

Top 17 Clinical Research Organizations (CRO) in 2023
In clinical research and treatment development, clinical research organizations (CROs) are frequently a sponsor’s most important partner and ally.
Depending on the nature of the clinical trial, and your existing capabilities as a sponsor to run the trial, the CRO company of your choice will typically be responsible for facilitating most of the micro and macro processes that go into designing and running a successful clinical trial.
When contracting a CRO to help you with your trial, you are transferring over a large portion of responsibility into the hands of your clinical research partner. The CRO of your choice will have the responsibility to control a variety of factors and processes of a clinical trial, and depending on their expertise, team structures, service offerings, internal resources and many other capabilities.
Your ability to find and contract a top CRO company that is the right fit for your unique trial will be a determinant of whether or not you will be able to operate a high-quality clinical trial that meets your expected timelines, budget and delivers a top-notch patient experience.
At ClaraHealth (a patient-centric recruitment acceleration platform) , we have put together an extensive list of the top CRO companies in the US and around the world.
This is not a cro rankings list, but rather a compiled list of some of the top clinical research organizations around the world. We have highlighted their strengths and core service offerings to make it easier for you to find the right fit clinical research partner.
In addition, we’ve put together a list of 9 fundamental questions to ask the prospective clinical research organization , which will help you to save time and ensure a right fit in picking the CRO.
Formerly known as Quintiles and IMS Health, IQVIA is one of the largest CROs in the world, with a large range of service offerings to help advance clinical research.
The company was founded in North Carolina in 1982, and has since grown to over 88,000 employees in more than 100 countries.
Some clinical trial solutions offered by IQVIA include:
- Assistance with protocol design
- Design of phase 1 clinical trials
- Assessment and improvement of phase 2 and 3 clinical trials
- Site identification & selection
- Patient recruitment
- Access to global laboratories via their wholly owned subsidiary Q2 Solutions
Parexel is a global clinical research organization that was founded in 1982, and specializes in conducting clinical studies on behalf of its pharmaceutical partners in order to accelerate and ensure the drug approval process of up-and-coming potential treatments. It currently operates in more than 50 countries, and is run by more than 18,000 employees around the world.
The company has a wide range of service offerings, covering nearly every type of clinical trial service to assist sponsors in running successful clinical studies.
Some clinical trial solutions offered by Parexel include:
- Clinical trial design and development for early phase, phase 2 & 3, and late phase clinical trials
- Clinical data management
- Decentralized clinical trials
- Clinical supply chain management
- Medical writing
- Regulatory affairs consulting
- Pharmacovigilance
3. PRA Health Sciences
PRA Health Sciences is one of the largest contract research organizations in the world. Founded in 1976 under the name “Anti-Inflammatory Drug Study Group”, the company was renamed to PRA in 1982. PRA Health Sciences employees more than 17,000 people, and provides coverage to more than 90 countries.
In 2021, PRA Health Sciences was acquired by the Ireland-headquartered global CRO leader ICON, which is also reviewed in this list.
Some clinical trial solutions offered by PRA Health Sciences include:
- Decentralized Clinical Trials Platform
- Protocol Consultation & Study Design
- Onsite Support services
- Customized Solutions for Biotech (such as asset valuation, regulatory strategy, engagement and support, drug development strategy and funding solutions)
- Clinical Diagnostics
- Site Commercial Solutions
- PRA’s Laboratories for Drug Development
Headquartered in Ireland, ICON was founded in 1990 in Dublin by co-founders John Climax and Ronan Lambre. The company has since grown to be one of the largest CROs in the world. As of September 2020, the company employs more than 15,000 people in 94 locations and across 40 countries.
ICON offers clinical research services which include consulting, clinical development and commercialization across a wide range of therapeutic areas.
In 2021, ICON acquired PRA Health Sciences, which is another CRO and global leader in clinical research services.
Some clinical trial solutions offered by ICON:
- Commercial Positioning
- Early Phase
- Functional Services Provision
- Laboratories
- Language Services
- Medical Imaging
- Real World Intelligence
- Site & Patient Solutions
- COVID-19 Clinical Operations
5. Syneos Health
Formerly known as InVentiv Health Incorporated and INC Research, Syneos Health is a publicly listed and global contract research organization. The company is based in Morrisville, North Carolina, and specializes in assisting companies with late-stage clinical trials. Syneos Health currently employs more than 25,000 people, and has offices across 91 locations.
In early 2018, INC Research was acquired inVentiv Health, and the merged company was named Syneos Health.
Some clinical trial solutions offered by Syneos Health include:
- Decentralized Clinical Trials Solutions
- Bioanalytical Solutions
- Phase II-III/Phase IIIb-IIIV
- Medical Device Diagnostics
- Clinical Data Management
- Clinical Project Management
- Clinical Monitoring
- Drug Safety & Pharmacovigilance
- Site and Patient Access
6. Labcorp Drug Development (Formerly Covance)
Formerly known as Covance and renamed to Labcorp Drug Development in early 2021, this CRO is one of the largest contract research organizations in the world. The company claims to provide the world’s largest central laboratory network, and has been rated as one of the best places to work for LGBTQ+ equality by the Human Rights Campaign organization in 2018 to 2021. Currently, Labcorp employs over 70,000 people and is able to support clinical research efforts in almost 100 countries around the world.
Some clinical trial solutions offered by Labcorp Drug Development include:
- Preclinical Services
- Clinical Trials
- Clinical Trial Laboratory Services
- Post-Marketing Solutions
- Medical Devices
- Data & Technology
Also known as Pharmaceutical Product Development, PPD is a large global contract research organization headquartered in Wilmington, North Carolina. Started as a one-person consulting firm in 1985, PPD has grown to over 27,000 employees worldwide, and provides a wide range of clinical research services to pharmaceutical and biotech companies.
Some clinical trial solutions offered by PPD include:
- Clinical Development
- Early Development
- Peri- and Post-Approval
- PPD Biotech
- PPD Laboratories
- Product Development and Consulting
- Site and Patient Centric Solutions
8. Fisher Clinical Services
Part of Thermo Fisher Scientific, Fisher Clinical Services is a global clinical research organization with headquarters in Center Valley, Philadelphia.
The company has been in the business of clinical supply chain management for over 20 years, and is focused exclusively on working with the packaging and distribution requirements of clinical trials across the globe.
Some clinical trial solutions offered by Fisher Clinical Services include:
- Biologistics Management
- Cell & Gene Therapy
- Clinical Ancillary Management
- Clinical Label Services
- Clinical Trial Packaging & Storage
- Clinical Supply Optimization Services
- Cold Chain Management & Expertise
- Direct-to-Patient
- Distribution & Logistics
- Strategic Comparator Sourcing
- Public Health Research
Established in 1997 under the name Kiecana Clinical Research, KCR is a full-service contract research organization that provides a variety of services for clinical monitoring, safety & pharmacovigilance, clinical project management, quality assurance and regulatory affairs.
KCR operates globally, and has offices in North America, Western Europe, Central Europe and Eartern Europe. The company currently employs more than 700 staff.
Some clinical trial solutions offered by KCR include:
- Trial Execution
10. Medpace
Founded in 1992 and based in Cincinnati, Ohio, Medpace is a midsize clinical contract research organization. The company has operations in over 45 countries, and employs over 2,800 people. Medpace provides support services for Phase I-IV clinical trials for pharmaceutical and biotechnology companies, which include central laboratory services and regulatory services.
Some clinical trial solutions offered by Medpace include:
- Biostatistics and Data Sciences
- Clinical Trial Management
- Drug Safety and Pharmacovigilance
- Medical Writing
- Quality Assurance
- Regulatory Affairs
- Risk-Based Monitoring
- Medpace Laboratories
11. Clintec
Now in business for over 22 years, Clintec is a medium-sized global contract research organization for pharmaceutical, biotech and medical device industries, with large expertise in oncology and rare diseases.
The company provides the flexibility and agility of a smaller-sized CRO, while also having a wide global coverage that large CRO companies are known for. Clintec is based in more than 50 countries, and was acquired by the leading global CRO IQVIA in late 2018.
Some clinical trial solutions offered by Clintec include:
- Project Management
- Data Management
- Biostatistics
- Global Feasibilities
- Patient Recruitment & Retention
12. Worldwide Clinical Trials
Bringing over 30 years of experience to the clinical research market, Worldwide Clinical Trials is a leading medium-sized global contract research organization. Founded by physicians with a dedication and commitment to advancing medical research, Worldwide Clinical Trials was the first customer-centric CRO.
Currently the company has coverage in more than 60 countries, and has extensive experience in a wide range of therapeutic areas, including central nervous system, metabolic, cardiovascular, oncology, rare diseases and general medicine.
Some clinical trial solutions offered by Worldwide Clinical Trials include:
- Bioanalytical Lab
- Early Phase Development
- Clinical Phase IIB-II Clinical Trials
- Phase IIIB-IV Clinical Trials
- Trial Management Technologies
Named #1 CRO in the world for operational excellence at the 2021 CRO Leadership Awards, CTI Clinical Trial And Consulting Services is a medium-sized global contract research organization that has been serving pharmaceutical companies since 1999.
Based in Covington, Kentucky, CTI has offices around the world in more than 60 countries, with coverage in North America, Europe, Latin America, Middle-East, Africa, and Asia-Pacific regions.
Some clinical trial solutions offered by CTI include:
- Feasibility
- Regulatory Affairs Study Start-Up
- Medical Monitoring
- Safety & Pharmacovigilance
- Clinical Services
14. Wuxi AppTec
Founded in 2000 as WuXi PharmaTech in the city of Wuxi, China, Wuxi AppTec has grown from a single laboratory into a leading global contract research organization with more than 28,000 employees, including 23,000 scientists and more than 30 research & development and manufacturing sites around the world.
With offices in Asia, U.S, Europe and the Middle East, the company is able to provide coverage to more than 30 countries around the world.
Some clinical trial solutions offered by Wuxi AppTec include:
- Small Molecule Drug R&D and Manufacturing
- Cell Therapy and Gene Therapy
- Drug R&D and Medical Device Testing
- Clinical Services (Phase I-IV)
15. Advanced Clinical
Founded in 1994 and based out of Deerfield, Illinois, Advanced Clinical is a midsize and full-service CRO that helps sponsors with running clinical trials. The company employs more than 700 staff, and offers a wide variety of services across many therapeutic areas. Advanced Clinical has global representation in over 50 countries around the world.
Some clinical trial solutions offered by Advanced Clinical include:
- eTMF & Document Management
- Global Medical Services
- Quality & Validation
16. Pharm-Olam
Pharm-Olam is a leading midsize CRO with global headquarters located in Houston, Texas and its European headquarters in Bracknell, United Kingdom. The company employs more than 800 staff, and has 25 offices around the world, with a global coverage in more than 60 countries.
The company has therapeutic expertise in 5 areas, including Rare & Orphan Disease, Infectious Disease & Vaccine, Oncology-Hematology, Allergy and Autoimmune.
Some clinical trial solutions offered by Pharm-Olam include:
- Study Feasibility
- Site Activation
- Patient Recruitment
- Medical Affairs
- Compliance & Training
- Clinical Monitoring & Operations
17. Clinipace
Founded in 2003 and based out of Morrisville, North Carolina, Clinipace is a global midsize full-service CRO with a focus on solution customization for clinical trials. The company has a large global coverage in more than 50 countries, and has offices in North America, South America, Europe and Asia-Pacific regions.
Clinipace’s therapeutic focus areas include Oncology, Nephrology and Urology, Rare Disease, Gastroenterology and Women’s Health. The company also has complete therapeutic expertise in Infectious Disease & Vaccines, Cardiology, CNS, Immunology, and Respiratory.
Some clinical trial solutions offered by Clinipace include:
- Clinical Analytics
- Clinical Technology and Ecosystem
- Functional Service Partnership (FSP)
- Regulatory & Strategic Product Development
9 Fundamental Questions To Ask A Top CRO Company Before Signing The Contract
1. which services does the cro provide.
CROs offload a lot of operational tasks from trial sponsors, which can touch any component of clinical trial operations. From formulating an overall study strategy and implementing technologies to support the operational processes of the trial, to picking and identifying sites, and supporting patients during the trial, the range of clinical services offered by a CRO tends to be vast and inclusive of all the typical services and support you will require for running a successful clinical trial.
However, not all CROs are the same in their service offerings, or are able to offer the same depth of capability within a seemingly same clinical trial support process. For this reason it is important to understand exactly which kind of clinical services and support you are looking to receive from the prospective CRO when running your clinical trial.
While services such as clinical monitoring and clinical trial management are offered by the majority of CROs, the specific needs of each trial are unique, and for this reason it is important to first identify what will be the unique services your trial requires. Completing this internal analysis first will help you to understand the extent to which a potential CRO partner will be able to provide all of these services.
Some CROs specialize in specific clinical trial functions which the company may label as a “core services”, in which case this is a sign the company will have more expertise, experience, and will be set up in a way to maximize their capabilities in providing support for these services compared to other services that the CRO offers.
For example, a CRO may include patient recruitment as part of its “core services”, which implies that they are highly skilled in and have the necessary infrastructure to design and implement a high-quality patient recruitment strategy.
Clara Health CRO Support Services: At Clara Health our specialty services include technology-augmented digital and patient advocacy recruitment, as well as patient support via our signature patient recruitment platform, which we use to upgrade clinical trials and deliver results sponsors look for in their recruitment and retention campaigns.
At Clara, we work alongside CROs to supplement and support clinical trials with modern and personalized capabilities that CROs do not typically have the bandwidth, corporate structure or infrastructure to support.
If you would like to learn more about exactly how our platform can upgrade your unique trial, feel free to book a Free 30 Minute Consultation Session Here with one of our in-house experts.
2. What Related Experience Does The CRO Have?
It is helpful to ask the prospective CRO company if they have any relevant experience in running clinical trials that would be an asset in designing and running your study. Previous experience in a related therapeutic area or in running a trial with a similar design allows CROs to have a deeper understanding into potential opportunities and challenges, increasing the likelihood of your clinical study being successful.
For example, if a sponsor is planning to run a trial in oncology, for the purpose of site identification and selection it would be valuable to partner with a CRO vendor that has expertise in this area, as they likely already have a good understanding of which sites will lead to optimal results.
However, it is also important to consider all factors when selecting a CRO vendor and not to rely on therapeutic experience as the sole qualifier for whether or not a potential CRO is a fit for your trial. While previous experience is beneficial, some sponsors close themselves off from working with vendors that have not worked in their therapeutic area, which significantly limits options when choosing a CRO partner that is truly a good fit for their clinical study.
This can impact the end result of your clinical study, as sponsors that are not successful in choosing a CRO vendor that is the right overall fit may face difficulties if the needs of their clinical study aren’t being properly met.
Clara Health: We have worked to provide support for clinical trials across a wide range of therapeutic areas and trial designs. Our specialty is filling in the gaps that CROs traditionally did not have to think about, which include digital patient recruitment, patient advocacy recruitment, and technology-augmented patient support.
Additionally, we are constantly building our proprietary data and running tests in a variety of therapeutic areas. These research efforts allow us to have a detailed understanding of the expected level of difficulty when recruiting particular patient populations, as well as allow us to predict with accuracy which segments of the targeted population will be likely to qualify in a particular study.
3. What Are The Communication Workflows & Expectations For Performing And Delivering Contracted Services?
It is important that you clarify what the expectations for communication will be between your prospective CRO vendor and your internal teams, as you will most likely be working with the CRO of your choice for the entire duration of your clinical trial.
There are a vast variety of factors and success determinants for a clinical trial, which are continuously undergoing change as the study unfolds. For this reason, it is recommended that you work with a CRO that is proactive in their communication, so that you are kept up to date with information about important changes as your clinical trial progresses.
A vendor that is proactive rather than reactive in their communication and approach to dealing with arising issues is one of the most important qualities in CRO. Challenging situations will naturally arise, and the promptness with which they are taken care of will significantly impact your clinical trial’s degree of success. Therefore, seeking a vendor that is able to match the standard of communication that you as a sponsor would like to experience throughout the duration of your partnership is one of the most critical steps in determining which CRO is the right fit for your clinical trial.
We’ve included a few additional questions pertaining to the communication structure and reporting expectations that you can ask a prospective CRO vendor to determine the degree of fit in this particular category:
Communication Expectations:
- If we were to move forward with you, which of your team members will be our main point of contact?
- How available will you be outside of the scheduled meetings to address any of our concerns or additional requests?
- What will be the frequency at which update meetings will be conducted, and who will be present at those meetings?
- Which clinical study processes will be reported on, and what will be the workflow for how we will receive this information?
- What will be the cadence at which we will receive progress reports?
- Would we be able to access metrics electronically via an interactive dashboard, or will you send us formal reports?
Clara Health: At Clara Health, we directly interact and actively work with several key stakeholders involved in running a clinical trial, which includes sponsors, CROs, sites, and patients. This unique position allows us to have a centralized perspective which helps us to see all the moving parts of a clinical trial at the same time, which helps to identify issues and relay this vital information and insight back to the sponsor (or other appropriate stakeholders) in the shortest time possible.
The ability to access this perspective allows us to gather the most accurate, complete, and up-to-date information about how the clinical trial is unfolding, and quickly becomes very valuable to sponsors for their clinical trial.
As an example, we may receive feedback from patients about having an unsatisfactory experience with a particular study site. We are able to aggregate and analyze this information, and relay our findings back to the sponsor and the study site to improve the experience for other patients.
4. What Is The CRO’s Client Satisfaction Record?
It is a good practice to request information or metrics from the prospective CRO vendor that can point to the degree of satisfaction of their past clients. Prior to signing the contract, vendors will naturally do their best to uplift their image and future value to you during their sales conversations with you and your team. It can be tricky to get an objective understanding of what the partnership experience will actually entail, especially when there are multiple vendors fighting for your commitment.
We recommend that you ask the prospective vendor to provide success metrics regarding areas of clinical trial operations that are going to be important for your trial.
For example, you may be interested in learning about the vendor’s relationship to finances, in which case it will be useful to ask them about situations in which they went over the planned budget, and investigate into the reasons behind that. Alternatively you may be concerned about potential delays in timelines, in which case it would be helpful to learn about metrics regarding the CRO’s ability to meet timeline expectations.
You may also request to talk to the prospective CRO’s past clients, which will help you to gain insight into what the relationship was like and give you the opportunity to examine if the way in which the particular CRO manages its relationships and performs its services meets the expectations that you would have for your potential relationship and for your clinical trial.
Clara Health: At Clara Health, our relationships with our partners and with our patients are most important to us. In the unique position where we fit in the clinical trial process, we have the opportunity to directly co-create the clinical trial patient experience with a variety of stakeholders, including sponsors, sites, CROs, and patients.
Our company’s values and culture have been directed and developed to be such that the client and patient experience is at the top of priority for all of our internal teams, and we work to provide the best quality of care to all stakeholders.
We have many testimonials from every type of partner we’ve worked with which we can happily share with you.
5. How Do You Adapt When Encountering Challenges With Running A Clinical Trial?
It is inevitable that challenges and unforeseen changes will arise throughout the operational clinical trial process, and for this reason it is important to work with a CRO vendor that can provide you with evidence of their flexibility and ability to adapt to sudden changes.
The ideal CRO partner is one that is highly consultative throughout the entire process, and has an ability and the initiative to deal with challenges at their seed stage, prior to them turning into major obstacles for the success of your trial.
CROs naturally have a large reach, and there are a lot of different clinical trial mechanisms and processes that are under their control. They are able to monitor and respond to what is going on in every key link in the chain of the clinical trial operation.
It is reasonable to expect this level of oversight from a CRO, and additional questions that can help you gain insight into this include:
- What are some examples where the CRO was effective at monitoring the health of clinical trials they’ve helped operate in the past?
- How quickly does the CRO respond to challenges or opportunities for improving the clinical trial experience?
- How well does the CRO gather & process information from study sites, study teams, patients & the sponsor, and what are their typical data analysis workflows?
It is also recommended to speak to the prospective CROs past clients to help you gain insight into how well they respond and adapt to the naturally arising challenges in clinical trials.
Clara Health: While CROs do have a large reach within the clinical trial, no CRO has complete visibility into every clinical process. They are not typically set up to support full visibility, which can manifest as a potential threat to your clinical trial as it unfolds. This is especially true for parts of the clinical trial processes that CROs naturally do not specialize and often subcontract, such as clinical trial recruitment.
At Clara, we are in a unique position in relation to other key partners involved in operating the clinical trial. We are in direct and frequent contact with patients, CROs, study sites, study teams, and the sponsor, and have a very deep understanding of the patient pipeline. This allows us the unique ability to go very deep into specific parts of the recruitment chain and investigate what is working and what is not working.
In addition, Clara functions as a resource for all partners in the clinical trial. For example, we work directly with site teams to ensure that they have access to a 3rd party that they can relay their needs to and receive fast support in case there is anything they require that can improve the patient recruitment process.
6. Which Parts Of Operating The Clinical Trial Will You Be Outsourcing?
Since there are so many processes and mechanisms that go into operating a clinical trial, CROs will always outsource some parts of running and managing the study. While you can expect that the prospective CRO will subcontract some of the work, it is important to find out which exact parts the clinical study will be outsourced.
There are certain basic and key clinical processes (such as site selection) that CROs almost always help with, and if you find that these parts of your trial are going to be subcontracted to another company, it is recommended to find out why the CROs operations are set up this way and how this would impact the service you will receive.
Ultimately what matters to you as a partner and client is that the quality of service and care that you will receive will be up to standard, and meet what was promised and what you are expecting. While this trust is important after you have signed the contract, it is recommended that prior to entering into such a significant commitment that you have evidence and the conviction that the CRO of your choice is truly the right fit and will deliver the quality of service that was being discussed.
Since it is impossible to predict exactly what the quality of this relationship and services performed will actually be like in practice, it is recommended that you understand the details of what will be done for your trial and how. Investigating how the CRO outsources and subcontracts services for a clinical trial will help you to gain necessary insight that you would need to make the correct vendor selection decision.
Clara Health: At Clara, we maximize the effectiveness of the digital component across the entire digital & recruitment spectrum, which is added on top of the existing capabilities of the CROs and other vendors involved in operating your clinical trial. In addition, we offer services that augment the CROs efforts, which has the potential to significantly improve the patient experience, operations flows, recruitment and retention performance, which is so important in ensuring the success of a clinical trial.
For example, if a CRO wants to have a great site relationship, we are able to come in as a third party on behalf of the sponsor and CRO and act as a resource and additional support for sites.
In another example, If a sponsor wants to have great relationships with the patient community, Clara is able to come in on behalf of the sponsor and develop these relationships while being perceived more neutrally by the patient community.
7. Do You Have Experience Running International Trials?
If you are planning on operating an international clinical trial, it is recommended to work with a CRO that has extensive experience in this area. While many CROs will offer near-global coverage, the level of experience with specific geographic locations can significantly vary from one vendor to another.
It is important to work with a CRO that has experience running clinical trials in the specific countries and regions you are planning to conduct your research in. Being compliant with the local rules and regulations for clinical testing is a very complex process that requires existing understanding and familiarity in order to ensure logistical smoothness and to mitigate legal risks. In operating a clinical trial, there are a multitude of clinical services and processes, which can greatly vary across the many regions in which you can conduct clinical testing.
A CRO that is lacking experience in operating international trials or operating in particular regions where you plan on conducting research may not be able to meet your desired quality and agility expectations, and therefore may not be the right fit for your international clinical trial.
Clara Health: In the past, we have provided international patient recruitment and digitally-augmented trial support services for clinical trials in the EU, Canada, UK, Australia and South America.
Clara Health is fully compliant to operate international studies everywhere in the world, with the exception of Russia and China.
8. What Is Your Relationship With Patients?
Patient-centric approach to designing and operating a clinical trial is becoming more and more crucial in the clinical research space. The ability of a sponsor and their CRO partner to understand the needs and characteristics of their target patient community is a significant determinant of whether or not the study will be a success.
A sponsor that has close and authentic relationships with the patient community tends to have a deeper understanding of how to create the best clinical trial experience that will attract patients and keep their interest throughout the clinical trial.
In addition, strong relationships with patients allow sponsors and CROs to forecast recruitment and patient retention pipeline with much higher accuracy. This ability is critical for ensuring the success of the trial and mitigating the risk of low enrollment. After an understanding of the patient population is acquired, sponsors gain the necessary insight to design a clinical trial that is not only favorable to their research results, but is also practical and will result in the enrollment numbers they are looking for.
While many CROs have already recognized the importance of patient-centricity and evolved the ways in which they design and operate clinical trials, other CROs have not yet made such a pivot in their values. It is important to understand the degree of importance the prospective CRO places on creating a favorable patient experience, and what kind of infrastructure the company has to support it.
At Clara, we recommend choosing a CRO partner that is adapting to the patient-centric model which is becoming more and more important for running a successful clinical trial.
Clara Health: Since early stages of our development, we’ve had a dedicated patient advocacy team that has been integral in shaping our company’s vision and operations. We have built our entire platform and recruitment infrastructure around creating the best experience for patients. Our teams, corporate values, service offerings and company infrastructure all work in the service of the patient.
In addition, over the many years of being in business we have heavily invested in building authentic patient community relationships that span across a variety of therapeutic areas. This has given us a unique ability to receive feedback directly from patients that is genuine and authentic around marketing materials, strategy for patient recruitment, and other services that we build for specific trials.
This ability to build partnerships with the patient community in an authentic way gives us a very unique ability to engage with the patient community on behalf of a pharmaceutical company, allowing our sponsor & CRO partners the opportunity to start conversations with patients through our in-house patient advocacy team.
If you would like to learn how Clara can help you to build a strong & authentic relationship with your target patient community, get in touch with us and we’d be happy to share our capabilities and previous results with you as they relate to your current or upcoming clinical trial.
9. How Is The CRO Going To Utilize Patient Input For Developing The Trial?
In the initial stages of clinical trial design, sponsors often determine the ideal patient profiles that would help them to drive the most favorable research outcomes for their study. While it is important for the success of your trial to determine who your ideal patients are, very often these projections do not match up with what is viable in practice.
At Clara, we often encounter study protocols that are not set up realistically for successful recruitment to be possible.
Common mistakes that are made when determining trial eligibility criteria and trial design include:
- Overestimating the interest in the clinical trial from the target patient population
- A lack of patient focus in the trial design
- A lack of convenience for patients in their participation
- Complicated and/or inefficient study experience flows
- Crafting the eligibility criteria around the patient population that is most likely to lead to favorable study outcomes, without conducting sufficient research to more accurately estimate the recruitment and retention difficulty of the group for a particular study
It is natural for there to be a “push & pull” between the research ideal and the real world practicality. It is important to determine the correct balance between these two sides for your trial, as going too far in either direction will decrease the chance of your clinical study’s success.
The nature of the industry as it is right now is such that there is excess research idealization and not enough emphasis on patient centricity. This distorted orientation has resulted in many clinical trials being unsuccessful, negatively impacting sponsors, patients and the entire clinical trials industry.
The ideal CRO partner should help you make sure that your protocol design sets your study up for success. The CRO should be able to help you determine the proper balance between the research ideal and the real world practicality, and back up their findings with sufficient research and patient data that can project your trial being a success.
Clara Health: When formulating a recruitment and retention plan for our clients, we begin with conducting thorough research into the target trial patient population. This allows us to get a clear understanding of which recruitment channels will yield the best results and what kind of marketing materials will resonate with the prospective study participants.
To ensure accuracy and real-world applicability of our research, we consult and collaborate with our internal patient advocacy and patient support teams, as well as with our clients and patients representing the target trial patient profiles. We then tie our findings back with any existing proprietary data that we have in connection with the therapeutic area or the prospective target patient group.
Our unique position within the clinical recruitment chain gives us the presence and deep-rooted access needed to effectively tap into any of the three patient traffic sources: digital recruitment, offline recruitment, or patient advocacy recruitment.
Once a recruitment campaign has gone live, we constantly monitor, analyze and optimize our performance to make sure that the processes we have in place are as efficient as possible and drive the greatest results. In addition, we have the capability to layer in any traditional advertising (such as billboard ads) if requested by the study sponsor.
1-888-664-9690
[email protected]
- Corporate Brochure
- Case Studies
- Testimonials
- Press Release
- Vascular Surgeons Email List
- Pathologists Email List
- Gastroenterologist Email List
- Chiropractors Email List
- Anesthesiologists Email List
- Plastic Surgeons Email List
- Pediatricians Email List
- Geriatricians Email List
- General Surgeons Email List
- Diabetes Specialist Email List
- Allergist / Immunologist Email List
- Arthritis Specialists Email List
- Cardiologist Email Lists
- Dermatologist Email List
- Neurologists Email List
- Orthopedic Surgeons Email List
- Urologists Email List
- Podiatrist Email List
- Dermatopathologist Email List
- Hematologists Email List
- Pulmonologists Email List
- Nephrologists Email List
- Oncologist Email List
- Rheumatologist Email List
- Radiologist Email List
- Optometrist Email List
- Ophthalmologist Email List
- Acupuncturist Email List
- Optician Email List
- Neonatologist Email List
- Neuropathologist Email List
- Hepatologist Email List
- Traumatologist Email List
- Gynecologists Email List
- Psychiatrists Email List
- Endocrinologist Email List
- Hygienists Email List
- Primary Care Physicians Email List
- General Practitioners Email List
- Osteopathic Physicians Email List
- Emergency Physicians Email List
- Bariatric Physician Email List
- Family Medicine Specialist Email List
- Epidemiologist Email List
- Clinical Lipidologist Email List
- Registered Dieticians Email List
- Psychologists Email List
- Neurosurgeon Email List
- Nutritionists Email List
- Audiologist Email List
- Hypnotists Email List
- ENT Specialists Email List
- Sleep Medicine Specialists Email List
- Sports Medicine Physician Email List
- Physiatrists Email List
- Physician Assistants (PAs) Email List
- Athletic Trainers (ATs) Email List
- Cosmetologists Email List
- Pediatric Allergists Email List
- Naturopathic Doctors Email List
- Veterinarian Email Lists
- Phlebotomists Email List
- Pediatric Radiologists Email List
- AMA Physicians Email List
- Paramedics and EMTs Email List
- Pediatric Neurologist Email List
- Preventive Medicine Specialist Email List
- Reproductive Endocrinologist Email List
- Interventional Cardiologists Email List
- Nuclear Medicine Specialist Email List
- Infectious Disease Specialist Email List
- Radiation Oncologists Email List
- Internal Medicine Specialist Email List
- Mental Health Specialists/Professionals Email List
- Addiction Medicine Specialist Email List
- Aerospace Medicine Physicians Email List
- Hematology & Oncology Specialist Email List
- Medical Geneticist Email List
- Addiction Psychiatrist Email List
- Physical Medicine Rehabilitation Email List
- Cardiac Care Nurses Email List
- Critical Care Nurses Email List
- Emergency Nurses Email List
- Geriatric Nurses Email List
- Home Healthcare Nurses Email List
- Military Nurses Email List
- Nurse Anesthetists Email List
- Nurse Practitioners Email List
- Registered Nurses Email List and Mailing List
- Neurology Nurses Email List
- Orthopedic Nurses Email List
- Occupational Health Nurses Email List
- Pediatric Nurse Practitioners Email List
- Dermatology Nurses Email List
- Perioperative Nurses Email List
- Midwife Nurses Email List
- Family Nurse Practitioners (FNP) Email List
- Public Health Nurses (PHNs) Email List
- Endocrinology Nurses Email List
- Ambulatory Care Nurses Email List
- Women’s Health Nurse Practitioners Email List
- Adolescent Medicine Nurses Email List
- Reproductive Health Nurses Email List
- Transplant Nurses Email List
- Dialysis Nurses Email List
- Allergy / Immunology Nurses Email List
- Family Planning Nurses Email List
- ENT Nurse Practitioners Email List
- HIV/AIDS Nurses Email List
- Community Health Nurses Email List
- School Nurses Email List
- Student Nurses Email List
- Nurse Manager Email List
- Oncology Nurses Email List
- Child Psychiatric Nurses Email List
- IV Certification Nurses Email List
- Licensed Practical Nurses Email List
- Licensed Vocational Nurses Email List
- Clinical Nurse Specialist Email List
- Acute Care Nurse Practitioners Email List
- Office Based Nurses Email List
- Certified Registered Nurse Anesthetist Email List
- Certified Nursing Assistants Email List
- Psychiatric-Mental Health Nurse Practitioners Email List
- Adult Care Nurse Practitioner Email List
- Registered Pharmacist Email List
- Hospital Druggist Email List
- Druggist Email List
- Community Pharmacists Email List
- Pharmacist Clinicians (PhC) Email List
- Ambulatory Care Pharmacists Email List
- Pharmaceutical Email & Mailing List
- Prosthodontists Email List
- Periodontist Email List
- Endodontists Email List
- Pediatric Dentists Email List
- Orthodontist Email List
- Oral Pathologists Email List
- Denturist Email List
- ADA Dentists Email List
- Dental Hygienist Specialists Email List
- Oral and Maxillofacial Radiologists Email List
- Oral and Maxillofacial Surgeons Email List
- Subacute Care Centers Email List
- Cancer Centers and Services Email List
- Ambulatory Surgery Centers Email List
- Diagnostic Imaging Centers Email List
- Urgent Care Centers Email List
- Trauma Centers Email List
- Nursing Homes Email List
- Nursing Home Administrators Email List
- Hospital Email List
- Assisted Living Facilities Email List
- Medical Institutions Email List
- X-Ray Laboratories Email List
- Primary Care Centers Email List
- Dental Clinics Email List
- Blood Bank Centers Email List
- Dental Laboratories Email List
- Clinics Email List
- Physical Fitness Facilities Email List
- Med Spa Email List
- Dental Equipment / Supplies Manufacturers Email List
- Biomedical Equipment Manufacturers Email List
- Electro-Medical Equipment Manufacturers Email List
- X-Ray Apparatus and Tubes Manufacturers Email List
- Ophthalmic Goods Manufacturers Email List
- Surgical Appliances Manufacturers Email List
- Surgical / Medical Instruments Manufacturers Email List
- Neuromodulation Devices Manufacturers Email List
- Cardiovascular Devices Manufacturers Email List
- Therapeutic Devices Manufacturers Email List
- Spine Devices Manufacturers Email List
- Laboratory Equipment and Supplies Email List
- Hospital Equipment and Supplies Email List
The Top 10 Clinical Research Companies in the USA
- Dec 02, 2022
- Posted By: admin

