
Subscribe or Renew

Create an E-mail Alert for This Article
Progress with treatments for alzheimer’s disease, permissions, information & authors, metrics & citations, continue reading this article.
Select an option below:
Create your account to get 2 free subscriber-only articles each month.
Already have an account, print subscriber, supplementary material, information, published in.
- Clinical Medicine General
- Dementia/Alzheimer Disease (Geriatrics/Aging)
- Dementia/Alzheimer Disease (Neurology/Neurosurgery)
- Psychiatry General
Affiliations
Export citation.
Select the format you want to export the citation of this publication.
- Hongqi Wang,
- Xiaodong Yan,
- Yiming Zhang,
- Peifu Wang,
- Shu-Tong Huang,
- Jin-Chong Luo,
- Guo-Hui Zhong,
- Li-Ping Teng,
- Cai-Yan Yang,
- Chun-Li Tang,
- Zhong-Bo Zhou,
- Neng Jiang,
- Xiao Huang,
- Jialing Zhao,
- Qinghua Wang,
- Tingqi Yan,
- Xiaofeng Zhu,
- Yanjiang Wang,
- Shaojie Yang,
- Weidong Le,
- Yang Xiang,
- Le-Quang Bao,
- Daniel Baecker,
- Do Thi Mai Dung,
- Nguyen Phuong Nhung,
- Nguyen Thi Thuan,
- Phuong Linh Nguyen,
- Phan Thi Phuong Dung,
- Tran Thi Lan Huong,
- Bakhtiyor Rasulev,
- Gerardo M. Casanola-Martin,
- Nguyen-Hai Nam,
- Hai Pham-The,
- Ernesto G. Miranda-Morales,
- Sorosh Husseinzadeh,
- Jia Yi Liew,
- Qing Chang,
- Balaji Krishnan,
- Angelo Gaitas,
- Michelle Felicella,
- Wei Qiao Qiu,
- Xiang Fang,
- Khalid Saad Alharbi,
- Mohammad Arshad Javed Shaikh,
- Syed Sarim Imam,
- Sultan Alshehri,
- Mohammed M. Ghoneim,
- Waleed Hassan Almalki,
- Sachin Kumar Singh,
- Deepak Kumar,
- Avvaru Praveen Kumar,
- Dinesh Kumar Chellappan,
- Keshav Raj Paudel,
- Gaurav Gupta,
- Caiyu Zhuang,
- Beibei Chen,
- Xiaolei Zhang,
- Yanfang Liao,
- Yifan Zhang,
- Huinian Lu,
- Zhiju Huang,
- Fengming Huang,
- Zhende Zhang,
- Zihao Wang,
- Yujie Huang,
- Guohui Zhong,
- Chengyun Pang,
- Chunli Tang,
- Fengling Zhang,
- Yaqian Yan,
- Zongji Chen,
- Sheng-Ta Tsai,
- Srinivasan Nithiyanantham,
- Senthil Kumaran Satyanarayanan,
- Kuan-Pin Su,
View Options
View options, content link.
Copying failed.
PREVIOUS ARTICLE
Next article, create your account for 2 free subscriber-only articles each month. get free access now..
New Alzheimer's studies reveal disease biology, risk for progression, and the potential for a novel blood test

The researchers examined blood plasma samples of three diagnostic groups of participants — cognitively normal, mildly cognitively impaired and dementia due to Alzheimer’s disease patients. | zorgens - stock.adobe.com
INDIANAPOLIS — The failure to diagnose Alzheimer's disease, the most common form of dementia in the elderly, at an early stage of molecular pathology is considered a major reason why treatments fail in clinical trials. Previous research to molecularly diagnose Alzheimer's disease yielded "A/T/N" central biomarkers based on the measurements of proteins, β-amyloid ("A") and tau ("T"), and "N" encompassing neurodegeneration. A/T/N can be measured in brain tissue, by in vivo brain imaging techniques, and by analysis of cerebrospinal fluid and plasma.
Alzheimer's disease is thought to be triggered by combinations of genetic and environmental risk factors. Blood-based biomarkers such as plasma microRNAs (miRNAs) — molecules that regulate genome-environment interactions and control the expression of genes governing brain functions which deteriorate in Alzheimer's — could offer advantages of cost-savings, accessibility and decreased invasiveness.
Two new papers by a team of researchers at Boston University, the Indiana University School of Medicine and the Alzheimer's Disease Neuroimaging Initiative (ADNI), and the German Center for Neurodegenerative Diseases (DZNE) in Goettingen, Germany, published in Alzheimer's & Dementia: The Journal of the Alzheimer's Association demonstrate that evaluating microRNAs in blood can be used not only to diagnose mild cognitive impairment (MCI) but also, critically, to predict the conversion from MCI to dementia due to Alzheimer's disease. Moreover, the researchers uncovered microRNA candidate molecular biomarkers that associate with current amyloid, tau, and neurodegeneration (A/T/N) Alzheimer's biomarkers.
The papers were recently published online:
- The plasma miRNAome in ADNI: Signatures to aid the detection of at-risk individuals
- Plasma miRNAs across the Alzheimer's disease continuum: Relationship to central biomarkers

The other senior authors are Andre Fischer, PhD, DZNE speaker and professor of epigenetics of neurodegenerative diseases at University Medical Center Goettingen, Germany; Kwangsik Nho, PhD , professor of radiology and imaging sciences at the IU School of Medicine; and Andrew J. Saykin, PsyD , Raymond C. Beeler Professor of Radiology and director of the Center for Neuroimaging and the Indiana Alzheimer's Disease Research Center at the IU School of Medicine. The work was funded by the National Institutes of Health's National Institute on Aging multisite project RF1AG078299, "MicroRNAs as Diagnostic and Prognostic Biomarker of Alzheimer’s Disease," that supports the teams of researchers in multiple institutions.
The researchers examined miRNA expression in the plasma samples of three diagnostic groups of participants — cognitively normal, mildly cognitively impaired and dementia due to Alzheimer's disease patients. They found that, when combined with neuropsychological testing, plasma microRNAome evaluation helps predict which aging individuals concerned about cognitive decline will progress to develop Alzheimer's.
"These findings provide a path toward a better understanding the molecular mechanisms driving plaques, tangles and atrophy, and may provide clues for the next generation of therapeutic targets," Saykin said.

While these are exciting times with novel therapies for Alzheimer's disease entering clinical care, the researchers note that those therapies only will work in a real-world setting if patients at risk are identified as early as possible.
"MicroRNAs are ideal biomarkers since they are not only very stable but also control entire molecular pathways thereby ensuring cellular homeostasis. As such one microRNA can simultaneously control many proteins belonging to a certain pathway," Fischer said. "Therefore, the analysis of a few microRNAs can inform about complex pathological changes reflecting multiple pathways, such as neuroinflammation, metabolic changes, or synapse dysfunction. Thus, we need biomarkers that allow screening applicable in a point-of-care setting. Our studies are an important step in this direction."
"We have laid the groundwork for further investigations into the role of microRNAs in Alzheimer’s disease pathogenesis," Nho said. "We envision that once specific miRNA signatures are further confirmed, the analysis of blood miRNAs will be transferred to simple assay formats enabling the adoption of blood miRNAome analysis in clinical practice."
The researchers said improved tools for the early detection of Alzheimer's are indispensable for developing prevention and treatment strategies for the disease that is causing enormous suffering and burdens health care systems around the world.
About the IU School of Medicine
The IU School of Medicine is the largest medical school in the U.S. and is annually ranked among the top medical schools in the nation by U.S. News & World Report. The school offers high-quality medical education, access to leading medical research and rich campus life in nine Indiana cities, including rural and urban locations consistently recognized for livability. According to the Blue Ridge Institute for Medical Research, the IU School of Medicine ranks No. 13 in 2023 National Institutes of Health funding among all public medical schools in the country.
About Boston University Chobanian & Avedisian School of Medicine
Originally established in 1848, the New England Female Medical College, became coed as Boston University School of Medicine in 1873. The school today is a leading academic medical center with an enrollment of more than 600 medical students and approximately 1,200 students pursuing Master's and PhD degrees. School of Medicine faculty attract more than $277 million in research awards annually, in Alzheimer's disease, arthritis, cardiovascular disease, cancer, infectious diseases, pulmonary disease, amyloidosis, and dermatology, among other areas. The school's 30 teaching affiliates include Boston Medical Center, Boston VA Healthcare System; St. Elizabeth's Medical Center in Brighton, Mass.; Kaiser Permanente in northern California; and many others.
About Deutsches Zentrum für Neurodegenerative Erkrankungen, DZNE (German Center for Neurodegenerative Diseases)
DZNE is a research institute for neurodegenerative diseases such as Alzheimer's, Parkinson's and ALS, which are associated with dementia, movement disorders and other serious health impairments. To date, there are no cures for these diseases, which represent an enormous burden for countless patients, their families and the healthcare system. DZNE is dedicated to the development and translation into practice of novel strategies for prevention, diagnosis, care and treatment. The institute comprises ten sites across Germany and collaborates with universities, university hospitals, research centers and other institutions in Germany and throughout the world. It is state-funded and a member of the Helmholtz Association and of the German Centers for Health Research.
Media Contact
Andrea zeek, related news, researchers discover new way inflammation impacts cell communication, new research explores the urea cycle’s strong connection to fatty liver disease.
New Alzheimer’s Studies Reveal Disease Biology, Risk for Progression, and Potential for Novel Blood Test
The failure to diagnose Alzheimer’s disease, the most common form of dementia in the elderly, at an early stage of molecular pathology is considered a major reason why treatments fail in clinical trials. Previous research to molecularly diagnose Alzheimer’s disease yielded “A/T/N” central biomarkers based on the measurements of proteins, β-amyloid (“A”) and tau (“T”), and “N” encompassing neurodegeneration. A/T/N can be measured in brain tissue, by in vivo brain imaging techniques, and by analysis of cerebrospinal fluid and plasma.
Alzheimer’s disease is thought to be triggered by combinations of genetic and environmental risk factors. Blood-based biomarkers such as plasma microRNAs (miRNAs)—molecules that regulate genome-environment interactions and control the expression of genes governing brain functions which deteriorate in Alzheimer’s—could offer advantages of cost-savings, accessibility and decreased invasiveness.
Two new papers by a team of researchers at Boston University Chobanian & Avedisian School of Medicine, the Indiana University School of Medicine and the Alzheimer’s Disease Neuroimaging Initiative (ADNI), and the German Center for Neurodegenerative Diseases (DZNE) in Goettingen, Germany, published in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association demonstrate that evaluating microRNAs in blood can be used not only to diagnose mild cognitive impairment (MCI) but also, critically, to predict the conversion from MCI to dementia due to Alzheimer’s disease. Moreover, the researchers uncovered microRNA candidate molecular biomarkers that associate with current Amyloid, Tau, and Neurodegeneration (A/T/N) Alzheimer’s biomarkers.

“Our papers are the result of a successful collaboration that tied the technology developed by professor Andre Fischer in Germany’s DZNE to reliably measure the levels of microRNA in human plasma, and the power of blood samples obtained from hundreds of ADNI participants participating in a simulated clinical trial taking place at about 60 medical centers across the US and Canada. Our discovery is important because, unlike the current A/T/N biomarkers, microRNAs may serve as blood molecular biomarkers years before Alzheimer’s disease manifests clinically, thus identifying the time window for effective prevention or early intervention to stop the progression of Alzheimer’s,” explained one of four senior authors Ivana Delalle , MD, PhD, professor of pathology & laboratory medicine at Boston University.
The other senior authors are Andre Fischer, PhD, DZNE speaker and professor of epigenetics of neurodegenerative diseases at University Medical Center Goettingen, Germany; Kwangsik Nho, PhD, professor of radiology and imaging sciences at the IU School of Medicine; and Andrew J. Saykin, PsyD, Raymond C. Beeler Professor of Radiology and director of the Center for Neuroimaging and the Indiana Alzheimer’s Disease Research Center at the IU School of Medicine. The work was funded by the National Institutes of Health’s National Institute on Aging multisite project RF1AG078299. “MicroRNAs as Diagnostic and Prognostic Biomarker of Alzheimer’s Disease” that supports the teams of researchers in multiple institutions.
The researchers examined miRNA expression in the plasma samples of three diagnostic groups of participants—cognitively normal, mildly cognitively impaired and dementia due to Alzheimer’s disease patients. They found that, when combined with neuropsychological testing, plasma microRNAome evaluation helps predict which aging individuals concerned about cognitive decline will progress to develop Alzheimer’s.
“These findings provide a path toward a better understanding the molecular mechanisms driving plaques, tangles and atrophy, and may provide clues for the next generation of therapeutic targets,” Saykin said.
While these are exciting times with novel therapies for Alzheimer’s disease entering clinical care, the researchers note that those therapies only will work in a real-world setting if patients at risk are identified as early as possible.
“MicroRNAs are ideal biomarkers since they are not only very stable but also control entire molecular pathways thereby ensuring cellular homeostasis. As such one microRNA can simultaneously control many proteins belonging to a certain pathway,” Fischer said. “Therefore, the analysis of a few microRNAs can inform about complex pathological changes reflecting multiple pathways, such as neuroinflammation, metabolic changes, or synapse dysfunction. Thus, we need biomarkers that allow screening applicable in a point-of-care setting. Our studies are an important step in this direction.”
“We have laid the groundwork for further investigations into the role of microRNAs in Alzheimer’s disease pathogenesis,” Nho said. “We envision that once specific miRNA signatures are further confirmed, the analysis of blood miRNAs will be transferred to simple assay formats enabling the adoption of blood miRNAome analysis in clinical practice.”
The researchers said improved tools for the early detection of Alzheimer’s are indispensable for developing prevention and treatment strategies for the disease that is causing enormous suffering and burdens health care systems around the world.
These findings appear online in Alzheimer’s & Dementia in two papers. Find them here and here .
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.
To assess a smoker’s lung cancer risk, think years — not packs

Eat this. Take that. Get skinny. Trust us.

High doses of Adderall may increase psychosis risk
Start of new era for alzheimer’s treatment.
Alvin Powell
Harvard Staff Writer
Expert discusses recent lecanemab trial, why it appears to offer hope for those with deadly disease
Researchers say we appear to be at the start of a new era for Alzheimer’s treatment. Trial results published in January showed that for the first time a drug has been able to slow the cognitive decline characteristic of the disease. The drug, lecanemab, is a monoclonal antibody that works by binding to a key protein linked to the malady, called amyloid-beta, and removing it from the body. Experts say the results offer hope that the slow, inexorable loss of memory and eventual death brought by Alzheimer’s may one day be a thing of the past.
The Gazette spoke with Scott McGinnis , an assistant professor of neurology at Harvard Medical School and Alzheimer’s disease expert at Brigham and Women’s Hospital , about the results and a new clinical trial testing whether the same drug given even earlier can prevent its progression.
Scott McGinnis
GAZETTE: The results of the Clarity AD trial have some saying we’ve entered a new era in Alzheimer’s treatment. Do you agree?
McGINNIS: It’s appropriate to consider it a new era in Alzheimer’s treatment. Until we obtained the results of this study, trials suggested that the only mode of treatment was what we would call a “symptomatic therapeutic.” That might give a modest boost to cognitive performance — to memory and thinking and performance in usual daily activities. But a symptomatic drug does not act on the fundamental pathophysiology, the mechanisms, of the disease. The Clarity AD study was the first that unambiguously suggested a disease-modifying effect with clear clinical benefit. A couple of weeks ago, we also learned a study with a second drug, donanemab, yielded similar results.
GAZETTE: Hasn’t amyloid beta, which forms Alzheimer’s characteristic plaques in the brain and which was the target in this study, been a target in previous trials that have not been effective?
McGINNIS: That’s true. Amyloid beta removal has been the most widely studied mechanism in the field. Over the last 15 to 20 years, we’ve been trying to lower beta amyloid, and we’ve been uncertain about the benefits until this point. Unfavorable results in study after study contributed to a debate in the field about how important beta amyloid is in the disease process. To be fair, this debate is not completely settled, and the results of Clarity AD do not suggest that lecanemab is a cure for the disease. The results do, however, provide enough evidence to support the hypothesis that there is a disease-modifying effect via amyloid removal.
GAZETTE: Do we know how much of the decline in Alzheimer’s is due to beta amyloid?
McGINNIS: There are two proteins that define Alzheimer’s disease. The gold standard for diagnosing Alzheimer’s disease is identifying amyloid beta plaques and tau neurofibrillary tangles. We know that amyloid beta plaques form in the brain early, prior to accumulation of tau and prior to changes in memory and thinking. In fact, the levels and locations of tau accumulation correlate much better with symptoms than the levels and locations of amyloid. But amyloid might directly “fuel the fire” to accelerated changes in tau and other downstream mechanisms, a hypothesis supported by basic science research and the findings in Clarity AD that treatment with lecanemab lowered levels of not just amyloid beta but also levels of tau and neurodegeneration in the blood and cerebrospinal fluid.
GAZETTE: In the Clarity AD trial, what’s the magnitude of the effect they saw?
McGINNIS: The relevant standards in the trial — set by the FDA and others — were to see two clinical benefits for the drug to be considered effective. One was a benefit on tests of memory and thinking, a cognitive benefit. The other was a benefit in terms of the performance in usual daily activities, a functional benefit. Lecanemab met both of these standards by slowing the rate of decline by approximately 25 to 35 percent compared to placebo on measures of cognitive and functional decline over the 18-month studies.

