- Research article
- Open access
- Published: 04 June 2021

Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews
- Israel Júnior Borges do Nascimento 1 , 2 ,
- Dónal P. O’Mathúna 3 , 4 ,
- Thilo Caspar von Groote 5 ,
- Hebatullah Mohamed Abdulazeem 6 ,
- Ishanka Weerasekara 7 , 8 ,
- Ana Marusic 9 ,
- Livia Puljak ORCID: orcid.org/0000-0002-8467-6061 10 ,
- Vinicius Tassoni Civile 11 ,
- Irena Zakarija-Grkovic 9 ,
- Tina Poklepovic Pericic 9 ,
- Alvaro Nagib Atallah 11 ,
- Santino Filoso 12 ,
- Nicola Luigi Bragazzi 13 &
- Milena Soriano Marcolino 1
On behalf of the International Network of Coronavirus Disease 2019 (InterNetCOVID-19)
BMC Infectious Diseases volume 21 , Article number: 525 ( 2021 ) Cite this article
17k Accesses
34 Citations
14 Altmetric
Metrics details
Navigating the rapidly growing body of scientific literature on the SARS-CoV-2 pandemic is challenging, and ongoing critical appraisal of this output is essential. We aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Nine databases (Medline, EMBASE, Cochrane Library, CINAHL, Web of Sciences, PDQ-Evidence, WHO’s Global Research, LILACS, and Epistemonikos) were searched from December 1, 2019, to March 24, 2020. Systematic reviews analyzing primary studies of COVID-19 were included. Two authors independently undertook screening, selection, extraction (data on clinical symptoms, prevalence, pharmacological and non-pharmacological interventions, diagnostic test assessment, laboratory, and radiological findings), and quality assessment (AMSTAR 2). A meta-analysis was performed of the prevalence of clinical outcomes.
Eighteen systematic reviews were included; one was empty (did not identify any relevant study). Using AMSTAR 2, confidence in the results of all 18 reviews was rated as “critically low”. Identified symptoms of COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%) and gastrointestinal complaints (5–9%). Severe symptoms were more common in men. Elevated C-reactive protein and lactate dehydrogenase, and slightly elevated aspartate and alanine aminotransferase, were commonly described. Thrombocytopenia and elevated levels of procalcitonin and cardiac troponin I were associated with severe disease. A frequent finding on chest imaging was uni- or bilateral multilobar ground-glass opacity. A single review investigated the impact of medication (chloroquine) but found no verifiable clinical data. All-cause mortality ranged from 0.3 to 13.9%.
Conclusions
In this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic were of questionable usefulness. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards.
Peer Review reports
The spread of the “Severe Acute Respiratory Coronavirus 2” (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [ 1 ]. The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [ 2 ], causing massive economic strain, and escalating healthcare and public health expenses [ 3 , 4 ].
The research community has responded by publishing an impressive number of scientific reports related to COVID-19. The world was alerted to the new disease at the beginning of 2020 [ 1 ], and by mid-March 2020, more than 2000 articles had been published on COVID-19 in scholarly journals, with 25% of them containing original data [ 5 ]. The living map of COVID-19 evidence, curated by the Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre), contained more than 40,000 records by February 2021 [ 6 ]. More than 100,000 records on PubMed were labeled as “SARS-CoV-2 literature, sequence, and clinical content” by February 2021 [ 7 ].
Due to publication speed, the research community has voiced concerns regarding the quality and reproducibility of evidence produced during the COVID-19 pandemic, warning of the potential damaging approach of “publish first, retract later” [ 8 ]. It appears that these concerns are not unfounded, as it has been reported that COVID-19 articles were overrepresented in the pool of retracted articles in 2020 [ 9 ]. These concerns about inadequate evidence are of major importance because they can lead to poor clinical practice and inappropriate policies [ 10 ].
Systematic reviews are a cornerstone of today’s evidence-informed decision-making. By synthesizing all relevant evidence regarding a particular topic, systematic reviews reflect the current scientific knowledge. Systematic reviews are considered to be at the highest level in the hierarchy of evidence and should be used to make informed decisions. However, with high numbers of systematic reviews of different scope and methodological quality being published, overviews of multiple systematic reviews that assess their methodological quality are essential [ 11 , 12 , 13 ]. An overview of systematic reviews helps identify and organize the literature and highlights areas of priority in decision-making.
In this overview of systematic reviews, we aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Methodology
Research question.
This overview’s primary objective was to summarize and critically appraise systematic reviews that assessed any type of primary clinical data from patients infected with SARS-CoV-2. Our research question was purposefully broad because we wanted to analyze as many systematic reviews as possible that were available early following the COVID-19 outbreak.
Study design
We conducted an overview of systematic reviews. The idea for this overview originated in a protocol for a systematic review submitted to PROSPERO (CRD42020170623), which indicated a plan to conduct an overview.
Overviews of systematic reviews use explicit and systematic methods for searching and identifying multiple systematic reviews addressing related research questions in the same field to extract and analyze evidence across important outcomes. Overviews of systematic reviews are in principle similar to systematic reviews of interventions, but the unit of analysis is a systematic review [ 14 , 15 , 16 ].
We used the overview methodology instead of other evidence synthesis methods to allow us to collate and appraise multiple systematic reviews on this topic, and to extract and analyze their results across relevant topics [ 17 ]. The overview and meta-analysis of systematic reviews allowed us to investigate the methodological quality of included studies, summarize results, and identify specific areas of available or limited evidence, thereby strengthening the current understanding of this novel disease and guiding future research [ 13 ].
A reporting guideline for overviews of reviews is currently under development, i.e., Preferred Reporting Items for Overviews of Reviews (PRIOR) [ 18 ]. As the PRIOR checklist is still not published, this study was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 statement [ 19 ]. The methodology used in this review was adapted from the Cochrane Handbook for Systematic Reviews of Interventions and also followed established methodological considerations for analyzing existing systematic reviews [ 14 ].
Approval of a research ethics committee was not necessary as the study analyzed only publicly available articles.
Eligibility criteria
Systematic reviews were included if they analyzed primary data from patients infected with SARS-CoV-2 as confirmed by RT-PCR or another pre-specified diagnostic technique. Eligible reviews covered all topics related to COVID-19 including, but not limited to, those that reported clinical symptoms, diagnostic methods, therapeutic interventions, laboratory findings, or radiological results. Both full manuscripts and abbreviated versions, such as letters, were eligible.
No restrictions were imposed on the design of the primary studies included within the systematic reviews, the last search date, whether the review included meta-analyses or language. Reviews related to SARS-CoV-2 and other coronaviruses were eligible, but from those reviews, we analyzed only data related to SARS-CoV-2.
No consensus definition exists for a systematic review [ 20 ], and debates continue about the defining characteristics of a systematic review [ 21 ]. Cochrane’s guidance for overviews of reviews recommends setting pre-established criteria for making decisions around inclusion [ 14 ]. That is supported by a recent scoping review about guidance for overviews of systematic reviews [ 22 ].
Thus, for this study, we defined a systematic review as a research report which searched for primary research studies on a specific topic using an explicit search strategy, had a detailed description of the methods with explicit inclusion criteria provided, and provided a summary of the included studies either in narrative or quantitative format (such as a meta-analysis). Cochrane and non-Cochrane systematic reviews were considered eligible for inclusion, with or without meta-analysis, and regardless of the study design, language restriction and methodology of the included primary studies. To be eligible for inclusion, reviews had to be clearly analyzing data related to SARS-CoV-2 (associated or not with other viruses). We excluded narrative reviews without those characteristics as these are less likely to be replicable and are more prone to bias.
Scoping reviews and rapid reviews were eligible for inclusion in this overview if they met our pre-defined inclusion criteria noted above. We included reviews that addressed SARS-CoV-2 and other coronaviruses if they reported separate data regarding SARS-CoV-2.
Information sources
Nine databases were searched for eligible records published between December 1, 2019, and March 24, 2020: Cochrane Database of Systematic Reviews via Cochrane Library, PubMed, EMBASE, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Web of Sciences, LILACS (Latin American and Caribbean Health Sciences Literature), PDQ-Evidence, WHO’s Global Research on Coronavirus Disease (COVID-19), and Epistemonikos.
The comprehensive search strategy for each database is provided in Additional file 1 and was designed and conducted in collaboration with an information specialist. All retrieved records were primarily processed in EndNote, where duplicates were removed, and records were then imported into the Covidence platform [ 23 ]. In addition to database searches, we screened reference lists of reviews included after screening records retrieved via databases.
Study selection
All searches, screening of titles and abstracts, and record selection, were performed independently by two investigators using the Covidence platform [ 23 ]. Articles deemed potentially eligible were retrieved for full-text screening carried out independently by two investigators. Discrepancies at all stages were resolved by consensus. During the screening, records published in languages other than English were translated by a native/fluent speaker.
Data collection process
We custom designed a data extraction table for this study, which was piloted by two authors independently. Data extraction was performed independently by two authors. Conflicts were resolved by consensus or by consulting a third researcher.
We extracted the following data: article identification data (authors’ name and journal of publication), search period, number of databases searched, population or settings considered, main results and outcomes observed, and number of participants. From Web of Science (Clarivate Analytics, Philadelphia, PA, USA), we extracted journal rank (quartile) and Journal Impact Factor (JIF).
We categorized the following as primary outcomes: all-cause mortality, need for and length of mechanical ventilation, length of hospitalization (in days), admission to intensive care unit (yes/no), and length of stay in the intensive care unit.
The following outcomes were categorized as exploratory: diagnostic methods used for detection of the virus, male to female ratio, clinical symptoms, pharmacological and non-pharmacological interventions, laboratory findings (full blood count, liver enzymes, C-reactive protein, d-dimer, albumin, lipid profile, serum electrolytes, blood vitamin levels, glucose levels, and any other important biomarkers), and radiological findings (using radiography, computed tomography, magnetic resonance imaging or ultrasound).
We also collected data on reporting guidelines and requirements for the publication of systematic reviews and meta-analyses from journal websites where included reviews were published.
Quality assessment in individual reviews
Two researchers independently assessed the reviews’ quality using the “A MeaSurement Tool to Assess Systematic Reviews 2 (AMSTAR 2)”. We acknowledge that the AMSTAR 2 was created as “a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions, or both” [ 24 ]. However, since AMSTAR 2 was designed for systematic reviews of intervention trials, and we included additional types of systematic reviews, we adjusted some AMSTAR 2 ratings and reported these in Additional file 2 .
Adherence to each item was rated as follows: yes, partial yes, no, or not applicable (such as when a meta-analysis was not conducted). The overall confidence in the results of the review is rated as “critically low”, “low”, “moderate” or “high”, according to the AMSTAR 2 guidance based on seven critical domains, which are items 2, 4, 7, 9, 11, 13, 15 as defined by AMSTAR 2 authors [ 24 ]. We reported our adherence ratings for transparency of our decision with accompanying explanations, for each item, in each included review.
One of the included systematic reviews was conducted by some members of this author team [ 25 ]. This review was initially assessed independently by two authors who were not co-authors of that review to prevent the risk of bias in assessing this study.
Synthesis of results
For data synthesis, we prepared a table summarizing each systematic review. Graphs illustrating the mortality rate and clinical symptoms were created. We then prepared a narrative summary of the methods, findings, study strengths, and limitations.
For analysis of the prevalence of clinical outcomes, we extracted data on the number of events and the total number of patients to perform proportional meta-analysis using RStudio© software, with the “meta” package (version 4.9–6), using the “metaprop” function for reviews that did not perform a meta-analysis, excluding case studies because of the absence of variance. For reviews that did not perform a meta-analysis, we presented pooled results of proportions with their respective confidence intervals (95%) by the inverse variance method with a random-effects model, using the DerSimonian-Laird estimator for τ 2 . We adjusted data using Freeman-Tukey double arcosen transformation. Confidence intervals were calculated using the Clopper-Pearson method for individual studies. We created forest plots using the RStudio© software, with the “metafor” package (version 2.1–0) and “forest” function.
Managing overlapping systematic reviews
Some of the included systematic reviews that address the same or similar research questions may include the same primary studies in overviews. Including such overlapping reviews may introduce bias when outcome data from the same primary study are included in the analyses of an overview multiple times. Thus, in summaries of evidence, multiple-counting of the same outcome data will give data from some primary studies too much influence [ 14 ]. In this overview, we did not exclude overlapping systematic reviews because, according to Cochrane’s guidance, it may be appropriate to include all relevant reviews’ results if the purpose of the overview is to present and describe the current body of evidence on a topic [ 14 ]. To avoid any bias in summary estimates associated with overlapping reviews, we generated forest plots showing data from individual systematic reviews, but the results were not pooled because some primary studies were included in multiple reviews.
Our search retrieved 1063 publications, of which 175 were duplicates. Most publications were excluded after the title and abstract analysis ( n = 860). Among the 28 studies selected for full-text screening, 10 were excluded for the reasons described in Additional file 3 , and 18 were included in the final analysis (Fig. 1 ) [ 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 ]. Reference list screening did not retrieve any additional systematic reviews.
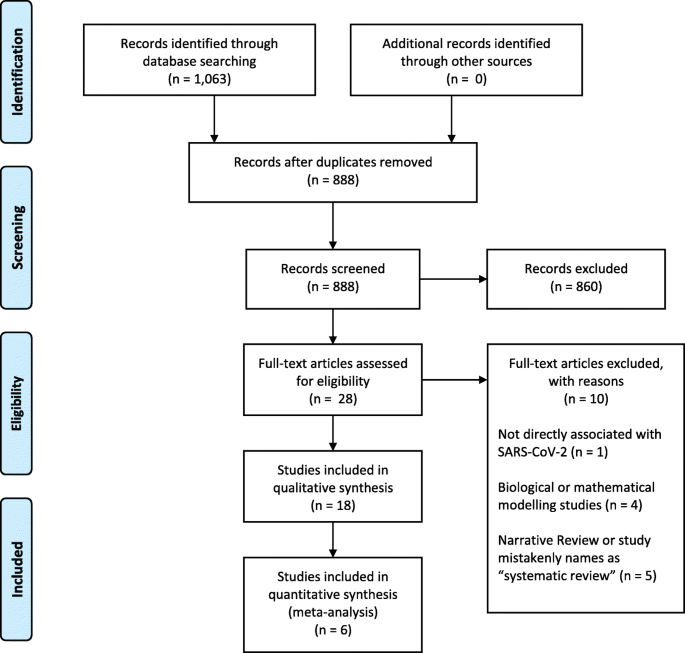
PRISMA flow diagram
Characteristics of included reviews
Summary features of 18 systematic reviews are presented in Table 1 . They were published in 14 different journals. Only four of these journals had specific requirements for systematic reviews (with or without meta-analysis): European Journal of Internal Medicine, Journal of Clinical Medicine, Ultrasound in Obstetrics and Gynecology, and Clinical Research in Cardiology . Two journals reported that they published only invited reviews ( Journal of Medical Virology and Clinica Chimica Acta ). Three systematic reviews in our study were published as letters; one was labeled as a scoping review and another as a rapid review (Table 2 ).
All reviews were published in English, in first quartile (Q1) journals, with JIF ranging from 1.692 to 6.062. One review was empty, meaning that its search did not identify any relevant studies; i.e., no primary studies were included [ 36 ]. The remaining 17 reviews included 269 unique studies; the majority ( N = 211; 78%) were included in only a single review included in our study (range: 1 to 12). Primary studies included in the reviews were published between December 2019 and March 18, 2020, and comprised case reports, case series, cohorts, and other observational studies. We found only one review that included randomized clinical trials [ 38 ]. In the included reviews, systematic literature searches were performed from 2019 (entire year) up to March 9, 2020. Ten systematic reviews included meta-analyses. The list of primary studies found in the included systematic reviews is shown in Additional file 4 , as well as the number of reviews in which each primary study was included.
Population and study designs
Most of the reviews analyzed data from patients with COVID-19 who developed pneumonia, acute respiratory distress syndrome (ARDS), or any other correlated complication. One review aimed to evaluate the effectiveness of using surgical masks on preventing transmission of the virus [ 36 ], one review was focused on pediatric patients [ 34 ], and one review investigated COVID-19 in pregnant women [ 37 ]. Most reviews assessed clinical symptoms, laboratory findings, or radiological results.
Systematic review findings
The summary of findings from individual reviews is shown in Table 2 . Overall, all-cause mortality ranged from 0.3 to 13.9% (Fig. 2 ).
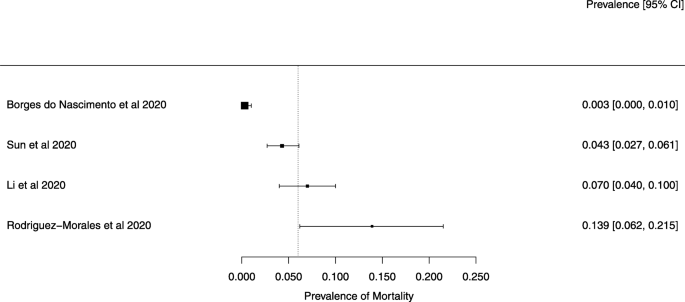
A meta-analysis of the prevalence of mortality
Clinical symptoms
Seven reviews described the main clinical manifestations of COVID-19 [ 26 , 28 , 29 , 34 , 35 , 39 , 41 ]. Three of them provided only a narrative discussion of symptoms [ 26 , 34 , 35 ]. In the reviews that performed a statistical analysis of the incidence of different clinical symptoms, symptoms in patients with COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%), gastrointestinal disorders, such as diarrhea, nausea or vomiting (5.0–9.0%), and others (including, in one study only: dizziness 12.1%) (Figs. 3 , 4 , 5 , 6 , 7 , 8 and 9 ). Three reviews assessed cough with and without sputum together; only one review assessed sputum production itself (28.5%).
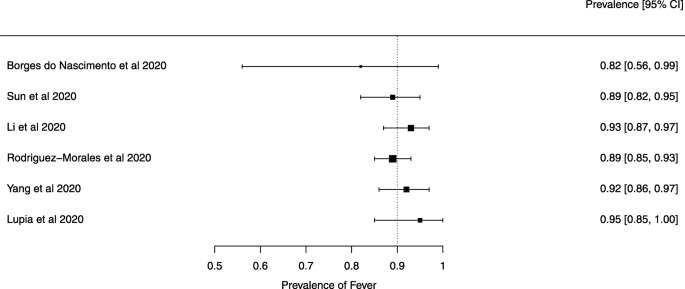
A meta-analysis of the prevalence of fever
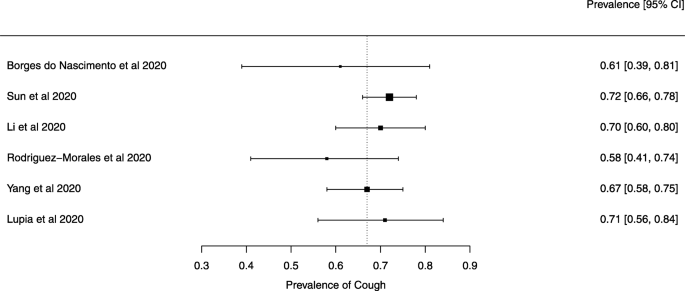
A meta-analysis of the prevalence of cough
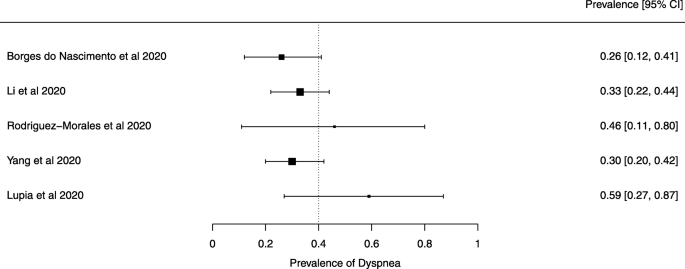
A meta-analysis of the prevalence of dyspnea
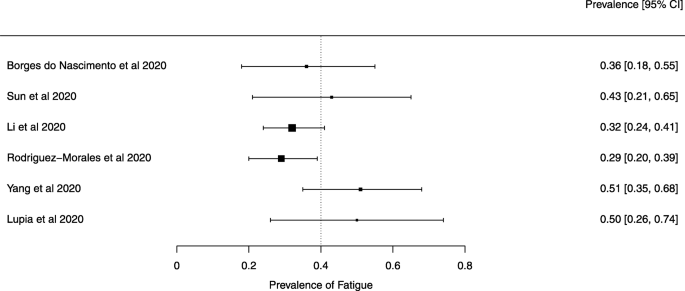
A meta-analysis of the prevalence of fatigue or myalgia
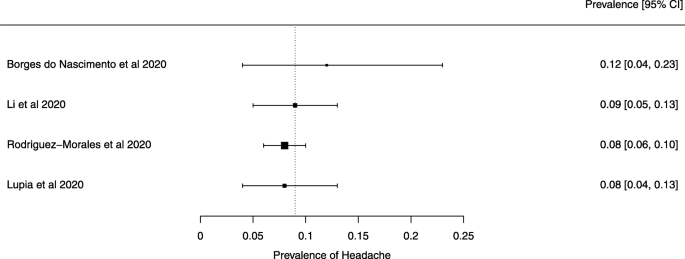
A meta-analysis of the prevalence of headache
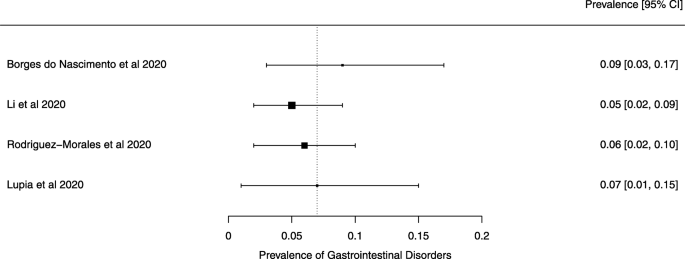
A meta-analysis of the prevalence of gastrointestinal disorders
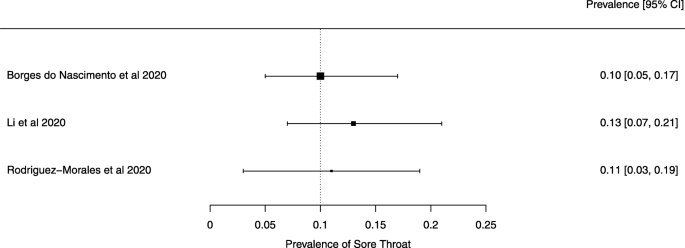
A meta-analysis of the prevalence of sore throat
Diagnostic aspects
Three reviews described methodologies, protocols, and tools used for establishing the diagnosis of COVID-19 [ 26 , 34 , 38 ]. The use of respiratory swabs (nasal or pharyngeal) or blood specimens to assess the presence of SARS-CoV-2 nucleic acid using RT-PCR assays was the most commonly used diagnostic method mentioned in the included studies. These diagnostic tests have been widely used, but their precise sensitivity and specificity remain unknown. One review included a Chinese study with clinical diagnosis with no confirmation of SARS-CoV-2 infection (patients were diagnosed with COVID-19 if they presented with at least two symptoms suggestive of COVID-19, together with laboratory and chest radiography abnormalities) [ 34 ].
Therapeutic possibilities
Pharmacological and non-pharmacological interventions (supportive therapies) used in treating patients with COVID-19 were reported in five reviews [ 25 , 27 , 34 , 35 , 38 ]. Antivirals used empirically for COVID-19 treatment were reported in seven reviews [ 25 , 27 , 34 , 35 , 37 , 38 , 41 ]; most commonly used were protease inhibitors (lopinavir, ritonavir, darunavir), nucleoside reverse transcriptase inhibitor (tenofovir), nucleotide analogs (remdesivir, galidesivir, ganciclovir), and neuraminidase inhibitors (oseltamivir). Umifenovir, a membrane fusion inhibitor, was investigated in two studies [ 25 , 35 ]. Possible supportive interventions analyzed were different types of oxygen supplementation and breathing support (invasive or non-invasive ventilation) [ 25 ]. The use of antibiotics, both empirically and to treat secondary pneumonia, was reported in six studies [ 25 , 26 , 27 , 34 , 35 , 38 ]. One review specifically assessed evidence on the efficacy and safety of the anti-malaria drug chloroquine [ 27 ]. It identified 23 ongoing trials investigating the potential of chloroquine as a therapeutic option for COVID-19, but no verifiable clinical outcomes data. The use of mesenchymal stem cells, antifungals, and glucocorticoids were described in four reviews [ 25 , 34 , 35 , 38 ].
Laboratory and radiological findings
Of the 18 reviews included in this overview, eight analyzed laboratory parameters in patients with COVID-19 [ 25 , 29 , 30 , 32 , 33 , 34 , 35 , 39 ]; elevated C-reactive protein levels, associated with lymphocytopenia, elevated lactate dehydrogenase, as well as slightly elevated aspartate and alanine aminotransferase (AST, ALT) were commonly described in those eight reviews. Lippi et al. assessed cardiac troponin I (cTnI) [ 25 ], procalcitonin [ 32 ], and platelet count [ 33 ] in COVID-19 patients. Elevated levels of procalcitonin [ 32 ] and cTnI [ 30 ] were more likely to be associated with a severe disease course (requiring intensive care unit admission and intubation). Furthermore, thrombocytopenia was frequently observed in patients with complicated COVID-19 infections [ 33 ].
Chest imaging (chest radiography and/or computed tomography) features were assessed in six reviews, all of which described a frequent pattern of local or bilateral multilobar ground-glass opacity [ 25 , 34 , 35 , 39 , 40 , 41 ]. Those six reviews showed that septal thickening, bronchiectasis, pleural and cardiac effusions, halo signs, and pneumothorax were observed in patients suffering from COVID-19.
Quality of evidence in individual systematic reviews
Table 3 shows the detailed results of the quality assessment of 18 systematic reviews, including the assessment of individual items and summary assessment. A detailed explanation for each decision in each review is available in Additional file 5 .
Using AMSTAR 2 criteria, confidence in the results of all 18 reviews was rated as “critically low” (Table 3 ). Common methodological drawbacks were: omission of prospective protocol submission or publication; use of inappropriate search strategy: lack of independent and dual literature screening and data-extraction (or methodology unclear); absence of an explanation for heterogeneity among the studies included; lack of reasons for study exclusion (or rationale unclear).
Risk of bias assessment, based on a reported methodological tool, and quality of evidence appraisal, in line with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method, were reported only in one review [ 25 ]. Five reviews presented a table summarizing bias, using various risk of bias tools [ 25 , 29 , 39 , 40 , 41 ]. One review analyzed “study quality” [ 37 ]. One review mentioned the risk of bias assessment in the methodology but did not provide any related analysis [ 28 ].
This overview of systematic reviews analyzed the first 18 systematic reviews published after the onset of the COVID-19 pandemic, up to March 24, 2020, with primary studies involving more than 60,000 patients. Using AMSTAR-2, we judged that our confidence in all those reviews was “critically low”. Ten reviews included meta-analyses. The reviews presented data on clinical manifestations, laboratory and radiological findings, and interventions. We found no systematic reviews on the utility of diagnostic tests.
Symptoms were reported in seven reviews; most of the patients had a fever, cough, dyspnea, myalgia or muscle fatigue, and gastrointestinal disorders such as diarrhea, nausea, or vomiting. Olfactory dysfunction (anosmia or dysosmia) has been described in patients infected with COVID-19 [ 43 ]; however, this was not reported in any of the reviews included in this overview. During the SARS outbreak in 2002, there were reports of impairment of the sense of smell associated with the disease [ 44 , 45 ].
The reported mortality rates ranged from 0.3 to 14% in the included reviews. Mortality estimates are influenced by the transmissibility rate (basic reproduction number), availability of diagnostic tools, notification policies, asymptomatic presentations of the disease, resources for disease prevention and control, and treatment facilities; variability in the mortality rate fits the pattern of emerging infectious diseases [ 46 ]. Furthermore, the reported cases did not consider asymptomatic cases, mild cases where individuals have not sought medical treatment, and the fact that many countries had limited access to diagnostic tests or have implemented testing policies later than the others. Considering the lack of reviews assessing diagnostic testing (sensitivity, specificity, and predictive values of RT-PCT or immunoglobulin tests), and the preponderance of studies that assessed only symptomatic individuals, considerable imprecision around the calculated mortality rates existed in the early stage of the COVID-19 pandemic.
Few reviews included treatment data. Those reviews described studies considered to be at a very low level of evidence: usually small, retrospective studies with very heterogeneous populations. Seven reviews analyzed laboratory parameters; those reviews could have been useful for clinicians who attend patients suspected of COVID-19 in emergency services worldwide, such as assessing which patients need to be reassessed more frequently.
All systematic reviews scored poorly on the AMSTAR 2 critical appraisal tool for systematic reviews. Most of the original studies included in the reviews were case series and case reports, impacting the quality of evidence. Such evidence has major implications for clinical practice and the use of these reviews in evidence-based practice and policy. Clinicians, patients, and policymakers can only have the highest confidence in systematic review findings if high-quality systematic review methodologies are employed. The urgent need for information during a pandemic does not justify poor quality reporting.
We acknowledge that there are numerous challenges associated with analyzing COVID-19 data during a pandemic [ 47 ]. High-quality evidence syntheses are needed for decision-making, but each type of evidence syntheses is associated with its inherent challenges.
The creation of classic systematic reviews requires considerable time and effort; with massive research output, they quickly become outdated, and preparing updated versions also requires considerable time. A recent study showed that updates of non-Cochrane systematic reviews are published a median of 5 years after the publication of the previous version [ 48 ].
Authors may register a review and then abandon it [ 49 ], but the existence of a public record that is not updated may lead other authors to believe that the review is still ongoing. A quarter of Cochrane review protocols remains unpublished as completed systematic reviews 8 years after protocol publication [ 50 ].
Rapid reviews can be used to summarize the evidence, but they involve methodological sacrifices and simplifications to produce information promptly, with inconsistent methodological approaches [ 51 ]. However, rapid reviews are justified in times of public health emergencies, and even Cochrane has resorted to publishing rapid reviews in response to the COVID-19 crisis [ 52 ]. Rapid reviews were eligible for inclusion in this overview, but only one of the 18 reviews included in this study was labeled as a rapid review.
Ideally, COVID-19 evidence would be continually summarized in a series of high-quality living systematic reviews, types of evidence synthesis defined as “ a systematic review which is continually updated, incorporating relevant new evidence as it becomes available ” [ 53 ]. However, conducting living systematic reviews requires considerable resources, calling into question the sustainability of such evidence synthesis over long periods [ 54 ].
Research reports about COVID-19 will contribute to research waste if they are poorly designed, poorly reported, or simply not necessary. In principle, systematic reviews should help reduce research waste as they usually provide recommendations for further research that is needed or may advise that sufficient evidence exists on a particular topic [ 55 ]. However, systematic reviews can also contribute to growing research waste when they are not needed, or poorly conducted and reported. Our present study clearly shows that most of the systematic reviews that were published early on in the COVID-19 pandemic could be categorized as research waste, as our confidence in their results is critically low.
Our study has some limitations. One is that for AMSTAR 2 assessment we relied on information available in publications; we did not attempt to contact study authors for clarifications or additional data. In three reviews, the methodological quality appraisal was challenging because they were published as letters, or labeled as rapid communications. As a result, various details about their review process were not included, leading to AMSTAR 2 questions being answered as “not reported”, resulting in low confidence scores. Full manuscripts might have provided additional information that could have led to higher confidence in the results. In other words, low scores could reflect incomplete reporting, not necessarily low-quality review methods. To make their review available more rapidly and more concisely, the authors may have omitted methodological details. A general issue during a crisis is that speed and completeness must be balanced. However, maintaining high standards requires proper resourcing and commitment to ensure that the users of systematic reviews can have high confidence in the results.
Furthermore, we used adjusted AMSTAR 2 scoring, as the tool was designed for critical appraisal of reviews of interventions. Some reviews may have received lower scores than actually warranted in spite of these adjustments.
Another limitation of our study may be the inclusion of multiple overlapping reviews, as some included reviews included the same primary studies. According to the Cochrane Handbook, including overlapping reviews may be appropriate when the review’s aim is “ to present and describe the current body of systematic review evidence on a topic ” [ 12 ], which was our aim. To avoid bias with summarizing evidence from overlapping reviews, we presented the forest plots without summary estimates. The forest plots serve to inform readers about the effect sizes for outcomes that were reported in each review.
Several authors from this study have contributed to one of the reviews identified [ 25 ]. To reduce the risk of any bias, two authors who did not co-author the review in question initially assessed its quality and limitations.
Finally, we note that the systematic reviews included in our overview may have had issues that our analysis did not identify because we did not analyze their primary studies to verify the accuracy of the data and information they presented. We give two examples to substantiate this possibility. Lovato et al. wrote a commentary on the review of Sun et al. [ 41 ], in which they criticized the authors’ conclusion that sore throat is rare in COVID-19 patients [ 56 ]. Lovato et al. highlighted that multiple studies included in Sun et al. did not accurately describe participants’ clinical presentations, warning that only three studies clearly reported data on sore throat [ 56 ].
In another example, Leung [ 57 ] warned about the review of Li, L.Q. et al. [ 29 ]: “ it is possible that this statistic was computed using overlapped samples, therefore some patients were double counted ”. Li et al. responded to Leung that it is uncertain whether the data overlapped, as they used data from published articles and did not have access to the original data; they also reported that they requested original data and that they plan to re-do their analyses once they receive them; they also urged readers to treat the data with caution [ 58 ]. This points to the evolving nature of evidence during a crisis.
Our study’s strength is that this overview adds to the current knowledge by providing a comprehensive summary of all the evidence synthesis about COVID-19 available early after the onset of the pandemic. This overview followed strict methodological criteria, including a comprehensive and sensitive search strategy and a standard tool for methodological appraisal of systematic reviews.
In conclusion, in this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all the reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic could be categorized as research waste. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards to provide patients, clinicians, and decision-makers trustworthy evidence.
Availability of data and materials
All data collected and analyzed within this study are available from the corresponding author on reasonable request.
World Health Organization. Timeline - COVID-19: Available at: https://www.who.int/news/item/29-06-2020-covidtimeline . Accessed 1 June 2021.
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/map.html . Accessed 1 June 2021.
Anzai A, Kobayashi T, Linton NM, Kinoshita R, Hayashi K, Suzuki A, et al. Assessing the Impact of Reduced Travel on Exportation Dynamics of Novel Coronavirus Infection (COVID-19). J Clin Med. 2020;9(2):601.
Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400. https://doi.org/10.1126/science.aba9757 .
Article CAS PubMed PubMed Central Google Scholar
Fidahic M, Nujic D, Runjic R, Civljak M, Markotic F, Lovric Makaric Z, et al. Research methodology and characteristics of journal articles with original data, preprint articles and registered clinical trial protocols about COVID-19. BMC Med Res Methodol. 2020;20(1):161. https://doi.org/10.1186/s12874-020-01047-2 .
EPPI Centre . COVID-19: a living systematic map of the evidence. Available at: http://eppi.ioe.ac.uk/cms/Projects/DepartmentofHealthandSocialCare/Publishedreviews/COVID-19Livingsystematicmapoftheevidence/tabid/3765/Default.aspx . Accessed 1 June 2021.
NCBI SARS-CoV-2 Resources. Available at: https://www.ncbi.nlm.nih.gov/sars-cov-2/ . Accessed 1 June 2021.
Gustot T. Quality and reproducibility during the COVID-19 pandemic. JHEP Rep. 2020;2(4):100141. https://doi.org/10.1016/j.jhepr.2020.100141 .
Article PubMed PubMed Central Google Scholar
Kodvanj, I., et al., Publishing of COVID-19 Preprints in Peer-reviewed Journals, Preprinting Trends, Public Discussion and Quality Issues. Preprint article. bioRxiv 2020.11.23.394577; doi: https://doi.org/10.1101/2020.11.23.394577 .
Dobler CC. Poor quality research and clinical practice during COVID-19. Breathe (Sheff). 2020;16(2):200112. https://doi.org/10.1183/20734735.0112-2020 .
Article Google Scholar
Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7(9):e1000326. https://doi.org/10.1371/journal.pmed.1000326 .
Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 1-purpose, eligibility, search and data extraction. Syst Rev. 2017;6(1):231. https://doi.org/10.1186/s13643-017-0617-1 .
Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Chapter V: Overviews of Reviews. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane. 2020. Available from www.training.cochrane.org/handbook .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane. 2020; Available from www.training.cochrane.org/handbook .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. The impact of different inclusion decisions on the comprehensiveness and complexity of overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):18. https://doi.org/10.1186/s13643-018-0914-3 .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. A decision tool to help researchers make decisions about including systematic reviews in overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):29. https://doi.org/10.1186/s13643-018-0768-8 .
Hunt H, Pollock A, Campbell P, Estcourt L, Brunton G. An introduction to overviews of reviews: planning a relevant research question and objective for an overview. Syst Rev. 2018;7(1):39. https://doi.org/10.1186/s13643-018-0695-8 .
Pollock M, Fernandes RM, Pieper D, Tricco AC, Gates M, Gates A, et al. Preferred reporting items for overviews of reviews (PRIOR): a protocol for development of a reporting guideline for overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):335. https://doi.org/10.1186/s13643-019-1252-9 .
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3(3):e123–30.
Krnic Martinic M, Pieper D, Glatt A, Puljak L. Definition of a systematic review used in overviews of systematic reviews, meta-epidemiological studies and textbooks. BMC Med Res Methodol. 2019;19(1):203. https://doi.org/10.1186/s12874-019-0855-0 .
Puljak L. If there is only one author or only one database was searched, a study should not be called a systematic review. J Clin Epidemiol. 2017;91:4–5. https://doi.org/10.1016/j.jclinepi.2017.08.002 .
Article PubMed Google Scholar
Gates M, Gates A, Guitard S, Pollock M, Hartling L. Guidance for overviews of reviews continues to accumulate, but important challenges remain: a scoping review. Syst Rev. 2020;9(1):254. https://doi.org/10.1186/s13643-020-01509-0 .
Covidence - systematic review software. Available at: https://www.covidence.org/ . Accessed 1 June 2021.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Borges do Nascimento IJ, et al. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J Clin Med. 2020;9(4):941.
Article PubMed Central Google Scholar
Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. https://doi.org/10.1186/s40249-020-00646-x .
Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–83. https://doi.org/10.1016/j.jcrc.2020.03.005 .
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–8. https://doi.org/10.1007/s00392-020-01626-9 .
Article CAS PubMed Google Scholar
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–83. https://doi.org/10.1002/jmv.25757 .
Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63(3):390–1. https://doi.org/10.1016/j.pcad.2020.03.001 .
Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med. 2020;75:107–8. https://doi.org/10.1016/j.ejim.2020.03.014 .
Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–1. https://doi.org/10.1016/j.cca.2020.03.004 .
Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–8. https://doi.org/10.1016/j.cca.2020.03.022 .
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–95. https://doi.org/10.1111/apa.15270 .
Lupia T, Scabini S, Mornese Pinna S, di Perri G, de Rosa FG, Corcione S. 2019 novel coronavirus (2019-nCoV) outbreak: a new challenge. J Glob Antimicrob Resist. 2020;21:22–7. https://doi.org/10.1016/j.jgar.2020.02.021 .
Marasinghe, K.M., A systematic review investigating the effectiveness of face mask use in limiting the spread of COVID-19 among medically not diagnosed individuals: shedding light on current recommendations provided to individuals not medically diagnosed with COVID-19. Research Square. Preprint article. doi : https://doi.org/10.21203/rs.3.rs-16701/v1 . 2020 .
Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55(5):586–92. https://doi.org/10.1002/uog.22014 .
Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JIP, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9(3):623.
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. https://doi.org/10.1016/j.tmaid.2020.101623 .
Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87–93. https://doi.org/10.2214/AJR.20.23034 .
Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020;92(6):612–7. https://doi.org/10.1002/jmv.25735 .
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. https://doi.org/10.1016/j.ijid.2020.03.017 .
Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur J Clin Investig. 2020;50(3):e13209. https://doi.org/10.1111/eci.13209 .
Article CAS Google Scholar
Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol Taiwanica. 2006;15(1):26–8.
Google Scholar
Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–7. https://doi.org/10.1097/01.mlg.0000249922.37381.1e .
Rajgor DD, Lee MH, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776–7. https://doi.org/10.1016/S1473-3099(20)30244-9 .
Wolkewitz M, Puljak L. Methodological challenges of analysing COVID-19 data during the pandemic. BMC Med Res Methodol. 2020;20(1):81. https://doi.org/10.1186/s12874-020-00972-6 .
Rombey T, Lochner V, Puljak L, Könsgen N, Mathes T, Pieper D. Epidemiology and reporting characteristics of non-Cochrane updates of systematic reviews: a cross-sectional study. Res Synth Methods. 2020;11(3):471–83. https://doi.org/10.1002/jrsm.1409 .
Runjic E, Rombey T, Pieper D, Puljak L. Half of systematic reviews about pain registered in PROSPERO were not published and the majority had inaccurate status. J Clin Epidemiol. 2019;116:114–21. https://doi.org/10.1016/j.jclinepi.2019.08.010 .
Runjic E, Behmen D, Pieper D, Mathes T, Tricco AC, Moher D, et al. Following Cochrane review protocols to completion 10 years later: a retrospective cohort study and author survey. J Clin Epidemiol. 2019;111:41–8. https://doi.org/10.1016/j.jclinepi.2019.03.006 .
Tricco AC, Antony J, Zarin W, Strifler L, Ghassemi M, Ivory J, et al. A scoping review of rapid review methods. BMC Med. 2015;13(1):224. https://doi.org/10.1186/s12916-015-0465-6 .
COVID-19 Rapid Reviews: Cochrane’s response so far. Available at: https://training.cochrane.org/resource/covid-19-rapid-reviews-cochrane-response-so-far . Accessed 1 June 2021.
Cochrane. Living systematic reviews. Available at: https://community.cochrane.org/review-production/production-resources/living-systematic-reviews . Accessed 1 June 2021.
Millard T, Synnot A, Elliott J, Green S, McDonald S, Turner T. Feasibility and acceptability of living systematic reviews: results from a mixed-methods evaluation. Syst Rev. 2019;8(1):325. https://doi.org/10.1186/s13643-019-1248-5 .
Babic A, Poklepovic Pericic T, Pieper D, Puljak L. How to decide whether a systematic review is stable and not in need of updating: analysis of Cochrane reviews. Res Synth Methods. 2020;11(6):884–90. https://doi.org/10.1002/jrsm.1451 .
Lovato A, Rossettini G, de Filippis C. Sore throat in COVID-19: comment on “clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis”. J Med Virol. 2020;92(7):714–5. https://doi.org/10.1002/jmv.25815 .
Leung C. Comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1431–2. https://doi.org/10.1002/jmv.25912 .
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. Response to Char’s comment: comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1433. https://doi.org/10.1002/jmv.25924 .
Download references
Acknowledgments
We thank Catherine Henderson DPhil from Swanscoe Communications for pro bono medical writing and editing support. We acknowledge support from the Covidence Team, specifically Anneliese Arno. We thank the whole International Network of Coronavirus Disease 2019 (InterNetCOVID-19) for their commitment and involvement. Members of the InterNetCOVID-19 are listed in Additional file 6 . We thank Pavel Cerny and Roger Crosthwaite for guiding the team supervisor (IJBN) on human resources management.
This research received no external funding.
Author information
Authors and affiliations.
University Hospital and School of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
Israel Júnior Borges do Nascimento & Milena Soriano Marcolino
Medical College of Wisconsin, Milwaukee, WI, USA
Israel Júnior Borges do Nascimento
Helene Fuld Health Trust National Institute for Evidence-based Practice in Nursing and Healthcare, College of Nursing, The Ohio State University, Columbus, OH, USA
Dónal P. O’Mathúna
School of Nursing, Psychotherapy and Community Health, Dublin City University, Dublin, Ireland
Department of Anesthesiology, Intensive Care and Pain Medicine, University of Münster, Münster, Germany
Thilo Caspar von Groote
Department of Sport and Health Science, Technische Universität München, Munich, Germany
Hebatullah Mohamed Abdulazeem
School of Health Sciences, Faculty of Health and Medicine, The University of Newcastle, Callaghan, Australia
Ishanka Weerasekara
Department of Physiotherapy, Faculty of Allied Health Sciences, University of Peradeniya, Peradeniya, Sri Lanka
Cochrane Croatia, University of Split, School of Medicine, Split, Croatia
Ana Marusic, Irena Zakarija-Grkovic & Tina Poklepovic Pericic
Center for Evidence-Based Medicine and Health Care, Catholic University of Croatia, Ilica 242, 10000, Zagreb, Croatia
Livia Puljak
Cochrane Brazil, Evidence-Based Health Program, Universidade Federal de São Paulo, São Paulo, Brazil
Vinicius Tassoni Civile & Alvaro Nagib Atallah
Yorkville University, Fredericton, New Brunswick, Canada
Santino Filoso
Laboratory for Industrial and Applied Mathematics (LIAM), Department of Mathematics and Statistics, York University, Toronto, Ontario, Canada
Nicola Luigi Bragazzi
You can also search for this author in PubMed Google Scholar
Contributions
IJBN conceived the research idea and worked as a project coordinator. DPOM, TCVG, HMA, IW, AM, LP, VTC, IZG, TPP, ANA, SF, NLB and MSM were involved in data curation, formal analysis, investigation, methodology, and initial draft writing. All authors revised the manuscript critically for the content. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Livia Puljak .
Ethics declarations
Ethics approval and consent to participate.
Not required as data was based on published studies.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix 1..
Search strategies used in the study.
Additional file 2: Appendix 2.
Adjusted scoring of AMSTAR 2 used in this study for systematic reviews of studies that did not analyze interventions.
Additional file 3: Appendix 3.
List of excluded studies, with reasons.
Additional file 4: Appendix 4.
Table of overlapping studies, containing the list of primary studies included, their visual overlap in individual systematic reviews, and the number in how many reviews each primary study was included.
Additional file 5: Appendix 5.
A detailed explanation of AMSTAR scoring for each item in each review.
Additional file 6: Appendix 6.
List of members and affiliates of International Network of Coronavirus Disease 2019 (InterNetCOVID-19).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Borges do Nascimento, I.J., O’Mathúna, D.P., von Groote, T.C. et al. Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews. BMC Infect Dis 21 , 525 (2021). https://doi.org/10.1186/s12879-021-06214-4
Download citation
Received : 12 April 2020
Accepted : 19 May 2021
Published : 04 June 2021
DOI : https://doi.org/10.1186/s12879-021-06214-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Coronavirus
- Evidence-based medicine
- Infectious diseases
BMC Infectious Diseases
ISSN: 1471-2334
- General enquiries: [email protected]
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
The COVID-19 research landscape
Measuring topics and collaborations using scientific literature.
Editor(s): Mittal., Vinay
a Institute of Medical Information, Chinese Academy of Medical Sciences
b Digital China Health Technologies Co. Ltd., Beijing, China.
∗Correspondence: Na Hong, Digital China Health Technologies Co. Ltd., Beijing 100080, China (e-mail: [email protected] ).
Abbreviations: ACE2 = Angiotensin Converting Enzyme 2, COVID-19 = Coronavirus Disease 2019, MeSH = Medical Subject Headings, MTI = Medical Text Indexer, SARS-COV-2 = severe acute respiratory syndrome coronavirus 2, VBA = Visual Basic for Applications.
How to cite this article: Wang J, Hong N. The COVID-19 research landscape: Measuring topics and collaborations using scientific literature. Medicine . 2020;99:43(e22849).
The authors report no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This is an open access article distributed under the terms of the Creative Commons Attribution-Non Commercial License 4.0 (CCBY-NC), where it is permissible to download, share, remix, transform, and buildup the work provided it is properly cited. The work cannot be used commercially without permission from the journal. http://creativecommons.org/licenses/by-nc/4.0
Objectives:
The Coronavirus Disease 2019 (COVID-19) caused heavy burdens and brought tremendous challenges to global public health. This study aimed to investigate collaboration relationships, research topics, and research trends on COVID-19 using scientific literature.
Method:
COVID-19-related articles published from January 1 to July 1, 2020 were retrieved from PubMed database. A total of 27,370 articles were included. Excel 2010, Medical Text Indexer (MTI), VOSviewer, and D3.js were used to summarize bibliometric features.
Results:
The number of the COVID-19 research publications has been continuously increasing after its break. United States was the most productive and active country for COVID-19 research, with the largest number of publications and collaboration relationships. Huazhong University of Science and Technology from China was the most productive institute on the number of publications, and University of Toronto from Canada ranked as Top 1 institute for global research collaboration. Four key research topics were identified, of which the topic of epidemiology and public health interventions has gathered highest attentions. Topic of virus infection and immunity has been more focused during the early stage of COVID-19 outbreak compared with later stage. The topic popularity of clinical symptoms and diagnosis has been steady.
Conclusions:
Our topic analysis results revealed that the study of drug treatment was insufficient. To achieve critical breakthroughs of this research area, more interdisciplinary, multi-institutional, and global research collaborations are needed.
1 Introduction
A novel coronavirus emerged and caused a rapid spread of phenomena in Wuhan, China, at the end of 2019. In February 11, 2020, the World Health Organization named this disease Coronavirus Disease 2019 (COVID-19). [1] With the global spread of COVID-19, it threatened human lives, caused heavy burdens, and brought tremendous challenges to social development. To support the public health decision-making and scientific countermeasures implementation, researchers around the world were racing to study on the disease transmission, diagnostic tests, treatments, vaccines, among others. With the joint efforts of researchers and clinicians around the world, more and more COVID-19-related articles have been published and the outputs of scientific research are constantly emerging. As of July 1, 2020, PubMed has included 27,370 published articles on COVID-19.
State of the art literature review about COVID-19 demonstrated that most available literature-based studies could be basically divided into 2 kinds. The first kind is systematic reviews or meta-analyses. Most of them focused on a certain specific subfields of COVID-19 research, such as drug therapy, diagnostic methods, or clinical symptoms. For example, Alzghari et al [2] performed a systematic review to investigate the effect of Tocilizumab on COVID-19, and Zhu et al [3] systematically reviewed the CT imaging features of COVID-19 to provide reference for clinical practice. The second kind is the bibliometric analysis which uses quantitative analysis methods to describe literature in a particular research domain. However, some of the bibliometric analysis were targeting at coronavirus, not just severe acute respiratory syndrome coronavirus 2 (SARS-COV-2), for the purpose of providing reference for COVID-19 research, and the time window was usually set for a long retrospective duration. [4–7] For example, Mao et al [7] analyzed coronavirus articles published from 2003 to 2020. Up to the investigation time of this study, there were limited number of bibliometric studies specific to COVID-19 and most of them were found and implemented at early stage of COVID-19 outbreak. [8,9] For example, Lou et al [8] executed a query in PubMed using keyword “COVID-19” and analyzed 183 related articles. Most of these previous literature-based studies of COVID-19 provided a specific review for COVID-19 research progresses or clinical observations; however, the description of a whole picture of COVID-19 scientific research using systematical methods was still insufficient.
Therefore, to answer who, what, where, and when questions of COVID-19 studies, we adopted a hybrid method that integrated multi-approaches, including bibliometrics, topic analysis, collaboration analysis, trends analysis, and visualization, to give a timely and systematic review of COVID-19 literatures. The analysis objectives include countries/regions, institutes, collaboration relationships, research topics, and research trends of COVID-19 studies.
2 Materials and methods
2.1 data source.
The data scope of this study is COVID-19-related articles published from January 1, to July 1, 2020. Since PubMed has served as the primary database for retrieving biomedical literature, it was selected as the only data source. [10] Ethical approval was not required because no human and animal subjects were enrolled.
2.2 Search strategy
The advanced search option was adopted, and the query “((novel coronavirus[Title/Abstract] OR COVID-19[Title/Abstract] OR 2019-nCov[Title/Abstract] OR SARS-Cov-2[Title/Abstract] OR COVID19[Title/Abstract] OR coronavirus disease 2019[Title/Abstract] OR coronavirus disease-19[Title/Abstract]) OR COVID-19[Supplementary Concept]) AND (“2020/01/01”: “2020/07/01”[dp])”was executed on July 1, 2020. In total, 27,370 COVID-19 articles were collected.
2.3 Data collection
All of the retrieved articles were downloaded and saved with PubMed default format. Microsoft Excel 2010 was used to pre-process the data and, in conjunction with Visual Basic for Applications (VBA), to extract analysis objects such as country/region names and institute names. The number of publications of a country is derived by counting the number of publications that contain at least one author's affiliation belongs to this country, and the first affiliation will be selected when an author has more than one affiliations.
2.4 Bibliometric and visualized analysis
MTI (National Library of Medicine, Bethesda, MD), [11] VOSviewer 1.6.15 (Leiden University, Leiden, Netherlands) [12] and D3.js (Mike Bostock, Observable, Inc., San Francisco, CA) [13] were used to carry out bibliometric and visual analysis of the publications. Since Medical Subject Headings (MeSH) represent much richer semantics that author-selected keywords, they were chosen as the object of topic analysis. MTI was used to extract MeSH terms from title and abstract of articles because newly created articles in PubMed will not be indexed with MeSH terms immediately. VOSviewer was used to generate collaborative network of countries/regions/institutes and co-occurrence network of MeSH terms. Finally, D3.js was used to visualize the internal hierarchy and the popularity trend of topics, which identified by MeSH terms co-occurrence clustering.
2.5 Analytical methods

