- Published: 18 April 2013

Neurogenomics of speech and language disorders: the road ahead
- Pelagia Deriziotis 1 &
- Simon E Fisher 1 , 2
Genome Biology volume 14 , Article number: 204 ( 2013 ) Cite this article
10k Accesses
25 Citations
8 Altmetric
Metrics details
Next-generation sequencing is set to transform the discovery of genes underlying neurodevelopmental disorders, and so offer important insights into the biological bases of spoken language. Success will depend on functional assessments in neuronal cell lines, animal models and humans themselves.
Introduction
The human capacity for complex spoken language is unique [ 1 ]. Speech endows us with the ability to verbally express our ideas, opinions and feelings, using rapid precise control of the oral motor structures (larynx, mouth, tongue) to convert our thoughts into streams of sound that can be decoded by others. While vocal communication in other species sometimes exploits simple mappings between sound and meaning, the reach of human language extends far beyond this, most notably through its extraordinary generative power. A discrete number of individual units of language can be combined into a limitless number of utterances, giving us the potential to express and comprehend an infinite array of concepts. Moreover, when growing up in a language-rich environment, any normal human infant becomes highly proficient in his or her native language with astonishing ease, and without the need for explicit teaching.
It has been argued for many years that inherited factors must make a key contribution to the acquisition of spoken language [ 2 ]. It is only in the past decade or so, with the rise of molecular genetics, that biologists have been able to provide the first robust empirical evidence regarding this issue. To begin investigating the pathways involved, research has focused on the roles of genes, proteins and cellular machinery in the etiology of language impairments, in which people mysteriously fail to develop normal skills despite adequate linguistic input and opportunity [ 3 ]. There is a diverse array of these language-related disorders, which usually appear in early childhood and often persist into later life, and they are common enough to have a major impact on modern society. Language problems are frequently observed co-occurring with other developmental disorders, such as autism and epilepsy [ 4 , 5 ].
Prior to the advent of molecular studies of language disorders, the importance of the genome was already evident from epidemiological analyses. These disorders typically cluster in families [ 6 – 9 ] and monozygotic twins display substantially higher rates of concordance than dizygotic twins [ 10 – 12 ]. Clearly, acquisition of fluent spoken language is also influenced by the environment and its interaction with our genes. However, beyond the obvious effects of impoverished language input (for example, due to hearing problems) there is little known regarding specific environmental risk factors that may disturb linguistic development [ 13 ].
Initial clues to the molecular bases of speech and language impairments came from low-density linkage screens [ 14 ], followed by targeted association studies of particular chromosome regions and/or focused mutation screens of candidate genes [ 15 ]. In addition, studies of chromosomal abnormalities are contributing to our understanding of such disorders, and genome-wide association scans using hundreds of thousands of single nucleotide polymorphisms (SNPs) are underway in several cohorts. However, it is evident that the future of gene discovery in language-related traits, as for many other complex phenotypes, lies in large-scale DNA sequencing of entire human genomes.
Traditional sequencing methods are slow, laborious and expensive; the original human genome sequencing project cost more than US$3 billion and took more than a decade to finish [ 16 ]. Dramatic technological advances have transformed the ability to analyze our genetic makeup at single nucleotide resolution and commercialization of these 'next-generation' platforms is growing fast. At the time of writing, a human genome can be entirely sequenced in a matter of days for only a few thousand dollars, and costs continue to fall at a remarkable rate. Nevertheless, excitement over the enormous potential of the new technologies must be tempered by acknowledging the associated analytical challenges. Already, our capacity to rapidly generate large swathes of sequence data from many individuals outstrips our capacity to infer the underlying biology of a trait using such information.
Here, we begin by summarizing approaches previously applied to identify and study the first genes implicated in speech and language disorders (Table 1 ). We go on to discuss the promise of next-generation sequencing (NGS) for uncovering the key genomic changes that affect our speech and language abilities, not only in relevant disorders, but also in the general population. We argue that it is essential to be able to assess the functional significance of identified variants if we are to understand their biological impact and elucidate their contributions to the human traits of interest. The success of such efforts will depend on synergies between diverse research techniques, including bioinformatics and experimental analyses using model systems, as well as integration of human genome sequences and functional gene network datasets (Figure 1 ).
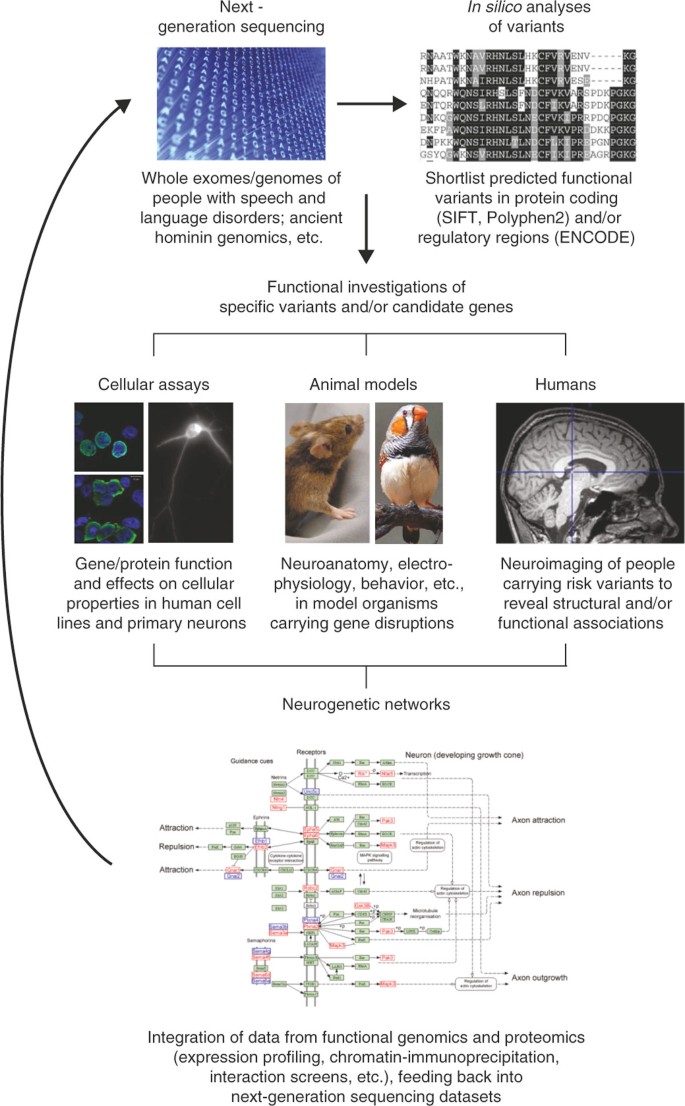
Neurogenomics of speech and language disorders . Next-generation sequencing will yield large datasets of genomic variants with potential relevance for speech and language. Identification of key variants is critically dependent on multidisciplinary studies of function in cell lines, animal models and humans, along with integration of data on neurogenetic networks, as detailed in the text. The image under 'Next-generation sequencing' comes from istockphoto.com (DNA code; File #9614920), the boxshade plot under ' In silico analyses' is a subpart taken from Figure 4 of [ 17 ], the lefthand bottom panel of 'Cellular assays' is a subpart taken from Supplementary Figure 5c of [ 68 ], the 'Neurogenetic networks' image is taken from Figure 4b of [ 82 ] and the Zebrafinch image is reproduced with permission from Geoffrey Dabb and Canberra Ornithologists Group.
Gene mapping in speech and language disorders
Speech apraxia.
The first gene to be clearly implicated in a speech and language disorder was FOXP2 . Disruptions of this gene cause a monogenic form of developmental verbal dyspraxia (DVD), also known as childhood apraxia of speech (CAS) [ 17 ], characterized by problems with the learning and execution of coordinated movement sequences of the mouth, tongue, lips and soft palate [ 18 , 19 ]. FOXP2 was discovered through molecular studies of a large three-generational pedigree (the KE family) in which half the members have CAS, accompanied by wide-ranging deficits in both oral and written language, affecting not only production but also comprehension [ 17 ]. Linkage mapping in this family identified a region on chromosome 7q31 that co-segregated perfectly with the disorder [ 20 ]. An unrelated child with similar speech and language deficits was found to carry a de novo balanced translocation involving the same interval, which directly interrupted the coding region of a novel gene, FOXP2 [ 17 , 21 ]. Screening of FOXP2 in the KE family revealed that all affected members had inherited a heterozygous point mutation yielding an amino acid substitution at a key residue of the encoded protein [ 17 ]. Subsequent studies identified additional etiological FOXP2 variants (nonsense mutations, translocations, deletions) in individuals and families with speech and language problems, typically including CAS as a core feature (reviewed by Fisher and Scharff [ 22 ]). Although etiological mutations of FOXP2 are rare [ 23 , 24 ], the gene provides a valuable molecular window into neurogenetic mechanisms contributing to human spoken language, as detailed elsewhere in this article.
Beyond FOXP2 , additional loci that may contribute to CAS have emerged from cases of chromosomal abnormalities, identified using cytogenetic screening and/or comparative genomic hybridization (CGH). One report described a family in which three affected siblings all carry an unbalanced 4q;16q translocation [ 25 ]. Another study defined a small region on 12p13.3, containing the ELKS/ERC1 gene, commonly deleted in nine unrelated patients with delayed speech development, most of whom had a formal diagnosis of CAS [ 26 ]. Interestingly, a key isoform encoded by ELKS/ERC1 appears to be expressed specifically in the brain, where it binds to RIM proteins. In neurons, RIMs act within the presynaptic active zone, a site that integrates synaptic vesicle exo/endocytosis with intracellular signaling in the nerve terminal [ 27 ]. Certain copy number variant (CNV) syndromes with complex variable phenotypes have been linked to increased risk of CAS, including 16p11.2 microdeletions [ 28 , 29 ] and 7q11.23 microduplications [ 30 ]. The rare metabolic disorder, galactosemia, is also associated with elevated incidence of CAS [ 31 ].
Specific language impairment
When a child is delayed or impaired in acquiring language, without any obvious physical or neurological cause (cleft lip/palate, intellectual disability (ID), autism, deafness, and so on) he or she is usually diagnosed with specific language impairment (SLI). Since it is defined using exclusionary criteria, SLI encompasses a range of different cognitive and behavioral profiles. The most common forms involve deficits in expressive language, either in isolation or accompanied by receptive problems.
The estimated prevalence of SLI is up to 7% in kindergarten children [ 32 ] and it shows familial clustering; twin studies consistently indicate high heritability [ 10 , 11 , 33 ]. In contrast to the rare cases of monogenic CAS discussed above, typical forms of SLI have a complex multifactorial basis [ 34 ]. Genome-wide linkage mapping in families with SLI have suggested the existence of multiple risk loci, on chromosomes 16q and 19q [ 35 – 38 ], as well as 2p and 13q [ 39 , 40 ]. Targeted analysis of 16q identified variants in two genes, ATP2C2 and CMIP , associated with deficits on a non-word repetition task, considered to be an index of impaired phonological short-term memory [ 15 , 41 ]. The ATP2C2 gene encodes a single subunit integral membrane P-type ATPase that catalyzes the ATP-driven transport of cytosolic calcium and manganese into the Golgi lumen [ 42 ]. This cellular role makes it a plausible candidate for SLI susceptibility, since intracellular calcium levels are intimately linked to multiple diverse aspects of neuronal function, ranging from migration to plasticity, while manganese dysregulation has been linked to neurodegenerative phenotypes. The product of CMIP contains pleckstrin homology and leucine-rich repeat domains, and is hypothesized to be an adaptor protein of the actin cytoskeleton, interacting with filamin A and RelA (an NF-kappaB subunit) [ 43 ]. Although little is known about CMIP at this stage, it is again a credible candidate for involvement in nervous system function, since cytoskeletal reorganization makes essential contributions to processes like neuronal migration and synapse formation/modification. Other candidate genes (such as CNTNAP2 ) have been implicated in SLI susceptibility through functional approaches [ 44 ], as highlighted elsewhere in this article.
Studies of isolated founder populations may also help pinpoint new genes contributing to language disorders. A notable example is Robinson Crusoe Island - an island of 633 residents lying west of Chile, South America - which was most recently colonized in the late 19th century [ 45 ]. Thirty-five percent of the colonizing children satisfy criteria for a diagnosis of SLI, substantially higher than the 4% prevalence rate for mainland Chile [ 45 ]. Initial molecular investigations identified several genomic regions of interest (on chromosomes 6, 7, 12, 13 and 17), but no specific risk genes have yet been discovered [ 46 ].
SLI has connections with another heritable neurodevelopmental trait, dyslexia, defined as specific significant impairments in reading and/or spelling that are not attributed to intelligence, visual acuity problems or inadequate learning opportunities. Although they do not display overt difficulties with speech or language, people with dyslexia often have subtle underlying deficits with aspects of linguistic processing [ 47 ]. Thus, genetic studies of dyslexia may be informative for understanding language pathways. We do not have space to discuss this here, and refer readers to other recent reviews [ 48 , 49 ].
Stuttering is a neurodevelopmental disorder that disturbs the flow of speech [ 50 ]. People who stutter are affected by uncontrollable repetitions and prolongations of syllables, and by involuntary silent pauses while speaking; these difficulties begin in childhood, persisting in about 20% of case referrals [ 51 ]. Most people who suffer from persistent stuttering nevertheless display normal linguistic proficiency [ 52 ]. Stuttering is thought to have a strong genetic basis [ 53 ]. Thus far, most genome-wide investigations of persistent familial stuttering have revealed only suggestive evidence of linkage, with loci distributed across at least ten chromosomes, and little overlap between different studies, indicating that this is a complex multifactorial trait [ 53 – 55 ].
One of the few reports of significant linkage focused on 46 consanguineous families from Pakistan, and highlighted chromosome 12q as a site of interest [ 56 ]. Subsequent analyses of the largest family from that study found that most affected relatives carried a coding variant in the 12q23.2 gene GNPTAB , which encodes two subunits of GlcNAc-phosphotransferase (GNPT) [ 57 ]. This putative risk variant (Q1200K), which altered a conserved residue of the protein, was identified in a number of other Pakistani cases, at higher frequency than Pakistani controls. GNPT is involved in addition of a mannose 6-phosphate tag to hydrolytic enzymes, allowing them to be targeted to lysosomes. Further screening of GNPTAB , as well as GNPTG and NAGPA , two closely related genes in this metabolic pathway, identified several different coding variants that were only present in cases and not controls [ 57 ]. The proposed risk variants are rare even among people who stutter, so it is likely that there are other unknown genes involved in stuttering.
The next generation: uncovering novel risk variants
While it is clear that exciting progress has been made, many of the genetic risk factors underlying speech and language disorders and/or normal linguistic variation remain to be discovered. At the time of writing, no study had yet reported the use of NGS methodologies to specifically investigate language-related traits. However, the advent of NGS has transformed the identification of genetic variants in other important neurodevelopmental phenotypes that co-occur with language deficits, such as ID and autism spectrum disorders (ASDs). Thus far, most such research has focused on sequencing protein-coding regions of the genome (the exome) to detect de novo variants in rare and common forms of these disorders [ 58 – 60 ]. Since de novo mutations have highly deleterious effects and are subject to strong negative selection, it is hypothesized that they might be important explanations of sporadic occurrences of disorder.
Whole-exome sequencing first proved effective in detecting causal de novo variants in rare reproductively lethal neurodevelopmental disorders, such as Kabuki syndrome [ 61 ], Bohring-Opitz syndrome [ 62 ] and KBG syndrome [ 63 ]. The study that pioneered this approach assessed 13 cases of Schinzel-Giedion syndrome, which is characterized by severe ID and typical facial features, and revealed de novo gain-of-function mutations independently occurring in a single gene, SETBP1 [ 64 ]. Interestingly, haploinsufficiency of SETBP1 has been identified in some cases of expressive speech impairment [ 65 ]. SETBP1 encodes a widely expressed nuclear protein that interacts with SET, an oncogene involved in DNA replication. Recent studies have shown that SET binding protein 1 (SETBP1) also includes three highly conserved AT-hooks (motifs that bind AT-rich DNA in a non-sequence-specific manner) and that it can act as a transcription factor, directly activating targets such as Hoxa9 and Hoxa10 [ 66 ]. Functional links between SETBP1 and brain development have yet to be explored.
NGS techniques are also shedding light on the roles of de novo changes in common non-syndromic disorders [ 59 ]. A pilot study of whole-exome sequencing in sporadic cases of non-syndromic ID and their parents (parent-child trios) reported nine non-synonymous de novo mutations in different genes in seven of ten probands [ 67 ]. Since then, multiple investigations have employed similar approaches to screen trios or quads (trio plus unaffected siblings), including four large-scale whole-exome sequencing efforts across about 1,000 ASD families [ 68 – 72 ] (reviewed by Buxbaum et al. [ 60 ]). One conclusion of this work was that the rate of de novo mutations was higher in ASD probands than controls, and it pointed to six genes of particular interest that had recurrent loss-of-function mutations.
A major advantage of focusing on de novo mutations is that it dramatically reduces the search space for potential causative variants; it is estimated that an average of approximately one de novo coding variant arises per genome per generation [ 59 ]. Interpretation of NGS data becomes more difficult when the search criteria are broadened to encompass all potential etiological coding variants that a proband carries, and it is even more challenging if one also considers non-exonic variations throughout the entire genome. It is not currently known if the genetic architecture underlying specific speech and language disorders includes a significant role for de novo mutations. Thus, it will be important to develop alternative study designs and analytic strategies (for example, Yu et al. [ 73 ] and Lim et al. [ 74 ]) for pinpointing causative mutations in NGS data from cases and families with language impairments.
Bridging the gap from genetic variants to biology
In the near future, NGS methods will become standard tools in molecular studies of speech and language disorders. As noted above, gene discovery strategies will need to move beyond the de novo paradigms that have been so successful for ID and ASD. Researchers will be faced with the major challenge of discerning which of the many plausibly causal variants carried by each affected person are physiologically relevant to their speech and/or language impairments. Fortunately, distinct fields combining computational and experimental methods can help ascertain the biological roles of detected variants and ultimately highlight genes important for our unique capacity for spoken language.
When focusing on protein-coding sequences, after initial filtering of identified variants from NGS data, it is possible to use predictive algorithms such as SIFT [ 75 ] and PolyPhen2 [ 76 ] to flag the most promising mutations for subsequent analyses. Computational methods such as these use known information on protein sequence and evolutionary history to rank them as benign, possibly damaging or probably damaging. Nonetheless, as cellular pathways harbor some degree of redundancy, not all loss-of-function mutations will contribute to a given disorder and such predictions should be treated with caution. For example, sequencing of FOXP2 in a cohort of CAS/DVD cases revealed a non-synonymous substitution near the N-terminus of the protein (Q17L) in one of the probands [ 24 ], a variant that is predicted to be damaging by both SIFT and PolyPhen2. However, follow-up functional experiments of the Q17L substitution using cell models did not find adverse effects on protein characteristics, in contrast to observations for other proband mutations [ 77 ]. Together with the fact that the Q17L proband has an affected sibling who does not carry the substitution, it seems unlikely that this particular change is etiological. Thus, although bioinformatic approaches help narrow down the list of variants from ongoing high-throughput genetic screens of speech and language phenotypes, experimental analyses in model systems are often crucial for determining causality, as well as offering deeper insights into mechanisms.
The value of functional approaches is particularly apparent from studies of how FOXP2 mutations lead to speech and language disorder [ 22 ]. FOXP2 encodes a forkhead-box transcription factor. Following homo- or hetero-dimerization with other forkhead box P (FOXP) family members [ 78 ], the protein binds DNA and represses transcription of its target genes [ 79 ]. Human neuron-like cells have been used to assess two different mutant FOXP2 proteins that co-segregate with disorder in CAS/DVD families: pFOXP2.R553H [ 17 ] and pFOXP2.R328X [ 24 ]. The functional assays demonstrated that these mutations severely disrupt nuclear localization, DNA-binding ability and transactivation potential of the protein [ 77 ]. Investigations into downstream targets of FOXP2 highlighted several neuronal pathways that it regulates. Independent high-throughput studies of promoter occupancy in cells and human fetal brain reported that FOXP2 directly regulates genes involved in neurite outgrowth, synaptic plasticity and axon guidance [ 80 , 81 ]. More recently, following genome-wide analyses of neural targets in vivo in mouse models, it has been shown that Foxp2 mutations can alter neurite outgrowth and branching in primary neurons [ 82 ].
A subset of FOXP2 targets are implicated in neurodevelopmental disorders that often co-occur with language deficits, such as the sushi repeat-containing protein X-linked 2 (SRPX2)-plasminogen activator receptor, urokinase-type (uPAR) complex in epilepsy and speech apraxia [ 83 ], DISC1 in schizophrenia [ 84 ] and MET in ASD [ 85 ]. The most rigorously studied FOXP2 target is CNTNAP2 , encoding contactin-associated protein-like 2 (CASPR2), a transmembrane scaffolding protein that clusters K + channels in myelinated axons [ 86 ]. CASPR2 is a member of the neurexin superfamily and, in addition to its role in mature neurons, it has been implicated in neuronal migration, dendritic arborization and spine development [ 87 ]. Homozygous loss-of-function CNTNAP2 mutations cause infant-onset epilepsy, learning deficits and language regression [ 88 ]. FOXP2 binds directly within the first intron of CNTNAP2 and is able to downregulate its expression [ 44 ]. Association analyses of quantitative phenotype data in 184 small SLI families identified a cluster of common intronic SNPs in CNTNAP2 that correlated significantly with reduced performance on linguistic tests, most strongly for the non-word repetition endophenotype [ 44 ]. The identity of the precise functional variant(s) in this region is not yet determined, but it is hypothesized that they affect the way that CNTNAP2 is regulated. Rare and common CNTNAP2 variants have also been implicated independently in ASDs [ 89 – 91 ], consistent with prior hypotheses that SLI and ASDs may involve some degree of shared genetic etiology. Beyond SLI, ASD and epilepsy, contributions of CNTNAP2 have been suggested for a range of other neurodevelopmental phenotypes, including schizophrenia [ 92 ], selective mutism [ 93 ] and Tourette syndrome [ 94 ].
A recent study of sporadic ASD demonstrates how the combination of NGS screens with functional experiments can shed light on language-related gene networks [ 68 ]. Whole-exome sequencing of parent-child trios identified a de novo frameshift mutation in an ASD proband, introducing a premature stop codon in FOXP1 [ 68 ]. The child was severely affected, with regression and language delays. FOXP1 is the most closely related gene to FOXP2 in the human genome and they can act synergistically to regulate shared targets in regions of co-expression [ 78 , 95 , 96 ]. Remarkably, the proband with the FOXP1 mutation also carried an extremely rare CNTNAP2 missense variant, inherited from his unaffected mother [ 68 ]. In cell-based functional analyses, the aberrant FOXP1 protein mislocalized to the cytoplasm and lost its transcriptional repressor properties; expression of the mutant FOXP1 isoform in cells elevated CNTNAP2 levels, unlike wild-type FOXP1 [ 68 ]. These data were consistent with a two-hit mechanism in which abnormal FOXP1 results in higher CNTNAP2 levels, amplifying any potentially deleterious effects of the missense CNTNAP2 variant of the proband [ 68 ]. Similar findings regarding multiple-hit mechanisms have emerged from independent studies of ASDs and other neurodevelopmental syndromes (for example, Leblond et al. [ 97 ]), suggesting that this may be an important model for genetic etiology of such disorders [ 98 ].
Previous screening of 49 children diagnosed with CAS/DVD did not detect any obviously etiological FOXP1 mutations [ 99 ]. However, studies of patients with mild to moderate ID and language impairment have detected rare de novo deletions and a nonsense FOXP1 variant [ 100 , 101 ]. High-throughput sequencing of balanced chromosomal abnormalities in neurodevelopmental disorders identified disruptions at the FOXP1 locus [ 102 ].
There has been little reported to date on functional analyses of other genes (such as ATP2C2 and CMIP ) associated with speech and language disorders, in part because no protein-coding variants have been pinpointed. As noted above, some cases of persistent stuttering carry coding variants in genes ( GNPTAB , GNPTG and NAGPA ) involved in lysosomal targeting of hydrolase enzymes. Interestingly, loss-of-function mutations of this pathway cause mucolipidosis disorders, which involve severe abnormalities affecting multiple systems, including skeletal, respiratory and cardiovascular tissues. Cell-based assays were recently used to analyze Mannose 6-phosphate-uncovering enzyme variants found in people who stutter, and were reported to yield incorrect protein folding, decreased enzymatic activity and degradation by the proteasome [ 103 ].
It is not always feasible to carry out experimental assessments of putative risk variants. The nature of assessment is highly dependent on the type of gene product; it is difficult to test protein function if there are no known measurable properties. In contrast to NGS technologies, functional experiments typically remain high cost, time-consuming and laborious, and are less amenable for upscaling. Nevertheless, as NGS reveals additional variants potentially implicated in language impairments and other neurodevelopmental traits, we will inevitably need access to high-throughput techniques for simultaneous mutation testing to define disease-causing variants across the genome [ 104 ]. Indeed, several multiplex approaches for characterizing the functional effects of genetic variation in proteins [ 105 ], mammalian regulatory elements [ 106 , 107 ] and RNA [ 108 ] have recently been developed. More and more emphasis will be placed on possible functional variants that lie outside protein-coding regions. Various efforts are underway to facilitate this transition, most notably the ENCODE project, which aims to characterize all functional elements at a genome-wide scale, including non-coding RNA and cis -regulatory elements [ 109 ]. RegulomeDB is of particular interest, as it combines data from the ENCODE project, GEO and published literature into a single, integrated database that can be used to query the functional significance of variants in both coding and non-coding regions of the genome [ 110 ].
Integrating data networks
Beyond establishing causality, functional characterization of candidate risk variants in model organisms may also help highlight pathways implicated in the origins and bases of language. For example, studies of FOXP2 across different species (mouse, bird, human) have given us initial clues into neurogenetic networks facilitating human spoken language [ 22 , 111 ]. FOXP2 expression is enriched in several brain areas, including the basal ganglia, deep cortical layers, thalamus and cerebellum [ 112 ], some of which display subtle structural and functional abnormalities in people carrying FOXP2 mutations [ 19 , 112 – 114 ]. From an evolutionary perspective, this is a highly conserved gene with regard to both the amino acid sequence of the encoded protein and the neural sites where it is expressed [ 95 , 115 ]. These data suggest that ancestral forms of FOXP2 were involved in important aspects of brain development long before the emergence of spoken language. There is evidence that the functions of the gene may have been modified during human evolution ([ 116 ]; also see below), but it remains clear that its roles in the human brain are built on evolutionarily ancient pathways [ 1 ].
Extensive characterization of rodent models carrying etiological Foxp2 variants indicates roles in synaptic plasticity, motor-skill learning, and processing and integration of auditory information [ 117 – 120 ]. When mice are heterozygous for the mutation that causes speech problems in the human KE family, they display decreased synaptic plasticity in corticostriatal circuits and motor-skill learning deficits [ 117 ]. These mouse findings are intriguing given that affected humans have problems learning to master the rapid coordinated orofacial movements underlying speech [ 121 ]. In vivo electrophysiology recordings in awake-behaving mice revealed more about the impacts of Foxp2 on corticostriatal circuitry; mice heterozygous for the KE mutation displayed higher basal striatal activity than wild-type controls, and medium spiny neurons showed aberrant negative modulation of their firing rates during motor-skill learning [ 118 ]. Separate studies used mouse models to explore whether impairments in auditory processing and auditory-motor integration might also be relevant to FOXP2 -related disorders [ 119 , 120 ]. Mice carrying the KE mutation were reported to have altered auditory brainstem responses to sound, although this finding was not replicated in mice carrying a different mutation associated with speech/language problems in another family [ 119 ]. Mice carrying either etiological mutation have deficits in learning to associate auditory stimuli with motor outputs [ 120 ].
Songbirds carry their own version of FOXP2 , referred to as FoxP2 , and it appears to make important contributions to the functions of a striatal nucleus called Area X [ 122 ]. In zebra finches, Area X is critical for auditory-guided vocal learning, a process in which young male birds learn their song by imitating an adult tutor. Vocal learning is also a key component of human speech acquisition. FoxP2 mRNA levels in Area X are enriched in young birds during the critical song-learning period [ 123 ] and show rapid downregulation when adult birds practice their songs outside the context of courtship [ 124 – 126 ]. Furthermore, selective knockdown of FoxP2 in Area X disrupts the song-learning process [ 127 ] and alters dendritic spine density in this region [ 128 ].
Functional studies of genes implicated in language-related disorders may also give us entry points into mechanisms involved in language function in the general population. As discussed above, variants of CNTNAP2 , a direct target of FOXP2, were associated with linguistic deficits in clinically distinct neurodevelopmental disorders [ 44 , 88 , 89 , 129 – 131 ]. Subsequent studies revealed that CNTNAP2 may contribute to language processing in healthy individuals [ 132 – 134 ]. The cluster of CNTNAP2 SNPs that is associated with language phenotypes in SLI and ASDs has also been reported to correlate with assessments of early language development in general population samples [ 132 ]. Neuroimaging genetics studies of common CNTNAP2 SNPs in healthy samples have proposed associations with functional brain measures related to language [ 133 , 134 ] and with altered structural connectivity patterns [ 135 ]. However, imaging genetics of language is a field that is only in its infancy; reports thus far involved small sample sizes with limited power, as well as a substantial multiple-testing burden, and results of different studies have been largely inconsistent. Additional analyses are required to elucidate how FOXP2 , CNTNAP2 and other language-related genes influence brain circuits at multiple levels of description - molecular, cellular, structural and functional.
Insights from ancient genomes
The reach of NGS technologies extends well beyond living species. These innovations have allowed molecular anthropologists to reconstruct large portions of nuclear genomes from extinct hominins that co-existed with our ancestors, such as Neanderthals [ 136 ] and Denisovans [ 137 ]. By comparing modern human sequences to ancient hominin genomes, as well as to our closest extant relatives, chimpanzees, it is possible to identify molecular variants that arose during human evolution, and roughly date them with regard to branches of the primate phylogenetic tree. As for other NGS projects, our capacity to generate large amounts of sequence data exceeds our ability to interpret it. So although scientists have successfully catalogued many of the DNA changes that occurred on our lineage, an extraordinary feat in itself, it is still a major challenge to determine which of these evolutionary events were relevant for the emergence of traits such as speech and language acquisition [ 1 ]. Here, success may depend on the integration of findings from evolutionary genomics with data from molecular studies of language-related disorders.
The best illustration of this approach comes again from work on the FOXP2 gene, which was targeted for evolutionary investigations, based on its prior link to a severe speech and language disorder. Comparative primate genomics suggests that FOXP2 probably underwent at least two interesting evolutionary events on the lineage that led to modern humans. After splitting from the chimpanzee (several million years ago) there were changes in the coding region of the locus that yielded two amino acid substitutions in the encoded protein [ 138 ]. Although these are minor changes outside the known functional domains, when such substitutions are inserted into the endogenous Foxp2 gene of a mouse, they have subtle detectable effects on brain structure and function, including altered connectivity and plasticity of corticostriatal circuits [ 116 ]. NGS approaches indicate that these amino acid substitutions are shared by Neanderthals [ 136 ] and Denisovans [ 137 ]. (It is worth emphasizing here that status of a single gene is not enough to determine whether or not a species can speak.) Researchers went on to identify a number of non-coding variants in intronic regions of FOXP2 that had occurred more recently on the human lineage, after splitting from Neanderthal/Denisovan a few hundred thousand years ago [ 139 ]. One of these changes lies in a region that underwent a recent selective sweep, and alters a putative binding site for the POU class 3 homeobox 2 (POU3F2) transcription factor, such that it may have affected regulation of FOXP2 expression; cell-based analyses are consistent with this hypothesis [ 139 ]. Thus, just like sequence-based analyses of language-related disorders, evaluation of the biological significance of interesting variants from ancient genomics requires functional studies using model systems.
The advent of whole genome NGS means that data generation will no longer be the limiting factor in understanding how genetic factors contribute to mechanisms underlying complex neurodevelopmental traits. Coupling NGS approaches to functional validation in model systems will facilitate network mapping and pathway investigation in speech and language disorders, and ultimately in normal linguistic development.
Abbreviations
autism spectrum disorder
childhood apraxia of speech
developmental verbal dyspraxia
forkhead box P
intellectual disability
- next-generation sequencing
specific language impairment.
Fisher SE, Marcus GF: The eloquent ape: genes, brains and the evolution of language. Nat Rev Genet. 2006, 7: 9-20.
PubMed CAS Google Scholar
Pinker S, Bloom P: Natural language and natural selection. Behav Brain Sci. 1990, 13: 707-784. 10.1017/S0140525X00081061.
Google Scholar
Bishop DV: Genes, cognition, and communication: insights from neurodevelopmental disorders. Ann N Y Acad Sci. 2009, 1156: 1-18. 10.1111/j.1749-6632.2009.04419.x.
PubMed CAS PubMed Central Google Scholar
Bishop DV: Overlaps between autism and language impairment: phenomimicry or shared etiology?. Behav Genet. 2010, 40: 618-629. 10.1007/s10519-010-9381-x.
Pal DK: Epilepsy and neurodevelopmental disorders of language. Curr Opin Neurol. 2011, 24: 126-131. 10.1097/WCO.0b013e328344634a.
PubMed Google Scholar
Neils J, Aram DM: Family history of children with developmental language disorders. Percept Mot Skills. 1986, 63: 655-658. 10.2466/pms.1986.63.2.655.
Tomblin JB: Familial concentration of developmental language impairment. J Speech Hear Disord. 1989, 54: 287-295.
Barry JG, Yasin I, Bishop DV: Heritable risk factors associated with language impairments. Genes Brain Behav. 2007, 6: 66-76. 10.1111/j.1601-183X.2006.00232.x.
Lewis BA, Freebairn LA, Hansen AJ, Miscimarra L, Iyengar SK, Taylor HG: Speech and language skills of parents of children with speech sound disorders. Am J Speech Lang Pathol. 2007, 16: 108-118. 10.1044/1058-0360(2007/015).
Lewis BA, Thompson LA: A study of developmental speech and language disorders in twins. J Speech Hear Res. 1992, 35: 1086-1094.
Bishop DV, North T, Donlan C: Genetic basis of specific language impairment: evidence from a twin study. Dev Med Child Neurol. 1995, 37: 56-71.
Bishop DV, Hayiou-Thomas ME: Heritability of specific language impairment depends on diagnostic criteria. Genes Brain Behav. 2008, 7: 365-372. 10.1111/j.1601-183X.2007.00360.x.
Bishop DV: Genetic and environmental risks for specific language impairment in children. Philos Trans R Soc Lond B Biol Sci. 2001, 356: 369-380. 10.1098/rstb.2000.0770.
Fisher SE, Lai CS, Monaco AP: Deciphering the genetic basis of speech and language disorders. Annu Rev Neurosci. 2003, 26: 57-80. 10.1146/annurev.neuro.26.041002.131144.
Newbury DF, Monaco AP: Genetic advances in the study of speech and language disorders. Neuron. 2010, 68: 309-320. 10.1016/j.neuron.2010.10.001.
International Human Genome Sequencing Consortium: Finishing the euchromatic sequence of the human genome. Nature. 2004, 431: 931-945. 10.1038/nature03001.
Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP: A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001, 413: 519-523. 10.1038/35097076.
Alcock KJ, Passingham RE, Watkins KE, Vargha-Khadem F: Oral dyspraxia in inherited speech and language impairment and acquired dysphasia. Brain Lang. 2000, 75: 17-33. 10.1006/brln.2000.2322.
Watkins KE, Vargha-Khadem F, Ashburner J, Passingham RE, Connelly A, Friston KJ, Frackowiak RSJ, Mishkin M, Gadian DG: MRI analysis of an inherited speech and language disorder: structural brain abnormalities. Brain. 2002, 125: 465-478. 10.1093/brain/awf057.
Fisher SE, Vargha-Khadem F, Watkins KE, Monaco AP, Pembrey ME: Localisation of a gene implicated in a severe speech and language disorder. Nat Genet. 1998, 18: 168-170. 10.1038/ng0298-168.
Lai CS, Fisher SE, Hurst JA, Levy ER, Hodgson S, Fox M, Jeremiah S, Povey S, Jamison DC, Green ED, Vargha-Khadem F, Monaco AP: The SPCH1 region on human 7q31: genomic characterization of the critical interval and localization of translocations associated with speech and language disorder. Am J Hum Genet. 2000, 67: 357-368. 10.1086/303011.
Fisher SE, Scharff C: FOXP2 as a molecular window into speech and language. Trends Genet. 2009, 25: 166-177. 10.1016/j.tig.2009.03.002.
Newbury DF, Bonora E, Lamb JA, Fisher SE, Lai CS, Baird G, Jannoun L, Slonims V, Stott CM, Merricks MJ, Bolton PF, Bailey AJ, Monaco AP, International Molecular Genetic Study of Autism Consortium: FOXP2 is not a major susceptibility gene for autism or specific language impairment. Am J Hum Genet. 2002, 70: 1318-1327. 10.1086/339931.
MacDermot KD, Bonora E, Sykes N, Coupe AM, Lai CS, Vernes SC, Vargha-Khadem F, McKenzie F, Smith RL, Monaco AP, Fisher SE: Identification of FOXP2 truncation as a novel cause of developmental speech and language deficits. Am J Hum Genet. 2005, 76: 1074-1080. 10.1086/430841.
Shriberg LD, Jakielski KJ, El-Shanti H: Breakpoint localization using array-CGH in three siblings with an unbalanced 4q;16q translocation and childhood apraxia of speech (CAS). Am J Med Genet. 2008, 146A: 2227-2233. 10.1002/ajmg.a.32363.
Thevenon J, Callier P, Andrieux J, Delobel B, David A, Sukno S, Minot D, Mosca Anne L, Marle N, Sanlaville D, Bonnet M, Masurel-Paulet A, Levy F, Gaunt L, Farrell S, Le Caignec C, Toutain A, Carmignac V, Mugneret F, Clayton-Smith J, Thauvin-Robinet C, Faivre L: 12p13.33 microdeletion including ELKS/ERC1, a new locus associated with childhood apraxia of speech. Eur J Hum Genet. 2013, 21: 82-88. 10.1038/ejhg.2012.116.
Wang Y, Liu X, Biederer T, Sudhof TC: A family of RIM-binding proteins regulated by alternative splicing: Implications for the genesis of synaptic active zones. Proc Natl Acad Sci USA. 2002, 99: 14464-14469. 10.1073/pnas.182532999.
Newbury DF, Mari F, Sadighi Akha E, Macdermot KD, Canitano R, Monaco AP, Taylor JC, Renieri A, Fisher SE, Knight SJ: Dual copy number variants involving 16p11 and 6q22 in a case of childhood apraxia of speech and pervasive developmental disorder. Eur J Hum Genet. 2012, 21: 361-365.
PubMed PubMed Central Google Scholar
Raca G, Baas BS, Kirmani S, Laffin JJ, Jackson CA, Strand EA, Jakielski KJ, Shriberg LD: Childhood Apraxia of Speech (CAS) in two patients with 16p11.2 microdeletion syndrome. Eur J Hum Genet. 2013, 21: 455-459. 10.1038/ejhg.2012.165.
Van der Aa N, Rooms L, Vandeweyer G, van den Ende J, Reyniers E, Fichera M, Romano C, Delle Chiaie B, Mortier G, Menten B, Destrée A, Maystadt I, Männik K, Kurg A, Reimand T, McMullan D, Oley C, Brueton L, Bongers EM, van Bon BW, Pfund R, Jacquemont S, Ferrarini A, Martinet D, Schrander-Stumpel C, Stegmann AP, Frints SG, de Vries BB, Ceulemans B, Kooy RF: Fourteen new cases contribute to the characterization of the 7q11.23 microduplication syndrome. Eur J Med Genet. 2009, 52: 94-100. 10.1016/j.ejmg.2009.02.006.
Shriberg LD, Potter NL, Strand EA: Prevalence and phenotype of childhood apraxia of speech in youth with galactosemia. J Speech Lang Hear Res. 2011, 54: 487-519. 10.1044/1092-4388(2010/10-0068).
Tomblin JB, Records NL, Buckwalter P, Zhang X, Smith E, O'Brien M: Prevalence of specific language impairment in kindergarten children. J Speech Lang Hear Res. 1997, 40: 1245-1260.
Tomblin JB, Buckwalter PR: Heritability of poor language achievement among twins. J Speech Lang Hear Res. 1998, 41: 188-199.
Newbury DF, Bishop DV, Monaco AP: Genetic influences on language impairment and phonological short-term memory. Trends Cogn Sci. 2005, 9: 528-534. 10.1016/j.tics.2005.09.002.
SLI Consortium: A genomewide scan identifies two novel loci involved in specific language impairment. Am J Hum Genet. 2002, 70: 384-398. 10.1086/338649.
SLI Consortium: Highly significant linkage to the SLI1 locus in an expanded sample of individuals affected by specific language impairment. Am J Hum Genet. 2004, 74: 1225-1238.
Monaco AP: Multivariate linkage analysis of specific language impairment (SLI). Ann Hum Genet. 2007, 71: 660-673. 10.1111/j.1469-1809.2007.00361.x.
Falcaro M, Pickles A, Newbury DF, Addis L, Banfield E, Fisher SE, Monaco AP, Simkin Z, Conti-Ramsden G: Genetic and phenotypic effects of phonological short-term memory and grammatical morphology in specific language impairment. Genes Brain Behav. 2008, 7: 393-402. 10.1111/j.1601-183X.2007.00364.x.
Bartlett CW, Flax JF, Logue MW, Vieland VJ, Bassett AS, Tallal P, Brzustowicz LM: A major susceptibility locus for specific language impairment is located on 13q21. Am J Hum Genet. 2002, 71: 45-55. 10.1086/341095.
Bartlett CW, Flax JF, Logue MW, Smith BJ, Vieland VJ, Tallal P, Brzustowicz LM: Examination of potential overlap in autism and language loci on chromosomes 2, 7, and 13 in two independent samples ascertained for specific language impairment. Hum Hered. 2004, 57: 10-20. 10.1159/000077385.
Newbury DF, Winchester L, Addis L, Paracchini S, Buckingham LL, Clark A, Cohen W, Cowie H, Dworzynski K, Everitt A, Goodyer IM, Hennessy E, Kindley AD, Miller LL, Nasir J, O'Hare A, Shaw D, Simkin Z, Simonoff E, Slonims V, Watson J, Ragoussis J, Fisher SE, Seckl JR, Helms PJ, Bolton PF, Pickles A, Conti-Ramsden G, Baird G, Bishop DV, Monaco AP: CMIP and ATP2C2 modulate phonological short-term memory in language impairment. Am J Hum Genet. 2009, 85: 264-272. 10.1016/j.ajhg.2009.07.004.
Xiang M, Mohamalawari D, Rao R: A novel isoform of the secretory pathway Ca2+, Mn(2+)-ATPase, hSPCA2, has unusual properties and is expressed in the brain. J Biol Chem. 2005, 280: 11608-11614. 10.1074/jbc.M413116200.
Kamal M, Valanciute A, Dahan K, Ory V, Pawlak A, Lang P, Guellaen G, Sahali D: C-mip interacts physically with RelA and inhibits nuclear factor kappa B activity. Mol Immunol. 2009, 46: 991-998. 10.1016/j.molimm.2008.09.034.
Vernes SC, Newbury DF, Abrahams BS, Winchester L, Nicod J, Groszer M, Alarcón M, Oliver PL, Davies KE, Geschwind DH, Monaco AP, Fisher SE: A functional genetic link between distinct developmental language disorders. N Engl J Med. 2008, 359: 2337-2345. 10.1056/NEJMoa0802828.
Villanueva P, de Barbieri Z, Palomino HM, Palomino H: [High prevalence of specific language impairment in Robinson Crusoe Island. A possible founder effect]. Rev Med Chil. 2008, 136: 186-192.
Villanueva P, Newbury DF, Jara L, De Barbieri Z, Mirza G, Palomino HM, Fernandez MA, Cazier JB, Monaco AP, Palomino H: Genome-wide analysis of genetic susceptibility to language impairment in an isolated Chilean population. Eur J Hum Genet. 2011, 19: 687-695. 10.1038/ejhg.2010.251.
Fisher SE, DeFries JC: Developmental dyslexia: genetic dissection of a complex cognitive trait. Nat Rev Neurosci. 2002, 3: 767-780.
Raskind WH, Peter B, Richards T, Eckert MM, Berninger VW: The genetics of reading disabilities: from phenotypes to candidate genes. Front Psychol. 2012, 3: 601-
Graham SA, Fisher SE: Decoding the genetics of speech and language. Curr Opin Neurobiol. 2013, 23: 43-51. 10.1016/j.conb.2012.11.006.
Prasse JE, Kikano GE: Stuttering: an overview. Am Fam Physician. 2008, 77: 1271-1276.
Drayna D, Kang C: Genetic approaches to understanding the causes of stuttering. J Neurodev Disord. 2011, 3: 374-380. 10.1007/s11689-011-9090-7.
Nippold MA: Stuttering and language ability in children: questioning the connection. Am J Speech Lang Pathol. 2012, 21: 183-196. 10.1044/1058-0360(2012/11-0078).
Kang C, Drayna D: Genetics of speech and language disorders. Annu Rev Genomics Hum Genet. 2011, 12: 145-164. 10.1146/annurev-genom-090810-183119.
Raza MH, Amjad R, Riazuddin S, Drayna D: Studies in a consanguineous family reveal a novel locus for stuttering on chromosome 16q. Hum Genet. 2012, 131: 311-313. 10.1007/s00439-011-1134-2.
Raza MH, Gertz EM, Mundorff J, Lukong J, Kuster J, Schaffer AA, Drayna D: Linkage analysis of a large African family segregating stuttering suggests polygenic inheritance. Hum Genet. 2012, 132: 385-396.
Riaz N, Steinberg S, Ahmad J, Pluzhnikov A, Riazuddin S, Cox NJ, Drayna D: Genomewide significant linkage to stuttering on chromosome 12. Am J Hum Genet. 2005, 76: 647-651. 10.1086/429226.
Kang C, Riazuddin S, Mundorff J, Krasnewich D, Friedman P, Mullikin JC, Drayna D: Mutations in the lysosomal enzyme-targeting pathway and persistent stuttering. N Engl J Med. 2010, 362: 677-685. 10.1056/NEJMoa0902630.
Gilissen C, Hoischen A, Brunner HG, Veltman JA: Unlocking Mendelian disease using exome sequencing. Genome Biol. 2011, 12: 228-10.1186/gb-2011-12-9-228.
Veltman JA, Brunner HG: De novo mutations in human genetic disease. Nat Rev Genet. 2012, 13: 565-575. 10.1038/nrg3241.
Buxbaum JD, Daly MJ, Devlin B, Lehner T, Roeder K, State MW: The autism sequencing consortium: large-scale, high-throughput sequencing in autism spectrum disorders. Neuron. 2012, 76: 1052-1056. 10.1016/j.neuron.2012.12.008.
Ng SB, Bigham AW, Buckingham KJ, Hannibal MC, McMillin MJ, Gildersleeve HI, Beck AE, Tabor HK, Cooper GM, Mefford HC, Lee C, Turner EH, Smith JD, Rieder MJ, Yoshiura K, Matsumoto N, Ohta T, Niikawa N, Nickerson DA, Bamshad MJ, Shendure J: Exome sequencing identifies MLL2 mutations as a cause of Kabuki syndrome. Nat Genet. 2010, 42: 790-793. 10.1038/ng.646.
Hoischen A, van Bon BW, Rodríguez-Santiago B, Gilissen C, Vissers LE, de Vries P, Janssen I, van Lier B, Hastings R, Smithson SF, Newbury-Ecob R, Kjaergaard S, Goodship J, McGowan R, Bartholdi D, Rauch A, Peippo M, Cobben JM, Wieczorek D, Gillessen-Kaesbach G, Veltman JA, Brunner HG, de Vries BB: De novo nonsense mutations in ASXL1 cause Bohring-Opitz syndrome. Nat Genet. 2011, 43: 729-731. 10.1038/ng.868.
Sirmaci A, Spiliopoulos M, Brancati F, Powell E, Duman D, Abrams A, Bademci G, Agolini E, Guo S, Konuk B, Kavaz A, Blanton S, Digilio MC, Dallapiccola B, Young J, Zuchner S, Tekin M: Mutations in ANKRD11 cause KBG syndrome, characterized by intellectual disability, skeletal malformations, and macrodontia. Am J Hum Genet. 2011, 89: 289-294. 10.1016/j.ajhg.2011.06.007.
Hoischen A, van Bon BW, Gilissen C, Arts P, van Lier B, Steehouwer M, de Vries P, de Reuver R, Wieskamp N, Mortier G, Devriendt K, Amorim MZ, Revencu N, Kidd A, Barbosa M, Turner A, Smith J, Oley C, Henderson A, Hayes IM, Thompson EM, Brunner HG, de Vries BB, Veltman JA: De novo mutations of SETBP1 cause Schinzel-Giedion syndrome. Nat Genet. 2010, 42: 483-485. 10.1038/ng.581.
Marseglia G, Scordo MR, Pescucci C, Nannetti G, Biagini E, Scandurra V, Gerundino F, Magi A, Benelli M, Torricelli F: 372 kb Microdeletion in 18q12.3 causing SETBP1 haploinsufficiency associated with mild mental retardation and expressive speech impairment. Eur J Med Genet. 2012, 55: 216-221. 10.1016/j.ejmg.2012.01.005.
Oakley K, Han Y, Vishwakarma BA, Chu S, Bhatia R, Gudmundsson KO, Keller J, Chen X, Vasko V, Jenkins NA, Copeland NG, Du Y: Setbp1 promotes the self-renewal of murine myeloid progenitors via activation of Hoxa9 and Hoxa10. Blood. 2012, 119: 6099-6108. 10.1182/blood-2011-10-388710.
Vissers LE, de Ligt J, Gilissen C, Janssen I, Steehouwer M, de Vries P, van Lier B, Arts P, Wieskamp N, del Rosario M, van Bon BW, Hoischen A, de Vries BB, Brunner HG, Veltman JA: A de novo paradigm for mental retardation. Nat Genet. 2010, 42: 1109-1112. 10.1038/ng.712.
O'Roak BJ, Deriziotis P, Lee C, Vives L, Schwartz JJ, Girirajan S, Karakoc E, Mackenzie AP, Ng SB, Baker C, Rieder MJ, Nickerson DA, Bernier R, Fisher SE, Shendure J, Eichler EE: Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet. 2011, 43: 585-589. 10.1038/ng.835.
O'Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, Levy R, Ko A, Lee C, Smith JD, Turner EH, Stanaway IB, Vernot B, Malig M, Baker C, Reilly B, Akey JM, Borenstein E, Rieder MJ, Nickerson DA, Bernier R, Shendure J, Eichler EE: Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012, 485: 246-250. 10.1038/nature10989.
Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, Ercan-Sencicek AG, DiLullo NM, Parikshak NN, Stein JL, Walker MF, Ober GT, Teran NA, Song Y, El-Fishawy P, Murtha RC, Choi M, Overton JD, Bjornson RD, Carriero NJ, Meyer KA, Bilguvar K, Mane SM, Sestan N, Lifton RP, Günel M, Roeder K, Geschwind DH, Devlin B, State MW: De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012, 485: 237-241. 10.1038/nature10945.
Neale BM, Kou Y, Liu L, Ma'ayan A, Samocha KE, Sabo A, Lin CF, Stevens C, Wang LS, Makarov V, Polak P, Yoon S, Maguire J, Crawford EL, Campbell NG, Geller ET, Valladares O, Schafer C, Liu H, Zhao T, Cai G, Lihm J, Dannenfelser R, Jabado O, Peralta Z, Nagaswamy U, Muzny D, Reid JG, Newsham I, Wu Y, et al: Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012, 485: 242-245. 10.1038/nature11011.
Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, Yamrom B, Lee YH, Narzisi G, Leotta A, Kendall J, Grabowska E, Ma B, Marks S, Rodgers L, Stepansky A, Troge J, Andrews P, Bekritsky M, Pradhan K, Ghiban E, Kramer M, Parla J, Demeter R, Fulton LL, Fulton RS, Magrini VJ, Ye K, Darnell JC, Darnell RB, Mardis ER, Wilson RK, Schatz MC, McCombie WR, Wigler M: De novo gene disruptions in children on the autistic spectrum. Neuron. 2012, 74: 285-299. 10.1016/j.neuron.2012.04.009.
Yu TW, Chahrour MH, Coulter ME, Jiralerspong S, Okamura-Ikeda K, Ataman B, Schmitz-Abe K, Harmin DA, Adli M, Malik AN, D'Gama AM, Lim ET, Sanders SJ, Mochida GH, Partlow JN, Sunu CM, Felie JM, Rodriguez J, Nasir RH, Ware J, Joseph RM, Hill RS, Kwan BY, Al-Saffar M, Mukaddes NM, Hashmi A, Balkhy S, Gascon GG, Hisama FM, LeClair E, et al: Using whole-exome sequencing to identify inherited causes of autism. Neuron. 2013, 77: 259-273. 10.1016/j.neuron.2012.11.002.
Lim ET, Raychaudhuri S, Sanders SJ, Stevens C, Sabo A, MacArthur DG, Neale BM, Kirby A, Ruderfer DM, Fromer M, Lek M, Liu L, Flannick J, Ripke S, Nagaswamy U, Muzny D, Reid JG, Hawes A, Newsham I, Wu Y, Lewis L, Dinh H, Gross S, Wang LS, Lin CF, Valladares O, Gabriel SB, dePristo M, Altshuler DM, Purcell SM, et al: Rare complete knockouts in humans: population distribution and significant role in autism spectrum disorders. Neuron. 2013, 77: 235-242. 10.1016/j.neuron.2012.12.029.
Ng PC, Henikoff S: SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003, 31: 3812-3814. 10.1093/nar/gkg509.
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR: A method and server for predicting damaging missense mutations. Nat Methods. 2010, 7: 248-249. 10.1038/nmeth0410-248.
Vernes SC, Nicod J, Elahi FM, Coventry JA, Kenny N, Coupe AM, Bird LE, Davies KE, Fisher SE: Functional genetic analysis of mutations implicated in a human speech and language disorder. Hum Mol Genet. 2006, 15: 3154-3167. 10.1093/hmg/ddl392.
Li S, Weidenfeld J, Morrisey EE: Transcriptional and DNA binding activity of the Foxp1/2/4 family is modulated by heterotypic and homotypic protein interactions. Mol Cell Biol. 2004, 24: 809-822. 10.1128/MCB.24.2.809-822.2004.
Shu W, Yang H, Zhang L, Lu MM, Morrisey EE: Characterization of a new subfamily of winged-helix/forkhead (Fox) genes that are expressed in the lung and act as transcriptional repressors. J Biol Chem. 2001, 276: 27488-27497. 10.1074/jbc.M100636200.
Spiteri E, Konopka G, Coppola G, Bomar J, Oldham M, Ou J, Vernes SC, Fisher SE, Ren B, Geschwind DH: Identification of the transcriptional targets of FOXP2, a gene linked to speech and language, in developing human brain. Am J Hum Genet. 2007, 81: 1144-1157. 10.1086/522237.
Vernes SC, Spiteri E, Nicod J, Groszer M, Taylor JM, Davies KE, Geschwind DH, Fisher SE: High-throughput analysis of promoter occupancy reveals direct neural targets of FOXP2, a gene mutated in speech and language disorders. Am J Hum Genet. 2007, 81: 1232-1250. 10.1086/522238.
Vernes SC, Oliver PL, Spiteri E, Lockstone HE, Puliyadi R, Taylor JM, Ho J, Mombereau C, Brewer A, Lowy E, Nicod J, Groszer M, Baban D, Sahgal N, Cazier JB, Ragoussis J, Davies KE, Geschwind DH, Fisher SE: Foxp2 regulates gene networks implicated in neurite outgrowth in the developing brain. PLoS Genet. 2011, 7: e1002145-10.1371/journal.pgen.1002145.
Roll P, Vernes SC, Bruneau N, Cillario J, Ponsole-Lenfant M, Massacrier A, Rudolf G, Khalife M, Hirsch E, Fisher SE, Szepetowski P: Molecular networks implicated in speech-related disorders: FOXP2 regulates the SRPX2/uPAR complex. Hum Mol Genet. 2010, 19: 4848-4860. 10.1093/hmg/ddq415.
Walker RM, Hill AE, Newman AC, Hamilton G, Torrance HS, Anderson SM, Ogawa F, Derizioti P, Nicod J, Vernes SC, Fisher SE, Thomson PA, Porteous DJ, Evans KL: The DISC1 promoter: characterization and regulation by FOXP2. Hum Mol Genet. 2012, 21: 2862-2872. 10.1093/hmg/dds111.
Mukamel Z, Konopka G, Wexler E, Osborn GE, Dong H, Bergman MY, Levitt P, Geschwind DH: Regulation of MET by FOXP2, genes implicated in higher cognitive dysfunction and autism risk. J Neurosci. 2011, 31: 11437-11442. 10.1523/JNEUROSCI.0181-11.2011.
Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, Pevny L, Stewart CL, Xu X, Chiu SY, Shrager P, Furley AJ, Peles E: Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol. 2003, 162: 1149-1160. 10.1083/jcb.200305018.
Anderson GR, Galfin T, Xu W, Aoto J, Malenka RC, Sudhof TC: Candidate autism gene screen identifies critical role for cell-adhesion molecule CASPR2 in dendritic arborization and spine development. Proc Natl Acad Sci USA. 2012, 109: 18120-18125. 10.1073/pnas.1216398109.
Strauss KA, Puffenberger EG, Huentelman MJ, Gottlieb S, Dobrin SE, Parod JM, Stephan DA, Morton DH: Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N Engl J Med. 2006, 354: 1370-1377. 10.1056/NEJMoa052773.
Alarcón M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, Sebat J, Wigler M, Martin CL, Ledbetter DH, Nelson SF, Cantor RM, Geschwind DH: Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008, 82: 150-159. 10.1016/j.ajhg.2007.09.005.
Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda M, Rea A, Guy M, Lin S, Cook EH, Chakravarti A: A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet. 2008, 82: 160-164. 10.1016/j.ajhg.2007.09.015.
Bakkaloglu B, O'Roak BJ, Louvi A, Gupta AR, Abelson JF, Morgan TM, Chawarska K, Klin A, Ercan-Sencicek AG, Stillman AA, Tanriover G, Abrahams BS, Duvall JA, Robbins EM, Geschwind DH, Biederer T, Gunel M, Lifton RP, State MW: Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am J Hum Genet. 2008, 82: 165-173. 10.1016/j.ajhg.2007.09.017.
Friedman JI, Vrijenhoek T, Markx S, Janssen IM, van der Vliet WA, Faas BH, Knoers NV, Cahn W, Kahn RS, Edelmann L, Davis KL, Silverman JM, Brunner HG, van Kessel AG, Wijmenga C, Ophoff RA, Veltman JA: CNTNAP2 gene dosage variation is associated with schizophrenia and epilepsy. Mol Psychiatry. 2008, 13: 261-266. 10.1038/sj.mp.4002049.
Stein MB, Yang BZ, Chavira DA, Hitchcock CA, Sung SC, Shipon-Blum E, Gelernter J: A common genetic variant in the neurexin superfamily member CNTNAP2 is associated with increased risk for selective mutism and social anxiety-related traits. Biol Psychiatry. 2011, 69: 825-831. 10.1016/j.biopsych.2010.11.008.