“In a perfect world, we’d have treatments that completely stop decline and even restore function. We’re not there yet, but this represents an important step toward that goal.”
Steven M. Smith
GAZETTE: What are the key questions that remain?
McGINNIS: An important question relates to the stages at which the interventions were done. The study was done in subjects with mild cognitive impairment and mild Alzheimer dementia. People who have mild cognitive impairment have retained their independence in instrumental activities of daily living — for example, driving, taking medications, managing finances, errands, chores — but have cognitive and memory changes beyond what we would attribute to normal aging. When people transition to mild dementia, they’re a bit further along. The study was for people within that spectrum but there’s some reason to believe that intervening even earlier might be more effective, as is the case with many other medical conditions.
We’re doing a study here called the AHEAD study that is investigating the effects of lecanemab when administered earlier, in cognitively normal individuals who have elevated brain amyloid, to see whether we see a preventative benefit. The hope is that we would at least see a delay to onset of cognitive impairment and a favorable effect not only on amyloid biomarkers, but other biomarkers that might capture progression of the disease.
GAZETTE: Is anybody in that study treatment yet or are you still enrolling?
McGINNIS: There’s a rolling enrollment, so there are people who are in the double-blind phase of treatment, receiving either the drug or the placebo. But the enrollment target hasn’t been reached yet so we’re still accepting new participants.
GAZETTE: Is it likely that we may see drug cocktails that go after tau and amyloid? Is that a future approach?
More like this
Newly identified genetic variant protects against Alzheimer’s
Using AI to target Alzheimer’s

Excessive napping and Alzheimer’s linked in study
McGINNIS: It has not yet been tried, but those of us in the field are very excited at the prospect of these studies. There’s been a lot of work in recent years on developing therapeutics that target tau, and I think we’re on the cusp of some important breakthroughs. This is key, considering evidence that spreading of tau from cell to cell might contribute to progression of the disease. Additionally, for some time, we’ve had a suspicion that we will likely have to target multiple different aspects of the disease process, as is the case with most types of cancer treatment. Many in our field believe that we will obtain the most success when we identify the most pertinent mechanisms for subgroups of people with Alzheimer’s disease and then specifically target those mechanisms. Examples might include metabolic dysfunction, inflammation, and problems with elements of cellular processing, including mitochondrial functioning and processing old or damaged proteins. Multi-drug trials represent a natural next step.
GAZETTE: What about side effects from this drug?
McGINNIS: We’ve known for a long time that drugs in this class, antibodies that harness the power of the immune system to remove amyloid, carry a risk of causing swelling in the brain. In most cases, it’s asymptomatic and just detected by MRI scan. In Clarity AD, while 12 to 13 percent of participants receiving lecanemab had some level of swelling detected by MRI, it was symptomatic in only about 3 percent of participants and mild in most of those cases.
Another potential side effect is bleeding in the brain or on the surface of the brain. When we see bleeding, it’s usually very small, pinpoint areas of bleeding in the brain that are also asymptomatic. A subset of people with Alzheimer’s disease who don’t receive any treatment are going to have these because they have amyloid in their blood vessels, and it’s important that we screen for this with an MRI scan before a person receives treatment. In Clarity AD, we saw a rate of 9 percent in the placebo group and about 17 percent in the treatment group, many of those cases in conjunction with swelling and mostly asymptomatic.
The scenario that everybody worries about is a hemorrhagic stroke, a larger area of bleeding. That was much less common in this study, less than 1 percent of people. Unlike similar studies, this study allowed subjects to be on anticoagulation medications, which thin the blood to prevent or treat clots. The rate of macro hemorrhage — larger bleeds — was between 2 and 3 percent in the anticoagulated participants. There were some highly publicized cases including a patient who had a stroke, presented for treatment, received a medication to dissolve clots, then had a pretty bad hemorrhage. If the drug gets full FDA approval, is covered by insurance, and becomes clinically available, most physicians are probably not going to give it to people who are on anticoagulation. These are questions that we’ll have to work out as we learn more about the drug from ongoing research.
GAZETTE: Has this study, and these recent developments in the field, had an effect on patients?
McGINNIS: It has had a considerable impact. There’s a lot of interest in the possibility of receiving this drug or a similar drug, but our patients and their family members understand that this is not a cure. They understand that we’re talking about slowing down a rate of decline. In a perfect world, we’d have treatments that completely stop decline and even restore function. We’re not there yet, but this represents an important step toward that goal. So there’s hope. There’s optimism. Our patients, particularly patients who are at earlier stages of the disease, have their lives to live and are really interested in living life fully. Anything that can help them do that for a longer period of time is welcome.
Share this article
You might like.
Far more cases get caught when screening guidelines consider duration of habit regardless of intensity, study finds — especially among Black patients

Popularity of newest diet drugs fuel ‘dumpster fire’ of risky knock-offs, questionable supplements, food products, experts warn

