Stem Cell Research & Therapy
Journal Abbreviation: STEM CELL RES THER Journal ISSN: 1757-6512
| Year | Impact Factor (IF) | Total Articles | Total Cites |
| 2023 (2024 update) | 7.1 | - | - |
| 2022 | - | - | |
| 2021 | 8.079 | - | 19072 |
| 2020 | 6.832 | 508 | 13356 |
| 2019 | 5.116 | 397 | 8268 |
| 2018 | 4.627 | 345 | 6132 |
| 2017 | 4.963 | 286 | 4578 |
| 2016 | 4.211 | 183 | 3159 |
| 2015 | 4.504 | 245 | 1970 |
| 2014 | 3.368 | 125 | 1153 |
| 2013 | 4.634 | 147 | 738 |
| 2012 | 3.652 | 46 | 280 |
| 2011 | 3.212 | 35 | 129 |
| 2010 | - | - |

You may also be interested in the following journals
- ► Stem Cells and Development
- ► Stem Cell Research
- ► Stem Cells Translational Medicine
- ► PLoS One
- ► Stem Cells
- ► Human Gene Therapy
- ► Cell Stem Cell
- ► New England Journal of Medicine
- ► Scientific Reports
- ► Cell Death and Differentiation
Top Journals in medicine
- New England Journal of Medicine
- Nature Reviews Drug Discovery
- Jama-Journal of The American Medical Association
- Nature Reviews Cancer
- Nature Reviews Immunology
- Lancet Oncology
- Nature Reviews Neuroscience
- Nature Medicine
- World Psychiatry
- Lancet Neurology
- Journal of Clinical Oncology
Journal Impact
Stem Cell Research and Therapy - Impact Score, Ranking, SJR, h-index, Citescore, Rating, Publisher, ISSN, and Other Important Details
Published By: BioMed Central Ltd.
Abbreviation: Stem Cell Res. Ther.
Impact Score The impact Score or journal impact score (JIS) is equivalent to Impact Factor. The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as indexed by Clarivate's Web of Science. On the other hand, Impact Score is based on Scopus data.
Important details.
| Stem Cell Research and Therapy | |
| Stem Cell Res. Ther. | |
| Journal | |
| Biochemistry, Genetics and Molecular Biology (miscellaneous) (Q1); Cell Biology (Q1); Medicine (miscellaneous) (Q1); Molecular Medicine (Q1) | |
| 7.16 | |
| 1.498 | |
| 106 | |
| 2140 | |
| BioMed Central Ltd. | |
| United Kingdom | |
| 17576512 | |
| 2010-2022 | |
| Q1 | |
| (Last 3 Year) | 11541 |
About Stem Cell Research and Therapy
Stem Cell Research and Therapy is a journal published by BioMed Central Ltd. . This journal covers the area[s] related to Biochemistry, Genetics and Molecular Biology (miscellaneous), Cell Biology, Medicine (miscellaneous), Molecular Medicine, etc . The coverage history of this journal is as follows: 2010-2022. The rank of this journal is 2140 . This journal's impact score, h-index, and SJR are 7.16, 106, and 1.498, respectively. The ISSN of this journal is/are as follows: 17576512 . The best quartile of Stem Cell Research and Therapy is Q1 . This journal has received a total of 11541 citations during the last three years (Preceding 2022).
Stem Cell Research and Therapy Impact Score 2022-2023
The impact score (IS), also denoted as the Journal impact score (JIS), of an academic journal is a measure of the yearly average number of citations to recent articles published in that journal. It is based on Scopus data.
Prediction of Stem Cell Research and Therapy Impact Score 2023
Impact Score 2022 of Stem Cell Research and Therapy is 7.16 . If a similar downward trend continues, IS may decrease in 2023 as well.
Impact Score Graph
Check below the impact score trends of stem cell research and therapy. this is based on scopus data..
| Year | Impact Score (IS) |
|---|---|
| 2023/2024 | Coming Soon |
| 2022 | 7.16 |
| 2021 | 7.42 |
| 2020 | 5.99 |
| 2019 | 5.26 |
| 2018 | 4.92 |
| 2017 | 5.17 |
| 2016 | 4.51 |
| 2015 | 4.51 |
| 2014 | 3.25 |
Stem Cell Research and Therapy h-index
The h-index of Stem Cell Research and Therapy is 106 . By definition of the h-index, this journal has at least 106 published articles with more than 106 citations.
What is h-index?
The h-index (also known as the Hirsch index or Hirsh index) is a scientometric parameter used to evaluate the scientific impact of the publications and journals. It is defined as the maximum value of h such that the given Journal has published at least h papers and each has at least h citations.
Stem Cell Research and Therapy ISSN
The International Standard Serial Number (ISSN) of Stem Cell Research and Therapy is/are as follows: 17576512 .
The ISSN is a unique 8-digit identifier for a specific publication like Magazine or Journal. The ISSN is used in the postal system and in the publishing world to identify the articles that are published in journals, magazines, newsletters, etc. This is the number assigned to your article by the publisher, and it is the one you will use to reference your article within the library catalogues.
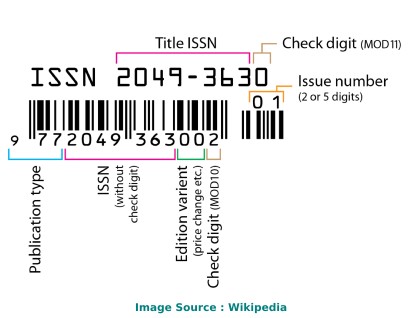
ISSN code (also called as "ISSN structure" or "ISSN syntax") can be expressed as follows: NNNN-NNNC Here, N is in the set {0,1,2,3...,9}, a digit character, and C is in {0,1,2,3,...,9,X}
Stem Cell Research and Therapy Ranking and SCImago Journal Rank (SJR)
SCImago Journal Rank is an indicator, which measures the scientific influence of journals. It considers the number of citations received by a journal and the importance of the journals from where these citations come.
Stem Cell Research and Therapy Publisher
The publisher of Stem Cell Research and Therapy is BioMed Central Ltd. . The publishing house of this journal is located in the United Kingdom . Its coverage history is as follows: 2010-2022 .
Call For Papers (CFPs)
Please check the official website of this journal to find out the complete details and Call For Papers (CFPs).
Abbreviation
The International Organization for Standardization 4 (ISO 4) abbreviation of Stem Cell Research and Therapy is Stem Cell Res. Ther. . ISO 4 is an international standard which defines a uniform and consistent system for the abbreviation of serial publication titles, which are published regularly. The primary use of ISO 4 is to abbreviate or shorten the names of scientific journals using the technique of List of Title Word Abbreviations (LTWA).
As ISO 4 is an international standard, the abbreviation ('Stem Cell Res. Ther.') can be used for citing, indexing, abstraction, and referencing purposes.
How to publish in Stem Cell Research and Therapy
If your area of research or discipline is related to Biochemistry, Genetics and Molecular Biology (miscellaneous), Cell Biology, Medicine (miscellaneous), Molecular Medicine, etc. , please check the journal's official website to understand the complete publication process.
Acceptance Rate
- Interest/demand of researchers/scientists for publishing in a specific journal/conference.
- The complexity of the peer review process and timeline.
- Time taken from draft submission to final publication.
- Number of submissions received and acceptance slots
- And Many More.
The simplest way to find out the acceptance rate or rejection rate of a Journal/Conference is to check with the journal's/conference's editorial team through emails or through the official website.
Frequently Asked Questions (FAQ)
What is the impact score of stem cell research and therapy.
The latest impact score of Stem Cell Research and Therapy is 7.16. It is computed in the year 2023.
What is the h-index of Stem Cell Research and Therapy?
The latest h-index of Stem Cell Research and Therapy is 106. It is evaluated in the year 2023.
What is the SCImago Journal Rank (SJR) of Stem Cell Research and Therapy?
The latest SCImago Journal Rank (SJR) of Stem Cell Research and Therapy is 1.498. It is calculated in the year 2023.
What is the ranking of Stem Cell Research and Therapy?
The latest ranking of Stem Cell Research and Therapy is 2140. This ranking is among 27955 Journals, Conferences, and Book Series. It is computed in the year 2023.
Who is the publisher of Stem Cell Research and Therapy?
Stem Cell Research and Therapy is published by BioMed Central Ltd.. The publication country of this journal is United Kingdom.
What is the abbreviation of Stem Cell Research and Therapy?
This standard abbreviation of Stem Cell Research and Therapy is Stem Cell Res. Ther..
Is "Stem Cell Research and Therapy" a Journal, Conference or Book Series?
Stem Cell Research and Therapy is a journal published by BioMed Central Ltd..
What is the scope of Stem Cell Research and Therapy?
- Biochemistry, Genetics and Molecular Biology (miscellaneous)
- Cell Biology
- Medicine (miscellaneous)
- Molecular Medicine
For detailed scope of Stem Cell Research and Therapy, check the official website of this journal.
What is the ISSN of Stem Cell Research and Therapy?
The International Standard Serial Number (ISSN) of Stem Cell Research and Therapy is/are as follows: 17576512.
What is the best quartile for Stem Cell Research and Therapy?
The best quartile for Stem Cell Research and Therapy is Q1.
What is the coverage history of Stem Cell Research and Therapy?
The coverage history of Stem Cell Research and Therapy is as follows 2010-2022.
Credits and Sources
- Scimago Journal & Country Rank (SJR), https://www.scimagojr.com/
- Journal Impact Factor, https://clarivate.com/
- Issn.org, https://www.issn.org/
- Scopus, https://www.scopus.com/
Note: The impact score shown here is equivalent to the average number of times documents published in a journal/conference in the past two years have been cited in the current year (i.e., Cites / Doc. (2 years)). It is based on Scopus data and can be a little higher or different compared to the impact factor (IF) produced by Journal Citation Report. Please refer to the Web of Science data source to check the exact journal impact factor ™ (Thomson Reuters) metric.
Impact Score, SJR, h-Index, and Other Important metrics of These Journals, Conferences, and Book Series
| Journal/Conference/Book Title | Type | Publisher | Ranking | SJR | h-index | Impact Score |
|---|---|---|---|---|---|---|
Check complete list
Stem Cell Research and Therapy Impact Score (IS) Trend
| Year | Impact Score (IS) |
|---|---|
| 2023/2024 | Updated Soon |
| 2022 | 7.16 |
| 2021 | 7.42 |
| 2020 | 5.99 |
| 2019 | 5.26 |
| 2018 | 4.92 |
| 2017 | 5.17 |
| 2016 | 4.51 |
| 2015 | 4.51 |
| 2014 | 3.25 |
Top Journals/Conferences in Biochemistry, Genetics and Molecular Biology (miscellaneous)
Top journals/conferences in cell biology, top journals/conferences in medicine (miscellaneous), top journals/conferences in molecular medicine.

- Paper Archives
- Journal Indexing
- Research Conference
- Add Journal
Searching By
- Search More ...

Studies on Stem Cells Research and Therapy (ISSN: 2641-3000)
Publisher Peertechz Publications Pvt. Ltd
ISSN-L 2641-3000
E-ISSN 2641-3000
IF(Impact Factor) 2024 Evaluation Pending
Website https://www.peertechz.com/journals/studi...
Description
Last modified: 2019-01-11 19:52:05
Advertisement
Stem Cells Journal List, Impact Factors
What are the best stem cells journal resources in 2023 and is there a stem cell journal list out there? I seem to have the only list, other than Google itself.
I’ve covered this topic for many years. In this post I have updated the material from over the years and dug more deeply into the question.
Looking for a stem cell journal? A regenerative medicine journal more focused on translation and clinical applications? Something more molecular or cellular? Developmental?
Where to go for unbiased info?
What about stem cells journal impact factors?
Some folks are trying to discount impact factors but scientists still focus a lot on impact factor.

If you need to publish your latest exciting stem cell manuscript or are wanting to read some stem cell and regenerative medicine articles, you need a stem cell and regenerative medicine journal list, and I’ve got the one for you. Google is also pretty helpful when searching for stem cell and regenerative medicine journals. I’ve posted an image montage from my web search for stem cell journals above.
In today’s post, I also included the name of the Editor where possible.
Older lists
In 2012, I published my first list of this kind, but somehow eight years have zoomed by. While I have published updated lists in ensuing years, now that it’s 2020, I figured that we need a new, updated list.
Since 2012 and even 2016 some journals have disappeared, while other new ones have popped up. For instance, npj Regenerative Medicine is a new one, but there are others too. According to Scopus, you can see the top 10 journals in terms of quantity of articles published with “Stem cells” in the title. This doesn’t take into account quality of articles or impact.
By the way, if you are looking for a fun, satirical read as well, I also did a satirical post on made up stem cell journals with humorous names. It still cracks me up.
2023 list: impact factor stem cells publishing
Note that I’m not endorsing these, but thought you’d find the list useful. If you see any you believe to be predatory on this list, please email me. What’s your favorite stem cell journal?
I have tried to include new impact factor (numbers as close to 2021 where possible and other comments reflecting my perspectives on or experiences with journals. Impact factor is a somewhat controversial metric these days, but it’s hard to find some other way to numerically compare journals. If we toss IF, do we just go on reputation within our field as we consider journals?
Keep in mind that broader journals like Science, Blood, PLOS ONE, Nature, Cell, and others publish many stem cell articles too. Also, there are a few stem cell-specific others out there I didn’t include because I haven’t had a chance to read up on them yet.
- Cell Stem Cell Impact Factor 21.4, tough to get into, great staff. Editor Sheila Chari.
- Stem Cells Impact Factor ~5.6, rigorous; fair review process.
- Stem Cells Translational Medicine Impact Factor~5.9, IF on the rise, good experiences here too.
- npj Regenerative Medicine Impact Factor ~ 7.0. As a relatively new journal it has a great impact factor.
- Stem Cells and Development Impact Factor ~3, good experiences.
- Stem Cell Reports Impact Factor ~ 5.5, down from over 7 in 2018. Editor Martin Pera.
- Development Impact Factor 5.8, rigorous, but fair.
- Regenerative Medicine Impact Factor ~2.9, good experiences with editors and reviewers.
- Stem Cell Research Impact Factor up to around 3.9.
- Current Stem Cell Reports Impact Factor 2.0.
- Cellular Reprogramming Impact Factor is 1.4. (used to be called Cloning and Stem Cells)
- Stem Cell Reviews and Reports Impact Factor ~3. Editor Mariusz Ratajczak.
- Journal of Stem Cells Impact Factor unclear.
- Journal of Stem Cells & Regenerative Medicine Impact Factor
- Stem Cells International (couldn’t access website)
- International Journal of Stem Cells Impact Factor unclear.
- World Journal of Stem Cells Impact Factor reported 3.4)
- Stem Cells and Cloning
- Stem Cell Research & Therapy Impact Factor 4.6, which is much higher than I recall in the past)
- Stem Cell Discovery (IF 0.6)
- Stem Cell Biology and Research
- American Journal of Stem Cell Research
Do you have a favorite stem cell or regenerative medicine journal not on this list? Let us know in the comments and say why you like it.
Cell biology journals that often publish stem cell work
Cell impact factor 39.7., nature impact factor 42.8, cell reports impact factor 8.1, molecular cell impact factor 15.6, cancer cell impact factor 26.6, nature cell biology impact factor 20.0, related posts, 2 thoughts on “stem cells journal list, impact factors”.
Hi Paul, Thank you for the list. I would like to add StemJournal to this list: https://stemjnl.org/ New journal. Editor in chief: Chad Cowan and Niels Geijsen. Just launched StemRxiv. Laurence
Hi Paul, Again, great work. I wanted to make your readers aware we have a complete line of VTM based COVID-19 Testing Supplies. Check out our video ad-https://youtu.be/ETmIvCwfsX0
Leave a Reply Cancel reply
Current Stem Cell Research and Therapy

Subject Area and Category
- Medicine (miscellaneous)
Bentham Science Publishers B.V.
Publication type
1574888X, 22123946
Information
How to publish in this journal
The set of journals have been ranked according to their SJR and divided into four equal groups, four quartiles. Q1 (green) comprises the quarter of the journals with the highest values, Q2 (yellow) the second highest values, Q3 (orange) the third highest values and Q4 (red) the lowest values.
| Category | Year | Quartile |
|---|---|---|
| Medicine (miscellaneous) | 2007 | Q3 |
| Medicine (miscellaneous) | 2008 | Q2 |
| Medicine (miscellaneous) | 2009 | Q1 |
| Medicine (miscellaneous) | 2010 | Q1 |
| Medicine (miscellaneous) | 2011 | Q1 |
| Medicine (miscellaneous) | 2012 | Q1 |
| Medicine (miscellaneous) | 2013 | Q1 |
| Medicine (miscellaneous) | 2014 | Q2 |
| Medicine (miscellaneous) | 2015 | Q1 |
| Medicine (miscellaneous) | 2016 | Q2 |
| Medicine (miscellaneous) | 2017 | Q2 |
| Medicine (miscellaneous) | 2018 | Q2 |
| Medicine (miscellaneous) | 2019 | Q2 |
| Medicine (miscellaneous) | 2020 | Q2 |
| Medicine (miscellaneous) | 2021 | Q2 |
| Medicine (miscellaneous) | 2022 | Q2 |
| Medicine (miscellaneous) | 2023 | Q2 |
The SJR is a size-independent prestige indicator that ranks journals by their 'average prestige per article'. It is based on the idea that 'all citations are not created equal'. SJR is a measure of scientific influence of journals that accounts for both the number of citations received by a journal and the importance or prestige of the journals where such citations come from It measures the scientific influence of the average article in a journal, it expresses how central to the global scientific discussion an average article of the journal is.
| Year | SJR |
|---|---|
| 2007 | 0.235 |
| 2008 | 0.403 |
| 2009 | 1.088 |
| 2010 | 1.182 |
| 2011 | 1.078 |
| 2012 | 1.065 |
| 2013 | 0.906 |
| 2014 | 0.813 |
| 2015 | 0.886 |
| 2016 | 0.723 |
| 2017 | 0.543 |
| 2018 | 0.595 |
| 2019 | 0.577 |
| 2020 | 0.802 |
| 2021 | 0.572 |
| 2022 | 0.552 |
| 2023 | 0.525 |
Evolution of the number of published documents. All types of documents are considered, including citable and non citable documents.
| Year | Documents |
|---|---|
| 2006 | 39 |
| 2007 | 31 |
| 2008 | 31 |
| 2009 | 33 |
| 2010 | 52 |
| 2011 | 35 |
| 2012 | 49 |
| 2013 | 58 |
| 2014 | 59 |
| 2015 | 69 |
| 2016 | 91 |
| 2017 | 67 |
| 2018 | 70 |
| 2019 | 95 |
| 2020 | 74 |
| 2021 | 66 |
| 2022 | 63 |
| 2023 | 100 |
This indicator counts the number of citations received by documents from a journal and divides them by the total number of documents published in that journal. The chart shows the evolution of the average number of times documents published in a journal in the past two, three and four years have been cited in the current year. The two years line is equivalent to journal impact factor ™ (Thomson Reuters) metric.
| Cites per document | Year | Value |
|---|---|---|
| Cites / Doc. (4 years) | 2006 | 0.000 |
| Cites / Doc. (4 years) | 2007 | 0.513 |
| Cites / Doc. (4 years) | 2008 | 1.886 |
| Cites / Doc. (4 years) | 2009 | 3.653 |
| Cites / Doc. (4 years) | 2010 | 3.791 |
| Cites / Doc. (4 years) | 2011 | 3.327 |
| Cites / Doc. (4 years) | 2012 | 3.974 |
| Cites / Doc. (4 years) | 2013 | 3.864 |
| Cites / Doc. (4 years) | 2014 | 2.876 |
| Cites / Doc. (4 years) | 2015 | 2.786 |
| Cites / Doc. (4 years) | 2016 | 2.306 |
| Cites / Doc. (4 years) | 2017 | 1.895 |
| Cites / Doc. (4 years) | 2018 | 2.063 |
| Cites / Doc. (4 years) | 2019 | 2.098 |
| Cites / Doc. (4 years) | 2020 | 3.062 |
| Cites / Doc. (4 years) | 2021 | 3.444 |
| Cites / Doc. (4 years) | 2022 | 3.184 |
| Cites / Doc. (4 years) | 2023 | 2.624 |
| Cites / Doc. (3 years) | 2006 | 0.000 |
| Cites / Doc. (3 years) | 2007 | 0.513 |
| Cites / Doc. (3 years) | 2008 | 1.886 |
| Cites / Doc. (3 years) | 2009 | 3.653 |
| Cites / Doc. (3 years) | 2010 | 4.011 |
| Cites / Doc. (3 years) | 2011 | 3.414 |
| Cites / Doc. (3 years) | 2012 | 3.925 |
| Cites / Doc. (3 years) | 2013 | 3.640 |
| Cites / Doc. (3 years) | 2014 | 2.789 |
| Cites / Doc. (3 years) | 2015 | 2.873 |
| Cites / Doc. (3 years) | 2016 | 2.269 |
| Cites / Doc. (3 years) | 2017 | 1.749 |
| Cites / Doc. (3 years) | 2018 | 1.956 |
| Cites / Doc. (3 years) | 2019 | 2.285 |
| Cites / Doc. (3 years) | 2020 | 3.353 |
| Cites / Doc. (3 years) | 2021 | 3.238 |
| Cites / Doc. (3 years) | 2022 | 2.996 |
| Cites / Doc. (3 years) | 2023 | 2.345 |
| Cites / Doc. (2 years) | 2006 | 0.000 |
| Cites / Doc. (2 years) | 2007 | 0.513 |
| Cites / Doc. (2 years) | 2008 | 1.886 |
| Cites / Doc. (2 years) | 2009 | 3.129 |
| Cites / Doc. (2 years) | 2010 | 4.328 |
| Cites / Doc. (2 years) | 2011 | 3.224 |
| Cites / Doc. (2 years) | 2012 | 3.333 |
| Cites / Doc. (2 years) | 2013 | 3.298 |
| Cites / Doc. (2 years) | 2014 | 2.692 |
| Cites / Doc. (2 years) | 2015 | 2.812 |
| Cites / Doc. (2 years) | 2016 | 2.117 |
| Cites / Doc. (2 years) | 2017 | 1.638 |
| Cites / Doc. (2 years) | 2018 | 2.076 |
| Cites / Doc. (2 years) | 2019 | 2.431 |
| Cites / Doc. (2 years) | 2020 | 3.242 |
| Cites / Doc. (2 years) | 2021 | 3.118 |
| Cites / Doc. (2 years) | 2022 | 2.357 |
| Cites / Doc. (2 years) | 2023 | 2.124 |
Evolution of the total number of citations and journal's self-citations received by a journal's published documents during the three previous years. Journal Self-citation is defined as the number of citation from a journal citing article to articles published by the same journal.
| Cites | Year | Value |
|---|---|---|
| Self Cites | 2006 | 0 |
| Self Cites | 2007 | 5 |
| Self Cites | 2008 | 1 |
| Self Cites | 2009 | 9 |
| Self Cites | 2010 | 9 |
| Self Cites | 2011 | 18 |
| Self Cites | 2012 | 39 |
| Self Cites | 2013 | 25 |
| Self Cites | 2014 | 15 |
| Self Cites | 2015 | 22 |
| Self Cites | 2016 | 13 |
| Self Cites | 2017 | 4 |
| Self Cites | 2018 | 19 |
| Self Cites | 2019 | 28 |
| Self Cites | 2020 | 18 |
| Self Cites | 2021 | 36 |
| Self Cites | 2022 | 12 |
| Self Cites | 2023 | 13 |
| Total Cites | 2006 | 0 |
| Total Cites | 2007 | 20 |
| Total Cites | 2008 | 132 |
| Total Cites | 2009 | 369 |
| Total Cites | 2010 | 381 |
| Total Cites | 2011 | 396 |
| Total Cites | 2012 | 471 |
| Total Cites | 2013 | 495 |
| Total Cites | 2014 | 396 |
| Total Cites | 2015 | 477 |
| Total Cites | 2016 | 422 |
| Total Cites | 2017 | 383 |
| Total Cites | 2018 | 444 |
| Total Cites | 2019 | 521 |
| Total Cites | 2020 | 778 |
| Total Cites | 2021 | 774 |
| Total Cites | 2022 | 704 |
| Total Cites | 2023 | 476 |
Evolution of the number of total citation per document and external citation per document (i.e. journal self-citations removed) received by a journal's published documents during the three previous years. External citations are calculated by subtracting the number of self-citations from the total number of citations received by the journal’s documents.
| Cites | Year | Value |
|---|---|---|
| External Cites per document | 2006 | 0 |
| External Cites per document | 2007 | 0.385 |
| External Cites per document | 2008 | 1.871 |
| External Cites per document | 2009 | 3.564 |
| External Cites per document | 2010 | 3.916 |
| External Cites per document | 2011 | 3.259 |
| External Cites per document | 2012 | 3.600 |
| External Cites per document | 2013 | 3.456 |
| External Cites per document | 2014 | 2.683 |
| External Cites per document | 2015 | 2.741 |
| External Cites per document | 2016 | 2.199 |
| External Cites per document | 2017 | 1.731 |
| External Cites per document | 2018 | 1.872 |
| External Cites per document | 2019 | 2.162 |
| External Cites per document | 2020 | 3.276 |
| External Cites per document | 2021 | 3.088 |
| External Cites per document | 2022 | 2.945 |
| External Cites per document | 2023 | 2.281 |
| Cites per document | 2006 | 0.000 |
| Cites per document | 2007 | 0.513 |
| Cites per document | 2008 | 1.886 |
| Cites per document | 2009 | 3.653 |
| Cites per document | 2010 | 4.011 |
| Cites per document | 2011 | 3.414 |
| Cites per document | 2012 | 3.925 |
| Cites per document | 2013 | 3.640 |
| Cites per document | 2014 | 2.789 |
| Cites per document | 2015 | 2.873 |
| Cites per document | 2016 | 2.269 |
| Cites per document | 2017 | 1.749 |
| Cites per document | 2018 | 1.956 |
| Cites per document | 2019 | 2.285 |
| Cites per document | 2020 | 3.353 |
| Cites per document | 2021 | 3.238 |
| Cites per document | 2022 | 2.996 |
| Cites per document | 2023 | 2.345 |
International Collaboration accounts for the articles that have been produced by researchers from several countries. The chart shows the ratio of a journal's documents signed by researchers from more than one country; that is including more than one country address.
| Year | International Collaboration |
|---|---|
| 2006 | 0.00 |
| 2007 | 22.58 |
| 2008 | 12.90 |
| 2009 | 27.27 |
| 2010 | 17.31 |
| 2011 | 14.29 |
| 2012 | 18.37 |
| 2013 | 31.03 |
| 2014 | 22.03 |
| 2015 | 34.78 |
| 2016 | 21.98 |
| 2017 | 23.88 |
| 2018 | 15.71 |
| 2019 | 23.16 |
| 2020 | 20.27 |
| 2021 | 19.70 |
| 2022 | 22.22 |
| 2023 | 18.00 |
Not every article in a journal is considered primary research and therefore "citable", this chart shows the ratio of a journal's articles including substantial research (research articles, conference papers and reviews) in three year windows vs. those documents other than research articles, reviews and conference papers.
| Documents | Year | Value |
|---|---|---|
| Non-citable documents | 2006 | 0 |
| Non-citable documents | 2007 | 0 |
| Non-citable documents | 2008 | 0 |
| Non-citable documents | 2009 | 0 |
| Non-citable documents | 2010 | 1 |
| Non-citable documents | 2011 | 4 |
| Non-citable documents | 2012 | 6 |
| Non-citable documents | 2013 | 8 |
| Non-citable documents | 2014 | 11 |
| Non-citable documents | 2015 | 13 |
| Non-citable documents | 2016 | 18 |
| Non-citable documents | 2017 | 26 |
| Non-citable documents | 2018 | 28 |
| Non-citable documents | 2019 | 24 |
| Non-citable documents | 2020 | 17 |
| Non-citable documents | 2021 | 16 |
| Non-citable documents | 2022 | 14 |
| Non-citable documents | 2023 | 9 |
| Citable documents | 2006 | 0 |
| Citable documents | 2007 | 39 |
| Citable documents | 2008 | 70 |
| Citable documents | 2009 | 101 |
| Citable documents | 2010 | 94 |
| Citable documents | 2011 | 112 |
| Citable documents | 2012 | 114 |
| Citable documents | 2013 | 128 |
| Citable documents | 2014 | 131 |
| Citable documents | 2015 | 153 |
| Citable documents | 2016 | 168 |
| Citable documents | 2017 | 193 |
| Citable documents | 2018 | 199 |
| Citable documents | 2019 | 204 |
| Citable documents | 2020 | 215 |
| Citable documents | 2021 | 223 |
| Citable documents | 2022 | 221 |
| Citable documents | 2023 | 194 |
Ratio of a journal's items, grouped in three years windows, that have been cited at least once vs. those not cited during the following year.
| Documents | Year | Value |
|---|---|---|
| Uncited documents | 2006 | 0 |
| Uncited documents | 2007 | 24 |
| Uncited documents | 2008 | 20 |
| Uncited documents | 2009 | 16 |
| Uncited documents | 2010 | 10 |
| Uncited documents | 2011 | 19 |
| Uncited documents | 2012 | 14 |
| Uncited documents | 2013 | 23 |
| Uncited documents | 2014 | 28 |
| Uncited documents | 2015 | 35 |
| Uncited documents | 2016 | 54 |
| Uncited documents | 2017 | 82 |
| Uncited documents | 2018 | 81 |
| Uncited documents | 2019 | 62 |
| Uncited documents | 2020 | 58 |
| Uncited documents | 2021 | 56 |
| Uncited documents | 2022 | 62 |
| Uncited documents | 2023 | 49 |
| Cited documents | 2006 | 0 |
| Cited documents | 2007 | 15 |
| Cited documents | 2008 | 50 |
| Cited documents | 2009 | 85 |
| Cited documents | 2010 | 85 |
| Cited documents | 2011 | 97 |
| Cited documents | 2012 | 106 |
| Cited documents | 2013 | 113 |
| Cited documents | 2014 | 114 |
| Cited documents | 2015 | 131 |
| Cited documents | 2016 | 132 |
| Cited documents | 2017 | 137 |
| Cited documents | 2018 | 146 |
| Cited documents | 2019 | 166 |
| Cited documents | 2020 | 174 |
| Cited documents | 2021 | 183 |
| Cited documents | 2022 | 173 |
| Cited documents | 2023 | 154 |
Evolution of the percentage of female authors.
| Year | Female Percent |
|---|---|
| 2006 | 20.37 |
| 2007 | 25.44 |
| 2008 | 28.75 |
| 2009 | 30.40 |
| 2010 | 27.65 |
| 2011 | 42.28 |
| 2012 | 38.93 |
| 2013 | 36.67 |
| 2014 | 38.70 |
| 2015 | 37.44 |
| 2016 | 42.17 |
| 2017 | 30.74 |
| 2018 | 39.50 |
| 2019 | 40.11 |
| 2020 | 46.67 |
| 2021 | 43.37 |
| 2022 | 40.77 |
| 2023 | 45.59 |
Evolution of the number of documents cited by public policy documents according to Overton database.
| Documents | Year | Value |
|---|---|---|
| Overton | 2006 | 3 |
| Overton | 2007 | 3 |
| Overton | 2008 | 0 |
| Overton | 2009 | 2 |
| Overton | 2010 | 1 |
| Overton | 2011 | 2 |
| Overton | 2012 | 1 |
| Overton | 2013 | 0 |
| Overton | 2014 | 0 |
| Overton | 2015 | 0 |
| Overton | 2016 | 0 |
| Overton | 2017 | 0 |
| Overton | 2018 | 0 |
| Overton | 2019 | 0 |
| Overton | 2020 | 0 |
| Overton | 2021 | 0 |
| Overton | 2022 | 0 |
| Overton | 2023 | 0 |
Evoution of the number of documents related to Sustainable Development Goals defined by United Nations. Available from 2018 onwards.
| Documents | Year | Value |
|---|---|---|
| SDG | 2018 | 10 |
| SDG | 2019 | 32 |
| SDG | 2020 | 22 |
| SDG | 2021 | 27 |
| SDG | 2022 | 17 |
| SDG | 2023 | 34 |
Leave a comment
Name * Required
Email (will not be published) * Required
* Required Cancel
The users of Scimago Journal & Country Rank have the possibility to dialogue through comments linked to a specific journal. The purpose is to have a forum in which general doubts about the processes of publication in the journal, experiences and other issues derived from the publication of papers are resolved. For topics on particular articles, maintain the dialogue through the usual channels with your editor.

