- Subscribe to journal Subscribe
- Get new issue alerts Get alerts

Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Overview of the Literature on Health Benefits
Singletary, Keith PhD
Keith Singletary, PhD, is Professor Emeritus of Nutrition, Department of Food Science & Human Nutrition, University of Illinois, Urbana, Illinois.
This study was supported by McCormick Science Institute.
Correspondence: Keith Singletary, PhD, Department of Food Science and Human Nutrition, University of Illinois, Urbana, IL 61801 ( [email protected] ).
Oregano is an herb that has been cultivated for centuries in the Mediterranean area, although it now can be found on most continents. Actually, there is not simply one "oregano," but rather several species that may contribute to the oregano used for culinary purposes. Origanum vulgare (also referred to as Spanish thyme and wild marjoram), a member of the plant family Lamiaceae, is generally the spice variety sold as oregano in Europe and the United States. Medicinal uses for oregano date back to the ancient Greek and Roman empires where applications of the leaves were used to treat such maladies as skin sores and relieve aching muscles and as an antiseptic. Oregano also has been used in traditional medicines for such ailments as asthma, cramping, diarrhea, and indigestion. In Greece, an oregano infusion is still used as a folk remedy against colds and upset stomach and to maintain general health. Based on the current scientific literature, oregano extracts and individual constituents consistently have demonstrated antimicrobial actions in vitro toward food-borne pathogens, although the capacity to counter human infections is not well studied. Oregano contains several potent antioxidants that may contribute to the findings in preliminary studies that oregano exhibits benefits toward the cardiovascular and nervous systems, relieves symptoms of inflammation, and modulates blood sugar and lipids. Well-controlled human studies substantiating these health effects are lacking
The low down on an Italian favorite that adds zest to meals and an herb that may have some interesting health benefits
Full Text Access for Subscribers:
Individual subscribers.

Institutional Users
Not a subscriber.
You can read the full text of this article if you:
- + Favorites
- View in Gallery
Readers Of this Article Also Read
Oregano: overview of the literature on health benefits, mediterranean diet and prevention of cardiovascular disease.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu
- Subscribe SciFeed
- Recommended Articles
- PubMed/Medline
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Essential oils of oregano: biological activity beyond their antimicrobial properties.

