Internet Explorer lacks support for the features of this website. For the best experience, please use a modern browser such as Chrome, Firefox, or Edge.


New Research Reveals Full Diversity of Killer Whales as Two Species Come into View on Pacific Coast
March 27, 2024
Long viewed as one worldwide species, killer whale diversity now merits more. Southern Resident Connections - Post 35
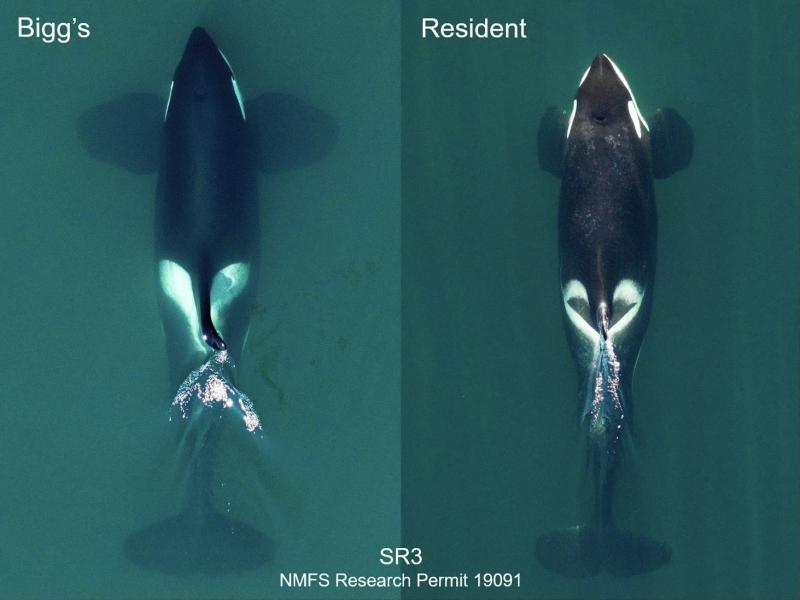
Scientists have resolved one of the outstanding questions about one of the world’s most recognizable creatures, identifying two well-known killer whales in the North Pacific Ocean as separate species.
Killer whales are one of the most widespread animals on Earth. They have long been considered one worldwide species known scientifically as Orcinus orca , with different forms in various regions known as “ecotypes.”
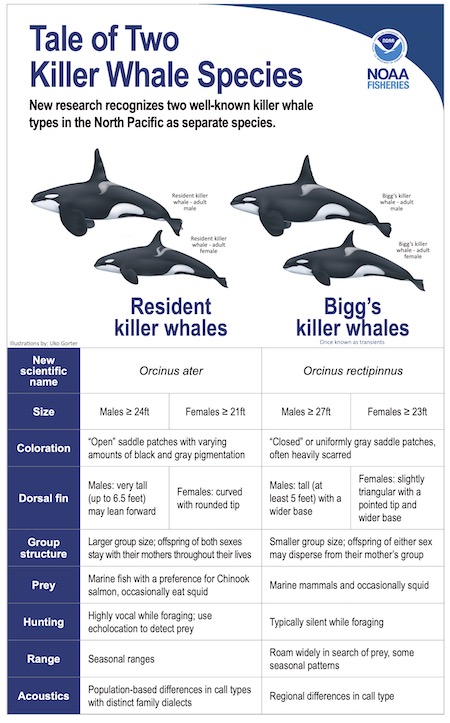
However, biologists have increasingly recognized the differences between resident and Bigg’s killer whales. Resident killer whales maintain tight-knit family pods and prey on salmon and other marine fish. Bigg’s killer whales roam in smaller groups, preying on other marine mammals such as seals and whales. (Killer whales actually belong to the dolphin family.) Bigg’s killer whales, sometimes called transients, are named for Canadian scientist Michael Bigg, the first to describe telltale differences between the two types.
He noted in the 1970s that the two animals did not mix with each other even when they occupied many of the same coastal waters. This is often a sign of different species.
The finding recognizes the accuracy of the listing of Southern Resident killer whales as a Distinct Population Segment warranting protection under the Endangered Species Act in 2005. At the time, NOAA described the distinct population segment as part of an unnamed subspecies of resident killer whales in the North Pacific.
Now a team of scientists from NOAA Fisheries and universities have assembled genetic, physical, and behavioral evidence. The data distinguish two of the killer whale ecotypes of the North Pacific Coast—residents and Bigg’s—as separate species.
“We started to ask this question 20 years ago, but we didn’t have much data, and we did not have the tools that we do now,” said Phil Morin, an evolutionary geneticist at NOAA Fisheries’ Southwest Fisheries Science Center and lead author of the new paper . “Now we have more of both, and the weight of the evidence says these are different species.”
Genetic data from previous studies revealed that the two species likely diverged more than 300,000 years ago and come from opposite ends of the killer whale family tree. That makes them about as genetically different as any killer whale ecotypes around the globe. Subsequent studies of genomic data confirm that they have evolved as genetically and culturally distinct groups, which occupy different niches in the same Northwest marine ecosystem.
“They’re the most different killer whales in the world, and they live right next to each other and see each other all the time,” said Barbara Taylor, a former NOAA Fisheries marine mammal biologist who was part of the science panel that assessed the status of Southern Residents. “They just do not mix.”
Recognizing New Species
The Taxonomy Committee of the Society of Marine Mammalogy will determine whether to recognize the new species in its official list of marine mammal species . The committee will likely determine whether to accept the new designations at its next annual review this summer.
The scientists proposed scientific names for the new species based on their earliest published descriptions in the 1800s. Neither will keep the ubiquitous worldwide moniker, orca . The team proposed to call resident killer whales Orcinus ater , a Latin reference to their dominant black coloring. Bigg’s killer whales would be called Orcinus rectipinnus , a combination of Latin words for erect wing, probably referring to their tall, sharp dorsal fin.
Both species names were originally published in 1869 by Edward Drinker Cope, a Pennsylvania scientist known more for unearthing dinosaurs than studying marine mammals. He was working from a manuscript that California whaling captain Charles Melville Scammon had sent to the Smithsonian Institution describing West Coast marine mammals, including the two killer whales. While Cope credited Scammon for the descriptions, Scammon took issue with Cope for editing and publishing Scammon’s work without telling him. (See accompanying story .)
The Smithsonian Institution had shared Scammon’s work with Cope, and a Smithsonian official later apologized to Scammon for what he called “Cope’s absurd blunder.”
Species Reflect Ecosystem
The contested question of whether Southern Residents were distinct enough to merit endangered species protections initially drove much of the research that helped differentiate the two species, said Eric Archer, who leads the Marine Mammal Genetics Program at the Southwest Fisheries Science Center and is a coauthor of the new research paper. The increasing processing power of computers has made it possible to examine killer whale DNA in ever finer detail. He said the findings not only validate protection for the animals themselves, but also help reveal different components of the marine ecosystems the whales depend on.
“As we better understand what makes these species special, we learn more about how they use the ecosystems they inhabit and what makes those environments special, too,” he said.
The new research synthesizes the earliest accounts of killer whales on the Pacific Coast with modern data on physical characteristics.
The team also use aerial imaging (called photogrammetry ), and measurement and genetic testing of museum specimens at the Smithsonian and elsewhere. While the two species look similar to the untrained eye, the evidence demonstrates they are very different species. The two species use different ecological niches, such as specializing in different prey, said Kim Parsons, a geneticist at the NOAA Fisheries Northwest Fisheries Science Center in Seattle and coauthor of the new research.
Recent research with drones that collect precise aerial photos has helped differentiate Bigg’s killer whales as longer and larger. This might better equip them to go after large marine mammal prey. The smaller size of residents is likely better suited to deep dives after their salmon prey, said John Durban, an associate professor at Oregon State University’s Marine Mammal Institute. His killer whale drone research is done collaboratively with Holly Fearnbach, a researcher at SR³.
The different prey of the two species may also help explain their different trajectories. Southern Residents are listed as endangered in part because of the scarcity of their salmon prey. Bigg’s killer whales, by contrast, have multiplied while feeding on plentiful marine mammals, including California sea lions.
While killer whales represent some of the most efficient predators the world has ever seen, Durban said science is still unraveling the diversity among them. The identification of additional killer whale species is likely to follow. One leading candidate may be “Type D” killer whales identified in the Southern Ocean around Antarctica.
Other killer whales in Antarctic waters also look very different from the best-known black and white killer whales. This reflects a wider diversity within the species, said Durban, who has used drones to study killer whales around the world. “The more we learn,” he said, “the clearer it becomes to me that at least some of these types will be recognized as different species in due course.”

Southern Resident Connections
Southern Resident killer whales are icons of a vibrant but struggling marine ecosystem that is important to us all. Join us in exploring the ecological connections that tie this system together, and the ways we are protecting and working to recover the whales we all care so much about.
Read more entries
More Information
- New Research Reveals Two Species of Killer Whale
- How Scientists Chose Names for Newly Identified Killer Whale Species
- Two Species of Killer Whale Infographic
- Marine Mammal Genetics Research
- 2004 Status Review of Southern Resident Killer Whales
- Saving the Southern Resident Killer Whales
- Listing of Southern Resident Killer Whale Under the ESA
- Killer Whale Ecotypes Poster
Recent News
Washington tribes restore salmon habitat on south fork nooksack river.

Reestablishing Connections for Fish and Tribes on Oregon’s North Santiam River

A Boater Drove Through a Pod of Killer Whales. Turns Out a Wildlife Videographer Was Filming.

Last updated by Southwest Fisheries Science Center on April 03, 2024
ORIGINAL RESEARCH article
Movements and social behavior of killer whales ( orcinus orca ) off the brazilian coast.

- 1 Projeto Baleia à Vista (ProBaV), Ilhabela, SP, Brazil
- 2 Departamento de Zoologia, Instituto de Biociências, Universidade de São Paulo, São Paulo, SP, Brazil
- 3 Department of Fisheries, Wildlife and Conservation Sciences, Marine Mammal Institute, Oregon State University, Newport, OR, United States
- 4 Orca Behavior Institute, Friday Harbor, WA, United States
- 5 Instituto Argonauta para Conservação Costeira e Marinha, Ubatuba, SP, Brazil
- 6 Departamento de Ciências Biológicas, Escola Nacional de Saúde Pública/ Fiocruz and Grupo de Estudos de Mamíferos Marinhos da Região dos Lagos (GEMM-Lagos), Rio de Janeiro, RJ, Brazil
Killer whales ( Orcinus orca ) are cosmopolitan apex predators that occupy important ecological roles and show some variations in feeding and social habits in coastal and pelagic environments worldwide. Although they have been regularly reported along the Brazilian coastline, their natural history in these tropical and subtropical waters remains poorly understood. Here, we provide new information on group size, behavior, movements and the first assessment of their social structure in Brazilian coast. From 2005 to 2021, 57 new records of sightings were opportunistically observed with estimated group sizes ranging from 1 to 11 individuals (mean = 5.61; SD = 2.91), and 47 individuals were photo-identified—28% adult females, 19% adult males, 19% juveniles, 17% calves and 17% adults of unknown sex. Thirty-one individuals (66%) were sighted just once and sixteen (34%) were resighted more than once (resighting rate = 0.30 ± 0.30 SD). Killer whales were observed feeding on rays four times (two out of which on butterfly rays Gymnura altavela ), twice on an unidentified fish school of fish, while attacks on marine mammals were recorded. Between 2020 and 2021, photo-identification results of 11 specific individuals revealed both long and short-distance movements from the southeastern and southern Brazilian coasts to the coast of Uruguay. Individuals seem to be resighted together over time, as suggested by the average half-weight association index (HWI = 0.29 ± 0.19 SD) and a permutation test rejecting the null hypothesis of random association ( CV real = 0.67 > CV mean = 0.01, p CV = 1.00), forming small groups of mixed age-sex that engage in both short- and long-term associations. These patterns suggest that they could form stable social units that also experience some degree of fission-fusion dynamics. While the nature of the opportunistic data hinders a definitive portrayal of the social structure of killer whales using the Brazilian coastal waters, these novel insights contribute to mapping the socio-ecology and behavioral diversity of one of the most widely distributed mammals.
Introduction
Killer whales ( Orcinus orca , Linnaeus, 1758) have a wide distribution throughout the world’s oceans. They are most commonly found in higher latitudes in temperate and sub-polar waters with high marine productivity but also occur in offshore and less productive tropical waters ( Leatherwood and Dalheim, 1978 ; Forney and Wade, 2006 ). They are considered top ocean predators, feeding on a variety of different prey, from invertebrates, fishes, and other marine mammals ( Ford, 2002 ). Within their monotypic genus ( Rice, 1998 ), killer whales from the both hemispheres form several ecotypes with distinguished external morphology, habitat use, behavior, social structure, acoustic repertoire, prey preferences, and genetics ( Ford et al., 1998 ; Baird and Whitehead, 2000 ; Pitman and Ensor, 2003 ; Visser, 2007 ; LeDuc et al., 2008 ; Foote et al., 2009 ; Pitman, 2011 ; Durban et al., 2016 ; Foote et al., 2016 ). In the south-western Atlantic, killer whales were recorded along all Argentinean coast, mainly in Peninsula Valdés ( Iñíguez, 2001 ). Small groups have been sighted around the Falkland (Malvinas) Islands ( Yates et al., 2007 ) and in Uruguay, were observed in coastal waters and also related with depredation on catches of the pelagic longline fishery in offshore waters ( Iriarte, 2006 ; Passadore et al., 2015 ).
Along most of the Brazilian coast, between latitudes 03°N to 32° S, killer whales have been reported as opportunistic sightings or stranding events of single individuals ( Lodi and Hetzel, 1998 ; Siciliano et al., 1999 ; Dantas, 2007 ). Capture records by ex-soviet pelagic fleets during the whaling period (1969/70-1978/79) were also recorded in the region ( Mikhalev et al., 1981 ). Their occurrence in coastal waters off south-eastern Brazil was associated with upwellings conditions and prey availability ( Siciliano et al., 1999 ) and records have increased in the last two decades as more search efforts were applied ( Pinedo et al., 2002 ; Santos and Netto, 2005 ; Meirelles et al., 2009 ; Santos and Silva, 2009 ; Santos et al., 2010 ; Batista et al., 2012 ; Ott et al., 2017 ; Lodi and Tardin, 2018 ; Santos et al., 2019 ; Renault-Braga et al., 2019 ). Few specimens had been studied in detail since fresh strandings are rare ( Lemos et al., 2013 ; Laeta et al., 2019 ; Groch et al., 2020 ) and Morin et al. (2015) cite the stranding of an Antarctic killer whale type C in Brazil but without providing detailed information. The depredation by killer whales in the tuna longline fishery that operates from the north to south of Brazil to Uruguay indicates the regular presence of killer whales in offshore waters ( Secchi and Vaske, 1998 ; Dalla Rosa and Secchi, 2007 ; Dantas, 2007 ; Passadore et al., 2012 ; Wedekin et al., 2014 ). Further evidence of their occurrence in offshore waters off Brazil comes from dedicated surveys and technical reports ( Ramos et al., 2010 ; Andriolo et al., 2015 ; Di Tullio et al., 2016 ; PMC-BS, 2017 ).
Despite these observations off Brazil, the natural history of killer whales in these tropical and subtropical waters remains poorly understood, and data deficiency precludes the assessment of their conservation status ( ICMBio, 2023 ). The first published record of killer whale movements in the region described a solitary male resighted over 11 years along the coast of Rio de Janeiro and Parana (22°S - 25°S) ( Santos and Silva, 2009 ; Lodi and Farias-Junior, 2011 ). In 2016 and 2017, the PMC-BS (Santos Basin Cetacean Monitoring Project) that monitors the cetacean populations in coastal and oceanic areas of the Santos Basin offshore sedimentary basin to assess possible impacts of oil activities and gas on these animals, tagged four individuals in southeastern Brazil. Their data showed animals that remained approximately 225 km from the coast for 3 to 13 days, and a solitary male that moved from southern Brazil to the southern coast of Uruguay and Argentina during 33 days ( PMC-BS, 2017 ). In addition, Santos et al. (2019) reported on a group of killer whales that travelled between Rio de Janeiro and São Paulo over a period of 27 days and Durban and Pitman (2012) , through satellite telemetry, identified movements of five Type B Antarctic killer whales between the Antarctic Peninsula and the oceanic waters of southern Brazil and Uruguay. Such scattered reports on movements and behavior, although precious, hinders our understanding of the complex relationships of killer whale groups of this region. In this paper, new observations including group size, behavior and movements are presented contributing with more pieces of this puzzle in order to have a better understanding of the whole picture. Also, considering that social structure synthesizes a vital class of ecological relationships ( Whitehead, 2008 ), can affect population dynamics, genetics ( Wilson, 1975 ; Strier, 1997 ) and, is an important factor for conservation ( Sutherland, 1998 ), using traditional photo-identification techniques, we present the first assessment of social structure of killer whales off the Brazilian coast.
Materials and methods
Data collection and study area.
The sighting data and images of killer whales in this study were recorded opportunistically from 2005 to 2021 by 3 different contributors: (i) from Baleia à Vista Project (ProBaV), a citizen science project that monitors the waters off the north coast of São Paulo, Brazil, around the region of Ilhabela Archipelago (23° 48.735' S, 45° 22.019' W) ( Cardoso et al., 2019a ; Cardoso et al., 2019b ; Athayde et al., 2020 ; Siciliano et al., 2020 ; Marcondes et al., 2021 ; Athayde et al., 2022 ), (ii) from Argonauta Institute, an institution dedicated to the conservation of coastal and marine ecosystems that also monitors the same region as ProBaV since 1998; and (iii) from occasional contributors: wildlife photographers and enthusiasts, divers, sailors, and others who had opportunistically encountered killer whales during their professional or recreational activities. Most of these contributor’s records were reported from southern and southeastern Brazil, except for five records that were made in La Paloma, on the coast off Uruguay ( Figure 1 ). The authors were contacted directly and the following information were collected: additional media (video/ photos), geographical coordinates, group size estimate and, species behavior observed during the sightings (travelling, feeding, courtship and resting). Permission for the academic use of their images and data were obtained.
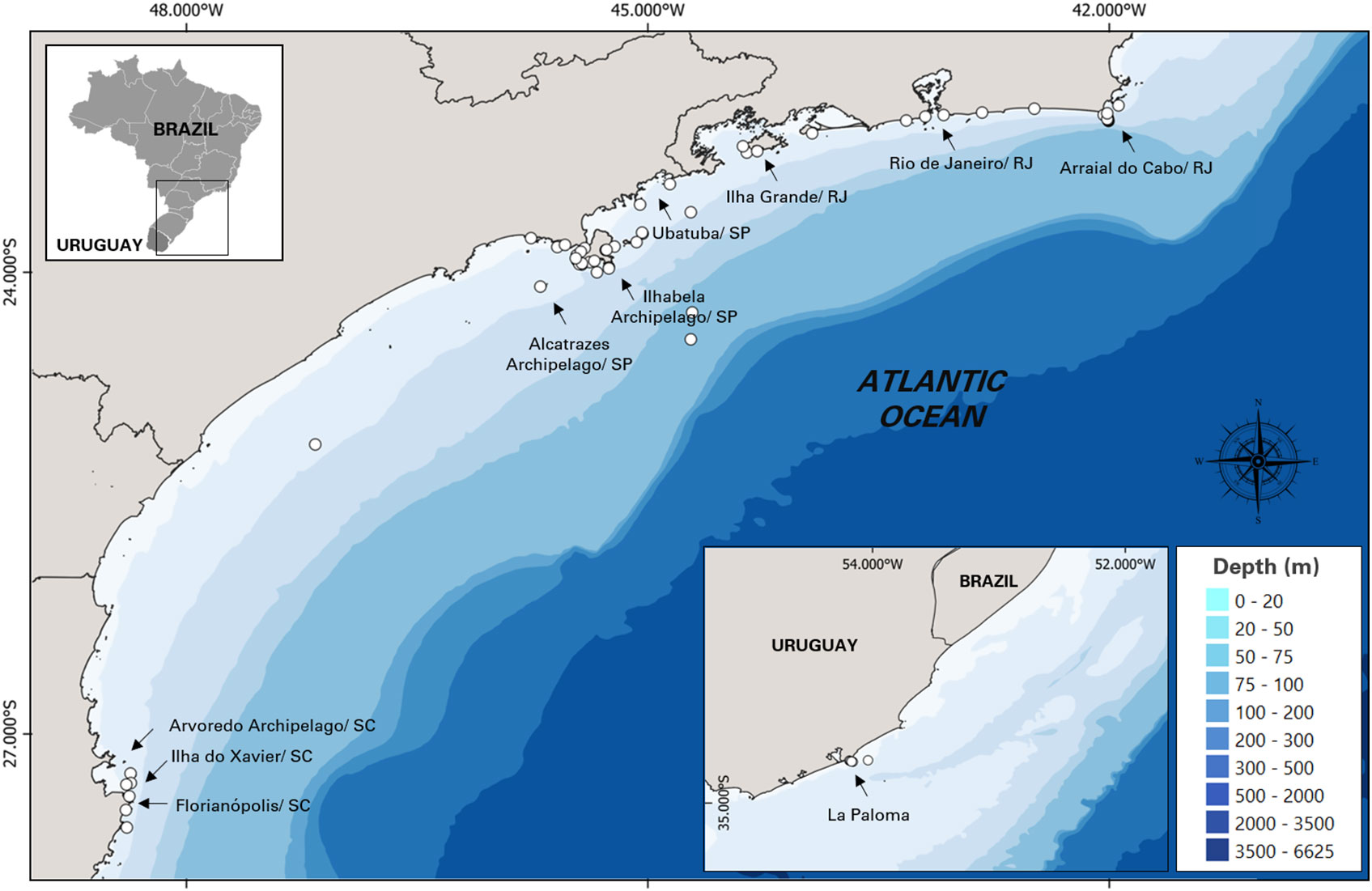
Figure 1 Study area in southeastern coast of Brazil and the 5 additional sightings of Uruguay showing the location of killer whale sightings (n = 57).
Photo and data analysis
Photos from ProBaV and Argonauta Institute were taken using various combinations of DSLR cameras and lenses and, in attempt to increase the chances of identifying individuals, when possible, pictures of both sides of the dorsal fins, eyepatches and saddle patches were taken in different angles. In some encounters, it was not achievable to photograph all individuals of the group. The images shared by contributors were added to this unified database. A total of 8.754 images were analyzed and for the entire data set, a quality rating between 1 (lowest) and 3 (highest) was assigned based on sharpness, contrast and angle of the dorsal fin and the eyepatch in relation to the camera ( Jourdain et al., 2017 ).
For the photo-identification analysis, only qualified images of a quality ≥ 2 were used. The individuals were photo-identified and cross compared by their natural marks, nicks, notches and scars and were included in the catalog ( Figure 2 ). Sex and stage of maturity were based on the external morphology as previously determined by Bigg et al. (1987) and classified in five different categories: 1) Adult males - males that have reached sexual maturity and clearly present a taller dorsal fin; 2) Adult females - individuals with smaller dorsal fin, where no development of secondary sexual characteristics over the years or by close and consistent association with the calf; 3) Juveniles - individuals of both sexes that have not reached mature size, measuring between 50% to 75% the size of an adult female, but larger than calves; 4) Calves - young killer whales measuring less than 50% the size of an adult female; 5) Unknow - when the distance of the image only allows identifying those animals were killer whales but not classifying the sex/ stage of maturity ( Visser, 2000b ; Tavares et al., 2016 ) or when it was not possible to differentiate females from males that had not yet had dorsal fin sprouting. Photo-identified individuals were assigned to alphanumeric labels (prefix BR followed by a number); calves accompanying females were assigned the same label plus a sequential letter. So, if the mother is BR01, the calf will be BR01A. If she is seen again at another time with a new calf, that one will be given the name BR01B and so on. This is a similar method used to catalog the transient killer whales of the west coast of the United States and Canada in Pacific waters ( Ford and Ellis, 1999 ) and for the Bryde’s whales ( Balaenoptera brydei ) studied in the same region ( Athayde et al., 2020 ). The resighting rate of identified killer whales was calculated by dividing individuals re-sighted in previous years (mt) by individuals identified each year (nt) ( Denkinger et al., 2020 ).

Figure 2 Individuals BR05 and BR16 photo-identified by their natural marks through the dorsal fin and eyepatch.
Group size, behavior, and seasonality analysis were recorded for all sightings from 2005 to 2021 from the three sources. The movement analysis considered only records of re-sightings of individuals to demonstrate their displacements. Maps for analysis and representation of movements and sightings were produced with QGIS 3.10 software using bathymetric data from GEBCO Compilation Group, (2021) .
Data social analysis
For the social analysis, we defined groups as two or more different individuals that were in visual range of the observers. To minimize spurious associations, we analyzed only individuals sighted at least three times and in encounters where it was possible to photo-identify at least two individuals in the group. Associations were estimated using the half-weight index (HWI), which represents the proportion of times individuals were seen together, attempting to minimize biases when not all individuals within each group have been identified ( Cairns and Schwager, 1987 ; Whitehead, 2008 ). The resulting association matrix was projected as a social network, in which numbered nodes representing the individuals were linked by weighted lines whose thicknesses were proportional to their association indices ( Whitehead and Dufault, 1999 ; Whitehead, 2009 ). A Monte Carlo permutation test was performed by permuting groups within samples ( Whitehead, 2009 ) to test the null hypothesis that individuals associate at random, using the coefficient of variation (CV) of the association matrix as a benchmark. The null hypothesis was rejected when the CV of the observed data was significantly greater than the CV of the permuted data ( Bejder et al., 1998 ; Whitehead, 2009 ), indicating that there were disproportionately larger and smaller observed indices than expected by chance, which are suggestive of preferred and avoided associations, respectively. The group data were permutated four times (1000, 5000, 10000 and 20000 iterations) until the overall p-value stabilized ( Bejder et al., 1998 ); we reported results generated with 20.000 permutations. To infer social units, if any, we projected the association matrix with hierarchical clustering analysis and used the maximum modularity Q, calculated by the leading eigenvector method ( Newman, 2006 ), to identify a suitable partition of the dendrogram. To test the statistical significance of this partition, we used a null model approach ( Farine and Whitehead, 2015 ), equivalent to the Monte Carlo permutations above ( Bejder et al., 1998 ), to generate an ensemble of 20000 permuted association matrices and build a benchmark distribution of Q -values; the observed Q -value was considered significant if falling outside of the 95% confidence intervals (CI) of the benchmark distribution ( Farine and Whitehead, 2015 ). Finally, we investigate the temporal patterns of associations. We calculated the standardized lagged association rate (SLAR) as the probability of two animals that are associated at a given time to be associated again after different time lags ( Whitehead, 1995 ). As a benchmark, we calculated the null association rates (NAR) in which individuals are assumed to associate at random. The standard errors of SLAR were estimated using the jackknife procedure ( Whitehead, 1995 ). Then, we fitted four exponential models to SLAR ( Whitehead, 1995 ) and selected the most parsimonious with quasi-Akaike Information Criterion (QAIC; Whitehead, 2007 ), to describe how associations decay over time. The first model, SLAR1, has no decay in association rates, suggesting permanent associations; the second model SLAR2, represents association rates that decay to zero, suggesting associations that happened for a given time but never again; SLAR3 represents rates that decay and level off after a given lag, suggesting both long-lasting and temporary associations; SLAR4 represents two exponential decays to zero, suggesting a level of disassociation at a shorter lag and another at longer time lag ( Whitehead, 1995 ). Social analyses were conducted using SOCPROG 2.9 ( Whitehead, 2009 ).
Group size, behavior and, seasonality
The opportunistic sightings from 2005 to 2021 yielded 57 new observations of killer whales, in groups from one to 11 individuals (mean = 5.61, SD = 2.91). Forty-seven individuals were photo-identified including: 28% adult females (n = 13), 19 % adult males (n = 9), 19% juveniles (n = 9; where 7 of unknow sex and 2 males), 17% calves (n = 8; where 7 of unknow sex and 1 male), and 17% of adults of unknown sex (n = 8). Travelling was the most common behavior observed (88%), followed by feeding (10%). In two occasions (July 20, 2019 and December 30, 2020), killer whales were observed hunting and sharing butterfly rays ( Gymnura altavela ) in the south of the Ilhabela Archipelago, São Paulo state, in association with frigatebirds ( Fregata magnificens ) and brown boobies ( Sula leucogaster ) ( Figure 3 ). In a third occasion (November 24, 2021), the group was hunting a stingray (unidentified species) in Arraial do Cabo, Rio de Janeiro. On January 10, 2021 and December 15, 2021, they were spotted surrounding a school of fish (unidentified species) around the region of the Arvoredo Archipelago in Santa Catarina state and Marambaia Island, Rio de Janeiro, respectively. On November 30, 2018, in the northeast of Ilhabela Archipelago, Bryde’s whales were observed by ProBaV feeding on a school of fish when killer whales approached the whales. No attempt to attack Bryde’s whales was observed and despite a brief interaction, killer whales seemed more interested in attacking a stingray (unidentified species) in the same area. After feeding on the stingray, they left the area. Only in one occasion (2%) it was possible to watch orcas in a video socializing in a possible courtship behavior off Ilhabela on December 7, 2012. Resting was not observed. killer Whales were sighted all year round on the coast, and the number of sightings seem to increase in the austral spring and summer (spring: 39%, summer: 33%, winter: 26%, fall: 2%; n=57 sightings).

