Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 24 March 2022

Tobacco and nicotine use
- Bernard Le Foll 1 , 2 ,
- Megan E. Piper 3 , 4 ,
- Christie D. Fowler 5 ,
- Serena Tonstad 6 ,
- Laura Bierut 7 ,
- Lin Lu ORCID: orcid.org/0000-0003-0742-9072 8 , 9 ,
- Prabhat Jha 10 &
- Wayne D. Hall 11 , 12
Nature Reviews Disease Primers volume 8 , Article number: 19 ( 2022 ) Cite this article
38k Accesses
86 Citations
106 Altmetric
Metrics details
- Disease genetics
- Experimental models of disease
- Preventive medicine
Tobacco smoking is a major determinant of preventable morbidity and mortality worldwide. More than a billion people smoke, and without major increases in cessation, at least half will die prematurely from tobacco-related complications. In addition, people who smoke have a significant reduction in their quality of life. Neurobiological findings have identified the mechanisms by which nicotine in tobacco affects the brain reward system and causes addiction. These brain changes contribute to the maintenance of nicotine or tobacco use despite knowledge of its negative consequences, a hallmark of addiction. Effective approaches to screen, prevent and treat tobacco use can be widely implemented to limit tobacco’s effect on individuals and society. The effectiveness of psychosocial and pharmacological interventions in helping people quit smoking has been demonstrated. As the majority of people who smoke ultimately relapse, it is important to enhance the reach of available interventions and to continue to develop novel interventions. These efforts associated with innovative policy regulations (aimed at reducing nicotine content or eliminating tobacco products) have the potential to reduce the prevalence of tobacco and nicotine use and their enormous adverse impact on population health.
Similar content being viewed by others

Associations between classic psychedelics and nicotine dependence in a nationally representative sample
Use of electronic cigarettes and heated tobacco products during the covid-19 pandemic.
Smoking cessation behaviors and reasons for use of electronic cigarettes and heated tobacco products among Romanian adults
Introduction.
Tobacco is the second most commonly used psychoactive substance worldwide, with more than one billion smokers globally 1 . Although smoking prevalence has reduced in many high-income countries (HICs), tobacco use is still very prevalent in low-income and middle-income countries (LMICs). The majority of smokers are addicted to nicotine delivered by cigarettes (defined as tobacco dependence in the International Classification of Diseases, Tenth Revision (ICD-10) or tobacco use disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)). As a result of the neuro-adaptations and psychological mechanisms caused by repeated exposure to nicotine delivered rapidly by cigarettes, cessation can also lead to a well-characterized withdrawal syndrome, typically manifesting as irritability, anxiety, low mood, difficulty concentrating, increased appetite, insomnia and restlessness, that contributes to the difficulty in quitting tobacco use 2 , 3 , 4 .
Historically, tobacco was used in some cultures as part of traditional ceremonies, but its use was infrequent and not widely disseminated in the population. However, since the early twentieth century, the use of commercial cigarettes has increased dramatically 5 because of automated manufacturing practices that enable large-scale production of inexpensive products that are heavily promoted by media and advertising. Tobacco use became highly prevalent in the past century and was followed by substantial increases in the prevalence of tobacco-induced diseases decades later 5 . It took decades to establish the relationship between tobacco use and associated health effects 6 , 7 and to discover the addictive role of nicotine in maintaining tobacco smoking 8 , 9 , and also to educate people about these effects. It should be noted that the tobacco industry disputed this evidence to allow continuing tobacco sales 10 . The expansion of public health campaigns to reduce smoking has gradually decreased the use of tobacco in HICs, with marked increases in adult cessation, but less progress has been achieved in LMICs 1 .
Nicotine is the addictive compound in tobacco and is responsible for continued use of tobacco despite harms and a desire to quit, but nicotine is not directly responsible for the harmful effects of using tobacco products (Box 1 ). Other components in tobacco may modulate the addictive potential of tobacco (for example, flavours and non-nicotine compounds) 11 . The major harms related to tobacco use, which are well covered elsewhere 5 , are linked to a multitude of compounds present in tobacco smoke (such as carcinogens, toxicants, particulate matter and carbon monoxide). In adults, adverse health outcomes of tobacco use include cancer in virtually all peripheral organs exposed to tobacco smoke and chronic diseases such as eye disease, periodontal disease, cardiovascular diseases, chronic obstructive pulmonary disease, stroke, diabetes mellitus, rheumatoid arthritis and disorders affecting immune function 5 . Moreover, smoking during pregnancy can increase the risk of adverse reproductive effects, such as ectopic pregnancy, low birthweight and preterm birth 5 . Exposure to secondhand cigarette smoke in children has been linked to sudden infant death syndrome, impaired lung function and respiratory illnesses, in addition to cognitive and behavioural impairments 5 . The long-term developmental effects of nicotine are probably due to structural and functional changes in the brain during this early developmental period 12 , 13 .
Nicotine administered alone in various nicotine replacement formulations (such as patches, gum and lozenges) is safe and effective as an evidence-based smoking cessation aid. Novel forms of nicotine delivery systems have also emerged (called electronic nicotine delivery systems (ENDS) or e-cigarettes), which can potentially reduce the harmful effects of tobacco smoking for those who switch completely from combustible to e-cigarettes 14 , 15 .
This Primer focuses on the determinants of nicotine and tobacco use, and reviews the neurobiology of nicotine effects on the brain reward circuitry and the functioning of brain networks in ways that contribute to the difficulty in stopping smoking. This Primer also discusses how to prevent tobacco use, screen for smoking, and offer people who smoke tobacco psychosocial and pharmacological interventions to assist in quitting. Moreover, this Primer presents emerging pharmacological and novel brain interventions that could improve rates of successful smoking cessation, in addition to public health approaches that could be beneficial.
Box 1 Tobacco products
Conventional tobacco products include combustible products that produce inhaled smoke (most commonly cigarettes, bidis (small domestically manufactured cigarettes used in South Asia) or cigars) and those that deliver nicotine without using combustion (chewing or dipping tobacco and snuff). Newer alternative products that do not involve combustion include nicotine-containing e-cigarettes and heat-not-burn tobacco devices. Although non-combustion and alternative products may constitute a lesser risk than burned ones 14 , 15 , 194 , no form of tobacco is entirely risk-free.
Epidemiology
Prevalence and burden of disease.
The Global Burden of Disease Project (GBDP) estimated that around 1.14 billion people smoked in 2019, worldwide, increasing from just under a billion in 1990 (ref. 1 ). Of note, the prevalence of smoking decreased significantly between 1990 and 2019, but increases in the adult population meant that the total number of global smokers increased. One smoking-associated death occurs for approximately every 0.8–1.1 million cigarettes smoked 16 , suggesting that the estimated worldwide consumption of about 7.4 trillion cigarettes in 2019 has led to around 7 million deaths 1 .
In most populations, smoking prevalence is much higher among groups with lower levels of education or income 17 and among those with mental health disorders and other co-addictions 18 , 19 . Smoking is also more frequent among men than women (Figs 1 – 3 ). Sexual and/or gender minority individuals have disproportionately high rates of smoking and other addictions 17 , 20 . In addition, the prevalence of smoking varies substantially between regions and ethnicities; smoking rates are high in some regions of Asia, such as China and India, but are lower in North America and Australia. Of note, the prevalence of mental health disorders and other co-addictions is higher in individuals who smoke compared with non-smokers 18 , 19 , 21 . For example, the odds of smoking in people with any substance use disorder is more than five times higher than the odds in people without a substance use disorder 19 . Similarly, the odds of smoking in people with any psychiatric disorder is more than three times higher than the odds of smoking in those without a psychiatric diagnosis 22 . In a study in the USA, compared with a population of smokers with no psychiatric diagnosis, subjects with anxiety, depression and phobia showed an approximately twofold higher prevalence of smoking, and subjects with agoraphobia, mania or hypomania, psychosis and antisocial personality or conduct disorders showed at least a threefold higher prevalence of smoking 22 . Comorbid disorders are also associated with higher rates of smoking 22 , 23 .
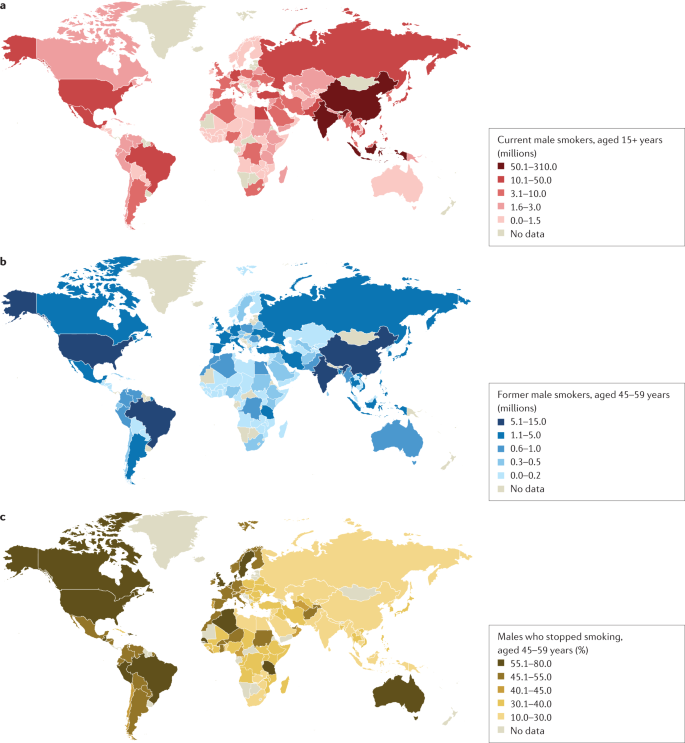
a | Number of current male smokers aged 15 years or older per country expressed in millions. b | Former male smokers aged 45–59 years per country expressed in millions. c | Former male smokers aged 45–59 years per country expressed as the percentage of smokers who stopped. The data shown are for male smokers for the period 2015–2019 from countries with direct smoking surveys. The prevalence of smoking among males is less variable than among females. Data from ref. 1 .
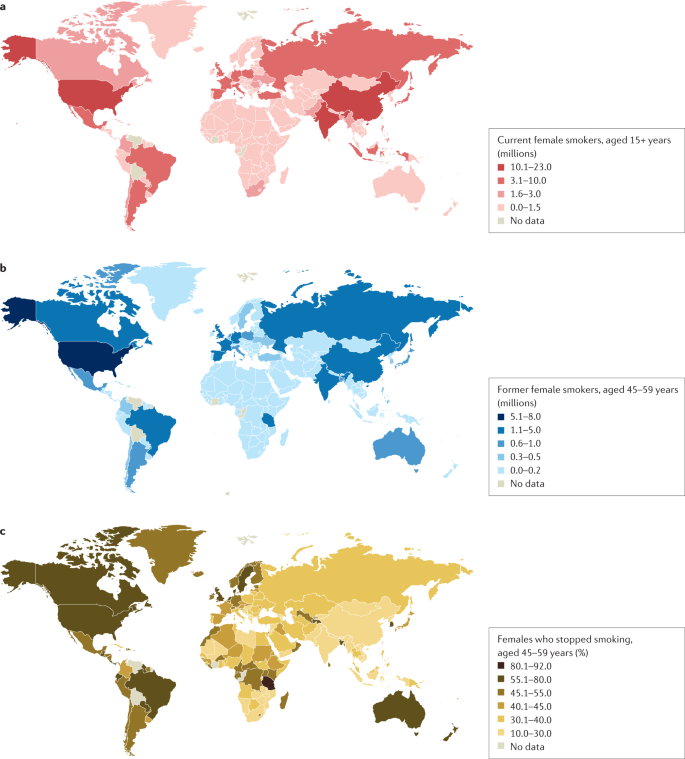
a | Number of current female smokers aged 15 years or older per country expressed in millions. b | Former female smokers aged 45–59 years per country expressed in millions. c | Former female smokers aged 45–59 years per country expressed as the percentage of smokers who stopped. The data shown are for female smokers for the period 2015–2019 from countries with direct smoking surveys. The prevalence of smoking among females is much lower in East and South Asia than in Latin America or Eastern Europe. Data from ref. 1 .
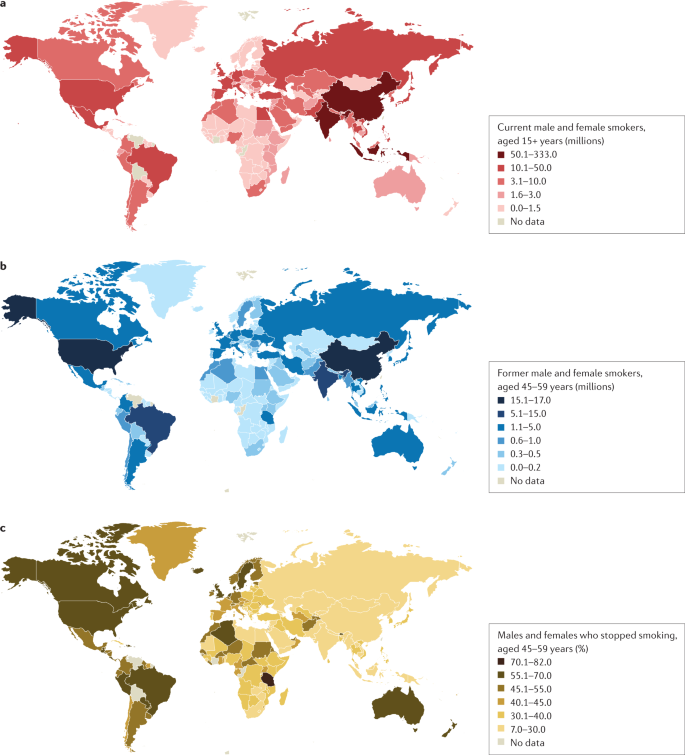
a | Number of current male and female smokers aged 15 years or older per country expressed in millions. b | Former male and female smokers aged 45–59 years per country expressed in millions. c | Former male and female smokers aged 45–59 years per country expressed as the percentage of smokers who stopped. The data shown are for the period 2015–2019 from countries with direct smoking surveys. Cessation rates are higher in high-income countries, but also notably high in Brazil. Cessation is far less common in South and East Asia and Russia and other Eastern European countries, and also low in South Africa. Data from ref. 1 .
Age at onset
Most smokers start smoking during adolescence, with almost 90% of smokers beginning between 15 and 25 years of age 24 . The prevalence of tobacco smoking among youths substantially declined in multiple HICs between 1990 and 2019 (ref. 25 ). More recently, the widespread uptake of ENDS in some regions such as Canada and the USA has raised concerns about the long-term effects of prolonged nicotine use among adolescents, including the possible notion that ENDS will increase the use of combustible smoking products 25 , 26 (although some studies have not found much aggregate effect at the population level) 27 .
Smoking that commences in early adolescence or young adulthood and persists throughout life has a more severe effect on health than smoking that starts later in life and/or that is not persistent 16 , 28 , 29 . Over 640 million adults under 30 years of age smoke in 22 jurisdictions alone (including 27 countries in the European Union where central efforts to reduce tobacco dependence might be possible) 30 . In those younger than 30 years of age, at least 320 million smoking-related deaths will occur unless they quit smoking 31 . The actual number of smoking-related deaths might be greater than one in two, and perhaps as high as two in three, long-term smokers 5 , 16 , 29 , 32 , 33 . At least half of these deaths are likely to occur in middle age (30–69 years) 16 , 29 , leading to a loss of two or more decades of life. People who smoke can expect to lose an average of at least a decade of life versus otherwise similar non-smokers 16 , 28 , 29 .
Direct epidemiological studies in several countries paired with model-based estimates have estimated that smoking tobacco accounted for 7.7 million deaths globally in 2020, of which 80% were in men and 87% were current smokers 1 . In HICs, the major causes of tobacco deaths are lung cancer, emphysema, heart attack, stroke, cancer of the upper aerodigestive areas and bladder cancer 28 , 29 . In some lower income countries, tuberculosis is an additional important cause of tobacco-related death 29 , 34 , which could be related to, for example, increased prevalence of infection, more severe tuberculosis/mortality and higher prevalence of treatment-resistant tuberculosis in smokers than in non-smokers in low-income countries 35 , 36 .
Despite substantial reductions in the prevalence of smoking, there were 34 million smokers in the USA, 7 million in the UK and 5 million in Canada in 2017 (ref. 16 ), and cigarette smoking remains the largest cause of premature death before 70 years of age in much of Europe and North America 1 , 16 , 28 , 29 . Smoking-associated diseases accounted for around 41 million deaths in the USA, UK and Canada from 1960 to 2020 (ref. 16 ). Moreover, as smoking-associated diseases are more prevalent among groups with lower levels of education and income, smoking accounts for at least half of the difference in overall mortality between these social groups 37 . Any reduction in smoking prevalence reduces the absolute mortality gap between these groups 38 .
Smoking cessation has become common in HICs with good tobacco control interventions. For example, in France, the number of ex-smokers is four times the number of current smokers among those aged 50 years or more 30 . By contrast, smoking cessation in LMICs remains uncommon before smokers develop tobacco-related diseases 39 . Smoking cessation greatly reduces the risks of smoking-related diseases. Indeed, smokers who quit smoking before 40 years of age avoid nearly all the increased mortality risks 31 , 33 . Moreover, individuals who quit smoking by 50 years of age reduce the risk of death from lung cancer by about two-thirds 40 . More modest hazards persist for deaths from lung cancer and emphysema 16 , 28 ; however, the risks among former smokers are an order of magnitude lower than among those who continue to smoke 33 .
Mechanisms/pathophysiology
Nicotine is the main psychoactive agent in tobacco and e-cigarettes. Nicotine acts as an agonist at nicotinic acetylcholine receptors (nAChRs), which are localized throughout the brain and peripheral nervous system 41 . nAChRs are pentameric ion channels that consist of varying combinations of α 2 –α 7 and β 2 –β 4 subunits, and for which acetylcholine (ACh) is the endogenous ligand 42 , 43 , 44 . When activated by nicotine binding, nAChR undergoes a conformational change that opens the internal pore, allowing an influx of sodium and calcium ions 45 . At postsynaptic membranes, nAChR activation can lead to action potential firing and downstream modulation of gene expression through calcium-mediated second messenger systems 46 . nAChRs are also localized to presynaptic membranes, where they modulate neurotransmitter release 47 . nAChRs become desensitized after activation, during which ligand binding will not open the channel 45 .
nAChRs with varying combinations of α-subunits and β-subunits have differences in nicotine binding affinity, efficacy and desensitization rate, and have differential expression depending on the brain region and cell type 48 , 49 , 50 . For instance, at nicotine concentrations found in human smokers, β 2 -containing nAChRs desensitize relatively quickly after activation, whereas α 7 -containing nAChRs have a slower desensitization profile 48 . Chronic nicotine exposure in experimental animal models or in humans induces an increase in cortical expression of α 4 β 2 -containing nAChRs 51 , 52 , 53 , 54 , 55 , but also increases the expression of β 3 and β 4 nAChR subunits in the medial habenula (MHb)–interpeduncular nucleus (IPN) pathway 56 , 57 . It is clear that both the brain localization and the type of nAChR are critical elements in mediating the various effects of nicotine, but other factors such as rate of nicotine delivery may also modulate addictive effects of nicotine 58 .
Neurocircuitry of nicotine addiction
Nicotine has both rewarding effects (such as a ‘buzz’ or ‘high’) and aversive effects (such as nausea and dizziness), with the net outcome dependent on dose and others factors such as interindividual sensitivity and presence of tolerance 59 . Thus, the addictive properties of nicotine involve integration of contrasting signals from multiple brain regions that process reward and aversion (Fig. 4 ).
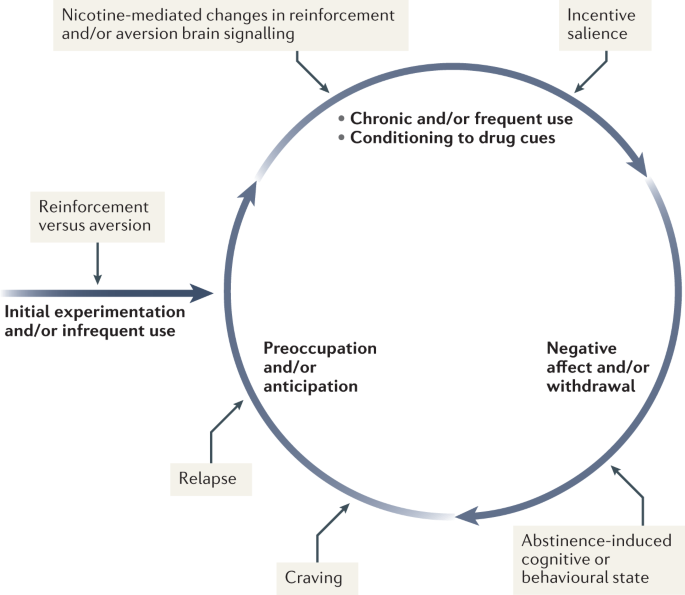
During initial use, nicotine exerts both reinforcing and aversive effects, which together determine the likelihood of continued use. As the individual transitions to more frequent patterns of chronic use, nicotine induces pharmacodynamic changes in brain circuits, which is thought to lead to a reduction in sensitivity to the aversive properties of the drug. Nicotine is also a powerful reinforcer that leads to the conditioning of secondary cues associated with the drug-taking experience (such as cigarette pack, sensory properties of cigarette smoke and feel of the cigarette in the hand or mouth), which serves to enhance the incentive salience of these environmental factors and drive further drug intake. When the individual enters into states of abstinence (such as daily during sleep at night or during quit attempts), withdrawal symptomology is experienced, which may include irritability, restlessness, learning or memory deficits, difficulty concentrating, anxiety and hunger. These negative affective and cognitive symptoms lead to an intensification of the individual’s preoccupation to obtain and use the tobacco/nicotine product, and subsequently such intense craving can lead to relapse.
The rewarding actions of nicotine have largely been attributed to the mesolimbic pathway, which consists of dopaminergic neurons in the ventral tegmental area (VTA) that project to the nucleus accumbens and prefrontal cortex 60 , 61 , 62 (Fig. 5 ). VTA integrating circuits and projection regions express several nAChR subtypes on dopaminergic, GABAergic, and glutamatergic neurons 63 , 64 . Ultimately, administration of nicotine increases dopamine levels through increased dopaminergic neuron firing in striatal and extrastriatal areas (such as the ventral pallidum) 65 (Fig. 6 ). This effect is involved in reward and is believed to be primarily mediated by the action of nicotine on α 4 -containing and β 2 -containing nAChRs in the VTA 66 , 67 .
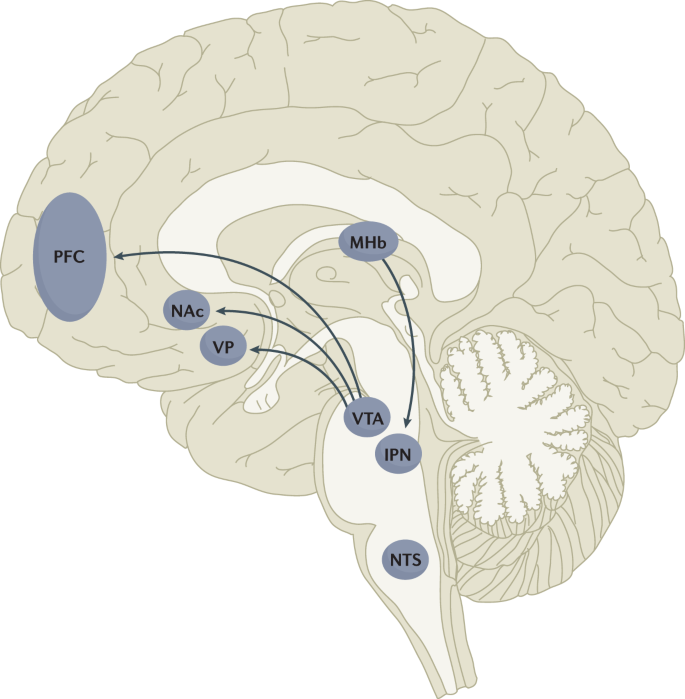
Multiple lines of research have demonstrated that nicotine reinforcement is mainly controlled by two brain pathways, which relay predominantly reward-related or aversion-related signals. The rewarding properties of nicotine that promote drug intake involve the mesolimbic dopamine projection from the ventral tegmental area (VTA) to the nucleus accumbens (NAc). By contrast, the aversive properties of nicotine that limit drug intake and mitigate withdrawal symptoms involve the fasciculus retroflexus projection from the medial habenula (MHb) to the interpeduncular nucleus (IPN). Additional brain regions have also been implicated in various aspects of nicotine dependence, such as the prefrontal cortex (PFC), ventral pallidum (VP), nucleus tractus solitarius (NTS) and insula (not shown here for clarity). All of these brain regions are directly or indirectly interconnected as integrative circuits to drive drug-seeking and drug-taking behaviours.
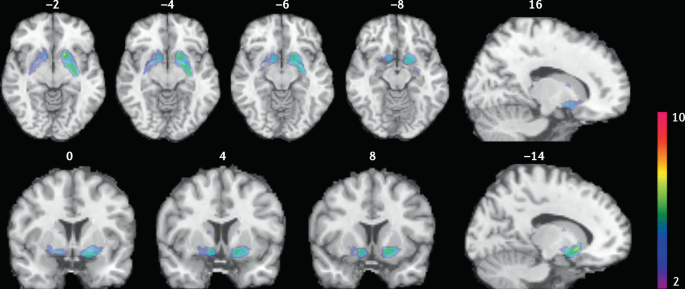
Smokers received brain PET scans with [ 11 C]PHNO, a dopamine D 2/3 PET tracer that has high sensitivity in detecting fluctuations of dopamine. PET scans were performed during abstinence or after smoking a cigarette. Reduced binding potential (BP ND ) was observed after smoking, indicating increased dopamine levels in the ventral striatum and in the area that corresponds to the ventral pallidum. The images show clusters with statistically significant decreases of [ 11 C]PHNO BP ND after smoking a cigarette versus abstinence condition. Those clusters have been superimposed on structural T1 MRI images of the brain. Reprinted from ref. 65 , Springer Nature Limited.
The aversive properties of nicotine are mediated by neurons in the MHb, which project to the IPN. Studies in rodents using genetic knockdown and knockout strategies demonstrated that the α 5 -containing, α 3 -containing and β 4 -containing nAChRs in the MHb–IPN pathway mediate the aversive properties of nicotine that limit drug intake, especially when animals are given the opportunity to consume higher nicotine doses 68 , 69 , 70 , 71 , 72 . In addition to nAChRs, other signalling factors acting on the MHb terminals in the IPN also regulate the actions of nicotine. For instance, under conditions of chronic nicotine exposure or with optogenetic activation of IPN neurons, a subtype of IPN neurons co-expressing Chrna5 (encoding the α 5 nAChR subunit) and Amigo1 (encoding adhesion molecule with immunoglobulin-like domain 1) release nitric oxide from the cell body that retrogradely inhibits MHb axon terminals 70 . In addition, nicotine activates α 5 -containing nAChR-expressing neurons that project from the nucleus tractus solitarius to the IPN, leading to release of glucagon-like peptide-1 that binds to GLP receptors on habenular axon terminals, which subsequently increases IPN neuron activation and decreases nicotine self-administration 73 . Taken together, these findings suggest a dynamic signalling process at MHb axonal terminals in the IPN, which regulates the addictive properties of nicotine and determines the amount of nicotine that is self-administered.
Nicotine withdrawal in animal models can be assessed by examining somatic signs (such as shaking, scratching, head nods and chewing) and affective signs (such as increased anxiety-related behaviours and conditioned place aversion). Interestingly, few nicotine withdrawal somatic signs are found in mice with genetic knockout of the α 2 , α 5 or β 4 nAChR subunits 74 , 75 . By contrast, β 2 nAChR-knockout mice have fewer anxiety-related behaviours during nicotine withdrawal, with no differences in somatic symptoms compared with wild-type mice 74 , 76 .
In addition to the VTA (mediating reward) and the MHb–IPN pathway (mediating aversion), other brain areas are involved in nicotine addiction (Fig. 5 ). In animals, the insular cortex controls nicotine taking and nicotine seeking 77 . Moreover, humans with lesions of the insular cortex can quit smoking easily without relapse 78 . This finding led to the development of a novel therapeutic intervention modulating insula function (see Management, below) 79 , 80 . Various brain areas (shell of nucleus accumbens, basolateral amygdala and prelimbic cortex) expressing cannabinoid CB 1 receptors are also critical in controlling rewarding effects and relapse 81 , 82 . The α 1 -adrenergic receptor expressed in the cortex also control these effects, probably through glutamatergic afferents to the nucleus accumbens 83 .
Individual differences in nicotine addiction risk
Vulnerability to nicotine dependence varies between individuals, and the reasons for these differences are multidimensional. Many social factors (such as education level and income) play a role 84 . Broad psychological and social factors also modulate this risk. For example, peer smoking status, knowledge on effect of tobacco, expectation on social acceptance, exposure to passive smoking modulate the risk of initiating tobacco use 85 , 86 .
Genetic factors have a role in smoking initiation, the development of nicotine addiction and the likelihood of smoking cessation. Indeed, heritability has been estimated to contribute to approximatively half of the variability in nicotine dependence 87 , 88 , 89 , 90 . Important advances in our understanding of such genetic contributions have evolved with large-scale genome-wide association studies of smokers and non-smokers. One of the most striking findings has been that allelic variation in the CHRNA5 – CHRNA3 – CHRNB4 gene cluster, which encodes α 5 , α 3 and β 4 nAChR subunits, correlates with an increased vulnerability for nicotine addiction, indicated by a higher likelihood of becoming dependent on nicotine and smoking a greater number of cigarettes per day 91 , 92 , 93 , 94 , 95 . The most significant effect has been found for a single-nucleotide polymorphism in CHRNA5 (rs16969968), which results in an amino acid change and reduced function of α 5 -containing nAChRs 92 .
Allelic variation in CYP2A6 (encoding the CYP2A6 enzyme, which metabolizes nicotine) has also been associated with differential vulnerability to nicotine dependence 96 , 97 , 98 . CYP2A6 is highly polymorphic, resulting in variable enzymatic activity 96 , 99 , 100 . Individuals with allelic variation that results in slow nicotine metabolism consume less nicotine per day, experience less-severe withdrawal symptoms and are more successful at quitting smoking than individuals with normal or fast metabolism 101 , 102 , 103 , 104 . Moreover, individuals with slow nicotine metabolism have lower dopaminergic receptor expression in the dopamine D2 regions of the associative striatum and sensorimotor striatum in PET studies 105 and take fewer puffs of nicotine-containing cigarettes (compared with de-nicotinized cigarettes) in a forced choice task 106 . Slower nicotine metabolism is thought to increase the duration of action of nicotine, allowing nicotine levels to accumulate over time, therefore enabling lower levels of intake to sustain activation of nAChRs 107 .
Large-scale genetic studies have identified hundreds of other genetic loci that influence smoking initiation, age of smoking initiation, cigarettes smoked per day and successful smoking cessation 108 . The strongest genetic contributions to smoking through the nicotinic receptors and nicotine metabolism are among the strongest genetic contributors to lung cancer 109 . Other genetic variations (such as those related to cannabinoid, dopamine receptors or other neurotransmitters) may affect certain phenotypes related to smoking (such as nicotine preference and cue-reactivity) 110 , 111 , 112 , 113 , 114 , 115 .
Diagnosis, screening and prevention
Screening for cigarette smoking.
Screening for cigarette smoking should happen at every doctor’s visit 116 . In this regard, a simple and direct question about a person’s tobacco use can provide an opportunity to offer information about its potential risks and treatments to assist in quitting. All smokers should be offered assistance in quitting because even low levels of smoking present a significant health risk 33 , 117 , 118 . Smoking status can be assessed by self-categorization or self-reported assessment of smoking behaviour (Table 1 ). In people who smoke, smoking frequency can be assessed 119 and a combined quantity frequency measure such as pack-year history (that is, average number of cigarettes smoked per day multiplied by the number of years, divided by 20), can be used to estimate cumulative risk of adverse health outcomes. The Association for the Treatment of Tobacco Use and Dependence recommends that all electronic health records should document smoking status using the self-report categories listed in Table 1 .
Owing to the advent of e-cigarettes and heat-not-burn products, and the popularity of little cigars in the US that mimic combustible cigarettes, people who use tobacco may use multiple products concurrently 120 , 121 . Thus, screening for other nicotine and tobacco product use is important in clinical practice. The self-categorization approach can also be used to describe the use of these other products.
Traditionally tobacco use has been classified according to whether the smoker meets criteria for nicotine dependence in one of the two main diagnostic classifications: the DSM 122 (tobacco use disorder) and the ICD (tobacco dependence) 123 . The diagnosis of tobacco use disorder according to DSM-5 criteria requires the presence of at least 2 of 11 symptoms that have produced marked clinical impairment or distress within a 12-month period (Box 2 ). Of note, these symptoms are similar for all substance use disorder diagnoses and may not all be relevant to tobacco use disorder (such as failure to complete life roles). In the ICD-10, codes allow the identification of specific tobacco products used (cigarettes, chewing tobacco and other tobacco products).
Dependence can also be assessed as a continuous construct associated with higher levels of use, greater withdrawal and reduced likelihood of quitting. The level of dependence can be assessed with the Fagerström Test for Nicotine Dependence, a short questionnaire comprising six questions 124 (Box 2 ). A score of ≥4 indicates moderate to high dependence. As very limited time may be available in clinical consultations, the Heaviness of Smoking Index (HSI) was developed, which comprises two questions on the number of cigarettes smoked per day and how soon after waking the first cigarette is smoked 125 . The HSI can guide dosing for nicotine replacement therapy (NRT).
Other measures of cigarette dependence have been developed but are not used in the clinical setting, such as the Cigarette Dependence Scale 126 , Hooked on Nicotine Checklist 127 , Nicotine Dependence Syndrome Scale 128 , the Wisconsin Inventory of Smoking Dependence Motives (Brief) 129 and the Penn State Cigarette Dependence Index 130 . However, in practice, these are not often used, as the most important aspect is to screen for smoking and encourage all smokers to quit smoking regardless of their dependence status.
Box 2 DSM-5 criteria for tobacco use disorder and items of the Fagerström Test for nicotine dependence
DSM-5 (ref. 122 )
Taxonomic and diagnostic tool for tobacco use disorder published by the American Psychiatric Association.
A problematic pattern of tobacco use leading to clinically significant impairment or distress as manifested by at least two of the following, occurring within a 12-month period.
Tobacco often used in larger amounts or over a longer period of time than intended
A persistent desire or unsuccessful efforts to reduce or control tobacco use
A great deal of time spent in activities necessary to obtain or use tobacco
Craving, or a strong desire or urge to use tobacco
Recurrent tobacco use resulting in a failure to fulfil major role obligations at work, school or home
Continued tobacco use despite having persistent or recurrent social or interpersonal problems caused or exacerbated by the effects of tobacco (for example, arguments with others about tobacco use)
Important social, occupational or recreational activities given up or reduced because of tobacco use
Recurrent tobacco use in hazardous situations (such as smoking in bed)
Tobacco use continued despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by tobacco use
Tolerance, defined by either of the following.
A need for markedly increased amounts of tobacco to achieve the desired effect
A markedly diminished effect with continued use of the same amount of tobacco
Withdrawal, manifesting as either of the following.
Withdrawal syndrome for tobacco
Tobacco (or a closely related substance, such as nicotine) taken to relieve or avoid withdrawal symptoms
Fagerström Test for Nicotine Dependence 124
A standard instrument for assessing the intensity of physical addiction to nicotine.
How soon after you wake up do you smoke your first cigarette?
Within 5 min (scores 3 points)
5 to 30 min (scores 2 points)
31 to 60 min (scores 1 point)
After 60 min (scores 0 points)
Do you find it difficult not to smoke in places where you should not, such as in church or school, in a movie, at the library, on a bus, in court or in a hospital?
Yes (scores 1 point)
No (scores 0 points)
Which cigarette would you most hate to give up; which cigarette do you treasure the most?
The first one in the morning (scores 1 point)
Any other one (scores 0 points)
How many cigarettes do you smoke each day?
10 or fewer (scores 0 points)
11 to 20 (scores 1 point)
21 to 30 (scores 2 points)
31 or more (scores 3 points)
Do you smoke more during the first few hours after waking up than during the rest of the day?
Do you still smoke if you are so sick that you are in bed most of the day or if you have a cold or the flu and have trouble breathing?
A score of 7–10 points is classified as highly dependent; 4–6 points is classified as moderately dependent; <4 points is classified as minimally dependent.
DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.
Young people who do not start smoking cigarettes between 15 and 25 years of age have a very low risk of ever smoking 24 , 131 , 132 . This age group provides a critical opportunity to prevent cigarette smoking using effective, evidence-based strategies to prevent smoking initiation and reduce escalation from experimentation to regular use 131 , 132 , 133 , 134 , 135 .