Graphical Abstract
1. Introduction
2. essential oils composition of oregano species, 3. biological activities of essential oils of oregano species, 3.1. antimicrobial effect of essential oils of oregano, 3.2. essential oils of oregano as antioxidants, 3.3. anti-inflammatory activity of essential oils of oregano species, 3.4. essential oils of oregano species and cardiovascular diseases, 3.5. essential oils of oregano and their effect on metabolic syndrome, 3.6. antiprolifertive and citotoxic activity of essential oils of oregano, 4. conclusions, acknowledgments, author contributions, conflicts of interest.
- Gooch, J.W. Essential oils. In Encyclopedic Dictionary of Polymers ; Gooch, J.W., Ed.; Springer: New York, NY, USA, 2011; p. 274. [ Google Scholar ]
- Dima, C.; Dima, S. Essential oils in foods: Extraction, stabilization, and toxicity. Curr. Opin. Food Sci. 2015 , 5 , 29–35. [ Google Scholar ] [ CrossRef ]
- Shaaban, H.A.E.; El-Ghorab, A.H.; Shibamoto, T. Bioactivity of essential oils and their volatile aroma components: Review. J. Essent. Oil Res. 2012 , 24 , 203–212. [ Google Scholar ] [ CrossRef ]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications ; CRC Press: Boca Raton, FL, USA, 2015; p. 975. ISBN 978-1-4200-6315-8. [ Google Scholar ]
- Franz, C.; Novak, J. 3 Sources of essential oils. In Handbook of Essential Oils: Science, Technology, and Applications ; Baser, K.H.C., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 39–82. ISBN 978-1-4200-6315-8. [ Google Scholar ]
- Patel, S.; Gogna, P. Tapping botanicals for essential oils: Progress and hurdles in cancer mitigation. Ind. Crop. Prod. 2015 , 76 , 1148–1163. [ Google Scholar ] [ CrossRef ]
- Zuzarte, M.; Salgueiro, L. Essential oils chemistry. In Bioactive Essential Oils and Cancer ; De Sousa, D.P., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 19–61. [ Google Scholar ]
- Islam, M.T.; Da Mata, A.M.O.F.; de Aguiar, R.P.S.; Paz, M.F.C.J.; de Alencar, M.V.O.B.; Ferreira, P.M.P.; de Carvalho Melo-Cavalcante, A.A. Therapeutic potential of essential oils focusing on diterpenes. Phytother. Res. 2016 , 30 , 1420–1444. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lenardão, E.J.; Savegnago, L.; Jacob, R.G.; Victoria, F.N.; Martinez, D.M. Antinociceptive effect of essential oils and their constituents: An update review. J. Braz. Chem. Soc. 2016 , 27 , 435–474. [ Google Scholar ] [ CrossRef ]
- Adorjan, B.; Buchbauer, G. Biological properties of essential oils: An updated review. Flavour Fragr. J. 2010 , 25 , 407–426. [ Google Scholar ] [ CrossRef ]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007 , 21 , 308–323. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010 , 15 , 9252–9287. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Calpouzos, L. Botanical aspects of oregano. Econ. Bot. 1954 , 8 , 222–233. [ Google Scholar ] [ CrossRef ]
- Kintzios, S.E. 21 Oregano. In Handbook of Herbs and Spices , 2nd ed.; Peter, K.V., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 417–436. [ Google Scholar ]
- Pascual, M.E.; Slowing, K.; Carretero, E.; Sánchez Mata, D.; Villar, A. Lippia : Traditional uses, chemistry and pharmacology: A review. J. Ethnopharmacol. 2001 , 76 , 201–214. [ Google Scholar ] [ CrossRef ]
- Leyva-López, N.; Nair, V.; Bang, W.Y.; Cisneros-Zevallos, L.; Heredia, J.B. Protective role of terpenes and polyphenols from three species of oregano ( Lippia graveolens , Lippia palmeri and Hedeoma patens ) on the suppression of lipopolysaccharide-induced inflammation in raw 264.7 macrophage cells. J. Ethnopharmacol. 2016 , 187 , 302–312. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Karioti, A.; Milošević-Ifantis, T.; Pachopos, N.; Niryiannaki, N.; Hadjipavlou-Litina, D.; Skaltsa, H. Antioxidant, anti-inflammatory potential and chemical constituents of Origanum dubium boiss., growing wild in cyprus. J. Enzym. Inhib. Med. Chem. 2015 , 30 , 38–43. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Economou, G.; Panagopoulos, G.; Tarantilis, P.; Kalivas, D.; Kotoulas, V.; Travlos, I.S.; Polysiou, M.; Karamanos, A. Variability in essential oil content and composition of Origanum hirtum L., Origanum onites L., Coridothymus capitatus (L.) and Satureja thymbra L. populations from the Greek island Ikaria. Ind. Crop. Prod. 2011 , 33 , 236–241. [ Google Scholar ] [ CrossRef ]
- Lukas, B.; Schmiderer, C.; Novak, J. Essential oil diversity of European Origanum vulgare L. (Lamiaceae). Phytochemistry 2015 , 119 , 32–40. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- González-Fuentes, F.; López-Gil, M.Á.; Mendoza, S.; Escarpa, A. Electrochemical screening of biomarkers in chemotype Mexican oregano oils on single-walled carbon nanotubes screen-printed electrodes. Electroanalysis 2011 , 23 , 2212–2216. [ Google Scholar ] [ CrossRef ]
- Arslan, M.; Uremis, I.; Demirel, N. Effects of sage leafhopper feeding on herbage colour, essential oil content and compositions of turkish and Greek oregano. Exp. Agric. 2012 , 48 , 428–437. [ Google Scholar ] [ CrossRef ]
- Asensio, C.M.; Grosso, N.R.; Juliani, H.R. Quality characters, chemical composition and biological activities of oregano ( Origanum spp.) essential oils from central and southern Argentina. Ind. Crop. Prod. 2015 , 63 , 203–213. [ Google Scholar ] [ CrossRef ]
- Azizi, A.; Yan, F.; Honermeier, B. Herbage yield, essential oil content and composition of three oregano ( Origanum vulgare L.) populations as affected by soil moisture regimes and nitrogen supply. Ind. Crop. Prod. 2009 , 29 , 554–561. [ Google Scholar ] [ CrossRef ]
- Baranauskienė, R.; Venskutonis, P.R.; Dambrauskienė, E.; Viškelis, P. Harvesting time influences the yield and oil composition of Origanum vulgare L. ssp. vulgare and ssp. hirtum . Ind. Crop. Prod. 2013 , 49 , 43–51. [ Google Scholar ] [ CrossRef ]
- Gerami, F.; Moghaddam, P.R.; Ghorbanim, R.; Hassani, A. Effects of irrigation intervals and organic manure on morphological traits, essential oil content and yield of oregano ( Origanum vulgare L.). An. Acad. Bras. Cienc. 2016 , 88 , 2375–2385. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- De Falco, E.; Mancini, E.; Roscigno, G.; Mignola, E.; Taglialatela-Scafati, O.; Senatore, F. Chemical composition and biological activity of essential oils of Origanum vulgare L. subsp. vulgare L. under different growth conditions. Molecules 2013 , 18 , 14948. [ Google Scholar ] [ PubMed ]
- Bolechowski, A.; Moral, R.; Bustamante, M.A.; Paredes, C.; Agullo, E.; Bartual, J.; Carbonell-Barrachina, A.A. Composition of oregano essential oil ( Origanum vulgare ) as affected by the use of winery-distillery composts. J. Essent. Oil Res. 2011 , 23 , 32–38. [ Google Scholar ] [ CrossRef ]
- Napoli, E.; Mazzaglia, A.; Restuccia, C.; Ragni, P.; Lanza, C.M.; Ruberto, G. The effect of γ-irradiation on chemical composition, microbial load and sensory properties of sicilian oregano. LWT Food Sci. Technol. 2016 , 72 , 566–572. [ Google Scholar ] [ CrossRef ]
- Morshedloo, M.R.; Craker, L.E.; Salami, A.; Nazeri, V.; Sang, H.; Maggi, F. Effect of prolonged water stress on essential oil content, compositions and gene expression patterns of mono- and sesquiterpene synthesis in two oregano ( Origanum vulgare L.) subspecies. Plant Physiol. Biochem. 2017 , 111 , 119–128. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Novak, I.; Sipos, L.; Kokai, Z.; Szabo, K.; Pluhar, Z.; Sarosi, S. Effect of the drying method on the composition of Origanum vulgare L. subsp hirtum essential oil analysed by gc-ms and sensory profile method. Acta Aliment. 2011 , 40 , 130–138. [ Google Scholar ] [ CrossRef ]
- Figiel, A.; Szumny, A.; Gutiérrez-Ortíz, A.; Carbonell-Barrachina, Á.A. Composition of oregano essential oil ( Origanum vulgare ) as affected by drying method. J. Food Eng. 2010 , 98 , 240–247. [ Google Scholar ] [ CrossRef ]
- Calín-Sánchez, Á.; Figiel, A.; Lech, K.; Szumny, A.; Martínez-Tomé, J.; Carbonell-Barrachina, Á.A. Dying methods affect the aroma of Origanum majorana L. analyzed by gc–ms and descriptive sensory analysis. Ind. Crop. Prod. 2015 , 74 , 218–227. [ Google Scholar ] [ CrossRef ]
- Sözmen, F.; Uysal, B.; Köse, E.O.; Aktaş, Ö.; Cinbilgel, I.; Oksal, B.S. Extraction of the essential oil from endemic Origanum bilgeri P.H.Davis with two different methods: Comparison of the oil composition and antibacterial activity. Chem. Biodivers. 2012 , 9 , 1356–1363. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hashemi, S.M.B.; Nikmaram, N.; Esteghlal, S.; Mousavi Khaneghah, A.; Niakousari, M.; Barba, F.J.; Roohinejad, S.; Koubaa, M. Efficiency of ohmic assisted hydrodistillation for the extraction of essential oil from oregano ( Origanum vulgare subsp. viride ) spices. Innov. Food Sci. Emerg. Technol. 2017 , 41 , 172–178. [ Google Scholar ] [ CrossRef ]
- Zheljazkov, V.D.; Astatkie, T.; Schlegel, V. Distillation time changes oregano essential oil yields and composition but not the antioxidant or antimicrobial activities. HortScience 2012 , 47 , 777–784. [ Google Scholar ]
- Stamenic, M.; Vulic, J.; Djilas, S.; Misic, D.; Tadic, V.; Petrovic, S.; Zizovic, I. Free-radical scavenging activity and antibacterial impact of Greek oregano isolates obtained by sfe. Food Chem. 2014 , 165 , 307–315. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Han, F.; Ma, G.-Q.; Yang, M.; Yan, L.; Xiong, W.; Shu, J.-C.; Zhao, Z.-D.; Xu, H.-L. Chemical composition and antioxidant activities of essential oils from different parts of the oregano. J. Zhejiang Univ. Sci. B 2017 , 18 , 79–84. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sarrazin, S.L.F.; Oliveira, R.B.; Barata, L.E.S.; Mourão, R.H.V. Chemical composition and antimicrobial activity of the essential oil of Lippia grandis schauer (verbenaceae) from the western amazon. Food Chem. 2012 , 134 , 1474–1478. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Betancourt, L.; Phandanauvong, V.; Patiño, R.; Ariza-Nieto, C.; Afanador-Téllez, G. Composition and bactericidal activity against beneficial and pathogenic bacteria of oregano essential oils from four chemotypes of Origanum and Lippia genus. Rev. Med. Vet. Zootec 2012 , 59 , 21–31. [ Google Scholar ]
- Ortega-Nieblas, M.M.; Robles-Burgueño, M.R.; Acedo-Félix, E.; González-León, A.; Morales-Trejo, A.; Vázquez-Moreno, L. Chemical composition and antimicrobial activity of oregano ( Lippia palmeri S. Wats) essential oil. Rev. Fitotec. Mex. 2011 , 34 , 11–17. [ Google Scholar ]
- Figueredo, G.; Ozcan, M.M.; Chalchat, J.C.; Bagci, Y.; Chalard, P. Chemical composition of essential oil of Hyssopus officinalis L. and Origanum acutidens . J. Essent. Oil Bear. Plant 2012 , 15 , 300–306. [ Google Scholar ] [ CrossRef ]
- Goze, I.; Alim, A.; Cetinus, S.A.; Cetin, A.; Durmus, N.; Atas, A.T.; Vural, N. In vitro antimicrobial, antioxidant, and antispasmodic activities and the composition of the essential oil of Origanum acutidens (hand.-mazz.) ietswaart. J. Med. Food 2010 , 13 , 705–709. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dambolena, J.S.; Zunino, M.P.; Lucini, E.I.; Olmedo, R.; Banchio, E.; Bima, P.J.; Zygadlo, J.A. Total phenolic content, radical scavenging properties, and essential oil composition of origanum species from different populations. J. Agric. Food Chem. 2010 , 58 , 1115–1120. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Al Hafi, M.; El Beyrouthy, M.; Ouaini, N.; Stien, D.; Rutledge, D.; Chaillou, S. Chemical composition and antimicrobial activity of Origanum libanoticum , Origanum ehrenbergii , and Origanum syriacum growing wild in Lebanon. Chem. Biodivers. 2016 , 13 , 555–560. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Waller, S.B.; Madrid, I.M.; Silva, A.L.; Dias de Castro, L.L.; Cleff, M.B.; Ferraz, V.; Meireles, M.C.A.; Zanette, R.; de Mello, J.R.B. In vitro susceptibility of Sporothrix brasiliensis to essential oils of Lamiaceae family. Mycopathologia 2016 , 181 , 857–863. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Karabörklü, S.; Ayvaz, A.; Yilmaz, S.; Akbulut, M. Chemical composition and fumigant toxicity of some essential oils against Ephestia kuehniella . J. Econ. Entomol. 2011 , 104 , 1212–1219. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ocak, I.; Celik, A.; Ozel, M.Z.; Korcan, E.; Konuk, M. Antifungal activity and chemical composition of essential oil of Origanum hypericifolium . Int. J. Food Prop. 2012 , 15 , 38–48. [ Google Scholar ] [ CrossRef ]
- Rodríguez-Solana, R.; Daferera, D.J.; Mitsi, C.; Trigas, P.; Polissiou, M.; Tarantilis, P.A. Comparative chemotype determination of lamiaceae plants by means of gc–ms, ft-ir, and dispersive-raman spectroscopic techniques and gc-fid quantification. Ind. Crop. Prod. 2014 , 62 , 22–33. [ Google Scholar ] [ CrossRef ]
- Stefanaki, A.; Cook, C.M.; Lanaras, T.; Kokkini, S. The oregano plants of chios island (Greece): Essential oils of Origanum onites L. growing wild in different habitats. Ind. Crop. Prod. 2016 , 82 , 107–113. [ Google Scholar ] [ CrossRef ]
- Ozkan, G.; Baydar, H.; Erbas, S. The influence of harvest time on essential oil composition, phenolic constituents and antioxidant properties of turkish oregano ( Origanum onites L.). J. Sci. Food Agric. 2010 , 90 , 205–209. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gendy, A.N.E.; Leonardi, M.; Mugnaini, L.; Bertelloni, F.; Ebani, V.V.; Nardoni, S.; Mancianti, F.; Hendawy, S.; Omer, E.; Pistelli, L. Chemical composition and antimicrobial activity of essential oil of wild and cultivated Origanum syriacum plants grown in Sinai, Egypt. Ind. Crop. Prod. 2015 , 67 , 201–207. [ Google Scholar ] [ CrossRef ]
- Viuda-Martos, M.; El Gendy, A.E.-N.G.S.; Sendra, E.; Fernández-López, J.; Abd El Razik, K.A.; Omer, E.A.; Pérez-Alvarez, J.A. Chemical composition and antioxidant and anti-listeria activities of essential oils obtained from some Egyptian plants. J. Agric. Food Chem. 2010 , 58 , 9063–9070. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Berrehal, D.; Boudiar, T.; Hichem, L.; Khalfallah, A.; Kabouche, A.; Al-Freihat, A.; Ghannadi, A.; Sajjadi, E.; Mehrabani, M.; Safaei-Ghomi, J.; et al. Comparative composition of four essential oils of oregano used in Algerian and Jordanian folk medicine. Nat. Prod. Commun. 2010 , 5 , 957–960. [ Google Scholar ] [ PubMed ]
- Werdin González, J.O.; Gutiérrez, M.M.; Murray, A.P.; Ferrero, A.A. Composition and biological activity of essential oils from labiatae against nezara viridula (hemiptera: Pentatomidae) soybean pest. Pest Manag. Sci. 2011 , 67 , 948–955. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Martucci, J.F.; Gende, L.B.; Neira, L.M.; Ruseckaite, R.A. Oregano and lavender essential oils as antioxidant and antimicrobial additives of biogenic gelatin films. Ind. Crop. Prod. 2015 , 71 , 205–213. [ Google Scholar ] [ CrossRef ]
- Olmedo, R.; Nepote, V.; Grosso, N.R. Antioxidant activity of fractions from oregano essential oils obtained by molecular distillation. Food Chem. 2014 , 156 , 212–219. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Quiroga, P.R.; Grosso, N.R.; Lante, A.; Lomolino, G.; Zygadlo, J.A.; Nepote, V. Chemical composition, antioxidant activity and anti-lipase activity of Origanum vulgare and Lippia turbinata essential oils. Int. J. Food Sci. Technol. 2013 , 48 , 642–649. [ Google Scholar ] [ CrossRef ]
- Wei, H.-K.; Chen, G.; Wang, R.-J.; Peng, J. Oregano essential oil decreased susceptibility to oxidative stress-induced dysfunction of intestinal epithelial barrier in rats. J. Funct. Foods 2015 , 18 , 1191–1199. [ Google Scholar ] [ CrossRef ]
- Elizalde, J.J.; Espinoza, M. Effect of ionizing irradiation on Origanum leaves ( Origanum vulgare L.) essential oil composition. J. Essent. Oil Bear. Plants 2011 , 14 , 164–171. [ Google Scholar ] [ CrossRef ]
- Gong, H.Y.; Liu, W.H.; Lv, G.Y.; Zhou, X. Analysis of essential oils of Origanum vulgare from six production areas of china and Pakistan. Rev. Bras. Farmacogn. 2014 , 24 , 25–32. [ Google Scholar ] [ CrossRef ]
- Thomidis, T.; Filotheou, A. Evaluation of five essential oils as bio-fungicides on the control of pilidiella granati rot in pomegranate. Crop Protect. 2016 , 89 , 66–71. [ Google Scholar ] [ CrossRef ]
- Verma, R.S.; Rahman, L.; Verma, R.K.; Chanotiya, C.S.; Chauhan, A.; Yadav, A.; Yadav, A.K.; Singh, A. Changes in the essential oil content and composition of Origanum vulgare L. during annual growth from kumaon himalaya. Curr. Sci. 2010 , 98 , 1010–1012. [ Google Scholar ]
- Pirigharnaei, M.; Zare, S.; Heidari, R.; Khara, J.; Sabzi, R.E.; Kheiry, F. The essential oil composition of Iranian oregano ( Origanum vulgare L.) populations in field and provenance from piranshahr district, west azarbaijan province, Iran. J. Food Agric. Environ. 2011 , 9 , 89–93. [ Google Scholar ]
- Fratini, F.; Mancini, S.; Turchi, B.; Friscia, E.; Pistelli, L.; Giusti, G.; Cerri, D. A novel interpretation of the fractional inhibitory concentration index: The case Origanum vulgare L. and Leptospermum scoparium J.R. et G. forst essential oils against Staphylococcus aureus strains. Microbiol. Res. 2017 , 195 , 11–17. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pesavento, G.; Calonico, C.; Bilia, A.R.; Barnabei, M.; Calesini, F.; Addona, R.; Mencarelli, L.; Carmagnini, L.; Di Martino, M.C.; Lo Nostro, A. Antibacterial activity of oregano, rosmarinus and thymus essential oils against Staphylococcus aureus and Listeria monocytogenes in beef meatballs. Food Control 2015 , 54 , 188–199. [ Google Scholar ] [ CrossRef ]
- Fouad, R.; Bousta, D.; Lalami, A.E.; Chahdi, F.O.; Amri, I.; Jamoussi, B.; Greche, H. Chemical composition and herbicidal effects of essential oils of Cymbopogon citratus (dc) stapf, Eucalyptus cladocalyx , Origanum vulgare L and Artemisia absinthium L cultivated in Morocco. J. Essent. Oil Bear. Plants 2015 , 18 , 112–123. [ Google Scholar ] [ CrossRef ]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical composition and bioactivity of different oregano ( Origanum vulgare ) extracts and essential oil. J. Sci. Food Agric. 2013 , 93 , 2707–2714. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ličina, B.Z.; Stefanović, O.D.; Vasić, S.M.; Radojević, I.D.; Dekić, M.S.; Čomić, L.R. Biological activities of the extracts from wild growing Origanum vulgare L. Food Control 2013 , 33 , 498–504. [ Google Scholar ] [ CrossRef ]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; Van Griensven, L.J. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010 , 15 , 7532–7546. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Stojković, D.; Glamočlija, J.; Ćirić, A.; Nikolić, M.; Ristić, M.; Šiljegović, J.; Soković, M. Investigation on antibacterial synergism of Origanum vulgare and Thymus vulgaris essential oils. Arch. Biol. Sci. 2013 , 65 , 639–643. [ Google Scholar ] [ CrossRef ]
- Boskovic, M.; Zdravkovic, N.; Ivanovic, J.; Janjic, J.; Djordjevic, J.; Starcevic, M.; Baltic, M.Z. Antimicrobial activity of thyme ( Thymus vulgaris ) and oregano ( Origanum vulgare ) essential oils against some food-borne microorganisms. Procedia Food Sci. 2015 , 5 , 18–21. [ Google Scholar ] [ CrossRef ]
- Mechergui, K.; Coelho, J.A.; Serra, M.C.; Lamine, S.B.; Boukhchina, S.; Khouja, M.L. Essential oils of Origanum vulgare L. subsp. glandulosum (desf.) ietswaart from Tunisia: Chemical composition and antioxidant activity. J. Sci. Food Agric. 2010 , 90 , 1745–1749. [ Google Scholar ] [ PubMed ]
- Mechergui, K.; Jaouadi, W.; Coelho, J.P.; Khouja, M.L. Effect of harvest year on production, chemical composition and antioxidant activities of essential oil of oregano ( Origanum vulgare subsp glandulosum (desf.) ietswaart) growing in north africa. Ind. Crop. Prod. 2016 , 90 , 32–37. [ Google Scholar ] [ CrossRef ]
- Béjaoui, A.; Chaabane, H.; Jemli, M.; Boulila, A.; Boussaid, M. Essential oil composition and antibacterial activity of Origanum vulgare subsp. glandulosum desf. at different phenological stages. J. Med. Food 2013 , 16 , 1115–1120. [ Google Scholar ] [ PubMed ]
- Kilic, Ö.; Özdemir, F.A. Variability of essential oil composition of Origanum vulgare L. subsp. gracile populations from Turkey. J. Essent. Oil Bear. Plants 2016 , 19 , 2083–2090. [ Google Scholar ] [ CrossRef ]
