- Market Access US & International
- Market Access Europe
- MDR / IVDR Consulting
- IVD Admission Strategy
- AI Medical Devices
- And more…
- Quality management systems (ISO 13485)
- ISO 13485 audits
- Quality management representative
- Quality management system as a service
- Software (IEC 62304, FDA)
- Risk Management (ISO 14971)
- Clinical Evaluation
- Performance evaluation of IVDs
- Electrical Safety & IEC 60601
- Human Factors / Usability (IEC 62366 and FDA)
- FDA relevant documents
- Human Factors Research
- Safety and EMC test laboratory
- Biological safety
- Computer System Validation
- Seminar Overview
- Digital Transformation
- Medical Device University
- Post Market Tools
- Our Mission
- Certificates
- Our Customers
- Current Vacancies
- Why the Johner Institute?
- Regulatory Affairs
- Laboratory products for “Research Use…

Laboratory products for “Research Use Only” (RUO) – often a dangerous claim
Manufactures use the “Research Use Only” (RUO) label to declare that their products should not be used in diagnostic procedures. This enables them to avoid the time-consuming and costly documentation required for conformity-assessed in vitro diagnostic medical devices (CE-IVDs). Nevertheless, some medical laboratories still use RUO products in diagnostic procedures, sometimes even with the knowledge of the manufacturers. This can have consequences – not just for manufacturers and operators but for patients as well.
In this article, you will learn
- what the “Research Use Only” (RUO) label means,
- what the requirements for RUO products are,
- how to avoid legal problems, and
- what alternatives there are to RUO products.
1. “Research Use Only” – what does it mean?
Products labeled “For Research Use Only” are hardly subject to any regulatory controls. Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR) aims to distance itself from RUO products clearly:
The scope of application of this Regulation should be clearly delimited from other legislation concerning products, such as medical devices, general laboratory products and products for research use only. IVDR Foreword (7)
a) Institutions affected
The following institutions, in particular, use RUO products:
- Medical laboratories can utilize RUO products, but doing so designates them as the manufacturer, carrying all the associated consequences.
- If medical laboratories utilize RUO products for purposes beyond research, this can potentially render them liable for damages and subject to criminal liability in the worst-case scenario.
You can find more information on “Lab Developed Tests” in our article The EU regulates medical laboratories – Are Laboratory Developed Tests still allowed?
- Manufacturers can incorporate RUO products as components in their IVD, but they are subsequently responsible for ensuring the conformity of the end device with the IVDR. The RUO labeling of the component is not mandatory.
- If manufacturers designate their devices as “RUO,” the intended use of these devices must be interpreted accordingly and, if required, substantiated. For instance, reasonably foreseeable misuse should be taken into account. The RUO label should not be applied to the device as a mere “protective claim,” as this may result in legal consequences.
b) Definition
There is no standardized definition for “Research Use Only” (RUO) products. Generally, they can be understood as products designed for analysis intended solely for scientific research purposes, as the name implies. Their main distinction from medical devices lies in their inability to be used for medical purposes.
Nevertheless, the interpretation of “Research Use Only” varies between Europe and the USA.
Definition in Europe
In Europe, the MEDDEV 2.14/2 guidance document ( IVD Guidance: Research Use Only products – A guide for manufacturers and notified bodies ) provides a definition of RUOs. This guidance was written within the framework of the now obsolete Directive 98/79/EC on in vitro diagnostic medical devices (IVDD) and, in the absence of an up-to-date replacement, it can still be considered the state of the art.
MEDDEV 2.14/2 states:
“for a product to be categorized as an RUO product it must have no intended medical purpose or objective.” Source: MEDDEV 2.14/2 rev.1
This means that an RUO product must not have a medical purpose, even not a rudimentary one.
This also applies to tests developed in-house (Laboratory Developed Tests) that are only used in a health institution for research purposes.
The IVDR also addresses RUO products. Article 1 (3) a) of the IVDR excludes RUO products from its scope:
This Regulation does not apply to: (a) products for general laboratory use or research-use only products, unless such products, in view of their characteristics, are specifically intended by their manufacturer to be used for in vitro diagnostic examination; Source: IVDR, Article 1 (3) a)
Furthermore, Article 2 (45) specifies:
“A device intended to be used for research purposes, without any medical objective , shall not be deemed to be a device for performance study;” IVDR, Article 2 (45)
Devices for performance studies are:
“‘device for performance study ’ means a device intended by the manufacturer to be used in a performance study” IVDR, Article 2 (45)
The IVDR thus distinguishes RUO products from IVDs and products for performance studies. The EU regulation also highlights the lack of a medical intended purpose for RUO products.
Definition in the USA
In 2013, the FDA published a guidance document on RUOs entitled “ Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only .”
This guidance defines RUO products as follows:
“ An RUO product is an IVD product that is in the laboratory research phase of development and is being shipped or delivered for an investigation that is not subject to part 812” [NB: Part 812 concerns the provision of devices for performance evaluation purposes as a preliminary step to IVDs] FDA guidance “Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only”, Chapter III A
Some examples of products that the FDA believes fall into this research phase of development are:
- Tests that are in development to identify test kit methodology, necessary components, and analytes to be measured.
- Instrumentation, software, or other electrical/mechanical components under development to determine correct settings, subcomponents, subassemblies, basic operational characteristics, and possible use methods.
- Reagents under development to determine production methods, purification levels, packaging needs, shelf life, storage conditions, etc.
However, the FDA further specifies:
“FDA also recognizes that there are certain products, such as instruments, systems, and reagents that are labeled for research use only and intended for use in the conduct of nonclinical laboratory research with goals other than the development of a commercial IVD product […].” FDA guidance “Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only”, Chapter III A
And subsequently gives examples of such research purposes in which the product itself is not the subject of research.
The FDA thus sees two “types” of RUO products: First, IVD devices whose development is ongoing and which are themselves the subject of the research purpose, and second, products for nonclinical research.
In both cases, the FDA requires a clearly visible RUO label to be affixed to the products. The RUO label is intended to prevent use for clinical diagnostics, patient management, and other investigations with a medical purpose.
c) What are the consequences of using the “Research Use Only” label?
Normally, IVDs are subject to regulatory requirements (for example, according to the IVDR or FDA) based on their risk class.
However, RUO products do not fall within the definition of “in vitro diagnostic medical devices” given by the IVDR or the relevant FDA regulations . This means that these regulations do not apply to RUO products.
‘ In vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software or system, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations, derived from the human body, solely or principally for the purpose of providing information on one or more of the following:
(a) concerning a physiological or pathological process or state; (b) concerning congenital physical or mental impairments; (c) concerning the predisposition to a medical condition or a disease; (d) to determine the safety and compatibility with potential recipients; (e) to predict treatment response or reactions; (f) to define or monitoring therapeutic measures.
Specimen receptacles shall also be deemed to be in vitro diagnostic medical devices.
Source: Article 2 IVDR
“In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions, including a determination of the state of health, in order to cure, mitigate, treat, or prevent disease or its sequelae. Such products are intended for use in the collection, preparation, and examination of specimens taken from the human body.”
Source: 21 CFR 809.3
However, RUO products do not automatically fall entirely outside the regulatory scope in the EU. Depending on the product, they may still have to comply with requirements that are not specifically intended for IVDs (such as the REACH regulation for chemicals or the Machinery Directive ).
Read more about the Machinery Directive: Which parts apply to medical devices .
Since RUO products are subject to considerably fewer controls than IVDs, it is necessary to severely restrict their use. Therefore, in particular they may not be used to
- make diagnoses and
- conduct performance studies.
2. Use and misuse of “Research Use Only” labels
A) what should ruo products be used for.
As the name “For Research Use Only” indicates, products with RUO labeling are intended for research purposes only. RUO products are particularly attractive for the research sector due to the simplified process and lower hurdles for placing them on the market.
MEDDEV. 2.14/2 rev.1 provides a precise list of areas where RUO products may potentially be used:
- basic research
- pharmaceutical research
- better identification and quantification of individual chemical substances or ligands in biological specimens
- in-house manufacturing of so called “Laboratory Developed Tests” for research purposes
And of areas where the use of RUOs is expressly not permitted:
- use of raw materials which are labeled “For Research Use Only” but which are incorporated into a finished product
- so called “research use products” being tested against a comparator IVD product that bears the CE mark
- products for market studies/feasibility studies
These products can be assigned a medical purpose.
b) What RUO products are often used for
However, the low hurdles are also the reason why RUO products are often used for purposes they are not intended for. This poses significant dangers for manufacturers, operators, and patients.
Sale of RUO products to medical laboratories
RUO products are sold by manufacturers to medical laboratories. Although doctors sometimes also conduct research, this is not really the main purpose of a medical laboratory.
Therefore, when discussing sales with doctors, it should always be assumed that there is a medical reason behind the use of the product. This means that anyone who knowingly sells RUO products to medical laboratories is potentially under suspicion of using the pretext “For Research Use Only” to ignore an intended medical purpose and thus avoid responsibility for a medical device.
Avoid reference to any specific diagnostic procedures in your advertising materials for products that clearly do not have a medical purpose. You should always stay on the technical or purely analytical level.
Use of RUO products in medical laboratories
The issue of selling RUOs to medical laboratories is not limited to manufacturers alone. The laboratories themselves may also not be acting in line with their status as operators and may, as a result, be liable under certain circumstances.
- Medical laboratories are free to develop in-house tests themselves. In such cases, RUO products are often used in diagnostic procedures. The laboratory bears full responsibility for these tests. Even under the IVDD, MEDDEV 2.14/2 saw this topic critical. However, with the IVDR, the EU is explicitly placing more restrictions on the routine use of such Lab Developed Tests.
Read more in our article The EU regulates medical laboratories – Are Laboratory Developed Tests still allowed?
- Due to the low regulatory hurdles, purchasing RUO products is very affordable. As a result, medical laboratories prefer them over expensive CE-IVD devices if they can achieve the same level of performance. Nevertheless, the use of RUO products for purposes other than research, even in cases where they provide similar results, is not permitted.

