

Organizational Structure
- Oncology Outreach
- Special Volunteers
- Brain Metastases & GBM Interest Group
- Hepatocellular Carcinoma Interest Group
- Quantitative Imaging in Radiation Therapy Interest Group
- Immunotherapy & Radiation Interest Group
- Sarcoma Interest Group
- Gastrointestinal Malignancy Interest Group
- TRT Dosimetry Interest Group
- RPT Interest Group
- RRP Funding Initiatives
- DCTD Funding Opportunities
- RRP Fiscal Year Grant Dollars
- Applying for an NIH Grant
- Information for New Grantees
- NCI Guide for Preparing P01 Applications
- Human Subjects Research
- NIH RePORTER
- Program Collaborations
- Specialized Initiatives
- Radiation Oncology-Biology Integration Network (ROBIN) U54
- Preclinical Chemo-Radiotherapy Testing Consortium (PCRTC) U01 Program
- Information Systems
- NIH Related Resources
- APS, AAPM & RRP Create Video for Medical Students on the Ascendancy of the Medical Physics in Radiotherapy
- RRP-SBIR Collaborations
- Cancer Therapy Evaluation Program
- National Clincal Trials Network
- NRG Oncology
- Imaging and Radiation Oncology Core
- Quality Assurance
- Consultation on Development of Experimental Cancer Drugs
- IMRT Guidelines (MS Word)
- Proton Guidelines (MS Word)
- Deprecated: Proton Guidelines (MS Word)
- Federal Resources
- Cancer Centers Supported by NCI
- Protection of Human Subjects
- Published Workshop and Working Group Reports
- Workshop Presentations and Reports
- Selected RRP Staff Publications
The Radiation Research Program (RRP) is divided into two highly collaboratively interactive branches:
- Clinical Radiation Oncology Branch (CROB)
- Radiotherapy Development Branch (RDB)
Interest Groups
- RRP staff manage and facilitate networking through topical and disease-site specific interest groups.
Description
The primary responsibility of the RRP is to the grantees and contractors of the NCI and NIH. In 2019, RRP staff managed a portfolio of 162 awarded grants, the bulk of which being through the R01 and R21 mechanisms. The RDB's primary focus is in radiation biology, cancer modeling, and pre-clinical research; while the CROB manages clinical and translational research in radiation oncology. Collectively, RRP staff are active in both the technical and physical aspects of radiation research with a substantial effort is devoted to supporting NCI, NIH, DHHS and government-wide activities such as technology development and assessment and comparative effectiveness research.
In addition to grant portfolio management, RRP program staff members share collective expertise in advising prospective applicants on the grants process and proposal development. RRP staff assist the broader NIH community as consultants and collaborators on radiation research-related issues with program staff in NIAID, NIBIB, NCATS, and NIA. RRP staff also serve as referees on manuscripts, and review for grants and contracts submitted to the Department of Defense, and coordinate joint activities with the Biomedical Advanced Research and Development Authority (BARDA), the National Aeronautics and Space Administration, and the Department of Energy.
Topical areas and lead contact information are listed in the organization chart below.
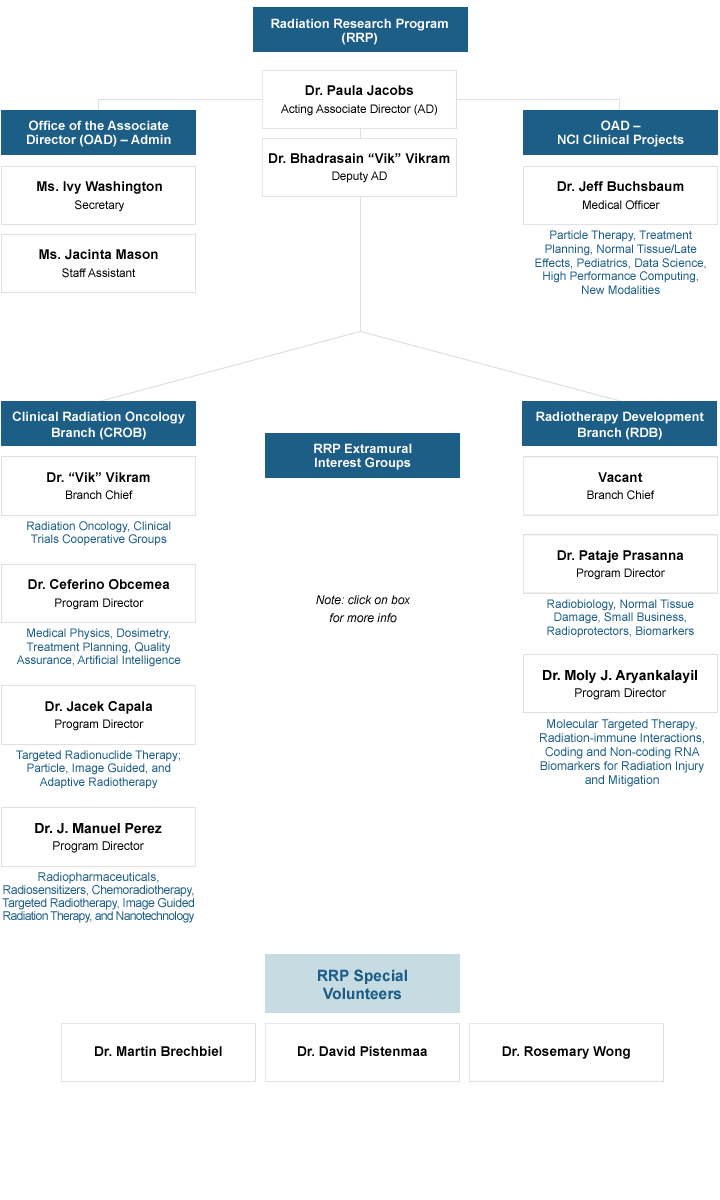
Molecular Radiation Therapeutics Branch
- Working Groups
- Contact RRP
- Accessibility
- Disclaimer Policy
- HHS Vulnerability Disclosure
- U.S. Department of Health and Human Services
- National Institutes of Health
- National Cancer Institute
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
An analysis of research activity in major UK cancer centres
Affiliation.
- 1 King's College London and Guy's and St Thomas' NHS Foundation Trust, United Kingdom. [email protected]
- PMID: 21094037
- DOI: 10.1016/j.ejca.2010.10.015
The organisation of cancer research is critical to its overall creativity and productivity. Cancer centres are a major organisational structure for this research, however, little is known about their effect on research or how national policy-making intersects with this complex policy nexus. This study of the evolution of United Kingdom cancer centres (UKCC), part of a wider European and United States programme, uses a bibliometric analysis of research activity prior to the creation of the NCRI and after its formation (1995-2004/5). In terms of critical research mass UKCC are very heterogeneous with a fourfold difference between the top and bottom quintiles. UK centres published just over one eighth of the total UKCC in 1995 but almost a quarter by 2004. This centrification occurred in the absence of any national strategy. Overall these centres conduct more fundamental (laboratory-based) research than that being conducted in the wider network but this hides major heterogeneity. UKCC collaborate with European investigators in 5-28% of all their outputs and with USA the range is between 6% and 21%. We have also derived new measures of research impact on clinical management and the general public as well as the impact of national policy on research assessment for certain types of cancer research.
Copyright © 2010 Elsevier Ltd. All rights reserved.
PubMed Disclaimer
Similar articles
- The actual citation impact of European oncological research. López-Illescas C, de Moya-Anegón F, Moed HF. López-Illescas C, et al. Eur J Cancer. 2008 Jan;44(2):228-36. doi: 10.1016/j.ejca.2007.10.020. Epub 2007 Nov 26. Eur J Cancer. 2008. PMID: 18039565
- [How to measure research emerging from hospitals? The case of French comprehensive cancer centres]. Bonastre J, de Pouvourville G. Bonastre J, et al. Bull Cancer. 2006 Nov;93(11):1144-51. Bull Cancer. 2006. PMID: 17145585 French.
- Analysing the attributes of Comprehensive Cancer Centres and Cancer Centres across Europe to identify key hallmarks. Kehrloesser S, Oberst S, Westerhuis W, Wendler A, Wind A, Blaauwgeers H, Burrion JB, Nagy P, Saeter G, Gustafsson E, De Paoli P, Lovey J, Lombardo C, Philip T, de Valeriola D, Docter M, Boomsma F, Saghatchian M, Svoboda M, Philip I, Monetti F, Hummel H, McVie G, Otter R, van Harten W. Kehrloesser S, et al. Mol Oncol. 2021 May;15(5):1277-1288. doi: 10.1002/1878-0261.12950. Epub 2021 Mar 30. Mol Oncol. 2021. PMID: 33734563 Free PMC article.
- Building PET research collaborations. Fleming IN, Gilbert FJ, Blower PJ. Fleming IN, et al. Nucl Med Commun. 2012 Jan;33(1):1-3. doi: 10.1097/MNM.0b013e32834c6411. Nucl Med Commun. 2012. PMID: 21946618 Review.
- Clinical Cancer Advances 2009: major research advances in cancer treatment, prevention, and screening--a report from the American Society of Clinical Oncology. Petrelli NJ, Winer EP, Brahmer J, Dubey S, Smith S, Thomas C, Vahdat LT, Obel J, Vogelzang N, Markman M, Sweetenham JW, Pfister D, Kris MG, Schuchter LM, Sawaya R, Raghavan D, Ganz PA, Kramer B. Petrelli NJ, et al. J Clin Oncol. 2009 Dec 10;27(35):6052-69. doi: 10.1200/JCO.2009.26.6171. Epub 2009 Nov 9. J Clin Oncol. 2009. PMID: 19901123 Review. No abstract available.
- Collective health research assessment: developing a tool to measure the impact of multistakeholder research initiatives. Kork AA, Antonini C, García-Torea N, Luque-Vílchez M, Costa E, Senn J, Larrinaga C, Bertorello D, Brichetto G, Zaratin P, Andreaus M. Kork AA, et al. Health Res Policy Syst. 2022 May 2;20(1):49. doi: 10.1186/s12961-022-00856-9. Health Res Policy Syst. 2022. PMID: 35501895 Free PMC article.
- Evaluating cancer research impact: lessons and examples from existing reviews on approaches to research impact assessment. Hanna CR, Boyd KA, Jones RJ. Hanna CR, et al. Health Res Policy Syst. 2021 Mar 11;19(1):36. doi: 10.1186/s12961-020-00658-x. Health Res Policy Syst. 2021. PMID: 33706777 Free PMC article. Review.
- The impact generated by publicly and charity-funded research in the United Kingdom: a systematic literature review. Gomes D, Stavropoulou C. Gomes D, et al. Health Res Policy Syst. 2019 Feb 28;17(1):22. doi: 10.1186/s12961-019-0425-2. Health Res Policy Syst. 2019. PMID: 30819185 Free PMC article.
- Differential research impact in cancer practice guidelines' evidence base: lessons from ESMO, NICE and SIGN. Pallari E, Fox AW, Lewison G. Pallari E, et al. ESMO Open. 2018 Jan 6;3(1):e000258. doi: 10.1136/esmoopen-2017-000258. eCollection 2018. ESMO Open. 2018. PMID: 29344408 Free PMC article.
- Tracing the indirect societal impacts of biomedical research: development and piloting of a technique based on citations. Jones TH, Hanney S. Jones TH, et al. Scientometrics. 2016;107:975-1003. doi: 10.1007/s11192-016-1895-4. Epub 2016 Mar 8. Scientometrics. 2016. PMID: 27340306 Free PMC article.
Publication types
- Search in MeSH
Related information
- Cited in Books
LinkOut - more resources
Full text sources.
- Elsevier Science
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
what is the organisational structure of Cancer Research UK?
Quick Reply
Related discussions.
- Seeking Summer Internships!
- Kallisto's Sunday Question: £ 1,000,000
- I'm interested in cancer biology but don't want to experiment on animals
- studying in uni of essex
- Good Unis for English Degree
- History and Art History Joint Honours
- Unit 7 Btec Applied science
- best cancer research institutes
- Occupational / Business Psychology MSc - Distance Learning - THOUGHTS?
- A level+ 2024 Results Day Countdown - Hub is open! #FindYourFuture
- Are my grades too low for a funded PhD in UK?
- Biomedical science or Biochemistry?
- psychology a level ocr
- Gap Year Internships
- TCD or UoM for Medicine?
- super curricular for biomed
- Greenwich or Kingston University
- EPQ advice plz
- Writing up dissertation
- Are there any scope for Public health in UK
Last reply 2 hours ago
Last reply 16 hours ago
Last reply 2 days ago
Last reply 3 days ago
Last reply 4 days ago
Last reply 5 days ago
Last reply 6 days ago
Last reply 1 week ago
Last reply 2 weeks ago
Articles for you

Why I chose a law degree and what studying law was like

Finding a university place in Ucas Clearing 2024: 10 top tips to help you get ready
Top 10 tips for Ucas Clearing 2024

Bringing business people into the classroom: what students learn from industry professionals
Building a Comprehensive Cancer Center: Overall Structure
- Open Access
- First Online: 29 October 2021
Cite this chapter
You have full access to this open access chapter

- Dolores Grosso 6 ,
- Mahmoud Aljurf 7 &
- Usama Gergis 6
6818 Accesses
1 Citations
According to the World Health Organization (WHO), cancer is the second leading cause of death globally, accounting for approximately 9.6 million deaths [1]. The WHO recommends that each nation has a national cancer control program (NCCP) to reduce the incidence of cancer and deaths related to cancer, as well as to improve the quality of life of cancer patients [2]. Comprehensive cancer centers form the backbone of a NCCP and are charged with developing innovative approaches to cancer prevention, diagnosis, and treatment [3]. This is accomplished through basic and clinical research, the provision of patient care, the training of new clinicians and scientists, and community outreach and education. Most comprehensive cancer centers are affiliated with university medical centers, but their cancer care initiatives may involve partnering outside the institution with other comprehensive cancer centers, community leaders, or members of industry [3]. When affiliated with a university medical center, cancer center executives must work in concert with their counterparts at the hospital, patient practice, medical school, and allied health science leaders resulting in an overlapping, often complicated reporting structure. Comprehensive cancer centers and the departments in the center receive funding for their services from various sources, including national and local grants, institutional funds, private donations, and industry [4].
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others

