- Mattress & Sleep
- Kids & Baby Gear
- Beauty & Grooming
- Tech & Electronics

The Best Natural Sleep Aids To Help You Fall (And Stay) Asleep
- Share to Facebook
- Share to Twitter
- Share to Linkedin
Creating the right environment is key to getting a good night’s sleep. It’s about more than just sticking to good habits—you also have to structure your surroundings to support them. “The best method for insomnia is not any prescription or any supplement,” says Dr. Matt Davis, a neurology and sleep medicine physician. “It is methodical, structured behavioral change to recondition your mind to sleep.” The best natural sleep aids help reinforce those behavioral changes by creating a more restful environment that signals to your body it’s time for sleep.
The best natural sleep aids help your body peacefully wind down.
Whether it’s a light therapy device , a sleep mask or aromatherapy , even small adjustments—like the scent of lavender or the gentle weight of a blanket—can help your mind and body naturally wind down. We’ve tried many of the products we recommend below, while others come highly rated by customers. Each product can be part of an effective bedtime routine and contribute to a more sleep-friendly environment, helping you fall asleep faster and stay asleep longer. Read on to learn about the best natural sleep aids that can reduce nighttime stress and improve overall sleep quality.
Best Natural Sleep Aids: Accessories
A relaxation device with soothing massage and heat, therabody smartgoggles.
I'm a writer and editor specializing in lifestyle, home, food, health, pets, and entertainment. I have a degree in Magazine Journalism from Syracuse University and my work has appeared in Country Living, The Pioneer Woman, Good Housekeeping, Cosmopolitan, House Beautiful, and more. When I'm not working, you'll probably find me hanging out with my dog.
For product reviews, gift ideas, and latest deals, Subscribe to the Forbes Finds newsletter .
Therabody’s SmartGoggles blend gentle eye and temple massage with heat and vibration to help you wind down before bed. The built-in biometric sensor adjusts treatments based on your heart rate, aiming to ease stress and promote relaxation. While they’re not designed to be worn through the night like a traditional sleep mask, the goggles can be a valuable part of your bedtime routine—especially if you struggle with headaches, screen-related eye strain or stress.
With three adjustable modes—SmartRelax, Focus and Sleep—you can find the setting that works best for you. The goggles feature weighted straps and a foldable build that makes them comfortable and easy to take on the go. Plus, if you like pairing sound with relaxation, you can connect them to the Therabody app to access soothing audio tracks and guided meditations. No matter how you use them, these goggles can help prepare your mind and body for a more restful night’s sleep.
A Cooling Blanket With Calming Pressure
Gravity cooling weighted blanket.
This weighted blanket is designed to provide gentle pressure that helps soothe your body and mind for better sleep. Acting like a full-body hug, the evenly distributed weight can help calm your nervous system and may help reduce stress, making it easier to fully unwind. It’s available in various weights, from 15 pounds up to 35 pounds, so it allows you to choose what’s most comfortable for you. This is key according to experts like Davis, who emphasizes the importance of personal preference with natural sleep accessories.
The blanket’s gridded stitching keeps the fine-grade glass beads evenly spread out, preventing clumping and providing consistent pressure through the night. The included duvet cover is also machine washable, making it easy to maintain, and the blanket comes in several sizes to fit different beds. Whether you’re looking for extra comfort at bedtime or a new way to relax, this is an easy product to add to your sleep toolkit.
A Gently Weighted Mask For Any Sleep Style
Nodpod sleep mask.
Think of this eye mask as a weighted blanket for your face—it uses microbead-filled pods that contour to your eyes and distribute gentle pressure, which is intended to create a calming effect. This soothing pressure can make it easier to naturally fall asleep and stay asleep, especially if you’re sensitive to light.
The mask’s strap-free design rests comfortably over your eyes and stays in place regardless of your sleep position. You can also chill the mask in the freezer to relieve headaches or tired eyes. It has a soft jersey cotton side and a plush microfleece side, so you have different (though equally comfy) options to choose from.
Best Natural Sleep Aids: Devices And Light Therapy
A sunrise alarm that makes mornings easier, hatch restore 2.
The Hatch Restore 2 is more than just an alarm clock—it’s a full sleep system that helps you establish a better routine from start to finish. You can customize your wind-down with relaxing sounds, guided meditations and soft lighting that signals to your body when it’s time to sleep. Personally, I love using my Hatch to unwind while sticking to a consistent sleep schedule. My nightly routine is set to deliver 30 minutes of red light while I read, followed by pink noise to help me drift off. This automatic setup lets me enjoy my book without disrupting melatonin production or staying up later than planned.
The Hatch Restore 2 is especially useful if you sleep in a very dark room . When morning comes, the simulated sunrise wakes you up gradually so you feel more refreshed. “Sunrise alarm clocks can absolutely help regulate and entrain a proper sleep-wake rhythm and thereby can help sleep quality at night,” says Davis. If you struggle to fall asleep or just want a calmer start to your day, this device can make both sleeping and waking up feel less stressful.
Wearable Light Therapy To Improve Sleep
Ayo premium blue light therapy glasses.
Struggling with grogginess and irregular sleep patterns? These circadian glasses offer a practical way to reset your body’s internal clock by simulating natural daylight. Davis explains that light is the most significant factor in regulating your brain’s sleep-wake cycle, and these glasses deliver targeted blue-enriched light to reduce melatonin and naturally boost energy. By using these glasses in the morning, you can fight daytime fatigue and improve your sleep at night. And because they’re wearable, they’re easy to use while you're doing other things.
Best Natural Sleep Aids: Aromatherapy And Relaxation Products
Lightly scented to promote relaxation, this works deep sleep pillow spray.
A soothing scent can make all the difference when it’s time to wind down. This Works Deep Sleep Pillow Spray blends lavender, chamomile and vetivert to create a calming environment for sleep—and yes, it works. While Davis notes that the benefits might partly be due to a placebo effect, he emphasizes that there’s “little to no risk or harm” in using natural sprays like this. If it helps you relax, it’s worth making it part of your nightly routine.
For me, this spray has become a nightly ritual. I’m sensitive to strong scents, but this one is subtle and instantly signals to my brain that it’s time for bed. It’s a simple habit that now helps me naturally ease into sleep.
A Soothing Nighttime Tea With Sleep-Friendly Ingredients
Yogi tea comforting chamomile tea.
There’s something so comforting about brewing a cup of tea as the day winds down. Chamomile, in particular, has long been cherished for its calming properties, making it a go-to for anyone looking to relax before bed. While experts like Davis remind us that the benefits might be mostly anecdotal, there’s little harm in sipping on this caffeine-free tea as part of your nightly ritual if it helps you relax.
Personally, I like to add a few drops of valerian root to my tea while winding down. I use MaryRuth Organics Valerian Root Liquid Drops , which are easy to mix into any drink. These natural supplements aren’t miracle cures, but they can be simple, soothing additions to a consistent bedtime routine.
Best Natural Sleep Aids: Apps and Digital Tools
A meditation app with sleep-focused content.
Calm is well known for its extensive library of meditations, but it’s the sleep-focused content that sets it apart as a natural sleep aid. With a premium subscription, you can access guided meditations, peaceful soundscapes and sleep stories narrated by familiar voices like Matthew McConaughey. Experts suggest that meditation apps like Calm can be effective if they help you unwind and quiet your mind—key factors in falling asleep. While it’s not a guaranteed fix for everyone, Calm’s range of options makes it easier to find what works for you.
The Best Affordable Mattresses That Don’t Skimp On Comfort Or Support
The best mattress under $2,000 is comfy, supportive and feature-rich, why trust forbes vetted.
The Forbes Vetted mattress and sleep team has built a comprehensive library of content to help our readers optimize their sleep health. This includes stories like the best sleep tech devices , the best products for hot sleepers and many more.
- Author of this piece and Forbes Vetted contributor Kelly O’Sullivan is a freelance writer and product reviewer experienced in covering health, wellness and sleep topics. She combines expert insights with her personal experience in creating a consistent sleep routine.
- This story was overseen and edited by mattress and sleep editors Bridget Chapman and McKenzie Dillon . Both are certified sleep science coaches with years of experience testing sleep products.
- We consulted with two medical experts: Dr. Matt Davis , a neurology and sleep medicine physician specializing in adult and pediatric sleep disorders; and Jamie Zeitzer , PhD, a professor of psychiatry and behavioral sciences (sleep medicine) at Stanford University and co-director of the Center for Sleep and Circadian Sciences. Their insights helped guide our selections and provided expert-backed advice on natural sleep aids.
- This story is updated regularly to reflect the latest sleep research and ensure our recommendations remain current and valuable.
How We Chose The Best Natural Sleep Aids
The best natural sleep aids is a broad category, so there weren’t strict criteria for each product. Our focus was on finding options that help create a relaxing environment and support healthy bedtime routines. Across the Forbes Vetted team, we’ve tested and reviewed several of these products ourselves, so we can personally vouch for their effectiveness.
- We prioritized trusted brands known for creating high-quality and effective products, like Hatch and Therabody.
- We used insights from Davis and Zeitzer to better understand how natural sleep aids work as part of a healthy bedtime routine.
- We included a range of options, from sleep devices to aromatherapy products, to suit different preferences and needs.
- We focused on products with positive customer feedback, sticking to natural sleep aids with an average rating of 4 stars or above.
Frequently Asked Questions (FAQs)
What is the most effective all-natural sleep aid.
The most effective natural sleep aid isn’t a supplement or remedy, but structured behavioral change . According to Davis, the key is reconditioning your mind to associate your bed only with sleep. That said, products like light therapy devices and sleep masks can be helpful additions to a routine by creating a sleep-friendly environment. Lavender and chamomile, while potentially more placebo than proven, can also help some people relax without causing harm.
What Is The Best Vitamin For Insomnia?
There isn’t strong evidence supporting specific vitamins as reliable solutions for insomnia. Many sleep supplements combine vitamins or herbs like valerian root, but the efficacy varies due to inconsistent dosing and limited research. Davis notes that while these supplements are generally safe, they might not address the root cause of insomnia. Prioritizing behavioral changes and establishing a consistent sleep routine is more effective in the long term.
What Is The Best Sleep Aid For Insomnia And Anxiety?
For managing insomnia and anxiety, cognitive behavioral therapy for insomnia (CBT-I) is considered the most effective approach. Davis explains that reconditioning your sleep habits is critical. Meditation apps, guided sleep exercises or weighted blankets can also support relaxation and reduce sleep-related anxiety. While these tools aren’t cures, they can be useful if they help calm your mind and prepare you for sleep.

- Editorial Standards
- Reprints & Permissions
September National Health Observances: Healthy Aging, Sickle Cell Disease, and More
Each month, we feature select National Health Observances (NHOs) that align with our priorities for improving health across the nation. In September, we’re raising awareness about healthy aging, sickle cell disease, substance use recovery, and HIV/AIDS.
Below, you’ll find resources to help you spread the word about these NHOs with your audiences.
- Healthy Aging Month Each September, we celebrate Healthy Aging Month to promote ways people can stay healthy as they age. Explore our healthy aging resources , bookmark the Healthy People 2030 and Older Adults page , share our Move Your Way® materials for older adults , and check out the Physical Activity Guidelines for Americans Midcourse Report . You can also share resources related to healthy aging from the National Institute on Aging — and register for the 2024 National Healthy Aging Symposium to hear from experts on innovations to improve the health and well-being of older adults.
- National Recovery Month The Substance Abuse and Mental Health Services Administration (SAMHSA) sponsors National Recovery Month to raise awareness about mental health and addiction recovery. Share our MyHealthfinder resources on substance use and misuse — and be sure to check out Healthy People 2030’s evidence-based resources related to drug and alcohol use .
- National Sickle Cell Awareness Month National Sickle Cell Awareness Month is a time to raise awareness and support people living with sickle cell disease. Help your community learn about sickle cell disease by sharing these resources from the National Heart, Lung, and Blood Institute (NHLBI) . You can also encourage new and expecting parents to learn about screening their newborn baby for sickle cell . And be sure to view our Healthy People 2030 objectives on improving health for people who have blood disorders .
- National HIV/AIDS and Aging Awareness Day (September 18) On September 18, we celebrate HIV/AIDS and Aging Awareness Day to encourage older adults to get tested for HIV. Share CDC’s Let’s Stop HIV Together campaign to help promote HIV testing, prevention, and treatment. MyHealthfinder also has information for consumers about getting tested for HIV and actionable questions for the doctor about HIV testing . Finally, share these evidence-based resources on sexually transmitted infections from Healthy People 2030.
- National Gay Men’s HIV/AIDS Awareness Day (September 27) National Gay Men’s HIV/AIDS Awareness Day on September 27 highlights the impact of HIV on gay and bisexual men and promotes strategies to encourage testing. Get involved by sharing CDC’s social media toolkit and HIV information to encourage men to get tested — and share our MyHealthfinder resources to help people get tested for HIV and talk with their doctor about testing .
We hope you’ll join us in promoting these important NHOs with your networks to help improve health across the nation!
The Office of Disease Prevention and Health Promotion (ODPHP) cannot attest to the accuracy of a non-federal website.
Linking to a non-federal website does not constitute an endorsement by ODPHP or any of its employees of the sponsors or the information and products presented on the website.
You will be subject to the destination website's privacy policy when you follow the link.
$500K BHE GT&S Foundation gift aids laboratory upgrades at WVU Statler College
Monday, August 26, 2024
A $500,000 gift to WVU from the BHE GT&S Foundation supports improvements to the reservoir engineering and core analysis lab in the Mineral Resources Building — shown on the far right — located on the Evansdale area of campus. (WVU Photo)
The West Virginia University Benjamin M. Statler College of Engineering and Mineral Resources is enhancing education and research with a $500,000 gift to support laboratory upgrades.
The gift from the BHE GT&S Foundation supports improvements to the reservoir engineering and core analysis lab, located in the Mineral Resources Building on the Evansdale Campus. Statler College students and faculty use the lab’s resources to measure rock properties for coursework and research projects focused on characterizing and modeling oil and natural gas reservoirs to improve recovery operations.
The BHE GT&S Foundation supports programs focused on health, education, community and the environment. The renovated facility will be known as the BHE GT&S Foundation Core/Reservoir Lab in recognition of the Foundation’s generosity.
Samuel Ameri , professor and chair of the petroleum and natural gas engineering program , said state-of-the-art teaching and research laboratories enable the petroleum and natural gas engineering program to recruit prospective students, provide experiential learning for students, foster research opportunities for students and faculty, and welcome industry leaders to host guest lectures and demonstrations.
“The petroleum and natural gas engineering program, already one of the highest caliber programs of its kind in the country, is now even better thanks to the generosity of the BHE GT&S Foundation,” Ameri said.
“Support like this helps our department recruit high-quality students from across the country and around the world, and it enables our faculty and staff to continue educating and graduating petroleum and natural gas engineers ready for the future. We value our relationship with the BHE GT&S Foundation, and we are truly grateful for the Foundation’s belief in our institution and support of our efforts to impact our industry in positive ways.”
Paul Ruppert earned his bachelor’s degree in petroleum and natural gas engineering from WVU in 1986, which launched his career in the natural gas industry. He is currently president of BHE GT&S, LLC, an interstate natural gas transmission and storage company headquartered in Richmond, Virginia, with operations in 10 states.
Ruppert said the lab has an important role in teaching students to understand and evaluate formation properties.
“I am proud to be a WVU alumnus and am pleased that the BHE GT&S Foundation can contribute to the University’s future success,” Ruppert said. “Everyone appreciates the important work that WVU does to educate future leaders and technical talent.”
The BHE GT&S Foundation gift was made through the WVU Foundation , the nonprofit organization that receives and administers private donations on behalf of the University.
MEDIA CONTACT: Cassie Rice Senior Communications Specialist WVU Foundation 304-554-0217; [email protected]
Call 1-855-WVU-NEWS for the latest West Virginia University news and information from WVUToday .
- UNC Chapel Hill

Acknowledge the CFAR
UNC Participation at IAS AIDS 2024 Conference in Munich
August 29, 2024
By Jordan Cobb

