

Top 17 Clinical Research Organizations (CRO) in 2023
In clinical research and treatment development, clinical research organizations (CROs) are frequently a sponsor’s most important partner and ally.
Depending on the nature of the clinical trial, and your existing capabilities as a sponsor to run the trial, the CRO company of your choice will typically be responsible for facilitating most of the micro and macro processes that go into designing and running a successful clinical trial.
When contracting a CRO to help you with your trial, you are transferring over a large portion of responsibility into the hands of your clinical research partner. The CRO of your choice will have the responsibility to control a variety of factors and processes of a clinical trial, and depending on their expertise, team structures, service offerings, internal resources and many other capabilities.
Your ability to find and contract a top CRO company that is the right fit for your unique trial will be a determinant of whether or not you will be able to operate a high-quality clinical trial that meets your expected timelines, budget and delivers a top-notch patient experience.
At ClaraHealth (a patient-centric recruitment acceleration platform) , we have put together an extensive list of the top CRO companies in the US and around the world.
This is not a cro rankings list, but rather a compiled list of some of the top clinical research organizations around the world. We have highlighted their strengths and core service offerings to make it easier for you to find the right fit clinical research partner.
In addition, we’ve put together a list of 9 fundamental questions to ask the prospective clinical research organization , which will help you to save time and ensure a right fit in picking the CRO.
Formerly known as Quintiles and IMS Health, IQVIA is one of the largest CROs in the world, with a large range of service offerings to help advance clinical research.
The company was founded in North Carolina in 1982, and has since grown to over 88,000 employees in more than 100 countries.
Some clinical trial solutions offered by IQVIA include:
- Assistance with protocol design
- Design of phase 1 clinical trials
- Assessment and improvement of phase 2 and 3 clinical trials
- Site identification & selection
- Patient recruitment
- Access to global laboratories via their wholly owned subsidiary Q2 Solutions
Parexel is a global clinical research organization that was founded in 1982, and specializes in conducting clinical studies on behalf of its pharmaceutical partners in order to accelerate and ensure the drug approval process of up-and-coming potential treatments. It currently operates in more than 50 countries, and is run by more than 18,000 employees around the world.
The company has a wide range of service offerings, covering nearly every type of clinical trial service to assist sponsors in running successful clinical studies.
Some clinical trial solutions offered by Parexel include:
- Clinical trial design and development for early phase, phase 2 & 3, and late phase clinical trials
- Clinical data management
- Decentralized clinical trials
- Clinical supply chain management
- Medical writing
- Regulatory affairs consulting
- Pharmacovigilance
3. PRA Health Sciences
PRA Health Sciences is one of the largest contract research organizations in the world. Founded in 1976 under the name “Anti-Inflammatory Drug Study Group”, the company was renamed to PRA in 1982. PRA Health Sciences employees more than 17,000 people, and provides coverage to more than 90 countries.
In 2021, PRA Health Sciences was acquired by the Ireland-headquartered global CRO leader ICON, which is also reviewed in this list.
Some clinical trial solutions offered by PRA Health Sciences include:
- Decentralized Clinical Trials Platform
- Protocol Consultation & Study Design
- Onsite Support services
- Customized Solutions for Biotech (such as asset valuation, regulatory strategy, engagement and support, drug development strategy and funding solutions)
- Clinical Diagnostics
- Site Commercial Solutions
- PRA’s Laboratories for Drug Development
Headquartered in Ireland, ICON was founded in 1990 in Dublin by co-founders John Climax and Ronan Lambre. The company has since grown to be one of the largest CROs in the world. As of September 2020, the company employs more than 15,000 people in 94 locations and across 40 countries.
ICON offers clinical research services which include consulting, clinical development and commercialization across a wide range of therapeutic areas.
In 2021, ICON acquired PRA Health Sciences, which is another CRO and global leader in clinical research services.
Some clinical trial solutions offered by ICON:
- Commercial Positioning
- Early Phase
- Functional Services Provision
- Laboratories
- Language Services
- Medical Imaging
- Real World Intelligence
- Site & Patient Solutions
- COVID-19 Clinical Operations
5. Syneos Health
Formerly known as InVentiv Health Incorporated and INC Research, Syneos Health is a publicly listed and global contract research organization. The company is based in Morrisville, North Carolina, and specializes in assisting companies with late-stage clinical trials. Syneos Health currently employs more than 25,000 people, and has offices across 91 locations.
In early 2018, INC Research was acquired inVentiv Health, and the merged company was named Syneos Health.
Some clinical trial solutions offered by Syneos Health include:
- Decentralized Clinical Trials Solutions
- Bioanalytical Solutions
- Phase II-III/Phase IIIb-IIIV
- Medical Device Diagnostics
- Clinical Data Management
- Clinical Project Management
- Clinical Monitoring
- Drug Safety & Pharmacovigilance
- Site and Patient Access
6. Labcorp Drug Development (Formerly Covance)
Formerly known as Covance and renamed to Labcorp Drug Development in early 2021, this CRO is one of the largest contract research organizations in the world. The company claims to provide the world’s largest central laboratory network, and has been rated as one of the best places to work for LGBTQ+ equality by the Human Rights Campaign organization in 2018 to 2021. Currently, Labcorp employs over 70,000 people and is able to support clinical research efforts in almost 100 countries around the world.
Some clinical trial solutions offered by Labcorp Drug Development include:
- Preclinical Services
- Clinical Trials
- Clinical Trial Laboratory Services
- Post-Marketing Solutions
- Medical Devices
- Data & Technology
Also known as Pharmaceutical Product Development, PPD is a large global contract research organization headquartered in Wilmington, North Carolina. Started as a one-person consulting firm in 1985, PPD has grown to over 27,000 employees worldwide, and provides a wide range of clinical research services to pharmaceutical and biotech companies.
Some clinical trial solutions offered by PPD include:
- Clinical Development
- Early Development
- Peri- and Post-Approval
- PPD Biotech
- PPD Laboratories
- Product Development and Consulting
- Site and Patient Centric Solutions
8. Fisher Clinical Services
Part of Thermo Fisher Scientific, Fisher Clinical Services is a global clinical research organization with headquarters in Center Valley, Philadelphia.
The company has been in the business of clinical supply chain management for over 20 years, and is focused exclusively on working with the packaging and distribution requirements of clinical trials across the globe.
Some clinical trial solutions offered by Fisher Clinical Services include:
- Biologistics Management
- Cell & Gene Therapy
- Clinical Ancillary Management
- Clinical Label Services
- Clinical Trial Packaging & Storage
- Clinical Supply Optimization Services
- Cold Chain Management & Expertise
- Direct-to-Patient
- Distribution & Logistics
- Strategic Comparator Sourcing
- Public Health Research
Established in 1997 under the name Kiecana Clinical Research, KCR is a full-service contract research organization that provides a variety of services for clinical monitoring, safety & pharmacovigilance, clinical project management, quality assurance and regulatory affairs.
KCR operates globally, and has offices in North America, Western Europe, Central Europe and Eartern Europe. The company currently employs more than 700 staff.
Some clinical trial solutions offered by KCR include:
- Trial Execution
10. Medpace
Founded in 1992 and based in Cincinnati, Ohio, Medpace is a midsize clinical contract research organization. The company has operations in over 45 countries, and employs over 2,800 people. Medpace provides support services for Phase I-IV clinical trials for pharmaceutical and biotechnology companies, which include central laboratory services and regulatory services.
Some clinical trial solutions offered by Medpace include:
- Biostatistics and Data Sciences
- Clinical Trial Management
- Drug Safety and Pharmacovigilance
- Medical Writing
- Quality Assurance
- Regulatory Affairs
- Risk-Based Monitoring
- Medpace Laboratories
11. Clintec
Now in business for over 22 years, Clintec is a medium-sized global contract research organization for pharmaceutical, biotech and medical device industries, with large expertise in oncology and rare diseases.
The company provides the flexibility and agility of a smaller-sized CRO, while also having a wide global coverage that large CRO companies are known for. Clintec is based in more than 50 countries, and was acquired by the leading global CRO IQVIA in late 2018.
Some clinical trial solutions offered by Clintec include:
- Project Management
- Data Management
- Biostatistics
- Global Feasibilities
- Patient Recruitment & Retention
12. Worldwide Clinical Trials
Bringing over 30 years of experience to the clinical research market, Worldwide Clinical Trials is a leading medium-sized global contract research organization. Founded by physicians with a dedication and commitment to advancing medical research, Worldwide Clinical Trials was the first customer-centric CRO.
Currently the company has coverage in more than 60 countries, and has extensive experience in a wide range of therapeutic areas, including central nervous system, metabolic, cardiovascular, oncology, rare diseases and general medicine.
Some clinical trial solutions offered by Worldwide Clinical Trials include:
- Bioanalytical Lab
- Early Phase Development
- Clinical Phase IIB-II Clinical Trials
- Phase IIIB-IV Clinical Trials
- Trial Management Technologies
Named #1 CRO in the world for operational excellence at the 2021 CRO Leadership Awards, CTI Clinical Trial And Consulting Services is a medium-sized global contract research organization that has been serving pharmaceutical companies since 1999.
Based in Covington, Kentucky, CTI has offices around the world in more than 60 countries, with coverage in North America, Europe, Latin America, Middle-East, Africa, and Asia-Pacific regions.
Some clinical trial solutions offered by CTI include:
- Feasibility
- Regulatory Affairs Study Start-Up
- Medical Monitoring
- Safety & Pharmacovigilance
- Clinical Services
14. Wuxi AppTec
Founded in 2000 as WuXi PharmaTech in the city of Wuxi, China, Wuxi AppTec has grown from a single laboratory into a leading global contract research organization with more than 28,000 employees, including 23,000 scientists and more than 30 research & development and manufacturing sites around the world.
With offices in Asia, U.S, Europe and the Middle East, the company is able to provide coverage to more than 30 countries around the world.
Some clinical trial solutions offered by Wuxi AppTec include:
- Small Molecule Drug R&D and Manufacturing
- Cell Therapy and Gene Therapy
- Drug R&D and Medical Device Testing
- Clinical Services (Phase I-IV)
15. Advanced Clinical
Founded in 1994 and based out of Deerfield, Illinois, Advanced Clinical is a midsize and full-service CRO that helps sponsors with running clinical trials. The company employs more than 700 staff, and offers a wide variety of services across many therapeutic areas. Advanced Clinical has global representation in over 50 countries around the world.
Some clinical trial solutions offered by Advanced Clinical include:
- eTMF & Document Management
- Global Medical Services
- Quality & Validation
16. Pharm-Olam
Pharm-Olam is a leading midsize CRO with global headquarters located in Houston, Texas and its European headquarters in Bracknell, United Kingdom. The company employs more than 800 staff, and has 25 offices around the world, with a global coverage in more than 60 countries.
The company has therapeutic expertise in 5 areas, including Rare & Orphan Disease, Infectious Disease & Vaccine, Oncology-Hematology, Allergy and Autoimmune.
Some clinical trial solutions offered by Pharm-Olam include:
- Study Feasibility
- Site Activation
- Patient Recruitment
- Medical Affairs
- Compliance & Training
- Clinical Monitoring & Operations
17. Clinipace
Founded in 2003 and based out of Morrisville, North Carolina, Clinipace is a global midsize full-service CRO with a focus on solution customization for clinical trials. The company has a large global coverage in more than 50 countries, and has offices in North America, South America, Europe and Asia-Pacific regions.
Clinipace’s therapeutic focus areas include Oncology, Nephrology and Urology, Rare Disease, Gastroenterology and Women’s Health. The company also has complete therapeutic expertise in Infectious Disease & Vaccines, Cardiology, CNS, Immunology, and Respiratory.
Some clinical trial solutions offered by Clinipace include:
- Clinical Analytics
- Clinical Technology and Ecosystem
- Functional Service Partnership (FSP)
- Regulatory & Strategic Product Development
9 Fundamental Questions To Ask A Top CRO Company Before Signing The Contract
1. which services does the cro provide.
CROs offload a lot of operational tasks from trial sponsors, which can touch any component of clinical trial operations. From formulating an overall study strategy and implementing technologies to support the operational processes of the trial, to picking and identifying sites, and supporting patients during the trial, the range of clinical services offered by a CRO tends to be vast and inclusive of all the typical services and support you will require for running a successful clinical trial.
However, not all CROs are the same in their service offerings, or are able to offer the same depth of capability within a seemingly same clinical trial support process. For this reason it is important to understand exactly which kind of clinical services and support you are looking to receive from the prospective CRO when running your clinical trial.
While services such as clinical monitoring and clinical trial management are offered by the majority of CROs, the specific needs of each trial are unique, and for this reason it is important to first identify what will be the unique services your trial requires. Completing this internal analysis first will help you to understand the extent to which a potential CRO partner will be able to provide all of these services.
Some CROs specialize in specific clinical trial functions which the company may label as a “core services”, in which case this is a sign the company will have more expertise, experience, and will be set up in a way to maximize their capabilities in providing support for these services compared to other services that the CRO offers.
For example, a CRO may include patient recruitment as part of its “core services”, which implies that they are highly skilled in and have the necessary infrastructure to design and implement a high-quality patient recruitment strategy.
Clara Health CRO Support Services: At Clara Health our specialty services include technology-augmented digital and patient advocacy recruitment, as well as patient support via our signature patient recruitment platform, which we use to upgrade clinical trials and deliver results sponsors look for in their recruitment and retention campaigns.
At Clara, we work alongside CROs to supplement and support clinical trials with modern and personalized capabilities that CROs do not typically have the bandwidth, corporate structure or infrastructure to support.
If you would like to learn more about exactly how our platform can upgrade your unique trial, feel free to book a Free 30 Minute Consultation Session Here with one of our in-house experts.
2. What Related Experience Does The CRO Have?
It is helpful to ask the prospective CRO company if they have any relevant experience in running clinical trials that would be an asset in designing and running your study. Previous experience in a related therapeutic area or in running a trial with a similar design allows CROs to have a deeper understanding into potential opportunities and challenges, increasing the likelihood of your clinical study being successful.
For example, if a sponsor is planning to run a trial in oncology, for the purpose of site identification and selection it would be valuable to partner with a CRO vendor that has expertise in this area, as they likely already have a good understanding of which sites will lead to optimal results.
However, it is also important to consider all factors when selecting a CRO vendor and not to rely on therapeutic experience as the sole qualifier for whether or not a potential CRO is a fit for your trial. While previous experience is beneficial, some sponsors close themselves off from working with vendors that have not worked in their therapeutic area, which significantly limits options when choosing a CRO partner that is truly a good fit for their clinical study.
This can impact the end result of your clinical study, as sponsors that are not successful in choosing a CRO vendor that is the right overall fit may face difficulties if the needs of their clinical study aren’t being properly met.
Clara Health: We have worked to provide support for clinical trials across a wide range of therapeutic areas and trial designs. Our specialty is filling in the gaps that CROs traditionally did not have to think about, which include digital patient recruitment, patient advocacy recruitment, and technology-augmented patient support.
Additionally, we are constantly building our proprietary data and running tests in a variety of therapeutic areas. These research efforts allow us to have a detailed understanding of the expected level of difficulty when recruiting particular patient populations, as well as allow us to predict with accuracy which segments of the targeted population will be likely to qualify in a particular study.
3. What Are The Communication Workflows & Expectations For Performing And Delivering Contracted Services?