Challenges in Running a Comprehensive Cancer Center
The cancer research network: a platform for epidemiologic and health services research on cancer prevention, care, and outcomes in large, stable populations.
Comprehensive cancer control in the U.S.: summarizing twenty years of progress and looking ahead
Introduction.
According to the World Health Organization (WHO), cancer is the second leading cause of death globally, accounting for approximately 9.6 million deaths [ 1 ]. The WHO recommends that each nation has a national cancer control program (NCCP) to reduce the incidence of cancer and deaths related to cancer, as well as to improve the quality of life of cancer patients [ 2 ]. Comprehensive cancer centers form the backbone of a NCCP and are charged with developing innovative approaches to cancer prevention, diagnosis, and treatment [ 3 ]. This is accomplished through basic and clinical research, the provision of patient care, the training of new clinicians and scientists, and community outreach and education. Most comprehensive cancer centers are affiliated with university medical centers, but their cancer care initiatives may involve partnering outside the institution with other comprehensive cancer centers, community leaders, or members of industry [ 3 ]. When affiliated with a university medical center, cancer center executives must work in concert with their counterparts at the hospital, patient practice, medical school, and allied health science leaders resulting in an overlapping, often complicated reporting structure. Comprehensive cancer centers and the departments in the center receive funding for their services from various sources, including national and local grants, institutional funds, private donations, and industry [ 4 ].
The structure of a comprehensive cancer center arises from the mission of the center and the framework required to support this mission. The overarching mission of a comprehensive cancer center is to reduce the incidence of cancer and increase the quality of life and survival rates in patients with malignancies. There are three primary areas of cancer care: research, clinical care, and education that coalesce to meet this goal. Multiple interconnected departments are required to meet the objectives of the cancer center. Department heads include physicians, scientists, or administrators, depending on the focus of the department. The department leaders report to the comprehensive cancer center director, who is assisted by deputy directors and hospital advisory boards. The comprehensive cancer center director is typically an accomplished individual trained in a specific area of cancer research, but who has a vision for the broad research and clinical base required of the cancer center. The cancer center director has a multitude of responsibilities, including setting departmental goals, coordinating efforts between departments, hiring and retaining scientific staff, obtaining national, state, and philanthropic funding, creating new programs, and monitoring the business aspects of the center.
Structure of a Comprehensive Cancer Center Based on Mission
Basic laboratory research.
Basic laboratory research generates the knowledge that forms the basis for applied science. This type of research focuses on the mechanistic understanding of biochemical, biologic, physiologic, and pharmacologic processes as they relate to cancer and cancer treatments [ 5 ]. Tools used in this type of research include laboratory techniques such as flow cytometry analysis, bioimaging, spectroscopy, and gene sequencing. Laboratory experiments with human cell lines or animal models may also be utilized in this type of research. Basic laboratory research requires trained scientists, laboratory space and equipment, storage facilities for cell samples and cell lines, and areas for the humane care and housing of research animals . In most comprehensive cancer centers, a centralized source of core services and equipment exists, which is accessible to all scientists. Gene expression analysis and next-generation sequencing are examples of services provided by a comprehensive cancer center’s core laboratory facility. Training of future generations of scientists is also a key function of laboratory scientists. Students in MD/PhD programs, clinical fellows requiring research experience, and postdoctoral scientists are examples of the many individuals trained in basic science in comprehensive cancer centers. The basic science division is composed of subspecialty areas such as immunology, cancer biology, or microbiology. Directors of these areas report to a director of basic science who in turn reports to the comprehensive cancer center director or deputy director.
Clinical Research in Human Subjects
Patients with cancer require multidisciplinary care to achieve optimal outcomes. Therefore, clinicians with expertise in medical, surgical, and radiation oncology participate in the direct care of patients with oncologic diagnoses and perform research in their specialty areas with the goal of improving cancer care. Examples of clinical research initiatives include those testing cancer prevention strategies [ 6 ], analyses of medication efficacy, trials comparing the benefits of various treatment modalities, and analyses of risk based on tumor genetic signature. Cancer research trials may be observational, analyzing cause and effect relationships, or interventional with the goal of evaluating the impact of a specific treatment [ 7 ]. Investigators in comprehensive cancer centers may participate with other institutions in national or international networks to analyze the outcomes of large numbers of combined patients providing more power to detect meaningful trends. Clinical research involves human subjects and, therefore, this type of research approach requires systems to be in place within the comprehensive cancer center to protect the safety, welfare, and rights of human research subjects.
Translational Research
Translational research is the integration of basic laboratory research with patient- and population-based research [ 8 ]. In this area, clinical research and basic research are complementary to each other with both areas contributing to a specific outcome. Ideally, translational research applies newly developed basic research understandings and applies them to early phase clinical research. This is a multistep, bidirectional process in which optimal treatments are refined over time by incremental discovery in both the clinical and laboratory settings. The ability to translate scientific data generated by the cancer center into actionable improvement in cancer care is central to the mission of the comprehensive cancer center. Therefore, a specific department of translational research exists in most cancer centers. Initiatives that foster working relationships between bench scientists and clinicians, such as scientific meetings, data sharing sessions, and availability of funding for multidisciplinary research, assist in the development of transitional research. Clinical trials, such as first-in-man or phase I studies, are developed by basic scientists and clinicians and are conducted within the comprehensive cancer center. The director of translational research reports directly to the comprehensive cancer center director or deputy director.
Population Health Research
The goal of population health science is to optimize health outcomes in specific populations. This type of research assesses trends in cancer incidence, identifies disparities in health care and suggests corrective actions, and examines cancer prevention, incidence, and treatment based on gender, race, or ethnicity, geographic location, or income. In doing so, population health scientists study community characteristics to inform the development of cancer care initiatives. In many comprehensive cancer centers , community outreach via education programs and free health services are offered through the population health department. The Framingham study is an early, important example of population health science which linked cigarette smoking, poor diet, and lack of exercise to the development of cardiovascular disease [ 9 ]. A more recent analysis of prostate cancer screening recommended different screening guidelines for African American versus Caucasian men, as African American men have a higher incidence and rate of death of prostate cancer than their Caucasian counterparts [ 10 ]. Population health scientists are in key positions to examine local health issues and can have direct, positive impacts on the health of their communities. The director of population health reports directly to the cancer center director or deputy director.
Protection of Human Subjects
Institutional review boards.
The primary group responsible for the oversight of clinical research in human subjects is the Institutional Review Board (IRB) that reviews, approves, and monitors the conduct of clinical trials. Physicians, nurses, pharmacists, administrators, and community members can all serve on an IRB. The IRB reviews informed consent documents, investigator brochures, and provides guidance to investigators. The IRB also serves a critical role in monitoring the compliance of researchers to the conditions set forth in their clinical trials as well as adherence to IRB regulations for patient safety, sponsor-investigator relationships, reporting of adverse events, and adherence to national guidelines. IRBs follow guidelines set forth by national regulatory institutions. In the United States, IRBs follow good clinical practice and clinical trial guidelines set forth by the Food and Drug Administration and assure that researchers are trained in the basic principles of human research [ 11 ]. Most IRBs are part of the academic medical center that is affiliated with the comprehensive cancer center, but commercial and free-standing IRBs exist as well.
Clinical Research Organizations
Comprehensive cancer centers may utilize either in-house or contracted organizations to assist in the conduct of clinical trials. These clinical research organizations (CROs) assist the investigator in maintaining good clinical practices in the conduct of the clinical trial [ 12 ]. A CRO can provide a diverse array of services that include clinical and regulatory support of clinical trials. Examples of clinical services include procurement and shipping of clinical samples and supplies, development of case report forms, data capture of trial outcomes, adverse event monitoring, recording and reporting, trial pre-screening, and assistance with patient education and consent. Regulatory support includes developing standard operating procedures for compliance monitoring, audits to assess for compliance to trial procedures, and support for changing and updating clinical trial documents. Regulatory staff additionally facilitate communication between the sponsors and investigators of clinical trials and assist with the registration of clinical trials and clinical trial results to public and national databases. The department head managing an in-house CRO or who contracts with hired CROs reports to the comprehensive cancer center director or deputy director.
Other Key Programs Supporting Cancer Research
The goal of comprehensive cancer centers is to apply resources to projects that are scientifically rigorous, are likely to advance cancer prevention, care, and quality of life, and have the potential for benefitting the largest amount of people. Towards that end, committees that evaluate the scientific merit, the financial feasibility, and the appropriateness of proposed research projects to the identified research needs of the population are required. Other supportive programs include an Office of Biostatistics to assist in formulating research plans as well as analyzing trial outcomes. An office of technology transfer is important in the identification of novel ideas, assistance with the development and application of these ideas, as well as protection of intellectual rights.
Clinical Care of Patients with Cancer
The complexity of cancer diagnostics, treatment, and follow-up requires care across multiple disciplines [ 13 ]. Surgeons, interventional radiologists, and clinical practitioners are utilized to obtain tissue for pathological analysis. Accurate cancer diagnosis and prognostication depends upon the availability of pathologists trained in the analysis of cancer cells and accompanying genetic and molecular profiling. Radiology services are required for cancer staging and surveillance. Clinicians experienced in the treatment and administration of chemotherapy, oncology-based pharmacists, radiation oncologists, and surgeons specializing in oncology are required for the administration of treatment and the monitoring of response. The framework for this treatment includes inpatient and outpatient treatment areas, support staff, insurance, budgetary and billing staff, housekeeping, supply chain management resources, and equipment. In free-standing comprehensive cancer centers, directors of these areas report to the cancer center director. However, in comprehensive cancer centers affiliated with university medical centers, services are shared across all disciplines, although oncology-dedicated subdivisions within these departments exist. Cancer-specific specialty services within various specialties, such as cardiology, renal, and pulmonary, have been developed for more optimal management of organ-specific toxicities related to cancer treatment. Clinicians providing cancer care in university medical center settings may have dual reporting relationships to both the comprehensive cancer center director and to hospital or university-based leadership.
Quality Monitoring in Cancer Care
Cancer care is a highly complex, high-risk, discipline characterized by rapid development of new therapies. To provide the safest and most effective care, comprehensive cancer centers must establish systems to assess and monitor the quality and safety of care. There are multiple components of a quality program, including the development of standardized processes to deliver care, monitoring adherence to established guidelines for care, assessment of compliance with established guidelines, and the development of procedures to improve care. Examples include the use of evidence-based clinical pathways when ordering chemotherapy [ 14 ], monitoring adherence to quality indicators, such as those developed by the Agency for Healthcare Research and Quality (AHRQ), and medical record auditing to monitor compliance to national best practice standards, such as those set forth by the Foundation for the Accreditation of Cellular Therapy (FACT) [ 15 ], in stem cell transplant programs. Because quality initiatives are integrated into every department in the cancer center, there is typically an executive level position in the cancer center overseeing all aspects of the quality program. This executive reports directly to the cancer center director or deputy director.
Improving the Quality of Life of Cancer Patients: Support Services
Social work.
Social work is a mandatory discipline in every comprehensive cancer center supporting every aspect of a patient’s cancer care experience. Social workers provide a wide array of patient services, including patient and family counseling and recognition of distress [ 16 ], assistance in finding financial reimbursement for medications and housing, end-of-life counseling and assistance with end-of-life issues [ 17 ]. Social workers have a broad array of responsibilities that may range from assistance in obtaining wigs, development of education programs for patients and families, or even coordinating fundraising services for patients and their families in the community. From the standpoint of continuity of care, social workers provide key information regarding the ability to obtain medications and information regarding health insurance issues as the patients move from inpatient to outpatient settings. Social workers increase the quality of cancer care by serving as a nonclinical support system.
Palliative Care
Palliative care is another aspect of cancer care that has the goal of increasing the patient’s quality of life. Palliative care specialists are physicians or advanced practice providers who address the needs of patients with life-threatening illnesses. The aim of palliative care providers is to manage symptoms and side effects of cancer care [ 18 ]. This may encompass direct interventions to treat pain, anxiety, or neuropathy related to cancer treatments. Palliative care specialists also address spiritual, social, and psychological issues with patients. In some cancer centers, oncology-specific psychiatrists are part of the palliative care team. The palliative care team, in conjunction with clinicians and social workers, also may serve as end-of-life counselors. Palliative care specialists work in a variety of settings and are often available for acute issues in the inpatient and outpatient settings.
Patients undergoing cancer care attempt to negotiate the complex health care system at a time of physical and psychological stress. Many cancer centers employ navigators to guide patients through the healthcare continuum. Navigators provide direct assistance to patients in making appointments, transferring records between offices, distributing directions to testing sites, coordinating family meetings, and providing a consistent contact for patients throughout cancer treatment. Navigators are also useful in providing consumer feedback to the cancer center to help improve services. Navigators have been shown to increase satisfaction and survival [ 19 ]. Most comprehensive cancer centers have some type of navigator services to support consistency and quality of care of patients with cancer. Registered nurses or specially trained lay people may serve as navigators in the comprehensive cancer center.
Survivorship
Survivorship refers to the physical, psychological, psychosocial, economic, and spiritual well-being of patients who have survived a cancer diagnosis [ 20 ]. Posttreatment survivorship goals include the transition back to a primary care provider for the majority of medical care, reintegration into the workforce, and return to family and social functions. This period of time in patient recovery may be marked by considerable anxiety related to both internal and external forces. Individuals recovered from cancer therapy have physical and mental challenges such as limited activity due to neuropathy, deconditioning, or osteoporosis, decreased self-confidence, or even fear of infection or relapse. Work supervisors may have concerns regarding the ability of returning employees to be fully productive. Family members, friends, and coworkers may have altered perceptions of cancer survivors resulting in relationship strain. Time missed from school or employment delays scholastic or career progression adding to frustration, stress, and anxiety. Comprehensive cancer centers support lifestyle reintegration through direct counseling and education from the clinical team, educational classes in the community sponsored by social workers, and the sponsorship of initiatives such as the buddy program, cancer survivor scholarships, beauty and support days, and job counselling.
Comprehensive cancer centers are not only central to the education of future scientists and health care providers, but also take part in the development and continuing education of employees, patients, and the public via community outreach programs. When affiliated with a university medical center, cancer centers participate in the education of medical students, house staff, laboratory-based future scientists, and students from across all health science disciplines. Care of patients with cancer and cancer research is intertwined with academic faculty support and career progression resulting in ongoing research in cancer specialty areas. Grand rounds programs with internal or external speakers educate staff and students to new scientific discovery. Cancer centers also form partnerships with community leaders, government agencies, and industry to develop community outreach programs to improve health literacy, develop early detection programs, and raise money for cancer research.
Comprehensive cancer centers are highly complex institutions responsible for the advancement of cancer research, clinical care, and education. A multitude of personnel with varying areas of expertise are responsible for the integration of all the critical cancer center activities described in this chapter. Therefore, a highly organized and functional framework is necessary to avoid overlap and address all aspects of the cancer center’s mission. Figure 2.1 displays the basic organization chart of a university-affiliated comprehensive cancer center.
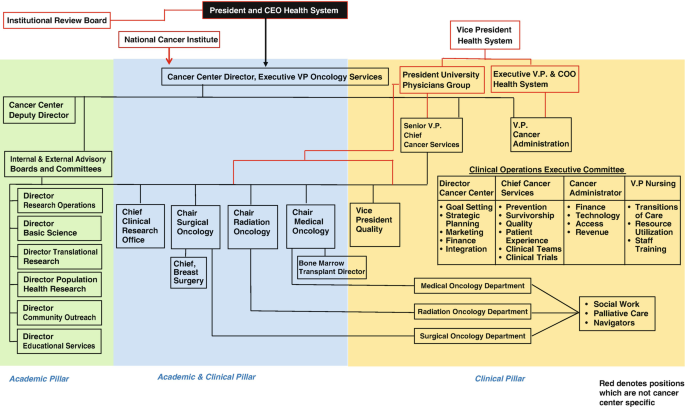
Structure of a comprehensive cancer center that is affiliated with a university medical center. In this example, the mission of the comprehensive cancer center is divided into three pillars. Personnel in the Academic Pillar provide academic leadership and planning of cancer center goals. The Academic and Clinical Pillar is composed of clinician leaders who assure that the goals of the cancer center are brought to individual departments, while the Clinical Pillar is composed primarily of clinicians and other key individuals providing direct patient care. Individuals in all three pillars ultimately report to the comprehensive cancer center director
World Health Organization. Cancer. 2020. Accessed May 6, 2020 at: https://www.who.int/health-topics/cancer#tab=tab_1 .
World Health Organization. National cancer care programmes. 2020. Accessed May 6, 2020 at: https://www.who.int/cancer/nccp/en/ .
National Cancer Institute. NCI-designated cancer centers. 2018. Accessed May 6, 2020 at: https://www.cancer.gov/research/nci-role/cancer-centers .
Eckhouse S, Sullivan R. A survey of public funding of cancer research in the European union. PLoS Med. 2006;3(7):e267.
Article Google Scholar
Association of American Medical Colleges-AAMC. Basic science. 2020. Accessed May 6, 2020 at: https://www.aamc.org/what-we-do/mission-areas/medical-research/basic-science .
McCaskill-Stevens W, Pearson DC, Kramer BS, Ford LG, Lippman SM. Identifying and creating the next generation of community-based cancer prevention studies: summary of a National Cancer Institute think tank. Cancer Prev Res (Phila). 2017;10(2):99–107.
Thiese MS. Observational and interventional study design types; an overview. Biochem Med. 2014;24(2):199–210.
Rubio DM, Schoenbaum EE, Lee LS, et al. Defining translational research: implications for training. Acad Med. 2010;85(3):470–5.
Mahmood S, Levy D, Vasan R, Wang T. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383(9921):P999–1008.
Shenoy D, Packianathan S, Chen AM, Vijayakumar S. Do African-American men need separate prostate cancer screening guidelines? BMC Urol. 2016;16(1):19.
U.S. Food and Drug Administration. Institutional review boards (IRBs) protection of human subjects in clinical trials. Sept 11, 2019. Accessed May 8, 2020 at: https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/institutional-review-boards-irbs-and-protection-human-subjects-clinical-trials .
World Health Organization. Handbook for good research clinical practice. 2002. Accessed May 11, 2020 at: https://www.who.int/medicines/areas/quality_safety/safety_efficacy/gcp1.pdf .
Taylor C, Munro AJ, Glynne-Jones R, et al. Multidisciplinary team working in cancer: what is the evidence? BMJ. 2010;340:c951.
Ellis PG. Development and implementation of oncology care pathways in an integrated care network: the via oncology pathways experience. J Oncol Pract. 2013;9(3):171–3.
Foundation for the Accreditation of Cellular Therapy. Setting the global standard for top quality patient care in cellular therapies. 2020. Accessed May 12, 2020 at: http://www.factwebsite.org/ .
Pirl WF, Fann JR, Greer JA, et al. Recommendations for the implementation of distress screening programs in cancer centers: report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer. 2014;120(19):2946–54.
Becker F. Oncology social workers’ role in patient-centered care. 2017. Accessed May 15, 2020 at: https://www.accc-cancer.org/acccbuzz/blog-post-template/accc-buzz/2017/03/15/oncology-social-workers-role-in-patient-centered-care .
National Institute of Health: National Cancer Institute. Palliative care in cancer. 2017. Accessed May 15, 2020 at: https://www.cancer.gov/about-cancer/advanced-cancer/care-choices/palliative-care-fact-sheet .
Riley S, Riley C. The role of patient navigation in improving the value of oncology care. J Clin Pathw. 2016;2(1):41–7.
Google Scholar
McCanney J, Winckworth-Prejsnar K, Schatz A, et al. Addressing survivorship in cancer care. J Natl Compr Cancer Netw. 2018;16(7):802–7.
Download references
Author information
Authors and affiliations.
Medical Oncology, Thomas Jefferson University Hospital, Philadelphia, PA, USA
Dolores Grosso & Usama Gergis
Adult Hematology and HSCT, Oncology Centre, King Faisal Specialist Hospital & Research Centre, Riyadh, Saudi Arabia
Mahmoud Aljurf
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Dolores Grosso .
Editor information
Editors and affiliations.
Oncology Centre, King Faisal Specialist Hosp and Res Ctr, Riyadh, Saudi Arabia
Blood and Marrow Transplant Program, Cleveland Clinic, Cleveland, OH, USA
Navneet S. Majhail
Stem Cell Transplantation, St George’s, University of London, London, UK
Mickey B.C. Koh
Blood and Marrow Transplantation, Mayo Clinic, Jacksonville, FL, USA
Mohamed A. Kharfan-Dabaja
Hematologic Malignancies and Cellular Therapy, Duke University, Durham, NC, USA
Nelson J. Chao
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Reprints and permissions
Copyright information
© 2022 The Author(s)
About this chapter
Grosso, D., Aljurf, M., Gergis, U. (2022). Building a Comprehensive Cancer Center: Overall Structure. In: Aljurf, M., Majhail, N.S., Koh, M.B., Kharfan-Dabaja, M.A., Chao, N.J. (eds) The Comprehensive Cancer Center. Springer, Cham. https://doi.org/10.1007/978-3-030-82052-7_2
Download citation
DOI : https://doi.org/10.1007/978-3-030-82052-7_2
Published : 29 October 2021
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-82051-0
Online ISBN : 978-3-030-82052-7
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Oops, you're using an old version of your browser so some of the features on this page may not be displaying properly.
MINIMAL Requirements: Google Chrome 24+ , Mozilla Firefox 20+ , Internet Explorer 11 , Opera 15–18 , Apple Safari 7 , SeaMonkey 2.15-2.23
- Meeting Calendar
- OncologyPRO
Organisational Structure
Composed by an Executive Board, a Council and several Committees and task forces, the organisational structure of ESMO enables participation and inclusion in the life of the Society.
Executive Board
The Executive Board is the executive body of the Society and responsible for the management and administration of the Society.
ESMO Council
The ESMO Council supports and advises the Executive Board and proposes strategic topics for discussion at the annual strategy meeting.
Annals of Oncology Editorial Board
Audit committee, cancer medicines committee.
The ESMO Cancer Medicines Committee works to address the topic of access to medicines at the European and global policy levels to ensure that all patients have access to the medicines they need, when they need them.
Compliance Committee
The general scope of the Compliance Committee is to develop compliance policies as requested by the Executive Board to mitigate risks to ESMO arising from the involvement of officers, staff and third parties in its activities.
Educational Committee
Esmo open editorial board, guidelines committee, iotech editorial board, leadership development committee.
Identifying and nurturing the future leaders in oncology
Membership Committee
National societies committee.
The ESMO National Societies Committee focuses on public policy, ESMO initiatives, and in reinforcing the specialisation of Medical Oncology.
Nomination Committee
The Nomination Committee is responsible for identifying the candidates for the Executive Board positions.

