An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

The 5-HT deficiency theory of depression: perspectives from a naturalistic 5-HT deficiency model, the tryptophan hydroxylase 2 Arg 439 His knockin mouse
Jacob p r jacobsen, ivan o medvedev, marc g caron.
- Author information
- Copyright and License information
Author for correspondence ( [email protected] ).
Present address: Cardiovascular Training Program, University of Washington, Seattle, WA 98104-2499, USA.
One contribution of 11 to a Theme Issue ‘ The neurobiology of depression—revisiting the serotonin hypothesis. I. Cellular and molecular mechanisms ’.
A decreased level of brain 5-hydroxytryptamine (5-HT) has been theorized to be a core pathogenic factor in depression for half a century. The theory arose from clinical observations that drugs enhancing extracellular levels of 5-HT (5-HT Ext ) have antidepressant effects in many patients. However, whether such drugs indeed correct a primary deficit remains unresolved. Still, a number of anomalies in putative biomarkers of central 5-HT function have been repeatedly reported in depression patients over the past 40 years, collectively indicating that 5-HT deficiency could be present in depression, particularly in severely ill and/or suicidal patients. This body of literature on putative 5-HT biomarker anomalies and depression has recently been corroborated by data demonstrating that such anomalies indeed occur consequent to severely reduced 5-HT Ext levels in a mouse model of naturalistic 5-HT deficiency, the tryptophan hydroxylase 2 His 439 knockin (Tph2KI) mouse. In this review, we will critically assess the evidence for 5-HT deficiency in depression and the possible role of polymorphisms in the Tph2 gene as a causal factor in 5-HT deficiency, the latter investigated from a clinical as well as preclinical angle.
Keywords: 5-HT, 5-HT deficiency, depression, Tph2, antidepressant, mouse model
1. Origin of the 5-HT deficiency theory of depression
The 5-hydroxytryptamine (5-HT) deficiency theory of depression originated from serendipitous clinical observations in the 1950s and was bolstered by subsequent clinical and basic science observations [ 1 ]. Iproniazid, a drug otherwise developed against tuberculosis, unexpectedly displayed antidepressant activity in tuberculosis patients also suffering from depression. Iproniazid was subsequently demonstrated to inhibit monoamine oxidase (MAO) A and B, the enzymes metabolizing 5-HT and the other monoamines, and to increase brain 5-HT levels. Imipramine, the prototypical tricyclic antidepressant (TCA), was originally being developed against schizophrenia. Imipramine failed as an antipsychotic, but serendipitously alleviated depression symptoms in schizophrenic patients with co-morbid depression and was subsequently successfully developed as an antidepressant. In the 1960s, imipramine and other TCAs were shown to block reuptake of 5-HT as well as noradrenaline [ 1 ]. Initially noradrenaline was believed to chiefly mediate the antidepressant effects of TCAs and MAOIs. From these notions arose the noradrenaline deficiency theory of depression [ 2 ]. Around the same time, reports were published indicating that the 5-HT precursor 5-hydroxytryptophan (5-HTP) had antidepressant effects [ 3 ] in humans and increased brain 5-HT levels in animals [ 4 ]. Furthermore, the TCA clomipramine showed equal antidepressant efficacy to other TCAs but inhibited 5-HT uptake with more than 100-fold higher affinity than the inhibition of noradrenaline uptake [ 5 ]. Such pharmacological observations in conjunction with the pathology findings mentioned below gave rise to a broader monoamine theory of depression [ 6 ] and eventually a related 5-HT deficiency theory of depression [ 7 , 8 ]. The observation that increasing 5-HT release confers antidepressant activity has been corroborated repeatedly, although the efficacy of, for instance, selective serotonin reuptake inhibitors (SSRIs) versus placebo may only be robust in moderately to severely depressed patients [ 9 – 11 ].
2. Depression: a heterogeneous disease with diverse pathogenesis?
That pharmacological enhancement of 5-HT function alleviates depression does not necessarily mean that depression is caused by a primary 5-HT deficit. Frequently in the lay media and scientific literature, it is assumed that 5-HT deficiency is a (or even the ) cause of depression. This assumption, whatever its heuristic attractiveness, may still be premature or at least an overgeneralization. The clinical presentation of depression is exceptionally heterogeneous [ 12 ] and the relatively new (1980) and broad term major depression encompasses several syndromes previously defined as separate, though related, disease entities, such as endogenous versus melancholic depression [ 13 ]. With such diverse manifestations and wide definitions for diagnosis, depression as a syndrome could represent several overlapping afflictions arising from distinct aetiologies. Indeed, depression symptomatology can arise secondarily to for instance diverse neurological (e.g. Alzheimer's [ 14 ], Parkinson's [ 15 ] and Creutzfeldt–Jakob's disease [ 16 ]), immunological (e.g. interferon treatment [ 17 ]) and endocrine (e.g. Cushings's disease [ 18 ]) anomalies. Life stressors, such as personal loss, social isolation, financial difficulties, job insecurity and childhood trauma [ 19 – 21 ], are well-established and important disposing factors for depression, clearly demonstrating the significance of environmental context. Concurrently, twin and adoption studies indicate an important genetic component in depression [ 22 ]. However, perhaps not surprisingly, disease-associations with specific genes, whether probed via genome-wide association studies or informed, targeted approaches, are at most moderate and not always reproducible across reports and populations [ 22 – 24 ].
3. Evidence for 5-HT deficiency in depression
In addition to the therapeutic action of augmenting 5-HT function—or more specifically, increasing extracellular 5-HT (5-HT Ext ), the 5-HT pool acting at the 5-HT receptors—there are several lines of evidence pointing towards the presence of a 5-HT deficit in at least some populations of depressed patients. In many studies, acute tryptophan depletion induces the recurrence of mild-depression symptoms in patients who have recovered following treatment with serotonergic antidepressants [ 25 – 27 ], presumably by lowering brain 5-HT Ext . Conceptually, tryptophan depletion appears to be a somewhat non-selective tool to probe the 5-HT deficiency hypothesis. Tryptophan is an essential amino acid, and the depletion could affect general protein synthesis. Further, less than 1 per cent of dietary tryptophan is converted to 5-HT [ 28 ], whereas 95 per cent is metabolized via the indoleamine 2,3-dioxygenase pathway giving rise to the neuroactive substances quinolinic and kynurenic acid that affect cholinergic and glutamatergic receptors, respectively [ 29 ]. Tryptophan depletion could conceivably affect the brain and mood by starving metabolism via this latter pathway, e.g. impairing quinolinic and kynurenic acid synthesis and neurobiological function [ 30 ]. Still, up to 50 per cent of patients treated with interferon-α develop depression symptoms and correlational evidence indicates that induction of indoleamine 2,3-dioxygenase by interferon-α and consequent depletion of tryptophan and hence presumably decreased 5-HT synthesis, is involved herein [ 31 , 32 ]. Chronic pharmacological depletion of 5-HT with the vesicular monoamine transporter inhibitor reserpine has also been reported to precipitate depression symptoms, though only in a subset of patients [ 33 ], a finding that has been taken as support for the 5-HT theory of depression [ 34 ]. However, reserpine, at least in high doses, not only depletes storage and extracellular levels of 5-HT, but also those of dopamine and noradrenaline [ 35 ], making it tenuous to ascribe reserpine's depressiogenic effect to one neurotransmitter only. The link between chronic reserpine treatment and depression also remains contentious, with later studies generally reporting smaller and less frequent depressiogenic effects [ 34 ], although differing dosing schedules could be a factor in the varying findings. In addition, one early study in fact reported weak antidepressant rather than pro-depressant effects of chronic reserpine treatment [ 36 ]. Thus, it remains to be established whether depression symptoms can indeed be elicited by deficient 5-HT neurotransmission.
Directly measuring brain 5-HT in humans can currently only be performed post-mortem. One early study reported decreased brain-stem levels of 5-HT in suicide victims [ 37 ], which is certainly an intriguing finding. Yet, animal studies show that tissue monoamine levels are not always reliable indicators of extracellular levels [ 38 – 40 ]. In lieu of tools for direct assessment, the integrity of the brain 5-HT system has been probed over the past four decades using several indirect measures, or biomarkers. Cerebrospinal fluid (CSF) levels of 5-HT's primary metabolite, 5-hydroxyindoleacetic acid (5-HIAA), appear to reflect brain 5-HIAA levels [ 41 ] and have been taken as an index of brain 5-HT neurotransmission. While reports of a correlation between low CSF 5-HIAA and major depression per se are inconsistent [ 42 – 44 ], the correlation between low CSF 5-HIAA and suicidality, aggression and impulsivity is more robust [ 43 , 45 , 46 ]. These traits tend to co-segregate—for instance in borderline personality disorder, a disease that commonly presents with co-morbid depression [ 47 ]—and may share neurobiology [ 48 ].
Acute stimulation of brain 5-HT release triggers systemic secretion of the neurohormone prolactin . The mechanisms involved in this phenomenon are complex and not precisely defined, but appear to involve modulation of hypothalamic GABA, vasoactive intestinal peptide and oxytocin neurotransmission [ 49 ]. The size of the plasma prolactin surge following a challenge with the 5-HT releaser fenfluramine has thus been employed as a brain 5-HT biomarker, where a blunted response is assumed to reflect lower brain 5-HT levels [ 49 ]. As with low CSF 5-HIAA, many studies report a blunted plasma prolactin response to fenfluramine in depressed patients [ 50 – 57 ] while some studies do not [ 58 – 61 ]. Given the heterogeneity of depression, a certain level of non-replication is not surprising. Further, a close reading of the mentioned negative studies reveals that two studies specifically assessed outpatients [ 59 , 61 ] that presumably are less severely ill, and two studies had substantial methodological problems, such as small number of subjects, minimal prolactin response to fenfluramine and use of non-matched controls [ 58 , 60 ]. Again as with CSF 5-HIAA, the blunting of the prolactin response to fenfluramine appears more profound in severely depressed subjects and where suicidality, impulsivity and/or aggression is present [ 51 , 53 , 62 – 64 ]. Increased cortical 5-HT 2A receptors (5-HT 2A R), ante- [ 65 ] and post-mortem [ 66 – 70 ], have also repeatedly been associated with depression or depression-related personality traits [ 71 , 72 ]. This link between increased frontal 5-HT 2A Rs and suicidality is widely quoted. By contrast, hippocampal 5-HT 1A Rs appear to be decreased in chronic depressed patients, indicating that general upregulation of 5-HT receptors (hypothetically as compensation to lower levels of 5-HT Ext ) does not occur in depression [ 73 ]. However, several groups report no association between frontal cortex 5-HT 2A R levels and suicide [ 74 , 75 ]. These discrepancies could be attributed to the hypothesis that elevated 5-HT 2A R levels more closely correlate with aggression [ 76 ] and that both age [ 76 ] and antidepressant treatment [ 77 ] may alter 5-HT 2A R levels, or, more parsimoniously, that 5-HT 2A R levels do not strongly associate with depression or suicidality.
Another reasonably robust 5-HT system-related finding in depression is a blunted hypothermic response to 5-HT 1A R agonists [ 64 , 78 – 80 ]. It is noteworthy that these studies examined either inpatients specifically or cohorts with a large proportion of inpatients, indicative of an association between the blunted 5-HT 1A R agonist hypothermic response and severe depression. Indeed, one study found a blunted hypothermic response only in depression patients with a history of suicidality [ 64 ]. The biological anomalies accounting for the blunted 5-HT 1A R agonist hypothermic response in depression are not clear, but are usually ascribed to deficiencies in 5-HT 1A R function rather than brain 5-HT deficiency per se . Admittedly, it is not immediately obvious that a blunted 5-HT 1A R agonist hypothermic response could result from brain 5-HT deficiency. For instance, chronic enhancement of 5-HT Ext actually results in blunted 5-HT 1A R-agonist-induced hypothermia in rodents and humans, likely through somatic 5-HT 1A R autoreceptor desensitization [ 81 , 82 ]. Further, as is detailed later in the text, the three other 5-HT biomarker anomalies reported in depression—low CSF 5-HIAA, a blunted plasma prolactin response to fenfluramine and increased frontal cortex 5-HT 2A Rs—had not until very recently been demonstrated to result from low endogenous 5-HT production and decreased 5-HT Ext [ 83 ] (see later text). The evidence for 5-HT deficiency in depression is summarized in table 1 .