Follow us on @ScimagoJR Scimago Lab , Copyright 2007-2024. Data Source: Scopus®

Cookie settings
Cookie Policy
Legal Notice
Privacy Policy

Current Stem Cell Research & Therapy
Impact Factor : 2.1
Indexed in: Scopus, SCI Expanded, MEDLINE/PubMed... View all
Volume 19 , Issues 11, 2024
This journal supports open access
Submission for General Articles
Submit to thematic issues.

Thematic Issue Issue: {[{issue.issue_title}]}
{[{issue.about_issue}]}
No Text Found
- Submit Abstracts
- Submit Manuscripts Online
- Thematic Issue Proposal
- Animated Abstract Submission

- About Journal
- Editorial Board
- Journal Insight
- Current Issue
- Volumes /Issues
- Author Guidelines
- Graphical Abstracts
- Fabricating and Stating False Information
- Research Misconduct
- Post Publication Discussions and Corrections
- Publishing Ethics and Rectitude
- Increase Visibility of Your Article
- Archiving Policies
- Peer Review Workflow
- Order Your Article Before Print
- Promote Your Article
- Manuscript Transfer Facility
- Editorial Policies
- Allegations from Whistleblowers
- Announcements
- Forthcoming Thematic Issues
- Guest Editor Guidelines
- Editorial Management
- Ethical Guidelines for New Editors
- Reviewer Guidelines
- Abstract Ahead of Print 1
- Article(s) in Press 47
- Free Online Copy
- Most Cited Articles
- Most Accessed Articles
- Highlighted Article
- Most Popular Articles
- Editor's Choice
- Thematic Issues
- Open Access Articles
- Open Access Funding
- Library Recommendation
- Trial Requests
- Advertise With Us
- Meet the Executive Guest Editor(s)
- Brand Ambassador
- Author's Comment & Reviews
- New Journals 2023
- New Journals 2024
- Alert Subscription
REVIEW article
Stem cell therapy: a new hope for stroke and traumatic brain injury recovery and the challenge for rural minorities in south carolina.

- Department of Neurology, Medical University of South Carolina, Charleston, SC, United States
Stroke and traumatic brain injury (TBI) are a significant cause of death and disability nationwide. Both are considered public health concerns in rural communities in the state of South Carolina (SC), particularly affecting the African American population resulting in considerable morbidity, mortality, and economic burden. Stem cell therapy (SCT) has emerged as a potential intervention for both diseases with increasing research trials showing promising results. In this perspective article, the authors aim to discuss the current research in the field of SCT, the results of early phase trials, and the utilization of outcome measures and biomarkers of recovery. We searched PubMed from inception to December 2023 for articles on stem cell therapy in stroke and traumatic brain injury and its impact on rural communities, particularly in SC. Early phase trials of SCT in Stroke and Traumatic Brain injury yield promising safety profile and efficacy results, but the findings have not yet been consistently replicated. Early trials using mesenchymal stem cells for stroke survivors showed safety, feasibility, and improved functional outcomes using broad and domain-specific outcome measures. Neuroimaging markers of recovery such as Functional Magnetic Resonance Imaging (fMRI) and electroencephalography (EEG) combined with neuromodulation, although not widely used in SCT research, could represent a breakthrough when evaluating brain injury and its functional consequences. This article highlights the role of SCT as a promising intervention while addressing the underlying social determinants of health that affect therapeutic outcomes in relation to rural communities such as SC. It also addresses the challenges ethical concerns of stem cell sourcing, the high cost of autologous cell therapies, and the technical difficulties in ensuring transplanted cell survival and strategies to overcome barriers to clinical trial enrollment such as the ethical concerns of stem cell sourcing, the high cost of autologous cell therapies, and the technical difficulties in ensuring transplanted cell survival and equitable healthcare.
Introduction
Stroke is the 6 th leading cause of mortality and a leading cause of morbidity in South Carolina and resulted in healthcare expenses of $1.3 billion in 2020 alone ( 1 ). The incidence of stroke varies by age, gender, race, and ethnicity. African American (AA) men are particularly vulnerable and have a 49 percent greater likelihood of dying from stroke than Caucasian Americans (CA) ( 2 ). A higher prevalence of stroke risk factors among AA and males compared to CA and females contributes to these disparities ( 2 ). A National Study of inpatient rehabilitation after the first stroke showed that AAs were younger and more disabled on admission, more likely to be discharged home and less likely to report independence on ADLs ( 3 ). Data from the Brain Attack Surveillance in Corpus Christi (BASIC) project also show that post-stroke Hispanic Americans scored worse on neurological, functional, and cognitive outcomes than CA ( 4 ).
Traumatic Brain Injury (TBI) is a significant cause of death and disability among the young population and is estimated to occur every 15 s in the United States ( 5 ). The economic impact of TBI is staggering, accruing an annual cost of over $77 billion in the United States ( 6 ). Between 2016 and 2018 about 4,310 TBI-related deaths were reported in South Carolina; this is 57.8% higher than the national average ( 7 ). These deaths were a combination of accidental, homicidal and suicidal causes. While the exact description of poor in South Carolina is unknown, health and economic barriers in this state may be more common than elsewhere. The degree of rurality played a role in higher incidences of TBI and increased barriers to emergency medical care ( 8 ). Racial and ethnic differences are apparent in acute and post-concussive management ( 9 ). During the early acute phase, there is a discrepancy in those taken to the hospital for evaluation ( 9 ). Afterward, there is a high risk of inadequate follow-up and management in the post-concussion period ( 10 ).
In addition, race and gender disparities in stroke and TBI care also play a significant role in patient outcomes. A report by the National Institute of Neurological Disorders and Stroke (NINDS) reveals that only 42% of the total population in clinical trials from 1985 to 2008 were women in acute stroke clinical trials ( 11 , 12 ). Numerous studies report reduced access to emergency stroke care, delayed hospital arrivals, and limited rehabilitation resources for AA compared to CA ( 13 ). These disparities are echoed in clinical trials, with non-White minorities significantly underrepresented, which affects the validation and generalizability of clinical trial outcomes ( 11 ).
Treatment options for acute ischemic stroke are approved by the Food and Drug Administration (FDA) including intravenous thrombolytics (IVT) and mechanical endovascular thrombectomy (MT) ( 14 ). However the time-sensitive nature and strict selection criteria often exclude acute stroke patients from receiving these treatments. After stroke completion, dedicated rehabilitation for survivors is the only option with a proven long-term patient benefit ( 15 ). Of those who develop motor weakness after stroke, only 50% achieve functional independence at 6 months. Maximum rehabilitation benefit occurs within the first months after stroke ( 16 ). Similarly treatment options for TBIs outside neurosurgical intensive care units are limited. Lifestyle modifications, medication management, cognitive rehabilitation, and surgeries have been explored with mixed results ( 17 ).
Stem cell therapy (SCT) has emerged as a potentially transformative intervention for ischemic stroke and TBI, with the ambitious aim of replacing or aiding the recovery of neurons and vascular cells affected by ischemic events. While there are no current FDA-approved SCT trials for stroke or TBI, increasing research over the past decade shows some promising trends ( 18 – 20 ) ( Figure 1 ).
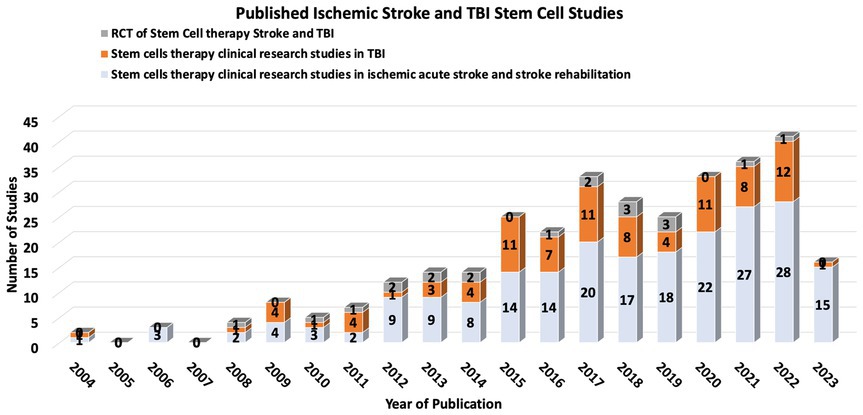
Figure 1 . Number of published Stroke and TBI from 2014-2023.
Stem cells in stroke and TBI clinical trials
Stem cell therapy is a potentially transformative intervention for ischemic stroke and TBI. Several clinical trials have addressed the utility of different stem cell types in ischemic stroke and TBI, including mesenchymal stem cells (MSCs), neural stem cells (NSCs), and induced pluripotent stem cells (iPSCs) ( 20 , 21 ). These trials vary widely in the design of stem cell sources, dosages, delivery routes, and timing of post-stroke therapy.
Results of early-phase SCT clinical trials present a promising safety profile, with no significant adverse effects directly attributable to the therapy ( 22 ). Some trials have shown improvements in neurological function and reductions in lesion volume, but these findings have yet to be consistently replicated across a spectrum of studies. The Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPs) committee has been formed to guide and bridge the gap between basic and clinical studies ( 23 ).
One noteworthy example is the multipotent adult progenitor cells in acute ischemic stroke (MASTERS) clinical trial, a phase 2 study exploring multipotent adult progenitor cells (MAPCs) in acute ischemic stroke ( 24 ). This trial enrolled 129 patients, allocating them to either a low or high dose of the cells or a placebo. While the treatment was deemed safe, no significant differences were observed in global recovery.
Stem cell therapy also may represent a breakthrough for stroke survivors, especially when combined with rehabilitation therapy ( 25 ). The two most extensive Randomized controlled trials (RCTs) for stem cell therapy in stroke rehabilitation and recovery in the US evaluated the impact of MSC in patients with stroke more than 6 months prior with safety endpoints and functional recovery endpoints. Both trials showed safety, feasibility and improved functional outcomes ( 26 ).
Stem cells and outcome measures
While early clinical trials for SCT in stroke have primarily focused on feasibility and safety, some studies have begun to evaluate efficacy ( 27 ). Selecting the appropriate patients and outcome measures to maximize stem-cell clinical trials, sensitivity, specificity and power is necessary. This is especially important in stroke and TBI, in which heterogeneous brain circuitry is affected, and plasticity is highly dynamic throughout various stages of the recovery process (e.g., acute, subacute, chronic) ( 28 ). The most frequently used outcome measures in stem cell clinical trials for stroke and TBI have included broad, domain-general actions of disability, such as the Glasgow Coma Scale (GCS), modified Rankin Scale (mRS), National Institutes of Health Stroke Scale (NIHSS) ( 29 ), European Stroke Scale (ESS) and Barthell Index (BI). These measures address broad aspects of functional impairment but lack specificity. Domain-specific outcome measures include the Fugl Meyer Assessment (FMA), Action Research Arm Test (ARAT), and performance on specific functional tasks. These measures may provide more targeted, sensitive measures of behavioral change ( 30 ) ( Figure 2 ).
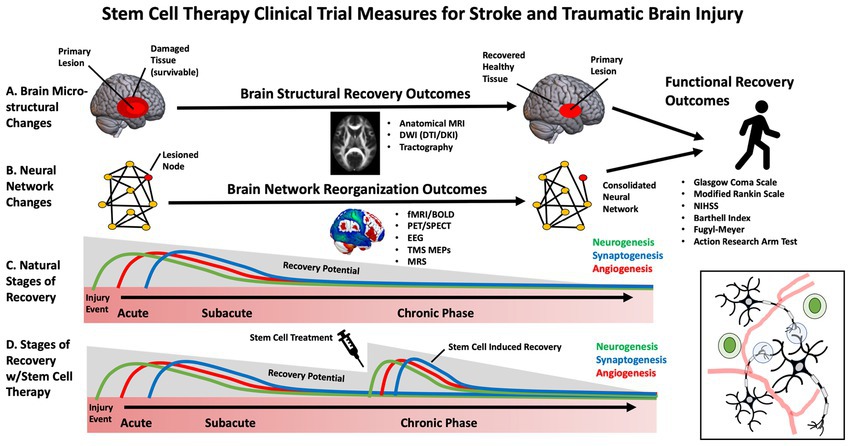
Figure 2 . Diagram of stem cell effects on brain injury recovery and outcome measures.
Biomarkers and mechanistic measures of brain recovery
While several stem-cell trials have focused on functional clinical outcome measures, there is an additional need to establish reliable biomarkers and mechanistic outcomes that capture brain-based changes during recovery ( 27 ). This enables effective translation between pre-clinical animal models and humans, allowing for more individualized and practical approaches to SCT ( 31 ). This is particularly important for disparities in stem cell clinical trials as it overcomes issues associated with language and cultural barriers that influence the reliability of subjective measures ( 13 ).
Currently there are no standardized or validated biomarkers for stroke or TBI stem cell treatments, making it difficult to determine which are optimal for clinical trials ( 32 ). Blood-based biomarkers have been investigated to measure growth factors and inflammation ( 33 ) which appear to be influenced by stem cell treatments in preclinical and clinical trials. Neurotrophic factors that support the survival and growth of brain tissue were explored in previous studies and included nerve growth factor (NGF), glial-derived neurotrophic factor (GDNF), and brain-derived neurotrophic factor (BDNF). Meanwhile, vascular endothelial growth factor (VEGF) and fibroblast growth factors (FGF) have been investigated as they may reflect vascular and tissue remodeling following injury ( 34 ). Serum-based inflammatory biomarkers can reflect the anti-inflammatory effects of stem cells and include inflammatory cytokines interleukins (IL; e.g., IL-2, IL-4, IL-6, IL1-beta, IL1-alpha, IL-10), tumor necrosis factor (TNF) alpha, and interferon-gamma among several others ( 34 ). Which of these growth factors and inflammatory biomarkers are the most sensitive and clinically meaningful within the context of stroke and TBI rehabilitation has yet to be determined still a matter of ongoing research ( 35 ). In addition to blood-based biomarkers, advances in brain imaging and non-invasive brain stimulation may prove to be useful tools in developing novel biomarkers for SCT clinical trials. These measures can be focused on changes to the primary site of injury or remote modifications, including reorganizing brain circuits affected by the injury. Recent advances in clinical neuroscience make it possible to non-invasively assess biological features of the brain, including structural integrity and neurophysiology ( 36 ). These novel tools, including neuroimaging and non-invasive brain stimulation to probe neural circuits, may be helpful in (1) developing more individualized approaches to stem cell treatment, (2) as an approach to stratify those who are most likely to benefit from a given therapy and (3) to understand how and where the cells are integrating into specific circuits or networks ( 37 ).
Brain imaging measures
Neuroimaging of the brain has undergone significant advancement over the past decades. These approaches measure the neural architecture and activity thought to underly functional recovery after stroke and TBI and may provide a more accurate measure of brain recovery than clinical assessment tools ( 38 ). Due to the non-invasive nature of these approaches and widespread accessibility across major medical centers, neuroimaging is one of the most widely used objective outcome measures for neurological clinical trials.
Structural neuroimaging can evaluate changes in the anatomical features of brain tissue, including volumetric measurements, morphology, and tissue microstructure. This has been primarily performed using MRI. Routine clinical scans including high-resolution T1 scans, diffusion-weighted imaging (DWI), susceptibility-weighted imaging (SWI) and T2 scans may be utilized to estimate gray and white matter volume, lesion volume, penumbra volume, and cortical gyrification indices and have been informative biomarkers in pre-clinical stem cell studies ( 39 ). These calculations can help monitor changes in lesion size and impact overall brain morphometry throughout the recovery period in future human trials ( 40 ). Meanwhile, advanced DWI sequences can track complex fiber pathways and detailed information about brain tissue microstructure ( 41 ). Early limitations associated with tractography derived from diffusion tensor imaging (DTI), such as complex fiber-crossing, have undergone rapid advancement with more sophisticated approaches, including diffusion kurtosis imaging (DKI) and constrained spherical deconvolution (CSD) ( 42 ). Many studies have examined how the integrity of the corticospinal tract measured using fractional anisotropy relates to motor impairment in the context of stroke ( 38 ). Although the precise biological correlates of these diffusion measures and their interpretation are still being investigated, these approaches hold promise as a sensitive measure of changes in the health of brain tissue in clinical trials with stem cell therapies.
There is a growing appreciation that brain injury and its functional consequences cannot simply be explained by damage to a single structure but rather by the connectivity of that structure to an integrated network ( 43 ). Neuroimaging is an effective tool to assess neural activity within these distributed brain networks. In the context of stroke and TBI, reorganization of neural networks may underly recover after rehabilitation ( 37 ). Thus, it will be essential to understand the impact of stem cell interventions on these large-scale networks. Determining neural activity-specific timescales and spatial resolutions for quantitative change provides a reliable measure of structural changes in the brain. Specific neuroimaging approaches can be tailored to brain assessments in the setting of stem cell infusion. Functional Magnetic Resonance Imaging (fMRI) and electroencephalography (EEG) are the most widely used approaches to study cortical networks. fMRI relies on an indirect measure of neural activation by assessing how blood oxygenation levels change over time. The resulting blood oxygen level-dependent signal (BOLD) is acquired by subtracting “resting state” activity from neural activity during or during task engagement. fMRI has been used to demonstrate neural plasticity within neural networks following brain injury ( 44 ). While less spatially precise, EEG can directly measure neuroelectric activity at a high temporal resolution which is easily scalable across medical centers. EEG may predict functional outcomes and may be correlated with mRS, the FM and the NIHSS ( 32 ). Transcranial Magnetic Stimulation (TMS) may be combined with other neuroimaging approaches to probe non-motor networks or with neurophysiological recordings to assess motor pathways. TMS motor evoked potentials (MEPs) have been used to assess corticospinal integrity following stroke and are a good prognostic indicator of the extent of functional recovery ( 45 ).
While the previously mentioned approaches may be effective at identifying network remodeling and neuroplasticity, these approaches cannot assess angiogenesis and neurogenesis associated with stem cell therapies. Imaging modalities such as Positron Emission Tomography (PET) can evaluate changes in vasculature and neuronal survivability by measuring regional cerebral blood flow (rCBF) and metabolic rate using radiotracers. Magnetic resonance spectroscopy (MRS) can also monitor changes in metabolite composition and concentration within brain tissue ( 46 , 47 ). This approach may provide a surrogate marker for cellular repair mechanisms and metabolic changes in the recovery process.
Stem cells therapy challenges
The current challenges of stem cell therapy for stroke and TBI are multifactorial and significant. First, the best source of MSCs for stroke treatment has yet to be established ( 48 , 49 ). Most preclinical studies used MSCs from healthy, young donors and about half of the clinical studies used autologous MSC ( 50 ). Harvesting stem cells from donors, especially neural stem cells (NSCs) or embryonic stem cells (ESCs), raises ethical concerns as well as concerns regarding the viability and effectiveness of stem cells from different donor types. Harvesting stem cells from donors, especially neural stem cells (NSCs) or embryonic stem cells (ESCs). Ethical issues arise primarily from the use of ESCs, which involves the destruction of embryos, and the use of NSCs, which often require fetal tissue. These ethical concerns can hinder research progress and limit the availability of stem cells for clinical use ( 51 , 52 ). Although the use of autologous MSC addresses this issue, MSC are costly and require several months for optimal production; this delays administration beyond desired treatment windows ( 49 , 53 ). The optimal timing for MSC administration is controversial; while very early transplantation within 48 h is recommended, some studies suggest benefits even 1 month post-stroke ( 54 ) ( Supplementary Table 1 ). The administration route presents another hurdle: systemic approaches like intravenous (IV) and intra-arterial (IA), compared to direct intrathecal (IC) approaches carry potential risks and benefits. For instance, IV administration may lead to pulmonary trapping of cells, whereas IC administration poses risks of infection and bleeding ( 55 ). Technical challenges include Tracking transplanted cells to ensure survival and overcoming potential immune rejection. Imaging modalities including magnetic resonance imaging (MRI), positron emission tomography (PET), single-photon emission computed tomography (SPECT/CT), or bioluminescence imaging (BLI) using green fluorescent protein-Luciferase (GFP-Luc) may be utilized for stem cells in vivo tracking ( 53 ). Safety concerns persist, especially the risk of undesirable tissue differentiation and oncogenesis, exacerbated when genetic manipulation or reprogramming is employed to augment MSCs, potentially causing unregulated cell proliferation. This is underscored by instances where stem cell transplants have induced tumorigenesis ( 56 , 57 ). Additionally, the survival and integration of transplanted cells into the host tissue remain significant hurdles. The hostile post-stroke environment, characterized by inflammation and scarring, can impede the survival and integration of transplanted cells ( 58 ).
Outcome evaluation measures should be clear and unified for patients receiving therapy whether functional, quality of life or cognitive preclinical and clinical study endpoints and outcome measurement methods were heterogeneous. Patient selection and treatment costs are other significant issues. Many stroke patients have comorbidities such as hypertension, diabetes and heart disease that may exert an impact on therapy efficacy ( 59 , 60 ). In 2018, the costs of producing autologous cell therapies were estimated to be US$ 94 per million cells for a dose of 2 million cells per kg, which is calculated to be US$ 13,160 per dose for an average-weight adult, which raises the question of whether stem cell therapy would benefit only the better socio-economic group ( 61 ). Comprehensive clinical research is essential to establish a clear transplantation protocol, considering the timing, route, and dosage for optimal therapeutic outcomes. This includes addressing the technical challenges of cell tracking, survival, and integration and ensuring ethical practices and cost-effectiveness to make stem cell therapy a viable option for a broader patient population.
Challenges in South Carolina
South Carolina (SC) is characterized by a predominantly rural demographic, with approximately 35% of its inhabitants residing in rural locales, a figure substantially higher than the national average ( 62 ). Health disparities are more frequent in rural populations due to diminished prevalence of health insurance coverage, inferior socioeconomic and educational strata, and distinct cultural and societal influences ( 63 ) Because there are barriers to clinical trial enrollment in these areas ( 64 ), continued efforts to determine the obstacles to CT enrollment in SC regarding accessibility (e.g., lack of awareness, physicians not broaching CT options, unavailability of health insurance) and cognitive/psychological impediments (e.g., deficits in subjective and objective knowledge, prevalent misconceptions, ingrained distrust, apprehensions, and perceived risk) are needed ( 65 ) ( Figure 3 ).
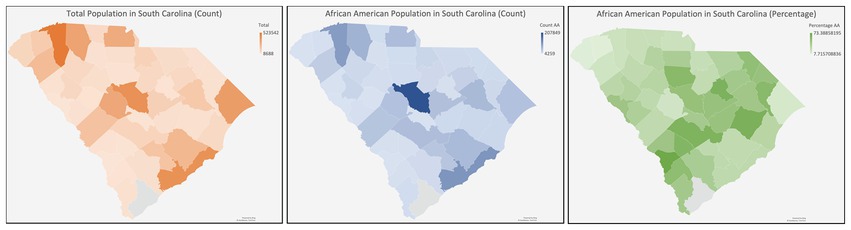
Figure 3 . Diagram of South Carolina with the racial distribution.
Several strategies can be applied to address enrollment barriers, including involving local physicians, community engagement/education, active recruitment, and financial incentives and support. A study monitoring community engagement with surgeons in the US Midwest found that most surgeons needed to be made aware of available trials and had no experience with the trial referral process ( 66 ). Furthermore, when later surveying patients following education, they described a more positive experience with their surgeons. This same study identified that facility communication and collaboration improved patient continuity of care. However, prior to seeing their referring physician, awareness of potential options extends to community and civic involvement ( 66 ). A study surveying 212 African Americans and Caucasians across rural and urban communities found that increased participation of churches/schools and family/friend referrals were more effective in rural communities versus in urban it was schools, media, and family/friends ( 67 ). This emphasizes the need to develop grassroots relationships in communities to foster a collaborative approach to medical access. This is further supported by another trial that identified low recruitment rates among rural and black individuals for palliative care clinical trials ( 68 ). In that study, recruitment strategies developed by community advisory groups aided in directing a more targeted approach to increase access and awareness of available trials. Of the 2,879 participants involved, 228 were eligible for potential trials. Of those who were enrolled in trials, only 12.7% consented when only a study coordinator was available, versus 58.8% when a community advisory group member was also present. This underlines the importance of embedded community allies in improving facility-community relationships. An analysis of recruitment strategies on pediatric RCTs in rural primary care clinics in 2022 found that utilizing traditional methods (i.e., posters, social media, press releases) was needed to complete enrollment for recruitment participation. In contrast, active enrollment (EMR-generated lists with staff follow-up) did ( 69 ). Furthermore, it reports that time to enrollment was quicker with active versus traditional methods. Lastly, financial barriers are among the most significant between rural and urban enrollment populations, as trials are ordinarily run in urban areas- thus requiring a considerable time commitment and financial commitment via travel, to participate in studies. Financial incentives alleviated these concerns and proved to be a significant motivator ( 67 ). Moreover, AAs exhibited a discernible gap in subjective and objective knowledge regarding CTs and an amplified perception of risk upon participation ( 65 , 70 ). Gender-specific data pertinent to CT enrollment remains limited; however, one survey delineating gender-related discrepancies in CT willingness unveiled that females exhibited an increased perception of potential harm from trials despite displaying heightened susceptibility to financial inducements ( 71 ). Subsequent studies discerned that clinician trust and the perceived prospective benefits (either personal or altruistic) notably influenced females’ participation in CTs ( 72 ).
Addressing these cognitive and psychological barriers requires culturally sensitive education and awareness campaigns. These campaigns should focus on dispelling misconceptions, building trust, and providing clear information about the benefits and risks of clinical trials.
Enrollment volumes for stem cell therapy clinical trials vary between states in the United States, influenced by infrastructure, funding, and public awareness. States with established stem cell research centers and robust healthcare infrastructure tend to have higher enrollment volumes. For example, California, home to the California Institute for Regenerative Medicine (CIRM) and multiple Alpha Stem Cell Clinics, sees a high volume of patient enrollment. These centers provide a collaborative infrastructure that accelerates the development and validation of stem cell therapies, making California a leader in this field. Significant state-specific funding can impact enrollment. For instance, the UC San Diego Alpha Stem Cell Clinic received a grant from CIRM to expand clinical trials, highlighting the state’s commitment to advancing stem cell research and increasing patient enrollment. States with leading academic and research institutions, such as Massachusetts and Texas often have more robust public awareness and better enrollment rates in clinical trials. Rural states or those with less developed healthcare infrastructures need help enrolling patients due to logistical issues like transportation and limited access to specialized healthcare facilities. Addressing these barriers through local physician involvement and community engagement is essential for improving enrollment rates.
Ethical considerations
In the United States, AAs have historically been subjected to experimental medical research while having limited access to quality healthcare ( 72 ). The unique socio-demographic landscape of SC, marked by its predominantly rural composition and higher-than-average AA population, necessitates a tailored approach to stem cell therapy research and application. It is not merely the impact of scientific innovation that must guide the research, but an acute understanding and acknowledgment of the existing health disparities that plague rural and AA communities in the state ( 73 ).The historical and systemic barriers these populations face, ranging from restricted access to healthcare, limited health insurance coverage, and socio-economic and educational challenges to deeply rooted cultural and societal norms, raise pivotal ethical questions ( 73 ).
Firstly, an ethical mandate is to ensure that the AA community is adequately represented in research trials, given its sizeable presence in SC. This is vital to ensure therapeutic efficacy and safety across the diverse genetic and socio-cultural landscapes. Beyond representation, the state also has higher rural demographics and inherent health disparities, emphasizing the need for equitable access. Given the intricate weave of socio-economic challenges, efforts must be undertaken to ensure that cost does not become a prohibitive barrier, particularly for the AA community and other marginalized groups in SC. Ethical considerations also extend to education and awareness campaigns. These campaigns must be culturally sensitive, addressing potential misconceptions and ensuring that the diverse populations of SC are informed and empowered to make decisions regarding stem cell therapies.
In conclusion, as SC navigates the promising terrain of stem cell research and application, it must do so with an ethical compass calibrated to its unique socio-demographic challenges. Only by doing so can the state ensure that the promise of stem cell therapies is a beacon of hope for all its residents, irrespective of race, socio-economic status, or geographical location.
Author contributions
GM: Methodology, Resources, Writing – original draft, Writing – review & editing. DL: Software, Writing – original draft, Writing – review & editing. PG: Writing – original draft, Writing – review & editing. MR: Writing – original draft, Writing – review & editing. JV: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank Dan Lackland and Mark Stacy for their advice and review of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1419867/full#supplementary-material
1. S.C. Department of Health and Environmental Control . (2020). *Stroke*. Retrieved from https://www.dph.sc.gov/diseases-conditions/conditions/heart-diseasestroke/stroke#:~:text=Stroke%20is%20South%20Carolina’s%20sixth,in%20South%20Carolina%20in%202020 .
Google Scholar
2. Feng, W, Nietert, PJ, and Adams, RJ. Influence of age on racial disparities in stroke admission rates, hospital charges, and outcomes in South Carolina. Stroke . (2009) 40:3096–101. doi: 10.1161/STROKEAHA.109.554535
PubMed Abstract | Crossref Full Text | Google Scholar
3. Odonkor, CA, Esparza, R, Flores, LE, Verduzco-Gutierrez, M, Escalon, MX, Solinsky, R, et al. Disparities in health Care for Black Patients in physical medicine and rehabilitation in the United States: a narrative review. PM&R . (2021) 13:180–203. doi: 10.1002/pmrj.12509
4. Reeves, SL, Brown, DL, Baek, J, Wing, JJ, Morgenstern, LB, and Lisabeth, LD. Ethnic differences in Poststroke quality of life in the brain attack surveillance in Corpus Christi (BASIC) project. Stroke . (2015) 46:2896–901. doi: 10.1161/STROKEAHA.115.010328
5. Johnson, WD, and Griswold, DP. Traumatic brain injury: a global challenge. Lancet Neurol . (2017) 16:949–50. doi: 10.1016/S1474-4422(17)30362-9
Crossref Full Text | Google Scholar
6. Dewan, MC, Rattani, A, Gupta, S, Baticulon, RE, Hung, Y-C, Punchak, M, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg . (2019) 130:1080–97. doi: 10.3171/2017.10.JNS17352
7. Daugherty, J, Zhou, H, Sarmiento, K, and Waltzman, D. Differences in state traumatic brain injury–related deaths, by principal mechanism of injury, intent, and percentage of population living in rural areas — United States, 2016–2018. MMWR Morb Mortal Wkly Rep . (2021) 70:1447–52. doi: 10.15585/mmwr.mm7041a3
8. Gontkovsky, ST, Sherer, M, Nick, TG, Nakase-Thompson, R, and Yablon, SA. Effect of urbanicity of residence on TBI outcome at one year post-injury. Brain Inj . (2006) 20:701–9. doi: 10.1080/02699050600744103
9. Bazarian, JJ, Pope, C, McClung, J, Cheng, YT, and Flesher, W. Ethnic and racial disparities in emergency Department Care for Mild Traumatic Brain Injury. Acad Emerg Med . (2003) 10:1209–17. doi: 10.1197/S1069-6563(03)00491-3
10. Tang, AR, Wallace, J, Grusky, AZ, Hou, BQ, Hajdu, KS, Bonfield, CM, et al. Investigation of factors contributing to racial differences in sport-related concussion outcomes. World Neurosurg . (2023) 173:e755–65. doi: 10.1016/j.wneu.2023.03.009
11. Burke, JF, Brown, DL, Lisabeth, LD, Sanchez, BN, and Morgenstern, LB. Enrollment of women and minorities in NINDS trials. Neurol Int . (2011) 76:354–60. doi: 10.1212/WNL.0b013e3182088260
12. Carcel, C, and Reeves, M. Under-enrollment of women in stroke clinical trials. Stroke . (2021) 52:452–7. doi: 10.1161/STROKEAHA.120.033227
13. Cruz-Flores, S, Rabinstein, A, Biller, J, Elkind, MSV, Griffith, P, Gorelick, PB, et al. Racial-ethnic disparities in stroke care: the American experience. Stroke . (2011) 42:2091–116. doi: 10.1161/STR.0b013e3182213e24
14. Powers, WJ, Rabinstein, AA, Ackerson, T, Adeoye, OM, Bambakidis, NC, Becker, K, et al. Guidelines for the early Management of Patients with Acute Ischemic Stroke: 2019 update to the 2018 guidelines for the early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American stroke. Stroke . (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
15. Lee, KB, Lim, SH, Kim, KH, Kim, KJ, Kim, YR, Chang, WN, et al. Six-month functional recovery of stroke patients: a multi-time-point study. Int J Rehab Res Internationale Zeitschrift fur Rehabilitationsforschung Revue internationale de recherches de readaptation . (2015) 38:173–80. doi: 10.1097/MRR.0000000000000108
16. Hatem, SM, Saussez, G, Della Faille, M, Prist, V, Zhang, X, Dispa, D, et al. Rehabilitation of motor function after stroke: a multiple systematic review focused on techniques to stimulate upper extremity recovery. Front Hum Neurosci . (2016) 10:442. doi: 10.3389/fnhum.2016.00442
17. Kowalski, RG, Hammond, FM, Weintraub, AH, Nakase-Richardson, R, Zafonte, RD, Whyte, J, et al. Recovery of consciousness and functional outcome in moderate and severe traumatic brain injury. JAMA Neurol . (2021) 78:548–57. doi: 10.1001/jamaneurol.2021.0084
18. Kawabori, M, Shichinohe, H, Kuroda, S, and Houkin, K. Clinical trials of stem cell therapy for cerebral ischemic stroke. Int J Mol Sci . (2020) 21:7380. doi: 10.3390/ijms21197380
19. Negoro, T, Okura, H, Maehata, M, Hayashi, S, Yoshida, S, and Takada, N. Trends in clinical trials for stroke by cell therapy: data mining ClinicalTrials.gov and the ICTRP portal site. NPJ Regen Med . (2019) 4:20. doi: 10.1038/s41536-019-0082-7
20. Trounson, A, and McDonald, C. Stem cell therapies in clinical trials: Progress and challenges. Cell Stem Cell . (2015) 17:11–22. doi: 10.1016/j.stem.2015.06.007
21. Prasad, K, Sharma, A, Garg, A, Mohanty, S, Bhatnagar, S, Johri, S, et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke. Stroke . (2018) 45:3618–24. doi: 10.1161/STROKEAHA.114.007028
22. Li, J, Zhang, Q, Wang, W, Lin, F, Wang, S, and Zhao, J. Mesenchymal stem cell therapy for ischemic stroke: a look into treatment mechanism and therapeutic potential. J Neurol . (2021) 268:4095–107. doi: 10.1007/s00415-020-10138-5
23. STEPS Participants . Stem cell therapies as an emerging paradigm in stroke (STEPS). Stroke . (2009) 40:510–5. doi: 10.1161/STROKEAHA.108.526863
24. Hess, DC, Wechsler, LR, Clark, WM, Savitz, SI, Ford, GA, Chiu, D, et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blindF, placebo-controlled, phase 2 trial. Lancet Neurol . (2017) 16:360–8. doi: 10.1016/S1474-4422(17)30046-7
25. Savitz, SI, Cramer, SC, Wechsler, L, Aronowski, J, Boltze, J, Borlongan, C, et al. Stem cells as an emerging paradigm in stroke 3. Stroke . (2014) 45:634–9. doi: 10.1161/STROKEAHA.113.003379
26. Borlongan, CV . Concise review: stem cell therapy for stroke patients: are we there yet? Stem Cells Transl Med . (2019) 8:983–8. doi: 10.1002/sctm.19-0076
27. Balkaya, M, and Cho, S. Optimizing functional outcome endpoints for stroke recovery studies. J Cereb Blood Flow Metab . (2019) 39:2323–42. doi: 10.1177/0271678X19875212
28. Jordan, HT, Che, J, Byblow, WD, and Stinear, CM. Fast outcome categorization of the upper limb after stroke. Stroke . (2022) 53:578–85. doi: 10.1161/STROKEAHA.121.035170
29. Kwah, LK, and Diong, J. National Institutes of Health stroke scale (NIHSS). Aust J Phys . (2014) 60:61. doi: 10.1016/j.jphys.2013.12.012
30. Gladstone, DJ, Danells, CJ, and Black, SE. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair . (2002) 16:232–40. doi: 10.1177/154596802401105171
31. Davis, KD, Aghaeepour, N, Ahn, AH, Angst, MS, Borsook, D, Brenton, A, et al. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: challenges and opportunities. Nat Rev Neurol . (2020) 16:381–400. doi: 10.1038/s41582-020-0362-2
32. Alt Murphy, M, Resteghini, C, Feys, P, and Lamers, I. An overview of systematic reviews on upper extremity outcome measures after stroke. BMC Neurol . (2015) 15:29. doi: 10.1186/s12883-015-0292-6
33. Palà, E, Bustamante, A, Jolkkonen, J, Hommel, M, Rosell, A, and Montaner, J. Blood-based biomarkers and stem cell therapy in human stroke: a systematic review. Mol Biol Rep . (2020) 47:6247–58. doi: 10.1007/s11033-020-05627-9
34. Taguchi, A, Sakai, C, Soma, T, Kasahara, Y, Stern, DM, Kajimoto, K, et al. Intravenous autologous bone marrow mononuclear cell transplantation for stroke: Phase1/2a clinical trial in a homogeneous Group of Stroke Patients. Stem Cells Dev . (2015) 24:2207–18. doi: 10.1089/scd.2015.0160
35. Turnbull, MT, Zubair, AC, Meschia, JF, and Freeman, WD. Mesenchymal stem cells for hemorrhagic stroke: status of preclinical and clinical research. NPJ Regen Med . (2019) 4:10. doi: 10.1038/s41536-019-0073-8
36. Nishimura, K, Cordeiro, JG, Ahmed, AI, Yokobori, S, and Gajavelli, S. Advances in traumatic brain injury biomarkers. Cureus . (2022) 14:e23804. doi: 10.7759/cureus.23804
37. Castellanos, NP, Leyva, I, Buldú, JM, Bajo, R, Paúl, N, Cuesta, P, et al. Principles of recovery from traumatic brain injury: reorganization of functional networks. NeuroImage . (2011) 55:1189–99. doi: 10.1016/j.neuroimage.2010.12.046
38. Jeurissen, B, and Szczepankiewicz, F. Multi-tissue spherical deconvolution of tensor-valued diffusion MRI. NeuroImage . (2021) 245:118717. doi: 10.1016/j.neuroimage.2021.118717
39. Hartman, RE, Nathan, NH, Ghosh, N, Pernia, CD, Law, J, Nuryyev, R, et al. A biomarker for predicting responsiveness to stem cell therapy based on mechanism-of-action: evidence from cerebral injury. Cell Rep . (2020) 31:107622. doi: 10.1016/j.celrep.2020.107622
40. Gauthier, LV, Taub, E, Mark, VW, Barghi, A, and Uswatte, G. Atrophy of spared gray matter tissue predicts poorer motor recovery and rehabilitation response in chronic stroke. Stroke . (2012) 43:453–7. doi: 10.1161/STROKEAHA.111.633255
41. Cheng, B, Dietzmann, P, Schulz, R, Boenstrup, M, Krawinkel, L, Fiehler, J, et al. Cortical atrophy and transcallosal diaschisis following isolated subcortical stroke. J Cereb Blood Flow Metab . (2019) 40:611–21. doi: 10.1177/0271678X19831583
42. Jensen, JH, and Helpern, JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed . (2010) 23:698–710. doi: 10.1002/nbm.1518
43. Price, CJ, Hope, TM, and Seghier, ML. Ten problems and solutions when predicting individual outcome from lesion site after stroke. NeuroImage . (2017) 145:200–8. doi: 10.1016/j.neuroimage.2016.08.006
44. Golestani, A-M, Tymchuk, S, Demchuk, A, and Goodyear, BG. Longitudinal evaluation of resting-state fMRI after acute stroke with hemiparesis. Neurorehabil Neural Repair . (2012) 27:153–63. doi: 10.1177/1545968312457827
45. Stinear, CM, Byblow, WD, Ackerley, SJ, Smith, M-C, Borges, VM, and Barber, PA. PREP2: a biomarker-based algorithm for predicting upper limb function after stroke. Ann Clin Transl Neurol . (2017) 4:811–20. doi: 10.1002/acn3.488
46. Paparella, I, Vanderwalle, G, Stagg, CJ, and Maquet, P. An integrated measure of GABA to characterize post-stroke plasticity. Neuroimage Clin . (2023) 39:103463. doi: 10.1016/j.nicl.2023.103463
47. Harston, GWJ, Okell, TW, Sheerin, F, Schulz, U, Mathieson, P, Reckless, I, et al. Quantification of serial cerebral blood flow in acute stroke using arterial spin labeling. Stroke . (2017) 48:123–30. doi: 10.1161/STROKEAHA.116.014707
48. Li, W, Shi, L, Hu, B, Hong, Y, Zhang, H, and Li, X. Mesenchymal stem cell-based therapy for stroke: current understanding and challenges. Front Cell Neurosci . (2021) 15:15. doi: 10.3389/fncel.2021.628940
49. Liu, H, Reiter, S, Zhou, X, Chen, H, Ou, Y, Lenahan, C, et al. Insight into the mechanisms and the challenges on stem cell-based therapies for cerebral ischemic stroke. Front Cell Neurosci . (2021) 15:15. doi: 10.3389/fncel.2021.637210
50. Block, TJ, Marinkovic, M, Tran, ON, Gonzalez, AO, Marshall, A, Dean, DD, et al. Restoring the quantity and quality of elderly human mesenchymal stem cells for autologous cell-based therapies. Stem Cell Res Ther . (2017) 8:239. doi: 10.1186/s13287-017-0688-x
51. Lo, B, and Parham, L. Ethical issues in stem cell research. Endocr Rev . (2009) 30:204–13. doi: 10.1210/er.2008-0031
52. Hyun, I . The bioethics of stem cell research and therapy. J Clin Invest . (2010) 120:71–5. doi: 10.1172/JCI40435
53. Manley, NC, and Steinberg, GK. Tracking stem cells for cellular therapy in stroke. Curr Pharm Des . (2012) 18:3685–93. doi: 10.2174/138161212802002643
54. Liaw, N, and Liebeskind, D. Emerging therapies in acute ischemic stroke. F1000Res . (2020) 9:9. doi: 10.12688/f1000research.21100.1
55. Levy, O, Kuai, R, Siren, EMJ, Bhere, D, Milton, Y, Nissar, N, et al. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv . (2024) 6:eaba6884. doi: 10.1126/sciadv.aba6884
56. Zhang, J, Huang, X, Wang, H, Liu, X, Zhang, T, Wang, Y, et al. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res Ther . (2015) 6:234. doi: 10.1186/s13287-015-0240-9
57. Ollier, MP, and Hartmann, L. Bidimensional immunoelectrophoresis in three stages (semi micromethod). Biomedicine . (1976) 25:184–7.
58. Hess, DC, and Borlongan, CV. Cell-based therapy in ischemic stroke. Expert Rev Neurother . (2008) 8:1193–201. doi: 10.1586/14737175.8.8.1193
59. Cipolla, MJ, Liebeskind, DS, and Chan, S-L. The importance of comorbidities in ischemic stroke: impact of hypertension on the cerebral circulation. J Cereb Blood Flow Metab . (2018) 38:2129–49. doi: 10.1177/0271678X18800589
60. Chen, J, Ye, X, Yan, T, Zhang, C, Yang, X-P, Cui, X, et al. Adverse effects of bone marrow stromal cell treatment of stroke in diabetic rats. Stroke . (2011) 42:3551–8. doi: 10.1161/STROKEAHA.111.627174
61. Krause, M, Phan, TG, Ma, H, Sobey, CG, and Lim, R. Cell-based therapies for stroke: are we there yet? Front Neurol . (2019) 10:10. doi: 10.3389/fneur.2019.00656
62. America’s Health Rankings analysis of U.S . (2023). Census Bureau, United Health Foundation, 485. Available at: AmericasHealthRankings.org . Accessed March 04, 2024.
63. U.S. Census Bureau . (2023). “U.S. Census Bureau.” Available at: https://www.census.gov/ . Accessed March 04, 2024.
64. Bergeron, CD, Foster, C, Friedman, DB, Tanner, A, and Kim, SH. Clinical trial recruitment in rural South Carolina: a comparison of investigators’ perceptions and potential participant eligibility. Rural Remote Health . (2013) 13:171–84. doi: 10.3316/informit.311580564006072
65. Kim, S-H, Tanner, A, Friedman, DB, Foster, C, and Bergeron, CD. Barriers to clinical trial participation: a comparison of rural and urban communities in South Carolina. J Community Health . (2014) 39:562–71. doi: 10.1007/s10900-013-9798-2
66. Ellis, SD, Geana, M, Mackay, CB, Moon, DJ, Gills, J, Zganjar, A, et al. Science in the heartland: exploring determinants of offering cancer clinical trials in rural-serving community urology practices. Urologic Oncol: Seminars and Original Investigations . (2019) 37:529.e9–529.e18. doi: 10.1016/j.urolonc.2019.03.004
67. Friedman, DB, Foster, C, Bergeron, CD, Tanner, A, and Kim, S-H. A qualitative study of recruitment barriers, motivators, and community-based strategies for increasing clinical trials participation among rural and urban populations. Am J Health Promot . (2015) 29:332–8. doi: 10.4278/ajhp.130514-QUAL-247
68. Gazaway, S, Bakitas, M, Underwood, F, Ekelem, C, Duffie, M, McCormick, S, et al. Community informed recruitment: a promising method to enhance clinical trial participation. J Pain Symptom Manag . (2023) 65:e757–64. doi: 10.1016/j.jpainsymman.2023.02.319
69. Darden, PM II, Davis, AM, Lee, JY, Bimali, M, Simon, AE, Atz, AM, et al. Active vs traditional methods of recruiting children for a clinical trial in rural primary care clinics: a cluster-randomized clinical trial. JAMA Netw Open . (2022) 5:e2244040. doi: 10.1001/jamanetworkopen.2022.44040
70. Kim, S-H, Tanner, A, Friedman, DB, Foster, C, and Bergeron, C. Barriers to clinical trial participation: comparing perceptions and knowledge of African American and white south Carolinians. J Health Commun . (2015) 20:816–26. doi: 10.1080/10810730.2015.1018599
71. Lobato, L, Bethony, JM, Pereira, FB, Grahek, SL, Diemert, D, and Gazzinelli, MF. Impact of gender on the decision to participate in a clinical trial: a cross-sectional study. BMC Public Health . (2014) 14:1156. doi: 10.1186/1471-2458-14-1156
72. Chu, SH, Kim, EJ, Jeong, SH, and Park, GL. Factors associated with willingness to participate in clinical trials: a nationwide survey study. BMC Public Health . (2015) 15:10. doi: 10.1186/s12889-014-1339-0
73. Stevenson, DK, Wong, RJ, Aghaeepour, N, Angst, MS, Darmstadt, GL, DiGiulio, DB, et al. Understanding health disparities. J Perinatol . (2019) 39:354–8. doi: 10.1038/s41372-018-0298-1
Keywords: stem cells, stroke, traumatic brain injury, disparities, South Carolina
Citation: Mohamed GA, Lench DH, Grewal P, Rosenberg M and Voeks J (2024) Stem cell therapy: a new hope for stroke and traumatic brain injury recovery and the challenge for rural minorities in South Carolina. Front. Neurol . 15:1419867. doi: 10.3389/fneur.2024.1419867
Received: 02 May 2024; Accepted: 16 July 2024; Published: 09 August 2024.
Reviewed by:
Copyright © 2024 Mohamed, Lench, Grewal, Rosenberg and Voeks. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ghada A. Mohamed, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