3. Consequences of incorrect classification
Lack of controls can have a negative effect on quality. As a result, the relevant authorities (e.g., authorities during inspections) take a closer look at whether a product is actually intended “For Research Use Only.”
Manufacturers should also be aware that simply sticking an RUO label on a product does not on its own mean that the product no longer has to comply with requirements for IVDs that would otherwise apply.
The RUO status is determined solely by the actual intended use of a device. To this end, authorities (both European and FDA) also use marketing material or other information as evidence.
Manufacturers and operators who misuse the RUO label could face severe penalties, as such behavior can cause serious harm to patients or even the general public.
a) Consequences for manufacturers and operators
Improperly selling IVDs with an RUO label or using RUO products for purposes other than research is not a trivial offense. Manufacturers who intentionally conceal or attempt to conceal a diagnostic purpose behind the RUO label should anticipate legal consequences in Germany. The same applies for operators who misuse RUO products. There is the possibility of a fine or even prison sentences. In addition, there is potential liability for harm suffered by patients.
b) Consequences in the USA
There are also severe penalties in the USA. If an RUO label is deemed to have been incorrectly used for a product, the product would be considered misbranded under sections 502(a) and 502(o) of 21 US Code, 352(a), 352(o) [A1] and would be considered adulterated under section 501(f) of 21 US Code 351(f).
c) Consequences for patients
However, the consequences can be even worse for patients. After all, the regulatory requirements for IVDs aren’t just plucked out of thin air to annoy manufacturers and operators. The regulations are intended to protect patients against incorrect results and subsequent wrong decisions. False-negative results can lull patients into a false sense of security and an existing undetected disease may worsen. One example would be the metastasis of an undetected cancer due to a test not performing as intended.
Some incorrect diagnoses could even be so severe that they can cause the death of a lot of people: an undetected viral infection can cost many lives in the early stages of an epidemic or pandemic, as the coronavirus pandemic sadly demonstrated.
4. Alternatives to “Research Use Only” products
To avoid legal problems and risks to third parties, manufacturers and users should use general laboratory equipment as an alternative to RUO products.
There are laboratory products that obviously have no specific medical purpose, such as
- pure chemicals,
- culture media,
- reaction vessels,
- washing solutions,
- qPCR cycler,
- sequencers,
- centrifuges.
Read more on the topic here: General laboratory equipment: What manufacturers and laboratories need to know to avoid problems and unnecessary expense
5. Ways to protect yourself
Manufacturers, operators, and patients can take the following steps to avoid legal and other negative consequences when using RUO products:
a) Manufacturers
In the case of manufacturers, it is particularly important that they narrowly define the intended purpose of their product.
Analyte specific reagents should only be labeled as RUO products for specific non-medical purposes.
SARS-CoV-2 and its mutations: a test kit that uses specific primers and probes to distinguish the variants B.1.1.7 (alpha variant) and B.1.351 (beta variant) from the initial variant following a positive result may be an RUO product if it is only intended to be used to determine the prevalence of the variant in the population.
A specific intended purpose in this case would be: “ Intended solely for epidemiological research for the purpose of surveying the prevalence of SARS-CoV-2 variants in the general population. ”
If a medical laboratory subsequently, based on new findings, used this test to provide the best possible treatment for infection by a specific variant, this would be an off-label use. The laboratory would then be responsible for the test’s conformity.
Tip: Provided the manufacturer did not advertise the product with this clinical benefit, it would be adequately protected.
b) Operators
Operators should record exactly for what they use IVDs and RUO products.
Medical laboratories are operators of medical devices and IVDs and, therefore, are responsible for only using medical devices according to their intended purpose and in accordance with the generally accepted rules of the technology. This is stipulated in Section 4 of the German Medizinprodukte-Betreiberverordnung (MPBetreibV).
To be on the safe side, laboratories should keep a record of which medical devices and IVDs are in operation and routine use. This record should include a reference to the applicable test procedure and the intended purpose of the IVD.
This record can also be used to identify investigational procedures for which there are no adequate CE-IVDs available on the market. The lack of alternatives would justify the use of RUOs in validated processes as in-house IVD , provided that the laboratory verifies and demonstrates that the general safety and performance requirements and the additional requirements of Article 5(5) of the IVDR are met.
Read more about the requirements for LDTs in our article .
c) Patients
Patients lack the knowledge to recognize what is and isn’t an RUO on their own. They are often given little to no information about the test they are undergoing. So, patients should follow this basic rule: ask your doctor or pharmacist!
- Patients can ask for the complete test report from the laboratory so that they can get a second opinion in case of doubt. The report should also indicate which specific test was performed.
- Patients should inform themselves about how “well” or “poorly” a test works, as well as the benefit-risk ratio.
- In the future, patients and doctors will also be able to get information about medical devices from EUDAMED and use this information to decide whether or not the test was performed with certified and thus legally compliant IVDs.
6. Conclusion
In the opinion of the EU Commission and the FDA, products “For Research Use Only” have no place in diagnostics. To be used for diagnostic purposes, products have to go through the necessary controls. But these controls do not apply to RUO products.
Anyone who ignores this prohibition and uses or sells RUO products for purposes other than pure research is playing with fire. Manufacturers and operators run the risk of legal trouble and could even endanger patients’ health. Therefore, RUO products should only be used for research purposes. For other uses, manufacturers and operators should use the alternatives mentioned.
If you, as a manufacturer or medical laboratory, find that an RUO product is particularly well-suited for in vitro diagnostics, consider whether further development and conformity assessment to make it an IVD is worthwhile.
Thanks to Dr. Boris Handorn , lawyer and partner at PRODUKTKANZLEI , Augsburg, for his valuable input on this article.
Benefit from the support of our IVD experts:
- They will help you qualify your devices or examination procedures, for example, with in-house workshops on approval strategy and in-house IVDs.
- They provide you with expert opinions on the qualification of your device, which you can submit to your customers and/or notified bodies.
- They support you in all activities up to the “certification” of your device (e.g., performance evaluation) and beyond (e.g., post-market surveillance).
Or use our e-learning platform : Learn how to meet the regulatory requirements and get access to our IVD-specific templates and tutorials on how to get your device approved.
Change history
- 2024-02-01 Complete revision; section “The thing with analyte-specific reagents” removed; shortening of chapter 4 (deletion of subchapters a) to c)); reference to article on general laboratory equipment
- 2021-11-16 First publication
More Articles
Medical Device Regulation MDR (2017/745) Status 2024 August 27, 2024
Electronic instructions for use for medical devices (EU law) August 22, 2024
I cannot access the specific URL you provided. However, if it’s about “For Research Use Only (RUO)” in regulatory affairs, a comment could emphasize the critical role of clear labeling and compliance in ensuring safety and integrity in research settings, promoting transparency and trust in scientific practices.
Hi RRMA Global,
Thank you for the comment! There seems to have been a mismatch in the links. These should now all be correct.
Kind regards Tea Bodrusic
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Post comment
Notify me when new comments are added.
We need your consent before you can continue on our website. If you are under 16 and wish to give consent to optional services, you must ask your legal guardians for permission. We use cookies and other technologies on our website. Some of them are essential, while others help us to improve this website and your experience. Personal data may be processed (e.g. IP addresses), for example for personalized ads and content or ad and content measurement. You can find more information about the use of your data in our privacy policy . You can revoke or adjust your selection at any time under Settings .
- External Media
Individual Privacy Preferences
Cookie Details Privacy Policy Imprint
If you are under 16 and wish to give consent to optional services, you must ask your legal guardians for permission. We use cookies and other technologies on our website. Some of them are essential, while others help us to improve this website and your experience. Personal data may be processed (e.g. IP addresses), for example for personalized ads and content or ad and content measurement. You can find more information about the use of your data in our privacy policy . Here you will find an overview of all cookies used. You can give your consent to whole categories or display further information and select certain cookies.
Accept all Save
Essential cookies enable basic functions and are necessary for the proper function of the website.
Show Cookie Information Hide Cookie Information
| Name | |
|---|---|
| Provider | Owner of this website, |
| Purpose | Saves the visitors preferences selected in the Cookie Box of Borlabs Cookie. |
| Cookie Name | borlabs-cookie |
| Cookie Expiry | 1 Year |
Content from video platforms and social media platforms is blocked by default. If External Media cookies are accepted, access to those contents no longer requires manual consent.
| Accept | |
|---|---|
| Name | |
| Provider | Google Ireland Limited, Gordon House, Barrow Street, Dublin 4, Ireland |
| Purpose | Used to unblock YouTube content. |
| Privacy Policy | |
| Host(s) | google.com |
| Cookie Name | NID |
| Cookie Expiry | 6 Month |
Privacy Policy Imprint
An Introduction to Research Use Only (RUO)
In this blog, we recap our eBook, “An Introduction to Research Use Only (RUO)” – Click HERE to download the entire publication.
Learn how it differs from adjacent labels, the FDA and EU guidance, its appropriate use, and the consequences of mislabeling products RUO.
Introduction
In the complex world of medical device development, regulation, and distribution, finding the appropriate label to put on a device may not be simple. When is one label appropriate over another? Does a device need to go through additional testing, verification, or validation? And what are the consequences of using the wrong label? In this eBook, we’ll cover the differences between Research Use Only (RUO) and a medical device – although, it’s generally a very clear distinction.
Using the right language and label is critical to complying with best practices. This is why Regulatory Affairs works with the regulatory bodies to ensure that the limitations of the product are properly documented. In a rush to get products to market, it may be tempting to use a Research Use Only (RUO) label to avoid additional regulatory processes while still empowering other researchers and developers. However, there are risks to using the RUO label inappropriately that can have serious consequences for developers, users, and patients. In fact, mislabeling a product is illegal, and punishable. You can see an example warning letter the FDA sent to Carolina Liquid Chemistries Corp after finding intentional mislabeling in 2019 here.
This introduction will provide an overview of the Research Use Only label, how it differs from similar, adjacent labels, its appropriate use, and the consequences of mislabeling products RUO.
What is Research Use Only (RUO)?
The label Research Use Only (RUO) is generally used to indicate products that are intended for scientific research only. They cannot be used for diagnostic or medical purposes. However, there is no standard definition of “research use only,” and the label has slightly different meanings in the European Union and the United States. With the IVDR regulations, RUO products that are being used in the LDT space are going to be revisited and potentially reclassified as a medical device. With this new classification, teams will likely need to follow design controls, best practices, and industry standards.
What is the FDA guidance on Research Use Only products?
Under the FDA’s guidance issued in 2013 , a product labeled Research Use Only is an In Vitro Diagnostic (IVD) product “that is in the laboratory research phase of development and is being shipped or delivered for an investigation that is not subject to part 812.” The agency includes in this category:
- “Tests that are in development to identify test kit methodology, necessary components, and analytes to be measured.
- “Instrumentation, software, or other electrical/mechanical components under development to determine correct settings, subcomponents, subassemblies, basic operational characteristics, and possible use methods.
- “Reagents under development to determine production methods, purification levels, packaging needs, shelf life, storage conditions, etc.”
The European guidance document MEDDEV 2.14/2 states that a product categorized as an RUO product “must have no intended medical purpose or objective.” The guidance does exempt some tests developed for in-house use as long as the products are not sold to other companies. Some examples of items that can be classified as “research use only” under this exemption include PCR enzymes, gel component agars, and primers.
RELATED: FDA released new draft guidance of premarket submissions for medical devices – are you ready?
What is the difference between ruo and ivd.
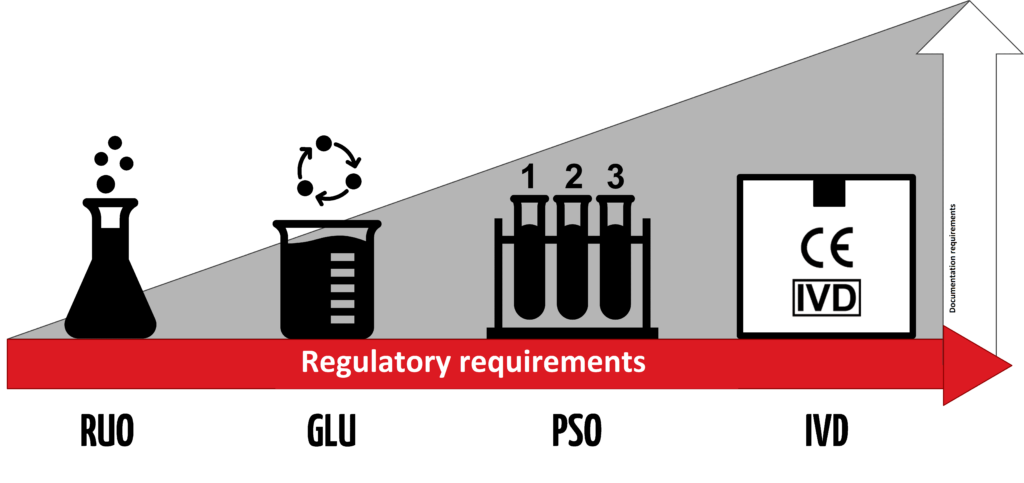
An IVD is an “In Vitro Diagnostic Medical Device,” and the general term applies to any device or product that either alone or with other products is intended to be used for diagnostic, monitoring, or compatibility purposes. There are four different regulatory levels for IVDs:
- Research Use Only (RUO)
- General Laboratory Use (GLU)
- For Performance Studies Only (PSO)
- In Vitro Diagnostic Medical Device (IVD)
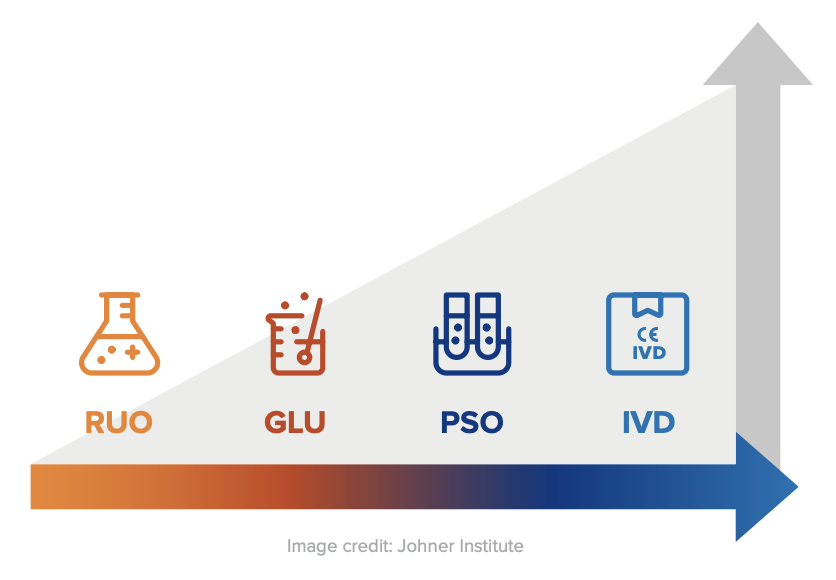
The simplest explanation for these different levels is that each increasing level requires more testing and oversight. Research Use Only products are at the lowest level of regulation, and In Vitro Diagnostic Medical Devices are at the highest level. Occasionally in the US, products will be labeled as “RUO IVD,” which means an in vitro device that is intended for research use only.
Products labeled with the “CE-IVD” label indicate that they have progressed through the applicable regulatory process and standards (such as IVDD or IVDR). These products are approved for diagnostic use and must include the IVD symbol to be used for medical purposes.
In the EU, as of May 2022, IVDs must comply with Regulation (EU) 2017/746 (IVDR) . The IVDR defines IVDs as follows:
“‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software or system, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations, derived from the human body, solely or principally for the purpose of providing information on one or more of the following:
(a) concerning a physiological or pathological process or state; (b) concerning congenital physical or mental impairments; (c) concerning the predisposition to a medical condition or a disease; (d) to determine the safety and compatibility with potential recipients; (e) to predict treatment response or reactions; (f) to define or monitoring therapeutic measures.”
All IVDs that comply with the IVDR must carry the CE Mark if marketed in the EU.
Research Use Only products are not subject to regulatory requirements in either the US or the EU, but because they don’t meet the same compliance standards as IVDs, they must be clearly labeled as RUO products and cannot be used for medical purposes.
A known exception is the lab developed test (LDT) pathway for clinical purposes.
What are the requirements for an RUO product?
In the US, RUO products are basically unregulated and do not need to meet any specific requirements to carry the RUO label. The FDA does not specify any restrictions or limitations on RUO products, provided they are clearly labeled “For Research Use Only. Not for use in diagnostic procedures.” For this reason, RUO products can be an excellent solution for laboratories that need research materials for testing and research purposes. Because products with the RUO label do not require extensive testing, verification, and validation, they tend to be more cost-effective for research purposes.
The EU rules are similar. Because RUO products do not have clinical applications, they are not considered medical devices, and there are no requirements for RUO products defined by either the IVDD or the IVDR. These products should not be marked with the IVD mark, and they should be clearly labeled as “Research Use Only.”
RELATED: See how Jama Software ® helped Össur improve the mobility of millions by replacing process rigidity with speed and agility.
Are there alternatives to ruo labels.
Given the significant differences between labeling a product as RUO and labeling a product as IVD, manufacturers and users can’t be too careful when it comes to assigning labels or using products for specific purposes. If there is a risk to using products labeled as RUO, manufacturers and users should opt for products that have attained a higher compliance level. For example, for a doctor’s office or home use, IVD is the right path. For clinical purposes or hospital labs, RUO could be used as LDT as long as they are CAP/CLIA certified, such was the case with COVID-19 testing kits when the pandemic first hit.
For products that meet a higher degree of compliance, it is possible to assign General Laboratory Use (GLU), Performance Studies Only (PSO), or even In Vitro Diagnostic Medical Device (IVD) labels. However, depending on the intended use for the Research Use Only products, pursuing these additional levels of compliance may or may not make sense.
What is CLIA certification?
CLIA stands for Clinical Laboratory Improvement Amendments. The Centers for Medicare & Medicaid Services (CMS) regulates all clinical laboratory testing performed on humans in the United States through CLIA.
What is a CAP accreditation?
CAP stands for The College of American Pathologists (CAP) . The purpose of CAP laboratory accreditation is to ensure laboratories provide precise test results for accurate patient diagnoses, meet CLIA and CAP requirements, and demonstrate compliance with professionally and scientifically sound and approved laboratory operating standards.
What are RUO products used for?
As the name implies, RUO projects should be used for research purposes only. They may be used for basic research, pharmaceutical research, or in-house manufacturing of “home brew kits” for research purposes and potentially for clinical applications via the LDT pathway. RUO products are specifically not to be used to make diagnoses, conduct performance studies, or as a substitute or comparator for a CE-IVD device. They may also not be used for market or feasibility studies. Raw ingredients labeled as RUO products may not be incorporated into a finished IVD product.
Learn more about the advantages and disadvantages of the RUO label (and more) by downloading the entire eBook HERE .
- Recent Posts
- [Webinar Recap] Key Systems Engineering Skills: Critical Thinking and Problem Framing - March 5, 2024
- Jama Connect® Features in Five: Medical Device & Life Sciences Solution 2.0 – Part 2 - July 28, 2023
- Jama Connect® Features in Five: Medical Device & Life Sciences Solution 2.0 – Part 1 - July 21, 2023
USA 135 SW Taylor Suite 200 Portland, Oregon, 97204
EUROPE Amsterdam Queens Tower Delflandlaan 1, 1062EA Amsterdam The Netherlands
© 2024 Jama Software
- JAMA CONNECT
- Product Overview
- Pricing and Licensing
- Why Jama Software®?
- Success Programs
- Education & Support
- Resource Library
- Discovery Center
- Guide to Requirements Management
- User Community
- Privacy Policy
- Privacy and Security
- Preferences
We use cookies to offer you a better browsing experience and analyse our website performance. We also use third-party cookies to further customise your experience showing relevant content while you are navigating on third-party platforms.