Evidence for 5-HT deficiency in depression.
In short, the association of 5-HT deficiency, or any other singular biochemical anomaly, with major depression as the all-encompassing syndrome is inconclusive. Given the great heterogeneity of symptomatology and disease severity between patients, compounded by the likely multitudes of aetiologies underlying depression as a syndrome, the difficulty of pinning the disease to a specific biochemical system is not surprising. Yet, when focusing the analysis on severe depression and/or suicidality, the combined constellation of 5-HT biomarker findings does suggest that 5-HT deficiency is associated with these depression subpopulations. While an association alone does not prove causality, it is piquing that SSRIs exactly demonstrate convincing superiority to placebo in severe depression only [ 9 – 11 ] and that in adult depression patients epidemiological studies find SSRIs to have a modest protective effect against suicide [ 93 ]. It is thus tempting to hypothesize that 5-HT deficiency is a significant causal factor in a specific subset of depression patients. This view would be consistent with the general clinical finding in double-blind SSRI antidepressant trials: at best, SSRIs produce a 10–20% greater response rate in patients receiving the drug versus those treated with placebo [ 94 , 95 ]. In fact, SSRIs fail to show superior antidepressant effect compared with placebo in 40 per cent of clinical trials [ 9 , 11 ]. A parsimonious explanation for the rather disappointing clinical data could be the simple fact that SSRIs are ineffective in a majority of patients and exhibit only antidepressant effects in individuals for whom 5-HT deficiency is a causal factor. On the other hand, because SSRIs and other 5-HTergic antidepressants depend on endogenous 5-HT to enhance 5-HT Ext , a large deficit in endogenous 5-HT could conceivably undermine the antidepressant response by limiting the ability of SSRIs to enhance 5-HT Ext.
4. Tryptophan hydroxylase
The rate-limiting step in 5-HT synthesis is the conversion of tryptophan to 5-HTP, catalysed by tryptophan hydroxylase (Tph) [ 96 , 97 ]. 5-HTP is subsequently converted to 5-HT by amino acid decarboxylase, the enzyme also converting l -DOPA to dopamine [ 98 ]. Tph exists in two isoforms (1 and 2) that show mostly non-overlapping distribution patterns [ 99 ]. Tph1 is largely responsible for 5-HT synthesis in the periphery and in the brain is found solely in the pineal gland [ 99 , 100 ]. Tph2 was only recently discovered [ 84 ] and is expressed in the brain exclusively in 5-HTergic neurons in the midbrain [ 84 , 100 ]. Tph2 is also found in enteric neurons [ 101 ], though gut 5-HT is predominantly synthesized via Tph1 [ 102 ]. (Tph1 −/− mice exhibit greater than 90% depletion of blood and gut 5-HT [ 84 , 100 , 102 ] while brain 5-HT levels are virtually unchanged [ 84 , 100 ].) Further, Tph1 mRNA is undetectable in cultured 5-HTergic neurons [ 103 ]. By contrast, Tph2 −/− mice have normal blood and gut levels of 5-HT [ 102 ], but greater than 95 per cent depletion of forebrain 5-HT [ 102 ]. A large body of literature, mostly pre-dating the discovery of Tph2, reported associations of TPH1 (then simply TPH ) with affective disorders and particularly suicide [ 85 – 87 ]. On the face of it, this can seem perplexing because we now know that the Tph isoform investigated, Tph1, does not play any significant role in brain 5-HT synthesis in the adult. However, an elegant study showed that mouse pups born to Tph1 −/− dams exhibit developmental anomalies in the brain and several other organs, irrespective of whether the pups were Tph1 −/− or +/− , indicating an important role for maternal peripheral, and hence Tph1-derived, 5-HT in brain development [ 88 ]. If these results are reproducible, they could provide one rationale for how TPH1 polymorphisms may influence brain development and thereby impact the occurrence of psychiatric disorders.
5. Tph2: depression and suicidality
The discovery of Tph2 as the enzyme catalysing brain 5-HT synthesis has spawned a vibrant field of research producing a multitude of reports that draw associations between genetic variants in the Tph2 gene and psychiatric disorders, including depression [ 104 , 105 ], bipolar disorder [ 89 ], attention deficit hyperactivity disorder [ 90 ] and, not surprisingly, suicidality as an independent dimensionality [ 91 , 106 – 108 ]. A comprehensive review of the TPH2 -depression association literature is beyond the scope of the present review. For the majority of TPH2 variants found linked to psychiatric disorders the functional implications are still unclear; for instance, when such variants are located in non-coding regions and analysis of the impact on gene transcription or translation is unavailable. We will therefore focus on published data relating depression-associated TPH2 variants with functional outcome measures.
Our group discovered a functional (i.e. protein sequence altering) single-nucleotide polymorphism (SNP) in exon 11 of TPH2 causing an amino acid substitution of Arg 441 to His 441 in a human geriatric sample in North Carolina, USA [ 104 ]. The Tph2 His 441 mutant was overrepresented among subjects with a history of depression when compared with subjects without. It is unlikely, though, that the Tph2 His 441 SNP is a general genetic risk factor for depression because this SNP was subsequently reported to be absent from several other geographical and ethnic populations [ 24 , 107 , 109 ]. Rather, these findings indicate that the Tph2 His 441 SNP may be a locally confined founder-mutation. The few other functional human Tph2 SNPs reported also frequently fail to appear consistently across cohorts and populations [ 110 ] or have been found within single families only [ 111 ]. Nevertheless, as pointed out earlier, it is hardly surprising that no single gene, let alone SNP, is a consistently high-risk factor for depression. Different variants in the TPH2 , and/or in other genes coding for proteins involved in 5-HT homeostasis, could all yield a similar outcome: reduced 5-HT Ext and/or aberrant 5-HT neurotransmission. An interesting comparison may be drawn between this notion and the genetics of phenylketonuria (PKU). In this disease, more than 500 distinct mutations in phenylalanine hydroxylase (PAH), another member of the small family of amino acid hydroxylases, may each give rise to varying degrees of reduced PAH activity, inversely corresponding to severity of PKU [ 112 ]. Typically, only a small subset of these PAH alleles are responsible for the majority of PKU cases, and the prevalent pathogenic PAH alleles are distinct between distinct geographical locales [ 112 ]. Functional in vitro analysis in PC12 cells demonstrated an approximately 80 per cent reduction in the 5-HT synthesizing capabilities of the Tph2 His 441 enzyme when compared with Tph2 Arg 441 . Our analysis did not find evidence of altered oligomerization and stability of the Tph2 His 441 enzyme. Two separate reports likewise found that 5-HT synthesis by the Tph2 His 441 enzyme was decreased by greater than 80 per cent using bacterial and immortalized human (HEK 293 cell) expression systems [ 92 , 113 ]. Coincidently, these findings are analogous to findings in PKU. Amino acid 441 in Tph2 is analogous to amino acid 408 in PAH and the mutation of PAH Arg 408 to Trp 408 causes a severe loss of function of PAH and is the most common pathogenic PAH mutation in PKU in western Europe [ 112 , 114 ].
Several other studies have addressed in a variety of ways the functional consequences of depression-related TPH2 variants. One study investigating Tph2 polymorphisms in several ethnic cohorts reported an association with anxiety, depression and suicidal behaviour in Finnish whites, US whites and African Americans and a specific TPH2 haplotype designated 212121. While the functionality of haplotype 212121 was not investigated on an enzymatic level, its association with CSF 5-HIAA was investigated in a sub-cohort of Finnish suicide attempters and non-psychiatric controls. The 212121 haplotype was associated with reduced CSF 5-HIAA, although only within control individuals without a psychiatric diagnosis. While not addressed by the authors directly, it is interesting to note that the data presented indicate that suicide attempters, regardless of haplotype, had lower CSF 5-HIAA when compared with controls who did not carry the 212121 haplotype [ 107 ].
A second study reported an association between a functional TPH2 SNP, leading to a Ser 41 to Tyr 41 substitution, and bipolar disorder in Taiwanese Han Chinese. When expressed in cells, the Tph2 Tyr 41 variant synthesized one-third less 5-HT when compared with the normal Tph2 Ser 41 enzyme [ 110 ]. The same study also investigated the potential effects of TPH2 promoter variants on transcription efficacy and found that the −703G/−473A haplotype associated with bipolar disorder displayed reduced transcription efficiency in an in vitro luciferase assay as well as reduced binding to the transcription factor POU3F2, when compared with the predominant −703T/−473T haplotype [ 110 ]. The latter promoter findings serve as a potential exemplar for how polymorphisms in upstream elements can affect Tph2 function and 5-HT synthesis. Other studies using similar approaches also reported effects on transcription efficiency of upstream SNPs in TPH2 [ 103 , 115 ] . It would be highly interesting to investigate whether such in vitro effects also translate to alterations in Tph2 protein expression and 5-HT levels, and 5-HT neurotransmission in vivo , particularly because, in contrast to functional Tph2 SNPs that generally are rare, non-coding SNPs appear more common. For instance, the T-703G SNP is detected in most cohorts examined [ 116 – 119 ].
A third study investigating the association of several TPH2 SNPs with bipolar disorder detected a rare (≪1%) functional Tph2 Pro 206 to Ser 206 mutation in both a German and a Russian sample [ 92 ]. The Tph2 Ser 206 variant was significantly overrepresented among bipolar patients in the German sample. This also appeared to be the case in the Russian sample; however, the number of subjects in the Russian sample was too small to allow for meaningful statistical analysis. Interestingly, a subsequent familial analysis of the relatives of some Tph2 Ser 206 carriers with bipolar disease suggested a non-random co-segregation of Tph2 Ser 206 with affective disorder. In vitro analysis in HEK 293 cells demonstrated that the catalytic activity of Tph2 Ser 206 was reduced to approximately 30 per cent of that of Tph2 Pro 206 .
A fourth study of a Norwegian sample reported a novel functional Tph2 Arg 303 to Trp 303 SNP present in a female ADHD patient, then subsequently also found the variant in her daughter who likewise suffered from ADHD [ 111 ]. In vitro analysis indicated that the Tph2 Trp 303 variant had a complete loss of function, suggesting that the two carriers would have suffered from 5-HT deficiency, although both appear to have been heterozygous carriers. While several other family members who did not carry the Trp 303 SNP also presented with a phenotype within the ADHD spectrum, only the mother–daughter pair met a formal ADHD diagnosis. Although such findings are anecdotal, they do provide an illustration of how a Tph2 loss-of-function may precipitate psychiatric disease in a context of familial disposition to ADHD [ 113 ].
In a fifth study [ 120 ], our group reported on investigations into the functional sequelae of a high-frequency (approx. 20% of US population) intronic TPH2 SNP (rs1386493, or ‘A-allele’) previously identified in samples of both psychiatric patients and non-psychiatric controls [ 105 , 121 ]. This SNP located in intron 5 of TPH2 lowers splicing fidelity, thereby leading to the formation of an alternatively spliced transcript, termed Q8N1X9 , which can be detected post-mortem in human brain-stem tissue. The Q8N1X9 transcript is translated into a truncated Tph2 protein (Tph2–TR), lacking part of the catalytic and the entire regulatory domain, which is devoid of catalytic activity. Interestingly, in cell culture, Tph2–TR acts as a dominant negative repressor when co-expressed with wild type (WT) Tph2, causing reduced 5-HT synthesis. The mechanism of repression is not clear at this point, but it is possible that Tph2–TR interferes with proper Tph2 tetramer formation. Sequence analysis using the STAR*D cohort [ 122 ] revealed that the A-allele was not overrepresented in depressed patients versus controls ( n > 1000). This finding was confirmed in another sample of 99 probands from the NIMH Depression Pedigree [ 123 ]. Hence, the A-allele may not by itself be a genetic risk factor for depression. However, it is possible that in conjunction with other genetic variants affecting 5-HT homeostasis, the A-allele could impact susceptibility to the development of psychiatric disorders.