Verkerk AJ, Mathews CA, Joosse M, Eussen BH, Heutink P, Oostra BA: CNTNAP2 is disrupted in a family with Gilles de la Tourette syndrome and obsessive compulsive disorder. Genomics. 2003, 82: 1-9. 10.1016/S0888-7543(03)00097-1.
Teramitsu I, Kudo LC, London SE, Geschwind DH, White SA: Parallel FoxP1 and FoxP2 expression in songbird and human brain predicts functional interaction. J Neurosci. 2004, 24: 3152-3163. 10.1523/JNEUROSCI.5589-03.2004.
Shu W, Lu MM, Zhang Y, Tucker PW, Zhou D, Morrisey EE: Foxp2 and Foxp1 cooperatively regulate lung and esophagus development. Development. 2007, 134: 1991-2000. 10.1242/dev.02846.
Leblond CS, Heinrich J, Delorme R, Proepper C, Betancur C, Huguet G, Konyukh M, Chaste P, Ey E, Rastam M, Anckarsäter H, Nygren G, Gillberg IC, Melke J, Toro R, Regnault B, Fauchereau F, Mercati O, Lemière N, Skuse D, Poot M, Holt R, Monaco AP, Järvelä I, Kantojärvi K, Vanhala R, Curran S, Collier DA, Bolton P, Chiocchetti A, et al: Genetic and functional analyses of SHANK2 mutations suggest a multiple hit model of autism spectrum disorders. PLoS Genet. 2012, 8: e1002521-10.1371/journal.pgen.1002521.
Ruzzo EK, Pappas AL, Goldstein DB: Modifier genetics in neuropsychiatric disease: challenges and opportunities. Genome Biol. 2012, 13: 150-10.1186/gb-2012-13-3-150.
Vernes SC, MacDermot KD, Monaco AP, Fisher SE: Assessing the impact of FOXP1 mutations on developmental verbal dyspraxia. Eur J Hum Genet. 2009, 17: 1354-1358. 10.1038/ejhg.2009.43.
Hamdan FF, Daoud H, Rochefort D, Piton A, Gauthier J, Langlois M, Foomani G, Dobrzeniecka S, Krebs MO, Joober R, Lafrenière RG, Lacaille JC, Mottron L, Drapeau P, Beauchamp MH, Phillips MS, Fombonne E, Rouleau GA, Michaud JL: De novo mutations in FOXP1 in cases with intellectual disability, autism, and language impairment. Am J Hum Genet. 2010, 87: 671-678. 10.1016/j.ajhg.2010.09.017.
Horn D, Kapeller J, Rivera-Brugués N, Moog U, Lorenz-Depiereux B, Eck S, Hempel M, Wagenstaller J, Gawthrope A, Monaco AP, Bonin M, Riess O, Wohlleber E, Illig T, Bezzina CR, Franke A, Spranger S, Villavicencio-Lorini P, Seifert W, Rosenfeld J, Klopocki E, Rappold GA, Strom TM: Identification of FOXP1 deletions in three unrelated patients with mental retardation and significant speech and language deficits. Hum Mutat. 2010, 31: E1851-1860. 10.1002/humu.21362.
Talkowski ME, Rosenfeld JA, Blumenthal I, Pillalamarri V, Chiang C, Heilbut A, Ernst C, Hanscom C, Rossin E, Lindgren AM, Pereira S, Ruderfer D, Kirby A, Ripke S, Harris DJ, Lee JH, Ha K, Kim HG, Solomon BD, Gropman AL, Lucente D, Sims K, Ohsumi TK, Borowsky ML, Loranger S, Quade B, Lage K, Miles J, Wu BL, Shen Y, et al: Sequencing chromosomal abnormalities reveals neurodevelopmental loci that confer risk across diagnostic boundaries. Cell. 2012, 149: 525-537. 10.1016/j.cell.2012.03.028.
Lee WS, Kang C, Drayna D, Kornfeld S: Analysis of mannose 6-phosphate uncovering enzyme mutations associated with persistent stuttering. J Biol Chem. 2011, 286: 39786-39793. 10.1074/jbc.M111.295899.
Cooper GM, Shendure J: Needles in stacks of needles: finding disease-causal variants in a wealth of genomic data. Nat Rev Genet. 2011, 12: 628-640. 10.1038/nrg3046.
Fowler DM, Araya CL, Fleishman SJ, Kellogg EH, Stephany JJ, Baker D, Fields S: High resolution mapping of protein sequence-function relationships. Nat Methods. 2010, 7: 741-746. 10.1038/nmeth.1492.
Patwardhan RP, Lee C, Litvin O, Young DL, Pe'er D, Shendure J: High-resolution analysis of DNA regulatory elements by synthetic saturation mutagenesis. Nat Biotech. 2009, 27: 1173-1175. 10.1038/nbt.1589.
CAS Google Scholar
Patwardhan RP, Hiatt JB, Witten DM, Kim MJ, Smith RP, May D, Lee C, Andrie JM, Lee SI, Cooper GM, Ahituv N, Pennacchio LA, Shendure J: Massively parallel functional dissection of mammalian enhancers in vivo. Nat Biotechnol. 2012, 30: 265-270. 10.1038/nbt.2136.
Pitt JN, Ferré-D'Amaré AR: Rapid construction of empirical RNA fitness landscapes. Science. 2010, 330: 376-379. 10.1126/science.1192001.
ENCODE Project Consortium, Dunham I, Kundaje A, Aldred SF, Collins PJ, Davis CA, Doyle F, Epstein CB, Frietze S, Harrow J, Kaul R, Khatun J, Lajoie BR, Landt SG, Lee BK, Pauli F, Rosenbloom KR, Sabo P, Safi A, Sanyal A, Shoresh N, Simon JM, Song L, Trinklein ND, Altshuler RC, Birney E, Brown JB, Cheng C, Djebali S, Dong X, et al: An integrated encyclopedia of DNA elements in the human genome. Nature. 2012, 489: 57-74. 10.1038/nature11247.
Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M, Karczewski KJ, Park J, Hitz BC, Weng S, Cherry JM, Snyder M: Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012, 22: 1790-1797. 10.1101/gr.137323.112.
White SA, Fisher SE, Geschwind DH, Scharff C, Holy TE: Singing mice, songbirds, and more: models for FOXP2 function and dysfunction in human speech and language. J Neurosci. 2006, 26: 10376-10379. 10.1523/JNEUROSCI.3379-06.2006.
Lai CS, Gerrelli D, Monaco AP, Fisher SE, Copp AJ: FOXP2 expression during brain development coincides with adult sites of pathology in a severe speech and language disorder. Brain. 2003, 126: 2455-2462. 10.1093/brain/awg247.
Belton E, Salmond CH, Watkins KE, Vargha-Khadem F, Gadian DG: Bilateral brain abnormalities associated with dominantly inherited verbal and orofacial dyspraxia. Hum Brain Mapp. 2003, 18: 194-200. 10.1002/hbm.10093.
Liegeois F, Baldeweg T, Connelly A, Gadian DG, Mishkin M, Vargha-Khadem F: Language fMRI abnormalities associated with FOXP2 gene mutation. Nat Neurosci. 2003, 6: 1230-1237. 10.1038/nn1138.
Ferland RJ, Cherry TJ, Preware PO, Morrisey EE, Walsh CA: Characterization of Foxp2 and Foxp1 mRNA and protein in the developing and mature brain. J Comp Neurol. 2003, 460: 266-279. 10.1002/cne.10654.
Enard W: FOXP2 and the role of cortico-basal ganglia circuits in speech and language evolution. Curr Opin Neurobiol. 2011, 21: 415-424. 10.1016/j.conb.2011.04.008.
Groszer M, Keays DA, Deacon RM, de Bono JP, Prasad-Mulcare S, Gaub S, Baum MG, French CA, Nicod J, Coventry JA, Enard W, Fray M, Brown SD, Nolan PM, Pääbo S, Channon KM, Costa RM, Eilers J, Ehret G, Rawlins JN, Fisher SE: Impaired synaptic plasticity and motor learning in mice with a point mutation implicated in human speech deficits. Curr Biol. 2008, 18: 354-362. 10.1016/j.cub.2008.01.060.
French CA, Jin X, Campbell TG, Gerfen E, Groszer M, Fisher SE, Costa RM: An aetiological Foxp2 mutation causes aberrant striatal activity and alters plasticity during skill learning. Mol Psychiatry. 2011, 17: 1077-1085.
Kurt S, Groszer M, Fisher SE, Ehret G: Modified sound-evoked brainstem potentials in Foxp2 mutant mice. Brain Res. 2009, 1289: 30-36.
Kurt S, Fisher SE, Ehret G: Foxp2 mutations impair auditory-motor association learning. PLoS One. 2012, 7: e33130-10.1371/journal.pone.0033130.
Watkins KE, Dronkers NF, Vargha-Khadem F: Behavioural analysis of an inherited speech and language disorder: comparison with acquired aphasia. Brain. 2002, 125: 452-464. 10.1093/brain/awf058.
Scharff C, Adam I: Neurogenetics of birdsong. Curr Opin Neurobiol. 2013, 23: 29-36. 10.1016/j.conb.2012.10.001.
Haesler S, Wada K, Nshdejan A, Morrisey EE, Lints T, Jarvis ED, Scharff C: FoxP2 expression in avian vocal learners and non-learners. J Neurosci. 2004, 24: 3164-3175. 10.1523/JNEUROSCI.4369-03.2004.
Miller JE, Spiteri E, Condro MC, Dosumu-Johnson RT, Geschwind DH, White SA: Birdsong decreases protein levels of FoxP2, a molecule required for human speech. J Neurophysiol. 2008, 100: 2015-2025. 10.1152/jn.90415.2008.
Teramitsu I, Poopatanapong A, Torrisi S, White SA: Striatal FoxP2 is actively regulated during songbird sensorimotor learning. PLoS One. 2010, 5: e8548-10.1371/journal.pone.0008548.
Teramitsu I, White SA: FoxP2 regulation during undirected singing in adult songbirds. J Neurosci. 2006, 26: 7390-7394. 10.1523/JNEUROSCI.1662-06.2006.
Haesler S, Rochefort C, Georgi B, Licznerski P, Osten P, Scharff C: Incomplete and inaccurate vocal imitation after knockdown of FoxP2 in songbird basal ganglia nucleus Area X. PLoS Biol. 2007, 5: e321-10.1371/journal.pbio.0050321.
Schulz SB, Haesler S, Scharff C, Rochefort C: Knockdown of FoxP2 alters spine density in Area X of the zebra finch. Genes Brain Behav. 2010, 9: 732-740. 10.1111/j.1601-183X.2010.00607.x.
Zweier C, de Jong EK, Zweier M, Orrico A, Ousager LB, Collins AL, Bijlsma EK, Oortveld MA, Ekici AB, Reis A, Schenck A, Rauch A: CNTNAP2 and NRXN1 are mutated in autosomal-recessive Pitt-Hopkins-like mental retardation and determine the level of a common synaptic protein in Drosophila. Am J Hum Genet. 2009, 85: 655-666. 10.1016/j.ajhg.2009.10.004.
Gregor A, Albrecht B, Bader I, Bijlsma EK, Ekici AB, Engels H, Hackmann K, Horn D, Hoyer J, Klapecki J, Kohlhase J, Maystadt I, Nagl S, Prott E, Tinschert S, Ullmann R, Wohlleber E, Woods G, Reis A, Rauch A, Zweier C: Expanding the clinical spectrum associated with defects in CNTNAP2 and NRXN1. BMC Med Genet. 2011, 12: 106-10.1186/1471-2350-12-106.
Newbury DF, Paracchini S, Scerri TS, Winchester L, Addis L, Richardson AJ, Walter J, Stein JF, Talcott JB, Monaco AP: Investigation of dyslexia and SLI risk variants in reading- and language-impaired subjects. Behav Genet. 2011, 41: 90-104. 10.1007/s10519-010-9424-3.
Whitehouse AJ, Bishop DV, Ang QW, Pennell CE, Fisher SE: CNTNAP2 variants affect early language development in the general population. Genes Brain Behav. 2011, 10: 451-456. 10.1111/j.1601-183X.2011.00684.x.
Whalley HC, O'Connell G, Sussmann JE, Peel A, Stanfield AC, Hayiou-Thomas ME, Johnstone EC, Lawrie SM, McIntosh AM, Hall J: Genetic variation in CNTNAP2 alters brain function during linguistic processing in healthy individuals. Am J Med Genet B Neuropsychiatr Genet. 2011, 156B: 941-948.
Kos M, van den Brink D, Snijders TM, Rijpkema M, Franke B, Fernandez G, Hagoort P: CNTNAP2 and language processing in healthy individuals as measured with ERPs. PLoS One. 2012, 7: e46995-10.1371/journal.pone.0046995.
Dennis EL, Jahanshad N, Rudie JD, Brown JA, Johnson K, McMahon KL, de Zubicaray GI, Montgomery G, Martin NG, Wright MJ, Bookheimer SY, Dapretto M, Toga AW, Thompson PM: Altered structural brain connectivity in healthy carriers of the autism risk gene, CNTNAP2. Brain Connect. 2011, 1: 447-459. 10.1089/brain.2011.0064.
Green RE, Krause J, Briggs AW, Maricic T, Stenzel U, Kircher M, Patterson N, Li H, Zhai W, Fritz MH, Hansen NF, Durand EY, Malaspinas AS, Jensen JD, Marques-Bonet T, Alkan C, Prüfer K, Meyer M, Burbano HA, Good JM, Schultz R, Aximu-Petri A, Butthof A, Höber B, Höffner B, Siegemund M, Weihmann A, Nusbaum C, Lander ES, Russ C, et al: A draft sequence of the Neandertal genome. Science. 2010, 328: 710-722. 10.1126/science.1188021.
Meyer M, Kircher M, Gansauge MT, Li H, Racimo F, Mallick S, Schraiber JG, Jay F, Prüfer K, de Filippo C, Sudmant PH, Alkan C, Fu Q, Do R, Rohland N, Tandon A, Siebauer M, Green RE, Bryc K, Briggs AW, Stenzel U, Dabney J, Shendure J, Kitzman J, Hammer MF, Shunkov MV, Derevianko AP, Patterson N, Andrés AM, Eichler EE, et al: A high-coverage genome sequence from an archaic Denisovan individual. Science. 2012, 338: 222-226. 10.1126/science.1224344.
Enard W, Przeworski M, Fisher SE, Lai CS, Wiebe V, Kitano T, Monaco AP, Paabo S: Molecular evolution of FOXP2, a gene involved in speech and language. Nature. 2002, 418: 869-872. 10.1038/nature01025.
Maricic T, Günther V, Georgiev O, Gehre S, Curlin M, Schreiweis C, Naumann R, Burbano HA, Meyer M, Lalueza-Fox C, de la Rasilla M, Rosas A, Gajovic S, Kelso J, Enard W, Schaffner W, Pääbo S: A recent evolutionary change affects a regulatory element in the human FOXP2 gene. Mol Biol Evol. 2012, 30: 844-852.
Falivelli G, De Jaco A, Favaloro FL, Kim H, Wilson J, Dubi N, Ellisman MH, Abrahams BS, Taylor P, Comoletti D: Inherited genetic variants in autism-related CNTNAP2 show perturbed trafficking and ATF6 activation. Hum Mol Genet. 2012, 21: 4761-4773. 10.1093/hmg/dds320.
Download references
Author information
Authors and affiliations.
Max Planck Institute for Psycholinguistics, 6525 XD, Nijmegen, The Netherlands
Pelagia Deriziotis & Simon E Fisher
Donders Institute for Brain, Cognition and Behaviour, Radboud University, 6525 EN, Nijmegen, The Netherlands
Simon E Fisher
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Simon E Fisher .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Rights and permissions.
Reprints and permissions
About this article
Cite this article.
Deriziotis, P., Fisher, S.E. Neurogenomics of speech and language disorders: the road ahead. Genome Biol 14 , 204 (2013). https://doi.org/10.1186/gb-2013-14-4-204
Download citation
Published : 18 April 2013
DOI : https://doi.org/10.1186/gb-2013-14-4-204
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- functional validation
- neurodevelopmental disorders
Genome Biology
ISSN: 1474-760X
- General enquiries: [email protected]
- Published: 26 January 2010
Recent advances in the genetics of language impairment
- Dianne F Newbury 1 ,
- Simon E Fisher 1 &
- Anthony P Monaco 1
Genome Medicine volume 2 , Article number: 6 ( 2010 ) Cite this article
16k Accesses
59 Citations
Metrics details
Specific language impairment (SLI) is defined as an unexpected and persistent impairment in language ability despite adequate opportunity and intelligence and in the absence of any explanatory medical conditions. This condition is highly heritable and affects between 5% and 8% of pre-school children. Over the past few years, investigations have begun to uncover genetic factors that may contribute to susceptibility to language impairment. So far, variants in four specific genes have been associated with spoken language disorders - forkhead box P2 ( FOXP2 ) and contactin-associated protein-like 2 ( CNTNAP2 ) on chromosome7 and calcium-transporting ATPase 2C2 ( ATP2C2 ) and c-MAF inducing protein ( CMIP ) on chromosome 16. Here, we describe the different ways in which these genes were identified as candidates for language impairment. We discuss how characterization of these genes, and the pathways in which they are involved, may enhance our understanding of language disorders and improve our understanding of the biological foundations of language acquisition.
Introduction
Language is a quintessential human trait that, for the most part, proceeds along a recognized trajectory with minimal explicit instruction [ 1 ]. In some cases, however, language acquisition is not so straightforward and language ability is delayed or permanently impaired. Sometimes these impairments form part of a recognized medical condition (such as learning deficit, autism or deafness), but often no obvious cause can be identified. In such cases, the language deficit is usually classified as specific language impairment (SLI) [ 2 ]. As such, SLI is usually diagnosed through exclusionary criteria rather than on the basis of any specific clinical test. SLI affects between 5% and 8% of English-speaking (primarily UK and US) pre-school children, and is a lifelong disability with an increased risk of behavioral disorders, social problems and literacy deficits [ 3 – 5 ]. The disorder shows significant overlap with associated developmental conditions, such as attention deficit hyperactivity disorder (ADHD), speech sound disorder (SSD), dyslexia and autism [ 6 ].
Over the past decade, researchers have begun to identify genetic factors that may have roles in the etiology of language disorders. It is hoped that the study of these genes will facilitate a better understanding of the cause of language impairments, leading to the development of improved diagnostic and treatment strategies for affected individuals. In turn, knowledge regarding the cause of such impairments may further our understanding of the biological pathways that underpin normal language acquisition [ 7 ].
Here, we focus on specific genes that have been identified to have a role in language impairment. Genetic linkage and association studies of SLI and related learning disorders are reviewed elsewhere [ 8 – 10 ].
Until recently, the only gene that had been directly implicated in the etiology of speech and language disorders was the FOXP2 gene on chromosome 7q (OMIM 605317). In 2001, a study by Lai and colleagues [ 11 ] implicated mutation of FOXP2 in a monogenic form of speech and language disorder found in a three-generation pedigree (the KE family) and in an unrelated individual with a chromosome translocation. In both cases, the disorder was characterized by verbal (or articulatory) dyspraxia, that is, difficulties controlling the movement and sequencing of orofacial muscles, causing deficits in the production of fluent speech. In-depth studies of the KE family showed that, in these individuals, speech production problems are accompanied by a complex array of linguistic deficits that include varying degrees of expressive and written language problems and, in some members, nonverbal cognitive impairments [ 12 ]. Subsequent screening studies have shown that although FOXP2 mutations are unlikely to be involved in the etiology of typical forms of SLI [ 13 , 14 ], heterozygous disruptions of this gene (point mutations or chromosomal rearrangements) invariably lead to syndromes that include aspects of verbal dyspraxia [ 15 – 21 ].
The FOXP2 gene encodes a transcription factor that regulates the expression of other genes. Downstream target screening studies have highlighted a variety of genes that may be regulated by FOXP2 and indicate that the effect of FOXP2 can vary greatly between tissues and developmental time points [ 22 – 24 ]. FOXP2 may thus be involved in a variety of biological pathways and cascades that may ultimately influence language development. Pathway analyses of the identified targets indicate an enrichment of genes involved in the functioning, development and patterning of the central nervous system. In analyses of human neuronal cell models, Vernes et al . [ 23 ] estimated that FOXP2 may bind directly to approximately 300 to 400 gene promoters in the human genome. Although statistically significant overlaps were seen between the individual studies of FOXP2 targets, there were also notable differences in the sets of downstream genes that were identified. This finding demonstrates the complexity of these regulatory pathways and the inherent difficulties of precisely defining them in the laboratory.
FOXP2 in the brain
The expression of FOXP2 is not limited to the brain but also seen in several other organs, primarily those derived from the foregut endoderm, such as the lungs and esophagus [ 25 ]. In the human brain, FOXP2 is expressed in a range of regions, including sensory and limbic nuclei, the cerebral cortex and several motor structures, particularly the striatum and cerebellum [ 26 , 27 ]. Within these anatomical areas, FOXP2 expression is often limited to selected subdivisions or neuron types (for example, deep layers of the cortex, medium spiny neurons in the striatum and Purkinje cells in the cerebellum).
Mice that are bred to carry disruptions of both copies of Foxp2 survive only a few weeks. They are small for their age and have widespread developmental delays, severe motor abnormalities and impaired cerebellar growth [ 28 – 32 ]. Given that total absence of functional Foxp2 results in lethality, in-depth behavioral investigations have focused on heterozygous mouse models, which carry a single working copy of Foxp2 . Note that this matches the heterozygous state of humans with FOXP2 mutations; no humans carrying homozygous mutations have ever been identified. In general, it found that these animals have normal motor skills and no obvious gross abnormalities. However, in-depth behavioral and morphological profiling has uncovered subtle deficits. Interestingly, two groups have reported that heterozygous pups produce fewer innate ultrasonic vocalizations than wild-type animals [ 28 , 30 ]. Other groups have questioned the reliability of this finding, instead describing deficits in motor skill learning [ 31 ], abnormal synaptic plasticity in striatal and cerebellar neural circuits [ 31 ] and differences in auditory brainstem responses [ 32 ] in heterozygous pups. In song-birds, it has been reported that reducing the expression of FoxP2 in an area of the brain necessary for vocal learning can interfere with the song learning process [ 33 ]. For an in-depth discussion of these animal studies, see [ 34 ].
Brain imaging studies of KE family members have also revealed structural and functional abnormalities in the cerebellum and striatum [ 12 , 35 , 36 ]. Affected individuals were found to have reduced gray matter densities in the caudate nucleus, the cerebellum, the inferior frontal gyrus and the lower primary motor cortex [ 12 , 35 ]. During the performance of language-related tasks, in contrast to the expected left-lateralized pattern of activation, affected members of the KE family showed bilateral, diffuse activation with little or no activity in the left inferior frontal cortex (which includes Broca's area, involved in speech production) and reduced activation in other speech-related cortical and sub-cortical brain regions. In addition, brain areas not usually activated during linguistic tasks, including the posterior parietal, occipital and postcentral regions, were found to be over-activated in affected individuals [ 36 ].
Evolution of FOXP2
Because of the proposed function of FOXP2 in speech and language development, this gene has been widely investigated from an evolutionary perspective. Versions of FOXP2 are found in many organisms and show striking similarities in terms of sequence and expression patterns across vertebrate species [ 26 , 27 , 33 , 37 – 39 ]. Aside from a difference in polyglutamine tract length, there are only three coding changes between the mouse and human versions of the FOXP2 gene, making it one of the most highly conserved genes found in comparisons of human-rodent genomes [ 38 , 39 ]. Interestingly, analyses of primates demonstrated that two of these three changes occurred in the human lineage after splitting from the chimpanzee and found additional signs that FOXP2 may have undergone accelerated evolution in humans [ 38 , 39 ]. Population modeling estimated that the gene was subject to positive selection approximately 200,000 years ago, a period that coincides with, or is subsequent to, the emergence of modern humans [ 38 , 39 ]. Note, however, that the errors attached to these estimates are large. Moreover, subsequent sequencing of paleontological samples has identified the human-specific coding changes of FOXP2 in Neanderthal tissues, which suggests a more ancient origin, given that Neanderthals split from humans at least 400,000 years ago [ 40 ]. Thus, the interpretation of these data is still under debate [ 41 ].
Two studies have investigated the functional differences between the human version of FOXP2 and that found in the chimpanzee. Enard et al . [ 42 ] reported that when human-specific coding changes were engineered in mice (partially 'humanizing' them at the locus), this resulted in an altered structure of innate pup vocalizations, decreased levels of exploration, decreased levels of dopamine in the brain and an increased dendrite length and synaptic plasticity in the striatum. These findings are intriguing, given that mice carrying disrupted versions of Foxp2 (described above) showed contrasting alterations in similar developmental areas. Konopka et al . [ 24 ] investigated potential differences in the functionality of the human and chimpanzee versions of FOXP2 [ 24 ]. They identified 116 genes that were differentially expressed between neuronal cell lines engineered to express either the human or the chimpanzee protein. They postulated that the identified set of genes may represent a biological network that could have a role in the evolution of human language, noting that the identified targets included genes involved in cerebellar motor function, craniofacial formation, cartilage and connective tissue formation [ 24 ].
In conclusion, although the exact contributions of FOXP2 to the development of speech and language remain unclear, the consensus from expression studies, neuro-imaging data and animal models is that this gene is of particular importance in the central nervous system, such that its dysfunction disturbs the development and function of the motor cortex, striatum and cerebellum. Investigations of the properties of FOXP2 and its downstream targets are beginning to identify networks of genes that could be crucial players in neural circuits that facilitate language acquisition.
The CNTNAP2 gene on chromosome 7q (OMIM 604569) was the first gene to be associated with genetically complex forms of SLI. This association was achieved through a candidate gene approach that arose from downstream target screening studies of FOXP2 [ 43 ]. Vernes et al . [ 43 ] discovered that FOXP2 directly binds a regulatory region of the CNTNAP2 gene. CASPR2, the protein encoded by CNTNAP2 , is a member of the neurexin family, a family that is particularly interesting from a functional point of view as members are known to interact with neuroligins to adhere presynaptic neuronal membranes to postsynaptic ones. In the case of CASPR2, the protein mediates interactions between neurons and glia during nervous system development and is also involved in localization of potassium channels within differentiating axons [ 44 , 45 ]. Furthermore, both neurexins and neuroligins have been strongly implicated in autistic disorder, a neurodevelopmental condition that shows strong overlap with SLI [ 46 – 52 ].
The regulation of CNTNAP2 by FOXP2 was verified both in neuronal cell lines and in vivo (in human fetal cortical slices). In both of these experiments, the level of FOXP2 was found to be inversely correlated with that of CASPR2 [ 43 ]. An association analysis of 38 single nucleotide polymorphisms (SNPs) across CNTNAP2 was performed in 184 families ascertained by the SLI Consortium (SLIC). These families were identified by various different groups from across the UK but all contained a proband who, currently or in the past, had expressive and/or receptive language abilities more than 2 standard deviations (SD) below that expected for their age [ 53 ]. In accordance with SLI diagnostic guidelines, individuals with autistic features, signs of mental retardation or co-occurring medical conditions were excluded from this cohort. Three quantitative measures of language were considered in this group; composite scores of expressive and receptive language ability were derived from the Clinical Evaluation of Language Fundamentals battery (CELF-R) [ 54 ]. In addition, a measure of non-word repetition [ 55 ] was collected for all probands and siblings. This test involves the repetition of nonsensical words of increasing length and complexity and the results from it have been shown to be highly heritable and a consistent marker of the presence of language impairment. Non-word repetition is considered to be a measure of phonological short-term memory, leading to the proposal that short-term memory deficits may underlie some aspects of language impairment (reviewed in [ 56 ]). Nine single SNPs in CNTNAP2 showed association primarily with the non-word repetition phenotype but also with expressive and receptive language measures. The most strongly associated SNP was rs17236239 ( P = 5.0 × 10 -5 ), a variant that falls within an intronic sequence near the middle of the gene. This same region has also been implicated in a quantitative language-related trait (age at first word) in autism [ 57 ]. The exact mechanism by which the identified SNPs alter CNTNAP2 function has yet to be elucidated, but the integration of evidence from these various routes of investigation makes CNTNAP2 a compelling candidate for language disorders.
The CNTNAP2 gene has recently been implicated in multiple neurodevelopmental disorders, including Gilles de la Tourette syndrome [ 58 ], schizophrenia [ 59 ], epilepsy [ 59 , 60 ], autism [ 57 , 61 – 65 ], ADHD [ 66 ] and mental retardation [ 45 ] (Table 1 ). This diverse range of studies provides evidence for the disruption of CNTNAP2 by copy number variants (CNVs), gross chromosomal rearrangements and mutations as well as association with common variants. It remains unclear how one gene can contribute to such an array of neurological conditions, although it should be noted that the implicated disorders are not completely disparate and can be expected to involve some shared neuropathology. Nonetheless, it is obvious that CNTNAP2 must have vital roles in neuronal development and that perturbations of the function of this gene significantly increase the chances of some form of neurological dysfunction. It is likely that the differences in outcome are decided by a complex function that includes the nature of the mutation and both the genetic and environmental background of the affected individual. For example, it is feasible to consider that gene deletions may have different effects from point mutations, and that the consequence of a point mutation will vary according to its location in the protein or its effect on gene expression. Equally, one can see how different combinations of point mutations or common variants across gene networks may have divergent outcomes that depend on the exact genes involved.
It is likely that a gene such as CNTNAP2 functions in overlapping and intersecting neurodevelopmental pathways and thus even a seemingly subtle disruption of its function may affect a variety of processes. The eventual outcome at the organ or organism level may in turn be modulated by the ability of downstream genes and proteins to compensate for these variations. We can therefore view CNTNAP2 as a neuronal buffer; subtle disruptions of this gene alone may be insufficient to cause disorder but may place a critical load on neurological systems, which manifest in different ways depending on the nature of additional load factors. Once a critical threshold of load is exceeded, it is likely that neurological imbalance will ensue.
ATP2C2 and CMIP
The calcium-transporting ATPase 2C2 ( ATP2C2 ) and cMAF inducing protein ( CMIP ) genes, both on chromosome 16q, were identified as SLI candidates by a positional cloning approach, which involved a genome-wide linkage study followed by a targeted high-density association investigation [ 53 , 67 – 70 ]. These phased investigations were performed using the SLIC sample, as described above [ 53 ]. Genome-wide linkage analyses in these families revealed a strong and consistent linkage signal on chromosome 16q with a measure of non-word repetition [ 53 , 67 – 69 ]. Association analyses of chromosome 16q indicated significant association with two clusters of SNPs, one between exons 2 and 5 of the CMIP gene (most significant P = 5.5 × 10 -7 ) and another 3 megabases distal between exons 7 and 12 of ATP2C2 (most significant P = 2.0 × 10 -5 ) [ 69 ]. Individuals carrying risk alleles at both these loci had an average non-word repetition score more than 1 SD below those carrying homozygous non-risk alleles. Association between ATP2C2 and performance on the non-word repetition task was subsequently replicated in a language-impaired sample selected from a population cohort (most significant P = 0.006) [ 69 ]. In this replication sample, some association was also observed with CMIP but in an opposite direction to that seen in the discovery cohort (most significant P = 0.02) [ 69 ]. Although this does not preclude the presence of a genuine association, as it may be caused by differences in linkage disequilibrium patterns, it does highlight the need for careful interpretation of this result as well as for further replication in additional cohorts.
Both ATP2C2 and CMIP show expression in the brain and, although little is known about their role in this tissue, hypothetical links can be made between their putative functions and language and memory-related processes. The CMIP protein forms part of the cellular scaffold linking the plasma membrane to the cytoskeleton [ 71 ], and cytoskeletal remodeling represents a critical step in neuronal migration and synaptic formation processes. In addition, CMIP has been shown to interact with filamin A and nuclear factor κB, both of which have important neurological functions [ 72 , 73 ]. ATP2C2 is responsible for the removal of calcium and manganese from the cytosol into the Golgi body [ 74 ]. Calcium is an important ion in the regulation of many neuronal processes, including working memory, synaptic plasticity and neuronal motility [ 75 ], and manganese dysregulation has been linked to neurological disorders [ 76 ]. Interestingly, in a recent meta-analysis of genetic data for ADHD, which shows significant co-morbidity with SLI, chromosome 16q was highlighted as the most consistently linked region for this disorder [ 77 ]. Concurrent genome-wide association studies described significant association with a variant in ATP2C2 [ 78 ], reinforcing the fact that, as discussed above, the correlation between genetic susceptibility and surface phenotype is far from straightforward.
As with CNTNAP2 , the specific causal variants and the underlying mechanisms by which ATP2C2 and CMIP might contribute to language impairment have yet to be elucidated. The characterization of these factors will not only provide definitive evidence for the involvement of these genes but may also lead to the identification of further neurological pathways that contribute to language acquisition. Given the proposed reliance of non-word repetition performance on short-term memory ability, one can postulate that the investigation of ATP2C2 and CMIP may provide a biological link between memory-related pathways and language acquisition. The fact that neither ATP2C2 nor CMIP have been identified as downstream targets of FOXP2 suggests that the eventual combination of information from converging routes of investigation will enable the characterization of overlapping and interacting neurological systems that serve the acquisition of language.
Conclusions
The past few years have seen exciting progress in the genetics of language impairment. The increased knowledge of the FOXP2 -dependent molecular networks has enabled the identification of brain regions and pathways that this gene may influence. Although FOXP2 mutations seem to contribute to only a relatively small number of language disorder cases, it seems likely that variations in the genes it controls, such as CNTNAP2 , may be implicated in common forms of language impairment. Thus, as our understanding of downstream targets grows, so will our list of potential candidate genes for SLI. The association of CNTNAP2 variations with an array of developmental disorders indicates that alternative deficits may arise from the dysfunction of a neurological network, demonstrating the complexity of brain development processes.
Although the expression of FOXP2 seems to be particularly important for neurological mechanisms relevant to motor skills, we predict that ATP2C2 and CMIP are likely to be involved in memory-related circuits. Thus, although language is unique to humans, we should not necessarily expect the pathways underlying it to be exclusive to humans. Processes such as memory and motor skills have key roles in language development, but they are certainly not specific to, and may not be completely essential for, language acquisition. Rather, we expect that a variety of pre-existing and diverse neurological pathways have been adapted to promote the development of human language [ 79 ]. Characterization of these pathways and the way they overlap and interact will be an enormous task but one that is becoming increasingly feasible thanks to advances in genetic techniques. Given the expected complexity of such pathways, it seems unlikely that the identification of genetic susceptibility factors will ever lead to the discovery of a 'cure' for SLI. Nonetheless, this is a worthwhile endeavor, as a better understanding of the causes of SLI will allow the development of better diagnostic systems and therapies for affected individuals. Furthermore, it is clear that the achievement of the ultimate goal - the elucidation of a genetic network underpinning language processes - will have an impact on our understanding not only of language impairment and acquisition, but also of human development, brain function and the neuropathology of associated developmental disorders.
Authors' information
DFN is a post-doctoral researcher in APM's lab. She leads the SLI research project and was involved in the positional cloning of ATP2C2 and CMIP . SEF is a Royal Society Research Fellow and Reader in Molecular Neuroscience at the WTCHG, where he pioneers investigations into molecular mechanisms underlying speech and language. After working with APM on the identification of FOXP2 , he became head of his own laboratory, which uses state-of-the-art methods to uncover how language-related genes influence the brain at multiple levels. APM is the head of the developmental neurogenetics group at the Wellcome Trust Centre for Human Genetics (WTCHG) in Oxford. His group works in two main areas: the genetics of neurodevelopmental disorders, including complex genetic diseases such as autism, specific language impairment and developmental dyslexia; and the positional cloning and functional characterization of monogenic neurological diseases, including chorea acanthocytosis, speech and language disorder and Menkes disease. All three authors are members of the SLI Consortium.
Abbreviations
attention deficit hyperactivity disorder
calcium-transporting ATPase 2C2
c-MAF inducing protein
contactin-associated protein-like 2
forkhead box P2
standard deviation
specific language impairment
SLI Consortium
single nucleotide polymorphism.
Pinker S: The Language Instinct: The New Science of Language and Mind. 1994, London: Allen Lane
Book Google Scholar
Bishop DV: Is specific language impairment a valid diagnostic category? Genetic and psycholinguistic evidence. Philos Trans R Soc Lond B Biol Sci. 1994, 346: 105-111. 10.1098/rstb.1994.0134.
Article PubMed CAS Google Scholar
Conti-Ramsden G, Durkin K: Language and independence in adolescents with and without a history of specific language impairment (SLI). J Speech Lang Hear Res. 2008, 51: 70-83. 10.1044/1092-4388(2008/005).
Article PubMed Google Scholar
Durkin K, Conti-Ramsden G: Language, social behavior, and the quality of friendships in adolescents with and without a history of specific language impairment. Child Dev. 2007, 78: 1441-1457. 10.1111/j.1467-8624.2007.01076.x.
Whitehouse AJ, Watt HJ, Line EA, Bishop DV: Adult psychosocial outcomes of children with specific language impairment, pragmatic language impairment and autism. Int J Lang Commun Disord. 2009, 44: 511-528. 10.1080/13682820802708098.
Article PubMed PubMed Central Google Scholar
Pennington BF, Bishop DV: Relations among speech, language and reading disorders. Annu Rev Psych. 2009, 60: 283-306. 10.1146/annurev.psych.60.110707.163548.
Article Google Scholar
Plomin R, Haworth CM, Davis OS: Common disorders are quantitative traits. Nat Rev Genet. 2009, 10: 872-878. 10.1038/nrg2715.
Lewis BA, Shriberg LD, Freebairn LA, Hansen AJ, Stein CM, Taylor HG, Iyengar SK: The genetic bases of speech sound disorders: evidence from spoken and written language. J Speech Lang Hear Res. 2006, 49: 1294-1312. 10.1044/1092-4388(2006/093).
Paracchini S, Scerri T, Monaco AP: The genetic lexicon of dyslexia. Annu Rev Genomics Hum Genet. 2007, 8: 57-79. 10.1146/annurev.genom.8.080706.092312.
Bishop DV: Genes, cognition, and communication: insights from neurodevelopmental disorders. Ann N Y Acad Sci. 2009, 1156: 1-18. 10.1111/j.1749-6632.2009.04419.x.
Article PubMed CAS PubMed Central Google Scholar
Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP: A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001, 413: 519-523. 10.1038/35097076.
Watkins KE, Dronkers NF, Vargha-Khadem F: Behavioural analysis of an inherited speech and language disorder: comparison with acquired aphasia. Brain. 2002, 125: 452-464. 10.1093/brain/awf058.
Newbury DF, Bonora E, Lamb JA, Fisher SE, Lai CS, Baird G, Jannoun L, Slonims V, Stott CM, Merricks MJ, Bolton PF, Bailey AJ, Monaco AP, International Molecular Genetic Study of Autism Consortium: FOXP2 is not a major susceptibility gene for autism or specific language impairment. Am J Hum Genet. 2002, 70: 1318-1327. 10.1086/339931.
O'Brien EK, Zhang X, Nishimura C, Tomblin JB, Murray JC: Association of specific language impairment (SLI) to the region of 7q31. Am J Hum Genet. 2003, 72: 1536-1543. 10.1086/375403.
Lai CS, Fisher SE, Hurst JA, Levy ER, Hodgson S, Fox M, Jeremiah S, Povey S, Jamison DC, Green ED, Vargha-Khadem F, Monaco AP: The SPCH1 region on human 7q31: genomic characterization of the critical interval and localization of translocations associated with speech and language disorder. Am J Hum Genet. 2000, 67: 357-368. 10.1086/303011.
MacDermot KD, Bonora E, Sykes N, Coupe AM, Lai CS, Vernes SC, Vargha-Khadem F, McKenzie F, Smith RL, Monaco AP, Fisher SE: Identification of FOXP2 truncation as a novel cause of developmental speech and language deficits. Am J Hum Genet. 2005, 76: 1074-1080. 10.1086/430841.
Feuk L, Kalervo A, Lipsanen-Nyman M, Skaug J, Nakabayashi K, Finucane B, Hartung D, Innes M, Kerem B, Nowaczyk MJ, Rivlin J, Roberts W, Senman L, Summers A, Szatmari P, Wong V, Vincent JB, Zeesman S, Osborne LR, Cardy JO, Kere J, Scherer SW, Hannula-Jouppi K: Absence of a paternally inherited FOXP2 gene in developmental verbal dyspraxia. Am J Hum Genet. 2006, 79: 965-972. 10.1086/508902.
Shriberg LD, Ballard KJ, Tomblin JB, Duffy JR, Odell KH, Williams CA: Speech, prosody, and voice characteristics of a mother and daughter with a 7;13 translocation affecting FOXP2. J Speech Lang Hear Res. 2006, 49: 500-525. 10.1044/1092-4388(2006/038).
Zeesman S, Nowaczyk MJ, Teshima I, Roberts W, Cardy JO, Brian J, Senman L, Feuk L, Osborne LR, Scherer SW: Speech and language impairment and oromotor dyspraxia due to deletion of 7q31 that involves FOXP2. Am J Med Genet A. 2006, 140: 509-514.
Lennon PA, Cooper ML, Peiffer DA, Gunderson KL, Patel A, Peters S, Cheung SW, Bacino CA: Deletion of 7q31.1 supports involvement of FOXP2 in language impairment: clinical report and review. Am J Med Genet A. 2007, 143A: 791-798. 10.1002/ajmg.a.31632.
Tomblin JB, O'Brien M, Shriberg LD, Williams C, Murray J, Patil S, Bjork J, Anderson S, Ballard K: Language features in a mother and daughter of a chromosome 7;13 translocation involving FOXP2. J Speech Lang Hear Res. 2009, 52: 1157-1174. 10.1044/1092-4388(2009/07-0162).
Spiteri E, Konopka G, Coppola G, Bomar J, Oldham M, Ou J, Vernes SC, Fisher SE, Ren B, Geschwind DH: Identification of the transcriptional targets of FOXP2, a gene linked to speech and language, in developing human brain. Am J Hum Genet. 2007, 81: 1144-1157. 10.1086/522237.
Vernes SC, Spiteri E, Nicod J, Groszer M, Taylor JM, Davies KE, Geschwind DH, Fisher SE: High-throughput analysis of promoter occupancy reveals direct neural targets of FOXP2, a gene mutated in speech and language disorders. Am J Hum Genet. 2007, 81: 1232-1250. 10.1086/522238.
Konopka G, Bomar JM, Winden K, Coppola G, Jonsson ZO, Gao F, Peng S, Preuss TM, Wohlschlegel JA, Geschwind DH: Human-specific transcriptional regulation of CNS development genes by FOXP2. Nature. 2009, 462: 213-217. 10.1038/nature08549.
Shu W, Lu MM, Zhang Y, Tucker PW, Zhou D, Morrisey EE: Foxp2 and Foxp1 cooperatively regulate lung and esophagus development. Development. 2007, 134: 1991-2000. 10.1242/dev.02846.
Lai CSL, Gerrelli D, Monaco AP, Fisher SE, Copp AJ: FOXP2 expression during brain development coincides with adult sites of pathology in a severe speech and language disorder. Brain. 2003, 126: 2455-2462. 10.1093/brain/awg247.
Takahashi K, Liu FC, Hirokawa K, Takahashi H: Expression of Foxp2, a gene involved in speech and language, in the developing and adult striatum. J Neurosci Res. 2003, 73: 61-72. 10.1002/jnr.10638.
Shu W, Cho JY, Jiang Y, Zhang M, Weisz D, Elder GA, Schmeidler J, De Gasperi R, Sosa MA, Rabidou D, Santucci AC, Perl D, Morrisey E, Buxbaum JD: Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. Proc Natl Acad Sci USA. 2005, 102: 9643-9648. 10.1073/pnas.0503739102.
French CA, Groszer M, Preece C, Coupe AM, Rajewsky K, Fisher SE: Generation of mice with a conditional Foxp2 null allele. Genesis. 2007, 45: 440-446. 10.1002/dvg.20305.
Fujita E, Tanabe Y, Shiota A, Ueda M, Suwa K, Momoi MY, Momoi T: Ultrasonic vocalization impairment of Foxp2 (R552H) knockin mice related to speech-language disorder and abnormality of Purkinje cells. Proc Natl Acad Sci USA. 2008, 105: 3117-3122. 10.1073/pnas.0712298105.
Groszer M, Keays DA, Deacon RM, de Bono JP, Prasad-Mulcare S, Gaub S, Baum MG, French CA, Nicod J, Coventry JA, Enard W, Fray M, Brown SD, Nolan PM, Pääbo S, Channon KM, Costa RM, Eilers J, Ehret G, Rawlins JN, Fisher SE: Impaired synaptic plasticity and motor learning in mice with a point mutation implicated in human speech deficits. Curr Biol. 2008, 18: 354-362. 10.1016/j.cub.2008.01.060.
Kurt S, Groszer M, Fisher SE, Ehret G: Modified sound-evoked brainstem potentials in Foxp2 mutant mice. Brain Res. 2009, 1289: 30-36. 10.1016/j.brainres.2009.06.092.
Haesler S, Wada K, Nshdejan A, Morrisey EE, Lints T, Jarvis ED, Scharff C: FoxP2 expression in avian vocal learners and non-learners. J Neurosci. 2004, 24: 3164-3175. 10.1523/JNEUROSCI.4369-03.2004.
Fisher SE, Scharff C: FOXP2 as a molecular window into speech and language. Trends Genet. 2009, 25: 166-177. 10.1016/j.tig.2009.03.002.
Belton E, Salmond CH, Watkins KE, Vargha-Khadem F, Gadian DG: Bilateral brain abnormalities associated with dominantly inherited verbal and orofacial dyspraxia. Hum Brain Mapp. 2003, 18: 194-200. 10.1002/hbm.10093.
Liégeois F, Baldeweg T, Connelly A, Gadian DG, Mishkin M, Vargha-Khadem F: Language fMRI abnormalities associated with FOXP2 gene mutation. Nat Neurosci. 2003, 6: 1230-1237. 10.1038/nn1138.
Campbell P, Reep RL, Stoll ML, Ophir AG, Phelps SM: Conservation and diversity of Foxp2 expression in muroid rodents: functional implications. J Comp Neurol. 2009, 512: 84-100. 10.1002/cne.21881.
Enard W, Przeworski M, Fisher SE, Lai CS, Wiebe V, Kitano T, Monaco AP, Pääbo S: Molecular evolution of FOXP2, a gene involved in speech and language. Nature. 2002, 418: 869-872. 10.1038/nature01025.
Zhang J, Webb DM, Podlaha O: Accelerated protein evolution and origins of human-specific features: FOXP2 as an example. Genetics. 2002, 162: 1825-1835.
PubMed CAS PubMed Central Google Scholar
Krause J, Lalueza-Fox C, Orlando L, Enard W, Green RE, Burbano HA, Hublin JJ, Hänni C, Fortea J, de la Rasilla M, Bertranpetit , Rosas A, Pääbo : The derived FOXP2 variant of modern humans was shared by Neandertals. Curr Biol. 2007, 17: 1908-1912. 10.1016/j.cub.2007.10.008.
Coop G, Bullaughey K, Luca F, Przeworski M: The timing of selection at the human FOXP2 gene. Mol Biol Evol. 2009, 25: 1257-1259. 10.1093/molbev/msn091.
Enard W, Gehre S, Hammerschmidt K, Hölter SM, Blass T, Somel M, Brückner MK, Schreiweis C, Winter C, Sohr R, Becker L, Wiebe V, Nickel B, Giger T, Müller U, Groszer M, Adler T, Aguilar A, Bolle I, Calzada-Wack J, Dalke C, Ehrhardt N, Favor J, Fuchs H, Gailus-Durner V, Hans W, Hölzlwimmer G, Javaheri A, Kalaydjiev S, Kallnik M, et al: A humanized version of Foxp2 affects cortico-basal ganglia circuits in mice. Cell. 2009, 137: 961-971. 10.1016/j.cell.2009.03.041.
Vernes SJ, Newbury DF, Abrahams BS, Winchester L, Nicod J, Groszer M, Alarcón M, Oliver PL, Davies KE, Geschwind DH, Monaco AP, Fisher SE: Afunctional genetic link between distinct developmental language disorders. N Engl J Med. 2008, 359: 2337-2345. 10.1056/NEJMoa0802828.
Poliak S, Gollan L, Martinez R, Custer A, Einheber S, Salzer JL, Trimmer JS, Shrager P, Peles E: Caspr2, a new member of the neurexin superfamily, is localised at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron. 1999, 24: 1037-1047. 10.1016/S0896-6273(00)81049-1.
Zweier C, de Jong EK, Zweier M, Orrico A, Ousager LB, Collins AL, Bijlsma EK, Oortveld MAW, Ekici AB, Reis A, Schenck A, Rauch A: CNTNAP2 and NRXN1 are mutated in autosomal-recessive Pitt-Hopkins-like mental retardation and determine the level of a common synaptic protein in Drosophila . Am J Hum Genet. 2009, 85: 655-666. 10.1016/j.ajhg.2009.10.004.
Jamain S, Quach H, Betancur C, Råstam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T, Paris Autism Research International Sibpair Study: Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003, 34: 27-29. 10.1038/ng1136.
Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP, Raynaud M, Ronce N, Lemonnier E, Calvas P, Laudier B, Chelly J, Fryns JP, Ropers HH, Hamel BC, Andres C, Barthélémy C, Moraine C, Briault S: X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet. 2004, 74: 552-557. 10.1086/382137.
Ylisaukko-oja T, Rehnström K, Auranen M, Vanhala R, Alen R, Kempas E, Ellonen P, Turunen JA, Makkonen I, Riikonen R, Nieminen-von Wendt T, von Wendt L, Peltonen L, Järvelä I: Analysis of four neuroligin genes as candidates for autism. Eur J Hum Genet. 2005, 13: 1285-1292. 10.1038/sj.ejhg.5201474.
Yamakawa H, Oyama S, Mitsuhashi H, Sasagawa N, Uchino S, Kohsaka S, Ishiura S: Neuroligins 3 and 4X interact with syntrophin-gamma2, and the interactions are affected by autism-related mutations. Biochem Biophys Res Commun. 2007, 355: 41-46. 10.1016/j.bbrc.2007.01.127.
Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N: Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci USA. 2008, 105: 1710-1715. 10.1073/pnas.0711555105.
Lawson-Yuen A, Saldivar JS, Sommer S, Picker J: Familial deletion within NLGN4 associated with autism and Tourette syndrome. Eur J Hum Genet. 2008, 16: 614-618. 10.1038/sj.ejhg.5202006.
Yan J, Feng J, Schroer R, Li W, Skinner C, Schwartz CE, Cook EH, Sommer SS: Analysis of the neuroligin 4Y gene in patients with autism. Psychiatr Genet. 2008, 18: 204-207. 10.1097/YPG.0b013e3282fb7fe6.
The SLI Consortium: A genomewide scan identifies two novel loci involved in specific language impairment. Am J Hum Genet. 2002, 70: 384-398. 10.1086/338649.
Article PubMed Central Google Scholar
Semel EM, Wiig EH, Secord W: Clinical Evaluation of Language Fundamentals-Revised. 1987, San Antonio: Psychological Corporation
Google Scholar
Gathercole SE, Willis CS, Baddeley AD, Emslie H: The children's test of nonword repetition: a test of phonological working memory. Memory. 1994, 2: 103-107. 10.1080/09658219408258940.
Archibald LM, Gathercole SE: Nonword repetition in specific language impairment: more than a phonological short-term memory deficit. Psychon Bull Rev. 2007, 14: 919-924.
Alarcón M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, Sebat J, Wigler M, Martin CL, Ledbetter DH, Nelson SF, Cantor RM, Geschwind DH: Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008, 82: 150-159. 10.1016/j.ajhg.2007.09.005.
Verkerk AJ, Matthews CA, Joosse M, Eussen BHJ, Heutink P, Oostra BA, the Tourette Syndrome Association International Consortium for Genetics: CNTNAP2 is disrupted in a family with Gilles de la Tourette syndrome and obsessive compulsive disorder. Genomics. 2003, 82: 1-9. 10.1016/S0888-7543(03)00097-1.
Friedman JI, Vrijenhoek T, Markx S, Janssen IM, Vliet van der WA, Faas BH, Knoers NV, Cahn W, Kahn RS, Edelmann L, Davis KL, Silverman JM, Brunner HG, van Kessel AG, Wijmenga C, Ophoff RA, Veltman JA: CNTNAP2 gene dosage variation is associated with schizophrenia and epilepsy. Mol Psychiatry. 2008, 13: 261-266. 10.1038/sj.mp.4002049.
Strauss KA, Puffenberger EG, Huentelman MJ, Gottlieb S, Dobrin SE, Parod JM, Stephan DA, Morton DH: Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N Engl J Med. 2006, 354: 1370-1377. 10.1056/NEJMoa052773.
Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda M, Rea A, Guy M, Lin S, Cook EH, Chakravarti A: A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet. 2008, 82: 160-164. 10.1016/j.ajhg.2007.09.015.
Bakkaloglu B, O'Roak BJ, Louvi A, Gupta AR, Abelson JF, Morgan TM, Chawarska K, Klin A, Ercan-Sencicek AG, Stillman AA, Tanriover G, Abrahams BS, Duvall JA, Robbins EM, Geschwind DH, Biederer T, Gunel M, Lifton RP, State MW: Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am J Hum Genet. 2008, 82: 165-173. 10.1016/j.ajhg.2007.09.017.
Rossi E, Verri AP, Patricelli MG, Destefani V, Ricca I, Vetro A, Ciccone R, Giorda R, Toniolo D, Maraschio P, Zuffardi O: A 12 Mb deletion at 7q33-q35 associated with autism spectrum disorders and primary amenorrhea. Eur J Med Genet. 2008, 51: 631-638. 10.1016/j.ejmg.2008.06.010.
Jackman C, Horn ND, Molleston JP, Sokol DK: Gene associated with seizures, autism, and hepatomegaly in an Amish girl. Pediatr Neurol. 2009, 40: 310-313. 10.1016/j.pediatrneurol.2008.10.013.
Poot M, Beyer V, Schwaab I, Damatova N, Van't Slot R, Prothero J, Holder SE, Haaf T: Disruption of CNTNAP2 and additional structural genome changes in a boy with speech delay and autism spectrum disorder. Neurogenetics. 2009, doi: 10.1007/s10048-009-0205-1.
Elia J, Gai X, Xie HM, Perin JC, Geiger E, Glessner JT, D'arcy M, Deberardinis R, Frackelton E, Kim C, Lantieri F, Muganga BM, Wang L, Takeda T, Rappaport EF, Grant SF, Berrettini W, Devoto M, Shaikh TH, Hakonarson H, White PS: Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes. Mol Psychiatry. 2009, doi:10.1038/mp.2009.57.
The SLI Consortium: Highly significant linkage to the SLI1 locus in an expanded sample of individuals affected by specific language impairment. Am J Hum Genet. 2004, 74: 1225-1238. 10.1086/421529.
Monaco AP, The SLI consortium (SLIC): Multivariate linkage analysis of specific language impairment (SLI). Ann Hum Genet. 2007, 71: 660-673. 10.1111/j.1469-1809.2007.00361.x.
Falcaro M, Pickles A, Newbury DF, Addis L, Banfield E, Fisher SE, Monaco AP, Simkin Z, Conti-Ramsden G, The SLI Consortium: Genetic and phenotypic effects of phonological short-term memory and grammatical morphology in specific language impairment. Genes Brain Behav. 2008, 7: 393-402. 10.1111/j.1601-183X.2007.00364.x.
Newbury DF, Winchester L, Addis L, Paracchini S, Buckingham LL, Clark A, Cohen W, Cowie H, Dworzynski K, Everitt A, Goodyer IM, Hennessy E, Kindley AD, Miller LL, Nasir J, O'Hare A, Shaw D, Simkin Z, Simonoff E, Slonims V, Watson J, Ragoussis J, Fisher SE, Seckl JR, Helms PJ, Bolton PF, Pickles A, Conti-Ramsden G, Baird G, Bishop DV, Monaco AP: CMIP and ATP2C2 modulate phonological short-term memory in language impairment. Am J Hum Genet. 2009, 85: 264-272. 10.1016/j.ajhg.2009.07.004.
Grimbert P, Valanciute A, Audard V, Pawlak A, Le gouvelo S, Lang P, Niaudet P, Bensman A, Guellaën G, Sahali D: Truncation of C-mip (Tc-mip), a new proximal signaling protein, induces c-maf Th2 transcription factor and cytoskeleton reorganization. J Exp Med. 2003, 198: 797-807. 10.1084/jem.20030566.
Grimbert P, Valanciute A, Audard V, Lang P, Guellaën G, Sahali D: The Filamin-A is a partner of Tc-mip, a new adapter protein involved in c-maf-dependent Th2 signaling pathway. Mol Immunol. 2004, 40: 1257-1261. 10.1016/j.molimm.2003.11.035.
Kamal M: C-mip interacts physically with RelA and inhibits nuclear factor kappa B activity. Mol Immunol. 2009, 46: 991-998. 10.1016/j.molimm.2008.09.034.
Missiaen L, Dode L, Vanoevelen J, Raeymaekers L, Wuytack F: Calcium in the Golgi apparatus. Cell Calcium. 2007, 41: 405-416. 10.1016/j.ceca.2006.11.001.
Zheng JQ, Poo MM: Calcium signalling in neuronal motility. Annu Rev Cell Dev Biol. 2007, 23: 375-404. 10.1146/annurev.cellbio.23.090506.123221.
Normandin L, Hazell AS: Manganese neurotoxicity: an update of pathophysiologic mechanisms. Metab Brain Dis. 2002, 17: 275-387. 10.1023/A:1021970120965.
Zhou K, Dempfle A, Arcos-Burgos M, Bakker SC, Banaschewski T, Biederman J, Buitelaar J, Castellanos FX, Doyle A, Ebstein RP, Ekholm J, Forabosco P, Franke B, Freitag C, Friedel S, Gill M, Hebebrand J, Hinney A, Jacob C, Lesch KP, Loo SK, Lopera F, McCracken JT, McGough JJ, Meyer J, Mick E, Miranda A, Muenke M, Mulas F, Nelson SF, et al: Meta-analysis of genome-wide linkage scans of attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008, 147B: 1392-1398. 10.1002/ajmg.b.30878.
Lesch KP, Timmesfeld N, Renner TJ, Halperin R, Röser C, Nguyen TT, Craig DW, Romanos J, Heine M, Meyer J, Freitag C, Warnke A, Romanos M, Schäfer H, Walitza S, Reif A, Stephan DA, Jacob C: Molecular genetics of adult ADHD: Converging evidence from genome-wide association and extended pedigree studies. J Neural Transm. 2008, 115: 1573-1585. 10.1007/s00702-008-0119-3.
Fisher SE, Marcus GF: The eloquent ape: genes, brains and the evolution of language. Nat Rev Genet. 2006, 7: 9-20. 10.1038/nrg1747.
Terracciano A, Sanna S, Uda M, Deiana B, Usala G, Busonero F, Maschio A, Scally M, Patriciu N, Chen WM, Distel MA, Slagboom EP, Boomsma DI, Villafuerte S, Sliwerska E, Burmeister M, Amin N, Janssens AC, van Duijn CM, Schlessinger D, Abecasis GR, Costa PT: Genome-wide association scan for five major dimensions of personality. Mol Psychiatry. 2008, doi: 10.1038/mp.2008.113
Download references
Acknowledgements
We thank the patients and families who contributed DNA to these research projects. SEF is a Royal Society Research Fellow and is funded by the Royal
Society, the Wellcome Trust and the Simons Foundation Autism Research Initiative. APM is funded by the Wellcome Trust.
Author information
Authors and affiliations.
Wellcome Trust Centre for Human Genetics, University of Oxford, Roosevelt Drive, Headington, Oxford, OX3 7BN, UK
Dianne F Newbury, Simon E Fisher & Anthony P Monaco
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Dianne F Newbury .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors' contributions
DFN drafted the manuscript; APM and SEF assisted with its preparation and provided vital edits. All authors read the final manuscript and agreed its content before publication.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Newbury, D.F., Fisher, S.E. & Monaco, A.P. Recent advances in the genetics of language impairment. Genome Med 2 , 6 (2010). https://doi.org/10.1186/gm127
Download citation
Published : 26 January 2010
DOI : https://doi.org/10.1186/gm127
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Attention Deficit Hyperactivity Disorder
- Language Acquisition
- Language Impairment
- Specific Language Impairment
Genome Medicine
ISSN: 1756-994X
- General enquiries: [email protected]
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