During the IAS AIDS conference held in Munich in July, UNC had over 15 faculty members and trainees attend to showcase the important work that is being done on HIV/AIDS within our university.
Here are some of the highlights from our UNC team in Munich:
Recent research has provided new insights into managing HIV-related comorbidities. Dr. Prema Menezes’ team discovered high rates of multimorbidity among older adults with HIV, noting that the types of comorbidities vary by gender. The TASKPEN study team, led by Dr. Jake Pry, Dr. Michael Herce, and Dr. Wilbroad Mutale, investigated how task-shifting and integration can enhance treatment for HIV, hypertension, and diabetes in Zambia.
Research also focused on the implementation and ethics of HIV interventions. Dr. Ramya Kumar, under the mentorship of Dr. Michael Herce and Dr. Maurice Musheke, examined female sex workers’ views on long-acting injectable PrEP in Zambia. Hadas Baron, from the PREPARE team led by Dr. Suzanne Day, presented research on adolescent perspectives from Botswana and Malawi regarding participation in clinical HIV studies during pregnancy, aiming to guide ethical research practices.
Social innovations in HIV research were highlighted by the SESH collaboration between UNC Project-China and Southern Medical University. Zhuoheng Yin showcased a project on PrEP distribution using a hybrid clinic-community model. Liyuan Zhang and Dorian Ho presented findings on improving integrated HIV/STI testing and service delivery through co-creation and mutual aid approaches. The SESH team also hosted a designathon in the Global Village, featuring a WHO/TDR guide on participatory health research.
We are looking forward to appreciating all the hard work our faculty and staff here at UNC will contribute to again next year! We are proud of the strong UNC contributions to the IAS 2024 conference.
To read more or see the full post by IGHID, click here .
Filed Under:
More from UNC Center for AIDS Research
- 2024-2025 NOSI: Secondary Data Analysis Award RFP
- 2024-2025 Traditional Developmental Award RFP
- Key Takeaways from IAS 2024
- Environment
- Science & Technology
- Business & Industry
- Health & Public Welfare
- Topics (CFR Indexing Terms)
- Public Inspection
- Presidential Documents
- Document Search
- Advanced Document Search
- Public Inspection Search
- Reader Aids Home
- Office of the Federal Register Announcements
- Using FederalRegister.Gov
- Understanding the Federal Register
- Recent Site Updates
- Federal Register & CFR Statistics
- Videos & Tutorials
- Developer Resources
- Government Policy and OFR Procedures
- Congressional Review
- My Clipboard
- My Comments
- My Subscriptions
- Sign In / Sign Up
- Site Feedback
- Search the Federal Register
This site displays a prototype of a “Web 2.0” version of the daily Federal Register. It is not an official legal edition of the Federal Register, and does not replace the official print version or the official electronic version on GPO’s govinfo.gov.
The documents posted on this site are XML renditions of published Federal Register documents. Each document posted on the site includes a link to the corresponding official PDF file on govinfo.gov. This prototype edition of the daily Federal Register on FederalRegister.gov will remain an unofficial informational resource until the Administrative Committee of the Federal Register (ACFR) issues a regulation granting it official legal status. For complete information about, and access to, our official publications and services, go to About the Federal Register on NARA's archives.gov.
The OFR/GPO partnership is committed to presenting accurate and reliable regulatory information on FederalRegister.gov with the objective of establishing the XML-based Federal Register as an ACFR-sanctioned publication in the future. While every effort has been made to ensure that the material on FederalRegister.gov is accurately displayed, consistent with the official SGML-based PDF version on govinfo.gov, those relying on it for legal research should verify their results against an official edition of the Federal Register. Until the ACFR grants it official status, the XML rendition of the daily Federal Register on FederalRegister.gov does not provide legal notice to the public or judicial notice to the courts.
Design Updates: As part of our ongoing effort to make FederalRegister.gov more accessible and easier to use we've enlarged the space available to the document content and moved all document related data into the utility bar on the left of the document. Read more in our feature announcement .
Agency Information Collection Activities; Submission to the Office of Management and Budget for Review and Approval; Request for Comment; Crash Injury Research and Engineering Network Data Collection
A Notice by the National Highway Traffic Safety Administration on 08/30/2024
This document has a comment period that ends in 29 days. (09/30/2024) Submit a formal comment
Thank you for taking the time to create a comment. Your input is important.
Once you have filled in the required fields below you can preview and/or submit your comment to the Transportation Department for review. All comments are considered public and will be posted online once the Transportation Department has reviewed them.
You can view alternative ways to comment or you may also comment via Regulations.gov at https://www.regulations.gov/commenton/NHTSA-2023-0065-0004 .
- What is your comment about?
Note: You can attach your comment as a file and/or attach supporting documents to your comment. Attachment Requirements .
this will NOT be posted on regulations.gov
- Opt to receive email confirmation of submission and tracking number?
- Tell us about yourself! I am... *
- First Name *
- Last Name *
- State Alabama Alaska American Samoa Arizona Arkansas California Colorado Connecticut Delaware District of Columbia Florida Georgia Guam Hawaii Idaho Illinois Indiana Iowa Kansas Kentucky Louisiana Maine Maryland Massachusetts Michigan Minnesota Mississippi Missouri Montana Nebraska Nevada New Hampshire New Jersey New Mexico New York North Carolina North Dakota Ohio Oklahoma Oregon Pennsylvania Puerto Rico Rhode Island South Carolina South Dakota Tennessee Texas Utah Vermont Virgin Islands Virginia Washington West Virginia Wisconsin Wyoming
- Country Afghanistan Åland Islands Albania Algeria American Samoa Andorra Angola Anguilla Antarctica Antigua and Barbuda Argentina Armenia Aruba Australia Austria Azerbaijan Bahamas Bahrain Bangladesh Barbados Belarus Belgium Belize Benin Bermuda Bhutan Bolivia, Plurinational State of Bonaire, Sint Eustatius and Saba Bosnia and Herzegovina Botswana Bouvet Island Brazil British Indian Ocean Territory Brunei Darussalam Bulgaria Burkina Faso Burundi Cambodia Cameroon Canada Cape Verde Cayman Islands Central African Republic Chad Chile China Christmas Island Cocos (Keeling) Islands Colombia Comoros Congo Congo, the Democratic Republic of the Cook Islands Costa Rica Côte d'Ivoire Croatia Cuba Curaçao Cyprus Czech Republic Denmark Djibouti Dominica Dominican Republic Ecuador Egypt El Salvador Equatorial Guinea Eritrea Estonia Ethiopia Falkland Islands (Malvinas) Faroe Islands Fiji Finland France French Guiana French Polynesia French Southern Territories Gabon Gambia Georgia Germany Ghana Gibraltar Greece Greenland Grenada Guadeloupe Guam Guatemala Guernsey Guinea Guinea-Bissau Guyana Haiti Heard Island and McDonald Islands Holy See (Vatican City State) Honduras Hong Kong Hungary Iceland India Indonesia Iran, Islamic Republic of Iraq Ireland Isle of Man Israel Italy Jamaica Japan Jersey Jordan Kazakhstan Kenya Kiribati Korea, Democratic People's Republic of Korea, Republic of Kuwait Kyrgyzstan Lao People's Democratic Republic Latvia Lebanon Lesotho Liberia Libya Liechtenstein Lithuania Luxembourg Macao Macedonia, the Former Yugoslav Republic of Madagascar Malawi Malaysia Maldives Mali Malta Marshall Islands Martinique Mauritania Mauritius Mayotte Mexico Micronesia, Federated States of Moldova, Republic of Monaco Mongolia Montenegro Montserrat Morocco Mozambique Myanmar Namibia Nauru Nepal Netherlands New Caledonia New Zealand Nicaragua Niger Nigeria Niue Norfolk Island Northern Mariana Islands Norway Oman Pakistan Palau Palestine, State of Panama Papua New Guinea Paraguay Peru Philippines Pitcairn Poland Portugal Puerto Rico Qatar Réunion Romania Russian Federation Rwanda Saint Barthélemy Saint Helena, Ascension and Tristan da Cunha Saint Kitts and Nevis Saint Lucia Saint Martin (French part) Saint Pierre and Miquelon Saint Vincent and the Grenadines Samoa San Marino Sao Tome and Principe Saudi Arabia Senegal Serbia Seychelles Sierra Leone Singapore Sint Maarten (Dutch part) Slovakia Slovenia Solomon Islands Somalia South Africa South Georgia and the South Sandwich Islands South Sudan Spain Sri Lanka Sudan Suriname Svalbard and Jan Mayen Swaziland Sweden Switzerland Syrian Arab Republic Taiwan, Province of China Tajikistan Tanzania, United Republic of Thailand Timor-Leste Togo Tokelau Tonga Trinidad and Tobago Tunisia Turkey Turkmenistan Turks and Caicos Islands Tuvalu Uganda Ukraine United Arab Emirates United Kingdom United States United States Minor Outlying Islands Uruguay Uzbekistan Vanuatu Venezuela, Bolivarian Republic of Viet Nam Virgin Islands, British Virgin Islands, U.S. Wallis and Futuna Western Sahara Yemen Zambia Zimbabwe
- Organization Type * Company Organization Federal State Local Tribal Regional Foreign U.S. House of Representatives U.S. Senate
- Organization Name *
- You are filing a document into an official docket. Any personal information included in your comment text and/or uploaded attachment(s) may be publicly viewable on the web.
- I read and understand the statement above.
- Preview Comment
This document has been published in the Federal Register . Use the PDF linked in the document sidebar for the official electronic format.
- Document Details Published Content - Document Details Agencies Department of Transportation National Highway Traffic Safety Administration Agency/Docket Number Docket No. NHTSA-2023-0065 Document Citation 89 FR 70687 Document Number 2024-19437 Document Type Notice Pages 70687-70690 (4 pages) Publication Date 08/30/2024 Published Content - Document Details
- View printed version (PDF)
- Document Dates Published Content - Document Dates Comments Close 09/30/2024 Dates Text Comments must be submitted on or before September 30, 2024. Published Content - Document Dates
This table of contents is a navigational tool, processed from the headings within the legal text of Federal Register documents. This repetition of headings to form internal navigation links has no substantive legal effect.
FOR FURTHER INFORMATION CONTACT:
Supplementary information:.
Comments are being accepted - Submit a public comment on this document .
FederalRegister.gov retrieves relevant information about this document from Regulations.gov to provide users with additional context. This information is not part of the official Federal Register document.
Crash Injury Research and Engineering Network Data Collection
- Sharing Enhanced Content - Sharing Shorter Document URL https://www.federalregister.gov/d/2024-19437 Email Email this document to a friend Enhanced Content - Sharing
- Print this document
Document page views are updated periodically throughout the day and are cumulative counts for this document. Counts are subject to sampling, reprocessing and revision (up or down) throughout the day.
This document is also available in the following formats:
More information and documentation can be found in our developer tools pages .
This PDF is the current document as it appeared on Public Inspection on 08/29/2024 at 8:45 am.
It was viewed 0 times while on Public Inspection.
If you are using public inspection listings for legal research, you should verify the contents of the documents against a final, official edition of the Federal Register. Only official editions of the Federal Register provide legal notice of publication to the public and judicial notice to the courts under 44 U.S.C. 1503 & 1507 . Learn more here .
Document headings vary by document type but may contain the following:
- the agency or agencies that issued and signed a document
- the number of the CFR title and the number of each part the document amends, proposes to amend, or is directly related to
- the agency docket number / agency internal file number
- the RIN which identifies each regulatory action listed in the Unified Agenda of Federal Regulatory and Deregulatory Actions
See the Document Drafting Handbook for more details.
Department of Transportation
National highway traffic safety administration.
- [Docket No. NHTSA-2023-0065]
National Highway Traffic Safety Administration (NHTSA), Department of Transportation (DOT).
Notice and request for comments on a request for approval of an information collection.
In compliance with the Paperwork Reduction Act of 1995 (PRA), this notice announces that the Information Collection Request (ICR) summarized below will be submitted to the Office of Management and Budget (OMB) for review and approval. The ICR describes the nature of the information collection and its expected burden. This document describes an information collection request for which NHTSA intends to seek a new OMB approval for NHTSA's Crash Injury Research and Engineering Network (CIREN) investigation-based crash data study. A Federal Register Notice with a 60-day comment period soliciting comments on the following information collection was published. Two comments were received, and burden estimates were adjusted based on the input.
Comments must be submitted on or before September 30, 2024.
Written comments and recommendations for the proposed information collection, including suggestions for reducing burden, should be submitted to the Office of Management and Budget at www.reginfo.gov/public/do/PRAMain . To find this particular information collection, select “Currently under Review—Open for Public Comment” or use the search function.
For additional information or access to background documents, contact Rodney Rudd, Office of Vehicle Safety Research, Human Injury Research Division (NSR-220), West Building, W46-324, 1200 New Jersey Avenue SE, Washington, DC 20590, (202) 366-5932.
Under the PRA ( 44 U.S.C. 3501 et seq. ), a Federal agency must receive approval from the Office of Management and Budget (OMB) before it collects certain information from the public and a person is not required to respond to a collection of information by a Federal agency unless the collection displays a valid OMB control number. In compliance with these requirements, this notice announces that the following information collection request will be submitted OMB.
Title: Crash Injury Research and Engineering Network (CIREN) Data Collection.
OMB Control Number: New.
Form Number: NHTSA Form 1770, NHTSA Form 1771, NHTSA Form 1772, NHTSA Form 1773, NHTSA Form 1774, NHTSA Form 1775, NHTSA Form 1776, NHTSA Form 1777, NHTSA Form 1778, NHTSA Form 1779, NHTSA Form 1780, NHTSA Form 1781, NHTSA Form 1782, NHTSA Form 1783, NHTSA Form 1784, NHTSA Form 1785, NHTSA Form 1786, NHTSA Form 1787, NHTSA Form 1788, NHTSA Form 1789, NHTSA Form 1790, NHTSA Form 1791, NHTSA Form 1792, NHTSA Form 1793, NHTSA Form 1794, NHTSA Form 1795, NHTSA Form 1796.
Type of Request: Request for approval of a new information collection.
Type of Review Requested: Regular.
Length of Approval Requested: Three years from date of approval.
Summary of the Collection of Information:
NHTSA proposes to collect information from the public as part of a study to improve NHTSA's understanding of injury causation in motor vehicle crashes. NHTSA is authorized, under 49 U.S.C. 30182 and 23 U.S.C. 403 to collect data on motor vehicle traffic crashes to aid in the identification of issues and the development, implementation, and evaluation of motor vehicle and highway safety countermeasures. For decades, NHTSA has been investigating crashes and collecting crash data through its investigation-based data collection systems. The Crash Injury Research and Engineering Network (CIREN) is a multidisciplinary, injury-focused crash data collection program using trauma centers under contract to NHTSA's Office of Vehicle Safety Research. NHTSA also investigates crashes through the Crash Investigation Sampling System (CISS), Special Crash Investigation (SCI), and specific issue-based Special Study data collection studies. Although each of these systems ( print page 70688) satisfy different purposes and collect data in different manners, they all utilize similar core data elements, procedures, information technology, and protocols for data collection.
NHTSA is seeking a new, independent approval of an information collection request for the CIREN program separate from NHTSA's other investigation-based crash data collection systems. The method of case subject identification and selection is unique for CIREN. CIREN collects a purposive sample of injured traffic crash victims from a small number of sites to extensively examine and document injury causation in motor vehicle crashes. The CIREN program enrolls case subjects (crash victims) who have been admitted to contracted level-one trauma centers for treatment of injuries sustained in a crash. CIREN requires case subjects admitted to the contracted trauma centers to consent to participate in the study, which facilitates detailed review and analysis of medical and engineering data by multidisciplinary teams to evaluate injury causation. The focus of the CIREN program has historically been on seriously injured occupants of recent model-year motor vehicles, though the program intends to expand to include pedestrians, pedalcyclists, and micromobility (non-motorist) users who have been injured in crashes.
Study personnel at each contracted CIREN site review trauma registry data to identify potential case subjects based on the study's inclusion criteria. Study teams obtain informed consent from eligible patients according to institutional policies and consent documents. Eligible patients who do not provide consent to participate in the study are dropped from consideration and no data are collected. Participation in CIREN does not affect the case subject's medical treatment. Observations from the CIREN program inform NHTSA research priorities and the data support improvements in motor vehicle safety. CIREN provides non-private data to the public through an online case viewer, database files, and reports.
After an eligible patient provides consent, study personnel retrieve the case subject's medical information and commence the crash investigation. Study personnel retrieve the medical information directly from the hospital's electronic medical record (EMR) system including case subject anthropometry, past medical history, radiological imaging and reports, operative procedure reports, and injury diagnoses. Study personnel also request emergency medical services (EMS) response reports from first responders. Study personnel also conduct an interview with the case subject (or a surrogate in cases where the case subject is unable to communicate) to develop an understanding about the crash circumstances. Study personnel may capture photographs of integumentary injuries ( e.g., lacerations, hematomas, abrasions) if the case subject agrees to have such photos taken. A trained crash investigator locates, visits, measures, and photographs the crash scene and the case subject's vehicle (or the striking vehicle for non-motorist case subjects). They also obtain the police crash report. These data are used to characterize the performance of vehicle safety systems and biomechanical responses of injured individuals in motor vehicle crashes.
Description of the Need for the Information and Proposed Use of the Information: NHTSA investigates real-world crashes and collects detailed crash and medical data in the CIREN program to identify human and vehicle factors related to injury causation in support of NHTSA research. Biomechanical engineers and medical doctors collaboratively review case evidence to establish injury causation scenarios. These detailed factors and scenarios inform research priorities. They may also guide the development and evaluation of effective safety countermeasures such as testing tools and criteria. The data collected also act as a sentinel, providing NHTSA with advanced notice of emerging crash injury problems, and are used to generate research hypotheses. These efforts give motor vehicle researchers an opportunity to specify areas in which improvements may be possible, design countermeasure programs, and evaluate the effects of existing and proposed safety measures. The resulting deidentified database provides NHTSA and the public with access to crash data which contains extensive medical detail, including medical imaging, which is a unique resource among available crash data systems. There is no other source for the biomechanics-focused data which is critical to support crash injury mitigation and prevention research.
60-Day Notice: A Federal Register notice with a 60-day comment period soliciting public comments on the following information collection was published on December 8, 2023 ( 88 FR 85725 ). Two individuals submitted comments in response to the notice, which are summarized below. [ 1 ]
The commenters, individuals who both had experience as former project coordinators for CIREN centers, described patient interaction times, for both obtaining consent and conducting the interview, shorter than the estimates included in the 60-day notice. In the 60-day notice, NHTSA estimated that it would approximately 30 minutes for the consent form and one hour for each interview. One of the commenters stated that time for obtaining consent took between five and ten minutes. The other commenter stated that the consent process involved five to ten minutes for describing the program, leaving the consent form with the patient, and returning to discuss the program further and answer any questions, adding an additional ten to fifteen minutes. Since the second commenter estimated that the total estimated time for consent could take up to 25 minutes, not including any time the patient read the consent form on their own, NHTSA has decided not to change its burden estimates for the consent form.
Both commenters also commented about the total to conduct interviews with patients. The first commented that the interviews normally take approximately ten to twenty minutes, with photographs taking about three minutes. The first commenter also stated that the longest interview took 30 minutes. The second commenter stated that interviews took approximately five to ten minutes, with photographs taking five to twenty minutes. The highest of the estimates provided by the commenters suggest that, at most, interviews take up to 35 minutes. This is less than the one-hour estimate NHTSA provided in its 60-day notice. After considering these comments, NHTSA has opted to retain its more conservative one-hour estimate for patient interviews to account for variability on interview lengths and to ensure that its estimate is not too low.
The second commenter noted that obtaining police reports could require several weeks of waiting and could involve CIREN contractor personnel checking in police report databases repeatedly by CIREN contractor personnel. NHTSA appreciates this comment and notes that the burden on CIREN contractor personnel is not counted in total burden hours as it is not a burden on a respondent.
The second commenter also noted that the process to obtain vehicle location information and inspection approval involves contact with the case subject's vehicle insurance provider. ( print page 70689) This was not considered in the original 60-day notice. For most CIREN cases, the case subject's vehicle has sustained sufficient damage to be deemed a total loss by the insurer and it becomes necessary to obtain approval from the insurer to conduct the vehicle inspection. This process requires contacting the claims adjuster to obtain permission as well as confirm the disposition of the vehicle ( i.e., salvage facility). The commenter stated that the amount of time spent getting insurance approval could be between 30 minutes to four hours collectively. While this estimate was provided from the perspective of the time the CIREN contractor personnel spent obtaining such information and approval, NHTSA does believe it to be a good indication of the time spent by the insurance provider as well. Accordingly, and based on this estimate, NHTSA estimates that insurance providers spend approximately two hours providing information and approval to inspect the case subject's vehicle. This burden estimate is included in the discussion of burden hours below. In response to this comment regarding insurer involvement, NHTSA is also updating the burden associated with tow facilities providing information. In the 60-day notice, NHTSA estimated that it would take the tow facility was five minutes of time to direct the investigator to the subject vehicle. Since part of the insurance approval process involves the insurance adjuster contacting the salvage facility in possession of the case subject's vehicle, NHTSA has increased the burden for the tow facilities by ten minutes to account for the interaction regarding inspection approval from the insurance provider.
Affected Public: The information collections affect people involved in select motor vehicle crashes admitted to contracted trauma centers for treatment; law enforcement jurisdictions that provide access to and a copy of crash reports from the investigated crashes; EMS providers responding to investigated crashes; insurance companies responsible for case subject vehicles; and tow or salvage facilities possessing case subject vehicles.
Estimated Number of Respondents: 1,394.
Study personnel screen trauma records for potentially eligible case subjects, and then approach potential case subjects to gain consent. It is estimated that 362 potential case subjects are approached for consent each year. Of those, an average of 258 provide consent and participate in the interview process. For each of the 258 consented case subjects, study personnel contact the police, EMS agencies, insurance companies, and a tow facility for report documentation and to coordinate the vehicle inspection. The combination of patients (362) and associated contacts (4 × 258) yields 1,394 total respondents each year, on average.
The 60-day notice indicated 1,136 respondents, which was increased to 1,394 in this notice due to the inclusion of insurance company involvement for each consented case subject (258). This increase was in response to a submitted comment noting the necessity to communicate with the insurance claim representative to receive permission to inspect the involved case vehicle.
Frequency: On occasion.
Number of Responses: One.
Estimated Total Annual Burden Hours: 1,059.
The CIREN program consists of six (6) information collections. The first information collection covers the consent process for individuals involved in crashes who are deemed potentially eligible for the study at contracted trauma centers. Based on historical data, approximately 362 potential case subjects are approached for study consent each year. The consent process generally requires thirty (30) minutes of the respondent's time during their acute hospital admission, which includes explanation of the study risks and benefits and review of consent language. This burden would apply for every patient approached for consent, regardless of their decision to participate in the study. The estimated total annual burden hours for seeking study consent from eligible case subjects is 181 hours (362 respondents × 0.5 hours).
The second information collection is from individuals who agree to participate in the study. After providing consent, CIREN contractor personnel conduct an interview that requires approximately one hour of the respondent's time during their acute hospital admission. The CIREN program has historically conducted interviews of approximately 258 case subjects per year. Therefore, the estimated total annual burden for case subject interviews is 258 hours (258 respondents × 1.0 hour).
The third and fourth information collections for CIREN is obtaining first responder reports to complete the cases. The reports are obtained from police and EMS agencies, and reports are only requested for crash subjects who have consented to participate in the study. NHTSA estimates each query to police agencies takes three (3) minutes (0.05 hours) and each query to EMS agencies takes six (6) minutes (0.1 hours). Therefore, the total estimated annual burden for crash reports is 13 hours (258 requests × 0.05 hours) and EMS reports is 26 hours (258 requests × 0.1 hours).
The fifth information collection for CIREN is gaining permission from the case vehicle's insurance company to inspect the vehicle. Most cases involve contacting the insurance claims representative to determine the location of the vehicle and obtain the necessary approval to perform the inspection. The insurance claims representative must then notify the salvage facility operator that the CIREN investigator has been approved to perform the inspection. NHTSA estimates this process takes an average of two (2) hours per case vehicle for which approval is sought. Therefore, the total estimated annual burden for insurance companies is 516 hours (258 requests × 2.0 hours). This step has been added based on comments received from the 60-day notice.
The sixth information collection for CIREN is associated with towing and salvage facility requests for access to case vehicles. Typically, a towing or salvage facility operator will provide the crash investigator permission to enter the facility to inspect the case-involved vehicle as well as provide guidance regarding the location of the vehicle. This process is estimated to take approximately five (5) minutes (0.08 hours) of staff time. The communication between the insurance claim representative and salvage facility operator is estimated to take approximately ten (10) minutes (0.17 hours) of staff time. CIREN averages 258 visits to towing and salvage facilities each year since most CIREN cases involve inspection of one case vehicle. The total annual burden for towing and salvage facilities is 64.5 hours (258 requests × 0.25 hours). This step was modified based on comments received from the 60-day notice.
Accordingly, NHTSA estimates that the total burden associated with the CIREN program is 1,059 hours (52 + 387 + 39 + 516 + 64.5). This represents an increase of 560 hours from what was in published in the 60-day notice, with the difference being associated with the inclusion of insurance company involvement. Table 1 includes a summary of the annual estimated burden hours. ( print page 70690)
| Information collection | Number of respondents | Number of responses (per respondent) | Burden per response | Burden per respondent | Total burden |
|---|---|---|---|---|---|
| Potential case subject consent | 362 | 362 (1) | 30 minutes | 30 minutes | 181 hours. |
| Case subject interview | 258 | 258 (1) | 1.0 hours | 1.0 hours | 258 hours. |
| Police report requests | 258 | 258 (1) | 3 minutes | 3 minutes | 13 hours. |
| EMS report requests | 258 | 258 (1) | 6 minutes | 6 minutes | 26 hours. |
| Insurance company | 258 | 258 (1) | 2.0 hours | 2.0 hours | 516 hours. |
| Access to towing/salvage facility | 258 | 258 (1) | 15 minutes | 15 minutes | 64.5 hours. |
| Total | 1,059 hours. |
Estimated Total Annual Burden Cost: $0.
There are no capital, start-up, or annual operation and maintenance costs involved in this collection of information. The respondents would not incur any reporting costs from the information collection beyond the opportunity or labor costs associated with the burden hours. The respondents also would not incur any recordkeeping burden or recordkeeping costs from the information collection.
Public Comments Invited: You are asked to comment on any aspects of this information collection, including (a) whether the proposed collection of information is necessary for the proper performance of the functions of the agency, including whether the information will have practical utility; (b) the accuracy of the agency's estimate of the burden of the proposed collection of information, including the validity of the methodology and assumptions used; (c) ways to enhance the quality, utility and clarity of the information to be collected; and (d) ways to minimize the burden of the collection of information on respondents, including the use of appropriate automated, electronic, mechanical, or other technological collection techniques or other forms of information technology, e.g., permitting electronic submission of responses.
Authority: The Paperwork Reduction Act of 1995; 44 U.S.C. chapter 35 , as amended; 49 CFR 1.49 ; and DOT Order 1351.29A.
Cem Hatipoglu,
Associate Administrator, Office of Vehicle Safety Research.
1. The comments are available at https://www.regulations.gov/comment/NHTSA-2023-0065-0002 and https://www.regulations.gov/comment/NHTSA-2023-0065-0003 .
[ FR Doc. 2024-19437 Filed 8-29-24; 8:45 am]
BILLING CODE 4910-59-P
- Executive Orders
Reader Aids
Information.
- About This Site
- Legal Status
- Accessibility
- No Fear Act
- Continuity Information
Industry Innovation Day Features Brain and Technology
Apr 24, 2023 —.