Where Dpro_t is the proportional frequency of the term in the t time window, D_t is the document frequency of the term, that is, the number of publications containing the term. DAll_t is the total number of publications and DAvg is the average number of publications on each time window. Topic popularity is measured by adding up proportional frequency of all the terms in this topic.
3.1 The Scale of COVID-19 publications
The number of COVID-19 research publications has been continuously increasing after its break. According to the growth trend from the view of global to country level, as shown in Figure 1 , United States overtook China Mainland as the largest contributor in publishing COVID-19-related articles in early May 2020. As of July 1, 2020, United States had published 5949 (21.7% of the total) articles, and China Mainland had published 4080 (14.9% of the total) articles in total that are much higher than any of the other countries. The following Italy (10.7%) and UK (8.4%) were also prolific among the top 10 countries ( Table 1 ). In addition, China Mainland had the highest rate of domestic collaboration (79.4%), whereas Australia had the lowest (34.8%) among the top 10 productive countries.

3.2 The collaborative network of countries/regions
Collaboration activities on country/region level were measured based on co-author analysis. As shown in Figure 2 , there were 76 countries/regions involved in COVID-19 research collaboration which divided into 3 clusters.

Cluster 1 (blue color) mainly included United States, China Mainland, Canada, and Australia, which were all ranked as Top 10 productive countries. When measuring the collaboration activities, our study further disclosed that United States and China Mainland played the leading role of the COVID-19 research. These two countries had strong internal co-authorship relations, and at the same time had strong external co-authorship relations with other countries/regions. Cluster 2 (green color) was composed with 27 European countries that included UK, Italy, Germany, and France, among others. There were frequent internal collaboration activities among these European countries. In addition, Cluster 3 (red color) included India, Brazil, and other countries of Asia, Africa, and South America with a relatively low frequency of internal collaboration.
Furthermore, total link strength analysis showed that United States was the most active country with the highest number of collaboration relationships with other countries/regions. United States and China Mainland had the largest number of link strength compared with other countries, with a total of 439 collaboration papers. However, Chinese researchers had mostly co-authored with their domestic collaborators, only 20.6% of the studies were collaborated with international researchers outside China Mainland ( Table 1 ).
3.3 The collaborative network of research institutes
The most productive institutes were located at United States, China Mainland, and Europe. There were 307 institutes that had published >10 articles. Table 2 lists the number of publications and internal collaboration publications for top 10 productive institutes. Huazhong University of Science and Technology (523), Wuhan University (340), and University of California (300) were ranked as Top 3 productive institutes by number of publications. Besides, the BMJ editors published 193 latest news and comments about COVID-19 research with the highest rate of internal collaboration of 100%.

Collaboration network among productive institutes was generated based on co-author analysis. Institutes were clearly separated into 5 clusters as shown in Figure 3 . Cluster 1 (red color) included 96 institutes which were mostly universities and hospitals of United States, as well as 10 universities from Canada, among which University of Toronto ranked as Top 1 institute for global research collaboration with the largest number of total link strength. Besides, University of California and University of Washington were also the collaboration centers with large number of co-authored articles. The universities, hospitals, and research institutes came from China composed Cluster 2 (blue color), from which Huazhong University of Science and Technology and Wuhan University had the largest number of link strength compared with other institutes, with a total of 60 collaboration papers. Furthermore, >100 institutes from Europe composed Cluster 3 (green color) and Cluster 4 (yellow color), of which universities and hospitals from Italy composed Cluster 4 and the remaining institutes composed Cluster 3. According to co-author analysis on these 2 clusters, University College London and University of Oxford were most active on research collaboration with other institutes. In addition, it was interesting to observe that Cluster 5 (purple color) contributed a relatively small volume of publications but was a self-centered research community mainly composed with 8 universities from Iran.

3.4 The identified COVID-19 research topics
To achieve better understanding of what are the researcher's focuses and research progress of COVID-19 with its break timeline, MeSH terms of each article were selected as the observation objects to measure the research topics and topic trends. On the analysis of selected 2000 MeSH terms with their frequency above 10, a MeSH terms co-occurrence network with 584 high-frequency terms were generated, as shown in Figure 4 . The network center nodes are COVID-19, severe acute respiratory syndrome coronavirus 2, and Coronavirus Infections. Four topics about COVID-19 research were obviously identified: epidemiology and public health interventions, virus infection and immunity, clinical symptoms and diagnosis, drug treatments, and clinical studies, as shown in Figure 5 .

3.4.1 Topic I: epidemiology and public health interventions
The research topic of epidemiology and public health interventions had gathered great attentions. It contained 281 of the 584 MeSH terms, indicating that the prevention and control of COVID-19 was the most concerned issue at all the stages of disease break. It mainly contained epidemic transmission dynamics, prevention and control measures and effect analysis at different regional levels (global, national, and urban), [14,15] epidemiological investigation, modeling, and trend prediction from the perspective of public health, [16,17] as well as various personal protective measures (Disinfection, Hand Hygiene, Masks, Personal Protective Equipment, Protective Devices), [18,19] and social prevention and control measures (Airway Management, Mass Screening, Social Distance, Social Isolation). [20] In addition, high attention had been paid to the psychological and mental state (Anxiety, Anxiety Disorders, Depression, Fear, Mental Disorders, Mental Health) of the general public, infected people, and medical workers. [21]
3.4.2 Topic II: virus infection and immunity
A total of 168 MeSH terms were included in this topic, which was mainly for the molecular biology and immunology studies of SARS-CoV-2 for the purpose of detection and prevention. Three subtopics of Topic II were identified based on content analysis. The first subtopic was the research on the pathogenesis of COVID-19 that included the replication process and infection mechanism of SARS-CoV-2 in human cells, with emphasis on the interaction between SARS-CoV-2 and biological enzymes (RNA-directed DNA polymerase, angiotensin-converting enzyme [ACE2], serine endopeptidases). [22,23] The second subtopic was the studies on the etiological detection methods of SARS-CoV-2 and the most important methods involved were real-time polymerase chain reaction and reverse transcriptase polymerase chain reaction (PCR). [24,25] In addition, COVID-19 vaccine development with the aim of inducing immune response composed the third subtopic. [26,27]
3.4.3 Topic III: clinical symptoms and diagnosis
A total of 111 MeSH terms were included in Topic III, which mainly covered clinical symptoms of COVID-19 patients and various testing methods used for diagnosis. The clinical symptoms (or complications) of COVID-19 mentioned in the literature mainly included: abdominal pain, cough, diarrhea, dyspnea, fatigue, fever, headache, leukopenia, lymphopenia, myalgia, nausea, pharyngitis, pleural effusion, pneumonia, pulmonary embolism, respiratory distress syndrome, respiratory insufficiency, vomiting, among others. [28,29] The diagnostic methods, mostly discussed in the literature, were routine blood tests (alanine transaminase, aspartate aminotransferases, biomarkers, C-reactive protein, leukocyte count, l -lactate dehydrogenase, lymphocyte count, neutrophils, platelet count) and imaging examinations (radiography, tomography, x-rays). [30]
3.4.4 Topic IV: drug treatments and clinical studies
Topic IV contained 24 MeSH terms, which was the smallest topic. The research content in this topic was mainly in vivo and in vitro trials of multiple drugs and their combinations for the purpose of treating COVID-19. The studied drugs involved antibacterial/antiviral drugs (azithromycin, favipiravir, lopinavir, remdesivir, ribavirin, ritonavir), antimalarials, and rheumatoid arthritis drugs (chloroquine, hydroxychloroquine, tocilizumab) among others. Because of the difference of clinical endpoint and experimental design, the trials results obtained so far are not consistent. For example, some researchers conclude that remdesivir can be used as potent drugs against COVID-19 [31] ; however, some studies show that remdesivir cannot significantly improve the symptoms of patients with severe COVID-19. [32] Chloroquine and hydroxychloroquine are in a similar situation to remdesivir. [33,34] Therefore, there is still no widely accepted standard on specific drugs or the best drug treatment options of COVID-19. [35–37]
3.5 Topic popularities and evolvements about COVID-19 research
Topic popularity of the above 4 COVID-19 topics was measured by using proportional frequency equation in Section 2, and the measured results, as shown in Figure 6 , were consistent with manually validation results by reviewing literature. According to trend analysis, the topic of epidemiology and public health interventions has gathered great attentions and continuously with high popularity. The characteristics of SARS-CoV-2, such as biological structure, genetic sequence, and infection mechanism, have been well studied, and beyond this, consensus has been reached on COVID-19 clinical symptoms and diagnostic methods.

On the topic tracking analysis of epidemiology and public health interventions, we found that most of the early studies and reports were mainly focus on China's epidemic prevention and control. [38,39] By implementing a series of preventive control and medical treatment measures, the pandemic in China had been effectively contained, but the number of confirmed cases outside China continued to increase, as did the corresponding research on epidemiology and public health interventions, which was consistent with the continuously high popularity trending curve of this topic (blue curve), as displayed in Figure 6 .
For virus infection and immunity study, the topic popularity decreased since early of February 2020. As studying the etiological characteristics of a novel virus, such as biological structure, genetic sequence, and infection mechanism, is the key to pandemic prevention and control, the trend curve of Topic II was in the highest position in the pre-outbreak period (January 2020). With the joint efforts of scientists around the world, substantial progress had been achieved in the understanding of SARS-CoV-2. For example, the genetic sequencing of SARS-CoV-2 was performed by Chinese scientists on January 7, 2020 and the results were timely shared with the WHO on January 12, 2020. Furthermore, the infection mechanism of SARS-CoV-2, especially its relationship with ACE2 was identified, and specific diagnostic PCR tests were produced. [40,41] The above achievements were mainly completed in January and February 2020, starting from February, the trend curve of Topic II gradually declined. However, the curve will remain at a high level because more and more attentions have been paid to vaccine-related research. According to literature reports, there are more than 100 candidate vaccine projects targeting COVID-19 worldwide, and some of them have entered clinical trials. [42,43]
With the continuous increase of confirmed and treated cases, clinicians achieved deeper understanding about COVID-19. Since March 2020, there has been a global consensus on the symptoms and diagnostic criteria for COVID-19. [28,44] In addition, the seventh and final edition of “Diagnosis and Treatment Protocol of COVID-19,” issued by the National Health Commission of the PRC, was also released on March 3, 2020. [45] As a result, the trend curve of Topic III starts to smooth out since March 2020 ( Fig. 6 ).
Although lopinavir/ritonavir was recommended as antiviral drug by the first edition of “Diagnosis and Treatment Protocol of COVID-19” on January 16, 2020 at the beginning of the pandemic, the widespread interest in using antiviral drugs to treat COVID-19 began with a report of the first diagnosed patient who benefit from remdesivir in United States, which was published in NEJM on January 31, 2020. [46] Therefore, the trend curve of Topic IV in Figure 6 has risen slightly since February 2020. However, the minimal topic size and low trend curve suggest that drug therapy remains the weak point in the response to COVID-19.
4 Discussion and conclusion
The number of COVID-19 publications has been growing dramatically since March 2020. According to our search strategy, as of the submission of this manuscript (July 13, 2020), the number of COVID-19 publications has exceeded 30,000. Given that COVID-19 pandemic has not been well contained at the global level, relevant research will continue to be carried out and the number of publications will increase accordingly. The methodology in this study can be easily implemented to analyze the future research status of COVID-19, or even applied to other fields.
Although United States and China were the most productive countries, they were not in the identical situation. Since the initial outbreak was in China, Chinese scholars quickly carried out a series of studies and published numerous articles in the early stages of the epidemic. However, Chinese scholars tend to collaborate with domestic scholars rather than aboard. Unlike China, United States has seen a significant increase in the number of publications since April 2020, and has quickly occupied the highest level of participation in global collaboration due to its strong scientific research strength and influence.
Collaboration at the institutional level has obvious geographical characteristics, especially the frequent internal collaborations among institutes located in China, as well as United States. For example, Huazhong University of Science and Technology and Wuhan University, which ranked first and second by the number of publications, co-authored a total of 60 articles, making up the most productive institute pair. Both universities are located in Wuhan and their affiliated hospitals, such as Tongji Hospital, Union Hospital, and Renmin Hospital, are major hospitals for treating COVID-19 patients. The front-line clinical medical workers in those hospitals have conducted a lot of research on virus detection, clinical diagnosis and treatment while fighting against the epidemic.
COVID-19 research topics are continuously evolving with their publication timeline, measuring these changes will help researchers and scientific policy makers understanding the status of COVID-19 research. As indicated by the trend curves of topic popularity, the prevention and control of COVID-19 remains the most important issue at present, and drug therapy remains the weak point in the response to COVID-19. In addition, more support should be given to vaccine research and development, because vaccines are the ultimate solution to the epidemic. [5]
This study provided an overall investigation of COVID-19 scientific progresses using multiple qualitative and quantitative analysis methods. The collaboration status of COVID-19 research at national and institutional levels was disclosed and 4 topics (epidemiology and public health interventions, virus infection and immunity, clinical symptoms and diagnosis, drug treatments, and clinical studies) were identified and interpreted. Our topic analysis results revealed that the study of drug treatment was insufficient. To achieve critical breakthroughs of this research area, more interdisciplinary, multi-institutional, and global research collaborations are needed.
4.1 Strengths and limitations
Publications on COVID-19 research were retrieved from PubMed, and the collaboration status and research trends of COVID-19 were measured via bibliometric and visualized analysis, which was considered to be relatively objective and comprehensive. Moreover, well curated MeSH terms were used as the object of topic analysis in this study, compared with author-selected keywords which were usually chosen by existing COVID-19-related bibliometric analysis. [4–7] Due to the limited number and randomness of author-selected keywords, the derived results, especially the co-occurrence analysis results, cannot reflect the real status of the COVID-19 research. Our MeSH terms-based methodology could better disclose the research topics and trends of COVID-19. However, limitations also exist in our research. On the one hand, PubMed was selected as the only data source, so some articles only indexed in other databases such as Web of Science and Scopus might be left out. On the other hand, for the sparisity reason of citation network of published COVID-19 articles, citation analysis has not been adopted in this study. In the future, studies based on citation analysis, such as identification of influential authors and highly-cited articles, will be conducted and included in our further analysis.
Author contributions
Conceptualization, N.H.; Data curation, J.W.; Software, J.W. and N.H.; Visualization, J.W. and N.H.; Writing—original draft, J.W. and N.H.; Writing—review & editing, J.W. and N.H. All authors have read and agreed to the published version of the manuscript.
Conceptualization: Na Hong.
Data curation: Junhui Wang.
Software: Junhui Wang, Na Hong.
Visualization: Junhui Wang, Na Hong.
Writing – original draft: Junhui Wang, Na Hong.
Writing – review & editing: Junhui Wang, Na Hong.
- Cited Here |
- Google Scholar
bibliometrics; collaboration analysis; COVID-19; topic analysis; trends analysis
- + Favorites
- View in Gallery
- Fact sheets
- Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- Endometriosis
- Excessive heat
- Mental disorders
- Polycystic ovary syndrome
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Coronavirus disease (COVID-19) /
Global research on coronavirus disease (COVID-19)
Section navigation.
- WHO COVID-19 Solidarity Therapeutics Trial
- "Solidarity II" global serologic study for COVID-19
- Accelerating a safe and effective COVID-19 vaccine
- Solidarity Trial Vaccines
WHO is bringing the world’s scientists and global health professionals together to accelerate the research and development process, and develop new norms and standards to contain the spread of the coronavirus pandemic and help care for those affected.
The R&D Blueprint has been activated to accelerate diagnostics, vaccines and therapeutics for this novel coronavirus.
The solidarity of all countries will be essential to ensure equitable access to COVID-19 health products.
WHO COVID-19 Research Database
The WHO COVID-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). It contained citations with abstracts to scientific articles, reports, books, preprints, and clinical trials on COVID-19 and related literature. The WHO Covid-19 Research Database was maintained by the WHO Library & Digital Information Networks and was funded by COVID-19 emergency funds. The database was built by BIREME, the Specialized Center of PAHO/AMRO. Its content spanned the time period March 2020 to June 2023. It has now been archived, and no longer searchable since January 2024.