Among those who take prescription amphetamines, 81% of cases of psychosis or mania could have been eliminated if they were not on the high dose, findings suggest
Harvard releases race data for Class of 2028
Cohort is first to be impacted by Supreme Court’s admissions ruling
Parkinson’s may take a ‘gut-first’ path
Damage to upper GI lining linked to future risk of Parkinson’s disease, says new study
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Cent Nerv Syst Dis
Current and Future Treatments in Alzheimer Disease: An Update
Konstantina g yiannopoulou.
1 Memory Center, Neurological Department, Henry Dunant Hospital Center, Athens, Greece
Sokratis G Papageorgiou
2 Cognitive Disorders/Dementia Unit, 2nd Neurological Department, National and Kapodistrian University of Athens, Attikon General University Hospital, Athens, Greece
Disease-modifying treatment strategies for Alzheimer disease (AD) are still under extensive research. Nowadays, only symptomatic treatments exist for this disease, all trying to counterbalance the neurotransmitter disturbance: 3 cholinesterase inhibitors and memantine. To block the progression of the disease, therapeutic agents are supposed to interfere with the pathogenic steps responsible for the clinical symptoms, classically including the deposition of extracellular amyloid β plaques and intracellular neurofibrillary tangle formation. Other underlying mechanisms are targeted by neuroprotective, anti-inflammatory, growth factor promotive, metabolic efficacious agents and stem cell therapies. Recent therapies have integrated multiple new features such as novel biomarkers, new neuropsychological outcomes, enrollment of earlier populations in the course of the disease, and innovative trial designs. In the near future different specific agents for every patient might be used in a “precision medicine” context, where aberrant biomarkers accompanied with a particular pattern of neuropsychological and neuroimaging findings could determine a specific treatment regimen within a customized therapeutic framework. In this review, we discuss potential disease-modifying therapies that are currently being studied and potential individualized therapeutic frameworks that can be proved beneficial for patients with AD.
Introduction
Alzheimer disease (AD) is one of the greatest medical care challenges of our century and is the main cause of dementia. In total, 40 million people are estimated to suffer from dementia throughout the world, and this number is supposed to become twice as much every 20 years, until approximately 2050. 1
Because dementia occurs mostly in people older than 60 years, the growing expansion of lifespan, leading to a rapidly increasing number of patients with dementia, 2 mainly AD, has led to an intensive growth in research focused on the treatment of the disease. However, despite all arduous research efforts, at the moment, there are no effective treatment options for the disease. 3 , 4
The basic pathophysiology and neuropathology of AD that drives the current research suggests that the primary histopathologic lesions of AD are the extracellular amyloid plaques and the intracellular Tau neurofibrillary tangles (NFTs). 5 The amyloid or senile plaques (SPs) are constituted chiefly of highly insoluble and proteolysis-resistant peptide fibrils produced by β-amyloid (Aβ) cleavage. Aβ peptides with Aβ38, Aβ40, and Aβ42 as the most common variants are produced after the sequential cleavage of the large precursor protein amyloid precursor protein (APP) by the 2 enzymes, β-secretase (BACE1) and γ-secretase. Nevertheless, Aβ is not formed if APP is first acted on and cleaved by the enzyme α-secretase instead of β-secretase. 6 According to the “amyloid hypothesis” Aβ production in the brain initiates a cascade of events leading to the clinical syndrome of AD. It is the forming of amyloid oligomers to which neurotoxicity is mainly attributed and initiates the amyloid cascade. The elements of the cascade include local inflammation, oxidation, excitoxicity (excessive glutamate), and tau hyperphosphorylation. 5 Tau protein is a microtubule-associated protein which binds microtubules in cells to facilitate the neuronal transport system. Microtubules also stabilize growing axons necessary for neuronal development and function. Abnormally hyperphosphorylated tau forms insoluble fibrils and folds into intraneuronic tangles. Consequently, it uncouples from microtubules, inhibits transport, and results in microtubule disassembly. 6 Although in the amyloid hypothesis, tau hyperphosphorylation was thought to be a downstream event of Aβ deposition, it is equally probable that tau and Aβ act in parallel pathways causing AD and enhancing each other’s toxic effects. 3 Progressive neuronal destruction leads to shortage and imbalance between various neurotransmitters (eg, acetylcholine, dopamine, serotonin) and to the cognitive deficiencies seen in AD. 5
All of the already established treatments that are used today try to counterbalance the neurotransmitter imbalance of the disease. The acetylocholinesterase inhibitors (AChEIs) which are approved for the treatment of AD are donepezil, galantamine, and rivastigmine. 4 , 5 Their development was based in the cholinergic hypothesis which suggests that the progressive loss of limbic and neocortical cholinergic innervation in AD is critically important for memory, learning, attention, and other higher brain functions decline. Furthermore, neurofibrillary degeneration in the basal forebrain is probably the primary cause for the dysfunction and death of cholinergic neurons in this region, giving rise to a widespread presynaptic cholinergic denervation. The AChEIs increase the availability of acetylcholine at synapses and have been proven clinically useful in delaying the cognitive decline in AD. 7
A further therapeutic agent approved for moderate to severe AD is the low-to-moderate affinity, noncompetitive N -methyl- d -aspartate (NMDA) receptor antagonist memantine. 4 , 5 Memantine binds preferentially to open NMDA receptor–operated calcium channels blocking NMDA-mediated ion flux and ameliorating the dangerous effects of pathologically elevated glutamate levels that lead to neuronal dysfunction. 8
In clinical trials, both Aβ and tau are prime targets for disease-modifying treatments (DMTs) in AD. From this point of view, AD could be prevented or effectively treated by decreasing the production of Aβ and tau; preventing aggregation or misfolding of these proteins; neutralizing or removing the toxic aggregate or misfolded forms of these proteins; or a combination of these modalities. 7
A number of additional pathogenic mechanisms have been described, possibly overlapping with Aβ plaques and NFT formation or induced by them, including inflammation, oxidative damage, iron deregulation, and cholesterol metabolism blood-brain barrier (BBB) dysfunction or α-synuclein toxicity. 9 - 13
This article will review current nonpharmacological and pharmacological management of the cognitive and behavioral symptoms of AD, with a focus on the medications that are currently FDA (Food and Drug Administration)–approved for the treatment of the cognitive and functional deficits of AD. 10 Pharmacological agents under research in phase 1, 2, and 3 clinical trials in AD will be summarized. 11 - 13
Current management of AD
A multifactorial tailored management of AD is attempted nowadays based in the following components:
- Open physician, caregiver, and patient communication: a sincere and successful conveying of information and feelings between them will offer opportune identifying of symptoms, exact evaluation and diagnosis, and suitable guidance.
- - Consistency and simplification of environment 10 ;
- - Established routines 10 ;
- - Communicative strategies such as calm interactions, providing pleasurable activities, using simple language and “saying no” only when safety is concerned 10 ;
- - Timely planning for legal and medical decisions and needs 10 ;
- - Cognitive behavioral therapy 14 , 15 ;
- - Exercise therapy, light therapy, music therapy. 14 , 15
- - Planned short rest periods for the caregiver;
- - Psychoeducation including preparing for effects of dementia on cognition, function and behaviors, expectations, avoiding situations that can worsen the symptoms or increasing the dangers for safety and well-being
- - Encouraging the development of support networks for the caregivers. 10
- Pharmacological interventions.
FDA-approved AD medications
The AChEIs donepezil, galantamine, rivastigmine, and the NMDA antagonist memantine are the only FDA-approved AD medications. 10
AChEIs attempt at reducing the breakdown of acetylcholine levels in the brain of the patients with AD by inhibiting the responsible enzyme acetylcholinesterase in the synaptic cleft. 5 Thus, AChEIs enhance central cholinergic neurotransmission and finally tend to mitigate decline in cognition at least during the first year of treatment. Further decline occurs, but even temporary discontinuation of these drugs results in rapid decline and is associated with greater risk of nursing home placement. 16
Initiation of AChEI treatment as soon as possible after the diagnosis is preferred as patients who started the AChEI 6 months later showed more rapid cognitive decline than those who started the drug immediately. 17 All 3 AChEIs have proved their treatment benefits in delaying decline, stabilizing, or even improving cognition and activities of daily living in randomized placebo-controlled trials up to 52 weeks duration. 10 , 18 Longer term open-label extension studies support also longer term treatment benefits. 10
Significant efficacy differences among the AChEIs have not been reported. Donepezil and rivastigmine have been approved by FDA for mild, moderate, and severe AD, whereas galantamine for mild and moderate AD. 18
The most common adverse effects are triggered by the cholinomimetic action of the AChEIs on the gastrointestinal tract and often include diarrhea, nausea, and vomiting. Rapid eye movement sleep behavior disorder has been also remarked in some individuals. Administration of the drug after a meal in the morning can minimize all of these adverse effects. The transdermal patch of rivastigmine can induce rash at the site of application. Adverse effects affect usually a 5% to 20% of patients but are mostly transient and mild. The AChEIs may also trigger bradycardia and increase the risk of syncope. Thus, AChEIs are contraindicated in conditions including severe cardiac arrhythmias, especially bradycardia or syncope. They are also contraindicated in active peptic ulcer or gastrointestinal bleeding history and uncontrolled seizures. Slow titration over months to years to a maximal tolerated of the indicated dose is important for the safety of the patients. 17 , 18
Pharmacokinetic characteristics differ among AChEIs: the primary route of elimination for donepezil and galantamine is hepatic metabolism, whereas for rivastigmine is liver and intestine metabolism. Donepezil and galantamine inhibit selectively and reversibly the acetylcholinesterase, whereas rivastigmine is a “pseudo-irreversible” inhibitor of acetylcholinesterase and butyrylcholinesterase. Donepezil has a long elimination half-life of 70 hours and galantamine of 6 to 8 hours. The elimination half-life of rivastigmine is very short (1-2 hours for oral and 3-4 hours for transdermal administration), but the duration of action is longer as acetylcholinesterase and butyrylcholinesterase are blocked for around 8.5 and 3.5 hours, respectively. 10 , 17 , 18
Memantine is a noncompetitive low-affinity NMDA-receptor open-channel blocker and affects glutamatergic transmission. 5 Its main elimination route is unchanged via the kidneys with a half-life of 70 hours. It has been approved by FDA for moderate and severe AD either as monotherapy or in combination with an AChEI. 3 Memantine monotherapy has demonstrated short- and long-term benefits for patients with moderate to severe AD as assessed by different scales evaluating activities of daily living, cognition, and behavioral and psychological symptoms of dementia (BPSD). 19
Memantine can be administered in combination with an AChEI, as they have complementary mechanisms of action. Their combination benefits patients with usually additive effects, without any increase in adverse effects. 14 , 15
Duration and persistence of monotherapy or combination treatment with higher doses in moderate or even in advanced dementia are associated with better global function and outcomes. 20
Medications for BPSD
Antipsychotics and antidepressants remain the main medications for BPSD. Selective serotonin reuptake inhibitors are preferred for treating depression and anxiety. Drugs with low anticholinergic effects and an acceptable tolerability, such as sertraline, citalopram, and escitalopram, are more appropriate. Antipsychotics should be administered only when a significant safety risk for the patient or for the caregivers by aggressive behaviors makes them necessary. Controversial and limited evidence cannot adequately support the use of benzodiazepines, anticonvulsants stimulants, or dextromethorphan/quinidine. Pharmacological approaches to managing BPSD are highly individualized and changeable, depending on patient’s comorbidities, stage of the disease, and symptoms’ severity. 21
Removal of superfluous and deleterious medications
Polypharmacy in older patients with dementia is usual (with a prevalence of 25%-98%). 22 Anticholinergics and sedatives are commonly used inappropriate medications. These drugs are prescribed despite strong evidence (Beers Criteria) that they should be avoided in cognitively vulnerable older persons because of their potential adverse cognitive effects. 23 Estrogen is another commonly prescribed potentially inappropriate medication despite evidence that its use is associated with increased cognitive decline in postmenopausal women. 24
Specific examples of usually prescribed potentially harmful medications in the elderly are diphenhydramine, often taken with acetaminophen for insomnia and pain, benzodiazepines for anxiety, anticholinergics (tolderodine, oxybutynin, tamsulosin) for urinary incontinence, biperiden, and pramipexole for extrapyramidal tremor 25 and sedative/hypnotics for sleep disorders. 26
Treating underlying medical conditions
Careful management of vascular risk factors (hyperlipidemia, diabetes, hypertension) is of paramount importance for patients with AD. Hydration, sleep, and nutrition status should also be closely monitored. Disorders in thyroid function or electrolytes, deficiencies in vitamin B 12 , folate, vitamin D, or systemic conditions and diseases that can affect cognition (infections, eg, urinary tract infection, pain, constipation) should be treated. 27
Current Landscape in Treatment Research for AD
No new drug has been approved by FDA for AD since 2003 and there are no approved DMTs for AD, despite many long and expensive trials. 22 , 28 As a matter of fact, more than 200 research projects in the last decade have failed or have been abandoned. 10 Nevertheless, drug pipeline for AD is still full of agents with mechanisms of action (MOA) that target either disease modification or symptoms. 4 , 10 Some of the recent failures of anti-amyloid agents in phase 3 clinical trials in patients with early-stage, mild, or mild-to-moderate stage AD were semagacestat, 29 bapineuzumab, 30 solanezumab 31 and in similar trials of β-secretase inhibitors (BACE) lanabecestat, 32 verubecestat, 33 and atabecestat. 34
The most popular and broadly accepted explanations for the multiple failures of clinical trials of DMT agents for AD include the too late starting of therapies in disease development, the inappropriate drug doses, the wrong main target of the treatment, and mainly an inadequate understanding of the pathophysiology of AD. 35 A novel approach to the problem seems more technical and mathematical than biological and suggests that the selected trials’ clinical endpoint may be extremely premature, and additionally, the variability in diagnostic markers and end points may result in inaccurate diagnosis of patients’ disease state and is finally a definite source of errors. 28 Given the fact that longer trial durations increase the probability of detecting a significant effect but at the same time increase tremendously the costs, the proposed solution seems to be the use of clinical trial simulators. 28 These simulators are constructed with mathematical, computational, and statistical tools and can predict the likelihood that a strategy and clinical end point selection of a given trial are proper or not, before the initiation of the trial. 36 They can also help in the perfecting of the design of the study; hence, they may augment the probability of success of estimated new drugs or save invaluable time and resources, by indicating earlier the forthcoming failure of any inappropriate therapy. 37 Although the use of clinical trial simulators is not frequent in recent research, 38 should this practice be abandoned, especially when potential treatments for diseases with slow progression and long duration, such as AD, are evaluated. 37
At the same time, current research remains focused on the development of therapeutic approaches to slow or stop the disease progression, taking into consideration every new aspect in the biology of the disease, the diagnostic markers, and the precise diagnosis of disease state of every individual and the design of clinical trials. Furthermore, drug development research for AD has become more complicated as preclinical and prodromal AD populations are potentially included in current trials, as well as traditionally included populations of all the clinical stages of AD dementia. 38 Consequently, current guidance provided by the FDA for AD clinical trials further includes use of fluid or neuroradiological biomarkers in disease staging for preclinical and prodromal AD trials and of a single primary outcome in prodromal AD trials. In addition, the use of clinical trial simulators, Bayesian statistics, and modifiable trial designs is strongly suggested. 4
The National Institute on Aging and the Alzheimer’s Association (NIA-AA) proposed a new framework for research, 39 which requires the application of amyloid, tau, and neurodegeneration biomarkers to clinical trials, succeeds in precise classification of patients in AD stages, and can be used to assist clinical trials design.
Tau positron emission tomography (tau PET), neurofilament light, and neurogranin are the new biomarkers that are increasingly used by clinical trials. 40
The above-mentioned biological and statistical advances that are recently integrated in clinical trials may comprise the final assets for succeeding in drug development. The current clinical trials in AD in phases 1, 2, and 3 4 , 11 - 13 are briefly discussed. The tested agents in these trials are classified either as potentially modifying the disease or as symptomatic for the cognitive enhancement, and for the relief of neuropsychiatric symptoms. The new directions in AD clinical trials, such as agents with novel MOA, advanced immunotherapies, the involvement of biomarkers in drug development, and repurposed agents, are highlighted.
A search for phases 1, 2, and 3 “recruiting” or “active but not recruiting” clinical trials for AD in clinicaltrials.gov (accessed August 19, 2019) showed 165 outcomes. The last annual review of the drug development pipeline for AD examined clinicaltrials.gov in February, 2019 (132 agents in 156 trials) and provides information and conclusions available at that time: 28 drugs in 42 clinical trials in phase 3 trials, 74 drugs in 83 phase 2 trials, and 30 drugs in 31 phase 1 trials. 4 The tested agents are classified as DMTs (73%), symptomatic cognitive enhancers (13%), and symptomatic for the treatment of BPSDs (11%). 4 The DMT agents were further separated into small molecules or biologics (monoclonal antibodies [mAbs] and other immunotherapies). The DMT agents were also classified according to their potential MOA as amyloid targeting, as tau-related targeting, and as having other MOA such as anti-inflammatory or metabolic protection, neuroprotection, and growth factor support. 4 The DMTs are suggested to be effective to delay or halt disease progression that would be expressed clinically with long-lasting benefits in cognition over many months to years. Symptomatic agents are supposed to show symptomatic benefits over weeks to many months in cognition improvement or BPSD elimination. 10
In this review, agents currently studied as potential DMTs will be discussed. Furthermore, an approach to a future “precision medicine” multifactorial therapeutic model based on biomarkers profile, genetic analysis, neuropsychologic evaluation, and neuroimaging accomplished with risk factors restriction will be attempted. 2 , 3
Currently studied DMTs for AD
Amyloid-related mechanisms—dmts.
The crucial step in AD pathogenesis is the production of amyloid (Aβ), which forms SPs (insoluble and proteolysis-resistant fibrils). The Aβ derives from a protein overexpressed in AD, APP through sequential proteolysis by β-secretase (BACE1) in the extracellular domain and γ-secretase in the transmembrane region. Full-length APP is first cleaved by α-secretase or β-secretase. The APP cleavage by α-secretase leads to nonamyloidogenic pathway, whereas APP cleavage by β-secretase (BACE1) leads to amyloidogenic pathway. Sequential cleavage of APP by BACE1 in the extracellular and γ-secretase in the transmembrane area results in the Aβ production. Major sites of γ-secretase cleavage usually occur in positions 40 and 42 of Aβ, thus Aβ40 and Aβ42 oligomers are the main products of the sequential APP cleavage, as the amyloidogenic pathway is favored in neurons because of the greater plentifulness of BACE1. On the contrary, the nonamyloidogenic processing is more favored in other cells without BACE1 predominance. 5
“Amyloid hypothesis” suggests that Aβ production in the brain triggers a cascade of pathophysiologic events leading to the clinical expression of AD. Aβ is a protein consisting of 3 main isoforms: Aβ38, Aβ40, and Aβ42. Aβ42 is the most aggregation-prone form and has the tendency to cluster into oligomers. Oligomers can form Aβ-fibrils that will eventually form amyloid plaques. Aβ40 is somewhat aggregation-prone and it is mostly found in the cerebral vasculature as a main component of “cerebral amyloid angiopathy.” Aβ40 usually constitutes more than 50% of total detected Aβ. Aβ38 is soluble, present in the vasculature of patients with sporadic and familial AD. Neurotoxicity is mainly attributed to the forming of amyloid oligomers, which finally initiates the amyloid cascade. 5
Oxidation, inflammation, excessive glutamate, and tau hyperphosphorylation are supposed to be the main pathophysiologic pillars of the cascade. Tau protein binds microtubules in cells to facilitate the neuronal transport system. Microtubules also stabilize growing axons. Hyperphosphorylated tau forms insoluble fibrils and folds into intraneuronic NFTs. Consequently, it inhibits neuronal transport and microtubule function. 2 Although in the initial amyloid hypothesis, tau hyperphosphorylation was thought to be a downstream event of Aβ deposition, it is now equally probable that tau and Aβ act in parallel pathways causing AD and enhancing each other’s toxic effects. 2 The result of massive neuronal destruction is the shortage and imbalance between neurotransmitters, such as acetylcholine, dopamine, serotonin, and to the cognitive and behavioral symptoms of AD. 5
Consequently, anti-amyloid DMTs have focused on 3 major MOAs: (1) reduction of Aβ42 production (γ-secretase inhibitors, β-secretase inhibitors, α-secretase potentiation), (2) reduction of Aβ-plaque burden (aggregation inhibitors, drugs interfering with metals), and (3) promotion of Aβ clearance (active or passive immunotherapy). 10
Reduction of Aβ42 production
γ-secretase inhibitors.
According to the amyloid hypothesis, amyloidogenic pathway is promoted after the sequential cleavage of APP by BACE1 and γ-secretase. Consequently, the inhibition of these enzymes has been considered as a major therapeutic target. Unluckily, concerning γ-secretase, in addition to APP, this particular enzyme acts on many other substances and cleaves different transmembrane proteins. Notch receptor 1, which is essential for control of normal cell differentiation and communication, is among them. 5 This fact is probably responsible for recent failures in clinical trials with γ-secretase inhibitors: semagacestat 29 was associated with worsening of activities in daily leaving and increased rates of infections and skin cancer, avagacestat 41 was associated with higher rate of cognitive decline and adverse dose-limiting effects (skin cancer) and tarenflurbil which showed low brain penetration. 42 Serious safety concerns for γ-secretase inhibitors remove γ-secretase from the role of appropriate target for the treatment of AD 43 until in depth studies on this key enzyme could help to therapeutically target γ-secretase in a safe way. 44 No γ-secretase modulators are currently studied in phase 1-3 clinical trials. 4
BACE inhibitors
Two BACE inhibitors are still elaborated: elenbecestat (E2609) in phase 2 and umibecestat (CNP520) in phase 3. 4 The later agent is studied in asymptomatic individuals at risk of developing AD (APOE4 homozygotes or APOE4 heterozygotes with elevated amyloid, detected by cerebrospinal fluid [CSF] biomarkers or amyloid PET). 45
Fluid and neuroimaging biomarkers indicative of AD pathology or neurodegeneration are integrated in this study.
However, the clinical trials with the BACE inhibitors lanabecestat, 32 verubecestat, 33 and atabecestat 34 have been recently discontinued due to unexpected difficulties. The phase 3 lanabecestat trial was discontinued due to lack of efficacy, whereas verubecestat and atabecestat trials were ceased due to ineffectiveness, as well as safety reasons (rash, falls, liver toxicity, and neuropsychiatric symptoms). 10 , 32 - 34 All agents showed significant and dose-dependent result of reducing CSF Aβ42, but without cognitive or functional benefit while many of them were poorly tolerated and some of them failed in subjects with prodromal AD. These results might support the suggestion that blocking the process of forming of Aβ may be not capable of halting the disease progression. 46
α-secretase modulators
According to the amyloid hypothesis, nonamyloidogenic pathway is promoted after the cleavage of APP by α-secretase. Consequently, the modulation of the enzyme has been considered as a major therapeutic target. However, little is known of the main signaling pathways that could stimulate cleavage of APP by α-secretase. Restricted, nowadays, knowledge assumes that α-secretase activation is promoted through the phosphatidylinositol 3-kinase (PI3K)/Akt pathway and may be through γ-aminobutyric acid (GABA) receptor signaling; thus, agents that activate the PI3K/Akt pathway or act as selective GABA receptor modulators are suggested as potential therapeutic drugs for AD. 47 , 48
Etazolate (EHT0202) stimulates the nonamyloidogenic α-secretase pathway acting as a selective modulator of GABA receptors. A previous, phase 2 trial has showed that the agent was safe and well tolerated in patients with mild to moderate AD. However, further evaluation of etazolate in phase 3 trials has not progressed. 48 Etazolate is currently evaluated in animal studies for its preventive effect in post-traumatic stress disorder. 49
Two α-secretase modulators that activate the PI3K/Akt pathway are studied in phase 2 clinical studies: APH-1105 and ID1201. APH-1105 is delivered intranasally and is assessed in mild to moderate AD. 4 ID1201 is a fruit extract of melia toosendan and also induces α-secretase activation. It is evaluated in mild AD. 47
Reduction of Aβ-plaque burden
Aggregation inhibitors (anti-amyloid aggregation agents).
Aggregation inhibitors interact directly with the Aβ peptide to inhibit Aβ42 fiber formation, thus they are considered potential therapeutic for AD.
The last Aβ42 aggregation inhibitor which was tested in humans was the oral agent scyllo-inositol (ELND005). A phase 2 clinical trial in patients with AD did not provide evidence to support a clinical benefit of ELND005 while severe toxicity issues (infections) forced the cessation of the study. Further development of the agent at a lower dose has not progressed in the last 8 years. 50
Nowadays, specific agents in the form of peptidomimetics that inhibit and partially reverse the aggregation of Aβ 42 are tested in transmission electron microscopic studies. KLVFF is a peptide sequence that resembles the hydrophobic central part of the Aβ and gradually replaces natural polypeptides. The KLVFF compound that mainly prevents the aggregation of Aβ 42 and can also dissolve the oligomerics to a limited extend is the final compound 18, which is resilient in proteolytic decomposition. 51
Another newly developed class of peptidomimetics are the “γ-AApeptides.” 52 One of them, compound γ-AA26, seems almost 100-fold as efficient as the compound 18 of the KLVFF in the inhibition of the aggregation of Aβ 42 . 52
In vivo animal studies will be developed to manifest the biological potential of peptidomimetics.
Reduction of Aβ-plaque burden via drugs interfering with metals
Abnormal accumulation or dyshomeostasis of metal ions such as iron, copper, and zinc has been associated with the pathophysiology of AD. 5
Deferiprone is an iron chelating agent which is studied in phase 2 trials in participants with mild and prodromal AD. 4 , 53
A metal protein–attenuating compound, PBT2, has recently progressed in phase 2 AD treatment trials, as it demonstrated promising efficacy data in preclinical studies. 54 In a 3-month phase 2 study, PBT2 succeeded in a 13% reduction of CSF Aβ and an executive function improvement in a dose-related pattern in patients with early AD. 55
Promotion of Aβ clearance (active or passive immunotherapy)
The 2 main immunotherapeutic approaches that intend to promote Aβ clearance and are currently tested in clinical and preclinical studies are active and passive immunization: 56
- Active immunization.
Aβ, phosphorylated tau (ptau) peptides, or specific artificial peptides such as polymerized British amyloidosis (ABri)-related peptide (pBri) 57 are used as immunogens. ABri is a rare hereditary amyloidosis associated with a mutation that results in the production of a highly amyloidogenic protein with a unique carboxyl terminus that has no homology to any other human protein. The pBri peptide corresponds to this terminus and induces an immune response that recognizes Aβ and ptau.
Antigen-presenting cells present the immunogens to B cells. Use of Ab or ptau peptides will produce antibodies to Ab or ptau epitopes, respectively. Use of pBri will produce antibodies to both Aβ and ptau epitopes. 56
- Passive immunization.
Monoclonal Abs to Ab, ptau, or b sheet epitopes are systemically and adequately for BBB penetration infused. As antibodies cross the BBB, they act to clear, degrade, or alternatively disaggregate or neutralize their targets. 56
- Stimulation of innate immunity either by active or passive immunization also ameliorates the pathology of the disease by promoting microglia and macrophage function. 56
Overall, Aβ-targeted strategies seem promising if used very early in the progression of the disease, before the presence of any symptoms; thus, they are developed in current trials in preclinical AD. Strategies that target tau pathology, although promising, bear the risk of toxicity at the moment. Nevertheless, it is hypothesized that, in sporadic late onset AD, ptau and Aβ pathologies could be evolved by separate pathways that can affect each other synergistically. 58 Consequently, it is possible that effective AD immunotherapies must be able to simultaneously target both ptau and Aβ pathologies. 56
Immunotherapeutic approaches have dominated in the past 15 years with negative results until now. However, lessons from these fails have altered the current immunotherapy development research for AD. 56
Active Aβ immunotherapy
Six active immunotherapy agents are currently studied in phase 1, 2, and 3 clinical trials:
CAD106 is an active Aβ immunotherapeutic agent, is studied in preclinical AD under the umbrella of the Alzheimer prevention initiative generation program, which comprises 2 phase 3 studies that evaluate simultaneously the safety and efficacy of CAD106 and umibecestat in asymptomatic individuals at risk of developing AD (60-75 years of age, APOE4 homozygotes, or APOE4 heterozygotes with elevated amyloid in CSF or in amyloid PET). 45
Subjects will be registered in generation study 1 (cohort 1: CAD106 or placebo, cohort 2: umibecestat or placebo) or generation study 2 (umibecestat 50 and 15 mg, or placebo). 45
ABvac40 is evaluated in a phase 2 study, as the first active vaccine against the C-terminal end of Aβ 40 . A phase 1 study was conducted with patients with mild to moderate AD aged 50 to 85 years. Neither incident vasogenic edema nor microhemorrhages were identified. Specific anti-Aβ 40 antibodies were developed in the 92% of the individuals receiving injections of ABvac40. 59
GV1001 peptide (tertomotide) was previously studied as a vaccine against various cancers, whereas now it is evaluated in a phase 2 study for AD. 60
ACC-001 (vanutide cridificar), an Aβ vaccine, was studied in phase 2a extension studies in subjects with mild to moderate AD. It was administered with QS-21 adjuvant. Long-term therapy with this combination was very well tolerated and produced the highest anti-Aβ IgG titers compared with other regimens. 61
UB-311, a synthetic peptide used as Aβ vaccine, has been advanced into an ongoing phase 2 study in patients with mild and moderate AD. In phase 1, it induced a 100% responder rate in patients with AD. The usual adverse effects were swelling in the injection site and agitation. A slower cognitive decline rate was observed in patients with mild AD. 62
Lu AF20513 epitope vaccine is estimated in a phase 1 study in mild AD. 63
The occurrence of encephalitis in previous studies (AN-1792) 64 led to the development of improved anti-Aβ active immunotherapy agents, more specific to Aβ sites less probable to activate T cells, currently studied in clinical trials. 5 , 6
Passive Aβ immunotherapy—via mAbs
Passive Ab immunotherapy via mAbs is the most active and promising class. Cerebral microhemorrhages and vasogenic edema are the main drawbacks in this group of agents. 5 Valuable learning gained from previous failed phase 3 trials of the first agents of this class, bapineuzumab 65 and solanezumab, 66 enlightened the mAbs’ research. Strict inclusion criteria were applied, such as biomarker proof of “amyloid positivity” and enrollment of individuals with preclinical stages of the disease. Furthermore, the design of the studies became more specific and targeted: the characteristics of amyloid-related imaging abnormalities were associated with the dose of antibodies and APOε4 genotyping, higher dosing necessity was recognized, and accurate measures for specific targets, such as reduction of Aβ plaque burden on amyloid PET, were required. 10
Many ongoing mAbs trials are in phase 3, including aducanumab, 67 gantenerumab, 68 and BAN2401 69 in prodromal and very mild AD, and crenezumab, 70 gantenerumab, and solanezumab 71 in studies for preclinical or at-risk populations. First results from aducanumab and BAN2401 trials suggested, at first, a treatment-related result of reducing in cerebral amyloid burden in agreement to deceleration of cognitive decline in patients with prodromal and very mild AD. 71 , 72 On the contrary, the initial trial of gantenerumab in prodromal AD was prematurely stopped for lack of efficacy, but exploratory analyses suggest that higher dosing of gantenerumab may be needed for clinical efficacy and an open-label extension for participating patients with mild AD is continued, simultaneously with a double-blind, placebo-controlled study in patients with mild AD. 4 , 68 Similarly, until now, solanezumab did not delay rates of brain atrophy. 73
Intravenous doses of LY3002813 (donanemab) and LY3372993 are studied in participants with mild cognitive impairment (MCI) and mild to moderate AD in separate phase 1 clinical studies. 4
Passive Aβ immunotherapy—via immunoglobulins
Anti-Aβ antibodies are included in naturally occurring autoantibodies. In contrast to mAbs, blood-derived human anti-Aβ immunoglobulin G (IgG) Abs are polyclonal, with lower avidity for single Aβ molecules, and higher for a broader range of epitopes, especially in Aβ oligomers and fibrils. The natural presence of antibodies against Aβ has been reported in intravenous immunoglobulin (IVIg); thus, IVIg has been considered as a possible AD treatment. Intravenous immunoglobulin is obtained from plasma of healthy donors and is made up of human Abs mainly of the IgG-type. 5 , 74
Nevertheless, the first completed phase 3 trial of IVIg as a treatment for AD demonstrated good tolerability but lack of efficacy of the agent on clinical stability or delay of cognitive or functional decline of participants with mild and moderate AD. 74
Another strategy directed at diminishing the accumulation of Aβ in the brain is based in altering the transportation of Aβ through the BBB. A recent therapeutic method performs plasma exchange (PE) with albumin replacement, inducing the shifting of the existing dynamic equilibrium between plasma and brain Aβ. This therapeutic method is based in the following considerations: (1) high levels of Aβ aggregation in the brain are accompanied by low levels of Aβ in CSF in AD, (2) albumin is the main protein transporter in humans, (3) albumin binds around the 90% of the circulating Aβ, and (4) albumin has proved Aβ-binding ability. Consequently, it is suggested that PE-mediated possession of albumin-bound Aβ would increase the shift of free Aβ from CSF to plasma to correct the imbalance between brain and blood Aβ levels. 75
A phase 3 trial called Alzheimer’s Management by Albumin Replacement (AMBAR) in mild and moderate AD assesses PE with several replacement volumes of albumin, with or without intravenous immunoglobulin. 76
Furthermore, an ongoing phase 2 study evaluates IVIg Octagram 10% in mild and moderate AD. 4
A novel immunotherapeutic strategy that targets simultaneously Aβ and tau is represented by the NPT088 agent. NPT088 is a mixture of the capsid protein of bacteriophage M13 (g3p) and human-IgG 1 -Fc. NPT088 reduced Aβ and ptau aggregates and improved cognition in aged Tg2576 mice. The agent is currently assessed in a phase 1 clinical study. 77
Tau-related mechanisms—DMTs
Anti-phospho-tau approaches consist a major potential treatment strategy, even if there are yet no agents with this specific MOA advanced in phase 3 studies.
Only 1 agent with tau-related mechanism is evaluated in phase 2/3, whereas 10 agents that target tau as one of their mechanisms are evaluated in phase 2, and 5 more agents with tau-related mechanism are assessed in phase 1 studies. 4
Prevention of ptau formation
The hyperphosphorylation of tau is induced by kinases. 78 Thus, kinase inhibitors are examined as potential therapeutic approaches targeting tau. Glycogen synthase kinase 3 (GSK3β) has become prominent as a possible therapeutic target. The most studied GSK3 inhibitor is lithium chloride, a therapeutic agent for affective disorders, which seems to prevent phosphorylation of tau in mouse models. Lithium is currently reassessed within the novel framework for drug research. 79
Another GSK-3 inhibitor, tideglusib, did not meet phase 2 clinical endpoints in patients with mild and moderate AD. 80
ANAVEX 2-73 is evaluated in a phase 2 trial, for eligible subjects AD MCI or mild AD. 81 ANAVEX 2-73 is also a GSK-3b inhibitor but additionally it is a high affinity sigma 1 receptor agonist and a low-affinity muscarinic agonist. 4 Results presented at 2019 Alzheimer’s Association International Conference (AAIC) revealed that patients treated with ANAVEX 2-73 had high levels of 2 gut microbiota families, Ruminococcaceae and Porphyromonadaceae, which were associated with improved activities of daily living. The effect might potentially be reversal of the microbiota imbalances and might have a homeostatic effect on the brain-gut-microbiota axis. 81
Inhibitors of tau aggregation
Methylene blue (MB), a known phenothiazine, is evaluated in AD studies as a potential tau aggregation inhibitor. The problem with this drug is that urine is colored blue, resulting in a lack of blinding. A monotherapy trial with MB on mild and moderate AD (NCT00515333) has demonstrated some clinical benefit in moderate, but not mild AD. 82 However, the methodology of the study, as blinding is impossible, has been highly criticized. 83
Methylene blue’s derivative TRx0237 (LMTX) which was studied in phase 3 failed finally to show efficacy, and based on the analysis of the results, a new phase 2/3 study named LUCIDITY was started 1 year ago in subjects with mild AD with a lower dose of the agent. 84
Microtubule stabilizers
The microtubule-stabilizing agent davunetide was studied in a phase 2 trial but it did not meet the clinical end points. 85
TPI-287 (abeotaxane), a small molecule derived from taxol, is a microtubule protein modulator. It was administered intravenously to patients with mild to moderate AD in a phase 1/2 study (NCT01966666). First results presented report that the agent was not well tolerated by the participants. 84
IONIS MAPTRx (BIIB080), a microtubule-associated protein tau RNA inhibitor, an antisense oligonucleotide, is assessed in a phase 2 clinical study that is still in the recruiting process of patients with mild AD (NCT02623699). 86
Targeting posttranslational modifications of Tau
Another tau modification that promotes aggregation besides phosphorylation is posttranslational modification by lysine acetylation. Thus, the use of inhibitors of tau acetylation is proposed as a possible therapeutic approach for AD.
Nilotinib is a c-Abl tyrosine kinase inhibitor which is used in patients with leukemia. It also appears to trigger intraneuronal autophagy to clear tau. It is now studied in a phase 2 trial in individuals with mild to moderate AD (NCT02947893). 4 , 83
Promotion of Tau clearance—immunotherapy
Recently emerged evidence in various animal models strongly suggests that targeting ptau epitopes is a practical approach to induce antibody responses that are able to promote tau clearance. 81 Hence, a number of active and passive immunotherapy projects have reached clinical trials for AD treatment. 83
Active immunotherapy
AADvac1 contains a synthetic tau peptide and is currently studied in a phase 2 clinical study in mild to moderate AD (NCT02579252). 4 , 10 , 83
Passive immunotherapy
ABBV-8E12 is a humanized anti-tau MAb assessed in a phase 2 clinical study in patients with early AD (NCT02880956). 87
BIIB092 is a humanized IgG4 MAb against tau fragments derived from the stem cells of a patient with familial AD. 84 A phase 2 clinical trial assesses the safety and efficacy of the agent in participants with AD MCI and mild AD. 4
RO7105705 (MTAU9937 A) is an anti-tau MAb which is assessed in a phase 2 study in individuals with prodromal and mild AD (NCT03289143). 83 , 88
Three other anti-tau mAbs (BIIB076, JNJ-63733657, and LY3303560) are currently assessed in phase 1 clinical trials. 4
DMTs with other mechanisms
Neuroprotection.
AGB101 (low-dose extended-release levetiracetam) is an SV2A modulator that is assessed in a phase 3 clinical trial as a repurposed agent (approved for use in another indication, not epilepsy but MCI due to AD). It is supposed to reduce neuronal hyperactivity induced by Aβ (NCT03486938) ( Diagram 1 ). 4

