Jim Clark ( Chemguide.co.uk )

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.


Chapter 4: Phase Diagrams
- Last updated
- Save as PDF
- Page ID 97982

- Joshua P. Steimel
- California State Polytechnic University, Humboldt
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
4.1 Lowest Energy Wins!
I have recorded a series of lectures to supplement the text which can be found in the playlist below:
https://www.youtube.com/playlist?lis...1CCqf55NMUHETA
Materials can exist as different phases , i.e. solid , liquid , gas , vapor , plasma and each of those phases are described by their own unique free energy curve. The thermodynamically stable phase is the one with the lowest free energy at any given temperature, pressure, composition, etc . And crossing points in the free energy curves will define the locations of phase transitions . We can see this schematically when considering a pure single component material going undergoing melting and we can plot the free energy as function of temperature and see the phase transition.

where \(G_l\) is the molar Gibbs free energy of the liquid phase and \(G_s\) is the molar Gibbs free energy of the solid phase. I will try to be as consistent as possible to keep liquid as blue color, like water.
We can also draw this out for the complete phase transition spectrum for a given material as the solid melts , the liquid boils , and the solid can sublimate directly to vapor.

However as we touched upon in structure materials can exhibit different crystalline forms in the solid state depending on the conditions of temperature and pressure.
What was the special name for these materials?
Polymorphs/allotropes.
Wouldn’t it be useful to have some type of diagram that would allow us to visualize these different phases....hmmm....!
4.2 Phase Diagrams of Single-Component Materials
Phase diagrams illustrate the phases of a system at equilibrium as a function of 2 or more thermodynamic variables.
Phase diagrams are also particularly useful because they o bey the laws of thermodynamics and there are constraints on the structure of phase diagrams, particularly the Gibbs Phase Rule.
4.3 Gibbs Phase Rule
The Gibbs Phase Rule determines how many phases can be in equilibrium simultaneously and when those phases are stable along a field, line, or point in the phase diagram. There are two criteria for phase equilibrium at a constant temperature and pressure are that the chemical potential of each component must be equal in each phase :
\begin{eqnarray} \mu^{\alpha}_{1}=\mu^{\beta}_{1}=\mu^{\gamma}_{1}=\mu^{P}_{1}...\\ \mu^{\alpha}_{2}=\mu^{\beta}_{2}=\mu^{\gamma}_{2}=\mu^{P}_{2}...\\ \mu^{\alpha}_{C}=\mu^{\beta}_{C}=\mu^{\gamma}_{C}=\mu^{P}_{C}... \end{eqnarray}
So we end up with C(P-1) equations. And we also have the second condition which is given by the Gibbs-Duhem equation
\begin{eqnarray} V^{\alpha}dP - S^{\alpha}dT - \sum_{i=1}^{c} n^{\alpha}_{i}d\mu^{\alpha}_{i}=0\\ V^{\beta}dP - S^{\beta}dT - \sum_{i=1}^{c} n^{\beta}_{i}d\mu^{\beta}_{i}=0\\ V^{\gamma}dP - S^{\gamma}dT - \sum_{i=1}^{c} n^{\gamma}_{i}d\mu^{\gamma}_{i}=0 \end{eqnarray}
So here we end up with P equations . So the degrees of freedom (DOF) is total number of variables - the total number of equations.
\begin{eqnarray} D = (CP +2) - (C(P-1) +P)\\ D = C - P + 2 \end{eqnarray}
where D is the degrees of freedom, C is the number of components, P is the number of phases. The 2 comes from T and P as independent variables.
So let’s do a couple of examples where we apply the Gibbs phase rule! Let’s look at the single-component phase diagram below:

What is the DOF for the 1st X location?
The DOF is 2.
\begin{eqnarray} D + P = C + 2\\ D +1 = 1 + 2 \end{eqnarray}
That means we can vary both T and P and still be in the liquid phase.
What is the DOF for the 2nd X location?
The DOF is 1. We can now only vary T or P freely the other is fixed.
