Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.


StatPearls [Internet].
Viral hepatitis.
Parth Mehta ; Lafaine M. Grant ; Anil Kumar Reddy Reddivari .
Affiliations
Last Update: March 10, 2024 .
- Continuing Education Activity
Hepatitis is defined as inflammation of the liver that can result from a variety of causes, such as heavy alcohol use, autoimmune disorders, drugs, or toxins. However, the most frequent cause of hepatitis is due to a viral infection, referred to as "viral hepatitis." Several different strains of viruses can cause hepatitis. In the United States, the most common types of viral hepatitis are hepatitis A, hepatitis B, and hepatitis C. The other types of viral hepatitis are hepatitis D and E, which are encountered less frequently.
The severity and duration of the hepatitis are variable depending on the pathogen and comorbidities. Based on the etiology of hepatitis, the severity can range from mild, nearly asymptomatic, to severe illness requiring liver transplantation. Hepatitis can be further classified into "acute" and "chronic" based on the duration of the inflammation in the liver. Depending on the type, hepatitis can be self-limited and resolve quickly, while others can become more chronic and last years. Some individuals may rapidly progress to fulminant liver failure, while others may be asymptomatic carriers.
This activity reviews the causes of viral hepatitis, its epidemiology, symptomatology, patient evaluation, treatment, prognosis, and preventive strategies. It also highlights the role and importance of a multidisciplinary healthcare team in caring for patients with viral hepatitis.
- Differentiate the etiology, epidemiology, and pathophysiology of the various types of viral hepatitis.
- Implement evidence-based treatment and preventive strategies for viral hepatitis.
- Identify common complications of viral hepatitis.
- Apply effective interprofessional team strategies for improving care coordination and communication to advance and improve outcomes for patients with viral hepatitis.
- Introduction
Hepatitis is defined as inflammation of the liver that can result from a variety of causes, such as heavy alcohol use, autoimmune disorders, drugs, or toxins. However, the most frequent cause of hepatitis is due to a viral infection, referred to as "viral hepatitis." In the United States, the most common types of viral hepatitis are hepatitis A, hepatitis B, and hepatitis C. The other types of viral hepatitis are hepatitis D and E, which are less frequently encountered. [1]
Based on the etiology of the hepatitis, the severity can range from mild and self-limiting to severe illness requiring liver transplantation. Hepatitis can be further classified into "acute" and "chronic" based on the duration of the inflammation in the liver. Inflammation lasting less than 6 months is defined as "acute," and inflammation lasting greater than 6 months is defined as "chronic." Although acute hepatitis is usually self-limiting, it may also cause fulminant liver failure, depending on the etiology. In contrast, chronic hepatitis may result in the development of liver fibrosis, cirrhosis, hepatocellular carcinoma, and features of portal hypertension, leading to significant morbidity and mortality. [2] [3]
The majority of cases of viral hepatitis result from hepatotropic viruses A, B, C, D, and E. It is unclear whether the hepatitis G virus (HGV) is pathogenic in humans. Other less common causes of viral hepatitis are cytomegalovirus (CMV), Epstein-Barr virus(EBV), herpes simplex virus (HSV), and varicella-zoster virus (VZV). These are non-heterotropic viruses that usually do not primarily target the liver and rarely cause hepatitis in the immunocompetent state.
Hepatitis A, B, C, and D are endemic to the United States, with hepatitis A, B, and C viruses causing 90% of acute viral hepatitis and hepatitis C being the most common cause of chronic hepatitis.
Hepatitis A
The hepatitis A virus (HAV) is an RNA virus from the Picornaviridae family. It is usually present in the highest concentration in the stool of infected individuals, with the greatest viral load shedding occurring during the end of the virus's incubation period. The most common mode of transmission of hepatitis A is via the fecal-oral route from contact with food, water, or objects contaminated by fecal matter from an infected individual. It is often encountered in developing countries with poor sanitation and an increased risk of fecal-oral spread. In the United States, the most common risk factor for contracting hepatitis A is international travel. Those who come in contact with infected individuals are also at risk, and the secondary infection rate for household contacts is approximately 20%. [4]
Hepatitis B
Hepatitis B virus (HBV) is a DNA virus and a member of the Hepadnaviridae family. The composition of the viral core is nucleocapsid, hepatitis B core antigen (HBcAg), which surrounds hepatitis B virus DNA, and DNA polymerase. The nucleocapsid is coated with the hepatitis B surface antigen (HBsAg), a viral surface polypeptide. Intact hepatitis B virus virion is known as the Dane particle. Hepatitis B virus is known to have 8 genotype variants but is not used in clinical practice to determine the severity of infection. The gene that codes for HBcAg also codes for hepatitis B e antigen (HBeAg).
HBV can be detected in serum, semen, vaginal mucus, saliva, and tears, even at a lower level but is not found in stool, urine, or sweat. It is transmitted parenterally and sexually when individuals come in contact with mucous membranes or body fluids of infected individuals. Intravenous drug users, men who have sex with men, healthcare workers with exposure to infected body fluids, patients who require frequent and multiple blood transfusions, people who have multiple sexual partners, prisoners, partners of HBV carriers, and persons born in endemic areas are all at high risk for contracting HBV infection.
Transfusion of blood and blood products, injection drug use with shared needles, needlesticks, or skin penetrating wounds caused by other instruments used by healthcare workers, and hemodialysis are all examples of parenteral and percutaneous exposures. The parenteral mode remains the dominant transmission mode globally and in the United States.
The virus can be transmitted perinatally as well. This occurs in infants of women who are HBeAg positive, and the infants have up to a 70% to 90% chance of infection. Up to 90% of those who become infected develop a chronic infection with HBV. [5]
Hepatitis C
Hepatitis C virus (HCV) is an RNA virus and is a member of the Flaviviridae family with 1 serotype but at least 6 major genotypes and more than 80 subtypes. The extensive genetic variability makes it challenging to develop a vaccine to prevent infection with HCV. Transmission can be parenteral, perinatal, and sexual. Those at the highest risk for infection include those injecting illicit drugs, as there tends to be sharing of contaminated needles. Others who are susceptible are those who require frequent blood transfusions and transplantation of organs from infected donors. Sexual and perinatal transmission is not very common. [6]
Hepatitis D
The hepatitis D virus (HDV) is an RNA virus and a single species in the Deltaviridae family. It contains the hepatitis D antigen and RNA strand and requires the presence and help of the hepatitis B virus for replication. It uses HBsAg as its envelope protein. Therefore, those who are infected with HDV also have coinfection with HBV as well. HDV has modes of transmission similar to HBV, but perinatal transmission is uncommon. [7]
Hepatitis E
The hepatitis E virus (HEV) is a non-enveloped RNA virus and a single species in the Caliciviridae family of the Herpesvirus genus. The primary transmission mode of the HEV is the fecal-oral route. Contaminated water is the most common source of infection. Person-to-person transmission is rare. However, occasionally, maternal-neonatal transmission can occur. [8]
Hepatitis G-Human Pegivirus
Hepatitis G virus ((HGV), now designated human pegivirus (HPgV-1), is an RNA virus and is a member of the Flaviviridae family. The primary mode of transmission is through infected blood and blood products, but sexual contact and vertical transmission occur as well. It is often manifested as a coinfection in those who have chronic hepatitis B or hepatitis C infections. It has been implicated in patients with acute and chronic liver disease, but the evidence to date has not established it as an agent that causes hepatitis by itself. [9]
- Epidemiology
Viral hepatitis is a major public health issue. Viral hepatitis infects millions of people annually, causing significant morbidity and mortality. Chronic hepatitis B and C infections can cause liver damage that includes liver fibrosis, cirrhosis, hepatocellular carcinoma, and features of portal hypertension. The World Health Organization (WHO) estimated that 1.3 million people died due to hepatitis in 2015, and 1 in 3 people in the world have had infections with either HBV or HCV. Viral hepatitis can cause up to 1.4 million deaths annually, and HBV and HCV are responsible for about 90% of those deaths. Reportedly, infection rates show that 2 billion people are infected with HBV, 185 million with HCV, and 20 million with HEV worldwide. [10] [11] [12]
Hepatitis A
In the US, approximately 24,900 new HAV infections are diagnosed each year. Typically, those with access to safe drinking water and sanitation services have low infection levels with HAV, with <50% of the population being endemic. Those who do not have access to safe drinking water and regions with low socioeconomic benefits have higher levels of HAV infection. HAV affects 90% of children in highly endemic regions, with more than 90% of the population being endemic. [13] [14] HAV's infection rate is much higher worldwide, but only 1.5 million cases are reported annually. Children who become infected early with asymptomatic exposure acquire lifelong immunity in highly endemic countries. [15]
Hepatitis B
Estimates are that about one-third of the world's population has had an infection with HBV, and around 5% of this population remains carriers of HBV. About 25% of these carriers develop chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. The documented number of global deaths annually from HBV infection is 780,000. [16] [17] In the United States, approximately 22,600 new infections were diagnosed in 2018, and it is estimated that 862,000 people have chronic HBV infection.
Hepatitis C
HCV is the most prevalent cause of parenteral hepatitis worldwide. It is prevalent in 0.5% to 2% of the population around the world, with IV drug users and hemophiliacs being the most commonly affected. However, with safer screening and viral elimination techniques for blood transfusion, the incidence of transfusion-associated HCV infection is decreasing. Approximately 71 million people globally have chronic HCV infection, and this accounts for close to 400,000 deaths every year. [18] [19] In the US, approximately 50,300 new HCV infections were diagnosed in 2018, and it is estimated that 2.4 million people have chronic HCV infection.
Hepatitis D
HDV infection occurs in patients who are positive for HBsAg (meaning that the person is infected with HBV and is contagious). HDV occurs as a coinfection with HBV or as a superinfection for patients with a chronic HBV infection. HDV infection is not reportable in the United States; therefore, accurate data is not readily available. It is estimated to affect 4% to 8% of cases of acute HBV and 5% of global chronic HBV patients. Approximately 18 million patients are infected with HDV globally. Prevalence remains high in South America and Africa as well among specific populations such as sex workers in Greece and Taiwan, where it has been well studied. [20]
Hepatitis E
HEV is associated with worldwide outbreaks of food and waterborne diseases. It is commonly seen in developing countries with restricted access to sanitation, clean water, and poor hygiene. About 20 million people are estimated to be infected with HEV globally, and there have been approximately 44,000 deaths due to HEV infection. In that regard, HEV carries a higher risk of mortality at about 3.3% compared to HAV infection despite a similar route of transmission and lack of chronicity. [21]
Human Pegivirus
Global infection with HPgV-1 is common, with a worldwide prevalence of around 3%, and detectable in all ethnicities. Researchers believe that 1% to 4% of blood donors worldwide are carriers of the virus. The general understanding is that around 25% of the global population carries an antibody to the virus. Infected blood products increase the prevalence of HPgV-1. Routine blood screening of donation for HPgV-1 is not done, presumably due to the lack of evidence for causing disease. Reports indicate that approximately 10% to 20% of adults with chronic HBV or HCV infection have HPgV-1, indicating that coinfection is a common occurrence. The coinfection does not increase the severity of the HBV or HCV disease. Vertical transmission of HPgV-1 from an infected mother to the newborn has been documented, but this is rare. [22]
- Pathophysiology
The pathophysiology of viral hepatitis varies with the type of hepatitis pathogen.
The incubation period of HAV is approximately 4 weeks. Acute infections with HAV are more severe, with higher mortality in adults than children. Symptoms of HAV infection include malaise, anorexia, nausea, vomiting, and jaundice. The illness lasts approximately 8 weeks and is self-limiting without treatment. Relapses are not common, and the infection does not result in chronic hepatitis. Less than 1% of cases result in fulminant hepatic failure. [23]
The incubation period of an acute HBV infection is approximately 12 weeks, with most patients experiencing mild illness, which is self-limiting within 6 months—less than 1% experience fulminant hepatic failure. After acute infection resolves, most adult patients and a small percentage of infected infants develop antibodies against the HBsAg and recover fully. However, a small percentage of adult patients and the majority of infected infants develop chronic infection. Approximately 10% to 30% of carriers become symptomatic from chronic infection. They may also have extrahepatic manifestations of the disease. About 20% of chronic hepatitis patients develop cirrhosis and hepatic decompensation, while 5% develop hepatocellular carcinoma. [24]
The incubation period of HCV is approximately 8 weeks. Most cases of acute HCV infection are asymptomatic. However, approximately 55% to 85% of patients develop chronic HCV infection and liver disease; 30% of those patients progress to developing cirrhosis. Patients who are infected with HCV and have a chronic infection are at high risk of developing hepatocellular carcinoma in the setting of cirrhosis. Before the availability of effective treatment for this infection, an estimated 20,000 deaths annually were attributable to chronic HCV infection. [25]
The incubation period for HDV is approximately 13 weeks. HDV infection causes hepatitis only in persons with acute or chronic HBV coinfection. Symptoms are similar to acute HBV infection. Still, patients with chronic HBV infection and HDV infection tend to progress more rapidly to cirrhosis than those patients with chronic HBV infection alone. Patients already infected with HBV may develop superinfection with the HDV. Superinfection can result in fulminant hepatic failure. [20]
The incubation period for HEV is approximately 2 to 10 weeks. Acute hepatitis E virus infection is less severe compared to acute HBV infection. Still, pregnant females infected in the third trimester have a >25% mortality associated with HEV infection. [26]
The incubation period of human pegivirus (HPgV-1) is approximately 14 to 20 days. The clinical picture can be similar to the picture of mild hepatitis infection with other hepatitis viruses, and patients can have normal or low aminotransferases without jaundice. Alanine aminotransferase (ALT) elevation is not proportional to the degree of viremia in HPgV-1, unlike HCV, where patients with high viral load can have higher alanine aminotransferase (ALT) levels. [22]
- History and Physical
The clinical presentation of viral hepatitis can be different in each individual, depending on the type of hepatitis virus causing the infection. Patients can be entirely asymptomatic or only mildly symptomatic at presentation. A small number of patients can present with rapid onset of fulminant hepatic failure.
Typically, patients with viral hepatitis go through 4 phases regardless of the type of hepatitis virus infection. Physical exam findings specific to each type of hepatitis are listed below, along with overall general findings on the physical exam.
- Phase 1 (Incubation/Viral Replication Phase) - Patients are usually asymptomatic in this phase, and laboratory studies are positive for markers of hepatitis.