Our Priorities MedTech Europe strives to support our dynamic sector in meeting the needs of patients and health systems. To achieve this, we focus on engaging with healthcare stakeholders on key issues from regulations and market access to digital health and Brexit, among others.
- COVID-19 Information Hub
- Interactions with the Medical Community
- Access to Medical Technology
- Medical Technology Regulations
- Digital Health
- International
- Environmental and Social Sustainability
- Market Data
- Research and Innovation
- Innovative Health Initiative (IHI)
Sector Groups MedTech Europe sector groups bring together company experts to drive forward key healthcare domains, helping to address issues facing these sectors and shaping their future. We have dedicated groups focused on cardiovascular health, ophthalmology, diabetes, orthopaedics, and AMR/HAI.
- Antimicrobial Resistance (AMR) and Healthcare Associated Infections (HAIS)
- Cardiovascular
- Homecare & Community Care
- Orthopaedic

Real stories of people’s lives transformed by medtech.

Your platform for dialogue about medical technologies.
Search on this website
Research Use Only Products

What are Research Use Only (RUO) products? Research Use Only (RUO) products are a distinct category of in vitro diagnostics (IVDs) exclusively tailored for laboratory research. RUOs encompass specialised reagents, equipment, and materials crucial for scientific investigations, contributing significantly to the development of cutting-edge tools and solutions for research applications.
Research Use Only (RUO) products play a crucial role in medical research and innovative management of many patients. These specialised products, which include laboratory reagents and equipment, are exclusively designed for research in controlled laboratory environments. As essential tools for medical and scientific investigations, experimentation, and analysis, RUOs contribute to developing innovative solutions and advancements in medical research.
For example: RUO products can be used for Fundamental Research, in Pharmaceutical Research to find new drug compounds, and for a better identification and quantification of individual chemical substances. In diagnostics research, RUO products are essential to the development of new diagnostic assays and tools.
Unlike in vitro diagnostic medical devices (IVDs), RUOs are dedicated to facilitating research initiatives and are not intended for direct medical procedures with human patients. RUOs are not defined in the EU’s In Vitro Diagnostic Medical Devices Regulation 2017/746 (IVDR); they are regulated by the EU General Product Safety Regulation and other applicable EU legislations. Manufacturers of RUO products clearly label them as “Research Use Only” and use the RUO label.
From a production and specifications general perspective, the knowledge and processes needed to manufacture RUOs are very similar to those needed to manufacture CE marked IVDs. Many companies which operate in the IVD space will have RUO products in their portfolio. RUOs will generally have a similar chemical and physical composition compared to IVDs, but their intended purpose will be different. While RUO or IVDs might seem similar in their appearance and specifications, unambiguous and documented evidence associating the use of devices with in vitro diagnostic examination procedures is required to qualify a device as an IVD.
RUOs provide researchers and scientists – including those operating in medical laboratories – with valuable resources to advance in the understanding of disease, in drug discovery, in the development of new therapies and diagnostic tools. Laboratories or research consortia often collaborate with RUO manufacturers to tailor products to meet specific research needs and requirements, fostering a collaborative environment and contributing to the continuous evolution of research tools and solutions.
One critical application of RUO is to enable medical laboratories to develop in-house assays to e.g. diagnose rare and emerging conditions or to improve the current knowledge and management of specific diseases for which no adequate CE marked IVDs exist. This not only fulfils a critical and imminent healthcare need but is also a key stepping stone in the eventual development of IVDs. A poignant example of this was the development of COVID-19 assays during the early phase of the pandemic – initially, reference laboratories developed in house assays test for the SARS-CoV-2 virus, and shortly afterwards, commercial IVDs began to reach the market in order to fulfil a critical need during the global health crisis. However, it is worth noting that the use of in-house assays is regulated in IVDR and is subject to certain conditions.
In essence, RUO products provide researchers and physicians with the necessary tools to conduct experiments and studies, contributing to the overall progress in medical research. Their intended use in laboratory settings supports the development of new technologies and innovative solutions for various research applications.
Share this page
Facts & Figures
The 2024 report is out!
Sign up for your monthly newsletter
By clicking the Subscribe button, you give consent to MedTech Europe AISBL to use of the information you provided and send you content on the services you selected. We will ensure that the information is processed confidentially, and will only share it with third party providers that assist in providing these services. These providers may be located outside the EU; in this case, we will ensure that they are subject to a legal framework adequate in safeguarding your data, in compliance with European data protection law. You can unsubscribe, change your preferences or update your information at any time by clicking on the unsubscribe button available on all messages. For more information on how MedTech Europe will handle your personal data, please refer to our Privacy Policy . You can contact us at [email protected] for further questions related to your privacy and your rights.

Research Use Only or IVD: What’s Right for Your Lab?
by Tina Sobania | Clinical , Molecular

Publish Date: September 13, 2018
There are many misconceptions in the clinical industry regarding laboratory quality control materials. With numerous products available and manufacturers using various labeling practices, how do you know what’s best for your laboratory?
To help clear up the confusion, we’re answering two important questions clinical laboratorians have about quality control products.
Are diagnostic system controls IVDs?
One common misconception is that materials used for quality control of diagnostic systems are not themselves in vitro diagnostics (IVDs). However, the U.S. Food & Drug Administration (FDA) has written regulations citing quality control material as medical devices. For example, 21 CFR 862.1660 , Mulit-Analyte Controls Unassayed under Clinical Chemistry, and more recently 21 CFR 866.3920 , classify Class II controls requiring FDA 510(k) review under microbiology.
It’s important to understand that if a manufacturer for controls of nucleic acid amplification states its product works with a specific instrument or assay in its labeling or marketing literature, the FDA considers the material to be a Class II IVD and requires a 510(k) review . The FDA has established special controls for this type of material to ensure the product is properly labeled, performs according to claims and remains stable. In addition, IVD material must be manufactured under the FDA’s current Good Manufacturing Practices (cGMP).
Should “Research Use Only” products be used for quality control?
The second misconception clinical laboratories should be aware of involves material labeled as Research Use Only (RUO). RUO labeling is intended for products that are still under development and are not commercially distributed. A developer would use this labeling to ship product for “investigation relating to product development” as explained by the FDA in guidance document, Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigation Use Only .
Another factor one must consider is products labeled RUO are not required to be manufactured in accordance with cGMP and FDA Quality System Regulation. Lack of manufacturing controls may be detrimental to the quality of the control material. As such, clinical laboratories using RUO quality control materials to ensure the quality of testing may be placing patients at unnecessary risk.
Key Takeaway
To maintain the highest possible quality of your diagnostic testing, it’s best to choose materials that have been manufactured by a cGMP compliant facility under the FDA QSR, and when necessary reviewed by the FDA. Materials clearly labeled as IVDs provide that assurance and lower your laboratory’s risk.
Follow the links below to find all the FDA regulations cited in this post.
- 21 CFR 862.1660 CFR – Code of Federal Regulations Title 21, Subchapter H – Medical Devices
- 21 CFR 866.3920 CFR – Code of Federal Regulations Title 21, Subchapter H – Medical Devices
- Distribution of In Vitro Diagnostic Products Labeled for Research Use Only of Investigation Use Only
Written by Tina Sobania
You may also like.

MLS and MLT Career Opportunities
As a manufacturer of quality controls used in clinical diagnostics, we see firsthand the difficulties that laboratory...

The Challenges of Diagnosing and Treating Secondary Infections
In the past two decades, there have been six major global outbreaks of infectious diseases. While these infections may...
Trackbacks/Pingbacks
- Put Your Best Plate Forward – #CreepyCultures is Back! – Microbiologics Blog - […] Read Next – Research Use Only or IVD: What’s Right for Your Lab? […]
- Clinical Case File: Group A Streptococcus – Microbiologics Blog - […] Read Next – Research Use Only or IVD: What’s Right for Your Lab? […]
- Dear Stanley: Molecular QC Best Practices – Microbiologics Blog - […] Read Next – Research Use Only or IVD: What’s Right for Your Lab? […]
- Our Top Posts from 2018 – Microbiologics Blog - […] 1. Research Use Only or IVD: What’s Right for Your Lab? […]
- LDT Oversight Deliberation Continues – Microbiologics Blog - […] Read Next – Research Use Only or IVD: What’s Right for Your Lab? […]

- Pharmaceutical
- Events and Webinars
- Uncategorized

1-800-599-2847 microbiologics.com [email protected]