NIH's Strategic Response to COVID-19
Explore the impact of covid-19 research.
Home Test to Treat Program Expands Nationwide
Shining a Light on Long COVID Brain Fog
Common Cold Virus May Increase Risk for Long COVID
SARS-CoV-2 Infection May Increase Risk of Heart Disease, Stroke
Severe COVID-19 May Cause Long-Term Immune System Changes
SARS-CoV-2 Antibodies From Vaccination During Pregnancy May Transfer to Fetuses
COVID-19 Datasets for Researchers
Find COVID-19 datasets, data tools, and publications to use in research.
- High Contrast
- Increase Font
- Decrease Font
- Default Font
- Turn Off Animations
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- NATURE PODCAST
- 17 December 2020
Coronapod: The big COVID research papers of 2020
- Benjamin Thompson ,
- Noah Baker &
- Traci Watson
You can also search for this author in PubMed Google Scholar
Benjamin Thompson, Noah Baker and Traci Watson discuss some of 2020's most significant coronavirus research papers.
In the final Coronapod of 2020, we dive into the scientific literature to reflect on the COVID-19 pandemic. Researchers have discovered so much about SARS-CoV-2 – information that has been vital for public health responses and the rapid development of effective vaccines. But we also look forward to 2021, and the critical questions that remain to be answered about the pandemic.
Papers discussed
A Novel Coronavirus from Patients with Pneumonia in China, 2019 - New England Journal of Medicine, 24 January
Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China - The Lancet , 24 January
A pneumonia outbreak associated with a new coronavirus of probable bat origin - Nature , 3 February
A new coronavirus associated with human respiratory disease in China - Nature , 3 February
Temporal dynamics in viral shedding and transmissibility of COVID-19 - Nature Medicine , 15 April
Spread of SARS-CoV-2 in the Icelandic Population - New England Journal of Medicine , 11 June
High SARS-CoV-2 Attack Rate Following Exposure at a Choir Practice — Skagit County, Washington, March 2020 - Morbidity & Mortality Weekly Report , 15 August
Respiratory virus shedding in exhaled breath and efficacy of face masks - Nature Medicine , 3 April
Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1 - New England Journal of Medicine , 13 April
Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period - Science , 22 May
Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe - Nature, 8 June
The effect of large-scale anti-contagion policies on the COVID-19 pandemic - Nature , 8 June
Retraction—Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis - The Lancet, 20 June
A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19 - New England Journal of Medicine , 3 June
Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19 - JAMA , 2 September
Immunological memory to SARS-CoV-2 assessed for greater than six months after infection - bioRxiv, 16 November
Coronavirus Disease 2019 (COVID-19) Re-infection by a Phylogenetically Distinct Severe Acute Respiratory Syndrome Coronavirus 2 Strain Confirmed by Whole Genome Sequencing - Clinical Infectious Diseases , 25 August
Nature’s COVID research updates – summarising key coronavirus papers as they appear
Never miss an episode: Subscribe to the Nature Podcast on Apple Podcasts , Google Podcasts , Spotify or your favourite podcast app. Head here for the Nature Podcast RSS feed .
doi: https://doi.org/10.1038/d41586-020-03609-2
Related Articles
- Public health

Molecular architecture of coronavirus double-membrane vesicle pore complex
Article 14 AUG 24
Long COVID lung damage linked to immune system response
News 18 JUL 24
Vaccines save lives: how can uptake be increased?
Editorial 09 JUL 24
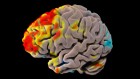
What accelerates brain ageing? This AI ‘brain clock’ points to answers
News 27 AUG 24

Mysterious Oropouche virus is spreading: what you should know
News Q&A 26 AUG 24

Extreme heat is a huge killer — these local approaches can keep people safe
News 22 AUG 24
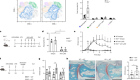
Recognition and control of neutrophil extracellular trap formation by MICL
Amplification of autoimmune organ damage by NKp46-activated ILC1
Article 13 AUG 24
Lecturer/Senior Lecturer at the Dyson School of Design Engineering
About the role: Do you want to change the world for the better, and do you believe this can be done through research and education? If so, grab you...
South Kensington, London (Greater) (GB)
Imperial College London (ICL)
Faculty Positions in Westlake University
Founded in 2018, Westlake University is a new type of non-profit research-oriented university in Hangzhou, China, supported by public a...
Hangzhou, Zhejiang, China
Westlake University
Call for Global Talents, Recruitment Information of Nankai University
Nankai University welcomes global outstanding talents to join for common development.
Tianjin, China
Nankai University
Director for Signature Research Programme in Cancer and Stem Cell Biology, DukeNUS Medical School &
The successful candidate will demonstrate their ability of growing, leading and mentoring faculty as well as developing and operationalising strategy.
Singapore (SG)
DukeNUS Medical School
Scientist / Postdoc (m/f/d): Analysis of Microscopic BIOMedical Images (AMBIOM)
A new project area in the institute is the development of artificial intelligence (AI)
Dortmund, Nordrhein-Westfalen (DE)
Leibniz-Institut für Analytische Wissenschaften – ISAS – e.V.
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- News & Views
- What are the latest...

What are the latest covid drugs and treatments?
- Related content
- Peer review
- Mun-Keat Looi , international features editor
- mlooi{at}bmj.com
Vaccines have taken up much of the spotlight, but where have we got to with covid-19 treatments, asks Mun-Keat Looi —and is there a global standard of care?
What are the best treatments for covid-19?
Written in cooperation with the World Health Organization, The BMJ ’s living systematic review is a meta-analysis comparing the effects of treatments for covid-19, 1 using data from more than 400 randomised clinical trials worldwide.
At the time of writing, it states that systemic corticosteroids (particularly dexamethasone), interleukin-6 receptor antagonists (such as tocilizumab), and Janus kinase inhibitors (such as baricitinib) reduce mortality and have other benefits in patients with severe covid-19, such as reducing the length of hospital stay and the time needed on a ventilator. It also notes that the antivirals molnupiravir (Lagevrio), nirmatrelvir/ritonavir (Paxlovid), and remdesivir (Veklury) have also been shown to be effective against non-severe covid-19.
How has treatment advice changed during the pandemic?
What is considered the “best” treatment continues to change as the pandemic progresses. Where previously the primary aim was to prevent death, the world’s exposure to covid-19 now means that outcomes are increasingly viewed in terms of reducing hospital admissions, disease severity, and perhaps even transmission.
Molnupiravir is a case in point. A study published in December 2022 involving 25 000 people confirmed that oral molnupiravir was associated with reduced viral detection and load, and patients recovered around four days more quickly than those who received usual care. However, it didn’t reduce hospital admissions or deaths among vaccinated high risk patients, which was the primary outcome the trial was set up to test. 2
Chris Butler, clinical director of the University of Oxford’s Primary Care Clinical Trials Unit and co-chief investigator of the study, tells The BMJ that although the trial found no benefit from molnupiravir for its primary outcome (to reduce the likelihood of hospital admission or death), it could have other benefits such as a faster recovery time and reduced follow-up with health services. “This could help to ease the burden on UK health services through the treatment of selected patients at home, during times of high disease burden and pressure on key services,” he says.
Janet Scott, clinical lecturer in infectious diseases at the University of Glasgow, says, “The vaccines are now doing their job and reducing the severity of infection in the high risk groups, so the benefit of molnupiravir is now more about time to recovery than reducing hospitalisation.” Whether the benefits are worth the £577 it costs for the five day course will depend on whether it reduces the number of people who go on to develop long covid, and those results are still being analysed.
“In my view there are currently two major challenges in covid-19 treatment,” adds Scott. “The prevention and treatment of long covid, and the prevention and treatment of acute covid-19 in the highest risk groups including immunosuppressed people. This immunosuppressed group is likely going to require a bespoke study focusing on this issue.”
Does the standard of care differ around the world?
Although there are recommended standard treatments for acute covid-19 in line with WHO’s advice, huge differences in access mean that countries and regions are not consistent.
“The consistency around the globe is probably not what we would want at this point,” says Janet Diaz, who leads clinical management at the WHO Health Emergencies Programme. “Of all the drugs that we have available, the one that’s most consistently available and used globally is corticosteroids—what we use for patients who have severe or critical covid-19. But I think for the remainder of the drugs that WHO has recommended—such as interleukin-6 receptor blockers, tocilizumab or baricitinib, and oral antivirals—the availability and access is limited in many low and middle income countries, and that has unfortunately probably impacted their use.”
There are many reasons behind this, but the upshot is that with limited access and supplies the cost becomes a major factor, as governments apply more scrutiny over evidence of efficacy. With antivirals, for instance, it comes down to how much a government has invested in buying up the various licensed therapies (mainly Paxlovid and molnupiravir), says Stephen Griffin, reader at the University of Leeds. He points out that the European Union still hasn’t approved molnupiravir, which shows mixed efficacy data.
Some places are still widely using drugs that have been shown to be ineffective, such as antibiotics and ivermectin—the latter still commonly used in Brazil, for instance. 3 Butler says that this variation in care can be justified to some extent by different vaccination rates, deprivation and nutrition, coinfection with other organisms, and problems in accessing modern antivirals. “But overall, I think there’s a lot of practice that is still not evidence based going on around the world,” he says.
Butler adds, “It’s also really important not to assume that the evidence from small trials done by the pharma company translates into evidence at scale in every other context in every other country, particularly since the phenotype of the illness varies so much: covid is a very different illness when the population is vaccinated and when there’s a different strain around.
“We’ve got to do the research to make sure that we are generating evidence from within the context. We need evidence from the intended use population before we start giving out drugs at scale.”
He cites inhaled budesonide, a steroid, which does have a benefit in terms of recovery and shows a high probability of reducing the need for hospital admission. 4 “That drug is being used in some places, though it wasn’t approved in the UK,” he says. “But it is an option in other places.”
Why don’t we have better data on covid treatments?
“We have few head-to-head trials of medications, or comparisons of different combinations of medications,” says Tari Turner, director of the National Covid-19 Clinical Evidence Taskforce at Monash University in Australia. “As a result, we have a small shopping list of effective drug treatment options, and little reliable information to guide decisions about which drugs should be used first or in which sequence or combination they should be used.”
Griffin says that the development of direct acting drugs was hampered by the initial response to covid-19, which focused on repurposing existing drugs since that was a faster route. “Back in 2020, we had to try and find any antiviral that worked against this virus—that’s why remdesivir and molnupiravir was used, as they had been tried before on different sorts of viruses,” he says. “There was data on things like interferon beta combined with lopinavir and ritonavir [having efficacy] in vitro, and there was a paper that showed favipiravir worked, but not very well.
“Basically, everything that was in a fairly bare antivirals cupboard was thrown at it in cell culture. That was fine at the time, as it identified lots of decent hits. But what they didn’t do was really carry through the validation process particularly well. And we ended up with things like hydroxychloroquine and ivermectin that, rather than repurposed, were mis-purposed.”
Antivirals have become caught up in this confusion because, says Griffin, their pricing in comparison with drugs such as ivermectin means that “some quarters believe that the pharmacy companies are trying to thrust expensive drugs down our throats, rather than use cheap, effective alternatives.” The monopoly of western drug companies—Pfizer with Paxlovid, for instance—hasn’t helped.
However, Diaz says that big pharma is playing its part. She says that the US Food and Drug Administration’s partnership for covid-19 drugs “has a therapeutic pillar, and many partners have been trying to advance on negotiations with manufacturers to have fair, transparent pricing and to secure doses and treatment courses for people in poorer, low middle income countries, and also to increase generic production of products.
“I think next year there will be more generic products on the horizon, which will be associated with better pricing of these drugs—and, I think at that point, more access.”
How might treatment advice change further in the coming months?
The BMJ ’s living systematic review is updated regularly as evidence continues to be published. 1 For instance, in December 2022 the Remap-Cap study of long term (180 day) outcomes in critically ill patients with covid-19 found that the benefit of interleukin-6 receptor antagonists persisted at six months. 5 Martin Landray, professor of medicine and epidemiology at Oxford Population Health, University of Oxford, says that while the results raised the possibility that antiplatelet treatment in patients with severe covid-19 would reduce long term mortality, this was not “conclusive.”
“It would be wise to wait for the results of the [10 times larger] study of aspirin in the Recovery trial,” he said. “These results, including around 18 months of follow-up, should be available early in 2023, along with the results for four treatments that have previously been shown to reduce 28 day mortality: dexamethasone, tocilizumab [an interleukin-6 receptor antagonist], baricitinib, and monoclonal antibody treatment.”
Do you have a “Covid Unanswered Question”? Email mlooi{at}bmj.com , and we’ll try to cover it in a future instalment.
Competing interests: None.
Provenance and peer review: Commissioned; externally peer reviewed.
This article is made freely available for personal use in accordance with BMJ's website terms and conditions for the duration of the covid-19 pandemic or until otherwise determined by BMJ. You may download and print the article for any lawful, non-commercial purpose (including text and data mining) provided that all copyright notices and trade marks are retained.
- Siemieniuk RAC ,
- Bartoszko JJ ,
- Zeraatkar D ,
- Gbinigie O ,
- Hentschke-Lopes M ,
- Botton MR ,
- Freitas M ,
- Mancuso ACB ,
- Higgins AM ,
- Lorenzi E ,
- Writing Committee for the REMAP-CAP Investigators
COVID-19 Research
Stanford Medicine scientists have launched dozens of research projects as part of the global response to COVID-19. Some aim to prevent, diagnose and treat the disease; others aim to understand how it spreads and how people’s immune systems respond to it.
Below is a curated selection, including summaries, of the projects.
To participate in research , browse COVID-19 studies . Our research registry also connects people like you with teams conducting research to make advances in health care. If you are eligible for a study, researchers may contact you to provide additional details on how to participate.
By participating in clinical research, you help accelerate medical science by providing valuable insights into potential treatments and methons of prevention.
Stanford COVID-19 Study Directory Stanford Medicine Research Registry
To improve our ability to determine who has COVID-19 and treat those infected.
Transmission
To better prevent and understand the transmission of the coronavirus.
Vaccination and Treatment
To improve our ability to prevent COVID-19 and treat those infected.
Epidemiology
To better understand how the coronavirus is spreading.
Data Science and Modeling
To better predict medical, fiscal and resource-related outcomes of the COVID-19 pandemic.
To better understand immune responses to the coronavirus.
Cardiovascular
To better understand the way the virus affects the cardiovascular system.
To better enable the workforce to achieve its goals during the COVID-19 pandemic.
Miscellaneous
A variety of other research projects related to the COVID-19 pandemic.
The list isn’t comprehensive and instead represents a portion of Stanford Medicine research on COVID-19. If you are a Stanford Medicine scientist and would like to see your research included here, please send a note to: [email protected].
The Stanford Institute for Human-Centered Artificial Intelligence has also created a webpage for COVID-19 research collaborations and other opportunities, such as research positions, internships and funding. If you would like to submit an opening please use the following form and they will post it on their website.
Support Stanford Medicine’s response to COVID-19 by making a gift .
COVID-19 Research Projects