Ethics of Modern Stem Cell Research and Therapy
- © 2024
- Ernst R. von Schwarz 0
Cedars Sinai Heart Inst, Ste 6215, Cedars Sinai Medical Center, Beverly Hills, USA
You can also search for this author in PubMed Google Scholar
- Discusses the FDA regulations for stem cell research
- Offers an authoritative and comprehensive overview of the ethical issues surrounding stem cell research
- Provides the expertise of a clinical cardiologist who has conducted clinical studies of stem cell therapy
26 Accesses
This is a preview of subscription content, log in via an institution to check access.
Access this book
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Other ways to access
Licence this eBook for your library
Institutional subscriptions
About this book
This book provides an authoritative and comprehensive overview on the ethical issues surrounding the most promising and most controversially discussed topic in modern biotechnology and medicine, stem cell therapy. It is written by a scientist who has been involved in the basic research of stem cell therapy for over 20 years and was part of the initial experimental studies demonstrating benefits and damage repair using stem cells, and who also is a clinical cardiologist involved in the clinical studies of stem cell therapy in patients with mainly cardiovascular and neurodegenerative diseases from its early stage until now.
The book starts with a brief overview of the history of stem cell research, the administrative and regulatory aspects including the federal governmental changes with every political administration over the last 20 years, and the stand of the FDA on research and therapy. It also discusses the issues of medical tourism, patient funded studies, false marketing claims, and the ethical and religious aspects of stem cell research, anti aging research, and immortality research including the Roman Catholic Churches view on embryonic stem cells.
Ethics of Modern Stem Cell Research and Therapy will be a valuable resource for clinicians, physicians, and researchers who are looking to either conduct or use stem cell therapies. It offers a one-stop guide to the current regulations of the FDA as well as the potential that stem cell therapies have on many degenerative diseases.
- Stem Cell Ethics
- Future Medicine
- Stem Cell Research
- Stem Cell Research FDA Regulations
- Unapproved Stem Cell Businesses
- Guidelines for Stem Cell Research
- Stem Cell Therapy
- Stem Cell Research Ethics
- Modern Medicine Ethics
- Stem Cell Research Regulations
Table of contents (6 chapters)
Front matter, background on ethics.
Ernst R. von Schwarz
Regulations and Guidelines
Ethical issues, religious views on stem cells, controversial remaining ethical questions, back matter, authors and affiliations, about the author.
Dr Ernst R von Schwarz, MD, PhD , is a triple board certified internist, cardiologist, heart transplant cardiologist and interventional cardiologist in Los Angeles, and a PhD in Roman Catholic Theology. He is former Professor of Medicine at Cedars Sinai Medical Center and Clinical Professor at the David Geffen School of Medicine at UCLA and UC Riverside. Dr Schwarz is world renowned as a clinical and academic heart specialist and serves as the Director of Cardiology and Director of the Heart Institute of the Southern California Hospital in Los Angeles, as well as Director and President of the Pacific Heart Medical Group in Murrieta, and CMO of the Dr von Schwarz Anti-Aging and Stem Cell Institute in Beverly Hills.
Dr Schwarz has published more than 150 scientific articles in international peer reviewed journals, several book chapters and books in Cardiology and Theology, and is a sought after speaker at international scientific conferences worldwide. Dr Schwarz is one of the thought leaders in modern future technologies including stem cell therapies for chronic diseases for the heart and other organs. Students from Universities from all over the world seek internships with Dr Schwarz on an ongoing basis.
Dr Schwarz studied Medicine at the Universities of Vienna in Austria and the Philipps University in Marburg, Germany, and he worked and earned academic positions at the RWTH University of Technology in Aachen, Germany, the University of Ife in Ile-Ife in Nigeria, a Harvard affiliated hospital in Jeddah, Saudi Arabia, the University of Texas in Galveston, Texas, and Cedars Sinai Medical Center and UCLA in L.A.. He resides in Los Angeles and in Germany and has clinical practices in Beverly Hills, Culver City, and Temecula, California.
Bibliographic Information
Book Title : Ethics of Modern Stem Cell Research and Therapy
Authors : Ernst R. von Schwarz
DOI : https://doi.org/10.1007/978-3-031-64843-4
Publisher : Springer Cham
eBook Packages : Medicine , Medicine (R0)
Copyright Information : The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG 2024
Hardcover ISBN : 978-3-031-64842-7 Published: 06 August 2024
Softcover ISBN : 978-3-031-64845-8 Due: 20 August 2025
eBook ISBN : 978-3-031-64843-4 Published: 05 August 2024
Edition Number : 1
Number of Pages : XIX, 152
Number of Illustrations : 1 illustrations in colour
Topics : Medicine/Public Health, general
- Publish with us
Policies and ethics
- Find a journal
- Track your research