To bring effective medicines and Innovations in healthcare industry , a Clinical Research Organization or Contract Research Organization (CRO) are a must for the Biotech, Medtech, and pharma industries. A CRO offers comprehensive support to their efforts to test, examine, refine and market the latest medicines and devices that help in improving Healthcare Industry.
In 2022, the global clinical research market stands at $63.6 billion , and by 2027, it is expected to reach around an exciting amount of 115.1 billion with a CAGR of 11.0% from 2022 to 2027.
This growth is powered by an increase in R&D activities and expenditures and a significant rise in clinical trials owing to the need for innovations in healthcare. For that reason, it is also essential to be aware of the most prominent global research organizations that offer vital support with their specialized clinical trial solutions. This post will walk you through the ten big Clinical Research Companies In USA . So, let’s begin:

- Founded: 1982
- Headquarters: Durham, North Carolina
- Annual Revenue : $13.87B
- Employee Size: 88,000
IQVIA is one of the largest Clinical Research companies in the world that works profusely to enhance healthcare by integrating the study of human science with discoveries in technology and data science to improve understanding of human health and provide better and quicker care.
It helps pharma companies, along with other medical organizations, to innovate and maximize positive outcomes. In addition to clinical research and development, IQVIA has also developed analytics and technology solutions to aid the medical sector in commercializing products and enhancing customer engagement.
Some clinical trial solutions given by IQVIA are as follows:
- Site identification & Selection
- Help with protocol design
- Design of clinical trial phase -1
- Investigation and improvements of the clinical trial in phases 2 and 3
- Accessibility to global laboratories
2. Pharmaceutical Product Development (PPD)

- Founded: 1985
- Headquarters: Wilmington, North Carolina
- Annual Revenue: $65.0M
- Employee Size: 24,000
PPD, also known as Pharmaceutical Product Development, is an extensive research organization headquartered in the United States but has a widespread global presence.
The company directs its attention to three prime areas: pharma development, lifecycle management services, and laboratory. Besides, it has clients and partners covering various sectors, including pharmaceutical companies, biotech firms, medical device manufacturers, and government or academic organizations.
Some clinical trials offered by PPD include:
- Early Development
- Consulting and Product Development
- PPD Biotech
- The site and patient-centered Solutions
- Clinical Development

- Headquarters: Waltham, Massachusetts
- Annual Revenue: $3B
- Employee Size: 18,000
Parexel is another important name in the list of Clinical Research Companies In USA that specializes on behalf of its pharmaceutical partners in carrying out clinical studies to boost and make certain that the medicine approval process takes place smoothly. It has a wide range of offerings that spreads across every type of clinical trial service to help sponsors work on creating successful trials.
Some of the clinical trial solutions which Paraxel offers:
- Management of clinical data
- Medical writing
- Regulatory affairs consulting
- Design and development of clinical trials at all phases
- Clinical supply chain management
With pride, the company operates in over 50 countries and owns over 95% of the top 200 selling biopharmaceuticals in today’s market.
4. PRA Health Services

- Founded: 1976
- Headquarters: Raleigh, North Carolina
- Annual Revenue: $64.3M
- Employee Size: 16,400
PRA Health Sciences is a contract research organization that provides coverage in more than 90 countries worldwide. It principally centers on contributing therapeutic and operational expertise through integrated applications and supplying local expertise in particular areas. Besides, it works in the early and end stages of clinical trial procedures and the domains of strategy, consultancy, technology, and bio-analytics.
Moreover, it works hard to speed up medicine development operations to introduce better and more effective medicine sooner in the market.
Let’s take a peek at the PRA Health Sciences clinical trial solutions:
- Onsite support services
- Personalized solutions for Biotech
- Clinical Diagnostics
- Site Commercial Solutions
- Protocol Consultation and Study Design
- PRA’s Laboratories for Medicine Development
5. Syneos Heath

- Founded: 1999
- Headquarters: Morrisville, North Carolina
- Annual Revenue: $5,213M
- Employee Size: 28,000
With the merger of inVentiv Health and INC Research, Syneos Health was created that combines every discipline involved in bringing new therapies or products to market, from clinical research to consulting and commercialization.
Though it provides clinical development services spanning all stages, it primarily owns a specialization in helping healthcare organizations with the late stages of clinical trials. Moreover, its commercial solutions cover communication, consulting, and medication adherence.
The clinical trial solutions it offers include:
- Early phase
- Decentralized clinical trial solutions
- Clinical data management
- Pharmaceutical
- Medical Device Diagnostics

- Founded: 1978
- Headquarters: Burlington, North Carolina
- Annual Revenue: $16.1B
- Employee Size: 70,000
Laboratory Corporation of America, or LabCorp, focuses on offering extensive clinical laboratory solutions and end-to-end medication and diagnostic development and commercialization.
The company develops special testing operations such as HIV genotyping, oncology testing, phenotyping, clinical trials, and diagnostic genetics. Moreover, its services are spread through care organizations, hospitals, government agencies, pharmaceutical companies, and physicians.
Some clinical trial solutions provided by LabCorp are:
- Preclinical Services
- Post-Marketing Solutions
- Medical Devices
- Clinical Trial Laboratory Services
- Clinical Trials
- Data & Technology
7. Charles River Lab

- Founded: 1947
- Headquarters: Wilmington, Massachusetts
- Annual Revenue: $690.4M
- Employee Size: 20,000
Charles River laboratories proudly span its capabilities throughout the medical R&D process from basic research to pre-clinical stage testing, manufacturing and commercialization within two significant services: Good Laboratory Practice (GLP) and non-GLP. It offers precise support to help its partners advance their research and efforts to introduce effective medicines in the market.
The company further serves biotechnology and pharmaceutical companies, hospitals, government agencies, and academic institutions.
Some of the clinical development solutions it offers include:
- Bioanalysis
- Clinical Kitting services
- Data management
- Quality control
- Stability testing
8. Fisher Clinical Services

- Founded: 1989
- Headquarters: Center Valley, Philadelphia
- Annual Revenue: $17B
- Employee Size: 50,000
Fisher Clinical Services is a part of Thermo Fisher Scientific that has been for over 20 years in the business of supply chain management. It focuses exclusively on working with distribution and packaging requirements of clinical trials happening around the globe.
The company is committed to providing high-value products which adhere to the standard of pharmaceutical companies and is reliable, sustainable and perform well.
Some of the clinical trial solutions offered by Fisher Clinical Services are:
- Cell & Gene Therapy
- Clinical Label Services
- Clinical Supply Optimization services
- Direct to patient
- Biologistics management
9. Medpace Holdings

- Founded: 1992
- Headquarters: Cincinnati, Ohio
- Revenue: $1B
- Employee Size: 1,001-5,000
Medpace offers support services for phases one to five of clinical trials for biotechnology, pharmaceutical companies, and medical device industries. It is a scientifically-driven organization that offers full service and helps industries make key differences with their contributions to the healthcare sector.
Some of the clinical research solutions that you receive from Medpace are:
- Medical Writing
- Quality Assurance
- Clinical Monitoring
- Clinical Trial Management
- Risk-based Monitoring
- Regulatory Affairs
- Biostatistics and Data sciences
10. Advanced Clinical

- Founded: 1994
- Headquartered: Deerfield, Illinois
- Annual Revenue: $106M
- Employee Size: 501-1,000
A mid-size and full-service Clinical Research organization that helps sponsors run clinical trials and offers various solutions across therapeutic fields. It allows clients to secure better outcomes through candid discussions, conversations, foresight, and innovative solutions.
The company works hard to improve every life touched by clinical research. As such, some clinical solutions provided by Advanced Clinical are:
- Project management
- Clinical monitoring
- eTMF and document management
- Quality & Validation
The Bottom Line
There you have it – the best Clinical Research Companies In USA to get support from while working on new medicine or medical devices.
CROs play a critical role in the pharmaceutical, biotech, and medical device industries to manage and lead their clinical trials now more than ever due to rapidly rising prices, regulations, and tighter deadlines. Also pharmaceutical companies approaching Clinical Research Organizations for their R&D activities to stay competitive, flexible, and profitable against all odds.

10 Best Clinical Research Organizations In The USA
With the growing population, diseases affecting mankind are also increasing. The old medications are being obsolete for certain diseases like bacterial infections, and new effective medicines are urgently needed. The clinical research institutions facilitate drug manufacture by running preclinical and clinical tests on the drugs. In addition, they also study safety, efficacy, and effectiveness of medical devices, diagnostic products, and treatment regimens intended for human health. The healthcare industry is booming with a lot of clinical research institutions in the US and all around the world.
Table of Contents
Top 10 American CROs
The United States of America has some of the top clinical research organizations in the world. Amongst them, here is the list of the 10 Best Clinical Research Organizations In The USA.
Annual Revenue: $9.6B Headquarters: Princeton, New Jersey Number of Employees: 50,000+ Total Funding: $0 Status: Public, Subsidiary of LabCorp Founded: 1996
In 2014, LabCorp acquired Covance for $6.1B. Covance, a global contract research organization under LabCorp, is the world’s leading healthcare diagnostics company, providing comprehensive clinical laboratory services and end-to-end solutions for drug and diagnostics development and commercialization. With its mission to bring new and innovative medicines to patients sooner, it has worked on more than 50 drugs available in the market today. It provides drug development services across all phases of development and multiple therapeutic areas.
Annual Revenue: $9.74B Headquarters: Durham, North Carolina Number of Employees: 55,000 Operating Income: $1.33B Status: Public, Independent Company Founded: 1982
Working to enhance healthcare by integrating health information technologies and clinical research, IQVIA explores a new path to better health outcomes via Human Data Science. It integrates the study of human science with breakthroughs in data science and technology to advance our understanding of human health, and help everyone make better, more insightful decisions.
3. Syneos Health
4. parexel international.
Annual Revenue: $2.4B Headquarters: Waltham, Massachusetts Number of Employees: 18,660 Total Funding: $0 Status: Public, Subsidiary of Pamplona Capital Management LLP Founded: 1982
5. PRA Health Sciences
Annual Revenue: $2.7B Headquarters: Raleigh, North Carolina Number of Employees: 16,400 Total Funding: $305.6M Status: Public, Subsidiary of Kohlberg Kravis Roberts & Co. L. P Founded: 1976
PRA participated in the pivotal or supportive trials and/or key NDA support services that led to the approval of 85 important products currently on the market. Its aim is to improve the drug development process for getting better medicines to market sooner.
6. Pharmaceutical Product Development
Annual Revenue: $1.9B Headquarters: Wilmington, North Carolina Number of Employees: 18,583 Total Funding: $0 Status: Private, Independent Company Founded: 1985
PPD applies innovative technologies, therapeutic expertise, and a firm commitment to provide comprehensive, integrated drug development, laboratory, and lifecycle management services. Its clients and partners include pharmaceutical, biotechnology, medical device, academic, and government organizations. It helps to bend the cost and time curve of drug development and optimize value in delivering life-changing therapies to improve health.
7. Charles River Laboratories
Annual Revenue: $2.27B Headquarters: Wilmington, Massachusetts Number of Employees: 14,700 Total Funding: $224M Status: Public, Independent Company Founded: 1947
Icon helps its clients to accelerate the development of drugs and devices that save lives and improve quality of life. It offers a full range of consulting, development, and commercialization services from a global network of offices in 37 countries.
9. WuXi AppTec
10. medpace holdings.
Annual Revenue: $704.6M Headquarters: Cincinnati, Ohio Number of Employees: 2,900 Total Funding: $161M Status: Public, Subsidiary of Cinven Limited Founded: 1992
Medpace provides services for Phase I-IV of drug and medical device development services, including regulatory services and central laboratory services. It combines scientific guidance, efficient clinical trial management, global regulatory leadership, and innovative technologies to deliver high-quality results.
Related Posts
Top 10 medical device companies in the world, top 10 jobs you can get in the biotechnology sector, top 10 biotechnology universities in the world.
Top 28 Companies in Pharmaceutical Clinical Trials