- Health Topics
- Drugs & Supplements
- Medical Tests
- Medical Encyclopedia
- About MedlinePlus
- Customer Support
FOXP2-related speech and language disorder
Description.
In addition to having problems with producing speech (expressive language), people with FOXP2 -related speech and language disorder may have difficulty with understanding speech (receptive language). Some also have trouble with other language-related skills, such as reading, writing, spelling, and grammar. In some affected individuals, problems with speech and language are the only features of the condition. Others also have delayed development in other areas, including motor skills such as walking and tying shoelaces, and autism spectrum disorders, which are conditions characterized by impaired communication and social interaction.
FOXP2 -related speech and language disorder appears to be a relatively uncommon cause of problems with speech and language development. The total prevalence of apraxia is estimated to be 1 to 2 in 1,000 people, and it is likely that FOXP2 -related speech and language disorder accounts for only a small portion of cases.
The genetic changes that underlie FOXP2 -related speech and language disorder disrupt the activity of the FOXP2 gene. Because forkhead box P2 is a transcription factor, these changes affect the activity of other genes in the developing brain. Researchers are working to determine which of these genes are involved and how changes in their activity lead to abnormal speech and language development.
Additional features that are sometimes associated with FOXP2 -related speech and language disorder, including delayed motor development and autism spectrum disorders, likely result from changes to other genes on chromosome 7. For example, in affected individuals with a deletion involving chromosome 7, a loss of FOXP2 is thought to disrupt speech and language development, while the loss of nearby genes accounts for other signs and symptoms. People with maternal UPD for chromosome 7 have FOXP2 -related speech and language disorder as part of a larger condition called Russell-Silver syndrome . In addition to speech and language problems, these individuals have slow growth, distinctive facial features, delayed development, and learning disabilities.
Learn more about the gene and chromosome associated with FOXP2-related speech and language disorder
- chromosome 7
Inheritance
When the condition is caused by rearrangements of the structure of chromosome 7, its pattern of inheritance can be complex and depends on the specific genetic change.
Other Names for This Condition
- Speech and language disorder with orofacial dyspraxia
- Speech-language disorder 1
Additional Information & Resources
Genetic testing information.
Genetic and Rare Diseases Information Center
Patient support and advocacy resources.
- National Organization for Rare Disorders (NORD)
Catalog of Genes and Diseases from OMIM
- SPEECH-LANGUAGE DISORDER 1; SPCH1
Scientific Articles on PubMed
- Feuk L, Kalervo A, Lipsanen-Nyman M, Skaug J, Nakabayashi K, Finucane B, Hartung D, Innes M, Kerem B, Nowaczyk MJ, Rivlin J, Roberts W, Senman L, Summers A, Szatmari P, Wong V, Vincent JB, Zeesman S, Osborne LR, Cardy JO, Kere J, Scherer SW, Hannula-Jouppi K. Absence of a paternally inherited FOXP2 gene in developmental verbal dyspraxia. Am J Hum Genet. 2006 Nov;79(5):965-72. doi: 10.1086/508902. Epub 2006 Sep 27. Citation on PubMed or Free article on PubMed Central
- Fisher SE, Vargha-Khadem F, Watkins KE, Monaco AP, Pembrey ME. Localisation of a gene implicated in a severe speech and language disorder. Nat Genet. 1998 Feb;18(2):168-70. doi: 10.1038/ng0298-168. Erratum In: Nat Genet 1998 Mar;18(3):298. Citation on PubMed
- Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001 Oct 4;413(6855):519-23. doi: 10.1038/35097076. Citation on PubMed
- MacDermot KD, Bonora E, Sykes N, Coupe AM, Lai CS, Vernes SC, Vargha-Khadem F, McKenzie F, Smith RL, Monaco AP, Fisher SE. Identification of FOXP2 truncation as a novel cause of developmental speech and language deficits. Am J Hum Genet. 2005 Jun;76(6):1074-80. doi: 10.1086/430841. Epub 2005 Apr 22. Citation on PubMed or Free article on PubMed Central
- Morgan A, Fisher SE, Scheffer I, Hildebrand M. FOXP2-Related Speech and Language Disorder. 2016 Jun 23 [updated 2023 Jan 26]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews(R) [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2024. Available from http://www.ncbi.nlm.nih.gov/books/NBK368474/ Citation on PubMed
- Tomblin JB, O'Brien M, Shriberg LD, Williams C, Murray J, Patil S, Bjork J, Anderson S, Ballard K. Language features in a mother and daughter of a chromosome 7;13 translocation involving FOXP2. J Speech Lang Hear Res. 2009 Oct;52(5):1157-74. doi: 10.1044/1092-4388(2009/07-0162). Citation on PubMed or Free article on PubMed Central
- Zeesman S, Nowaczyk MJ, Teshima I, Roberts W, Cardy JO, Brian J, Senman L, Feuk L, Osborne LR, Scherer SW. Speech and language impairment and oromotor dyspraxia due to deletion of 7q31 that involves FOXP2. Am J Med Genet A. 2006 Mar 1;140(5):509-14. doi: 10.1002/ajmg.a.31110. Citation on PubMed