- Phase 2 (Prodromal Phase) - Patients in this phase may present with anorexia, nausea, vomiting, malaise, pruritus, urticaria, arthralgias, and fatigue. These patients are often diagnosed as having gastroenteritis or other viral infections.
- Phase 3 (Icteric Phase) - Patients in this phase present with dark-colored urine and pale-colored stool. Some patients develop jaundice and right upper quadrant pain coupled with liver enlargement. Liver enzymes will be elevated.
- Phase 4 (Convalescent Phase) - Patients typically start noticing the resolution of symptoms, and laboratory studies show liver enzymes returning to normal levels. [27]
Patients who are infected with the HAV usually present with symptoms similar to gastroenteritis or a viral respiratory infection, including symptoms of fatigue, nausea, vomiting, fever, jaundice, anorexia, and dark urine. Symptoms usually start after the approximately 4-week incubation period, and they resolve spontaneously in most patients. [4]
Patients with HBV infection enter the prodromal phase after the incubation period of approximately 12 weeks and have symptoms of anorexia, malaise, and fatigue, which are the most common initial clinical symptoms. Some patients may experience right upper quadrant pain due to hepatic inflammation. A small percentage of patients experience fever, arthralgias, or rash. Once these patients progress to the icteric phase, they develop jaundice and painful hepatomegaly, dark-colored urine, and pale-colored stools. After the icteric phase, the clinical course can be variable, where some patients experience rapid symptom improvement. In contrast, others develop a prolonged illness with a slow resolution with periodic flareups. A small number of patients have rapid progression of the disease that can lead to fulminant hepatic failure over a few days to weeks. [27]
After the incubation period of approximately 8 weeks, patients infected with HCV develop symptoms similar to those of the acute infection phase in those with HBV infection. These include symptoms of anorexia, malaise, and fatigue. However, 80% of patients remain asymptomatic and do not develop jaundice. [6]
The majority of patients who have a simultaneous infection with HBV and HDV have a self-limited infection. Symptoms are similar to acute HBV infection. Chronic hepatitis B virus carriers who develop superinfection with HDV tend to have more severe acute hepatitis, and the majority of these patients will develop chronic HDV infection. Chronic infection with both HBV and HDV can lead to fulminant liver failure, severe chronic active hepatitis, and progression to cirrhosis in a majority of patients compared to those patients who only have chronic HBV infection. [28]
Patients with acute HEV infection develop an acute self-limited illness similar to HAV infection. Fulminant hepatic failure is rare, but patients with HEV infection who are pregnant have a higher mortality rate. [29]
Human Pegivirus
Patients with HPgV-1 infection can have a mild infection without jaundice, but most patients are asymptomatic. There have been few reports of fulminant and chronic hepatitis as well as hepatic fibrosis also, but it is not typical and not clearly due to HPgV-1 infection. The patient may also have an elevation of alkaline phosphatase (ALP) and gamma-glutamyl transpeptidase (GGT), but most patients infected with HPgV-1 have normal liver function tests. [22]
General Physical Examination
Physical findings vary in individual patients depending on the time of presentation. Patients may present with a low-grade fever. Patients may show signs of dehydration, especially if they have been having vomiting and loss of appetite. Findings like a dry mucous membrane, tachycardia, and delayed capillary refill can be observed. During the icteric phase, patients can have jaundiced skin or icteric sclerae and sometimes urticarial rashes. Tender hepatomegaly may be present. In advanced liver failure, signs of ascites, pedal edema, and malnutrition may be observed. [30]
Initial evaluation of a patient suspected of having viral hepatitis includes checking a hepatic function panel. A patient with severe disease may have elevated total bilirubin levels. Typically, alkaline phosphatase (ALP) levels remain in the reference range, but if elevated significantly, the clinician should also consider biliary obstruction. In advanced liver disease, prothrombin time (PT) and international normalized ratio (INR) may be prolonged, and leukopenia and thrombocytopenia may be present. Anemia may also be present in those with advanced liver disease from gastrointestinal blood loss due to portal hypertensive sequelae (eg varices). Elevated creatinine may be an indication of the severity of the acute liver injury with secondary renal impairment. A patient who presents with altered mental status should have serum ammonia level checked, as this is usually elevated in the presence of hepatic encephalopathy. [31]
In addition to these initial routine laboratory tests, other specific tests are available to evaluate for the type of viral hepatitis as follows:
The standard test for diagnosing acute infection with HAV is the presence of immunoglobulin M (IgM) antibodies to HAV. IgM antibody disappears a few months after the acute infection. Immunoglobulin G (IgG) antibody to HAV is detected in the later stages of infection. The presence of IgG antibodies to the HAV means that the patient has been infected with the HAV in the past, as recently as approximately 2 months to as long ago as several decades. Therefore, IgG antibodies to HAV do not indicate acute infection and should not be used to screen for such. Most patients with IgG antibodies to HAV have lifelong immunity against the HAV infection [23] .
Acute hepatitis B infection
The first serum marker to appear in a patient with an acute HBV infection is the HBsAg. This antigen indicates that the patient has hepatitis B viremia, but it does not differentiate between an acute versus chronic infection, especially in the absence of symptoms. When the patient exhibits signs of acute hepatitis, the presence of HBsAg strongly suggests acute HBV infection. However, it does not rule out chronic HBV infection with a flare or superinfection. HBsAg disappears approximately 6 months after the acute infection in those patients who clear the HBV. If HBsAg remains present for more than 6 months, it indicates a chronic infection. For those who clear the hepatitis B viremia, the antibody to HBsAg (anti-HBs) appears. This antibody may persist for a lifetime and offers immunity to the patients from subsequent exposure to HBV.
The first antibody to appear is the IgM antibody to hepatitis B core antigen (HBcAg). The presence of IgM anti-HBc also means that the patient has an acute hepatitis B virus infection, and this is required to make the diagnosis. Once the IgM anti-HBc disappears within a few weeks, IgG anti-HBc, which usually remains present for life, is detected. Total assay for antibody to hepatitis B core antigen (anti-HBc) can detect both IgM and IgG antibodies and indicates that the patient has a history of infection with HBV at some time in the past, as it remains positive both in patients who clear the virus and those who have a persistent infection.
Hepatitis B e antigen (HBeAg) is also present early in the infection and indicates that the virus is replicating. After the viral replication diminishes, HBeAg becomes undetectable, and antibodies to HBeAg (anti-HBe) appear and can persist in the blood indefinitely (see Table. Interpretation of Hepatitis B Screening Results).
Chronic Hepatitis B infection
Patients with chronic HBV infection will have a positive HBsAg. These patients may be inactive carriers of HBV or may have active chronic hepatitis. All patients with chronic HBV infection have the presence of anti-HBc. HBeAg may or may not be present in patients with active chronic hepatitis. When present, it is indicative of viral replication.
Similarly, HBV DNA may or may not be detectable, but high levels can indicate active chronic hepatitis. Patients with chronic HBV infection usually have an absence of anti-HBs. Still, the presence of anti-HBs with positive HBsAg in patients with chronic HBV infection indicates that the antibody was unsuccessful in inducing viral clearance.
Evaluating HBV infection can be complex, and some uncommon scenarios should be considered while investigating. Testing may be negative for HBsAg and anti-HBs but positive for anti-HBc. This may occur when the result is a false positive but may also happen in the setting of the period where the patient has cleared HBsAg from the blood but anti-HBs have not yet appeared. Some patients who have cleared HBV infection but have lost the anti-HBs over time can have a negative test for HBsAg and anti-HBs but positive for anti-HBc. Also, patients infected with HBV in the remote past may develop a core mutant variant of HBV where testing is negative for HBeAg and positive for anti-HBe even though the virus is active and replicating. In the setting of the aforementioned laboratory results, checking for viral replication with HBV DNA PCR assay is generally recommended.
Patients who receive a vaccine for the hepatitis B virus develop protective anti-HBs as a response to recombinant HBsAg in the vaccine. There are no hepatitis B virus DNA or other hepatitis B virus-associated proteins in the vaccine. Therefore, patients who receive the vaccine are not positive for anti-HBc unless they have a previous infection with the hepatitis B virus. [32]
The Centers for Disease Control and Prevention (CDC) updated guidance in 2020 includes recommendations for 1-time screening for HCV infection for all adults 18 years and older and pregnant women during each pregnancy, except for instances when the prevalence of HCV infection is <0.1%. [33] Testing guidance otherwise remains the same for those with identifiable risk factors such as birth years between 1945 and 1965, blood product transfusion or organ transplant before 1992, those on chronic hemodialysis, and those with a history of injection drug use. [34] [35]
HCV exposure can be detected with antibodies to the HCV (anti-HCV). These assays can detect antibodies within 4 to 10 weeks of infection. However, anti-HCV can remain negative for a few months after an acute HCV infection, although once it appears in the blood, it generally remains present for life. Unlike anti-HBs, anti-HCV is not a protective antibody and does not offer immunity with subsequent exposure to HCV.
In the setting of a positive hepatitis C antibody test, HCV RNA testing is necessary to confirm infection. It is the most specific test for HCV infection and can detect acute infection even before antibodies develop. It is also helpful in discriminating between true infection and false-positive cases, indeterminate results, and rare cases where perinatal transmission may have taken place. (see Table. Hepatitis C Stages and Serological Markers). HCV RNA testing also provides information on viral load and HCV genotype, which can help tailor treatment and predict response to therapy. In recent years, testing for HCV genotype has become less important as some of the available treatment options are pan-genotypic (able to effectively treat infection regardless of the genotype). Knowing the genotype may be more relevant in previously failed treatment patients. During the initial evaluation, patients should be asked about prior treatment as this may play a role in medical regimen selection, and testing for viral resistance may be indicated.
Assessment of hepatic fibrosis is an important step in evaluating those confirmed to have HCV infection. The degree of fibrosis plays a role in determining the treatment regimen and duration if there is advanced (bridging) fibrosis or cirrhosis. Fibrosis stage can be assessed by indirect markers readily found in routine blood tests such as the AST/ALT ratio, the AST to platelet ratio index (APRI), and the Fibrosis-4 score (online calculators available). Noninvasive assessment of fibrosis can also be obtained via ultrasound-based transient elastography. Liver biopsy has previously been accepted as the gold standard of fibrosis assessment. However, it is an invasive procedure with a small risk of complications not limited to bleeding and infection. In addition, the high cost and sampling error makes it a less desirable option. [36] [37] [38] Liver biopsy is generally reserved for cases of concern for a competing or concomitant diagnosis. (see Image . Liver Biopsy Hematoxylin and Eosin Stain).
Liver enzyme testing can indicate the presence of liver injury but is not very accurate in predicting the severity of the disease. Liver function testing can be more valuable in monitoring therapeutic response to HCV treatment, with improvement in aminotransferase levels indicating patients responding to the treatment while worsening of aminotransferase levels post HCV treatment may indicate a relapse. However, it is more common to use HCV RNA as the test of choice to monitor the response to treatment. [39] [40]
HDV infection is diagnosed by checking for IgM and IgG antibodies to the HDV (anti-HDV). IgM antibody to HbcAg (anti-HBc) must be checked to differentiate a coinfection where the patients test positive for IgM anti-HBc and superinfection, where the patients test negative for IgM anti-HBc. (see Table. Hepatitis D Stages, Serological Markers, and the Three Infective Patterns). HDV RNA test can be done but is not routinely performed. [41]
HEV infection is diagnosed by checking for IgM and IgG antibodies to the HEV (anti-HEV). Also, HEV RNA can be checked in the serum and stool of infected patients. [42]
HPgV-1 is usually identified by checking the hepatitis G RNA PCR. Most patients develop antibodies, which are detectable after the clearance of the virus. The coexistence of HPgV-1 RNA and antibody to HPgV-1 is not common. Following the clearance of the virus, the antibody appears, and patients fully recover. Patients with persistent HPgV-1 RNA may have no biochemical or histological signs of liver disease. A small percentage of patients with chronic HPgV-1 may go on to develop liver fibrosis. [9]
- Treatment / Management
The general management of acute viral hepatitis is supportive, and most individuals can be safely monitored in the outpatient setting. The infection is usually self-limited in most cases. Efforts should be made to prevent disease transmission to those in close contact with the patient.
Patients who are experiencing significant nausea or vomiting and those at greatest risk for dehydration should be admitted for intravenous fluids for hydration. Potentially hepatotoxic substances, such as alcohol, should be avoided, and medications that are metabolized through the liver, such as acetaminophen, should be used with caution. The development of rare complications like liver abscess or acute liver failure requires admission and appropriate management. Referral to specialty services such as gastroenterology or hepatology should be considered for cases that are atypical in severity or duration.
Treatment for acute HAV infection is supportive. There is no antiviral therapy for HAV infection. Patients who have intractable nausea or vomiting, as well as those who are showing signs of liver failure, should be admitted and closely monitored. [4]
Treatment of HBV infection falls into 2 categories: acute HBV infection and chronic HBV infection.
Treatment of acute HBV infection is supportive and similar to the treatment of acute HAV infection unless a more severe infection is present. Medication treatment should be considered for those who present with acute liver failure (INR >1.5, bilirubin >10 mg/dL), a severe protracted course (>4 weeks), those who are immunocompromised or have coinfection with other viruses such as HCV or HDV. If therapy is instituted, nucleoside or nucleotide analogs are generally recommended. Historically, the nucleoside analog lamivudine has been used with favorable results. [43] However, due to concerns for the development of drug resistance, another nucleoside or nucleotide analog is preferred, especially if the duration of treatment may be long-term based on the clinical scenario. Interferon therapy should be avoided due to the risk of hepatic necroinflammation and worsening liver condition and the potential adverse effects.
Chronic hepatitis B infection
The primary goal of treatment for chronic HBV infection is inhibiting viral replication. This is manifested by suppression of HBV DNA and subsequent loss of hepatitis B e antigen (HBeAg). Secondary goals include reducing symptoms and preventing the progression of chronic hepatitis to cirrhosis or developing hepatocellular carcinoma. First-line agents include pegylated interferon alfa-2a (PEG-IFN) and oral nucleoside or nucleotide analogs. Therapy should be selected based on individual patient profiles, patient preference, safety, efficacy, treatment cost, and drug resistance risks.