The pharmaceutical clinical trials industry plays an indispensable role across the global healthcare spectrum. Made of numerous companies, their primary responsibility involves conducting research, managing clinical trials, and ensuring the safety and efficacy of medical treatments. These corporations frequently collaborate with pharmaceutical and medical device manufacturers, healthcare organizations, and research institutes. As innovation intensifies and international healthcare challenges elevate, the pharmaceutical clinical trials industry is set to register unprecedented expansion, while contributing towards the introduction of advanced medical treatments and therapies.
Top 28 Pharmaceutical Clinical Trials Companies
- Website: parexel.com
- Headquarters: Newton, Massachusetts, United States
- Founded: 1982
- Headcount: 10001+
- Latest funding type: Acquired
Parexel is a global company that offers services and solutions in the clinical research and healthcare industry. They provide expertise in various areas such as clinical trial management, regulatory outsourcing, medical monitoring, and consulting. They work with pharmaceutical companies, medical device manufacturers, and other healthcare organizations to support the development and advancement of new medicines and therapies.
2. Worldwide Clinical Trials
- Website: worldwide.com
- Headquarters: Research Triangle Park, North Carolina, United States
- Founded: 1986
- Headcount: 1001-5000
Worldwide.com is a global company specializing in clinical trial services and real-world evidence. With over 35 years of experience, they offer a range of services including clinical monitoring, data management, drug safety, patient recruitment, and project management. They focus on therapeutic areas such as cardiovascular, metabolic, neuroscience, oncology, and rare diseases. Their innovative lab services and personalized approach make them a trusted partner in the pharmaceutical industry.
- Website: medpace.com
- Headquarters: Cincinnati, Ohio, United States
- Founded: 1992
- Latest funding type: Ipo
Medpace is a full-service Contract Research Organization (CRO) that accelerates the advancement of safe and effective medical therapeutics. With a global reach, Medpace offers a wide range of services including clinical trial management, patient recruitment, and drug safety monitoring.
4. Premier Research
- Website: premier-research.com
- Headquarters: Morrisville, North Carolina, United States
- Founded: 1989
- Latest funding type: Series Unknown
Premier Research is a clinical research organization that helps innovative biotech and specialty pharma companies transform ideas and breakthrough science into new medical treatments. They offer a range of services, including product development consulting, global regulatory consulting, nonclinical studies, quality assurance, and project management.
5. Novotech
- Website: novotech-cro.com
- Headquarters: Sydney, Nsw, Australia
- Founded: 1996
- Latest funding type: Debt Financing
Novotech is a leading Asia Pacific biotech specialist CRO that offers cost competitive solutions for clinical research. They provide a full range of services including medical and regulatory consulting, patient recruitment, site selection, and clinical operations. With expertise in infectious diseases, vaccines, and biometrics, Novotech offers high-quality research and data management. They have a large homogenous population and locations in various countries.
6. Clinipace
- Website: clinipace.com
- Founded: 2003
- Headcount: 501-1000
Clinipace is a global CRO that specializes in clinical research and development. They provide personalized solutions to biotech and pharmaceutical companies, offering expertise in clinical technology, analytics, regulatory and strategic product development. With a focus on collaboration and control, Clinipace guides their clients through the entire clinical development program.
7. Crown Bioscience
- Website: crownbio.com
- Headquarters: San Diego, California, United States
- Founded: 2006
Crown Bioscience is a Contract Research Organization (CRO) that empowers customers to improve human health. They engage scientific talent, uphold quality standards, and pursue innovation to accelerate and de-risk drug development. Their services include biomarker discovery, efficacy testing, DMPK, and pharmacology.
8. Veeda Clinical Research Limited
- Website: veedacr.com
- Headquarters: Ahmedabad, Gujarat, India
- Founded: 2004
- Latest funding type: Private Equity
Veeda Clinical Research is a company that provides quality research solutions for sponsors and regulatory authorities. They conduct early phase clinical trials in healthy volunteers and patients, specializing in ophthalmology trials. Additionally, they offer bioequivalence and bioavailability studies, drug discovery and development services, clinical endpoint phase III studies, and safety monitoring services.
9. Quanticate
- Website: quanticate.com
- Headquarters: Hitchin, Hertfordshire, United Kingdom
- Founded: 1994
- Headcount: 201-500
Quanticate is a global biometric Clinical Research Organization (CRO) offering statistical and data expertise. They help pharmaceutical and biotech companies bring their drugs to market by providing fast and reliable access to expert biometric teams. Their services include biostatistics, statistical programming, clinical data management, and biostatistical consultancy.
10. Nucleus Network
- Website: nucleusnetwork.com.au
- Headquarters: Melbourne, Victoria, Australia
Nucleus Network is a company that offers clinical research services focusing on early phase and vaccine trials. They provide state-of-the-art facilities, experienced staff, and innovative approaches to help pharmaceutical and biotech companies accelerate their drug development processes.
11. Richmond Pharmacology
- Website: richmondpharmacology.com
- Headquarters: London, London, United Kingdom
- Founded: 2001
- Headcount: 51-200
Richmond Pharmacology is a Clinical Research Organisation that conducts early phase clinical trials in a wide range of medical conditions with patients and healthy volunteers. They work with international pharmaceutical and biotech organizations to drive innovation in the field of clinical research.
12. Integrium, LLC
- Website: integrium.com
- Headquarters: Tustin, California, United States
- Founded: 1998
Integrium is a full-service Clinical Proof of Concept (PoC) firm specializing in various therapeutic areas, including cardiovascular, metabolic disease, and dermatology research. We assist pharmaceutical companies in designing and executing clinical trials to achieve their objectives on time and with expected quality. With cutting-edge technology and unparalleled services, we play a crucial role in advancing new medical advancements. Our team of experts works collaboratively with clients, investigators, and vendors to ensure the successful implementation of clinical trials.
13. Mithra Biotechnology Inc.
- Website: mithracro.com
- Headquarters: 新北市 New Taipei City, Taiwan, Taiwan
- Founded: 1988
MithraCro is a biotechnology company specializing in pharmaceutical analysis services, with expertise in bioequivalence testing, clinical trial services, drug metabolism research, and protein analysis.
14. Pearl Therapeutics
- Website: devprobiopharma.com
- Headquarters: Morristown, New Jersey, United States
- Founded: 2020
- Headcount: 11-50
DevPro Biopharma is a clinical research and development accelerator that specializes in transforming molecules into medicines. They offer comprehensive services to assist in the development of pharmaceutical products.
15. Almac Group
- Website: almacgroup.com
- Headquarters: Craigavon, County Armagh, United Kingdom
- Founded: 1968
- Headcount: 5001-10000
- Latest funding type: Grant
Almac Group provides a range of services in the pharmaceutical industry, including storage and distribution, product launch and distribution, API development and manufacture, clinical trial assays, genomic services, clinical testing, and clinical supply chain management. They offer flexible solutions to meet the needs of their clients and ensure the successful launch and distribution of pharmaceutical products. Almac Group is committed to exceptional quality and customer satisfaction.
16. 4B Technologies, Ltd
- Website: 4btechnologies.com
- Headquarters: 苏州市, Jiangsu, China
- Founded: 2017
- Latest funding type: Series B
4B Technologies is a biopharmaceutical company with a leadership team composed of elite professionals from various fields. They are dedicated to developing innovative and effective treatments for neurological diseases.
17. TechnoDerma Medicines
- Website: tkskin.com
- Headquarters: Xuzhou, Jiangsu, China
- Founded: 2014
- Latest funding type: Series A
Techonderma Medicines, Inc. is a private clinical-stage biopharmaceutical company focused on developing innovative therapies for various skin conditions.
- Website: caidya.com
Caidya is a clinical research organization (CRO) that offers personalized services and expertise in various therapeutic areas. They specialize in conducting regional or multinational studies using efficient operational models, local market knowledge, and advanced technologies. Caidya is committed to transparency, clinical development expertise, and personalized experiences for their clients.
19. Novum Pharmaceutical Research Services
- Website: novumprs.com
- Headquarters: Pittsburgh, Pennsylvania, United States
- Founded: 1972
Novum is a global clinical research organization (CRO) with a focus on delivering high-quality research outcomes and exceptional customer satisfaction. With nearly fifty years of experience, Novum offers a comprehensive suite of services in early clinical development, bioanalytical testing, and phase II-IV clinical trial management. The company's dedicated and skilled team, extensive training programs, and global resources enable efficient project optimization and streamline timelines and budgets.
20. PharPoint Research, Inc.
- Website: pharpoint.com
- Headquarters: Durham, North Carolina, United States
- Founded: 2007
PharPoint Research provides drug development solutions to clients of all sizes. Contact us to speak with a representative about how PharPoint can help with your current or upcoming study needs.
21. NC Coast Clinical Research Initiative
- Website: nccoastclinicalresearch.com
- Headquarters: United States
- Founded: 2012
NC Coast Clinical Research is a consortium of 60+ CROs and 100+ clinical research support companies, providing comprehensive integrated drug development, laboratory and lifecycle management services.
22. Quinta-analytica
- Website: quinta.cz
- Headquarters: Praha 10, Hlavni Mesto Praha, Czechia
- Founded: 1997
Quinta is an innovative and trusted world leader in pharmaceutical analysis, R&D, and clinical testing in both human and veterinary medicinal products.
23. LINK Medical Research
- Website: linkmedical.eu
- Headquarters: Oslo, Oslo, Norway
- Founded: 1995
LINK Medical Research is a leading contract research organization (CRO) in Northern Europe. They offer a strategic partnership and provide highly competent teams and evidence-based documentation for superior clinical outcomes. From early phase development to post-marketing, they offer expert guidance through every stage of product development. With a well-integrated local presence in the Nordics, UK, and Germany, they make the journey to market simple and cost-effective.
24. Medlab Clinical Ltd
- Website: medlab.co
- Headquarters: Botany, New South Wales, Australia
Medlab.co is a company that specializes in drug delivery technology. They have a proprietary NanoCelle® platform with 57 patents worldwide. They offer partnering opportunities, clinical trials, and a range of products. Medlab.co is committed to transforming medicine and improving patient outcomes.
25. Clexio Biosciences
- Website: clexio.com
- Headquarters: Petach Tikva, Israel, Israel
- Founded: 2018
Clexio Biosciences is a multi-asset company dedicated to developing novel therapies for patients suffering from neurological and psychiatric conditions.
26. Suzhou Abogen Biosciences
- Website: abogenbio.com
- Headquarters: 苏州市, 江苏省, China
- Founded: 2019
- Latest funding type: Series C
Abogenbio is a biotechnology company focused on innovative drug development and research. With a team culture of openness and collaboration, we strive to bring hope and healing to patients through the creation of groundbreaking medicines. We are dedicated to fostering a fearless and accountable environment where partners can thrive and pave the way for a brighter future.
27. Abond CRO Inc.
- Website: abondcro.com
- Headquarters: Allendale, Michigan, United States
- Founded: 1974
ABOND is a full-service CRO that offers consulting, clinical operations, data management, and statistical analysis. Our commitment to honesty, focus, and responsibility sets us apart. We provide reliable outcomes and are always answerable for our work.
28. Novatek Pharmaceuticals
- Website: novatekpharmaceuticals.com
Novatek Pharmaceuticals Inc. is a startup based in Houston, TX that provides scientific guidance and conducts clinical research for immunomodulatory drugs related to cancers and infectious diseases. They are currently raising Series A for clinical trials and pipeline development.
Want to find more pharmaceutical clinical trials companies?
If you want to find more companies that offer comprehensive clinical trial services and research solutions you can do so with Inven . This list was built with Inven and there are hundreds of companies like these globally.
With Inven you'll also get to know the company's:
- Ownership: Which of these are private equity backed? Which are family-owned?
- Contact data: Who are the owners and CEO's? What are their emails and phone numbers?
- Financials: How do these companies perform financially? What are their revenues and profit margins?
...and a lot more!
Find companies 10x faster with Inven

Keep on reading
More articles.


Velocity is the world's leading integrated site organization.
Sponsors and cros trust velocity to deliver high-quality clinical trial data and patient care with unprecedented efficiency., simplify everything from site selection to study close-out.
Velocity unifies operational processes to provide world-class sites, reliable enrollment, and predictably high performance for your trials.
The right sites. The right investigators. The right partner for you.
Strategically located to give you access to diverse specialty populations, Velocity's sites are supported by next-gen technologies and patient engagement capabilities. Welcome to recruitment and retention reimagined.
Scale for a purpose: Supporting research programs worldwide
From the leading pharma companies, to the most pioneering biotech startups, Velocity supports those who are exploring new frontiers in human health.
Whether you’re ready to conduct a single-site study or a complex, high-volume clinical trial, contact Velocity.

Paul Evans Featured in Business North Carolina Article on AI in the State’s Clinical Research Industry
Paul Evans was recently featured in a Business North Carolina article discussing the emerging impact of artificial intelligence on North Carolina’s clinical research industry. As Paul notes, “Our industry is … Read more

Drs. Essink and Overcash Author Article Featured in Nature Communications for Moderna COVE Study
Brandon Essink, MD, CPI, and J. Scott Overcash, MD, were authors of a recent Nature Communications article, “Long-term safety and effectiveness of mRNA-1273 vaccine in adults: COVE trial open-label and … Read more

VISION Reaches $2 Million in Patient Stipends Paid Globally
Since launching in-app stipend payments, Velocity has paid more than $2 million to patients globally through VISION. The mobile app has also contributed to more than 2,500 patient randomizations, empowering … Read more

Kris Kowdley, MD, Supported Research Leading to Accelerated FDA Approval of Gilead’s Livdelzi® for Primary Biliary Cholangitis
Another milestone for patients with primary biliary cholangitis (PBC) — Gilead’s Livdelzi® (seladelpar) has received accelerated FDA approval as a second-line treatment for PBC. Kris Kowdley, MD, AGAF, FAASLD, FACP, … Read more
Quality. Continuity. Velocity.
To provide the best experiences, we use technologies like cookies to store and/or access device information. Consenting to these technologies will allow us to process data such as browsing behavior or unique IDs on this site. Not consenting or withdrawing consent, may adversely affect certain features and functions. Cookie Policy (US) Cookie Policy (EU) Cookie Policy (UK)
- Reach Our Healthcare List Expert +1 (786) 408 5757
- Browse Data Cards
- GET A QUOTE

The Top Players in Clinical Research: A Review of the Leading Companies
Top 10 leading clinical research companies in the usa.
The landscape of drug discovery and development is intricate, demanding significant resources at each stage of the process. As pharmaceutical companies strive to bring groundbreaking therapies to market, they increasingly rely on the expertise of Contract Research Organizations (CROs). These organizations play a vital role in supporting drug manufacturers throughout the entire drug development journey, from initial discovery to final approval. These CROs offer a comprehensive range of services, including clinical trial management, data research, and project management. Their critical role has become even more evident during the pandemic-induced challenges, where the need for rapid vaccine and drug development resulted in a surge of clinical trials.

The Impact of Covid-19 on Clinical Trials
At the outset of the pandemic, the clinical research landscape faced initial setbacks. However, the urgency to combat Covid-19 created an unprecedented rise in the number of clinical trials. The pressing demand for effective vaccines and treatments drove pharmaceutical companies and CROs to collaborate more than ever before. Looking ahead, the future of CROs appears promising, fueled by the rise of technologies that enable decentralized clinical trials. These modern approaches leverage digital tools and remote patient monitoring, reducing the need for physical site visits and expanding the reach of clinical research.
The Growth of the Global CRO Services Market
The significance of CROs is evident as the global CRO services market continues to expand exponentially. Projections indicate that this market, which was valued at US$76.6 billion , is expected to reach an impressive US$127.8 billion by 2028. This growth reflects the pivotal role CROs play in revolutionizing drug development and medical research.
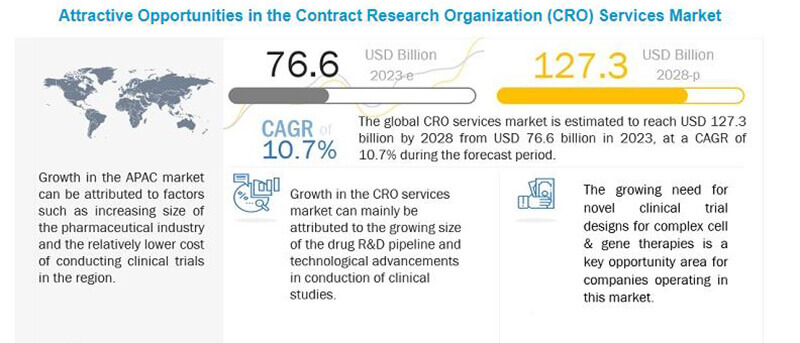
Source: Markets&Markets
Unveiling the Best 10 Clinical Research Organizations in the Field
Join us as we embark on this insightful journey, where we shine a spotlight on the ten biggest CROs shaping the pharmaceutical landscape. –
Source: Lablcorp
Labcorp, a leading provider of comprehensive drug development solutions for various industries, achieved a significant milestone in 2015 with its acquisition of Covance for an impressive $6 billion. This strategic union brought together Covance's expertise in drug development and Labcorp's unparalleled medical testing capabilities, propelling the company to become the world's foremost healthcare diagnostics company.
Over the past decade, Labcorp has continued to expand its influence through a series of strategic acquisitions. Notable additions to their portfolio include LipoScience, Inc., Bode Technology Group, Sequenom, MNG Laboratories, and Personal Genome Diagnostics (PGDx). These acquisitions have further strengthened Labcorp's position in the market, broadening its scope and enhancing its ability to provide cutting-edge solutions.
Looking forward to 2023, Labcorp unveiled its plans to spin off its Contract Research Organization (CRO) segment, creating a separate, independent publicly traded entity known as Fortrea.
Source: IQVIA
In 2016, two industry giants, Quintiles and IMS Health, joined forces and rebranded as IQVIA, establishing the largest Contract Research Organization (CRO) globally. With a presence in over 100 countries, IQVIA brings together cutting-edge advancements in data science, technology, and human science expertise, offering clients a comprehensive end-to-end clinical and commercial service. Through a series of strategic acquisitions of smaller specialist companies, IQVIA has strengthened its position and continues to lead the way in the world of CROs.
In the year 2022, IQVIA demonstrated remarkable growth and success, generating revenues of $14.494b . This impressive growth, amounting to 3.29% on a reported basis and 7.8% at constant currency , highlighted the company's continued upward trajectory. Despite uncertain market conditions, IQVIA achieved record bookings and surpassed the goals set in their Vision 22 plan, underscoring their commitment to excellence and innovation.
Source: Parexel
Parexel continues to hold its position as one of the largest CROs globally. Specializing in Phase I to IV clinical development services, Parexel plays a vital role in expediting and ensuring the smooth progress of the drug approval process. With a comprehensive array of service offerings, the company caters to nearly every aspect of clinical trial management, supporting sponsors in conducting successful clinical studies.
In a significant development in November 2021, Parexel was acquired by EQT Private Equity and Goldman Sachs for a remarkable $8.5 billion . Despite this transition, the commitment to putting patients first remains unwavering, ensuring that Parexel maintains its patient-centric trajectory. The newly constituted board of directors brings together a wealth of experience in the life sciences industry, further strengthening Parexel's position as a leader in the field.
4. Pharmaceutical Product Development (PPD)
Source: PPD
Pharmaceutical Product Development (PPD) stands as a leading global Contract Research Organization (CRO) with an extensive workforce of over 30,000 professionals worldwide.
The acquisition of Evidera in 2016 marked a significant milestone for PPD, solidifying their position as a leader in real-world research. Leveraging Evidera's expertise in real-world evidence, PPD has enhanced its capabilities in providing life science companies with a crucial element of the clinical development process, maintaining a competitive edge in the industry. Subsequent strategic acquisitions of Synexus (now Accelerated Enrollment Solutions) and Bioclinica, both patient recruitment and clinical research site businesses, respectively, have further augmented PPD's offerings. These collective advancements have propelled PPD's core organic growth to "high teens" in the segment, as the company achieved remarkable full-year 2022 results. With over $7.00 billion in revenue and a significant contribution of over $2.00 to adjusted earnings per share, PPD remains a dominant force in the CRO landscape, upholding their mission to facilitate groundbreaking clinical research and development.
5. Syneos Health
Source: Syneos Health
Founded in 1999, Syneos Health has its headquarters in Morrisville, North Carolina. With an impressive annual revenue of $5,213 million and a workforce of 28,000 employees, Syneos Health is a leading international Contract Research Organization (CRO) that offers a wide range of services spanning all aspects of bringing new therapies and products to the market.
While Syneos Health provides clinical development services across all stages of drug development, it has particularly specialized expertise in assisting healthcare organizations during the late stages of clinical trials. Some of them include Early phase trials, Late phase trials, Decentralized clinical trial solutions, Clinical data management, Pharmaceutical trial services, Medical device diagnostics trial services, and more.
Source: ICON
Operating across 46 locations worldwide, ICON is a top-tier Contract Research Organization (CRO) that provides a comprehensive range of services, including consulting, clinical development, and commercialization services.
In a pivotal move in 2016, ICON formed strategic partnerships with Genomics England for the UK's ambitious 100,000 Genomes Project and IBM Watson for oncology research support. These collaborations expanded ICON's service offerings and strengthened their presence in the fields of genomic science and oncology research, creating new opportunities for clinical research jobs in these cutting-edge sectors.
Additionally, the year 2022 marked a significant milestone for ICON, as it achieved impressive financial results. With full-year revenues amounting to US$7.7 billion , the company witnessed substantial growth of 41.2% compared to the previous year. On a constant currency basis, the revenue surge reached an outstanding 45%, reflecting ICON's continued success in advancing clinical research and serving as a leading partner for the life sciences industry.
7. Charles River Laboratories
Source: Charles River Lab.
Since its inception in 1947, this company has grown into an exceptional industry leader, specializing in cutting-edge cell and gene therapies. Not only does it provide vital lab services to the pharmaceutical, medical device, and biotech industries, but it also stands out with its impressive global presence. With a robust network spanning over 110+ facilities scattered robust network spanning over 110+ facilities scattered across 20+ countries, the company offers unmatched accessibility and support to clients worldwide, solidifying its reputation as a trailblazer in the field.
As of the latest data available in 2023, the company boasts an impressive annual revenue of 4.092B, a 12.73% increase year-over-year , reflecting its strong position in the market and significant contributions to the life sciences industry.
Source: CTI
CTI Clinical Trial and Consulting Services has been making waves in the industry since its establishment in 1999. With a remarkable track record of driving over 150 new drug and medical device approvals, the company has firmly established itself as a leader in the field.
Boasting a vast international footprint, CTI operates offices in more than 60 locations across the globe, ensuring its presence and support in key regions such as North America, Europe, Latin America, Middle-East, Africa, and Asia-Pacific. In a strategic move in 2021, CTI further bolstered its position by announcing the acquisition of Dynakin, a European-based strategic consulting company. This acquisition aimed to fortify CTI's standing as a comprehensive and dynamic full-service clinical research organization, ready to take on the challenges of a rapidly evolving healthcare landscape.

