
- Table of Contents
- Random Entry
- Chronological
- Editorial Information
- About the SEP
- Editorial Board
- How to Cite the SEP
- Special Characters
- Advanced Tools
- Support the SEP
- PDFs for SEP Friends
- Make a Donation
- SEPIA for Libraries
- Entry Contents

Bibliography
Academic tools.
- Friends PDF Preview
- Author and Citation Info
- Back to Top
Ethics of Stem Cell Research
Human embryonic stem cell (HESC) research offers much hope for alleviating the human suffering brought on by the ravages of disease and injury. HESCs are characterized by their capacity for self-renewal and their ability to differentiate into all types of cells of the body. The main goal of HESC research is to identify the mechanisms that govern cell differentiation and to turn HESCs into specific cell types that can be used for treating debilitating and life-threatening diseases and injuries.
Despite the tremendous therapeutic promise of HESC research, the research has met with heated opposition because the harvesting of HESCs involves the destruction of the human embryo. HESCs are derived in vitro around the fifth day of the embryo’s development (Thomson et al . 1998). A typical day-5 human embryo consists of 200–250 cells, most of which comprise the trophoblast, which is the outermost layer of the blastocyst. HESCs are harvested from the inner cell mass of the blastocyst, which consists of 30–34 cells. The derivation of HESC cultures requires the removal of the trophoblast. This process of disaggregating the blastocyst’s cells eliminates its potential for further development. Opponents of HESC research argue that the research is morally impermissible because it involves the unjust killing of innocent human beings.
Scientists recently succeeded in converting adult human skin cells into cells that appear to have the properties of HESCs by activating four genes in the adult cells (Takahashi et al . 2007; Yu et al . 2007). The reprogrammed cells—“induced pluripotent stem cells” (iPSCs)—could ultimately eliminate the need for HESCs. However, at present, the consensus in the scientific community is that both HESC and iPSC research should be pursued, as we do not yet know whether iPSCs have the same potential as HESCs or whether it is safe to transplant them into humans. Thus, the controversies around HESC research will continue, at least in the near-term.
While the principal source of the controversy surrounding HESC research lies in competing views about the value of human embryonic life, the scope of ethical issues in HESC research is broader than the question of the ethics of destroying human embryos. It also encompasses questions about, among other things, whether researchers who use but do not derive HESCs are complicit in the destruction of embryos, whether there is a moral distinction between creating embryos for research purposes and creating them for reproductive ends, the permissibility of cloning human embryos to harvest HESCs, and the ethics of creating human/non-human chimeras. This entry provides an overview of all but the last two issues just listed; cloning and human-non-human chimeras are addressed in separate entries.
1.1 When does a human being begin to exist?
1.2 the moral status of human embryos, 1.3 the case of “doomed embryos”, 2. the ethics of using human embryonic stem cells in research, 3. the ethics of creating embryos for stem cell research and therapy, 4. stem cell-derived gametes, 5. stem cell-derived organoids, gastruloids, and synthetic embryos, cited resources, other resources, related entries, 1. the ethics of destroying human embryos for research.
The potential therapeutic benefits of HESC research provide strong grounds in favor of the research. If looked at from a strictly consequentialist perspective, it’s almost certainly the case that the potential health benefits from the research outweigh the loss of embryos involved and whatever suffering results from that loss for persons who want to protect embryos. However, most of those who oppose the research argue that the constraints against killing innocent persons to promote social utility apply to human embryos. Thus, as long as we accept non-consequentialist constraints on killing persons, those supporting HESC research must respond to the claim that those constraints apply to human embryos.
In its most basic form, the central argument supporting the claim that it is unethical to destroy human embryos goes as follows: It is morally impermissible to intentionally kill innocent human beings; the human embryo is an innocent human being; therefore it is morally impermissible to intentionally kill the human embryo. It is worth noting that this argument, if sound, would not suffice to show that all or even most HESC research is impermissible, since most investigators engaged in HESC research do not participate in the derivation of HESCs but instead use cell lines that researchers who performed the derivation have made available. To show that researchers who use but do not derive HESCs participate in an immoral activity, one would further need to establish their complicity in the destruction of embryos. We will consider this issue in section 2. But for the moment, let us address the argument that it is unethical to destroy human embryos.