- Grondona, E.; Gatti, G.; Lopez, A.G.; Sanchez, L.R.; Rivero, V.; Pessah, O.; Zunino, M.P.; Ponce, A.A. Bio-efficacy of the essential oil of oregano ( Origanum vulgare lamiaceae. Ssp hirtum ). Plant Food Hum. Nutr. 2014 , 69 , 351–357. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Paraskevakis, N.; Tsiplakou, E.; Daferera, D.; Sotirakoglou, K.; Polissiou, M.; Zervas, G. Changes in essential oil content and composition of Origanum vulgare spp. hirtum during storage as a whole plant or after grinding and mixing with a concentrate ruminant diet. J. Essent. Oil Res. 2015 , 27 , 264–270. [ Google Scholar ]
- Karamanos, A.J.; Sotiropoulou, D.E.K. Field studies of nitrogen application on Greek oregano ( Origanum vulgare ssp. hirtum (link) ietswaart) essential oil during two cultivation seasons. Ind. Crop. Prod. 2013 , 46 , 246–252. [ Google Scholar ]
- Tibaldi, G.; Fontana, E.; Nicola, S. Growing conditions and postharvest management can affect the essential oil of Origanum vulgare L. ssp. hirtum (link) ietswaart. Ind. Crop. Prod. 2011 , 34 , 1516–1522. [ Google Scholar ] [ CrossRef ]
- Spagnoletti, A.; Guerrini, A.; Tacchini, M.; Vinciguerra, V.; Leone, C.; Maresca, I.; Simonetti, G.; Sacchetti, G.; Angiolella, L. Chemical composition and bio-efficacy of essential oils from italian aromatic plants: Mentha suaveolens , Coridothymus capitatus , Origanum hirtum and Rosmarinus officinalis . Nat. Prod. Commun. 2016 , 11 , 1517–1520. [ Google Scholar ]
- Bonfanti, C.; Iannì, R.; Mazzaglia, A.; Lanza, C.M.; Napoli, E.M.; Ruberto, G. Emerging cultivation of oregano in sicily: Sensory evaluation of plants and chemical composition of essential oils. Ind. Crop. Prod. 2012 , 35 , 160–165. [ Google Scholar ] [ CrossRef ]
- Mancini, E.; Camele, I.; Elshafie, H.S.; de Martino, L.; Pellegrino, C.; Grulova, D.; de Feo, V. Chemical composition and biological activity of the essential oil of Origanum vulgare ssp. hirtum from different areas in the southern apennines (Italy). Chem. Biodivers. 2014 , 11 , 639–651. [ Google Scholar ] [ PubMed ]
- Sarikurkcu, C.; Zengin, G.; Oskay, M.; Uysal, S.; Ceylan, R.; Aktumsek, A. Composition, antioxidant, antimicrobial and enzyme inhibition activities of two Origanum vulgare subspecies (subsp. vulgare and subsp. hirtum ) essential oils. Ind. Crop. Prod. 2015 , 70 , 178–184. [ Google Scholar ] [ CrossRef ]
- Vale-Silva, L.; Silva, M.-J.; Oliveira, D.; Gonçalves, M.-J.; Cavaleiro, C.; Salgueiro, L.; Pinto, E. Correlation of the chemical composition of essential oils from Origanum vulgare subsp. virens with their in vitro activity against pathogenic yeasts and filamentous fungi. J. Med. Microbiol. 2012 , 61 , 252–260. [ Google Scholar ] [ PubMed ]
- Vazirian, M.; Mohammadi, M.; Farzaei, M.H.; Amin, G.; Amanzadeh, Y. Chemical composition and antioxidant activity of origanum vulgare subsp. vulgare essential oil from Iran. Res. J. Pharmacogn. 2015 , 2 , 41–46. [ Google Scholar ]
- Nurzynska-Wierdak, R.; Bogucka-Kocka, A.; Sowa, I.; Szymczak, G. The composition of essential oil from three ecotypes of Origanum vulgare L. ssp vulgare cultivated in Poland. Farmacia 2012 , 60 , 571–577. [ Google Scholar ]
- Rodriguez-Garcia, I.; Silva-Espinoza, B.A.; Ortega-Ramirez, L.A.; Leyva, J.M.; Siddiqui, M.W.; Cruz-Valenzuela, M.R.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Oregano essential oil as an antimicrobial and antioxidant additive in food products. Crit. Rev. Food Sci. Nutr. 2016 , 56 , 1717–1727. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Adame-Gallegos, J.R.; Andrade-Ochoa, S.; Nevarez-Moorillon, G.V. Potential use of Mexican oregano essential oil against parasite, fungal and bacterial pathogens. J. Essent. Oil Bear. Plant 2016 , 19 , 553–567. [ Google Scholar ] [ CrossRef ]
- Jayasena, D.D.; Jo, C. Essential oils as potential antimicrobial agents in meat and meat products: A review. Trends Food Sci. Technol. 2013 , 34 , 96–108. [ Google Scholar ] [ CrossRef ]
- Arana-Sánchez, A.; Estarrón-Espinosa, M.; Obledo-Vázquez, E.N.; Padilla-Camberos, E.; Silva-Vázquez, R.; Lugo-Cervantes, E. Antimicrobial and antioxidant activities of Mexican oregano essential oils ( Lippia graveolens h. B.K.) with different composition when microencapsulated inβ-cyclodextrin. Lett. Appl. Microbiol. 2010 , 50 , 585–590. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Benavides, S.; Villalobos-Carvajal, R.; Reyes, J.E. Physical, mechanical and antibacterial properties of alginate film: Effect of the crosslinking degree and oregano essential oil concentration. J. Food Eng. 2012 , 110 , 232–239. [ Google Scholar ] [ CrossRef ]
- Emiroğlu, Z.K.; Yemiş, G.P.; Coşkun, B.K.; Candoğan, K. Antimicrobial activity of soy edible films incorporated with thyme and oregano essential oils on fresh ground beef patties. Meat Sci. 2010 , 86 , 283–288. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hernández, H.; Fraňková, A.; Sýkora, T.; Klouček, P.; Kouřimská, L.; Kučerová, I.; Banout, J. The effect of oregano essential oil on microbial load and sensory attributes of dried meat. J. Sci. Food Agric. 2017 , 97 , 82–87. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010 , 48 , 749–762. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Halliwell, B.; Gutteridge, J.M.C. Chapter 4: Cellular responses to oxidative stress: Adaptation, damage, repair, senescence and death. In Free Radicals in Biology and Medicine ; Halliwell, B., Gutteridge, J.M.C., Eds.; Oxford University Press: Oxford, UK, 2007; Volume 4, pp. 187–267. ISBN 978-0-19-856869-8. [ Google Scholar ]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul. Pharmacol. 2015 , 71 , 40–56. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gupta, R.K.; Patel, A.K.; Shah, N.; Chaudhary, A.; Jha, U.; Yadav, U.C. Oxidative stress and antioxidants in disease and cancer: A review. Asian Pac. Cancer Prev. 2014 , 15 , 4405–4409. [ Google Scholar ] [ CrossRef ]
- Cazzola, R.; Cestaro, B. Chapter 9: Antioxidant spices and herbs used in diabetes. In Diabetes: Oxidative Stress and Dietary Antioxidants ; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 89–97. [ Google Scholar ]
- Gonzalez-Burgos, E.; Gomez-Serranillos, M.P. Terpene compounds in nature: A review of their potential antioxidant activity. Curr. Med. Chem. 2012 , 19 , 5319–5341. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Conforti, F.; Marrelli, M.; Menichini, F.; Tundis, R.; Statti, G.A.; Solimene, U.; Menichini, F. Chemical composition and protective effect of oregano ( Origanum heracleoticum L.) ethanolic extract on oxidative damage and on inhibition of NO in LPS-stimulated raw 264.7 macrophages. J. Enzym. Inhib. Med. Chem. 2011 , 26 , 404–411. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tuttolomondo, T.; La Bella, S.; Licata, M.; Virga, G.; Leto, C.; Saija, A.; Trombetta, D.; Tomaino, A.; Speciale, A.; Napoli, E.M.; et al. Biomolecular characterization of wild sicilian oregano: Phytochemical screening of essential oils and extracts, and evaluation of their antioxidant activities. Chem. Biodivers. 2013 , 10 , 411–433. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tan, C.; Wei, H.; Sun, H.; Ao, J.; Long, G.; Jiang, S.; Peng, J. Effects of dietary supplementation of oregano essential oil to sows on oxidative stress status, lactation feed intake of sows, and piglet performance. Biomed. Res. Int. 2015 , 2015 , 9. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ferguson, L.R. Role of plant polyphenols in genomic stability. Mut. Res. Fund. Mol. Mutagen. 2001 , 475 , 89–111. [ Google Scholar ] [ CrossRef ]
- León-González, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem. Pharmacol. 2015 , 98 , 371–380. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Puerto, M.; Pichardo, S.; Jos, Á.; Cameán, A.M. In vitro pro-oxidant/antioxidant role of carvacrol, thymol and their mixture in the intestinal caco-2 cell line. Toxicol. In Vitro 2015 , 29 , 647–656. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Terenina, M.B.; Misharina, T.A.; Krikunova, N.I.; Alinkina, E.S.; Fatkulina, L.D.; Vorob’yova, A.K. Oregano essential oil as an inhibitor of higher fatty acid oxidation. Appl. Biochem. Microbiol. 2011 , 47 , 445–449. [ Google Scholar ] [ CrossRef ]
- Loizzo, M.R.; Menichini, F.; Conforti, F.; Tundis, R.; Bonesi, M.; Saab, A.M.; Statti, G.A.; Cindio, B.d.; Houghton, P.J.; Menichini, F.; et al. Chemical analysis, antioxidant, antiinflammatory and anticholinesterase activities of Origanum ehrenbergii boiss and Origanum syriacum L. essential oils. Food Chem. 2009 , 117 , 174–180. [ Google Scholar ] [ CrossRef ]
- Suhaj, M. Spice antioxidants isolation and their antiradical activity: A review. J. Food Comp. Anal. 2006 , 19 , 531–537. [ Google Scholar ] [ CrossRef ]
- Olmedo, R.H.; Nepote, V.; Grosso, N.R. Preservation of sensory and chemical properties in flavoured cheese prepared with cream cheese base using oregano and rosemary essential oils. LWT Food Sci. Technol. 2013 , 53 , 409–417. [ Google Scholar ] [ CrossRef ]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical profile, antioxidant and antibacterial activity of thyme and oregano essential oils, thymol and carvacrol and their possible synergism. J. Essent. Oil Bear. Plants 2015 , 18 , 1013–1021. [ Google Scholar ] [ CrossRef ]
- El Babili, F.; Bouajila, J.; Souchard, J.P.; Bertrand, C.; Bellvert, F.; Fouraste, I.; Moulis, C.; Valentin, A. Oregano: Chemical analysis and evaluation of its antimalarial, antioxidant, and cytotoxic activities. J. Food Sci. 2011 , 76 , C512–C518. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Alinkina, E.S.; Misharina, T.A.; Fatkullina, L.D. Antiradical properties of oregano, thyme, and savory essential oils. Appl. Biochem. Microbiol. 2013 , 49 , 73–78. [ Google Scholar ] [ CrossRef ]
- Quiroga, P.R.; Riveros, C.G.; Zygadlo, J.A.; Grosso, N.R.; Nepote, V. Antioxidant activity of essential oil of oregano species from Argentina in relation to their chemical composition. Int. J. Food Sci. Technol. 2011 , 46 , 2648–2655. [ Google Scholar ] [ CrossRef ]
- Borgarello, A.V.; Mezza, G.N.; Soltermann, A.T.; Pramparo, M.C. Use of a free radical scavenging method on extracts obtained by molecular distillation from oregano essential oil. Lat. Am. Appl. Res. 2014 , 44 , 25–30. [ Google Scholar ]
- Karakaya, S.; El, S.N.; Karagözlü, N.; Şahin, S. Antioxidant and antimicrobial activities of essential oils obtained from oregano ( Origanum vulgare ssp. hirtum ) by using different extraction methods. J. Med. Food 2011 , 14 , 645–652. [ Google Scholar ] [ PubMed ]
- Boroski, M.; Giroux, H.J.; Sabik, H.; Petit, H.V.; Visentainer, J.V.; Matumoto-Pintro, P.T.; Britten, M. Use of oregano extract and oregano essential oil as antioxidants in functional dairy beverage formulations. LWT Food Sci. Technol. 2012 , 47 , 167–174. [ Google Scholar ] [ CrossRef ]
- Marrelli, M.; Conforti, F.; Formisano, C.; Rigano, D.; Arnold, N.A.; Menichini, F.; Senatore, F. Composition, antibacterial, antioxidant and antiproliferative activities of essential oils from three origanum species growing wild in Lebanon and Greece. Nat. Prod. Res. 2016 , 30 , 735–739. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Castilho, P.C.; Savluchinske-Feio, S.; Weinhold, T.S.; Gouveia, S.C. Evaluation of the antimicrobial and antioxidant activities of essential oils, extracts and their main components from oregano from Madeira island, Portugal. Food Control 2012 , 23 , 552–558. [ Google Scholar ] [ CrossRef ]
- Almeida, A.P.; Rodriguez-Rojo, S.; Serra, A.T.; Vila-Real, H.; Simplicio, A.L.; Delgadilho, I.; Da Costa, S.B.; Da Costa, L.B.; Nogueira, I.D.; Duarte, C.M.M. Microencapsulation of oregano essential oil in starch-based materials using supercritical fluid technology. Innov. Food Sci. Emerg. Technol. 2013 , 20 , 140–145. [ Google Scholar ] [ CrossRef ]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008 , 454 , 428–435. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Robbins Basic Pathology ; Elsevier Health Sciences: New York, NY, USA, 2013. [ Google Scholar ]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005 , 352 , 1685–1695. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Trinchieri, G. Cancer and inflammation: An old intuition with rapidly evolving new concepts. Annu. Rev. Immunol. 2012 , 30 , 677–706. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Arranz, E.; Jaime, L.; López de las Hazas, M.C.; Reglero, G.; Santoyo, S. Supercritical fluid extraction as an alternative process to obtain essential oils with anti-inflammatory properties from marjoram and sweet basil. Ind. Crop. Prod. 2015 , 67 , 121–129. [ Google Scholar ] [ CrossRef ]
- Han, X.; Parker, T.L. Anti-inflammatory, tissue remodeling, immunomodulatory, and anticancer activities of oregano ( Origanum vulgare ) essential oil in a human skin disease model. Biochim. Open 2017 , 4 , 73–77. [ Google Scholar ] [ CrossRef ]
- Lima, M.D.S.; Quintans-Júnior, L.J.; De Santana, W.A.; Martins Kaneto, C.; Pereira Soares, M.B.; Villarreal, C.F. Anti-inflammatory effects of carvacrol: Evidence for a key role of interleukin-10. Eur. J. Pharmacol. 2013 , 699 , 112–117. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cho, S.; Choi, Y.; Park, S.; Park, T. Carvacrol prevents diet-induced obesity by modulating gene expressions involved in adipogenesis and inflammation in mice fed with high-fat diet. J. Nutr. Biochem. 2012 , 23 , 192–201. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rajan, B.; Ravikumar, R.; Premkumar, T.; Devaki, T. Carvacrol attenuates n-nitrosodiethylamine induced liver injury in experimental wistar rats. Food Sci. Hum. Wellness 2015 , 4 , 66–74. [ Google Scholar ] [ CrossRef ]
- Silva, F.V.; Guimarães, A.G.; Silva, E.R.S.; Sousa-Neto, B.P.; Machado, F.D.F.; Quintans-Júnior, L.J.; Arcanjo, D.D.R.; Oliveira, F.A.; Oliveira, R.C.M. Anti-inflammatory and anti-ulcer activities of carvacrol, a monoterpene present in the essential oil of oregano. J. Med. Food 2012 , 15 , 984–991. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- De Souza Siqueira Quintans, J.; Menezes, P.P.; Santos, M.R.V.; Bonjardim, L.R.; Almeida, J.R.G.S.; Gelain, D.P.; Araújo, A.A.d.S.; Quintans-Júnior, L.J. Improvement of p -cymene antinociceptive and anti-inflammatory effects by inclusion in β-cyclodextrin. Phytomedicine 2013 , 20 , 436–440. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Abbas, M.A.; Taha, M.O.; Zihlif, M.A.; Disi, A.M. β-caryophyllene causes regression of endometrial implants in a rat model of endometriosis without affecting fertility. Eur. J. Pharmacol. 2013 , 702 , 12–19. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Alberti, T.; Barbosa, W.; Vieira, J.; Raposo, N.; Dutra, R. (−)-β-caryophyllene, a cb2 receptor-selective phytocannabinoid, suppresses motor paralysis and neuroinflammation in a murine model of multiple sclerosis. Int. J. Mol. Sci. 2017 , 18 , 691. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Klauke, A.L.; Racz, I.; Pradier, B.; Markert, A.; Zimmer, A.M.; Gertsch, J.; Zimmer, A. The cannabinoid cb2 receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur. Neuropsychopharmacol. 2014 , 24 , 608–620. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Park, K.-R.; Nam, D.; Yun, H.-M.; Lee, S.-G.; Jang, H.-J.; Sethi, G.; Cho, S.K.; Ahn, K.S. B-caryophyllene oxide inhibits growth and induces apoptosis through the suppression of pi3k/akt/mtor/s6k1 pathways and ros-mediated mapks activation. Cancer Lett. 2011 , 312 , 178–188. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A review. Cogent. Food Agric. 2016 , 2 . [ Google Scholar ] [ CrossRef ]
- Halliwell, B.; Gutteridge, J.M.C. Chapter 9: Reactive species and disease: Fact, fiction or filibuster. In Free Radicals in Biology and Medicine ; Halliwell, B., Gutteridge, J.M.C., Eds.; Oxford University Press: Oxford, UK, 2007; Volume 4, pp. 488–613. ISBN 978-0-19-856869-8. [ Google Scholar ]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013 , 13 , 709–721. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hopkins, P.N. Molecular biology of atherosclerosis. Physiol. Rev. 2013 , 93 , 1317–1542. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dantas, B.P.V.; Alves, Q.L.; de Assis, K.S.; Ribeiro, T.P.; de Almeida, M.M.; de Vasconcelos, A.P.; de Araújo, D.A.M.; de Andrade Braga, V.; de Medeiros, I.A.; Alencar, J.L.; et al. Participation of the trp channel in the cardiovascular effects induced by carvacrol in normotensive rat. Vascul. Pharmacol. 2015 , 67–69 , 48–58. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ocaña-Fuentes, A.; Arranz-Gutiérrez, E.; Señorans, F.J.; Reglero, G. Supercritical fluid extraction of oregano ( Origanum vulgare ) essentials oils: Anti-inflammatory properties based on cytokine response on THP-1 macrophages. Food Chem. Toxicol. 2010 , 48 , 1568–1575. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Watanabe, H.; Murakami, M.; Ohba, T.; Takahashi, Y.; Ito, H. Trp channel and cardiovascular disease. Pharmacol. Ther. 2008 , 118 , 337–351. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Oz, M.; Lozon, Y.; Sultan, A.; Yang, K.-H.S.; Galadari, S. Effects of monoterpenes on ion channels of excitable cells. Pharmacol. Ther. 2015 , 152 , 83–97. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Saljoughian, S.; Roohinejad, S.; Bekhit, A.E.-D.A.; Greiner, R.; Omidizadeh, A.; Nikmaram, N.; Mousavi Khaneghah, A. The effects of food essential oils on cardiovascular diseases: A review. Crit. Rev. Food Sci. Nutr. 2017 . [ Google Scholar ] [ CrossRef ]
- Alves-Silva, J.M.; Zuzarte, M.; Marques, C.; Salgueiro, L.; Girão, H. Protective effects of terpenes on the cardiovascular system: Current advances and future perspectives. Curr. Med. Chem. 2016 , 23 , 4559–4600. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Federation, I.D. The idf Worldwide Definition of the Metabolic Syndrome ; International Diabetes Federation: Brussels, Belgium, 2016; Volume 2017. [ Google Scholar ]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A consensus statement from the international diabetes federation. Diabet. Med. 2006 , 23 , 469–480. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mnafgui, K.; Kchaou, M.; Ben Salah, H.; Hajji, R.; Khabbabi, G.; Elfeki, A.; Allouche, N.; Gharsallah, N. Essential oil of Zygophyllum album inhibits key-digestive enzymes related to diabetes and hypertension and attenuates symptoms of diarrhea in alloxan-induced diabetic rats. Pharm. Biol. 2016 , 54 , 1326–1333. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Aidi Wannes, W.; Marzouk, B. Research progress of Tunisian medicinal plants used for acute diabetes. J. Acute Dis. 2016 , 5 , 357–363. [ Google Scholar ] [ CrossRef ]
- Russo, G.L.; Russo, M.; Ungaro, P. AMP-activated protein kinase: A target for old drugs against diabetes and cancer. Biochem. Pharmacol. 2013 , 86 , 339–350. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yen, H.-F.; Hsieh, C.-T.; Hsieh, T.-J.; Chang, F.-R.; Wang, C.-K. In vitro anti-diabetic effect and chemical component analysis of 29 essential oils products. J. Food Drug Anal. 2015 , 23 , 124–129. [ Google Scholar ] [ CrossRef ]
- Dham, S.; Shah, V.; Hirsch, S.; Banerji, M.A. The role of complementary and alternative medicine in diabetes. Curr. Diab. Rep. 2006 , 6 , 251–258. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sebai, H.; Selmi, S.; Rtibi, K.; Souli, A.; Gharbi, N.; Sakly, M. Lavender ( Lavandula stoechas L.) essential oils attenuate hyperglycemia and protect against oxidative stress in alloxan-induced diabetic rats. Lipids Health Dis. 2013 , 12 , 189. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Alef, B.; Abdennacer, B.; Mohamed, B. A-amylase inhibitory activities of Origanum glandolosum , a North African endemic species. Int. J. Adv. Res. 2013 , 1 , 25–32. [ Google Scholar ]
- Bayramoglu, G.; Senturk, H.; Bayramoglu, A.; Uyanoglu, M.; Colak, S.; Ozmen, A.; Kolankaya, D. Carvacrol partially reverses symptoms of diabetes in stz-induced diabetic rats. Cytotechnology 2014 , 66 , 251–257. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bhalla, Y.; Gupta, V.K.; Jaitak, V. Anticancer activity of essential oils: A review. J. Sci. Food Agric. 2013 , 93 , 3643–3653. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017 , 22 , 70. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Begnini, K.R.; Nedel, F.; Lund, R.G.; Carvalho, P.H.D.; Rodrigues, M.R.A.; Beira, F.T.A.; Del-Pino, F.A.B. Composition and antiproliferative effect of essential oil of Origanum vulgare against tumor cell lines. J. Med. Food 2014 , 17 , 1129–1133. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Misharina, T.A.; Burlakova, E.B.; Fatkullina, L.D.; Alinkina, E.S.; Vorob’eva, A.K.; Medvedeva, I.B.; Erokhin, V.N.; Semenov, V.A.; Nagler, L.G.; Kozachenko, A.I. Effect of oregano essential oil on the engraftment and development of lewis carcinoma in f1 dba c57 black hybrid mice. Appl. Biochem. Microbiol. 2013 , 49 , 432–436. [ Google Scholar ] [ CrossRef ]