What is the DOF for the 3rd X location?
The DOF is 0. We can not move anywhere. This is an invariant or triple point.
Now there is one unique point, an invariant point , called the triple point that can allow the 3 phases to co-exist, i.e. any change in the variables will cause the equilibrium to shift to either 1 or 2 phases in equilibrium. The slope of the 2-phase line on the P vs. T diagram is determined by the Clausius-Clapeyron equation evaluated at the coexistence curve:
\begin{equation} \frac{dP}{dT} = \frac{\Delta \overline{S}^{S \rightarrow L}}{\Delta \overline{ V}^{S \rightarrow L}} = \frac{\Delta \overline{S}_{m}}{\Delta \overline{V}_{m}} =\frac{\Delta \overline{H}_{m}}{T_{m} \Delta\overline{ V}_{m}} \end{equation}
We know that typically the enthalpy of melting is positive so the slope of the P-T diagram for the solid-liquid coexistence curve should be positive. Let’s look at iron on the lecture slides.
4.4 Multi-Component Phase Diagrams
So far we have only dealt with phase diagrams of pure components but typically you will deal with either binary, ternary, quaternary, etc. phase diagrams .
Let’s take a look at a relatively simple phase diagram, a Binary Lens phase diagram which holds for ideal solution scenarios.
(Side Note: When I say something is simple please do not interpret this as the concept being easy. These concepts are very difficult but what I mean by the problem is simple I want to encourage and show you that all these problems can be solved. When we say that this or that problem is really difficulty that problem is a given a special mystique and students might be wary about going about soling the problem or learning that concepts. By saying something is simple I want to remove that mystique.)
Note here that we are not varying pressure now because typically we are working in a constant pressure environment , although that is not necessarily always the case. Now this will change our Gibbs phase rule condition to :
\begin{equation} \mu = \bigg (\frac{\partial G}{\partial n}\bigg)_{T,P} \end{equation}
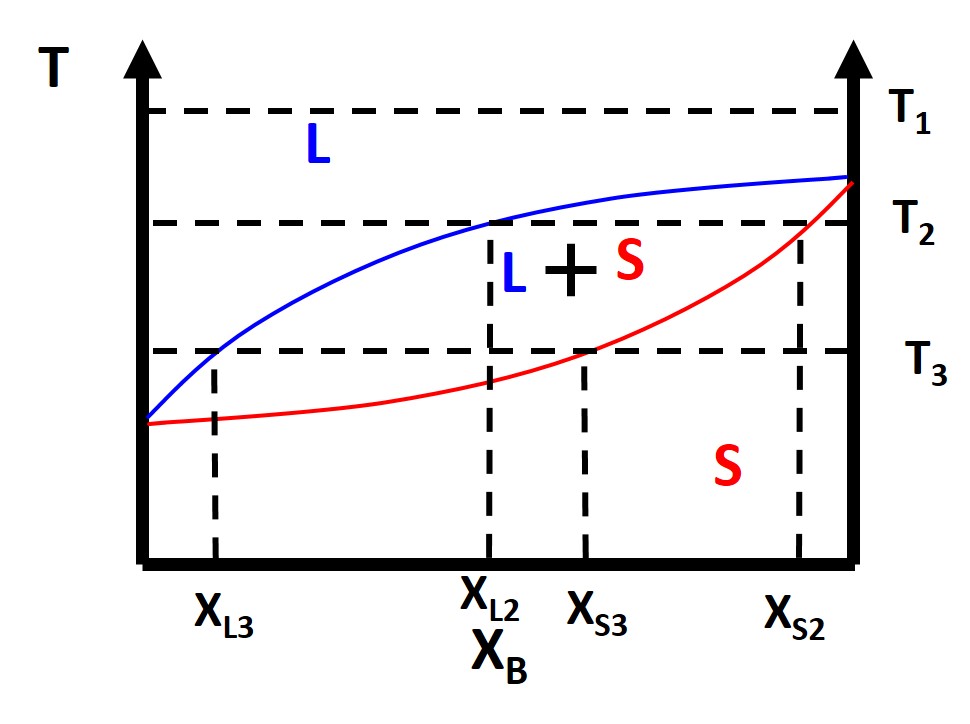
Figure \(\PageIndex{4}\): Binary Lens Phase Diagram