Figure 3 Killer whales hunting a butterfly ray ( Gymnura altavela ) off the Ilhabela Archipelago, São Paulo, on (A) July 20 2019 and on (B, C) December 30 2020 in association with Fregata magnificens .
Re-sightings
Out of the 47 photo-identified individuals, 31 (66%) were sighted just once and 16 (34%) were resighted more than once, where: 1 (2%) was resighted thirteen times, 1 (2%) eleven times, 1 (2%) ten times, 1 (2%) nine times, 3 (6%) eight times, 1 (2%) seven times, 2 (4%) four times, 4 (8%) three times and, 2 (4%) twice. The mean of resighting rate for the cataloged killer whales was 0.30 (SD = 0.30), noting that in the last 4 years more than 50% of individuals had been sighted in previous years (2018 - 56%; 2019 - 63%; 2020 - 53%; 2021 – 83%) ( Table 1 ).
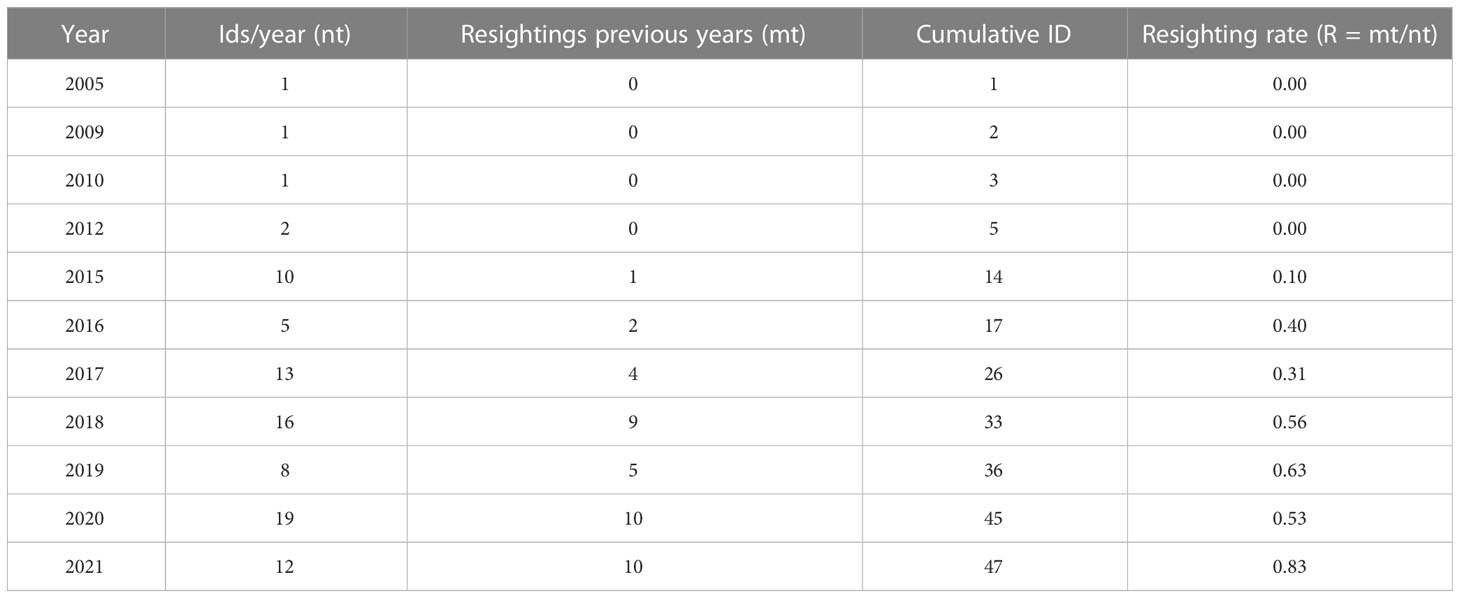
Table 1 Resighting rates per year from 2005 to 2021.
Two animals were resighted equal to or greater than 10 years apart. The adult female BR22, where the first sighting was November, 22 2010 and last on November 22, 2020 (10 years) and the adult male BR14 where the first sighting was July, 04 2005 and last on August 10, 2018 (13 years). A previously known adult male was sighted once traveling alone (BR01). Published articles reported 14 sightings of this male ( Santos and Silva, 2009 ; Lodi and Farias-Junior, 2011 ) over 11 years and here we report one additional sighting on July 7, 2009.
Based on these re-sightings, between 2020 and 2021, it was possible to track the movements of 11 specific individuals: BR03, BR04, BR04A, BR05, BR06, BR08, BR12, BR13, BR16, BR16A and BR22 in different regions ( Figure 4 ). On October 29, 2020, individuals BR04, BR05 and BR06 were sighted with other non-photo-identified individuals near to Ilha do Xavier in the state of Santa Catarina. Seventeen days later, between November 15 and November 22 of 2020, BR03, BR05, BR06, BR08, BR13 and BR16A were sighted in Arraial do Cabo in Rio de Janeiro. On this last occasion, these individuals were seen together with the adult female BR22, which was swimming with 4 other non-photo-identified individuals, in a group of 11 animals. On December 27 of 2020, a group of killer whales were reported in Ilha Grande in Rio de Janeiro and the individuals BR03, BR04A, BR06, and BR08 were identified. On December 28 and 29, BR05, BR16 and, BR16A were sighted between the region of Ubatuba, São Paulo and Ilhabela Archipelago. On December 30, all the killer whales of the group were photo-identified by ProBaV in the Ilhabela Archipelago (BR03, BR04, BR04A, BR05, BR06, BR08, BR12, BR13, BR16 and BR16A). On January 9 and 10, 2021, BR04, BR04A, and BR05, were sighted in Santa Catarina, in the region of Ilha do Arvoredo. Seven months later, on August 18 of 2021, a group of 5 killer whales were sighted in Marica, Rio de Janeiro, where was possible to identify 3 individuals: BR04, BR12 and BR13. The individuals BR05 and BR13 were sighted in La Paloma on September 16, in the coast of Uruguay and 69 days later, on November 24, BR05, BR06, BR12 and BR13 were sighted again on Arraial do Cabo, RJ (approximately 920nm of distance). On December 14, 2021, BR12, BR13, BR16, BR16A were sighted swimming close to the Ipanema beach, Rio de Janeiro and on December 15, individuals BR05, BR08, BR12 were sighted around Marambaia Island. It is important to note that, except for the record of December, 30 of 2020, in all these occasions, more animals were part of the group, but only some of them were photo-identified ( Table 2 ).
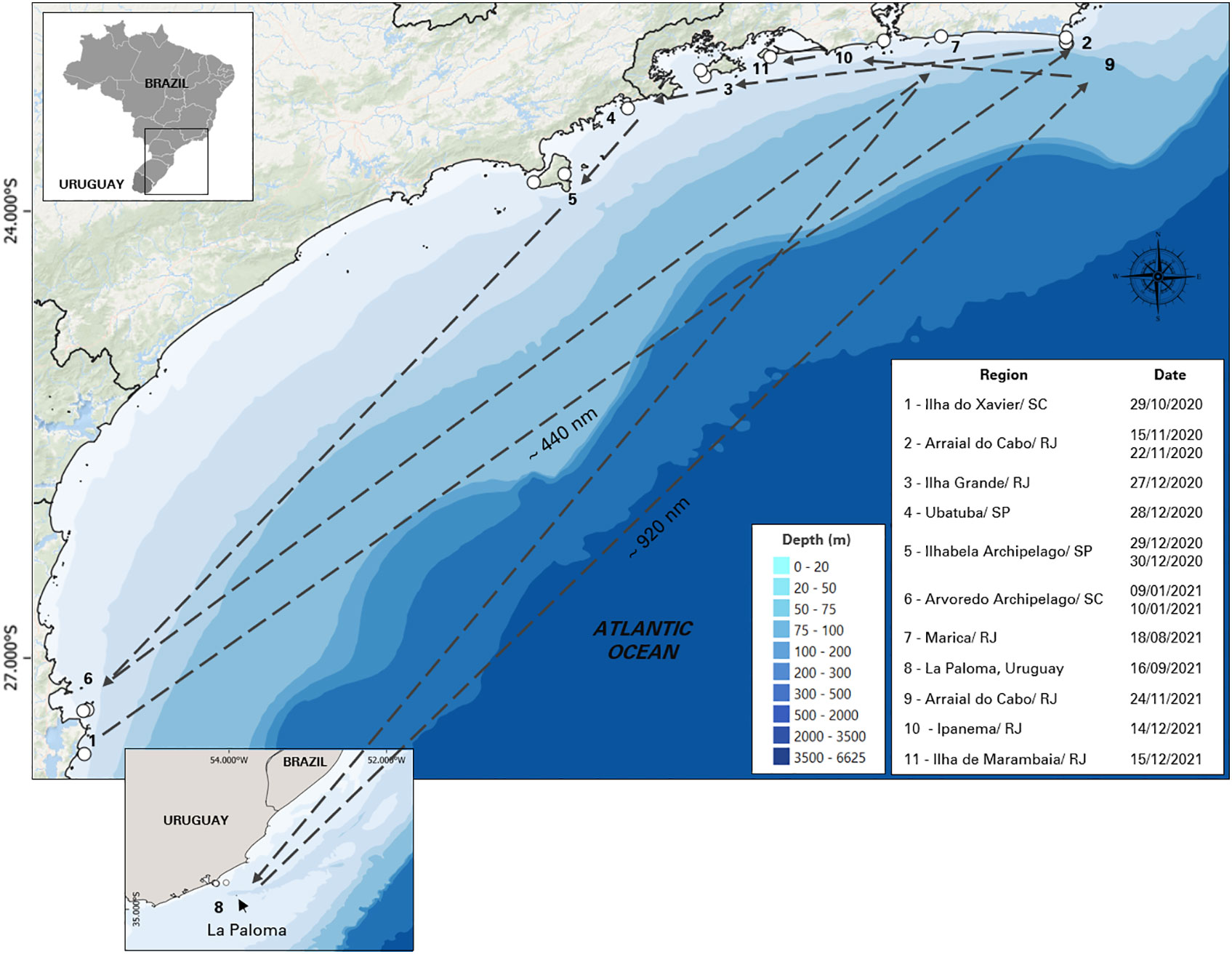
Figure 4 Movements of 11 photo-identified individual killer whales ( Orcinus orca ) between October 2020 and December 2021 along the southeastern-south Brazilian coast and Uruguay.
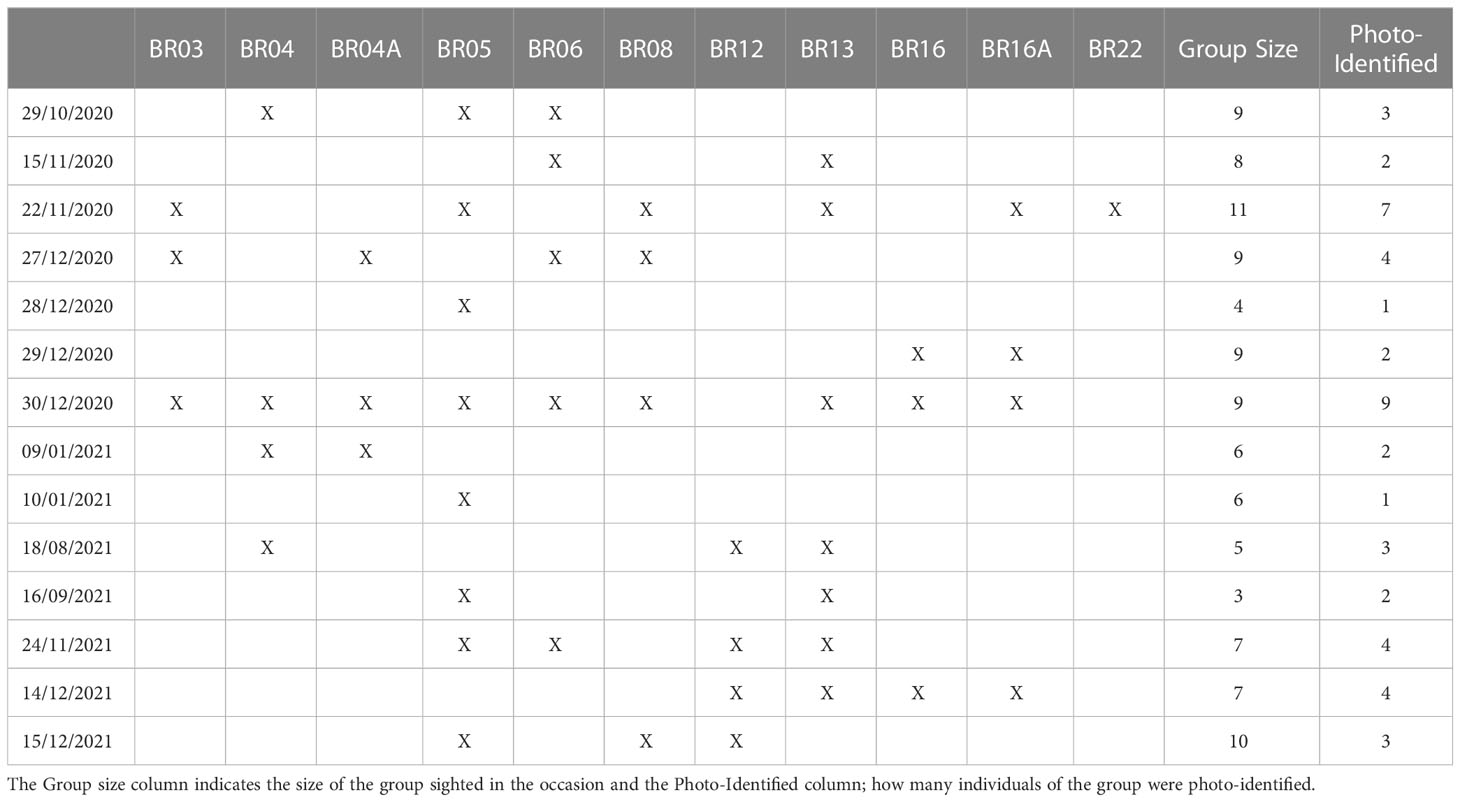
Table 2 Sighting of 11 specific individual killer whales ( Orcinus orca ) between 2020 and 2021.
In addition to the sighting of individuals BR05 and BR13 on 16 September, 2021 in La Paloma, Uruguay, a third individual sighted on January 22, 2017 in Florianópolis, Santa Catarina, Brazil, in a group with 5 other animals, was also sighted 3 times in Uruguay: the adult male BR34 was sighted alone on September 1 2015, on 26 November, 2017 with another individual non-photo identified and on October 9 2021, with individuals BR38 and BR39.
Social associations
The social analysis was conducted for 12 out of the 47 photo-identified individuals that were sighted at least three times, and during encounters when it was possible to photo-identify at least 2 individuals in the group. The mean half-weight association index was 0.29 ± 0.19 SD, with values ranging from 0 to 0.75 ( Table 3 ). Among the sex and maturity classes, on average, associations with juveniles (0.38 ± 0.03 SD) and with adult females (0.30 ± 0.10 SD) tended to be higher than those with calves (0.22 ± 0.03 SD), and adult males (0.17 ± 0.07 SD).
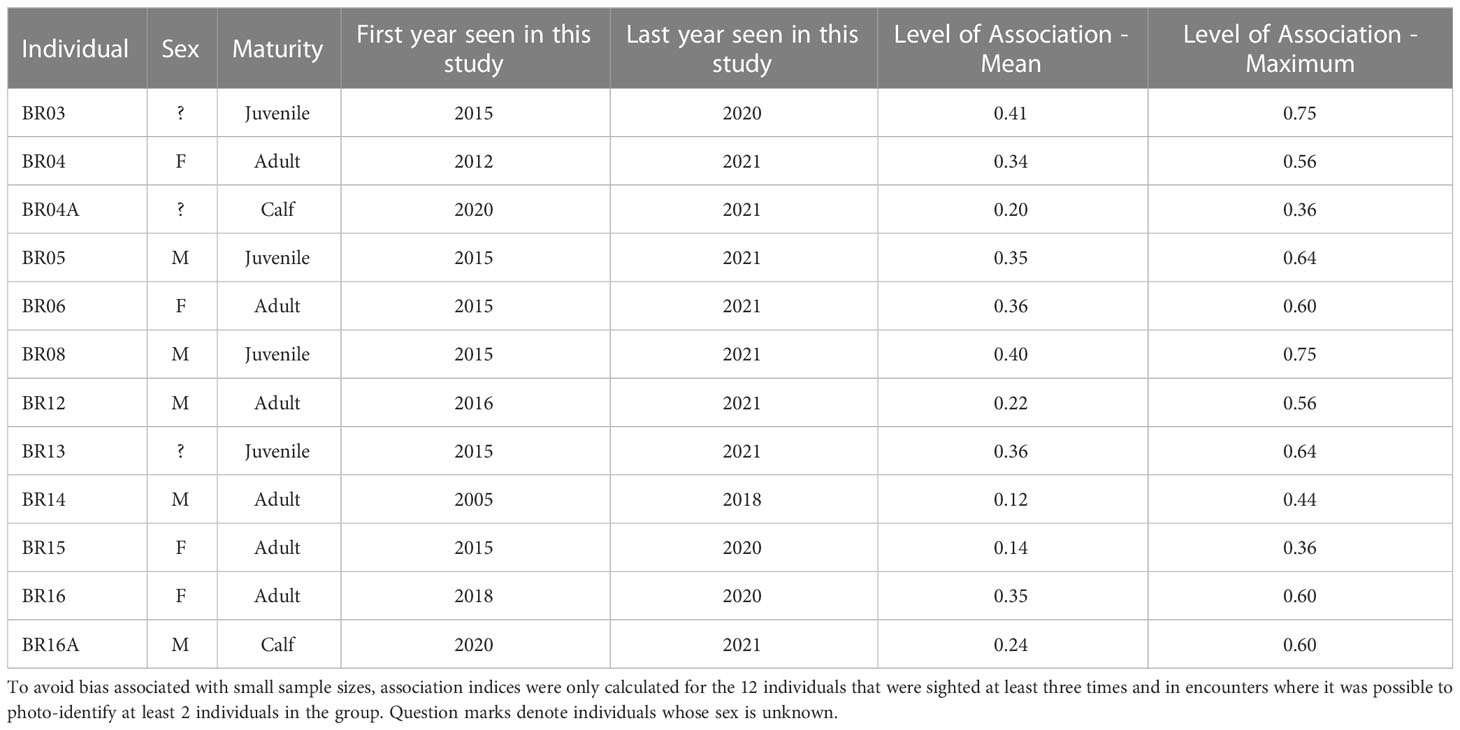
Table 3 Sighting history and associations among killer whale individuals in the Brazilian coastal waters.
The Monte Carlo permutation test allowed us to reject the null hypothesis that individuals associated randomly (CVreal = 0.67 > CVmean = 0.01, pCV = 1.00); therefore there are some preferred or avoided associations among these subset of 12 individuals. Social preferences may happen among individuals of different sexes and maturity classes. For example, the adult male BR14 and the adult female BR15 were seen associated with BR03, BR04, BR16 and BR08 on August 10 2018, and individuals BR16 and BR14 were photographed together on November 7, 2018. The hierarchical cluster analysis produced a dendrogram with a cophenetic correlation coefficient value of 0.82592 ( Figure 5A ) visually suggesting that there could be different social units with mixed sexes and maturity classes. Values above 0.8 are considered a good representation of social matrix ( Bridge, 1993 ). However, the social network depicting associations among individuals ( Figure 5B ) was highly connected (proportion of realized links and possible links = 0.833) and showed no reliable partitions since the modularity was low (<0.3; Newman, 2004 ) and nonsignificant ( Q = 0.034, 95% CI = 0.008 –0.088).
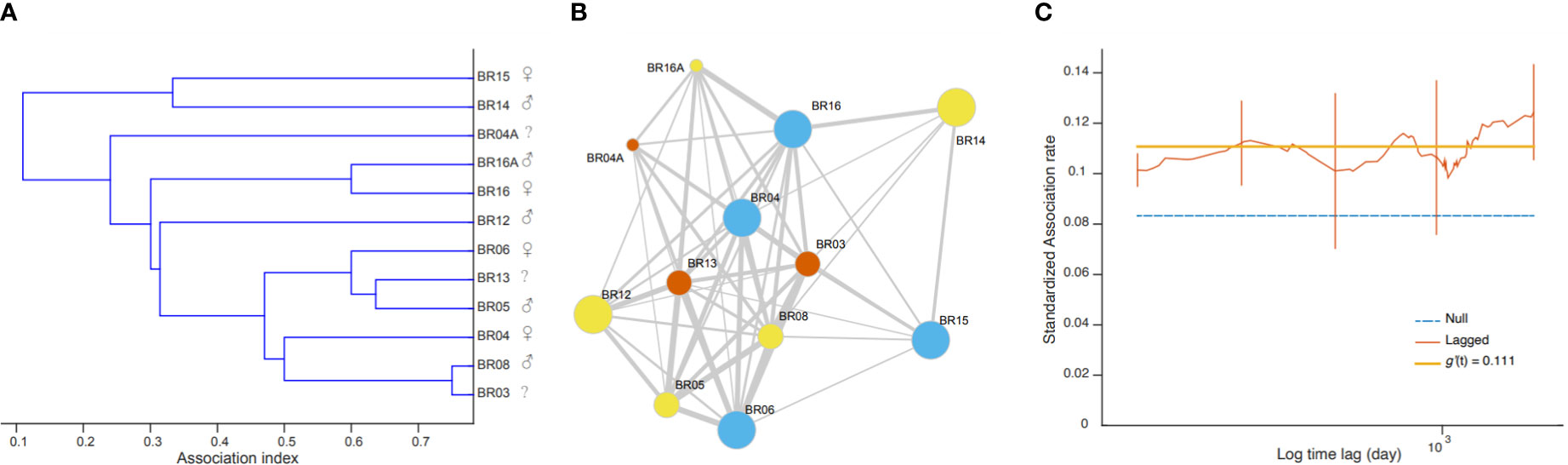
Figure 5 Depiction of the social structure of killer whales off the coast of Brazil. (A) The dendrogram from a hierarchical cluster analysis with the average linkage method. Sexes are indicated when known. (B) The social network in which nodes representing individuals are linked by their association indices with no clear partition in social units. Node colors denote sex (orange = females, blue = males, yellow = unknown), sizes denote maturity (large = adult, medium = juvenile, small = calf). (C) Standardized lagged association rates were, for most part, above the null rate and relatively stable over time, as suggested by the best-fitting model.
The lagged association rate (SLAR) was, for the most part, greater than the null association rate (NAR), suggesting that, overall, individuals continue to associate over time ( Figure 5C ). The best fitting model was SLAR 1, with association rates of 0.111 ± 0.018 SE and no decay over time ( Table 4 ), reinforcing that there are some long-lasting associations within this subset of 12 individuals. SLAR2 also received some support of the data (ΔQAIC< 2; Whitehead 2007 ), which indicate that association rates could decay down to zero. This suggest that there are some casual acquaintances, that is, associations that eventually happen among some individuals then never again. Taken together, the social analyses suggest that the most re-sighted individuals can engage in both long- and short-term associations, that are not defined by the sex or maturity classes.
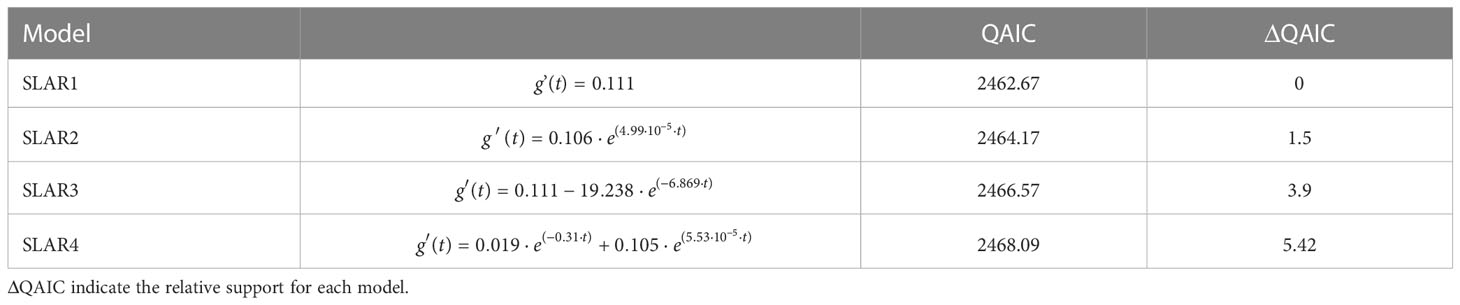
Table 4 Exponential decay models fit to Standardized Lagged Association Rates (SLAR) ranked by the lowest quasi-Akaike Information Criteria (QAIC).
There is a remarkable lack of consistent data on killer whales’ population characteristics, social structure and behavior in tropical and subtropical waters worldwide. Despite the opportunistic nature of our sighting data, our findings suggest that killer whales are present regularly along the coast of western south Atlantic, and provide novel insights on the occurrence, movements, foraging and social behavior.
These data suggest that killer whales are apparently more frequently sighted off southeastern Brazil during the austral spring and summer (see also Siciliano et al., 1999 )—although caution is needed when interpreting seasonality because nautical activity, thus opportunistic sightings, increase in the region during these seasons. Remarkably, the re-sightings of the same 11 individuals in the same region over the years, including re-sightings of 3 individuals off the coast of Brazil and Uruguay, suggest that killer whales are widely distributed in this region and yet can show some degree of site fidelity to specific locations along this coast. Similar wide-ranging movements have been observed across the world. For example, between Mexico and Peru ( Guerrero-Ruiz et al., 2005 ; Pacheco et al., 2019 ), Antarctic and New Zealand ( Eisert et al., 2015 ), and between Alaska and California ( Goley and Straley, 1994 ; Dahlheim et al., 2008 ). In the latter, some social units range more widely and transit hundreds of kilometers along the coast, while other units how higher fidelity to certain regions ( Ford and Ellis, 1999 ).
These movements and occurrence of killer whales are typically associated to prey preferences and availability. In the southeastern coast of Brazil, there are upwelling systems (such as the rich waters of the Cabo Frio) that attract a variety of seasonally abundant sharks, rays, fish, and cetaceans ( Siciliano et al., 1999 ). Previous studies on killer whales off Brazil have shown both predation and the presence in the stomach contents of a wide range of prey, such as rays, sharks, teleosts, salps, penguins, Burmeister’s porpoise ( Phocoena spinipinnis ), franciscana dolphins ( Pontoporia blainvillei ) and minke whale ( Balaenoptera sp. ) (See Table S1 on Supplementary Data ) ( Castello, 1977 ; Bittencourt, 1983 ; Castello and Pinedo, 1986 ; Dalla Rosa, 1995 ; Lodi and Hetzel, 1998 ; Secchi and Vaske Jr., 1998 ; Ott and Danilewicz, 1998 ; Fernandes, 2001 ; Santos and Haimovici, 2001 ; Santos and Netto, 2005 ; Dalla Rosa and Secchi, 2007 , Monteiro, 2008 ; Troina et al. 2020 ). Two videos released on the internet, one on September 11, 2019 in Bahia and the other on November 5, 2021, in Arraial do Cabo – Rio de Janeiro, show respectively, killer whales in a potential hunt and an attack on humpback whales (unpublished data). This region is also regularly used by Bryde’s Whales ( Gonçalves et al., 2015 ; Lodi et al., 2015 ; Athayde et al., 2020 ) and as a migration route for humpback whales ( Megaptera novaeangliae ) and southern right whales ( Eubalaena australis ) ( Groch et al., 2005 ; Siciliano et al., 2019 ; Renault-Braga et al., 2019 )—all of which could be potential targets of killer whales. For instance, in 2008, a newborn female southern right whale was found dead with several tooth marks of adult killer whale in the body ( Ott et al., 2017 ) and in 2012, killer whales were observed harassing a group of sperm whales ( Physeter macrocephalus ) to prey on a sperm whale calf ( Andriolo et al., 2015 ; Sucunza et al., 2022 ). By contrast, on September 19, 2019, while conducting regular monitoring of southern right whales in the south of Brazil, researchers observed a group of at least four killer whales in direct interaction with a mother-calf pair of southern right whales. Despite the close proximity of the killer whales to the whales, no attack occurred ( Renault-Braga et al., 2019 ). In our observations, killer whales were only seen feeding on elasmobranchs and fish: four times on rays (such as Gymnura altavela ) and twice on a school of unidentified fish. No attacks on marine mammals have been recorded and just as observed with the mother-calf pair in 2019, despite the opportunity to prey on two Bryde's whales on November 30, 2018, north of the Ilhabela Archipelago, the group of eight killer whales preferred to attack a stingray. The killer whales found in these waters seem to be more generalist or opportunistic foragers ( Lodi and Hetzel, 1998 ; Siciliano et al., 1999 ), in agreement with suggestions that killer whales in tropical waters tend to be less specialized on certain prey items than those found in temperate waters because diet breadth should increase as the availability of the most highly profitable prey decreases ( Baird, 2002 ).
Prey type and availability typically shape group size and stability ( Beck et al., 2012 ). Corroborating this idea, the mean size of the group of killer whales observed in this study was 5.61 individuals (SD = 2.91), similarly to previous observations off Brazil (4.3 individuals; Lodi and Hetzel, 1998 ; 3.9 individuals; Siciliano et al., 1999 ). This average group size is also similar to observations in other sub-tropical and tropical waters where killer whales show generalist feeding behaviors: Caribbean: 4.1 individuals ( Bolaños-Jiménez et al., 2023 ), Galápagos: 4 individuals ( Denkinger et al., 2020 ), Mexico Central Pacific coast: 4.6 individuals ( Vargas-Bravo et al., 2020 ), Peru: 4.5 individuals ( Garcia-Godos, 2004 ) and 4.3 individuals ( Testino et al., 2019 ), Costa Rica: 3.4 individuals ( Castro-Azofeifa, 2021 ), Hawaii: 4.2 individuals ( Baird et al., 2006 ), Tropical West Africa: 5.6 individuals ( Weir et al., 2010 ), Eastern Tropical Pacific: 5.4 individuals ( Wade and Gerrodette, 1993 ), and Mozambique Channel: 6.1 individuals ( Terrapon et al., 2021 ). By contrast, in temperate and polar zones, group size and is more variable and vary with the target prey type. For instance, the Antarctic Type A killer whales feeds mainly on Antarctic Minke whales and form groups of 13.6 individuals on average; the Type B (11.8 individuals) tend to prey upon pinnipeds, whales and penguins; and the Type C, (46.1 individuals) apparently feeds mainly on fish ( Pitman and Ensor, 2003 ). In Norwegian waters, the mean size for herring-feeding killer whale groups is 15 individuals ( Simila et al., 1996 ) while seal-feeding groups are 5 individuals ( Jourdain et al., 2017 ); in addition, the group sizes of killer whales hunting seals off Scotland are smaller (5.8 individuals) than those on the Icelandic herring grounds (14.8 individuals) ( Beck et al., 2012 ). These observations in Antarctic, Norway, Scotland and Icelandic are similar to those from the well-studied North Pacific population, with a proportional smaller more consistent group size of mammal-eating transient Killer whales (4.2 individuals; Baird and Dill, 1996 ) compared with fish- eating resident killer whales that often occur in groups of dozens to hundreds of individuals ( Ford et al., 1998 ). According to Baird and Dill (1996) the small size of the mammal-eating groups could contribute to the management and maximization of energy intake. Associations with other groups, increasing the size of the group, would be useful to increase the success rates of prey difficult to capture or large prey allowing additional individuals to feed without increasing competition. Still in these zones, it is also possible to find more diverse groups where whales seem to be more generalists, as in Chilean Patagonia (4.2 individuals; Häussermann et al., 2013 and 5 individuals; Capella et al., 2014 ) and in New Zealand (mean of 12 individuals) where 27 different species of prey have been recorded, being ray the most common type of prey and, the diet varying according to the 3 sub-populations proposed for the region ( Visser, 2007 ). Taken together, our data and this literature suggest that killer whales of southeastern Brazil are generalist foragers; however, one cannot yet discard the possibility that there may be specific groups with a specialized diet in the region.
Despite the paucity of encounters, our data provide novel insights on the social patterns of killer whales in the western south Atlantic waters. The association patterns among the most re-sighted 12 killer whale individuals suggest that they can form social groups of mixed age-sex, and engage in both long-term and more labile social associations. It is possible that these individuals are part of a single or more relatively stable social units in which members occasionally associate with each other with no clear preferences for sex and maturity stage. This pattern resembles the social structure of transient killer whales in the North Pacific ( Ford and Ellis, 1999 ; Baird and Whitehead, 2000 ), Marion Islands ( Reisinger et al., 2017 ), and Galápagos Islands ( Denkinger et al., 2020 ), Argentinean Patagonia ( Iñiguez et al., 2005 ) and, Mexico Pacific coast ( Guerrero-Ruíz, 2013 ). However, the nature and scarcity of our association data is such that it remains premature to make such a conclusion. An increased and more homogeneous sampling effort in space and time to complement our current opportunistic and citizen science sampling will be crucial to further reveal the structure into social units, and to estimate the rates at which different units associate. Beyond odontocetes, such as killer and sperm whales, social patterns combining permanent and temporary associations can be found in several animal populations with different degrees of fission-fusion dynamics, such as orangutans, bats, elephants and hyenas ( Kerth and König, 1999 ; Van Schaik, 1999 ; Wittemyer et al., 2005 ; Smith et al., 2008 ). Social plasticity in groups formation can reflect the underlying social and ecological conditions that modulate the costs and benefits that individual member experience. As in the abovementioned populations, associations among multiple social units of killer whales, temporarily increasing the size of the group, can be advantageous for mating opportunities, care of the young, or to increase foraging success on large prey that is difficult to capture; on the other hand, individuals in larger groups pay the cost of increased probability of competition for prey access ( Hoelzel, 1993 ; Baird and Dill, 1996 ). In Galápagos, the average group size was estimated to be 4 animals. However, when attacking baleen whales, the groups appeared to consist of at least 5-10 animals, and even up to 25 animals when they attacked sperm whales ( Denkinger et al., 2020 ). In northern Patagonia, the associations between killer whales are mostly long-term, but individuals from different groups have been observed to leave their maternal groups and form new groups ( Iñíguez et al., 2005 ). This fluidity has also been identified along the Mexico Pacific coast, where individuals have been found to have indirect relationships with each other through key associations ( Guerrero-Ruíz, 2013 ). In our study, we observed solitary adult males (BR01) that were not seen associated with any group and males that in certain sightings were alone or that were associated with different groups on different occasions, as is the case of males BR12, BR14 and, BR34 ( Figure 6 ). Solitary adult males have also been recorded preying in the region, including in association with the tuna longline fishery ( Secchi and Vaske, 1998 ; Santos and Netto, 2005 ). According to Baird and Dill (1996) , dispersal of individuals likely to occur due to the energetic benefits of foraging alone or in small groups and other groups may also allow them for a period of time to increased mating opportunities. In our study, we did not observe male-male pairs as described in Argentina ( Hoelzel, 1991 ) and the Galápagos Islands ( Denkinger et al., 2020 ).