Genetics Home Reference has merged with MedlinePlus. Genetics Home Reference content now can be found in the "Genetics" section of MedlinePlus. Learn more
The information on this site should not be used as a substitute for professional medical care or advice. Contact a health care provider if you have questions about your health.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 27 July 2021
Association between genes regulating neural pathways for quantitative traits of speech and language disorders
- Penelope Benchek 1 na1 ,
- Robert P. Igo Jr. ORCID: orcid.org/0000-0002-0024-1993 1 na1 na2 ,
- Heather Voss-Hoynes 1 na1 ,
- Yvonne Wren ORCID: orcid.org/0000-0002-1575-453X 2 ,
- Gabrielle Miller 3 ,
- Barbara Truitt 1 ,
- Wen Zhang 4 ,
- Michael Osterman 1 ,
- Lisa Freebairn 3 ,
- Jessica Tag 3 ,
- H. Gerry Taylor 5 , 6 ,
- E. Ricky Chan 1 ,
- Panos Roussos ORCID: orcid.org/0000-0002-4640-6239 4 , 7 ,
- Barbara Lewis 3 , 8 na3 ,
- Catherine M. Stein ORCID: orcid.org/0000-0002-9763-5023 1 na3 &
- Sudha K. Iyengar 1 na3
npj Genomic Medicine volume 6 , Article number: 64 ( 2021 ) Cite this article
2424 Accesses
8 Citations
4 Altmetric
Metrics details
- Genome-wide association studies
- Psychiatric disorders
Speech sound disorders (SSD) manifest as difficulties in phonological memory and awareness, oral motor function, language, vocabulary, reading, and spelling. Families enriched for SSD are rare, and typically display a cluster of deficits. We conducted a genome-wide association study (GWAS) in 435 children from 148 families in the Cleveland Family Speech and Reading study (CFSRS), examining 16 variables representing 6 domains. Replication was conducted using the Avon Longitudinal Study of Parents and Children (ALSPAC). We identified 18 significant loci (combined p < 10 −8 ) that we pursued bioinformatically. We prioritized 5 novel gene regions with likely functional repercussions on neural pathways, including those which colocalized with differentially methylated regions in our sample. Polygenic risk scores for receptive language, expressive vocabulary, phonological awareness, phonological memory, spelling, and reading decoding associated with increasing clinical severity. In summary, neural-genetic influence on SSD is primarily multigenic and acts on genomic regulatory elements, similar to other neurodevelopmental disorders.
Similar content being viewed by others
Multi-level evidence of an allelic hierarchy of USH2A variants in hearing, auditory processing and speech/language outcomes