Coronavirus Disease 2019 (COVID-19)
- Author: David J Cennimo, MD, FAAP, FACP, FIDSA, AAHIVS; Chief Editor: Michael Stuart Bronze, MD more...
- Sections Coronavirus Disease 2019 (COVID-19)
- Practice Essentials
- Route of Transmission
- Epidemiology
- Physical Examination
- Complications
- Approach Considerations
- Laboratory Studies
- CT Scanning
- Chest Radiography
- Medical Care
- Antiviral Agents
- Immunomodulators and Other Investigational Therapies
- Investigational Antibody-Directed Therapies
- Antithrombotics
- Renin Angiotensin System Blockade and COVID-19
- Diabetes and COVID-19
- Therapies Determined Ineffective
- QT Prolongation with Potential COVID-19 Pharmacotherapies
- Investigational Devices
- Guidelines Summary
- CDC Evaluating and Testing Persons Under Investigation (PUI) for COVID-19 Clinical Guidelines
- CDC Sample Collection and Testing Guidelines for COVID-19
- Guidance for Hospitals on Containing Spread of COVID-19
- American Academy of Pediatrics Guidance on Management of Infants Born to Mothers with COVID-19
- NIH Coronavirus Disease 2019 (COVID-19) Treatment Guidelines
- Infectious Diseases Society of America (IDSA) Management Guidelines
- Thromboembolism Prevention and Treatment
- Medication Summary
- Corticosteroids
- Immunomodulators
- Complement Inhibitors
- COVID-19, Monoclonal Antibodies
- Questions & Answers
- Media Gallery
Coronavirus disease 2019 (COVID-19) is defined as illness caused by a novel coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; formerly called 2019-nCoV), which was first identified amid an outbreak of respiratory illness cases in Wuhan City, Hubei Province, China. [ 1 ] It was initially reported to the WHO on December 31, 2019. On January 30, 2020, the WHO declared the COVID-19 outbreak a global health emergency. [ 2 , 3 ] On March 11, 2020, the WHO declared COVID-19 a global pandemic, its first such designation since declaring H1N1 influenza a pandemic in 2009. [ 4 ]
Illness caused by SARS-CoV-2 was termed COVID-19 by the WHO, the acronym derived from "coronavirus disease 2019." The name was chosen to avoid stigmatizing the virus's origins in terms of populations, geography, or animal associations. [ 5 , 6 ] On February 11, 2020, the Coronavirus Study Group of the International Committee on Taxonomy of Viruses issued a statement announcing an official designation for the novel virus: severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). [ 7 ]
The CDC estimates that SARS-CoV-2 entered the United States in late January or early February 2020, establishing low-level community spread before being noticed. [ 8 ] Since that time, the United States has experienced widespread infections, with over 97.6 million reported cases and over 1,131,000 deaths reported as of June 8, 2023 as reported by the CDC COVID data tracker . According to the CDC, 75% of people who have died of the virus in the United States as of April 5, 2023 are aged 65 years or older . According to the New York Times , the CDC reports that 1 in 100 older Americans has died from the virus. For people younger than 65, the ratio is about 1 in 1,400.
Ending the COVID-19 Public Health Emergency and Continued Surveillance
On May 11, 2023, the federal COVID-19 public health emergency (PHE) ended; however, COVID-19 continues to be a health risk. [ 9 ]
Going forward, hospitalizations are the main data to track COVID-19 trends by geographic regions in the United States. Additionally, the tracker provides emergency department visits of COVID-19, which is an excellent early indicator of spread.
A voluntary network of laboratories that submit test data to the CDC will provide valuable information as another early indicator of spread, along with other respiratory diseases (eg, influenza, RSV).
Additionally, wastewater surveillance provides current levels compared with past levels of infection.
Monitoring continues for new variants to enable testing of vaccines and therapies for efficacy.
Early Pandemic Recommendations
Early in the pandemic (April 3, 2020), the CDC issued a recommendation that the general public, even those without symptoms, should wear face coverings in public settings where social-distancing measures were difficult to maintain to abate the spread of COVID-19. [ 10 ] For high-risk individuals, these recommendations remain to avoid infection from COVID-19 and other infections (eg, RSV, influenza).
The CDC postulated that large numbers of patients could require medical care concurrently, resulting in overloaded public health and healthcare systems and, potentially, elevated rates of hospitalizations and deaths. The CDC advised that nonpharmaceutical interventions (NPIs) are the most important response strategy for delaying viral spread and reducing disease impact. Unfortunately, these concerns were proven accurate.
The feasibility and implications of suppression and mitigation strategies was rigorously analyzed and was encouraged or enforced by many governments to slow or halt viral transmission. Population-wide social distancing plus other interventions (eg, home self-isolation, school and business closures) are strongly advised. These policies were required for periods to avoid rebound viral transmission. [ 11 ]
As the United States experienced surges of COVID-19 infections, the CDC intensified its recommendations for transmission mitigation. They recommended all unvaccinated individuals wear masks in public indoor settings. On the basis of evidence regarding emerging variants of concern (See Virology ), CDC recommended that persons who were fully vaccinated also wear masks in public indoor settings in areas with substantial or high transmission. Fully vaccinated individuals might consider wearing a mask in public indoor areas, regardless of transmission level, if they or someone in their home was immunocompromised, was at increased risk for severe disease, or was unvaccinated (including young children who were ineligible for vaccination). [ 12 ] Recommendations for high risk individuals remains in place to guard against all infections, including COVID-19.
The CDC recommended physical distancing, avoiding nonessential indoor spaces, postponing travel until fully vaccinated, enhanced ventilation, and hand hygiene. [ 13 , 14 ]
According to the CDC, individuals at high risk for infection include persons in areas with ongoing local transmission, healthcare workers caring for patients with COVID-19, close contacts of infected persons, and travelers returning from locations where local spread has been reported.
The CDC has published a summary of evidence of comorbidities that are supported by meta-analysis/systematic review that have a significant association with risk of severe COVID-19 illness. These include the following conditions [ 15 ] :
- Cancer
- Cerebrovascular disease
- Chronic kidney disease
- COPD (chronic obstructive pulmonary disease)
- Diabetes mellitus, type 1 and type 2
- Heart conditions (eg, heart failure, coronary artery disease, cardiomyopathies)
- Immunocompromised state from solid organ transplant
- Obesity (BMI 30 kg/m 2 or greater)
- Pregnancy
- Smoking, current or former
Comorbidities that are supported by mostly observational (eg, cohort, case-control, or cross-sectional) studies include the following [ 15 ] :
- Children with certain underlying conditions
- Down syndrome
- HIV (human immunodeficiency virus)
- Neurologic conditions, including dementia
- Overweight (BMI 25 to less than 30 kg/m 2 )
- Other lung disease (including interstitial lung disease, pulmonary fibrosis, pulmonary hypertension)
- Sickle cell disease
- Solid organ or blood stem cell transplantation
- Substance use disorders
- Use of corticosteroids or other immunosuppressive medications
Comorbidities that are supported by mostly case series, case reports, or, if other study design or the sample size is small include the following [ 15 ] :
- Cystic fibrosis
- Thalassemia
Comorbidities supported by mixed evidence include the following [ 15 ] :
- Asthma
- Hypertension
- Immune deficiencies
- Liver disease
Such individuals should consider the following precautions [ 15 ] :
- Stock up on supplies.
- Avoid close contact with sick people.
- Wash hands often.
- Stay home as much as possible in locations where COVID-19 is spreading.
- Develop a plan in case of illness.
Signs and symptoms
In a study that included 172 patients diagnosed with COVID-19 in January 2022, the estimated median incubation period was 2.8 days (SD, 1.20) among those infected with the Omicron variant (primarily sublineage BA.1). Most infections fell between 1 and 6 days. The distribution was significantly longer in patients with the Alpha variant (4.5 days), and the researchers’ previous study that used contact tracing data estimated a median incubation period of 3.7 days for the Delta variant. [ 16 ]
The following symptoms may indicate COVID-19 [ 17 ] :
- Fever or chills (43-45%)
- Cough (63-83%)
- Shortness of breath or difficulty breathing (45.6%) [ 18 ]
- Fatigue (63%)
- Muscle or body aches (36-63%)
- Headache (34-70%)
- New loss of taste (54.2%) or smell (70.2%)
- Sore throat (52.9%)
- Congestion (67.8%) or runny nose (60.1%)
- Nausea or vomiting (31.6%) [ 19 ]
- Diarrhea (17.8%) [ 19 ]
Other reported symptoms have included the following:
- Sputum production
- Respiratory distress
- Neurologic (eg, headache, altered mentality)
The most common serious manifestation of COVID-19 appears to be pneumonia.
A complete or partial loss of the sense of smell (anosmia) has been reported as a potential history finding in patients eventually diagnosed with COVID-19 [ 20 ] ; however, rates of smell or taste dysruption have decreased as the pandemic has progressed. A study of 616,318 patients with COVID-19 found that 3431 had an associated disturbance in smell or taste; of those, the odds ratios were 0.50 among those infected with the Alpha variant; 0.44 among those infected with Delta; and 0.17 among those infected with Omicron (December 27, 2021–February 7, 2022). [ 21 ]
COVID-19 should be considered a possibility (1) in patients with respiratory tract symptoms and newly onset fever or (2) in patients with severe lower respiratory tract symptoms with no clear cause. Suspicion is increased if such patients have been in an area with community transmission of SARS-CoV-2 or have been in close contact with an individual with confirmed or suspected COVID-19 in the preceding 14 days.
Microbiologic (PCR or antigen) testing is required for definitive diagnosis.
Patients who do not require emergency care are encouraged to contact their healthcare provider by phone. Patients with suspected COVID-19 who present to a healthcare facility should trigger infection-control measures. These patients should be evaluated in a private room with the door closed (an airborne infection isolation room is ideal) and instructed to wear a surgical mask. All other standard contact and airborne precautions should be observed, and treating healthcare personnel should wear eye protection. [ 22 ]
Utilization of programs established by the FDA to allow clinicians access to investigational therapies during the pandemic has been essential. The expanded access (EA) and emergency use authorization (EUA) programs allowed for rapid deployment of potential therapies for investigation and investigational therapies with emerging evidence. A review by Rizk et al describes the role for each of these measures and their importance to providing medical countermeasures in the event of infectious disease and other threats. [ 23 ]
Pharmacologic therapies for COVID-19 disease that have been approved by the FDA or are available by EUA include the following:
- Antiviral agents: Remdesivir, nirmatrelvir/ritonavir, and molnupiravir
- Corticosteroids: Dexamethasone (or equivalent) for patients who require conventional oxygen
- Antithrombotics: Heparin (therapeutic or prophylactic) in hospitalized patients
- Immunomodulators: Baricitinib, tocilizumab, abatacept, anakinra, or infliximab for hospitalized patients requiring oxygen
- Complement inhibitors: Vilobelimab for hospitalized patients requiring oxygen
- Vaccines are available to decrease risk of hospitalization and severe disease
See Treatment and Medication for more detail.
Coronaviruses comprise a vast family of viruses, seven of which are known to cause disease in humans. Some coronaviruses that typically infect animals have evolved to infect humans. SARS-CoV-2 is likely one such virus, postulated to have originated in a large animal and seafood market.
Severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) also are caused by coronaviruses that “jumped” from animals to humans. More than 8000 individuals developed SARS, nearly 800 of whom died of the illness (mortality rate, approximately 10%), before it was controlled in 2003. [ 24 ] MERS continues to resurface in sporadic cases. In late February 2022, a total of 2585 laboratory-confirmed cases of Middle East respiratory syndrome (MERS) were reported worldwide (890 associated deaths; case-fatality ratio, 34.4%). [ 25 ]
The principal mode by which people are infected with SARS-CoV-2 is through exposure to respiratory droplets carrying infectious virus (generally within a space of 6 feet). [ 26 ] Additional methods include contact transmission (eg, shaking hands) and airborne transmission [ 27 ] of droplets that linger in the air over long distances (usually greater than 6 feet). [ 28 , 29 , 30 ] Virus released in respiratory secretions (eg, during coughing, sneezing, talking) can infect other individuals via contact with mucous membranes.
On July 9, 2020, the WHO issued an update stating that airborne transmission may play a role in the spread of COVID-19, particularly involving “super spreader” events in confined spaces such as bars, although they stressed a lack of such evidence in medical settings. Thus, they emphasized the importance of social distancing and masks in prevention. [ 31 ]
"Lack of common terminology" regarding transmission of pathogens through the air
On April 18, 2024, the World Health Organization (WHO) published a global technical consultation report that introduced updated terminology for pathogens that are transmitted through the air, including those that cause COVID-19, influenza, measles, Middle East respiratory syndrome (MERS), severe acute respiratory syndrome (SARS), and tuberculosis. [ 26 ]
During 2021-2023, WHO collaborated with experts from various disciplines and specialties as well as Africa Centres for Disease Control and Prevention; Chinese Center for Disease Control and Prevention; European Centre for Disease Prevention and Control; and United States Centers for Disease Control and Prevention.
Their work “addressed a lack of common terminology” regarding transmission of pathogens through the air that highlighted issues associated with the term “airborne transmission” during the COVID-19 pandemic.
The groups reached consensus on the term ‘infectious respiratory particles’ (IRPs), which describes pathogens of various particle sizes that can be spread through the air at short and long ranges. “Transmission through the air” may be used as an umbrella term to describe spread of IRPs through the air by either airborne transmission or direct deposition modes.
Surface transmission
The virus can also persist on surfaces to varying durations and degrees of infectivity, although this is not believed to be the main route of transmission. [ 29 ] One study found that SARS-CoV-2 remained detectable for up to 72 hours on some surfaces despite decreasing infectivity over time. Notably, the study reported that no viable SARS-CoV-2 was measured after 4 hours on copper or after 24 hours on cardboard. [ 32 ]
In a separate study, Chin and colleagues found the virus was very susceptible to high heat (70°C). At room temperature and moderate (65%) humidity, no infectious virus could be recovered from printing and tissue papers after a 3-hour incubation period or from wood and cloth by day 2. On treated smooth surfaces, infectious virus became undetectable from glass by day 4 and from stainless steel and plastic by day 7. “Strikingly, a detectable level of infectious virus could still be present on the outer layer of a surgical mask on day 7 (~0.1% of the original inoculum),” the researchers write. [ 33 ] Contact with fomites is thought to be less significant than person-to-person spread as a means of transmission. [ 29 ]
Viral shedding
The duration of viral shedding varies significantly and may depend on severity. A 2022 sytematic review and meta-analysis of 20 studies (866 participants) found that after symptom onset, the mean duration of RT-PCR positivity was 27.9 days, whereas the mean duration of isolation of replicant competent virus was 7.3 days. The mean duration of SARS-CoV-2 shedding was 26.5 days among immunocompetent individuals and 36.3 days among immunocompromised individuals. The mean duration of infectivity was 6.3 days among immunocompetent participants and 29.5 days among immunocompromised participants. The longest duration of infectivity was 18 days after symptom onset among immunocompetent patients compared with a maximum of 112 days among immunocompromised patients. [ 34 ]
Among 137 survivors of COVID-19, viral shedding based on testing of oropharyngeal samples ranged from 8 to 37 days, with a median of 20 days. [ 35 ] A different study found that repeated viral RNA tests using nasopharyngeal swabs were negative in 90% of cases among 21 patients with mild illness, whereas results were positive for longer durations in patients with severe COVID-19. [ 36 ] In an evaluation of patients recovering from severe COVID-19, Zhou and colleagues found a median shedding duration of 31 days (range, 18-48 days). [ 37 ] These studies have all used PCR detection as a proxy for viral shedding. The Korean CDC, investigating a cohort of patients who had prolonged PCR positivity, determined that infectious virus was not present. [ 38 ] These findings were incorporated into the CDC guidance on the duration of isolation following COVID-19 infection .
Additionally, patients with profound immunosuppression (eg, following hematopoietic stem-cell transplantation, receiving cellular therapies) may shed viable SARS-CoV-2 for at least 2 months. [ 39 , 40 ]
SARS-CoV-2 has been found in the semen of men with acute infection, as well as in some male patients who have recovered. [ 41 ]
Asymptomatic/presymptomatic SARS-CoV-2 infection and its role in transmission
Oran and Topol published a narrative review of multiple studies on asymptomatic SARS-CoV-2 infection. Such studies and news articles reported rates of asymptomatic infection in several worldwide cohorts, including resident populations from Iceland and Italy, passengers and crew aboard the cruise ship Diamond Princess, homeless persons in Boston and Los Angeles, obstetric patients in New York City, and crew aboard the USS Theodore Roosevelt and Charles de Gaulle aircraft carriers, among several others. Almost half (40-45%) of SARS-CoV-2 infections were asymptomatic. [ 42 ]
Johansson et al from the CDC assessed transmission from presymptomatic, never symptomatic, and symptomatic individuals across various scenarios to determine the infectious period of transmitting SARS-CoV-2. Results from their base case determined 59% of all transmission came from asymptomatic transmission, 35% from presymptomatic individuals and 24% from individuals who never developed symptoms. They estimate at least 50% of new infections came from exposure to individuals with infection, but without symptoms. [ 43 ]
Zou and colleagues followed viral expression through infection via nasal and throat swabs in a small cohort of patients. They found increases in viral loads at the time that the patients became symptomatic. One patient never developed symptoms but was shedding virus beginning at day 7 after presumed infection. [ 44 ]
Coronavirus outbreak and pandemic
As of April 5, 2023, confirmed COVID-19 infections numbered over 762 million individuals worldwide and have resulted in nearly 7 million deaths. [ 45 ] Additionally, the World Health Organization estimates the full death toll associated directly or indirectly with the pandemic is approximately 15 million.
In the United States, more than 1,131,000 deaths have occurred from COVID-19 as of June 8, 2023. [ 46 ] The pandemic caused approximately 375,000 deaths in the United States during 2020. The age-adjusted death rate increased by 15.9% in 2020, making it the third leading cause of death after heart disease and cancer. [ 47 ] During 2021, COVID-19 was associated with 416,893 deaths in the United States, and was again the third leading cause of death . Approximately 75% of deaths in the United States from COVID-19 occurred in individuals aged 65 years and older . [ 48 ]
Racial health disparities
Communities of color have been disproportionally devastated by COVID-19 in the United States and in Europe. Data from New Orleans illustrated these disparities. African Americans represent 31% of the population but 76.9% of the hospitalizations and 70.8% of the deaths. [ 49 ]
A systematic review of 52 studies found racial and ethnic minority groups were at higher risk for COVID-19 infection and hospitalization, confirmed diagnosis, and death. Most of the studies listed factors such as low education level, poverty, poor housing conditions and overcrowded households, low household income, and not speaking the national language in a country as risk factors for COVID-19 incidence/infection, death, and confirmed diagnosis. [ 50 ]
Data suggest the cumulative effects of health disparities are the driving force. The prevalence of chronic (high-risk) medical conditions is higher, and access to healthcare may be less available. Finally, socioeconomic status may decrease the ability to isolate and avoid infection. [ 51 , 52 ]
A prospective cohort study surveyed 170 adult patients who had recovered from COVID-19 1 year prior, during March and April of 2020. The patients participated in a telephone survey during March and April of 2021.
Almost half (79 patients; 46.5%) were of Hispanic ethnicity and 27.1% (46 patients) were African American. Job loss after COVID-19 diagnosis was highest among Hispanics (31/79; 39.2%) and African Americans (16/46; 34.7%). Hispanic individuals (31/79; 39.2%) and African Americans (17/46; 36.9%) also reported the most financial distress after COVID-19 diagnosis.
Compared with Whites, Hispanics were more likely to experience job loss (odds ratio [OR], 4.456), as were African Americans (OR, 4.465). [ 53 ]
Hobbs et al compared MIS-C cases (38 cases) and COVID-19 hospitalizations (74 children) among non-Hispanic Black and White children in a defined catchment 16-county area of Mississippi.
Compared with White children, Black children had an almost fivefold cumulative incidence of MIS-C (40.7 vs 8.3 cases per 100,000 SARS-CoV-2 infections). The cumulative incidence of hospitalization for COVID-19 was almost twice as high in Black children compared with White children (62.3 among Black vs 33.1 among White children per 100,000 SARS-CoV-2 infections). [ 54 ]
Ward et al conducted a retrospective analysis of COVID-19 cases reported to the Alaska Department of Health and Social Services from March 12, 2020-December 31, 2021. The age-adjusted COVID-19 incidence among American Indian (AI)/Alaska Native (AN) individuals (26,583 per 100,000 standard population) was approximately twice the rate among White individuals (11,935).
The age-adjusted COVID-19-associated hospitalization rate (273; rate ratio [RR], 2.72) and the age-adjusted COVID-19 related mortality rate (104; RR, 2.86) among AI/AN individuals were nearly three times those of White study participants. [ 55 ]
The overall age-adjusted death rate increased by 15.9% in 2020. Death rates were highest among non-Hispanic Black persons and non-Hispanic American Indian or Alaska Native persons. [ 47 ]
CDC maintains a COVID-19 Data Tracker for near real time updates.
Young Adults
Outcomes from COVID-19 disease in young adults have been described by Cunningham and colleagues. Of 3200 adults aged 18 to 34 years hospitalized in the United States with COVID-19, 21% were admitted to the ICU, 10% required mechanical ventilation, and 3% died. Comorbidities included obesity (33%; 25% overall were morbidly obese), diabetes (18%), and hypertension (16%). Independent predictors of death or mechanical ventilation included hypertension, male sex, and morbid obesity. Young adults with multiple risk factors for poor outcomes from COVID-19 compared similarly to middle-aged adults without such risk factors. [ 56 ]
A study from South Korea found that older children and adolescents are more likely to transmit SARS CoV-19 to family members than are younger children. The researchers reported that the highest infection rate (18.6%) was in household contacts of patients with COVID-19 aged 10 to 19 years, and the lowest rate (5.3%) was in household contacts of those aged 0 to 9 years. [ 57 ] Teenagers have been the source of clusters of cases, illustrating the role of older children. [ 58 ]
COVID-19 in children
Data continue to emerge regarding the incidence and effects of COVID-19, especially for severe disease. [ 59 ] A severe multisystem inflammatory syndrome linked to COVID-19 infection has been described in children. [ 60 , 61 , 62 , 63 , 64 , 59 ]
The American Academy of Pediatrics (AAP) reports children represent 18.3% of all COVID-19 cases in the 49 states reporting by age; nearly 15 million children have tested positive in the United States since the onset of the pandemic as of October 27, 2022. This represents an overall rate of 19,787 cases per 100,000 children. During the 2-week period of October13-27, 2022, there was less than a 1% increase in the cumulated number of children who tested positive, representing 47,261 new confirmed cases. In the week from October 20-27, 2022, cases in children numbered 14,868 and represented 11.1% of the new weekly cases. [ 65 ]
AAP has issued interim guidance for follow-up care of children following a SARS-CoV-2 infection.
In the United States, a modeling study found one child loses a parent or caregiver for every four COVID-19 associated deaths. From April 1, 2020 through June 30, 2021, more than 140,000 children younger than 18 years in the United States lost a parent, custodial grandparent, or grandparent caregiver who provided the child’s home and basic needs, including love, security, and daily care. Overall, approximately one of 500 US children has experienced COVID-19-associated orphanhood or the death of a grandparent caregiver. Racial, ethnic, and geographic disparities in COVID-19-associated death of caregivers were also seen – children of racial and ethnic minorities accounted for 65% of those who lost a primary caregiver due to the pandemic. [ 66 ]
As of late June 2022, approximately 85,825,048 cases of SARS-CoV-2 infection and 1,007,964 associated deaths have been reported in the United States. [ 67 ] Persons younger than 21 years constitute 26% of the US population. [ 68 ]
Clinical characteristics and outcomes of hospitalized children and adolescents aged 1 month to 21 years with COVID-19 in the New York City area have been described. These observations alerted clinicians to rare, but severe illness in children. Of 67 children who tested positive for COVID-19, 21 (31.3%) were managed as outpatients. Among 46 hospitalized patients, 33 (72%) were admitted to the general pediatric medical unit and 13 (28%) to the pediatric intensive care unit (PICU). Obesity and asthma were highly prevalent, but not significantly associated with PICU admission ( P = .99).
Admission to the pediatric intensive care unit (PICU) was significantly associated with higher C-reactive protein, procalcitonin, and pro-B type natriuretic peptide levels and platelet counts ( P < .05 for all). Patients in the PICU were more likely to require high-flow nasal cannula ( P = .0001) and were more likely to have received remdesivir through compassionate release ( P < .05). Severe sepsis and septic shock syndromes were observed in 7 (53.8%) patients in the PICU. ARDS was observed in 10 (77%) PICU patients, 6 (46.2%) of whom required invasive mechanical ventilation for a median of 9 days. Of the 13 patients in the PICU, 8 (61.5%) were discharged home, and 4 (30.7%) patients remained hospitalized on ventilatory support at Day 14. One patient died after withdrawal of life-sustaining therapy associated with metastatic cancer. [ 69 ]
A case series of 91 children who tested positive for COVID-19 in South Korea showed 22% were asymptomatic during the entire observation period. Among 71 symptomatic cases, 47 children (66%) had unrecognized symptoms before diagnosis, 18 (25%) developed symptoms after diagnosis, and 6 (9%) were diagnosed at the time of symptom onset. Twenty-two children (24%) had lower respiratory tract infections. The mean (SD) duration of the presence of SARS-CoV-2 RNA in upper respiratory samples was 17.6 (6.7) days. These results lend more data to unapparent infections in children that may be associated with silent COVID-19 community transmission. [ 70 ]
An Expert Consensus Statement has been published that discusses diagnosis, treatment, and prevention of COVID-19 in children.
Multisystem inflammatory syndrome in children
Media reports and a health alert from the New York State Department of Health drew initial attention to a newly recognized multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 . Since then, MIS-C cases have been reported across the United States and Europe, and the American Academy of Pediatrics has published interim guidance .
Symptoms are reminiscent of Kawasaki disease , atypical Kawasaki disease, or toxic shock syndrome . All patients had persistent fevers, and more than half had rashes and abdominal complaints. Interestingly, respiratory symptoms were rarely described. Many patients did not have PCR results positive for COVID-19, but many had strong epidemiologic links with close contacts who tested positive. Furthermore, many had antibody tests positive for SARS-CoV-2. These findings suggest recent past infection, and this syndrome may be a postinfectious inflammatory syndrome. The CDC case definition requires:
An individual younger than 21 years presenting with fever ≥38.0°C for ≥24 hours, laboratory evidence of inflammation (including an elevated C-reactive protein [CRP], erythrocyte sedimentation rate [ESR], fibrinogen, procalcitonin, D-dimer, ferritin, lactic acid dehydrogenase [LDH], or interleukin 6 [IL-6], elevated neutrophils, reduced lymphocytes, and low albumin), and evidence of clinically severe illness requiring hospitalization, with multisystem (≥2) organ involvement (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic, or neurological); AND
- No alternative plausible diagnoses; AND
- Positive for current or recent SARS-CoV-2 infection by RT-PCR, serology, or antigen test; or exposure to a suspected or confirmed COVID-19 case within the 4 weeks prior to the onset of symptoms.
Jiang and colleagues reviewed the literature on MIS-C noting the multiple organ system involvement. Unlike classic Kawasaki Disease, the children tended to be older and those of Asian ethnicity tended to be spared. [ 71 ]
A case series compared 539 patients who had MIS-C with 577 children and adolescents who had severe COVID-19. The patients with MIS-C were typically younger (predominantly aged 6-12 years) and more likely to be non-Hispanic Black. They were less likely to have an underlying chronic medical condition, such as obesity. Severe cardiovascular or mucocutaneous involvement was more common in those with MIS-C. Patients with MIS-C also had higher neutrophil to lymphocyte ratios, higher CRP levels, and lower platelet counts than those with severe COVID-19. [ 72 ]
COVID-19 in pregnant individuals and neonates
Pregnant women are at increased risk for severe COVID-19–related illness, and COVID-19 is associated with an increased risk for adverse pregnancy outcomes including intrauterine growth restriction, premature rupture of membranes and preterm delivery, fetal distress, spontaneous abortion, and stillbirth, and maternal and neonatal complications. [ 73 , 74 , 75 ]
The Centers for Disease Control and Prevention reported in November 2021 that maternal COVID-19 infection increases risk for stillbirth compared with women without COVID-19. From March 2020 to September 2021, 8154 stillbirths were reported, affecting 0.65% of births by women without COVID and 1.26% of births by women with COVID, for a relative risk (RR) of 1.90. The magnitude of association was higher during the period of SARS-CoV-2 B.1.617.2 (Delta) variant predominance than during the pre-Delta period. [ 76 ]
A multicenter study involving 16 Spanish hospitals reported outcomes of 242 pregnant patients diagnosed with COVID-19 during the third trimester from March 13 to May 31, 2020. They and their 248 newborns were monitored until the infant was 1 month old. Pregnant patients with COVID-19 who were hospitalized had a higher risk for cesarean birth ( P = 0.027). Newborns whose mothers were hospitalized for COVID-19 infection had a higher risk for premature delivery ( P = 0.006). No infants died and no vertical or horizontal transmission was detected. Exclusive breastfeeding was reported for 41.7% of infants at discharge and 40.4% at 1 month. [ 77 ]
A cohort study of pregnant patients (n = 64) with severe or critical COVID-19 disease hospitalized at 12 US institutions between March 5, 2020, and April 20, 2020 has been published. At the time of the study, most (81%) received hydroxychloroquine; 7% of those with severe disease and 65% with critical disease received remdesivir. All of those with critical disease received either prophylactic or therapeutic anticoagulation. One case of maternal cardiac arrest occurred, but there were no cases of cardiomyopathy or death. Half (n = 32) delivered during their hospitalization (34% severe group; 85% critical group). Additionally, 88% with critical disease delivered preterm during their disease course, with 16 of 17 (94%) pregnant women giving birth through cesarean delivery. Overall, 15 of 20 (75%) with critical disease delivered preterm. There were no stillbirths or neonatal deaths or cases of vertical transmission. [ 78 ]
Adhikari and colleagues published a cohort study evaluating 252 pregnant patients with COVID-19 in Texas. Maternal illness at initial presentation was asymptomatic or mild in 95% of them, and 3% developed severe or critical illness. Compared with COVID negative pregnancies, there was no difference in the composite primary outcome of preterm birth, preeclampsia with severe features, or cesarean delivery for abnormal fetal heart rate. Early neonatal SARS-CoV-2 infection occurred in six of 188 tested infants,(3%) primarily born to asymptomatic or mildly symptomatic mothers. There were no placental pathologic differences by illness severity. [ 79 ]
Breastfeeding
A study by Chambers and colleagues found human milk is unlikely to transmit SARS-CoV-2 from infected mothers to infants. The study included 64 milk samples provided by 18 mothers infected with COVID-19. Samples were collected before and after COVID-19 diagnosis. No replication-competent virus was detectable in any of their milk samples compared with samples of human milk that were experimentally infected with SARS-CoV-2. [ 80 ]
Mothers or birthing parents who have been infected with SARS CoV-2 may have neutralizing antibodies expressed in their milk. In an evaluation of 1-7 milk samples over 2 months from 64 women, 75% contained SARS-CoV-2-specific IgA and 7% persited for at least 2 months. These results support recommendations to continue breastfeeding/chestfeeding with masking during mild-to-moderate maternal COVID-19 illness. [ 81 ]
COVID-19 in patients with HIV
Data for people with HIV and coronavirus are emerging. A multicenter registry has published outcomes for 286 patients with HIV who tested positive for COVID-19 between April 1 and July 1, 2020. Patient characteristics included mean age of 51.4 years, 25.9% were female, and 75.4% were African-American or Hispanic. Most patients (94.3%) were on antiretroviral therapy, 88.7% had HIV virologic suppression, and 80.8% had comorbidities. Within 30 days of positive SARS-CoV-2 testing, 164 (57.3%) patients were hospitalized, and 47 (16.5%) required ICU admission. Mortality rates were 9.4% (27/286) overall, 16.5% (27/164) among those hospitalized, and 51.5% (24/47) among those admitted to an ICU. [ 82 ]
Multiple case series have subsequently been published. Most suggest similar outcomes in patients living with HIV as the general patient population. [ 83 , 84 ] Severe COVID-19 has been seen, however, suggesting that neither antiretroviral therapy of HIV infection are protective. [ 82 , 85 ]
A systematic review and meta-analysis of 43 studies including 692,032 COVID-19 cases found that 9097 (1.3%) were among people living with HIV (PLWH); the global prevalence of PLWH among cases of COVID-19 was 2%, and the highest prevalence occurred in sub-Saharan Africa. The relative risk (RR) for severe COVID-19 in PLWH was significant only in Africa, at 1.14, whereas the RR for mortality was 1.5 worldwide, suggesting that HIV infection may be associated with increased death from COVID-19. [ 86 ]
COVID-19 in clinicians
Among a sample of healthcare providers who routinely cared for patients with COVID-19 in 13 US academic medical centers from February 1, 2020, 6% had evidence of previous SARS-CoV-2 infection, with considerable variation by location that generally correlated with community cumulative incidence. Among participants who had positive test results for SARS-CoV-2 antibodies, approximately one third did not recall any symptoms consistent with an acute viral illness in the preceding months, nearly one half did not suspect that they previously had COVID-19, and approximately two thirds did not have a previous positive test result demonstrating an acute SARS-CoV-2 infection. [ 87 ]
During January to December 2020, the estimated 2020 age-adjusted death rate increased for the first time since 2017, with an increase of 15.9% compared with 2019, from 715.2 to 828.7 deaths per 100,000 population. COVID-19 was the underlying or a contributing cause of 377,883 deaths (91.5 deaths per 100,000). COVID-19 death rates were highest among males, older adults, non-Hispanic American Indian or Alaska Native (AI/AN) persons, and Hispanic persons. Age-adjusted death rates was highest among Black (1105.3) and AI/AN persons (1024). [ 47 ]
Mortality and diabetes
Type 1 and type 2 diabetes are both independently associated with a significant increased odds of in-hospital death with COVID-19. In a nationwide analysis in England of 61,414,470 individuals in the registry alive as of February 19, 2020, 0.4% had a recorded diagnosis of type 1 diabetes and 4.7% of type 2 diabetes. A total of 23,804 COVID-19 deaths in England were reported as of May 11, 2020; one third were in people with diabetes, including 31.4% with type 2 diabetes and 1.5% with type 1 diabetes. Upon multivariate adjustment, the odds of in-hospital COVID-19 death were 3.5 for those with type 1 diabetes and 2.03 for those with type 2 diabetes, compared with deaths among individuals without known diabetes. Further adjustment for cardiovascular comorbidities found the odds ratios were still significantly elevated in both type 1 (2.86) and type 2 (1.81) diabetes. [ 88 ]
The CDC estimates diabetes is associated with a 20% increased odds of in-hospital mortality. [ 47 ]
Hospitalization and cardiometabolic conditions
O’Hearn et al estimate nearly 2 in 3 adults hospitalized for COVID-19 in the United States have associated cardiometabolic conditions including total obesity (BMI 30 kg/m2 or greater), diabetes mellitus, hypertension, and heart failure. [ 89 ]
The full genome of SARS-CoV-2 was first posted by Chinese health authorities soon after the initial detection, facilitating viral characterization and diagnosis. The CDC analyzed the genome from the first US patient who developed the infection on January 24, 2020, concluding that the sequence is nearly identical to the sequences reported by China. [ 1 ] SARS-CoV-2 is a group 2b beta-coronavirus that has at least 70% similarity in genetic sequence to SARS-CoV. [ 90 ] Like MERS-CoV and SARS-CoV, SARS-CoV-2 originated in bats. [ 1 ]
Viral variants emerge when the virus develops one or more mutations that differentiate it from the predominant virus variants circulating in a population. The CDC surveillance of SARS-CoV-2 variants includes US COVID-19 cases caused by variants. The site also includes which mutations are associated with particular variants. The CDC has launched a genomic surveillance dashboard and a website tracking US COVID-19 case trends caused by variants . Researchers are studying how variants may or may not alter the extent of protection by available vaccines. For more information, see the Medscape topic COVID-19 Variants .
Variants of Concern in the United States
As mentioned, viruses such as SARS-CoV-2 are constantly changing. Among the hundreds of variants detected in the first year of the pandemic, the ones that are most concerning are the so-called variants of concern (VOCs) . Researchers are continually studying how variants may or may not alter the extent of protection by avaible vaccines and antibody-directed therapies . As of January 2023, there are no active EUAs for SARS-CoV-2 directed monoclonal antibodies owing to current circulating variants that are non-susceptible.
The Omicron variant (B.1.1.529), initially identified in South Africa, was declared a variant of concern in the United States by the CDC November 30, 2021. This VOC contains several dozen mutations, including a large number in the spike gene, more than previous VOCs. These mutations include several associated with increased transmission. The Omicron variant quickly became dominant in the United States. As of January 8, 2022, it accounted for over 98% of circulating virus, compared with less than 8% on December 11, 2021.
Antiviral agent effectiveness
An in vitro study published in December 2021 indicate that remdesivir, nirmatrelvir, molnupiravir, EIDD-1931, and GS-441524 (oral prodrug of remdesivir) retain their activity against the VOCs Alpha, Beta, Gamma, Delta, and Omicron. [ 91 ]
Vaccine effectiveness
A preprinted, nonpeer reviewed article of routine surveillance data from South Africa suggests the Omicron variant may evade immunity from prior infection. Among 2,796,982 individuals with laboratory-confirmed SARS-CoV-2 who had a positive test result for SARS-CoV-2 at least 90 days before November 27, 2021, there were 35,670 suspected reinfections identified. [ 92 ]
In another preprinted article, neutralization performed with sera from double or triple BNT162b2-vaccinated individuals (6, 0.5 or 3 months after last vaccination/booster) revealed an 11.4-, 37.0- and 24.5-fold reduction, respectively. Sera from double mRNA-1273-vaccinated and additionally BNT162b2-vaccinated individuals (sampled 6 or 0.5 months after last vaccination/booster) showed a 20- and 22.7-fold reduction in the neutralization capacity. [ 93 ]
The Delta variant (B.1.617.2) that was first identified in India became the dominant variant in the United States in mid-July 2021. This variant increases ACE binding and transmissibility. An approximate 6.8-fold decreased neutralization for mRNA vaccines and convalescent plasma was observed with the Delta variant. [ 94 , 95 ] However, a study completed by Public Health England found the BNT162b2 vaccine was only slightly reduced from 93.7% with the B.1.1.7 variant to 88% for the Delta variant 2 weeks after the second dose. [ 96 ] As the Omicron variant transmission increased rapidly in December 2021, the Delta variant now accounts for less than 2% of cases in the United States.
Older Variants Monitored in the United States
Alpha
The CDC tracks variant proportions circulating in the United States and estimates the B.1.1.7 variant (Alpha) that was first detected in the United Kingdom accounted for over 44% of cases from January 2 to March 27, 2021. On April 7, 2021, the CDC announced B.1.1.7 was the dominant strain circulating in the United States. It was the dominant strain until mid-July 2021, when the Delta variant became the dominant strain.
At the same time that the transmission of the wild type virus was dropping, the variant increased, suggesting that the same recommendations (eg, masks, social distancing) may not be enough. The UK variant is also infecting more children (aged 19 years and younger) than the wild type, indicating that it may be more transmissible in children. This has raised concerns because a relative sparing of children has been observed to date. This variant is hypothesized to have a stronger ACE binding than the original variant, which was felt to have trouble infecting younger individuals as they express ACE to a lesser degree. [ 97 ]
The E484K mutation was found initially in the South Africa VOC (B.1.351 [Beta]) and also with the Brazil variants in late 2020, and was observed in the UK variant in early February 2021.
Position 484 and 501 mutations that are both present in the South African variant, and the combination is a concern that immune escape may occur. These mutations, among others, have combined to create the VOC B.1.351. [ 98 ]
The Brazil VOC P.1 (Gamma) was responsible for an enormous second surge of infections. Sabino et al describe resurgence of COVID-19 in Manaus, Brazil in January 2021, despite a high seroprevalence. A study of blood donors indicated that 76% of the population had been infected with SARS-CoV-2 by October 2020. Hospitalizations for COVID-19 in Manaus numbered 3431 in January 1 to 19, 2021 compared with 552 for December 1 to 19, 2020. Hospitalizations had remained stable and low for 7 months prior to December 2020. Several postulated variables regarding this resurgence include waning titers to the original viral lineage and the high prevalence of the P.1 variant, which was first discovered in Manaus. [ 99 ] In addition, researchers are monitoring emergence of a second variant in Brazil, P.2, identified in Rio de Janeiro. As of September 21, 2021, the CDC lists P.2 as a variant being monitored.
Epsilon
VOCs B.1.427 (Epsilon) and B.1.429 (Epsilon) emerged in California. These variants accounted for 2.9% and 6.9% of variants circulating in the United States between January 2 to March 27, 2021. An approximate 20% increase in transmission has been observed with this variant.
CDC. 2019 Novel Coronavirus, Wuhan, China. CDC. Available at https://www.cdc.gov/coronavirus/2019-ncov/about/index.html . Aug. 11, 2022; Accessed: February 20, 2024.
Gallegos A. WHO Declares Public Health Emergency for Novel Coronavirus. Medscape Medical News. Available at https://www.medscape.com/viewarticle/924596 . January 30, 2020; Accessed: February 29, 2024.
Ramzy A, McNeil DG. W.H.O. Declares Global Emergency as Wuhan Coronavirus Spreads. The New York Times. Available at https://nyti.ms/2RER70M . January 30, 2020; Accessed: February 29, 2024.
The New York Times. Coronavirus Live Updates: W.H.O. Declares Pandemic as Number of Infected Countries Grows. The New York Times. Available at https://www.nytimes.com/2020/03/11/world/coronavirus-news.html#link-682e5b06 . March 11, 2020; Accessed: February 29, 2024.
Coronavirus Updates: The Illness Now Has a Name: COVID-19. The New York Times. Available at https://www.nytimes.com/2020/02/11/world/asia/coronavirus-china.html . February 11, 2020; Accessed: February 29, 2024.
WHO Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. Available at https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 . February 11, 2020; Accessed: February 29, 2024.
Gorbalenya AE. Severe acute respiratory syndrome-related coronavirus – The species and its viruses, a statement of the Coronavirus Study Group. Available at https://read.qxmd.com/doi/10.1101/2020.02.07.937862 . February 11, 2020; Accessed: February 29, 2024.
CDC COVID-19 Response Team, Jorden MA, Rudman SL, et al. Evidence for Limited Early Spread of COVID-19 Within the United States, January-February 2020. MMWR. Available at https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments . May 29, 2020; Accessed: February 29, 2024.
End of the Federal COVID-19 Public Health Emergency (PHE) Declaration. CDC Centers for Disease Control and Prevention. Available at https://www.cdc.gov/coronavirus/2019-ncov/your-health/end-of-phe.html . 2023 May 05; Accessed: February 29, 2024.
CDC. Coronavirus Disease 2019 (COVID-19): Recommendations for Cloth Face Covers. Centers for Disease Control and Prevention. Available at https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover.html . April 3, 2020; Accessed: February 29, 2024.
Ferguson NM, Laydon D, Nedjati-Gilani G, Imai N, Ainslie K, Baguelin M, et al. Impact of non-pharmaceutical interventions (NPIs) to reduce COVID-19 mortality and healthcare demand. Imperial College COVID-19 Response Team. Available at https://www.imperial.ac.uk/media/imperial-college/medicine/sph/ide/gida-fellowships/Imperial-College-COVID19-NPI-modelling-16-03-2020.pdf . 2020 Mar 16; Accessed: February 29, 2024.
Christie A, Brooks JT, Hicks LA, et al. Guidance for Implementing COVID-19 Prevention Strategies in the Context of Varying Community Transmission Levels and Vaccination Coverage. MMWR Morb Mortal Wkly Rep . 2021. 70:1044–1047. [Full Text] .
Honein MA, Christie A, Rose DA, et al. Summary of guidance for public health strategies to address high levels of community transmission of SARS-CoV-2 and related deaths, December 2020. MMWR Morb Mortal Wkly Rep . 2020 Dec 04. [Full Text] .
Centers for Disease Control and Prevention. How to Protect Yourself & Others. Centers for Disease Control and Prevention. Available at https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html . November 29, 2021; Accessed: February 29, 2024.
CDC. Underlying medical conditions associated with high risk for severe COVID-19: Information for healthcare providers. Centers for Disease Control and Prevention. Available at https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html . 2021 Mar 29; updated Feb. 9, 2023; Accessed: February 29, 2024.
Tanaka H, Ogata T, Shibata T, Nagai H, Takahashi Y, Kinoshita M, et al. Shorter Incubation Period among COVID-19 Cases with the BA.1 Omicron Variant. Int J Environ Res Public Health . 2022 May 23. 19 (10): [QxMD MEDLINE Link] .
Clinical characteristics of COVID-19. European Centre for Disease Prevention and Control. Available at https://www.ecdc.europa.eu/en/covid-19/latest-evidence/clinical . Updated: 30 May 2023; Accessed: February 20, 2024.
Hentsch L, Cocetta S, Allali G, Santana I, Eason R, Adam E, et al. Breathlessness and COVID-19: A Call for Research. Respiration . 2021. 100 (10):1016-1026. [QxMD MEDLINE Link] .
Zoghi G, Moosavy SH, Yavarian S, HasaniAzad M, Khorrami F, Sharegi Brojeni M, et al. Gastrointestinal implications in COVID-19. BMC Infect Dis . 2021 Nov 4. 21 (1):1135. [QxMD MEDLINE Link] .
Rabin RC. Lost Sense of Smell May Be Peculiar Clue to Coronavirus Infection. The New York Times. Available at https://www.nytimes.com/2020/03/22/health/coronavirus-symptoms-smell-taste.html . March 22, 2020; Accessed: February 29, 2024.
Coelho DH, Reiter ER, French E, Costanzo RM. Decreasing Incidence of Chemosensory Changes by COVID-19 Variant. Otolaryngol Head Neck Surg . 2022 May 3. 1945998221097656. [QxMD MEDLINE Link] .
CDC. 2019 Novel Coronavirus, Wuhan, China: Interim Healthcare Infection Prevention and Control Recommendations for Patients Under Investigation for 2019 Novel Coronavirus. CDC. Available at https://www.cdc.gov/coronavirus/2019-ncov/infection-control.html . January 18, 2020; Accessed: December 1, 2021.
Rizk JG, Forthal DN, Kalantar-Zadeh K, Mehra MR, Lavie CJ, Rizk Y, et al. Expanded Access Programs, compassionate drug use, and Emergency Use Authorizations during the COVID-19 pandemic. Drug Discov Today . 2020 Nov 27. [QxMD MEDLINE Link] . [Full Text] .
Abutaleb Y. How the new coronavirus differs from SARS, measles and Ebola. The Washington Post. Available at https://www.washingtonpost.com/health/how-the-new-coronavirus-differs-from-sars-measles-and-ebola/2020/01/23/aac6bb06-3e1b-11ea-b90d-5652806c3b3a_story.html . January 23, 2020; Accessed: February 20, 2024.
World Health Organization. MERS situation update, February 2022. WHO Regional Office for the Eastern Mediterranean. Available at https://www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html . February 2022; Accessed: May 17, 2022.
WHO. Leading health agencies outline updated terminology for pathogens that transmit through the air. News Release. World Health Organization. Available at https://www.who.int/news/item/18-04-2024-leading-health-agencies-outline-updated-terminology-for-pathogens-that-transmit-through-the-air . April 18, 2024; Accessed: April 25, 2024.
Handiso TB, Jifar MS, Nuriye Hagisso S. Coronavirus's (SARS-CoV-2) airborne transmission. SAGE Open Med . 2022. 10:20503121221094185. [QxMD MEDLINE Link] .
Centers for Disease Control and Prevention. COVID-19 Overview and Infection Prevention and Control Priorities in non-U.S. Healthcare Settings. Centers for Disease Control and Prevention. Available at https://www.cdc.gov/coronavirus/2019-ncov/hcp/non-us-settings/overview/index.html . Updated Oct 27, 2023; Accessed: February 29, 2024.
CDC. Coronavirus Disease 2019: Scientific Brief: SARS-CoV-2 and Potential Airborne Transmission. Centers for Disease Control and Prevention. Centers for Disease Control and Prevention. Available at https://www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-sars-cov-2.html . 2020 Oct 05; Accessed: December 1, 2021.
Morawska L, Milton DK. It is Time to Address Airborne Transmission of COVID-19. Clin Infect Dis . 2020 Jul 6. [QxMD MEDLINE Link] .
Mask use in the context of COVID-19 (1 December 2020). World Health Organization. Available at https://www.who.int/publications/i/item/advice-on-the-use-of-masks-in-the-community-during-home-care-and-in-healthcare-settings-in-the-context-of-the-novel-coronavirus-(2019-ncov)-outbreak . December 1, 2020; Accessed: February 29, 2024.
van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med . 2020 Mar 17. [QxMD MEDLINE Link] .
Chin AWH, Chu JTS, Perera MRA, et al. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe . April 2, 2020. [Full Text] .
Rahmani A, Dini G, Leso V, Montecucco A, Kusznir Vitturi B, Iavicoli I, et al. Duration of SARS-CoV-2 shedding and infectivity in the working age population: a systematic review and meta-analysis. Med Lav . 2022 Apr 26. 113 (2):e2022014. [QxMD MEDLINE Link] .
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet . 2020 Mar 11. [QxMD MEDLINE Link] .
Liu Y, Yan LM, Wan L, Xiang TX, Le A, Liu JM, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis . 2020 Mar 19. [QxMD MEDLINE Link] .
Zhou B, She J, Wang Y, Ma X. The duration of viral shedding of discharged patients with severe COVID-19. Clin Infect Dis . 2020 Apr 17. [QxMD MEDLINE Link] .
Mole B. Recovered COVID-19 patients test positive but not infectious, data finds. Ars Technica. Available at https://arstechnica.com/science/2020/05/feared-reactivation-of-covid-19-infections-disputed-by-new-data/ . May 19, 2020; Accessed: February 29, 2024.
Aydillo T, Gonzalez-Reiche AS, Aslam S, van de Guchte A, Khan Z, Obla A, et al. Shedding of Viable SARS-CoV-2 after Immunosuppressive Therapy for Cancer. N Engl J Med . 2020 Dec 24. 383 (26):2586-2588. [QxMD MEDLINE Link] . [Full Text] .
Baang JH, Smith C, Mirabelli C, Valesano AL, Manthei DM, Bachman M, et al. Prolonged SARS-CoV-2 replication in an immunocompromised patient. J Infect Dis . 2020 Oct 22. [QxMD MEDLINE Link] . [Full Text] .
Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical Characteristics and Results of Semen Tests Among Men With Coronavirus Disease 2019. JAMA Netw Open . 2020 May 1. 3 (5):e208292. [QxMD MEDLINE Link] .
Oran DP, Topol EJ. Prevalence of Asymptomatic SARS-CoV-2 Infection : A Narrative Review. Ann Intern Med . 2020 Sep 1. 173 (5):362-367. [QxMD MEDLINE Link] . [Full Text] .
Johansson MA, Quandelacy TM, Kada S, Prasad PV, Steele M, Brooks JT, et al. SARS-CoV-2 Transmission From People Without COVID-19 Symptoms. JAMA Netw Open . 2021 Jan 4. 4 (1):e2035057. [QxMD MEDLINE Link] . [Full Text] .
Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med . 2020 Feb 19. [QxMD MEDLINE Link] .
WHO Coronavirus Disease (COVID-19) Dashboard. World Health Organization. Available at https://covid19.who.int/ . 2023 Apr 05; Accessed: February 29, 2024.
CDC. Coronavirus Disease 2019 (COVID-19) – Cases in the U.S. Centers for Disease Control and Prevention (CDC). Available at https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days . 2023 Mar 29; Accessed: April 5, 2023.
Ahmad FB, Cisewski JA, Miniño A, Anderson RN. Provisional Mortality Data - United States, 2020. MMWR Morb Mortal Wkly Rep . 2021 Apr 9. 70 (14):519-522. [QxMD MEDLINE Link] . [Full Text] .
Ahmad FB, Cisewski JA, Anderson RN. Provisional Mortality Data - United States, 2021. MMWR Morb Mortal Wkly Rep . 2022 Apr 29. 71 (17):597-600. [QxMD MEDLINE Link] . [Full Text] .
Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and Mortality among Black Patients and White Patients with Covid-19. N Engl J Med . 2020 Jun 25. 382 (26):2534-2543. [QxMD MEDLINE Link] . [Full Text] .
Khanijahani A, Iezadi S, Gholipour K, Azami-Aghdash S, Naghibi D. A systematic review of racial/ethnic and socioeconomic disparities in COVID-19. Int J Equity Health . 2021 Nov 24. 20 (1):248. [QxMD MEDLINE Link] .