Figure 6 The adult male BR12, sighted in association with a mother-calf pair (BR29 and BR29A) on July, 3 2016 in Florianópolis, SC (A) and on November, 22 2018 in Arraial do Cabo, RJ (B) with a group of other eight killer whales (BR03, BR04, BR05, BR06, BR08 BR13 and BR16), suggesting associations with reproductive purposes or energetic benefits.
This is the first integrated assessment of the movements and social behavior of killer whales in tropical and subtropical waters of Brazil. Our data suggest site fidelity by various groups that experience some degree of fission-fusion dynamics and engage in long and short-term movements in the waters of southern and southeastern Brazilian coast. Such movements seem to be erratic and driven by foraging opportunities, likely related to the productivity of western tropical South Atlantic and subtropical waters. Killer whales exhibit a generalist feeding behavior in this area; however, the possibility of there being groups with specialized diets in the region is not ruled out. More systematic surveys, covering both inshore and offshore waters over longer periods, are required to further clarify the status, social patterns, movements in western south Atlantic as well as an analysis of stable isotopes and fatty acids from biopsies to better characterize the diet of killer whales in the region. Given this, it appears premature to classify the killer whales in this particular region as belonging to any current or identified ecotype or a new yet to be defined.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the animal study because the analysis is based in photos of killer whales opportunistically captured.
Author contributions
AA: first authorship. SS: senior authorship. All authors contributed to the article and approved the submitted version.
Acknowledgments
These sightings and this study would not have been possible without the help of the ProBaV crew: Captain Wagner Braz da Silva, Master Marcone dos Santos and Master Natan Santos. We would like to thank Viva Instituto Verde e Azul and Mar e Vida Ecotrip, Capitão Ximango, Monika Wieland Shields, Sara Hysong-Shimazu and, all ProBaV’s contributors for the partnership. Also, Adrien Caradec, Alex Pretto, Aline Bassi, Bruno Oliveira, Cadu Lonias, Captain Nills, Cibele Sanches, Diana Figueroa, Eduardo Honuma, Fernando Viek, Gabriel Klabin, Gisele Matarozzo, Laihany Jacob, Leandro Borba from Fauna Marina Uruguay, Leandro Matheus, Lilian Fontalba, Luiza Perin, Maria Eduarda Barros, Maria Luciene da Silva, Matias Gomes, Mayara Lays, Mercedes Rios, Marcos Paulo Zettritz, Nilson Mattos, Olivar Bouças, Paulo Magalhães, Rafael Rodrigues, Sergio Lobo, Tycho Fernandes and, Victor Aune for shared with us photos and sightings of killer whales contributing to the science and for a better understanding and conservation of this species. Also, SS is supported by CNPq (Bolsa de Produtividade em Pesquisa: 306076/2019-5).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1206796/full#supplementary-material
Andriolo A., Reis S. S., Amorim T. O., Sucunza F., de Castro F. R., Maia Y. G., et al. (2015). Killer whale ( Orcinus orca ) whistles from the western south Atlantic ocean include high frequency signals. J. Acous. Soc. America. 138 (3), 1696–1701. doi: 10.1121/1.4928308
CrossRef Full Text | Google Scholar
Athayde A., Cardoso J., Francisco A., Siciliano S. (2020). Bryde's whales ( Balaenoptera brydei ) off the north coast of São Paulo, Brazil: first photo-identification study. Aquat. Mammals. 46 (5), 1–5. doi: 10.1578/AM.46.5.2020.488
Athayde A., Cardoso J., Francisco A., Siciliano S. (2022). A vessel collision report for Bryde's whales ( Balaenoptera brydei ) off the northern coast of São Paulo, Brazil. Aquat. Conserv.: Mar. Freshw. Ecosys. 33 (2), 1–5. doi: 10.1002/aqc.3906
Baird R. W. (2002). Killer whales of the world natural history and conservation (Stillwater, Minnesota: Voyageur Press).
Google Scholar
Baird R. W., Dill L. M. (1996). Ecological and social determinants of group size in transient killer whales. Behav. Ecol. 7, 408–416. doi: 10.1093/beheco/7.4.408
Baird R. W., McSweeney D. J., Bane C., Barlow J., Salden D. R., Antoine L. K., et al. (2006). Killer whales in Hawaiian waters: information on population identity and feeding habits. Pacific Sci. 60, 523–530. doi: 10.1353/psc.2006.0024
Baird R. W., Whitehead H. (2000). Social organization of mammal-eating killer whales: group stability and dispersal patterns. Can. J. Zool. 78, 2096–2105. doi: 10.1139/z00-155
Batista R. L. G., Schiavetti A., Santos U. A., Reis M. S. S. (2012). Cetaceans registered on the coast of Ilhéus (Bahia), northeastern Brazil. Biota Neotrop. 12 (1), 31–38. doi: 10.1590/S1676-06032012000100003
Beck S., Kuningas S., Esteban R., Foote A. D. (2012). The influence of ecology on sociality in the killer whale ( Orcinus orca ). Behav. Ecol. 23 (2), 246–253. doi: 10.1093/beheco/arr151
Bejder L., Fletcher D., Bräger S. (1998). A method for testing association patterns of social animals. Anim. Behav. 56, 719–725. doi: 10.1006/anbe.1998.0802
PubMed Abstract | CrossRef Full Text | Google Scholar
Bigg M. A., Ellis G. M., Ford J. K. B., Balcomb K. C. (1987). Killer whales: a study of their identification, genealogy and natural history in British Columbia and Washington state (Nanaimo, B.C: Phantom Press and Publishers), 79.
Bittencourt M. L. (1983). Orcinus orca "Baleia assassina" (Cetacea, Delphinidae), primeiro registro para o litoral norte catarinense, com notas osteológicas. [ Orcinus orca "Killer whale" (Cetacea, Delphinidae), first record for the north coast of Santa Catarina, with osteological notes]. Arq. Biol. Tecnol. 26 (1), 77–103.
Bolaños-Jiménez J., Kiszka J. J., Bouveret B., Ferrer G. R., Ramos E. A., Henriquez A., et al (2023). The Killer Whale in the Caribbean Sea: An Updated Review of Its Ecology, Exploitation, and Interactions with Fisheries. Aquat. Mamm. 49, 184–194. doi: 10.1578/AM.49.2.2023.184
Bridge P. D. (1993). “Classification,” in Biological data analysis . Ed. Fry J. C. (Oxford, UK: Oxford University Press), 219–242.
Cairns S. J., Schwager S. J. (1987). A comparison of association indices. Anim. Behav. 35, 1454–1469. doi: 10.1016/S0003-3472(87)80018-0
Capella J., Abramson J. Z., Vilina Y., Gibbons J. (2014). Observations of killer whales ( Orcinus orca ) in the fjords of Chilean Patagonia. Polar Biol. 37, 1533–1539. doi: 10.1007/s00300-014-1535-5
Cardoso J., Francisco A., De Souza S. P., Siciliano S. (2019a). Rough-toothed dolphins ( Steno bredanensis ) along southeastern Brazil: report of an anomalous pigmented juvenile and description of social and feeding behaviors. Aquat. Mamm. J. 45 (1), 30–36. doi: 10.1578/AM.45.1.2019.30
Cardoso J., Francisco A., Siciliano S., Moreira S. C. (2019b). A stop for a snack: apparent humpback whale ( Megaptera novaeangliae ) feeding behavior and association with gillnets during migration off south-eastern Brazil. Boletim Do Laboratório Hidrobiologia (UFAMA IMPRESSO) 29, 41–49. doi: 10.18764/1981-6421e2019.6
Castello H. P. (1977). Food of a killer whale: eagle sting-ray, Myliobatis , found in the stomach of a stranded Orcinus orca . Sci. Rep. Whales Res. Inst. 29, 107–111.
Castello H. P., Pinedo M. C. (1986). “Sobre unos avistajes en el mar de distintas especies de cetaceos en el sur del brasil [About sightings in the sea of different species of cetaceans in the south of Brazil],” in Actas de la primera reunion de trabajo de expertos en mamiferos acuaticos de America del sur (Buenos Aires), 61–68.
Castro-Azofeifa C. (2021). Avistamientos de Orcinus orca (Linnaeus 1758) (Cetartiodactyla: Odontoceti: Delphinidae) en el pacífico costarricense, (1990- 2020) [Sightings of Orcinus orca (Linnaeus 1758) (Cetartiodactyla: Odontoceti: Delphinidae) in the Costa Rican pacific ocean, (1990-2020)]. Rev. Cienc. Marinas y Costeras 13 (2), 29–47. doi: 10.15359/revmar.13-2.3
Dahlheim M., Schulman-Janiger A., Black N. A., Ternullo R., Ellifrit D., Balcomb K. C. (2008). Eastern Temperate north pacific offshore killer whales ( Orcinus orca ): occurrence, movements, and insights into feeding ecology. Mar. Mamm. Sci. 24, 719–729. doi: 10.1111/j.1748-7692.2008.00206.x
Dalla Rosa L. (1995). Interações com a pesca de espinhel e informações sobre a dieta alimentar de orca, Orcinus orca (Linnaeus 1758) (Cetacea, Delphinidae), no sul e sudeste do Brasil. [Interactions with longline fisheries and information on the diet of killer whales, Orcinus orca (Linnaeus 1758) (Cetacea, Delphinidae), in southern and southeastern Brazil] (Rio Grande, RS, Brasil: Monografia de Graduação do Curso de Oceanologia. Fundação Universidade de Rio Grande), 40.
Dalla Rosa L., Secchi E. R. (2007). Killer whale ( Orcinus orca ) interactions with the tuna and swordfish longline fishery off southern and southeastern Brazil: a comparison with shark interactions. J. Mar. Biol. Assoc. United Kingdom 87, 135–140. doi: 10.1017/S0025315407054306
Dantas W. (2007). Interações entre orcas Orcinus orca (Linnaeus 1758) e falsas-orcas Pseudorca crassidens (Owen 1846) com a pesca de espinhel pelágico monofilamento no atlântico oeste tropical. [Interactions between killer whales Orcinus orca (Linnaeus 1758) and false killer whales Pseudorca crassidens (Owen 1846) with pelagic monofilament longline fisheries in the tropical Western Atlantic] (Recife, Pernambuco, Brazil: Universidade Federal Rural de Pernambuco). Master Thesis dissertation.
Denkinger J., Alarcon D., Espinosa B., Fowler L., Manning C., Oña J., et al. (2020). Social structure of killer whales ( Orcinus orca ) in a variable low-latitude environment, the Galápagos archipelago. Mar. Mamm. Sci. 36, 774–785. doi: 10.1111/mms.12672
Di Tullio J. C., Gandra T. B. R., Zerbini A. N., Secchi E. R. (2016). Diversity and distribution patterns of cetaceans in the subtropical southwestern Atlantic outer continental shelf and slope. PloS One 11 (5), e0155841. doi: 10.1371/journal.pone.0155841
Durban J. W., Fearnbach H., Burrows D. G., Ylitalo G. M., Pitman R. L. (2016). Morphological and ecological evidence for two sympatric forms of type b killer whale around the Antarctic peninsula. Polar Biol . 40, 231−236. doi: 10.1007/s00300-016-1942-x
Durban J. W., Pitman R. L. (2012). Antarctic Killer whales make rapid, round-trip movements to subtropical waters: evidence for physiological maintenance migrations? Biol. Lett. 8, 39–45. doi: 10.1098/rsbl.2011.0875
Eisert R., Ovsyanikova E., Visser I., Ensor P., Currey R., Sharp B. (2015). Seasonal site fidelity and movement of type-c killer whales between Antarctica and new zealand. paper SC/66a/SM/69 (Alaska: International Whaling Commission. Anchorage).
Farine D. R., Whitehead H. (2015). Constructing, conducting and interpreting animal social network analysis. J. Anim. Ecol. 84 (5), 1144–1163. doi: 10.1111/1365-2656.12418
Fernandes T. (2001). Ocorrência e monitoramento de cetáceos na região de arraial do cabo - RJ.[Occurrence and monitoring of cetaceans in the region of arraial do cabo – RJ]. Brasil. UFRJ: Master Thesis dissertation . Rio de Janeiro, Brasil: Departamento de Biologia Marinha, UFRJ.
Foote A. D., Newton J., Piertney S. B., Willerslev E., Gilbert M. T. P. (2009). Ecological, morphological and genetic divergence of sympatric north Atlantic killer whale populations. Mol. Ecol. 18, 5207–5217. doi: 10.1111/j.1365-294X.2009.04407.x
Foote A., Vijay N., Ávila-Arcos M., Baird R., Durban J. W., Fumagalli M., et al. (2016). Genome-culture coevolution promotes rapid divergence of killer whale ecotypes. Nat. Commun. 7, 11693. doi: 10.1038/ncomms11693
Ford J. K. B. (2002). “Killer whale: Orcinus orca . Pp. 669-676,” in Encyclopedia of marine mammals . Eds. Perrin W. F., Wursigand B., Thewissen J. G. M. First Edition. (San Diego: Academic Press), p. 1414.
Ford J. K. B., Ellis G. M. (1999). Transients: Mammal-Hunting Killer Whales . (Vancouver, British Columbia: UBC Press), 96 pp.
Ford J. K. B., Ellis G. M., Barrett-Lennard L. G., Morton A. B., Palm R. S., Balcomb I. K. C. (1998). Dietary specialization in two sympatric populations of killer whales ( Orcinus orca ) in coastal British Columbia and adjacent waters. J. Zool. 76, 1456–1471. doi: 10.1139/z98-089
Forney K. A., Wade P. (2006). “Worldwide distribution and abundance of killer whales,” in Whales, whaling and ocean ecosystems . Eds. Estes J. A., Brownell R. L., DeMaster D. P., Doak D. F., Williams T. M. (Berkeley: University of California Press), 1–25.
Garcia-Godos I. (2004). Killer whale ( Orcinus orca ) occurrence off Peru 1995–2003. Latin Am. J. Aquat. Mamm. 3, 177–180. doi: 10.5597/lajam00064
GEBCO Compilation Group (2021). GEBCO 2021 grid . doi: 10.5285/c6612cbe-50b3-0cffe053-6c86abc09f8f
Goley P. D., Straley J. M. (1994). Attack on grey whales in Monterey bay, California, by killer whales previously identified in glacier bay, Alaska. Can. J. Zool. 72 (8), 1528–1530. doi: 10.1139/z94-202
Gonçalves L. R., Augustowski M., Andriolo A. (2015). Occurrence, distribution and behavior of Bryde’s whales (Cetacea: Mysticeti) off south-east Brazil. J. Mar. Biol. Assoc. United Kingdom 96 (4), 943–954. doi: 10.1017/S0025315415001812
Groch K. R., Jerdy H., Marcondes M. C., Barbosa A. L., Ramos G. C. H., Pavanelli L., et al. (2020). Cetacean morbillivirus infection in a killer whale ( Orcinus orca ) from Brazil. J. Comp. Pathol. 181, 26–32. doi: 10.1016/j.jcpa.2020.09.012
Groch K. R., Palazzo J. T. Jr., Flores P. A. C., Adler F. R., Fabian M. E. (2005). Recent rapid increases in the right whale ( Eubalaena australis ) population of southern Brazil. LAJAM. 4, 41–47. doi: 10.5597/lajam00068
Guerrero-Ruiz M., García-Godos I., Urbán J. (2005). Photographic match of a killer whale ( Orcinus orca ) between Peruvian and Mexican waters. Aquat. Mammals. 31 (4), 438–411. doi: 10.1578/AM.31.4.2005.438
Guerrero-Ruíz M. E. (2013). Identidad poblacional y estructura social de la orca Orcinus orca (Linnaeus 1758) en el pacífico mexicano [Population identity and social structure of the killer whale Orcinus orca (Linnaeus 1758) in the Mexican pacific] (Tesis de doctoral) (La Paz, México: Universidad Autónoma de Baja California Sur).
Häussermann V., Acevedo J., Försterra G., Bailey M., Aguayo-Lobo. A. (2013). Killer whales in Chilean Patagonia: additional sightings, behavioural observations, and individual identifications. Rev. Biol. Mar. Oceanogr. 48, 73–85. doi: 10.4067/S0718-19572013000100007
Hoelzel A. R. (1991). Killer whale predation on marine mammals at Punta norte, Argentina; food sharing, provisioning and foraging strategy. Behav. Ecol. Sociobiol. 29, 197–204. doi: 10.1007/BF00166401
Hoelzel A. R. (1993). Foraging behaviour and social group dynamics in puget sound killer whales. Anim. Behav. 45 (3), 581–591. doi: 10.1006/anbe.1993.1068
ICMBio (2023) Sistema de avaliação do risco de extinção da biodiversidade - SALVE . Available at: https://salve.icmbio.gov.br .
Iñíguez M. A. (2001). Seasonal distribution of killer whales ( Orcinus orca ) in northern Patagonia, Argentina. Aquat. Mammals. 27 (2), 154–161.
Iñíguez M. A., Tossenberger V. P., Gasparrou C. (2005). Socioecology of killer whales (Orcinus orca ) in northern Patagonia, Argentina. Paper SC/57/SM5 presented to IWC Sci. Committee (Ulsan, Korea), 9.
Iriarte V. (2006). Killer whale ( Orcinus orca ) occurrence at Isla de Lobos, Uruguay. Latin Am. J. Aquat. Mammals. 5 (1), 73–76. doi: 10.5597/lajam00096
Jourdain E., Vongraven D., Bisther A., Karoliussen R. (2017). First longitudinal study of seal-feeding killer whales ( Orcinus orca ) in Norwegian coastal waters. PloS One 12, e0180099. doi: 10.1371/journal.pone.0180099
Kerth G., König B. (1999). Fission, fusion and nonrandom associations in female bechstein’s bats ( Myotis bechsteinii ). Behaviour 136 (9), 1187–1202.
Laeta M., Kompanje E. J. O., Watson A., Souza S. M. F. M., Dittmar K., Cuenca S. C., et al. (2019). Osteochondromatosis (multiple cartilaginous exostoses) in an immature killer whale ( Orcinus orca ). Dis. Aquat. Organ. 34 (3), 209–213. doi: 10.3354/dao03372
Leatherwood J. S., Dalheim M. E. (1978). Worldwide distribution of pilot whales and killer whales. Nav. Ocean Syst. Cent. Tech. Rep. 443, 1–39.
LeDuc R. G., Robertson K. M., Pitman R. L. (2008). Mitochondrial sequence divergence among Antarctic killer whale ecotypes is consistent with multiple species. Biol. Lett. 4, 426–429. doi: 10.1098/rsbl.2008.0168
Lemos L. S., Moura J. F., Hauser-Davis R. A., Campos R. C., Siciliano S. (2013). Small cetaceans found stranded or accidentally captured in southeastern Brazil: bioindicators of essential and non-essential trace elements in the environment. Ecotoxicol. Environ. Saf. 97, 166–175. doi: 10.1016/j.ecoenv.2013.07.025
Lodi L., Farias-Junior S. (2011). Movements of a solitary adult male killer whale, Orcinus orca (Cetacea, Delphinidae), along the coast of south-eastern Brazil. Pan-Am J. Aquat. Sci. 6, 325–328.
Lodi L., Hetzel B. (1998). Orcinus orca (Cetacea, Delphinidae) em águas costeiras do estado do Rio de Janeiro. Bioikos. 12 (1), 46–r54.
Lodi L., Tardin R. (2018). Citizen science contributes to the understanding of the occurrence and distribution of cetaceans in southeastern Brazil–a case study. Ocean Coast. Manage. 158, 45–55. doi: 10.1016/j.ocecoaman.2018.03.029
Lodi L., Tardin R. H., Hetzel B., Maciel I. S., Figueiredo L. D., Simão S. M. (2015). Bryde’s whale (Cetartiodactyla: Balaenopteridae) occurrence and movements in coastal areas of southeastern Brazil. Zoologia Curitiba. 32 (2), 171–175. doi: 10.1590/S1984-46702015000200009
Marcondes M. C. C., Cheeseman T., Jackson J. A., Friedlaender A. S., Pallin L., Olio M., et al. (2021). The southern ocean exchange: porous boundaries between humpback whale breeding populations in southern polar waters. Sci. Rep. 11, 23618. doi: 10.1038/s41598-021-02612-5
Meirelles A. C. O., Monteiro-Neto C., Martins A. M. A., Costa A. F., Barros H. M. D. R., Alves A. M. D. O. (2009). Cetacean strandings on the coast of Ceará, northeastern Brazil, (1992-2005). J. Mar. Biol. Assoc. United Kindom. 89 (5), 1083–1090. doi: 10.1017/S0025315409002215
Mikhalev Y. A., Ivashin M. V., Sausin V. P., Zelemaya F. E. (1981). The distribution and biology of killer whales in the southern hemisphere. Rep. Int. Whal. Commn. 31, 551–566.
Monteiro D. (2008). Fatores determinantes da captura incidental de aves e tartarugas marinhas e da interação com orcas/falsas-orcas, na pescaria com espinhel pelágico no sudeste-sul do brasil [Determining factors of incidental capture of birds and sea turtles and interaction with killer whales/false killer whales, in the pelagic longline fishery in southeastern-south brazil.] (Rio Grande, Brazil: Universidade Federal do Rio Grande/FURG). Master Thesis dissertation.
Morin P. A., Parsons K. M., Archer F. I., Ávila-Arcos M. C., Barrett-Lennard L. G., et al. (2015). Geographic and temporal dynamics of a global radiation and diversification in the killer whale. Mol. Ecol. 24. doi: 10.1111/mec.13284
Newman M. E. J. (2004). Analysis of weighted networks. Phys. Rev. E. 70, 56131. doi: 10.1103/PhysRevE.70.056131
Newman M. E. J. (2006). Modularity and community structure in networks. Proc. Natl. Acad. Sci. U.S.A. 103, 8577–8582. doi: 10.1073/pnas.0601602103
Ott P. H., Danilewicz D. (1998). Presence of franciscana dolphins ( Pontoporia blainvillei ) in the stomach of a killer whale ( Orcinus orca ) stranded in southern Brazil. Mammalia 62 (4), 605–609.
Ott P. H., Sucunza F., Wickert J., Danilewicz D., Tavares M. (2017). Evidences of attack of a killer whale on a calf southern right whale in southern Brazil. Mastozoología Neotropical 24 (1), 235–240. Available at: https://www.redalyc.org/pdf/457/45753369020.pdf .
Pacheco A. S., Castro C., Carnero-Huaman R., Villagra D., Pinilla S., Denkinger J., et al. (2019). Sightings of an adult male killer whale match humpback whale breeding seasons in both hemispheres in the Eastern tropical pacific. Aquat. Mammals. 45, 320–326. doi: 10.1578/AM.45.3.2019.320
Passadore C., Domingo A., Secchi E. R. (2015). Depredation by killer whale ( Orcinus orca ) and false killer whale ( Pseudorca crassidens ) on the catch of the Uruguayan pelagic longline fishery in southwestern Atlantic ocean. ICES J. Mar. Sci. 72 (5), 1653–1666. doi: 10.1093/icesjms/fsu251
Passadore C., Domingo A., Szephegyi M., Secchi E. R. (2012). Influence of environmental and longline fishing operational variables on the presence of killer whales ( Orcinus orca ) in south-western Atlantic. J. Mar. Biol. Assoc. United Kingdom 94 (6), 1267–1276. doi: 10.1017/S002531541200166X
Pinedo M. C., Polacheck T., Barreto A. S., Lammardo M. P. (2002). A note on vessel of opportunity sighting surveys for cetaceans in the shelf edge region off the southern coast of Brazil. J. Cetacean Res. Manag. 4 (3), 323–329. doi: 10.47536/jcrm.v4i3.846
Pitman R. L. (2011). Antarctic Killer whales: top of the food chain at the bottom of the world. J. Am. Cetac. Soc 40 (1), 39–45.
Pitman R. L., Ensor P. (2003). Three forms of killer whales ( Orcinus orca ) in Antarctica. J. Cetacean Res. Manag. 5, 131–139. doi: 10.47536/jcrm.v5i2.813
PMC-BS (2017) 2° relatório anual – ciclos 1 a 4 (Volume unico) [2nd annual report - cycles 1 to 4 (Single volume)] . Available at: https://sispmcprd.petrobras.com.br/sispmc/faces/informacoes/relatorios.xhtml?name=relatorios .
Ramos R. M. A., Siciliano S., Ribeiro R. (2010). Monitoramento da biota marinha em navios de sísmica: seis anos de pesquisa, (2001-2007). [Marine biota monitoring on seismic vessels: six years of research, (2001-2007)] (Vitória, ES, Brasil: Everest Tecnologia em Serviços).
Reisinger R. R., Beukes C., Hoelzel A. R., de Bruyn P. J. N. (2017). Kinship and association in a highly social apex predator population, killer whales at Marion island. Behav. Ecol. 28 (3), 750e759. doi: 10.1093/beheco/arx034
Renault-Braga E. P., Medeiros C. R. M., Leite H. N., Groch K. R., Maciel L. R. L. D., Albernaz T. L. First record of direct interaction between a potential predator – Orcinus orca (Odontoceti, Delphinidae) – and a mother-calf pair of Eubalaena australis (Mysticeti, Balaenidae) in Brazilian calving ground). Pan-American J. Aquat. Sci. 17 (3), 319–325.
Rice D. W. (1998). Marine mammals of the world: systematics and distribution (Lawrence, Kansas, USA: Society for Marine Mammalogy).
Santos M. C. O., Brito J. L., Flach L., Oshima J. E. F., Figueiredo G. C., Carvalho R. R., et al. (2019). Cetacean movements in coastal waters of the southwestern Atlantic ocean. Biota Neotropica 19 (2), e20180670. doi: 10.1590/1676-0611-bn-2018-0670
Santos R. A., Haimovici M. (2001). Cephalopods in the diet of marine mammals caught stranded or incidentally caught along southeastern and southern Brazil (21-34° s). Fish. Res. 52 (1-2), 99–112. doi: 10.1016/S0165-7836(01)00234-X
Santos M. C. O., Netto D. F. (2005). Killer whale ( Orcinus orca ) predation on franciscana dolphin ( Pontoporia blainvillei ) in Brazilian waters. Latin Am. J. Aquat. Mamm. 4 (1), 69–72. doi: 10.5597/lajam00072
Santos M. C. O., Siciliano S., Vicente A. F. C., Alvarenga F. S., Zampirolli E., Souza S. P., et al. (2010). Cetacean records along São Paulo state coast, southeastern Brazil. Braz. J. Oceanogr. 58 (2), 123–142. doi: 10.1590/S1679-87592010000200004
Santos M. C. O., Silva E. (2009). Records of a male killer whale ( Orcinus orca ) off southeastern Brazil. Braz. J. Oceanogr. 57, 65– 68. doi: 10.1590/S1679-87592009000100007
Secchi E. R., Vaske T. Jr. (1998). Killer whale ( Orcinus orca ) sightings and depredation on tuna and swordfish longline catches in southern Brazil. Aquat. Mammals. 24 (2), 117–122.
Siciliano S., Brito J. L., Azevedo A. F. (1999). Seasonal occurrence of killer whales ( Orcinus orca ) in waters of Rio de Janeiro, Brazil. Mamm. Biol. 64 (4), 251–255.
Siciliano S., Cardoso J., Francisco A., de Souza S. P., Hauser-Davis R. A., Iwasa-Arai T. (2020). Epizoic barnacle ( Xenobalanus globicipitis ) infestations in several cetacean species in south-eastern Brazil. Mar. Biol. Res. 16, 1–13. doi: 10.1080/17451000.2020.1783450
Siciliano S., Cardoso J., Francisco A., Moreira S. C. (2019). Stop for a snack: evidence of humpback whale ( Megaptera novaeangliae ) feeding behavior and association with gillnets during migration off southeastern Brazil. Boletim do Laboratório Hidrobiologia. 29, 41–49. doi: 10.18764/1981-6421e2019.6
Simila T., Holst J. C., Christensen I. (1996). Occurrence and diet of killer whales in northern Norway: seasonal patterns relative to the distribution and abundance of Norwegian spring-spawning herring. Can. J. Fish. Aquat. Sci. 53 (4), 769–779. doi: 10.1139/f95-253
Smith J. E., Kolowski J. M., Graham K. E., Dawes S. E., Holekamp K. E. (2008). Social and ecological determinants of fission–fusion dynamics in the spotted hyaena. Anim. Behav. 76 (3), 619–636. doi: 10.1016/j.anbehav.2008.05.001
Strier K. B. (1997). Behavioral ecology and conservation biology of primates and other animals. Adv. Study Behav. 26, 101–158. doi: 10.1016/S0065-3454(08)60378-2
Sucunza F., Andriolo A., Dalla Rosa L., Castro F. R., Danilewicz D., Zerbini A. N. (2022). Sperm whale, Physeter macrocephalus, harassment by killer whales, Orcinus orca , in the western south Atlantic ocean. Latin Am. J. Aquat. Mammals. 17 (2), 129–132. doi: 10.5597/lajam00286
Sutherland W. J. (1998). The importance of behavioural studies in conservation biology. Anim. Behav. 56, 801–809. doi: 10.1006/anbe.1998.0896
Tavares S. B., Samarra F. I. P., Miller P. J. O. (2016). A multilevel society of herring-eating killer whales indicates adaptation to prey characteristics. Behav. Ecol. 28 (2), 500–514. doi: 10.1093/beheco/arw179
Terrapon M., Kiszka J. J., Wagner J. (2021). Observations of killer whale ( Orcinus orca ) feeding behavior in the tropical waters of the northern Mozambique channel island of Mayotte, southwest Indian ocean. Aquat. Mammals. 47 (2), 196–205. doi: 10.1578/AM.47.2.2021.196
Testino J. P., Petit A., Acorta B., Pacheco A. S., Silva S., Alfaro-Shigueto J., et al. (2019). Killer whale ( Orcinus orca ) occurrence and interactions with marine mammals off Peru. Pacific Sci. 73 (2), 261–273. doi: 10.2984/73.2.7
Troina G. C., Botta S., Dehairs F., Di Tullio J. C., Elskens M., Secchi E. R. (2020). Skin 13C and 15N reveal spatial and temporal patterns of habitat and resources use by free-ranging odontocetes from the southwestern Atlantic ocean. Mar. Biol. 167, 186. doi: 10.1007/s00227-020-03805-8
Van Schaik C. P. (1999). The socioecology of fission-fusion sociality in orangutans. Primates 40, 69–86. doi: 10.1007/BF02557703
Vargas-Bravo M. H., Elorriaga-Verplancken F. R., Olivos-Ortiz A., Morales-Guerrero B., Liñán-Cabello M. A., Ortega-Ortiz C. D. (2020). Ecological aspects of killer whales from the Mexican central pacific coast: revealing a new ecotype in the Eastern tropical pacific. Mar. Mam. Sci. 1–16. doi: 10.1111/mms.12748
Visser I. N. (2000b). Orca (Orcinus orca) in new Zealand waters (Auckland: University of Auckland), 193. Ph.D. Dissertation.
Visser I. N. (2007). “Killer whales in new Zealand waters: status and distribution with comments on foraging,” in 59th Annual meeting of the International Whaling Commission Scientific Committee. Paper SC/59/SM19. (Anchorage, Alaska: International Whaling Commission).
Wade P. R., Gerrodette T. (1993). Estimates of cetacean abundance and distribution in the eastern tropical pacific. Rep. Int. Whaling Commun. 43, 477–493.
Wedekin L. L., Rossi-Santos M. R., Baracho C., Cypriano-Souza A. L., Simões-Lopes P. C. (2014). Cetacean records along a coastal offshore gradient in the vitória-trindade chain, western south Atlantic ocean. Braz. J. Biol. 74 (1), 137–144. doi: 10.1590/1519-6984.21812
Weir C. R., Collins T., Carvalho I., Rosenbaum H. C. (2010). Killer whales ( Orcinus orca ) in Angolan and gulf of Guinea waters, tropical West Africa. J. Mar. Biol. Assoc. United Kingdom 90, 1601–1611. doi: 10.1017/S002531541000072X
Whitehead H. (1995). Investigating structure and temporal scale in social organizations using identified individuals. Behav. Ecol. 6, 199–208. doi: 10.1093/beheco/6.2.199
Whitehead H. (2007). Selection of models of lagged identification rates and lagged association rates using AIC and QAIC. Commun. Stat. Simul. Comput. 36, 1233–1246. doi: 10.1080/03610910701569531
Whitehead H. (2008). Analyzing animal societies: quantitative methods for vertebrate social analysis (Chicago and London: The University of Chicago Press).
Whitehead H. (2009). SOCPROG programs: analysing animal social structures. Behav. Ecol. Sociobiol. 63, 765–778. doi: 10.1007/s00265-008-0697-y
Whitehead H., Dufault S. (1999). Techniques for analyzing vertebrate social structure using identified individuals. Adv. Stud. Behav. 28, 33–74. doi: 10.1016/S0065-3454(08)60215-6
Wilson E. O. (1975). Sociobiology: the new synthesis (Cambridge: Belknap Press).
Wittemyer G., Douglas-Hamilton I., Getz W. M. (2005). The socioecology of elephants: analysis of the processes creating multitiered social structures. Anim. Behav. 69 (6), 1357–1371. doi: 10.1016/j.anbehav.2004.08.018
Yates O., Black A. D., Palavecino P. (2007). Site fidelity and behaviour of killer whales ( Orcinus orca ) at Sea lion island in the southwest Atlantic. LAJAM 6 (1), 89–95. doi: 10.5597/lajam00112
Keywords: killer whales, Orcinus orca , Brazil, photo-identification, social behavior, cetaceans, movements
Citation: Athayde A, Cantor M, Cardoso J, Francisco A, Santos FPd, Crespo H, de Morais MV, Albaladejo MdC, Gallo Neto H and Siciliano S (2023) Movements and social behavior of killer whales ( Orcinus orca ) off the Brazilian coast. Front. Mar. Sci. 10:1206796. doi: 10.3389/fmars.2023.1206796
Received: 16 April 2023; Accepted: 07 June 2023; Published: 20 July 2023.
Reviewed by:
Copyright © 2023 Athayde, Cantor, Cardoso, Francisco, Santos, Crespo, de Morais, Albaladejo, Gallo Neto and Siciliano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aline Athayde, [email protected]
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Killer Whales: Behavior, Social Organization, and Ecology of the Oceans’ Apex Predators
- First Online: 03 August 2019
Cite this chapter

- John K. B. Ford 3 , 4
Part of the book series: Ethology and Behavioral Ecology of Marine Mammals ((EBEMM))
3052 Accesses
19 Citations
59 Altmetric
The killer whale—the largest of the dolphins and the top marine predator––has a cosmopolitan distribution throughout the world’s oceans. Although globally it could be considered a generalist predator with a diverse diet, it is deeply divided into ecotypes, many of which have distinct foraging strategies involving only a narrow range of prey species. These ecotypes, which often exist in sympatry, are believed to arise from culturally driven dietary specializations that develop within matrilineal social groups and are transmitted among matriline members and across generations by social learning. Specializations are maintained by behavioral conformity and social insularity of lineages, which result in reproductive isolation and, ultimately, genetic divergence of ecotypes. Ecotypes have distinct patterns of seasonal distribution, group size, social organization, foraging behavior, and acoustic activity that are related to the type of prey being sought. Sophisticated cooperative foraging tactics have evolved in some ecotypes, and prey sharing within matrilineal social groups is common. Remarkable behavioral and demographic attributes have been documented in one well-studied ecotype, including lifelong natal philopatry without dispersal of either sex from the social group, vocal dialects that encode genealogical relatedness within lineages, and multi-decade long post-reproductive periods of females. Cultural traditions of killer whales, including foraging specializations, can be deeply rooted and resistant to change, which may limit the ability of ecotypes to adapt to sudden environmental variability.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others

You Are What You Eat: Foraging Specializations and Their Influence on the Social Organization and Behavior of Killer Whales