Source: Medpace
MEDPACE is a global organization with a workforce of over 5,000 employees spread across 40 countries. In 2022, Medpace reported an impressive full-year revenue of $1.46 billion, reflecting a substantial 27.8% increase from the previous year. In an earnings call, Jesse Geiger, the President at Medpace, highlighted the company's ability to grow its workforce by 15.8% despite facing challenges in a competitive labor environment. In 2023, Medpace places significant emphasis on employee retention and ongoing recruitment as top priorities to support its future business endeavors.
10. Fisher Clinical Services
Source: Thermo Fisher
Fisher Clinical Services is a vital division of Thermo Fisher Scientific with a rich history of over two decades in supply chain management. The company's core focus centers on efficiently handling the distribution and packaging requirements for clinical trials conducted worldwide.
With an impressive annual revenue of $20 billion and a workforce of 70,000 employees, Fisher Clinical Services is dedicated to delivering high-value products that align with the rigorous standards of pharmaceutical companies, ensuring reliability, sustainability, and optimal performance. The company plays a crucial role in supporting the success of clinical trials across the globe.
In conclusion, the best Clinical Research Companies in the USA serve as indispensable partners for pharmaceutical, biotech, and medical device industries embarking on new medicine and medical device development. With the complexities of clinical trials escalating, CROs play a pivotal role in managing and leading these trials efficiently amidst rising prices, stringent regulations, and tighter deadlines.
As the pharmaceutical landscape continues to evolve, pharmaceutical companies are increasingly turning to Clinical Research Organizations for their R&D activities to maintain competitiveness, flexibility, and profitability. With their proven track record, comprehensive services, and commitment to excellence, the best Clinical Research Companies in the USA stand as crucial allies in the quest to advance medical science and improve patient outcomes. Through fruitful partnerships with CROs, the pharmaceutical, biotech, and medical device industries continue to push the boundaries of innovation and deliver life-changing products to those in need.
Healthcare Databases
- Healthcare Email List
Doctors Email List
- Nurses Email List
- Medical Executives Email List
- Physician Email List
- Dentist Email List
- Pharmacist Email List
- Healthcare SIC Code
- Healthcare NAICS Code
- Chiropractors Email List
- Veterinarian Email List
- Radiologists Email List
- Psychiatrists Email List
- Pediatrician Email List
- Cardiologists Email List
- Neurologist Email List
- Dermatologist Email List
- Oncologists Email List
Medical Services
- Hospitals Email List
- Pharmacy Email Database
- Nursing Homes Email List
- X-Ray Laboratories Mailing List
- Pharmaceutical Email List
- Assisted Living Email List
- Dental Clinics Email List
- Clinics Email List
- Physical Fitness Facilities List
HealthcareMailing
- +1 (786) 408 5757
- [email protected]

- Privacy Policy
31 Biotech Companies Bolstering Life Sciences
These top biotech companies are impacting medical research and drug discovery.
While biotech has long been a vital component of the global tech market, this pivotal sector has seemingly reached new heights over the past decade. And although organizations around the world have tried to rule the life sciences space, the United States remains the undisputed biotech leader.
Biotech Companies to Know
Flatiron health.
According to reports , the nation’s life sciences sector alone generates over $112 billion in revenue. In light of biotech’s mounting economic impact, it’s no surprise that tech hubs across the country are witnessing a surge in life sciences companies , bolstering the nation’s status as a biotech epicenter.
Over the past couple years, the importance of the biotech industry has been even further amplified, as the world raced to create a COVID-19 vaccine.
While focus has been on combating COVID-19, the country’s biotech leaders continue to dedicate themselves to addressing other pressing medical issues. Backed by teams of world-renowned scientists and technologists, these organizations are addressing challenges across the healthcare spectrum. Whether they’re formulating new cancer treatments or harnessing the power of human genetics, the nation’s biotech companies are making a monumental impact on the future of medical research and drug discovery.
We’ve rounded up the top biotech companies to give you a glimpse into the burgeoning life sciences sector.
Location: New York, New York
Pfizer is a biotech and pharmaceutical company that develops digital solutions and medical treatments. It specializes in the development and production of therapies, vaccines and drugs for disease prevention, treatment and overall wellness. The company is best known for developing the first FDA-authorized Covid-19 vaccine in its partnership with BioNTech, during which it used remote site monitoring and real-time predictive modeling to support large-scale clinical trials.
Formation Bio
Location: New York, New York Formation Bio is a drug development company that uses AI to discover new drugs, and then to develop them into pharmaceutical products that are viable to bring to market. By leaning on AI for every stage of the drug development process, from research to trial design to marketing, the company is able to radically decrease the timeline that new pharmaceutical development typically follows.
Endpoint Clinical, Inc.
Location: Wakefield, Massachusetts
Endpoint Clinical makes tech that life sciences teams rely on to keep clinical trials for medications, medical devices and other biotech products running on time, on budget and in compliance. Using interactive response technology, or IRT, Endpoint facilitates logistics including patient randomization and equipment supply chains to keep the patient-participant and drug supply sides of medication and biotech device trials on course.
Novo Nordisk
Location: Bagsværd, Denmark
Novo Nordisk has been around since the 1920s, and today focuses on developing treatments and pharmaceuticals for chronic illnesses such as diabetes and hemophilia. The company has roughly 860 research and development employees working in the United States and is conducting clinical trials in more than four dozen countries.
Location: Boston, Massachusetts
Biotech firm Asimov is a genetic design company. It uses machine learning, computer-aided design and a niche of biology known as synthetic biology — a field that redesigns existing organisms for new uses — to genetically engineer therapeutics like biologics and gene therapies. Asimov markets a platform that includes host cells, a genetic parts library, technical guides and design software for creating genetic systems in various types of cells.
Location: Menlo Park, California
GRAIL is on a mission to detect cancer at earlier stages so that it can be more easily cured. The company focuses on seeking cancer signals in the blood, guided by the belief that tumors release cell-free nucleic acids into the bloodstream, which are thought to be a direct measure of cancer and can potentially be detected before the onset of symptoms. GRAIL intends to foster a deeper understanding of cancer biology through its high-intensity sequencing assays and population-scale clinical studies.
Schrödinger, Inc.
Location: Cambridge, Massachusetts
Schrödinger is powering drug research with its computer simulations platform. Founded in 1990, the company employs physics-based methods to evaluate chemical matter and compounds before synthesis. As a result, researchers should see faster lead discoveries, accurate property descriptions and access to large-scale molecular exploration. The company also provides integrated data and visualization tools
Location: San Diego, California
Illumina seeks to make “genomics more useful for all, for a better understanding of human health .” The company develops products for cancer research, reproductive health, genetic and rare diseases and microbial genomics.
Location: Lexington, Massachusetts
Founded in the 18th century, Takeda is a global pharmaceutical company with a strong emphasis on research and development. The company focuses its research in several areas: oncology, rare diseases, plasma-derived therapies, vaccines, neuroscience and gastroenterology. In the U.S. alone, Takeda has created over 50 products and service programs that benefit patients and physicians.
Viome Life Sciences
Location: Bellevue, Washington
Viome Life Sciences believes microbial and human gene expression are altering how biological activities and human health are connected. The company uses an mRNA platform to digitize human data to prevent, diagnose and treat chronic illnesses and diseases. Through the platform, consumers can receive personalized recommendations — like dietary restrictions and exercise plans — on how to live a healthy life.
Location: San Francisco, California
Invitae provides genetic testing services, the Invitae Digital Health platform to coordinate patient care, biopharma partnerships. Its products have applications for oncology, reproductive health, pediatrics, cardiology and pathology.
Variant Bio
Location: Seattle, Washington
Variant Bio uses innovative sequence technology and studies the genetics of individuals with “exceptional health-related traits.” The company’s goal: to transform drug development as we know it today and, ultimately, find better ways to treat diseases.
Benchling is dedicated to speeding up life sciences research through its suite of unified applications, which are housed within the company’s life sciences research and development cloud. The cloud serves as a platform for centralizing and standardizing all R&D data, enabling users to track every workflow, automatically interlink related data and easily and quickly export data. Benchling’s applications can be used for the research of antibodies, cell therapy, proteins and peptides, gene therapy, vaccines and more.
Location: San Mateo, California
Helix created an end-to-end genomics platform in an effort to enable health systems, life sciences companies and payers to advance genomic research. The company’s platform allows users to deliver actionable genetic insights and conduct large-scale genetic analyses. Helix’s Exome+ assay is designed to facilitate the discovery and analysis of rare and novel variants, genome-wide imputation, polygenic risk score calculation, ancestry inference and more.
Charles River Laboratories
Location: Wilmington, Massachusetts
Charles River Laboratories ’ focus is on the quick, efficient and safe discovery and development of drugs and therapeutics. The company provides products and services that take its clients from the basic research stage to clinical development and commercialization. Its customers come from the pharmaceutical, biotech, agrochemical, and academic sectors.
Location: Rahway, New Jersey
Merck ’s efforts center around biopharmaceutical research for preventing and treating disease in people and animals with a focus on oncology, vaccines, infectious diseases, COVID-19, cardio-metabolic disorders and discovery and development.
Recommended Reading 40 AI in Healthcare Examples Improving the Future of Medicine
Flatiron Health aims to reinvent cancer care by learning from the experiences of cancer patients. The company’s Flatiron HC platform can help providers with administrative tasks, matching patients to clinical trials they’re eligible for and accessing patient information whenever or wherever they need.
Imagen Technologies
Imagen Technologies is dedicated to making diagnostic care more accessible while eliminating errors in radiology. The company’s FDA-cleared Computer Assisted Detection and Diagnosis software applies AI technology to medical image analysis in an effort to improve patient outcomes.
Location: Chicago, Illinois
CancerIQ is dedicated to helping providers use genetic information to predict and prevent disease. The company provides user-friendly screening tools that allow healthcare providers to offer patients easy risk assessment questionnaires in waiting rooms and track patient outcomes over time to keep them engaged and informed. CancerIQ’s screening solution enables providers to replace long paper forms and quickly identify patients eligible for genetic counseling, genetic testing or an MRI.
Location: Palo Alto, California
BridgeBio works in medicine development for patients who have genetic diseases and cancers with clear genetic drivers. The company’s partners have included St. Jude Children’s Research Hospital and The University of Texas MD Anderson Cancer Center.
Location: Chicago, Illinois
Neurocern aims to leverage neuroinformatics and data analytics to improve the quality of life and longevity of neurological patients. The company’s technology is designed to identify different types of dementia more effectively than other forms of diagnosis. Neurocern is guided by the belief that analytics can connect siloed markets in new ways to improve long-term care outcomes and ultimately find cures for neurodegenerative diseases.
Location: Foster City, California
Notable ’s platform aims to dramatically reduce the cost and time associated with the traditional drug development process. Powered by machine learning, automation and high-throughput flow cytometry, the company’s precision medicine platform is capable of determining which drugs or drug combinations would be most effective for specific types of cancers. Notable’s mission is to change the way clinicians and prescribers select treatments for the millions of individuals suffering from hematological cancers.
Recommended Reading Radar Can Detect Your Heartbeat. Really.
Atomwise developed deep learning technology for the discovery of structure-based small molecule drugs. The company’s AtomNet technology utilizes a statistical approach in order to extract insights from millions of experimental affinity measurements and thousands of protein structures to predict the binding of small molecules to proteins. Atomwise’s technology removes some of the physical barriers that previously limited the success of drug discovery, and has the ability to analyze a very large chemical space to identify a small subset with high specificity for synthesis and testing.
Location: Austin, Texas
Natera is a genetic testing and diagnostics company dedicated to changing how doctors and patients manage genetic disease. The company’s solutions include personalized cancer care management, health assessments for transplant patients and women’s health testing. Natera aims to deliver technology and finely-tuned workflows that drive superior clinical and analytics performance.
ATX Therapeutics
ATX Therapeutics is dedicated to helping people more easily manage chronic health conditions such as cancer and heart disease. The company offers evidence-based digital therapeutics intervention programs, which are based on individual patients’ health history, needs and abilities. ATX Therapeutics’ programs are designed to be integrated into patient lifestyles and provider workflows to deliver a fully integrated healthcare experience.
Location: Greenwood Village, Colorado
VieCure aims to make genomic-based cancer care more accessible for patients and providers. The company’s platform operates as a point-of-care clinical decision support system that combines clinical knowledge with patient data to help oncologists generate personalized treatment plans and manage patients’ care. VieCure intends for its platform to serve as a value-added extender for every oncologist, nurse, therapist and clinical researcher.
Paige seeks to transform the diagnosis and treatment of cancer with its AI-native digital pathology ecosystem. The company’s AI suite is intended to provide data-driven insights to pathologists, clinicians and pharmaceutical teams. Paige’s aim is to propel cancer care with more powerful and efficient tools for diagnosis, treatment selection and drug development.
Location: Santa Monica, California
Quantgene aims to transform the future of medicine by unlocking the deep human genome. The company’s platform combines deep genomic sequencing and AI to detect mutational patterns of disease down to a single molecule, thus helping inform early cancer detection, prediction of disease onset and non-invasive treatment monitoring. Additionally, Quantgene’s Serenity Medical Intelligence is intended to extract vital health data from patients’ DNA so they can help protect themselves from chronic diseases, drug interactions and lifestyle risks.
Location: Cambridge, Massachusetts
Moderna is a pharmaceutical company working to use mRNA technology to develop medicines, vaccines and therapeutics. Infectious diseases, immuno-oncology, cardiovascular disease and autoimmune diseases have been among Moderna’s areas of focus.
Recommended Reading Topv 16 Machine Learning Companies in Healthcare
Strata Oncology
Location: Ann Arbor, Michigan
Strata Oncology is a genomic testing company working on precision medicine for cancer patients. The company’s StrataNGS genomic profiling test is effective for smaller tumor tissue samples compared to other tests, making testing that can inform immunotherapy decisions more accessible to patients with advanced solid tumors.
Myriad Genetics
Location: Salt Lake City, Utah
Myriad Genetics aims to provide accurate genetic insights to improve disease diagnosis, treatment and prevention. The company offers multiple testing options, including MyRisk hereditary cancer risk testing and Prequel prenatal screening.
Margo Steines, Ana Gore and Da’Zhane Johnson contributed reporting to this story.
Recent Biotech Articles

Top 10 largest clinical research organizations
We take a look at the 10 biggest contract research organizations in the pharma sector in 2022.

The drug discovery process is complex. The clinical stage is particularly resource-intensive, something that has led to the rise in demand for contract research organizations (CROs) that can support drug manufacturers at each stage of the process, from discovery to approval. The core activities a CRO can provide include (but are not limited to) clinical trial management, data research and project management.
Despite an initial downturn at the start of the pandemic, Covid-19 created a spike in the number of clinical trials taking place due to the need for vaccines and drugs to tackle the virus. In the coming years, we expect the rise of technologies that enable decentralized clinical trials to help expand the market share of CROs.
Currently the global CRO services market is projected to grow from US$73.38 bn to $163.48 bn by 2029. Here Pharma IQ takes a look at the 10 biggest CROs in pharma today.
Founded in 1982, IQVIA is an American multinational company formed through the merger of Quintiles, a leading provider of product development and integrated healthcare services, and IMS Health, a global information and technology services company. The latter has enabled company to have a strong focus on digital solutions and analytics. In 2017, Quintiles IMS rebranded to IQVIA.
Laboratory Corporation of America Holdings, also known as Labcorp, is an American company that operates one of the largest clinical laboratory networks in the world. In an average week Labcorp processes tests on more than 3 million patient specimens. In 2020 the company earned revenue in excess of $14 bn.
Syneos Health
Founded in 1999, Syneos Health was created following the merger of two biopharmaceutical companies: INC Research and inVentive health. Today it has offices in more than 110 countries and offers services as a CRO as well as consultancy.
Founded in 1985 as a one-person consultancy firm, Pharmaceutical Product Development (PPD) is a global contract research organization that provides drug development, lab and lifecycle management services. In 2020 the company made US$4.7bn and the following year it became part of Thermo Fisher Scientific.
Headquartered in Dublin, Icon provides strategic management and support for clinical development from the compound selection stage through to clinical trials. Its services include clinical trials management, biometric activities, investigator recruitment and outcomes research.
Parexel was founded in 1982 and acquired by private equity firm Pamplona Capital in 2017 in a deal worth $5 bn. In 2021 it was bought by EQT Private Equity and Goldman Sachs Asset Management. The company conducts clinical trials and operates in more than 50 countries. It makes around $3 bn in revenue annually.
Charles River Laboratories
Founded in 1947, today this company specializes in cell and gene therapies as well as lab services for the pharmaceutical, medical device and biotech industries. As of 2021 it operates more than 90 facilities in 20 countries and has an annual revenue of $3.54 bn.
Award-winning MedPace has offices on six continents, with headquarters in Ohio where it has a clinical research campus and a number of clinical and bioanalytical laboratories. It provides clinical trial services for Phase I-IV studies in the biotech, pharma and medical device industries. Its revenue has been growing steadily year- on-year and is currently almost $1 bn.
CTI Clinical Trial and Consulting Services
This organization was founded in 1999 to provide clinical trial services and bring new drugs to market. It operates in more than 60 countries and since its inception has contributed to the approval of more than 150 new drugs and medical devices around the world.
WuXi AppTec
Founded in 2000 in Shanghai, WuXi AppTec is the newest company in our top 10. It provides services across the entire development cycle including small molecule R&D and manufacturing, biologics R&D and manufacturing, cell and gene therapy. It currently operates in 18 locations across China, Iceland and the US.
Quick links
- How to improve the success rate of clinical trials
- Webinar: The technology enabling decentralized clinical trials
- The challenges of handling and delivering highly potent oncology drugs
Get exclusive access to member-only articles, reports, videos, interviews, webinars and other premium content from industry experts and thought leaders by signing up to Pharma IQ here .
Upcoming Events
Digit pharma & health 2024.
September 10 - 12, 2024 Hilton Düsseldorf

Temperature Control and Logistics North America Summit
September 10 - 12, 2024 Falls Church, VA

AI for Pharma & Healthcare
24 - 26 September, 2024 Amsterdam Marriott Hotel

SmartLab Exchange Europe
February 25 - 26, 2025 Novotel Amsterdam City, Netherlands

Pharma Contract Manufacturing
26 - 27 March, 2025 Berlin

SmartLab Exchange USA 2025
08 - 09 April, 2025 Le Méridien, Fort Lauderdale, Florida, USA

Subscribe to our Free Newsletter
Insights from the world’s foremost thought leaders delivered to your inbox.
Latest Webinars
Pharma iq's power list 2022: in conversation with pharma's top leaders.
2022-10-18 02:00 PM - 03:00 PM BST

A post-pandemic 3D view of the patient and supply journey
2022-06-01 04:30 PM - 05:30 PM CET

Discover how targeted radiotherapy induced toxicity can be identified with imaging
2022-04-28 01:00 PM - 02:00 PM EST

RECOMMENDED

FIND CONTENT BY TYPE
- Infographics
Pharma IQ COMMUNITY
- Advertise With Us
- User Agreement
- Cookie Policy
- Become a Member Today
- Pharma IQ App

ADVERTISE WITH US
Reach Pharmaceuticals & Biotechnology professionals through cost-effective marketing opportunities to deliver your message, position yourself as a thought leader, and introduce new products, techniques and strategies to the market.
JOIN THE Pharma IQ COMMUNITY
Join Pharma & Biotech today and interact with a vibrant network of professionals, keeping up to date with the industry by accessing our wealth of articles, videos, live conferences and more.

Pharma IQ, a division of IQPC
Careers With IQPC | Contact Us | About Us | Cookie Policy
Become a Member today!
PLEASE ENTER YOUR EMAIL TO JOIN FOR FREE
Already an IQPC Community Member? Sign in Here or Forgot Password Sign up now and get FREE access to our extensive library of reports, infographics, whitepapers, webinars and online events from the world’s foremost thought leaders.
We respect your privacy, by clicking 'Subscribe' you will receive our e-newsletter, including information on Podcasts, Webinars, event discounts, online learning opportunities and agree to our User Agreement. You have the right to object. For further information on how we process and monitor your personal data click here . You can unsubscribe at any time.
22 Top Health Care Research Companies 2021

A Quirk's resource guide covering health care research firms
SPONSORED CONTENT
Health care research can be complicated due to the size of the industry and the sensitivity of the data involved. More importantly, it has immediate and important consequences for patients, caregivers, practitioners and others – making the quality of the research critical.
Companies specializing in health care research are accustomed to the difficulties of working in this industry and utilize a variety of methods to gain access to hard-to-find respondents efficiently and in compliance with information privacy regulations. Whether your research involves medical devices, rare-disease patients or physicians, the following companies feature an array of specialties to assist with any step of the research process.