Pegylated interferon (PEG-IFN) treatment duration is 48 weeks for both HBeAg positive and negative chronic hepatitis. It is best used in younger patients and those with uncomplicated disease who prefer a finite treatment duration. Advantages of pegylated interferon (PEG-IFN) treatment include high rates of seroconversion (loss of HBVeAg) within 1 year of therapy, loss of HBVsAg 3 years after treatment, and absence of resistance. However, it has many adverse effects, including flu-like symptoms, fatigue, weight loss, depression, and bone marrow suppression, and is generally not well-tolerated. Given these considerations, compliance remains a considerable issue. Pegylated interferon (PEG-IFN) treatment is contraindicated in patients who have uncontrolled psychiatric disorders, autoimmune conditions, pregnancy, decompensated cirrhosis, and blood dyscrasias.
Treatment with oral nucleoside or nucleotide analog agents is usually for an indefinite period, as withdrawal of treatment usually results in relapse. The advantages of treating with an oral regimen include ease of administration, fewer adverse effects, and an excellent safety profile. The nucleotide analog tenofovir is contraindicated in children and, in rare cases, can cause renal insufficiency, decreased bone density, Fanconi syndrome, and proximal tubular acidosis. Dose adjustment is required for these medications in patients with renal insufficiency. Patients for whom pegylated interferon (PEG-IFN) is contraindicated usually tolerate these oral agents well. These agents have a potent antiviral effect, with viral suppression seen in more than 90% of patients over 5 years and subsequent fibrosis regression. The effectiveness of combination therapy with 2 oral agents or 1 oral agent with pegylated interferon (PEG-IFN) is not well established. [44]
Treatment of HCV infection may also be divided into acute HCV infection and chronic HCV infection.
Acute hepatitis C infection
Acute HCV infection is infrequently detected as most cases are asymptomatic. However, when detected, immediate treatment is recommended. [45] The treatment is similar to that outlined below for chronic HCV infection.
Chronic hepatitis C infection
The primary goal of treatment for chronic HCV infection is the eradication of HCV. A cure of the viral infection, also known as a sustained virologic response (SVR), is defined as the lack of viral detection in the blood 12 weeks after receiving anti-HCV therapy. Patients who achieve this endpoint have a 99% or greater chance of maintaining the cure with a later appearance of viremia attributed to reinfection. [46] Cure of the hepatitis C infection reverses liver inflammation, fibrosis, and associated changes related to portal hypertension. [47] [48] There is also ample evidence that the elimination of HCV results in a decreased risk of developing hepatocellular carcinoma and an overall reduction in all-cause mortality. [48] [49] Patients also generally report improvement in symptoms of fatigue and general well-being. Treatment of HCV infection is also an indirect path to treating extrahepatic manifestations of the virus, such as glomerulonephropathies, cryoglobulinemic vasculitis, and non-Hodgkin lymphoma. The treatment regimen selection is determined by the patient's treatment history (whether naive or experienced), the degree of fibrosis or the presence of cirrhosis, and whether there are any complications of cirrhosis. Additional treatment selection considerations are given to unique patient populations such as those with HIV/HCV coinfection, decompensated cirrhosis, those after liver or kidney transplant, children, and during pregnancy.
Historically, pegylated interferon(PEG-IFN) alfa and ribavirin were the mainstay of treatment. The number of patients eligible for treatment was limited due to the potential adverse effects of interferon, such as debilitating fatigue and flu-like symptoms, bone marrow suppression with resulting thrombocytopenia and leukopenia, retinopathy, and worsening of psychiatric disorders. Ribavirin therapy carried the risk of hemolytic anemia requiring growth factor or transfusion. For eligible patients, poor tolerability limited the course of treatment, and the overall cure rate was low.
Since the introduction of the second generation of direct-acting antiviral (DAA) agents in 2013 and 2014, interferon-based therapy has become obsolete. It is now contraindicated in the management of HCV infection. Ribavirin use can be considered in particular circumstances, such as decompensated cirrhosis or those with viral resistance to prior therapy. With the availability of an all-oral regimen that is well tolerated and minimal pretreatment testing required, an increased number of patients are eligible for anti-HCV treatment.
For most treatment-naive patients without cirrhosis, the preferred regimen includes the pan-genotypic option of glecaprevir/pibrentasvir administered as 3 combination pills 3 times daily for 8 weeks or sofosbuvir/velpatasvir administered as 1 pill daily for 12 weeks. The choice of regimen is mainly influenced by the preference for the duration of therapy versus the number of pills and payer coverage. Both regimens have sustained virologic response (SVR) rates exceeding 95% and are well tolerated. Both combinations are safe to use in patients with renal insufficiency; glecaprevir/pibrentasvir should be avoided in decompensated cirrhosis. There are some potential severe drug interactions with either regimen, so it is necessary to run a drug interaction check before prescribing so that any necessary adjustments can be made. Additional regimens include sofosbuvir/daclatasvir (for genotypes 1, 2, 3, and 4), ledipasvir/sofosbuvir (for genotypes 1, 4, 5, and 6), and elbasvir/grazoprevir (for genotypes 1 and 4). These second-line agents require additional genotype and resistance testing and are indicated for patients with specific favorable clinical profiles. Sofosbuvir/daclatasvir is not available in the United States. These factors render them suboptimal options for consideration.
Those patients who are treatment-experienced, have decompensated cirrhosis, or are otherwise part of a unique population (HCV with HIV coinfection, solid organ transplant recipient, organs from HCV-viremic donors) require more complex evaluation and management and should be referred for expert care. Recommendations for management are outlined in the updated guidance by the joint association of The Infectious Diseases Society of America and the American Association for the Study of Liver Diseases. [45] Dynamic changes in recommendation can be viewed online at https://www.hcvguidelines.org/.
Patients who are coinfected with HDV and HBV usually receive treatment with pegylated interferon (PEG-IFN). Oral nucleoside and nucleotide analogs have limited or no efficacy in these patients. Pegylated interferon remains the only effective treatment. In 1 study, patients who had HDV infection with coexisting HIV infection had therapy with tenofovir, which showed good efficacy. Still, the exact mechanism of this efficacy is unknown, and more studies are required. [50]
Treatment for acute HEV infection is supportive. Immunosuppressed patients and solid organ transplant recipients can develop chronic HEV infection and can have treatment with ribavirin. Pegylated interferon (PEG-IFN) has been used successfully but has been associated with many adverse effects, such as cholestasis. [51]
There is currently no recommended treatment for HPgV-1. Patients who develop liver cirrhosis have treatment with similar treatment modalities used to treat cirrhosis of any other etiology. [22]
- Differential Diagnosis
The clinical presentation of viral hepatitis can be nonspecific, so other etiologies of hepatitis and liver disorders may present with similar symptoms and signs commonly found in patients with viral hepatitis. Patients with acute and chronic active viral hepatitis infections may present with malaise, fatigue, low-grade fever, anorexia, nausea, vomiting, and weight loss. Physical exam may be unremarkable, or there may be the presence of right upper quadrant tenderness with hepatomegaly, urticarial rash, and possibly evidence of dehydration. In advanced stages of liver disease from chronic viral hepatitis, patients may present with hematemesis, ascites, pedal edema, or hepatic encephalopathy.
Liver and non-liver-related disorders with similar presentation to viral hepatitis include drug-induced hepatitis, viral or bacterial gastroenteritis, acute cholecystitis, acute cholelithiasis, hereditary hemochromatosis, and malignancies such as pancreatic cancer, lymphoma, hepatocellular cancer, and peptic ulcer disease. Patients with severe congestive heart failure can present with pedal edema, ascites, and hepatomegaly due to hepatic congestion. Patients who present with symptoms of end-stage liver disease (variceal bleeding, volume overload, and encephalopathy) may have liver decompensation from etiologies other than chronic viral hepatitis, such as metabolic dysfunction-associated steatohepatitis and autoimmune or alcohol-related hepatitis.
Hereditary hemochromatosis is an autosomal recessive disease that disrupts the body's iron regulation, and excess iron becomes deposited in various organs of the body, including the liver. Diagnosis is usually made by checking serum iron, serum ferritin, and serum transferrin levels. A liver biopsy may become necessary to evaluate the degree of fibrosis and to differentiate it from other disorders of the liver, including viral or autoimmune hepatitis. Patients can present with joint pain, and some patients complain of pain in the knuckles of the first 2 fingers, called the "iron fist" sign. This sign is specific to hereditary hemochromatosis but is not present in all the patients. [52]
Patients with drug-induced hepatitis and congenital hepatopathies can also have a similar presentation, and a careful history is integral while evaluating the patients. Drug-induced liver injuries have become more common, and more than a thousand drugs have been identified, with studies underway to find out more about them. Patients with drug-induced liver injuries can be completely asymptomatic with only lab abnormalities of elevated aminotransferases or with acute or chronic hepatitis or acute liver failure. Drug-induced liver injuries remain 1 of the biggest causes of emergency liver transplants. The unregulated use of herbal and dietary supplements has created a new challenge to identify and treat patients promptly. [53]
To differentiate the type of hepatitis, a careful history, laboratory studies, and, when necessary, a liver biopsy should be obtained. The evaluation section of this review provides further information on how to diagnose and differentiate these conditions.
The differential diagnoses for viral hepatitis are as follows:
- Liver abscess
- Hepatocellular cancer
- Pancreatic cancer
- Drug-induced hepatitis
- Autoimmune hepatitis
- Acute cholangitis
- Acute cholecystitis and biliary colic
- Pancreatitis
- Gastroenteritis
- Cholelithiasis
- Peptic ulcer disease
- Small-bowel obstruction
The prognosis of viral hepatitis depends on the type of virus causing the infection.
HAV infection is usually a mild self-limiting illness. Patients infected with HAV develop lifelong immunity against subsequent infection from HAV. Overall, mortality is low, and complications, including relapse, jaundice, and fulminant liver failure, are rare. Patients who are immunocompromised, older adults, and young children are at higher risk compared to healthy adults. [4]
Patients with HBV infection are at risk of developing chronic hepatitis and cirrhosis, as well as hepatocellular carcinoma as a consequence. Fulminant hepatic failure happens in about 0.5% to 1% of the patients with HBV infection. However, when there is fulminant hepatic failure, the mortality rate is approximately 80%. Chronic liver disease from chronic HBV infection is responsible for about 650,000 fatalities per year globally. [54]
Patients infected with HCV will develop a chronic infection in 50% to 60% of cases. These patients are at risk of progression to cirrhosis and developing hepatocellular carcinoma. Chronic HCV infection was previously a leading cause of liver transplantation. The hepatitis C-related mortality rate in the United States increased annually until 2013. With the introduction of highly effective anti-HCV therapy in 2013 and 2014, the mortality rate and the need for liver transplants have declined significantly. However, mortality remains elevated in developing countries. [55]
Patients with chronic HBV infection who later acquire superimposed HDV infection generally develop chronic HDV infection. This typically results in the development of more severe hepatitis compared to patients who only have chronic HBV infection. Many of the coinfected patients will progress to end-stage liver disease and cirrhosis. [56]
HEV infection is a mild self-limiting illness comparable to HAV infection. However, a mortality rate of 15% to 25% may be seen in pregnant patients. The exact mechanism of how it triggers such severe infection with a resulting high mortality rate in pregnant patients is not clear. [57]
With human pegivirus-1, most patients are asymptomatic in acute infection with normal aminotransferase levels. Fulminant hepatic failure and chronic infections are rare, and often, the etiology of HPgV-1 is in question. Although coinfection with HBV and HCV is common, it does not increase the severity of the disease. [9]
- Complications
Complications of viral hepatitis include chronic infection with chronic active hepatitis, acute or subacute hepatic necrosis, liver failure, cirrhosis, hepatic encephalopathy, and hepatocellular carcinoma in patients with HBV or HCV infection. The complications of viral hepatitis can be treated in some cases with antiviral therapy helpful in eradicating or ameliorating the extrahepatic complications of viral hepatitis.
Patients with chronic HBV infection are at risk of developing hepatocellular carcinoma, even in the absence of cirrhosis. Hepatitis B-associated hepatocellular carcinoma is responsible for 45% of primary liver cancer worldwide. About 1% of patients can also develop fulminant hepatic failure, and the mortality rate is approximately 80% in those patients.
About >50% of patients infected with HCV develop chronic infection, with approximately 20% of those patients developing cirrhosis, and some will eventually progress to hepatocellular carcinoma. The complications of cirrhosis include hepatic encephalopathy, portal hypertension, ascites, spontaneous bacterial peritonitis, variceal bleeding, hepatorenal syndrome, and progression to hepatocellular carcinoma.
Liver-directed treatment of complications is, in large part, related to the complications of cirrhosis and is best managed by experts in cirrhosis care. Liver transplantation is curative for the complications of cirrhosis in eligible patients.
For patients who develop hepatic encephalopathy, the first-line therapy is oral lactulose. When symptoms are uncontrolled by lactulose, rifaximin is FDA-approved for addition to lactulose therapy. The administration of somatostatin or octreotide and prophylactic antibiotics is the standard of care for active gastrointestinal bleeding. Patients with variceal bleeding require immediate resuscitation, protection of the airway, endoscopy with sclerotherapy, or band ligation of the varices. Transjugular intrahepatic portosystemic shunt (TIPS) placement is rescue therapy for variceal bleeding not controlled endoscopically.
Ascites is initially managed with restriction of dietary sodium intake of 2000 mg daily followed by oral diuretic agents (commonly spironolactone and furosemide). Paracentesis is reserved for those uncontrolled by initial measures or who are severely symptomatic and in need of immediate relief. Transjugular intrahepatic portosystemic shunt (TIPS) can also be considered for ascites otherwise challenging to control. Ascites may be complicated by the development of spontaneous bacterial infection termed spontaneous bacterial peritonitis. Treatment generally involves intravenous antibiotics and requires subsequent antibiotic maintenance for secondary prophylaxis against repeat infection.
Patients who develop hepatocellular carcinoma may be candidates for locally directed therapies (e.g., radiofrequency ablation, transarterial chemoembolization), systemic chemotherapy, or surgical resection in carefully selected patients.
Patients with chronic HCV infection may also develop extrahepatic complications, including coagulopathies and cryoglobulinemia, which can lead to rash, vasculitis, and glomerulonephritis secondary to the deposition of immune complexes in the small vessels. Other associated disorders include non-Hodgkin lymphoma, focal lymphocytic sialadenitis, autoimmune thyroiditis, porphyria cutanea tarda, and lichen planus. [58] Correction of severe coagulopathy can be considered in the setting of active bleeding or the need for invasive procedures such as placement of a transjugular intrahepatic portosystemic shunt (TIPS) or other surgeries.
