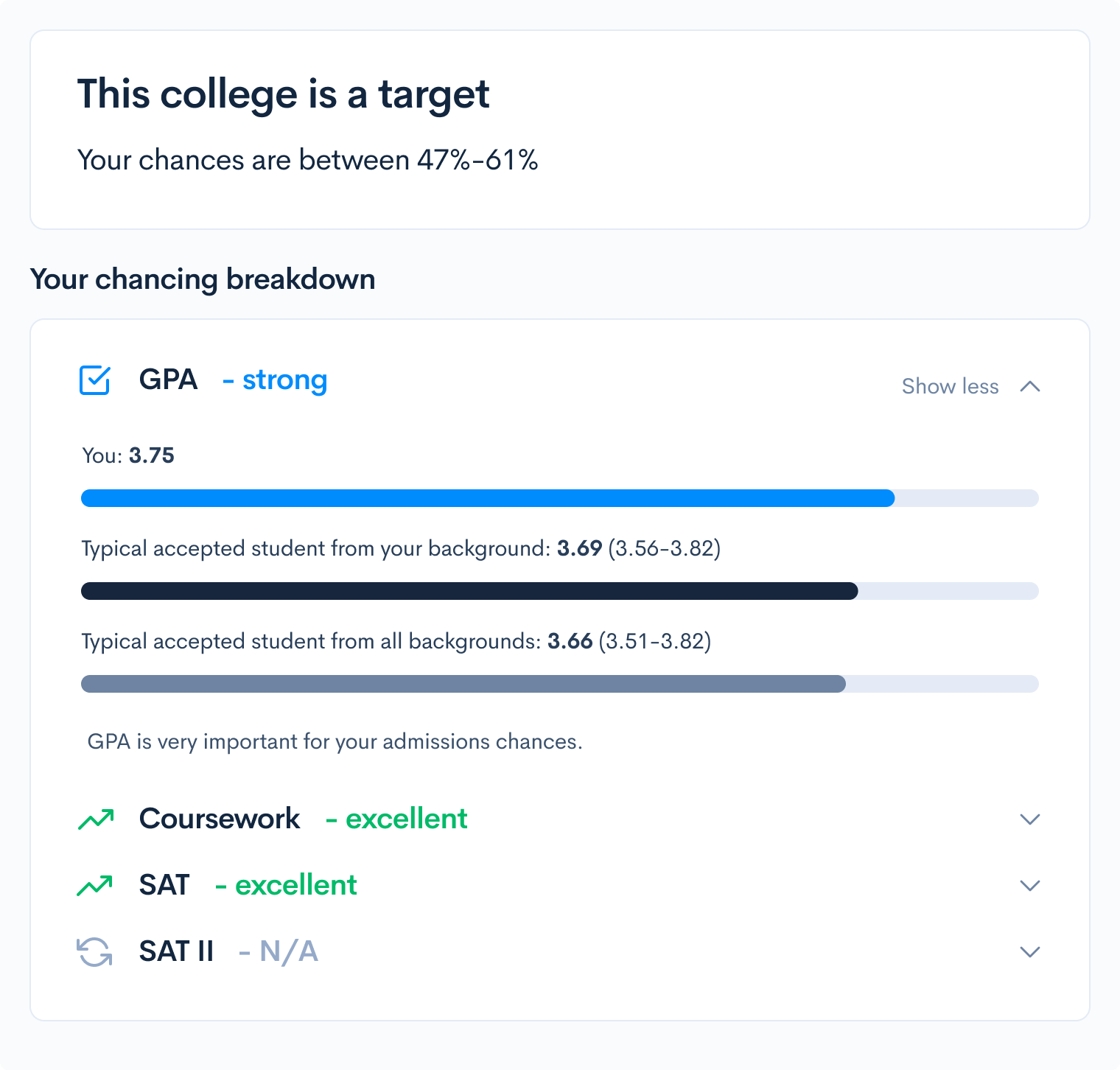
What are your chances of acceptance?
Calculate for all schools, your chance of acceptance.

Your chancing factors
Extracurriculars.
10 Hardest AP Chemistry Exam Questions
Do you know how to improve your profile for college applications.
See how your profile ranks among thousands of other students using CollegeVine. Calculate your chances at your dream schools and learn what areas you need to improve right now — it only takes 3 minutes and it's 100% free.
Show me what areas I need to improve
What’s Covered:
How will ap scores impact my college chances, overview of the ap chemistry exam, 10 hardest ap chemistry questions.
In 2020, 56.1% of test takers passed the AP Chemistry exam with a 3 or higher. As you can see, the AP Chemistry exam is one of the more difficult exams administered by the College Board. But, with the right study tools, such as CollegeVine’s Ultimate Guide to the AP Chemistry Exam , and through lots of practice, you will be more than prepared to pass the exam!
In this article, we will review some of the more difficult questions you may encounter on the exam followed by detailed explanations describing how to solve them.
The score you get on an AP exam, whether it be a 2 or a 5, won’t really impact your chances of admission into a particular college. Most colleges will not even review your scores , especially since you don’t have to send them in if you don’t want to!
College admissions will instead take a more holistic approach when reviewing your college application. Course rigor, GPA, essays, and extracurriculars are more likely to be valued when reviewing your application. When it comes to course rigor, colleges want to see how many APs you take and what grades you get in the classes rather than the exam scores, which are used to determine course credit and placement after you get into college.
If you are interested in understanding how your course load impacts your chances at college, use our free chancing engine . This tool will help you determine your chances of acceptance at over 500 colleges based on your grades, test scores, extracurriculars and demographics. Keep in mind that your essays and letters of recommendation are also important in the college admissions process!
The AP Chemistry exam assesses your ability to understand science practices in relation to four big ideas: scale, proportion and quantity; structure and properties; transformations; and energy. The exam is 3 hours and 15 minutes long and is comprised of two separate sections:
Section I: Multiple Choice Questions
- 60 questions
- A calculator is permitted
- Worth 50% of your exam score
Section II: Free Response Questions(FRQs)
- 3 Long-Answer Questions
- 4 Short-Answer Questions
- 105 minutes
- A calculator is NOT permitted
The AP Chemistry exam is broken up into nine units with the following percentage breakdowns:
- Atomic Structure and Properties (7-9%)
- Molecular and Ionic Compound Structure and Properties (7-9%)
- Intermolecular Forces and Properties (18-22%)
- Chemical Reactions (7-9%)
- Kinetics (7-9%)
- Thermodynamics (7-9%)
- Equilibrium (7-9%)
- Acids and Bases (11-15%)
- Applications of Thermodynamics (7-9%)
In this article, we will be focusing on official CollegeBoard AP Chemistry Exam questions. This means that you can expect to see the same level of difficulty on the exam you take.
CollegeBoard is also known to provide answer options that are meant to confuse you into choosing the wrong answer instead of the correct one. But fear not! We will show you how to carefully read the question and make sure that you are confident in the answer you choose!
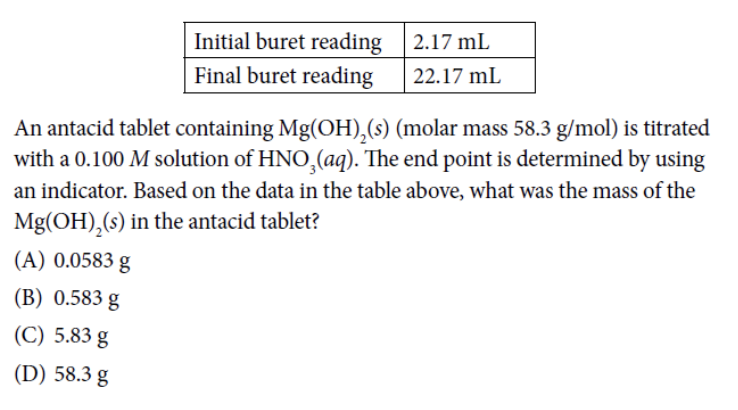
Answer: (A)
In this first question, we see that a base Mg(OH) 2 is being titrated by an acid HNO 3 . In order to determine the mass of the initial base, we need to perform a series of titration calculations. This is a great example of when your labs come in handy, since the lab can help you visualize what is going on.
First, we need to determine the reaction that is occurring when Mg(OH) 2 is reacted with HNO 3 . We first write out the equation we know, which is that the base and acid react to create water and an aqueous solution of the remaining ions:
Mg(OH) 2 + HNO 3 → H 2 O + Mg 2 + + NO 3 –
We now need to balance the above reaction. Hydrogen is already balanced, so the next element we move to is Oxygen. We see that Oxygen is unbalanced with five oxygen on the left and four oxygen on the right. We can balance Oxygen by:
Mg(OH) 2 + 2HNO 3 → 2H 2 O + Mg 2 + + 2NO 3 –
This gives us 8 oxygen molecules on either side. This simple balancing act has now balanced the entire equation with one Mg molecule, 8 O molecules, 4 H molecules and 2 N molecules on either side.
Now we return to the titration problem. Since we know that the acid is being used as the titration solution, we know that HNO 3 is in the buret. We also know that 20 mL is needed to get to the end point since:
22.17 mL – 2.17 mL = 20 mL
Now that we know the molar mass of the base (58.3 grams), the molarity of the acid solution, and the amount of acid needed to titrate the bass and the balanced equation, we can perform a simple stoichiometric equation. Make sure to balance all your units and note that it takes 2 moles of HNO 3 for every one mole of Mg(OH) 2 !
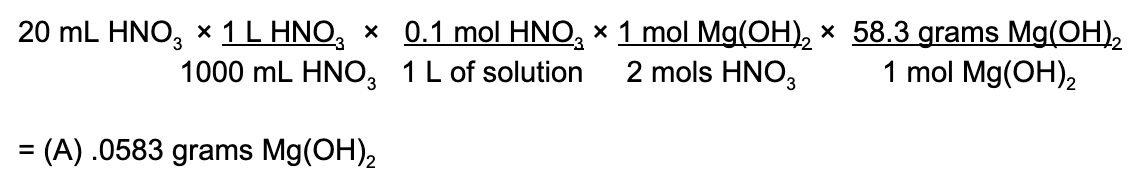
Answer: (E)
Geometry of a molecule or compound is an important topic covered on the AP Chemistry exam so it is important that you know your tetrahedrals from your trigonal bipyramidials! An easy hint is that you can almost always determine what type of shape a compound is based on the number of lone electron pairs and molecules that are attached to the central atom.
That being said, a planar compound is one where the atoms surrounding the central molecule arrange themselves so that they exist on a singular, two-dimensional plane. In order to determine which of these is NOT planar (remember to read extra carefully!!!), we’ll draw out each of the molecules. Make sure to follow the quartet rule when drawing out each of the compounds!
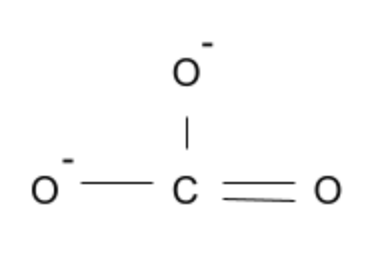
This carbonate ion, while experiencing negative charges on the oxygen, is planar since all oxygens are in the same plane as one another.
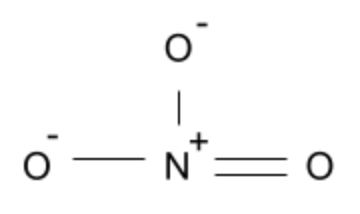
The nitrate ion is also planar since the oxygen atoms are in the same plane as one another and are not impacted by lone pairs (similar to the Carbonate ion).
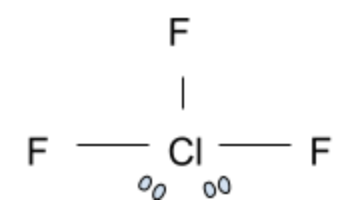
Now this is a tricky compound. At first glance, you would assume that ClF 3 is not planar due to the impact of the two electron pairs present. However, the chlorine trifluoride compound is actually a T-shaped geometrical structure. In this shape, the 3 Fluorine atoms are in the same plane and form a T shape with the Chlorine atom. Surprisingly, this is not the correct answer.
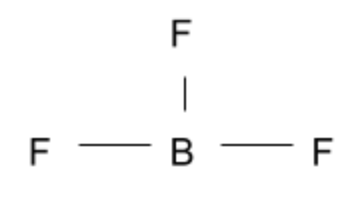
This compound is another simple answer we can cross off since the three Fluorine atoms are in the same plane as one another.
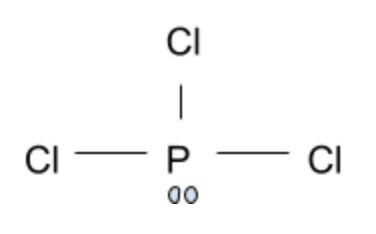
This leaves us with phosphorus trichloride. The electron pair causes a disruption in what would be expected of a planar compound. Each individual chlorine atom experiences great repulsion from the lone electron pair, thereby causing the Chlorine atoms to bend closer together. This gives this compound a trigonal pyramidal shape instead of a planar geometry.
Therefore, the compound that is not planar is (E) PCl 3 .
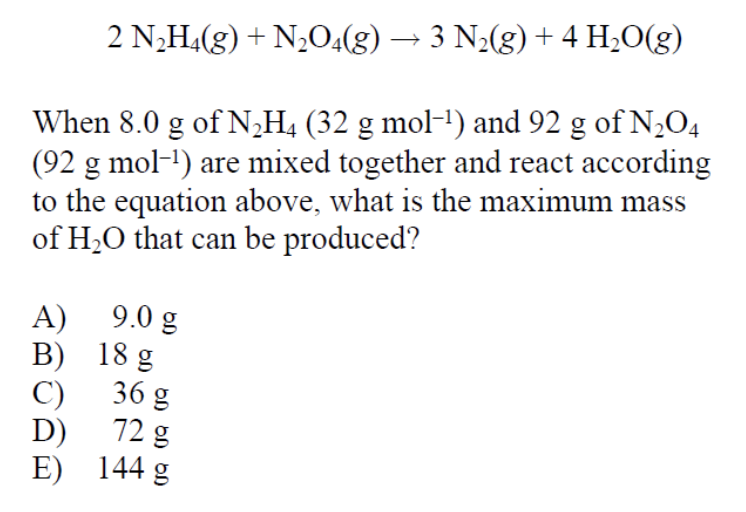
This is a tricky question since we are given the initial mass of both reactants and are asked to find the maximum mass of a product, in this case water. This means this is a limiting reactant problem in which a limiting reactant limits how much a product can be produced.
Let’s perform stoichiometric equations on 8 grams of N 2 H 4 as well as 92 grams of N 2 O 4 to determine which of these reactants is the limiting reactant. But first, always, always, always double check that the provided equation is balanced! (Thankfully in our case, it is).
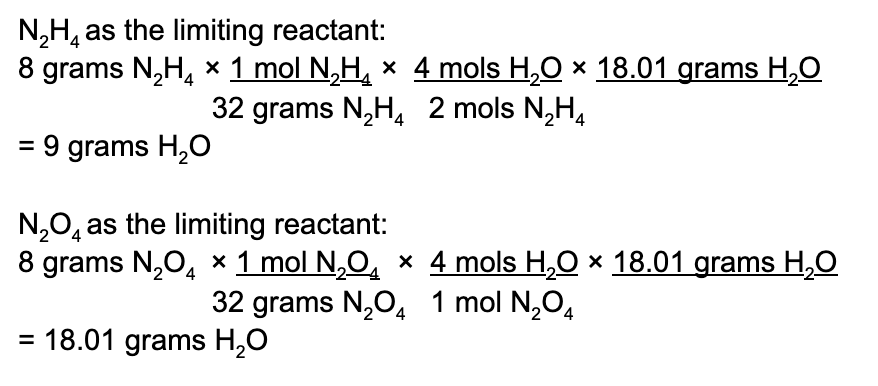
Here we see that while N 2 O 4 may produce the most amount of water, this is an inaccurate representation of what happens. N 2 H 4 acts as a limiting reactant and limits how much water is produced. N 2 O 4 is in excess. Therefore, the maximum amount of water produced is 9 grams.