Genetic architecture of childhood speech disorder: a review
A genome-wide association study reveals a polygenic architecture of speech-in-noise deficits in individuals with self-reported normal hearing
Introduction.
Communication disorders are highly prevalent in the United States with approximately one in twelve children ages 3–17 years demonstrating a disorder 1 . The most common difficulties are a speech problem (5%) or language problem (3.3%). Speech sound disorders (SSD) refer to difficulties producing certain sound past the age that a child is expected to acquire the sound, and include both errors of articulation or phonetic structure (errors due to poor motor abilities associated with the production of speech sounds) and phonological errors (errors in applying linguistic rules to combine sounds to form words). SSD has a prevalence of approximately 16% in children 3 years of age 2 , with an estimated 3.8% of children persisting with speech delay at 6 years of age 3 . More than half of these children encounter later academic difficulties in language, reading, and spelling 4 , 5 , 6 , 7 , 8 . Because of the clinical heterogeneity of speech problems and their correlation with other communication domains, endophenotypes are key to the study of genetic underpinnings 9 .
Vocabulary is core to speech acquisition 10 . Children with difficulties in speech sound development often have difficulties with oral language and later reading and spelling disability 2 , 4 , 5 , 6 , 11 . Thus, speech, language, reading, and spelling measures are highly correlated and often have common genetic associations 7 , 8 . Moreover, speech and other communication phenotypes follow a developmental trajectory, where some speech and language disorders resolve with age, whereas others persist; genetic influences on the less easily resolved manifestations are generally stronger 12 , 13 . Because of the common genetic underpinnings and pathologic associations between speech and other communication phenotypes, it is conceivable that genetic replication interweaves with different communication measures. Indeed, various studies have examined candidate gene associations associated with both binary traits and quantitative endophenotypes, and have identified several strong candidates 14 , though a clear model of genetic susceptibility has not emerged. Of seven known GWASs, none overlap in their top results (multiple genes with p < 5 × 10 −5 , see Table 2 in the Graham and Fisher meta-analysis paper 13 ), because they focused on several phenotypes (word reading, vocabulary, receptive and expressive language, nonword repetition, and language impairment (LI) binary trait), or these measures were assessed at different ages (either pre-school or early school-age) 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 . Because these studies only present results from one or a few measures and/or a binary trait, it is difficult to dissect the complexity of shared genetic influences. Most have not focused on children with SSD, particularly measures of articulation. Our sample represents a unique set of deeply phenotyped individuals with information on six domains that form the core of speech and language.
SSDs are likely due to deficits in both motor ability and broader neural dysfunction. While motor deficits contribute to problems in speech production, abnormalities in other neural systems likely influence the formation of phonological representation, which is common to SSD as well as reading and LI. We hypothesize that genomic factors associated with variation in speech production, phonological representation, and language may point to neural pathways common to speech, language, reading, and spelling ability. To address this hypothesis, we examined endophenotypes representing motor speech, vocabulary, phonological memory, phonological awareness, reading, spelling, and language, in order to characterize genetic commonality across these domains and fully characterize the complexity of SSD. We conducted a GWAS in the Cleveland Family Speech and Reading Study (CFSRS), a cohort ascertained through a proband with SSD, and replicated findings in a population-based cohort. We also conducted a methylome-wide study (i.e., MWAS) to determine the functional implications of these genetic associations. We utilized a family-based cohort as our discovery sample because we hypothesized it would be enriched for disease-associated variants 23 , 24 . In these analyses, we identified new candidate genes for correlated communication endophenotypes, and bioinformatic annotation of these loci revealed that regulation of neural pathways is associated with variation in these measures.
Study population
The CFSRS sample included 435 subjects from 148 families (Table 1 ). Of these, 27% had SSD only, 4% had LI only, 16% had SSD + LI without CAS, and 11% had CAS (Table 1 ) diagnosed by a speech-language pathologist. There was a high rate of comorbidities, especially among the probands (Table 1 , Supplementary Table 4 ). Of the subjects in the ALSPAC sample, the prevalence of speech problems by parental report varied from 4 to 6% (Supplementary Table 5 ).
Genetic correlation analysis reveals new relationships among endophenotypes
Genetic correlation analysis revealed that while many of the patterns of correlation were consistent with phenotypic correlations we have previously reported 8 , polygenic correlations enable a deeper understanding of these measures, which will inform the examination of replication of association effects both within the CFSRS data set and with measures from ALSPAC (Fig. 1 ). For example, while previous studies have demonstrated a strong genetic correlation between reading and spelling measures, polygenic correlation analysis additionally reveals correlations between those skills and Elision. Not surprisingly, expressive and receptive language (measured by the CELF), are strongly correlated with vocabulary (EOWPVT and PPVT) in addition to reading (WRMT-AT and WRMT-ID). Vocabulary is also strongly correlated with listening comprehension (WIAT-LC).
Figure 1 shows cross-trait correlation results for each pair of tests using GCTA’s bivariate REML analysis 69 . The cross-trait correlation was tested under the null hypothesis of 0 correlation. Circles shown are for results significant at p < 0.05, with increasing diameter/color corresponding with increasing correlation (circles omitted otherwise). Traits: Phonological memory MSW.PPC, MSW, NSW, NSW.PPC), Spelling (TWS), Reading (WRMT-ID, WRMT-AT, WIAT-RC), Language (CELF.E, CELF.R, WIAT.LC), Vocabulary (EOWPVT, PPVT), phonological awareness (Elision).
The most significant findings from GWAS reveal five new candidate genes
Single marker association tests significant at p < 10 −5 were examined further and integrated with data on gene expression and regulation, as detailed below. Other GWAS of neuropsychiatric disease and behavioral traits have similarly found that noncoding regions harbor a significant proportion of risk alleles 25 (Supplementary Fig. 1 ).
Of five top loci, all had enhancers or promoters for muscle, brain, and/or neuronal progenitor cells, four out of five had significant methylation and meQTL effects, and three out of five had eQTLs for brain and/or skeletal-muscle tissue (Table 2 , Fig. 2 , Supplementary Fig. 2 , Supplementary Table 6 ). EpiXcan analysis suggested that the SNP in the chromosome 1 IFI6 region is associated with expression in the DLPF cortex (Elision p = 0.018, TWS p = 0.008; Supplementary Tables 7 and 8 ). The first region on chromosome 14, including NFKBIA and PPP2R3C , shows significant chromatin interaction mapping in adult cortex tissue. NFKBIA , which codes for a component of the NF-κB pathway, is associated with neurogenesis, neuritogenesis, synaptic plasticity, learning, and memory 26 . The second region on chromosome 14 includes PP2R3C , which is within the topologically associating domain (TAD) boundary of the NFKBIA locus in the Hippocampus and DLPFC. EpiXcan analysis showed NFKBIZ , a gene in the same pathway as NFKIBA , is also associated with expression in the DLPFC (Elision p = 0.000452, TWS p = 0.004939; Supplementary Tables 7 and 8 ). Further, there was significant colocalization at the MON1B/SYCE1L locus on chromosome 16, with differential gene expression of SYCE1L in multiple brain tissues and skeletal muscle localizing with our SNP association signature (Supplementary Fig. 3 ) and borderline significant colocalization with MON1B expression. The SETD3 locus also showed colocalization with gene expression in skeletal muscle and brain tissue (Supplementary Fig. 3 ).
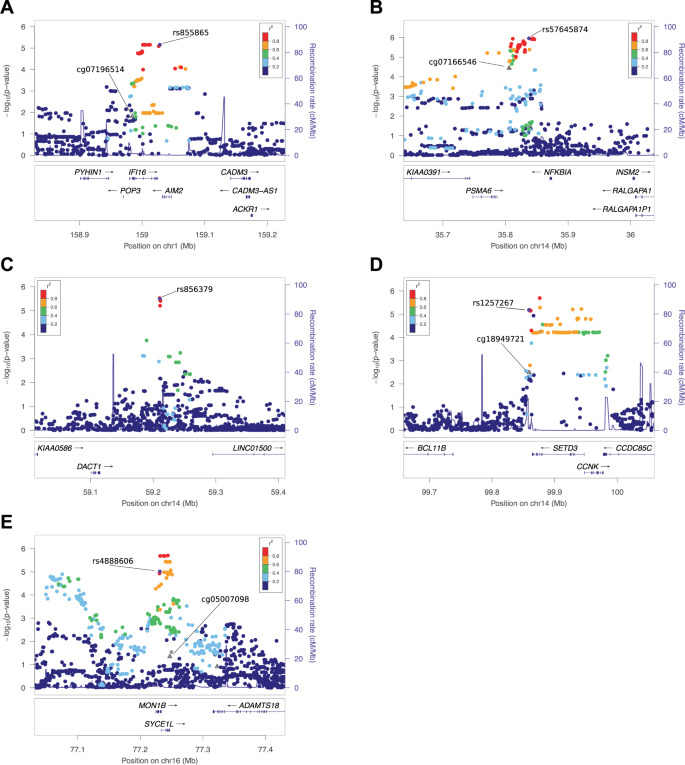
Figure 2 shows association results for the top loci. p -Values displayed are for CFSRS and are for the test for which the top SNP was observed. Circles show p -values for SNP associations and triangles show p -values for methylation associations (specifically those for which the top SNP is an meQTL). The plot shows the top SNP for each region ±200 kb. a IFI16 region. rs855865 was associated with NSW in CFSRS ( p = 7 × 10 −6 ) and with vocabulary (WISC-V) in ALSPAC ( p = 0.01). This region also includes an meQTL (rs12124059, p = 4 × 10 −8 ) for methylation marker cg07196514, and this methylation marker (cg07196514) was also associated with NSW ( p = 0.018). b NFKBIA region. rs57645874 was associated with Elision in CFSRS ( p = 1 × 10 −6 ) and with reading accuracy (NARA-A) in ALSPAC ( p = 0.02). This region also contains an meQTL, rs4981288, for cg07166546 ( p = 2 × 10 −50 ), and this methylation marker was associated with Elision ( p = 3 × 10 −5 ), TWS ( p = 0.0005) and WRMT-ID ( p = 0.002). c DACT1 region. rs856379 was associated with MSW in CFSRS ( p = 3 × 10 −6 ) and with nonword reading (ALSPACread) in ALSPAC ( p = 0.036). This SNP is an meQTL for methylation marker cg13972423 (p = 3 × 10 −5 ). d SETD3 region rs1257267 was associated with WRMT-AT in CFSRS ( p = 6.58 × 10 −6 ) and with nonsense word repetition (CNrep5) in ALSPAC ( p = 0.05). While only 1 SNP replicated between CFSRS and ALSPAC, 14 additional SNPs showed association in CFSRS at p < 10 −5 . This SNP (rs1257267) is an meQTL for cg18949721 ( p = 4 × 10 −12 ), and methylation marker cg18949721 was also associated with WRMT-AT ( p = 0.003). e MON1B region. rs4888606 was associated with MSW in CFSRS ( p = 9 × 10 −6 ) and with nonword reading (ALSPACread) in ALSPAC ( p = 0.046). While only 1 SNP replicated between CFSRS and ALSPAC, 18 additional SNPs showed association in CFSRS at p < 10 −5 . This SNP (rs4888606) falls in an intron of MON1B and is an meQTL for cg06128999 ( p = 4 × 10 −23 ) and cg05007098 ( p = 1 × 10 −15 ); these 2 methylation markers were also associated with MSW ( p = 0.045 and p = 0.12, respectively). Functional annotation is in Supplementary Fig. 2 .
Replication of previous communication disorder loci
In the replication phase, we focused on gene-level replication because of the differences in SNP coverage between our study and the original findings. ATP2C2 was associated with single word reading (WRMT-ID, p = 7.6 × 10 −8 ), nonword reading (WRMT-AT, p = 4.6 × 10 −5 ), and phonological awareness (Elision, p = 4.6 × 10 −5 ), consistent with prior literature 27 (Supplementary Figs. 4 and 5 ). Similarly, CYP19A1 was associated with nonword reading (WRMT-AT, p = 2.8 × 10 −5 ), phonological awareness ( p = 3.3 × 10 −4 ), and single-word reading (WRMT-ID, p = 5.0 × 10 −4 ), validating a previous association 28 . CNTNAP2 was associated with receptive language (CELF-R, p = 5.2 × 10 −6 ), and diadochokinetic rate (DDK, p = 2.9 × 10 −5 ), replicating a previous association 27 . While SNPs within ROBO1 and ROBO2 were not significantly associated with our measures, SNPs in the intergenic region were associated with single word reading (WRMT-ID, p = 3.6 × 10 −6 ); ROBO1 was originally associated with dyslexia while ROBO2 was originally associated with expressive vocabulary 22 , 29 . Finally, SNPs within the DCDC2-KIAA0319-TTRAP and in FOXP2 regions were associated with various traits at p < 0.01. Within the ALSPAC cohort, a different pattern of replication emerged (Supplementary Fig. 6 ), with sometimes different SNPs and/or different phenotypes than those associated with CFSRS.
In addition, we examined loci (genes and/or SNPs) associated in recently published GWAS studies of language and reading 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 (Supplementary Data 2 and 3 ); we restricted our examination to the CFSRS data, since the ALSPAC data were included in some of the original studies. In these analyses, we often observed cross-trait replication, with most genes originally associated with dyslexia, and associated with other traits in our sample. These included ZNF385D 16 , which was associated with all CFSRS traits at p < 0.005, CDH13 21 , associated with all CFSRS traits at p < 0.005, GRIN2B 17 , associated with spelling (TWS), expressive vocabulary (EOWPVT), and phonological awareness (Elision) at p < 0.0005 and all CFSRS traits at p < 0.05, NKAIN 17 , associated with receptive language (CELF-R, at 9.7 × 10 −5 (rs16928927 p = 1 × 10 −4 ) and reading comprehension (WIAT-RC, p = 4 × 10 −4 ), and MACROD2 19 associated with all CFSRS traits at p < 0.005).
Polygenic risk scores are associated with increasing clinical severity
In Fig. 3 , we illustrate polygenic risk scores (PRS) for six endophenotypes representing the major domains that we analyzed (receptive language, expressive vocabulary, phonological awareness, phonological memory, spelling, and reading decoding), by quintile, across the clinical subgroups to illustrate the connection between clinical diagnosis and genetic underpinnings of these traits (all endophenotypes are illustrated in Supplementary Fig. 7 ). Generally, we found that polygenic load, indicated by increasing risk scores, was associated with clinical severity ( p < 1 × 10 −8 by ANOVA), with typical children having the lowest scores, followed by children with SSD-only, and children with SSD + LI and CAS having the greatest scores. The exception to this trend is receptive language, where the genetic load is greatest for children with LI, for whom receptive language is a focal deficit. Thus, in general, an increase in PRS scores is associated with greater clinical severity.
We constructed polygenic risk scores for 587 individuals who were both genotyped and had clinical subgroup information available. Polygenic risk scores are displayed by quantile across the clinical subgroups for six endophenotypes representing the major domains ( a Receptive language; b Expressive vocabulary; c Phonological awareness; d Phonological memory; e Spelling; f Reading decoding).
Communication disorders are genetically complex, manifested by a variety of deficiencies in articulation, vocabulary, receptive and expressive language, phonological awareness, reading decoding and comprehension, and spelling. This study ascertained children through an earlier-presenting clinical disorder and examined several key communication measures, and is thus one of the first studies of its kind. This study is also novel in that it is the first GWAS to include a measure of phonological awareness, as well as a motor speech measure. By analyzing several endophenotypes together, we can draw conclusions about the common genetic basis across these seemingly dissimilar skills. Here, we have identified five new candidate regions, some containing multiple genes, that have connections to neurological function and regulation of neurological pathways. We also found that increased polygenic load is associated with more severe communication disorders. Finally, by examining genetic correlations among these traits, we conclude that different domains of communication have some common genetic influences. All of these aspects together add new clarity regarding the genetic underpinnings of speech and language skills.
First, the novel candidate genes that we have identified all have roles in neurological function as evidenced by expression levels of those genes in brain and/or neural tissue, and associations with other communication and/or psychiatric phenotypes. Colocalization analysis provided the strongest evidence for two loci, MON1B/SYCE1L and SETD3 , showing that our association effects localized with gene expression in brain and skeletal tissue. This commonality between communication traits and brain and neural pathways was also demonstrated by a mouse study of vocalization 30 , and pleiotropy between the brain, learning, and psychiatric phenotypes was recently demonstrated by a large GWAS of brain phenotypes 31 . The existence of enhancers, promoters, and methylation effects in the associated regions further emphasizes the importance of regulatory effects on these traits. Deletions spanning SETD3 and CCNK have been associated with syndromic neurodevelopmental disorders 32 and variants in SETX , within this same family of genes, have been associated with CAS 33 . In addition, CCNK is in the FOXP2 pathway in brain tissue 34 , 35 , 36 . NFKBIA is involved in the regulation of the NF-κB pathway, which is involved a number of brain-related processes including neurogenesis, neuritogenesis, synaptic plasticity, learning, and memory 37 . PPP2R3C has been associated with schizophrenia 38 . IFI6 expression has been associated with autism 39 and overexpression of IFI6 in the brain is present in chronic neurodegeneration 40 . Finally, DACT1 may be involved in excitatory synapse organization and dendrite formation during neuronal differentiation 41 and is mainly expressed within the first two trimesters of pregnancy, just before the first evidence of speech processing is observed in preterm neonates 42 . DACT1 was associated with several endophenotypes in our sample. Interestingly, SETD3, NFKBIA , and IFI6 are all also tied to the immune system, and a recent study identified an excess of T cells in the brains of individuals with autism 43 .
Second, understanding the genetic architecture across these endophenotypes is essential for understanding how loci are associated with different measures in different study cohorts or across the developmental trajectory. Strong genetic correlations are observed between spelling, reading comprehension and decoding, expressive and receptive language, vocabulary, and phonological awareness. The strongest replications were for a variety of measures collected in CFSRS with ALSPAC from older youth. Consistent with these findings, we previously demonstrated that spelling at later ages has a higher estimated heritability than spelling at school-age 12 . Measures administered in older youth may also be more sensitive to variations in clinical manifestation of SSD. Examination of the ALSPAC measures suggests that many of those administered at younger ages may have tapped different domains than intended, or may have been less sensitive to later emerging reading and spelling skills. Methods of cohort ascertainment may also be important in comparing our findings to those of other studies. Our families were ascertained through a child with SSD whereas other studies ascertained subjects through LI or dyslexia. These different ascertainment schemes affect both the available measures, as well as the distribution of scores and power to detect association. Since dyslexia emerges later than SSD, longitudinal studies that ascertain through a proband with SSD will be able to capture variants associated with SSD, LI, and dyslexia, as there is high comorbidity. In addition to the plethora of studies ascertaining children at a variety of ages, which has an impact on the heritability of traits 7 , these studies use a wide variety of measures, even for the same endophenotype. Moreover, these studies have been conducted in populations that speak different languages of varying orthographic transparency, which makes them difficult to compare. As noted by Carrion-Castillo et al. 15 , most of the novel loci identified through GWAS have been unique to each study, and these aforementioned issues may explain the lack of replication. Thus, examination of the genetic correlation matrix is essential for the interpretation of results across studies, as it is nearly impossible to analyze the same exact traits, as we have demonstrated with our replication study cohort (ALSPAC).
Third, we replicated candidate genes that had been previously primarily associated with reading and/or LI: CNTNAP2, ATP2C2 , and CYP19A1 . These analyses extend previous findings to show that these genes are associated with articulation ( CNTNAP2 ) and phonological awareness ( ATP2C2 and CYP19A1 ). This further illustrates the pleiotropic nature of these genes. While we did not observe an association with SNPs within the coding regions of ROBO1 and ROBO2 , we did observe significant associations with SNPs between these two genes, which may have regulatory influences on ROBO1/ROBO2 . We also replicated ( p < 5 × 10 −3 ) loci identified in recent GWAS of reading and/or language traits. Similar to another association study between FOXP2 variants and language 44 , we did not observe a statistically significant association between FOXP2 and measures in CFSRS, though there was a replication of some traits at a less stringent ( p < 0.01) level 44 .
Finally, our analysis of PRSs shows strong associations between these risk scores and clinical outcomes of increasing severity. Because of the strong significance of these findings, this suggests that the genetic architecture of communication disorders are maybe largely polygenic, which may additionally explain the lack of replication and/or genome-wide significance. While other studies have examined PRSs associated with language 17 , 45 , ours is the first to examine the polygenic risk associated with other communication endophenotypes. It is noteworthy that our associated SNPs fell outside of gene coding regions but resided in regulatory regions, even having potential regulatory effects themselves as further evidenced by colocalization analysis. This further illustrates the genetic complexity of communication disorders; perhaps the search for single gene dysfunction is misplaced, and rather regulatory functions are more relevant.
This study has several limitations. The sample size of the CFSRS cohort was modest, potentially reducing power. There was no clear correspondence between measures obtained in ALSPAC with those in CFSRS, necessitating consideration of cross-trait replication. We restricted analyses in both cohorts to individuals of European descent because of the low sample size in other ethnic groups, reducing generalizability.
In summary, this first GWAS of communication measures ascertained through families with SSD identified five new candidate genes, all with potential relevance in central nervous system function. Polygenic risk is strongly associated with more severe speech and language outcomes. Careful consideration of genetic correlation among domains of verbal and written language shows that these loci have general effects on communication, not specific to any single domain, suggesting a common genetic architecture. Further research is needed to more closely examine the impact of regulatory variants on these outcomes.
Subject ascertainment—CFSRS
From the CFSRS 46 , 47 , 48 , 49 , 50 , 51 , we examined 435 individuals from 148 families who had both DNA and endophenotype data available (Table 1 ). As previously described, families were ascertained through a proband with SSD identified from caseloads of speech-language pathologists in the Greater Cleveland area and referred to the study. All participants met inclusion criteria based on information provided by a parent in an interview or via questionnaire including normal hearing acuity; fewer than six episodes of otitis media prior to age 3; monolingual English speaker; absence of a history of neurological disorders other than childhood apraxia of speech (CAS), such as cerebral palsy or autism spectrum disorder; and a diagnosis of an SSD or suspected CAS by a local speech-language pathologist or neurologist. Diagnosis of CAS, one severe type of SSD, was confirmed by an experienced licensed speech-language pathologist upon enrollment into the study. Socioeconomic status was determined at the initial assessment based on parent education levels and occupations using the Hollingshead Four Factor Index of Social Class 52 . This study was approved by the Institutional Review Board of Case Medical Center and University Hospitals and all parents provided informed consent and children older than 5 years provided assent.
Communication measures in CFSRS
We studied many endophenotypes covering domains that are common to speech, language, and reading, We examined diadochokinetic rates using the Robbins and Klee Oral Speech Motor Control Protocol 53 or Fletcher Time-by-Count Test of Diadochokinetic Syllable Rate 54 . The merged variable is referred to as DDK. Expressive vocabulary was assessed with the Expressive One Word Picture Vocabulary Test-Revised ( EOWPVT 55 ) and receptive vocabulary with the Peabody Picture Vocabulary Test—Third Edition (PPVT 56 ) , and phonological memory with the Nonsense Word Repetition (NSW 57 ), Multisyllabic Word Repetition (MSW 57 ), and Rapid Color Naming 58 task. In addition to examining the total number of words correct for the MSW and NSW, we also examined the percent phonemes correct for both of these tasks (NSW-PPC and MSW-PPC, respectively). Phonological awareness was assessed using the Elision subtest of the Comprehensive Test of Phonological Processing —Second Edition 59 , which measures the ability to remove phonological segments from spoken words to form other words. Reading was assessed using the Woodcock Reading Mastery Test-Revised, Word Attack subtest (WRMT-AT ) (reading of nonsense words) and Word Identification Subtest (WRMT-ID) (reading of real words), the Reading Comprehension subtest (WIAT-RC) , and Listening Comprehension subtest (WIAT-LC) of the Wechsler Individual Achievement Test 60 . Spelling was assessed on the Test of Written Spelling-3 (TWS) using the total score 61 . The expressive and receptive language was assessed using the Test of Language Development (TOLD 62 ) and Clinical Evaluation of Language Fundamentals-Revised or Clinical Evaluation of Language Fundamentals-Preschool according to age (CELF 63 ) referred to as the CELF-E (expressive) and CELF-R (receptive), respectively. Additional details about these measures are provided in the Supplementary Note. For each of our tests, we selected the first available assessment for each individual (Supplementary Table 1 ).
For the following tests—NSW, NSW PPC, MSW, and MSW PPC—we did not have population normed data, therefore, we converted all scores to age-adjusted z -scores using CFSRS controls. Here, controls were defined as individuals without SSD, LI or CAS. To age-adjust we chose the first available observation for each of the four tests for every control within the CFSRS to determine the effect of age. The age-adjusted score is simply the standardized residual of the score with the effect of age and age-squared regressed out (where the age effect is determined by controls and subsequent adjustment is applied to all participants) 48 , 64 . Age and age-squared are both used to determine the effect of age, as there is a nonlinear relationship between age and each of the above four tests. If applicable, test scores were transformed to an approximately normal distribution using the Box–Cox power transformation 39 . Because measures were already age-normed or age-adjusted, age was not included additionally as a covariate in GWAS or other analytical models.
GWAS analysis
DNA was extracted from buffy coats or saliva samples as previously described 6 . All genotyping was performed using the Illumina Omni 2.5 platform. Standard QC procedures were applied, including filtering based on call rate, Hardy–Weinberg equilibrium (HWE), chromosome (autosomes only), minor allele frequency (MAF), and Mendelian errors. Principal components analysis (PCA) was conducted using markers that attained MAF ≥ 0.01, sample and variant call rate ≥ 0.98 and p ≥ 0.0001 from an exact test of HWE while omitting genomic regions with long-range linkage disequilibrium (LD) 65 . Genotyped data were later imputed to Phase 3, cosmopolitan reference option, of the 1000 Genomes Project panel using the University of Michigan Imputation server 66 which implements minimac3 67 . Following imputation, all markers with imputation quality score R 2 < 0.6 and MAF < 0.05 in our population were removed. Samples were processed and typed for the Illumina Methylation450 chip by the CWRU School of Medicine Genomics Core.
Principal components (PC) obtained from PCA and the genetic relationship matrix (GRM) were generated using genotyped markers that met QC criteria. We used PC-AiR and PC-Relate from the Bioconductor package GENESIS version 2.6.1 68 to generate our PCs and GRM, respectively. PC-AiR accounts for sample relatedness to provide ancestry inference that is not confounded by family structure, while PC-Relate uses the ancestry representative PCs from PC-AiR to provide relatedness estimates due only to the recent family (pedigree) structure.
To examine cross-trait correlation, we used GCTA version 1.24.4 69 to run a bivariate REML analysis for each pair of tests and tested for genetic correlations equal to 0. GCTA’s bivariate REML analysis estimates the genetic variance of each test and the genetic covariance between the two tests that can be captured by all SNPs 70 . Here we included all SNPs with MAF ≥ 0.01. The genetic variance/covariance calculated was adjusted for sex and the first two PCs.
We used RVTests, version 2.0 71 to conduct our GWAS for each of the 16 communication phenotypes, assuming an additive effect of alleles and restricting to all common SNPs with MAF > 0.05. Phenotypes were transformed using a Box-Cox transformation (MASS, R) when applicable (Supplementary Table 2 ). We specifically relied on RVTest’s Grammar-gamma test 72 , which performs a linear mixed model association test while allowing for genotype dosages and accounting for family structure using the GRM. Because each of our tests was age-normed we included only sex and the first two PCs as covariates in our regression models.
PRS analysis
In addition, we generated endophenotype-based PRS in the European subset of the CFSRS where genotype data, as well as clinical group data (no disorder, SSD only, language impairment (LI) only, SSD + LI, CAS) were available. This analysis was done to elucidate the connection between the genetic architecture of these endophenotypes and standard clinical diagnosis seen in clinics. Risk scores were derived from association statistics from our CFSRS GWASs and were constructed using PLINK 1.9 73 (clump and score functions). Regions were considered if at least one variant in the region met the threshold for inclusion as a risk variant ( p < 0.001). Clumping of variants was done in selected regions around the variant showing the strongest association in the region, removing other variants in linkage disequilibrium ( r 2 > 0.5). We used a linear mixed model to model the relationship between PRS and clinical group, controlling for sex and familial relationship (based on family ID ) . Nested model comparison (the full model with the clinical group included versus the reduced model with clinical group removed) using the chi-squared test was implemented to determine if the clinical group explained a significant amount of variability in polygenic risk. These PRSs were used to examine the hypothesis that an increase in PRS score would associate with more complex clinical phenotypes when comparing SSD only versus SSD + LI and CAS.
Statistical analysis of methylome-wide data
Quality control and normalization of raw methylation data (as Illumina.idat files) were carried out using the Bioconductor package RnBeads version 2.3.3 for R 74 . We removed methylation probes in non-CpG contexts, with nearby SNPs, on the X and Y chromosomes, and probes with low variability (SD < 0.005), leaving a total of 470,870 CpG markers with detection p value < 0.05. We normalized signal intensity by means of the BMIQ algorithm 75 , which adjusts for differences between Infinium I and II loci, and adjusted background by the methylumi NOOB procedure, as implemented in RnBeads. Our final data set was scaled to proportion of methylated DNA strand ( β ) values. Duplicate pairs were verified through concordance of genotypes for 65 SNPs on the Methylation450 chip. The final data set typed for the Methylation450 panel comprised 713 unique individuals, plus 60 duplicate samples.
The source of DNA for the MWAS came from saliva samples. Because our sample included salivary DNA samples, we were unable to adjust for cell-type composition using a blood-sample-based reference. Instead, we conducted principal components analysis (PCA) on genomewide methylation as follows: We selected 287,720 CpG sites with SD ≥ 0.02 across the entire sample and normalized the beta values for each site to mean = 0, SD = 1, creating an m × n matrix X, where m is the number of markers and n the number of samples. The eigenvectors from the matrix X′X/( m − 1), an n × n matrix, were obtained using the eigen() function in R, to be used as PC covariates in methylome-wide association studies (MWAS). We regressed our SSD outcomes on each of the first 20 PCs, and included significantly associated PCs in MWAS. Phenotypes were adjusted for between one and four PCs.
We tested for association between CpG beta values and endophenotypes using the linear mixed model approach of GRAMMAR-Gamma 72 as implemented in RVtests 71 . Because our phenotypes were age-normed, we did not adjust for age, but rather for sex and one to four PCs.
We conducted a targeted cis-methylation QTL analysis over 521 CpG sites within 50 kilobasepairs (kb) of 162 candidate SNPs (Supplementary Data 1 ), using Matrix eQTL version 2.2 76 to find the effect of genotype on the extent of methylation in a sample of 597 individuals with both epigenetic and imputed genotype data. All pairs of SNPs and CpG sites within 100 kb were considered to be in cis. Methylation was expressed as M values, where M = log( β /(1 − β )), which extends the range of possible values to (−∞,∞), making the values suitable as an outcome for linear regression.
Replication dataset—ALSPAC
To replicate our GWAS findings, we obtained data from the Avon Longitudinal Study of Parents and Children (ALSPAC). The ALSPAC study was a prospective population-based birth cohort of babies born from >14,000 pregnancies between April 1991–December 1992, who were followed prospectively with a wide battery of developmental tests, parental questionnaires, child-completed questionnaires, and health outcomes 77 , 78 , 79 . Pregnant women residents in Avon, the UK with expected dates of delivery from 1st April 1991 to 31st December 1992 were invited to take part in the study. The initial number of pregnancies enrolled is 14,541 (for these at least one questionnaire has been returned or a “Children in Focus” clinic had been attended by 19/07/99). Of these initial pregnancies, there was a total of 14,676 fetuses, resulting in 14,062 live births and 13,988 children who were alive at 1 year of age. The study website contains details of all the data that is available through a fully searchable data dictionary ( http://www.bris.ac.uk/alspac/researchers/data-access/data-dictionary ). Blood samples were also collected for biomarker and genetic analyses.
Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and Institutional Review Board of Case Medical Center and University Hospitals. Because this was a birth cohort, all children were included, regardless of diagnosis. We obtained both parental report data on speech development in the children, and also communication measures similar to those that we analyzed (see Communication Measures above and Supplementary Table 3 ). As this was a longitudinal study, different measures were given at different ages, and when the same domain was tested at two different ages, the identical measure was not used. At some ages, only random subsets were selected, so the sample size available from each age is not the same. In Supplementary Table 3 , we list the measures given in the CFSRS battery along with the most similar measure given in ALSPAC. Because all the children were the same age when specific assessments were given, no age adjustment was needed. There were no equivalent measures for RAN and Elision.
Genotype QC was performed previously by ALSPAC 16 . We restricted our ALSPAC sample to unrelated individuals by randomly removing one from a pair of twins, when applicable. PCs were generated using Hail 0.1 software, to accommodate the format of files obtained from ALSPAC, using a standard PCA approach 80 . In generating the PCs we first removed long-range LD regions and restricted to variants with a MAF > 0.01, an imputation quality score of >0.95, and variants not in LD ( r 2 < 0.2; following the same process as with PLINK’s –indep-pairwise default procedure). Genetic association testing was performed using linear regression in Hail 0.1 when outcome measures were continuous and using logistic regression in Hail 0.1 when outcome measures were binary. We restricted our analyses to variants with a MAF > 0.01 and an imputation quality score of >0.6; we used a lower MAF threshold because we hypothesized that causal variants might be rarer in a population-based cohort compared to a cohort that was ascertained through a trait of interest. Covariates adjusted for included sex and the first two PCs. Age was not a consideration as ALSPAC is a longitudinal birth cohort study and age differences were negligible for any given measure.
Functional annotation and results integration
In this analysis, we considered CFSRS the discovery sample, since families were ascertained through a child with SSD, and used ALSPAC as the replication sample. We identified associated loci with SNPs significant at p < 10 −5 in CFSRS and p < 0.05 in ALSPAC, with effects in the same direction.
Because the majority of our findings are intergenic and/or fall in noncoding regions, we relied on annotation tools FUMA version 1.35d and HaploReg to characterize which genes our variants might affect, as well as variants’ functionality. We utilized FUMA 81 for mapping genes to our variants based on genomic proximity, eQTL evidence, and chromatin interactions evidence. Default settings in FUMA were used, with the exception of tissue specificity. We hypothesized that gene expression and regulation would be most relevant in the brain and neural tissues, as well as muscles related to speech. In FUMA, we focused on eQTL and chromatin interaction evidence in our target tissues (brain and muscle). Additional details are found in the Supplement. HaploReg v.4.1 was used to examine the chromatin state evidence predicting whether the variant fell in a promoter or enhancer region. Using HaploReg v4.1 we examined histone marks indicating enhancer/promoter for brain tissues, neural tissues (including neuronal progenitor cells) and skeletal muscle tissue.
In order to further prioritize and synthesize our findings, we annotated associated loci as described above, including annotation of associated effects of these loci in the literature, and incorporated supportive findings from our MWAS (Supplementary Data 1 ). We generated a simple locus priority score as the sum of the number of times a locus included an enhancer and/or promoter, included an eQTL, was previously associated with a communication disorder and/or neuropsychiatric disorder, showed eQTL or chromatin state evidence specific to brain and/or neural tissues, mapped to a gene that was a FOXP2 target in brain tissue 34 , 35 , 36 , and an meQTL in that region (at p < 5 × 10 −5 ) with an associated methylation site (at p < 0.05) with the same phenotype as the associated GWAS loci, as determined using the bioinformatic resources described above.
We applied the EpiXcan pipeline 82 to train gene expression predictors in human brain tissue. For genotypes and gene expression, we used psychENCODE data from the dorsolateral prefrontal cortex (DLPFC) 83 . We restricted our analysis to 924 Caucasian samples. We initially computed eQTL summary statistics using the R package Matrix eQTL version 2.2 76 , followed by estimation of SNP priors through the qtlBHM Bayesian hierarchical model 84 using the Roadmap Epigenomics Project chromatin states for DLPFC (‘BRN_DL_PRFRNTL_CRTX’). In total, 363,955 predictors for 18,425 genes were recruited in the EpiXcan psychENCODE model. We then applied the S-PrediXcan method 85 using the EpiXcan psychENCODE model as well as the SNP covariance matrix on the GWAS summary statistics. These analyses were based on genome-wide association results from two phenotypes from our GWAS, TWS, and Elision; these traits were chosen because they had the greatest number of unique significantly associated loci. Detailed results are in Supplementary Tables 7 and 8 .
Chromatin interaction mapping was performed in FUMA using Hi-C data from PsychENCODE 83 (Hi-C based enhancer-promoter interactions), Schmitt et al. 86 (Hi-C based (significant loops) of cell line GSE87112, tissues Dorsolateral Prefrontal Cortex, Hippocampus and Neural progenitor cell) and Giusti-Rodriguez et al. 87 (Hi-C data (significant loops after Bonferroni correction (Pbon < 0.001)) of adult and fetal cortex). Chromatin interactions were filtered by FDR < 1 × 10 −6 .
We primarily focused on loci with priority scores >5, and for loci with priority scores equal to 5, we examined loci with compelling evidence in the communication disorders literature and/or our own methylation data. These loci were then examined using colocalization analysis in LocusFocus 88 , as described below, which facilitates the exploration of a GWAS signal and the degree of colocalization with eQTLs in relevant tissue.
We used LocusFocus version 1.4.9 88 to explore our GWAS signals in their degree of colocalization with expression quantitative trait loci (eQTL) for genes within ±200 kb of the lead SNP in the relevant GTEx tissues. The aim of this method is to annotate GWAS-derived associations to the most probable gene(s) and tissue(s) that may be driving that signal. This method uses the Simple Sum method to assess the degree of colocalization of any two given datasets. The Simple Sum region used for calculating colocalization of eQTLs and GWAS signals is ±100 kb of the lead SNP (i.e., GWAS signals and eQTLs within 100 kb of the lead SNP for genes within 200 kb of lead SNP). When applied to GTEx, LocusFocus presents the degree of colocalization of genes nearby the GWAS association for all the tissues selected in an interactive heatmap plot. Here we selected 14 tissues, including all brain tissue available for GTEx v7 (brain_spinal_cord_cervical, nucleus_accumbens_basal_ganglia, cerebellar_hemisphere, hippocampus, caudate_basal_ganglia, anterior_cingulate_cortex, cortex, hypothalamus, amygdala, frontal_cortex, substantia_nigra, putamen_basal_ganglia, cerebellum), as well as, skeletal_muscle. We also brought in psychEncode eQTL data 83 (FDR < 0.05 and a filter requiring genes to have an expression > 0.1 fragments per KB per million reads (FPKM) in at least 10 samples) in as a secondary dataset to examine colocalization with our GWAS signal and eQTLs within psychEncode data. Here, we pulled eQTLs, within 100 kb of our lead SNP for genes within 200 kb of our lead SNP.
Examination of previously identified candidate genes for communication disorders
In order to examine whether our GWAS replicated previous findings (either from published GWAS in language and reading phenotypes and/or targeted candidate gene studies of these phenotypes), we took a twofold approach. If the original papers provided rs IDs, we looked up our results at those specific SNPs. If the papers did not provide that level of detail, we instead examined all SNPs with MAF > 5% in the gene regions ±5 kb.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Data from the Cleveland Family Speech and Reading study are not available for broad genetic data sharing because study subjects did not provide informed consent for such data sharing, over 80% specifically saying that they wanted to be recontacted for additional use of the data. The IRB governing this study has imposed a restriction stating that the consent forms did not adequately cover the issue of deposition of the data into public repositories and that participants needed to be reconsented. In an effort to reconsent them, we have attempted to recontact these participants on numerous occasions, but have only been marginally successful. Summary statistics are not provided because of concerns that subjects can be identified from summary statistics, based on published literature demonstrating this is possible, and because these phenotypes are sufficiently rare and participants were ascertained in a narrow geographic region. Please contact the corresponding author, Sudha Iyengar, [email protected], to request summary statistics. These can be shared on request but will require an IRB application, and submission of names of individuals who will use the data to our IRB.
Code availability
All software versions are identified within the Methods. If there is no version number, then that software package only has one (current) version. There were no custom scripts created for the analyses conducted in this paper. Only one variable was created in this analysis (DDK) and those methods are fully described with the Measures.
American Speech-Language Association. Almost 8 percent of US children have a communication or swallowing disorder (2015).
Catts, H. W., Adlof, S. M., Hogan, T. P. & Weismer, S. E. Are specific language impairment and dyslexia distinct disorders? J. Speech Lang. Hear. Res. 48 , 1378–1396 (2005).
Article PubMed Google Scholar
Shriberg, L., Tomblin, J. & McSweeny, J. Prevalence of speech delay in 6-year-old children and comorbidity with language impairment. J. Speech Lang. Hear. Res. 42 , 1461–1481 (1999).
Article CAS PubMed Google Scholar
Al Otaiba, S., Puranik, C., Zilkowski, R. & Curran, T. Effectiveness of early phonological awareness interventions for students with speech or language impairments. J. Spec. Educ. 43 , 107–128 (2009).
Article PubMed PubMed Central Google Scholar
Larivee, L. C. H. W. Early reading achievement in children with expressive phonological disorders. Am. J. Speech Lang. Pathol. 8 , 119–128 (1999).
Google Scholar
Scarborough, H. In Specific Reading Disabilities: A View of the Spectrum (eds Shapiro, B. K., Accardo, P. J., Capute, J.) 75–119 (York Press, 1990).
Lewis, B. A. et al. The genetic bases of speech sound disorders: evidence from spoken and written language. J. Speech Lang. Hear. Res. 49 , 1294–1312 (2006).
Stein, C. M. et al. Pleiotropic effects of a chromosome 3 locus on speech-sound disorder and reading. Am. J. Hum. Genet. 74 , 283–297 (2004).
Article CAS PubMed PubMed Central Google Scholar
Gottesman, I. I. & Gould, T. D. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry 160 , 636–645 (2003).
McLeod, S. B. E. In Children’s Speech: An Evidence-Based Approach to Assessment and Intervention (eds McLeod, S., Baker, E.) 181–184 (Pearson Education, 2017).
Lemons, C. J. & Fuchs, D. Phonological awareness of children with Down syndrome: its role in learning to read and the effectiveness of related interventions. Res. Dev. Disabil. 31 , 316–330 (2010).
Lewis, B. A. et al. Heritability and longitudinal outcomes of spelling skills in individuals with histories of early speech and language disorders. Learn. Individ. Differ. 65 , 1–11 (2018).
Stevenson, J., Graham, P., Fredman, G. & McLoughlin, V. A twin study of genetic influences on reading and spelling ability and disability. J. Child Psychol. Psychiatry Allied Discip. 28 , 229–247 (1987).
Article CAS Google Scholar
Graham, S. A. & Fisher, S. E. Understanding language from a genomic perspective. Annu. Rev. Genet. 49 , 131–160 (2015).
Carrion-Castillo, A. et al. Evaluation of results from genome-wide studies of language and reading in a novel independent dataset. Genes Brain Behav. 15 , 531–541 (2016).
Eicher, J. D. et al. Genome-wide association study of shared components of reading disability and language impairment. Genes Brain Behav. 12 , 792–801 (2013).
Gialluisi, A. et al. Genome-wide association scan identifies new variants associated with a cognitive predictor of dyslexia. Transl. Psychiatry 9 , 77 (2019).
Article PubMed PubMed Central CAS Google Scholar
Gialluisi, A. et al. Genome-wide screening for DNA variants associated with reading and language traits. Genes Brain Behav. 13 , 686–701 (2014).
Harlaar, N. et al. Genome-wide association study of receptive language ability of 12-year-olds. J. Speech Lang. Hear. Res. 57 , 96–105 (2014).
Kornilov, S. A. et al. Genome-wide association and exome sequencing study of language disorder in an isolated population. Pediatrics https://doi.org/10.1542/peds.2015-2469 (2016).
Luciano, M. et al. A genome-wide association study for reading and language abilities in two population cohorts. Genes Brain Behav. 12 , 645–652 (2013).
St Pourcain, B. et al. Common variation near ROBO2 is associated with expressive vocabulary in infancy. Nat. Commun. 5 , 4831 (2014).
Morris, N., Elston, R. C., Barnholtz-Sloan, J. S. & Sun, X. Novel approaches to the analysis of family data in genetic epidemiology. Front. Genet. 6 , 27 (2015).
Ott, J., Kamatani, Y. & Lathrop, M. Family-based designs for genome-wide association studies. Nat. Rev. Genet. 12 , 465–474 (2011).
Goriounova, N. A. & Mansvelder, H. D. Genes, cells and brain areas of intelligence. Front. Hum. Neurosci. 13 , 44–44 (2019).
Zhang, Y. & Hu, W. NFκB signaling regulates embryonic and adult neurogenesis. Front. Biol. (Beijing) 7 , https://doi.org/10.1007/s11515-11012-11233-z (2012).
Newbury, D. F. & Monaco, A. P. Genetic advances in the study of speech and language disorders. Neuron 68 , 309–320 (2010).
Anthoni, H. et al. The aromatase gene CYP19A1: several genetic and functional lines of evidence supporting a role in reading, speech and language. Behav. Genet. 42 , 509–527 (2012).
Hannula-Jouppi, K. et al. The axon guidance receptor gene ROBO1 is a candidate gene for developmental dyslexia. PLoS Genet. 1 , e50 (2005).
Ashbrook, D. G. et al. Born to cry: a genetic dissection of infant vocalization. Front. Behav. Neurosci. 12 , 250–250 (2018).
Zhao, B. et al. Genome-wide association analysis of 19,629 individuals identifies variants influencing regional brain volumes and refines their genetic co-architecture with cognitive and mental health traits. Nat. Genet. 51 , 1637–1644 (2019).
Fan, Y. et al. De novo mutations of CCNK cause a syndromic neurodevelopmental disorder with distinctive facial dysmorphism. Am. J. Hum. Genet. 103 , 448–455 (2018).
Worthey, E. A. et al. Whole-exome sequencing supports genetic heterogeneity in childhood apraxia of speech. J. Neurodev. Disord. 5 , 29 (2013).
MacDermot, K. et al. Identification of FOXP2 truncation as a novel cause of developmental speech and language deficits. Am. J. Hum. Genet. 76 , 1074–1080 (2005).
Spiteri, E. et al. Identification of the transcriptional targets of FOXP2, a gene linked to speech and language, in developing human brain. Am. J. Hum. Genet. 81 , 1144–1157 (2007).
Vernes, S. C. et al. High-throughput analysis of promoter occupancy reveals direct neural targets of FOXP2, a gene mutated in speech and language disorders. Am. J. Hum. Genet. 81 , 1232–1250 (2007).
Lanzillotta, A. et al. NF-κB in innate neuroprotection and age-related neurodegenerative diseases. Front. Neurol. 6 , 98–98 (2015).
Gusev, A. et al. Transcriptome-wide association study of schizophrenia and chromatin activity yields mechanistic disease insights. Nat. Genet. 50 , 538–548 (2018).
El-Ansary, A. & Al-Ayadhi, L. GABAergic/glutamatergic imbalance relative to excessive neuroinflammation in autism spectrum disorders. J. Neuroinflamm. 11 , 189–189 (2014).
Nazmi, A. et al. Chronic neurodegeneration induces type I interferon synthesis via STING, shaping microglial phenotype and accelerating disease progression. Glia 67 , 1254–1276 (2019).
Okerlund, N. D. et al. Dact1 is a postsynaptic protein required for dendrite, spine, and excitatory synapse development in the mouse forebrain. J. Neurosci. 30 , 4362–4368 (2010).
Le Gruen, Y., Leroy, F., Philippe C. IMAGEN Consortium, Mangin, J-F., Dehaene-Lambertz, G., Frouin V. Enhancer locus in ch14q23.1 modulates brain asymmetric temporal regions involved in language processing cerebral cortex. 30 , 5322–5332 (2020).
DiStasio, M. M., Nagakura, I., Nadler, M. J. & Anderson, M. P. T lymphocytes and cytotoxic astrocyte blebs correlate across autism brains. Ann. Neurol. 86 , 885–898 (2019).
Mueller, K. L. et al. Common genetic variants in FOXP2 are not associated with individual differences in language development. PLoS ONE 11 , e0152576 (2016).
Nudel, R. et al. Language deficits in specific language impairment, attention deficit/hyperactivity disorder, and autism spectrum disorder: an analysis of polygenic risk. Autism Res. https://doi.org/10.1002/aur.2211 (2019).
Lewis, B. & Freebairn, L. Speech production skills of nuclear family members of children with phonology disorders. Speech Lang. 41 , 45–61 (1998).
Article Google Scholar
Lewis, B., Freebairn, L. & Taylor, H. Follow-up of children with early expressive phonology disorders. J. Learn. Disabil. 33 , 433–444 (2000).
Lewis, B. A. et al. Literacy outcomes of children with early childhood speech sound disorders: impact of endophenotypes. J. Speech Lang. Hear. Res. 54 , 1628–1643 (2011).
Lewis, B. A. et al. Family pedigrees of children with suspected childhood apraxia of speech. J. Commun. Disord. 37 , 157–175 (2004).
Lewis, B. A., Freebairn, L. A., Hansen, A. J., Iyengar, S. K. & Taylor, H. G. School-age follow-up of children with childhood apraxia of speech. Lang. Speech Hear. Servic. Schools 35 , 122–140 (2004).
Lewis, B. A. et al. Speech and language skills of parents of children with speech sound disorders. Am. J. Speech Lang. Pathol. 16 , 108–118 (2007).
Hollingshead, A. Four Factor Index of Social Class . (Department of Sociology, Yale University, New Haven, CT, 1975).
Robbins, J. & Klee, T. Clinical assessment of oropharyngeal motor development in young children. J. Speech Hear. Res. 52 , 271–277 (1987).
Fletcher, D. The Fletcher Time-by-Count Test of Diadochokinetic Syllable Rate . (C.C. Publications, Inc., Tigard, OR, 1977).
Gardner, M. Expressive One Word Picture Vocabulary Test-Revised . (Academic Therapy Publications, Novato, CA, 1990).
Dunn, L. & Dunn, L. Peabody Picture Vocabulary Test—Third Edition . (American Guidance Service, Inc., Circle Pines, MN, 1997).
Catts, H. Speech production/phonological deficits in reading disordered children. J. Learn. Disabil. 19 , 504–508 (1986).
Denkla, M. & Rudel, R. Rapid ‘automatized’ naming (R.A.N.): dyslexia differentiated from other learning disabilities. Neuropsychologia 14 , 471–479 (1976).
Wagner, R. T., Rashotte, J., Pearson, C. & Comprehensive, N. A. Test of Phonological Processing . (Pearson, London, England, 2013).
Wechsler, D. Wecshler Intelligence Scale for Children-Third Edition. (The Psychological Coporation, San Antonio, TX, 1991).
Larsen, S. H. D. Test of Language Development . (The Psychological Corporation, San Antonio, TX, 1994).
Newcomer, P. & Hammill, D. Test of Language Development—Primary , Second Edition . (Pro-Ed., 1988).
Semel, E., Wiig, E. & Secord, W. Clinical Evaluation of Language Fundamentals—Revised . (The Psychological Corporation, 1987).
Wellman, R. L. et al. Narrative ability of children with speech sound disorders and the prediction of later literacy skills. Lang. Speech Hear. Serv. Schools 42 , 561–579 (2011).
Novembre, J. et al. Genes mirror geography within Europe. Nature 456 , 98–101 (2008).
Das, S. et al. Next-generation genotype imputation service and methods. Nat. Genet. 48 , 1284–1287 (2016).
Howie, B. N., Donnelly, P. & Marchini, J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS. Genet. 5 , e1000529 (2009).
GENESIS: GENetic EStimation and Inference in Structured samples (GENESIS): Statistical methods for analyzing genetic data from samples with population structure and/or relatedness. R package version (2019).
Yang, J., Lee, S. H., Goddard, M. E. & Visscher, P. M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 88 , 76–82 (2011).
Lee, S. H., Yang, J., Goddard, M. E., Visscher, P. M. & Wray, N. R. Estimation of pleiotropy between complex diseases using single-nucleotide polymorphism-derived genomic relationships and restricted maximum likelihood. Bioinformatics (Oxford, England) 28 , 2540–2542 (2012).
CAS Google Scholar
Zhan, X., Hu, Y., Li, B., Abecasis, G. R. & Liu, D. J. RVTESTS: an efficient and comprehensive tool for rare variant association analysis using sequence data. Bioinformatics (Oxford, England) 32 , 1423–1426 (2016).
Svishcheva, G. R., Axenovich, T. I., Belonogova, N. M., van Duijn, C. M. & Aulchenko, Y. S. Rapid variance components-based method for whole-genome association analysis. Nat. Genet. 44 , 1166–1170 (2012).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81 , 559–575 (2007).
Assenov, Y. et al. Comprehensive analysis of DNA methylation data with RnBeads. Nat. Methods 11 , 1138–1140 (2014).
Teschendorff, A. E. et al. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics 29 , 189–196 (2013).
Shabalin, A. A. Matrix eQTL: ultra fast eQTL analysis via large matrix operations. Bioinformatics 28 , 1353–1358 (2012).
Fraser, A. et al. Cohort Profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int. J. Epidemiol. 42 , 97–110 (2013).
Golding, J., Pembrey, M. & Jones, R. ALSPAC—the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr. Perinatal Epidemiol. 15 , 74–87 (2001).
Boyd, A. et al. Cohort Profile: the ‘children of the 90s’–the index offspring of the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiol. 42 , 111–127 (2013).
Price, A. L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38 , 904–909 (2006).
Watanabe, K., Taskesen, E., van Bochoven, A. & Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 8 , 1826–1826 (2017).
Zhang, W. et al. Integrative transcriptome imputation reveals tissue-specific and shared biological mechanisms mediating susceptibility to complex traits. Nat. Commun. 10 , 3834 (2019).
Wang, D. et al. Comprehensive functional genomic resource and integrative model for the human brain. Science https://doi.org/10.1126/science.aat8464 (2018).
Li, Y. I. et al. RNA splicing is a primary link between genetic variation and disease. Science 352 , 600–604 (2016).
Barbeira, A. N. et al. Exploring the phenotypic consequences of tissue specific gene expression variation inferred from GWAS summary statistics. Nat. Commun. 9 , 1825 (2018).
Schmitt, A. D. et al. A compendium of chromatin contact maps reveals spatially active regions in the human genome. Cell Rep. 17 , 2042–2059 (2016).
Giusti-Rodríguez, P. et al. Using three-dimensional regulatory chromatin interactions from adult and fetal cortex to interpret genetic results for psychiatric disorders and cognitive traits. Preprint at bioRxiv https://doi.org/10.1101/406330 (2019).
Panjwani, N. et al. LocusFocus: web-based colocalization for the annotation and functional follow-up of GWAS. PLoS Comput. Biol. 16 , e1008336 (2020).
Download references
Acknowledgements
We would like to thank the families who have so generously participated in this study for many years. This research was supported by the Genomics Core Facility of the CWRU School of Medicine’s Genetics and Genome Sciences Department. This work made use of the High-Performance Computing Resource in the Core Facility for Advanced Research Computing at Case Western Reserve University. This work was supported by NIH grant R01DC000528 awarded to Dr. Lewis and R01DC012380 awarded to Dr. Iyengar. We are extremely grateful to all the families who took part in the ALSPAC study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. The UK Medical Research Council and Wellcome (Grant ref: 217065/Z/19/Z) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and Dr. Sudha Iyengar will serve as guarantor for the contents of this paper. GWAS data for ALSPAC was generated at the Genotyping Facilities at Wellcome Sanger Institute.
Author information
These authors contributed equally: Penelope Benchek, Robert P. Igo Jr., Heather Voss-Hoynes
Deceased: Robert P. Igo Jr.
These authors jointly supervised this work: Barbara Lewis, Catherine M. Stein, Sudha K. Iyengar
Authors and Affiliations
Department of Population & Quantitative Health Sciences, Case Western Reserve University, Cleveland, OH, USA
Penelope Benchek, Robert P. Igo Jr., Heather Voss-Hoynes, Barbara Truitt, Michael Osterman, E. Ricky Chan, Catherine M. Stein & Sudha K. Iyengar
Bristol Dental School, Faculty of Health Sciences, University of Bristol, and Bristol Speech and Language Therapy Research Unit, North Bristol NHS Trust, Bristol, UK
Yvonne Wren
Department of Psychological Sciences, Case Western Reserve University, Cleveland, OH, USA
Gabrielle Miller, Lisa Freebairn, Jessica Tag & Barbara Lewis
Department of Psychiatry, Friedman Brain Institute, and Department of Genetics and Genomic Science and Institute for Multiscale Biology, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Wen Zhang & Panos Roussos
Department of Pediatrics, Case Western Reserve University, and Rainbow Babies & Children’s Hospital, University Hospital Case Medical Center, Cleveland, OH, USA
H. Gerry Taylor
Nationwide Children’s Hospital Research Institute and Department of Pediatrics, The Ohio State University, Columbus, OH, USA
Mental Illness Research, Education, and Clinical Center (VISN 2 South), James J. Peters VA Medical Center, Bronx, NY, USA
Panos Roussos
Cleveland Hearing and Speech Center, Cleveland, OH, USA
Barbara Lewis
You can also search for this author in PubMed Google Scholar
Contributions
C.M.S., P.B., B.L. and S.K.I. conceptualized and designed the study, drafted the initial paper, and reviewed and revised the paper. P.B., R.P.I., H.V.-H., B.T., W.Z., M.O., E.R.C. and P.R. conducted the statistical analyses. R.P.I., H.V.-H. and H.G.T. helped conceptualize the study and critically reviewed the manuscript for important intellectual content. G.M., Y.W., L.F., J.T. and B.L. collected the data and revised and reviewed the paper. All authors approved the final manuscript as submitted. Dr. Igo passed away prior to the final submission of this paper. He provided edits and written comments on the penultimate version in May 2020 and passed away unexpectedly in July 2020. He was responsible for the methylation (MWAS) analyses and provided invaluable guidance on the GWAS analyses.
Corresponding authors
Correspondence to Catherine M. Stein or Sudha K. Iyengar .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