It is important that you clarify what the expectations for communication will be between your prospective CRO vendor and your internal teams, as you will most likely be working with the CRO of your choice for the entire duration of your clinical trial.
There are a vast variety of factors and success determinants for a clinical trial, which are continuously undergoing change as the study unfolds. For this reason, it is recommended that you work with a CRO that is proactive in their communication, so that you are kept up to date with information about important changes as your clinical trial progresses.
A vendor that is proactive rather than reactive in their communication and approach to dealing with arising issues is one of the most important qualities in CRO. Challenging situations will naturally arise, and the promptness with which they are taken care of will significantly impact your clinical trial’s degree of success. Therefore, seeking a vendor that is able to match the standard of communication that you as a sponsor would like to experience throughout the duration of your partnership is one of the most critical steps in determining which CRO is the right fit for your clinical trial.
We’ve included a few additional questions pertaining to the communication structure and reporting expectations that you can ask a prospective CRO vendor to determine the degree of fit in this particular category:
Communication Expectations:
- If we were to move forward with you, which of your team members will be our main point of contact?
- How available will you be outside of the scheduled meetings to address any of our concerns or additional requests?
- What will be the frequency at which update meetings will be conducted, and who will be present at those meetings?
- Which clinical study processes will be reported on, and what will be the workflow for how we will receive this information?
- What will be the cadence at which we will receive progress reports?
- Would we be able to access metrics electronically via an interactive dashboard, or will you send us formal reports?
Clara Health: At Clara Health, we directly interact and actively work with several key stakeholders involved in running a clinical trial, which includes sponsors, CROs, sites, and patients. This unique position allows us to have a centralized perspective which helps us to see all the moving parts of a clinical trial at the same time, which helps to identify issues and relay this vital information and insight back to the sponsor (or other appropriate stakeholders) in the shortest time possible.
The ability to access this perspective allows us to gather the most accurate, complete, and up-to-date information about how the clinical trial is unfolding, and quickly becomes very valuable to sponsors for their clinical trial.
As an example, we may receive feedback from patients about having an unsatisfactory experience with a particular study site. We are able to aggregate and analyze this information, and relay our findings back to the sponsor and the study site to improve the experience for other patients.
4. What Is The CRO’s Client Satisfaction Record?
It is a good practice to request information or metrics from the prospective CRO vendor that can point to the degree of satisfaction of their past clients. Prior to signing the contract, vendors will naturally do their best to uplift their image and future value to you during their sales conversations with you and your team. It can be tricky to get an objective understanding of what the partnership experience will actually entail, especially when there are multiple vendors fighting for your commitment.
We recommend that you ask the prospective vendor to provide success metrics regarding areas of clinical trial operations that are going to be important for your trial.
For example, you may be interested in learning about the vendor’s relationship to finances, in which case it will be useful to ask them about situations in which they went over the planned budget, and investigate into the reasons behind that. Alternatively you may be concerned about potential delays in timelines, in which case it would be helpful to learn about metrics regarding the CRO’s ability to meet timeline expectations.
You may also request to talk to the prospective CRO’s past clients, which will help you to gain insight into what the relationship was like and give you the opportunity to examine if the way in which the particular CRO manages its relationships and performs its services meets the expectations that you would have for your potential relationship and for your clinical trial.
Clara Health: At Clara Health, our relationships with our partners and with our patients are most important to us. In the unique position where we fit in the clinical trial process, we have the opportunity to directly co-create the clinical trial patient experience with a variety of stakeholders, including sponsors, sites, CROs, and patients.
Our company’s values and culture have been directed and developed to be such that the client and patient experience is at the top of priority for all of our internal teams, and we work to provide the best quality of care to all stakeholders.
We have many testimonials from every type of partner we’ve worked with which we can happily share with you.
5. How Do You Adapt When Encountering Challenges With Running A Clinical Trial?
It is inevitable that challenges and unforeseen changes will arise throughout the operational clinical trial process, and for this reason it is important to work with a CRO vendor that can provide you with evidence of their flexibility and ability to adapt to sudden changes.
The ideal CRO partner is one that is highly consultative throughout the entire process, and has an ability and the initiative to deal with challenges at their seed stage, prior to them turning into major obstacles for the success of your trial.
CROs naturally have a large reach, and there are a lot of different clinical trial mechanisms and processes that are under their control. They are able to monitor and respond to what is going on in every key link in the chain of the clinical trial operation.
It is reasonable to expect this level of oversight from a CRO, and additional questions that can help you gain insight into this include:
- What are some examples where the CRO was effective at monitoring the health of clinical trials they’ve helped operate in the past?
- How quickly does the CRO respond to challenges or opportunities for improving the clinical trial experience?
- How well does the CRO gather & process information from study sites, study teams, patients & the sponsor, and what are their typical data analysis workflows?
It is also recommended to speak to the prospective CROs past clients to help you gain insight into how well they respond and adapt to the naturally arising challenges in clinical trials.
Clara Health: While CROs do have a large reach within the clinical trial, no CRO has complete visibility into every clinical process. They are not typically set up to support full visibility, which can manifest as a potential threat to your clinical trial as it unfolds. This is especially true for parts of the clinical trial processes that CROs naturally do not specialize and often subcontract, such as clinical trial recruitment.
At Clara, we are in a unique position in relation to other key partners involved in operating the clinical trial. We are in direct and frequent contact with patients, CROs, study sites, study teams, and the sponsor, and have a very deep understanding of the patient pipeline. This allows us the unique ability to go very deep into specific parts of the recruitment chain and investigate what is working and what is not working.
In addition, Clara functions as a resource for all partners in the clinical trial. For example, we work directly with site teams to ensure that they have access to a 3rd party that they can relay their needs to and receive fast support in case there is anything they require that can improve the patient recruitment process.
6. Which Parts Of Operating The Clinical Trial Will You Be Outsourcing?
Since there are so many processes and mechanisms that go into operating a clinical trial, CROs will always outsource some parts of running and managing the study. While you can expect that the prospective CRO will subcontract some of the work, it is important to find out which exact parts the clinical study will be outsourced.
There are certain basic and key clinical processes (such as site selection) that CROs almost always help with, and if you find that these parts of your trial are going to be subcontracted to another company, it is recommended to find out why the CROs operations are set up this way and how this would impact the service you will receive.
Ultimately what matters to you as a partner and client is that the quality of service and care that you will receive will be up to standard, and meet what was promised and what you are expecting. While this trust is important after you have signed the contract, it is recommended that prior to entering into such a significant commitment that you have evidence and the conviction that the CRO of your choice is truly the right fit and will deliver the quality of service that was being discussed.
Since it is impossible to predict exactly what the quality of this relationship and services performed will actually be like in practice, it is recommended that you understand the details of what will be done for your trial and how. Investigating how the CRO outsources and subcontracts services for a clinical trial will help you to gain necessary insight that you would need to make the correct vendor selection decision.
Clara Health: At Clara, we maximize the effectiveness of the digital component across the entire digital & recruitment spectrum, which is added on top of the existing capabilities of the CROs and other vendors involved in operating your clinical trial. In addition, we offer services that augment the CROs efforts, which has the potential to significantly improve the patient experience, operations flows, recruitment and retention performance, which is so important in ensuring the success of a clinical trial.
For example, if a CRO wants to have a great site relationship, we are able to come in as a third party on behalf of the sponsor and CRO and act as a resource and additional support for sites.
In another example, If a sponsor wants to have great relationships with the patient community, Clara is able to come in on behalf of the sponsor and develop these relationships while being perceived more neutrally by the patient community.
7. Do You Have Experience Running International Trials?
If you are planning on operating an international clinical trial, it is recommended to work with a CRO that has extensive experience in this area. While many CROs will offer near-global coverage, the level of experience with specific geographic locations can significantly vary from one vendor to another.
It is important to work with a CRO that has experience running clinical trials in the specific countries and regions you are planning to conduct your research in. Being compliant with the local rules and regulations for clinical testing is a very complex process that requires existing understanding and familiarity in order to ensure logistical smoothness and to mitigate legal risks. In operating a clinical trial, there are a multitude of clinical services and processes, which can greatly vary across the many regions in which you can conduct clinical testing.
A CRO that is lacking experience in operating international trials or operating in particular regions where you plan on conducting research may not be able to meet your desired quality and agility expectations, and therefore may not be the right fit for your international clinical trial.
Clara Health: In the past, we have provided international patient recruitment and digitally-augmented trial support services for clinical trials in the EU, Canada, UK, Australia and South America.
Clara Health is fully compliant to operate international studies everywhere in the world, with the exception of Russia and China.
8. What Is Your Relationship With Patients?
Patient-centric approach to designing and operating a clinical trial is becoming more and more crucial in the clinical research space. The ability of a sponsor and their CRO partner to understand the needs and characteristics of their target patient community is a significant determinant of whether or not the study will be a success.
A sponsor that has close and authentic relationships with the patient community tends to have a deeper understanding of how to create the best clinical trial experience that will attract patients and keep their interest throughout the clinical trial.
In addition, strong relationships with patients allow sponsors and CROs to forecast recruitment and patient retention pipeline with much higher accuracy. This ability is critical for ensuring the success of the trial and mitigating the risk of low enrollment. After an understanding of the patient population is acquired, sponsors gain the necessary insight to design a clinical trial that is not only favorable to their research results, but is also practical and will result in the enrollment numbers they are looking for.
While many CROs have already recognized the importance of patient-centricity and evolved the ways in which they design and operate clinical trials, other CROs have not yet made such a pivot in their values. It is important to understand the degree of importance the prospective CRO places on creating a favorable patient experience, and what kind of infrastructure the company has to support it.
At Clara, we recommend choosing a CRO partner that is adapting to the patient-centric model which is becoming more and more important for running a successful clinical trial.
Clara Health: Since early stages of our development, we’ve had a dedicated patient advocacy team that has been integral in shaping our company’s vision and operations. We have built our entire platform and recruitment infrastructure around creating the best experience for patients. Our teams, corporate values, service offerings and company infrastructure all work in the service of the patient.
In addition, over the many years of being in business we have heavily invested in building authentic patient community relationships that span across a variety of therapeutic areas. This has given us a unique ability to receive feedback directly from patients that is genuine and authentic around marketing materials, strategy for patient recruitment, and other services that we build for specific trials.
This ability to build partnerships with the patient community in an authentic way gives us a very unique ability to engage with the patient community on behalf of a pharmaceutical company, allowing our sponsor & CRO partners the opportunity to start conversations with patients through our in-house patient advocacy team.
If you would like to learn how Clara can help you to build a strong & authentic relationship with your target patient community, get in touch with us and we’d be happy to share our capabilities and previous results with you as they relate to your current or upcoming clinical trial.
9. How Is The CRO Going To Utilize Patient Input For Developing The Trial?
In the initial stages of clinical trial design, sponsors often determine the ideal patient profiles that would help them to drive the most favorable research outcomes for their study. While it is important for the success of your trial to determine who your ideal patients are, very often these projections do not match up with what is viable in practice.
At Clara, we often encounter study protocols that are not set up realistically for successful recruitment to be possible.
Common mistakes that are made when determining trial eligibility criteria and trial design include:
- Overestimating the interest in the clinical trial from the target patient population
- A lack of patient focus in the trial design
- A lack of convenience for patients in their participation
- Complicated and/or inefficient study experience flows
- Crafting the eligibility criteria around the patient population that is most likely to lead to favorable study outcomes, without conducting sufficient research to more accurately estimate the recruitment and retention difficulty of the group for a particular study
It is natural for there to be a “push & pull” between the research ideal and the real world practicality. It is important to determine the correct balance between these two sides for your trial, as going too far in either direction will decrease the chance of your clinical study’s success.
The nature of the industry as it is right now is such that there is excess research idealization and not enough emphasis on patient centricity. This distorted orientation has resulted in many clinical trials being unsuccessful, negatively impacting sponsors, patients and the entire clinical trials industry.
The ideal CRO partner should help you make sure that your protocol design sets your study up for success. The CRO should be able to help you determine the proper balance between the research ideal and the real world practicality, and back up their findings with sufficient research and patient data that can project your trial being a success.
Clara Health: When formulating a recruitment and retention plan for our clients, we begin with conducting thorough research into the target trial patient population. This allows us to get a clear understanding of which recruitment channels will yield the best results and what kind of marketing materials will resonate with the prospective study participants.
To ensure accuracy and real-world applicability of our research, we consult and collaborate with our internal patient advocacy and patient support teams, as well as with our clients and patients representing the target trial patient profiles. We then tie our findings back with any existing proprietary data that we have in connection with the therapeutic area or the prospective target patient group.
Our unique position within the clinical recruitment chain gives us the presence and deep-rooted access needed to effectively tap into any of the three patient traffic sources: digital recruitment, offline recruitment, or patient advocacy recruitment.
Once a recruitment campaign has gone live, we constantly monitor, analyze and optimize our performance to make sure that the processes we have in place are as efficient as possible and drive the greatest results. In addition, we have the capability to layer in any traditional advertising (such as billboard ads) if requested by the study sponsor.
Top 15 Clinical Research Companies: Leaders in Medical Innovation
What’s on this page:

In the healthcare industry, the number of contract research organizations in the US has reached 2,823 in 2023. This marks a subtle but significant increase of 0.9% compared to the previous year.
This increase signals a vital trend: the growing complexity of finding the best clinical research companies in a crowded field. These organizations aren’t just businesses; they’re important in advancing medicine and developing drugs and therapies.
With such an important task, choosing the right company becomes essential. In this guide, we’ve looked closely at many companies along with their strengths and weaknesses and made a list of the top clinical research organizations.
By the end, you’ll know which company is the best fit for your needs.
Quick List of Top 15 Clinical Research Companies
Here is a quick overview of the best companies of clinical research:
- IQVIA: Best for data-driven insights and advanced analytics in healthcare research.
- ICON: Best for comprehensive clinical development services and therapeutic expertise.
- Parexel: Best for global biopharmaceutical services, emphasizing regulatory and clinical trial excellence.
- Syneos Health: Best for integrated biopharmaceutical solutions and clinical-commercial capabilities.
- PPD: Best for drug development services with innovative, technology-enhanced trial strategies.
- Labcorp: Best for comprehensive clinical testing and diagnostics services with global reach.
- Medpace: Best for expertise in clinical research and regulatory affairs for pharmaceutical companies.
- Charles River Laboratories: Best for preclinical research and development services, including animal testing and research models.
- PRA Health Sciences: Best for clinical trial expertise and integrated solutions for biopharmaceutical development.
- AdvanCell: Best for innovative cell and tissue-based research solutions for life science industries.
- Dynata: Best for data-driven insights and market research services for informed decision-making.
- Covance: Best for end-to-end drug development solutions, from preclinical to post-marketing.
- MedNet: Best for technology solutions and eClinical platforms for streamlined clinical trials.
- Fisher Clinical Services Inc: Best for global logistics and supply chain services for clinical trial materials.
- Worldwide Clinical Trials: Best for specialized CRO offering personalized clinical research solutions.
3 Best Clinical Research Organizations: Comparison Chart
Here’s a comparison table to highlight the key features and differences among the best companies of clinical research. This table aims to provide a quick overview of each company’s unique strengths and areas of expertise in the pharmaceutical and healthcare research sector.
| Data-driven insights, advanced analytics | Healthcare research, Data analytics | Technology-driven healthcare research services | |
| Comprehensive clinical development, expertise | Clinical development, Therapeutic research | Clinical trial and development services | |
| Regulatory expertise, clinical trial excellence | Biopharmaceutical services | Global clinical trial management |
3 Top Clinical Research Organization List For Advanced Medical Discoveries

Now, we’ll explore the top clinical research organizations (CROs) dedicated to advancing medical discoveries. Let’s jump into the details of these exceptional organizations.
IQVIA is a global leader in clinical research and healthcare data analytics. They play a crucial role in the medical field by providing comprehensive data, advanced analytics, and expert insights. This helps pharmaceutical and healthcare companies make smarter, more effective decisions.
Why is IQVIA among the best? Their strength lies in their vast database and advanced technology, which enable them to analyze complex healthcare data efficiently. This leads to a better understanding of diseases, more effective treatments, and faster drug development.
IQVIA’s work is essential because it speeds up the process of bringing new medicines to the market, ultimately benefiting patients worldwide. In short, IQVIA is a key catalyst in advancing global healthcare.