Discover your chances at hundreds of schools
Our free chancing engine takes into account your history, background, test scores, and extracurricular activities to show you your real chances of admission—and how to improve them.
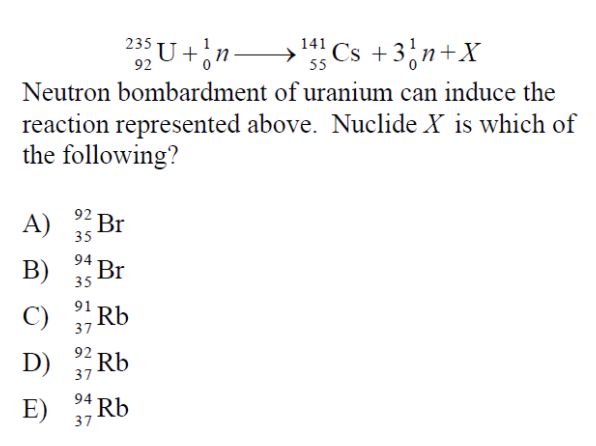
Answer: (D)
This question is difficult since it’s not a problem that is often seen in class. The main thing we need to understand is that regardless of how difficult this question looks, ultimately, it’s another balancing problem!
In this reaction, neutrons are being used to induce a fission reaction. A neutron is the same mass as a proton, however, has no charge (no electrons or protons).
Remember that the upper number next to the element represents the atomic mass while the bottom number represents the atomic number.
To simplify the reaction we can expand the equation to account for the three neutrons that are being displaced:
92 235 U + 1 0 n → 55 141 Cs + 0 1 n + 0 1 n + 0 1 n + ? ? X
This means that we can perform simple subtraction to determine what ? ? X is since the law of conservation of mass must be obeyed:
(92)-(55) (235+1) – (141+1+1+1) X = 37 92 X
By checking a periodic table of elements, we see that Rb has an atomic mass of 92 and an atomic number of 37.
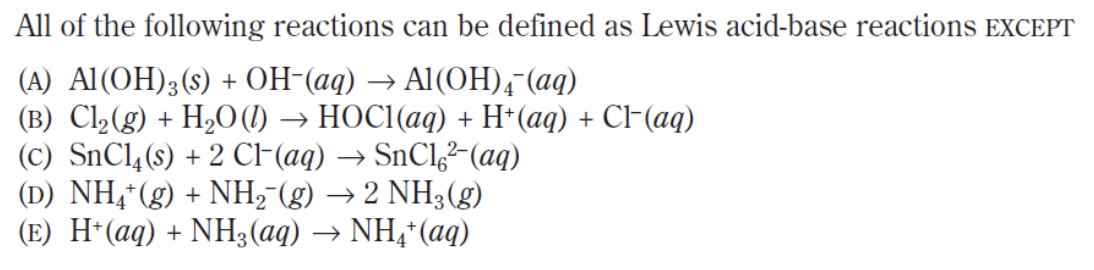
Answer: (B)
This is another EXCEPT question so we have to be extra careful as to what is being asked of us! Remember that a Lewis acid-base reaction is one in which a Lewis-base donates it’s extra pair of electrons to a Lewis acid.
In the five options above, OH – , CL – , NH 2 – and NH 3 have electron pairs that are capable of donating their additional electrons to the Lewis acid AL(OH) 3 , SnCl 4 , NH 4 + and H + respectively. In option (b), H 2 O acts as a nucleophile and binds with Cl 2 and breaks the intramolecular Cl 2 bond. While doing so, the additional hydrogen atom breaks off in order to stay true to the octet rule. In doing so, the reaction fails to create a complete covalent bond between the two molecules.
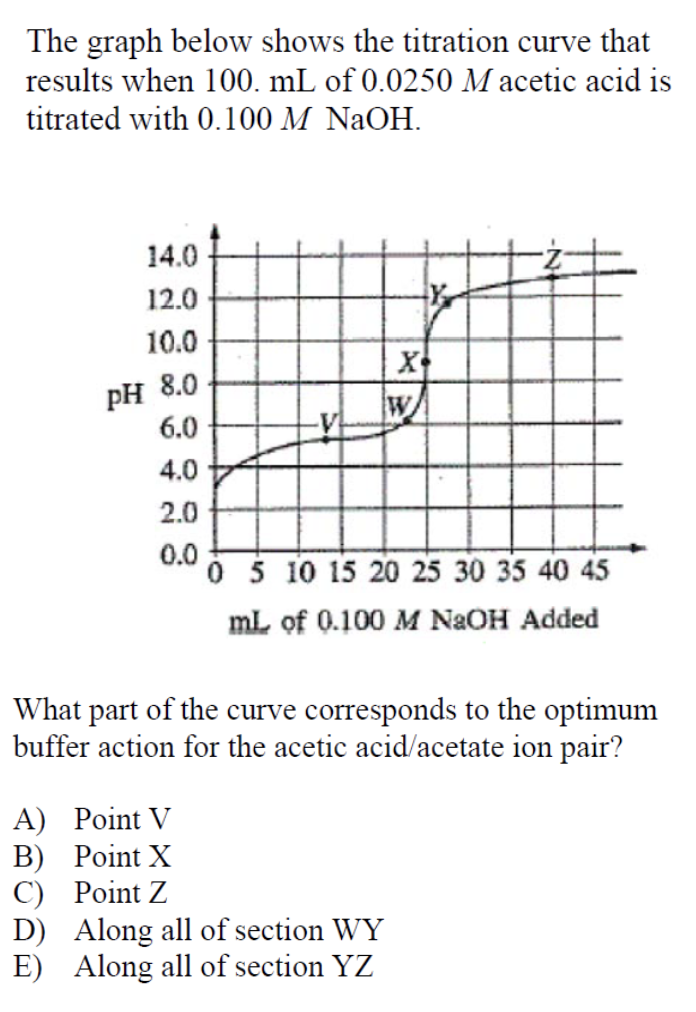
This question is difficult because it requires you to understand how a buffer and titration works and know how to approximate data based on a graph.
The optimum buffer action occurs when the amounts of acid and its conjugate base are approximately equal. In the case of our situation, this happens when half of the weak acid has been neutralized to form its conjugate base.
It may seem like the best answer for this question is point W, the point when there is enough base to neutralize the acid (also known as the equivalence point). But this is incorrect! This point basically neutralizes the entire solution and that’s not what we’re looking for! At this point, the strong base is in excess in relation to the weak acid to guarantee that all of the weak acid is neutralized (as seen by the pH value of 8.0 at point W).
We are looking for the point when the buffer action is optimal, which as we defined before, is when there are equal amounts of acid and its conjugate base. This point occurs approximately midway between the equivalence point (at which the slope of the graph is nearly infinite) and the starting point. In relation to the graph this occurs at point V.