Meta Lab's Thomas Reardon (pictured on screen) and Chris Rozell, professor and Julian T. Hightower Chair in the School of Electrical and Computer Engineering
More than 150 people attended Industry Innovation Day and the GVU Spring Research Showcase on April 19 held at the Technology Square Research Building conference center on the Georgia Tech campus. This year’s event centered around the brain and neuro-related technologies, and touched on topics ranging from brain computer interaction, cognitive aids, psychology, the future of work, artificial intelligence and various other topics that surfaced due to audience questions. The event was sponsored by the Georgia Tech Institute of People and Technology (IPaT) and the Georgia Tech Neuro community.
The keynote speaker this year was Thomas Reardon, vice president and head of neural interfaces at Meta Reality Labs. Reardon is a highly regarded neuroscientist and entrepreneur who founded CTRL-labs which was acquired by Meta (Facebook) in 2019. He currently leads a team of computational neuroscientists and biotech engineers working to connect neurons to machines via a novel non-invasive neural interface technology. Reardon’s talk topic for this year’s annual Industry Innovation Day was “Consumer Neural Interfaces: View from Meta Reality Labs.” In addition to providing an informative lecture about neural technology, he briefly displayed some of the capabilities of his Meta team’s wrist-mounted, non-invasive device that was able to translate neuro hand activity into its corresponding robotic hand movements.
“Our undergraduate degree in neuroscience is one of the Institute’s most popular degrees,” said Julia Kubanek, vice president for interdisciplinary research at Georgia Tech, who gave several introductory remarks. “The neuroscience area integrates many disciplines across campus such as the arts, humanities, social science, computer science, engineering, business, design, and the basic sciences and is a great example of the true integration of interdisciplinary research in many forms across Georgia Tech. We are particularly grateful for the participation today of companies and other organizations that collaborate with the Georgia Tech community of researchers.”
Leigh McCook, interim executive director of IPaT, emphasized the need to have dialogue and conversations between industry partners and community partners with Georgia Tech researchers to develop supportive research projects and create greater impact in the area of the human technology frontier.
“The neuro space and the IPaT space are natural partners for each other,” said Chris Rozell, professor and Julian T. Hightower Chair in the School of Electrical and Computer Engineering. “This is a perfect day to come together and talk about what the human frontier looks like. Georgia Tech hired its first neuro engineer more than 30 years ago long before it was cool to be an engineer studying neuro. Today, we have more than a 100 faculty spanning six colleges studying neuro-related topics with the additional involvement of Emory University and Georgia State University. We’ve had an incredible trajectory over the last decade and we’ve fostered a growing and active community.”
Following Reardon’s keynote were two interactive panel sessions. The first panel was focused on “brain computer input and output” was led by moderator Michelle LaPlaca, professor in the Wallace H. Coulter Department of Biomedical Engineering. Her research interests are in neurotrauma, injury biomechanics, and neuroengineering as they relate to traumatic brain injury.
The panelists were:
Melody Moore Jackson, professor in the School of Interactive Computing Omer Inan, professor in the School of Electrical and Computer Engineering Carlos Bremer, president North America Division - Global VP of Knowledge at brain4care Isaac Clements, CTO and co-founder of BioCircuit Technologies The second panel was focused on the “future of cognitive and psychological aids” and was moderated by Maribeth Gandy Coleman, director of research at the Institute for People and Technology where her work has been focused on the intersection of technology for mobile/wearable computing, augmented reality, AI, human computer interaction, healthcare, assistive technology, and gaming.
Jennifer R. DuBose, director of the SimTigrate Design Lab Tansu Celikel, chair of the School of Psychology Deborah Backus, vice president of research and innovation, Shepherd Center Barbara Olasov Rothbaum, director of the Emory Healthcare veterans program and chair in neuropsychopharmacology, Emory University School of Medicine “I am really pleased with the connections we were able to foster today,” said Clint Zeagler, co-director of strategic partnerships for IPaT and principal research scientist. “Key to translational and impactful research outcomes are transdisciplinary collaboration across campus and with industry and corporate partners. Events like this with both academic and industry experts allow for deep conversations and spark interesting and innovative projects.”
Walter Rich

This website uses cookies. For more information, review our Privacy & Legal Notice Questions? Please email [email protected]. More Info Decline --> Accept

Data Science Across NIAID
Researchers across NIAID use data science to accelerate research into understanding, treating, and preventing infectious, immunologic, and allergic diseases. Learn more about data science research at each of the offices and centers listed below.
Bioinformatics and Computational Biosciences Branch
The Bioinformatics and Computational Biosciences Branch (BCBB) serves as a centralized resource for data science and emerging technologies. We utilize cutting-edge techniques like machine learning and network analysis to support, enable, and advance biomedical discovery for NIAID researchers and collaborators.
Integrated Data Sciences Section
The Research Technologies Branch (RTB) Integrated Data Sciences Section (IDSS) provides scientific consultation, training and workshops, computational and data science support, and technology collaboration. IDSS works with NIAID investigators to provide bioinformatics, data science, and computational biology expertise at all project stages, from experimental design through manuscript preparation.
Office of Data Science and Emerging Technologies
The Office of Data Science and Emerging Technologies (ODSET) coordinates the development and implementation of NIAID’s data science strategy across its entire global portfolio of research and training programs. The overall goal of the office is to enable the efficient use of data and computational methods to better understand, treat, and ultimately prevent infectious, immunologic, and allergic diseases.
Office of Genomics and Advanced Technologies
Advanced technologies research fields, such as genomics, proteomics, and bioinformatics, hold great promise for developing new diagnostics, therapeutics, and vaccines to treat and prevent infectious and immune-mediated diseases. Sophisticated tools are being used to determine the genetic make-up of disease-causing pathogens, to analyze discrepancies among pathogen strains, and to evaluate how immune system responses differ.
Related Technologies at NIAID
Bioinformatics.
NIAID is a leader in the field of bioinformatics and our work is growing and expanding as technologies advance. NIAID supported contracts combined with internally developed technologies offer services related to bioinformatics.
- HIV & AIDS
- History & Facts
- Risk Factors & Spread
- Among Specific Individuals & Communities
- Pregnancy, Children & HIV
- Screening & Testing
- Overview of Treatment
- Drug Treatments
- What Does HIV do to Your Body?
- Heart Problems
- Lung Problems
- Pain & Nerve Problems
- Vision Problems
- Opportunistic Infections
- What Complications Are Caused by HIV and AIDS?
- HIV With Other Conditions
- Living With
- Dating & Relationships
- Support & Resources
- Is HIV Preventable?
- PrEP and PEP
- Vaccines Potential
- Appointment Prep
- View Full Guide
HIV: Latest Research

HIV research has made remarkable progress since scientists first identified the disease in the 1980s. There are new prevention methods and therapies to extend the lives of those living with the disease. So what does the future hold for HIV research? Here’s a look at what’s on the horizon.
HIV Prevention
Stopping the spread of HIV is an important step toward ending the outbreak of the disease around the world. Today, there are several methods to slow HIV, and scientists are working on new tools.
HIV is constantly evolving into new strains. This makes it hard to develop a vaccine , but scientists are making progress. The National Institutes of Health (NIH) is researching two HIV vaccines and testing them in people around the world. The goal of these vaccines is to turn on an immune response to a wide range of HIV strains.
Another vaccine candidate from IAVI and Scripps Research works by prompting the immune system to turn on in response to different versions and mutations of HIV. Early research results show it’s 97% effective.
Long-acting prevention
Researchers are also working on HIV prevention methods that last for months or even years. They could offer new choices for protecting yourself against the virus or improve products that you already use.
- Vaginal ring: This flexible silicone ring steadily releases the anti-HIV drug dapivirine. You replace it monthly. Women ages 18 to 45 who took part in two large clinical trials lowered their chance of HIV infection by about 30% when using it.
- Injections: The FDA has approved cabotegravir ( Apretude ) to protect you from HIV for as long as 1 to 6 months. You get it as a shot once every 8 weeks. Studies show it may be just as safe and work better than the daily oral drug emtricitabine /tenofovir.
- Implants: One promising new technology is long-acting implants in your arm. The matchstick-sized implant slowly releases an anti-HIV drug and could offer protection against HIV for 1 year or longer. Several of these implants are in the works but are still in the early stages of development.
- Oral pills: Researchers are also studying a pill that could protect you from HIV for 30 days. Two other HIV prevention pills, Truvada and Descovy , have been around for years, but you take them daily. Research shows that although these drugs lower your chance of getting HIV by anywhere from 74% to 99%, many people aren’t aware of them or are afraid they would be shamed for taking them.
- Monoclonal antibodies: These lab-created immune system proteins may work to prevent HIV. Scientists are looking at how a mix of assorted antibodies could be a tool in long-term HIV prevention and treatment.
HIV Treatment
There’s no cure for HIV, but medicine can help you manage the disease and ward off other health problems. Scientists and drugmakers continue to develop new treatments for people living with HIV , turning their focus to long-acting therapies.
Once-monthly HIV therapy
In January 2021, the FDA approved the first long-acting injectable treatment for adults with HIV. Cabenuva is a combo of two drugs ( cabotegravir and rilpivirine ) that you take as a shot once a month or every two months. It’s considered a breakthrough in treatment since most HIV drugs require an oral daily dose.
One small survey of people living with HIV shows that more people prefer long-acting shots than pills you take every day. Most (73%) who responded to the survey said they were definitely or probably interested in trying an injectable. This type of medicine could help with issues of missed doses and medical privacy.
Twice Yearly HIV Treatment
Lenacapavir ( Sunlenca ) is the first of a new class of drugs called capsid inhibitors to be FDA-approved and is for treating adults with HIV that is not adequately controlled by their current treatment regimen. It blocks the HIV virus’ protein shell called the capsid which interrupts an important step in the virus life cycle. Sunlenca’s starting dose is given as oral tablets and subcutaneous injections which is followed by maintenance injections every six months. It is given in combination with other antiretroviral(s).
Other HIV treatments
The FDA has also recently approved two other drugs to treat HIV in kids and adults.
- Dolutegravir ( Tivicay ) for children: There are 1.8 million children (birth to 14 years old) living with HIV. This drug is the first integrase inhibitor (a class of anti-HIV drugs) dissolved in water that’s available for children as young as 4 weeks old.
- Fostemsavir ( Rukobia ): This medicine is an attachment inhibitor (antiretroviral drug) for adults who haven’t had success with other HIV treatments .

Top doctors in ,
Find more top doctors on, related links.
- HIV & AIDS Health Resources News
- HIV & AIDS Health Resources Reference
- HIV & AIDS Health Resources Slideshows
- HIV & AIDS Health Blogs
- HIV & AIDS Health Resources Medications
- Find a Doctor
- Crisis Assistance
- HIV Myths and Facts
- Molluscum Contagiosum
- Sexual Conditions
- More Related Topics

An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Office of AIDS Research
Mobile app for infusion pump software wins oar’s hiv prize in the 2024 debut challenge.

Fiscal Year 2026 NIH HIV/AIDS Professional Judgment Budget: Enhancing Equity and Maintaining Momentum

Clinical guidelines update: Expert panel releases new recommendations to prevent anal cancer for people with HIV
Explore NIH HIV Research Portfolio with New Data Tools

Perinatal HIV clinical guidelines update infant feeding options.

Welcome to OAR
The Office of AIDS Research (OAR) coordinates HIV/AIDS research across the National Institutes of Health (NIH). The NIH provides the largest public investment in HIV/AIDS research globally.
As HIV crosses nearly every area of medicine and scientific investigation, the response to the HIV pandemic requires a multi-Institute, multidisciplinary, global research program. OAR provides scientific coordination and management of this research program.
OAR Spotlight

NIH had a broad presence at the 25th International AIDS Conference, or AIDS 2024. The NIH Office of AIDS Research and partners across NIH participated in several sessions and presented new discoveries in HIV science. Leadership from OAR and the National Institute of Allergy and Infectious Diseases held an “Ask the Experts” session, giving attendees an opportunity to learn and ask questions about the NIH HIV research program. Learn about on-site NIH activities .
This page last reviewed on July 1, 2024
Meet the Director

Recent Updates
Research news.

- Patient Care & Health Information
- Diseases & Conditions
Acquired immunodeficiency syndrome (AIDS), is an ongoing, also called chronic, condition. It's caused by the human immunodeficiency virus, also called HIV. HIV damages the immune system so that the body is less able to fight infection and disease. If HIV isn't treated, it can take years before it weakens the immune system enough to become AIDS . Thanks to treatment, most people in the U.S. don't get AIDS .
HIV is spread through contact with genitals, such as during sex without a condom. This type of infection is called a sexually transmitted infection, also called an STI. HIV also is spread through contact with blood, such as when people share needles or syringes. It is also possible for a person with untreated HIV to spread the virus to a child during pregnancy, childbirth or breastfeeding.
There's no cure for HIV / AIDS . But medicines can control the infection and keep the disease from getting worse. Antiviral treatments for HIV have reduced AIDS deaths around the world. There's an ongoing effort to make ways to prevent and treat HIV / AIDS more available in resource-poor countries.
Products & Services
- A Book: Mayo Clinic Family Health Book
- Assortment of Pill Aids from Mayo Clinic Store
The symptoms of HIV and AIDS vary depending on the person and the phase of infection.
Primary infection, also called acute HIV
Some people infected by HIV get a flu-like illness within 2 to 4 weeks after the virus enters the body. This stage may last a few days to several weeks. Some people have no symptoms during this stage.
Possible symptoms include:
- Muscle aches and joint pain.
- Sore throat and painful mouth sores.
- Swollen lymph glands, also called nodes, mainly on the neck.
- Weight loss.
- Night sweats.
These symptoms can be so mild that you might not notice them. However, the amount of virus in your bloodstream, called viral load, is high at this time. As a result, the infection spreads to others more easily during primary infection than during the next stage.
Clinical latent infection, also called chronic HIV
In this stage of infection, HIV is still in the body and cells of the immune system, called white blood cells. But during this time, many people don't have symptoms or the infections that HIV can cause.
This stage can last for many years for people who aren't getting antiretroviral therapy, also called ART. Some people get more-severe disease much sooner.
Symptomatic HIV infection
As the virus continues to multiply and destroy immune cells, you may get mild infections or long-term symptoms such as:
- Swollen lymph glands, which are often one of the first symptoms of HIV infection.
- Oral yeast infection, also called thrush.
- Shingles, also called herpes zoster.
Progression to AIDS
Better antiviral treatments have greatly decreased deaths from AIDS worldwide. Thanks to these lifesaving treatments, most people with HIV in the U.S. today don't get AIDS . Untreated, HIV most often turns into AIDS in about 8 to 10 years.
Having AIDS means your immune system is very damaged. People with AIDS are more likely to develop diseases they wouldn't get if they had healthy immune systems. These are called opportunistic infections or opportunistic cancers. Some people get opportunistic infections during the acute stage of the disease.
The symptoms of some of these infections may include:
- Fever that keeps coming back.
- Ongoing diarrhea.
- Swollen lymph glands.
- Constant white spots or lesions on the tongue or in the mouth.
- Constant fatigue.
- Rapid weight loss.
- Skin rashes or bumps.
When to see a doctor
If you think you may have been infected with HIV or are at risk of contracting the virus, see a healthcare professional as soon as you can.
More Information
- Early HIV symptoms: What are they?
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
HIV is caused by a virus. It can spread through sexual contact, shooting of illicit drugs or use of shared needles, and contact with infected blood. It also can spread from parent to child during pregnancy, childbirth or breastfeeding.
HIV destroys white blood cells called CD4 T cells. These cells play a large role in helping the body fight disease. The fewer CD4 T cells you have, the weaker your immune system becomes.
How does HIV become AIDS?
You can have an HIV infection with few or no symptoms for years before it turns into AIDS . AIDS is diagnosed when the CD4 T cell count falls below 200 or you have a complication you get only if you have AIDS , such as a serious infection or cancer.
How HIV spreads
You can get infected with HIV if infected blood, semen or fluids from a vagina enter your body. This can happen when you:
- Have sex. You may become infected if you have vaginal or anal sex with an infected partner. Oral sex carries less risk. The virus can enter your body through mouth sores or small tears that can happen in the rectum or vagina during sex.
- Share needles to inject illicit drugs. Sharing needles and syringes that have been infected puts you at high risk of HIV and other infectious diseases, such as hepatitis.
- Have a blood transfusion. Sometimes the virus may be transmitted through blood from a donor. Hospitals and blood banks screen the blood supply for HIV . So this risk is small in places where these precautions are taken. The risk may be higher in resource-poor countries that are not able to screen all donated blood.
- Have a pregnancy, give birth or breastfeed. Pregnant people who have HIV can pass the virus to their babies. People who are HIV positive and get treatment for the infection during pregnancy can greatly lower the risk to their babies.
How HIV doesn't spread
You can't become infected with HIV through casual contact. That means you can't catch HIV or get AIDS by hugging, kissing, dancing or shaking hands with someone who has the infection.
HIV isn't spread through air, water or insect bites. You can't get HIV by donating blood.
Risk factors
Anyone of any age, race, sex or sexual orientation can have HIV / AIDS . However, you're at greatest risk of HIV / AIDS if you:
- Have unprotected sex. Use a new latex or polyurethane condom every time you have sex. Anal sex is riskier than is vaginal sex. Your risk of HIV increases if you have more than one sexual partner.
- Have an STI . Many STIs cause open sores on the genitals. These sores allow HIV to enter the body.
- Inject illicit drugs. If you share needles and syringes, you can be exposed to infected blood.
Complications
HIV infection weakens your immune system. The infection makes you much more likely to get many infections and certain types of cancers.
Infections common to HIV/AIDS
- Pneumocystis pneumonia, also called PCP. This fungal infection can cause severe illness. It doesn't happen as often in the U.S. because of treatments for HIV / AIDS . But PCP is still the most common cause of pneumonia in people infected with HIV .
- Candidiasis, also called thrush. Candidiasis is a common HIV -related infection. It causes a thick, white coating on the mouth, tongue, esophagus or vagina.
- Tuberculosis, also called TB. TB is a common opportunistic infection linked to HIV . Worldwide, TB is a leading cause of death among people with AIDS . It's less common in the U.S. thanks to the wide use of HIV medicines.
- Cytomegalovirus. This common herpes virus is passed in body fluids such as saliva, blood, urine, semen and breast milk. A healthy immune system makes the virus inactive, but it stays in the body. If the immune system weakens, the virus becomes active, causing damage to the eyes, digestive system, lungs or other organs.
- Cryptococcal meningitis. Meningitis is swelling and irritation, called inflammation, of the membranes and fluid around the brain and spinal cord, called meninges. Cryptococcal meningitis is a common central nervous system infection linked to HIV . A fungus found in soil causes it.
Toxoplasmosis. This infection is caused by Toxoplasma gondii, a parasite spread primarily by cats. Infected cats pass the parasites in their stools. The parasites then can spread to other animals and humans.
Toxoplasmosis can cause heart disease. Seizures happen when it spreads to the brain. And it can be fatal.
Cancers common to HIV/AIDS
- Lymphoma. This cancer starts in the white blood cells. The most common early sign is painless swelling of the lymph nodes most often in the neck, armpit or groin.
- Kaposi sarcoma. This is a tumor of the blood vessel walls. Kaposi sarcoma most often appears as pink, red or purple sores called lesions on the skin and in the mouth in people with white skin. In people with Black or brown skin, the lesions may look dark brown or black. Kaposi sarcoma also can affect the internal organs, including the lungs and organs in the digestive system.
- Human papillomavirus (HPV)-related cancers. These are cancers caused by HPV infection. They include anal, oral and cervical cancers.
Other complications
- Wasting syndrome. Untreated HIV / AIDS can cause a great deal of weight loss. Diarrhea, weakness and fever often happen with the weight loss.
- Brain and nervous system, called neurological, complications. HIV can cause neurological symptoms such as confusion, forgetfulness, depression, anxiety and difficulty walking. HIV -associated neurological conditions can range from mild symptoms of behavior changes and reduced mental functioning to severe dementia causing weakness and not being able to function.
- Kidney disease. HIV -associated nephropathy (HIVAN) is swelling and irritation, called inflammation, of the tiny filters in the kidneys. These filters remove excess fluid and waste from the blood and pass them to the urine. Kidney disease most often affects Black and Hispanic people.
- Liver disease. Liver disease also is a major complication, mainly in people who also have hepatitis B or hepatitis C.
There's no vaccine to prevent HIV infection and no cure for HIV / AIDS . But you can protect yourself and others from infection.
To help prevent the spread of HIV :
Consider preexposure prophylaxis, also called PrEP. There are two PrEP medicines taken by mouth, also called oral, and one PrEP medicine given in the form of a shot, called injectable. The oral medicines are emtricitabine-tenofovir disoproxil fumarate (Truvada) and emtricitabine-tenofovir alafenamide fumarate (Descovy). The injectable medicine is called cabotegravir (Apretude). PrEP can reduce the risk of sexually transmitted HIV infection in people at very high risk.
PrEP can reduce the risk of getting HIV from sex by about 99% and from injecting drugs by at least 74%, according to the Centers for Disease Control and Prevention. Descovy hasn't been studied in people who have sex by having a penis put into their vaginas, called receptive vaginal sex.
Cabotegravir (Apretude) is the first U.S. Food and Drug Administration-approved PrEP that can be given as a shot to reduce the risk of sexually transmitted HIV infection in people at very high risk. A healthcare professional gives the shot. After two once-monthly shots, Apretude is given every two months. The shot is an option in place of a daily PrEP pill.
Your healthcare professional prescribes these medicines to prevent HIV only to people who don't already have HIV infection. You need an HIV test before you start taking any PrEP . You need to take the test every three months for the pills or before each shot for as long as you take PrEP .
You need to take the pills every day or closely follow the shot schedule. You still need to practice safe sex to protect against other STIs . If you have hepatitis B, you should see an infectious disease or liver specialist before beginning PrEP therapy.
Use treatment as prevention, also called TasP. If you have HIV , taking HIV medicines can keep your partner from getting infected with the virus. If your blood tests show no virus, that means your viral load can't be detected. Then you won't transmit the virus to anyone else through sex.
If you use TasP , you must take your medicines exactly as prescribed and get regular checkups.
- Use post-exposure prophylaxis, also called PEP, if you've been exposed to HIV . If you think you've been exposed through sex, through needles or in the workplace, contact your healthcare professional or go to an emergency room. Taking PEP as soon as you can within the first 72 hours can greatly reduce your risk of getting HIV . You need to take the medicine for 28 days.
Use a new condom every time you have anal or vaginal sex. Both male and female condoms are available. If you use a lubricant, make sure it's water based. Oil-based lubricants can weaken condoms and cause them to break.
During oral sex, use a cut-open condom or a piece of medical-grade latex called a dental dam without a lubricant.
- Tell your sexual partners you have HIV . It's important to tell all your current and past sexual partners that you're HIV positive. They need to be tested.
- Use clean needles. If you use needles to inject illicit drugs, make sure the needles are sterile. Don't share them. Use needle-exchange programs in your community. Seek help for your drug use.
- If you're pregnant, get medical care right away. You can pass HIV to your baby. But if you get treatment during pregnancy, you can lessen your baby's risk greatly.
- Consider male circumcision. Studies show that removing the foreskin from the penis, called circumcision, can help reduce the risk of getting HIV infection.
- About HIV and AIDS . HIV.gov. https://www.hiv.gov/hiv-basics/overview/about-hiv-and-aids/what-are-hiv-and-aids. Accessed Oct. 18, 2023.
- Sax PE. Acute and early HIV infection: Clinical manifestations and diagnosis. https://www.uptodate.com/contents/search. Accessed Oct. 18, 2023.
- Ferri FF. Human immunodeficiency virus. In: Ferri's Clinical Advisor 2024. Elsevier; 2024. https://www.clinicalkey.com. Accessed Oct. 18, 2023.
- Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV . HIV.gov. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/immunizations. Accessed Oct. 18, 2023.
- AskMayoExpert. Human immunodeficiency virus (HIV) infection. Mayo Clinic; 2023.
- Elsevier Point of Care. Clinical Overview: HIV infection and AIDS in adults. https://www.clinicalkey.com. Accessed Oct. 18, 2023.
- Male circumcision for HIV prevention fact sheet. Centers for Disease Control and Prevention. https://www.cdc.gov/nchhstp/newsroom/fact-sheets/hiv/male-circumcision-HIV-prevention-factsheet.html. Accessed Oct. 19, 2023.
- Acetyl-L-carnitine. Natural Medicines. https://naturalmedicines.therapeuticresearch.com. Accessed. Oct. 19, 2023.
- Whey protein. Natural Medicines. https://naturalmedicines.therapeuticresearch.com. Accessed. Oct. 19, 2023.
- Saccharomyces boulardii. Natural Medicines. https://naturalmedicines.therapeuticresearch.com. Accessed Oct. 19, 2023.
- Vitamin A. Natural Medicines. https://naturalmedicines.therapeuticresearch.com. Accessed Oct. 19, 2023.
- Red yeast rice. Natural Medicines. https://naturalmedicines.therapeuticresearch.com. Accessed Oct. 19, 2023.
Associated Procedures
- Liver function tests
News from Mayo Clinic
- Mayo Clinic Minute: Know your status -- the importance of HIV testing June 25, 2024, 04:00 p.m. CDT
- Unlocking the mechanisms of HIV in preclinical research Jan. 10, 2024, 03:30 p.m. CDT
- Mayo Clinic expert on future of HIV on World AIDS Day Dec. 01, 2023, 05:15 p.m. CDT
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
5X Challenge
Thanks to generous benefactors, your gift today can have 5X the impact to advance AI innovation at Mayo Clinic.
- Fact sheets
Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- Endometriosis
- Excessive heat
- Mental disorders
- Polycystic ovary syndrome
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Observatory
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Fact sheets /