In summary, the evidence for associations of functional coding and non-coding polymorphisms in TPH2 with depression and other psychiatric diseases is growing. A picture is emerging that Tph2 variants collectively are common correlates of depression but that no singular variant is a major determinant in the disease. Intriguingly, distinct Tph2 variants appear to be important in distinct populations and several of these variants have been independently shown to cause loss-of-function of the Tph2 enzyme. It seems quite reasonable that a multitude of different mutations in TPH2 as well as mutations in genes for other proteins important for 5-HT homeostasis, e.g. the r1 protein, a repressor for MAO A [ 124 ], may converge on the same functional endpoint: reduced 5-HT synthesis and consequent brain 5-HT deficiency. In turn, there exists a multipronged body of evidence in support for 5-HT deficiency as an important vulnerability factor in depression [ 83 , 125 , 126 ]. Thus, TPH2 gene variants leading to impaired Tph2 function could, alone or in conjunction with other genetic vulnerability factors, predispose to depression precipitated by for instance life stressors [ 19 , 20 ]. Finally, future studies simultaneously assessing multiple SNPs in 5-HT system genes in depression patients and healthy controls coupled with functional evaluation of disease-associated SNPs could provide a clearer picture of the degree to which 5-HT deficiency associates with depression.
6. Consequences of naturally occurring murine Tph2 functional variants for the 5-HT system
That 5-HT deficiency necessarily follows decreased Tph2 function per se in all cases may not be a given. While the effective absence of brain 5-HT in Tph2 −/− mice demonstrates that Tph2 is indeed responsible for brain 5-HT synthesis [ 102 ], there is a clear conceptual distinction between complete and partial loss of function. For instance, Tph2 +/− mice have normal brain tissue levels of 5-HT and 5-HIAA [ 127 ]. Further, the 5-HT system contains several feedback systems: most notably somatic 5-HT 1A and terminal 5-HT 1B autoreceptors that provide homeostatic regulation of 5-HT Ext [ 128 ]. Through these and additional mechanisms normal 5-HT release and 5-HT Ext levels, and thereby 5-HT neurotransmission, could be preserved despite reduced Tph2-mediated 5-HT synthesis. Because brain 5-HT function cannot directly be assessed in humans, animal models are indispensible for critically evaluating the functional consequences of hypomorphic Tph2 variants on 5-HT neurotransmission.
7. Naturally occurring murine Tph2 functional polymorphisms
As a potentially interesting parallel to human TPH2 polymorphisms present in specific individuals and seemingly in distinct geographical populations, our group discovered that different commonly used laboratory mouse strains carry different coding variants of tph2 [ 129 ]. Mouse strains examined carrying the Pro 447 coding variant, i.e. C57Bl/6 and 129X1/SvJ mice, displayed two- to threefold faster 5-HT synthesis, as measured by 5-HTP accumulation during amino acid decarboxylase inhibition, and twofold higher brain 5-HT tissue levels when compared with strains carrying the Arg 447 variant, i.e. DBA/2 and Balb/Cj mice. Another group subsequently independently confirmed these findings on inter-strain differences in 5-HT synthesis and tissue levels [ 130 ]. This group further demonstrated that the DBA/2 and Balb/Cj strains carrying the catalytically less active Tph2 Arg 447 variant were insensitive to the SSRI citalopram in the forced swim test , a mouse model used to assess the antidepressant potential of compounds, which is generally responsive to pro-5-HTergics [ 131 ]. In contrast, the C57Bl/6 and 129X1/SvJ strains carrying the catalytically more active Tph2 Pro 447 variant did display antidepressant-like responses to citalopram in the forced swim test [ 130 ]. Moreover, likely underlying the behavioural findings, microdialysis studies showed that C57Bl/6 mice had higher baseline 5-HT Ext and a numerically more pronounced 5-HT Ext response to acute citalopram when compared with DBA/2 and Balb/Cj mice [ 132 ]. However, while strain comparison data are informative and can be used to model aspects of genetic variability, a caveat inherent in this approach is the difficulty of attributing phenotypic differences to one gene, given the obvious multiple genetic differences between strains.
Other factors further complicate the interpretation of strain comparison data. One study did not find the Tph2 Pro 447 variant to be a determinant for SSRI antidepressant-like behavioural responsiveness in the forced swim test [ 131 ], i.e. finding DBA/2 and Balb/Cj mice to display SSRI antidepressant-like responses to SSRIs. Another study likewise found that the Tph2 Pro 447 variant did not determine SSRI antidepressant-like responses in the tail-suspension test , a model conceptually related to the forced swim test [ 133 ]. The reason for these discrepant findings is not clear but could relate to differences in specific methodology as well as housing conditions and ambient stress in the colony. It should also be noted that many presumably homogenous inbred mouse strains exist in several sub-strains, founded from a limited number of founder breeders bred separately for decades within individual academic laboratories and commercial breeding facilities. Resulting sub-strain differences could affect behavioural responses.
To address the significance of genetic context on the functional consequences of the Tph2 Arg 447 variant for 5-HT synthesis and 5-HT tissue levels, our laboratory generated congenic mice lines by backcrossing Tph2 Arg 447 or Pro 447 onto two mouse lines, C57Bl/6 and Balb/Cj, usually carrying the opposite Tph2 variant, Pro 447 or Arg 447 , respectively [ 134 ]. We found that Tph2 variant indeed determined 5-HT synthesis, as assessed by in vivo 5-HTP accumulation under amino acid decarboxylase inhibition. Surprisingly, however, 5-HT brain tissue levels were generally not affected by whether C57Bl/6 and Balb/Cj mice harboured one or the other Tph2 variant. The reasons for this remain obscure at this point. Formation and degradation of 5-HT is a dynamic process and preserved 5-HT levels in the face of reduced synthesis could be due to decreased catabolism by MAO. However, investigations of MAO (A and B) mRNA expression and enzymatic activity did not detect any compensatory changes. Further, whether mice carried Tph2 Pro 447 or Arg 447 had no effect on baseline immobility in the tail-suspension test and did not affect the antidepressant-like (reduced immobility) response to escitalopram in either mouse strain. A previous study, describing C57Bl/6 mice carrying either Tph2 Pro 447 or Arg 447 , also found no effect of Tph2 variant on 5-HT brain tissue levels or anxiety and depression-like behaviours [ 135 ]. In apparent contradiction to our results, this previous study found no difference in 5-HT synthesis rate. However, methodological differences could likely play into these discrepant results. In contrast to our study, the assessment of 5-HT synthesis rate was performed using an in vitro approach in which brain tissue was homogenized, and then 5-HT synthesis assayed by addition of exogenous tryptophan, amino acid decarboxylase inhibitor and cofactors. It is not clear whether such an approach mimics the biochemical conditions under which Tph2 catalyses 5-HTP formation in vivo . In any case, it is important here to note that neither the previous, nor our, study assessed the levels of 5-HT Ext , the functionally receptor-active pool of 5-HT. While tissue levels of monoamines under specific conditions (see for instance later text) can reflect functionality, in many cases they do not, sometimes to a surprising degree. For instance, both serotonin transporter (SERT) −/− and overexpressing knockin (KI) mice have decreased tissue levels of 5-HT, but, as expected, increased [ 136 ] and decreased [ 38 ] 5-HT Ext , respectively. Therefore, although tissue 5-HT and the assessed depression behaviours were not affected, any firm conclusion on the relative importance of the murine Tph2 Pro 447 and Arg 447 variants for 5-HT neurotransmission cannot be made.

8. Murine 5-HT system functional differences not related to Tph2 or 5-HT synthesis
Obviously, many other factors could contribute to decreased 5-HT levels and function. In a report co-authored by one of us, it was reported that the NMRI strain from a specific supplier of mice was completely insensitive to several different SSRIs in the tail-suspension test [ 137 ]. The NMRI mice had only approximately 50 per cent the tissue 5-HT and 5-HT Ext levels of C57Bl/6 mice, a strain that strongly responded to SSRIs. NMRI mice also displayed a relatively blunted increase in 5-HT Ext after acute SSRI treatment when compared with C57Bl/6 mice. However, unexpectedly, 5-HT synthesis (5-HTP accumulation) was equal (frontal cortex) or even higher (hippocampus) in the NMRI compared with the C57Bl/6 mice. Both strains carried the Tph2 Pro 447 variant. A salient finding in this study was that acutely co-administering a low dose of 5-HTP with the SSRI conferred sensitivity in the tail-suspension test in the NMRI mice and likewise augmented the SSRI-induced 5-HT Ext response to a level equivalent to the one observed in C57Bl/6 mice. Combined with the constraints inherent in strain comparison studies, these data indicate that low 5-HT levels can occur, irrespective of Tph2 activity and that low 5-HT levels can have functional consequences that can be reversed by enhancing 5-HT levels using exogenous 5-HTP.
9. The Tph2 His 439 knockin mouse: a naturalistic murine model of 5-HT deficiency
Following the discovery by our laboratory of the rare Arg 441 to His 441 Tph2 SNP in a cohort of Eastern US geriatric depression patients, we generated a KI mouse by homologous recombination carrying the Arg 441 to His 441 substitution at the analogous location (amino acid 439) in the mouse Tph2 [ 138 ]. Characterization of this mouse model, henceforth referred to as the Tph2KI mouse, revealed that in vivo 5-HT synthesis (5-HTP accumulation) and 5-HT tissue levels were decreased by 60–80%, depending on region. 5-HIAA levels were even more depleted, measuring less than 5 per cent of WT ( figure 2 ). Tph2 and SERT gene expression and SERT protein levels were not affected in the Tph2KI mice. Behaviourally, the Tph2KI mice presented with an intriguing baseline phenotype ( figure 1 ), namely apparent exaggerated emotional responses when challenged in paradigms relevant for depression, anxiety and aggression. Specifically, immobility in the tail-suspension test was increased, indicative of a depression-like phenotype. Latency to enter the lit area in the light–dark box , a paradigm assessing conflict anxiety/phobia was increased, indicating an anxiety-like phenotype. In the dyadic test , a paradigm assessing social interaction and aggression in a novel environment, the Tph2KI mice displayed significantly increased attack behaviour, indicating an aggressive phenotype. In a later study, we found that marble burying , a putative anxiety-like behaviour [ 139 ], was increased in the Tph2KI mice [ 83 ]. We have also found that Tph2 mice lever-press less for sucrose rewards ( figure 1 c ), although these mice do not differ from WT in sucrose drinking in a standard free-drinking two-bottle choice paradigm (J. Jacobsen 2011, unpublished data), indicating a defined anhedonic phenotype. Further studies are underway to address the essential question of whether 5-HT deficiency in the Tph2KI mice imparts increased vulnerability to chronic stressors, i.e. modelled in mice by chronic mild stress and social defeat . Although rodent depression models bear limited resemblance to depression in humans, the findings obtained thus far are highly intriguing. As outlined earlier, a collective body of clinical evidence indicates that depression, particularly when severe and/or coinciding with suicidality, appears to be associated with indices of low endogenous 5-HT function. Further, crossing Tph2KI mice to heterozygous glycogen synthase kinase 3β (GSK3β) knockout (KO) mice reversed the depression/anxiety/aggression-like phenotype, suggesting that signalling pathways involving GSK3β could be centrally involved in mediating the effects of endogenous 5-HT deficiency [ 138 ].