- Deterrence and Patient Education
Patient education is crucial in preventing and controlling viral hepatitis. Patients with chronic viral hepatitis should be educated on the risk and mode of transmission, as well as the need for routine monitoring to assess the necessity for treatment. Patients who have evidence of disease progression, especially with evidence of cirrhosis with or without complications, should be referred to a gastroenterologist or hepatologist promptly. Instruction should be provided to all patients about not sharing items such as toothbrushes, razors, or needles that can result in blood exposure and, therefore, transmission to others. Fruits and vegetables should be eaten after being cooked or washed and peeled. Patients traveling to endemic areas should be advised not to drink untreated water or ingest shellfish or raw seafood. Patients should avoid using hepatotoxic agents such as alcohol. Acetaminophen, which is metabolized through the liver, should be used with caution but avoided in the setting of severe liver injury. Patients with hepatitis A infection should not handle food for others until they stop shedding the virus.
All pregnant women should be screened for HBV infection and HIV infection. Treatment of HBV during pregnancy will depend on the viral load and HIV status. Neonates born to mothers with chronic HBV infection should receive immunoglobulin and HBV vaccination according to standard protocol to prevent the transmission of the virus.
Healthcare workers should maintain strict universal infection control practices. Those workers at risk of viral hepatitis infection should undergo vaccination against HAV and HBV. Where there is a risk of infection with HCV, appropriate health education, testing, and treatment, if needed, should be offered. Healthcare professionals should be educated on identifying individuals at risk for viral hepatitis and offer testing to treat if infection is identified. While it is important to identify those at increased risk of viral hepatitis infection, healthcare workers should be educated regarding the recommendations for universal screening for HBV and HCV for all adults aged 18 and older in the United States.
Vaccinations are available for the prevention of some types of hepatitis; preventing hepatitis is much easier than treating hepatitis. Viral hepatitis is preventable with vaccination.
Individuals who provide services to adults with a high risk for HAV infection (eg, non-residential group homes) and those traveling to areas where HAV is endemic should be offered the HAV vaccine 1 month before anticipated exposure risk. Other individuals who should be offered the HAV vaccine are hemophiliacs whether or not they receive clotting products, those who use illicit drugs, men who have sex with men, patients with chronic liver disease, patients who are awaiting liver transplant or are recipients of a liver transplant, and individuals who work with hepatitis virus-infected primates in laboratories.
Patients can receive inactivated HAV vaccine through the intramuscular route, with a booster dose recommended after 6 months. Patients exposed to HAV can also be given postexposure prophylaxis with hepatitis A immunoglobulin and should have it within 48 hours of exposure for higher effectiveness. Typically, it is recommended for individuals in close contact with the patients of HAV infection at home or in daycare centers. [59]
The HBV vaccine is recommended for all infants as a part of the routine immunization schedule. It should also be given to adults at high risk of infection, including patients on dialysis, healthcare workers who are at risk of exposure to blood and body fluids, people with sexual partners who have HBV infection, sexually active persons who are not in a long-term monogamous relationship, persons who are being evaluated or treated for sexually transmitted diseases, men who have sex with men, individuals who share needles or syringes, household contacts of individuals who have HBV infection, residents and staff of facilities for developmentally disabled persons and in correctional facilities. Patients with chronic liver disease should also be offered the HBV vaccine. Neonates who are born to mothers who have HBV infection should be given HBV vaccination within 12 hours of birth to prevent the transmission of the virus. Plasma-derived or recombinant HBV vaccines are highly effective, with more than a 95% seroconversion rate. Infants are given initial vaccination at birth with repeat vaccination at 1 to 2 months and 6 to 18 months. For the adults, after the initial vaccination, it is repeated at 1 month and 6 months. Some clinicians recommend a booster dose at 5 to 10 years. Individuals can also receive postexposure prophylaxis with hepatitis B immunoglobulin. Patients requiring frequent transfusion of blood products or factor concentrates, such as patients with hemophilia, should also receive the vaccination. However, the higher usage of recombinant factors and better techniques for viral detection have decreased the risk of infection significantly. [60]
Vaccines against HCV are currently unavailable, and immunoglobulin has not been proven effective in preventing transmission. It is, therefore, of high importance to put infection control practices in place to prevent the contamination of blood, organs, and semen and prevent the transmission of the virus into donor pools with better screening and improved viral elimination techniques. [60] Pregnant mothers testing positive for HCV should be offered treatment after delivery. Infants born to mothers with HCV infection should have testing done at 2 months and then at 18 months to detect infection.
Since HDV occurs as a coinfection when HBV infection is present, transmission can be prevented by immunizing patients against HBV. Currently, it is not possible to prevent HDV superinfection in patients with chronic HBV infection.
A vaccine for HEV infection does not exist at this time, and immunoglobulin administration does not prevent the disease. [61]
Patients who are transplant recipients or get frequent transfusions, injection drug users, hemodialysis patients, and men who have sex with men are the groups that are at increased risk. However, there is currently no vaccine available for hepatitis G. [22]
- Enhancing Healthcare Team Outcomes
Viral hepatitis is a complex disease requiring an interprofessional approach from healthcare professionals. Strategies to prevent viral hepatitis through patient education and vaccination are essential, and closer monitoring for disease progression and complications is paramount. To enhance patient-centered care, these strategies require significant interprofessional communication and care coordination by physicians, including primary care physicians and specialists, nurses, pharmacists, and other health professionals. The healthcare team must work closely with the patient to ensure they understand their disease, comply with medications, vaccines, and other precautions, and note progress or lack thereof. Pharmacists are a crucial part of the team by ensuring the medications at the correct dose are in the therapy regimen and that there are no drug-drug interactions. Any issues noted by any member of the interprofessional healthcare team should be documented and shared promptly. These measures will help improve patient outcomes, improve patient safety, and enhance team performance.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Hepatitis D Stages, Serological Markers, and the Three Infective Patterns. The three infective patterns vary in appearance and levels of HDV RNA, HDAg, and anti-HDV and HBV markers. Contributed by U Masood, MD
Hepatitis C Stages and Serological Markers. Hepatitis C virus RNA (HCV RNA) testing is necessary to confirm infection. It is the most specific test for hepatitis C virus infection and can detect acute infection even before antibodies develop. (more...)
Interpretation of Hepatitis B Screening Results. Hepatitis B e antigen (HBeAg) is present early in the infection and indicates that the virus is replicating. After the viral replication diminishes, HBeAg becomes undetectable, and antibodies to (more...)
Liver Biopsy Hematoxylin and Eosin Stain. Liver Biopsy hematoxylin and eosin stain showing chronic inflammation in a distorted portal space, a feature of chronic hepatitis. Contributed by F Farci, MD
Disclosure: Parth Mehta declares no relevant financial relationships with ineligible companies.
Disclosure: Lafaine Grant declares no relevant financial relationships with ineligible companies.
Disclosure: Anil Kumar Reddy Reddivari declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Mehta P, Grant LM, Reddivari AKR. Viral Hepatitis. [Updated 2024 Mar 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- [What General/Family Medicine Practitioner should Know about Viral Hepatitis]. [Acta Med Croatica. 2016] [What General/Family Medicine Practitioner should Know about Viral Hepatitis]. Vučak J, Vučak E. Acta Med Croatica. 2016 Apr; 70(2):87-95.
- Natural history of chronic hepatitis B virus infection in adults with emphasis on the occurrence of cirrhosis and hepatocellular carcinoma. [J Gastroenterol Hepatol. 2000] Natural history of chronic hepatitis B virus infection in adults with emphasis on the occurrence of cirrhosis and hepatocellular carcinoma. Chu CM. J Gastroenterol Hepatol. 2000 May; 15 Suppl:E25-30.
- Review Impact of etiological treatment on prognosis. [Hepatol Int. 2018] Review Impact of etiological treatment on prognosis. Su CW, Yang YY, Lin HC. Hepatol Int. 2018 Feb; 12(Suppl 1):56-67. Epub 2017 Jul 12.
- Review Overview of Complications in Cirrhosis. [J Clin Exp Hepatol. 2022] Review Overview of Complications in Cirrhosis. Premkumar M, Anand AC. J Clin Exp Hepatol. 2022 Jul-Aug; 12(4):1150-1174. Epub 2022 May 14.
- Ultrasound Shear Wave Elastography for Liver Disease. A Critical Appraisal of the Many Actors on the Stage. [Ultraschall Med. 2016] Ultrasound Shear Wave Elastography for Liver Disease. A Critical Appraisal of the Many Actors on the Stage. Piscaglia F, Salvatore V, Mulazzani L, Cantisani V, Schiavone C. Ultraschall Med. 2016 Feb; 37(1):1-5. Epub 2016 Feb 12.
Recent Activity
- Viral Hepatitis - StatPearls Viral Hepatitis - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- Fact sheets
- Facts in pictures
Publications
- Questions and answers
- Tools and toolkits
- Endometriosis
- Excessive heat
- Mental disorders
- Polycystic ovary syndrome
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Health topics /
Hepatitis is an inflammation of the liver that is caused by a variety of infectious viruses and noninfectious agents leading to a range of health problems, some of which can be fatal. There are five main strains of the hepatitis virus, referred to as types A, B, C, D and E. While they all cause liver disease, they differ in important ways including modes of transmission, severity of the illness, geographical distribution and prevention methods. In particular, types B and C lead to chronic disease in hundreds of millions of people and together are the most common cause of liver cirrhosis, liver cancer and viral hepatitis-related deaths. An estimated 354 million people worldwide live with hepatitis B or C, and for most, testing and treatment remain beyond reach.
Some types of hepatitis are preventable through vaccination. A WHO study found that an estimated 4.5 million premature deaths could be prevented in low- and middle-income countries by 2030 through vaccination, diagnostic tests, medicines and education campaigns. WHO’s global hepatitis strategy, endorsed by all WHO Member States, aims to reduce new hepatitis infections by 90% and deaths by 65% between 2016 and 2030.
Many people with hepatitis A, B, C, D or E exhibit only mild symptoms or no symptoms at all. Each form of the virus, however, can cause more severe symptoms. Symptoms of hepatitis A, B and C may include fever, malaise, loss of appetite, diarrhoea, nausea, abdominal discomfort, dark-coloured urine and jaundice (a yellowing of the skin and whites of the eyes). In some cases, the virus can also cause a chronic liver infection that can later develop into cirrhosis (a scarring of the liver) or liver cancer. These patients are at risk of death.
Hepatitis D (HDV) is only found in people already infected with hepatitis B (HBV); however, the dual infection of HBV and HDV can cause a more serious infection and poorer health outcomes, including accelerated progression to cirrhosis. Development of chronic hepatitis D is rare.
Hepatitis E (HEV) begins with mild fever, reduced appetite, nausea and vomiting lasting for a few days. Some persons may also have abdominal pain, itching (without skin lesions), skin rash or joint pain. They may also exhibit jaundice, with dark urine and pale stools, and a slightly enlarged, tender liver (hepatomegaly), or occasionally acute liver failure.
Safe and effective vaccines are available to prevent hepatitis B virus (HBV). This vaccine also prevents the development of hepatitis D virus (HDV) and given at birth strongly reduces transmission risk from mother to child. Chronic hepatitis B infection can be treated with antiviral agents. Treatment can slow the progression of cirrhosis, reduce incidence of liver cancer and improve long term survival. Only a proportion of people with chronic hepatitis B infection will require treatment. A vaccine also exists to prevent infections of hepatitis E (HEV), although it is not currently widely available. There are no specific treatments for HBV and HEV and hospitalization is not usually required. It is advised to avoid unnecessary medications due to the negative effect on liver function caused by these infections.
Hepatitis C (HCV) can cause both acute and chronic infection. Some people recover on their own, while others develop a life-threatening infection or further complications, including cirrhosis or cancer. There is no vaccine for hepatitis C. Antiviral medicines can cure more than 95% of persons with hepatitis C infection, thereby reducing the risk of death from cirrhosis and liver cancer, but access to diagnosis and treatment remains low.
Hepatitis A virus (HAV) is most common is low- and middle-income countries due to reduced access to clean and reliable water sources and the increased risk of contaminated food. A safe and effective vaccine is available to prevent hepatitis A. Most HAV infections are mild, with the majority of people recovering fully and developing immunity to further infection. However, these infections can also rarely be severe and life threatening due to the risk of liver failure.
- Hepatitis A
- Hepatitis B
- Hepatitis C
- Hepatitis D
- Hepatitis E
- Immunization coverage
- What is hepatitis?
- Severe acute hepatitis of unknown cause in children
- Hepatitis B: How can I protect myself?
- Guidelines on hepatitis
- Global reporting system for hepatitis
- Acute hepatitis of unknown aetiology
- Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections, for the period 2022–2030 (WHA75.20)
- WHA67.6: hepatitis
- WHA63.18: viral hepatitis
- Global Hepatitis Programme
WHO updates HIV testing guidance: more self-testing, integration, and prevention support
Antiretrovirals in Pregnancy Research Toolkit
WHO prequalifies the first self-test for hepatitis C virus
New report flags major increase in sexually transmitted infections, amidst challenges in HIV and hepatitis
Elimination of mother-to-child transmission of HIV and syphilis in Belize, Jamaica and Saint Vincent and the Grenadines
WHO sounds alarm on viral hepatitis infections claiming 3500 lives each day
WHO publishes new guidelines on hepatitis B
Reaching men with person-centred health services through evidence-based approaches and interventions
WHO commends Egypt for its progress on the path to eliminate hepatitis C
INFOSAN Quarterly Summary, 2023 #2
WHO launches “One life, one liver” campaign on World Hepatitis Day
Public notice and comment on new members of the Global Validation Advisory Committee (GVAC)
High-level resource mobilization conference to eliminate viral hepatitis
WHO announces the update of hepatitis B guidelines on testing and treatment
New WHO study: Making diagnosis of hepatitis C more accessible and closer to the community using point-of-care HCV viral load assays
WHO position paper on hepatitis A vaccines published
WHO publishes new guidelines on HIV, hepatitis and STIs for key populations
WHO and UNAIDS support countries to introduce virtual interventions and HIV self-testing
WHO and ANRS Emerging Infectious Diseases strengthen their collaboration in the area of HIV, viral hepatitis and sexually transmitted infections
WHO publishes updated guidance on hepatitis C infection – with new recommendations on treatment of adolescents and children, simplified service delivery and diagnostics