Supplementary information
Supplementary information, supplementary data 1, supplementary data 2, supplementary data 3, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Benchek, P., Igo, R.P., Voss-Hoynes, H. et al. Association between genes regulating neural pathways for quantitative traits of speech and language disorders. npj Genom. Med. 6 , 64 (2021). https://doi.org/10.1038/s41525-021-00225-5
Download citation
Received : 05 January 2021
Accepted : 22 June 2021
Published : 27 July 2021
DOI : https://doi.org/10.1038/s41525-021-00225-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Genetic aspects of speech disorders in children.
- E. A. Morozova
- M. V. Belousova
- V. V. Bogolyubova
Neuroscience and Behavioral Physiology (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Sustainability
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
Scientists discover how mutations in a language gene produce speech deficits
Press contact :, media download.
*Terms of Use:
Images for download on the MIT News office website are made available to non-commercial entities, press and the general public under a Creative Commons Attribution Non-Commercial No Derivatives license . You may not alter the images provided, other than to crop them to size. A credit line must be used when reproducing images; if one is not provided below, credit the images to "MIT."
Previous image Next image
Mutations of a gene called Foxp2 have been linked to a type of speech disorder called apraxia that makes it difficult to produce sequences of sound. A new study from MIT and National Yang Ming Chiao Tung University sheds light on how this gene controls the ability to produce speech.
In a study of mice, the researchers found that mutations in Foxp2 disrupt the formation of dendrites and neuronal synapses in the brain’s striatum, which plays important roles in the control of movement. Mice with these mutations also showed impairments in their ability to produce the high-frequency sounds that they use to communicate with other mice.
Those malfunctions arise because Foxp2 mutations prevent the proper assembly of motor proteins, which move molecules within cells, the researchers found.
“These mice have abnormal vocalizations, and in the striatum there are many cellular abnormalities,” says Ann Graybiel, an MIT Institute Professor, a member of MIT’s McGovern Institute for Brain Research, and an author of the paper . “This was an exciting finding. Who would have thought that a speech problem might come from little motors inside cells?”
Fu-Chin Liu PhD ’91, a professor at National Yang Ming Chiao Tung University in Taiwan, is the senior author of the study, which appears today in the journal Brain . Liu and Graybiel also worked together on a 2016 study of the potential link between Foxp2 and autism spectrum disorder. The lead authors of the new Brain paper are Hsiao-Ying Kuo and Shih-Yun Chen of National Yang Ming Chiao Tung University.
Speech control
Children with Foxp2-associated apraxia tend to begin speaking later than other children, and their speech is often difficult to understand. The disorder is believed to arise from impairments in brain regions, such as the striatum, that control the movements of the lips, mouth, and tongue. Foxp2 is also expressed in the brains of songbirds such as zebra finches and is critical to those birds’ ability to learn songs.
Foxp2 encodes a transcription factor, meaning that it can control the expression of many other target genes. Many species express Foxp2, but humans have a special form of Foxp2. In a 2014 study , Graybiel and colleagues found evidence that the human form of Foxp2, when expressed in mice, allowed the mice to accelerate the switch from declarative to procedural types of learning.
In that study, the researchers showed that mice engineered to express the human version of Foxp2, which differs from the mouse version by only two DNA base pairs, were much better at learning mazes and performing other tasks that require turning repeated actions into behavioral routines. Mice with human-like Foxp2 also had longer dendrites — the slender extensions that help neurons form synapses — in the striatum, which is involved in habit formation as well as motor control.
In the new study, the researchers wanted to explore how the Foxp2 mutation that has been linked with apraxia affects speech production, using ultrasonic vocalizations in mice as a proxy for speech. Many rodents and other animals such as bats produce these vocalizations to communicate with each other.
While previous studies, including the work by Liu and Graybiel in 2016, had suggested that Foxp2 affects dendrite growth and synapse formation, the mechanism for how that occurs was not known. In the new study, led by Liu, the researchers investigated one proposed mechanism, which is that Foxp2 affects motor proteins.
One of these molecular motors is the dynein protein complex, a large cluster of proteins that is responsible for shuttling molecules along microtubule scaffolds within cells.
“All kinds of molecules get shunted around to different places in our cells, and that's certainly true of neurons,” Graybiel says. “There’s an army of tiny molecules that move molecules around in the cytoplasm or put them into the membrane. In a neuron, they may send molecules from the cell body all the way down the axons.”
A delicate balance
The dynein complex is made up of several other proteins. The most important of these is a protein called dynactin1, which interacts with microtubules, enabling the dynein motor to move along microtubules. In the new study, the researchers found that dynactin1 is one of the major targets of the Foxp2 transcription factor.
The researchers focused on the striatum, one of the regions where Foxp2 is most often found, and showed that the mutated version of Foxp2 is unable to suppress dynactin1 production. Without that brake in place, cells generate too much dynactin1. This upsets the delicate balance of dynein-dynactin1, which prevents the dynein motor from moving along microtubules.
Those motors are needed to shuttle molecules that are necessary for dendrite growth and synapse formation on dendrites. With those molecules stranded in the cell body, neurons are unable to form synapses to generate the proper electrophysiological signals they need to make speech production possible.
Mice with the mutated version of Foxp2 had abnormal ultrasonic vocalizations, which typically have a frequency of around 22 to 50 kilohertz. The researchers showed that they could reverse these vocalization impairments and the deficits in the molecular motor activity, dendritic growth, and electrophysiological activity by turning down the gene that encodes dynactin1.
Mutations of Foxp2 can also contribute to autism spectrum disorders and Huntington’s disease, through mechanisms that Liu and Graybiel previously studied in their 2016 paper and that many other research groups are now exploring. Liu’s lab is also investigating the potential role of abnormal Foxp2 expression in the subthalamic nucleus of the brain as a possible factor in Parkinson’s disease.
The research was funded by the Ministry of Science and Technology of Taiwan, the Ministry of Education of Taiwan, the U.S. National Institute of Mental Health, the Saks Kavanaugh Foundation, the Kristin R. Pressman and Jessica J. Pourian ’13 Fund, and Stephen and Anne Kott.
Share this news article on:
Related links.
- Ann Graybiel
- McGovern Institute
- Department of Brain and Cognitive Sciences
Related Topics
- Brain and cognitive sciences
- Neuroscience
Related Articles
How Huntington’s disease affects different neurons

Neuroscientists get at the roots of pessimism
Distinctive brain pattern helps habits form

Neuroscientists identify key role of language gene
Previous item Next item
More MIT News

No detail too small
Read full story →
Study assesses seizure risk from stimulating the thalamus
Atoms on the edge

New filtration material could remove long-lasting chemicals from water

Keeping the cosmos clean

Sam Madden named faculty head of computer science in EECS
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
A forkhead-domain gene is mutated in a severe speech and language disorder
Affiliation.
- 1 Wellcome Trust Centre for Human Genetics, University of Oxford, Roosevelt Drive, Oxford OX3 7BN, UK.
- PMID: 11586359
- DOI: 10.1038/35097076
Individuals affected with developmental disorders of speech and language have substantial difficulty acquiring expressive and/or receptive language in the absence of any profound sensory or neurological impairment and despite adequate intelligence and opportunity. Although studies of twins consistently indicate that a significant genetic component is involved, most families segregating speech and language deficits show complex patterns of inheritance, and a gene that predisposes individuals to such disorders has not been identified. We have studied a unique three-generation pedigree, KE, in which a severe speech and language disorder is transmitted as an autosomal-dominant monogenic trait. Our previous work mapped the locus responsible, SPCH1, to a 5.6-cM interval of region 7q31 on chromosome 7 (ref. 5). We also identified an unrelated individual, CS, in whom speech and language impairment is associated with a chromosomal translocation involving the SPCH1 interval. Here we show that the gene FOXP2, which encodes a putative transcription factor containing a polyglutamine tract and a forkhead DNA-binding domain, is directly disrupted by the translocation breakpoint in CS. In addition, we identify a point mutation in affected members of the KE family that alters an invariant amino-acid residue in the forkhead domain. Our findings suggest that FOXP2 is involved in the developmental process that culminates in speech and language.
PubMed Disclaimer
- Talk of genetics and vice versa. Pinker S. Pinker S. Nature. 2001 Oct 4;413(6855):465-6. doi: 10.1038/35097173. Nature. 2001. PMID: 11586336 No abstract available.
Similar articles
- Localisation of a gene implicated in a severe speech and language disorder. Fisher SE, Vargha-Khadem F, Watkins KE, Monaco AP, Pembrey ME. Fisher SE, et al. Nat Genet. 1998 Feb;18(2):168-70. doi: 10.1038/ng0298-168. Nat Genet. 1998. PMID: 9462748
- Deciphering the genetic basis of speech and language disorders. Fisher SE, Lai CS, Monaco AP. Fisher SE, et al. Annu Rev Neurosci. 2003;26:57-80. doi: 10.1146/annurev.neuro.26.041002.131144. Epub 2003 Jan 8. Annu Rev Neurosci. 2003. PMID: 12524432 Review.
- Genetics. First gene linked to speech identified. Balter M. Balter M. Science. 2001 Oct 5;294(5540):32. doi: 10.1126/science.294.5540.32a. Science. 2001. PMID: 11588230 No abstract available.
- Molecular evolution of FOXP2, a gene involved in speech and language. Enard W, Przeworski M, Fisher SE, Lai CS, Wiebe V, Kitano T, Monaco AP, Pääbo S. Enard W, et al. Nature. 2002 Aug 22;418(6900):869-72. doi: 10.1038/nature01025. Epub 2002 Aug 14. Nature. 2002. PMID: 12192408
- FOXP2 as a molecular window into speech and language. Fisher SE, Scharff C. Fisher SE, et al. Trends Genet. 2009 Apr;25(4):166-77. doi: 10.1016/j.tig.2009.03.002. Epub 2009 Mar 21. Trends Genet. 2009. PMID: 19304338 Review.
- Pleiotropy in FOXC1-attributable phenotypes involves altered ciliation and cilia-dependent signaling. Havrylov S, Chrystal P, van Baarle S, French CR, MacDonald IM, Avasarala J, Rogers RC, Berry FB, Kume T, Waskiewicz AJ, Lehmann OJ. Havrylov S, et al. Sci Rep. 2024 Aug 31;14(1):20278. doi: 10.1038/s41598-024-71159-y. Sci Rep. 2024. PMID: 39217245 Free PMC article.
- Persistent vocal learning in an aging open-ended learner reflected in neural FoxP2 expression. Moussaoui B, Ulmer K, Araya-Salas M, Wright TF. Moussaoui B, et al. BMC Neurosci. 2024 Jul 4;25(1):31. doi: 10.1186/s12868-024-00879-8. BMC Neurosci. 2024. PMID: 38965498 Free PMC article.
- Genetic variability of FOXP2 and its targets CNTNAP2 and PRNP in frontotemporal dementia: A pilot study in a southern Italian population. Crocco P, De Rango F, Bruno F, Malvaso A, Maletta R, Bruni AC, Passarino G, Rose G, Dato S. Crocco P, et al. Heliyon. 2024 May 22;10(11):e31624. doi: 10.1016/j.heliyon.2024.e31624. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38828303 Free PMC article.
- What do mammals have to say about the neurobiology of acoustic communication? Salles A, Neunuebel J. Salles A, et al. Mol Psychol. 2023;2:5. doi: 10.12688/molpsychol.17539.1. Epub 2023 May 4. Mol Psychol. 2023. PMID: 38827277 Free PMC article.
- Wings of Change: aPKC/FoxP-dependent plasticity in steering motor neurons underlies operant self-learning in Drosophila . Ehweiner A, Duch C, Brembs B. Ehweiner A, et al. F1000Res. 2024 Jun 11;13:116. doi: 10.12688/f1000research.146347.1. eCollection 2024. F1000Res. 2024. PMID: 38779314 Free PMC article.
Publication types
- Search in MeSH
Related information
- Cited in Books
- Gene (GeneRIF)
- MedGen (Bookshelf cited)
- MedGen (OMIM)
- Nucleotide (RefSeq)
- OMIM (cited)
- Protein (RefSeq)
LinkOut - more resources
Full text sources.
- Nature Publishing Group
- Ovid Technologies, Inc.
Other Literature Sources
- The Lens - Patent Citations
- MedlinePlus Health Information
Molecular Biology Databases
- GlyGen glycoinformatics resource

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

Specializing in intensive episodes of care for adults and children.

Our Philosophy
Here at Because You Can, our staff of experienced doctors, speech-language pathologists, occupational therapists, behavior analysts, and teachers believe everyone is unique and learns in a different way. Whether recovering from a major incident or just learning to communicate, we believe that intensity is the key to success.
Adults and children with communication disorders are often given limited opportunities to participate in therapy with a therapist who specializes in their unique diagnosis. Here at Because You Can, we believe that a diagnosis is just a starting point, and that each individual should have the opportunity to grow in their own unique way.
Specialties
augmentative and alternative communication (aac) therapy.
Applied Behavioral Analysis (ABA) Therapy
ADHD/Executive Functioning
Articulation
Complex Communication Needs
Dysarthria
Literacy Evaluations
Math (K-College)
Occupational Therapy
Phonologic al Delays/Disorders
Receptive and Expressive Language Delays/Disorders
Reading (K-College)
Social Skills
Call to schedule an evaluation and let us help you help your loved one to meet their full potential!
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2024.