Communication Committee
Women for oncology committee.
Meet the members of the ESMO Women for Oncology Committee
Young Oncologists Committee
Meet the ESMO Young Oncologists Committee
This site uses cookies. Some of these cookies are essential, while others help us improve your experience by providing insights into how the site is being used.
For more detailed information on the cookies we use, please check our Privacy Policy .
Necessary cookies enable core functionality. The website cannot function properly without these cookies, and you can only disable them by changing your browser preferences.
Global site navigation
- Capital Market
- Celebrity biographies
- Messages - Wishes - Quotes
- Fashion and style
- Celebrities
- Relationships
Local editions
- Habari za Kenya Swahili
What type of ownership is Cancer Research UK? Explained
There are medical research organizations that are state-owned while some are independent. Cancer Research UK is a renowned medical research institute, a forefront runner in the fight against cancer in the region; but what type of ownership is Cancer Research UK?

Cancer Research UK is a company limited by guarantee. Guarantors own the company and are expected to pay a certain amount of money should the company go into debt. A Council of Trustees , which is also the body’s Board of Directors, controls the institution. Professor Sir Leszek Borysiewicz chairs the board and Carolyn Bradley is his deputy chair. As of June 21st, 2020, the other trustees include:
- Catherine Brown
- Professor Moira Whyte
- Professor Dame Amanda Fisher
- Professor Sir Mike Richards
- Tracy De Groose
- Professor Nic Jones
- Professor Sir Bruce Ponder
- Peter Chambré
- Professor Stephen T Holgate
- Andrew Palmer
- David Lindsell
What type of organization structure is Cancer Research UK ?

Dr Pepper commercial cast: Fansville's actors and actresses and their roles

The Council of Trustees governs the organization and has power over the company, the company’s property, and its funds. The board works together with the Cancer Research UK’s Chief Executive Officer, Michelle Mitchell. She spearheads the Senior Management Team.
The team comprises of the following:
- Chief Scientist
- Executive Director of Corporate Resources
- Executive Director of Philanthropy & Partnerships
- Chief Information Officer
- Executive Director of Human Resources
- Executive Director of Policy and Information
- Executive Director of Strategy and International Partnerships
- Executive Director of Fundraising and Marketing
- Chief Clinician and
- Executive Director of Research & Innovation / CEO of Cancer Research Technology
Is Cancer Research UK a nonprofit organization?

It is in fact the world’s major charity in the battle against cancer. There are no shareholders deriving any benefits. The founders set out to assist individuals affected by the disease and not as a profit-making venture . The situation is still the same to date.

Jackie DeAngelis bio: husband, wedding, education, political party, family
The organization uses its money to conduct research and raise awareness about cancer. It also uses the money to cater to the marketing and creation of extra means of fundraising and to operate its events and shops.
What is the aim of Cancer Research UK?
Imagine a future where cancer is curable. The organization is not just imagining it; it is endeavoring to make it a reality. It has made great strides in cancer diagnosis, treatment, and prevention . Over the years, survival rates have been twofold because of its research. It aims at ensuring the survival of individuals from cancer. Professor Alexander Haddow, who started cancer research on how to kill cancer cells using nitrogen mustard chemical, was a Cancer Research UK scientist.
What has Cancer Research UK achieved?
The number of individuals who have a reason to smile today because of this organization has increased over the years. It was among the pioneers who made the nexus between skin cancer and exposure to the sun.

JTV hosts salaries and net worth 2021: Who is the wealthiest?
It funded Renato Dulbecco who arrived at how cancer alters and copies itself in cells. Its two scientists Tim Hunt and Paul Nurse were very influential in cancer remedy. They found the protein that facilitates cell development. Cancer Research UK has gone above and beyond to raise awareness about tobacco smoke and the risk of lung cancer even for passive smokers. Smoking rates have declined, which could minimize the cases of lung cancer.
The organization’s cancer research funding comes from donor funds. Be it from individuals or from groups, the organization relies on well-wishers and philanthropy to run. It has shops where products used in cancer care like relieving scar gels and mastectomy swimsuits get purchased. Flowers, hampers, and fundraising merchandise like T-shirts are available too.
The products are also available online from stores like eBay. Cancer Research UK also organizes events like Race For Life where proceeds go towards research. It also accepts gifts from wills as part of donations. It is battling two hundred types of cancer and every pound goes a long way into helping the fight.

Meet 30-year-old medical scientist giving her best to make the world a better place
Cancer Research UK is among other cancer research charities in the world . These others include Breast Cancer Research Foundation and Ovarian Cancer Research Fund. The organization’s cancer research stakeholders consist of the Government, its Directors, Trustees, Corporate partners like Tesco, Employees, Doctors, and Nurses, Patients, Volunteers, and Donors.
Over the years, Cancer Research UK has made Immense contributions to the fight against cancer. If you have been wondering what type of ownership is Cancer Research UK, you now know it's de facto owners, structure, and achievements.
Source: TUKO.co.ke
Winnie Mwangi (SEO writer) Winnie Aswani is a seasoned reporter. A graduate of Masinde Muliro University with a degree in social work and community development. She is passionate about digital marketing, content writing and volunteer services. Solve day-to-day challenges with her informative pieces.
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Institutes of Health (US); Biological Sciences Curriculum Study. NIH Curriculum Supplement Series [Internet]. Bethesda (MD): National Institutes of Health (US); 2007.