Implementing the global health sector strategies on HIV, viral hepatitis and sexually transmitted infections,...
This report is the first of a series of biennial progress reports on the implementation of the Global health sector strategies on, respectively, HIV, viral...

Consolidated guidelines on differentiated HIV testing services
These guidelines outline a public health approach to strengthening and expanding HIV testing services (HTS). They present and discuss key updates...

Fourth Consultation of the Regional Expert Panel for Verification of Hepatitis B Control in the South-East...
Hepatitis B control had gained momentum in the WHO South-East (SE) Asia Region as its public health burden had been recognized even prior to the COVID-19...

Recommended package of enabling and health interventions for HIV, viral hepatitis and STI prevention,...
In 2022, WHO published the Consolidated guidelines on HIV, viral hepatitis and STI prevention, diagnosis, treatment and care for key populations. These...

World Hepatitis Day 2024
It's time for action, infographics.
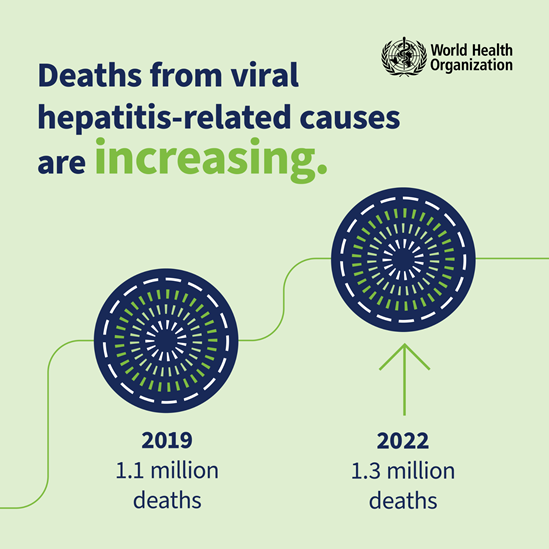
Deaths from viral hepatitis-related causes are increasing.

Most countries overpay for viral hepatitis medicines.

6000 people are newly infected with viral hepatitis each day.

Most people with chronic viral hepatitis don't realize they have it.

World Hepatitis Day 2024 – It’s time for action

WHO guidelines for key populations: what is new and what is important

Bringing hepatitis care closer to communities

Hepatitis can’t wait
Tackling health facility intersectional stigma faced by key populations in Ghana
Donors making a difference: WHO, communities and partners collaborate to end infectious diseases
Donors making a difference: the resilient spirit of women providing and receiving health care
WHO Youtube Channel

Hepatitis: WHO videos playlis
Related topics.
Sexually transmitted infections (STIs)
Universal health coverage

Viral Hepatitis Clinical Presentation
- Author: Naga Swetha Samji, MD; Chief Editor: BS Anand, MD more...
- Sections Viral Hepatitis
- Pathophysiology
- Epidemiology
- Patient Education
- Physical Examination
- Approach Considerations
- Hepatitis A
- Hepatitis B
- Hepatitis C
- Hepatitis D and E
- Histologic Findings
- Acute Hepatitis A
- Acute Hepatitis B
- Chronic Hepatitis B
- Acute Hepatitis C
- Chronic Hepatitis C
- Treatment of Hepatitis D and E
- Medication Summary
- Interferons
- Corticosteroids
- Vaccines, Viral, Prevention
- Immune Globulins
- Questions & Answers
- Media Gallery
The clinical presentation of infectious hepatitis varies with the individual, as well as with the specific causative virus. Some patients may be entirely asymptomatic or only mildly symptomatic at presentation. Others may present with rapid onset of fulminant hepatic failure (FHF). The classic presentation of infectious hepatitis involves four phases, as follows:
Phase 1 (viral replication phase): Patients are asymptomatic; laboratory studies demonstrate serologic and enzyme markers of hepatitis
Phase 2 (prodromal phase): Patients experience anorexia, nausea, vomiting, alterations in taste, arthralgias, malaise, fatigue, urticaria, and pruritus, and some develop an aversion to cigarette smoke; when seen by a healthcare provider during this phase, patients are often diagnosed as having gastroenteritis or a viral syndrome
Phase 3 (icteric phase): Patients may note dark urine, followed by pale-colored stools; in addition to the predominant gastrointestinal (GI) symptoms and malaise, patients become icteric and may develop right upper quadrant pain with hepatomegaly
Phase 4 (convalescent phase): Symptoms and icterus resolve, liver enzymes return to normal
The incubation period of hepatitis A virus (HAV) is 2-7 weeks (average, 28 days). Clinical symptoms then develop, often with a presentation similar to that of gastroenteritis or a viral respiratory infection. The most common signs and symptoms include fatigue, nausea, vomiting, fever, hepatomegaly, jaundice, dark urine, anorexia, and rash.
HAV infection usually occurs as a mild self-limited disease and confers lifelong immunity to the virus. Chronic HAV infection does not occur. [ 9 ]
The incubation period for hepatitis B virus (HBV) is 30-180 days (average, approximately 75 days). Patients then enter the prodromal or preicteric phase, characterized by the gradual onset of anorexia, malaise, and fatigue. During this phase, as the liver becomes inflamed, the liver enzymes start to elevate, and the patient may experience right upper quadrant pain. About 15% of patients develop an illness resembling serum sickness. These patients may experience fever, arthritis, arthralgias, or an urticarial rash.
As the disease progresses to the icteric phase, the liver becomes tender, and jaundice develops. Patients may note that their urine darkens and their stools lighten in color. Other symptoms in this stage include nausea, vomiting, and pruritus.
From this point on, the clinical course may be highly variable. Whereas some patients experience fairly rapid improvements in their symptoms, others go on to experience prolonged disease with slow resolution. Still others may have symptoms that periodically improve, only to worsen later (relapsing hepatitis). Finally, there is an unfortunate subset of patients in whom the disease rapidly progresses to FHF; this may occur over days to weeks.
The incubation period for hepatitis C virus (HCV) is 15-150 days, with symptoms developing anywhere from 5-12 weeks after exposure. During acute HCV infection, symptoms may appear similar to those of HBV infection. In up to 80% of cases, however, the patients are asymptomatic and do not develop icterus. [ 1 , 18 ]
Hepatitis D
The incubation period of hepatitis D virus (HDV) is approximately 35 days. Patients simultaneously infected with HBV and HDV often have an acute, self-limited infection. [ 48 , 55 ] Fewer than 5% of these patients develop chronic HDV infection.
Chronic HBV carriers who become superinfected with HDV tend to have a more severe acute hepatitis; 80% of these patients go on to develop chronic HDV infection. Chronic infection with HBV and HDV may lead to fulminant acute hepatitis and severe chronic active hepatitis with progression to cirrhosis. [ 48 , 55 ] Over the long term, as many as 70-80% of these patients have evidence of chronic liver disease with cirrhosis, compared with only 15-30% of patients with chronic HBV alone.