QUICK LINKS
CATEGORIES RESOURCES ABOUT US CONTACT US SITE MAP PRIVACY POLICY
- Environment
- Science & Technology
- Business & Industry
- Health & Public Welfare
- Topics (CFR Indexing Terms)
- Public Inspection
- Presidential Documents
- Document Search
- Advanced Document Search
- Public Inspection Search
- Reader Aids Home
- Office of the Federal Register Announcements
- Using FederalRegister.Gov
- Understanding the Federal Register
- Recent Site Updates
- Federal Register & CFR Statistics
- Videos & Tutorials
- Developer Resources
- Government Policy and OFR Procedures
- Congressional Review
- My Clipboard
- My Comments
- My Subscriptions
- Sign In / Sign Up
- Site Feedback
- Search the Federal Register
This site displays a prototype of a “Web 2.0” version of the daily Federal Register. It is not an official legal edition of the Federal Register, and does not replace the official print version or the official electronic version on GPO’s govinfo.gov.
The documents posted on this site are XML renditions of published Federal Register documents. Each document posted on the site includes a link to the corresponding official PDF file on govinfo.gov. This prototype edition of the daily Federal Register on FederalRegister.gov will remain an unofficial informational resource until the Administrative Committee of the Federal Register (ACFR) issues a regulation granting it official legal status. For complete information about, and access to, our official publications and services, go to About the Federal Register on NARA's archives.gov.
The OFR/GPO partnership is committed to presenting accurate and reliable regulatory information on FederalRegister.gov with the objective of establishing the XML-based Federal Register as an ACFR-sanctioned publication in the future. While every effort has been made to ensure that the material on FederalRegister.gov is accurately displayed, consistent with the official SGML-based PDF version on govinfo.gov, those relying on it for legal research should verify their results against an official edition of the Federal Register. Until the ACFR grants it official status, the XML rendition of the daily Federal Register on FederalRegister.gov does not provide legal notice to the public or judicial notice to the courts.
Design Updates: As part of our ongoing effort to make FederalRegister.gov more accessible and easier to use we've enlarged the space available to the document content and moved all document related data into the utility bar on the left of the document. Read more in our feature announcement .
Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only: Guidance for Industry and Food and Drug Administration Staff; Availability
A Notice by the Food and Drug Administration on 11/25/2013
This document has been published in the Federal Register . Use the PDF linked in the document sidebar for the official electronic format.
- Document Details Published Content - Document Details Agencies Department of Health and Human Services Food and Drug Administration Agency/Docket Number Docket No. FDA-2011-D-0305 Document Citation 78 FR 70306 Document Number 2013-28084 Document Type Notice Pages 70306-70307 (2 pages) Publication Date 11/25/2013 Published Content - Document Details
- View printed version (PDF)
- Document Dates Published Content - Document Dates Dates Text Submit either electronic or written comments on this guidance at any time. General comments on Agency guidance documents are welcome at any time. Published Content - Document Dates
This table of contents is a navigational tool, processed from the headings within the legal text of Federal Register documents. This repetition of headings to form internal navigation links has no substantive legal effect.
FOR FURTHER INFORMATION CONTACT:
Supplementary information:, i. background, ii. significance of guidance, iii. electronic access, iv. paperwork reduction act of 1995, v. comments.
This feature is not available for this document.
Additional information is not currently available for this document.
- Sharing Enhanced Content - Sharing Shorter Document URL https://www.federalregister.gov/d/2013-28084 Email Email this document to a friend Enhanced Content - Sharing
- Print this document
Document page views are updated periodically throughout the day and are cumulative counts for this document. Counts are subject to sampling, reprocessing and revision (up or down) throughout the day.
This document is also available in the following formats:
More information and documentation can be found in our developer tools pages .
This PDF is the current document as it appeared on Public Inspection on 11/22/2013 at 8:45 am.
If you are using public inspection listings for legal research, you should verify the contents of the documents against a final, official edition of the Federal Register. Only official editions of the Federal Register provide legal notice of publication to the public and judicial notice to the courts under 44 U.S.C. 1503 & 1507 . Learn more here .
Document headings vary by document type but may contain the following:
- the agency or agencies that issued and signed a document
- the number of the CFR title and the number of each part the document amends, proposes to amend, or is directly related to
- the agency docket number / agency internal file number
- the RIN which identifies each regulatory action listed in the Unified Agenda of Federal Regulatory and Deregulatory Actions
See the Document Drafting Handbook for more details.
Department of Health and Human Services
Food and drug administration.
- [Docket No. FDA-2011-D-0305]
Food and Drug Administration, HHS.
The Food and Drug Administration (FDA) is announcing the availability of the guidance entitled “Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only.” This guidance document is intended for manufacturers and distributors of “for research use only” (RUO) and “for investigational use only” (IUO) in vitro diagnostic (IVD) products and any other entities who label IVD products, as well as FDA staff.
Submit either electronic or written comments on this guidance at any time. General comments on Agency guidance documents are welcome at any time.
Submit written requests for single copies of the guidance document entitled “Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only” to the Division of Small Manufacturers, International and Consumer Assistance, Center for Devices and Radiological Health (CDRH) Food and Drug Administration, 10903 New Hampshire Ave., Bldg. 66, Rm. 4613, Silver Spring, MD 20993-0002; or the Office of Communication, Outreach and Development (HFM-40), Center for Biologics Evaluation and Research (CBER), Food and Drug Administration, 1401 Rockville Pike, Suite 200N, Rockville, MD 20852-1448. Send one self-addressed adhesive label to assist that office in processing your request, or fax your request to 301-847-8149. See the SUPPLEMENTARY INFORMATION section for information on electronic access to the guidance.
Submit electronic comments to http://www.regulations.gov . Submit written comments concerning this guidance to the Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852. Identify comments with the docket number found in brackets in the heading of this document.
Elizabeth Mansfield, Center for Devices and Radiological Health, Food and Drug Administration, 10903 New Hampshire Ave., Bldg. 66, Rm. 5676, Silver Spring, MD 20993-0002, 301-796-4664.
For questions relating to devices regulated by CBER, contact: Stephen Ripley, Center for Biologics Evaluation and Research (HFM-17), Food and Drug Administration, 1401 Rockville Pike, Suite 200N, Rockville, MD 20852-1448, 301-827-6210.
This guidance document is intended for manufacturers, and any other entities legally responsible for the labeling of IVD products that are distributing such products they have labeled RUO or IUO (subsequently referred to collectively as “manufacturers”). This guidance is intended to provide the current thinking of CDRH and CBER on when IVD products are properly labeled RUO and IUO.
This guidance is being issued because FDA is concerned that the distribution of unapproved and uncleared IVD products labeled RUO or IUO, but intended for purposes other than research or investigation (for example, for clinical diagnostic use [ 1 ] ), has led, in some cases, to the diagnostic use of products with unproven performance characteristics, and with manufacturing controls that are inadequate to ensure consistent manufacturing of the finished product. Use of such tests for clinical diagnostic purposes may mislead healthcare providers and cause serious adverse health consequences to patients who are not aware that they are being diagnosed with or treated based on the results of tests with research or investigational products. This guidance is thus intended to remind manufacturers that RUO and IUO labeling must be consistent with the manufacturer's intended use of the device.
In the Federal Register of June 1, 2011 ( 76 FR 31615 ), FDA announced the availability of the draft guidance document under the title “Draft Guidance for Industry and Food and Drug Administration Staff: Commercially Distributed In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only: Frequently Asked Questions.” Interested persons were invited to comment by August 30, 2011. The FDA received 55 sets of comments regarding the guidance. As a result of these comments, FDA revised the guidance and changed its format. Due to these revisions, FDA also changed the name of the guidance document.
This guidance is being issued consistent with FDA's good guidance practices regulation ( 21 CFR 10.115 ). The guidance represents the Agency's current thinking on “Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only.” It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. An alternative approach may be used if such approach satisfies the requirements of the applicable statute and regulations.
Persons interested in obtaining a copy of the guidance may do so by using the Internet. A search capability for all CDRH guidance documents is available at http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/default.htm . Guidance documents are also available at http://www.regulations.gov or from CBER at http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/default.htm . To receive “Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only” you may either send an email request to [email protected] to receive an electronic copy of the document or send a fax request to 301-847-8149 to receive a hard copy. Please use the document number 1723 to identify the guidance you are requesting.
This guidance refers to previously approved collections of information found in FDA regulations. These collections of information are subject to review by the Office of Management and Budget (OMB) under the Paperwork Reduction Act of 1995 ( 44 U.S.C. 3501-3520 ). The collections of information in 21 CFR part 809.10 and part 812 have been approved under OMB control numbers 0910-0485 and 0910-0078, respectively.
Interested persons may submit either electronic comments regarding this document to http://www.regulations.gov or written comments to the Division of Dockets Management (see ADDRESSES ). It ( print page 70307) is only necessary to send one set of comments. Identify comments with the docket number found in brackets in the heading of this document. Received comments may be seen in the Division of Dockets Management between 9 a.m. and 4 p.m., Monday through Friday, and will be posted to the docket at http://www.regulations.gov .
Dated: November 19, 2013.
Leslie Kux,
Assistant Commissioner for Policy.
1. Throughout this guidance document, references to “clinical diagnostic use” and “use in clinical diagnosis” include use in making medical treatment decisions.
[ FR Doc. 2013-28084 Filed 11-22-13; 8:45 am]
BILLING CODE 4160-01-P
- Executive Orders
Reader Aids
Information.
- About This Site
- Legal Status
- Accessibility
- No Fear Act
- Continuity Information
Thermo Fisher Scientific
Molecular testing content for clinical lab professionals and clinicians
Paper Overview: Regulatory Guidance for Clinical Laboratories
- Pharmacogenomics Testing’s Evolving Landscape: Regulatory Changes, Reimbursement Challenges, and Legislative Advances
- Double Detection: The Case for Including Wound Testing in UTI Labs
- Unraveling Solid Tumor Genetics with Whole-genome Copy Number Analysis

For readers outside of the U.S., this content can be used as a case study resource.
Understanding the regulatory environment is critical for the successful creation and launch of diagnostic tests—both in-vitro diagnostics (IVDs) and laboratory developed tests (LDTs). The latest educational paper published by the Arizona State University College of Health Solutions describes the regulatory landscape for laboratories establishing an LDT in the U.S., and how to navigate requirements set forth by the Food and Drug Administration (FDA) and Centers for Medicare & Medicaid Services (CMS). Let’s take a closer look at the key takeaways from this paper.
How does the FDA distinguish between IVDs and LDTs?
Laboratories that design and implement diagnostic tests must first understand how the FDA distinguishes between IVDs and LDTs (Table 1). FDA regulation of IVDs and LDTs has fueled an ongoing, two-decade debate. IVD tests manufactured for use in multiple laboratories, including clinical assays, are subject to FDA regulation such as premarket notification, approval or clearance. On the other hand, LDTs, based on in-house protocols and used at a single site, are exempt from such oversight.
Table 1. Differences between IVD tests registered with the U.S. FDA and LDTs.
| Developed for sale to diagnostic laboratories, health clinics, or consumers | Developed by individual laboratories; not transferred, licensed, or sold |
| Standardized instrument qualification procedures and training required | Instrument qualification and training requirements established by individual laboratories |
| Must be pre-validated with a data analysis and bioinformatics report | Often developed in-house by necessity—no standard assay available |
| Must be clinically validated | Must be clinically verified and can be implemented quickly for emergency use* |
*Must comply with the Clinical Laboratory Improvement Amendments (CLIA) of the U.S. Centers for Medicare and Medicaid Services.
What does the FDA say about RUOs, IUOs, and EUAs?It’s important to note that the FDA permits use of non-approved assays for research use only (RUO) or investigational use only (IUO) if they are not part of any clinical diagnostic procedures. It is illegal to make a clinical diagnosis with RUO or IUO tests because they lack comprehensive performance characterization, quality system conformity and cGMP manufacturing compliance. During a declared public health emergency, the FDA may grant Emergency Use Authorization (EUA) for unapproved medical products (including diagnostic tests). EUAs compress the development time (validation and clinical testing) required by manufacturers to provide comprehensive evidence of safety and effectiveness, compared to conventional approval. Following the EUA path is risky, and so it’s necessary to have a plan in place for market approval when the health emergency is over.How are LDTs regulated at clinical laboratories?While the FDA maintains authority over manufacturers in a device-centric manner, CMS uses a process-centric focus towards laboratories where the tests are performed. Unlike the FDA, the Clinical Laboratory Improvement Amendments (CLIA) program through CMS focuses on laboratory quality and competence, rather than the assays themselves. Any facility that performs diagnostic tests on human specimens must obtain the appropriate CLIA certificate from CMS. Analytical validation studies are required at each location to ensure assays perform as intended. CLIA-certified clinical laboratories must obtain certification for each type of assay they perform, which is assigned a complexity level by the FDA. By definition, LDTs are highly complex and must meet the most stringent criteria.Arizona State University College of Health Solutions is dedicated to translating scientific health research and discovery into practical interventions. Be sure to read ASU’s educational paper for more information on regulatory guidance for laboratories that design and implement tests for clinical use .In case you missed it, check out these related and informative resources:
- An introduction to diagnostic testing in laboratories
- Preparing for and implementing a laboratory-developed test
- Reimbursement for laboratory-developed and in vitro diagnostic tests
Intellectual property associated with laboratory-developed tests (LDTs) and in vitro diagnostic (IVD) tests
- The Simple, Sensible, Salient & Still Spell-Binding Seven Questions About Laboratory-Developed Tests
- Challenges of Establishing Laboratory-Developed Tests
| FDA | United States Food and Drug Administration | FDA has oversight of biomedical products in the USA.[1] |
| IVD | in vitro Diagnostic | Diagnostic tests must be approved as a medical device by FDA if it is [2] |
| LDT | Laboratory-developed test | LDTs are based on in-house protocols, and are exempt from premarket review and manufacturing oversight from FDA, as long as the test is [3] |
| RUO | Research Use Only | A non-IVD product in the laboratory research phase of development that |
| IUO | Investigational Use Only | A product being shipped or delivered for product testing and process evaluation, but |
| EUA | Emergency Use Authorization | A type of FDA authorization for medical products, including IVDs, during a declared |
| CMS | Centers for Medicare & Medicaid Services | A government organization that provides health coverage. Clinical labs must obtain a certificate of compliance in order to bill the CMS for their services.[4] |
| CLIA | Clinical Laboratory Improvement Amendments | The CMS regulates all laboratory testing (except research) performed on humans in the U.S. through the Clinical Laboratory Improvement Amendments (CLIA). |
- https://www.fda.gov/about-fda/what-we-do
- 21 CFR 809.3
- 42 CFR Part 493
- https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Mol Ther Methods Clin Dev
- v.24; 2022 Mar 10
Consensus guidelines for the validation of qRT-PCR assays in clinical research by the CardioRNA consortium
David de gonzalo-calvo.
1 Translational Research in Respiratory Medicine, University Hospital Arnau de Vilanova and Santa Maria, IRBLleida, 25198 Lleida, Spain
2 CIBER of Respiratory Diseases (CIBERES), Institute of Health Carlos III, 28029 Madrid, Spain
Monica Marchese
3 Integrated Bed to Bench Operations (IBBO), Luxembourg Institute of Health, 1445 Strassen, Luxembourg
Jan Hellemans
4 Biogazelle, 9052 Zwijnaarde, Belgium
5 Laboratoire National de Santé (LNS), 3555 Dudelange, Luxembourg
Nanna Lond Skov Frisk
6 Department of Science and Environment, Roskilde University, 4000 Roskilde, Denmark
7 Department of Clinical Immunology, Naestved Hospital, 4700 Naestved, Denmark
Louise Torp Dalgaard
Päivi lakkisto.
8 Minerva Foundation Institute for Medical Research, 00290 Helsinki, Finland
9 Department of Clinical Chemistry, University of Helsinki and Helsinki University Hospital, 00029 Helsinki, Finland
10 National Measurement Laboratory, LGC, Teddington TW11 0LY, UK
Andreas Scherer
11 Institute for Molecular Medicine Finland FIMM, Helsinki Institute for Life Science HiLIFE, University of Helsinki, 00014 Helsinki, Finland
12 European Research Infrastructure for Translational Medicine EATRIS, 1081 HZ Amsterdam, the Netherlands
María Laura Garcia Bermejo
13 Biomarkers and Therapeutic Targets Group, Ramon y Cajal Health Research Institute (IRYCIS), RedinRen, 28034 Madrid, Spain
Yvan Devaux
14 Cardiovascular Research Unit, Department of Population Health, Luxembourg Institute of Health, 1A-B rue Edison, 1445 Strassen, Luxembourg
Despite promising findings, quantitative PCR (qPCR)-based tests for RNA quantification have experienced serious limitations in their clinical application. The noticeable lack of technical standardization remains a huge obstacle in the translation of qPCR-based tests. The incorporation of qPCR-based tests into the clinic will benefit from guidelines for clinical research assay validation. This will ultimately impact the clinical management of the patient, including diagnosis, prognosis, prediction, monitoring of the therapeutic response, and evaluation of toxicity. However, clear assay validation protocols for biomarker investigation in clinical trials using molecular assays are currently lacking . Here, we will focus on the necessary steps, including sample acquisition, processing and storage, RNA purification, target selection, assay design, and experimental design, that need to be taken toward the appropriate validation of qRT-PCR assays in clinical research. These recommendations can fill the gap between research use only (RUO) and in vitro diagnostics (IVD). Our contribution provides a tool for basic and clinical research for the development of validated assays in the intermediate steps of biomarker research. These guidelines are based on the current understanding and consensus within the EU-CardioRNA COST Action consortium ( www.cardiorna.eu ). Their applicability encompasses all clinical areas.
Graphical abstract