Effective prevention of cigarette uptake requires a comprehensive package of cost-effective policies 134 , 136 , 137 to synergistically reduce the population prevalence of cigarette smoking 131 , 135 . These policies include high rates of tobacco taxation 30 , 134 , 137 , 138 , widespread and rigorously enforced smoke-free policies 139 , bans on tobacco advertising and promotions 140 , use of plain packaging and graphic warnings about the health risks of smoking 135 , 141 , mass media and peer-based education programmes to discourage smoking, and enforcement of laws against the sale of cigarettes to young people below the minimum legal purchase age 131 , 135 . These policies make cigarettes less available and affordable to young people. Moreover, these policies make it more difficult for young people to purchase cigarettes and make smoking a much less socially acceptable practice. Of note, these policies are typically mostly enacted in HICs, which may be related to the declining prevalence of smoking in these countries, compared with the prevalence in LMICs.
Pharmacotherapy
Three evidence-based classes of pharmacotherapy are available for smoking cessation: NRT (using nicotine-based patches, gum, lozenges, mini-lozenges, nasal sprays and inhalers), varenicline (a nAChR partial agonist), and bupropion (a noradrenaline/dopamine reuptake inhibitor that also inhibits nAChR function and is also used as an antidepressant). These FDA-approved and EMA-approved pharmacotherapies are cost-effective smoking cessation treatments that double or triple successful abstinence rates compared with no treatment or placebo controls 116 , 142 .
Combinations of pharmacotherapies are also effective for smoking cessation 116 , 142 . For example, combining NRTs (such as the steady-state nicotine patch and as-needed NRT such as gum or mini-lozenge) is more effective than a single form of NRT 116 , 142 , 143 . Combining NRT and varenicline is the most effective smoking cessation pharmacotherapy 116 , 142 , 143 . Combining FDA-approved pharmacotherapy with behavioural counselling further increases the likelihood of successful cessation 142 . Second-line pharmacotherapies (for example, nortriptyline) have some potential for smoking cessation, but their use is limited due to their tolerability profile.
All smokers should receive pharmacotherapy to help them quit smoking, except those in whom pharmacotherapy has insufficient evidence of effectiveness (among adolescents, smokeless tobacco users, pregnant women or light smokers) or those in whom pharmacotherapy is medically contraindicated 144 . Table 2 provides specific information regarding dosing and duration for each FDA-approved pharmacotherapy. Extended use of pharmacotherapy beyond the standard 12-week regimen after cessation is effective and should be considered 116 . Moreover, preloading pharmacotherapy (that is, initiating cessation medication in advance of a quit attempt), especially with the nicotine patch, is a promising treatment, although further studies are required to confirm efficacy.
Cytisine has been used for smoking cessation in Eastern Europe for a long time and is available in some countries (such as Canada) without prescription 145 . Cytisine is a partial agonist of nAChRs and its structure was the precursor for the development of varenicline 145 . Cytisine is at least as effective as some approved pharmacotherapies for smoking cessation, such as NRT 146 , 147 , 148 , and the role of cytisine in smoking cessation is likely to expand in the future, notably owing to its much lower cost than traditional pharmacotherapies. E-cigarettes also have the potential to be useful as smoking cessation devices 149 , 150 . The 2020 US Surgeon General’s Report concluded that there was insufficient evidence to promote cytisine or e-cigarettes as effective smoking cessation treatments, but in the UK its use is recommended for smoking cessation (see ref. 15 for regularly updated review).
Counselling and behavioural treatments
Psychosocial counselling significantly increases the likelihood of successful cessation, especially when combined with pharmacotherapy. Even a counselling session lasting only 3 minutes can help smokers quit 116 , although the 2008 US Public Health Service guidelines and the Preventive Services Task Force 151 each concluded that more intensive counselling (≥20 min per session) is more effective than less intensive counselling (<20 min per session). Higher smoking cessation rates are obtained by using behavioural change techniques that target associative and self-regulatory processes 152 . In addition, behavioural change techniques that will favour commitment, social reward and identity associated with changed behaviour seems associated with higher success rates 152 . Evidence-based counselling focuses on providing social support during treatment, building skills to cope with withdrawal and cessation, and problem-solving in challenging situations 116 , 153 . Effective counselling can be delivered by diverse providers (such as physicians, nurses, pharmacists, social workers, psychologists and certified tobacco treatment specialists) 116 .
Counselling can be delivered in a variety of modalities. In-person individual and group counselling are effective, as is telephone counselling (quit lines) 142 . Internet and text-based intervention seem to be effective in smoking cessation, especially when they are interactive and tailored to a smoker’s specific circumstances 142 . Over the past several years, the number of smoking cessation smartphone apps has increased, but there the evidence that the use of these apps significantly increases smoking cessation rates is not sufficient.
Contingency management (providing financial incentives for abstinence or engagement in treatment) has shown promising results 154 , 155 but its effects are not sustained once the contingencies are removed 155 , 156 . Other treatments such as hypnosis, acupuncture and laser treatment have not been shown to improve smoking cessation rates compared with placebo treatments 116 . Moreover, no solid evidence supports the use of conventional transcranial magnetic stimulation (TMS) for long-term smoking cessation 157 , 158 .
Although a variety of empirically supported smoking cessation interventions are available, more than two-thirds of adult smokers who made quit attempts in the USA during the past year did not use an evidence-based treatment and the rate is likely to be lower in many other countries 142 . This speaks to the need to increase awareness of, and access to, effective cessation aids among all smokers.
Brain stimulation
The insula (part of the frontal cortex) is a critical brain structure involved in cigarette craving and relapse 78 , 79 . The activity of the insula can be modulated using an innovative approach called deep insula/prefrontal cortex TMS (deep TMS), which is effective in helping people quit smoking 80 , 159 . This approach has now been approved by the FDA as an effective smoking cessation intervention 80 . However, although this intervention was developed and is effective for smoking cessation, the number of people with access to it is limited owing to the limited number of sites equipped and with trained personnel, and the cost of this intervention.
Quality of life
Generic instruments (such as the Short-Form (SF-36) Health Survey) can be used to evaluate quality of life (QOL) in smokers. People who smoke rate their QOL lower than people who do not smoke both before and after they become smokers 160 , 161 . QOL improves when smokers quit 162 . Mental health may also improve on quitting smoking 163 . Moreover, QOL is much poorer in smokers with tobacco-related diseases, such as chronic respiratory diseases and cancers, than in individuals without tobacco-related diseases 161 , 164 . The dimensions of QOL that show the largest decrements in people who smoke are those related to physical health, day-to-day activities and mental health such as depression 160 . Smoking also increases the risk of diabetes mellitus 165 , 166 , which is a major determinant of poor QOL for a wide range of conditions.
The high toll of premature death from cigarette smoking can obscure the fact that many of the diseases that cause these deaths also produce substantial disability in the years before death 1 . Indeed, death in smokers is typically preceded by several years of living with the serious disability and impairment of everyday activities caused by chronic respiratory disease, heart disease and cancer 2 . Smokers’ QOL in these years may also be adversely affected by the adverse effects of the medical treatments that they receive for these smoking-related diseases (such as major surgery and radiotherapy).
Expanding cessation worldwide
The major global challenge is to consider individual and population-based strategies that could increase the substantially low rates of adult cessation in most LMICs and indeed strategies to ensure that even in HICs, cessation continues to increase. In general, the most effective tools recommended by WHO to expand cessation are the same tools that can prevent smoking initiation, notably higher tobacco taxes, bans on advertising and promotion, prominent warning labels or plain packaging, bans on public smoking, and mass media and educational efforts 29 , 167 . The effective use of these policies, particularly taxation, lags behind in most LMICs compared with most HICs, with important exceptions such as Brazil 167 . Access to effective pharmacotherapies and counselling as well as support for co-existing mental health conditions would also be required to accelerate cessation in LMICs. This is particularly important as smokers living in LMICs often have no access to the full range of effective treatment options.
Regulating access to e-cigarettes
How e-cigarettes should be used is debated within the tobacco control field. In some countries (for example, the UK), the use of e-cigarettes as a cigarette smoking cessation aid and as a harm reduction strategy is supported, based on the idea that e-cigarette use will lead to much less exposure to toxic compounds than tobacco use, therefore reducing global harm. In other countries (for example, the USA), there is more concern with preventing the increased use of e-cigarettes by youths that may subsequently lead to smoking 25 , 26 . Regulating e-cigarettes in nuanced ways that enable smokers to access those products whilst preventing their uptake among youths is critical.
Regulating nicotine content in tobacco products
Reducing the nicotine content of cigarettes could potentially produce less addictive products that would allow a gradual reduction in the population prevalence of smoking. Some clinical studies have found no compensatory increase in smoking whilst providing access to low nicotine tobacco 168 . Future regulation may be implemented to gradually decrease the nicotine content of combustible tobacco and other nicotine products 169 , 170 , 171 .
Tobacco end games
Some individuals have proposed getting rid of commercial tobacco products this century or using the major economic disruption arising from the COVID-19 pandemic to accelerate the demise of the tobacco industry 172 , 173 . Some tobacco producers have even proposed this strategy as an internal goal, with the idea of switching to nicotine delivery systems that are less harmful ( Philip Morris International ). Some countries are moving towards such an objective; for example, in New Zealand, the goal that fewer than 5% of New Zealanders will be smokers in 2025 has been set (ref. 174 ). The tobacco end-game approach would overall be the best approach to reduce the burden of tobacco use on society, but it would require coordination of multiple countries and strong public and private consensus on the strategy to avoid a major expansion of the existing illicit market in tobacco products in some countries.
Innovative interventions
The COVID-19 pandemic has shown that large-scale investment in research can lead to rapid development of successful therapeutic interventions. By contrast, smoking cessation has been underfunded compared with the contribution that it makes to the global burden of disease. In addition, there is limited coordination between research teams and most studies are small-scale and often underpowered 79 . It is time to fund an ambitious, coordinated programme of research to test the most promising therapies based on an increased understanding of the neurobiological basis of smoking and nicotine addiction (Table 3 ). Many of those ideas have not yet been tested properly and this could be carried out by a coordinated programme of research at the international level.
GBD 2019 Tobacco Collaborators. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet 397 , 2337–2360 (2021). This study summarizes the burden of disease induced by tobacco worldwide .
Google Scholar
West, R. Tobacco smoking: health impact, prevalence, correlates and interventions. Psychol. Health 32 , 1018–1036 (2017).
PubMed PubMed Central Google Scholar
West, R. The multiple facets of cigarette addiction and what they mean for encouraging and helping smokers to stop. COPD 6 , 277–283 (2009).
PubMed Google Scholar
Fagerström, K. Determinants of tobacco use and renaming the FTND to the Fagerström test for cigarette dependence. Nicotine Tob. Res. 14 , 75–78 (2012).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General (Centers for Disease Control and Prevention, 2014).
Doll, R. & Hill, A. B. Smoking and carcinoma of the lung; preliminary report. Br. Med. J. 2 , 739–748 (1950).
CAS PubMed PubMed Central Google Scholar
Royal College of Physicians. Smoking and health. Summary of a report of the Royal College of Physicians of London on smoking in relation to cancer of the lung and other diseases (Pitman Medical Publishing, 1962).
Henningfield, J. E., Smith, T. T., Kleykamp, B. A., Fant, R. V. & Donny, E. C. Nicotine self-administration research: the legacy of Steven R. Goldberg and implications for regulation, health policy, and research. Psychopharmacology 233 , 3829–3848 (2016).
Le Foll, B. & Goldberg, S. R. Effects of nicotine in experimental animals and humans: an update on addictive properties. Hand. Exp. Pharmacol. https://doi.org/10.1007/978-3-540-69248-5_12 (2009).
Article Google Scholar
Proctor, R. N. The history of the discovery of the cigarette–lung cancer link: evidentiary traditions, corporate denial, global toll. Tob. Control. 21 , 87–91 (2012).
Hall, B. J. et al. Differential effects of non-nicotine tobacco constituent compounds on nicotine self-administration in rats. Pharmacol. Biochem. Behav. 120 , 103–108 (2014).
Musso, F. et al. Smoking impacts on prefrontal attentional network function in young adult brains. Psychopharmacology 191 , 159–169 (2007).
CAS PubMed Google Scholar
Goriounova, N. A. & Mansvelder, H. D. Short- and long-term consequences of nicotine exposure during adolescence for prefrontal cortex neuronal network function. Cold Spring Harb. Perspect. Med. 2 , a012120 (2012).
Fagerström, K. O. & Bridgman, K. Tobacco harm reduction: the need for new products that can compete with cigarettes. Addictive Behav. 39 , 507–511 (2014).
Hartmann-Boyce, J. et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 9 , CD010216 (2021).
Jha, P. The hazards of smoking and the benefits of cessation: a critical summation of the epidemiological evidence in high-income countries. eLife https://doi.org/10.7554/eLife.49979 (2020).
Article PubMed PubMed Central Google Scholar
Palipudi, K. M. et al. Social determinants of health and tobacco use in thirteen low and middle income countries: evidence from Global Adult Tobacco Survey. PLoS ONE 7 , e33466 (2012).
Goodwin, R. D., Pagura, J., Spiwak, R., Lemeshow, A. R. & Sareen, J. Predictors of persistent nicotine dependence among adults in the United States. Drug Alcohol. Depend. 118 , 127–133 (2011).
Weinberger, A. H. et al. Cigarette use is increasing among people with illicit substance use disorders in the United States, 2002-14: emerging disparities in vulnerable populations. Addiction 113 , 719–728 (2018).
Evans-Polce, R. J., Kcomt, L., Veliz, P. T., Boyd, C. J. & McCabe, S. E. Alcohol, tobacco, and comorbid psychiatric disorders and associations with sexual identity and stress-related correlates. Am. J. Psychiatry 177 , 1073–1081 (2020).
Hassan, A. N. & Le Foll, B. Survival probabilities and predictors of major depressive episode incidence among individuals with various types of substance use disorders. J. Clin. Psychiatry https://doi.org/10.4088/JCP.20m13637 (2021).
Article PubMed Google Scholar
Smith, P. H., Mazure, C. M. & McKee, S. A. Smoking and mental illness in the U.S. population. Tob. Control. 23 , e147–e153 (2014).
Bourgault, Z., Rubin-Kahana, D. S., Hassan, A. N., Sanches, M. & Le Foll, B. Multiple substance use disorders and self-reported cognitive function in U.S. adults: associations and sex-differences in a nationally representative sample. Front. Psychiatry https://doi.org/10.3389/fpsyt.2021.797578 (2022).
Reitsma, M. B. et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and initiation among young people in 204 countries and territories, 1990-2019. Lancet Public Health 6 , e472–e481 (2021).
Warner, K. E. How to think–not feel–about tobacco harm reduction. Nicotine Tob. Res. 21 , 1299–1309 (2019).
Soneji, S. et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 171 , 788–797 (2017).
Levy, D. T. et al. Examining the relationship of vaping to smoking initiation among US youth and young adults: a reality check. Tob. Control. 28 , 629–635 (2019).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking — 50 years of progress: a report of the Surgeon General (Centers for Disease Control and Prevention, 2014).
Jha, P. & Peto, R. Global effects of smoking, of quitting, and of taxing tobacco. N. Engl. J. Med. 370 , 60–68 (2014). This review covers the impact of tobacco, of quitting smoking and the importance of taxation to impact prevalence of smoking .
Jha, P. & Peto., R. in Tobacco Tax Reform: At the Crossroads of Health and Development . (eds Marquez, P. V. & Moreno-Dodson, B.) 55–72 (World Bank Group, 2017).
Jha, P. et al. 21st-century hazards of smoking and benefits of cessation in the United States. N. Engl. J. Med. 368 , 341–350 (2013).
Banks, E. et al. Tobacco smoking and all-cause mortality in a large Australian cohort study: findings from a mature epidemic with current low smoking prevalence. BMC Med. 13 , 38 (2015).
Pirie, K. et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet 381 , 133–141 (2013).
Jha, P. et al. A nationally representative case-control study of smoking and death in India. N. Engl. J. Med. 358 , 1137–1147 (2008).
Chan, E. D. et al. Tobacco exposure and susceptibility to tuberculosis: is there a smoking gun? Tuberculosis 94 , 544–550 (2014).
Wang, M. G. et al. Association between tobacco smoking and drug-resistant tuberculosis. Infect. Drug Resist. 11 , 873–887 (2018).
Jha, P. et al. Social inequalities in male mortality, and in male mortality from smoking: indirect estimation from national death rates in England and Wales, Poland, and North America. Lancet 368 , 367–370 (2006).
Jha, P., Gelband, H, Irving, H. & Mishra, S. in Reducing Social Inequalities in Cancer: Evidence and Priorities for Research (eds Vaccarella, S et al.) 161–166 (IARC, 2018).
Jha, P. Expanding smoking cessation world-wide. Addiction 113 , 1392–1393 (2018).
Jha, P. Avoidable global cancer deaths and total deaths from smoking. Nat. Rev. Cancer 9 , 655–664 (2009).
Wittenberg, R. E., Wolfman, S. L., De Biasi, M. & Dani, J. A. Nicotinic acetylcholine receptors and nicotine addiction: a brief introduction. Neuropharmacology 177 , 108256 (2020).
Boulter, J. et al. Functional expression of two neuronal nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc. Natl Acad. Sci. USA 84 , 7763–7767 (1987).
Couturier, S. et al. A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homo-oligomeric channel blocked by α-BTX. Neuron 5 , 847–856 (1990).
Picciotto, M. R., Addy, N. A., Mineur, Y. S. & Brunzell, D. H. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog. Neurobiol. 84 , 329–342 (2008).
Changeux, J. P. Structural identification of the nicotinic receptor ion channel. Trends Neurosci. 41 , 67–70 (2018).
McKay, B. E., Placzek, A. N. & Dani, J. A. Regulation of synaptic transmission and plasticity by neuronal nicotinic acetylcholine receptors. Biochem. Pharmacol. 74 , 1120–1133 (2007).
Wonnacott, S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 20 , 92–98 (1997).
Wooltorton, J. R., Pidoplichko, V. I., Broide, R. S. & Dani, J. A. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J. Neurosci. 23 , 3176–3185 (2003).
Gipson, C. D. & Fowler, C. D. Nicotinic receptors underlying nicotine dependence: evidence from transgenic mouse models. Curr. Top. Behav. Neurosci. 45 , 101–121 (2020).
Hamouda, A. K. et al. Potentiation of (α4)2(β2)3, but not (α4)3(β2)2, nicotinic acetylcholine receptors reduces nicotine self-administration and withdrawal symptoms. Neuropharmacology 190 , 108568 (2021).
Lallai, V. et al. Nicotine acts on cholinergic signaling mechanisms to directly modulate choroid plexus function. eNeuro https://doi.org/10.1523/ENEURO.0051-19.2019 (2019).
Benwell, M. E., Balfour, D. J. & Anderson, J. M. Evidence that tobacco smoking increases the density of (-)-[3H]nicotine binding sites in human brain. J. Neurochem. 50 , 1243–1247 (1988).
Perry, D. C., Davila-Garcia, M. I., Stockmeier, C. A. & Kellar, K. J. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J. Pharmacol. Exp. Ther. 289 , 1545–1552 (1999).
Marks, M. J. et al. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J. Neurosci. 12 , 2765–2784 (1992).
Le Foll, B. et al. Impact of short access nicotine self-administration on expression of α4β2* nicotinic acetylcholine receptors in non-human primates. Psychopharmacology 233 , 1829–1835 (2016).
Meyers, E. E., Loetz, E. C. & Marks, M. J. Differential expression of the beta4 neuronal nicotinic receptor subunit affects tolerance development and nicotinic binding sites following chronic nicotine treatment. Pharmacol. Biochem. Behav. 130 , 1–8 (2015).
Zhao-Shea, R., Liu, L., Pang, X., Gardner, P. D. & Tapper, A. R. Activation of GABAergic neurons in the interpeduncular nucleus triggers physical nicotine withdrawal symptoms. Curr. Biol. 23 , 2327–2335 (2013).
Jensen, K. P., Valentine, G., Gueorguieva, R. & Sofuoglu, M. Differential effects of nicotine delivery rate on subjective drug effects, urges to smoke, heart rate and blood pressure in tobacco smokers. Psychopharmacology 237 , 1359–1369 (2020).
Villanueva, H. F., James, J. R. & Rosecrans, J. A. Evidence of pharmacological tolerance to nicotine. NIDA Res. Monogr. 95 , 349–350 (1989).
Corrigall, W. A., Coen, K. M. & Adamson, K. L. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 653 , 278–284 (1994).
Nisell, M., Nomikos, G. G., Hertel, P., Panagis, G. & Svensson, T. H. Condition-independent sensitization of locomotor stimulation and mesocortical dopamine release following chronic nicotine treatment in the rat. Synapse 22 , 369–381 (1996).
Rice, M. E. & Cragg, S. J. Nicotine amplifies reward-related dopamine signals in striatum. Nat. Neurosci. 7 , 583–584 (2004).
Mameli-Engvall, M. et al. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron 50 , 911–921 (2006).
Picciotto, M. R., Higley, M. J. & Mineur, Y. S. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76 , 116–129 (2012).
Le Foll, B. et al. Elevation of dopamine induced by cigarette smoking: novel insights from a [11C]-+-PHNO PET study in humans. Neuropsychopharmacology 39 , 415–424 (2014). This brain imaging study identified the brain areas in which smoking elevates dopamine levels .
Maskos, U. et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436 , 103–107 (2005). This article discusses the implication of the β 2 - containing nAChRs in the VTA in mammalian cognitive function .
Picciotto, M. R. et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391 , 173–177 (1998). This article discusses the implication of the β 2 - containing nAChRs in addictive effects of nicotine .
Fowler, C. D., Lu, Q., Johnson, P. M., Marks, M. J. & Kenny, P. J. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature 471 , 597–601 (2011). This article discusses the implication of the α5 nicotinic receptor located in the MHb in a mechanism mediating the aversive effects of nicotine .
Elayouby, K. S. et al. α3* Nicotinic acetylcholine receptors in the habenula-interpeduncular nucleus circuit regulate nicotine intake. J. Neurosci. https://doi.org/10.1523/JNEUROSCI.0127-19.2020 (2020).
Ables, J. L. et al. Retrograde inhibition by a specific subset of interpeduncular α5 nicotinic neurons regulates nicotine preference. Proc. Natl Acad. Sci. USA 114 , 13012–13017 (2017).
Frahm, S. et al. Aversion to nicotine is regulated by the balanced activity of β4 and α5 nicotinic receptor subunits in the medial habenula. Neuron 70 , 522–535 (2011).
Jackson, K. J. et al. Role of α5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J. Pharmacol. Exp. Ther. 334 , 137–146 (2010).
Tuesta, L. M. et al. GLP-1 acts on habenular avoidance circuits to control nicotine intake. Nat. Neurosci. 20 , 708–716 (2017).
Salas, R., Pieri, F. & De Biasi, M. Decreased signs of nicotine withdrawal in mice null for the β4 nicotinic acetylcholine receptor subunit. J. Neurosci. 24 , 10035–10039 (2004).
Salas, R., Sturm, R., Boulter, J. & De Biasi, M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J. Neurosci. 29 , 3014–3018 (2009).
Jackson, K. J., Martin, B. R., Changeux, J. P. & Damaj, M. I. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J. Pharmacol. Exp. Ther. 325 , 302–312 (2008).
Le Foll, B. et al. Translational strategies for therapeutic development in nicotine addiction: rethinking the conventional bench to bedside approach. Prog. Neuropsychopharmacol. Biol. Psychiatry 52 , 86–93 (2014).
Naqvi, N. H., Rudrauf, D., Damasio, H. & Bechara, A. Damage to the insula disrupts addiction to cigarette smoking. Science 315 , 531–534 (2007). This article discusses the implication of the insular cortex in tobacco addiction .
Ibrahim, C. et al. The insula: a brain stimulation target for the treatment of addiction. Front. Pharmacol. 10 , 720 (2019).
Zangen, A. et al. Repetitive transcranial magnetic stimulation for smoking cessation: a pivotal multicenter double-blind randomized controlled trial. World Psychiatry 20 , 397–404 (2021). This study validated the utility of deep insula/prefrontal cortex rTMS for smoking cessation .
Le Foll, B., Forget, B., Aubin, H. J. & Goldberg, S. R. Blocking cannabinoid CB1 receptors for the treatment of nicotine dependence: insights from pre-clinical and clinical studies. Addict. Biol. 13 , 239–252 (2008).
Kodas, E., Cohen, C., Louis, C. & Griebel, G. Cortico-limbic circuitry for conditioned nicotine-seeking behavior in rats involves endocannabinoid signaling. Psychopharmacology 194 , 161–171 (2007).
Forget, B. et al. Noradrenergic α1 receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology 35 , 1751–1760 (2010).
Garrett, B. E., Dube, S. R., Babb, S. & McAfee, T. Addressing the social determinants of health to reduce tobacco-related disparities. Nicotine Tob. Res. 17 , 892–897 (2015).
Polanska, K., Znyk, M. & Kaleta, D. Susceptibility to tobacco use and associated factors among youth in five central and eastern European countries. BMC Public Health 22 , 72 (2022).
Volkow, N. D. Personalizing the treatment of substance use disorders. Am. J. Psychiatry 177 , 113–116 (2020).
Li, M. D., Cheng, R., Ma, J. Z. & Swan, G. E. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction 98 , 23–31 (2003).
Carmelli, D., Swan, G. E., Robinette, D. & Fabsitz, R. Genetic influence on smoking–a study of male twins. N. Engl. J. Med. 327 , 829–833 (1992).
Broms, U., Silventoinen, K., Madden, P. A. F., Heath, A. C. & Kaprio, J. Genetic architecture of smoking behavior: a study of Finnish adult twins. Twin Res. Hum. Genet. 9 , 64–72 (2006).
Kendler, K. S., Thornton, L. M. & Pedersen, N. L. Tobacco consumption in Swedish twins reared apart and reared together. Arch. Gen. Psychiat 57 , 886–892 (2000).
Saccone, N. L. et al. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res. 69 , 6848–6856 (2009).
Bierut, L. J. et al. Variants in nicotinic receptors and risk for nicotine dependence. Am. J. Psychiatry 165 , 1163–1171 (2008). This study demonstrates that nAChR gene variants are important in nicotine addiction .
Bierut, L. J. et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum. Mol. Genet. 16 , 24–35 (2007).
Berrettini, W. et al. α-5/α-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol. Psychiatry 13 , 368–373 (2008).
Sherva, R. et al. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with ‘pleasurable buzz’ during early experimentation with smoking. Addiction 103 , 1544–1552 (2008).
Thorgeirsson, T. E. et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat. Genet. 42 , 448–453 (2010).
Ray, R., Tyndale, R. F. & Lerman, C. Nicotine dependence pharmacogenetics: role of genetic variation in nicotine-metabolizing enzymes. J. Neurogenet. 23 , 252–261 (2009).
Bergen, A. W. et al. Drug metabolizing enzyme and transporter gene variation, nicotine metabolism, prospective abstinence, and cigarette consumption. PLoS ONE 10 , e0126113 (2015).
Mwenifumbo, J. C. et al. Identification of novel CYP2A6*1B variants: the CYP2A6*1B allele is associated with faster in vivo nicotine metabolism. Clin. Pharmacol. Ther. 83 , 115–121 (2008).
Raunio, H. & Rahnasto-Rilla, M. CYP2A6: genetics, structure, regulation, and function. Drug Metab. Drug Interact. 27 , 73–88 (2012).
CAS Google Scholar
Patterson, F. et al. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin. Pharmacol. Ther. 84 , 320–325 (2008).
Rodriguez, S. et al. Combined analysis of CHRNA5, CHRNA3 and CYP2A6 in relation to adolescent smoking behaviour. J. Psychopharmacol. 25 , 915–923 (2011).
Strasser, A. A., Malaiyandi, V., Hoffmann, E., Tyndale, R. F. & Lerman, C. An association of CYP2A6 genotype and smoking topography. Nicotine Tob. Res. 9 , 511–518 (2007).
Liakoni, E. et al. Effects of nicotine metabolic rate on withdrawal symptoms and response to cigarette smoking after abstinence. Clin. Pharmacol. Ther. 105 , 641–651 (2019).
Di Ciano, P. et al. Influence of nicotine metabolism ratio on [11C]-(+)-PHNO PET binding in tobacco smokers. Int. J. Neuropsychopharmacol. 21 , 503–512 (2018).
Butler, K. et al. Impact of Cyp2a6 activity on nicotine reinforcement and cue-reactivity in daily smokers. Nicotine Tob. Res. https://doi.org/10.1093/ntr/ntab064 (2021).
Benowitz, N. L., Swan, G. E., Jacob, P. 3rd, Lessov-Schlaggar, C. N. & Tyndale, R. F. CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clin. Pharmacol. Ther. 80 , 457–467 (2006).
Liu, M. et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat. Genet. 51 , 237–244 (2019).
McKay, J. D. et al. Large-scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility across histological subtypes. Nat. Genet. 49 , 1126–1132 (2017).
Chukwueke, C. C. et al. The CB1R rs2023239 receptor gene variant significantly affects the reinforcing effects of nicotine, but not cue reactivity, in human smokers. Brain Behav. 11 , e01982 (2021).
Ahrens, S. et al. Modulation of nicotine effects on selective attention by DRD2 and CHRNA4 gene polymorphisms. Psychopharmacology 232 , 2323–2331 (2015).
Harrell, P. T. et al. Dopaminergic genetic variation moderates the effect of nicotine on cigarette reward. Psychopharmacology 233 , 351–360 (2016).
Lerman, C. et al. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: results of two randomized clinical trials. Neuropsychopharmacology 31 , 231–242 (2006).
Le Foll, B., Gallo, A., Le Strat, Y., Lu, L. & Gorwood, P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav. Pharmacol. 20 , 1–17 (2009).
Chukwueke, C. C. et al. Exploring the role of the Ser9Gly (rs6280) dopamine D3 receptor polymorphism in nicotine reinforcement and cue-elicited craving. Sci. Rep. 10 , 4085 (2020).
The Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff A clinical practice guideline for treating tobacco use and dependence: 2008 update: a U.S. Public Health Service report. Am. J. Prev. Med. 35 , 158–176 (2008).
Hackshaw, A., Morris, J. K., Boniface, S., Tang, J. L. & Milenković, D. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ 360 , j5855 (2018).
Qin, W. et al. Light cigarette smoking increases risk of all-cause and cause-specific mortality: findings from the NHIS cohort study. Int. J. Env. Res. Public Health https://doi.org/10.3390/ijerph17145122 (2020).
Rodu, B. & Plurphanswat, N. Mortality among male smokers and smokeless tobacco users in the USA. Harm Reduct. J. 16 , 50 (2019).
Kasza, K. A. et al. Tobacco-product use by adults and youths in the United States in 2013 and 2014. N. Engl. J. Med. 376 , 342–353 (2017).
Richardson, A., Xiao, H. & Vallone, D. M. Primary and dual users of cigars and cigarettes: profiles, tobacco use patterns and relevance to policy. Nicotine Tob. Res. 14 , 927–932 (2012).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental disorders 5th edn (American Psychiatric Association, 2013).
World Health Organization. Tobacco fact sheet. WHO https://www.who.int/news-room/fact-sheets/detail/tobacco (2021).
Heatherton, T. F., Kozlowski, L. T., Frecker, R. C. & Fagerström, K. O. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br. J. Addict. 86 , 1119–1127 (1991).
Heatherton, T. F., Kozlowski, L. T., Frecker, R. C., Rickert, W. & Robinson, J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br. J. Addict. 84 , 791–799 (1989).
Etter, J. F., Le Houezec, J. & Perneger, T. V. A self-administered questionnaire to measure dependence on cigarettes: the cigarette dependence scale. Neuropsychopharmacology 28 , 359–370 (2003).
DiFranza, J. R. et al. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch. Pediatr. Adolesc. Med. 156 , 397–403 (2002).
Shiffman, S., Waters, A. & Hickcox, M. The Nicotine Dependence Syndrome Scale: a multidimensional measure of nicotine dependence. Nicotine Tob. Res. 6 , 327–348 (2004).
Smith, S. S. et al. Development of the Brief Wisconsin Inventory of Smoking Dependence Motives. Nicotine Tob. Res. 12 , 489–499 (2010).
Foulds, J. et al. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking E-cigarette users. Nicotine Tob. Res. 17 , 186–192 (2015).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Preventing tobacco use among youth and young adults: a report of the Surgeon General (Centers for Disease Control and Prevention, 2012).
World Health Organization. Tobacco control to improve child health and development. Thematic brief (WHO, 2021).
Lantz, P. M. et al. Investing in youth tobacco control: a review of smoking prevention and control strategies. Tob. Control. 9 , 47–63 (2000).
Leão, T., Kunst, A. E. & Perelman, J. Cost-effectiveness of tobacco control policies and programmes targeting adolescents: a systematic review. Eur. J. Public Health 28 , 39–43 (2018).
Royal College of Physicians. Smoking and health 2021: a coming of age for tobacco control? (RCP, 2021).
Higashi, H. et al. Cost effectiveness of tobacco control policies in Vietnam: the case of population-level interventions. Appl. Health Econ. Health Policy 9 , 183–196 (2011).
Ranson, M. K., Jha, P., Chaloupka, F. J. & Nguyen, S. N. Global and regional estimates of the effectiveness and cost-effectiveness of price increases and other tobacco control policies. Nicotine Tob. Res. 4 , 311–319 (2002).
International Agency for Research on Cancer. I ARC Handbooks of Cancer Prevention: Tobacco control Vol. 14 (IARC, 2011).
Frazer, K. et al. Legislative smoking bans for reducing harms from secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database Syst. Rev. 2 , CD005992 (2016).
Hoffman, S. J. & Tan, C. Overview of systematic reviews on the health-related effects of government tobacco control policies. BMC Public Health 15 , 744 (2015).
McNeill, A. et al. Tobacco packaging design for reducing tobacco use. Cochrane Database Syst. Rev. 4 , CD011244 (2017).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Smoking cessation: a report of the Surgeon General (Department of Health and Human Services, 2020).
Lindson, N. et al. Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Database Syst. Rev. 4 , CD013308 (2019).
Krist, A. H. et al. Interventions for tobacco smoking cessation in adults, including pregnant persons: US Preventive Services Task Force recommendation statement. JAMA 325 , 265–279 (2021).
Tutka, P. & Zatonski, W. Cytisine for the treatment of nicotine addiction: from a molecule to therapeutic efficacy. Pharmacol. Rep. 58 , 777–798 (2006).
Courtney, R. J. et al. Effect of cytisine vs varenicline on smoking cessation: a randomized clinical trial. JAMA 326 , 56–64 (2021).
Walker, N. et al. Cytisine versus nicotine for smoking cessation. N. Engl. J. Med. 371 , 2353–2362 (2014). This study validated the utility of cytisine for smoking cessation .
West, R. et al. Placebo-controlled trial of cytisine for smoking cessation. N. Engl. J. Med. 365 , 1193–1200 (2011).
Hajek, P. et al. E-cigarettes compared with nicotine replacement therapy within the UK Stop Smoking Services: the TEC RCT. Health Technol. Assess. 23 , 1–82 (2019).
Walker, N. et al. Nicotine patches used in combination with e-cigarettes (with and without nicotine) for smoking cessation: a pragmatic, randomised trial. Lancet Respir. Med. 8 , 54–64 (2020).
Siu, A. L., U.S. Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 163 , 622–634 (2015).
Black, N. et al. Behaviour change techniques associated with smoking cessation in intervention and comparator groups of randomized controlled trials: a systematic review and meta-regression. Addiction 115 , 2008–2020 (2020).
Center for Substance Abuse and Treatment. Detoxification and Substance Abuse Treatment (Center for Substance Abuse and Treatment, 2006).
Cahill, K., Hartmann-Boyce, J. & Perera, R. Incentives for smoking cessation. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD004307.pub5 (2015).
Secades-Villa, R., Aonso-Diego, G., García-Pérez, Á. & González-Roz, A. Effectiveness of contingency management for smoking cessation in substance users: a systematic review and meta-analysis. J. Consult. Clin. Psychol. 88 , 951–964 (2020).
Cahill, K. & Perera, R. Competitions and incentives for smoking cessation. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD004307.pub4 (2011).
Trojak, B. et al. Transcranial magnetic stimulation combined with nicotine replacement therapy for smoking cessation: a randomized controlled trial. Brain Stimul. 8 , 1168–1174 (2015).
Wing, V. C. et al. Brain stimulation methods to treat tobacco addiction. Brain Stimul. 6 , 221–230 (2013).
Dinur-Klein, L. et al. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol. Psychiatry 76 , 742–749 (2014).
Goldenberg, M., Danovitch, I. & IsHak, W. W. Quality of life and smoking. Am. J. Addict. 23 , 540–562 (2014).
Heikkinen, H., Jallinoja, P., Saarni, S. I. & Patja, K. The impact of smoking on health-related and overall quality of life: a general population survey in Finland. Nicotine Tob. Res. 10 , 1199–1207 (2008).