DMTs with other mechanisms. DMTs indicate disease-modifying therapies; hMSCs, human mesenchymal stem cells.
BHV4157 (troriluzole) is a glutamate modulator that reduces synaptic levels of glutamate and is assessed in a phase 3 clinical trial (NCT03605667). 4
Icosapent ethyl is the eicosapentaenoic acid (EPA) omega-3 fatty acid in a purified form. It is supposed to protect neurons from disease pathology and is assessed in a phase 3 clinical trial (NCT02719327). 4
There are also 2 biologics and 24 small molecules with neuroprotection as one of their mechanisms 4 assessed in phase 2 clinical studies and 8 small molecules in phase 1 clinical trials. 4
Anti-inflammatory effects
Although neuroinflammation has been proposed as a possible mechanism for the pathogenesis of AD more than 30 years ago, only recently research is spurred into neuroiflammation probably due to 2 enlightening discoveries: first, there is evidence that activated glial cells are involved in the formation of the brain lesions in AD and second, epidemiological studies revealed that patients with rheumatoid arthritis, who are treated with anti-inflammatory drugs for decades, are spared from AD. 89 Further exploration of the inflammatory mechanisms in the disease showed that activation of glial cells, microglia, and astrocytes induces the production of inflammatory cytokines, mainly interleukin 1β (IL-1β) and tumor necrosis factor α (TNF-α). More specifically, TNF-α signaling has been proved to exacerbate both Aβ aggregation and tau phosphorylation in vivo, 90 whereas its levels have been found elevated in brain and plasma of patients with AD. 91
According to the previously mentioned neuroinflammatory mechanisms, it is established by multiple biomarker and epidemiological studies of Aβ levels in the CSF and the brain that nonsteroidal anti-inflammatory drugs, complement activation blockers, and other anti-inflammatory agents could postpone the clinical onset of AD if they are timely and for a long time applied, such as in rheumatoid arthritis. 89
Furthermore, the already existing TNF-α inhibitors (TNFIs), which are FDA-approved biologic drugs (mAbs) for the treatment of rheumatoid arthritis, Crohn disease, psoriatic arthritis, and other peripheral inflammatory diseases, are studied as a potential therapeutic strategy for AD. The TNF-α–specific mAbs are the agents infliximab, adalimumab, golimumab, and certolizumab, whereas etanercept is a recombinant fusion protein, which is also a TNFI. 91 The limited BBB penetration of these agents is the main drawback for their development. Peripheral targeting of TNF-α activity is the one proposed method for the tackling of the problem and reengineering of the TNFIs to enable BBB penetration is the other. 91 To sum up, large-scale randomized controlled trials assessing the safety and the effectiveness of TNFIs on patients with AD are warranted.
The following are the anti-inflammatory agents currently assessed in phase 3 clinical trials:
- ALZT-OP1a plus ALZT-OP1b (cromolyn plus ibuprofen) is a combination of a mast cell stabilizer and an anti-inflammatory agent, respectively, assessed in a phase 3 clinical trial (NCT02547818). 4
- COR388 targets a periodontal pathogen acting as bacterial protease inhibitor that reduces neuroinflammation and consequently hippocampal degeneration and is currently assessed in a phase 3 clinical trial (NCT03823404). 4
- Masitinib acts on mast cells as a selective tyrosine kinase inhibitor and a modulator of neuroinflammation. It is assessed in a phase 3 clinical trial (NCT01872598). 4
The following are the anti-inflammatory agents studied in phase 2:
- Elderberry Juice improves the mitochondrial function acting as powerful antioxidant rich in anthocyanins (NCT02414607) and GRF6019, a human plasma protein fraction administered with infusions, based on the hypothesis that brain neuroinflammation can be counteracted by young blood parabiosis (NCT03520998, NCT03765762). 4
- Anti-inflammatory agents studied in phase 1 are the mAbs AL002, AL003 (NCT03635047, NCT03822208). 4
Growth factor promotion
NDX-1017 is an hepatocyte growth factor with the role to regenerate neurons, which is studied in a phase 1 clinical trial (NCT03298672). 4
Metabolic effects
Losartan plus amlodipine plus atorvastatin plus exercise is a combination repurposed agent suggested to succeed significant reduction of the vascular risk capable of preserving cognitive function. It is assessed in a phase 3 clinical trial (NCT02913664). 4
Candesartan, an angiotensin receptor blocker; formoterol, a β 2 adrenergic receptor agonist; and intranasal insulin glulisine, which rises brain insulin signaling, are currently studied in phase 2 clinical trials (NCT02646982, NCT02500784, NCT02503501, respectively), whereas intranasal insulin aspart is assessed in a phase 1 clinical study. 4
Stem cell therapies
AstroStem is a stem-cell-based treatment administered 10 times intravenously, which consists of stem cells derived from autologous adipose tissue. AstroStem is currently assessed in a phase 2 study (NCT03117738), whereas hMSCs (human mesenchymal stem cells) treatment is assessed in a phase 1 study (NCT02600130). 4
Symptomatic agents
Symptomatic treatments are agents that target and improve the clinical symptoms of the disease, either cognitive or BPSD, without modifying the pathological steps leading to AD or acting on the evolution of the disease, as DMTs are supposed to do.
Overall, there are 33 symptomatic agents in current trials: 19 agents aim to improve cognition and 14 target BPSD.
Eleven of them are studied in phase 3: 3 cognitive intensifiers and 8 acting on BPSD.
Twenty symptomatic agents are in phase 2: 14 cognitive intensifiers and 6 acting on BPSD.
There are also 2 cognitive intensifiers being studied in phase 1. 4
Arduous research efforts persist to develop effective DMTs for AD, as well as symptomatic therapeutics. A plethora of continuing phase 1, 2, and 3 human studies are focused on various treatment targets in AD. Given the recent experience of a high proportion of lack of success in AD clinical trials on therapeutic agents, more recent trials appear robustly empowered by the integration of developments in biomarkers of AD, of the targeting of a single primary outcome, especially in prodromal AD studies, of the enrollment of earlier populations and the innovative trial designs. 91 - 93
At the same time, innovative research targets the development of more sophisticated diagnostic tools (neuroimaging, fluid, proteomic, and genomic AD biomarkers), whereas prevention studies for the disease are also ongoing. 10
If all these research efforts come to fruition, an effective “precision medicine” context could be applied in every patient with AD in the near future: risk factor elimination, comorbid disease treatment, and personalized advice for lifestyle modification will be provided. An AD biomarkers and neuropsychological evaluation profile will be outlined. Afterward, the patient may start a combination of DMTs tailored to meet his genetic, neuroimaging, biochemical, and neuropsychological requirements. 3 , 94
Furthermore and beyond any DMT perspective, clinicians should always maintain a patient/caregiver-targeted dealing with AD. Establishing a strong therapeutic alliance with the patient and his or her caregivers with a holistic and realistic approach involving psychoeducation, behavioral, and environmental techniques; advanced planning for future care needs; and appropriate pharmaceutical treatment is not only an efficient but also an ethical care in AD.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: SGP conceptualized the study, developed the proposal and coordinated the project. KGY completed initial data entry and analysis, and wrote the report. Both authors read and approved the final manuscript.


From Blood Tests to Brain Scans: How AI is Revolutionizing Alzheimer’s Research
How advanced technology offers new hope in the fight against a devastating disease.
MRI of the human brain (.gif courtesy of iStock). In this collaboration between USC doctors and engineers, the AI algorithm was trained, in part, from tens of thousands of MRI scans from patients worldwide.
There are more than 55 million people globally with Alzheimer’s disease . Fifty-five million that are struggling to remember.
Enter AI, a technology that famously struggles to forget . What if it could remember hundreds of thousands of MRI scans and blood tests? And then, apply that knowledge to how we comprehend, diagnose and treat one of the leading killers in the United States?
That’s exactly the case with research being done in the Keck School of Medicine of USC and the USC Viterbi School of Engineering , led by Paul Thompson , the associate director of the USC Mark and Mary Stevens Neuroimaging and Informatics Institute and a professor in the Keck School of Medicine.
The Promise of AI in Early Detection

Paul Thompson
In 2019, Thompson and his team focused on identifying potential blood-based markers for early Alzheimer’s detection by using machine learning.
This approach revealed that factors such as cardiovascular health, hormone levels and immune response played significant roles in Alzheimer’s development, alongside the well-known amyloid plaques and tau proteins. Today, the research has advanced to using more sophisticated AI techniques like convolutional neural networks, vision transformers and federated learning.
These methods can analyze vast amounts of neuroimaging data, genetic information and other biomarkers to predict disease progression and identify early signs of Alzheimer’s with unprecedented accuracy. According to Thompson, one algorithm learned from reviewing more than 85,721 MRI scans from 50,876 patients, while another learned from poring over the 3 billion letters of the human genome to find signs of Alzheimer’s.
“Deep learning methods allow us to predict clinical decline and discover genomic markers associated with Alzheimer’s,” Thompson said. “These AI models are over 90% accurate in detecting Alzheimer’s from brain scans, a significant improvement from traditional methods.”
The Power of Collaboration: AI for AD
The Artificial Intelligence for Alzheimer’s Disease Consortium (AI for AD), spearheaded by Thompson, is leading this transformative journey. Funded by the National Institutes of Health (NIH) with an $18 million grant, the project spans 12 research sites across the U.S., with USC as the lead site.
“AI for AD is a major project trying to discover ways to treat people with Alzheimer’s disease, discover new medicines and find the best medications that exist today using AI in various exciting ways,” said Thompson, who also holds an appointment at the USC Viterbi School of Engineering.
Federated Learning: A Secure, Collaborative Approach
Data remains the key.
With hypothetical access to every relevant blood test and MRI, Thompson said, “AI would make it easier get the best treatments more quickly and not waste time on ones more likely to fail or have challenging side effects.”
But how to maintain patient privacy?

Jose-Luis Ambite
A key player in this research, Jose-Luis Ambite , a USC Viterbi associate research professor of computer science and a principal scientist at the USC Information Sciences Institute (ISI) , has been instrumental in developing the AI technology. Ambite detailed how federated learning , a method allowing multiple sites to collaborate on training AI models without sharing sensitive patient data, is pivotal to their success. This approach not only maintains privacy but also enhances the model’s performance.
“Federated learning enables us to train a neural network on data from multiple hospitals without actually sharing the data,” Ambite said. “This is crucial for medical data due to privacy concerns and the difficulty of sharing such sensitive information.”
Ambite also discussed the challenge of ensuring the security and accuracy of the AI models.“ We encrypt the parameters of the neural network so that even if someone intercepts the data, they can’t access the sensitive information. This method has proven to be both secure and efficient, adding only a minimal overhead to the computational process.”
AI’s Role in Personalized Treatment and Drug Discovery
One of the most promising aspects of the current research is its potential to personalize treatment for Alzheimer’s patients. By integrating various types of data, AI can help determine the most effective treatments for individuals based on their unique genetic and clinical profiles.
“If we can predict how aggressive Alzheimer’s is and which treatments are best, we can personalize care,” Thompson said. “AI can help classify dementia, identify subtypes and stages, and even determine the likelihood of cognitive decline in specific domains.”
As an example, imagine “Mary,” age 62.
Mary shows signs of forgetfulness, is socially withdrawn and no longer expresses interest in activities she previously enjoyed. She gets a range of clinical tests, brain scans and blood tests, and an AI method detects vascular disease and elevated levels of brain amyloid, both of which can be treated if caught early. During the treatment for brain amyloid, an AI method checks Mary’s scans for side effects and shows her brain aging rate has slowed, motivating her to stick with the treatment plan.
Beyond diagnosis and treatment, the consortium’s work also extends to drug discovery. AI can identify new genomic markers that could become targets for future therapies. The consortium is now collaborating with pharmaceutical companies like Biogen to develop innovative imaging techniques that make it easier to track Alzheimer’s progression and test new drugs.
“Biogen funded us to develop an MRI-based test of amyloid plaques using a technique called neural style transfer,” said Thompson. “This AI-driven method could create affordable and widely accessible amyloid scans, making it possible to prescreen patients for clinical trials.”
A Glimpse Into the Future
Looking ahead, both Thompson and Ambite are optimistic about the future of AI in Alzheimer’s research. Ambite highlighted the potential of multimodal AI, which combines various types of medical data, such as brain imaging, genetic information and clinical records, to improve diagnosis and treatment predictions.
“Combining different types of data will enhance the AI’s ability to provide accurate diagnoses and effective treatment plans,” Ambite said. “We are already seeing the benefits of this approach, and it’s only going to get better as we continue to refine our models.”
Thompson echoed this sentiment while emphasizing the importance of making these AI tools both widely available and affordable. As these technologies continue to evolve, they hold the promise of not only improving diagnostics and treatments but also offering hope to millions affected by this debilitating disease.
“Our goal is to ensure that these advancements reach as many patients as possible, regardless of their location or resources,” he said. “We want to see these tools used globally to improve the lives of those affected by Alzheimer’s.”
Published on September 19th, 2024
Last updated on September 19th, 2024
Share this Story
Related Stories

ABOUT THE SCHOOL
- 115 Year Celebration
- About Andrew Viterbi
- Diversity Equity & Inclusion
- Facts and Numbers
- Faculty Directory
- Ginsburg Hall
- USC Michelson Center
FROM THE DEAN
- Dean's Message
- Dean's Report
- Initiatives and Priorities
- Engineering +
- Strategic Plan
NEWS | MEDIA | EVENTS
- Keynote Lecture Series
- Media Contact & Press Releases
- Media Coverage
- Public Image Archive
- Publications
- Social Media
- Viterbi News Now
SCHOOL OF ADVANCED COMPUTING
- Thomas Lord Department of Computer Science
- Ming Hsieh Department of Electrical and Computer Engineering
- Division of Computing Education (DCE)
- Information Technology Program (ITP)
- Interdisciplinary Data Science (IDS)
- Information Science Institute (ISI)
- Institute for Creative Technologies (ICT)
- More to come soon
DEPARTMENTS AND ACADEMIC PROGRAMS
- Aerospace and Mechanical Engineering
- Astronautical Engineering
- Alfred E. Mann Department of Biomedical Engineering
- Mork Family Department of Chemical Engineering and Materials Science
- Sonny Astani Department of Civil and Environmental Engineering
- Daniel J. Epstein Department of Industrial and Systems Engineering
- Engineering in Society Program
- Information Technology Program
EXECUTIVE AND CONTINUING EDUCATION
- Aviation Safety and Security Program
- Corporate and Professional Programs
ONLINE ACCESS
- Graduate Programs - DEN@Viterbi
SPECIALIZED GRADUATE PROGRAMS
- Financial Engineering Program
- Green Technologies Program
- Data Science Program
- Progressive Degree Program
- Systems Architecting and Engineering Program
RESOURCES AND INITIATIVES
- Academic Integrity
- Accreditation
- Awards Office
- John Brooks Slaughter Center for Engineering Diversity
- Division of Engineering Education
- Globalization
- K-12 Outreach
- USC Experts Directory
- Women in Science and Engineering
FIRST YEAR APPLICANTS

MASTER'S APPLICANTS

PHD APPLICANTS

TRANSFER APPLICANTS

RESEARCH ENVIRONMENT
- Search Faculty Research Areas
- Departments, Research Institutes and Centers
- Research Infrastructure
- Research Initiatives
- Research Vision
- Student Research
- Summer Undergraduate Research Experience
TECHNOLOGY INNOVATION AND ENTREPRENEURSHIP
- NSF I-Corps Hub: West Region
- Office of Technology Innovation and Entrepreneurship
- USC Stevens Center for Innovation
- Viterbi News Network
- Diversity Equity Inclusion
- Dean’s Message
- Dean’s Report
- Media Contact & Press Releases
- More to Come Soon
- Biomedical Engineering
- Informatics Program
- Graduate Programs – DEN@Viterbi
- First Year Applicants
- Master’s Applicants
- PHD Applicants
- Transfer Applicants
- Competitions
- Entrepreneurship
- I-Corps Node
- Viterbi Startup Garage
- Viterbi Student Innovation Institute (VSI2)
- Viterbi Venture Fund

Masks Strongly Recommended but Not Required in Maryland
Respiratory viruses continue to circulate in Maryland, so masking remains strongly recommended when you visit Johns Hopkins Medicine clinical locations in Maryland. To protect your loved one, please do not visit if you are sick or have a COVID-19 positive test result. Get more resources on masking and COVID-19 precautions .
- Vaccines
- Masking Guidelines
- Visitor Guidelines
News & Publications
Research aims to uncover the mysteries of alzheimer’s disease.
Physicians often detect early warning signs of dementia in their aging patients from the stories shared by a patient’s family members. They learn about situations that are out of character, such as paying a bill multiple times, misplacing keys and repeating stories. Often a patient has become more irritable and anxious.
When the clinical examination also suggests that a longtime patient’s behavior or disposition has changed, the next step is to rule out possible causes such as medication issues, poor sleep or traumatic life events, according to Esther Oh , co-director of the Johns Hopkins Memory and Alzheimer’s Treatment Center.
“When there’s clearly some functional impairment going on, I try to figure out why,” she says. “How older patients experience cognitive problems is complex.”
If physicians suspect a patient may have mild cognitive impairment due to Alzheimer’s disease or a related brain disease, they can order a variety of labs including blood tests, MRIs and CT scans, and may refer the patient to Oh and her colleagues in psychiatry, geriatrics or neurology at the Memory and Alzheimer’s Treatment Center.
There, patients can receive individualized treatment based on their needs, including medications targeting memory and cognition and treatments for mood, behavioral and sleep changes. Additionally, patients, caregivers and family members can get guidance and support that helps improve their quality of life, plan for the future and manage inevitable crises.
Much is still unknown about the disease. At this time, there’s no cure for Alzheimer’s, no proven way of slowing down its progression and no treatment available to reverse the deterioration that occurs in the brain.
Best practices for managing the disease include physical and social activity, healthy lifestyle and diet, and a well-structured environment. Older adults who engage in these behaviors appear to have less risk of cognitive and functional decline.
While the diagnosis is grim, Johns Hopkins clinicians and researchers are broadening Alzheimer’s and dementia research and expanding treatment options in a number of ways: searching for biological markers (biomarkers) that could predict Alzheimer’s; determining how to target certain proteins that are present in the brains of patients with the disease; defining the different kinds of Alzheimer’s to tailor future treatment and research; developing new drugs; and piloting a home-based care program.