Hepatitis E
The incubation period of hepatitis E virus (HEV) is 2-9 weeks (average, 45 days). HEV usually causes an acute self-limited disease similar to HAV infection. Fulminant disease does occur in about 10% of cases. In women who are pregnant, HEV infection has a case-fatality rate of 15-20%. [ 34 ] No reports exist of chronic infection with HEV. [ 34 ]
Physical findings in patients with hepatitis vary with the type of hepatitis and the time of presentation.
Patients often present with low-grade fever. Those experiencing significant vomiting and anorexia may show signs of dehydration, such as tachycardia, dry mucous membranes, loss of skin turgor, and delayed capillary refill.
Patients in the icteric phase may have icterus of the sclerae or mucous membranes, or discoloration of the tympanic membranes. The skin may be jaundiced and may reveal macular, papular, or urticarial rashes.
In viral hepatitis, the liver may be tender and diffusely enlarged with a firm, sharp, smooth edge. If the patient has a nodular liver or a mass is palpated, clinicians should suspect an abscess or tumor.
Wasley A, Grytdal S, Gallagher K, Centers for Disease Control and Prevention (CDC). Surveillance for acute viral hepatitis--United States, 2006. MMWR Surveill Summ . 2008 Mar 21. 57(2):1-24. [QxMD MEDLINE Link] . [Full Text] .
Previsani N, Lavanchy D, World Health Organization. Hepatitis B (WHO/CDS/CSR/LYO/2002.2). 2002. [Full Text] .
Fattovich G. Natural history of hepatitis B. J Hepatol . 2003. 39 suppl 1:S50-8. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Terrault NA, Bzowej NH, Chang KM, et al, for the American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology . 2016 Jan. 63(1):261-83. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. 2015 Mar. [QxMD MEDLINE Link] . [Full Text] .
Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci . 2006. 3(2):47-52. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Ghany MG, Morgan TR, AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology . 2020 Feb. 71(2):686-721. [QxMD MEDLINE Link] . [Full Text] .
Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol . 2006 Oct. 45(4):529-38. [QxMD MEDLINE Link] .
Centers for Disease Control and Prevention. Viral Hepatitis Surveillance United States, 2014 (revised: 9/26/16). Available at https://www.cdc.gov/hepatitis/statistics/2014surveillance/pdfs/2014hepsurveillancerpt.pdf . Accessed: June 12, 2017.
Keeffe EB, Dieterich DT, Han SH, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol . 2008 Dec. 6(12):1315-41; quiz 1286. [QxMD MEDLINE Link] . [Full Text] .
Sorrell MF, Belongia EA, Costa J, et al. National Institutes of Health consensus development conference statement: management of hepatitis B. Hepatology . 2009 May. 49(5 suppl):S4-S12. [QxMD MEDLINE Link] .
[Guideline] Lok AS, McMahon BJ. AASLD practice guideline update: chronic hepatitis B: update 2009. Hepatology . 2009 Sep. 50(3):661-2. [QxMD MEDLINE Link] . [Full Text] .
Keeffe EB, Dieterich DT, Han SH, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States. Clin Gastroenterol Hepatol . 2004 Feb. 2(2):87-106. [QxMD MEDLINE Link] .
Tabor E. The role of tumor suppressor genes in the development of hepatocellular carcinoma. In: Okuda K, Tabor E, eds. Liver Cancer . New York, NY: Churchill Livingstone; 1997. 89-95.
Chen CJ, Yang HI, Iloeje UH, for the REVEAL-HBV Study Group. Hepatitis B virus DNA levels and outcomes in chronic hepatitis B. Hepatology . 2009 May. 49(5 suppl):S72-84. [QxMD MEDLINE Link] .
Liaw YF, Sung JJ, Chow WC, et al, for the Cirrhosis Asian Lamivudine Multicentre Study Group. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med . 2004 Oct 7. 351(15):1521-31. [QxMD MEDLINE Link] . [Full Text] .
Di Marco V, Marzano A, Lampertico P, et al, for the Italian Association for the Study of the Liver (AISF) Lamivudine Study Group, Italy. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology . 2004 Oct. 40(4):883-91. [QxMD MEDLINE Link] . [Full Text] .
World Health Organization. Hepatitis C. Fact sheet no 164. Available at https://www.who.int/mediacentre/factsheets/fs164/en/ . Updated: April 2017; Accessed: June 12, 2017.
Centers for Disease Control and Prevention. Viral hepatitis: hepatitis C FAQs for health professionals. Available at https://www.cdc.gov/hepatitis/hcv/hcvfaq.htm . Updated: January 27, 2017; Accessed: June 12, 2017.
[Guideline] World Health Organization. Guidelines for the screening, care and treatment of persons with chronic hepatitis C infection: updated version (April 2016). 2016 Apr. [QxMD MEDLINE Link] . [Full Text] .
World Health Organization. Hepatitis E. Fact sheet no 280. Available at https://www.who.int/mediacentre/factsheets/fs280/en/ . Updated: July 2016; Accessed: June 13, 2017.
Kumar A, Beniwal M, Kar P, Sharma JB, Murthy NS. Hepatitis E in pregnancy. Int J Gynaecol Obstet . 2004 Jun. 85(3):240-4. [QxMD MEDLINE Link] .
Kamar N, Selves J, Mansuy JM, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med . 2008 Feb 21. 358(8):811-7. [QxMD MEDLINE Link] . [Full Text] .
Gerolami R, Moal V, Colson P. Chronic hepatitis E with cirrhosis in a kidney-transplant recipient. N Engl J Med . 2008 Feb 21. 358(8):859-60. [QxMD MEDLINE Link] . [Full Text] .
Kamar N, Mansuy JM, Cointault O, et al. Hepatitis E virus-related cirrhosis in kidney- and kidney-pancreas-transplant recipients. Am J Transplant . 2008 Aug. 8(8):1744-8. [QxMD MEDLINE Link] . [Full Text] .
Centers for Disease Control and Prevention. Viral hepatitis. Available at https://www.cdc.gov/hepatitis/abc/index.htm . Updated: May 26, 2016; Accessed: June 13, 2017.
World Health Organization. Hepatitis A. Fact sheet no 328. Available at https://www.who.int/mediacentre/factsheets/fs328/en/ . Updated: July 2016; Accessed: June 12, 2017.
Centers for Disease Control and Prevention. Hepatitis A questions and answers for health professionals. Available at https://www.cdc.gov/hepatitis/hav/havfaq.htm . Updated: July 13, 2016; Accessed: June 13, 2017.
Centers for Disease Control and Prevention. Surveillance for Viral Hepatitis – United States, 2015 (updated: May 11, 2017). Available at https://www.cdc.gov/hepatitis/statistics/2015surveillance/commentary.htm . Accessed: June 12, 2017.
World Health Organization. Hepatitis B. Fact sheet no 204. Available at https://www.who.int/mediacentre/factsheets/fs204/en/ . Updated: April 2017; Accessed: June 12, 2017.
Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature . 2009 Oct 8. 461(7265):798-801. [QxMD MEDLINE Link] . [Full Text] .
Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature . 2009 Sep 17. 461(7262):399-401. [QxMD MEDLINE Link] .
World Health Organization. Hepatitis D. Fact sheet. Available at https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-d . July 2016; Accessed: June 12, 2017.
Adhami T, Levinthal G. Hepatitis E and hepatitis G/GBV-C. The Cleveland Clinic Disease Management Project . May 29, 2002.
Previsani N, Lavanchy D, World Health Organization. Hepatitis E (WHO/CDS/CSR/EDC/2001.12.). 2001.
Chivero ET, Stapleton JT. Tropism of human pegivirus (formerly known as GB virus C/hepatitis G virus) and host immunomodulation: insights into a highly successful viral infection. J Gen Virol . 2015 Jul. 96(pt 7):1521-32. [QxMD MEDLINE Link] . [Full Text] .
Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet . 2016 Sep 10. 388(10049):1081-8. [QxMD MEDLINE Link] . [Full Text] .
World Health Organization. World hepatitis day: 28 July. Hepatitis A & E. 2014. Available at https://www.who.int/campaigns/hepatitis-day/2014/hepatitis-a-e.pdf?ua=1 . Accessed: June 12, 2017.
Thomas E, Yoneda M, Schiff ER. Viral hepatitis: past and future of HBV and HDV. Cold Spring Harb Perspect Med . 2015 Feb 2. 5(2):a021345. [QxMD MEDLINE Link] . [Full Text] .
Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet . 2011 Aug 13. 378(9791):571-83. [QxMD MEDLINE Link] . [Full Text] .
Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat . 2004 Mar. 11(2):97-107. [QxMD MEDLINE Link] .
National Heart, Lung, and Blood Institute. What are the risks of a blood transfusion?. Available at https://www.nhlbi.nih.gov/health/health-topics/topics/bt/risks . Updated: January 30, 2012; Accessed: June 12, 2017.
Centers for Disease Control and Prevention. Healthcare-associated hepatitis B and C outbreaks reported to the Centers for Disease Control and Prevention (CDC) 2008-2015 (updated: July 14, 2016). Available at https://www.cdc.gov/hepatitis/outbreaks/healthcarehepoutbreaktable.htm . Accessed: June 12, 2017.
Alter MJ. Epidemiology of hepatitis C. Hepatology . 1997 Sep. 26(3 suppl 1):62S-65S. [QxMD MEDLINE Link] .
Krawitt EL. Autoimmune hepatitis: classification, heterogeneity, and treatment. Am J Med . 1994 Jan 17. 96(1A):23S-26S. [QxMD MEDLINE Link] .
Koneru A, Nelson N, Hariri S, et al. Increased hepatitis C virus (HCV) detection in women of childbearing age and potential risk for vertical transmission - United States and Kentucky, 2011-2014. MMWR Morb Mortal Wkly Rep . 2016 Jul 22. 65(28):705-10. [QxMD MEDLINE Link] . [Full Text] .
Dienstag JL. Sexual and perinatal transmission of hepatitis C. Hepatology . 1997 Sep. 26(3 suppl 1):66S-70S. [QxMD MEDLINE Link] .
Adhami T, Levinthal G. Hepatitis D. The Cleveland Clinic Disease Management Project . May 29, 2002.
Noureddin M, Gish R. Hepatitis delta: epidemiology, diagnosis and management 36 years after discovery. Curr Gastroenterol Rep . 2014 Jan. 16(1):365. [QxMD MEDLINE Link] . [Full Text] .
Kushner T, Serper M, Kaplan DE. Delta hepatitis within the Veterans Affairs medical system in the United States: Prevalence, risk factors, and outcomes. J Hepatol . 2015 Sep. 63(3):586-92. [QxMD MEDLINE Link] . [Full Text] .
Sultanik P, Pol S. Hepatitis delta virus: epidemiology, natural course and treatment. J Infect Dis Ther . 2016 Mar 3. 4:271. [Full Text] .
Khuroo MS, Kamili S, Khuroo MS. Clinical course and duration of viremia in vertically transmitted hepatitis E virus (HEV) infection in babies born to HEV-infected mothers. J Viral Hepat . 2009 Jul. 16(7):519-23. [QxMD MEDLINE Link] .
Mathur P, Arora NK, Panda SK, Kapoor SK, Jailkhani BL, Irshad M. Sero-epidemiology of hepatitis E virus (HEV) in urban and rural children of North India. Indian Pediatr . 2001 May. 38(5):461-75. [QxMD MEDLINE Link] .
Previsani N, Lavanchy D, World Health Organization. Hepatitis C (WHO/CDS/CSR/LYO/2003). 2002.
Previsani N, Lavanchy D, World Health Organization. Hepatitis D (WHO/CDS/CSR/NCS/2001.1). 2001.
Smith BD, Morgan RL, Beckett GA, et al, for the Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep . 2012 Aug 17. 61(RR-4):1-32. [QxMD MEDLINE Link] . [Full Text] .
Waknine Y. FDA approves first rapid HCV test. Medscape Medical News. June 28, 2010. Available at https://www.medscape.com/viewarticle/724236 . Accessed: June 30, 2010.
Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology . 2008 Apr. 134(4):960-74. [QxMD MEDLINE Link] . [Full Text] .
Bonder A, Afdhal N. Utilization of FibroScan in clinical practice. Curr Gastroenterol Rep . 2014 Feb. 16(2):372. [QxMD MEDLINE Link] .
Tapper EB, Castera L, Afdhal NH. FibroScan (vibration-controlled transient elastography): where does it stand in the United States practice. Clin Gastroenterol Hepatol . 2015 Jan. 13(1):27-36. [QxMD MEDLINE Link] . [Full Text] .
Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol . 1995 Dec. 19 (12):1409-17. [QxMD MEDLINE Link] .
Schmilovitz-Weiss H, Ben-Ari Z, Sikuler E, et al. Lamivudine treatment for acute severe hepatitis B: a pilot study. Liver Int . 2004 Dec. 24 (6):547-51. [QxMD MEDLINE Link] .
Fattovich G, Giustina G, Sanchez-Tapias J, et al. Delayed clearance of serum HBsAg in compensated cirrhosis B: relation to interferon alpha therapy and disease prognosis. European Concerted Action on Viral Hepatitis (EUROHEP). Am J Gastroenterol . 1998 Jun. 93 (6):896-900. [QxMD MEDLINE Link] .
Janssen HL, van Zonneveld M, Senturk H, et al, for the HBV 99-01 Study Group, Rotterdam Foundation for Liver Research. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet . 2005 Jan 8-14. 365 (9454):123-9. [QxMD MEDLINE Link] .
Lau GK, Piratvisuth T, Luo KX, et al, for the Peginterferon Alfa-2a HBeAg-Positive Chronic Hepatitis B Study Group. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med . 2005 Jun 30. 352 (26):2682-95. [QxMD MEDLINE Link] .
Marcellin P, Lau GK, Bonino F, et al, for the Peginterferon Alfa-2a HBeAg-Negative Chronic Hepatitis B Study Group. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med . 2004 Sep 16. 351 (12):1206-17. [QxMD MEDLINE Link] .
Dienstag JL. Benefits and risks of nucleoside analog therapy for hepatitis B. Hepatology . 2009 May. 49 (5 suppl):S112-21. [QxMD MEDLINE Link] .
Brouwer WP, Xie Q, Sonneveld MJ, et al, for the ARES Study Group. Adding pegylated interferon to entecavir for hepatitis B e antigen-positive chronic hepatitis B: a multicenter randomized trial (ARES study). Hepatology . 2015 May. 61(5):1512-22. [QxMD MEDLINE Link] . [Full Text] .
Marcellin P, Ahn SH, Ma X, et al, for the Study 149 Investigators. Combination of tenofovir disoproxil fumarate and peginterferon α-2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology . 2016 Jan. 150(1):134-44.e10. [QxMD MEDLINE Link] . [Full Text] .
Wursthorn K, Lutgehetmann M, Dandri M, et al. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology . 2006 Sep. 44(3):675-84. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology . 2018 Apr. 67 (4):1560-99. [QxMD MEDLINE Link] . [Full Text] .
Jaeckel E, Cornberg M, Wedemeyer H, et al, for the German Acute Hepatitis C Therapy Group. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med . 2001 Nov 15. 345 (20):1452-7. [QxMD MEDLINE Link] .
Nelson DR, Davis GL, Jacobson I, et al. Hepatitis C virus: a critical appraisal of approaches to therapy. Clin Gastroenterol Hepatol . 2009 Apr. 7 (4):397-414; quiz 366. [QxMD MEDLINE Link] .
[Guideline] American Association for the Study of Liver Diseases, Infectious Diseases Society of America. Simplified HCV treatment* for treatment-naive adults without cirrhosis. HCV Guidance: recommendations for testing, managing, and treating hepatitis C. Available at https://www.hcvguidelines.org/treatment-naive/simplified-treatment . Updated: August 27, 2020; Accessed: December 9, 2021.
Lawitz E, Poordad FF, Pang PS, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet . 2014 Feb 8. 383(9916):515-23. [QxMD MEDLINE Link] .
Afdhal N, Zeuzem S, Kwo P, et al, for the ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med . 2014 May 15. 370(20):1889-98. [QxMD MEDLINE Link] . [Full Text] .
Nkuize M, Serste T, Buset M, Mulkay JP. Combination ledipasvir-sofosbuvir for the treatment of chronic hepatitis C virus infection: a review and clinical perspective. Ther Clin Risk Manag . 2016. 12:861-72. [QxMD MEDLINE Link] . [Full Text] .
Feld JJ, Jacobson IM, Hezode C, et al, for the ASTRAL-1 Investigators. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med . 2015 Dec 31. 373(27):2599-607. [QxMD MEDLINE Link] . [Full Text] .
Everson GT, Towner WJ, Davis MN, et al. Sofosbuvir with velpatasvir in treatment-naive noncirrhotic patients with genotype 1 to 6 hepatitis C virus infection: a randomized trial. Ann Intern Med . 2015 Dec 1. 163(11):818-26. [QxMD MEDLINE Link] .
Agarwal K, Castells L, Mullhaupt B, et al. Sofosbuvir/velpatasvir for 12 weeks in genotype 1-4 HCV-infected liver transplant recipients. J Hepatol . 2018 Sep. 69(3):603-7. [QxMD MEDLINE Link] . [Full Text] .
Mir F, Kahveci AS, Ibdah JA, Tahan V. Sofosbuvir/velpatasvir regimen promises an effective pan-genotypic hepatitis C virus cure. Drug Des Devel Ther . 2017. 11:497-502. [QxMD MEDLINE Link] . [Full Text] .
Zeuzem S, Foster GR, Wang S, et al. Glecaprevir-pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med . 2018 Jan 25. 378(4):354-69. [QxMD MEDLINE Link] . [Full Text] .
Forns X, Lee SS, Valdes J, et al. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis . 2017 Oct. 17(10):1062-8. [QxMD MEDLINE Link] .
Xu HQ, Wang CG, Xiao P, Gao YH. Efficacy and safety of glecaprevir/pibrentasvir for chronic hepatitis C patients: a systematic review and meta-analysis. J Clin Transl Hepatol . 2020 Sep 28. 8(3):267-76. [QxMD MEDLINE Link] . [Full Text] .
Buti M, Gordon SC, Zuckerman E, et al. Grazoprevir, elbasvir, and ribavirin for chronic hepatitis C virus genotype 1 infection after failure of pegylated interferon and ribavirin with an earlier-generation protease inhibitor: final 24-week results from C-SALVAGE. Clin Infect Dis . 2016 Jan 1. 62(1):32-6. [QxMD MEDLINE Link] .
Zeuzem S, Ghalib R, Reddy KR, et al. Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection: a randomized trial. Ann Intern Med . 2015 Jul 7. 163(1):1-13. [QxMD MEDLINE Link] .
Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med . 2014 Apr 24. 370(17):1594-603. [QxMD MEDLINE Link] . [Full Text] .
Klibanov OM, Gale SE, Santevecchi B. Ombitasvir/paritaprevir/ritonavir and dasabuvir tablets for hepatitis C virus genotype 1 infection. Ann Pharmacother . 2015 May. 49(5):566-81. [QxMD MEDLINE Link] .
Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med . 2014 May 22. 370(21):1973-82. [QxMD MEDLINE Link] . [Full Text] .
Niro GA, Ciancio A, Gaeta GB, et al. Pegylated interferon alpha-2b as monotherapy or in combination with ribavirin in chronic hepatitis delta. Hepatology . 2006 Sep. 44 (3):713-20. [QxMD MEDLINE Link] .
Bourliere M, Gordon SC, Flamm SL, et al, for the POLARIS-1 and POLARIS-4 Investigators. Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection. N Engl J Med . 2017 Jun 1. 376(22):2134-46. [QxMD MEDLINE Link] . [Full Text] .
Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet . 2014 Nov 15. 384(9956):1756-65. [QxMD MEDLINE Link] .
Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al, for the AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med . 2014 Jan 16. 370(3):211-21. [QxMD MEDLINE Link] . [Full Text] .
Nishiguchi S, Kuroki T, Nakatani S, et al. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet . 1995 Oct 21. 346 (8982):1051-5. [QxMD MEDLINE Link] .
Bruno S, Stroffolini T, Colombo M, et al, for the Italian Association of the Study of the Liver Disease (AISF). Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology . 2007 Mar. 45 (3):579-87. [QxMD MEDLINE Link] .
Poynard T, McHutchison J, Davis GL, et al. Impact of interferon alfa-2b and ribavirin on progression of liver fibrosis in patients with chronic hepatitis C. Hepatology . 2000 Nov. 32(5):1131-7. [QxMD MEDLINE Link] .
Mallet V, Gilgenkrantz H, Serpaggi J, et al. Brief communication: the relationship of regression of cirrhosis to outcome in chronic hepatitis C. Ann Intern Med . 2008 Sep 16. 149 (6):399-403. [QxMD MEDLINE Link] .
Da BL, Lourdusamy V, Kushner T, Dieterich D, Saberi B. Efficacy of sofosbuvir/velpatasvir/voxilaprevir in direct-acting antiviral experienced patients with hepatitis C virus. Eur J Gastroenterol Hepatol . 2021 Jun 1. 33(6):859-61. [QxMD MEDLINE Link] .
Lau DT, Doo E, Park Y, et al. Lamivudine for chronic delta hepatitis. Hepatology . 1999 Aug. 30 (2):546-9. [QxMD MEDLINE Link] .
Yurdaydin C, Bozkaya H, Onder FO, et al. Treatment of chronic delta hepatitis with lamivudine vs lamivudine + interferon vs interferon. J Viral Hepat . 2008 Apr. 15 (4):314-21. [QxMD MEDLINE Link] .
Fiore AE, Wasley A, Bell BP, for the Advisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep . 2006 May 19. 55(RR-7):1-23. [QxMD MEDLINE Link] . [Full Text] .
Vento S, Garofano T, Renzini C, et al. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med . 1998 Jan 29. 338(5):286-90. [QxMD MEDLINE Link] . [Full Text] .
Advisory Committee on Immunization Practices. Recommended adult immunization schedule: United States, 2009*. Ann Intern Med . 2009 Jan 6. 150 (1):40-4. [QxMD MEDLINE Link] .
Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC). Update: Prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep . 2007 Oct 19. 56 (41):1080-4. [QxMD MEDLINE Link] .
Centers for Disease Control and Prevention. Hepatitis B FAQs for health professionals. Available at https://www.cdc.gov/hepatitis/HBV/HBVfaq.htm#recctbl . Updated: August 4, 2016; Accessed: June 13, 2017.
Halperin SA, Ward B, Cooper C, et al. Comparison of safety and immunogenicity of two doses of investigational hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligodeoxyribonucleotide and three doses of a licensed hepatitis B vaccine in healthy adults 18-55 years of age. Vaccine . 2012 Mar 28. 30(15):2556-63. [QxMD MEDLINE Link] .
Chang MH, Chen TH, Hsu HM, et al, for the Taiwan Childhood HCC Study Group. Prevention of hepatocellular carcinoma by universal vaccination against hepatitis B virus: the effect and problems. Clin Cancer Res . 2005 Nov 1. 11 (21):7953-7. [QxMD MEDLINE Link] .
Moorman AC, de Perio MA, Goldschmidt R, et al. Testing and clinical management of health care personnel potentially exposed to hepatitis C virus - CDC guidance, United States, 2020. MMWR Recomm Rep . 2020 Jul 24. 69(6):1-8. [QxMD MEDLINE Link] . [Full Text] .
McHutchison JG, Dusheiko G, Shiffman ML, et al, for the TPL102357 Study Group. Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med . 2007 Nov 29. 357 (22):2227-36. [QxMD MEDLINE Link] .
Centers for Disease Control and Prevention. HIV and viral hepatitis. Available at https://www.cdc.gov/hiv/pdf/library/factsheets/hiv-viral-hepatitis.pdf . June 2017; Accessed: June 13, 2017.
Brown RS Jr, McMahon BJ, Lok AS, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: A systematic review and meta-analysis. Hepatology . 2016 Jan. 63 (1):319-33. [QxMD MEDLINE Link] .
- Viral Hepatitis. Hepatitis A virus as viewed through electron microscopy.
- Viral Hepatitis. Liver biopsy specimen showing ground-glass appearance of hepatocytes in a patient with hepatitis B.
- Viral Hepatitis. Liver biopsy with hematoxylin stain showing stage 4 fibrosis (ie, cirrhosis) in a patient with hepatitis B.
- Viral Hepatitis. Liver biopsy with trichrome stain showing stage 3 fibrosis in a patient with hepatitis B.
- Viral Hepatitis. Hepatic carcinoma, primary. Large multifocal hepatocellular carcinoma in an 80-year-old man without cirrhosis.
- Viral Hepatitis. Triple-phase computed tomography scan depicting liver cancer, revealing classic findings of enhancement during the arterial phase and delayed hypointensity during the portal venous phase.
- Table 1. Diagnostic Tests for Hepatitis B
- Table 2. Histologic Grading for Hepatitis C–Induced Liver Disease
|
|
|
|
HBsAg | + | + | + |
Anti-HBs | - | - | - |
HBeAg | + | - | - |
Anti-HBe | - | + | + |
Anti-HBc | + | + | + |
IgM anti-HBc | - | - | - |
HBV DNA | >2 × 10 IU/mL* (>10 copies/mL) | >2 × 10 IU/mL (>10 copies/mL) | < 2 × 10 IU/mL (< 10 copies/mL) |
ALT level | Elevated | Elevated | Normal |
ALT = alanine aminotransferase; anti-HBc = antibody to hepatitis B core antigen; anti-HBe = antibody to HBeAg; anti-HBs = antibody to HBsAg; CHB = chronic hepatitis B; HBV = hepatitis B virus; HBeAg = hepatitis B e antigen; HBsAg = hepatitis B surface antigen; IgM = immunoglobulin M. *Increasingly, experts in the field use IU/mL rather than copies/mL. | |||
|
|
|
|
1 – Minimal | Mild | Scant | None |
2 – Mild | Mild | Mild | Scant |
3 – Moderate | Moderate | Moderate | Spotty |
4 – Severe | Marked | Marked | Confluent |