We can also take a look at the free energy diagrams as well to conceptualize the phase diagrams. At \(T_1\) everything is as usual in terms of our free energy diagram with liquid phase being lower energy than the solid phase. Things start to get a little funky at where we have the two phase region of a solid and liquid. What happens here? Why does this occur? Well at compositions less than \(X_{L2}\) and greater than \(X_{S2}\) everything is normal. What happens is in between these compositions. You see that we can draw a common tangent line (purple). When you have a common tangent line the system can phase separate from a 1-component system (liquid) to a weighted mixture of two components (solid and liquid) which has a lower free energy than the starting 1-component phase. This is because the requirement for equilibrium as we previously mentioned is that the chemical potential of each component must be equal in all phases and we have the expression that the chemical potential \(\mu\) is
\begin{equation} \bigg (\frac{\partial G}{\partial X}\bigg)_{X = X^\alpha} = \bigg (\frac{\partial G}{\partial X}\bigg)_{X = X^\beta} \end{equation}
So common tangent rule will allow us to find your equilibrium volume fraction of species in each phase via the common tangents in free energy curves and the slope is essential the chemical potential so when the slope is equal. So between the common tangent points equilibrium will have the components in both phases, i.e. mixed as given below

4.5 The Lever Rule:
Typically we are also interested in finding at a particular composition and temperature what will be the relative fractions of the different phases of the material , or in the case of a simple lens diagram, what fractions will be solid and liquid. To calculate these phase fractions we will use the lever rule . Once you pick a temperature of interest, \(T_1\), and a composition, \(X^o_B\) then you draw a horizontal isotherm which connects or ties together the boundaries of a two phase regions. These horizontal isotherms are also called tie lines .
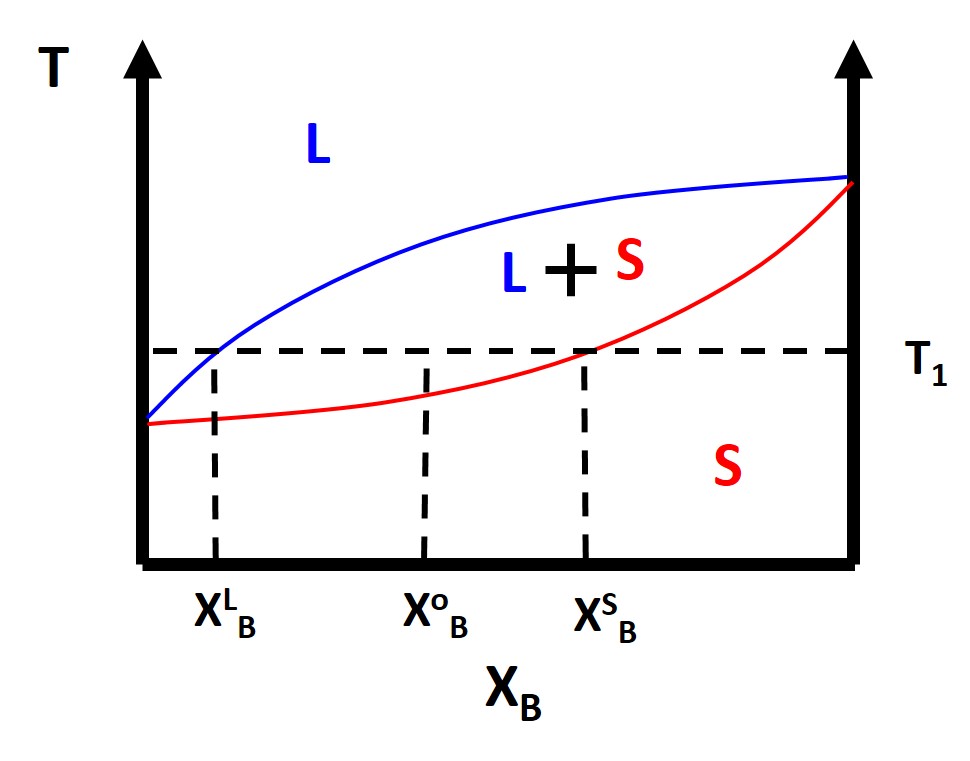