- Bostancioglu, R.B.; Kurkcuoglu, M.; Baser, K.H.C.; Koparal, A.T. Assessment of anti-angiogenic and anti-tumoral potentials of Origanum onites L. essential oil. Food Chem. Toxicol. 2012 , 50 , 2002–2008. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mosby. Mosby’s Medical Dictionary ; Elsevier: St. Louis, MO, USA, 2017; p. 757. ISBN 978-0-323-41425-8. [ Google Scholar ]
- Ceker, S.; Nardemir, G.; Alpsoy, L.; Agar, G.; Mete, E. Anti-genotoxic and anti-oxidant effects of Origanum rotundifolium on human lymphocytes in vitro. J. Essent. Oil Bear. Plant 2012 , 15 , 415–423. [ Google Scholar ] [ CrossRef ]
- Mossa, A.-T.H.; Refaie, A.A.; Ramadan, A.; Bouajila, J. Antimutagenic effect of Origanum majorana L. essential oil against prallethrin-induced genotoxic damage in rat bone marrow cells. J. Med. Food 2013 , 16 , 1101–1107. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hussain, A.I.; Anwar, F.; Rasheed, S.; Nigam, P.S.; Janneh, O.; Sarker, S.D. Composition, antioxidant and chemotherapeutic properties of the essential oils from two Origanum species growing in Pakistan. Rev. Bras. Farmacogn. Braz. J. Pharmacogn. 2011 , 21 , 943–952. [ Google Scholar ] [ CrossRef ]
- De Kok, T.M.; de Waard, P.; Wilms, L.C.; van Breda, S.G. Antioxidative and antigenotoxic properties of vegetables and dietary phytochemicals: The value of genomics biomarkers in molecular epidemiology. Mol. Nutr. Food Res. 2010 , 54 , 208–217. [ Google Scholar ] [ CrossRef ] [ PubMed ]
Click here to enlarge figure
| Oregano Species | Origin | Components | Yield * | Reference |
|---|---|---|---|---|
| H. patens | Mexico | Thymol, trans-piperitol, carvacrol acetate, carvacrol, camphene, β-myrcene, γ-terpinene, cis-p-mentha-1(7), 8-dien-2-ol, α-muurolene, α-calacorene, bulnesol, cadalene, viridiflorol. | NR | [ ] |
| L. grandis | Brazil | Carvacrol (37.12%), p-cymene (11.64%), thymol (7.83%), β-caryophyllene (3.93%). | 2.7% | [ ] |
| L. graveolens | Mexico | Thymol, carvacrol acetate, carvacrol, camphene, β-myrcene, γ-terpinene, cis-p-mentha-1(7),8-dien-2-ol, viridiflorol. | NR | [ ] |
| L. origanoides | Colombia | Thymol (78.7%), p-cymene (6.6%), γ-terpinene (2.7%), trans-β-caryophyllene (2.1%). | NR | [ ] |
| L. palmeri | Mexico | Thymol, α-cedrene, trans-piperitol, eugenol, carvacrol acetate, β-selinene, γ-cadinene, spathulenol. | NR | [ ] |
| O. acutidens | Mexico | Carvacrol (8.76–24.57%), p-cymene (14.25–22.37%), thymol (15.11–21.39%). | 5.0–6.0% | [ ] |
| Turkey | Carvacrol (76.21%), p-cymene (7.42%), borneol (3.19%), γ-terpinene (1.38%). | 1.45% | [ ] | |
| Turkey | Carvacrol (65.13%), meta-cymene (9.15%), trans-β-caryophyllene (4.43%), γ-terpinene (3.54%). | 3.1% | [ ] | |
| O. x applii | Argentina | Thymol (30.77%), trans-sabinene hydrate (29.63%), γ-terpinene (4.4%), terpinen-4-ol (3.23%). | 1.83 ± 0.27 mg/g dw | [ ] |
| O. ehrenbergii | Lebanon | Carvacrol (79.0%), p-cymene (4.4%), carvacrol methyl ether (2.7%), γ-terpinene (2.6%). | 3.19% | [ ] |
| O. bilgeri | Turkey | Carvacrol (84.30–90.20%), p-cymene (3.40–5.85%), γ-terpinene (0.47–1.20%), thymol (0.69–1.08%). | 0.54–0.57% | [ ] |
| O. libanoticum | Lebanon | β-Caryophyllene (26.8%), caryophyllene oxide (22.6%), germacrene D (17.2%), thymol methyl ether (10.5%). | 0.16% | [ ] |
| O. majorana | Brazil | 1,8-Cineole (20.9%), terpinen-4-ol(20.4%), γ-terpinene (8.5%), p-cymene (7.0%). | NR | [ ] |
| O. x majoricum | Colombia | trans-Sabinene hydrate (14.5%), γ-terpinene (14.0%), carvacrol methyl ether (6.0%), terpinen-4-ol (6.0%). | NR | [ ] |
| Turkey | Limonene (88.01%), thymol (11.98%). | NR | [ ] | |
| Argentina | trans-Sabinene hydrate (24.3–28.1%), thymol (12.1–17.4%), γ-terpinene (7.0–7.5%). | NR | [ ] | |
| Argentina | trans-Sabinene hydrate (36.77%), thymol (17.77%), γ-terpinene (5.9%), α-terpinene (3.9%). | 3.9 ± 0.25 mg/g dw | [ ] | |
| O. hypericifolium | Turkey | p-Cymene (34.33%), carvacrol (21.76%), thymol (19.54%), γ-terpinene (13.91%). | 2.9% | [ ] |
| O. onites | Greece | Carvacrol (79.63%), γ-terpinene (3.89%), p-cymene (3.51%), β-caryophyllene (2.24%). | 3.62% | [ ] |
| Greece | Carvacrol (62.6%), p-cymene (8.87%), γ-terpinene (8.45%), β-myrcene (2.92%). | NR | [ ] | |
| Greece | Carvacrol (69.0–92.6%), p-cymene (0.5–9.5%), γ-terpinene (0.3–7.9%), borneol (0.8–5.5%). | 3.0–7.0% | [ ] | |
| Turkey | Carvacrol (85.86%), γ-terpinene (4.43%), β-phellandrene (3.20%), p-cymene (1.83%). | 4.7 ± 0.06% | [ ] | |
| Turkey | Carvacrol (83.97–88.65%), thymol (0.80–7.48%), γ-terpinene (2.63–6.15%), p-cymene (1.52–3.16%). | 2.5–3.2% | [ ] | |
| O. syriacum | Egypt | Carvacrol (81.38%), p-cymene (8.48%), γ-terpinene (1.98%), β-myrcene (1.32%). | 5.5% | [ ] |
| Egypt | Thymol (31.73%), γ-terpinene (14.32%), linalool (9.44%), terpinen-4-ol (7.68%). | 4.63% | [ ] | |
| Egypt | Thymol (21.04%), γ-terpinene (18.96%), terpinen-4-ol (17.20%), α-terpinene (7.41%). | 0.6% | [ ] | |
| Lebanon | Carvacrol (60.8%), p-cymene (8.4%), thymol (7.9%), γ-terpinene (7.5%). | 1.65% | [ ] | |
| O. syriacum ssp. syriacum | Jordan | Thymol (51.8%), carvacrol (34.4%), p-cymene (3.9%). | 2.0–2.2% | [ ] |
| Jordan | Thymol (72.4%), γ-terpinene (7.8%), p-cymene (5.4%), carvacrol (3.5%). | 2.0–2.2% | ||
| O. vulgare L. | Argentina | p-Cymene (26.00%), γ-terpinene (21.89%), terpinen-4-ol (16.29%), β-caryophyllene (8.25%). | NR | [ ] |
| Argentina | Carvacrol (26.70%), p-cymene (15.20%), γ-terpinene (15.10%), terpinene (7.50%). | NR | [ ] | |
| Argentina | γ-Terpinene (25.1%), terpinen-4-ol (16.7%), carvacrol (16.2%), α-terpinene (8.54%). | NR | [ ] | |
| Argentina | γ-Terpinene (32.1%), α-terpinene (15.1%), p-cymene (8.0%), thymol (8.0%). | NR | [ ] | |
| Argentina | Carvacrol (81.92%), γ-terpinene (4.49%), thymol (3.5%), p-cymene (3.07%). | NR | [ ] | |
| Brazil | Carvacrol (73.9%), γ-terpinene (3.6%), thymol (3.0%), β-caryophyllene (2.8%). | NR | [ ] | |
| Chile | cis-β-Terpineol (16.49%), thymol (13.26%), terpinen-4-ol (10.24%), α-terpineol (4.35%). | NR | [ ] | |
| China | Carvacrol (30.73%), thymol (18.81%), p-cymene (10.88%), β-caryophyllene (8.21%). | NR | [ ] | |
| China | β-Citronellol (85.3%), citronellol acetate (5.2%), β-citronellal (1.2%). | 0.7% | [ ] | |
| China | Thymol (42.9%), citronellol (12.2%), β-caryophyllene (7.8%), p-cymen-2-ol (7.5%). | 0.3% | [ ] | |
| China | β-Citronellol (75.0%), geraniol (7.7%), citronellol acetate (3.4%). | 0.3% | [ ] | |
| China | 1,8-Cineole (20.8%), β-caryophyllene (10.2%), eugenol methyl ether (9.8%), citronellol (8.8%). | 0.3% | [ ] | |
| China | Caryophyllene oxide (32.9%), β-caryophyllene (17.7%), citronellol (10.2%), germacrene D (9.8%). | 0.1% | [ ] | |
| Colombia | Thymol (21.5%), p-cymene (21.0%), γ-terpinene (20.3%), α-terpinene (5.9%). | NR | [ ] | |
| Greece | Carvacrol (63.03%), thymol (15.09%), p-cymene (10.47%), γ-terpinene (3.43%). | NR | [ ] | |
| India | Carvacrol (35.02–62.81%), p-cymene (8.60–46.59%), γ-terpinene (2.49–19.11%). | 0.20–1.30% | [ ] | |
| Iran | Carvacrol (29.85%), γ-terpinene (20.94%), α-himachalene (12.17%), β-pinene (11.67%). | 0.80% | [ ] | |
| Iran | Carvacrol (23.54%), γ-terpinene (20.50%), thymol (15.41%), germacrene D-4-ol (9.26%). | 1.26% | [ ] | |
| Iran | Carvacrol (59.37%), γ-terpinene (18.36%), cedrene (6.65%). | 1.66% | [ ] | |
| Iran | Carvacrol (58.51%), humulene (11.46%), γ-terpinene (9.56%). | 0.93% | [ ] | |
| Iran | Carvacrol (67.09%), γ-terpinene (7.71%), humulene (7.67%). | 1.36% | [ ] | |
| Italy | Cavacrol (65.94%), p-cymene (9.33%), γ-terpinene (5.25%), β-caryophyllene (3.72%). | NR | [ ] | |
| Italy | Carvacrol (71.8%), p-cymene (11.6%), β-caryophyllene (2.7%), linalool (1.8%). | NR | [ ] | |
| Morocco | Carvacrol (34.0%), γ-terpinene (21.6%), p-cymene (9.4%), thymol (3.3%). | 2.7% | [ ] | |
| Pakistan | β-Citronellol (72.7%), thymol (7.2%), citronellol acetate (5.9%). | 0.3% | [ ] | |
| Poland | Carvacrol (26.38–36.72%), thymol (16.59–25.58%), γ-terpinene (10.06–16.11%), p-cymene (6.09–6.76%). | NR | [ ] | |
| Portugal | Carvacrol (14.5%), β-fenchyl alcohol (12.8%), γ-terpinene (11.6%), δ-terpineol (7.5%). | NR | [ ] | |
| Serbia | Sabinene (10.2%), terpinen-4-ol (9.3%), 1,8-cineole (5.8%), γ-terpinene (5.6%). | 0.17% | [ ] | |
| Serbia | Carvacrol (64.5%), p-cymene (10.9%), γ-terpinene (10.8%), thymol (3.5%). | 1.5% | [ ] | |
| Serbia | Carvacrol (64.5%), p-cymene (10.9%), γ-terpinene (10.8%), thymol (3.5%). | NR | [ ] | |
| Serbia | Carvacrol (77.6%), p-cymene (5.14%), trans-β-caryophyllene (2.45%), linalool (2.44%). | NR | [ ] | |
| Spain | Terpinen-4-ol (24.57%), carvacrol (16.09%), thymol (9.03%), γ-terpinene (6.20%). | 516 mg/plant | [ ] | |
| USA | Carvacrol (17.9–81.8%), p-cymene (2.62–25.7%), γ-terpinene (2.5–19.4%), β-myrcene (0.58–6.06%). | 0.114–2.312% | [ ] | |
| O. vulgare L. ssp. glandulosum | Algeria | Thymol (34.2%), carvacrol (30.5%), γ-terpinene (13.4%), p-cymene (6.6%). | 2.0–2.2% | [ ] |
| Algeria | Thymol (51.1%), γ-terpinene (14.5%), p-cymene (7.5%), carvacrol (6.8%). | 2.0–2.2% | [ ] | |
| Tunisia | p-Cymene (35.7–46.3%), thymol (18.4–39.1%), γ-terpinene (11.7–24.2%), carvacrol (1.7–15.1%). | 2.5–4.6% | [ ] | |
| Tunisia | Thymol (31.8–46.1%), p-cymene (11.5–35.7%), γ-terpinene (24.0–27.1%), α-terpinene (1.9–3.2%). | 4.3–5.8% | [ ] | |
| Tunisia | Carvacrol (65.01%), p-cymene (9.00%), γ-terpinene (4.25%), borneol (3.19%). | 1.87–3.42% | [ ] | |
| O. vulgare L. ssp. gracile | Iran | Carvacrol (46.86%), γ-terpinene (14.16%), p-cymene (11.63%), carvacrol methyl ether (5.97%). | ≈2.0% | [ ] |
| Turkey | Thymol (7.02–40.04%), carvacrol (8.21–33.21%), γ-terpinene (9.15–27.82%), p-cymene (3.07–23.52%). | 0.25–0.50% | [ ] | |
| O. vulgare L. ssp. hirtum | Argentina | trans-Sabinene hydrate (22.9%), thymol (18.6%), γ-terpinene (7.1%), terpinen-4-ol (6.2%). | NR | [ ] |
| Argentina | trans-Sabinene hydrate (17.9%), thymol (17.1%), terpinen-4-ol (9.5%), γ-terpinene (8.0%). | NR | [ ] | |
| Argentina | γ-Terpinene (13.7%), terpinen-4-ol (11.2%), α-terpinene (9.9%), trans-sabinene hydrate (8.3%). | NR | [ ] | |
| Colombia | Carvacrol (90.3%), thymol (3.5%), p-cymene (2.7%), γ-terpinene (1.0%). | NR | [ ] | |
| Greece | Carvacrol (70.38%), p-cymene (8.17%), γ-terpinene (7.78%), β-myrcene (2.37%). | NR | [ ] | |
| Greece | Carvacrol (90.29%), γ-terpinene (3.09%), p-cymene (2.25%), β-caryophyllene (1.81%). | 7.77% | [ ] | |
| Greece | Carvacrol (81.28–91.21%), p-cymene (1.52–6.40%), γ-terpinene (0.49–4.01%), β-caryophyllene (0.94–2.03%). | 4.71–5.00% | [ ] | |
| Greece | Carvacrol (56.46–82.70%), p-cymene (9.54–21.40%), β-disavolene (1.09–3.06%). | 0.63–4.25% | [ ] | |
| Hungary | Carvacrol (82.75%), p-cymene (6.58%), γ-terpinene (5.78%). | 4.46% | [ ] | |
| Italy | terpinen-4-ol (13.27–17.51%), γ-terpinene (14.58–14.95%), carvacrol (12.31–14.58%), p-cymene (8.43–10.07%). | 0.063–0.165% | [ ] | |
| Italy | Thymol (37.9%), γ-terpinene (24.5%), p-cymene (16.3%), α-terpinene (4.3%). | NR | [ ] | |
| Italy | γ-Terpinene (29.41%), thymol (26.86%), p-cymene (8.20%), α-terpinene (5.93%). | 5.4% | [ ] | |
| Italy | Thymol (37.22%), γ-terpinene (26.37%), p-cymene (6.83%), α-terpinene (4.02%). | 2.4% | [ ] | |
| Italy | Thymol (36.46%), γ-terpinene (20.77%), p-cymene (8.31%), carvacrol methyl ether (6.21%). | 3.6% | [ ] | |
| Italy | Thymol (30.25%), γ-terpinene (25.89%), p-cymene (7.62%), carvacrol methyl ether (5.63%). | 4.2% | [ ] | |
| Italy | Thymol y carvacrol (65.3–84.7%), linalool (0.1–2.6%), carvacrol methyl ether (0.4–1.9%). | 1.0–2.7% | [ ] | |
| Italy | Thymol (18.16–56.37%), γ-terpinene (12.70–32.70%), p-cymene (8.22–10.30%). | 1.7–4.5% | [ ] | |
| Lithuania | Carvacrol (72.4–88.2%), γ-terpinene (4.1–8.7%), p-cymene (2.0–3.2%), β-caryophyllene (0.9–3.0%). | 35.50–325.45 dm /ha | [ ] | |
| Serbia | Carvacrol (74.65%), p-cymene (5.87%), γ-terpinene (5.04%), trans-β-caryophyllene (1.76%). | 1.34% | [ ] | |
| Turkey | Linalool (96.31%), β-caryophyllene (1.27%). | 7.31% | [ ] | |
| Turkey | Carvacrol (80.09%), γ-terpinene (12.01%), p-cymene (1.72%), α-terpinene (1.58%). | 5.9 ± 0.02% | [ ] | |
| O. vulgare L. ssp. virens | Argentina | trans-Sabinene hydrate (27.77%), thymol (26.1%), γ-terpinene (5.9%), α-terpinene (4.17%). | 2.17 ± 0.32 mg/g dw | [ ] |
| Iran | (Z)-α-Bisabolene (39.17%), sabinene (11.52%), carvacrol (5.23%), β-bisabolene (4.24%). | ≈0.3% | [ ] | |
| Portugal | α-Terpineol (0.1–65.1%), γ-terpinene (0.3–34.25), linalool (2.0–27.4%), carvacrol (0–34.2%), E-caryophyllene (2.4–11.0%). | 0.8–1.2% | [ ] | |
| O. vulgare L. ssp. vulgare | Argentina | trans-Sabinene hydrate (23.4–27.2%), thymol (14.4–17.2%), terpinen-4-ol (7.8–11.0%), γ-terpinene (7.3–9.8%). | NR | [ ] |
| Argentina | trans-Sabinene hydrate (32.47%), thymol (20.5%), γ-terpinene (15.47%), terpinen-4-ol (5.03%). | 1.97 ± 0.22 mg/g dw | [ ] | |
| Iran | Thymol (37.13%), γ-terpinene (9.67%), carvacrol (9.57%), carvacrol methyl ether (6.88%). | 0.5% | [ ] | |
| Italy | Spathulenol (18.6%), carvacrol (11.7%), β-caryophyllene (8.8%), terpinen-4-ol (5.6%). | 0.13% | [ ] | |
| Italy | Carvacrol (14.3%), spathulenol (9.4%), β-caryophyllene (5.3%), terpinen-4-ol (5.0%). | 0.18% | [ ] | |
| Lithuania | Sabinene (6.6–28.2%), β-caryophyllene (7.3–15.5%), E-β-ocimene (4.4–15.1%), allo-ocimene (7.7–12.1%). | 3.08–36.65 dm /ha | [ ] | |
| Turkey | Thymol (58.31%), carvacrol (16.11%), p-cymene (13.45%), γ-terpinene (4.64%). | 5.09% | [ ] | |
| Poland | Sabinene (10.85–25.46%), Z-(β)-ocimene (9.10–16.33%), germacrene D (9.36–15.34%), E-caryophyllene (9.38–12.87%). | 0.66–0.86% | [ ] |
| Oregano Species | Biological Activity | Effect | Reference |
|---|---|---|---|
| H. patens | Anti-inflammatory | Reduction on the levels of NO and ROS produced in murine macrophage cells. | [ ] |
| L. palmeri | Anti-inflammatory | Inhibition on the production of ROS and NO by LPS-stimulated RAW 264.7 macrophages | [ ] |
| L. graveolens | Antioxidant | Radical scavenging activity against DPPH | [ ] |
| Anti-inflammatory | Reduction on the levels of NO and ROS produced in LPS-stimulated murine macrophage cells | [ ] | |
| O. acutidens | Antioxidant | Showed scavenging activity against DPPH radical | [ ] |
| O. compactum | Antioxidant | ABTS radical-scavenging activity | [ ] |
| Cytotoxic | Nontoxic when used in MCF-7 cells | [ ] | |
| O. dictamnus | Antioxidant | Ferric reducing/antioxidant power | [ ] |
| Antiproliferative | Inhibit colon carcinoma (LoVo) and hepatocarcinoma (HepG2) cell proliferation | [ ] | |
| O. ehrenbergii | Antioxidant | DPPH radical-scavenging activity | [ ] |
| O. glandulosum | Antioxidant | Showed antiradical activity | [ , ] |
| O. heracleoticum | Anti-inflammatory | Inhibition of NO production | [ ] |
| O. libanoticum | Antioxidant | Ferric reducing/antioxidant power | [ ] |
| Antiproliferative | Inhibit HepG2 cell proliferation | [ ] | |
| O. majorana | Anti-inflammatory | Reduction in the secretion of inflammatory cytokines (TNF-α, IL-1β and IL-6) in THP-1 cells | [ ] |
| Anti-genotoxic | Reduces the chromosomal aberration in bone marrow cells of rats | [ ] | |
| Cytotoxic | Inhibit cell viability of human breast (MCF-7) and prostate (LNCaP) cancer cell lines. | [ ] | |
| O. microphyllum | Antioxidant | Showed ferric reducing power | [ ] |
| O. minutiflorum | Antioxidant | Retard lipidic oxidation | [ ] |
| O. onites | Antioxidant | Showed free radical scavenging against DPPH radical | [ ] |
| Anti-angiogenic | Blocks in vitro tube formation | [ ] | |
| O. rotundifolium | Anti-genotoxic | Reduces the effect of Aflatoxin B (AFB ) in human peripheral lymphocytes | [ ] |
| O. syriacum | Antioxidant | DPPH radical-scavenging activity | [ ] |
| O. virens | Antioxidant | Showed scavenging activity against DPPH radical | [ ] |
| O. vulgare subsp. hirtum | Antioxidant | Total reducing capacity (Folin-Ciocalteu method), radical-scavenging activity in the UV radiation-induced peroxidation in liposomal membranes | [ ] |
| Antioxidant | Reduces 8-hydroxy-deoxyguanosine and thiobarbituric acid reactive substances. | [ ] | |
| Antioxidant | DPPH and ABTS radical-scavenging activity | [ , , , ] | |
| Antiproliferative | Inhibit human lung adenocarcinoma epithelial (A549) cell proliferation | [ ] | |
| Cytotoxic | Decrease cell viability in a concentration-dependent manner on human keratinocyte (HaCaT) and lung cancer (A549) cell lines | [ ] | |
| Hypoglycemic | α-Amylase and α-glucosidase inhibitory activity | [ ] | |
| O. vulgare subsp. vulgare | Antioxidant | Radical scavenging activity (DPPH, ABTS and FRAP assays). Total reducing capacity (Folin-Ciocalteu method) | [ , , , , , , , ] |
| Antioxidant | Prevent autoxidation of polyunsaturated fatty acid esters | [ ] | |
| Anti-inflammatory | Reduced synthesis of TNF-α, IL-1β, and IL-6 cytokines. Increased synthesis of cytokine IL-10 | [ ] | |
| Anti-inflammatory | Inhibition of the levels of inflammatory biomarkers (MCP-1, VCAM-1 and ICAM-1) on activated-primary human neonatal fibroblasts | [ ] | |
| Antiproliferative | Inhibit human breast adenocarcinoma (MCF-7) and human colon adenocarcinoma (HT-29) cell proliferation | [ ] | |
| Antitumor | Decrease the sizes of tumors in disease mice | [ ] | |
| Hypoglycemic | Inhibits α-amylase and α-glucosidase activity | [ , ] |
Share and Cite
Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017 , 22 , 989. https://doi.org/10.3390/molecules22060989
Leyva-López N, Gutiérrez-Grijalva EP, Vazquez-Olivo G, Heredia JB. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules . 2017; 22(6):989. https://doi.org/10.3390/molecules22060989
Leyva-López, Nayely, Erick P. Gutiérrez-Grijalva, Gabriela Vazquez-Olivo, and J. Basilio Heredia. 2017. "Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties" Molecules 22, no. 6: 989. https://doi.org/10.3390/molecules22060989
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 06 April 2022
Natural diversity in phenolic components and antioxidant properties of oregano ( Origanum vulgare L.) accessions, grown under the same conditions
- Ghazaleh Jafari Khorsand 1 ,
- Mohammad Reza Morshedloo 1 ,
- Hasan Mumivand 2 ,
- Zohreh Emami Bistgani 3 ,
- Filippo Maggi 4 &
- Abdolvahab Khademi 5
Scientific Reports volume 12 , Article number: 5813 ( 2022 ) Cite this article
5237 Accesses
30 Citations
11 Altmetric
Metrics details
- Biochemistry
- Plant sciences
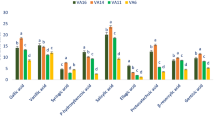
Oregano ( Origanum vulgare L.) is a rich source of biologically active components such as phenolic compounds. Here, seven pot grown O. vulgare accessions belonging to three subspecies (subsp. virens , subsp. vulgare and subsp. gracile ) were investigated for their content in sixteen bioactive phenolic compounds as well as their antioxidant capacities (DPPH • and FRAP tests), total phenolic content (TPC) and total flavonoid content (TFC) in order to identify the most suitable ones on an industrial level. HPLC analyses showed that rosmarinic acid (659.6–1646.9 mg/100 g DW) was by far the most abundant constituent, followed by luteolin (46.5–345.4 mg/100 g DW), chicoric acid (36.3–212.5 mg/100 g DW), coumarin (65.7–193.9 mg/100 g DW) and quercetin (10.6–106.1 mg/100 g DW), with variability in concentration depending on the accession and subspecies. The highest level of rosmarinic acid and TPC was obtained from Ardabil accession (subsp. virens ). There was a significant and positive correlation between rosmarinic acid and antioxidant activity (r = 0.46). TFC significantly correlated to TPC (r = 0.57) as well as to chicoric acid (r = 0.73). Cluster (CA) and principal component (PCA) analyses classified the investigated accessions in three different groups. Such natural variabilities in phenolics provide the possibility of using elite plants for nutraceutical and pharmaceutical industries and domestication of highly antioxidative accessions of oregano.
Similar content being viewed by others

