Module 11: Schizophrenia Spectrum and Other Psychotic Disorders
Case studies: schizophrenia spectrum disorders, learning objectives.
- Identify schizophrenia and psychotic disorders in case studies

Case Study: Bryant
Thirty-five-year-old Bryant was admitted to the hospital because of ritualistic behaviors, depression, and distrust. At the time of admission, prominent ritualistic behaviors and depression misled clinicians to diagnose Bryant with obsessive-compulsive disorder (OCD). Shortly after, psychotic symptoms such as disorganized thoughts and delusion of control were noticeable. He told the doctors he has not been receiving any treatment, was not on any substance or medication, and has been experiencing these symptoms for about two weeks. Throughout the course of his treatment, the doctors noticed that he developed a catatonic stupor and a respiratory infection, which was identified by respiratory symptoms, blood tests, and a chest X-ray. To treat the psychotic symptoms, catatonic stupor, and respiratory infection, risperidone, MECT, and ceftriaxone (antibiotic) were administered, and these therapies proved to be dramatically effective. [1]
Case Study: Shanta
Shanta, a 28-year-old female with no prior psychiatric hospitalizations, was sent to the local emergency room after her parents called 911; they were concerned that their daughter had become uncharacteristically irritable and paranoid. The family observed that she had stopped interacting with them and had been spending long periods of time alone in her bedroom. For over a month, she had not attended school at the local community college. Her parents finally made the decision to call the police when she started to threaten them with a knife, and the police took her to the local emergency room for a crisis evaluation.
Following the administration of the medication, she tried to escape from the emergency room, contending that the hospital staff was planning to kill her. She eventually slept and when she awoke, she told the crisis worker that she had been diagnosed with attention-deficit/hyperactive disorder (ADHD) a month ago. At the time of this ADHD diagnosis, she was started on 30 mg of a stimulant to be taken every morning in order to help her focus and become less stressed over the possibility of poor school performance.
After two weeks, the provider increased her dosage to 60 mg every morning and also started her on dextroamphetamine sulfate tablets (10 mg) that she took daily in the afternoon in order to improve her concentration and ability to study. Shanta claimed that she might have taken up to three dextroamphetamine sulfate tablets over the past three days because she was worried about falling asleep and being unable to adequately prepare for an examination.
Prior to the ADHD diagnosis, the patient had no known psychiatric or substance abuse history. The urine toxicology screen taken upon admission to the emergency department was positive only for amphetamines. There was no family history of psychotic or mood disorders, and she didn’t exhibit any depressive, manic, or hypomanic symptoms.
The stimulant medications were discontinued by the hospital upon admission to the emergency department and the patient was treated with an atypical antipsychotic. She tolerated the medications well, started psychotherapy sessions, and was released five days later. On the day of discharge, there were no delusions or hallucinations reported. She was referred to the local mental health center for aftercare follow-up with a psychiatrist. [2]
Another powerful case study example is that of Elyn R. Saks, the associate dean and Orrin B. Evans professor of law, psychology, and psychiatry and the behavioral sciences at the University of Southern California Gould Law School.
Saks began experiencing symptoms of mental illness at eight years old, but she had her first full-blown episode when studying as a Marshall scholar at Oxford University. Another breakdown happened while Saks was a student at Yale Law School, after which she “ended up forcibly restrained and forced to take anti-psychotic medication.” Her scholarly efforts thus include taking a careful look at the destructive impact force and coercion can have on the lives of people with psychiatric illnesses, whether during treatment or perhaps in interactions with police; the Saks Institute, for example, co-hosted a conference examining the urgent problem of how to address excessive use of force in encounters between law enforcement and individuals with mental health challenges.
Saks lives with schizophrenia and has written and spoken about her experiences. She says, “There’s a tremendous need to implode the myths of mental illness, to put a face on it, to show people that a diagnosis does not have to lead to a painful and oblique life.”
In recent years, researchers have begun talking about mental health care in the same way addiction specialists speak of recovery—the lifelong journey of self-treatment and discipline that guides substance abuse programs. The idea remains controversial: managing a severe mental illness is more complicated than simply avoiding certain behaviors. Approaches include “medication (usually), therapy (often), a measure of good luck (always)—and, most of all, the inner strength to manage one’s demons, if not banish them. That strength can come from any number of places…love, forgiveness, faith in God, a lifelong friendship.” Saks says, “We who struggle with these disorders can lead full, happy, productive lives, if we have the right resources.”
You can view the transcript for “A tale of mental illness | Elyn Saks” here (opens in new window) .
- Bai, Y., Yang, X., Zeng, Z., & Yang, H. (2018). A case report of schizoaffective disorder with ritualistic behaviors and catatonic stupor: successful treatment by risperidone and modified electroconvulsive therapy. BMC psychiatry , 18(1), 67. https://doi.org/10.1186/s12888-018-1655-5 ↵
- Henning A, Kurtom M, Espiridion E D (February 23, 2019) A Case Study of Acute Stimulant-induced Psychosis. Cureus 11(2): e4126. doi:10.7759/cureus.4126 ↵
- Modification, adaptation, and original content. Authored by : Wallis Back for Lumen Learning. Provided by : Lumen Learning. License : CC BY: Attribution
- A tale of mental illness . Authored by : Elyn Saks. Provided by : TED. Located at : https://www.youtube.com/watch?v=f6CILJA110Y . License : Other . License Terms : Standard YouTube License
- A Case Study of Acute Stimulant-induced Psychosis. Authored by : Ashley Henning, Muhannad Kurtom, Eduardo D. Espiridion. Provided by : Cureus. Located at : https://www.cureus.com/articles/17024-a-case-study-of-acute-stimulant-induced-psychosis#article-disclosures-acknowledgements . License : CC BY: Attribution
- Elyn Saks. Provided by : Wikipedia. Located at : https://en.wikipedia.org/wiki/Elyn_Saks . License : CC BY-SA: Attribution-ShareAlike
- A case report of schizoaffective disorder with ritualistic behaviors and catatonic stupor: successful treatment by risperidone and modified electroconvulsive therapy. Authored by : Yuanhan Bai, Xi Yang, Zhiqiang Zeng, and Haichen Yangcorresponding. Located at : https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5851085/ . License : CC BY: Attribution

Schizophrenia case studies: putting theory into practice
This article considers how patients with schizophrenia should be managed when their condition or treatment changes.
DR P. MARAZZI/SCIENCE PHOTO LIBRARY
Treatments for schizophrenia are typically recommended by a mental health specialist; however, it is important that pharmacists recognise their role in the management and monitoring of this condition. In ‘ Schizophrenia: recognition and management ’, advice was provided that would help with identifying symptoms of the condition, and determining and monitoring treatment. In this article, hospital and community pharmacy-based case studies provide further context for the management of patients with schizophrenia who have concurrent conditions or factors that could impact their treatment.
Case study 1: A man who suddenly stops smoking
A man aged 35 years* has been admitted to a ward following a serious injury. He has been taking olanzapine 20mg at night for the past three years to treat his schizophrenia, without any problems, and does not take any other medicines. He smokes 25–30 cigarettes per day, but, because of his injury, he is unable to go outside and has opted to be started on nicotine replacement therapy (NRT) in the form of a patch.
When speaking to him about his medicines, he appears very drowsy and is barely able to speak. After checking his notes, it is found that the nurses are withholding his morphine because he appears over-sedated. The doctor asks the pharmacist if any of the patient’s prescribed therapies could be causing these symptoms.
What could be the cause?
Smoking is known to increase the metabolism of several antipsychotics, including olanzapine, haloperidol and clozapine. This increase is linked to a chemical found in cigarettes, but not nicotine itself. Tobacco smoke contains aromatic hydrocarbons that are inducers of CYP1A2, which are involved in the metabolism of several medicines [1] , [2] , [3] . Therefore, smoking cessation and starting NRT leads to a reduction in clearance of the patient’s olanzapine, leading to increased plasma levels of the antipsychotic olanzapine and potentially more adverse effects — sedation in this case.
Patients who want to stop, or who inadvertently stop, smoking while taking antipsychotics should be monitored for signs of increased adverse effects (e.g. extrapyramidal side effects, weight gain or confusion). Patients who take clozapine and who wish to stop smoking should be referred to their mental health team for review as clozapine levels can increase significantly when smoking is stopped [3] , [4] .
For this patient, olanzapine is reduced to 15mg at night; consequently, he seems much brighter and more responsive. After a period on the ward, he has successfully been treated for his injury and is ready to go home. The doctor has asked for him to be supplied with olanzapine 15mg for discharge along with his NRT.
What should be considered prior to discharge?
It is important to discuss with the patient why his dose was changed during his stay in hospital and to ask whether he intends to start smoking again or to continue with his NRT. Explain to him that if he wants to begin, or is at risk of, smoking again, his olanzapine levels may be impacted and he may be at risk of becoming unwell. It is necessary to warn him of the risk to his current therapy and to speak to his pharmacist or mental health team if he does decide to start smoking again. In addition, this should be used as an opportunity to reinforce the general risks of smoking to the patient and to encourage him to remain smoke-free.
It is also important to speak to the patient’s community team (e.g. doctors, nurses), who specialise in caring for patients with mental health disorders, about why the olanzapine dose was reduced during his stay, so that they can then monitor him in case he does begin smoking again.
Case 2: A woman with constipation
A woman aged 40 years* presents at the pharmacy. The pharmacist recognises her as she often comes in to collect medicine for her family. They are aware that she has a history of schizophrenia and that she was started on clozapine three months ago. She receives this from her mental health team on a weekly basis.
She has visited the pharmacy to discuss constipation that she is experiencing. She has noticed that since she was started on clozapine, her bowel movements have become less frequent. She is concerned as she is currently only able to go to the toilet about once per week. She explains that she feels uncomfortable and sick, and although she has been trying to change her diet to include more fibre, it does not seem to be helping. The patient asks for advice on a suitable laxative.
What needs to be considered?
Constipation is a very common side effect of clozapine . However, it has the potential to become serious and, in rare cases, even fatal [5] , [6] , [7] , [8] . While minor constipation can be managed using over-the-counter medicines (e.g. stimulant laxatives, such as senna, are normally recommended first-line with stool softeners, such as docusate, or osmotic laxatives, such as lactulose, as an alternative choice), severe constipation should be checked by a doctor to ensure there is no serious bowel obstruction as this can lead to paralytic ileus, which can be fatal [9] . Symptoms indicative of severe constipation include: no improvement or bowel movement following laxative use, fever, stomach pain, vomiting, loss of appetite and/or diarrhoea, which can be a sign of faecal impaction overflow.
As the patient has been experiencing this for some time and is only opening her bowels once per week, as well as having other symptoms (i.e. feeling uncomfortable and sick), she should be advised to see her GP as soon as possible.
The patient returns to the pharmacy again a few weeks later to collect a prescription for a member of their family and thanks the pharmacist for their advice. The patient was prescribed a laxative that has led to resolution of symptoms and she explains that she is feeling much better. Although she has a repeat prescription for lactulose 15ml twice per day, she says she is not sure whether she needs to continue to take it as she feels better.
What advice should be provided?
As she has already had an episode of constipation, despite dietary changes, it would be best for the patient to continue with the lactulose at the same dose (i.e. 15ml twice daily), to prevent the problem occurring again. Explain to the patient that as constipation is a common side effect of clozapine, it is reasonable for her to take laxatives before she gets constipation to prevent complications.
Pharmacists should encourage any patient who has previously had constipation to continue taking prescribed laxatives and explain why this is important. Pharmacists should also continue to ask patients about their bowel habits to help pick up any constipation that may be returning. Where pharmacists identify patients who have had problems with constipation prior to starting clozapine, they can recommend the use of a prophylactic laxative such as lactulose.
Case 3: A mother is concerned for her son who is talking to someone who is not there
A woman has been visiting the pharmacy for the past 3 months to collect a prescription for her son, aged 17 years*. In the past, the patient has collected his own medicine. Today the patient has presented with his mother; he looks dishevelled, preoccupied and does not speak to anyone in the pharmacy.
His mother beckons you to the side and expresses her concern for her son, explaining that she often hears him talking to someone who is not there. She adds that he is spending a lot of time in his room by himself and has accused her of tampering with his things. She is not sure what she should do and asks for advice.
What action can the pharmacist take?
It is important to reassure the mother that there is help available to review her son and identify if there are any problems that he is experiencing, but explain it is difficult to say at this point what he may be experiencing. Schizophrenia is a psychotic illness which has several symptoms that are classified as positive (e.g. hallucinations and delusions), negative (e.g. social withdrawal, self-neglect) and cognitive (e.g. poor memory and attention).
Many patients who go on to be diagnosed with schizophrenia will experience a prodromal period before schizophrenia is diagnosed. This may be a period where negative symptoms dominate and patients may become isolated and withdrawn. These symptoms can be confused with depression, particularly in younger people, though depression and anxiety disorders themselves may be prominent and treatment for these may also be needed. In this case, the patient’s mother is describing potential psychotic symptoms and it would be best for her son to be assessed. She should be encouraged to take her son to the GP for an assessment; however, if she is unable to do so, she can talk to the GP herself. It is usually the role of the doctor to refer patients for an assessment and to ensure that any other medical problems are assessed.
Three months later, the patient comes into the pharmacy and seems to be much more like his usual self, having been started on an antipsychotic. He collects his prescription for risperidone and mentions that he is very worried about his weight, which has increased since he started taking the newly prescribed tablets. Although he does not keep track of his weight, he has noticed a physical change and that some of his clothes no longer fit him.
What advice can the pharmacist provide?
Weight gain is common with many antipsychotics [10] . Risperidone is usually associated with a moderate chance of weight gain, which can occur early on in treatment [6] , [11] , [12] . As such, the National Institute for Health and Care Excellence recommends weekly monitoring of weight initially [13] . As well as weight gain, risperidone can be associated with an increased risk of diabetes and dyslipidaemia, which must also be monitored [6] , [11] , [12] . For example, the lipid profile and glucose should be assessed at 12 weeks, 6 months and then annually [12] .
The pharmacist should encourage the patient to attend any appointments for monitoring, which may be provided by his GP or mental health team, and to speak to his mental health team about his weight gain. If he agrees, the pharmacist could inform the patient’s mental health team of his weight gain and concerns on his behalf. It is important to tackle weight gain early on in treatment, as weight loss can be difficult to achieve, even if the medicine is changed.
The pharmacist should provide the patient with advice on healthy eating (e.g. eating a balanced diet with at least five fruit and vegetables per day) and exercising regularly (e.g. doing at least 150 minutes of moderate-intensity activity or 75 minutes of vigorous-intensity activity per week), and direct him to locally available services. The pharmacist can record the adverse effect on the patient’s medical record, which will help flag this in the future and thus help other pharmacists to intervene should he be prescribed risperidone again.
*All case studies are fictional.