These guidelines provide a tool for the development of validated assays in research. We have focused on the necessary steps that need to be taken toward the appropriate clinical research validation of qRT-PCR assays. These recommendations can fill the gap between research use only and in vitro diagnostics.
Introduction
A literature search on biomarkers and cardiovascular diseases (CVDs) highlights the poor correlation between the efforts performed in the initial steps of the development of quantitative PCR (qPCR) assay-based biomarkers, i.e., discovery and preclinical stages and their incorporation into clinical practice. There are a number of barriers that contribute to this poor implementation. The lack of technical standardization constitutes a key limitation in the incorporation of qPCR-assay-based biomarkers into the clinic. Limitations are also linked to the absence of consensus reference values, poor harmonization of the study populations, and the barriers in collaboration between academia, physicians, and industry. For instance, despite the thousands of noncoding RNA (ncRNA)-based biomarker studies published to date, there is a paucity of potential indicators that have been successfully translated into clinical practice, mainly due to the lack of reproducibility of research findings. Kok et al. 1 nicely illustrate the situation for coronary artery disease (CAD)-associated circulating microRNA (miRNA) biomarkers based on a literature review yielding 13 miRNAs found to be up- or downregulated in more than one study, of which more than half (7 out of 13) showed a contradictory result between studies (e.g., for miR-21, two studies showed upregulation and one study showed downregulation). This lack of reproducibility has also been addressed in several publications, 2 , 3 , 4 with reported causes ranging from technical analytical aspects to variable patient inclusion criteria and underpowered studies to sample quality. As such, the field of in vitro diagnostics (IVD)-grade quantitative reverse transcription PCR (qRT-PCR) assays for clinical use, initially developed in research laboratories, is still in its infancy. The incorporation of novel molecular biomarkers for clinical decision-making and patient management, i.e., diagnosis, prognosis, prediction, and monitoring of the therapeutic response or toxicity, need clear assay validation guidelines to be followed in the context of clinical research.
In this context, basic and clinical researchers often resort to the use of laboratory-developed assays with variable and undefined quality, commercial research use only (RUO) assays or, in the best-case scenarios, laboratory-developed assays validated in accordance with guidance such as minimum information for the publication of quantitative real-time PCR experiments (MIQE) guidelines. 5 The difference between such assays and certified IVD assays is significant. Laboratory-developed assays for clinical research are typically less controlled and standardized and do not need to comply with regulations, such as the European In Vitro Diagnostic Regulation (IVDR 2017/746). The European regulatory framework, based on the aforementioned IVDR and the Clinical Trials Regulation 2014/536, leaves a gray area relative to the status of laboratory assays that are used in the context of clinical trials. Poorly validated assays are not appropriate for large-scale clinical biomarker studies. Therefore, researchers would benefit from guidelines on the validation of what we refer to as clinical research (CR) assays, an assay type filling the gap between RUO and IVD that addresses the specific needs of researchers in the development of biomarkers. To some degree, such CR assays are similar to laboratory-developed test (LDT) assays in that they have undergone more thorough validation without reaching the status of a certified IVD assay.
Analytical precision (or precision): closeness of two or more measurements to each other
Analytical sensitivity: the ability of a test to detect the analyte
Analytical specificity: the ability of a test to distinguish target from nontarget analytes
Analytical trueness / analytical accuracy: closeness of a measured value to the true value
Clinical research: in this article, “clinical research” encompasses clinical studies involving patients and/or healthy controls and their biomaterials, of which the objectives are related to therapeutic interventions (clinical trials), diagnostic or prognostic developments, understanding of disease mechanisms. Good Clinical Laboratory Practice (GCLP) standards typically apply to laboratory work in clinical research
Context of use: statement that describes the appropriate use of a product or test
Fit-for-purpose: a conclusion that the level of validation associated with a medical product development tool is sufficient to support its context of use
Negative predictive value: the predictive ability of a test to identify the absence of the disease in individuals with negative test results
Positive predictive value: the predictive ability of a test to identify disease in individuals with positive results
True positive rate / Sensitivity: proportion of positives that are correctly identified
True negative rate / Specificity: proportion of negatives that are correctly identified
By defining a CR level validation, researchers can more easily license out RUO assays that are affordable and easy to obtain in the early stages of biomarker research to diagnostic test manufacturers or clinical laboratory providers. This progression is visually represented in Figure 1 . Here, we will focus on the necessary steps that need to be taken toward the appropriate validation of qRT-PCR workflows for CR and clinical use. Overall, the objective of this review is not to provide regulatory guidance for compliance with agency requirements. The aim is to provide supplementary practical and technical support in the specific context of qRT-PCR for which existing regulations are not always easy to apply or are unknown to researchers usually working outside of the regulated frameworks.

Workflow from research-use assays to in vitro diagnostic tests suitable for clinical practice
CR, clinical research; COU, context of use; IVD, in vitro diagnostics; LDT, laboratory-developed test, RUO: research use only.
Considerations for biomarker identification, validation, and clinical use
A biomarker is a characteristic, a measurable indicator of normal or pathologic biological processes, the responses to an exposure or intervention (including therapeutic interventions), or the risk of developing a medical condition or disease.
According to their intended use, biomarkers can be structured into several categories: susceptibility/risk, diagnostic, monitoring, prognostic, predictive, pharmacodynamics/response, and safety. Thus, with the right set of biomarkers, questions can be addressed such as how a condition will develop (prognostic), who will benefit from a treatment (predictive/stratification), will a treatment be efficacious (pharmacodynamic or surrogate) and beneficial (monitoring/response), will it be safe or toxic (safety), and how stable the health condition of a patient would be.
In general, the validation of a biomarker includes an evaluation of the analytical performance (trueness, precision, and analytical sensitivity and specificity) and the clinical performance (specificity, sensitivity, and predictive values). Analytical trueness (or analytical accuracy) refers to the closeness of a measured value to the true value, while analytical precision refers to the closeness of two or more measurements to each other and includes establishing the repeatability and reproducibility of the test. Analytical sensitivity is the ability of a test to detect the analyte (usually the minimum detectable concentration or LOD), and analytical specificity is the ability of a test to distinguish the target from nontarget analytes (in qPCR assays, the detection of a target sequence rather than other, nonspecific sequences).
Clinical performance is the ability of a test to correctly discriminate between the presence or absence of disease, and it is evaluated using measures of diagnostic accuracy. 6 The diagnostic sensitivity of a test is reflected in the true positive rate (TPR), meaning the correct identification of subjects with the disease, while the diagnostic specificity of a test is measured as the true negative rate (TNR), meaning the correct identification of subjects without disease. Positive predictive value (PPV) is the predictive ability of a test to identify disease in individuals with positive results, while negative predictive value (NPV) is the predictive ability of a test to identify the absence of disease in individuals with negative test results. Predictive values are dependent on the prevalence of disease.
The thresholds of these performance characteristics depend on the context of use (COU) and adhere to the “fit-for-purpose” (FFP) concept and must ideally be decided prior to the test. Properly defined, FFP is “a conclusion that the level (or rigor) of validation associated with a medical product development tool (assay) is sufficient to support its COU,” 7 where validation is the process of testing an assay performance, including the measures of the calibration of the instruments, the standardization of the experimental processes, the accuracy, the precision, and the reproducibility. 8
The COU elements are laid out in the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) guidelines and provide an informative and structured framework for identifying a biomarker's utility. 9 , 10 COU elements include (1) what aspect of the biomarker is measured and in what form, (2) what is the clinical purpose of the measurements, and (3) what is the interpretation and decision/action based on the measurements.
Biomarkers that are expected to support clinical decision-making have to be validated according to a formal qualification process, which concludes that a biomarker allows a specific interpretation and an application according to its COU in clinical product development. One example of the use of a therapeutic COU is in the allocation to specific treatment regimens. Biomarkers can influence the decision of the cessation of a patient's participation in a clinical trial, can establish a drug's proof of concept in a patient population, support clinical dose selection, and serve to enrich clinical trials for populations of interest, and can help evaluate treatment responses. This concept can be generalized to all phases of discovery research and drug development with the term FFP.
Hence, the intended use of a biomarker determines the choice of analytical methods as well as the stringency of the performance criteria of the biomarker during its validation process. An adjustment of methods with regard to new findings during the FFP validation process may be necessary and may concern the selection of preanalytical conditions. A validation phase with preliminary performance acceptance criteria, followed by larger sample sizes and off-site tests of the biomarker assay, is essential for the determination of the robustness and usability in more extended settings.
Nonclinical biomarkers (RUOs), which are the output of the FFP validation process, do not require regulatory submission. RUO biomarkers can be used as “good-enough” biomarkers internally or possibly for publication. However, full validation will be required in case the biomarker will be developed further toward clinical use, e.g., as a companion diagnostic.
For regulatory acceptance, a biomarker needs to follow and fully comply with the path of a qualification that outlines how a biomarker will be used in the clinical setting. The outcome will be a valid biomarker within its COU, one that is measured in an analytical test system with well-established performance characteristics and for which there is an established framework or body of evidence that elucidates the physiological, toxicological, pharmacological, or clinical significance of the test results. 9 , 10 , 11
Controlled experimental environments, which include experimental designs with predefined acceptance criteria, preanalytical requirements, qualified equipment, trained operators, analytical performance, and data stewardship, are prerequisites for reliable and thereby meaningful measurement of biomarkers.
Guidelines for validation of CR-grade qPCR assays
Many references on validation requirements exist, ranging from academic guidelines and international standards such as ICH and ISO to national regulatory requirements such as the 21 CFR Part 820 (US) and IVDR 2017/746 (EU). 8 , 12 , 13 A practical challenge for the use of many of these standards is the fact that they have not been tailored to the specifics of PCR-based tests. ISO 20395:2019 (Biotechnology — Requirements for evaluating the performance of quantification methods for nucleic acid target sequences — qPCR and digital PCR [dPCR]), which was recently published, addresses specific underpinning requirements. Inspired by these standards, we present guidelines for the practical analytical validation of CR-grade qPCR assays in clinical samples.
It is worth noting that the concept of a guideline explicitly allows researchers to deviate from them as a function of the specific needs and characteristics of their tests. A few examples where a risk analysis could alter the validation of qPCR assays include the following: (1) the specificity of an assay is typically a crucial assay property (for reference genes, this requirement may be relaxed since their measurements are required to represent the total amount of RNA, not necessarily the amount of a specific gene); (2) the limit of detection (LOD) is an important property for assays testing weakly expressed genes or for tests optimized to work with minimal input quantities (for tests where the analyte is highly abundant, defining the LOD addresses specific points in standards and regulatory requirements without significantly contributing to the quality of the assay); or (3) the capacity of an assay to accurately quantify small expression differences requires a thorough validation when used for biomarkers that rely on such small expression differences. On the other hand, less extensive validation experiments may be sufficient for tests that rely on the detection of large expression differences.
Method development for RUO
Even when aiming for a higher level of analytical assay validation, it is useful to start with RUO-level validation. Such validation is fast and easy and allows for the elimination of inferior designs before proceeding to the more extensive CR (or ultimately IVD)-level validation.
Prior to the initiation of the analytical validation, the qPCR master mix components and the thermocycler and cycling conditions must be selected, optimized, and then fixed. Frequently, these decisions are implicit by relying on standard procedures used within the laboratory, but one needs to be aware that the obtained validation results only apply to that setting. As there are considerable differences between qPCR master mixes with regard to assay performance, polymerase reliability, and optimized conditions, defining the conditions is crucial. Importantly, validated results only apply to the settings used, meaning that revalidation is required whenever the setup changes.
RUO-level analytical validation (for genes of interest and reference genes) should at least cover the following aspects: (1) correct amplification on a positive control sample (for example, Cq values below 28), (2) lack of amplification on a negative control sample, (3) evidence of RT and amplification efficiency, and (4) specificity of an amplified product (melting temperature and/or amplicon length analysis). An experimental workflow providing data for validation could be performed as follows:
- i. In general, RNA, rather than cDNA, is preferred as a positive control. The RNA may be derived from a positive sample or generated by in vitro transcription from a vector or synthetic sequence.
- ii. cDNA sample. Ideally derived from an RNA sample that has undergone the same process (collection, extraction, RT) as clinical samples and that is known to express the gene of interest. Alternatively, this could also be a commercial sample available in large quantities and with sufficient and similar RNA quality to the intended test samples. Clinical biobanks, under ISO certification, may be a source of samples if no commercial controls are available.
- iii. A cloned and sequence-verified DNA fragment containing the transcript of interest.
- iv. A synthetic DNA fragment containing the transcript sequence of interest.
- i. No-template control testing for contamination and primer-dimer formation (e.g., water or carrier RNA).
- ii. Genomic DNA testing for gDNA coamplification. A RT reaction without reverse transcriptase added will also test for gDNA contamination of the RNA eluate.
- iii. “Extraction blank” to control for contamination at the extraction stage.
- iv. Optional: cDNA from other species to test for cross-species reactivity (important in preclinical studies where human genes are used in a hybrid context).
- v. Optional: cDNA from a specific RNA sample known not to contain the transcript or variant of interest (this is the ideal negative control because of its complex matrix similar to real samples).
- ◦ cDNA, if the gene of interest is highly expressed.
- ◦ Plasmid or long double-stranded synthetic DNA fragments.
- ◦ Short single-stranded chemically synthesized templates, e.g., 60-mer oligonucleotides of the first and last 30 nucleotides of the amplicon. This solution can be applied also for genes with very low or rare expression. Of note, for probe-based assays, the oligonucleotide (oligo) should also contain the probe binding site.
- ◦ In-vitro -transcribed synthetic RNA fragments representing the entire amplicon.
- ◦ RNA from cell lines, patient samples, and/or reference materials.