NIH Curriculum Supplement Series [Internet].
Understanding cancer.
In simple terms, cancer is a group of more than 100 diseases that develop across time and involve the uncontrolled division of the body's cells. Although cancer can develop in virtually any of the body's tissues, and each type of cancer has its unique features, the basic processes that produce cancer are quite similar in all forms of the disease.
Cancer begins when a cell breaks free from the normal restraints on cell division and begins to follow its own agenda for proliferation ( Figure 3 ). All of the cells produced by division of this first, ancestral cell and its progeny also display inappropriate proliferation. A tumor , or mass of cells, formed of these abnormal cells may remain within the tissue in which it originated (a condition called in situ cancer), or it may begin to invade nearby tissues (a condition called invasive cancer). An invasive tumor is said to be malignant , and cells shed into the blood or lymph from a malignant tumor are likely to establish new tumors ( metastases ) throughout the body. Tumors threaten an individual's life when their growth disrupts the tissues and organs needed for survival.
The stages of tumor development. A malignant tumor develops across time, as shown in this diagram. This tumor develops as a result of four mutations, but the number of mutations involved in other types of tumors can vary. We do not know the exact number (more...)
What happens to cause a cell to become cancerous? Thirty years ago, scientists could not offer a coherent answer to this question. They knew that cancer arose from cells that began to proliferate uncontrollably within the body, and they knew that chemicals, radiation, and viruses could trigger this change. But exactly how it happened was a mystery.
Research across the last three decades, however, has revolutionized our understanding of cancer. In large part, this success was made possible by the development and application of the techniques of molecular biology, techniques that enabled researchers to probe and describe features of individual cells in ways unimaginable a century ago. Today, we know that cancer is a disease of molecules and genes, and we even know many of the molecules and genes involved. In fact, our increasing understanding of these genes is making possible the development of exciting new strategies for avoiding, forestalling, and even correcting the changes that lead to cancer.
- Unraveling the Mystery of Cancer
People likely have wondered about the cause of cancer for centuries. Its name derives from an observation by Hippocrates more than 2,300 years ago that the long, distended veins that radiate out from some breast tumors look like the limbs of a crab. From that observation came the term karkinoma in Greek, and later, cancer in Latin.
With the work of Hooke in the 1600s, and then Virchow in the 1800s, came the understanding that living tissues are composed of cells, and that all cells arise as direct descendants of other cells. Yet, this understanding raised more questions about cancer than it answered. Now scientists began to ask from what kinds of normal cells cancer cells arise, how cancer cells differ from their normal counterparts, and what events promote the proliferation of these abnormal cells. And physicians began to ask how cancer could be prevented or cured.
Clues from epidemiology
One of the most important early observations that people made about cancer was that its incidence varies between different populations. For example, in 1775, an extraordinarily high incidence of scrotal cancer was described among men who worked as chimney sweeps as boys. In the mid-1800s, lung cancer was observed at alarmingly high rates among pitch blende miners in Germany. And by the end of the 19th century, using snuff and cigars was thought by some physicians to be closely associated with cancers of the mouth and throat.
These observations and others suggested that the origin or causes of cancer may lie outside the body and, more important, that cancer could be linked to identifiable and even preventable causes. These ideas led to a widespread search for agents that might cause cancer. One early notion, prompted by the discovery that bacteria cause a variety of important human diseases, was that cancer is an infectious disease. Another idea was that cancer arises from the chronic irritation of tissues. This view received strong support with the discovery of X-rays in 1895 and the observation that exposure to this form of radiation could induce localized tissue damage, which could lead in turn to the development of cancer. A conflicting view, prompted by the observation that cancer sometimes seems to run in families, was that cancer is hereditary.
Such explanations, based as they were on fragmentary evidence and incomplete understanding, helped create the very considerable confusion about cancer that existed among scientists well into the mid-twentieth century. The obvious question facing researchers—and no one could seem to answer it—was how agents as diverse as this could all cause cancer. Far from bringing science closer to understanding cancer, each new observation seemed to add to the confusion.
Yet each new observation also, ultimately, contributed to scientists' eventual understanding of the disease. For example, the discovery in 1910 that a defined, submicroscopic agent isolated from a chicken tumor could induce new tumors in healthy chickens showed that a tumor could be traced simply and definitively back to a single cause. Today, scientists know this agent as Roussarcoma virus, one of several viruses that can act as causative factors in the development of cancer.
Although cancer-causing viruses are not prime agents in promoting most human cancers, their intensive study focused researchers' attention on cellular genes as playing a central role in the development of the disease.
Likewise, investigations into the association between cancer and tissue damage, particularly that induced by radiation, revealed that while visible damage sometimes occurs, something more subtle happens in cells exposed to cancer-causing agents. One clue to what happens came from the work of Herman Muller, who noticed in 1927 that X-irradiation of fruit flies often resulted in mutant offspring. Might the two known effects of X-rays, promotion of cancer and genetic mutation, be related to one another? And might chemical carcinogens induce cancer through a similar ability to damage genes?
Support for this idea came from the work of Bruce Ames and others who showed in 1975 that com pounds known to be potent carcinogens (cancer-causing agents) generally also were potent mutagens (mutation-inducing agents), and that compounds known to be only weak carcinogens were only weak mutagens. Although scientists know today that many chemicals do not follow this correlation precisely, this initial, dramatic association between mutagenicity and carcinogenicity had widespread influence on the development of a unified view of the origin and development of cancer.
Finally, a simple genetic model, proposed by Alfred Knudson in 1971, provided both a compelling explanation for the origins of retinoblastoma, a rare tumor that occurs early in life, and a convincing way to reconcile the view of cancer as a disease produced by external agents that damage cells with the observation that some cancers run in families. Knudson's model states that children with sporadic retinoblastoma (children whose parents have no history of the disease) are genetically normal at the moment of conception, but experience two somatic mutations that lead to the development of an eye tumor. Children with familial retinoblastoma (children whose parents have a history of the disease) already carry one mutation at conception and thus must experience only one more mutation to reach the doubly mutated configuration required for a tumor to form. In effect, in familial retinoblastoma, each retinal cell is already primed for tumor development, needing only a second mutational event to trigger the cancerous state. The difference in probabilities between the requirement for one or two mutational events, happening randomly, explains why in sporadic retinoblastoma, the affected children have only one tumor focus, in one eye, while in familial retinoblastoma, the affected children usually have multiple tumor foci growing in both eyes.
Although it was years before Knudson's explanation was confirmed, it had great impact on scientists' understanding of cancer. Retinoblastoma, and by extension, other familial tumors, appeared to be linked to the inheritance of mutated versions of growth-suppressing genes. This idea led to the notion that cells in sporadically arising tumors might also have experienced damage to these critical genes as the cells moved along the path from the normal to the cancerous state.
Clues from cell biology
Another field of study that contributed to scientists' growing understanding of cancer was cell biology. Cell biologists studied the characteristics of cancer cells, through observations in the laboratory and by inferences from their appearance in the whole organism. Not unexpectedly, these investigations yielded a wealth of information about normal cellular processes. But they also led to several key understandings about cancer, understandings that ultimately allowed scientists to construct a unified view of the disease.
One such understanding is that cancer cells are indigenous cells—abnormal cells that arise from the body's normal tissues. Furthermore, virtually all malignant tumors are monoclonal in origin, that is, derived from a single ancestral cell that somehow underwent conversion from a normal to a cancerous state. These insights, as straight for ward as they seem, were surprisingly difficult to reach. How could biologists describe the cell pedigree of a mass of cells that eventually is recognized as a tumor?
One approach to identifying the origin of cancer cells came from attempts to transplant tissues from one person to another. Such transplants work well between identical twins, but less well as the people involved are more distantly related. The barrier to successful transplantation exists because the recipient's immune system can distinguish between cells that have always lived inside the self and cells of foreign origin. One practical application of this discovery is that tissues can be classified as matching or nonmatching before a doctor attempts to graft a tissue or organ into another person's body. Such tissue-typing tests, when done on cancer cells, reveal that the tumor cells of a particular cancer patient are always of the same transplantation type as the cells of normal tissues located elsewhere in the person's body. Tumors, therefore, arise from one's own tissues, not from cells introduced into the body by infection from another person.
How do we know that tumors are monoclonal? Two distinct scenarios might explain how cancers develop within normal tissues. In the first, many individual cells become cancerous, and the resulting tumor represents the descendants of these original cells. In this case, the tumor is polyclonal in nature ( Figure 4 ). In the second scenario, only one cell experiences the original transformation from a normal cell to a cancerous cell, and all of the cells in the tumor are descendants of that cell.
Two schemes by which tumors can develop. Most—if not all—human cancer appears to be monoclonal.
Direct evidence supporting the monoclonal origin of virtually all malignant tumors has been difficult to acquire because most tumor cells lack obvious distinguishing marks that scientists can use to demonstrate their clonal relationship. There is, however, one cellular marker that scientists can use as an indication of such relationships: the inactivated X chromosome that occurs in almost all of the body cells of a human female. X-chromosome inactivation occurs randomly in all cells during female embryonic development. Because the inactivation is random, the female is like a mosaic in terms of the X chromosome, with different copies of the X turned on or off in different cells of the body. Once inactivation occurs in a cell, all of the future generations of cells coming from that cell have the same chromosome inactivated in them as well (either the maternal or the paternal X). The observation that all the cells within a given tumor invariably have the same X chromosome inactivated suggests that all cells in the tumor must have descended from a single ancestral cell.
Cancer, then, is a disease in which a single normal body cell undergoes a genetic transformation into a cancer cell. This cell and its descendants, proliferating across many years, produce the population of cells that we recognize as a tumor, and tumors produce the symptoms that an individual experiences as cancer.
Even this picture, although accurate in its essence, did not represent a complete description of the events involved in tumor formation. Additional research revealed that as a tumor develops, the cells of which it is composed become different from one another as they acquire new traits and form distinct subpopulations of cells within the tumor. As shown in Figure 5 , these changes allow the cells that experience them to compete with increasing success against cells that lack the full set of changes. The development of cancer, then, occurs as a result of a series of clonal expansions from a single ancestral cell.
A series of changes leads to tumor formation. Tumor formation occurs as a result of successive clonal expansions. This figure illustrates only three such changes; the development of many cancers likely involves more than three.
A second critical understanding that emerged from studying the biology of cancer cells is that these cells show a wide range of important differences from normal cells. For example, cancer cells are genetically unstable and prone to rearrangements, duplications, and deletions of their chromosomes that cause their progeny to display unusual traits. Thus, although a tumor as a whole is monoclonal in origin, it may contain a large number of cells with diverse characteristics.
Cancerous cells also look and act differently from normal cells. In most normal cells, the nucleus is only about one-fifth the size of the cell; in cancerous cells, the nucleus may occupy most of the cell's volume. Tumor cells also often lack the differentiated traits of the normal cell from which they arose. Whereas normal secretory cells pro duce and release mucus, cancers derived from these cells may have lost this characteristic. Likewise, epithelial cells usually contain large amounts of keratin, but the cells that make up skin cancer may no longer accumulate this protein in their cytoplasms.
The key difference between normal and cancerous cells, however, is that cancer cells have lost the restraints on growth that characterize normal cells. Significantly, a large number of cells in a tumor are engaged in mitosis, whereas mitosis is a relatively rare event in most normal tissues. Cancer cells also demonstrate a variety of unusual characteristics when grown in culture; two such examples are a lack of contact inhibition and a reduced dependence on the presence of growth factors in the environment. In contrast to normal cells, cancer cells do not cooperate with other cells in their environment. They often proliferate indefinitely in tissue culture. The ability to divide for an apparently unlimited number of generations is another important characteristic of the cancerous state, allowing a tumor composed of such cells to grow without the constraints that normally limit cell growth.
A unified view
By the mid-1970s, scientists had started to develop the basis of our modern molecular understanding of cancer. In particular, the relationship Ames and others had established between mutagenicity and carcinogenicity pro vided substantial support for the idea that chemical carcinogens act directly through their ability to damage cellular genes. This idea led to a straightforward model for the initiation of cancer: Carcinogens induce mutations in critical genes, and these mutations direct the cell in which they occur, as well as all of its progeny cells, to grow abnormally. The result of this abnormal growth appears years later as a tumor. The model could even explain the observation that cancer sometimes appears to run in families: If cancer is caused by mutations in critical genes, then people who inherit such mutations would be more susceptible to cancer's development than people who do not.
As exciting as it was to see a unified view of cancer begin to emerge from the earlier confusion, cancer researchers knew their work was not finished. The primary flaw in their emerging explanation was that the nature of these cancer-causing mutations was unknown. Indeed, their very existence had yet to be proven. Evidence from work with cancer-causing viruses suggested that only a small number of genes were involved, and evidence from cell biology pointed to genes that normally control cell division. But now scientists asked new questions: Exactly which genes are involved? What are their specific roles in the cell? and How do their functions change as a result of mutation?
It would take another 20 years and a revolution in the techniques of biological research to answer these questions. However, today our picture of the causes and development of cancer is so detailed that scientists find themselves in the extraordinary position of not only knowing many of the genes involved but also being able to target prevention, detection, and treatment efforts directly at these genes.
- Cancer as a Multistep Process
A central feature of today's molecular view of cancer is that cancer does not develop all at once, but across time, as a long and complex succession of genetic changes. Each change enables precancerous cells to acquire some of the traits that together create the malignant growth of cancer cells.
Two categories of genes play major roles in triggering cancer. In their normal forms, these genes control the cell cycle , the sequence of events by which cells enlarge and divide. One category of genes, called proto-oncogenes , encourages cell division. The other category, called tumor suppressor genes , inhibits it. Together, proto-oncogenes and tumor suppressor genes coordinate the regulated growth that normally ensures that each tissue and organ in the body maintains a size and structure that meets the body's needs.
What happens when proto-oncogenes or tumor suppressor genes are mutated? Mutated proto oncogenes become oncogenes, genes that stimulate excessive division. And mutations in tumor suppressor genes inactivate these genes, eliminating the critical inhibition of cell division that normally prevents excessive growth. Collectively, mutations in these two categories of genes account for much of the uncontrolled cell division that occurs in human cancers ( Figure 6 ).
Some Genes Involved in Human Cancer
The role of oncogenes
How do proto-oncogenes, or more accurately, the oncogenes they become after mutation, contribute to the development of cancer? Most proto-oncogenes code for proteins that are involved in molecular pathways that receive and process growth-stimulating signals from other cells in a tissue. Typically, such signaling begins with the production of a growth factor, a protein that stimulates division. These growth factors move through the spaces between cells and attach to specific receptor proteins located on the surfaces of neighboring cells. When a growth-stimulating factor binds to such a receptor, the receptor conveys a stimulatory signal to proteins in the cytoplasm. These proteins emit stimulatory signals to other proteins in the cell until the division-promoting message reaches the cell's nucleus and activates a set of genes that help move the cell through its growth cycle.
Oncogenes, the mutated forms of these proto oncogenes, cause the proteins involved in these growth-promoting pathways to be overactive. Thus, the cell proliferates much faster than it would if the mutation had not occurred. Some oncogenes cause cells to overproduce growth factors. These factors stimulate the growth of neighboring cells, but they also may drive excessive division of the cells that just produced them. Other oncogenes produce aberrant receptor proteins that release stimulatory signals into the cytoplasm even when no growth factors are present in the environment. Still other oncogenes disrupt parts of the signal cascade that occurs in a cell's cytoplasm such that the cell's nucleus receives stimulatory messages continuously, even when growth factor receptors are not prompting them.
The role of tumor suppressor genes
To become cancerous, cells also must break free from the inhibitory messages that normally counterbalance these growth-stimulating pathways. In normal cells, inhibitory messages flow to a cell's nucleus much like stimulatory messages do. But when this flow is interrupted, the cell can ignore the normally powerful inhibitory messages at its surface.
Scientists are still trying to identify the normal functions of many known tumor suppressor genes. Some of these genes apparently code for proteins that operate as parts of specific inhibitory pathways. When a mutation causes such proteins to be inactivate or absent, these inhibitory pathways no longer function normally. Other tumor suppressor genes appear to block the flow of signals through growth-stimulating pathways; when these genes no longer function properly, such growth-promoting pathways may operate without normal restraint. Mutations in all tumor suppressor genes, however, apparently inactivate critical tumor suppressor proteins, depriving cells of this restraint on cell division.
The body's back-up systems
In addition to the controls on proliferation afforded by the coordinated action of proto-oncogenes and tumor suppressor genes, cells also have at least three other systems that can help them avoid runaway cell division. The first of these systems is the DNA repair system. This system operates in virtually every cell in the body, detecting and correcting errors in DNA. Across a lifetime, a person's genes are under constant attack, both by carcinogens imported from the environment and by chemicals produced in the cell itself. Errors also occur during DNA replication. In most cases, such errors are rapidly corrected by the cell's DNA repair system. Should the system fail, however, the error (now a mutation) becomes a permanent feature in that cell and in all of its descendants.
The system's normally high efficiency is one reason why many years typically must pass before all the mutations required for cancer to develop occur together in one cell. Mutations in DNA repair genes themselves, however, can undermine this repair system in a particularly devastating way: They damage a cell's ability to repair errors in its DNA. As a result, mutations appear in the cell (including mutations in genes that control cell growth) much more frequently than normal.
A second cellular back-up system prompts a cell to commit suicide (undergo apoptosis ) if some essential component is damaged or its control system is deregulated. This observation suggests that tumors arise from cells that have managed to evade such death. One way of avoiding apoptosis involves the p53 protein. In its normal form, this protein not only halts cell division, but induces apoptosis in abnormal cells. The product of a tumor suppressor gene, p53 is inactivated in many types of cancers.
This ability to avoid apoptosis endangers cancer patients in two ways. First, it contributes to the growth of tumors. Second, it makes cancer cells resistant to treatment. Scientists used to think that radiation and chemotherapeutic drugs killed cancer cells directly by harming their DNA. It seems clear now that such therapy only slightly damages the DNA in cells; the damaged cells, in response, actively kill themselves. This discovery suggests that cancer cells able to evade apoptosis will be less responsive to treatment than other cells.
A third back-up system limits the number of times a cell can divide, and so assures that cells cannot reproduce endlessly. This system is governed by a counting mechanism that involves the DNA segments at the ends of chromosomes. Called telomeres, these segments shorten each time a chromo some replicates. Once the telomeres are shorter than some threshold length, they trigger an internal signal that causes the cell to stop dividing. If the cells continue dividing, further shortening of the telomeres eventually causes the chromosomes to break apart or fuse with one another, a genetic crisis that is inevitably fatal to the cell.
Early observations of cancer cells grown in culture revealed that, unlike normal cells, cancer cells can proliferate indefinitely. Scientists have recently discovered the molecular basis for this characteristic—an enzyme called telomerase, that systematically replaces telomeric segments that are trimmed away during each round of cell division. Telomerase is virtually absent from most mature cells, but is present in most cancer cells, where its action enables the cells to proliferate endlessly.
The multistep development of cancer
Cancer, then, does not develop all at once as a massive shift in cellular functions that results from a mutation in one or two wayward genes. Instead, it develops step-by-step, across time, as an accumulation of many molecular changes, each contributing some of the characteristics that eventually pro duce the malignant state. The number of cell divisions that occur during this process can be astronomically large—human tumors often become apparent only after they have grown to a size of 10 billion to 100 billion cells. As you might expect, the time frame involved also is very long— it normally takes decades to accumulate enough mutations to reach a malignant state.
Understanding cancer as a multistep process that occurs across long periods of time explains a number of long-standing observations. A key observation is the increase in incidence with age. Cancer is, for the most part, a disease of people who have lived long enough to have experienced a complex and extended succession of events. Because each change is a rare accident requiring years to occur, the whole process takes a very long time, and most of us die from other causes before it is complete.
Understanding cancer in this way also explains the increase in cancer incidence in people who experience unusual exposure to carcinogens, as well as the increased cancer risk of people who inherit predisposing mutations. Exposure to carcinogens increases the likelihood that certain harmful changes will occur, greatly increasing the probability of developing cancer during a normal life span. Similarly, inheriting a cancer -susceptibility mutation means that instead of that mutation being a rare event, it already has occurred, and not just in one or two cells, but in all the body's cells. In other words, the process of tumor formation has leapfrogged over one of its early steps. Now the accumulation of changes required to reach the malignant state, which usually requires several decades to occur, may take place in one or two.
Finally, understanding the development of cancer as a multistep process also explains the lag time that often separates exposure to a cancer-causing agent and the development of cancer. This explains, for example, the observation that severe sunburns in children can lead to the development of skin cancer decades later. It also explains the 20-to 25-year lag between the onset of widespread cigarette smoking among women after World War II and the massive increase in lung cancer that occurred among women in the 1970s.
- The Human Face of Cancer
For most Americans, the real issues associated with cancer are personal. More than 8 million Americans alive today have a history of cancer (National Cancer Institute, 1998; Rennie, 1996). In fact, cancer is the second leading cause of death in the United States, exceeded only by heart disease.
Who are these people who develop cancer and what are their chances for surviving it? Scientists measure the impact of cancer in a population by looking at a combination of three elements: (1) the number of new cases per year per 100,000 persons ( incidence rate ), (2) the number of deaths per 100,000 persons per year ( mortality rate ), and (3) the proportion of patients alive at some point after their diagnosis of cancer ( survival rate ). Data on incidence, mortality, and survival are collected from a variety of sources. For example, in the United States there are many statewide cancer registries and some regional registries based on groups of counties, many of which surround large metropolitan areas. Some of these population-based registries keep track of cancer incidence in their geographic areas only; others also collect follow-up information to calculate survival rates.
In 1973, the National Cancer Institute began the Surveillance, Epidemiology, and End Results (SEER) Program to estimate cancer incidence and patient survival in the United States. SEER collects cancer incidence data in 11 geographic areas and two supplemental registries, for a combined population of approximately 14 percent of the entire U.S. population. Data from SEER are used to track cancer incidence in the United States by primary cancer site, race, sex, age, and year of diagnosis. For example, Figure 7 shows SEER data for the age-adjusted cancer incidence rates for the 10 most common sites for Caucasian and African-American males and females for the period 1987–1991.
Age-Adjusted Cancer Incidence Rates, 1987–1991
Cancer among children is relatively rare. SEER data from 1991 showed an incidence of only 14.1 cases per 100,000 children under age 15. Nevertheless, after accidents, cancer is the second leading cause of childhood death in the United States. Leukemias (4.3 per 100,000) and cancer of the brain and other nervous system organs (3.4 per 100,000) account for more than one-half of the cancers among children.
Everyone is at some risk of developing cancer. Cancer researchers use the term lifetime risk to indicate the probability that a person will develop cancer over the course of a lifetime. In the United States, men have a 1 in 2 lifetime risk of developing cancer, and women have a 1 in 3 risk.
For a specific individual, however, the risk of developing a particular type of cancer may be quite different from his or her lifetime risk of developing any type of cancer. Relative risk compares the risk of developing cancer between persons with a certain exposure or characteristic and persons who do not have this exposure or characteristic. For example, a person who smokes has a 10- to 20-fold higher relative risk of developing lung cancer compared with a person who does not smoke. This means that a smoker is 10- to 20-times more likely to develop lung cancer than a nonsmoker.
Scientists rely heavily on epidemiology to help them identify factors associated with the development of cancer. Epidemiologists look for factors that are common to cancer victims' histories and lives and evaluate these factors in the light of current understandings of the disease. With enough study, researchers may assemble evidence that a particular factor "causes" cancer, that is, that exposure to it increases significantly the probability of the disease developing. Although this information cannot be used to predict what will happen to any one individual exposed to this risk factor, it can help people make choices that reduce their exposure to known carcinogens (cancer-causing agents) and increase the probability that if cancer develops, it will be detected early (for example, by getting regular check-ups and participating in cancer screening programs).
As noted above, hereditary factors also can contribute to the development of cancer. Some people are born with mutations that directly promote the unrestrained growth of certain cells or the occurrence of more mutations. These mutations, such as the mutation identified in the 1980s that causes retinoblastoma, confer a high relative cancer risk. Such mutations are rare in the population, however, accounting for the development of fewer than 5 percent of the cases of fatal cancer.
Hereditary factors also contribute to the development of cancer by dictating a person's general physiological traits. For example, a person with fair skin is more susceptible to the development of skin cancer than a person with a darker complexion. Likewise, a person whose body metabolizes and eliminates a particular carcinogen relatively inefficiently is more likely to develop types of cancer associated with that carcinogen than a person who has more efficient forms of the genes involved in that particular metabolic process. These inherited characteristics do not directly promote the development of cancer; each person, susceptible or not, still must be exposed to the related environ mental carcinogen for cancer to develop. Nevertheless, genes probably do contribute in some way to the vast majority of cancers.
One question often asked about cancer is "How many cases of cancer would be expected to occur naturally in a population of individuals who somehow had managed to avoid all environmental carcinogens and also had no mutations that predisposed them to developing cancer?" Comparing populations around the world with very different cancer patterns has led epidemiologists to suggest that perhaps only about 25 percent of all cancers are "hard core"—that is, would develop anyway, even in a world free of external influences. These cancers would occur simply because of the production of carcinogens within the body and because of the random occurrence of unrepaired genetic mistakes.
Although cancer continues to be a significant health issue in the United States, a recent report from the American Cancer Society (ACS), National Cancer Institute (NCI), and Centers for Disease Control and Prevention (CDC) indicates that health officials are making progress in controlling the disease. In a news bulletin released on 12 March 1998, the ACS, NCI, and CDC announced the first sustained decline in the cancer death rate, a turning point from the steady increase observed throughout much of the century. The report showed that after increasing 1.2 percent per year from 1973 to 1990, the incidence for all cancers combined declined an average of 0.7 percent per year from 1990 to 1995. The overall cancer death rate also declined by about 0.5 percent per year across this period.
The overall survival rate for all cancer sites combined also continues to increase steadily, from 49.3 percent in 1974–1976 to 53.9 percent in 1983–1990 ( Figure 8 ). In some cases—for example, among children age 15 and younger—survival rates have increased dramatically.
Five-Year Relative Survival Rates for Selected Cancer Sites, All Races
- New Hope for Treating Cancer
What explanation can we offer for the steady increase in survival rates among cancer patients? One answer likely is the improvements scientists have made in cancer detection. These improvements include a variety of new imaging techniques as well as blood and other tests that can help physicians detect and diagnose cancer early. Although many Americans regularly watch for the early symptoms of cancer, by the time symptoms occur many tumors already have grown quite large and may have metastasized. Likewise, many cancers have no symptoms. Clearly, great effort is needed to educate Americans that cancer screening (checking for cancer in people with no symptoms) is key to early detection.
Another explanation for increased survival is improved treatment. Today, the traditional workhorses of cancer treatment—surgery, radiation, and chemotherapy—are being used in ways that are increasingly specific to the type of cancer involved. In fact, many cases of cancer now are being fully cured.
But is this the best we can do? What will the future bring? Hellman and Vokes, in their 1996 article in Scientific American , note that war often serves as a metaphor for cancer research. In 1971, two days before Christmas, President Richard M. Nixon signed the National Cancer Act, committing the United States to a "war" on cancer. Although the analogy is not perfect, Hellman and Vokes suggest that it can help us understand our current position with respect to cancer prevention, detection, and treatment. Looking at the "map" of cancer research after almost 30 years of "war," we can see that we have made some modest advances. But these successes do not reveal the tremendous developments that lie ahead of us by virtue of the new, strategic position we have achieved. In fact, most scientists expect that our newly gained understanding of the molecular basis of cancer will eventually give rise to a whole generation of exciting new techniques, not only for detecting and treating cancer but also for preventing it.
A key area of interest lies in learning how to exploit the molecular abnormalities of cancer cells to bring about their destruction. For example, understanding the role of oncogenes in the development of cancer suggests new targets for anticancer therapies. Some drug companies are working on drugs designed to shut down abnormal receptor proteins. Other potential targets are the aberrant proteins within the cytoplasm that transmit stimulatory signals even without being stimulated by surface receptors.
As in the case of oncogenes, a better understanding of the role of tumor suppressor genes in preventing runaway cell division may help scientists develop new therapies directed at these genes. For example, various studies have shown that introducing a normal tumor suppressor gene into a cell can help restore the cell to normalcy. Similarly, a therapy capable of restoring a cell's capacity for apoptosis would improve significantly the effectiveness of current cancer treatments. Even telomerase represents an important potential target for scientists looking for new and more powerful treatments for cancer. If telomerase could be blocked in cancer cells, their telomeres would continue to shorten with each division until their own proliferation pushed them into a genetic crisis and death.
One bold new research initiative that offers significant promise is the Cancer Genome Anatomy Project (CGAP). The project's goal is to identify all the genes responsible for the establishment and growth of human cancer. The work is based on a simple concept: Although almost every cell in the body contains the full set of human genes, only about one-tenth of them are expressed in any particular type of cell. Thus, different types of cells— for example, muscle cells and skin cells—can be distinguished by their patterns of gene expression.
Establishing for a particular cell the repertoire of genes expressed, together with the amount of nor mal or altered gene product produced by each expressed gene, yields a powerful "fingerprint" or "signature" for that cell type. Not unexpectedly, during the transformation of a normal cell to a cancer cell, this signature changes. Some changes are quantitative. That is, gene A may be expressed in both cells, but at greatly different levels, or it may be expressed in one cell but not the other. Other changes are qualitative: Gene B may be expressed at the same level in both cells, but pro duce an altered product in the cancerous cell.
Scientists expect that being able to "read" these signatures—in other words, being able to compare the signatures of cells in their normal and cancerous states—will change cancer detection, diagnosis, and treatment in many exciting ways. Specifically, studying the exact sequence of molecular changes a cell undergoes during its transformation to a cancerous state will help scientists identify new molecular-level targets for prevention, detection, and treatment. One observation scientists have recently made is that cells surrounding an incipient tumor also may undergo changes that indicate that cancer is present. For example, early tobacco-induced molecular changes in the mouth may predict the risk of developing lung cancer, and cancers of the urinary tract may be signaled by molecularly-altered cells that are shed in the urine. Reading the signatures of these easily accessed cells may enable scientists to develop simple, non-invasive tests that will allow early detection of cancerous or precancerous cells hidden deep within the body.
Reading such signatures will also enhance the specificity of cancer diagnosis by allowing scientists to differentiate among tumors at the molecular level. By assessing the meaning of individual changes in a cell's signature, scientists will be able to determine which cancers are most likely to progress and which are not—a dilemma that confronts doctors in the treatment of prostate cancer—thereby allowing patients to avoid the harmful consequences of unnecessary treatment.
Finally, molecular fingerprinting will allow researchers to develop new treatments specifically targeted at cellular subtypes of different cancers. Often, patients suffering from tumors that by traditional criteria are indistinguishable, nevertheless experience quite different outcomes despite having received the same treatment. Research indicates that these different outcomes sometimes are related to the presence or absence of particular gene products. In the future, such molecular characteristics likely will be used to identify patients who would benefit from one type of treatment as compared with another.
The ultimate goal of such work, of course, is to push back the detection and diagnosis of cancer to its earliest stages of development. For the first time in the history of humankind, scientists can now envision the day when medical intervention for cancer will become focused at identifying incipient disease and preventing its progression to overt disease, rather than treating the cancer after it is well established.
- Cancer and Society
But what does this mean for society? The financial costs of cancer loom large, not only for the individual but also for the community. The NCI estimates overall annual costs for cancer at about $107 billion. This cost includes $37 billion for direct medical costs, $11 billion for morbidity costs (cost of lost productivity), and $59 billion for mortality costs. Interestingly, treatment for breast, lung, and prostate cancers account for more than one-half of the direct medical costs.
Although early detection and successful treatment can reduce cancer deaths, the most desirable way to reduce them is prevention. In fact, scientists estimate that as many as one-half of the deaths from cancer in the United States and Europe, two areas with closely tracked cancer rates, could theoretically be prevented.
Nevertheless, the widespread persistence of unhealthful habits suggests that many Americans remain unconvinced about the power of prevention as a defense against cancer. Part of the reason may be that the only data we have about factors related to cancer are drawn from whole populations. These data cannot tell us who will develop cancer. Nor can they tell us whether healthful choices prevented its appearance in a particular individual.
Unhealthful habits also may persist because of the long time that elapses between the exposures that trigger the development of cancer and its actual appearance as disease. Conversely, there is a time lag between the institution of a beneficial personal habit (such as quitting smoking) or public policy (such as banning use of a known carcinogen) and its positive impact on personal and public health.
In their article "Strategies for Minimizing Cancer Risk," Willett, Colditz, and Mueller propose four levels on which to focus cancer prevention efforts. The first level is that of the individual. These authors argue that because most of the actions that can prevent cancer must be taken by individuals, dissemination of accurate information directly to the American public, together with peer support for behavioral changes, are critical.
A second level is health care providers, who are in a position to provide both counseling and screening to individuals under their care. Here, dissemination of accurate and timely information also is key.
A third level of prevention is the national level, where government agencies can impose regulations that help minimize the public's exposure to known carcinogens and implement policies that improve public health. Examples include regulating industries to cease using potent carcinogens and providing community facilities for safe physical activity.
Finally, a fourth level of prevention is at the international level, where the actions of developed countries can affect the incidence of cancer worldwide. Unfortunate examples of this include promoting the exportation of tobacco products and moving hazardous manufacturing processes to unregulated developing countries.
How do we think about devising and implementing measures to improve personal and public health in a pluralist society? One way to address this question is by attending to the ethical and public policy issues raised by our understanding and treatment of cancer.
A history of severe sunburns is strongly linked to the development of skin cancer later in life.
Ethics is the study of good and bad, right and wrong. It has to do with the actions and character of individuals, families, communities, institutions, and societies. During the last 2,500 years, Western philosophy has developed a variety of powerful methods and a reliable set of concepts and technical terms for studying and talking about the ethical life. Generally speaking, we apply the terms "right" and "good" to actions and qualities that foster the interests of individuals, families, communities, institutions, and society. Here, an "interest" refers to a participant's share in a situation. The terms "wrong" or "bad" apply to actions and qualities that impair interests. Often there are competing, well-reasoned answers to questions about what is right and wrong and good and bad about an individual's or group's conduct or actions.
Ethical considerations are complex, multifaceted, and raise many questions. In the United States, for example, we value protecting individuals from preventable harms. We support restrictions on who can purchase cigarettes and where smoking can occur. We inform pregnant women of the risks of drinking and smoking. However, we also value individual freedom and autonomy. We do not ban cigarettes outright; instead, we allow individuals over 18 years of age to take personal risks and be exposed to the related consequences. We permit pregnant women to buy and use liquor and cigarettes.
The inevitability of ethical tradeoffs is not simply a mark of the discussions in the United States. When considering differing health policy issues between and among countries, one cannot avoid encountering a pluralism of ethical considerations. Developing countries, whose health standards often differ from those in the United States, provide different cultural approaches to cancer and different standards for marketing and using tobacco and other known carcinogens. These different approaches raise a variety of ethical questions. For example, is there any legal and ethical way for people in the United States to prevent the widespread use of tobacco in other countries, a practice that contributes to the rise of lung cancer worldwide? Is there any legal and ethical way to govern other choices of individuals (for example, poor diet and lack of exercise) that contribute to cancer?
Typically, answers to such questions all involve an appeal to values. A value is something that has significance or worth in a given situation. One of the exciting events to witness in any discussion in ethics in a pluralist society is the varying ways in which the individuals involved assign value to things, persons, and states of affairs. Examples of values that students may appeal to in discussions of ethical issues include autonomy, freedom, privacy, sanctity of life, protecting another from harm, promoting another's good, justice, fairness, relationships, scientific knowledge, and technological progress.
Acknowledging the complex, multifaceted nature of ethical discussions is not to suggest that "anything goes." Experts generally agree on the following features of ethics. First, ethics is a process of rational inquiry. It involves posing clearly formulated questions and seeking well-reasoned answers to those questions. Well-reasoned answers to ethical questions constitute arguments . Ethical analysis and argument, then, result from successful ethical inquiry.
Second, ethics requires a solid foundation of information and rigorous interpretation of that information. For example, one must have a solid understanding of cancer to discuss the ethics of requiring protective covering to be worn to prevent skin cancer. Ethics is not strictly a theoretical discipline but is concerned in vital ways with practical matters.
Third, because tradeoffs among interests are complex, constantly changing, and sometimes uncertain, there are often competing, well-reasoned answers to questions about what is right and wrong and good and bad. This is especially true in a pluralist society.
Public policy is a set of guidelines or rules that results from the actions or lack of actions of government entities. Government entities act by making laws. In the United States, laws can be made by each of the three branches of government: by legislatures (statutory law), by courts (case law), and by regulatory agencies (regulatory law). Regulatory laws are written by the executive branch of the government, under authorization by the legislative branch. All three types of law are pertinent to how we respond to cancer. When laws exist to regulate behavior, public policy is called de jure public policy.
Whether one makes public policy involves at least the following five considerations:
- -the costs of implementing particular policies (including financial, social, and personal costs),
- the urgency of implementing a new policy,
- how effective a particular policy is likely to be,
- -whether appropriate means exist to implement the policy, and
- social, cultural, and political factors.
For example, many argue that there is overwhelming evidence to support increased public policy restrictions on access to and use of cigarettes. Cigarette smoking is said to be linked to 85–90 percent of lung cancer cases. In 1998, 171,500 new cases of lung cancer were predicted. Of these, 160,100 were expected to end in death. Public policy prohibitions on cigarette use and access may be seen to satisfy four of the five criteria: (1) the cost of the policy would be minimal because cigarette access and use restrictions are in place, (2) the urgency of the situation is serious given the large number of deaths, (3) prohibiting purchase by minors and raising the prices (through taxation) are seen as effective, and (4) means are already in place for additional restrictions. The challenge in this era of high economic interest in cigarette production is the social, cultural, and political considerations (5).
Where do we spend our money? A consequence of allowing unhealthful habits, such as smoking, is that public funds may be spent on cancer treatments instead of on other societal benefits, such as improved school facilities.
It is important to recognize that sometimes the best public policy is not to enact a law in response to a controversy, but rather to allow individuals, families, communities, and societies to act in the manner they choose. Clearly, de jure public policy can only go so far in regulating people's behaviors. De jure public policy in the United States offers no match for the addictive power of nicotine and the marketing clout of the tobacco industry. In addition, any decline in cigarette use brought about by de jure public policy in the United States has been more than offset in recent years by a rapid increase of cigarette consumption elsewhere in the world.
When no laws exist to regulate behavior, public policy is called de facto (actual) public policy. With regard to lung cancer prevention programs, many think that other approaches are needed: improved general education and cultivation of an antismoking ethos. In any discussion of society's response to a social problem, it is important to think about other ways to address the problem.
Knowledge, choice, behavior, and human welfare
We can conclude that science plays an important role in assisting individuals to make choices about enhancing personal and public welfare. Science provides evidence that can be used to support ways of understanding and treating human disease, ill ness, deformity, and dysfunction. But the relationships between scientific information and human choices, and between choices and behaviors, are not linear. Human choice allows individuals to choose against sound knowledge, and choice does not necessarily lead to particular actions.
Nevertheless, it is increasingly difficult for most of us to deny the claims of science. We are continually presented with great amounts of relevant scientific and medical knowledge that is publicly accessible. We are fortunate to have available a large amount of convincing data about the development, nature, and treatment of particular cancers. As a consequence, we might be encouraged to think about the relationships among knowledge, choice, behavior, and human welfare in the following ways:

One of the goals of this module is to encourage students to think in terms of these relationships, now and as they grow older.
- Baron-Faust R. Breast cancer: What every woman should know. New York: Hearst Books; 1995.
- Biological Sciences Curriculum Study. Teaching tools. Dubuque, IA: Kendall/Hunt Publishing Company; 1999.
- Bonwell CC, Eison JA. Active learning: Creating excitement in the classroom. Washington, DC: The George Washington University: School of Education and Human Development; 1991. (ASHE-ERIC Higher Education Report No. 1)
- Brody CM. Collaborative or cooperative learning? Complementary practices for instructional reform. The Journal of Staff, Program, & Organizational Development. 1995; 12 (3):134–143.
- Knapp MS, Shields PM, Turnbull BJ. Academic challenge in high-poverty classrooms. Phi Delta Kappan. 1995; 76 (10):770–776.
- McKinnell RG, Parchment RE, Perantoni AO, Pierce GB. The biological basis of cancer. New York: Cambridge University Press; 1998.
- Murphy GP, Morris LB, Lange D. Informed decisions: The complete book of cancer diagnosis, treatment, and recovery. Viking Penguin: The American Cancer Society; 1997.
- National Cancer Institute. New report on declining cancer incidence and death rates: Report shows progress in controlling cancer. March 12, 1998. [Online press release]. Available http://nci .nih.gov/massmedia /pressreleases/deathrate.html .
- National Institutes of Health. Congressional justification. Bethesda, MD: Author; 1996.
- National Institutes of Health (NIH). Jun, 1999. [Online]. Available http://www .nih.gov .
- National Research Council. National science education standards. Washington, DC: National Academy Press; 1996.
- Moore JA. Science as a way of knowing: The foundations of modern biology. Cambridge, MA: Harvard University Press; 1993.
- Patterson JT. The dread disease: Cancer and modern American culture. Cambridge, MA: Harvard University Press; 1987.
- Perkins D. Smart schools: Better thinking and learning for every child. New York: The Free Press; 1992.
- Project Kaleidoscope. What works: Building natural science communities. Vol. 1. Washington, DC: Stamats Communications, Inc; 1991.
- Rennie J, Rusting, R Making headway against cancer. Scientific American. 1996 September; 275 (3):56. [ PubMed : 8701294 ]
- Rennie J. Editorial. Scientific American. 1996 September; 275 (3):6.
- Roblyer MD, Edwards J, Havriluk MA. Integrating educational technology into teaching. Upper Saddle River, NJ: Prentice-Hall, Inc; 1997.
- Saunders WL. The constructivist perspective: Implications and teaching strategies for science. School Science and Mathematics. 1992; 92 (3):136–141.
- Sizer TR. New York: Houghton Mifflin Co; Horace's school: Redesigning the American high school. 1992
- Trichopoulos D, Li FP, Hunter DJ. What causes cancer? Scientific American. 1996 September; 275 (3):80. [ PubMed : 8701297 ]
- Varmus H, Weinberg RA. Genes and the biology of cancer. New York: Scientific American Library; 1993.
- Vogelstein B, Kinzler KW. The genetic basis of cancer. New York: McGraw Hill; 1998.
- Weinberg RA. Racing to the beginning of the road: The search for the origin of cancer. New York: Harmony Books; 1996.
- Weinberg RA. How cancer arises. Scientific American. 1996 September; 275 (3):62. [ PubMed : 8701295 ]
- Willett WC, Colditz GA, Mueller NE. Strategies for minimizing cancer risk. Scientific American. 1996 September; 275 (3):88. [ PubMed : 8701299 ]
The following glossary was modified from the glossary on the National Cancer Institute's Web site, available from http://www.nci.nih.gov .
Type of blood cancer that originates in lymphatic cells of the bone marrow.
Type of blood cancer that involves accumulation of myeloid cells in the bone marrow and bloodstream.
Cancer that begins in cells that line certain internal organs.
Noncancerous tumor.
Protein often found in abnormal amounts in the blood of patients with liver cancer.
Mutagenesis assay (a measure of mutagenic ability) that involves specially engineered strains of bacteria. Because of the relationship between mutagenicity and carcinogenicity, the test is used as a rapid and relatively inexpensive first screening of untested chemicals that are suspected to be carcinogens.
Term used to describe cancer cells that divide rapidly and bear little or no resemblance to normal cells.
Blood vessel formation, which usually accompanies the growth of malignant tissue.
Type of cancer that begins in the lining of blood vessels.
Normal cellular process involving a genetically programmed series of events leading to the death of a cell.
Presenting no signs or symptoms of disease.
Hereditary disorder characterized by problems with muscle coordination, immunodeficiency, inadequate DNA repair, and an increased risk of developing cancer.
Benign (noncancerous) condition in which tissue has certain abnormal features.
Small, round cell found in the lower part, or base, of the epidermis, the outer layer of the skin.
Type of skin cancer that arises from the basal cells.
Not cancerous; does not invade nearby tissue or spread to other parts of the body.
A noncancerous growth that does not spread to other parts of the body.
Use of the body's immune system, either directly or indirectly, to fight cancer or to lessen side effects that may be caused by some cancer treatments. Also known as immuno-therapy, biotherapy, or biological response modifier therapy.
Removal of a sample of tissue, which is then examined under a microscope to check for cancer cells.
Soft, spongy tissue in the center of large bones that produces white blood cells, red blood cells, and platelets.
Removal of a small sample of bone marrow (usually from the hip) through a needle for examination under a microscope to see whether cancer cells are present.
Removal of a sample of tissue from the bone marrow with a large needle. The cells are checked to see whether they are cancerous. If cancerous plasma cells are found, the pathologist estimates how much of the bone marrow is affected. Bone marrow biopsy is usually done at the same time as bone marrow aspiration.
Procedure in which doctors replace marrow destroyed by treatment with high doses of anticancer drugs or radiation. The replacement marrow may be taken from the patient before treatment or may be donated by another person.
Technique to create images of bones on a computer screen or on film. A small amount of radioactive material is injected and travels through the bloodstream. It collects in the bones, especially in abnormal areas of the bones, and is detected by a scanner.
Internal radiation therapy using an implant of radioactive material placed directly into or near the tumor.
Gene located on chromosome 17 that normally helps restrain cell growth. Inheriting an altered version of BRCA1 predisposes an individual to breast, ovarian, or prostate cancer.
Gene located on chromosome 13 that scientists believe may account for 30 to 40 percent of all inherited breast cancer.
Surgery to rebuild a breast's shape after a mastectomy.
Type of non-Hodgkin lymphoma that most often occurs in young people between the ages of 12 and 30. The disease usually causes a rapidly growing tumor in the abdomen.
Term for a group of more than 100 diseases in which abnormal cells divide without control. Cancer cells can invade nearby tissues and can spread through the bloodstream and lymphocytic system to other parts of the body.
Any substance that is known to cause cancer.
Process by which normal cells are transformed into cancer cells.
Cancer that begins in the lining or covering of an organ.
Cancer that involves only the cells in which it began and has not spread to other tissues.
Laboratory test to measure the level of carcinoembryonic antigen (CEA), a substance that is sometimes found in an increased amount in the blood of patients with certain cancers.
Sequence of events by which cells enlarge and divide. Includes stages typically named G1, S, G2, and M.
Use of natural or laboratory-made substances to prevent cancer.
Treatment with anticancer drugs.
Type of blood cancer that involves overproduction of mature lymphocytes.
Type of blood cancer that involves accumulation of granulocytes (a type of white blood cell) in the bone marrow and bloodstream.
Research study that involves patients. Each study is designed to find better ways to prevent, detect, diagnose, or treat cancer and to answer scientific questions.
Procedure that uses a flexible fiber optic endoscope to examine the internal surface of the colon along its entire length.
Treatment in which two or more chemicals are used to obtain more effective results.
X-ray procedure that uses a computer to produce a detailed picture of a cross section of the body; also called CAT or CT scan.
Inhibition of cell division in normal (noncancerous) cells when they contact a neighboring cell.
See computed tomography.
Poisonous to cells. In chemotherapy, used to describe an agent that is poisonous to cancer cells.
Process of identifying a disease by the signs and symptoms.
Abnormal cells that are not cancer.
Atypical moles; moles whose appearance is different from that of common moles. Dysplastic nevi are generally larger than ordinary moles and have irregular and indistinct borders. Their color often is not uniform and ranges from pink or even white to dark brown or black; they usually are flat, but parts may be raised above the skin surface.
Confined to a specific area; an encapsulated tumor remains in a compact form.
Having to do with the mucous membrane that lines the cavity of the uterus.
Smoke that comes from the burning end of a cigarette and smoke that is exhaled by smokers. Also called ETS or secondhand smoke. Inhaling ETS is called involuntary or passive smoking.
Study of the factors that affect the prevalence, distribution, and control of disease.
Upper or outer layer of the two main layers of cells that make up the skin.
Virus that has been associated with the development of infectious mononucleosis and also with Burkitt lymphoma.
Female hormone produced by the ovary. Responsible for secondary sex characteristics and cyclic changes in the lining of the uterus and vagina.
Study of the causes of abnormal condition or disease.
Inherited condition in which several hundred polyps develop in the colon and rectum. These polyps have a high potential to become malignant.
Test to reveal blood hidden in the feces, which may be a sign of colon cancer.
Parts of fruits and vegetables that cannot be digested. Also called bulk or roughage.
Benign uterine tumor made up of fibrous and muscular tissue.
Treatment that alters genes (the basic units of heredity found in all cells in the body). In studies of gene therapy for cancer, researchers are trying to improve the body's natural ability to fight the disease or to make the tumor more sensitive to other kinds of therapy.
Inherited; having to do with information that is passed from parents to children through DNA in the genes.
Describes how closely a cancer resembles normal tissue of its same type, along with the cancer's probable rate of growth.
System for classifying cancer cells in terms of how malignant or aggressive they appear microscopically. The grading of a tumor indicates how quickly cancer cells are likely to spread and plays a role in treatment decisions.
Member of the herpes family of viruses. One type of herpes virus is sexually transmitted and causes sores on the genitals.
Treatment of cancer by removing, blocking, or adding hormones.
Viruses that generally cause warts. Some papillomaviruses are sexually transmitted. Some of these sexually transmitted viruses cause wartlike growths on the genitals, and some are thought to cause abnormal changes in cells of the cervix.
Precancerous condition in which there is an increase in the number of normal cells lining an organ.
Tests that produce pictures of areas inside the body.
Treatment that uses the body's natural defenses to fight cancer. Also called biotherapy or biological modifier response therapy.
Number of new cases of a disease diagnosed each year.
Number of new cases per year per 100,000 persons.
Preneoplastic change in the genetic material of cells caused by a chemical carcinogen. Cancer develops when initiated cells are subsequently exposed to the same or another carcinogen.
Cancer that has remained within the tissue in which it originated.
As related to cancer, the spread of cancer cells into healthy tissue adjacent to the tumor.
Cancer that has spread beyond the layer of tissue in which it developed.
Insoluble protein that is the major constituent of the outer layer of the skin, nails, and hair.
Area of abnormal tissue change.
Cancer of the blood cells.
Probability that a person, over the course of a lifetime, will develop cancer.
Rare family predisposition to multiple cancers, caused by an alteration in the p53 tumor suppressor gene.
An enclosed space bounded by an epithelial membrane; for example, the lumen of the gut.
Cancerous; can invade nearby tissue and spread to other parts of the body.
Skin pigment (substance that gives the skin its color). Dark-skinned people have more melanin than light-skinned people.
Cell in the skin that produces and contains the pigment called melanin.
Cancer of the cells that produce pigment in the skin. Melanoma usually begins in a mole.
Cancer growth (secondary tumors) that is anatomically separated from the site at which the original cancer developed.
To spread from one part of the body to another. When cancer cells metastasize and form secondary tumors, the cells in the metastatic tumor are like those in the original (primary) tumor.
Area on the skin (usually dark in color) that contains a cluster of melanocytes. See also nevus.
Population of cells that was derived by cell division from a single ancestral cell.
Number of deaths per 100,000 persons per year.
Any substance that is known to cause mutations.
Process by which mutations occur.
Change in the way cells function or develop, caused by an inherited genetic defect or an environmental exposure. Such changes may lead to cancer.
The largest of the 24 separate institutes, centers, and divisions of the National Institutes of Health. The NCI coordinates the federal government's cancer research program.
One of eight health agencies of the Public Health Service (the Public Health Service is part of the U.S. Department of Health and Human Services). Composed of 24 separate institutes, centers, and divisions, NIH is the largest biomedical research facility in the world.
Cell death.
Abnormal new growth of cells.
New growth of tissue. Can be referred to as benign or malignant.
Medical term for a spot on the skin, such as a mole. A mole is a cluster of melanocytes that usually appears as a dark spot on the skin.
One of the several types of lymphoma (cancer that develops in the lymphocytic system) that are not Hodgkin lymphoma. Hodgkin lymphoma is rare and occurs most often in people aged 15 to 34 and in people over 55. All other lymphomas are grouped together and called non-Hodgkin lymphoma.
Skin cancer that does not involve melanocytes. Basal cell cancer and squamous cell cancer are nonmelanoma skin cancers.
The Office of Science Education of the National Institutes of Health (NIH) coordinates science education activities at NIH and sponsors science education projects in-house.
Gene that normally directs cell growth but also can promote or allow the uncontrolled growth of cancer if damaged (mutated) by an environmental exposure to carcinogens or if damaged or missing because of an inherited defect.
Having the capacity to cause cancer.
Doctor who specializes in treating cancer. Some oncologists specialize in a particular type of cancer treatment. For example, a radiation oncologist specializes in treating cancer with radiation.
Study of tumors encompassing their physical, chemical, and biologic properties.
Surgical removal of one or both ovaries.
Gene that normally inhibits the growth of tumors, which can prevent or slow the spread of cancer.
Treatment that does not alter the course of a disease, but improves the quality of life.
Population of cells that was derived by cell division from more than one ancestral cell.
Mass of tissue that projects into the colon.
Term used to describe a condition that may or is likely to become cancer.
Growths in the colon that often become cancerous.
Female hormone produced by the ovaries and placenta; responsible for preparing the uterine lining for implantation of an early embryo.
Probable outcome or course of a disease; the chance of recovery.
Expression of the cancerous potential of initiated cells after exposure to the same or a different carcinogen.
Treatment administered or taken to prevent disease.
Gene that, when converted to an oncogene by a mutation or other change, can cause a normal cell to become malignant. Normal oncogenes function to control normal cell growth and differentiation.
Treatment with high-energy rays (such as X-rays) to kill cancer cells. The radiation may come from outside the body (external radiation) or from radioactive materials placed directly in the tumor (implant radiation). Also called radiotherapy.
Giving off radiation.
Radioactive gas that is released by uranium, a substance found in soil and rock. When too much radon is breathed in, it can damage lung cells and lead to lung cancer.
Comparison of the risk of developing cancer in persons with a certain type of exposure or characteristic with the risk in persons who do not have this exposure or characteristic.
Disappearance of the signs and symptoms of cancer. When this happens, the disease is said to be "in remission." A remission can be temporary or permanent.
Eye cancer caused by the loss of both copies of the tumor suppressor gene RB ; the inherited form typically occurs in childhood because one gene is missing from the time of birth.
Small RNA virus that has an RNA genome. Acts as a template for the production of the DNA that is integrated into the DNA of the host cell. Many retroviruses are believed to be oncogenic.
Something that increases the chance of developing a disease.
Chicken retrovirus that was the first virus shown to cause a malignancy.
Malignant tumor that begins in connective and supportive tissue.
Checking for disease when there are no symptoms.
Metastasis.
Surveillance, Epidemiology, and End Results Program of the National Cancer Institute. Started in 1973, SEER collects cancer incidence data in nine geographic areas with a combined population of approximately 9.6 percent of the total population of the United States.
Problem that occurs when treatment affects healthy cells. Common side effects of cancer treatment are fatigue, nausea, vomiting, decreased blood cell counts, hair loss, and mouth sores.
Any of the body cells except the reproductive cells.
Scale for rating sun-screens. Sunscreens with an SPF of 15 or higher provide the best protection from the sun's harmful rays.
Type of skin cancer that arises from the squamous cells.
Extent of a cancer, especially whether the disease has spread from the original site to other parts of the body.
Doing exams and tests to learn the extent of the cancer, especially whether it has spread from its original site to other parts of the body.
Cells from which all blood cells develop.
Substance that blocks the effect of the sun's harmful rays. Using lotions or creams that contain sunscreens can protect the skin from damage that may lead to cancer. See also SPF.
Proportion of patients alive at some point after their diagnosis of a disease.
Enzyme that is present and active in cells that can divide without apparent limit (for example, cancer cells and cells of the germ line). Telomerase replaces the missing repeated sequences of each telomere.
End of a chromosome. In vertebrate cells, each telomere consists of thousands of copies of the same DNA sequence, repeated again and again. Telomeres become shorter each time a cell divides; when one or more telomeres reaches a minimum length, cell division stops. This mechanism limits the number of times a cell can divide.
Male sex hormone.
Change that a normal cell undergoes as it becomes malignant.
Abnormal mass of tissue that results from excessive cell division. Tumors perform no useful body function. They may be either benign (not cancerous) or malignant (cancerous).
Substance in blood or other body fluids that may suggest that a person has cancer.
Gene in the body that can suppress or block the development of cancer.
Invisible rays that are part of the energy that comes from the sun. UV radiation can burn the skin and cause melanoma and other types of skin cancer. UV radiation that reaches the earth's surface is made up of two types of rays, UVA and UVB rays. UVB rays are more likely than UVA rays to cause sunburn, but UVA rays pass further into the skin. Scientists have long thought that UVB radiation can cause melanoma and other types of skin cancer. They now think that UVA radiation also may add to skin damage that can lead to cancer. For this reason, skin specialists recommend that people use sunscreens that block or absorb both kinds of UV radiation.
Process by which one of the two X chromosomes in each cell from a female mammal becomes condensed and inactive. This process assures that most genes on the X chromosome are expressed to the same extent in both males and females.
High-energy radiation used in low doses to diagnose diseases and in high doses to treat cancer.
Hereditary disease characterized by extreme sensitivity to the sun and a tendency to develop skin cancers. Caused by inadequate DNA repair.
- Cite this Page National Institutes of Health (US); Biological Sciences Curriculum Study. NIH Curriculum Supplement Series [Internet]. Bethesda (MD): National Institutes of Health (US); 2007. Understanding Cancer.
In this Page
Related items in bookshelf.
- All Textbooks
Related information
- PubMed Links to PubMed
Recent Activity
- Understanding Cancer - NIH Curriculum Supplement Series Understanding Cancer - NIH Curriculum Supplement Series
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Together we are beating cancer
About cancer
Cancer types
- Breast cancer
- Bowel cancer
- Lung cancer
- Prostate cancer
Cancers in general
- Clinical trials
Causes of cancer
Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
Get involved
- Make a donation
By cancer type
- Leave a legacy gift
- Donate in Memory
Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay For Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our We Are campaign
Our research
- Brain tumours
- Skin cancer
- All cancer types
By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
Funding for researchers
Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
Applying for funding
- Start your application online
- How to make a successful application
- Funding committees
- Successful applicant case studies
How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
Shop online
- Wedding favours
- Cancer Care
- Flower Shop
Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
- Cancer news
- Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
ABOUT CANCER
GET INVOLVED
NEWS & RESOURCES
FUNDING & RESEARCH
You are here