Useful resources
- Mind — Schizophrenia
- Rethink Mental Illness — Schizophrenia
- Mental Health Foundation — Schizophrenia
- Royal College of Psychiatrists — Schizophrenia
- NICE guidance [CG178] — Psychosis and schizophrenia in adults: prevention and management
- NICE guidance [CG155] — Psychosis and schizophrenia in children and young people: recognition and management
- British Association for Psychopharmacology — Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology
About the author
Nicola Greenhalgh is lead pharmacist, Mental Health Services, North East London NHS Foundation Trust
[1] Chiu CC, Lu ML, Huang MC & Chen KP. Heavy smoking, reduced olanzapine levels, and treatment effects: a case report. Ther Drug Monit 2004;26(5):579–581. doi: 10.1097/00007691-200410000-00018
[2] de Leon J. Psychopharmacology: atypical antipsychotic dosing: the effect of smoking and caffeine. Psychiatr Serv 2004;55(5):491–493. doi: 10.1176/appi.ps.55.5.491
[3] Mayerova M, Ustohal L, Jarkovsky J et al . Influence of dose, gender, and cigarette smoking on clozapine plasma concentrations. Neuropsychiatr Dis Treat 2018;14:1535–1543. doi: 10.2147/NDT.S163839
[4] Ashir M & Petterson L. Smoking bans and clozapine levels. Adv Psychiatr Treat 2008;14(5):398–399. doi: 10.1192/apt.14.5.398b
[5] Young CR, Bowers MB & Mazure CM. Management of the adverse effects of clozapine. Schizophr Bull 1998;24(3):381–390. doi: 10.1093/oxfordjournals.schbul.a033333
[6] Taylor D, Barnes TRE & Young AH. The Maudsley Prescribing Guidelines in Psychiatry . 13th edn. London: Wiley Blackwell; 2018
[7] Oke V, Schmidt F, Bhattarai B et al . Unrecognized clozapine-related constipation leading to fatal intra-abdominal sepsis — a case report. Int Med Case Rep J 2015;8:189–192. doi: 10.2147/IMCRJ.S86716
[8] Hibbard KR, Propst A, Frank DE & Wyse J. Fatalities associated with clozapine-related constipation and bowel obstruction: a literature review and two case reports. Psychosomatics 2009;50(4):416–419. doi: 10.1176/appi.psy.50.4.416
[9] Medicines and Healthcare products Regulatory Agency. Clozapine: reminder of potentially fatal risk of intestinal obstruction, faecal impaction, and paralytic ileus. 2020. Available from: https://www.gov.uk/drug-safety-update/clozapine-reminder-of-potentially-fatal-risk-of-intestinal-obstruction-faecal-impaction-and-paralytic-ileus (accessed April 2020)
[10] Leucht S, Cipriani A, Spineli L et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013;382(9896):951–962. doi: 10.1016/S0140-6736(13)60733-3
[11] Bazire S. Psychotropic Drug Directory . Norwich: Lloyd-Reinhold Communications LLP; 2018
[12] Cooper SJ & Reynolds GP. BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. J Psychopharmacol 2016;30(8):717–748. doi: 10.1177/0269881116645254
[13] National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. Clinical guideline [CG178]. 2014. Available from: https://www.nice.org.uk/guidance/cg178 (accessed April 2020)
You might also be interested in…

Nearly half of long-term antidepressant users could safely taper off medication using helpline

Boots UK shuts online mental health service to new patients

Eating disorders: identification, treatment and support
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Understanding Schizophrenia: A Case Study

Schizophrenia is characterized mainly, by the gross distortion of reality, withdrawal from social interaction, disorganization and fragmentation of perception, thoughts and emotions. Insight is an important concept in clinical psychiatry, a lack of insight is particularly common in schizophrenia patient. Previous studies reported that between 50-80% of patients with schizophrenia do not believe, they have a disorder. By the help of psychological assessment, we can come to know an individual's problems especially in cases, where patient is hesitant or has less insight into illness. Assessment is also important for the psychological management of the illness. Knowing the strengths and weaknesses of that particular individual with psychological analysis tools can help to make better plan for the treatment. The present study was designed to assess the cognitive functioning, to elicit severity of psychopathology, understanding diagnostic indicators, personality traits that make the individual vulnerable to the disorder and interpersonal relationship in order to plan effective management. Schizophrenia is a chronic disorder, characterized mainly by the gross distortion of reality, withdrawal from social interaction, and disorganization and fragmentation of perception, thought and emotion. Approximately, 1% world population suffering with the problem of Schizophrenia. Both male and female are almost equally affected with slight male predominance. Schizophrenia is socioeconomic burden with suicidal rate of 10% and expense of 0.02-1.65% of GDP spent on treatment. Other co-morbid factors associated with Schizophrenia are diabetes, Obesity, HIV infection many metabolic disorders etc. Clinically, schizophrenia is a syndrome of variables symptoms, but profoundly disruptive, psychopathology that involves cognition, emotion, perception, and other aspects of behavior. The expression of these manifestations varies across patients and over the time, but the effect of the illness is always severe and is usually long-lasting. Patients with schizophrenia usually get relapse after treatment. The most common cause for the relapse is non-adherent with the medication. The relapse rate of schizophrenia increases later time on from 53.7% at 2 years to
Related Papers
Bangladesh Journal of Psychiatry
Luna Krasota Nur Laila
Schizophrenia is a chronic psychiatric illness with high rate of relapse which is commonly associated with noncompliance of medicine, as well as stress and high expressed emotions. The objective of the study was to determine the factors of relapse among the schizophrenic patients attending in outpatient departments of three tertiary level psychiatric facilities in Bangladesh. This was a cross sectional study conducted from July, 2001 to June, 2002. Two hundred patients including both relapse and nonrelapse cases of schizophrenia and their key relatives were included by purposive sampling. The results showed no statistically significant difference in terms of relapse with age, sex, religion, residence, occupation and level of education (p>0.05), but statistically significant difference was found with marital status and economic status (p<0.01). The proportion of non-compliance was found to be 80% and 14%, of high expressed emotion was 17% and 2% and of the occurrence of stressf...
Ashok Kumar Patel
BMC Psychiatry
Bonginkosi Chiliza
Revista Brasileira de Psiquiatria
Helio Elkis
OBJECTIVES: The heterogeneity of clinical manifestations in schizophrenia has lead to the study of symptom clusters through psychopathological assessment scales. The objective of this study was to elucidate clusters of symptoms in patients with refractory schizophrenia which may also help to assess the patients' therapeutical response. METHODS: Ninety-six treatment resistant patients were evaluated by the anchored version Brief Psychiatric Rating Scale (BPRS-A) as translated into Portuguese. The inter-rater reliability was 0.80. The 18 items of the BPRS-A were subjected to exploratory factor analysis with Varimax rotation. RESULTS: Four factors were obtained: Negative/Disorganization, composed by emotional withdrawal, disorientation, blunted affect, mannerisms/posturing, and conceptual disorganization; Excitement, composed of excitement, hostility, tension, grandiosity, and uncooperativeness, grouped variables that evoke brain excitement or a manic-like syndrome; Positive, compo...
Nicholas Tarrier
Annals of Clinical and Laboratory Research
James Mwaura
Sou Agarwal
Schizophrenia Bulletin
Joseph Goldberg
International journal of mental health nursing
Inayat ullah Shah
Despite a large body of research evaluating factors associated with the relapse of psychosis in schizophrenia, no studies in Pakistan have been undertaken to date to identify any such factors, including specific cultural factors pertinent to Pakistan. Semistructured interviews and psychometric measures were undertaken with 60 patients diagnosed with schizophrenia (49 male and 11 female) and their caregivers at four psychiatric hospitals in the Peshawar region in Pakistan. Factors significantly associated with psychotic relapse included treatment non-adherence, comorbid active psychiatric illnesses, poor social support, and high expressed emotion in living environments (P < 0.05). The attribution of symptoms to social and cultural values (97%) and a poor knowledge of psychosis by family members (88%) was also prevalent. In addition to many well-documented factors associated with psychotic relapse, beliefs in social and cultural myths and values were found to be an important, and p...
Octavian Vasiliu
Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
RELATED PAPERS
International Journal of Medicine and Public Health
amresh srivastava
Epidemiologia e psichiatria sociale
Rita Roncone
Progress in Neuro-Psychopharmacology and Biological Psychiatry
Archives of Psychiatric Nursing
Karen Schepp
Derege Kebede
ncbi.nlm.nih.gov
Swapnesh Tiwari
Actas españolas de psiquiatría
Enrique Echeburúa
European Psychiatry
Schizophrenia Research
Jonathan Rabinowitz
Adellah Sariah
Rikus Knegtering
Actas espanolas de psiquiatria
Manuel Bousono
The Journal of Nervous and Mental Disease
Andrea Affaticati , Rebecca Ottoni
Psychiatry Research
Massimo Tusconi
zewdu shewangizaw
IOSR Journals
Annals of General Psychiatry
Andreas Schreiner
Journal of psychiatry & neuroscience: JPN
Lawrence Annable
ROMANIAN JOURNAL …
Cornelia Rada
Ifeta Licanin
American family physician
Stephen Schultz
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
CASE REPORT article
Early-onset schizophrenia with predominantly negative symptoms: a case study of a drug-naive female patient treated with cariprazine.

- Institute of Genomic Medicine and Rare Disorders, Semmelweis University, Budapest, Hungary
Schizophrenia is a chronic and severe mental disorder characterized by positive, negative, and cognitive symptoms. Negative symptoms are usually present from the prodromal phase; early diagnosis and management of negative symptoms is a major health concern since an insidious onset dominated by negative symptoms is associated with a worse outcome. Antipsychotic medications, which are effective for treating positive symptoms, are generally ineffective for treating negative or cognitive symptoms. We present a 23-year-old woman showing severe symptoms at her first visit to our department. The patient’s parents reported that their daughter had experienced several years of psychosocial decline and putative psychiatric symptoms, but no medical attention had been previously sought; as such, the diagnosis of schizophrenia with predominantly negative symptoms was very much delayed. Early onset of schizophrenia, longer duration of untreated psychosis, and severe negative symptoms, which have limited treatment options, suggested a poor prognosis. We initiated monotherapy with cariprazine, a novel antipsychotic that has recently been proven efficacious in treating schizophrenia with predominantly negative symptoms. This report describes a 52-week cariprazine treatment regimen and follows the patient’s impressive clinical improvement confirmed by PANSS and CGI scores, and psychological tests.
Schizophrenia is a severe, chronic, and heterogeneous mental disorder that often has debilitating long-term outcomes. Its lifetime prevalence rate is estimated to be approximately 1% worldwide in the adult population ( Lehman et al., 2010 ). Onset generally occurs in late adolescence or early adulthood, with an average age of 18 years for men and 25 years for women. 1 The term early-onset schizophrenia (EOS) is used to refer to patients who are diagnosed with the disorder before this age. EOS is a severe, frequently disabling, and chronic condition with a prevalence approaching 0.5% in those younger than 18 years ( Hafner and Van der Heiden, 1997 ).
Schizophrenia is accompanied by a distortion of personality that affects fundamental mental and social functions, making everyday life extremely difficult for patients. Clinical symptoms are often classified in three main domains: positive symptoms, such as hallucinations, delusions, suspiciousness/persecution; negative symptoms, such as emotional withdrawal, blunted affect, and passive social withdrawal; and cognitive symptoms, such as impaired perception, learning, thinking, and memorizing. EOS may be accompanied by greater symptom severity, premorbid developmental impairment, ‘soft’ neurological signs (eg, clumsiness, motor incoordination), and a higher rate of substance abuse ( Hsiao and McClellan, 2008 ; Clemmensen et al., 2012 ; Immonen et al., 2017 ). Accordingly, diagnosis of EOS is often difficult and frequently delayed since onset is more commonly insidious than acute, which makes it difficult to differentiate EOS from underlying cognitive deficits, premorbid functional impairment, or other abnormalities ( Russell, 1994 ; Bartlet, 2014 ). Given this common delay in recognition of the disorder, the duration of untreated psychosis is often very long, further contributing to a poor outcome ( Penttila et al., 2014 ).
Although various hypotheses have been developed, the etiopathogenesis of schizophrenia and EOS is not fully understood ( McGuffin, 2004 ; Klosterkotter et al., 2011 ). 2 Among the rising and falling neurochemical theories, the dopamine hypothesis has remained a primary hypothesis guiding the treatment of schizophrenia. There are four dopaminergic pathways in the human brain: the mesolimbic, the mesocortical, the tuberoinfundibular, and the nigrostriatal. Positive symptoms of schizophrenia are associated with the hyperdopaminergic state of D 2 receptors in the mesolimbic area, while negative and cognitive symptoms are believed to be related to the hypodopaminergic dysregulation of the prefrontal cortex ( Stahl, 2003 ).
Negative symptoms of schizophrenia, which affect up to 60% of patients with schizophrenia ( Rabinowitz et al., 2013 ), form a complex clinical constellation of symptoms that challenge both diagnosis and treatment. By definition, negative symptoms mean the absence of normal functions. Negative symptoms are classified by their etiology as primary negative symptoms, which are core features of the disease itself, and secondary negative symptoms, which are consequences of positive symptoms, antipsychotic treatment, depression or extrapyramidal side effects. Five constructs have been accepted by general consensus as key aspects of negative symptoms: blunted affect, alogia, anhedonia, asociality, and avolition ( Marder and Galderisi, 2017 ). Patients with predominant negative symptoms lose their motivation, cannot function at school or work, and their interpersonal relationships severely decay. Due to impaired daily functioning and social amotivation, they may need constant care.
Although early intervention is associated with improvement in negative symptoms ( Boonstra et al., 2012 ), this may be challenging since negative symptoms develop slowly and may be difficult to detect or differentiate from other clinical features ( Kirkpatrick et al., 2001 ; Galderisi et al., 2018 ). Moreover, a more insidious onset predicts poorer outcome and more severe negative symptoms ( Kao and Liu, 2010 ; Immonen et al., 2017 ; Murru and Carpiniello, 2018 ). Diagnosis of patients with predominantly negative symptoms (lacking manifest psychotic signs) is often delayed, resulting in a longer duration of untreated psychosis. The length of untreated psychosis is closely related to poorer functional outcome ( Perkins et al., 2005 ).
Negative symptoms have traditionally had minimal response to antipsychotic treatment. First-generation antipsychotics are effective in treating positive symptoms, but negative symptom improvement is only evident when symptoms are secondary to positive symptoms. It was initially hoped that second-generation antipsychotics would target both positive and negative symptoms, but efficacy data have been disappointing. This was a large meta-analysis where only four second-generation drugs (amisulpride, risperidone, olanzapine, and clozapine) resulted to be more efficacious than first-generation antipsychotics in the overall change of symptoms, including positive and negative symptoms. The other examined second-generation antipsychotics were only as efficacious as first-generation antipsychotic agents ( Leucht et al., 2009 ). These studies were mainly conducted in patients with general symptoms of schizophrenia, therefore a secondary effect on negative symptoms could not be ruled out. Therefore negative symptom improvement cannot be considered a core component of atypicality ( Veerman et al., 2017 ). Previous studies have demonstrated that no drug had a beneficial effect on negative symptoms when compared to another drug ( Arango et al., 2013 ; Millan et al., 2014 ; Fusar-Poli et al., 2015 ), meaning that head to head comparisons of different agents among each other did not result in superiority of one drug to another. The latest comparison ( Krause et al., 2018 ) evaluated all studies that have been performed in the negative symptom population so far, and has found that amisulpride claimed superiority only to placebo, olanzapine was superior to haloperidol, but only in a small trial (n = 35), and cariprazine outperformed risperidone in a large well-controlled trial.
Hence cariprazine emerged as an agent of particular interest in regard to negative symptoms. Cariprazine is a dopamine D 3 /D 2 receptor partial agonist and serotonin 5-HT 1A receptor partial agonist. It has been hypothesized that cariprazine is the only antipsychotic that can block D 3 receptors in the living brain, thereby exhibiting functions that are related to D 3 blockade (e.g., improvement of negative symptoms) ( Stahl, 2016 ). In that large clinical trial including 460 patients with predominant negative symptoms and stable positive symptoms of schizophrenia, cariprazine was significantly more effective than risperidone in improving negative symptoms and patient functioning ( Nemeth et al., 2017 ).