Fear of Killer Whales Drives Extreme Synchrony in Deep Diving Beaked Whales

Social Strategies of a Consummate Marine Predator: Mammal-Hunting Killer Whales
Baird RW, Dill LM (1996) Ecological and social determinants of group size in transient killer whales. Behav Ecol 7:408–416
Google Scholar
Baird RW, Whitehead H (2000) Social organization of mammal-eating killer whales: group stability and dispersal patterns. Can J Zool 78:2096–2105
Baird RW, McSweeney DJ, Bane C, Barlow J, Salden DR, Antoine LRK, LeDuc RG, Webster DL (2006) Killer whales in Hawaiian waters: information on population identity and feeding habits. Pac Sci 60:523–530
Barrett-Lennard LG (2000) Population structure and mating systems of northeastern Pacific killer whales. Ph.D. dissertation. University of British Columbia, Vancouver
Barrett-Lennard LG, Ford JKB, Heise KA (1996) The mixed blessing of echolocation: differences in sonar use by fish-eating and mammal-eating killer whales. Anim Behav 51:553–565
Barrett-Lennard LG, Matkin CO, Durban JW, Saulitis EL, Ellifrit D (2011) Predation on gray whales and prolonged feeding on submerged carcasses by transient killer whales at Unimak Island, Alaska. Mar Ecol Prog Ser 421:229–241
Bigg MA (1982) An assessment of killer whale stocks off Vancouver Island, British Columbia. Report of the International Whaling Commission 32:655–666
Bigg MA, Olesiuk PF, Ellis GM, Ford JKB, Balcomb KC (1990) Social organization and genealogy of resident killer whales ( Orcinus orca ) in the coastal waters of British Columbia and Washington state. Report of the International Whaling Commission 12:383–405
Brent LJ, Franks DW, Foster EA, Balcomb KC, Cant MA, Croft DP (2015) Ecological knowledge, leadership, and the evolution of menopause in killer whales. Curr Biol 25:746–750
CAS PubMed Google Scholar
Connor RC, Wells R, Mann J, Read A (2000) The bottlenose dolphin: social relationships in a fission-fusion society. In: Mann J, Connor RC, Tyack P, Whitehead H (eds) Cetacean societies: field studies of whales and dolphins. University of Chicago Press, Chicago, pp 91–126
Deecke VB, Ford JKB, Slater PJ (2005) The vocal behaviour of mammal-eating killer whales: communicating with costly calls. Anim Behav 69:395–405
Deecke VB, Nykänen M, Foote AD, Janik VM (2011) Vocal behaviour and feeding ecology of killer whales Orcinus orca around Shetland, UK. Aquat Biol 13:79–88
Duignan PJ, Hunter JE, Visser IN, Jones GW, Nutman A (2000) Stingray spines: a potential cause of killer whale mortality in New Zealand. Aquat Mamm 26:143–147
Durban JW, Fearnbach H, Burrows DG, Ylitalo GM, Pitman RL (2017) Morphological and ecological evidence for two sympatric forms of type B killer whale around the Antarctic Peninsula. Polar Biol 40:231–236
Esteban R, Verborgh P, Gauffier P, Giménez J, Foote AD, de Stephanis R (2016) Maternal kinship and fisheries interaction influence killer whale social structure. Behav Ecol Sociobiol 70:111–122
Filatova OA, Samarra FI, Deecke VB, Ford JK, Miller PJ, Yurk H (2015) Cultural evolution of killer whale calls: background, mechanisms and consequences. Behaviour 152:2001–2038
Foote AD, Newton J, Piertney SB, Willerslev E, Gilbert MTP (2009) Ecological, morphological and genetic divergence of sympatric North Atlantic killer whale populations. Mol Ecol 18:5207–5217
Foote AD, Morin PA, Pitman RL, Ávila-Arcos MC, Durban JW, van Helden A, Sinding MHS, Gilbert MTP (2013) Mitogenomic insights into a recently described and rarely observed killer whale morphotype. Polar Biol 36:1519–1523
Foote AD, Vijay N, Ávila-Arcos MC, Baird RW, Durban JW, Fumagalli M, Gibbs RA, Hanson MB, Korneliussen TS, Martin MD, Robertson KM (2016) Genome-culture coevolution promotes rapid divergence of killer whale ecotypes. Nat Commun 7:11693
CAS PubMed PubMed Central Google Scholar
Ford JKB (1991) Vocal traditions among resident killer whales ( Orcinus orca ) in coastal waters of British Columbia. Can J Zool 69:1454–1483
Ford JKB (2014) Marine mammals of British Columbia. Royal BC Museum handbook, mammals of BC, vol 6. Royal BC Museum, Victoria, p 460
Ford JKB, Ellis GM (1999) Transients: mammal-hunting killer whales of British Columbia, Washington, and Southeastern Alaska. UBC Press, Vancouver, p 96
Ford JKB, Ellis GM (2006) Selective foraging by fish-eating killer whales Orcinus orca in British Columbia. Mar Ecol Prog Ser 316:185–199
Ford JKB, Ellis GM (2014) You are what you eat: ecological specializations and their influence on the social organization and behaviour of killer whales. In: Yamagiwa J, Karczmarski L (eds) Primates and cetaceans: field research and conservation of complex mammalian societies. Springer, New York, NY, pp 75–98
Ford JKB, Ellis GM, Barrett-Lennard LG, Morton AB, Palm RS, Balcomb KC III (1998) Dietary specialization in two sympatric populations of killer whales ( Orcinus orca ) in coastal British Columbia and adjacent waters. Can J Zool 76:1456–1471
Ford JKB, Ellis GM, Balcomb KC (2000) Killer whales: the natural history and genealogy of Orcinus orca in British Columbia and Washington, vol 102, 2nd edn. UBC Press, Vancouver
Ford JKB, Ellis GM, Durban JW (2007) An assessment of the potential for recovery of west coast transient killer whales using coastal waters of British Columbia. Research document 2007/088, Fisheries and Oceans Canada. Canadian Science Advisory Secretariat, Ottawa
Ford JKB, Ellis GM, Olesiuk PF, Balcomb KC (2010) Linking killer whale survival and prey abundance: food limitation in the oceans’ apex predator? Biol Lett 6:139–142
PubMed Google Scholar
Ford JKB, Ellis GM, Matkin CO, Wetklo MH, Barrett-Lennard LG, Withler RE (2011) Shark predation and tooth wear in a population of northeastern Pacific killer whales. Aquat Biol 11:213–224
Ford JKB, Reeves RR (2008) Fight or flight: antipredator strategies of baleen whales. Mammal Rev 38(1):50–86
Ford MJ, Parsons KM, Ward EJ, Hempelmann JA, Emmons CK, Hanson MB, Balcomb KC, Park LK (2018) Inbreeding in an endangered killer whale population. Anim Conserv. https://doi.org/10.1111/acv.12413
Foster EA, Franks DW, Mazzi S, Darden SK, Balcomb KC, Ford JKB, Croft DP (2012) Adaptive prolonged postreproductive life span in killer whales. Science 337:1313–1313
Guinet C (1991) L’orque ( Orcinus orca ) autour de l’Archipel Crozet comparaison avec d’autres localités. Rev Ecol (Terre Vie) 46:1991
Guinet C (1992) Comportement de chasse des orques Orcinus orca autour des Îles Crozet. Can J Zool 70:1656–1667
Guinet C, Bouvier J (1995) Development of intentional stranding hunting techniques in killer whale ( Orcinus orca ) calves at Crozet Archipelago. Can J Zool 73(1):27–33
Guinet C, Domenici P, De Stephanis R, Barrett-Lennard L, Ford JKB, Verborgh P (2007) Killer whale predation on bluefin tuna: exploring the hypothesis of the endurance-exhaustion technique. Mar Ecol Prog Ser 347:111–119
Guinet C, Tixier P, Gasco N, Duhamel G (2014) Long-term studies of Crozet Island killer whales are fundamental to understanding the economic and demographic consequences of their depredation behaviour on the Patagonian toothfish fishery. ICES J Mar Sci 72:1587–1597
Hoelzel AR (1991) Killer whale predation on marine mammals at Punta Norte, Argentina; food sharing, provisioning and foraging strategy. Behav Ecol Sociobiol 29:197–204
Hoelzel AR, Moura AE (2016) Killer whales differentiating in geographic sympatry facilitated by divergent behavioural traditions. Heredity 117:481–482
Hoelzel AR, Hey J, Dahlheim ME, Nicholson C, Burkanov V, Black N (2007) Evolution of population structure in a highly social top predator, the killer whale. Mol Biol Evol 24:1407–1415
Iñíguez, M., Tossenberger, V.P., and Gasparrou, C. 2005. Socioecology of killer whales ( Orcinus orca ) in northern Patagonia, Argentina. Unpublished paper to the IWC Scientific Committee, 9 p. Ulsan, Korea, June 2005. (SC/57/SM5)
Ivkovich T, Filatova OA, Burdin AM, Sato H, Hoyt E (2010) The social organization of resident-type killer whales ( Orcinus orca ) in Avacha Gulf, Northwest Pacific, as revealed through association patterns and acoustic similarities. Mammalian Biology-Zeitschrift für Säugetierkunde 75:198–210
Jourdain E, Vongraven D, Bisther A, Karoliussen R (2017) First longitudinal study of seal-feeding killer whales ( Orcinus orca ) in Norwegian coastal waters. PLoS One 12(6):e0180099
PubMed PubMed Central Google Scholar
Kassen R (2002) The experimental evolution of specialists, generalists, and the maintenance of diversity. J Evol Biol 15:173–190
Lacy RC, Williams R, Ashe E, Balcomb KC III, Brent LJ, Clark CW, Croft DP, Giles DA, MacDuffee M, Paquet PC (2017) Evaluating anthropogenic threats to endangered killer whales to inform effective recovery plans. Sci Rep 7:14119
Lopez JC, Lopez D (1985) Killer whales ( Orcinus orca ) of Patagonia, and their behavior of intentional stranding while hunting nearshore. J Mammal 66:181–183
Matkin CO, Saulitis E (1994) Killer whale ( Orcinus orca ): biology and management in Alaska. Prepared for US Marine Mammal Commission by North Gulf Oceanic Society, Homer, AK
Matkin CO, Testa JW, Ellis GM, Saulitis EL (2014) Life history and population dynamics of southern Alaska resident killer whales ( Orcinus orca ). Mar Mamm Sci 30:460–479
Miller PJO, Shapiro AD, Tyack PL, Solow AR (2004) Call-type matching in vocal exchanges of free-ranging resident killer whales, Orcinus orca . Anim Behav 67:1099–1107
Moore SE, Francine JK, Bowles AE, Ford JKB (1988) Analysis of calls of killer whales, Orcinus orca , from Iceland and Norway. Rit Fiskideildar 11:225–250
Morin PA, Archer FI, Foote AD, Vilstrup J, Allen EE, Wade P, Durban J, Parsons K, Pitman R, Li L, Bouffard P, Abel Nielsen SC, Rasmussen M, Willerslev E, Gilbert MTP, Harkins T (2010) Complete mitochondrial genome phylogeographic analysis of killer whales ( Orcinus orca ) indicates multiple species. Genome Res 20(7):908–916
Morin PA, Parsons KM, Archer FI, Ávila-Arcos MC, Barrett-Lennard LG, Dalla Rosa L, Duchêne S, Durban JW, Ellis GM, Ferguson SH, Ford JKB, Ford MJ, Garilao C, Gilbert MTP, Kaschner K, Matkin CO, Peterson SD, Robertson KM, Visser IN, Wade PR, Ho SYW, Foote AD (2015) Geographic and temporal dynamics of a global radiation and diversification in the killer whale. Mol Ecol 24:3964–3979
Moura AE, Kenny JG, Chaudhuri RR, Hughes MA, Reisinger RR, De Bruyn PJN, Dahlheim ME, Hall N, Hoelzel AR (2015) Phylogenomics of the killer whale indicates ecotype divergence in sympatry. Heredity 114:48
Olesiuk PF, Ellis GM, Ford JKB (2005) Life history and population dynamics of northern resident killer whales ( Orcinus orca ) in British Columbia. Research Document 2005/045. Canadian Science Advisory Secretariat, Fisheries & Oceans Canada, Ottawa, ON
Pitman RL, Durban JW (2012) Cooperative hunting behavior, prey selectivity and prey handling by pack ice killer whales ( Orcinus orca ), type B, in Antarctic Peninsula waters. Mar Mamm Sci 28:16–36
Pitman RL, Durban JW, Greenfelder M, Guinet C, Jorgensen M, Olson PA, Plana J, Tixier P, Towers JR (2011) Observations of a distinctive morphotype of killer whale ( Orcinus orca ), type D, from subantarctic waters. Polar Biol 34(2):303–306
Pitman RL, Ensor P (2003) Three forms of killer whales ( Orcinus orca ) in Antarctic waters. J Cetacean Res Manag 5(2):131–140
Pitman RL, Fearnbach H, Durban JW (2018) Abundance and population status of Ross Sea killer whales ( Orcinus orca , type C) in McMurdo Sound, Antarctica: evidence for impact by commercial fishing? Polar Biol 41:781–792
Riesch R, Barrett-Lennard LG, Ellis GM, Ford JKB, Deecke VB (2012) Cultural traditions and the evolution of reproductive isolation: ecological speciation in killer whales? Biol J Linn Soc 106:1–17
Saulitis E, Matkin C, Barrett-Lennard L, Heise K, Ellis G (2000) Foraging strategies of sympatric killer whale ( Orcinus orca ) populations in Prince William Sound, Alaska. Mar Mamm Sci 16:94–109
Scammon CM (1874) The marine mammals of the north-western coast of North America: described and illustrated; together with an account of the American whale-fishery. JH Carmany, San Francisco
Similä T, Ugarte F (1993) Surface and underwater observations of cooperatively feeding killer whales in northern Norway. Can J Zool 71:1494–1499
Similä T, Ugarte F (1999) Patterns in social organisation and occurrence among killer whales photo-identified in northern Norway. European Research on Cetaceans 12:220. In: Evan PGH, Parsons ECM (eds) Proceedings of the twelfth annual conference of the European Cetacean Society, Monaco, 20–24 Jan 1998. Artes Graficas Soler, Valencia
Similä T, Holst JC, Christensen I (1996) Occurrence and diet of killer whales in northern Norway: seasonal patterns relative to the distribution and abundance of Norwegian spring-spawning herring. Can J Fish Aquat Sci 53:769–779
Simon M, Ugarte F, Wahlberg M, Miller L (2006) Icelandic killer whales Orcinus orca use a pulsed call suitable for manipulating the schooling behaviour of herring Clupea harengus . Bioacoustics 16:57–74
Strager H (1995) Pod-specific call repertoires and compound calls of killer whales, Orcinus orca Linnaeus, 1758, in the waters of northern Norway. Can J Zool 73:1037–1047
Stredulinsky EH (2016) Determinants of group splitting: an examination of environmental, demographic, genealogical and state-dependent factors of matrilineal fission in a threatened population of fish-eating killer whales ( Orcinus orca ). MSc thesis. University of Victoria, BC
Tavares SB, Samarra FI, Miller PJ (2017) A multilevel society of herring-eating killer whales indicates adaptation to prey characteristics. Behav Ecol 28:500–514
Ternullo R, Black N (2002) Predation behavior of transient killer whales in Monterey Bay, California. In: Fourth international Orca symposium and workshop. CEBC-CNRS, Villiers en Bois, pp 156–159
Tixier P, Gasco N, Guinet C (2014) Killer whales of the Crozet Islands: photoidentification catalogue 2014. Villiers en Bois: Centre d’Etudes Biologiques de Chizé-CNRS, vol 10, p m9. https://www.researchgate.net/publication/268978107_Tixier_et_al_CROZET_KILLER_WHALES_PHOTO_ID_CATALOGUE_2014_POSTER
Tosh CA, De Bruyn PJ, Bester MN (2008) Preliminary analysis of the social structure of killer whales, Orcinus orca , at subantarctic Marion Island. Mar Mamm Sci 24:929–940
Towers JR, Ellis GM, Ford JKB (2015) Photo-identification catalogue and status of the northern resident killer whale population in 2014. Canadian Technical Report of Fisheries and Aquatic Science 3139:vi + 75
Visser IN (2000) Orca ( Orcinus orca ) in New Zealand waters. Ph.D. dissertation, University of Auckland, New Zealand
Visser IN, Smith TG, Bullock ID, Green GD, Carlsson OG, Imberti S (2008) Antarctic Peninsula killer whales ( Orcinus orca ) hunt seals and a penguin on floating ice. Mar Mamm Sci 24:225–234
Whitehead H, Ford JKB (2018) Consequences of culturally-driven ecological specialization: killer whales and beyond. J Theor Biol 456:279–294
Wright BM, Stredulinsky EH, Ellis GM, Ford JKB (2016) Kin-directed food sharing promotes lifetime natal philopatry of both sexes in a population of fish-eating killer whales, Orcinus orca . Anim Behav 115:81–95
Yano K, Dahlheim ME (1995) Behavior of killer whales Orcinus orca during longline fishery interactions in the southeastern Bering Sea and adjacent waters. Fish Sci 61:584–589
CAS Google Scholar
Yurk H, Barrett-Lennard L, Ford JKB, Matkin CO (2002) Cultural transmission within maternal lineages: vocal clans in resident killer whales in southern Alaska. Anim Behav 63:1103–1119
Download references
Acknowledgment
Many thanks to John Durban, Eve Jourdain (Norwegian Orca Survey) and Jared Towers for kindly allowing use of their photographs.
Author information
Authors and affiliations.
Department of Zoology, University of British Columbia, Vancouver, BC, Canada
John K. B. Ford
Pacific Biological Station, Fisheries and Oceans Canada, Nanaimo, BC, Canada
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to John K. B. Ford .
Editor information
Editors and affiliations.
Texas A&M University at Galveston, Galveston, TX, USA
Bernd Würsig
Rights and permissions
Reprints and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Ford, J.K.B. (2019). Killer Whales: Behavior, Social Organization, and Ecology of the Oceans’ Apex Predators. In: Würsig, B. (eds) Ethology and Behavioral Ecology of Odontocetes. Ethology and Behavioral Ecology of Marine Mammals. Springer, Cham. https://doi.org/10.1007/978-3-030-16663-2_11
Download citation
DOI : https://doi.org/10.1007/978-3-030-16663-2_11
Published : 03 August 2019
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-16662-5
Online ISBN : 978-3-030-16663-2
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
US Mainland
Initiatives, publications, collaboration, biology and behavior of killer whales.
Since it’s inception Cascadia Research biologists working in the field have taken photos of killer whales when encountered and contributed them to a variety of research projects being undertaken along the west coast of North America. Starting in 2003 Cascadia began a variety of research efforts focused on killer whales, most in collaboration with the Northwest Fisheries Science Center of NOAA Fisheries and a continuation of long-term studies of killer whales being undertaken by Robin Baird. Recent projects have focused on studying the underwater acoustics and behavior of Southern Resident killer whales using DTAGs , diving behavior of mammal-eating killer whales , movement patterns of mammal-eating killer whales , and a study of diet and behavioral cues of predation in fish-eating killer whales . This latter project was begun in 2002 in collaboration with Dr. Brad Hanson of the Northwest Fisheries Science Center and has involved collecting fecal samples (for genetic analysis of diet) and prey remains left behind foraging whales. As part of the effort to examine diet and underwater behavior, with Greg Marshall and Dr. Mike Heithaus of the National Geographic Television Remote Imaging Program a Crittercam was deployed on a fish-eating “southern resident” killer whale (K25) in 2002, obtaining the first underwater video footage collected from a killer whale. Unfortunately the whale was not foraging while the camera was attached, but the video footage obtained did demonstrate that this technique should be useful to examine underwater feeding.
Books, book chapters and popular articles

Predators, prey, and play: killer whales and other marine mammals . An article by R.W. Baird published in Whalewatcher in 2011. Download PDF copy
Peer-reviewed publications
- Harassment and killing of porpoises (“phocoenacide”) by fish-eating Southern Resident killer whales ( Orcinus orca ). Paper published by D.A. Giles and others, published in Marine Mammal Science in 2023. Download PDF copy
- Endangered predators and endangered prey: seasonal diet of Southern Resident killer whales. Paper by M.B. Hanson, and others, published in PLoS One in 2021. Download PDF copy
- Killer whale predatory scarring on mysticetes: a comparison of rake marks among blue, humpback, and gray whales in the Eastern North Pacific. Paper by E Corsi, J. Calambokidis, K.R. Flynn and G.H Steiger, published in Marine Mammal Science in 2021. View a read-only copy
- Pathology findings and correlation with body condition index in stranded killer whales (Orcinus orca) in the Northeastern Pacific and Hawaii from 2004 to 2013. Paper by S. Raverty and others, published in PLoS ONE in 2020. Download PDF copy
- Killer whale genomes reveal a complex history of recurrent admixture and vicariance. Paper by A.D. Foote, M.D. Martin, M. Louis, G. Pacheco, K.M. Robertson, M.S. Sinding, A.R. Amaral, R.W. Baird and others, published in Molecular Ecology in 2019. View a read-only copy.
- Noise levels received by endangered killer whales Orcinus orca before and after implementation of vessel regulations. Paper by M. M. Holt, M. B. Hanson, D. A. Giles, C. K. Emmons, J. T. Hogan, published in Endangered Species Research in 2017. Download PDF copy
- Estimation of a killer whale ( Orcinus orca ) population’s diet using sequencing analysis of DNA from feces . Paper by M.J. Ford, J. Hempelmann, M.B. Hanson, K.L. Ayres, R.W. Baird and others, published in PLoS One in 2015. Download PDF copy
- Changes in the occurrence and behavior of mammal-eating killer whales in southern British Columbia and Washington State, 1987-2010. Paper by J. Houghton, R.W. Baird, C.K. Emmons and M.B. Hanson, published in Northwest Science in 2015. Download PDF copy
- Distinguishing the impacts of inadequate prey and vessel traffic on an Endangered killer whale ( Orcinus orca ) population Paper by K.L. Ayres, R.K. Booth, J.A. Hempelmann, K.S. Koski, C.K. Emmons, R.W. Baird and others, published in PLoS One in 2012. Download PDF copy
- Inferred paternity and male reproductive success in a killer whale ( Orcinus orca ) population. Paper by M.J. Ford, M.B. Hanson, J.A. Hempelmann, K.L. Ayres, C.K. Emmons, G.S. Schorr, R.W. Baird and others in Journal of Heredity in 2011. Download PDF copy
- Species and stock identification of prey consumed by endangered southern resident killer whales in their summer range . Paper by M.B. Hanson, R.W. Baird, J.K.B. Ford, J. Hempelmann-Halos, D.M. Van Doornik, J.R. Candy, C.K. Emmons, G.S. Schorr, B. Gisborne and others in Endangered Species Research in 2010. Download PDF copy
- Effects of age, sex and reproductive status on persistent organic pollutant concentrations in “Southern Resident” killer whales. Paper by M.M. Krahn, M.B. Hanson, G.S. Schorr, C.K. Emmons, D.G. Burrows, J.L. Bolton, R.W. Baird and G.M.Ylitalo, published in Marine Pollution Bulletin in 2009. Download PDF copy
- Vulnerability of a killer whale social network to disease outbreaks. Paper by P.R. Guimaraes Jr., M.A. de Menezes, R.W. Baird, D. Lusseau, P. Guimaraes, and S.F. dos Reis, published in Physical Review E. in 2007. Download PDF copy
- Persistent organic pollutants and stable isotopes in biopsy samples (2004/2006) from Southern Resident killer whales. Paper by M.M. Krahn, M.B. Hanson, R.W. Baird, R.H. Boyer, D.G. Burrows, C.K. Emmons, J.K.B. Ford, L.L. Jones, D.P. Noren, P.S. Ross, G.S. Schorr, and T.K. Collier, published in Marine Pollution Bulletin in 2007. Download PDF copy
- Killer whales in Hawaiian waters: information on population identity and feeding habits. Paper by R.W. Baird, D.J. McSweeney, C. Bane, J. Barlow, D.R. Salden, L.K. Antoine, R.G. LeDuc, and D.L. Webster, published in Pacific Science in 2006. Download PDF copy
- Suspected surplus killing of harbor seal pups ( Phoca vitulina ) by killer whales ( Orcinus orca ). Paper by J.K. Gaydos, S. Raverty, R.W. Baird and R.W. Osborne, published in Northwestern Naturalist in 2005. Download PDF copy
- Factors influencing the diving behaviour of fish-eating killer whales: sex differences and diel and interannual variation in diving rates. Paper by R.W. Baird, M.B. Hanson and L.M. Dill published in the Canadian Journal of Zoology in 2005. Download PDF copy
- Low worldwide genetic diversity in the killer whale ( Orcinus orca ): implications for demographic history. Paper by A.R. Hoelzel, A. Natoli, M.E. Dahlheim, C. Olavarria, R.W. Baird and N.A. Black published in the Proceedings of the Royal Society of London in 2002. Download PDF copy
- Social organization of mammal-eating killer whales: group stability and dispersal patterns. Paper by R.W. Baird and H. Whitehead published in the Canadian Journal of Zoology in 2000. Download PDF copy
- Status of killer whales in Canada . Paper by R.W. Baird published in the Canadian Field-Naturalist in 2001. Download PDF copy
- Birth of a “resident” killer whale off Victoria, British Columbia, Canada. Note by P.J. Stacey and R.W. Baird, published in Marine Mammal Science in 1997. Download Adobe PDF copy
- Ecological and social determinants of group size in transient killer whales. Paper by R.W. Baird and L.M. Dill published in Behavioral Ecology in 1996. Download Adobe PDF copy
- Levels of organochlorine compounds, including PCDDs and PCDFs, in the blubber of cetaceans from the west coast of North America. Paper by W.M. Jarman and colleagues published in Marine Pollution Bulletin in 1996. Download Adobe PDF copy
- Occurrence and behaviour of transient killer whales: seasonal and pod-specific variability, foraging behaviour and prey handling. Paper by R.W. Baird and L.M. Dill published in Canadian Journal of Zoology in 1995. Download Adobe PDF copy
- Possible indirect interactions between transient and resident killer whales: implications for the evolution of foraging specializations in the genus Orcinus . Paper by R.W. Baird, P.A. Abrams and L.M. Dill published in Oecologia in 1992. Download Adobe PDF copy
- A review of killer whale interactions with other marine mammals: predation to co-existence. Paper by T.A. Jefferson, P.J. Stacey and R.W. Baird published in Mammal Review in 1991. Download Adobe PDF copy
- Observations on the reactions of sea lions, Zalophus californianus and Eumetopias jubatus , to killer whales, Orcinus orca , evidence of “prey” having a “search image” for predators. Paper by R.W. Baird and P.J. Stacey published in Canadian Field-Naturalist in 1989. Download Adobe PDF copy
- Variation in saddle patch pigmentation in populations of killer whales ( Orcinus orca ) from British Columbia, Alaska, and Washington State. Paper by R.W. Baird and P.J. Stacey published in Canadian Journal of Zoology in 1988. Download Adobe PDF copy
Reports, conference presentations, and a few other things
- Diet studies of “southern resident” killer whales: prey sampling and behavioral cues of predation. Report by R.W. Baird and M.B. Hanson to NOAA, 2004. Download Adobe PDF copy
- Studies of foraging in “southern resident” killer whales during July 2002: dive depths, bursts in speed, and the use of a “Crittercam” system for examining sub-surface behavior. Report submitted in February 2003 to the National Marine Mammal Laboratory, Seattle, WA, by R.W. Baird, M.B. Hanson, E.E. Ashe, M.R. Heithaus and G.J. Marshall. Download PDF copy
- Diving behaviour of killer whales . Abstract of a presentation by R.W. Baird, L.M. Dill and M.B. Hanson, to the World Marine Mammal Conference, held in Monaco in 1998.
- Information on killer whale reactions to suction-cup tagging can also be found in an Abstract to a presentation at a workshop on “Methods for Assessing Behaviorial Impacts On Marine Mammals from Human Activities”, held in Monaco in 1998.
- Management of killer whale/boat interactions in Haro Strait . Abstract of a presentation authored by R.W. Baird, R. Otis and R.W. Osborne, from a workshop on “Whale Watching Research” held in Monaco in 1998.
- Diving behavior of fish-eating killer whales off southern Iceland. Poster Presentation by J.L. Schorr, R.W. Baird, J.J. Foster, and M.B. Hanson at the 14th Biennial Conference of the Society of Marine Mammalogy, Vancouver, British Columbia, Canada 28 Nov – 3 Dec, 2001. Download PDF copy
- Preliminary calibration of velocity meters on a captive killer whale. Report prepared by R.W. Baird for the Free Willy Keiko Foundation, Newport, Oregon, in 1998. Download PDF copy
- Orca Survey field guide to transients of the Haro Strait area. Text from a catalogue authored by A.M. van Ginneken, D.K. Ellifrit and R.W. Baird, published by the Center for Whale Research , Friday Harbor, WA in 1998.
- Foraging behaviour and ecology of transient killer whales. Ph.D. Thesis of R.W. Baird, completed in 1994. Most of the thesis has been published in the above-noted papers in Behavioral Ecology, Oecologia and Canadian Journal of Zoology, but the epilogue (which focuses on the question of whether “transient” killer whales are a different species from the fish-eating “residents”) and some of the appendices are not published.
- Transient killer whale ( Orcinus orca ) harassment, predation, and “surplus killing” of marine birds in British Columbia Abstract of a conference presentation by P.J. Stacey, R.W. Baird and A.B. Hubbard-Morton, published in the Pacific Seabird Group Bulletin in 1990. Download Adobe PDF copy
- Foraging and feeding behavior of transient killer whales. Article by R.W. Baird and P.J. Stacey published in Whalewatcher, the Journal of the American Cetacean Society, in 1988. Download Adobe PDF
share this!
March 28, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
New research reveals full diversity of killer whales as two species come into view on Pacific Coast
by NOAA Headquarters
Scientists have resolved one of the outstanding questions about one of the world's most recognizable creatures, identifying two well-known killer whales in the North Pacific Ocean as separate species. The research is published in the journal Royal Society Open Science .
Killer whales are one of the most widespread animals on Earth. They have long been considered one worldwide species known scientifically as Orcinus orca, with different forms in various regions known as "ecotypes."
However, biologists have increasingly recognized the differences between resident and Bigg's killer whales. Resident killer whales maintain tight-knit family pods and prey on salmon and other marine fish. Bigg's killer whales roam in smaller groups, preying on other marine mammals such as seals and whales. (Killer whales actually belong to the dolphin family.) Bigg's killer whales, sometimes called transients, are named for Canadian scientist Michael Bigg, the first to describe telltale differences between the two types.
He noted in the 1970s that the two animals did not mix with each other even as they occupied many of the same coastal waters, which is often a sign of different species.
The finding recognizes the accuracy of the listing of Southern Resident killer whales as a Distinct Population Segment warranting protection under the Endangered Species Act in 2005. At the time, NOAA described the distinct population segment as part of an unnamed subspecies of resident killer whales in the North Pacific.
Now a team of scientists from NOAA Fisheries and universities have assembled genetic, physical, and behavioral evidence. The data distinguish two of the killer whale ecotypes of the North Pacific Coast—residents and Bigg's—as separate species.
"We started to ask this question 20 years ago, but we didn't have much data, and we did not have the tools that we do now," said Phil Morin, an evolutionary geneticist at NOAA Fisheries' Southwest Fisheries Science Center and lead author of the new paper. "Now we have more of both, and the weight of the evidence says these are different species."

Genetic data from previous studies revealed that the two species likely diverged more than 300,000 years ago and come from opposite ends of the killer whale family tree. That makes them about as genetically different as any killer whale ecotypes around the globe. Subsequent studies of genomic data confirm that they have evolved as genetically and culturally distinct groups, which occupy different niches in the same Northwest marine ecosystem.
"They're the most different killer whales in the world, and they live right next to each other and see each other all the time," said Barbara Taylor, a former NOAA Fisheries marine mammal biologist who was part of the science panel that assessed the status of Southern Residents. "They just do not mix."
Recognizing new species
The Taxonomy Committee of the Society of Marine Mammalogy will determine whether to recognize the new species in its official list of marine mammal species . The committee will likely determine whether to accept the new designations at its next annual review this summer.
The scientists proposed scientific names for the new species based on their earliest published descriptions in the 1800s. Neither will keep the ubiquitous worldwide moniker, orca. The team proposed to call resident killer whales Orcinus ater, a Latin reference to their dominant black coloring. Bigg's killer whales would be called Orcinus rectipinnus, a combination of Latin words for erect wing, probably referring to their tall, sharp dorsal fin.
Both species names were originally published in 1869 by Edward Drinker Cope, a Pennsylvania scientist known more for unearthing dinosaurs than studying marine mammals. He was working from a manuscript that California whaling captain Charles Melville Scammon had sent to the Smithsonian Institution describing West Coast marine mammals, including the two killer whales. While Cope credited Scammon for the descriptions, Scammon took issue with Cope for editing and publishing Scammon's work without telling him. (See accompanying story.)
The Smithsonian Institution had shared Scammon's work with Cope, and a Smithsonian official later apologized to Scammon for what he called "Cope's absurd blunder."
Species reflect ecosystem
The contested question of whether Southern Residents were distinct enough to merit endangered species protections initially drove much of the research that helped differentiate the two species, said Eric Archer, who leads the Marine Mammal Genetics Program at the Southwest Fisheries Science Center and is a co-author of the new research paper.
The increasing processing power of computers has made it possible to examine killer whale DNA in ever finer detail. He said the findings not only validate protection for the animals themselves, but also help reveal different components of the marine ecosystems the whales depend on.
"As we better understand what makes these species special, we learn more about how they use the ecosystems they inhabit and what makes those environments special, too," he said.
The new research synthesizes the earliest accounts of killer whales on the Pacific Coast with modern data on physical characteristics. They also use aerial imaging (called photogrammetry), and measurement and genetic testing of museum specimens at the Smithsonian and elsewhere.
While the two species look similar to the untrained eye, the evidence demonstrates they are very different species. They use different ecological niches, such as specializing in different prey, said Kim Parsons, a geneticist at the NOAA Fisheries Northwest Fisheries Science Center in Seattle and co-author of the new research.
Recent research with drones and precise aerial photos has helped differentiate Bigg's killer whales as longer and larger. This might better equip them to go after large marine mammal prey. The smaller size of residents is likely better suited to deep dives after their salmon prey, said John Durban, an associate professor at Oregon State University's Marine Mammal Institute. He leads killer whale drone research with Holly Fearnbach, a researcher at SR³.
The different prey of the two species may also help explain their different trajectories. Southern Residents are listed as endangered in part because of the scarcity of their salmon prey. Bigg's killer whales, by contrast, have multiplied while feeding on plentiful marine mammals, including California sea lions.
While killer whales represent some of the most efficient predators the world has ever seen, Durban said science is still unraveling the diversity among them. The identification of additional killer whale species is likely to follow. One leading candidate may be "Type D" killer whales identified in the Southern Ocean around Antarctica.
Other killer whales in Antarctica also look very different from the best-known black and white killer whales. This reflects a wider diversity within the species, said Durban, who has used drones to study killer whales around the world. "The more we learn," he said, "the clearer it becomes to me that at least some of these types will be recognized as different species in due course."
Journal information: Royal Society Open Science
Provided by NOAA Headquarters
Explore further
Feedback to editors

Hillside erosion worsening in California due to wildfires and intense rain
31 minutes ago

Cells use alternative splicing to regulate gene expression, research suggests
2 hours ago

Global South cities lack cooling green spaces

Inside the 'golden age' of alien hunting at the Green Bank Telescope
3 hours ago

Data from space probes show that Alfvén waves drive the acceleration and heating of the solar wind
Aug 31, 2024

Saturday Citations: Corn sweat! Nanoplastics! Plus: Massive objects in your area are dragging spacetime

How fruit flies use internal representations of head direction to support goal-directed navigation

Study finds RNA molecule controls butterfly wing coloration

Doughnut-shaped region found inside Earth's core deepens understanding of planet's magnetic field
Aug 30, 2024

Study combines data and molecular simulations to accelerate drug discovery
Relevant physicsforums posts, the predictive brain (stimulus-specific error prediction neurons).
18 hours ago
Any suggestions to dampen the sounds of a colostomy bag?
Will cryosleep ever be a reality, any stereo audio learning resources for other languages.
Aug 25, 2024
Cannot find a comfortable side-sleeping position
Therapeutic interfering particle.
Aug 24, 2024
More from Biology and Medical
Related Stories

Killer whales use specialized hunting techniques to catch marine mammals in the open ocean
Mar 20, 2024

Shark-bitten orcas in the Northeastern Pacific could be a new population of killer whale
Mar 15, 2024

Orcas covered in scars left by 'cookiecutter sharks' may be new population, study says
Mar 23, 2024

As chinook salmon get thinner and fewer, southern resident killer whales struggle to find enough food
Sep 21, 2022

Why are killer whales harassing and killing porpoises without eating them?
Sep 28, 2023
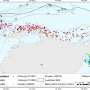
Study shows links between Australia's false killer whales and endangered groups from Hawaii
Mar 16, 2023
Recommended for you

Biodiversity loss: Many students of environment-related subjects are partly unaware of the causes

Groups of weaver birds found to have their own distinct nest-building styles

New discoveries about how mosquitoes mate may help the fight against malaria

Heat waves impair bumblebees' ability to detect floral scents, study finds
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.11(23); 2021 Dec
Killer whale ( Orcinus orca ) population dynamics in response to a period of rapid ecosystem change in the eastern North Atlantic
Eve jourdain.
1 Norwegian Orca Survey, Andenes Norway
2 Department of Biosciences, University of Oslo, Oslo Norway
Tiffany Goh
3 Sea Mammal Research Unit, Scottish Oceans Institute, University of St Andrews, Fife UK
Sanna Kuningas
4 Natural Resources Institute Finland, Helsinki Finland

Tiu Similä
5 Whale2Sea, Andenes Norway
Dag Vongraven
6 Norwegian Polar Institute, Tromsø Norway
Richard Karoliussen
Anna bisther.
7 Reportagebörsen, Gothenburg Sweden
Philip S. Hammond
Associated data.
Capture histories of the killer whales photo‐identified in 1988–2019 in northern Norway, used to fit capture–recapture models, are available from Figshare data repository: https://doi.org/10.6084/m9.figshare.15112737 .
This study investigates survival and abundance of killer whales ( Orcinus orca ) in Norway in 1988–2019 using capture–recapture models of photo‐identification data. We merged two datasets collected in a restricted fjord system in 1988–2008 (Period 1) with a third, collected after their preferred herring prey shifted its wintering grounds to more exposed coastal waters in 2012–2019 (Period 2), and investigated any differences between these two periods. The resulting dataset, spanning 32 years, comprised 3284 captures of 1236 whales, including 148 individuals seen in both periods. The best‐supported models of survival included the effects of sex and time period, and the presence of transients (whales seen only once). Period 2 had a much larger percentage of transients compared to Period 1 (mean = 30% vs. 5%) and the identification of two groups of whales with different residency patterns revealed heterogeneity in recapture probabilities. This caused estimates of survival rates to be biased downward (females: 0.955 ± 0.027 SE, males: 0.864 ± 0.038 SE) compared to Period 1 (females: 0.998 ± 0.002 SE, males: 0.985 ± 0.009 SE). Accounting for this heterogeneity resulted in estimates of apparent survival close to unity for regularly seen whales in Period 2. A robust design model for Period 2 further supported random temporary emigration at an estimated annual probability of 0.148 (± 0.095 SE). This same model estimated a peak in annual abundance in 2015 at 1061 individuals (95% CI 999–1127), compared to a maximum of 731 (95% CI 505–1059) previously estimated in Period 1, and dropped to 513 (95% CI 488–540) in 2018. Our results indicate variations in the proportion of killer whales present of an undefined population (or populations) in a larger geographical region. Killer whales have adjusted their distribution to shifts in key prey resources, indicating potential to adapt to rapidly changing marine ecosystems.
Mark–recapture models fitted to a 32‐year photo‐identification dataset suggest constant, high apparent survival rates for killer whales in Norway but reveal temporal variations in abundance and movement patterns in response to a period of ecosystem change. These results reflected variations in the proportion present of an undefined population (or populations) in a larger geographical region of the north‐eastern Atlantic. Our results show that killer whales have adjusted their distribution to shifts in key prey resources, indicating potential to adapt to rapidly changing marine ecosystems.