Defining and Refining
Dementia is a general term that refers to memory loss and decline of other cognitive abilities that limit independence in day-to-day function. Alzheimer’s is the most common brain disease that causes dementia among older adults, accounting for 60%–80% of cases. It affects an estimated one in nine people age 65 and older — 6.2 million Americans. This number is projected to grow to 12.7 million by 2050, according to the Alzheimer’s Association.
Almost two-thirds of the cases are in women, and people of color are at a higher risk of developing Alzheimer’s.
Alzheimer’s is a progressive, neurodegenerative disease that occurs when nerve cells in the brain die. It affects memory, thinking and behavior. But, unlike other forms of dementia, it does not affect patients’ motor function until late stages of the disease.
Alzheimer’s experts think individuals may experience different versions of the disease.
“It’s probably not one kind of Alzheimer’s disease, it’s probably many,” says psychiatrist Paul Rosenberg , co-director of the Johns Hopkins Memory and Alzheimer’s Treatment Center. “What we want to do is find the subtypes so we can find better treatments.”
Cancer treatment, for example, is specific to the kind and subtype of cancer. Breast cancer is treated differently from colon cancer, and within breast cancer, different subtypes mean different treatments. This is the direction in which Johns Hopkins researchers hope to move Alzheimer’s treatment.
Rosenberg and his colleagues, including Memory and Alzheimer’s Treatment Center director Kostas Lyketsos , are crunching data in the Richman Family Precision Medicine Center of Excellence in Alzheimer’s Disease to do just that. The goal is to find characteristics that can allow physicians to predict which patients will develop Alzheimer’s, as well as determine what clinical data is necessary to differentiate subtypes of the disease.
The Precision Medicine Center’s patient registry includes more than 130,000 medical records that researchers hope can help define clinical subgroups of patients with dementia, determine when symptoms first develop and when diagnoses occur, among other factors. Additionally, center researchers are developing a collection of unique blood biomarkers that could help target future treatments to subgroups of patients.
Other projects include:
- Analyzing hundreds of MRIs from patients with dementia to look for variations in the size of different structures that could indicate subgroups.
- Studying changes in biomarkers over time to try to measure the progression of dementia.
- Using person-specific stem cells — which are made using a person’s own blood — to create different brain cells in the lab with the potential to predict response to specific medications that may have a role in improving cognitive decline.
- Treating brain vascular disease — an important contributor to dementia — by repurposing an existing drug, atorvastatin, which is typically used to lower cholesterol. Researchers are studying the drug’s effect on the brain’s circulatory system using a new MRI technique.
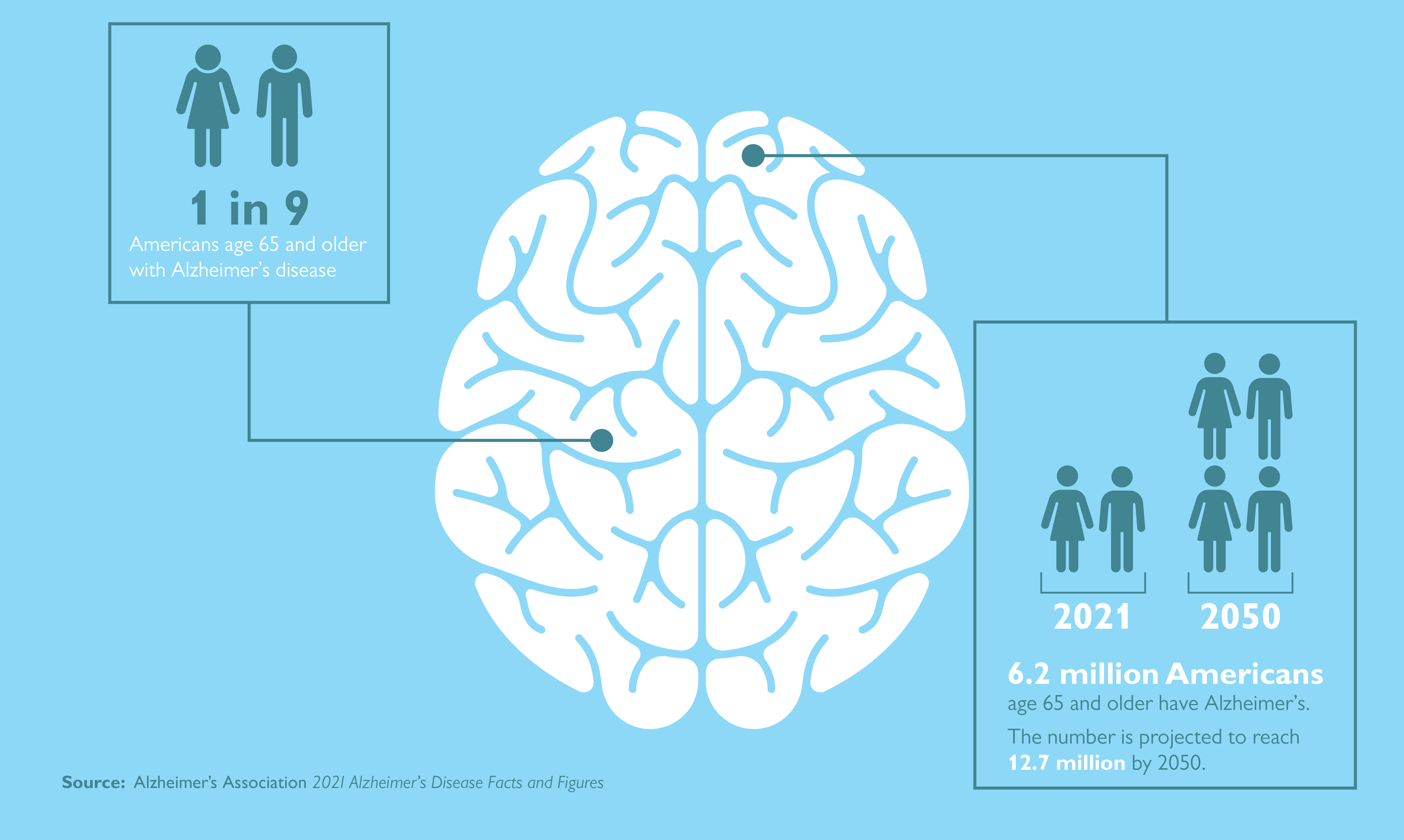
New Drugs in Development
Other Johns Hopkins researchers are testing a drug that has the potential to slow the progression of Alzheimer’s. Marilyn Albert , director of the Johns Hopkins Alzheimer’s Disease Research Center , Michela Gallagher , a professor of neuroscience at the Johns Hopkins University School of Medicine, and Arnold Bakker , director of the Johns Hopkins Psychiatric Neuroimaging Core , hope to receive FDA approval for the drug for patients in the earliest stages of the disease.
The drug targets two proteins: tau, which serves many functions in healthy neurons but can accumulate into tangles in cells, and amyloid, which forms plaques between cells. Gallagher theorized that periods of hyperactivity in the brain, such as seizures, push those proteins around the brain, spreading more tangles and plaques. She identified a compound, levetiracetam, that calms these hyperactive periods and is now approved by the FDA to treat seizures in patients with epilepsy when used with other medications. She and Albert are testing a time-release capsule version of levetiracetam taken at breakfast by patients in early stages of the disease.
The study , sponsored by AgeneBio , involves more than 164 people taking the drug for 78 weeks at 27 research sites across the country. Preliminary results are expected in fall 2022.
In addition to Albert and Gallagher’s drug, referred to as AGB101, there are more than 100 other Alzheimer’s drugs being tested at Johns Hopkins and elsewhere, according to the Alzheimer’s Association.
Universal Screening
As researchers wrestle with how to catch the disease in its early stages, the question of whether to do universal screening for cognitive impairment remains. Such screening is required as part of the Medicare Annual Wellness visit, initiated in 2011 as part of the Affordable Care Act. While primary care providers must perform this screening for Medicare patients, specialists at Johns Hopkins believe this kind of test can be useful in more targeted ways.
“There could be a place for targeted universal screening, but we have to be very careful,” Oh says. “The question remains, what are you going to do with that information?”
In some scenarios, screening may be appropriate, she says. For example, a patient undergoing surgery who has possible cognitive impairment could be screened in order to make sure they understand the procedure as well as post-op instructions. It could help providers manage possible complications, such as delirium after surgery, which occurs more commonly in individuals with dementia.
The Alzheimer’s Association recommends evaluation for people with memory concerns or cognitive complaints as well as for those with non-memory triggers, including personality change, depression, deterioration of chronic disease without explanation, and falls or balance issues. It is also recommended if a family member or loved one reports cognitive impairment.
Like Oh, Paul Rosenberg and Kostas Lyketsos don’t see a role for universal screening as there’s no demonstrated benefit from it.
“Until we have a safe and effective therapy that people can afford, it is just not ethical to do the tests,” Rosenberg says.
Related Reading
Alzheimer’s disease: frustration and hope.
While Johns Hopkins clinicians help patients with Alzheimer's disease maximize quality of life, researchers are gaining insights that could lead to better treatment.
Stress, Alzheimer’s and Aging
New research suggests that if you are caring for a spouse with Alzheimer’s, you have a higher risk of developing the disease yourself.

Cornell Chronicle
- Architecture & Design
- Arts & Humanities
- Business, Economics & Entrepreneurship
- Computing & Information Sciences
- Energy, Environment & Sustainability
- Food & Agriculture
- Global Reach
- Health, Nutrition & Medicine
- Law, Government & Public Policy
- Life Sciences & Veterinary Medicine
- Physical Sciences & Engineering
- Social & Behavioral Sciences
- Coronavirus
- News & Events
- Public Engagement
- New York City
- Photos of the Week
- Big Red Sports
- Freedom of Expression
- Student Life
- University Statements
- Around Cornell
- All Stories
- In the News
- Expert Quotes
- Cornellians
Immune cells key in blood vessel damage, neurodegeneration
By bridget kuehn weill cornell medicine.
A new study helps explain why having ApoE4 – the gene variant most closely linked to Alzheimer’s disease – increases the risk of neurodegeneration and white matter damage.
Researchers at Weill Cornell Medicine discovered that immune cells in the brain called border-associated macrophages (BAMs) are a source of ApoE4 protein and contribute to damaging blood vessels and brain tissue.
The study , published Sept. 18 in Nature Neuroscience, may help scientists identify new approaches to preventing or treating Alzheimer’s disease in people who carry the ApoE4 gene and other forms of age-related brain disease.
The APOE gene encodes apolipoprotein E (ApoE), which has many roles in the brain. It also has several common variants (ApoE2, ApoE3 and ApoE4), of which ApoE4 increases the risk for Alzheimer’s disease up to 12-fold. ApoE4 also increases the risk of damage to the white matter that underlies vascular dementia, the second-most common cause of cognitive impairment after Alzheimer’s disease. However, how ApoE4 produces these damaging effects on the brain is not completely clear.
“Our study points to border-associated macrophages as a critical mediator of these deleterious effects and helps us understand how ApoE4 may contribute to damaging blood vessels and brain white matter in patients with Alzheimer’s disease or other forms of age-related brain disease,” said the study’s co-senior author, Laibaik Park , associate professor of research in neuroscience at the Feil Family Brain and Mind Research Institute at Weill Cornell Medicine.”
“We previously showed in another model that the amyloid beta protein that builds up in the brains of patients with Alzheimer’s disease interacts with a protein receptor on BAMs,” said Antoine Anfray, instructor in neuroscience at the Brain and Mind Research Institute, and first author of the study. This triggers a chain reaction that damages blood vessels, preventing them from clearing amyloid, which leads to degeneration of brain tissue.
In their latest study, the researchers show that pre-clinical models genetically engineered to express the human ApoE4 variant developed impaired blood vessels and tissue damage in their brains, while those with the more benign ApoE3 remain healthy. They found that BAMs with the ApoE4 variant produce inflammatory oxygen free radicals, which damage the blood vessels. As a result, blood flow needed to remove waste and repair damage to the brain tissue is limited.
Surprisingly, when the animal models with the ApoE4 variant had their BAMs removed, they didn’t experience this damaging cascade. The study also showed that the BAMs are not only the mediators of the damage induced by ApoE4, but also the source of ApoE4 causing the damage. Accordingly, reducing ApoE4 expression in BAMs eliminated the harmful vascular effects.
“These findings show BAMs are both the source and the target of the ApoE4 necessary for damage to blood vessels,” said study senior author Dr. Costantino Iadecola , director and chair of the Brain and Mind Research Institute and the Anne Parrish Titzell Professor of Neurology at Weill Cornell Medicine.
The investigators further confirmed that ApoE4 and BAMs transferred to animal models which didn’t have the ApoE4 variant developed blood vessel and tissue damage. Alternatively, transplanting BAMs from animals with the ApoE3 variant to animals with the ApoE4 variant reversed the damage.
The findings may help explain why some patients are more likely to experience harmful swelling and bleeding in the brain when treated with amyloid-removing antibody drugs like Lecanemab, a complication most frequent in patients with ApoE4. This complication, termed amyloid related imaging abnormality (ARIA), requires the treatment to be stopped, limiting its benefits in slowing the progression of early-stage Alzheimer's disease.
Understanding how blood vessels are more vulnerable in some patients may help scientists develop ways to prevent this adverse effect by suppressing ApoE4 production by BAMs. Iadecola and Park are working on developing such interventions, but they caution that more work is needed before the findings can be applied in the clinic.
For now, they are looking for ways to block the receptors that mediate ApoE4-related blood vessel damage to reduce or prevent the genetic variant’s harmful effects on the amyloid-beta clearance pathway.
“We now know that ApoE4 from border-associated macrophages increases blood vessel damage, but the next step would be to actually find a way to target the macrophages to enhance amyloid and tau clearance,” Iadecola said. “Can genetically switching the ApoE4 to the ApoE3 genetic variant remove amyloid build up better? That will be proof-of-concept.”
Funding for this work came from the National Institutes of Health and from a fellowship of the BrightFocus Foundation.
Bridget Kuehn is a freelance writer for Weill Cornell Medicine.
Media Contact
Barbara prempeh.
Get Cornell news delivered right to your inbox.
You might also like

Gallery Heading
Recent breakthroughs in Alzheimer’s research provide hope for patients
While there is no cure, researchers say a newly approved drug, advanced testing, and increasing knowledge about the disease may improve patients’ lives..

A few years ago, Lori Weiss, a high school math and engineering teacher, noticed it was taking her longer to do her lesson plans and grading. She also repeatedly needed to ask for help using spreadsheets she’d once mastered and she struggled to answer her students’ questions.
The symptoms were all too familiar to Weiss. Not only had she cared for her grandfather with Alzheimer’s disease when she was a teenager, she’d also watched her mother slowly lose her memory to the disease for nearly two decades. She had aunts, uncles, and a cousin as well who were diagnosed with the neurological disorder, which gradually steals a person’s memory and cognitive abilities.
“It’s rampant in my family,” Weiss says.
Weiss decided to speak with her primary care physician, who referred her to a neurologist for testing. In 2020, at the age of 62, Weiss was diagnosed with mild cognitive impairment. Two years after that, a PET scan revealed amyloid plaques, a buildup of toxic proteins in the brain that disrupt neural function and are a hallmark of Alzheimer’s disease.
Soon, Weiss began to lose her sense of direction, which prompted fears that she might be forced into a full-time care facility at a young age.
“Losing my freedom was just more than I could handle,” she says. Around that time, a friend saw a TV advertisement for a clinical trial for a drug that would attempt to slow progression of the disease using manmade monoclonal antibodies to attack and remove the amyloid plaques in the brain.
“I don’t even think that I thought twice about” enrolling in the trial, says Weiss, who has been receiving monthly infusions of the drug, called donanemab , near her home in Portland, Oregon, for about a year. “I just said, ‘Yeah, sign me up!’”
Although donanemab is not approved by the Food and Drug Administration (FDA), it uses a similar approach to the drug lecanemab, which received accelerated FDA approval on Jan. 6, and which showed biological and clinical benefits for patients in trials. In November, drugmaker Eli Lilly and Company announced promising results for donanemab, but last week, the FDA denied the company’s request for accelerated approval, saying it needed more data for participants receiving the drug for at least 12 months.
Nonetheless, this recent progress has given people like Weiss hope that previous generations have not had.
“Alzheimer’s research is getting to a place where cancer research was maybe 30, 40 years ago.” Anton Porsteinsson, MD, University of Rochester Medical Center in New York
Participating in the clinical trial “has had a huge impact,” Weiss says. “It’s given me the drive to do things while I can; it’s given me the desire to talk to more people about getting treatment, getting diagnosed early, and getting in drug trials.”
Weiss says that since she’s begun taking donanemab, she’s regained her sense of direction and has not noticed significant cognitive decline. For her, even the hope that the trial has given her has made all the difference.
“For my husband and I, it’s totally changed our lives. Instead of living in fear … we treat each day like it’s Valentine’s Day,” Weiss says. Getting diagnosed early has “given me so much more life. [I thought] getting the disease was a death sentence for me, but I’m taking a water painting class, I’m in a walking group and a music group. I thrive on my relationships with my Alzheimer’s friends and other friends, and I’m connected with my family. I feel like I’m living my life. It’s so much better than I imagined.”