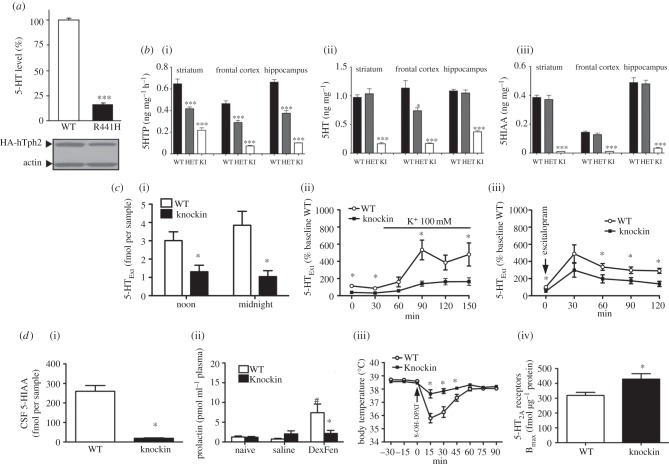
5-HT biochemical and biomarker profile of Tph2KI mice. ( a ) 5-HT synthesis, here assessed by 5-HT levels in cell culture, is decreased by approximately 80 per cent in PC12 cells expressing human Tph2 His 441 (R441H) or WT Tph2 (adapted from [ 104 ]). ( b ) (i) 5-HT synthesis as assessed by 5-HTP accumulation, (ii) 5-HT tissue levels and (iii) levels of the primary metabolite 5-HIAA are drastically reduced in the Tph2KI mice carrying the equivalent murine mutation Tph2 His 439 to the human Tph2 His 441 (adapted from [ 138 ]). ( c ) Microdialysis data showing reduced frontal cortex diurnal 5-HT Ext (i) and reduced 5-HT Ext increases in the Tph2KI mice in response to K depolarization (ii) or SERT blockade with high dose escitalopram (iii) (adapted from [ 83 ]). Together, these biochemical findings demonstrate qualitatively and dynamically preserved but quantitatively reduced 5-HT system function in the Tph2KI mice ( a , b ). ( d ) (i) Reduced CSF 5-HIAA, (ii) blunted fenfluramine (DexFen)-stimulated plasma prolactin, (iii) blunted 5-HT 1A R agonist(8-OH-DPAT)-induced hypothermia and (iv) increased frontal cortex 5-HT 2A Rs in Tph2KI mice (adapted from [ 83 ]). This biomarker profile is remarkably similar to findings reported in depression/suicidality.
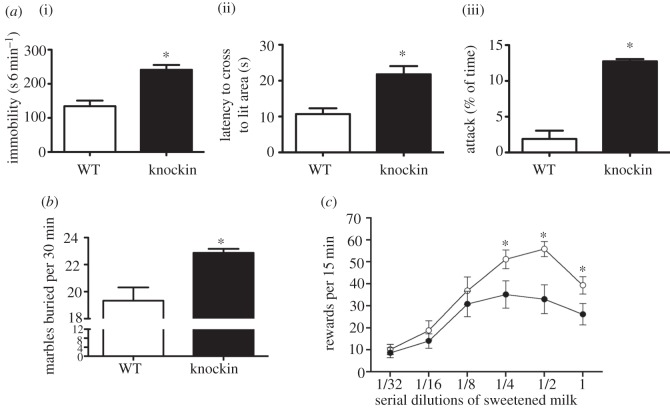
Depression-relevant behaviours in Tph2KI mice. ( a ) (i) Increased tail-suspension immobility (depression-like behaviour, i.e. behavioural despair), (ii) increased latency to cross into lit area in the light-dark box test (anxiety-like behaviour) and (iii) increased male aggression during an encounter with an unknown male in a non-territorial arena (dyadic test) in the Tph2KI (knockin) mice (adapted from Beaulieu et al . [ 138 ]). ( b ) The Tph2KI mice also displayed increased anxiety in the marble burying test (adapted from Jacobsen et al . [ 83 ]). Given random access to various dilutions of sweetened condensed milk both WT and Tph2KI mice displayed inverted U-shaped lever-press responses to obtain the reward. ( c ) However, the Tph2KI mice manifested reduced lever-pressing for sweetened condensed milk, indicating an anhedonic phenotype in this paradigm (previous unpublished data). Filled circles denote knockin, open circles denote WT.
In a subsequent series of experiments, we explored the consequences of endogenous 5-HT deficiency in the Tph2KI mice for 5-HT neurotransmission in more detail [ 83 ]. Using microdialysis, we showed that brain 5-HT Ext indeed was decreased in the Tph2KI mice by 60–70% ( figure 2 ). This is an important, and not necessarily obvious, finding because 5-HT homeostasis appears to be tightly regulated, for instance by presynaptic 5-HT 1A and 5-HT 1B autoreceptors as well as by neural networks involving post-synaptic 5-HT 2A Rs and 5-HT 2C Rs [ 128 , 140 – 144 ]. In fact, although we did not explore this complex and multi-faceted aspect comprehensively, we found little evidence of compensatory homeostatic alterations among 5-HT system elements in the face of 5-HT deficiency in the Tph2KI mice. 5-HT 1A R levels were unchanged in the dorsal raphe as well as the hippocampus and hypothalamus, indicating neither pre- nor post-synaptic compensatory upregulation of 5-HT 1A Rs. Likewise, 5-HT 1A R G-protein coupling, as assessed by agonist-induced GTPγ 35 S binding, in the same regions was unaltered. There was also no change in MAO A and B mRNA levels or MAO catalytic activity. SERT levels as assessed by ligand binding were normal, confirming SERT level data from the initial report obtained by Western blotting [ 138 ]. This apparent lack of homeostatic presynaptic compensations to lifelong endogenous 5-HT deficiency in the TphKI mice is by itself a salient finding. It could signify that the mammalian organism is vulnerable to dysfunction in the brain 5-HT system because the organism does not seem to compensate for decreased availability of brain 5-HT below a certain threshold. (Recall, the moderate 5-HT synthesis deficit consequent to the hypofunctional murine Tph2 Arg 447 variant did appear to be compensated for, at least when assessed on the 5-HT tissue level [ 134 , 135 ].) This would possibly apply, irrespective of the root cause of 5-HT deficiency: whether by hypomorphic Tph2 variants or by different anomalies in 5-HT system elements. This notion is important, and has potential translational relevance, given the multiple studies reporting associations of TPH2 polymorphisms with depression and other psychiatric disorders. The 5-HT biochemical and biomarker phenotypes of the Tph2KI mice are summarized in figure 2 .
10. The Tph2 knockin mouse: a model of reduced, but not abolished, 5-HT neurotransmission
An important distinction between the Tph2KI mice and other murine models of 5-HT disruption, e.g. Tph2 KO mice [ 102 , 145 ], 5-HT neuron-specific vesicular monoamine transporter 2 KO mice [ 146 ], Pet-1 KO mice [ 147 ] and Lmx1b KO mice [ 148 ], lies in the nature of the 5-HT deficiency. In the Tph2KI mouse, 5-HT neurotransmission is qualitatively and dynamically preserved, yet quantitatively reduced. Cell signalling elicited via the fifteen or so brain 5-HT receptors will still occur, albeit to a lesser degree. In contrast, in the other available models mentioned, 5-HT levels and neurotransmission are essentially abolished. Abolishment of function is of course biologically distinct from the reduction of function observed in the Tph2KI mice. This could perhaps account for the at times discrepant findings between different 5-HT deficiency models, e.g. a depressive-like phenotype in the Tph2KI mice versus an antidepressant-like phenotype in the 5-HT neuron-specific vesicular monoamine transporter 2 KO mice [ 146 ] and non-depressant phenotype in the Tph2 KO mice [ 102 ]. (For an excellent and comprehensive review of the neurochemical and behavioural phenotypes of the various murine 5-HT deficiency models, see [ 149 ].) Moreover, complete abolishment of brain 5-HT levels or function is not known to occur in humans, while substantially reduced brain 5-HT levels does seem to be relatively frequent, as judged from biomarkers, and to be associated with depression-related traits in humans [ 43 , 45 , 46 , 78 , 80 , 83 , 150 ].
11. The Tph2 knockin mice recapitulate core clinical 5-HT-related biomarker findings in depression
In contrast to the unchanged 5-HT 1A R functionality in Tph2KI mice, when quantifying frontal 5-HT 2A Rs with ligand binding we found a significant increase, around 20 per cent, that was accompanied by increased 5-HT 2A R functionality as assessed by 5-HT 2A R agonist or 5-HTP SSRI-induced head-twitches, 5-HT 2A R-dependent behaviours. This selective increase in 5-HT 2A Rs consequent to 5-HT deficiency is intriguing because it parallels findings from presumably depressed suicidal individuals, both in terms of the region in question as well as the moderate magnitude of change [ 65 – 70 ]. We also investigated whether three additional biochemical anomalies associated with depression and 5-HT function—discussed earlier—could be found in the Tph2KI mice. For two of these, decrements had long been assumed, but never actually shown, to reflect brain 5-HT deficiency, namely CSF 5-HIAA- and fenfluramine-induced plasma prolactin. We found that CSF 5-HIAA was reduced by approximately 90 per cent in Tph2KI compared with WT mice. This demonstrates that endogenous 5-HT deficiency indeed can manifest as low CSF 5-HIAA. Notably, the decrement in CSF 5-HIAA levels in the Tph2KI mice was within the range of decrement encountered in clinical populations, for instance in suicidal patients [ 46 ]. In the Tph2KI mice, the plasma prolactin response to fenfluramine was essentially abolished, while in WT mice a robust plasma prolactin surge was observed. Again, similar essentially abolished fenfluramine prolactin responses have been reported in depressed inpatients [ 150 ]. Another study also reported completely abolished plasma prolactin responses to the SSRI citalopram in currently depressed and recovered depression outpatients [ 55 ]. SSRIs produce a less pronounced acute 5-HT Ext surge when compared with fenfluramine; SSRIs could therefore conceivably reveal less-marked brain 5-HT deficits. (SSRIs produced a minimal plasma prolactin response in both WT and Tph2KI mice, hindering assessment of SSRI-induced prolactin responses as a brain 5-HT probe in our model system; J. Jacobsen 2011, unpublished data). A fourth 5-HT system anomaly that has been reported in depression is blunted 5-HT 1A R-agonist-induced hypothermia [ 78 – 80 ]. As mentioned earlier, this has been ascribed to 5-HT 1A R dysfunction rather than any changes in 5-HT levels . When challenged with the 5-HT 1A R agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT), WT mice exhibited a 3°C decrease in body temperature. In the Tph2KI mice, the temperature drop was markedly attenuated, to 1°C. Yet again, qualitatively, these findings closely parallel clinical findings in depressed inpatients [ 79 ], although the 5-HT 1A R agonist hypothermic responses in humans are of a generally smaller magnitude. The finding that 5-HT 1A R-agonist-induced hypothermia is blunted but 5-HT 1A R function seemingly unimpaired in Tph2KI mice, in conjunction with previous reports that pharmacological or toxin-induced depletion of brain 5-HT attenuates 8-OH-DPAT hypothermia [ 151 , 152 ], suggest that 5-HT 1A R-agonist-induced hypothermia could be a novel biomarker of low endogenous brain 5-HT Ext . We are currently pursuing this angle pre-clinically in the laboratory. One caveat stems from apparent species differences within the mechanisms underlying 5-HT 1A R-agonist-induced hypothermia, involving pre-synaptic 5-HT 1A receptors in the mouse, but apparently post -synaptic 5-HT 1A receptors in the rat [ 152 ]. Still, if the mouse data translate to humans, the implication is that the observed blunted hypothermic responses in depression patients could be a consequence of 5-HT deficiency rather than dysfunction in pre-synaptic 5-HT 1A receptors. Conceivably, 5-HT 1A agonist-induced hypothermia could be used as a clinical research (if not general practice) tool, perhaps together with other non-invasive 5-HT biomarkers, such as fenfluramine-induced plasma prolactin, to identify patients more likely to have low 5-HT, which, in turn, could influence treatment response to specific classes of existing and novel antidepressants.
12. Conclusions and perspectives
Because depression symptomatology can occur subsequent to diverse disorders, e.g. endocrine, immunological, neurological [ 14 – 18 ], it is tempting to speculate that depression can be a sequelae of cell biological distortions in general and not only from deficits in discrete systems, perhaps particularly when additional physiological and/or social vulnerability factors are present. Thus, while it seems rather unlikely that 5-HT deficiency or dysfunction is the cause of major depression, the clinical evidence in toto points to the presence of 5-HT deficiency in at least some depressed individuals, perhaps most convincingly when suicidality or aggression is present. 5-HT deficiency could conceivably arise from multiple different defects in one or more of the various components of the 5-HT system. The multiple distinct mutations in TPH2 may be a representation of such a notion.
The Tph2KI mice display marked functional 5-HT deficiency, seemingly without pre-synaptic compensation, although post-synaptic 5-HT receptor function may be enhanced for specific 5-HT receptors, e.g. 5-HT 2A R. While the mice appear, breed and grow normal, they show a startling range of behavioural, biochemical and physiological anomalies, which parallel findings in depression patients. Notwithstanding the requisite reservations for the validity of animal models of such a uniquely human condition as depression, these characteristics of the Tph2KI mice offer support to the notion of 5-HT deficiency as one, although hardly the only, important disposing factor in depression.