Contributor Information and Disclosures
Naga Swetha Samji, MD Physician, Bellin Clinic, Bellin Health Systems Naga Swetha Samji, MD is a member of the following medical societies: American College of Gastroenterology , American College of Physicians , Wisconsin Medical Society Disclosure: Nothing to disclose.
Adrienne M Buggs, MD, FACEP, FAAEM Medical Policy Advisor, Office of Merchant Mariner Credentialing, United States Coast Guard Adrienne M Buggs, MD, FACEP, FAAEM is a member of the following medical societies: American Academy of Emergency Medicine , American College of Emergency Physicians , American College of Occupational and Environmental Medicine Disclosure: Nothing to disclose.
Praveen K Roy, MD, MSc Clinical Assistant Professor of Medicine, University of New Mexico School of Medicine Praveen K Roy, MD, MSc is a member of the following medical societies: Alaska State Medical Association , American Gastroenterological Association Disclosure: Nothing to disclose.
BS Anand, MD Professor, Department of Internal Medicine, Division of Gastroenterology, Baylor College of Medicine BS Anand, MD is a member of the following medical societies: American Association for the Study of Liver Diseases , American College of Gastroenterology , American Gastroenterological Association , American Society for Gastrointestinal Endoscopy Disclosure: Nothing to disclose.
Eugene Hardin, FAAEM, FACEP Former Chair and Associate Professor, Department of Emergency Medicine, Charles Drew University of Medicine and Science; Former Chair, Department of Emergency Medicine, Martin Luther King Jr/Drew Medical Center
Disclosure: Nothing to disclose.
Douglas M Heuman, MD, FACP, FACG, AGAF Chief of GI, Hepatology, and Nutrition at North Shore University Hospital/Long Island Jewish Medical Center; Professor, Department of Medicine, Hofstra North Shore-LIJ School of Medicine
Douglas M Heuman, MD, FACP, FACG, AGAF is a member of the following medical societies: American Association for the Study of Liver Diseases , American College of Physicians , and American Gastroenterological Association
Disclosure: Novartis Grant/research funds Other; Bayer Grant/research funds Other; Otsuka Grant/research funds None; Bristol Myers Squibb Grant/research funds Other; Scynexis None None; Salix Grant/research funds Other; MannKind Other
Julian Katz, MD Clinical Professor of Medicine, Drexel University College of Medicine
Julian Katz, MD is a member of the following medical societies: American College of Gastroenterology , American College of Physicians , American Gastroenterological Association , American Geriatrics Society , American Medical Association , American Society for Gastrointestinal Endoscopy , American Society of Law, Medicine & Ethics , American Trauma Society , Association of American Medical Colleges , and Physicians for Social Responsibility
Joseph K Lim, MD Associate Professor of Medicine, Director, Yale Viral Hepatitis Program, Section of Digestive Diseases, Yale University School of Medicine
Joseph K Lim, MD is a member of the following medical societies: American Association for the Study of Liver Diseases , American College of Gastroenterology , American College of Physicians , American Gastroenterological Association , and American Society for Gastrointestinal Endoscopy
Robert M McNamara, MD, FAAEM Chair and Professor, Department of Emergency Medicine, Temple University School of Medicine
Robert M McNamara, MD, FAAEM is a member of the following medical societies: American Academy of Emergency Medicine , American Medical Association , Pennsylvania Medical Society , and Society for Academic Emergency Medicine
Sandeep Mukherjee, MB, BCh, MPH, FRCPC Associate Professor, Department of Internal Medicine, Section of Gastroenterology and Hepatology, University of Nebraska Medical Center; Consulting Staff, Section of Gastroenterology and Hepatology, Veteran Affairs Medical Center
Sandeep Mukherjee, MB, BCh, MPH, FRCPC is a member of the following medical societies: Royal College of Physicians and Surgeons of Canada
Disclosure: Merck Honoraria Speaking and teaching; Ikaria Pharmaceuticals Honoraria Board membership
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Salary Employment
Rajeev Vasudeva, MD, FACG Clinical Professor of Medicine, Consultants in Gastroenterology, University of South Carolina School of Medicine
Rajeev Vasudeva, MD, FACG is a member of the following medical societies: American College of Gastroenterology , American Gastroenterological Association , American Society for Gastrointestinal Endoscopy , Columbia Medical Society, South Carolina Gastroenterology Association, and South Carolina Medical Association
Disclosure: Pricara Honoraria Speaking and teaching; UCB Consulting fee Consulting
David C Wolf, MD, FACP, FACG, AGAF Medical Director of Liver Transplantation, Westchester Medical Center; Professor of Clinical Medicine, Division of Gastroenterology and Hepatobiliary Diseases, Department of Medicine, New York Medical College
David C Wolf, MD, FACP, FACG, AGAF is a member of the following medical societies: American Association for the Study of Liver Diseases , American College of Gastroenterology , American College of Physicians , and American Gastroenterological Association
What would you like to print?
- Print this section
- Print the entire contents of
- Print the entire contents of article

- Hepatitis in Pregnancy
- Treatment Recommendations for HIV-Infected Patients With Co-infections
- Pediatric Vaccines: Global Brands and Country Availability
- Pediatric Hepatitis B
- Polyarteritis Nodosa
- Viral Hepatitis
- Fast Five Quiz: Hepatitis B Epidemiology and Prevention
- US FDA Declines Expanded Use Of Dynavax's Hepatitis B Vaccine on Insufficient Data
- Don't Forget Adult Hepatitis Vaccinations
- Combination Therapy Looks Promising for Hepatitis D

- Drug Interaction Checker
- Pill Identifier
- Calculators

- 2002964662-overviewDiseases & Conditions Diseases & Conditions Pediatric Hepatitis B

Got any suggestions?
We want to hear from you! Send us a message and help improve Slidesgo
Top searches
Trending searches

62 templates

49 templates

american history
85 templates

el salvador
34 templates

pink flowers
255 templates

22 templates
Liver Diseases: Hepatitis B
It seems that you like this template, liver diseases: hepatitis b presentation, premium google slides theme, powerpoint template, and canva presentation template.
Hepatitis B is a viral infection that affects the liver. It is transmitted through blood or bodily fluids, and can range from a mild illness lasting only a few weeks to a lifelong condition. There's a vaccine for this disease, so most people won't suffer complications, which include cirrhosis or even liver cancer. However, having as much information as possible at hand won't hurt, so prepare an informative slideshow about hepatitis B with this editable template. Its palette revolves around dark red tones, and the text included in the different sections has been generated by AI. While it's not specificaly about hepatitis B, it will give you some tips when it comes to structuring a presentation.
Features of this template
- 100% editable and easy to modify
- 20 different slides to impress your audience
- Contains easy-to-edit graphics such as graphs, maps, tables, timelines and mockups
- Includes 500+ icons and Flaticon’s extension for customizing your slides
- Designed to be used in Google Slides, Canva, and Microsoft PowerPoint
- 16:9 widescreen format suitable for all types of screens
- Includes information about fonts, colors, and credits of the resources used
What are the benefits of having a Premium account?
What Premium plans do you have?
What can I do to have unlimited downloads?
Don’t want to attribute Slidesgo?
Gain access to over 29700 templates & presentations with premium from 1.67€/month.
Are you already Premium? Log in

Register for free and start downloading now
Related posts on our blog.

How to Add, Duplicate, Move, Delete or Hide Slides in Google Slides

How to Change Layouts in PowerPoint

How to Change the Slide Size in Google Slides
Related presentations.

Premium template
Unlock this template and gain unlimited access


- Upload Ppt Presentation
- Upload Pdf Presentation
- Upload Infographics
- User Presentation
- Related Presentations

Viral Hepatitis
By: a.k7778hotmail.com Views: 4726
Ulcerative Colitis
By: ueANJU Views: 2022

Pancreatitis
By: Joand1503 Views: 1732

Peptic ulcer disease
By: JenniferDwayne Views: 4313

By: drdwayn Views: 3409

The Large Intestine
By: drdwayn Views: 2451

- Occupation :
- Specialty :
HEALTH A TO Z
- Eye Disease
- Heart Attack
- Medications

Oct 01, 2012
1.34k likes | 3.17k Views
Hepatitis. RM SBI3U Mr. Watts. Definition. HEPATITIS- inflammation of the liver. - Hepatitis is a viral infection in the liver that causes the liver to swell and become inflamed. -Many different forms of the hepatitis virus (A,B,C,D,E) which vary in severity. History.
Share Presentation
- fun time final round
- viral infection
- infectious hepatitis
- hepatitis b foundation
- severity increases