HIV and AIDS
- HIV remains a major global public health issue, having claimed an estimated 42.3 million lives to date. Transmission is ongoing in all countries globally.
- There were an estimated 39.9 million people living with HIV at the end of 2023, 65% of whom are in the WHO African Region.
- In 2023, an estimated 630 000 people died from HIV-related causes and an estimated 1.3 million people acquired HIV.
- There is no cure for HIV infection. However, with access to effective HIV prevention, diagnosis, treatment and care, including for opportunistic infections, HIV infection has become a manageable chronic health condition, enabling people living with HIV to lead long and healthy lives.
- WHO, the Global Fund and UNAIDS all have global HIV strategies that are aligned with the SDG target 3.3 of ending the HIV epidemic by 2030.
- By 2025, 95% of all people living with HIV should have a diagnosis, 95% of whom should be taking lifesaving antiretroviral treatment, and 95% of people living with HIV on treatment should achieve a suppressed viral load for the benefit of the person’s health and for reducing onward HIV transmission. In 2023, these percentages were 86%, 89%, and 93% respectively.
- In 2023, of all people living with HIV, 86% knew their status, 77% were receiving antiretroviral therapy and 72% had suppressed viral loads.
Human immunodeficiency virus (HIV) is a virus that attacks the body’s immune system. Acquired immunodeficiency syndrome (AIDS) occurs at the most advanced stage of infection.
HIV targets the body’s white blood cells, weakening the immune system. This makes it easier to get sick with diseases like tuberculosis, infections and some cancers.
HIV is spread from the body fluids of an infected person, including blood, breast milk, semen and vaginal fluids. It is not spread by kisses, hugs or sharing food. It can also spread from a mother to her baby.
HIV can be prevented and treated with antiretroviral therapy (ART). Untreated HIV can progress to AIDS, often after many years.
WHO now defines Advanced HIV Disease (AHD) as CD4 cell count less than 200 cells/mm3 or WHO stage 3 or 4 in adults and adolescents. All children younger than 5 years of age living with HIV are considered to have advanced HIV disease.
Signs and symptoms
The symptoms of HIV vary depending on the stage of infection.
HIV spreads more easily in the first few months after a person is infected, but many are unaware of their status until the later stages. In the first few weeks after being infected people may not experience symptoms. Others may have an influenza-like illness including:
- sore throat.
The infection progressively weakens the immune system. This can cause other signs and symptoms:
- swollen lymph nodes
- weight loss
Without treatment, people living with HIV infection can also develop severe illnesses:
- tuberculosis (TB)
- cryptococcal meningitis
- severe bacterial infections
- cancers such as lymphomas and Kaposi's sarcoma.
HIV causes other infections to get worse, such as hepatitis C, hepatitis B and mpox.
Transmission
HIV can be transmitted via the exchange of body fluids from people living with HIV, including blood, breast milk, semen, and vaginal secretions. HIV can also be transmitted to a child during pregnancy and delivery. People cannot become infected with HIV through ordinary day-to-day contact such as kissing, hugging, shaking hands, or sharing personal objects, food or water.
People living with HIV who are taking ART and have an undetectable viral load will not transmit HIV to their sexual partners. Early access to ART and support to remain on treatment is therefore critical not only to improve the health of people living with HIV but also to prevent HIV transmission.
Risk factors
Behaviours and conditions that put people at greater risk of contracting HIV include:
- having anal or vaginal sex without a condom;
- having another sexually transmitted infection (STI) such as syphilis, herpes, chlamydia, gonorrhoea and bacterial vaginosis;
- harmful use of alcohol or drugs in the context of sexual behaviour;
- sharing contaminated needles, syringes and other injecting equipment, or drug solutions when injecting drugs;
- receiving unsafe injections, blood transfusions, or tissue transplantation; and
- medical procedures that involve unsterile cutting or piercing; or accidental needle stick injuries, including among health workers.
HIV can be diagnosed through rapid diagnostic tests that provide same-day results. This greatly facilitates early diagnosis and linkage with treatment and prevention. People can also use HIV self-tests to test themselves. However, no single test can provide a full HIV positive diagnosis; confirmatory testing is required, conducted by a qualified and trained health worker or community worker. HIV infection can be detected with great accuracy using WHO prequalified tests within a nationally approved testing strategy and algorithm.
Most widely used HIV diagnostic tests detect antibodies produced by a person as part of their immune response to fight HIV. In most cases, people develop antibodies to HIV within 28 days of infection. During this time, people are in the so-called “window period” when they have low levels of antibodies which cannot be detected by many rapid tests, but they may still transmit HIV to others. People who have had a recent high-risk exposure and test negative can have a further test after 28 days.
Following a positive diagnosis, people should be retested before they are enrolled in treatment and care to rule out any potential testing or reporting error. While testing for adolescents and adults has been made simple and efficient, this is not the case for babies born to HIV-positive mothers. For children less than 18 months of age, rapid antibody testing is not sufficient to identify HIV infection – virological testing must be provided as early as birth or at 6 weeks of age. New technologies are now available to perform this test at the point of care and enable same-day results, which will accelerate appropriate linkage with treatment and care.
HIV is a preventable disease. Reduce the risk of HIV infection by:
- using a male or female condom during sex
- being tested for HIV and sexually transmitted infections
- having a voluntary medical male circumcision
- using harm reduction services for people who inject and use drugs.
Doctors may suggest medicines and medical devices to help prevent HIV infection, including:
- antiretroviral drugs (ARVs), including oral Pre-Exposure Prophylaxis (PrEP) and long acting products
- dapivirine vaginal rings
- injectable long acting cabotegravir.
ARVs can also be used to prevent mothers from passing HIV to their children.
People taking antiretroviral therapy (ART) and who have no evidence of virus in the blood will not pass HIV to their sexual partners. Access to testing and ART is an important part of preventing HIV.
Antiretroviral drugs given to people without HIV can prevent infection
When given before possible exposures to HIV it is called pre-exposure prophylaxis (PrEP) and when given after an exposure it is called post-exposure prophylaxis (PEP). People can use PrEP or PEP when the risk of contracting HIV is high; people should seek advice from a clinician when thinking about using PrEP or PEP.
There is no cure for HIV infection. It is treated with antiretroviral drugs, which stop the virus from replicating in the body.
Current antiretroviral therapy (ART) does not cure HIV infection but allows a person’s immune system to get stronger. This helps them to fight other infections.
Currently, ART must be taken every day for the rest of a person’s life.
ART lowers the amount of the virus in a person’s body. This stops symptoms and allows people to live full and healthy lives. People living with HIV who are taking ART and who have no evidence of virus in the blood will not spread the virus to their sexual partners.
Pregnant women with HIV should have access to, and take, ART as soon as possible. This protects the health of the mother and will help prevent HIV transmission to the fetus before birth, or through breast milk.
Advanced HIV disease remains a persistent problem in the HIV response. WHO is supporting countries to implement the advanced HIV disease package of care to reduce illness and death. Newer HIV medicines and short course treatments for opportunistic infections like cryptococcal meningitis are being developed that may change the way people take ART and prevention medicines, including access to injectable formulations, in the future.
More information on HIV treatments
WHO response
Global health sector strategies on HIV, viral hepatitis, and sexually transmitted infections for the period 2022–2030 ( GHSSs ) guide strategic responses to achieve the goals of ending AIDS, viral hepatitis B and C, and sexually transmitted infections by 2030.
WHO’s Global HIV, Hepatitis and STIs Programmes recommend shared and disease-specific country actions supported by WHO and partners. They consider the epidemiological, technological, and contextual shifts of previous years, foster learning, and create opportunities to leverage innovation and new knowledge.
WHO’s programmes call to reach the people most affected and most at risk for each disease, and to address inequities. Under a framework of universal health coverage and primary health care, WHO’s programmes contribute to achieving the goals of the 2030 Agenda for Sustainable Development.
- Global HIV, Hepatitis and STIs Programmes
- Global Health Sector Strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022–2030 (GHSS)
- GHSS report on progress and gaps 2024
- HIV country profiles
- HIV statistics, globally and by WHO region, 2024
To revisit this article, visit My Profile, then View saved stories .
- The Big Story
- Newsletters
- Steven Levy's Plaintext Column
- WIRED Classics from the Archive
- WIRED Insider
- WIRED Consulting
There’s New Hope for an HIV Vaccine
Since it was first identified in 1983, HIV has infected more than 85 million people and caused some 40 million deaths worldwide.
While medication known as pre-exposure prophylaxis , or PrEP, can significantly reduce the risk of getting HIV, it has to be taken every day to be effective. A vaccine to provide lasting protection has eluded researchers for decades. Now, there may finally be a viable strategy for making one.
An experimental vaccine developed at Duke University triggered an elusive type of broadly neutralizing antibody in a small group of people enrolled in a 2019 clinical trial. The findings were published today in the scientific journal Cell .
“This is one of the most pivotal studies in the HIV vaccine field to date,” says Glenda Gray, an HIV expert and the president and CEO of the South African Medical Research Council, who was not involved in the study.
A few years ago, a team from Scripps Research and the International AIDS Vaccine Initiative (IAVI) showed that it was possible to stimulate the precursor cells needed to make these rare antibodies in people. The Duke study goes a step further to generate these antibodies, albeit at low levels.
“This is a scientific feat and gives the field great hope that one can construct an HIV vaccine regimen that directs the immune response along a path that is required for protection,” Gray says.
Vaccines work by training the immune system to recognize a virus or other pathogen. They introduce something that looks like the virus—a piece of it, for example, or a weakened version of it—and by doing so, spur the body’s B cells into producing protective antibodies against it. Those antibodies stick around so that when a person later encounters the real virus, the immune system remembers and is poised to attack.
While researchers were able to produce Covid-19 vaccines in a matter of months, creating a vaccine against HIV has proven much more challenging. The problem is the unique nature of the virus. HIV mutates rapidly, meaning it can quickly outmaneuver immune defenses. It also integrates into the human genome within a few days of exposure, hiding out from the immune system.
“Parts of the virus look like our own cells, and we don’t like to make antibodies against our own selves,” says Barton Haynes, director of the Duke Human Vaccine Institute and one of the authors on the paper.
The particular antibodies that researchers are interested in are known as broadly neutralizing antibodies, which can recognize and block different versions of the virus. Because of HIV’s shape-shifting nature, there are two main types of HIV and each has several strains. An effective vaccine will need to target many of them.
Some HIV-infected individuals generate broadly neutralizing antibodies, although it often takes years of living with HIV to do so, Haynes says. Even then, people don’t make enough of them to fight off the virus. These special antibodies are made by unusual B cells that are loaded with mutations they’ve acquired over time in reaction to the virus changing inside the body. “These are weird antibodies,” Haynes says. “The body doesn’t make them easily.”

Haynes and his colleagues aimed to speed up that process in healthy, HIV-negative people. Their vaccine uses synthetic molecules that mimic a part of HIV’s outer coat, or envelope, called the membrane proximal external region. This area remains stable even as the virus mutates. Antibodies against this region can block many circulating strains of HIV.
The trial enrolled 20 healthy participants who were HIV-negative. Of those, 15 people received two of four planned doses of the investigational vaccine, and five received three doses. The trial was halted when one participant experienced an allergic reaction that was not life-threatening. The team found that the reaction was likely due to an additive in the vaccine, which they plan to remove in future testing.
Still, they found that two doses of the vaccine were enough to induce low levels of broadly neutralizing antibodies within a few weeks. Notably, B cells seemed to remain in a state of development to allow them to continue acquiring mutations, so they could evolve along with the virus. Researchers tested the antibodies on HIV samples in the lab and found that they were able to neutralize between 15 and 35 percent of them.
Jeffrey Laurence, a scientific consultant at the Foundation for AIDS Research (amfAR) and a professor of medicine at Weill Cornell Medical College, says the findings represent a step forward, but that challenges remain. “It outlines a path for vaccine development, but there’s a lot of work that needs to be done,” he says.
For one, he says, a vaccine would need to generate antibody levels that are significantly higher and able to neutralize with greater efficacy. He also says a one-dose vaccine would be ideal. “If you’re ever going to have a vaccine that’s helpful to the world, you’re going to need one dose,” he says.
Targeting more regions of the virus envelope could produce a more robust response. Haynes says the next step is designing a vaccine with at least three components, all aimed at distinct regions of the virus. The goal is to guide the B cells to become much stronger neutralizers, Haynes says. “We’re going to move forward and build on what we have learned.”
You Might Also Like …
In your inbox: The best and weirdest stories from WIRED’s archive
How the brain decides what to remember
The Big Story: Meet Priscila, queen of the rideshare mafia
Silicon Valley's soulless plutocrats flip for Donald Trump
Event: Join us for The Big Interview on December 3 in San Francisco

- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Research & training, advances in hiv/aids research.
For an update on what medical science is doing to fight the global HIV/AIDS pandemic, read a Parade article by NIH Director Francis S. Collins and NIAID Director Anthony S. Fauci, AIDS in 2010: How We're Living with HIV .
Over the past several decades, researchers have learned a lot about the human immunodeficiency virus (HIV) and the disease it causes, acquired immunodeficiency syndrome (AIDS). But still more research is needed to help the millions of people whose health continues to be threatened by the global HIV/AIDS pandemic.
At the National Institutes of Health, the HIV/AIDS research effort is led by the National Institute of Allergy and Infectious Diseases (NIAID). A vast network of NIAID-supported scientists, located on the NIH campus in Bethesda, Maryland, and at research centers around the globe, are exploring new ways to prevent and treat HIV infection, as well as to better understand the virus with the goal of finding a cure. For example, in recent months, NIAID and its partners made progress toward finding a vaccine to prevent HIV infection. Check out other promising areas of NIAID-funded research on HIV/AIDS at http://www.niaid.nih.gov/topics/hivaids/Pages/Default.aspx .
Other NIH institutes, including the Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Institute on Alcohol Abuse and Alcoholism, also support research to better control and ultimately end the HIV/AIDS pandemic. Some of these researchers have found a simple, cost-effective way to cut HIV transmission from infected mothers to their breastfed infants. Others have developed an index to help measure the role of alcohol consumption in illness and death of people with HIV/AIDS.
Find out more about these discoveries and what they mean for improving the health of people in the United States and all around the globe.
This page last reviewed on August 20, 2015
Connect with Us
- More Social Media from NIH
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.

CDC provides national leadership for HIV prevention research, including the development and evaluation of HIV biomedical and behavioral interventions to prevent HIV transmission and reduce HIV disease progression in the United States and internationally. CDC’s research efforts also include identifying those scientifically proven, cost-effective, and scalable interventions and prevention strategies to be implemented as part of a high-impact prevention approach for maximal impact on the HIV epidemic.
The AIDS epidemic, although first recognized only 20 years ago, has had a profound impact in communities throughout the United States.
The Serostatus Approach to Fighting the HIV Epidemic: Prevention Strategies for Infected Individuals R. S. Janssen, D. R. Holtgrave, and K. M. De Cock led the writing of this commentary. R. O. Valdiserri, M. Shepherd, and H. D. Gayle contributed ideas and helped with writing and reviewing the manuscript.

CDC has provided funding to HIV partners to help implement programs that will help curb the increase of HIV infections. These programs facilitated with our partners and grantees are critical in the goal of eliminating HIV infection in the United States.

CDC has researched several HIV prevention interventions that have proven effective in helping to prevent HIV infection in certain populations and communities.

CDC has worked with key cities to create effective policies and programs to curb the tide of HIV infections in those cities. These cities have higher rates of HIV due to a number of factors therefore making them key locations for studies.

The Medical Monitoring Project (MMP) is a surveillance system designed to learn more about the experiences and needs of people who are living with HIV. It is supported by several government agencies and conducted by state and local health departments along with the Centers for Disease Control and Prevention.
- Assessment of 2010 CDC-funded Health Department HIV Testing Spending and Outcomes [PDF – 359 KB]
- HIV Testing Trends in the United States, 2000-2011 [PDF – 1 MB]
- HIV Testing at CDC-Funded Sites, United States, Puerto Rico, and the U.S. Virgin Islands, 2010 [PDF – 691 KB]
- HIV Prevention Funding Allocations at CDC-Funded State and Local Health Departments, 2010 [PDF – 792 KB]
Cost-effectiveness of HIV Prevention
- The cost-effectiveness of HIV prevention efforts has long been a criterion in setting program priorities. The basic principle is straightforward: choose those options that provide the greatest outcome for the least cost.
- The fact sheet Projecting Possible Future Courses of the HIV Epidemic in the United States compares the cost-effectiveness of three different prevention investment scenarios.
The HIV/AIDS Prevention Research Synthesis (PRS) Project identifies evidence-based HIV behavioral interventions (EBIs) listed in the Compendium of Evidence-Based HIV Behavioral Interventions to help HIV prevention planners and providers in the United States choose the interventions most appropriate for their communities.
- On January 1, 2012, CDC began a new 5-year HIV prevention funding cycle with health departments, awarding $339 million annually.
- The STD/HIV National Network of Prevention Training Centers provides training for health departments and CBOs on the HIV prevention interventions.
- HIV Nexus: Resources for Clinicians
- Ending the HIV Epidemic (EHE)
- Public Health Partners
- Let’s Stop HIV Together
- @StopHIVTogether
- Get Email Updates
- Send Feedback
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 24 November 2021
A study of awareness on HIV/AIDS among adolescents: A Longitudinal Study on UDAYA data
- Shobhit Srivastava ORCID: orcid.org/0000-0002-7138-4916 1 ,
- Shekhar Chauhan ORCID: orcid.org/0000-0002-6926-7649 2 ,
- Ratna Patel ORCID: orcid.org/0000-0002-5371-7369 3 &
- Pradeep Kumar ORCID: orcid.org/0000-0003-4259-820X 1
Scientific Reports volume 11 , Article number: 22841 ( 2021 ) Cite this article
12k Accesses
9 Citations
11 Altmetric
Metrics details
- Health care
- Health services
- Public health
Acquired Immunodeficiency Syndrome caused by Human Immunodeficiency Virus (HIV) poses a severe challenge to healthcare and is a significant public health issue worldwide. This study intends to examine the change in the awareness level of HIV among adolescents. Furthermore, this study examined the factors associated with the change in awareness level on HIV-related information among adolescents over the period. Data used for this study were drawn from Understanding the lives of adolescents and young adults, a longitudinal survey on adolescents aged 10–19 in Bihar and Uttar Pradesh. The present study utilized a sample of 4421 and 7587 unmarried adolescent boys and girls, respectively aged 10–19 years in wave-1 and wave-2. Descriptive analysis and t-test and proportion test were done to observe changes in certain selected variables from wave-1 (2015–2016) to wave-2 (2018–2019). Moreover, random effect regression analysis was used to estimate the association of change in HIV awareness among unmarried adolescents with household and individual factors. The percentage of adolescent boys who had awareness regarding HIV increased from 38.6% in wave-1 to 59.9% in wave-2. Among adolescent girls, the percentage increased from 30.2 to 39.1% between wave-1 & wave-2. With the increase in age and years of schooling, the HIV awareness increased among adolescent boys ([Coef: 0.05; p < 0.01] and [Coef: 0.04; p < 0.01]) and girls ([Coef: 0.03; p < 0.01] and [Coef: 0.04; p < 0.01]), respectively. The adolescent boys [Coef: 0.06; p < 0.05] and girls [Coef: 0.03; p < 0.05] who had any mass media exposure were more likely to have an awareness of HIV. Adolescent boys' paid work status was inversely associated with HIV awareness [Coef: − 0.01; p < 0.10]. Use of internet among adolescent boys [Coef: 0.18; p < 0.01] and girls [Coef: 0.14; p < 0.01] was positively associated with HIV awareness with reference to their counterparts. There is a need to intensify efforts in ensuring that information regarding HIV should reach vulnerable sub-groups, as outlined in this study. It is important to mobilize the available resources to target the less educated and poor adolescents, focusing on rural adolescents.
Similar content being viewed by others

Predictors of never testing for HIV among sexually active individuals aged 15–56 years in Rwanda
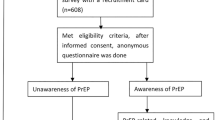
Awareness and use of HIV pre-exposure prophylaxis and factors associated with awareness among MSM in Beijing, China