Balancing equations always proves to be time consuming and difficult, especially when performed under pressure. However, it is almost guaranteed that these types of problems will appear on the exam.
This type of question is best solved as reduction and oxidation half reactions To do this, we need to determine which elements are being reduced and which elements are being oxidized. We see that the chromium ion gains electrons, therefore becoming reduced, while sulfur loses electrons when it becomes a solid, therefore becoming oxidized.
Reduction: Cr 2 O 7 – (aq) + H + (aq) → Cr 3+ (aq) + H 2 O(l)
Oxidation: H 2 S(g) → S(s)
Next, we must balance all the atoms in the above equation. We can account for missing Hydrogen ions by adding in protons:
Reduction: Cr 2 O 7 – (aq) + 14H + (aq) → 2Cr 3+ (aq) + 7H 2 O(l)
Oxidation: H 2 S(g) → S(s) + 2H +
From here, we must determine how many electrons are needed to balance out the charges of either side, which we can do by adding 6 electrons to the left side of the reduction equation and 2 electrons the the right side of the oxidation equation:
Reduction: Cr 2 O 7 – (aq) + 14H + (aq) + 6e – → Cr 3+ (aq) + 7H 2 O (l)
Oxidation: H 2 S(g) → S(s) + 2H + + 2e –
The next goal is to cancel out the electrons from either side. We can do this by multiplying the oxidation equation by 3. This then becomes:
Oxidation: 3H 2 S (g) → 3S(s) + 6H + + 6e –
Now we can add the respective equations to get:
Cr 2 O 7 – (aq) + 14H + (aq) + 3H 2 S (g) + 6e – → Cr 3+ (aq) + 7H 2 O (l) + 3S(s) + 6H + + 6e –
The last step is to cancel out any like terms on opposite sides:
Cr 2 O 7 – (aq) + 8H + (aq) + 3H 2 S (g) → Cr 3+ (aq) + 7H 2 O (l) + 3S(s)
Therefore, the coefficient of H + is 8!
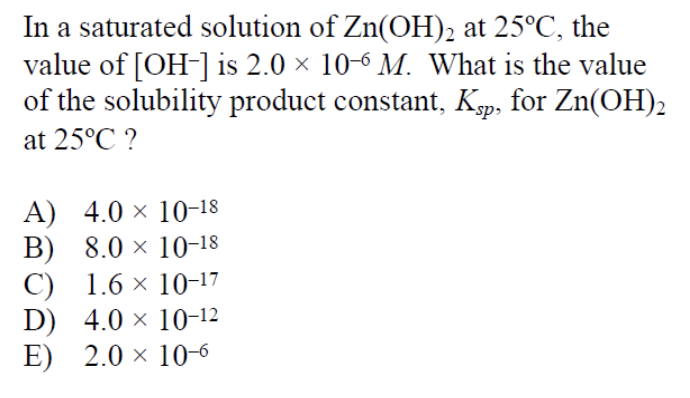
This question requires us to remember concepts of solubility constant as well as how reactions work in order to conserve mass. The first thing we must do is to write out the reaction that is described in the problem statement. The thing that makes this question difficult is writing out the correct reaction. Why you may ask? It’s because the correct equation involves balancing out the charges of the atoms!
Zn(OH) 2 → 2OH – + Zn 2+
We know that K sp is written as the product of the concentrations of the aqueous ions in a solution. Since OH – has a coefficient of 2, we must square the concentration of the base. Therefore:
K sp = [OH – ] 2 [Zn 2+ ]
From here, we are dealing with easy math! We know that the hydroxide ion and Zinc need to dissolve in proportional concentrations. Therefore, we can reduce the equation in terms of x . But, don’t forget to include the coefficient of 2 in front of the hydroxide ion as well!
K sp = [2x] 2 [x]
We are given the concentration of the hydroxide ion as 2.0 x 10 -6 M. This means that:
2x = 2.0 x 10 -6 M
x = 1.0 x 10 -6 M
Plugging these values into the above equation gives us:
K sp = [2.0 x 10 -6 ] 2 [1.0 x 10 -6 ] = 4.0 x 10 -18 M
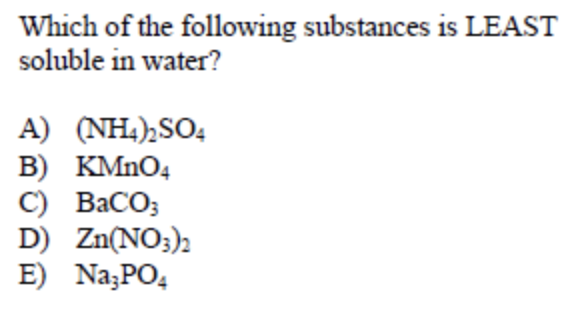
Answer: (C)
This type of question seems difficult to look at since you are immediately presented with numerous compounds you may have not seen before. With this type of question, it is important to remember characteristics about specific compounds such as halides and sulfates.
One fact to remember about carbonates (CO 3 2- ) is that they are most often insoluble. They are especially insoluble when paired with Group II atoms such as Barium, Calcium and Strontium. Therefore, BaCO 3 is the least soluble in water of the groupings.
Question 10

This is another one of those questions where it is extremely helpful if you are able to remember labs shown or conducted in your class. This question also requires you to remember qualitative characteristics of compounds to deduce what ions could be present in an unknown solution.
The initial white precipitate formed corresponds to a chloride precipitate since hydrochloric acid was used to treat the unknown solution. However, we know that BaCl 2 is soluble, so we can deduce that Barium is not in the initial solution since a solid forms after the treatment.
This leaves us with AgCl, HgCl 2 and PbCl 2 as potential precipaties. Since a bright yellow precipitate formed after the introduction of the potassium iodide solution, we know that this is a characteristic of lead iodide. Therefore, lead is present in the solution. This leaves us with answer choice (B) as the right answer.
To verify that B is correct, we must determine that silver is also present in the unknown solution. We know this to be true because silver easily dissolves in ammonia, as described in the problem statement.
As you prepare for this exam, remember to:
Know Your Labs!
Many of the multiple choice questions and free response questions you’ll come across will describe lab scenarios. These scenarios may have been ones you have previously seen in class or in a video. It’ll be easier for you to understand and correctly answer the question at hand if you are able to visualize what is being described based on something you have seen previously in class!
Practice, Practice, Practice!
You may know the concepts very well, but you also need to be able to apply the concepts in a timed setting. Make sure you take multiple practice tests administered through CollegeBoard or through your school. You might be able to answer every question on the test perfectly, but you need to be sure that you can finish the multiple choice section in 90 minutes! Remember, that leaves just about one and a half minutes to read the question and determine the answer. Over time, you will get better at quickly answering questions and determining what the question is asking for on your first try!
The Night Before…
Review your important equations! Know what each equation on the formula sheet means. It’s one thing to know what the variables on the equation sheet mean, but it’s another to know why you would use an equation and how it can help you solve any question at hand.
People will always tell you to get a good eight hours of sleep and eat a healthy breakfast. While this may seem like basic info that is not relevant to the test material you are memorizing, it is important that you are awake and ready to sit through a three hour exam!
Related CollegeVine Blog Posts


G.C.E. Advanced Level (A/L) Chemistry Past Papers and Answers

Advanced Level Chemistry MCQ 1984
Advanced level chemistry mcq 1985, advanced level chemistry mcq 1986, advanced level chemistry mcq 1987, advanced level chemistry mcq 1988, advanced level chemistry mcq 1989, advanced level chemistry ii 1989, advanced level chemistry mcq 1990, advanced level chemistry ii 1990, advanced level chemistry mcq 1991, advanced level chemistry ii 1991, advanced level chemistry mcq 1992, advanced level chemistry ii 1992, advanced level chemistry mcq 1993, advanced level chemistry ii 1993, advanced level chemistry mcq 1994, advanced level chemistry ii 1994, advanced level chemistry mcq 1995, advanced level chemistry ii 1995, advanced level chemistry mcq 199 6, advanced level chemistry ii 1996, advanced level chemistry mcq 1997, advanced level chemistry ii 1997, advanced level chemistry mcq 1998, advanced level chemistry ii 1998, advanced level chemistry mcq 1999, advanced level chemistry ii 1999, advanced level chemistry mcq 2000, advanced level chemistry ii 2000, advanced level chemistry mcq 2012, advanced level chemistry ii 2012, a dvanced level chemistry 2012 marking scheme, advanced level chemistry mcq 2013, advanced level chemistry ii 2013, a dvanced level chemistry 2013 marking scheme, advanced level chemistry mcq 2014, advanced level chemistry ii 2014, a dvanced level chemistry 2014 marking scheme, advanced level chemistry mcq 2015, advanced level chemistry ii 2015, a dvanced level chemistry 2015 marking scheme, advanced level chemistry mcq 2016, advanced level chemistry ii 2016, a dvanced level chemistry 2016 marking scheme, advanced level chemistry mcq 2017, advanced level chemistry ii 2017, a dvanced level chemistry 2017 marking scheme, advanced level chemistry mcq 2018, advanced level chemistry ii 2018, a dvanced level chemistry 2018 marking scheme, advanced level chemistry i 2019 | new & old syllabus, advanced level chemistry ii 2019 | new & old syllabus, advanced level chemistry 2019 marking scheme | new & old syllabus, advanced level chemistry i 2020 | new & old syllabus, advanced level physics ii 2020 | new & old syllabus, advanced level chemistry 2020 marking scheme | new & old syllabus, advanced level chemistry i 2021, advanced level chemistry ii 2021, advanced level chemistry 2021 marking scheme, related posts.
- Aptitude Tests (33)
- Architecture (10)
- Articles (1)
- B.Design (6)
- Biology Marking (14)
- Biology Marking (English Medium) (5)
- Biology Notes (5)
- Biology Papers (45)
- Biology Papers (English Medium) (5)
- Chemistry Marking (19)
- Chemistry Marking (English Medium) (7)
- Chemistry Notes (16)
- Chemistry Papers (54)
- Chemistry Papers (English Medium) (5)
- Colombo Uni (5)
- Combined Maths Marking (14)
- Combined Maths Marking (English Medium) (3)
- Combined Maths Notes (5)
- Combined Maths Papers (97)
- Combined Maths Papers (English Medium) (21)
- Fashion Design (13)
- General English (5)
- German Tech (2)
- GIT Notes (10)
- GIT Papers (8)
- Higher Mathematics (7)
- ICT Marking (11)
- ICT Marking (English Medium) (3)
- ICT Notes (37)
- ICT Papers (11)
- ICT Papers (English Medium) (9)
- Irrigation Technology (1)
- Japura Uni (1)
- Kelaniya Uni (9)
- Model Papers (67)
- Moratuwa Uni (7)
- NDES / TTI (17)
- Nursing (3)
- Ocean Uni (1)
- Open Uni (2)
- Palath Papers (96)
- Physical Science (2)
- Physics Marking (18)
- Physics Marking (English Medium) (6)
- Physics Notes (5)
- Physics Papers (49)
- Physics Papers (English Medium) (5)
- Rajarata Uni (4)
- Resource Books (4)
- Resource Books (English medium) (4)
- Sabaragamuwa Uni (1)
- Scholarships (1)
- School Papers (138)
- UNIVOTEC (5)
- Uva Uni (15)
- Vidyapeeta (1)

Choose Your Test
- Search Blogs By Category
- College Admissions
- AP and IB Exams
- GPA and Coursework
Every AP Chemistry Practice Test Available: Free and Official
Advanced Placement (AP)

What's the best way to study for AP Chemistry? Practice, practice, practice. This article will provide you with links to every practice test and quiz for AP Chemistry that's available online , including full official and unofficial tests, shorter quizzes that cover each topic area, and other prep services you can access with a subscription!
Official AP Chemistry Practice Exams
Official exams are the best practice materials because they help you make accurate predictions of your performance on the real test. They will also get you used to the test format so that you're not caught off guard by the structure of the final exam.
Unfortunately, for AP Chemistry, most of the available official practice materials are for the old version of the test (pre-2013), but these can still be useful for practice. You should be able to get newer practice tests from your teacher or through review books.
I'd recommend starting with the unofficial practice materials listed later on in this article and then using official tests in the final stages of your studying. That way you'll be in the best position to estimate your ultimate AP score, and you won't squander limited resources.
Old Official Released Exams:
- 2008 AP Chemistry Exam
- 2002 AP Chemistry Exam (multiple choice only)
- 1999 AP Chemistry Exam
- 1994 AP Chemistry Exam
These official exams come from before 2013 (when significant changes were made to the AP Chemistry curriculum), so they're formatted slightly differently from the current test. They have 75 multiple-choice questions (there are now 60) and six free-response questions (there are now seven). There are also five answer choices for each multiple-choice question, whereas now there are only four.
The old AP Chemistry exam emphasized calculations and factual knowledge over a strong understanding of fundamental concepts and mastery of scientific practices. The questions on these tests will still help you practice your skills; just make sure you also use more recent materials for an accurate preview of what to expect on test day.
Current AP Chemistry Course and Exam Description (multiple choice practice included)
Go to page 216 of this course description to review sample multiple-choice and free-response questions for the current exam. This is not a full practice test (it only has 15 multiple-choice questions and two free-response questions total), but it's directly from the College Board, so it's the most accurate and up-to-date representation of the format and content of the test.
Free-Response Questions 2014-2021
These free response questions are from the most up-to-date version of the test. I would advise you to save most of them for later on in the year when you're more serious about practicing for the real AP exam. There are seven questions from each year.
Practice Tests from Your Teacher
Since there aren't any full AP Chemistry practice tests available online that reflect the current format of the exam (well, any that we can legally link to in this blog post), you can also ask your teacher for additional practice materials. AP teachers often have access to extra practice tests from the College Board that are available for classroom use.