Case Description
The 23-year-old female patient visited the Institute of Rare Diseases at our university with her parents. They had suspected for a long time that something was wrong with their daughter, but this was the first time they had asked for medical help. The patient was quiet and restrained since she did not speak much, her parents told us her story instead. Initially, the patient had done very well in a bilingual secondary school and was socially active with friends and peers. At the age of 15 years, her academic performance started to deteriorate, with her first problems associated with difficulty learning languages and memorizing. Her school grades dropped, and her personality started to gradually change. She became increasingly irritated, and was verbally and physically hostile toward her classmates, resorting to hitting and kicking at times. She was required to repeat a school year and subsequently dropped out of school at the age of 18 because she was unable to complete her studies. During these years, her social activity greatly diminished. She lived at home with her parents, did not go out with friends, or participate in relationships. Most of the time she was silent and unsociable, but occasionally she had fits of laughter without reason. Once the patient told her mother that she could hear the thoughts of others and was probably hearing voices as well. Slowly, her impulse-control problems faded; however, restlessness of the legs was quite often present.
Our patient’s medical history was generally unremarkable. She lacked neurological or psychiatric signs. She had a tonsillectomy and adenotomy at age 7 years. Epilepsy was identified in the patient’s family history (father’s uncle). On physical examination, there were no signs of internal or neurological disease; body mass index was 21.5 (normal weight).
During the first psychiatric interview and examination, we found that our patient was alert and vigilant, but had trouble relating due to decreased integrity of consciousness. Her attention could be aroused or partially directed, and she had difficulty keeping a target idea. Autopsychic and allopsychic orientations were preserved. Longer thinking latencies and slowed movement responses were observed, sometimes with even cataleptic impressions. Cognitive functions, such as thinking, memory, and concept formation, were severely impaired, and we were unable to carry out some of our neurocognitive tests -such as the Addenbrooke’s Cognitive Examination ( Hsieh et al., 2013 ), the Toulouse-Pieron attention test (Kanizsa G1951), Bells test ( Gauthier et al., 1989 ) and the Trail Making Test- because of the patient’s denial of symptoms and refusal to cooperate.
She often looked aside and laughed frequently, suggesting the presence of perceptual disturbances, but she denied her symptoms when asked. In contrast to the periodic inappropriate laughing, apathy and anhedonia were markedly present. During the examination, the patient could not recall anything she would do or even think of with pleasure. According to the heteroanamnesis, she lost her interest in activities she used to like, did not go out with friends anymore, and showed no signs of joy or intimacy towards her family members either.
Along with the affective hyporesponsiveness, amotivation and a general psychomotor slowing were observed. Hypobulia, void perspectives, and lack of motivation were explored. Parental statements indicated that the patient’s social activity had continued to diminish, and her appearance and personal physical hygiene had deteriorated. When we initiated a conversation, the patient was negativistic and agitated. Her critical thinking ability was reduced, which led to inappropriate behavior (she, e.g., unexpectedly stood up and left the room while the examination was still ongoing). Considering her status, she was admitted to the clinic after her first visit.
After several differential diagnostic tests were performed (e.g., routine diagnostic laboratory parameters, immune serological analyses, electroencephalogram, magnetic resonance imaging, genetic testing), all the possible common and rare disorders, such as Huntington’s disease, Niemann Pick C disease, mitochondrial disorders, and autoimmune diseases, were ruled out.
At first contact, to differentiate the symptoms and severity of putative schizophrenia, we mapped the positive, negative, and general symptoms, as well as a clinical impression, using the Positive and Negative Syndrome Scale (PANSS), the Scale for Assessment of Negative Symptoms, and the Clinical Global Impressions-Severity (CGI-S) ( Groth-Marnat, 2009 ).
The patient had a very high PANSS total score, which corresponded to being considered “severely ill” or “among the most severely ill’ on the CGI-S ( Leucht et al., 2005 ). The PANSS score was derived dominantly from the negative items of the scale. Overall, her negative symptoms fulfilled criteria for predominantly negative symptoms, meaning that positive symptomatology was reduced, while negative symptoms were more explicit and dominated the clinical picture ( Riedel et al., 2005 ; Olie et al., 2006 ; Mucci et al., 2017 ). Baseline rating scale sores are presented in Table 1 .
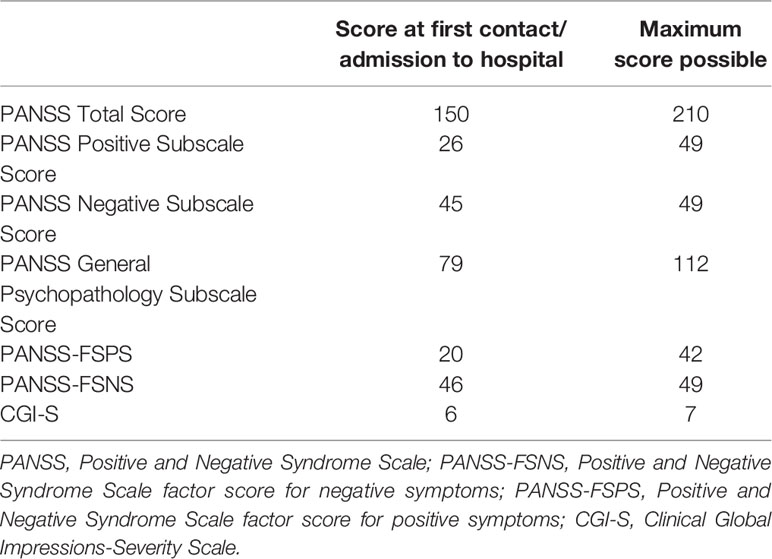
Table 1 Summary of symptom scale scores at the time of admission to the hospital.
The diagnosis of EOS with predominantly negative symptoms was given and treatment with the antipsychotic agent cariprazine was initiated. The patient was hospitalized for 2 weeks following her arrival at the clinic. Cariprazine was started at the dose of 1.5 mg/day and titrated up to 4.5 mg/day over a 2-week period: the patient received 1.5 mg/day for the first 3 days, 3 mg/day from day 4 to day 12, and eventually 4.5 mg/day from day 13 onward. During these 2 weeks, which were spent in hospital, the patient’s explicit negative symptoms such as poverty of speech, psychomotor retardation, poor eye contact, and affective nonresponsiveness improved; however, delusions and hallucinatory perceptions did not fade significantly.
Two weeks after discharge, we saw the patient for her first outpatient visit. Significant clinical improvement was observed. The patient calmly cooperated during the examination, with no signs of agitation. She was oriented to time, place, and self, attention could be drawn and directed, and she was able to keep a target idea and change the subject. Although according to the family, perceptual disturbances were still present, laughing with no reason and looking aside were much less frequent, and restlessness of the legs had stopped; these symptoms were not observed during the examination. Psychomotoric negativism had improved greatly, the patient was more communicative, and she paid more attention to the activities of family members. The pace of speech was close to normal: the thinking latencies and slowed movement responses as observed at admission were not seen anymore. The patient had adequate reaction time to questions asked and could focus in the interview. Mild obstipation and somnolence in the evening were her main complaints. Apart from some tick-like eye closures, there was no pathological finding during physical and neurological examination. At this point, cariprazine was reduced to 3 mg per day.
At her second outpatient visit, which occurred 8 weeks after treatment initiation, further improvement was observed. According to her mother, the patient was more active and open at home. Neurological examination found that the alternating movements of her fingers were slightly slowed. Cariprazine 3 mg/day was continued with concomitant anticholinergic medication.
At the third outpatient visit, which occurred 16 weeks after the first contact, the patient’s overall symptoms, including cognitive functions, such as memory and abstract thinking, as well as functions in activities of daily living, had improved remarkably. She had started to participate in the family’s daily life, even taking responsibility for some household duties; further, she went to the hairdresser for the first time in years, a step forward from her previous state of self-neglect. She was probably still having auditory hallucinations, which she considered natural, and some extrapyramidal symptom (EPS)-like ruminating movements, like to-and-fro swinging of her trunk, were observed. She did not look aside any more and tics were no longer present. Compared with previous visits overall, she was very relaxed, retained eye contact, cooperated, and communicated adequately during the interview. She started to develop insight into her condition, and she told us that her “thoughts were not healthy.” At the last two visits, the synkinesis of the arms was reduced.
After 16 weeks of treatment, the patient’s PANSS Negative Subscale Score and PANSS factor score for negative symptoms (PANSS-FSNS) score were reduced by 44.44% and 41.31%, respectively. Recent studies have demonstrated that linking the percentage improvement of PANSS with CGI-S and -Improvement (CGI-I) scores shows that a 25–50% reduction of PANSS scores corresponds to clinically meaningful change ( Correll et al., 2011 ; Fusar-Poli et al., 2015 ). In acutely ill patients with predominantly positive symptoms who are more likely to respond well to treatment, the 50% cutoff would be a more clinically meaningful criterion; however, since even slight improvement might represent a clinically significant effect in a patient with atypical schizophrenia, the use of 25% cutoff is justified ( Correll et al., 2011 ; Fusar-Poli et al., 2015 ).
In this regard, the 44.44% (change from baseline: −20) and 41.31% (change from baseline: −19) improvement demonstrated on PANSS Negative Symptom subscale and PANSS-FSNS, respectively, are considered a clearly clinically relevant change. Beyond the impaired synkinesis and alternating movement of the arms and fingers, there were no other treatment-related physical dysfunctions. Change from baseline on the PANSS and CGI scales are shown over the course of treatment in Table 2 .
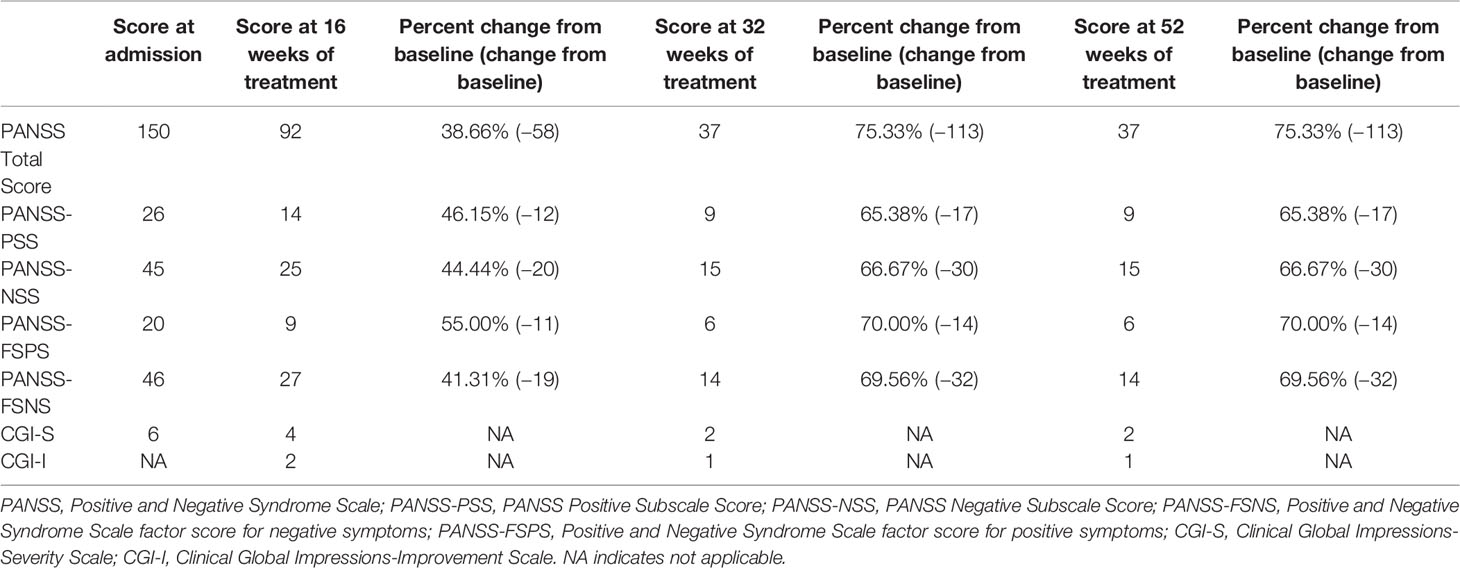
Table 2 Summary of symptom scale scores at weeks 16, 32, and 52.
Since our patient’s symptoms demonstrated strong improvement and tolerability was favorable, cariprazine therapy was continued. Improvement in both negative and positive symptoms was maintained over the course of treatment. At her later visits (32 and 52 weeks), PANSS total score was reduced to a level that was close to the minimum, and the decrease in negative symptom scores was considerable (PANSS-NSS=66.67% and PANS-FSNS=70.00% at both time points). The patient’s progress was also reflected in clinical and functional measurements, with the CGI-S score reduced to 2 (borderline mentally ill) and a CGI-I score of 1 (very much improved) indicating notable improvement.
Cariprazine has demonstrated broad spectrum efficacy in the treatment of positive and negative symptoms of schizophrenia. In a field where no treatment is available for difficult-to-treat negative symptoms, this case is unique and may have important implications for schizophrenia treatment. Despite experiencing approximately 8 years of untreated symptoms and functional impairment associated with predominantly negative symptom EOS, our 23-year-old female patient showed considerable symptomatic and functional improvement after several weeks of treatment with cariprazine. Given that the duration of untreated negative symptoms is associated with worse functional outcomes ( Boonstra et al., 2012 ), the remarkable improvement seen in this case shows how valuable cariprazine could be for patients with similar symptom presentations. Although it is not possible to generalize the observations and findings of this single case, it has the novelty of detecting a potential effect of cariprazine in a drug-naïve patient with marked negative symptoms of early-onset schizophrenia. To our knowledge, no cariprazine-related data has been published in this type of patients. A single case study is obviously far from being predictive for the efficacy of a drug, however, the results seen with this case are promising. With a dose recommended for patients with negative symptoms, our patient’s clinical condition, including positive, negative, and cognitive symptoms, as well as social functioning have improved notably, with the effect maintained for over 12 months. Generally, cariprazine has been well tolerated, with mild EPS observed after 8 weeks, but no metabolic, cardiac, or other side effects.
This case report suggests that the management of patients with EOS and prominent negative symptoms is achievable in everyday practice with cariprazine. More real-world clinical experience is needed to support this finding.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
All authors listed have made substantial, direct, and intellectual contribution to the work and approved it for publication.
This work was supported from Research and Technology Innovation Fund by the Hungarian National Brain Research Program (KTIA_NAP_ 2017-1.2.1-NKP-2017-00002). Editorial support for this case report was supported by funding from Gedeon Richter. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Acknowledgments
We are thankful to the patient and her family for giving us the opportunity to share her story in the form of a publication. Also, we acknowledge editorial assistance was provided by Carol Brown, MS, ELS, of Prescott Medical Communications Group, Chicago, Illinois, USA, a contractor of Gedeon Richter plc.
- ^ http://www.schizophrenia.com/szfacts.htm
- ^ https://www.nimh.nih.gov/health/topics/schizophrenia/index.shtml
Arango, C., Garibaldi, G., Marder, S. R. (2013). Pharmacological approaches to treating negative symptoms: a review of clinical trials. Schizophr. Res. 150 (2-3), 346–352. doi: 10.1016/j.schres.2013.07.026
PubMed Abstract | CrossRef Full Text | Google Scholar
Bartlet, J. (2014). Childhood-onset schizophrenia: what do we really know? Health Psychol. Behav. Med. 2 (1), 735–747. doi: 10.1080/21642850.2014.927738
Boonstra, N., Klaassen, R., Sytema, S., Marshall, M., De Haan, L., Wunderink, L., et al. (2012). Duration of untreated psychosis and negative symptoms–a systematic review and meta-analysis of individual patient data. Schizophr. Res. 142 (1-3), 12–19. doi: 10.1016/j.schres.2012.08.017
Clemmensen, L., Vernal, D. L., Steinhausen, H. C. (2012). A systematic review of the long-term outcome of early onset schizophrenia. BMC Psychiatry 12, 150. doi: 10.1186/1471-244X-12-150
Correll, C. U., Kishimoto, T., Nielsen, J., Kane, J. M. (2011). Quantifying clinical relevance in the treatment of schizophrenia. Clin. Ther. 33 (12), B16–B39. doi: 10.1016/j.clinthera.2011.11.016
Fusar-Poli, P., Papanastasiou, E., Stahl, D., Rocchetti, M., Carpenter, W., Shergill, S., et al. (2015). Treatments of Negative Symptoms in Schizophrenia: Meta-Analysis of 168 Randomized Placebo-Controlled Trials. Schizophr. Bull. 41 (4), 892–899. doi: 10.1093/schbul/sbu170
Galderisi, S., Mucci, A., Buchanan, R. W., Arango, C. (2018). Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry 5 (8), 664–677. doi: 10.1016/S2215-0366(18)30050-6
Gauthier, L., Dehaut, F., Joanette, Y. (1989). A quantitative and qualitative test for visual neglect. Int. J. Clin. Neuropsychol. 11 (2), 49–54. doi: 10.1016/j.neuropsychologia.2010.04.018
CrossRef Full Text | Google Scholar
Groth-Marnat, G. (2009). “Handbook of Psychological Assessment”. Fifth ed. (Hoboken, NJ: Wiley).