GeneReviews ® [Internet].
- GeneReviews by Title
- GeneReviews Advanced Search
FOXP2- Related Speech and Language Disorder
Angela Morgan , BSpPath (Aud Hons), PhD, Simon E Fisher , DPhil, Ingrid Scheffer , MBBS, PhD, FRACP, FAA, and Michael Hildebrand , PhD.
Initial Posting: June 23, 2016 ; Last Update: January 26, 2023 .
Estimated reading time: 31 minutes
Clinical characteristics.
FOXP2- related speech and language disorder ( FOXP2- SLD) is caused by heterozygous FOXP2 pathogenic variants (including whole- or partial-gene deletions). The core phenotype of FOXP2- SLD is childhood apraxia of speech (CAS), a disorder of speech motor programming or planning that affects the production, sequencing, timing, and stress of sounds, and the accurate sequencing of speech sounds into syllables and syllables into words. CAS also interferes nonselectively with multiple other aspects of language, including phonology, grammar, and literacy. Additional findings in FOXP2- SLD can include oral-motor dyspraxia (difficulty planning or programming oral movements on command); dysarthria; moderate-to-severe receptive and expressive language disorder; reading and spelling impairments; and fine motor difficulties. Nonverbal (performance) IQ is typically relatively preserved compared to verbal IQ; gross motor skills are normal. Autistic features or a diagnosis of autism spectrum disorder have been reported in some individuals.
Diagnosis/testing.
The diagnosis of FOXP2- SLD is established in a proband with suggestive findings and a heterozygous pathogenic (or likely pathogenic) variant in FOXP2 identified by molecular genetic testing.
Management.
Treatment of manifestations: There is no cure for FOXP2 -SLD. Supportive care to improve quality of life, maximize function, and reduce complications is recommended. This ideally involves multidisciplinary care by speech-language pathologists (to individualize care, which may include use of nonverbal support or alternative means of communication), developmental pediatricians (to help guide parents through appropriate behavior management strategies and individualized education plans), occupational therapists (to address fine motor impairments), and mental health specialists (to address issues such as anxiety and depression, which can occur).
Surveillance: To monitor existing manifestations, the individual's response to supportive care, and the emergence of new manifestations, the following evaluations are recommended: follow-up evaluations with standardized tests by a speech-language pathologist; review of educational progress/needs; review of mental health if anxiety and/or depression have been issues or have emerged as issues.
Genetic counseling.
FOXP2 -SLD is inherited in an autosomal dominant manner. About half of individuals diagnosed with FOXP2- SLD have the disorder as the result of a de novo pathogenic variant. If a parent of the proband has the FOXP2 pathogenic variant identified in the proband, the risk to sibs of inheriting the pathogenic variant is 50%. Once the FOXP2 pathogenic variant has been identified in an affected family member, prenatal and preimplantation genetic testing are possible.
- GeneReview Scope
This chapter addresses the core phenotype (speech and language disorder) and additional (variable) findings associated with intragenic FOXP2 pathogenic variants. The generally more severe phenotype associated with large copy number variants (i.e., contiguous gene deletions), structural variants (i.e., translocations or inversions) , or maternal uniparental disomy of chromosome 7 involving FOXP2 and additional adjacent genes – referred to in this GeneReview as FOXP2 -plus-related disorder – is outside the scope of this chapter. (See also Genetically Related Disorders .)
No consensus clinical diagnostic criteria for FOXP2 -related speech and language disorder ( FOXP2- SLD) have been published.
Suggestive Findings
FOXP2- SLD should be suspected in a child with the following clinical findings and family history.
Clinical Findings
Childhood apraxia of speech (CAS) [ American Speech-Language-Hearing Association 2007 ] (also known as developmental verbal dyspraxia, verbal dyspraxia, or speech dyspraxia)
- Children with CAS have difficulties in automatically and accurately sequencing speech sounds into words with the correct prosody.
- The diagnosis of CAS is made by assessment by a speech-language pathologist (also known as a speech and language therapist in the UK or speech pathologist in Australia). CAS is challenging to diagnose in a child younger than age three years; speech development is delayed in these children, and thus key manifestations are typically not seen or able to be elicited until the child has acquired sufficient speech to complete the verbal assessment tasks.
Additional Clinical Findings
- Delayed speech development
- Poor oral-motor function (e.g., excessive drooling, early feeding difficulties)
- Oral-motor difficulties and/or oral-motor dyspraxia
- Receptive and expressive language impairment
- Low average IQ, typically with poorer verbal IQ compared to nonverbal IQ (and average nonverbal IQ reported in some)
- Reading and spelling impairment
- Fine and gross motor impairment
Family History
Family history is consistent with autosomal dominant inheritance (e.g., affected males and females in multiple generations). Absence of a known family history does not preclude the diagnosis.
Establishing the Diagnosis
The diagnosis of FOXP2- SLD is established in a proband with suggestive findings and a heterozygous pathogenic (or likely pathogenic) variant in FOXP2 identified by molecular genetic testing (see Table 1 ).
Note: (1) Per ACMG/AMP variant interpretation guidelines, the terms "pathogenic variants" and "likely pathogenic variants" are synonymous in a clinical setting, meaning that both are considered diagnostic and both can be used for clinical decision making [ Richards et al 2015 ]. Reference to "pathogenic variants" in this section is understood to include any likely pathogenic variants. (2) Identification of a heterozygous FOXP2 variant of uncertain significance does not establish or rule out the diagnosis.
Molecular genetic testing approaches can include a combination of gene-targeted testing (multigene panel, single-gene testing) and comprehensive genomic testing (exome sequencing, genome sequencing). Gene-targeted testing requires the clinician to determine which gene(s) are likely involved (see Option 1 ), whereas genomic testing may not (see Option 2 ).
Single-gene testing. Sequence analysis of FOXP2 is performed first to detect small intragenic deletions/insertions and missense, nonsense, and splice site variants. Note: Depending on the sequencing method used, single-exon, multiexon, or whole-gene deletions/duplications may not be detected. If no variant is detected by the sequencing method used, the next step is to perform gene-targeted deletion/duplication analysis to detect exon and whole-gene deletions or duplications.
An inherited disorders of speech delay and developmental delay / intellectual disability multigene panel that includes FOXP2 and other genes of interest (see Differential Diagnosis ) is most likely to identify the genetic cause of the condition while limiting identification of variants of uncertain significance and pathogenic variants in genes that do not explain the underlying phenotype. Note: (1) The genes included in the panel and the diagnostic sensitivity of the testing used for each gene vary by laboratory and are likely to change over time. (2) Some multigene panels may include genes not associated with the condition discussed in this GeneReview . (3) In some laboratories, panel options may include a custom laboratory-designed panel and/or custom phenotype-focused exome analysis that includes genes specified by the clinician. (4) Methods used in a panel may include sequence analysis, deletion/duplication analysis, and/or other non-sequencing-based tests.
For an introduction to multigene panels click here . More detailed information for clinicians ordering genetic tests can be found here .
Exome sequencing is most commonly used; genome sequencing is also possible.
For an introduction to comprehensive genomic testing click here . More detailed information for clinicians ordering genomic testing can be found here .
Molecular Genetic Testing Used in FOXP2- Related Speech and Language Disorder
View in own window
| Gene | Method | Proportion of Probands with a Pathogenic Variant Detectable by Method |
|---|---|---|
| Sequence analysis | ~70% | |
| Gene-targeted deletion/duplication analysis | ~30% |
See Table A. Genes and Databases for chromosome locus and protein.
See Molecular Genetics for information on variants detected in this gene.
Sequence analysis detects variants that are benign, likely benign, of uncertain significance, likely pathogenic, or pathogenic. Variants may include small intragenic deletions/insertions and missense, nonsense, and splice site variants; typically, exon or whole-gene deletions/duplications are not detected. For issues to consider in interpretation of sequence analysis results, click here .
Data derived from the subscription-based professional view of Human Gene Mutation Database [ Stenson et al 2020 ]
Gene-targeted deletion/duplication analysis detects intragenic deletions or duplications. Methods used may include a range of techniques such as quantitative PCR, long-range PCR, multiplex ligation-dependent probe amplification (MLPA), and a gene-targeted microarray designed to detect single-exon deletions or duplications. This percentage is an estimate derived from data regarding intragenic deletion and duplications as well as whole gene deletions from the Human Gene Mutation Database [ Stenson et al 2020 ]. This may not necessarily distinguish between large intragenic deletions and contiguous gene deletions involving FOXP2 ( FOXP2 -plus-related disorder). Thus, if the individual's phenotype suggests FOXP2 -plus-related disorder, a chromosomal microarray is recommended.
- Clinical Characteristics
Clinical Description
Available evidence to date suggests that FOXP2- related speech and language disorder ( FOXP2- SLD) is caused by heterozygous FOXP2 pathogenic variants (including whole- or partial-gene deletions). FOXP2- SLD has a core phenotype: childhood apraxia of speech (CAS), a disorder of speech motor programming or planning that affects the production, sequencing, timing, and stress of sounds, and the accurate sequencing of speech sounds into syllables and syllables into words. In addition, CAS interferes nonselectively with multiple other aspects of language, including phonology, grammar, and literacy.
The interactions between these communication disorder subtypes are not well understood. Language and literacy difficulties may be influenced by or even result from CAS, or these phenotypes may actually be features of the same broad communication disorder.
Additional findings in FOXP2- SLD can include oral-motor dyspraxia (difficulty planning or programming oral movements on command); dysarthria (a neuromuscular-based speech disorder that may affect nasal resonance, voice quality, prosody, and breath support for speech); moderate-to-severe receptive and expressive language disorder; reading and spelling impairments; and fine motor difficulties. "Autistic features" or a diagnosis of autism spectrum disorder have been reported in a quarter of affected individuals. Typically mild dysmorphology has been reported in a few individuals.
In FOXP2- SLD nonverbal IQ (performance) is typically relatively preserved compared to verbal IQ; gross motor skills are delayed or impaired in the early years of development.
Sleep issues are present in some.
To date, FOXP2- SLD has been described in approximately 30 families (see Supplementary Table 1 in Morison et al [2022] for a summary of all reported families). The following description is based on the findings in these families.
Childhood apraxia of speech. First words typically appear between ages 18 months and seven years in children with FOXP2- SLD [ Vargha-Khadem et al 1995 , MacDermot et al 2005 , Laffin et al 2012 , Reuter et al 2017 , Morison et al 2022 ]. FOXP2- SLD is typically diagnosed around age three to four years, but may be considered earlier when the family history is positive.
In the first decade of life, speech is highly unintelligible, even to close friends and family. Although speech development and intelligibility improve over time, speech never develops to the same level as age-matched peers, and intelligibility may remain reduced in adulthood [ Morison et al 2022 ]. In contrast, for typically developing children, speech sound acquisition is mastered by around age eight years [ Dodd et al 2003 ], with intelligibility as high as 97% as early as age three years [ Flipsen 2006 ].
Although CAS comprises certain core features, it is important to note that the severity and features of CAS change across the life span [ Royal College of Speech and Language Therapists 2011 ], and while referred to as "core" features, they are not necessarily present in all individuals with CAS [ American Speech-Language-Hearing Association 2007 ]. Core features, agreed upon by a consensus panel, include the following:
- Inconsistent speech errors (e.g., producing the same syllable or word differently across repetitions of the same word, such as "ubella," "umbrella," and "umbarella" for umbrella)
- Lengthened and disrupted coarticulatory transitions (e.g., oral groping behaviors during speech; difficulty sequencing phonemes and syllables; difficulty maintaining syllable integrity; hypernasality, thought to be due to incoordination of the velum for denoting oral-nasal contrasts; slowed and disrupted diadochokinetic sequences, e.g., when asked to repeat "pa-ta-ka")
- Inappropriate prosody (e.g., lexical stress errors, equal stress across words giving a robotic-sounding presentation)
In addition, children with CAS tend to lag behind their peers in acquiring the sounds of their language system; hence, their phonetic inventory may be reduced for the child's age. Children with CAS may use a more restricted range of consonants and vowels than age-matched peers. For example, they will simplify syllable shapes, reducing a consonant-consonant-vowel (CCV) shape (e.g., "sta") or a consonant-consonant-consonant-vowel (CCCV) shape ("stra") to a consonant-vowel (CV) shape ("sa").
Although CAS is distinct from other speech disorders (e.g., stuttering, phonologic disorder) and language disorders (e.g., developmental language disorder), these additional diagnoses can co-occur with CAS [ Morison et al 2022 ].
Additional common speech- and language-related comorbidities in FOXP2- SLD can include the following, irrespective of underlying genetic alteration:
- Oral-motor dyspraxia, an inability or difficulty in planning or programming of oral movements on command, including single movements in isolation (e.g., commands such as "blow," "bite," "stick out your tongue") or sequences of oral movements (e.g., commands such as "bite and blow," "touch your bottom lip with your tongue and then blow a kiss"). Both oral dyspraxia [ Vargha-Khadem et al 1998 , Alcock et al 2000 , Lai et al 2000 , MacDermot et al 2005 , Turner et al 2013 ] and more general oral-motor deficits (e.g., difficulty performing isolated tongue movements) have been reported in FOXP2- SLD [ Morison et al 2022 ].
- Dysarthria is infrequent relative to CAS and phonologic errors [ Morison et al 2022 ]. Typical dysarthric features include hypernasality, impaired laryngeal quality, and difficulties modulating pitch and loudness [ Turner et al 2013 , Morison et al 2022 ].
- Moderate-to-severe receptive and expressive language disorder [ Vargha-Khadem et al 1995 , Morison et al 2022 ]. Expressive language is usually poorer than receptive language, with expressive language likely confounded by the presence of CAS. Impaired performance across both semantic and syntactic language domains has been reported in FOXP2- SLD [ Watkins et al 2002 , Vargha-Khadem et al 2005 , Turner et al 2013 , Morison et al 2022 ]. Affected semantic domains include naming accuracy and lexical decision making; affected syntactic domains include past tense production for regular and irregular verbs.
- Reading and spelling impairments are typically evident once literacy develops [ Vargha-Khadem et al 2005 ]. Difficulties with real word and nonword reading, spelling, and phonologic awareness skills are common in FOXP2- SLD [ Watkins et al 2002 , Turner et al 2013 , Morison et al 2022 ].
Other features of FOXP2- SLD
- IQ. Generally stronger nonverbal (performance) IQ compared to verbal IQ [ Vargha-Khadem et al 1995 , Watkins et al 2002 , Turner et al 2013 , Reuter et al 2017 , Morison et al 2022 ], although both verbal and nonverbal (performance) IQ may be impaired [ Watkins et al 2002 , Reuter et al 2017 , Morison et al 2022 ].
- Fine or gross motor impairments are highly prevalent in the early years of life but typically improve and even resolve with physiotherapy and occupational therapy input. These motor impairments are relatively mild compared to the marked speech production deficits [ Lai et al 2000 , Morison et al 2022 ].
- Autism spectrum disorder or features of autism have been observed in about 25% of affected individuals [ Reuter et al 2017 , Morison et al 2022 ].
- Mental health. Some individuals report anxiety and depression, but it is not clear whether this is part of FOXP2 -SLD or occurs secondary to the other communication and developmental challenges [ Morison et al 2022 ].
- Mild physical features or dysmorphology have been reported in a small number of individuals, although no clear pattern has been identified [ Morison et al 2022 ]. Physical features have included: high-arched palate; horizontal eyebrows; ear features (i.e., simply folded ears, prominent ears, anteverted ears); periorbital fullness; nasal features (i.e., upturned nose, prominent nose, hypoplastic alae nasi, high nasal root, rounded, fleshy, or prominent nasal tip); short/flat philtrum; prominent eyes; retrognathia; full lips or thin upper lip [ Reuter et al 2017 , Morison et al 2022 ]. In individual instances, mild finger pads, tapering fingers, single palmar crease, and clinodactyly were also noted [ Morison et al 2022 ]. Submucous cleft palate was reported in one individual [ Liégeois et al 2016 ].
Neuroimaging. Routine clinical brain MRIs of individuals with FOXP2 -SLD typically appear normal on visual inspection [ Vargha-Khadem et al 1998 ].
Genotype-Phenotype Correlations
The specific genetic alteration responsible for FOXP2 -SLD does not predict clinical severity.
Of note, large copy number abnormalities and more complex variants (including deletions, translocations, and inversions) affecting one allele also lead to speech and language issues as well as other features similar to FOXP2- plus-related disorder (see Genetically Related Disorders ).
The penetrance for FOXP2 -SLD is high – close to 100% – based on the findings in individuals reported to date [ Morison et al 2022 ].
Nomenclature
Prior to the discovery of causative pathogenic variants in FOXP2 , the locus "speech language disorder-1 (SPCH1)" was assigned to the chromosome region linked to the CAS phenotype [ Fisher et al 1998 ] .
FOXP2- SLD may also be referred to as " FOXP2 -only speech and language disorder" to distinguish the condition from FOXP2 -plus-related disorder (a generally more severe phenotype associated with large copy number variants, structural variants, or maternal uniparental disomy of chromosome 7 involving FOXP2 and additional adjacent genes; see Genetically Related Disorders ).
"Speech and language impairment" is a synonymous term for "speech and language disorder." The term "speech and language delay" should be avoided unless there is a clear clinical justification for use of this term, which implies a child will "catch up" to peers.
The population prevalence of CAS has not been determined by any epidemiologic study. The most commonly referenced estimate of prevalence is 1-2:1,000 [ Shriberg et al 1997 ].
In a cohort with a severe speech disorder, one of 49 individuals had a confirmed FOXP2 pathogenic variant [ MacDermot et al 2005 ].
Three recent studies performed molecular genetic testing on probands clinically diagnosed with CAS [ Eising et al 2019 , Hildebrand et al 2020 , Kaspi et al 2022 ]. Within these three cohorts (total: 121 individuals), none of the probands had a pathogenic FOXP2 variant. Hence, pathogenic FOXP2 variants are rare, even in cohorts selected for CAS.
- Genetically Related (Allelic) Disorders
No phenotypes other than those discussed in this GeneReview are known to be associated with germline intragenic FOXP2 pathogenic variants.
Large copy number variants (i.e., contiguous gene deletions), structural variants (i.e., translocations or inversions), or maternal uniparental disomy of chromosome 7 involving FOXP2 and additional adjacent genes are associated with a generally more severe phenotype referred to in this GeneReview as FOXP2 -plus-related disorder.
Like children with FOXP2 -related speech and language disorder ( FOXP2 -SLD), children with FOXP2 -plus-related disorder have childhood apraxia of speech (CAS), and their first words are reported to appear between ages 18 months and seven years [ Feuk et al 2006 , Zeesman et al 2006 , Lennon et al 2007 , Rice et al 2012 , Zilina et al 2012 ]. No data are available to determine what proportion of CAS is caused by disruption of FOXP2 only ( FOXP2 -SLD) or large copy number variants or structural variants involving FOXP2 ( FOXP2 -plus-related disorder) .
In addition to CAS, clinical features reported in FOXP2 -plus-related disorder include:
- Oral-motor deficits (commonly reported) [ Lennon et al 2007 , Laffin et al 2012 , Zilina et al 2012 ]
- "Global" developmental delay (presumably involving speech, cognitive, gross, and fine motor abilities) [ Feuk et al 2006 ]
- Autism spectrum disorder (ASD) [ Feuk et al 2006 , Zilina et al 2012 ] or presence of features of autism such as repetitive behaviors and unusual interests [ Zeesman et al 2006 ]. Of note, many individuals with FOXP2- SLD are explicitly stated not to meet diagnostic criteria for ASD [ Feuk et al 2006 , Lennon et al 2007 , Rice et al 2012 ], suggesting that these features may relate to disruption of neighboring genes on chromosome 7.
- Facial dysmorphology [ Zeesman et al 2006 , Lennon et al 2007 , Zilina et al 2012 , Reuter et al 2017 ]. Oral structures are typically intact in the absence of a cleft lip or palate, but a high-arched palate has been reported in one individual [ Palka et al 2012 ].
Sporadic tumors occurring as single tumors in the absence of any other findings of FOXP2 -SLD frequently harbor a somatic pathogenic variant in FOXP2 that is not present in the germline. In these circumstances predisposition to these tumors is not heritable.
- Differential Diagnosis
The prelinguistic developmental history of children with childhood apraxia of speech (CAS) (e.g., restricted babbling or feeding difficulties) is very similar to that seen in other neurodevelopmental speech or language conditions (e.g., developmental language disorder, phonologic disorder) or even other neurodevelopmental disorders in which language impairment may occur such as autism spectrum disorder. Hence, early signs are not usually sufficiently discriminating to enable a differential diagnosis prior to a child gaining some speech production abilities.
While CAS is rare, it may also be observed in a range of other conditions. The following chromosomal and single-gene disorders may be considered in the differential diagnosis.
Chromosomal disorders associated with CAS include:
- 16p11.2 recurrent deletion . The 16p11.2 recurrent deletion phenotype is characterized by motor speech disorder, language disorder (broadly impaired receptive, expressive, and pragmatic domains), motor coordination difficulties, psychiatric conditions, and autistic features. While most, if not all, individuals with the 16p11.2 recurrent deletion experience some degree of developmental delay, the severity varies significantly. The majority of children (~80%) with the 16p11.2 recurrent deletion present with a motor speech disorder, such as CAS and dysarthria. CAS is particularly prevalent (found in 77% of affected children) and often co-occurs with other speech sound disorders, such as articulation and phonologic disorders. Additional features include epilepsy or recurrent seizures and dysmorphic features (e.g., low-set ears, partial syndactyly).
- 7q11.23 duplication syndrome is characterized by delayed motor, speech, and social skills in early childhood; neurologic abnormalities (hypotonia, adventitious movements, and abnormal gait and station); speech sound disorders including motor speech disorders (CAS and/or dysarthria) and phonologic disorders; behavior issues (especially social anxiety disorder / social phobia); autism spectrum disorder; and, in some individuals, intellectual disability. Distinctive craniofacial features (macrocephaly, brachycephaly, broad forehead, straight eyebrows, deep-set eyes, long eyelashes, broad nasal tip, low insertion of the columella, short philtrum, thin vermilion of the upper lip, high-arched palate, and minor ear anomalies) are common.
- Koolen-de Vries syndrome (KdVS) is characterized by developmental delay / intellectual disability, neonatal/childhood hypotonia, dysmorphisms, congenital malformations, and behavioral features. Psychomotor developmental delay is noted in all individuals from an early age. Communication disorder is a core feature of KdVS, with a common speech and language phenotype seen. This includes an overriding "double hit" of oral hypotonia and apraxia in infancy and preschool, associated with severely delayed speech development. CAS is common in the preschool years. KdVS is caused by either a heterozygous 500- to 650-kb deletion at chromosome 17q21.31 that includes KANSL1 (~95% of affected individuals) or a heterozygous intragenic pathogenic variant in KANSL1 . Note: The 17q21.31 deletion cannot be identified by analysis of G-banded chromosomes or other cytogenetic banding techniques.
Single-gene disorders with robust evidence of CAS involvement are summarized in Table 2 .
Single-Gene Disorders with Childhood Apraxia of Speech in the Differential Diagnosis of FOXP2- Related Speech and Language Disorder
| Gene | Disease Name | MOI | Speech & Language Phenotype | Other Features |
|---|---|---|---|---|
| -related disorder | AD | |||
| Snijders Blok-Campeau syndrome (OMIM ) | AD | |||
| -related neurodevelopmental disorder | XL | -NDD typically occurs in females & very rarely in males). | ||
| AR | ) | |||
| -related speech disorders & epilepsy | AD | Epilepsy (present in ~90% of affected persons) | ||
| AD | See . | See . | ||
| syndrome | AD | |||
| ( -related disorder) | AD | Severe speech & language acquisition delays or difficulties incl CAS | NDD w/wide spectrum of cognitive dysfunction, DD, hypotonia, ASD, & behavioral issues | |
| syndrome (See -Related Neurodevelopmental Disorders.) | AD | |||
| haploinsufficiency disorder | AD | |||
| -related neurodevelopmental disorder | AD | |||
| -related absent or severely delayed speech (See .) | AD | 22q13.3 deletions involving & pathogenic variants are known to be assoc w/Phelan-McDermid syndrome. In the authors' experience, children w/intragenic pathogenic variants are much more mildly affected than children w/22q13.3 deletions. | ||
| AD | ||||
| related syndrome | AD | |||
| related NDD w/impaired speech & hyperkinetic movements (OMIM ) | AR | Speech impairment ranging from severely affected (minimally verbal) to verbal (w/CAS) |
AD = autosomal dominant; ADHD = attention-deficit/hyperactivity disorder; AR = autosomal recessive; ASD = autism spectrum disorder; CAS = childhood apraxia of speech; DD = developmental delay; ID = intellectual disability; MOI = mode of inheritance; NDD = neurodevelopmental disorder
Snijders Blok et al [2018] , Eising et al [2019]
Kaspi et al [2022]
Shriberg et al [2011]
A heterozygous intragenic pathogenic variant in KANSL1 is identified in ~5% of affected individuals. Most individuals with Koolen-de Vries syndrome have the disorder as the result of a heterozygous deletion at chromosome 17q21.31 that includes KANSL1 [ St John et al 2022b ].
St John et al [2022a]
Author, personal observation; Brignell et al [2021]
Eising et al [2019] , Granadillo et al [2020]
Khan et al [2019] , Christensen et al [2022] , Kamal et al [2022]
No clinical practice guidelines for FOXP2- related speech and language disorder ( FOXP2- SLD) have been published.
Evaluations Following Initial Diagnosis
To establish the extent of disease and management needs for an individual with FOXP2- SLD, the following evaluations conducted by a trained and specialized speech-language pathologist are recommended:
- Detailed developmental history including early oral-motor and feeding abilities, speech sound development, motor milestones, and cognitive development
- Family history of speech disorder
- Oral-facial structural examination to determine if any structural abnormalities are present
- Speech sound assessment including a test of single words, sounds in isolation, and connected speech to determine the child's phonetic inventory (i.e., has the child acquired age-appropriate speech sounds) and to determine if the child has phonologic errors, apraxic errors, dysfluency (stuttering), dysarthric errors, or a combination of these speech disorder diagnoses. The presence of resonance or nasality deficits signals the need to consider whether structurally based velopharyngeal port incompetence is present by referral to an ear, nose, and throat specialist and possibly videopalatography.
- Examination of facial asymmetry, reduced or increased oral-facial tone, and/or poor coordination of neuromuscular oral movements (e.g., "try to lick your nose with your tongue," "move your tongue quickly side to side," "blow a kiss" [lip protrusion], "smile" [lip retraction], etc.)
- Examination for evidence of oral-motor dyspraxia (i.e., can the individual perform oral movements on command in isolation [e.g., "bite" or "blow"] or in sequence [e.g., "kiss and blow"; "kiss, blow, and bite"])
- Language assessment to determine the presence of receptive and/or expressive language impairments across the domains of semantics, syntax, and morphology
- Literacy assessment or preliteracy (phonologic awareness) for evidence of reading and spelling difficulties so that appropriate support can be arranged
Additional evaluations include the following:
- Referral to a neuropsychologist or clinical psychologist to determine the extent of any coexisting cognitive and learning impairments and to assess for the presence of behaviors associated with autism spectrum disorder, attention-deficit/hyperactivity disorder, anxiety, or depression
- Referral to a physical therapist if gross motor movement difficulties are reported and to an occupational therapist if fine motor movement difficulties are observed
- Consultation with a medical geneticist, certified genetic counselor, or certified advanced genetic nurse to inform affected individuals and their families about the nature, mode of inheritance, and implications of FOXP2 -SLD to facilitate medical and personal decision making
- Assessment of the need for family support (see Resources and Parent to Parent )
Treatment of Manifestations
There is no cure for FOXP2 -SLD.
Supportive care to improve quality of life, maximize function, and reduce complications is recommended. This ideally involves multidisciplinary care by specialists in relevant fields.
Speech and language disorder. A speech-language pathologist will utilize treatments targeted to the specific findings in an affected individual; thus, a thorough initial assessment to establish the extent of the condition and management needs for an individual is important.
No single recommended treatment exists. The optimal approach should be determined based on the individual's presentation, but guidance on CAS therapies is as follows [ American Speech-Language-Hearing Association 2007 , Royal College of Speech and Language Therapists 2011 , Murray et al 2014 , Murray et al 2015 , Morgan et al 2018 ].
- Consider evaluation for nonverbal support or alternative means of communication (e.g., augmentative and alternative communication [AAC]) for individuals with severe speech and expressive language difficulties. An AAC evaluation can be completed by a speech-language pathologist who has expertise in the area. The evaluation will consider cognitive abilities and sensory impairments to determine the most appropriate form of communication. AAC devices can range from low-tech, such as picture exchange communication, to high-tech, such as voice-generating devices. Contrary to popular belief, AAC devices do not hinder verbal development of speech, but rather support optimal speech and language development.
- In terms of verbal development, difficulties with motor planning (apraxia) are severe in the early years of life, and intensive evidence-based motor speech therapies should be applied [ Morgan et al 2018 ]. Early phonologic awareness tasks should be implemented to support speech and later literacy development. Therapies addressing both receptive and expressive semantics and grammar are also recommended. The optimal intervention will be tailored to the child's specific profile as it changes during development.
Developmental Delay / Intellectual Disability Management Issues
The following information represents typical management recommendations for individuals with developmental delay / intellectual disability in the United States; standard recommendations may vary from country to country.
Ages 0-3 years. Referral to an early intervention program is recommended for access to occupational, physical, speech-language, and feeding therapy as well as infant mental health services, special educators, and sensory impairment specialists. In the US, early intervention is a federally funded program available in all states that provides in-home services to target individual therapy needs.
Ages 3-5 years. In the US, developmental preschool through the local public school district is recommended and results from referral to Child Find programs. Before placement, an evaluation is made to determine needed services and therapies and an individualized education plan (IEP) is developed for those who qualify based on established motor, language, social, or cognitive delay. The early intervention program typically assists with this transition. Developmental preschool is center based; for children too medically unstable to attend, home-based services are provided.
All ages. Consultation with a developmental pediatrician is recommended to ensure the involvement of appropriate community, state, and educational agencies (US) and to support parents in maximizing quality of life. Some issues to consider:
- An IEP provides specially designed instruction and related services to children who qualify.
- IEP services will be reviewed annually to determine whether any changes are needed.
- Special education law requires that children participating in an IEP be in the least restrictive environment feasible at school and included in general education as much as possible, when and where appropriate.
- PT, OT, and speech services will be provided in the IEP to the extent that the need affects the child's access to academic material. Beyond that, private supportive therapies based on the affected individual's needs may be considered. Specific recommendations regarding type of therapy can be made by a developmental pediatrician.
- As a child enters the teen years, a transition plan should be discussed and incorporated in the IEP. For those receiving IEP services, the public school district is required to provide services until age 21.
- Vocational opportunities and programming including vocational rehabilitation should be considered early with a focus on achievement of meaningful employment
- A 504 plan (Section 504: a US federal statute that prohibits discrimination based on disability) can be considered for those who require accommodations or modifications such as front-of-class seating, assistive technology devices, classroom scribes, extra time between classes, modified assignments, and enlarged text.
- Developmental Disabilities Administration (DDA) enrollment is recommended. DDA is a US public agency that provides services and support to qualified individuals. Eligibility differs by state but is typically determined by diagnosis and/or associated cognitive/adaptive disabilities.
- Families with limited income and resources may also qualify for supplemental security income (SSI) for their child with a disability.
Fine motor dysfunction. Occupational therapy is recommended for difficulty with fine motor skills that affect adaptive function such as self-feeding, grooming, dressing, and writing.
Oral motor dysfunction . Feeding therapy (typically from a speech-language pathologist or occupational therapist) is recommended to help improve coordination of oral movement skills for feeding or sensory-related feeding issues using relevant approaches including postural modification and altering the consistency of food and fluid [ Morgan et al 2012 ]. Mothers may need support from a breastfeeding or lactation consultant in the early weeks or months of life.
Gross motor dysfunction. Physical therapy may be recommended for difficulty with crawling, walking, running, and building strength resulting from hypotonia.
Social/Behavioral Concerns
Children may qualify for and benefit from interventions used in treatment of autism spectrum disorder, including applied behavior analysis (ABA). ABA therapy is targeted to the individual child's behavioral, social, and adaptive strengths and weaknesses and typically performed one on one with a board-certified behavior analyst.
Consultation with a developmental pediatrician may be helpful in guiding parents through appropriate behavior management strategies or providing prescription medications, such as medication used to treat attention-deficit/hyperactivity disorder, when necessary.
Concerns about anxiety can be addressed by a developmental specialist or psychiatrist.
Surveillance
To monitor existing manifestations, the individual's response to supportive care, and the emergence of new manifestations, the following evaluations are recommended:
- Routine care by a general pediatrician
- Follow-up evaluations with standardized tests by a speech-language pathologist
- Review educational progress/needs
- Review mental health if anxiety and/or depression have been issues or have emerged as issues
Evaluation of Relatives at Risk
It is appropriate to clarify the genetic status of apparently asymptomatic sibs of an affected individual by molecular genetic testing for the FOXP2 pathogenic variant in the family to identify as early as possible those who would benefit from prompt evaluation by a speech-language pathologist and initiation of treatment.
See Genetic Counseling for issues related to testing of at-risk relatives for genetic counseling purposes.
Therapies Under Investigation
Search ClinicalTrials.gov in the US and EU Clinical Trials Register in Europe for access to information on clinical studies for a wide range of diseases and conditions. Note: There may not be clinical trials for this disorder.
- Genetic Counseling
Genetic counseling is the process of providing individuals and families with information on the nature, mode(s) of inheritance, and implications of genetic disorders to help them make informed medical and personal decisions. The following section deals with genetic risk assessment and the use of family history and genetic testing to clarify genetic status for family members; it is not meant to address all personal, cultural, or ethical issues that may arise or to substitute for consultation with a genetics professional . —ED.
Mode of Inheritance
FOXP2- related speech and language disorder ( FOXP2- SLD) is caused by a heterozygous pathogenic variant in FOXP2 and inherited in an autosomal dominant manner.
Risk to Family Members
Parents of a proband
- About half of individuals diagnosed with FOXP2- SLD have the disorder as the result of a de novo pathogenic variant. In a cohort of 17 families with FOXP2- SLD, eight individuals represented simplex cases (i.e., the only affected family member) and had the disorder as the result of a de novo pathogenic variant [ Morison et al 2022 ].
- About half of individuals diagnosed with FOXP2- SLD have an affected parent [ Morison et al 2022 ].
- If the proband appears to be the only affected family member, molecular genetic testing is recommended for the parents of the proband to confirm their genetic status and to allow reliable recurrence risk counseling.
- The proband has a de novo pathogenic variant.
- The proband inherited a pathogenic variant from a parent with germline (or somatic and germline) mosaicism. Note: Testing of parental leukocyte DNA may not detect all instances of somatic mosaicism and will not detect a pathogenic variant that is present in the germ cells only.
Sibs of a proband. The risk to the sibs of a proband depends on the genetic status of the proband's parents:
- If a parent of the proband has the FOXP2 pathogenic variant identified in the proband, the risk to the sibs of inheriting the pathogenic variant is 50%.
- The penetrance of FOXP2- SLD in heterozygous family members approaches 100% [ Morison et al 2022 ]. Although interfamilial variability is observed in FOXP2- SLD, minimal clinical variability is observed among affected family members [ Morison et al 2022 ].
- If the FOXP2 pathogenic variant found in the proband cannot be detected in the leukocyte DNA of either parent, the recurrence risk to sibs is slightly greater than that of the general population because of the possibility of parental mosaicism [ Morison et al 2022 ].
Offspring of a proband. Each child of an individual with FOXP2 -SLD has a 50% chance of inheriting the FOXP2 pathogenic variant.
Related Genetic Counseling Issues
See Management, Evaluation of Relatives at Risk for information on evaluating at-risk relatives for the purpose of early diagnosis and treatment.
Family planning
- The optimal time for determination of genetic risk and discussion of the availability of prenatal/preimplantation genetic testing is before pregnancy.
- It is appropriate to offer genetic counseling (including discussion of potential risks to offspring and reproductive options) to young adults who are affected or at risk of having a child with FOXP2- SLD.
Prenatal Testing and Preimplantation Genetic Testing
Once the FOXP2 pathogenic variant has been identified in an affected family member, prenatal and preimplantation genetic testing are possible.
Differences in perspective may exist among medical professionals and within families regarding the use of prenatal testing. While most centers would consider use of prenatal testing to be a personal decision, discussion of these issues may be helpful.
GeneReviews staff has selected the following disease-specific and/or umbrella support organizations and/or registries for the benefit of individuals with this disorder and their families. GeneReviews is not responsible for the information provided by other organizations. For information on selection criteria, click here .
- Apraxia Kids Phone: 412-785-7072 Email: [email protected] apraxia-kids.org
- Dyspraxia Foundation United Kingdom Phone: 01462 454986; 01462 454986 dyspraxiafoundation.org.uk
- Molecular Genetics
Information in the Molecular Genetics and OMIM tables may differ from that elsewhere in the GeneReview: tables may contain more recent information. — ED.
FOXP2-Related Speech and Language Disorder: Genes and Databases
| Gene | Chromosome Locus | Protein | Locus-Specific Databases | HGMD | ClinVar |
|---|---|---|---|---|---|
| .1 |
Data are compiled from the following standard references: gene from HGNC ; chromosome locus from OMIM ; protein from UniProt . For a description of databases (Locus Specific, HGMD, ClinVar) to which links are provided, click here .
OMIM Entries for FOXP2-Related Speech and Language Disorder ( View All in OMIM )
| SPEECH-LANGUAGE DISORDER 1; SPCH1 | |
| FORKHEAD BOX P2; FOXP2 |
Molecular Pathogenesis
FOXP2 encodes the Forkhead box protein P2, a transcription factor with zinc finger (residues 346 to 371) and DNA-binding (residues 504 to 594) functional domains. FOXP2 is thought to form homodimers (and heterodimers with FOXP1 or FOXP4 ) via a protein region that includes a leucine zipper and zinc finger. Forkhead box proteins are transcription factors that likely regulate hundreds of downstream target genes, some of which will be critical for development of speech and language. FOXP2 pathogenic missense variants, including those that disrupt the DNA-binding domain (e.g., p.Arg553His [ Vernes et al 2006 ]), produce abnormal gene products that cannot bind DNA targets properly, as shown by functional studies of some variants that cause FOXP2 -related speech and language disorder [ Deriziotis et al 2014 , Estruch et al 2018 , Hickey et al 2019 , den Hoed et al 2021 ].
Other pathogenic missense variants may be associated with dominant-negative effects, which to date have not been demonstrated in published reports.
Mechanism of disease causation. Loss of function, haploinsufficiency
FOXP2 -specific laboratory technical considerations. Missense variants that directly affect the DNA-binding domain are likely to be pathogenic, while missense variants outside this domain are unlikely to be pathogenic.
- Chapter Notes
Author Notes
Angela T Morgan is a speech pathologist with more than 25 years of experience in speech phenotyping in genetic conditions. She works closely with the coauthors of this review both in identifying genes that cause severe speech disorder and in characterizing speech and language in known genetic conditions.
- University of Melbourne profile
- Murdoch Children's Research Institute profile and publications
Prof Morgan ( [email protected] ) is actively involved in clinical research regarding individuals with speech disorder. She would be happy to communicate with persons who have any questions regarding diagnosis of genetic conditions related to severe speech presentations.
Prof Morgan ( [email protected] ) is also interested in hearing from clinicians treating families affected by severe speech disorder or speech apraxia in whom no causative variant has been identified through molecular genetic testing of the genes known to be involved in this group of disorders.
Contact Dr Simon Fisher ( [email protected] ) to inquire about review of FOXP2 variants of uncertain significance.
Acknowledgments
Thank you sincerely to the families who have taken part in our research. Thank you to Dr Christiane Zweier and Prof Faraneh Vargha-Khadem for past collaborative work in phenotyping individuals with FOXP2 -related speech and language disorder. AM, IS, MH, and SF received funding from the National Health and Medical Research Council Centre of Research Excellence (APP1116976).
Revision History
- 26 January 2023 (bp) Comprehensive update posted live
- 2 February 2017 (am) Revision: based on Reuter et al [2017]
- 23 June 2016 (bp) Review posted live
- 4 August 2015 (am, msh) Original submission
Literature Cited
- Alcock KJ, Passingham RE, Watkins KE, Vargha-Khadem F. Oral dyspraxia in inherited speech and language impairment and acquired dysphasia. Brain Lang. 2000; 75 :17–33. [ PubMed : 11023636 ]
- American Speech-Language-Hearing Association. Childhood apraxia of speech [Position Statement]. Available online . 2007. Accessed 1-17-23.
- Brignell A, Gu C, Holm A, Carrigg B, Sheppard DA, Amor DJ, Morgan AT. Speech and language phenotype in Phelan-McDermid (22q13.3) syndrome. Eur J Hum Genet. 2021; 29 :564–74. [ PMC free article : PMC8115660 ] [ PubMed : 33293697 ]
- Christensen MB, Levy AM, Mohammadi NA, Niceta M, Kaiyrzhanov R, Dentici ML, Al Alam C, Alesi V, Benoit V, Bhatia KP, Bierhals T, Boßelmann CM, Buratti J, Callewaert B, Ceulemans B, Charles P, De Wachter M, Dehghani M, D'haenens E, Doco-Fenzy M, Geßner M, Gobert C, Guliyeva U, Haack TB, Hammer TB, Heinrich T, Hempel M, Herget T, Hoffmann U, Horvath J, Houlden H, Keren B, Kresge C, Kumps C, Lederer D, Lermine A, Magrinelli F, Maroofian R, Vahidi Mehrjardi MY, Moudi M, Müller AJ, Oostra AJ, Pletcher BA, Ros-Pardo D, Samarasekera S, Tartaglia M, Van Schil K, Vogt J, Wassmer E, Winkelmann J, Zaki MS, Zech M, Lerche H, Radio FC, Gomez-Puertas P, Møller RS, Tümer Z. Biallelic variants in ZNF142 lead to a syndromic neurodevelopmental disorder. Clin Genet. 2022; 102 :98–109. [ PMC free article : PMC9546172 ] [ PubMed : 35616059 ]
- den Hoed J, Devaraiu K, Fisher SE. Molecular networks of the FOXP2 transcription factor in the brain. EMBO Rep. 2021; 22 :e52803. [ PMC free article : PMC8339667 ] [ PubMed : 34260143 ]
- Deriziotis P, O'Roak BJ, Graham SA, Estruch SB, Dimitropoulou D, Bernier RA, Gerdts J, Shendure J, Eichler EE, Fisher SE. De novo TBR1 mutations in sporadic autism disrupt protein functions. Nat Commun. 2014; 5 :4954. [ PMC free article : PMC4212638 ] [ PubMed : 25232744 ]
- Dodd B, Holm A, Hua Z, Crosbie S. Phonological development: a normative study of British English-speaking children. Clin Linguist Phon. 2003; 17 :617–43. [ PubMed : 14977026 ]
- Eising E, Carrion-Castillo A, Vino A, Strand EA, Jakielski KJ, Scerri TS, Hildebrand MS, Webster R, Ma A, Mazoyer B, Francks C, Bahlo M, Scheffer IE, Morgan AT, Shriberg LD, Fisher SE. A set of regulatory genes co-expressed in embryonic human brain is implicated in disrupted speech development. Mol Psychiatry. 2019; 24 :1065–78. [ PMC free article : PMC6756287 ] [ PubMed : 29463886 ]
- Estruch SB, Graham SA, Quevedo M, Vino A, Dekkers DHW, Deriziotis P, Sollis E, Demmers J, Poot RA, Fisher SE. Proteomic analysis of FOXP proteins reveals interactions between cortical transcription factors associated with neurodevelopmental disorders. Hum Mol Genet. 2018; 27 :1212–27. [ PubMed : 29365100 ]
- Feuk L, Kalervo A, Lipsanen-Nyman M, Skaug J, Nakabayashi K, Finucane B, Hartung D, Innes M, Kerem B, Nowaczyk MJ, Rivlin J, Roberts W, Senman L, Summers A, Szatmari P, Wong V, Vincent JB, Zeesman S, Osborne LR, Cardy JO, Kere J, Scherer SW, Hannula-Jouppi K. Absence of a paternally inherited FOXP2 gene in developmental verbal dyspraxia. Am J Hum Genet. 2006; 79 :965–72. [ PMC free article : PMC1698557 ] [ PubMed : 17033973 ]
- Fisher SE, Vargha-Khadem F, Watkins KE, Monaco AP, Pembrey ME. Localisation of a gene implicated in a severe speech and language disorder. Nat Genet. 1998; 18 :168–70. [ PubMed : 9462748 ]
- Flipsen P. Measuring the intelligibility of conversational speech in children. Clin Linguist Phon. 2006; 20 :303–12. [ PubMed : 16644588 ]
- Granadillo JL, Stegmann APA, Guo H, Xia K, Angle B, Bontempo K, Ranells JD, Newkirk P, Costin C, Viront J, Stumpel CT, Sinnema M, Panis B, Pfundt R, Krapels IPC, Klaassens M, Nicolai J, Li J, Jiang Y, Marco E, Canton A, Latronico AC, Montenegro L, Leheup B, Bonnet C. M Amudhavalli S, Lawson CE, McWalter K, Telegrafi A, Pearson R, Kvarnung M, Wang X, Bi W, Rosenfeld JA, Shinawi M. Pathogenic variants in TNRC6B cause a genetic disorder characterised by developmental delay/intellectual disability and a spectrum of neurobehavioural phenotypes including autism and ADHD. J Med Genet. 2020; 57 :717–24. [ PubMed : 32152250 ]
- Hickey SL, Berto S, Konopka G. Chromatin decondensation by FOXP2 promotes human neuron maturation and expression of neurodevelopmental disease genes. Cell Rep. 2019; 27 :1699–711.e9. [ PMC free article : PMC6794152 ] [ PubMed : 31067457 ]
- Hildebrand MS, Jackson VE, Scerri TS, Van Reyk O, Coleman M, Braden RO, Turner S, Rigbye KA, Boys A, Barton S, Webster R, Fahey M, Saunders K, Parry-Fielder B, Paxton G, Hayman M, Coman D, Goel H, Baxter A, Ma A, Davis N, Reilly S, Delatycki M, Liégeois FJ, Connelly A, Gecz J, Fisher SE, Amor DJ, Scheffer IE, Bahlo M, Morgan AT. Severe childhood speech disorder: gene discovery highlights transcriptional dysregulation. Neurology. 2020; 94 :e2148–e2167. [ PubMed : 32345733 ]
- Kamal N, Khamirani HJ, Mohammadi S, Dastgheib SA, Dianatpour M, Tabei SMB. ZNF142 mutation causes neurodevelopmental disorder with speech impairment and seizures: novel variants and literature review. Eur J Med Genet. 2022; 65 :104522. [ PubMed : 35618198 ]
- Kaspi A, Hildebrand MS, Jackson VE, Braden R, van Reyk O, Howell T, Debono S, Lauretta M, Morison L, Coleman MJ, Webster R, Coman D, Goel H, Wallis M, Dabscheck G, Downie L, Baker EK, Parry-Fielder B, Ballard K, Harrold E, Ziegenfusz S, Bennett MF, Robertson E, Wang L, Boys A, Fisher SE, Amor DJ, Scheffer IE, Bahlo M, Morgan AT. Genetic aetiologies for childhood speech disorder: novel pathways co-expressed during brain development. Mol Psychiatry. 2022. Epub ahead of print. [ PMC free article : PMC10208970 ] [ PubMed : 36117209 ]
- Khan K, Zech M, Morgan AT, Amor DJ, Skorvanek M, Khan TN, Hildebrand MS, Jackson VE, Scerri TS, Coleman M, Rigbye KA, Scheffer IE, Bahlo M, Wagner M, Lam DD, Berutti R, Havránková P, Fečíková A, Strom TM, Han V, Dosekova P, Gdovinova Z, Laccone F, Jameel M, Mooney MR, Baig SM, Jech R, Davis EE, Katsanis N, Winkelmann J. Recessive variants in ZNF142 cause a complex neurodevelopmental disorder with intellectual disability, speech impairment, seizures, and dystonia. Genet Med. 2019; 21 :2532–42. [ PMC free article : PMC6821592 ] [ PubMed : 31036918 ]
- Laffin JJ, Raca G, Jackson CA, Strand EA, Jakielski KJ, Shriberg LD. Novel candidate genes and regions for childhood apraxia of speech identified by array comparative genomic hybridization. Genet Med. 2012; 14 :928–36. [ PMC free article : PMC3563158 ] [ PubMed : 22766611 ]
- Lai CS, Fisher SE, Hurst JA, Levy ER, Hodgson S, Fox M, Jeremiah S, Povey S, Jamison DC, Green ED, Vargha-Khadem F, Monaco AP. The SPCH1 region on human 7q31: genomic characterization of the critical interval and localization of translocations associated with speech and language disorder. Am J Hum Genet. 2000; 67 :357–68. [ PMC free article : PMC1287211 ] [ PubMed : 10880297 ]
- Lennon PA, Cooper ML, Peiffer DA, Gunderson KL, Patel A, Peters S, Cheung SW, Bacino CA. Deletion of 7q31.1 supports involvement of FOXP2 in language impairment: clinical report and review. Am J Med Genet A. 2007; 143A :791–8. [ PubMed : 17330859 ]
- Liégeois FJ, Hildebrand MS, Bonthrone A, Turner SJ, Scheffer IE, Bahlo M, Connelly A, Morgan AT. Early neuroimaging markers of FOXP2 intragenic deletion. Sci Rep. 2016; 6 :35192. [ PMC free article : PMC5062117 ] [ PubMed : 27734906 ]
- MacDermot KD, Bonora E, Sykes N, Coupe AM, Lai CS, Vernes SC, Vargha-Khadem F, McKenzie F, Smith RL, Monaco AP, Fisher SE. Identification of FOXP2 truncation as a novel cause of developmental speech and language deficits. Am J Hum Genet. 2005; 76 :1074–80. [ PMC free article : PMC1196445 ] [ PubMed : 15877281 ]
- Morgan AT, Dodrill P, Ward EC. Interventions for oropharyngeal dysphagia in children with neurological impairment. Cochrane Database Syst Rev. 2012; 10 :CD009456. [ PubMed : 23076958 ]
- Morgan AT, Murray E, Liégeois FJ. 2018. Intervention for childhood apraxia of speech. Cochrane Database of Systematic Reviews. 30;5:CD006278. [ PMC free article : PMC6494637 ] [ PubMed : 29845607 ]
- Morison L, Meffert E, Stampfer M, Steiner-Wilke I, Vollmer B, Schulze K, Briggs T, Braden R, Vogel AP, Thompson-Lake D, Patel C, Blair E, Goel H, Turner S, Moog U, Riess A, Liegeois F, Koolen DA, Amor DJ, Kleefstra T, Fisher SE, Zweier C, Morgan AT. In-depth characterisation of a cohort of individuals with missense and loss-of-function variants disrupting FOXP2. J Med Gen. 2022. Epub ahead of print. [ PMC free article : PMC10314088 ] [ PubMed : 36328423 ]
- Murray E, McCabe P, Ballard KJ. A systematic review of treatment outcomes for children with childhood apraxia of speech. Am J Speech Lang Pathol. 2014; 23 :486–504. [ PubMed : 24686844 ]
- Murray E, McCabe P, Ballard KJ. A randomized controlled trial for children with childhood apraxia of speech comparing Rapid Syllable Transition treatment and the Nuffield Dyspraxia Programme - Third edition. J Speech Lang Hear Res. 2015;58:669-86. [ PubMed : 25807891 ]
- Palka C, Alfonsi M, Mohn A, Cerbo R, Guanciali Franchi P, Fantasia D, Morizio E, Stuppia L, Calabrese G, Zori R, Chiarelli F, Palka G. Mosaic 7q31 deletion involving FOXP2 gene associated with language impairment. Pediatrics. 2012; 129 :e183–8. [ PubMed : 22144704 ]
- Rice GM, Raca G, Jakielski KJ, Laffin JJ, Iyama-Kurtycz CM, Hartley SL, Sprague RE, Heintzelman AT, Shriberg LD. Phenotype of FOXP2 haploinsufficiency in a mother and son. Am J Med Genet A. 2012; 158A :174–81. [ PMC free article : PMC3319495 ] [ PubMed : 22106036 ]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17 :405–24. [ PMC free article : PMC4544753 ] [ PubMed : 25741868 ]
- Reuter MS, Riess A, Moog U, Briggs TA, Chandler KE, Rauch A, Stampfer M, Steindl K, Glaser D, Joset P, Study DDD, Krumbiegel M, Rabe H, Schulte-Mattler U, Bauer P, Beck-Wödl S, Kohlhase J, Reis A, Zweier C. FOXP2 variants in 14 individuals with developmental speech and language disorders broaden the mutational and clinical spectrum. J Med Genet. 2017; 54 :64–72. [ PubMed : 27572252 ]
- Royal College of Speech and Language Therapists. Policy statement on developmental verbal dyspraxia. Available online . 2011. Accessed 1-17-23.
- Shriberg LD, Aram DM, Kwiatkowski J. Developmental apraxia of speech: I. Descriptive and theoretical perspectives. J Speech Lang Hear Res. 1997; 40 :273–85. [ PubMed : 9130199 ]
- Shriberg LD, Potter NL, Strand EA. Prevalence and phenotype of childhood apraxia of speech in youth with galactosemia. J Speech Lang Hear Res. 2011; 54 :487–519. [ PMC free article : PMC3070858 ] [ PubMed : 20966389 ]
- Snijders Blok L, Rousseau J, Twist J, Ehresmann S, Takaku M, Venselaar H, Rodan LH, Nowak CB, Douglas J, Swoboda KJ, Steeves MA, Sahai I, Stumpel CTRM, Stegmann APA, Wheeler P, Willing M, Fiala E, Kochhar A, Gibson WT, Cohen ASA, Agbahovbe R, Innes AM, Au PYB, Rankin J, Anderson IJ, Skinner SA, Louie RJ, Warren HE, Afenjar A, Keren B, Nava C, Buratti J, Isapof A, Rodriguez D, Lewandowski R, Propst J, van Essen T, Choi M, Lee S, Chae JH, Price S, Schnur RE, Douglas G, Wentzensen IM, Zweier C, Reis A, Bialer MG, Moore C, Koopmans M, Brilstra EH, Monroe GR, van Gassen KLI, van Binsbergen E, Newbury-Ecob R, Bownass L, Bader I, Mayr JA, Wortmann SB, Jakielski KJ, Strand EA, Kloth K, Bierhals T, Roberts JD, Petrovich RM, Machida S, Kurumizaka H, Lelieveld S, Pfundt R, Jansen S, Deriziotis P, Faivre L, Thevenon J, Assoum M, Shriberg L, Kleefstra T, Brunner HG, Wade PA, Fisher SE, Campeau PM, et al. CHD3 helicase domain mutations cause a neurodevelopmental syndrome with macrocephaly and impaired speech and language. Nat Commun. 2018; 9 :4619. [ PMC free article : PMC6218476 ] [ PubMed : 30397230 ]
- Stenson PD, Mort M, Ball EV, Chapman M, Evans K, Azevedo L, Hayden M, Heywood S, Millar DS, Phillips AD, Cooper DN. The Human Gene Mutation Database (HGMD®): optimizing its use in a clinical diagnostic or research setting. Hum Genet. 2020; 139 :1197–207. [ PMC free article : PMC7497289 ] [ PubMed : 32596782 ]
- St John M, Amor DJ, Morgan AT. Speech and language development and genotype-phenotype correlation in 49 individuals with KAT6A syndrome. Am J Med Genet A. 2022a; 188 :3389–3400. [ PubMed : 35892268 ]
- St John M, van Reyk O, Koolen DA, de Vries BBA, Amor DJ, Morgan AT. Expanding the speech and language phenotype in Koolen-de Vries syndrome: late onset and periodic stuttering a novel feature. Eur J Hum Genet. 2022b. Epub ahead of print. [ PMC free article : PMC10172335 ] [ PubMed : 36529818 ]
- Turner SJ, Hildebrand MS, Block S, Damiano J, Fahey M, Reilly S, Bahlo M, Scheffer IE, Morgan AT. Small intragenic deletion in FOXP2 associated with childhood apraxia of speech and dysarthria. Am J Med Genet A. 2013; 161A :2321–6. [ PubMed : 23918746 ]
- Vargha-Khadem F, Gadian DG, Copp A, Mishkin M. FOXP2 and the neuroanatomy of speech and language. Nat Rev Neurosci. 2005; 6 :131–8. [ PubMed : 15685218 ]
- Vargha-Khadem F, Watkins K, Alcock K, Fletcher P, Passingham R. Praxic and nonverbal cognitive deficits in a large family with a genetically transmitted speech and language disorder. Proc Natl Acad Sci U S A. 1995; 92 :930–3. [ PMC free article : PMC42734 ] [ PubMed : 7846081 ]
- Vargha-Khadem F, Watkins KE, Price CJ, Ashburner J, Alcock KJ, Connelly A, Frackowiak RS, Friston KJ, Pembrey ME, Mishkin M, Gadian DG, Passingham RE. Neural basis of an inherited speech and language disorder. Proc Natl Acad Sci U S A. 1998; 95 :12695–700. [ PMC free article : PMC22893 ] [ PubMed : 9770548 ]
- Vernes SC, Nicod J, Elahi FM, Coventry JA, Kenny N, Coupe AM, Bird LE, Davies KE, Fisher SE. Functional genetic analysis of mutations implicated in a human speech and language disorder. Hum Mol Genet. 2006; 15 :3154–67. [ PubMed : 16984964 ]
- Watkins KE, Dronkers NF, Vargha-Khadem F. Behavioural analysis of an inherited speech and language disorder: comparison with acquired aphasia. Brain. 2002; 125 :452–64. [ PubMed : 11872604 ]
- Zeesman S, Nowaczyk MJ, Teshima I, Roberts W, Cardy JO, Brian J, Senman L, Feuk L, Osborne LR, Scherer SW. Speech and language impairment and oromotor dyspraxia due to deletion of 7q31 that involves FOXP2. Am J Med Genet A. 2006; 140 :509–14. [ PubMed : 16470794 ]
- Zilina O, Reimand T, Zjablovskaja P, Männik K, Männamaa M, Traat A, Puusepp-Benazzouz H, Kurg A, Ounap K. Maternally and paternally inherited deletion of 7q31 involving the FOXP2 gene in two families. Am J Med Genet A. 2012; 158A :254–6. [ PubMed : 22105961 ]
GeneReviews® chapters are owned by the University of Washington. Permission is hereby granted to reproduce, distribute, and translate copies of content materials for noncommercial research purposes only, provided that (i) credit for source ( http://www.genereviews.org/ ) and copyright (© 1993-2024 University of Washington) are included with each copy; (ii) a link to the original material is provided whenever the material is published elsewhere on the Web; and (iii) reproducers, distributors, and/or translators comply with the GeneReviews® Copyright Notice and Usage Disclaimer . No further modifications are allowed. For clarity, excerpts of GeneReviews chapters for use in lab reports and clinic notes are a permitted use.
For more information, see the GeneReviews® Copyright Notice and Usage Disclaimer .
For questions regarding permissions or whether a specified use is allowed, contact: ude.wu@tssamda .
- Cite this Page Morgan A, Fisher SE, Scheffer I, et al. FOXP2-Related Speech and Language Disorder. 2016 Jun 23 [Updated 2023 Jan 26]. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2024.
- PDF version of this page (548K)
In this GeneReview
Bulk download.
- Bulk download GeneReviews data from FTP
GeneReviews Links
- GeneReviews Glossary
- Resource Materials NEW FEATURE
- New in GeneReviews
- Author List
- For Current/Prospective Authors
- GeneReviews Personnel
- Download/Link to GeneReviews
Tests in GTR by Gene
Related information.
- OMIM Related OMIM records
- PMC PubMed Central citations
- PubMed Links to PubMed
- Gene Locus Links
Similar articles in PubMed
- Review WARS2 Deficiency. [GeneReviews(®). 1993] Review WARS2 Deficiency. Mroczek M, Busra A, Houlden H, Efthymiou S, Nagy S. GeneReviews(®). 1993
- Review GRIN2A-Related Disorders. [GeneReviews(®). 1993] Review GRIN2A-Related Disorders. Strehlow V, Myers KA, Morgan AT, Scheffer IE, Lemke JR. GeneReviews(®). 1993
- Review CSF1R-Related Disorder. [GeneReviews(®). 1993] Review CSF1R-Related Disorder. Dulski J, Sundal C, Wszolek ZK. GeneReviews(®). 1993
- Review Ataxia-Telangiectasia. [GeneReviews(®). 1993] Review Ataxia-Telangiectasia. Veenhuis S, van Os N, Weemaes C, Kamsteeg EJ, Willemsen M. GeneReviews(®). 1993
- Small intragenic deletion in FOXP2 associated with childhood apraxia of speech and dysarthria. [Am J Med Genet A. 2013] Small intragenic deletion in FOXP2 associated with childhood apraxia of speech and dysarthria. Turner SJ, Hildebrand MS, Block S, Damiano J, Fahey M, Reilly S, Bahlo M, Scheffer IE, Morgan AT. Am J Med Genet A. 2013 Sep; 161A(9):2321-6. Epub 2013 Aug 5.
Recent Activity
- FOXP2-Related Speech and Language Disorder - GeneReviews® FOXP2-Related Speech and Language Disorder - GeneReviews®
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