Identification of adolescent girls and young women for targeted HIV prevention: a new risk scoring tool in KwaZulu Natal, South Africa
Introduction.
Acquired Immunodeficiency Syndrome (AIDS) caused by Human Immunodeficiency Virus (HIV) poses a severe challenge to healthcare and is a significant public health issue worldwide. So far, HIV has claimed almost 33 million lives; however, off lately, increasing access to HIV prevention, diagnosis, treatment, and care has enabled people living with HIV to lead a long and healthy life 1 . By the end of 2019, an estimated 38 million people were living with HIV 1 . More so, new infections fell by 39 percent, and HIV-related deaths fell by almost 51 percent between 2000 and 2019 1 . Despite all the positive news related to HIV, the success story is not the same everywhere; HIV varies between region, country, and population, where not everyone is able to access HIV testing and treatment and care 1 . HIV/AIDS holds back economic growth by destroying human capital by predominantly affecting adolescents and young adults 2 .
There are nearly 1.2 billion adolescents (10–19 years) worldwide, which constitute 18 percent of the world’s population, and in some countries, adolescents make up as much as one-fourth of the population 3 . In India, adolescents comprise more than one-fifth (21.8%) of the total population 4 . Despite a decline projection for the adolescent population in India 5 , there is a critical need to hold adolescents as adolescence is characterized as a period when peer victimization/pressure on psychosocial development is noteworthy 6 . Peer victimization/pressure is further linked to risky sexual behaviours among adolescents 7 , 8 . A higher proportion of low literacy in the Indian population leads to a low level of awareness of HIV/AIDS 9 . Furthermore, the awareness of HIV among adolescents is quite alarming 10 , 11 , 12 .
Unfortunately, there is a shortage of evidence on what predicts awareness of HIV among adolescents. Almost all the research in India is based on beliefs, attitudes, and awareness of HIV among adolescents 2 , 12 . However, few other studies worldwide have examined mass media as a strong predictor of HIV awareness among adolescents 13 . Mass media is an effective channel to increase an individuals’ knowledge about sexual health and improve understanding of facilities related to HIV prevention 14 , 15 . Various studies have outlined other factors associated with the increasing awareness of HIV among adolescents, including; age 16 , 17 , 18 , occupation 18 , education 16 , 17 , 18 , 19 , sex 16 , place of residence 16 , marital status 16 , and household wealth index 16 .
Several community-based studies have examined awareness of HIV among Indian adolescents 2 , 10 , 12 , 20 , 21 , 22 . However, studies investigating awareness of HIV among adolescents in a larger sample size remained elusive to date, courtesy of the unavailability of relevant data. Furthermore, no study in India had ever examined awareness of HIV among adolescents utilizing information on longitudinal data. To the author’s best knowledge, this is the first study in the Indian context with a large sample size that examines awareness of HIV among adolescents and combines information from a longitudinal survey. Therefore, this study intends to examine the change in the awareness level of HIV among adolescents. Furthermore, this study examined the factors associated with a change in awareness level on HIV-related information among adolescents over the period.
Data and methods
Data used for this study were drawn from Understanding the lives of adolescents and young adults (UDAYA), a longitudinal survey on adolescents aged 10–19 in Bihar and Uttar Pradesh 23 . The first wave was conducted in 2015–2016, and the follow-up survey was conducted after three years in 2018–2019 23 . The survey provides the estimates for state and the sample of unmarried boys and girls aged 10–19 and married girls aged 15–19. The study adopted a systematic, multi-stage stratified sampling design to draw sample areas independently for rural and urban areas. 150 primary sampling units (PSUs)—villages in rural areas and census wards in urban areas—were selected in each state, using the 2011 census list of villages and wards as the sampling frame. In each primary sampling unit (PSU), households to be interviewed were selected by systematic sampling. More details about the study design and sampling procedure have been published elsewhere 23 . Written consent was obtained from the respondents in both waves. In wave 1 (2015–2016), 20,594 adolescents were interviewed using the structured questionnaire with a response rate of 92%.
Moreover, in wave 2 (2018–2019), the study interviewed the participants who were successfully interviewed in 2015–2016 and who consented to be re-interviewed 23 . Of the 20,594 eligible for the re-interview, the survey re-interviewed 4567 boys and 12,251 girls (married and unmarried). After excluding the respondents who gave an inconsistent response to age and education at the follow-up survey (3%), the final follow-up sample covered 4428 boys and 11,864 girls with the follow-up rate of 74% for boys and 81% for girls. The effective sample size for the present study was 4421 unmarried adolescent boys aged 10–19 years in wave-1 and wave-2. Additionally, 7587 unmarried adolescent girls aged 10–19 years were interviewed in wave-1 and wave-2 23 . The cases whose follow-up was lost were excluded from the sample to strongly balance the dataset and set it for longitudinal analysis using xtset command in STATA 15. The survey questionnaire is available at https://dataverse.harvard.edu/file.xhtml?fileId=4163718&version=2.0 & https://dataverse.harvard.edu/file.xhtml?fileId=4163720&version=2.0 .
Outcome variable
HIV awareness was the outcome variable for this study, which is dichotomous. The question was asked to the adolescents ‘Have you heard of HIV/AIDS?’ The response was recorded as yes and no.
Exposure variables
The predictors for this study were selected based on previous literature. These were age (10–19 years at wave 1, continuous variable), schooling (continuous), any mass media exposure (no and yes), paid work in the last 12 months (no and yes), internet use (no and yes), wealth index (poorest, poorer, middle, richer, and richest), religion (Hindu and Non-Hindu), caste (Scheduled Caste/Scheduled Tribe, Other Backward Class, and others), place of residence (urban and rural), and states (Uttar Pradesh and Bihar).
Exposure to mass media (how often they read newspapers, listened to the radio, and watched television; responses on the frequencies were: almost every day, at least once a week, at least once a month, rarely or not at all; adolescents were considered to have any exposure to mass media if they had exposure to any of these sources and as having no exposure if they responded with ‘not at all’ for all three sources of media) 24 . Household wealth index based on ownership of selected durable goods and amenities with possible scores ranging from 0 to 57; households were then divided into quintiles, with the first quintile representing households of the poorest wealth status and the fifth quintile representing households with the wealthiest status 25 .
Statistical analysis
Descriptive analysis was done to observe the characteristics of unmarried adolescent boys and girls at wave-1 (2015–2016). In addition, the changes in certain selected variables were observed from wave-1 (2015–2016) to wave-2 (2018–2019), and the significance was tested using t-test and proportion test 26 , 27 . Moreover, random effect regression analysis 28 , 29 was used to estimate the association of change in HIV awareness among unmarried adolescents with household factors and individual factors. The random effect model has a specific benefit for the present paper's analysis: its ability to estimate the effect of any variable that does not vary within clusters, which holds for household variables, e.g., wealth status, which is assumed to be constant for wave-1 and wave-2 30 .
Table 1 represents the socio-economic profile of adolescent boys and girls. The estimates are from the baseline dataset, and it was assumed that none of the household characteristics changed over time among adolescent boys and girls.
Figure 1 represents the change in HIV awareness among adolescent boys and girls. The percentage of adolescent boys who had awareness regarding HIV increased from 38.6% in wave-1 to 59.9% in wave-2. Among adolescent girls, the percentage increased from 30.2% in wave-1 to 39.1% in wave-2.
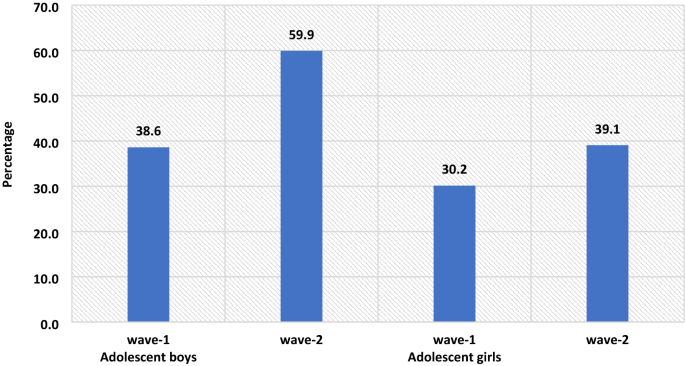
The percenate of HIV awareness among adolescent boys and girls, wave-1 (2015–2016) and wave-2 (2018–2019).
Table 2 represents the summary statistics for explanatory variables used in the analysis of UDAYA wave-1 and wave-2. The exposure to mass media is almost universal for adolescent boys, while for adolescent girls, it increases to 93% in wave-2 from 89.8% in wave-1. About 35.3% of adolescent boys were engaged in paid work during wave-1, whereas in wave-II, the share dropped to 33.5%, while in the case of adolescent girls, the estimates are almost unchanged. In wave-1, about 27.8% of adolescent boys were using the internet, while in wave-2, there is a steep increase of nearly 46.2%. Similarly, in adolescent girls, the use of the internet increased from 7.6% in wave-1 to 39.3% in wave-2.
Table 3 represents the estimates from random effects for awareness of HIV among adolescent boys and girls. It was found that with the increases in age and years of schooling the HIV awareness increased among adolescent boys ([Coef: 0.05; p < 0.01] and [Coef: 0.04; p < 0.01]) and girls ([Coef: 0.03; p < 0.01] and [Coef: 0.04; p < 0.01]), respectively. The adolescent boys [Coef: 0.06; p < 0.05] and girls [Coef: 0.03; p < 0.05] who had any mass media exposure were more likely to have an awareness of HIV in comparison to those who had no exposure to mass media. Adolescent boys' paid work status was inversely associated with HIV awareness about adolescent boys who did not do paid work [Coef: − 0.01; p < 0.10]. Use of the internet among adolescent boys [Coef: 0.18; p < 0.01] and girls [Coef: 0.14; p < 0.01] was positively associated with HIV awareness in reference to their counterparts.
The awareness regarding HIV increases with the increase in household wealth index among both adolescent boys and girls. The adolescent girls from the non-Hindu household had a lower likelihood to be aware of HIV in reference to adolescent girls from Hindu households [Coef: − 0.09; p < 0.01]. Adolescent girls from non-SC/ST households had a higher likelihood of being aware of HIV in reference to adolescent girls from other caste households [Coef: 0.04; p < 0.01]. Adolescent boys [Coef: − 0.03; p < 0.01] and girls [Coef: − 0.09; p < 0.01] from a rural place of residence had a lower likelihood to be aware about HIV in reference to those from the urban place of residence. Adolescent boys [Coef: 0.04; p < 0.01] and girls [Coef: 0.02; p < 0.01] from Bihar had a higher likelihood to be aware about HIV in reference to those from Uttar Pradesh.
This is the first study of its kind to address awareness of HIV among adolescents utilizing longitudinal data in two indian states. Our study demonstrated that the awareness of HIV has increased over the period; however, it was more prominent among adolescent boys than in adolescent girls. Overall, the knowledge on HIV was relatively low, even during wave-II. Almost three-fifths (59.9%) of the boys and two-fifths (39.1%) of the girls were aware of HIV. The prevalence of awareness on HIV among adolescents in this study was lower than almost all of the community-based studies conducted in India 10 , 11 , 22 . A study conducted in slums in Delhi has found almost similar prevalence (40% compared to 39.1% during wave-II in this study) of awareness of HIV among adolescent girls 31 . The difference in prevalence could be attributed to the difference in methodology, study population, and study area.
The study found that the awareness of HIV among adolescent boys has increased from 38.6 percent in wave-I to 59.9 percent in wave-II; similarly, only 30.2 percent of the girls had an awareness of HIV during wave-I, which had increased to 39.1 percent. Several previous studies corroborated the finding and noticed a higher prevalence of awareness on HIV among adolescent boys than in adolescent girls 16 , 32 , 33 , 34 . However, a study conducted in a different setting noticed a higher awareness among girls than in boys 35 . Also, a study in the Indian context failed to notice any statistical differences in HIV knowledge between boys and girls 18 . Gender seems to be one of the significant determinants of comprehensive knowledge of HIV among adolescents. There is a wide gap in educational attainment among male and female adolescents, which could be attributed to lower awareness of HIV among girls in this study. Higher peer victimization among adolescent boys could be another reason for higher awareness of HIV among them 36 . Also, cultural double standards placed on males and females that encourage males to discuss HIV/AIDS and related sexual matters more openly and discourage or even restrict females from discussing sexual-related issues could be another pertinent factor of higher awareness among male adolescents 33 . Behavioural interventions among girls could be an effective way to improving knowledge HIV related information, as seen in previous study 37 . Furthermore, strengthening school-community accountability for girls' education would augment school retention among girls and deliver HIV awareness to girls 38 .
Similar to other studies 2 , 10 , 17 , 18 , 39 , 40 , 41 , age was another significant determinant observed in this study. Increasing age could be attributed to higher education which could explain better awareness with increasing age. As in other studies 18 , 39 , 41 , 42 , 43 , 44 , 45 , 46 , education was noted as a significant driver of awareness of HIV among adolescents in this study. Higher education might be associated with increased probability of mass media and internet exposure leading to higher awareness of HIV among adolescents. A study noted that school is one of the important factors in raising the awareness of HIV among adolescents, which could be linked to higher awareness among those with higher education 47 , 48 . Also, schooling provides adolescents an opportunity to improve their social capital, leading to increased awareness of HIV.
Following previous studies 18 , 40 , 46 , the current study also outlines a higher awareness among urban adolescents than their rural counterparts. One plausible reason for lower awareness among adolescents in rural areas could be limited access to HIV prevention information 16 . Moreover, rural–urban differences in awareness of HIV could also be due to differences in schooling, exposure to mass media, and wealth 44 , 45 . The household's wealth status was also noted as a significant predictor of awareness of HIV among adolescents. Corroborating with previous findings 16 , 33 , 42 , 49 , this study reported a higher awareness among adolescents from richer households than their counterparts from poor households. This could be because wealthier families can afford mass-media items like televisions and radios for their children, which, in turn, improves awareness of HIV among adolescents 33 .
Exposure to mass media and internet access were also significant predictors of higher awareness of HIV among adolescents. This finding agrees with several previous research, and almost all the research found a positive relationship between mass-media exposure and awareness of HIV among adolescents 10 . Mass media addresses such topics more openly and in a way that could attract adolescents’ attention is the plausible reason for higher awareness of HIV among those having access to mass media and the internet 33 . Improving mass media and internet usage, specifically among rural and uneducated masses, would bring required changes. Integrating sexual education into school curricula would be an important means of imparting awareness on HIV among adolescents; however, this is debatable as to which standard to include the required sexual education in the Indian schooling system. Glick (2009) thinks that the syllabus on sexual education might be included during secondary schooling 44 . Another study in the Indian context confirms the need for sex education for adolescents 50 , 51 .
Limitations and strengths of the study
The study has several limitations. At first, the awareness of HIV was measured with one question only. Given that no study has examined awareness of HIV among adolescents using longitudinal data, this limitation is not a concern. Second, the study findings cannot be generalized to the whole Indian population as the study was conducted in only two states of India. However, the two states selected in this study (Uttar Pradesh and Bihar) constitute almost one-fourth of India’s total population. Thirdly, the estimates were provided separately for boys and girls and could not be presented combined. However, the data is designed to provide estimates separately for girls and boys. The data had information on unmarried boys and girls and married girls; however, data did not collect information on married boys. Fourthly, the study estimates might have been affected by the recall bias. Since HIV is a sensitive topic, the possibility of respondents modifying their responses could not be ruled out. Hawthorne effect, respondents, modifying aspect of their behaviour in response, has a role to play in HIV related study 52 . Despite several limitations, the study has specific strengths too. This is the first study examining awareness of HIV among adolescent boys and girls utilizing longitudinal data. The study was conducted with a large sample size as several previous studies were conducted in a community setting with a minimal sample size 10 , 12 , 18 , 20 , 53 .
The study noted a higher awareness among adolescent boys than in adolescent girls. Specific predictors of high awareness were also noted in the study, including; higher age, higher education, exposure to mass media, internet use, household wealth, and urban residence. Based on the study findings, this study has specific suggestions to improve awareness of HIV among adolescents. There is a need to intensify efforts in ensuring that information regarding HIV should reach vulnerable sub-groups as outlined in this study. It is important to mobilize the available resources to target the less educated and poor adolescents, focusing on rural adolescents. Investment in education will help, but it would be a long-term solution; therefore, public information campaigns could be more useful in the short term.
WHO. HIV/AIDS . https://www.who.int/news-room/fact-sheets/detail/hiv-aids (2020).
Singh, A. & Jain, S. Awareness of HIV/AIDS among school adolescents in Banaskantha district of Gujarat. Health Popul.: Perspect. Issues 32 , 59–65 (2009).
Google Scholar
WHO & UNICEF. adolescents Health: The Missing Population in Universal Health Coverage . 1–31 https://www.google.com/search?q=adolescents+population+WHO+report&rlz=1C1CHBF_enIN904IN904&oq=adolescents+population+WHO+report&aqs=chrome..69i57.7888j1j7&sourceid=chrome&ie=UTF-8 (2018).
Chauhan, S. & Arokiasamy, P. India’s demographic dividend: state-wise perspective. J. Soc. Econ. Dev. 20 , 1–23 (2018).
Tiwari, A. K., Singh, B. P. & Patel, V. Population projection of India: an application of dynamic demographic projection model. JCR 7 , 547–555 (2020).
Troop-Gordon, W. Peer victimization in adolescence: the nature, progression, and consequences of being bullied within a developmental context. J. Adolesc. 55 , 116–128 (2017).
PubMed Google Scholar
Dermody, S. S., Friedman, M., Chisolm, D. J., Burton, C. M. & Marshal, M. P. Elevated risky sexual behaviors among sexual minority girls: indirect risk pathways through peer victimization and heavy drinking. J. Interpers. Violence 35 , 2236–2253 (2020).
Lee, R. L. T., Loke, A. Y., Hung, T. T. M. & Sobel, H. A systematic review on identifying risk factors associated with early sexual debut and coerced sex among adolescents and young people in communities. J. Clin. Nurs. 27 , 478–501 (2018).
Gurram, S. & Bollampalli, B. A study on awareness of human immunodeficiency virus among adolescent girls in urban and rural field practice areas of Osmania Medical College, Hyderabad, Telangana, India. (2020).
Jain, R., Anand, P., Dhyani, A. & Bansal, D. Knowledge and awareness regarding menstruation and HIV/AIDS among schoolgoing adolescent girls. J. Fam. Med. Prim Care 6 , 47–51 (2017).
Kawale, S., Sharma, V., Thaware, P. & Mohankar, A. A study to assess awareness about HIV/AIDS among rural population of central India. Int. J. Commun. Med. Public Health 5 , 373–376 (2017).
Lal, P., Nath, A., Badhan, S. & Ingle, G. K. A study of awareness about HIV/AIDS among senior secondary school Children of Delhi. Indian J. Commun. Med. 33 , 190–192 (2008).
CAS Google Scholar
Bago, J.-L. & Lompo, M. L. Exploring the linkage between exposure to mass media and HIV awareness among adolescents in Uganda. Sex. Reprod. Healthcare 21 , 1–8 (2019).
McCombie, S., Hornik, R. C. & Anarfi, J. K. Effects of a mass media campaign to prevent AIDS among young people in Ghana . Public Health Commun. 163–178 (Routledge, 2002). https://doi.org/10.4324/9781410603029-17 .
Sano, Y. et al. Exploring the linkage between exposure to mass media and HIV testing among married women and men in Ghana. AIDS Care 28 , 684–688 (2016).
Oginni, A. B., Adebajo, S. B. & Ahonsi, B. A. Trends and determinants of comprehensive knowledge of HIV among adolescents and young adults in Nigeria: 2003–2013. Afr. J. Reprod. Health 21 , 26–34 (2017).
Rahman, M. M., Kabir, M. & Shahidullah, M. Adolescent knowledge and awareness about AIDS/HIV and factors affecting them in Bangladesh. J. Ayub. Med. Coll. Abbottabad 21 , 3–6 (2009).
Yadav, S. B., Makwana, N. R., Vadera, B. N., Dhaduk, K. M. & Gandha, K. M. Awareness of HIV/AIDS among rural youth in India: a community based cross-sectional study. J. Infect. Dev. Countries 5 , 711–716 (2011).
Alhasawi, A. et al. Assessing HIV/AIDS knowledge, awareness, and attitudes among senior high school students in Kuwait. MPP 28 , 470–476 (2019).
Chakrovarty, A. et al. A study of awareness on HIV/AIDS among higher secondary school students in Central Kolkata. Indian J. Commun. Med. 32 , 228 (2007).
Katoch, D. K. S. & Kumar, A. HIV/AIDS awareness among students of tribal and non-tribal area of Himachal Pradesh. J. Educ. 5 , 1–9 (2017).
Shinde, M., Trivedi, A., Shinde, A. & Mishra, S. A study of awareness regarding HIV/AIDS among secondary school students. Int. J. Commun. Med. Public Health https://doi.org/10.18203/2394-6040.ijcmph20161611 (2016).
Article Google Scholar
Council, P. UDAYA, adolescent survey, Bihar and Uttar Pradesh, 2015–16. Harvard Dataverse https://doi.org/10.7910/DVN/RRXQNT (2018).
Kumar, P. & Dhillon, P. Household- and community-level determinants of low-risk Caesarean deliveries among women in India. J. Biosoc. Sci. 53 , 55–70 (2021).
Patel, S. K., Santhya, K. G. & Haberland, N. What shapes gender attitudes among adolescent girls and boys? Evidence from the UDAYA Longitudinal Study in India. PLoS ONE 16 , e0248766 (2021).
CAS PubMed PubMed Central Google Scholar
Fan, C., Wang, L. & Wei, L. Comparing two tests for two rates. Am. Stat. 71 , 275–281 (2017).
MathSciNet Google Scholar
Kim, T. K. T test as a parametric statistic. Korean J. Anesthesiol. 68 , 540–546 (2015).
PubMed PubMed Central Google Scholar
Bell, A., Fairbrother, M. & Jones, K. Fixed and random effects models: making an informed choice. Qual. Quant. 53 , 1051–1074 (2019).
Jarrett, R., Farewell, V. & Herzberg, A. Random effects models for complex designs. Stat. Methods Med. Res. 29 , 3695–3706 (2020).
MathSciNet CAS PubMed PubMed Central Google Scholar
Neuhaus, J. M. & Kalbfleisch, J. D. Between- and within-cluster covariate effects in the analysis of clustered data. Biometrics 54 , 638–645 (1998).
CAS PubMed MATH Google Scholar
Kaur, G. Factors influencing HIV awareness amongst adolescent women: a study of slums in Delhi. Demogr. India 47 , 100–111 (2018).
Alene, G. D., Wheeler, J. G. & Grosskurth, H. Adolescent reproductive health and awareness of HIV among rural high school students, North Western Ethiopia. AIDS Care 16 , 57–68 (2004).
CAS PubMed Google Scholar
Oljira, L., Berhane, Y. & Worku, A. Assessment of comprehensive HIV/AIDS knowledge level among in-school adolescents in eastern Ethiopia. J. Int. AIDS Soc. 16 , 17349 (2013).
Samkange-Zeeb, F. N., Spallek, L. & Zeeb, H. Awareness and knowledge of sexually transmitted diseases (STDs) among school-going adolescents in Europe: a systematic review of published literature. BMC Public Health 11 , 727 (2011).
Laguna, E. P. Knowledge of HIV/AIDS and unsafe sex practices among Filipino youth. in 15 (PAA, 2004).
Teitelman, A. M. et al. Partner violence, power, and gender differences in South African adolescents’ HIV/sexually transmitted infections risk behaviors. Health Psychol. 35 , 751–760 (2016).
Oberth, G. et al. Effectiveness of the Sista2Sista programme in improving HIV and other sexual and reproductive health outcomes among vulnerable adolescent girls and young women in Zimbabwe. Afr. J. AIDS Res. 20 , 158–164 (2021).
DeSoto, J. et al. Using school-based early warning systems as a social and behavioral approach for HIV prevention among adolescent girls. Preventing HIV Among Young People in Southern and Eastern Africa 280 (2020).
Ochako, R., Ulwodi, D., Njagi, P., Kimetu, S. & Onyango, A. Trends and determinants of Comprehensive HIV and AIDS knowledge among urban young women in Kenya. AIDS Res. Ther. 8 , 11 (2011).
Peltzer, K. & Supa, P. HIV/AIDS knowledge and sexual behavior among junior secondary school students in South Africa. J. Soc. Sci. 1 , 1–8 (2005).
Shweta, C., Mundkur, S. & Chaitanya, V. Knowledge and beliefs about HIV/AIDS among adolescents. WebMed Cent. 2 , 1–9 (2011).
Anwar, M., Sulaiman, S. A. S., Ahmadi, K. & Khan, T. M. Awareness of school students on sexually transmitted infections (STIs) and their sexual behavior: a cross-sectional study conducted in Pulau Pinang, Malaysia. BMC Public Health 10 , 47 (2010).
Cicciò, L. & Sera, D. Assessing the knowledge and behavior towards HIV/AIDS among youth in Northern Uganda: a cross-sectional survey. Giornale Italiano di Medicina Tropicale 15 , 29–34 (2010).
Glick, P., Randriamamonjy, J. & Sahn, D. E. Determinants of HIV knowledge and condom use among women in madagascar: an analysis using matched household and community data. Afr. Dev. Rev. 21 , 147–179 (2009).
Rahman, M. Determinants of knowledge and awareness about AIDS: Urban-rural differentials in Bangladesh. JPHE 1 , 014–021 (2009).
Siziya, S., Muula, A. S. & Rudatsikira, E. HIV and AIDS-related knowledge among women in Iraq. BMC. Res. Notes 1 , 123 (2008).
Gao, X. et al. Effectiveness of school-based education on HIV/AIDS knowledge, attitude, and behavior among secondary school students in Wuhan, China. PLoS ONE 7 , e44881 (2012).
ADS CAS PubMed PubMed Central Google Scholar
Kotecha, P. V. et al. Reproductive health awareness among urban school going adolescents in Vadodara city. Indian J. Psychiatry 54 , 344–348 (2012).
Dimbuene, T. Z. & Defo, K. B. Fostering accurate HIV/AIDS knowledge among unmarried youths in Cameroon: do family environment and peers matter?. BMC Public Health 11 , 348 (2011).
Kumar, R. et al. Knowledge attitude and perception of sex education among school going adolescents in Ambala District, Haryana, India: a cross-sectional study. J. Clin. Diagn. Res. 11 , LC01–LC04 (2017).
ADS PubMed PubMed Central Google Scholar
Tripathi, N. & Sekher, T. V. Youth in India ready for sex education? Emerging evidence from national surveys. PLoS ONE 8 , e71584 (2013).
Rosenberg, M. et al. Evidence for sample selection effect and Hawthorne effect in behavioural HIV prevention trial among young women in a rural South African community. BMJ Open 8 , e019167 (2018).
Gupta, P., Anjum, F., Bhardwaj, P., Srivastav, J. & Zaidi, Z. H. Knowledge about HIV/AIDS among secondary school students. N. Am. J. Med. Sci. 5 , 119–123 (2013).
Download references
This paper was written using data collected as part of Population Council’s UDAYA study, which is funded by the Bill and Melinda Gates Foundation and the David and Lucile Packard Foundation. No additional funds were received for the preparation of the paper.
Author information
Authors and affiliations.
Ph.D. Research Scholar, Department of Survey Research & Data Analytics, International Institute for Population Sciences, Mumbai, India
Shobhit Srivastava & Pradeep Kumar
Ph.D. Research Scholar, Department of Family and Generations, International Institute for Population Sciences, Mumbai, India
Shekhar Chauhan
Ph.D. Research Scholar, Department of Public Health and Mortality Studies, International Institute for Population Sciences, Mumbai, India
Ratna Patel
You can also search for this author in PubMed Google Scholar
Contributions
Conception and design of the study: S.S. and P.K.; analysis and/or interpretation of data: P.K. and S.S.; drafting the manuscript: S.C., and R.P.; reading and approving the manuscript: S.S., P.K., S.C. and R.P.
Corresponding author
Correspondence to Pradeep Kumar .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Srivastava, S., Chauhan, S., Patel, R. et al. A study of awareness on HIV/AIDS among adolescents: A Longitudinal Study on UDAYA data. Sci Rep 11 , 22841 (2021). https://doi.org/10.1038/s41598-021-02090-9
Download citation
Received : 05 June 2021
Accepted : 29 September 2021
Published : 24 November 2021
DOI : https://doi.org/10.1038/s41598-021-02090-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