Considerations for sample material for qPCR dilution series
The last option is applicable to one-step qRT-PCR.
- ii. Six-point (or more) 10-fold dilution series, ideally spanning the 10 1 to 10 6 copies/qPCR reaction range. If the input quantities for this dilution series are well known (because of the use of qualified reference samples or because of calibration by means of dPCR), the dilution series could also be used for absolute quantification.
- • In total, 21–33 reactions/assay.
- • Perform triplicate qPCR reactions for all samples.
- • Amplicon size analysis should be performed (agarose gel or microfluidic electrophoresis). Positive control samples should yield a single sized product of correct length. For negative controls with a signal, it may support troubleshooting by differentiating between primer-dimer formation and template contamination.
- • Evaluate amplification plots for correct amplification in positive control samples.
- i. Evaluate melting curves.
- ii. Positive control samples should yield a characteristic Tm peak. Different tools enable the prediction of the Tm peak (or multiple peaks for some amplicons with nonuniform GC% distributions) as a reference value.
- iii. Negative samples should display no primer-dimer peak (typically broader peak at low Tm) nor a template Tm peak.
- • Evaluate negative control samples. Ideally, no amplification is observed. In cases where the sequence of interest is very highly expressed, a low level of false positive signals (high Cq value) might be tolerated.
- i. Linearity in a standard curve. Deviations from linearity may be observed at the ends of the dilution series, e.g., because of plateauing due to contamination or primer-dimer formation or because of inhibition due to the use of too much cDNA. The testable range is restricted to the linear range of the dilution series.
- ii. Amplification efficiency. Ideally in the 90%–110% range. Efficiencies of 80%–90% or 110%–120% are suboptimal but acceptable for some assays, e.g., when the effect size (difference) is sufficiently large and such small technical imperfections do not interfere with their separation.
CR-level validation
A CR assay consists of the entire workflow from RNA template measurement to data analysis. The standardized treatment of samples at each step is imperative to ensure biomarker performance.
Reverse transcription
During reverse transcription, the complementary DNA template is synthesized. Ideally, this reaction should generate a 1-to-1 DNA complementary to the RNA template, but this is very rarely achievable. Depending on the type and quality of RNA investigated, a reverse transcription priming method should be selected. The RT reaction may use random primers (6–9 mers), oligo dT primers, a combination of both of the aforementioned primers, or target primers, corresponding to those used in the qPCR reaction. Validation experiments should include the test of saturation by an RNA template, which is performed as multiple cDNA synthesis reactions with increasing amounts of purified RNA, including an RNA spike-in (an external control) followed by qPCR for the intended targets. Here, there should be linearity between the Cq levels of the targets and the input amount of RNA within a range corresponding to (patho)physiological levels of the RNA template. Alternatively, increasing amounts of synthetic target RNA can be added to multiple reverse transcription reactions of one specific RNA sample. Similarly, to test for reaction inhibition during cDNA synthesis, we also recommend adding a synthetic spike-in RNA molecule during reverse transcription that is different from the one used for spike-in during RNA isolation. The amount of RNA spike-in should generally be in the linear dynamic range of the assay, which would often be in the attomol range of added spike-in. This is fundamental to correct for differences in the RT reaction efficiency.
Target selection
Different approaches lead to the identification of target sequences. Target selection is beyond the scope of these guidelines but depends on the following questions: is one interested in the gene with all its isoforms or in a subset of transcripts encoding a particular protein? Does the assay have to detect a specific allele or fusion event? Are there any regions to be avoided for assay design because of homology with mouse sequences that would interfere with analysis of murine xenografts?
Reference genes
Importantly, most qPCR tests measuring gene expression levels rely on reference genes for data normalization. Assay validation is thus not limited to the assays measuring the target of interest but must include assays for selected reference genes as well. Since the quality of the final test results strongly depends on the quality of normalization by means of the selected reference genes, it is important to ensure that multiple and stably expressed reference genes are selected. A pilot study evaluating eight candidate reference genes in 12 representative samples and analyzed by a tool such as geNorm 14 or NormFinder 15 can provide the needed assurance that the reference genes are unaffected by the experimental conditions and are stable in the population and sample type of interest. Additionally, for small RNAs determined in body fluids, a set of reference small RNAs has already been suggested. 16 Nevertheless, a critical evaluation of these reference small RNAs is fundamental for each experimental condition and study population.
Assay design
To increase the success rate of analytical assay validation, the basics of a design by tools such as Primer3 17 should be complemented with additional bioinformatic analyses testing for the specificity, transcript coverage, and absence of secondary structures and common single-nucleotide polymorphisms (SNPs) in primer or probe annealing regions. Such analyses may be integrated in assay design by programs such as primerXL, 18 performed independently with online tools such as BiSearch (for specificity) 19 and UNAfold (secondary structures), 20 or performed manually in genome browsers (transcript coverage and SNP overlap).
Despite the benefit of such in silico examinations, they cannot provide a full guarantee of optimal performance when put in practice in the lab. Therefore, multiple designs can be fed into downstream analytical validation, allowing for the selection of the best performing assay and increasing the likelihood of identifying at least one assay meeting all requirements.
Experimental design for CR assay validation of qPCR-based tests
We provide an example of a design that may be used for most gene expression tests ( Figure 3 ). This design may, however, have to be modified to meet the requirements of certain specific analytical COUs, e.g., when dealing with transcript or allele-specific assays, when working in a multispecies context, or when aiming for multiplexing. The proposed number of biological replicates may also be increased, e.g., for biomarkers with a small effect size.

Clinical research qPCR assay development
The researcher also needs to predefine the assay performance acceptance criteria. These might relate to parts of the method (for instance, the qPCR amplification efficiency) but should always include criteria for the full workflow starting from the matrix to the end result. The latter are typical test-related criteria such as accuracy, precision, specificity, sensitivity, LOD, and limit of quantification (LOQ). The results of this validation have to be properly documented, e.g., electronically.
Due to the lack of suitable reference samples, the accuracy of normalized gene expression levels is difficult to determine. Inspired by the mixing experiments proposed in the microarray quality control (MAQC) studies but enhanced to ensure the testability of differential expression for any gene, we propose an approach relying on known mixtures of in-vitro -transcribed RNAs to support the accuracy analysis of qRT-PCR tests. To make this experiment representative of the actual analysis applying reference gene normalization, it includes both transcripts for the gene(s) of interest (GOI) and for the reference gene(s).
To systematically assess the robustness of the test for RNA integrity, we propose the evaluation of a series of RNA samples with varying degrees of artificial degradation. This degradation can be achieved via heating, sonication, UV radiation, or incubation with ribonucleases. 21 , 22 If samples are known to systematically yield high-quality RNA, and its integrity is systematically tested, one may consider skipping this robustness analysis. Evaluation of the quality of RNA can be achieved by spectrophotometry and by microfluidic electrophoretic methods based on the RNA integrity number (RIN) or an equivalent metric. It is more difficult to evaluate the quality of ncRNA, such as miRNAs, but this can be done either by verifying the presence of ubiquitous ncRNA molecules (e.g., RNU-24, miR-16, miR-221) or by measuring quality scores that have been described in specific COUs. 23 , 24
Consensus experimental design for a standard gene expression assay is presented hereafter (for absolute and relative quantification). The recommended acceptance criteria for precision and accuracy depends on the intended purpose of the assay:
- i. GOI template containing amplicon sequences for the GOIs: promotor – GOI1 – GOI2 – … – GOIn – control. If a large number of assays for different GOIs are to be tested, one may consider making different GOI templates, with each containing a subset of GOI amplicon sequences.
- ii. REF template containing amplicon sequences for the reference genes: promotor – REF1 – REF2 – … – REFn – control.
- • Optional: determine the copy number of the synthetic templates by means of dPCR using the control assay. If dPCR is not available, one has to rely on the quantities described by the oligo producer to estimate the template copy number.
- • Create a dilution series for both GOI and REF templates spanning the 5 × 10 6 to 5 copies/PCR reaction range.
- • Confirm the correctness of the dilution series by testing the control assay. With qPCR, the correctness of fold dilutions can be verified. If dPCR is available, the accuracy of the dilution points can also be assessed. If these deviate more than 2-fold (or any fold difference that is relevant for the intended purpose), a new dilution series should be made.
- • Heat-treat an RNA sample for different times (75°C for 1–10 min/μg RNA). Ideally, the same representative RNA sample (same matrix and extraction method) should be used. If such RNA is already too degraded to be used as a starting point for the RNA integrity series or the available material is too limiting for these experiments, one may have to rely on commercial samples such as the MAQC RNA.
- • Assess RNA integrity by means of microfluidic electrophoresis (see above). The process of artificial RNA degradation and integrity assessment may have to be iterated until proper timings have been found to generate the targeted RNA integrity range. Create cDNA for the selected RNA samples.
- • For DNA analysis, an equivalent test can be set up.
- • In vitro transcription of the two templates containing the GOI and REF, respectively (two separate reactions).
- i. A: mixture containing equal amounts of GOI and REF RNA.
- ii. B: mixture containing the same amount of REF RNA as sample A but only 1/1,000 of GOI RNA.
- i. C: 90% A + 10% B
- ii. D: 75% A + 25% B
- iii. E: 50% A + 50% B
- iv. F: 25% A + 75% B
- v. G: 10% A + 90% B
- • Spike mock RNA with synthetic RNA mixes A–G. The mock RNA provides for a more natural, complex RNA background. Any RNA sample void of amplifiable sequences may be suitable. RNA from bacteriophage MS2 is often a good candidate mock RNA sample.
- • Synthesize cDNA (by performing RT) for all spiked RNA samples and for unspiked mock RNA (sample H).
- • For gDNA analyses, an equivalent test, not needing in vitro transcription and cDNA synthesis, can be set up.
- • Four representative clinical samples with sufficient material should be selected for at least 2 extractions. If material for individual samples is limiting, one may consider homogeneous pooling and mixing of samples to obtain samples with sufficient material to support repeat extraction.
- • Perform repeat extraction, reflecting the different sources of variation (day of extraction, extraction kit lot number, person executing the extraction).
- • Perform cDNA synthesis independently for the two sets of 4 RNA extracts.
- • In total, 48–96 reactions/assay.
- i. Dilution series of synthetic DNA templates (see Preparation 1).
- ii. No-template control (NTC) with carrier RNA.
- iii. 5 cDNA samples from RNA integrity series.
- iv. 3 cDNA samples from representative clinical samples.
- • Assess specificity on amplicons from the 3 representative cDNA samples using size and, for assays using intercalating dyes, melt curve analysis.
- i. Determine slope, intercept, linear dynamic range, coefficient of variation (r 2 ), and amplification efficiency from dilution series data.
- ii. Determine qPCR repeatability for cDNA from representative clinical samples and for the different samples of the dilution series. The latter may reveal concentration-dependent repeatability, typically showing increasing variability as concentrations approach the detection limit.
- iii. Assessment of the absence or degree of primer-dimer formation on the NTC sample.
- iv. Assess the robustness of the assay against RNA degradation. A robust assay would show identical normalized relative quantity (NRQ) values for artificially degraded RNA as for intact, undegraded RNA. Lower NRQ values for degraded samples would reveal an impact of certain degrees of RNA degradation.
- • In total, 48 reactions/assay.
- i. On 12 samples: an NTC, the 7 cDNA samples (A–G) derived from the RNA/cDNA titration series (see Preparation 3), and 4 cDNA samples derived from the same repeat extraction and reverse transcription.
- ii. Using assays for both the reference genes and a set of GOIs.
- iii. Triplicate qPCR reactions (or other replicate number as will be used in later testing—more replicates may improve data quality).
- iv. Repeat the analysis of the 12 samples twice within the same run. One set containing the 4 cDNA samples of one extraction and reverse transcription round, and the other set containing those of repeat extraction and reverse transcription.
- i. Calculate normalized expression levels for the GOIs by normalizing their relative quantities with the geometric mean of the relative quantities of the selected reference genes. 25
Relative input quantities
| Sample | Expected expression level |
|---|---|
| A | 1.00 |
| C | 0.9001 |
| D | 0.75025 |
| E | 0.50005 |
| F | 0.25075 |
| G | 0.10009 |
| B | 0.001 |
- iii. Determine repeatability by comparing the normalized expression levels for the two repeats within the same run.
- i. Prepared by a different person.
- ii. On a different day.
- iii. Measured on a different qPCR instrument.
- • Determine between-run precision of the assay by comparing the NRQ values for the two repeats between the runs. The results for samples A–G only reflect the repeatability of the qPCR measurements, including pipetting errors. The results for the other 4 samples also reflect the variability of other parts of the workflow (RNA extraction and cDNA synthesis).
- 8 .Validation of a run.
Quality control steps to monitor relative quantification assay performance and accept or reject a run include the following: (1) cDNA prepared from a reference RNA sample should show Cq < 30; (2) reverse transcription NTC sample should give indeterminate Cq or Cq at least 5 Cq units higher than the highest Cq value of the positive test samples used in the assay; (3) NTC sample should give indeterminate Cq or Cq at least 5 Cq units higher than the highest Cq value of the positive test samples used in the assay; (4) if amplification efficiencies are remeasured, they should fall within their predefined acceptance range (typically 90%–110%); or (5) if multiple reference genes are used to normalize data, their stability measures should fall within their predefined acceptance range (typically geNorm M value < 0.5). 14 , 25
Quality control steps to monitor absolute quantification assay performance and accept or reject a run include the following: (1) NTC sample gives an undetermined result or a result below the lowest point of the calibration curve, (2) the calibration curve R 2 >99%, (3) the calibration curve slope is between −2.9 and −3.8, or (4) there are at least four consecutive data points in the calibration curve (defining the “validated range” for the run).
The guidelines proposed above, which are summarized in Figure 4 , are not mandatory and are proposed to assist researchers in validating their assays before implementation in CR and clinical trials. They are not intended to impose formal validation requirements but rather to describe the consensus obtained within the European CardioRNA consortium (CardioRNA COST Action CA17129) on a relevant set of validation experiments. These guidelines are focused on the analytical validation of singleplex gene expression assays. Several aspects are explicitly not covered: multiplex assays, data analysis, cross-species amplification (xenograft, infectious), preamplification, genotyping or allele-specific PCRs, or dPCR. These assays may require other approaches that are inspired by these guidelines or are completely tailor made.