IMAGES
VIDEO
COMMENTS
The discovery of FOXP2 as the first gene implicated in speech and language disorders provided a window into the biology of speech and language. FOXP2 , a transcription factor, is involved in the downstream control of many other genes important for a huge range of biological processes.
The first gene to be implicated in a speech and language disorder was identified by the investigation of a large family affected by a distinctive form of speech impairment known as verbal dyspraxia. Verbal dyspraxia is characterized by difficulties in the control of orofacial muscles leading to a deficit in the production of fluent speech.
One notable success in this area was the discovery that heterozygous disruptions of the FOXP2 gene cause a rare mendelian speech and language disorder. 5-9 Point mutations and chromosomal ...
Two of these genes, FOXP2 and GRIN2A, are OMIM morbid genes strongly related to speech and language development. ATP2C 2 is identified as a susceptibility locus (OMIM #606711). To date, FOXP2 is well-known as monogenic cause for the autosomal dominant disorder Speech-language disorder 1 ( SPCH1; OMIM # 602081).
Vocal communication mediated by speech and language is a uniquely human trait, and has served an important evolutionary role in the development of our species. Deficits in speech and language functions can be of numerous types, including aphasia, stuttering, articulation disorders, verbal dyspraxia, and specific language impairment; language ...
A forkhead-domain gene is mutated in a severe speech and language disorder. Nature 2001;413:519-23. Article CAS Google Scholar
Speech apraxia. The first gene to be clearly implicated in a speech and language disorder was FOXP2.Disruptions of this gene cause a monogenic form of developmental verbal dyspraxia (DVD), also known as childhood apraxia of speech (CAS) [], characterized by problems with the learning and execution of coordinated movement sequences of the mouth, tongue, lips and soft palate [18, 19].
Developmental speech and language disorders cover a wide range of childhood conditions with overlapping but heterogeneous phenotypes and underlying etiologies. This characteristic heterogeneity hinders accurate diagnosis, can complicate treatment strategies, and causes difficulties in the identification of causal factors. Nonetheless, over the last decade, genetic variants have been identified ...
Until recently, the only gene that had been directly implicated in the etiology of speech and language disorders was the FOXP2 gene on chromosome 7q (OMIM 605317). In 2001, a study by Lai and colleagues [] implicated mutation of FOXP2 in a monogenic form of speech and language disorder found in a three-generation pedigree (the KE family) and in an unrelated individual with a chromosome ...
Rare genetic variants that disrupt speech development provide entry points for deciphering the neurobiological foundations of key human capacities. The value of this approach is illustrated by FOXP2, a transcription factor gene that was implicated in speech apraxia, and subsequently investigated using human cell-based systems and animal models.
The inheritance pattern of FOXP2-related speech and language disorder depends on its genetic cause.Mutations within the FOXP2 gene and deletions of genetic material from chromosome 7 that include FOXP2 have an autosomal dominant pattern of inheritance, which means one copy of the altered gene or chromosome in each cell is sufficient to cause the disorder.
Speech sound disorders (SSD) manifest as difficulties in phonological memory and awareness, oral motor function, language, vocabulary, reading, and spelling. Families enriched for SSD are rare ...
In this review, we summarize advances in the genetic investigation of stuttering, speech-sound disorder (SSD), specific language impairment (SLI), and developmental verbal dyspraxia (DVD). We discuss how the identification and study of specific genes and pathways, including , , , , and lysosomal enzymes, may advance our under-.
The first gene to be implicated in a speech and language disorder was identified by the investigation of a large family affected by a distinctive form of speech impairment known as verbal dyspraxia. Verbal dyspraxia is characterized by difficulties in the control of orofacial muscles leading to a deficit in the production of fluent speech.
Speech control. Children with Foxp2-associated apraxia tend to begin speaking later than other children, and their speech is often difficult to understand. The disorder is believed to arise from impairments in brain regions, such as the striatum, that control the movements of the lips, mouth, and tongue.
Methods: DNA samples were collected from 359 individuals for the genome-wide association study and from 12 severely affected individuals for whole exome sequencing. Multifaceted phenotypes, representing major domains of expressive language functioning, were derived from collected speech samples. Results: Gene-based analyses revealed a ...
We have studied a unique three-generation pedigree, KE, in which a severe speech and language disorder is transmitted as an autosomal-dominant monogenic trait. Our previous work mapped the locus responsible, SPCH1, to a 5.6-cM interval of region 7q31 on chromosome 7 (ref. 5). We also identified an unrelated individual, CS, in whom speech and ...
It is the first gene to be linked to an inherited form of language and speech disorder. 4 The discovery of a mutation in FOXP2 in a family with a speech and language disorder opens a new window to understanding of the genetic cascades and neural circuits that underlie speech and language via molecular approaches.
Prelock PA, Hutchins T, Glascoe FP (2008) Speech-language impairment: How to identify the most common and least diagnosed disability of childhood. The Medscape Journal of Medicine 10(6): 136. Google Scholar. Rinaldi S, Caselli MC, Cofelice V, et al. (2021) Efficacy of the treatment of developmental language disorder: A systematic review.
A speech sound disorder means difficulty producing and organizing certain sounds. SSDs can be broadly categorized into two types: articulation disorders and phonological disorders. Articulation disorders occur when there are difficulties in producing speech sounds, leading to challenges in pronouncing certain consonants or vowels.. Phonological disorders, on the other hand, involve patterns of ...
AAC is used by those with a wide range of speech and language impairments, including congenital impairments such as cerebral palsy, intellectual impairment, genetic disorders or autism, and acquired conditions such as amyotrophic lateral sclerosis, stroke, traumatic brain injury, or Parkinson's disease.
Call to schedule an evaluation and let us help you help your loved one to meet their full potential! Because You Can is a speech therapy center servicing adults and children. Evaluations and intensive therapy for speech and language disorders, dyslexia, AAC, aphasia, apraxia, articulation, receptive and expressive language delays/disorders ...
FOXP2-related speech and language disorder (FOXP2-SLD) is caused by heterozygous FOXP2 pathogenic variants (including whole- or partial-gene deletions). The core phenotype of FOXP2-SLD is childhood apraxia of speech (CAS), a disorder of speech motor programming or planning that affects the production, sequencing, timing, and stress of sounds, and the accurate sequencing of speech sounds into ...
TN 33 (08-23) DI 22510.055 Pediatric Consultative Examination (CE) Report Content Guidelines for Speech and Language (SL) Impairments in Children from Birth to Attainment of Age 3 . Use the following guidelines to provide the minimum content in a CE report for a child disability case. Each Disability Determination Service (DDS) will notify medical sources of any additional requirements.
TN 33 (08-23) DI 22510.060 Pediatric Consultative Examination (CE) Report Content Guidelines for Speech and Language (SL) Impairments in Children Age 3 and Older . Use the following guidelines to provide the minimum content in a CE report for a child disability case. Each Disability Determination Service (DDS) will notify medical sources of any additional requirements.
Angela Pizon-Moore is a speech-language pathologist in Tampa, FL. Practice Areas: • Speech Language Pathology • Apraxia • Articulation and Phonological Process Disorders • Augmentative Alternative Communication • Aural (re)habilitation • Cognitive-Communication Disorders • Fluency and fluency disorders • Language acquisition.