Presentation Transcript
Hepatitis RM SBI3U Mr. Watts
Definition HEPATITIS- inflammation of the liver - Hepatitis is a viral infection in the liver that causes the liver to swell and become inflamed. -Many different forms of the hepatitis virus (A,B,C,D,E) which vary in severity
History - 1947 Mark McCallum released the first evidence of infectious hepatitis and serum hepatitis in his research. - 1967 Dr. Blumberg discovered the Australian antigen which lead to the discovery of hepatitis B, in 1969 Blumberg assisted in the development of the blood test used to detect hepatitis B as well as the vaccine. - Hepatitis C was discovered in 1989 - Hepatitis D was discovered in 1977 - Hepatitis E was discovered sometime between 1971-1976
Causes Hepatitis A: -found in feces -both hepatitis A & E are spread through fecal-oral contamination or close contact (unprotected sex) • Hepatitis B: • - found in blood and certain body fluids • spread when blood or fluid from infected person enters body of person not immune • spread by unprotected sex, sharing needles, and through infected mother to child.
Hepatitis C: • found in blood and certain body fluids • Spread when HCV infected blood/fluid enters another person • Spread through sharing needles or infected mother to child but rarely is spread through unprotected sex. • Hepatitis D: • person must also be infected with hepatitis B • Spread through sharing needles and/or exposure to blood/blood products.
Symptoms • Jaundice • Clay coloured stools • Abdominal Pain • Dark coloured urine • Loss of apetite • Other flu like symptoms
Effects • Hepatitis A: • Recovery from HAV = lifetime immunity • 15-50 days for symptoms to develop • Kills 2% of those over 50 • Patient is contagious until jaundice appears • Can cause liver failure • Hepatitis B: • - babies born to mothers with HBV have higher chance of developing chronic HBV later in life which can lead to cirrhosis and liver cancer. • Chronic HBV can attack the liver for years without detection • 90% of healthy adults will recovery and develop antibody against it.
Hepatitis C: • 70-80% of people progress to chronic infection • 1-5% of infected people die as a result of chronic liver diseases, diseases from liver damage, cancer of liver • Hepatitis D: • Infects those carrying hepatitis B • Up to 20% of hepatitis D infections are rapidly fatal • Doesn’t have necessary viral equipment to replicate itself, depends on HBV for replication • Hepatitis E: • - severity increases with age • Most found in developing countries • Death rate in pregnant women 15-20% • not long lasting
Diagnosis • All forms of hepatitis can be detected and diagnosed through a blood test. • Two tests are necessary to confirm diagnosis of hepatitis C and a measure of liver enzymes through a blood test and sometimes a biopsy.
Treatment • Hepatitis A&B: • -Hepatitis A is prevented by good sanitation • Both hepatitis A and B are treated with rest and relaxation and no alcohol for several months • people with chronic hep. B should get vaccinated for hep. A • Hepatitis C: • can be waited out depending on liver damage seen through biopsy • Doctors will monitor condition, antiviral medication exist to help fight hep C (peginterferonaifa, ribavirin) Hepatitis D: - Liver transplant is effective treatment for severe chronic hepatitis D. Hepatitis E: - get enough calories, drink enough fluids, and avoid harmful liver meds.
Future Outlook • 7 drugs approved in U.S. For chronic hepatitis B • Medical people working on more effective and less toxic antivirals • 2 new drugs, telaprevirand boceprevir have been found and are providing hope for more cures to hepatitis C.
Fun Time!! What is the difference between acute and chronic hepatitis? Acute lasts less than 6 months whereas chronic lasts longer than 6 months Who was the doctor who won the Nobel Prize for Medicine through his work with hepatitis B? Dr. Blumberg To be diagnosed with hepatitis D you must have also been diagnosed with? Hepatitis B The word hepatitis means? Inflammation of the liver. Name 3 symptoms of hepatitis Jaundice, clay coloured stool, abdominal pain, dark coloured urine, loss of appetite, flu like symptoms.
Fun Time Final Round What were Mark McCallum’s original names for hepatitis A&B? Infectious and serum hepatitis What 2 symptoms are only associated with chronic hepatitis? Jaundice and abdominal pain. In severe cases of hepatitis D what is the most effective treatment? Liver transplant
Works Cited Youngson, R.M. 2005, Encyclopedia of Family Health Third Edition, Tarrytown, NY, Marshall Cavendish Corporation Pubmed Health, Hepatitis, 11,11,11, www.ncbi.nlm.nih.gov/pubmedhealth/PMH0002139/ Health Canada, Hepatitis, 11,11,11, www.hc-sc.gc.ca/hc-ps/dc-ma/hep-eng.php Hepatitis B Foundation, Hepatitis B Reference Summary, 11, 11, 11, http://www.hepb.org/hepatitisbcd/modules/infectd/id450101/id459101g.html Public Health Agency of Canada, Hepatitis E Fact Sheet, 11, 11, 11, www.phac-aspc.gc.ca/hcai-iamss/bbp-pts/hepatitis/hep_e-eng.php Public Health Agency of Canada, Hepatitis G Fact Sheet, 11, 11, 11, http://www.phac-aspc.gc.ca/hcai-iamss/bbp-pts/hepatitis/hep_g-eng.php Public Health Agency of Canada, Hepatitis C Fact Sheet, 11, 11, 11, http://www.phac-aspc.gc.ca/hcai-iamss/bbp-pts/hepatitis/hep_c-eng.php Washington State Department of Health, Delta Hepatitis (hepatitis D), 11, 11, 11, www.doh.wa.gov/EHSPHL/factsheet/hepd.htm Hepatitis B Foundation, Meet Dr. Blumberg, 7, 12, 11 http://www.hepb.org/about/blumberg.htm
Picture Citations http://kirstyne.files.wordpress.com/2007/09/infection.gif http://www.beltina.org/pics/hepatitis_prevention.jpg http://www.mysafetysign.com/img/lg/S/Unsafe-Drinking-Water-Notice-Sign-S-2828.gif http://www.asianweek.com/wp-content/uploads/2010/08/Dr.Blumberg.jpg http://www.hongkiat.com/blog/wp-content/uploads/homersimpson-css.gif http://www.vaccineinformation.org/photos/hepbuta002.jpg
- More by User
HEPATITIS . NEW YORK STATE OFFICE OF ALCOHOLISM AND SUBSTANCE ABUSE SERVICES. Workbook prepared by the Office of the Medical Director and the Bureau of Treatment: Steven Kipnis MD, FACP, FASAM Robert Killar, CASAC Patricia Lincourt, LCSW. THE LIVER. WEDGE SHAPED ORGAN
1.3k views • 99 slides

HEPATITIS. Iva Pitner Mentor: A. Žmegač Horvat. HEPATITIS = inflammation of liver. HEPATITIS - causes. ACUTE: Viral hepatitis Non - viral infection Alcohol Toxins Drugs Ischemic hepatits Autoimmune Metabolic diseases. CHRONIC: Viral hepatitis Alcohol Drugs
913 views • 19 slides

HEPATITIS. WHAT IS HEPATITIS?
HEPATITIS. WHAT IS HEPATITIS?. Hepatitis is the inflammation of the liver caused by viruses, bacterial infections, or continuous exposure to alcohol, drugs, or toxic chemicals, such as those found in aerosol sprays and paint thinners. . OTHER FACTS ABOUT HEPATITIS A.
1.72k views • 34 slides

HEPATITIS. Diah Puspita Rini, dr., SpPK. Hepatitis is inflammation of the liver which can be caused by viruses, medications, or toxic agents . Non viral : miliary TB, staphylococcal bacteriemia, salmonelloses, amebiasis, drugs, etc. Viral hepatitis :, Hepatitis A,B,C,D,E
926 views • 33 slides

Hepatitis. Dr. Meg- angela Christi M. Amores. Hepatitis. Inflammation of the liver Acute Viral Hepatitis Toxic and Drug-induced Hepatitis Chronic Hepatitis. Acute Viral Hepatitis. Almost all cases of acute viral hepatitis are caused by one of five viral agents: hepatitis A virus (HAV)
899 views • 24 slides
Hepatitis:. Hepatitis is the inflammation of liver caused by immune response against : liver parenchyma induced by viral infections , or some intracellular pathogens that survive in Kupffer cells causing granulomatous infections (Typhoid fever, brucellosis, Q fever, T.B).
2.08k views • 15 slides
Hepatitis. - Hepato = liver - itis = inflammation. Learning Objectives:. Identify the different mode of transmissions for each of the Hepatitis types. Discuss 2 ways of preventing the spread of Hepatitis. Name three symptoms of Hepatitis.
806 views • 27 slides

HEPATITIS. Definition & causative organisms. Infections of the liver caused by a group of viruses having an affinity for the liver Infection of the hepatocytes produces necrosis and inflammation of the liver Hepatitis virus A,B,C,D,E (G -no acute or chronic illness)…..
1.69k views • 18 slides
HEPATITIS. DR.AMANULLAH ABBASI FCPS, MRCP SENIOR REGISTRAR WARD-7 JPMC. The liver is designed to maintain body’s chemical and metabolic homeostasis. THE LIVER. - The liver is the largest organ in the body, occupying The entire upper right quadrant of the abdomen.
1.02k views • 58 slides
Hepatitis. 19 Muharram 1428 7 th February 2007 SBM 2044. http://www.sumanasinc.com/webcontent/anisamples/microbiology/herpessimplex.html. Hepatitis. Hepatitis is an inflammation of liver It is usually caused by viral infections, toxic agents or drugs but may be an autoimmune response.
1.58k views • 42 slides

Hepatitis. Acute hepatitis: It is an acute parenchymal damage can be caused by many agents. Epidemiology of hepatitis A :. It occurs world wide It is prevalent in areas of poor sanitation and hygiene. More common in children. There was an outbreak in Mexico
762 views • 41 slides

HEPATITIS. Dr. FERDA ÖZKAN. Objectives and Aim. To learn the types of inflammatory diseases of liver To learn the inflammatory changes in liver. I nflammatory diseases. Viral Hepatitis Autoimmune Hepatitis Fulminant Hepatitis Postnecrotic Cirrhosis Liver Abscesses .
1.86k views • 90 slides

Hepatitis. Hepatitis inflammation of the liver. Can have many causes drugs toxins alcohol viral infections (A, B, C, D, E) other infections (parasites, bacteria) physical damage. Liver. Functions Stores sugar needed for energy Absorbs good nutrients
664 views • 43 slides
HEPATITIS. NEW YORK STATE OFFICE OF ALCOHOLISM AND SUBSTANCE ABUSE SERVICES. Workbook prepared by the Office of the Medical Director and the Bureau of Treatment: Steven Kipnis MD, FACP, FASAM Robert Killar, CASAC Patricia Lincourt, LCSW. THE LIVER. WEDGE SHAPED ORGAN
1.29k views • 99 slides

Hepatitis. Christine Lytrang and Janett Rodriguez. What is Hepatitis?. Swelling and inflammation of the liver. It is not a condition, but is often used to Refer to a viral infection of the liver. Hepatitis is caused from?
427 views • 8 slides

861 views • 19 slides

HEPATITIS. Sahar Zakaria Lecterer of Tropical Medicin e. Clinical Terms. Hepatiti s : inflammation of liver; presence of inflammatory cells in organ tissue Acute Viral Hepatitis : symptoms last less than 6 months
1k views • 56 slides

Power Point Presentation


IMAGES
COMMENTS
The vaccine provides long-term protection against clinical illness and chronic hepatitis B virus infection. Because of the vaccine, cases of acute hepatitis B infections have notably decreased in the United States. Contact C-ASSIST Family Health Clinic Today! Call (313) 670-9943 for more information.
The clinical presentation of viral hepatitis can be different in each individual, depending on the type of hepatitis virus causing the infection. Patients can be entirely asymptomatic or only mildly symptomatic at presentation. A small number of patients can present with rapid onset of fulminant hepatic failure.
Hepatitis means inflammation of the liver. The liver is one of the largest and most important organs in a person's body. The liver is about the size of a football and, on average, weighs about 3 pounds. It is located on the upper right side of a person's body, behind the lower ribs. Almost all the blood in a person's body passes through the liver.
Hepatitis A is caused by the hepatitis A virus (HAV). There were an estimated 4,500 acute infections in 2022. Hepatitis B. Hepatitis B is caused by the hepatitis B virus (HBV). There were an estimated 13,888 acute infections in 2022. There were an estimated 640,000 adults with chronic HBV infection during January 2017-March 2020. Hepatitis C
Safe and effective vaccines are available to prevent hepatitis B virus (HBV). This vaccine also prevents the development of hepatitis D virus (HDV) and given at birth strongly reduces transmission risk from mother to child. Chronic hepatitis B infection can be treated with antiviral agents.
The incubation period of hepatitis A virus (HAV) is 2-7 weeks (average, 28 days). Clinical symptoms then develop, often with a presentation similar to that of gastroenteritis or a viral respiratory infection. The most common signs and symptoms include fatigue, nausea, vomiting, fever, hepatomegaly, jaundice, dark urine, anorexia, and rash.
Hepatitis refers to an inflammatory condition of the liver. It is commonly the result of a viral infection, but there are other possible causes of hepatitis. These include autoimmune hepatitis and ...
Hepatitis is an inflammation of the liver often caused by a virus. Learn about viral hepatitis, statistics, surveillance, resources, populations and impact. View All For Everyone. Basics Outbreak Linked to Frozen Organic Strawberries Outbreak Linked to Fresh Organic Strawberries Prevention Approaches for Viral Hepatitis in Gay and Bisexual Men ...
SEMINAR PRESENTATION ON PROMOTING THE INTRODUCTION OF HEPATITIS B BIRTH DOSE VACCINE BY FRONT LINE HEALTHCARE WORKERS IN CAMEROON FACILITATED BY CARE FOR SOCIAL WELFARE ... • Hepatitis B is a the best way to prevent and eliminate mother to child transmission at birth.
Premium Google Slides theme, PowerPoint template, and Canva presentation template. Hepatitis B is a viral infection that affects the liver. It is transmitted through blood or bodily fluids, and can range from a mild illness lasting only a few weeks to a lifelong condition. There's a vaccine for this disease, so most people won't suffer ...
Symptoms of chronic viral hepatitis can take decades to develop. Slide 9-. Sign & symptoms Nausea Vomiting Diarrhea Dark urine (bilirubinuria) Pale stool jaundice Fever Headache Abdominal pain Hepatomegaly and splenomegaly Weight loss / lost of appetite. Slide 10-.
AcceptClose. Join us to raise awareness of viral hepatitis on #WorldHepatitisDay - 28 July.
Resources from non-federal partners. The Hepatitis B Foundation offers free educational materials for people seeking more information on hepatitis B.. The Hepatitis B United coalition hosts free webinars and a Resource Repository of hepatitis B, hepatitis D, and liver cancer-related resources.. The Asian Liver Center at Stanford University is a nonprofit that addresses the disproportionately ...
A • Hepatitis C: • can be waited out depending on liver damage seen through biopsy • Doctors will monitor condition, antiviral medication exist to help fight hep C (peginterferonaifa, ribavirin) Hepatitis D: - Liver transplant is effective treatment for severe chronic hepatitis D. Hepatitis E: - get enough calories, drink enough fluids ...
QîŽ ¯µ ppt/slides/slide19.xmlì]ënã8-þÝ ì; Ø ‰bQ¤$*èª ®µ ôT *éý/Kt¬-YÒP² l£ }‡}Ã}'9¤¤ÄŽsqªÓ W†)"ES ynßÇCšq~üËÕ²Dk.Ú¢®ÞMð©9A¼Ê꼨.ßM~¹H 6Am—VyZÖ 7¹æíä/ïÿõ_~lÎÚ2GðtÕž¥ï&‹®kΦÓ6 ...
The present case highlights a rare presentation of EBV-induced cholestasis. The classical history and physical exam findings in our case of malaise, low grade fevers, splenomegaly, and cervical lymphadenopathy were diagnostic cues leading to a higher suspicion of EBV-related cholestasis. ... 14 In cases complicated by fulminant hepatitis or ...
AcceptClose. Join us to raise awareness of viral hepatitis on #WorldHepatitisDay - 28 July.
â 3ø|ôýÛ™J„´Ì ˆ &!Cœ[«'044g%1'R1 {K©KbáS¯ÂL"GÈ[ò°ÝjõÂ' ×ñú3ñr¹,(» t]2a«$šqb¡w" Ê4ÙÔg²)Í ¤ñÑÏZ 6ºà™[ ºÓŒ9Kl~hµPsí·¯7s Š ÃH ˆÁa½Q óŸbã ðEøª1I²]êÒ€ m‡ èß¹ÿÐùØÖ"Z9éÁKó›7ÎÒ|úÆé°) u¨ªæ^Ãi7p ¼È º,ÉŠ¡9'"å'gL£h ³A`Ô•¤¿ V„È[ik+Í ...