- News Releases
Potential new approach to enhancing stem-cell transplants
Albert Einstein College of Medicine
Credit: Albert Einstein College of Medicine
August 8, 2024—(BRONX NY)— A discovery by a three-member Albert Einstein College of Medicine research team may boost the effectiveness of stem-cell transplants, commonly used for patients with cancer, blood disorders, or autoimmune diseases caused by defective stem cells, which produce all the body’s different blood cells. The findings, made in mice, were published today in the journal Science .
“Our research has the potential to improve the success of stem-cell transplants and expand their use,” explained Ulrich Steidl, M.D., Ph.D. , professor and chair of cell biology , interim director of the Ruth L. and David S. Gottesman Institute for Stem Cell Research and Regenerative Medicine , and the Edward P. Evans Endowed Professor for Myelodysplastic Syndromes at Einstein , and deputy director of the National Cancer Institute-designated Montefiore Einstein Comprehensive Cancer Center (MECCC).
Dr. Steidl, Einstein’s Britta Will, Ph.D. , and Xin Gao, Ph.D . , a former Einstein postdoctoral fellow, now at the University of Wisconsin in Madison, are co-corresponding authors on the paper.
Mobilizing Stem Cells Stem-cell transplants treat diseases in which an individual’s hematopoietic (blood-forming) stem cells (HSCs) have become cancerous (as in in leukemia or myelodysplastic syndromes) or too few in number (as in bone marrow failure and severe autoimmune disorders). The therapy involves infusing healthy HSCs obtained from donors into patients. To harvest those HSCs, donors are given a drug that causes HSCs to mobilize, or escape, from their normal homes in the bone marrow and enter the blood, where HSCs can be separated from other blood cells and then transplanted. However, drugs used to mobilize HSCs often don’t liberate enough of them for the transplant to be effective.
“It’s normal for a tiny fraction of HSCs to exit the bone marrow and enter the blood stream, but what controls this mobilization isn’t well understood,” said Dr. Will, associate professor of oncology and of medicine , and the Diane and Arthur B. Belfer Faculty Scholar in Cancer Research at Einstein, and the co-leader of the Stem Cell and Cancer Biology research program at MECCC. “Our research represents a fundamental advance in our understanding, and points to a new way to improve HSC mobilization for clinical use.”
Tracking Trogocytosis The researchers suspected that variations in proteins on the surface of HSCs might influence their propensity to exit the bone marrow. In studies involving HSCs isolated from mice, they observed that a large subset of HSCs display surface proteins normally associated with macrophages, a type of immune cell. Moreover, HSCs with these surface proteins largely stayed in the bone marrow, while those without the markers readily exited the marrow when drugs for boosting HSCs mobilization were given.
After mixing HSCs with macrophages, the researchers discovered that some HSCs engaged in trogocytosis, a mechanism whereby one cell type extracts membrane fractions of another cell type and incorporates them into their own membranes. Those HSCs expressing high levels of the protein c-Kit on their surface were able to carry out trogocytosis, causing their membranes to be augmented with macrophage proteins—and making them far more likely than other HSCs to stay in the bone marrow. The findings suggest that impairing c-Kit would prevent trogocytosis, leading to more HSCs being mobilized and made available for transplantation.
“Trogocytosis plays a role in regulating immune responses and other cellular systems, but this is the first time anyone has seen stem cells engage in the process. We are still seeking the exact mechanism for how HSCs regulate trogocytosis,” said Dr. Gao, assistant professor of pathology and laboratory medicine at the University of Wisconsin-Madison, Madison, WI.
The researchers intend to continue their investigation into this process: “Our ongoing efforts will look for other functions of trogocytosis in HSCs, including potential roles in blood regeneration, eliminating defective stem cells and in hematologic malignancies,” added Dr. Will.
The study originated in the laboratory of the late Paul S. Frenette, M.D. , a pioneer in hematopoietic stem cell research and founding director of the Ruth L. and David S. Gottesman Institute for Stem Cell Biology and Regenerative Medicine Research at Einstein. Other key contributors include Randall S. Carpenter, Ph.D., and Philip E. Boulais, Ph.D., both postdoctoral scientists at Einstein.
The Science paper is titled, “Regulation of the hematopoietic stem cell pool by c-Kit-associated trogocytosis.” Additional authors are Huihui Li, Ph.D., and Maria Maryanovich, Ph.D., both at Einstein, Christopher R. Marlein, Ph.D., at Einstein and FUJIFILM Diosynth Biotechnologies, Wilton, England, and Dachuan Zhang, Ph.D., at Einstein and Shanghai Jiao Tong University School of Medicine, Shanghai, China, Matthew Smith at the University of Wisconsin-Madison, and David J. Chung, M.D., Ph.D., at Memorial Sloan Kettering Cancer Center, New York, NY.
The study was funded by grants from the National Institutes of Health (U01DK116312, R01DK056638, R01DK112976, R01HL069438, DK10513, CA230756, R01HL157948 and R35CA253127).
About Albert Einstein College of Medicine Albert Einstein College of Medicine is one of the nation’s premier centers for research, medical education and clinical investigation. During the 2023-24 academic year, Einstein is home to 737 M.D . students, 209 Ph.D . students, 124 students in the combined M.D./Ph.D. program , and approximately 239 postdoctoral research fellows . The College of Medicine has more than 2,000 full-time faculty members located on the main campus and at its clinical affiliates . In 2023, Einstein received more than $192 million in awards from the National Institutes of Health. This includes the funding of major research centers at Einstein in cancer, aging, intellectual development disorders, diabetes, clinical and translational research, liver disease, and AIDS. Other areas where the College of Medicine is concentrating its efforts include developmental brain research, neuroscience, cardiac disease, and initiatives to reduce and eliminate ethnic and racial health disparities. Its partnership with Montefiore , the University Hospital and academic medical center for Einstein, advances clinical and translational research to accelerate the pace at which new discoveries become the treatments and therapies that benefit patients. For more information, please visit einsteinmed.edu , follow us on Twitter , Facebook , Instagram , LinkedIn , and view us on YouTube .
Method of Research
Experimental study
Subject of Research
Article title.
Potential New Approach to Enhancing Stem-Cell Transplants
Article Publication Date
Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for the use of any information through the EurekAlert system.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Onco Targets Ther
A Concise Review: The Role of Stem Cells in Cancer Progression and Therapy
Hasaan hayat.
1 Precision Health Program, Department of Radiology, College of Human Medicine, Michigan State University, East Lansing, MI, USA
2 Lyman Briggs College, Michigan State University, East Lansing, MI, USA
Hanaan Hayat
3 Institute for Quantitative Health Science and Engineering, Michigan State University, East Lansing, MI, USA
Bennett Francis Dwan
4 College of Natural Science, Michigan State University, East Lansing, MI, USA
Mithil Gudi
Jack owen bishop.
5 Department of Radiology, College of Human Medicine, Michigan State University, East Lansing, MI, USA
The properties of cancer stem cells (CSCs) have recently gained attention as an avenue of intervention for cancer therapy. In this review, we highlight some of the key roles of CSCs in altering the cellular microenvironment in favor of cancer progression. We also report on various studies in this field which focus on transformative properties of CSCs and their influence on surrounding cells or targets through the release of cellular cargo in the form of extracellular vesicles. The findings from these studies encourage the development of novel interventional therapies that can target and prevent cancer through efficient, more effective methods. These methods include targeting immunosuppressive proteins and biomarkers, promoting immunization against tumors, exosome-mediated CSC conversion, and a focus on the quiescent properties of CSCs and their role in cancer progression. The resulting therapeutic benefit and transformative potential of these novel approaches to stem cell-based cancer therapy provide a new direction in cancer treatment, which can focus on nanoscale, molecular properties of the cellular microenvironment and establish a more precision medicine-oriented paradigm of treatment.
Introduction
Conventionally, cancer therapy has relied on various pharmacological and radiation-based interventions, often through means of chemotherapy and radiotherapy. 1–3 Current challenges in the clinical success of cancer therapy result from limitations in the interventional mechanisms themselves. Often, this is due to patient incompatibility with treatment, a unique disease phenotype or rapid drug resistance. 4 This results in low rates of patient remission and greater rates of mortality. 4 A new and developing area of research has opened up the realm of cancer therapy through a deeper focus on a novel interventional paradigm for cancer: stem cell therapy. Although stem cell therapy has remained an ongoing area of research with many new developments in cell-based therapies (CBT) for different diseases including autoimmune disorders and regenerative medicine, the molecular relationship between cancer stem cells (CSCs) and cancer pathogenesis has now grown into a budding realm of interest. 5 This is due to various studies that have highlighted the critical role of CSCs in promoting a tumorigenic environment. 6 A greater focus on researching the role of stem cells including CSCs in cancer progression and development will permit the creation of novel therapies and technologies that can target cancers at earlier stages of pathogenesis. This can also allow for long-term resolutions to many cancers because the highly transformative properties of stem cells can be repurposed for targeting cancer cells through genetic or phenotypic alteration, a mechanism which is in contrast to short-term remedies like chemotherapy. 7 The current reliance on radiation therapy for cancer and the use of chemo drugs which impact healthy, endogenous cellular functions results in greater, more lethal side effects. 8 , 9 To circumnavigate this issue, a focus on genetic and molecular therapies that do not impact normal, healthy cell function can support the development of longitudinal therapies for cancer with reduced side effects and morbidity rates. This approach is made feasible through studying the interactions amongst components of the tumor microenvironment, particularly with a focus on stem cell interactions and cell-conversion in cancer pathogenesis. Hence, due to its potential to transform the current narrative in the approach to cancer therapy, here we explore the current state of the role of stem cells in cancer progression and therapy. Particularly, we summarize numerous studies that explore potential areas of focus when targeting stem cells for cancer therapy, including targeting markers on CSCs, stem cells for immunization against tumors, using stem cells as carriers of therapeutic cargo, quiescent properties of stem cells, and targeting exosomes to prevent (Epithelial Mesenchymal Transition) EMT and metastasis.
Targeting Cell-Surface and Intracellular Markers of Cancer Stem Cells (CSCs) for Cancer Therapy
Primary cancer cells have been found to derive from stem cells, with a subsection of these cells named “cancer stem cells” (CSC’s). CSCs replicate similar characteristics to regular stem cells, such as the ability to proliferate in their microenvironments. 10 CSCs sustain the cancer by promoting proliferation, and therefore must be targeted when attempting to eliminate cancer for successful and long-lasting results. 11 As do most healthy cells and hematopoietic stem cells, pancreatic, liver, and lung CSCs overexpress CD-47 on their cell surface, which is an immunosuppressive defense signal that inhibits attacks from macrophages on cancer cells. 12 Normally, CD-47 plays a positive role of defense in protecting healthy cells in organs and tissue from harmful attack by macrophages. 13 , 14 Similarly, in CSC’s, the CD-47 protein acts as an immune checkpoint blockade for any targeted attempts to diminish the CSC’s by activated macrophages in the tumor microenvironment. 15 , 16 CD-47 elicits its effects via interaction with signal regulatory protein alpha (SIRPα) to inhibit phagocytosis of normal cells, which suppresses the phagocytic activity of immune cells, particularly macrophage phagocytosis. 17 CD-47 is overexpressed in many human malignancies. 18 One study performed simultaneous silencing of CD-47 and PD-L1 in order to enhance immunotherapy against circulating tumor cells. 19 Inhibiting PD-L1 allowed immune cells to locate tumor cells more adequately, and blockade of CD-47 permitted macrophage-mediated destruction of the tumor cells. In vitro flow cytometry confirmed overexpression of CD-47 and PD-L1 in the tumor cell line. 19 Compared to the blank controls or single-antibody group, dual inhibition of these immunosuppressive proteins resulted in a more potent reduction of solid tumors in mice.
In order to target CD-47 and silence its downstream effects, various forms of pharmacological and nanomedicine-based approaches have been established. An antibody named Hu5F9-G4 that targets CD-47, allowing macrophages to destroy the cancer cells, has been developed. Another similar antibody, Rituximab, which has been known to positively amplify destruction signals inhibited by CD-47, is highly active and is well tolerated as first-line single-agent therapy for indolent non-Hodgkin lymphoma (NHL). 20 Using the application of both antibodies, Hu5F9-G4 and Rituximab, the results of a clinically evaluated study on the treatment outcome in patients of NHL concluded that at least 50% of the test subjects had eliminated most symptoms of cancer. 21 The authors did not report directly on tumor size, but clinical evaluation of response to treatment indicated that more than 60% of patients had complete or partial response to the drug, a metric that relates to change in tumor size. PET-CT of patients indicated partial to complete remission of the lymphoma in male and female adult subjects. 21 This method of targeting CD-47 protein on Cancer Stem Cells has been shown to result in no detectable side effects in human beings, and thus may pave new routes for immunotherapy towards many forms of cancer by targeting cancer stem cells. 22 This is because in the past, various CSCs in cancers such as pancreatic, lung, and breast cancers have been proven to express CD-47. 23–25 Hence, targeting this immune blockade molecule expressed on CSCs may provide a new avenue of cancer treatment.
However, current limitations to therapies targeting CD47 result from its presence on an abundance of normal, healthy cells. Thus, the attack on host cells as a side effect of treatment is a potential roadblock in CD47-based therapy. To circumnavigate this issue, several CSC relevant markers have been elucidated in efforts to target CSCs specifically without damaging native cells. CD44 and CD133 are widely used and accepted as relevant cell-surface markers for CSCs. 26–30 CD44 has implications in being a CSC marker for breast, pancreatic, and head and neck cancers. 28 The enrichment of CD44 cells after drug treatment indicates higher rates of proliferation and a greater resistance to drug-induced death, helping dictate CD44 as a negative prognostic factor. 31 CD133 holds implications in acting as a CSC marker for brain, colon, and prostate cancers. 32 CD133 has been shown to upregulate the FLIP (FLICE-like inhibitory protein) which aids in CSC resistance to apoptosis. 32 CD133 is also a vastly recognized chemo-resistant CSC surface antigen, thus aiding in the prognosis of treatment. By inhibiting CD133, the cell’s proliferation pathways are also inhibited. A larger presence of CD133 on the tumor may require alternative chemotherapeutic agents, or a different treatment. 32
The benefit of identifying CSC-surface molecules lies in their potential to act as targets for new cytotoxic therapies, 33 often those which are mediated by neutralizing antibodies, in combination with CSC-directed therapy. Several studies have recently explored this form of combined therapy which prevents the repeated formation of tumors and inhibits recurrent population of CSCs. One such study evaluated the effect of a combined therapy on drug-resistant triple-negative breast cancer (TNBC) cells by targeting the TGF-β of the CSC. 34 Particularly, the group demonstrated that the TNBC CSC population maintained a unique ability to upregulate IL-8 in response to TGF- β signaling following chemotherapy with Paclitaxel (a mechanism which contributes to drug resistance). Using a TGF- β type 1 receptor kinase inhibitor, a TGF- β type II receptor neutralizing antibody, and SMAD-4 siRNA as forms of combined therapy with Paclitaxel, the subsequent recruitment of IL-8 following chemotherapy was blocked and the expansion of the chemotherapy-resistant CSC populations was inhibited. This study shows the ability of combined therapy to both inhibit primary mammosphere and further prevent drug resistance in the CSC population of the TNBC population. Besides above-mentioned markers, Aldehyde dehydrogenase 1 (ALDH1) has also been reported as a marker of cancer stem cells could be targeted for molecular therapy. 35 , 36 Table 1 provides a list of common cell-surface and intracellular markers pertaining more specifically to CSCs.
A List of Common Cell-Surface and Intracellular Markers Pertaining to CSCs
| Marker | Cancers (Found in) | Location |
|---|---|---|
| CD44 | Melanoma, Oral Squamous Cell Carcinoma, Primary Pancreatic Cancer | Cell Surface |
| CD133 | Colorectal Cancer, Breast Cancer | Cell Surface |
| ALDH1 | Invasive Ductal Carcinoma (Breast Cancer), Ovary Adenocarcinoma, Liver Hepatocellular Carcinoma | Intracellular |
| TGF-β | Glioblastoma, Breast Cancer | Cell Surface |
Stem Cells for Immunization Against Tumors
At the beginning of the 20th century, Frederick Schöne noted that fetal tissue vaccination could suppress transplanted tumor growth in mice. 37 However, it took many more years for other groups to further investigate the potential of this discovery. In the 1960s and 70s, research in this area resumed, and investigators reported mice immunized against embryonic material could prevent tumor growth, priming their bodies to recognize and fight cancer cells. However, these results tended to be weak and hard to reproduce. 38–40 Furthermore, ethical concerns and technological limitations during this time period made further research in humans impossible. With recent progress involving embryonic cell lines, research into this area has been revisited. These include studies that found very similar RNA transcript profiles and surface antigen expression between embryonic cells and different cancer cell lines, including pancreatic cancer, prostate cancer, breast cancer, myeloid leukemia, and glioblastoma. 41 , 42 Furthermore, ES and cancer cells have both been shown to exhibit similar markers of stemness, particularly when these cancer cells are less differentiated, or more immature. 42 In this study by Ben-Porath et al, poorly differentiated breast tumors were shown to display an ESC-like expression signature, more so than further differentiated tumors. As ESCs are known to exhibit stemness, this shows the possibility that more immature tumors (less differentiated) may exhibit higher tumor stemness than mature tumors. On this point of stemness, stemness is usually described as the ability for stem cells to balance between a few different processes: proliferation, quiescence, regeneration, and differentiation. 43 Stem cells rely on interactions with and signals within their microenvironment to determine which of these processes to undergo. 44 Cancer cells exhibit stemness as well, and can use this stemness to survive stress and treatment, and preserve their lineage. 43 Discovering the similarity in gene and surface antigen expression between embryonic stem cells (ESCs) and cancer cells, we now understood why these cells could potentially be used to as an anti-tumor vaccination. Moreover, since the discovery of induced pluripotent stem cells, groups have shown that the transcriptomes and antigens of ESCs and iPSCs are almost identical, 45 and that induced pluripotent stem cells (iPSCs) also show potential as an immunization agent. In fact, iPSC is most likely advantageous compared to ESCs for this purpose, as using iPSCs from a specific patient would be more representative of the patient’s own immunogens. 46 Furthermore, a theoretically unlimited number of iPSCs can be generated from each patient, given enough time. In 2018, a study used tumor-specific antigens and tumor-associated antigens expressing iPSCs to prime the immune systems of mice, followed by the transplantation of different tumors, including melanoma and breast cancer. 47 Significant regression of these tumors was found when compared to the control group. This result was attributed to the upregulation in mature antigen-presenting cells in the lymph nodes, which led to an increase in helper and cytotoxic T-cells. This group then isolated T-cells and tumor-experienced lymphocytes (TELs) from mice with tumor and that had received vaccination and transferred them to mice with tumor that had not received vaccination. Both of these groups experienced tumor regression. However, it is important to note that this iPSC vaccination was only able to slow or prevent the growth of tumors that were transplanted after vaccination; it was not effective in preventing the growth of tumors that have already been established in vivo. This continues to be the shortcoming of using stem cells as a therapeutic treatment; their effect is diminished when tumor transplant and growth takes place before the vaccination. For this reason, they tend to provide better results when used as a prophylactic treatment, as opposed to a therapeutic treatment after disease onset. The safe application of ESC and iPSC–based technologies requires the use of methods of iPSCs production and their directed differentiation which minimize both the possibility of mutations in cell genomes under in vitro culturing and the probability of malignant transformation of the injected cells. 48 Some have suggested that vaccinations including CSC lysates would improve outcomes, such as a vaccination that Lin et al formulated. 49 , 50 They combined CSC dendritic cells, which present tumor-associated antigens to T cells, with melanoma and carcinoma tumor models, which showed promise in increasing protective immunity against tumor cell challenge. However, as pointed out in a recent review by Chu et al, the isolation of enough number of CSCs (in this case, CSC-DCs) from tumor tissues is very challenging, which poses difficulties in access and quantity for possible future study in larger animal models, or in the clinic. 51 As discussed earlier, iPSCs can be made in large quantities and from each individual patient, but they do not provide adequate tumor suppression when tumor has been established. Combination vaccines with CSCs and tumor cells show promise but provide isolation and collection challenges. A very recent study, published in 2020, provides improvement on cancer vaccines for tumor rejection. 52 In this study, this group enriched a whole-cell melanoma vaccine with stem cells (this vaccine also contained a molecular adjuvant, cytokine Hyper-IL6). One vaccine was enriched with melanoma stem-like cells from B16F10 melanospheres, while the other vaccine contained mouse-induced pluripotent stem cells (miPSCs). While both vaccines showed impressive reductions in tumor growth, and in disease-free and overall survival of the immunized mice, the most effective vaccine was the one containing miPSCs. This study provides exciting evidence that vaccines containing iPSCs with tumor cells can be just as effective if not more effective than those containing the harder to obtain tumor stem cells. However, once again, this study was only done with immunization before tumor transplant, so no conclusions about treatment after transplant and tumor establishment can be made. Currently, we can only suggest stem cell vaccine for cancer treatment in addition to other treatments such as surgery, radiation, and chemotherapy, and not as a standalone therapy option, as it has only been seen as effective before tumor formation and progression. 47 , 51 , 53
Stem Cells as Therapeutic Carriers
Genetic modification enhances the therapeutic potential for stem cells in oncology by facilitating precise secretion of bioactive mediators. Typically derived from bone marrow, endogenous mesenchymal stem cells (MSCs) migrate towards sites of damaged tissue. MSC tropism is propagated by a cascade of signaling mechanisms and chemokines which trigger the recruitment of MSCs towards sites of damaged tissue. 54 , 55 MSCs are able to mobilize effectively as they express numerous chemokine receptors including: CCR1, CCR2, CCR4, CCR7, CCR8, CCR9, CCR10, CXCR1, CXCR2, CXCR3, CXCR4, CXCR5, CXCR6, and CX3CR1. 56 Additionally, MSCs possess the ability to produce a diverse array of cell adhesion molecules which facilitate the engraftment to specific target tissue. 57 Upon transplantation, MSCs will migrate away from the initial injection site into a tumor microenvironment (TME) before engrafting to various target cells. Therefore, the transduction of MSCs and other multipotent stem cells could potentially facilitate the decisive delivery of a therapeutic payload within a tumor microenvironment. Specifically, virally transduced MSCs and Neural Stem Cells (NSCs) have exhibited the expression of chemotactic cytokines, interleukins, interferons, growth factors and prodrug-converting enzymes. 58 , 59 The latter of which constitutes the technique known as gene-directed enzyme prodrug therapy (GDEPT). This treatment method allows various non-toxic prodrugs to be converted into their active forms via non-endogenous enzymes produced by genetically modified stem cells. 60 The aggregate of these characteristics makes GDEPT uniquely qualified to treat gliomas, medulloblastomas, and other brain tumors. Another benefit of this therapy stems from the ability of MSCs to manipulate tight junctions within the blood brain barrier (BBB), temporarily inhibiting its exclusion properties and allowing for the seamless traversal of MSCs into the cortex; MSCs then utilize tumor-tropism mechanisms to infiltrate and destroy tumor cells in the brain. 59 , 61
Another means by which stem cells can serve as therapeutic carriers is by the precise delivery of nanoparticles (NPs) bearing anti-cancer drugs and various other oncolytic mediators. NPs have long been used in the distribution of drugs used to treat cancer. However, the applicability of NPs is limited due to the lack of accurate targeting, their tendency to be internalized by a wide variety of normally function cells, and their rapid excretion from the body. 62 One study analyzing the nanodrug deposits provided by MSCs internalized within mice found that NPs exhibited more accurate delivery of therapeutics in a developed orthotopic lung tumor. 63 An additional study conducted using rats has demonstrated that MSCs infiltrate tumor tissue uniformly and that this infiltration leads to a more uniform distribution of a therapeutic payload. However, in the same study, they found no evidence to suggest MSCs could engage in long-distance tropism for a series of gliomas. 64 Despite this, NPs conjugated to anti-cancer agents can be delivered into a tumor microenvironment reliably using stem cell-mediated tumor tropic delivery. Furthermore, MSCs retain their inherent ability to sense tumors and respond to chemokines following the anchoring of nanoparticles to their surface. In fact, there is no significant difference in tumor tropism between traditional MSCs and those bound to NPs. 65 However, in the latter case, the half-life of the nanoparticle is increased exponentially. 66 , 67
MSCs, with their innate tumor tropism characteristics, are ideal agents for this style of theranostic-based therapy. This is because the inherent tumor tropism presents various biomarkers that can be used as targets for nanoparticles which can then be imaged in vivo using clinically relevant imaging modalities such as Magnetic Resonance Imaging (MRI) and Nuclear imaging. 53 , 68–70 Nouri et al demonstrate that theranostic MSCs are a reliable cell-based, non-viral or viral vectors for suicide gene therapy of cancer using enzyme/prodrug systems. 71 MSCs were used as a medium to perform the first comparative study that illustrated the impact of subtle differences among various enzyme/prodrug systems such as thymidine kinase/ganciclovir (TK/GCV), yeast cytosine deaminase/5-fluorocytosine (yCD/5-FC) and nitroreductase/CB1954 (NTR/CB1954) on the therapeutic outcome. MSCs were genetically modified to stably express a panel of four suicide genes including TK (TK007 and TKSR39 mutants), yeast cytosine deaminase: uracil phosphoribosyltransferase (yCD:UPRT) and nitroreductase (NTR). Then, they evaluated the anticancer efficacies of the genetically engineered MSCs using SKOV3 cell models in vivo. In addition, all MSCs were engineered to stably express luciferase gene making them suitable for quantitative imaging and dose–response relationship studies in vivo. The study results demonstrated that yCD:UPRT/5-FC was the most effective enzyme/prodrug system among the ones tested with this theranostic imaging platform. 71
It is worth noting that theranostics can be applied to nanodrug therapy for cancer. Liu et al reported that a silica-based multifunctional NP system encapsulated a chemotherapeutic agent and magnetic cores and coated with a specific antibody against the lung CSCs was systematically studied in vivo. These NPs were systematically administered and activated for targeted chemotherapy and thermotherapy by using an externally applied alternating magnetic field (AMF). 72 The application of an AMF causes localized induced hyperthermia in the areas in which the nanoparticles accumulate. 73 This can kill the tumor cells either directly or indirectly. The cytotoxic thermal effects of the localized hyperthermia from the NPs directly ablate the tumor cells. There is also an indirect mechanism which supports dendritic cell activation and immune cell migration to the tumor microenvironment. This is caused by the hyperthermia which induces the production of Heat Shock Protein (HSP) and a subsequent increase in presentation of these antigens on the cell surface, which induces dendritic cells and triggers a greater host immune response towards the cancer cells. 74 The antibody-modified NPs targeted to lung CSCs with extended accumulation in tumors after systemic injection. In in vivo models, this hyperthermia and chemotherapeutic combined therapy significantly suppressed tumor growth and metastasis in lung CSC xenograft-bearing mice, with minimal side effects and adverse effects. 72 This work demonstrated the feasibility of developing multifunctional nanomedicine targeting CSCs for effective cancer treatment, which can be monitored by magnetic particle imaging or MRI. 75–77 Figure 1 highlights some of the aforementioned mechanisms of using stem cells as carriers of therapeutic payload and alternative therapeutic carriers such as NPs for tumor treatment.

Therapeutic carrier models for cancer treatment. (Left) Cancer stem cells (CSCs) loaded with therapeutic cargo can be transplanted or delivered to site of tumor formation (tumor bed). (Right) Nanoparticles (NPs) targeting the CSCs can deliver chemotherapeutic payload and induce hyperthermia with an external applied magnetic field (AMF).
Quiescent Properties in Treatment
Anti-cancer therapies often utilize a combination of procedures and drugs that target gatherings of tumor cells. CSCs have long been understood now as a group of cells that fuel the growth of tumors and have properties that allow them to persist through the electromagnetic and chemical treatment that are common in contemporary practice. 78 CSCs separate themselves among cancer cells due to their ability to maintain a long, slow growing quiescent state. This dormancy allows for the cells to be preserved in spite of conventional cancer treatments that are used to combat their progeny as well as posing long-term tumorigenic potential. 78 Previous studies have shown that there maintains a population of chemotherapy-resistant cancer cells that demonstrated unique properties of self-renewal and increased potential for tumor formation. In these studies, CSC-marker expressing cells have survived treatment such as neoadjuvant chemotherapy whereas cells without CSC markers were destroyed. 79
Their unique properties have only added on to the need for the development of new therapeutic strategies that exceed the scope of conventional antiproliferative agents and treatments. Much research is focused on CSC’s quiescent properties as a potential target for treatment. Research has shown that this quiescent function is not simply a dormant state, but rather it is actively maintained by the cell by downregulating known regulators of the cell cycle such as cyclin A2 and E2 as well as mitotic regulators such as survivin. 80 There are generally three main approaches through which research is attempting to eradicate these quiescent stem cells in tumors. The first involves driving quiescent cells to reenter a normal cell cycle state by stopping cellular mechanisms that drive quiescence and then attack them with chemotherapeutic agents that only function on proliferating cells. 81 This theory is primarily supported by the notion that cell quiescence is defined as being in a reversible G0 state that requires maintenance. 81 For example, Fbxw7 has been understood to play a crucial role in the maintenance of quiescence, but its ablation along with the introduction of Imatinib treatment, a tyrosine-kinase inhibitor, is shown to interrupt the quiescent state of the studied leukemia-initiating cells (LICs) and contribute to their depletion. 82 The second method that has been proposed is one where pharmacological intervention is used to maintain CSCs in their quiescent state throughout the lifetime of their patient so that they will not cause any future tumor growth or metastasis. This strategy attempts to lock these quiescent cells in the G0 state. One such way to accomplish this is to inhibit Src kinase signaling along with inhibiting MEK1/2 as they are both factors in cell cycle progression. This can be achieved through pharmacological means and the prevention of cell cycle progression will induce apoptosis or maintenance in this state without growth. 83 The final school of thought looks to eradicate CSCs while they are still in their dormant state. 81 However, there are issues with these three potential methods which further highlight why research in these fields has been unsuccessful. Clinical evidence has been inconclusive on whether activating these quiescent cells in order to target them with conventional therapy is even able to be controlled. In addition, these quiescent stem cells are known to be extremely heterogeneous which indicates that activating them could exacerbate the condition by giving them an increased arsenal of mechanisms through which the cancer can develop. 84 Difficulties in the latter two methods are due to the need of a more comprehensive understanding of the various pathways and factors associated in CSC quiescence. Normal stem cells are a valuable resource in understanding CSC signaling as they are known to share several pathways such as Notch, Hedgehog, WNT/B-catenin, and NFkB. 81 , 85
Multiple myeloma (MM) is a common cancer of the blood which has an alarming 5-year survival rate of about 54% along with high chances of recurrence and need for further treatment. 86 Its resistance to treatments has been attributed to its development of drug resistance. A proposed model of MM malignant stem cell progression suggests that there are two states that cells are in - a quiescent state and a proliferative state. The quiescent state has exhibit properties of increased adhesion and minimal proliferation. Those in the quiescent state also have increased drug-resistant properties. There also exists a small population of cells that are in the proliferative state. These cells have high capacity for growth and mutation as a result of their increased chromosomal instability. 87 Impairment of MM cell survival through inducing cell cycle arrest was conducted through the use of anti-sense oligonucleotides (ASOs) to target human interferon regulatory factor 4 (IRF4). IRF4 has been identified as a critical MM cell factor for survival with an important role in disease development and progression. This treatment also reduced mRNA levels and levels of the MYC gene, which has a known impact in stem cell progression and has been studied thoroughly as an oncogene. Increased cleavage rates, apoptosis rates, and decreased colony formation all suggested success in disrupting cell growth. Furthermore, ASO-mediated IRF4 treatment led to cell cycle arrest in G1 and decreased proportion of cells in G2/M phase which signifies significant reduction in cell viability. 86 Seeing as though the G1 phase length plays an important factor in maintaining a quiescent state, this ASO treatment could help decrease the risk of quiescent MM cells leading to disease relapse. Further study is necessary to find an effective solution to both quiescent and proliferative states of MM, but studies that combat CSC quiescence show promise for a solution to decrease relapse rates.
Much of the difficulty in identifying, understanding, and treating CSCs originates from their extensive plasticity and asymmetry driven by intra-tumoral heterogeneity. 34 Their plasticity allows them to evade therapies by presenting various phenotypes and providing the ability to inhabit different tumor microenvironments. Normal cancer cells within a tumor mass can convert into CSCs in response to chemotherapy and can gain drug resistance as a result of a change in gene expression. 88 One such mechanism of drug resistance includes an increase in the expression of ATP-Binding cassette (ABC) transporters that permit greater drug efflux rates. CSCs can also be found in either a quiescent state that is difficult to eradicate or a proliferative state that prioritizes growth and metastasis. This quiescent state, when met with increased drug efflux mechanisms in CSC populations, often contributes to greater relapsed tumors. In hypoxic conditions, cells seeded in the core of the tumor are found to transition to the edge of the tumor where they become more quiescent. 89 This is because cells typically found seeded in the tumor core are more proliferative, which is why there is greater cell density in that region as compared to the edge cells which are more invasive, quiescent, and resistant. 89 As a result of this core-to-edge migration of tumor cells in response to hypoxia, particularly due to the induction of HIF-1α and HIF-2α, a novel front for cancer intervention and therapy has been unlocked. 89 However, the therapeutic potential and efficacy of a combined therapy targeting HIF-1α and HIF-2α in tumor cells, likely to prevent core to edge migration of progressive tumoral cells when presented with hypoxia, needs to be further studied to gain momentum as an effective therapy. The cell division of these quiescent cells can also be either symmetric or asymmetric. Symmetric division either creates a pair of quiescent daughter cells or a pair that is more proliferative and differentiates. 90 Asymmetric division results in one quiescent cell and transient amplifying cell that is very proliferative and contributes to most of the tumor. 90 It is widely inferred that this asymmetric division is more dangerous for cancer growth and it should be targeted for effective cancer treatment.
CSC Exosomes, New Targets for Cancer Therapy?
Stem cells have been found to play the role of mediators of conversion of healthy cells to cancerous cells. 91 This is done through the synthesis and release of exosomes– extracellular vesicles of cellular cargo that are released by a cell for various purposes. 92 Exosomes have been previously found to contain various cargo including miRNA, siRNA, transcription factors, and other proteins. Through the use of these extracellular vesicles, cells are able to communicate and exert transformative influence through phenotypic and genotypic alterations of surrounding cells. 93 Often used to encourage differentiation, proliferation, or inhibition thereof of cells in the surrounding environment, exosomes role in mediating the transformation of regular, healthy cells into cancerous cells is an area of great research due to its potential to catalyze interventional mechanisms for cancer therapy through targeting exosomes.
Stem cell-derived extracellular vesicles can provide powerful alternatives to cell-based therapies since the former acts as a noninvasive method for in vivo modulation of gene expression, inhibition of cell surface receptors and intracellular signaling molecules, and initiation of cell differentiation or death. 92 Instead of transplanting differentiated stem cells or donor cells into the patient, the delivery of extracellular vesicles can allow for transformation of native cells in the subject and transformation of cells that have been transplanted prior. This eliminates the need for surgery and removes the necessity to probe the patient for cell transplantation, which requires some invasive protocol. Stem cell-derived extracellular vesicles can prove to be a valuable tool in cell-based therapy by perhaps altering the nature of the cells that are native to the microenvironment of the subject.
There are various mechanisms through which exosomes released by CSCs exert influence on neighboring cells. One such method is through induction of Epithelial to Mesenchymal Transition (EMT). This is induced through the release of transcription factors such as Snail, Twist, and FOXC2 that are carried by the extracellular vesicle to neighboring cells where these transcription factors exert their effects phenotypically and genotypically. 94 In mammary epithelial stem cells, the influence of these factors on neighboring healthy cells is apparent by the CD44 (high)/CD24 (low) antigen phenotype which is typical of CSCs. 95 This can contribute to the development of severe tumorigenicity amongst the cells, as noted by the study. Another study observed the role of CD-103+exosomes released by CSCs which promoted EMT in clear cell renal cell carcinoma (CCRCC) cells. 96 Particularly, the group observed the importance of one critical factor in promoting EMT in CCRCC: MiR-19b-3p. 96 This was done through the repression of PTEN, a protein which is apparently expressed and has been previously studied to play a key role in the promotion of EMT. 97 The study was able to confirm the role of MiR-19b-3p in promoting EMT through suppression of PTEN by infecting ACHN and 786-O cells with a miR-19b-3p lentivirus. 96 Like the exosomes released by the CSCs, this encouraged the migration and invasion of the CCRCC cells. These findings implicate the role of CD103, since it mediates entry of the miRNA into neighboring cells via use of exosomes, in acting as a potential biomarker or target for cancer therapy. Further studies focusing on CSC-derived exosomes include macrovesicles derived from CD105+ cells which have been found to promote angiogenesis and metastasis with a distinct miRNA profile inside of the vesicles, resulting from presence of CD105. 98 This unique composition encouraged greater tumorigenicity amongst the cells, favoring further growth and invasion. Although there are various studies investigating the role of CSC-derived exosomes in promoting tumor development, limited information currently exists on the CSC-specificity of these exosomes and further research must be done in this area to determine entirely which exosomes are specific to only CSCs, or whether it is the content within that can be used as a biomarker. This further emphasizes the importance of researching the role of the CSC-influenced tumor microenvironment and its various components (eg, exosomes) in promoting and accelerating cancer development.
Conclusion and Perspectives
The aforementioned studies permit insight into a previously unexplored interface between stem cells and cancer progression and treatment. Silencing cell surface markers on CSCs can promote immune recognition of tumor sites and inhibit binding of tumor cargo to healthy cells, thus preventing their transformation. Furthermore, iPSCs can be used to vaccinate and immunize individuals against tumor cells in the future due to surface-antigen similarity between iPSCs and cancer cells. In addition to these mechanisms, stem cells have been shown to transfer various genetic and molecular cargo intercellularly through exosomes, another area for drug targeting and disease intervention in cancer. As a result of these various interactions between cancer cells, stem cells and their individual components, there is now a greater need to explore the influence that stem cells have on tumorigenesis. This will enable the innovation and translation of theranostics that are more efficient in nature and do not result in an abundance of unwanted side effects as a result of treatment.
Current organoid models are limited by their inability to mimic mature organ architecture and associated tissue microenvironments. Multilayer bladder ‘assembloids’ were recently created by reconstituting tissue stem cells with stromal components to represent an organized architecture with an epithelium surrounding stroma and an outer muscle layer. 99 A urothelial carcinoma assembloids platform has been developed by the same group. These assembloids exhibit characteristics of mature adult bladders and tumors in cell composition and gene expression, and recapitulate in vivo tissue dynamics. This will help advance functional studies in the context of the increasingly recognized importance of tissue stroma and microenvironments. 99–101
The authors report no conflicts of interest in this work.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 05 August 2024

Role of N6-methyladenosine in tumor neovascularization
- Lu Zhao 1 , 2 na1 ,
- Qinshan Li 3 , 4 na1 ,
- Tongliang Zhou 1 ,
- Xuan Liu 1 ,
- Jing Guo 1 ,
- Qing Fang 1 ,
- Xiaoxue Cao 1 , 5 ,
- Qishun Geng 1 , 5 ,
- Yang Yu 1 ,
- Songjie Zhang 1 ,
- Tingting Deng 1 ,
- Xing Wang 1 , 6 ,
- Yi Jiao 1 , 6 ,
- Mengxiao Zhang 1 ,
- Honglin Liu ORCID: orcid.org/0000-0002-2122-7250 1 , 2 ,
- Haidong Tan 7 &
- Cheng Xiao ORCID: orcid.org/0000-0002-5601-9670 1 , 2
Cell Death & Disease volume 15 , Article number: 563 ( 2024 ) Cite this article
303 Accesses
Metrics details
- Epigenetics
- Tumour angiogenesis
Tumor neovascularization is essential for the growth, invasion, and metastasis of tumors. Recent studies have highlighted the significant role of N6-methyladenosine (m 6 A) modification in regulating these processes. This review explores the mechanisms by which m 6 A influences tumor neovascularization, focusing on its impact on angiogenesis and vasculogenic mimicry (VM). We discuss the roles of m 6 A writers, erasers, and readers in modulating the stability and translation of angiogenic factors like vascular endothelial growth factor (VEGF), and their involvement in key signaling pathways such as PI3K/AKT, MAPK, and Hippo. Additionally, we outline the role of m 6 A in vascular-immune crosstalk. Finally, we discuss the current development of m 6 A inhibitors and their potential applications, along with the contribution of m 6 A to anti-angiogenic therapy resistance. Highlighting the therapeutic potential of targeting m 6 A regulators, this review provides novel insights into anti-angiogenic strategies and underscores the need for further research to fully exploit m 6 A modulation in cancer treatment. By understanding the intricate role of m 6 A in tumor neovascularization, we can develop more effective therapeutic approaches to inhibit tumor growth and overcome treatment resistance. Targeting m 6 A offers a novel approach to interfere with the tumor’s ability to manipulate its microenvironment, enhancing the efficacy of existing treatments and providing new avenues for combating cancer progression.
Similar content being viewed by others