Zelner J, Trangucci R, Naraharisetti R, Cao A, Malosh R, Broen K, et al. Racial disparities in COVID-19 mortality are driven by unequal infection risks. Clin Infect Dis . 2020 Nov 21. [QxMD MEDLINE Link] . [Full Text] .
Tai DBG, Shah A, Doubeni CA, Sia IG, Wieland ML. The Disproportionate Impact of COVID-19 on Racial and Ethnic Minorities in the United States. Clin Infect Dis . 2020 Jun 20. [QxMD MEDLINE Link] . [Full Text] .
Millet C, Racoosin E, Narvar S, Horani G, et al. The Racial Divide: A Follow Up Study on Racial Disparity Amongst COVID-19 Survivors in an Urban Community. J Community Hosp Intern Med Perspect . 2022 May 2. 12(3):66-70. [QxMD MEDLINE Link] . [Full Text] .
Hobbs CV, Kim SS, Vemula P, Inagaki K, et al. Active Surveillance With Seroprevalence-based Infection Rates Indicates Racial Disparities With Pediatric SARS-CoV-2 Requiring Hospitalization in Mississippi, March 2020-February 2021. Pediatr Infect Dis J . 2022 Jun 7. [QxMD MEDLINE Link] .
Ward LA, Black KP, Britton CL, Tompkins ML, Provost EM. COVID-19 Cases, Hospitalizations, and Deaths Among American Indian or Alaska Native Persons - Alaska, 2020-2021. MMWR Morb Mortal Wkly Rep . 2022 Jun 3. 71 (22):730-733. [QxMD MEDLINE Link] .
Cunningham JW, Vaduganathan M, Claggett BL, Jering KS, Bhatt AS, Rosenthal N, et al. Clinical Outcomes in Young US Adults Hospitalized With COVID-19. JAMA Intern Med . 2020 Sep 9. [QxMD MEDLINE Link] . [Full Text] .
Park YJ, Choe YJ, Park O, and the, COVID-19 National Emergency Response Center, Epidemiology and Case Management Team. Contact Tracing during Coronavirus Disease Outbreak, South Korea, 2020. Emerg Infect Dis . 2020 Oct. 26 (10):2465-2468. [QxMD MEDLINE Link] . [Full Text] .
Schwartz NG, Moorman AC, Makaretz A, Chang KT, Chu VT, Szablewski CM, et al. Adolescent with COVID-19 as the Source of an Outbreak at a 3-Week Family Gathering - Four States, June-July 2020. MMWR Morb Mortal Wkly Rep . 2020 Oct 9. 69 (40):1457-1459. [QxMD MEDLINE Link] . [Full Text] .
Zhu F, Ang JY. COVID-19 Infection in Children: Diagnosis and Management. Curr Infect Dis Rep . 2022 Apr 11. 1-12. [QxMD MEDLINE Link] .
Waghmare A, Hijano DR. SARS-CoV-2 Infection and COVID-19 in Children. Clin Chest Med . 2023 Jun. 44 (2):359-371. [QxMD MEDLINE Link] .
Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlini S, et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Children and Adolescents: A Systematic Review. JAMA Pediatr . 2020 Apr 22. [QxMD MEDLINE Link] .
Aronoff SC, Hall A, Del Vecchio MT. The Natural History of SARS-Cov-2 Related Multisystem Inflammatory Syndrome in Children (MIS-C): A Systematic Review. J Pediatric Infect Dis Soc . 2020 Sep 14. [QxMD MEDLINE Link] . [Full Text] .
Hoang A, Chorath K, Moreira A, Evans M, Burmeister-Morton F, Burmeister F, et al. COVID-19 in 7780 pediatric patients: A systematic review. EClinicalMedicine . 2020 Jul. 24:100433. [QxMD MEDLINE Link] . [Full Text] .
Ahmed M, Advani S, Moreira A, Zoretic S, Martinez J, Chorath K, et al. Multisystem inflammatory syndrome in children: A systematic review. EClinicalMedicine . 2020 Sep 4. 100527. [QxMD MEDLINE Link] . [Full Text] .
Children and COVID-19: State-Level Data Report. American Academy of Pediatrics. Available at https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/ . 2022 Oct 27; Accessed: November 7, 2022.
Hillis SD, Blenkinsop A, Villaveces A, Annor FB, Liburd L, Massetti GM, et al. COVID-19-Associated Orphanhood and Caregiver Death in the United States. Pediatrics . 2021 Oct 7. [QxMD MEDLINE Link] . [Full Text] .
CDC. COVID Data Tracker. Centers for Disease Control and Prevention. Available at https://covid.cdc.gov/covid-data-tracker/#datatracker-home . May 11, 2023; Accessed: March 6, 2024.
Bixler D, Miller AD, Mattison CP, and the, Pediatric Mortality Investigation Team. SARS-CoV-2-Associated Deaths Among Persons Aged MMWR Morb Mortal Wkly Rep</i>. 2020 Sep 18. 69 (37):1324-1329. [QxMD MEDLINE Link] . [Full Text] .
Chao JY, Derespina KR, Herold BC, Goldman DL, Aldrich M, Weingarten J, et al. Clinical Characteristics and Outcomes of Hospitalized and Critically Ill Children and Adolescents with Coronavirus Disease 2019 at a Tertiary Care Medical Center in New York City. J Pediatr . 2020 Aug. 223:14-19.e2. [QxMD MEDLINE Link] . [Full Text] .
Han MS, Choi EH, Chang SH, Jin BL, Lee EJ, Kim BN, et al. Clinical Characteristics and Viral RNA Detection in Children With Coronavirus Disease 2019 in the Republic of Korea. JAMA Pediatr . 2020 Aug 28. [QxMD MEDLINE Link] . [Full Text] .
Jiang L, Tang K, Levin M, Irfan O, Morris SK, Wilson K, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis . 2020 Nov. 20 (11):e276-e288. [QxMD MEDLINE Link] .
Feldstein LR, Tenforde MW, Friedman KG, and the, Overcoming COVID-19 Investigators. Characteristics and Outcomes of US Children and Adolescents With Multisystem Inflammatory Syndrome in Children (MIS-C) Compared With Severe Acute COVID-19. JAMA . 2021 Feb 24. [QxMD MEDLINE Link] . [Full Text] .
Ko JY, DeSisto CL, Simeone RM, Ellington S, Galang RR, Oduyebo T, et al. Adverse Pregnancy Outcomes, Maternal Complications, and Severe Illness Among US Delivery Hospitalizations With and Without a Coronavirus Disease 2019 (COVID-19) Diagnosis. Clin Infect Dis . 2021 Jul 15. 73 (Suppl 1):S24-S31. [QxMD MEDLINE Link] . [Full Text] .
Wang H, Li N, Sun C, Guo X, Su W, Song Q, et al. The association between pregnancy and COVID-19: A systematic review and meta-analysis. Am J Emerg Med . 2022 Apr 6. 56:188-195. [QxMD MEDLINE Link] .
Ayala-Ramírez P, González M, Escudero C, Quintero-Arciniegas L, Giachini FR, Alves de Freitas R, et al. Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Pregnancy. A Non-systematic Review of Clinical Presentation, Potential Effects of Physiological Adaptations in Pregnancy, and Placental Vascular Alterations. Front Physiol . 2022. 13:785274. [QxMD MEDLINE Link] .
DeSisto CL, Wallace B, Simeone RM, Polen K, Ko JY, Meaney-Delman D, et al. Risk for Stillbirth Among Women With and Without COVID-19 at Delivery Hospitalization - United States, March 2020-September 2021. MMWR Morb Mortal Wkly Rep . 2021 Nov 26. 70 (47):1640-1645. [QxMD MEDLINE Link] . [Full Text] .
Marín Gabriel MA, Reyne Vergeli M, Caserío Carbonero S, Sole L, Carrizosa Molina T, Rivero Calle I, et al. Maternal, Perinatal and Neonatal Outcomes With COVID-19: A Multicenter Study of 242 Pregnancies and Their 248 Infant Newborns During Their First Month of Life. Pediatr Infect Dis J . 2020 Sep 11. [QxMD MEDLINE Link] .
Pierce-Williams RAM, Burd J, Felder L, Khoury R, Bernstein PS, Avila K, et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: a United States cohort study. Am J Obstet Gynecol MFM . 2020 Aug. 2 (3):100134. [QxMD MEDLINE Link] . [Full Text] .
Adhikari EH, Moreno W, Zofkie AC, MacDonald L, McIntire DD, Collins RRJ, et al. Pregnancy Outcomes Among Women With and Without Severe Acute Respiratory Syndrome Coronavirus 2 Infection. JAMA Netw Open . 2020 Nov 2. 3 (11):e2029256. [QxMD MEDLINE Link] .
Chambers C, Krogstad P, Bertrand K, Contreras D, Tobin NH, Bode L, et al. Evaluation for SARS-CoV-2 in Breast Milk From 18 Infected Women. JAMA . 2020 Aug 19. [QxMD MEDLINE Link] . [Full Text] .
Pace RM, Williams JE, Järvinen KM, Meehan CL, Martin MA, Ley SH, et al. Milk From Women Diagnosed With COVID-19 Does Not Contain SARS-CoV-2 RNA but Has Persistent Levels of SARS-CoV-2-Specific IgA Antibodies. Front Immunol . 2021. 12:801797. [QxMD MEDLINE Link] . [Full Text] .
Dandachi D, Geiger G, Montgomery MW, and the, HIV-COVID-19 consortium. Characteristics, Comorbidities, and Outcomes in a Multicenter Registry of Patients with HIV and Coronavirus Disease-19. Clin Infect Dis . 2020 Sep 9. [QxMD MEDLINE Link] . [Full Text] .
Okoh AK, Bishburg E, Grinberg S, Nagarakanti S. COVID-19 Pneumonia in Patients With HIV: A Case Series. J Acquir Immune Defic Syndr . 2020 Sep 1. 85 (1):e4-e5. [QxMD MEDLINE Link] . [Full Text] .
Karmen-Tuohy S, Carlucci PM, Zervou FN, Zacharioudakis IM, Rebick G, Klein E, et al. Outcomes Among HIV-Positive Patients Hospitalized With COVID-19. J Acquir Immune Defic Syndr . 2020 Sep 1. 85 (1):6-10. [QxMD MEDLINE Link] . [Full Text] .
Ho HE, Peluso MJ, Margus C, Matias Lopes JP, He C, Gaisa MM, et al. Clinical outcomes and immunologic characteristics of Covid-19 in people with HIV. J Infect Dis . 2020 Jun 30. [QxMD MEDLINE Link] . [Full Text] .
Oyelade T, Alqahtani JS, Hjazi AM, Li A, Kamila A, Raya RP. Global and Regional Prevalence and Outcomes of COVID-19 in People Living with HIV: A Systematic Review and Meta-Analysis. Trop Med Infect Dis . 2022 Feb 3. 7 (2): [QxMD MEDLINE Link] .
Self WH, Tenforde MW, Stubblefield WB, and the, CDC COVID-19 Response Team IVY Network. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network – 13 academic medical centers, April-June 2020. MMWR Morb Mortal Wkly Rep . 2020 Aug 31. 69: [Full Text] .
Barron E, Bakhai C, Kar P, Weaver A, Bradley D, Ismail H, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol . 2020 Aug 13. [QxMD MEDLINE Link] . [Full Text] .
O'Hearn M, Liu J, Cudhea F, Micha R, Mozaffarian D. Coronavirus Disease 2019 Hospitalizations Attributable to Cardiometabolic Conditions in the United States: A Comparative Risk Assessment Analysis. J Am Heart Assoc . 2021 Feb. 10 (5):e019259. [QxMD MEDLINE Link] . [Full Text] .
Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis . 2020 Jan 14. 91:264-266. [QxMD MEDLINE Link] .
Vangeel L, De Jonghe S, Maes P, Slechten B, Raymenants J, André E, et al. Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 omicron and other variants of concern. bioRxiv . 2021 Dec 28. [Full Text] .
Pulliam JRC, van Schalkwyk C, Govender N, von Gottberg A, Cohen C, Groome MJ, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. medRxiv . 2021 Dec 02. [Full Text] .
Wilhelm A, Widera M, Grikscheit K, Toptan T, Schenk B, Pallas C, et al. Reduced neutralization of SARS-CoV-2 omicron variant by vaccine sera and monoclonal antibodies. medRxiv . 2021 Dec 13. [Full Text] .
Cherian S, Potdar V, Jadhav S, Yadav P, Gupta N, Das M, et al. Convergent evolution of SARS-CoV-2 spike mutations, L452R, E484Q and P681R, in the second wave of COVID-19 in Maharashtra, India. bioRxiv . 2021 May 03. [Full Text] .
Edara VV, Lai L, Sahoo MK, Floyd K, Sibai M, Solis D, et al. Infection and vaccine-induced neutralizing antibody responses to the SARS-CoV-2 B.1.617.1 variant. bioRxiv . 2021 May 10. [Full Text] .
Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med . 2021 Jul 21. [QxMD MEDLINE Link] . [Full Text] .
Volz E, Mishra S, Chand M, Barrett JC, Johnson R, Geidelberg L, et al. Report 42 - Transmission of SARS-CoV-2 Lineage B.1.1.7 in England: Insights from linking epidemiological and genetic data. Imperial College London, MRC Centre for Global Infectious Disease Analysis. Available at https://www.imperial.ac.uk/media/imperial-college/medicine/mrc-gida/2020-12-31-COVID19-Report-42-Preprint-VOC.pdf . 2020 Dec 31; Accessed: December 1, 2021.
Nelson G, Oleksandr B, Spilman P, Niazi K, Rabizadeh S, Soon-Shiong P. Molecular dynamic simulation reveals E484K mutation enhances spike RBD-ACE2 affinity and the combination of E484K, K417N and N401Y mutations (501Y.V2 variant) induces conformational change greater than N401Y mutant alone, potentially resulting in an escape mutant. bioRxiv . 2021 Jan 13. [Full Text] .
Sabino EC, Buss LF, Carvalho MPS, Prete CA, Crispim MAE, Fraiji NA, et al. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet . 2021 Jan 27. [Full Text] .
CDC. Symptoms of Coronavirus. CDC. Available at https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html . May 13, 2020 (updated March 22, 2022); Accessed: April 29, 2022.
Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med . 2020 Mar 10. [QxMD MEDLINE Link] .
Deo R, Choudhary MC, Moser C, and the, ACTIV-2/A5401 Study Team†. Symptom and Viral Rebound in Untreated SARS-CoV-2 Infection. Ann Intern Med . 2023 Feb 21. [QxMD MEDLINE Link] . [Full Text] .
Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA . 2020 Feb 24. [QxMD MEDLINE Link] .
Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA . 2020 Aug 25. 324 (8):782-793. [QxMD MEDLINE Link] .
Burke RM, Killerby ME, Newton S, Ashworth CE, Berns AL, Brennan S, et al. Symptom Profiles of a Convenience Sample of Patients with COVID-19 - United States, January-April 2020. MMWR Morb Mortal Wkly Rep . 2020 Jul 17. 69 (28):904-908. [QxMD MEDLINE Link] .
CDC. Healthcare Workers: Information on COVID-19. CDC. Available at https://www.cdc.gov/coronavirus/2019-ncov/hcp/index.html . March 31, 2021 (updated); Accessed: December 1, 2021.
Williamson EJ, Walker AJ, Bhaskaran K, et al. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature . 2020 Jul 8. [QxMD MEDLINE Link] .
Hewitt J, Carter B, Vilches-Moraga A, et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health . 2020 Jun 30. [QxMD MEDLINE Link] .
Ellinghaus D, Degenhardt F, Bujanda L, et al. The ABO blood group locus and a chromosome 3 gene cluster associate with SARS-CoV-2 respiratory failure in an Italian-Spanish genome-wide association analysis. medRxiv. Available at https://www.medrxiv.org/content/10.1101/2020.05.31.20114991v1 . June 2, 2020; Accessed: December 1, 2021.
Ellinghaus D, Degenhardt F, Bujanda L, et al. Genomewide Association Study of Severe Covid-19 With Respiratory Failure. NEJM . 2020 Jun 17. [QxMD MEDLINE Link] . [Full Text] .
Spinato G, Fabbris C, Polesel J, Cazzador D, Borsetto D, Hopkins C, et al. Alterations in Smell or Taste in Mildly Symptomatic Outpatients With SARS-CoV-2 Infection. JAMA . 2020 Apr 22. [QxMD MEDLINE Link] .
Luers JC, Rokohl AC, Loreck N, Wawer Matos PA, Augustin M, Dewald F, et al. Olfactory and Gustatory Dysfunction in Coronavirus Disease 19 (COVID-19). Clin Infect Dis . 2020 May 1. [QxMD MEDLINE Link] .
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet . 2020 Jan 24. [QxMD MEDLINE Link] .
Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med . 2020 Feb 28. [QxMD MEDLINE Link] .
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet . 2020 Feb 15. 395 (10223):507-513. [QxMD MEDLINE Link] .
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA . 2020 Feb 7. [QxMD MEDLINE Link] .
CDC. Long-Term Effects of COVID-19. Centers for Disease Control and Prevention. Available at https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects.html . 2020 Nov 13; Accessed: December 1, 2021.
[Guideline] COVID-19 Treatment Guidelines Panel. Clinical Spectrum of SARS-CoV-2 Infection. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ . March 1 2024 (updated); Accessed: February 29, 2024.
[Guideline] COVID-19 rapid guideline: managing the long-term effects of COVID-19. National Institute for Health and Care Excellence (NICE). Available at https://www.nice.org.uk/guidance/NG188 . 2020 Dec 18; Accessed: December 1, 2021.
Datta SD, Talwar A, Lee JT. A Proposed Framework and Timeline of the Spectrum of Disease Due to SARS-CoV-2 Infection: Illness Beyond Acute Infection and Public Health Implications. JAMA . 2020 Dec 8. 324 (22):2251-2252. [QxMD MEDLINE Link] . [Full Text] .
McFadyen JD, Stevens H, Peter K. The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications. Circ Res . 2020 Jul 31. 127 (4):571-587. [QxMD MEDLINE Link] . [Full Text] .
Kotecha T, Knight DS, Razvi Y, Kumar K, Vimalesvaran K, Thornton G, et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J . 2021 Feb 18. [QxMD MEDLINE Link] . [Full Text] .
Sciscent BY, Eisele CD, Ho L, King SD, Jain R, Golamari RR. COVID-19 reinfection: the role of natural immunity, vaccines, and variants. J Community Hosp Intern Med Perspect . 2021. 11 (6):733-739. [QxMD MEDLINE Link] .
Pérez-Lago L, Kestler M, Sola-Campoy PJ, et al. SARS-CoV-2 superinfection and reinfection with three different strains. Transbound Emerg Dis . 2021 Oct 23. [QxMD MEDLINE Link] .
CDC. 2019 Novel Coronavirus, Wuhan, China: Frequently Asked Questions and Answers. CDC. Available at https://www.cdc.gov/coronavirus/2019-ncov/faq.html . October 19, 2021; Accessed: December 1, 2021.
FDA. Coronavirus (COVID-19) Update: Daily Roundup. fda.gov. Available at https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-daily-roundup . March 23, 2020; Accessed: December 1, 2021.
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med . 2020 Mar 13. [QxMD MEDLINE Link] .
Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis . 2020 Feb 24. [QxMD MEDLINE Link] .
Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. AJR Am J Roentgenol . 2020 Mar 3. 1-6. [QxMD MEDLINE Link] .
Bai HX, Hsieh B, Xiong Z, Halsey K, Choi JW, Tran TML, et al. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology . 2020 Mar 10. 200823. [QxMD MEDLINE Link] .
ACR. ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. American College of Radiology. Available at https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection . March 22, 2020; Accessed: December 1, 2021.
Hu Z, Song C, Xu C, Jin G, Chen Y, Xu X, et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci . 2020 Mar 4. [QxMD MEDLINE Link] .
Wang Y, Liu Y, Liu L, Wang X, Luo N, Ling L. Clinical outcome of 55 asymptomatic cases at the time of hospital admission infected with SARS-Coronavirus-2 in Shenzhen, China. J Infect Dis . 2020 Mar 17. [QxMD MEDLINE Link] .
Li M, Lei P, Zeng B, Li Z, Yu P, Fan B, et al. Coronavirus Disease (COVID-19): Spectrum of CT Findings and Temporal Progression of the Disease. Acad Radiol . 2020 Mar 20. [QxMD MEDLINE Link] .
Wong HYF, Lam HYS, Fong AH, Leung ST, Chin TW, Lo CSY, et al. Frequency and Distribution of Chest Radiographic Findings in COVID-19 Positive Patients. Radiology . 2019 Mar 27. 201160. [QxMD MEDLINE Link] .
Bogoch II, Watts A, Thomas-Bachli A, Huber C, Kraemer MUG, Khan K. Pneumonia of Unknown Etiology in Wuhan, China: Potential for International Spread Via Commercial Air Travel. J Travel Med . 2020 Jan 14. [QxMD MEDLINE Link] .
Veklury (remdesivir) [package insert]. Foster City, CA: Gilead Sciences, Inc. February 2024. Available at [Full Text] .
RECOVERY Collaborative Group., Horby P, Lim WS, Emberson JR, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med . 2021 Feb 25. 384 (8):693-704. [QxMD MEDLINE Link] . [Full Text] .
WHO. The use of non-steroidal anti-inflammatory drugs (NSAIDs) in patients with COVID-19. WHO. Available at https://www.who.int/news-room/commentaries/detail/the-use-of-non-steroidal-anti-inflammatory-drugs-(nsaids)-in-patients-with-covid-19 . April 19, 2020; Accessed: December 1, 2021.
[Guideline] Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. IDSA. Available at https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ . 2021 Oct 01; updated 6/26/2023; Accessed: February 29, 2024.
Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, O’Mera MJ, et al. A SARS-CoV-2-Human Protein-Protein Interaction Map Reveals Drug Targets and Potential Drug-Repurposing. bioRxiv. Available at https://www.biorxiv.org/content/10.1101/2020.03.22.002386v1 . 2020 Mar 22; Accessed: December 1, 2021.
Arshad U, Pertinez H, Box H, Tatham L, Rajoki RKR, Curley P, et al. Prioritisation of potential anti-SARS-CoV-2 drug repurposing opportunities based on ability to achieve adequate target site concentrations derived from their established human pharmacokinetics. medRxiv . 2020 Apr 22. [Full Text] .
World Health Organization. “Solidarity” clinical trial for COVID-19 treatments. WHO. Available at https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments . 2020 Oct 16; Accessed: December 1, 2021.
WHO Solidarity Trial Consortium. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med . 2020 Dec 2. [QxMD MEDLINE Link] . [Full Text] .
Kalil AC. Treating COVID-19-Off-Label Drug Use, Compassionate Use, and Randomized Clinical Trials During Pandemics. JAMA . 2020 Mar 24. [QxMD MEDLINE Link] . [Full Text] .
Rome BN, Avorn J. Drug Evaluation during the Covid-19 Pandemic. N Engl J Med . 2020 Apr 14. [QxMD MEDLINE Link] . [Full Text] .
Frellick M. Key Drugs Join PPEs on List of Front-Line Shortages. Medscape Medical News . 2020 Apr 02. Available at https://www.medscape.com/viewarticle/928039 .
Live Updates: Which Drugs Are In Shortage Because of COVID-19?. GoodRx. Available at https://www.goodrx.com/blog/covid-19-drug-shortages-updates/ . July 13, 2020; Accessed: December 1, 2021.
Wilson FP. Hydroxychloroquine for COVID-19: What’s the Evidence? (Commentary). Medscape Medical News and Perspectives . 2020 Mar 25. Available at https://www.medscape.com/viewarticle/927342 .
CDC. Coronavirus Disease 2019 (COVID-19): Community-Related Exposures. Centers for Disease Control and Prevention. Available at https://www.cdc.gov/coronavirus/2019-ncov/php/public-health-recommendations.html . March 1, 2021 (updated); Accessed: December 1, 2021.
CDC. Coronavirus Disease 2019 (COVID-19): Travel-Associated Exposures. Centers for Disease Control and Prevention. Available at https://www.cdc.gov/coronavirus/2019-ncov/php/risk-assessment.html . November 5, 2021 (updated); Accessed: December 1, 2021.
Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med . 2020. [Full Text] .
Smith JD, MacDougall CC, Johnstone J, Copes RA, Schwartz B, Garber GE. Effectiveness of N95 respirators versus surgical masks in protecting health care workers from acute respiratory infection: a systematic review and meta-analysis. CMAJ . 2016 May 17. 188 (8):567-574. [QxMD MEDLINE Link] .
TLR 2/6/9 agonist PUL-042. NCI Drug Dictionary. Available at https://www.cancer.gov/publications/dictionaries/cancer-drug/def/tlr-2-6-9-agonist-pul-042 . Accessed: December 1, 2021.
The Use PUL-042 Inhalation Solution to Prevent COVID-19 in Adults Exposed to SARS-CoV-2. ClinicalTrials.gov. Available at https://clinicaltrials.gov/ct2/show/NCT04313023 . 2020 Mar 24; Accessed: December 1, 2021.
The Use of PUL-042 Inhalation Solution to Reduce the Severity of COVID-19 in Adults Positive for SARS-CoV-2 Infection. ClinicalTrials.gov. Available at https://clinicaltrials.gov/ct2/show/NCT04312997?term=PUL-042&cond=COVID&cntry=US&draw=2&rank=2 . 2020 May 07; Accessed: December 1, 2021.
Holshue ML, DeBolt C, Lindquist S, and, the Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med . 2020 Mar 5. 382 (10):929-936. [QxMD MEDLINE Link] .
National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. U.S. Department of Health and Human Services. Available at https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins . 2020 Feb 25; Accessed: March 24, 2020.
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med . 2020 Oct 8. [QxMD MEDLINE Link] . [Full Text] .
Harrington DP, Baden LR, Hogan JW. A Large, Simple Trial Leading to Complex Questions. N Engl J Med . 2020 Dec 2. [QxMD MEDLINE Link] . [Full Text] .
Ader F, Bouscambert-Duchamp M, Hites M, and the, DisCoVeRy Study Group. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect Dis . 2021 Sep 14. [QxMD MEDLINE Link] . [Full Text] .
Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med . November 5, 2020. 383:1827-1837. [QxMD MEDLINE Link] . [Full Text] .
Spinner CD, Gottlieb RL, Criner GJ, and the, GS-US-540-5774 Investigators. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA . 2020 Aug 21. [QxMD MEDLINE Link] . [Full Text] .
Gottlieb RL, Vaca CE, Paredes R, and the, GS-US-540-9012 (PINETREE) Investigators. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N Engl J Med . 2021 Dec 22. [QxMD MEDLINE Link] . [Full Text] .
Veklury (remdesivir) reduced risk of mortality in hospitalized COVID-19 patients across all variant time periods in a real world study of more than 500,000 patients. Gilead . 2023 Jan 21. Available at https://www.gilead.com/news-and-press/press-room/press-releases/2023/2/veklury-remdesivir-reduced-risk-of-mortality-in-hospitalized-covid19-patients-across-all-variant-time-periods-in-a-real-world-study-of-more-than-5 .
Amstutz A, et al. Effects of remdesivir in patients hospitalised with COVID-19: a systematic review and individual patient data meta-analysis of randomized controlled trials. Lancet Respir Med . 2023 Feb 21. [Full Text] .
Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of Remdesivir (GS-5734) in Participants From Birth to < 18 Years of Age With Coronavirus Disease 2019 (COVID-19) (CARAVAN). ClinicalTrials.gov. Available at https://www.clinicaltrials.gov/ct2/show/NCT04431453 . 2020 Jun 01; Accessed: December 1, 2021.
Chiotos K, Hayes M, Kimberlin DW, Jones SB, James SH, Pinninti SG, et al. Multicenter interim guidance on use of antivirals for children with COVID-19/SARS-CoV-2. J Pediatric Infect Dis Soc . 2020 Sep 12. [QxMD MEDLINE Link] . [Full Text] .
Burwick RM, Yawetz S, Stephenson KE, Collier AY, Sen P, Blackburn BG, et al. Compassionate Use of Remdesivir in Pregnant Women with Severe Covid-19. Clin Infect Dis . 2020 Oct 8. [QxMD MEDLINE Link] . [Full Text] .
McCoy JA, Short WR, Srinivas SK, Levine LD, Hirshberg A. Compassionate use of remdesivir for treatment of severe coronavirus disease 2019 in pregnant women at a United States academic center. Am J Obstet Gynecol MFM . 2020 Aug. 2 (3):100164. [QxMD MEDLINE Link] . [Full Text] .
Hammond J, Leister-Tebbe H, Gardner A, and the, EPIC-HR Investigators. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med . 2022 Feb 16. [QxMD MEDLINE Link] . [Full Text] .
Pfizer announces additional phase 2/3 study results confirming robust efficacy of novel COVID-19 oral antiviral treatment candidate in reducing risk of hospitalization or death. Pfizer, Inc . 2021 Dec 14. Available at https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-additional-phase-23-study-results .
A post-exposure prophylaxis study of PF-07321332/ritonavir in adult household contacts of an individual with symptomatic COVID-19. ClinicalTrials.gov. Available at https://www.clinicaltrials.gov/ct2/show/NCT05047601 . 2021 Dec 01; Accessed: December 14, 2021.
Xie Y, Choi T, Al-Aly Z. Nirmatrelvir and the risk of post-acute sequelae of COVID-19. medRxiv . November 5, 2022. [Full Text] .
Liu J, Pan X, Zhang S, Li M, Ma K, Fan C, et al. Efficacy and safety of Paxlovid in severe adult patients with SARS-Cov-2 infection: a multicenter randomized controlled study. Lancet Reg Health West Pac . 2023 Feb 6. 100694. [QxMD MEDLINE Link] . [Full Text] .
Wong CKH, Lau KTK, Au ICH, Lau EHY, Poon LLM, Hung IFN, et al. Viral burden rebound in hospitalised patients with COVID-19 receiving oral antivirals in Hong Kong: a population-wide retrospective cohort study. Lancet Infect Dis . 2023 Feb 13. [QxMD MEDLINE Link] . [Full Text] .
Zheng Q, Ma P, Wang M, Cheng Y, Zhou M, Ye L, et al. Efficacy and safety of Paxlovid for COVID-19:a meta-analysis. J Infect . 2023 Jan. 86 (1):66-117. [QxMD MEDLINE Link] . [Full Text] .
Jayk Bernal A, Gomes da Silva MM, Musungaie DB, and the, MOVe-OUT Study Group. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med . 2022 Feb 10. 386:509-520. [QxMD MEDLINE Link] . [Full Text] .
Xie Y, Bowe B, Al-Aly Z. Molnupiravir and risk of hospital admission or death in adults with covid-19: emulation of a randomized target trial using electronic health records. BMJ . 2023 Mar 7. 380:e072705. [QxMD MEDLINE Link] . [Full Text] .
Najjar-Debbiny R, Gronich N, Weber G, Khoury J, Amar M, Stein N, et al. Effectiveness of Molnupiravir in High-Risk Patients: A Propensity Score Matched Analysis. Clin Infect Dis . 2023 Feb 8. 76 (3):453-460. [QxMD MEDLINE Link] . [Full Text] .
Merck provides update on phase 3 MOVe-AHEAD trial evaluating Lagevrio (molnupiravir) for post-exposure prophylaxis for prevention of COVID-19. Merck . 2023 Feb 21. Available at https://www.merck.com/news/merck-provides-update-on-phase-3-move-ahead-trial-evaluating-lagevrio-molnupiravir-for-post-exposure-prophylaxis-for-prevention-of-covid-19/ .
Ingraham NE, Lotfi-Emran S, Thielen BK, Techar K, Morris RS, Holtan SG, et al. Immunomodulation in COVID-19. Lancet Respir Med . 2020 May 4. [QxMD MEDLINE Link] . [Full Text] .
Rizk JG, Kalantar-Zadeh K, Mehra MR, Lavie CJ, Rizk Y, Forthal DN. Pharmaco-Immunomodulatory Therapy in COVID-19. Drugs . 2020 Sep. 80 (13):1267-1292. [QxMD MEDLINE Link] . [Full Text] .
Peterson JH, Paranjape NS, Grundlingh N, Priestley JL. Outcomes and Adverse Effects of Baricitinib Versus Tocilizumab in the Management of Severe COVID-19. Crit Care Med . 2023 Mar 1. 51 (3):337-346. [QxMD MEDLINE Link] . [Full Text] .
Cawcutt KA, Kalil AC. Baricitinib or Tocilizumab? Treatment of Patients Hospitalized With Severe COVID-19. Crit Care Med . 2023 Mar 1. 51 (3):413-415. [QxMD MEDLINE Link] .
Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis . 2020 Apr. 20 (4):400-402. [QxMD MEDLINE Link] . [Full Text] .
Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet . 2020 Feb 15. 395 (10223):e30-e31. [QxMD MEDLINE Link] . [Full Text] .
Stebbing J, Krishnan V, de Bono S, Ottaviani S, Casalini G, Richarson PJ, et al. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. 2020 Apr 15. [Full Text] .
Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med . 2020 Dec 11. [QxMD MEDLINE Link] . [Full Text] .
Kalil AC, Stebbing J. Baricitinib: the first immunomodulatory treatment to reduce COVID-19 mortality in a placebo-controlled trial. Lancet Respir Med . 2021 Aug 31. [QxMD MEDLINE Link] . [Full Text] .
Marconi VC, Ramanan AV, de Bono S, Kartman CE, Krishnan V, Liao R, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med . 2021 Aug 31. [QxMD MEDLINE Link] . [Full Text] .
Lilly and Incyte’s baricitinib reduced deaths among patients with COVID-19 receiving invasive mechanical ventilation. Lilly. Available at https://investor.lilly.com/news-releases/news-release-details/lilly-and-incytes-baricitinib-reduced-deaths-among-patients . 2021 Aug 03; Accessed: December 1, 2021.
Guimarães PO, Quirk D, Furtado RH, and the STOP-COVID Trial Investigators. Tofacitinib in patients hospitalized with COVID-19 pneumonia. N Engl J Med . 2021 Jun 16. [QxMD MEDLINE Link] . [Full Text] .
Wagner JL, Veve MP, Barber KE. Using IL-6 inhibitors to treat COVID-19. ContagionLive. Available at https://www.contagionlive.com/publications/contagion/2020/august/using-il6-inhibitors-to-treat-covid19?ekey=c2NiZXJnbWFuQG5lYnJhc2thbWVkLmNvbQ . 2020 Aug 17; Accessed: December 1, 2021.
[Guideline] NIH. The COVID-19 Treatment Guidelines Panel’s Statement on the Use of Tocilizumab (and Other Interleukin-6 Inhibitors) for the Treatment of COVID-19. COVID-19 Treatment Guidelines. Available at https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/interleukin-6-inhibitors/ . 2021 Mar 05; Accessed: December 1, 2021.
Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N Engl J Med . 2021 Jan 7. 384 (1):20-30. [QxMD MEDLINE Link] . [Full Text] .
Rosas IO, Diaz G, Gottlieb RL, Lobo SM, Robinson P, Hunter BD, et al. Tocilizumab and remdesivir in hospitalized patients with severe COVID-19 pneumonia: a randomized clinical trial. Intensive Care Med . 2021 Nov. 47 (11):1258-1270. [QxMD MEDLINE Link] . [Full Text] .
REMAP-CAP Investigators., Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med . 2021 Apr 22. 384 (16):1491-1502. [QxMD MEDLINE Link] . [Full Text] .
RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet . 2021 May 1. 397 (10285):1637-1645. [QxMD MEDLINE Link] . [Full Text] .
Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, et al. Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia. N Engl J Med . 2021 Apr 22. 384 (16):1503-1516. [QxMD MEDLINE Link] . [Full Text] .
Rubin EJ, Longo DL, Baden LR. Interleukin-6 Receptor Inhibition in Covid-19 - Cooling the Inflammatory Soup. N Engl J Med . 2021 Apr 22. 384 (16):1564-1565. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] NIH. Interleukin-1 Inhibitors. COVID-19 Treatment Guidelines. Available at https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/interleukin-1-inhibitors/ . 2020 Jul 17; Accessed: September 8, 2021.
[Guideline] Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. World Health Organization. Available at https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected . 2020 Mar 13; Accessed: March 24, 2020.
Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet . 2020 Feb 15. 395 (10223):473-475. [QxMD MEDLINE Link] .
[Guideline] Alhazzani W, Evans L, Alshamsi F, Møller MH, Ostermann M, Prescott HC, et al. Surviving Sepsis Campaign Guidelines on the Management of Adults With Coronavirus Disease 2019 (COVID-19) in the ICU: First Update. Crit Care Med . 2021 Mar 1. 49 (3):e219-e234. [QxMD MEDLINE Link] . [Full Text] .
WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA . 2020 Sep 2. [QxMD MEDLINE Link] . [Full Text] .
Prescott HC, Rice TW. Corticosteroids in COVID-19 ARDS: Evidence and Hope During the Pandemic. JAMA . 2020 Sep 2. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] WHO. Corticosteroids for COVID-19 – Living Guidance. World Health Organization. Available at https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1 . 2020 Sep 02; Accessed: December 1, 2021.
Afzali B, Noris M, Lambrecht BN, Kemper C. The state of complement in COVID-19. Nat Rev Immunol . 2022 Feb. 22 (2):77-84. [QxMD MEDLINE Link] . [Full Text] .
Carvelli J, Demaria O, Vély F, Batista L, Chouaki Benmansour N, Fares J, et al. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature . 2020 Dec. 588 (7836):146-150. [QxMD MEDLINE Link] . [Full Text] .
Aiello S, Gastoldi S, Galbusera M, Ruggenenti P, Portalupi V, Rota S, et al. C5a and C5aR1 are key drivers of microvascular platelet aggregation in clinical entities spanning from aHUS to COVID-19. Blood Adv . 2022 Jan 8. 6 (3):866-881. [QxMD MEDLINE Link] . [Full Text] .
Vlaar APJ, Witzenrath M, van Paassen P, and the, PANAMO study group. Anti-C5a antibody (vilobelimab) therapy for critically ill, invasively mechanically ventilated patients with COVID-19 (PANAMO): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med . 2022 Dec. 10 (12):1137-1146. [QxMD MEDLINE Link] . [Full Text] .
Strich JR, Tian X, Samour M, King CS, Shlobin O, Reger R, et al. Fostamatinib for the treatment of hospitalized adults with COVD-19 A randomized trial. Clin Infect Dis . 2021 Sep 1. [QxMD MEDLINE Link] . [Full Text] .
Reis G, Moreira Silva EAS, Medeiros Silva DC, and the, TOGETHER Investigators. Early Treatment with Pegylated Interferon Lambda for Covid-19. N Engl J Med . 2023 Feb 9. 388 (6):518-528. [QxMD MEDLINE Link] . [Full Text] .
Kalil AC, Mehta AK, Patterson TF, and the, ACTT-3 study group members. Efficacy of interferon beta-1a plus remdesivir compared with remdesivir alone in hospitalised adults with COVID-19: a double-bind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med . 2021 Oct 18. [QxMD MEDLINE Link] . [Full Text] .
Schönbeck U, Libby P. Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents?. Circulation . 2004 Jun 1. 109 (21 Suppl 1):II18-26. [QxMD MEDLINE Link] . [Full Text] .
Hariyanto TI, Kurniawan A. Statin therapy did not improved the in-hospital outcome of coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr . 2020 Aug 26. 14(6):1613-1615. [QxMD MEDLINE Link] . [Full Text] .
Kow CS, Hasan SS. Meta-analysis of Effect of Statins in Patients with COVID-19. Am J Cardiol . 2020 Aug 12. [QxMD MEDLINE Link] . [Full Text] .
Castiglione V, Chiriacò M, Emdin M, Taddei S, Vergaro G. Statin therapy in COVID-19 infection. Eur Heart J Cardiovasc Pharmacother . 2020 Jul 1. 6 (4):258-259. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] NIH. Covid-19 Treatment Guidelines - Adjunctive Therapy. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/adjunctive-therapy/ . 2020 Jul 17; Accessed: December 1, 2021.
Yao JS, Paguio JA, Dee EC, Tan HC, Moulick A, Milazzo C, et al. The minimal effect of zinc on the survival of hospitalized patients with Covid-19: an observational study. Chest . 2020 Jul 22. [QxMD MEDLINE Link] . [Full Text] .
Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. JAMA Netw Open . 2020 Sep 1. 3 (9):e2019722. [QxMD MEDLINE Link] . [Full Text] .
Sahota O. Understanding vitamin D deficiency. Age Ageing . 2014 Sep. 43 (5):589-91. [QxMD MEDLINE Link] . [Full Text] .
Bishop CW, Ashfaq A, Melnick JZ, Vazquez-Escarpanter E, Fialkow JA, Strugnell SA, et al. Results from the REsCUe trial: a randomized controlled trial with extended-release calcifediol in symptomatic outpatients with COVID-19. medRXiv . 2022 Feb 05. [Full Text] .
NIH COVID-19 Treatment Guidelines Panel. Statement on the emergency use authorization of convalescent plasma for the treatment of COVID-19. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/statement-on-convalescent-plasma-eua/ . 2022 Dec 01; Accessed: February 1, 2023.
Senefeld JW, Franchini M, Mengoli C, Cruciani M, Zani M, Gorman EK, et al. COVID-19 Convalescent Plasma for the Treatment of Immunocompromised Patients: A Systematic Review and Meta-analysis. JAMA Netw Open . 2023 Jan 3. 6 (1):e2250647. [QxMD MEDLINE Link] . [Full Text] .
Writing Committee for the REMAP-CAP Investigators, Estcourt LJ, Turgeon AF, McQuilten ZK, et al. Effect of Convalescent Plasma on Organ Support-Free Days in Critically Ill Patients With COVID-19: A Randomized Clinical Trial. JAMA . 2021 Oct 4. [QxMD MEDLINE Link] .
A Study to Investigate the Prevention of COVID-19 with VYD222 in Adults With Immune Compromise and in Participants Aged 12 Years or Older Who Are at Risk of Exposure to SARS-CoV-2. ClinicalTrials.gov. Available at https://classic.clinicaltrials.gov/ct2/show/NCT06039449 . 2024 January 18; Accessed: April 1, 2024.
Levin MJ, Ustianowski A, De Wit S, and the, PROVENT Study Group. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for Prevention of Covid-19. N Engl J Med . 2022 Jun 9. 386 (23):2188-2200. [QxMD MEDLINE Link] . [Full Text] .
Young-Xu Y, Epstein L, Marconi VC, Davey V, Zwain G, Smith J, et al. Tixagevimab/cilagavimab for prevention of COVID-19 during the omicron surge: Retrospective analysis of national VA electronic data. medRxiv . 2022 May 29. [Full Text] .
Westendorf K, Žentelis S, Wang L, Foster D, Vaillancourt P, Wiggin M, et al. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. Cell Rep . 2022 May 17. 39 (7):110812. [QxMD MEDLINE Link] . [Full Text] .
Gupta A, Gonzalez-Rojas Y, Juarez E, and the, COMET-ICE Investigators. Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N Engl J Med . 2021 Nov 18. 385 (21):1941-1950. [QxMD MEDLINE Link] . [Full Text] .
GSK and Vir submit emergency use authorization application to FDA for intramuscular administration of sotrovimab for the early treatment of COVID-19. GlaxoSmithKline . 2022 Jan 13. Available at https://us.gsk.com/en-us/media/press-releases/gsk-and-vir-submit-emergency-use-authorization-application-to-fda-for-intramuscular-administration-of-sotrovimab-for-the-early-treatment-of-covid-19/ .
O'Brien MP, Forleo-Neto E, Musser BJ, and the, Covid-19 Phase 3 Prevention Trial Team. Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19. N Engl J Med . 2021 Aug 4. [QxMD MEDLINE Link] . [Full Text] .
Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N Engl J Med . 2020 Dec 17. [QxMD MEDLINE Link] . [Full Text] .
RECOVERY Collaborative Group, Horby PW, Mafham M, Peto L, et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomized, controlled, open-label, platform trial. medRxiv . 2021 Jun 16. [Full Text] .
Dougan M, Nirula A, Azizad M, and the, BLAZE-1 Investigators. Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19. N Engl J Med . 2021 Oct 7. 385 (15):1382-1392. [QxMD MEDLINE Link] . [Full Text] .
Cohen MS, Nirula A, Mulligan MJ, and the, BLAZE-2 Investigators. Effect of Bamlanivimab vs Placebo on Incidence of COVID-19 Among Residents and Staff of Skilled Nursing and Assisted Living Facilities: A Randomized Clinical Trial. JAMA . 2021 Jul 6. 326 (1):46-55. [QxMD MEDLINE Link] . [Full Text] .
Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 Vaccines at Pandemic Speed. N Engl J Med . 2020 May 21. 382 (21):1969-1973. [QxMD MEDLINE Link] . [Full Text] .
Koirala A, Joo YJ, Khatami A, Chiu C, Britton PN. Vaccines for COVID-19: The current state of play. Paediatr Respir Rev . 2020 Sep. 35:43-49. [QxMD MEDLINE Link] . [Full Text] .
Iba T, Levy JH, Levi M, Connors JM, Thachil J. Coagulopathy of Coronavirus Disease 2019. Crit Care Med . 2020 Sep. 48 (9):1358-1364. [QxMD MEDLINE Link] . [Full Text] .
Katneni UK, Alexaki A, Hunt RC, Schiller T, DiCuccio M, Buehler PW, et al. Coagulopathy and Thrombosis as a Result of Severe COVID-19 Infection: A Microvascular Focus. Thromb Haemost . 2020 Aug 24. [QxMD MEDLINE Link] . [Full Text] .
Ferguson J, Volk S, Vondracek T, Flanigan J, Chernaik A. Empiric therapeutic anticoagulation and mortality in critically ill patients with respiratory failure from SARS-CoV-2: A retrospective cohort study. J Clin Pharmacol . 2020 Sep 3. [QxMD MEDLINE Link] . [Full Text] .
Paranjpe I, Fuster V, Lala A, Russak AJ, Glicksberg BS, Levin MA, et al. Association of Treatment Dose Anticoagulation With In-Hospital Survival Among Hospitalized Patients With COVID-19. J Am Coll Cardiol . 2020 Jul 7. 76 (1):122-124. [QxMD MEDLINE Link] . [Full Text] .
NIH ACTIV-4B COVID-19 outpatient thrombosis prevention trial ends early. Brigham Health . 2021 Jun 22. Available at https://www.brighamandwomens.org/about-bwh/newsroom/press-releases-detail?id=3925 .
REMAP-CAP Investigators, ACTIV-4a Investigators, ATTACC Investigators, Goligher EC, Bradbury CA, McVerry BJ, et al. Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. N Engl J Med . 2021 Aug 4. [QxMD MEDLINE Link] . [Full Text] .
ATTACC Investigators, ACTIV-4a Investigators, REMAP-CAP Investigators, Lawler PR, Goligher EC, Berger JS, et al. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. N Engl J Med . 2021 Aug 4. [QxMD MEDLINE Link] . [Full Text] .
Assessing Safety, Hospitalization and Efficacy of rNAPc2 in COVID-19 (ASPEN). ClinicalTrials.gov. Available at https://www.clinicaltrials.gov/ct2/show/NCT04655586 . 2021 Nov 03; Accessed: December 6, 2021.
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature . 2020 Mar. 579 (7798):270-273. [QxMD MEDLINE Link] . [Full Text] .
Lopes RD, Macedo AVS, de Barros E Silva PGM, Moll-Bernardes RJ, Feldman A, D'Andréa Saba Arruda G, et al. Continuing versus suspending angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: Impact on adverse outcomes in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)--The BRACE CORONA Trial. Am Heart J . 2020 Aug. 226:49-59. [QxMD MEDLINE Link] . [Full Text] .
Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, et al. Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease. Circulation . 2020 Mar 21. [QxMD MEDLINE Link] . [Full Text] .
Fang L, Karakiulakis G, Roth M. Correction to Lancet Respir Med 2020; 8: e21. Lancet Respir Med . 2020 Jun. 8 (6):e54. [QxMD MEDLINE Link] . [Full Text] .
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med . 2005 Aug. 11 (8):875-9. [QxMD MEDLINE Link] . [Full Text] .
Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon S. Renin–Angiotensin–Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med . 2020 March 30. [QxMD MEDLINE Link] . [Full Text] .
Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension . 2004 May. 43 (5):970-6. [QxMD MEDLINE Link] . [Full Text] .
Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation . 2005 May 24. 111 (20):2605-10. [QxMD MEDLINE Link] . [Full Text] .
Klimas J, Olvedy M, Ochodnicka-Mackovicova K, Kruzliak P, Cacanyiova S, Kristek F, et al. Perinatally administered losartan augments renal ACE2 expression but not cardiac or renal Mas receptor in spontaneously hypertensive rats. J Cell Mol Med . 2015 Aug. 19 (8):1965-74. [QxMD MEDLINE Link] . [Full Text] .
Walters TE, Kalman JM, Patel SK, Mearns M, Velkoska E, Burrell LM. Angiotensin converting enzyme 2 activity and human atrial fibrillation: increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. Europace . 2017 Aug 1. 19 (8):1280-1287. [QxMD MEDLINE Link] . [Full Text] .
Burchill LJ, Velkoska E, Dean RG, Griggs K, Patel SK, Burrell LM. Combination renin-angiotensin system blockade and angiotensin-converting enzyme 2 in experimental myocardial infarction: implications for future therapeutic directions. Clin Sci (Lond) . 2012 Dec. 123 (11):649-58. [QxMD MEDLINE Link] .
Bozkurt B, Kovacs R, Harrington B. Joint HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19. J Card Fail . 2020 May. 26 (5):370. [QxMD MEDLINE Link] . [Full Text] .
Sama IE, Ravera A, Santema BT, van Goor H, Ter Maaten JM, Cleland JGF, et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur Heart J . 2020 May 14. 41 (19):1810-1817. [QxMD MEDLINE Link] . [Full Text] .
Baral R, Tsampasian V, Debski M, Moran B, Garg P, Clark A, et al. Association Between Renin-Angiotensin-Aldosterone System Inhibitors and Clinical Outcomes in Patients With COVID-19: A Systematic Review and Meta-analysis. JAMA Netw Open . 2021 Mar 1. 4 (3):e213594. [QxMD MEDLINE Link] . [Full Text] .
Torres A, Blasi F, Dartois N, Akova M. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax . 2015 Oct. 70 (10):984-9. [QxMD MEDLINE Link] . [Full Text] .
Drucker DJ. Coronavirus infections and type 2 diabetes-shared pathways with therapeutic implications. Endocr Rev . 2020 Apr 15. [QxMD MEDLINE Link] . [Full Text] .
Muniyappa R, Gubbi S. COVID-19 Pandemic, Corona Viruses, and Diabetes Mellitus. Am J Physiol Endocrinol Metab . 2020 Mar 31. [QxMD MEDLINE Link] . [Full Text] .
DPP4 dipeptidyl peptidase 4. National Center for Biotechnology Information (NCBI). Available at https://www.ncbi.nlm.nih.gov/gene/1803 . 2020 Apr 20; Accessed: 2020 Apr 21.
RECOVERY Collaborative Group. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomized, controlled, open-label, platform trial. The Lancet . 2020 Oct 05. [Full Text] .
Momekov G, Momekova D. Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: antiviral levels are not likely attainable with known dosing regimens. Biotechnology & Biotechnological Equipment. 2020 . 2020. 34(1):469-74. [Full Text] .
Chaccour C, Hammann F, Ramón-García S, Rabinovich NR. Ivermectin and Novel Coronavirus Disease (COVID-19): Keeping Rigor in Times of Urgency. Am J Trop Med Hyg . 2020 Apr 16. [QxMD MEDLINE Link] . [Full Text] .
López-Medina E, López P, Hurtado IC, Dávalos DM, Ramirez O, Martínez E, et al. Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19: A Randomized Clinical Trial. JAMA . 2021 Apr 13. 325 (14):1426-1435. [QxMD MEDLINE Link] . [Full Text] .
Lim SCL, Hor CP, Tay KH, and the, I-TECH Study Group. Efficacy of Ivermectin Treatment on Disease Progression Among Adults With Mild to Moderate COVID-19 and Comorbidities: The I-TECH Randomized Clinical Trial. JAMA Intern Med . 2022 Feb 18. [QxMD MEDLINE Link] . [Full Text] .
Reis G, Silva EASM, Silva DCM, and the, TOGETHER Investigators. Effect of Early Treatment with Ivermectin among Patients with Covid-19. N Engl J Med . 2022 May 5. 386 (18):1721-1731. [QxMD MEDLINE Link] . [Full Text] .
Naggie S, Boulware DR, Lindsell CJ, for the, Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators. Effect of Ivermectin vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA . 2022 Oct 25. 328 (16):1595-1603. [QxMD MEDLINE Link] . [Full Text] .
Naggie S, Boulware DR, Lindsell CJ, for the, Accelerating Covid-19 Therapeutic Interventions and Vaccines (ACTIV)-6 Study Group and Investigators. Effect of Higher-Dose Ivermectin for 6 Days vs Placebo on Time to Sustained Recovery in Outpatients With COVID-19: A Randomized Clinical Trial. JAMA . 2023 Feb 20. [QxMD MEDLINE Link] . [Full Text] .
Bramante CT, Huling JD, Tignanelli CJ, for the COVID-OUT Trial Team. Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19. N Eng J Med . 2022. 387:599-610. [Full Text] .
Reis G, Dos Santos Moreira-Silva EA, Silva DCM, and the, TOGETHER investigators. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health . 2022 Jan. 10 (1):e42-e51. [QxMD MEDLINE Link] . [Full Text] .
McCarthy MW, Naggie S, Boulware DR, and the, Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)-6 Study Group and Investigators. Effect of Fluvoxamine vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA . 2023 Jan 24. 329 (4):296-305. [QxMD MEDLINE Link] .
Golan Y, Campos JAS, Woolson R, Cilla D, Hanabergh R, Gonzales-Rojas Y, et al. Favipiravir in Patients With Early Mild-to-moderate Coronavirus Disease 2019 (COVID-19): A Randomized Controlled Trial. Clin Infect Dis . 2023 Feb 8. 76 (3):e10-e17. [QxMD MEDLINE Link] . [Full Text] .
Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med . 2012 May 17. 366 (20):1881-90. [QxMD MEDLINE Link] . [Full Text] .
Simpson TF, Kovacs RJ, Stecker EC. Ventricular Arrhythmia Risk Due to Hydroxychloroquine-Azithromycin Treatment For COVID-19. American College of Cardiology; Cardiology Magazine. Available at https://www.acc.org/latest-in-cardiology/articles/2020/03/27/14/00/ventricular-arrhythmia-risk-due-to-hydroxychloroquine-azithromycin-treatment-for-covid-19 . 2020 Mar 29; Accessed: April 1, 2020.
[Guideline] Roden DM, Harrington RA, Poppas A, Russo AM. Considerations for Drug Interactions on QTc in Exploratory COVID-19 (Coronavirus Disease 2019) Treatment. Circulation . 2020 Apr 8. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Giudicessi JR, Noseworthy PA, Friedman PA, Ackerman MJ. Urgent guidance for navigating and circumventing the QTc prolonging and torsadogenic potential of possible pharmacotherapies for COVID-19. Mayo Clin Proc . 2020 Apr 07. [Full Text] .
Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, et al. Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol . 2020 May 1. [QxMD MEDLINE Link] . [Full Text] .
Chorin E, Dai M, Shulman E, Wadhwani L, et al. The QT Interval in Patients with SARS-CoV-2 Infection Treated with Hydroxychloroquine/Azithromycin. medRxiv . 2020 Apr 03. [Full Text] .
Borba M, de Almeida Val F, Sampaio VS, Alexandre MA, Melo GC, Brito M, et al. Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS-CoV-2) infection: Preliminary safety results of a randomized, double-blinded, phase IIb clinical trial (CloroCovid-19 Study). MedRxiv . 2020 Apr 11. [Full Text] .
Lane JCE, Weaver J, Kostka K, Duarte-Salles T, Abrahao MTF, Alghoul H, et al. Safety of hydroxychloroquine, alone and in combination with azithromycin, in light of rapid wide-spread use for COVID-19: a multinational, network cohort and self-controlled case series study. medRxiv . 2020 Apr 10. [Full Text] .
Supady A, Weber E, Rieder M, Lother A, Niklaus T, Zahn T, et al. Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): a single centre, open-label, randomised, controlled trial. Lancet Respir Med . 2021 Jul. 9 (7):755-762. [QxMD MEDLINE Link] . [Full Text] .
Matson J, Lange P, Honore PM, Chung KK. Adverse outcomes with extracorporeal adsorbent blood treatments in toxic systemic inflammation: a perspective on possible mechanisms. Ann Intensive Care . 2022 Nov 12. 12 (1):105. [QxMD MEDLINE Link] . [Full Text] .
Zhang Q, et al. Cellular nanosponges inhibit SARS-CoV-2 infectivity. Nano Lett . 2020 Jun 17. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Centers for Disease Control and Prevention. Interim guidelines for collecting, handling, and testing clinical specimens from persons for coronavirus disease 2019 (COVID-19). CDC. Available at https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html . 2020 Nov 05; Accessed: November 16, 2020.
[Guideline] Center for Clinical Standards and Quality/Quality, Safety & Oversight Group. Guidance for Infection Control and Prevention Concerning Coronavirus Disease (COVID-19): FAQs and Considerations for Patient Triage, Placement and Hospital Discharge. Available at https://www.cms.gov/files/document/qso-20-13-hospitalspdf.pdf-2 . March 4, 2020; Accessed: March 4, 2020.
Puopolo KM, Hudak ML, Kimberlin DW, Cummings J. Management of Infants Born to Mothers with COVID-19. American Academy of Pediatrics. Available at https://downloads.aap.org/AAP/PDF/COVID%2019%20Initial%20Newborn%20Guidance.pdf . April 2, 2020; Accessed: April 3, 2020.
[Guideline] AAP. FAQs: Management of Infants Born to Mothers with Suspected or Confirmed COVID-19. American Academy of Pediatrics. Available at https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/faqs-management-of-infants-born-to-covid-19-mothers/ . May 21, 2020; Accessed: May 27, 2020.
[Guideline] COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/ . 2021 Mar 05; Accessed: March 12, 2021.
[Guideline] Moores LK, Tritschler T, Brosnahan S, Carrier M, Collen JF, Doerschug K, et al. Prevention, diagnosis and treatment of venous thromboembolism in patients with COVID-19: CHEST Guideline and Expert Panel Report. Chest . 2020 Jun 2. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost . 2020 May. 18 (5):1023-1026. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] COVID-19 Treatment Guidelines Panel. Antithrombotic Therapy in Patients with COVID-19. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/antithrombotic-therapy/ . 2021 Feb 11; Accessed: June 28, 2021.
World Health Organization. Roadmap to improve and ensure good indoor ventilation in COVID-19 context (1 March 2021). World Health Organization. Available at https://www.who.int/publications/i/item/9789240021280 . March 1, 2021; Accessed: February 20, 2024.
Lim EHT, Vlaar APJ, Bos LDJ, and the, Amsterdam UMC COVID-19 Biobank Investigators. Anti-C5a antibody vilobelimab treatment and the effect on biomarkers of inflammation and coagulation in patients with severe COVID-19: a substudy of the phase 2 PANAMO trial. Respir Res . 2022 Dec 24. 23 (1):375. [QxMD MEDLINE Link] . [Full Text] .
Management Strategies in Children and Adolescents with Mild to Moderate COVID-19. American Academy of Pediatri cs. Available at https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/outpatient-covid-19-management-strategies-in-children-and-adolescents/ . Updated February 8, 2023; Accessed: March 6, 2024.
Huang YH, Kuo HC. Kawasaki Disease, MIS-C and COVID-19. Children (Basel) . 2023 Sep 22. 10 (10): [QxMD MEDLINE Link] . [Full Text] .
Simbar M, Nazarpour S, Sheidaei A. Evaluation of pregnancy outcomes in mothers with COVID-19 infection: a systematic review and meta-analysis. J Obstet Gynaecol . 2023 Dec. 43 (1):2162867. [QxMD MEDLINE Link] .
Rojas-Suarez J, Miranda J. Coronavirus Disease-2019 in Pregnancy. Clin Chest Med . 2023 Jun. 44 (2):373-384. [QxMD MEDLINE Link] .
He YF, Liu JQ, Hu XD, Li HM, Wu N, Wang J, et al. Breastfeeding vs. breast milk transmission during COVID-19 pandemic, which is more important?. Front Pediatr . 2023. 11:1253333. [QxMD MEDLINE Link] . [Full Text] .
Weiss A, Donnachie E, Beyerlein A, Ziegler AG, Bonifacio E. Type 1 Diabetes Incidence and Risk in Children With a Diagnosis of COVID-19. JAMA . 2023 Jun 20. 329 (23):2089-2091. [QxMD MEDLINE Link] .
Lippi G, Sanchis-Gomar F, Henry BM. COVID-19 and its long-term sequelae: what do we know in 2023?. Pol Arch Intern Med . 2023. 133: [Full Text] .
Brolly J, Chadwick DR. COVID-19 infection in people living with HIV. Br Med Bull . 2023 Sep 12. 147 (1):20-30. [QxMD MEDLINE Link] . [Full Text] .
- The heart is normal in size. There are diffuse, patchy opacities throughout both lungs, which may represent multifocal viral/bacterial pneumonia versus pulmonary edema. These opacities are particularly confluent along the periphery of the right lung. There is left midlung platelike atelectasis. Obscuration of the left costophrenic angle may represent consolidation versus a pleural effusion with atelectasis. There is no pneumothorax.
- The heart is normal in size. There are bilateral hazy opacities, with lower lobe predominance. These findings are consistent with multifocal/viral pneumonia. No pleural effusion or pneumothorax are seen.
- The heart is normal in size. Patchy opacities are seen throughout the lung fields. Patchy areas of consolidation at the right lung base partially silhouettes the right diaphragm. There is no effusion or pneumothorax. Degenerative changes of the thoracic spine are noted.
- The same patient as above 10 days later.
- The trachea is in midline. The cardiomediastinal silhouette is normal in size. There are diffuse hazy reticulonodular opacities in both lungs. Differential diagnoses include viral pneumonia, multifocal bacterial pneumonia or ARDS. There is no pleural effusion or pneumothorax.
- Axial chest CT demonstrates patchy ground-glass opacities with peripheral distribution.
- Coronal reconstruction chest CT of the same patient above, showing patchy ground-glass opacities.
- Axial chest CT shows bilateral patchy consolidations (arrows), some with peripheral ground-glass opacity. Findings are in peripheral and subpleural distribution.
- Table 1. SARS-CoV-2 Monoclonal Antibodies – inactive EUAs
| Antibody | Description |
|---|---|
| Evusheld (tixagevimab/cilgavimab) | EUA for preexposure prophylaxis halted in January 2023 owing to Omicron XBB VOCs. Initial authorization was based on the phase 3 PROVENT in unvaccinated individuals with comorbidities and a retrospective cohort study of veterans who were immunosuppressed. , ] |
| Bebtelovimab | Data supporting the treatment EUA were primarily based on analyses from the phase 2 BLAZE-4 trial conducted before the emergence of the Omicron BQ.1 and BQ.1.1 VOCs. Most participants were infected with the Delta (49.8%) or Alpha (28.6%) VOCs. ] |
| Sotrovimab | EUA stopped owing to resistance to Omicron BA.2 subvariant. Initial IV and IM authorization based on COMET-ICE and COMET-TAIL studies. , ] |
| Casirivimab/imdevimab | EUA stopped in January 2022, as the Omicron variant is not susceptible. The EUA for treatment was supported by US trials and the UK RECOVERY trial. , , ] |
| Bamlanivimab/etesevimab | EUA revoked in April 2021 as the Delta VOC emerged. Initial EUA was supported by Phase 3 BLAZE-1 trial for treatment and the BLAZE-2 trial for postexposure prophylaxis. , ] |