The composition of the liquid and solid phases, \(X^L_B\) and \(X^S_B\), respectively are simply where the tie line intersects the liquidus or solidus lines , again respectively. The liquids line defines the two phase coexistence between a liquid and a solid phase. The solidus line represent the two phase coexistence between a solid and another solid phase, for binary phase diagrams. We can also calculate the fraction of phases that are present by using the lever rule. What you do is simply take the length of the tie line from the composition, \(X^o_B\) , to the phase boundary for the other phase and divide by the total tie line length. For example:
\begin{eqnarray} f^{S} = \frac{X^{o}_{B}-X^{L}_{B}}{X^{S}_{B}-X^{L}_{B}}\\ f^{L} = \frac{X^{S}_{B}-X^{o}_{B}}{X^{S}_{B}-X^{L}_{B}} \end{eqnarray}
where \(f^S\) and \(f^L\) are the phase fractions of solid and liquid respectively. This is typically the simplest type of phase diagram that you will find and it is actually the phase diagram for Cu-Ni. This also hold for ideal solution scenarios but typically most binary solutions or alloys will not be miscible at all compositions and temperatures. We know from structure that different materials have different crystal structures so those crystal structures might not always be compatible.
4.6 Phase Transitions Congruent vs Incongruent
A phase transition can occur congruently or incongruently . A congruent phase transition occurs when there is a complete transformation from one phase to another with no change in composition as seen in the examples below. An incongruent phase transition is a partial transformation from one phase to another and there will be a change in composition like we just saw with the lens diagram.
4.7 Eutectic Phase Diagram
So far we have only discussed the simple binary lens phase diagram which only applied to several real experimental systems. There is another relatively simple phase diagram that corresponds to many more experimental systems and that is the binary eutectic phase diagram .
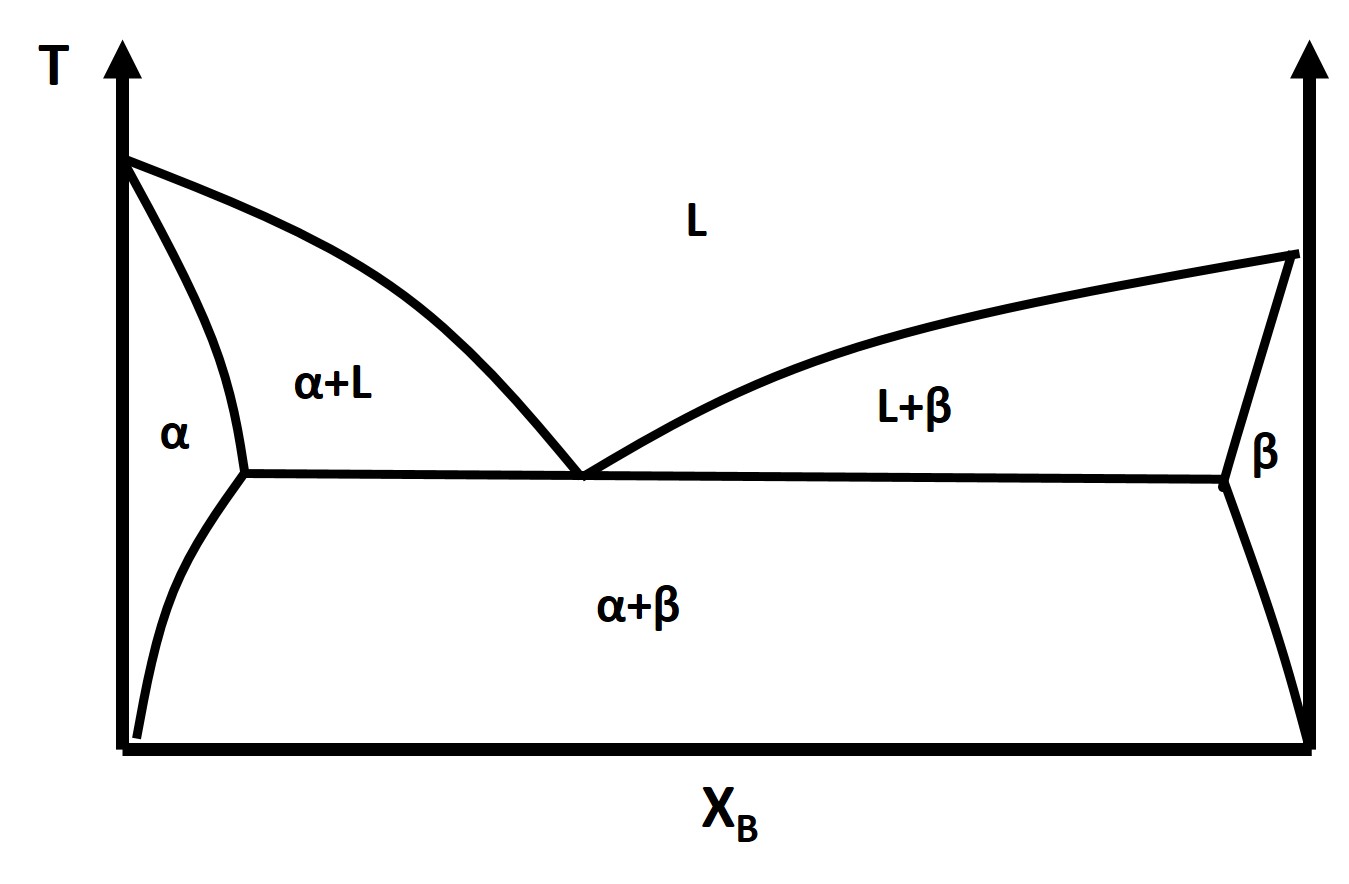
The binary eutectic phase diagram has several distinctive features one being a solid-solid phase mixture, limit of solubility at different temperatures, and an invariant point in the phase diagram, the eutectic point.