Angiogenic signaling pathways and anti-angiogenic therapy for cancer
Targeting PAK4 to reprogram the vascular microenvironment and improve CAR-T immunotherapy for glioblastoma
An agonistic anti-Tie2 antibody suppresses the normal-to-tumor vascular transition in the glioblastoma invasion zone
Neovascularization is a rate-limiting step in tumor progression.
m 6 A modification participates in various aspects of cancer biology.
Tumor neovascularization induces an immunosuppressive microenvironment.
Immunosuppressive cells promote tumor neovascularization.
Open Questions
What is the role of m 6 A modification in different modes of tumor neovascularization and associated pathways?
What is the relationship between m 6 A modification and anti-angiogenic drug resistance?
Can anti-angiogenic therapy be combined with immunotherapy by targeting m 6 A regulators?
Can m 6 A targeting effectively improve the limited efficacy of current anti-angiogenic therapy?
Introduction
Tumor neovascularization ensures the acquisition of adequate oxygen and nutrients required for sustained tumor growth [ 1 ]. Notably, solid tumors tend to grow around blood vessels and cannot expand beyond 2 mm 3 without vascularization [ 2 , 3 ]. The induction of the “angiogenic switch”, which depends on a balance of angiogenic and anti-angiogenic factors, is a rate-limiting step in tumorigenesis, triggering exponential tumor growth [ 4 , 5 ]. Neovascularization, considered as a hallmark of cancer, is indispensable for tumor proliferation, invasion, and metastasis [ 5 ]. Consequently, targeting tumor neovascularization has emerged as a crucial component of cancer therapy. Existing anti-angiogenic strategies primarily focus on the vascular endothelial growth factor (VEGF) or VEGF receptor (VEGFR) signaling pathway. Despite advancements, these approaches yield transitory benefits and often fail to achieve long-term clinical responses [ 6 ]. Increasing evidence indicates that tumor neovascularization is a complex process involving multiple components, underscoring the need to elucidate the underlying mechanisms to improve anti-angiogenic therapy efficacy [ 7 , 8 , 9 ].
Recently, researchers have proposed that non-mutational epigenetic reprogramming facilitates the acquisition of hallmark capabilities by tumors [ 10 , 11 ]. Epigenetics refers to the study of heritable alterations that do not involve changes to the DNA sequence, including DNA and RNA methylation, nucleosome remodeling, and histone modifications [ 11 , 12 ]. Over 170 RNA modifications have been identified, including N6-methyladenosine (m 6 A), 5-methylcytosine (m 5 C), N7-methylguanosine, and N1-methyladenosine [ 13 ]. The most prevalent RNA modification among these is m 6 A, first described in 1974, which occurs as an RNA methylation at the sixth nitrogen atom of adenosine [ 14 ]. m 6 A modification is a dynamic and reversible process that is installed by methyltransferases (“writers”), removed by demethylases (“erasers”), and recognized by RNA-binding proteins (“readers”) [ 15 ]. m 6 A participates in multiple aspects of RNA metabolism processes, including splicing, translation, stability, degradation, and nuclear export. It plays an essential role in reshaping the tumor microenvironment (TME), regulating cancer metabolism, and facilitating carcinogenesis [ 12 , 15 , 16 , 17 ].
Recent evidence highlights the role of m 6 A in regulating tumor neovascularization. Our previous review linked m 6 A to immune reprogramming [ 16 ]. In this review, we aim to explore the regulatory role of m 6 A in tumor neovascularization, offering a comprehensive grasp of its significance in cancer therapy. This review introduces the diverse role of m 6 A in multiple modes of neovascularization and associated signaling pathways. Additionally, we concisely outline its contribution to vascular-immune crosstalk. Finally, we discuss the current development of m 6 A inhibitors and their potential clinical applications. This review clarifies the underlying mechanism of tumor neovascularization and provides novel insights into targeting m 6 A in anti-angiogenic therapy.
Tumor neovascularization
In 1971, Folkman proposed that solid tumor growth is always accompanied by the formation of new blood vessels, suggesting that inhibiting tumor vascularization could suppress tumor growth [ 2 ]. Since then, serveral modes of tumor neovascularization have been identified, including sprouting angiogenesis, vasculogenesis, intussusceptive angiogenesis, vasculogenic mimicry (VM), vessel co-option, and cancer stem cell (CSC)-derived vasculogenesis (Fig. 1 ) [ 7 , 8 ]. The first three modes occur in both normal tissues and tumors, whereas the latter three are specific to tumor neovascularization. Among them, angiogenesis and VM are the most extensively studied.
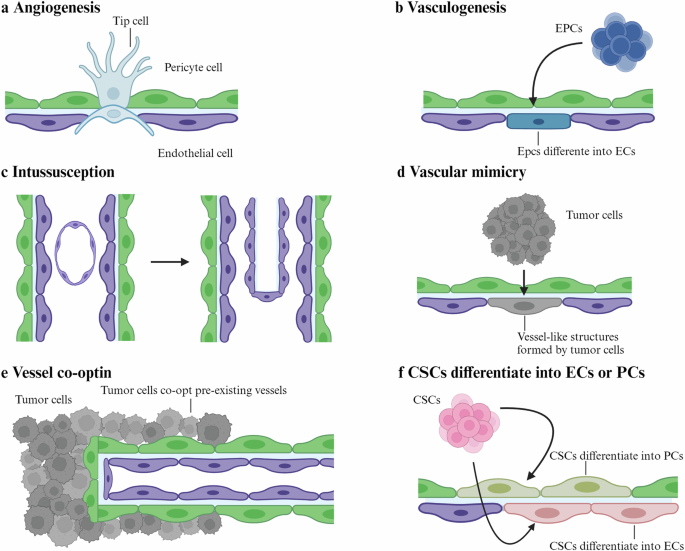
There are several modes of tumor neovascularization. a Angiogenesis: blood vessels form from preexisting vessels through sprouting; b vasculogenesis: endothelial progenitor cells derived from the bone marrow are recruited and differentiate into endothelial cells to form blood vessels; c intussusception: transcapillary tissue pillars insert into the lumen of existing vessels, undergo vascular splitting, and eventually fuse to remodel the vascular network; d vascular mimicry: tumor cells form vessel-like structures; e vessel co-option: tumor cells hijack the existing vasculature and migrate along the vessel surface or infiltrate non-malignant tissues between vessels; f CSC differentiate into ECs or PCs: cancer stem cell differentiate into endothelial cells or pericytes. CSCs cancer stem cells, ECs endothelial cells, EPCs endothelial progenitor cells, PCs pericytes.
Angiogenesis
Angiogenesis is the traditional process by which new blood vessels form from preexisting vessels through sprouting. Initially, endothelial cells (ECs) loosen their junctions, increasing permeability and releasing plasma proteins. Subsequently, ECs sprout, with tip cells penetrating the basement membrane, and an imbalance between matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinase (TIMP) leads to extracellular matrix (ECM) degradation. Finally, ECs proliferate and migrate, accompanied by pericyte recruitment, resulting in the formation of new blood vessels [ 7 , 18 ].
Vasculogenic mimicry (VM)
The classical theory of tumor angiogenesis proposes that blood vessels are generated through ECs sprouting. However, in 1999, a paradigm shift occurred with the introduction of the concept of VM in a study focused on melanoma [ 19 ]. Unlike traditional angiogenesis, VM is independent of ECs which forms vessel-like structures by tumor cells [ 8 ]. Subsequent studies revealed that VM occurs not only in melanoma but also in glioma, hepatocellular carcinoma (HCC), and prostate cancer [ 20 , 21 , 22 ]. Mechanistically, VM involves the epithelial-mesenchymal transition (EMT) process and differentiation of CSCs [ 23 , 24 ]. During VM, epithelial cell markers like E-cadherin are downregulated, whereas mesenchymal cell markers like VE-cadherin, vimentin are upregulated [ 24 , 25 ].
m 6 A components
M 6 a writers.
m 6 A writers are methyltransferases responsible for installing m 6 A and modifications on RNA.The core components include methyltransferase-like 3 (METTL3), methyltransferase-like 14 (METTL14), and Wilms tumor 1-associated protein (WTAP). METTL3 and its homolog METTL14 form an asymmetric heterodimer in a 1:1 ratio [ 26 ]. METTL3 functions as the catalytic subunit, whereas METTL14 acts as an allosteric activator, enhancing METTL3’s catalytic activity by providing an RNA-binding scaffold [ 27 ]. With the assistance of WTAP, an adapter subunit, the METTL3- METTL14 complex is located in nuclear speckles [ 28 ]. Additionally, RNA-binding motif protein 15 (RBM15) and its paralog RBM15B interact with WTAP-METTL3, recruiting it to proximal m 6 A consensus sites [ 29 ]. Other writer components, including vir-like m 6 A methyltransferase associated (VIRMA), zinc finger CCCH-type containing 13 (ZC3H13), and cbl proto-oncogene like 1 (CBLL1), also interact with WTAP [ 15 ]. Among them, VIRMA mediates m 6 A in the 3′ untranslated region (3′UTR) and near the stop codon by acting as a scaffold to hold WTAP/CBLL1/ZC3H13 together [ 30 ]. Methyltransferase-like 16 (METTL16) is an independent RNA methyltransferase that installs m 6 A on molecules such as U6 small nuclear RNA (snRNA), methionine adenosyltransferase 2A ( MAT2A ) mRNA, and metastasis-associated lung adenocarcinoma transcript 1( MALAT1 ) [ 31 ].
In various tumor types, METTL3 typically functions as an oncogenic factor, including acute myeloid leukemia (AML), lung cancer, HCC, among others, while exhibiting an anti-oncogenic role in endometrial cancer [ 32 ]. Additionally, METTL3 is implicated in therapy resistance across cancers. For instance, it promotes chemoresistance in HCC, breast cancer, and colorectal cancer (CRC), and reduces the effectiveness of immunotherapy in CRC and melanoma [ 33 ]. However, despite METTL14 lacking catalytic activity and functions as an allosteric activator of METTL3, its impact on tumors may not consistently align with METTL3. METTL14 exerts an oncogenic effect in HCC and AML, but suppresses tumor progression in CRC and renal cell carcinoma (RCC) [ 32 , 34 ]. Additionally, decreased METTL14 has been reported to promote radiotherapy resistance in esophageal squamous cell carcinoma (ESCC) and sorafenib resistance in HCC [ 33 ]. This discrepancy could arise from the distinct target sites methylated by each protein within the writer complex. m 6 A methylation is preferentially enriched at DRACH (D = A, G, or U; R = G or A; H = A, C or U) motifs, suggesting numerous potential methylation sites exist within transcripts. However, the writer complex exhibits site- and transcript-specific selectivity, which may be influenced by transcription factors or histone marks that recruit the writer complex to specific genomic loci [ 15 , 35 ].
m 6 A erasers
The reversible nature of m 6 A modification is regulated by m 6 A erasers, which are limited in specific tissues and are context-dependent [ 35 ]. m 6 A demethylation is catalyzed by two enzymes, fat mass and obesity-associated (FTO) and AlkB homolog 5 (ALKBH5), both part of the ALKB enzyme family [ 36 ]. FTO is the first identified m 6 A eraser that is associated with obesity and energy homeostasis [ 37 ]. Initially, FTO was identified as a nucleus m 6 A demethylase, but later studies suggested its primary target might be N6,2′-O-dimethyladenosine (m 6 A m ) rather than m 6 A [ 37 ]. m 6 A typically occurs at internal sites within mRNA, whereas m 6 A m is located near the 5′cap structure. Subsequent studies indicated that FTO regulates m 6 A m modification of snRNA, thereby affecting the alternative splicing of mRNA [ 38 ]. Despite a lower response rate, FTO can demethylate m 6 A and exhibit an oncogenic role. In AML, FTO is abnormally localized in the cytoplasm and exerts oncogenic effects by altering m 6 A demethylation [ 39 ]. ALKBH5, another m 6 A demethylase, is highly expressed in the testis, lungs, and germ cells, but weakly in cardiac and cerebral tissues [ 40 , 41 ]. Unlike FTO, ALKBH5 has no activity toward m 6 A m and can be induced under hypoxia conditions in tumors, promoting self-renewal of glioblastoma and breast CSCs [ 42 ].
m 6 A readers
m 6 A readers are RNA-binding proteins that specifically recognize and bind to m 6 A-modified RNA molecules. The YTH domain-containing proteins, including YTH domain-containing 1–2 (YTHDC1-2) and YTH domain family 1–3 (YTHDF1–3), were the first identified m 6 A readers. YTHDC1 promotes RNA splicing and nuclear export, and YTHDC2 weakly binds m 6 A and promotes mRNA translation and degradation. The YTHDF proteins have three highly similar paralogues: YTHDF1 enhances mRNA translation, YTHDF2 promotes mRNA degradation, and YTHDF3 performs both functions [ 43 ]. Additionally, m 6 A can indirectly recruit RNA-binding proteins by remodeling RNA structure, a phenomenon known as the“m 6 A-switch” [ 15 ]. The insulin-like growth factor 2 mRNA binding protein (IGF2BP) family and heterogeneous nuclear ribonucleoprotein (HNRNP) family belong to this category. IGF2BP1-3 promote mRNA stability, HNRNPC/G facilitate RNA splicing, and HNRNPA2B1 promotes both RNA splicing and degradation [ 15 ].
m 6 A and tumor neovascularization
M 6 a and angiogenesis.
Among m 6 A writers, METTL3 and METTL14 are primarily investigated for their roles in tumor angiogenesis (Table 1 ). Firstly, they facilitate the expression of angiogenic factors in various cancers. For example, METTL3 directly promotes the expression of hypoxia-inducible factor 1-alpha (HIF-1α), VEGFA and tyrosine kinase (TEK) in bladder cancer (BLCA) [ 44 , 45 ]. Similarly, METTL14 induces the expression of basic leucine zipper ATF-like transcription factor 2 (BATF2), which indirectly upregulates VEGFA secretion and promotes angiogenesis in tongue squamous cell carcinoma (TSCC) [ 46 ]. Beyond VEGFA, METTL3 also regulates ECM components to modulate angiogenesis [ 47 ]. In HCC and prostate cancer, METTL3 stimulates angiogenesis by increasing the expression of MMP2 and MMP9 [ 48 , 49 , 50 ]. Moreover, in CRC, METTL3 enhances plasminogen activator, urokinase (PLAU) expression, which activates angiogenic factors stored in the ECM, thereby facilitating angiogenesis [ 51 , 52 ]. Additionally, METTL3 regulates cell cycle-associated proteins. In gastric cancer (GC) and in head and neck squamous cell carcinoma (HNSCC), METTL3 upregulates centromere protein F (CENPF), ensuring an adequate blood supply for rapidly dividing tumor cells [ 53 , 54 ]. METTL3 and METTL14 also affect metabolism and inflammation, indirectly regulating angiogenesis. In GC, METTL3 increases hepatoma-derived growth factor (HDGF) expression, promoting glycolysis, which subsequently contributes to angiogenesis and liver metastasis [ 55 ]. In RCC, METTL14 activates TNF receptor-associated factor 1 (TRAF1), and indirectly facilitates angiogenesis [ 56 ].
The regulation of tumor angiogenesis by m 6 A erasers varies across different cancer types. In multiple myeloma (MM), ALKBH5 promotes angiogenesis by elevating salvador family WW domain-containing protein 1 (SAV1) expression and activating the Hippo pathway [ 57 ]. Conversely, in BLCA, ALKBH5 suppresses angiogenesis and hematogenous metastasis by inhibiting lncBLACAT3 expression, which inactivates the NF-κB pathway [ 58 ]. In RCC, FTO promotes angiogenesis by inhibiting von Hippel-Lindau tumor suppressor (VHL) expression, whereas in intrahepatic cholangiocarcinoma (ICC), FTO suppresses angiogenesis by inducing TEA domain transcription factor 2 (TEAD2) expression [ 59 , 60 ]. These findings demonstrate that the effects of m 6 A erasers on angiogenesis are specific to the cancer type.
Correlations between tumor angiogenesis and m 6 A readers have been observed in both the IGF2BP and YTH domain-containing proteins, which exhibit opposing functions. The IGF2BP family exerts a pro-angiogenic effect by enhancing the stability of downstream genes. For instance, in lung cancer, IGF2BP2 increases the stability of fms-related tyrosine kinase 4 ( FLT4 ; also known as VEGFR3) or thymidine kinase 1 ( TK1 ) mRNA, leading to tumor angiogenesis and aggressiveness [ 61 , 62 ]. Similarly, in CRC, IGF2BP2 and IGF2BP3 stabilize cyclin D1 and VEGF mRNA, thereby promoting angiogenesis [ 63 , 64 ]. In contrast, the YTH domain-containing proteins have been demonstrated to suppress tumor angiogenesis. In lung cancer, YTHDC2 enhances the translation efficiency of lncZNRD1-AS1, further suppressing angiogenesis and tumorigenesis through the miR-942/Tensin 1 axis [ 65 ]. Additionally, YTHDF2 inhibits angiogenesis in clear cell RCC (cRCC) and HCC by facilitating the degradation of target genes. In cRCC, YTHDF2 inhibits angiogenesis by increasing circPOLR2A degradation [ 66 ]. In HCC, it increases the degradation of IL11 and serpin family E member 2 (SERPINE2) and thus contributing to vascular normalization [ 67 ].
m 6 A and vasculogenic mimicry
The well-studied m 6 A regulator METTL3, is associated with vasculogenic mimicry (VM) in CRC, glioma, and HCC [ 68 , 69 , 70 ]. In CRC, METTL3 promotes VM indirectly by targeting Eph receptor A2 (EphA2) and VEGFA, enhancing their stability through IGF2BP2 and IGF2BP3, respectively [ 69 ]. Elevated METTL3 levels in glioma contribute to VM through targeting HOXA transcript antisense RNA myeloid-specific 1 (HOTAIRM1) [ 70 ]. Similarly, in HCC, inhibition of METTL3 impairs VM-related tumor vasculature formation, indicating a positive correlation between m 6 A levels and VM [ 68 ]. These findings suggest that METTL3 regulates target genes at the translational level, ultimately inducing VM.
However, the effect of METTL3 on VM in glioblastomas (GBM) appears to be different. One study found that METTL3 enhances the stability of BUD13 homolog (BUD13), promoting the translation of cyclin-dependent kinase 12 (CDK12) and muscleblind-like splicing regulator 1 (MBNL1). This cascade results in the upregulation of MMP2 and laminin subunit gamma 2 (LAMC2), promoting VM [ 71 ]. Conversely, another study indicates that reduced METTL3 facilitates VM and is correlated with higher histopathological grade and lower overall survival [ 72 ]. The contradictory results may be attributed to differences in cell line selection and sample size. Further research is needed to clarify the specific role of m 6 A on VM in different tumors.
m 6 A and stemness-associated factors
M 6 a and oct4.
Octamer-binding transcription factor 4 ( Oct4 ), a member of the POU transcription factor family, is essential for stemness maintenance and differentiation of CSCs [ 73 ]. Recent studies have also linked it to angiogenesis [ 74 ]. Our previous research demonstrated that Oct4 regulates the differentiation of liver CSCs into tumor ECs [ 75 ]. YTHDF2 interacts with the 5′UTR of Oct4 mRNA to increase its expression, thus maintaining stemness and promoting lung metastasis in HCC [ 76 ]. ALKBH5 is positively correlated with Oct4 in MM and non-small cell lung cancer. Suppression of ALKBH5 reduces Oct4 expression and inhibits CSC characteristics, suggesting that ALKBH5 may induce neovascularization through CSC-derived vasculogenesis manner in these tumors [ 57 , 77 ].
m 6 A and Sox2
SRY-box transcription factor 2 ( Sox2 ) is a transcription factor that is essential for the self-renewal and pluripotency of stem cells. It has been demonstrated that Sox2 is capable of promoting tumor neovascularization. In ESCC, Sox2 promotes angiogenesis by inducing suprabasin expression [ 78 ]. Furthermore, it contributes to VM in CRC [ 79 ]. On the other hand, tumor neovascularization increases Sox2 expression, which in turn helps to maintain the CSC phenotype. In skin tumors, CSCs are found in proximity to ECs and reside within a perivascular microenvironment [ 80 ]. Besides, in retinoblastoma, VEGF has been found to stimulate Sox2 expression and enhance tumor invasiveness [ 81 ]. These findings demonstrate a reciprocal relationship between Sox2 and tumor neovascularization, where both factors reinforce the aggressive behavior of the tumor.
Sox2 is a downstream target of METTL3, with IGF2BP2 recognizing methylated Sox2 transcripts and preventing their degradation [ 82 , 83 ]. METTL3 sustains Sox2 expression through an m 6 A-mediated mechanism, thereby maintaining stemness and metastasis in CRC [ 82 ]. In GBM, METTL3 binds to the 3′UTR of Sox2 mRNA, thus maintaining stemness and radioresistance [ 83 ]. Additionally, ALKBH5 has been reported to facilitate Sox2 expression in lung cancer, MM and endometrial cancer [ 57 , 84 , 85 ]. For instance, in lung cancer, ALKBH5 counteracts YTHDF2-mediated degradation of Sox2 and thereby promoting tumor aggressiveness [ 84 ].
m 6 A and tumor neovascularization-associated pathways
M 6 a and vegf.
VEGF, an extensively studied angiogenic factor, is produced by various cell types. The VEGF family consists of six members, with VEGFA being the most critical for tumor neovascularization. VEGFR are categorized into three subtypes, with VEGFR1 and VEGFR2 being the most prevalent in vascular ECs. VEGFR1 is responsible for hematopoiesis, and VEGFR2 is involved in vasculogenesis and angiogenesis. VEGFR3, mainly expressed in lymphatic ECs, is associated with lymphangiogenesis [ 86 ]. When VEGF binds to VEGFR, it triggers TEK phosphorylation, activating intracellular signaling pathways that regulate the proliferation, migration, survival, and penetration of vascular ECs, ultimately leading to neovascularization [ 18 ]. Moreover, VEGF infulences tumor neovascularization thorugh various downstream signaling pathways, including PI3K/AKT, MAPK, PLC, and SRC [ 87 ].
In lung cancer, METTL3 binds to the A859 site within the internal ribosome entry site of VEGFA 5’UTR, recruiting YTHDC2/eIF4GI complex to promote VEGFA translation and increase its expression [ 88 ]. This promoting effect of METTL3 on VEGFA is also observed in CRC, pancreatic cancer and BLCA [ 45 , 69 , 89 ]. However, in sorafenib-resistant HCC, METTL3 exerts an opposite effect. Depletion of METTL3 increases the expression of VEGFA and other angiogenic factors [ 90 ]. This may result from the alterations in the recognition site of the m 6 A-modified target gene following drug resistance, which affecting their binding capacity. In RCC, FTO is mutually exclusive with VHL. Elevated FTO inhibits VHL expression and increases VEGFA secretion [ 59 ]. IGF2BP3 has been reported to promote angiogenesis by interacting with VEGFA in CRC and GC [ 63 , 69 , 91 ]. Notably, it is IGF2BP3, rather than other IGF2BP members, that specifically binds to VEGFA [ 69 ]. Further research is required to elucidate the selectivity of m 6 A readers.
m 6 A and EGFR
Epidermal growth factor receptor (EGFR) is a membrane receptor on the surface of epidermal cells that belongs to the tyrosine kinase receptor family. Upon activation by EGF, it initiates tyrosine kinase activity and activates downstream signaling such as PI3K/AKT, MAPK, and JAK/STAT pathways, which regulate various biological processes [ 92 ].
In breast cancer with brain metastases, YTHDF3 enhances the translation of EGFR and VEGFA mRNA by binding to eukaryotic translation initiation factor 3 subunit A (eIF3a). Suppressing YTHDF3 reduces blood vessel density and impairs brain endothelial tube formation, thereby decreasing brain metastasis and prolonging survival [ 93 ]. Under hypoxic conditions, YTHDF2 is downregulated in HCC. However, when overexpressed, it binds to the 3’UTR of the EGFR mRNA and accelerates its degradation. Consequently, this process suppresses the MAPK/ERK pathway, thereby inhibiting cell proliferation and tumor growth [ 94 ].
m 6 A and PI3K/AKT
The PI3K/AKT signaling pathway is crutial in cancer development and progression. PI3K consists of a regulatory subunit (p85) and a catalytic subunit (p110). When activated, PI3K converts PIP2 to PIP3, subsequently activating PDK1 and AKT, while PTEN can counteract these effects. Upon activation, AKT stimulates mTOR, which in turn phosphorylates downstream substrates and regulates diverse biological processes [ 95 ]. The PI3K/AKT pathway is widely involved in tumor neovascularization. For instance, the inactivation of the p110 subunit impedes functional vessel formation, thereby restraining tumor growth. Activated AKT in tumor ECs results in an increase in nitric oxide levels, forstering vascular permebility. Conversely, the deletion of PTEN delays pericyte maturation, resulting in defective vascular remodeling [ 96 , 97 , 98 ].
In CRC, METTL3 promotes VM by activating the PI3K/AKT and ERK1/2 pathways [ 69 ]. Similarly, in GC, METTL3 promotes tumor angiogenesis through the ADAM metallopeptidase with thrombospondin type 1 motif 9 (ADAMTS9)-mediated PI3K/AKT pathway [ 99 ]. In pancreatic cancer, METTL3 increases the stability of lncLIFR-AS1 and indirectly promotes VEGFA expression. This, in turn, activates the AKT/mTOR pathway and further promotes tumor progression [ 89 ]. Using a bioinformatics database, Chen et al. found that METTL3 also regulates the PI3K/AKT pathway in BLCA, silencing METTL3 exerts an inhibitory effect on angiogenesis [ 45 ]. Furthermore, METTL14 is upregulated in sunitinib-resistant RCC compared to sensitive ones. It increases TRAF1 mRNA stability in an IGF2BP2-dependent manner, activating the AKT/mTOR/HIF-1α pathway and facilitating angiogenesis [ 56 ]. Similarly, in lung adenocarcinoma, IGF2BP2 upregulation in metastatic subpopulations is associated with a poor prognosis. It enhances FLT4 mRNA stability and activates the PI3K/AKT pathway, thereby promoting angiogenesis [ 61 ].
m 6 A and MAPK
MAPK, a serine-threonine protein kinase, regulates biological processes such as cell proliferation, differentiation, and migration. The MAPK family comprises ERK, p38, JNK, and BMK1, which represent four distinct MAPK pathways [ 100 ]. Among these, the Ras/Raf/MEK/ERK pathway has been extensively investigated and is closely linked to cancer [ 101 ].
Phosphatidylethanolamine binding protein 1 (PEBP1) inhibits the Raf/MEK/ERK pathway by binding to Raf and disrupting the Raf/MEK complex. In cRCC, YTHDF2 increases PEBP1 expression, leading to ERK pathway inactivation. Specifically, circPOLR2A facilitates the interaction between PEBP1 and ubiquitin protein ligase E3C (UBE3C), thereby promoting PEBP1 degradation. YTHDF2 negatively regulates circPOLR2A, resulting in ERK pathway inactivation and ultimately suppressing angiogenesis, metastasis, and tumor growth [ 66 ]. In GC, METTL3 upregulates CENPF expression through HNRNPA2B1. Elevated CENPF binds to focal adhesion kinase (FAK) and promotes its nuclear export, thereby activating the MAPK pathway and thus promoting angiogenesis and liver metastasis [ 53 ]. Similarly, METTL3, WTAP, and YTHDC1 are involved in MAPK pathway activation, enhancing tumor neovascularization in CRC [ 51 , 69 , 102 ].
m 6 A and Hippo
The Hippo pathway, comprising FZD2, LATS1/2, SAV1, and YAP/TAZ, is essential for regulating CSC maintenance, angiogenesis, and drug resistance. FZD2 has been reported to induce VM and maintain stemness in HCC, while YAP is associated with angiogenesis and VM in various cancers [ 103 , 104 ]. Recent studies indicate that the Hippo pathway is involved in m 6 A-mediated tumor neovascularization, especially in VM.
YAP exhibits widespread association with VM and can be mediated by m 6 A methylation. In HCC, METTL3 promotes YAP mRNA splicing and enhances its translation efficiency, thereby facilitating VM [ 68 ]. In pancreatic cancer, inhibition of YTHDF2 increases YAP expression and promotes EMT [ 105 ]. Using verteporfin, a YAP inhibitor, neovascularization is suppressed, as evidenced by reduced levels of angiopoietin-2 (Ang2), MMP2 and VE-cadherin [ 106 ]. Given that EMT serves as the mechanism underlying VM, it is speculated that YTHDF2 may inhibit VM in pancreatic cancer by suppressing the Hippo pathway. Similarly, in CRC, IGF2BP2 binds to YAP mRNA to promote its translation, and verteporfin reduces the number of cancer-associated fibroblasts, inhibiting angiogenesis and tumor progression [ 107 , 108 , 109 ]. Apart form YAP, SAV1 has been implicated in promoting stem cell phenotype and neovascularization in MM. Inhibition of ALKBH5 decreases SAV1 mRNA stability, suppresses the Hippo pathway and angiogenesis [ 57 ]. Considering the intimate connection among CSC, EMT and VM, it is worthwhile to investigate the role of m 6 A in regulating tumor neovascularization, especially in VM through the Hippo pathway.
m 6 A and Wnt/β-catenin
The Wnt signaling pathway is important for regulating CSC maintenance and tumor metastasis, with three distinct pathways indentified: Wnt/β-catenin, Wnt/planar cell polarity, and Wnt/calcium. In the classical Wnt/β-catenin pathway, Wnt binds to the Frizzled receptor and activates the TCF/LEF transcription factor. Subsequently, TCF/LEF binds to β-catenin and promotes the transcription of various downstream genes [ 110 ].
In CRC, upregulated circ3823 functions as a competing endogenous RNA, disrupting the suppressive effect of miR-30c-5p on TCF7, consequently activating the Wnt/β-catenin pathway. Suppression of YTHDF3 or ALKBH5 increases circ3823 expression, thus promoting angiogenesis, metastasis, and tumor growth [ 111 ] (Fig. 2 ).
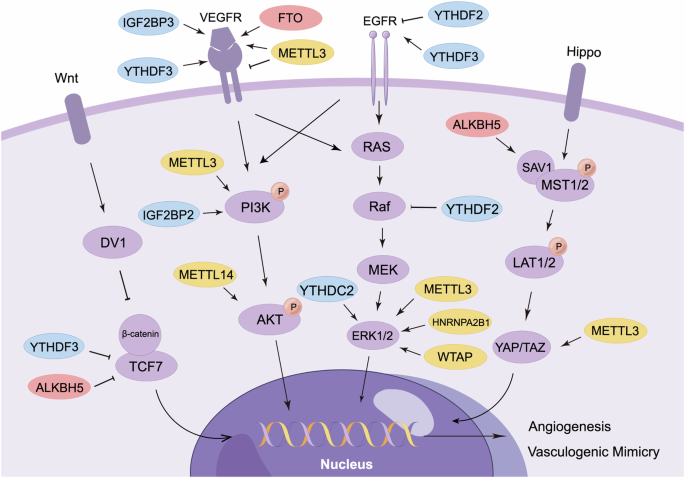
m 6 A “writers” (yellow circles), “erasers” (red circles), and “readers” (blue circles) selectively target specific signaling components, leading to the activation/inactivation of multiple intracellular signaling pathways (including Wnt/β-catenin, VEGFR, EGFR, Hippo, PI3K/AKT and MAPK pathways) associated with tumor neovascularization.
m 6 A in tumor vascular-immune crosstalk
Tumor angiogenesis creates an immunosuppressive microenvironment, aiding tumors in evading immune surveillance. Additionally, immunosuppressive cells can stimulate blood vessel formation, establishing abberrant communication between vascular and immune cells [ 112 ]. The synergistic effect of combining anti-angiogenic therapy with immunotherapy has demonstrated significant efficacy in treating various cancers, including RCC, HCC, GC, and endometrial cancer [ 113 , 114 , 115 , 116 ]. Recent studies highlight the role of m 6 A in regulating both tumor neovascularization and the immune microenvironment [ 117 ].
m 6 A-mediated tumor vasculature effects on immune cells
Immune cells extravasation into the TME requires adherence to ECs. However, ECs create an immune barrier by expressing programmed cell death-1 ligand 1 (PD-L1) and FAS ligand (FASL), as well as inhibiting adhesion factors such as intercellular adhesion molecule 1 (ICAM1), vascular cell adhesion molecule 1 (VCAM1), and P-selectin (Fig. 3 ) [ 118 , 119 ]. Additionally, angiogenic factors like VEGF impede dendritic cells (DCs) maturation, impairing antigen presentation, suppressing tumor-specific cytotoxic T lymphocytes (CTLs) activation, or promoting the accumulation of immunosuppressive cells such as myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) [ 120 ]. METTL3 and IGF2BP3 promote VEGF expression in various cancers, potentially contributing to the immunosuppressive TME [ 44 , 63 , 69 , 88 , 91 ].
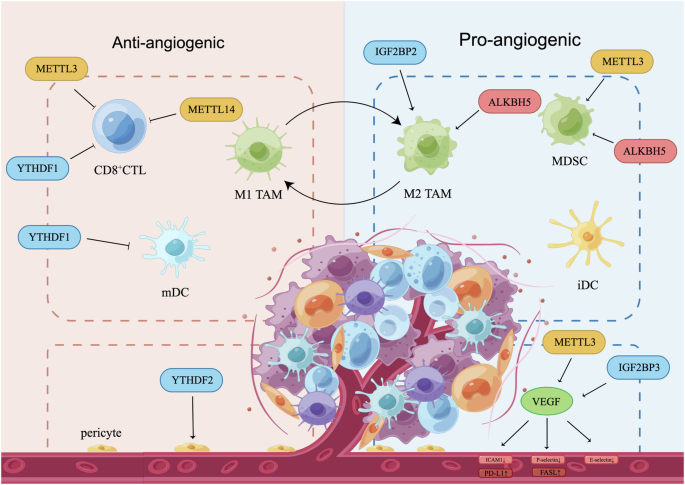
Tumor neovascularization contributes to the establishment of an immunosuppressive tumor microenvironment, while immunosuppressive cells facilitate tumor angiogenesis through the secretion of pro-angiogenic factors. Immune effector cells like CD8 + CTL, M1 TAM, mDC contribute to the establishment of an anti-angiogenic TME, whereas immunosuppressive cells, such as M2-like TAM, MDSC, and iDC, promote angiogenesis. Meanwhile, VEGF reduces the expression of endothelial adhesion molecules (ICAM1, P-selectin, E-selectin) expression, inhibits DC maturation and CTL activation, increases the abundance of MDSC, consequently impedes the infiltration of immune cells. Furthermore, reduced pericyte coverage hinders blood vessel integrity and immune infiltration. m 6 A regulators play a role in modulating immune-vascular crosstalk by influencing various components such as immune cells, vascular-associated cells, angiogenic factors, and endothelial adhesion molecules. CTL cytotoxic T lymphocyte, FASL FAS ligand, iDC immature DC, M1 TAM M1-like tumor-associated macrophage, M2 TAM, M2-like tumor-associated macrophage, mDC mature DC, MDSC myeloid-derived suppressor cell, PD-L1 programmed cell death-1 ligand 1, ICAM1 intercellular adhesion molecule 1, VEGF vascular endothelial growth factor.
Tumor blood vessels exhibit chaotic, leaky, and highly permeable characteristics, leading to increased interstitial fluid pressure that hampers immune cell infiltration. Inhomogeneous blood flow reduces perfusion and oxygenation, adversely impacting the delivery of anticancer drugs [ 121 ]. Pericytes are crucial for maintaining blood vessel structural integrity and have dual effects on tumor neovascularization. Generally, pericytes produce angiogenic factors that stimulate neovascularization [ 122 , 123 ]. However, under certain conditions, increased pericyte coverage can normalize tumor vasculature [ 5 ]. In HCC, low levels of YTHDF2 due to hypoxia result in decreased pericyte coverage. Elevated YTHDF2 facilitates the degradation of IL11 and SERPINE2, reducing vascular density and permeability, thereby favorably impacting vascular normalization [ 67 ].
m 6 A-mediated immune cell effects on tumor neovascularization
Immune cells regulate tumor angiogensis through the secretion of cytokines and chemokines, while m 6 A modification indirectly modulates this process by recruiting and activating immune cells. Tumor-associated macrophages (TAMs) exhibit distinct M1 or M2 phenotype, with M1-like TAMs promoting inflammation and inhibiting angiogenesis, and M2-like TAMs displaying an immunosuppressive phenotype that fosters angiogenesis [ 124 ]. ALKBH5 promotes M2-like TAMs recruitment in glioma [ 125 ]. In pancreatic cancer, lncPACERR promotes M2-like TAMs polarization via IGF2BP2, thereby driving tumor progression [ 126 ]. The impact of DCs on tumor angiogenesis depends on their maturation status. Mature DCs (mDCs) inhibit tumor angiogenesis, while immature DCs (iDCs) exhibit a pro-angiogenic effect [ 112 , 120 ]. In GC, YTHDF1 deletion increases mDCs recruitment, implying a pro-angiogenic role of YTHDF1 [ 127 ]. In addition, other immunosuppressive cells such as MDSCs, Tregs, and Tie2-expressing macrophages also contribute to tumor angiogenesis [ 9 , 112 ]. In CRC, METTL3 recruits MDSCs and promotes tumor growth [ 128 ]. Consistently, in cervical cancer, METTL3 positively correlates with CD33 + MDSCs and predicts unfavorable prognosis [ 129 ]. Conversely, in ICC, ALKBH5 decreases MDSC-like cell accumulation, enhances PD-L1 expression, thus facilitating immune infiltration [ 130 ].
Considering adaptive immunity, CD8 + CTLs release IFN-γ to normalize blood vessels, while helper T (Th) cells, including Th1, Th2, and Th17 subsets, actively participate in angiogenesis by releasing chemokines and activating M2-like TAMs [ 9 , 112 ]. In CRC and melanoma, deletion of METTL3 and METTL14 increases CD8 + CTL levels and enhances response to anti-PD1 therapy [ 131 ]. Similarly, silencing of YTHDF1 in GC increases CD8 + CTL and mDCs proportions, thereby restoring sensitivity to immunotherapy (Table 2 ) [ 127 ].
m 6 A methylation in cancer therapy
Clinical treatment prospects of new strategies.
The advancement of targeted therapy strategies focused on m 6 A regulators is rapidly progressing, driven by their crucial regulatory functions and precise recognition abilities. Dysregulation of enzymes and binding proteins involved in RNA methylation has been linked to various human cancers, suggesting a promising novel approach for clinical intervention [ 34 ].
m 6 A inhibitors
Recent studies indicate that inhibitors targeting RNA methylation regulatory factors have potential in cancer therapy. For instance, SAH and the broad-spectrum 2-OG oxygenase inhibitor IOX1 inhibit cancer development by targeting METTL3-METTL14 and ALKBH5, respectively [ 132 ]. Additionally, inhibitors like FB23-2, FG-2216/IOX3, Rhein, Entacapone, and meclofenamic acid inhibit FTO activity, preventing the self-renewal and tumorigenic properties of AML and GBM [ 132 , 133 , 134 ]. In mouse xenograft models, FTO inhibition resulted in reduced tumor growth and prolonged survival.
Clinical trials and preclinical research
Ongoing clinical trials of m 6 A emphasize the potential of targeting m 6 A modifications in cancer treatment. STC-15, as an oral small molecule inhibitor targeting METTL3, is a first-in-class inhibitor of RNA modification. In November 2022, it advanced into Phase I clinical trials, representing the first RNA methyltransferase inhibitor to enter clinical development. STC-15 has demonstrated efficacy in suppressing AML or suppress tumor growth through anticancer immune responses, promising a novel avenue for cancer therapy [ 135 ].
Another promising candidate is STM2457, a highly selective METTL3 inhibitor with minimal effects on other methyltransferases, indicating its potential as a targeted cancer therapy. Preclinical research indicates that STM2457 specifically inhibits key stem cell populations in AML without significant toxicity to normal haematopoiesis, promoting cell differentiation, inducing apoptosis, and inhibiting tumor growth [ 136 ]. These findings support targeting m 6 A modification as a promising strategy for anticancer therapy.
Potential of post-transcriptional modifications as biomarkers
Post-transcriptional modifications (PTMs) have been demonstrated to be associated with various human diseases. RNA modifications can be potential biomarkers for monitoring cancer progression through regulating mRNA stability, translation efficiency, and other RNA metabolic processes. Distinct modification patterns in different cancer types influence tumor cell behaviors such as proliferation, apoptosis, invasiveness, and metabolic activity, which makes them important indicators in cancer research. For instance, the m 5 C-based signature is an independent prognostic factor associated with immunotherapy efficacy and drug susceptibility in RCC [ 137 ]. The interaction network of m 6 A/m 5 C/m 1 A regulated genes is reported to assess the prognosis of HCC [ 138 ]. These imply the potential of RNA modification in clinical applications.
Clinically, detecting m 6 A often requires high-throughput sequencing (such as MeRIP-seq) and specific immunoprecipitation techniques [ 15 ]. These methods help clinicians analyze m 6 A levels in samples to assess tumor status and treatment response. As biomarkers, m 6 A modifications have the advantage of dynamically reflecting biological changes within tumor cells, allowing real-time monitoring of tumor progression and treatment response through non-invasive samples (such as blood) [ 139 ]. For instance, in gastrointestinal cancer, m 6 A levels were elevated compared to adjacent tissues and the serum of healthy individuals, and decreased post-surgery [ 140 ]. However, the dynamic and variable nature of m 6 A and other RNA modifications can complicate result interpretation. Moreover, the need for specialized techniques and equipment limits the widespread clinical application of m 6 A as a biomarker. Overall, research on RNA modifications as potential cancer biomarkers is still in its early stages, requiring further study to overcome current technical barriers and enhance their clinical accuracy and feasibility.
Conclusions and perspectives
This review emphasizes the significant role of m 6 A modifications in tumor neovascularization. We aim to explore how m 6 A influences various modes of neovascularization and its interactions with multiple signaling pathways and components of the TME. By inhibiting key m 6 A writers or erasers, it may be possible to suppress pro-angiogenic factors, thereby reducing tumor vascularization and growth. This approach could potentially enhance the efficacy of existing treatments, such as VEGF inhibitors.
Although studies using m 6 A inhibitors to directly target tumor neovascularization are limited, growing evidence suggests that m 6 A is widely involved in anti-angiogenic resistance. Specifically, in HCC and RCC, both of which are characterized by high vascular density. Increased m 6 A levels contribute to resistance against sorafenib, apatinib, and lenvatinib in HCC. Under normoxic conditions, METTL3 increases resistance to sorafenib and lenvatinib through the Wnt/β-catenin pathway, while WTAP facilitates lenvatinib resistance under hypoxic conditions. Knockdown of METTL14 restores sensitivity to sorafenib by upregulating hepatocyte nuclear factor 3 gamma (HNF3γ). In RCC, downregulation of METTL14 enhances sensitivity to sunitinib by reducing TRAF1 expression. YTHDC1 increases sensitivity to sunitinib by inhibiting histone deacetylase 2 (HDAC2). Conversely, in pazopanib-resistant cRCC, YTHDF2 fails to recognize lncIGFL2AS1 for degradation, promoting drug resistance. These findings suggest targeting m 6 A might provide a novel approach to anti-angiogenic therapy. In conclusion, the regulatory role of m 6 A in tumor neovascularization is critical and warrants further investigation to develop potential treatment strategies. Additional research is needed to fully understand its clinical potential, especially its interactions with various pathways and immune cells. Despite the limited studies targeting tumor neovascularization with m 6 A inhibitors, growing evidence suggests that this approach may offer promising therapeutic potential.
Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–87.
Article CAS PubMed Google Scholar
Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6.
de Heer EC, Jalving M, Harris AL. HIFs, angiogenesis, and metabolism: elusive enemies in breast cancer. J Clin Investig. 2020;130:5074–87.
Article PubMed PubMed Central Google Scholar
Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603.
Article CAS PubMed PubMed Central Google Scholar
Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307.
Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77:1745–70.
De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17:457–74.
Article PubMed Google Scholar
Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46.
Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27.
An Y, Duan H. The role of m6A RNA methylation in cancer metabolism. Mol Cancer. 2022;21:14.
Song P, Tayier S, Cai Z, Jia G. RNA methylation in mammalian development and cancer. Cell Biol Toxicol. 2021;37:811–31.
Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci USA. 1974;71:3971–5.
Zaccara S, Ries RJ, Jaffrey SR. Reading, writing and erasing mRNA methylation. Nat Rev Mol Cell Biol. 2019;20:608–24.
Cao X, Geng Q, Fan D, Wang Q, Wang X, Zhang M, et al. m(6)A methylation: a process reshaping the tumour immune microenvironment and regulating immune evasion. Mol Cancer. 2023;22:42.
Uddin MB, Wang Z, Yang C. The m(6)A RNA methylation regulates oncogenic signaling pathways driving cell malignant transformation and carcinogenesis. Mol Cancer. 2021;20:61.
Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–84.
Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–52.
Li X, Xue Y, Liu X, Zheng J, Shen S, Yang C, et al. ZRANB2/SNHG20/FOXK1 axis regulates vasculogenic mimicry formation in glioma. J Exp Clin Cancer Res. 2019;38:68.
Wang M, Zhao X, Zhu D, Liu T, Liang X, Liu F, et al. HIF-1alpha promoted vasculogenic mimicry formation in hepatocellular carcinoma through LOXL2 up-regulation in hypoxic tumor microenvironment. J Exp Clin Cancer Res. 2017;36:60.
Luo Y, Yang Z, Yu Y, Zhang P. HIF1alpha lactylation enhances KIAA1199 transcription to promote angiogenesis and vasculogenic mimicry in prostate cancer. Int J Biol Macromol. 2022;222:2225–43.
Yao XH, Ping YF, Bian XW. Contribution of cancer stem cells to tumor vasculogenic mimicry. Protein Cell. 2011;2:266–72.
Luo Q, Wang J, Zhao W, Peng Z, Liu X, Li B, et al. Vasculogenic mimicry in carcinogenesis and clinical applications. J Hematol Oncol. 2020;13:19.
Cai HP, Wang J, Xi SY, Ni XR, Chen YS, Yu YJ, et al. Tenascin-cmediated vasculogenic mimicry formation via regulation of MMP2/MMP9 in glioma. Cell Death Dis. 2019;10:879.
Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–5.
Wang X, Feng J, Xue Y, Guan Z, Zhang D, Liu Z, et al. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature. 2016;534:575–8.
Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–89.
Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, Guttman M, et al. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537:369–73.
Yue Y, Liu J, Cui X, Cao J, Luo G, Zhang Z, et al. VIRMA mediates preferential m(6)A mRNA methylation in 3’UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 2018;4:10.
Satterwhite ER, Mansfield KD. RNA methyltransferase METTL16: targets and function. Wiley Interdiscip Rev RNA. 2022;13:e1681.
Wang T, Kong S, Tao M, Ju S. The potential role of RNA N6-methyladenosine in cancer progression. Mol Cancer. 2020;19:88.
Lin H, Wang Y, Wang P, Long F, Wang T. Mutual regulation between N6-methyladenosine (m6A) modification and circular RNAs in cancer: impacts on therapeutic resistance. Mol Cancer. 2022;21:148.
Chen XY, Zhang J, Zhu JS. The role of m(6)A RNA methylation in human cancer. Mol Cancer. 2019;18:103.
Shi H, Wei J, He C. Where, when, and how: context-dependent functions of rna methylation writers, readers, and erasers. Mol Cell. 2019;74:640–50.
Deng X, Su R, Weng H, Huang H, Li Z, Chen J. RNA N(6)-methyladenosine modification in cancers: current status and perspectives. Cell Res. 2018;28:507–17.
Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–7.
Mauer J, Sindelar M, Despic V, Guez T, Hawley BR, Vasseur JJ, et al. FTO controls reversible m(6)Am RNA methylation during snRNA biogenesis. Nat Chem Biol. 2019;15:340–7.
Wei J, Liu F, Lu Z, Fei Q, Ai Y, He PC, et al. Differential m(6)A, m(6)A(m), and m(1)A demethylation mediated by FTO in the cell nucleus and cytoplasm. Mol Cell. 2018;71:973–85.e5.
Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29.
Qu J, Yan H, Hou Y, Cao W, Liu Y, Zhang E, et al. RNA demethylase ALKBH5 in cancer: from mechanisms to therapeutic potential. J Hematol Oncol. 2022;15:8.
He L, Li H, Wu A, Peng Y, Shu G, Yin G. Functions of N6-methyladenosine and its role in cancer. Mol Cancer. 2019;18:176.
Jiang X, Liu B, Nie Z, Duan L, Xiong Q, Jin Z, et al. The role of m6A modification in the biological functions and diseases. Signal Transduct Target Ther. 2021;6:74.
Liu H, Gu J, Huang Z, Han Z, Xin J, Yuan L, et al. Fine particulate matter induces METTL3-mediated m(6)A modification of BIRC5 mRNA in bladder cancer. J Hazard Mater. 2022;437:129310.
Wang G, Dai Y, Li K, Cheng M, Xiong G, Wang X, et al. Deficiency of Mettl3 in bladder cancer stem cells inhibits bladder cancer progression and angiogenesis. Front Cell Dev Biol. 2021;9:627706.
Wen H, Tang J, Cui Y, Hou M, Zhou J. m6A modification-mediated BATF2 suppresses metastasis and angiogenesis of tongue squamous cell carcinoma through inhibiting VEGFA. Cell Cycle. 2023;22:100–16.
Sang QX. Complex role of matrix metalloproteinases in angiogenesis. Cell Res. 1998;8:171–7.
Liu J, Jiang K. METTL3-mediated maturation of miR-589-5p promotes the malignant development of liver cancer. J Cell Mol Med. 2022;26:2505–19.
Chen YT, Xiang D, Zhao XY, Chu XY. Upregulation of lncRNA NIFK-AS1 in hepatocellular carcinoma by m(6)A methylation promotes disease progression and sorafenib resistance. Hum Cell. 2021;34:1800–11.
Zheng Y, Qi F, Li L, Yu B, Cheng Y, Ge M, et al. LncNAP1L6 activates MMP pathway by stabilizing the m6A-modified NAP1L2 to promote malignant progression in prostate cancer. Cancer Gene Ther. 2023;30:209–18.
Yu T, Liu J, Wang Y, Chen W, Liu Z, Zhu L, et al. METTL3 promotes colorectal cancer metastasis by stabilizing PLAU mRNA in an m6A-dependent manner. Biochem Biophys Res Commun. 2022;614:9–16.
Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol. 2010;11:23–36.
Xu P, Yang J, Chen Z, Zhang X, Xia Y, Wang S, et al. N6-methyladenosine modification of CENPF mRNA facilitates gastric cancer metastasis via regulating FAK nuclear export. Cancer Commun. 2023;43:685–705.
Article Google Scholar
Guo YQ, Wang Q, Wang JG, Gu YJ, Song PP, Wang SY, et al. METTL3 modulates m6A modification of CDC25B and promotes head and neck squamous cell carcinoma malignant progression. Exp Hematol Oncol. 2022;11:14.
Wang Q, Chen C, Ding Q, Zhao Y, Wang Z, Chen J, et al. METTL3-mediated m(6)A modification of HDGF mRNA promotes gastric cancer progression and has prognostic significance. Gut. 2020;69:1193–205.
Chen Y, Lu Z, Qi C, Yu C, Li Y, Huan W, et al. N(6)-methyladenosine-modified TRAF1 promotes sunitinib resistance by regulating apoptosis and angiogenesis in a METTL14-dependent manner in renal cell carcinoma. Mol Cancer. 2022;21:111.
Yu T, Yao L, Yin H, Teng Y, Hong M, Wu Q. ALKBH5 promotes multiple myeloma tumorigenicity through inducing m(6)A-demethylation of SAV1 mRNA and myeloma stem cell phenotype. Int J Biol Sci. 2022;18:2235–48.
Xie J, Zhang H, Wang K, Ni J, Ma X, Khoury CJ, et al. M6A-mediated-upregulation of lncRNA BLACAT3 promotes bladder cancer angiogenesis and hematogenous metastasis through YBX3 nuclear shuttling and enhancing NCF2 transcription. Oncogene. 2023;42:2956–70.
Xiao Y, Thakkar KN, Zhao H, Broughton J, Li Y, Seoane JA, et al. The m(6)A RNA demethylase FTO is a HIF-independent synthetic lethal partner with the VHL tumor suppressor. Proc Natl Acad Sci USA. 2020;117:21441–9.
Rong ZX, Li Z, He JJ, Liu LY, Ren XX, Gao J, et al. Downregulation of fat mass and obesity associated (FTO) promotes the progression of intrahepatic cholangiocarcinoma. Front Oncol. 2019;9:369.
Fang H, Sun Q, Zhou J, Zhang H, Song Q, Zhang H, et al. m(6)A methylation reader IGF2BP2 activates endothelial cells to promote angiogenesis and metastasis of lung adenocarcinoma. Mol Cancer. 2023;22:99.
Ma YS, Shi BW, Guo JH, Liu JB, Yang XL, Xin R, et al. microRNA-320b suppresses HNF4G and IGF2BP2 expression to inhibit angiogenesis and tumor growth of lung cancer. Carcinogenesis. 2021;42:762–71.
Yang Z, Wang T, Wu D, Min Z, Tan J, Yu B. RNA N6-methyladenosine reader IGF2BP3 regulates cell cycle and angiogenesis in colon cancer. J Exp Clin Cancer Res. 2020;39:203.
Bian Y, Wang Y, Xu S, Gao Z, Li C, Fan Z, et al. m(6)A Modification of long non-coding RNA HNF1A-AS1 facilitates cell cycle progression in colorectal cancer via IGF2BP2-mediated CCND1 mRNA stabilization. Cells. 2022;11:3008.
Wang J, Tan L, Yu X, Cao X, Jia B, Chen R, et al. lncRNA ZNRD1-AS1 promotes malignant lung cell proliferation, migration, and angiogenesis via the miR-942/TNS1 axis and is positively regulated by the m(6)A reader YTHDC2. Mol Cancer. 2022;21:229.
Xu Z, Chen S, Liu R, Chen H, Xu B, Xu W, et al. Circular RNA circPOLR2A promotes clear cell renal cell carcinoma progression by facilitating the UBE3C-induced ubiquitination of PEBP1 and, thereby, activating the ERK signaling pathway. Mol Cancer. 2022;21:146.
Hou J, Zhang H, Liu J, Zhao Z, Wang J, Lu Z, et al. YTHDF2 reduction fuels inflammation and vascular abnormalization in hepatocellular carcinoma. Mol Cancer. 2019;18:163.
Qiao K, Liu Y, Xu Z, Zhang H, Zhang H, Zhang C, et al. RNA m6A methylation promotes the formation of vasculogenic mimicry in hepatocellular carcinoma via Hippo pathway. Angiogenesis. 2021;24:83–96.
Liu X, He H, Zhang F, Hu X, Bi F, Li K, et al. m6A methylated EphA2 and VEGFA through IGF2BP2/3 regulation promotes vasculogenic mimicry in colorectal cancer via PI3K/AKT and ERK1/2 signaling. Cell Death Dis. 2022;13:483.
Wu Z, Lin Y, Wei N. N6-methyladenosine-modified HOTAIRM1 promotes vasculogenic mimicry formation in glioma. Cancer Sci. 2022;114:129–41.
Liu M, Ruan X, Liu X, Dong W, Wang D, Yang C, et al. The mechanism of BUD13 m6A methylation mediated MBNL1-phosphorylation by CDK12 regulating the vasculogenic mimicry in glioblastoma cells. Cell Death Dis. 2022;13:1017.
Tao M, Li X, He L, Rong X, Wang H, Pan J, et al. Decreased RNA m(6)A methylation enhances the process of the epithelial mesenchymal transition and vasculogenic mimicry in glioblastoma. Am J Cancer Res. 2022;12:893–906.
CAS PubMed PubMed Central Google Scholar
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5:8.
Hess DL, Kelly-Goss MR, Cherepanova OA, Nguyen AT, Baylis RA, Tkachenko S, et al. Perivascular cell-specific knockout of the stem cell pluripotency gene Oct4 inhibits angiogenesis. Nat Commun. 2019;10:967.
Liu HL, Tang HT, Yang HL, Deng TT, Xu YP, Xu SQ, et al. Oct4 regulates the transition of cancer stem-like cells to tumor endothelial-like cells in human liver cancer. Front Cell Dev Biol. 2020;8:563316.
Zhang C, Huang S, Zhuang H, Ruan S, Zhou Z, Huang K, et al. YTHDF2 promotes the liver cancer stem cell phenotype and cancer metastasis by regulating OCT4 expression via m6A RNA methylation. Oncogene. 2020;39:4507–18.
Liu X, Wang Z, Yang Q, Hu X, Fu Q, Zhang X, et al. RNA demethylase ALKBH5 prevents lung cancer progression by regulating EMT and stemness via regulating p53. Front Oncol. 2022;12:858694.
Takahashi K, Asano N, Imatani A, Kondo Y, Saito M, Takeuchi A, et al. Sox2 induces tumorigenesis and angiogenesis of early-stage esophageal squamous cell carcinoma through secretion of Suprabasin. Carcinogenesis. 2020;41:1543–52.
Chen J, Chen S, Zhuo L, Zhu Y, Zheng H. Regulation of cancer stem cell properties, angiogenesis, and vasculogenic mimicry by miR-450a-5p/SOX2 axis in colorectal cancer. Cell Death Dis. 2020;11:173.
Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A, et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478:399–403.
Garcia JR, Gombos DS, Prospero CM, Ganapathy A, Penland RL, Chevez-Barrios P. Expression of angiogenic factors in invasive retinoblastoma tumors is associated with increase in tumor cells expressing stem cell marker Sox2. Arch Pathol Lab Med. 2015;139:1531–8.
Li T, Hu PS, Zuo Z, Lin JF, Li X, Wu QN, et al. METTL3 facilitates tumor progression via an m(6)A-IGF2BP2-dependent mechanism in colorectal carcinoma. Mol Cancer. 2019;18:112.
Visvanathan A, Patil V, Arora A, Hegde AS, Arivazhagan A, Santosh V, et al. Essential role of METTL3-mediated m(6)A modification in glioma stem-like cells maintenance and radioresistance. Oncogene. 2018;37:522–33.
Zhang D, Ning J, Okon I, Zheng X, Satyanarayana G, Song P, et al. Suppression of m6A mRNA modification by DNA hypermethylated ALKBH5 aggravates the oncological behavior of KRAS mutation/LKB1 loss lung cancer. Cell Death Dis. 2021;12:518.
Chen G, Liu B, Yin S, Li S, Guo Y, Wang M, et al. Hypoxia induces an endometrial cancer stem-like cell phenotype via HIF-dependent demethylation of SOX2 mRNA. Oncogenesis. 2020;9:81.
Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176:1248–64.
Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J Intern Med. 2013;273:114–27.
Zhang H, Zhou J, Li J, Wang Z, Chen Z, Lv Z, et al. N6-methyladenosine promotes translation of VEGFA to accelerate angiogenesis in lung cancer. Cancer Res. 2023;83:2208–25.
Chen JQ, Tao YP, Hong YG, Li HF, Huang ZP, Xu XF, et al. M(6)A-mediated up-regulation of LncRNA LIFR-AS1 enhances the progression of pancreatic cancer via miRNA-150-5p/ VEGFA/Akt signaling. Cell Cycle. 2021;20:2507–18.
Lin Z, Niu Y, Wan A, Chen D, Liang H, Chen X, et al. RNA m(6) A methylation regulates sorafenib resistance in liver cancer through FOXO3-mediated autophagy. EMBO J. 2020;39:e103181.
Jiang L, Li Y, He Y, Wei D, Yan L, Wen H. Knockdown of m6A reader IGF2BP3 inhibited hypoxia-induced cell migration and angiogenesis by regulating hypoxia inducible factor-1alpha in stomach cancer. Front Oncol. 2021;11:711207.
Levantini E, Maroni G, Del Re M, Tenen DG. EGFR signaling pathway as therapeutic target in human cancers. Semin Cancer Biol. 2022;85:253–75.
Chang G, Shi L, Ye Y, Shi H, Zeng L, Tiwary S, et al. YTHDF3 induces the translation of m(6)A-enriched gene transcripts to promote breast cancer brain metastasis. Cancer Cell. 2020;38:857–71.e7.
Zhong L, Liao D, Zhang M, Zeng C, Li X, Zhang R, et al. YTHDF2 suppresses cell proliferation and growth via destabilizing the EGFR mRNA in hepatocellular carcinoma. Cancer Lett. 2019;442:252–61.
Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18:26.
Figueiredo AM, Villacampa P, Dieguez-Hurtado R, Jose Lozano J, Kobialka P, Cortazar AR, et al. Phosphoinositide 3-kinase-regulated pericyte maturation governs vascular remodeling. Circulation. 2020;142:688–704.
Soler A, Serra H, Pearce W, Angulo A, Guillermet-Guibert J, Friedman LS, et al. Inhibition of the p110alpha isoform of PI 3-kinase stimulates nonfunctional tumor angiogenesis. J Exp Med. 2013;210:1937–45.
Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci. 2011;4:51.
Wang N, Huo X, Zhang B, Chen X, Zhao S, Shi X, et al. METTL3-mediated ADAMTS9 suppression facilitates angiogenesis and carcinogenesis in gastric cancer. Front Oncol. 2022;12:861807.
McCubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal. 2006;8:1775–89.
Ullah R, Yin Q, Snell AH, Wan L. RAF-MEK-ERK pathway in cancer evolution and treatment. Semin Cancer Biol. 2022;85:123–54.
Ye M, Chen J, Yu P, Hu C, Wang B, Bao J, et al. WTAP activates MAPK signaling through m6A methylation in VEGFA mRNA-mediated by YTHDC1 to promote colorectal cancer development. FASEB J. 2023;37:e23090.
Azad T, Ghahremani M, Yang X. The role of YAP and TAZ in angiogenesis and vascular mimicry. Cells. 2019;8:407.
Ou H, Chen Z, Xiang L, Fang Y, Xu Y, Liu Q, et al. Frizzled 2-induced epithelial-mesenchymal transition correlates with vasculogenic mimicry, stemness, and Hippo signaling in hepatocellular carcinoma. Cancer Sci. 2019;110:1169–82.
Chen J, Sun Y, Xu X, Wang D, He J, Zhou H, et al. YTH domain family 2 orchestrates epithelial-mesenchymal transition/proliferation dichotomy in pancreatic cancer cells. Cell Cycle. 2017;16:2259–71.
Wei H, Wang F, Wang Y, Li T, Xiu P, Zhong J, et al. Verteporfin suppresses cell survival, angiogenesis and vasculogenic mimicry of pancreatic ductal adenocarcinoma via disrupting the YAP-TEAD complex. Cancer Sci. 2017;108:478–87.
Naktubtim C, Payuhakrit W, Uttarawichien T, Hassametto A, Suwannalert P. YAP, a novel target regulates F-actin rearrangement-associated CAFs transformation and promotes colorectal cancer cell progression. Biomed Pharmacother. 2022;155:113757.
Shen Y, Wang X, Liu Y, Singhal M, Gurkaslar C, Valls AF, et al. STAT3-YAP/TAZ signaling in endothelial cells promotes tumor angiogenesis. Sci Signal. 2021;14:eabj8393.
Cui J, Tian J, Wang W, He T, Li X, Gu C, et al. IGF2BP2 promotes the progression of colorectal cancer through a YAP-dependent mechanism. Cancer Sci. 2021;112:4087–99.
Zhang Y, Wang X. Targeting the Wnt/beta-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13:165.
Guo Y, Guo Y, Chen C, Fan D, Wu X, Zhao L, et al. Circ3823 contributes to growth, metastasis and angiogenesis of colorectal cancer: involvement of miR-30c-5p/TCF7 axis. Mol Cancer. 2021;20:93.
Lee WS, Yang H, Chon HJ, Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med. 2020;52:1475–85.
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380:1116–27.
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–905.
Kawazoe A, Fukuoka S, Nakamura Y, Kuboki Y, Wakabayashi M, Nomura S, et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21:1057–65.
Makker V, Rasco D, Vogelzang NJ, Brose MS, Cohn AL, Mier J, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20:711–8.
Li X, Ma S, Deng Y, Yi P, Yu J. Targeting the RNA m(6)A modification for cancer immunotherapy. Mol Cancer. 2022;21:76.
Huinen ZR, Huijbers EJM, van Beijnum JR, Nowak-Sliwinska P, Griffioen AW. Anti-angiogenic agents - overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat Rev Clin Oncol. 2021;18:527–40.
Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol. 2018;15:310–24.
Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325–40.
Viallard C, Larrivee B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20:409–26.
Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153:139–52.
Zhou W, Chen C, Shi Y, Wu Q, Gimple RC, Fang X, et al. Targeting glioma stem cell-derived pericytes disrupts the blood-tumor barrier and improves chemotherapeutic efficacy. Cell Stem Cell. 2017;21:591–603.e4.
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55.
Wei C, Wang B, Peng D, Zhang X, Li Z, Luo L, et al. Pan-cancer analysis shows That ALKBH5 is a potential prognostic and immunotherapeutic biomarker for multiple cancer types including gliomas. Front Immunol. 2022;13:849592.
Liu Y, Shi M, He X, Cao Y, Liu P, Li F, et al. LncRNA-PACERR induces pro-tumour macrophages via interacting with miR-671-3p and m6A-reader IGF2BP2 in pancreatic ductal adenocarcinoma. J Hematol Oncol. 2022;15:52.
Bai X, Wong CC, Pan Y, Chen H, Liu W, Zhai J, et al. Loss of YTHDF1 in gastric tumors restores sensitivity to antitumor immunity by recruiting mature dendritic cells. J Immunother Cancer. 2022;10:e003663.
Chen H, Pan Y, Zhou Q, Liang C, Wong CC, Zhou Y, et al. METTL3 inhibits antitumor immunity by targeting m(6)A-BHLHE41-CXCL1/CXCR2 axis to promote colorectal cancer. Gastroenterology. 2022;163:891–907.
Ni HH, Zhang L, Huang H, Dai SQ, Li J. Connecting METTL3 and intratumoural CD33(+) MDSCs in predicting clinical outcome in cervical cancer. J Transl Med. 2020;18:393.
Qiu X, Yang S, Wang S, Wu J, Zheng B, Wang K, et al. M(6)A demethylase ALKBH5 regulates PD-L1 expression and tumor immunoenvironment in intrahepatic cholangiocarcinoma. Cancer Res. 2021;81:4778–93.
Wang L, Hui H, Agrawal K, Kang Y, Li N, Tang R, et al. m(6) A RNA methyltransferases METTL3/14 regulate immune responses to anti-PD-1 therapy. EMBO J. 2020;39:e104514.
Geng Q, Cao X, Fan D, Wang Q, Wang X, Zhang M, et al. Potential medicinal value of N6-methyladenosine in autoimmune diseases and tumours. Br J Pharmacol. 2023.
Su R, Dong L, Li Y, Gao M, Han L, Wunderlich M, et al. Targeting FTO suppresses cancer stem cell maintenance and immune evasion. Cancer Cell. 2020;38:79–96.e11.
Xiao L, Li X, Mu Z, Zhou J, Zhou P, Xie C, et al. FTO inhibition enhances the antitumor effect of temozolomide by targeting MYC-miR-155/23a cluster-MXI1 feedback circuit in glioma. Cancer Res. 2020;80:3945–58.
ST Ltd. Homepage. https://www.stormtherapeutics.com/clinical-trials/ .
Yankova E, Blackaby W, Albertella M, Rak J, De Braekeleer E, Tsagkogeorga G, et al. Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature. 2021;593:597–601.
Zhang R, Gan W, Zong J, Hou Y, Zhou M, Yan Z, et al. Developing an m5C regulator-mediated RNA methylation modification signature to predict prognosis and immunotherapy efficacy in rectal cancer. Front Immunol. 2023;14:1054700.
Li D, Li K, Zhang W, Yang KW, Mu DA, Jiang GJ, et al. The m6A/m5C/m1A regulated gene signature predicts the prognosis and correlates with the immune status of hepatocellular carcinoma. Front Immunol. 2022;13:918140.
Ge L, Zhang N, Chen Z, Song J, Wu Y, Li Z, et al. Level of N6-methyladenosine in peripheral blood RNA: a novel predictive biomarker for gastric cancer. Clin Chem. 2020;66:342–51.
Konno M, Koseki J, Asai A, Yamagata A, Shimamura T, Motooka D, et al. Distinct methylation levels of mature microRNAs in gastrointestinal cancers. Nat Commun. 2019;10:3888.
Download references
Acknowledgements
We thank Figdraw ( www.figdraw.com ) and Biorender ( www.biorender.com ) for expert assistance in the pattern drawing.
This study was financially supported by the National Natural Science Foundation of China (Grant numbers: 82173378, U22A20374, 81960476), National High Level Hospital Clinical Research Funding (No. 2023-NHLHCRF-DJMS-06), and Elite Medical Professionals Project of China-Japan Friendship Hospital (No. ZRJY2021-TD02).
Author information
These authors contributed equally: Lu Zhao, Qinshan Li.
Authors and Affiliations
Institute of Clinical Medicine, China-Japan Friendship Hospital, Beijing, China
Lu Zhao, Tongliang Zhou, Xuan Liu, Jing Guo, Qing Fang, Xiaoxue Cao, Qishun Geng, Yang Yu, Songjie Zhang, Tingting Deng, Xing Wang, Yi Jiao, Mengxiao Zhang, Honglin Liu & Cheng Xiao
China-Japan Friendship Hospital, Capital Medical University, Beijing, China
Lu Zhao, Honglin Liu & Cheng Xiao
Institute of Precision Medicine of Guizhou Province, Department of Obstetrics and Gynecology, Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, 550004, China
Department of Clinical Biochemistry, School of Clinical Laboratory Science, Guizhou Medical University, Guiyang, Guizhou, 550004, China
Graduate School of Peking Union Medical College, Chinese Academy of Medical Sciences/Peking Union Medical College, Beijing, China
Xiaoxue Cao & Qishun Geng
China-Japan Friendship Clinical Medical College, Beijing University of Chinese Medicine, Beijing, China
Xing Wang & Yi Jiao
Department of Hepatobiliary Surgery, China-Japan Friendship Hospital, Beijing, 100029, China
Haidong Tan
You can also search for this author in PubMed Google Scholar
Contributions
CX and HLL conceptualized the paper, HLL gave the outline of the paper. LZ searched and sorted out relevant literatures, LZ and QSL co-wrote the manuscript. YY, XXC, and SJZ each created Figs. 1 – 3 , respectively, and QSG enhanced their presentation. TLZ prepared Table 1 , XL and JG revised Table 1 in the revised manuscript. QF and TTD jointly prepared and revised Table 2 . YJ polished the language of the article, XW and MXZ revised the manuscript. HDT supervised the revised manuscript and LZ wrote the final version of the manuscript.
Corresponding authors
Correspondence to Honglin Liu , Haidong Tan or Cheng Xiao .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Francesca Pentimalli
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Zhao, L., Li, Q., Zhou, T. et al. Role of N6-methyladenosine in tumor neovascularization. Cell Death Dis 15 , 563 (2024). https://doi.org/10.1038/s41419-024-06931-z
Download citation
Received : 18 October 2023
Revised : 14 July 2024
Accepted : 22 July 2024
Published : 05 August 2024
DOI : https://doi.org/10.1038/s41419-024-06931-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
COVID-19: Long-term effects
Some people continue to experience health problems long after having COVID-19. Understand the possible symptoms and risk factors for post-COVID-19 syndrome.
Most people who get coronavirus disease 2019 (COVID-19) recover within a few weeks. But some people — even those who had mild versions of the disease — might have symptoms that last a long time afterward. These ongoing health problems are sometimes called post- COVID-19 syndrome, post- COVID conditions, long COVID-19 , long-haul COVID-19 , and post acute sequelae of SARS COV-2 infection (PASC).
What is post-COVID-19 syndrome and how common is it?
Post- COVID-19 syndrome involves a variety of new, returning or ongoing symptoms that people experience more than four weeks after getting COVID-19 . In some people, post- COVID-19 syndrome lasts months or years or causes disability.
Research suggests that between one month and one year after having COVID-19 , 1 in 5 people ages 18 to 64 has at least one medical condition that might be due to COVID-19 . Among people age 65 and older, 1 in 4 has at least one medical condition that might be due to COVID-19 .
What are the symptoms of post-COVID-19 syndrome?
The most commonly reported symptoms of post- COVID-19 syndrome include:
- Symptoms that get worse after physical or mental effort
- Lung (respiratory) symptoms, including difficulty breathing or shortness of breath and cough
Other possible symptoms include:
- Neurological symptoms or mental health conditions, including difficulty thinking or concentrating, headache, sleep problems, dizziness when you stand, pins-and-needles feeling, loss of smell or taste, and depression or anxiety
- Joint or muscle pain
- Heart symptoms or conditions, including chest pain and fast or pounding heartbeat
- Digestive symptoms, including diarrhea and stomach pain
- Blood clots and blood vessel (vascular) issues, including a blood clot that travels to the lungs from deep veins in the legs and blocks blood flow to the lungs (pulmonary embolism)
- Other symptoms, such as a rash and changes in the menstrual cycle
Keep in mind that it can be hard to tell if you are having symptoms due to COVID-19 or another cause, such as a preexisting medical condition.
It's also not clear if post- COVID-19 syndrome is new and unique to COVID-19 . Some symptoms are similar to those caused by chronic fatigue syndrome and other chronic illnesses that develop after infections. Chronic fatigue syndrome involves extreme fatigue that worsens with physical or mental activity, but doesn't improve with rest.
Why does COVID-19 cause ongoing health problems?
Organ damage could play a role. People who had severe illness with COVID-19 might experience organ damage affecting the heart, kidneys, skin and brain. Inflammation and problems with the immune system can also happen. It isn't clear how long these effects might last. The effects also could lead to the development of new conditions, such as diabetes or a heart or nervous system condition.
The experience of having severe COVID-19 might be another factor. People with severe symptoms of COVID-19 often need to be treated in a hospital intensive care unit. This can result in extreme weakness and post-traumatic stress disorder, a mental health condition triggered by a terrifying event.
What are the risk factors for post-COVID-19 syndrome?
You might be more likely to have post- COVID-19 syndrome if:
- You had severe illness with COVID-19 , especially if you were hospitalized or needed intensive care.
- You had certain medical conditions before getting the COVID-19 virus.
- You had a condition affecting your organs and tissues (multisystem inflammatory syndrome) while sick with COVID-19 or afterward.
Post- COVID-19 syndrome also appears to be more common in adults than in children and teens. However, anyone who gets COVID-19 can have long-term effects, including people with no symptoms or mild illness with COVID-19 .
What should you do if you have post-COVID-19 syndrome symptoms?
If you're having symptoms of post- COVID-19 syndrome, talk to your health care provider. To prepare for your appointment, write down:
- When your symptoms started
- What makes your symptoms worse
- How often you experience symptoms
- How your symptoms affect your activities
Your health care provider might do lab tests, such as a complete blood count or liver function test. You might have other tests or procedures, such as chest X-rays, based on your symptoms. The information you provide and any test results will help your health care provider come up with a treatment plan.
In addition, you might benefit from connecting with others in a support group and sharing resources.
- Long COVID or post-COVID conditions. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects.html. Accessed May 6, 2022.
- Post-COVID conditions: Overview for healthcare providers. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html. Accessed May 6, 2022.
- Mikkelsen ME, et al. COVID-19: Evaluation and management of adults following acute viral illness. https://www.uptodate.com/contents/search. Accessed May 6, 2022.
- Saeed S, et al. Coronavirus disease 2019 and cardiovascular complications: Focused clinical review. Journal of Hypertension. 2021; doi:10.1097/HJH.0000000000002819.
- AskMayoExpert. Post-COVID-19 syndrome. Mayo Clinic; 2022.
- Multisystem inflammatory syndrome (MIS). Centers for Disease Control and Prevention. https://www.cdc.gov/mis/index.html. Accessed May 24, 2022.
- Patient tips: Healthcare provider appointments for post-COVID conditions. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/post-covid-appointment/index.html. Accessed May 24, 2022.
- Bull-Otterson L, et al. Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥ 65 years — United States, March 2020 — November 2021. MMWR Morbidity and Mortality Weekly Report. 2022; doi:10.15585/mmwr.mm7121e1.
Products and Services
- A Book: Endemic - A Post-Pandemic Playbook
- Begin Exploring Women's Health Solutions at Mayo Clinic Store
- A Book: Future Care
- Antibiotics: Are you misusing them?
- COVID-19 and vitamin D
- Convalescent plasma therapy
- Coronavirus disease 2019 (COVID-19)
- COVID-19: How can I protect myself?
- Herd immunity and respiratory illness
- COVID-19 and pets
- COVID-19 and your mental health
- COVID-19 antibody testing
- COVID-19, cold, allergies and the flu
- COVID-19 tests
- COVID-19 drugs: Are there any that work?
- COVID-19 in babies and children
- Coronavirus infection by race
- COVID-19 travel advice
- COVID-19 vaccine: Should I reschedule my mammogram?
- COVID-19 vaccines for kids: What you need to know
- COVID-19 vaccines
- COVID-19 variant
- COVID-19 vs. flu: Similarities and differences
- COVID-19: Who's at higher risk of serious symptoms?
- Debunking coronavirus myths
- Different COVID-19 vaccines
- Extracorporeal membrane oxygenation (ECMO)
- Fever: First aid
- Fever treatment: Quick guide to treating a fever
- Fight coronavirus (COVID-19) transmission at home
- Honey: An effective cough remedy?
- How do COVID-19 antibody tests differ from diagnostic tests?
- How to measure your respiratory rate
- How to take your pulse
- How to take your temperature
- How well do face masks protect against COVID-19?
- Is hydroxychloroquine a treatment for COVID-19?
- Loss of smell
- Mayo Clinic Minute: You're washing your hands all wrong
- Mayo Clinic Minute: How dirty are common surfaces?
- Multisystem inflammatory syndrome in children (MIS-C)
- Nausea and vomiting
- Pregnancy and COVID-19
- Safe outdoor activities during the COVID-19 pandemic
- Safety tips for attending school during COVID-19
- Sex and COVID-19
- Shortness of breath
- Thermometers: Understand the options
- Treating COVID-19 at home
- Unusual symptoms of coronavirus
- Vaccine guidance from Mayo Clinic
- Watery eyes
Related information
- Post-COVID Recovery & COVID-19 Support Group - Related information Post-COVID Recovery & COVID-19 Support Group
- Rehabilitation after COVID-19 - Related information Rehabilitation after COVID-19
- Post-COVID-19 syndrome could be a long haul (podcast) - Related information Post-COVID-19 syndrome could be a long haul (podcast)
- COVID-19 Coronavirus Long-term effects
Help transform healthcare
Your donation can make a difference in the future of healthcare. Give now to support Mayo Clinic's research.
- Open access
- Published: 13 August 2024
Mechanical force regulates the paracrine functions of ADSCs to assist skin expansion in rats
- Zhixin Xue 1 ,
- Delin Hu 1 na1 ,
- Haojing Tang 1 ,
- Mingheng Xue 1 ,
- Yufan Zhu 1 ,
- Ye Li ORCID: orcid.org/0000-0002-0255-3915 1 &
- Yunjun Liao 1
Stem Cell Research & Therapy volume 15 , Article number: 250 ( 2024 ) Cite this article
Metrics details
In the repair of massive tissue defects using expanded large skin flaps, the incidence of complications increases with the size of the expanded area. Currently, stem cell therapy has limitations to solve this problem. We hypothesized that conditioned medium of adipose-derived stem cells (ADSC-CM) collected following mechanical pretreatment can assist skin expansion.
Rat aortic endothelial cells and fibroblasts were cultured with ADSC-CM collected under 0%, 10%, 12%, and 15% stretching force. Ten-milliliter cylindrical soft tissue expanders were subcutaneously implanted into the backs of 36 Sprague-Dawley rats. The 0% and 10% stretch groups were injected with ADSC-CM collected under 0% and 10% stretching force, respectively, while the control group was not injected. After 3, 7, 14, and 30 days of expansion, expanded skin tissue was harvested for staining and qPCR analyses.
Endothelial cells had the best lumen formation and highest migration rate, and fibroblasts secreted the most collagen upon culture with ADSC-CM collected under 10% stretching force. The skin expansion rate was significantly increased in the 10% stretch group. After 7 days of expansion, the number of blood vessels in the expanded area, expression of the angiogenesis-associated proteins vascular endothelial growth factor, basic fibroblast growth factor, and hepatocyte growth factor, and collagen deposition were significantly increased in the 10% stretch group.
Conclusions
The optimal mechanical force upregulates specific paracrine proteins in ADSCs to increase angiogenesis and collagen secretion, and thereby promote skin regeneration and expansion. This study provides a new auxiliary method to expand large skin flaps.
Massive soft tissue defects caused by excision surgery, trauma, burns, and chronic ulcers seriously increase morbidity and mortality, which remains one of the most challenging problems in plastic surgery [ 1 ]. Unfortunately, traditional skin flap transplantation has drawbacks including additional damage of the donor site and functional and aesthetic deficiencies.
Tissue expansion can eliminate donor site complications related to long-distance tissue transplantation, and expanded skin tissue has a similar quality as skin in the defective area. Therefore, this technique has been widely used for breast reconstruction, genitourinary system reconstruction, and treatment of giant congenital naevi and other diseases [ 2 ]. Nevertheless, as the size of the expanded area increases, the blood supply becomes insufficient and the necrosis rate gradually increases [ 3 ]. In addition, some therapeutic factors, such as postoperative radiotherapy for breast cancer, [ 4 ] greatly increase the incidence of complications. Mesenchymal stem cells (MSCs) are a promising treatment option due to their unique tissue regenerative capacity. Compared with other types of MSCs, adipose-derived stem cells (ADSCs) have received much attention due to their abundance and simple isolation procedure. However, although stem cells are promising for tissue repair, their use is hindered by problems such as immune rejection and tumorigenicity [ 5 ]. In addition, the migration ability, survival rate, and differentiation ability of MSCs decrease after transplantation, which limits their therapeutic potential [ 6 ]. Therefore, the development of novel therapies that are safe and effective for expansion of large skin flaps has been intensely pursued.
An increasing body of evidence demonstrates that paracrine function is central to the effects of MSC-based therapy [ 7 ]. MSCs can release a variety of functional molecules such as growth factors, inflammatory cytokines, chemokines, and extracellular matrix components. Some studies have shown that conditioned medium of ADSCs (ADSC-CM) has the potential to promote tissue regeneration [ 8 , 9 ]. However, the composition of the stem cell secretome is affected by numerous factors, including the tissue source, microenvironment, biological behaviors, and physical and chemical stimulation, which hampers its application in regenerative medicine [ 10 ]. It may be possible to customize the secretome and develop a cell-free therapy by modulating these factors [ 11 ].
Many studies have shown that MSCs can be pretreated with hypoxia, genetic engineering, and physical and chemical stimulation to amplify or inhibit specific biomolecules and thereby achieve the desired therapeutic effect [ 12 , 13 ]. Among the numerous pretreatment methods, mechanical force has the advantages of simple application, quantitative comparability, and no by-products. MSCs are sensitive to mechanical induction [ 14 ]. Mechanical stimulation can induce ADSCs to secrete growth factors, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF), which promote angiogenesis [ 15 , 16 ]. Previous study found that mechanical forces regulate the migration, differentiation, and paracrine functions of ADSCs to varying degrees [ 17 ]. Thus, we hypothesized that application of a specific amount of mechanical force can modulate the paracrine functions of ADSCs to promote skin regeneration. Green nanomaterial is a new field that makes use of sustainable processes and non-toxic ingredients, making it an ideal choice for medical applications [ 18 ]. Many studies have shown that nanomaterials can bind to extracellular vesicles obtained by cell paracrine and show great potential in tumor therapy [ 19 ]. In addition, the development of nano-scaffolds highlights their great potential in plastic and reconstructive surgery. Therefore, we expect that the combination of mechanically treated ADSC-CM and nanomaterials will make a breakthrough in repairing massive soft tissue defects in the future.
We hypothesized that ADSC-CM collected under specific mechanical forces can promote skin regeneration by facilitating angiogenesis and collagen secretion. To investigate this, we applied different degrees of mechanical force to ADSCs and explored the relationship between the magnitude of mechanical force and the paracrine functions of ADSCs. A rat skin expansion model was used in which ADSC-CM was subcutaneously injected into the expanded area to explore the role of ADSC-CM in skin expansion and the underlying mechanism (Fig. 1 ).
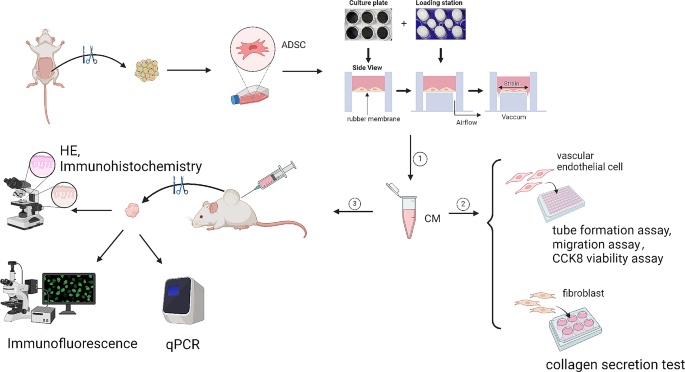
Schematic presentation of the experimental procedure
Materials and methods
This study was approved by the Nanfang Hospital Animal Ethics Committee and was conducted according to the guidelines of the National Health and Medical Research Council of China. All animals were purchased from Nanfang Hospital Animal Center (Guangzhou, China), without specific pathogens, and were excluded from abnormal or dying animals in the acclimatization period before the experiment. All surgical procedures were performed according to the aseptic principle. The work has been reported in line with the ARRIVE guidelines 2.0.
Cell isolation and culture
ADSCs were isolated as previously described [ 20 ]. After shaving, two male Sprague-Dawley (SD) rats (6-weeks-old, n = 2) were sacrificed and subcutaneous fat was harvested. Approximately 4 g of fat was acquired from each rat and stored in a sterile 50 mL centrifuge tube. Subsequently, red blood cells were removed by washing three times with phosphate-buffered saline (PBS, pH 7.4). The isolated fat was cut into small pieces and then digested with 0.2% type Ι collagenase (Solarbio Technology Co., Ltd, Beijing, China) for 60 min at 37 °C with continuous stirring. After digestion, the adipose cell suspension was centrifuged at 800 g for 5 min, and then the cell pellet was resuspended in PBS and filtered through a 100 μm mesh cell strainer. After further centrifugation at 800 g for 5 min, the cell pellet was resuspended in complete growth medium, which comprised Dulbecco’s modified Eagle’s medium: Nutrient Mixture F-12 (DMEM/F12; Gibco, Carlsbad Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin-streptomycin (Gibco). The cell suspension was then placed in 100 mm culture dishes and incubated at 37 °C with 5% CO 2 . The medium was changed every 3 days and cells were passaged when they reached 90% confluency. ADSCs were used at passage 2–5.
Rat aortic endothelial cells and dermal fibroblasts (Procell Life Science & Technology Co., Ltd, Wuhan, China) were cultured in Dulbecco’s modified Eagle medium-low glucose (Gibco) containing 10% FBS and 1% penicillin-streptomycin (Gibco). Cells were maintained at 37 °C in a humidified atmosphere of 5% CO 2 and 95% air.
Cell stretching and conditioned medium collection
ADSCs were seeded in a silicone rubber membrane coated with type I collagen (Col I) at a density of 1 × 10 4 cells/cm 2 in DMEM/F12 supplemented with 10% FBS. After continuous culture for 24 h, the medium was removed and 2 mL of fresh DMEM/F12 was added to starve cells. After cell starvation, all samples were exposed to static stretch for 6 h using a FX-4000T™ Flexcell ® Tension Plus™ unit (Flexcell International Corporation, Burlington, NC, USA), [ 21 ] and the conditioned medium was collected. Before the mechanical stretch experiment, the stretching range, frequency, time, and load model were set using the software, and the equipment automatically calculated the required vertical displacement of the rubber membrane, which ensured that cell length was elongated a certain amount in the circumferential direction. We reviewed various cell stretching experiments and found that when the cell elongation rate was between 10% and 15%, the cells maintained a physiological stretching state and avoided apoptosis. Additionally, this range of elongation induced corresponding changes in paracrine, promoting different cellular functions. Based on these findings, four groups (0% stretch, 10% stretch, 12% stretch, and 15% stretch) were included [ 22 , 23 , 24 , 25 ].
Cell viability assay
The Cell Counting Kit-8 (CCK-8; Shanghai, Biosharp, China) was used to evaluate the effects of ADSC-CM collected under different mechanical forces on endothelial cell proliferation. Serum-free DMEM or ADSC-CM collected under 0%, 10%, 12%, or 15% stretching force was added into the culture medium, and the cells were incubated for 0, 24, 48 h. After incubation, PBS was used to wash cells for 3 times, and CCK-8 solution (10 µL; 1:10 diluted) was added into the fresh culture medium at 37 °C for 2 h. Finally, the optical absorbance for each sample was measured at 450 nm using an ELISA reader (Thermo Fisher, Massachusetts, USA).
Migration assay
The scratch wound assay was performed to evaluate the effect of ADSC-CM collected under different mechanical forces on endothelial cell migration. Rat aortic endothelial cells were seeded in a 6-well plate at a density of 3 × 10 5 cells/well. After the cells had reached 90% confluency, a sterile 200 µL pipette tip was used to scratch them vertically. Each scratch was required to be straight and the same in each well. The cells were then washed twice with PBS, and serum-free DMEM or ADSC-CM collected under 0%, 10%, 12%, or 15% stretching force was added. Images were acquired 0, 12, and 24 h after scratching.
Tube formation assay
To evaluate angiogenesis induced by ADSC-CM, rat aortic endothelial cells were seeded into a 96-well plate coated with Matrigel (BD Biosciences, CA, USA) at a density of 3 × 10 4 cells/well. Matrigel was dissolved overnight at 4°C and placed in each well on ice in advance. ADSC-CM collected under 0%, 10%, 12%, or 15% stretching force or serum-free DMEM was added to each well, and then the cells were cultured for 30 min at 37 °C. The polygonal structures of endothelial cells were observed using an optical microscope 6 h after cell seeding. The tube formation ability was evaluated by calculating the total tube length and total number of nodes.
Collagen secretion assay
To evaluate secretion of collagen induced by ADSC-CM, rat dermal fibroblasts were seeded in a 6-well plate at a density of 5 × 10 5 cells/well. After the cells had reached 90% confluency, the medium was replaced by serum-free DMEM or ADSC-CM collected under 0%, 10%, 12%, or 15% stretching force, and the culture was continued for 24 h. Collagen secretion by fibroblasts was measured by quantitative real-time PCR.
Skin expansion model
Thirty-six SD rats (6-week-old, male, 250–300 g) were used for in vivo experiments. For sample size calculation, we calculated that the sample size of 3 achieves 90.6% power in 1 weeks and the sample size of 3 achieves 95.2% power with a significance level (α) of 0.05 using a two-sided paired t-test in 30 days according to our pre-experimental results. Three samples per time point were selected in 3, 7, 14, and 30 days. Rats were maintained by routine breeding in the Laboratory Animal Center at Southern Medical University and maintained on a standard chow diet ad libitum in a 12-h light/dark cycle. Three rats were housed per cage. All experimental procedures were approved by the Institutional Animal Care and Use Committee, and were in accordance with their recommendations. Rats were anesthetized with 2% isoflurane, and 10 mL silicon expanders (Guangzhou Wanhe Plastic Material, Guangzhou, China) were subcutaneously implanted on the dorsal side. Thereafter, 15 mL of 0.9% saline was injected through the port of the tissue expander to maintain the same expansion tension in all groups. Rats were allocated randomly to the 10% stretch group ( N = 12), 0% stretch group ( N = 12), and control group ( N = 12) by random number table. In the 0% and 10% stretch groups, ADSC-CM collected under 0% and 10% stretching force, respectively, was subcutaneously injected into the expanded area as soon as the expander was implanted. In the control group, the expander was implanted but no injection was performed. In each group, rats were randomly assigned to four time points, namely, 3, 7, 14, and 30 days ( N = 3 per time point). At each time point, full thickness skin specimens were collected from the expanded area to observe the effect of mechanically preconditioned ADSC-CM on skin regeneration. After the samples were collected, the rats were immediately euthanized by inhaling excessive isoflurane and cervical dislocation, and the respiratory rate and heart rate were monitored.
Histological examination
Skin specimens were fixed in 4% paraformaldehyde overnight at low temperature. Subsequently, samples were washed using precooled PBS, dehydrated with different concentrations of ethyl alcohol, embedded in paraffin, and cut into 4 μm thick sections for hematoxylin and eosin (H&E) staining. A microscope (Olympus Corp., Tokyo, Japan) was used to obtain photomicrographs (magnification, ×5). Five H&E-stained regions per group were randomly selected by author in a single-blinded fashion to measure the full thickness of skin using ImageJ software (NIH, Bethesda, MD, USA).
Immunofluorescence and immunohistochemistry
For immunofluorescence assays, after dewaxing, antigen retrieval, dehydration, and blocking, paraffin sections were incubated with an anti-rat CD31 primary antibody (diluted 1:100; Abcam, Cambridge, England) at 4 °C overnight to investigate the level of newly formed vessels. Then, the secondary antibody was applied and sections were incubated for 1 h in the dark. Finally, nuclei were counterstained with DAPI for 15 min. Stained cells were photographed using a fluorescence microscope. To assess angiogenesis, the central and side regions of each sample were compared. To quantitate angiogenesis, the numbers of new capillaries (CD31-positive) in central regions per sample were counted by author in a single-blinded fashion in 100× magnification field.
For immunohistochemistry assays, the paraffin sections were dewaxed, dehydrated, and incubated overnight at 4 °C with anti-Col I (diluted 1:100, Abcam) and anti-type III collagen (Col III; diluted 1:100, Abcam) primary antibodies. After washing, the secondary antibody (diluted 1:100; Thermo Fisher, Massachusetts, USA) was added, and sections were incubated for 1 h at room temperature. Stained cells were developed with diaminobenzidine and counterstained with hematoxylin. To quantitate collagen deposition, the author calculated the average optical density in five fields per sample in a single blind way in 100× magnification field using ImageJ software (NIH, Bethesda, MD, USA).
Quantitative real-time PCR
The total RNA was extracted from the skin samples and fibroblasts with TRIzol ® Reagent (Invitrogen, Carlsbad, CA, USA). cDNA was synthesized using EasyScript ® First-Strand cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China) according to the manufacturer’s instructions. Quantitative real-time PCR was performed using the PRISM ® 7500 Sequence Detection System (ABI, Massachusetts, USA) and FastStart Universal SYBR Green Master Mix (Vazyme Biotech Co., Ltd., Nanjing, China).
Fibroblast samples were tested for type I collagen (Col I), and type III collagen (Col III), and the skin samples were tested for the following genes: VEGF, bFGF, hepatocyte growth factor (HGF) and platelet-derived growth factor (PDGF). Relative expression normalized to expression of β-actin (endogenous loading control) was calculated using the 2 −ΔΔCt method. The primer–probe sequences were as follows: β-actin (150 bp), forward 5′-AGGGAAATCGTGCGTGACAT-3′ and reverse 5′-GAACCGCTCATTGCCGATAG-3′; Col I (200 bp), forward 5′-CCTGACGGTGCTATTTAACA-3′ and reverse 5′-GGAAAATGGTGCTCTGAAAC-3′; Col III (170 bp), forward 5′-CCTGAAGATGTCCTTGATGTAC-3′ and reverse 5′- GCCTTGAATTCTCCCTCATT-3′; VEGF (232 bp), forward 5′-AGATTCTGCAAGAGCACC-3′ and reverse 5′-AAGGTCCTCCTGAGCTAT-3′; HGF (160 bp), forward 5′-AAACAAGGTCTGGACTCACATG-3′ and reverse 5′- CCAAGGAACGAGAGGATTCC-3′; bFGF (170 bp), forward 5′-AAGGATCCCAAGCGGCTCTA-3′ and reverse 5′-TCGCACACACTCCCTTGATG-3′; and PDGF (190 bp), forward 5′-ACTCCATCCGCTCCTTTGA-3′ and reverse 5′-GTCTTGCACTCGGCGATTAC-3′.
Statistical analysis
Statistical analyses were performed using SPSS version 25.0 (IBM, Inc., Armonk, NY, USA) with a one-way analysis of variance. Two groups were compared using the least significant difference method or Mann-Whitney U-test. p < 0.05 was considered statistically significant.
ADSC-CM collected under mechanical force promotes migration and angiogenic tube formation of rat aortic endothelial cells
To study the effect of ADSC-CM on angiogenesis and determine the most suitable mechanical pretreatment, we investigated the effects of ADSC-CM collected under different stretching forces on rat aortic endothelial cells. Cells were cultured in ADSC-CM or serum-free DMEM, and the effects of ADSC-CM on proliferation, migration and angiogenic tube formation of these cells were compared. The scratch wound assay demonstrated that migration of these cells was most promoted in the 10% stretch group (8.05 ± 2.20, n = 3, p < 0.0001; 15.55 ± 0.70, n = 3, p < 0.0001) (Fig. 2 a b) (Additional file 1). The results of the CCK8 experiment showed that the absorbance changes in each stretching group were not significantly different from those in the control group. (0.953 ± 0.112, n = 3 , p > 0.05; 0.968 ± 0.121 , n = 3 , p > 0.05; 0.989 ± 0.147 , n = 3 , p > 0.05; 0.853 ± 0.073 , n = 3 , p > 0.05;0.920 ± 0.074 , n = 3 , p > 0.05 ) (Fig. 2 c) After cells began to form capillaries, calculation of the length of tubes and number of nodes showed that lumen formation at 6 h was best in the 10% stretch group (1554.00 ± 79.6806, n = 3, p < 0.01; 47504.33 ± 1742.71, n = 3, p < 0.0001) (Fig. 2 d, e, f) (Additional file 2).