HIV/AIDS epidemiology, pathogenesis, prevention, and treatment
The HIV-1 pandemic is a complex mix of diverse epidemics within and between countries and regions of the world, and is undoubtedly the defining public-health crisis of our time. Research has deepened our understanding of how the virus replicates, manipulates, and hides in an infected person. Although our understanding of pathogenesis and transmission dynamics has become more nuanced and prevention options have expanded, a cure or protective vaccine remains elusive. Antiretroviral treatment has transformed AIDS from an inevitably fatal condition to a chronic, manageable disease in some settings. This transformation has yet to be realised in those parts of the world that continue to bear a disproportionate burden of new HIV-1 infections and are most a% ected by increasing morbidity and mortality. This Seminar provides an update on epidemiology, pathogenesis, treatment, and prevention interventions pertinent to HIV-1.
HIV pandemic
An estimated 38·6 (33·4–46·0) million people live with HIV-1 worldwide, while about 25 million have died already. 1 In 2005 alone, there were 4·1 million new HIV-1 infections and 2·8 million AIDS deaths. 1 These estimates mask the dynamic nature of this evolving epidemic in relation to temporal changes, geographic distribution, magnitude, viral diversity, and mode of transmission. Today, there is no region of the world untouched by this pandemic ( figure 1 ). 2

HSex=heterosexual. MSM=Men who have sex with men. IDU=injection drug users. Based on Joint UNAIDS and WHO AIDS epidemic update December, 2005.
Heterosexual transmission remains the dominant mode of transmission and accounts for about 85% of all HIV-1 infections. Southern Africa remains the epicentre of the pandemic and continues to have high rates of new HIV-1 infections. 3 Although overall HIV-1 prevalence remains low in the emerging epidemics in China and India, the absolute numbers, which are fast approaching those seen in southern Africa, are of concern. 1 Outside of sub-Saharan Africa, a third of all HIV-1 infections are acquired through injecting drug use, most (an estimated 8·8 million) of which are in eastern Europe and central and southeast Asia. 1 The rapid spread of HIV-1 in these regions through injecting drug use is of importance, since it is a bridge for rapid establishment of more generalised epidemics.
A defining feature of the pandemic in the current decade is the increasing burden of HIV-1 infections in women, 4 which has additional implications for mother-to-child transmission. Women now make up about 42% of those infected worldwide; over 70% of whom live in sub-Saharan Africa. 1 Overall, a quarter of all new HIV-1 infections are in adults aged younger than 25 years. 1 HIV-1 infection rates are three to six times higher in female adolescents than in their male counterparts, 1 , 5 – 7 and this difference is attributed to sexual coupling patterns of young women with older men. Population prevalence of HIV-1 infection, concurrent sexual relationships, partner change, sexual practices, the presence of other sexually transmitted diseases, 8 – 11 and population mobility patterns 12 – 14 for economic and other reasons (eg, natural disasters and wars) further increase the probability of HIV-1 acquisition. 3 , 15 Emerging data accord with strong links between risk of sexual HIV-1 acquisition and episodic recreational drug or alcohol use. 16
Although sub-Saharan Africa continues to bear a disproportionate burden of HIV-1 infections, there is now an increasing number of countries reporting stabilisation or declines in prevalence (eg, Zambia, Tanzania, Kenya, Ghana, Rwanda, Burkina Faso, and Zimbabwe). 1 There is some evidence to attribute these reductions to effective changes in sexual behaviour, such as postponement of sexual debut, reduction in casual relationships, and more consistent condom use in casual relationships. 17 , 18 However, increasing morbidity and mortality rates associated with a maturing HIV-1 epidemic need to be considered when interpreting these data. 19 For example, the death of a few high-risk individuals who are key to transmission chains could exert a major effect on sexual networks and result in major reductions in infection rates. 20 Additionally, since most HIV-1 estimates are based on surveys in antenatal populations, increasing morbidity and mortality could cause the numbers of women in this group to decrease, and thus lead to underestimates of the true prevalence in these countries. 19
Although the relative contribution of cell-free virus compared with cell-associated virus in HIV-1 transmission remains unclear, there is growing evidence that viral load is predictive of transmission risk. 21 , 22 The highest levels of viraemia are seen during acute infection and advanced HIV-1 disease. 22 Further, co-infections with other sexually transmitted diseases in asymptomatic HIV-1 infected people can increase viral shedding to levels similar to those seen during acute infection. 23 Thus, sexually transmitted diseases could enhance HIV-1 transmission to rates similar to those seen during primary infection. 24 This observation could help to explain why the efficiency of HIV-1 transmission exceeds, in some settings, the earlier mathematical projections. 25 Thus, identification and treatment of recently infected people is an important means to reduce transmission. However, most people are unaware of their HIV-1 status during these crucial first months of infection. Several screening strategies based on laboratory testing and clinical algorithms are being developed and tested 26 for efficient identification of early infection before antibody development. 27 Additionally, a more aggressive management of sexually transmitted infections in settings with generalised epidemics has the potential to affect current epidemic trajectories. 24
Based on their genetic make-up, HIV-1 viruses are divided into three groups (eg, M [main], N, and O group, figure 2 ). These HIV-1 groups and HIV-2 probably result from distinct cross-species transmission events. 28 Pandemic HIV-1 has diversified into at least nine subtypes ( figures 1 and and2) 2 ) and many circulating recombinant forms, 29 , 30 which encode genetic structures from two or more subtypes (eg, A/E=CRF01; A/G=CRF02). The continuously evolving HIV-1 viral diversity poses an immense challenge to the development of any preventive or therapeutic intervention. 29

The HIV-1 pandemic is largely due to viral isolates belonging to the HIV-1 M-group, with HIV-1 subtype C being the most prevalent (red). Recombinant circulating forms cluster with the M-group but have been omitted for clarity. HIV-1 M group and the contemporary SIV strains identified in wild chimpanzees in Cameroon (SIVcpzLB7/EK5 28 ) are highlighted. HIV-1 sequences cluster closely with SIV from chimpanzees (SIVcpz), whereas HIV-2 resembles SIV from macaques and sooty mangabeys (SIVmac/SIVsm).
In terms of viral diversity, subtype C viruses continue to dominate and account for 55–60% of all HIV-1 infections worldwide ( figure 1 ). 30 Non-subtype B isolates might differ in their virological characteristics from the subtype B isolates (eg, viral load, chemokine co-receptor usage, transcriptional activation in specific biological compartments). 31 – 33 However, the clinical consequences of subtype variations remain unclear.
Infection with two or more genetically distinct viruses could lead to new recombinant viruses. Recombination takes place at a higher rate than initially predicted, 30 and circulating recombinant forms account for as much as 20% of infections in some regions (eg, southeast Asia). 31 These findings are in agreement with the occurrence of co-infections with multiple distinct isolates in a close temporal context. 34 – 36 Further, superinfections in which time points of virus acquisition are months to years apart have been described, although at a much lower frequency than co-infections. 34 , 37 – 39 Collectively, these observations challenge the assumption that HIV-1 acquisition happens only once with a singular viral strain and that, thereafter, the infected individual is protected from subsequent infections. 40 This lack of immunisation has substantial implications for vaccine development. Emerging evidence suggests that clinical progression to AIDS might be more rapid in individuals with dual infections, 35 and encouraging safer sex practices in viraemic HIV-1-infected people might be appropriate to keep recurrent exposure to new viral strains to a minimum.
Pathogenesis of HIV-1
The worldwide spread of HIV-1 indicates that the virus effectively counteracts innate, adapted, and intrinsic immunity. 41 , 42 Despite its modest genome size (less than 10 kb) and its few genes ( figure 3 ), HIV-1 excels in taking advantage of cellular pathways while neutralising and hiding from the different components of the immune system. 43 – 45 Notably, our understanding of pathogenesis is often derived from studies of subtype B viruses and non-human primate studies.

(A) Envelope glycoproteins gp120/41 form the spikes on the virion’s surface. During maturation the gag protein is cleaved and Gag p24 forms the core. The viral genome, viral reverse transcriptase (RT), integrase as well as a number of host proteins are encapsidated. (B) Dir erent steps of the viral life cycle on a cellular level and the potential targets for treatment interventions. (C) HIV-1 has evolved strategies to counteract the restriction factors TRIM5α and APOBEC3G/3F. If left unchecked by HIV-1 Vif, APOBEC3G/3F is encapsidated into the egressing virion, and on infection of a target cell leads to G-to-A hypermutations in the viral genome. Rhesus TRIM5α inhibits HIV-1 replication early after infection of the target cell before the step of reverse transcription.
The HIV-1 life cycle is complex ( figure 3 ) and its duration and outcome is dependent on target cell type and cell activation. 46 In the early steps, HIV-1 gains access to cells without causing immediate lethal damages but the entry process can stimulate intracellular signal cascades, which in turn might facilitate viral replication. 47 , 48 The two molecules on the HIV-1 envelope, the external glycoprotein (gp120) and the transmembrane protein (gp41), form the spikes on the virion’s surface. 49 During the entry process, gp120 attaches to the cell membrane by first binding to the CD4+ receptor. Subsequent interactions between virus and chemokine co-receptors (eg, CCR5, CXCR4) trigger irreversible conformational changes. 49 , 50 The actual fusion event takes place within minutes by pore formation, 50 , 51 and releases the viral core into the cell cytoplasm. After the core disassembles, the viral genome is reverse transcribed into DNA by the virus’ own reverse transcriptase enzyme. 46 Related yet distinct viral variants can be generated during this process since reverse transcriptase is error prone and has no proofreading activity. 46 At the midpoint of infection, the viral protein integrase in conjunction with host DNA repair enzymes inserts the viral genome into gene-rich, transcriptionally active domains of the host’s chromosomal DNA. 52 – 54 An integrase binding host factor, LEDGF/p75 (lens epithelium-derived growth factor), facilitates integration, 55 , 56 which marks the turning point by irreversibly transforming the cell into a potential virus producer. In the late steps, production of viral particles needs host driven as well as virus driven transcription. 46 Viral proteins are transported to and assemble in proximity to the cell membrane. Virus egress from the cell is not lytic and takes advantage of the vesicular sorting pathway (ESCRT-I, II, III), which normally mediates the budding of endosomes into multivesicular bodies. 57 , 58 HIV-1 accesses this protein-sorting pathway by binding TSG101 via its late domain, a short sequence motif in p6 of Gag. 59 , 60 Cleavage of the Gag-Pol poly-protein by the viral protease produces mature infectious virions. 46 , 61
Since cytoplasmic molecules of the producer cell and components from its cell surface lipid bilayer are incorporated into the new viral particle, virions bear characteristics of the cells in which they were produced. 62 Incorporated host molecules can determine the virus’ phenotype in diverse ways (eg, shape the replicative features in the next cycle of infection or mediate immune activation of bystander cells 62 ).
Studies of the early events that happen after HIV-1 breaches the mucosal barrier suggest the existence of a window period in which viral propagation is not yet established and host defences could potentially control viral expansion. 63 The important co-receptors for HIV-1 infection are two chemokine receptors—CCR5 and CXCR4. Independently of the transmission route, most new infections are established by viral variants that rely on CCR5 usage. 64 CXCR4-tropic viruses generally appear in late stages of infection and have been associated with increased pathogenicity and disease progression. 65
Compelling evidence from non-human primate models (eg, simian immunodeficiency virus [SIV] infection of rhesus macaques) suggest that vaginal transmission results in infection of a small number of CD4+ T lymphocytes, macrophages, and dendritic cells located in the lamina propria. 63 Potential pathways for virus transmission involve endocytosis, transcytosis, and virus attachment to mannose C-type lectin receptors (eg, DC-SIGN) located on dendritic cells and macrophages. 66 The initial replication takes place in the regional lymph organs (eg, draining lymph nodes) and is composed of few viral variants, and leads to modest primary amplification. With migration of infected T lymphocytes or virions into the bloodstream, secondary amplification in the gastrointestinal tract, spleen, and bone marrow results in massive infection of susceptible cells. In close temporal relation with the resulting peak of viraemia (eg, 10 6 to 10 7 copies per mL plasma), clinical symptoms can be manifest during primary HIV-1 infection ( figure 4 ). The level of viraemia characteristic for the chronic phase of infection in an individual (viral set point) differs from the peak viraemia by one or two orders of magnitude. This reduction is largely attributed to HIV-1 specific CD8+ responses but target cell limitation could also play a part. The viral population is most homogeneous early after transmission, but as viral quasi-species diversify in distinct biological compartments, mutant viruses that are resistant to antibody neutralisation, cytotoxic T cells, or antiretroviral drugs are generated and archived in long-lived cells (ie, viral reservoirs).