The solubility limit is the maximum amount of solute that you can integrate into the structure (or phase) or the solvent to form a solid solution. The solubility limit is a function of temperature and must be defined at a given temperature.
What is the solubility limit of B into A at \(T_1\) ?
What about A into B?
Identify the Solidus Line
The eutectic point is an invariant point , D = 0, and it describes the constant composition transformation from a pure liquid phase to a two phase solid solution/mixture or alternatively
\begin{equation} L \rightleftarrows \alpha + \beta \end{equation}
There are also a number of different invariant points beyond the simple eutectic.
4.8 Eutectic, Eutectoid, Peritectic, Peritectoid, and Monotectic
We have already discussed one invariant point, the eutectic. There is also another invariant point called the eutectoid . The eutectoid describes the constant composition transformation from a pure solid phase to two phase solid solution/mixture as described below
\begin{equation} \gamma \rightleftarrows \alpha + \beta \end{equation}
There is also a peritectic invariant point. The peritectic describes where a solid and liquid phase mixture will transform into a pure solid phase which is described by the equation below
\begin{equation} L +\alpha \rightleftarrows \beta \end{equation}
Finally the last invariant point is described by the peritectoid which is a transformation of two solid phases in a mixture to a single solid phase again described by the equation below
\begin{equation} \gamma + \alpha \rightleftarrows \beta \end{equation}
There is also a monotectic invariant point that does not appear in to many phase diagrams but this invariant point describes the transformation from a pure liquid to another liquid and solid phase which is described by the equation below:
\begin{equation} L_{1} \rightleftarrows L_{2} + \alpha \end{equation}
Let’s do a couple of examples were we identify the invariant points in a couple of phase diagrams and calculate the fraction of phases at a given composition and temperature.