Unofficial Free AP Chemistry Practice Exams
There are also a bunch of unofficial resources for AP Chemistry practice questions on various online learning platforms and independent sites. Few of these offer complete tests in the same format as the real exam, but they do provide a large repository of practice questions (mainly multiple-choice). These are great if you're looking for questions in specific topic areas or are studying early on in the year and want to avoid certain concepts that you haven't learned in class yet.
Just be wary of using these resources too much in your studying, and make sure you supplement them with official College Board materials at regular intervals. Unofficial practice questions often lack many of the nuances of real test questions. In a lot of cases, they will test straightforward factual recall whereas on the real test you'll have to do more complex analyses of unfamiliar experimental scenarios.
Varsity Tutors Diagnostic Tests
There are six diagnostic tests here with 50-60 questions each at varying difficulty levels. You'll also be timed as you take the tests so you can get a better sense of your pacing. Questions are multiple-choice only , so this won't give you any free-response practice. There are also tons of practice quizzes divided by topic, so you can use those to tackle tricky subject areas. We'd also recommend trying out their AP Chemistry practice app (it's free).

Albert Quizzes
This site includes quizzes for each concept broken down according to the major units of the course. This site will track your progress and tell you what percentage of questions you got right from each difficulty level (questions are organized into easy, medium, and hard categories). You can also access additional questions, including free-response, if you pay $25 to set up an account.
ScienceGeek
Here you'll find tons of review questions and activities, with lengthy practice quizzes for each unit of the course. This is one of the few resources that has non-multiple-choice questions that you can check automatically online.
This website offers 46 different multiple-choice practice tests (each is quite a bit shorter than the actual exam), and lots more downloadable practice questions organized by topic. You’ll be able to see whether or not you’ve answered correctly, as well as explanations of each question and answer.
PracticeQuiz
This quiz includes 58 free AP Chemistry practice multiple-choice questions.
There's a lot of stuff here, but if you're just looking for practice tests, you can find them at the end of the list of resources for each unit. There are multiple-choice and free-response tests for most units with accompanying answer keys.

Unofficial Paid/Subscription AP Chemistry Practice Exams
Here are some additional resources that will cost you some money, but they might be worth it because they provide full properly-formatted AP Chemistry practice tests.
Peterson's ($39-$49 per month)
- Two full-length practice tests (up to date format and content)
- Answer explanations
- Automatically tells you what you still need to study based on your results
- Also includes test prep for other AP exams
Sterling Test Prep (price varies)
On this site, you can buy individual practice tests for each topic in AP Chemistry. All of them together cost almost $100, so that might not be feasible, but you can get each specialized practice test for about $3 each (most have around 60 questions). You can also just get the Sterling book of practice questions , which many students seem to find helpful.
Review Books (price varies)
Review books can be great resources because many of them include instructions for how to structure your studying in addition to focused content overviews. For AP Chemistry, we recommend the 5 Steps to a 5 and Crash Course books. You can click on the link in the title of this section to read my full article on the best review books for this course.
Barron’s AP Chemistry , and The Princeton Review AP Chemistry Prep book are also excellent resources. In addition, each of these includes several different practice tests you can use to help you be prepared for test day!

How to Use AP Chemistry Practice Tests
Practice tests are great study tools for AP tests, and they're especially helpful for a subject like Chemistry that involves a lot of calculations and experimental analysis. In the next couple of subsections, we'll tell you how to use practice tests throughout the school year to prepare for the AP Chemistry exam.
First Semester: Using Practice Tests for Your Class
It's not practical to take full practice tests during the first semester of AP Chemistry because you haven't covered enough of the course material yet. Focus on official free-response questions and unofficial topic-specific practice tests that address aspects of the curriculum that you've learned already. It's a great idea to start early and do consistent reviews so that your knowledge base remains strong throughout the year.
Since chemistry is a subject that builds on the fundamental concepts learned in the first few months of class, it's vital that those early lessons are solidified in your memory. This way, more complex material that you learn in the second semester won't fly over your head. You can also consider getting a prep book; most of them have practice questions organized by chapter for selective review of different concepts.
Second Semester: Preparing for the AP Test
During your second semester, you can start to take full practice tests to predict your AP score-range. At this point, you've learned most of the material that will be covered in the class, so your scores should accurately reflect your abilities. Every time you take a full practice test, keep track of the areas where you need more practice.
As we mentioned at the beginning of this article, we would recommend saving the most up-to-date official practice materials for later in the semester so that the format of the current test stays fresh in your mind. As you take each test, circle any questions where you were unsure about your answer. Even if your choice ends up being correct, you should still plan to go over these concepts, so you don't feel shaky about them on the real AP test.
After you've finished taking the test (with realistic time constraints!), categorize your mistakes by topic area, and use their distribution to inform the rest of your studying. The purpose of taking practice tests is to diagnose your weaknesses so you can address them as efficiently as possible. DON'T go from one test to the next without taking a deeper look at what went wrong! You'll end up wasting your time, and your second practice test is unlikely to demonstrate much improvement.
Spend at least a couple of hours after each practice test doing practice problems and reviewing concepts that you didn't quite understand when they came up on the test. When you feel satisfied that you have a better handle on the background information and solution methods, you can take a second practice test to see how much you've improved.
The process as a whole should work like this:
- Take and score first practice test (4 hours)
- Evaluate mistakes (1.5 hours)
- Practice problems and study content to improve weak areas (2.5 hours)
- Take and score second practice test (4 hours)
- Reevaluate your progress and repeat steps if necessary!
One cycle through all of these steps will take around 8-10 hours, but you can repeat the steps ad infinitum until you're satisfied with your scores. If you find that you're not improving between practice tests, you'll need to reevaluate your study strategy. To master a complex subject like chemistry, you need to have a strong grasp of the fundamental concepts. Then, you can build on that understanding for more difficult problems. Be sure to do lots of practice problems where you're required to justify your answers!

Practice tests are essential study tools, especially for AP Chemistry. Doing practice problems that align with the format and content of the real exam will help you to gain familiarity with the material and feel less stressed on test day.
Try to start your studying with unofficial practice tests to build up a strong knowledge base, and then move onto official practice tests when you're ready to estimate your real AP score level.
As you take practice tests, assess your mistakes and plan out your study time according to which areas need the most work. Make sure you start with basic concepts and then work your way up to more complex problems. Use these practice materials to detect gaps in your knowledge, and fill them before you take the test!

What's Next?
Want to learn a bit more about the test before you start practicing? Read our expert guide to the AP Chemistry exam , which includes sample questions and study tips!
If you want a complete overview of the concepts that will be covered on the test, check out our ultimate study guide for AP Chemistry . We also have a specific guide to balancing chemical equations , if that's something you need extra help with.
Wondering how you can see chemistry in action in your day-to-day life? If you're looking for chemistry you can taste, we recommend this article on vegetable oil substitutes . If you're thinking more along the lines of something to play with, we have three different recipes for homemade slime . And if you need to clean things up afterwards, be sure to read our article on muriatic acid and how to safely use it .
These recommendations are based solely on our knowledge and experience. If you purchase an item through one of our links, PrepScholar may receive a commission.
Trending Now
How to Get Into Harvard and the Ivy League
How to Get a Perfect 4.0 GPA
How to Write an Amazing College Essay
What Exactly Are Colleges Looking For?
ACT vs. SAT: Which Test Should You Take?
When should you take the SAT or ACT?
Get Your Free

Find Your Target SAT Score
Free Complete Official SAT Practice Tests
How to Get a Perfect SAT Score, by an Expert Full Scorer
Score 800 on SAT Math
Score 800 on SAT Reading and Writing
How to Improve Your Low SAT Score
Score 600 on SAT Math
Score 600 on SAT Reading and Writing
Find Your Target ACT Score
Complete Official Free ACT Practice Tests
How to Get a Perfect ACT Score, by a 36 Full Scorer
Get a 36 on ACT English
Get a 36 on ACT Math
Get a 36 on ACT Reading
Get a 36 on ACT Science
How to Improve Your Low ACT Score
Get a 24 on ACT English
Get a 24 on ACT Math
Get a 24 on ACT Reading
Get a 24 on ACT Science
Stay Informed
Get the latest articles and test prep tips!

Samantha is a blog content writer for PrepScholar. Her goal is to help students adopt a less stressful view of standardized testing and other academic challenges through her articles. Samantha is also passionate about art and graduated with honors from Dartmouth College as a Studio Art major in 2014. In high school, she earned a 2400 on the SAT, 5's on all seven of her AP tests, and was named a National Merit Scholar.
Ask a Question Below
Have any questions about this article or other topics? Ask below and we'll reply!
- Chemistry Essay Writing Guide for Students:...
Chemistry Essay Writing Guide for Students: Topics, Outline, Questions
The modern world considers chemistry to be the science of existence and life. This science and its wonders are evident in our daily lives. Chemistry scholars handle crucial experiments and create numerous write-ups throughout their courses. They also develop solutions to daily life issues, and their works serve people from every background.
Chemistry essay questions are marvelous for those who know a little about the subject, but they can be reasonably compound for anyone unfamiliar. Fortunately, this detailed guide covers the crucial areas when preparing for this paper, including the critical research areas, topic suggestions, and practical writing tips.
Interesting Chemistry Research Areas
Chemistry isn't only limited to the periodic table elements or the study of acid dissolution. Choosing an exciting research area that can reveal your innovative approach to lab work or study is advisable.
Here are the main areas of chemistry to explore:
- Nanochemistry – This cool and relatively new research area comprises nanoscience and chemistry. These cover genome and synthesis studies and building blocks. It's applied in military weapons and carbon nanotubes.
- Biomolecular Research – Also called a high-throughput screening system, this challenging field of science entails antibody identification, drug discovery, and examinations of genes that could facilitate breakthrough solutions. The task is easier when you follow a clear structure and support every challenging experiment with citation.
- Organic Chemistry – This is the scientific study of carbon-containing organic compounds' composition, structure, and various properties. This branch of chemistry is relevant to dentists, chemical engineers, veterinarians, and experts working with living organisms.
- Analytical Chemistry – This chemistry field is a safe bet for analyzing various compounds and their attributes. Most college or uni professors recommend that students select a single chemical property in their writing assignments. However, notable chemistry essay examples like volumetric analysis research allow you to show an element's state using several equivalence measure points.
- Biochemistry – This term may sound vague, but its supremacy lies in the excellent coverage of diverse subjects like healthcare, environmental protection, opioid usage, and genetics. The field is especially relevant thanks to the vast helpful topics covered and exploration of Biological reactions.
Examples of Chemistry Essay Topics
Your essay topic is arguably the most crucial segment of your chemistry paper. It keeps your chemistry answers focused while also guiding your audience through your arguments. When your professor hasn't issued a topic suggestion, creating your fascinating topic can be challenging.
Here are some topic suggestions to guide you:
- An Evaluation of Compounds That Cause Specific Food Allergies
- The Influence of Plastic Packaging On Food
- The Chemistry Behind Dental Fillings
- Analyzing The Use of Sugars in Batteries
- Unmasking The Chemistry in Liquid Bandages
- Pheromones – How Do They Affect Humans
- Plastic Packaging – Analyzing Its Impact On the Environment
- What's The Role of Chemistry in Genetic Medicine?
- Comparison Between Branded and Generic Drugs
- Lava Rocks – An Overview of Its Chemical Composition
- Vitamin Deficiency in Human Beings – What Are the Causes and Consequences?
- The Chemistry Behind Diet Soda and Its' Impact On Human Bone Structure
- How to Counter Electrodes in Solar Cell Applications
- An Evaluation of Preservatives in Carbonated Drinks
- The Effects of Acid Rain On Plants
Write a Perfect Chemistry Essay Outline
How do you write a chemistry essay perfectly? The answer lies in the outline. To write exceptionally, you must explain every concept in your paper. A perfect essay outline will keep the ideas in a logical flow and limit reader confusion.
Generally, a chemistry essay outline comprises the following three key elements:
Essay Introduction
Your essay intro should provide a general overview of your essay's content through the thesis statement. You don't have to explain your points in detail, but make sure you convey the information.
Begin with a relevant disturbing point, controversial fact, or an attractive quote to grab the reader's attention
Before starting your chemistry essay body, evaluate the arguments you intend to make and choose the most vital points. This positively impacts your assignment and showcases your proficiency.
Every paragraph should start with the main point followed by supporting evidence without diverting from the topic. Also, explain each point exhaustively to convince your readers to adopt your viewpoint and use the appropriate citations.
Typically, this section shares your final thoughts regarding the subject of discussion, and you're free to give your personal opinion. With a chemistry essay, you must base your final thoughts on accurate results based on lab experiments. But if there's no experiment involved, you can summarize the main points. Avoid repetitions or including new information in your conclusion.
Practical Tips for Successful Research and Writing
Consider these tips if you wish to create a winning chemistry essay:
Follow the Complete Outline
Use a framework that includes an arguable thesis statement that supports or criticizes a particular scientific method or makes an assumption. Maintain a structured plan, and avoid including too many ideas in one assignment. Outlining the main points first can help you get it right with the outline.
Consider Format Clarity and Transitions
Make the structure clear and use transitional phrases to maintain flow. Begin the paragraphs with a topic sentence that sums up the chemistry paper's main idea.
Avoid Colloquial Language and Personal Pronouns
Deliver your content in an active voice and keep the assignment structured from the intro section with a hook sentence to your conclusory remarks, which should comprise an experiment outcome and a call to action.
Use Reliable Sources
Pick content and inspiration from authoritative sources and avoid exact phrasing. Instead, paraphrase the information and deliver it in your own words. Otherwise, you may submit plagiarized content, and your professor won't be impressed with this.
Include Relevant References and Visual Materials
If possible, add visual materials to your essay and include appendix information.
How to Get Chem Help for All Your Assignments
The above tips can help you write great chemistry essays. However, it won't be as cut and dry, especially if you're doing it for the first time or don't feel confident in your prowess on the subject. The challenges have led many to ask, "is there an app to solve chemistry problems?"
Well, several applications can help you learn chemistry, but none will guarantee to provide correct answers to every question or create high-scoring essays. Instead, you can hire academic writing professionals like us to guide you and help you with the most complex chemistry questions.
This way, you'll be sure of plagiarism-free assignments and professional assistance from experienced writers. You're also guaranteed quality, reliability, security, and confidentiality. So reach out to us today.