Effects of ADSC-CM collected under different mechanical forces on rat aortic endothelial cells and fibroblasts. a Migration of rat aortic endothelial cells at 12 and 24 h in the scratch wound assay. Scale bar, 200 μm. b Quantitative analysis of the scratch wound assay. c Cell proliferation ability of rat aortic endothelial cells at 24, 48 h in the CCK8 viability assay. d Tube formation ability of rat aortic endothelial cells at 6 h in the tube formation assay. Scale bar, 200 μm. e f Quantitative analysis of the tube formation assay. g qPCR analysis of Col I and Col III secretion by fibroblasts. Each experiment was independently repeated three times. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001
ADSC-CM collected under mechanical force promotes collagen secretion by rat fibroblasts
To study the effect of ADSC-CM on collagen secretion, we observed the effect of ADSC-CM collected under different stretching forces on rat fibroblasts. Fibroblasts in the 10% stretch group secreted more Col I and Col III (3.19 ± 0.64, n = 3, p < 0.0001; 4.79 ± 0.21, n = 3, p < 0.0001) (Fig. 2 g).
ADSC-CM collected under mechanical force improves skin expansion
Based on the results of the in vitro experiments, ADSC-CM collected under 0% and 10% stretching force was applied to the expanded area in rats. Gross observation of the expanded tissue is shown in Fig. 3 a. In each group, images of the expanded skin at different time points were superimposed and the expansion rate on day 30 was calculated (58.65 ± 1.44, n = 3, p < 0.001) (Fig. 3 b, c) (Additional file 3). The expansion rate was significantly higher in the 10% stretch group than in the other groups, but did not significantly differ between the 0% stretch and control groups.