About IQVIA
- Founding Team: Dennis Gillings
- Founding Year: 1982
- Company Size: 86,000
Features of IQVIA
IQVIA, a prominent player in the life sciences sector, is dedicated to advancing healthcare through connected intelligence. Here are some key features of IQVIA in the world of clinical research:
Innovative Clinical Development
IQVIA is reimagining clinical development by intelligently connecting data, technology, and analytics. This approach leads to faster decision-making and reduced risk, enabling the delivery of life-changing therapies more quickly.
Efficient Payment Systems for Clinical Trials
They have simplified the process of paying sites involved in clinical trials. IQVIA offers the capability to make payments within 30 days, even in challenging locations. This significantly reduces the administrative burden of managing clinical trial payments by up to 90%.
Decentralized Trials Expertise
The company has conducted over 500 studies in more than 75 countries, covering over 30 indications using decentralized trial methodologies. This demonstrates their capability in managing complex, multinational clinical trials.
Global Reach and Impact
With a presence in various regions, including Australia, New Zealand, the Middle East, and Africa, IQVIA’s global footprint allows it to drive healthcare innovations worldwide.
AI and Technology Integration
The company is at the forefront of integrating AI and other technologies in healthcare. Their Healthcare-grade AI promises precision, speed, scale, trust, and reliability, essential for advancing health and improving patient outcomes.
- Extensive, reliable healthcare data enhances market research quality.
- Utilizes AI and machine learning for advanced healthcare insights.
- Specialized focus yields a deep understanding of healthcare dynamics.
- Broad international presence enables diverse and large-scale studies.
- Offers advanced tools for insightful healthcare data analysis.
- Advanced tools can be challenging to use without training.
- Handling sensitive health data raises privacy and security issues.
Our Review of IQVIA
IQVIA, a prominent player in the healthcare and life sciences industry, presents a mixed bag of strengths and weaknesses. On the positive side, we appreciate IQVIA’s extensive expertise in data analytics and healthcare consulting.
Their comprehensive research and analysis have undoubtedly driven valuable insights and innovations in the sector. Moreover, their global presence allows for diverse perspectives and access to critical healthcare data.
However, we must also acknowledge some shortcomings. IQVIA’s services can be prohibitively expensive for smaller organizations, limiting accessibility. Additionally, the sheer volume of data can sometimes lead to information overload, making it challenging to extract actionable insights.
ICON is a prominent company in the field of clinical research, playing a significant role in advancing medical science. They specialize in designing and conducting clinical trials for new medicines and treatments.
The work of ICON is crucial because they help determine the safety and effectiveness of these potential medical breakthroughs. They are considered one of the best in clinical research due to their high standards of accuracy, reliability, and ethical practices.
ICON’s expertise ensures that the clinical trials they manage are conducted efficiently and effectively, leading to faster approval of new treatments. This directly impacts patient care, as it allows quicker access to new, potentially life-saving medicines.
In essence, ICON’s contribution is vital in driving forward medical innovations.
- Founding Team: John Climax and Ronan Lambe
- Founding Year: 1990
- Company Size: 41,160
Features of ICON
Here are some of the key features of ICON in clinical research:
Diverse Clinical and Scientific Operations
ICON offers a wide range of clinical and scientific operations services, ensuring comprehensive support for various aspects of clinical trials. This includes everything from study design to execution and data analysis.
Decentralized Clinical Trial Solutions
They provide end-to-end services, operational models, and technology to deliver customized solutions for decentralized clinical trials. This approach is increasingly important in today’s clinical research landscape, offering flexibility and efficiency.
Specialized Therapeutic Areas
ICON has expertise across multiple therapeutic areas including cardiovascular, central nervous system, endocrine & metabolic disorders, infectious diseases, internal medicine & immunology, oncology, and more. This broad expertise allows them to handle a wide range of clinical research projects.
Innovative Solutions for Biotech
ICON provides full-service outsourcing and flexible support customized to the specific needs of biotech companies. This includes due diligence and asset valuation, which are critical for biotech firms navigating the complex landscape of drug development.
Advanced Medical Imaging Solutions
Their expert medical imaging solutions support all stages of clinical research, improving decision-making, increasing efficiency, and reducing trial costs.
- Decades of expertise ensure high-quality clinical research.
- Offers wide-reaching capabilities for multi-regional clinical studies.
- Deep understanding of global regulations enhances compliance and efficiency.
- Invests in new technologies for more efficient trial processes.
- Broad range of specialties contributes to comprehensive service offerings.
- Managing multi-regional trials can lead to logistical challenges.
- Rapid growth may strain resources and affect service quality.
Our Review of ICON
When we researched ICON, we found both commendable aspects and areas for improvement. On the positive side, we appreciate their commitment to clinical research and their global presence, which allows for diverse study options. Their experienced team and advanced technology contribute to reliable data collection and analysis.
However, there are some drawbacks to consider. We have noticed occasional delays in project timelines, which can be frustrating. Additionally, the cost of their services tends to be on the higher side, making it a potential barrier for smaller research endeavors.
Parexel is a globally recognized company in clinical research, known for its important role in developing new medical treatments. They are one of the biggest clinical research organizations. Parexel conducts clinical trials, crucial steps in testing the safety and effectiveness of new drugs.
The work of Parexel is essential because it bridges the gap between medical research and the availability of new treatments to patients. One of the reasons they stand out as one of the best in this field is their rigorous approach to research.
Their commitment to quality and their global network also enables diverse and large-scale studies, setting them apart from others in the field. These strengths allow Parexel to deliver reliable and valuable data, accelerating the process of bringing new, effective medicines to the market.
Simply put, Parexel is a key player in transforming medical research into real-world health solutions.
About Parexel
- Founding Team: Josef von Rickenbach and Anne B. Sayigh
- Company Size: 18,900
Features of Parexel
Parexel, a global biopharmaceutical services organization, offers a range of features in clinical research. Here are some key aspects of their approach:
Patient-Centric Approach
Parexel emphasizes a patient-first strategy in their clinical trials. This approach results in deeper and more relevant insights for trial design and execution. This ensures that the trials are more aligned with patient needs and experiences.
Innovative Trial Designs
Parexel employs innovative trial designs to optimize trials for maximum impact. This includes advanced modeling and simulation to predict drug effects ahead of time, which can save time, money, and resources.
Regulatory Compliance and Market Access
Parexel designs studies and endpoints with market access in mind, ensuring that they satisfy global regulations. This approach helps in getting treatments to patients safely and quickly.
Patient Advocacy and Engagement
The company includes patient advocates in their council, using their experiences to improve trial designs. This inclusion demonstrates their commitment to understanding and incorporating patient perspectives in clinical research.
Focus on Speed and Precision
Parexel aims to design neuroscience trials with speed and precision, utilizing the right experts and specializations. This focus is crucial in delivering effective treatments on time.
- Provides advanced technology and analytics for efficient data management.
- Extensive network provides global insights with regional knowledge.
- Expertise in navigating complex regulatory environments worldwide.
- Broad experience across various therapeutic areas ensures versatile solutions.
- Focuses on patient engagement for more effective trial outcomes.
- Rapid expansion can lead to challenges in resource management.
- Concentration in specific areas could pose risks in market shifts.
Our Review of Parexel
Parexel is a notable player in the field of clinical research and pharmaceutical services. We’ve thoroughly analyzed their offerings and found both strengths and areas that need improvement.
On the positive side, Parexel excels in its commitment to innovation and technology. We appreciate their continuous efforts to simplify clinical trials and drug development processes, making them more efficient.
However, we also noticed some downsides. Communication with clients could be more transparent, with clearer updates on project progress. Additionally, there’s room for improvement in terms of ensuring consistency in service quality across different projects.
Other 12 Companies of Clinical Research

In the world of clinical research, beyond the well-known names, there are 12 other companies making significant contributions. Let’s explore their vital role in advancing healthcare.
1. Syneos Health
Syneos Health helps develop medicines by managing clinical trials for new drugs. They’re essential because they ensure medicines are safe and effective. Syneos Health stands out in clinical research for its comprehensive services and global reach, making drug development smoother and faster.
About Syneos Health
- Founding Team: Colin Shannon
- Founding Year: 1980
- Company Size: 28,000
PPD is a group that tests new drugs to see if they’re good and safe. This is crucial for getting new treatments to people. They stand out for their thorough research and global reach.
- Founding Team: Fred Eshelman
- Founding Year: 1985
- Company Size: 40,000+3
Labcorp does important tests and research for health. They’re needed because they help find out if new treatments are good. They’re among the best for their big labs and fast results.
About Labcorp
- Founding Team: Matthew Benger
- Founding Year: 1978
- Company Size: 75,5000
Medpace focuses on making sure new health treatments are safe. This is key for better medicine. They’re a top choice because of their focus on quality and detail in research.
About Medpace
- Founding Team: August Troendle
- Founding Year: 1992
- Company Size: 5,400
5. Charles River Laboratories
Charles River Laboratories tests drugs and does research to help pets and people stay healthy. They’re essential for safe, new treatments. Their expertise makes them a leader in the field.
About Charles River Laboratories
- Founding Team: Henry Foster
- Founding Year: 1947
- Company Size: 21,400
6. PRA Health Science
PRA Health Science works on finding out if new medicines are safe. This helps everyone get better treatments. They’re known for their excellent research and care in studies.
About PRA Health Science
- Founding Year: 1976
- Company Size: 17,000+
7. AdvanCell
AdvanCell specializes in new treatments, checking if they’re safe and working. Their work is vital for progress in medicine. They’re recognized for their innovation in research.
About AdvanCell
- Founding Team: Andrew Adamovich
Dynata gathers data for health studies. They’re needed for understanding what works in healthcare. They’re a top name for their accurate and wide-reaching data collection.

About Dynata
- Founding Team: Mike Petrullo
- Founding Year: 1940
- Company Size: 5000-10000
Covance helps with drug tests and research to fight diseases. Their role is key for new treatments. They’re celebrated for their comprehensive services and global impact.
About Covance
- Founding Team: Fred Cummings
- Founding Year: 1981
- Company Size: 50,000
MedNet provides software for managing clinical trials. This helps in making research easier and faster. They’re among the best for their tech solutions in research.
About MedNet
- Founding Team: John “Rob” Robertson
- Founding Year: 1996
- Company Size: 51-200
11. Fisher Clinical Services Inc.
Fisher Clinical Services Inc. manages the logistics of clinical trials, ensuring that treatments are tested efficiently. Their work is crucial for the progress of medicine, and they are renowned for their reliability and global network.
About Fisher Clinical Services Inc.
- Founding Team: John Pickering
- Founding Year: 1989
12. Worldwide Clinical Trials
Worldwide Clinical Trials conducts essential research to evaluate new medical treatments. Their work is critical for advancing healthcare. They are distinguished by their global expertise and commitment to innovation in clinical research.
About Worldwide Clinical Trials
- Founding Team: Neal Cutler
- Founding Year: 1986
- Company Size: 3,147
What To Consider When Choosing the Best Clinical Research Companies?
Choosing the right clinical research company (CRC) is crucial for the success of any clinical trial. Here’s a detailed guide on what to consider:

Expertise and Specialization
Always ensure the CRC has expertise in your specific therapeutic area. Companies with experience in similar drug trials or medical devices can better navigate the complexities of your project.
Regulatory Compliance
The CRC must adhere to regulatory guidelines like FDA (US) , EMA (Europe), and others. Check their track record in meeting these standards to avoid compliance issues.
Reputation and Track Record
You should research the company’s history. Look for testimonials, case studies, and reviews from past clients. A company with a strong reputation is likely to deliver quality results.
Project Management Capabilities
Effective project management is key. Assess their ability to manage timelines, budgets, and communication. A CRC that provides transparent, regular updates is preferable.
Patient Recruitment Strategies
Patient recruitment can be challenging. Evaluate their strategies for participant recruitment and retention. Consider their demographic reach and methods for ensuring a diverse participant pool.
Data Management and Analysis
The CRC should have strong systems for data collection, management, and analysis. Ask about their use of Electronic Data Capture (EDC) systems and how they handle data security and confidentiality.
Cost and Financial Terms
Get a clear understanding of the cost structure. Consider the value for money rather than just the lowest cost. Ensure there are no hidden fees and clarify what is included in the quoted price.
How Heartbeat AI Can Help You Get the Best List of Clinical Research Organizations?
Heartbeat AI, with its advanced features, can significantly assist in identifying the best list of clinical research organizations (CROs). Here’s how its various features contribute to this process:

Data Analysis and Processing
Heartbeat AI excels in analyzing vast amounts of data. When it comes to selecting CROs, it can process and analyze information from numerous sources, including past performance records, clinical trial reports, and regulatory compliance data. This thorough analysis helps in identifying CROs with a proven track record of success and reliability.
Machine Learning Algorithms
These algorithms enable Heartbeat AI to learn from historical data and improve its recommendations over time. By understanding trends and patterns in the successful execution of clinical trials, it can better predict which CROs are likely to meet your specific needs.
Predictive Analytics
Heartbeat AI uses predictive models to forecast future trends and outcomes based on historical data. This can be invaluable in predicting the success rate of CROs in upcoming projects, thus aiding in making more informed choices.
Customization and Personalization
The AI can be customized to your specific requirements. If you’re focusing on a specific therapeutic area or clinical trial phase, Heartbeat AI can prioritize specialized CROs in these fields.
Real-time Data Updates
The healthcare and pharmaceutical landscapes are constantly changing. Heartbeat AI’s ability to integrate and analyze real-time data ensures that the recommendations are based on the most current information available.
Integration with External Databases
Heartbeat AI can integrate with various external databases and platforms. This enables it to pull in comprehensive information about CROs from diverse sources, enhancing the accuracy of its recommendations.
Claim $500 of Free Data
Summing up, we’ve explored the best clinical research companies, diving into their features, strengths, weaknesses, and more. Clinical research is vital in healthcare; it’s key for advancing medical knowledge and developing new treatments.
With this guide, you’re equipped to find the right clinical research company that meets your specific needs. Whether it’s for innovative therapies, drug development, or medical advancements, choosing the right partner is crucial. This guide serves as a valuable resource to help you make an informed decision in the complex world of clinical research.
Frequently Asked Question
What services do companies of clinical research offer.
Clinical research organizations offer a wide range of services, including protocol development, patient recruitment, data collection and analysis, regulatory compliance, and more.
What is the role of a clinical research coordinator?
A clinical research coordinator is responsible for managing various aspects of a clinical trial, including patient recruitment, data collection, and ensuring compliance with protocols.
What is informed consent in clinical research?
Informed consent is the process by which participants in a clinical trial are fully informed about the study’s purpose, risks, and benefits. They voluntarily agree to participate based on this information.
9 Notable AI Companies in Clinical Research to Watch in 2023
The clinical trial is a critical stage of drug development workflow, with an estimated average success rate of about 11% for drug candidates moving from Phase 1 towards approval. Even if the drug candidate is safe and efficacious, clinical trials might fail due to the lack of financing, insufficient enrollment, or poor study design .
Artificial Intelligence (AI) is increasingly perceived as a source of opportunities to improve the operational efficiency of clinical trials and minimize clinical development costs. Typically AI vendors offer their services and expertise in the three main areas. AI start-ups in the first area help to unlock information from disparate data sources, such as scientific papers, medical records, disease registries, and even medical claims by applying Natural Language Processing (NLP). This can support patient recruitment and stratification, site selection, and improve clinical study design and understanding of disease mechanisms. As an example, about 18 % of clinical studies fail due to insufficient recruitment, as a 2015 study reported.
Another aspect of success in clinical trials is improved patient stratification. Since trial patients are expensive - the average cost of enrolling one patient was $15,700-26,000 in 2017 -- it is important to be able to predict which patient will have greater benefit or risk from treatment. AI-driven companies operate with multiple data types, such as Electronic Health Records (EHR), omics, and imaging data to reduce population heterogeneity and increase clinical study power. Vendors could use speech biomarkers to identify neurological disease progression, imaging analyses to track treatment progression, or genetic biomarkers to identify patients with more severe symptoms.
RELATED: The Rise of Decentralized Clinical Trials: 10 Companies Pushing the Field Forward
AI is also streamlining the operational processes of clinical trials. AI vendors help to track patient health from their homes, monitor treatment response, and patient adherence to the trial procedures. By doing that AI companies decrease the risk of patient dropouts, which accounted for 30% on average. Usually, the Phase 3 clinical study stage requires 1000-3000 participants, with a part of them taking a placebo. That’s why the development of synthetic control arms - AI models that could replace the placebo-control groups of individuals thus reducing the number of individuals required for clinical trials - might become a novel trend.
Below we summarize a list of notable AI vendors providing advanced tools for clinical development.
ConcertAI (formerly, Concerto HealthAI) is a US-based company founded in 2017. The company provides real-world evidence (RWE) services for precision oncology. It has established the broadest clinical network through partnerships and licensing with community oncology networks, thus getting access to Electronic Medical Records, Results of NGS diagnostics, and patient-reported outcomes. Concerto then analyzes such data and generates evidence for new therapeutic approaches. In 2023, ConcertAI launched its CTO 2.0 solution to enhance the design and execution of clinical trials by leveraging expanded data assets from public sources, which include site- and physician-level trial information. Through collaborations with data partners, the system offers operational trial metrics and site profile data, emphasizing the capabilities and performance of study centers. Furthermore, the CTO 2.0 integrates social determinants of health information at the site, physician, and patient levels, aiming to automate trial site selection using a data-driven methodology. The platform's SaaS technology aligns with FDA mandates, enabling research scalability through advanced clinical informatics and data standards. The company raised a total of $300 million from a number of investors, with the latest $150 million Series C round from Sixth Street.
Saama is a Silicon Valley-based company that was founded in 1997, but it raised its first venture capital in 2015. The company has raised more than $500 million in venture capital, including the latest mega-round of $430 million from Carlyle and venture funds from Merck, Pfizer, Amgen, McKesson, and others, with a transfer of company control.
Continue reading
This content available exclusively for BPT Mebmers
Topics: Clinical Trials
Get Exclusive Insights Into Your Inbox join 6700+ BPT insiders
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- NATURE INDEX
- 13 March 2024
How AI is being used to accelerate clinical trials
- Matthew Hutson 0
Matthew Hutson is a science writer based in New York City.
You can also search for this author in PubMed Google Scholar
Credit: Taj Francis
For decades, computing power followed Moore’s law, advancing at a predictable pace. The number of components on an integrated circuit doubled roughly every two years. In 2012, researchers coined the term Eroom’s law (Moore spelled backwards) to describe the contrasting path of drug development 1 . Over the previous 60 years, the number of drugs approved in the United States per billion dollars in R&D spending had halved every nine years. It can now take more than a billion dollars in funding and a decade of work to bring one new medication to market. Half of that time and money is spent on clinical trials, which are growing larger and more complex. And only one in seven drugs that enters phase I trials is eventually approved.