Influence of climate variation on phenolic composition and antioxidant capacity of Medicago minima populations
Phenolic profiles and antioxidant activities in selected drought-tolerant leafy vegetable amaranth

Screening of tarragon accessions based on physiological and phytochemical responses under water deficit
Introduction.
Herbs represent an extensive variety of aromatic plants utilized for food flavoring and therapeutic goals 1 . The fragrances, flavors, and therapeutic characteristics of herbs are related to their secondary metabolites 2 . These chemicals, mostly within the essential oil or extract, are synthesized in the plant during the course of metabolism 3 . The genus Origanum (Lamiaceae) includes some well-known annual, perennial, and shrubby herbs with wide morphological and chemotype diversity throughout the world 4 , 5 . Most types of the genus are distributed in the Mediterranean, Euro-Siberian, and Iran-Siberian regions 1 . Origanum vulgare L., commonly known as oregano, is generally recognized as a renowned condiment and culinary herb in the world 1 . Based on the recent classification of the World Flora Online (WFO; www.worldfloraonline.org ), five different subspecies have been reported for O. vulgare (i.e. subsp. glandulosum , subsp. gracile , subsp. hirtum , subsp. virens and subsp. vulgare ). Of these, the subsp. vulgare , gracile and virens are wildly distributed in Iran 6 . Among the mentioned Iranian subspecies, O. vulgare subsp. gracile is a rich source of phenolic monoterpenes such as carvacrol 7 . The aerial parts of oregano contain a wide array of chemical constituents, including phenolics, terpenes, flavonoids, glucosides, sterols, tannins, resins and mucilages 1 , 8 , 9 . The plant is commonly used as a flavoring herb in food production and industry and to flavor salads, soups, fish, pizza, processed meats, and other eatables 9 . In addition, oregano, as a medicinal plant, has been traditionally used as an expectorant, anti-flatulence, appetizer, diuretic and sedative agent 2 . The plant extract has strong antibacterial and antifungal properties due to its richness in phenolic compounds 6 . Rosmarinic acid, luteolin, quercetin, apigenin, scutellarein and their derivatives are the major phenolic acids and flavonoids that have been detected in oregano species 10 .
Through inhibiting the initiation of oxidizing chain reaction, antioxidants inhibit or delay the oxidation of molecules. Because of its high phenolic content, oregano is considered as one of the most favorite natural antioxidants 11 . The antioxidant capacity of O . vulgare extract is mainly related to its phenolic constituents 8 , 12 . The phenolic antioxidants take over various pharmacological properties, such as antidiabetic, antiulcer, antiviral, cytotoxic, antitumor and anti-inflammatory activities 13 , and are mainly responsible for the health effects of O. vulgare . Potential anticancer characteristics of phenolic acids and flavonoids have been previously reported by Shukla and Gupta (2010) 14 .
Our previous investigations showed that there is a great variability in terms of essential oil compounds among the different subspecies and/or accessions of oregano herb 5 , 15 . However, there is also a big challenge as to whether there is variation in phenolics and antioxidant capacity among the accessions and subspecies of oregano. A previous study has shown a high variability in rosmarinic acid content and oxygen radical absorbance capacity (ORAC) and total phenolic content (TPC) of European oregano accessions 11 . As a result, it can be presumed that such variability in rosmarinic acid and other phenolic compounds can also be seen in Iranian oregano accessions. Considering the great importance of phenolic compounds in human nutrition and their health effects, the current study aimed to investigate the variability in main phenolic compounds (including rosmarinic acid, chicoric acid, apigenin, luteolin and others), total phenolic content (TPC), total flavonoids contents (TFC) and antioxidant capacity among different accessions of Iranian oregano belonging to three subspecies (subsp. virens , subsp. vulgare and subsp. gracile ). This research is a continuation of an ongoing oregano breeding program, pointing out the elite accessions of oregano in terms of phenolic components for domestication. In the meantime, the results generated from the current study will be useful to introduce the elite accessions with high antioxidant capacity into food and pharmaceutical industries.
Materials and methods
Reagents and standards.
HPLC grade rosmarinic acid, chlorogenic acid, cinnamic acid, quercetin, caffeic acid, syringic acid, benzoic acid, vanillic acid, gallic acid, apigenin, chicoric acid, luteolin, kaempferol, 2,4-dihydroxybenzoic acid, naringenin and coumarin standards were purchased from Sigma-Aldrich (MO, USA). Other chemicals and solvents were analytical grade and were purchased from Merck (Darmstadt, Germany).
Plant and soil materials
Seeds of the seven accessions of O. vulgare belonging to three subspecies (subsp. virens , subsp. vulgare and subsp. gracile ) were obtained from the seed gene bank of the Research Institute of Forest and Rangeland in Tehran, Iran. The seed gene bank declared that seeds of all accessions were obtained under national and international guidelines and the seed were prepared under the supervision and permission of Maragheh University and all authors comply with all the local and national guidelines. The voucher specimens of the plants were deposited at the herbarium of Department of Horticultural Sciences, University of Tehran, Karaj, Iran. Geological characteristics of the seed, collection sites, subspecies and their voucher numbers are presented in Table 1 .
Oregano seeds were sown in a plastic germination tray filled with coco peat:perlite mixture (70:30, w:w) and kept in a glass greenhouse at the University of Maragheh, Maragheh, Iran. On 12 April 2020, the seedlings with about 10 cm height were transferred into 7 L pots. The growth medium was composed of combined proportions of field soil, silt, leaf mold and perlite (45:25:20:10, v:v). The soil contained 0.09% N, 21.8 mg kg −1 available P, and 487 mg kg −1 available K; the medium pH was 7.2 and EC was adjusted to be 1.2 dS m −1 . During the growth seasons, the plants were irrigated regularly. The pots were placed in the greenhouse under natural daylight with a maximum and minimum day temperature of 33.5 and 17.5 °C, respectively. To warrant the well growth of the plants, the pots were fed with half Hoagland-based solution six times during the growth period (Jons, 2014). The harvest was performed at full flowering stage on 15 August 2020. The plants were cut from 5 cm above the soil and dried in oven at 40 °C, after which their dry drug weight (flowering aerial part) was measured (g/pot). For each accession, 12 individual plants were grown and each of the four harvested oregano plants was bulked together to obtain three replications (n = 3) for the extraction purpose.
Plant extraction procedure
For extraction, 200 mg of leaf and inflorescence powder from each dried sample was dissolved in 20 mL of 80% methanol (methanol–water mixture in 80:20 proportion) and shaken for 72 h at room temperature (25 °C). Then, the supernatant was filtered using a Whatman filter paper (No. 4) and the residue was re-extracted with the same method 16 .
Determination of total phenolic compounds
In order to determine the total phenolic content of oregano accessions, the Folin-Ciocalteu method was used (Spanos and Wrolstad 1990). For this purpose, 10 μL of plant extract were added to 500 μL of 10% Folin-Ciocalteu's reagent. Next, 500 μL of 1% saturated sodium carbonate solution were added to the mixture and incubated for 2 h at 25 °C. After incubation, the solution absorbance was read at 765 nm using a microplate reader (Spectromax-M5-USA). Gallic acid with different concentrations was used to make the calibration curve. The total phenolics content was reported as mg gallic acid equivalent (GAE) g −1 dry weight 17 .
Determination of total flavonoids
According to the outline described by Quettier-Deleu et al. (2000) 18 , total flavonoids content of oregano extracts was measured using AlCl 3 reagent. Briefly, 2 mL of 2% methanolic aluminum chloride solution was added to 2 mL of the extract and then stirred slightly. The procedure was continued by adding 6 mL of 5% potassium acetate solution. The mixture was incubated for 40 min at 25 °C and after that the absorbance of samples was read at 415 nm using a microplate reader (Spectromax-M5-USA). For quantification of absorbance, quercetin was used as a standard. The amount of flavonoids in each extract was expressed in terms of mg quercetin equivalent (QE) g −1 dry weight.
RP-HPLC analysis of phenolic compounds
To identify the phenolic components, oregano extracts were analyzed using high-performance liquid chromatography (HPLC, Shimadzu, Japan) coupled with a photodiode array detector (SPD-M20A, Shimadzu, Japan). Phenolics were separated using a phenomenex column (Gemini-5 µm NX-C18 110 Å, LC Column 250 × 4.6 mm). The mobile phase was composed of solvent B (20 mM phosphoric acid) and solvent D (methanol). The program began with isocratic elution with 10% B (1.5 min), then a linear gradient was used until 40 min, increasing B to 95%, 5 min in 95% B, decreased to 10% in 30 s, 6 min in 10%. The flow rate was 1 mL min −1 . Ten μL from each sample were injected into instrument. Scanning was performed from wavelength range of 200 to 400 nm. The identification of phenolic components was performed by comparing their pure standard retention time and the UV spectra with those of standards. For quantification of the components, different concentrations of each standard (10–80 μg mL −1 ) were injected under the same operative conditions of extracts and the calibration curve of each compound was plotted. The results were exposed as mg of each compound per 100 g of dry weight (mg/100 g DW).
DPPH-radical scavenging activity
The method described by Chou et al., (2011) 8 was used to capture DPPH (2,2-diphenyl-1-picrylhyrazyl) free radical scavenging activity of oregano extracts. Toward that end, different concentrations of oregano extract (10 μL) were added to 990 μL of 0.1 mM methanolic solution of DPPH. After 30 min of incubation under darkness at room temperature, the absorbance was recorded at 517 nm using a microplate reader (Spectromax-M5-USA). Ascorbic acid was used as a positive control. Ethanolic solution of DPPH was used as control. DPPH radical scavenging activity (%) = [(A control – A final DPPH ) / A control ] × 100.
Linear regression analysis was used to determine the EC 50 (half maximal effective concentration) value of the extracts.
Ferric-reducing antioxidant power (FRAP) assay
Ferric-reducing antioxidant power (FRAP) of the extracts was determined using the method explained by Benzie and Strain (1996) 19 with minor modifications. Following the procedure, the FRAP reagent was prepared by mixing 100 mL of acetate buffer (300 mM; pH 3.6), 10 mL of TPTZ (10 mM in HCl), and 10 mL of FeCl 3 .6H 2 O (20 mM in water). The fresh mixture was incubated at 37 °C until use. Acetate buffer was prepared by mixing 0.31 g of sodium acetate trihydrate with 1.6 mL of glacial acetic acid and reaching the volume of 100 mL using doubly distilled water. The reaction was done by mixing 380 μL of FRAP reagent and 20 μL of plant extracts in the wells and incubating at 37 °C for 10 min. The absorbance of samples was read at 593 nm using Spectromax-M5 microplate reader (USA). Quantification was done when the calibration curve of Fe 2 SO 4 .7H 2 O (25–1000 µM) was plotted. The FRAP antioxidant activity was expressed in terms of μM Fe (II).
Statistical analysis
Analysis of variance (ANOVA) and least significant difference (LSD) test at a 5% probability level was performed using SAS statistical software (version 9.1, SAS Inst., USA). Pearson correlation coefficients were computed using IBM SPSS (SPSS, version 22, USA). Cluster analysis and principal component analysis (PCA) were performed using Xlstat software, 2018.
The results from ANOVA showed that there were significant differences (p < 0.01) among oregano accessions in terms of drug yield (flowering aerial parts). As depicted in Fig. 1 , a high variability was observed among the oregano accessions in terms of drug yield, ranging from 32.3 to 50.5 g/pot. According to mean compression values, the highest drug yield (50.5 g/pot) was obtained for Gilan accession (subsp. virens ), followed by Kaleybar (43.2 g/pot; subsp. vulgare ) and Ardabil (37.5 g/pot; subsp. virens ) accessions. However, Arasbaran (32.3 g/pot; subsp. vulgare ), Baneh (33.7 g/pot; subsp. gracile ) and Mazandaran (33.8 g/pot; subsp. virens ) accessions yielded the lowest drug weights in comparison with others.
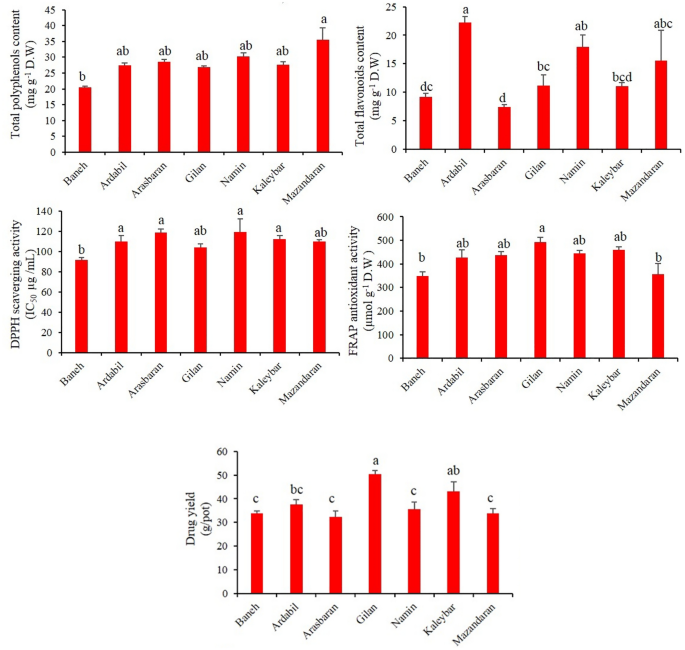
Mean values (n = 3) of yield and antioxidant biochemicals in seven oregano accessions. The error bars represent standard errors of the means. Different letters indicate significant differences ( p < 0.05) among the treatments within each genotype (LSD test at 5% level).
Total polyphenol and flavonoid contents
As depicted in Fig. 1 , based on the mean compression of TPC in oregano accessions, a high variability was observed. According to the results, total polyphenols contents varied from 20.3 to 35.5 mg GAE g −1 DW. The highest and lowest TPC were observed in Mazandaran (subsp. virens ) and Baneh (subsp. gracile ) accessions, respectively. This creates a variance of roughly 44% between the lowest and highest concentration levels of TPC. However, there was no significant difference among the Ardabil, Arasbaran, Gilan, Namin and Kaleybar accessions in terms of TPC (Fig. 1 ). According to the ANOVA results, TFC were largely variable among the investigated accessions ( p < 0.01). Overall, Ardabil and Arasbaran accessions had the highest and lowest TFC (22.2 and 7.7 mg QE g −1 DW, respectively) (Fig. 1 ). This creates about a three-fold variation among the mentioned accessions.
Phenolic constituents
To identify the phenolic components in different oregano accessions, RP-HPLC analysis was performed. In total, sixteen major phenolic metabolites, including ten phenolic acids (rosmarinic, chlorogenic, cinnamic, caffeic, syringic, benzoic, vanillic, gallic, chicoric, and 2,4-dihydroxybenzoic acids), five flavonoids (quercetin, apigenin, luteolin, naringenin, kaempferol) and coumarin were identified. Table 2 illustrates the phenolic components of the oregano plants. Among the investigated phenolics, rosmarinic acid was identified as the major component in all extracts. According to the ANOVA results, significant differences ( p < 0.01) were found among different oregano accessions and subspecies in terms of rosmarinic acid content. The maximum amount of rosmarinic acid (1646.9 ± 29.3 mg/100 g DW) was obtained from Ardebil accession ( O. vulgare subsp. virence ), while the minimum content (659.6 ± 66.6 mg/100 g DW) was found in Baneh population ( O. vulgare subsp. gracile ). Namin (1606.7 ± 120.0 mg/100 g DW), Arasbaran, (1484.2 ± 200.1 mg/100 g DW) and Kaleybar (1164.8 ± 28.6 mg/100 g DW) accessions also had a high amount of rosmarinic acid. Luteolin was the second major phenolic compound of oregano. The highest (345.4 ± 56.1 mg/100 g DW) and lowest (77 ± 42.1 mg/100 g DW) amount of luteolin were observed in Namin and Mazandaran accessions, respectively. Chicoric acid (ranging from 36 ± 3 to 212 ± 1.53 mg/100 g DW), coumarin (ranging from 65 ± 3 to 193.9 ± 8.9 mg/100 g DW), quercetin (ranging from 10 ± 3.9 to 106 ± 15.1 mg/100 g DW) and vanillic acid (ranging from 21.6 ± 8.4 to 89.7 ± 3.1 mg/100 g DW) were the other dominant phenolics detected in all accessions. The other phenolic components were recorded in lower amounts in all oregano plants.
Antioxidant properties
Figure 1 shows the free radical-scavenging activities of the seven oregano accessions which were assessed using the DPPH method. According to the obtained results, there was not a high difference among the oregano accessions in terms of DPPH antioxidant activity. However, the maximum antioxidant capacity belonged to Baneh population (IC 50 = 91.3 µg mL −1 ). It is notable to mention that the IC 50 content (50% reduction in DPPH concentration) for ascorbic acid as the positive control was 9.95 µg mL −1 . On the other hand, no significant difference was found among the oregano accessions in terms of DPPH antioxidant capacity. According to the mean compression, the FRAP activity of oregano accessions ranged from 347.7 to 493.6 (µmol g −1 DW). Overall, Gilan accession (subsp. virens ), with 493.6 µmol g −1 DW, showed the highest FRAP antioxidant activity; however, there were no statistically significant differences between other investigated accessions (Fig. 1 ). The FRAP activity of ascorbic acid as the positive control was 1946.6 µmol g −1 .
Multivariate analysis of phenolic antioxidants
Cluster analysis (CA) and principal components analysis (PCA) were performed using a data matrix composed of 133 data points (19 variables × 7 observations) to display the grouping among oregano accessions based on their phenolic components and antioxidant attributes (Fig. 2 ). The first two components explained 69.03% of the total variance. According to the CA and PCA analyses, the mentioned accessions were classified into three different groups (Fig. 2 ). The first cluster was composed of three accessions (namely, Mazandaran (subsp. virens ), Gilan (subsp. virens ) and Baneh (subsp. gracile ) including the lowest rosmarinic acid and luteolin content, and DPPH radical scavenging activity, and higher amounts of apigenin. Furthermore, Kaleybar accession is in close angle with kaempferol and placed in a distinct group (Fig. 2 ). However, Arasbaran (subsp. vulgare ), Ardabil (subsp. virens ) and Namin (subsp. virens ) accessions possessed the highest rosmarinic acid, luteolin, vanillic acid, chicoric acid, coumarin and DPPH radical scavenging activity and were placed in the same group. Simple correlation analysis showed a significant relationship between the TFC and TPC content (r = 0.58, p < 0.01), as well as to chicoric acid (r = 0.73, p < 0.01). Furthermore, correlation analysis revealed that rosmarinic acid content is moderately correlated with DPPH antioxidant activity (r = 0.46, p < 0.05). Moreover, there were negative correlations between vanillic acid and caffeic acid content (r = −0.92, p < 0.01), and syringic acid (r = −0.93, p < 0.01). Statistical analysis also revealed moderate and significant relationships between the chlorogenic acid and caffeic acid (r = 0.67), syringic acid and benzoic acid (r = 0.70), syringic acid and naringenin (r = 0.67) (Table 3 ).
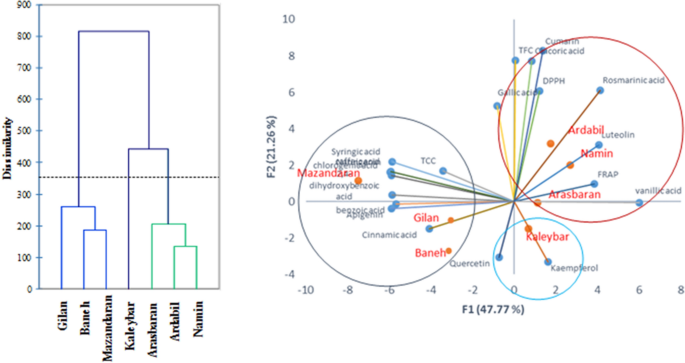
Agglomerative hierarchical clustering and principal component analysis (PCA) classification of oregano accessions based on their phenolic compounds and antioxidant properties. TPC: Total phenol content, TFC: Total flavonoids content.
O. vulgare is a popular medicinal herb, which is frequently used as antioxidant, antifungal, antimicrobial, expectorant, stimulant, carminative, anti-cancer and ant-aging agent among other applications in the food and beverage industries 7 , 9 . Natural antioxidants isolated from oregano extracts have been found to have many health effects such as mental well-being and decreasing postsurgical wounds infection 6 , 9 . The quality of oregano plants based on antioxidant efficacy is significantly influenced by subspecies and geographical origin. Such variation could be due to the plant genetics and environmental conditions, such as light intensity, soil conditions and water availability 20 . The results of the present work showed a high variation in drug yield of Iranian oregano accessions. Concerning the planting in the same controlled environmental and growing conditions, such differences in drug yield may be related to the genetic factors of oregano subspecies 15 . Genetic breeding in oregano subspecies can potentially aid in achieving superior agronomic properties, higher yields and desirable cultivars rich in phenolic compositions 5 , 11 . Therefore, accessions with higher biomass yield can be considered for higher production of secondary metabolites in breeding programs.
O. vulgare is a rich source of phenolic acids and flavonoids with a strong antioxidant activity, which qualify the plant as a potential functional food and medicine 10 , 11 , 21 . Polyphenols can fluctuate broadly in their arrangement and overall classification; however, all of them share the common structure of comprising a minimum of one aromatic ring and one or more hydroxyl groups 22 . As a matter of fact, phenolic compounds are considered as important ingredients of plants, fruits and vegetables 23 . Here, a high variation in TPC and TFC was observed among the investigated accessions belonging to three different subspecies. The highest TPC was found in Mazandaran (subsp. virens ) accession, which was about 42% higher than that of Baneh (subsp. gracile ) accession. Regarding flavonoids, the highest concentration was obtained from Ardabil (subsp. virens ), while the lowest was seen in Arasbaran (subsp. vulgare ) accession. Among these accessions, the highest TFC was about three times higher than the lowest one. In agreement with the present study, a comparative study on European oregano demonstrated that there is a high variation among the oregano accessions in terms of TPC and antioxidant activity 11 . Compared with our study, Dambolena et al. (2010) 24 found a smaller variation in the TPC of oregano subspecies; however, they reported a range of 17 to 19.4 mg GAE/g DW for TPC that was in agreement with our findings. Yan et al. (2016) 11 reported a higher amount of TPC (ranging from 93 to 135 mg GAE/g DW) in European oregano subspecies; however, the TPC in our subspecies is comparable to those reported by Pizzale et al. (2002) 25 and Capecka et al. (2005) 26 . These disparate results may imply that several factors, such as extraction methods, genetic controls, environmental parameters, growing and post-harvesting conditions as well as gene expression patterns may affect plant metabolism 27 .
Oregano contains different classes of polyphenolic compounds with multiple biological effects, such as antioxidant activity 28 . In the present study, a high antioxidant activity was observed among the oregano accessions and was confirmed by the abundance of their polyphenol compounds. The highest DPPH scavenging activities was detected in the Namin accession; however, the highest FRAP activity was obtained in Gilan accession. Interestingly, both accessions belonged to the virens subspecies. In accordance with our results, a high variation in antioxidant activity has previously been reported for oregano accessions 11 . Consistent with our results, the study of Lamien-Meda et al. (2010) 29 showed a similar range for DPPH antioxidant activity among the 19 sage ( Salvia officinalis L.) accessions (ranging from 64 to 132 mg TE/g DW). However, the upper range in FRAP activity was observed in our study. The total and specific polyphenol compounds are the key accountable agents for the antioxidant activity of oregano species 30 . Previous studies on oregano showed a high antioxidant activity of dry leaves in olive oil as well as a high increase in oxidative stability of fried chips 9 .
Rosmarinic acid is one of the major phenolic acids which have been reported in the oregano plant 31 . This component is also found in other species of the Lamiaceae family, such as thyme ( Thymus daenensis and Thymus vulgaris ) 32 , basil ( Ocimum spp.) 23 , mint ( Mentha spp.) 33 and sage ( Salvia officinalis ) (Lamien-Meda et al., 2010). In the current study, a high variation in rosmarinic acid content was found among the oregano subspecies (ranged from 659.6 to 1646.9 mg/100 g DW). An approximately similar variation (ranging from 7.2 to 41.3 mg/g DW) was previously reported in oregano accessions by Yan et al. (2016) 11 . Interestingly, based on a previous study 34 , the average content of rosmarinic acid for subspecies vulgare was 18.2 mg/g DW which was in good agreement with our results. This variation in rosmarinic acid content provides valuable data for oregano breeders for achieving high quality plants with high antioxidant capacity for food and medicinal applications. Results also demonstrate that the main bioactive component of accession with the lowermost to the uppermost concentration varied among the oregano accessions, for instance about 6.6-fold for luteolin, tenfold for quercetin, threefold for coumarin and 5.8-fold for chicoric acid (Table 2 ). Our findings agree with those of others, specifying that oregano species were reported to be rich sources of phenolic acids such as rosmarinic acid 11 . In accordance with our results on phenolics, literature review illustrates the existence of diverse phenolic constituents in the Oregano species, such as rosmarinic acid, hydroxycinnamic acid, apigenin, luteolin, quercetin, scutellarein, apigenin-7-O-glucoside, luteolin-7-O-glucoside, and luteolin-7-O-glucuronide 11 , 18 , 35 . The composition of phenolic compounds in oregano depends on the accession, subspecies, genotype, and geographical and environmental conditions. It has been shown that the flavonoid and phenolic compounds of oregano can be used to differentiate between oregano chemotypes within the same species. For instance, the concentration of rosmarinic acid has been reported to vary between the chemotype within the species of O. vulgare subp. hirtum , O. vulgare subsp. vulgare and O. syriacum 34 .
Correlation analysis showed some relations among phenolic compounds and antioxidant attributes. However, there was not a significant correlation among the TFC and antioxidant activity. The results demonstrated a moderate correlation among DPPH antioxidant activity and rosmarinic acid, but no significant correlation was observed among FRAP activity and rosmarinic acid content. Similar to our results, Yan et al. (2016) 11 reported that there is no correlation between the antioxidant capacity measured by oxygen radical absorbance capacity and the concentration of rosmarinic acid. It can be specified that other phenolics and constituents can also be considered as antioxidant agents. In another study, the authors ascribed the antioxidant capacity of the similar fairly extract to the amount of rosmarinic acid and other bioactive polyphenol constituents 36 .
Oregano is a widely used medicinal herb due to its special phenolic compounds, aroma transfer and medicinal properties. In this study, the genetic variation in phenolic components and antioxidant activity of Iranian oregano accessions was investigated. A high variation was observed between the highest and lowest quantity of concentration of rosmarinic acid and other phenolics. These results might be utilized for breeding oregano plants containing high amount of phenolic components and exhibiting high antioxidant activity. Upgraded species and accessions with great polyphenolic compounds and small progressive duration is of high interest to plant producers. The extracts of herbs as well as other plant materials are gradually gaining attention in numerous industrial applications based on natural products. As a result, identification of different accessions which are richest in special components is very important for food and pharmaceutical industries. Finally, the survey of our study offers a broad-ranging variability among the Iranian oregano populations for utilizing elite accessions and subspecies (especially Ardabil and Namin accessions belonging to virens subspecies) based on phenolic components and antioxidant attributes for breeding programs and domestication purposes.
Peter, K. V. Handbook of Herbs and Spices (Elsevier, 2012).
Book Google Scholar
Bouyahya, A. et al. Anti-inflammatory and analgesic properties of Moroccan medicinal plants: Phytochemistry, in vitro and in vivo investigations, mechanism insights, clinical evidences and perspectives. J. Pharm. Anal. 12 , 35–57 (2021).
Article Google Scholar
Azimychetabi, Z., Nodehi, M. S., Moghadam, T. K. & Motesharezadeh, B. Cadmium stress alters the essential oil composition and the expression of genes involved in their synthesis in peppermint ( Mentha piperita L.). Ind. Crops Prod. 168 , 113602 (2021).
Article CAS Google Scholar
Gök, H. N. et al. Profiling the annual change of the neurobiological and antioxidant effects of five Origanum species in correlation with their phytochemical composition. Food Chem. 368 , 130775 (2022).
Morshedloo, M. R. et al. Effect of prolonged water stress on essential oil content, compositions and gene expression patterns of mono-and sesquiterpene synthesis in two oregano ( Origanum vulgare L.) subspecies. Plant Physiol. Biochem. 111 , 119–128 (2017).
Morshedloo, M. R., Pirali Hamedani, M. & Yazdani, D. An over review to Origanum vulgare L. and its pharmacological properties. J. Med. Plants 17 , 15–31 (2018).
Google Scholar
Emrahi, R., Morshedloo, M. R., Ahmadi, H., Javanmard, A. & Maggi, F. Intraspecific divergence in phytochemical characteristics and drought tolerance of two carvacrol-rich Origanum vulgare subspecies: subsp. hirtum and subsp. gracile . Ind. Crops Prod. 168 , 1135 (2021).
Chou, T.-H., Ding, H.-Y., Chan, L.-P., Liang, J.-Y. & Liang, C.-H. Novel phenolic glucoside, origanoside, protects against oxidative damage and modulates antioxidant enzyme activity. Food Res. Int. 44 , 1496–1503 (2011).
Charles, D. J. Antioxidant Properties of Spices, Herbs and Other Sources. (Springer, 2012).
Leyva-López, N., Nair, V., Bang, W. Y., Cisneros-Zevallos, L. & Heredia, J. B. Protective role of terpenes and polyphenols from three species of Oregano ( Lippia graveolens , Lippia palmeri and Hedeoma patens ) on the suppression of lipopolysaccharide-induced inflammation in RAW 2647 macrophage cells. J. Ethnopharmacol. 187 , 302–312 (2016).
Yan, F. et al. Antioxidant capacity variation in the oregano ( Origanum vulgare L.) collection of the German National Genebank. Ind. Crops Prod. 92 , 19–25 (2016).
Liang, C.-H. et al. Free radical scavenging activity of 4-(3, 4-dihydroxybenzoyloxymethyl) phenyl-O-β-D-glucopyranoside from Origanum vulgare and its protection against oxidative damage. J. Agric. Food Chem. 60 , 7690–7696 (2012).
Saxena, M., Saxena, J. & Pradhan, A. Flavonoids and phenolic acids as antioxidants in plants and human health. Int. J. Pharm. Sci. Rev. Res. 16 , 130–134 (2012).
CAS Google Scholar
Shukla, S. & Gupta, S. Bioactive Foods in Promoting Health 663–689 (Elsevier, 2010).
Morshedloo, M. R., Salami, S. A., Nazeri, V., Maggi, F. & Craker, L. Essential oil profile of oregano ( Origanum vulgare L.) populations grown under similar soil and climate conditions. Ind. Crops Prod. 119 , 183–190 (2018).
Samuelsson, G. & Bohlin, L. Drugs of Natural Origin: A Treatise of Pharmacognosy (CRC Press Inc., 2017).
Ahmadi, H., Babalar, M., Sarcheshmeh, M. A. A., Morshedloo, M. R. & Shokrpour, M. Effects of exogenous application of citrulline on prolonged water stress damages in hyssop ( Hyssopus officinalis L.): Antioxidant activity, biochemical indices, and essential oils profile. Food Chem. 333 , 127433 (2020).
Gutiérrez-Grijalva, E. P. et al. Flavonoids and phenolic acids from oregano: Occurrence, biological activity and health benefits. Plants 7 , 2 (2018).
Benzie, I. F. & Strain, J. J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 239 , 70–76 (1996).
Morshedloo, M. R., Moghadam, M. R. F., Ebadi, A. & Yazdani, D. Genetic relationships of Iranian Hypericum perforatum L. wild populations as evaluated by ISSR markers. Plant Systemat. Evol. 301 , 657–665 (2015).
Oniga, I. et al. Origanum vulgare ssp. vulgare: Chemical composition and biological studies. Molecules 23 , 2077 (2018).
Singla, R. K. et al. Natural polyphenols: Chemical classification, definition of classes, subcategories, and structures. J. AOAC International , 102 (5), 1397–1400 (2019).
Moghaddam, M. & Mehdizadeh, L. Variability of total phenolic, flavonoid and rosmarinic acid content among Iranian basil accessions. LWT-Food Sci. Technol. 63 , 535–540 (2015).
Dambolena, J. S. et al. Total phenolic content, radical scavenging properties, and essential oil composition of Origanum species from different populations. J. Agric. Food Chem. 58 , 1115–1120 (2010).
Pizzale, L., Bortolomeazzi, R., Vichi, S., Überegger, E. & Conte, L. S. Antioxidant activity of sage ( Salvia officinalis and S. fruticosa ) and oregano ( Origanum onites and O. indercedens ) extracts related to their phenolic compound content. J. Sci. Food Agric. 82 , 1645–1651 (2002).
Capecka, E., Mareczek, A. & Leja, M. Antioxidant activity of fresh and dry herbs of some Lamiaceae species. Food Chem. 93 , 223–226 (2005).
Fereidoonfar, H., Salehi-Arjmand, H., Khadivi, A., Akramian, M. & Safdari, L. Chemical variation and antioxidant capacity of sumac ( Rhus coriaria L.). Ind. Crops Prod. 139 , 111518 (2019).
Žitek, T., Borjan, D., Golle, A., Knez, Ž & Knez, M. Optimization of extraction of phenolic compounds with antimicrobial properties from Origanum vulgare . Processes 9 , 1032 (2021).
Lamien-Meda, A. et al. Investigation of antioxidant and rosmarinic acid variation in the sage collection of the genebank in Gatersleben. J. Agric. Food Chem. 58 , 3813–3819 (2010).
Alirezalu, A. et al. Physicochemical characterization, antioxidant activity, and phenolic compounds of hawthorn ( Crataegus spp.) fruits species for potential use in food applications. Foods 9 , 436 (2020).
Kosakowska, O. et al. Antioxidant and antibacterial activity of essential oils and hydroethanolic extracts of Greek oregano ( O. vulgare L. subsp. hirtum (Link) Ietswaart) and common oregano ( O. vulgare L. subsp. vulgare ). Molecules 26 , 988 (2021).
Bistgani, Z. E. et al. Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crops Prod. 135 , 311–320 (2019).
Petersen, M. & Simmonds, M. S. Rosmarinic acid. Phytochemistry 62 , 121–125 (2003).
Shen, D. et al. LC-MS method for the simultaneous quantitation of the anti-inflammatory constituents in oregano ( Origanum species). J. Agric. Food Chem. 58 , 7119–7125 (2010).
Rothwell, J. A. et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013 , bat070 (2013).
Gonçalves, S. et al. Phenolic profile, antioxidant activity and enzyme inhibitory activities of extracts from aromatic plants used in Mediterranean diet. J. Food Sci. Technol. 54 , 219–227 (2017).
Download references
Author information
Authors and affiliations.
Department of Horticultural Sciences, Faculty of Agriculture, University of Maragheh, Maragheh, Iran
Ghazaleh Jafari Khorsand & Mohammad Reza Morshedloo
Department of Horticultural Sciences, Faculty of Agriculture, Lorestan University, Khorramabad, Iran
Hasan Mumivand
Agricultural Research Education and Extension Organization (AREEO), Isfahan Agricultural and Natural Resources Research and Education Center, Isfahan, Iran
Zohreh Emami Bistgani
School of Pharmacy, Chemistry Interdisciplinary Project (ChIP), University of Camerino, Camerino, Italy
Filippo Maggi
Department of Mathematics and Statistics, University of Massachusetts Amherst, Amherst, USA
Abdolvahab Khademi
You can also search for this author in PubMed Google Scholar
Contributions
G.J.K: Investigation, Data analysis, Resources, Writing—review & editing. M.R.M.: Conceptualization, Supervision, Methodology, Funding acquisition, Original draft. H.M.: Data curation, Supervision, Visualization, Review & editing. Z.E.B.: Methodology, Writing—review & editing. A.K.: Investigation, Statistical analysis, Review & editing. F.M.: Formal analysis, Methodology, Supervision, Writing—review & editing.
Corresponding author
Correspondence to Mohammad Reza Morshedloo .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Jafari Khorsand, G., Morshedloo, M.R., Mumivand, H. et al. Natural diversity in phenolic components and antioxidant properties of oregano ( Origanum vulgare L.) accessions, grown under the same conditions. Sci Rep 12 , 5813 (2022). https://doi.org/10.1038/s41598-022-09742-4
Download citation
Received : 19 October 2021
Accepted : 29 March 2022
Published : 06 April 2022
DOI : https://doi.org/10.1038/s41598-022-09742-4

Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Image analysis-based discoloration rate quantification and kinetic modeling for shelf-life prediction in herb-coated pear slices.
- Prasad Rasane
- Essam A. Ali
Scientific Reports (2024)
Impacts of Preharvest Treatment with Salicylic Acid and Melatonin in Suppressing Gray Mold (Botrytis cinerea Pers.) in Bell Pepper
- Saideh Nasiri
- Mehdi Rezaei
- Shideh Mojerlou
Journal of Crop Health (2024)
A Comprehensive Review of Essential Oils and Their Pharmacological Activities in Neurological Disorders: Exploring Neuroprotective Potential
- Mohammad Qneibi
- Sosana Bdir
- Mira Hallak
Neurochemical Research (2024)
Diuretic and Neurotropic Activity of Oreganol A, a Component of Oregano
- V. A. Kurkin
- E. N. Zaitceva
- A. V. Dubishchev
Pharmaceutical Chemistry Journal (2023)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

Our Research
Msi funded paper: potential health benefits of oregano, singletary, k.

August 2010-- An overview of the potential health benefits of oregano, funded by the McCormick Science Institute. An evaluation of the research is presented. Read the entire study (PDF)
Oregano is an herb that has been cultivated for centuries in the Mediterranean area, although it now can be found on most continents. Actually, there is not simply one ‘‘oregano,’’ but rather several species that may contribute to the oregano used for culinary purposes. Origanum vulgare (also referred to as Spanish thyme and wild marjoram), a member of the plant family Lamiaceae, is generally the spice variety sold as oregano in Europe and the United States.
Medicinal uses for oregano date back to the ancient Greek and Roman empires where applications of the leaves were used to treat such maladies as skin sores and relieve aching muscles and as an antiseptic. Oregano also has been used in traditional medicines for such ailments as asthma, cramping, diarrhea, and indigestion. In Greece, an oregano infusion is still used as a folk remedy against colds and upset stomach and to maintain general health. Based on the current scientific literature, oregano extracts and individual constituents consistently have demonstrated antimicrobial actions in vitro toward food-borne pathogens, although the capacity to counter human infections is not well studied.
Oregano contains several potent antioxidants that may contribute to the findings in preliminary studies that oregano exhibits benefits toward the cardiovascular and nervous systems, relieves symptoms of inflammation, and modulates blood sugar and lipids. Well-controlled human studies substantiating these health effects are lacking.
Scientific Overviews
December, 2022
September, 2022
April, 2022
January, 2022
August, 2021
November, 2020
February, 2020
January, 2019
April, 2018
April, 2017
March, 2016
January, 2016
August, 2014
August, 2011
February, 2011
September, 2010
February, 2010
December, 2008
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Effects of oregano on performance and immunmodulating factors in weaned piglets
Affiliation.
- 1 a Institute of Animal Nutrition , Friedrich-Loeffler-Institute (FLI), Federal Research Institute for Animal Health , Braunschweig , Germany.
- PMID: 24228909
- DOI: 10.1080/1745039X.2013.858897
Many health effects can be attributed to the Mediterranean herb oregano (Origanum vulgare L.) and several studies demonstrated the improving effect on performance, changes in blood count, antibacterial, antifungal and immunmodulating abilities. The majority of these investigations were carried out with processed essential oil, while whole plant material was only used in a few studies. Thus, the aim of the present experiment was to test the effect of increasing proportions of dried oregano in piglet feed on health and performance, with a special focus on immune modulation. A total of 80 male castrated weaned piglets (body weight [BW] 7.9 kg ± 1.0 kg) were used in a feeding experiment lasting 5 weeks. They were assigned to 4 experimental groups: a control diet, and three diets with an oregano supplementation at 2 g, 4 g and 8 g per kg feed, respectively, corresponding to 23.5 mg, 46.9 mg and 93.9 mg carvacrol/kg DM. After 3 weeks, half of each group was challenged with 5 µg lipopolysaccharides (LPS) per kg BW. Blood samples were collected 2 h after LPS stimulation and analysed for T-cell phenotypes, granulocyte activity, clinical-chemistry as well as white and red blood count. The results indicate no effects of oregano on performance. In contrast, oregano altered the lymphocyte proportion and the ratio of CD4(+) and CD8(+) T-cells as well as the triglyceride concentration in the serum of non-stimulated and in LPS-stimulated piglets. In conclusion, whole plant supplementation of oregano to piglet feed altered immune-related parameters, but did not modulate the acute inflammatory response induced by LPS stimulation.
PubMed Disclaimer
Similar articles
- Effect of the dietary oregano (Origanum vulgare) on Cu and Zn balance in weaned piglets. Untea A, Criste R, Panaite T, Costache I. Untea A, et al. J Trace Elem Med Biol. 2011 Jan;25 Suppl 1:S35-40. doi: 10.1016/j.jtemb.2010.10.011. Epub 2010 Dec 17. J Trace Elem Med Biol. 2011. PMID: 21168317
- Effect of a post-weaning diet supplemented with functional feed additives on ileal transcriptome activity and serum cytokines in piglets challenged with lipopolysaccharide. Bissonnette N, Jiang XR, Matte JJ, Guay F, Talbot G, Bontempo V, Gong J, Wang Q, Lessard M. Bissonnette N, et al. Vet Immunol Immunopathol. 2016 Dec;182:136-149. doi: 10.1016/j.vetimm.2016.10.004. Epub 2016 Oct 17. Vet Immunol Immunopathol. 2016. PMID: 27863544
- Effect of dietary dried oregano leaves on growth performance, carcase characteristics and serum cholesterol of female early maturing turkeys. Bampidis VA, Christodoulou V, Florou-Paneri P, Christaki E, Chatzopoulou PS, Tsiligianni T, Spais AB. Bampidis VA, et al. Br Poult Sci. 2005 Oct;46(5):595-601. doi: 10.1080/00071660500256057. Br Poult Sci. 2005. PMID: 16359114 Clinical Trial.
- Effects of dietary nucleotide supplementation on growth performance and hormonal and immune responses of piglets. Superchi P, Saleri R, Borghetti P, De Angelis E, Ferrari L, Cavalli V, Amicucci P, Ossiprandi MC, Sabbioni A. Superchi P, et al. Animal. 2012 Jun;6(6):902-8. doi: 10.1017/S1751731111002473. Animal. 2012. PMID: 22558960 Clinical Trial.
- The effects of superoxide dismutase-rich melon pulp concentrate on inflammation, antioxidant status and growth performance of challenged post-weaning piglets. Ahasan ASML, Invernizzi G, Farina G, Pilotto A, Barbé F, Bontempo V, Rossi R, Bellagamba F, Lecchi C, Savoini G, Agazzi A. Ahasan ASML, et al. Animal. 2019 Jan;13(1):136-143. doi: 10.1017/S1751731118001234. Epub 2018 Jun 18. Animal. 2019. PMID: 29909802
- Influence of Different Plant Extracts on CYP-Mediated Skatole and Indole Degradation in Pigs. Marro P, Wesoly R, Stefanski V. Marro P, et al. Animals (Basel). 2024 Mar 13;14(6):888. doi: 10.3390/ani14060888. Animals (Basel). 2024. PMID: 38539986 Free PMC article.
- Evaluation of oral supplementation of free and nanoencapsulated Minthostachys verticillata essential oil on immunological, biochemical and antioxidants parameters and gut microbiota in weaned piglets. Montironi ID, Arsaute S, Roma DA, Cecchini ME, Pinotti A, Mañas F, Bessone FA, de Moreno de LeBlanc A, Alustiza FE, Bellingeri RV, Cariddi LN. Montironi ID, et al. Vet Res Commun. 2024 Jun;48(3):1641-1658. doi: 10.1007/s11259-024-10347-7. Epub 2024 Mar 8. Vet Res Commun. 2024. PMID: 38453821
- Alternatives to antibiotics in pig production: looking through the lens of immunophysiology. Liu HY, Zhu C, Zhu M, Yuan L, Li S, Gu F, Hu P, Chen S, Cai D. Liu HY, et al. Stress Biol. 2024 Jan 2;4(1):1. doi: 10.1007/s44154-023-00134-w. Stress Biol. 2024. PMID: 38163818 Free PMC article. Review.
- Pharmacological Applications and Action Mechanisms of Phytochemicals as Alternatives to Antibiotics in Pig Production. Li L, Sun X, Zhao D, Dai H. Li L, et al. Front Immunol. 2021 Dec 9;12:798553. doi: 10.3389/fimmu.2021.798553. eCollection 2021. Front Immunol. 2021. PMID: 34956234 Free PMC article. Review.
- Encapsulated Mixture of Methyl Salicylate and Tributyrin Modulates Intestinal Microbiota and Improves Growth Performance of Weaned Piglets. Wei Y, Mao J, Liu J, Zhang Y, Deng Z, Lv J, He M, Liu J, Wang H. Wei Y, et al. Microorganisms. 2021 Jun 21;9(6):1342. doi: 10.3390/microorganisms9061342. Microorganisms. 2021. PMID: 34205785 Free PMC article.
- Search in MeSH
LinkOut - more resources
Full text sources.
- Taylor & Francis
Other Literature Sources
- scite Smart Citations
Research Materials
- NCI CPTC Antibody Characterization Program
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Microbiol
Bactericidal Property of Oregano Oil Against Multidrug-Resistant Clinical Isolates
1 Wellman Center for Photomedicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States
Tianhong Dai
Clinton k. murray.
2 First Area Medical Laboratory, JBSA-Fort Sam Houston, Houston, TX, United States
Development of non-antibiotic alternatives to treat infections caused by multidrug-resistant (MDR) microbes represents one of the top priorities in healthcare and community settings, especially in the care of combat trauma-associated wound infections. Here, we investigate efficacy of oregano oil against pathogenic bacteria including MDR isolates from the combat casualties in vitro and in a mouse burn model. Oregano oil showed a significant anti-bacterial activity against 11 MDR clinical isolates including four Acinetobacter baumannii , three Pseudomonas aeruginosa , and four methicillin-resistant Staphylococcus aureus (MRSA) obtained from combat casualties and two luminescent strains of PA01 and MRSA USA300, with a MIC ranging from 0.08 mg/ml to 0.64 mg/ml. Oregano oil also effectively eradicated biofilms formed by each of the 13 pathogens above at similar MICs. Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) revealed that oregano oil damaged bacterial cells and altered the morphology of their biofilms. While efficiently inactivating bacteria, there was no evidence of resistance development after up to 20 consecutive passages of representative bacterial strains in the presence of sublethal doses of oregano oil. In vivo study using the third-degree burn wounds infected with PA01 or USA300 demonstrated that oregano oil, topically applied 24 h after bacterial inoculation, sufficiently reduced the bacterial load in the wounds by 3 log 10 in 1 h, as measured by drastic reduction of bacterial bioluminescence. This bactericidal activity of oregano oil concurred with no significant side effect on the skin histologically or genotoxicity after three topical applications of oregano oil at 10 mg/ml for three consecutive days. The investigation suggests potentials of oregano oil as an alternative to antibiotics for the treatment of wound-associated infections regardless of antibiotic susceptibility.
Introduction
Skin wound infection is a widespread problem in both civilian and military healthcare settings. Skin wounds are particularly prone to bacterial infections because the wounds provide an ideal medium for bacterial proliferation and a portal of entry into the bloodstream and are direct exposure to the “dirty” environment. The infections can be readily treated with a variety of antibiotics if the bacteria involved are susceptible. However, there are only extremely limited or no treatment options when antibiotic resistant strains are involved in the wound infections, which occurs at a worrying speed. If the wound infection cannot be eliminated in a timely fashion, the infection would alter cellular metabolisms and induce persistent inflammation systemically that can predispose the patients to various complications and life-threatening sepsis ( Fitzwater et al., 2003 ; Wang et al., 2018 ). For instance, burn wound infection outbreaks caused by multidrug-resistant (MDR) organisms emerged as a serious problem early in the course of Iraq military operations despite that the United States military has provided rapid and highly effective care for wounded soldiers ( Scott et al., 2007 ; Vento et al., 2013 ). As a matter of fact, skin infections caused by MDR bacteria are the most common cause of morbidity and mortality in patients infected with MDR microbes and represent almost 61% of deaths of this infected population ( Gomez et al., 2009 ).
Extensive uses of broad spectrum antibiotics are the single most important factor in evolution of bacterial resistance ( Hampton, 2013 ). A number of studies have shown that the most frequently identified MDR strains of bacteria in nosocomial infections and on the battlefield are Gram-negative bacteria Acinetobacter baumannii and Pseudomonas aeruginosa , and Gram-positive bacterium methicillin-resistant Staphylococcus aureus (MRSA) ( Scott et al., 2007 ; Calhoun et al., 2008 ; Li et al., 2014 ; Levin-Reisman et al., 2017 ). In addition, bacterial biofilms formed by MDR bacteria are the major obstacles in treatment of burn wounds ( Bloemsma et al., 2008 ; Jiang et al., 2017 ). Bacteria within biofilms can be as much as 1,000 times more resistant to antibiotics and are responsible for recurrent antibiotic-resistant infections elsewhere in the body upon dissemination from the site of the biofilm ( Ceri et al., 1999 ; Caraher et al., 2007 ). Currently, the only effective treatments available to fight these infections are older drugs like colistin, which are highly toxic and detrimental to the overall health of the patients ( Crane et al., 2009 ). There is a pressing need for the development of non-antibiotic approaches to combat MDR microbes.
Essential oils (EOs) are a mixture of volatile constituents produced by aromatic plant/herbs. There are about 3,000 well-recognized EOs, of which 300 are generally recognized as safe (GRAS) to humans by the United States Food and Drug Administration (U.S. FDA) and have broad applications in food preservation, additives, and favors, perfume, cosmetic industries, antiseptic oral solutions, toothpastes, cleaner, and air fresheners for centuries ( Pandey et al., 2017 ; Sakkas and Papadopoulou, 2017 ). These natural products are of particular interest as “green” antimicrobial agents because of their low-cost, biocompatibility, potential antibiofilm properties, and friendly to eukaryote cells and environment ( Burt, 2004 ; Nostro et al., 2007 ; Kavanaugh and Ribbeck, 2012 ). Among these safe EOs, oregano oil has been shown to have a variety of activities such as antioxidant ( Yan et al., 2016 ), anti-inflammatory ( Ocana-Fuentes et al., 2010 ; Shen et al., 2010 ), anti-fungal ( Akgul and Kivanc, 1988 ; Soylu et al., 2007 ), and anti-allergic ( Benito et al., 1996 ). Its antimicrobial effect has been demonstrated in vitro cell culture, food systems studies ( Lopez-Reyes et al., 2010 ; Soylu et al., 2010 ; Munhuweyi et al., 2017 ), and in vivo systemic infections ( Manohar et al., 2001 ; Preuss et al., 2005 ). In the present study, we investigate effectiveness of oregano oil in inactivation of MDR bacteria isolated from combat casualties in vitro and bioluminescent strains of P. aeruginosa (PA01) and MRSA (USA300) in mouse burn wounds. Our study showed that oregano oil effectively inactivated various pathogenic bacteria and their biofilms irrespective of their antibiotic susceptibility. The study is the first in vivo attempt on the use of oregano oil for the treatment of burn wounds infected with clinically important MDR bacteria.
Materials and Methods
Chemical constituents of oregano oil.
Oregano oil was purchased from Bulk Apothecary (Aurora, OH, United States) and used throughout the study. To define the constituents of oregano oil, gas chromatography/mass spectrometry (GC-MS) analyses were carried out using an Agilent 6980 GC coupled to an Agilent 5973N MS and a fused-silica capillary column (HP-5MS: 30 m × 0.25 mm i.d., film thickness 0.25 μm). The initial column temperature was set at 60°C for 10 min and then increased at 3°C/min till 220°C. The temperature was held at 220°C for 10 min and raised to 240°C by increments of 1°C/min; the injector port temperature was 250°C with the carrier gas of helium at a flow rate of 0.8 ml/min. Ionization voltage of MS in the EI-mode was 70 eV and ionization source temperature at 250°C with a mass range of 35–465 amu. The volatile components were identified by comparison of their retention indices relative to n -alkanes (C6-C28) and mass spectra with those of authentic compounds by means of NIST and Wiley databases and with the Adams library spectra.
Bacterial Strains
The antibacterial activity of oregano oil was tested against a panel of MDR bacteria isolated from combat casualties, including seven Gram-negative strains A. baumannii (AF0004, AF0005, IQ0012, and IQ0013) and P. aeruginosa (AF0001, IQ0042, and IQ0046), and four Gram-positive MRSA (AF0003, IQ0064, IQ0103, and IQ0211). All the bacterial isolates were obtained from San Antonio Military Medical Center under a Materials Transfer Agreement and demonstrated MDR according to the microbiology tests performed at the United States Army Institute of Surgical Research ( Table Table2 2 ). Amongst the A. baumannii and P. aeruginosa tested, only strains of IQ0012, IQ0013, and IQ0042 were susceptible to imipenem; the rest of strains were resistant to all antibiotics tested. The five MRSA strains were resistant to amikacin, ampicillin, cefazolin, cefoxitin, and erythromycin, but susceptible to gentamicin, levofloxacin, nitrofurantoin, and tetracycline. In addition, luminescent strains of P. aeruginosa (PA01) and MRSA USA300 were used in the in vivo study, allowing real-time monitoring of infection in the mouse burn wounds in vivo via bioluminescence imaging ( Dai et al., 2013 ; Zhang et al., 2014 ; Wang et al., 2016 ).
MIC (mg/ml) of the oregano EO and results of antibiotic susceptibility testing for the pathogens.
| Species | Strain no. | Oregano | AMK | AMP | ATM | CFZ | FOX | CRO | CXM | CIP | ERY | GEN | IPM | LVX | MEM | NIT | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | R/S | R/S | R/S | R/S | R/S | R/S | R/S | R/S | R/S | R/S | R/S | R/S | R/S | R/S | R/S | ||
| AF0004 | 0.16 | R | R | R | R | R | R | R | R | R | R | R | R | R | R | ||
| IQ0012 | 0.08 | R | R | R | R | R | R | R | R | R | S | R | R | R | R | ||
| AF0005 | 0.16 | R | R | R | R | R | R | R | R | R | R | R | R | R | R | ||
| IQ0013 | 0.16 | R | R | R | R | R | R | R | R | R | S | R | R | R | R | ||
| AF0001 | 0.64 | R | R | R | R | R | R | R | R | R | R | R | R | R | R | ||
| IQ0042 | 0.56 | R | R | R | R | R | R | R | R | R | S | R | R | R | R | ||
| IQ0046 | 0.64 | R | R | R | R | R | R | R | R | R | R | R | R | R | R | ||
| PA01 | 0.56 | R | R | R | R | R | R | R | R | R | R | R | R | R | R | ||
| Methicillin-resistant | AF0003 | 0.16 | R | R | R | R | R | R | S | S | S | S | |||||
| IQ0064 | 0.32 | R | R | R | R | R | R | S | S | S | S | ||||||
| IQ0103 | 0.32 | R | R | R | R | R | R | S | S | S | S | ||||||
| IQ0211 | 0.16 | R | R | R | R | R | R | S | S | S | S | ||||||
| USA300 | 0.32 | R | R | R | R | R | R | S | S | S | S | ||||||
Determinations of Minimum Inhibitory Concentration (MIC)
In order to determine a MIC, a broth microdilution assay was employed as previously described ( Joshi et al., 2010 ; Gao et al., 2011 ). Stock solution of oregano oil was prepared at 40 mg/ml in DMSO and twofold dilutions (0.04–1.28 mg/ml) of the stock EO in brain heart infusion (BHI) medium were added into 96-well plates for bactericidal tests. In each well, 20 μL of the suspensions containing 10 8 CFU/ml of bacteria was added to 180 μL of the above medium containing oregano oil at varying concentrations. Medium supplemented with a similar amount of DMSO only severed as controls. The microplates were incubated at 37°C for 24 h and examined for bacterial growth. The first well without turbidity was determined as a MIC value. All assays were performed in triplicate.
Antibiofilm Activity
Bacteria were incubated in trypticase soy broth (TSB) with 0.1% glucose at 37°C for 18 h, after which the cultures were harvested by centrifugation and washed twice with PBS. Bacterial suspensions with an optical density of OD 600 equal to 0.1 in TSB were added to 96-well plates at 100 μL/well, followed by incubation at 37°C under static condition for 24 h to form biofilms. Oregano oil was added at indicated concentrations and incubated with the biofilms for 1 h, after which biofilms were washed twice with PBS and bacterial viability was determined using an Alamar Blue assay. TSB without bacteria was used as a negative control. All experiments were performed in triplicate.
Assessment of Possible Resistance Development to Oregano Oil
To study any potential development of resistance to oregano oil, three representative strains ( A. baumannii AF0005, P. aeruginosa IQ0042, and MRSA IQ0064) were propagated for 20 generations in the presence of sublethal doses of oregano oil as previously described ( Li et al., 2014 ). Briefly, 200-μL aliquots of the bacterial suspensions (10 7 CFU/ml) were inoculated into 96-well plates and exposed to the sub-MIC (2/3 MIC) of oregano oil at 37°C for 1 h, and the resultant bacteria were labeled as the first generation and tested for a MIC as above. The second generation was obtained by exposing the first generation to its sub-MIC for 1 h and determined for its MIC again. The procedure was repeated for up to 20 times. Oregano-resistance was determined by any significant increases in the MIC of successive generations.
Transmission Electron Microscopy (TEM)
To determine bactericidal mechanism of oregano oil, A. baumannii AF0005 and P. aeruginosa IQ0042 were investigated as the representative strains for oregano-induced ultrastructural damages using transmission electron microscopy (TEM). Bacterial suspensions were fixed in 2.5% glutaraldehyde plus 2% paraformaldehyde overnight at 4°C. Fixed cells were collected by centrifugation at 4,000 × g for 5 min and rinsed with 0.1 M sodium cacodylate buffer (pH 7.2) for three times. After the final wash, hot agar was added to each pellet and the cell pellets were post-fixed in 2% osmium tetroxide for 1 h, dehydrated with a graded ethanol series, embedded in fresh Epon, and then polymerized at 60°C for 48 h. Ultra-thin sections were cut on ultramicrotome and collected onto 200 mesh bare copper grids. Samples were stained for 30 min with uranyl acetate and lead citrate and examined with a CM-10 TEM (Philips, Eindhoven, Netherlands).
Scanning Electron Microscopy (SEM)
To investigate the ultrastructural changes of bacterial biofilms caused by oregano oil, scanning electron microscopy (SEM) was performed using the representative strains of P. aeruginosa IQ0042 and MRSA IQ0064. Briefly, biofilms of IQ0042 and IQ0064 were grown for 24 h on sterilized squares of ACLAR 33C (Electron Microscopy Sciences, Hatfield, PA, United States), and treated for 1 h with oregano oil at 0.75 mg/ml or 0.3 mg/ml, respectively. Untreated and oregano-treated biofilms were fixed at 4°C for 24 h in 0.1 M sodium cacodylate buffer containing 2.5% glutaraldehyde, 0.15% alcian blue, and 0.15% safranin O. The fixed biofilms were washed with 0.1 M sodium cacodylate buffer, infiltrated with 2% osmium tetroxide for 2 h, and dehydrated to 100% ethanol. The biofilms were dried using a critical-point dryer (Tousimis Research Corporation, Rockville, MD, United States), mounted on specimen stubs, sputter-coated with 10 nm Cressington 208 platinum (Cressington Scientific Instruments, Watford, United Kingdom), and examined on a S4800 SEM (Hitachi Ltd., Tokyo, Japan). Micrographs were acquired under high vacuum using an accelerating voltage of 3.0 kV.
Female BALB/c mice at 8 weeks of age and 17–19 g were purchased from Charles River Laboratories (Wilmington, MA, United States). All animal procedures were approved by the Institutional Animal Care and Used Committees (IACUC) of Massachusetts General Hospital (Protocol 2014N000009) and were in accordance with guidelines of the National Institutes of Health.
Treatment of Burn Infection in Mice by Oregano Oil
Mice were anesthetized with an intraperitoneal injection of ketamine-xylazine cocktail and shaved on the lower dorsal skin. The burn was introduced by a brass block (1 cm 2 ) heated to thermal equilibration with boiling water prior to application of its extremity onto the shaved skin for 5 s, which generated a third-degree burn wound. Sterile saline was intraperitoneally administered at 0.5 ml/mouse to support fluid balance during recovery. Aliquots of 50 μL bacterial suspensions containing 5 × 10 6 CFU in PBS were inoculated onto the burn 30 min after the injury and remained in place while the mice recovered from anesthesia. Luminescent strains of P. aeruginosa PA01 and MRSA USA300 were used as the causative pathogens in the study. At 24 h after bacterial inoculation when the biofilms were formed in the wounds, oregano oil was diluted with grape seed oil, which is commonly used for EO dilution in aromatherapy, at a final concentration of 5 or 10 mg/ml, and topically applied to the infected wounds.
Bioluminescence emission of the bacteria in the wounds was recorded in real time by a Lumina in vivo image system (IVIS) (PerkinElmer, Waltham, MA, United States). Bioluminescence images were acquired (60 s exposure, medium binning) at different time points after infection. During imaging, mice were anesthetized in chambers containing 2.0% isoflurane inhalant mixed with oxygen via an IVIS manifold placed within the imaging chamber. Bioluminescence was quantified with the Living Image software (Xenogen).
For measurement of bacterial burden, the infected burn wounds were collected after sacrifice of the mice on day 7 of bacterial inoculation. The collected tissues were homogenized in 2 ml sterile PBS. The resultant homogenate was serially diluted and spotted onto BHI agar plate containing Skirrow’s supplement (10 μg/ml vancomycin, 5 μg/ml trimethoprim lactate, and 2500 IU/L polymyxin B). The plates were then incubated at 37°C for 24 h and bacterial colonies were enumerated in a treatment-blind fashion.
Gram stain was carried out as previously described, with some modifications, to corroborate formation of PA01 biofilms in the infected wounds ( Christensen et al., 2013 ; Wang et al., 2016 ). Briefly, at 24 h after bacterial inoculation, the infected wounds were excised, fixed in 10% phosphate-buffered formalin for 2 days, and then embedded in paraffin. Tissue sections were cut at 5 μm, de-paraffined, and rehydrated, followed by staining with 0.8% crystal violet in 1% sodium bicarbonate for 1 min and then in gram’s iodine for 2 min. After decolorization with acetone/alcohol = 1:1 (v/v), the tissue sections were counterstained with 0.1% safranin O for 2 min, washed, air dried, and mounted with permount (Fisher Scientific, Waltham, MA, United States). Sections were visualized by Hamamatsu NanoZoomer 2.0 HT and the images were processed using NDP viewer software.
Toxicity of Oregano Oil to Mouse Skin in vivo
To evaluate any possible toxicity of oregano oil to the skin in vivo , mice were shaved on the low dorsal skin 24 h prior to application of oregano oil. Oregano oil at 10 mg/ml was applied topically on the shaved area once a day for three consecutive days. Mice treated with PBS served as negative controls. The mice were sacrificed 24 h after the final oregano oil application, and the skin was cross-sectioned using 8 mm biopsy punch for standard histological examination. The tissue sections stained with hematoxylin and eosin (HE) were visualized by Hamamatsu Nanozommer 2.0 HT and the images were processed using NDP viewer software.
DNA damage in oregano oil-treated skin was assessed using the DeadEnd Fluorometric TUNEL system (Promega, Madison, WI, United States), in which damage DNA undergoes end labeling with fluorophore as per the manufacturer’s instruction. Briefly, after deparaffinization and rehydration, the tissue sections were incubated with Proteinase K for 10 min to permeabilize the cells, washed, and stained with the TUNEL reaction mixture for 1 h at 37°C in a humidifies chamber. The sections were counterstained with DAPI to mark cell nuclei. Fluorescence images were captured using a FluoView FV1000-MPE confocal microscopy (Olympus Corporation, Tokyo, Japan). For the positive control, tissue sections were pre-treated with 10 unit/ml of RQ1 RNase-free DNase I for 10 min to induce DNA fragmentation before the sections were assayed by the TUNEL staining kit.
Statistical Analyses
Data are presented as means ± standard deviations (SDs). Statistical significance was assessed with two-tailed Student’s t -test between two groups or one-way ANOVA for multiple group comparison. P -values of < 0.05 were considered statistically significant. All statistical analyses were performed using GraphPad Prism 7.0 (GraphPad Software).
GC/MS Analysis of Constituents in Oregano Oil
Chemical ingredients of commercial oregano oil were identified by GC/MS analysis ( Table Table1 1 ). The twenty constituents accounted for 97.45% of the total amount of ingredients in oregano oil. The phenols carvacrol (72.25%) and thymol (6.62%), as well as the monoterpene hydrocarbons p -cymene (5.21%), γ-terpinene (4.12%), and α-pinene (1.21%) were the predominant components of oregano oil.
Chemical composition (%) of the oregano EO determined by GC-MS analyses.
| No. | Constituents | RI | RI | Peak area (%) |
|---|---|---|---|---|
| 1 | α-Thujene | 926 | 924 | 0.21 |
| 2 | α-Pinene | 939 | 932 | 1.21 |
| 3 | Camphene | 953 | 946 | 0.13 |
| 4 | 1-Octen-3-ol | 982 | 974 | 0.23 |
| 5 | Myrcene | 991 | 988 | 2.12 |
| 6 | α-Phellandrene | 1002 | 1002 | 0.62 |
| 7 | α-Terpinene | 1018 | 1014 | 0.10 |
| 8 | Cymene | 1026 | 1020 | 5.21 |
| 9 | Limonene | 1031 | 1024 | 0.56 |
| 10 | γ-Terpinene | 1060 | 1054 | 4.12 |
| 11 | Terpinolene | 1092 | 1086 | 0.10 |
| 12 | Linalool | 1100 | 1095 | 1.21 |
| 13 | Borneol | 1165 | 1165 | 0.35 |
| 14 | Terpinen-4-ol | 1178 | 1174 | 0.56 |
| 15 | Thymol | 1295 | 1289 | 6.62 |
| 16 | Carvacrol | 1315 | 1298 | 72.25 |
| 17 | Caryophyllene | 1426 | 1417 | 0.66 |
| 18 | α-Humulene | 1456 | 1452 | 0.12 |
| 19 | β-Bisabolene | 1509 | 1505 | 0.35 |
| 20 | Isocaryophyllene oxide | 1585 | 1582 | 0.72 |
| Total | 97.45 | |||
Oregano Oil Effectively Inactivated Bacteria in vitro Irrespective of Antibiotic Sensitivity
The MIC values of oregano oil against the 13 bacterial strains are shown in Table Table2 2 . Oregano oil showed a significant antibacterial activity over PBS controls against A. baumannii strains of AF0004, AF0005, IQ0012, and IQ0013 and MRSA strains of AF0003 and IQ0211, with the MICs ranging from 0.08 to 0.16 mg/ml. The MICs were significantly lower than those MICs ranging from 0.32 to 0.64 mg/ml against strains of P. aeruginosa and MRSA strains IQ0064, IQ0103, and USA300 ( Table Table1 1 ). Oregano oil also exhibited similar antibacterial activities against established biofilms (24-h-old) formed by the 13 bacterial strains within 1 h, with complete inactivation of the biofilms of A. baumannii , P. aeruginosa , and MRSA at the concentrations of 0.3, 1.0, and 0.4 mg/ml, respectively, in good agreement with the MIC values for planktonic bacterial cells ( Table Table1 1 and Figure Figure1 1 ). The results clearly suggest that oregano oil can overcome the obstacles of biofilms and kill bacteria within as sufficiently as planktonic bacteria, in contrast to antibiotics that kills bacterial biofilms poorly.