Macroscopic observation of tissue after expansion in the presence of ADSC-CM collected under mechanical force. a Macroscopic observation of expanded tissue. b Superimposed schematic diagram of expanded skin over 30 days. c Evaluation of the skin expansion rate. *** p < 0.001
ADSC-CM collected under mechanical force improves vascularization of expanded skin
We performed immunofluorescence staining for CD31 to determine the blood vessel density of central regions. The number of blood vessels was significantly higher in the 10% stretch group than in the other groups at 3 and 7 days, but did not significantly differ among the groups at 14 days (69.50 ± 3.27, n = 3, p < 0.0001; 68.17 ± 4.12, n = 3, p < 0.0001; 38.17 ± 4.49, n = 3, p < 0.05; 37.50 ± 4.18, n = 3, p < 0.05) (Fig. 4 ).
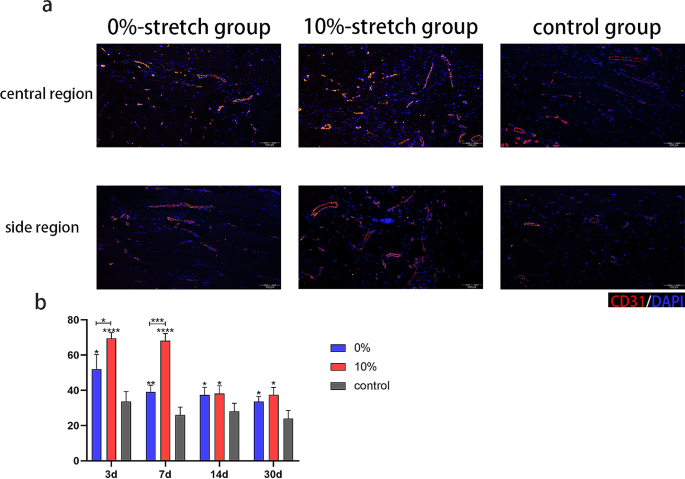
Assessment of vascularization in skin after expansion in the presence of ADSC-CM collected under mechanical force. a Evaluation of angiogenesis by immunofluorescence staining for CD31 after 7 days of expansion in the central and side regions respectively. Scale bar, 100 μm. b Quantitative analysis of the capillary density of central regions. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001
ADSC-CM collected under mechanical force increases expression of angiogenesis-associated proteins
To elucidate the mechanism by which ADSC-CM collected under 10% stretching force improves vascularization of expanded skin, we investigated expression of selected proteins in expanded skin by qPCR. Expression of VEGF, HGF, and bFGF was significantly higher in the 10% stretch group than in the other groups. (8.16 ± 1.40, n = 3, p < 0.0001; 3.28 ± 0.03, n = 3, p < 0.0001; 2.34 ± 0.20, n = 3, p < 0.01; 1.55 ± 0.22, n = 3, p > 0.05) (Fig. 5 ).