And while Alzheimer’s researchers are careful to emphasize that they are still a long way from a cure, many say the hope is not a false one. The field has had several breakthroughs in recent years, from identifying easier and cheaper ways to diagnose the disease early to better understanding how individuals with the disease might require a variety of interventions.
“Alzheimer’s research is getting to a place where cancer research was maybe 30, 40 years ago,” says Anton Porsteinsson, MD, director of the Alzheimer’s Disease Care, Research and Education Program at the University of Rochester Medical Center in New York. “I think we’re at a point where we’re going to see a logarithmic increase in discovery.”
Fighting a complex disease
Alzheimer’s disease, which was discovered in 1906 and is now the seventh leading cause of death in the United States, has long boggled the scientific community. Though research over the decades has identified characteristics of the disease — such as the presence of amyloid plaques between neurons and the buildup, known as tangles, of another toxic protein, tau, inside neurons — questions remain about what causes the disease and how best to treat it in a clinically meaningful way.
“It’s a complex disease. It’s not just a single molecule that’s gone awry. It’s not an infection that has a viral particle,” says Ronald C. Petersen, MD, PhD, director of the Mayo Clinic Alzheimer’s Disease Research Center in Rochester, Minnesota. “We’ve defined it by the presence of amyloid , neuritic plaques , and neurofibrillary tangles , but that’s just the tip of the iceberg.”
Many researchers now believe that the precursors to developing Alzheimer’s begin to accumulate in the brain 10 or more years before symptoms begin to show.
Alzheimer’s disease progression affects the brain much like a forest fire, with many factors affecting how it spreads, says Rudolph E. Tanzi, PhD, director of the Genetics and Aging Research Unit at Massachusetts General Hospital in Boston.
Amyloid plaques and tau tangles can build up over years, at some point triggering an inflammatory response that can quickly destroy brain cells. These conditions can be influenced by a range of factors, from genetic predisposition to environmental exposures to lifestyle, he explains.
That’s why the solution to treating — or ideally, preventing — Alzheimer’s disease will likely require a combination of interventions, Petersen says.
One important part of the puzzle — and a part that has been the focus of much pharmaceutical development — is targeting the amyloid plaques.
This approach has been controversial. In 2021, the FDA granted accelerated approval to the anti-amyloid drug aducanumab, sold as Aduhelm, despite objections from an advisory committee and outcry from the scientific community that the lack of clinical benefit made the drug’s high cost, initially set at $56,000 a year and later reduced to $28,000 a year, unjustifiable. A Congressional investigation found numerous flaws and irregularities in the process the FDA used when approving the drug.
Lecanemab, on the other hand, has been met with more optimism in the Alzheimer’s research community because its clinical trials demonstrated an actual clinical benefit to patients early in the disease progression.
“The field is feeling that, finally, we have a drug that didn’t have the controversy aducanumab had,” Petersen says. “It looks like it does what it’s supposed to do biologically [and] this looks like it could be meaningful for patients.”
In clinical trials , lecanemab showed a modest but tangible decrease in cognitive decline (of 27%) over 18 months in Alzheimer’s patients who were early in the disease’s progression, compared with patients who were given a placebo. Though it’s far from a cure, experts say it could give patients months of retaining memory and cognition that they might otherwise lose, a prospect that could be meaningful for patients and their families who have no other options.
But this drug, too, has stirred some controversy because of its high price tag and potentially deadly side effects, including swelling and bleeding in the brain. The pharmaceutical company Eisai has priced lecanemab, sold as Leqembi, at $26,000 a year, and the Centers for Medicare and Medicaid Services has yet to decide if it will cover the drug.
“It’s very expensive,” Tanzi says, explaining that patients who take the drug will also need several MRIs to check for brain bleeds on top of the cost of the infusions. “There is a health care disparity this could create; those who want to remove amyloid can pay out of pocket [but] the average person can’t afford that. The wealthy can protect themselves.”
Equity starting in research
The high costs of treatment could also exacerbate existing racial disparities when it comes to Alzheimer’s outcomes. Although Black Americans are about twice as likely as White Americans to have Alzheimer’s, and Hispanics are about 1.5 times as likely to have it, White people make up a disproportionate majority of clinical trial participants and non-White people report greater barriers to diagnosis and access to care, according to the Alzheimer’s Association .
“Most of the research operations are either based at large academic institutions or private professional research sites,” Porsteinsson explains about pharmaceutical company trials. “The temptation [for researchers] is to go where the treatment is ‘easiest’; where you’ve recruited before.”
In its clinical trial recruitment for lecanemab, the University of Rochester succeeded in increasing the representation of Hispanic participants, but struggled to include a representative number of Black patients.
“We can’t just wait until the brain deteriorates.” Rudolph E. Tanzi, PhD, Massachusetts General Hospital in Boston
“If we want to go after historically underrepresented groups in research, first we need to recognize they’re underrepresented for a reason,” Porsteinsson says. “There might have been a poor experience with researchers coming [into their community], doing a study [the researchers] needed, and then basically leaving. There isn’t an ongoing commitment.”
He says that if Alzheimer’s treatments are going to be meaningful to all people affected by the disease, it will take a concerted effort to include more diversity in clinical trial participants, not only in race and ethnicity, but in health status and inclusion of people with comorbidities. Often, trials tend to select for the healthiest patients possible, he explains.
“[We must] secure making our research more representative of the American population,” Porsteinsson says. “It’s going to take an investment in infrastructure and it’s going to take an investment of time.”
A stage set for discovery
Alzheimer’s disease already affects more than six million people living in the United States, and that number is projected to grow to 13 million by 2050. It’s also an incredibly financially costly disease, with an economic impact of $321 billion in health care costs in 2022, expected to rise to $1 trillion by 2050, according to the Alzheimer’s Association. That prospect prompted the U.S. Congress to approve an additional $226 million to the National Institutes of Health for Alzheimer’s research in December, bringing the annual federal funding outlay to more than $3.7 billion .
Experts say it is not in vain. Research efforts, particularly those at teaching hospitals, have helped unlock mysteries about the genetic underpinnings of the disease, ways to identify biomarkers in the blood that can more easily diagnose the disease in its earliest states, and complex treatment approaches that use lifestyle interventions and a combination of drug therapies.
Tanzi believes that the future of Alzheimer’s treatment and prevention will be similar to current management of heart disease and diabetes. It could mean more regular screenings and early interventions, such as taking anti-amyloid drugs and incorporating lifestyle and diet changes before the disease gets out of control. And for those already diagnosed, it means using a combination of therapies that target different aspects of the disease, such as neuroinflammation and plaque buildup.
“We can’t just wait until the brain deteriorates,” he says.
With the current momentum, Porsteinsson hopes that young and aspiring physician-scientists will be inspired to join the field and continue the research for generations to come.
“What many medical students and young doctors have historically been hesitant about is that dementia is very nebulous, there is a lot of gray there. … They felt things were pretty bleak, too uncertain, and there was too little you could offer,” he says. “Now, I think we are at the dawn of a very different era.”

Several experimental medicines aim to block the activity of these enzymes. They're known as beta- and gamma-secretase inhibitors. Recent studies showed that the beta-secretase inhibitors did not slow cognitive decline. They also were associated with significant side effects in those with mild or moderate Alzheimer's. This has decreased enthusiasm for the medicines.
Keeping tau from tangling
A vital brain cell transport system collapses when a protein called tau twists into tiny fibers. These fibers are called tangles. They are another common change in the brains of people with Alzheimer's. Researchers are looking at a way to prevent tau from forming tangles.
Tau aggregation inhibitors and tau vaccines are currently being studied in clinical trials.
Reducing inflammation
Alzheimer's causes chronic, low-level brain cell inflammation. Researchers are studying ways to treat the processes that lead to inflammation in Alzheimer's disease. The medicine sargramostim (Leukine) is currently in research. The medicine may stimulate the immune system to protect the brain from harmful proteins.
Researching insulin resistance
Studies are looking into how insulin may affect the brain and brain cell function. Researchers are studying how insulin changes in the brain may be related to Alzheimer's. However, a trial testing of an insulin nasal spray determined that the medicine wasn't effective in slowing the progression of Alzheimer's.
Studying the heart-head connection
Growing evidence suggests that brain health is closely linked to heart and blood vessel health. The risk of developing dementia appears to increase as a result of many conditions that damage the heart or arteries. These include high blood pressure, heart disease, stroke, diabetes and high cholesterol.
A number of studies are exploring how best to build on this connection. Strategies being researched include:
- Current medicines for heart disease risk factors. Researchers are looking into whether blood pressure medicines may benefit people with Alzheimer's. They're also studying whether the medicines may reduce the risk of dementia.
- Medicines aimed at new targets. Other studies are looking more closely at how the connection between heart disease and Alzheimer's works at the molecular level. The goal is to find new potential medicines for Alzheimer's.
- Lifestyle choices. Research suggests that lifestyle choices with known heart benefits may help prevent Alzheimer's disease or delay its onset. Those lifestyle choices include exercising on most days and eating a heart-healthy diet.
Studies during the 1990s suggested that taking hormone replacement therapy during perimenopause and menopause lowered the risk of Alzheimer's disease. But further research has been mixed. Some studies found no cognitive benefit of taking hormone replacement therapy. More research and a better understanding of the relationship between estrogen and cognitive function are needed.
Speeding treatment development
Developing new medicines is a slow process. The pace can be frustrating for people with Alzheimer's and their families who are waiting for new treatment options.
To help speed discovery, the Critical Path for Alzheimer's Disease (CPAD) consortium created a first-of-its-kind partnership to share data from Alzheimer's clinical trials. CPAD 's partners include pharmaceutical companies, nonprofit foundations and government advisers. CPAD was formerly called the Coalition Against Major Diseases.
CPAD also has collaborated with the Clinical Data Interchange Standards Consortium to create data standards. Researchers think that data standards and sharing data from thousands of study participants will speed development of more-effective therapies.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
- Treatments and research. Alzheimer's Association. https://www.alz.org/alzheimers-dementia/research_progress/treatment-horizon. Accessed March 23, 2023.
- Cummings J, et al. Alzheimer's disease drug development pipeline: 2022. Alzheimer's and Dementia. 2022; doi:10.1002/trc2.12295.
- Burns S, et al. Therapeutics of Alzheimer's disease: Recent developments. Antioxidants. 2022; doi:10.3390/antiox11122402.
- Plascencia-Villa G, et al. Lessons from antiamyloid-beta immunotherapies in Alzheimer's disease. Handbook of Clinical Neurology. 2023; doi:10.1016/B978-0-323-85555-6.00019-9.
- Brockmann R, et al. Impacts of FDA approval and Medicare restriction on antiamyloid therapies for Alzheimer's disease: Patient outcomes, healthcare costs and drug development. The Lancet Regional Health. 2023; doi:10.1016/j.lana. 2023.100467 .
- Wojtunik-Kulesza K, et al. Aducanumab — Hope or disappointment for Alzheimer's disease. International Journal of Molecular Sciences. 2023; doi:10.3390/ijms24054367.
- Can Alzheimer's disease be prevented? Alzheimer's Association. http://www.alz.org/research/science/alzheimers_prevention_and_risk.asp. Accessed March 23, 2023.
- Piscopo P, et al. A systematic review on drugs for synaptic plasticity in the treatment of dementia. Ageing Research Reviews. 2022; doi:10.1016/j.arr. 2022.101726 .
- Facile R, et al. Use of Clinical Data Interchange Standards Consortium (CDISC) standards for real-world data: Expert perspectives from a qualitative Delphi survey. JMIR Medical Informatics. 2022; doi:10.2196/30363.
- Imbimbo BP, et al. Role of monomeric amyloid-beta in cognitive performance in Alzheimer's disease: Insights from clinical trials with secretase inhibitors and monoclonal antibodies. Pharmacological Research. 2023; doi:10.1016/j.phrs. 2022.106631 .
- Conti Filho CE, et al. Advances in Alzheimer's disease's pharmacological treatment. Frontiers in Pharmacology. 2023; doi:10.3389/fphar. 2023.1101452 .
- Potter H, et al. Safety and efficacy of sargramostim (GM-CSF) in the treatment of Alzheimer's disease. Alzheimer's and Dementia. 2021; doi:10.1002/trc2.12158.
- Zhong H, et al. Effect of peroxisome proliferator-activated receptor-gamma agonists on cognitive function: A systematic review and meta-analysis. Biomedicines. 2023; doi:10.3390/biomedicines11020246.
- Grodstein F. Estrogen and cognitive function. https://www.uptodate.com/contents/search. Accessed March 23, 2023.
- Mills ZB, et al. Is hormone replacement therapy a risk factor or a therapeutic option for Alzheimer's disease? International Journal of Molecular Sciences. 2023; doi:10.3390/ijms24043205.
- Custodia A, et al. Biomarkers assessing endothelial dysfunction in Alzheimer's disease. Cells. 2023; doi:10.3390/cells12060962.
- Overview. Critical Path for Alzheimer's Disease. https://c-path.org/programs/cpad/. Accessed March 29, 2023.
- Shi M, et al. Impact of anti-amyloid-β monoclonal antibodies on the pathology and clinical profile of Alzheimer's disease: A focus on aducanumab and lecanemab. Frontiers in Aging and Neuroscience. 2022; doi:10.3389/fnagi. 2022.870517 .
- Leqembi (approval letter). Biologic License Application 761269. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761269. Accessed July 7, 2023.
- Van Dyck CH, et al. Lecanemab in early Alzheimer's disease. New England Journal of Medicine. 2023; doi:10.1056/NEJMoa2212948.
- Leqembi (prescribing information). Eisai; 2023. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=761269. Accessed July 10, 2023.
- Kisunla (approval letter). Biologic License Application 761248. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process. Accessed July 9, 2024.
- Sims JR, et al. Donanemab in early symptomatic Alzheimer disease: The TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023; doi:10.1001/jama.2023.13239.
- Kisunla (prescribing information). Eli Lilly and Company; 2024. https://www.lilly.com/. Accessed July 3, 2024.
Products and Services
- A Book: Mayo Clinic on Alzheimer's Disease
- Assortment of Products for Independent Living from Mayo Clinic Store
- A Book: Day to Day: Living With Dementia
- A Book: Mayo Clinic on Healthy Aging
- Give today to find Alzheimer's cures for tomorrow
- Alzheimer's sleep problems
- Alzheimer's 101
- Understanding the difference between dementia types
- Alzheimer's disease
- Alzheimer's genes
- Alzheimer's drugs
- Alzheimer's prevention: Does it exist?
- Alzheimer's stages
- Antidepressant withdrawal: Is there such a thing?
- Antidepressants and alcohol: What's the concern?
- Antidepressants and weight gain: What causes it?
- Antidepressants: Can they stop working?
- Antidepressants: Side effects
- Antidepressants: Selecting one that's right for you
- Antidepressants: Which cause the fewest sexual side effects?
- Anxiety disorders
- Atypical antidepressants
- Caregiver stress
- Clinical depression: What does that mean?
- Corticobasal degeneration (corticobasal syndrome)
- Depression and anxiety: Can I have both?
- Depression, anxiety and exercise
- What is depression? A Mayo Clinic expert explains.
- Depression in women: Understanding the gender gap
- Depression (major depressive disorder)
- Depression: Supporting a family member or friend
- Diagnosing Alzheimer's
- Did the definition of Alzheimer's disease change?
- How your brain works
- Intermittent fasting
- Lecanemab for Alzheimer's disease
- Male depression: Understanding the issues
- MAOIs and diet: Is it necessary to restrict tyramine?
- Marijuana and depression
- Mayo Clinic Minute: 3 tips to reduce your risk of Alzheimer's disease
- Mayo Clinic Minute: Alzheimer's disease risk and lifestyle
- Mayo Clinic Minute: New definition of Alzheimer's changes
- Mayo Clinic Minute: Women and Alzheimer's Disease
- Memory loss: When to seek help
- Monoamine oxidase inhibitors (MAOIs)
- Natural remedies for depression: Are they effective?
- Nervous breakdown: What does it mean?
- New Alzheimers Research
- Pain and depression: Is there a link?
- Phantosmia: What causes olfactory hallucinations?
- Positron emission tomography scan
- Posterior cortical atrophy
- Seeing inside the heart with MRI
- Selective serotonin reuptake inhibitors (SSRIs)
- Serotonin and norepinephrine reuptake inhibitors (SNRIs)
- Sundowning: Late-day confusion
- Treatment-resistant depression
- Tricyclic antidepressants and tetracyclic antidepressants
- Video: Alzheimer's drug shows early promise
- Vitamin B-12 and depression
- Young-onset Alzheimer's
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
- Alzheimer s treatments What s on the horizon
5X Challenge
Thanks to generous benefactors, your gift today can have 5X the impact to advance AI innovation at Mayo Clinic.
Call our 24 hours, seven days a week helpline at 800.272.3900

- Professionals

- Younger/Early-Onset Alzheimer's
- Is Alzheimer's Genetic?
- Women and Alzheimer's
- Creutzfeldt-Jakob Disease
- Dementia with Lewy Bodies
- Down Syndrome & Alzheimer's
- Frontotemporal Dementia
- Huntington's Disease
- Mixed Dementia
- Normal Pressure Hydrocephalus
- Posterior Cortical Atrophy
- Parkinson's Disease Dementia
- Vascular Dementia
- Korsakoff Syndrome
- Traumatic Brain Injury (TBI)
- Know the 10 Signs
- Difference Between Alzheimer's & Dementia
- 10 Steps to Approach Memory Concerns in Others
- Medical Tests for Diagnosing Alzheimer's
- Why Get Checked?
- Visiting Your Doctor
- Life After Diagnosis
- Stages of Alzheimer's
Earlier Diagnosis
- Part the Cloud
- Research Momentum
Our Commitment to Research
- TrialMatch: Find a Clinical Trial
- What Are Clinical Trials?
- How Clinical Trials Work
- When Clinical Trials End
- Why Participate?
- Talk to Your Doctor
- Clinical Trials: Myths vs. Facts
Can Alzheimer's Disease Be Prevented?
Brain donation.
- Navigating Treatment Options
- Aducanumab Discontinued as Alzheimer's Treatment
- Donanemab Approved for Treatment of Early Alzheimer's Disease
- Lecanemab Approved for Treatment of Early Alzheimer's Disease
- Medicare Treatment Coverage
- Questions for Your Doctor
- Medications for Memory, Cognition and Dementia-Related Behaviors
- Treatments for Behavior
- Treatments for Sleep Changes
- Alternative Treatments
- Facts and Figures
- Assessing Symptoms and Seeking Help
- Now is the Best Time to Talk about Alzheimer's Together
- Get Educated
- Just Diagnosed
- Sharing Your Diagnosis
- Changes in Relationships
- If You Live Alone
- Treatments and Research
- Legal Planning
- Financial Planning
- Building a Care Team
- End-of-Life Planning
- Programs and Support
- Overcoming Stigma
- Younger-Onset Alzheimer's
- Taking Care of Yourself
- Reducing Stress
- Tips for Daily Life
- Helping Family and Friends
- Leaving Your Legacy
- Live Well Online Resources
- Make a Difference
- Daily Care Plan
- Communication and Alzheimer's
- Food and Eating
- Art and Music
- Incontinence
- Dressing and Grooming
- Dental Care
- Working With the Doctor
- Medication Safety
- Accepting the Diagnosis
- Early-Stage Caregiving
- Middle-Stage Caregiving
- Late-Stage Caregiving
- Aggression and Anger
- Anxiety and Agitation
- Hallucinations
- Memory Loss and Confusion
- Sleep Issues and Sundowning
- Suspicions and Delusions
- In-Home Care
- Adult Day Centers
- Long-Term Care
- Respite Care
- Hospice Care
- Choosing Care Providers
- Geriatric Care
- Finding a Memory Care-Certified Nursing Home or Assisted Living Community
- Changing Care Providers
- Working with Care Providers
- Creating Your Care Team
- Long-Distance Caregiving
- Community Resource Finder
- Be a Healthy Caregiver
- Caregiver Stress
- Caregiver Stress Check
- Caregiver Depression
- Changes to Your Relationship
- Grief and Loss as Alzheimer's Progresses
- Home Safety
- Dementia and Driving
- Technology 101
- Preparing for Emergencies
- Managing Money Online Program
- Planning for Care Costs
- Paying for Care
- Health Care Appeals for People with Alzheimer's and Other Dementias
- Social Security Disability
- Medicare Part D Benefits
- Tax Deductions and Credits
- Planning Ahead for Legal Matters
- Legal Documents
- ALZ Talks Virtual Events
- ALZNavigator™
- Veterans and Dementia
- The Knight Family Dementia Care Coordination Initiative
- Online Tools
- Asian Americans and Pacific Islanders and Alzheimer's
- Native Americans and Alzheimer's
- Black Americans and Alzheimer's
- Hispanic Americans and Alzheimer's
- LGBTQ+ Community Resources for Dementia
- Educational Programs and Dementia Care Resources
- Brain Facts
- 50 Activities
- For Parents and Teachers
- Resolving Family Conflicts
- Holiday Gift Guide for Caregivers and People Living with Dementia
- Trajectory Report
- Resource Lists
- Search Databases
- Publications
- Favorite Links
- 10 Healthy Habits for Your Brain
- Stay Physically Active
- Adopt a Healthy Diet
- Stay Mentally and Socially Active
- Online Community
- Support Groups
- Find Your Local Chapter
Any Given Moment
New ideas study.
- Bruce T. Lamb, Ph.D., Chair
- Christopher van Dyck, M.D.
- Cynthia Lemere, Ph.D.
- David Knopman, M.D.
- Lee A. Jennings, M.D. MSHS
- Karen Bell, M.D.
- Lea Grinberg, M.D., Ph.D.
- Malú Tansey, Ph.D.
- Mary Sano, Ph.D.
- Oscar Lopez, M.D.
- Suzanne Craft, Ph.D.
- RFI Amyloid PET Depletion Following Treatment
- Criteria for Diagnosis and Staging
- About Our Grants
- Andrew Kiselica, Ph.D., ABPP-CN
- Arjun Masurkar, M.D., Ph.D.
- Benjamin Combs, Ph.D.
- Charles DeCarli, M.D.
- Damian Holsinger, Ph.D.
- David Soleimani-Meigooni, Ph.D.
- Donna M. Wilcock, Ph.D.
- Elizabeth Head, M.A, Ph.D.
- Fan Fan, M.D.
- Fayron Epps, Ph.D., R.N.
- Ganesh Babulal, Ph.D., OTD
- Hui Zheng, Ph.D.
- Jason D. Flatt, Ph.D., MPH
- Jennifer Manly, Ph.D.
- Joanna Jankowsky, Ph.D.
- Luis Medina, Ph.D.
- Marcello D’Amelio, Ph.D.
- Marcia N. Gordon, Ph.D.
- Margaret Pericak-Vance, Ph.D.
- María Llorens-Martín, Ph.D.
- Nancy Hodgson, Ph.D.
- Shana D. Stites, Psy.D., M.A., M.S.
- Walter Swardfager, Ph.D.
- ALZ WW-FNFP Grant
- Capacity Building in International Dementia Research Program
- ISTAART IGPCC
- Alzheimer’s Disease Strategic Fund: Endolysosomal Activity in Alzheimer’s (E2A) Grant Program
- Imaging Research in Alzheimer’s and Other Neurodegenerative Diseases
- Supporting Research in Health Disparities, Policy and Ethics in Alzheimer’s Disease and Dementia Research (HPE-ADRD)
- Zenith Fellow Awards
- Alzheimer's Association Research Fellowship (AARF) I alz.org
- Alzheimer's Association Research Fellowship to Promote Diversity (AARF-D)
- Alzheimer's Association Research Grant (AARG)
- 2024 Part the Cloud Translational (PTC) Gene Targeting Challenge
- National Academy of Neuropsychology & Alzheimer’s Association Funding Opportunity
- Part the Cloud-Gates Partnership Grant Program: Bioenergetics and Inflammation
- Pilot Awards for Global Brain Health Leaders (Invitation Only)
- Robert W. Katzman, M.D., Clinical Research Training Scholarship
- Funded Studies
- How to Apply
- Portfolio Summaries
- Annual Conference: AAIC
- Alzheimer's & Dementia
- Alzheimer's & Dementia: DADM
- Alzheimer's & Dementia: TRCI
- Alzheimer’s & Dementia: Behavior and Socioeconomics of Aging
- Professional Society: ISTAART
- International Network to Study SARS-CoV-2 Impact on Behavior and Cognition
- Alzheimer’s Association Business Consortium (AABC)
- Global Biomarker Standardization Consortium (GBSC)
- Global Alzheimer’s Association Interactive Network
- International Alzheimer's Disease Research Portfolio
- Alzheimer’s Disease Neuroimaging Initiative Private Partner Scientific Board (ADNI-PPSB)
- Research Roundtable
- About WW-ADNI
- North American ADNI
- European ADNI
- Australia ADNI
- Taiwan ADNI
- Argentina ADNI
- WW-ADNI Meetings
- Submit Study
- RFI Inclusive Language Guide
- Scientific Conferences
- AUC for Amyloid and Tau PET Imaging
- Make a Donation
- Walk to End Alzheimer's
- The Longest Day
- RivALZ to End ALZ
- Ride to End ALZ
- Donate Gold & Sterling Silver
- Tribute Pages
- Gift Options to Meet Your Goals
- Founders Society
- Fred Bernhardt
- Anjanette Kichline
- Lori A. Jacobson
- Pam and Bill Russell
- Gina Adelman
- Franz and Christa Boetsch
- Adrienne Edelstein
- For Professional Advisors
- Free Planning Guides
- Contact the Planned Giving Staff
- Workplace Giving
- Do Good to End ALZ
- Donate a Vehicle
- Donate Stock
- Donate Cryptocurrency
- Donor-Advised Funds
- Use of Funds
- Giving Societies
- Why We Advocate
- Ambassador Program
- About the Alzheimer’s Impact Movement
Research Funding
- Improving Care
- Support for People Living With Dementia
- Public Policy Victories
- Planned Giving
- Community Educator
- Community Representative
- Support Group Facilitator or Mentor
- Faith Outreach Representative
- Early Stage Social Engagement Leaders
- Data Entry Volunteer
- Tech Support Volunteer
- Other Local Opportunities
- Visit the Program Volunteer Community to Learn More
- Become a Corporate Partner
- A Family Affair
- A Message from Elizabeth
- The Belin Family
- The Eliashar Family
- The Fremont Family
- The Freund Family
- Jeff and Randi Gillman
- Harold Matzner
- The Mendelson Family
- Patty and Arthur Newman
- The Ozer Family
- Salon Series
- No Shave November
- Other Philanthropic Activities
- Still Alice
- The Judy Fund E-blast Archive
- The Judy Fund in the News
- The Judy Fund Newsletter Archives
- Sigma Kappa Foundation
- Alpha Delta Kappa
- Parrot Heads in Paradise
- Tau Kappa Epsilon (TKE)
- Sigma Alpha Mu
- Alois Society Member Levels and Benefits
- Alois Society Member Resources
- Zenith Society
- Founder's Society
- Joel Berman
- JR and Emily Paterakis
- Legal Industry Leadership Council
- Accounting Industry Leadership Council