Further studies are in progress in our laboratory to address the question of whether 5-HT deficiency is a vulnerability factor to depression-like behaviours. Specifically, we are investigating whether the Tph2 mice are more susceptible to chronic social and environmental stressors. We are also exploring the consequences of 5-HT deficiency in the Tph2KI mouse on neurogenesis, an important line of studies given the hypothesized framework connecting low 5-HT, decreased neurotrophic stimulation, hippocampal dysfunction and depression [ 153 ]. We are further investigating whether behavioural aberrations in the Tph2KI mice can be reversed by reinstating 5-HT Ext in the adult mouse, thereby seeking to define whether these aberrations are state, owing to 5-HT deficiency per se , or trait, a consequence of developmental alterations.
Importantly, conformation that the predicted anomalies in putative 5-HT biomarkers indeed occur in the Tph2KI mice, where 5-HT deficiency in contrast to the human can be unequivocally established, lends credence to the large body of clinical literature relying on these 5-HT biomarkers. Previously, that blunted fenfluramine-induced plasma prolactin response and decreased CSF 5-HIAA indeed would result from low brain 5-HT Ext had not been established in any model. Further, that two of the most commonly reported 5-HT-system-related biochemical anomalies—increased frontal cortex 5-HT 2A R and blunted 5-HT 1A R-agonist-induced hypothermia—indeed occur consequent to congenital 5-HT deficiency was not necessarily predictable and had likewise not been demonstrated before. The similarities between depression in humans and the phenotype of the Tph2KI mice are summarized in table 2 .
Similarities between findings in depression patients and Tph2KI mice.
a Reflects the opinion of the authors.
In conclusion, the Tph2KI mouse model of naturalistic 5-HT deficiency has already yielded important contributions to the fields of 5-HT and depression. Future studies will be able to address core questions regarding 5-HT, stress and depression biology in a pre-clinical setting with fidelity hitherto not possible. The generation of additional naturalistic models of 5-HT deficiency and of other neurotransmitter systems where reduced function is believed to have important neuropsychopathophysiological consequences is a worthwhile approach in an attempt to further our understanding of the underpinnings of brain disorders.
Acknowledgements
We acknowledge Dr Benjamin Sachs for helpful comments and discussion and Meghan Rudder for careful editing of the manuscript. This work was supported in part by grants from the National Institutes of Health (RO1 MH-079201, and P50 MH-060451). Support from the Lennon Family Foundation to M.G.C. for the initial part of this work is also greatly appreciated. We also acknowledge the generous support of H. Lundbeck A/S for unrestricted gifts to Duke University. J.P.R.J. is the grateful recipient of an individual grant from The Lundbeck Foundation of Denmark.
- 1. Lopez-Munoz F., Alamo C. 2009. Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today. Curr. Pharm. Des. 15, 1563–1586 10.2174/138161209788168001 ( doi:10.2174/138161209788168001 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Schildkraut J. J. 1965. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am. J. Psychiatry 122, 509–522 [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Persson T., Roos B. E. 1967. 5-hydroxytryptophan for depression. Lancet 2, 987–988 10.1016/S0140-6736(67)90824-0 ( doi:10.1016/S0140-6736(67)90824-0 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Udenfriend S., Weissbach H., Bogdanski D. F. 1957. Increase in tissue serotonin following administration of its precursor 5-hydroxytryptophan. J. Biol. Chem. 224, 803–810 [ PubMed ] [ Google Scholar ]
- 5. Millan M. J., Gobert A., Lejeune F., Newman-Tancredi A., Rivet J. M., Auclair A., Peglion J. L. 2001. S33005, a novel ligand at both serotonin and norepinephrine transporters. I. Receptor binding, electrophysiological, and neurochemical profile in comparison with venlafaxine, reboxetine, citalopram, and clomipramine. J. Pharmacol. Exp. Ther. 298, 565–580 [ PubMed ] [ Google Scholar ]
- 6. Coppen A. 1967. The biochemistry of affective disorders. Br. J. Psychiatry 113, 1237–1264 10.1192/bjp.113.504.1237 ( doi:10.1192/bjp.113.504.1237 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Lapin I. P., Oxenkrug G. F. 1969. Intensification of the central serotoninergic processes as a possible determinant of the thymoleptic effect. Lancet 1, 132–136 10.1016/S0140-6736(69)91140-4 ( doi:10.1016/S0140-6736(69)91140-4 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Mendels J., Stinnett J. L., Burns D., Frazer A. 1975. Amine precursors and depression. Arch. Gen. Psychiatry 32, 22–30 10.1001/archpsyc.1975.01760190024002 ( doi:10.1001/archpsyc.1975.01760190024002 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Kirsch I., Deacon B. J., Huedo-Medina T. B., Scoboria A., Moore T. J., Johnson B. T. 2008. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 5, e45. 10.1371/journal.pmed.0050045 ( doi:10.1371/journal.pmed.0050045 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Papakostas G. I., Fan H., Tedeschini E. 2011. Severe and anxious depression: combining definitions of clinical sub-types to identify patients differentially responsive to selective serotonin reuptake inhibitors. Eur. Neuropsychopharmacol. 22, 347–355 10.1016/j.euroneuro.2011.09.009 ( doi:10.1016/j.euroneuro.2011.09.009 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Khan A., Leventhal R. M., Khan S. R., Brown W. A. 2002. Severity of depression and response to antidepressants and placebo: an analysis of the Food and Drug Administration database. J. Clin. Psychopharmacol. 22, 40–45 10.1097/00004714-200202000-00007 ( doi:10.1097/00004714-200202000-00007 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Chen L., Eaton W. W., Gallo J. J., Nestadt G. 2000. Understanding the heterogeneity of depression through the triad of symptoms, course and risk factors: a longitudinal, population-based study. J. Affect. Disord. 59, 1–11 10.1016/S0165-0327(99)00132-9 ( doi:10.1016/S0165-0327(99)00132-9 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Taylor M. A., Fink M. 2008. Restoring melancholia in the classification of mood disorders. J. Affect. Disord. 105, 1–14 10.1016/j.jad.2007.05.023 ( doi:10.1016/j.jad.2007.05.023 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Burns A., Jacoby R., Levy R. 1990. Psychiatric phenomena in Alzheimer's disease. III. Disorders of mood. Br. J. Psychiatry 157, 92–94 [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Veazey C., Aki S. O., Cook K. F., Lai E. C., Kunik M. E. 2005. Prevalence and treatment of depression in Parkinson's disease. J. Neuropsychiatry Clin. Neurosci. 17, 310–323 10.1176/appi.neuropsych.17.3.310 ( doi:10.1176/appi.neuropsych.17.3.310 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Wall C. A., Rummans T. A., Aksamit A. J., Krahn L. E., Pankratz V. S. 2005. Psychiatric manifestations of Creutzfeldt–Jakob disease: a 25-year analysis. J. Neuropsychiatry Clin. Neurosci. 17, 489–495 10.1176/appi.neuropsych.17.4.489 ( doi:10.1176/appi.neuropsych.17.4.489 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Dieperink E., Willenbring M., Ho S. B. 2000. Neuropsychiatric symptoms associated with hepatitis C and interferon alpha: a review. Am. J. Psychiatry 157, 867–876 10.1176/appi.ajp.157.6.867 ( doi:10.1176/appi.ajp.157.6.867 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Krystal A., Krishnan K. R., Raitiere M., Poland R., Ritchie J. C., Dunnick N. R., Hanada K., Nemeroff C. B. 1990. Differential diagnosis and pathophysiology of Cushing's syndrome and primary affective disorder. J. Neuropsychiatry Clin. Neurosci. 2, 34–43 [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Kendler K. S., Karkowski L. M., Prescott C. A. 1999. Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry 156, 837–841 [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Kendler K. S., Kuhn J., Prescott C. A. 2004. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am. J. Psychiatry 161, 631–636 10.1176/appi.ajp.161.4.631 ( doi:10.1176/appi.ajp.161.4.631 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Meltzer H., Bebbington P., Brugha T., Jenkins R., McManus S., Stansfeld S. 2010. Job insecurity, socio-economic circumstances and depression. Psychol. Med. 40, 1401–1407 10.1017/S0033291709991802 ( doi:10.1017/S0033291709991802 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Levinson D. F. 2006. The genetics of depression: a review. Biol. Psychiatry 60, 84–92 10.1016/j.biopsych.2005.08.024 ( doi:10.1016/j.biopsych.2005.08.024 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Rietschel M., et al. 2010. Genome-wide association-, replication-, and neuroimaging study implicates HOMER1 in the etiology of major depression. Biol. Psychiatry 68, 578–585 10.1016/j.biopsych.2010.05.038 ( doi:10.1016/j.biopsych.2010.05.038 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Delorme R., et al. 2006. No human tryptophan hydroxylase-2 gene R441H mutation in a large cohort of psychiatric patients and control subjects. Biol. Psychiatry 60, 202–203 10.1016/j.biopsych.2005.12.014 ( doi:10.1016/j.biopsych.2005.12.014 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Leyton M., Young S. N., Benkelfat C. 1997. Relapse of depression after rapid depletion of tryptophan. Lancet 349, 1840–1841 10.1016/S0140-6736(05)61726-6 ( doi:10.1016/S0140-6736(05)61726-6 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Delgado P. L. 2006. Monoamine depletion studies: implications for antidepressant discontinuation syndrome. J. Clin. Psychiatry 67(Suppl. 4), 22–26 [ PubMed ] [ Google Scholar ]
- 27. Porter R. J., Phipps A. J., Gallagher P., Scott A., Stevenson P. S., O'Brien J. T. 2005. Effects of acute tryptophan depletion on mood and cognitive functioning in older recovered depressed subjects. Am. J. Geriatr. Psychiatry 13, 607–615 10.1176/appi.ajgp.13.7.607 ( doi:10.1176/appi.ajgp.13.7.607 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Bender D. A. 1983. Biochemistry of tryptophan in health and disease. Mol. Aspects Med. 6, 101–197 10.1016/0098-2997(83)90005-5 ( doi:10.1016/0098-2997(83)90005-5 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Erhardt S., Olsson S. K., Engberg G. 2009. Pharmacological manipulation of kynurenic acid: potential in the treatment of psychiatric disorders. CNS Drugs 23, 91–101 10.2165/00023210-200923020-00001 ( doi:10.2165/00023210-200923020-00001 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. van Donkelaar E. L., Blokland A., Ferrington L., Kelly P. A., Steinbusch H. W., Prickaerts J. 2011. Mechanism of acute tryptophan depletion: is it only serotonin? Mol. Psychiatry 16, 695–713 10.1038/mp.2011.9 ( doi:10.1038/mp.2011.9 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Muller N., Schwarz M. J. 2007. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol. Psychiatry 12, 988–1000 10.1038/sj.mp.4002006 ( doi:10.1038/sj.mp.4002006 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Raison C. L., Demetrashvili M., Capuron L., Miller A. H. 2005. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS drugs 19, 105–123 10.2165/00023210-200519020-00002 ( doi:10.2165/00023210-200519020-00002 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Akiskal H. S., McKinney W. T., Jr 1973. Depressive disorders: toward a unified hypothesis. Science 182, 20–29 10.1126/science.182.4107.20 ( doi:10.1126/science.182.4107.20 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Baumeister A. A., Hawkins M. F., Uzelac S. M. 2003. The myth of reserpine-induced depression: role in the historical development of the monoamine hypothesis. J. Hist. Neurosci. 