A premise of the argument against killing embryos is that human embryos are human beings. The issue of when a human being begins to exist is, however, a contested one. The standard view of those who oppose HESC research is that a human being begins to exist with the emergence of the one-cell zygote at fertilization. At this stage, human embryos are said to be “whole living member[s] of the species homo sapiens … [which] possess the epigenetic primordia for self-directed growth into adulthood, with their determinateness and identity fully intact” (George & Gomez-Lobo 2002, 258). This view is sometimes challenged on the grounds that monozygotic twinning is possible until around days 14–15 of an embryo’s development (Smith & Brogaard 2003). An individual who is an identical twin cannot be numerically identical to the one-cell zygote, since both twins bear the same relationship to the zygote, and numerical identity must satisfy transitivity. That is, if the zygote, A, divides into two genetically identical cell groups that give rise to identical twins B and C, B and C cannot be the same individual as A because they are not numerically identical with each other. This shows that not all persons can correctly assert that they began their life as a zygote. However, it does not follow that the zygote is not a human being, or that it has not individuated. This would follow only if one held that a condition of an entity’s status as an individual human being is that it be impossible for it to cease to exist by dividing into two or more entities. But this seems implausible. Consider cases in which we imagine adult humans undergoing fission (for example, along the lines of Parfit’s thought experiments, where each half of the brain is implanted into a different body) (Parfit 1984). The prospect of our going out of existence through fission does not pose a threat to our current status as distinct human persons. Likewise, one might argue, the fact that a zygote may divide does not create problems for the view that the zygote is a distinct human being.
There are, however, other grounds on which some have sought to reject that the early human embryo is a human being. According to one view, the cells that comprise the early embryo are a bundle of homogeneous cells that exist in the same membrane but do not form a human organism because the cells do not function in a coordinated way to regulate and preserve a single life (Smith & Brogaard 2003, McMahan 2002). While each of the cells is alive, they only become parts of a human organism when there is substantial cell differentiation and coordination, which occurs around day-16 after fertilization. Thus, on this account, disaggregating the cells of the 5-day embryo to derive HESCs does not entail the destruction of a human being.
This account is subject to dispute on empirical grounds. That there is some intercellular coordination in the zygote is revealed by the fact that the development of the early embryo requires that some cells become part of the trophoblast while others become part of the inner cell mass. Without some coordination between the cells, there would be nothing to prevent all cells from differentiating in the same direction (Damschen, Gomez-Lobo and Schonecker 2006). The question remains, though, whether this degree of cellular interaction is sufficient to render the early human embryo a human being. Just how much intercellular coordination must exist for a group of cells to constitute a human organism cannot be resolved by scientific facts about the embryo, but is instead an open metaphysical question (McMahan 2007a).
Suppose that the 5-day human embryo is a human being. On the standard argument against HESC research, membership in the species Homo sapiens confers on the embryo a right not to be killed. This view is grounded in the assumption that human beings have the same moral status (at least with respect to possessing this right) at all stages of their lives.
Some accept that the human embryo is a human being but argue that the human embryo does not have the moral status requisite for a right to life. There is reason to think that species membership is not the property that determines a being’s moral status. We have all been presented with the relevant thought experiments, courtesy of Disney, Orwell, Kafka, and countless science fiction works. The results seem clear: we regard mice, pigs, insects, aliens, and so on, as having the moral status of persons in those possible worlds in which they exhibit the psychological and cognitive traits that we normally associate with mature human beings. This suggests that it is some higher-order mental capacity (or capacities) that grounds the right to life. While there is no consensus about the capacities that are necessary for the right to life, some of the capacities that have been proposed include reasoning, self-awareness, and agency (Kuhse & Singer 1992, Tooley 1983, Warren 1973).