Plasma viraemia (top), and dynamic changes of the CD4+ T-lymphocyte compartments (bottom). Primary infection characterised by high plasma viraemia (red line, top), low CD4 cells (green line, bottom), and absence of HIV-1 specific antibodies (orange line, bottom). Viraemia drops as cytotoxic CD8+ T-lymphocytes (CTL) develop (blue line, bottom) and an individual viral-load set point is reached during chronic infection. Viral set points dir er greatly among individuals (eg, red dotted line, top) and predict disease progression. Viral diversity increases through out the disease (closed circles, top). The risk of transmission is highest in the first weeks when viraemia peaks (closed circles, top). GALT=gut-associated lymphoid tissues.
A pronounced depletion of activated as well as memory CD4+ T cells located in the gut-associated lymphoid tissues has been seen in individuals identified early after infection. 67 The preferential depletion of the CD4+ cells in the mucosal lymphoid tissues remains despite years of antiretroviral treatment, a striking observation that contrasts with the fact that the number of CD4+ T lymphocytes in the peripheral blood can return to normal under antiretroviral treatment.
A gradual destruction of the naive and memory CD4+ T-lymphocyte populations is the hallmark of HIV-1 infection, with AIDS being the last disease stage ( figure 4 ). 68 Despite the frequent absence of symptoms during early and chronic phase, HIV-1 replication is dynamic throughout the disease. The half-life of a single virion is so short that half the entire plasma virus population is replaced in less than 30 minutes, 69 and the total number of virions produced in a chronically infected person can reach more than 10¹P particles per day. 69 , 70 The turnover rates of lymphocyte populations are upregulated many fold during HIV-1 infection, whereas cell proliferation decreases once viral replication is reduced by antiretroviral treatment. 71 , 72 Different depletion mechanisms have been proposed, with an emerging consensus favouring generalised immune activation as cause for constant depletion of the CD4+ cell reservoir. 73
Immune activation predicts disease progression 74 and, thus, seems to be a central feature of pathogenic HIV-1 infections. Recently, Nef proteins from SIV lineages that are non-pathogenic in their natural hosts (eg, African green monkeys) have proved to down-regulate CD3-T-cell receptors, resulting in reduced cell activation and apoptosis. 75 HIV-1 Nef fails to quench T-cell activation, possibly leading to the high degree of immune activation seen in infected people.
Understanding the mechanisms that lead to protection or long-lasting control of infection will guide vaccine development by providing correlates of protection. Natural resistance to HIV-1 infection is rare and varies greatly between individuals. Two groups—long-term non-progressors and highly exposed persistently seronegative individuals—have been studied widely to identify innate and acquired protective determinants ( table 1 ). 76 Host resistance factors consist of human leucocyte antigen (HLA) haplotypes, autoantibodies, mutations in the promoter regions, and coding regions of the co-receptors CCR5 and CCR2, as well as the up-regulation of chemokine production ( table 1 ). 76 , 77 Indeed, individuals encoding a truncated CCR5 version (CCR5Δ32) have slower disease progression (heterozygote) or are resistant to CCR5-using viruses (homozygote). 78 The CCL3L1 gene encodes MIP1α, a CCR5 co-receptor ligand and chemokine with antiviral activity. Recent findings show that CCL3L1 gene copies vary individually and higher numbers of gene duplications result in reduced susceptibility to infection, 77 , 79 possibly by competitive saturation of CCR5 co-receptor. Cytotoxic T-lymphocyte responses, helper T-cell functions, and humoral responses are some of the acquired factors that modulate the risk of transmission in highly exposed persistently seronegative individuals, 76 and could also contribute to spontaneous control of replication in long-term non-progressors. The putative protective role of cytotoxic T-lymphocyte activity has been suggested in seronegative sex workers and in some long-term non-progressors. 76 , 80
Some of the host factors affecting susceptibility to HIV-1 infection
| Innate | Genetic | Acquired | Intrinsic |
|---|---|---|---|
| Autoantibodies | HLA haplotype | Cytotoxic T-cell activity | APOBEC3G/3F |
| Chemokines | CCR5 gene/promoter | Helper T-cell function | TRIM5α |
| Cytokines | CCR2 gene/promoter | Neutralising antibodies | |
| CCL3L1 gene copy number |
HLA=human leucocyte antigen. CCR5=chemokine receptor 5. CCR2=chemokine receptor 2. CCL3L1=CC chemokine ligand like-1, APOBEC3G/3F=apolipoprotein B mRNA editing complex 3.
Mammalian cells are not welcoming micro-environments, but rather deploy a defensive web to curb endogenous and exogenous viruses. HIV-1’s ability to circumvent these defences is as impressive as its efficiency to exploit the cellular machinery. APOBEC3G/3F and TRIM5α are recently described intrinsic restriction factors that are constitutively expressed in many cells. 81 , 82 Both gene loci have been under strong selective pressure throughout primate evolution, 83 indicating an ancient need to neutralise foreign DNA and maintain genome stability that precedes the current HIV-1 pandemic.
APOBEC3 enzymes (A3) belong to the superfamily of cytidine deaminases, 84 a group of intracellular proteins with DNA/RNA editing activity. 84 , 85 Most representatives of the APOBEC3 group have some mutagenic potential and restrict endogenous retroviruses and mobile genetic elements. The deaminases A3G, A3F, and A3B have potent antiviral activity, with the first two being expressed in cells that are susceptible to HIV-1 infection (T-lymphocytes, macrophages). HIV-1 evades APOBEC3 mutagenesis by expressing Vif, which leads to APOBEC3G/3F but not A3B degradation. 42 , 86 – 90
We still need to establish how the mechanisms of DNA editing and antiviral activity are interwoven, since some antiviral activity can be maintained despite defective DNA editing. 91 The early replication block in non-stimulated CD4+ T cells has been attributed to low molecular mass complexes of APOBEC3G. 92 Hypermutated genomes in HIV-1 infected patients 93 and mutations in Vif resulting in abrogated or differential APOBEC3 neutralisation capacity have been described. 94 , 95 The degree to which APOBEC3G/3F mRNA expression predicts clinical progression remains an area of intensive investigation. 96 , 97
Several representatives of the heterogeneous family of tripartite motif proteins (TRIM) inhibit retroviruses in a species-specific manner. 81 , 98 TRIM5α from rhesus macaques and African green monkeys inhibit HIV-1 replication, whereas the human homologue is inactive against SIV and HIV-1, leading to the recorded susceptibility of human cells to both viruses. 81 Rhesus TRIM5α recognises the capsid domain of HIV-1 Gag and manipulates the kinetics of HIV-1 core disassembly within minutes after cell entry. 99 , 100 Thus, experimental approaches to render HIV-1 resistant to rhesus TRIM5α could lead to immunodeficiency viruses capable of replicating in rhesus macaques. Such a non-primate model would allow testing of antiviral treatment and vaccine interventions with HIV-1 viruses instead of SIV or SIV/HIV chimeric viruses.
Clinical management
The diagnosis of HIV-1 infection is based on the detection of specific antibodies, antigens, or both, and many commercial kits are available. Serological tests are generally used for screening. A major advance has been the availability of rapid HIV-1 antibody tests. These assays are easy to do and provide results in as little as 20 minutes, 101 enabling specimen collection and proper diagnosis at the same visit. Rapid tests are important tools for surveillance, screening, and diagnosis, and can be reliably done on plasma, serum, whole blood, or saliva by health-care providers with little laboratory expertise. The two limitations of these serological tests are detection of infection during primary infection when antibodies are absent, and in infants younger than 18 months who might bear maternal HIV-1 antibodies. In these instances direct virus detection is the only option (eg, quantification of viral RNA [standard] or p24 antigen in heat denatured serum [less expensive]).
For staging purposes, measurement of CD4+ cells and viraemia is required. Plasma viral load is widely used to monitor therapeutic success on antiretroviral treatment. Several commercially available tests provide sensitive quantification of plasma HIV-1 RNA copies. The newer versions of the Amplicor and Quantiplex (Roche, Indianapolis, IN, USA, and Bayer Diagnostics, Walpole, MA, USA, respectively) assays have overcome initial suboptimum performance for non-B subtypes. 102 While the viral load determines the rate of destruction of the immune system, the number of CD4+ cells reveals the degree of immunodeficiency and is, therefore, used to assess the stage of infection. CD4+ cell counts together with clinical manifestations (eg, occurrence of opportunistic infections) are key criteria for HIV-1 disease classification. Flow cytometry analysis is the standard method for CD4+ cells quantification.
Standard methods for quantifying viral load and CD4+ cell counts need advanced laboratory infrastructures, and assays require a specimen to be tested within a short time of collection. These requirements pose challenges for resource-constrained settings. The use of dried blood spot specimen has resolved some of the difficulties associated with transportation of samples needed for virological assessments. 103 Measurement of reverse transcriptase activity in plasma samples, simplification of gene amplification methods (eg, Taqman technology), and paper-strip quantification (dipstick assays) might provide cost-effective alternatives for the future. 104 – 106 Similarly microcapilliary flow-based systems, CD4+ chips, or total white counts (panleucocyte gating) provide alternatives for establishment of the level of immunodeficiency in resource-limited settings. 107 – 110
Drug treatment
Antiretroviral compounds.
Antiretroviral treatment is the best option for longlasting viral suppression and, subsequently, for reduction of morbidity and mortality. However, current drugs do not eradicate HIV-1 infection and lifelong treatment might be needed.
20 of the 21 antiretroviral drugs currently approved by the US Food and Drug Administration target the viral reverse transcriptase or protease ( table 2 ). Eight nucleoside/nucleotide analogues and three non-nucleoside reverse transcriptase inhibitors inhibit viral replication after cell entry but before integration. Fixed-dose combination tablets simplify treatment regimens by reducing the daily pill burden, and drugs with long half-lives allow once or twice daily dosing. Eight protease inhibitors prevent the maturation of virions resulting in production of non-infectious particles. The recently approved darunavir (June, 2006) is the first of its class that retains activity against viruses with reduced susceptibility to protease inhibitors. Enfuvirtide targets a gp41 region of the viral envelope and stops the fusion process before the cell is infected. This drug needs to be injected twice daily and its use is reserved for treatment of heavily drug-experienced patients since it can help overcome existing drug resistance. 111 , 112 Development of new antiretrovirals focuses on molecules that target entry, reverse transcription, integration, or maturation. Compounds that have been designed to inhibit resistant viruses are urgently needed since many patients treated during the past decades harbour viral strains with reduced susceptibilities to many if not all available drugs ( table 3 ).
Antiretroviral drugs currently approved by US Food and Drug Administration
| Entry | Reverse transcriptase | Protease | |||
|---|---|---|---|---|---|
| Nucleoside | Nucleotide | Non-nucleoside | |||
| Single compound tablets | Enfuvirtide | Abacavir | Tenofovir | Delaviridine | (Fos)-Amprenavir |
| Didanosine | Efavirenz | Atazanavir | |||
| Emtricitabine | Nevirapine | Darunavir | |||
| Lamivudine | Indinavir | ||||
| Stavudine | Nelfinavir | ||||
| Zalcitabine | Ritonavir | ||||
| Zidovudine | Saquinavir | ||||
| Tripanavir | |||||
| Fixed-dose combination tablets | Abacavir/lamivudine (Epzicom) | Lopinavir/ritonavir | |||
| Zidovudine/lamivudine (Combivir) | |||||
| Tenofovir/emtricitabine (Truvada) | |||||
| Abacavir/lamivudine/zidovudine (Trizavir) | |||||
| Tenofovir/emtricitabine/efavirenz (Atripla) | |||||
Drugs belong to five drug classes and target three dir erent viral steps (entry, reverse transcription, or protease). Availability of these drugs in resource-limited countries is subject to country specific licensing agreements.
Antiretrovirals currently in phase II/III of clinical development
| Drug | Mechanism | Activity against PI and RT resistant strains | |
|---|---|---|---|
| Maraviroc | MVC | CCR5 inhibitor | Yes, but not X4 variants |
| Vicriviroc | SCH D | Yes, but not X4 variants | |
| Etravirine | TMC-125 | Non-nucleoside reverse transcriptase inhibitor | Yes, also NNRTI-resistant strains |
| TMC-278 | Yes, also NNRTI-resistant strains | ||
| n/a | MK-0518 | Integrase strand transfer inhibitor | Yes |
| n/a | GS-9137 | Yes |
The goal of antiretroviral treatment is to decrease the morbidity and mortality that is generally associated with HIV-1 infection. A combination of three or more active drugs is needed to achieve this aim in most patients. Effective treatment returns to near normal the turnover rates of both CD4+ and CD8+ T-cell populations. 72 Potent but well tolerated drugs with long half-lives and simplified regimens improve the options for first-line and second-line chemotherapeutic interventions.
Combination antiretroviral treatment
High rate of viral replication, low fidelity of reverse transcription, and the ability to recombine are the viral characteristics that lead to the diversity of HIV-1 species (quasi-species) in chronically infected individuals. This high genetic variability provided the rationale for highly active antiretroviral treatments (HAART). By combination of several potent antiretroviral agents, viral replication is suppressed to such low levels that emergence of drug resistant HIV-1 variants was, if not prevented, at least delayed. By doing so, CD4+ T-lymphocyte numbers increase, leading to a degree of immune reconstitution that is sufficient to reverse clinically apparent immunodeficiency. Widespread introduction of HAART in industrialised countries resulted in a striking decrease in morbidity and mortality, putting forward the hope that HIV-1 infection can be transformed into a treatable chronic disease. 113 – 115
A set of criteria composed of plasma viraemia concentration, absolute or relative CD4+ cell counts, and clinical manifestations, is used to recommend initiation of HAART. The benefits of treatment clearly outweigh the potential side-effects in patients with clinical signs of immunodeficiency (eg, AIDS defining illnesses) or with CD4+ numbers less than 200 per μL (recommendation of US Department of Health and Human Services, October, 2005). However, the best time point to begin treatment remains controversial in asymptomatic patients with modest depletion of CD4+ T cells (eg, more than 350 per μL) and modest levels of viraemia (eg, less than 100 000 copies per mL). 116 Studies with clinical endpoints supporting the validity of early versus late interventions in asymptomatic patients are difficult to do and insufficient clinical data are currently available. Early depletion of gut CD4+ T lymphocytes, 117 increasing viral diversity, and the poor regenerative abilities of key populations of the immune system provide arguments for beginning treatment as early as possible. The wide application of this principle is restricted by long-term drug toxicities that lead to reduction of quality of life, and by treatment costs. Toxicities (eg, renal, hepato, mitochondrial), metabolic changes (eg, lipodystrophy, diabetes mellitus), and immune reconstitution disease are some of the long-term problems that complicate decade-long HAART. 118 – 121
One strategy addressing life-long daily compliance to HAART has been structured treatment interruptions. The rationale for this approach was based on the premise that the body’s own immune system could keep the virus in check if exposed to a very modest level of viral replication. If successful, this strategy could limit drug toxicity and reduce treatment costs. 122 Although preliminary findings for this strategy were mixed in terms of benefits, 123 – 125 the recent early closure of the SMART trial was based on increased morbidity and mortality in the treatment interruption arm. 126 Thus, in the absence of clinical benefits, most investigators strongly discourage treatment interruptions except as needed to address treatment intolerance.
HAART in resource-constrained settings
The transformation of AIDS into a chronic disease in industrialised countries has yet to be realised in resource-constrained settings. Access to HAART is an absolute humanitarian necessity to avert mortality in people who are central to the future survival of their countries. 127 Despite restricted health infrastructures and diverse co-morbidities in these regions, remarkable therapeutic success rates have been shown, with adherence rates at least comparable with those reported in industrialised countries. 128 – 131 WHO and UNAIDS treatment guidelines focusing on resource-limited settings suggest use of standard first-line regimen followed by a set of more expensive second-line options 132 and proposes the use of standardised decision-making steps (eg, when to start, to substitute for side-effects, to switch for virological failure). 132 , 133 In many countries, treatment options are limited not only by the costs of HAART but also by restrictive licensing policies, and current estimates suggest that 80% of people infected with HIV-1 with a clinical need for treatment do not yet have access to antiretroviral drugs. 1 Thus, efforts and strategies to further scale up treatment access are crucial, 134 – 137 since antiretroviral treatment is also an effective intervention for prevention. 138
Drug resistance
Emergence of drug resistance is the most common reason for treatment failure. Insufficient compliance, drug side-effects, or drug-drug interactions can lead to suboptimum drug concentrations, resulting in viral rebound. Viral resistance has been described to every antiretroviral drug and therefore poses a serious clinical as well as public-health problem. 139 HIV-1 subtypes differ in the sequence of mutations leading to drug resistance, and some naturally occurring polymorphisms might actually modulate resistance. 140 , 141 Drug-resistant HIV-1 is transmissible and can be detected in up to 20% of newly infected individuals in countries with broad access to antiretrovirals. 34 The prevalence of drug resistance in the untreated population remains low in regions with poor access to treatment. 142
Short-term antiretroviral-based interventions are effective in prevention of mother-to-child transmission. However, these interventions could result in drug resistant viral variants in the mother, baby, or both. 143 Around half the women who received one dose of nevirapine to prevent mother-to-child transmission harbour viruses resistant to non-nucleoside reverse transcriptase inhibitors (NNRTI). 144 , 145 These resistant viruses replicate efficiently and can be transmitted by breast milk, 146 and minor resistant populations present long after the intervention can possibly decrease the effectiveness of subsequent NNRTI-based treatment regimens. 147 The combination of short-course zidovudine, lamivudine, and nevirapine prevents peripartum transmission while reducing the risk of nevirapine resistant viruses. 148
Viral reservoirs
Viral reservoirs consist of anatomical sanctuaries and a small pool of infected long-lived memory T lymphocytes. HIV-1 latency in long-lived cell populations (eg, memory T lymphocytes, macrophages) poses an obstacle to eradication because current antiviral combination treatments fail to eliminate integrated proviruses from resting cells. Different strategies, including immune-modulatory molecules (interleukin 2, anti-CD3 mAb, interleukin 7), have been used to reactivate resting cells in the setting of HAART. Histone deacetylase-1 inhibitors, like valproic acid, release an inherent transcriptional block and by doing so facilitate viral long terminal repeat-driven expression. 149 Augmenting standard antiretroviral treatment with enfuviridine and valproic acid reduced the number of latently infected CD4+ T cells (29–84%), but to establish the relative contribution of each drug with respect to the final outcome is difficult. 150
Mother-to-child transmission
Prevention of mother-to-child transmission has seen advances in both industrialised and resource-constrained settings. 151 – 153 Intrapartum transmission has been reduced by increasing access to interventions such as one dose of nevirapine to mother and newborn baby. 154 Concerns about drug-resistant viral strains have led to several trials with combination treatments to reduce transmission during the intrapartum period. 148 , 152 , 155 In some settings, elective delivery by caesarean section can further reduce HIV-1 transmission during the intrapartum period, but the benefits of the intervention could be countered by post-partum sepsis and increasing maternal mortality. 156
Because HIV-1 can be transmitted by breastfeeding, replacement feeding is recommended in many settings. Poor access to clean running water precludes, however, the use of formula feeding under these circumstances, 157 and exclusive breastfeeding with abrupt weaning is one option for reducing transmission. 158 A potential novel intervention still being tested is the daily use of antiretrovirals during breastfeeding. More attention is starting to focus on the pregnant mother, especially initiation of antiretroviral therapy in mothers with low CD4+ counts during pregnancy and thereafter. 159 , 160 Only limited data are available regarding the health of uninfected children born to HIV-1-positive mothers. 161 In a European cohort of exposed-uninfected children, no serious clinical manifestations were apparent, at least in the short term to medium term (median follow-up 2 years). 162
Sexual transmission
Reduction of heterosexual transmission is crucial for control of the epidemic in many parts of the world. 1 , 163 Prevention is achieved through reduction in the number of discordant sexual acts or reduction of the probability of HIV-1 transmission in discordant sexual acts. The first can be achieved through abstinence and sex between concordantly seronegative individuals. Abstinence and lifelong monogamous relationships might not be adequate solutions for many people and therefore several interventions aimed at lowering the risk of transmission per discordant sexual act are in the process of clinical testing. Male and female condoms provide a proven and affordable prevention option. 164 , 165 In combination, these options are also more commonly referred to as the ABC (abstinence, be faithful, condom use) approach.
Other biomedical prevention interventions include male circumcision, antiretrovirals for prevention (eg, pre-exposure or post-exposure), chemoprophylactic treatment of herpes simplex virus-2 (HSV-2), microbicides, and vaccines. Results from one of three independent phase III male circumcision trials underway in South Africa, Kenya, and Uganda has helped to allay some of the ambivalence around the protective effect of male circumcision. 8 , 166 The findings from the South African trial show a 60% protective effect of male circumcision. 167 The possible mechanism relates to the fact that the foreskin has apocrine glands that secrete lysozymes but also Langerhans cells expressing CD4 and other receptors. 168 , 169 These skin-specific dendritic cells can uptake virus and are believed to play a part in transport of the virus to susceptible T cells. Immunofluorescence studies of foreskin mucosa suggest that these tissues might be more susceptible to HIV-1 infection than cervical mucosa. 170 Findings from this proof-of-concept trial need to be compared with evidence from the two trials still underway in Kenya and Uganda, and to acceptability data, behaviour change after circumcision, surgical complication rates, and logistics of undertaking the procedures before policy formulation and wide-scale access as a prevention strategy. 171 – 173
Since high plasma viraemia increases the risk of transmission by as much as an order of magnitude, 21 does reducing viral load in the infected partner through, for example, antiretroviral treatment reduce the risk of HIV-1 transmission in the uninfected sexual partner? A trial to explore this question is currently being run jointly by the HIV Prevention Trials Network ( www.hptn.org ) and the Adult Clinical Trials Group. Mathematical projections estimate up to 80% HIV-1 reduction, 174 , 175 but scarce observational data currently exist. 176 Post-exposure prophylaxis is recommended after occupational (eg, needle stick) 177 and non-occupational (eg, rape, sexual abuse) 178 exposure, although data for efficacy and optimum drug combinations are few. 179 Some clinical trials assessing the benefits of once daily pre-exposure chemoprophylaxis with antiretroviral compounds with long biological half-life (eg, tenofovir) have been put on hold or stopped. 175 , 180 Neither the overall idea of pre-exposure prophylaxis nor the drug itself, which is well tolerated, was at the root of the protests. Concerns were centred on clinical trials in resource-poor settings and the perceived scarcity of adequate interventions protecting these vulnerable populations.
HSV-2 might increase both the risk of transmitting and acquiring HIV-1. 181 , 182 Antivirals (eg, aciclovir, valaciclovir) are effective in reducing viral shedding 183 – 185 and HSV-2 transmission in discordant heterosexual couples. 182 The future of HSV-2 prevention might reside in the vaccine that is currently under development. 186 Whether prophylactic use of aciclovir in populations with high HSV-2 prevalence and incidence rates results in reduced HIV-1 incidence rates remains unresolved but several trials addressing this issue are underway, including HPTN039.
Gender disparities lie at the centre of women’s vulnerability. Prevention options need to be provided that can be used by women independently of their male sexual partner’s knowledge or consent. 187 Notwithstanding that redressing these disparities is a long-term challenge, several preventive interventions can be implemented in the interim on the basis of our incomplete understanding at a biological level of HIV-1 risk for women. For example, there seems to be a correlation between levels of sexual hormones (eg, progesterone) and transmission risk. 188 Observational studies also highlight the relation between abnormal vaginal flora and increased risk of HIV-1 infection. 189 , 190 The high prevalence of vaginal infections such as bacterial vaginosis (30–50%), vulvovaginal candidosis (10–13%), and trichomonas vaginalis (7–23%) in African women is associated with a substantial risk of HIV-1 acquisition. 189 In addition to increasing access to female condoms and treatment of other sexually transmitted infections, trials are underway to assess the use of other barrier methods such as cervical caps, invisible condoms, diaphragms, and diaphragms combined with micro bicides. 190 The control of vaginal infections is a potentially important method for decreasing HIV-1 acquisition that has yet to be tested. Periodic presumptive treatment for vaginal infections is being explored as an HIV-1 prevention strategy. 191
Microbicides
Microbicides are an additional important biomedical intervention technology that is covert and under women’s control. 192 These topical products potentially could be used to prevent rectal and vaginal transmission of HIV-1, but proof of concept has been elusive. Although the three phase III trial results of the first microbicidal product (nonoxynol-9) done in the mid-1980s and 1990s did not show protective effects, 193 , 194 they have informed the medical knowledge in terms of product selection, clinical testing, and safety assessments. The past 5 years have seen major advances in investment and product development. 66 , 195 , 197 Early clinical testing of multiple products including the launch of advanced clinical trials for five different products is continuing ( table 4 ). The development of antiretroviral gels increased the specificity of these third generation microbicides in relation to surfactants, vaginal enhancers, or entry inhibitors that have dominated the product pipeline so far ( figure 5 ). The first antiretroviral gel to undergo early testing is tenofovir gel, and the findings in terms of safety profile, tolerance, low systemic absorption, and slight adverse events are promising. 192 As with vaccines, a major obstacle is the absence of a surrogate marker of protection. Additional challenges are adherence to product use and the high rates of pregnancy in trial participants.