Find Local Resources
Let us connect you to professionals and support options near you. Please select an option below:
Use Current Location Use Map Selector
Search Alzheimer’s Association

Double Your Impact for World Alzheimer's Day
Invest in big, bold ideas with part the cloud.
Be part of an important effort to supercharge the development of genetic-based Alzheimer's therapies. Join the Gene Targeting Challenge.
Research and Progress
This is a time of unprecedented promise in the quest to end alzheimer’s..
Today, we are growing philanthropic support for Alzheimer's research, fostering a dynamic community of Alzheimer's scientists and securing increased federal funding for research – all of which are instrumental to finding new treatments to stop, slow and prevent Alzheimer’s disease.
Promising Research and Treatments
In the century since Alzheimer’s was first described, scientists have made remarkable strides in understanding the disease.
What if we could diagnose Alzheimer's before symptoms started? Research around earlier diagnosis is among the most active areas in Alzheimer's science.
While Alzheimer's prevention has no definitive answers yet, research has shown that we can take action to reduce our risk of developing it.
Play a Role in Progress
A donation today supports vital research toward methods of treatment, prevention and, ultimately, a cure.
Learn how to donate your brain and help impact Alzheimer's research. Both healthy people and those with dementia can donate.
Clinical Trials
Don’t just hope for a cure, help us find one. Participating in a clinical trial, whether you’re living with the disease or not, can make a big difference.
The New IDEAS Study is designed to evaluate the use of PET scans in diverse populations living with dementia and Alzheimer's.
U.S. POINTER
U.S. POINTER is a lifestyle intervention clinical trial to support brain health and prevent cognitive decline. Learn about study locations and eligibility.
Advancing Research Around the Globe
The Alzheimer's Association is the world's largest nonprofit funder of Alzheimer's research, currently investing more than $430 million in over 1,110 active best-of-field projects in 56 countries spanning six continents.
Our commitment to accelerating the global effort to eliminate Alzheimer's disease and other dementias is at the core of all we do.
Through our many initiatives and worldwide reach, the Alzheimer’s Association leads the charge in Alzheimer’s care, support, research and advocacy.
Discover how the Alzheimer’s Association connects scientists and fuels diverse research in an effort to change the trajectory of the disease.
The Alzheimer’s Association is an unrelenting advocate for public policies that increase critical research and support for all those affected.
Keep Up With Alzheimer’s News and Events
The first survivor of alzheimer's is out there, but we won't get there without you., learn how alzheimer’s disease affects the brain..
Take the Brain Tour
Don't just hope for a cure. Help us find one.

Focus on Alzheimer's Disease and Related Dementias
Alzheimer's Disease-Related Dementias Summit 2025
Save the Date: March 25-26, 2025
For the most current updates on NINDS activities related to ADRD, including upcoming activities, and research opportunities sign up for our listserv.

What is AD/ADRD?
Alzheimer's Disease and Alzheimer's Disease Related Dementias (AD/ADRD) refers to the most common forms of dementia. Dementia likely affects more than 6 million people in the U.S. and more than 55 million people worldwide . Currently, there are no known treatments to prevent or stop the progression of dementia. The toll on individuals, caregivers and society is enormous and will increase as the population ages unless effective interventions are developed. NINDS collaborates with NIH ’s National Institute on Aging ( NIA ), the lead NIH Institute for Alzheimer’s disease (AD) research and for NIH's response to the National Plan to Address Alzheimer’s Disease , to establish research priorities and fund biomedical research to decrease the burden of dementia on individuals, families, and communities.
While AD is the most common dementia diagnosis, ADRDs share many cognitive and pathological features with and can be difficult to distinguish from AD. In fact, more often than not, patients with a clinical diagnosis of Alzheimer’s disease have different mixtures of brain pathologies, complicating both the diagnosis, as well as treatment. A special video testimonial to raise awareness of the disease burden on patients with AD/ADRD and their caregivers titled “Voices of AD/ADRD” was presented at the NINDS ADRD Summit 2022 , and can be viewed here: Voices of AD/ADRD
In the National Plan to Address Alzheimer’s Disease , ADRDs include:
- Frontotemporal degeneration (FTD)
- Lewy body dementia (LBD)
- Vascular contributions to cognitive impairment and dementia (VCID)
- Multiple-Etiology Dementias (MED)
NINDS and NIA continue to partner in AD/ADRD research planning and implementation, and we urge the research community to join in our efforts to accelerate scientific progress toward reducing the enormous burden and cost of dementia.
No RFA is needed to apply!! NINDS special AD/ADRD payline applies to investigator-Initiated research applications to the NIH Parent R01 and the NINDS R21 ( PA-21-219 )
NINDS AD/ADRD Payline Information
Funding Opportunity Announcements
Currently active , basic disease mechanisms.
Interaction Between Environmental Factors and Lewy Body Dementia (R01 - Clinical Trial Not Allowed) (PAR-24-249) Due dates: October 04, 2024 Contact PO: David Jett
Mechanistic Investigations into ADRD Associated Protein Structures in Biological Settings (R01 - Clinical Trial Not Allowed) (PAR-24-234) Due dates: October 04, 2024 Contact PO : George Umanah
Investigating Distinct and Overlapping Mechanisms in TDP-43 Proteinopathies, including in LATE, FTD, and other ADRDs (R01 - Clinical Trial Not Allowed) (PAR-24-148) Due dates: June 04, 2024 and October 04, 2024 ; Contact PO: Linda McGavern
Mechanistic Investigations into ADRD Multiple Etiology Dementias (R01 - Clinical Trial Not Allowed) (PAR-24-147) Due dates: June 04, 2024 and October 04, 2024 ; Contact PO: Linda McGavern
Mechanistic and Hemodynamic Basis of Diffuse White Matter Disease in Vascular Contributions to Cognitive Impairment and Dementia (VCID) (R01 - Clinical Trial Not Allowed) (PAR-24-196) Due date: October 04, 2024 ; Contact PO: Roderick Corriveau
Protective Strategies to Reduce Amyloid Related Imaging Abnormalities (ARIA) after anti-Aβ immunotherapy (R01 - Clinical Trial Not Allowed) (PAR-24-198) Due date: October 04, 2024 ; Contact PO: Francesca Bosetti
Neuropathological Interactions Between COVID-19 and ADRD (R01 - Clinical Trial Not Allowed) (PAR-24-203) Due date: October 04, 2024 ; Contact PO: William P. Daley
ADRD Risk and Disease Following Nervous System Exposures at Biological Interfaces with the Environment (R01 - Clinical Trial Not Allowed) (PAR-24-270) Due date: November 19, 2024 ; Contact PO: David Jett
Clinical Trial Readiness to Understand and Develop Solutions to Social, Ethical, Behavioral Implications and Barriers to Health Equity in ADRD (R01 - Clinical Trial Not Allowed) (RFA-NS-25-013) Due date: October 04, 2024 ; Contact POs: Richard T. Benson, Rebecca Hommer and Carolina Mendoza-Puccini Email: [email protected]
Safety and Efficacy of Amyloid-Beta Directed Antibody Therapy in Mild Cognitive Impairment and Dementia with Evidence of Lewy Body Dementia and Amyloid-Beta Pathology (U01 - Clinical Trial Required) (RFA-NS-25-010) Due date: January 24, 2025 ; Contact PO: Rebecca Hommer
Notice of Special Interest (NOSI): Administrative Supplement Program to Add Fluid-based Biomarkers and APOE Genotyping to NINDS ADRD Human Subjects Research Grants (NOT-NS-24-109) Expiration date: November 12, 2024 ; Contact PO: Amber McCartney
NINDS Alzheimer’s Disease-Related Dementias (ADRD) Advanced Postdoctoral Career Transition Award (K99/R00 Independent Clinical Trial Not Allowed) (PAR-24-213) Due date: NIH Standard due dates ; Contact PO: Amber McCartney
NINDS Alzheimer’s Disease-Related Dementias (ADRD) Advanced Postdoctoral Career Transition Award to Promote Diversity (K99/R00 Independent Clinical Trial Not Allowed) (PAR-24-212) Due date: NIH Standard due dates ; Contact PO: Amelie Gubitz, and Lauren Ullrich Email: [email protected]
Notice of Special Interest (NOSI): Administrative Supplements to Promote Diversity for NINDS ADRD Awardees (NOT-NS-24-071) Expiration date: February 15, 2027 ; Contact PO: Amber McCartney
Translational
Functional Target Validation for Alzheimer's Disease-Related Dementias (R61/R33 Clinical Trial Not Allowed) (RFA-NS-25-011) Due date: November 08, 2024 ; Contact PO: Pascal Laeng
Optimization of Genome Editing Therapeutics for Alzheimer's Disease-Related Dementias (ADRD) (U01 - Clinical Trials Not Allowed) (RFA-NS-24-037) Due date: November 19, 2024 ; Contact PO: Timothy LaVaute
Advancing Research on Alzheimer's Disease (AD) and AD-Related Dementias (ADRD) (Small Business Innovation Research; R43/R44 Clinical Trial Optional) (PAR-22-196) Due date: NIH Standard due dates ; Contact PO: Annette Gilchrist
Advancing Research on Alzheimer's Disease (AD) and AD-Related Dementias (ADRD) (Small Business Technology Transfer; R41/R42 Clinical Trial Optional) (PAR-22-197) Due date: NIH Standard due dates ; Contact PO: Annette Gilchrist
ADRD Models
Development and Validation of Human Cellular Models for Alzheimer's Disease-Related Dementias (ADRD) (R01 - Clinical Trial Not Allowed) (RFA-NS-24-032) Due dates: June 20, 2024 and October 21, 2024 ; Contact POs: Linda McGavern and Frank Shewmaker
Planned Initiatives
We are excited to share with you the research concepts approved by NINDS Council. Please note that this page will be periodically updated with links when funding opportunities are published. These are also announced via our email listserv (to join the NINDS AD/ADRD listserv, please email [email protected] ).
Please note that an approved concept listed below does not necessarily indicate an award mechanism or funding allocation is imminent or will happen. The NOFO is only official when published in the NIH Guide .
Integrative Multiomics Profiling for Lewy Body Dementia- This initiative is designed to support research to conduct genetic and molecular characterization of LBD. This may include whole genome sequencing, longitudinal bulk-transcriptomics, longitudinal matched plasma, and cerebrospinal fluid (CSF) proteomic analysis, and post-mortem single-cell RNA sequencing, which will be generated and paired with harmonized longitudinal clinical data. Contact PO: Christine Swanson-Fischer
IND -enabling Studies and Clinical Trials for Genome Editing Therapeutics for Alzheimer's Disease and Alzheimer's Disease-Related Dementias - This initiative support Investigational New Drug (IND) enabling studies for the preparation and submission of an IND for a genome editing therapeutics for ADRD, and optional small delayed-onset first in human Phase I clinical trial. Contact PO: Timothy LaVaute
Recently Closed Funding Opportunities
VCID Center Without Walls for Understanding and Leveraging Small Vessel Cerebrovascular Disease Mechanisms in ADRD (R01 - Clinical Trial Not Allowed) (RFA-NS-24-027)
Blood Brain Barrier Response to Antibodies Targeting Beta-Amyloid (R01 - Clinical Trial Not Allowed) (PAR-23-140)
Role of Environmental Stress in the Health Inequities of Alzheimer's Disease-Related Dementias (ADRD) (R01 - Clinical Trial Not Allowed) (RFA-NS-24-024)
Mechanistic Investigations into ADRD Multiple Etiology Dementias (R01 - Clinical Trial Not Allowed) (PAR-23-211)
Investigating Distinct and Overlapping Mechanisms in TDP-43 Proteinopathies, including in LATE, FTD, and other ADRDs (R01 - Clinical Trial Not Allowed) (PAR-23-212)
Neuropathological Interactions Between COVID-19 and ADRD (R01 - Clinical Trial Not Allowed) (PAR-23-214)
Mechanisms of Cognitive Fluctuations in ADRD Populations (R01 - Clinical Trial Optional) (RFA-NS-25-014)
Efficacy and Safety of Amyloid-Beta Directed Antibody Therapy in Mild Cognitive Impairment and Dementia with Evidence of Both Amyloid-Beta and Vascular Pathology (U01 - Clinical Trial Required) (RFA-NS-24-013)
Tools and Resources to Understand the Vascular Pathophysiology of in vivo Neuroimaging Findings in ARIA (U24 - Clinical Trials Not Allowed) (RFA-NS-24-034) Using Multimodal Biomarkers to Differentially Diagnose ADRDs for Clinical Trials (U19 Clinical Trial Optional) (RFA-NS-24-001)
Validating digital health technologies for monitoring biomarkers in ADRD clinical trials (R61/R33 - Clinical Trials Optional) (RFA-NS-24-026)
Assessment of TBI-related ADRD Pathology Related to Cognitive Impairment and Dementia Outcomes (U01 - Clinical Trial Not Allowed) (RFA-NS-24-003)
Administrative Supplements to Promote Diversity for NINDS AD/ADRD Awardees (NOT-NS-21-047)
NINDS Institutional AD/ADRD Research Training Program (T32 Clinical Trial Not Allowed) (PAR-23-113)
Simultaneous and Synergistic Multi-Target Validation for Alzheimer’s Disease-Related Dementias (R61/R33 Clinical Trial not allowed) (PAR-23-195)
Early-Stage Therapy Development for Alzheimer's Disease-Related Dementias (ADRD) (R61/R33 - Clinical Trial Not Allowed) (RFA-NS-24-010)
Optimization of Genome Editing Therapeutics for Alzheimer's Disease-Related Dementias (ADRD) (U01 - Clinical Trials Not Allowed) (RFA-NS-24-009)
Center without Walls for PET Ligand Development for Alzheimer's disease-related dementias (ADRDs) (U19 - Clinical Trial Optional) (RFA-NS-19-014)
Development & Characterization of Experimental models of post-TBI ADRD (R01 - Clinical Trial Not Allowed) (PAR-23-218)
Development and Validation of Models for ADRD (R61/R33 - Clinical Trial Not Allowed) (PAR-23-154)
NINDS Leads ADRD Research Priority-Setting Planning Efforts
NINDS-led ADRD summits represent a continuous decade long planning effort. ADRD summits occur every three years and respond to the National Plan to Address Alzheimer’s Disease (“National Plan”) that was released in 2012 and updated annually. These Summits set national research recommendations with timelines that reflect critical scientific priorities for research on ADRD. During each ADRD Summit planning process, the established prioritized recommendations are updated, and developed further, under the leadership of the ADRD Summit Steering Committee, which includes a Working Group of the NANDS Council. The Committee solicits input from nationally and internationally recognized dementia-science experts, as well as public and private stakeholders. The resulting recommendations guide ADRD research for the next several years. Links to NANDS Council-approved ADRD Summit Reports are provided below.
ADRD Summit 2022 FACA Report; (pdf, 3804 KB) ADRD Summit 2022 Research Milestones and Success Criteria (pdf, 512 KB)
ADRD Summit 2019 FACA Report (pdf, 2131 KB) ; ADRD Summit 2019 Research Milestones and Success Criteria
ADRD Summit 2016 FACA Report (pdf, 922 KB) ; ADRD Summit 2016 Research Milestones and Success Criteria ; Proceedings Article
ADRD Summit 2013 FACA Report (pdf, 980 KB) ; ADRD Summit 2013 Research Milestones and Success Criteria ; Proceedings Article
Rod Corriveau | Program Director and ADRD Lead [email protected]
Sara Dodson | Senior Health Science Policy Analyst [email protected]
Erin Bryant | Office of Neuroscience Communications & Engagement (ONCE) [email protected]
Amber McCartney | Scientific Project Manager [email protected]
Arvind Shukla | Health Program Specialist [email protected]
Herson Rene Astacio Cuevas | Health Program Specialist [email protected]
Kiara Bates | Program Specialist [email protected]
AD+ADRD Research Implementation Milestones database The AD+ADRD Research Implementation Milestones database is a research framework detailing specific steps and success criteria towards achieving the goal of the National Plan to Address Alzheimer's Disease : to treat and prevent AD and ADRDs by 2025. This database includes research milestones and responsive activities for the NIH triennial AD, ADRD, and Dementia Care, Services and Supports research summits.
International Alzheimer's and Related Dementias Research Portfolio IADRP reports categories of funded research supported by public and private organizations both in the US and abroad all categorized using the Common Alzheimer's and Related Dementias Research Ontology (CADRO)
NIH Estimates of Funding for Various Research, Condition, and Disease Categories
| Research/Disease Areas* | FY 2020 | FY 2021 | FY 2022 | FY 2023 (Estimated) | FY 2024 (Estimated) |
|---|---|---|---|---|---|
| Alzheimer's Disease Including Alzheimer's Disease Related Dementias (AD/ADRD) | $2,869 | $3,251 | $3,514 | $3,749 | $3,767 |
| Alzheimer's Disease | $2,683 | $3,059 | $3,314 | $3,502 | $3,515 |
| Frontotemporal Dementia | $166 | $164 | $169 | $174 | $177 |
| Lewy Body Dementia | $84 | $113 | $118 | $124 | $124 |
| Vascular Contributions to Cognitive Impairment and Dementia | $362 | $455 | $445 | $461 | $464 |
*Dollars in millions and rounded To learn more about NIH Investment in AD/ADRD, please visit the Categorical Spending site and enter "Alzheimer's Disease Including Alzheimer's Disease Related Dementias".