Moayeri, F., Hsueh, Y. A., Dunt, D. & Clarke, P. Smoking cessation and quality of life: insights from analysis of longitudinal Australian data, an application for economic evaluations. Value Health 24 , 724–732 (2021).
Taylor, G. M. et al. Smoking cessation for improving mental health. Cochrane Database Syst. Rev. 3 , CD013522 (2021).
López-Nicolás, Á., Trapero-Bertran, M. & Muñoz, C. Smoking, health-related quality of life and economic evaluation. Eur. J. Health Econ. 19 , 747–756 (2018).
Morris, A. Linking nicotine addiction and T2DM. Nat. Rev. Endocrinol. 16 , 6 (2020).
Willi, C., Bodenmann, P., Ghali, W. A., Faris, P. D. & Cornuz, J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. Jama 298 , 2654–2664 (2007).
World Health Organization. WHO report on the global tobacco epidemic (WHO, 2019).
Donny, E. C. et al. Randomized trial of reduced-nicotine standards for cigarettes. N. Engl. J. Med. 373 , 1340–1349 (2015). This study tested the impact of reducing the quantity of nicotine present in cigarettes on smoking .
Benowitz, N. L. & Henningfield, J. E. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N. Engl. J. Med. 331 , 123–125 (1994).
Benowitz, N. L. & Henningfield, J. E. Reducing the nicotine content to make cigarettes less addictive. Tob. Control. 22 , i14–i17 (2013).
Gottlieb, S. & Zeller, M. A nicotine-focused framework for public health. N. Engl. J. Med. 377 , 1111–1114 (2017).
Hall, W. & West, R. Thinking about the unthinkable: a de facto prohibition on smoked tobacco products. Addiction 103 , 873–874 (2008).
Ioannidis, J. P. A. & Jha, P. Does the COVID-19 pandemic provide an opportunity to eliminate the tobacco industry? Lancet Glob. Health 9 , e12–e13 (2021).
Smokefree. Smokefree 2025. Smokefree https://www.smokefree.org.nz/smokefree-in-action/smokefree-aotearoa-2025 (2021).
Morgan, C. J., Das, R. K., Joye, A., Curran, H. V. & Kamboj, S. K. Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict. Behav. 38 , 2433–2436 (2013).
Elsaid, S., Kloiber, S. & Le Foll, B. Effects of cannabidiol (CBD) in neuropsychiatric disorders: a review of pre-clinical and clinical findings. Prog. Mol. Biol. Transl. Sci. 167 , 25–75 (2019).
Butler, K. & Le Foll, B. Novel therapeutic and drug development strategies for tobacco use disorder: endocannabinoid modulation. Expert Opin. Drug Discov. 15 , 1065–1080 (2020).
D’Souza, D. C. et al. Efficacy and safety of a fatty acid amide hydrolase inhibitor (PF-04457845) in the treatment of cannabis withdrawal and dependence in men: a double-blind, placebo-controlled, parallel group, phase 2a single-site randomised controlled trial. Lancet Psychiatry 6 , 35–45 (2019).
Robinson, J. D. et al. Pooled analysis of three randomized, double-blind, placebo controlled trials with rimonabant for smoking cessation. Addict. Biol. 23 , 291–303 (2018).
Gueye, A. B. et al. The CB1 neutral antagonist AM4113 retains the therapeutic efficacy of the inverse agonist rimonabant for nicotine dependence and weight loss with better psychiatric tolerability. Int. J. Neuropsychopharmacol. https://doi.org/10.1093/ijnp/pyw068 (2016).
Yammine, L. et al. Exenatide adjunct to nicotine patch facilitates smoking cessation and may reduce post-cessation weight gain: a pilot randomized controlled trial. Nicotine Tob. Res. 23 , 1682–1690 (2021).
Eren-Yazicioglu, C. Y., Yigit, A., Dogruoz, R. E. & Yapici-Eser, H. Can GLP-1 be a target for reward system related disorders? A qualitative synthesis and systematic review analysis of studies on palatable food, drugs of abuse, and alcohol. Front. Behav. Neurosci. 14 , 614884 (2020).
Vanderkam, P. et al. Effectiveness of drugs acting on adrenergic receptors in the treatment for tobacco or alcohol use disorders: systematic review and meta-analysis. Addiction 116 , 1011–1020 (2021).
Sokoloff, P. & Le Foll, B. The dopamine D3 receptor, a quarter century later. Eur. J. Neurosci. 45 , 2–19 (2017).
David, S. P., Lancaster, T., Stead, L. F., Evins, A. E. & Prochaska, J. J. Opioid antagonists for smoking cessation. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD003086.pub3 (2013).
Ray, L. A. et al. Efficacy of combining varenicline and naltrexone for smoking cessation and drinking reduction: a randomized clinical trial. Am. J. Psychiatry 178 , 818–828 (2021).
Mooney, M. E. et al. Bupropion and naltrexone for smoking cessation: a double-blind randomized placebo-controlled clinical trial. Clin. Pharmacol. Ther. 100 , 344–352 (2016).
Justinova, Z., Le Foll, B., Redhi, G. H., Markou, A. & Goldberg, S. R. Differential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on nicotine versus cocaine self-administration and relapse in squirrel monkeys. Psychopharmacology 233 , 1791–1800 (2016).
Le Foll, B., Wertheim, C. E. & Goldberg, S. R. Effects of baclofen on conditioned rewarding and discriminative stimulus effects of nicotine in rats. Neurosci. Lett. 443 , 236–240 (2008).
Franklin, T. R. et al. The GABA B agonist baclofen reduces cigarette consumption in a preliminary double-blind placebo-controlled smoking reduction study. Drug Alcohol. Depend. 103 , 30–36 (2009).
Lotfy, N., Elsawah, H. & Hassan, M. Topiramate for smoking cessation: systematic review and meta-analysis. Tob. Prev. Cessat. 6 , 14 (2020).
Shanahan, W. R., Rose, J. E., Glicklich, A., Stubbe, S. & Sanchez-Kam, M. Lorcaserin for smoking cessation and associated weight gain: a randomized 12-week clinical trial. Nicotine Tob. Res. 19 , 944–951 (2017).
Higgins, G. A., Fletcher, P. J. & Shanahan, W. R. Lorcaserin: a review of its preclinical and clinical pharmacology and therapeutic potential. Pharmacol. Ther. 205 , 107417 (2020).
Stead, L. F. & Lancaster, T. Interventions to reduce harm from continued tobacco use. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD005231.pub2 (2007).
Download references
Acknowledgements
B.Le F. is supported by a clinician-scientist award from the Department of Family and Community Medicine at the University of Toronto and the Addiction Psychiatry Chair from the University of Toronto. The funding bodies had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication. The authors thank H. Fu (University of Toronto) for assistance with Figs 1–3.
Author information
Authors and affiliations.
Translational Addiction Research Laboratory, Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health, University of Toronto, Toronto, Ontario, Canada
Bernard Le Foll
Departments of Family and Community Medicine, Psychiatry, Pharmacology and Toxicology, University of Toronto, Toronto, Ontario, Canada
Department of Medicine, University of Wisconsin, Madison, WI, USA
Megan E. Piper
University of Wisconsin Center for Tobacco Research and Intervention, Madison, WI, USA
Department of Neurobiology and Behaviour, University of California Irvine, Irvine, CA, USA
Christie D. Fowler
Section for Preventive Cardiology, Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway
Serena Tonstad
Department of Psychiatry, Washington University School of Medicine, St. Louis, MO, USA
Laura Bierut
Institute of Mental Health, Peking University Sixth Hospital, Peking University, Beijing, China
National Institute on Drug Dependence, Peking University Health Science Center, Beijing, China
Centre for Global Health Research, Unity Health Toronto, University of Toronto, Toronto, Ontario, Canada
- Prabhat Jha
National Centre for Youth Substance Use Research, The University of Queensland, St Lucia, Queensland, Australia
Wayne D. Hall
Queensland Alliance for Environmental Health Sciences, The University of Queensland, Woolloongabba, Queensland, Australia
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (B.Le F.); Epidemiology (P.J. and W.D.H.); Mechanisms/pathophysiology (C.D.F., L.B., L.L. and B.Le F.); Diagnosis, screening and prevention (P.J., M.E.P., S.T. and B.Le F.); Management (M.E.P., S.T., W.D.H., L.L. and B.Le F.); Quality of life (P.J. and W.D.H.); Outlook (all); Conclusions (all). All authors contributed substantially to the review and editing of the manuscript.
Corresponding author
Correspondence to Bernard Le Foll .
Ethics declarations
Competing interests.
B.Le F. has obtained funding from Pfizer (GRAND Awards, including salary support) for investigator-initiated projects. B.Le F. has received some in-kind donations of cannabis product from Aurora and medication donation from Pfizer and Bioprojet and was provided a coil for TMS study from Brainsway. B.Le F. has obtained industry funding from Canopy (through research grants handled by CAMH or the University of Toronto), Bioprojet, ACS, Indivior and Alkermes. B.Le F. has received in-kind donations of nabiximols from GW Pharma for past studies funded by CIHR and NIH. B.Le F. has been an advisor to Shinoghi. S.T. has received honoraria from Pfizer the manufacturer of varenicline for lectures and advice. All other authors declare no competing interests.
Peer review
Peer reviewer information.
Nature Reviews Disease Primers thanks Jamie Brown, Elisardo Iglesias and Ming Li for their contribution to the peer review of this work.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
CTRI website: https://d3futrf33lk36a.cloudfront.net/wp-content/uploads/sites/240/2019/04/Meds-Chart.pdf
Philip Morris International: https://www.pmi.com/
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Le Foll, B., Piper, M.E., Fowler, C.D. et al. Tobacco and nicotine use. Nat Rev Dis Primers 8 , 19 (2022). https://doi.org/10.1038/s41572-022-00346-w
Download citation
Accepted : 07 February 2022
Published : 24 March 2022
DOI : https://doi.org/10.1038/s41572-022-00346-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Integrated mrna- and mirna-sequencing analyses unveil the underlying mechanism of tobacco pollutant-induced developmental toxicity in zebrafish embryos.
- Jiasheng Chen
- Xiaoping Zhong
Journal of Translational Medicine (2024)
Influence of substance use on male reproductive health and offspring outcomes
- Jamie O. Lo
- Jason C. Hedges
- Charles A. Easley
Nature Reviews Urology (2024)
Reductions in smoking due to ratification of the Framework Convention for Tobacco Control in 171 countries
- Guillermo Paraje
- Mauricio Flores Muñoz
Nature Medicine (2024)
Impact of benzoic acid and 2,2’-methylenebis (6-tert-butyl-4-methylphenol) on the metabolome of flue-cured tobacco and rhizosphere microbial communities: implications for continuous cropping obstacles
- Qiulian Peng
- Jiayan Zhang
Plant and Soil (2024)
Nicotinic regulation of microglia: potential contributions to addiction
- Alexa R. Soares
- Marina R. Picciotto
Journal of Neural Transmission (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
The impact of peer pressure on cigarette smoking among high school and university students in Ethiopia: A systemic review and meta-analysis
Roles Conceptualization, Data curation, Methodology, Software, Writing – review & editing
* E-mail: [email protected]
Affiliation College of Health Sciences, Debre Markos University, Debre Markos, Ethiopia
Roles Formal analysis, Resources, Supervision
Roles Data curation, Formal analysis, Investigation, Methodology, Validation
Roles Data curation, Formal analysis, Project administration, Software, Supervision
Roles Formal analysis, Visualization, Writing – original draft
Roles Data curation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing
Affiliation Department of Nursing, College of Nursing, University of Saskatchewan, Regina, Canada
Roles Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Validation, Visualization
Roles Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization
Roles Data curation, Investigation, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing
Roles Investigation, Project administration, Software, Supervision, Validation, Writing – review & editing
Roles Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
Roles Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
Roles Methodology, Supervision, Writing – review & editing
Affiliations Colleges of Nursing, University of Saskatchewan, Saskatoon, Canada, School of Life Sciences and Bioengineering, Nelson Mandela African Institute of Science and Technology, Arusha City, Tanzania
Roles Methodology, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing
Affiliations School of Science and Health, Western Sydney University, Penrith, NSW, Australia, Translational Health Research Institute, Western Sydney University, Penrith, NSW, Australia, Discipline of Child and Adolescent Health, Sydney Medical School, Faculty of Medicine and Health, The University of Sydney, Westmead, NSW, Australia, Oral Health Services, Sydney Local Health District and Sydney Dental Hospital, NSW Health, Surry Hills, NSW, Australia
- Cheru Tesema Leshargie,
- Animut Alebel,
- Getiye Dejenu Kibret,
- Molla Yigzaw Birhanu,
- Henok Mulugeta,
- Patricia Malloy,
- Fasil Wagnew,
- Atsede Alle Ewunetie,
- Daniel Bekele Ketema,

- Published: October 11, 2019
- https://doi.org/10.1371/journal.pone.0222572
- Reader Comments
Cigarettes and their by-products (i.e., smoke; ash) are a complex, dynamic, and reactive mixture of around 5,000 chemicals. Cigarette smoking potentially harms nearly every organ of the human body, causes innumerable diseases, and impacts the health of smokers and those interacting with the smokers. Smoking brings greater health problems in the long-term like increased risk of stroke and brain damage. For students, peer pressure is one of the key factors contributing to cigarette smoking. Therefore, this systematic review and meta-analysis assessed the impact of peer pressure on cigarette smoking among high school and university students in Ethiopia.
An extensive search of key databases including Cochrane Library, PubMed, Google Scholar, Hinari, Embase and Science Direct was conducted to identify and access articles published on the prevalence of cigarette smoking by high school and university students in Ethiopia. The search period for articles was conducted from 21 st September, 2018 to 25 th December 25, 2018. All necessary data were extracted using a standardized data extraction checklist. Quality and risk of bias of studies were assessed using standardized tools. Heterogeneity between the included studies was assessed using Cochrane Q-test statistic and I 2 test. To estimate the pooled prevalence of cigarette smoking, a random effects model was fitted. The impact of peer pressure on cigarette smoking was determined and was reported in Odds Ratio (OR) with 95% Confidence Interval (CI). Meta-analysis was conducted using Stata software.
From 175 searched articles, 19 studies fulfilled the eligibility criteria and were included in this study. The pooled prevalence of cigarette smoking among Ethiopian high school and university students was 15.9% (95% CI: 12.21, 19.63). Slightly higher prevalence of cigarette smoking was noted among university students [17.35% (95% CI: 13.21, 21.49)] as compared to high school students [12.77% (95% CI: 6.72%, 18.82%)]. The current aggregated meta-analysis revealed that peer pressure had a significant influence on cigarette smoking (OR: 2.68 (95% CI: 2.37, 3.03).
More than one sixth of the high school and university students in Ethiopia smoke cigarette. Students who had peer pressure from their friends were more likely to smoke cigarette. Therefore, school-based intervention programs are needed to reduce the high prevalence of cigarette smoking among students in Ethiopia.
Citation: Leshargie CT, Alebel A, Kibret GD, Birhanu MY, Mulugeta H, Malloy P, et al. (2019) The impact of peer pressure on cigarette smoking among high school and university students in Ethiopia: A systemic review and meta-analysis. PLoS ONE 14(10): e0222572. https://doi.org/10.1371/journal.pone.0222572
Editor: Wisit Cheungpasitporn, University of Mississippi Medical Center, UNITED STATES
Received: March 15, 2019; Accepted: September 3, 2019; Published: October 11, 2019
Copyright: © 2019 Leshargie et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the manuscript and its Supporting Information files.
Funding: The authors received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Abbreviations: CI, Confidence Interval; HIV, Human Immune Deficiency Virus; OR, Odd Ration; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SE, Standard Error; SNNPR, South Nation and Nationalities People of the Region; RR, Relative Risk; WHO, World Health Organization
Introduction
Smoking cigarettes yields a complex, dynamic and reactive mixture of around 5,000 chemicals [ 1 – 3 ]. Globally, it is one of the leading preventable causes of respiratory tract complications, disability, and early deaths related to complications [ 4 – 7 ]. It accounts for six of the eight leading causes of morbidity and mortality [ 5 ]. Essentially, it is a legal drug that kills many of its users when used exactly as intended by manufacturers. Currently, the World Health Organization (WHO) estimates that the use of both smoking and smokeless tobacco account for around 6 million deaths worldwide annually, of which 600,000 deaths were among non-smokers due to exposure to the smoke [ 8 ]. More than 30% of world’s adult population are consumers of tobacco, which leads to a warning that a billion people will die of adverse health effects related to the tobacco epidemic within the 21st century unless effective preventative measures are undertaken [ 3 ].
Smoking affects almost every organ in the human body (such as circulatory, respiratory, gastrointestinal and musculoskeletal systems), increases the risk for several diseases, and reduces the health of smokers in general [ 9 , 10 ]. The key effect of smoking cigarettes is primarily on the lungs with approximately 85% of chronic obstructive pulmonary disease (COPD) and lung cancer and about 33% of other cancers (i.e., esophagus, oral cavity, uterus, stomach, and pancreas) related to smoking [ 9 – 11 ].
Normal adolescent developmental stage is affected by high level of peer pressure that can influence risk-taking behaviors including substance use [ 12 ]. Globally, especially in low- and middle-income countries, an estimated 80% of the one billion adolescent smokers are suffering from tobacco-related morbidity and mortality [ 7 ]. Cigarette smoking negatively influences the physical and mental health of an individual [ 13 ]. This is particularly true for high school and university students who already face major health challenges such as stress [ 14 ]. Smoking is also associated with poor educational performance, high-risk drinking behavior, illegal drug use, and high-risk sexual behaviors [ 14 , 15 ]. Peer pressure is widely recognized as a crucial factor affecting young people's early experimentation with tobacco and their willingness to continue smoking [ 16 ]. Several students attending higher education institutions practice cigarette smoking for several reasons, such as a way to cope with stress [ 17 ]. Factors that contribute to the continued use of tobacco include being male, drinking alcohol, having a friend who drinks alcohol, having a friend who smokes, having family members who smoke and being older in age, to mention some [ 18 ].
In sub-Saharan Africa, the prevalence of smoking is increasing and is projected to continue to increase [ 19 , 20 ]. The current data in the region reveals substantial variation in smoking rates among countries ranging from 1.8% in Zambia to 25.8% in Sierra Leone [ 21 ]. In Ethiopia, cigarette smoking is among one of the most commonly used substances, which leads to addiction [ 22 ]. It has deleterious effects on the health of the young users, significantly reduces academic performance in students and increases risk of contracting HIV and other sexually transmitted diseases. Several primary studies on the prevalence and associated factors of cigarette smoking among high school and university students have been conducted in Ethiopia [ 23 – 37 ]. According to earlier reviews of the literature, prevalence of smoking in Ethiopia ranges from 2.99% in Addis Ababa [ 38 ] to 28.6% in Hawassa and Jima University [ 30 ]. Therefore, this systematic review and meta-analysis aimed to review the pooled prevalence of cigarette smoking among high school and university students in Ethiopia and the impact of peer pressure on cigarette smoking among high school and university students in Ethiopia.
Method and materials
This systematic review is based on the Preferred Reporting Items of Systematic Reviews and Meta-Analysis (PRISMA) checklist guidelines to ensure scientific rigor [ 39 ] ( S1 Table ). Prospective registration of systematic review and meta-analysis promotes transparency, helps reduce potential for bias, and improves review’s credibility. However, this meta-analysis and systematic review was not registered on the prosperous, and we have acknowledged this gap in the limitation section.
This systematic review and meta-analysis reports data from Ethiopia. Ethiopia is located in the north-eastern part of the African continent or what is known as the “Horn of Africa”. The country is divided into nine regional states and two administrative cities [ 40 ] containing a total of 108,386,391 million population with a national density of 94 people per square kilometer, 2019 [ 41 ]. Ethiopia shares land borders with five countries: Sudan , Somalia , Djibouti , Eritrea , and Kenya [ 42 ].
Inclusion and exclusion criteria
Eligibility criteria..
This systematic review and meta-analysis included studies only conducted in Ethiopia that assessed the prevalence of cigarette smoking. Published articles were reviewed and rated for inclusion. Full articles were retrieved if a specific outcome of interest (smoking status) was defined. This review included all observational study designs (cross-sectional studies, case-control studies, and cohort studies). However, case reports or case series, duplicate reports, and inconsistent outcome measures were excluded. Moreover, we excluded articles that were published in a language other than English. Documents that were not accessible after contacting the principal investigator three times by email were also excluded. Articles that reported measures other than Relative Risk (RR) or equivalent values, or from which an Odds Ratio (OR) could not be calculated were also excluded from consideration, The eligibility criteria for each individual article were checked by three authors independently (CT, AA1, and AA2). If there was a disagreement between the two authors, a third person (UGM) resolved the disagreement. All reviewers came together in person and discussed the assessment results.
Information sources
This systematic review and meta-analysis were conducted by considering all the available studies (both published and open grey reports), governmental and other stakeholder annual reports, and national surveys on children and adolescents which have data on cigarette smoking among high school and university students in Ethiopia. An extensive search was done from the following international databases, including Cochrane Library, PubMed, Google Scholar, Hinari , Embase, CINAHL, Web of Science, and Science Direct to access articles conducted on the prevalence of smoking cigarette. The following keywords “prevalence”, ("cigarette smoking" OR ("cigarette"[All Fields] AND "smoking"[All Fields]) OR "cigarette smoking"[All Fields]) AND substance[All Fields]) AND (high[All Fields] AND ("schools"[MeSH Terms] OR "schools"[All Fields] OR "school"[All Fields]) AND ("universities"[MeSH Terms] OR "universities"[All Fields] OR "university"[All Fields])) AND ("students"[MeSH Terms] OR "students"[All Fields]) AND ("Ethiopia"[MeSH Terms] OR "Ethiopia"[All Fields]) were used to obtain published articles. Boolean operators particularly pairing aspects of “OR” or “AND” were used as search terms to separate articles. The search for all articles was conducted from 21 st September, 2018 to 25 th December, 2018 ( S2 Table ).
This systematic review and meta-analysis had two outcomes. The first outcome was the pooled prevalence of cigarette smoking among high school and university students in Ethiopia, which was calculated by dividing the number of smokers to the total students (sample size) multiplied by 100. The second outcome was the impact of peer pressure on cigarette smoking practice. We adjusted the effect size into Odd Ratio (OR) since all the studies were cross sectional and the appropriate effect size estimate for cross sectional design is OR to estimate the impact of peer pressure on cigarette smoking.
Data extraction
The necessary data (primary author, publication year, region, study design, sample size, prevalence of cigarette smoking) were extracted from the eligible articles by two authors (CT, AA and AA1) independently using prepiloted data extraction format prepared in Microsoft ™ Excel spreadsheet ( S3 Table ). Any disagreements between the three reviewers in the review process were discussed with the three reviewer team members (GD, DB and PM) until consensus was reached. Moreover, the data of kappa of agreement during the systematic searches was also used to solve the disagreements among two independent reviewers (CT and AA4). The kappa agreement was interpreted as less than chance agreement if less than 0, slight agreement if 0.01–0.20, fair agreement if 0.21–0.40, moderate agreement if 0.41–0.60, substantial agreement if 0.61–0.80 and moderate agreement if the kappa was 0.81–0.99 [ 43 ].
The four authors (CT, FW, MA and AA1) also independently extracted data on the association of cigarette smoking and peer pressure. If studies did not report OR, RR, or equivalent measures, raw data were screened to determine whether OR could be calculated. When the studies reported both the crude OR/RRs and the adjusted OR/RRs, the adjusted figures were extracted.
Quality assessment of the included studies
We assessed the quality of the included studies according to the Newcastle-Ottawa Scale (NOS) [ 44 ] ( S4 Table ). The NOS has three main domains and uses a star-based grading system with each study scoring a maximum of 10 stars. The first domain focuses on the methodological quality of the study (sample size, response rate, and sampling technique) with the possibility of a five-star grading (1 = poor to 5 = excellent). The second domain of the tool deals with the comparability of the study cases or cohorts, with the possibility of two stars. The last domain deals with the outcomes and statistical analysis of the study with a possibility of three stars. Three authors (MA, UGM, and DB) independently assessed the quality of each included study using the NOS. Any disagreement between the three authors was resolved by requesting other two authors (MY and PP) to independently assess the methodological quality to reach a consensus. Finally, studies with stars of ≥ 7 out of 10 were considered to be of a high quality [ 45 ]. Moreover, we assessed the quality of each included articles using National Institutes of Health (NIH) ( S5 Table ) which is a more detail tool on quality assessment than NOS. The tool has 14 criteria to assess the article independently with a response of “Yes, No and Not Applicable”. Articles with NIH assessment result of 85% and more (that means number of articles with yes divided by total criteria minus not applicable) were considered as good quality.
Risk of bias
For each included study, the risk of bias was assessed independently by two authors (UGM and CT). Risk of bias assessment was carried out using Holly 2012 tool which contain 10 recommended criteria for the internal and external validity tool [ 46 ]. This tool includes: representation of the population, sampling frame, methods of participants’ selection, non-response bias, data collection directly from subjects, acceptability of case definition, reliability and validity of study tools, mode of data collection, length of prevalence period; and appropriateness of numerator and denominator. Each item was classified as low and high risk of bias. Unclear assessment was classified as high risk of bias. The overall score of the risk of bias was then categorized according to the number of high risk item scores for bias per study: low (≤ 2), moderate (3–4), and high (≥ 5) ( S6 Table ).
Statistical data analysis
Standard error for all included studies was computed using the binomial distribution formula. Heterogeneity across studies were assessed by determining the p-values of Cochrane Q-test and I 2 -test statistics [ 47 ]. For meta-analysis result with significant heterogeneity, univariate meta-regression was used to assess the source of heterogeneity across each study. A funnel plot was also used for visual assessment of the publication bias. Asymmetry of the funnel plot is an indicator of potential publication bias. Furthermore, Egger’s test was used to determine if there was significant publication bias, and a p -value less than 0.10 was considered to indicate the presence of significant publication bias [ 48 ]. We selected Egger`s test to assess the publication bias because, the value of Egger`s test is more specific than Begg`s test [ 49 , 50 ]. We conducted the log relative risk to assess the effect of peer pressure on students’ cigarette smoking status. Furthermore, sensitivity analysis using a random effects model was performed to assess the influence of a single study on the pooled prevalence estimates. Subgroup analysis was used to minimize the random variations between the point estimates of the primary study subgroup, and analysis was done based on study settings (i.e., institution). Univariable meta-regression analysis was also conducted with year of publication and the outcome variable. All data manipulation and statistical analysis were performed using Stata ™ software (Version 14; Stata Corp, College Station, TX).
The electronic database search identified a total of 179 published articles. Of these, 121 duplicate articles were removed. Furthermore, 28 articles were removed after reviewing the titles and the abstract as they were not relevant to the focus of the review. Finally, one article was excluded due to inaccessibility of the full text despite three requests to the primary author on data, and 10 articles were excluded after reviewing their full text. Finally, 19 articles met all the prior criteria and were included in this analysis ( Fig 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0222572.g001
Overview of the original included articles
All of the 19 articles included in this study were published between 1999 to 2017 in peer-reviewed journals. A total of 16,486 study participants were included in this systematic review and meta-analysis. The smallest sample size was 155 from a study conducted at Bahir Dar University [ 36 ], and the largest sample size was 1,984 in a study conducted in Gondar Medical College, Amhara Region [ 34 ]. All included studies were cross-sectional in design. The characteristics of the studies included in this review are described in ( Table 1 ) .
https://doi.org/10.1371/journal.pone.0222572.t001
Quality assessment result of the included articles
The qualities of individual articles were assessed using different tools; namely NOS and NIH quality assessment tools. Accordingly, NOS assessment result all articles had good quality using the NOS criteria. However, when assessed using NIH quality assessment tool, 1 (5.3%) study [ 36 ] was categorized as poor and the rest [ 11 , 15 , 23 – 35 , 37 , 38 , 51 ] were categorized as good quality ( S5 Table ).
Kappa agreement
Disagreements between the two reviewers during data extraction process were assessed using the Kappa agreement. Therefore, a = 9 and b = 2 represent the number of times the two reviewers agreed while c = 1 and d = 7 represent the number of times the two reviewers disagree. If there are no disagreements, b and c would be zero, and the reviewers agreement (po) is 1, or 100%. If there are no agreements, a and d would be zero, and the reviewers agreement (po) is 0. Interobserver agreement was 68% that indicate a substantial agreement between the two main reviewers who extracted data.
Risk of bias was performed for each included study using the risk of bias assessment tool that includes ten different items [ 46 ]. From the 19 included studies, the risk of bias summary assessment revealed that 94.7% of the included studies had a low risk of bias [ 15 , 23 – 35 , 37 , 38 , 51 ] while only one (5.3%) of the included studies had a moderate risk of bias [ 36 ].
Prevalence of cigarette smoking
The overall pooled prevalence of cigarette smoking in Ethiopia using the 19 studies was 16.31% (95% CI: 12.17, 20.45). A random-effects model was used because of the significant heterogeneity ( I 2 = 98.1%, p-value <0.001) across the studies ( Fig 2 ). Additionally, univariate meta-regression analysis was conducted to identify possible sources of heterogeneity. The different covariates included in the analysis were publication year and sample size. However, none of these variables were found to be statistically significant.
https://doi.org/10.1371/journal.pone.0222572.g002
The existence of publication bias was assured by funnel plot asymmetry. The funnel plot graph indicates that there is a significant variability within the findings of the 19 individual primary articles included in this meta-analysis ( Fig 3 ). The publication bias checked by objective measurement namely Egger’s tests also showed a statistically significant publication bias ( Egger's test p-value = 0 . 001 ). To handle the observed publication bias, we performed the trim and fill analysis, which is a nonparametric methods for estimating the number of missing studies that might exist and helps in reducing and adjusting publication bias in meta-analysis.
https://doi.org/10.1371/journal.pone.0222572.g003
Assessment of heterogeneity
We used I 2 statistics to investigate the presence of variation across the included studies. Accordingly, the result of I 2 statistics using a random effects model revealed a significant heterogeneity across the included studies ((I 2 = 98.1%, p-value <0 . 001 ).
Subgroup analysis
The findings from the subgroup analysis showed that the highest and lowest cigarette smoking was observed among university students 17.35% (95% CI: 12.97, 22.16) and high school students 13.76% (95% CI: 7.24, 20.27), respectively ( Fig 4 ).
https://doi.org/10.1371/journal.pone.0222572.g004
Similarly, the regional subgroup analysis result revealed the pooled prevalence of smoking from highest to lowest was [20.11% (95% CI: 11.39, 28.84)] in Ethio-Somalia and Harari region, [18.96% (95% CI: -0.03, 38.01)] in Tigray region, [17.35% (95% CI: 13.21, 21.49)] in South Nation Nationality and People of Ethiopia (SNNPE), [15.34% (95% CI: 10.84, 19.83)] in Amhara region, [14.98% (95% CI: 7.37, 22.55)] in Oromia region, and [5.9% (95% CI: 0.02, 11.79)] in Addis Ababa region ( Fig 5 ).
https://doi.org/10.1371/journal.pone.0222572.g005
The linear trend of cigarette smoking status of students in Ethiopia
The cumulative univariate meta-analysis on cigarette smoking status among high school and university with the year of 1984–2017 was performed. The result from cumulative univariate meta-analysis showed the trend in prevalence estimates of cigarette smoking status among high school and university over time. The finding revealed that there is more or less constant trend ( Fig 6 ).
https://doi.org/10.1371/journal.pone.0222572.g006
The univariate meta-regression using bubble plot was also performed. The bubble plot figure indicates that the trend was slight increment ( Fig 7 ).
https://doi.org/10.1371/journal.pone.0222572.g007
The effect of peer pressure on cigarette smoking status
Five of the 19 included studies reported the effect of peer pressure on cigarette smoking. From this, three studies [ 11 , 30 , 37 ] showed a positive effect of peer pressure on cigarette smoking, while the other two studies [ 31 , 51 ] showed no relationship between peer pressure and cigarette smoking. However, the aggregated meta-analysis revealed a higher odds of cigarette smoking among students who experienced peer pressure than those who didn’t (OR: 2.68, 95% CI: 2.37, 3.03) ( Fig 8 ).
https://doi.org/10.1371/journal.pone.0222572.g008
Cigarette smoking has major health and social consequences, and it reduces the educational performance of students [ 52 , 53 ]. This systematic review and meta-analysis, therefore, was conducted to assess the pooled prevalence of cigarette smoking and its association with peer pressure among high school and university students in Ethiopia. Accordingly, the pooled prevalence of cigarette smoking among Ethiopian high school and university students was 15.92%. This finding is lower than a study conducted among students in South Africa which reported a prevalence of 16.9% [ 50 ]. Conversely, the current reported pooled prevalence of cigarette smoking was higher than a study conducted among government and private schools and college students in Bengaluru, India (12.8%) [ 54 ] and amongst university students in Iran (13.8%) [ 55 ].
In this review, the pooled prevalence of cigarette smoking was lower than a study finding observed among Kenyan secondary school students (38.6%) and Cameroon university students (93.1%) [ 56 , 57 ]. In addition, our finding was slightly lower than a study conducted among high school students in Shiraz- Iran (19.7%) [ 58 ]. This might be due to the difference between sample size and socio-demographic nature of the two study populations. There is also cultural variation among the study communities. Moreover, the higher prevalence of cigarette smoking in the current study could be due to the dominance of male participants as evidence suggests that males tend towards different types of substance abuse than females [ 59 , 60 ].
Similarly, the current pooled prevalence of cigarette smoking is also lower than a systematic review conducted in Africa [ 50 ] and the Middle East [ 61 ]. This variation might be due to the differences in the study period and sample size between these two studies. In addition, the previous review was conducted only among university students, while the current review included both high school and university students.
The current review also considered subgroup analysis to appreciate the variability or heterogenic characteristics of the included studies. Accordingly, a higher prevalence was observed among university students (17.35%) than high school students (12.77%). This could be because most high school students live with their families which may limit them from cigarette smoking because of parental control. Additionally, in most cases, students during their high school time live with families and that may not encourage smoking cigarette. On the contrary, when they join to the university, almost all students become independent of their family supervision. This independency and pressure from their friends increases the proportion of students who smokes cigarette [ 62 ]. Educational institutions can be a challenging environment and everyone copes with stress in different ways [ 17 ]. Moreover, as students enter to university, they start a new life away from their families in a different and strange environment which can contribute to their behavior or involvement in substance abuse like cigarette smoking [ 55 ]. Evidence also supports that as the level of education increase, the proportion of smoking increases [ 63 , 64 ].
A subgroup analysis by regions of the country also showed a higher prevalence of cigarette smoking among universities in other category (i.e., Harar region, Somalia region and Oromia region). This finding might be due to typical local practices of substances like cigarette and khat in these regions. Therefore, the government, school management, local communities and other concerned bodies need to implement school-based intervention programs in order to reduce the pooled prevalence of cigarette smoking.
Students who felt peer pressure were more likely to smoke cigarette than those who had no peer pressure. This finding was similar to a study conducted in Kenyan students and Shiraz- Iran [ 57 ] where peer pressure was found to have a significant (positive) effect on the likelihood of cigarette smoking [ 56 , 58 ]. Peer group pressure is widely known as a decisive factor which affects the early onset of experimentation with tobacco and the individual’s subsequent willingness to continue smoking [ 16 ]. Similarly, other systematic reviews state the most common factors influencing students’ smoking status was having smoker friends [ 55 , 65 ]. Therefore, the school management needs to implement youth association focusing on counseling and rehabilitation service for to seize students already practicing smoking and also those who are not practicing yet now.
Strengths and limitations of the study
This review has several strengths including: this review focus on the adolescent and young adult populations who are vulnerable to initiating substance use/abuse behaviors. In addition, this review rigorous adherence to the PRISMA checklist which improves its quality for the readers. Moreover, this finding will give an insight into developing a health promotion policy for the country. Whereas, on top of the above strength, this review has the following limitations: This review included studies that were published only in English language which may limit the number of studies that were reported in other languages. Moreover, the other limitation of this review was the risk of self-report bias introduced from the original studies included in the review. On top of these the protocol of this manuscript was not registered online before conducting it.
Conclusions
This systematic review and meta-analysis indicate that the prevalence of cigarette smoking among Ethiopian high school and university students was high. More than one sixth of the high school and university students smoke cigarettes. This higher cigarette smoking proportion of students was influenced by peer pressure. Variations were also observed in the prevalence of cigarette smoking by different regions in the country. Therefore, school-based intervention programs aimed at prevention of cigarette smoking is recommended. In particular, educational programs on how to resist and handle peer pressure are essential to prevent cigarette smoking among high school and university students in Ethiopia.
Supporting information
S1 table. prisma 2009 checklist..
https://doi.org/10.1371/journal.pone.0222572.s001
S2 Table. Searches for databases.
https://doi.org/10.1371/journal.pone.0222572.s002
S3 Table. Data extraction tools Smoke.
https://doi.org/10.1371/journal.pone.0222572.s003
S4 Table. Quality assessments.
https://doi.org/10.1371/journal.pone.0222572.s004
S5 Table. NIH quality assessments.
https://doi.org/10.1371/journal.pone.0222572.s005
S6 Table. Risk of bias for each study.
https://doi.org/10.1371/journal.pone.0222572.s006
Acknowledgments
The authors of this work would like to forward great and deepest gratitude for Debre Markos University for creating convenient environment and internet service. Furthermore, the authors would like also to forward special acknowledgement for authors of primary studies.