Need help with an assignment , essay, or online class ?

Sims 4 - How to Do Homework Guide Updated in 2021
The modern world considers chemistry to be the science of existence and life. This science and its wonders are evident in our daily lives. Chemistry scholars handle crucial ex...

Khan Academy Answers: Tips, Tricks and Important Hacks

What Is LockDown Browser? Everything You Need to Know
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar
Student Sri Lanka Education
Courses, Classes, Jobs, O/L A/L Exams, Universities
A/L Chemistry Vivarana books by Ranga Gunarathna
November 9, 2021 By Hiran 1 Comment
Ranga Gunarathna has published a series of Chemistry vivarana (Analysis) books. These books contain complete examination papers (MCQ, structured essay and essay questions) and answers (marking schemes), explanations and answers to those questions.
You can order these books online from daraz .
- Questions, Answers and Analysis
- self-study books
- Includes additional MCQ papers
- Compiled by Ranga Gunarathna
- Complete Exam paper with Answers (Marking scheme), analysis of questions with clear explanations
A/L Chemistry Past Paper vivarana books
2021 A/L vivarana – coming soon
2020 A/L vivarana
2018- 2019 A/L vivarana
2016 – 2017 vivarana
2014-2015 vivarana
2011-2012-2013 vivarana
2006-2010 vivarana

Chemistry books for A/L students
Share this:
- Click to share on Twitter (Opens in new window)
- Click to share on Facebook (Opens in new window)
No related posts.
Reader Interactions
September 26, 2023 at 8:57 pm
Please add English medium chemistry books, specially past papers vivarana

Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Notify me of follow-up comments by email.
Notify me of new posts by email.

Chemistry Paper 3 WASSCE (PC), 2021
- Subject Home
| Menu |
2. The following tests were carried out on solid C . Complete the table.
|
|
|
|
| (a) | + distilled water, stirred and filtered | partly soluble | …………………. |
(b)(i)
(ii)
(iii)
(iv)
(v) | A portion of the filtrate +
A portion of the filtrate +
A portion of the filtrate + A portion of the filtrate + A portion of the filtrate + | White (chalky) precipitate
…………………………..
………………………….. …………………………..
…………………………..
………………………….. |
………………….
………………….
…………………………..
………………………….
NO - present
|
(i)
(ii) |
Residue from (a) above + HCl
Gas form (i) bubbled through lime water |
Effervescence of colourless, odourless gas
………………………………..
|
……………………….
………………………. |
| A portion of the solution |
……………………………. |
……………………. Zn present |
[21 marks]
Observation
This question tested the knowledge of qualitative analysis. Majority of the candidates responded to this question. However, their performance was below average.
In part (a), majority of the candidates could not write correct inference from the given observation.
In part (b), majority of the candidates could not write correct observations and inferences.
In part (c), majority of the candidates could not confirm the gas when bubbled through lime water.
In part (d), majority of the candidates could not write correct observations and inferences.
The expected answers include:
| Year | |
|---|---|
| A/L 1980 | |
| A/L 1981 | |
| A/L 1981 E | |
| A/L 1982 | |
| A/L 1983 | |
| A/L 1984 | |
| A/L 1985 | |
| A/L 1986 | |
| A/L 1987 | |
| A/L 1988 | |
| A/L 1989 | |
| A/L 1990 | |
| A/L 1991 | |
| A/L 1992 | |
| A/L 1993 | |
| A/L 1994 | |
| A/L 1995 | |
| A/L 1996 | |
| A/L 1997 | |
| A/L 1998 | |
| A/L 1999 | |
| A/L 2000 | |
| A/L 2001 | |
| A/L 2002 | |
| A/L 2003 | |
| A/L 2004 | |
| A/L 2005 | |
| A/L 2006 | |
| A/L 2007 | |
| A/L 2008 | |
| A/L 2009 | |
| A/L 2010 | |
| A/L 2011 | |
| A/L 2012 | |
| A/L 2013 | |
| A/L 2014 | |
| A/L 2015 | |
| A/L 2016 | |
| A/L 2017 | |
| A/L 2018 | |
| A/L 2019 | |
| A/L 2019 Model | |
| A/L 2020 | |
| A/L 2021 |
| Lesson | |
|---|---|
| රසායනික ගණනය කිරීම් - Chemical Calculations | |
| ඔක්සිකරණය සහ ඔක්සිහරණය - Oxidation & Reduction | |
| පරමාණුක ව්යුහය - Atomic Structure | |
| රසායනික බන්ධන - Chemical Bonding | |
| පදාර්ථයේ වායු අවස්ථාව - Gas state of matter | |
| ශක්ති විද්යාව - Energetics | |
| ආවර්තික ගුණ විචලනය - Periodic trends | |
| අකාබනික රසායනය : s-ගොනුව - Inorganic Chemistry : s-block | |
| අකාබනික රසායනය : p-ගොනුව ( Inorganic Chemistry : p-block) | |
| අකාබනික රසායනය : d-ගොනුව ( Inorganic Chemistry : d-block) | |
| අකාබනික සංයෝගවල ගුණාත්මක විශ්ලේෂණය - Qualitative analysis of inorganic compounds | |
| කාබනික රසායනයේ මූලික සංකල්ප - Basic concepts of Organic Chemistry | |
| හයිඩ්රෝකාබන - Hydrocarbons | |
| ඔක්සිජන් අඩංගු කාබනික සංයෝග - Organic compounds containing oxygen | |
| නයිට්රිජන් අඩංගු කාබනික සංයෝග සහ හේලෝහයිඩ්රෝකාබන - Organic compounds containing nitrogen & halo-hydrocarbons | |
| කාබනික පරිවර්තන - Organic conversions | |
| කාබනික සංයෝග විශ්ලේෂණය - Analysis of organic compounds | |
| රසායනික චාලන විද්යාව - Chemical kinetics | |
| රසායනික සමතුලිතතාව - Chemical Equilibrium | |
| ද්රාව්යතා ගුණිතය - Solubility Product | |
| අම්ල-භෂ්ම සමතුලිතතාව - Acid-base Equilibrium | |
| කලාප සමතුලිතතාව - Phase Equilibrium | |
| අකාබනික සංයෝගවල ප්රමාණාත්මක විශ්ලේෂණය - Quantitative analysis of inorganic compounds | |
| විද්යුත් රසායනය - Electrochemistry | |
| කාර්මික රසායනය - Industrial Chemistry | |
| පාරිසරික රසායනය - Enviorenmental Chemistry |
| Tag | |
|---|---|
| කාබනික සංයෝගවල IUPAC නාමකරණය | |
| අණුක සූත්ර | |
| න්යෂ්ටික ප්රතික්රියා තුලිත කිරීම | |
| ආරෝපණ ආශ්රිත ගණනය කිරීම් |
WAEC Past Questions and Answers (PDF) Free Download

WAEC Past Questions and Answers are available here, in PDF format. You can download the WACE past questions for all available subjects. See the list of available subjects for the WAEC Past Papers and how to download them.
We have WAEC Past Questions and Answers for all the most popular WAEC subjects. Most of the WAEC past papers start from the most recent WAEC exam, down to a couple of years back.
So, you will have the material that will help you study many WAEC past questions and the answers to the questions as well. To download the Past questions (PDF), see below.
Recommended for WAEC students : Study our more complete WAEC Past Questions and Answers for FREE .
But if you don't want that, download the free limited PDF versions below.
Download WAEC Past Questions and Answers [PDF]
- WAEC mathematics past questions & answers PDF (free)
- WAEC past questions and answers on physics
- WAEC chemistry past questions and answers (PDF) free
- WAEC Biology past questions and answers download pdf
- WAEC Past Questions and Answers - Accounts (PDF)
- WAEC Economics Past Questions and Answers
- WAEC English Language past questions and answers PDF
Study all WAEC past questions
You can also study all WAEC Past Questions and Answers for different subjects for FREE. The link takes you to more Past Questions and Answers on WAEC for all available subjects and different years of the past questions.
All the best!
How to register for WAEC GCE 2019
January 03 2019

WAEC Timetable Aug - Oct 2021 Exam (updated +PDF download)
September 03 2021

WAEC Syllabus 2023/2024 For All Subjects (PDF Download)
March 20 2023
Add a Comment
Notice: Posting irresponsibily can get your account banned!
Comments, Page 1/10
Please what of literature, animal husbandry, geography, civic and more 🥺🥺🥺
Please sir, i need chemistry, Physycs, mathmahics,biology
i need pass question and and answer for any subject@@@
English mathematics physic chemistry biology civil education
I hate reading
I need general science subject pdf
Please add me to the WhatsApp 09045547416
I love reading the paper and it helps me to know more about the system
Biology, English, literature and government past question waec paper
I need past questions and answers on the Core subjects plus E-biology
PLS I NEED GCE QUESTIONS FOR THE FOLLOWING COMMERCE CRS CIVIC ENGLISH MATHS ACCOUNTING ECONOMICS
I need c r s past questions and answers,
Please send more pass questions on the five main requirements subjects
I need English language
I need last year past question all the subject
i need pasco for business management
Please I need past questions for maths English chemistry biology health science food and nutrition Civic education marketing and physics
Featured Posts
Latest posts.