Nature Index 2024 Health sciences
Some researchers are hoping that the fruits of Moore’s law can help to curtail Eroom’s law. Artificial intelligence (AI) has already been used to make strong inroads into the early stages of drug discovery , assisting in the search for suitable disease targets and new molecule designs. Now scientists are starting to use AI to manage clinical trials, including the tasks of writing protocols, recruiting patients and analysing data.
Reforming clinical research is “a big topic of interest in the industry”, says Lisa Moneymaker, the chief technology officer and chief product officer at Saama, a software company in Campbell, California, that uses AI to help organizations automate parts of clinical trials. “In terms of applications,” she says, “it’s like a kid in a candy store.”
Trial by design
The first step of the clinical-trials process is trial design. What dosages of drugs should be given? To how many patients? What data should be collected on them? The lab of Jimeng Sun, a computer scientist at the University of Illinois Urbana-Champaign, developed an algorithm called HINT (hierarchical interaction network) that can predict whether a trial will succeed, based on the drug molecule, target disease and patient eligibility criteria. They followed up with a system called SPOT (sequential predictive modelling of clinical trial outcome) that additionally takes into account when the trials in its training data took place and weighs more recent trials more heavily. Based on the predicted outcome, pharmaceutical companies might decide to alter a trial design, or try a different drug completely.
A company called Intelligent Medical Objects in Rosemont, Illinois, has developed SEETrials, a method for prompting OpenAI’s large language model GPT-4 to extract safety and efficacy information from the abstracts of clinical trials. This enables trial designers to quickly see how other researchers have designed trials and what the outcomes have been. The lab of Michael Snyder, a geneticist at Stanford University in California, developed a tool last year called CliniDigest that simultaneously summarizes dozens of records from ClinicalTrials.gov, the main US registry for medical trials, adding references to the unified summary. They’ve used it to summarize how clinical researchers are using wearables such as smartwatches, sleep trackers and glucose monitors to gather patient data. “I’ve had conversations with plenty of practitioners who see wearables’ potential in trials, but do not know how to use them for highest impact,” says Alexander Rosenberg Johansen, a computer-science student in Snyder’s lab. “Best practice does not exist yet, as the field is moving so fast.”
Most eligible
The most time-consuming part of a clinical trial is recruiting patients, taking up to one-third of the study length. One in five trials don’t even recruit the required number of people, and nearly all trials exceed the expected recruitment timelines. Some researchers would like to accelerate the process by relaxing some of the eligibility criteria while maintaining safety. A group at Stanford led by James Zou, a biomedical data scientist, developed a system called Trial Pathfinder that analyses a set of completed clinical trials and assesses how adjusting the criteria for participation — such as thresholds for blood pressure and lymphocyte counts — affects hazard ratios, or rates of negative incidents such as serious illness or death among patients. In one study 2 , they applied it to drug trials for a type of lung cancer. They found that adjusting the criteria as suggested by Trial Pathfinder would have doubled the number of eligible patients without increasing the hazard ratio. The study showed that the system also worked for other types of cancer and actually reduced harmful outcomes because it made sicker people — who had more to gain from the drugs — eligible for treatment.
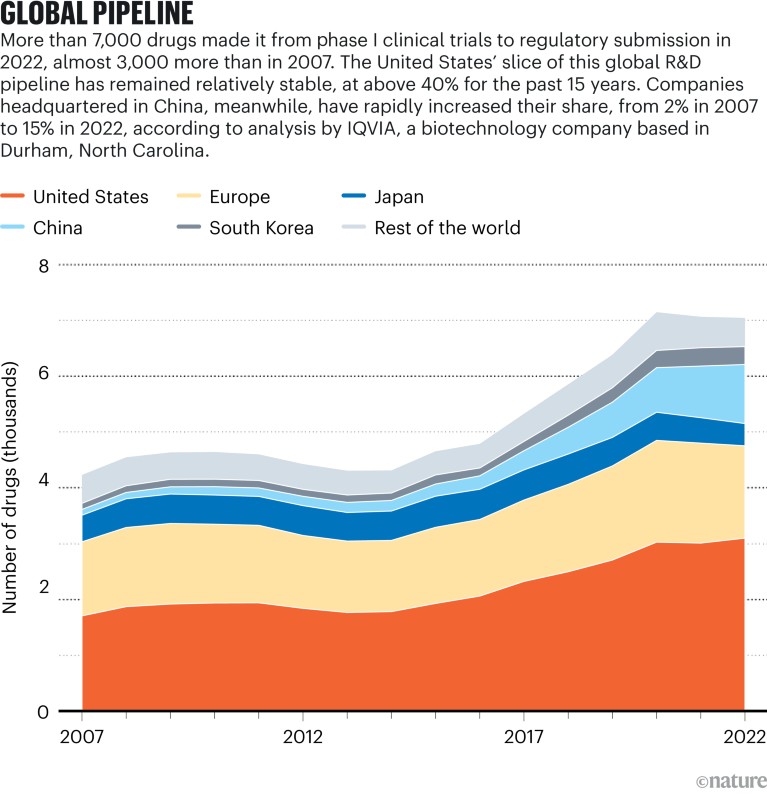
Sources: IQVIA Pipeline Intelligence (Dec. 2022)/IQVIA Institute (Jan. 2023)
AI can eliminate some of the guesswork and manual labour from optimizing eligibility criteria. Zou says that sometimes even teams working at the same company and studying the same disease can come up with different criteria for a trial. But now several firms, including Roche, Genentech and AstraZeneca, are using Trial Pathfinder. More recent work from Sun’s lab in Illinois has produced AutoTrial, a method for training a large language model so that a user can provide a trial description and ask it to generate an appropriate criterion range for, say, body mass index.
Once researchers have settled on eligibility criteria, they must find eligible patients. The lab of Chunhua Weng, a biomedical informatician at Columbia University in New York City (who has also worked on optimizing eligibility criteria), has developed Criteria2Query. Through a web-based interface, users can type inclusion and exclusion criteria in natural language, or enter a trial’s identification number, and the program turns the eligibility criteria into a formal database query to find matching candidates in patient databases.
Weng has also developed methods to help patients look for trials. One system, called DQueST, has two parts. The first uses Criteria2Query to extract criteria from trial descriptions. The second part generates relevant questions for patients to help narrow down their search. Another system, TrialGPT, from Sun’s lab in collaboration with the US National Institutes of Health, is a method for prompting a large language model to find appropriate trials for a patient. Given a description of a patient and clinical trial, it first decides whether the patient fits each criterion in a trial and offers an explanation. It then aggregates these assessments into a trial-level score. It does this for many trials and ranks them for the patient.
Helping researchers and patients find each other doesn’t just speed up clinical research. It also makes it more robust. Often trials unnecessarily exclude populations such as children, the elderly or people who are pregnant, but AI can find ways to include them. People with terminal cancer and those with rare diseases have an especially hard time finding trials to join. “These patients sometimes do more work than clinicians in diligently searching for trial opportunities,” Weng says. AI can help match them with relevant projects.
AI can also reduce the number of patients needed for a trial. A start-up called Unlearn in San Francisco, California, creates digital twins of patients in clinical trials. Based on an experimental patient’s data at the start of a trial, researchers can use the twin to predict how the same patient would have progressed in the control group and compare outcomes. This method typically reduces the number of control patients needed by between 20% and 50%, says Charles Fisher, Unlearn’s founder and chief executive. The company works with a number of small and large pharmaceutical companies. Fisher says digital twins benefit not only researchers, but also patients who enrol in trials, because they have a lower chance of receiving the placebo.
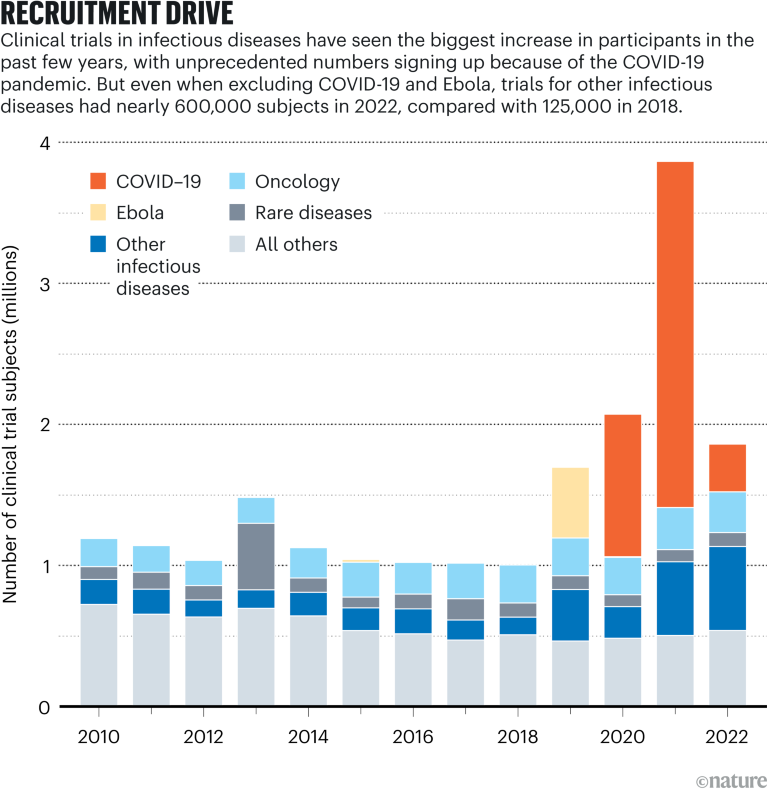
Source: Citeline Trialtrove/IQVIA Institute (Jan. 2023)
Patient maintenance
The hurdles in clinical trials don’t end once patients enrol. Drop-out rates are high. In one analysis of 95 clinical trials, nearly 40% of patients stopped taking the prescribed medication in the first year. In a recent review article 3 , researchers at Novartis mentioned ways that AI can help. These include using past data to predict who is most likely to drop out so that clinicians can intervene, or using AI to analyse videos of patients taking their medication to ensure that doses are not missed.
Chatbots can answer patients’ questions, whether during a study or in normal clinical practice. One study 4 took questions and answers from Reddit’s AskDocs forum and gave the questions to ChatGPT. Health-care professionals preferred ChatGPT’s answers to the doctors’ answers nearly 80% of the time. In another study 5 , researchers created a tool called ChatDoctor by fine-tuning a large language model (Meta’s LLaMA-7B) on patient-doctor dialogues and giving it real-time access to online sources. ChatDoctor could answer questions about medical information that was more recent than ChatGPT’s training data.
Putting it together
AI can help researchers manage incoming clinical-trial data. The Novartis researchers reported that it can extract data from unstructured reports, as well as annotate images or lab results, add missing data points (by predicting values in results) and identify subgroups among a population that responds uniquely to a treatment. Zou’s group at Stanford has developed PLIP, an AI-powered search engine that lets users find relevant text or images within large medical documents. Zou says they’ve been talking with pharmaceutical companies that want to use it to organize all of the data that comes in from clinical trials, including notes and pathology photos. A patient’s data might exist in different formats, scattered across different databases. Zou says they’ve also done work with insurance companies, developing a language model to extract billing codes from medical records, and that such techniques could also extract important clinical trial data from reports such as recovery outcomes, symptoms, side effects and adverse incidents.
To collect data for a trial, researchers sometimes have to produce more than 50 case report forms. A company in China called Taimei Technology is using AI to generate these automatically based on a trial’s protocol.
Increased trial complexity
The cost of drug development continues to rise, and the size and complexity of clinical trials is a major factor. In the past two decades, the number of countries in which a clinical trial is conducted has more than doubled, and the average number of data points collected has grown dramatically. There are more endpoints — outcomes of a clinical trial that help to determine the efficacy and safety of an experimental therapy — and procedures to measure these outcomes, such as blood tests and heart-activity assessments. By comparison, eligibility criteria for participants, which include demographics such as age and sex and whether a participant is a healthy or a patient volunteer, have remained relatively consistent.
Category | 2001–05 | 2011–15 | 2016–20 | 20-year overall rise |
|---|---|---|---|---|
Endpoints* | 7 | 13 | 22 | 214% |
Procedures* | 110 | 187 | 263 | 139% |
Eligibility criteria* | 31 | 30 | 30 | −3% |
Countries* | 6 | 9 | 15 | 150% |
Data points collected* | 494,236 | 929,203 | 3,453,133 | 599% |
Source: Tufts Center for the Study of Drug Development. *Mean of total numbers
A few companies are developing platforms that integrate many of these AI approaches into one system. Xiaoyan Wang, who heads the life-science department at Intelligent Medical Objects, co-developed AutoCriteria, a method for prompting a large language model to extract eligibility requirements from clinical trial descriptions and format them into a table. This informs other AI modules in their software suite, such as those that find ideal trial sites, optimize eligibility criteria and predict trial outcomes. Soon, Wang says, the company will offer ChatTrial, a chatbot that lets researchers ask about trials in the system’s database, or what would happen if a hypothetical trial were adjusted in a certain way.
The company also helps pharmaceutical firms to prepare clinical-trial reports for submission to the US Food and Drug Administration (FDA), the organization that gives final approval for a drug’s use in the United States. What the company calls its Intelligent Systematic Literature Review extracts data from comparison trials. Another tool searches social media for what people are saying about diseases and drugs in order to demonstrate unmet needs in communities, especially those that feel underserved. Researchers can add this information to reports.
Zifeng Wang, a student in Sun’s lab, in Illinois, says he’s raising money with Sun and another co-founder, Benjamin Danek, for a start-up called Keiji AI. A product called TrialMind will offer a chatbot to answer questions about trial design, similar to Xiaoyan Wang’s. It will do things that might normally require a team of data scientists, such as write code to analyse data or produce visualizations. “There are a lot of opportunities” for AI in clinical trials, he says, “especially with the recent rise of larger language models.”
At the start of the pandemic, Saama worked with Pfizer on its COVID-19 vaccine trial. Using Saama’s AI-enabled technology, SDQ, they ‘cleaned’ data from more than 30,000 patients in a short time span. “It was the perfect use case to really push forward what AI could bring to the space,” Moneymaker says. The tool flags anomalous or duplicate data, using several kinds of machine-learning approaches. Whereas experts might need two months to manually discover any issues with a data set, such software can do it in less than two days.
Other tools developed by Saama can predict when trials will hit certain milestones or lower drop-out rates by predicting which patients will need a nudge. Its tools can also combine all the data from a patient — such as lab tests, stats from wearable devices and notes — to assess outcomes. “The complexity of the picture of an individual patient has become so huge that it’s really not possible to analyse by hand anymore,” Moneymaker says.
Xiaoyan Wang notes that there are several ethical and practical challenges to AI’s deployment in clinical trials. AI models can be biased . Their results can be hard to reproduce . They require large amounts of training data, which could violate patient privacy or create security risks. Researchers might become too dependent on AI. Algorithms can be too complex to understand. “This lack of transparency can be problematic in clinical trials, where understanding how decisions are made is crucial for trust and validation,” she says. A recent review article 6 in the International Journal of Surgery states that using AI systems in clinical trials “can’t take into account human faculties like common sense, intuition and medical training”.
Moneymaker says the processes for designing and running clinical trials have often been slow to change, but adds that the FDA has relaxed some of its regulations in the past few years, leading to “a spike of innovation”: decentralized trials and remote monitoring have increased as a result of the pandemic, opening the door for new types of data. That has coincided with an explosion of generative-AI capabilities . “I think we have not even scratched the surface of where generative-AI applicability is going to take us,” she says. “There are problems we couldn’t solve three months ago that we can solve now.”
Nature 627 , S2-S5 (2024)
doi: https://doi.org/10.1038/d41586-024-00753-x
This article is part of Nature Index 2024 Health sciences , an editorially independent supplement. Advertisers have no influence over the content.
Scannell, J. W., Blanckley, A., Boldon, H. & Warrington, B. Nature Rev. Drug. Discov. 11 , 191–200 (2012).
Article PubMed Google Scholar
Liu, R. et al. Nature 592 , 629–633 (2021).
Blaschke, T. F., Osterberg, L., Vrijens, B. & Urquhart, J. Annu. Rev. Pharmacol. Toxicol. 52 , 275–301 (2012).
Ayers, J. W. et al. JAMA Intern. Med. 183 , 589–596 (2023).
Li, Y. et al. Cureus 15 , e40895 (2023).
PubMed Google Scholar
Chopra, H. et al. Int. J. Surg. 109 , 4211–4220 (2023).
Download references
Related Articles