1. INTRODUCTION
Life history and other population parameters are key elements in status assessments of animal populations. In particular, mortality rate, population size, and geographic range are the main criteria for evaluation of a species’ extinction risk (IUCN, 2019 ), because small populations characterized by restricted geographical ranges are less buffered against losses and face an increased risk of extinction (Purvis et al., 2000 ). Time series of abundance estimates can indicate the extent to which a population may be in decline and estimates of survival and birth rates are important components in the evaluation of conservation measures. Information on abundance is also needed to assess how predators may affect prey populations and how they may respond to fluctuations in prey availability (e.g., Millon et al., 2014 ; Morissette et al., 2010 ).
For a wide range of taxa, survival and abundance have been routinely estimated using capture–recapture methods applied to photo‐identification data (e.g., birds: Dugger et al., 2004 ; felids: Oliver et al., 2011 ; reptiles: Sreekar et al., 2013 ; sharks: Gore et al., 2016 ). In cetacean research, photo‐identification is a noninvasive way to consistently “capture” (first photographic record) and “recapture” (subsequent photographic records) individually recognizable animals over time, using long‐lasting natural markings (Hammond, 1990 ). This technique offers the possibility to include images from participants other than primary research teams (e.g., citizen science), thus increasing sample size at reduced costs and allowing for data collection in regions for which funding may be limited (Gibson et al., 2020 ). Best practices in image manipulation, scoring, and cataloguing are important to allow generation of robust capture history datasets for analysis (Urian et al., 2015 ). Capture–recapture models fitted to capture histories generated from photo‐identification data have been used to obtain estimates of survival rates and abundance for a range of cetacean species (e.g., Arso Civil et al., 2019 ; Pace et al., 2017 ; Ramp et al., 2006 ; Schleimer et al., 2019 ; Zeh et al., 2002 ).
Photo‐identification was first applied to killer whales ( Orcinus orca ) in the north‐eastern Pacific in 1973 (Bigg, 1982 ) and has since led to robust estimates of life‐history parameters for a number of discrete populations worldwide (Durban et al., 2010 ; Esteban et al., 2016 ; Fearnbach et al., 2019 ; Jordaan et al., 2020 ; Kuningas et al., 2014 ; Olesiuk et al., 1990 , 2005 ; Pitman et al., 2018 ; Tixier et al., 2015 , 2017 ). As time series of photo‐identification data have become increasingly available, they have played a central role in identifying population trends and conservation status. For example, a small population size (≤100 individuals), low declining survival rates, and/or low‐to‐no reproductive output were used as basis for management advice on killer whales at Crozet (Guinet et al., 2015 ; Poncelet et al., 2010 ; Tixier et al., 2015 , 2017 ), Gibraltar (Esteban et al., 2016 ), Prince William Sound, Alaska (AT1 group, Matkin et al., 2008 ), and for the Southern resident population in British Columbia, Canada (COSEWIC, 2008 ). These studies provided an understanding of underlying threats to long‐term survival of killer whales and also emphasized the need to account for intrapopulation heterogeneity in behavior when assessing demographic trajectories in this species, otherwise risking false trends being detected (see Esteban et al., 2016 ; Tixier et al., 2015 , 2017 ).
In Norway, photo‐identification studies of killer whales were initiated in the 1980s (Lyrholm, 1988 ). In this part of the world, killer whales have long been known to mainly feed on Atlantic herring ( Clupea harengus ) and, more specifically, to follow seasonal movements of the Norwegian Spring Spawning stock (hereafter referred to as the NSS herring; Christensen, 1982 , 1988 ; Jonsgård & Lyshoel, 1970 ; Similä et al., 1996 ). The NSS herring has gone through major changes in abundance and distribution over the past decades, with recruitment of abundant year classes to the spawning stock often resulting in changes in wintering locations (Dragesund et al., 1997 ; Huse et al., 2010 ). Throughout the 1990s, the NSS herring (and killer whales) consistently wintered in the fjord system of Tysfjord‐Vestfjord, where they were readily accessible for study, and killer whales were photo‐identified annually from 1986 through 2003 (Bisther & Vongraven, 1995 ; Kuningas et al., 2014 ; Similä et al., 1996 ). From these 18 years of data, population size, survival, and reproductive rates were estimated for the first time for killer whales in Norway, which were comparable to other apparently healthy killer whale populations (Kuningas et al., 2014 ). From 2002 onward, as the inshore winter distribution of NSS herring progressively shifted to a new area further offshore (Holst et al., 2004 ; Huse et al., 2010 ), lower numbers of killer whales entered the fjords each year until 2008, after which data collection was interrupted for a few years. After the NSS herring started wintering in coastal fjords of Vesterålen and Troms, annual winter photo‐identification surveys were resumed from 2013 (see Jourdain & Vongraven, 2017 ).
Other major ecological changes occurred in the Norwegian Sea over the past two decades and may have impacted killer whales. The north‐eastern Atlantic mackerel ( Scomber scombrus ) increased in biomass (from ~2 Mt in 2007 to 9 Mt in 2014) and expanded its geographic range north‐ and westward (ICES, 2013 ; Nøttestad, Utne, et al., 2015 ). The NSS herring declined from ~12 Mt in 2009 to 5 Mt in 2014 and changed feeding and wintering distributions (ICES, 2013 , 2018 ). A number of cetacean predators of herring (e.g., pilot whales Globicephala melas and humpback whales Megaptera novaeangliae ) seem to have increased in occurrence in the Norwegian Sea (Leonard & Øien, 2020b ; Nøttestad, Krafft, et al., 2015 ), while other abundant baleen whales (e.g., common minke whales Balaenoptera acutorostrata acutorostrata and fin whales Balaenoptera physalus ) may have switched from mainly feeding on planktonic prey to pelagic fish such as herring (see Nøttestad, Krafft, et al., 2015 ; Nøttestad, Sivle, Krafft, Langård, et al., 2014 ), implying possible variations in resource competition (see Jourdain & Vongraven, 2017 ). Recent studies in seasons and locations not previously investigated have documented new prey types, that is, Atlantic salmon ( Salmo salar ; Vester & Hammerschmidt, 2013 ), Atlantic mackerel (Nøttestad, Sivle, Krafft, Langard, et al., 2014 ), harbor porpoise ( Phocoena phocoena ; Cosentino, 2015 ), lumpfish ( Cyclopterus lumpus ; Jourdain et al., 2019 ), and pinnipeds (Jourdain et al., 2017 ; Vongraven & Bisther, 2014 ) for killer whales in Norway, including for individuals known as herring‐eaters (see Jourdain et al., 2019 , 2020 ). These new observations could be the result of enhanced research effort but could also reflect behavioral responses to a changing marine ecosystem. Recent toxicological assessments, which analyzed both fish specialists and individuals who consumed various proportions of fish and pinnipeds, showed that killer whales in Norway carried higher pollution levels than previously assumed, with possible impact on survival and population growth (Andvik et al., 2020 ). Estimates from line‐transect surveys in the Northeast Atlantic are insufficiently precise to explore whether killer whale abundance in this area may have changed in the last 20 years (2002–2007: 18,821 and 95% CI: 11,525–30,735; 2008–2013: 9563 and 95% CI: 4713–19,403 in Leonard & Øien, 2020b ; 2014–2018: 15,056 and 95% CI: 8423–26,914 in Leonard & Øien, 2020a ). To investigate how killer whales may have responded to this period of rapid ecosystem change in the Norwegian Sea, new estimates of population parameters are needed.
In this study, we fitted capture–recapture models to a photo‐identification dataset spanning a 32‐year period to generate population parameters for killer whales in northern Norwegian waters. The objectives were (1) to estimate survival rates for the period 1988–2019, including investigating any difference between time periods (i.e., 1988–2008 and 2012–2019) and possible underlying factors and (2) to estimate the size of the population at recent herring wintering grounds in 2012–2019 for comparison with estimates published for the period 1986–2003 (Kuningas et al., 2014 ). The overall aim was to improve understanding of how killer whales may respond to shifting prey populations in rapidly changing Arctic marine ecosystems.
2. MATERIAL AND METHODS
2.1. study area and data collection.
Annual photo‐identification surveys were conducted independently by three teams of investigators in Tysfjord‐Ofotfjord‐Vestfjord, in Lofoten (68°19′34.52″N, 15°56′44.38″E) from 1988 to 2008; off Andøya, in Vesterålen (69°16′29.47″N, 16°25′2.29″E) from 2013 to 2018; off Vengsøya‐Kvaløya, in Troms (69°48′52.06″N, 18°38′54.31″E) from 2015 to 2016; and in Kvænangen (70°4′36.22″N, 21°11′29.13″E) from 2017 to 2019, resulting in a study area of ~900 km 2 for the entire study period (Table (Table1; 1 ; Figure Figure1). 1 ). During 1988–2008 (referred to as Period 1), fieldwork took place between October and January. Data collection shifted to November–February in 2012–2019 (referred to as Period 2) in response to the later arrival of herring and killer whales in the fjords. Hereafter, each field season is referred to by the initial year (e.g., winter 2012–2013 is designated as 2012).
Total numbers of observation days, killer whale encounters (when known), photographs (total collected regardless of quality), individual killer whales identified (IDs), and of fair‐to‐excellent quality (see Section 2) identifications including resightings, as contributed by the three research teams (DV/AB: Dag Vongraven and Anna Bisther, TS/SK: Tiu Similä and Sanna Kuningas, NOS: Norwegian Orca Survey) and citizen‐science (CS) in 1988–2019 in the study area, and which contributed to building the capture histories of the 1236 killer whales included in this study
| Source | Years | Days | Encounters | Photographs | IDs | Identifications |
|---|---|---|---|---|---|---|
| TS/SK | 1988–2008 | 272 | 318 | 12,420 | 316 | 2415 |
| DV/AB | 1990–1996 | 103 | N/A | N/A | 179 | 452 |
| NOS | 2013–2019 | 151 | 422 | 94,900 | 1032 | 1843 |
| CS | 2012–2019 | 303 | N/A | 66,900 | 694 | 1345 |
A number of observation days overlapped between DV/AB and TS/SK because the two research teams operated independently but at the same time of year and within the same region.

Map showing the study areas where photo‐identification data were collected in northern Norway in 1988–2019: the red‐shaded area in region A indicates where killer whale encounters occurred in 1988–2008 and red plots in regions B (2013–2018), C (2015–2016) and D (2017–2019) indicate exact location at start of killer whale encounters. Killer whale photographs provided by citizen‐science originated from areas B, C, and D in 2012–2019 (not plotted). Adjacent coastal (zones 1–4) and offshore (zone 5) regions from which opportunistic photo‐identifications were available are also shown
In both periods, surveys were carried out opportunistically or using sighting reports obtained from other vessels in the area, with the aim of maximizing the number of killer whales found. A similar approach was maintained in 2013–2014 when whale‐watching rigid inflatable boats were used as research platforms. When a group (defined as individuals in apparent association and acting in a coordinated manner during the observation period) was encountered, left‐sided identification photographs were taken following the protocols described by Bigg ( 1982 ; Figure Figure2). 2 ). Efforts focused on photographing as many individuals as possible in each encountered group (hereafter referred to as an encounter), regardless of individuals’ size, behavior, or distinctiveness to minimize heterogeneity of capture probabilities (Hammond, 2010 ). When all or most individuals in the encounter were believed to have been photographed, the research vessel left the animals and resumed its search for other killer whale groups. Photographs were taken with SLR cameras and Kodak T MAX or Ilford HP5 400 ASA films in 1988–2000 and DSLR cameras in 2001–2019, all equipped with 200‐ or 300‐mm lenses. Supplementary photographs, with reliable information on date and time, collected from wildlife photographers and members of the general public within the study area were also used for identification purposes in 2012–2019 (Table (Table1; 1 ; Figure Figure1 1 ).

Sample of identification photographs showing the persistence of scarring and pigmentation patterns of the saddle patch and nicks in the dorsal fin and thus their reliability for long‐term re‐identification of individual killer whales in Norway. Distinctively taller dorsal fin for NKW‐693, compared to NKW‐443 and NKW‐619 for which dorsal fin did not develop over the course of the study, further illustrates how sex could be readily determined based on morphological features for most identified individuals
2.2. Photo‐identification
2.2.1. photograph processing.
Processing photographs required the films to be inspected using a stereoscopic microscope until 2000, after which digital images were viewed and enhanced in Adobe Photoshop. The three teams of investigators followed similar photo‐identification protocols. For each encounter, individuals were identified from left‐sided photographs using nicks, shape, and size of the dorsal fin, alongside scarring and pigmentation patterns of the adjacent gray saddle patch as per Bigg ( 1982 ; Figure Figure2). 2 ). The best photograph of each individual from each encounter was selected and rated for (1) quality (poor, fair, good, excellent) based on combined criteria of sharpness, contrast, and size of the dorsal fin relative to the frame; (2) angle of the killer whale relative to the photographer (parallel, slight angle, angle); and (3) proportion of the saddle patch visible (top 1/3, top 2/3, or fully visible). Each identified individual was matched against an existing catalogue of previously identified individuals (see published catalogue 2007–2021: Jourdain & Karoliussen, 2021 ). If a match was found, the individual received a new record of where and when it was seen together with corresponding photograph scoring. If no match was found, the previously unidentified individual was assigned a unique identification (ID) number, added to the ID catalogue and received its first sighting record. A database listing individuals’ sighting histories was held independently by each research team (Table (Table1). 1 ). For each encounter, unidentifiable individuals lacking features for reliable long‐term identification were differentiated from each other using temporary subtle skin markings (e.g., body scars, lesions) and pigmentation of the eye patch from photographs of fair‐to‐excellent quality. This information on the number of identified and unidentified individuals in an encounter was used to estimate the proportion of identifiable individuals in the population and correct capture–recapture estimates of abundance (see below).
2.2.2. Comparing ID catalogues
To build a database common to all three studies, images of individual killer whales were systematically cross‐matched across all three ID catalogues (Table (Table1). 1 ). Potential matches were evaluated by five of the authors (EJ, TG, TS, SK, DV) and by an external analyst with >40 years of experience with photo‐identifying killer whales (Graeme Ellis). A match was considered certain only when accepted by all. Individuals found in more than one catalogue were renamed to a unique ID number and their sighting histories, as logged independently by the different investigators, were combined.
2.3. Characterization of individuals
2.3.1. determining sex and age class.
Using clear morphological evidence of physical maturity, adult males were identified based on a distinctively taller dorsal fin (Bigg, 1982 ; Olesiuk et al., 1990 ; Figure Figure2). 2 ). Other individuals of apparent mature size, seen in close and consistent association with a calf (in echelon position) on at least two encounter days, or showing no development of the dorsal fin in at least 3 years, were categorized as adult females (Figure (Figure2). 2 ). Individuals for which sex could not be determined were categorized as “unknowns.” These individuals could be either subadult males or females, or adult females.
2.3.2. Assessing ranging patterns
To assess how the study area compared to ranging capacities of killer whales identified from annual winter surveys, we compiled additional photographic records collected from citizen‐science in adjacent coastal and offshore regions for these individuals (Figure (Figure1 1 ).
2.4. Mark–recapture analyses
2.4.1. data selection.
To be considered marked (re‐identifiable) and to be retained for analysis, an individual had to have a minimum of one primary feature, defined as (a) at least three scars on the saddle patch; or (b) at least two nicks in the dorsal fin, or a minimum of two secondary features. Secondary features were defined as (i) one or two scars on the saddle patch, (ii) a single nick in the dorsal fin, and (iii) distinctive pigmentation of the saddle patch (Figure S1 ). In addition, only identifications from photographs of fair‐to‐excellent quality of killer whales describing a parallel or only slight angle relative to the photographer, and for which the full dorsal fin and at least the top 2/3 of the saddle patch were visible were retained for analysis (Figure S1 ). Individuals (including calves) lacking permanent markings were excluded from all analyses but were used to estimate the proportion of identifiable individuals in the population (see below).
2.4.2. Cormack–Jolly–Seber (CJS) models
To estimate annual survival probabilities using CJS models (Lebreton et al., 1992 ), capture histories were built by pooling sightings recorded during the same annual winter season and by treating each year as a sampling occasion. Prior to running models, we ran goodness‐of‐fit (GOF) tests implemented in the R (version 4.0.2; R Core Team, 2018 ) package R2ucare (Gimenez et al., 2018 ) to test for lack of fit of the global CJS model. Through specialized interpretable test components, this approach can identify features of the data that underlie departure from model assumptions. In particular, component Test 3.SR tests for equal probability of recapture between newly and previously captured individuals (Pradel et al., 1997 ), and Test 2.CT tests for equal recapture probability between individuals encountered and not encountered in a given sampling occasion (Pradel, 1993 ). These tests can identify features of the data that are typically caused by a transience effect, resulting from the presence of transient individuals (defined as having been seen only once), and trap‐dependence, in which recapture probability is influenced by whether or not an individual was captured during the previous sampling session, respectively. However, these features could also be the result of other features of the data. The global test, combining all test components, was used to assess the general goodness of fit of the CJS model.
CJS models were fitted to annual sex‐specific capture histories for 1988–2019 (thus excluding unknown sex animals) to estimate adult apparent survival probability ( φ ; incorporating any permanent emigration) between years and recapture probability ( p ) for each year. Gap years (2009, 2010, 2011 with no data available) were included in the full time series by fixing recapture probability to 0 for these years. A set of candidate models was constructed in which apparent survival and recapture probabilities were (using conventional notation): constant over time (.), varied annually ( t ), or displayed a linear temporal trend ( T ) (Lebreton et al., 1992 ). In addition to incorporating a temporal trend, we explored the effect of modeling Period 1 and Period 2 as distinct time periods ( period ). A sex‐effect ( s ) on both survival and recapture probabilities was also tested. GOF Test 2.CT indicated a behavioral (“trap”) response (see Section 3 ) and justified testing the effect of trap‐dependence ( td ) when modeling recapture probabilities. Trap‐dependence was implemented using an individual time‐varying covariate comprising dummy variables (0 and 1) depending on whether or not an individual was seen on the previous occasion. Lack of fit in GOF Test 3.SR (see Section 3) justified testing the effect of transience on estimates of apparent survival. This was achieved by building time‐since‐marking models with two classes ( trans ), in which survival probability was estimated for the first annual interval after first capture (first class) and also for all subsequent annual intervals (second class). Additive (+) and interactive (*) models were constructed to test for combinations of effects on φ and p . Overdispersion in the data was evaluated by calculating the variance inflation factor ( ĉ , “c‐hat”) as global GOF test X 2 /degrees of freedom (Lebreton et al., 1992 ).
The probability of apparent survival is the product of surviving from one sampling occasion to the next and of returning to the study area. We investigated whether differences in residency patterns could influence estimates of apparent survival in Period 2 (2012–2019). Residency groups were identified by categorizing individuals following methods described by Schleimer et al. ( 2019 ).
Sighting histories of all individuals (males, females, unknowns) in 2012–2019 were used to calculate individuals’ yearly (YSR) and seasonal (SSR) sighting rates with: YSR = number of years in which seen/total number of years since first identification, and SSR = number of days in which seen/total number of days since first identification in a given season. Individuals first identified in 2018 and 2019 were excluded due to insufficient years with sighting data to reliably evaluate residency patterns. Agglomerative Hierarchical Clustering (AHC) was conducted using the hclust function in R to classify individuals based on similar sighting rates. To allow for direct comparison of the two rates, YSR and SSR were standardized (relative to the median and the median absolute deviation) beforehand using the scale function in R. In the AHC, Euclidean distance was chosen as a measure of dissimilarity and to compute proximity matrices between individuals using Ward's method. This clustering method merges the closest individuals (data points) into clusters based on a proximity matrix. The most appropriate number of residency groups was chosen based on obvious main clusters identified visually in the resulting dendrogram.
CJS models were fitted separately to the residency groups identified by the AHC analysis. For each group, both survival and recapture probabilities were allowed to be constant over time (.), vary annually ( t ), or display a linear temporal trend ( T ).
2.4.3. Robust design models
Robust design (RD) models were fitted to the capture histories of all individuals (males, females, unknowns) in Period 2 to estimate the annual number of killer whales using the study area and to evaluate the extent of temporary emigration from the study area between years (Kendall et al., 1997 ). Each annual winter season (year) was considered as a primary sampling occasion. Each survey area was covered in every week in all years, so weeks within each winter season were treated as secondary sampling occasions (Table (Table2). 2 ). Candidate models were built to incorporate effects that were constant over time (.), varied over time ( t ), had a linear temporal trend ( T ), and/or a transience effect ( trans ) on survival probabilities (φ; GOF Test 3.SR was marginally significant—see Section 3). Capture and recapture probabilities were assumed equal in all models ( p = c ) and were modeled to vary by primary sampling occasion alone ( session ) or by both primary and secondary sampling occasion ( session*time ). The probability of temporary emigration from the study area between years (primary occasions) was modeled using the parameters γ′ (probability of being outside the study area conditional on being outside the study area in the previous year) and γ″ (probability of being outside the study area conditional on being inside the study area in the previous year). γ″ can thus be interpreted as the annual probability of temporary emigration and 1 – γ′ as the annual probability of re‐immigration. Temporary emigration was modeled as: random (γ′ = γ″), Markovian (γ′ ≠ γ″), or no emigration (γ′ = 1; γ″ = 0). Temporary emigration parameters were modeled as either constant (.) or varying over time ( t ).
Total number of captures in each secondary occasion (weeks within field seasons) that made up each primary period (years) in the dataset 2012–2019 used for fitting robust design models to the capture histories of all individuals (i.e., males, females, unknowns)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2012 | 18 | 20 | |||||||||||||||
| 2013 | 11 | 13 | 21 | 22 | 45 | ||||||||||||
| 2014 | 8 | 16 | 11 | 6 | 16 | 28 | 37 | 38 | |||||||||
| 2015 | 18 | 17 | 45 | 99 | 78 | 22 | 31 | 13 | 31 | 35 | 121 | 44 | 40 | 24 | 37 | 17 | 16 |
| 2016 | 29 | 108 | 133 | 123 | 33 | 8 | 38 | 32 | 22 | 27 | |||||||
| 2017 | 48 | 61 | 82 | 104 | 13 | 14 | 11 | 19 | |||||||||
| 2018 | 50 | 47 | 28 | 111 | 121 | 47 | 25 | 13 | 18 | 22 | 28 | ||||||
| 2019 | 77 | 155 | 85 | 61 | 33 | 16 | 25 |
2.4.4. POPAN models
To obtain an alternative estimate of the size of the “super‐population,” defined as the total number of killer whales that was in the study area at some point in time, the POPAN parameterization of the Jolly–Seber model (Schwarz & Arnason, 1996 ) was fitted to the capture histories of all individuals (males, females, unknowns) in Period 1 (1988–2008) and in Period 2 (2012–2019). Other parameters in the POPAN model are the probability of apparent survival ( φ ), capture ( p ), and recruitment into the study area from the super‐population ( pent ). All these parameters were modeled as constant (.), varying over time ( t ), or as a trend over time ( T ). Because GOF Test 3.SR was significant (marginally for Period 2—see Section 3), we also built time‐since‐marking models with two classes ( trans ) to account for transience effects in survival probabilities, with additive and interactive effects with ( t ) and ( T ). Estimates of annual population size were also derived from the models.
2.4.5. Model selection, adjustment for overdispersion, and model‐averaging
The support that candidate CJS and POPAN models received from the data was assessed using quasi‐likelihood AIC for small sample size (QAICc), obtained by adjusting AICc for overdispersion using estimated ĉ (except for the high residency group which did not show overdispersion—see Section 3). Estimates of parameters of interest were obtained by model‐averaging over models within delta‐QAICc ≤10 of the lowest QAICc, considered to receive some support from the data (Burnham & Anderson, 2002 ). GOF tests are unavailable for RD models so neither overall model fit nor overdispersion in the data could be assessed. Therefore, AIC C was used to assess relative model fit.
All capture–recapture analyses were conducted using the package RMark v.2.2.7. (Laake & Rexstad, 2008 ) in R.
2.4.6. Proportion of identifiable individuals
The proportion of identifiable individuals in the population in each year, θ , was estimated by fitting a binomial generalized linear model with logit link function to the number of identified and unidentified individuals in encountered groups, where this could be determined. Total population size was estimated as:
where N ^ is the capture–recapture estimate of the number of identifiable animals, with coefficient of variation (CV) estimated using the delta method as:
and 95% confidence intervals calculated assuming a log normal distribution as N total /c to c* N total , where:
3.1. Data summary
A total of 672 observation days (unique dates) in 1988–2019 resulted in 6055 identifications and 3284 annual captures of 1236 individual killer whales throughout the study period (Table (Table1; 1 ; Figures Figures1, 1 , ,2, 2 , ,3, 3 , S2 ). Comparing the three ID catalogues (see Table Table1), 1 ), 179 matches were identified between the catalogues held by TS/SK and DV/AB, 72 matches between Norwegian Orca Survey (NOS) and DV/AB and 151 matches between NOS and TS/SK, including 148 individuals seen in both periods (Table S3 ; Figures Figures2 2 and and3). 3 ). Overall, 691 (56%) individuals were seen in two or more years (Table S3 ; Figure Figure4). 4 ). While the rate of new identifications leveled off toward the end of Period 1, it increased again after fieldwork was resumed at newly established herring wintering grounds from 2012 (Table (Table1; 1 ; Figures Figures1 1 and and3). 3 ). The proportion of transients (animals seen only once) relative to the total number of identified individuals in each year peaked in 2015 and 2016 and averaged 30% (SD = 18%) in Period 2, compared to 5% (SD = 5%) in Period 1 (Figure (Figure5). 5 ). Sex was reliably determined for 960 of the 1236 individuals, including 719 males and 241 females, while 276 were categorized as unknowns (Table S4 ). Such a disproportionate sex ratio in the ID catalogue may be explained by adult males being more identifiable due to their tendency to bear more markings than adult females and subadults; only 6% of the unmarked individuals in 2015–2019 were adult males. In 1988–2019, 71 (6%) killer whales identified at herring wintering grounds had also been photo‐identified in at least one adjacent region, in one or multiple years (Table (Table3; 3 ; Figure Figure1 1 ).

Number of killer whales identified for the first time (black) and previously identified (gray) in each year (bar plots) and the cumulative discovery curve of new individuals in 1988–2019. The red solid line indicates the transition to Period 2 (2012–2019)

Frequency of capture (number of years in which seen) for the 1236 individual killer whales identified in 1988–2019

Number of individuals seen in only 1 year (transients, gray) relative to the total number of identified individuals (black and gray combined) in each year. The red solid line indicates the transition to Period 2 (2012–2019)
Number of photo‐identified killer whales that were opportunistically photo‐identified in adjacent coastal (zones 1–4) and offshore (zone 5) regions in addition to their winter records in the study area. Zones (Z) match region subdivision from Figure Figure1 1
| Z1 | Z2 | Z3 | Z4 | Z5 | Any zone | M/F/U | High/Low | |
|---|---|---|---|---|---|---|---|---|
| Period 1 | 0 | 0 | 26 | 0 | 0 | 26 | 12/13/1 | – |
| Period 2 | 3 | 28 | 11 | 0 | 7 | 45 | 26/17/2 | 18/22 |
| Full series | 3 | 28 | 37 | 0 | 7 | 71 | 39/30/2 | – |
The sex ratio (males/females/unknowns) and the number of individuals assigned to each of the high and low residency groups (Period 2) are also shown. Some individuals may have been seen in multiple zones meaning that “Any Zone” is not the sum of the Z‐columns
3.2. Apparent survival rates 1988–2019
GOF tests 2.CT and 3.SR indicated a lack of fit of the CJS model (Table (Table4), 4 ), which was addressed by fitting models that included the effects of trap‐dependence on recapture probability and transience on apparent survival probability. CJS models accounting for combined (additive or interactive) effects of sex, transience, and blocks of time or linear temporal trend on survival carried all the QAICc weight (Table (Table5). 5 ). Model‐averaged estimates of apparent survival declined from 0.998 (± 0.002 SE) for females and 0.985 (± 0.009 SE) for males in Period 1 to 0.955 (± 0.027 SE) and 0.864 (± 0.038 SE), respectively, in Period 2 (values averaged across years for each sex and in each period; Figure Figure6a). 6a ). Preliminary modeling of the two periods separately indicated that the data gap between periods did not influence estimates of survival in the two periods when modeling the entire dataset. Average apparent survival was lower for transients (geometric mean Period 1: 0.907 ± 0.043 SE, Period 2: 0.697 ± 0.070 SE). The models for recapture probabilities that accounted for additive effects of time and trap‐dependence received all the QAICc weight (Table (Table5). 5 ). Model‐averaged recapture probabilities varied considerably throughout the study period, reaching maxima (>0.53) at the beginning and toward the end of the time series (Figure (Figure6b 6b ).
Results of the four directional goodness‐of‐fit tests (GOF), the global combined test of overall CJS model fit, and the variance inflation factor (ĉ, “c‐hat”) calculated as X 2 /degrees of freedom
| Dataset | 3.SR | 3.SM | 2.CT | 2.CL | Global test | ĉ |
|---|---|---|---|---|---|---|
| MF | = 76.903 df = 23 < .001 | = 31.840 df = 33 = .525 | = 140.885 df = 26 < .001 | = 75.809 df = 41 = .001 | = 325.437 df = 123 < .001 | 2.64 |
| MFU | = 80.808 df = 15 < .001 | = 18.045 df = 18 = .453 | = 71.112 df = 17 < .001 | = 53.407 df = 33 = .014 | = 223.372 df = 83 < .001 | 2.69 |
| MFU | = 20.273 df = 6 = .002 | = 10.207 df = 6 = .116 | = 7.190 df = 5 = .207 | = 4.025 df = 4 = .403 | =41.695 df = 21 = .005 | 1.98 |
| HIGH | = 0 df = 1 = 1 | = 5.322 df = 4 = .256 | = 3.434 df = 4 = .488 | = 1.323 df = 3 = .724 | =10.079 df = 12 = .609 | 0.84 |
| LOW | = 7.926 df = 5 = .160 | = 10.383 df = 6 = .109 | = 7.110 df = 5 = .213 | = 10.778 df = 4 = .029 | = 36.197 df = 20 = .015 | 1.81 |
Datasets are MF: sex‐specific, MFU: males, females, unknowns, HIGH: high residency group, LOW: low residency group.
Summary of the best‐supported candidate CJS models (≤10 ΔQAICc) for 1988–2019 used for model‐averaging the probability of apparent survival ( φ ) and of recapture ( p ) accounting for additive (+) or interactive (*) effects of time ( t ), a linear temporal trend ( T ), sex ( s ), periods of time ( period ), transience ( trans ), and/or trap‐dependence ( td )
| Model | QAICc | ΔQAICc | QAIC weight | Q deviance | Number of parameters |
|---|---|---|---|---|---|
| 2666.064 | 0 | 0.472 | 2584.732 | 40 | |
| 2667.297 | 1.233 | 0.255 | 2585.964 | 40 | |
| 2667.573 | 1.509 | 0.222 | 2594.493 | 36 | |
| 2670.499 | 4.434 | 0.051 | 2597.418 | 36 |

Model‐averaged probabilities of (a) apparent survival and (b) recapture with 95% CI for adult male (black) and female (gray) killer whales in 1988–2019, as estimated from the four best‐supported CJS models listed in Table Table5. 5 . The red solid line indicates the transition to Period 2 (2012–2019)
3.3. Residency groups and survival rates 2012–2019
Out of the 902 killer whales encountered in 2012–2019 and first identified before 2018 (see Section 2), the AHC results (Figure (Figure7) 7 ) indicated two main clusters in which 159 individuals were assigned to a first cluster characterized by high yearly (0.823 ± 0.012 SE) and seasonal (0.037 ± 0.001 SE) sighting rates, hereafter referred to as the “High residency group.” The remaining 743 whales were assigned to a second cluster characterized by low yearly (0.322 ± 0.006) and seasonal (0.009 ± 0.0002 SE) sighting rates, hereafter referred to as the “Low residency group.” A Mann–Whitney Wilcoxon test for non‐normally distributed data confirmed a significant difference in both yearly ( W = 1850.5, p < .001) and seasonal ( W = 601, p < .001) sighting rates between the two residency groups. Figure Figure7 7 could be interpreted as showing three clusters, rather than two. We estimated apparent survival rates independently for the three indicated clusters. Results (not shown) indicated that estimated survival was the same for two of the clusters and were thus no more informative that the results for two clusters.