Bacterial viability of 24 h-old-biofilms formed by MDR strains of A. baumannii (A) , P. aeruginosa (B) , and MRSA (C) after 1 h treatment with oregano oil at indicated concentrations. The viability of bacterial biofilms was determined using an Alamar Blue assay. Data represent means ± SDs. ∗∗∗ p < 0.001 and ∗∗∗∗ p < 0.0001 in the presence vs. absence of oregano oil. ns, no significance.
TEM and SEM Illustrated Ultrastructural Damages of Bacteria
Transmission electron microscopy showed ultrastructural damages of A. baumannii AF0005 ( Figure Figure2B 2B ) and P. aeruginosa IQ0042 cells ( Figure Figure2D 2D ) after exposure to oregano oil for 1 h at 0.16 mg/ml and 0.56 mg/ml, respectively. The cell wall and membrane damages were apparent in A. baumannii AF0005 and P. aeruginosa IQ0042 cells with a severe leakage of intracellular substances resulting in cell membrane shrinking and separating from cell wall ( Figures 2B,D , arrows). Moreover, cytoplasmic vacuoles in A. baumannii AF0005 ( Figure Figure2B 2B , asterisk) and many stainless-vesicles in P. aeruginosa IQ0042 ( Figure Figure2D 2D , asterisk) were observed. Intracellular structural discontinuation such as dissociation between cell wall and membrane was also seen in A. baumannii AF0005 ( Figure Figure2B 2B , oval). In comparison, untreated A. baumannii AF0005 ( Figure Figure2A 2A ) and P. aeruginosa IQ0042 cells ( Figure Figure2C 2C ) had intact, clear cell wall and membrane and dense and homogeneous cytoplasm. However, we did not find any significant differences in the ultrastructure between the control and oregano oil-treated MRSA USA300 by TEM (data not shown), which probably hints at different responses of MRSA from A. baumannii or P. aeruginosa . As with biofilms, dense and thick bacterial biofilm was observed on the dentin surface, comprised of numerous layers of densely concentrated cocci in 24-h-old P. aeruginosa IQ0042 ( Figure Figure2E 2E ) and MRSA IQ0064 ( Figure Figure2G 2G ) biofilms. The biofilms of P. aeruginosa IQ0042 ( Figure Figure2E 2E ) and MRSA IQ0064 ( Figure Figure2G 2G ) were treated with oregano oil at 1 mg/ml and 0.4 mg/ml for 1 h, respectively. The cells decohered in the extracellular polymeric matrix, dead bacteria were readily seen all over, and biofilms were destroyed completely in oregano oil-treated samples ( Figures 2F,H , arrows). There were only a few bacteria scantly growing on the dentin surface owing to intensive cell death ( Figures 2F,H ).

Representative TEM images of planktonic cells (A–D) and SEM images of bacterial biofilms (E–H) with (B,D,E,F) or without (A,C,E,G) oregano oil treatment for 1 h. Oregano oil was used at 0.16 mg/ml for A. baumannii AF0005 cells, 0.56 mg/ml for P. aeruginosa IQ0042 cells, 1.0 mg/ml for P. aeruginosa IQ0042 biofilms, and 0.4 mg/ml for MRSA IQ0064 biofilm. (A,B) A. baumannii AF0005 cells; (C,D) P. aeruginosa IQ0042 cells; (E,F) P. aeruginosa IQ0042 biofilms; and (G,H) MRSA IQ0064 biofilms. Shown are cell wall and membrane damages (B,D; arrows) ; dissociation between cell wall and membrane (B; oval) , cytoplasmic vacuoles and bubbles (B,D; asterisk) , and cell collapse (F,H, arrows) . The number of bacteria was drastically reduced after oregano oil treatment in (F,H) as compared to untreated controls (E,G) .
No Evidence of Resistant Development to Oregano Oil
Risk of resistant development was evaluated with three representative strains: A. baumannii AF0005, P. aeruginosa IQ0042, and MRSA IQ0064. The bacteria were cultured up to 20 successive passages in the presence of sub-lethal doses of oregano oil for bacterial inactivation. As shown in Figure Figure3A 3A , A. baumannii AF0005, P. aeruginosa IQ0042, and MRSA IQ0064 retained susceptibility to the original MIC values of 0.16, 0.32, or 0.56 mg/ml, respectively, after 20 cycles of treatment. Moreover, there were no statistically significant differences in the survival rates of all the bacterial strains among the cycles 0, 1, and 20 in the three tested strains, after exposure to oregano oil at the MICs for 24 h ( Figures Figures3B 3B – D ). The results indicate that resistance to oregano oil of the bacterial strains did not take place under this condition.

Possible development of bacterial resistance to oregano oil. (A) A. baumannii AF0005, P. aeruginosa IQ0042, and MRSA IQ0064 were passaged for 20 generations each in the presence of indicated MIC doses of oregano oil. The inhibition rate of A. baumannii AF0005 (B) , P. aeruginosa IQ0042 (C) , or MRSA IQ0064 (D) was unaltered when the bacteria of indicated generations were exposed to oregano oil at an initial MIC concentration. ns, no significance.
Oregano Oil Significantly Reduced Bacterial Burden in Burn Wounds Infected With PA01
Gram stain of histological longitudinal section ( Figure Figure4A 4A ) and crossing section ( Figure Figure4B 4B ) of a representative skin specimen demonstrated the presence of PA01 biofilms at 24 h after bacterial inoculation, as evidenced by abundant bacteria densely clustered together (red) in a highly hydrated extracellular matrix on the surface of skin and in the epidermis ( Figure Figure4A 4A , outline in red). In addition, highly resilient microbial assemblies were readily found in the dermis suggesting that the PA01 could infect not only the epidermis but also the dermis within 24 h ( Figure Figure4B 4B ). In successive bacterial luminescence images of representative wounds infected with 5 × 10 6 CFU of PA01, oregano oil treatment at 10 mg/ml almost completely eradicated bacterial luminescence in 60 min, while luminescence remained unchanged during the same period in untreated mice ( Figures 4C,D ). Moreover, there was no recurrence of infection in the following days in oregano oil-treated mice, whereas the mice remained significantly infected in untreated mice in the same experimental period ( Figures 4C,D ). An average reduction in bacterial luminescence of 2.9 log 10 and 3.5 log 10 were achieved in 60 min at a concentration of 5 or 10 mg/ml of oregano oil, respectively ( Figure Figure4E 4E ). On the contrary, bacterial luminescence of the wounds in the absence of oregano oil treatment was almost unaltered with only 0.08 log 10 reduction during the equivalent period ( Figure Figure4E 4E , P < 0.0001). A time course study of the mean bacterial luminescence from days 2 to 7 after bacterial inoculation corroborated that the treatment consistently and significantly lowered the luminescence compared to untreated mice during the whole period of the experiment regardless of whether oregano oil was used at 5 or 10 mg/ml ( Figure Figure4F 4F ). The mean areas under the curve (AUC) of the bioluminescence time course were 6.9 × 10 9 and 2.4 × 10 9 for oregano oil-treated groups at 5 and 10 mg/ml, respectively, but it was 5.9 × 10 10 for untreated mice ( P < 0.0001; Figure Figure4G 4G ), representing an 8.6-fold or a 24.6-fold reduction of the AUC in infected burns by oregano oil. We next excised the infected wounds on day 7 to determine bacterial CFU remaining in the wounds. There were 6.7 × 10 6 and 2.4 × 10 6 CFU in the wounds treated with oregano oil at a concentration of 5 or 10 mg/ml, respectively, which was significantly lower than the bacterial burden of 6.6 × 10 7 CFU/wound in untreated mice ( Figure Figure4H 4H ).

Oregano oil treatment of PA01 infections in the burn wounds. (A,B) Gram-stained longitudinal section (A) and crossing section (B) of a representative wound showing the presence of PA01 biofilms outlined in red. The skin sample was harvested 24 h after bacterial inoculation. (C,D) Successive bacterial luminescence images of representative wounds infected with 5 × 10 6 CFU of luminescent PA01 with (D) and without (C) oregano oil at 10 mg/ml. The oregano oil was topically applied onto the wounds at 24 h after bacterial inoculation. (E) A dose response of mean bacterial luminescence of the wounds infected with 5 × 10 6 CFU of PA01 in the presence or absence of oregano oil treatment at 5 or 10 mg/ml. (F) Time courses of mean bacterial luminescence of the infected wounds in the presence or absence of oregano oil treatment at 5 or 10 mg/ml from days 2 to 7. (G) Mean areas under the bacterial luminescence curves (F) , representing the overall bacterial burden of infected wounds. (H) . The wounds were treated with grape seed oil (control) or oregano oil 24 h after infection and bacterial CFU were quantified on day 7 after bacterial inoculation. RLU, relative luminescence units; A.U., arbitrary units. The data represent means ± SDs ( n = 8). ∗∗ p < 0.01, ### or ∗∗∗ p < 0.001 and #### or ∗∗∗∗ p < 0.0001 in the presence vs. absence of oregano oil. ns, no significance.
Oregano Oil Significantly Reduced Bacterial Burden in Burn Wounds Infected With USA300
As shown in Figure Figure5A 5A , an average reduction in bacterial luminescence of 2.9 log 10 was attained when the infected wounds were treated with oregano oil at 5 mg/ml for 40 min, which was highly significant compared to only 0.4 log 10 decline of the bioluminescence in the absence of oregano oil during the same period ( P < 0.0001; Figure Figure5A 5A ). The diminished bacterial luminescence was persistent for 7 days after a single oregano oil treatment at 5 mg/ml ( Figure Figure5B 5B ). The mean AUC of the bioluminescence were 3.7 × 10 7 in oregano oil-treated group, but it was 1.8 × 10 9 for untreated mice ( P < 0.0001; Figure Figure5C 5C ), a 48.6-fold reduction of the AUC by oregano oil treatment. In accordance with this, a single dose of oregano oil treatment diminished bacterial load to 2.7 × 10 7 CFU/mouse that was 18-fold lower than 4.8 × 10 8 CFU/mouse in the untreated group ( P < 0.0001; Figure Figure5D 5D ).

Oregano oil treatment of MRSA USA300 in infected wounds. (A) The change in mean bacterial luminescence of the wounds infected with 5 × 10 6 CFU of USA300 with or without 5 mg/ml oregano oil treatment. (B) Time courses of mean bacterial luminescence of the infected wounds with and without oregano oil treatment. (C) Mean areas under the bacterial luminescence curves in (B) . (D) The wounds were treated with grape seed oil (control) or oregano oil once 24 h after infection and bacterial CFU were quantified on day 7 after bacterial inoculation. The data represent means ± SDs ( n = 8). ∗∗ p < 0.01, ∗∗∗ p < 0.001, and ∗∗∗∗ p < 0.0001 in the presence vs. absence of 5 mg/ml of oregano oil.
No Side Effects in Mouse Skin Caused by Oregano Oil
There was no noticeable skin reaction, as visualized with the naked eye, after three consecutive days of oregano oil treatment at 10 mg/ml ( Figure Figure6B 6B ) when compared to untreated skin ( Figure Figure6A 6A ). On the histological levels, the skin maintained an undisturbed structure with a clear layer of healthy epidermal cells on the top of the dermis, indistinguishable to mock-treated skins ( Figures 6C,D ). Genotoxicity was next evaluated by a TUNEL assay to examine any DNA damage induced by oregano oil. In comparison with mock-treated controls, there was no any apparent increase of DNA staining in oregano oil-treated skin ( Figures 6E,F ), while the staining was readily seen in the positive control in which skin section was treated with DNase I ( Figure Figure6G 6G ). The absence of any oregano oil-induced skin reaction and DNA damage after a 3-day treatment suggests that topical application of oregano oil is neither cytotoxic nor genotoxic to the host.