12, 207–220 10.1076/jhin.12.2.207.15535 ( doi:10.1076/jhin.12.2.207.15535 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Yoshitake T., Yoshitake S., Fujino K., Nohta H., Yamaguchi M., Kehr J. 2004. High-sensitive liquid chromatographic method for determination of neuronal release of serotonin, noradrenaline and dopamine monitored by microdialysis in the rat prefrontal cortex. J. Neurosci. Methods 140, 163–168 10.1016/j.jneumeth.2004.04.041 ( doi:10.1016/j.jneumeth.2004.04.041 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Davies D. L., Shepherd M. 1955. Reserpine in the treatment of anxious and depressed patients. Lancet 269, 117–120 10.1016/S0140-6736(55)92118-8 ( doi:10.1016/S0140-6736(55)92118-8 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Shaw D. M., Camps F. E., Eccleston E. G. 1967. 5-Hydroxytryptamine in the hind-brain of depressive suicides. Br. J. Psychiatry 113, 1407–1411 10.1192/bjp.113.505.1407 ( doi:10.1192/bjp.113.505.1407 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Jennings K. A., et al. 2006. Increased expression of the 5-HT transporter confers a low-anxiety phenotype linked to decreased 5-HT transmission. J. Neurosci. 26, 8955–8964 10.1523/JNEUROSCI.5356-05.2006 ( doi:10.1523/JNEUROSCI.5356-05.2006 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Jacobsen J. P., Mork A. 2004. The effect of escitalopram, desipramine, electroconvulsive seizures and lithium on brain-derived neurotrophic factor mRNA and protein expression in the rat brain and the correlation to 5-HT and 5-HIAA levels. Brain Res. 1024, 183–192 10.1016/j.brainres.2004.07.065 ( doi:10.1016/j.brainres.2004.07.065 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Gainetdinov R. R., Wetsel W. C., Jones S. R., Levin E. D., Jaber M., Caron M. G. 1999. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science 283, 397–401 10.1126/science.283.5400.397 ( doi:10.1126/science.283.5400.397 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Wester P., Bergstrom U., Eriksson A., Gezelius C., Hardy J., Winblad B. 1990. Ventricular cerebrospinal fluid monoamine transmitter and metabolite concentrations reflect human brain neurochemistry in autopsy cases. J. Neurochem. 54, 1148–1156 10.1111/j.1471-4159.1990.tb01942.x ( doi:10.1111/j.1471-4159.1990.tb01942.x ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Meltzer H. 1989. Serotonergic dysfunction in depression. Br. J. Psychiatry 8, 25–31 [ PubMed ] [ Google Scholar ]
- 43. Asberg M. 1997. Neurotransmitters and suicidal behavior. The evidence from cerebrospinal fluid studies. Ann. N.Y. Acad. Sci. 836, 158–181 10.1111/j.1749-6632.1997.tb52359.x ( doi:10.1111/j.1749-6632.1997.tb52359.x ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Placidi G. P., Oquendo M. A., Malone K. M., Huang Y. Y., Ellis S. P., Mann J. J. 2001. Aggressivity, suicide attempts, and depression: relationship to cerebrospinal fluid monoamine metabolite levels. Biol. Psychiatry 50, 783–791 10.1016/S0006-3223(01)01170-2 ( doi:10.1016/S0006-3223(01)01170-2 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Asberg M., Thoren P., Traskman L., Bertilsson L., Ringberger V. 1976. ‘Serotonin depression’: a biochemical subgroup within the affective disorders? Science 191, 478–480 10.1126/science.1246632 ( doi:10.1126/science.1246632 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Brown G. L., Ebert M. H., Goyer P. F., Jimerson D. C., Klein W. J., Bunney W. E., Goodwin F. K. 1982. Aggression, suicide, and serotonin: relationships to CSF amine metabolites. Am. J. Psychiatry 139, 741–746 [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Leichsenring F., Leibing E., Kruse J., New A. S., Leweke F. 2011. Borderline personality disorder. Lancet 377, 74–84 10.1016/S0140-6736(10)61422-5 ( doi:10.1016/S0140-6736(10)61422-5 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Malkesman O., Pine D. S., Tragon T., Austin D. R., Henter I. D., Chen G., Manji H. K. 2009. Animal models of suicide-trait-related behaviors. Trends Pharmacol. Sci. 30, 165–173 10.1016/j.tips.2009.01.004 ( doi:10.1016/j.tips.2009.01.004 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Emiliano A. B., Fudge J. L. 2004. From galactorrhea to osteopenia: rethinking serotonin-prolactin interactions. Neuropsychopharmacology 29, 833–846 10.1038/sj.npp.1300412 ( doi:10.1038/sj.npp.1300412 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Siever L. J., Murphy D. L., Slater S., de la Vega E., Lipper S. 1984. Plasma prolactin changes following fenfluramine in depressed patients compared to controls: an evaluation of central serotonergic responsivity in depression. Life Sci. 34, 1029–1039 10.1016/0024-3205(84)90016-X ( doi:10.1016/0024-3205(84)90016-X ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Coccaro E. F., Siever L. J., Klar H. M., Maurer G., Cochrane K., Cooper T. B., Mohs R. C., Davis K. L. 1989. Serotonergic studies in patients with affective and personality disorders. Correlates with suicidal and impulsive aggressive behavior. Arch. Gen. Psychiatry 46, 587–599 10.1001/archpsyc.1989.01810070013002 ( doi:10.1001/archpsyc.1989.01810070013002 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. O'Keane V., Dinan T. G. 1991. Prolactin and cortisol responses to d-fenfluramine in major depression: evidence for diminished responsivity of central serotonergic function. Am. J. Psychiatry 148, 1009–1015 [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Mann J. J., McBride P. A., Malone K. M., DeMeo M., Keilp J. 1995. Blunted serotonergic responsivity in depressed inpatients. Neuropsychopharmacology 13, 53–64 10.1016/0893-133X(95)00016-7 ( doi:10.1016/0893-133X(95)00016-7 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Cleare A. J., Bearn J., Allain T., McGregor A., Wessely S., Murray R. M., O'Keane V. 1995. Contrasting neuroendocrine responses in depression and chronic fatigue syndrome. J. Affect. Disord. 34, 283–289 10.1016/0165-0327(95)00026-J ( doi:10.1016/0165-0327(95)00026-J ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Bhagwagar Z., Whale R., Cowen P. J. 2002. State and trait abnormalities in serotonin function in major depression. Br. J. Psychiatry 180, 24–28 10.1192/bjp.180.1.24 ( doi:10.1192/bjp.180.1.24 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Abel K. M., O'Keane V., Murray R. M., Cleare A. J. 1997. Serotonergic function and negative and depressive symptomatology in schizophrenia and major depression. Psychoneuroendocrinology 22, 539–548 10.1016/S0306-4530(97)00050-4 ( doi:10.1016/S0306-4530(97)00050-4 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Lichtenberg P., Shapira B., Gillon D., Kindler S., Cooper T. B., Newman M. E., Lerer B. 1992. Hormone responses to fenfluramine and placebo challenge in endogenous depression. Psychiatry Res. 43, 137–146 10.1016/0165-1781(92)90128-P ( doi:10.1016/0165-1781(92)90128-P ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Maes M., D'Hondt P., Suy E., Minner B., Vandervorst C., Raus J. 1991. HPA-axis hormones and prolactin responses to dextro-fenfluramine in depressed patients and healthy controls. Progr. Neuro-psychopharmacol. Biol. Psychiatry 15, 781–790 10.1016/0278-5846(91)90007-N ( doi:10.1016/0278-5846(91)90007-N ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Kavoussi R. J., Kramer J., Hauger R. L., Coccaro E. F. 1998. Prolactin response to D-fenfluramine in outpatients with major depression. Psychiatry Res. 79, 199–205 10.1016/S0165-1781(98)00039-0 ( doi:10.1016/S0165-1781(98)00039-0 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Weizman A., Mark M., Gil-Ad I., Tyano S., Laron Z. 1988. Plasma cortisol, prolactin, growth hormone, and immunoreactive beta-endorphin response to fenfluramine challenge in depressed patients. Clin. Neuropharmacol. 11, 250–256 10.1097/00002826-198806000-00007 ( doi:10.1097/00002826-198806000-00007 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Asnis G. M., Eisenberg J., van Praag H. M., Lemus C. Z., Friedman J. M., Miller A. H. 1988. The neuroendocrine response to fenfluramine in depressives and normal controls. Biol. Psychiatry 24, 117–120 10.1016/0006-3223(88)90133-3 ( doi:10.1016/0006-3223(88)90133-3 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Fava M., Vuolo R. D., Wright E. C., Nierenberg A. A., Alpert J. E., Rosenbaum J. F. 2000. Fenfluramine challenge in unipolar depression with and without anger attacks. Psychiatry Res. 94, 9–18 10.1016/S0165-1781(00)00120-7 ( doi:10.1016/S0165-1781(00)00120-7 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Newman M. E., Shapira B., Lerer B. 1998. Evaluation of central serotonergic function in affective and related disorders by the fenfluramine challenge test: a critical review. Int. J. Neuropsychopharmacol. 1, 49–69 10.1017/S1461145798001072 ( doi:10.1017/S1461145798001072 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Pitchot W., Hansenne M., Pinto E., Reggers J., Fuchs S., Ansseau M. 2005. 5-Hydroxytryptamine 1A receptors, major depression, and suicidal behavior. Biol. Psychiatry 58, 854–858 10.1016/j.biopsych.2005.05.042 ( doi:10.1016/j.biopsych.2005.05.042 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Bhagwagar Z., Hinz R., Taylor M., Fancy S., Cowen P., Grasby P. 2006. Increased 5-HT(2A) receptor binding in euthymic, medication-free patients recovered from depression: a positron emission study with [(11)C]MDL 100,907. Am. J. Psychiatry 163, 1580–1587 10.1176/appi.ajp.163.9.1580 ( doi:10.1176/appi.ajp.163.9.1580 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Shelton R. C., Sanders-Bush E., Manier D. H., Lewis D. A. 2009. Elevated 5-HT 2A receptors in postmortem prefrontal cortex in major depression is associated with reduced activity of protein kinase A. Neuroscience 158, 1406–1415 10.1016/j.neuroscience.2008.11.036 ( doi:10.1016/j.neuroscience.2008.11.036 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Pandey G. N., Dwivedi Y., Rizavi H. S., Ren X., Pandey S. C., Pesold C., Roberts R. C., Conley R. R., Tamminga C. A. 2002. Higher expression of serotonin 5-HT(2A) receptors in the postmortem brains of teenage suicide victims. Am. J. Psychiatry 159, 419–429 10.1176/appi.ajp.159.3.419 ( doi:10.1176/appi.ajp.159.3.419 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Turecki G., et al. 1999. Prediction of level of serotonin 2A receptor binding by serotonin receptor 2A genetic variation in postmortem brain samples from subjects who did or did not commit suicide. Am. J. Psychiatry 156, 1456–1458 [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Arora R. C., Meltzer H. Y. 1989. Serotonergic measures in the brains of suicide victims: 5-HT2 binding sites in the frontal cortex of suicide victims and control subjects. Am. J. Psychiatry 146, 730–736 [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Stanley M., Mann J. J. 1983. Increased serotonin-2 binding sites in frontal cortex of suicide victims. Lancet 1, 214–216 10.1016/S0140-6736(83)92590-4 ( doi:10.1016/S0140-6736(83)92590-4 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. Frokjaer V. G., et al. 2010. Familial risk for mood disorder and the personality risk factor, neuroticism, interact in their association with frontolimbic serotonin 2A receptor binding. Neuropsychopharmacology 35, 1129–1137 10.1038/npp.2009.218 ( doi:10.1038/npp.2009.218 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 72. Frokjaer V. G., et al. 2008. Frontolimbic serotonin 2A receptor binding in healthy subjects is associated with personality risk factors for affective disorder. Biol. Psychiatry 63, 569–576 10.1016/j.biopsych.2007.07.009 ( doi:10.1016/j.biopsych.2007.07.009 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Drevets W. C., Thase M. E., Moses-Kolko E. L., Price J., Frank E., Kupfer D. J., Mathis C. 2007. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl. Med. Biol. 34, 865–877 10.1016/j.nucmedbio.2007.06.008 ( doi:10.1016/j.nucmedbio.2007.06.008 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Audenaert K., Peremans K., Goethals I., van Heeringen C. 2006. Functional imaging, serotonin and the suicidal brain. Acta Neurol. Belg. 106, 125–131 [ PubMed ] [ Google Scholar ]