Partner content: A holistic approach to health in Japan
Partner content: Can we increase the safety of carbon nanotubes?
Partner content: Building for a digitized, personalized future
Partner content: Translating basic research into medical advances
Partner content: Meds, meals and microbes: gut health on the cancer front line
Partner content: A hotspot for research and development of medical AI
Partner content: What are we learning from the world’s myopia capital?
Partner content: Systems biology: a network view of disease could unearth hidden targets
Partner content: Learning from the pupil: the power of pupillometry
Partner content: Does green tea offer cognitive and sleep benefits?
Partner content: A team effort delivers a new tool for fighting neuroblastoma
Partner content: Shaping the future of medical science - A multidisciplinary paradigm
Partner content: Harnessing AI to see a patient’s unique patterns
Partner content: Improving the outlook for chronic kidney disease
Partner content: From medical robots to a mouse metaverse
Partner content: What comes after clinical trials?
Partner content: Making vitamin D detection much more accessible
Partner content: A new injectable gel could seal wounds after cancer surgery
Partner content: The surprising interplay between metabolism and mind
Partner content: Detecting atrial fibrillation while there’s still time to act
- Therapeutics
- Machine learning
- Research data
- Medical research
Long-lasting heart-failure treatment could be a game-changer
News & Views 11 SEP 24

Blocking an inflammatory protein slows the pace of ageing
News & Views 29 JUL 24

A Trojan horse for thirsty tumours
Outlook 11 JUL 24

Red light, green light: flickering fluorophores reveal biochemistry in cells
Technology Feature 12 SEP 24

How a struggling biotech company became a university ‘spin-in’
Career Q&A 10 SEP 24

A day in the life of the world’s fastest supercomputer
News Feature 04 SEP 24

This AI chatbot got conspiracy theorists to question their convictions
News 12 SEP 24
Artificial intelligence can help to make animal research redundant
Correspondence 10 SEP 24

Back to the future: two books that tried to predict how science would evolve
News & Views 10 SEP 24
Institute for Systems Genetics, Tenure Track Faculty Positions
The Institute for Systems Genetics at NYU Langone Health has tenure track faculty positions (assistant professor level) at the new SynBioMed Center.
New York City, New York (US)
NYU Langone Health
Faculty Position
The Institute of Cellular and Organismic Biology (ICOB), Academia Sinica, Taiwan, is seeking candidates to fill multiple tenure-track faculty position
Taipei (TW)
Institute of Cellular and Organismic Biology, Academia Sinica
Postdoctoral Associate
Houston, Texas (US)
Baylor College of Medicine (BCM)
Associate or Senior Editor, Nature Energy
Job Title: Associate or Senior Editor, Nature Energy Location: New York, Jersey City, Philadelphia or London — Hybrid Working Application Deadline:...
Springer Nature Ltd
Open Rank Tenure-track/Tenured Faculty Positions in the Department of Genetic and Genomic Sciences
Seeking expertise in areas including Cancer Genetics; Artificial Intelligence; Drug Development & Clinical Trials; Functional Genomics; & Gene Editing
Mount Sinai Department of Genetics and Genomic Sciences
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
How artificial intelligence can power clinical development
Clinical development and the randomized clinical trial (RCT) remain largely unaffected by the unprecedented wave of innovation in pharmaceutical R&D fueled by developments in artificial intelligence (AI), including generative AI (gen AI) and foundational models. However, they are under pressure from the rise of precision medicine and a more competitive development landscape. So far, the adoption of AI for clinical development has emphasized operational excellence and acceleration, but advances in scientific AI have made this the time to leverage modern analytical tools and novel sources of data to design more precise, efficient trials with greater success rates.
About the authors
This article is a collaborative effort by Chris Anagnostopoulos, David Champagne , Thomas Devenyns, Alex Devereson , and Heikki Tarkkila, representing views from McKinsey’s Life Sciences Practice.
The introduction of RCTs in the mid-20th century ushered in the modern era of evidence-based drug development. Their rigidity and simplicity were welcome defenses against an overreliance on anecdotes and case studies and have unarguably served patients well, supporting the introduction of countless safe, effective therapies. Yet RCTs are starting to be seen as bottlenecks that lengthen the time for therapies to gain approval and can increase costs. 1 Donald A Berry, “The brave new world of clinical cancer research: Adaptive biomarker-driven trials integrating clinical practice with clinical research,” Molecular Oncology , 2015, Volume 9, Issue 5; Michael Baumann et al., “How much does it cost to research and develop a new drug? A systematic review and assessment,” PharmacoEconomics , 2021, Volume 39, Issue 11. Meanwhile, as gen AI breaks milestone after milestone, patients eagerly await the translation of such unprecedented technological progress into pharmaceutical R&D that could deliver faster access to better treatments.
In addition to rising expectations about timelines, clinical development is also facing increasing demands to generate targeted and meaningful data. As precision medicine becomes mainstream, RCTs often must prove not only the general efficacy of a treatment but also whether it will benefit a specific segment of the patient population. The smaller that segment, the harder it can be to enroll enough patients in trials. In addition, the bar for what constitutes a clinically meaningful treatment effect is creeping higher to meet standards set by regulators and payers as well as competitive pressure. In most therapeutic areas today, there are, on average, 40 percent more assets per indication than in 2006, 2 McKinsey research on data from EvaluatePharma, March 2019. raising the need for greater differentiation. And clinicians and patients alike need more evidence to make decisions about the best available treatment at a given time.
All this comes as researchers accelerate drug discovery by making greater use of AI and gen AI, such as DeepMind’s AI-enabled platform AlphaFold, which can predict the 3-D structure of molecules 3 “ AI in biopharma research: A time to focus and scale ,” McKinsey, October 10, 2022. leading to a pipeline of more and better-designed preclinical assets with a better target validation. Companies are also striving to improve their operational processes with a view to speeding up the time it takes to apply for a first-in-human study. But if clinical development fails to keep pace, the benefits to patients of faster drug discovery will inevitably be delayed, and delivering on the promise of AI-enabled acceleration may flounder.
While the RCT will remain a central pillar of clinical development, the tide is changing. Regulators have recently issued guidance on appropriate use of real-world data (RWD). 4 See, for example, the US Food and Drug Administration’s real-world evidence activities responding to the 21st Century Cures Act. The healthcare data ecosystem is booming, leveraging privacy-respecting technology to make patient-level data safely available for research. And evidence generation from multiple data sources is now possible using causal machine learning, which aims to distinguish correlation from causation via a combination of biostatistics with machine learning (ML) and, increasingly, gen AI and foundational models.
Despite this level of promise, only a handful of established companies are deploying AI and data-driven approaches systematically in their clinical development. The focus so far has been on improving operational excellence and increasing acceleration rather than helping to inform trial design strategy. The remainder of this white paper dives deeper into the context, gives tangible example use cases and associated impact, and identifies challenges companies face when adopting AI for clinical development. Rather than staying at a high level, it aims to bring the topic to life through relevant details and case examples.