Dendrogram showing the results of the Agglomerative Hierarchical Cluster (AHC) analysis conducted on individuals seen in 2012–2019 on the basis of dissimilarity in yearly (YSR) and seasonal sighting rates (SSR). Sex ratio (females/males/unknowns) for each cluster is also shown
No lack of fit of the CJS models was detected from the GOF tests run on each of the two residency groups (Table (Table4). 4 ). For the High residency group, the most supported CJS model received 73% of the AICc weight and estimated apparent survival at 1 (95% CI: 0.99–1.00) (Table (Table6; 6 ; Figure Figure8a). 8a ). This is explained by all 159 killer whales in this residency group still being alive at the end of this short second study period (2012–2019). Model‐averaged estimates of apparent survival were much lower for the whales assigned to the Low residency group (geometric mean: 0.731 ± 0.075 SE; Figure Figure8a). 8a ). In this group, individuals also had consistently lower recapture probabilities than the high residency group, confirming reduced fidelity to the area for these whales (Figure (Figure8b 8b ).
Most‐supported CJS model for the High residency group and best‐supported candidate models (≤10 ΔQAICc) for the Low residency group used for model‐averaging the probability of apparent survival ( φ ) and of recapture ( p ), both allowed to be constant (.), vary by time ( t ) or display a linear temporal trend ( T )
| Model | AICc/QAICc | ΔAICc/ΔQAICc | AIC/QAIC weight | Deviance | Number of parameters |
|---|---|---|---|---|---|
| . | 616.180 | 0 | 0.734 | 76.375 | 7 |
| 1329.559 | 0 | 0.625 | 109.296 | 8 | |
| 1331.589 | 2.030 | 0.226 | 109.293 | 9 | |
| 1333.116 | 3.557 | 0.106 | 100.600 | 14 | |
| 1336.539 | 6.980 | 0.019 | 116.276 | 8 | |
| 1338.005 | 8.446 | 0.009 | 115.709 | 9 | |
| 1338.340 | 8.781 | 0.008 | 130.196 | 2 | |

Non‐sex‐specific probabilities of (a) survival and (b) recapture with 95% CI for the High residency group (black) and the Low residency group (gray), as obtained from the best‐supported CJS models listed in Table Table6. 6 . Note: there were no captures in the High residency group in 2012
3.4. Temporary emigration
Robust design models that included random temporary emigration, either constant or time varying, carried most of the AIC weight (74%), although models featuring Markovian temporary emigration also received some support from the data (26% of the AIC weight, Table Table7). 7 ). Models with no temporary emigration had no support. Only models with capture probability varying by both primary and secondary sampling occasions were supported. Model‐averaged survival probabilities were similar to those obtained with the CJS (Figure S5 a). Model‐averaged capture probabilities varied considerably within and between primary periods, ranging from 0.013 (± 0.006 SE) to 0.392 (± 0.030 SE; Figure S5 b). Average annual probability of emigration and re‐immigration were γ″ = 0.148 (± 0.095 SE) and 1 – γ′ = 0.760 (± 0.215 SE), respectively.
Summary of the best‐supported candidate models (≤10 ΔQAICc) obtained when fitting robust design models to the dataset 2012–2019 and used for model‐averaging the probability of apparent survival ( φ ), the probability of being outside the study area conditional on being outside the study area in the previous year ( γ ′) and the probability of being outside the study area conditional on being inside the study area in the previous year ( γ″ )
| Model | AICc | ΔAICc | AIC weight | Deviance | Number of parameters |
|---|---|---|---|---|---|
| ′ | −1371.205 | 0.000 | 0.364 | 5025.278 | 91 |
| ′ | −1369.319 | 1.885 | 0.142 | 5039.940 | 85 |
| ′ | −1368.863 | 2.341 | 0.113 | 5014.786 | 97 |
| . ≠ ′ . | −1368.590 | 2.615 | 0.098 | 5038.545 | 86 |
| ≠ ′ . | −1368.502 | 2.703 | 0.094 | 5025.846 | 92 |
| ′ | −1367.015 | 4.190 | 0.045 | 5042.245 | 85 |
| ′ | −1366.219 | 4.985 | 0.030 | 5030.263 | 91 |
| . ≠ ′ . | −1365.801 | 5.404 | 0.024 | 5028.547 | 92 |
| ′ | −1365.063 | 6.142 | 0.017 | 5046.321 | 84 |
| = ′ | −1364.977 | 6.227 | 0.016 | 5042.157 | 86 |
| . ≠ ′ . | −1364.780 | 6.424 | 0.015 | 5044.480 | 85 |
| ≠ ′ . | −1364.531 | 6.674 | 0.013 | 5016.975 | 98 |
| ′ | −1362.946 | 8.258 | 0.006 | 5042.061 | 87 |
| . ≠ ′ | −1362.856 | 8.348 | 0.006 | 5031.491 | 92 |
| ′ | −1362.848 | 8.356 | 0.006 | 5035.768 | 90 |
| ≠ ′ . | −1362.400 | 8.805 | 0.004 | 5034.083 | 91 |
| ≠ ′ . | −1361.586 | 9.619 | 0.003 | 5043.421 | 87 |
For apparent survival, single, additive (+) or interactive (*) effects were modeled as constant (.), time‐specific ( t ), with a linear temporal trend ( T ) and accounting for transience ( trans ). All listed supported models had capture probabilities varying by primary and secondary sampling occasion.
3.5. Population size
When fitted to data from Period 1, POPAN models that accounted for a temporal trend ( T ) in recruitment from the super‐population into the study area ( pent ) received >87% of the QAICc weight (Table (Table8). 8 ). Models with pent ( t ) were unable to estimate all parameters and were therefore excluded from consideration. When fitted to data from Period 2, POPAN models that included a temporal trend ( T ) or a time effect ( t ) on pent received equal support from the data (48% and 51% of the QAICc weight, respectively), while models with constant pent carried low weight (<2% of the QAICc weight; Table Table9 9 ).
Summary of the best‐supported candidate models (≤10 ΔQAICc) obtained when fitting POPAN models to Period 1 (1988–2008) and used for model‐averaging the probability of apparent survival ( φ ), of recapture ( p ) and of recruitment into the study area from the super‐population ( pent ), accounting for single, additive (+) or interactive (*) effects of time ( t ), a linear temporal trend ( T ), and transience ( trans ) or set constant (.)
| Model | QAICc | ΔQAICc | QAICc weight | deviance | Number of parameters |
|---|---|---|---|---|---|
| 1508.323 | 0 | 0.403 | 158.738 | 25 | |
| 1509.484 | 1.161 | 0.226 | 157.817 | 26 | |
| 1510.157 | 1.834 | 0.161 | 158.490 | 26 | |
| 1511.415 | 3.092 | 0.086 | 157.662 | 27 | |
| 1512.243 | 3.920 | 0.057 | 164.736 | 24 | |
| 1513.402 | 5.079 | 0.032 | 163.817 | 25 | |
| 1514.056 | 5.733 | 0.023 | 164.471 | 25 | |
| 1515.32 | 6.997 | 0.012 | 163.653 | 26 |
Summary of the best‐supported candidate models (≤10 ΔQAICc) obtained when fitting POPAN models to Period 2 (2012–2019) and used for model‐averaging the probability of apparent survival ( φ ), of recapture ( p ) and of recruitment into the study area from the super‐population ( pent ), accounting for single, additive (+) or interactive (*) effects of time ( t ), a linear temporal trend ( T ) and transience ( trans ) or set constant (.)
| Model | QAICc | ΔQAICc | QAICc weight | deviance | Number of parameters |
|---|---|---|---|---|---|
| 1922.582 | 0 | 0.264 | −1744.355 | 24 | |
| 1923.441 | 0.859 | 0.172 | −1741.459 | 23 | |
| 1924.060 | 1.479 | 0.126 | −1730.686 | 18 | |
| 1924.799 | 2.217 | 0.087 | −1752.353 | 19 | |
| 1924.862 | 2.280 | 0.084 | −1742.075 | 18 | |
| 1925.144 | 2.562 | 0.073 | −1741.793 | 18 | |
| 1925.900 | 3.319 | 0.050 | −1730.872 | 17 | |
| 1925.961 | 3.380 | 0.049 | −1730.811 | 17 | |
| 1926.848 | 4.267 | 0.031 | −1752.354 | 17 | |
| 1926.851 | 4.269 | 0.031 | −1742.124 | 14 | |
| 1927.901 | 5.319 | 0.018 | −1730.900 | 13 | |
| 1929.439 | 6.857 | 0.009 | −1735.461 | 13 | |
| 1930.719 | 8.137 | 0.005 | −1734.181 | 12 | |
| 1932.375 | 9.793 | 0.002 | −1720.347 | 11 |
In Period 1, the proportion of identifiable individuals in the population was 0.556 (± 0.052 SE) for 1990–1995 and 0.656 (± 0.034 SE) for 1997–2003 (see Kuningas et al., 2014 ), leading to an average across years of 0.606 (± 0.043 SE). In Period 2, the estimated proportion of identifiable individuals in the population varied from 0.687 (± 0.021 SE) in 2019 to 0.744 (± 0.023 SE) in 2015 (Table (Table10 10 ).
Estimated proportion of identifiable killer whales in the population for each annual winter season
| Year | Identifiable proportion (± SE) | Number of encounters |
|---|---|---|
| 2012 | 0.716 (± 0.017) | – |
| 2013 | 0.716 (± 0.017) | – |
| 2014 | 0.716 (± 0.017) | – |
| 2015 | 0.744 (± 0.023) | 18 |
| 2016 | 0.730 (± 0.016) | 20 |
| 2017 | 0.716 (± 0.012) | 19 |
| 2018 | 0.702 (± 0.014) | 20 |
| 2019 | 0.687 (± 0.021) | 31 |
| Average/total | 0.716 (± 0.017) | 108 |
Annual abundance, estimated from the RD models corrected for the proportion of identifiable individuals, peaked in 2015 at 1061 whales (95% CI: 999–1127) and dropped to 513 whales (95% CI: 488–540) in 2018 (Figure (Figure9; 9 ; Table S6 ). Large standard errors for estimates in 2012–2014 indicated low precision, most likely as a result of a relatively small number of identifications for these years (Figure S2 ). Annual abundance estimates obtained from POPAN models were comparable to those obtained from the RD, but less precise (Table S7 ).

Model‐averaged estimates of the number of identifiable killer whales (gray) and total abundance (corrected for the proportion of identifiable individuals, black), with 95% CI, that used the study area during the winter months between 2012 and 2019, as estimated from the best‐supported robust design models listed in Table Table7 7
Super‐population size in Period 1 (i.e., number of killer whales present in the study area at some point between 1988 and 2008), obtained by model‐averaging the most supported POPAN models (Table (Table8) 8 ) and corrected for the average proportion of identifiable individuals across years (0.606, see above) was 886 (95% CI: 789–994). In Period 2, and accounting for an average proportion of identifiable individuals of 0.716 (see Table Table10), 10 ), super‐population size was 1894 (95% CI: 1806–1986). Other parameters estimated with POPAN models are given in the supplementary material (Figure S8 ).
4. DISCUSSION
Using a dataset on individual killer whales identified over three decades in northern Norway, we document survival rates and abundance estimates for the period 1988–2019. Models fitted to data from the two time periods 1988–2008 and 2012–2019 independently indicated changes in killer whales’ residency patterns, rather than a decrease in apparent survival suggested by a model fitted to the full time series.
4.1. Validation of method assumptions
To meet the assumption of correct mark recognition, only good quality photographs of reliably marked individuals were used (i.e., with multiple marks, see Figure S1 ), thus minimizing the risk of erroneous identifications. In addition, the photo‐identification work was carried out by the same experienced analysts throughout the study period, further minimizing inconsistencies during cataloguing and scoring of images (Urian et al., 2015 ). Lack of CJS model fit to the data was tested to facilitate model development and obtain robust estimates of apparent survival (Gimenez et al., 2018 ). In the full dataset, Test 2.CT (Table (Table4) 4 ) indicated evidence of trap‐dependence in recapture probabilities, which was incorporated in the CJS models (Table (Table5). 5 ). In our study, a behavioral trap response is unlikely because killer whales were photographically and not physically captured. Instead, heterogeneity in recapture probabilities could have been generated by sampling a restricted part of the range of the study population, by individual‐ or sex‐specific differences in behavior, or by nonrandom temporary emigration (Pradel & Sanz‐Aguilar, 2012 ). In most datasets, Test 3.SR indicated a transience effect that could have been caused by the presence of transient individuals (Pradel et al., 1997 ; Table Table4). 4 ). The strong support for time‐since‐marking models to account for transience (Tables (Tables5 5 and and7) 7 ) and the much lower estimates of apparent survival for transient individuals (see Section 3), confirmed that this was an effective way of dealing with this lack of fit of the CJS model.
4.2. Survival rates
In Period 1, estimates of apparent survival probability for adult killer whales of both sexes exceeded 0.98 and were higher for females than males throughout the study period (Figure (Figure6a). 6a ). These results are consistent with estimates from other killer whale populations (Esteban et al., 2016 ; Fearnbach et al., 2019 ; Jordaan et al., 2020 ; Olesiuk et al., 1990 , 2005 ) and with previous analysis of similar data from northern Norway 1986–2003 (Kuningas et al., 2014 ). A contributing factor to the sex‐specific difference in survival is the extended postreproductive lifespan in females (Foster et al., 2012 ), which results in a longer mean life expectancy at birth for females than males (46 vs. 31 years in Northern Resident killer whales in British Columbia, Olesiuk et al., 2005 ). From 2012, after the NSS herring established new wintering grounds (Huse et al., 2010 ), apparent survival dropped for adult females (geometric mean: from 0.998 ± 0.002 SE to 0.955 ± 0.027 SE), and even more so for adult males (geometric mean: from 0.985 ± 0.009 SE to 0.864 ± 0.038 SE; Figure Figure6a). 6a ). In such long‐lived species, a decrease in survival of this magnitude is highly unlikely to reflect natural variation in mortality but could be indicative of anthropogenic mortality. For example, survival estimates of killer whales at the Crozet Islands dropped from 0.99 to 0.92 (equivalent to an increase in apparent mortality rate from 1 to 8%) after the illegal Patagonian toothfish longline fisheries started in 1996 (Tixier et al., 2017 ). In this region, where killer whales depredate longlines as a feeding strategy, illegal vessels were reported to have used lethal means to repel the depredating whales, which led to an increased mortality risk (Poncelet et al., 2010 ; Tixier et al., 2017 ). In our study region, there is no evidence of increased mortality to explain the decline in apparent survival rates between the two periods. Thus, it is likely that the detected trend was a result of other features of the data.
Our analysis revealed important differences between the two periods. There was a substantially higher number of individual killer whales identified in 2012–2019 ( n = 1032) compared to 1988–2008 ( n = 352), despite Period 2 being much shorter. Notably, a much higher percentage of animals in Period 2 were transients (i.e., seen only once) compared to Period 1 (30% vs. 5%) (Figure (Figure5). 5 ). Lower recapture probabilities for the Low residency group (geometric mean: 0.239 ± 0.059 SE) compared to the High residency (geometric mean: 0.714 ± 0.050 SE) in Period 2 confirmed variation in residency patterns as a source of heterogeneity (Figure (Figure8b). 8b ). Not accounting for this heterogeneity in recapture probabilities when modeling the full time series caused the decline in apparent survival probabilities seen in Figure Figure6a. 6a . In Period 2, while the maximal apparent survival probabilities estimated for the High residency group were comparable to those estimated for Period 1, survival probabilities for the Low residency group were much lower (0.726 ± 0.074 SE; Figure Figure8a). 8a ). This low apparent survival may result almost entirely from movement patterns of these animals modeled as permanent emigration. In support of this explanation, the drop in estimated apparent survival was greater for males (∆φ = 0.12) than females (∆φ = 0.04) in the full time series (Figure (Figure6a), 6a ), which likely is a consequence of most transients (75%) and individuals in the Low residency group (58%; Figure Figure7) 7 ) being males. This is most likely an artifact in the data rather than emigration being more pronounced in males. It takes several years to reliably sex an individual as female (see Section 2), while adult males can be sexed upon first sighting based on their tall dorsal fin. Therefore, transient females would not have been identified up in the data. However, the short length of Period 2 (8 years) relative to the lifespan of a killer whale requires our results for this second period to be interpreted with caution. Even if it seems clear that true survival for the High residency group has not declined, we cannot entirely rule out a decline in true survival for the Low residency group.
4.3. Movement patterns and abundance
Robust design (RD) models for Period 2 indicated most support for random temporary emigration (Table (Table7). 7 ). When random, and not Markovian, temporary emigration is not expected to bias survival estimates in CJS models, explaining the similarity in estimates of apparent survival from the RD and CJS models (Schaub et al., 2004 ). Five times more individuals showing low fidelity to the study area (Low residency group), compared to the 159 whales regularly seen (High residency group; Figure Figure7), 7 ), further confirms movement in and out of the study area as an important characteristic of the study population.
Opportunistic photo‐identifications provided further corroboration of these movements; 71 of the individuals identified from winter surveys in the study area had also been photographed in other regions of the Norwegian coast and even offshore (Table (Table3; 3 ; Figure Figure1). 1 ). In 2012–2019, of the 523 (51%) identified individuals never seen again after the first year of capture (Figures (Figures4 4 and and5), 5 ), seven were photographed near Jan Mayen in summer in 2015 and 2016, confirming an offshore origin for at least some transient individuals. Notably, the single winter records of these whales were from 2015, the peak year of the number of transients and estimated abundance (Figures (Figures5 5 and and9), 9 ), and during which sampling covered open waters northwest of Andøya (Figure (Figure1). 1 ). Therefore, it appears likely that high herring abundance in coastal but open areas in some years, rather than in the inshore fjord system, attracted animals from elsewhere (including offshore) that had previously not been available to be sampled. This explanation is supported by the observation that 60% of the transients seen between 2012 and 2018 were identified at Andøya, even though this area contributed <30% of all captures for this period (EJ, unpublished data). The appearance of large numbers of humpback whales (and fin whales) at the newly established herring wintering grounds, which were not observed at former inshore locations in 1986–2006 (Jourdain & Vongraven, 2017 ), lends further support to this explanation.
As the photo‐identification study continues in these dynamic herring wintering grounds, sighting frequencies of individual killer whales are expected to vary over time. For example, a number of transients could be re‐identified in the future if coastal but open areas were to be surveyed again. Thus, what may appear as permanent emigration in the low estimates of apparent survival for the Low residency group in Period 2 could, in the future, contribute to temporary emigration in RD models.
Estimates of the number of killer whales that used the study area in a particular annual winter season varied considerably among years between 2012 and 2019 (Figure (Figure9). 9 ). Substantial fluctuations in killer whale abundance in the study area were also documented in 1990–2003 (Kuningas et al., 2014 ). As discussed above, this variability is likely linked to prey availability and associated killer whale movements. Killer whales are able to scout large areas to track the dynamic distribution of their herring prey and likely adjust their winter distribution accordingly (Similä & Stenersen, 2004 ). For example, Vengsøyfjord (surveyed in 2015–2016) and Kvænangen (surveyed in 2017–2019) held different herring year‐classes after the older year‐class started wintering offshore from 2017 (ICES, 2018 ). While some killer whales were still found in the fjords post‐2017, possibly owing to benefits from using the shallow bottom topography for hunting wintering herring (Nøttestad, 2002 ), others may have followed the larger, more profitable portion of the stock offshore. This hypothesis is supported by the lower abundance estimates in 2018–2019 compared to 2015–2017 (Figure (Figure9 9 ).
Estimated annual killer whale abundance peaked at 731 individuals (95% CI: 505–1059, Kuningas et al., 2014 ) in Period 1, compared to 1061 (95% CI: 999–1127) in Period 2. Super‐population size estimated from POPAN models also increased from 911 (95% CI: 812–1022) in Period 1 to 1896 (95% CI: 1806–1991) in Period 2. As discussed above, it seems likely that this increase in abundance resulted from killer whales responding to the shifting of herring wintering grounds from the strictly inshore fjord system throughout Period 1 to both coastal (Vengsøyfjord and Kvænangen) and open waters (off Andøya) in Period 2 (Huse et al., 2010 ) (Figure (Figure1 1 ).
A recent period of population growth also cannot be ruled out. Indeed, a demographic rebound following the end of the culling in 1982 (Øien, 1988 ) and the recovery of the NSS herring after a nearly total collapse in the late 1960s (Dragesund et al., 1997 ) may have been expected for killer whales in Norway. However, the combination of limited sampling and dynamic herring wintering grounds preclude the estimation of any meaningful trend in abundance, even in the sampled areas. In addition to a rebound to a precommercial fisheries ecosystem, killer whale population dynamics may be further affected by other ecological changes that are influenced by global warming. For example, the north‐eastern Atlantic mackerel, also a prey of killer whales in the study region (Nøttestad, Sivle, Krafft, Langard, et al., 2014 ), has greatly increased in biomass in the Norwegian Sea (Nøttestad, Utne, et al., 2015 ). Total super‐population size in 2012–2019 represented roughly 10 to 20% of killer whale abundance estimated from Norwegian shipboard surveys (Leonard & Øien, 2020a , 2020b ). The large‐scale migration of the NSS herring, which couples offshore and coastal ecosystems, and the documented wide‐ranging capacities of killer whales in Norway (Table (Table5; 5 ; Dietz et al., 2020 ; Similä & Stenersen, 2004 ; Vogel et al., 2021 ) suggest that the killer whales studied in northern Norway are part of a larger population of the Norwegian Sea and the wider Northeast Atlantic.
5. CONCLUSIONS
Re‐identification of individuals over multiple decades confirmed capture–recapture analysis as a suitable tool to monitor long‐term changes in the dynamics of killer whales in Norway. Our results show that the NSS herring remains a major ecological driver of killer whale dynamics in this region. We show that killer whales adjust their movement to shifting prey resources, indicating potential to adapt to rapidly changing marine ecosystems, as previously shown in other regions (e.g., Canadian Arctic, Ferguson et al., 2010 ). By shaping killer whale movement patterns, distributional prey shifts may also influence contact zones between killer whale groups, with possible implications for genetic population structure. Overall, killer whale abundance in northern Norwegian coastal waters shows an increase between 1988 and 2008 and post‐2012, although lower estimates in 2018–2019 may indicate a recent change. The variation in estimated annual abundance reflects variation in the proportion present in this area of an undefined population (or populations) in a larger geographical region. Our dataset is rare in its temporal extent and in its documentation of individual killer whales through a period characterized by marked ecosystem change. These data increase in value with each year that photo‐identification surveys are maintained. While the focus of this study was to explore population dynamics, this dataset is well suited to research questions at the individual level. Future studies should investigate how killer whales may be impacted by declining herring biomass (ICES, 2013 , 2018 ), in the context of expected bottom‐up regulatory effects (Ford et al., 2010 ), and develop population models incorporating the effects of various stressors to inform conservation policy and any necessary management actions.
CONFLICT OF INTEREST
Authors have no competing interests to declare.
AUTHOR CONTRIBUTION
Eve Marie Jourdain: Conceptualization (lead); Data curation (equal); Formal analysis (lead); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (equal); Resources (equal); Software (equal); Visualization (equal); Writing‐original draft (lead); Writing‐review & editing (lead). Tiffany Goh: Formal analysis (equal); Methodology (equal); Software (equal); Visualization (equal); Writing‐review & editing (equal). Sanna Kuningas: Data curation (equal); Funding acquisition (equal); Investigation (equal); Resources (equal); Writing‐review & editing (equal). Tiu Similä: Data curation (equal); Funding acquisition (equal); Investigation (equal); Resources (equal); Writing‐review & editing (equal). Dag Vongraven: Data curation (equal); Funding acquisition (equal); Investigation (equal); Resources (equal); Writing‐review & editing (equal). Richard Karoliussen: Data curation (supporting); Funding acquisition (equal); Investigation (equal); Resources (equal); Writing‐review & editing (supporting). Anna Bisther: Data curation (equal); Funding acquisition (equal); Investigation (equal); Resources (equal); Writing‐review & editing (equal). Philip Hammond: Conceptualization (equal); Formal analysis (equal); Methodology (lead); Project administration (equal); Software (equal); Supervision (lead); Validation (lead); Writing‐original draft (equal); Writing‐review & editing (equal).
Supporting information
Acknowledgments.
Fieldwork conducted by TS and colleagues in 1986–2003 was funded by the Norwegian Research Council, WWF Sweden, the Academy of Finland, Tampereen Särkänniemi, Sea World Inc., the Alfred Kordelin Foundation, the Norwegian Institute of Marine Research, Canon and Discovery Initiatives. Data collection conducted by DV and AB in 1990–1993 was funded by the Norwegian Research Council as part of the national research program on marine mammals. Fieldwork and study conducted by SK received financial support by the University of St Andrews studentship, SMRU Ltd., the Russell Trust Award and from the Finnish‐Norwegian Cultural Foundation. Sea Safari Andenes (became Whale 2Sea in 2021) and Lofoten Opplevelser were the platforms for data collection in 2013–2014. Fieldwork conducted by Norwegian Orca Survey (EJ, RK) in 2015–2019 was funded by the Sea World and Busch Gardens Conservation fund, Sea World and Parks Entertainment, Spare Bank‐1 Nord Norge and private donations, and sponsored by Regatta Norway and Steiner. In 2015–2019, invaluable collaborations with whale watching companies Sea Safari Andenes, Green Gold of Norway, Valhalla, Northern Explorers, Arctic Whale Tours and Tromsø Friluftsenter resulted in substantial numbers of additional killer whale identification‐photographs collected and contributed to this study. In addition, many citizen‐scientists shared their killer whale photographs with Norwegian Orca Survey in 2013–2019, some of which contributed to building the sighting histories used in this study. We wish to address a particular thank you to Marten Bril (Whale 2Sea) who dedicated much effort, all year round, in collecting killer whale photographs to be used in this study during all these years. Special thanks also go to Krisztina Balotay, Julie Guiderdoni, Rodolphe Tanneau, Jacques de Vos, Sven Gust, Piet van den Bemd, Espen Bergersen, Inaki Aizpurua Quiroga, Kevin Ochoa, and Fredrik Broms who collected killer whale photographs over multiple winter seasons. We thank Patrick Miller for sharing killer whale photographs collected near Jan Mayen in June 2015 and June 2016. We also thank Mònica Arso Civil and Anna Schleimer for advice on analysis. Last, we are very grateful to Graeme Ellis who accepted to review killer whale identification photographs and greatly assisted with data validation.
Jourdain, E. , Goh, T. , Kuningas, S. , Similä, T. , Vongraven, D. , Karoliussen, R. , Bisther, A. , & Hammond, P. S. (2021). Killer whale ( Orcinus orca ) population dynamics in response to a period of rapid ecosystem change in the eastern North Atlantic . Ecology and Evolution , 11 , 17289–17306. 10.1002/ece3.8364 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
DATA AVAILABILITY STATEMENT
- Andvik, C. , Jourdain, E. , Ruus, A. , Lyche, J. L. , Karoliussen, R. , & Borgå, K. (2020). Preying on seals pushes killer whales from Norway above pollution effects thresholds . Scientific Reports , 10 , 1–10. 10.1038/s41598-020-68659-y [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Arso Civil, M. , Cheney, B. , Quick, N. J. , Islas‐Villanueva, V. , Graves, J. A. , Janik, V. M. , Thompson, P. M. , & Hammond, P. S. (2019). Variations in age‐ and sex‐specific survival rates help explain population trend in a discrete marine mammal population . Ecology and Evolution , 9 , 533–544. 10.1002/ece3.4772 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bigg, M. (1982). An assessment of killer whale Orcinus orca stocks off Vancouver Island, British Columbia . Report of the International Whaling Commission , 32 , 655–666. [ Google Scholar ]
- Bisther, A. , & Vongraven, D. (1995). Studies of social ecology of Norwegian killer whales ( Orcinus orca ). In Blix A. S., Walløe L., & Ulltang Ø. (Eds.), Whales, seals, fish and man (pp. 169–176). Elsevier Science. [ Google Scholar ]
- Burnham, K. P. , & Anderson, D. R. (2002). Model selection and multimodal inference: A practical information‐theoretic approach . Springer‐Verlag. [ Google Scholar ]
- Christensen, I. (1982). Killer whales in Norwegian coastal waters . Report of the International Whaling Commission , 32 , 633–641. [ Google Scholar ]
- Christensen, I. (1988). Distribution, movements and abundance of killer whales ( Orcinus orca ) in Norwegian coastal waters, 1982–1987, based on questionnaire surveys . Rit Fiskideildar , 11 , 79–88. [ Google Scholar ]
- Cosentino, A. M. (2015). First record of Norwegian killer whales attacking and feeding on a harbour porpoise . Marine Biodiversity Records , 8 , E108. 10.1017/S1755267215000895 [ CrossRef ] [ Google Scholar ]
- Cosewic . (2008). COSEWIC assessment and update status report on the killer whale Orcinus orca , Southern Resident population, Northern Resident population, West Coast Transient population, Offshore population and Northwest Atlantic / Eastern Arctic population, in Canada . Committee on the Status of Endangered Wildlife in Canada. Ottawa. viii +65 p. [ Google Scholar ]
- Dietz, R. , Rikardsen, A. H. , Biuw, M. , Kleivane, L. , Noer, C. L. , Stalder, D. , van Beest, F. M. , Rigét, F. F. , Sonne, C. , Hansen, M. , Strager, H. , & Olsen, M. T. (2020). Migratory and diurnal activity of North Atlantic killer whales ( Orcinus orca ) off northern Norway . Journal of Experimental Marine Biology and Ecology , 533 , 151456. 10.1016/j.jembe.2020.151456 [ CrossRef ] [ Google Scholar ]
- Dragesund, O. , Johannessen, A. , & Ulltang, Ø. (1997). Variation in migration and abundance of Norwegian Spring Spawning herring ( Clupea harengus L.) . Sarsia , 82 , 97–105. [ Google Scholar ]
- Dugger, K. M. , Faaborg, J. , Arendt, W. J. , & Hobson, K. A. (2004). Understanding survival and abundance of overwintering warblers: does rainfall matter? The Condor , 106 , 744–760. 10.1093/condor/106.4.744 [ CrossRef ] [ Google Scholar ]
- Durban, J. , Ellifrit, D. , Dahlheim, M. , Waite, J. , Matkin, C. , Barrett‐Lennard, L. , Ellis, G. , Pitman, R. , Leduc, R. , & Wade, P. (2010). Photographic mark‐recapture analysis of clustered mammal‐eating killer whales around the Aleutian Islands and Gulf of Alaska . Marine Biology , 157 , 1591–1604. 10.1007/s00227-010-1432-6 [ CrossRef ] [ Google Scholar ]
- Esteban, R. , Verborgh, P. , Gauffier, P. , Giménez, J. , Guinet, C. , & De Stephanis, R. (2016). Dynamics of killer whale, bluefin tuna and human fisheries in the Strait of Gibraltar . Biological Conservation , 194 , 31–38. 10.1016/j.biocon.2015.11.031 [ CrossRef ] [ Google Scholar ]
- Fearnbach, H. , Durban, J. W. , Ellifrit, D. K. , & Pitman, R. L. (2019). Abundance of type A killer whales ( Orcinus orca ) in the coastal waters off the western Antarctic Peninsula . Polar Biology , 42 , 1477–1488. 10.1007/s00300-019-02534-z [ CrossRef ] [ Google Scholar ]
- Ferguson, S. H. , Higdon, J. W. , & Chmelnitsky, E. G. (2010). The rise of killer whales as a major Arctic predator. In Ferguson S. H., Loseto L. L., & Mallory M. L. (Eds.), A little less arctic: Top predators in the World’s Largest Northern Inland Sea, Hudson Bay (pp. 117–136). Springer Netherlands. [ Google Scholar ]
- Ford, J. K. B. , Ellis, G. M. , Olesiuk, P. F. , & Balcomb, K. C. (2010). Linking killer whale survival and prey abundance: Food limitation in the Oceans’ apex predator? Biology Letters , 6 , 139–142. 10.1098/rsbl.2009.0468 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Foster, E. A. , Franks, D. W. , Mazzi, S. , Darden, S. K. , Balcomb, K. C. , Ford, J. K. B. , & Croft, D. P. (2012). Adaptive prolonged postreproductive life span in killer whales . Science , 337 , 1313. 10.1126/science.1224198 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gibson, C. E. , Williams, D. , Dunlop, R. , & Beck, S. (2020). Using social media as a cost‐effective resource in the photo‐identification of a coastal bottlenose dolphin community . Aquatic Conservation: Marine and Freshwater Ecosystems , 30 , 1702–1710. 10.1002/aqc.3356 [ CrossRef ] [ Google Scholar ]
- Gimenez, O. , Lebreton, J.‐D. , Choquet, R. , & Pradel, R. (2018). R2ucare: An R package to perform goodness‐of‐fit tests for capture–recapture models . Methods in Ecology and Evolution , 9 , 1749–1754. 10.1111/2041-210X.13014 [ CrossRef ] [ Google Scholar ]
- Gore, M. A. , Frey, P. H. , Ormond, R. F. , Allan, H. , & Gilkes, G. (2016). Use of photo‐identification and mark‐recapture methodology to assess basking shark ( Cetorhinus maximus ) populations . PLoS One , 11 , e0150160. 10.1371/journal.pone.0150160 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Guinet, C. , Tixier, P. , Gasco, N. , & Duhamel, G. (2015). Long‐term studies of Crozet Island killer whales are fundamental to understanding the economic and demographic consequences of their depredation behaviour on the Patagonian Toothfish fishery . ICES Journal of Marine Science , 72 , 1587–1597. 10.1093/icesjms/fsu221 [ CrossRef ] [ Google Scholar ]
- Hammond, P. S. (1990). Capturing whales on film—Estimating cetacean population parameters from individual recognition data . Mammal Review , 20 , 17–22. 10.1111/j.1365-2907.1990.tb00099.x [ CrossRef ] [ Google Scholar ]
- Hammond, P. S. (2010). Estimating the abundance of marine mammals. In Boyd I. L., Bowen W. D., & Iverson S. J. (Eds.), Marine mammal ecology and conservation (pp. 42–67). Oxford University Press. [ Google Scholar ]
- Holst, J. C. , Røttingen, I. , & Melle, W. (2004). The herring. In Hr S. (Ed.), The Norwegian sea ecosystem (pp. 203–226). Tapir Academic Press. [ Google Scholar ]
- Huse, G. , Fernö, A. , & Holst, J. C. (2010). Establishment of new wintering areas in herring co‐occurs with peaks in the ‘first time/repeat spawner’ ratio . Marine Ecology Progress Series , 409 , 189–198. 10.3354/meps08620 [ CrossRef ] [ Google Scholar ]
- ICES . (2013). Report of the Working Group on Widely Distributed Stocks (WGWIDE), 27 August ‐ 2 September 2013 . ICES, Headquarters. 950 p. [ Google Scholar ]
- ICES . (2018). Report of the Working Group on Widely Distributed Stocks (WGWIDE), 28 August‐ 3 September 2018 . 488 p. [ Google Scholar ]
- IUCN, Standards and Petitions Committee (2019). Guidelines for using the IUCN Red List categories and criteria. Version 14. Prepared by the standards and petitions committee . [ Google Scholar ]
- Jonsgård, Å. , & Lyshoel, P. B. (1970). A contribution to the knowledge of the biology of the killer whale Orcinus orca L . Nytt Magasin for Zoologi , 18 , 41–48. [ Google Scholar ]
- Jordaan, R. K. , Oosthuizen, W. C. , Reisinger, R. R. , & De Bruyn, P. J. N. (2020). Abundance, survival and population growth of killer whales Orcinus orca at subantarctic Marion Island . Wildlife Biology , 4 . 10.2981/wlb.00732 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jourdain, E. , Andvik, C. , Karoliussen, R. , Ruus, A. , Vongraven, D. , & Borgå, K. (2020). Isotopic niche differs between seal and fish‐eating killer whales ( Orcinus orca ) in northern Norway . Ecology and Evolution , 10 , 4115–4127. 10.1002/ece3.6182 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jourdain, E. , & Karoliussen, R. (2021). Photo‐identification catalogue of Norwegian killer whales 2007‐2021 . Figshare. Dataset. 10.6084/m9.figshare.4205226.v4 [ CrossRef ] [ Google Scholar ]
- Jourdain, E. , Karoliussen, R. , De Vos, J. , Zakharov, S. E. , & Tougard, C. (2019). Killer whales ( Orcinus orca ) feeding on lumpfish ( Cyclopterus lumpus ) in northern Norway . Marine Mammal Science , 36 ( 1 ), 1–14. 10.1111/mms.12618 [ CrossRef ] [ Google Scholar ]
- Jourdain, E. , & Vongraven, D. (2017). Humpback whale ( Megaptera novaeangliae ) and killer whale ( Orcinus orca ) feeding aggregations for foraging on herring ( Clupea harengus ) in Northern Norway . Mammalian Biology ‐ Zeitschrift Für Säugetierkunde , 86 , 27–32. 10.1016/j.mambio.2017.03.006 [ CrossRef ] [ Google Scholar ]
- Jourdain, E. , Vongraven, D. , Bisther, A. , & Karoliussen, R. (2017). First longitudinal study of seal‐feeding killer whales ( Orcinus orca ) in Norwegian coastal waters . PLoS One , 12 , e0180099. 10.1371/journal.pone.0180099 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kendall, W. L. , Nichols, J. D. , & Hines, J. E. (1997). Estimating temporary emigration using capture‐recapture data with Pollock’s Robust Design . Ecology , 78 , 563–578. [ Google Scholar ]
- Kuningas, S. , Similä, T. , & Hammond, P. S. (2014). Population size, survival and reproductive rates of Northern Norwegian killer whales ( Orcinus orca ) in 1986–2003 . Journal of the Marine Biological Association of the United Kingdom , 94 , 1277–1291. 10.1017/S0025315413000933 [ CrossRef ] [ Google Scholar ]
- Laake, J. , & Rexstad, E. (2008). RMark—An alternative approach to building linear models in MARK. In Cooch G. C. W. E. (Ed.), Program MARK: A gentle introduction (p. 111). Colorado State University, Fort Collins, CO. [ Google Scholar ]
- Lebreton, J.‐D. , Burnham, K. P. , Clobert, J. , & Anderson, D. R. (1992). Modeling survival and testing biological hypotheses using marked animals: A unified approach with case studies . Ecological Monographs , 62 , 67–118. 10.2307/2937171 [ CrossRef ] [ Google Scholar ]
- Leonard, D. , & Øien, N. (2020a). Estimated abundances of cetacean species in the Northeast Atlantic from Norwegian Shipboard Surveys Conducted in 2014–2018 . NAMMCO Scientific Publications , 11 , 2014–2018. 10.7557/3.4694 [ CrossRef ] [ Google Scholar ]
- Leonard, D. , & Øien, N. (2020b). Estimated abundances of cetacean species in the Northeast Atlantic from two multiyear surveys conducted by Norwegian vessels between 2002–2013 . NAMMCO Scientific Publications , 11 , 2002–2013. 10.7557/3.4695 [ CrossRef ] [ Google Scholar ]
- Lyrholm, T. (1988). Photoidentification of individual killer whales, Orcinus orca , off the coast of Norway, 1983–1986 . Rit Fiskideildar , 11 , 89–94. [ Google Scholar ]
- Matkin, C. O. , Saulitis, E. , Ellis, G. M. , Olesiuk, P. , & Rice, S. D. (2008). Ongoing population‐level impacts on killer whales Orcinus orca following the ‘Exxon Valdez’ oil spill in Prince William Sound, Alaska . Marine Ecology Progress Series , 356 , 269–281. 10.3354/meps07273 [ CrossRef ] [ Google Scholar ]
- Millon, A. , Petty, S. J. , Little, B. , Gimenez, O. , Cornulier, T. , & Lambin, X. (2014). Dampening prey cycle overrides the impact of climate change on predator population dynamics: A long‐term demographic study on tawny owls . Global Change Biology , 20 , 1770–1781. 10.1111/gcb.12546 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Morissette, L. , Kaschner, K. , & Gerber, L. R. (2010). Ecosystem models clarify the trophic role of whales off Northwest Africa . Marine Ecology Progress Series , 404 , 289–302. 10.3354/meps08443 [ CrossRef ] [ Google Scholar ]
- Nøttestad, L. (2002). Killer whales ( Orcinus orca L.) and saithe ( Pollachius virens L.) trap herring ( Clupea harengus L.) in shallow water by taking advantage of steep bottom topograhy. In International Council for the Exploration of the Sea (Hillerød, Denmark). [ Google Scholar ]
- Nøttestad, L. , Krafft, B. A. , Anthonypillai, V. , Bernasconi, M. , Langård, L. , Mørk, H. L. , & Fernö, A. (2015). Recent changes in distribution and relative abundance of cetaceans in the Norwegian Sea and their relationship with potential prey . Frontiers in Ecology and Evolution , 2 . 10.3389/fevo.2014.00083 [ CrossRef ] [ Google Scholar ]
- Nøttestad, L. , Sivle, L. D. , Krafft, B. A. , Langård, L. , Anthonypillai, V. , Bernasconi, M. , Langøy, H. , & Axelsen, B. E. (2014). Ecological aspects of fin whale and humpback whale distribution during summer in the Norwegian Sea . Marine Ecology , 35 , 221–232. 10.1111/maec.12075 [ CrossRef ] [ Google Scholar ]
- Nøttestad, L. , Sivle, L. D. , Krafft, B. A. , Langard, L. , Anthonypillai, V. , Bernasconi, M. , Langoy, H. , & Ferno, A. (2014). Prey selection of offshore killer whales Orcinus orca in the Northeast Atlantic in late summer: Spatial associations with mackerel . Marine Ecology Progress Series , 499 , 275–283. 10.3354/meps10638 [ CrossRef ] [ Google Scholar ]
- Nøttestad, L. , Utne, K. R. , Óskarsson, G. J. , Jónsson, S. Þ. , Jacobsen, J. A. , Tangen, Ø. , Anthonypillai, V. , Aanes, S. , Vølstad, J. H. , & Bernasconi, M. (2015). Quantifying changes in abundance, biomass, and spatial distribution of Northeast Atlantic mackerel ( Scomber scombrus ) in the Nordic seas from 2007 to 2014 . ICES Journal of Marine Science , 73 , 359–373. 10.1093/icesjms/fsv218 [ CrossRef ] [ Google Scholar ]
- Øien, N. (1988). The distribution of killer whales ( Orcinus orca ) in the North Atlantic based on Norwegian catches, 1938–1981, and incidental sightings, 1967–1987 . Rit Fiskideildar , 11 , 65–78. [ Google Scholar ]
- Olesiuk, P. F. , Bigg, M. A. , & Ellis, G. M. (1990). Life history and population dynamics of resident killer whales Orcinus orca in the coastal waters of British Columbia and Washington State . Report of the International Whaling Commission , 12 , 209–243. [ Google Scholar ]
- Olesiuk, P. F. , Ellis, G. M. , & Ford, J. K. B. (2005). Life history and population dynamics of Northern Resident killer whales ( Orcinus orca ) in British Columbia . Research Document 2005/045, Canadian Science Advisory Secretariat, Fisheries and Oceans, Canada. 75 p. [ Google Scholar ]
- Oliver, L. J. , Morgan, B. J. T. , Durant, S. M. , & Pettorelli, N. (2011). Individual heterogeneity in recapture probability and survival estimates in cheetah . Ecological Modelling , 222 , 776–784. 10.1016/j.ecolmodel.2010.11.021 [ CrossRef ] [ Google Scholar ]
- Pace, R. M. , Corkeron, P. J. , & Kraus, S. D. (2017). State–space mark–recapture estimates reveal a recent decline in abundance of North Atlantic right whales . Ecology and Evolution , 7 , 8730–8741. 10.1002/ece3.3406 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pitman, R. L. , Fearnbach, H. , & Durban, J. W. (2018). Abundance and population status of Ross Sea killer whales ( Orcinus orca , Type C) in McMurdo Sound, Antarctica: Evidence for impact by commercial fishing? Polar Biology , 41 , 781–792. 10.1007/s00300-017-2239-4 [ CrossRef ] [ Google Scholar ]
- Poncelet, E. , Barbraud, C. , & Guinet, C. (2010). Population dynamics of killer whales ( Orcinus orca ) in the Crozet Archipelago, southern Indian Ocean: A mark–recapture study from 1977 to 2002 . Journal of Cetacean Research and Management , 11 , 41–48. [ Google Scholar ]
- Pradel, R. (1993). Flexibility in survival analysis from recapture data: Handling trap‐dependence. In North J. D. L. P. M. (Ed.), Marked individuals in the study of bird populations (pp. 29–37). Birkhäuser Verlag. [ Google Scholar ]
- Pradel, R. , Hines, J. E. , Lebreton, J.‐D. , & Nichols, J. D. (1997). Capture‐recapture survival models taking account of transients . Biometrics , 53 , 60–72. 10.2307/2533097 [ CrossRef ] [ Google Scholar ]
- Pradel, R. , & Sanz‐Aguilar, A. (2012). Modeling trap‐awareness and related phenomena in capture‐recapture studies . PLoS One , 7 , e32666. 10.1371/journal.pone.0032666 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Purvis, A. , Gittleman, J. L. , Cowlishaw, G. , & Mace, G. M. (2000). Predicting extinction risk in declining species . Proceedings of the Royal Society of London. Series B: Biological Sciences , 267 , 1947–1952. 10.1098/rspb.2000.1234 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- R Core Team . (2018). R: A language and environment for statistical computing . R Foundation for Statistical Computing. https://www.R‐project.org/ [ Google Scholar ]
- Ramp, C. , Bérubé, M. , Hagen, W. , & Sears, R. (2006). Survival of adult blue whales Balaenoptera musculus in the Gulf of St. Lawrence, Canada . Marine Ecology Progress Series , 319 , 287–295. 10.3354/meps319287 [ CrossRef ] [ Google Scholar ]
- Schaub, M. , Gimenez, O. , Schmidt, B. R. , & Pradel, R. (2004). Estimating survival and temporary emigration in the multistate capture‐recapture framework . Ecology , 85 , 2107–2113. 10.1890/03-3110 [ CrossRef ] [ Google Scholar ]
- Schleimer, A. , Ramp, C. , Delarue, J. , Carpentier, A. , Bérubé, M. , Palsbøll, P. J. , Sears, R. , & Hammond, P. S. (2019). Decline in abundance and apparent survival rates of fin whales ( Balaenoptera physalus ) in the northern Gulf of St. Lawrence . Ecology and Evolution , 9 , 4231–4244. 10.1002/ece3.5055 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schwarz, C. J. , & Arnason, A. N. (1996). A general methodology for the analysis of capture‐recapture experiments in open populations . Biometrics , 52 , 860–873. 10.2307/2533048 [ CrossRef ] [ Google Scholar ]
- Similä, T. , Holst, J. C. , & Christensen, I. (1996). Occurrence and diet of killer whales in Northern Norway: Seasonal patterns relative to the distribution and abundance of Norwegian Spring‐Spawning Herring . Canadian Journal of Fisheries and Aquatic Sciences , 53 , 769–779. 10.1139/f95-253 [ CrossRef ] [ Google Scholar ]
- Similä, T. , & Stenersen, J. (2004). Norwegian killer whales . Tringa forlag. [ Google Scholar ]
- Sreekar, R. , Purushotham, C. B. , Saini, K. , Rao, S. N. , Pelletier, S. , & Chaplod, S. (2013). Photographic capture‐recapture sampling for assessing populations of the Indian gliding lizard Draco dussumieri . PLoS One , 8 , e55935. 10.1371/journal.pone.0055935 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tixier, P. , Authier, M. , Gasco, N. , & Guinet, C. (2015). Influence of artificial food provisioning from fisheries on killer whale reproductive output . Animal Conservation , 18 , 207–218. 10.1111/acv.12161 [ CrossRef ] [ Google Scholar ]
- Tixier, P. , Barbraud, C. , Pardo, D. , Gasco, N. , Duhamel, G. , & Guinet, C. (2017). Demographic consequences of fisheries interaction within a killer whale ( Orcinus orca ) population . Marine Biology , 164 , 170. 10.1007/s00227-017-3195-9 [ CrossRef ] [ Google Scholar ]
- Urian, K. , Gorgone, A. , Read, A. , Balmer, B. , Wells, R. S. , Berggren, P. , Durban, J. , Eguchi, T. , Rayment, W. , & Hammond, P. S. (2015). Recommendations for photo‐identification methods used in capture‐recapture models with cetaceans . Marine Mammal Science , 31 , 298–321. 10.1111/mms.12141 [ CrossRef ] [ Google Scholar ]
- Vester, H. , & Hammerschmidt, K. (2013). First record of killer whales ( Orcinus orca ) feeding on Atlantic salmon ( Salmo salar ) in Northern Norway suggest a multi‐prey feeding type . Marine Biodiversity Records , 6 , 1–5. 10.1017/S1755267212001030 [ CrossRef ] [ Google Scholar ]
- Vogel, E. F. , Biuw, M. , Blanchet, M. A. , Jonsen, I. D. , Mul, E. , Johnsen, E. , Hjøllo, S. S. , Olsen, M. T. , Dietz, R. , & Rikardsen, A. (2021). Killer whale movements on the Norwegian shelf are associated with herring density . Marine Ecology Progress Series , 665 , 217–231. 10.3354/meps13685 [ CrossRef ] [ Google Scholar ]
- Vongraven, D. , & Bisther, A. (2014). Prey switching by killer whales in the North‐East Atlantic: Observational evidence and experimental insights . Journal of the Marine Biological Association of the United Kingdom , 94 , 1357–1365. 10.1017/S0025315413001707 [ CrossRef ] [ Google Scholar ]
- Zeh, J. , Poole, D. , Miller, G. , Koski, W. , Baraff, L. , & Hugh, D. (2002). Survival of bowhead whales, Balaena mysticetus , estimated from 1981–1998 photoidentification data . Biometrics , 58 , 832–840. 10.1111/j.0006-341X.2002.00832.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
International Trade, Noise Pollution, and Killer Whales
Orcinus Orca is the world’s largest predator, and simultaneously a significant tourist asset and cultural icon for much of the Pacific Northwest. In the past two decades, the Southern Resident Killer whale (SRKW) population has declined by more than 25 percent, putting them at risk of extinction. The cause of this decline is hotly debated. This paper employs novel data, an innovative noise pollution model, and quasi-experimental methods borrowed from environmental economics to solve this puzzle. We find consistent evidence that vessel noise pollution from international shipping has lowered fertility and raised the mortality of the SRKW significantly. Had noise pollution remained at its pre-1998 levels, the SRKW population would be 30% larger. Noise pollution is a growing threat to marine mammals worldwide.
Funding provided by the SSHRC of Canada. The views expressed herein are those of the authors and do not necessarily reflect the views of the National Bureau of Economic Research.
MARC RIS BibTeΧ
Download Citation Data
- data appendix
- Additional details
More from NBER
In addition to working papers , the NBER disseminates affiliates’ latest findings through a range of free periodicals — the NBER Reporter , the NBER Digest , the Bulletin on Retirement and Disability , the Bulletin on Health , and the Bulletin on Entrepreneurship — as well as online conference reports , video lectures , and interviews .