Google Scholar
Hafner, H., Van der Heiden, W. (1997). Epidemiology of schizophrenia. Can. J. Psychiatry 42 (2), 139–151. doi: 10.1177/070674379704200204
Hsiao, R., McClellan, J. (2008). Substance abuse in early onset psychotic disorders. J. Dual Diagn. 4 (1), 87–99. doi: 10.1300/J374v04n01_06
Hsieh, S., Schubert, S., Hoon, C., Mioshi, E., Hodges, J. R. (2013). Validation of the Addenbrooke’s Cognitive Examination III in frontotemporal dementia and Alzheimer’s disease. Dement Geriatr. Cognit. Disord. 36 (3-4), 242–250. doi: 10.1159/000351671
Immonen, J., Jaaskelainen, E., Korpela, H., Miettunen, J. (2017). Age at onset and the outcomes of schizophrenia: A systematic review and meta-analysis. Early Interv Psychiatry 11 (6), 453–460. doi: 10.1111/eip.12412
Kao, Y. C., Liu, Y. P. (2010). Effects of age of onset on clinical characteristics in schizophrenia spectrum disorders. BMC Psychiatry 10, 63. doi: 10.1186/1471-244X-10-63
Kirkpatrick, B., Buchanan, R. W., Ross, D. E., Carpenter, J. (2001). A separate disease within the syndrome of schizophrenia. Arch. Gen. Psychiatry 58 (2), 165–1671. doi: 10.1001/archpsyc.58.2.165
Klosterkotter, J., Schultze-Lutter, F., Bechdolf, A., Ruhrmann, S. (2011). Prediction and prevention of schizophrenia: what has been achieved and where to go next? World Psychiatry 10 (3), 165–174. doi: 10.1007/s00406-018-0869-3
Krause, M., Zhu, Y., Huhn, M., Schneider-Thoma, J., Bighelli, I., Nikolakopoulou, A., et al. (2018). Antipsychotic drugs for patients with schizophrenia and predominant or prominent negative symptoms: a systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 268 (7), 625–639. doi: 10.1007/s00406-018-0869-3
Lehman, A. F., Lieberman, J. A., Dixon, L. B., McGlashan, T. H., Miller, A. L., Perkins, D. O., et al. (2010). “Practice guideline for the treatment of patients with schizophrenia”, 2nd ed. American Psychiatric Association. Avaialble at: https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf .
Leucht, S., Kane, J. M., Kissling, W., Hamann, J., Etschel, E., Engel, R. R. (2005). What does the PANSS mean? Schizophr. Res. 79 (2-3), 231–238. doi: 10.1016/j.schres.2005.04.008
Leucht, S., Corves, C., Arbter, D., Engel, R. R., Li, C., Davis, J. M. (2009). Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 373 (9657), 31–41. doi: 10.1016/S0140-6736(08)61764-X
Marder, S. R., Galderisi, S. (2017). The current conceptualization of negative symptoms in schizophrenia. World Psychiatry 16 (1), 14–24. doi: 10.1002/wps.20385
McGuffin, P. (2004). Nature and nurture interplay: schizophrenia. Psychiatr. Prax 31 Suppl 2, S189–S193. doi: 10.1055/s-2004-834565
Millan, M. J., Fone, K., Steckler, T., Horan, W. P. (2014). Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur. Neuropsychopharmacol. 24 (5), 645–692. doi: 10.1016/j.euroneuro.2014.03.008
Mucci, A., Merlotti, E., Ucok, A., Aleman, A., Galderisi, S. (2017). Primary and persistent negative symptoms: Concepts, assessments and neurobiological bases. Schizophr. Res. 186, 19–28. doi: 10.1016/j.schres.2016.05.014
Murru, A., Carpiniello, B. (2018). Duration of untreated illness as a key to early intervention in schizophrenia: A review. Neurosci. Lett. 669, 59–67. doi: 10.1016/j.neulet.2016.10.003
Nemeth, G., Laszlovszky, I., Czobor, P., Szalai, E., Szatmari, B., Harsanyi, J., et al. (2017). Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet 389 (10074), 1103–1113. doi: 10.1016/S0140-6736(17)30060-0
Olie, J. P., Spina, E., Murray, S., Yang, R. (2006). Ziprasidone and amisulpride effectively treat negative symptoms of schizophrenia: results of a 12-week, double-blind study. Int. Clin. Psychopharmacol. 21 (3), 143–151. doi: 10.1097/01.yic.0000182121.59296.70
Penttila, M., Jaaskelainen, E., Hirvonen, N., Isohanni, M., Miettunen, J. (2014). Duration of untreated psychosis as predictor of long-term outcome in schizophrenia: systematic review and meta-analysis. Br. J. Psychiatry 205 (2), 88–94. doi: 10.1192/bjp.bp.113.127753
Perkins, D. O., Gu, H., Boteva, K., Lieberman, J. A. (2005). Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am. J. Psychiatry 162 (10), 1785–1804. doi: 10.1176/appi.ajp.162.10.1785
Rabinowitz, J., Werbeloff, N., Caers, I., Mandel, F. S., Stauffer, V., Menard, F., et al. (2013). Negative symptoms in schizophrenia–the remarkable impact of inclusion definitions in clinical trials and their consequences. Schizophr. Res. 150 (2-3), 334–338. doi: 10.1016/j.schres.2013.06.023
Riedel, M., Muller, N., Strassnig, M., Spellmann, I., Engel, R. R., Musil, R., et al. (2005). Quetiapine has equivalent efficacy and superior tolerability to risperidone in the treatment of schizophrenia with predominantly negative symptoms. Eur. Arch. Psychiatry Clin. Neurosci. 255 (6), 432–437. doi: 10.1007/s00406-005-0622-6
Russell, ,. A. T. (1994). The Clinical Presentation of Childhood-Onset Schizophrenia. Schizophr. Bull. 20 (4), 631–646. doi: 10.1093/schbul/20.4.631
Stahl, S. M. (2003). Describing an atypical antipsychotic: Receptor binding and its role in pathophysiology. Prim Care Companion J. Clin. Psychiatry 5 (Suppl 3), 9–13.
Stahl, S. M. (2016). Mechanism of action of cariprazine. CNS Spectr. 21 (2), 123–127. doi: 10.1017/S1092852916000043
Veerman, S. R. T., Schulte, P. F. J., de Haan, L. (2017). Treatment for Negative Symptoms in Schizophrenia: A Comprehensive Review. Drugs 77 (13), 1423–1459. doi: 10.1007/s40265-017-0789-y
Keywords: cariprazine, schizophrenia, negative symptoms, early-onset schizophrenia, second-generation antipsychotic
Citation: Molnar MJ, Jimoh IJ, Zeke H, Palásti Á and Fedor M (2020) Early-Onset Schizophrenia With Predominantly Negative Symptoms: A Case Study of a Drug-Naive Female Patient Treated With Cariprazine. Front. Pharmacol. 11:477. doi: 10.3389/fphar.2020.00477
Received: 24 October 2019; Accepted: 26 March 2020; Published: 23 April 2020.
Reviewed by:
Copyright © 2020 Molnar, Jimoh, Zeke, Palásti and Fedor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Judit Molnar, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Physiother Can
- v.62(4); Fall 2010
Language: English | French
Case Report: Schizophrenia Discovered during the Patient Interview in a Man with Shoulder Pain Referred for Physical Therapy
Purpose: The purpose of this case report is to demonstrate the importance of a thorough patient interview. The case involves a man referred for physical therapy for a musculoskeletal dysfunction; during the patient interview, a psychiatric disorder was recognized that was later identified as schizophrenia. A secondary purpose is to educate physical therapists on the recognizable signs and symptoms of schizophrenia.
Client description: A 19-year-old male patient with chronic shoulder, elbow, and wrist pain was referred for physical therapy. During the interview, the patient reported that he was receiving signals from an electronic device implanted in his body.
Measures and outcome: The physical therapist's initial assessment identified a disorder requiring medical referral. Further management of the patient's musculoskeletal dysfunction was not appropriate at this time.
Intervention: The patient was referred for further medical investigation, as he was demonstrating signs suggestive of a psychiatric disorder. The patient was diagnosed with schizophrenia by a psychiatrist and was prescribed Risperdal.
Implications: This case study reinforces the importance of a thorough patient interview by physical therapists to rule out non-musculoskeletal disorders. Patients seeking neuromusculoskeletal assessment and treatment may have undiagnosed primary or secondary psychiatric disorders that require recognition by physical therapists and possible medical referral.
RÉSUMÉ
Objectif : L'objectif de cette étude de cas consiste à démontrer l'importance de réaliser des entrevues en profondeur avec les patients. Le cas étudié concerne un homme dirigé vers la physiothérapie en raison d'une dysfonction musculosquelettique. Au cours de l'entrevue avec ce patient, un problème psychiatrique a été décelé; par la suite, de la schizophrénie a été diagnostiquée. Le deuxième objectif de cette étude de cas est d'éduquer et de sensibiliser les physiothérapeutes aux signes et aux symptômes aisément reconnaissables de la schizophrénie.
Description du client : Le patient est un jeune homme de 19 ans qui souffre de douleurs chroniques à l'épaule, au coude et au poignet et qui avait été dirigé en physiothérapie. Au cours de l'entrevue, le patient a déclaré qu'il recevait des signaux provenant d'un appareil électronique implanté dans son corps.
Mesures et résultats : L'évaluation préliminaire du physiothérapeute a permis d'identifier un problème qui nécessitait que le patient soit redirigé vers un médecin. Une gestion plus poussée de la dysfonction musculosquelettique de ce patient a été jugée inappropriée à cette étape.
Intervention : Le patient a été dirigé vers une investigation médicale plus approfondie, puisqu'il manifestait des signes de possibles problèmes psychiatriques. Le patient a par la suite été diagnostiqué comme schizophrène et on lui a prescrit du Risperdal.
Implication : Cette étude de cas vient réaffirmer l'importance, pour le physiothérapeute, de procéder à des entrevues approfondies avec les patients pour s'assurer qu'il n'y a pas d'autres problèmes que les seules dysfonctions musculosquelettiques. Les patients qui souhaitent obtenir une évaluation et un traitement musculosquelettique peuvent souffrir aussi d'un problème psychiatrique primaire ou secondaire non diagnostiqué qui exige d'être reconnu par le physiothérapeute et qui nécessitera vraisemblablement une attention médicale ultérieure.
INTRODUCTION
A recent US study demonstrated that less than one-third of diagnoses provided to physical therapists by primary-care physicians are specific. 1 The same study illustrated that physical therapists must assume a greater diagnostic role and must routinely provide medical screening and differential diagnosis of pathology during the examination. 1 Similarly, studies conducted in Australia and Canada have concluded that the majority of referrals for physical therapy are not provided with a specific diagnosis. 2 , 3 Medical screening is important, since physical therapists are increasingly functioning as the primary contact for patients with neuromusculoskeletal dysfunctions, 4 , 5 which means a greater likelihood of encountering patients with non-musculoskeletal disorders, including psychiatric disorders.
As demonstrated by the World Health Organization's International Classification of Functioning, Disability and Health, it is imperative to take an individual's psychological state into account, since disorders in this area can lead to disability. 6 Many psychiatric conditions are commonly encountered in physical therapy practice; for example, depression, anxiety, and fear-avoidance have all been associated with low back, neck, and widespread musculoskeletal pain. 7 – 9 These psychiatric disorders have been identified both as risk factors for musculoskeletal dysfunction and as an important secondary psychosocial aspect of disablement. 7 – 10 It is therefore important for physical therapists to consider the primary and secondary roles of psychopathology in disability.
Although various models of primary-care physical therapy have demonstrated physical therapists' expertise in the realm of neuromusculoskeletal dysfunctions, there is a need for increased competencies in academic, clinical, and affective domains. 5 Few et al. propose a hypothesis-oriented algorithm for symptom-based diagnosis through which physical therapists can arrive at a diagnostic impression. 11 This algorithm takes into account the various causes of pathology, including psychogenic disorders. 11 Although additional research is necessary to validate Few et al.'s algorithm, it provides one model that considers underlying pathologies in determining the appropriateness of physical therapy intervention. 11 The present case report further illustrates the importance of considering the patient's affective and psychological state in order to more effectively screen for and identify psychiatric disorders that require medical referral.
The purpose of this case report is to demonstrate the importance of a thorough patient interview. We present the case of a man, referred for physical therapy for a musculoskeletal dysfunction, who was determined during the patient interview to have an undiagnosed psychiatric disorder, later identified as schizophrenia. In addition, this report is intended to educate physical therapists about the recognizable signs and symptoms of schizophrenia.
CASE DESCRIPTION
The patient was a 19-year-old male university student. His recreational activities included skateboarding, snowboarding, break dancing, and weight training. The patient first sought medical attention from a sport medicine physician in January 2006, when he reported right lateral wrist pain since falling and hitting the ulnar aspect of his wrist while skateboarding in October 2005. Plain film radiographs taken after the injury were negative, and the patient did not receive any treatment. The physician found no wrist swelling, minimal tenderness over the ulnar aspect of the right wrist, full functional strength, and minimally restricted range of motion (ROM). The patient was given ROM exercises and was diagnosed with a right wrist contusion.
Over the next 22 months, the patient returned to the same sport medicine clinic 10 times, reporting pain in his wrist, shoulder, elbow, knee, ankle, and neck. He stated that the elbow, wrist, and shoulder injuries were due to falls while skateboarding and snowboarding or to overuse during weight training; some injuries had no apparent cause. Over the course of his medical care, the patient followed up with three different physicians at the same clinic. He was diagnosed by these physicians, in order of occurrence, with (1) right wrist contusion and sprain; (2) right wrist impingement and left wrist strain; (3) right shoulder supraspinatus tendinopathy; (4) right peroneal overuse injury and strain; (5) disuse adhesions of the right peroneals and right hip adhesions; (6) right ankle neuropathic pain secondary to nerve injury and sprain and right-knee patellofemoral pain syndrome (PFPS); (7) neuropathic pain of the right peroneal nerve; (8) trauma-induced left-knee PFPS; (9) ongoing post-traumatic left-knee PFPS; and (10) right levator scapula strain, chronic right infraspinatus strain, right elbow ulnar ridge contusion, and right wrist chronic distal ulnar impingement secondary to malaligned triangular fibrocartilage complex (TFCC).
After his tenth visit to a physician, the patient was referred for physical therapy for chronic right levator scapula strain and right supraspinatus strain. During the interview, the patient stated that he had right shoulder pain because of a snowboarding injury sustained 1 year earlier and because of a fall onto the lateral right shoulder 2 years ago. Aggravating activities to the shoulder included pull-ups, rowing, and free weights. No position or movement alleviated his pain, and the pain did not fluctuate over the course of the day. His sleep was disturbed only when lying on the right shoulder. The patient was in generally good health, but he said that his right wrist and left knee occasionally felt cold for no apparent reason. He denied experiencing any loss of sensation, decreased blood flow, or numbness or tingling in the knee and wrist. The patient said he believed that his knee and wrist became cold as a result of electromagnetic impulses sent to the joint via an electrical implant in his body and that this device was the cause of his ongoing shoulder pain.