Chief Executive and Executive Board
Michelle Mitchell OBE
Chief Executive Officer
Michelle Mitchell OBE joined in November 2018. She is responsible for the overall leadership and management of CRUK, the world’s leading charitable funder of cancer research.
Since taking the helm, Michelle has helped put CRUK on a strong footing to achieve its vision of bringing about a world where everyone can lead longer, better lives, free from the fear of cancer. Having navigated the COVID-19 pandemic, she set the organisation on an ambitious new course with its long-term strategy, launched shortly after CRUK celebrated its 20th anniversary. In 2022/23, Michelle led the charity in delivering one of the strongest impact and financial performances in its history.
Michelle is a senior voluntary sector leader who has transformed a number of not-for-profit organisations to better their purpose and global impact. She has a successful track record of delivering long term value, collaboration and innovation.
She is a member of the National Cancer Board (England) and the NHS Genomics Board (England). Before joining Cancer Research UK, Michelle was CEO of the MS Society for five years and a founding member of the Progressive MS Alliance: a global scientific and research joint venture and launched the STOP MS £100m appeal. Prior to that, she was Director General at Age UK, the UK’s largest older people’s charity where she worked for nearly a decade and was at the forefront of national debates on health, care and welfare.
Michelle has extensive non-executive experience, which has included NED at NHS England, which sets the priorities and direction for the NHS in England and a Trustee of the King’s Fund, a leading health policy think-tank.
Michelle has a BA in Economics, an MA in Politics and Public Administration and an International Executive Diploma from INSEAD. She is an Honorary Fellow of the University of Cambridge’s Homerton College.
Michelle was awarded an OBE in 2015 for services to the charity sector.
Angela Morrison
Chief Operating Officer
Angela joined Cancer Research UK in April 2021 as Chief Operating Officer and leads the Charity's finance, people, technology, general council and change teams, working across our organisation to ensure we have appropriate governance and an operating model that enables us to be as effective and efficient as we can at delivering on our mission to beat cancer.
Angela is also a Non Executive Board Member of HM Land Registry, where she sits on the Audit and Change Committees alongside providing support to the operations, transformation and technology teams.
Angela has extensive experience as a commercial executive, most recently at Debenhams where she led their retail, supply chain and technology teams and prior to that Direct Line Group, Sainsburys and Asda/Walmart. In these organisations she led and managed both technical and business change, and as part of the broader executive led the organisations through their cultural transformations.
She was the executive sponsor for Corporate Social Responsibility at both Debenhams and Direct Line and for a number of years sat on the Advisory Board for The Tech Partnership (previously E-skills), working with peers and counterparts in the information technology (IT) service sector to improve IT skills in education and the workplace.
Angela has a degree in Electrical and Electronic Engineering from the University of Bristol.
Philip Almond
Executive Director, Marketing, Fundraising and Engagement
Phil joined Cancer Research UK in December 2019 and leads the Charity's Fundraising and Marketing teams, working with our supporters, donors and staff to raise the funds which will help us beat cancer sooner.
Prior to joining CRUK he was Chief Marketing Officer at the BBC and before that had a long career at Diageo, where among other roles he led the company's UK consumer and customer marketing teams, and the Baileys and Smirnoff brands globally.
Phil and his team are responsible for the brand, communications and revenue generation to allow the Charity to meet its ambitious goals.
Phil has a Masters in English from the University of Cambridge and an MBA from the London Business School.
Iain Foulkes PhD
Executive Director, Research and Innovation / CEO of Cancer Research Horizons
Iain was appointed to the CRUK Board in August 2009. He oversees CRUK's portfolio of research across discovery science, translational research, clinical and population research. This research portfolio includes the international Cancer Grand Challenge programme, a large clinical trial portfolio and oversight of CRUK’s Institutes and Centres.
As CEO of Cancer Research Horizons, he is also responsible for ensuring research outputs are developed and commercialised, often in partnership with biotech and pharmaceutical companies to ensure rapid and effective patient impact.
Iain is a member of several boards including The Francis Crick Institute, the Scotland Institute (formerly the Beatson Institute), American Friends of Cancer Research, and is a Non-Executive Director at the British Medical Journal.
Executive Director, Strategy and Philanthropy
Nick is the Executive Director of Strategy and Philanthropy at Cancer Research UK, where he is responsible for developing the organisation’s long-term strategy and leads the Philanthropy directorate – a strategic growth priority for the charity.
Nick has been a member of the Executive Board for eight years, and has also held roles as Executive Director, International Partnerships; Interim Executive Director of Policy and Information, and as Director of Strategy.
Prior to Cancer Research UK, Nick spent 10 years as a management consultant at The Boston Consulting Group, Braxton Associates and Deloitte where he supported clients in the pharmaceutical and healthcare sectors on strategy, organisation and change projects. He has a Masters in Manufacturing Engineering from the University of Cambridge, and an MBA from INSEAD business school.
Ian Walker Bsc, PhD, MBA
Executive Director for Policy, Information and Communications
Ian was appointed to the Board in 2021 and is responsible for developing and implementing our strategic priorities across policy, information and communications.
Ian has over 15 years of combined experience dedicated to advancements in cancer prevention, diagnosis, and treatment.
First appointed as a CRUK Director in 2013, Ian has held several high impact roles within the charity across basic, translational and clinical research. H e brings a wealth of relevant experience to the board, having advised on several of the charity’s key campaigns to inform government policy around the provision of life-saving cancer research, including the push for the protection and restoration of clinical research activity within the NHS during the COVID-19 pandemic.
Ian is a trustee for the Association of Medical Research Charities (AMRC) and has previously held a Non-executive role in the biotechnology sector.
Ian holds a Biochemistry BSc with honours and a PhD from the University of Leeds. He also holds an MBA with distinction from Warwick Business School.
Clinical and Scientific Advisors
Professor Ketan (KJ) Patel PhD FRS FMedSci MRCP
Professor Patel was appointed as CRUK’s Chief Scientist in October 2022. He provides scientific leadership to the charity’s ambitions, working with our research community to deliver our research strategy which focuses on the importance of discovery science to unlock new and better ways to beat cancer.
He is Director of the MRC Weatherall Institute of Molecular Medicine and the MRC Molecular Haematology Unit at the University of Oxford. Prior to this, he was a tenured principal investigator at the MRC Laboratory of Molecular Biology (LMB) in Cambridge, and Professor for molecular medicine and stem cell genomics at the University of Cambridge.
Professor Patel trained in medicine in London, and his postdoctoral research led to the discovery that BRCA2 works by repairing damaged DNA.
His subsequent work has remained focussed on DNA damage, and specifically on how living cells repair DNA crosslinks. These crosslinks can be exploited to kill cancer cells, for example the drug cisplatin causes crosslinking in cancer cells, but they can also occur naturally. Specific defects in the molecular machinery that repairs DNA crosslinking is associated with the illness Fanconi anaemia. KJ’s recent work has shown that metabolism releases aldehydes that are a potent source of such endogenous DNA damage.
Cancer Research UK has funded Professor Patel’s work in the past, including his research into cancer cachexia, the debilitating wasting condition many people with cancer experience in the later stages of their disease, which we still don’t fully understand. His findings have also contributed to the organisation's improved understanding of what drives the development of cancer, such as how alcohol exposure can cause cancer, opening up new opportunities for prevention, detection and treatment.
Professor Patel is a Fellow of both the Royal Society and Academy of Medical Sciences UK and is also a member of the European Molecular Biology Organisation.
Professor Charles Swanton MBPhD FRCP FMedSci FRS
Charles was appointed Chief Clinician for Cancer Research UK in October 2017. He has responsibility for the strategy and shape of the Charity's clinical activities, both in clinical research and in the wider context of cancer prevention, diagnosis, and treatment.
Charles completed his MBPhD in 1999 at the Imperial Cancer Research Fund Laboratories and Cancer Research UK clinician/scientist medical oncology training in 2008. He combines his laboratory research at the Francis Crick Institute and University College London Cancer Institute with clinical duties at UCLH and as director of the CRUK Lung Cancer Centre, focussed on how tumours evolve over space and time. Charles has demonstrated ubiquitous cancer branched evolution, how it is shaped by genome instability, therapy and immunity, and how this can be exploited for therapy, demonstrating how inflammation drives cancer initiation.
Charles was made Fellow of the Royal College of Physicians in April 2011, appointed Fellow of the Academy of Medical Sciences in 2015, Napier Professor in Cancer by the Royal Society in 2016, appointed Cancer Research UK’s Chief Clinician in 2017, and elected Fellow of the Royal Society in 2018.
Charles was awarded the Stand up to Cancer Translational Cancer Research Prize (2015), Glaxo Smithkline Biochemical Society Prize (2016), San Salvatore prize for Cancer Research (2017), the Ellison-Cliffe Medal, Royal Society of Medicine (2017), recipient of the Gordon Hamilton Fairley Medal (2018), the Massachusetts General Hospital Cancer Centre Kraft Prize for Excellence in Cancer Research (2018), the International Association for the Study of Lung Cancer (IASLC) Paul A. Bunn Scientific Award for achievements in lung cancer (2018), the ESMO Translation Award (2019), the Weizmann Institute Sergio Lombroso Award in Cancer Research (2021), the Memorial Sloan Kettering Paul Marks Prize for Cancer Research (2021), UCLH Celebrating Excelence Award for Contribution to World Class Research (2022), Inductee to OncLive’s Giants of Cancer Care award (2023), and the SpringerNature CDD Award (2023).
Get in touch
If you require further information or have any comments please get in touch.
020 7242 0200
- Internal Audit Charter
- Our fundraising promise
- Our Members
- Privacy statement
- Scientific Executive Board
- Safeguarding at Cancer Research UK
- Tax Strategy
- Cancer Research UK Pension Scheme
- Council Committees
- Annual Supporter Update
- Full Disclosure of interactions with Industry
- Non-Trustee Committee Members
- Our Strategy to beat cancer
- Responsible Organisation at Cancer Research UK
- Cancer Strategy in England