- Username Password Remember me Sign in New here ? Join Us

Chemistry 2021 WAEC Past Questions
The hydrolysis of proteins by diluting mineral acids produces
- C. amino acids
- D. fatty acids
Which of the following oxide causes acid rain?
- C. H\(_{2}\)O\(_{2}\)
- D. NO\(_{2}\)
The ratio of carbon atoms of hydrogen atoms in a hydrocarbon is 1:2. If its molecular mass is 56, what is its molecular formula?
- A. C\(_{3}\)H\(_{6}\)
- B. C\(_{4}\)H\(_{8}\)
- C. C\(_{2}\)H\(_{4}\)
- D. CH\(_{2}\)
What is the relative molecular mass of the compound below?
[H = 1.0; C = 12.0; O = 16.0]
Cathodic protection of metals is based on
- A. standard electrode potential of hydrogen
- B. its electrical conductivity
- C. nature of oxides formed
- D. relative tendencies of oxidation
- Mathematics
- English Language
- Animal Husbandry
- Literature in English
- Accounts - Principles of Accounts
- Christian Religious Knowledge (CRK)
- Agricultural Science
- Islamic Religious Knowledge (IRK)
- Civic Education
- Further Mathematics
- Home Economics
- Book Keeping
- Data Processing
- Catering Craft Practice
- Computer Studies
- Physical Education
- Office Practice
- Technical Drawing
- Food and Nutrition
- Home Management

- Form 1 Mathematics Notes
- Form 2 Mathematics Notes
- Form 3 Mathematics Notes
- Form 4 Mathematics Notes
- Form 1 Mathematics Topical Questions and Answers
- Form 2 Mathematics Topical Questions and Answers
- Form 3 Mathematics Topical Questions and Answers
- Form 4 Mathematics Topical Questions and Answers
- Form 1 Functional Writing Notes
- Form 2 Functional Writing Notes
- Form 3 Functional Writing Notes
- Form 4 Functional Writing Notes
- Poetry Notes
- Grammar Notes
- Oral Literature Notes
- Oral Skills Notes
- Guide to Blossoms of the Savannah Summarized Notes - Easy Elimu
- A Doll's House
- The Pearl Study Guide
- Memories We Lost and Other Stories Study Guide
- Inheritance Study Guide
- A Silent song and Other Stories Guide
- Fathers of Nations Guide
- An Artist of the Floating World Guide
- The Samaritan Guide
- Sarufi na Matumizi ya Lugha
- Isimu Jamii Notes
- Fasihi Notes
- Ushairi Notes
- Mwongozo wa Kuandika Insha
- Tumbo Lililoshiba na Hadithi Nyingine
- Mwongozo wa Kigogo
- Mwongozo wa Chozi La Heri - Chozi la Heri Notes PDF
- Mwongozo wa Bembea ya Maisha - Bembea ya Maisha Notes PDF
- Mwongozo wa Nguu za Jadi
- Mwongozo wa Mapambazuko ya Machweo na Hadithi Nyingine
- Biology Form 1 Notes
- Biology Form 2 Notes
- Biology Form 3 Notes
- Biology Form 4 Notes
- Biology Essays
- Form 1 Biology Topical Revision Questions and Answers
- Form 2 Biology Topical Revision Questions and Answers
- Form 3 Biology Topical Revision Questions and Answers
- Form 4 Biology Topical Revision Questions and Answers
- Form 1 Chemistry Notes
- Form 2 Chemistry Notes
- Form 3 Chemistry Notes
- Form 4 Chemistry Notes
- All Chemistry Practicals Notes for KCSE and MOCKS
- Form 1 Chemistry Topical Revision Questions and Answers
- Form 2 Chemistry Topical Revision Questions and Answers
- Form 3 Chemistry Topical Revision Questions and Answers
- Form 4 Chemistry Topical Revision Questions and Answers
- IRE Form 1 Notes
- IRE Form 2 Notes
- IRE Form 3 Notes
- IRE Form 4 Notes
- Physics Form 1 Notes
- Physics Form 2 Notes
- Physics Form 3 Notes
- Physics Form 4 Notes
- CRE Form 1 Notes
- CRE Form 2 Notes
- CRE Form 3 Notes
- CRE Form 4 Notes
- Geography Form 1 Notes
- Geography Form 2 Notes
- Geography Form 3 Notes
- Geography Form 4 Notes
- History Form 1 Notes
- History Form 2 Notes
- History Form 3 Notes
- History Form 4 Notes
- Business Studies Form 1 Notes
- Business Studies Form 2 Notes
- Business Studies Form 3 Notes
- Business Studies Form 4 Notes
- Home Science Form 2 Notes
- Home Science Form 3 Notes
- Home Science Form 4 Notes
- Home Science Form 1 Notes
- Agriculture Form 1 Notes
- Agriculture Form 2 Notes
- Agriculture Form 3 Notes
- Agriculture Form 4 Notes
- Agriculture KCSE 2019 Project
- Computer Studies Form 1 Notes
- Computer Studies Form 2 Notes
- Computer Studies Form 3 Notes
- Computer Studies Form 4 Notes
- KCSE 2017 Reports
- 2018 Pre-Mocks
- 2019 Pre-Mocks
- 2022 Pre Mocks
- 2021/2022 Pre-Mock Past Papers
- 2023 Pre Mocks
- 2017 Mock Past Papers
- 2019 Mock Past Papers
- 2020 Mock Past Papers
- Mock Exam Papers 2021/2022 - Easy Elimu
- Mock Exam 2022 Questions and Answers
- Alliance Boys High School
- Maranda High School
- Form 1 Past Papers
- Form 2 Past Papers
- Form 3 Past Papers
- Form 4 Past Papers
- 2019 KCSE Prediction Papers
- 2020 KCSE Prediction Papers
- 2021 KCSE Prediction Papers
- 2022 KCSE Prediction Questions and Answers - EasyElimu
- KCSE Prediction 2023
- 2020 Post Mock Past Papers
- 2021/2022 Post Mocks
- 2023 Post Mocks
- Play Group: Activities, Homework and Syllabus
- 2023 PP1 Exams
- 2023 PP2 Exams
- Grade 1 Notes
- 2023 Grade 1 Exams
- Grade 2 Notes
- 2023 Grade 2 Exams
- Grade 3 Notes
- 2023 Grade 3 Exams
- Grade 4 Notes
- 2023 Grade 4 Exams
- Grade 5 Notes
- 2023 Grade 5 Exams
- Grade 6 Notes
- KPSEA Exams
- 2023 Grade 6 Exams
- Class 6 : Notes, Revision Papers and Syllabus
- Class 7 : Notes, Revision Papers and Syllabus
- Class 8 Notes
- 2023 Class 8 Exams
- 2023 Kcpe Prediction
- Grade 7 Notes
- 2023 Grade 7 Exams
- Pre Mock Exams 2024
- KCSE 2024 Prediction Exams
- 2019 - 2024 Mock Exams
- KCSE 2008 - 2023 Exams with Answers
- Form 4 End Term 2 Exams 2024
- Form 3 End Term 2 Exams 2024
- Form 2 End Term 2 Exams 2024
- Form 1 End Term 2 Exams 2024
- The Samaritan Essay Questions with Answers
- Fathers of Nations Excerpts with Answers
- Kiswahili Setbooks
- Form 1 - 4 High School Notes
Chemistry Paper 3 Questions and Answers - KCSE 2021 Past Papers
« Previous Topic Chemistry Paper 2 Questions and Answers - KCSE 2021 Past Papers
Next Topic » Physics Paper 1 Questions and Answers - KCSE 2021 Past Papers
- Solution A: 0.10 M solution of a monobasic acid A;
- Solution B: Sodium hydroxide solution:
- Solution C: containing 10.0g of acid C per litre of solution. You are required to:
- Standardise solution B using solution A;
- Determine the number of moles of sodium hydroxide that react with one mole of acid C.
PROCEDURE I Fill the burette with solution A. Using a pipette and pipette filler, place 25.0cm of solution B into 250 ml conical flask. Titrate solution B with solution A using phenolphthalein indicator and record your results in Table 1. Repeat the titration and complete Table 1.
| I | II | III | |
| Final burette reading | |||
| Initial burette reading | |||
| Volume of solution A used, cm |
- average volume of solution A used. (1 mark)
- number of moles of solution A in the average volume used. (1 mark)
- number of moles of sodium hydroxide (N) in 25.0cm of solution B. (1 mark)
- concentration of sodium hydroxide in moles per litre. (1 mark) PROCEDURE II Clean the burette and fill it with solution C. Using a pipette and pipette filler, place 25.0cm of solution B into a 250 ml conical flask. Titrate solution B with solution C using phenolphthalein indicator and record your results in Table 2. Repeat the titration and complete Table 2.
- average volume of solution Cused. (1 mark)
- concentration in moles per litre, of solution C, given that the relative formula mass of acid C is 210.0. (1 mark)
- number of moles of acid C in the average volume used. (1 mark)
- Write the ratio of moles of acid Cto moles of sodium hydroxide (N) in the 25.0cm of solution B. (1 mark)
- Determine the number of moles of sodium hydroxide that react with one mole of acid C. (1 mark)
- Fill a 250 ml beaker with about 200 cm 3 of tap water and heat the water until it boils.
- Place all solid D provided in a dry test tube and insert a thermometer into the solid.
- Place the test tube in the boiling water and allow the solid to heat until it all melts.
- When the temperature of the melted solid is approximately 90°C, remove the test tube, wipe the sides with tissue paper and then place the test tube into an empty 250 ml beaker
- Start the stop watch or clock when the temperature of the melted solid is 85.0°C.
| Time, s | 0 | 30 | 60 | 90 | 120 | 150 | 180 | 210 | 240 | 270 | 300 |
| Temperature, °C |
- On the grid provided, plot a graph of temperature (vertical axis) against time. (3 marks)
- Using the graph in (b), determine the freezing point of solid D. (1 mark)
| Observations | Inferences |
| (2 marks) | (1 mark) |
| Observations | Inferences |
| (1 marks) | (1 mark) |