4.8.2 Ternary and Quaternary Phase Diagrams
So far we have dealt with relatively simple binary phase diagrams however there are also much more complicated ternary and quaternary phase diagrams. These types of phase diagrams are crucial, particularly in the field of ceramics. We can perform the same type of analysis on these diagrams but of course it becomes more complex.
4.9 Stable vs. Metastable Phase Boundaries: Cahn-Hilliard Spinodal Decomposition
We just touched upon the concept of stability and metastable with martensite. So let us define the conditions for stability . For a closed system at constant temperature and pressure the Gibbs free energy is minimized with respect to fluctuations in other extensive variables, particularly fluctuations in composition . To be stable against these fluctuations we have an additional condition on the second derivative of the free energy which is given by Le Chatelier’s principle : A system perturbed by a small fluctuation will elicit a thermodynamic driving force to return to the stable equilibrium state . Or in terms of fluctuations in composition the condition for stability is
\begin{equation} \frac{\partial^{2}G}{\partial X^{2}_{B}} > 0 \end{equation}
Let’s take a look at this condition in the graph below
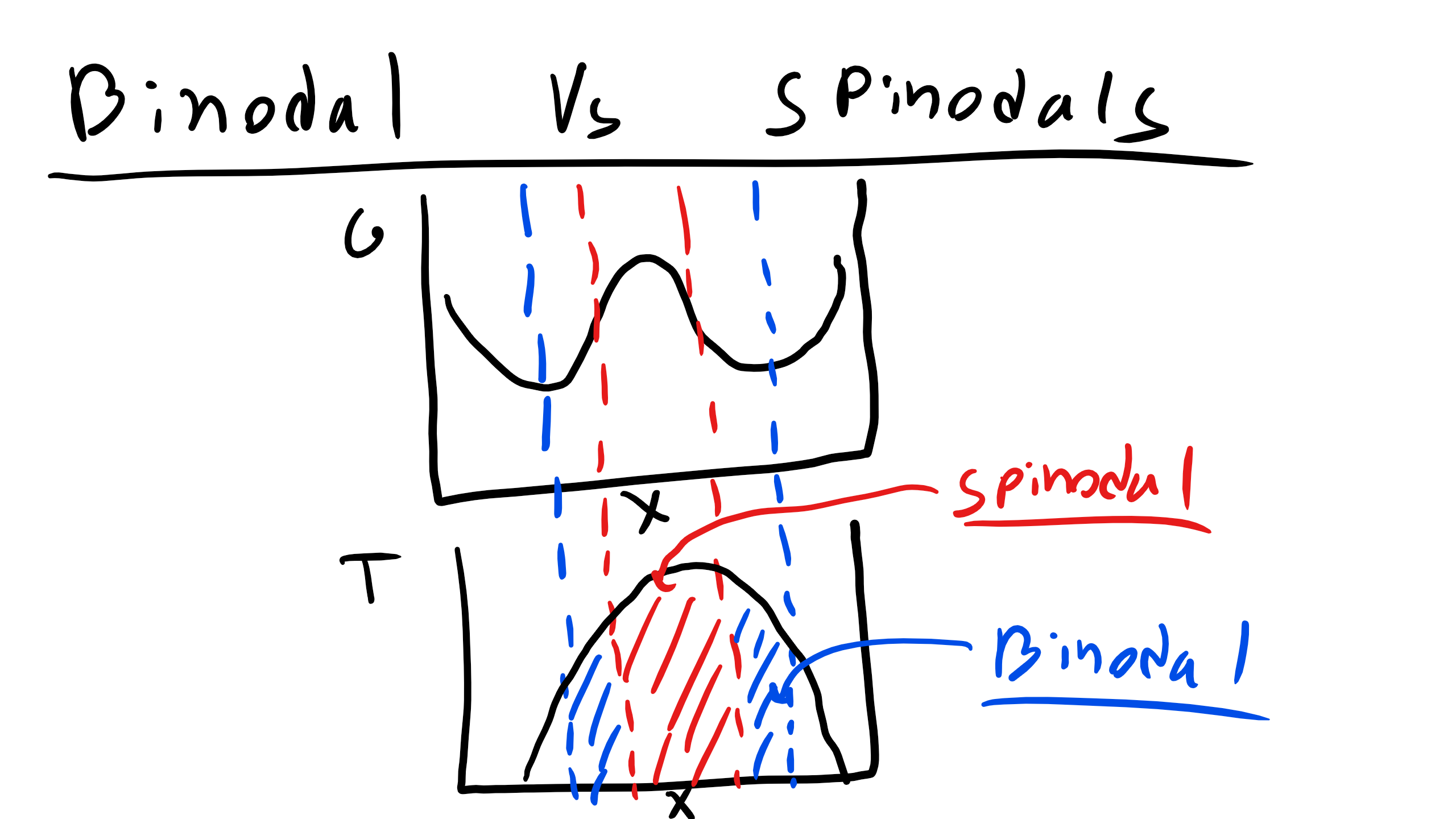
We can clearly see here that we have several special points binodals and spinodals .
Binodals are defined by the following mathematical relations
\begin{eqnarray} \frac{\partial G}{\partial X_{B}} =0\\ \frac{\partial^{2}G}{\partial X^{2}_{B}} > 0 \end{eqnarray}
while spinodals are defined by
\begin{equation} \frac{\partial^{2}G}{\partial X^{2}_{B}} = 0 \end{equation}
We can also see a point in this diagram that is unstable where
\begin{eqnarray} \frac{\partial G}{\partial X_{B}} =0\\ \frac{\partial^{2}G}{\partial X^{2}_{B}} < 0 \end{eqnarray}
The spinodal points will define the spinodal boundary and the binodal points will define the boundaries of the 2-phase regions . Systems that are initially unstable will undergo spinodal decomposition while systems that are initially stable or metastable will evolve via nucleation and mechanisms. Spinodal decomposition is a homogeneous transformation while nucleation and growth is typically dominated by a heterogeneous process.