Online Help
Our 24/7 cancer helpline provides information and answers for people dealing with cancer. We can connect you with trained cancer information specialists who will answer questions about a cancer diagnosis and provide guidance and a compassionate ear.
Chat live online
Select the Live Chat button at the bottom of the page
Call us at 1-800-227-2345
Available any time of day or night
Our highly trained specialists are available 24/7 via phone and on weekdays can assist through online chat. We connect patients, caregivers, and family members with essential services and resources at every step of their cancer journey. Ask us how you can get involved and support the fight against cancer. Some of the topics we can assist with include:
- Referrals to patient-related programs or resources
- Donations, website, or event-related assistance
- Tobacco-related topics
- Volunteer opportunities
- Cancer Information
For medical questions, we encourage you to review our information with your doctor.
Our Research Programs
What does it take to outsmart cancer? Research.
The American Cancer Society (ACS) has helped make possible almost every major cancer breakthrough since 1946. Since then, we've invested more than $5 billion in cancer research, making us the largest nonprofit funder of cancer research in the United States, outside of the federal government.
We remain committed to finding more – and better – ways to improve the quality of life for cancer patients.
Save the Date: June 25-27, 2025 Cancer Research Prevention Conference in London
Investing in Research Pays Off
See how. Check out easy-to-understand summaries of recent studies.