- 75. Stockmeier C. A., Dilley G. E., Shapiro L. A., Overholser J. C., Thompson P. A., Meltzer H. Y. 1997. Serotonin receptors in suicide victims with major depression. Neuropsychopharmacology 16, 162–173 10.1016/S0893-133X(96)00170-4 ( doi:10.1016/S0893-133X(96)00170-4 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. Oquendo M. A., Russo S. A., Underwood M. D., Kassir S. A., Ellis S. P., Mann J. J., Arango V. 2006. Higher postmortem prefrontal 5-HT2A receptor binding correlates with lifetime aggression in suicide. Biol. Psychiatry 59, 235–243 10.1016/j.biopsych.2005.06.037 ( doi:10.1016/j.biopsych.2005.06.037 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 77. Peroutka S. J., Snyder S. H. 1980. Long-term antidepressant treatment decreases spiroperidol-labeled serotonin receptor binding. Science 210, 88–90 10.1126/science.6251550 ( doi:10.1126/science.6251550 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. Lesch K. P. 1991. 5-HT1A receptor responsivity in anxiety disorders and depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 15, 723–733 10.1016/0278-5846(91)90001-H ( doi:10.1016/0278-5846(91)90001-H ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Shapira B., Newman M. E., Gelfin Y., Lerer B. 2000. Blunted temperature and cortisol responses to ipsapirone in major depression: lack of enhancement by electroconvulsive therapy. Psychoneuroendocrinology 25, 421–438 10.1016/S0306-4530(99)00067-0 ( doi:10.1016/S0306-4530(99)00067-0 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Cowen P. J., Power A. C., Ware C. J., Anderson I. M. 1994. 5-HT1A receptor sensitivity in major depression. A neuroendocrine study with buspirone. Br. J. Psychiatry 164, 372–379 10.1192/bjp.164.3.372 ( doi:10.1192/bjp.164.3.372 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Lerer B., Gelfin Y., Gorfine M., Allolio B., Lesch K. P., Newman M. E. 1999. 5-HT1A receptor function in normal subjects on clinical doses of fluoxetine: blunted temperature and hormone responses to ipsapirone challenge. Neuropsychopharmacology 20, 628–639 10.1016/S0893-133X(98)00106-7 ( doi:10.1016/S0893-133X(98)00106-7 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 82. Li Q., Wichems C., Heils A., Van De Kar L. D., Lesch K. P., Murphy D. L. 1999. Reduction of 5-hydroxytryptamine (5-HT)(1A)-mediated temperature and neuroendocrine responses and 5-HT(1A) binding sites in 5-HT transporter knockout mice. J. Pharmacol. Exp. Ther. 291, 999–1007 [ PubMed ] [ Google Scholar ]
- 83. Jacobsen J. P., Siesser W. B., Sachs B. D., Peterson S., Cools M. J., Setola V., Folgering J. H., Flik G., Caron M. G. In press. Deficient serotonin neurotransmission and depression-like serotonin biomarker alterations in tryptophan hydroxylase 2 (Tph2) loss-of-function mice. Mol. Psychiatry. 10.1038/mp.2011.50 ( doi:10.1038/mp.2011.50 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 84. Walther D. J., Peter J. U., Bashammakh S., Hortnagl H., Voits M., Fink H., Bader M. 2003. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299, 76. 10.1126/science.1078197 ( doi:10.1126/science.1078197 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 85. Mann J. J., Malone K. M., Nielsen D. A., Goldman D., Erdos J., Gelernter J. 1997. Possible association of a polymorphism of the tryptophan hydroxylase gene with suicidal behavior in depressed patients. Am. J. Psychiatry 154, 1451–1453 [ DOI ] [ PubMed ] [ Google Scholar ]
- 86. Bellivier F., et al. 1998. Association between the tryptophan hydroxylase gene and manic-depressive illness. Arch. Gen. Psychiatry 55, 33–37 10.1001/archpsyc.55.1.33 ( doi:10.1001/archpsyc.55.1.33 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 87. Roy A., Nielsen D., Rylander G., Sarchiapone M., Segal N. 1999. Genetics of suicide in depression. J. Clin. Psychiatry 60(Suppl. 2), 12–17; discussion 18–20, 113–116 [ PubMed ] [ Google Scholar ]
- 88. Cote F., Fligny C., Bayard E., Launay J. M., Gershon M. D., Mallet J., Vodjdani G. 2007. Maternal serotonin is crucial for murine embryonic development. Proc. Natl Acad. Sci. USA 104, 329–334 10.1073/pnas.0606722104 ( doi:10.1073/pnas.0606722104 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 89. Harvey M., Gagne B., Labbe M., Barden N. 2007. Polymorphisms in the neuronal isoform of tryptophan hydroxylase 2 are associated with bipolar disorder in French Canadian pedigrees. Psychiatry Genet. 17, 17–22 10.1097/YPG.0b013e3280111877 ( doi:10.1097/YPG.0b013e3280111877 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 90. Sheehan K., Lowe N., Kirley A., Mullins C., Fitzgerald M., Gill M., Hawi Z. 2005. Tryptophan hydroxylase 2 (TPH2) gene variants associated with ADHD. Mol. Psychiatry 10, 944–949 10.1038/sj.mp.4001698 ( doi:10.1038/sj.mp.4001698 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Ke L., Qi Z. Y., Ping Y., Ren C. Y. 2006. Effect of SNP at position 40237 in exon 7 of the TPH2 gene on susceptibility to suicide. Brain Res. 1122, 24–26 10.1016/j.brainres.2006.09.007 ( doi:10.1016/j.brainres.2006.09.007 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Cichon S., et al. 2008. Brain-specific tryptophan hydroxylase 2 (TPH2): a functional Pro206Ser substitution and variation in the 5′-region are associated with bipolar affective disorder. Hum. Mol. Genet. 17, 87–97 10.1093/hmg/ddm286 ( doi:10.1093/hmg/ddm286 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Stone M., Laughren T., Jones M. L., Levenson M., Holland P. C., Hughes A., Hammad T. A., Temple R., Rochester G. 2009. Risk of suicidality in clinical trials of antidepressants in adults: analysis of proprietary data submitted to US Food and Drug Administration. BMJ 339, b2880. 10.1136/bmj.b2880 ( doi:10.1136/bmj.b2880 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 94. Walsh B. T., Seidman S. N., Sysko R., Gould M. 2002. Placebo response in studies of major depression: variable, substantial, and growing. JAMA 287, 1840–1847 10.1001/jama.287.14.1840 ( doi:10.1001/jama.287.14.1840 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 95. Gibbons R. D., Brown C. H., Hur K., Davis J. M., Mann J. J. 2012. Suicidal thoughts and behavior with antidepressant treatment: reanalysis of the randomized placebo-controlled studies of fluoxetine and venlafaxine. Arch. Gen. Psychiatry 339, 1. 10.1001/archgenpsychiatry.2011.2048 ( doi:10.1001/archgenpsychiatry.2011.2048 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 96. Grahame-Smith D. G. 1964. Tryptophan hydroxylation in brain. Biochem. Biophys. Res. Commun. 16, 586–592 10.1016/0006-291X(64)90197-4 ( doi:10.1016/0006-291X(64)90197-4 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 97. Gal E. M., Morgan M., Chatterjee S. K., Marshall F. D., Jr 1964. Hydroxylation of tryptophan by brain tissue in vivo and related aspects of 5-hydroxytryptamine metabolism. Biochem. Pharmacol. 13, 1639–1653 10.1016/0006-2952(64)90218-7 ( doi:10.1016/0006-2952(64)90218-7 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 98. Manegold C., Hoffmann G. F., Degen I., Ikonomidou H., Knust A., Laass M. W., Pritsch M., Wilichowski E., Horster F. 2009. Aromatic L-amino acid decarboxylase deficiency: clinical features, drug therapy and follow-up. J. Inherit. Metab. Dis. 32, 371–380 10.1007/s10545-009-1076-1 ( doi:10.1007/s10545-009-1076-1 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 99. Gutknecht L., Kriegebaum C., Waider J., Schmitt A., Lesch K. P. 2009. Spatio-temporal expression of tryptophan hydroxylase isoforms in murine and human brain: convergent data from Tph2 knockout mice. Eur. Neuropsychopharmacol. 19, 266–282 10.1016/j.euroneuro.2008.12.005 ( doi:10.1016/j.euroneuro.2008.12.005 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 100. Cote F., et al. 2003. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc. Natl Acad. Sci USA 100, 13 525–13 530 10.1073/pnas.2233056100 ( doi:10.1073/pnas.2233056100 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 101. Neal K. B., Parry L. J., Bornstein J. C. 2009. Strain-specific genetics, anatomy and function of enteric neural serotonergic pathways in inbred mice. J. Physiol. 587, 567–586 10.1113/jphysiol.2008.160416 ( doi:10.1113/jphysiol.2008.160416 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 102. Savelieva K. V., Zhao S., Pogorelov V. M., Rajan I., Yang Q., Cullinan E., Lanthorn T. H. 2008. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS ONE 3, e3301. 10.1371/journal.pone.0003301 ( doi:10.1371/journal.pone.0003301 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 103. Scheuch K., Lautenschlager M., Grohmann M., Stahlberg S., Kirchheiner J., Zill P., Heinz A., Walther D. J., Priller J. 2007. Characterization of a functional promoter polymorphism of the human tryptophan hydroxylase 2 gene in serotonergic raphe neurons. Biol. Psychiatry 62, 1288–1294 10.1016/j.biopsych.2007.01.015 ( doi:10.1016/j.biopsych.2007.01.015 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 104. Zhang X., Gainetdinov R. R., Beaulieu J. M., Sotnikova T. D., Burch L. H., Williams R. B., Schwartz D. A., Krishnan K. R., Caron M. G. 2005. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron 45, 11–16 10.1016/j.neuron.2004.12.014 ( doi:10.1016/j.neuron.2004.12.014 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 105. Zill P., Baghai T. C., Zwanzger P., Schule C., Eser D., Rupprecht R., Moller H. J., Bondy B., Ackenheil M. 2004. SNP and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene provide evidence for association with major depression. Mol. Psychiatry 9, 1030–1036 10.1038/sj.mp.4001525 ( doi:10.1038/sj.mp.4001525 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 106. De Luca V., Mueller D. J., Tharmalingam S., King N., Kennedy J. L. 2004. Analysis of the novel TPH2 gene in bipolar disorder and suicidality. Mol. Psychiatry 9, 896–897 10.1038/sj.mp.4001531 ( doi:10.1038/sj.mp.4001531 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 107. Zhou Z., Roy A., Lipsky R., Kuchipudi K., Zhu G., Taubman J., Enoch M. A., Virkkunen M., Goldman D. 2005. Haplotype-based linkage of tryptophan hydroxylase 2 to suicide attempt, major depression, and cerebrospinal fluid 5-hydroxyindoleacetic acid in 4 populations. Arch. Gen. Psychiatry 62, 1109–1118 10.1001/archpsyc.62.10.1109 ( doi:10.1001/archpsyc.62.10.1109 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 108. Lopez de Lara C., Brezo J., Rouleau G., Lesage A., Dumont M., Alda M., Benkelfat C., Turecki G. 2007. Effect of tryptophan hydroxylase-2 gene variants on suicide risk in major depression. Biol. Psychiatry 62, 72–80 10.1016/j.biopsych.2006.09.008 ( doi:10.1016/j.biopsych.2006.09.008 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 109. Garriock H. A., et al. 2005. Lack of association of TPH2 exon XI polymorphisms with major depression and treatment resistance. Mol. Psychiatry 10, 976–977 10.1038/sj.mp.4001712 ( doi:10.1038/sj.mp.4001712 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 110. Lin Y. M., Chao S. C., Chen T. M., Lai T. J., Chen J. S., Sun H. S. 2007. Association of functional polymorphisms of the human tryptophan hydroxylase 2 gene with risk for bipolar disorder in Han Chinese. Arch. Gen. Psychiatry 64, 1015–1024 10.1001/archpsyc.64.9.1015 ( doi:10.1001/archpsyc.64.9.1015 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 111. McKinney J., Johansson S., Halmoy A., Dramsdahl M., Winge I., Knappskog P. M., Haavik J. 2008. A loss-of-function mutation in tryptophan hydroxylase 2 segregating with attention-deficit/hyperactivity disorder. Mol. Psychiatry 13, 365–367 10.1038/sj.mp.4002152 ( doi:10.1038/sj.mp.4002152 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 112. Scriver C. R. 2007. The PAH gene, phenylketonuria, and a paradigm shift. Hum. Mutat. 28, 831–845 10.1002/humu.20526 ( doi:10.1002/humu.20526 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 113. McKinney J. A., Turel B., Winge I., Knappskog P. M., Haavik J. 2009. Functional properties of missense variants of human tryptophan hydroxylase 2. Hum. Mutat. 