- View Article
- Google Scholar
- 7. World Health Organization. WHO Report on the global tobacco epidemic, 2015. Geneva, Switzerland: WHO. 2015.
- 8. World Health Organization: WHO Global Report on Trends in Prevalence of Tobacco Smoking. Geneva, SW: WHO 2015.
- 13. Fit for Work team: Smoking and its impact on mental health. March 6, 2017.
- PubMed/NCBI
- 22. Kebede Y, Abula T: Substance Abuse For the Ethiopian Health Center Team. 1st edition. Addis Ababa: Ethiopian Public Health training Initiative 2005.
- 25. Gezahegn T, Andualem D, Mitiku TH: Substance Use and Associated Factors among University Students in Ethiopia: A Cross-Sectional Study. Hindawi Publishing Corporation 2014.
- 40. Ethiopia—Historical Attractions, Regions, Cities and Population.
- 44. Newcastle: Newcastle-Ottawa Scale customized for cross-sectional studies. In. available from https://static-content.springer.com/esm/…/12889_2012_5111_MOESM3_ESM.doc . 2012.
- 52. US Department of Health and Human Services: How Tobacco Smoke Causes Disease: What It Means to You. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health,. 2010.
- 53. US Department of Health and Human Services: The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 2014.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Smoking research: basic research, intervention, prevention, and new trends
Affiliation.
- 1 Western Psychiatric Institute and Clinic, University of Pittsburgh, PA 15213-2593.
- PMID: 2700344
Smoking is a behavior that is influenced by a variety of factors that cut across methodologies, disciplines, and content areas within health psychology. The present article is designed to show the diversity and richness of smoking research by examining smoking from four perspective: basic laboratory research, intervention, prevention and deterrence, and new directions in smoking research. Methodologies that were derived from such varied sources as psychopharmacology, behavioral pharmacology, behavior therapy, clinical psychology, public health and health promotion, and social and developmental psychology have been used to study the smoking problem. The subject populations in these investigations ranged from animal models, to the individual smoker attempting to quit, to communities involved in health promotion and public health approaches. Future research should seek to provide new and improved examples of interdisciplinary research within the field of health psychology to multidisciplinary approaches from the basic and applied sciences.
PubMed Disclaimer
Similar articles
- Preventing tobacco use among lesbian, gay, bisexual, and transgender youths. Remafedi G, Carol H. Remafedi G, et al. Nicotine Tob Res. 2005 Apr;7(2):249-56. doi: 10.1080/14622200500055517. Nicotine Tob Res. 2005. PMID: 16036282
- "Not lighting up": a case study of a woman who quit smoking. Giarelli E, Ledbetter N, Mahon S, McElwain D. Giarelli E, et al. Oncol Nurs Forum. 2004 May 12;31(3):E54-63. doi: 10.1188/04.ONF.E54-E63. Print 2004 May. Oncol Nurs Forum. 2004. PMID: 15152275 Review.
- Adolescent cigarette smoking: prevalence, causes, and intervention approaches. Botvin GJ, Epstein JA, Botvin EM. Botvin GJ, et al. Adolesc Med. 1998 Jun;9(2):299-313, vi. Adolesc Med. 1998. PMID: 10961237 Review.
- Public health approaches to tobacco use prevention and cessation in the U.S. Glynn TJ, Manley MW, Gerlach KK, Shopland DR. Glynn TJ, et al. J Public Health Manag Pract. 1996 Spring;2(2):17-26. J Public Health Manag Pract. 1996. PMID: 10186665
- [Smoking from the perspective of positive psychology]. Mojs E, Stanisławska-Kubiak M, Skommer M, Wójciak R. Mojs E, et al. Przegl Lek. 2009;66(10):765-7. Przegl Lek. 2009. PMID: 20301932 Polish.
- Predicting nicotine dependence profiles among adolescent smokers: the roles of personal and social-environmental factors in a longitudinal framework. Kleinjan M, Vitaro F, Wanner B, Brug J, Van den Eijnden RJ, Engels RC. Kleinjan M, et al. BMC Public Health. 2012 Mar 16;12:196. doi: 10.1186/1471-2458-12-196. BMC Public Health. 2012. PMID: 22424115 Free PMC article.
- Seeing, wanting, owning: the relationship between receptivity to tobacco marketing and smoking susceptibility in young people. Feighery E, Borzekowski DL, Schooler C, Flora J. Feighery E, et al. Tob Control. 1998 Summer;7(2):123-8. doi: 10.1136/tc.7.2.123. Tob Control. 1998. PMID: 9789929 Free PMC article.
Publication types
- Search in MeSH
Related information
Grants and funding.
- CA38243/CA/NCI NIH HHS/United States
- DA-HI04174/DA/NIDA NIH HHS/United States
- N02-CN-64094/CN/NCI NIH HHS/United States
LinkOut - more resources
Full text sources.
- Ovid Technologies, Inc.
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Research article
- Open access
- Published: 30 June 2021
The negative impact of chronic tobacco smoking on adult neuropsychological function: a cross-sectional study
- Mohammed Sh. Nadar ORCID: orcid.org/0000-0003-4281-5630 1 ,
- Abdullah M. Hasan 2 &
- Mohammed Alsaleh 2
BMC Public Health volume 21 , Article number: 1278 ( 2021 ) Cite this article
5769 Accesses
15 Citations
2 Altmetric
Metrics details
The evidence on the effects of chronic tobacco smoking on neuropsychological functions is conflicting. The literature remains limited by inconsistent accounting for potentially confounding biomedical and psychiatric conditions. This study aimed to assess the neuropsychological functions of adult chronic tobacco smokers in comparison to group-matched non-smokers.
The study included 73 smokers and 84 group-matched non-smokers. The data was collected during the year 2019. After an initial interview to collect demographics and smoking profile, the subjects undertook neuropsychological assessments that targeted a wide range of cognitive domains.
The performance of smokers was poorer on almost all neuropsychological domains, namely selective attention ( p ≤ .001, p = .044), alternating attention ( p = .002) working memory ( p ≤ .001), Short-term memory ( p = .006 and .003), Long-term memory ( p ≤ .001), processing accuracy ( p ≤ .001), and executive function ( p = .011 and .026). Smokers were intact on processing speed. Smoking accumulation and lower age onset of regular smoking were correlated with lower neuropsychological function.
Our findings add to the growing body of evidence suggesting that chronic tobacco smoking impacts cognition negatively.
Peer Review reports
In 2015, around a quarter (24.9%) of the global population were current users of some form of tobacco [ 1 ]. Smoking is one form of tobacco exposure that is prevalent across the world.
The harmful impact of chronic tobacco smoking on physical health is well documented and includes cardiovascular diseases, respiratory diseases, and various forms of cancer [ 2 ]. Additionally, chronic smoking is implicated in the pathogenesis of neuropsychological dysfunction and has been directly linked to increased risk of depression and cognitive impairment [ 1 , 3 , 4 ].
A large number of studies have examined the effects of smoking on neuropsychological function across multiple variables. Compared to non-smokers, chronic smoking was cumulatively reported to have detrimental effects on various neuropsychological domains, including general intellectual abilities, processing speed, attention, memory, cognitive flexibility and executive functions [ 2 , 3 , 5 ]. The effects of smoking on cognition are believed to vary based on the dose and onset of regular use. Although the majority of studies provide evidence on the effect of chronic tobacco smoking on neuropsychological impairments, conflicting evidence also exists. For example, a 10-year longitudinal study of 1436 older adults found that smokers were less likely to develop cognitive impairment than those who had never smoked [ 6 ]. Another study consisting of 2553 adults found smokers to have lower rates of cognitive impairment compared to non-smokers [ 7 ], and both studies concluded that smoking may be protective of cognitive function. Several other studies also support the notion that cigarette smoking seems not to affect cognition or to have a positive effect on some aspects of cognitive function of smokers [ 8 , 9 , 10 , 11 ].
Based on the current literature, it is difficult to draw solid conclusions on the impact of smoking on neuropsychological function [ 2 ]. The influence of tobacco smoking on specific domains of cognition is complex and much remains to be known about its impact on neuropsychology and cognition. The literature remains limited by inconsistent accounting for potentially confounding biomedical and psychiatric conditions. For example, many of the studies did not account for confounding variables such as psychiatric disorders and comorbid substance abuse (i.e., alcohol, cannabis, and other drugs). In other studies, the duration of tobacco use was not taken into account [ 12 ]. Other studies varied in terms of which subcategories of specific neuropsychological domains were tested. Some controversy also remains regarding smoking effect on specific cognitive functions, and individual differences in smoking cognitive effects. Consequently, it is essential to continue to investigate the association between chronic tobacco smoking and potential neuropsychological impairments while controlling for possible confounding variables. This cross-sectional study aimed to assess the neuropsychological functions of chronic tobacco smokers in comparison to group-matched non-smokers. It was hypothesized that chronic tobacco smokers would have significantly poorer global neuropsychological functions compared to non-smokers.
Participants and procedure
We recruited the participants via social medial and local adverts during the year 2019. The participants were interviewed regarding cigarette smoking and use of other tobacco products. We excluded participants who reported current use of other substances that are known to affect cognition (i.e., alcohol, cannabis, and psychotropic medications, except for caffeine) up to 3 months prior to study enrollment. Since psychiatric illness is strongly correlated to cognitive impairment, we excluded subjects with any known psychiatric problems or mental diseases. Participants with any medical condition or history of serious head injury that are known to influence cognition were also excluded. To be included in the smoking group, the participant must have been a smoker for at least 10 years and smoked a minimum of one pack per day. The smokers and non-smokers were matched as a group for age, sex, ethnicity, educational, and socioeconomic status. Past smokers and second-hand smokers were excluded from the non-smokers’ group.
The study was approved by the Institutional Review Board, and all participants signed a written consent form to participate in the study before data collection. An initial interview collected information related to socio-demographic, health, and smoking profiles. After the interview session, all participants completed a comprehensive battery of outcome measures assessing neuropsychological functions that target a wide range of cognitive domains. All the measures used in this study have well-established and comprehensive psychometric properties, and were used in other studies on neuropsychological function (a brief description of each measure is provided in Table 1 ). In addition to the neuropsychological measures, we used the Grooved Pegboard (Lafayette Instrument, Lafayette, IN) as a measure of fine motor dexterity, which requires visual-spatial and motor coordination. The test battery was administered by a trained researcher, and the entire battery required about 60–70 min to complete. The participants were allowed short breaks between tests, and smokers were free to smoke during the breaks if desired. The sequence of outcome measures was administered consistently across all participants.
After completion of all measures, the participants were presented with two questions which they were required to answer by “Yes” or “No”. 1) “Do you believe that smoking increases the risk of physical health problems, such as getting heart disease, lung disease, stroke and cancer?”, and 2) “Do you believe that smoking increases the risk of cognitive health problems, such as reduced memory, attention, and concentration?”.
Statistical analysis
All statistical analysis was performed with Statistical Package for the Social Sciences (SPSS – Windows version 25; SPSS Inc., Chicago, IL). Comparison between groups of participants in terms of demographic characteristics and quantitative outcome measures were performed using t-tests for normally distributed data. Where homogeneity of variance was violated in a given model (Levine’s test), we used the Mann-Whitney U test for skewed data. Two-tailed statistics were used, and statistical significance was set at P < 0.05. Cohen’s D effect sizes were reported when significant effects of group on a cognitive variable were identified. Pearson correlations were used to assess the influences of the smoking variables of accumulation (number of years smoking), Cigarettes/day, and Pack-Years of smoking and decline in cognitive function.
The final sample included 73 smokers (M = 52.1 years, SD = 7.2) and 84 non-smokers (M = 51.7 years, SD = 8.1). Independent t-tests showed that both groups were comparable across participant socio-demographic and health characteristics. Table 2 shows the general characteristics of the study population.
Data for all neuropsychological tasks are summarized in Table 3 . In the Montreal Cognitive Assessment (MoCA), both groups performed above the cutoff score of 26/30, indicating global cognition scores within the “normal” range. Nonetheless, the smokers’ group scored significantly lower than the non-smokers’ group ( p = .042) with a moderate effect size of .49.
The Word condition (W) and Color condition (C) component of the Stroop Color and Word Test revealed no significant differences between both groups, demonstrating comparable processing speed abilities. The Color-word condition (CW) yielded a significant difference between groups ( p ≤ .001, d = .62) demonstrating better selective attention of non-smokers. The Comprehensive Trail making test (CTMT) Trial 1 subtest, which measures processing speed, revealed no significant differences between groups, but the Trial 5 subtest revealed a significant difference ( p = .002, d = .47) representing healthier alternating attention function of non-smokers. For the Wisconsin Card Sorting Test-64 (WCST-64), a small but significant effect size was detected in favor of the non-smokers’ group, showing a slightly better executive function capacity for non-smokers as evident by fewer Perseveration errors ( p < .011, d = .34) and Non-perseveration errors ( p < .026, d = 0.23).
In the Contextual Memory Test, the non-smokers’ group performed significantly better with small effect size for short-term memory ( p = .006, d = .38), and with moderate effect size for long-term memory ( p ≤ .001, d = .66). The Digit Span Task revealed comparable short-term memory results to the Contextual Memory Test, where the non-smokers’ group performed better than the smoking group ( p = .003, d = .40). For working memory, a significant and large effect size of .75 was detected in favor of the non-smokers’ group ( p ≤ .001).
The total number of items processed in the d2 Test of Attention was similar in both groups, indicating parallel processing speed. However, when factoring in the errors in performance, the smoking group had significantly poorer processing accuracy than their non-smokers’ counterpart in both measures of error (OE-Omission errors: p ≤ .001, d = .55, CE-Commission errors: p ≤ .001, d = .54, and TE-Total errors: p ≤ .001, d = .67). The overall TP-Total performance of the d2 Test of Attention was significant with a small effect size ( p = .044, d = .19) reflecting superior selective attention ability of the non-smokers’ group. As per the psychomotor domain, the non-smokers’ group outperformed the smoking group in the Grooved Pegboard test of fine motor dexterity ( p = .007, d = .37).
A set of Pearson correlations were conducted to explore the relationship between smoking variables with scores of the neuropsychological measures. Higher smoking accumulation (total lifetime years of smoking) was significantly correlated with the lower neuropsychological performance of selective attention of the Stroop Color and Word Test ( r = − 0.58; p = .009), alternating attention of the Comprehensive Trail making test (CTMT) ( r = − 0.32; p = .027), and working memory of the Digit Span task ( r = − 0.57; p ≤ .001). There was an association between lifetime years of smoking and poorer performance on the Grooved Pegboard psychomotor measure of fine motor dexterity ( r = −.41; p = .047). The age onset of regular smoking was also correlated with the lower performance in the same measures above; namely selective attention ( r = − 0.41; p = .037), alternating attention ( r = − 0.29; p = .048), working memory ( r = − 0.62; p ≤ .001), and also processing accuracy of the d2 Test of Attention ( r = − 0.46; p = .039). There were no other significant correlations between the number of cigarettes smoked per day or Pack-Years and neuropsychological performance.
When asking the participants about the effects of smoking on health, 100% of non-smokers and 92% of smokers believe that smoking has negative effects on physical health, while 37% of non-smokers and 19% of smokers believed that smoking has negative effects on cognitive health.
The current study aimed to assess and compare the neuropsychological functions of chronic tobacco smokers in comparison to non-smokers. As hypothesized, the results indicated that chronic tobacco smokers had significantly poorer neuropsychological functions compared to their group-matched non-smokers. The poorer performance was apparent in almost all cognitive domains, namely attention, memory, processing accuracy, and executive function, but not processing speed.
With respect to global neuropsychological function, the performance of smokers in the Montreal Cognitive Assessment (MoCA) was not impaired since they scored within the “normal” range. Nonetheless, their performance was significantly weaker compared to their matched non-smokers. Despite the “normal” results of the MoCA, the more dedicated measures in this study clearly revealed a sub-optimal neuropsychological performance of our smokers’ group.
The effects of smoking on memory performance is inconsistent in the literature, with studies reporting significant differences between smokers and non-smokers in some memory measures [ 4 , 21 , 22 , 23 , 24 , 25 ] while others reporting insignificant differences [ 10 , 21 , 22 , 26 , 27 , 28 ]. Our results showed the neuropsychological domain of memory to be affected in our smoking sample and with group differences of moderate to strong effect sizes, as our non-smokers’ group outperformed the smokers in all components of memory measures. The non-smokers’ group had better short-term and long-term memory as indicated by their superior capacity to recall information in the Contextual Memory Test, which involves visual presentation of pictures, as well as the Digit Span Task, which involves auditory and verbal presentation of numbers. Working memory was particularly compromised in our chronic tobacco smokers, as evident by the largest effect size (d = .75) amongst the neuropsychological measures employed in this study. Similar results for working memory impairments were found in middle-aged adults [ 22 , 29 ], young adults [ 30 , 31 , 32 , 33 ] and even adolescent smokers [ 34 ]. Based on the compromised overall memory performance of smokers, it is plausible to state that chronic tobacco smoking may predispose the development of dementia.
While the effects of tobacco smoking on memory have been widely studied, their effects on attention have been less investigated. Attention is central for learning and memory since encoding information requires attention in the first place. The smokers in our study were less able to block irrelevant information and focus their selective attention on the task at hand. This was strongly demonstrated in the Stroop Color and Word Test and less significantly demonstrated in the d2 Test of Attention. Alternating attention was also affected in our smokers’ CTMT test, indicating more difficulty to disengage and reengage the focus of attention in response to environmental stimuli, in comparison to the non-smokers’ group. Our findings on the domain of attention are consistent with the literature where attention was found to be affected in smokers [ 28 , 31 ], but contrasted several other studies where attention was not affected [ 10 , 26 , 33 , 34 ]. Given the intertwined relationship between attention and memory, it is reasonable to suggest that smoking may diminish memory as a result of decreasing attentional capacities, reflected in reduced ability to resist distraction and blocking irrelevant stimuli.
Executive function involves the simultaneous use of a set of cognitive abilities to allow the individual to perform higher-level complex tasks. The findings in our study detected a significant executive function difference in favor of the non-smokers’ group, who performed better in both subtests of the WCST-64. These results are consistent with previous literature findings of executive function limitation among smokers [ 9 , 10 , 22 , 23 , 25 , 26 , 31 , 35 ], indicating that chronic tobacco smokers have inferior mental flexibility and abstract thinking compared to non-smokers.
Interestingly, processing speed was not significantly affected in the smoking group across all the four subtests we used in this study, albeit two of the subtests were statistically borderline ( p = .052, .055). These findings agree with few studies [ 10 , 26 , 31 , 33 ], but contrast with most other studies that assessed processing speed for their smoking participants [ 4 , 21 , 22 , 36 , 37 , 38 ]. Despite this finding, the processing accuracy of our smokers’ sample was significantly lower than non-smokers ( p ≤ .001). That is, the smokers’ processing speed matched that of non-smokers, but with a significantly higher number of errors. The substandard performance of smokers on measures of processing accuracy, as evident by a significantly higher number of cognitive errors, should be added to a growing list of neuropsychological sequelae associated with persistent smoking.
Within the smoker group, smoking onset was correlated with lower performance on working memory, selective attention, alternating attention, and the number of errors made. This means that individuals who start smoking at a younger age are at a greater risk of developing neuropsychological dysfunction. The impairments appear to manifest very early in smokers, as demonstrated in the inferior working memory of adolescents with a mean of only 4 years of smoking [ 34 ]. Additional support on the effects of smoking on the young brain was objectively shown in functional magnetic resonance imaging where young adult smokers had reduced prefrontal cortex activation during attentional tasks when compared with non-smokers [ 39 ]. This pronounced negative effects of smoking on the young brain might be explained by the fact that the prefrontal cortex has not completed its maturation, as the prefrontal cortex is one of the last brain areas to mature and is still developing during adolescence and early adulthood [ 40 ]. This stage of ongoing development makes the brain more susceptible to the influence of tobacco smoking and other psychoactive substances [ 40 ].
Correspondingly, smoking accumulation in this study similarly affected neuropsychological function, meaning the longer an individual smoked during their lifetime, the more prone they become to cognitive dysfunction. These results are consistent with other studies that reported total lifetime years of smoking to be correlated with inferior neuropsychological efficiency [ 22 ] and executive function [ 27 ]. Interestingly, the number of cigarettes per day or pack-years were not associated with neuropsychological performance in this study, contrasting the main findings of a recent review conducted by Conti et al. (2019), in which several studies included in their review reported a negative link between the number of pack-years and neuropsychological function [ 2 ]. This discrepancy in results may be confounded by the considerable variations in the number of pack-years reported across studies (ranging from 4.26 to 73.73) [ 2 ].
The magnitude of differences between smokers and non-smokers in this study extend beyond neuropsychological function to include the psychomotor domain as well. Our non-smokers’ group outperformed the smoking group in the Grooved Pegboard test of fine motor dexterity, albeit with a relatively small effect size of .37. Durazzo et al. [ 22 ] also reported significantly poorer fine motor dexterity performance in their smoking sample, but with much larger effect sizes (i.e., .72). The psychomotor function was also negatively correlated with smoking accumulation, where longer lifetime smoking was associated with reduced fine motor dexterity.
The question arises related to the reasons for heterogeneity in study outcomes between studies of comparable designs. For example, processing speed was reported in some studies to be reduced among smokers [ 4 , 21 , 22 , 36 , 37 , 38 ] but not in other studies [ 10 , 26 , 31 , 33 ]. The same point can be raised for memory and attention. Several reasons could explain such variations in the performance; 1) The outcome measures used to assess the target variable (i.e., processing speed) are different across studies, which may vary in their sensitivity and other psychometric properties. 2) Some studies did not report which version of the tests they used (e.g., original version, modified version, pen and paper, or electronic version). Depending on the assessment used, it could involve a visual task, an auditory task, or even a motor task. An assessment that involves the use of pen and paper will recruit a variety of motor and cognitive pathways in the brain to facilitate writing or tracing, while an electronic version of the same assessment will involve different brain pathways. Such variations in assessment components could yield different results. These factors make it less accurate to perform cross-study comparisons or to compare the study scores with normative data. A possible solution to help makes the results more comparable across studies, and thus more clinically meaningful, is to use complete reporting of the specific testing procedures (e.g., test version, subtests used, and scoring methods) and to use consistent and systematic data collection and analysis procedures.
The participants awareness about the negative effects of smoking on physical health were very high. However, their awareness about the negative effects of smoking on neuropsychological health was low, particularly among smokes (37% of non-smokers and 19% of smokers). This very low awareness of the negative consequences of smoking on neuropsychological function among smokers and non-smokers is a major public health issue that should be properly addressed.
Since tobacco smoking remains highly prevalent across the globe and is known to be influenced by public perception of risk and associations with negative outcomes [ 31 ], it is imperative to invest further in policy initiatives to control smoking. The emphasis of public health campaigns primarily focuses on physical health, and less commonly address neuropsychological health. Increasing public awareness should go beyond the already established physical health consequences to include the negative impact of smoking on neuropsychological function. We hope that raising awareness about the wider effects of tobacco smoking on cognition could help encourage people to stop smoking.
Limitations and future research
There are several limitations and areas for improvement in future research. Although we attempted group matching for demographics and health variables, the self-reported health & medical profile can be problematic due to matters of inaccuracy of some participants. Future research should adopt standardized screening measures to provide accurate objective measures. The scope of neuropsychological impairment associated with chronic cigarette smoking has yet to be fully delineated. Large-scale prospective studies with more consistent, highly sensitive, and robust cognitive outcome measures are required to determine the true links between smoking and neuropsychological dysfunction.
Chronic tobacco smoking seems to be a prospective risk factor for neuropsychological impairment, as expressed in our data by reduced attention, memory, executive function, and processing accuracy of smokers compared to their group-matched non-smokers. The cognitive performance of participants decreased as the number of years smoking increased. Similarly, the younger the age when regular smoking started, the lower was the cognitive performance. The consequences of smoking go beyond neuropsychological performance to encompass fine motor dexterity tasks as well.
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
Montreal Cognitive Assessment
Comprehensive Trail making test
Wisconsin Card Sorting Test-64
World Health Organization, “WHO global report on trends in prevalence of tobacco use 2000–2025,” 2019.
Google Scholar
Conti AA, Lauren M, Serenella T, Steele JD, Baldacchino A. Chronic tobacco smoking and neuropsychological impairments: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2019;96:143–54.
Article CAS PubMed Google Scholar
Waisman Campos M, Serebrisky D, Castaldelli-Maia JM. Smoking and cognition. Curr Drug Abuse Rev. 2016;9(76):1–4.
Richards M, Jarvis MJ, Thompson N, Wadsworth ME. Cigarette smoking and cognitive decline in midlife: evidence from a prospective birth cohort study. Am J Public Health. 2003;93(6):994–8. https://doi.org/10.2105/AJPH.93.6.994 .
Article PubMed PubMed Central Google Scholar
Durazzo TC, Meyerhoff DJ, Nixon SJ. Chronic cigarette smoking: implications for neurocognition and brain neurobiology. Int J Environ Res Public Health. 2010;7(10):3760–91. https://doi.org/10.3390/ijerph7103760 .
Wang C-C, Lu T-H, Liao W-C, Yuan S-C, Kuo P-C, Chuang H-L, et al. Cigarette smoking and cognitive impairment: a 10-year cohort study in Taiwan. Arch Gerontol Geriatr. 2010;51(2):143–8.
Article PubMed Google Scholar
Momtaz YA, Ibrahim R, Hamid TA, Chai ST. Smoking and cognitive impairment among older persons in Malaysia. Am J Alzheimers Dis Other Dement. 2015;30(4):405–11. https://doi.org/10.1177/1533317514552318 .
Article Google Scholar
Pandey K, Panday D, Sapkota N, Dhami A, Sarraf A, Shrestha S, et al. Effect of smoking in cognition. J Pulm Respir Med. 2017;3(399):2.
Razani J, Boone K, Lesser I, Weiss D. Effects of cigarette smoking history on cognitive functioning in healthy older adults. Am J Geriatr Psychiatry. 2004;12(4):404–11. https://doi.org/10.1097/00019442-200407000-00008 .
Caspers K, Arndt S, Yucuis R, McKirgan L, Spinks R. Effects of alcohol- and cigaretteuse disorders on global and specific measures of cognition in middle-age adults. J Stud Alcohol Drugs. 2010;71(2):192–200. https://doi.org/10.15288/jsad.2010.71.192 .
Heishman S, Kleykamp B, Singleton E. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl.). 2010;210(4):453–69. https://doi.org/10.1007/s00213-010-1848-1 .
Wagner M, Schulze-Rauschenbach S, Petrovsky N, Brinkmeyer J, von der Goltz C, Gründer G, et al. Neurocognitive impairments in non-deprived smokers-results from a population-based multi-center study on smoking-related behavior. Addict Biol. 2013;18(4):752–61. https://doi.org/10.1111/j.1369-1600.2011.00429.x .
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. https://doi.org/10.1111/j.1532-5415.2005.53221.x .
Golden CJ, Freshwater SM. The Stroop color word test: a manual for clinical and experimental uses. Los Angeles: Western Psychological Services; 2002.
Moses JA. Test review-Comprehensive Trail Making Test (CTMT). Arch Clin Neuropsychol. 2004;19(5):703–8. https://doi.org/10.1016/j.acn.2004.02.004 .
Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin card sorting test-, 64 card version: WCST-64. Lutz: PAR; 2000.
Toglia JP. Contextual memory test. San Antonio: Therapy Skill Builders; 1993.
Wechsler D. Wechsler adult intelligence scale: administration and scoring manual. 3rd ed. San Antonio: The Psychological Corporation; 1997.
Bates ME, Lemay EP Jr. The d2 test of attention: construct validity and extensions in scoring techniques. J Int Neuropsychol Soc. 2004;10(3):392–400. https://doi.org/10.1017/S135561770410307X .
Ruff RM, Parker SB. Gender-and age-specific changes in motor speed and eye-hand coordination in adults: normative values for the Finger Tapping and Grooved Pegboard Tests. Perceptual Motor Skills. 1993;76(3_suppl):1219–30.
Nooyens AC, Gelder BM v, Verschuren WM. Smoking and cognitive decline among middle-aged men and women: the Doetinchem cohort study. Am J Public Health. 2008;98(12):2244–50. https://doi.org/10.2105/AJPH.2007.130294 .
Durazzo T, Meyerhoff D, Nixon S. A comprehensive assessment of neurocognition in middle-aged chronic cigarette smokers. Drug Alcohol Depend. 2012;122(1):105–11. https://doi.org/10.1016/j.drugalcdep.2011.09.019 .
Sabia S, Marmot M, Dufouil C, Singh-Manoux A. Smoking history and cognitive function in middle age from the Whitehall II study. Arch Intern Med. 2008;168(11):1165–73. https://doi.org/10.1001/archinte.168.11.1165 .
Reitz C, Luchsinger J, Tang M-X, Mayeux R. Effect of smoking and time on cognitive function in the elderly without dementia. Neurology. 2005;65(6):870–5. https://doi.org/10.1212/01.wnl.0000176057.22827.b7 .
Sabia S, Elbaz A, Dugravot A, Head J, Shipley M, Hagger-Johnson G, et al. Impact of smoking on cognitive decline in early old age: the Whitehall II cohort study. Arch Gen Psychiatry. 2012;69(6):627–35. https://doi.org/10.1001/archgenpsychiatry.2011.2016 .
Paul RH, Brickman AM, Cohen RA, Williams LM, Niaura R, Pogun S, et al. Cognitive status of young and older cigarette smokers: data from the international brain database. J Clin Neurosci. 2006;13(4):457–65. https://doi.org/10.1016/j.jocn.2005.04.012 .
Durazzo TC, Rothlind JC, Gazdzinski S, Banys P, Meyerhoff DJ. A comparison of neurocognitive function in nonsmoking and chronically smoking short-term abstinent alcoholics. Alcohol. 2006;39(1):1–11. https://doi.org/10.1016/j.alcohol.2006.06.006 .
Bashir S, Alghamdi F, Alhussien A, Alohali M, Alatawi A, Almusned T, et al. Effect of smoking on cognitive functioning in Young Saudi Adults. Med Sci Monitor Basic Res. 2017;23:31.
George TP, Vessicchio JC, Termine A, Sahady DM, Head CA, Pepper WT, et al. Effects of smoking abstinence on visuospatial working memory function in schizophrenia. Neuropsychopharmacology. 2002;26(1):75–85. https://doi.org/10.1016/S0893-133X(01)00296-2 .
Sutherland MT, Ross TJ, Shakleya DM, Huestis MA, Stein EA. Chronic smoking, but not acute nicotine administration, modulates neural correlates of working memory. Psychopharmacology. 2011;213(1):29–42.
Chamberlain SR, Odlaug BL, Schreiber LR, Grant JE. Association between tobacco smoking and cognitive functioning in young adults. Am J Addict. 2012;21:S14–9.
Spilich GJ, June L, Renner J. Cigarette smoking and cognitive performance. Br J Addict. 1992;87(9):1313–26. https://doi.org/10.1111/j.1360-0443.1992.tb02740.x .
Fried PA, Watkinson B, Gray R. Neurocognitive consequences of cigarette smoking in young adults – a comparison with pre-drug performance. Neurotoxicology. 2006;28(4):517–25. https://doi.org/10.1016/j.ntt.2006.06.003 .
Article CAS Google Scholar
Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005;57(1):56–66. https://doi.org/10.1016/j.biopsych.2004.10.022 .
Whalley LJ, Fox HC, Deary IJ, Starr JM. Childhood IQ, smoking, and cognitive change from age 11 to 64 years. Addict Behav. 2005;30(1):77–88. https://doi.org/10.1016/j.addbeh.2004.04.014 .
Valentine G, Sofuoglu M. Cognitive effects of nicotine: recent Progress. Curr Neuropharmacol. 2018;16(4):403–14. https://doi.org/10.2174/1570159X15666171103152136 .
Article CAS PubMed PubMed Central Google Scholar
Starr JM, Deary IJ, Fox HC, Whalley LJ. Smoking and cognitive change from age 11 to 66 years: a confirmatory investigation. Addict Behav. 2007;32(1):63–8. https://doi.org/10.1016/j.addbeh.2006.03.020 .
Corley J, Gow AJ, Starr JM, Deary IJ. Smoking, childhood IQ, and cognitive function in old age. J Psychosom Res. 2012;73(2):132–8. https://doi.org/10.1016/j.jpsychores.2012.03.006 .
Musso F, Bettermann F, Vucurevic G, Stoeter P, Konrad A, Winterer G. Smoking impacts on prefrontal attentional network function in young adult brains. Psychopharmacology. 2007;191(1):159–69.
Goriounova NA, Mansvelder HD. Short- and long-term consequences of nicotine exposure during adoles¬cence for prefrontal cortex neuronal network function. Cold Spring Harbor Perspect Med. 2012;2(12):a012120.
Download references
Acknowledgements
Not applicable.
Author information
Authors and affiliations.
Occupational Therapy Department, Faculty of Allied Health Sciences, Kuwait University, Jabriah, Kuwait
Mohammed Sh. Nadar
School of Pharmacy and Pharmaceutical Sciences, Cardiff University, Cardiff, UK
Abdullah M. Hasan & Mohammed Alsaleh
You can also search for this author in PubMed Google Scholar
Contributions
MN designed the study, coordinated the implementation of the study and data collection, and drafted the manuscript. AH and MA managed the literature review and references cited. AH and MA reviewed and revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Correspondence to Mohammed Sh. Nadar .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by Kuwait University Health Sciences Center Ethical Committee. All participants signed a written consent form to participate in the study before data collection.

Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Nadar, M.S., Hasan, A.M. & Alsaleh, M. The negative impact of chronic tobacco smoking on adult neuropsychological function: a cross-sectional study. BMC Public Health 21 , 1278 (2021). https://doi.org/10.1186/s12889-021-11287-6
Download citation
Received : 12 August 2020
Accepted : 15 June 2021
Published : 30 June 2021
DOI : https://doi.org/10.1186/s12889-021-11287-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Working memory
- Cognitive impairment
- Chronic smoking
BMC Public Health
ISSN: 1471-2458
- General enquiries: [email protected]
- Open access
- Published: 09 August 2024
Modeling the population health impact of accurate and inaccurate perceptions of harm from nicotine
- Thaddaeus Hannel 1 ,
- Lai Wei 1 ,
- Raheema S. Muhammad-Kah 1 ,
- Edward G. Largo 1 &
- Mohamadi Sarkar 1
Harm Reduction Journal volume 21 , Article number: 145 ( 2024 ) Cite this article
302 Accesses
66 Altmetric
Metrics details
Scientific evidence clearly demonstrates that inhaling the smoke from the combustion of cigarettes is responsible for most of the harm caused by smoking, and not the nicotine. However, a majority of U.S. adults who smoke inaccurately believe that nicotine causes cancer which may be a significant barrier, preventing switching to potentially reduced risk, non-combustible products like electronic nicotine delivery systems (ENDS) and smokeless tobacco (ST). We assessed the population health impact associated with nicotine perceptions.
Using a previously validated agent-based model to the U.S. population, we analyzed nationally representative data from the Population Assessment of Tobacco and Health (PATH) study to estimate base case rates of sustained (maintained over four waves) cessation and switching to non-combustible product use, by sex. Nicotine perception scenarios were determined from PATH data. The overall switch rate from smoking in Wave 4 to non-combustible product use in Wave 5 (3.94%) was stratified based on responses to the nicotine perception question “Do you believe nicotine is the chemical that causes most of the cancer caused by smoking cigarettes?”, (four-item scale from “Definitely not” to “Definitely yes”). The relative percent change between the overall and stratified rates, corresponding to each item, was used to adjust the base case rates of switching, to determine the impact, if all adults who smoke exhibited switching behaviors based on responses to the nicotine perceptions question. The public health impact of nicotine perceptions was estimated as the difference in all-cause mortality between the base case and the four nicotine perception scenarios.
Switch rates associated with those who responded, “Definitely not” (8.39%) resulted in a net benefit of preventing nearly 800,000 premature deaths over an 85-year period. Conversely switch rates reflective of those who responded, “Definitely yes” (2.59%) resulted in a net harm of nearly 300,000 additional premature deaths over the same period.
Conclusions
Accurate knowledge regarding the role of nicotine is associated with higher switch rates and prevention of premature deaths. Our findings suggest that promoting public education to correct perceptions of harm from nicotine has the potential to benefit public health.