According to the patient, this device had been implanted into his body 2 years earlier by a government organization (the Central Intelligence Agency, the US government, or the US Army) to control his actions. Electromagnetic impulses generated by the implant had caused his falls and injuries; they also caused his joints to become cold or painful when he was doing something “they” did not want him to do, such as break dancing, snowboarding, skateboarding, or exercising. The patient also believed that many other people unknowingly had implants; he claimed that friends, neighbours, professors, and strangers were “working with them” and that they “emotionally abuse[d]” him by giving signs such as kicking a leg back to let him know he was being watched. Furthermore, he indicated that he often received commands telling him to harm his friends or family and that these orders came either from the electrical implant or from the people he claimed were emotionally abusing him. He therefore distanced himself from some friends because he did not want to follow through with these commands. I asked the patient if he felt he would harm himself or others because of his psychotic-like symptoms. He denied any desire to inflict harm on himself or others. Had he posed a threat to himself or others, he would have been “formed” (i.e., committed to a psychiatric facility by the appropriate medical professional).
The patient's past medical and family history were unremarkable. He did not use any prescription or over-the-counter medications, but he felt his thoughts about electrical implants were decreased by the use of marijuana, which he used socially. He was a non-smoker and a social consumer of alcohol. He had a normal gait and appeared comfortable in an unsupported seated position. He denied any weight changes, bowel or bladder problems, night pain, or difficulty breathing.
PHYSICAL EXAMINATION
The patient reported a maximum verbal numeric pain rating scale (NPRS) score of 8/10 and a minimum score of 0/10, with pain usually present in the shoulder. In a double-blind, placebo-controlled, multi-centre chronic pain study, when the baseline NPRS raw score fluctuated by 0 points, the sensitivity and specificity were 95.32% and 31.80% respectively; 12 , 13 when there was a 4-point raw score change, the sensitivity and specificity were 35.92% and 96.92% respectively. 12 The patient stated that when he experienced shoulder pain, it was located on the anterior, posterior, and lateral aspects of his shoulder and radiated down to his elbow and wrist. He reported 0/10 shoulder pain while seated.
Standing posture was assessed in the frontal and sagittal planes. 14 The patient had a mild forward head posture and internally rotated glenohumeral joints in the sagittal plane. The frontal-plane analysis revealed a slight elevation of the right shoulder and level iliac crests. Such visual assessment of cervical and lumbar lordosis has an intrarater reliability of k =0.50 but an interrater reliability of k =0.16. 15
In the frontal plane, the right scapula was abducted four finger-widths from the mid-thoracic spine, and the left scapula was abducted three finger-widths. The scapulas were superiorly rotated bilaterally. Surface palpation of the acromial angle, inferior angle, and spine of the scapula differed less than 0.98 cm, 0.46 cm, and 0.67 cm, respectively, from the actual bony location, with a 95% confidence interval. 16 There was visible hypertrophy of the pectoralis major muscle bilaterally. Active and passive ROM were tested for the shoulders as recommended by Magee. 14 The patient had full bilateral active ROM, with minimal pain at end-range flexion and abduction that was not increased with overpressure in accordance with Magee. 14 He had full passive ROM with no pain reported.
Manual muscle testing based on Hislop and Montgomery revealed 4/5 strength of external rotation at 0° and 45° of abduction, with pain reported along the anterolateral shoulder. 17 Testing also showed 3/5 strength and no pain with resisted abduction with the arm at the side at approximately 30° of abduction. 18 Manual muscle testing is a useful clinical assessment tool, although a recent literature review suggested that further testing is required for scientific validation. 18 Palpation of the shoulder, as described by Hoppenfeld, revealed slight tenderness over the greater tubercle, as well as along the length of the levator scapula muscle. 19
Special tests were negative for the sulcus sign, Speed's test, the drop arm test, and the empty can test, as described by Magee. 14 Research shows that Speed's test has a sensitivity and specificity of 32% and 61% for biceps and labral pathology respectively; 20 the drop arm test has a sensitivity of 27% and a specificity of 88% as a specific test for rotator cuff tears, and the empty can test has a sensitivity of 44% and a specificity of 90% in diagnosing complete or partial rotator cuff tears. 20 , 21 The Neer and Hawkins-Kennedy impingement tests were both negative. 14 According to a meta-analysis by Hegedus et al., the Neer test is 79% sensitive and 53% specific, while the Hawkins-Kennedy test is 79% sensitive and 59% specific, for impingement. 21
I (NS) diagnosed the patient with mild supraspinatus tendinosis, with no evidence of tearing of the rotator cuff muscles, based on the following findings drawn from the patient interview: shoulder pain aggravated by pull-ups, rowing, and free weights; increased pain when lying on the affected shoulder. Additional significant findings from the physical examination included full shoulder active ROM with minimal pain at end-range flexion and abduction; pain along the anterior lateral shoulder with resisted testing of external rotation at 0° and 45° of abduction; negative drop arm and empty can tests; and tenderness over the greater tubercle of the humerus. The musculoskeletal dysfunction did not explain the level of pain reported by the patient (maximum NPRS 8/10), nor was the physical examination able to reproduce the exact location of the reported shoulder pain or the elbow, wrist, and knee pain described by the patient.
I was concerned about a serious pathology or a psychological disorder, given that this 19-year-old had made 10 medical appointments over 22 months for 6 different regions of the body; in my experience of examining and treating patients between the ages of 18 and 25, the frequency of the appointments and the variation in afflicted body parts are not typical of a young patient. The patient's description of his shoulder pain, in terms of location and severity, was not reproducible by physical examination. Throughout our interview, the patient did not maintain good eye contact, spoke in a monotone voice, and had an overall flat affect. Even when he described his beliefs about implants and government control, his voice and demeanour remained expressionless. The patient described persecutory delusions, command hallucinations, and social isolation from friends and family, all of which are signs of psychosis according to the Diagnostic and Statistical Manual of Mental Health . 22
Based on the findings from the patient interview and the physical examination, the patient did have symptoms consistent with a known musculoskeletal dysfunction; however, the undiagnosed and uncontrolled psychiatric symptoms made it more appropriate to refer him back to the physician for evaluation and treatment of his psychosis than to provide physical therapy intervention for his shoulder dysfunction. Furthermore, because research shows that the rate of suicide among patients with schizophrenia can range from 2% or 4% to as high as 15% 23 , 24 and that the rate of suicide is highest among patients close to the date of diagnosis, early recognition is crucial. 23
INTERVENTION
Based on the findings from the patient interview and the signs and symptoms of psychiatric disorders, I explained to the patient that there was a need for further medical investigation. Although the patient did not agree with this initial assessment, he did consent to a follow-up with the referring physician.
I spoke to the referring physician in person and explained to him my findings from the patient interview, specifically the patient's belief that he had electrical implants in his body. I also pointed out the patient's affect and the limited physical findings during the physical examination. I provided the physician with some direct quotes from the patient to demonstrate the level of psychosis he was presenting with. I stated my conclusion that the patient was suffering from some form of psychosis that precluded physical therapy treatment for his shoulder at that time. The referring physician was quite concerned about the patient and called him during our meeting to arrange a follow-up medical appointment.
The physician examined the patient, made similar observations, concurred with my assessment, and concluded that the patient was experiencing some form of psychosis. The plan of care involved referral to a psychiatrist, follow-up with the physician, and explaining to the patient that physical therapy would not be appropriate at this time because of the presence of a serious psychiatric disorder. The patient did not believe that he had a psychiatric disorder, but he was willing to follow up with a psychiatrist. The physician noted that the patient was not a threat to himself or others and that he did not report having homicidal or suicidal thoughts.
The patient followed up with the psychiatrist 11 days after his appointment with the physician. He was diagnosed with schizophrenia and started on a daily dose of risperidone (Risperdal). The patient was also instructed to follow up with the psychiatrist every second week to ensure compliance with the medication and to discuss progress. Further details of the psychiatric assessment and treatment were not available for this case report. Outcomes are also unavailable for this case report, since follow-up by the physical therapist was not possible.
Case Summary
This case report describes a 19-year-old man referred to physical therapy with shoulder, wrist, and knee pain who was later diagnosed with a psychiatric disorder. After completing a thorough patient interview and physical examination, I concluded that the patient was suffering from an undiagnosed psychiatric disorder that required medical referral. The interview revealed that the patient had delusions about electrical devices' being implanted in his body and was experiencing various forms of hallucination. The patient was promptly referred for medical consult and was diagnosed with schizophrenia by a psychiatrist.
Patient Symptoms and Schizophrenia
Schizophrenia is a psychiatric disorder affecting between 0.5% and 1.5% of adults worldwide, with a slightly greater prevalence in men. 22 The age of onset may be from 5 to 60 years; however, more than 50% of first episodes occur between the ages of 15 and 24. 22 , 25 , 26 An earlier onset is more common among men, while later onset is more common among women. 25 Schizophrenia shows a higher incidence in individuals born in urban areas than in those born in rural areas. 22 , 25 Because the patient in the present case fell into several of these categories (male, born in an urban area, experienced onset of symptoms around age 17) and presented with clear symptoms of a psychiatric disorder (delusions, hallucinations), schizophrenia seemed the most likely diagnosis.
The signs and symptoms of schizophrenia are classified as either positive or negative. 22 Positive symptoms are an excess of normal function and include delusions, hallucinations, and disorganized speech; 22 , 27 negative symptoms are a deficiency of normal function and include limited goal-directed behaviour (avolition), limited fluency and productivity of speech and thought, and a flat affect. 22 , 27 The diagnosis of schizophrenia requires the presence of at least two of these positive or negative symptoms lasting at least 6 months. 22 , 27 In this case, the patient presented with delusions (e.g., electrical implants trying to control his and others' actions), including persecutory delusions (e.g., “they are emotionally abusing me”), hallucinations (e.g., hearing voices, seeing signs), and a flat affect. Since the patient was enrolled in university at the time of diagnosis, his cognitive function is assumed to be well preserved. The patient reported no change in symptoms for 2 years.
Schizophrenia is subdivided into five types: paranoid, disorganized, catatonic, undifferentiated, and residual (see Table Table1 1 ). 22 , 28 Based on these observations and on the literature, the patient's symptoms were suggestive of paranoid schizophrenia, 22 which is the most prevalent form of schizophrenia in most parts of the world. 22
Schizophrenia Subtypes 6
| Subtype | Primary Symptoms | Features |
|---|---|---|
| Paranoid | 1. Persecutory or grandiose delusions 2. Auditory hallucinations 3. Delusions and hallucinations organized around a central theme | 1. Normal affect and cognition 2. Late onset 3. Best prognosis of the subtypes |
| Disorganized | 1. Disorganized speech 2. Disorganized behaviour 3. Flat affect | 1. Disorganized hallucinations or delusions 2. Insidious onset 3. No remission |
| Catatonic | 1. Motor immobility 2. Purposeless and excessive motor activity 3. Inappropriate or bizarre postures maintained 4. Echolalia | 1. Risk of malnutrition, hyperpyrexia, or self-inflicted injuries 2. May pose threat to self and others 3. Mutism |
| Undifferentiated | 1. Symptoms meet the basic criteria for schizophrenia | N/A |
| Residual | 1. At least one episode of schizophrenia 2. Presence of negative symptoms 3. Two or more attenuated positive symptoms | 1. Can be transition between full-blown episode and complete remission 2. Can be present for years, with or without exacerbations |
The aetiology of schizophrenia remains unknown. 29 , 30 There is a strong genetic predisposition. 29 , 30 Patients who experience the onset of schizophrenia before age 22 are 10 times more likely to have a history of a complicated caesarean birth than patients with a later onset of schizophrenia, which suggests a possible neurodevelopmental factor in early-onset schizophrenia. 31 Mild childhood head injuries may play a role in the expression of schizophrenia in families with a strong genetic predisposition to this disorder. 32 Psychological stress has also been implicated in the onset of schizophrenia, since it often precipitates the first psychotic episode or increases the likelihood of a relapse. 33 , 34 In this case, the patient described a family “break-up” which may have precipitated the onset of psychosis. Details about his childhood head injuries and the circumstances of his birth were not obtained. After being diagnosed with schizophrenia, the patient revealed to the referring physician that his father had experienced something similar when he was younger, which may point to a genetic predisposition.
There are no conclusive diagnostic tests for schizophrenia. 22 However, imaging studies have suggested neurophysiologic changes as an associated finding. Volumetric magnetic resonance imaging (MRI) studies of patients with schizophrenia have demonstrated an overall reduction in grey matter; an increase in white matter; decreased size of the amygdala, hippocampus, and parahippocampus; an overall reduction in brain volume; and larger lateral ventricles relative to a control group. 35 – 37
Psychiatric Disorders as They Relate to Musculoskeletal Dysfunction
As primary-care practitioners, physical therapists may encounter patients with possible psychiatric disorders such as schizophrenia. However, the physical therapy literature on psychiatric disorders as they relate to musculoskeletal disorders focuses mainly on low back pain (LBP). 7 , 8 In an examination of a large number of physical and psychological factors, one prospective case-control study points to the importance of psychological variables as a risk factor for chronic LBP and widespread musculoskeletal pain. 8 Previous research has also concurred with this study in implicating psychological variables as risk factors for LBP and neck pain. 9 , 10 These articles provide a link between psychological disorders and patients seeking physical therapy for musculoskeletal dysfunctions.
In this case report, the physical examination was suggestive of a mild supraspinatus tendinosis, but this did not explain the severity of pain reported by the patient or the referral of pain to the elbow, wrist, and knee. One of the limitations of the physical examination was that there was not sufficient time to perform physical examination of the elbow, wrist, and knee. The patient's undiagnosed and uncontrolled psychiatric symptoms took priority over the musculoskeletal dysfunction and required immediate medical referral without physical therapy intervention. Because of the inconsistencies between interview and physical examination, as well as the patient's perception that an electrical implant was causing his musculoskeletal pain, there is a possibility that at least some of his musculoskeletal symptoms may have been manifestations of his psychiatric disorder.
Effective Patient Interviews
The medical literature indicates that 50% of all mental illness is recognized during the interview process as part of medical assessment by the primary-care physician. 38 As physical therapists embrace their role as providers of primary care, 4 , 5 they must rely on their skills in patient interviewing and physical examination to rule out medical pathology. Improved assessment skills by the physical therapist may help to identify primary or secondary medical pathologies that have not previously been diagnosed. Within the peer-reviewed literature, a number of case studies demonstrate identification of non-musculoskeletal or visceral pathology that can manifest as musculoskeletal disorders; 39 – 41 these case studies are examples of how physical therapists can perform an initial assessment, identify a medical pathology that precludes treatment, and make an appropriate referral. During a patient interview, physical therapists must be well aware of the psychological and psychosocial aspects of the examination to identify relevant aspects of the patient's demeanour (e.g., appropriate self-care) and emotional state (e.g., inappropriate affect). The patient interview should consist of non-leading, open-ended questions about how pain in multiple areas is related and how it is caused. Furthermore, physical therapists should avoid rationalizing the patient's symptoms during the interview process. At a minimum, patients should be permitted to speak about and describe their symptoms in a way that is meaningful to them.