Checklist for reference procedure
We envisage reviewing these guidelines in 3 years based on the feedback received from the CR community, the practical experience gained in using these guidelines, the new consensus in the scientific community, and new technological developments and approaches.
Conclusions, remarks, and perspectives
The increase in RNA-focused research in the last decade has led to great advances in the general and specific knowledge of the transcriptome and pathophysiological mechanisms of diseases, leading to the identification of new biomarkers that are useful in clinical practice. Despite the promising findings, qPCR-based tests for RNA quantification have experienced serious limitations in their direct clinical application. The development and commercialization of novel qRT-PCR-based tools is a laborious process, and successful assay validation requires substantial resources. Ultimately, the establishment and application of evidence-based recommendations for CR assays may reduce the time and cost of obtaining new assays from the research laboratory to clinical practice and the market.
These recommendations can fill the gap between RUO and IVD. They are the output of a collective effort of the EU-CardioRNA consortium with collaboration and endorsement by the European Research Infrastructure for Translational Medicine (EATRIS) ( https://www.EATRIS.eu ) and the Biobanking and BioMolecular Resources Research Infrastructure (BBMRI) ( www.BBMRI.eu ), with both being part of the EU-AMRI alliance (European Alliance of Medical Research Infrastructures), whose vision is to alleviate the attrition rate of biomarkers early in their development to assure accuracy and to help save costs, time, and expectancies of patients and clinicians. We are confident that application of these guidelines will result in more effective biomarkers development for many diseases but that are, above all, useful in clinical practice.
Acknowledgments
This manuscript is based upon work from EU-CardioRNA COST Action CA17129 ( www.cardiorna.eu ) supported by COST (European Cooperation in Science and Technology). D.d.G.-C. wants particularly to acknowledge Marta Molinero and Jo Vandesompele for their technical support. D.d.G.-C. has received financial support from the Instituto de Salud Carlos III (Miguel Servet 2020: CP20/00041), which is co-funded by the European Social Fund (ESF)/"Investing in your future.” This work is supported by the Instituto de Salud Carlos III (PI20/00577), which is co-funded by European Regional Development Fund (ERDF)/“A way to make Europe.” CIBERES is an initiative of the Instituto de Salud Carlos III. Y.D. is funded by the EU Horizon 2020 project COVIRNA (grant agreement 101016072), the National Research Fund (grants C14/BM/8225223, C17/BM/11613033, and COVID-19/2020-1/14719577/miRCOVID), the Ministry of Higher Education and Research, and the Heart Foundation-Daniel Wagner of Luxembourg. P.L. is funded by the Finnish Cultural Foundation, The Finnish Foundation for Cardiovascular Research, The Finnish Society of Clinical Chemistry, and the Finnish Foundation for Laboratory Medicine.
Author contributions
D.d.G.-C., M.M., J.H., F.B., N.L.S.F., A.S., L.T.D., and P.L. designed the article and wrote the manuscript. C.F., M.L.G.B., and Y.D. revised the manuscript. All authors read and approved the final manuscript.
Declaration of interests
D.d.G.-C. holds a patent on miRNAs as biomarkers. Y.D. holds patents related to diagnostic and therapeutic applications of RNAs. The other authors declare no competing interests.
- Blog | The CURRENT
Healthcare and Life Sciences Taking Research Use Only Products from the Research Lab to the IVD Market
Dick Rubin has over 30 years of experience in the lab instrument industry, an educational background in chemistry and a certificate in FDA regulatory affairs. As a Senior Manager of Engineering Solutions at Plexus, Dick works with Plexus engineering staff to draft development solutions and supports active development projects to ensure Plexus’ commitment to flawless execution.
Q: What trends are you noticing in the market right now?
A: Scientific innovation across the life sciences industry is driving the rapid expansion of the instrumentation market with products that provide better results, faster. A pull for more point-of-care and home-use technology is also driving innovation. In particular, we’re seeing more companies looking to move instruments from Research Use Only (RUO) applications to pursue in vitro diagnostic (IVD) applications with the same instrument.
This is a big undertaking, which is why companies find value in working with Plexus. Commercializing an RUO solution for the IVD market comes with challenges, such as greater regulatory demands and finding the optimum balance between high quality and cost-effectiveness. Realizing such products requires a unique, technical skill set and strong understanding of the regulatory environment.
Q: How do you help companies make the leap from the lab to the clinical market?
A: With expertise across the product lifecycle, from initial design to manufacturing to aftermarket services, Plexus can partner with the customer’s clinical team to address any documentation gaps before categorizing the assay, or instrument, as an IVD device capable of clinical applications.
Our team brings deep familiarity with the complex regulatory environment that life sciences products need to navigate for market clearance. We have decades of experience developing healthcare and life sciences products ranging from Class I to Class III, and continually invest in our regulatory and compliance expertise to ensure the safety, dependability and quality of the products we support.
Q: Can you describe an instance where you helped a customer bring an RUO instrument to market?
A: We recently helped a customer transition an RUO instrument to IVD application for COVID-19 in the near term, and other diagnostic applications afterward. This involved critical analysis and partial redesigning of a system that was originally built for lab use to obtain IVD certification. It may sound simple, but one thing that’s frequently missed in RUO product development is a rigorous quality system for documentation of initial design inputs, and the subsequent crosscheck to ensure design outputs meet the input objectives.
For this application especially, time was precious. We leveraged as much of the system’s existing components as possible in the redesign to save time. Using a systems engineering approach, we brought our full depth of experience to the table to get this project to market quickly. We also worked to ensure the customer met all regulatory requirements, including EMC, electrical safety and IEC 62304.
Related Articles:
Aftermarket services for life sciences: finding the right partner for products in the market, life science commercialization: getting complex instruments to market faster, a valuable framework to evaluate maturity of technology for life sciences.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
Bioresearch Monitoring Inspections in Vitro Diagnostics Devices
TABLE OF CONTENTS
Introduction
Nature, Scope, & Purpose
Exemptions from 21 CFR 812
Labeling Requirements
Prohibited Labeling Information
Specimen Testing & Sampling Reqmnts
The Sponsor's Investigational Plan
Proposed Intended Use of the IVD and Clinical Data
Performance Characteristics and the Clinical Data
Factors Affecting the Quality of the Results of the Clinical Investigation
INTRODUCTION
The purpose of this document is to provide a written reference for Food and Drug Administration (FDA) Investigators conducting bioresearch monitoring (BIMO) inspections involving in vitro diagnostic (IVD) devices. The following material presents key aspects of existing compliance approaches to BIMO IVD inspections.
This guide was prepared by the FDA, Office of Regulatory Affairs (ORA) and the Center for Devices and Radiological Health (CDRH) with input from the Center for Biologics Evaluation and Research (CBER).
NATURE, SCOPE, AND PURPOSE
The purpose of bioresearch monitoring inspections is to ensure that data and information contained in premarket applications are scientifically valid and accurate. Another objective of the program is to ensure that human subjects are protected from undue hazard or risk during the course of scientific investigations. Legal authority for these inspections is found in Section 704 of the Federal Food, Drug and Cosmetic Act (the Act) which gives FDA authority to inspect facilities where devices are "held."
IVD products are those reagents, instruments, and systems intended for use in the diagnosis of disease or other conditions, including a determination of the state of health, in order to cure, mitigate, treat, or prevent disease or its sequelae. Such products are intended for use in the collection, preparation, and examination of specimens taken from the human body. These products are devices as defined in section 201(h) of the Act.
EXEMPTIONS FROM 21 CFR 812
Section 21 CFR 812.2(c)(3) exempts investigations of IVD devices from the specific regulations of 21 CFR 812, Investigational Device Exemptions, under certain conditions. Furthermore, because these are clinical investigations, good laboratory practices (GLP) regulations do not apply and should not be used as a basis for citations on the form FDA-483. Although the design control section of the Quality System Regulation applies to investigational devices, Quality System Regulation deviations should only be cited during Quality System Regulation inspections. In order to be exempt from 21 CFR 812 the sponsor must comply with the labeling requirements of 21 CFR 809.10(c) and the testing requirements of 21 CFR 812.2(c)3).
LABELING REQUIREMENTS
IVDs shipped solely for research purposes must be labeled: "For Research Use Only, Not for use in diagnostic procedures." If an IVD is labeled "For Research Use Only," the research that may be performed is limited to the laboratory research phase needed to identify test kit methods, components, and analytes to be measured. An IVD labeled for research use as described above is mislabeled if used for a clinical study for even one patient if the results are reported to the patient's physician or to the patient's medical records. Research use devices are not to be used to assess the patient's condition regardless of whether or not a confirmatory test or procedure is used. IVDs shipped for clinical investigations must be labeled: "For Investigational Use Only. The performance characteristics of this product have not been established. The regulations define an investigation as a clinical investigation or research involving one or more subjects to determine the safety or effectiveness of a device. (See draft CPG Commercialization of In Vitro Diagnostic (IVD) Devices Labeled for Research Use Only or Investigational Use Only, dated January 5, 1998. This CPG will not be implemented until finalized).
PROHIBITED LABELING INFORMATION
Labeling cannot include any representation that the IVD is safe or effective because this is a determination that only the FDA can make based on the review of data gathered through the clinical investigation and supplied by the sponsor to FDA.
Labeling cannot include performance characteristics or expected range because they will be established by the research and/or clinical investigation.
SPECIMEN TESTING AND SAMPLING REQUIREMENTS
The testing must be noninvasive, must not require an invasive sampling procedure that presents significant risk, must not introduce energy into the patient, and must not be used as a diagnostic procedure without confirmation of the diagnosis by an established diagnostic product or procedure.
21 CFR 812.3(k) defines noninvasive devices or procedures as those that do not penetrate or pierce the skin, mucous membranes, ocular cavity or urethra or do not enter body orifices beyond specified limits. However, the regulation defines simple venipuncture to obtain blood specimens and the use of surplus samples of body fluids or tissues left over from samples taken for non-investigational purposes as noninvasive.
Procedures like amniocentesis, lumbar puncture, and tissue biopsy, are examples of invasive sampling procedures that present significant risk. If they are performed solely for the investigation, then the IVD would not be exempt from the IDE regulations. If samples from these procedures are left over from samples originally taken for non-investigative purposes, then the sampling is considered noninvasive. However, the initial procedure should have been indicated for the patient's condition by current medical practice and not performed to obtain specimens surreptitiously for the clinical investigation.
In order to be exempt from 21 CFR 812, the investigational device cannot be used as a diagnostic procedure without confirmation of the diagnosis by another, medically established diagnostic product or procedure. Disease diagnosis usually involves a number of observations and factors including signs and symptoms, medical history, and a battery of tests. There are few tests that are pathognomonic, i.e., are considered "gold standards," for diagnosis of a disease and, therefore, the diagnosis is established from a number of factors. Moreover, a sponsor or investigator may consider the investigational IVD to be more accurate, precise, sensitive, specific, etc., than current medically established products or procedures. This is generally the goal for producing a new product.
Nevertheless, the diagnosis itself must be confirmed in the established way to meet the requirements for exemption from the IDE regulation.
THE SPONSOR'S INVESTIGATIONAL PLAN
In order to obtain valid scientific data to support its submission to the FDA and to maintain the integrity of that data, the sponsor should have an investigational plan including a protocol or other effective means to communicate procedures, etc., to its investigators. Non-adherence to such a protocol should be noted on an FDA 483.
Purpose: The purpose of the plan is to establish and support claims and information in proposed labeling, including intended use; statements about reagents, instruments, and specimens; the procedure; limitations of the procedure; expected values; and specific performance characteristics; and to support a determination of safety and effectiveness and/or substantial equivalence.
Description: Such a study must be carried out in a scientifically sound manner. Therefore, to assure useful results and the integrity of the data and to be able to present their plan to an Institutional Review Board (IRB), the sponsor should develop an investigational plan. A good plan will include all information, procedures, reporting forms, etc., required by the clinical investigator to gather valid data for the sponsor to submit to FDA. These would include such things as a statement of purpose, a protocol, a description of the device, monitoring procedures, labeling, consent materials, IRB information, and additional records and reports. Additional records could include a certification program that ensures that the sponsor is controlling the distribution of the investigational and/or research device and is using it in scientifically sound research and investigations. The investigator should sign an investigator's agreement acknowledging his/her responsibilities.
At this phase there should be no promotional/advertising material. Advertisements to recruit subjects should be reviewed by the IRB to ensure that information is not misleading and that patient's rights and welfare are protected.
IRB and Informed Consent: The IRB must review and approve the protocol and consent materials before the study can begin. 21 CFR 56, Institutional Review Boards, and 21 CFR 50, Informed Consent, do not specifically exempt IVDs and, therefore, are applicable.
Because most IVD research and investigations do not require an IDE and are minimal risk, the IRB may use expedited review procedures to review most IVD research and investigational proposals. The IRB must document why expedited review was used for approving the IVD investigation.
The IRB may exempt the study from informed consent if it finds that the research presents no more than minimal risk of harm to subjects and involves no procedures for which written consent is normally required. For example, an IRB may exempt a study from informed consent if left-over specimens will be used, provided that patient confidentiality is maintained.
Protocol: While the protocol does not need FDA approval, it is an essential tool for the sponsor to communicate accurately to the IRB and the clinical investigator. Although the sponsor is exempt from labeling requirements if it meets the requirements of 21 CFR 809.10(c), the labeling or its equivalent supplies the clinical investigator with important information about the test procedure. Without a protocol, or similar tool, the sponsor runs the risk of getting invalid results from the investigation. The protocol and the labeling should reflect all the steps the clinical investigator must take to obtain useful information for the sponsor. They should describe such things as specimen collection, instrumentation, reagents, calibration, quality control, step-by-step procedures, calculations, storage conditions, stability of various components both before and after opening and/or reconstituting, reporting procedures, and the necessary reporting forms, etc., for obtaining accurate and precise results and communicating them to the sponsor.
PROPOSED INTENDED USE OF THE IVD AND THE CLINICAL DATA
For Diagnosis or Differential Diagnosis of a Disease or Medical or Physiological Condition: The sponsor may use the data to establish expected values or ranges and cut-off values. The sponsor's proposed labeling will designate concentrations that characterize the healthy and affected populations. These are usually expressed as diagnostic cut-off values. This information will determine the clinical usefulness of the test results and will affect the rates of true and false results. Since treatment may be based on a diagnosis from an IVD, expected values should be established with accurate information. For example, an IVD to measure blood glucose levels will have a normal range for healthy individuals. Values outside the normal range will be used in the diagnosis of diabetes.
In many cases, the sponsor may simply compare the performance of the investigational device to a device already cleared with the same intended use, using left-over patient specimens. When the patient's diagnosis is necessary, it must have been established by some medically acceptable scientific method. In those cases, the sponsor and investigator must record enough of the patient's medical history to determine the diagnosis and any other conditions that might impinge on the performance of the IVD.
To Monitor a Patient's Therapy or to Follow
Their Progress After Treatment: Records should establish which patients are on the therapy or have had the treatment. For example, if the IVD measured a tumor marker to assure total removal of the tumor and/or monitor its reoccurrence, then records should reflect the patient's diagnosis and treatment and the pre-treatment levels of the marker.
Screening and Prognosis: Screening is performed to identify risk factors in health promotion and disease prevention. For example, cholesterol screening may be performed on a random population to identify individuals with this risk factor for heart disease.
Prognosis means determining the intensity or stage of a disease and predicting the expected course of a disease.
Generally firms do not develop an IVD specifically for screening or prognosis. IVDs intended to diagnose or monitor are used instead and the results translated into screening or prognostic terms. If the intended use is, or includes screening, then the investigation should reflect the anticipated screening population, generally healthy adults. If it is for prognosis, then the screening population should consist almost exclusively of those with the disease. Prognostic claims should be established with patient outcome data.
Home Use and Physician Office Lab Devices
Versus Professional Lab Devices: If a device is intended for use outside the professional laboratory setting, the Office of Device Evaluation may require other types of studies, e.g., analyses performed by the actual users.
The FDA investigator should be alert to any special instructions, e.g., patient instruction and preparation, when he or she is inspecting such studies.
PERFORMANCE CHARACTERISTICS AND THE CLINICAL DATA
Labeling: The sponsor may use the investigational data to support the performance characteristics section of the product's proposed labeling. This section of the labeling describes how well the device performed during the clinical investigation and describes such things as the accuracy, precision, sensitivity, and specificity of the IVD. The sponsor is establishing the purported quality of the device and therefore should assure that the data are valid. Accuracy or bias describes how well the IVD result compares to the actual concentration in the patient's specimen. Precision describes how well the IVD repeats test results on the same material. Sensitivity describes the lowest concentration at which the IVD gives acceptable results.
Specificity is the ability of the IVD to accurately measure the analyte of interest in the presence of potential interfering substances. The performance characteristics should be related to a generally accepted method and use biological specimens from normal and abnormal populations. The sponsor should define these populations. Too few patients in any one group may not provide the sponsor with the statistical power to make a claim in their labeling.
FACTORS AFFECTING THE QUALITY OF THE RESULTS OF THE CLINICAL INVESTIGATION
There are many factors that may affect the quality and validity of the data collected to support the claims and statements discussed above. The sponsor and investigator should control these factors using QC and QA methods applicable to diagnostic and analytical laboratories. These factors are usually categorized as pre-analytical, analytical, and post-analytical.
Factors: Pre-analytical considerations center around the patient, his or her preparation, and the specimen.
Analytical considerations include everything surrounding the actual measurement process. Post-analytical considerations center around the proper calculation and reporting of results. Although elements of QC and QA principles outlined in FDA's Good Laboratory Practices or Quality Systems GMP Regulation may apply, the regulations themselves do not. FDA Investigators should base any inspectional observations on whether the sponsor or clinical investigator followed the protocol and labeling specified for the investigation.
Although 21 CFR Part 812, Investigational Device Exemptions (IDE), is used as guidance when reviewing inspectional reports, the IDE regulations themselves do not apply to in vitro diagnostic devices and should not be used as a reference when documenting observations on the FDA-483.
CONCLUSION In summary, IVDs for clinical investigations or research must meet the labeling requirements in 21 CFR 809.10(c). Labeling must not contain performance claims, diagnostic ranges, indications of safety and effectiveness, etc. The sponsor must control the distribution of the device to avoid the appearance of commercializing an uncleared or unapproved medical device. They must also meet the requirements of 21 CFR 56, Institutional Review Boards, and 21 CFR 50, Protection of Human Subjects. Additionally, if a protocol or its equivalent exists, the FDA Investigator should assure that the clinical investigator has followed it. The clinical investigator should have followed the specific inclusion and exclusion criteria for patients assuring that the diagnosis for each patient is accurate by a cleared IVD or other standard of diagnosis. They should assure the integrity of the data, specifically, that the analyses were actually performed according to instructions that accompany the kit and that the data were recorded and reported accurately. Raw data should exist to support the data submitted in reports and applications to the Agency. Although many of these requirements resemble GLPs, the observations should be in terms of adherence to the sponsor's protocol. Should you have comments or questions regarding In Vitro diagnostic bioresearch monitoring inspections or this guide, please contact Robert Fish at:
Center for Devices and Radiological Health Office of Compliance Division of Bioresearch Monitoring Program Enforcement Branch II, HFZ-312 28 Gaither Road Rockville, MD 20850 (301) 594-4723
REFERENCES This reference is intended to be used in conjunction with the: -Compliance Program Guidance Manuals for Institutional Review Boards; Sponsors, Contract Research Organization and Monitors; and Clinical Investigators (CP 7348.809; 7348.810; and 7348. 811), -21 CFR Part 809 - In Vitro Diagnostic Products for Human Use -21 CFR Part 812.2 (c)(3), 812.3(k) - IVD exemptions -21 CFR Part 50 - Protection of Human Subjects -21 CFR Part 56 - Institutional Review Boards -Investigations Operations Manual (IOM), and -Applicable Compliance Policy Guides (CPG) for devices (beginning with the numbers 7124 and 7133).
Guidances are posted to the CDRH and ORA Internet World Wide Web Home Pages at http://www.fda.gov . See IOM Chapter 10, References, for additional information.
Return to: Page Top | Inspection Start