A Mozambican marine protected area provides important habitat for vulnerable pelagic sharks

Three decades of nearshore surveys reveal long-term patterns in gray whale habitat use, distribution, and abundance in the Northern California Current
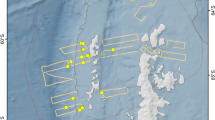
Return of large fin whale feeding aggregations to historical whaling grounds in the Southern Ocean
Introduction.
High trophic-level consumers, or top predators, play an important ecological role through top-down forcing 1 , 2 , 3 . Regulation of prey density via direct consumption by predators is the most common form of top-down control documented in ecological literature 4 , 5 , 6 . Yet there is also increasing recognition for non-lethal or behaviorally mediated mechanisms, which can similarly shape ecosystem function and structure 7 , 8 , 9 , 10 , 11 . Non-consumptive mechanisms include ‘risk effects’ in which prey are not removed, but respond behaviorally to the presence of a predator by reducing activity or shifting habitats to reduce risk 9 . Risk effects can result in the ecological equivalent of density reduction and may have negative impacts on fitness including decreased reproductive success through loss of foraging opportunities, increased stress, and increased energy demands associated with predator avoidance 12 , 13 , 14 . Top-down forcing can result in trophic cascades where changes in predator forcing alter the densities of intermediate and lower level consumers down through multiple trophic levels 15 . Risk effects can also initiate trophic cascades 8 , 16 , 17 . For instance, under threat from a potential predator, behavioral responses of a risk-averse intermediate consumer can result in the local release of its food base 18 .
In marine ecosystems predator-prey interactions and resulting ecological effects have received relatively more focus 2 , 6 , 19 , whereas much less is known about the interactions among top consumers 20 , which likely have similar top-down implications for marine ecosystems 21 . Large-bodied upper trophic-level consumers have few natural predators. However, competition within predator guilds can lead to complex interactions and strongly affect the distribution and abundance of the predator populations 5 , 21 , 22 , 23 . Relatively common in terrestrial systems, intraguild predation among top predators can potentially reduce exploitation competition for food resources and confer energetic benefits for the prevailing consumer 23 , 24 . Even in cases where the rate of killing is very low 8 , indirect dominance effects can profoundly influence the behavior and fitness of sub-dominant predators 25 , 26 , 27 . In spite of their ubiquity where well-studied 22 , 23 , 28 , the frequency and importance of lethal and sub-lethal interactions among top marine predators remain difficult to measure in the oceanic realm and therefore are potentially underrepresented 3 .
Many marine top predators exhibit migratory behavior and seasonal aggregations at foraging areas 29 . Concentrated seasonal foraging is crucial in supporting migratory behavior in many consumers 30 , 31 and, conversely, the seasonal influx of predators can have strong regulatory and behavioral effects on local prey populations 3 . Perturbations in such predator-prey systems may therefore be impactful for both prey and predator with potentially cascading effects 1 . In cases of co-occurrence between top predators at such sites, the effect of intraguild interactions on local ecosystem dynamics remains relatively unknown.
Here we document and investigate interactions between two top ocean predators, white sharks ( Carcharodon carcharias ) and killer whales ( Orcinus orca ). In the northeastern Pacific (NEP) white sharks aggregate seasonally at Southeast Farallon Islands (SEFI), Año Nuevo Island (ANI), and other pinniped rookeries off the west coast of North America 32 , 33 . The sharks’ timing and observed foraging is associated with the seasonal haulout of juvenile elephant seals ( Mirounga angustirostrous ), a preferred prey 34 , 35 , 36 , 37 consumed prior to offshore migration 32 , 38 . Although white sharks also forage or scavenge on cetaceans 39 , teleosts, other elasmobranchs 40 and various pinnipeds, their seasonal targeting of elephant seals 41 provides a consistent source of caloric capital to fuel extended oceanic migrations 42 , 43 .
White sharks and killer whales exhibit a high degree of niche overlap along the western shores of North America. The coastal distribution of NEP white sharks extends from northern Mexico to Canada (and in El Nino years up into Alaskan waters), entirely within the coastal distribution of NEP killer whales that range from Mexico to the Aleutian islands of Alaska 44 (Fig. 1 ). NEP killer whale populations form distinct and stable social groups (pods), which differ in specialization of prey choice (ecotypes). Three recognized NEP ecotypes, ‘resident,’ ‘transient,’ and ‘offshore’, exhibit genetic and phenotypic differentiation 45 , 46 , 47 , 48 . Transient pods typically feed on marine mammals including elephant seals and sea lions, whereas resident pods target teleosts, mainly salmonids 45 , 49 . The offshore ecotype is least known, but thought to primarily target teleosts including salmonids and elasmobranchs such as Pacific sleeper sharks 45 , 50 . All three killer whale ecotypes overlap spatially with NEP white sharks and share similar prey resources, and thus may be considered part of the same ecological guild 51 . Regional overlap is highest during fall and early winter, when NEP white sharks show high site fidelity and extended residency periods at SEFI or other coastal aggregation sites near pinniped rookeries 32 for approximately 4–4.5 months (Fig. 1 ).
Spatial and temporal overlap of two top predators, white sharks ( Carcharodon carcharias ), and killer whales ( Orcinus orca ), and their shared prey, juvenile elephant seals ( Mirounga angustirostrous ), in the Northeastern Pacific (see Supplement) and at Southeast Farallon Islands (SEFI). Seasonally concentrated activity of each species at SEFI (*) evident from ( A ) weekly M . angustirostrous counts between March and December (1987–2013), ( B ) daily mean number of tagged C . carcharias detected (2007–2013) with shaded standard error and, ( C ) monthly frequency of O . orca observed (1987–2013). Note the two predators co-occur only during the fall peak. Map was created using R software (v3.5.1; https://www.R-project.org/ ).
Despite an extensive overlap in distributional range and trophic niche, observations of direct interactions between killer whales and white sharks are extremely rare, but have been recorded off California, South Africa, and Southern Australia 52 , 53 . A clear understanding of the ecological relationship between these two top predators has remained elusive. An interaction between these top predators in the NEP was documented on Oct 4, 1997, at SEFI in which a white shark was killed and partially consumed (liver only) by transient killer whales. Immediately following this event, observations of white sharks during regular surveys at SEFI declined precipitously; only two predations by sharks were observed in the remaining eight weeks of study at SEFI 52 .
In the current study we use a combination of an extensive electronic tagging dataset of white sharks throughout Central California together with long-term observational surveys of shark-pinniped predatory interactions and killer whale occurrences at SEFI to elucidate the frequency and consequences of rarely observed intraguild interactions between white sharks and killer whales. We reveal in detail the immediate behavioral nature of the predator interactions, as well as resulting effects that white shark redistribution has on the predator-prey relationship between white sharks and elephant seals.
Materials and Methods
White shark tagging.
Between 2006 and 2013, we tagged 165 white sharks ( Carcharodon carcharias ) with acoustic tags (V16-4H-A69, Vemco; transmitting at 158 Db every 60–180 s for >1400 days) in the vicinities of Southeast Farallon Island, Tomales Point, and Año Nuevo Island using previously described methods 32 . Briefly, white sharks were attracted to the research boat using a seal decoy made from outdoor carpet. Upon investigation by a shark, the decoy was retrieved using a fishing rod, reel and monofilament line. A small amount of olfactory attractant served to retain the shark near the boat, while the tag was applied using 3 to 4 m pole to insert a titanium dart tethered to the tag beneath the sharks’ dorsal skin. All methods were carried out in accordance with relevant guidelines and regulations, and all experimental protocols were approved under Stanford University animal care protocol 10765. The annual probability of tag loss (shedding) was estimated at 0.32 (95% CI = 0.26, 0.39) 54 , and annual incremental tagging (average 20 per year) provided a relatively constant flux of tagged individuals in the study system. We maintained continuous coverage in tag detection throughout the study period using sub-surface moored acoustic receivers (VR3, Vemco) stationed at the same three tagging locations 32 . Equivalent acoustic receivers (VR3, Vemco) deployed off Point Reyes by other research teams provided opportunistic detections supplementing the data collected at the primary shark aggregation sites.
Long-term surveys at Southeast Farallon Island
We recorded the number of hauled-out elephant seals from weekly elephant seal population census surveys conducted throughout the duration of the study (1987–2013) under NMFS Permit No. 373-1868. Surveys occurred between 1000 and 1600, throughout the year. The number of individuals hauled-out on SEFI were tallied with respect to age class as previously described in depth 55 . During the same period, we recorded the number of predations by white sharks from annual ‘shark watch’ surveys conducted from a lighthouse platform at the highest peak of SEFI (elevation 90 m). Between September 1 and November 30, trained observers continually scanned the waters around the islands for the occurrence of predation events during all daylight hours, unless visibility dropped below 1.6 km, winds exceeded 25 knots, or rain was persistent 37 . Observation hours averaged 569.7 hours per year (SD = 93.6). Predation observations included prey species when identifiable. Observational survey data were non-experimental, and carried out in accordance with relevant guidelines and regulations.
Killer whale observations were aggregated from a variety of sources, including the ‘shark watch’ surveys, island cetacean surveys 56 , opportunistic island-based observations, and reports received via a network of wildlife-viewing tour boats during daily radio contact. Killer whale observations were not procedurally standardized, however were relatively consistent throughout the year, except between September 1 and November 30 each year when observation effort increased during the standardized ‘shark watch’ survey. When possible, an estimate of the minimum distance between killer whale pods from the nearest point of the island was recorded. If observation notes indicated killer whales were inside one of the island bays, then a minimum distance of <1 km was assigned. For survey observations with no killer whale sightings, we assumed a distance value of >15 km, a distance beyond the maximum range included in SEFI cetacean surveys 56 . Where distance was missing from killer whale sightings, we assumed an average distance obtained from all other distance values. Accounts of killer whale behavior and photographs of individual killer whales including dorsal fin and saddle pigmentation were collected from tour boat operators and Island staff. Photos were compared to killer whale ID catalogues to match individuals and determine the pod’s ecotype and size.
We hypothesized that shark predations on elephant seals would decline in years when killer whales occurred at SEFI. We initially fit a log-log regression model to the positive predator-prey relationship 37 between annual predation rate (number of predations per observation hour) by sharks and mean fall (September 1–November 30) elephant seal counts to determine if deviations corresponded with killer whale occurrences. To test whether the presence of killer whales disrupted the seasonality of shark predations on elephant seals, we modeled daily predation events using a Generalized Additive Mixed Model. We assumed that the realized number of predation events followed a Poisson distribution and that the expected number of predations per day was a smooth function (cyclic cubic regression spline) of date included as ordinal days within a season from September 1 to November 30. We also constrained the spline to start and end at similar values. We treated sighting distance as a factor variable and as a quadratic function of survey observation effort in hours 37 . We also hypothesized that the shape of the functional relationship between daily shark predations on seals and date would change in relation to the distance of sighted killer whales, and consequently affect the average seasonal predation rate by white sharks. Therefore we included an interaction term between the spline of date and distance. We also expected to have between-year variability in predation rate. This could arise from annual variability in the number or behavior of individual sharks and seals. To control for this variability and ensure unbiased parameter estimates on the other terms, we included season (categorical) as a random effect, modeled as a smooth term with a random effect spline basis 57 . For model fitting we used a restricted maximum likelihood approach with the package “mgcv” in R 57 .
To test the hypothesis that killer whale activity in close proximity to SEFI elicited avoidance behavior, we looked at the number of tagged sharks detected per day at each site (SEFI, ANI, and TOM) between 2006 and 2013. We compared this metric during periods when killer whales were observed at SEFI against the mean value for all other years.
Results and Discussion
Multi-predator community at southeast farallon island (sefi).
Long-term intensive monitoring surveys, combined with electronic tagging and observational studies, revealed the frequency and modality of cryptic interactions amongst marine predator populations at SEFI. Seasonality in white shark and killer whale presence matched annual cycles in prey aggregations, namely juvenile (age 0–3) northern elephant seals ( Mirounga angustirostris ) that first haulout during spring molt (peaking in April and May) and then again in the fall (peaking in October and November) (Fig. 1 ). An estimated 219 adult and sub-adult white sharks ((130, 275) 95% credible intervals) aggregate and feed at SEFI and adjacent elephant seal rookeries around Point Reyes during this fall haulout period 58 . Long-term (1972–2010) birth rates of elephant seals at SEFI are variable (median = 198 births/year; mean = 232; SD = 132) and have decreased to a relatively stable level over the past decade, while the regional population continues to rapidly increase 59 . Additionaly, California sea lions ( Zalophus californianus ), Steller sea lions ( Eumetopias jubatus ), harbor seals ( Phoca vitulina ricardii ), and northern fur seals ( Callorhinus ursinus ) also haul out at SEFI at various times of the year 55 .
Killer whale pod observations occurred year-round on 57 occasions between 1987 and 2013 (Fig. 1C ). These sparse occurrences peaked in May, concurrent with gray whale calf migrations, followed by a secondary fall peak during October and November (Fig. 1C ). The co-occurrence of white sharks and killer whales was confined to fall, coincident with the peak in adult white shark activity at SEFI (Fig. 1B ). During the fall overlap (September–November) killer whale pods were recorded during daily surveys ( mean = 7.7 hr/day; weather permitting) from the island lighthouse at various distances from SEFI on 18 out of 1998 survey days in eight different years: 1992, 1995–1998, 2000, 2001, 2009, and 2013 (Table 1 ). The recorded duration of these 18 visits ranged from a maximum of 5.5 hours to less than an hour. When killer whale ecotype could reliably be identified (n = 5), mammal-eating transient pods were the most common visitors to SEFI (four of five), while offshore individuals were identified on a single occasion in 2009 when both offshores and transients were observed (Table 1 ).
White sharks aggregated at SEFI annually, where the observed number of predations by sharks peaked during October and November (Fig. 2B ). During the fall surveys, a mean of 40 observed predations (±16 SD; N = 27 years) by sharks occurred annually on elephant seals and unidentified pinniped prey. In years when killer whales were not observed or were sighted 3 or more km from shore (N = 19), the distribution of predation events on pinnipeds peaked between mid-October and mid-November (Fig. 2B ). In years when killer whales were sighted <3 km from shore (N = 9), this predation rate was depressed and truncated (Fig. S3 and Table S1 ). Therefore, annual predation rates on pinnipeds were significantly impacted when killer whale activity occurred at a distance threshold <3 km from the SEFI seal haul-out.
Predator-prey relationship between white sharks ( Carcharodon carcharias ) and elephant seals ( Mirounga angustirostrous ) altered by the presence of killer whales ( Orcinus orca ) at Southeast Farallon Island (SEFI). ( A ) Annual predation rate by C . carcharias as a function of mean fall (Sept. – Nov.) M . angustirostrous counts fit with a log-log regression line (dashed black line) showing confidence interval (dashed blue lines). Points are years where no flight response was detected, and triangles are the years in which a flight response was observed, near or before the peak of the C . carcharias season (≤November 2, inverted triangles), and near the end of the season (≥November 19, upright triangles). For comparison, an equivalent regression fit excluding flight years is shown with red dotted lines. ( B ) Seasonal C . carcharias kill rate as a function of the observed distance of O . orca activity to SEFI. The distribution of observed predations was reduced and truncated as a function of O . orca proximity to the common foraging ground (distance given in legend in km).
Overall, the observed annual rate of predation by sharks was positively correlated with the abundance of elephant seals present (R 2 = 0.191, p = 0.023) (Fig. 2A ). However, in years when killer whales occurred in close proximity to the island during or before peak shark abundance, the observed rate of predation by sharks deviated most from this relationship dropping 3.5 to 7-fold from the long-term average of 6.02 ± 2.4 predations per 100hrs (SD), to 1.73, 1.29, and 0.84 respectively in 1997, 2009, and 2013 (Fig. 2B ). In 2000 by contrast, killer whales also occurred close to SEFI, but much later in the season (November 18; Table 1 ) resulting in no deviation from the expected annual predation rate (Fig. 2A ).
Displacement of white sharks and flight response
Acoustic tag detections documented the abrupt and consistent flight of white sharks from SEFI in 2009, 2011, and 2013 (Figs 3 and SI ). In the best-documented instance, killer whales from two separate pods (offshore and transient ecotypes; Table 1 ) arrived at SEFI on November 2, 2009, when 17 previously tagged white sharks were present. Killer whales were present at SEFI for just over 2.5 hours between 12:48 and 15:30 local time, remained on the western side of SEFI during approach and initiated three separate killing bouts on pinnipeds, then departed to the north. There were no observations of direct predation on white sharks, and all tagged animals were later confirmed alive through acoustic detections; still predations on untagged white sharks could not be ruled out.
The flight response of white sharks ( Carcharodon carcharias ) triggered by the presence of killer whales ( Orcinus orca ) at a common foraging site, Southeast Farallon Islands (SEFI). ( A ) Mean daily number of acoustic tagged C . carcharias detected (2007–2013; excluding 2009; shaded standard error) at Central California receivers colored by location: Tomales Point (green), Southeast Farallon Islands (orange and orange/yellow), Año Nuevo Island (blue), and Point Reyes (purple). ( B ) The number of tagged C . carcharias detected per day at each site (respectively colored) during the 2009 season showing the sudden departure of all tagged individuals from SEFI in response to O . orca (Nov 2) presence. Note the subsequent influx around Año Nuevo Island where the shaded orange area represents individuals present at SEFI during killer whale interactions. ( C ) Detections of each tagged shark at color-coded locations are shown along the horizontal timeline illustrating the abrupt departure from SEFI by tagged C . carcharias following O . orca presence (between vertical black lines) and subsequent avoidance. Solid orange diamonds indicate the western SEFI receiver while orange with yellow centers indicate the eastern receiver. ( D ) Precise receiver locations are indicated by the right corner of each solid diamond and the left corner of the yellow filled diamond.
Desertion of SEFI by all tagged sharks followed the foraging behavior of killer whales close to SEFI. Regular daily detections of 17 tagged animals at two stationary acoustic receivers moored on eastern and western sides of SEFI (SI) discontinued abruptly following the appearance of killer whales (Fig. 3 ). Overall, the mean number of white sharks detected per day at SEFI declined from a seasonal maximum to zero for the remainder of the season. Declines in detections followed a spatial gradient, immediately subsiding at the western receiver most proximal to killer whale observations, followed by a tapering of detections over the following hours at the eastern receiver (Fig. 3 ). Seven hours and 50 minutes following the event, no tagged sharks remained within receiver range at SEFI and 16 individuals (of 17 displaced tagged sharks) were not detected at SEFI again until the following season (July 2010 or later). One individual returned a week later (November 8), and was detected at SEFI three times over 73 minutes, before departing and being re-detected at Año Nuevo Island (ANI) on November 24.
Anomalous shark absences at SEFI for the remainder of the 2009 season resulted in influxes of displaced individuals at mainland aggregation sites. Within 2 to 13 days of departing SEFI following the killer whale disturbance at SEFI, seven tagged individuals relocated nearly 90 km to the south at ANI. Three individuals were redetected at Tomales Point for extended periods, before two of these continued to ANI (Fig. 3 ). These influxes at mainland sites resulted in daily totals of individual sharks detected at ANI increasing sharply from 4 day −1 on 2 November to 10 day −1 by 14 November and peaking at 16 day −1 by 23 November. In contrast, SEFI remained virtually shark-free for the remaining season. Three tagged individuals not initially present during the killer whale event were detected subsequently at SEFI, though for abbreviated durations (0.25, 1, and 11 hours, respectively) compared to mean SEFI residency periods of 35 days 32 . Two of these three sharks were then subsequently detected at mainland aggregation sites (Fig. 3 ).
Acoustic tag records provided a clear ‘signature’ for understanding and estimating flight responses of sharks relative to other killer whale occurrences at SEFI during years when sufficient active tags were present (see Table 1 ). The well-documented incident in 2009 was consistent with previous observations of cessation of seal predation at SEFI by white sharks following brief killer whale visits 52 , 60 . Acoustic tag records revealed two additional similar signatures at SEFI: November 20, 2011, with 10 tagged individual present, and October 31, 2013, with 3 tagged individuals present (see SI). Inclement weather resulting in poor visibility precluded visual confirmation of killer whales for the former event (no surveys that day) in 2011. On the 2013 occasion, 13 killer whales were observed during regular shark visual surveys from SEFI. In both cases following typical flight responses, no further tag detections were recorded at SEFI for the remainder of the season (see SI). Similarly, no further predations of pinnipeds by white sharks were observed during the remaining visual surveys in 2011 and 2013, and only a single predation during the remaining season in 2009 near Mid-Farallon Island, 3.4 km northwest of SEFI. In summary, a white shark flight response from SEFI related to killer whale occurrence was identified in four separate years, along with a fifth flight response with unconfirmed attribution (Table 1 ). While intensive observer survey data are lacking at TOM and ANI, no equivalent flight response was ever apparent in acoustic tagging data with continuous coverage between 2006 and 2013.
Ecological roles and context of killer whale-shark interactions
Transient killer whales were present in the two flight response years when ecotypes could be reliably determined (1997, 2009), whereas offshore individuals were identified in addition to transients in the 2009 disturbance. In determining the ecological relationship between white sharks and killer whales, understanding whether their interactions are defined by predator-prey or competitive aggression interactions depends on killer whale ecotype. Mammal-eating transient killer whales 45 are direct competitors, but also pose a predatory threat as illustrated in the 1997 event 52 (see introduction). Interactions with the offshore ecotype are potentially predatory, as well as competitive. Offshore killer whales are known to forage on teleosts and elasmobranchs, the latter forming a potentially important dietary component as evidenced by apical teeth worn flat, presumably from the abrasive shark skin 50 , and observations of repeated feeding on Pacific sleeper sharks, Somniosus pacificus 61 . Residents are likely a weak competitor (for teleosts) and potentially not a predation threat 45 , 49 . Whether white sharks might distinguish a predatory versus competitive threat remains unknown, but the result may be the same. Like predation pressure, interspecific competitive aggression can similarly drive behavior that reduces encounter rates, shape habitat use, and shift activity schedules 8 , 62 . Only one direct predation on white sharks by killer whales was ever confirmed on white sharks at SEFI 52 , yet white sharks vacating SEFI, effectively freed up potential pinniped resources for the killer whales and restricted white shark access to those resources.
Risk effects among top ocean predators
This study demonstrates the occurrence of risk effects among upper trophic level marine predators. The key interactions surrounding the phenomena remained cryptic and rarely observed despite intensive long-term visual surveys and multi-year continuous electronic tracking coverage. In the rare instances when both predators co-occurred at SEFI, antagonistic interactions between them resulted in the extended displacement of foraging white sharks via risk effects (Fig. 3 ), and in turn reduced local predation pressure on seals (Fig. 2 ). Despite exceptionally brief killer whale visits to SEFI (2.4–5 hr) during well-documented events near the peak white shark foraging season, the observed predation rate on pinnipeds by white sharks during those years decreased (Fig. 2 ). It is unlikely that killer whale predation on pinnipeds could compensate for the reduction in predation by white sharks following their displacement. Of the predation events observed at the surface in 15,383 hours of lighthouse surveys between 1987 and 2013, 912 were attributed to white sharks and only 5 events on 3 dates to killer whales.
Killer whales exert top-down effects in various systems by directly reducing meso-predator density through consumption 1 , 4 as well as eliciting shifts in prey behaviors and distributions due to risk effects 63 , 64 . Similarly, large sharks can have a direct regulatory influence over their prey populations 6 , 65 , 66 , and induce food-safety tradeoffs 67 including avoidance behavior 68 , 69 . This study suggests that intraguild interactions between killer whales and white sharks may result in cascading effects at lower trophic levels by reducing consumptive (and possibly non-consumptive) effects on elephant seals. Quantifying the indirect population-level effects killer whales induce on white sharks may have on elephant seals locally and regionally remains an important future direction. Northern elephant seals are undergoing rapid habitat expansion and population growth, following long-term human exploitation and extreme depletion 59 . Any population regulatory effects white sharks, killer whales, and their interactions have on elephant seals could become more significant as elephant seals approach an equilibrium level.
Occasional consumption of the highly-caloric liver of white sharks may confer ancillary energetic benefits to the killer whale. The fitness loss to white sharks from direct lethal interactions with killer whales is unambiguous. But avoidance behaviors in response to killer whale presence could also impact white shark fitness by restricting spatiotemporal access and activity to habitats that are sub-optimal or more competitive (more densely populated by conspecifics). Intimidation and predation risk pervasively affects entire populations, not just the individuals directly killed 16 . Potential consequences of displacement to white sharks should be evaluated within the ecological context of their migratory phenology. Fall-time aggregations and site fidelity of NEP white sharks along the central California coast immediately precede extensive offshore migrations to relatively oligotrophic waters 32 , 60 , 70 . Despite spending one third of their time in coastal California habitats, adults assimilate nearly half their protein from coastal foraging 41 . Concentrated energy acquisition during this coastal phase is stored in the oil-rich liver mass and is expended during long migrations (1000–3000 km) to seasonal offshore subtropical habitats 43 , where males increase diving activity extensively 32 , 38 . Disruptions of foraging prior to migration is known to negatively impact migratory performance in numerous long-distance migrating species 30 , 71 , 72 . Future efforts should aim to measure the impact and ecological implications of these risk effects on white shark fitness and elephant seal population dynamics.
Data Availibility