Combustible cigarettes are the most harmful of all tobacco products and the leading cause of preventable death in the U.S [ 1 ] which have been attributed to more than 400,000 premature deaths every year [ 2 ]. The morbidity and mortality from smoking cigarettes are primarily due to serious diseases such as lung cancer, chronic obstructive pulmonary disease (COPD), and heart disease [ 1 ]. The harm from cigarette smoking is caused by inhaling the smoke from combustion, which contains more than 7,000 chemicals. The Food and Drug Administration (FDA) has identified a number of these chemicals in cigarette smoke as harmful and potentially harmful constituents (HPHCs) and classified many of them as carcinogens, respiratory or cardiovascular toxicants, reproductive and developmental toxicants, or addictive [ 3 ]. Non-combustible tobacco products such as electronic nicotine delivery systems (ENDS) Footnote 1 ; oral tobacco products e.g., smokeless tobacco, snus, and nicotine pouches; and heated tobacco products either do not contain or have substantially lower levels of these HPHCs compared to cigarettes and therefore can be potentially less risky than cigarettes. Public health authorities, including the FDA, acknowledge that not all tobacco products carry the same amount of risk but instead exist on a risk continuum, with combustible cigarettes considered the most risky and non-combustible products considered much less. Adults who smoke, particularly those who don’t want to quit completely, may reduce their risks of smoking-related diseases by switching from cigarettes to non-combustible products. Therefore, making available products that deliver nicotine in a less risky manner to adults who smoke, particularly those who don’t want to quit, is critical to reducing the risk of smoking-related diseases for such individuals. However, despite the knowledge that smoking cigarettes is harmful, there are nearly 30 million adults in the U.S. that continue to smoke [ 4 ]. One of many potential factors preventing adults who smoke from switching to potentially reduced risk alternatives is misperceptions of nicotine harm.
The vast majority of adults who smoke believe that nicotine is the chemical in cigarettes that causes cancer [ 5 , 6 , 7 ]. For example, researchers from FDA report that about 75% of people either were unsure of the relationship between nicotine and cancer or incorrectly believed that nicotine causes cancer [ 8 ]. The researchers posit that the incorrect beliefs regarding nicotine causing cancer, could discourage adults who smoke from switching to nicotine-containing non-combustible alternatives that may reduce their risks to smoking-related diseases. The observations regarding misperceptions of the harm from nicotine have been consistently reported across many studies. In a U.S. based study, 65% of people who smoke believed nicotine causes lung cancer and 71% believed it caused oral cancer [ 9 ]. Furthermore, a nationally representative study found that two-thirds of adults who smoke responded that “nicotine is a cause of cancer” [ 10 , 11 ]. An International Tobacco Control (ITC) survey conducted from 2002 to 2008 found that more than half of U.S. adults who smoke incorrectly reported that nicotine is the cause of cancer, and this proportion significantly increased over time [ 6 ].
Nicotine misperceptions also impact attitudes and beliefs towards nicotine replacement therapy (NRT). Shiffman et al. found that a majority of adults who currently smoke and those who had formerly smoked (defined as having quit smoking within the last year) perceived NRT products to be just as harmful as smoking and they were significantly less likely to use NRT in future quit attempts [ 12 ]. Moreover, Snell et al., report that nicotine misperceptions could impede efforts to encourage people who smoke to transition to potentially reduced risk sources of nicotine, either to support cessation efforts or to replace cigarette smoking [ 13 ]. However, the net impact of nicotine misperceptions on a population level has not been investigated.
The purpose of this research was to estimate the public health impact associated with varying adults who smoke perceptions of nicotine harm using a computational health impact model. To our knowledge, this is the first analysis of the potential public health impact that focuses on outcomes based on real-world evidence regarding adults who smoke holding either accurate or inaccurate risk perceptions of nicotine. We applied a validated agent-based model to simulate individuals representative of the U.S. population and associated smoking behaviors [ 14 ].We projected changes in smoking prevalence and all-cause mortality outcomes (the number of premature deaths) among adults who smoke over an 85-year period and compared the outcomes associated with accurate perceptions of nicotine harm versus misperceptions.
We started with a base case scenario which represents the status quo. In the base case, the rate of switching to non-combustible use is representative of a population of adults who smoke with their existing perceptions of nicotine harm. We compared the base case to four nicotine perception scenarios in which the rate of switching to non-combusted use is adjusted based on stratified transition probabilities associated with the responses to the question “Do you believe nicotine is the chemical that causes most of the cancer caused by smoking cigarettes?” from Wave 4 data of the Population Assessment of Tobacco and Health (PATH) Study (R04_AC9120, four-item scale from “Definitely not” to “Definitely yes”). Each nicotine perception scenario modeled the population impact expected to occur if all adults who smoke held the same level of perception of nicotine harm as those in each response group from “Definitely not” to “Definitely yes”. We modeled nicotine perception scenarios corresponding to each response rather than dichotomizing them into correct versus incorrect, as other researchers have done [ 8 , 10 , 11 ] to determine the population health impact associated with each response on this perception scale. The public health impact of nicotine perceptions was estimated as the difference in all-cause mortality and cigarette prevalence among adults who smoke, between the base case and each nicotine perception scenario.
In this research, we use a validated Agent-Based Population Model (ABM) as previously described [ 14 , 15 , 16 ] which projects future cigarette prevalence and all-cause mortality beginning in the year 2000. The ABM begins by initializing a hypothetical population of 2.81 million agents (1/100th of the year 2000 U.S. population) that is representative of the U.S. population for age, sex and tobacco use status. The model is iterated 100 times to simulate the entire U.S. population. The initial population mirrors the age and sex distribution data from the year 2000 U.S. Census. Each agent in the initial population was assigned to one of three tobacco use statuses representative of people who have never smoked, people who currently smoke and people who formerly smoked. Tobacco use status was assigned by sex and ages ≥ 18 using information from the National Health Interview Survey (NHIS) Sample Adult Questionnaire data for the year 2000. In our analysis of NHIS data, we defined status of individuals who were currently and previously smoking based on every day or some days definitions and having smoked 100 cigarettes criteria, commonly used by the Centers for Disease Control and Prevention (CDC) to estimate tobacco use prevalence for the U.S. adult population [ 17 ]. Since NHIS does not provide tobacco use information for ages < 18 years, tobacco use status assigned to the younger U.S. population, ages 10–17, by sex were estimated from the 2000 National Youth Tobacco Survey (NYTS). The NYTS is a nationally representative survey of middle and high school students focused exclusively on patterns of tobacco use. In analysis of the NYTS, we used past 30 days and lifetime use of 100 cigarettes or more to define use statuses.
Each agent in the initial population was assigned tobacco use history which was updated over the 100-year simulation timeframe from year 2001 to 2100. Agents who were representative of individuals who smoke or who previously smoked were assigned with their associated years of smoking and/or years stopped smoking and the age(s) at which the agent initiated and/or stopped smoking. Age and sex-specific probabilities from U.S. birth cohort smoking history data developed by Jeon et al. were used to assign when agents in the current smoking or former smoking statuses in the model’s starting population initiated or stopped smoking [ 18 ]. The age and sex specific cigarette smoking initiation and cessation probabilities were generated by Tam et al. who used NHIS surveys administered from 1964 to 2015 to estimate birth cohort smoking histories [ 19 ] and details of the methodological approach and the resulting data are available on the Cancer Intervention and Surveillance Modelling Network website (CISNET, https://resources.cisnet.cancer.gov/projects/ - Publication Support and Modeling Resources).
Once the initial population was generated, the following algorithms were executed in 1-year time intervals throughout the simulation time frame:
Mortality sub-model
A mortality sub-model was used to estimate the survival probability of each agent based on their age, sex and current or former tobacco use history. The mortality sub-model was developed using data from a Kaiser Permanente (KP) Medical Care Program Cohort study [ 20 ], which included number of deaths, person- years, smoking status, age, sex, years smoked, and years since quitting smoking. The KP Study data were adjusted using the Human Mortality Database (HMD) to be representative of the U.S. population in the Year 2000. Mortality rates throughout the simulation time frame were further adjusted to account for expected age-specific changes in mortality over time using the methodology described by Carter et al., [ 21 ].
Transition sub-model
At each time interval within a simulation, agents were provided with an option to change or maintain their current tobacco use status. These decisions were governed by the agent’s defined current tobacco use status, age and sex specific transition probabilities.
Population update
The age of agents who survive at each time interval was increased by 1-year increments. New agents are added to the population each year to account for birth and net immigration based on U.S. Census projections [ 22 ]. We assigned agents who entered the population via immigration with tobacco use status and history similar to that used for the initial population.
We projected ABM scenarios through the year 2100. The public health impact of varying perceptions of nicotine harm was quantified as the difference in adult prevalence and cumulative all-cause deaths for ages 35–85 between the base case and nicotine perception scenarios. We used the 35–85 age range since it is expected that most smoking mortalities occur within this range [ 1 ].
Base case scenario inputs
The base case scenario models smoking prevalence and all-cause mortality where cigarette smoking continues to be the predominant tobacco use behavior. Transition probabilities, by and sex, for initiation from individuals that never smoked to currently smoking to smoking cessation to a state where they were established as previously smoking were obtained from CISNET, where the smoking history of U.S. birth cohorts was reported using NHIS data [ 18 ]. CISNET transition probabilities are available by age (0–99), sex and year through 2015. CISNET initiation probabilities by sex and age are updated each year in the model from 2001 to 2015, at which point they were held constant for the remaining simulation timeframe. Cessation probabilities in CISNET are based on at least two years of successful smoking cessation. This is an important consideration since minimal relapse has been reported [ 23 ] after a period of two years which minimizes the relapse transition between individuals who were previously smoking back to smoking cigarettes. The CISNET smoking cessation probabilities were updated by model each year between 2001 and 2013 with the corresponding yearly estimates. Beginning in the year 2014, former smoking state was split to differentiate individuals that successfully quit smoking and do not use non-combustible products from those who successfully quit smoking but who now use non-combustible products. The non-combustible use status represents the proportion of people who previously smoked who completely switched to non-combustible product use. Figure 1 provides a diagram of the base case tobacco use states and transitions.

Schematic of the base case tobacco use states and transitions. Footnote: Never = Individuals who have never smoked cigarettes; Current = Individuals who are currently smoking cigarettes; Former = Individuals who have previously smoked cigarettes; NCP = non-combustible products
To differentiate individuals who previously smoked from those who use non-combustible products, beginning in 2014 transitions from current smoking to former smoking and current smoking to non-combustible product use were estimated using data from Wave 1 (W1) 2013/2014 to Wave 5 (W5) 2018/2019 of the PATH study which is funded by the FDA Center for Tobacco Products (CTP) and administered by the National Institute on Drug Abuse (NIDA). The PATH study was designed to generate longitudinal epidemiologic data on tobacco-use behavior and health in the U.S. population [ 24 ].
As of 2022, five waves of PATH data are publicly available for analyses [ 25 ]. In our analysis of PATH, we identified adults who smoke ( n = 6,349) in W1 as those who reported smoking at least 100 cigarettes in their lifetime and currently smoke every day or some days. Adults who previously smoked were identified as W1 adults who smoke who no longer smoked in subsequent waves and did not use ENDS (defined in PATH to include products such as e-cigarettes, e-hookahs, e-cigars, e-pipes, personal vaporizers, vape pens, and hookah pens) or smokeless tobacco (ST, defined in PATH to include moist loose snus, snuff, dip and spit or chewing tobacco or snus every day or some days). Non-combustible product use was identified as W1 adults who smoke who no longer smoked but also indicated every day or someday use of ENDS and ST in subsequent waves. We calculated successful quitting and switching as people who smoked in W1 who transitioned to either former smoking or non-combustible product use in W2 (2015/2016) and maintained their status through W5 (an approximate 3-year of follow up). W1 adults who smoke who indicated they had not used ENDS or ST every day or some days in W2 but had used in any subsequent wave, W3 through W5, were excluded from our analysis. This was done to ensure that the transition rates reflected long-term sustained behavior. Estimates from five waves of PATH were used to obtain successful quitting/switching probabilities, which follows a similar methodology as CISNET successful smoking cessation calculation. We observed higher successful cigarette cessation probabilities based on the PATH study data which aligns with observations from existing research that indicates a recent increasing trend in cigarette cessation [ 26 ]. We applied PATH cessation probabilities to account for recent changes in smoking prevalence observed between 2014 and 2018 since they were not captured in the CISNET cessation rates.
The all-cause mortality probability assigned to use of non-combustible products was based on the excess relative risk (ERR) of non-combustible product use compared to cigarette smoking. We applied an ERR of 10%, which reflects a reasonable aggregate estimate for the various non-combustible tobacco products. An ERR of 10% is slightly higher than the estimate of 9% for current ST product use relative to smoking [ 27 ]. Applying a multi-criteria decision analysis (MCDA) model developed by an international expert panel convened by the Independent Scientific Committee on Drugs [ 28 ], the authors assigned the relative importance of different types of harm related to the use of nicotine-containing products compared to cigarette smoking. The panel estimated a relative risk of 5% for snus and 4% for ENDS products relative to smoking. An ERR value of 10% represents a conservative choice for non-combustible use in the model given that this tobacco use state is representative of exclusive or other combinations of use of ST and ENDS. Additionally, we do not allow transitioning to former non-combustible use, therefore agents entering the non-combustible use state carry their former smoking risk in addition to the ERR for current non-combustible use throughout the remaining simulation timeframe.
Nicotine perception scenarios
Nicotine perception scenarios were simulated in which all adults who smoke transition to non-combustible product use at rates associated with different levels of perception of nicotine harm. PATH W4 (2016–2018) data were used to assess adults who smoke perceptions of nicotine harm based on their responses to the question “Do you believe nicotine is the chemical that causes most of the cancer caused by smoking cigarettes?” (R04_AC9120, four-item scale from “Definitely not” to “Definitely yes”). We choose W4 as the baseline wave to analyze the levels of nicotine perception because, in addition to also being a true cross-sectional wave due to sample replenishment, it is more recent compared to W1 (2013–2014) and may reflect the current state of nicotine perception among adults who smoke.
Nicotine perception scenario inputs were developed by first estimating the overall rate of switching from smoking to non-combustible product use based on longitudinal analysis of PATH W4 (2016–2018) and W5 (2018–2019). The analysis included W4 adults who smoke (18+) that responded to the nicotine perception question (i.e., “Definitely not”, “Probably not”, “Probably yes” or “Definitely yes”) ( n = 6886) following the same definitions used to define individuals who smoke and individuals who use non-combustible products in the base case. The overall switching rate was then stratified by W4 adults who smoke perceptions of nicotine harm. We did not estimate switching rates by age or sex to preserve sample size for each of the nicotine perception response groups.
A total of four nicotine perception scenarios were simulated corresponding to each of the four responses to the question “Do you believe nicotine is the chemical that causes most of the cancer caused by smoking cigarettes?”. The model inputs corresponding to each nicotine perception scenario were estimated by adjusting the base case sustained switching rates with the relative percent change between the W4 to W5 overall and stratified transition rates corresponding to each different level of nicotine perception. Relative percent changes were calculated as the stratified rate associated with one level of the nicotine harm perception minus the overall rate divided by the overall Footnote 2 rate of smoking to non-combustible product use between W4 and W5. Relative percent changes were used to adjust the base case rates by sex to avoid over estimation of switching behavior. Switching rates between W4 to W5 would not be expected to be comparable to the sustained 1-year switching rate used in the base case since W5 is a follow up study conducted approximately two years after W4 data collection and may not reflect sustained switching. Therefore, we only applied relative percentage changes between the overall and stratified switching rates to the base case to evaluate the population impact associated with various levels of nicotine harm perception. In each scenario we assume that all adults who smoke will exhibit the switching behavior associated with the specific response groups.
The difference in overall prevalence and all-cause mortality between the base case and each nicotine perception scenario was used to quantify the population health impact corresponding to adults who smoke perceptions of nicotine harm.
Base case PATH estimates
Based on our analysis of PATH W1 to W5, 3.92% male and 4.35% female adults who smoke transitioned to former smoking in W2 and remained through W5. Adults who smoked in W1 transitioned to non-combustible product use in W2 and maintained use of non-combustible products through W5 at a rate of 1.32% and 0.44%, male and female respectively.
Nicotine perception scenario PATH estimates
Table 1 shows the demographics characteristics of W4 adults who smoke overall and by perception of nicotine harm. We note that striking differences were observed among the different age and race/ethnicity subgroups in response to the nicotine perceptions question. While the nicotine misperceptions of harm were high across all age subgroups, they trended to be higher among the 45 + age subgroup. More than 70% of adults who smoke in the 45 + age subgroup responded “Definitely yes” or “Probably yes” regarding harm from nicotine, compared to ~ 50–55% for those in the lower age groups. Additionally, a higher proportion (~ 76%) of adults who smoke in the Non-Hispanic Black and Hispanic subgroups indicated higher perceptions of nicotine harm compared to the Non-Hispanic Whites (~ 58%). The data in Table 1 are important as the observed differences may result in different rates of switching to non-combustible product use between demographic subgroups. Modeling can theoretically be used to investigate population health impacts by demographic characteristics, however the sample sizes needed to provide robust input estimates have limited most models to include only age and sex variables. We focused our simulations on the overall group of W4 adults who smoke (18+) due to sample size and model limitations (i.e., current model is not capable of utilizing population inputs by race or economic status) however it is important to consider the data in Table 1 when interpreting the results.
The overall transition rate for W4 adults who smoke who completely switched to non-combustible product use by W5 was estimated to be 3.94%. Figure 2 shows the proportions of W4 adults who smoke that comprise of each response group and their associated transition rates to non-combustible product use in W5. As shown in Figs. 2 , 6 .61% of W4 adults who smoke ( n = 515 shown in Table 1 ) responded “Definitely not”. Of those adults who smoke who responded, “Definitely not”, 8.39% quit smoking and switched to non-combustible product use by W5, more than twice the overall rate. This is in contrast with 18.52% ( n = 1338 in Table 1 ) of adults who smoke who responded, “Definitely yes”, of which 2.59% switched to exclusive non-combustible product use at W5.

Proportions of Wave 4 adults who smoke that comprise each response group and their associated switch rates to non-combustible product use in Wave 5. Footnote: Analysis based on PATH data from Waves 4 and 5. Error bars represent 95% confidence intervals for each response group switching rate
Table 2 provides the base case transition rate inputs by sex, relative percent change adjustments and the final rates used in each of the four nicotine perception scenarios. As shown in Table 2 , the switch rates among adults switching from smoking cigarettes to using non-combustible products who had accurate nicotine risk perceptions (responding “Definitely not”) was more than twice compared to those who had nicotine risk misperceptions (responding “Definitely yes”) Also included in Table 2 are relative percent change adjustments corresponding to 95% confidence limits of the transition rates calculated as the ratio of the upper and lower 95% confidence limit of the transition rate associated with one level of the nicotine harm perception over the overall transition rate of smoking to non-combustible product use between W4 and W5. Sensitivity scenarios were conducted based on these 95% confidence limits (Table 2 ) of the stratified transition rates.
Table 3 shows adults who smoke prevalence projections between 2014 and 2100 for the base case and nicotine perception scenarios. Under the base case scenario, which reflects a mix of nicotine perceptions, the model predicts a relative change of -58.9% (i.e., a 58.9% reduction) in adult smoking prevalence between the years 2014 and 2100. Adult smoking prevalence decreases further over the same period when the transition rate to non-combustible product use is adjusted to correspond to nicotine harm perceptions held by all the adults who smoke in the simulation similar to the “Definitely not” and “Probably not” response groups with relative changes of -63.7% and − 60.2%, respectively. The model predicted slightly higher smoking prevalence in the nicotine perception scenarios if all adults who smoke in the simulation held nicotine harm perceptions similar to the “Probably yes” and “Definitely yes” response groups resulting in relative changes of -57.8% and − 57.1%, respectively.
Figure 3 shows the predicted population health impact as cumulative premature deaths prevented through the year 2100. The nicotine perception scenarios in Fig. 3 resulting in cumulative premature deaths prevented greater than zero correspond to a net population health benefit. On the other hand, differences less than zero indicate an increase in smoking attributable mortality. Additionally, cumulative premature deaths prevented equal to zero indicate no change from the base case would be expected. The error bars in Fig. 3 show the results of additional sensitivity simulations corresponding to adjustments from the relative percent change of the overall W4-W5 switch rate and the 95% confidence limits of the stratified switch rates by nicotine perception response groups. The scenarios corresponding to “Definitely not” and “Probably not” both result in positive net public health gains of approximately 800,000 and 200,000 premature deaths prevented over the simulation time frame, respectively.

Predicted population health impact as cumulative premature deaths prevented through to the year 2100. Footnote: The error bars indicate the results of additional sensitivity simulations corresponding to adjustments from the relative percent change of the overall W4-W5 switch rate and the 95% confidence limits of the stratified switch rates by nicotine perception response groups. The scenarios corresponding to “Definitely not” and “Probably not” both result in positive net public health gains of approximately 800,000 and 200,000 premature deaths prevented over the simulation time frame, respectively
Table 4 shows the predicted population health impact as cumulative premature deaths prevented from 2025 to 2100 in 25-year intervals. As shown in Table 4 the population health risks and benefits associated with varying perceptions of nicotine harm are seen as early as 2025.
Figure 4 show the ranges corresponding to sensitivity scenarios in which the adjustments to the base case rates are based on the 95% confidence intervals of the switching rates (see Table 2 ) for the “Definitely not” and Definitely yes” response groups. The upper and lower 95% confidence limits associated with the “Definitely not” response group results in a net population health benefit between 373,000 and 1.3 million premature deaths prevented (shaded region in Fig. 4 labeled Definitely not). The upper and lower 95% confidence limit corresponding to the “Definitely yes” response group results in a net population health risk between approximately 40,000 and 509,000 additional premature deaths (shaded region in Fig. 4 labeled Definitely yes). Figure 4 illustrates that if all the adults who smoke exhibit the switching behavior corresponding to those who responded “Definitely not” then a net population benefit could be expected. On the other hand, the behavior associated with adults who smoke with misperceptions regarding the role of nicotine in the harm caused by smoking result in net population risk. The shaded regions in Fig. 4 do not overlap indicating a clear difference in the health outcomes of these two groups.

Ranges of cumulative premature deaths prevented corresponding to sensitivity scenarios for the “Definitely not” and Definitely yes” response groups. Footnote: The adjustments to the base case rates are based on the 95% confidence intervals of the switching rates for the “Definitely not” and Definitely yes” response groups. The upper and lower 95% confidence limits associated with the “Definitely not” response group results in a net population health benefit between 373,000 and 1.3 million premature deaths prevented (shaded region labeled “Definitely not”). The upper and lower 95% confidence limit corresponding to the “Definitely yes” response group results in a net population health risk between approximately 40,000 and 509,000 additional premature deaths (shaded region labeled “Definitely yes”). Note: The shaded regions do not overlap, indicating a clear difference in the outcomes of these two groups
The objective of this study was to estimate the public health impact associated with varying perceptions of nicotine harm using computational modelling. While it is imperative for individuals to understand that nicotine is addictive, it is equally important for them to understand that nicotine is not the main reason why cigarette smoking is harmful to health. A clear trend in the rate of switching to non-combustible product use was observed across the nicotine harm perception response scale. We forecasted substantial benefits to public health if adults who smoke have correct perceptions of nicotine harm and negative consequences from continuing to maintain misperceptions regarding nicotine. Applying to all adults who smoke, switch rates associated with those who responded that nicotine is “Definitely not” the chemical that causes the cancer caused by smoking, resulted in preventing nearly 800,000 premature deaths with an upper estimate of 1.3 million over an 85-year period to 2100. The lower switch rate associated with those who responded “Definitely yes” correspondingly resulted in increasing deaths attributable to smoking by an additional 300,000 by the year 2100 if all adults who smoke held this misperception. Our findings indicate that, if left unchecked, increases in the proportion of adults who smoke who hold misperceptions of nicotine harm could have negative impacts on harm reduction efforts and public health. This is particularly important for “older” and Non-Hispanic Black and Hispanic adults who smoke. Our observations of higher prevalence of nicotine misperceptions, particularly in those individuals in the older age subgroups (Table 1 ) are comparable to those reported recently by Rubenstein et al. [ 29 ]. As indicated by the authors, the higher levels of nicotine misperceptions in these individuals, could be a barrier to switching to non-combustible products. Overall, our results illustrate the potential long-term public health gains that could be made through campaigns aimed at correcting perceptions of nicotine harm among all adults who smoke rather than the current status quo which has been described as a “quarantine of information” [ 30 ]. The trend from risk to benefit across the nicotine perception scenarios indicates that even a modest shift in perceptions of nicotine harm could have positive impacts on public health.
Our analysis of PATH W4 data are consistent with previous research indicating that a majority of adults who smoke (63% responded “Definitely” or “Probably yes”) have misperceptions regarding the role of nicotine as a cause of cancer from smoking [ 5 , 6 , 8 , 10 , 11 , 13 ]. Borland et al. reported nicotine harm perceptions are continuing to trend in the wrong direction with a significant decline observed (OR = 0.97, p = 0.002) among people reporting that nicotine is not the chemical that causes most of the cancer [ 6 ].
Incorrectly believing that nicotine causes cancer could discourage adults who smoke from switching to safer nicotine containing alternatives from cigarettes. Nicotine misperceptions could be erroneously associated with perceived harm of non-combustible products. Wilson et al. found that correct perceptions of nicotine harm were associated with correctly perceiving e-cigarettes to be less harmful than smoking [ 31 ]. Studies have shown that adults who smoke who perceive ENDs to be less harmful than smoking are more likely to switch. Kim et al. reported that switching to ENDS was nearly three times higher for adults who smoke who perceived ENDS to be less harmful than smoking [ 32 ]. Similarly, Snell et al. report that misperceiving nicotine to be a main cause of smoking-related cancers was associated with lower odds for ENDS use (adjusted odds ratio (AOR) = 0.59; 95% confidence interval (CI)I: 0.49, 0.71; p < 0.01) [ 13 ]. Therefore, the findings from our analyses support the notion reported in published literature. Substantial benefit can be manifested if all adults who smoke accurately understand that nicotine is not responsible for the harm related to smoking related diseases.
Moreover, misperceptions regarding the harm from nicotine also impacts the attitudes and beliefs of adults who smoke toward NRTs. Shiffman et al. report that a majority of adults who smoke (66%) perceived NRT products to be just as harmful as smoking or were unsure [ 12 ]. These individuals were less likely to have used NRT in the past (odds ratio (OR) = 0.45, 95% CI: 0.39–0.53) and less likely to consider using NRT during future quit attempts (OR = 0.60, 95% CI = 0.51–0.71). Additionally, Snell et al. reported lower odds of NRT use (AOR: 0.84; 95% CI: 0.71, 0.99; p = 0.04) among those individuals who made a quit attempt during the study period [ 13 ]. Therefore, nicotine misperceptions could not only impede efforts to encourage adults who smoke to transition from cigarettes to potentially reduced risk sources of nicotine, but also to support cessation efforts.
The results of our findings should be considered in the context of the limitations of the model due to the assumptions. In this study we made several assumptions to simplify the modeling framework. For example, we assumed that initiation rates for smoking to be unchanged between both the base case and nicotine perception scenarios. Additionally, we assumed that the prevalence of immigrating populations, who were smoking and had previously smoked, to be the same as the starting population. This can be a limitation of our approach, since immigrating populations can be vastly different between countries, for example in New Zealand “Asians” including mostly Chinese and Indian people have the lowest smoking prevalence in NZ. Very few Chinese women smoke.( https://www.smokefree.org.nz/smoking-its-effects/facts-figures.Accessed-May , 2024).The model outcomes are not expected to be greatly impacted since these assumptions impact both the base case and the nicotine perception scenarios and the population health impact is evaluated by comparing the outcomes between the scenarios. Thus, changes to total prevalence and all-cause deaths would be impacted to a similar extent in both the base case and nicotine perception scenarios. However, it is important to consider that correcting the misperceptions should be targeted to adults who smoke and minimize any “spillover” reach to youth. Additionally, as the focus of this research is on cigarette smoking prevalence and smoking attributable all-cause mortality, we did not consider relapse from non-combustible product use back to cigarette smoking. Because the base case rate of switching to non-combustible product use is based on long-term analysis, spanning over roughly four years, (PATH W2-W5), this is a reasonable assumption since minimal relapse back to smoking is expected after two years [ 33 ]. Moreover, we assumed cessation from non-combustible product use as nonexistent, a conservative assumption, since it excludes any additional potential population health benefit resulting from long term cessation of non-combustible product s. We also made a simplifying assumption that the impact of initiation to non-combustible product use among those not currently using tobacco products was minimal. While potential increases in initiation of non-combustible products due to corrected perceptions of nicotine harm alone would be expected to decrease the overall net benefit, a concurrent decrease in smoking initiation and potential increases to non-combustible product cessation could act to counterbalance impacts due to initiation by people who have never used tobacco. Various population health models examining the impact of ENDS on the population have shown that the public health benefit associated with rates of smoking cessation and switching generally far outweighs possible increases to initiation [ 34 , 35 , 36 , 37 ]. In a recent model evaluating the impact of introducing a novel oral nicotine product, we found that initiation would have to increase by more than 3000% before the benefit of adults who smoke switching was outweighed [ 38 ]. Finally, we acknowledge that, as cited by some researchers [ 39 , 40 ], number of premature deaths due to an exposure may not be precisely assessed, even under optimal conditions. However, such an approach is reasonable and has been used by authoritative bodies like the Food and Drug Administration [ 41 ] to make regulatory decisions regarding public health impact.
The benefit associated with correcting perceptions of nicotine harm is dependent on the relative risk of the non-combustible product category as compared to smoking. In our model, we assumed that the ERR of non-combustible products was a single value of 0.1. This estimate is derived from the epidemiological evidence for smokeless tobacco products [ 42 ] which is a reasonable and conservative assumption, representative of other non-combustible products. Current estimates of the relative risk of the various non-combustible products (e.g., snus and ENDS products) have been reported to be slightly lower than smokeless tobacco [ 28 , 42 , 43 ].
Accurate knowledge regarding the role of nicotine is associated with higher switch rates and prevention of premature deaths. Our findings suggest that promoting public education to correct nicotine misperceptions has potential to benefit public health. Despite the inherent uncertainty of a modeling approach, this research highlights the potential public health benefit of a harm reduction strategy for current adults who smoke, especially those who don’t want to stop smoking. Providing information to adults who smoke about the role of nicotine and the relative risk of non-combustible products compared to smoking would be crucial to removing barriers to adults who smoke transitioning to non-combustible products, potentially lower harm sources of nicotine. However, dissemination of this information should be considered in the context of interpretation by individuals who have never used tobacco, including youth. In conclusion, correcting the inaccurate perceptions of the harm from nicotine may promote switching to non-combustible products among adults who smoke but don’t want to stop smoking. The switching behavior will accelerate the decline in smoking prevalence and has the potential to substantially reduce premature smoking-related mortality.
Data availability
No datasets were generated or analysed during the current study.
Electronic nicotine delivery systems, e-cigarettes, e-vapor products.
Relative Change = (Stratified Rate – Overall)/Overall.
United States. Public Health Service. Office of the Surgeon General. The health consequences of smoking–50 years of progress: a report of the surgeon general. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General. 2014. 2 volumes p.
CDC. Smoking-attributable mortality, years of potential life lost, and productivity losses–United States, 2000–2004. MMWR Morb Mortal Wkly Rep. 2008/11/15 ed2008. pp. 1226-8.
FDA. Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke; Established List: Food and Drug Administration, HHS (Notice; establishment of a list). Food and Drug Administration Federal Register. 2012a. pp. 20034-7.
Cornelius M, Loretan C, Wang T, Jamal A, Homa D. Tobacco product use among adults — United States, 2020. MMWR Morb Mortal Wkly Rep. 2022;71:397–405.
Article PubMed PubMed Central Google Scholar
Siahpush M, McNeill A, Hammond D, Fong GT. Socioeconomic and country variations in knowledge of health risks of tobacco smoking and toxic constituents of smoke: results from the 2002 International Tobacco Control (ITC) Four Country Survey. Tob Control. 2006;15(Suppl 3):iii65–70.
Borland R, Cooper J, McNeil A, O’Connor R, Cummings K. Trends in beliefs about the harmfulness and use of stop-smoking medications and smokeless tobacco products among cigarettes smokers: findings from the ITC four-country survey. Harm Reduct J. 2011;8(21).
Villanti AC, Byron MJ, Mercincavage M, Pacek LR. Misperceptions of Nicotine and Nicotine reduction: the Importance of Public Education to maximize the benefits of a Nicotine Reduction Standard. Nicotine Tob Res. 2019;21(Suppl 1):S88–90.
O’Brien EK, Nguyen AB, Persoskie A, Hoffman AC. U.S. adults’ addiction and harm beliefs about nicotine and low nicotine cigarettes. Prev Med. 2017;96:94–100.
Article PubMed Google Scholar
Mooney ME, Leventhal AM, Hatsukami DK. Attitudes and knowledge about nicotine and nicotine replacement therapy. Nicotine Tob Res. 2006;8(3):435–46.
Bansal MA, Cummings KM, Hyland A, Giovino GA. Stop-smoking medications: who uses them, who misuses them, and who is misinformed about them? Nicotine Tob Res. 2004;6(Suppl 3):S303–10.
Cummings KM, Hyland A, Giovino GA, Hastrup JL, Bauer JE, Bansal MA. Are smokers adequately informed about the health risks of smoking and medicinal nicotine? Nicotine Tob Res. 2004;6(Suppl 3):S333–40.
Shiffman S, Ferguson SG, Rohay J, Gitchell JG. Perceived safety and efficacy of nicotine replacement therapies among US smokers and ex-smokers: relationship with use and compliance. Addiction. 2008;103(8):1371–8.
Snell LM, Colby SM, DeAtley T, Cassidy R, Tidey JW. Associations between Nicotine Knowledge and Smoking Cessation behaviors among US adults who smoke. Nicotine Tob Res. 2022;24(6):855–63.
Article CAS PubMed Google Scholar
Muhammad-Kah R, Hannel T, Wei L, Pithawalla YB, Gogova M. A computational model assessing population impact of a new tobacco product. Qeios. 2023.
Lee PN, Abrams D, Bachand A, Baker G, Black R, Camacho O, et al. Estimating the Population Health Impact of recently introduced modified risk Tobacco products: a comparison of different approaches. Nicotine Tob Res. 2021;23(3):426–37.
Muhammad-kah M, Pithawalla YB, Wei L, Hannel T, Gogova M, Boone EL, editors. Presentation and poster session on advances in nonparametric statistics: An agent based modeling approach for tobacco product risk assessments. Joint Statistical Meetings (JSM 2016); 2016; Chicago.
Centers for Disease Control and Prevention. NCHS Vital Health Statistics Series 10. In: Health NIo, editor 2017.
Jeon J, Holford TR, Levy DT, Feuer EJ, Cao P, Tam J, et al. Smoking and Lung Cancer Mortality in the United States from 2015 to 2065: a comparative modeling Approach. Ann Intern Med. 2018;169(10):684–93.
Tam J, Levy DT, Jeon J, Clarke J, Gilkeson S, Hall T, et al. Projecting the effects of tobacco control policies in the USA through microsimulation: a study protocol. BMJ Open. 2018;8(3):e019169.
Friedman G, Tekawa I, Sadler M, Sidney S. Smoking and mortality: the Kaiser Permanente experience. Smoking and Tobacco Control Monograph No 8: changes in cigarette-related Disease risks and their implications for Prevention and Control. Bethesda, MD: National Cancer Institute, U.S. National Institutes of Health; 1997. pp. 477–500.
Google Scholar
Carter LR, Lee RD. Modeling and forecasting U.S. sex differentials in mortality. Int J Forecast. 1992;8(3):393–411.
Census Bureau US. 2008 National Population Projections Tables United States Census Bureau [ https://www.census.gov/data/tables/2008/demo/popproj/2008-summary-tables.html .
Anderson CM, Burns DM, Dodd KW, Feuer EJ. Chapter 2: birth-cohort-specific estimates of smoking behaviors for the U.S. population. Risk Anal. 2012;32(0 1):S14–24.
PubMed PubMed Central Google Scholar
Hyland A, Ambrose BK, Conway KP, Borek N, Lambert E, Carusi C, et al. Design and methods of the Population Assessment of Tobacco and Health (PATH) study. Tob Control. 2017;26(4):371–8.
Health USDo A, HSNIoHNIoD H, USDo, Food HS, Products DACT. Population Assessment of Tobacco and Health (PATH) study [United States] Public-Use Files. Inter-university Consortium for Political and Social Research [distributor]; 2022.
Mendez D, Le TTT, Warner KE. Monitoring the increase in the U.S. Smoking Cessation Rate and its implication for future smoking prevalence. Nicotine Tob Res. 2022;24(11):1727–31.
Muhammad-Kah RS, Pithawalla YB, Boone EL, Wei L, Jones MA, Black RA et al. A Computational Model for Assessing the Population Health Impact of Introducing a Modified Risk Claim on an Existing Smokeless Tobacco Product. Int J Environ Res Public Health. 2019;16(7).
Nutt DJ, Phillips LD, Balfour D, Curran HV, Dockrell M, Foulds J, et al. Estimating the harms of nicotine-containing products using the MCDA approach. Eur Addict Res. 2014;20(5):218–25.