MARKING SCHEME
| 24.0 | CT 1 | |||
| I | II | III | D1 | |
| Final burette reading | A1 | |||
| Initial burette reading | PA1 | |||
| Volume of solution A used, cm | FA1 |
- Correct working correct ans (b)(i)
- = correct ans (b)(i) x 0.1 = correct ans (b)(ii) 1000
- Ratio A:N b1:1 ans (b) (iii)
- = 1000 x ans (b)(iii) = ans (b)(iv) 25
PROCEDURE II
| 16.5 | CT1 | |||
| I | II | III | D1 | |
| Final burette reading | A1 | |||
| Initial burette reading | PA1 | |||
| Volume of solution A used, cm | FA1 |
- Correct working correct ans (d)(i)
- = 10 / 210 = 0.0476
- = correct ans (d)(i) x 0.0476 = correct ans (d)(iii) 1000
- ans (d)(iii) : ans(b)(iii)
- = ans(b)(iii) = ans (e)(ii) ans(d)(iii)
| Time, s | 0 | 30 | 60 | 90 | 120 | 150 | 180 | 210 | 240 | 270 | 300 |
| Temperature, °C | 85.0 | 82.0 | 80.5 | 78.0 | 75.5 | 72.0 | 69.0 | 69.0 | 69.0 | 67.5 | 65.0 |
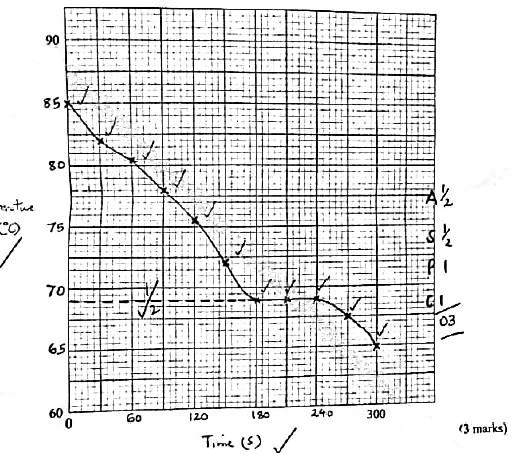
- showing correct reading
| Observations | Inferences |
| No effervescence E dissolves to form a blue solution (1 marks) | CO / SO absent Cu present (1 mark) |
| Observations | Inferences |
| No white ppt formed | SO absent |
| Observations | Inferences |
| No yellow ppt formed No white ppt formed | I absent Cl /Br absent |
| Observations | Inferences |
| Blue ppt formed insoluble in excess | Cu present |
| Observations | Inferences |
| Blue ppt formed that dissolves in excess to form a deep blue solution | Cu present |
| Observations | Inferences |
| Blue solution changes to green Brown residue Green filtrate Boiling tube becomes warm | Cu displaced by G/G is more reactive than Cu/G is oxidized by Cu /Cu are reduced by G/ Cu are displaced by Fe |
| Observations | Inferences |
| Green ppt formed insoluble in excess | Fe |
| Observations | Inferences |
| Green solution changes to brown/Yellow Effervescence | Fe oxidized to Fe Fe formed |
Download Chemistry Paper 3 Questions and Answers - KCSE 2021 Past Papers .
Why download.
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students
Related items
- Arabic Paper 2 Questions and Answers - KCSE 2021 Past Papers
- Arabic Paper 1 Questions and Answers - KCSE 2021 Past Papers
- Aviation Technology Paper 2 Questions and Answers - KCSE 2021 Past Papers
- Aviation Technology Paper 1 Questions and Answers - KCSE 2021 Past Papers
- Woodwork Paper 1 Questions and Answers - KCSE 2021 Past Papers
access all the content at an affordable rate or Buy any individual paper or notes as a pdf via MPESA and get it sent to you via WhatsApp
What does our community say about us?
- KCSE Revision Questions
- Privacy Policy
- Mobile App Privacy Policy
- High Schools in Kenya
- Teacher Resources
- Questions and Answers
- Online Tuition and Classes in Kenya
Copyright © 2022 EasyElimu
Sign up now
Fill in the form below to sign up:.
By clicking on the "Sign up" button, you agree to our Terms and Privacy Policy
Already a member? Login
Password Reset
Enter your phone number to reset password:.
- Reset by Phone Number
- Reset by Email
A 6-digit reset code will be sent to your phone number
Cancel? Return to login
A 6-digit reset code will be sent to your email
Login to your account
Forgot Password?
Sign up as a new member

Paper 1 | Objectives | 50 Questions
WASSCE/WAEC MAY/JUNE
Type: Question Paper
Answers provided
- DESCRIPTION
- STUDENTS REVIEWS (0)
No description provided
This paper is yet to be rated
Yet to recieve reviews
Add a review
Share This Paper
Recent posts, mtn ghana bright scholarships 2022 for ghana undergraduate students.
Did you apply the MTN Ghana Bright Scholarships 2022 for Ghana Undergraduate Students?
26 Scientific Ways to Cope With Exam Stress
Effective ways of coping with exam stress and test anxiety which is supported by Science
Four Tips How to Score 320 in JAMB UTME 2022
Adequate preparation and effective revision strategies tips to get a high score 320 in JAMB in 2022
11 Hot Tips And Guide For Successful Exam Preparation
The best study methods and strategies and tips for successful exam preparation for good grades
MENA Scholarship For Africa 2022
Middle East and North Africa MENA Scholarship Program (MSP) initiative provides scholarships
Paper 1 | Objectives
Recommended for you.
Mathematics (Core)
Agricultural Science
Write to us
Past Questions
Daily Grove
Year :
Title : .
| # | Question | Ans |
|---|---|---|
| 1. | sucrose glucose amino acids fatty acids | |
| 2. | CO NO H\(_{2}\)O\(_{2}\) NO\(_{2}\) | |
| 3. | C\(_{3}\)H\(_{6}\) C\(_{4}\)H\(_{8}\) C\(_{2}\)H\(_{4}\) CH\(_{2}\) | |
| 4. | 137 136 64 59 | |
| 5. | standard electrode potential of hydrogen its electrical conductivity nature of oxides formed relative tendencies of oxidation | |
| 6. | solid MnO\(_{2}\) acidified KMnO\(_{4}\) acidified FeSO\(_{4}\) saturated NaCl(aq) | |
| 7. | primary and dihydric secondary and monohydric tertiary and dihydric secondary and dihydric | |
| 8. | NaCl and KNO\(_{3}\) KCL and NaNO\(_{3}\) K\(_{2}\)SO\(_{4}\) and BaCl\(_{2}\) NH\(_{4}\)NO\(_{3}\) and CO\(_{3}\) | |
| 9. | I II III IV | |
| 10. | H of a given volume of strong acid solution (y-axis) with the volume of strong base titrated against its (x-axis)? I II III IV |
Preview displays only 10 out of the 50 Questions
WAEC Chemistry Questions and Answers 2022/2023 [Theory & OBJ] Expo
- January 18, 2022
What is better than getting free WAEC Chemistry questions 2021 which also comes with WAEC Chemistry answers 2021 for theory and OBJ before the exam start?
I am sure you will be very happy to get our free WAEC material which contains both WAEC 2021 Chemistry questions for Objective and essay (paper 1 & paper 2) and the answers for 2021 a day before the exam.
This material is 100% free and it contains the 2021 WAEC Chemistry OBJ questions and answers sample and the possible WAEC 2021 Chemistry theory questions and answers that may likely appear on the exam day.
Free and Legit WAEC Chemistry Expo Runs 2021
Note: this material is not aimed at leaking the WAEC Chemistry questions and answers for 2021.
But it is an expo material that will tell you all the WAEC Chemistry expo hints for the 2021 exam which will help you to score A in this subject.
Please, please, please, do not pay money to anyone online for the WAEC Chemistry Expo runs 2021.
There are so many expo sites claiming to be WAEC expo runs website online that which promises WAEC candidates correct WAEC Chemistry questions and answers expo before the exam when they pay to subscribe.
![WAEC Chemistry Questions and Answers 2022/2023 [Theory & OBJ] Expo 1 WAEC Chemistry Questions and Answers [Theory & OBJ] Expo Guide For Chemistry Paper 1 & 2 (Objective & essay Solution).](https://studentmajor.com/wp-content/uploads/2020/03/WAEC-answers-5-232x300.jpg)
But I will advise you to get your mind away from such a website and make use of our free material below.
If you have ever planned of making such payment, then know that you will be scammed and they will end up sending nothing or they will send you fake WAEC Chemistry questions and answers that will make you fail.
WAEC 2021 Chemistry Questions and Answers for Theory and OBJ.
Exam Scheme, Format, and instructions:
This exam paper follows the following format and exam scheme: Paper 1 (Objective) and paper 2 (theory).
Paper one contains 50 OBJ questions (to answers all questions) while paper 2 is essay/theory questions (candidates are to answer the number of questions as indicated on the exam paper)
Success hints:
- Go to your exam hall early (1 hour before exam)
- Follow the WAEC timetable daily to avoid missing a paper.
- Pray before and after each paper.
- Follow the exam instructions on the question paper.
- Shade your answers properly using an HB pencil.
- Fill in your details properly before you start answering your questions.
- Attempt all questions before submitting them.
Sample WAEC Chemistry OBJ questions and Answers 2021 for paper 1.
Below are possible WAEC Chemistry Objective questions 2021 that may appear in this year’s exam.
There are not the actual chemistry questions but, sample questions for practices which might possibly be repeated in this year’s exam.
1. Which of the statements below is correct about Isotopes of the same element?
- Have the Same number of Protons, neutrons, and electrons.
- Same Protons and neutrons but a different number of electrons.
- Same number Protons and electrons but a different number of neutrons.
- The number of Neutrons and electrons is the same but the number of protons differs.
2. The chemical bond that is formed by the transfer of electrons is known as?
- Covalent Bond
- Dative Bond
- Metallic bond
3. Two electrons can fill the same orbital if only?
- They have different Angular momentum quantum numbers.
- Magnetic quantum numbers differ
- Principal quantum numbers are different.
- Spin quantum numbers not the same
4. Which of the substances listed below is not a hydrocarbon?
5. The complete ionization of A substance into hydroxonium ions indicates:
- Strong acid.
- Strong base.
6. One of the solutions below that can resist changes in pH when a small quantity of a base or acid is added is?
- Buffer solution
- Neutral solution
- Saturated solution
- Supersaturated solution
7. Write a balanced chemical equation for a 2.47g dry pure copper (II) oxide which is completely reduced copper using a laboratory gas. If the mass of the residue left is found to be 1.97g.
- Cu + O → CuO.
- 2Cu+O 2 → 2CuO
The correct answer is B. 2Cu+O 2 → 2CuO. See how this question is solved in the number one (No.1) question in the theory section.
Use this question to answer question 2 & 3
8. Calculate the mass of anhydrous sodium-trioxocarbonate (IV) present in a 300cm 3 of 0.1M; (Na =23, C=12, O=16).
The correct answer is A. 3.18g. See solving in No.2 ques. in the theory section.
9. The number of Na 2 CO 3 particles present in the solution
- 6.02 X 10 23
- 0.1 X 6.02 10 23
- 1.81 X 10 22
- 1.81 X 10 23
- 6.02 X 10 22
The correct answer is C. 1.81 X 10 22.
See how to solve this question in the number No.2 question in the theory section.
Free WAEC Chemistry questions and answers for 2021 is loading>>>>
Keep refreshing this page for the free and correct WAEC 2021 Chemistry questions and answers. We will update it here if we have any.
- Download WAEC syllabus for chemistry
WAEC Chemistry Theory Questions and answers 2021 samples
These are possible WAEC Chemistry theory questions and answers 2021 that may appear in this year’s exam.
There are not actual questions and answers but, sample questions for practices.
![WAEC Chemistry Questions and Answers 2022/2023 [Theory & OBJ] Expo 2 Correct WAEC Chemistry Theory answers to OBJ paper 1 and 2 solved solution](https://studentmajor.com/wp-content/uploads/2020/03/Waec-answer-2-300x300-1.jpg)
2.47g of dry pure copper (II) oxide was completely reduced to a copper using laboratory gas. The mass of the residue left was found to be 1.97g. Write a chemical equation for this reaction.
Molar mass of copper atoms =63.5
No. of moles of copper atoms =1.97
=63.5 = 0.03 mole
Molar mass of oxygen atoms = 16
No. of moles Oxygen atom = (2.47 – 1.97) = 0.5
Mole ratio of Cu to O = 0.03 : 0.03
= 1 : 1
Therefore, the equation is Cu + O → CuO.
But Oxygen is diatomic, so we have 2Cu+O 2 → 2CuO
2. Calculate the;
- mass of anhydrous sodiumtrioxocarbonate (IV) present in a 300cm 3 of 0.1M;
- Number of Na 2 CO 3 particles present in the solution (Na =23, C=12, O=16)
a. Molar concentration of Na 2 CO 3 =0.1M
Molar mass of Na 2 CO 3 = 106gmol -1
Mass concentration = Molar concentration X Molar mass
=10.6gdm -3
i.e 1000cm3 of 0.1M solution contain 10.6g of Na 2 CO 3
Therfore,300cm 3 of 0.1M solution will contain
= 300 X 10.6/1000
=3.18g of Na 2 CO 3
b. Number of Na 2 CO 3 particles
=Molar concentration X 6.02 X 10 23
=0.1 X 6.02 10 23
=6.02 X 10 22
Now, 100cm 3 of 0.1M solution contains 6.02X10 22 Na 2 CO 3 particles
Therefore, 300cm 3 of 0.1M solution will contain 300X6.02 X10 22 /1000
= 1.81 X 10 22 particular of Na 2 CO 3 .
Free WAEC Chemistry questions and answers for 2021 is loading…………….. Keep refreshing this page for the free and correct WAEC 2021 Chemistry questions and answers. We will update it here if we have any.
Final note: we don’t encourage exam malpractice. So study hard for your exam and you will score better and feel proud of yourself. For a proper study, use the following materials:
- WAEC Chemistry past questions and answers
- 2021 WAEC syllabus for Chemistry
- Check our revealed secrets of scoring A in your 9 subjects here.
- Study with WAEC Chemistry e-learning portal.
15 comments
This is good, and I hope it will help a lot But please, I need your assistance on the following subject before the exam day : Chemistry, Elective Mathematics
I need answers or assistance On;maths, English, physics, chemistry, biology, animal husbandry.
Please I need the question and answers for maths, English, chemistry, physics, geography, marketing, biology, Civic and Agriculture. Thank you
Yes I need it
I need WAEC answer before on the Examination day in Chemistry and physics
I also need pass answers on 2019/2020 on chemistry and physics
I need physics, biology, chemistry and English lang.
I need answers for 2021 Mathematics English Computer Chemistry Physics Economics Geography
I need answers for 2021 waec with the following subject: Mathematics English Physics Chemistry Biology Geography Civic Education Animal husbandry Agriculture Science
I need answers for 2021waec with the following subject Mathematics English Physic Chemistry Biology Geography Economic Civic education Marketing
I need waec 2021questions and answers for math English biology chemistry agric civic physics Yoruba and economics
I need answers before the exam,I need ur WhatsApp number pls
I need waec 2021 answers to the following subjects, English, Mathematics, Chemistry, Biology, Physics
I need waec 2021 answers to the following subjects, English, mathematics, chemistry, biology, physics, agricultural science, economic, Civic education, Fisheries.
I need answers to 2021 wace questions in the following subjects, English, maths, chemistry, biology and physics
I need questions and answers in 2021waec in the following subjects, math, chemistry, physics, biology, geography and agricultural science.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
20 Quick Certifications That Pay Well Today
Upth internship result list 2022/2023 -names of successful intense, you may also like, waec biology questions and answers 2022/2023 [obj & theory expo].
- December 20, 2021
NECO Animal Husbandry Practical Questions & Answers 2022 [15th July, 2022]
- February 23, 2022
WAEC GCE Commerce Questions, Answers 2020/2021 & Syllabus
- April 19, 2022
NECO Data Processing Questions and Answers 2022/2023 (Essay and Objectives)
Neco chemistry practical apparatus & answers to questions 2022/2023 (complete solution), neco mathematics questions and answers 2022/2023 | obj & theory.
- April 7, 2022
Jamb Admission
Latest nigerian school news, verified complete waec 2021 chemistry theory questions and answers.
WAEC Chemistry Obj and Theory/Essay 2021/2022 Free Questions & Answers Posted Online. May/June First series Free Expo subscription Runz. Chemistry waec syllabus, waec expo, Free verified complete waec questions and answers on Chemistry, waec gce 2021/2022 Chemistry full verified questions and answers , waec Chemistry past questions, Chemistry wassce past questions site, waec gce expo Chemistry website, 2021/2022 free waec expo Chemistry website posted on WhatsApp.