Applied Marketing Science (AMS)
Founded 1989 | 40 employees John Mitchell, President and Managing Principal

Phone +1-781-250-6300 ams-insights.com/insights-for-health care-markets

Ascribe Services
Founded 1999 Chrissy Stevens, Vice President, Services

Phone +1-877-241-9112 ext. 55 goascribe.com/products-services/ascribe-services

Decision Analyst Inc.
Founded 1978 | 150 employees Sara Sutton, Senior Vice President

Phone +1-817-640-6166 www.decisionanalyst.com/industry/medical

Founded 1980 | 250+ employees Steve Raebel, President

Phone +1-800-863-4353 www.fieldwork.com/medical-research-recruitment

Holden Healthcare
Founded 2006 | 50 employees Jeffrey Kelsch, Managing Partner

Phone +1-208-809-7117 E-mail [email protected] www.holdendata.com

Insights Opinion
Founded 2015 | 50 employees Sharoz Ghauri, CEO

Phone +1-646-475-7865 (U.S.) +91-120-498-7860 (India) www.insightsopinion.com

Ironwood Insights Group LLC
Founded 2017 | 300 employees David Bryant, VP Data Analytics

Phone +1-602-661-0807 ext. 2140 E-mail [email protected] www.ironwoodinsights.com

Just The Facts Inc.
Founded 1994 Bruce Tincknell, Managing Director

Phone +1-847-506-0033 www.justthefacts.com Testimonials www.justthefacts.com/testimonials-x-30

Kantar, Profiles Health Division
Founded 1996 | 600 employees in 25 countries Jennifer Carrea, Managing Director, Americas and Global Health, Kantar Profiles Division

hubs.ly/H0SLGCb0

Founded 2002 | 35 employees Paul Golota, CEO and Co-founder

Phone +1-866-963-3000 www.medsurvey.com

Mindspot Research
Founded 2006 | 8 employees Lynnette Leathers, CEO and Lead Moderator

Phone +1-407-730-4603 www.mindspotresearch.com

Murray Hill National
Founded 2013 | 35 employees Susan Owens, COO

Phone +1-972-707-7645 www.murrayhillnational.com

Olson Research Group Inc.
Founded 1995 | 45 employees Charles Olson, CEO

Phone +1-267-487-5500 www.olsonresearchgroup.com

Precision Research Inc.
Founded 1959 | 25-49 employees Scott Adleman, President

Phone +1-847-257-0827 RFQ [email protected] www.preres.com

Rare Patient Voice LLC
Founded 2013 | 14 full-time, 50 part-time patient advocates Wes Michael, President and Founder
Rare Patient Voice recruits hard-to-find patients and caregivers (rare and non-rare) in the U.S., Canada, U.K., France, Germany, Italy and Spain for qualitative and quantitative market research. We attend patient events such as conferences and walks to build our panels so that our patients are authentic and we can complete recruits within two weeks. We focus on recruiting only. With 100,000 patients and caregivers across 618 diseases/conditions, our panels include: all cancer types, Crohn’s, cystic fibrosis, diabetes, epilepsy, Fabry, Gaucher, hemophilia, HIV, Huntington’s, lupus, mental health, multiple sclerosis, Parkinson’s, primary immunodeficiency, PNH, pulmonary hypertension, sickle cell and spinal muscular atrophy. To get a bid with feasibility and costs at any time: pam.rarepatientvoice.com. Learn more about Rare Patient Voice and our services on our YouTube channel: www.youtube.com/watch?v=vq6n_JxtIDg&t=2s
Phone +1-410-218-0527 www.rarepatientvoice.com

RC Horowitz & Company Inc.
Founded 1983 | 5 employees Rob Horowitz, President

Phone +1-212-779-0033; +1-866-704-8000 www.rchorowitz.com

Reckner Healthcare
Founded 1991 | 225 employees Jason Gamber, Vice President

Phone +1-215-822-6220 recknerhealth care.com

Research America
Founded 1985 | 300 employees Robert Porter, CEO

Phone +1-610-356-1800 www.ResearchAmericaInc.com

Schlesinger Group
Founded 1966 | 1,200 employees Rob Ramirez, Chief Client Officer

Phone +1-203-899-0475 SchlesingerGroup.com

Shapiro+Raj
Founded 1955 | 80 employees Zain Raj, CEO

Phone +1-312-965-2319 www.shapiroraj.com

Founded 2000 | 1,400 employees Frédéric-Charles Petit, CEO

Phone +1-203-834-8585 www.tolunacorporate.com

WebMD/Medscape Market Research
Founded 1995 | 1,800 employees Audrey Rosen, Vice President, Market Research

Phone +1-212-624-3780 www.MedscapeMarketResearch.com
Quirk's Time Capsule – June 2002 Related Categories: Health Care (Healthcare), Health Care (Healthcare) Research Health Care (Healthcare), Health Care (Healthcare) Research, Research Industry, Data Analysis, Internet/Web, Media Research-Print/Publication, Newspapers/Magazines, Online Research, Publishing, Qualitative Research
Survey Monitor May/June 2024 Related Categories: Health Care (Healthcare), Health Care (Healthcare) Research Health Care (Healthcare), Health Care (Healthcare) Research, Research Industry, Grocery/Supermarkets, Restaurants/Food Service, Shopper Insights, Artificial Intelligence / AI, Beverage, Consumer Research, Consumers, Entertainment, Environmental, Media Research-Digital, Physicians, Transportation, Travel
Survey Monitor March/April 2024 Related Categories: Health Care (Healthcare), Health Care (Healthcare) Research Health Care (Healthcare), Health Care (Healthcare) Research, Air Travelers, College Students, Education, Employees, Entrepreneurs/Small Business, Generation Baby Boomers, High-Tech, Higher Education, Research Industry, Shopper Insights, Travel
9 Top Physician Research Companies Related Categories: Health Care (Healthcare), Health Care (Healthcare) Research, Health Care-Rare Patients Health Care (Healthcare), Health Care (Healthcare) Research, Health Care-Rare Patients, Physicians, Research Industry, Data Security, Medical, Nurses, Patients , Focus Group-Online, Journey Mapping

Top 10 Companies Sponsoring Clinical Trials
Clinical trials are critical for advancing medical research and developing new treatments and therapies. These trials require substantial resources, and leading companies play a crucial role by sponsoring and supporting these endeavors. Several prominent companies have recognized the importance of clinical trials and have actively contributed to their success.
Pharmaceutical giants like Pfizer, Johnson & Johnson, and Novartis are among the leading companies sponsoring clinical trials. These companies invest significant financial resources and scientific expertise to bring new drugs and treatments to the market. By sponsoring clinical trials, they gain valuable insights into the efficacy and safety of their products, which helps them obtain regulatory approvals and ensure patient well-being. These companies often collaborate with academic institutions, healthcare organizations, and research centers to conduct large-scale trials and engage diverse patient populations.
Biotechnology companies such as Amgen, Gilead Sciences, and Moderna also play a vital role in sponsoring clinical trials. With a focus on innovative therapies and cutting-edge research, biotech companies contribute to advancing medical science. They often specialize in developing novel drugs and therapies, particularly in areas with unmet medical needs. By sponsoring clinical trials, these companies not only test the effectiveness of their experimental treatments but also support the scientific community in discovering new possibilities for disease management and prevention.
Medical device manufacturers like Medtronic, Abbott Laboratories, and Philips are another significant group of companies that sponsor clinical trials. They design and produce devices such as pacemakers, imaging systems, and surgical instruments. These companies sponsor clinical trials to evaluate the safety and efficacy of their devices in real-world settings. Through these trials, they gather data on device performance, patient outcomes, and usability, ensuring that their products meet the highest standards and address the needs of healthcare professionals and patients.
The importance of leading companies sponsoring clinical trials cannot be overstated. Their financial investments, research capabilities, and industry knowledge enable the successful completion of trials, leading to improved medical interventions and better patient care. Additionally, their involvement fosters collaborations between academia, healthcare providers, and industry, creating a rich ecosystem for scientific progress. By supporting clinical trials, these companies contribute to the growth of medical knowledge, the development of innovative treatments, and the overall improvement of global healthcare.
1. Pfizer: A Leader in Clinical Research
When it comes to clinical research, one name that stands out is Pfizer . They're making groundbreaking contributions through their involvement in clinical trials. Pfizer, a global pharmaceutical giant, has been at the forefront of clinical research for decades. They're driven by a passion for innovation and a commitment to improving lives through medical advancements. With their extensive resources and expertise, Pfizer has successfully conducted numerous notable clinical trials.
One remarkable example is the clinical trial that led to the development of the Pfizer-BioNTech COVID-19 vaccine . This breakthrough vaccine revolutionized the fight against the pandemic and offered hope to millions worldwide. It's a shining testament to Pfizer's dedication to tackling global health challenges head-on.
2. Johnson & Johnson: Advancing Healthcare through Trials
When it comes to advancing healthcare through clinical trials, Johnson & Johnson is dedicated to making a positive impact on people's lives by driving innovation through their commitment to clinical research. Johnson & Johnson is a renowned pharmaceutical and medical device company with a rich history of groundbreaking contributions to the world of healthcare. Their commitment to clinical research is unwavering, and they continuously strive to develop innovative solutions that address unmet medical needs.
One of the key areas where Johnson & Johnson has made a significant impact is in the field of oncology. Through their clinical trials, they have pioneered breakthrough treatments and therapies for various types of cancer . Their dedication to improving cancer care has offered hope and better outcomes for patients fighting this formidable disease.
In addition to oncology, Johnson & Johnson has been involved in numerous other clinical trials, exploring treatments for cardiovascular diseases, neurological conditions, and infectious diseases, to name just a few. Their diverse portfolio of trials showcases their wide-ranging expertise and commitment to advancing healthcare across various therapeutic areas.
3. Novartis: Pioneering Breakthroughs in Medicine
When it comes to pioneering breakthroughs in medicine, Novartis is at the forefront of clinical trials, driving innovation and transforming the lives of patients worldwide.
Novartis is a global healthcare company with a relentless commitment to improving patient outcomes through its involvement in clinical trials. They have made significant contributions to the development of innovative therapies and treatments across various therapeutic areas.
In the realm of oncology, Novartis has been a game-changer. They have conducted groundbreaking clinical trials that have revolutionized cancer care. Through their research, they have introduced targeted therapies and immunotherapies that are changing the way we fight cancer, offering new hope and improved survival rates for patients.
They have also made notable advancements in areas such as cardiovascular diseases, neurology, ophthalmology , and rare genetic disorders. Their commitment to diverse therapeutic areas demonstrates their dedication to addressing unmet medical needs and improving the lives of patients with various conditions.
4. Merck & Co.: A Legacy of Clinical Excellence
Merck & Co . has a long-standing commitment to advancing healthcare and has left an indelible mark on the world of clinical trials. Merck & Co. has been a driving force in the pharmaceutical industry for decades. Their involvement in clinical trials spans a wide range of therapeutic areas, from infectious diseases to cardiovascular health and beyond. With a legacy built on scientific rigor and a dedication to improving patient lives, Merck & Co. continues to make significant contributions to medical advancements.
One notable example of their groundbreaking research is the development of the human papillomavirus (HPV) vaccine . Through meticulous clinical trials, Merck & Co. brought this life-saving vaccine to the world, helping to prevent HPV-related cancers and protect countless lives. This achievement exemplifies their unwavering commitment to disease prevention and public health.
Merck & Co.'s commitment to clinical excellence and its transformative trials have made a lasting impact on global health.
5. Eli Lilly and Company: Transforming Lives through Research
Eli Lilly and Company 's dedication to clinical research is unwavering, and they have made significant contributions to the world of healthcare.
Are you curious about clinical trials?
One remarkable example of their impact is their work in the field of mental health. Eli Lilly and Company has conducted groundbreaking trials that have led to the development of medications for conditions such as depression , anxiety, and schizophrenia. Their research has provided hope to individuals and families affected by these challenging conditions.
Beyond mental health , Eli Lilly and Company has also made significant advancements in areas such as diabetes , oncology, and immunology. Through their clinical trials, they have introduced life-changing treatments that have the potential to save lives and improve the quality of life for countless patients.
6. Bristol-Myers Squibb: Advancing Cancer and Immunotherapy Research
When it comes to advancing cancer and immunotherapy research, Bristol-Myers Squibb (BMS) has a strong focus on these critical areas of healthcare and are committed to making significant strides in the fight against cancer.
Bristol-Myers Squibb has dedicated considerable resources and expertise to the field of oncology. They are driven by a passion to improve the lives of cancer patients and develop innovative treatments that target the disease at its core. Through its extensive research efforts, BMS has become a trailblazer in the fight against cancer.
BMS is also at the forefront of immunotherapy research, which harnesses the power of the immune system to combat cancer. Their trials focus on developing therapies that activate the body's own defense mechanisms to recognize and destroy cancer cells. This cutting-edge approach has revolutionized cancer treatment and provided new hope for patients.
When it comes to key clinical trials, BMS has numerous noteworthy examples such as the trial involving the development of a groundbreaking immunotherapy drug that targets specific proteins found on cancer cells. This therapy has shown remarkable efficacy in treating certain types of cancer, improving patient outcomes, and extending survival rates.
7. Sanofi: Innovating Healthcare through Clinical Trials
When it comes to innovating healthcare through clinical trials, Sanofi is committed to pushing the boundaries of medical research and developing life-changing treatments.
Sanofi has a deep-rooted commitment to clinical research and drug development. They believe in the power of scientific exploration to transform healthcare and improve patient outcomes. With its strong focus on research and innovation, Sanofi continues to make significant contributions to the medical world.
One area where Sanofi has made notable strides is in the field of rare diseases. Through their clinical trials, they have developed groundbreaking therapies that address the unmet medical needs of individuals with rare and complex conditions. These treatments have brought new hope and improved quality of life to patients who previously had limited options.
But Sanofi's impact extends beyond rare diseases. They have also conducted noteworthy clinical trials in areas such as cardiovascular health, diabetes, and immunology. Their dedication to diverse therapeutic areas demonstrates their commitment to improving healthcare across a wide spectrum of conditions.
8. AstraZeneca: Revolutionizing Medicine through Collaborative Trials
When it comes to revolutionizing medicine through collaborative trials, AstraZeneca is a standout. They believe in the power of partnerships and collaborative research to drive medical advancements.
AstraZeneca takes a unique approach to clinical research by fostering collaboration. They understand that by working together with scientists, healthcare professionals, and research institutions, they can accelerate the pace of medical breakthroughs. Through their collaborative trials, AstraZeneca aims to tackle complex diseases and develop innovative solutions that improve patient lives.
One area where AstraZeneca has made significant strides is in the field of respiratory medicine. Through their clinical trials, they have developed groundbreaking treatments for conditions such as asthma and chronic obstructive pulmonary disease (COPD). These treatments have provided relief and improved quality of life for millions of individuals affected by respiratory disorders.
AstraZeneca has also conducted notable clinical trials in areas such as cardiovascular health, oncology, and autoimmune diseases. Their commitment to diverse therapeutic areas demonstrates their dedication to addressing a wide range of medical needs.
9. AbbVie: Leading the Way in Biopharmaceutical Research
AbbVie is dedicated to pushing the boundaries of biopharmaceutical research and clinical trials scientific innovation and developing transformative treatments.
AbbVie has a strong focus on biopharmaceutical research, utilizing the power of biotechnology to develop cutting-edge therapies. Their commitment to scientific excellence is unwavering, and they continuously strive to address unmet medical needs and improve patient outcomes. Through their clinical trials, AbbVie is shaping the future of medicine.
One area where AbbVie has made significant strides is in the field of immunology. Their clinical trials have resulted in breakthrough treatments for autoimmune diseases such as rheumatoid arthritis and psoriasis . These therapies have provided much-needed relief and improved the quality of life for patients living with these chronic conditions.
AbbVie has also conducted notable clinical trials in areas such as oncology, neuroscience, and gastroenterology . Their diverse portfolio of trials demonstrates their commitment to tackling a wide range of medical challenges and improving healthcare across various therapeutic areas.
10. Gilead Sciences: Innovators in Infectious Disease and Beyond
When it comes to innovating in the field of infectious disease research and beyond, Gilead Sciences is a name that stands out. They are dedicated to tackling some of the most challenging health issues of our time.
Gilead Sciences has made significant contributions to infectious disease research, particularly in areas like HIV/AIDS and viral hepatitis. They have developed groundbreaking treatments that have revolutionized the lives of patients affected by these diseases. Gilead Sciences' relentless pursuit of scientific innovation has brought new hope to millions worldwide.
One key area where Gilead Sciences has made an impact is in the treatment of HIV/AIDS . Through their clinical trials, they have developed antiretroviral therapies that have transformed HIV/AIDS from a fatal disease to a manageable chronic condition. Their commitment to research and development has saved countless lives and improved the quality of life for individuals living with HIV/AIDS.
Gilead Sciences have also conducted key clinical trials in other therapeutic areas such as oncology, respiratory diseases, and liver diseases. Their diverse portfolio of trials showcases their dedication to addressing a wide range of health challenges.
The top 10 companies we've explored in this article play a pivotal role in advancing medical research and driving innovation in healthcare. From Pfizer to AstraZeneca, these companies are at the forefront of clinical trials, pushing the boundaries of scientific discovery and improving patient outcomes.
Their research has led to groundbreaking advancements in various therapeutic areas, from oncology to infectious diseases, immunology, and rare genetic disorders. Through their transformative trials, they have developed life-saving treatments, innovative therapies, and preventive interventions that have transformed the lives of countless individuals worldwide.
Therapeutic Expertise
Participate

Explore end-to-end solutions throughout development — from portfolio optimization and regulatory strategy, to Phase I-IV clinical trials, market access planning, and more.
- Portfolio management and asset valuation
- Early development and innovation
- Integrated clinical development
- Approval and access
- Value substantiation lifecycle management
HOW WE DO IT
- Delivery models
- Operational excellence
- Medical affairs
- Building patient insights into assets, profile and claims
- Portfolio optimization
- Asset valuation and indication prioritization
- Early evidence review
- Model-based drug development
- Integrated development strategy and planning
- Phase I Clinical Trials
- Proof of Concept Studies: Phase IB-IIA
- Patient Engagement Strategy and Enrollment Solutions
- Patient Inclusion
- Site Alliance Network and KOL Engagement
- Protocol Optimization
- Regulatory Strategy
- Market Access Strategy and Delivery
- Biomarker and Genomic Medicine Strategy
- Clinical Trial Supply & Logistics
- Medical Communications
- Phase IIB-IV Clinical Trials
- Operationalize post-trial access with a proven platform approach
- Protocol-Driven, Customized Site Solution Strategy
- Regulatory Strategy, Submissions, Compliance, and Outsourcing
- Clinical Development Technology Optimization
- Real World Evidence
- Global Regulatory Submissions and Outsourcing
- Compliance and Risk Management
- Real-World Evidence, Market Access Strategy and Planning
- Regulatory Compliance, Drug Safety and Pharmacovigilance
- Lifecycle Optimization
- Gain an advantage through FSP
- Leveraging AI and digital in clinical development

Parexel Biotech provides the end-to-end capabilities you’ll need to succeed. We're fast, flexible, and dedicated to impacting patient lives.

Utilize our expertise across therapeutic areas, combining innovative trial designs, leading clinical and regulatory expertise, global reach, and a passion for changing patient lives.
Neuroscience
- General medicine
- Infectious disease & vaccines
- Inflammation & immunology
Cross-Therapeutic Expertise
- Cell & gene therapies
- Rare diseases

Our experts help you stay at the forefront of the industry - and ahead of change.
New Medicines, Novel Insights
- Advancing precision oncology
- Advancing rare disease drug development
- Accelerating development of cell and gene therapies
- Achieving patient-guided drug development
Discussions on Diversity
- Chapter 1 Bridging the Gap
- Chapter 2 Beyond the Binary
The Regulatory Navigator
- Gene therapy: are high costs and manufacturing complexities impeding progress?
- New FDA draft guidance: Implications for simplifying interchangeability for biosimilars in the US
- Key implications for nonclinical development: FDA guidance on human gene therapy products incorporating human genome editing
Latest Report

New Medicines, Novel Insights: Advancing precision oncology

Thinking about joining a clinical trial? Learn the drug development process, what it’s like to participate, how to find a trial, and answers to frequently asked questions.
INTERESTED IN PARTICIPATING?
- Healthy Volunteers
- Patient Volunteers
- Patient Advocacy Groups
HEAR FROM REAL PATIENTS
- Patient Stories
TRIAL SITES

Want to collaborate with us to offer clinical trials at your site? We would welcome the opportunity to discuss.
We are one of the largest CROs in the world, speeding life-changing medicine to market by engaging patients With Heart ™. Learn about who we are, what we do, and what we believe.
- Leadership team
- Global reach
- Our DE&I strategy
- Compliance, tax & privacy
- Meet us at an event
- Board of Directors
- Our ESG strategy
- Compliance & Ethics
- Tax Strategy
- Privacy Policy
- Cookies and other web tracking
- Supplier Data Privacy Requirements
- Terms of Use
- French Gender Pay Data
- UK Gender Pay Data
- UK Modern Slavery Act Statement
- Data Privacy Framework
- Supplier Diversity Policy
What can we help you find today?
The perspectives and opinions expressed in this material represent those of the patient advocate only and should not be considered a solicitation, promotion or advertisement for any services of Parexel, or any drugs or therapies, including those under development. Participating in clinical trials for investigational medicines offers patients potential benefits, such as access to cutting-edge treatments and expert medical care, while contributing to medical research. However, risks may include side effects, unpredictable outcomes, and time commitment. Careful assessment of these factors helps patients make informed decisions. The content of this material, including graphics, images and text, is provided for informational purposes only and does not constitute medical advice, diagnosis or treatment. Please consult your healthcare professional for medical advice. The patient advocate has provided their consent for the use and distribution of this content.
Patient Story
- Cell & Gene Therapy
Rare Diseases
- Inflammation & Immunology
- Neurosciences
One night, while watching TV with her husband, Robyn felt a lump in her neck.
She had large B-cell lymphoma — an aggressive cancer diagnosed in 150,000 patients each year globally.
As a doctor, she knew the risk. She got a CAT scan, looked at the images, and her world stopped.
Four years later the lymphoma returned. Robyn’s care team prescribed a further six cycles of a brutal chemotherapy, followed by an autologous stem cell transplant (ASCT) and external beam radiation. Robyn’s ASCT treatment was further complicated by septic shock requiring a stay in intensive care.
Robyn entered remission again, but it was short-lived, with the lymphoma returning nine months later. Treatment options for Robyn were now bleak and limited.
Robyn immediately sought treatment. With her husband by her side, she underwent many rounds of chemotherapy and went into remission.
Feeling defeated, she found a clinical trial for car t-cell therapy, a new treatment that reengineers white blood cells to target and eradicate cancer., she thought this was her last chance., a week after receiving treatment, her lymph nodes shrunk. within three months, there was no evidence of the disease., today, she’s cancer free — and sharing her experiences to help us better meet the needs of patients like her in our cell and gene therapy trials., lives can change when you design cell and gene therapy trials with speed and precision..
- Utilize the right experts, focused on the right indications
- Access even hard-to-find patient populations
- Pinpoint the perfect sites for your trial
- Satisfy global regulations, to get your treatment to market safely and quickly
What we do, we do
Our Experts
Our cell and gene therapy specialists collaborate to help get your innovative treatments to patients like Robyn faster.
MORE EXPERTS
Steve Winitsky, M.D.
Vice President, Tech...
Stefan Zietze
Executive Director, ...
Hicham El Bariqi
Director, Cell & Gen...
Jingke Yang, M.D., Ph.D.
Global TA Section He...
Chris Learn, Ph.D., PMP

Vice President, Cell and Gene Therapy, Center of Excellence
With 20+ years of trial execution and team management experience, Chris leads development for our cell and gene therapeutic area. He reinforces our patient-first focus to help ensure your trials are designed to meet patient needs.
"Being able to capture and understand the patient’s perspective — not just as a patient, but as a person — and use their insight to guide my decisions is what it means to work With Heart™."
Our diverse experiences ensure you get the expertise you need, no matter the indication.
Our experience with CAGT clinical trial sites around the world allows us to accelerate study start-ups.
- North America
- South America
- Middle East & Africa
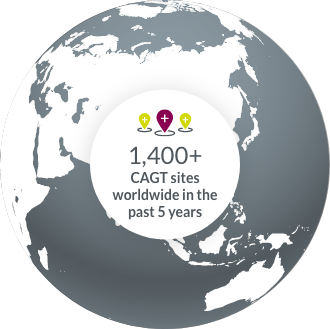
Access to global EMR data enables us to pinpoint the perfect sites for your trial.

Our cross-functional team overcomes any technical, logistical, and strategic challenges.
Our highly tailored recruitment and retention strategies drive access to even hard-to-find patient populations.