Schizophrenia and Primary Care
Schizophrenia is most often initially recognized by the primary-care physician. 42 Psychiatrists, psychologists, and even the lay community have also been noted in the literature as making the initial identification. 43 – 45 Although conspicuously absent from the literature on the initial identification of schizophrenia, physical therapists are in a position to be important first-contact care providers who can make the initial identification of schizophrenia, and other psychiatric disorders, through effective patient interviews. Although labelling patients as having a psychiatric disorder is outside physical therapists' scope of practice, the diagnostic process is not exclusive to any one profession. In this case, the process of diagnosis, which involves assessing the patient, grouping findings, interpreting the data, and identifying the patient's problems, led me to conclude that the primary dysfunction was psychiatric in nature. 46 This process, which Few et al. call “diagnostic reasoning,” is well within physical therapists' scope of practice and is something we constantly engage in during our daily clinical practice. 11 Diagnostic reasoning involves taking into account all of the possible pathological structures and determining the most likely cause of the patient's symptoms. In practice, expert clinicians do not follow standardized protocols; 46 rather, they pay attention to cues provided by the patient, recognize patterns, and test hypotheses to arrive at a probable cause for the patient's symptoms. 11
IMPLICATIONS AND FUTURE DIRECTIONs
The medical literature has identified gaps in the knowledge of primary-care physicians, specifically a lack of awareness of the symptoms and epidemiology of schizophrenia. 28 To facilitate early recognition, referral, and diagnosis of schizophrenia, the medical literature has suggested increased collaboration among family physicians and mental-health professionals, as well as ongoing mental-health training for family physicians. 47 , 48 Physical therapists should also heed these suggestions. A study in the physical therapy literature recommends mental-health training for recognizing the symptoms of depression in a population with LBP; 7 the same study, conducted in Australia, concluded that physical therapists' ability to recognize depressive symptoms in an outpatient setting was poor. 7
An initial step to address these gaps could be a position paper that draws on the medical literature to inform physical therapists about the presence, prevalence, signs, and symptoms of common psychiatric disorders. As well, future research needs to focus on the incidence of musculoskeletal signs and symptoms in patients with common psychiatric disorders.
KEY MESSAGES
What is already known on this topic.
To the authors' knowledge, there are no known studies in the literature describing a case of a patient referred to physical therapy for musculoskeletal dysfunction who was later diagnosed with schizophrenia.
What This Study Adds
This case report contributes to the existing literature on physical therapists functioning as competent providers of primary care who have the knowledge and skills needed to rule out non-musculoskeletal pathology. It also educates physical therapists about the signs and symptoms of schizophrenia.
Shah N, Nakamura Y. Case report: schizophrenia discovered during the patient interview in a man with shoulder pain referred for physical therapy. Physiother Can. 2010;62:308–315
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 15 August 2024
Exploration of first onsets of mania, schizophrenia spectrum disorders and major depressive disorder in perimenopause
- Lisa M. Shitomi-Jones ORCID: orcid.org/0009-0008-7433-0498 1 ,
- Clare Dolman ORCID: orcid.org/0000-0001-7466-1651 2 ,
- Ian Jones ORCID: orcid.org/0000-0001-5821-5889 1 , 3 ,
- George Kirov ORCID: orcid.org/0000-0002-3427-3950 1 ,
- Valentina Escott-Price ORCID: orcid.org/0000-0003-1784-5483 1 ,
- Sophie E. Legge ORCID: orcid.org/0000-0001-6617-0289 1 &
- Arianna Di Florio ORCID: orcid.org/0000-0003-0338-2748 1
Nature Mental Health ( 2024 ) Cite this article
1598 Accesses
1157 Altmetric
Metrics details
- Bipolar disorder
- Schizophrenia
Although the relationship between perimenopause and changes in mood has been well established, knowledge of risk of a broad spectrum of psychiatric disorders associated with reproductive aging is limited. Here we investigate whether the perimenopause (that is, the years around the final menstrual period (FMP)) is associated with increased risk of developing psychiatric disorders compared with the late reproductive stage. Information on menopausal timing and psychiatric history was obtained from nurse-administered interviews and online questionnaires from 128,294 female participants within UK Biobank. Incidence rates of psychiatric disorders during the perimenopause (4 years surrounding the FMP) were compared with the reference premenopausal period (6–10 years before the FMP). The rates were calculated for major depressive disorder (MDD), mania, schizophrenia spectrum disorders and other diagnoses. Overall, of 128,294 participants, 753 (0.59%) reported their first onset of a psychiatric disorder during the late reproductive stage (incidence rate 1.53 per 1,000 person-years) and 1,133 (0.88%) during the perimenopause (incidence rate 2.33 per 1,000 person-years). Compared with the reference reproductive period, incidence rates of psychiatric disorders significantly increased during the perimenopause (incidence rate ratio (RR) of 1.52, 95% confidence interval (CI) 1.39–1.67) and decreased back down to that observed in the premenopausal period in the postmenopause (RR of 1.09 (95% CI 0.98–1.21)). The effect was primarily driven by increased incidence rates of MDD, with an incidence RR of 1.30 (95% CI 1.16–1.45). However, the largest effect size at perimenopause was observed for mania (RR of 2.12 (95% CI 1.30–3.52)). No association was found between perimenopause and incidence rates of schizophrenia spectrum disorders (RR of 0.95 (95% CI 0.48–1.88)). In conclusion, perimenopause was associated with an increased risk of developing MDD and mania. No association was found between perimenopause and first onsets of schizophrenia spectrum disorders.
Similar content being viewed by others

Major depression, physical health and molecular senescence markers abnormalities

Key subphenotypes of bipolar disorder are differentially associated with polygenic liabilities for bipolar disorder, schizophrenia, and major depressive disorder

Epidemiologic and genetic associations of female reproductive disorders with depression or dysthymia: a Mendelian randomization study
There are estimated to be greater than 945 million women and those assigned female at birth aged between 40 and 60 years in the world 1 . During perimenopause (the years around the final menstrual period (FMP)), approximately 80% of people develop neuropsychiatric symptoms, most commonly hot flushes, cognitive dysfunction, sleep disturbances and mood-related symptoms 2 . It has been suggested that perimenopause is also a high-risk period for the onset or exacerbation of psychiatric disorders, including major depressive disorder (MDD), schizophrenia spectrum disorders and bipolar disorder; although, research thus far has predominately measured only depressive symptoms 3 , 4 , 5 , 6 , 7 . A two- to four-times greater risk of a depressive episode during perimenopause compared with the reproductive stage has been observed by the limited studies that have investigated MDD, even after controlling for other predictors and perimenopausal symptoms 8 , 9 , 10 . However, failure to account for multiple testing, retrospective design and focus on mild symptomatology have limited the generalizability of the results. Very little research has been conducted on the association between perimenopause and onset of disorders such as schizophrenia and bipolar disorder. Observations thus far have been limited to risk of recurrence in women with preexisting disorders and have shown that midlife in women is associated with worsening bipolar symptoms 11 , as well as an increased risk of hospitalizations for psychosis compared with men of the same age 12 . A major limitation of researching the association between reproductive aging and psychiatric disorders is related to the difficulties in obtaining reliable evaluations of ovarian aging, especially in epidemiological studies, with most studies using age as a proxy for age at FMP 13 . However, there is an over 20-year range variation in age at menopause, with research advocating that chronological age is not a valid proxy for menopausal status 14 , 15 , 16 . The UK Biobank represents a unique opportunity to study the effects of reproductive aging, with information on menopausal timing and deep longitudinal data collected on over 200,000 female participants recruited at 40–69 years of age 17 . Here, we focus on ‘late’ first onsets, because studies on the effect of reproductive aging on first-onset severe mental illness (that is, schizophrenia spectrum disorders and bipolar disorder) are lacking. This is likely due to the low prevalence and, therefore, difficulty to detect any effect in epidemiological studies specifically designed to study menopause. In this article, we aim to exploit a unique and specific asset of the UK Biobank: the combination of questions on first-onset psychiatric illness and age at FMP in an epidemiological study large enough to detect less prevalent, devastating outcomes, such as first incidence of bipolar disorder/schizophrenia, at a population level. The aim of this study is to test the hypothesis that perimenopause is a time of increased rate of first-onset psychiatric disorders, including MDD, mania and schizophrenia spectrum disorders, compared with the late reproductive stage.
A total of 128,294 participants from the UK Biobank met inclusion criteria, as shown in Fig. 1 . The mean age at recruitment into this study was 59.6 years (standard deviation (s.d.) of 5.69 years) with an average follow-up period of 2.98 years (s.d. of 3.92 years) and mean age at menopause of 50.6 years of age (range of 40–68 years; s.d. of 4.00 years), as displayed in Supplementary Fig. 1 . Demographic characteristics of female participants, including self-reported ethnicity, are presented in Table 1 . As presented in Table 2 , a total of 753 participants (0.59%) had a first onset of a psychiatric disorder 6–10 years before the FMP (premenopause period), 1,133 participants (0.88%) between 2 years prior and 2 years following FMP (perimenopause) and 637 participants (0.50%) 6–10 years following the FMP (postmenopause), with incidence rates per 1,000 person-years of 1.53, 2.33 and 1.66, respectively. Trends in the number of new onsets of each psychiatric disorder for both female and male participants are displayed in Fig. 2 .
Please note that each exclusion criterion was implemented sequentially, with the number of participants removed at each stage calculated on the basis of the participants remaining after the previous criteria had been applied.

a , Female participants for MDD (left), mania (middle left), schizophrenia spectrum disorders (middle right) and other diagnoses (right). b , Male participants for MDD (left), mania (middle left), schizophrenia spectrum disorders (middle right) and other diagnoses (right). Please note that not all participants were followed up for 10 years after the FMP (or matched ‘FMP’ proxy for male participants), and thus, later numbers of new onsets are likely to be underestimated.
The perimenopause was associated with a significant increase in the incidence rates of psychiatric disorders compared with the premenopause period (incidence rate ratio (RR) of 1.52 (95% confidence interval (CI) 1.39–1.67); Table 2 ). During the postmenopause, the incidence rate decreased back down to that observed in the premenopause period (RR of 1.09 (95% CI 0.98–1.21)).
Disorder-specific analyses
MDD was found to have a higher incidence rate during the perimenopause compared with the premenopause stage, with a RR of 1.30 (95% CI 1.16–1.45) (Fig. 3 and Table 2 ). The incidence rate of MDD became significantly lower in the postmenopause compared with the premenopause (RR of 0.68 (95% CI 0.59–0.78)).

The dots indicate the incidence RR calculated relative to the premenopausal period (6–10 years before FMP). The whiskers indicate 95% CIs. Total sample sizes analyzed for each disorder are as follows: MDD ( n = 39,800), mania ( n = 128,105), schizophrenia spectrum disorders ( n = 128,170) and other diagnoses ( n = 127,292). Perimenopause is between 2 years before and 2 years after FMP, and postmenopause is 6–10 years after FMP.
The incidence rate of mania significantly increased in the perimenopause compared with the premenopause (RR of 2.12 (95% CI 1.30–3.52)), to then return to the premenopause level during the postmenopause (RR of 0.98 (95% CI 0.51–1.86)).
Perimenopause was not found to be significantly associated with a change in incidence rate of schizophrenia spectrum disorders compared with the premenopause stage (RR of 0.95 (95% CI 0.48–1.88)), though rates were found to be lower during the postmenopause (RR of 0.37 (95% CI 0.12–0.96)).
Both perimenopause and postmenopause were found to be significantly associated with an increased incidence rate of other diagnoses compared with the premenopause, with incidence RRs of 2.10 (95% CI 1.78–2.49) and 2.08 (95% CI 1.74–2.49), respectively.
Sensitivity analysis
Sensitivity analyses did not detect any effect of potential confounders on results. Compared with the premenopause, an association between perimenopause and an increased incidence rate of psychiatric disorders was observed in those with low and high Townsend Deprivation Indexes, those of healthy, preobese and obese body mass index (BMI) categories, across all six alcohol intake frequency categories and in both previous and never smokers (Supplementary Table 1 ). Perimenopause was not found to be significantly associated with an increase in incidence rate of psychiatric disorders compared with the premenopause in those with underweight BMI or current smokers.
Analysis of male participants
Demographic characteristics of male participants are available in Supplementary Table 2 . No individual disorder had any significant peak of incidence at the interval period that matched ‘perimenopause’ (Extended Data Fig. 1 ). First onsets of MDD had a similar pattern in male and female participants; although, this did not reach statistical significance in the male sample during the ‘perimenopause’ proxy (RR of 1.13 (95% CI 1.00–1.29); Extended Data Fig. 2 and Supplementary Table 3 ). In male participants, no increase in incidence rate was observed during the ‘perimenopause’ proxy compared with the ‘premenopause’ proxy for mania (RR of 0.88 (95% CI 0.55–1.41)) nor schizophrenia spectrum disorders (RR of 0.57 (95% CI 0.26–1.22); Supplementary Table 3 ). Similarly to the female sample, both the perimenopause and postmenopause proxies in male participants were associated with an increased rate of other diagnoses compared with the premenopause proxy, with incidence RRs of 1.44 (95% CI 1.20–1.74) and 1.36 (95% CI 1.11–1.67), respectively.
Our results show that the perimenopause is a period of increased risk of first-onset psychiatric disorders, with compelling evidence for a specific link with mania. We found that participants without previous history of mania were over twice as likely to develop mania for the first time in the perimenopause than in the late reproductive stage. The increased risk was specific for the perimenopause, as the rates of first-onset mania returned to premenopause levels in the postmenopause.
Main findings
Our results highlight the importance of considering the FMP rather than chronological age in both clinical practice and research concerning mental health and reproductive aging. Previous studies using chronological age had not been able to detect the effect of perimenopause on mania/bipolar disorder 18 . Given the 20-year range variation in age at FMP 14 , inferring age at menopause on solely chronological age can lead to errors and, in research, to false negatives. The disease-specific and narrow time window (4 years) of increased risk for mania also suggests that specific changes associated with the perimenopause may trigger mania in people without previous psychiatric history of mania. The lack of any association in male participants corroborates the idea that sex-specific factors are associated with the first occurrence of mania at the time of the perimenopause. For bipolar disorder and mania, evidence suggests a trimodal distribution of age of onset, reflecting possible biological heterogeneity 19 . By selecting a period of 4–10 years prior the FMP as reference, our analyses focused on the late onset group, providing for the first time direct evidence of a possible link between mania and the hormonal fluctuations of the perimenopause. Intriguingly, one of the most robust pieces of evidence in psychiatry is that of the association between mania and the first few weeks after childbirth, with a 23 times increased risk of first-onset mania in the 30 days postpartum compared with 12 months after giving birth 20 . Although the effect we found for perimenopause is much smaller than that observed for childbirth, our results support the theory that there is a link between mania/bipolar disorder and reproductive events beyond childbirth. It has been suggested that menopausal mood disorders could be predicted by sensitivity to estradiol, with 27% of individuals sensitive to either withdrawal (7%) or absolute change (20%) in estradiol levels 21 . As the postpartum period is associated with a sharp decline in estradiol levels, it may be that individuals with an estradiol-sensitive predisposition may have already developed an onset of mania during postpartum, reducing the number of first onsets at perimenopause.
Contrary to mania, we did not find any specific association between schizophrenia spectrum disorders and the perimenopause. While the lack of effect may be due to the small sample size, we did find that the incidence rate of schizophrenia spectrum disorder was significantly lower in the postmenopause compared with the premenopausal stage. Our findings, therefore, do not support the widely discussed hypothesis that hypoestrogenism may trigger the first onset of schizophrenia but are in line with those of a large collaborative study on over 130,000 incident cases of schizophrenia, showing a decline in new onsets in women after the age of 40 years 22 .