The main difficulty for those who appeal to such mental capacities as the touchstone for the right to life is that early human infants lack these capacities, and do so to a greater degree than many of the nonhuman animals that most deem it acceptable to kill (Marquis 2002). This presents a challenge for those who hold that the non-consequentialist constraints on killing human children and adults apply to early human infants. Some reject that these constraints apply to infants, and allow that there may be circumstances where it is permissible to sacrifice infants for the greater good (McMahan 2007b). Others argue that, while infants do not have the intrinsic properties that ground a right to life, we should nonetheless treat them as if they have a right to life in order to promote love and concern towards them, as these attitudes have good consequences for the persons they will become (Benn 1973, Strong 1997).
Some claim that we can reconcile the ascription of a right to life to all humans with the view that higher order mental capacities ground the right to life by distinguishing between two senses of mental capacities: “immediately exercisable” capacities and “basic natural” capacities. (George and Gomez-Lobo 2002, 260). According to this view, an individual’s immediately exercisable capacity for higher mental functions is the actualization of natural capacities for higher mental functions that exist at the embryonic stage of life. Human embryos have a “rational nature,” but that nature is not fully realized until individuals are able to exercise their capacity to reason. The difference between these types of capacity is said to be a difference between degrees of development along a continuum. There is merely a quantitative difference between the mental capacities of embryos, fetuses, infants, children, and adults (as well as among infants, children, and adults). And this difference, so the argument runs, cannot justify treating some of these individuals with moral respect while denying it to others.
Given that a human embryo cannot reason at all, the claim that it has a rational nature has struck some as tantamount to asserting that it has the potential to become an individual that can engage in reasoning (Sagan & Singer 2007). But an entity’s having this potential does not logically entail that it has the same status as beings that have realized some or all of their potential (Feinberg 1986). Moreover, with the advent of cloning technologies, the range of entities that we can now identify as potential persons arguably creates problems for those who place great moral weight on the embryo’s potential. A single somatic cell or HESC can in principle (though not yet in practice) develop into a mature human being under the right conditions—that is, where the cell’s nucleus is transferred into an enucleated egg, the new egg is electrically stimulated to create an embryo, and the embryo is transferred to a woman’s uterus and brought to term. If the basis for protecting embryos is that they have the potential to become reasoning beings, then, some argue, we have reason to ascribe a high moral status to the trillions of cells that share this potential and to assist as many of these cells as we reasonably can to realize their potential (Sagan & Singer 2007, Savulescu 1999). Because this is a stance that we can expect nearly everyone to reject, it’s not clear that opponents of HESC research can effectively ground their position in the human embryo’s potential.
One response to this line of argument has been to claim that embryos possess a kind of potential that somatic cells and HESCs lack. An embryo has potential in the sense of having an “active disposition” and “intrinsic power” to develop into a mature human being (Lee & George 2006). An embryo can mature on its own in the absence of interference with its development. A somatic cell, on the other hand, does not have the inherent capacity or disposition to grow into a mature human being. However, some question whether this distinction is viable, especially in the HESC research context. While it is true that somatic cells can realize their potential only with the assistance of outside interventions, an embryo’s development also requires that numerous conditions external to it are satisfied. In the case of embryos that are naturally conceived, they must implant, receive nourishment, and avoid exposure to dangerous substances in utero . In the case of spare embryos created through in vitro fertilization—which are presently the source of HESCs for research—the embryos must be thawed and transferred to a willing woman’s uterus. Given the role that external factors—including technological interventions—play in an embryo’s realizing its potential, one can question whether there is a morally relevant distinction between an embryo’s and somatic cell’s potential and thus raise doubts about potentiality as a foundation for the right to life (Devolder & Harris 2007).