Reload this page after 30 seconds. jambadmission.com.ng is the best website for posting and getting WAEC 2021/2022 Chemistry Obj & Essay Questions And Answers. Chemistry Obj & Essay questions and Answers would alive a day before Examination.
We have made available a download link to Download our WAEC CBT Software for Android and PC so that you can practice it for this upcoming WAEC 2021/2022 examination . Instead of you buying the WAEC WASSCE past questions and answers which cost N500 each per subject, kindly go for this WAEC CBT application software.
WAEC Chemistry Obj and Theory/Essay 2021/2022 Free Questions & Answers Posted Online
We assure you A’s and B’s in all your WAEC subject papers. Questions and answers arrive a day before examination. Bank Payment: (i) ALL SUBJECTS + practicals + VIP Treat: CONTACT US – 08133139104. —————————— (ii) 7/8/9 Subjects WITH OR WITHOUT Practicals : N5,000. After Payment you will instantly be added to the WhatsApp group chat where you will be getting questions and answers a day before the examination.
WAEC 2021/2022 ACCOUNT DETAILS.
We will send u the bank account Details instantly, contact us now on WhatApp: 08133139104 –AFTER BANK PAYMENT: Send (i) Depositor’s name (ii)Teller no/Id (iii)Amount Paid(s). (iv)Subjects. (v) Phone number to To 08133139104 via WhatsApp/SMS
Download our FREE WAEC CBT Software for Android and PC
Always participate in the activities of this site cause we also have Online PDF WAEC WASSCE Past Questions and answers for you to read and revise with well explained answered. We also have CBT software for Jamb also, if you writing this year’s 2021/2022 JAMB UTME EXAMINATION then you should navigate to our page and download JAMB CBT SOFTWARE.
How to get good grades in 2021/2022 WAEC Chemistry
- Revise past WAEC questions and answers on Chemistry (both in hard and soft copy)
- Download our WAEC CBT software with over 200,000 questions and answers
- Attend any nereby WAEC tutorial lesson
Incoming search: WAEC Chemistry objectives and theory free questions now available online. 2021/2022 Chemistry expo runs answers on WhatsApp. WAEC Chemistry answer, 2021/2022 free waec Chemistry answer, WAEC Chemistry answer, 100% 2021/2022 waec Chemistry free expo/runz, 2021/2022 waec ECONOMICS STUDIES free answer,Chemistry PRACTICAL waec 2021/2022 expo, waec 2021/2022 expo Chemistry answer, live 2021/2022 waec Chemistry PRACTICAL answer now available, how to get 2021/2022 waec Chemistry OBJECTIVES & ESSAY question and answer, 2021/2022 waec Chemistry OBJECTIVES & ESSAY question and answers. Download waec Chemistry STUDIES OBJECTIVES & ESSAY waec answer.. Waec Biology PRACTICAL expo, waec 2021/2022 civic expo, waec Chemistry & ESSAY runz/expo, 2021/2022 waec Chemistry & ESSAY expo now available, 2021/2022 waec Chemistry obj and theory answer. Correct Real Waec 2021/2022 Chemistry STUDIES OBJECTIVES & ESSAY Prose And Objective CORRECT WAEC Chemistry Prose OBJ Essay Questions And Answer Expo/Runs
Be the first to comment
Leave a reply cancel reply.
Your email address will not be published.
Notify me of follow-up comments by email.
Notify me of new posts by email.
Copyright © 2023 | All Rights Reserved.

IMAGES
VIDEO
COMMENTS
Directions: Questions 1-3 are long free-response questions that require about 23 minutes each to answer and are worth 10 points each. Questions 4-7 are short free-response questions that require about 9 minutes each to answer and are worth 4 points each. For each question, show your work for each part in the space provided after that part.
AP Chemistry Exam Questions - AP Central - College Board
The AP Chemistry exam assesses your ability to understand science practices in relation to four big ideas: scale, proportion and quantity; structure and properties; transformations; and energy. The exam is 3 hours and 15 minutes long and is comprised of two separate sections: Section I: Multiple Choice Questions. 60 questions.
AP Chemistry 2022 Free-Response Questions
G.C.E. Advanced Level (A/L) Chemistry Past Papers and ...
Free-Response Questions 2014-2021 . These free response questions are from the most up-to-date version of the test. I would advise you to save most of them for later on in the year when you're more serious about practicing for the real AP exam. There are seven questions from each year. Practice Tests from Your Teacher
Question 2 deals with the atomic structure of silicon and the properties of silicon-containing compounds. In part (a)(i), the student is asked to interpret a mass spectrum to determine the number of subatomic particles in the most abundant isotope of Si (SPQ-1.B, 5.D). Part (a)(ii) asks for the ground-state electron configuration of Si (SAP-1.A ...
Chemistry scholars handle crucial experiments and create numerous write-ups throughout their courses. They also develop solutions to daily life issues, and their works serve people from every background. Chemistry essay questions are marvelous for those who know a little about the subject, but they can be reasonably compound for anyone unfamiliar.
A/L Chemistry Vivarana books by Ranga Gunarathna
then allowed to cool. A portion of the filtrate +. H 2 SO 4(aq) A portion of the filtrate +. FeSO 4(aq) + Conc. H 2 SO 4 gently down the. sides of the test tube. White (chalky) precipitate. White precipitate soluble.
Most comprehensive collection of Answers has been provided for Past Paper Questions of A/L Chemistry Since 1980. Freedom of Learning. Learn anywhere any time! Now you can learn Advanced level chemistry for totally Free! Pioneer of using ICT to teach chemistry in sinhala medium. All lessons and answers are explained according to the current ...
SEPTEMBER 2021 EXAMINATIONS CHEMISTRY Time Allowed: 3 Hours SECTION A: MULTIPLE CHOICE QUESTIONS Answer all questions in this section. Use the OMR answer sheet provided to answer the questions. Follow the instructions on the OMR sheet. SECTION B: ESSAY QUESTIONS Answer FOUR questions; ONE question from each course.
CLICK TO SEE ANSWERS. (Chemistry - NECO 2021) Direct Mobile/SMS: N800 MTN CARD. Password/Link: N500 MTN CARD. Forward Subject Name, MTN CARD PINS, Phone number to: 08074021548 via TEXT MESSAGE ...
Overview. Question 1 presents a suite of questions on the reactions and structure of methanoic acid, HCOOH. Part (a) asks the student to write the equilibrium constant expression for the acid ionization reaction of HCOOH. This question addresses Learning Objective SAP-9.C and Science Practice 5.B from the AP Chemistry Course and Exam Description.
WAEC Past Questions and Answers (PDF) Free Download
Chemistry Paper 1 Questions and Answers - KCSE 2021 Past Papers. The atomic number of phosphorus is 15. Draw a dot (•) and cross (x) diagram for the compound formed when phosphorus reacts with chlorine, atomic number 17. (1 mark) One of the regions in the non-luminous flame is the unburnt gas region.
View Answer & Discuss (3) WAEC 2021. 3. The ratio of carbon atoms of hydrogen atoms in a hydrocarbon is 1:2. If its molecular mass is 56, what is its molecular formula? A. C 3 3 H 6 6. B. C 4 4 H 8 8. C. C 2 2 H 4 4.
Chemistry Paper 3 Questions and Answers - KCSE 2021 Past Papers. Download PDF for future reference Get on Whatsapp for 50/-. QUESTIONS. You are provided with: Solution A: 0.10 M solution of a monobasic acid A; Solution B: Sodium hydroxide solution: Solution C: containing 10.0g of acid C per litre of solution. You are required to:
Chemistry. Paper 1 | Objectives | 50 Questions WASSCE/WAEC MAY/JUNE. Year: 2021 Level: SHS Time: Type: Question Paper Answers provided
For the correct answer: 1 point. Electron flow should be indicated only in a counter-clockwise direction in the external circuit, from the Cl2 anode to the Mg cathode. (b) For the correct answer and calculated value: No, because 2.0 V is less than 3.73 V, which is the minimum voltage needed for electrolysis to occur.
The chemistry questions and answers for NECO 2021 will be in two papers. The first paper (paper 1) is the OBJ (objective) and the second paper is paper 2 (theory, essay, calculations and show working). So in this aspect, I will supply you with the basic NECO chemistry questions 2021 samples with correct NECO chemistry answers 2021 to the NECO ...
1.81 X 10 23. 6.02 X 10 22. The correct answer is C. 1.81 X 1022. See how to solve this question in the number No.2 question in the theory section. Free WAEC Chemistry questions and answers for 2021 is loading>>>>. Keep refreshing this page for the free and correct WAEC 2021 Chemistry questions and answers.
Reload this page after 30 seconds. jambadmission.com.ng is the best website for posting and getting WAEC 2021/2022 Chemistry Obj & Essay Questions And Answers. Chemistry Obj & Essay questions and Answers would alive a day before Examination.. We have made available a download link to Download our WAEC CBT Software for Android and PC so that you can practice it for this upcoming WAEC 2021/2022 ...