Contributor Information and Disclosures
David J Cennimo, MD, FAAP, FACP, FIDSA, AAHIVS Associate Professor of Medicine and Pediatrics, Adult and Pediatric Infectious Diseases, Rutgers New Jersey Medical School David J Cennimo, MD, FAAP, FACP, FIDSA, AAHIVS is a member of the following medical societies: American Academy of HIV Medicine , American Academy of Pediatrics , American College of Physicians , American Medical Association , HIV Medicine Association , Infectious Diseases Society of America , Medical Society of New Jersey , Pediatric Infectious Diseases Society Disclosure: Nothing to disclose.
Scott J Bergman, PharmD, FCCP, FIDSA, BCPS, BCIDP Antimicrobial Stewardship Program Coordinator, Infectious Diseases Pharmacy Residency Program Director, Department of Pharmaceutical and Nutrition Care, Division of Infectious Diseases, Nebraska Medicine; Clinical Associate Professor, Department of Pharmacy Practice, College of Pharmacy, University of Nebraska Medical Center Scott J Bergman, PharmD, FCCP, FIDSA, BCPS, BCIDP is a member of the following medical societies: American Association of Colleges of Pharmacy , American College of Clinical Pharmacy , American Pharmacists Association , American Society for Microbiology , American Society of Health-System Pharmacists , Infectious Diseases Society of America , Society of Infectious Diseases Pharmacists Disclosure: Received research grant from: Merck & Co., Inc.
Keith M Olsen, PharmD, FCCP, FCCM Dean and Professor, College of Pharmacy, University of Nebraska Medical Center Disclosure: Nothing to disclose.
Mary L Windle, PharmD Adjunct Associate Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference Disclosure: Nothing to disclose.
Michael Stuart Bronze, MD David Ross Boyd Professor and Chairman, Department of Medicine, Stewart G Wolf Endowed Chair in Internal Medicine, Department of Medicine, University of Oklahoma Health Science Center; Master of the American College of Physicians; Fellow, Infectious Diseases Society of America; Fellow of the Royal College of Physicians, London Michael Stuart Bronze, MD is a member of the following medical societies: Alpha Omega Alpha , American College of Physicians , American Medical Association , Association of Professors of Medicine , Infectious Diseases Society of America , Oklahoma State Medical Association , Southern Society for Clinical Investigation Disclosure: Nothing to disclose.
Molly Marie Miller, PharmD Clinical Infectious Diseases Pharmacist Practitioner, Nebraska Medicine Molly Marie Miller, PharmD is a member of the following medical societies: Society of Infectious Diseases Pharmacists Disclosure: Nothing to disclose.
What would you like to print?
- Print this section
- Print the entire contents of
- Print the entire contents of article