30, 787–794 10.1002/humu.20956 ( doi:10.1002/humu.20956 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 114. Scriver C. R., et al. 2003. PAHdb 2003: What a locus-specific knowledgebase can do? Hum. Mut. 21, 333–344 10.1002/humu.10200 ( doi:10.1002/humu.10200 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 115. Chen G. L., Vallender E. J., Miller G. M. 2008. Functional characterization of the human TPH2 5′ regulatory region: untranslated region and polymorphisms modulate gene expression in vitro . Hum. Genet. 122, 645–657 10.1007/s00439-007-0443-y ( doi:10.1007/s00439-007-0443-y ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 116. Walitza S., et al. 2005. Transmission disequilibrium of polymorphic variants in the tryptophan hydroxylase-2 gene in attention-deficit/hyperactivity disorder. Mol. Psychiatry 10, 1126–1132 10.1038/sj.mp.4001734 ( doi:10.1038/sj.mp.4001734 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 117. Kim Y. K., Lee H. J., Yang J. C., Hwang J. A., Yoon H. K. 2009. A tryptophan hydroxylase 2 gene polymorphism is associated with panic disorder. Behav. Genet. 39, 170–175 10.1007/s10519-008-9254-8 ( doi:10.1007/s10519-008-9254-8 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 118. Yoon H. K., Kim Y. K. 2009. TPH2–703G/T SNP may have important effect on susceptibility to suicidal behavior in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 403–409 10.1016/j.pnpbp.2008.12.013 ( doi:10.1016/j.pnpbp.2008.12.013 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 119. Stefulj J., Mokrovic G., Hranilovic D., Bordukalo-Niksic T., Bakula M., Kubat M., Jernej B. 2011. Functional promoter polymorphism of the neuronal isoform of tryptophan hydroxylase (Tph2) in suicide. Psychiatry Res. 186, 446–447 10.1016/j.psychres.2010.08.034 ( doi:10.1016/j.psychres.2010.08.034 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 120. Zhang X., et al. 2010. A functional alternative splicing mutation in human tryptophan hydroxylase-2. Mol. Psychiatry 16, 1169–1176 10.1038/mp.2010.99 ( doi:10.1038/mp.2010.99 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 121. Haghighi F., et al. 2008. Genetic architecture of the human tryptophan hydroxylase 2 gene: existence of neural isoforms and relevance for major depression. Mol. Psychiatry 13, 813–820 10.1038/sj.mp.4002127 ( doi:10.1038/sj.mp.4002127 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 122. Rush A. J., Trivedi M., Fava M. 2003. Depression, IV: STAR*D treatment trial for depression. Am. J. Psychiatry 160, 237. 10.1176/appi.ajp.160.2.237 ( doi:10.1176/appi.ajp.160.2.237 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 123. Levinson D. F., et al. 2003. Genetics of recurrent early-onset depression (GenRED): design and preliminary clinical characteristics of a repository sample for genetic linkage studies. Am. J. Med. Genet. B 119, 118–130 10.1002/ajmg.b.20009 ( doi:10.1002/ajmg.b.20009 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 124. Johnson S., et al. 2011. The reduction of r1, a novel repressor protein for monoamine oxidase a, in major depressive disorder. Neuropsychopharmacology 36, 2139–2148 10.1038/npp.2011.105 ( doi:10.1038/npp.2011.105 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 125. Jans L. A., Riedel W. J., Markus C. R., Blokland A. 2007. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol. Psychiatry 12, 522–543 10.1038/sj.mp.4001920 ( doi:10.1038/sj.mp.4001920 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 126. Cowen P. J. 2008. Serotonin and depression: pathophysiological mechanism or marketing myth? Trends Pharmacol. Sci. 29, 433–436 10.1016/j.tips.2008.05.004 ( doi:10.1016/j.tips.2008.05.004 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 127. Alenina N., et al. 2009. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc. Natl Acad. Sci. USA 106, 10 332–10 337 10.1073/pnas.0810793106 ( doi:10.1073/pnas.0810793106 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 128. Sharp T., Boothman L., Raley J., Queree P. 2007. Important messages in the ‘post’: recent discoveries in 5-HT neurone feedback control. Trends Pharmacol. Sci. 28, 629–636 10.1016/j.tips.2007.10.009 ( doi:10.1016/j.tips.2007.10.009 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 129. Zhang X., Beaulieu J. M., Sotnikova T. D., Gainetdinov R. R., Caron M. G. 2004. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science 305, 217. 10.1126/science.1097540 ( doi:10.1126/science.1097540 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 130. Cervo L., et al. 2005. Genotype-dependent activity of tryptophan hydroxylase-2 determines the response to citalopram in a mouse model of depression. J. Neurosci. 25, 8165–8172 10.1523/jneurosci.1816-05.2005 ( doi:10.1523/jneurosci.1816-05.2005 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 131. Lucki I., Dalvi A., Mayorga A. J. 2001. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology 155, 315–322 10.1007/s002130100694 ( doi:10.1007/s002130100694 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 132. Calcagno E., Canetta A., Guzzetti S., Cervo L., Invernizzi R. W. 2007. Strain differences in basal and post-citalopram extracellular 5-HT in the mouse medial prefrontal cortex and dorsal hippocampus: relation with tryptophan hydroxylase-2 activity. J. Neurochem. 103, 1111–1120 10.1111/j.1471-4159.2007.04806.x ( doi:10.1111/j.1471-4159.2007.04806.x ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 133. Crowley J. J., Blendy J. A., Lucki I. 2005. Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test. Psychopharmacology 183, 257–264 10.1007/s00213-005-0166-5 ( doi:10.1007/s00213-005-0166-5 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 134. Siesser W. B., Zhang X., Jacobsen J. P., Sotnikova T. D., Gainetdinov R. R., Caron M. G. 2010. Tryptophan hydroxylase 2 genotype determines brain serotonin synthesis but not tissue content in C57Bl/6 and BALB/c congenic mice. Neurosci. Lett. 481, 6–11 10.1016/j.neulet.2010.06.035 ( doi:10.1016/j.neulet.2010.06.035 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 135. Tenner K., Qadri F., Bert B., Voigt J. P., Bader M. 2008. The mTPH2 C1473G single nucleotide polymorphism is not responsible for behavioural differences between mouse strains. Neurosci. Lett. 431, 21–25 10.1016/j.neulet.2007.11.012 ( doi:10.1016/j.neulet.2007.11.012 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 136. Shen H. W., et al. 2004. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology 29, 1790–1799 10.1038/sj.npp.1300476 ( doi:10.1038/sj.npp.1300476 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 137. Jacobsen J. P., Nielsen E. O., Hummel R., Redrobe J. P., Mirza N., Weikop P. 2008. Insensitivity of NMRI mice to selective serotonin reuptake inhibitors in the tail suspension test can be reversed by co-treatment with 5-hydroxytryptophan. Psychopharmacology 199, 137–150 10.1007/s00213-008-1142-7 ( doi:10.1007/s00213-008-1142-7 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 138. Beaulieu J. M., Zhang X., Rodriguiz R. M., Sotnikova T. D., Cools M. J., Wetsel W. C., Gainetdinov R. R., Caron M. G. 2008. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc. Natl Acad. Sci. USA 105, 1333–1338 10.1073/pnas.0711496105 ( doi:10.1073/pnas.0711496105 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 139. Deacon R. M. 2006. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat. Protoc. 1, 122–124 10.1038/nprot.2006.20 ( doi:10.1038/nprot.2006.20 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 140. De Groote L., Olivier B., Westenberg H. G. 2002. The effects of selective serotonin reuptake inhibitors on extracellular 5-HT levels in the hippocampus of 5-HT(1B) receptor knockout mice. Eur. J. Pharmacol. 439, 93–100 10.1016/S0014-2999(02)01417-6 ( doi:10.1016/S0014-2999(02)01417-6 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 141. Hjorth S., Sharp T. 1991. Effect of the 5-HT1A receptor agonist 8-OH-DPAT on the release of 5-HT in dorsal and median raphe-innervated rat brain regions as measured by in vivo microdialysis. Life Sci. 48, 1779–1786 10.1016/0024-3205(91)90216-X ( doi:10.1016/0024-3205(91)90216-X ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 142. Hjorth S., Westlin D., Bengtsson H. J. 1997. WAY100635-induced augmentation of the 5-HT-elevating action of citalopram: relative importance of the dose of the 5-HT1A (auto)receptor blocker versus that of the 5-HT reuptake inhibitor. Neuropharmacology 36, 461–465 10.1016/S0028-3908(97)00050-6 ( doi:10.1016/S0028-3908(97)00050-6 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 143. de Groote L., Klompmakers A. A., Olivier B., Westenberg H. G. 2003. An evaluation of the effect of NAS-181, a new selective 5-HT(1B) receptor antagonist, on extracellular 5-HT levels in rat frontal cortex. Naunyn Schmiedebergs Arch. Pharmacol. 367, 89–94 10.1007/s00210-002-0685-0 ( doi:10.1007/s00210-002-0685-0 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 144. Martin-Ruiz R., Puig M. V., Celada P., Shapiro D. A., Roth B. L., Mengod G., Artigas F. 2001. Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. J. Neurosci. 21, 9856–9866 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 145. Waider J., Araragi N., Gutknecht L., Lesch K. P. 2011. Tryptophan hydroxylase-2 (TPH2) in disorders of cognitive control and emotion regulation: a perspective. Psychoneuroendocrinology 36, 393–405 10.1016/j.psyneuen.2010.12.012 ( doi:10.1016/j.psyneuen.2010.12.012 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 146. Narboux-Neme N., et al. 2011. Severe serotonin depletion after conditional deletion of the vesicular monoamine transporter 2 gene in serotonin neurons: neural and behavioral consequences. Neuropsychopharmacology 36, 2538–2550 10.1038/npp.2011.142 ( doi:10.1038/npp.2011.142 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 147. Hendricks T. J., et al. 2003. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron 37, 233–247 10.1016/S0896-6273(02)01167-4 ( doi:10.1016/S0896-6273(02)01167-4 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 148. Zhao Z. Q., Scott M., Chiechio S., Wang J. S., Renner K. J., Gereau R. W. T., Johnson R. L., Deneris E. S., Chen Z. F. 2006. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J. Neurosci. 26, 12 781–12 788 10.1523/JNEUROSCI.4143-06.2006 ( doi:10.1523/JNEUROSCI.4143-06.2006 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 149. Fernandez S. P., Gaspar P. 2012. Investigating anxiety and depressive-like phenotypes in genetic mouse models of serotonin depletion. Neuropharmacology 62, 144–154 10.1016/j.neuropharm.2011.08.049 ( doi:10.1016/j.neuropharm.2011.08.049 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 150. Correa H., et al. 2000. Prolactin response to D-fenfluramine and suicidal behavior in depressed patients. Psychiatry Res. 93, 189–199 10.1016/S0165-1781(00)00114-1 ( doi:10.1016/S0165-1781(00)00114-1 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 151. Goodwin G. M., De Souza R. J., Green A. R. 1985. The pharmacology of the hypothermic response in mice to 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT). A model of presynaptic 5-HT1 function. Neuropharmacology 24, 1187–1194 10.1016/0028-3908(85)90153-4 ( doi:10.1016/0028-3908(85)90153-4 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 152. Bill D. J., Knight M., Forster E. A., Fletcher A. 1991. Direct evidence for an important species difference in the mechanism of 8-OH-DPAT-induced hypothermia. Br. J. Pharmacol. 103, 1857–1864 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 153. Duman R. S., Malberg J., Nakagawa S. 2001. Regulation of adult neurogenesis by psychotropic drugs and stress. J. Pharmacol. Exp. Ther. 299, 401–407 [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (603.0 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
VIDEO