What can we do to help you change patient lives?
See CAGT capabilities Visit all therapeutic areas Explore CAGT careers
Discover other patient stories
Sara's only symptom was fatigue. But after some routine tests, she was diagnosed with breast cancer.
When Austin was 3 years old, his parents realized something was wrong. Multiple falls, concussions, and a broken arm led to a diagnosis.
Tina had lost her father to Crohn's disease. Now she faced her own diagnosis — and it derailed her life.
While running a triathlon, Andrea stumbled and realized something was wrong. She thought it was just an injury. But it was much more.
We focus on patients, because they inspire us to deliver better trials, faster than ever . So we can make a difference for more patients like Robyn.
Who we are,, parexel is proudly among the world’s largest clinical research organizations.
A dedicated CRO providing the full range of Phase I to IV clinical development services and leveraging the breadth of our clinical, regulatory and therapeutic expertise , our team of more than 21,000 global professionals works in partnership with biopharmaceutical leaders and sites to design and deliver clinical trials with patients in mind , to make clinical research a care option for anyone, anywhere.
What can we help you find?

We believe research is the will to conquer the impossible and that science will lead the way.
The journey to discovery is guided by science — and inspired by patients
Every day our scientists, from all backgrounds and disciplines, work together to use the power of leading-edge science to save and improve lives.
Watch now: Merck Research Laboratories
Our research in action
People employed in R&D
Invested in R&D in 2023
Publications by our scientists
Our areas of focus
We follow the science and apply our expertise on core therapeutic areas where we have the greatest potential to help save and improve lives.
Our team of researchers and scientists are pushing the boundaries of cancer research to discover more effective anticancer therapies.
Our work in oncology
Vaccines are one of the greatest public health success stories — and we’ve been discovering, developing, supplying and delivering vaccines to help prevent disease for over 130 years.
Our work in vaccines
Infectious diseases

Our focus has always been on the prevention and treatment of diseases that threaten people and communities around the world.
Our work in infectious diseases
Cardio-metabolic disorders
We have a long history of making an impact in cardio-metabolic disorders, such as type 2 diabetes and cardiovascular disease.
Our work in cardio-metabolic disorders

We’re at the forefront of research, seeking to translate scientific discoveries into treatments for people with immune-mediated inflammatory disorders.
Our work in immunology
Neuroscience

We're tackling the complexities and therapeutic challenges of some of the most devastating neurological disorders.
Our work in neuroscience
We have a proud legacy of turning breakthrough science into medicines and vaccines that save and improve lives around the world.
Check out our pipeline
Publications
As part of our commitment to unparalleled research, our scientists and postdoctoral research fellows are encouraged to be active members of the scientific community.
Explore our publications
Clinical trials
Our progress is due in large part to the important and tough scientific questions we set out to answer with our clinical trials.
Learn about clinical trials
Business development & licensing
With a robust pipeline and portfolio fueled by both our own discoveries and external innovation, we know that collaborations are critical to advancing bold science.
Collaborate with us
Discovery & development

Journey of a molecule
Creating a medicine or vaccine is a journey — from the lab to patients.
Meet our scientists
Innovation is fueled by diverse expertise and backgrounds. Learn more about our R&D leaders pushing scientific boundaries at Merck.

Dean Li Executive vice president and president, Merck Research Laboratories

Eliav Barr Senior vice president, head of global clinical development and chief medical officer

George Addona Senior vice president and head, discovery, preclinical development and translational medicine
Where we work
Research based on innovation is at the core of who we are and what we do. It starts with the capabilities and expertise at our facilities, but we're open to collaboration anywhere the science leads us.
South San Francisco
Visit all of our R&D locations
Read our R&D stories

What is One Pipeline?
How we’re advancing the best internal and external science to progress our pipeline for patients
How Merck scientists are driving next-generation cancer research
Our scientists are accelerating research by looking to improve anti-tumor immune response, targeting specific cancer cells and helping inhibit cancer growth

In our commitment to R&D, the numbers speak for themselves
We follow the science where we can make the greatest difference

Meet two women at the forefront of our HIV research
Two esteemed scientists share their motivations and hopes for the future of HIV research

Vaccines: Our history, our legacy
We've been working to discover and develop vaccines for more than a century
Careers in R&D
We’re calling all aspiring world-class scientists who want to be a part of cutting-edge work to save and improve lives.
Related links

We’re here to help you get the information you need to guide you on your health journey.

Find our latest financials, events & presentations, news, stock information, and contacts.

We aspire to be the premier research-intensive biopharmaceutical company.
Forward-looking statement of Merck & Co., Inc., Rahway, N.J., USA
This website of Merck & Co., Inc., Rahway, N.J., USA (the “company”) includes “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. These statements are based upon the current beliefs and expectations of the company’s management and are subject to significant risks and uncertainties. There can be no guarantees with respect to pipeline candidates that the candidates will receive the necessary regulatory approvals or that they will prove to be commercially successful. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements. Risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of pharmaceutical industry regulation and health care legislation in the United States and internationally; global trends toward health care cost containment; technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; the company’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of the company’s patents and other protections for innovative products; and the exposure to litigation, including patent litigation, and/or regulatory actions. The company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise. Additional factors that could cause results to differ materially from those described in the forward-looking statements can be found in the company’s Annual Report on Form 10-K for the year ended December 31, 2023 and the company’s other filings with the Securities and Exchange Commission (SEC) available at the SEC’s Internet site (www.sec.gov). No Duty to Update The information contained in this website was current as of the date presented. The company assumes no duty to update the information to reflect subsequent developments. Consequently, the company will not update the information contained in the website and investors should not rely upon the information as current or accurate after the presentation date.
You are leaving Merck.com
Welcome to merck.com.
By continuing, you will be directed to a site intended only for residents of the United States and Canada. We are called MSD everywhere, except in the United States and Canada where we are known as Merck & Co Inc, Rahway, NJ USA.
Medical Research
Scientists and physicians in academic medicine conduct groundbreaking biomedical research that improves our knowledge of human health and promotes the development of treatments from bench to bedside to community.

Through policy and advocacy initiatives, data and research projects, professional learning and networking opportunities, and the development of tools and resources, the AAMC supports the entire spectrum of medical research from basic discovery and translational science to clinical and population health research, research policies and regulations that promote robust and ethical science and minimize administrative burden and a diverse biomedical research workforce and an inclusive and equitable research environment across all career stages.
On this page:
Science policy updates .
AAMC updates on federal science policy and regulatory topics impacting institutions and researchers can be found below.
The AAMC submitted a letter to FDA and HHS on draft guidance.
- May 3, 2024
AAMC submitted comments to the Department of Justice regarding access to bulk sensitive personal data by countries of concern.
- April 26, 2024
AAMC submitted comments to the National Institutes of Health (NIH) on the updated NIH Strategic Plan for Data Science, 2023-2028
- March 15, 2024
For more on the latest legislative and regulatory activities affecting academic medicine, check out Washington Highlights . For the latest news, current trends, and ongoing conversations about the most important topics in academic medicine, visit AAMCNews .
Meet our New Chief Scientific Officer

As chief scientific officer, Elena Fuentes-Afflick, MD, MPH, leads AAMC programs that support medical research and the training of physician-scientists and researchers in academic medicine. In this role, she provides leadership and vision for addressing research and science policy and other related critical issues facing academic medicine, medical schools, teaching health systems, and teaching hospitals. Learn more about Dr. Fuentes-Afflick .
Connect with Colleagues
The AAMC convenes several affinity groups focused on connecting individuals who work in biomedical research or support researchers at their institution. Affinity groups include councils, professional development groups, and other organizations that provide individuals at member institutions access to professional growth, leadership development, networking, and collaboration opportunities.
Find information about how to join each group on the pages below.
GRAND convenes research leaders in discussion of issues critical to the research enterprise and linking research advancements with improvements to health.
GREAT provides a forum for discussion of the research training enterprise with leadership of biomedical graduate, postdoctoral, and MD-PhD programs.
CFAS represents the collective interests of medical school faculty and academic societies on a range of cross-cutting issues.
FOCI provides a national forum for leadership who oversee and manage conflict of interest related to research, medical education, and clinical decision-making.
The GDI unites expertise, experience, and innovation to guide the advancement of diversity, equity, and inclusion throughout medicine and biomedical sciences.
The research subgroup of COF promotes compliance and ethical conduct in academic medicine with a specific focus on research and laboratory issues.
Attend an Event
The AAMC is committed to providing professional learning opportunities for biomedical researchers in every stage of their career. Find information about the AAMC’s webinars, meetings, and more below.
Related AAMC Initiatives
The AAMC engages in other work that may be of interest to researchers and scientists:
An optimal research environment that drives impactful biomedical discovery is supportive, diverse, equitable, and inclusive.
The Center sparks community-centered, multi-sector research, collaboration, and action to make the case for policies and practices that ensure all communities have an equal opportunity to thrive.

- All subject areas
- Agricultural and Biological Sciences
- >> Animal Science and Zoology
- >> Aquatic Science
- >> Ecology, Evolution, Behavior and Systematics
- >> Food Science
- >> Forestry
- >> Plant Science
- >> Soil Science
- Arts and Humanities
- >> Classics
- >> History
- >> Literature and Literary Theory
- >> Philosophy
- >> Religious Studies
- Biochemistry, Genetics and Molecular Biology
- Business, Management and Accounting
- Computer Science
- Earth and Planetary Sciences
- >> Atmospheric Science
- >> Geology
- >> Oceanography
- >> Paleontology
- >> Space and Planetary Science
- Economics, Econometrics and Finance
- Engineering
- >> Aerospace Engineering
- >> Architecture
- >> Automotive Engineering
- >> Biomedical Engineering
- >> Building and Construction
- >> Civil and Structural Engineering
- >> Industrial and Manufacturing Engineering
- >> Mechanical Engineering
- >> Ocean Engineering
- Environmental Science
- Mathematics
- >> Anatomy
- >> Anesthesiology and Pain Medicine
- >> Cardiology and Cardiovascular Medicine
- >> Critical Care and Intensive Care Medicine
- >> Dermatology
- >> Epidemiology
- >> Gastroenterology
- >> Geriatrics and Gerontology
- >> Infectious Diseases
- >> Nephrology
- >> Obstetrics and Gynecology
- >> Oncology
- >> Ophthalmology
- >> Otorhinolaryngology
- >> Pathology and Forensic Medicine
- >> Pediatrics, Perinatology and Child Health
- >> Psychiatry and Mental Health
- >> Public Health, Environmental and Occupational Health
- >> Pulmonary and Respiratory Medicine
- >> Radiology, Nuclear Medicine and Imaging
- >> Rheumatology
- >> Surgery
- >> Urology
- Pharmacology, Toxicology and Pharmaceutics
- Physics and Astronomy
- Social Sciences
- >> Anthropology
- >> Communication
- >> Demography
- >> Education
- >> Gender Studies
- >> Geography, Planning and Development
- >> Law
- >> Library and Information Sciences
- >> Sociology and Political Science
- Overall Rank
- Research Rank
- Innovation Rank
- Societal Rank
- All sectors
- Universities
- All regions and countries
- Asiatic Region
- Eastern Europe
- Latin America
- Middle East
- Northern America
- Pacific Region
- Western Europe
- ARAB COUNTRIES
- IBEROAMERICA
- NORDIC COUNTRIES
- Afghanistan
- Bosnia and Herzegovina
- Brunei Darussalam
- Burkina Faso
- Côte d’Ivoire
- Czech Republic
- Democratic Republic Congo
- French Guiana
- Kosovo (UNMIK)
- Multinational
- Netherlands
- New Zealand
- North Korea
- Philippines
- Puerto Rico
- Russian Federation
- Saint Kitts and Nevis
- Saudi Arabia
- South Africa
- South Korea
- Switzerland
- Syrian Arab Republic
- Trinidad and Tobago
- United Arab Emirates
- United Kingdom
- United States
- Vatican City State
| Best quartile | |||||
|---|---|---|---|---|---|
| 1 (5) | USA | ||||
| 2 (10) | USA | ||||
| 3 (19) | USA | ||||
| 4 (34) | USA | ||||
| 5 (40) | USA | ||||
| 6 (46) | USA | ||||
| 7 (47) | USA | ||||
| 8 (68) | USA | ||||
| 9 (71) | USA | ||||
| 10 (76) | USA | ||||
| 11 (107) | USA | ||||
| 12 (113) | USA | ||||
| 13 (131) | USA | ||||
| 14 (133) | USA | ||||
| 15 (146) | USA | ||||
| 16 (149) | USA | ||||
| 17 (150) | USA | ||||
| 18 (158) | USA | ||||
| 19 (164) | USA | ||||
| 20 (168) | USA | ||||
| 21 (172) | USA | ||||
| 22 (196) | USA | ||||
| 23 (199) | USA | ||||
| 24 (215) | USA | ||||
| 25 (225) | USA | ||||
| 26 (233) | USA | ||||
| 27 (244) | USA | ||||
| 28 (255) | USA | ||||
| 29 (263) | USA | ||||
| 30 (264) | USA | ||||
| 31 (272) | USA | ||||
| 32 (275) | USA | ||||
| 33 (276) | USA | ||||
| 34 (289) | USA | ||||
| 35 (291) | USA | ||||
| 36 (302) | USA | ||||
| 37 (303) | USA | ||||
| 38 (304) | USA | ||||
| 39 (312) | USA | ||||
| 40 (315) | USA | ||||
| 41 (316) | USA | ||||
| 42 (348) | USA | ||||
| 43 (363) | USA | ||||
| 44 (378) | USA | ||||
| 45 (384) | USA | ||||
| 46 (391) | USA | ||||
| 47 (405) | USA | ||||
| 48 (409) | USA | ||||
| 49 (417) | USA | ||||
| 50 (456) | USA | ||||
| 51 (480) | USA | ||||
| 51 (480) | USA | ||||
| 53 (498) | USA | ||||
| 54 (518) | USA | ||||
| 55 (555) | USA | ||||
| 56 (560) | USA | ||||
| 57 (562) | USA | ||||
| 58 (578) | USA | ||||
| 59 (584) | USA | ||||
| 60 (606) | USA | ||||
| 61 (636) | USA | ||||
| 62 (640) | USA | ||||
| 62 (640) | USA | ||||
| 64 (652) | USA | ||||
| 65 (672) | USA | ||||
| 66 (699) | USA | ||||
| 67 (726) | USA | ||||
| 68 (730) | USA | ||||
| 69 (748) | USA | ||||
| 70 (755) | USA | ||||
| 71 (772) | USA | ||||
| 72 (790) | USA | ||||
| 73 (797) | USA | ||||
| 74 (806) | USA | ||||
| 75 (809) | USA | ||||
| 76 (817) | USA | ||||
| 77 (830) | USA | ||||
| 78 (846) | USA | ||||
| 78 (846) | USA | ||||
| 80 (850) | USA | ||||
| 81 (863) | USA | ||||
| 82 (877) | USA | ||||
| 83 (886) | USA | ||||
| 84 (927) | USA | ||||
| 85 (948) | USA | ||||
| 86 (954) | USA | ||||
| 87 (963) | USA | ||||
| 88 (969) | USA | ||||
| 89 (988) | USA | ||||
| 90 (992) | USA | ||||
| 91 (1010) | USA | ||||
| 92 (1014) | USA | ||||
| 93 (1020) | USA | ||||
| 94 (1042) | USA | ||||
| 95 (1055) | USA | ||||
| 96 (1061) | USA | ||||
| 97 (1070) | USA | ||||
| 98 (1078) | USA | ||||
| 99 (1086) | USA | ||||
| 99 (1086) | USA | ||||
| 101 (1090) | USA | ||||
| 102 (1094) | USA | ||||
| 103 (1100) | USA | ||||
| 104 (1115) | USA | ||||
| 105 (1150) | USA | ||||
| 106 (1169) | USA | ||||
| 107 (1171) | USA | ||||
| 108 (1208) | USA | ||||
| 109 (1218) | USA | ||||
| 110 (1225) | USA | ||||
| 111 (1246) | USA | ||||
| 112 (1260) | USA | ||||
| 113 (1265) | USA | ||||
| 114 (1266) | USA | ||||
| 115 (1295) | USA | ||||
| 116 (1306) | USA | ||||
| 117 (1309) | USA | ||||
| 118 (1313) | USA | ||||
| 119 (1335) | USA | ||||
| 119 (1335) | USA | ||||
| 121 (1339) | USA | ||||
| 122 (1352) | USA | ||||
| 123 (1411) | USA | ||||
| 124 (1420) | USA | ||||
| 125 (1421) | USA | ||||
| 126 (1430) | USA | ||||
| 127 (1444) | USA | ||||
| 127 (1444) | USA | ||||
| 129 (1464) | USA | ||||
| 130 (1507) | USA | ||||
| 131 (1522) | USA | ||||
| 132 (1526) | USA | ||||
| 133 (1549) | USA | ||||
| 134 (1563) | USA | ||||
| 135 (1572) | USA | ||||
| 136 (1620) | USA | ||||
| 137 (1659) | USA | ||||
| 138 (1685) | USA | ||||
| 138 (1685) | USA | ||||
| 138 (1685) | USA | ||||
| 141 (1706) | USA | ||||
| 142 (1713) | USA | ||||
| 143 (1715) | USA | ||||
| 144 (1719) | USA | ||||
| 145 (1743) | USA | ||||
| 146 (1821) | USA | ||||
| 147 (1837) | USA | ||||
| 148 (1857) | USA | ||||
| 149 (1888) | USA | ||||
| 150 (1924) | USA | ||||
| 151 (1945) | USA | ||||
| 152 (1951) | USA | ||||
| 153 (1982) | USA | ||||
| 154 (1996) | USA | ||||
| 155 (2001) | USA | ||||
| 156 (2045) | USA | ||||
| 157 (2060) | USA | ||||
| 158 (2084) | USA | ||||
| 159 (2093) | USA | ||||
| 160 (2106) | USA | ||||
| 161 (2115) | USA | ||||
| 162 (2117) | USA | ||||
| 163 (2131) | USA | ||||
| 164 (2168) | USA | ||||
| 165 (2210) | USA | ||||
| 166 (2250) | USA | ||||
| 167 (2257) | USA | ||||
| 168 (2270) | USA | ||||
| 169 (2275) | USA | ||||
| 170 (2322) | USA | ||||
| 171 (2330) | USA | ||||
| 172 (2352) | USA | ||||
| 173 (2365) | USA | ||||
| 174 (2372) | USA | ||||
| 175 (2399) | USA | ||||
| 175 (2399) | USA | ||||
| 177 (2404) | USA | ||||
| 178 (2428) | USA | ||||
| 178 (2428) | USA | ||||
| 180 (2445) | USA | ||||
| 181 (2468) | USA | ||||
| 182 (2477) | USA | ||||
| 183 (2483) | USA | ||||
| 184 (2490) | USA | ||||
| 185 (2508) | USA | ||||
| 186 (2538) | USA | ||||
| 187 (2568) | USA | ||||
| 188 (2572) | USA | ||||
| 189 (2593) | USA | ||||
| 190 (2630) | USA | ||||
| 191 (2639) | USA | ||||
| 192 (2662) | USA | ||||
| 193 (2668) | USA | ||||
| 194 (2686) | USA | ||||
| 195 (2692) | USA | ||||
| 196 (2783) | USA | ||||
| 197 (2796) | USA | ||||
| 198 (2820) | USA | ||||
| 199 (2822) | USA | ||||
| 200 (2830) | USA | ||||
| 201 (2855) | USA | ||||
| 202 (2862) | USA | ||||
| 203 (2893) | USA | ||||
| 204 (2903) | USA | ||||
| 205 (2926) | USA | ||||
| 206 (2940) | USA | ||||
| 207 (2944) | USA | ||||
| 207 (2944) | USA | ||||
| 209 (2954) | USA | ||||
| 210 (2978) | USA | ||||
| 211 (3003) | USA | ||||
| 212 (3020) | USA | ||||
| 213 (3048) | USA | ||||
| 214 (3056) | USA | ||||
| 215 (3060) | USA | ||||
| 216 (3085) | USA | ||||
| 217 (3092) | USA | ||||
| 218 (3102) | USA | ||||
| 218 (3102) | USA | ||||
| 220 (3110) | USA | ||||
| 221 (3116) | USA | ||||
| 222 (3238) | USA | ||||
| 223 (3241) | USA | ||||
| 224 (3295) | USA | ||||
| 225 (3315) | USA | ||||
| 226 (3358) | USA | ||||
| 227 (3364) | USA | ||||
| 228 (3374) | USA | ||||
| 229 (3383) | USA | ||||
| 230 (3396) | USA | ||||
| 231 (3513) | USA | ||||
| 232 (3530) | USA | ||||
| 233 (3613) | USA | ||||
| 233 (3613) | USA | ||||
| 235 (3631) | USA | ||||
| 236 (3650) | USA | ||||
| 237 (3679) | USA | ||||
| 238 (3709) | USA | ||||
| 239 (3715) | USA | ||||
| 240 (3727) | USA | ||||
| 240 (3727) | USA | ||||
| 242 (3770) | USA | ||||
| 243 (3780) | USA | ||||
| 244 (3809) | USA | ||||
| 244 (3809) | USA | ||||
| 246 (3862) | USA | ||||
| 247 (3925) | USA | ||||
| 248 (3929) | USA | ||||
| 249 (3940) | USA | ||||
| 250 (3947) | USA | ||||
| 251 (3957) | USA | ||||
| 252 (3972) | USA | ||||
| 253 (3991) | USA | ||||
| 254 (4040) | USA | ||||
| 255 (4068) | USA | ||||
| 256 (4131) | USA | ||||
| 257 (4171) | USA | ||||
| 258 (4175) | USA | ||||
| 259 (4187) | USA | ||||
| 260 (4216) | USA | ||||
| 261 (4226) | USA | ||||
| 262 (4257) | USA | ||||
| 263 (4293) | USA | ||||
| 264 (4300) | USA | ||||
| 265 (4354) | USA | ||||
| 266 (4373) | USA | ||||
| 267 (4398) | USA | ||||
| 268 (4442) | USA | ||||
| 269 (4484) | USA | ||||
| 269 (4484) | USA | ||||
| 271 (4494) | USA | ||||
| 272 (4514) | USA | ||||
| 273 (4526) | USA | ||||
| 274 (4530) | USA | ||||
| 275 (4564) | USA | ||||
| 276 (4647) | USA | ||||
| 277 (4705) | USA | ||||
| 278 (4776) | USA | ||||
| 279 (4869) | USA | ||||
| 279 (4869) | USA | ||||
| 281 (4894) | USA | ||||
| 282 (5120) | USA | ||||
| 283 (5130) | USA | ||||
| 284 (5141) | USA | ||||
| 285 (5154) | USA | ||||
| 286 (5240) | USA | ||||
| 287 (5338) | USA | ||||
| 288 (5364) | USA | ||||
| 289 (5493) | USA | ||||
| 290 (5534) | USA | ||||
| 291 (5549) | USA | ||||
| 292 (5582) | USA | ||||
| 293 (5615) | USA | ||||
| 294 (5647) | USA | ||||
| 295 (5652) | USA | ||||
| 296 (5664) | USA | ||||
| 296 (5664) | USA | ||||
| 298 (5739) | USA | ||||
| 299 (5743) | USA | ||||
| 300 (5756) | USA | ||||
| 301 (5769) | USA | ||||
| 302 (5775) | USA | ||||
| 303 (5821) | USA | ||||
| 304 (5908) | USA | ||||
| 305 (5921) | USA | ||||
| 306 (5943) | USA | ||||
| 307 (5958) | USA | ||||
| 307 (5958) | USA | ||||
| 309 (6110) | USA | ||||
| 310 (6128) | USA | ||||
| 311 (6199) | USA | ||||
| 312 (6267) | USA | ||||
| 313 (6274) | USA | ||||
| 313 (6274) | USA | ||||
| 315 (6299) | USA | ||||
| 316 (6341) | USA | ||||
| 317 (6438) | USA | ||||
| 318 (6552) | USA | ||||
| 319 (6606) | USA | ||||
| 320 (6839) | USA | ||||
| 321 (6864) | USA | ||||
| 322 (6953) | USA | ||||
| 323 (6970) | USA | ||||
| 324 (7154) | USA | ||||
| 325 (7322) | USA | ||||
| 326 (7358) | USA | ||||
| 327 (7415) | USA | ||||
| 328 (7575) | USA | ||||
| 329 (7669) | USA | ||||
| 330 (8167) | USA | ||||
- Infographics
- Methodology
- Edit Cookie Consent
- Legal Notice
- Privacy Policy
- Overall Ranking
- University Rankings
- Government Rankings
- Health Rankings
- Private Rankings
- Other Rankings
- Research Rankings
- Innovation Rankings
- Societal Rankings