Our results corroborate findings from previous studies that have observed an increased risk of depression in the perimenopause 3 , 4 , 8 , 9 , 10 . The effect was smaller than that for mania, but the CIs were narrower, as there were more people who developed major depression than people who developed mania. Interestingly, while rates of first-onset MDD decreased in the postmenopause, rates of first-onset depressive symptoms remained high in the postmenopause (Supplementary Table 4 ). The mechanisms underpinning the link between first-onset depressive symptoms and reproductive aging may, therefore, be complex and include not only hormonal changes associated with the perimenopause, but also biopsychosocial challenges associated with aging. Depression is likely an umbrella term for several conditions with heterogeneous disease pathways. It is then possible that onsets at different reproductive stages are linked to different mechanisms. Given that our study ascertained age at first diagnosis by a health care professional, it is also possible that some participants who experienced depressive symptoms during the perimenopause delayed seeking help or that their symptoms had become severe enough to seek help only in the postmenopause. Curiously, although the male analysis revealed a similar trend of depression risk, upon closer inspection of the number of onsets around the FMP proxy (Fig. 2 ), they lack the sharp peak present in the female analysis. The present study observed a decline in risk of a first onset of MDD during the postmenopausal period compared with premenopause in both the female and male proxy analysis. Although older populations often have high prevalence of depression 23 , with the course of MDD becoming poorer with age 24 , our results are in line with previous studies which observe a decline in first-ever onsets of depression in the decade following the average age at FMP (~50 years of age) 25 , 26 , 27 , 28 . As such, it may be that while the onset of depression occurs during perimenopause or earlier in life, some individuals may remain depressed during the postmenopause, leading to a high prevalence.
Strengths and limitations
Using data from the UK Biobank has allowed us to use a reported age at menopause to determine reproductive timing and, thus, estimate time periods to capture the pre-, peri- and postmenopausal life stages. This is a major strength over many previous studies, which have often used chronological age as a proxy for menopause despite the large variability in reproductive timing between individuals 14 . However, the UK Biobank does not include additional information on the menstrual cycle, which would provide more fine grain information on the stages of reproductive aging and menopausal symptoms. Aware of this UK Biobank limitation and of the variability in the length of the early menopause transition, we focused our analyses on the late menopausal transition and the early postmenopause, as: (1) they have less variability in their length 29 and (2) previous studies have suggested that the late menopause transition is the period of highest risk of new-onset depression 9 . We then chose the reference period of 6–10 years before the FMP, as only very few people will experience a late menopausal transition longer than 6 years 29 .
Another unique strength of the UK Biobank is that the large sample size, the collection on information on FMP and the cohort design allow inference on a range of psychiatric disorders, including less prevalent and more severe ones, such as mania and psychosis. However, due to the particularly low prevalence of schizophrenia spectrum disorders, we were not able to run fine-grained analyses considering the different disorders separately. Further replication of our results should be carried out before definitive conclusions can be drawn. Additionally, representativeness of UK Biobank has been an area of controversy, with diverging views on the validity of its measures of association 30 , 31 . Our sensitivity analyses show the robustness of our findings across several socioeconomic, health and lifestyle characteristics. The increased incidence of psychiatric conditions at perimenopause were present across different levels of the Townsend Deprivation Index, BMI categories, alcohol intake frequencies and in previous and never smokers. Additionally, we note that our study design selects for those without a diagnosis of severe mental illness before 10 years before the FMP and, thus, will be ‘healthier’ by definition as is the design of the study, rather than due to an unintended selection bias.
We emphasize that the incidence and prevalence estimates of our study are not the focus of the paper and should not be taken as representative of the UK population as a whole. Moreover, our study focused on the effect of the perimenopause on the first incidence of psychiatric disorders. By using as a reference group people 10 years before their FMP, by design, we selected a population that has reached the middle age without any psychiatric diagnosis. This study design has the advantage of excluding the well-established peak of severe psychiatric disorders in early adulthood, which would mask the effect of the perimenopause.
Directions for future work
Our study focused on the first onset of psychiatric disorders during the perimenopause. The study of recurrence of preexisting psychiatric disorders was beyond the scope of this paper and would have required a different study design. Further research focusing on large cohorts of people with previous history of mental illness is necessary to improve their risk prediction and mitigation associated with reproductive aging.
Conclusions
Our results highlight the importance of diagnostic accuracy in the assessment of psychiatric phenomena associated with aging. First, research and clinicians should consider interpersonal variability in reproductive aging rather than use demographic age as a proxy. Second, differential diagnosis is key, and not all psychiatric symptoms with onset at the perimenopause should be considered depressive symptoms etiologically related to the perimenopause. Mechanistic research needs to differentiate between clinical manifestation that are mostly epiphenomena from those that may be triggered by the physiological changes associated with reproductive aging. Clinically, the link with mania was particularly striking and significant: although first-onset bipolar disorder is usually associated with younger age, the perimenopause represents a period of increased risk of manic onset in those without previous psychiatric history of mania. Primary care physicians should be aware of this late onset presentation of bipolar disorder to avoid the risks associated with a delayed diagnosis and misdiagnosis with depression. Antidepressants without mood stabilizers can in fact precipitate manic episodes in those with bipolar diathesis 32 .
Study design and participants
The initial sample consisted of 502,357 individuals from the UK Biobank 17 . The UK Biobank is a large-scale biomedical database designed to enable research into genetic and environmental determinants of disease. Participants were recruited at 40–69 years of age between 2006 and 2010 and undertook extensive data collection. Measurements included assessment center visits, genotyping and longitudinal follow-up of health outcomes including linkage to medical records. The North West Multi-Centre Ethics Committee granted ethical approval to the UK Biobank, and all participants provided written informed consent. This study was conducted under project number 13310.
Psychiatric diagnoses were assessed using interviews and a self-report web-based questionnaire (the UK Biobank mental health questionnaire). Interviews were conducted by trained nurses and included questions on past and current medical conditions and date of diagnosis. The mental health questionnaire was based on the World Health Organization’s Composite International Diagnostic Interview Short Form 33 and integrated elements of other validated tools, such as the Patient Health Questionnaire (9-question version), the Generalized Anxiety Disorder Questionnaire 7-item 34 and bespoke additional questions. Although some participants also have linked medical primary health care records and hospital admission records, these data were not used in the present study as only more recent records are available electronically; thus, admission dates were not trusted as valid proxies for first onsets of psychiatric disorders.
Sample demographic variables include ethnicity. This was self-reported via touchscreen questionnaire at the initial assessment center visit by the question: ‘What is your ethnic background?’.
Selection of individuals for analysis
Figure 1 illustrates sample selection. The UK Biobank dataset was restricted to participants of female sex, as reported in the NHS central registry at the time of recruitment; although, in some instances this was updated by the participant (field identification (ID) 31). Of the 502,357 participants in the UK Biobank, 273,293 (54.4%) were of female sex. The sample was then limited to the 170,556 participants (62.4%) that had reported menopause occurrence (field ID 2724), which is defined as the cessation of menstrual periods for 12 months 29 . At each assessment center visit, participants were asked to self-report if menopause had occurred via touchscreen questionnaire (field ID 2724). Of the 22,971 individuals (13.5%) who had answered this question at multiple assessment center visits, 42 individuals (0.18%) who answered ‘yes’ but then responded ‘no’ to the same question at a subsequent visit were excluded, leaving 170,514 participants in the sample. Age at menopause, defined as age at the FMP, was asked via touchscreen questionnaire (field ID 3581) if the participant had responded ‘yes’ when asked if menopause had occurred. As this question was administered at each assessment center visit, 13,311 participants (7.81%) answered the question more than once. Of these, 442 participants (3.32%) reported different ages when asked again, and 330 (2.48%) whose reported ages at menopause varied by two or more years were excluded from the study due to potential unreliability. For the 112 individuals (0.84%) with multiple reported ages at menopause that did not vary by two or more years, the response from the earliest available assessment center visit was taken to reduce the probability of recall bias. A total of 10,726 participants (6.30%) had no age at menopause reported, having responded, ‘do not know’ or ‘prefer not to answer’ and were subsequently excluded from the study. A further 6,487 (4.07%) reported an age at menopause (field ID 3581) before 40 years of age and were excluded, as primary ovarian insufficiency is associated with major depression 35 , leaving 152,971 participants in the sample. A total of 15,490 participants (10.1%) who reported a hysterectomy (field ID 3591) or a bilateral oophorectomy (field ID 2834) were excluded from the study as these procedures have also been linked with an increased risk of depression 36 , 37 , 38 . Those who had undergone uterine ablations (8,988) were excluded, as this can cause cessation of menstrual periods (field ID 41272, OPCS4 codes: Q07, Q08, Q10, Q16). As contraceptive use can also cause cessation of menstrual periods, 199 participants (0.15%) who reported using a hormonal intrauterine device (IUD) or who reported starting oral contraceptives at the same age they experienced menopause were excluded from analysis, resulting in a sample size of 128,294.
Diagnostic criteria
First onsets of MDD, mania, schizophrenia spectrum disorders and an ‘other diagnoses’ category were investigated in the present study. These disorders were chosen on the basis of previous literature investigating the effect of childbirth 20 , as these events both cause changes in sex hormone levels, and thus, new onsets of disorders during these periods may share hormone-driven etiological pathways 2 , 21 .
The diagnosis of MDD was based on answers from the online mental health questionnaire and mapped on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, (DSM-5) criteria. Such approach has been previously validated by Cai and colleagues 39 , who found this approach to have higher SNP-based heritability than any other definition of depression available in the UK Biobank, including medical record-based criteria. Details of this diagnostic criteria are available in Supplementary Note: Diagnostic Criteria . Briefly, a classification of MDD required at least two cardinal symptoms, as well as at least five total symptoms, and excluded individuals with a history of substance abuse and/or manic/psychotic conditions. Age at first onset was defined as the age at first episode of depression (field ID 20433). Analyses of individuals self-reporting depressive symptoms are available in Supplementary Note: Diagnostic Criteria (Supplementary Table 4 ).
The diagnoses of mania/bipolar/manic–depression (henceforth referred to as ‘mania’), schizophrenia spectrum disorders and an ‘other diagnoses’ category were based on the nurse-conducted interviews (field IDs 20002 and 20009). Individuals who reported having schizophrenia at interview were combined with individuals who reported a diagnosis of schizophrenia or ‘any other type of psychosis or psychotic illness’ in those who completed the mental health questionnaire (field IDs 20544 and 20461) to form a ‘schizophrenia spectrum disorders’ group. The ‘other diagnoses’ group included participants who reported any of the following conditions at a nurse-conducted interview: ‘anxiety/panic attacks’, ‘substance abuse/dependency’, ‘post-traumatic stress disorder’, anorexia/bulimia/other eating disorder’, ‘stress’, ‘obsessive compulsive disorder’ or ‘insomnia’.
Finally, a combined ‘psychiatric disorder’ group was formed of all the above diagnoses combined. In the case of the combined ‘psychiatric disorder’ group, if multiple diagnoses were present in a single participant, the earliest age at onset was prioritized.
For each diagnosis group, individuals were removed from analysis if they met the diagnostic criteria but did not have onset age data available, as detailed in Supplementary Note: Diagnostic Criteria .
Statistical analysis
Age at first onset of any given psychiatric disorder relative to age at FMP was calculated as self-reported age at menopause (field ID 3581) subtracted from the age at onset. These values were grouped to form the following three life stages: premenopause (6–10 years before the FMP), perimenopause (2 years before and 2 years following FMP) and postmenopause (6–10 years after the FMP). The values between 2 and 6 years from the FMP were excluded to increase distinction between the time periods and to minimize the likelihood of misclassification due to inaccuracies in menopausal timing. The premenopause represents the reference life stage against which the other life stages were compared. The late reproductive stage (6–10 years before the FMP) was used as the refence premenopausal period to reduce the effect of recall bias and to minimize inclusion of adolescent and postnatal onsets within the reference period.
A Kaplan–Meier survival analysis was used for each diagnosis group to determine the likelihood of disorder onset at each life stage. This was conducted using the ‘survival’ R package in RStudio, version 4.2.1 (refs. 40 , 41 ). Incidence rates in person-years for each time period were calculated as the number of cases divided by four times the total number of cases and controls, as each time period was 4 years long. Incidence RRs were calculated to compare the rate of first onsets in the perimenopause and postmenopause compared with the premenopausal stage, using the two-sided ‘rateratio.test’ R package 42 , with a confidence level of 0.95. The false discovery rate correction was applied to correct for 50 tests (10 tests in Table 2 , 30 tests in Supplementary Table 1 , 8 tests in Supplementary Table 3 and 2 tests in Supplementary Table 4 ) 43 . We provide P values both with and without correction for multiple testing.
Sensitivity analyses
Previous literature has observed that the UK Biobank participants differ from UK Census data on several socioeconomic, health and lifestyle characteristics 30 . Participants have been found to be more likely to live in less deprived geographical areas and less likely to be obese, to currently smoke or to drink alcohol daily. To consider the effect of this sampling bias, we investigated the effects of Townsend Deprivation Index, BMI, smoking status and alcohol intake frequency on the findings of the present study by stratifying the sample on these characteristics. Further details are available in Supplementary Note: Sensitivity Analysis .
Analyses of male participants
It is possible that the observed effects may not be driven by the hormonal fluctuations that characterized the perimenopause. Rather, they could be driven by age or by unknown ascertainment and assessment bias. The same analyses were, therefore, conducted in male participants enrolled in UK Biobank.
First, we matched male participants to female participants on the basis of their most recent age that they came into the assessment center (field ID 21003). Then, for each male participant, we constructed a timeline centered around the age at the FMP of their matched female participant. So, for example, if we matched a female and male participant based on the age at the last assessment of 62 and the female participant had an age at the FMP of 50 years, we then centered the timeline of the male participant around 50. The three time periods considered as a proxy in this case would be 40–44 years for premenopause, 48–52 years for perimenopause and 56–60 years for postmenopause. This was carried out to account for the variation and distribution of menopausal timing in female participants to consider the effect of attrition based on the age of most recent follow-up and to create equal sample sizes to homogenize the statistical power. This resulted in 128,294 male participants being included in analysis. All following statistical analyses mirrored the analyses conducted in the female sample.
Patient and public involvement
People with lived experience were involved in the design of the study, in the interpretation of the results and in the writing of the manuscript. C.D., a researcher with lived experience and coauthor on the publication, provided expertise and voiced the issues experienced by people affected by severe mental illness. In her roles within the charities Bipolar UK and Action on Postpartum Psychosis, C.D. has provided support to women with lived experience of illness during the perimenopause over several years and has worked as patient and public involvement lead. Moreover, the design of the current study was informed by a Bipolar UK survey designed and conducted by C.D., which received over 1,000 responses,
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All the data used in this study, both raw and derived, are available from the UK Biobank ( https://www.ukbiobank.ac.uk/ ). This study was conducted under project number 13310. Our access to the data does not allow for data redistribution.
Code availability
Code used for analysis is available online at https://lms-j.github.io/perimeno-first-onsets/ .
World Population Prospects 2022. United Nations, Department of Economic and Social Affairs, Population Division https://population.un.org/wpp/Download/Standard/Population/ (2022).
Brinton, R. D., Yao, J., Yin, F., Mack, W. J. & Cadenas, E. Perimenopause as a neurological transition state. Nat. Rev. Endocrinol. 11 , 393–405 (2015).
Article PubMed PubMed Central Google Scholar
Tangen, T. & Mykletun, A. Depression and anxiety through the climacteric period: an epidemiological study (HUNT-II). J. Psychosom. Obstet. Gynecol. 29 , 125–131 (2008).
Article Google Scholar
Lin, H.-L., Hsiao, M.-C., Liu, Y.-T. & Chang, C.-M. Perimenopause and incidence of depression in midlife women: a population-based study in Taiwan. Climacteric 16 , 381–386 (2013).
Article PubMed Google Scholar
Timur, S. & Sahin, N. H. The prevalence of depression symptoms and influencing factors among perimenopausal and postmenopausal women. Menopause 17 , 545–551 (2010).
Bromberger, J. T. et al. Depressive symptoms during the menopausal transition: the Study of Women’s Health Across the Nation (SWAN). J. Affect. Disord. 103 , 267–272 (2007).