- Search forums
- Medical Devices, Medical Information Technology, Medical Software and Health Informatics
- Medical Device Related Regulations
- EU Medical Device Regulations
RUO (Research Use Only) vs. IVD (In Vitro Diagnostic) - Differences
- Thread starter QA Bee
- Start date Jul 23, 2014
- Jul 23, 2014
Can we use a RUO kit in an IVD registered equipment? Will the IVD regulations (both in US and EU) permit this? I understand that ASR products have to be claimed as IVD as soon as it's used on IVD equipment, so wondering if same rule applies with RUO products,
Re: RUO vs. IVD For the EU: IVD's that are placed on the market must comply with the IVD directive and carry the CE mark. An RUO by definition is intended for research use only, not clinical use and therefore does not meet the requirements of the directive. If you want to add an RUO kit to IVD equipment and use it in a clinical setting then it must comply with the directive.
maria elena
- Oct 25, 2019
which are the regulations to follow to place an RUO reagent kit in Europe and USA? Thanks! Maria Elena
Involved In Discussions
- Jul 16, 2020
maria elena said: which are the regulations to follow to place an RUO reagent kit in Europe and USA? Thanks! Maria Elena Click to expand...
LukasLosigkeit
Starting to get involved.
- Jul 27, 2020
In practice, RUOs are used in laboratories for diagnostics. However, the laboratories then validate each RUO batch and associated procedures prior to use (Europe).
LukasLosigkeit said: In practice, RUOs are used in laboratories for diagnostics. However, the laboratories then validate each RUO batch and associated procedures prior to use (Europe). Click to expand...
- Jul 29, 2020
akp060 said: For USA, you can follow recommendations HERE . I am not sure if you would require Registration/Listing though. Any comments about registration/listing requirements, Exempt or Subject to Enforcement Discretion? Click to expand...
- Aug 23, 2022
Hi, I know this thread is relatively old now. But a follow-up question if anyone knows the answer. Can a manufacturer sell a device which is cleared for use labelled as "IVD" within its scope of the intended use but also sell the same device not with IVD label but with "RUO" label to allow it's exploration in the market? Thanks
REG12 said: Hi, I know this thread is relatively old now. But a follow-up question if anyone knows the answer. Can a manufacturer sell a device which is cleared for use labelled as "IVD" within its scope of the intended use but also sell the same device not with IVD label but with "RUO" label to allow it's exploration in the market? Thanks Click to expand...
Thank you Lukas for explaining that. I understand products being technically identical and being sold as RUO and CE marked. But can they be one single product designed through new product development procedure, but have two different labellings?
Similar threads
- Feb 20, 2023
- RegulatoryUS_EU
- Jul 12, 2023
- CE Marking (Conformité Européene) / CB Scheme
- US Medical Device Regulations
- maxoverclock
- May 7, 2024
- Sep 13, 2023
- This site uses cookies to help personalise content, tailor your experience and to keep you logged in if you register. By continuing to use this site, you are consenting to the use of cookies. Accept Learn more…

IMAGES
VIDEO
COMMENTS
Regulatory Requirements for Research Use Only and Investigational Use Only IVD products Section 520(g) of the FD&C Act, 21 U.S.C. 360j(g), provides for the exemption of devices
Provides the current thinking of CDRH and CBER on when IVD products are properly labeled 'for research use only' (RUO) or 'for investigational use only' (IUO).
Clinical laboratory professionals may not pause to remember that these labels stand for In Vitro Diagnostics (IVD) and Research Use Only (RUO). Even clinical laboratory professionals who are familiar with these regulatory designations for assays or instruments sometimes do not realize the full significance that these labels have for certain ...
In Europe, the MEDDEV 2.14/2 guidance document (IVD Guidance: Research Use Only products - A guide for manufacturers and notified bodies) provides a definition of RUOs. This guidance was written within the framework of the now obsolete Directive 98/79/EC on in vitro diagnostic medical devices (IVDD) and, in the absence of an up-to-date ...
01. Introduction. This document has been developed as a result of the outcome of initial discussions on "research only products" at the Medical Devices Expert Group (MDEG) meeting of July 2003. It aims to clarify a number of issues raised by Competent Authorities with regard to products labeled as "For Research Use Only" (RUO) and their ...
- A product in the laboratory research phase, not represented as an IVD, that is prominently labeled: "For Research Use Only. Not for use in diagnostic procedures;" and
(IVD) products intended for research use only (RUO) or investigational use only (IUO). In addition, the guidance serves as a warning that products so labeled should not be used in clinical diagnosis or patient management. The College of American Pathologists (CAP), celebrating 50 years as the gold standard in laboratory
There are four different regulatory levels for IVDs: Research Use Only (RUO) General Laboratory Use (GLU) For Performance Studies Only (PSO) In Vitro Diagnostic Medical Device (IVD) The simplest explanation for these different levels is that each increasing level requires more testing and oversight. Research Use Only products are at the lowest ...
Research Use Only (RUO) products play a crucial role in medical research and innovative management of many patients. These specialised products, which include laboratory reagents and equipment, are exclusively designed for research in controlled laboratory environments. As essential tools for medical and scientific investigations ...
Research product (Research Use Only/RUO) is medical device and in-vitro medical device product that is in research development stage and has not been approved to be used for clinical purposes; or which is declared RUO by the authorized body in country of origin of the manufacturer. Category 3. 1.
The 21 CFR 809.10 and 21 CFR 864 define four types of IVDs: General Purpose Reagent (GPR), Investigational Use Only (IUO), Analyte Specific Reagent (ASR) and Research Use Only (RUO). This is why in the U.S., RUOs are also called RUO IVDs - In contrast, in EU, only the term RUO prevails. As per the 21 CFR, RUO products are IVD products in the ...
In addition, IVD material must be manufactured under the FDA's current Good Manufacturing Practices (cGMP). Should "Research Use Only" products be used for quality control? The second misconception clinical laboratories should be aware of involves material labeled as Research Use Only (RUO). RUO labeling is intended for products that are ...
Except as a component of the approved/cleared test (Name of approved/cleared test), analytical and performance characteristics of this ASR are not established". The Shop. U.S. regulations define four other IVD types: Research Use Only (RUO), Investigational Use Only (IUO), General Purpose Reagent (GPR), and Analyte Specific Reagent (ASR).
Save for later. NEW YORK (GenomeWeb News) - The Food and Drug Administration has released a guidance document that lays out and clarifies the rules for how in vitro diagnostic products for research use only (RUO) and investigational use only (IUO) may be used, labeled, or marketed. FDA created the guidance on RUOs and IUOs, which has been ...
Thermo Fisher Scientific GMP products can support your efforts to produce products that function consistently as intended. We follow quality standards in manufacturing, testing, documentation, and proven use. Our - CTS products, intended for use in GMP production, are manufactured at sites that are FDA registered, ISO 13485 certified, and ...
IVD devices that are under study, including IVD devices that are exempt from the IDE regulations, must comply with labeling requirements under 21 CFR 809.10(c)(2). ... "For Research Use Only. Not ...
To receive "Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only" you may either send an email request to [email protected] to receive an electronic copy of the document or send a fax request to 301-847-8149 to receive a hard copy. Please use the document number 1723 to identify the ...
Research Use Only: A non-IVD product in the laboratory research phase of development that cannot be used in diagnostic procedures. IUO: Investigational Use Only: A product being shipped or delivered for product testing and process evaluation, but not yet commercialized. ...
These recommendations can fill the gap between research use only (RUO) and in vitro diagnostics (IVD). Our contribution provides a tool for basic and clinical research for the development of validated assays in the intermediate steps of biomarker research. ... (IVD)-grade quantitative reverse transcription PCR (qRT-PCR) assays for clinical use ...
In particular, we're seeing more companies looking to move instruments from Research Use Only (RUO) applications to pursue in vitro diagnostic (IVD) applications with the same instrument. This is a big undertaking, which is why companies find value in working with Plexus. Commercializing an RUO solution for the IVD market comes with ...
If an IVD is labeled "For Research Use Only," the research that may be performed is limited to the laboratory research phase needed to identify test kit methods, components, and analytes to be ...
the FDA in a research context or as part of product development or a clinical trial. However, the assay cannot be used legally for clinical diagnostic procedures or any purpose other than research or investigation. The assay must be prominently labeled for Research Use Only (RUO) or Investigational Use Only (IUO) prior to shipment or delivery to a
Trusted Information Resource. Jul 23, 2014. #2. Re: RUO vs. IVD. For the EU: IVD's that are placed on the market must comply with the IVD directive and carry the CE mark. An RUO by definition is intended for research use only, not clinical use and therefore does not meet the requirements of the directive. If you want to add an RUO kit to IVD ...