Data underlying this study and described above in Methods are archived (open access) at https://osf.io/b7su4/?view_only=f4f874c19e044ea5951a9ac355954d9f . These include (1) aggregated phenological acoustic tag detection data (2007–2013), and raw tag detection data (October 14 to November 30, 2009) (2) weekly census data of juvenile elephant seals at SEFI (1987–2013), and (3) killer whale observations at SEFI aggregated by month (1987–2013).
Estes, J. A., Tinker, M. T., Williams, T. M. & Doak, D. F. Killer Whale Predation on Sea Otters Linking Oceanic and Nearshore Ecosystems. Science 282 , 473–476 (1998).
Article ADS CAS Google Scholar
Estes, J. A. et al . Trophic Downgrading of Planet Earth. Science 333 , 301–306 (2011).
Heithaus, M. R., Frid, A., Wirsing, A. J. & Worm, B. Predicting ecological consequences of marine top predator declines. Trends in Ecology & Evolution 23 , 202–210 (2008).
Article Google Scholar
Williams, T. M., Estes, J. A., Doak, D. F. & Springer, A. M. Killer Appetites: Assessing the Role of Predators in Ecological Communities. Ecology 85 , 3373–3384 (2004).
Ritchie, E. G. & Johnson, C. N. Predator interactions, mesopredator release and biodiversity conservation. Ecology Letters 12 , 982–998 (2009).
Baum, J. K. & Worm, B. Cascading top-down effects of changing oceanic predator abundances. Journal of Animal Ecology 78 , 699–714 (2009).
Werner, E. E. & Peacor, S. D. A review of trait-mediated indirect interactions in ecological communities. Ecology 84 , 1083–1100 (2003).
Creel, S. & Christianson, D. Relationships between direct predation and risk effects. Trends in Ecology & Evolution 23 , 194–201 (2008).
Heithaus, M. R., Wirsing, A. J., Burkholder, D., Thomson, J. & Dill, L. M. Towards a predictive framework for predator risk effects: the interaction of landscape features and prey escape tactics. Journal of Animal Ecology 78 , 556–562 (2009).
Laundré, J. W., Hernández, L. & Ripple, W. J. The landscape of fear: ecological implications of being afraid. Open Ecology Journal 3 , 1–7 (2010).
Burkholder, D. A., Heithaus, M. R., Fourqurean, J. W., Wirsing, A. & Dill, L. M. Patterns of top-down control in a seagrass ecosystem: could a roving apex predator induce a behaviour-mediated trophic cascade? Journal of Animal Ecology 82 , 1192–1202 (2013).
Clinchy, M., Zanette, L., Boonstra, R., Wingfield, J. C. & Smith, J. N. M. Balancing food and predator pressure induces chronic stress in songbirds. Proceedings of the Royal Society of London B: Biological Sciences 271 , 2473–2479 (2004).
Mukherjee, S., Heithaus, M. R., Trexler, J. C., Ray-Mukherjee, J. & Vaudo, J. Perceived Risk of Predation Affects Reproductive Life-History Traits in Gambusia holbrooki, but Not in Heterandria formosa. PLOS ONE 9 , e88832 (2014).
Article ADS Google Scholar
Allen, A. N., Schanze, J. J., Solow, A. R. & Tyack, P. L. Analysis of a Blainville’s beaked whale’s movement response to playback of killer whale vocalizations. Marine Mammal Science 1 , 154–168 (2013).
Google Scholar
Pace, M. L., Cole, J. J., Carpenter, S. R. & Kitchell, J. F. Trophic cascades revealed in diverse ecosystems. Trends in Ecology & Evolution 14 , 483–488 (1999).
Article CAS Google Scholar
Preisser, E. L., Bolnick, D. I. & Benard, M. F. Scared to death? The effects of intimidation and consumption in predator-prey interactions. Ecology 86 , 501–509 (2005).
Suraci, J. P., Clinchy, M., Dill, L. M., Roberts, D. & Zanette, L. Y. Fear of large carnivores causes a trophic cascade. Nature Communications 7 , 10698 (2016).
Beckerman, A. P., Uriarte, M. & Schmitz, O. J. Experimental evidence for a behavior-mediated trophic cascade in a terrestrial food chain. PNAS 94 , 10735–10738 (1997).
McCauley, D. J. et al . Marine defaunation: Animal loss in the global ocean. Science 347 , 1255641 (2015).
Heithaus, M. R. Predator–prey and competitive interactions between sharks (order Selachii) and dolphins (suborder Odontoceti): a review. Journal of Zoology 253 , 53–68 (2001).
Polis, G. A. & Holt, R. D. Intraguild predation: The dynamics of complex trophic interactions. Trends in Ecology & Evolution 7 , 151–154 (1992).
Polis, G. A., Myers, C. A. & Holt, R. D. The Ecology and Evolution of Intraguild Predation: Potential Competitors That Eat Each Other. Annual Review of Ecology and Systematics 20 , 297–330 (1989).
Palomares, F. & Caro, T. M. Interspecific Killing among Mammalian Carnivores. The American Naturalist 153 , 492–508 (1999).
Glen, A. S. & Dickman, C. R. Complex interactions among mammalian carnivores in Australia, and their implications for wildlife management. Biological Reviews 80 , 387–401 (2005).
Laundré, J. W., Hernández, L. & Altendorf, K. B. Wolves, elk, and bison: reestablishing the ‘landscape of fear’ in Yellowstone National Park, USA. Can. J. Zool. 79 , 1401–1409 (2001).
Heithaus, M. R. & Dill, M. L. Does tiger shark predation risk influence foraging habitat use by bottlenose dolphins at multiple spatial scales? Oikos 114 , 257–264 (2006).
Wirsing, A. J., Heithaus, M. R., Frid, A. & Dill, L. M. Seascapes of fear: evaluating sublethal predator effects experienced and generated by marine mammals. Marine Mammal Science 24 , 1–15 (2008).
Lourenço, R., Penteriani, V., Rabaça, J. E. & Korpimäki, E. Lethal interactions among vertebrate top predators: a review of concepts, assumptions and terminology. Biol Rev 89 , 270–283 (2014).
Block, B. A. et al . Tracking apex marine predator movements in a dynamic ocean. Nature 475 , 86–90 (2011).
Dingle, H. Migration: the biology of life on the move . (Oxford University Press, USA, 1996).
Newton, I. The migration ecology of birds . (Academic Press, 2010).
Jorgensen, S. J. et al . Philopatry and migration of Pacific white sharks. Proc R Soc B 277 , 679–688 (2010).
Hoyos-Padilla, E. M., Klimley, A. P., Galván-Magaña, F. & Antoniou, A. Contrasts in the movements and habitat use of juvenile and adult white sharks (Carcharodon carcharias) at Guadalupe Island, Mexico. Animal Biotelemetry 4 , 14 (2016).
Le Beouf, B. J. & Laws, R. M. Elephant Seals: An Introduction to the Genus. In Elephant Seals: Population Ecology , Behavior , and Physiology 1–26 (University of California Press, 1994).
Klimley, A. P. et al . The hunting strategy of white sharks (Carcharodon carcharias) near a seal colony. Mar Biol 138 , 617–636 (2001).
Anderson, S. D., Becker, B. H. & Allen, S. G. Observations and Prey of White Sharks, Carcharodon carcharias, at Point Reyes National Seashore: 1982-2004. California Fish and Game 94 , 33 (2008).
Brown, A. C., Lee, D. E., Bradley, R. W. & Anderson, S. Dynamics of White Shark Predation on Pinnipeds in California: Effects of Prey Abundance. Copeia 2010 , 232–238 (2010).
Jorgensen, S. J. et al . Eating or Meeting? Cluster Analysis Reveals Intricacies of White Shark (Carcharodon carcharias) Migration and Offshore Behavior. PLoS ONE 7 , e47819 (2012).
Long, D. J. & Jones, R. E. White shark predation and scavenging on cetaceans in the eastern North Pacific Ocean. Great white sharks: the biology of Carcharodon carcharias 293–307 (1996).
Klimley, A. P. The areal distribution and autoecology of the white shark, Carcharodon carcharias, off the west coast of North America. Memoirs of the Southern California Academy of Sciences 9 , 15–40 (1985).
Carlisle, A. B. et al . Using stable isotope analysis to understand the migration and trophic ecology of northeastern Pacific white sharks (Carcharodon carcharias). PLoS ONE 7 , e30492 (2012).
Carey, F. G. et al . Temperature and activities of a white shark, Carcharodon carcharias. Copeia 1982 , 254–260 (1982).
Raye, G. D., Jorgensen, S. J., Krumhansl, K., Ezcurra, J. M. & Block, B. A. Travelling light: white sharks (Carcharodon carcharias) rely on body lipid stores to power ocean-basin scale migration. Proc. R. Soc. B 280 , 20130836 (2013).
Herman, D. P. et al . Feeding ecology of eastern North Pacific killer whales Orcinus orca from fatty acid, stable isotope, and organochlorine analyses of blubber biopsies. Mar Ecol Prog Ser 302 , 275–291 (2005).
Ford, J. K. B. et al . Dietary specialization in two sympatric populations of killer whales (Orcinus orca) in coastal British Columbia and adjacent waters. Can J Zool 76 , 1456–1471 (1998).
Morin, P. A. et al . Complete mitochondrial genome phylogeographic analysis of killer whales (Orcinus orca) indicates multiple species. Genome Res. 20 , 908–916 (2010).
de Bruyn, P. J. N., Tosh, C. A. & Terauds, A. Killer whale ecotypes: is there a global model? Biological Reviews 88 , 62–80 (2013).
Hoelzel, A. R. & Moura, A. E. Resource specialisation and the divergence of killer whale populations. Heredity (Edinb) 115 , 93–95 (2015).
Ford, J. K. B., Ellis, G. M. & Balcomb, K. C. Killer whales: The natural history and genealogy of Orcinus orca in British Columbia and Washington . (Univ of Washington Pr, 2000).
Ford, J. K. et al . Shark predation and tooth wear in a population of northeastern Pacific killer whales. (2011).
Simberloff, D. & Dayan, T. The Guild Concept and the Structure of Ecological Communities. Annual Review of Ecology and Systematics 22 , 115–143 (1991).
Pyle, P., Schramm, M. J., Keiper, C. & Anderson, S. D. Predation on a white shark (Carcharodon carcharias) by a killer whale (Orcinus orca) and a possible case of competitive displacement. Marine Mammal Science 15 , 563–568 (1999).
Best, P. B., Meÿer, M. A. & Lockyer, C. Killer whales in South African waters—a review of their biology. African Journal of Marine Science 32 , 171–186 (2010).
Chapple, T. K. et al . A novel application of multi-event modeling to estimate class segregation in a highly migratory oceanic vertebrate. Ecology 97 , 3494–3502 (2016).
Sydeman, W. J. & Allen, S. G. Pinniped population dynamics in central California: correlations with sea surface temperatures and upwelling indices. Marine Mammal Science 15 , 446–461 (1999).
Pyle, P. & Gilbert, L. Occurrence patterns and trends of cetaceans recorded from Southeast Farallon Island, California, 1973 to 1994. Northwestern Naturalist 1–8 (1996).
Wood, S. N. Generalized Additive Models: An Introduction with R , Second Edition . (CRC Press, 2017).
Chapple, T. K. et al . A first estimate of white shark, Carcharodon carcharias, abundance off Central California. Biol lett 7 , 581–583 (2011).
Lowry, M. S. et al . Abundance, Distribution, and Population Growth of the Northern Elephant Seal (Mirounga angustirostris) in the United States from 1991 to 2010. Aquatic Mammals 40 , 20–31 (2014).
Weng, K. C. et al . Migration and habitat of white sharks (Carcharodon carcharias) in the eastern Pacific Ocean. Mar Biol 152 , 877–894 (2007).
Deecke, V. B., Ford, J. K. B. & Slater, P. J. B. The vocal behaviour of mammal-eating killer whales: communicating with costly calls. Animal Behaviour 69 , 395–405 (2005).
Peiman, K. S. & Robinson, B. W. Ecology and Evolution of Resource-Related Heterospecific Aggression. The Quarterly Review of Biology 85 , 133–158 (2010).
Jefferson, T. A., Stacey, P. J. & Baird, R. W. A review of Killer Whale interactions with other marine mammals: predation to co-existence. Mammal Review 21 , 151–180 (1991).
Breed, G. A. et al . Sustained disruption of narwhal habitat use and behavior in the presence of Arctic killer whales. PNAS 114 , 2628–2633 (2017).
Wirsing, A. J., Heithaus, M. R. & Dill, L. M. Tiger shark (Galeocerdo cuvier) abundance and growth in a subtropical embayment: evidence from 7 years of standardized fishing effort. Mar Biol 149 , 961–968 (2006).
Heithaus, M. R., Wirsing, A. J. & Dill, L. M. The ecological importance of intact top-predator populations: a synthesis of 15 years of research in a seagrass ecosystem. Mar. Freshwater Res. 63 , 1039–1050 (2012).
Wirsing, A. J., Heithaus, M. R. & Dill, L. M. Fear factor: do dugongs (Dugong dugon) trade food for safety from tiger sharks (Galeocerdo cuvier)? Oecologia 153 , 1031–1040 (2007).
Wirsing, A. J., Cameron, K. E. & Heithaus, M. R. Spatial responses to predators vary with prey escape mode. Animal Behaviour 79 , 531–537 (2010).
Moxley, J. H. The Abundance and Behavioral Ecology of Cape Cod Gray Seals Under Predation Risk From White Sharks. (2016).
Boustany et al . Expanded niche for white sharks. Nature 415 , 35–36 (2002).
Fontaine, M. Physiological Mechanisms in the Migration of Marine and Amphihaline Fish. Advances in Marine Biology 13 , 241–355 (1976).
Alerstam, T., Hedenström, A. & Åkesson, S. Long-distance migration: evolution and determinants. Oikos 103 , 247–260 (2003).
Download references
Acknowledgements
All research activities were conducted under permits and authorizations granted by the Greater Farallones National Marine Sanctuary, the California department of Fish and Wildlife, U.S. department of fish and Wildlife, and U.S. National Park Service, in accordance with relevant guidelines and regulations. We would like to thank numerous individuals who assisted in field work, lab work, data processing, editing and inspiration including J. Moskito, A. Shulman-Janiger, J. Benson, J. Barlow, R. Elliot, A. Carlisle, C. Perle, A. Brown, C. Logan, J. Cornelius, B. Becker, S. McAfee, C. Farwell, C. Harrold, J. O’Sullivan, J. Ganong, A. Swithenbank, M. Castleton, A.P. Klimley, T. Brandt, R. Repass and many more. We thank Wyliecat, R. Repass, T. O’Leary, Bodega Marine Lab, and Farallon Patrol for vessel assistance. We also thank Farallon Islands National Wildlife Refuge staff, and biologists who collected data over several decades. The research on SEFI was made possible by a cooperative agreement with the United States Fish and Wildlife Service. Financial support was provided by the Monterey Bay Aquarium, Sloan, Moore and David and Lucile Packard Foundations and Stanford University. Additional funding for Point Blue’s Farallon research was provided by the Shark Trust, Baker Trust, and the Bently, Marisla, Mead, Campini, Bernice Barbour, Kimball, Volgenau, and RHE Charitable Foundations, and by individual donors. FF acknowledges the Lenfest Ocean Program and the Bertarelli Foundation. For JT and RWB this is Point Blue Contribution # 2051.
Author information
Authors and affiliations.
Monterey Bay Aquarium, 886 Cannery Row, Monterey, CA, 93940, USA
Salvador J. Jorgensen, Scot Anderson, Paul Kanive & Jerry H. Moxley
Department of Biology, Stanford University Pacific Grove, California, 93950, USA
Francesco Ferretti, Taylor Chapple & Barbara A. Block
Point Blue Conservation Science, 3820 Cypress Drive #11, Petaluma, CA, 94954, USA
James R. Tietz & Russell W. Bradley
Fish and Wildlife Management, Montana State University, PO Box 173460, Bozeman, MT, 59717, USA
Paul Kanive
You can also search for this author in PubMed Google Scholar
Contributions
S.J.J., S.A., B.A.B., R.W.B., T.C. and J.R.T. designed the study. S.J.J., S.A., B.A.B., R.W.B., T.C., P.K. and J.R.T. conducted field work. S.J.J., F.F. and J.H.M. analyzed the data and drafted the figures. S.J.J. wrote the manuscript. All authors contributed data and edited the manuscript.
Corresponding author
Correspondence to Salvador J. Jorgensen .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Jorgensen, S.J., Anderson, S., Ferretti, F. et al. Killer whales redistribute white shark foraging pressure on seals. Sci Rep 9 , 6153 (2019). https://doi.org/10.1038/s41598-019-39356-2
Download citation
Received : 30 July 2018
Accepted : 18 January 2019
Published : 16 April 2019
DOI : https://doi.org/10.1038/s41598-019-39356-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Premières images détaillées d'orques chassant des grands requins blancs.
- Jack Dutton
Nature Africa (2022)
North Pacific warming shifts the juvenile range of a marine apex predator
- Kisei R. Tanaka
- Kyle S. Van Houtan
- Salvador J. Jorgensen
Scientific Reports (2021)
New insights into the trophic ecology of the scalloped hammerhead shark, Sphyrna lewini, in the eastern tropical Pacific Ocean
- Colombo Estupiñán-Montaño
- Elena Tamburin
- Antonio Delgado-Huertas
Environmental Biology of Fishes (2021)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Manage Account
- Press and Journal ePaper
- Evening Express ePaper
- Newsletters
Are there more killer whales than usual in Aberdeenshire waters, and how can you spot one? We ask an expert
Dr Kevin Robinson, lead researcher and director of the Cetacean Research and Rescue Unit (CRRU), gave us the low-down on these impressive predators.

Photos of killer whales spotted off the coast of Aberdeenshire have flooded social media this year — but are there more orcas than usual in our waters?
The summer months are always busy for wildlife enthusiasts, who spend their days outdoors clutching binoculars or massive cameras hoping to catch a glimpse of something special.
Last year, basking sharks were in the spotlight due to a rise in sightings, particularly after a couple of paddleboarders shared a “lucky” encounter.
But this year, it seems as though orcas have stolen the show, with so many posts sharing sightings from Troup Head in Macduff and Kinnaird Head in Fraserburgh, to further south in Stonehaven and even Cove — leaving some onlookers “speechless”.
For many, seeing orcas in the wild is an incredible experience, but it seems no one expects to see them quite so close to home.

Dr Kevin Robinson, lead researcher and director of the Cetacean Research and Rescue Unit ( CRRU ) based in Banff, gave us the low-down on these impressive predators.
And his answers might surprise you.
Read on to find out:
- If there are more killer whales off the coast of Aberdeenshire
- Where they come from, and what brings them to Scotland
- When and where to try spot some
So, are there more killer whales than usual in Aberdeenshire waters?
Orcas are well-known to frequent Shetland, Orkney and the Caithness coast.
But do all the posts about killer whales being spotted off Aberdeenshire mean there’s more suddenly visiting our waters?
Well, the short answer is no… but the long answer is interesting.
Kevin has worked with CRRU in the Banff area for 28 years now and says orcas are actually “pretty normal” visitors to our waters — which may be a bit surprising to hear.

So if there haven’t been more killer whales visiting the area, why are we only just now spotting them and sharing these sightings?
The lead researcher has put it down to people simply noticing them a bit more than before, especially now with Facebook groups dedicated to reporting sightings of marine mammals .
“I think very often it’s a reflection of public interest more than the actual occurrence of orcas,” he went on to say. “We’ve been seeing them in this region for the last 20 years on a regular basis.
“They’re just not very predictable on the southern coastline.
“But there’s been a couple of years in a row now where spottings have reoccurred at the same time. So that’s raised heads and people think ‘oh, this is unusual’.”
Where do the orcas come from and what brings them to Aberdeenshire and the Moray Firth?
Kevin explained that all the animals cited in the region are “regulars” and belong to the Icelandic population.
He said there are about 60 or so killer whales from the Icelandic group that come to Scotland throughout the year, and it’s all the same family groups that they tend to see.

And, there haven’t been any new orcas in the region that his team doesn’t already know about.
But why do they travel hundreds of miles south from Iceland and end up on the north-east coast?
The predators make the trip to hunt seals.
Kevin said people have noticed the animals more towards Fraserburgh in recent years although they are “quite regular and common”. The creel fishermen from the harbour are aware of them, and a few people have reported their sightings to CRRU over the years too.
Are the sightings in Cove and Stonehaven rare?
The black and white whales have been known to travel further south, and the recent sightings in Cove and Stonehaven aren’t so unusual, according to Kevin.
“We do see them quite commonly heading down past Newburgh, Aberdeen and even further south,” he explained. “But with an ever-changing environment, changes in sea temperatures, changes in the availability of prey… these are highly adaptable animals.
“They’re basically opportunists. So wherever they find prey patches or sources they’re going to look in those areas.
“If they don’t find them in the regular areas, then they’re probably going to pursue their prey, or extend their search patterns further.”
And he reiterated that while there isn’t a bigger occurrence of killer whales, there’s “certainly more awareness of them”.
Kevin added: “When a walrus turned up and sat on the end of the pier at Crovie 20 years ago, it was quite the local tourist attraction.
“But now, when someone posts a picture on Facebook, then a thousand people roll up to see it — which can actually be a hindrance for the poor animals in the long run.”
When and where might you be able to catch a glimpse of killer whales?
CRRU has recorded over 16 different whale and dolphin species along the southern Moray Firth coastline (between Fraserburgh and Lossiemouth) over the last 26 years.
But, Kevin says unlike minke whales and other species the team study, they unfortunately cannot predict when or where orcas will pop up.

This year, they have also spotted a lot of Risso’s dolphins, a species they don’t often see, as well as a fin whale — which is the first time the team has seen the second-biggest mammal on Earth in the area.
And basking sharks arrived very early this year because of the warmer weather.
Although orcas are unpredictable, they can be found in Scottish waters all year round, according to Kevin — but they do travel quite long distances and sightings can be quite few and far between.
They can be seen from the shore, and Kevin says once you know what you’re looking for they can be quite easy to track along the coast.
It just requires a lot of patience and potentially long hours watching the sea, particularly in areas like Kinnaird Head where they’ve been known to frequent.
He said: “During the winter months, fewer people are watching or the sea is too inclement. So it can look as if there are more sightings in certain months.
“But that really is all about the sea conditions and whether people are going to be bothered to sit on a cold, icy Kinnaird Head in December. But, there are quite a few dedicated people who do record them all year round now.
“We are seeing increased numbers of posts on social media about these animals. And as I said, it can be a little bit misleading but suffice to say, there’s been quite a bit of activity this year for orcas.”
Here one day, gone the next: how far can they travel?
The coastal mammals have a big range, move big distances, and spend a lot of time traveling in their pod to different hunting spots.
One week they could be off the coast of Fraserburgh, and the next they could be in Shetland before heading to the Caithness coastline.

Kevin finished: “One argument against keeping these animals in captivity is that in a day, even if they’re not feeding or specifically going anywhere, they cover tens, and even hundreds, of miles in a day.
“Sometimes just in their social groups, but they certainly will be targeting certain areas where they know they can find prey.
“And if they don’t find prey in those areas, maybe they’ll wander a bit further until they do find that what they’re looking for.
“They’re certainly a very exciting apex predator to look out for along the coastline.
“You just have to put in a concerted effort — there’s people that will just go to these headlands to look out during their lunchbreaks or during the evening or early morning and just enjoy sitting on the coast. And they will get to see these things.”
Read more about killer whales being spotted in north-east:
- ‘It was amazing’: Killer whales captured on camera near Kinnaird Head in Fraserburgh
- Spectacular photos of killer whale pod off Fraserburgh coast with new baby
- Why arrival of Orcas in Moray Firth has surprised and enthralled sealife watchers
More from Environment

Why are Newburgh seal disturbances down? Meet the people working behind the scenes to…

Debate: How would you like to see Aberdeen LEZ fine money spent by the…

Driven grouse shooting: 'No place in modern society', or a vital tradition? Locals have…

Britain in Bloom judges reveal what makes amazing Forres flower displays among best in…

Warning Moray brown bin charges could increase again despite income soaring to record levels

Why Newburgh diver Lauren loves plunging into shark-infested waters

How Highland Council is seeking to secure at least £4 million community benefit boost…

'We are crying out for this help': Anger as move to help Highland communities…

Balnagown Estate: Mohamed Al Fayed's family continuing tycoon's work on Highland property nearly a…

'Their best bet is with their mums': New Arc's advice to avoid accidentally 'kidnapping'…
Conversation.
Comments are currently disabled as they require cookies and it appears you've opted out of cookies on this site. To participate in the conversation, please adjust your cookie preferences in order to enable comments.

IMAGES
VIDEO
COMMENTS
Resident killer whales maintain tight-knit family pods and prey on salmon and other marine fish. Bigg's killer whales roam in smaller groups, preying on other marine mammals such as seals and whales. (Killer whales actually belong to the dolphin family.) Bigg's killer whales, sometimes called transients, are named for Canadian scientist ...
Genomic and demographic analyses of the 'Southern Resident' killer whales in the North Pacific find that strong inbreeding depression is inhibiting growth of this small and isolated population ...
The Southern Resident killer whale population will decline slowly for a generation or two before accelerating toward extinction, suggests a model-based population viability analysis accounting for ...
Killer whales (Orcinus orca) are currently recognized as a single ecologically and morphologically diverse, globally distributed species. Multiple morphotypes or ecotypes have been described, often associated with feeding specialization, and several ...
While the nature of the opportunistic data hinders a definitive portrayal of the social structure of killer whales using the Brazilian coastal waters, these novel insights contribute to mapping the socio-ecology and behavioral diversity of one of the most widely distributed mammals.
Killer whales ( Orcinus orca) are the largest members of the dolphin family, with males reaching up to 9 meters long and potentially weighing well over 6 tons. Killer whales are easily identifiable by their striking black and white coloration, with a white underside and 'eye patch' marking above the eyes and a grey 'saddle patch' behind ...
The critically endangered, Southern Resident killer whale population of the northeastern Pacific Ocean provides a data-rich case to explore anthropogenic threats on population viability.
The impetus for studying the social dynamics and intelligence of killer whales is manifold. As apex predators, these marine mammals play a pivotal role in maintaining the health and stability of ...
The killer whale—the largest of the dolphins and the top marine predator--has a cosmopolitan distribution throughout the world's oceans. Although globally it could be considered a generalist predator with a diverse diet, it is deeply divided...
Starting in 2003 Cascadia began a variety of research efforts focused on killer whales, most in collaboration with the Northwest Fisheries Science Center of NOAA Fisheries and a continuation of long-term studies of killer whales being undertaken by Robin Baird. Recent projects have focused on studying the underwater acoustics and behavior of ...
Persistent polychlorinated biphenyls still contribute to killer whale declines three decades after having being banned.
New research reveals full diversity of killer whales as two species come into view on Pacific Coast. Scientists have resolved one of the outstanding questions about one of the world's most ...
This study investigates survival and abundance of killer whales (Orcinus orca) in Norway in 1988-2019 using capture-recapture models of photo‐identification data. We merged two datasets collected in a restricted fjord system in ...
Killer whale is an apex predator which plays an important role in marine ecosystems. Inthe Russian waters, killer whales are represented by two ecotypes — fish-eaters (R-type) and marine-mammal ...
In this study, we examined the year-round distribution of killer whales in the northern Gulf of Alaska from 2016 to 2020 using passive acoustic monitoring.
Introduction Orcas ( Orcinus orca, also known as killer whales) are part of the worldwide commercial trade in cetaceans and are the third most commonly confined cetacean species after bottlenose dolphins ( Tursiops truncatus) and beluga whales ( Delphinapterus leucas ).
The killer whale, Orcinus orca, is the largest and most widely geographically distributed species of dolphin (Delphinidae). Killer whales are found from the Arctic to the Antarctic, and all waters in between, occurring in the greatest densities at high latitudes ( Forney & Wade, 2007 ).
Humpback whales (Megaptera novaeangliae) are known to interfere with attacking killer whales (Orcinus orca). To investigate why, we reviewed accounts of 115 interactions between them. Humpbacks initi...
Orcinus Orca is the world's largest predator, and simultaneously a significant tourist asset and cultural icon for much of the Pacific Northwest. In the past two decades, the Southern Resident Killer whale (SRKW) population has declined by more than 25 percent, putting them at risk of extinction. The cause of this decline is hotly debated. This paper employs novel data, an innovative noise ...
Reports of killer whales (Orcinus orca) preying on large whales have been relatively rare, and the ecological significance of these attacks is controversial. Here we report on numerous observations o...
Extensive research on killer whale acoustic behavior has taken place in the Northeast Pacific where resident fish-eating, transient mammal-eating and offshore killer whales can be found, the three ...
The distribution, seasonality and schooling behaviour of killer whales Orcinus orca in South African waters have been investigated from 785 records compiled between 1963 and 2009, and their size, morphometrics, growth, reproduction, food and feeding behaviour described from the examination of 54 individuals, 36 of which were landed at the ...
Killer whales have become something of a menace to seafarers around the world, with orca attacks on boats and yachts increasing every year leaving some sailors fearing for their lives. Now, as attacks begin once again off the coast of Spain, new research suggests that the animals may simply be using our boats for "target practice." Orcas off the coast of Spain this week struck another boat ...
Any population regulatory effects white sharks, killer whales, and their interactions have on elephant seals could become more significant as elephant seals approach an equilibrium level ...
Killer whales spotted in southern Moray Firth. Image: Cetacean Research & Rescue Unit (CRRU)