Gene expression in skin after expansion in the presence of ADSC-CM collected under mechanical force. Evaluation of expression of the angiogenesis-related genes VEGF, bFGF, HGF, and PDGF by qPCR after 7 days of expansion. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001
ADSC-CM collected under mechanical force increases collagen deposition in expanded skin
To evaluate the amount of collagen in expanded skin, immunohistochemical and histological analyses were performed at each time point. On day 30, the levels of Col I and Col III were increased in the 10% stretch group, but did not significantly differ between the 0% stretch and control groups. (44.54 ± 0.51, n = 3, p < 0.0001; 33.33 ± 2.06, n = 3, p < 0.0001) (Fig. 6 ) Skin thickness did not significantly differ among the three groups. (2.18 ± 0.39, n = 3, p > 0.05) (Additional file 4).

Assessment of collagen deposition in skin after expansion in the presence of ADSC-CM collected under mechanical force. a Immunohistochemistry of Col I after 30 days of expansion. Scale bar, 100 μm. b Immunohistochemistry of Col III after 30 days of expansion. Scale bar, 100 μm. c Quantitative analysis of the levels of Col I and Col III. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001
In this study, we confirmed that mechanical force can regulate the paracrine functions of ADSCs, determined the best mechanical condition to optimize the secretome of ADSCs in order to promote angiogenesis and collagen deposition, and applied ADSC-CM to an expanded area in order to promote skin regeneration (Fig. 7 ). Our results strongly advocate the application of ADSC-CM collected following mechanical pretreatment for skin expansion because it increases angiogenesis and the collagen content in newly formed skin, does not cause excessive thickening of skin flaps, and thus provides better conditions for expansion of ultra-thin large skin flaps to repair massive soft tissue defects.

An illustration of the possible mechanism by which mechanically preconditioned ADSC-CM assists skin expansion
Tissue expansion plays an important role in reconstruction of massive skin defects [ 26 ]. Application of a tissue expander to exert mechanical force regulates the behavior and function of cells, and thereby promotes tissue regeneration [ 27 ]. However, the expansion process takes a long time when simple mechanical stretching is used to stimulate cell proliferation and tissue regeneration. In addition, excessive pressure may cause tissue necrosis due to insufficient blood supply. Therefore, some studies have used MSCs [ 28 ] or acellular agents, such as cell-free fat extract, [ 29 ] to assist tissue regeneration. However, immune rejection of stem cells and quality control of cell-free liquids hinder their clinical application. Several studies have confirmed that mechanical stimulation is a simple and effective intervention that can control the functions of cells according to its magnitude [ 14 , 30 , 31 ]. Previous studies showed that the paracrine functions of ADSCs can be regulated by changing the mechanical environment to promote wound repair [ 32 ]. In the current study, we demonstrated that the paracrine functions of ADSCs can be regulated by adjusting the degree of mechanical force, the secretome can be altered to achieve the desired therapeutic effect, and a higher skin expansion rate can be achieved using ADSC-CM collected under the most suitable mechanical force. To explore the reasons for the increase in the skin expansion rate, we focused on the level of vascularization, which plays an indispensable role in skin regeneration.
Angiogenesis is key to tissue expansion. Tissue necrosis usually occurs when the speed of neovascularization is insufficient for tissue expansion [ 33 ]. Therefore, a strategy is needed to promote angiogenesis for tissue expansion. Conditioned medium of MSCs exposed to a suitable level of mechanical force has better angiogenic activity. Nasser et al. demonstrated that secretion of VEGF depends on the stiffness of the matrix and is maximal when MSCs are seeded on hydrogel matrices with a stiffness of 5.0 kPa [ 34 ]. Chen et al. found that activation of the Wnt/β-catenin signaling pathway in MSCs exposed to laminar shear stress increases secretion of proteins related to angiogenesis [ 35 ]. Previous studies showed that when a full-thickness skin defect is repaired using a hydrogel containing ADSCs and with a stiffness gradient, expression of VEGF in the wound area, vascularization, and wound healing increase [ 32 ]. In the current study, we confirmed that ADSC-CM collected following mechanical pretreatment promoted angiogenesis during tissue expansion, possibly due to increased expression of the angiogenesis-related proteins VEGF, bFGF, and HGF. Tissue regeneration requires a variety of biological processes in addition to angiogenesis; therefore, we also studied changes of extracellular matrix components during expansion.
Collagen is the main component of the extracellular matrix in skin; therefore, its secretion must increase during expansion of large skin flaps. Previous studies revealed that mechanically stimulated ADSCs promote collagen deposition in newly formed skin [ 32 ]. In the current study, we demonstrated that ADSC-CM collected following mechanical pretreatment promoted collagen expression in regenerated skin. According to histological analysis, the increase of collagen expression did not significantly increase the thickness of the expanded skin flap, which also satisfies the clinical need for construction of an ultra-thin skin flap. These effects may be due to increased expression of growth factors such as bFGF, which promotes proliferation of fibroblasts and regulates expression of collagen [ 36 ]. However, the specific proteins involved and their relationship with the optimal degree of mechanical force must be further confirmed experimentally.
The optimal degree of mechanical force to promote the paracrine functions of ADSCs was determined in this study, and the therapeutic effect of ADSC-CM collected under stretching force was preliminarily confirmed by in vivo experiments. Compared to the method of using a tissue expander alone to promote tissue regeneration, incorporating ADSC-CM enhances blood vessel and collagen production during pre-expansion. This reduces the risk of ischemic necrosis and is more advantageous for pre-expanding super-large skin flaps to repair extensive soft tissue defects and refractory wounds. Currently in clinical practice, we mainly rely on the expansive force of expanders to facilitate skin regeneration and achieve large skin flaps. Based on our research findings, while cell-free therapy cannot completely replace current treatments, its auxiliary application can significantly enhance skin expansion efficacy, decrease necrosis rates, and improve overall treatment outcomes. Meanwhile, in contrast with simple stem cell therapy, application of ADSC-CM obtained following mechanical pretreatment avoids safety problems. ADSC-CM does not require immune compatibility to avoid rejection or strictly controlled sterile conditions for its administration, unlike cell-based treatments. Additionally, it minimizes the potential for tumor formation and embolism development associated with stem cell injections [ 37 ]. In addition, the quantifiable nature of mechanical force ensures the uniformity and consistency of product content and treatment outcomes. Finally, the study also inspires us to explore the relationship between the optimal degree of mechanical force and intracapsular pressure in the expander in order to reduce the incidence of complications during skin expansion and guide the clinical application of tissue expanders. In the future, ADSC-CM can be combined with rapidly developing green nanomaterials to achieve new breakthroughs in plastic surgery. For example, the combination of mechanically treated CM with biodegradable nano-scaffolds offers great potential. nano-scaffolds provide a biocompatible framework for supporting cell adhesion, proliferation and differentiation, while ADSC-CM promotes the production of blood vessels and collagen. This combined method is very effective for repairing massive tissue defects and complex wounds, and represents one of the important future directions of ADSC-CM application.
There are also some issues to be resolved in the mechanical pretreatment of ADSCs to promote their paracrine functions and thereby skin expansion. It is unclear which components of ADSC-CM play a key role; therefore, it is necessary to further analyze these components and explore the underlying molecular mechanism. In addition, expression of growth factors differs according to the degree of mechanical force applied, and the effects on other aspects of tissue regeneration must be studied.
In conclusion, application of mechanical force to ADSCs increases angiogenesis and collagen secretion by regulating their paracrine functions and thereby promotes skin regeneration and assists skin expansion. This study provides a new strategy to optimize cell-free therapy in the field of tissue regeneration.
Data availability
The data used to support the findings of this study are included within the article.
Abbreviations
Conditioned Medium of Adipose-Derived Stem Cells
Adipose-Derived Stem Cells
Mesenchymal Stem Cells
Phosphate-Buffered Saline
Dulbecco’s Modified Eagle’s Medium: Nutrient Mixture F-12
Dulbecco’s modified Eagle’s Medium
Fetal Bovine Serum
Carbon dioxide
Normal Control
Hematoxylin and Eosin
4,6-Diamidino-2-Phenylindole
Complementary DNA
Polymerase Chain Reaction
quantitative Real-Time Polymerase Chain Reaction
Sprague-Dawley
Vascular Endothelial Growth Factor
basic Fibroblast Growth Factor
Hepatocyte Growth Factor
Platelet-Derived Growth Factor
Collagen type 1
Collagen type 3
Wingless/Integrated
Tong X, Lu J, Zhang W, Wang S, Huang R, Zhang X, et al. Efficacy and safety of external tissue expansion technique in the treatment of soft tissue defects: a systematic review and meta-analysis of outcomes and complication rates. Burns Trauma. 2022;10:tkac045.
Article Google Scholar
Wang HD, Ibrahim Z, Quan A, Bai J, Ostrander BT, Redett RJ. Pediatric tissue expansion: predictors of premature expander removal in a single surgeon’s experience with 472 expanders. Plast Reconstr Surg. 2020;145(3):755–62.
Article CAS Google Scholar
Handschel J, Schultz S, Depprich RA, Smeets R, Sproll C, Ommerborn MA, et al. Tissue expanders for soft tissue reconstruction in the head and neck area–requirements and limitations. Clin Oral Investig. 2013;17(2):573–8.
Naoum GE, Salama L, Niemierko A, Vieira BL, Belkacemi Y, Colwell AS, et al. Single Stage Direct-to-Implant breast Reconstruction has lower complication Rates Than tissue expander and Implant and comparable rates to Autologous Reconstruction in patients receiving Postmastectomy Radiation. Int J Radiat Oncol Biol Phys. 2020;106(3):514–24.
Lukomska B, Stanaszek L, Zuba-Surma E, Legosz P, Sarzynska S, Drela K. Challenges and controversies in Human mesenchymal stem cell therapy. Stem Cells Int. 2019;2019:9628536.
Turinetto V, Vitale E, Giachino C. Senescence in human mesenchymal stem cells: functional changes and implications in Stem Cell-based therapy. Int J Mol Sci. 2016;17(7).
Han Y, Yang J, Fang J, Zhou Y, Candi E, Wang J, et al. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct Target Ther. 2022;7(1):92.
Irons RF, Cahill KW, Rattigan DA, Marcotte JH, Fromer MW, Chang S, et al. Acceleration of diabetic wound healing with adipose-derived stem cells, endothelial-differentiated stem cells, and topical conditioned medium therapy in a swine model. J Vasc Surg. 2018;68(6S):S115–25.
He Y, Xia J, Chen H, Wang L, Deng C, Lu F. Human adipose liquid extract induces angiogenesis and adipogenesis: a novel cell-free therapeutic agent. Stem Cell Res Ther. 2019;10(1):252.
Daneshmandi L, Shah S, Jafari T, Bhattacharjee M, Momah D, Saveh-Shemshaki N, et al. Emergence of the Stem Cell Secretome in Regenerative Engineering. Trends Biotechnol. 2020;38(12):1373–84.
Kandoi LPK, Misra S, Verma RSVKR. The mesenchymal stem cell secretome: a new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019;46:1–9.
Su Y, Xu C, Cheng W, Zhao Y, Sui L, Zhao Y. Pretreated mesenchymal stem cells and their secretome: enhanced immunotherapeutic strategies. Int J Mol Sci. 2023;24(2).
Gorgun C, Ceresa D, Lesage R, Villa F, Reverberi D, Balbi C, et al. Dissecting the effects of preconditioning with inflammatory cytokines and hypoxia on the angiogenic potential of mesenchymal stromal cell (MSC)-derived soluble proteins and extracellular vesicles (EVs). Biomaterials. 2021;269:120633.
Vining KH, Mooney DJ. Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol. 2017;18(12):728–42.
Yang H, Cheam NMJ, Cao H, Lee MKH, Sze SK, Tan NS, et al. Materials stiffness-dependent redox metabolic reprogramming of mesenchymal stem cells for Secretome-based therapeutic angiogenesis. Adv Healthc Mater. 2019;8(20):e1900929.
Ogle ME, Doron G, Levy MJ, Temenoff JS. Hydrogel Culture Surface Stiffness modulates mesenchymal stromal cell secretome and alters senescence. Tissue Eng Part A. 2020;26(23–24):1259–71.
Li Y, Wu M, Zhang Z, Xia J, Wang Z, Chen X, et al. Application of External Force regulates the Migration and differentiation of adipose-derived Stem/Progenitor cells by altering tissue stiffness. Tissue Eng Part A. 2019;25(23–24):1614–22.
Khalilov R, Bakishzade A, Nasibova A. Future prospects of biomaterials in nanomedicine. Adv Biol Earth Sci. 2024;9:5–10.
Rosic G, Selakovic D, Omarova S. CANCER SIGNALING, CELL/GENE THERAPY, DIAGNOSIS AND ROLE OF NANOBIOMATERIALS. Adv Biol Earth Sci. 2024;9.
Luo Y, Yi X, Liang T, Jiang S, He R, Hu Y, et al. Autograft microskin combined with adipose-derived stem cell enhances wound healing in a full-thickness skin defect mouse model. Stem Cell Res Ther. 2019;10(1):279.
Sha Y, Zhang B, Chen L, Hong H, Chi Q. Mechano Growth factor accelerates ACL repair and improves cell mobility of mechanically injured human ACL fibroblasts by targeting Rac1-PAK1/2 and RhoA-ROCK1 pathways. Int J Mol Sci. 2022;23(8).
Lin LQ, Zeng HK, Luo YL, Chen DF, Ma XQ, Chen HJ, et al. Mechanical stretch promotes apoptosis and impedes ciliogenesis of primary human airway basal stem cells. Respir Res. 2023;24(1):237.
Shan S, Fang B, Zhang Y, Wang C, Zhou J, Niu C, et al. Mechanical stretch promotes tumoricidal M1 polarization via the FAK/NF-κB signaling pathway. FASEB J off Publ Fed Am Soc Exp Biol. 2019;33(12):13254–66.
CAS Google Scholar
Liu J, Li Q, Liu S, Gao J, Qin W, Song Y, et al. Periodontal Ligament Stem cells in the Periodontitis Microenvironment are sensitive to static mechanical strain. Stem Cells Int. 2017;2017:1380851.
Ma H, Wang L, Sun H, Yu Q, Yang T, Wang Y, et al. MIR-107/HMGB1/FGF-2 axis responds to excessive mechanical stretch to promote rapid repair of vascular endothelial cells. Arch Biochem Biophys. 2023;744:109686.
Xiong Z, Chen Y, Xu P, Chen C, Xie Y, Chang Y, et al. Regional-controlled tissue expanders increase skin expansion and thickness compared to standard tissue expanders in a rat model. Plast Reconstr Surg. 2022;150(6):1273–84.
He Y, Li J, Liang Z, Tang H, Shi J, Cai J, et al. Internal expansion preconditioning of recipient site increases Fat Graft Retention by enriching stem Cell Pool and Inducing Browning in rats. Plast Reconstr Surg; 2023.
Zhou SB, Chiang CA, Liu K, Li QF. Intravenous transplantation of bone marrow mesenchymal stem cells could effectively promote vascularization and skin regeneration in mechanically stretched skin. Br J Dermatol. 2015;172(5):1278–85.
Deng M, Wang X, Yu Z, Cai Y, Liu W, Zhou G, et al. Cell-free fat extract promotes tissue regeneration in a tissue expansion model. Stem Cell Res Ther. 2020;11(1):50.
Liang X, Huang X, Zhou Y, Jin R, Li Q. Mechanical stretching promotes skin tissue regeneration via enhancing mesenchymal stem cell homing and transdifferentiation. Stem Cells Transl Med. 2016;5(7):960–9.
Driskill JH, Pan D. Control of stem cell renewal and fate by YAP and TAZ. Nat Rev Mol Cell Biol. 2023;24(12):895–911.
Ma Y, Wang Y, Chen D, Su T, Chang Q, Huang W, et al. 3D bioprinting of a gradient stiffened gelatin-alginate hydrogel with adipose-derived stem cells for full-thickness skin regeneration. J Mater Chem B. 2023;11(13):2989–3000.
Wang Q, Zhou L, Wang T, Guo X, Yu H, Wang J. Assisting Rapid Soft-tissue expansion with adipose-derived stem cells: an experimental study in a Pig Model. Plast Reconstr Surg. 2018;142(5):e674–84.
Xue Z, Liao Y, Li Y. Effects of microenvironment and biological behavior on the paracrine function of stem cells. Genes Dis. 2024;11(1):135–47.
Chen W-T, Hsu W-T, Yen M-H, Changou CA, Han C-L, Chen Y-J et al. Alteration of mesenchymal stem cells polarity by laminar shear stimulation promoting β-catenin nuclear localization. Biomaterials. 2019;190–1.
Yang Y, Xia T, Zhi W, Wei L, Weng J, Zhang C, et al. Promotion of skin regeneration in diabetic rats by electrospun core-sheath fibers loaded with basic fibroblast growth factor. Biomaterials. 2011;32(18):4243–54.
Fuentes P, Torres MJ, Arancibia R, Aulestia F, Vergara M, Carrión F, et al. Dynamic culture of mesenchymal Stromal/Stem cell spheroids and secretion of paracrine factors. Front Bioeng Biotechnol. 2022;10:916229.
Download references
Acknowledgements
Not applicable.
This study was supported by College Students’ Innovative Entrepreneurial Training Plan Program (202312121015, S202312121094, 202312121227, 202312121313, 202312121314, 202312121317, 202312121321), National Nature Science Foundation of China (82202474, 82360615), Clinical Program of Nanfang Hospital, Southern Medical University (2022CR007), First People’s Hospital of Yunnan Province (KHYJ-2023-5-02, 2023-KHRCBZ-B14), Guangdong Basic and Applied Basic Research Foundation (2021A1515110440) and Science and Technology Projects in Guangzhou (2024A04J5192, 2023A04J2350, 2023A04J2349, 2023A04J2347, 2023A04J2271).
Author information
Ye Li and Yunjun Liao contributed equally to this work.
Authors and Affiliations
Department of Plastic and Cosmetic Surgery, Nanfang Hospital, Southern Medical University, 1838 Guangzhou North Road, Guangzhou, 510515, Guangdong, P. R. China
Zhixin Xue, Delin Hu, Haojing Tang, Mingheng Xue, Yufan Zhu, Ye Li & Yunjun Liao
You can also search for this author in PubMed Google Scholar
Contributions
ZX carried out the experiments, data analyses and manuscript writing. DH, YZ, participated in the in vitro experiments. MX, HT, participated in the in vivo experiments. YL and YL designed the study and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Ye Li or Yunjun Liao .
Ethics declarations
Ethics approval and consent to participate.
The project entitled “Mechanical force regulates the paracrine functions of ADSCs to assist skin expansion in rats” was approved by the he Nanfang Hospital Animal Ethics Committee (Approval Number: NFYY20230425034; Date of approval: 2023-07-19).
Consent for publication
All authors agreed to the publication of this paper.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, supplementary material 3, supplementary material 4, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Xue, Z., Hu, D., Tang, H. et al. Mechanical force regulates the paracrine functions of ADSCs to assist skin expansion in rats. Stem Cell Res Ther 15 , 250 (2024). https://doi.org/10.1186/s13287-024-03822-0
Download citation
Received : 11 April 2024
Accepted : 30 June 2024
Published : 13 August 2024
DOI : https://doi.org/10.1186/s13287-024-03822-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Mechanical force
- ADSC paracrine
- Skin expansion
- Angiogenesis
- Collagen secretion
Stem Cell Research & Therapy
ISSN: 1757-6512
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

IMAGES
COMMENTS
Timothy O'Brien, Editor-in-Chief, Stem Cell Research & Therapy "The study of stem cells is one of the most exciting areas of contemporary biomedical research. ... Citation Impact 2023 Journal Impact Factor: 7.1 5-year Journal Impact Factor: 7.9 Source Normalized Impact per Paper (SNIP): 1.277 SCImago Journal Rank (SJR): 1.798.
In recent years, stem cell therapy has become a very promising and advanced scientific research topic. The development of treatment methods has evoked great expectations. This paper is a review focused on the discovery of different stem cells and the potential therapies based on these cells. The genesis of stem cells is followed by laboratory steps of controlled stem cell culturing and derivation.
These stem cells play a crucial role in tissue regeneration and repair, r... Reyhaneh Toghiani, Vajihe Azimian Zavareh, Hanyieh Najafi, Mina Mirian, Negar Azarpira, Samira Sadat Abolmaali, Jaleh Varshosaz and Ali Mohammad Tamaddon. Stem Cell Research & Therapy 2024 15 :240. Research Published on: 30 July 2024.
The Impact IF 2022 of Stem Cell Research and Therapy is 7.16, which is computed in 2023 as per its definition. Stem Cell Research and Therapy IF is decreased by a factor of 0.26 and approximate percentage change is -3.5% when compared to preceding year 2021, which shows a falling trend. The impact IF, also denoted as Journal impact score (JIS), of an academic journal is a measure of the yearly ...
Scope. Stem Cell Research & Therapy is the major forum for translational research into stem cell therapies. An international peer-reviewed journal, it publishes high-quality open access research articles with a special emphasis on basic, translational and clinical research into stem cell therapeutics and regenerative therapies, including animal ...
Stem Cell Research & Therapy Impact Factor, IF, number of article, detailed information and journal factor. ISSN: 1757-6512. ... Journal Impact. Enter journal title, issn or abbr in this box to search. Stem Cell Research & Therapy. Journal Abbreviation: STEM CELL RES THER Journal ISSN: 1757-6512. Year: Impact Factor (IF) Total Articles: Total ...
In vivo stem cells can be broadly divided into three types based on their origin: embryonic (ESCs), fetal (FSCs), and adult stem cells (ASCs, among them mesenchymal stem cells, or MSCs). Embryonic stem cells (ESCs) are derived from the inner cell mass on the pre-implantation embryo, known as a blastocyst, post-fertilization. These pluripotent ...
The latest impact score (IS) of the Stem Cell Research and Therapy is 7.16.It is computed in the year 2023 as per its definition and based on Scopus data. 7.16 It is decreased by a factor of around 0.26, and the percentage change is -3.5% compared to the preceding year 2021, indicating a falling trend.The impact score (IS), also denoted as the Journal impact score (JIS), of an academic journal ...
Introduction. Clinical and preclinical studies have identified stem cell therapy (SCT) as an emerging and promising treatment method for several diseases (), including but not limited to age-related macular degeneration, corneal injury, diabetes mellitus, epidermolysis bullosa, knee osteoarthritis, myocardial infarction, and fire burn (1-5).However, in the era of evidence-based medicine, the ...
About. Stem Cell Research & Therapy is the major forum for translational research into stem cell therapies. An international peer-reviewed journal, it publishes high-quality open access research articles with a special emphasis on basic, translational and clinical research into stem cell therapeutics and regenerative therapies, including animal models and clinical trials.
Top authors and change over time. The top authors publishing in Stem Cell Research & Therapy (based on the number of publications) are: Rocky S. Tuan (22 papers) published 1 paper at the last edition the same number as at the previous edition,; Timothy O'Brien (16 papers) absent at the last edition,; Patricia R. M. Rocco (16 papers) published 1 paper at the last edition, 1 less than at the ...
Studies on Stem Cells Research and Therapy (ISSN: 2641-3000) ... (Impact Factor) ... Stem cells can be considered as the foundation cells for every organ and tissue in our bodies due to its two key abilities- a. the ability to self-renew, dividing in a manner that makes copies of themselves. b. Ability to differentiate, giving rise to the ...
Stem Cell Research and Therapy had the most articles, totaling 33. Stem Cell Reviews and Reports ranked second, with 23 papers. The third was Frontiers in Immunology, with 25 papers. The following journals were International Journal of Molecular Sciences and Cells, with 20 and 18 papers, respectively. These journals are mainly focused on cell ...
Stem cell-based therapies. Stem cell-based therapies are defined as any treatment for a disease or a medical condition that fundamentally involves the use of any type of viable human stem cells including embryonic stem cells (ESCs), iPSCs and adult stem cells for autologous and allogeneic therapies ().Stem cells offer the perfect solution when there is a need for tissue and organ ...
Editor Sheila Chari. Stem Cells Impact Factor ~5.6, rigorous; fair review process. Stem Cells Translational Medicine Impact Factor~5.9, IF on the rise, good experiences here too. npj Regenerative Medicine Impact Factor ~ 7.0. As a relatively new journal it has a great impact factor. Stem Cells and Development Impact Factor ~3, good experiences.
Stem Cell Research & Therapy is the major forum for translational research into stem cell therapies. An international peer-reviewed journal, it publishes high-quality open access research articles with a special emphasis on basic, translational and clinical research into stem cell therapeutics and regenerative therapies, including animal models and clinical trials.
Topic (s) Covered. Current Stem Cell Research & Therapy publishes high quality frontier reviews, original research articles, drug clinical trial studies and guest edited issues on all aspects of basic research on stem cells and their uses in clinical therapy. The journal is essential reading for all researchers and clinicians involved in stem ...
The Role of Various Factors in Neural Differentiation of Human Umbilical Cord Mesenchymal Stem Cells with a Special Focus on the Physical Stimulants, Current Stem Cell Research & Therapy, 19, 2 ...
Scope. Current Stem Cell Research & Therapy publishes high quality frontier reviews, original research articles, drug clinical trial studies and guest edited issues on all aspects of basic research on stem cells and their uses in clinical therapy. The journal is essential reading for all researchers and clinicians involved in stem cells research.
Current Stem Cell Research & Therapy. Impact Factor: 2.1. Indexed in: Scopus, SCI Expanded, MEDLINE/PubMed... View all. Volume 19 , Issues 11, 2024. Request Failed! Current Stem Cell Research & Therapy publishes reviews, research articles, drug clinical trial on all aspects of basic research on stem cells and their uses in clinical therapy.
Stem cells therapy challenges. The current challenges of stem cell therapy for stroke and TBI are multifactorial and significant. First, the best source of MSCs for stroke treatment has yet to be established (48, 49). Most preclinical studies used MSCs from healthy, young donors and about half of the clinical studies used autologous MSC ...
present a title that includes, if appropriate, the study design e.g.: "A versus B in the treatment of C: a randomized controlled trial", "X is a risk factor for Y: a case control study", "What is the impact of factor X on subject Y: A systematic review" or for non-clinical or non-research studies: a description of what the article reports
4. Research on Stem Cell Therapies: The Long-Term Social Impact. Those familiar with stem cell-based therapies may understand the long and difficult road that precedes a promising discovery at the laboratory level, at which point researchers will seek to translate it to clinical practice in the form of an Advanced Therapeutic Medicinal Product (ATMP).
It also discusses the issues of medical tourism, patient funded studies, false marketing claims, and the ethical and religious aspects of stem cell research, anti aging research, and immortality research including the Roman Catholic Churches view on embryonic stem cells. Ethics of Modern Stem Cell Research and Therapy will be a valuable ...
A discovery by a three-member Albert Einstein College of Medicine research team may boost the effectiveness of stem-cell transplants, commonly used for patients with cancer, blood disorders, or ...
The study originated in the laboratory of the late Paul S. Frenette, M.D., a pioneer in hematopoietic stem cell research and founding director of the Ruth L. and David S. Gottesman Institute for ...
Abstract. The properties of cancer stem cells (CSCs) have recently gained attention as an avenue of intervention for cancer therapy. In this review, we highlight some of the key roles of CSCs in altering the cellular microenvironment in favor of cancer progression. We also report on various studies in this field which focus on transformative ...
SRY-box transcription factor 2 (Sox2) is a transcription factor that is essential for the self-renewal and pluripotency of stem cells. It has been demonstrated that Sox2 is capable of promoting ...
The experience of having severe COVID-19 might be another factor. People with severe symptoms of COVID-19 often need to be treated in a hospital intensive care unit. This can result in extreme weakness and post-traumatic stress disorder, a mental health condition triggered by a terrifying event.
Background In the repair of massive tissue defects using expanded large skin flaps, the incidence of complications increases with the size of the expanded area. Currently, stem cell therapy has limitations to solve this problem. We hypothesized that conditioned medium of adipose-derived stem cells (ADSC-CM) collected following mechanical pretreatment can assist skin expansion. Methods Rat ...