N9=nonoxonol-9. CS=cellulose sulphate.
Summary of microbicides currently undergoing advanced clinical testing
| Phase | Mode of action | Effective against | |
|---|---|---|---|
| Carraguard | III | Seaweed polymer, entry inhibitor | HIV-1/HIV-2, human papilloma virus, herpes simplex virus |
| Cellulose sulphate | III | Entry inhibitor | Broad range of sexually transmitted diseases; also active as contraceptive |
| PRO2000 | III | Entry inhibitor | HIV-1/HIV-2, human papillomavirus, chlamydia |
| Savvy (C31G) | III | Surfactant | Broad range of sexually transmitted diseases; also active as contraceptive |
| Tenofovir gel | IIb | Nucleotide reverse transcriptase inhibitor | HIV-1/HIV-2 |
| Buffer gel+PRO2000 | IIb/III | Natural vaginal defence/entry inhibitor | HIV-1/HIV-2, chlamydia, gonorrhoea, human papillomavirus, bacterial vaginosis |
| Tenofovir gel+oral | II | Nucleotide reverse transcriptase inhibitor | HIV-1/HIV-2 |
A safe, protective, and inexpensive vaccine would be the most efficient and possibly the only way to curb the HIV pandemic. 198 Despite intensive research, development of such a candidate vaccine remains elusive. Safety concerns prohibit the use of live-attenuated virus as immunogen. 199 Many different approaches with recombinant technologies have been pursued over the past two decades. Initially, efforts were focused on generating neutralising antibodies with recombinant monomeric envelope gp120 (AIDSVAX) as immunogen. 200 , 201 This vaccine did not induce neutralising antibodies and, not unexpectedly, the phase III trials failed to show protection. 202 , 203 Antibody mediated HIV-1 neutralisation is complicated by the high genetic diversity of the variable Env regions, epitopes masked by a carbohydrate shield (glycosylation), and conformational or energetic constraints. 204 Since CD8 T-cell responses control to some extent viral replication in vivo, recent vaccine development has focused on eliciting cellular immune responses. Overcoming pre-existing immunity against replication incompetent immunogenic vectors (eg, recombinant adenovirus type 5) is one of the challenges. 205 Safety and immunogenicity studies using replication defective vaccine vectors are continuing after preliminary studies in non-human primates showed some protection. 204 The immune system generally fails to spontaneously clear HIV-1 and the true correlates of protection continue to be ill defined. 198 , 206 However, the general belief is that approaches aimed at eliciting both humoral and cell mediated immunity are most promising to prevent or at least control retroviral infection. 198
Conclusions
An important gateway to both prevention and care is knowledge of HIV-1 status. 207 Fear of knowledge of status, including stigma and discrimination, has discouraged many from seeking voluntary counselling and testing services. 208 As access to antiretroviral interventions (prevention of mother-to-child transmission, antiretroviral treatment) increases, the opportunities for HIV-1 testing will grow and create opportunities for a prevention-care continuum, with the voluntary counselling and testing services as a point of entry. These changes will result in a shift in prevention efforts from a focus on individuals not infected with HIV-1 to a more effective continuum of prevention that includes uninfected, recently infected, infected, and asymptomatic people, as well as those with advancing HIV disease and on antiretroviral therapy.
HIV/AIDS is an exceptional epidemic that demands an exceptional response. Much progress has been made in a short space of time, despite many scientific and programmatic challenges ( figure 6 ). In the absence of a protective vaccine or a cure, prevention and access to antiretroviral treatments are the best options to slow down the HIV-1 pandemic. Broad implementation of these principles needs improved infrastructures in resource-constrained regions, which have been and will continue to be most affected. The fact that HIV-1 is predominantly sexually transmitted and disproportionately affects populations that are already socially or economically marginalised, or both, poses many ethical, social, economic, and political challenges.

Estimates place the cross species transmission events leading to the worldwide spread of HIV-1 to the early decades of the 20th century. Numbers circled by a hexagon identify the specific year of an event. PEPFAR=President’s Emergency Plan for AIDS Relief.
In view of the immediacy of the problem, and the fact that both research and programmes are mainly funded by the public sector, there is a greater demand from civil society for co-ownership of research and accountability for use of public funds. On the one hand, this co-ownership defines a changing role and responsibility of science in society, and on the other hand, shows a necessary synergy between activism and science. This partnership has been invaluable for antiretroviral drug development, treatment access in resource-constrained settings, and the scale-up of interventions to reduce mother-to-child transmission.
The increasing number of infected women and the disproportionate burden of infection in resource-constrained settings creates a scientific imperative to ensure research is done for people and in settings who stand to benefit most. The most affected countries face many other economic, political, and development challenges, which have raised issues in undertaking multicentre and multicountry research. Research addressing women-specific topics (such as effect of sexual hormones on transmission and disease progression, viral diversity, and antiretroviral potency) and women-specific prevention interventions including microbicides is crucial. We are probably at one of the most hopeful and optimistic points in our response to the pandemic. There is definitely more attention being directed to HIV-1, more resources ( panel ), more civil society mobilisation, more governments speaking up, more possibilities for treatment, and more evidence about what prevention and treatment strategies will work than in previous years. The unrelenting growth of the pandemic tells us that current strategies are not enough. Clearly, we need to do some things differently, while also increasing the scale and magnitude of current strategies in keeping with the pandemic.
PanelOnline resources
Epidemiology.
http://www.unaids.org/en/HIV_data/default.asp
Treatment recommendations
Centers for Disease Control and Prevention
http://www.cdc.gov/hiv/topics/treatment/index.htm
HIV-1 drug resistance
Stanford University HIV Drug Resistance Database
http://hivdb.stanford.edu/index.html
International AIDS Society–USA
http://www.iasusa.org/resistance_mutations/index.html
Microbicide
Alliance for Microbicide Development
http://www.microbicide.org
HIV Prevention Trials Network
http://www.hptn.org/index.htm
International AIDS Vaccine Initiative
http://www.iavi.org
Search strategy and selection criteria
A comprehensive literature review was undertaken by searching the PubMed database online, for English language publications between January, 2000, and June, 2006. The database search terms included keywords such as “HIV/AIDS”, “epidemiology”, “prevention”, “pathogenesis”, “HSV-2”, “male circumcision”, “PMTCT”, “scaling up treatment”, “resource constrained settings”, “antiretroviral pre-exposure prophylaxis”, “HAART”, “restriction”, “host factor”, “HIV pathogenesis”, “resistance”, “latency”. Various combinations of these words were entered. All duplicate articles were removed. A subset of relevant articles was chosen and full-text manuscripts were summarised.
Acknowledgments
We thank P D Bieniasz, W Cates, L Chakrabarti, C Cheng-Mayer, J Coovadia, H Gayle, P A Fryd, R Gray, S Abdool Karim, L Kuhn, K Mayer, P Mane, L C F Mulder, L Myer, and M Wawer for helpful discussions. M Boettiger and C Baxter assisted with literature searches. This work was supported by NIH grant RO1AI064001 (VS), by grant 1 U19AI51794 (QAK) from CAPRISA that forms part of the Comprehensive International Program of Research on AIDS (CIPRA) funded by the National Institute of Allergy and infectious Disease (NIAID), National Institutes of Health (NIH) and the US Department of Health and Human Services (DHHS) and grant D43 TW00231 (QAK) from the Columbia University-Southern African Fogarty AIDS International Training and Research Program.
Conflict of interest statement
D D Ho sits on the scientific advisory boards for Monogram, Osel, Achillion, Valiant, Oyagen, Lavipharm, and XTL. Products or work from these companies are not discussed in the review. He holds patents on vaccine candidates. The other authors declare no conflict of interest.
- Subscribe to journal Subscribe
- Get new issue alerts Get alerts
- Submit your manuscript
Secondary Logo
Journal logo, current issue.

October 01, 2024 - Volume 38 - Issue 12
- Editor-in-Chief: Michael S. Saag Editors: Roel A. Coutinho Mathias D. Lichterfeld Sarah L. Rowland-Jones
- ISSN: 0269-9370
- Online ISSN: 1473-5571
- Frequency: 15 / year
- Ranking: 21 of 41 Virology 47 of 132 Infectious Diseases 93 of 181 Immunology
- Impact Factor: 3.4
Featured Supplement

Open Access Special Issue

December 15, 2021 - Volume 35 - Supplement 2
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Current Issue Highlights
- CLINICAL SCIENCE

Choice of antiretroviral therapy has low impact on weight gain
AIDS. 38(12):1731-1739, October 01, 2024.
- Abstract Abstract
- Permissions
CONCISE COMMUNICATION

Efficacy and safety of tesamorelin in people with HIV on integrase inhibitors
AIDS. 38(12):1758-1764, October 01, 2024.
- EPIDEMIOLOGY AND SOCIAL

Accuracy of nine-item Patient Health Questionnaire against psychiatric diagnosis for depression among people with HIV
AIDS. 38(12):1765-1773, October 01, 2024.
Go to Full Text of this Article
- EDITORIAL COMMENTS
Deconvoluting the contribution of antiretroviral choice in weight gain
AIDS. 38(12):1794-1795, October 01, 2024.
- RESEARCH LETTERS

Increasing HIV prevalence rate among men who have sex with men: results of a comparison of two national surveys
AIDS. 38(12):1799-1801, October 01, 2024.
- Correspondence
Impact of COVID-19 vaccination on HIV parameters in people with HIV on antiretroviral therapy
AIDS. 38(12):1807-1808, October 01, 2024.
- CLINICAL SCIENCE: CONCISE COMMUNICATION
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Research Progress in the Epidemiology of HIV/AIDS in China
Affiliation.
- 1 Department of Epidemiology, School of Public Health, and The Key Laboratory of Public Health Safety of Ministry of Education; Shanghai Institute of Infectious Diseases and Biosecurity; and Yiwu Research Institute of Fudan University, Fudan University, Shanghai, China.
- PMID: 34888119
- PMCID: PMC8633551
- DOI: 10.46234/ccdcw2021.249
After thirty-two years since the first domestic outbreak of human immunodeficiency virus (HIV)/ acquired immune deficiency syndrome (AIDS) among injection drug users (IDUs) and almost two decades of comprehensive response efforts by the Chinese government, HIV/AIDS remains a major public health problem. The increasing burden of HIV/AIDS and comorbidities, the emergence of new HIV subtypes and/or circulating recombinant forms and drug mutations, the changing transmission networks, and the urgency of immediate antiretroviral therapy initiation upon an HIV diagnosis are increasingly challenging and altogether likely to have significant impact on the HIV epidemic in China. Upon the call for the global AIDS response to end AIDS by 2030, China needs to develop an innovative and pragmatic roadmap to address these challenges. This review is intended to provide a succinct overview of what China has done in efforts to achieve the global goal of ending AIDS by 2030 and the recently proposed "95-95-95-95" target (95% combination prevention, 95% detection, 95% treatment, 95% viral suppression), and to summarize the most recent progresses in the epidemiological research of HIV/AIDS in China with the aim of providing insights on the next generation of HIV control and prevention approaches and to shed light on upgrading the national strategy to end AIDS in this country.
Keywords: Antiretroviral treatment; Epidemiology; HIV; Mortality.; Prevention; Testing.
Copyright and License information: Editorial Office of CCDCW, Chinese Center for Disease Control and Prevention 2021.
PubMed Disclaimer
Similar articles
- Tuberculosis. Bloom BR, Atun R, Cohen T, Dye C, Fraser H, Gomez GB, Knight G, Murray M, Nardell E, Rubin E, Salomon J, Vassall A, Volchenkov G, White R, Wilson D, Yadav P. Bloom BR, et al. In: Holmes KK, Bertozzi S, Bloom BR, Jha P, editors. Major Infectious Diseases. 3rd edition. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2017 Nov 3. Chapter 11. In: Holmes KK, Bertozzi S, Bloom BR, Jha P, editors. Major Infectious Diseases. 3rd edition. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2017 Nov 3. Chapter 11. PMID: 30212088 Free Books & Documents. Review.
- Optimizing Treatment for Adults with HIV/AIDS in China: Successes over Two Decades and Remaining Challenges. Cao W, Hsieh E, Li T. Cao W, et al. Curr HIV/AIDS Rep. 2020 Feb;17(1):26-34. doi: 10.1007/s11904-019-00478-x. Curr HIV/AIDS Rep. 2020. PMID: 31939111 Free PMC article. Review.
- Prevention and control of HIV/AIDS in China: lessons from the past three decades. Xu JJ, Han MJ, Jiang YJ, Ding HB, Li X, Han XX, Lv F, Chen QF, Zhang ZN, Cui HL, Geng WQ, Zhang J, Wang Q, Kang J, Li XL, Sun H, Fu YJ, An MH, Hu QH, Chu ZX, Liu YJ, Shang H. Xu JJ, et al. Chin Med J (Engl). 2021 Nov 10;134(23):2799-2809. doi: 10.1097/CM9.0000000000001842. Chin Med J (Engl). 2021. PMID: 34759226 Free PMC article.
- Injection drug use and HIV/AIDS transmission in China. Chu TX, Levy JA. Chu TX, et al. Cell Res. 2005 Nov-Dec;15(11-12):865-9. doi: 10.1038/sj.cr.7290360. Cell Res. 2005. PMID: 16354561 Review.
- HIV / AIDS in China: migrant population, drug injection responsible for increased transmission. Thomas J. Thomas J. AIDSlink. 1998 Jan-Feb;(49):12-4. AIDSlink. 1998. PMID: 12293301
- Adolescent HIV prevent and care framework: A global scoping review protocol- BSGH 006. Abu-Ba'are GR, Shamrock OW, Rodriguez D, Agbemedu GRK, Nelson LE. Abu-Ba'are GR, et al. PLoS One. 2024 Aug 19;19(8):e0289994. doi: 10.1371/journal.pone.0289994. eCollection 2024. PLoS One. 2024. PMID: 39159161 Free PMC article.
- Analysis of risky sexual behaviors among male college students who were sexually active in Sichuan, China: a cross-sectional survey. Dai Y, Li Y, Zhou D, Zhang J. Dai Y, et al. AIDS Res Ther. 2024 Jul 26;21(1):46. doi: 10.1186/s12981-024-00636-1. AIDS Res Ther. 2024. PMID: 39061023 Free PMC article.
- Spatiotemporal Analysis of HIV/AIDS Incidence in China From 2009 to 2019 and Its Association With Socioeconomic Factors: Geospatial Study. Xie Z, Chen B, Duan Z. Xie Z, et al. JMIR Public Health Surveill. 2024 Jun 7;10:e56229. doi: 10.2196/56229. JMIR Public Health Surveill. 2024. PMID: 38848123 Free PMC article.
- Evaluating the spatial accessibility and spatial layout optimization of HIV/AIDS healthcare services in Shandong Province, China. Zhang C, Yan Y, Zhu X, Li L, Li Y, Wang G, He F, Song Y, Liu Y, Zhang N. Zhang C, et al. Sci Rep. 2024 May 17;14(1):11258. doi: 10.1038/s41598-024-61484-7. Sci Rep. 2024. PMID: 38755199 Free PMC article.
- Real experience of caregivers of patients with HIV/AIDS from the perspective of iceberg theory: a qualitative research. Tang J, Ren J, Wang H, Shi M, Jia X, Zhang L. Tang J, et al. BMJ Open. 2024 May 7;14(5):e079474. doi: 10.1136/bmjopen-2023-079474. BMJ Open. 2024. PMID: 38719298 Free PMC article.
National Center for AIDS & STD Control and Prevention, The Chinese Center for Disease Control and Prevention (CDC). Annals of Information on Comprehensive Prevention and Treatment for AIDS, STD and Hepatitis C; 2020 July 9; Beijing, China.
- Gao DS, Zou ZY, Dong B, Zhang WJ, Chen TQ, Cui WX, et al Secular trends in HIV/AIDS mortality in China from 1990 to 2016: gender disparities. PLoS One. 2019;14(7):e0219689. doi: 10.1371/journal.pone.0219689. - DOI - PMC - PubMed
- Dong MJ, Peng B, Liu ZF, Ye QN, Liu H, Lu XL, et al The prevalence of HIV among MSM in China: a large-scale systematic analysis. BMC Infect Dis. 2019;19(1):1000. doi: 10.1186/s12879-019-4559-1. - DOI - PMC - PubMed
- Feng YB, Bu K, Li M, Zhang XY, Jin SS, Wang L Meta-analysis of HIV infection incidence and risk factors among men who have sex with men in China. Chin J Epidemiol. 2015;36(7):752–8. doi: 10.3760/cma.j.issn.0254-6450.2015.07.019. - DOI - PubMed
- Cui Y, Guo W, Li D, Wang L, Shi CX, Brookmeyer R, et al Estimating HIV incidence among key affected populations in China from serial cross-sectional surveys in 2010-2014. J Int AIDS Soc. 2016;19(1):20609. doi: 10.7448/IAS.19.1.20609. - DOI - PMC - PubMed
Related information
Grants and funding, linkout - more resources, full text sources.
- Europe PubMed Central
- PubMed Central
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

IMAGES
VIDEO
COMMENTS
The best natural sleep aids is a broad category, so there weren't strict criteria for each product. Our focus was on finding options that help create a relaxing environment and support healthy ...
National Gay Men's HIV/AIDS Awareness Day on September 27 highlights the impact of HIV on gay and bisexual men and promotes strategies to encourage testing. Get involved by sharing CDC's social media toolkit and HIV information to encourage men to get tested — and share our MyHealthfinder resources to help people get tested for HIV and ...
The West Virginia University Benjamin M. Statler College of Engineering and Mineral Resources is enhancing education and research with a $500,000 gift to support laboratory upgrades.. The gift from the BHE GT&S Foundation supports improvements to the reservoir engineering and core analysis lab, located in the Mineral Resources Building on the Evansdale Campus.
During the IAS AIDS conference held in Munich in July, UNC had over 15 faculty members and trainees attend to showcase the important work that is being done on HIV/AIDS within our university. Here are some of the highlights from our UNC team in Munich: Recent research has provided new insights into managing HIV-related comorbidities. Dr.
'Evolved behaviour aids survival' The findings also suggest that members of the same family use similar vocal labels and sound similar to each other, with the academics comparing it to the use ...
If you are using public inspection listings for legal research, you should verify the contents of documents against a final, official edition of the Federal Register. Only official editions of the Federal Register provide legal notice of publication to the public and judicial notice to the courts under 44 U.S.C. 1503 & 1507 .
More than 150 people attended Industry Innovation Day and the GVU Spring Research Showcase on April 19 held at the Technology Square Research Building conference center on the Georgia Tech campus. This year's event centered around the brain and neuro-related technologies, and touched on topics ranging from brain computer interaction, cognitive aids, psychology, the future of work, artificial ...
AIDS Research Advisory Committee. AIDS Vaccine Research Subcommittee. Autoimmune Diseases Committee. Board of Scientific Counselors Members. Executive Committee. ... Advanced technologies research fields, such as genomics, proteomics, and bioinformatics, hold great promise for developing new diagnostics, therapeutics, and vaccines to treat and ...
HIV research has made remarkable progress since scientists first identified the disease in the 1980s. There are new prevention methods and therapies to extend the lives of those living with the ...
HIV and AIDS information and facts. Read latest medical articles and view educational videos on AIDS and HIV symptoms and treatments. Stay informed about new developments on the AIDS/HIV front.
ELISA: The First HIV Blood Test. In CDC's immunology lab, scientists began working with AIDS specimens as early as July 1981 to understand how the immune systems of young, healthy men were so compromised by the mystery illness. In a photograph from 1983 displayed here, a female CDC research chemist conducts tests on biological fluids from ...
Interview with Dr. Anthony Fauci on progress made during the past four decades of the HIV/AIDS pandemic and ongoing efforts to end this threat. 18m 44s Download. The dramatic saga of the acquired ...
Welcome to OAR. The Office of AIDS Research (OAR) coordinates HIV/AIDS research across the National Institutes of Health (NIH). The NIH provides the largest public investment in HIV/AIDS research globally. As HIV crosses nearly every area of medicine and scientific investigation, the response to the HIV pandemic requires a multi-Institute ...
Scientists have conducted research studies that has led to the conclusion that rapid progression before administration of cART stops the recovery of CD4 + T-cells once the suppressive response to HIV-1 through cART is achieved. These findings have implications in public health policy making, clinical outcomes and science research.
HIV/AIDS research includes all medical research which attempts to prevent, treat, or cure HIV/AIDS, along with fundamental research about the nature of HIV as an infectious agent, and about AIDS as the disease caused by HIV. Many governments and research institutions participate in HIV/AIDS research.
Research Foundation to Cure AIDS is the first 501(c)(3) non-for-profit organization with a royalty-free license to research, develop and commercialize a cell engineering technology in the field of curing AIDS on a pro bono basis. Initial stem cell cures of HIV/AIDS. In 2007, ...
Untreated HIV / AIDS can cause a great deal of weight loss. Diarrhea, weakness and fever often happen with the weight loss. Brain and nervous system, called neurological, complications. HIV can cause neurological symptoms such as confusion, forgetfulness, depression, anxiety and difficulty walking.
Human immunodeficiency virus (HIV) is a virus that attacks the body's immune system. Acquired immunodeficiency syndrome (AIDS) occurs at the most advanced stage of infection. HIV targets the body's white blood cells, weakening the immune system. This makes it easier to get sick with diseases like tuberculosis, infections and some cancers.
HIV Glasgow 2024: The Lancet HIV will be at HIV Drug Therapy Glasgow, November 10-13. If you would like to meet to discuss your research or possible publications, please contact [email protected]. HIVR4P 2024: Deputy Editor Adrian Gonzalez Lopez will be at the 5th HIV Research for Prevention Conference, October 6-10. If you would like ...
A few years ago, a team from Scripps Research and the International AIDS Vaccine Initiative (IAVI) showed that it was possible to stimulate the precursor cells needed to make these rare antibodies ...
At the National Institutes of Health, the HIV/AIDS research effort is led by the National Institute of Allergy and Infectious Diseases (NIAID). A vast network of NIAID-supported scientists, located on the NIH campus in Bethesda, Maryland, and at research centers around the globe, are exploring new ways to prevent and treat HIV infection, as ...
Research. CDC provides national leadership for HIV prevention research, including the development and evaluation of HIV biomedical and behavioral interventions to prevent HIV transmission and reduce HIV disease progression in the United States and internationally. CDC's research efforts also include identifying those scientifically proven ...
Almost all the research in India is based on beliefs, attitudes, and awareness of HIV among adolescents 2,12. However, few other studies worldwide have examined mass media as a strong predictor of ...
The HIV-1 pandemic is a complex mix of diverse epidemics within and between countries and regions of the world, and is undoubtedly the defining public-health crisis of our time. Research has deepened our understanding of how the virus replicates, manipulates, and hides in an infected person. Although our understanding of pathogenesis and ...
Publishing the very latest ground breaking research on HIV and AIDS. Read by all the top clinicians and researchers, AIDS has the highest impact of all AIDS-related journals. With 18 issues per year, AIDS guarantees the authoritative presentation of significant advances. The Editors, themselves noted international experts who know the demands of your work, are committed to making AIDS the most ...
Upon the call for the global AIDS response to end AIDS by 2030, China needs to develop an innovative and pragmatic roadmap to address these challenges. This review is intended to provide a succinct overview of what China has done in efforts to achieve the global goal of ending AIDS by 2030 and the recently proposed "95-95-95-95" target (95% ...