Maartens, L. W. F., Knottnerus, J. A. & Pop, V. J. Menopausal transition and increased depressive symptomatology: a community based prospective study. Maturitas 42 , 195–200 (2002).
Freeman, E. W., Sammel, M. D., Boorman, D. W. & Zhang, R. Longitudinal pattern of depressive symptoms around natural menopause. JAMA Psychiatry 71 , 36–43 (2014).
Schmidt, P. J., Haq, N. & Rubinow, D. R. A longitudinal evaluation of the relationship between reproductive status and mood in perimenopausal women. Am. J. Psychiatry 161 , 2238–2244 (2004).
Bromberger, J. T. & Kravitz, H. M. Mood and menopause: findings from the Study of Women’s Health Across the Nation (SWAN) over 10 years. Obstet. Gynecol. Clin. 38 , 609–625 (2011).
Perich, T., Ussher, J. & Meade, T. Menopause and illness course in bipolar disorder: a systematic review. Bipolar Disord. 19 , 434–443 (2017).
Sommer, I. E. et al. Women with schizophrenia-spectrum disorders after menopause: a vulnerable group for relapse. Schizophr. Bull. 49 , 136–143 (2023).
Culbert, K. M., Thakkar, K. N. & Klump, K. L. Risk for midlife psychosis in women: critical gaps and opportunities in exploring perimenopause and ovarian hormones as mechanisms of risk. Psychol. Med. 52 , 1612–1620 (2022).
Brinton, R. D. in Brocklehurst’s Textbook of Geriatric Medicine and Gerontology 8th edn, Ch. 13 (eds Fillit H., Rockwood K. and Young J.) 76–81 (Elsevier, 2017).
Gold, E. B. The timing of the age at which natural menopause occurs. Obstet. Gynecol. Clin. North Am. 38 , 425–440 (2011).
Chan, S., Gomes, A. & Singh, R. S. Is menopause still evolving? Evidence from a longitudinal study of multiethnic populations and its relevance to women’s health. BMC Womens Health 20 , 74 (2020).
Sudlow, C. et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12 , e1001779 (2015).
Kennedy, N. et al. Gender differences in incidence and age at onset of mania and bipolar disorder over a 35-year period in Camberwell, England. Am. J. Psychiatry 162 , 257–262 (2005).
Bolton, S., Warner, J., Harriss, E., Geddes, J. & Saunders, K. E. A. Bipolar disorder: trimodal age-at-onset distribution. Bipolar Disord. 23 , 341–356 (2021).
Munk-Olsen, T., Laursen, T. M., Pedersen, C. B., Mors, O. & Mortensen, P. B. New parents and mental disorders: a population-based register study. JAMA 296 , 2582–2589 (2006).
Gordon, J. L., Sander, B., Eisenlohr-Moul, T. A. & Tottenham, L. S. Mood sensitivity to estradiol predicts depressive symptoms in the menopause transition. Psychol. Med. 51 , 1733–1741 (2021).
van der Werf, M. et al. Systematic review and collaborative recalculation of 133,693 incident cases of schizophrenia. Psychol. Med. 44 , 9–16 (2014).
Zenebe, Y., Akele, B., W/Selassie, M. & Necho, M. Prevalence and determinants of depression among old age: a systematic review and meta-analysis. Ann. Gen. Psychiatry 20 , 55 (2021).
Schaakxs, R. et al. Associations between age and the course of major depressive disorder: a 2-year longitudinal cohort study. Lancet Psychiatry 5 , 581–590 (2018).
Zisook, S. et al. Effect of age at onset on the course of major depressive disorder. Am. J. Psychiatry 164 , 1539–1546 (2007).
Zhu, T. et al. Admixture analysis of age at onset in major depressive disorder. Gen. Hosp. Psychiatry 34 , 686–691 (2012).
Pedersen, C. B. et al. A comprehensive nationwide study of the incidence rate and lifetime risk for treated mental disorders. JAMA Psychiatry 71 , 573–581 (2014).
Plana-Ripoll, O. et al. Temporal changes in sex- and age-specific incidence profiles of mental disorders—a nationwide study from 1970 to 2016. Acta Psychiatr. Scand. 145 , 604–614 (2022).
Harlow, S. D. et al. Executive summary of the stages of reproductive aging workshop +10: addressing the unfinished agenda of staging reproductive aging. J. Clin. Endocrinol. Metab. 97 , 1159–1168 (2012).
Fry, A. et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am. J. Epidemiol. 186 , 1026–1034 (2017).
Keyes, K. M. & Westreich, D. UK Biobank, big data, and the consequences of non-representativeness. Lancet 393 , 1297 (2019).
Viktorin, A. et al. The risk of switch to mania in patients with bipolar disorder during treatment with an antidepressant alone and in combination with a mood stabilizer. Am. J. Psychiatry 171 , 1067–1073 (2014).
Kessler, R. C., Andrews, G., Mroczek, D., Ustun, B. & Wittchen, H.-U. The World Health Organization Composite International Diagnostic Interview short-form (CIDI-SF). Int. J. Methods Psychiatr. Res. 7 , 171–185 (1998).
Kroenke, K., Spitzer, R. L., Williams, J. B. W. & Löwe, B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen. Hosp. Psychiatry 32 , 345–359 (2010).
Schmidt, P. J. et al. Depression in women with spontaneous 46, XX primary ovarian insufficiency. J. Clin. Endocrinol. Metab. 96 , E278–E287 (2011).
Choi, H. G., Rhim, C. C., Yoon, J. Y. & Lee, S. W. Association between hysterectomy and depression: a longitudinal follow-up study using a national sample cohort. Menopause 27 , 543–549 (2020).
Kim, H. et al. Increased risk of depression before and after unilateral or bilateral oophorectomy: a self-controlled case series study using a nationwide cohort in South Korea. J. Affect. Disord. 285 , 47–54 (2021).
Rocca, W. A. et al. Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause 25 , 1275–1285 (2018).
Cai, N. et al. Minimal phenotyping yields genome-wide association signals of low specificity for major depression. Nat. Genet. 52 , 437–447 (2020).
Therneau, T. A Package for Survival Analysis in R. https://CRAN.R-project.org/package=survival (2022).
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing https://www.R-project.org/ (2022).
Fay, M. rateratio.test: exact rate ratio test. https://CRAN.R-project.org/package=rateratio.test (2022).
Benjamini, Y. & Yekutieli, D. The Control of the False Discovery Rate in Multiple Testing Under Dependency. Ann. Stat. https://doi.org/10.1214/aos/1013699998 (2001).
Download references
Acknowledgements
We are thankful for funding from the Medical Research Council (grant MR/W004658/1), awarded to Cardiff University and the National Centre for Mental Health, for supporting this research. A.D.F. is also funded by the European Research Council (grant 947763). S.E.L. is supported by the National Institute of Mental Health (grant R01MH124873). V.E.-P. is supported by the Medical Research Council (grants UKDRI-3003 and MR/Y004094/1). We are also grateful to the participants and staff of the UK Biobank for their time in producing the data used in this study. We thank M. Hamshere for her comments and suggestions, which helped to refine this manuscript.
Author information
Authors and affiliations.
Centre for Neuropsychiatric Genetics and Genomics, Division of Psychological Medicine and Clinical Neurosciences, School of Medicine, Cardiff University, Cardiff, UK
Lisa M. Shitomi-Jones, Ian Jones, George Kirov, Valentina Escott-Price, Sophie E. Legge & Arianna Di Florio
Bipolar UK, London, UK
Clare Dolman
National Centre for Mental Health, School of Medicine, Cardiff University, Cardiff, UK
You can also search for this author in PubMed Google Scholar
Contributions
A.D.F., C.D., G.K. and I.J. conceptualized the study and acquired funding. Data curation and data analysis were carried out by L.M.S.-J. and supervised by S.E.L., V.E.-P. and A.D.F. L.M.S.-J. and S.E.L. had access to and verified the data. L.M.S.-J. and A.D.F. prepared the manuscript. Review and editing of the manuscript were conducted by L.M.S.-J., C.D., G.K., I.J., V.E.-P., S.E.L. and A.D.F. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. The study sponsor had no role in study design, data collection, data analysis, interpretation of data, manuscript preparation or submission for publication.
Corresponding author
Correspondence to Arianna Di Florio .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Mental Health thanks Veerle Bergink and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 incidence rate ratios of psychiatric disorders for male participants during matched ‘perimenopause’ and ‘postmenopause’ proxies, relative to the matched ‘premenopause’ period proxy..
Note that age-based classifications were based on values from matched female participants. Dots indicate the incidence rate ratio calculated relative to the premenopausal period proxy (6-10 years before the assigned FMP). Whiskers indicate 95% confidence intervals. Total sample sizes analysed for each disorder are as follows: major depressive disorder (n = 37 719); mania (n = 128 131), schizophrenia spectrum disorders (n = 128 108); other diagnoses (n = 127 449). Perimenopause = Between −2 years before and 2 years after FMP; Postmenopause = 6-10 years after FMP.
Extended Data Fig. 2 Incidence rate ratios of psychiatric disorders in female participants during the perimenopause (2 years prior - 2 years following FMP) compared to male participants during the matched ‘perimenopause’ proxy (2 years prior - 2 years following assigned FMP).
Note that age at FMP in male participants was based on values from matched female participants. Dots indicate the incidence rate ratio calculated relative to the premenopausal period (6-10 years before FMP). Whiskers indicate 95% confidence intervals. Sample sizes analysed for each disorder are as follows: major depressive disorder (n female = 39 800, n male = 37 719); mania (n female = 128 105, n male = 128 131), schizophrenia spectrum disorders (n female = 128 170, n male = 128 108); other diagnoses (n female = 127 292, n male = 127 449). Perimenopause = Between −2 years before and 2 years after FMP; Postmenopause = 6-10 years after FMP.
Supplementary information
Supplementary information.
Supplementary methods (diagnostic criteria and sensitivity analysis), Tables 1–4 and Figs. 1 and 2.
Reporting Summary
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Shitomi-Jones, L.M., Dolman, C., Jones, I. et al. Exploration of first onsets of mania, schizophrenia spectrum disorders and major depressive disorder in perimenopause. Nat. Mental Health (2024). https://doi.org/10.1038/s44220-024-00292-4
Download citation
Received : 13 November 2023
Accepted : 24 June 2024
Published : 15 August 2024
DOI : https://doi.org/10.1038/s44220-024-00292-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
COMMENTS
PDF | On Jan 1, 2015, Ms. Vipasha Kashyap published Case Study of a Young Patient with Paranoid Schizophrenia | Find, read and cite all the research you need on ResearchGate
Case Study: Bryant. Thirty-five-year-old Bryant was admitted to the hospital because of ritualistic behaviors, depression, and distrust. At the time of admission, prominent ritualistic behaviors and depression misled clinicians to diagnose Bryant with obsessive-compulsive disorder (OCD). Shortly after, psychotic symptoms such as disorganized ...
Here, we present three case studies with varying presentation of childhood-onset psychosis/schizophrenia and associated management issues. Keywords: Childhood onset, psychosis, schizophrenia, very early onset Go to:
In this article, hospital and community pharmacy-based case studies provide further context for the management of patients with schizophrenia who have concurrent conditions or factors that could impact their treatment.
We report a case involving a young African male adult with gainful employment and no history of substance abuse who was diagnosed with schizophrenia. The patient was referred to the psychiatric unit of a hospital, and interventions by clinical pharmacists during management of the patient's medical problems contributed to overall improved ...
The second section consists of 12 case studies, which are presented in a detailed and articulate manner, spanning four continents. Each case study illustrates in detail a particular sociocultural context that affects the healing process for schizophrenia. Readers can select which case studies to read based on their interest.
ing for the studies. This document represents a synthesis of current scientific knowledge and rational clinical practice on the treatment of patients with schizophrenia.
w examines contextual variables that affect an individual diagnosed with schizophrenia, his family, and his recovery. The therapeutic experience of one client diagnosed with schizophrenia and his family is presented. The client's symptoms were tracked using a pre- and posttest from the Sixteen Personality Factor Questionnaire (16 PF) assessment tool over the 15-week treatment period. There ...
The case medical history was identified with schizophrenia characterized by a history of characterized by a history of psychotic episodes in which he displayed signs and symptoms of schizophrenia, but the patient is currently having no positive symptoms (delusions, hallucinations, disorganized speech, or behavior).
The DSM-5 diagnostic criteria for schizophrenia require two or more symptoms to include delusions, hallucinations, disorganized speech, grossly disorganized, and negative
Case Study: Kelsey Patterson. The case of Kelsey Patterson, who was executed in 2004, is one of the most compelling examples of what can happen when the mental health system fails to provide adequate care and in doing so, puts the public at risk. For more than two decades, Patterson struggled with paranoid schizophrenia.
1. Abstract. This single case study describes a structural therapy perspective applied to a family and examines. interaction patterns within the clinical setting and the home; the therapeutic view ...
Schizophrenia has long puzzled researchers in the fields of psychiatric medicine and anthropology. Why is it that the rates of developing schizophrenia-long the...
A B S T R A C T Introduction: An individual with paranoid schizophrenia exhibits a preoccupation with one or more delusions and experiences frequent auditory hallucinations. This study aimed to describe the diagnosis and treatment of paranoid schizophrenia in young men. Case presentation: A 20-year-old man was found sitting in the middle of traffic on a bustling route, prompting the cops to ...
toms of Schizophrenia. Precursors to hospitalizations included feelings of paranoia and impulsiveness regarding thoughts of arm to self or others. On one occasion, Mr. N attempted suicide by cutting his wrists because the v
Schizophrenia is a chronic disorder, characterized mainly by the gross distortion of reality, withdrawal from social interaction, and disorganization and fragmentation of perception, thought and emotion. Approximately, 1% world population suffering with the problem of Schizophrenia. Both male and female are almost equally affected with slight ...
The various classifications of schizophrenia, ability to receive treatments, and the consideration of various influences and base-line factors help researchers determine what classification a patient falls into. In this case, the patient displays symptoms of paranoid schizophrenia. References Hansell, J. &Damour, L. (2008).
Schizophrenia is a chronic and severe mental disorder characterized by positive, negative, and cognitive symptoms. Negative symptoms are usually present from the prodromal phase; early diagnosis and management of negative symptoms is a major health concern since an insidious onset dominated by negative symptoms is associated with a worse outcome. Antipsychotic medications, which are effective ...
Case study History: A 25 years old male, the eldest among his three siblings, belonging to a middle socio-economic class was diagnosed with paranoid schizophrenia (ICD10 classification).
d: Effective psychosocial treatment like cognitive-behavioural therapy (CBT) is needed for quick recovery from schizophrenia. The applicability of CBT for schizophrenia has many applications to social work practice. Aim: The aim of the present study was to manage auditory hallucination using cognitive behavioural case work study approach. Methodology: It uses a single subject design and ...
A case study in adolescent's schizophrenia Matt, a 15 years, 6 month old boy, was referred simultaneously by his General Practitioner and the crisis intervention team. He had been found in a park wandering and had been reported to the police by onlookers. When the police arrived on the scene and questioned him, he had been unable to provide a satisfactory explanation for how he was not at ...
ventions with schizophrenia patients are well researched areas. The current research focuses on application of these interventions and challenges while using them with paranoid schizophrenia patient. The study included in depth exploration of symptomatology of the paranoid schizophrenia patient, individual and group therapy interventions with the patient, and challenges for
The patient was diagnosed with schizophrenia by a psychiatrist and was prescribed Risperdal. Implications: This case study reinforces the importance of a thorough patient interview by physical therapists to rule out non-musculoskeletal disorders.
The authors investigate first onsets of psychiatric disorders during perimenopause, finding higher incidence rates of major depressive disorder and mania.