Rubenstein D, Denlinger-Apte RL, Cornacchione Ross J, McClernon FJ. Adoption of E-Cigarettes among older adults who smoke to reduce harm and narrow age-related disparities: an application of the Health Belief Model. Nicotine Tob Res. 2023;25(6):1212–4.
Kozlowski LT, Sweanor D. Withholding differential risk information on legal consumer nicotine/tobacco products: the public health ethics of health information quarantines. Int J Drug Policy. 2016;32:17–23.
Wilson S, Partos T, McNeill A, Brose LS. Harm perceptions of e-cigarettes and other nicotine products in a UK sample. Addiction. 2019;114(5):879–88.
Kim S, Shiffman S, Sembower MA. US adult smokers’ perceived relative risk on ENDS and its effects on their transitions between cigarettes and ENDS. BMC Public Health. 2022;22(1):1771.
Herd N, Borland R, Hyland A. Predictors of smoking relapse by duration of abstinence: findings from the International Tobacco Control (ITC) Four Country Survey. Addiction. 2009;104(12):2088–99.
Vugrin ED, Rostron BL, Verzi SJ, Brodsky NS, Brown TJ, Choiniere CJ, et al. Modeling the potential effects of new tobacco products and policies: a dynamic population model for multiple product use and harm. PLoS ONE. 2015;10(3):e0121008.
Cherng ST, Tam J, Christine PJ, Meza R. Modeling the effects of E-cigarettes on Smoking Behavior: implications for future adult smoking prevalence. Epidemiology. 2016;27(6):819–26.
Levy DT, Borland R, Lindblom EN, Goniewicz ML, Meza R, Holford TR, et al. Potential deaths averted in USA by replacing cigarettes with e-cigarettes. Tob Control. 2018;27(1):18–25.
Warner KE, Mendez D. E-cigarettes: comparing the possible risks of increasing smoking initiation with the potential benefits of increasing Smoking Cessation. Nicotine Tob Res. 2019;21(1):41–7.
Muhammad-Kah M, Hannel T, Cheng H, Sarkar M, editors. Assessing the Potential Population Health Impact of a Market Authorization of an Oral Nicotine Pouch Product in the U.S. Society for Research on Nicotine and Tobacco; 2021 February 24–27, 2021; Virtual Conference.
Morfeld P, Erren TC. Mortality and attributable fraction in COVID-19 analysis: avoiding Research Waste and negligence. Am J Public Health. 2020;110(11):1644–5.
Robins JM, Greenland S. Estimability and estimation of excess and etiologic fractions. Stat Med. 1989;8(7):845–59.
Le TT, Mendez D. An estimation of the harm of menthol cigarettes in the United States from 1980 to 2018. Tob Control. 2021.
Fisher MT, Tan-Torres SM, Gaworski CL, Black RA, Sarkar MA. Smokeless tobacco mortality risks: an analysis of two contemporary nationally representative longitudinal mortality studies. Harm Reduct J. 2019;16(1):27.
Murkett R, Rugh M, Ding B. Nicotine products relative risk assessment: a systematic review and meta-analysis. F1000Research. 2020;9.
Download references
Acknowledgements
The authors would like to acknowledge the following individuals from Altria Client Services LLC., Elizabeth Becker, Lincoln Edwards and Brandon Newmyer for reviewing the early draft and providing helpful comments.
No external funding was received for this research.
Author information
Authors and affiliations.
Altria Client Services LLC Center for Research and Technology, 601 E. Jackson Street, 23219, Richmond, VA, USA
Thaddaeus Hannel, Lai Wei, Raheema S. Muhammad-Kah, Edward G. Largo & Mohamadi Sarkar
You can also search for this author in PubMed Google Scholar
Contributions
TH and RMK developed the concept, LW generated the model inputs from PATH dataset, EL and MS provided strategic direction, all authors contributed towards the writing and review of the manuscript.
Corresponding author
Correspondence to Mohamadi Sarkar .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
We confirm that the corresponding author has read the journal policies and submit this manuscript in accordance with those policies.
Competing interests
The authors are employees of Altria Client Services LLC.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Hannel, T., Wei, L., Muhammad-Kah, R.S. et al. Modeling the population health impact of accurate and inaccurate perceptions of harm from nicotine. Harm Reduct J 21 , 145 (2024). https://doi.org/10.1186/s12954-024-01059-x
Download citation
Received : 14 November 2023
Accepted : 14 July 2024
Published : 09 August 2024
DOI : https://doi.org/10.1186/s12954-024-01059-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Nicotine perceptions
- Misperceptions
- Public health impact
- Population modeling
- Tobacco harm reduction
Harm Reduction Journal
ISSN: 1477-7517
- Submission enquiries: [email protected]

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
Tobacco, Nicotine, and E-Cigarettes Research Report What research is being done on tobacco use?
New scientific developments can improve our understanding of nicotine addiction and spur the development of better prevention and treatment strategies.
Genetics and Epigenetics
An estimated 50-75 percent of the risk for nicotine addiction is attributable to genetic factors. 221 A cluster of genes (CHRNA5-CHRNA3-CHRNB4) on chromosome 15 that encode the α5, α3, and β4 protein subunits that make up the brain receptor for nicotine 221–223 are particularly implicated in nicotine dependence and smoking among people of European descent. Variation in the CHRNA5 gene influences the effectiveness of combination NRT, but not varenicline. 224 Other research has identified genes that influence nicotine metabolism and therefore, the number of cigarettes smoked, 225 responsiveness to medication, 204,205 and chances of successfully quitting. 226 For example, the therapeutic response to varenicline is associated with variants for the CHRNB2, CHRNA5, and CHRNA4 genes, while bupropion-related cessation is linked with variation in genes that affect nicotine metabolism. 227
Smoking can also lead to persistent changes in gene expression (epigenetic changes), which may contribute to associated medical consequences over the long term, even following cessation. 228 Epigenetic changes may serve as a potential biomarker for prenatal tobacco smoke exposure. Researchers found tobacco-specific changes at 26 sites on the epigenome, and this pattern predicted prenatal exposure with 81 percent accuracy. 229 A large scale meta-analysis of data on epigenetic changes associated with prenatal exposure to cigarette smoke also identified many epigenetic changes that persisted into later childhood. 230 More research is needed to understand the long-term health impacts of these changes.
Neuroimaging
Cutting-edge neuroimaging technologies have identified brain changes associated with nicotine dependence and smoking. Using functional magnetic resonance imaging (fMRI), scientists can visualize smokers’ brains as they respond to cigarette-associated cues that can trigger craving and relapse. 231 Such research may lead to a biomarker for relapse risk and for monitoring treatment progress, as well as point to regions of the brain involved in the development of nicotine addiction. 29
A neuroimaging technology called default-mode or resting-state fMRI (rs-fMRI) reveals intrinsic brain activity when people are alert but not performing a particular task. Using this technique, researchers are examining the neurobiological profile associated with withdrawal and how nicotine impacts cognition. 232 Comparisons between smokers and nonsmokers suggest that chronic nicotine may weaken connectivity within brain circuits involved in planning, paying attention, and behavioral control—possibly contributing to difficulty with quitting. 233 fMRI studies also reveal the impact of smoking cessation medications on the brain—particularly how they modulate the activity of different brain regions to alleviate withdrawal symptoms and reduce smoking. A review of these studies suggested that NRT enhances cognition during withdrawal by modulating activity in default-network regions, but may not affect neural circuits associated with nicotine addiction. 234
Some imaging techniques allow researchers to visualize neurotransmitters and their receptors, further informing our understanding of nicotine addiction and its treatment. 27 Using these techniques, researchers have established that smoking increases the number of brain receptors for nicotine. Individuals who show greater receptor upregulation are less likely to stop smoking. 28 Combining neuroimaging and genetics may yield particularly useful information for improving and tailoring treatment. For example, nonsmoking adolescents with a particular variant in the CHRNA5-CHRNA3-CHRNB4 gene cluster (which is associated with nicotine dependence and smoking) showed reduced brain activity in response to reward in the striatum as well as the orbitofrontal and anterior cingulate cortex. This finding suggests that genetics can influence how the brain processes rewards which may influence vulnerability to nicotine dependence. 235 Neuroimaging genetics also shows that other genes, including ones that influence dopamine neurotransmission, influence reward sensitivity and risk for addiction to nicotine. 236

- Center for Financial Research
- Researchers
- Research Fellows
- Senior Advisor
- Special Advisor
- 2023 Fellows
- 2022 Fellows
- Visiting Scholar
Working Papers
- 23rd Bank Research Conference
- Conference Videos
- Speaker Information
- Poster Videos
- Poster Session Videos
- Previous Conference Programs
- Other Conferences
- 2021-2022 FDIC Academic Challenge
- 2020-2021 FDIC Academic Challenge
- Prior Years
- Research Assistants
- Internships
- Visiting Scholars
The FDIC is a preeminent banking research institution. The FDIC established the Center for Financial Research to promote research on topics important to the FDIC's mission including deposit insurance, bank supervision, making large and complex financial institutions resolvable, and resolution of failed financial institutions. The Center has an active seminar series and maintains contacts with preeminent scholars in the industry, academics, and the public sector. Its research follows banking industry developments, risk measurement and management methods, regulatory policy, and related topics. The Center sponsors an annual Bank Research Conference, hosts short-term visiting scholars, and manages a Visiting Scholars Program. The work of our researchers helps the FDIC maintain a safe, sound, and vibrant banking sector.
Papers, Studies, and Survey Reports
The Center publishes working papers, staff studies, survey reports, and other analyses to prompt discussion among the FDIC's many stakeholders to expand knowledge and understanding of issues that affect the banking system.
The Center hosts an annual Bank Research Conference and other events throughout the year to foster dialogue among banking regulators and supervisors, academics, and the private sector.
The Center includes a team of highly qualified economists and researchers, who conduct and publish empirical and theoretical research on the banking industry, bank regulation, and deposit insurance. They also develop statistical and financial models to support FDIC operations. The Center is also supported by advisors, scholars, and fellows who advise senior management and coauthor research papers with economists.
Career opportunities are available for interns, fellowships, and economists.
For additional information about the FDIC Center for Financial Research, please contact us .
Have a Paper You Want to Present at the FDIC?
The FDIC offers a seminar series to present interesting and informative papers. If you would like to present a paper, please e-mail your paper and available presentation dates to [email protected] .
Presenters will be reimbursed for their travel expenses.

About the Symposium
Staff Studies
September 19-20, 2024
Last Updated: May 20, 2024
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.5(10); 2019 Oct
Current advances in research in treatment and recovery: Nicotine addiction
Judith j. prochaska.
1 Stanford Prevention Research Center, Department of Medicine, Stanford University, Stanford, CA, USA.
Neal L. Benowitz
2 Program in Clinical Pharmacology, Division of Cardiology, and the Center for Tobacco Control Research and Education, Department of Medicine, University of California, San Francisco, San Francisco, CA, USA.
This review covers the changing tobacco product marketplace and evidence-based approaches to prevent and treat nicotine addiction.
The health harms of combusted tobacco use are undeniable. With market and regulatory pressures to reduce the harms of nicotine delivery by combustion, the tobacco product landscape has diversified to include smokeless, heated, and electronic nicotine vaping products. Products of tobacco combustion are the main cause of smoking-induced disease, and nicotine addiction sustains tobacco use. An understanding of the biology and clinical features of nicotine addiction and the conditioning of behavior that occurs via stimuli paired with frequent nicotine dosing, as with a smoked cigarette, is important for informing pharmacologic and behavioral treatment targets. We review current advances in research on nicotine addiction treatment and recovery, with a focus on conventional combustible cigarette use. Our review covers evidence-based methods to treat smoking in adults and policy approaches to prevent nicotine product initiation in youth. In closing, we discuss emerging areas of evidence and consider new directions for advancing the field.
INTRODUCTION
“ To lower nicotine too much might end up destroying the nicotine habit in a large number of consumers and prevent it from ever being acquired by new smokers. ”
– British American Tobacco Company internal document, June 1959 ( 1 ).
Combusted tobacco use remains a major cause of premature disability and death around the world ( 2 ). Cigarette smoke contains an estimated 7000 different chemical compounds, of which at least 70 are proven or suspected human carcinogens including arsenic, benzene, formaldehyde, lead, nitrosamines, and polonium 210. Tobacco smoke also contains poisonous gasses: carbon monoxide, hydrogen cyanide, butane, toluene, and ammonia. Little cigars and water pipes deliver similar toxicants.
Tobacco smoking causes about half a million U.S. deaths annually, of which 50,000 are among nonsmokers exposed to secondhand smoke ( 3 , 4 ). More than half of all long-term smokers die from a tobacco-caused disease, with an average loss of at least 10 years of life ( 3 ). Smoking causes 87% of lung cancer deaths, 61% of pulmonary disease deaths [chronic obstructive pulmonary disease (COPD) and emphysema], and one in three cancer deaths. In the 50 years following the U.S. Surgeon General’s first report on tobacco (1964–2014), 20 million Americans died from smoking, and an estimated 1 billion people will die worldwide this century ( 3 , 5 ). For every person who dies from smoking, at least 30 people live with serious smoking-related illnesses costing >$300 billion annually, with nearly $170 billion in direct medical costs and $156 billion in lost worker productivity ( 3 , 6 ).
The health harms of combusted tobacco use are now undeniable ( 7 ). With market and regulatory pressures to reduce the harms of nicotine delivery by combustion, the tobacco product landscape has diversified ( Table 1 ). Nicotine now comes in smokeless tobacco prepackaged pouches (i.e., snus tobacco), in electronic devices that heat nicotine to an inhalable aerosol from a plug of tobacco (i.e., heated or heat-not-burn tobacco) or from an e-liquid (nicotine vaping device; e.g., e-cigarette, vape pen, and pod), and in pharmaceutical-grade nicotine replacement therapies (NRTs) (i.e., gum, lozenge, patch, nasal spray, mouth spray, and inhaler). Cigars come in a variety of sizes down to little filtered cigars, some discernible from cigarettes only by their tobacco leaf wrapper. Despite the diversification, conventional combusted cigarettes remain, by far, the most common nicotine product used by adults in the United States and in most places globally. Worldwide, there are approximately 1 billion smokers ( 5 ).
| Cigarette | Tobacco rolled in paper for smoking | A typical cigarette weighs <1 g; regular length (70 mm long), king (84 mm), 100s (100 mm), and 120s (120 mm) | Acidic, inhalable, pH 5.5–6 | Average in rod, 13.5 mg (range: 11.9–14.5 mg); nicotine yield to the smoker: 1–1.5 mg/cigarette |
| Cigar | Air-cured, fermented tobacco wrapped in material made at least, in part, of tobacco leaf | Small filtered cigars (0.9–1.3 g tobacco), cigarillos (1.3–2.5 g tobacco), and large (premium) cigars | pH 6.5–8.0 inhalable and/or buccal depending on product pH | Nicotine content ranges from 10 to 444 mg and dependent on weight of the cigar |
| Blunt | Cannabis filled in a hollowed-out cigarillo shell | No pH data available | Nicotine intake much lower than from cigarette or cigar smoking, but, based on animal studies, could enhance rewarding effects of delta 9-tetrahydrocannbinaol | |
| Smokeless tobacco | Tobacco inserted between lip and gum or snorted into the nose rather than smoked by the user | Snuff (ground tobacco), snus (ground tobacco in a tea bag–like pouch), chew (shredded tobacco) | Products range from more acidic, pH 5.2–7.1, to more alkaline for greater buccal absorption, pH 7.6–8.6 | Nicotine concentrations vary, range of 0.2 to 34 mg/g, the more alkaline products are capable of delivering higher levels of nicotine |
| Waterpipe/Hookah | Charcoal-heated flavored tobacco passed through a water-filled chamber that cools the smoke | Water tobacco is a mixture of dried fruit, molasses and glycerin, and conventional tobacco leaf | pH 3.8–5.8 | Average of 1.13 mg/g and high of 3.30 mg/g for product containing nicotine; nicotine-free for herbal (nontobacco) varieties |
| Heated tobacco | Electronic devices that heat reconstituted tobacco sticks treated with a glycerin humectant to deliver an aerosol | IQOS, Glo, and Ploom Tech | pH 5.5–6 | Nicotine delivery can match that of conventional cigarettes |
| E-cigarette | Electric devices that produce an aerosol from a liquid that typically contains nicotine, propylene glycol, vegetable glycerin, and flavorings | Cigalikes/e-pens, tank systems, pods/nicotine salts (e.g., benzoate and lactate) | Free base e-liquid: alkaline, pH 7–9; nicotine salts: acidic, inhalable, pH 3.5–6.8 | E-liquid nicotine content from 0 to 100 mg/ml. Nicotine delivery can match that of conventional cigarette but varies by device design (heating temperature), e-liquid nicotine content, and user behavior |
While products of tobacco combustion are the main cause of smoking-induced disease, nicotine addiction sustains tobacco use ( 8 ). Nicotine addiction, in the form of cigarette smoking, causes more harm to public health than any other drug addiction. Reflected in the quote above, at least since the 1950s, the tobacco industry has researched and recognized, decades before it became generally understood in the scientific community, that nicotine is an addictive drug and central to their business ( 9 ). An understanding of the clinical features of nicotine addiction and the behavioral conditioning that occurs with frequent nicotine dosing is important for informing pharmacologic and behavioral treatment targets.
We review current advances in research on nicotine addiction treatment and recovery. The “Tobacco Product Use and Nicotine Addiction” section covers the changing landscape of nicotine products with comparison of use patterns among adults and adolescents in the United States. The pharmacology of nicotine and effects on the brain are then reviewed, with consideration of particularly vulnerable populations. The “Treating Nicotine Addiction in Adults, with a Focus on Conventional Cigarettes” section focuses on treatment of nicotine addiction with attention to counseling and behavioral approaches and cessation medications. The tobacco treatment literature is far more developed for combusted cigarettes and relatively sparse in other product areas. We focus on adults given developmental differences in adolescents’ preferred nicotine product type, use patterns, addiction profile, and treatment efficacy. The tobacco treatment literature with adolescents largely consists of failed smoking cessation trials ( 10 ), and while youth nicotine vaping is drawing public health concern and policy attention, no study, to date, has evaluated an intervention to treat e-cigarette use in adolescents. The “Tobacco Control Population-Based and Policy Approaches” section gives greater attention to use in youth with review of the evidence for tobacco control policy interventions. The “Discussion: What Evidence Is Needed” section closes with discussion of emerging areas and consideration of new directions for advancing the field.
TOBACCO PRODUCT USE AND NICOTINE ADDICTION
Patterns of tobacco use in the united states.
In 2017, 47.4 million U.S. adults (19%) reported every day or some day use of a tobacco product, which includes e-cigarettes (in the United Stated, electronic nicotine delivery systems are classified and regulated as tobacco products) ( 11 ). Among U.S. adult tobacco users, 87% (41.1 million) smoked combusted tobacco products ( 11 ). The prevalence of tobacco product use was 14% (34.3 million) cigarettes; 4% (9.3 million) cigars, cigarillos, and little filtered cigars; 3% (6.9 million) e-cigarettes; 2% (5.1 million) smokeless tobacco; and 1% (2.6 million) pipes, water pipes, or hookah ( 11 ). Among cigarette smokers, 76% smoked daily ( 12 ).
In contrast, among U.S. adolescents, e-cigarettes exceed conventional cigarette use. In 2018, past 30-day e-cigarette use was reported by 21% of high school (3.05 million) and 5% of middle school (570,000) students, and combustible cigarette use was reported by 8% of high school (1.1 million) and 2% of middle school (200,000) students ( 13 ). From 2017 to 2018, e-cigarette use increased by 78% among high school and 49% among middle school students. Preliminary U.S. data for 2019 indicate that e-cigarette use has climbed further to 27.5% among high school students with most reporting use of sweet-flavored (65.9% fruit, 38.7% candy, and 4.2% chocolate) and minty/menthol–flavored (63.9%) e-cigarette products, while use of combustible cigarettes has further declined to 5.8% ( 14 ). To address youth e-cigarette use, the U.S. Food and Drug Administration (FDA) is considering banning all unauthorized non–tobacco-flavored e-cigarettes.
Among adolescents who use tobacco, 7 in 10 use a flavored product ( 15 ). For youth, flavored tobacco products are highly appealing and decrease perceptions of harm and addictiveness ( 16 ). With the explicit intent of protecting youth from smoking initiation, in 2009, the U.S. Congress banned characterizing flavors from traditional cigarettes except for menthol ( 17 ). The 2009 flavored cigarette ban reduced the U.S. youth smoking prevalence; however, menthol cigarette use among adolescent smokers has increased ( 18 ). In 2013, the FDA concluded that menthol cigarettes lead to increased smoking initiation among youth and young adults, greater addiction, and decreased success in quitting smoking ( 19 ). In 2017, menthol cigarettes comprised 36% of the U.S. cigarette market, the highest proportion on record ( 20 ).
Dual use of tobacco products is also on the rise ( 21 , 22 ). The most recent surveillance data show that 3.7% (9 million) of adults ( 11 ), 11% (1.7 million) of high school students, and 2% (270,000) of middle school students ( 13 ) use two or more tobacco products. The most prevalent dual tobacco combination for adults and adolescents was combustible cigarettes and e-cigarettes, followed by cigarettes and cigars for adults, and e-cigarettes and cigars for adolescents. Motivations for dual use among adults include use of smokeless tobacco and e-cigarettes in places where combustible cigarette smoking is prohibited, as a form of harm reduction, and to support quitting smoking ( 23 ).
While e-cigarette use may represent harm reduction for adult smokers, few would suggest a benefit of nicotine vaping in adolescence when the brain is still developing. Unknown is the extent to which e-cigarette use among youth is a fad, will lead some to long-lasting primary nicotine addiction, and/or may be a gateway to cigarette smoking. The National Academies of Sciences 2017 review concluded that there is substantial evidence that e-cigarette use increases the risk of ever smoking combustible cigarettes among youth and young adults, but whether this is trial use versus sustained use could not be determined from the literature ( 24 ). Two recent prospective observational studies of U.S. adolescents reported that among never smokers, e-cigarette use was significantly associated with both initiating and continuing combustible cigarette use ( 25 , 26 ). A potential confounder is cannabis use, which has a high concordance with e-cigarette use. A third prospective study examined the question of whether adolescents are engaging in e-cigarette trial use versus dependent use ( 27 ). The sample was adolescent past-month e-cigarette users reporting 10+ uses in their lifetime at baseline. At 12-month follow-up, 80% continued their e-cigarette use, daily use increased from 14.5 to 29.8%, and the adolescents tended to “graduate up” to higher–nicotine content pod-type devices such as JUUL. The youths’ top e-cigarette flavor preferences—fruit, mint/menthol, and candy—remained stable over time. The adolescents’ self-rated level of e-cigarette addiction correlated significantly with their level of nicotine exposure, as measured by the nicotine metabolite biomarker of urinary cotinine ( 28 ).
The increase in e-cigarette use in youth over the past 5 years has been mirrored by a decrease in cigarette smoking, which raises the question of whether vaping may be diverting some youth away from cigarette smoking. Whether the greater e-cigarette than conventional cigarette use among adolescents will lead to sustained population declines in adult smoking is as yet unknown.
Nicotine addiction: Definitions, biology, clinical features, and vulnerable groups
Defining nicotine addiction.
In this review, we refer to the compulsive use of nicotine and tobacco products as an addiction, based on the definition provided in the 1988 U.S. Surgeon General’s report, referring to “behavior of repetitively ingesting mood-altering substances by individuals” ( 29 ). However, it should be noted that definitions such as that from the World Health Organization (WHO) define addiction as “a behavioral pattern in which the use of a given psychoactive drug is given a sharply higher priority over other behaviors that once had a significantly higher value” ( 30 ). There is no question that cigarette smoking fits both definitions, but with the advent of noncombusted forms of nicotine (like e-cigarettes), which are considered to be much less harmful than cigarette smoking (but not necessarily safe), some clinicians and vapers (those who use e-cigarettes) object to the term addiction because they view pure nicotine dependence as not being detrimental to health. Thus, while we use the term addiction to refer to being unable or unwilling to stop when it is clearly in one’s interest to do so, we acknowledge some controversy as to its application to noncombusted tobacco product use.
Today, the health harms of smoking are well known, and most smokers want to quit. However, most attempts to quit smoking fail. The statistics are striking: (i) 68% of smokers in the United States want to stop smoking, and 55% quit for 24 hours in a given year (many more smokers attempt to quit but are unable to make it a full day) ( 31 ); (ii) 60% of those who quit for a day relapse by 1 week; and (iii) only 7% of quit attempts are sustained 6 months, and 45% of those end in relapse ( 31 ).
Ultimately, more than 90% of smokers try to quit; most make multiple quit attempts, and about half quit smoking long term, although most do not achieve abstinence until after age 30. That most smokers attempt to quit each year and less than 4% of quit attempts are sustained long term illustrates the loss of control of drug use with addiction. Factors that influence the development and maintenance of nicotine addiction are complex and include the drug’s pharmacologic effects and tobacco product design; genetics; learned factors, such as conditioning of stimuli through frequent nicotine dosing; and sociocultural exposures including family and peer use and pervasive tobacco marketing and retail availability ( 8 ).
Nicotine and its pharmacology
Nicotine is an alkaloid that occurs in highest concentrations in leaves of the tobacco plant ( Nicotiana tabacum ). Approximately 95% of the alkaloid content of tobacco is nicotine, along with 5% of minor alkaloids including anabasine, anatabine, and norcotinine. Easy to extract, nicotine from tobacco plants is used almost exclusively in nicotine medications and e-cigarettes.
Nicotine chemistry and pharmacokinetics
Nicotine is a tertiary amine that can exist in a charged (ionized) or uncharged (unionized) form, depending on pH. Nicotine is a weak base with a p K a (where K a is the acid dissociation constant) of 8.0 such that, at physiological pH (7.4), 69% is ionized and 31% is unionized. The unionized (also known as free base) form of nicotine passes readily though membranes, such as the buccal mucosa, such that the pH of smokeless tobacco influences the rate and extent of systemic nicotine absorption. The more alkaline (higher pH), the more rapidly nicotine is absorbed from smokeless tobacco. Cigarette smoke has an acidic pH of about 5.5 to 6, so little nicotine is absorbed through the mouth, while large cigars have an alkaline pH, facilitating oral absorption. The differences in pH of tobacco products depends on the strains of tobacco used and curing processes, as well as on chemicals used in processing. The pH of nicotine solutions also influences the pharmacology of e-cigarettes. The earliest forms of e-cigarette liquid (e-liquid) contained mostly nicotine in free base form (pH 7 to 9), which results in a considerable nicotine-related harshness during inhalation. Recently, e-liquids have used nicotine salts (such as benzoate or lactate), with acidic pH (5.5), similar to that of cigarettes. This results in less irritation with inhalation and has been implicated in the current popularity of e-cigarette use in never-smoker adolescents ( 32 ).
When cigarette smoke is inhaled, nicotine moves quickly to the lungs, arterial blood, and the brain in only 15 to 20 s ( 33 ), where it exerts its addiction-related effects. Rapidity of delivery to the brain is thought to be an important factor in the abuse liability of inhaled nicotine compared to other routes of nicotine administration. The importance of rapid delivery relates to higher arterial concentrations, nearly immediate psychological effects, and the ability to titrate doses to desired effects. Higher arterial levels also allow the smoker to overcome effects of tolerance to the desired psychological effects of nicotine. Inhaled nicotine from e-cigarettes potentially carries a similar abuse liability to that of tobacco cigarettes, but empirical data, to date, suggest that it is not the case. It appears that the dependence liability of inhaled nicotine is also influenced by other constituents of tobacco smoke, such as chemicals that inhibit monoamine oxidase (MAO), an enzyme that degrades neurotransmitters released by nicotine, discussed in more detail later. Furthermore, dependence on nicotine from medications (e.g., nicotine patches, gum, and lozenge) that deliver nicotine slowly appears to be low.
On average, smokers absorb 1 to 1.5 mg of nicotine from a cigarette ( 33 ). Nicotine has an average half-life of 2 hours, but the half-life can be affected by genetic and environmental factors. With regular smoking, nicotine levels rise in the blood over 4 to 6 hours, plateau throughout the day, and then decline overnight. Thus, although each cigarette produces a spike of arterial nicotine with a rapid decline between cigarettes, in a regular daily smoker, the brain is exposed to nicotine for 24 hours each day. This duration of exposure has implications for the development of tolerance and withdrawal symptoms, as discussed later.
Nicotine is primarily metabolized (via oxidation) by the liver enzyme, CYP2A6 ( 34 ). The main proximate metabolite is cotinine, which has been widely used as a biomarker of nicotine exposure. CYP2A6 activity is strongly influenced by genetic and environmental factors. Genetic variants associated with a slow rate of nicotine metabolism are more common in people of Asian and African descent compared to Caucasians. Environmental influences on nicotine metabolism include estrogen: Premenopausal women metabolize nicotine faster than men; women taking estrogen-containing birth control pills metabolize nicotine faster than women who do not; and pregnant women metabolize nicotine fastest of all. Various foods and medications can also affect nicotine metabolism. The rate of metabolism affects smoking behavior, with faster metabolizers smoking more cigarettes per day (presumably to titrate desirable nicotine levels in blood) ( 35 ).
Brain mechanisms
Nicotine acts on nicotinic acetylcholine receptors (nAChRs) that are found throughout the nervous system. Acetylcholine is a neurotransmitter that acts on nearly every organ in the body, and similarly, nicotine affects nearly every organ in the body. Many subtypes of nAChRs are present in the brain. Each receptor is composed of five subunits. Eleven nAChR subunits are expressed in the brain, including α2 to α7, α9, α10, and β2 to β4 ( 36 ). Nicotinic receptors can be heteromeric, with α and β subunits, or homomeric, with five α7 subunits. The most abundant nAChRs in the brain are α4β2 and α7 (homomeric). The α4β2 nAChR can also contain α5 and/or α6 subunits, which alter receptor physiology and contribute to differences in susceptibility to nicotine dependence. Another widespread receptor subtype is α3β4, which mediates cardiovascular and other autonomic effects of nicotine.
When nicotine binds to the outside of a nAChR, an ion channel opens, allowing entry of calcium, sodium, or potassium ions. Initially, the receptor is activated, which is then followed by desensitization. nAChRs can exist in three conformational states: closed, in the resting state; open, allowing ion entry and membrane depolarization; and desensitized, where the receptor is unresponsive to nAChR agonists ( 37 ). The sensitivity to nicotine and the pharmacodynamics of response (such as duration of desensitization) vary based on the particular receptor type, which translates into differential development and time course of tolerance to different nicotine effects.
Mood, cognitive, and relaxation effects of smoking are thought to occur via nicotine’s stimulation of presynaptic nAChRs ( 8 ). Activation of these receptors results in facilitation of release of various neurotransmitters, including (i) dopamine, which is known to signal pleasure and is released by all drugs of abuse; (ii) norepinephrine and acetylcholine, which enhance vigilance and cognitive function; (iii) glutamate, which enhances memory and learning; (iv) serotonin, which affects mood; and (v) γ-aminobutyric acid (GABA) and endorphins, which ameliorate stress and anxiety.
The neural connections involving nicotine actions are complex. Nicotine affects the mesolimbic dopamine system, which is central in the neurobiology of addiction. Nicotine binds to nAChRs in the ventral tegmental area, which then activate dopamine neurons in the nucleus accumbens. The firing of dopamine neurons is modulated by GABAergic and glutaminergic neurons such that glutaminergic neurons enhance firing, while GABAergic neurons inhibit firing. The high-affinity α4β2 nAChRs are located on the inhibitory GABAergic neurons, while the α7 nAChRs are located on the excitatory glutaminergic nAChRs. The actions of nicotine on the inhibitory GABAergic neurons desensitize rapidly, while the actions on the α7 nAChR desensitize more slowly. Thus, over time, nicotine exposure results in a greater and persistent activation of dopamine neurons, actions that may promote the rewarding effects of nicotine ( 38 ). Nicotine may also interact with other drugs of abuse via interactions with opioid and cannabinoid receptor pathways ( 39 , 40 ). The importance of various nAChR subunits has been determined using genetic knockout mice. The β2 nAChR subunit is necessary for nicotine-related reward, while the β4 subunit influences nicotine withdrawal symptoms ( 41 ). The α6 nAChR subunit is important in activation of dopaminergic neurons, while the α5 subunit modulates the aversive effects of nicotine ( 42 ). Aversion to nicotine appears to be an important determinant of dependence, as people with genetic variants of the α5 nAChR subunit associated with less aversiveness are at higher risk of nicotine dependence ( 43 ).
With prolonged exposure to nicotine, structural changes occur in the brain. Most notably, there is up-regulation of nAChRs, with greater density of nAChRs in many parts of the brain. This up-regulation has been thought to be a response to nAChR desensitization, but more recent studies suggest that up-regulation occurs by a chaperoning mechanism ( 44 ). That is, nicotine appears to bind to nAChRs in the cell to facilitate assembly and chaperoning the receptors to the cell membrane. Up-regulation of nAChRs is thought to be related to the development of physical dependence, including the withdrawal symptoms that occur when nicotine exposure stops. Presumably, the up-regulated receptors that are inactive in the presence of nicotine become sensitive again during nicotine abstinence.
Two other neurotransmitter systems appear to play important roles in nicotine dependence. Hypocretins are neuropeptides that regulate the effects of nicotine on reward centers in the brain, found to influence nicotine self-administration in animals ( 45 ). The insular cortex contains a high density of hypocretin-1–containing neurons. Immediate and sustained reduction in craving and withdrawal symptoms has been observed in hospitalized smokers following stroke damage to the insular cortex compared to hospitalized smokers without brain lesions ( 46 ).
Tolerance develops to many of the effects of nicotine with repeated exposures. In time, the brain adapts to the persistent effects to normalize brain function and related behavior. When nicotine exposure is stopped, brain function is disrupted and put in a state of withdrawal. Nicotine withdrawal results in activation of the corticotropin-releasing factor (CRF) system involved in the hypothalamus pituitary adrenal stress response. Withdrawal symptoms, such as anxiety and stress, are thought to be mediated, at least in part, by a relative underactivity of the dopaminergic system and hyperactivity of the CRF system. Antagonists of the CRF receptor reduce the anxiogenic effects of nicotine withdrawal and reduce self-administration of nicotine in the withdrawal state ( 47 ).
Dependence on nicotine appears to be augmented by other chemicals in cigarette smoke. Acetaldehyde, for example, increases self-administration of nicotine in animals. Particular chemicals in cigarette smoke inhibit the activity of the enzyme MAO in the brain ( 48 ). MAO catalyzes the breakdown of dopamine, norepinephrine, and serotonin, which are neurotransmitters that mediate nicotine reward. In animals, administration of drugs that inhibit MAO enhances nicotine self-administration. MAO-inhibiting medications have been used to treat depression. As discussed later, people with psychiatric illness, including depression, are more likely to smoke and to be more highly dependent. One theory is that MAO inhibition from smoking may have beneficial effects in depressed smokers. However, while acute smoking abstinence is associated with depressive symptoms and anxiety, prolonged quitting generally improves mood, including among smokers with psychiatric disorders such as depression ( 49 ).
Clinical features of nicotine addiction
Positive psychoactive effects of nicotine include pleasure, stimulation, and mood modulation, with reduced anxiety and stress ( 8 ). A smoker often reports pleasure and stimulation with the first cigarette of the day, stimulation and increased concentration from smoking during repetitive tasks during the day, and relaxation at times of stress and at bedtime. However, tolerance develops to many of nicotine’s effects such that even within the day, the pleasure experienced from each cigarette diminishes. As nicotine levels decline, withdrawal symptoms develop, reversing nicotine’s positive effects. Thus, an abstinent smoker may feel anxious, irritable, and depressed and have problems concentrating. Hedonic dysregulation (a reduced ability to experience pleasure) may be experienced, presumably related to a relative deficiency of dopaminergic activity. Nicotine increases metabolic rate and suppresses appetite, resulting in smokers, on average, weighing less than nonsmokers. During nicotine withdrawal, smokers typically experience hunger and gain weight.
Some of the perceived benefits of nicotine are mediated by the reduction of adverse effects of nicotine withdrawal (termed negative reinforcement). Thus, the pharmacologic role of nicotine in addiction is a combination of providing positive and negative reinforcement ( Fig. 1 ). For daily smokers, there is a daily cycle during which nicotine levels rise in the blood, substantial tolerance develops during the day, and smoking occurs to relieve withdrawal symptoms. Some highly addicted smokers wake at night to smoke because of withdrawal symptoms. In contrast, some light and intermittent smokers smoke in response to particular cues, without experiencing withdrawal symptoms, and are thought to smoke just for positive reinforcement.