- Today's news
- Reviews and deals
- Climate change
- 2024 election
- Fall allergies
- Health news
- Mental health
- Sexual health
- Family health
- So mini ways
- Unapologetically
- Buying guides
Entertainment
- How to Watch
- My Portfolio
- Latest News
- Stock Market
- Biden Economy
- Stocks: Most Actives
- Stocks: Gainers
- Stocks: Losers
- Trending Tickers
- World Indices
- US Treasury Bonds Rates
- Top Mutual Funds
- Options: Highest Open Interest
- Options: Highest Implied Volatility
- Basic Materials
- Communication Services
- Consumer Cyclical
- Consumer Defensive
- Financial Services
- Industrials
- Real Estate
- Stock Comparison
- Advanced Chart
- Currency Converter
- Credit Cards
- Balance Transfer Cards
- Cash-back Cards
- Rewards Cards
- Travel Cards
- Credit Card Offers
- Best Free Checking
- Student Loans
- Personal Loans
- Car insurance
- Mortgage Refinancing
- Mortgage Calculator
- Morning Brief
- Market Domination
- Market Domination Overtime
- Asking for a Trend
- Opening Bid
- Stocks in Translation
- Lead This Way
- Good Buy or Goodbye?
- Financial Freestyle
- Capitol Gains
- Living Not So Fabulously
- Decoding Retirement
- Fantasy football
- Pro Pick 'Em
- College Pick 'Em
- Fantasy baseball
- Fantasy hockey
- Fantasy basketball
- Download the app
- Daily fantasy
- Scores and schedules
- GameChannel
- World Baseball Classic
- Premier League
- CONCACAF League
- Champions League
- Motorsports
- Horse racing
- Newsletters
New on Yahoo
- Privacy Dashboard
Yahoo Finance
27 largest biotech companies in the us.
In this article, we take a look at 27 largest biotech companies in the US. You can skip our detailed analysis of the biotech industry and go directly to the 5 Largest Biotech Companies in the US . The largest biotech companies in the US have a total market cap of $88 trillion.
Latest Developments in the Biotech Industry
The biotechnology industry is making significant developments in food and agriculture to help minimize health risks and combat environmental threats. For example, a French biotech company called Enterome has collaborated with Nestlé to produce an immunity drug for the treatment of food allergies. Similarly, companies like UPSIDE Foods and BioBetter Limited are producing cultivated meat , which is sustainable and climate-friendly, and has gotten approved for safety by the U.S. Food and Drug Administration (FDA).
Experts in biotechnology are also producing various profitable products that are encouraging growth in the pharmaceutical sector. Researchers at the Massachusetts Institute of Technology have developed a vaccine printer that can make hundreds of vaccine patches during a day. This biotechnological invention can help combat the spread of the Ebola virus in countries that are still vulnerable to the epidemic such as Uganda and the Democratic Republic of Congo. CureVac N.V. (NASDAQ: CVAC ) had begun developing RNA printing technology in March 2022 to help the invention of this vaccine printer.
To further enhance the integration of artificial intelligence with biotechnology, researchers at Insilico Medicine are using ChatGPT to pace up the process of discovering potential drug targets for treatment. Similarly, scientists are using software such as Scilife, Veeva Vault, and Ace, which support innovation in biotechnology by improving content and quality management in laboratories.
Currently, the most common diseases in the US are cancer, obesity, and heart-related diseases. Various biotechnology companies are making huge investments to combat these diseases such as Guardant Health, Inc. (NASDAQ: GH ), which developed liquid biopsy for cancer detection; and Verve Therapeutics, Inc. (NASDAQ: VERV ), which is developing gene editing medicines for cardiovascular diseases.
In order to combat diabetes, Novo Nordisk A/S (NYSE: NVO ) produced medicines called Ozempic and Wegovy , which quickly garnered mass popularity and celebrity endorsements. These are also prescribed by American doctors for weight loss and fighting cardiovascular issues. Digital health management companies such as WeightWatchers and Lark Health are also using biotechnology to help patients cope with chronic diseases by offering weight loss programs and health monitoring devices.
Outlook of the Biotech Industry
The United States is the global leader in biotechnology, with Massachusetts being the biggest biotech hub in the world. It is closely followed by China, with the UK and the rest of Europe trailing behind. In response to China’s heavy investment in the biotechnology sector, the Biden government announced its creation of the National Biotechnology and Biomanufacturing Initiative , to support its bio-economy already worth $1 trillion in 2022.
Between 2022 and 2023, the S&P Biotechnology index increased by 8.86%. However, since the Federal Reserve is expected to increase interest rates through 2023, the value of biotech stocks might decrease compared to previous years, where the industry was supplied with huge investments and cheap capital to encourage the development of Covid 19 treatment.
Between 2022 and 2030, the global market is forecasted to grow at a compound annual rate of 13.9%, ultimately reaching $3879 billion in 2030. The development of genomic techniques to combat chronic diseases, enhancing the portability of biotechnological instruments, and the integration of robotics and biotechnology are predicted to be some of the key drivers of this industrial growth during the coming years.
Tonhom1009/Shutterstock.com
Our Methodology
For our list of the largest biotech companies in the US, we've ranked them in ascending order based on their annual revenue for the latest year. We've also included foreign companies that either trade or operate in the US.
Here are the 27 largest biotech companies in the US:
27. Vertex Pharmaceuticals Incorporated (NASDAQ: VRTX )
2022 Revenue: $8.93 billion
This biotech company specializes in the development and production of small-molecule drugs to treat serious ailments. The revenue for Vertex Pharmaceuticals increased by 17.4% in the fourth quarter of 2022 compared to the previous year, and the company is expected to make further gains after receiving FDA’s approval to expand its cystic fibrosis medicine.
26. Biogen Inc. (NASDAQ: BIIB )
2022 Revenue: $10.17 billion
Biogen Inc. (NASDAQ:BIIB) is a Massachusetts-based company that develops and delivers treatment for complex neurological illnesses such as Alzheimer’s and multiple sclerosis. Biogen Inc. (NASDAQ:BIIB) was the first company to gain approval for its treatment of spinal muscular atrophy.
25. CSL Limited
2022 Revenue: $10.49 billion
CSL Limited focuses on biotechnological advancement for combating rare diseases such as hereditary angioedema, producing vaccines for influenza, and treating iron deficiency in serious health conditions such as heart failure.
24. Regeneron Pharmaceuticals, Inc. (NASDAQ: REGN )
2022 Revenue: $12.17 billion
Regeneron Pharmaceuticals is a New York-based company that produces life-saving drugs for the treatment of serious diseases. Its highest-selling product is Libtayo, a medicine for skin cancer, which drove sales of approximately $448 million in 2022.
23. Otsuka Holdings Co., Ltd.
2022 Revenue: $13.02 billion
Otsuka Holdings is a Japanese company that conducts business in the global drugs and biotechnology industry. Otsuka Holdings Co. produces pharmaceuticals, neutraceuticals, and consumer products. In 2022, the company made a profit of $1.1 billion .
22. Laboratory Corporation of America Holdings (NYSE: LH )
2022 Revenue: $14.87 billion
Laboratory Corporation of America Holdings (NYSE:LH) is a biotech and healthcare company that has a network of 36 laboratories in America, which are used for diagnostics and drug development. Laboratory Corporation of America Holdings (NYSE:LH) is further expanding its business by buying Enzo Biochem, Inc. (NYSE: ENZ )’s clinical lab business for $146 million while also launching an independent clinical development business called Fortrea .
21. Teva Pharmaceutical Industries Limited (NYSE: TEVA )
2022 Revenue: $14.92 billion
Teva Pharmaceutical Industries Limited (NYSE:TEVA) produces generic drugs and consumer healthcare solutions. Teva Pharmaceutical Industries Limited (NYSE:TEVA) has a portfolio of 3600 medicines and health products, which are supplied to more than 200 million people in 60 countries across the world.
20. Baxter International Inc. (NYSE: BAX )
2022 Revenue: $15.11 billion
Baxter International Inc. produces healthcare solutions for the treatment of nutritional and renal ailments among others. In the last quarter of 2022, its biggest stakeholder was Generation Investment Management, which held more than 10 million shares of the company, worth a total of $553 million.
19. Viatris Inc. (NASDAQ: VTRS )
2022 Revenue: $16.26 billion
Viatris Inc. (NASDAQ:VTRS) is a Pennsylvania-based company that produces medicines, generic drugs, and pharmaceutical ingredients. Merely two years after its incorporation, Viatris Inc. (NASDAQ:VTRS) bought Oyster Point Pharma and Famy Life Sciences for $750 million and now aims to become a globally leading company in ophthalmology.
18. BioNTech SE (NASDAQ: BNTX )
2022 Revenue: $18.43 billion
BioNTech SE is a German biotech and immunotherapy company that is traded publicly in the US. During the Covid-19 pandemic, the company collaborated with Pfizer Inc. (NYSE: PFE ) to utilize its innovative genetic technology for the production of vaccines, which helped nearly 5 billion people in 181 countries in fighting coronavirus.
17. Novo Nordisk A/S (NYSE:NVO)
2022 Revenue: $25.05 billion
Novo Nordisk is a Danish biotech and pharmaceutical company that has established production plants in nine countries across the world, including the US. Its primary focus is on producing medicine to treat obesity, diabetes, and hemophilia among other illnesses. Its most popular medicines are Ozempic and Wegovy.
16. Amgen, Inc. (NASDAQ: AMGN )
2022 Revenue: $26.32 billion
Amgen is one of the world’s biggest independent biotech and biopharmaceutical firms, that produces medicine for serious illnesses such as cancer and anemia. Amgen’s earnings for Q1 2023 amounted to $6.11 billion, a 2% decline compared to first-quarter earnings in 2022.
15. Gilead Sciences, Inc. (NASDAQ: GILD )
2022 Revenue: $27.28 billion
Gilead Sciences, Inc. (NASDAQ:GILD) produces medicine and therapy for the treatment of cancers, viral diseases, and inflammation. Gilead Sciences, Inc. (NASDAQ:GILD) helped roughly 12 million people across the world in recovering from the coronavirus disease.
14. Eli Lilly and Company (NYSE: LLY )
2022 Revenue: $28.54 billion
Eli Lilly and Company is famous for being the first biotech company to mass-produce Penicillin, which is the first antibiotic, and insulin. It is also the largest producer of psychiatric medicine such as Prozac and Zyprexa. The greatest share of Eli Lilly’s revenue is driven by the sale of Trulicity and Humalog, which treat diabetes.
13. Takeda Pharmaceutical Company Limited (NYSE: TAK )
2022 Revenue: $29.59 billion
Takeda Pharmaceutical is the largest Asian company in the biopharma sector. In March 2023, Takeda Pharmaceutical received an investment of $960,000 from the Global Health Innovative Technology (GHIT) Fund for the development of antimalarial medicine.
12. GSK plc (NYSE: GSK )
2022 Revenue: $38.94 billion
GSK is a global biotech and pharmaceutical company that produces vaccines, general medicine for primary care, and specialty medicine and therapy for cancer, HIV, and respiratory diseases. It is expanding its biotech operation in Canada by acquiring Bellus Health for $2 billion.
11. AstraZeneca PLC (NASDAQ: AZN )
2022 Revenue: $44.35 billion
AstraZeneca is a biotech company that produces medicine, vaccines, and immune therapy, specifically for type 2 diabetes, respiratory problems, and cancers . The company earned a profit of $1.8 billion in Q1 of 2023.
10. Thermo Fisher Scientific Inc. (NYSE: TMO )
2022 Revenue: $44.91 billion
Thermo Fisher Scientific is a biotechnology and healthcare distributor company that produces scientific instruments, software, and chemicals. In Q4 of 2022, its biggest stakeholder was Farallon Capital, which owned 1 million company shares, amounting to $639 million in total.
9. Bristol-Myers Squibb Company (NYSE: BMY )
2022 Revenue: $46.15 billion
Bristol-Myers Squibb ranks among the most prominent global companies in the biotechnology and pharmaceutical industries. The company has made a collaboration of $1 billion with Tubulis, a German biotechnology company, to develop and produce potent therapies for solid tumors and cancers.
8. Sanofi (NASDAQ: SNY )
2022 Revenue: $47.62 billion
Sanofi is a pharmaceutical and biotech company that produces vaccines and affordable medicine to provide quality healthcare in developing countries. The French company has acquired Provention Bio, Inc. (NASDAQ: PRVB ), thus adding TZIELD–an innovative therapy for type 1 diabetes–to its portfolio.
7. Novartis AG (NYSE: NVS )
2022 Revenue: $51.82 billion
Novartis AG (NYSE:NVS) is one of the leading research and development companies that produces transformative treatments for serious medical issues. Novartis AG (NYSE:NVS) witnessed an increase in profit during the first quarter of 2023, which was mainly supported by sales of cardiovascular and cancer medicines.
6. Bayer AG
2022 Revenue: $53.67 billion
Bayer is a German pharmaceutical and biotech company that also produces consumer healthcare products along with chemicals and seeds for agriculture. According to the CEO, the company’s sales increased by 9% in 2022, even though it was a difficult year for businesses amid the energy crisis and inflation.
Click to continue reading and see the 5 Largest Biotech Companies in the US .
Suggested Articles:
Top 10 Growth Stocks in Biotech
12 Most Profitable Biotech Stocks Today
10 Most Promising Biotech Stocks According to Analysts
Disclosure: None. 27 Largest Biotech Companies in the US is originally published on Insider Monkey.
- ICH GCP (De)
- ICH GCP (En)
- ICH GCP (Es)
- ICH GCP (Fr)
- ICH GCP (It)
- ICH GCP (Pt)
- ICH GCP (Ru)
- AUSTRALIA (NHMRC)
- JAPAN (PMDA)
- US Clinical Trials Registry
- EU Clinical Trials Registry
- Pharmaceutical Companies
- Clinical Research Labs
- Service Companies
- Clinical Research Events
- Publications
- Researchers
List of Pharmaceutical Companies in United States
Featured pharmaceutical companies.

We have been committed to improving lives since the company was founded in 1896 in Basel, Switzerland. Today, Roche creates innovative medicines and diagnostic tests that help millions of patients globally. Roche was one of the first companies to...