Creating value beyond the hype
Let’s deliver on the promise of technology from strategy to scale.
Expanding adoption of AI and RWD for clinical development
Today AI can draw upon growing volumes of RWD. Electronic health records and claims data from certain geographies are widely available, and novel data sources—including biobanks, data omics panels, population-wide genomic studies, patient registries, and imaging and digital pathology—are increasing in number and diversity. All these sources reflect a notable change in patients’ ability to share their data for the purpose of advancing research and treatments in privacy-respecting ways, including technology that enables the training of ML models in a manner that respects patients’ privacy.
In addition, new tools can systematically capture knowledge from unstructured data. Large language models (for example, BioGPT) are able to convert unstructured physician notes into high-quality structured data. Similarly, these models can search the vast corpus of published literature and identify connections between biological entities on a large scale, generating high-quality input for knowledge graphs that better represent the totality of available evidence on a given indication across domains, including genes, targets, proteins, pathways, and phenotypes.
Most pharma companies using AI and RWD in clinical development tend to do so only in isolated use cases, and they rarely deploy both in combination. For example, they might use machine learning to select trial sites or to predict patient enrollment rates. They might use RWD to measure disease prevalence—the size of an eligible population—to understand the natural course of a disease among untreated patients, or to construct an external control arm for regulatory purposes. While these use cases deliver value, a great deal more is now possible. Similarly, while the level of funding and investment going into AI-enabled drug discovery companies has tripled in the last five years, that same trend is not true for equivalent companies in the clinical development space.
Four use cases that demonstrate the potential of AI
Companies that are deploying AI combined with RWD are seeing impactful results. While RWD can be valuable supporting evidence in health authority submission packages, the most successful companies focus their use of AI and RWD to make better and more informed decisions to support the success of clinical development programs in every step, from asset and portfolio strategy to protocol and trial design:
- At the stage of defining an asset strategy, AI and RWD can reveal which indications are most promising indications to pursue for novel assets. Several leading biopharma companies identified multiple new indications for existing assets in this way, and an early-stage biotech used AI and RWD to assess whether to shift its indication selection strategy regarding a novel asset.
- AI and RWD can support decisions about the target patient population of a clinical trial, with subgroup discovery, refine trial eligibility criteria and help remove patients that are highly unlikely to benefit from the treatment, and shorten the length of trials. One early-stage biotech could better characterize “super-progressors” (that is, patients whose disease was likely to progress faster within the time frame of a clinical trial) and design a trial with similar expected benefits in a faster time frame.
- For decisions about portfolio strategy, AI and RWD can help companies identify the right combination of drugs for an indication or for the right patients. One biopharma company leveraged a prospective observational data set to generate evidence supportive of earlier positioning of its third-line treatment. Another was able to identify “super-responders” for several drugs in its portfolio—insights that helped the company position its assets optimally in a crowded indication.
- At the step of selecting and optimizing the end points of a clinical trial, AI and RWD can help a company identify patient attributes that closely track the primary end point over time. One biopharma company replaced a rare disease’s existing end point, which was an infrequent event, with end points that occurred with greater frequency or could be measured with blood tests. This cut the length of trials by 15 to 30 percent.
The following discussion looks in greater depth at four detailed use cases, illustrating the wealth of information that AI can unlock.
Indication selection for asset strategy
Selecting which indications to target with a specific molecule is one of the most important decisions a biopharma company makes. This decision is often informed by a combination of input from key opinion leaders, literature reviews, omics analysis (for example, genome-wide association studies), RCT data, and competitor decisions. Such decisions rarely are fully data driven, typically prove hard to integrate, and cover only part of the available evidence base, resulting in a subjective and suboptimal synthesis. In contrast, strategies informed by RWD and AI are objective and comprehensive in their use of multiple data sets and foundational models built on clinical data continually redefining the art of the possible.
For already-approved therapies, companies can use RWD to understand the therapies’ likely efficacy on alternative indications. One approach is examining the outcomes of patients who were prescribed the drug on a spontaneous basis by their physician; another is determining a drug’s average effect on patients exposed to the treatment because of incidental comorbidities. AI techniques can then extrapolate any findings to a set of patients whose characteristics may be distributed differently, by leveraging observed correlations between patient characteristics and outcomes.
Where companies are looking to expand the indication of an approved asset or identify for a novel asset, RWD and AI can estimate the biological proximity of one indication to another from a patient and clinical perspective—that is, whether the patient experiences similar symptoms, comorbidities, lab characteristics, and treatment journeys. Foundational models that treat medical events as words and patient medical histories as documents can uncover the semantic similarity of different events, including diagnoses. Each indication, from what is likely to be a sizable list of those with biological proximity, is scored according to its similarity to one or more anchor references—perhaps the indication for which the asset was originally approved or, in the case of a novel asset, one for which there is strong preclinical evidence of efficacy of the asset’s mechanism of action (MoA).
The scoring can also incorporate information from molecular knowledge graphs that show new connections—for example, between entities such as proteins or human biological pathways that have already been identified in literature or public data. Exhibit 1 shows a potential outcome of this approach: it can reveal the indications most strongly connected to each of the reference indications, serving as an anchor into the existing evidence base. The indications with the most connections to these references are further prioritized by unmet medical need, strategic fit, and technical feasibility, resulting in an evidence-based indication selection.
These analytical approaches can identify novel indications that can be rapidly validated via in vitro or animal models, can increase the confidence in selecting indications with a high probability of success, and can derisk resource allocation accordingly. The analysis provides a clearer and more holistic evidence base for investors, shareholders, and R&D leaders, and it can reduce the opportunity cost of blind alleys by helping new treatments reach patients faster.
Subgroup discovery for trial design
Once an asset has been matched with an indication, pharmaceutical companies pursue an all-comer clinical development and trial design strategy—that is, one that includes all patients except those deemed high-risk based on input from key opinion leaders and conventional wisdom. 5 The use of the all-comer strategy may reflect a low level of confidence in existing hypotheses that currently seek to explain response heterogeneity in patient subpopulations. As a result, patients unlikely to respond to a treatment may be included in the trial. A notable exception to this method is in precision oncology, where researchers use specific biomarkers discovered preclinically (such as genetic mutations) to stratify patients according to their probability of progression or to predict a patient’s response to different treatments. This approach is revolutionizing oncology treatments, but even it can identify only a fraction of possible patient subpopulations.
In contrast, AI and omics-rich RWD can examine thousands of genetic and/or phenotypic attributes to pinpoint the combinations most likely to influence prognostic or predictive scores, explain response heterogeneity, and improve a trial’s technical probability of success. Even in situations where the patient cohorts are modestly sized, foundational models trained on broad RWD can be used as a starting point for more bespoke modeling at the indication level.
One large biopharmaceutical company used RWD to remove likely nonresponders from a trial and to reduce its expected duration by between 5 and 10 percent without compromising its probability of success, which would allow the treatment to reach patients faster. Another company leveraged hospital episode RWD to identify super-responders to its comparator arm for an asset in Phase I, ensuring the trial was designed in a manner that focused on patients without access to effective treatments.
AI for subgroup discovery can also directly support the strategic trade-off between the size of the eligible population and the level of the treatment effect in a smaller population, in the form of an efficient frontier (Exhibit 2). The different subpopulations that are marked as blue on the exhibit are those for which it is impossible to simultaneously increase their breadth without reducing the drug’s expected effect. This approach widens the overlap between patients with access to this new treatment and patients who are most likely to benefit significantly from it.
Subgroup discovery and comparative efficacy for portfolio optimization
Not only can AI be used to discover subgroups to inform the design of a trial, it also can contribute to the wider portfolio strategy. The product pipelines and portfolios of many biopharmaceutical companies contain multiple assets aimed to treat a similar set of patients, often because companies lack sufficient evidence to inform the portfolio strategy differently, especially for new MoAs. However, the systematic analysis of all available data sources can predict the response of different patient subgroups (or disease endotypes), which could help companies hone their portfolio strategy—in some cases, resulting in a double-digit percentage improvement in net present value. This strategic stance can support targeted trials that produce the type of precision evidence that clinicians need to make the best possible treatment decisions for their patients amid a proliferation of novel assets.
In addition, several analytical approaches and data sources can be combined to determine the efficacy of novel MoAs in the portfolio relative to each other or to approved treatments of different patient subgroups. Enriching RWD with data from molecular knowledge graphs can enable foundational-model-powered representations of treatments that represent their associated biological pathways. From this, the company can estimate the efficacy of a novel treatment by observing the outcomes of approved treatments that share similar biological action mechanisms with the novel treatment. This exercise can be repeated across multiple disease endotypes or patient subgroups, identified through a combination of patient-level attributes (Exhibit 3).
End point optimization in clinical development
In many trials, there is no single, established end point. In others, it can be hard to know which of several would best measure the intended action of the drug. Some end points may take a long time to manifest, leading to extended development timelines. Others may detect progression that is more reliable for some types of patients than for others or that may be particularly invasive, increasing the trial’s burden on patients.
AI used on rich, patient-level longitudinal data sets can be deployed to identify patient attributes that closely track the primary end point over time. Such an application can control multiple confounding factors to ensure the association persists in future trials where the patient population may differ, thereby establishing potential novel end points.
Here, X-rays, CT and MRI scans, and other imaging techniques are often valuable sources of data for monitoring biomarkers in a noninvasive manner (as is the case with using MRIs to help detect Alzheimer’s disease 6 Ruth Stephen et al., “Change in CAIDE dementia risk score and neuroimaging biomarkers during a 2-year multidomain lifestyle randomized controlled trial: Results of a post-hoc subgroup analysis,” Journals of Gerontology: Series A , 2021, Volume 76, Issue 8. ). Foundational models using medical imaging are increasingly able to extract imaging biomarkers curated by human expertise from raw images at scale. They can even discover altogether novel, deeply hidden visual signatures of disease activity and severity with better predictive characteristics.
In all cases, the association between novel end points and desired patient outcomes such as survival or quality of life must persist even under treatment. The clinical data and the information present in RWD must be combined with a biological understanding of the mechanisms affected by the considered treatment.
Tackling the organizational challenges
Inevitably, supplementing a century-long, RCT-dependent approach to clinical development with a new, analytics-driven one using novel data poses organizational challenges. Here are some ways to tackle them.
Embed AI into clinical development
The governance processes and incentive structures in place in most biopharmaceutical companies encourage default solutions in clinical development at the asset team level and beyond. One consequence is the prevalence of all-comer trials, which target the broadest possible population without leveraging evidence about nonresponders and super-responders—a method that can increase trials’ duration and decrease their probability of success, resulting in longer waits for patients who would benefit from the treatment.
R&D leaders can help change that culture by encouraging the use of analytical methods to integrate all sources of available evidence when making key decisions. Individual incentives can help. A stronger step is to strengthen the development governance model to require that all key decisions be supported by AI.
Develop internal capabilities and talent
External providers can supply off-the-shelf AI solutions to support some use cases. But with so many new solutions emerging, no provider can yet support them all. Even if they could, companies would be unwise to become dependent upon them for critical strategic decisions. Providers with such rich intellectual property might eventually become competitors.
Biopharmaceutical companies could therefore consider building their own AI capabilities and products. However, significant investment is needed to acquire the relevant data sets; build up the necessary data science, data engineering, and business translator expertise; and establish agile product development processes that ensure use cases deliver business value in a timely and cost-efficient manner.
Companies will also have to work hard to attract and retain top data scientists and data engineers, as the biopharmaceutical industry is not typically their first choice. Measures that can help include offering competitive remuneration packages, building an innovative employer brand, and locating in key talent hubs. So, too, can a focus on the industry’s value proposition—offering employees the chance to help people lead longer and healthier lives.
Develop a targeted data strategy
With so much data becoming available, companies should consider formulating a detailed and iterative data strategy for each disease area. The purchase of certain commercial data sets, such as electronic health records, claims, and sometimes omics, can be relatively straightforward, even in therapeutic areas such as oncology, where biomarker-rich data are required. But other types of data sets—registries, biobanks, and clinical trials from other pharma companies—can involve complex negotiations and contracts, and companies will have to partner with a range of different data providers.
In addition, each data set is likely to be structured differently, with varying levels of quality and completeness. This means companies will likely need data curation and analytical capabilities for tasks such as combining different data sets to plug gaps in evidence and meeting regulatory expectations for the use of real-world data.
Build scalable products
AI’s power lies in its application across the clinical development portfolio, not restricted to isolated use cases. Achieving this kind of scale can be hard, due to limitations of the code base, data platforms, the company’s data engineering capabilities, and the analytical stack needed to collect, combine, and analyze data. As a result, pilots often remain just that—never reused or deployed against other assets in development.
To counter this, analytical use cases should be built as products, meaning solutions built in a manner that makes them easy to reuse and scale for different use cases, even at the proof-of-concept phase. The code base should be well annotated, for example, and deploy common, reusable analytical components that are either developed internally, open-source, or bought off the shelf. Shared ML development standards and coding frameworks can help by speeding up development work across different use cases because data scientists and engineers are familiar with them. Companies also need a mature and flexible ML operations stack—that is, the operational components needed to streamline the process of taking ML models to production and maintaining and monitoring them.
By harnessing novel data and the power of AI, biopharmaceutical companies can move beyond RCTs to vastly improve clinical development. The benefits accrue at each stage of the development process, from formulating asset strategy to designing the protocol and trial planning. For patients, the results can speed up access to new treatments, thanks to an accelerated development timeline, and make treatments more likely to elicit the strongest response.
High-impact solutions are already being implemented, and more will follow. Companies can benefit from beginning to build the capabilities they need and scaling them across the portfolio and their assets’ life cycles. The clinical development and trial design system is ripe for change, and the value to patients will be vast.
Chris Anagnostopoulos is an associate partner in McKinsey’s Athens office, David Champagne and Alex Devereson are partners in the London office, Thomas Devenyns is an associate partner in the Geneva office, and Heikki Tarkkila is an associate partner in the Helsinki office.
Explore a career with us
Related articles.

Generating real-world evidence at scale using advanced analytics

How AI could revolutionize drug discovery

AI in biopharma research: A time to focus and scale

IMAGES
VIDEO
COMMENTS
This is not a cro rankings list, but rather a compiled list of some of the top clinical research organizations around the world. We have highlighted their strengths and core service offerings to make it easier for you to find the right fit clinical research partner.
Quick List of Top 15 Clinical Research Companies. Here is a quick overview of the best companies of clinical research: IQVIA: Best for data-driven insights and advanced analytics in healthcare research. ICON: Best for comprehensive clinical development services and therapeutic expertise.
9 Notable AI Companies in Clinical Research to Watch in 2023. The clinical trial is a critical stage of drug development workflow, with an estimated average success rate of about 11% for drug candidates moving from Phase 1 towards approval.
AI-driven companies operate with multiple data types, such as Electronic Health Records (EHR), omics, and imaging data to reduce population heterogeneity and increase clinical study power.
A company called Intelligent Medical Objects in Rosemont, Illinois, has developed SEETrials, a method for prompting OpenAI’s large language model GPT-4 to extract safety and efficacy...
AI is accelerating drug discovery, research, and clinical trials. We look at why clinical development also needs to keep pace with artificial intelligence.