Nicotine dependence severity is best measured by the number of cigarettes smoked per day and the time to first cigarette upon wakening. The two items make up the heaviness of smoking index (HSI) ( 50 ). Number of cigarettes smoked per day is a measure of both daily nicotine intake and the frequency of nicotine self-administration. Time to first cigarette is a measure of physical dependence and the intensity of withdrawal symptoms after overnight abstinence. The two HSI items significantly correlate with biomarkers of tobacco exposure, accounting for 20 to 30% of the variance in measures of alveolar carbon monoxide, nicotine, and urinary cotinine ( 50 ). Research conducted by Altria with funding from Philip Morris USA concluded that the HSI items were the most important factors correlating with biomarkers of exposure ( 51 ). The HSI is associated with smoking-induced deprivation, measured as prioritization of cigarettes over household essentials such as food ( 52 ). Both items are used for dosing nicotine replacement medications, discussed in the “Tobacco Control Population-Based and Policy Approaches” section, with higher doses for heavier smokers and those who smoke within 30 min of waking. HSI scores predict difficulty with quitting smoking ( 53 ) and the likelihood of developing tobacco-related diseases, such as heart disease, COPD, and lung cancer ( 54 , 55 ). Smoking affects gene expression, and the two HSI items correlate with candidate genes previously associated with cocaine, alcohol, and heroin addiction ( 56 ). The rate of nicotine metabolism also correlates significantly with the HSI ( 57 ). The HSI items have demonstrated very good test-retest reliability among adolescents and adults ( 58 , 59 ). The HSI items come from the longer Fagerström Test for Cigarette Dependence ( 60 ). A similar instrument has been developed to assess severity of dependence on e-cigarettes ( 61 ) with demonstrated validity, including among adolescents ( 28 ).
While nicotine is necessary for tobacco dependence, conditioned behavior is also an important factor and has strong implications for behavioral treatment. When a person quits smoking, cravings for cigarettes persist long after nicotine withdrawal symptoms have resolved ( 62 ). A smoker typically associates smoking with particular situations, moods, or environmental factors that become cues to smoke. Thus, smokers often smoke after a meal, with coffee or alcohol, while driving, and/or with friends who smoke. Smoking a cigarette reverses the negative mood, anxiety, and irritability of nicotine withdrawal ( 62 ). This repeated experience can generalize to a condition in which anxiety or depression from any cause becomes a cue to smoke. The act of smoking, with the handling, hit to the throat, and taste and smell of cigarettes, which are often associated with the neurochemical effects of smoking, signals reward and becomes a cue to smoke. Exposure to tobacco advertising, particularly prevalent at point-of-sale retail and in popular media (e.g., movies, TV, and music), and exposure to others smoking can also elicit craving and smoking behavior ( 63 – 65 ).
Vulnerability to nicotine addiction
Not all smokers become regular, daily, or addicted users. The younger a person starts smoking cigarettes, the greater the risk of stronger physiological addiction to nicotine. Smoking co-occurs with mental illness and other addictive disorders, suggesting greater vulnerability, and research suggests the potential for a gateway effect. Genetic factors also influence the risk of nicotine dependence.
Adolescents and the developing brain
Nearly all (9 in 10) individuals who smoke started by the age of 18. Adolescence is a critical window for brain development, with the brain not reaching full maturity until the mid-20s. Adolescence is a period of enhanced neuroplasticity during which the underdeveloped neural networks necessary for adult-level judgment (the prefrontal cortical regions) cannot yet properly regulate impulses and emotion ( 66 ). As a consequence, adolescents are highly vulnerable to drug experimentation and addiction ( 67 ).
Nicotine exposure during adolescence may have lasting adverse consequences for brain development. In animals, nicotine exposure during adolescence produces permanent changes in brain structure and function, including enhanced self-administration of nicotine and other drugs as adults ( 68 ). In humans, adolescents experience symptoms of dependence at lower levels of nicotine exposure than adults ( 69 , 70 ). Earlier onset of daily smoking is associated with higher nicotine dependence scores ( 71 ) and heavier and longer smoking careers compared to late-onset smokers ( 72 , 73 ). Individuals who begin smoking as teens are more likely to become lifelong smokers than those who start smoking in their 20s or later ( 74 – 76 ). In a study of 1200 individuals, those who initiated smoking before age 13 had the lowest likelihood of quitting, followed by those who initiated between ages 14 and 17, while adult initiators (18+) had the highest likelihood of quitting ( 77 ). A number of studies have yielded similar results ( 78 ). The findings have implications for policy interventions aimed at preventing initiation in youth.
Smoking among people with mental illness
Mental illness commonly co-occurs with tobacco addiction ( 79 , 80 ), including major depression, bipolar disorder, posttraumatic stress disorder (PTSD), and schizophrenia. Evidence that nicotine may improve cognitive function and sensory gating and reduce psychotic symptoms has led to the self-medication hypothesis, which posits that people with psychiatric disorders smoke to lessen their symptoms ( 81 ). The tobacco industry funded research in support of the self-medication hypothesis ( 82 ). Bidirectional models maintain that smoking and psychiatric symptoms influence each other ( 83 ), and studies indicate that early-onset smoking may predispose to depression, anxiety disorders, and schizophrenia ( 80 ). There is also evidence of modest shared genetic susceptibility to tobacco dependence and mental illness.
Cigarette smoking induces the metabolism of some psychiatric medications leading to lower blood levels, with reduced sedation, and may explain, in part, the improvements observed in cognitive function ( 84 ). Studies in youth and adults, cross-sectional and prospective, have found that current smoking is predictive of future suicidal behavior—independent of depressive symptoms, previous suicidal acts, and other substance use—and that longer lifetime smoking (>40 years versus ≤10 years) is associated with a twofold higher odds of suicide ( 80 ). Notably, quitting smoking appears to mitigate the risk ( 85 ).
The self-medication hypothesis—that people smoke to manage their mental health symptoms—drove concerns that treating smoking would worsen mental health. This belief and the perceptions that smoking is a chronic, rather than acute, concern have been substantial barriers to addressing tobacco use in psychiatric settings ( 86 ). Newer research, however, indicates that quitting smoking is associated with improvements in mental health, including reductions in depression, anxiety, psychotic symptoms, emotional lability, and PTSD symptoms ( 87 – 89 ). In a randomized trial with smokers recruited from inpatient psychiatry, the tobacco cessation intervention was associated with a significantly lower likelihood of rehospitalization out to 18-month follow-up ( 90 ). A meta-analysis of 26 longitudinal studies assessing mental health before smoking cessation and at least 6 weeks after abstinence found reduced depression, anxiety, and stress and greater overall well-being compared with continuing to smoke ( 49 ). The effects were comparable for those with and without psychiatric disorders. If people self-medicate nicotine for acute relief, it does not appear to produce sustained benefits and is a form of self-medication that should be discouraged.
Smoking among people with other addictions
Three in four adults with alcohol use disorder and 9 in 10 adults with drug use disorders smoke tobacco ( 91 ). Early-onset smoking is a significant predictor of lifetime drinking, more excessive alcohol consumption, and the subsequent development of lifetime alcohol abuse and dependence ( 72 ). Nicotine addiction, in the form of cigarette smoking, causes greater morbidity and mortality than any other single drug addiction and the combination of all other risks ( 92 ). Among individuals treated for alcohol dependence, tobacco-related diseases were responsible for half of all deaths, greater than alcohol-related causes ( 93 ).
While the causal progression remains under debate, tobacco use has been implicated as a “gateway” to other drugs of abuse ( 94 ). Possible mechanisms include nicotine enhancing the rewarding effects of other drugs, nicotine reducing the negative effects of another drug (for example, less sedation with alcohol use), and shared genetic susceptibility. Mice given nicotine in their drinking water for a week had an increased response to cocaine; nicotine caused epigenetic changes in DNA, in particular, affecting expression of the FosB gene found related to addiction ( 95 ). In human adults, cocaine users who smoked cigarettes before starting cocaine had a two- to threefold greater likelihood of cocaine dependence compared to those who tried cocaine before smoking cigarettes and compared to those who never became regular smokers ( 95 ). The inference was that brain changes due to early exposure to nicotine made it more likely that the individuals would become addicted to cocaine.
While concomitant smoking and drug use are common, treating smoking may improve sobriety outcomes in the long term. A meta-analysis of randomized controlled tobacco cessation trials with smokers in treatment for substance use disorders found that tobacco cessation interventions were associated with a 25% increased likelihood of sobriety from alcohol and drugs relative to usual care ( 96 ).
Genetic factors
Genetic factors also influence the risk of nicotine dependence. The strongest genetic factor associated with nicotine dependence involves the CHRNA5 gene, which encodes the α5 nAChR subunit ( 43 , 56 ). The rs16969968 single-nucleotide polymorphism on chromosome 15 is associated with greater risk of becoming dependent, a lower likelihood of smoking cessation, and increased risk of lung cancer and COPD. The nAChR with a reduced function α5 subunit is thought to result in less aversiveness to nicotine and greater nicotine intake and, therefore, greater dependence. The other major genetic risk factor for nicotine dependence is the CYP2A6 gene, which is associated with the rate of nicotine metabolism and greater nicotine dependence including smoking more cigarettes per day, more rapid onset of withdrawal symptoms during abstinence, and lower quit rates ( 35 , 97 ).
TREATING NICOTINE ADDICTION IN ADULTS, WITH A FOCUS ON CONVENTIONAL CIGARETTES
As discussed, tobacco dependence is characterized as a physiological dependence (addiction to nicotine) and behavioral (or conditioned) habit of using tobacco. Hence, for maximal effectiveness, as recommended by U.S. Clinical Practice Guidelines, tobacco dependence treatment engages a multipronged approach ( 98 ). Addiction can be treated with FDA-approved medications for smoking cessation; the behavioral habit can be treated through counseling and behavior change programs, and policy interventions can promote smoke-free environments, discussed in the “Tobacco Control Population-Based and Policy Approaches” section.
Counseling and psychosocial treatments
Brief cessation advice.
With 7 in 10 tobacco users seeing a healthcare provider in a given year, opportunities exist for brief cessation clinical advice. Treating smoking is relevant to all areas of medicine, and the evidence in support of brief clinical cessation advice is strong ( 99 ). The U.S. Preventive Services Task Force gives a “grade A” recommendation for clinician-delivered brief tobacco cessation interventions ( 100 ). Counseling by nonphysician health providers, including nurses ( 101 ), oral health professionals ( 102 ), and pharmacists ( 103 ), also increases quit rates.
The gold standard for brief cessation advice is the National Cancer Institute’s (NCI) 5A’s to (i) ask all patients about use of all forms of tobacco; (ii) advise tobacco users to quit; (iii) assess patient readiness to quit; (iv) assist in the quit attempt with counseling, medications, and referrals; and (v) arrange follow-up. The 5 A’s increase patient treatment engagement, quit attempts, and tobacco abstinence ( 104 ).
Recognizing time constraints in the clinical setting, an alternate approach with evidence is Ask-Advise-Refer (AAR), whereby clinicians ask about tobacco use, advise tobacco users to quit, and then refer patients to an outside entity for assistance and follow-up, such as a tobacco quitline (1-800-QUIT-NOW) ( 105 , 106 ). Further adaptation is Ask-Advise-Connect (AAC), the distinction being that the referral is provided in the form of a direct connection, such as a fax or other electronic referral ( 107 ). Comparison of AAR to the 5 A’s delivered in 68 dental clinics found comparable quit rates, and both approaches were better than usual care ( 106 ). Sustained quit rates at long-term follow-up, however, were under 4% in all three study arms. A recognized standard of care, brief provider advice is effective for engaging patients in treatment and supporting quitting ( 98 ); however, to further improve sustained abstinence rates, more intensive interventions are needed.
Intensive counseling
Clinical practice guidelines recommend intensive cessation counseling offered in person, individually or in groups, in clinical, behavioral, or community settings for treating smoking ( 98 ). The counseling framework tends to be cognitive behavioral and motivational, although, increasingly, other clinical approaches (e.g., mindfulness, acceptance, and commitment therapy) are being incorporated. A systematic review of 49 randomized trials with some 19,000 participants concluded that intensive counseling only (without medications) delivered by a cessation counselor on a one-to-one basis was more effective than minimal contact (i.e., brief advice and self-help materials) and had greater effects when combined with cessation medications ( 108 ). Intensive individual and group counseling treatments also have demonstrated effectiveness in workplace settings ( 109 ). Access to intensive counseling may be limited because of travel, time, cost, or privacy concerns. To overcome these barriers, tobacco quitlines were developed to improve accessibility and reach of tobacco cessation counseling treatment.
Tobacco quitlines
Tobacco quitlines are staffed by trained counselors or coaches who provide information, individual counseling, local referrals, self-help materials, and, in some cases, limited supplies of free cessation medications. The effectiveness of tobacco quitlines is well demonstrated ( 110 ). Quitline services are available at no cost to U.S. residents and accessed via a toll-free national portal (1-800-QUIT-NOW or 1-855-DÉJELO-YA), which links callers to their state quitline based on their area code. While specific services vary by state, by county, and over time, most state quitlines provide at least one counseling session to any adult tobacco user who calls, and many states provide a multi-call program that includes reactive and proactive calls. The reactive approach relies upon smokers to initiate the calls, whereas the proactive approach makes outbound calls to engage tobacco users. In meta-analyses, better outcomes are seen with multi-call versus single-call protocols ( 110 ) and with proactive versus reactive quitline services ( 111 ).
Although free, convenient, and confidential, quitlines in most states reach an average of only 1% of smokers annually ( 112 ). Even among smokers aware of quitlines and making a quit attempt, reach is only about 8% ( 113 ). The Centers for Disease Control and Prevention’s (CDC) Tips From Former Smokers (Tips) tobacco education campaign, developed to encourage quitting and raise awareness of state quitline services and conducted annually since 2012, has generated hundreds of thousands of additional calls to state quitlines ( 114 ). The Tips campaign and its impact on quitting are discussed under media campaigns in the “Tobacco Control Population-Based and Policy Approaches” section. To further expand reach, some state quitlines have incorporated mobile health technologies.
Mobile technologies: Internet, text, and social
Mobile technologies, such as internet interventions, email, chat, and texting, are being leveraged for health promotion at a low cost, with broad reach potential and with evidence of efficacy.
Internet-delivered tobacco cessation interventions have existed for more than 25 years and have continued to develop in sophistication, level of interaction, and complexity of functionality, as well as treatment efficacy. In 2011, the Community Preventive Services Task Force deemed the evidence insufficient to recommend internet-based interventions for tobacco cessation ( 115 ). Two years later, a 2013 review concluded that internet-based interventions can assist in achieving long-term smoking cessation, particularly interactive programs tailored to the individual ( 116 ). A 2016 review noted significant improvements in internet-based smoking cessation interventions with evidence of superior efficacy relative to print materials and equivalent efficacy to telephone and in-person counseling ( 117 ). Relative to quitlines, internet-delivered interventions have 27 times greater national reach [annually, 11 million for internet versus 400,000 for quitlines ( 112 )] and at a lower cost per quit [e.g., $291 for internet versus $900 for quitlines ( 118 )].
A model example of an internet-delivered tobacco cessation program is NCI’s Smokefree.gov , which combines evidence-based guidelines for quitting smoking, tailored to readiness to quit, with availability of professional assistance via instant messaging and telephone (1-877-44U-QUIT). The site has also tailored offerings for veterans, women, adolescents, Spanish-speaking smokers, and older adults. SmokefreeTXT is an additional mobile service that provides encouragement, advice, and tips for young adults to quit smoking. Smokefree smartphone apps are offered to provide motivational reminders and help with tracking progress with quitting smoking. The Smokefree.gov site had 3.6 million visitors in 2016 ( 118 ) and received high user satisfaction ratings ( 119 ). Randomized trial evidence supports Smokefree.gov as a population-based intervention for smoking cessation ( 118 , 120 ).
Mobile technologies
Mobile phone–based tobacco cessation interventions that send automated low-cost messages (i.e., texts) were deemed to have sufficient evidence of efficacy to be recommended by the Community Preventive Services Task Force ( 121 ). Trials in New Zealand and the United Kingdom evaluated text messages sent daily up to the quit day that tapered to a maintenance phase; texts included general information, motivational messages, quitting advice, and distraction strategies, and effects on quit rates were significant relative to no-text controls ( 122 , 123 ). A 2016 review found significant short-term effects of text-based smoking cessation interventions, although they were not sustained at long-term (>6 months) follow-up ( 124 ). Given the chronic, relapsing nature of nicotine addiction, more intensive extended interventions may be needed.
With the potential for more dynamic interactions, smoking cessation apps (applications) are available for download from digital marketplaces (e.g., iTunes and Google Play) for use on smartphones, tablets, and other handheld devices. A 2014 search identified 546 smoking cessation apps in the Apple Store and on Google Play that were downloaded some 3.2 million times in the United States and 20 million times worldwide ( 125 ). Broad reach and high scalability make apps particularly well suited for serving remote and resource-poor settings. Advantages include low or no cost to the user, self-tracking and tailored feedback functionalities, and use of images and video for enhanced health literacy. However, a 2015 review of 225 Android apps for quitting smoking found that most provide simplistic tools (e.g., calculators and trackers); use of tailoring was limited, although positively related to app popularity and user ratings of quality ( 126 ). Evaluation of intervention effects on quitting smoking is sorely needed. Notably, one randomized trial found that a simpler, direct texting program outperformed a smoking cessation app ( 127 ).
Social media
Social media, such as Twitter and Facebook, are being explored for delivering cessation treatment. In the United States, 74% of online adults use social media, 80% of whom are seeking health information, and a majority access the sites daily ( 128 ). A promising technology, efforts to sustain engagement are key and can be challenging; like predecessor technologies such as bulletin boards and listservs, initial interest may be high but then tends to wane ( 129 , 130 ). There is preliminary evidence, however, of good acceptability and efficacy. Using Twitter, small, private groups of 20 smokers, who interact for 100 days, have been studied. The intervention (Tweet2Quit) seeds the groups with twice-daily automessages to encourage group sharing and support. In a randomized trial, the Tweet2Quit Twitter groups added to Smokefree.gov and the nicotine patch fostered peer-to-peer support for quitting and significantly doubled the likelihood of reported sustained abstinence relative to the website and patch alone ( 131 ). Similar efforts are being developed on Facebook, with a focus on engaging young adults into cessation treatment. In a randomized trial, a novel Facebook smoking cessation intervention increased abstinence at the end of treatment, although effects were not sustained out to 1-year follow-up ( 132 ).
Social media can provide varying degrees of anonymity, which may be attractive. Having tried and failed to quit smoking in the past, smokers may not initially publicize their quit attempts within their main social circle ( 133 ). With social media sites that are largely uncurated or expert moderated, however, users should be forewarned that inaccurate information may be posted. For example, online communities may encourage use of non–evidence-based treatments (e.g., laser, herbs, acupuncture, or hypnosis for quitting smoking) ( 129 ). A heterogeneous group of emerging applications and knowledge gaps remain concerning best strategies for maximizing the reach and efficacy of mobile technologies for treating nicotine addiction as well as the comparative effectiveness relative to in-person approaches.
Monetary incentives
Monetary incentives that reward outcome (i.e., quitting smoking) or engagement (e.g., treatment participation) have been evaluated in 33 trials, with a meta-analysis finding evidence of increased abstinence that persisted after the incentives ceased ( 134 ). The level of the incentives ranged from zero (self-deposits) to between $45 and $1185, with no clear direction of effect by level of incentive. Conditional payments (i.e., payment for abstinence) outperformed nonconditional payments. Findings from a subgroup analysis of eight trials conducted with smokers with substance use problems were consistent with the overall analysis. A summary of nine trials with pregnant smokers reported more than twofold greater odds of abstinence at longest follow-up assessment (up to 24 weeks postpartum). The findings are particularly important given the substantial health harms of smoking to mother and baby and that, currently, there is no other effective cessation intervention for pregnant smokers.
Pharmacotherapies to aid smoking cessation
While counseling and psychosocial treatments help promote cessation, medications that address the neuropharmacological effects of nicotine and nicotine withdrawal further enhance the likelihood of quitting. E-cigarettes, which allow continued self-administration of nicotine without combustion, can also promote quitting smoking.
Smoking cessation guidelines, such as those from the U.S. Public Health Service and National Cancer Center Network, recommend smoking cessation medications for all daily smokers where feasible and safe ( 98 , 135 ). Pharmacotherapy can be considered for nondaily smokers as well, although there are few clinical trials to guide treatment in this group. The mechanism of benefit in nondaily smokers would be reduction of nicotine reward from cigarettes by nicotinic receptor desensitization or antagonism, as discussed below. Table 2 presents the FDA-approved smoking cessation medications, including dosing guidelines, advantages, disadvantages, adverse effects, and precautions. FDA-approved medications are NRT in the form of gum, patches, lozenge, nasal spray and inhaler, varenicline, and bupropion. Nicotine gums, lozenges, and patches are available over the counter in the United States, while the nicotine nasal spray, nicotine inhaler, varenicline, and bupropion are by prescription only. Nicotine mouth spray is available outside of the United States and has evidence of acceptability, efficacy, and safety, including with minimal behavioral support ( 136 ).
| Product | , Generic OTC 2 mg, 4 mg original, cinnamon, fruit, mint | , Generic Mini OTC 2 mg, 4 mg; cherry, mint | , OTC (NicoDerm CQ, generic) 7 mg, 14 mg, 21 mg (24-hr release) | Rx Metered spray 10 mg/mL nicotine solution | Rx 10 mg cartridge delivers 4 mg inhaled vapor | , Generic Rx 150 mg sustained- release tablet | Rx 0.5 mg, 1 mg tablet |
| Precautions | and breastfeeding | and breastfeeding | and breastfeeding | and breastfeeding | and breastfeeding | and breastfeeding Boxed warning removed 12/2016
| and breastfeeding Boxed warning removed 12/2016 |
| Dosing | 4 mg 2 mg Weeks 1–6: 1 piece q 1–2 hours Weeks 7–9: 1 piece q 2–4 hours Weeks 10–12: 1 piece q 4–8 hours | 4 mg 2 mg Weeks 1–6: 1 lozenge q 1–2 hours Weeks 7–9: 1 lozenge q 2–4 hours Weeks 10–12: 1 lozenge q 4–8 hours | : 21 mg/day x 4–6 weeks 14 mg/day x 2 weeks 7 mg/day x 2 weeks : 14 mg/day x 6 weeks 7 mg/day x 2 weeks | 1–2 doses/hour (8–40 doses/day) One dose = 2 sprays (one in nostril); each spray delivers 0.5 mg of nicotine to the nasal mucosa | 6–16 cartridges/day Individualize dosing; initially use 1 cartridge q 1–2 hours | 150 mg po q AM x 3 days, then 150 mg po bid to quit date | Days 1–3: 0.5 mg po q AM Days 4–7: 0.5 mg po bid Weeks 2–12: 1 mg po bid to quit date |
| Adverse Effects | |||||||
| Advantages | |||||||
| Disadvantages | (see Precautions) | (see Precautions) | |||||
| Cost/day | 2 mg or 4 mg: $1.90–$3.60 (9 pieces) | 2 mg or 4 mg: $3.33–$3.60 (9 pieces) | $1.52–$2.90 (1 patch) | $8.72 (8 doses) | $14.88 (6 cartridges) | $2.58–$8.25 (2 tablets) | $15.14 (2 tablets) |
Abbreviations: MAO, monoamine oxidase; NRT, nicotine replacement therapy; OTC, over-the-counter (nonprescription product); Rx, prescription product.
For complete prescribing information and a comprehensive listing of warnings and precautions, please refer to the manufacturers’ package inserts.
Copyright © 1999-2019 The Regents of the University of California. All rights reserved. Updated January 9, 2019.
1 Marketed by GlaxoSmithKline.
2 Marketed by Pfizer.
3 The U.S. Clinical Practice Guideline states that pregnant smokers should be encouraged to quit without medication based on insufficient evidence of effectiveness and theoretical concerns with safety. Pregnant smokers should be offered behavioral counseling interventions that exceed minimal advice to quit.
4 In July 2009, the FDA mandated that the prescribing information for all bupropion- and varenicline-containing products include a black-boxed warning highlighting the risk of serious neuropsychiatric symptoms, including changes in behavior, hostility, agitation, depressed mood, suicidal thoughts and behavior, and attempted suicide. Clinicians should advise patients to stop taking varenicline or bupropion SR and contact a health care provider immediately if they experience agitation, depressed mood, or any changes in behavior that are not typical of nicotine withdrawal, or if they experience suicidal thoughts or behavior. If treatment is stopped due to neuropsychiatric symptoms, patients should be monitored until the symptoms resolve. Based on results of a mandated clinical trial, the FDA removed this boxed warning in December 2016.
5 Approximate cost based on the recommended initial dosing for each agent and the wholesale acquisition cost from Red Book Online. Thomson Reuters, December 2018.
In general, medications serve to make smokers more comfortable while they learn to live and cope with daily cues/triggers and life stressors without smoking cigarettes. There are three main mechanisms by which medications can facilitate smoking cessation: (i) reduction of nicotine withdrawal symptoms, (ii) reduction of the rewarding effects of nicotine from smoking by blocking or desensitizing nicotine receptors, and (iii) providing an alternative source of nicotine with the desired pharmacologic effect previously provided by nicotine from cigarettes. NRT medications are not as satisfying as cigarette smoking because of slower absorption of nicotine; nicotine delivery from e-cigarettes can resemble that of a cigarette, and these devices tend to be much more satisfying. Most smoking cessation medications are recommended for 8 to 12 weeks, although use for 6 months or longer may be necessary to achieve optimal quit rates. It makes sense to use medications to support smoking cessation for as long as the individual feels at risk for relapse. For those switching to e-cigarettes as a less harmful substitute for cigarette smoking, use sometimes continues for many months or years.
Nicotine replacement therapy
Nicotine medications consist of purified nicotine that is administered to ameliorate symptoms of physical dependence on nicotine. The particular actions of different products vary according to route of administration and rate of nicotine absorption into the bloodstream. For example, nicotine patches deliver nicotine slowly, relieving nicotine withdrawal symptoms and reducing positive effects of cigarette smoking, without providing much, if any, direct positive effects of nicotine. Nicotine gums, lozenges, sprays, and inhalers deliver nicotine more rapidly, providing some acute nicotine effects that may serve as a substitute for smoking a cigarette. Combining a short-acting (gum, lozenge, spray, or inhaler) with a long-acting (nicotine patch) NRT results in superior quit rates compared to any NRT product alone and is recommended as a first-line treatment ( 137 ).
NRT products are marketed in different strengths, with higher doses recommended for more dependent smokers based on the number of cigarettes smoked per day or time to first cigarette upon wakening. A 2019 Cochrane review concluded that 4-mg gum is more effective than 2-mg gum in more highly dependent smokers and that 21-mg patch is more effective than 14-mg patch in general ( 137 ). While clinical trials do not demonstrate superiority of 42- to 21-mg dose nicotine patch, some clinicians do use high-dose patch for smokers with particularly severe withdrawal symptoms. Tapering of nicotine doses over time is an option for nicotine patches but does not appear to affect outcome in clinical trials.
All forms of NRT have shown similar efficacy in clinical trials ( 137 ), increasing quit rates by 50 to 100% compared to behavioral treatment alone. For the NRTs, compliance is greatest with nicotine patches, lower with gum and lozenge, and lowest with the nasal spray and inhaler. Nicotine patches are usually placed on the skin in the morning and deliver nicotine over 16 to 24 hours. Some smokers experience nicotine patch–related insomnia and/or abnormal dreams and do better removing the patch at bedtime. Use of patches for 16 or 24 hours is equally effective in promoting quitting smoking. The pharmacokinetics of nicotine gum, lozenge, and inhaler are similar, with gradual absorption of relatively low doses of nicotine over 15 to 30 min. Use every 1 to 2 hours provides the best pharmacologic response. The nicotine inhaler is a plastic device inhaled like a cigarette but delivers nicotine to the oropharyngeal area rather than to the lungs, which explains its slow absorption. The main advantage of the inhaler is providing a hand-to-mouth experience similar to smoking. All oral nicotine products have an alkaline pH, which results in a high proportion of nicotine in the free base form, which is rapidly absorbed across mucous membranes. Acidic beverages (e.g., coffee, citrus juice, sodas, and many alcohol beverages) reduce the pH and reduce nicotine absorption and should be avoided for >10 min before using oral NRT products. The nicotine nasal spray is absorbed much faster than the other rapid-release products, most closely resembling a cigarette. More dependent smokers may find nicotine nasal spray to be more effective than other NRT products for smoking cessation. The spray is associated with more local toxicity, including a burning sensation, watery eyes, and sneezing; however, tolerance develops to these effects with regular use of the spray over 1 to 2 days.
Overall, NRT products are well tolerated and present few safety concerns. Safety concerns with NRT are primarily skin irritation with patches, gastrointestinal symptoms with oral products, and nasal/throat burning and irritation with nasal spray. Nicotine’s cardiovascular effects raised concern about NRT cardiovascular safety. Nicotine enhances sympathetic neural activity, resulting in increased heart rate, constriction of blood vessels, induction of proatherogenic lipid profiles (lower high-density lipoprotein cholesterol), development of insulin resistance, and possible promotion of arrhythmias ( 138 ). Cigarette smoke delivers not only nicotine but also many oxidants, prothrombotic and other toxic chemicals, making smoking much more toxic than nicotine alone. Clinical trials and other studies of NRT in patients with cardiovascular disease find no increase in adverse cardiovascular events due to NRT ( 139 , 140 ).
Varenicline
Varenicline is a partial agonist at the nicotinic α4β2 receptor, the major receptor mediating nicotine addiction. Varenicline both activates (about 50% of the maximal effect of nicotine) and blocks the effects of nicotine on the α4β2 receptor ( 141 ). The agonist effect serves to reduce withdrawal symptoms, while the antagonist effects reduce the rewarding effects of nicotine from cigarette smoke. Varenicline treatment before smoking cessation is often associated with reduced smoking, presumably because smoking is less satisfying, an effect that can promote subsequent cessation.
In clinical trials, varenicline is more effective than bupropion or nicotine patch in promoting smoking cessation and is comparably effective to combined NRT ( 142 ). The EAGLES trial, the largest smoking cessation trial conducted with 8000 smokers, directly compared varenicline, bupropion, nicotine patch, and placebo. Varenicline outperformed all conditions; bupropion and nicotine patch were comparable to each other and were significantly better than placebo ( 143 ). EAGLES included smokers without and with psychiatric diagnoses. Quit rates were higher in those without psychiatric diagnoses, but the relative efficacy of the various treatments was similar. Extended treatment with varenicline for 6 months is superior to the standard 12-week treatment and is recommended for smokers who feel at risk of relapse ( 144 ).
The most common adverse effect of varenicline is nausea, which is dose related and to which tolerance develops over time. Concern about nausea is the rationale for starting at lower doses (0.5 mg once to twice daily) for a week before starting the full dose (1.0 mg twice daily). Some smokers cannot tolerate the normal dose but do well on continued use of the lower dose. Anecdotal reports of neuropsychiatric adverse effects of varenicline used for smoking cessation have been reported, prompting a black box warning in the label after the drug was marketed (for both varenicline and bupropion). The putative neuropsychiatric side effects included depression, psychosis, and suicide, with potentially higher risk in smokers with psychiatric disease. However, the EAGLES trial found no evidence of increased neuropsychiatric adverse events for varenicline or bupropion relative to nicotine patch or placebo, in smokers with or without psychiatric illness, and in 2016, the black box warnings were removed for both varenicline and bupropion ( 143 ). Varenicline has been shown to enhance smoking cessation in patients with cardiovascular disease, including stable coronary heart disease and acute coronary syndrome ( 145 , 146 ). Concern was raised about possible cardiovascular toxicity due to the nicotine-like effects of varenicline and anecdotal reports of adverse cardiovascular events, but several meta-analyses, a large retrospective cohort study, and clinical trials in smokers with cardiovascular disease, as well as the EAGLES trial, showed no increase in cardiovascular risk ( 147 , 148 ). Varenicline has also been found efficacious for cessation of smokeless tobacco use ( 149 ).
Bupropion is a stimulant drug originally marketed as an antidepressant. Bupropion blocks neuronal uptake of dopamine and norepinephrine and has antagonist activity on the α4β2 nicotinic receptor. By blocking reuptake, bupropion increases brain levels of dopamine and norepinephrine, simulating effects of nicotine. Bupropion is marketed for smoking cessation as a sustained-release preparation. The drug works in both depressed and non-depressed smokers. The usual duration of bupropion treatment is 12 weeks, but extended bupropion therapy for a year reduces relapse and enhances long-term quit rates ( 150 ). With lower quit rates, bupropion is considered to be second-line, after combination NRT and varenicline.
The main adverse effects of bupropion relate to its nervous system stimulant actions. Some smokers are intolerant to bupropion because of anxiety, agitation, and insomnia. Bupropion reduces the seizure threshold and should not be used in smokers who are at risk for seizures. In overdose, bupropion causes tachycardia and hypertension, but there is no evidence of increased cardiovascular events in smokers with preexisting stable cardiovascular disease ( 151 , 152 ).
Combination pharmacotherapy
Combined NRT with patch and a more immediate acting product results in higher quit rates than single NRT [Cochrane meta-analysis: risk ratio (RR), 1.34; 95% confidence interval (CI), 1.18 to 1.48] ( 137 ). The combination of varenicline and nicotine patch has been evaluated with mixed results ( 153 ). The mechanism for why NRT should augment effects of varenicline is unclear, but the combination appears to be safe. The combination can be considered in a smoker who does not quit with dual NRT or varenicline. Bupropion in combination with nicotine patch or dual NRT increases quit rates compared to these drugs given alone ( 154 ). One trial reported promising results with the combination of varenicline and bupropion, although neuropsychiatric adverse effects were greater in the first 2 weeks compared to varenicline alone ( 155 ).
Preloading pharmacotherapy
Many smokers would like to quit but are not prepared to commit to a quit date when seen by a healthcare provider. Starting pharmacotherapy while the smoker is still smoking with the expectation that quitting will be easier at a later date has been studied with the use of nicotine patches and varenicline. The pharmacological basis for this approach is that NRT, by desensitizing nicotinic receptors and reducing withdrawal symptoms between cigarettes, and varenicline, by antagonizing effects of nicotine from cigarettes and also providing relief of withdrawal symptoms, will reduce satisfaction from smoking and decrease the number of cigarettes smoked per day. Preloading trials with nicotine patches have shown mixed benefit on quitting with a weak overall effect, although some trials showed large beneficial effects ( 156 , 157 ). Varenicline trials have shown benefit with a flexible quit date, and this approach is approved by the FDA ( 158 ). The attraction of precessation pharmacotherapy is that the clinician can now approach every patient who smokes, regardless of whether they are prepared to quit at the time of the visit, with a pharmacological intervention along with communication that this will help with quitting smoking in time, just as the clinician would advise every patient with hypertension to take medication to prevent future disease. In this regard, a small trial involving heavy smokers with COPD, who were initially unprepared to quit, prescribed varenicline for as long as they wanted, without a fixed quit date, and by 18 months, most had quit ( 159 ).
Gradual reduction
Meta-analysis finds similar quit rates for gradual reduction in cigarettes smoked per day before quitting as compared to abrupt quitting ( 160 ). Even in trials that found that abrupt quitting resulted in higher quit rates, many in the gradual reduction group successfully quit. Precessation varenicline with instructions to reduce cigarettes per day by 50% at 4 weeks, 75% at 8 weeks, and completely quit at 12 weeks showed substantial benefit compared to placebo ( 161 ).
Targeted pharmacotherapy
Personalized medicine aims to use individual patient characteristics to select the most effective and/or safest medications for their medical problem. With long-term quit rates of 30% or less in most smoking cessation trials, there is interest in individualizing treatment to enhance efficacy. A promising approach involves phenotyping based on an individual rate of nicotine metabolism. Rapid metabolizers of nicotine, on average, smoke more cigarettes and take in more nicotine per day compared to slower metabolizers, presumably to maintain desired levels of nicotine in the body ( 35 ). Rapid metabolizers also have more severe withdrawal symptoms when not smoking ( 97 ). The nicotine metabolite ratio is a phenotypic marker of the rate of nicotine metabolism, which can be measured in blood, saliva, or urine ( 162 , 163 ). In a prospective clinical trial, smokers were stratified as slow or normal metabolizers and treated with nicotine patch, varenicline, or placebo. In slow metabolizers, varenicline and nicotine patch were equally effective [odds ratio (OR), 1.13; P = 0.56], but in rapid metabolizers, varenicline was more effective (OR, 2.17; P < 0.001) ( 164 ). Side effects were greater for varenicline in slow metabolizers. The results indicate that slow metabolizers can be successfully treated with nicotine patch, at lower cost and with fewer side effects, but normal metabolizers are better treated with varenicline. More research is needed for confirmation.