- COVID-19 Variants
- COVID-19 Pulmonary Management
- COVID-19 Reinfections
- Diagnostics for Coronavirus Disease 2019 (COVID-19) Patients
- COVID-19 Treatment: Investigational Drugs and Other Therapies
- Long Coronavirus 2019 (COVID-19)
- Symptoms and Management of Coronavirus Disease 2019 (COVID-19) FAQ
- Picture of COVID-19 in Europe Is Complex
- Sleep Disturbances Increase Susceptibility to COVID-19
- Global Consensus Reached on COVID-19 Vaccine Updates
- Drug Interaction Checker
- Pill Identifier
- Calculators

- 2010comirnaty-covid-19-vaccine-mRNA-pfizer-4000140Drugs Drugs COVID-19 vaccine, mRNA-Pfizer

- 2010covid-19-vaccine-adjuvanted-novavax-4000146Drugs Drugs COVID-19 vaccine, adjuvanted-Novavax
- 2010spikevax-covid-19-vaccine-mRNA-moderna-4000149Drugs Drugs COVID-19 vaccine, mRNA-Moderna
EDITORIAL article
Editorial: coronavirus disease (covid-19): the impact and role of mass media during the pandemic.

- 1 Department of Social and Organizational Psychology, Iscte-University Institute of Lisbon, CIS-IUL, Lisbon, Portugal
- 2 Department of Psychology and Social Work, Mid Sweden University, Östersund, Sweden
- 3 Department of Psychiatry and Psychotherapy, Medical School and University Hospital, Eberhard Karls University of Tübingen, Tübingen, Germany
Editorial on the Research Topic Coronavirus Disease (COVID-19): The Impact and Role of Mass Media During the Pandemic
The outbreak of the coronavirus disease 2019 (COVID-19) has created a global health crisis that had a deep impact on the way we perceive our world and our everyday lives. Not only has the rate of contagion and patterns of transmission threatened our sense of agency, but the safety measures to contain the spread of the virus also required social and physical distancing, preventing us from finding solace in the company of others. Within this context, we launched our Research Topic on March 27th, 2020, and invited researchers to address the Impact and Role of Mass Media During the Pandemic on our lives at individual and social levels.
Despite all the hardships, disruption, and uncertainty brought by the pandemic, we received diverse and insightful manuscript proposals. Frontiers in Psychology published 15 articles, involving 61 authors from 8 countries, which were included in distinct specialized sections, including Health Psychology, Personality and Social Psychology, Emotion Science, and Organizational Psychology. Despite the diversity of this collective endeavor, the contributions fall into four areas of research: (1) the use of media in public health communication; (2) the diffusion of false information; (3) the compliance with the health recommendations; and (4) how media use relates to mental health and well-being.
A first line of research includes contributions examining the use of media in public health communication. Drawing on media messages used in previous health crises, such as Ebola and Zika, Hauer and Sood describe how health organizations use media. They offer a set of recommendations for COVID-19 related media messages, including the importance of message framing, interactive public forums with up-to-date information, and an honest communication about what is known and unknown about the pandemic and the virus. Following a content analysis approach, Parvin et al. studied the representations of COVID-19 in the opinion section of five Asian e-newspapers. The authors identified eight main issues (health and drugs, preparedness and awareness, social welfare and humanity, governance and institutions, the environment and wildlife, politics, innovation and technology, and the economy) and examined how e-newspapers from these countries attributed different weights to these issues and how this relates to the countries' cultural specificity. Raccanello et al. show how the internet can be a platform to disseminate a public campaign devised to inform adults about coping strategies that could help children and teenagers deal with the challenges of the pandemic. The authors examined the dissemination of the program through the analysis of website traffic, showing that in the 40 days following publication, the website reached 6,090 visits.
A second related line of research that drew the concern of researchers was the diffusion of false information about COVID-19 through the media. Lobato et al. examined the role of distinct individual differences (political orientation, social dominance orientation, traditionalism, conspiracy ideation, attitudes about science) on the willingness to share misinformation about COVID-19 over social media. The misinformation topics varied between the severity and spread of COVID-19, treatment and prevention, conspiracy theories, and miscellaneous unverifiable claims. Their results from 296 adult participants (Mage = 36.23; 117 women) suggest two different profiles. One indicating that those reporting more liberal positions and lower social dominance were less willing to share conspiracy misinformation. The other profile indicated that participants scoring high on social dominance and low in traditionalism were more willing to share both conspiracy and other miscellaneous claims, but less willing to share misinformation about the severity and spread of COVID-19. Their findings can have relevant contributions for the identification of specific individual profiles related to the widespread of distinct types of misinformation. Dhanani and Franz examined a sample of 1,141 adults (Mage = 44.66; 46.9% female, 74.7% White ethnic identity) living in the United States in March 2020. The authors examined how media consumption and information source were related to knowledge about COVID-19, the endorsement of misinformation about COVID-19, and prejudice toward Asian Americans. Higher levels of trust in informational sources such as public health organizations (e.g., Center for Disease Control) was associated with greater knowledge, lower endorsement of misinformation, and less prejudice toward Asian Americans. Media source was associated with distinct levels of knowledge, willingness to endorsement misinformation and prejudice toward American Asians, with social media use (e.g., Twitter, Facebook) being related with a lower knowledge about COVID-19, higher endorsement of misinformation, and stronger prejudice toward Asian Americans.
A third line of research addressed the factors that could contribute to compliance with the health recommendations to avoid the spread of the disease. Vai et al. studied early pre-lockdown risk perceptions about COVID-19 and the trust in media sources among 2,223 Italians (Mage = 36.4, 69.2% female). They found that the perceived usefulness of the containment measures (e.g., social distancing) was related to threat perception and efficacy beliefs. Lower threat perception was associated with less perception of utility of the containment measures. Although most participants considered themselves and others capable of taking preventive measures, they saw the measures as generally ineffective. Participants acknowledged using the internet as their main source of information and considered health organizations' websites as the most trustworthy source. Albeit frequently used, social media was in general considered an unreliable source of information. Tomczyk et al. studied knowledge about preventive behaviors, risk perception, stigmatizing attitudes (support for discrimination and blame), and sociodemographic data (e.g., age, gender, country of origin, education level, region, persons per household) as predictors of compliance with the behavioral recommendations among 157 Germans, (age range: 18–77 years, 80% female). Low compliance was associated with male gender, younger age, and lower public stigma. Regarding stigmatizing attitudes, the authors only found a relation between support for discrimination (i.e., support for compulsory measures) and higher intention to comply with recommendations. Mahmood et al. studied the relation between social media use, risk perception, preventive behaviors, and self-efficacy in a sample of 310 Pakistani adults (54.2% female). The authors found social media use to be positively related to self-efficacy and perceived threat, which were both positively related to preventive behaviors (e.g., hand hygiene, social distancing). Information credibility was also related to compliance with health recommendations. Lep et al. examined the relationship between information source perceived credibility and trust, and participants' levels of self-protective behavior among 1,718 Slovenians (age range: 18–81 years, 81.7% female). The authors found that scientists, general practitioners (family doctors), and the National Institute of Public Health were perceived as the more credible source of information, while social media and government officials received the lowest ratings. Perceived information credibility was found to be associated with lower levels of negative emotional responses (e.g., nervousness, helplessness) and a higher level of observance of self-protective measures (e.g., hand washing). Siebenhaar et al. also studied the link between compliance, distress by information, and information avoidance. They examined the online survey responses of 1,059 adults living in Germany (Mage = 39.53, 79.4% female). Their results suggested that distress by information could lead to higher compliance with preventive measures. Distress by information was also associated with higher information avoidance, which in turn is related to less compliance. Gantiva et al. studied the effectiveness of different messages regarding the intentions toward self-care behaviors, perceived efficacy to motivate self-care behaviors in others, perceived risk, and perceived message strength, in a sample of 319 Colombians (age range: 18–60 years, 69.9% female). Their experiment included the manipulation of message framing (gain vs. loss) and message content (economy vs. health). Participants judged gain-frame health related messages to be stronger and more effective in changing self-behavior, whereas loss-framed health messages resulted in increased perceived risk. Rahn et al. offer a comparative view of compliance and risk perception, examining three hazard types: COVID-19 pandemic, violent acts, and severe weather. With a sample of 403 Germans (age range: 18–89 years, 72% female), they studied how age, gender, previous hazard experience and different components of risk appraisal (perceived severity, anticipated negative emotions, anticipatory worry, and risk perception) were related to the intention to comply with behavioral recommendations. They found that higher age predicted compliance with health recommendations to prevent COVID-19, anticipatory worry predicted compliance with warning messages regarding violent acts, and women complied more often with severe weather recommendations than men.
A fourth line of research examined media use, mental health and well-being during the COVID-19 pandemic. Gabbiadini et al. addressed the use of digital technology (e.g., voice/video calls, online games, watching movies in party mode) to stay connected with others during lockdown. Participants, 465 Italians (age range: 18–73 years, 348 female), reported more perceived social support associated with the use of these digital technologies, which in turn was associated with fewer feelings of loneliness, boredom, anger, and higher sense of belongingness. Muñiz-Velázquez et al. compared the media habits of 249 Spanish adults (Mage = 42.06, 53.8% female) before and during confinement. They compared the type of media consumed (e.g., watching TV series, listening to radio, watching news) and found the increased consumption of TV and social networking sites during confinement to be negatively associated with reported level of happiness. People who reported higher levels of well-being also reported watching less TV and less use of social networking sites. Majeed et al. , on the other hand, examined the relation between problematic social media use, fear of COVID-19, depression, and mindfulness. Their study, involving 267 Pakistani adults (90 female), suggested trait mindfulness had a buffer effect, reducing the impact of problematic media use and fear of COVID-19 on depression.
Taken together, these findings highlight how using different frames for mass media gives a more expansive view of its positive and negative roles, but also showcase the major concerns in the context of a pandemic crisis. As limitations we highlight the use of cross-sectional designs in most studies, not allowing to establish true inferences of causal relationships. The outcome of some studies may also be limited by the unbalanced number of female and male participants, by the non-probability sampling method used, and by the restricted time frame in which the research occurred. Nevertheless, we are confident that all the selected studies in our Research Topic bring important and enduring contributions to the understanding of how media, individual differences, and social factors intertwine to shape our lives, which can also be useful to guide public policies during these challenging times.
Author Contributions
PA: conceptualization, writing the original draft, funding acquisition, writing—review, and editing. FE: conceptualization, writing—review, and editing. MP: writing—review and editing. NP: conceptualization, writing the original draft, writing—review, and editing. All authors approved the submitted version.
PA and NP received partial support to work on this Research Topic through Fundação para a Ciência e Tecnologia (FCT) with reference to the project PTDC/CCI-INF/29234/2017. MP contribution was supported by the German Research Foundation (DFG, PA847/22-1 and PA847/25-1). The authors are independent of the funders.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to express our gratitude to all the authors who proposed their work, all the researchers who reviewed the submissions to this Research Topic, and to Rob Richards for proofreading the Editorial manuscript.
Keywords: COVID-19, coronavirus disease, mass media, health communication, prevention, intervention, social behavioral changes
Citation: Arriaga P, Esteves F, Pavlova MA and Piçarra N (2021) Editorial: Coronavirus Disease (COVID-19): The Impact and Role of Mass Media During the Pandemic. Front. Psychol. 12:729238. doi: 10.3389/fpsyg.2021.729238
Received: 22 June 2021; Accepted: 30 July 2021; Published: 23 August 2021.
Edited and reviewed by: Eduard Brandstätter , Johannes Kepler University of Linz, Austria
Copyright © 2021 Arriaga, Esteves, Pavlova and Piçarra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrícia Arriaga, patricia.arriaga@iscte-iul.pt
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
The COVID-19 research landscape: Measuring topics and collaborations using scientific literature
Affiliations.
- 1 Institute of Medical Information, Chinese Academy of Medical Sciences.
- 2 Digital China Health Technologies Co. Ltd., Beijing, China.
- PMID: 33120818
- PMCID: PMC7581087
- DOI: 10.1097/MD.0000000000022849
Objectives: The Coronavirus Disease 2019 (COVID-19) caused heavy burdens and brought tremendous challenges to global public health. This study aimed to investigate collaboration relationships, research topics, and research trends on COVID-19 using scientific literature.
Method: COVID-19-related articles published from January 1 to July 1, 2020 were retrieved from PubMed database. A total of 27,370 articles were included. Excel 2010, Medical Text Indexer (MTI), VOSviewer, and D3.js were used to summarize bibliometric features.
Results: The number of the COVID-19 research publications has been continuously increasing after its break. United States was the most productive and active country for COVID-19 research, with the largest number of publications and collaboration relationships. Huazhong University of Science and Technology from China was the most productive institute on the number of publications, and University of Toronto from Canada ranked as Top 1 institute for global research collaboration. Four key research topics were identified, of which the topic of epidemiology and public health interventions has gathered highest attentions. Topic of virus infection and immunity has been more focused during the early stage of COVID-19 outbreak compared with later stage. The topic popularity of clinical symptoms and diagnosis has been steady.
Conclusions: Our topic analysis results revealed that the study of drug treatment was insufficient. To achieve critical breakthroughs of this research area, more interdisciplinary, multi-institutional, and global research collaborations are needed.
PubMed Disclaimer
Conflict of interest statement
The authors report no conflicts of interest.
The growth trend on number…
The growth trend on number of publications about COVID-19 research.
The collaboration network on COVID-19…
The collaboration network on COVID-19 research across countries/regions.
The collaboration network on COVID-19 research across institutes.
The MeSH terms co-occurrence network…
The MeSH terms co-occurrence network on COVID-19 research.
The hierarchy of four identified…
The hierarchy of four identified COVID-19 topics.
Trends of topic popularity.
Similar articles
- Mapping the situation of research on coronavirus disease-19 (COVID-19): a preliminary bibliometric analysis during the early stage of the outbreak. Zyoud SH, Al-Jabi SW. Zyoud SH, et al. BMC Infect Dis. 2020 Aug 1;20(1):561. doi: 10.1186/s12879-020-05293-z. BMC Infect Dis. 2020. PMID: 32738881 Free PMC article.
- [An analysis of global research on SARS-CoV-2]. Zhang L, Li B, Jia P, Pu J, Bai B, Li Y, Zhu P, Li L, Zeng G, Zhao X, Dong S, Liu M, Zhang N. Zhang L, et al. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2020 Apr 25;37(2):236-245. doi: 10.7507/1001-5515.202002034. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2020. PMID: 32329275 Free PMC article. Review. Chinese.
- Research on the global trends of COVID-19 associated acute kidney injury: a bibliometric analysis. Zhao WJ, Tan RZ, Gao J, Su H, Wang L, Liu J. Zhao WJ, et al. Ren Fail. 2024 Dec;46(1):2338484. doi: 10.1080/0886022X.2024.2338484. Epub 2024 Jun 4. Ren Fail. 2024. PMID: 38832469 Free PMC article. Review.
- Rules for scientific progress while living with the COVID-19 Pandemic: from 'benchside' to 'fireside.'. [No authors listed] [No authors listed] Cancer Biol Ther. 2020 Jul 2;21(7):581-582. doi: 10.1080/15384047.2020.1747775. Epub 2020 Mar 30. Cancer Biol Ther. 2020. PMID: 32223689 Free PMC article. No abstract available.
- Indian Publications on SARS-CoV-2: A bibliometric study of WHO COVID-19 database. N VR, Patil SB. N VR, et al. Diabetes Metab Syndr. 2020 Sep-Oct;14(5):1171-1178. doi: 10.1016/j.dsx.2020.07.007. Epub 2020 Jul 10. Diabetes Metab Syndr. 2020. PMID: 32673837 Free PMC article.
- Bibliometric study of the scientific productivity of the COVID-19 impact on constructs affecting happiness in university students. Bedoya-Gonzales M, Yucra-Mamani Y, Aragón-Cruz W, Barrientos-Paredes K, Gómez-Bailón P, Laura-Chauca S, Fuentes-López J, Flores-Gutiérrez C, Cossio-Bolaños M, Gomez-Campos R. Bedoya-Gonzales M, et al. J Educ Health Promot. 2024 Mar 28;13:96. doi: 10.4103/jehp.jehp_615_23. eCollection 2024. J Educ Health Promot. 2024. PMID: 38726077 Free PMC article. Review.
- Evolution of global scientific collaboration in mRNA vaccine research: Insights from bibliometric and social network analysis (2010~2023). Chen J, Pan L, Lu Y, Zhang T, Xu D, Yan S, Ouyang Z. Chen J, et al. Hum Vaccin Immunother. 2023 Dec 15;19(3):2276624. doi: 10.1080/21645515.2023.2276624. Epub 2023 Nov 14. Hum Vaccin Immunother. 2023. PMID: 37964602 Free PMC article.
- COVID-19 imaging, where do we go from here? Bibliometric analysis of medical imaging in COVID-19. Wen R, Zhang M, Xu R, Gao Y, Liu L, Chen H, Wang X, Zhu W, Lin H, Liu C, Zeng X. Wen R, et al. Eur Radiol. 2023 May;33(5):3133-3143. doi: 10.1007/s00330-023-09498-z. Epub 2023 Mar 9. Eur Radiol. 2023. PMID: 36892649 Free PMC article.
- The most influential COVID-19 articles: A systematic review. Ahmad SJ, Degiannis K, Borucki J, Pouwels S, Rawaf DL, Head M, Li CH, Archid R, Ahmed AR, Lala A, Raza W, Mellor K, Wichmann D, Exadaktylos A. Ahmad SJ, et al. New Microbes New Infect. 2023 Mar;52:101094. doi: 10.1016/j.nmni.2023.101094. Epub 2023 Feb 11. New Microbes New Infect. 2023. PMID: 36816491 Free PMC article. Review.
- Current global research landscape on COVID-19 and cancer: Bibliometric and visualization analysis. Zyoud SH, Koni A, Al-Jabi SW, Amer R, Shakhshir M, Al Subu R, Salameh H, Odeh R, Musleh S, Abushamma F, Abu Taha A. Zyoud SH, et al. World J Clin Oncol. 2022 Oct 24;13(10):835-847. doi: 10.5306/wjco.v13.i10.835. World J Clin Oncol. 2022. PMID: 36337308 Free PMC article.
- WHO Director-General's remarks at the media briefing on 2019-nCoV. Available online: www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-med... (July 12, 2020).
- Alzghari SK, Acuna VS. Supportive treatment with tocilizumab for COVID-19: a systematic review. J Clin Virol 2020;127:104380. - PMC - PubMed
- Zhu J, Zhong Z, Li H, et al. CT imaging features of 4121 patients with COVID-19: a meta-analysis. J Med Virol 2020;92:891–902. - PMC - PubMed
- Zhou Y, Chen L. Twenty-year span of global coronavirus research trends: a bibliometric analysis. Int J Environ Res Public Health 2020;17:3082. - PMC - PubMed
- Zhai F, Zhai Y, Cong C, et al. Research progress of coronavirus based on bibliometric analysis. Int J Environ Res Public Health 2020;17:3766. - PMC - PubMed
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Europe PubMed Central
- Ovid Technologies, Inc.
- PubMed Central
- Wolters Kluwer

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Agriculture & Environment
- Architecture & Design
- Arts & Humanities
- Business & Management
- Campus Affairs
- Law & Policy
- Science & Technology
- Social Sciences
- Sports & Recreation
- Story Archive
- For Journalists
Study uncovers the basis of COVID-19-related brain fog

Neurological symptoms have been widely observed in COVID-19 patients, with many survivors exhibiting persistent neurological and cognitive impairment. New research from the University of Minnesota found that COVID-19 triggers inflammation in the brain, which is linked to many COVID-related symptoms such as fatigue and “brain fog.” Published in Frontiers in Microbiology , the researchers examined the specific ways the virus impacts the brain, developing a preclinical model to accurately mimic the effects of COVID-19 on humans to explore the impact of age and sex on the brain-related impacts of the disease.
The study was led by Maxim Cheeran in the College of Veterinary Medicine, Ling Li in the College of Pharmacy and Walter Low in the Medical School. The findings include:
- SARS-CoV-2 infection triggered a neuroinflammatory response, despite the lack of detectable virus in the brain.
- Older age males showed a higher viral load and more pronounced symptoms.
- Older age also led to increased neurological inflammation.
The researchers also found several biological pathways that the virus impacts in the brain, including overactive or misdirected immune response, disruption to the protective blood-brain barrier, damage to cells lining blood vessels, and impacts to how nerve cells are formed and function. These impacts can impair brain function and may even lead to permanent damage.
“There are still things we don’t know about how coronavirus infection affects the brain, and our research is beginning to unravel the mystery,” said Cheeran. “We now know that viral infection in the lungs can cause inflammatory changes in the brain, affected by the age and sex of the patient. With this model, we can further examine the connections between the infected lung and the brain, and start development on targeted treatments to mitigate the impact of COVID-19 on the brain.”
In addition to new targeted therapies, these findings allow for more accurate vaccine development and continued research. The research team has a study underway that could shed light on the persistence of these symptoms in individuals experiencing long COVID, including the long-term impacts on neurocognitive behavior and memory loss.
This work was supported in part by grants from the National Institutes of Health/National Institute on Aging and the SURRGE award program of the University of Minnesota’s College of Pharmacy.
About the College of Veterinary Medicine The University of Minnesota College of Veterinary Medicine affects the lives of animals and people every day through educational, research, service, and outreach programs. Established in 1947, the University of Minnesota College of Veterinary Medicine is Minnesota’s only veterinary college. Fully accredited, the college has graduated over 4,000 veterinarians and hundreds of scientists. The college is also home to the Veterinary Medical Center, the Veterinary Diagnostic Laboratory, the Leatherdale Equine Center and The Raptor Center. Learn more at vetmed.umn.edu. About the College of Pharmacy Founded in 1892, the University of Minnesota College of Pharmacy is the only pharmacy school in Minnesota, with campuses in the Twin Cities and in Duluth. The College of Pharmacy improves health through innovative education, pioneering research and interdisciplinary practice development that attends to the diverse needs of the people of Minnesota and the world. Learn more at pharmacy.umn.edu.
About the Medical School The University of Minnesota Medical School is at the forefront of learning and discovery, transforming medical care and educating the next generation of physicians. Our graduates and faculty produce high-impact biomedical research and advance the practice of medicine. We acknowledge that the U of M Medical School is located on traditional, ancestral and contemporary lands of the Dakota and the Ojibwe, and scores of other Indigenous people, and we affirm our commitment to tribal communities and their sovereignty as we seek to improve and strengthen our relations with tribal nations. Learn more at med.umn.edu.
- Categories:
- Link to share current story on Twitter
- Link to share current story on Facebook
- Link to share current story via email
Media Contacts
Rachel cain, more stories in health.

Changing minds about substance use
A new graduate helped launch a nonprofit to educate high school students about addiction.

Talking back-to-school nutrition with U of M
Back-to-school means new schedules and routines for the whole family, and meal times are no exception.
Editor's picks

The technicolor world of life after glaciers
As the Andean glaciers retreat, Mariana Cardenas studies how lichens pave the way for new ecosystems.

Athletes with U of M connections in the Paris Paralympics
As the Paralympic Games in Paris commence, you can follow four athletes with ties to the University of Minnesota.

Twelve great U of M things to do at the fair
The University of Minnesota is a mainstay at the Minnesota State Fair. Here are a dozen attractions that highlight the U’s countless contributions to the state.
Confused by the Latest COVID-19 Medical Study? Read This
BY KATHY KATELLA January 6, 2021

Note: Information in this article was accurate at the time of original publication. Because information about COVID-19 changes rapidly, we encourage you to visit the websites of the Centers for Disease Control & Prevention (CDC), World Health Organization (WHO), and your state and local government for the latest information.
What exactly is a clinical trial? What the heck is a meta-analysis? Why are people talking about preprints—and do I really need to know?
You’re ahead of the game if you know any of these terms. But there is more to learn. Research is racing along at a dizzying pace as scientists seek to understand and identify solutions to the COVID-19 pandemic. Where knowing a few random terms might have been helpful before the pandemic, now that level of knowledge just skims the surface.
F. Perry Wilson, MD , a Yale nephrologist, teaches a free online course called, " Understanding Medical Research: Your Facebook Friend is Wrong " on the Coursera platform that has attracted 38,000 followers since it was launched in March.
The first thing people need to know, Wilson says, is that research results reported in the media have been interpreted by journalists (who may also cover politics, sports, and other topics)—even science journalists may be looking at the data through a particular filter. “And then that information is filtered through the lens of social media, with your friends, family, and coworkers offering their personal insights,” he says. “This is like a game of telephone, and a lot can change in the process.”
The rapidity at which new information is released can compound the confusion, says Jaimie Meyer, MD, MS , a Yale Medicine infectious diseases specialist. “People who don’t live in the world of scientific research need to know that normally the pace is extremely slow, and it’s slow, in part, because it’s careful,” she says. “But now data is coming in so quickly that you may be reacting to things that aren’t fully vetted, and you have to interpret everything in that light.”
Both doctors agree that it could be helpful for people to know more about medical studies, including the terminology researchers use—and it might even help to look up an actual study in some cases.
We put together a vocabulary to make it easier.
Launching a study
A medical study starts with a question, along with a carefully formatted plan to answer it. It also takes time, input, and, in most cases, money. Here are some basic terms to know.
Hypothesis: A question (and proposed solution/answer) that serves as a starting point for investigation. It could take years to test a hypothesis. Example: Will patients who take a new drug (tested in the study) have significantly lower blood pressure than those who don’t take it?
Placebo : A pill, liquid, or powder, for example, that looks like the drug being studied but is made of inactive ingredients. Placebos are used to ensure that participants can’t tell if they are receiving a medication, since that might affect how they report their symptoms.
Research grant or funding: Money to fund part or all of a study. Money can come from a variety of sources, including academia, the government (such as the National Institutes of Health [NIH]), a philanthropic source, a pharmaceutical company, and even crowdfunding.
“There's no doubt that different funders have different priorities and those priorities are not always exclusively in the best interest of people’s health and well-being,” says Dr. Wilson. For that reason, he advises looking at how a study was funded, what role the funder may have played in the study, and whether the funder contributed to decisions about whether to publish the results.
What you want to evaluate here, Dr. Meyer says, is whether the people analyzing, interpreting, and presenting the results have any real or perceived conflicts of interest that might affect how those tasks are done.
Types of studies
There are different approaches to research, and knowing what kind of study you are looking at can help you put its results into context. For example, studying a new treatment in a lab may provide important information about its potential, but there may still be questions about how that treatment will perform when used to treat people in a clinical setting. Here are some types of studies.
Basic science study (also called “bench research”): A study conducted in a lab that does not involve human subjects; it may involve cells or animals, for example.
Preclinical research: The trial process begins with what’s called “preclinical research.” This is when drugs or products are first tested on cell cultures and animals—not humans. If it passes the preclinical phase, it can move on to clinical trials.
Clinical trial (also known as an interventional study): A research study in which one or more human subjects are assigned to one or more interventions (these could include a treatment first studied in a laboratory). An intervention is a procedure or treatment such as a drug, nutritional supplement, vaccine, behavior (like exercise or diet), device, or anything else that is provided for clinical research purposes.
Phases of clinical trials: Studies that focus on drugs or biological products undergo phases based on definitions developed by the Food and Drug Administration (FDA):
- Phase I: An experimental treatment is given to a handful of patients. Researchers may assess how participants’ bodies react to the treatment, watch for side effects, determine a safe dose, and decide on the best way to give the medication (orally or by infusion).
- Phase II: The drug or treatment is tried on a larger group of patients (the number varies, but it can be up to several hundred). Researchers aim to determine the effect the treatment has on a disease and the rest of the body. Often, these studies are too small to tell if the treatment affects certain outcomes (like death), but they may show an improvement in laboratory values, symptoms, or other markers.
- Phase III: Larger groups (from hundreds to up to tens of thousands) are given the treatment, and researchers compare its effectiveness with placebo or standard treatments. Phase III trials are designed to test whether the treatment improves very important outcomes (like death, or remission of a disease), says Dr. Wilson. Once this is complete, a new drug application can be filed with the FDA, which also reviews all other available research. If the FDA approves it, doctors can use the treatment on patients. The standard approval procedure at the FDA requires two Phase III trials showing efficacy. You can get approval with just one trial if the treatment addresses a rare disease or if there are no existing treatments for the condition, adds Dr. Wilson.
- Phase IV: Once the product reaches the public, researchers look to see what happens to people who get the medication or intervention in the “real world.” This phase provides additional information about long-term risks and benefits, and the best way to prescribe the drug.
Observational study: Researchers observe a group of individuals and measure their outcomes. Participants are not given any special interventions.
Cohort study: A type of observational study defined by similarities in the group being studied (for example a study of older Americans living alone, or a study of smokers). Cohort studies follow that group to observe what outcomes happen to them.
Randomized controlled trial: A study that randomly assigns participants into different groups. It is known as “the gold standard” because it is a way to reduce bias by balancing certain characteristics participants might have (like gender, ethnicity, or income) between groups.
Systematic review: A review of all previous research designed to answer a specific research question.
Meta-analysis: Like a systematic review, a meta-analysis analyzes data from multiple studies that have been done on a particular subject to derive conclusions based on the whole body of research. But a meta-analysis combines the data from those prior studies to arrive at an “average” effect of the intervention.
Single- and double-blind studies: Two different approaches to testing a treatment or other intervention. Participants in a single-blind study don’t know which treatment, if any, they are getting. In a double-blind study, neither the subjects nor the researchers know which treatments participants are receiving.
Conducting a study
To conduct a medical study properly, researchers need to determine what kinds of trial participants to recruit. They also need someone to lead the study. Here are some basic terms.
Principal investigator (PI): The person who acquires funding for the study, designs the study, and carries out the plan for the research. He or she is responsible for all of the oversight of the study. He or she is usually the first or last author on the paper when it is published.
Healthy volunteer/patient volunteer: The former is a study participant volunteer who has no health problems. The latter has a health problem and participates to help researchers better understand, diagnose, treat, or cure that problem.
Eligibility criteria: Key requirements for clinical trial participants that could include such things as age, or health or gender status. A study may have inclusion criteria that a person must meet to participate, and exclusion criteria that bars them from joining the study.
Dr. Meyer notes that elderly people and women who are pregnant or of “childbearing potential” are commonly excluded from clinical trials, which can lead to potential questions about a drug's effectiveness in those populations. “If you fall into one of these groups, you may not know for sure whether a drug tested in a trial is safe or effective in someone like you,” she says.
Control Group: A group of clinical trial participants who are assigned a placebo or an intervention other than the primary one being tested (researchers may test against the existing standard of care or “treatment as usual”) so that researchers can compare the two.
Randomization: The process of assigning clinical trial participants to treatment or control groups randomly, using the element of chance to reduce bias. “Randomization is now usually done by a computer, but one can think of it as a “coin flip,” says Dr. Wilson.
Variable: An attribute that varies among study participants. In a study that involves people in general, participants may have variable attributes in such areas as income, gender, health status, weight, or whether or not the participants are smokers.
Adverse event: A negative change in the health of a study participant. This may or may not have been caused by the intervention given in the study.
Serious adverse events are pre-defined and, if they occur during the course of a study, an oversight committee can pause the study. “This is especially important to understand as multiple clinical trials of COVID-19 vaccines were paused at one point,” says Dr. Meyer, “but this is a normal part of carrying out clinical trials.”
Publication of the study
Usually, it can take a decade from the moment the researcher asks the initial question to the time a fully researched and vetted paper is published, and publication is by no means the final word on the topic. For instance, there may need to be multiple studies of a particular drug before the FDA will approve it and doctors will use it to treat patients. It’s important to keep in mind, especially as scientists work more quickly toward solutions for COVID-19, that some published research is released before it’s fully reviewed, in a form known as preprints.
Peer review: A review of a study by multiple experts who provide criticism and, in most cases, ask for revisions. Reviewers are often anonymous to the authors of the paper and are almost never paid for their services. The peer review cycle may be repeated multiple times.
Preprint: An early draft of a research study that has not yet gone through a formal peer-review process. The use of preprints has exploded during the pandemic, say the doctors. “You’re seeing things that are very raw. Often through the process of rejection and revision, these papers are substantially revised,” says Dr. Meyer.
While preprints can provide the research community with a wealth of data and opportunities for collaboration, lay readers need to be wary, says Dr. Meyer. Dr. Wilson agrees. “I have seen the media use these pre-peer-reviewed studies as the basis for stories. Often, the good stories will say that the study has not yet been peer-reviewed, but even that tends to get lost in the interpretation.”
Rapid review: An approach to reviewing a medical study that follows the principles of a formal peer review process, but that is completed more quickly. Because reviewers are asked to complete reviews on an expedited track, journal editors use this judiciously, applying it only to those papers that have urgent implications for basic science or clinical care.
Impact factor (IF): A number that reflects the yearly average number of citations that articles published in the previous two years in a given journal received. The impact factor is a way to show a journal’s importance, especially when compared to others in the same field. A higher IF generally indicates that a journal has greater importance “One thing people might not be aware of is that there are a ton of journals out there, and there is a huge difference between them in terms of quality, scope, and impact,” says Dr. Meyer. Some journals are not peer-reviewed, and some are frankly biased, she says. “ The Journal of the American Medical Association [ JAMA ] and The Lancet are seen as very high-impact journals, and they will only accept manuscripts and data of the highest quality.”
Open access: An approach to publishing a study that makes it free to anyone to read. The downside is that journals that provide open access may charge researchers high fees to publish a study, says Dr. Wilson. “There is controversy as some of these journals may offer to publish a study without conducting adequate peer reviews—a practice called predatory publishing,” he says.
How to read the actual study
You can go beyond the headlines about new research and read the actual studies. Some journals may charge fees to read them, but there are a variety of ways to get around the paywalls. You can check your local library, which may subscribe to some medical journals. Another option is PubMed , a government database of citations and abstracts that often includes links to the full-text articles on the publishers' websites.(PubMedCentral is an archive for free full-text biomedical and life sciences journal articles.) Yet another approach is to email one of the study’s authors directly—the first or last author listed usually includes his or her email with the abstract, which is free to read.
Abstract: A brief overview of a published paper. “If you really want to understand a study, you should at least read the abstract,” says Dr. Meyer. “It’s a quick read, and it’s raw, not refined by other people’s viewpoints.”
Introduction: Essentially the background, including previous research and the rationale behind the decision to conduct the study. The hypothesis and objectives are usually included here.
Methods: Includes such essential details as the eligibility criteria for the participants (if it’s a clinical study) and how they were recruited, how the study was conducted, and what equipment, instruments, and procedures were used.
Results: Just the raw data, sometimes accompanied by a chart, graph, or table. If something changed during the time the study was being conducted—if some participants left, for instance—this section should provide an explanation.
Conclusion/Discussion: An interpretation of the results that may include opinions from the study’s authors. The strengths and limitations of the study may be included here, as well as recommendations for further research.
When one study contradicts another
Even if you understand how studies work, it can be confusing when one study contradicts another, or experts disagree. But that’s normal, according to the doctors. “Everything we do in science really needs to be interpreted in terms of all of the things coming out, not just the one sexy paper,” says Dr. Meyer. The picture can change long after a study is published, as more studies are completed and doctors put a new treatment that has been studied into practical use, she says.
But, if a piece of research does catch your attention—or you want to know more about vaccines and potential treatments for COVID-19, for example—Dr. Wilson says it still might be useful to do a deep dive and read a few studies.
“If you find something that could make a difference in your own life, I would encourage you to go to the primary source,” he says. “We need to embrace the scientific method now, more than ever. We've got a challenge in front of us that I think we have a good chance of beating, but we can only do that with the best quality science.”
For more information, read our COVID-19 glossary.
Information provided in Yale Medicine articles is for general informational purposes only. No content in the articles should ever be used as a substitute for medical advice from your doctor or other qualified clinician. Always seek the individual advice of your health care provider with any questions you have regarding a medical condition.
More news from Yale Medicine

Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Browse by collection
- BMJ Journals
You are here
- Volume 10, Issue 11
- The Philippine COVID-19 Outcomes: a Retrospective study Of Neurological manifestations and Associated symptoms (The Philippine CORONA study): a protocol study
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0001-5621-1833 Adrian I Espiritu 1 , 2 ,
- http://orcid.org/0000-0003-1135-6400 Marie Charmaine C Sy 1 ,
- http://orcid.org/0000-0002-1241-8805 Veeda Michelle M Anlacan 1 ,
- http://orcid.org/0000-0001-5317-7369 Roland Dominic G Jamora 1
- 1 Department of Neurosciences , College of Medicine and Philippine General Hospital, University of the Philippines Manila , Manila , Philippines
- 2 Department of Clinical Epidemiology, College of Medicine , University of the Philippines Manila , Manila , Philippines
- Correspondence to Dr Adrian I Espiritu; aiespiritu{at}up.edu.ph
Introduction The SARS-CoV-2, virus that caused the COVID-19 global pandemic, possesses a neuroinvasive potential. Patients with COVID-19 infection present with neurological signs and symptoms aside from the usual respiratory affectation. Moreover, COVID-19 is associated with several neurological diseases and complications, which may eventually affect clinical outcomes.
Objectives The Philippine COVID-19 Outcomes: a Retrospective study Of Neurological manifestations and Associated symptoms (The Philippine CORONA) study investigators will conduct a nationwide, multicentre study involving 37 institutions that aims to determine the neurological manifestations and factors associated with clinical outcomes in COVID-19 infection.
Methodology and analysis This is a retrospective cohort study (comparative between patients with and without neurological manifestations) via medical chart review involving adult patients with COVID-19 infection. Sample size was determined at 1342 patients. Demographic, clinical and neurological profiles will be obtained and summarised using descriptive statistics. Student’s t-test for two independent samples and χ 2 test will be used to determine differences between distributions. HRs and 95% CI will be used as an outcome measure. Kaplan-Meier curves will be constructed to plot the time to onset of mortality (survival), respiratory failure, intensive care unit (ICU) admission, duration of ventilator dependence, length of ICU stay and length of hospital stay. The log-rank test will be employed to compare the Kaplan-Meier curves. Stratified analysis will be performed to identify confounders and effects modifiers. To compute for adjusted HR with 95% CI, crude HR of outcomes will be adjusted according to the prespecified possible confounders. Cox proportional regression models will be used to determine significant factors of outcomes. Testing for goodness of fit will also be done using Hosmer-Lemeshow test. Subgroup analysis will be performed for proven prespecified effect modifiers. The effects of missing data and outliers will also be evaluated in this study.
Ethics and dissemination This protocol was approved by the Single Joint Research Ethics Board of the Philippine Department of Health (SJREB-2020–24) and the institutional review board of the different study sites. The dissemination of results will be conducted through scientific/medical conferences and through journal publication. The lay versions of the results may be provided on request.
Trial registration number NCT04386083 .
- adult neurology
- epidemiology
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjopen-2020-040944
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Strengths and limitations of this study
The Philippine COVID-19 Outcomes: a Retrospective study Of Neurological manifestations and Associated symptoms Study is a nationwide, multicentre, retrospective, cohort study with 37 Philippine sites.
Full spectrum of neurological manifestations of COVID-19 will be collected.
Retrospective gathering of data offers virtually no risk of COVID-19 infection to data collectors.
Data from COVID-19 patients who did not go to the hospital are unobtainable.
Recoding bias is inherent due to the retrospective nature of the study.
Introduction
The COVID-19 has been identified as the cause of an outbreak of respiratory illness in Wuhan, Hubei Province, China, in December 2019. 1 The COVID-19 pandemic has reached the Philippines with most of its cases found in the National Capital Region (NCR). 2 The major clinical features of COVID-19 include fever, cough, shortness of breath, myalgia, headache and diarrhoea. 3 The outcomes of this disease lead to prolonged hospital stay, intensive care unit (ICU) admission, dependence on invasive mechanical ventilation, respiratory failure and mortality. 4 The specific pathogen that causes this clinical syndrome has been named SARS-CoV-2, which is phylogenetically similar to SARS-CoV. 4 Like the SARS-CoV strain, SARS-CoV-2 may possess a similar neuroinvasive potential. 5
A study on cases with COVID-19 found that about 36.4% of patients displayed neurological manifestations of the central nervous system (CNS) and peripheral nervous system (PNS). 6 The associated spectrum of symptoms and signs were substantially broad such as altered mental status, headache, cognitive impairment, agitation, dysexecutive syndrome, seizures, corticospinal tract signs, dysgeusia, extraocular movement abnormalities and myalgia. 7–12 Several reports were published on neurological disorders associated with patients with COVID-19, including cerebrovascular disorders, encephalopathy, hypoxic brain injury, frequent convulsive seizures and inflammatory CNS syndromes like encephalitis, meningitis, acute disseminated encephalomyelitis and Guillain-Barre syndrome. 7–16 However, the estimates of the occurrences of these manifestations were based on studies with a relatively small sample size. Furthermore, the current description of COVID-19 neurological features are hampered to some extent by exceedingly variable reporting; thus, defining causality between this infection and certain neurological manifestations is crucial since this may lead to considerable complications. 17 An Italian observational study protocol on neurological manifestations has also been published to further document and corroborate these findings. 18
Epidemiological data on the proportions and spectrum of non-respiratory symptoms and complications may be essential to increase the recognition of clinicians of the possibility of COVID-19 infection in the presence of other symptoms, particularly neurological manifestations. With this information, the probabilities of diagnosing COVID-19 disease may be strengthened depending on the presence of certain neurological manifestations. Furthermore, knowledge of other unrecognised symptoms and complications may allow early diagnosis that may permit early institution of personal protective equipment and proper contact precautions. Lastly, the presence of neurological manifestations may be used for estimating the risk of certain important clinical outcomes for better and well-informed clinical decisions in patients with COVID-19 disease.
To address this lack of important information in the overall management of patients with COVID-19, we organised a research study entitled ‘The Philippine COVID-19 Outcomes: a Retrospective study Of Neurological manifestations and Associated symptoms (The Philippine CORONA Study)’.
This quantitative, retrospective cohort, multicentre study aims: (1) to determine the demographic, clinical and neurological profile of patients with COVID-19 disease in the Philippines; (2) to determine the frequency of neurological symptoms and new-onset neurological disorders/complications in patients with COVID-19 disease; (3) to determine the neurological manifestations that are significant factors of mortality, respiratory failure, duration of ventilator dependence, ICU admission, length of ICU stay and length of hospital stay among patients with COVID-19 disease; (4) to determine if there is significant difference between COVID-19 patients with neurological manifestations compared with those COVID-19 patients without neurological manifestations in terms of mortality, respiratory failure, duration of ventilator dependence, ICU admission, length of ICU stay and length of hospital stay; and (5) to determine the likelihood of mortality, respiratory failure and ICU admission, including the likelihood of longer duration of ventilator dependence and length of ICU and hospital stay in COVID-19 patients with neurological manifestations compared with those without neurological manifestations.
Scope, limitations and delimitations
The study will include confirmed cases of COVID-19 from the 37 participating institutions in the Philippines. Every country has its own healthcare system, whose level of development and strategies ultimately affect patient outcomes. Thus, the results of this study cannot be accurately generalised to other settings. In addition, patients with ages ≤18 years will be excluded in from this study. These younger patients may have different characteristics and outcomes; therefore, yielded estimates for adults in this study may not be applicable to this population subgroup. Moreover, this study will collect data from the patient records of patients with COVID-19; thus, data from patients with mild symptoms who did not go to the hospital and those who had spontaneous resolution of symptoms despite true infection with COVID-19 are unobtainable.
Methodology
To improve the quality of reporting of this study, the guidelines issued by the Strengthening the Reporting of Observational Studies in Epidemiology Initiative will be followed. 19
Study design
The study will be conducted using a retrospective cohort (comparative) design (see figure 1 ).
- Download figure
- Open in new tab
- Download powerpoint
Schematic diagram of the study flow.
Study sites and duration
We will conduct a nationwide, multicentre study involving 37 institutions in the Philippines (see figure 2 ). Most of these study sites can be found in the NCR, which remains to be the epicentre of the COVID-19 pandemic. 2 We will collect data for 6 months after institutional review board approval for every site.
Location of 37 study sites of the Philippine CORONA study.
Patient selection and cohort description
The cases will be identified using the designated COVID-19 censuses of all the participating centres. A total enumeration of patients with confirmed COVID-19 disease will be done in this study.
The cases identified should satisfy the following inclusion criteria: (A) adult patients at least 19 years of age; (B) cases confirmed by testing approved patient samples (ie, nasal swab, sputum and bronchoalveolar lavage fluid) employing real-time reverse transcription PCR (rRT-PCR) 20 from COVID-19 testing centres accredited by the Department of Health (DOH) of the Philippines, with clinical symptoms and signs attributable to COVID-19 disease (ie, respiratory as well as non-respiratory clinical signs and symptoms) 21 ; and (C) cases with disposition (ie, discharged stable/recovered, home/discharged against medical advice, transferred to other hospital or died) at the end of the study period. Cases with conditions or diseases caused by other organisms (ie, bacteria, other viruses, fungi and so on) or caused by other pathologies unrelated to COVID-19 disease (ie, trauma) will be excluded.
The first cohort will involve patients with confirmed COVID-19 infection who presented with any neurological manifestation/s (ie, symptoms or complications/disorder). The comparator cohort will compose of patients with confirmed COVID-19 infection without neurological manifestation/s.
Sample size calculation
We looked into the mortality outcome measure for the purposes of sample size computation. Following the cohort study of Khaledifar et al , 22 the sample size was calculated using the following parameters: two-sided 95% significance level (1 – α); 80% power (1 – β); unexposed/exposed ratio of 1; 5% of unexposed with outcome (case fatality rate from COVID19-Philippines Dashboard Tracker (PH) 23 as of 8 April 2020); and assumed risk ratio 2 (to see a two-fold increase in risk of mortality when neurological symptoms are present).
When these values were plugged in to the formula for cohort studies, 24 a minimum sample size of 1118 is required. To account for possible incomplete data, the sample was adjusted for 20% more. This means that the total sample size required is 1342 patients, which will be gathered from the participating centres.
Data collection
We formulated an electronic data collection form using Epi Info Software (V.7.2.2.16). The forms will be pilot-tested, and a formal data collection workshop will be conducted to ensure collection accuracy. The data will be obtained from the review of the medical records.
The following pertinent data will be obtained: (A) demographic data; (B) other clinical profile data/comorbidities; (C) neurological history; (D) date of illness onset; (E) respiratory and constitutional symptoms associated with COVID-19; (F) COVID-19 disease severity 25 at nadir; (G) data if neurological manifestation/s were present at onset prior to respiratory symptoms and the specific neurological manifestation/s present at onset; (H) neurological symptoms; (i) date of neurological symptom onset; (J) new-onset neurological disorders or complications; (K) date of new neurological disorder or complication onset; (L) imaging done; (M) cerebrospinal fluid analysis; (N) electrophysiological studies; (O) treatment given; (P) antibiotics given; (Q) neurological interventions given; (R) date of mortality and cause/s of mortality; (S) date of respiratory failure onset, date of mechanical ventilator cessation and cause/s of respiratory failure; (T) date of first day of ICU admission, date of discharge from ICU and indication/s for ICU admission; (U) other neurological outcomes at discharge; (V) date of hospital discharge; and (W) final disposition. See table 1 for the summary of the data to be collected for this study.
- View inline
Data to be collected in this study
Main outcomes considered
The following patient outcomes will be considered for this study:
Mortality (binary outcome): defined as the patients with confirmed COVID-19 who died.
Respiratory failure (binary outcome): defined as the patients with confirmed COVID-19 who experienced clinical symptoms and signs of respiratory insufficiency. Clinically, this condition may manifest as tachypnoea/sign of increased work of breathing (ie, respiratory rate of ≥22), abnormal blood gases (ie, hypoxaemia as evidenced by partial pressure of oxygen (PaO 2 ) <60 or hypercapnia by partial pressure of carbon dioxide of >45), or requiring oxygen supplementation (ie, PaO 2 <60 or ratio of PaO 2 /fraction of inspired oxygen (P/F ratio)) <300).
Duration of ventilator dependence (continuous outcome): defined as the number of days from initiation of assisted ventilation to cessation of mechanical ventilator use.
ICU admission (binary outcome): defined as the patients with confirmed COVID-19 admitted to an ICU or ICU-comparable setting.
Length of ICU stay (continuous outcome): defined as the number of days admitted in the ICU or ICU-comparable setting.
Length of hospital stay (continuous outcome): defined as the number of days from admission to discharge.
Data analysis plan
Statistical analysis will be performed using Stata V.7.2.2.16.
Demographic, clinical and neurological profiles will be summarised using descriptive statistics, in which categorical variables will be expressed as frequencies with corresponding percentages, and continuous variables will be pooled using means (SD).
Student’s t-test for two independent samples and χ 2 test will be used to determine differences between distributions.
HRs and 95% CI will be used as an outcome measure. Kaplan-Meier curves will be constructed to plot the time to onset of mortality (survival), respiratory failure, ICU admission, duration of ventilator dependence (recategorised binary form), length of ICU stay (recategorised binary form) and length of hospital stay (recategorised binary form). Log-rank test will be employed to compare the Kaplan-Meier curves. Stratified analysis will be performed to identify confounders and effects modifiers. To compute for adjusted HR with 95% CI, crude HR of outcomes at discrete time points will be adjusted for prespecified possible confounders such as age, history of cardiovascular or cerebrovascular disease, hypertension, diabetes mellitus, and respiratory disease, COVID-19 disease severity at nadir, and other significant confounding factors.
Cox proportional regression models will be used to determine significant factors of outcomes. Testing for goodness of fit will be done using Hosmer-Lemeshow test. Likelihood ratio tests and other information criteria (Akaike Information Criterion or Bayesian Information Criterion) will be used to refine the final model. Statistical significance will be considered if the 95% CI of HR or adjusted HR did not include the number one. A p value <0.05 (two tailed) is set for other analyses.
Subgroup analyses will be performed for proven prespecified effect modifiers. The following variables will be considered for subgroup analyses: age (19–64 years vs ≥65 years), sex, body mass index (<18.5 vs 18.5–22.9 vs ≥23 kg/m 2 ), with history of cardiovascular or cerebrovascular disease (presence or absence), hypertension (presence or absence), diabetes mellitus (presence or absence), respiratory disease (presence or absence), smoking status (smoker or non-smoker) and COVID-19 disease severity (mild, severe or critical disease).
The effects of missing data will be explored. All efforts will be exerted to minimise missing and spurious data. Validity of the submitted electronic data collection will be monitored and reviewed weekly to prevent missing or inaccurate input of data. Multiple imputations will be performed for missing data when possible. To check for robustness of results, analysis done for patients with complete data will be compared with the analysis with the imputed data.
The effects of outliers will also be assessed. Outliers will be assessed by z-score or boxplot. A cut-off of 3 SD from the mean can also be used. To check for robustness of results, analysis done with outliers will be compared with the analysis without the outliers.
Study organisational structure
A steering committee (AIE, MCCS, VMMA and RDGJ) was formed to direct and provide appropriate scientific, technical and methodological assistance to study site investigators and collaborators (see figure 3 ). Central administrative coordination, data management, administrative support, documentation of progress reports, data analyses and interpretation and journal publication are the main responsibilities of the steering committee. Study site investigators and collaborators are responsible for the proper collection and recording of data including the duty to maintain the confidentiality of information and the privacy of all identified patients for all the phases of the research processes.
Organisational structure of oversight of the Philippine CORONA Study.
This section is highlighted as part of the required formatting amendments by the Journal.
Ethics and dissemination
This research will adhere to the Philippine National Ethical Guidelines for Health and Health-related Research 2017. 26 This study is an observational, cohort study and will not allocate any type of intervention. The medical records of the identified patients will be reviewed retrospectively. To protect the privacy of the participant, the data collection forms will not contain any information (ie, names and institutional patient number) that could determine the identity of the patients. A sequential code will be recorded for each patient in the following format: AAA-BBB where AAA will pertain to the three-digit code randomly assigned to each study site; BBB will pertain to the sequential case number assigned by each study site. Each participating centre will designate a password-protected laptop for data collection; the password is known only to the study site.
This protocol was approved by the following institutional review boards: Single Joint Research Ethics Board of the DOH, Philippines (SJREB-2020-24); Asian Hospital and Medical Center, Muntinlupa City (2020- 010-A); Baguio General Hospital and Medical Center (BGHMC), Baguio City (BGHMC-ERC-2020-13); Cagayan Valley Medical Center (CVMC), Tuguegarao City; Capitol Medical Center, Quezon City; Cardinal Santos Medical Center (CSMC), San Juan City (CSMC REC 2020-020); Chong Hua Hospital, Cebu City (IRB 2420–04); De La Salle Medical and Health Sciences Institute (DLSMHSI), Cavite (2020-23-02-A); East Avenue Medical Center (EAMC), Quezon City (EAMC IERB 2020-38); Jose R. Reyes Memorial Medical Center, Manila; Jose B. Lingad Memorial Regional Hospital, San Fernando, Pampanga; Dr. Jose N. Rodriguez Memorial Hospital, Caloocan City; Lung Center of the Philippines (LCP), Quezon City (LCP-CT-010–2020); Manila Doctors Hospital, Manila (MDH IRB 2020-006); Makati Medical Center, Makati City (MMC IRB 2020–054); Manila Medical Center, Manila (MMERC 2020-09); Northern Mindanao Medical Center, Cagayan de Oro City (025-2020); Quirino Memorial Medical Center (QMMC), Quezon City (QMMC REB GCS 2020-28); Ospital ng Makati, Makati City; University of the Philippines – Philippine General Hospital (UP-PGH), Manila (2020-314-01 SJREB); Philippine Heart Center, Quezon City; Research Institute for Tropical Medicine, Muntinlupa City (RITM IRB 2020-16); San Lazaro Hospital, Manila; San Juan De Dios Educational Foundation Inc – Hospital, Pasay City (SJRIB 2020-0006); Southern Isabela Medical Center, Santiago City (2020-03); Southern Philippines Medical Center (SPMC), Davao City (P20062001); St. Luke’s Medical Center, Quezon City (SL-20116); St. Luke’s Medical Center, Bonifacio Global City, Taguig City (SL-20116); Southern Philippines Medical Center, Davao City; The Medical City, Pasig City; University of Santo Tomas Hospital, Manila (UST-REC-2020-04-071-MD); University of the East Ramon Magsaysay Memorial Medical Center, Inc, Quezon City (0835/E/2020/063); Veterans Memorial Medical Center (VMMC), Quezon City (VMMC-2020-025) and Vicente Sotto Memorial Medical Center, Cebu City (VSMMC-REC-O-2020–048).
The dissemination of results will be conducted through scientific/medical conferences and through journal publication. Only the aggregate results of the study shall be disseminated. The lay versions of the results may be provided on request.
Protocol registration and technical review approval
This protocol was registered in the ClinicalTrials.gov website. It has received technical review board approvals from the Department of Neurosciences, Philippine General Hospital and College of Medicine, University of the Philippines Manila, from the Cardinal Santos Medical Center (San Juan City) and from the Research Center for Clinical Epidemiology and Biostatistics, De La Salle Medical and Health Sciences Institute (Dasmariñas, Cavite).
Acknowledgments
We would like to thank Almira Abigail Doreen O Apor, MD, of the Department of Neurosciences, Philippine General Hospital, Philippines, for illustrating figure 2 for this publication.
- Adhikari SP ,
- Wu Y-J , et al
- Department of Health
- Philippine Society for Microbiology and Infectious Diseases
- Hu Y , et al
- Li Yan‐Chao ,
- Bai Wan‐Zhu ,
- Hashikawa T ,
- Wang M , et al
- Paterson RW ,
- Benjamin L , et al
- Hall JP , et al
- Varatharaj A ,
- Ellul MA , et al
- Mahammedi A ,
- Vagal A , et al
- Collantes MEV ,
- Espiritu AI ,
- Sy MCC , et al
- Merdji H , et al
- Sharifi Razavi A ,
- Poyiadji N ,
- Noujaim D , et al
- Zhou H , et al
- Moriguchi T ,
- Goto J , et al
- Nicholson TR , et al
- Ferrarese C ,
- Priori A , et al
- von Elm E ,
- Altman DG ,
- Egger M , et al
- Li J , et al
- Centers for Disease Control and Prevention
- Khaledifar A ,
- Hashemzadeh M ,
- Solati K , et al
- McGoogan JM
- Philippine Research Ethics Board
VMMA and RDGJ are joint senior authors.
AIE and MCCS are joint first authors.
Twitter @neuroaidz, @JamoraRoland
Collaborators The Philippine CORONA Study Group Collaborators: Maritoni C Abbariao, Joshua Emmanuel E Abejero, Ryndell G Alava, Robert A Barja, Dante P Bornales, Maria Teresa A Cañete, Ma. Alma E Carandang-Concepcion, Joseree-Ann S Catindig, Maria Epifania V Collantes, Evram V Corral, Ma. Lourdes P Corrales-Joson, Romulus Emmanuel H Cruz, Marita B Dantes, Ma. Caridad V Desquitado, Cid Czarina E Diesta, Carissa Paz C Dioquino, Maritzie R Eribal, Romulo U Esagunde, Rosalina B Espiritu-Picar, Valmarie S Estrada, Manolo Kristoffer C Flores, Dan Neftalie A Juangco, Muktader A Kalbi, Annabelle Y Lao-Reyes, Lina C Laxamana, Corina Maria Socorro A Macalintal, Maria Victoria G Manuel, Jennifer Justice F Manzano, Ma. Socorro C Martinez, Generaldo D Maylem, Marc Conrad C Molina, Marietta C Olaivar, Marissa T Ong, Arnold Angelo M Pineda, Joanne B Robles, Artemio A Roxas Jr, Jo Ann R Soliven, Arturo F Surdilla, Noreen Jhoanna C Tangcuangco-Trinidad, Rosalia A Teleg, Jarungchai Anton S Vatanagul and Maricar P Yumul.
Contributors All authors conceived the idea and wrote the initial drafts and revisions of the protocol. All authors made substantial contributions in this protocol for intellectual content.
Funding Philippine Neurological Association (Grant/Award Number: N/A). Expanded Hospital Research Office, Philippine General Hospital (Grant/Award Number: N/A).
Disclaimer Our funding sources had no role in the design of the protocol, and will not be involved during the methodological execution, data analyses and interpretation and decision to submit or to publish the study results.
Map disclaimer The depiction of boundaries on the map(s) in this article does not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area or of its authorities. The map(s) are provided without any warranty of any kind, either express or implied.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Springer Nature - PMC COVID-19 Collection

How the COVID-19 pandemic has changed research?
Hassan karimi-maleh.
1 School of Resources and Environment, University of Electronic Science and Technology of China, Xiyuan Ave, P.O. Box 611731, Chengdu, People’s Republic of China
Elena Niculina Dragoi
2 Cristofor Simionescu Faculty of Chemical Engineering and Environmental Protection, Gheorghe Asachi Technical University, Bld. D Mangeron No. 73, 700050 Iasi, Romania
Eric Lichtfouse
3 Aix Marseille Univ, CNRS, IRD, INRAE, CEREGE, Aix-en-Provence, France
4 State Key Laboratory of Multiphase Flow in Power Engineering, Xian Jiaotong University, Xian, 710049 Shaanxi People’s Republic of China
Adversity and challenges are life's way of creating strength. Adversity creates challenge, and challenge creates change, and change is absolutely necessary for growth. If there is no change and challenge, there can be no growth and development. Willie Jolley
The coronavirus disease pandemic (COVID-19) started in 2019 and induced long-lasting effects on many aspects of life. Every one of us felt how the quiet existence was transformed into a chaotic state full of uncertainties, doubts, and fear for one’s safety. This led to many societal changes, some influenced by objective facts and events, others by human risk perception and behavior modifications. Although risk perception tends to be biased and the responses of individuals to the perceived threat are very different, jumping from lack of precautions and a false feeling of security to unnecessary scares and stigmatization of risks groups will impact human activities in all areas for many years to come (Brug et al. 2009 ). Here, we review the positive and negative outcomes of the pandemic on academia and scientific enterprises.
Research disruption
The government's drastic measures, especially in the early days of the pandemic, led to the closure of many laboratories. The discontinuation of experiments caused the loss of data and, in turn, shrank financial and material resources. In addition, the potential for scientific innovation was significantly hampered by travel restrictions, and by less face-to-face meetings, conferences, and workshops (Subramanya et al. 2020 ). Although few argue the importance of precautions to slow the spread of diseases, the pandemic policies significantly disrupted both professional and personal lives. The adverse effects of the pandemic on academia and scientific enterprises resulted from the closure of laboratories, the reduction of avenues for conducting research in a collaborative and direct manner, and the limitation of direct dissemination of results to peers. These major issues prompted changes in research time allocation, publication behavior, and funding in a domino fall-like way.
Impact on publication
Publishing is essential for researchers because, whatever criticisms are currently raised against the use of publication metrics, an academic career is closely correlated to the quality and frequency of publications. During the first COVID year of 2020, the average self-reported number of publication metrics for the USA and Europe was slightly lower than in 2019 (Gao et al. 2021 ). However, this perceived reduction is not general, and publication number varied with country, institution, and discipline. For example, medical-based publications showed a 6.5-fold increase, while non-medical publications decreased by 10–12% (Riccaboni and Verginer 2022 ). In the engineering field, for the School of Resources and Environment of the University of Electronic Science and Technology of China, a Scopus search indicates a slight reduction of publication number in 2020, of 163, compared to 165 in 2019, while in 2021, the number raised to 243. The same search procedure applied to the Cristofor Simionescu Faculty of Chemical Engineering and Environmental Protection from Gheorghe Asachi Technical University shows a rise from 36 articles in 2019 to 63 articles in 2020 and 73 articles in 2021.
Less experimental time
A strong impact of the discipline field on research time was observed. For instance, research time declined by 30–40% versus pre-pandemic levels in research heavily relying on physical laboratories and experiments such as biological sciences and chemical engineering (Myers et al. 2020 ). This reduction is not only due to the lack of on-site access but also to staff shortage and supply-chain issues for materials, spare parts, and protective equipment (Sohrabi et al. 2021 ). As the measures relaxed and more protective equipment became available, laboratory work improved slowly to return to a ‘new normal’ functioning where the measures and the management of the protective equipment are still essential (Yang et al. 2022 ; Ufnalska and Lichtfouse 2021 ; Gorrasi et al. 2021 ). Nevertheless, the self-reported working hours decreased by 11%, and the reduction of time allocated to research was about 24% (Myers et al. 2020 ). Consequently, most tasks were performed at home, sometimes in unsuitable conditions, with spouses and kids wandering around. The work at home focused more on data analysis, manuscript, and proposal writing.
Research advancement was slowed down, particularly for early-stage career scientists, due to reduced laboratory access, less direct teamwork, and meeting cancelation. The delay or cancellation of research opportunities and the impaired ability to collect and analyze data led to a decreased ability to work. According to a survey in the UK, 50% of responders reported being very stressed, and 75% were apprehensive about their future plans (Byrom 2020 ). This survey also revealed that only 12% of final-year doctoral students had an option to extend their studies, which put additional pressure on an already at-risk group.
Fund redirection
The pandemic also reduced the number of projects. For example, in the USA and Europe, the number of respondents claiming that they had no new project increased from 9% in 2019 to 27% in 2020 (Gao et al. 2021 ). Research topics were also strongly modified by redirecting funds toward COVID issues, with many classical clinical trials being temporarily stopped in vulnerable, low-income countries (Subramanya et al. 2020 ). Specifically, in July 2020, about 1200 clinical trials were suspended because clinical scientists had no or reduced access to healthcare research infrastructures (Riccaboni and Verginer 2022 ). Moreover, drastic budget cuts also occurred in other areas, such as cancer research. For instance, 45 million pounds were cut in the UK, inducing a substantial decline in fellowships and research programs for hundreds of scientists (Burki 2021 ). Here, early-career scientists are at risk because institutions are not hiring new personnel.
The redeployment of private and public funds to COVID-related concerns has substantially increased the number of investigations in this field. For instance, in May 2020, shortly after the pandemic outbreak, 1,221 COVID studies were declared in the international clinical trial registry (Bramstedt 2020 ). Nevertheless, research misconduct increased rapidly as an unwanted consequence of the rapid pace imposed by the pandemic and available funds. Indeed, 33 articles were already considered unsuitable in May 2020 (Bramstedt 2020 ). Ironically, the overflow of funds is as much a curse as the lack of funds, as it uncovers new problems and exacerbates existing issues. Crowdfunding, a popular fund-raising means commonly used by the public, was explored by academia for the first time during the pandemic. However, researchers did not favor this approach due to the somewhat different rules that must be applied and the limited amount of money compared with the standard sources (Sultan et al. 2022 ).
The personal living conditions of scientists have also dropped research efficiency. Indeed, the balance between work and free time has been utterly disrupted during the pandemic. Moreover, researchers who did not fit the classical profile of the ideal academic career—the traditional man with his traditional wife—have been under additional pressure in the context of unrealistic expectations for tenure or promotion (King and Frederickson 2021 ). In addition, female academics had difficulty balancing the expected primary role of caregiver with the role of the scientist, leading to an overall reduction in female publications compared with men (Alam et al. 2021 ). For example, in the first ten weeks of lockdown, the academic productivity of women dropped 13.2% compared to that of male academics in the USA. This productivity gap occurred in various countries and was more pronounced for assistant professors and top-ranked institutions (Cui et al. 2022 ). In several academic journals, the reduction in the proportion of published articles by women was confirmed in the summer of 2020 (Pereira 2021 ). Analyzing tweets, similar trends were observed in social media (Kim and Patterson 2022 ).
Elitism discrimination, a form of inequality, was exacerbated by the pandemic. In this context, elitism discrimination indicates the cases where results or scientists from less prestigious institutions are considered of lower quality. One example is the discrepancy between the number of vaccines approved by the European Medicines Agency versus the World Health Organization, where some vaccines were considered inadequate for the former. This has led to confusion, skepticism, and an increased sense of injustice (Sikimić 2022 ).
Online adaptation
To mitigate research issues arising from the pandemic, research institutions strongly reinforced techniques allowing online work and collaboration by video-conferencing. For example, new portals for sharing scientific data, such as the European COVID-19 Data Portal, emerged, and conferences and workshops were held online (Korbel and Stegle 2020 ). Social media were also found to facilitate the dissemination of information. However, curating data effectively and extracting meaningful information from social media remains a challenge.
Despite shutdowns, electronic communications systems allowed researchers to participate in various collaborative endeavors (Korbel and Stegle 2020 ). Due to its effectiveness, electronic communication was initially targeted at COVID research and then rapidly transferred to most research disciplines. Sometimes, work unfinished in the lab was enhanced by exchanging information with theoretical researchers, improving the quality of published articles. In other words, online work allows more time to think compared with experimental work, where scientists, in particular students, tend to jump rapidly from one experiment to another without taking the time to explore the meaning of their results in depth.
Figure 1 depicts the main changes induced by the pandemic in 2020. Before the pandemic, collaboration was done face-to-face with information shared within local groups. At that time, computers were mainly used to improve presentation. Although online tools were already available, e.g., for online teaching and research discussions, they were rarely used by universities. Moreover, data storage database processing was done mainly in local servers and computers. During the pandemic, we observed sharp and rapid changes such as an intense development of online tools for meetings, teaching, cloud storing, data sharing, and social media. As a result, platforms such as Zoom, Google Meet, or Microsoft Teams registered an unprecedented rise in the number of users and services provided. This allowed a tighter connection between people in different areas and demonstrated that even the most change-resistant institutions could adopt new technologies when needed.

Research before the pandemic in 2019 involved mainly local meetings, teaching, experiments, computers, and servers. The pandemic in 2020 has fostered remote meetings, distant teaching, international collaboration, and the use of cloud services
The conference format underwent significant changes during the pandemic. Due to the various restrictions, most topical conferences were suspended or transformed into online meetings. On-site laboratory and project meetings were rapidly converted into online sessions. Indeed, these types of gatherings are essential for learning, dissemination, and creating collaboration. Virtual meetings presented advantages such as easy accessibility to many individuals located anywhere, and reduced meeting organization and participant accommodation costs (Reinhard et al. 2021 ). These meetings have fostered international collaboration. Moreover, virtual conferences display a much lower environmental price (Donlon 2021 ). Virtual conferences also save much traveling time. These benefits make virtual meetings attractive to young scientists and underfunded academics from developing countries. Social media tools allow for the improvement of the attractiveness of these events. For example, backchannels on Twitter enhance immersion and communication, live streams increase awareness, and video recordings and archiving perpetuate information availability (Atkinson 2009 ). However, a virtual conference environment does not provide the same level of social networking, camaraderie, and connection that an in-person conference can offer (Reinhard et al. 2021 ). Nevertheless, virtual conferencing must not be dismissed, and a mixed format of both online and in-person meetings is promising for future research.
Overall, although the COVID pandemic induced adverse effects on many societal aspects, the lockdowns stimulated a rapid adaptation of research with the development of online practices that will undoubtedly improve research.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hassan Karimi-Maleh, Email: nc.ude.ctseu@nassah .
Elena Niculina Dragoi, Email: [email protected] .
Eric Lichtfouse, Email: [email protected] .
- Alam A, Rampes S, Ma D. The impact of the COVID-19 pandemic on research. Trans Perioper Pain Med. 2021; 8 :312–314. [ Google Scholar ]
- Atkinson C (2009) The backchannel: How audiences are using Twitter and social media and changing presentations forever. New Riders
- Bramstedt KA. The carnage of substandard research during the COVID-19 pandemic: a call for quality. J Med Ethics. 2020; 46 :803–807. doi: 10.1136/medethics-2020-106494. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brug J, Aro AR, Richardus JH. Risk perceptions and behaviour: towards pandemic control of emerging infectious diseases : international research on risk perception in the control of emerging infectious diseases. Int J Behav Med. 2009; 16 :3–6. doi: 10.1007/s12529-008-9000-x. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Burki TK. Cuts in cancer research funding due to COVID-19. Lancet Oncology. 2021; 22 :E6–E6. doi: 10.1016/S1470-2045(20)30749-X. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Byrom N. The challenges of lockdown for early-career researchers. Elife. 2020; 9 :e59634. doi: 10.7554/eLife.59634. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cui RM, Ding H, Zhu F. Gender inequality in research productivity during the COVID-19 pandemic, M&Som-Manufacturing & Service. Oper Manag. 2022; 24 :707–726. [ Google Scholar ]
- Donlon E. Lost and found: the academic conference in pandemic and post-pandemic times. Irish Educ Stud. 2021; 40 :367–373. doi: 10.1080/03323315.2021.1932554. [ CrossRef ] [ Google Scholar ]
- Gao J, Yin Y, Myers KR, Lakhani KR, Wang D. Potentially long-lasting effects of the pandemic on scientists. Nat Commun. 2021; 12 :6188. doi: 10.1038/s41467-021-26428-z. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gorrasi G, Sorrentino A, Lichtfouse E. Back to plastic pollution in COVID times. Environ Chem Lett. 2021; 19 :1–4. doi: 10.1007/s10311-020-01129-z. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kim E, Patterson S (2022) The pandemic and gender inequality in academia. PS Polit Sci Polit 55:109–116
- King MM, Frederickson ME. The pandemic penalty: the gendered effects of COVID-19 on scientific productivity. Socius. 2021; 7 :23780231211006977. doi: 10.1177/23780231211006977. [ CrossRef ] [ Google Scholar ]
- Korbel JO, Stegle O. Effects of the COVID-19 pandemic on life scientists. Genome Biol. 2020; 21 :113. doi: 10.1186/s13059-020-02031-1. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pereira MdM (2021) Researching gender inequalities in academic labor during the COVID-19 pandemic: avoiding common problems and asking different questions. Gender Work Org 28:498–509 [ PMC free article ] [ PubMed ]
- Myers KR, Tham WY, Yin Y, Cohodes N, Thursby JG, Thursby MC, Schiffer P, Walsh JT, Lakhani KR, Wang D. Unequal effects of the COVID-19 pandemic on scientists. Nat Hum Behav. 2020; 4 :880–883. doi: 10.1038/s41562-020-0921-y. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Reinhard D, Stafford MC, Payne TC. COVID-19 and academia: considering the future of academic conferencing. J Crim Just Educ. 2021; 32 :171–185. doi: 10.1080/10511253.2020.1871047. [ CrossRef ] [ Google Scholar ]
- Riccaboni M, Verginer L. The impact of the COVID-19 pandemic on scientific research in the life sciences. PLoS ONE. 2022; 17 :e0263001. doi: 10.1371/journal.pone.0263001. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sikimić V (2022) How to improve research funding in academia? Lessons from the COVID-19 crisis. Front Res Metrics Anal, p 7 [ PMC free article ] [ PubMed ]
- Sohrabi C, Mathew G, Franchi T, Kerwan A, Griffin M, Soleil CDMJ, Ali SA, Agha M, Agha R. Impact of the coronavirus (COVID-19) pandemic on scientific research and implications for clinical academic training—a review. Int J Surg. 2021; 86 :57–63. doi: 10.1016/j.ijsu.2020.12.008. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Subramanya SH, Lama B, Acharya KP. Impact of COVID-19 pandemic on the scientific community. Qatar Med J. 2020; 2020 :21. doi: 10.5339/qmj.2020.21. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sultan A, Juneja A, Kaur G. The after-effects of COVID-19 on academia and scientific community: Researchers perspective. Southeast Asian J Case Report Rev. 2022; 9 :15–17. doi: 10.18231/j.sajcrr.2022.005. [ CrossRef ] [ Google Scholar ]
- Ufnalska S, Lichtfouse E. Unanswered issues related to the COVID-19 pandemic. Environ Chem Lett. 2021; 19 :3523–3524. doi: 10.1007/s10311-021-01249-0. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yang S, Cheng Y, Liu T, Huang S, Yin L, Pu Y, Liang G (2022) Impact of waste of COVID-19 protective equipment on the environment, animals and human health: a review. Environ Chem Lett, pp 1–20 [ PMC free article ] [ PubMed ]

IMAGES
COMMENTS
Covid-19 has affected humanity in a major way. An extremely dangerous virus, hitherto unknown to humanity, had to be studied and contained in order to overcome the pandemic. Research on Covid-19 had surged in the early days with an unprecedented surge in the publications on that specific topic.
The spread of the "Severe Acute Respiratory Coronavirus 2" (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [].The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [], causing massive economic strain ...
elated articles published from January 1 to July 1, 2020 were retrieved from PubMed database. A total of 27,370 articles were included. Excel 2010, Medical Text Indexer (MTI), VOSviewer, and D3.js were used to summarize bibliometric features. Results: The number of the COVID-19 research publications has been continuously increasing after its break. United States was the most productive and ...
The impact on research in progress prior to COVID-19 was rapid, dramatic, and no doubt will be long term. The pandemic curtailed most academic, industry, and government basic science and clinical ...
Scroll down the page to view all COVID-19 articles, stories, and resources from across NIH. You can also select a topic from the list to view resources on that topic. - Any -. Aging. Cancer. Children. Clinical Trials. Immune Responses. Long COVID.
The outbreak of COVID-19 has proven to be an unprecedented disaster for the whole world. The virus has inflicted billion of lives across the globe in all aspects—physically, psychologically, as well as socially. Compared to the previous strains of β-CoV genera- MERS and SARS, SARS-CoV-2 has significantly higher transmissibility and worst ...
The WHO Covid-19 Research Database was maintained by the WHO Library & Digital Information Networks and was funded by COVID-19 emergency funds. The database was built by BIREME, the Specialized Center of PAHO/AMRO. Its content spanned the time period March 2020 to June 2023. It has now been archived, and no longer searchable since January 2024.
Here the authors show that, in convalescent COVID-19 patients, memory T cell responses are detectable up to 317 days post-symptom onset, in which the presence of stem cell-like memory T cells ...
Learn how NIH is supporting research in COVID-19 testing, treatments, and vaccines. Learn how NIH is supporting research in COVID-19 testing, treatments, and vaccines. ... Find COVID-19 datasets, data tools, and publications to use in research. EXPLORE COVID-19 DATA For NIH Staff NIH Strategic Response to COVID-19
Download MP3. In the final Coronapod of 2020, we dive into the scientific literature to reflect on the COVID-19 pandemic. Researchers have discovered so much about SARS-CoV-2 - information that ...
COVID-19: Emergence, Spread, Possible Treatments, and Global Burden. The Coronavirus (CoV) is a large family of viruses known to cause illnesses ranging from the common cold to acute respiratory tract infection. The severity of the infection may be visible as pneumonia, acute respiratory syndrome, and even death.
MEDICAL TECHNOLOGY. Computational approaches to innovate treatment and diagnosis of infectious diseases. 16 October 2020 | This article collection explores computational approaches applied to the study of infectious diseases, including COVID-19. These methods are creating a new research field that takes advantage of the great progress from molecular and structural biology, immunology ...
Example of research topic about medicine (e.g., COVID 19) - 25556766. It is true that theory is of utmost relevance, but what many Excel courses fail to address is the fact that they focus too much on theory and little o …
What are the best treatments for covid-19? Written in cooperation with the World Health Organization, The BMJ's living systematic review is a meta-analysis comparing the effects of treatments for covid-19,1 using data from more than 400 randomised clinical trials worldwide. At the time of writing, it states that systemic corticosteroids (particularly dexamethasone), interleukin-6 receptor ...
1. Safety and immunogenicity study of 2019-nCoV vaccine (mRNA-1273) for prophylaxis of SARS-CoV-2 infection (COVID-19) This clinical trial is designed to assess the safety, reactogenicity, and immunogenicity of mRNA-1273. It encodes for a full-length, prefusion stabilized spike (S) protein of SARS-CoV-2.
COVID-19 Research. Stanford Medicine scientists have launched dozens of research projects as part of the global response to COVID-19. Some aim to prevent, diagnose and treat the disease; others aim to understand how it spreads and how people's immune systems respond to it. Below is a curated selection, including summaries, of the projects.
DOI: 10.1056/NEJMe2402224. Nirmatrelvir-ritonavir (Paxlovid [Pfizer]) is used as first-line therapy for nonhospitalized persons with Covid-19 1 on the basis of the results of the Evaluation of ...
Coronavirus disease 2019 (COVID-19) is defined as illness caused by a novel coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; formerly called 2019-nCoV), which was first identified amid an outbreak of respiratory illness cases in Wuhan City, Hubei Province, China. [ 1] It was initially reported to the WHO on ...
A fourth line of research examined media use, mental health and well-being during the COVID-19 pandemic. Gabbiadini et al. addressed the use of digital technology (e.g., voice/video calls, online games, watching movies in party mode) to stay connected with others during lockdown. Participants, 465 Italians (age range: 18-73 years, 348 female ...
The COVID-19 research landscape: Measuring topics and collaborations using scientific literature Medicine (Baltimore). 2020 Oct 23;99(43): e22849. doi ... The number of the COVID-19 research publications has been continuously increasing after its break. United States was the most productive and active country for COVID-19 research, with the ...
Neurological symptoms have been widely observed in COVID-19 patients, with many survivors exhibiting persistent neurological and cognitive impairment. New research from the University of Minnesota found that COVID-19 triggers inflammation in the brain, which is linked to many COVID-related symptoms such as fatigue and "brain fog."
Research is racing along at a dizzying pace as scientists seek to understand and identify solutions to the COVID-19 pandemic. Where knowing a few random terms might have been helpful before the pandemic, now that level of knowledge just skims the surface. F. Perry Wilson, MD, a Yale nephrologist, teaches a free online course called ...
Introduction The SARS-CoV-2, virus that caused the COVID-19 global pandemic, possesses a neuroinvasive potential. Patients with COVID-19 infection present with neurological signs and symptoms aside from the usual respiratory affectation. Moreover, COVID-19 is associated with several neurological diseases and complications, which may eventually affect clinical outcomes. Objectives The ...
Less experimental time. A strong impact of the discipline field on research time was observed. For instance, research time declined by 30-40% versus pre-pandemic levels in research heavily relying on physical laboratories and experiments such as biological sciences and chemical engineering (Myers et al. 2020).This reduction is not only due to the lack of on-site access but also to staff ...
The authors also studied a vaccinated cohort including 14,035,286 adults, of whom 866,469 had a confirmed COVID-19 diagnosis, with an average age of 53 years and 52.1 per cent female (7,308,556 ...