4.10 First Order vs Second Order Phase Transitions
This discussion brings us nicely to a discussion on the order of phase transformations . So far we have really been focused on first order phase transformations like a crystal melting, water boiling, allotropes, etc, these are all first order phase transformations.
Why is that?
Well when we discuss order we are talking about whether the transformation is accompanied by a discontinuity in a first, second, or higher order derivative of Gibbs free energy .
The s econd order transition s are also called continuous phase transitions because the first derivative of Gibbs is continuous and it is not until there is a second order derivative that a discontinuity is observed. Some examples of second order transitions are ODT (Order Disorder Transitions), glass transitions, superconducting transitions, ferromagnetic to paramagnetic transitions .
We can see how these transitions look like by looking at Gibbs free energy.

Remember from thermo that:
\begin{eqnarray} S = -\bigg(\frac{\partial G}{\partial T}\bigg)_{P,n}\\ C_{p} = -T\bigg(\frac{\partial^{2} G}{\partial T^{2}}\bigg)_{P,n} \end{eqnarray}
Chemistry Homework: Reading the Phase Diagram

What educators are saying
Also included in.

Description
This homework page is included in the lesson: Phase Changes, Phase Diagrams, & Heating/Cooling Curves . This homework page can be used for homework, class practice, or a quiz.
With this homework page, students will practice reading a phase diagram. There are 3 different phase diagrams and 15 questions.
A complete KEY is included.
Objectives:
- Practice reading a phase diagram
- Identify and define the triple point and critical point on a phase diagram
- Relate temperature, energy, and pressure to phases changes
Prior Knowledge:
- Understand the concepts of phases of matter and how they relate to energy
- A good understanding of phase changes related to energy changes, changes in attractive forces, and particle movement and arrangement
This homework is appropriate for grades 9-12 chemistry, or physical science.