An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.

News & Events
Read the latest scientific news and updates on Alzheimer’s disease and related dementias.

alzheimers.gov
An official website of the U.S. government, managed by the National Institute on Aging at the National Institutes of Health

- Health A to Z
- Alternative Medicine
- Blood Disorders
- Bone and Joint
- Cardiovascular Diseases
- Child Health
- Coronavirus
- Dental Care
- Digestive System
- Disabilities
- Drug Center
- Ear, Nose, and Throat
- Environmental Health
- Exercise And Fitness
- First Aid and Emergencies
- General Health
- Health Technology
- Hearing Loss
- Hypertension
- Infectious Disease
- LGBTQ Health
- Liver Health
- Men's Health
- Mental Health
- Pain Management
- Public Health
- Pulmonology
- Senior Health
- Sexual Health
- Skin Health
- Women's Health
- News For Medical Professionals
- Our Products
- Consumer News
- Physician's Briefing
- HealthDay TV
- Wellness Library
- HealthDay Living
- Conference Coverage
- Custom Products
- Health Writing
- Health Editing
- Video Production
- Medical Review
Could 'Brain Training' Exercises Help Slow Alzheimer's Symptoms?

Key Takeaways
Memory training can help seniors fight thinking declines
Seniors who got memory training had less decline than those who didn’t
Protection from the training lasted at least five years
TUESDAY, Sept. 17, 2024 (HealthDay News) -- Brain training aimed at improving memory can ward off symptoms of Alzheimer’s disease for years, a new study claims.
Seniors experienced a slower decline in their memory and thinking abilities after undergoing brain training, compared to others who didn’t get the training, researchers found.
This benefit persisted for five years after the seniors got the brain training, results show.
"These results are important because this kind of intervention is non-pharmacological -- there are no drugs involved -- and can have a significant impact on the lives of those affected,” said lead researcher Sylvie Belleville , research chair in cognitive neuroscience of aging and brain plasticity at the University of Montreal.
For the study, 145 seniors with mild cognitive impairment were recruited from memory clinics in Montreal and Quebec City between 2012 and 2015.
One-third of the seniors were randomly assigned to receive training in memory strategies. They worked on things like memorizing the names of people, remembering lists of items or tasks and focusing their attention to better memorize.
Another third underwent training to help their overall psychological well-being, such as techniques in anger management and problem-solving. The final third received no training at all.
The initial results “showed that early intervention can improve cognitive function in people at risk of Alzheimer's disease,” Belleville said in a university news release. “We had also observed cerebral changes showing these people had compensated mentally for their memory loss.”
In the latest paper, published Sept. 12 in the journal Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring , researchers recontacted study participants five years after that experiment, to see if the benefits held over time without repeated training.
The research team “still observed that the benefits on memory and that a measure of dementia had not changed at all in the treated group, whereas there was a decline in the untreated group,” Belleville said.
Those with memory training had less memory decline, and they also scored better on a screening test for cognitive ability, results showed.
The findings "underscore the potential of cognitive training as a preventive approach for cognitively vulnerable older adults, reducing cognitive decline and potentially delaying the onset of dementia," the study concluded.
"Furthermore, it is noteworthy that these enduring effects were achieved through a relatively brief, cost-effective intervention that can be readily implemented as a preventive measure for at-risk individuals,” researchers added.
More information
Harvard Medical School has more on training the brain .
SOURCE: University of Montreal, news release, Sept. 12, 2024
What This Means For You
Seniors should talk with their doctor about potential ways to train their brain to improve their memory and thinking abilities.
Related Stories


An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Advances in Alzheimer's Disease & Related Dementias Research
NIA is the primary Federal agency supporting and conducting Alzheimer’s disease research. The Institute also supports much work on cognitive health and related dementias. Below is a listing of some of the most significant NIA-supported research findings about cognitive health, Alzheimer’s disease, and related dementias from the last ten years. Advances such as these continue to push researchers ever closer to one day discovering how we may effectively prevent and treat dementia.
| Advance | Description of research finding | Link to publication |
|---|---|---|
| Training on specific reasoning and speed of processing tasks—two key indicators of cognition—can improve performance on those tasks under controlled conditions. Benefits on reasoning were shown to last at least five years, while benefits on speed of processing persisted for up to ten years. | (Jan. 2014) | |
| The REACH II (Resources for Enhancing Alzheimer’s Caregiver Health) study found the first effective support intervention to improve the health and well-being of Alzheimer’s caregivers in an ethnically-diverse population. The intervention is currently being translated through the Veterans Administration, with participating centers in fifteen states. | (May 2017) (June 2016) | |
| Recent NIA-supported research has greatly advanced the ability to detect changes that can occur years, even decades, before the first symptoms of Alzheimer’s and related dementias appear. For example, researchers are now able to image both beta amyloid and tau in living humans and can detect changes in these factors before symptom onset. Researchers have also characterized changes in the sense of smell as an early indication of cognitive impairment. | (April 2017) (May 2015) | |
| Researchers have identified more than 25 additional genes involved in Alzheimer’s disease and what role they may play. Discovering these pathways will help researchers identify possible targets for drug and nondrug interventions to stop or prevent the disease. For example, a number of genes involved in inflammation have recently been associated with Alzheimer’s and may serve as therapeutic targets in the future. | (Jan. 2013) |
Advancing Research Through Collaborations
One way NIA supports Alzheimer’s research is by collaborating with external groups, including other federal agencies, biopharmaceutical companies, and non-profits. Find a listing of some of the largest collaborations below.
| Collaboration | Description of collaboration | Link to publication |
|---|---|---|
| ADNI is a public-private partnership established to develop a multi-site longitudinal, prospective, naturalistic study of normal cognitive aging, mild cognitive impairment, and early Alzheimer’s disease. Now in its 13 year, ADNI continues to develop and integrate new technologies to achieve these goals. For example, research from ADNI led to the development of methods for early detection of Alzheimer’s. |
(Jan. 2014) | |
| AMP is a bold venture between NIH, ten biopharmaceutical companies, and multiple non-profit organizations to transform the current model for developing new diagnostics and treatments by jointly identifying and validating promising biological targets of disease. AMP AD is particularly focused on developing new diagnostics and therapies for Alzheimer’s disease. The program seeks to shorten the time between the discovery of potential new drug targets and the development of new drugs for Alzheimer’s treatment and prevention. AMP AD integrates analysis of large-scale molecular data from human brain samples with network modeling approaches and experimental validation while enabling rapid, broad sharing of data and analytical tools across the entire research community. | ||
| API is an international effort to help identify pre-symptomatic treatments or interventions that will postpone, slow, or prevent Alzheimer’s disease progression. This focus on prevention launched a new approach to Alzheimer’s research by evaluating the most promising therapies at the earliest possible stage of the disease process in cognitively normal people who, based on age and genetic background, are at the highest risk of developing Alzheimer’s symptoms. The goal of API is to identify pre-symptomatic treatments or interventions that will postpone, slow, or prevent disease progression. |
Last updated: January 31, 2024
nia.nih.gov
An official website of the National Institutes of Health
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Dementia articles from across Nature Portfolio
Dementia is a syndrome that involves severe loss of cognitive abilities as a result of disease or injury. Dementia caused by traumatic brain injury is often static, whereas dementia caused by neurodegenerative disorders, such as Alzheimer's disease, is usually progressive and can eventually be fatal.

The planetary health diet is associated with slower cognitive decline — but tied to income
Income is a modifying factor in the association between increased adherence to the planetary health diet and slower cognitive decline observed in a sample of 11,737 Brazilian civil servants who were followed for 8 years. Thus, addressing the barriers posed by low income is vital when promoting healthy eating patterns.
Related Subjects
- Alzheimer's disease
Latest Research and Reviews
Synergistic association of Aβ and tau pathology with cortical neurophysiology and cognitive decline in asymptomatic older adults
Gallego-Rudolf et al. report accelerated brain activity with initial amyloid-β deposition in asymptomatic individuals. In those where tau also starts accumulating, brain activity decelerates, correlating with subsequent cognitive decline.
- Jonathan Gallego-Rudolf
- Alex I. Wiesman
- Sylvain Baillet
Heteromeric amyloid filaments of ANXA11 and TDP-43 in FTLD-TDP Type C
- Diana Arseni
- Takashi Nonaka
- Benjamin Ryskeldi-Falcon
Digital speech hearing screening using a quick novel mobile hearing impairment assessment: an observational correlation study
- Russell Banks
- Barry R. Greene
- Alvaro Pascual-Leone
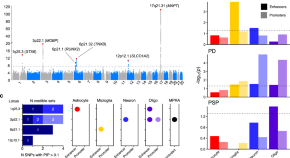
Genetic, transcriptomic, histological, and biochemical analysis of progressive supranuclear palsy implicates glial activation and novel risk genes
The authors present the largest genome-wide association study to date for a rare Parkinsonian disorder, progressive supranuclear palsy (PSP). They include follow-up investigations of the identified susceptibility loci, functional consequences, and cell-specific pathologies, providing insights into genetic and molecular mechanisms underlying PSP.
- Kurt Farrell
- Jack Humphrey
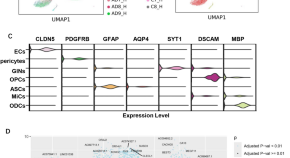
Microvascular and cellular dysfunctions in Alzheimer’s disease: an integrative analysis perspective
- Xiaoyan Hao
- Changhe Shi

Glucose metabolism and smaller hippocampal volume in elderly people with normal cognitive function
- Ayano Shima
- Moeko Noguchi-Shinohara
- Yutaka Kiyohara
News and Comment
Preparing for disease-modifying dementia therapies in the uk.
Although lecanemab has been licensed for use in the UK, the systems to deliver this or similar disease-modifying therapies do not exist. These systems need to be developed urgently, but not at the expense of post-diagnostic care.
- Claudia Cooper
- Charles R. Marshall
- Sube Banerjee
Blood profile indicates central inflammation in frontotemporal lobar degeneration
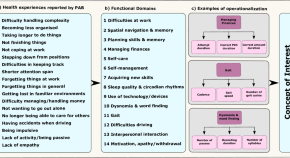
Regulatory considerations for developing remote measurement technologies for Alzheimer’s disease research
The Remote Assessment of Disease and Relapse – Alzheimer’s Disease (RADAR-AD) consortium evaluated remote measurement technologies (RMTs) for assessing functional status in AD. The consortium engaged with the European Medicines Agency (EMA) to obtain feedback on identification of meaningful functional domains, selection of RMTs and clinical study design to assess the feasibility of using RMTs in AD clinical studies. We summarized the feedback and the lessons learned to guide future projects.
- Gül Erdemli
- Margarita Grammatikopoulou
- Anna-Katharine Brem

Proteomic aging signatures predict disease risk and mortality across diverse populations
In a large human population study of proteomic aging, we developed a proteomics-based age clock for UK Biobank participants and validated its accuracy in the China Kadoorie Biobank and FinnGen. Proteomic aging is associated with mortality, risk of 18 chronic diseases and numerous age-related traits, including cognitive function.
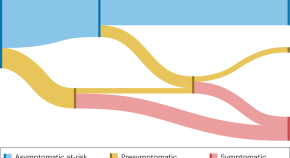
Disentangling clinical and biological trajectories of neurodegenerative diseases
In recent years, we have seen a shift towards defining sporadic neurodegenerative diseases as a biological continuum. Here, we discuss the risks associated with this shift, emphasize the importance of maintaining a strong connection between disease definitions and subsequent clinical outcomes, and suggest clinicobiological frameworks to disentangle multiple discrete nosological entities.
- Nicolas Villain
- Vincent Planche
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
COMMENTS
Alzheimer's disease articles from across Nature Portfolio. Alzheimer's disease is a progressive neurodegenerative disease that impairs memory and cognitive judgment and is often accompanied by ...
Previous research to molecularly diagnose Alzheimer's disease yielded "A/T/N" central biomarkers based on the measurements of proteins, β-amyloid ("A") and tau ("T"), and "N" encompassing ...
Beyond Alzheimer's disease, time-saved models could be applied to other progressive conditions, including Parkinson's disease and amyotrophic lateral sclerosis (ALS). ... Medical research ...
1. Introduction. Alzheimer's disease (AD) is a polygenic and multifactorial disease characterized by the deposition of amyloid-β (Aβ) fibrils in the brain, leading to the formation of plaques and neurofibrillary tangles (NFTs), and ultimately resulting in dendritic dysfunction, neuronal cell death, memory loss, behavioral changes, and organ shutdown [1,2,3,4,5].
NIH has released Advancing Alzheimer's Disease and Related Dementias Research for All Populations: Prevent.Diagnose. Treat. Care. (PDF, 17M), a 2022 scientific progress report. The report features science advances and related efforts made between March 2021 and early 2022 in areas including drug development, lifestyle interventions, biomarker research, and more.
Abstract. An estimated 50 million people worldwide have dementia, mostly due to Alzheimer's disease. The inexorable progression of Alzheimer's disease exerts a huge toll on patients, families ...
A number of disease-modifying therapeutics in current AD clinical trials are attempting to target ... Hope Center for Neurological Disorders, Knight Alzheimer's Disease Research Center ...
NIH has released Advancing Alzheimer's Disease and Related Dementias Research for All Populations: Prevent.Diagnose. Treat. Care (PDF, 17.5M), a 2022 scientific progress report. This report provides a comprehensive overview of the meaningful progress researchers made from April 2021 through March 2022 to address the enormous challenges of Alzheimer's and related dementia diseases.
Previous research to molecularly diagnose Alzheimer's disease yielded "A/T/N" central biomarkers based on the measurements of proteins, β-amyloid ("A") and tau ("T"), and "N" encompassing neurodegeneration. A/T/N can be measured in brain tissue, by in vivo brain imaging techniques, and by analysis of cerebrospinal fluid and plasma.
Our discovery is important because, unlike the current A/T/N biomarkers, ... PsyD, Raymond C. Beeler Professor of Radiology and director of the Center for Neuroimaging and the Indiana Alzheimer's Disease Research Center at the IU School of Medicine. The work was funded by the National Institutes of Health's National Institute on Aging ...
NIH has released Advancing Alzheimer's Disease and Related Dementias Research for All Populations: Prevent. Diagnose. Treat. Care. (PDF, 17M), a 2022 scientific progress report. The report features science advances and related efforts made between March 2021 and early 2022 in areas including drug development, lifestyle interventions ...
The Journal of Alzheimer's Disease is an international multidisciplinary journal to facilitate progress in understanding the etiology, ... including the latest journal articles, special issues, and related books and digital library content. ... Sage Research Methods Supercharging research opens in new tab;
Researchers say we appear to be at the start of a new era for Alzheimer's treatment. Trial results published in January showed that for the first time a drug has been able to slow the cognitive decline characteristic of the disease. The drug, lecanemab, is a monoclonal antibody that works by binding to a key protein linked to the malady ...
Introduction. Alzheimer disease (AD) is one of the greatest medical care challenges of our century and is the main cause of dementia. In total, 40 million people are estimated to suffer from dementia throughout the world, and this number is supposed to become twice as much every 20 years, until approximately 2050. 1 Because dementia occurs mostly in people older than 60 years, the growing ...
Seven recent papers amplify advances in Alzheimer's research. New findings from big-data and open-science research are revealing clues about the molecular mechanisms of Alzheimer's disease and new ways to discover potential therapeutic targets and biomarkers. These new discoveries were made by six research teams participating in the ...
The first survivor of Alzheimer's is out there, but we won't get there without you. Donate Now. Learn how Alzheimer's disease affects the brain. Take the Brain Tour. Don't just hope for a cure. Help us find one. Learn More. Home Office. 225 N. Michigan Ave. Floor 17 Chicago, IL 60601.
The Artificial Intelligence for Alzheimer's Disease Consortium (AI for AD), spearheaded by Thompson, is leading this transformative journey. ... One of the most promising aspects of the current research is its potential to personalize treatment for Alzheimer's patients. By integrating various types of data, AI can help determine the most ...
Dementia is a general term that refers to memory loss and decline of other cognitive abilities that limit independence in day-to-day function. Alzheimer's is the most common brain disease that causes dementia among older adults, accounting for 60%-80% of cases. It affects an estimated one in nine people age 65 and older — 6.2 million ...
What thrilled Sperling, who won the award for her work on clinical trials of Alzheimer's treatments, was a sense of hope, which has been conspicuously missing from research into the disease for ...
It also has several common variants (ApoE2, ApoE3 and ApoE4), of which ApoE4 increases the risk for Alzheimer's disease up to 12-fold. ApoE4 also increases the risk of damage to the white matter that underlies vascular dementia, the second-most common cause of cognitive impairment after Alzheimer's disease.
Jan. 24, 2023. Lori Weiss, 65, a retired teacher, has early onset Alzheimer's disease. She is enrolled in a drug clinical trial and is hopeful about recent progress in disease research. Courtesy of Lori Weiss. A few years ago, Lori Weiss, a high school math and engineering teacher, noticed it was taking her longer to do her lesson plans and ...
Current medicines for heart disease risk factors. ... the 1990s suggested that taking hormone replacement therapy during perimenopause and menopause lowered the risk of Alzheimer's disease. But further research has been mixed. Some studies found no cognitive benefit of taking hormone replacement therapy. More research and a better understanding ...
Research and Progress This is a time of unprecedented promise in the quest to end Alzheimer's. Today, we are growing philanthropic support for Alzheimer's research, fostering a dynamic community of Alzheimer's scientists and securing increased federal funding for research - all of which are instrumental to finding new treatments to stop, slow and prevent Alzheimer's disease.
NIA is currently supporting over 400 active clinical trials on Alzheimer's disease and dementia in many areas of research. See the comprehensive list. ... Closed-loop transcranial Alternating Current Stimulation (tACS) at 40 Hz to modulate brain oscillations and cognition, as an individualized and potential disease-modifying precision therapy ...
Alzheimer's Disease and Alzheimer's Disease Related Dementias (AD/ADRD) refers to the most common forms of dementia. Dementia likely affects more than 6 million people in the U.S. and more than 55 million people worldwide. Currently, there are no known treatments to prevent or stop the progression of dementia.
Read the latest scientific news and updates on Alzheimer's disease and related dementias. Read the latest scientific news and updates on Alzheimer's disease and related dementias. ... During the Aug. 5 meeting of the Advisory Council on Alzheimer's Research, Care, and Services, NIA Director Richard J. Hodes, M.D., unveiled NIH's FY 2026 ...
In the latest paper, published Sept. 12 in the journal Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring, researchers recontacted study participants five years after that experiment, to see if the benefits held over time without repeated training.
ADNI is a public-private partnership established to develop a multi-site longitudinal, prospective, naturalistic study of normal cognitive aging, mild cognitive impairment, and early Alzheimer's disease. Now in its 13 th year, ADNI continues to develop and integrate new technologies to achieve these goals. For example, research from ADNI led ...
Dementia is a syndrome that involves severe loss of cognitive abilities as a result of disease or injury. Dementia caused by traumatic brain injury is often static, whereas dementia caused by ...