Some grant that human embryos lack the properties essential to a right to life, but hold that they possess an intrinsic value that calls for a measure of respect and places at least some moral constraints on their use: “The life of a single human organism commands respect and protection … no matter in what form or shape, because of the complex creative investment it represents and because of our wonder at the divine or evolutionary processes that produce new lives from old ones.” (Dworkin l992, 84). There are, however, divergent views about the level of respect embryos command and what limits exist on their use. Some opponents of HESC research hold that the treatment of human embryos as mere research tools always fails to manifest proper respect for them. Other opponents take a less absolutist view. Some, for example, deem embryos less valuable than more mature human beings but argue that the benefits of HESC research are too speculative to warrant the destruction of embryos, and that the benefits might, in any case, be achieved through the use of noncontroversial sources of stem cells (e.g., adult stem cells) (Holm 2003).
Many, if not most, who support the use of human embryos for HESC research would likely agree with opponents of the research that there are some circumstances where the use of human embryos would display a lack of appropriate respect for human life, for example, were they to be offered for consumption to contestants in a reality TV competition or destroyed for the production of cosmetics. But proponents of the research hold that the value of human embryos is not great enough to constrain the pursuit of research that may yield significant therapeutic benefits. Supporters of the research also frequently question whether most opponents of the research are consistent in their ascription of a high value to human embryos, as opponents generally display little concern about the fact that many embryos created for fertility treatment are discarded.
When spare embryos exist after fertility treatment, the individuals for whom the embryos were created typically have the option of storing for them for future reproductive use, donating them to other infertile couples, donating them to research, or discarding them. Some argue that as long as the decision to donate embryos for research is made after the decision to discard them, it is morally permissible to use them in HESC research even if we assume that they have the moral status of persons. The claim takes two different forms. One is that it is morally permissible to kill an individual who is about to be killed by someone else where killing that individual will help others (Curzer, H. 2004). The other is that researchers who derive HESCs from embryos that were slated for destruction do not cause their death. Instead, the decision to discard the embryos causes their death; research just causes the manner of their death (Green 2002).
Both versions of the argument presume that the decision to discard spare embryos prior to the decision to donate them to research entails that donated embryos are doomed to destruction when researchers receive them. There are two arguments one might marshal against this presumption. First, one who wants to donate embryos to research might first elect to discard them only because doing so is a precondition for donating them. There could be cases in which one who chooses the discard option would have donated the embryos to other couples were the research donation option not available. The fact that a decision to discard embryos is made prior to the decision to donate the embryos thus does not establish that the embryos were doomed to destruction before the decision to donate them to research was made. Second, a researcher who receives embryos could choose to rescue them, whether by continuing to store them or by donating them to infertile couples. While this would violate the law, the fact that it is within a researcher’s power to prevent the destruction of the embryos he or she receives poses problems for the claim that the decision to discard the embryos dooms them or causes their destruction.
Assume for the sake of argument that it is morally impermissible to destroy human embryos. It does not follow that all research with HESCs is impermissible, as it is sometimes permissible to benefit from moral wrongs. For example, there is nothing objectionable about transplant surgeons and patients benefiting from the organs of murder and drunken driving victims (Robertson 1988). If there are conditions under which a researcher may use HESCs without being complicit in the destruction of embryos, then those who oppose the destruction of embryos could support research with HESCs under certain circumstances.
Researchers using HESCs are clearly implicated in the destruction of embryos where they derive the cells themselves or enlist others to derive the cells. However, most investigators who conduct research with HESCs obtain them from an existing pool of cell lines and play no role in their derivation. One view is that we cannot assign causal or moral responsibility to investigators for the destruction of embryos from which the HESCs they use are derived where their “research plans had no effect on whether the original immoral derivation occurred.” (Robertson 1999). This view requires qualification. There may be cases in which HESCs are derived for the express purpose of making them widely available to HESC investigators. In such instances, it may be that no individual researcher’s plans motivated the derivation of the cells. Nonetheless, one might argue that investigators who use these cells are complicit in the destruction of the embryos from which the cells were derived because they are participants in a research enterprise that creates a demand for HESCs. For these investigators to avoid the charge of complicity in the destruction of embryos, it must be the case that the researchers who derived the HESCs would have performed the derivation in the absence of external demand for the cells (Siegel 2004).