Chemistry Corner
**************************************************************************************
Check out these other products that you may be interested in:
States of Matter & the Kinetic-Molecular Theory: Gases
States of Matter and the Kinetic Molecular Theory: Liquids
States of Matter and the Kinetic Molecular Theory: Solids
Phase Changes, Phase Diagrams, & Heating/Cooling Curves
*************************************************************************************
Get TPT credit to use on your Future Purchases!
Go to your “My Purchases” page and click on “Provide Feedback” button. Your feedback is greatly appreciated! Click here for more information.
Become a follower to receive updates about new products as I add them.
LICENSING TERMS: By downloading this product, you own a license for one teacher only for personal use in your classroom. Licenses are non-transferable, meaning they cannot be passed from one teacher to another. No part of this resource is to be shared with colleagues or used by an entire grade level, school, or district without purchasing the proper number of licenses. I you are a coach, principal or district interested in transferable licenses to accommodate yearly staff changes, please contact TpT for Schools at [email protected] or find more information under “Schools” on the Teachers Pay Teachers site.
COPYRIGHT TERMS: ©Chemistry Corner. Please note – all material included in this resource belongs to Chemistry Corner. By downloading, you have a license to use the material, but you do not own the material. This resource, or any portion of this resource, may not be uploaded to the internet in any form, including classroom/personal websites or network drives, unless the site is password protected and can only be accessed by students —no other teachers or anyone else on the internet.
Questions & Answers
- We're hiring
- Help & FAQ
- Privacy policy
- Student privacy
- Terms of service
- Tell us what you think

IMAGES
VIDEO
COMMENTS
Mechanical engineering expert. View the full answer. Previous question Next question. Transcribed image text: 2 Reading Phase Diagrams [10 pts] For each of the following provide: i) The phases present ii) The composition of each phase iii) The relative wt% of each phase a) 15 wt% Sn-85 wt% Pb at 100°C b) 1.25 kg Sn and 1.4 kg Pb at 200°C c ...
This is a chemistry tutorial video that goes through how to read, analyze, and interpret a phase diagram. There are several examples of different questions you might be asked on phase diagrams...
Reading phase diagrams of a two-component system 1. Mark all fields on the phase diagram. 2. Draw the liquidus and solidus lines and mark characteristic points on the phase diagram 3. Draw a cooling curve for the composition. 4.
Chemical Engineering questions and answers. 1 Reading Phase Diagrams [9 pts] For each of the following provide: i) The phases present ii) The composition of each phase iii) The relative wt% of each phase a) 15 wt% Sn - 85 wt% Pb at 100 °C b) 1.88 kg Cu and 2.12 kg Zn at 500°C c) 30 wt% Pb and 70 wt% Mg at 550 °C Composition (at% Sn) 60 0 20 ...
Use this diagram for questions (1) – (9) (1) Label each region of the graph as solid, liquid, or gas. (2) Label the triple point, normal melting point, and normal boiling point on the graph and estimate their values in the spaces below.
A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapor (gas) states of a pure substance.
Phase diagrams illustrate the phases of a system at equilibrium as a function of 2 or more thermodynamic variables. Phase diagrams are also particularly useful because they obey the laws of thermodynamics and there are constraints on the structure of phase diagrams, particularly the Gibbs Phase Rule.
This worksheet assesses students' knowledge of phase diagrams by examining both a generic and specific diagram. A complete answer key is provided at the end. This worksheet can be used in any Chemistry class, regardless of the students' ability level.
With this homework page, students will practice reading a phase diagram. There are 3 different phase diagrams and 15 questions. A complete KEY is included. Objectives: Practice reading a phase diagram; Identify and define the triple point and critical point on a phase diagram; Relate temperature, energy, and pressure to phases changes; Prior ...
Research your phase diagram(s). In Volume 4 of ASM Handbook, locate a binary phase diagram for the two primary constituents of the alloy system(s) you are studying. For example, if you are studying bronze, find the phase diagram for copper-tin alloys.