Toxicity evaluation of oregano oil to normal mouse skins. The dorsal skin of mice was topically treated with (A,C,E) or without 10 mg/ml oregano oil (B,D,F) once a day for three consecutive days. On day 4, the skins were photographed (A,B) , followed by histological examination (C,D) . The skin sections were also TUNEL stained (E,F) . DNase I treated skin samples (G) were TUNEL stained in parallel as positive-staining controls.
In seeking non-antibiotic microbicides, we have screened dozens of EOs from Chinese indigenous aromatic plants/spices because EOs have been long recognized as one of the most promising natural products for safe microbicides in folk medicines ( Lu et al., 2013a , b , c ). The selection was initially based on their antiseptic applications in the food industry and in agricultures after an extensive database search. Among the dozen EOs tested, about one third showed significant antibacterial activities against clinically and agricultural important microbes ( Lu et al., 2013a , b , c ). Oregano oil stood out as one of the best ones in terms of safety and efficacy. We thus detailed the bactericidal activity of oregano oil against 11 MDR clinical isolates of P. aeruginosa, A. baumannii and MRSA as well as two bioluminescent strains of P. aeruginosa PA01 and MRSA USA300 in the current study. Oregano oil effectively killed all the bacterial strains tested, with the MICs ranging from 0.08 to 0.64 mg/ml and at an order of sensitivity of A. baumannii > MRSA > P. aeruginosa ( Table Table1 1 ). The finding is in agreement with previous studies demonstrating that oregano oil and its main component carvacrol had a higher MIC against P. aeruginosa compared to other species, such as S. spp. ( Nostro et al., 2007 ), Chromobacterium violaceum , Salmonella typhimurium , and S. aureus ( Burt et al., 2014 ). Similar to the clinical isolates, oregano oil also inactivated standard strains of A. baumannii ATCC 19606 ( Rosato et al., 2010 ), P. aeruginosa ATCC 27853 and S. aureus ATCC 29213 ( Bouhdid et al., 2009 ), with the MICs 0.15 mg/mL, 1 mg/mL, and 0.33 mg/mL, respectively, which are comparable to our investigation. Previous studies suggested that Gram-negative bacteria appeared to be more resistant than Gram-positive bacteria in response to EO ( Tepe et al., 2005 ; Longaray Delamare et al., 2007 ; Gilles et al., 2010 ). This relative resistance of Gram-negative over Gram-positive bacteria may be ascribed to their cell wall structure and outer membrane arrangement. The outer membrane of Gram-negative bacteria is rich in lipopolysaccharide molecules, relatively impermeable to lipophilic compounds, thereby presenting a barrier to penetration of EO antimicrobial substances ( Gao et al., 2011 ). It may be also associated with the enzymes in the periplasmic space, which are capable of breaking down the antimicrobial substances upon their entrance of the cells ( Nikaido, 1996 ). However, our studies disagreed with these observations and found Gram-negative A. baumannii was more sensitive to oregano oil than Gram-positive MRSA. This different outcome suggests that antibacterial activity of oregano oil may not depend on the type of Gram reaction in contrast to other EOs, a possibility that is supported by the studies of Gao et al. (2011) . In their studies, Gram-negative bacteria Klebsiella pneumoniae was the most sensitive bacteria whereas the Gram-positive bacteria Listeria monocytogenes was the most resistant strain to the Sphallerocarpus gracilis seed EO ( Gao et al., 2011 ).
The possibility that the cell wall and membrane were primary targets of oregano oil was supported by TEM imaging of the ultrastructure of the bacteria. We found damages of the cell wall and membranes, occurrent with cytoplasmic vacuoles, stainless-vesicles, and disruption and discontinuation of the intracellular structures in a large number of bacterial cells after oregano oil treatment ( Figures 2B,E ). This finding is consistent with an association of the antibacterial activity of oregano oil/carvacrol with disturbance of membrane embedded proteins and disruption of lipids, RNA synthesis, ATPase activity, and efflux pump previously demonstrated ( Simoes et al., 2009 ; Tapia-Rodriguez et al., 2017 ). Moreover, oregano oil may cause an imbalance in intracellular osmotic pressure owing to a leakage of cytoplasmic contents following cell wall and membrane damages, and formation of cytoplasmic vacuoles, eventually inducing cell necrosis, although more investigations are required to conclude the mechanism in detail.
Biofilms are sessile organizations of bacterial cells with a strong adherence to surfaces. Biofilm-associated microbial cells are well protected by an extracellular matrix that comprises exopolysaccharides, proteins and DNA and is poorly permeable ( Donlan, 2002 ; Husain et al., 2015 ). Systemic antibiotics administered to treat bacterial infections frequently fails at least in part due to the poor permeability of biofilms. Interestingly, oregano oil was capable of biofilm-killing at least at an early stage (24-h-old biofilms) as efficiently as planktonic cells. Biofilms of A. baumannii , P. aeruginosa , and MRSA were eliminated by oregano oil at a concentration of 0.3, 1.0, or 0.4 mg/ml, respectively, similar to the corresponding MICs attained in planktonic cells. The similarity can be extended to the order of sensitivity with P. aeruginosa biofilms more resistant than MRSA biofilms than A. baumannii biofilms ( Figure Figure1 1 and Table Table1 1 ). This may be attributed to the superior permeability and lipid solubility of oregano oil to bacterial cell membrane and wall ( Magi et al., 2015 ; Khan et al., 2017 ). Likewise, the effectiveness of oregano oil to inactivate S. aureus , S. epidermidis , and P. aeruginosa biofilms was also found at similar MICs as those against planktonic cells ( Nostro et al., 2007 ; dos Santos Rodrigues et al., 2017 ; Tapia-Rodriguez et al., 2017 ). SEM observations confirmed the physical damage and considerable morphological alteration in the P. aeruginosa IQ0042 ( Figures 2E,F ) and MRSA IQ0064 ( Figures 2G,H ) biofilms following oregano oil treatment. These observations raise an intriguing possibility that EO may have advantages over water soluble antibiotics in treatment of biofilms because bacteria living in the biofilms are well known to be more resistant to antibiotics (up to 1,000 times) than their planktonic counterparts, in part owing to poor permeability of biofilms to the antibiotics ( Ceri et al., 1999 ; Caraher et al., 2007 ).
One concern of using oregano oil as an alternative for the treatment of infections in clinics will be whether MDR bacteria can develop resistance to oregano oil. Although this remains largely unaddressed to date, our results suggest that resistance may not be readily developed because 20 passages in the presence of sublethal concentrations of oregano oil did not alter their susceptibility to the oil ( Figure Figure3A 3A ). Moreover, oregano oil has been used in food prevention and other antiseptic application for centuries and no resistance has been reported so far. It is commonly believed that EOs act at multiple sites within bacterial cells (cell membrane, cell wall, structural proteins, enzymes, nucleic acids, unsaturated lipids, etc.) and would be less likely to induce the development of resistance ( Burt, 2004 ; Simoes et al., 2009 ; Tapia-Rodriguez et al., 2017 ). On the contrary, the MIC of conventional antibiotics could gradually increase with a treatment length due to their single action to inactivate the bacteria ( Baym et al., 2016 ; Levin-Reisman et al., 2017 ).
The bactericidal activity of oregano oil was corroborated in mouse burn models using model bioluminescent strains of Gram-negative P. aeruginosa PA01 and Gram-positive MRSA USA300. When applied at 24 h after bacterial inoculation forming early stage biofilms, oregano oil effectively reduced the bacterial burden by 25-folds for PA01 and 49-folds for USA300, respectively, in comparison to untreated wounds. While efficiently inactivating bacteria, oregano oil exhibited no cytotoxicity or genotoxicity to the skin, in good agreement with its long record of safety. Moreover, oregano oil did not adversely affect human keratinocytes ( Babili et al., 2011 ) and was safe when administered orally in mice ( Manohar et al., 2001 ; Preuss et al., 2005 ; Feng et al., 2017 ).
In summary, we reported here the effectiveness of oregano oil against a panel of MDR bacteria isolated from combating casualties and demonstrated for the first time efficacy of oregano oil for the treatment of burn infections in mice. The study serves as an initial effort in the pursuit of a novel therapeutic option for wound infections, especially those caused by MDR bacteria.
Author Contributions
ML designed and performed all the experiments, analyzed the data, and wrote the manuscript. TD supervised and designed the experiments, analyzed the data, and wrote the paper. CM isolated and characterized all the clinical bacteria and wrote the paper. and MW designed and supervised the study, analyzed the data, and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Drs. Michael R. Hamblin and Ji Wang for stimulating comments and discussions from Massachusetts General Hospital and the staff at the photopathology core at Wellman Center for Photomedicine for assisting transmission electron microscopy and histopathology.
Funding. This study was supported in part by FA9550-16-1-00173, Department of Defense/Air Force Office of Scientific Research Military Photomedicine Program and Department funds to MW and TD.
- Akgul A., Kivanc M. (1988). Inhibitory effects of selected Turkish spices and oregano components on some foodborne fungi. Int. J. Food Microbiol. 6 263–268. 10.1016/0168-1605(88)90019-0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Babili F. E., Bouajila J., Souchard J. P., Bertrand C., Bellvert F., Fouraste I., et al. (2011). Oregano: chemical analysis and evaluation of its antimalarial, antioxidant, and cytotoxic activities. Food Sci. 3 512–518. 10.1111/j.1750-3841.2011.02109.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baym M., Lieberman T. D., Kelsic E. D., Chait R., Gross R., Yelin I., et al. (2016). Spatiotemporal microbial evolution on antibiotic landscapes. Science 353 1147–1151. 10.1126/science.aag0822 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Benito M., Jorro G., Morales C., Pelaez A., Fernandez A. (1996). Labiatae allergy: systemic reactions due to ingestion of oregano and thyme. Ann. Allergy Asthma Immunol. 76 416–418. 10.1016/S1081-1206(10)63456-4 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bloemsma G. C., Dokter J., Boxma H., Oen I. M. (2008). Mortality and causes of death nin a burn centre. Burns 34 1103–1107. 10.1016/j.burns.2008.02.010 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bouhdid S., Abrini J., Zhiri A., Espuny M. J., Manresa A. (2009). Investigation of functional and morphological changes in Pseudomonas aeruginosa and Staphylococcus aureus cells induced by Origanum compactum essential oil. J. Appl. Microbiol. 106 1558–1568. 10.1111/j.1365-2672.2008.04124.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Burt S. (2004). Essential oils: their antibacterial properties and potential applications in foods – A review. Int. J. Food Microbiol. 94 223–253. 10.1016/j.ijfoodmicro.2004.03.022 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Burt S. A., Ojo-Fakunle V. T. A., Woertman J., Veldhuizen E. J. A. (2014). The natural antimicrobial carvacrol inhibits quorum sensing in chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. PLoS One 9 : e93414 . 10.1371/journal.pone.0093414 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Calhoun J. H., Murray C. K., Manring M. M. (2008). Multidrug-resistant organisms in military wounds from iraq and afghanistan. Clin. Orthop. 466 1356–1362. 10.1007/s11999-008-0212-9 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Caraher E., Reynolds G., Murphy P., McClean S., Callaghan M. (2007). Comparison of antibiotic susceptibility of Burkholderia cepacia complex organisms when grown planktonically or as biofilm in vitro. Eur. J. Clin. Microbiol. Infect. Dis. 26 213–216. 10.1007/s10096-007-0256-x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ceri H., Olson M., Stremick C., Read R., Morck D., Buret A. (1999). The calgary biofilm device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37 1771–1776. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Christensen L., Breiting V., Bjarnsholt T., Eickhardt S., Hogdall E., Janssen M., et al. (2013). Bacterial infection as a likely cause of adverse reactions to polyacrylamide hydrogel fillers in cosmetic surgery. Clin. Infect. Dis. 56 1438–1444. 10.1093/cid/cit067 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Crane D. P., Gromov K., Li D., Soballe K., Wahnes C., Buechner H., et al. (2009). Efficacy of colistin-impregnated beads to prevent multidrug-resistant A. baumannii implant-associated osteomyelitis. J. Orthop. Res. 27 1008–1015. 10.1002/jor.20847 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dai T., Gupta A., Huang Y., Yin R., Murray C. K., Vrahas M. S., et al. (2013). Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: efficacy, safety, and mechanism of action. Antimicrob. Agents Chemother. 57 1238–1245. 10.1128/AAC.01652-12 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Donlan R. (2002). Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8 881–890. 10.3201/eid0809.020063 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- dos Santos Rodrigues J. B., de Carvalho R. J., de Souza N. T., Oliveira K. d. S., Franco O. L., Schaffner D., et al. (2017). Effects of oregano essential oil and carvacrol on biofilms of Staphylococcus aureus from food-contact surfaces. Food Control 73 1237–1246. 10.1016/j.foodcont.2016.10.043 [ CrossRef ] [ Google Scholar ]
- Feng J., Zhang S., Shi W., Zubcevik N., Miklossy J., Zhang Y. (2017). Selective essential oils from spice or culinary herbs have high activity against stationary phase and biofilm borrelia burgdorferi. Front. Med. 4 : 169 . 10.3389/fmed.2017.00169 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fitzwater J., Purdue G., Hunt J., O’Keefe G. (2003). The risk factors and time course of sepsis and organ dysfunction after burn trauma. J. Trauma-Injury Infect. Crit. Care 54 959–966. 10.1097/01.TA.0000029382.26295.AB [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gao C., Tian C., Lu Y., Xu J., Luo J., Guo X. (2011). Essential oil composition and antimicrobial activity of Sphallerocarpus gracilis seeds against selected food-related bacteria. Food Control 22 517–522. 10.1016/j.foodcont.2010.09.038 [ CrossRef ] [ Google Scholar ]
- Gilles M., Zhao J., An M., Agboola S. (2010). Chemical composition and antimicrobial properties of essential oils of three Australian Eucalyptus species. Food Chem. 119 731–737. 10.1016/j.foodchem.2009.07.021 [ CrossRef ] [ Google Scholar ]
- Gomez R., Murray C. K., Hospenthal D. R., Cancio L. C., Renz E. M., Holcomb J. B., et al. (2009). Causes of mortality by autopsy findings of combat casualties and civilian patients admitted to a burn unit. J. Am. Coll. Surg. 208 348–354. 10.1016/j.jamcollsurg.2008.11.012 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hampton T. (2013). Report reveals scope of US antibiotic resistance threat. JAMA 310 1661–1663. 10.1001/jama.2013.280695 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Husain F. M., Ahmad I., Khan M. S., Ahmad E., Tahseen Q., Khan M. S., et al. (2015). Sub-MICs of Mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and biofilm of ram-negative bacteria. Front. Microbiol. 6 : 420 . 10.3389/fmicb.2015.00420 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jiang B., Yin S., You B., Huang G., Yang Z., Zhang Y., et al. (2017). A 5-year survey reveals increased susceptibility to glycopeptides for methicillin-resistant Staphylococcus aureus isolates from Staphylococcus aureus bacteremia patients in a Chinese burn center. Front. Microbiol. 8 : 2531 . 10.3389/fmicb.2017.02531 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Joshi S. C., Verma A. R., Mathela C. S. (2010). Antioxidant and antibacterial activities of the leaf essential oils of Himalayan Lauraceae species. Food Chem. Toxicol. 48 37–40. 10.1016/j.fct.2009.09.011 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kavanaugh N. L., Ribbeck K. (2012). Selected antimicrobial essential oils eradicate Pseudomonas spp. and Staphylococcus aureus biofilms. Appl. Environ. Microbiol. 78 4057–4061. 10.1128/AEM.07499-11 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Khan I., Bahuguna A., Kumar P., Bajpai V. K., Kang S. C. (2017). Antimicrobial potential of carvacrol against uropathogenic Escherichia coli via membrane disruption, depolarization, and reactive oxygen species generation. Front. Microbiol. 8 : 2421 . 10.3389/fmicb.2017.02421 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Levin-Reisman I., Ronin I., Gefen O., Braniss I., Shoresh N., Balaban N. Q. (2017). Antibiotic tolerance facilitates the evolution of resistance. Science 355 826–830. 10.1126/science.aaj2191 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Li X., Robinson S. M., Gupta A., Saha K., Jiang Z., Moyano D. F. (2014). Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano 8 10682–10686. 10.1021/nn5042625 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Longaray Delamare A. P., Moschen-Pistorello I. T., Artico L., Atti-Serafini L., Echeverrigaray S. (2007). Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem. 100 603–608. 10.1016/j.foodchem.2005.09.078 [ CrossRef ] [ Google Scholar ]
- Lopez-Reyes J. G., Spadaro D., Gullino M. L., Garibaldi A. (2010). Efficacy of plant essential oils on postharvest control of rot caused by fungi on four cultivars of apples in vivo. Flavour Fragrance J. 25 171–177. 10.1002/ffj.1989 [ CrossRef ] [ Google Scholar ]
- Lu M., Han Z., Xu Y., Yao L. (2013a). In vitro and in vivo anti-tobacco mosaic virus activities of essential oils and individual compounds. J. Microbiol. Biotechnol. 23 771–778. 10.4014/jmb.1210.10078 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lu M., Han Z., Yao L. (2013b). In vitro and in vivo antimicrobial efficacy of essential oils and individual compounds against Phytophthora parasitica var. nicotianae. J. Appl. Microbiol. 115 187–198. 10.1111/jam.12208 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lu M., Han Z., Xu Y., Yao L. (2013c). Effects of essential oils from Chinese indigenous aromatic plants on mycelial growth and morphogenesis of three phytopathogens. Flavour Fragrance J. 28 84–92. 10.1002/ffj.3132 [ CrossRef ] [ Google Scholar ]
- Magi G., Marini E., Facinelli B. (2015). Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci . Front. Microbiol. 6 : 165 . 10.3389/fmicb.2015.00165 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Manohar V., Ingram C., Gray J., Talpur N., Echard B., Bagchi D., et al. (2001). Antifungal activities of origanum oil against Candida albicans . Mol. Cell Biochem. 228 111–117. 10.1023/A:1013311632207 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Munhuweyi K., Caleb O. J., Lennox C. L., van Reenen A. J., Opara U. L. (2017). In vitro and in vivo antifungal activity of chitosan-essential oils against pomegranate fruit pathogens. Postharvest. Biol. Technol. 129 9–22. 10.1016/j.postharvbio.2017.03.002 [ CrossRef ] [ Google Scholar ]
- Nikaido H. (1996). Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol. 178 5853–5859. 10.1128/jb.178.20.5853-5859.1996 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nostro A., Roccaro A. S., Bisignano G., Marino A., Cannatelli M. A., Pizzimenti F. C., et al. (2007). Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med. Microbiol. 56 519–523. 10.1099/jmm.0.46804-0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ocana-Fuentes A., Arranz-Gutierrez E., Senorans F. J., Reglero G. (2010). Supercritical fluid extraction of oregano ( Origanum vulgare ) essentials oils: anti-inflammatory properties based on cytokine response on THP-1 macrophages. Food Chem. Toxicol. 48 1568–1575. 10.1016/j.fct.2010.03.026 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pandey A. K., Kumar P., Singh P., Tripathi N. N., Bajpai V. K. (2017). Essential oils: sources of antimicrobials and food preservatives. Front. Microbiol. 7 : 2161 . 10.3389/fmicb.2016.02161 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Preuss H., Echard B., Dadgar A., Talpur N., Manohar V., Enig M., et al. (2005). Effects of essential oils and monolaurin on Staphylococcus aureus : in vitro and in vivo studies. Toxicol. Mech. Methods 15 279–285. 10.1080/15376520590968833 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rosato A., Piarulli M., Corbo F., Muraglia M., Carone A., Vitali M. E., et al. (2010). In vitro synergistic action of certain combinations of gentamicin and essential oils. Curr. Med. Chem. 17 3289–3295. 10.2174/092986710792231996 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sakkas H., Papadopoulou C. (2017). Antimicrobial activity of basil, oregano, and thyme essential oils. J. Microbiol. Biotechnol. 28 429–438. 10.4014/jmb.1608.08024 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Scott P., Deye G., Srinivasan A., Murray C., Moran K., Hulten E., et al. (2007). An outbreak of multidrug-resistant Acinetobacter baumannii -calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin. Infect. Dis. 44 1577–1584. 10.1086/518170 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shen D., Pan M., Wu Q., Park C., Juliani H. R., Ho C., et al. (2010). LC-MS method for the simultaneous quantitation of the anti-inflammatory constituents in oregano ( Origanum Species). J. Agric. Food Chem. 58 7119–7125. 10.1021/jf100636h [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Simoes M., Bennett R. N., Rosa E. A. S. (2009). Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat. Prod. Rep. 26 746–757. 10.1039/b821648g [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Soylu E. M., Kurt S., Soylu S. (2010). In vitro and in vivo antifungal activities of the essential oils of various plants against tomato grey mould disease agent Botrytis cinerea . Int. J. Food Microbiol. 143 183–189. 10.1016/j.ijfoodmicro.2010.08.015 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Soylu S., Yigitbas H., Soylu E. M., Kurt S. (2007). Antifungal effects of essential oils from oregano and fennel on Sclerotinia sclerotiorum . J. Appl. Microbiol. 103 1021–1030. 10.1111/j.1365-2672.2007.03310.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tapia-Rodriguez M. R., Hernandez-Mendoza A., Gonzalez-Aguilar G. A., Martinez-Tellez M. A., Martins C. M., Ayala-Zavala J. F. (2017). Carvacrol as potential quorum sensing inhibitor of Pseudomonas aeruginosa and biofilm production on stainless steel surfaces. Food Control 75 255–261. 10.1016/j.foodcont.2016.12.014 [ CrossRef ] [ Google Scholar ]
- Tepe B., Daferera D., Sokmen A., Sokmen M., Polissiou M. (2005). Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem. 90 333–340. 10.1016/j.foodchem.2003.09.013 [ CrossRef ] [ Google Scholar ]
- Vento T. J., Cole D. W., Mende K., Calvano T. P., Rini E. A., Tully C. C., et al. (2013). Multidrug-resistant gram-negative bacteria colonization of healthy US military personnel in the US and Afghanistan. BMC Infect. Dis. 13 : 68 . 10.1186/1471-2334-13-68 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wang Y., Beekman J., Hew J., Jackson S., Issler-Fisher A. C., Parungao R., et al. (2018). Burn injury: challenges and advances in burn wound healing, infection, pain and scarring. Adv. Drug Deliv. Rev. 123 3–17. 10.1016/j.addr.2017.09.018 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wang Y., Wu X., Chen J., Amin R., Lu M., Bhayana B., et al. (2016). Antimicrobial blue light inactivation of ram-negative pathogens in biofilms: in vitro and in vivo studies. J. Infect. Dis. 213 1380–1387. 10.1093/infdis/jiw070 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yan F., Azizi A., Janke S., Schwarz M., Zeller S., Honermeier B. (2016). Antioxidant capacity variation in the oregano ( Origanum vulgare L.) collection of the German National Genebank. Ind. Crops Prod. 92 19–25. 10.1016/j.indcrop.2016.07.038 [ CrossRef ] [ Google Scholar ]
- Zhang Y., Zhu Y., Gupta A., Huang Y., Murray C. K., Vrahas M. S., et al. (2014). Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: implications for prophylaxis and treatment of combat-related wound infections. J. Infect. Dis. 209 1963–1971. 10.1093/infdis/jit842 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]

COMMENTS
While most studies are focused on the potential beneficial effects of individual EOs, or the combination of them, it is important to mention that some efforts have been extended on the study of the bioactive properties of EOs from different species of oregano. Among the most notorious studies reported to date, anti-inflammatory properties of ...
Oregano transcends its culinary appeal and emerges as a powerhouse of health benefits (Singletary, 2010). From digestive support to immunity enhancement, bone health, vitality, weight management ...
Oregano is one such herb that has been approved in the United States as a spice and natural flavor that can reduce oxidation. A recent study reported that oregano extract can maintain the physicochemical, sensory acceptance, reduce lipid and protein oxidation of lamb meat after frozen storage after 120 days 23. In addition to maintaining the ...
1. Introduction. Origanum vulgare L., commonly oregano, is one of the most renowned aromatic species, with a strong traditional background as a spice and medicinal plant, but also as a well-established source of valuable plant-based drugs in modern phytotherapy. This Lamiaceae plant is native to Europe, the North of the African continent, and most of temperate Asia, but the hotspot of its ...
Oregano extract (OE) and essential oil (OEO) are two such agents that have shown promise as natural food preservatives. Additionally, oregano is being evaluated for its positive effect on gastrointestinal health, suggesting an additional benefit of food preservation with oregano. This review will describe in vitro studies related to the anti ...
Oregano contains several potent antioxidants that may contribute to the findings in preliminary studies that oregano exhibits benefits toward the cardiovascular and nervous systems, relieves symptoms of inflammation, and modulates blood sugar and lipids. Well-controlled human studies substantiating these health effects are lacking.
Substances. Essential oils of oregano are widely recognized for their antimicrobial activity, as well as their antiviral and antifungal properties. Nevertheless, recent investigations have demonstrated that these compounds are also potent antioxidant, anti-inflammatory, antidiabetic and cancer suppressor agents ….
This review discusses the antimicrobial and antioxidant activity of oregano essential oil (OEO) and its potential as a food additive. Oregano is a plant that has been used as a food seasoning since ancient times. The common name of oregano is given to several species: Origanum (family: Lamiaceae) and Lippia (family: Verbenaceae), amongst others ...
Based on the current scientific literature, oregano extracts and individual constituents consistently have demonstrated antimicrobial actions in vitro toward food-borne pathogens, although the capacity to counter human infections is not well studied. Oregano is an herb that has been cultivated for centuries in the Mediterranean area, although it now can be found on most continents. Actually ...
Essential oils of oregano are widely recognized for their antimicrobial activity, as well as their antiviral and antifungal properties. Nevertheless, recent investigations have demonstrated that these compounds are also potent antioxidant, anti-inflammatory, antidiabetic and cancer suppressor agents. These properties of oregano essential oils are of potential interest to the food, cosmetic and ...
This research is a continuation of an ongoing oregano breeding program, pointing out the elite accessions of oregano in terms of phenolic components for domestication. ... Previous studies on ...
Although research has often focused on the study of essential oils , they represent only one of the main groups of phytochemicals found in oregano. Thus, the study of hydrophilic compounds such as phenolic compounds is frequently ignored. Flavonoids (FL) and phenolic acids (PA) are the main types of phenolic compounds present in oregano .
Abstract. The oregano, Origanum onites L., essential oil (EO) was tested in laboratory behavioural bioassays for repellent activity against Amblyomma americanum (L.) and Aedes aegypti (L.). The O. onites EO was characterised using GC-FID and GC-MS. Carvacrol (75.70%), linalool (9.0%), p-cymene (4.33%) and thymol (1.9%) were the most abundant compounds.At a concentration of 0.413 mg oil/cm 2 of ...
Oregano has made headlines in recent years due to market surveys and research publications attempting to quantify how much adulteration exists in the oregano market. Deriving from the genus Oreganum, oregano is an important culinary herb, with the most critical volatile oils that impart its distinct flavor found in the leaves of the plant ...
The purpose of our study was to analyze the chemical composition, and antimicrobial and antioxidant activities of four oregano essential oils (OEOs) from Poland, Europe, Turkey and the USA. ... (71.42-80.44%). OEOs high in carvacrol should be the subject of further research as potential antimicrobial and antioxidant agents. Keywords ...
The oregano composition depends on the specie, climate, altitude, time of recollection and the stage of growth. Some of the properties of this plant's extracts are being currently studied due to the growing interest for substituting synthetic additives commonly found in foods. Oregano has a good antioxidant capacity and also presents ...
Singletary, K. share. August 2010-- An overview of the potential health benefits of oregano, funded by the McCormick Science Institute. An evaluation of the research is presented. Read the entire study (PDF) Oregano is an herb that has been cultivated for centuries in the Mediterranean area, although it now can be found on most continents.
This study sought to assess the effectiveness of oregano leaves and lemongrass extract as organic household insect repellant against flies. The objectives were to determine the resistance rate of ...
Another test-tube study found that oregano was effective against 23 species of bacteria . Furthermore, a test-tube study compared the antimicrobial activity of oregano, sage and thyme essential oils.
The results indicate no effects of oregano on performance. In contrast, oregano altered the lymphocyte proportion and the ratio of CD4 (+) and CD8 (+) T-cells as well as the triglyceride concentration in the serum of non-stimulated and in LPS-stimulated piglets. In conclusion, whole plant supplementation of oregano to piglet feed altered immune ...
In addition, difference in the chemical composition of oregano oil from previously published papers with our study may is regarding the type of oregano subspecies. Two percent of α-terpinene, monoterpenes derived from Thymus vulgaris were reported with stronger repellency activity than DEET against Culex pipiens . In our findings, oregano ...
Chemical Constituents of Oregano Oil. Oregano oil was purchased from Bulk Apothecary (Aurora, OH, United States) and used throughout the study. To define the constituents of oregano oil, gas chromatography/mass spectrometry (GC-MS) analyses were carried out using an Agilent 6980 GC coupled to an Agilent 5973N MS and a fused-silica capillary column (HP-5MS: 30 m × 0.25 mm i.d., film thickness ...