Featured Research Studies

Cancer Facts & Figures for Asian American, Native Hawaiian, & Other Pacific Islander People
This inaugural report provides an overview of cancer occurrence, risk factors, and screening for disaggregated Asian American, Native Hawaiian and other Pacific Islander groups in the United States.

Global Cancer Facts & Figures
According to the International Agency for Research on Cancer (IARC), in 2022, there were 20 million new cancer cases and 9.7 million cancer deaths worldwide. See how cancer affects 185 countries across every continent.
We're Finding Answers That Save Lives
Whether we're conducting research or funding it, our goal remains the same: to free the world from the pain and suffering of cancer.

All Cancer Facts & Figures Reports
Learn 2023 estimated new cancer cases and deaths, and stats for breast, colorectal, and global cancer, as well as for African Americans and Hispanics/Latinos.

Cancer Prevention Studies-3 (CPS-3)
See how 300,000 volunteer participants in this active population study are helping us move closer to a world without cancer.
Currently Funded Cancer Research
Learn more about our substantial current spending on research.
Explore Our Research Programs
Our work covers the full realm of cancer research.

Surveillance & Health Equity Science (SHES)
We conduct and publish research on cancer prevention, surveillance, health services, and disparities, including the ACS Cancer Facts & Figures reports.

Population Science (PopSci)
We conduct and publish research about cancer risk factors and the quality of life of cancer survivors, including the ACS Cancer Prevention Studies, CPS-II, and CPS-3.

Extramural Discovery Science (EDS)
We fund high impact and innovative research for any type of cancer and from bench to bedside by supporting scientists across the United States with research grants.
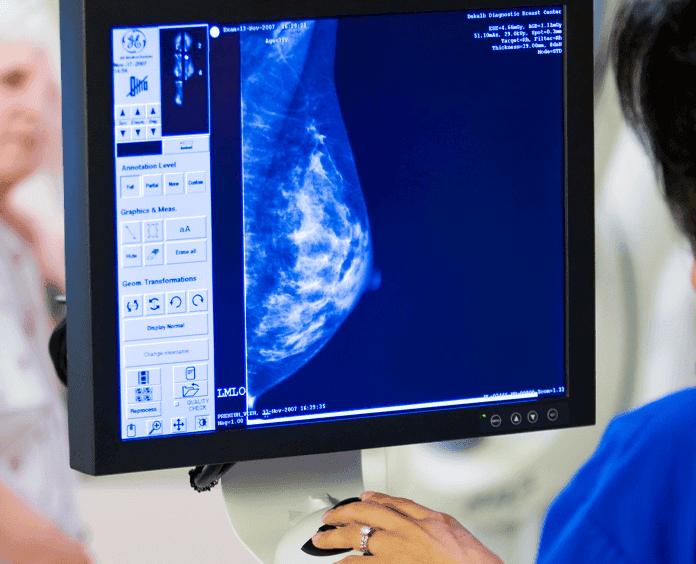
Early Cancer Detection Science
We oversee the development, review, and update of evidence-based cancer screening guidelines using rigorous international standards.

Center for Diversity in Cancer Research (DICR) Training
We fund hands-on research programs for some of today's minority and disadvantaged students to improve the diversity of cancer research and care in the future.
Glossary for Nonscientists
Featured term: cancer survivor.
Anyone who has been diagnosed with cancer, regardless of whether they are actively receiving treatment.
Our Scientists are Helping to End Cancer

Interactive and Multimedia Platforms

Cancer Statistics Center
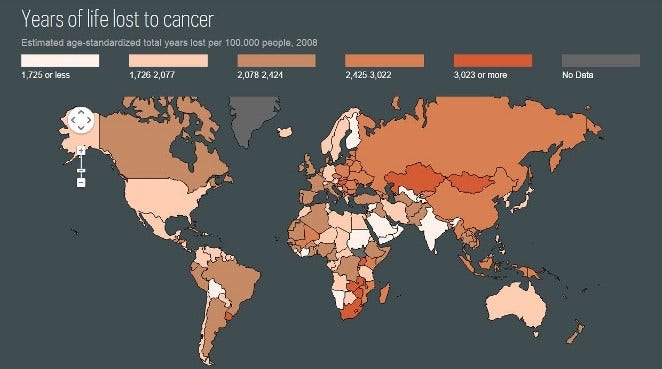
Cancer Atlas

ACS Research Podcasts
ACS Recorded Webinars
Help us end cancer as we know it, for everyone..


Organisation Structure

Related Items

About The Institute
Collaborations
About the Manchester Cancer Research Centre
Annual Report
Scientific Advisory Board
Accessibility
Use of Animals in Our Research
Equality, Diversity and Inclusion
Statement of Research Integrity
Related Links
Cancer Research UK Manchester Centre
Manchester Cancer Research Centre
CRUK Lung Cancer Centre of Excellence
Download our Annual Report

Cancer Research UK Manchester Institute The University of Manchester Wilmslow Road Manchester M20 4BX
Telephone 0161 306 0871 Email [email protected]

IMAGES
VIDEO
COMMENTS
How we are structured. The Institute of Cancer Research, London, operates as a research institute, a higher education institution and an exempt charity. Its structure and governance arrangements reflect these multiple organisational roles. The ICR is a company limited by guarantee (company number 00534147), incorporated in 1954. It is also a ...
As the UK's largest charity, we recognise the positive role we can play in developing and embedding EDI best practice across all our business operations, including our public engagement and outreach work. A number of bodies work together to ensure that we make the best use of the funds we receive and continue to carry out world-class research.
According to the World Health Organization (WHO), cancer is the second leading cause of death globally, accounting for approximately 9.6 million deaths [1]. The WHO recommends that each nation has a national cancer control program (NCCP) to reduce the incidence of cancer and deaths related to cancer, as well as to improve the quality of life of cancer patients [2]. Comprehensive cancer centers ...
Our strategy puts discovery research and excellence at the heart of what we'll do. We must understand the mechanisms of how cancer develops and progresses to unlock new ways to prevent, detect and treat it. It's built around 4 objectives - to discover, detect, prevent, and treat - so that progress in understanding the fundamental biology of ...
NCI Organization. As the nation's leading federal agency for cancer research, NCI has 30 divisions, offices, and centers who work together to build, maintain, and enhance a cohesive and comprehensive cancer research agenda. Their work ranges from cutting-edge research on cancer causes, treatment, and prevention; to training the next generation ...
The primary responsibility of the RRP is to the grantees and contractors of the NCI and NIH. In 2019, RRP staff managed a portfolio of 162 awarded grants, the bulk of which being through the R01 and R21 mechanisms. The RDB's primary focus is in radiation biology, cancer modeling, and pre-clinical research; while the CROB manages clinical and ...
Audit Committee The Audit Committee provides structured systematic oversight of the Charity's governance, risk management and internal controls practices that may have an impact on the Charity's ability to meet its aims; provides Council with objective advice, guidance and support regarding the adequacy and effectiveness of the organisation's systems and processes of governance, risk ...
The organisation of cancer research is critical to its overall creativity and productivity. Cancer centres are a major organisational structure for this research, however, little is known about their effect on research or how national policy-making intersects with this complex policy nexus. This stu …
A. n7569403. 1. its neither a tall or a flat structure. its a matrix structure. this is mainly the structure that most charities or non profitable businesses have. its not as much about someone leading someone else.in a matrix structure the business or charity has many objectives that they wish to meet, therefore they would make different ...
The American Cancer Society, Inc., is a 501 (c) (3) nonprofit corporation governed by a single Board of Directors that is responsible for setting policy, establishing long-term goals, monitoring general operations, and approving the organizational outcomes and allocation of resources. The Board is composed entirely of volunteers from the ...
Research Basic Laboratory Research. Basic laboratory research generates the knowledge that forms the basis for applied science. This type of research focuses on the mechanistic understanding of biochemical, biologic, physiologic, and pharmacologic processes as they relate to cancer and cancer treatments [].Tools used in this type of research include laboratory techniques such as flow cytometry ...
A world where: This strategy offers the one thing we all seek: hope. Hope that the more we discover about the causes of cancer, the more this can be used to prevent it. Hope that discoveries will also lead to a better understanding of how cancer affects our bodies, leading to a better quality of life. Hope that more people will be able to live ...
Appointed 2016. Peter Chambré is a Trustee of Cancer Research UK and Chair of Cancer Research Horizons, the commercialisation arm of Cancer Research UK. He is also Chair of Immatics N.V., a company developing new cancer immunotherapy treatments. He has been a director of a number of public and private healthcare and life science companies and ...
Organisational Structure. Translational Research and Precision Medicine Working Group. ESMO-Magnitude of Clinical Benefit Scale Working Group. ESMO Clinical Research Observatory Task Force (ECRO) Notice of the 2024 Ordinary Annual General Assembly. Stepping into the shoes of a Society with a global footprint.
Cancer Research UK's constitution provides for the appointment of 100 Members of the charity. Members are similar to the shareholders of a company. They are entitled to attend all general meetings of Cancer Research UK (including the AGM) and their most significant formal duty is the election of Trustees. ...
Explained. There are medical research organizations that are state-owned while some are independent. Cancer Research UK is a renowned medical research institute, a forefront runner in the fight against cancer in the region; but what type of ownership is Cancer Research UK? Cancer Research UK is a company limited by guarantee.
The image below shows the current organisational structure of Cancer Research UK. The UK Cancer Research Hierarchy Structure is a flat structure and a matrix structure. This is because even with the Board of Trustees having the majority of the Authority in decision-making, the actual mission delegations and the actual day-to-day operation of ...
Understanding Cancer. In simple terms, cancer is a group of more than 100 diseases that develop across time and involve the uncontrolled division of the body's cells. Although cancer can develop in virtually any of the body's tissues, and each type of cancer has its unique features, the basic processes that produce cancer are quite similar in ...
Iain Foulkes PhD. Executive Director, Research and Innovation / CEO of Cancer Research Horizons. Iain was appointed to the CRUK Board in August 2009. He oversees CRUK's portfolio of research across discovery science, translational research, clinical and population research. This research portfolio includes the international Cancer Grand ...
Genetics and Epidemiology . The Division of Genetics and Epidemiology conducts high-quality laboratory, epidemiological and clinical research to understand the genes, behaviours and exposures that influence cancer risk, and translate discoveries into clinical practice. View list of teams View list of researchers .
The American Cancer Society (ACS) has helped make possible almost every major cancer breakthrough since 1946. Since then, we've invested more than $5 billion in cancer research, making us the largest nonprofit funder of cancer research in the United States, outside of the federal government. We remain committed to finding more - and better ...
CRUK Lung Cancer Centre of Excellence. Download our Annual Report. Contact. Cancer Research UK Manchester Institute. The University of Manchester. Wilmslow Road. Manchester M20 4BX. Telephone0161 306 0871. [email protected].
Participates in providing timely, informative, and accurate communication to the IRB as required. Facilitates and participates in the preparation for and implementation of scheduled and unscheduled meetings with external and internal monitors and auditors (e.g., sponsors, FDA, IRB, QA).