AstraZeneca
We are a global, science-led biopharmaceutical business and our innovative medicines are used by millions of patients worldwide. Our purpose and values help explain why we exist, what we hope to accomplish, the behaviours we value, how we will ac...
Local, small- and mid-size Pharmaceutical Companies in United States
Alnylam has led the translation of RNAi (RNA interference) from Nobel Prize-winning discovery into an innovative, entirely new class of medicines. Founded in 2002 by a team of distinguished life sciences leaders, Alnylam’s vision is to harness the... View full profile
- United States
Amneal's Generics Division focuses on a broad range of therapeutic areas, including solid oral dosage products and alternative dosage form products. The company's Specialty Pharma Division is focused on the development of proprietary branded pharmac... View full profile
Azurity Pharmaceuticals is a privately held, specialty pharmaceutical company that focuses on innovative products that meet the needs of patients with underserved conditions. As an industry leader in providing unique, accessible, and high-quality med... View full profile
At Bora Pharmaceuticals, we insist on high quality, reliability, and efficiency without any compromises. Dedication to these high standards is how we create a long lasting company culture that is the bedrock for our growth. We cover the entire pharma... View full profile
Cycle Pharmaceuticals is a global, privately-owned and patient-dedicated biotechnology company headquartered in Cambridge in the UK. Our mission is to utilise the latest pharmaceutical technologies to deliver superior drug treatments to better serve... View full profile
- United Kingdom
Endo is a specialty pharmaceutical company committed to helping everyone we serve live their best life through the delivery of quality, life-enhancing therapies. Our decades of proven success come from a global team of passionate employees collaborat... View full profile
Genentech, Inc., is an American biotechnology corporation which became a subsidiary of Roche in 2009. Genentech Research and Early Development operates as an independent center within Roche. Historically, the company is regarded as the world's firs... View full profile
MSN Group is the fastest growing research-based pharmaceutical company based out of India. Founded in 2003 with a mission to make health care affordable, this Hyderabad-based venture has nine API and five finished dosage facilities established across... View full profile
Purdue Pharma and its subsidiaries develop, manufacture and market medications and consumer health products to meet the evolving needs of healthcare professionals, patients, consumers and caregivers. If consummated, Purdue’s plan of reorganization w... View full profile
Taro is a research-based international pharmaceutical company that was established on the principal that research and development would be the cornerstone of its growth strategy. Providing quality products through scientific innovations, diligence an... View full profile
TIB Molbiol is a medium-sized biotech company who has supplied the global market for over 30 years with reagents for research and medical diagnostics. We are known for our innovation, product quality, design and time to market. Our customers are mai... View full profile
Vertex creates new possibilities in medicine to cure diseases and improve people's lives. We work with leading researchers, doctors, public health experts and other collaborators who share our vision for transforming the lives of people with serious... View full profile
Zealand Pharma A/S was founded in 1998 and is a biotechnology company focused on the discovery, design and development of innovative peptide-based medicines. We intend to be a leader in specialty medicines focusing on metabolic and gastrointestinal... View full profile
Global Pharmaceutical Companies in United States
Abbott Laboratories is an American multinational medical devices and health care company with headquarters in Abbott Park, Illinois, United States. The company was founded by Chicago physician Wallace Calvin Abbott in 1888 to formulate known drugs;... View full profile
- Czech Republic
- Netherlands
- New Zealand
- Philippines
- Saudi Arabia
- South Africa
- South Korea
- Switzerland
We’re a company that takes on the toughest health challenges. But we do more than treat diseases—we aim to make a remarkable impact on people’s lives. We are AbbVie, a highly focused research-driven biopharmaceutical company. Our ~47,000 employees... View full profile
- Bosnia & Herzegovina
- United Arab Emirates
People living with rare and devastating diseases are our Guiding Star. We believe it is our responsibility to listen to, understand, and change the lives of patients and those who work tirelessly to help them. To transform the lives of people affec... View full profile
Our purpose is a better health and a better quality of life for patients, caregivers and healthcare providers. Serving patients is our inspiration and we are passionate about improving their lives. Alfasigma has the ambition of becoming one of the le... View full profile
Amgen is one of the world’s leading biotechnology companies. Amgen is a values-based company, deeply rooted in science and innovation to transform new ideas and discoveries into medicines for patients with serious illnesses. At Amgen, we believe in... View full profile
Angelini Pharma is the pharmaceutical division of Angelini Group. The Group started almost 100 years ago as a small pharmaceutical laboratory, and over the years has grown into a leading international group in healthcare, present in Pharmaceuticals... View full profile
We are a global specialty and branded pharmaceutical company, improving the health of patients across the world through our high quality and affordable medicines. Active at every stage of the value chain, we are uniquely diversified by geography, prod... View full profile
The Astellas Group’s business philosophy has three elements — raison d'être, mission and beliefs. Raison D'être Contribute toward improving the health of people around the world through the provision of innovative and reliable pharmaceutical products... View full profile
We are a global, science-led biopharmaceutical business and our innovative medicines are used by millions of patients worldwide. Our purpose and values help explain why we exist, what we hope to accomplish, the behaviours we value, how we will achi... View full profile
Founded in 1986 by Mr. P. V. Ramprasad Reddy, Mr. K. Nityananda Reddy and a small group of highly committed professionals, Aurobindo Pharma was born of a vision. The company commenced operations in 1988-89 with a single unit manufacturing Semi-Synthe... View full profile
Bausch Health is focused on improving people’s lives with our health care products. We are delivering on our commitments to patients, health care providers, other stakeholders and society, as we build an innovative company dedicated to advancing glob... View full profile
Everything we do converges at the critical intersection where products that save and sustain lives meet the healthcare professionals and caregivers who make it all happen. Now, we support more patients through every point of their journey, with a bro... View full profile
Bayer is a Life Science company with a more than 150-year history and core competencies in the areas of health care and agriculture. With our innovative products, we are contributing to finding solutions to some of the major challenges of our time. View full profile
- Dominican Republic
- El Salvador
At Biogen, our mission is clear: we are pioneers in neuroscience. Since our founding in 1978 as one of the world’s first global biotechnology companies, Biogen has led innovative scientific research with the goal over the last decade to defeat deva... View full profile
Boehringer Ingelheim is a global group of companies embracing many cultures and diverse societies. Learn more about the financial highlights, the corporate vision, the organisation, the Board of Managing Directors and the company's history as well... View full profile
We are a global biopharmaceutical company whose mission is to discover, develop and deliver innovative medicines that help patients prevail over serious diseases. At Bristol Myers Squibb, we work every day to transform patients’ lives through science... View full profile
We want to be recognized as an international group focused on research, able to develop and commercialize innovative therapeutic solutions that improve people's quality of life. We intend to maintain a high quality entrepreneurial team, characterized... View full profile
At Curium, we have a singular focus – to develop, manufacture and supply world-class radiopharmaceutical products to customers around the globe. Drawing on years of proven experience, we aim to drive the nuclear medicine industry forward through exce... View full profile
Daiichi Sankyo Company, Limited (Japanese: 第一三共株式会社, Hepburn: Daiichi Sankyō Kabushiki-gaisha) is a global pharmaceutical company and the second-largest pharmaceutical company in Japan. It achieved JPY 981.8 billion in revenue in 2019. The... View full profile
We give first thought to patients and their families, and to increasing the benefits health care provides. A human health care company capable of making a meaningful contribution under any health care system while observing the highest legal and ethi... View full profile
Lilly was founded in 1876 by Colonel Eli Lilly, a man committed to creating high-quality medicines that met real needs in an era of unreliable elixirs peddled by questionable characters. His charge to the generations of employees who have followed... View full profile
Ever Pharma is a fully integrated specialty pharmaceuticals company focused on the research, development, production, and commercialization of products in the areas of neurology and specialty injectables, covering oncology, intensive care and hormone... View full profile
Ferring is a research-driven, specialty biopharmaceutical group committed to helping people build healthy families and live better lives. Ferring is a leader in reproductive medicine and maternal health, and in specialty areas within gastroenterology... View full profile
We are proud of the fact that Richter is a specialised Hungarian-based pharmaceutical company built on innovation and whose main goal is to help the public treat illnesses with innovative products. Our company is a major player in the Hungarian econo... View full profile
- Turkmenistan
At Gilead, we’re committed to creating possible. For more than 30 years, we’ve pursued the impossible, chased it down, tackled it for answers and surrounded it for a way in. We have worked tirelessly to bring forward medicines for life-threatening... View full profile
We are a science-led global healthcare company with a special purpose: to help people do more, feel better, live longer. We have 3 global businesses that research, develop and manufacture innovative pharmaceutical medicines, vaccines and consumer h... View full profile
- Trinidad & Tobago
Glenmark Pharmaceuticals was founded in 1977 by Gracias Saldanha as a generic drug and active pharmaceutical ingredient manufacturer. He named the company after his two sons. The company initially sold its products in India, Russia, and Africa. The c... View full profile
Clinical trials help physicians, investigators and regulators understand whether a potential treatment may safely and effectively treat a specific disease or condition and can provide patients an opportunity to access investigational treatments that... View full profile
Incyte is a global biopharmaceutical company founded on the premise that investment in strong science and the relentless pursuit of R&D excellence can translate into new solutions that can positively affect patients’ lives. Our drug discovery and... View full profile
Italfarmaco SpA is a private Italian multinational company located in Milan, operating in Italy and abroad in both the pharmaceutical and fine chemical industries through its controlled and/or participated companies. The Group has modern and sophisti... View full profile
Jazz Pharmaceuticals plc (NASDAQ: JAZZ) is a global biopharmaceutical company whose purpose is to innovate to transform the lives of patients. We are focused on developing life-changing medicines for people with serious diseases — often with limite... View full profile
At Johnson & Johnson, we believe good health is the foundation of vibrant lives, thriving communities and forward progress. That’s why for more than 130 years, we have aimed to keep people well at every age and every stage of life. Today, as the... View full profile
The Kyowa Kirin Group is a Japanese pharma group that develops innovative specialty drugs, to raise the health and well-being of people around the world. The Kyowa Kirin Group companies strive to contribute to the health and well-being of people arou... View full profile
Lupin is a global pharmaceutical company offering a wide range of products such as Branded & Generic Formulations, Biotechnology Products, Active Pharmaceutical Ingredients (APIs) and Specialty. Lupin is a significant player in the therapy areas... View full profile
Mallinckrodt Pharmaceuticals is a multibillion dollar specialty pharmaceutical company focused on our mission: Managing Complexity. Improving Lives. We provide medicines to address unmet patient needs, stemming from 150 years of using our unique stre... View full profile
The Menarini Group is present in 140 countries worldwide, a guarantee of internationally recognised quality. The achieved results are proof of an efficient strategy based on Research, Innovation and Internationalisation, along with the ability to r... View full profile
We aspire to be the premier research-intensive biopharmaceutical company in the world For more than 125 years, Merck (known as MSD outside of the U.S. and Canada) has been inventing for life, bringing forward medicines and vaccines for many of the wo... View full profile
Novartis is structured to deliver innovative products, exploit global scale, and respond to new opportunities and risks. Our purpose is to reimagine medicine to improve and extend people's lives. We use innovative science and technology to address... View full profile
We are a global healthcare company, founded in 1923 and headquartered just outside Copenhagen, Denmark. Our purpose is to drive change to defeat diabetes and other serious chronic diseases such as obesity, and rare blood and rare endocrine diseases... View full profile
Our five values -Ownership, Integrity, Leadership, Sustainability and Entrepreneurship- guide our patient-oriented corporate culture. We aspire to embody these values in everything we do. We are the largest privately owned and independent plasma fr... View full profile
ONO has taken a new step beyond the major milestone of the 300th anniversary, since its establishment in 1717 as an apothecary by Ichibei Fushimiya in Doshomachi, Osaka. Upholding the corporate philosophy “Dedicated to Man’s Fight against Disease and... View full profile
Otsuka Pharmaceutical Co., Ltd. (大塚製薬株式会社, Ōtsuka Seiyaku Kabushiki-gaisha) (TYO: 4578), abbreviated OPC, is a pharmaceutical company headquartered in Tokyo, Osaka and Naruto, Japan. The company was established August 10, 1964. View full profile
Over 130 years of creating Quality, Affordable Self-Care Products to trusted retailers, pharmacies and e-commerce channels. Perrigo's branded and private label self-care products are available at retailers, pharmacies, and e-commerce outlets in the U... View full profile
At Pfizer, we apply science and our global resources to bring therapies to people that extend and significantly improve their lives. We strive to set the standard for quality, safety, and value in the discovery, development, and manufacture of heal... View full profile
Recipharm is a leading global pharmaceutical Contract Development and Manufacturing Organisation (CDMO). We provide pharmaceutical companies around the world with tailor-made development and manufacturing services, including a wide variety of drug do... View full profile
Recordati è una multinazionale farmaceutica italiana fondata nel 1926 con sede a Milano. Sviluppa, produce e commercia prodotti farmaceutici o di chimica farmaceutica. Il 96,4% del fatturato è dato nel 2017 dal settore farmaceutico mentre il restante... View full profile
Regeneron (NASDAQ: REGN) is a leading biotechnology company that invents life-transforming medicines for people with serious diseases. Founded and led for over 30 years by physician-scientists, our unique ability to repeatedly and consistently tran... View full profile
We have been committed to improving lives since the company was founded in 1896 in Basel, Switzerland. Today, Roche creates innovative medicines and diagnostic tests that help millions of patients globally. Roche was one of the first companies to b... View full profile
- Cote d'Ivoire
Sanofi is a global biopharmaceutical company focused on human health. Sanofi engages in the research and development, manufacturing and marketing of pharmaceutical drugs principally in the prescription market, but the firm also develops over-the-co... View full profile
As a specialized pharmaceutical company, Santen continues to make utmost efforts to contribute to ophthalmic treatment around the world. View full profile
Shionogi is enhancing our strengths as a drug discovery-based pharmaceutical company to create innovative products and services and to deliver value in collaboration with a diverse array of partners. By delivering value to tackle social issues and ad... View full profile
To broadly contribute to society through value creation based on innovative research and development activities for the betterment of healthcare and fuller lives of people worldwide. Management Mission To contribute to healthcare and people's well-... View full profile
Sun Pharmaceutical Industries Ltd. (Sun Pharma) is the fourth largest specialty generic pharmaceutical company in the world with global revenues of over US$ 4.5 billion. Supported by more than 40 manufacturing facilities, we provide high-quality, aff... View full profile
- Central African Republic
With over a century of history, Taisho Pharmaceutical Holdings has the largest share of Japan’s over-the-counter pharmaceutical market and a strong presence in health food, cosmetics, and other fields. The Company's mission is to contribute to societ... View full profile
Takeda is a patient-focused, values-based, R&D-driven global biopharmaceutical company committed to bringing Better Health and a Brighter Future to people worldwide. Our passion and pursuit of potentially life-changing treatments for patients a... View full profile
Our mission is to be a global leader in generics and biopharmaceuticals, improving the lives of patients across the world. In a complex world, Teva's mission is simple: to improve the lives of patients across the globe. We believe that everyone shoul... View full profile
A global biopharma company, focusing on neurology and immunology. Our business is strong. Total revenue grew to €4.9 billion in 2019. We are more than 7,600 people in all four corners of the globe, inspired by patients and driven by science. UCB’s am... View full profile
At Viatris, we have one of the strongest global commercial infrastructures in the industry, with a balanced reach across North America, Europe, the Asia Pacific region and emerging markets. Our high quality global manufacturing, scientific excellen... View full profile
Xellia Pharmaceuticals is a specialty pharmaceutical company focused on providing important anti-infective treatments against serious and often life-threatening infections. Xellia is wholly owned by Novo Holdings A/S since 2013 and headquartered in C... View full profile
List of Pharmaceutical Companies by location
- Afghanistan
- Congo, DR of
- Latest News
- Emergencies
- Environment
- Ask the Law
- Visa+Immigration
- Phone+Internet
- Reader Queries
- Safety+Security
- Banking & Insurance
- Corporate Tax
- Travel & Tourism
- Corporate News
- Electronics
- Home and Kitchen
- Consumables
- Saving and Investment
- Budget Living
- Expert Columns
- Community Tips
- Cryptocurrency
- Cooking and Cuisines
- Guide to Cooking
- Art & People
- Friday Partner
- Daily Crossword
- Word Search
- Philippines
- Australia-New Zealand
- Corrections
- Special Reports
- Pregnancy & Baby
- Learning & Play
- Child Health
- For Mums & Dads
- UAE Success Stories
- Premiere League
- Photos & Videos
- Course Reviews
- Learn to Play
- Schedule | Medal Tally
- South Indian
- Health+Fitness
- Best Of Bollywood
- Entertainment
- Special Features
- Gratuity Calculator
- Notifications
- Prayer Times
Gulf Medical University leads major collaboration with top Chinese universities
Zhejiang University PhD students take research internships at Gulf Medical University

A group of six female PhD students from the Zhejiang University School of Medicine (China) have successfully completed their research internships at the Gulf Medical University led Thumbay Research Institute for Precision Medicine in the UAE. The students' summer 2024 internship provided a unique opportunity to gain hands-on experience with state-of-the-art technological platforms, including Affymetrix, Next-Generation Sequencing (NGS), confocal microscopy, liquid biopsy techniques, and zebra fish facilities.
The program was instrumental in enriching their knowledge and practical skills in the field of translational cancer research, particularly in cancer immunobiology.
To honour the students' dedication and achievements, Gulf Medical University hosted a special event attended by GMU Chancellor, Professor Hossam Hamdy, and Professor Salem Chouaib, Director of the Thumbay Research Institute for Precision Medicine, along with other senior faculty members. During the event, the students were awarded certificates in recognition of their hard work and the promising contributions they are poised to make in the field of medical research.
This recognition reflects the valuable experience the students gained at the Thumbay Research Institute for Precision Medicine, which provided them with an environment that promotes innovation and collaboration. It further promoted them to explore different aspects of cancer research and clinical applications, and further develop the skills and knowledge essential for their future in medical research.
During their four-week internship, the students were not only trained in advanced research methodologies but were also provided with the opportunity to engage in patient clinicals at Thumbay University Hospital and Thumbay Physical Therapy and Rehabilitation Hospital. This comprehensive programme was tailored to cater to the diverse research interests of the students, giving them exposure to both the research and clinical aspects of medical science.
In his address, Professor Hossam Hamdy commended the students for their commitment to advancing their research capabilities and emphasized the importance of such international collaborations in strengthening global scientific progress.
“In their time with us, I have been impressed by the commitment of these students from Zhejiang University School of Medicine. Over the past month, they have not only advanced their research capabilities but have also shown a level of enthusiasm and hard work that serves as a model and inspiration for all of us at Gulf Medical University. The quality of research produced in such a short time is remarkable, and it demonstrates the collaborative spirit nurtured here at the university. Our faculty approached this collaboration with an open mind, adapting to the students' needs and aligning their objectives with our own capabilities. This flexible, student-centered approach allowed us to provide a supportive environment where the students could thrive. I'm confident that the experiences gained here—working with state-of-the-art technologies like Zebrafish, NGS, and confocal microscopy—will be invaluable in their future careers.”
Professor Hamdy added, “Beyond the lab, the students have had the opportunity to immerse themselves in a new culture and build friendships with people from different nationalities. This cultural exchange is just as important as the scientific knowledge they’ve acquired, and it lays the foundation for future collaborations between our institutions. I want to extend my gratitude to the management of Zhejiang University School of Medicine for entrusting us with their students. We are already looking forward to welcoming the next group and continuing this fruitful partnership.”
Also, Professor Salem Chouaib highlighted the role of Thumbay Research Institute of Precision Medicine in providing a robust platform for young researchers to develop and refine their skills. “We are delighted to welcome such a diverse and talented group of students to our team. Our goal at the institute is to provide a platform that not only broadens technical knowledge but also promoted innovative thinking and problem-solving skills. These are the tools that will empower to tackle the complex challenges of the future, and I am confident that they are well on their way to doing just that.”
Professor Chouaib further stated, “I am genuinely proud of what all have accomplished during this internship. Their hard work, dedication, and enthusiasm have truly shone through these weeks. I’ve heard wonderful things about their progress, and it’s clear that each of them has made significant strides in their research journey.”
The students, who were provided with accommodation at Gulf Medical University, expressed their gratitude for the opportunity to learn and grow in a supportive and intellectually stimulating environment. They shared their experiences with faculty, peers, and guests at the event, reflecting on how the internship had broadened their perspectives and deepened their understanding of translational research.
The successful completion of this programme is an important step in the students’ academic journey and highlights Gulf Medical University's support in the development of future researchers. Going forward, the university is poised to continue its partnership with Zhejiang University and other renowned institutions around the world to further medical research.
More From GN Focus
Revolutionary cartilage transplant saves 24-year-old

Oak brings wood-fired restaurant to Dubai

Burjeel Holdings awarded Most Honored Company

ASUS unveils Zenbook S14 and new Vivobook S15
Us debate fails to throw up clear winner, t20 cricket fuelling the rise of women’s sport, uae and cyprus presidents discuss strategic relations, follow these new tips to avoid ever-rising crypto scams, 6 firms fined sr91m over price fixing in saudi arabia.

Get Breaking News Alerts From Gulf News
We’ll send you latest news updates through the day. You can manage them any time by clicking on the notification icon.

APA Services pushed for new rule strengthening mental health care access
- Mental Health
- Managed Care and Insurance

The Biden administration finalized a new rule on September 9 designed to ensure that Americans with private health insurance can more easily access mental health services. This will help to rectify a stark disparity highlighted in a 2024 study showing that patients were 10.6 times more likely to go out of network for psychological care than for specialty medical care.
The new rule aims to hold insurance companies accountable for practices preventing patients from accessing mental health care as easily as medical care. It mandates that insurance companies assess whether networks include enough mental health providers to meet patient needs, and how that compares to network access for medical patients. This is crucial, as inadequate networks have left many mental health patients feeling like they are navigating “ghost” networks, where finding an in-network provider is nearly impossible. Most of the new requirements will roll out in 2026.
APA Services, the companion advocacy organization to APA, was a major proponent of this new rule and waged a campaign to encourage members to submit comments to the U.S. Department of Labor in support of the rule. In fact, more than 15% of the total number of all federal comments submitted were from psychologists. In addition, 28 of APA’s state, provincial, and territorial psychological associations submitted comments as well.
Katherine B. McGuire, MSc, APA Services chief advocacy officer, noted that the rule’s publication is the fruit of long years of advocacy led by APA Services staff in close coordination with other national behavioral health consumer and provider organizations. “We would simply not be where we are today without the years of diligent work that we and the mental health community devoted to sounding the alarm that stronger enforcement was needed.”
Key points of the new parity rule
- Psychologists’ advocacy: APA Services has long advocated for action to address the suffering caused by inadequate mental health care access. The new rule is seen as a major step toward resolving the mental health crisis.
- Regulatory tools: New tools for regulators include requiring insurers to gather and assess data on whether mental health patients have less access to care compared to medical patients. Insurers must take corrective steps to address significant disparities.
- Network adequacy: Agencies supporting parity have recognized that low pay for mental health providers is one major cause of inadequate insurance networks. The rule ensures that insurance networks take reasonable corrective actions if outcomes data shows that mental health patients have less access to care than medical patients.
The 2008 Mental Health Parity and Addiction Equity Act was a significant step towards ensuring that insurance providers offer equal coverage for mental and medical health services. However, as many providers know, the law’s promise has often fallen short, particularly in areas like insurance network adequacy. Patients frequently struggle to find mental health providers who accept their insurance, while providers struggle with administrative demands seemingly unrelated to clinical care and low reimbursements that have not kept pace with inflation or the rising costs of doing business.
Jared Skillings, PhD, ABPP, APA’s chief of professional practice, highlighted the ongoing challenges. “The American Psychological Association knows that many Americans have faced unnecessary suffering because they have been unable to get timely or adequate mental health care through their insurance. We have been hearing for years about the challenges that psychologists face, including bureaucratic hurdles and low reimbursement.” Skillings emphasized the urgency of addressing these issues. “Ignoring mental health costs lives. That’s why we have fought for this action and change. We are optimistic that the new Parity Rule will be a major step toward addressing the nation’s mental health crisis.”
Skillings pointed out that some insurance companies attribute the main cause of network inadequacy to a workforce shortage. However, APA and recent studies disagree, identifying low reimbursement rates as a significant factor. “There is a large pool of psychologists ready to work with insurance companies if treated fairly,” said Skillings.
Six Things Psychologists are Talking About
The APA Monitor on Psychology ® sister e-newsletter offers fresh articles on psychology trends, new research, and more.
Welcome! Thank you for subscribing.
Speaking of Psychology
Subscribe to APA’s audio podcast series highlighting some of the most important and relevant psychological research being conducted today.
Subscribe to Speaking of Psychology and download via:

Contact APA Office of Public Affairs
Related and recent.

IMAGES
VIDEO
COMMENTS
1. IQVIA. Formerly known as Quintiles and IMS Health, IQVIA is one of the largest CROs in the world, with a large range of service offerings to help advance clinical research. The company was founded in North Carolina in 1982, and has since grown to over 88,000 employees in more than 100 countries.
1. IQVIA. IQVIA is a global leader in clinical research and healthcare data analytics. They play a crucial role in the medical field by providing comprehensive data, advanced analytics, and expert insights. This helps pharmaceutical and healthcare companies make smarter, more effective decisions.
Founded: 1982 Headquarters: Waltham, Massachusetts Annual Revenue: $3B Employee Size: 18,000 Parexel is another important name in the list of Clinical Research Companies In USA that specializes on behalf of its pharmaceutical partners in carrying out clinical studies to boost and make certain that the medicine approval process takes place smoothly. It has a wide range of offerings that spreads ...
The United States of America has some of the top clinical research organizations in the world. Amongst them, here is the list of the 10 Best Clinical Research Organizations In The USA. 1. Covance. Source. Annual Revenue: $9.6B. Headquarters: Princeton, New Jersey. Number of Employees: 50,000+. Total Funding: $0.
IQVIA is a globally renowned medical research companies in USA that stands out for its exceptional contributions to the healthcare and pharmaceutical industries. As one of the leading providers of advanced analytics, technology solutions, and contract research services, IQVIA plays a pivotal role in shaping the future of therapeutic development
Top 28 Pharmaceutical Clinical Trials Companies. 1. Parexel. Parexel is a global company that offers services and solutions in the clinical research and healthcare industry. They provide expertise in various areas such as clinical trial management, regulatory outsourcing, medical monitoring, and consulting.
The company partners with pharmaceutical and biotechnology companies to research new drugs, medical devices, and diagnostics that could improve human health and wellbeing. Velocity's unified research site solutions deliver the right patients, investigators, and research staff for clinical trials across the U.S. and Europe.
Top 10 Leading Clinical Research Companies In The USA. The landscape of drug discovery and development is intricate, demanding significant resources at each stage of the process. As pharmaceutical companies strive to bring groundbreaking therapies to market, they increasingly rely on the expertise of Contract Research Organizations (CROs).
PWNHealth. According to reports, the nation's life sciences sector alone generates over $112 billion in revenue. In light of biotech's mounting economic impact, it's no surprise that tech hubs across the country are witnessing a surge in life sciences companies, bolstering the nation's status as a biotech epicenter.
Laboratory Corporation of America Holdings, also known as Labcorp, is an American company that operates one of the largest clinical laboratory networks in the world. In an average week Labcorp processes tests on more than 3 million patient specimens. In 2020 the company earned revenue in excess of $14 bn. Syneos Health.
Our Experience Reckner SM approach delivers a collaborative research process unparalleled in the industry. With Experience Reckner SM and Respondent First SM, your data quality is higher, your respondents more engaged and your insights more meaningful. Phone +1-215-822-6220. recknerhealth care.com.
Biotechnology companies such as Amgen, Gilead Sciences, and Moderna also play a vital role in sponsoring clinical trials. With a focus on innovative therapies and cutting-edge research, biotech companies contribute to advancing medical science. They often specialize in developing novel drugs and therapies, particularly in areas with unmet ...
Our Methodology. 20 fastest-growing biotech companies in the US. Merck & Co., Inc. (NYSE:MRK) $680 million. Baxter International Inc. (NYSE:BAX) prominent medtech company. Sanofi (NASDAQ:SNY ...
But it was much more. We focus on patients, because they inspire us to deliver So we can make a difference for more patients like Tina. For over 35 years Parexel has been a trusted global CRO and biopharmaceutical services company. Learn more about how we can help.
We're calling all aspiring world-class scientists who want to be a part of cutting-edge work to save and improve lives. At Merck, we follow the science. Learn about Merck's research and products by getting to know the science, strategy and brains behind the innovations.
Medical Research. Scientists and physicians in academic medicine conduct groundbreaking biomedical research that improves our knowledge of human health and promotes the development of treatments from bench to bedside to community. Through policy and advocacy initiatives, data and research projects, professional learning and networking ...
USA. 20 (168) Whitehead Institute for Biomedical Research. USA. 21 (172) Memorial Sloan-Kettering Cancer Center. USA. 22 (196) University of Texas M.D. Anderson Cancer Center.
List of largest biomedical companies by revenue. The following is a list of independent pharmaceutical, biotechnology and medical companies listed on a stock exchange (as indicated) that have generated a revenue of at least US$ 10 billion, ranked by their revenue in the respective financial year. It does not include biotechnology companies that ...
Here are the 27 largest biotech companies in the US: 27. Vertex Pharmaceuticals Incorporated (NASDAQ: This biotech company specializes in the development and production of small-molecule drugs to ...
Medical research in the United States remains the primary source of new discoveries, drugs, devices, and clinical procedures for the world, although the US lead in these categories is declining. For example, whereas the United States funded 57% of medical research in 2004, in 2011 that had declined to 44%. Basic research and product development ...
The top companies hiring now for medical research jobs in United States are Tandem Clinical Research, Forensic Psychology and Neuropsychology Service, P.C., NBR, Alliance for Multispecialty Research, LLC, Boys Town, NOVUM PHARMACEUTICAL RESEARCH SERVICES, NEXTSTAGE CLINICAL RESEARCH LLC, Gastro Florida, CenExel IResearch, Agendia Inc.
List of Pharmaceutical Companies and representative ofices of global pharmaceutical companies in United States. ... TIB Molbiol is a medium-sized biotech company who has supplied the global market for over 30 years with reagents for research and medical diagnostics. We are known for our innovation, product quality, design and time to market ...
Zhejiang University PhD students take research internships at Gulf Medical University Six female PhD students from Zhejiang University School of Medicine completed a research internship programme ...
This will help to rectify a stark disparity highlighted in a 2024 study showing that patients were 10.6 times more likely to go out of network for psychological care than for specialty medical care. The new rule aims to hold insurance companies accountable for practices preventing patients from accessing mental health care as easily as medical ...