Cytisine is an alkaloid extracted from the seeds of Cytisus laburnum , commonly known as golden chain or golden rain, a common garden plant in central and southern Europe. Cytisine has been used for smoking cessation in eastern and central European countries for more than 50 years. Cytisine, like varenicline, is a partial agonist at the α4β2 nAChR. Thus, it has nicotine-like effects, while at the same time it desensitizes and/or blocks the effects of nicotine from tobacco on the α4β2 nAChR. The recommended treatment regimen involves tapering doses over 25 days, a treatment course that is shorter than the 12 weeks recommended for most other smoking cessation medications, with significant effects relative to placebo (meta-analysis; RR, 1.74; 95% CI, 1.38 to 2.19) ( 165 ). The cost of cytisine in Europe is several-fold less than that of other smoking cessation medications. The drug is well tolerated, with the most common side effects being nausea, vomiting, dyspepsia, and dry mouth. Clinical trials of cytisine for FDA-approved use in the United States are underway.
Second-line smoking cessation medications
While not approved by the FDA, nortriptyline and clonidine have demonstrated efficacy in clinical trials for smoking cessation ( 166 , 167 ). These drugs are used primarily by smoking cessation specialists for patients who have not responded to other treatment. Nortriptyline is a tricyclic antidepressant that blocks neuronal reuptake of norepinephrine, thereby increasing levels of the neurotransmitter in the brain. These actions simulate some of the actions of nicotine on brain neurotransmitters. Clonidine is a central α2 adrenergic receptor agonist that reduces sympathetic activity, resulting in sedation and anxiolysis. The benefit of clonidine is thought to be mediated by its anxiolytic and calming effects and appears to be most useful in smokers with anxiety as a major withdrawal symptom.
Smoking cessation pharmacotherapies in development or that have failed
A number of medications have been considered as possible candidates for smoking cessation ( 168 ). While animal and/or small studies in people show effects on nicotine reward or smoking behavior, none of these medications alone has been shown in adequately sized clinical trials to be effective in promoting cessation, including (i) serotonin agonists (lorcaserin), (ii) acetylcholinesterase inhibitors (galantamine and rivastigmine), (iii) drugs affecting GABA receptors (baclofen, topiramate, and gabapentin), and (iv) N -methyl- d -aspartate (NMDA) receptor modulators (cycloserine, memantine, and N -acetylcysteine).
A promising new medication is lorcaserin, a selective 5-hydroxytryptamine 2c receptor agonist. The drug induces food satiety by increasing pro-opiomelanocortin production in the hypothalamus and is FDA approved for weight loss in overweight individuals. Lorcaserin has also been reported to reduce nicotine self-administration in rodents. Because weight gain after stopping smoking is common and sometimes triggers relapse, lorcaserin alone or in combination with other smoking cessation medications has been of interest. In a placebo-controlled trial combining lorcaserin (10 mg twice daily) with varenicline, the combination significantly increased 3-month continuous abstinence (OR, 3.0; 95% CI, 1.5 to 6.2) versus placebo ( 169 ), and weight gain was significantly less.
Medications evaluated in clinical trials and judged ineffective for quitting smoking include mecamylamine, serotonin-specific uptake inhibitors, anxiolytics (benzodiazepines and buspirone), MAO inhibitors (moclobemide and selegiline), modafenil, naltrexone, rimonabant, silver acetate, ondansetron, lobeline, nicotine vaccines, and Nicobrevin (quinine, methyl valerate, camphor, and eucalyptus oil).
E-cigarettes
A general discussion of e-cigarettes and other tobacco products for harm reduction, including consideration of benefits versus risks, is presented in the “Discussion: What Evidence Is Needed” section. Here, we specifically discuss evidence regarding e-cigarettes for smoking cessation. To date, no e-cigarette company has undergone FDA review and approval for use of e-cigarettes as a therapeutic aid for quitting smoking. Less than a handful of randomized controlled trials of e-cigarettes for smoking cessation have been published, and none has been conducted in the United States; hence, most of the evidence to date is observational.
E-cigarettes produce an aerosol from a liquid that typically contains nicotine. The e-cigarette concept is to deliver nicotine by an inhaled route without generating products of tobacco combustion. NRT medications can aid cessation as discussed previously, but most smokers do not find NRT very satisfying, and quit rates are modest. The performance of e-cigarettes as nicotine delivery devices has evolved over time. The earliest devices looked like cigarettes but delivered very low levels of nicotine. The two clinical trials performed with these devices were encouraging, but the quality of evidence was low ( 170 ). Recently, a randomized clinical trial with 886 smokers treated in the United Kingdom’s National Health Service evaluated a second-generation e-cigarette refillable tank–type device to patients’ choice of NRT provided free of cost for up to 3 months ( 171 ). All received standard behavioral support. At 1 year, the sustained abstinence rate in the e-cigarette group was twofold greater than the NRT group (RR, 1.83; CI, 1.30 to 2.58). Among participants randomized to the e-cigarette arm who quit smoking, 80% were still using e-cigarettes at 1 year; in comparison, among those randomized to the NRT arm, continued use of NRT was 9% for those who quit smoking. While e-cigarettes were found to significantly increase smoking cessation, some have expressed concern about the unknown health risks of long-term e-cigarette use. Adverse effects reported during the trial included greater throat or mouth irritation in the e-cigarette group and more nausea in the NRT group. Overall, adverse effects were minor in severity.
Population-based observational studies report different results depending on the intention of the smokers to quit, how e-cigarettes are used, and where the study was conducted. A four-country comparison found the likelihood of quitting with e-cigarettes to differ by the regulatory environment ( 172 ). In Canada and Australia, which have more restrictive e-cigarette regulations, e-cigarette use was associated with a significantly lower likelihood of quitting smoking relative to unassisted quitting (i.e., no medication or e-cigarette use), whereas in the United States and United Kingdom, which have less restrictive e-cigarette regulatory environments, e-cigarette use was associated with increased quitting, consistent with other reports ( 173 , 174 ). The United Kingdom estimates that, annually, 22,000 to 57,000 long-term cigarette quitters are associated with e-cigarette use, more than quits attributed to NRT or other forms of pharmacotherapy ( 175 ). In the United States and United Kingdom, daily use of e-cigarettes is associated with a greater likelihood of quitting smoking than nondaily use ( 176 , 177 ). In a study from France, e-cigarette use was associated with not only higher smoking cessation rates but also greater relapse to smoking ( 178 ).
In conclusion, with respect to e-cigarettes, there is evidence that e-cigarettes can aid smoking cessation. This can occur both in the general population, where e-cigarette use is adopted as an acceptable and safer alternative to cigarette smoking, and in the context of a health service. The risks of long-term e-cigarette use are still unknown, and some medical professionals oppose the use of e-cigarettes for that reason. As discussed in the “Discussion: What Evidence Is Needed” section, there are also concerns about the use of e-cigarettes by children possibly creating a new epidemic of primary nicotine addiction, leading some U.S. public health professionals to conclude that the potential benefits of e-cigarettes for smoking cessation in adults are outweighed by the risks to youth.
TOBACCO CONTROL POPULATION-BASED AND POLICY APPROACHES
U.S. population-based and policy approaches successful for tobacco control include mass media tobacco education campaigns, expanded healthcare coverage for tobacco cessation treatment, excise taxation on tobacco products, clean air laws, and Tobacco 21 policies, which raise the minimum legal age to purchase tobacco products to age 21 ( 92 ). Other population-based interventions to reduce tobacco use have faced challenges in the United States at the federal level (e.g., pictorial warnings on products, regulation of advertising, and promotion at point of sale), and even state tobacco taxes and clean air laws have slowed ( 179 , 180 ). In contrast, interventions in the tobacco retail environment are increasing rapidly at the local level ( 181 ). Also gaining traction at the FDA, and discussed in the “Discussion: What Evidence Is Needed” section, is an effort to reduce the amount of nicotine in combusted tobacco products to reduce its addictive effects.
Mass media tobacco education campaigns
An important component of comprehensive tobacco control programs, mass media tobacco education campaigns are composed of paid and earned media on TV, radio, community placements (e.g., billboards and bus shelters), magazines, newspapers, and digital/social media platforms. Well-designed mass media campaigns implemented with sufficient reach, intensity, and duration can help counter pro-tobacco marketing, build support for tobacco control policies, increase awareness of tobacco’s harmful effects, promote quitting, and reduce smoking prevalence ( 182 ). Here, we describe the success of two ongoing U.S. campaigns.
Tips from former smokers
The CDC’s Tips national mass media tobacco education campaign has been implemented annually since 2012. Tips profiles real people living with serious long-term health consequences from smoking and secondhand smoke exposure based on evidence that messages graphically depicting the physical consequences of smoking-related diseases can encourage quit attempts ( 182 , 183 ). While Tips primarily targets adult smokers, secondary audiences include family members, healthcare providers, and faith communities able to reach people who smoke. Campaign goals include building public awareness of tobacco’s harms to self and others, encouraging smokers to quit, and making free help available (e.g., national quitline). Tips has been effective at increasing population-level quit intentions, quit attempts, and sustained quits, with effectiveness persisting over time ( 184 ). In 2016, Tips ads featured Rebecca, a former smoker with depression. In a national evaluation, greater exposure to the Rebecca ads was associated with a greater likelihood of intending to quit and with making a quit attempt specifically among smokers with mental health conditions ( 185 ). National media campaigns are an important population-level strategy for reaching specific population groups, such as people living with mental health conditions, who are experiencing tobacco-related disparities.
The FDA’s Real Cost campaign is a national tobacco education campaign aimed at preventing tobacco initiation and established tobacco use in youth ages 12 to 17. The campaign is disseminated on national TV and radio, via the internet/social media, in magazines and movie theaters, and on posters distributed to schools. The Real Cost’s central theme in 2014–2016 was “Every cigarette costs you something,” with attention to teen-relevant concerns (e.g., cosmetic effects, loss of control, and toxic chemicals). Between 2014 and 2016, the Real Cost campaign estimated that 350,000 fewer adolescents initiated cigarette smoking ( 186 ). This time period was also when e-cigarettes surpassed combustible cigarettes in popularity among U.S. youth. In 2018, the Real Cost campaign shifted to a focus on e-cigarette prevention in youth.
Tobacco taxes
In the United States, tobacco tax increases have produced the desired effects of both dissuading young people from starting to smoke and encouraging smokers of all ages to quit, with the Community Preventive Services Task Force deeming the evidence strong ( 187 ). Given limited resources, at some point, the health, financial, and social costs of smoking outweigh the perceived benefits or drive of the addiction. Increasing tobacco taxes is suggested as a population-level strategy for reducing smoking among individuals with alcohol, drug, and mental health disorders ( 188 ). With tobacco tax increases should be the availability and promotion of cessation treatments via insurance coverage and resources such as the state quitlines.
Healthcare coverage for tobacco cessation treatments
Healthcare reform legislation can increase receipt of tobacco cessation treatment for smokers from disparity groups. The U.S. Affordable Care Act (ACA) mandated comprehensive coverage for tobacco treatment for most private health plans and newly eligible Medicaid beneficiaries in states that expanded Medicaid, including at least two tobacco cessation attempts per year and four tobacco cessation counseling sessions (each 10+ min long) and prohibited cost-sharing and previous authorization restrictions for FDA-approved tobacco cessation medication. The ACA also removed coverage limits and preexisting condition exclusions. Concerning, however, was the ACA’s allowance for states to decide whether employers could charge smokers up to 50% more in premiums. Several states rejected the surcharge outright, while other states capped the maximum penalty at a lower level. National data from 2011 to 2014 indicate that in the first year of implementation, penalized smokers were less likely to be insured and the penalty did not encourage cessation ( 189 ). Charging smokers higher insurance premiums could discourage getting health insurance or lead to concealment of one’s smoking status; either would reduce opportunities for treatment. Tobacco cessation treatments are cost effective. In Massachusetts, for every $1 spent on cessation services for state Medicaid program beneficiaries, more than $3 was saved ( 190 ).
Smoke-free air
The Community Preventive Services Task Force deemed smoke-free air policies to have strong evidence for reducing youth initiation of tobacco use, increasing quitting among smokers, reducing exposure to secondhand smoke, reducing tobacco-related morbidity and mortality, and reducing healthcare costs ( 191 ). Furthermore, smoke-free policies do not adversely affect businesses. Smoke-free air policies in the home similarly reduce harmful secondhand smoke exposure, increase quit attempts and abstinence, and decrease cigarette consumption in adult smokers ( 192 ). A U.S. study found that statewide smoking bans in restaurants and bars were associated with reduced smoking among those with psychiatric conditions ( 193 ). Psychiatric facilities are increasingly adopting smoking bans, although still not mandated nationally.
Tobacco 21 legislation
Given that few people start smoking after age 20 and that brain development continues through the mid-20s, with early drug exposure predictive of greater likelihood of chronic, addictive use, legislation has sought to raise the minimum tobacco sales age to 21 (i.e., Tobacco 21). The Institute of Medicine concluded, based on simulation models, that Tobacco 21 laws would reduce smoking and related mortality ( 194 ). Lacking a federal Tobacco 21 law, states and local jurisdictions have passed legislation, with regional differences in coverage. As of January 2019, most U.S. residents aged 18 to 20 were not covered by a Tobacco 21 policy, with the largest gaps in coverage in the South ( 195 ). As of 1 June 2019, 14 states and >400 local jurisdictions have passed Tobacco 21 legislation; 16 of the non-adopting states preempt lower levels of government from implementing these regulations. Analyzing national data, a recent study found that Tobacco 21 policies were associated with a significant absolute 3% reduction in the prevalence of smoking among 18 to 20 year olds ( 196 ). Surveys indicate that two-thirds to three-quarters of U.S. adults are in favor of raising the minimum age of tobacco sales to 21 ( 197 , 198 ).
Tobacco retailer restrictions
Tobacco products are readily accessible for open sale in retail outlets throughout the United States and globally. In the United States, there are an estimated 375,000 tobacco retailers ( 199 ); this equates to 27 tobacco retail locations for every McDonald’s restaurant. The tobacco retail environment contributes to tobacco-related disparities. Tobacco retailers concentrate disproportionately in disadvantaged areas ( 200 ). Even after adjusting for the density of retailers, cigarettes and little cigars/cigarillos cost less in these areas. The same is true for areas with a higher proportion of African American residents ( 201 ).
In its blueprint to end the U.S. tobacco epidemic, the Institute of Medicine recommended that governments develop, implement, and evaluate legal mechanisms for restructuring retail tobacco sales and restricting the number of tobacco outlets ( 202 ). In response, there has been a rapid rise in planning and implementation of retail interventions by states and communities ( 181 ). For example, at least two states and >200 localities restrict the sale of flavored tobacco (45 communities restrict the sale of menthol cigarettes); dozens have set a minimum price and pack size for little cigars/cigarillos, and at least three prohibit price discounts and coupon redemption ( 203 ). By restricting the sales and distribution of tobacco, the long-term goal of these interventions is to reduce tobacco use and inequities in the retail environment. With a focus on youth, a global study of bans on tobacco point-of-sale ads in retail environments reported lower odds of ever smoking, lower smoking prevalence, and less daily smoking ( 204 ). A growing evidence base is informing best practices for state and local programs aimed at countering tobacco industry influence at the point of sale.
DISCUSSION: WHAT EVIDENCE IS NEEDED
Tobacco use remains the leading preventable cause of death in the United States and worldwide. While important public health gains have been achieved in reducing the prevalence of cigarette smoking, because of population growth and diversification of product, the absolute number of tobacco users in the United States has stayed relatively constant over the last 50 years, at about 40 million. Furthermore, dual use of tobacco products is on the rise ( 21 , 22 ), and declines in smoking have not been equitable for all groups. Disproportionately affected by tobacco-related morbidity and mortality are people of certain racial/ethnic groups (e.g., African Americans and American Indian/Alaska Native people), individuals of lower income and lower education, and people with mental illness and substance use disorders.
Among adolescents, cigarette smoking has declined to under 10%; however, the use of e-cigarettes has increased markedly, with 27.5% of high school students reporting past 30-day use. Today, more young people in the United States are exposing their brains to nicotine than in recent years. Although free of the toxins from combustion, e-cigarettes typically still contain nicotine, the main psychoactive and addictive component in tobacco.
Our review covered evidence-based methods to treat smoking in adults and policy approaches to prevent nicotine product use in youth. The smoking cessation treatments with evidence in adults include seven FDA-approved cessation medications ( Table 2 ), individual and group counseling, quitlines and other mobile technologies, and monetary incentives. At the population level, mass media education campaigns, product regulations, health insurance coverage of cessation treatments, and enactment of tobacco control policies (e.g., clean air, Tobacco 21, flavor bans, and retailer density restrictions) are promising interventions. Most efficacious are combinations of medication and behavioral treatments leveraged in an environment with strong tobacco control policies. Notably absent are evidence-based treatments for stopping e-cigarette use, particularly in adolescents, an area of public health interest.
The changing marketplace and the challenges of treating addiction necessitate the sustained efforts of clinical providers, policymakers, and researchers. Investment in comprehensive tobacco cessation treatment at the state and federal levels and continued research in the development of novel behavioral and medication treatments, diagnostics for personalized medicine, technological innovations for broader reach, and evidence-based policies are warranted. Here, we briefly highlight some areas for further investigation.
Candidate new mechanisms of action for cessation pharmacotherapy
As reviewed above, existing medications aid in smoking cessation, but none has high success rates during a single course of treatment. As we learn more about the effects of nicotine on the brain and the mechanisms of addiction, we may gain insight into new molecular targets for nicotine addiction. In addition, combinations of treatments with different actions, as exemplified by varenicline plus lorcaserin to both promote quitting and prevent associated weight gain, need to be explored.
Long-term effects of alternative/harm reduction products
The potential harms to health from various harm reduction products could not be extensively discussed here, but assessment of harm is a critical component of a reasoned benefit versus risk analysis. On the basis of current evidence, it is believed that e-cigarettes and heated tobacco will be very much less harmful than cigarette smoking, but how much less harmful is unknown. Heated tobacco products have been successfully marketed in Japan where 4.7% of the population used the products in 2017, although 72% of heated tobacco users also continued to smoke cigarettes ( 205 ). The prevalence of cigarette smoking has declined substantially in recent years in Japan, and although speculated that heated tobacco use is responsible for that decline, this is unproven. Heated tobacco products are marketed in many other countries and are approved for use in the United States, but so far, uptake has been limited. As yet, there are no data on abuse liability and no trials of heated tobacco for combustible cigarette cessation, and we are unaware of any data on youth uptake of IQOS.
Considerable national and international debate has also occurred regarding the use of smokeless tobacco for harm reduction ( 206 , 207 ). While the use of some forms of smokeless tobacco is associated with oral, esophageal, and pancreatic cancer and other adverse health effects, low nitrosamine smokeless tobacco is associated with much lower risk ( 208 , 209 ). In Sweden, snus (ground tobacco in a teabag-like pouch placed between lip and gum) is manufactured and marketed under strict quality standards, resulting in low levels of nitrosamines (potential carcinogens) ( 210 ). In Sweden, 20% of men and 8% of women use snus, while the smoking prevalence is lower than in other countries. The incidence and mortality from smoking-related diseases is significantly lower in Sweden than in other European countries ( 211 ). Epidemiologic studies indicate that the health risks of Swedish snus use are low, including a small, if any, increase in cancer and cardiovascular disease risk and no increased risk of lung disease. On the basis of these observations, some public health experts advocate that smokeless tobacco be encouraged as an alternative to cigarette smoking. The potential harm reduction benefit of smokeless tobacco most likely varies by country and cultural norms. In Sweden, there is a long tradition of smokeless tobacco use, and most men use snus without a transition to cigarette smoking. However, in the United States, where smokeless tobacco use is much less widely accepted, there is concern that smokeless tobacco use is a gateway to smoking among youth ( 212 ). There is also concern that smokeless tobacco could reduce smoking cessation in dual users, because smokeless tobacco could be used in circumstances where smoking is prohibited. Controlled clinical trials of smokeless tobacco as an approach to aid smoking cessation or in switching from cigarettes to smokeless tobacco have shown modest benefits, similar to NRT ( 213 , 214 ).
Further mechanistic and epidemiologic studies are needed to help inform harm reduction public policy. In addition, likely an area of research development and interest in the very near future are study of cessation treatments for those users who want to quit their e-cigarette, heated tobacco, or snus use. Given the mechanism of nicotine addiction, it would seem reasonable that medications helpful in quitting smoking would prove efficacious; however, no randomized controlled trial to address these questions has been conducted to date.
Understanding and treating dual tobacco use
As mentioned at the start, dual use of tobacco products is on the rise ( 21 , 22 ), and rates of dual use are threefold greater for high school students (11%) ( 13 ) than adults (3.7%) ( 11 ), with smoking cigarettes and vaping e-cigarettes the most common combination. Analysis of survey data from the United States, United Kingdom, Canada, and Australia concluded that adults who smoke cigarettes and e-cigarettes concurrently should be considered a distinct group given higher levels of nicotine dependence and generally more pro-attitudes toward both smoking and vaping ( 215 ). Dual use may represent greater dependence and compulsion to dose nicotine in settings where smoking is prohibited or may reflect motivation to quit combustible cigarettes ( 23 ). In a nationally representative study, interest in quitting and attempts to quit were comparable among dual tobacco–using adults and cigarette-only users ( 216 ). The research on dual tobacco use is still nascent. Greater and more detailed study is needed to understand use patterns of two or more tobacco products; the implication of different types of combinations; and the relationship of dual use to addiction, biomarkers of harm, and success with quitting.
Tobacco-drug co-use and translational potential
Treatment studies of cannabis use disorder in adults suggest that about half of participants also currently smoke tobacco. Among adolescents ( 217 , 218 ) and adults ( 219 ), persistent tobacco use is associated with poorer treatment outcomes for cannabis use disorders, and individuals who use both cannabis and tobacco in combination have higher rates of psychiatric and psychosocial problems as compared to individuals who smoke cannabis only ( 219 ). Blunt smoking (i.e., cannabis smoked in a cigar shell) is associated with greater difficulty controlling cannabis use ( 220 ) and high levels of toxicant exposures (e.g., carbon monoxide and carcinogens) ( 221 ), as compared to joint smoking. Despite decades of research on cannabis and tobacco use separately, there is little treatment research addressing the co-use of cannabis and tobacco. In addition, although currently the co-use of cannabis and nicotine by vaping is relatively rare and primarily occurs among established tobacco or cannabis users, given the growth in popularity of both cannabis and nicotine vaping, it is likely to increase and expand to tobacco/cannabis naïve individuals. Study of the behavioral co-use patterns and pharmacologic effects, with an understanding of addiction potential and quantified toxicant exposures, and the potential for pulmonary injury is needed.
There is a high concordance of tobacco use with virtually all other drugs of abuse, including cannabis, alcohol, opiates, and stimulants. Neurobiology research has found interacting neural circuits between nicotine and other abused substances. Such research may lead to discovery of medications that simultaneously treat multiple drugs of abuse. Likewise, studies of the genetics of addiction to nicotine and other substances of abuse, as well as genetic signals of concordance of nicotine addiction with other addictions and mental illnesses, may lead to the discovery of similar therapeutic targets.
Vulnerable populations
Smoking cessation treatment has been particularly challenging in some populations, including among people with mental illness, those with other substance use disorders, adolescents, pregnant smokers, and light and nondaily smokers. In addition, cessation success varies by race and ethnicity, as seen with lower quit rates in African American and American Indian/Alaska Native smokers. State data from Alaska indicate that the proportion of people who have quit smoking among those who have ever smoked (i.e., the quit ratio) is 41% for Alaska Native adults compared to 62% for Alaskan adults of other races/ethnicities ( 222 ). This means that for the Alaska Native community, there are more current than former smokers. Behavioral interventions that are culturally relevant for specific populations and individualized pharmacotherapy approaches are needed. As an example, with funding from the National Heart, Lung, and Blood Institute (NHLBI), our research is testing the efficacy of internet-assisted tobacco cessation counseling in the remote region of Norton Sound with Alaska Native men and women ( 223 ). The treatment includes combination NRT, and we are evaluating the nicotine metabolism ratio in predicting treatment outcome. To promote cessation in groups particularly vulnerable to tobacco use, emerging research has supported the value of targeted communication ( 185 ) and regulatory policies such as reducing nicotine levels in cigarettes ( 224 ), discussed next.
Regulation of cigarette addictiveness: Very low nicotine content cigarettes
In 1994, Benowitz and Henningfield proposed the idea of federal regulation of the nicotine content of cigarettes to reduce levels over time, resulting in lower intake of nicotine and a lower level of nicotine dependence ( 225 ). When nicotine levels get very low, cigarettes would be much less addictive. Now, 25 years later, the concept of regulating combustible tobacco to very low levels of nicotine content is being seriously considered.
Very low nicotine content cigarettes (VLNCs) are engineered to have reduced yields of nicotine in the tobacco contained in the cigarette rod. These cigarettes deliver much lower levels of nicotine than earlier cigarettes that were marketed as “light” or “ultralight” but which in practice allowed smokers to obtain levels of nicotine similar to regular “full-flavor” cigarettes through compensation behaviors, such as blocking ventilation holes or inhaling more deeply ( 225 ). Reducing the nicotine content of cigarettes to approximately 0.5 mg per cigarette is believed to render cigarettes minimally addictive and lead to lower levels of consumption, making it easier for smokers to quit ( 225 ). Randomized trials examining the effects of VLNCs have shown reductions in smoking and dependence and increases in quit attempts for VLNCs in comparison with standard nicotine cigarettes. A 6-week trial found decreases in nicotine exposure and dependence on nicotine for VLNCs, decreases in craving during abstinence from smoking, and decreases in the number of cigarettes smoked without significantly increasing levels of expired carbon monoxide or total puff volume, which suggests minimal compensation behavior ( 226 ). In a randomized, parallel-arm, semi-blinded study of adult cigarette smokers, participants receiving 0.05 mg/g cigarettes showed greater relief of withdrawal from usual-brand cigarettes than the nicotine lozenge, significantly higher abstinence at the 6-week follow-up than the 0.3 mg/g cigarette, and a similar rate of cessation as the nicotine lozenge ( 227 ). At 12-month follow-up, however, findings were not sustained ( 228 ).
In clinical trials, VLNCs generally have lower acceptability than commercially available cigarettes, and these trials have encountered problems with nonadherence (with upward of 70% of participants substituting traditional cigarette brands for VLNCs) and study dropout rates of 25 to 45% ( 229 , 230 ). Combining VLNCs with nicotine patches may aid with the transition to VLNCs and increase compliance, but doing so was not found to improve long-term quit rates. If the nicotine content in all cigarettes was reduced to make them less addictive, either through federal regulation or by the tobacco industry’s own initiative, then problems with adherence and attrition could be less of an issue and long-term cessation rates could be higher.
A series of laboratory and experimental studies have tested VLNCs with smokers, with mental illness (depression and schizophrenia) and substance use (opioid use) disorders finding VLNCs less satisfying than usual brand cigarettes and leading to reduced smoking while decreasing craving, withdrawal, and depressive symptoms and without leading to compensatory smoking ( 224 ). In one study that found negative cognitive performance associated with VLNCs, use of the nicotine patch reversed the decrements ( 231 ). The findings support FDA-mandated reduction in the nicotine content of cigarettes to a minimally addictive level to reduce cigarette use among smokers with mental illness.
The Family Smoking Prevention and Tobacco Control Act bars the FDA from completely removing nicotine from cigarettes. The FDA, however, is allowed to reduce the amount of nicotine in cigarettes to very low levels. In July 2017, the FDA indicated that it would issue an Advance Notice of Proposed Rulemaking to seek input on the potential public health benefits and any possible adverse effects from lowering the nicotine content of cigarettes ( 232 ). The process of review continues. The WHO emphasizes that a nicotine reduction strategy ought to cover all combustible tobacco products, not just cigarettes; include provision of tobacco cessation treatment; and consider toxicant exposures from switching to noncombustible forms of tobacco to sustain nicotine intake and for what duration ( 233 ).
The future of e-cigarettes and public health impact
The overall impact of e-cigarettes on public health remains a question of debate. While e-cigarettes may have adverse effects on respiratory health and possibly other diseases, the harm is generally accepted to be much less than that of cigarette smoking ( 24 ). Thus, if smokers were to switch completely to e-cigarettes, then smoking-related disease is predicted to decrease substantially. Population-based models of the impact of e-cigarette use predict an overall health benefit, because many smokers will quit, while those who continue vaping or take up e-cigarettes anew experience much less harm ( 234 ). On the other hand, many parents, pediatricians, public health officials, and others are extremely concerned about youth uptake of e-cigarettes and are encouraging local communities to ban e-cigarette sales. E-cigarette use in youth shows exposure to toxins with concern about the long-term health effects from sustained use ( 235 ). The overall benefit versus risk for a community is likely to depend on the prevalence of cigarette smoking in the community. Where smoking prevalence is high, the potential benefits of e-cigarettes in reducing smoking are high. Where smoking prevalence is low, the benefit is low and the potential risk of e-cigarettes to youth becomes the major community concern.
Another consideration regarding e-cigarettes is a role that it may play in a broader public health regulatory intervention. Reducing the nicotine content of combustible tobacco would make the products less satisfying to smokers. The availability of less harmful noncombusted sources of nicotine, such as e-cigarettes, could help a smoker transfer their nicotine addiction from combustibles to e-cigarettes. Presumably, many, if not most, people would stop smoking, and the result would be prevention of most tobacco-related disease. In time, a former smoker who switched to e-cigarettes could quit nicotine use or remain a long-term e-cigarette user but with much less harm than from smoking cigarettes.
In closing, with the evolving nicotine product market, critically important is the need for evidence to inform innovations in tobacco control policies and tobacco treatment approaches (behavioral, pharmacologic, and technology based), with consideration of the risks and benefits for all populations affected.
Acknowledgments
We thank N. Addo and A. Chieng for editorial assistance. Funding: J.J.P.’s time in writing this manuscript was supported by grants from the NHLBI (R01HL117736) and the NCI (P01CA225597). N.L.B.’s time in writing this manuscript was supported by NHLBI grant nos. R01HL117736 and U54HL147127. Author contributions: J.J.P. and N.L.B. drafted sections of the manuscript and edited and reviewed the manuscript in full to final version. Competing interests: J.J.P. and N.L.B. have served as expert witnesses against the tobacco companies in lawsuits for which they have received fees for the work and have provided consultation to Pfizer and Achieve Life Sciences, which make medications for quitting smoking. The authors declare no other competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper. Additional data related to this paper may be requested from the authors.
REFERENCES AND NOTES

COMMENTS
Background and objectives: Despite reductions in prevalence in recent years, tobacco smoking remains one of the main preventable causes of ill-health and premature death worldwide.This paper reviews the extent and nature of harms caused by smoking, the benefits of stopping, patterns of smoking, psychological, pharmacological and social factors that contribute to uptake and maintenance of ...
We identified three outcomes with a 4-star association with smoking: COPD (72% increase in risk based on the BPRF, 0.54 ROS), lower respiratory tract infection (54%, 0.43) and pancreatic cancer ...
Almost 35 years ago, the Office of the Surgeon General of the United States Health Service reviewed over 7000 research papers on the topic of smoking and health, and publicly recognized the role of smoking in various diseases, including lung cancer. Since then, numerous studies have been published that substantiate the strong association of ...
We identified three outcomes with a 4-star association with smoking: COPD (72% increase in risk based on the BPRF, 0.54 ROS), lower respiratory tract infection (54%, 0.43) and pancreatic cancer (52%, 0.42). In the present study, we provide detailed results for one example 4-star association: current smoking and COPD.
Decades after its ill effects on human health were first documented, tobacco smoking remains one of the major global drivers of premature death and disability. In 2017, smoking was responsible for ...
The 2014 Surgeon General's report estimates that cigarette smoking causes more than 480,000 deaths each year in the United States. 1 This widely cited estimate of the mortality burden of smoking ...
Abstract. Tobacco smoking is a major determinant of preventable morbidity and mortality worldwide. More than a billion people smoke, and without major increases in cessation, at least half will ...
Tobacco smoking: Health impact, prevalence, correlates and interventions. Background and objectives: Despite reductions in prevalence in recent years, tobacco smoking remains one of the main preventable causes of ill-health and premature death worldwide. This paper reviews the extent and nature of harms caused by smoking, the benefits of ...
About the Journal. Nicotine & Tobacco Research aims to provide a forum for empirical findings, critical reviews, and conceptual papers on the many aspects of nicotine and tobacco, including research from the biobehavioral, neurobiological, molecular biologic, epidemiological, prevention, and treatment arenas. Find out more here.
In their Article in The Lancet Public Health, Marissa Reitsma and colleagues1 report their comprehensive analysis of smoking tobacco use in young people from more than 3000 tobacco surveys from 204 countries and territories around the world. The result is an invaluable overview of an epidemic that causes millions of deaths every year. Their detailed mapping of the prevalence of smoking tobacco ...
Objectives: Although the global COVID-19 pandemic has increased interest in research involving high-risk smokers, studies examining changed smoking behaviours, cessation intentions and associated psychological states among smokers are still scarce. This study aimed to systematically review the literature related to this subject. Design: A systematic review of published articles on cigarettes ...
Most Americans recognize that smoking causes serious diseases, yet many Americans continue to smoke. One possible explanation for this paradox is that perhaps Americans do not accurately perceive the extent to which smoking increases the probability of adverse health outcomes. This paper examines the accuracy of Americans' perceptions of the absolute risk, attributable risk, and relative ...
Background Cigarettes and their by-products (i.e., smoke; ash) are a complex, dynamic, and reactive mixture of around 5,000 chemicals. Cigarette smoking potentially harms nearly every organ of the human body, causes innumerable diseases, and impacts the health of smokers and those interacting with the smokers. Smoking brings greater health problems in the long-term like increased risk of ...
The Doll and Hill paper showed that cigarette smoking was far more commonly reported among patients with lung cancer than those with other diseases or those free of disease. ... research to better understand how risks are perceived and internalized by individuals and governments is needed to ensure that epidemiological studies on the ...
Abstract. Smoking is a behavior that is influenced by a variety of factors that cut across methodologies, disciplines, and content areas within health psychology. The present article is designed to show the diversity and richness of smoking research by examining smoking from four perspective: basic laboratory research, intervention, prevention ...
Background The evidence on the effects of chronic tobacco smoking on neuropsychological functions is conflicting. The literature remains limited by inconsistent accounting for potentially confounding biomedical and psychiatric conditions. This study aimed to assess the neuropsychological functions of adult chronic tobacco smokers in comparison to group-matched non-smokers. Method The study ...
While the health effects of cigarette smoking are well recognized and documented, the environmental impacts of tobacco are less appreciated and often overlooked. Here, we evaluate tobacco's global footprint across its entire supply chain, looking at resource needs, waste, and emissions of the full cradle-to-grave life cycle of cigarettes. The cultivation of 32.4 Mt of green tobacco used for ...
estimated that smoking increases the risk of. coronary heart disease about 2-4 times, stroke 2-4 times, lung cancer 25 times in. men, and 25.7 times in women. Be sides, smoking can lead to an ...
In 2014, the Nation marked the 50th anniversary of the first Surgeon General's Report on Smoking and Health. In 1964, more than 40 percent of the adult population smoked. Once the link between smoking and its medical consequences—including cancers and heart and lung diseases—became a part of the public consciousness, education efforts and public policy changes were enacted to reduce the ...
Background Scientific evidence clearly demonstrates that inhaling the smoke from the combustion of cigarettes is responsible for most of the harm caused by smoking, and not the nicotine. However, a majority of U.S. adults who smoke inaccurately believe that nicotine causes cancer which may be a significant barrier, preventing switching to potentially reduced risk, non-combustible products like ...
Prevalence and burden of tobacco smoking. In 2012, approximately 45,500 deaths (18% of all deaths in Canada) were attributed to tobacco smoking [].Smoking continues to be a leading cause of preventable death and disability [2, 3].Among smoking-related deaths, most were attributable to cancers, cardiovascular disease, and respiratory diseases [1, 4].
Data have been collected via several methods, including paper- and web-based questionnaires, computer-assisted interviews, clinic visits and data linkage. ... previous research has shown that smoking is associated with increased time to conceive (Wootton et al., 2023). Our results show the opposite (i.e., smoking leading to younger age at first ...
Cutting-edge neuroimaging technologies have identified brain changes associated with nicotine dependence and smoking. Using functional magnetic resonance imaging (fMRI), scientists can visualize smokers' brains as they respond to cigarette-associated cues that can trigger craving and relapse. 231 Such research may lead to a biomarker for ...
There is also emerging data suggesting that e-cigarettes may facilitate smoking cessation but further research is needed to compare the effectiveness and safety of e-cigarettes compared to other nicotine replacement therapies [13,14]. Consistent with the U.S. FDA regulatory oversight and the U.S. Surgeon General report, it may be prudent to ...
The FDIC is a preeminent banking research institution. The FDIC established the Center for Financial Research to promote research on topics important to the FDIC's mission including deposit insurance, bank supervision, making large and complex financial institutions resolvable, and resolution of failed financial institutions.
Tobacco rolled in paper for smoking: A typical cigarette weighs <1 g; regular length (70 mm long), king (84 mm), 100s (100 mm), and 120s (120 mm) ... use and high levels of toxicant exposures (e.g., carbon monoxide and carcinogens) , as compared to joint smoking. Despite decades of research on cannabis and tobacco use separately, there is ...