The issue about complicity goes beyond the question of an HESC researcher’s role in the destruction of the particular human embryo(s) from which the cells he or she uses are derived. There is a further concern that research with existing HESCs will result in the future destruction of embryos: “[I]f this research leads to possible treatments, private investment in such efforts will increase greatly and the demand for many thousands of cell lines with different genetic profiles will be difficult to resist.” (U.S. Conference of Catholic Bishops 2001). This objection faces two difficulties. First, it appears to be too sweeping: research with adult stem cells and non-human animal stem cells, as well as general research in genetics, embryology, and cell biology could be implicated, since all of this research might advance our understanding of HESCs and result in increased demand for them. Yet, no one, including those who oppose HESC research, argues that we should not support these areas of research. Second, the claim about future demand for HESCs is speculative. Indeed, current HESC research could ultimately reduce or eliminate demand for the cells by providing insights into cell biology that enable the use of alternative sources of cells (Siegel 2004).
While it might thus be possible for a researcher to use HESCs without being morally responsible for the destruction of human embryos, that does not end the inquiry into complicity. Some argue that agents can be complicit in wrongful acts for which they are not morally responsible. One such form of complicity arises from an association with wrongdoing that symbolizes acquiescence in the wrongdoing (Burtchaell 1989). The failure to take appropriate measures to distance oneself from moral wrongs may give rise to “metaphysical guilt,” which produces a moral taint and for which shame is the appropriate response (May 1992). The following question thus arises: Assuming it is morally wrongful to destroy human embryos, are HESC researchers who are not morally responsible for the destruction of embryos complicit in the sense of symbolically aligning themselves with a wrongful act?
One response is that a researcher who benefits from the destruction of embryos need not sanction the act any more than the transplant surgeon who uses the organs of a murder or drunken driving victim sanctions the homicidal act (Curzer 2004). But this response is unlikely to be satisfactory to opponents of HESC research. There is arguably an important difference between the transplant case and HESC research insofar as the moral wrong associated with the latter (a) systematically devalues a particular class of human beings and (b) is largely socially accepted and legally permitted. Opponents of HESC research might suggest that the HESC research case is more analogous to the following kind of case: Imagine a society in which the practice of killing members of a particular racial or ethnic group is legally permitted and generally accepted. Suppose that biological materials obtained from these individuals subsequent to their deaths are made available for research uses. Could researchers use these materials while appropriately distancing themselves from the wrongful practice? Arguably, they could not. There is a heightened need to protest moral wrongs where those wrongs are socially and legally accepted. Attempts to benefit from the moral wrong in these circumstances may be incompatible with mounting a proper protest (Siegel 2003).
But even if we assume that HESC researchers cannot avoid the taint of metaphysical guilt, it is not clear that researchers who bear no moral responsibility for the destruction of embryos are morally obligated not to use HESCs. One might argue that there is a prima facie duty to avoid moral taint, but that this duty may be overridden for the sake of a noble cause.
Most HESCs are derived from embryos that were created for infertility treatment but that were in excess of what the infertile individual(s) ultimately needed to achieve a pregnancy. The HESCs derived from these leftover embryos offer investigators a powerful tool for understanding the mechanisms controlling cell differentiation. However, there are scientific and therapeutic reasons not to rely entirely on leftover embryos. From a research standpoint, creating embryos through cloning technologies with cells that are known to have particular genetic mutations would allow researchers to study the underpinnings of genetic diseases in vitro . From a therapeutic standpoint, the HESCs obtained from leftover IVF embryos are not genetically diverse enough to address the problem of immune rejection by recipients of stem cell transplants. (Induced pluripotent stem cells may ultimately prove sufficient for these research and therapeutic ends, since the cells can (a) be selected for specific genetic mutations and (b) provide an exact genetic match for stem cell recipients.) At present, the best way to address the therapeutic problem is through the creation of a public stem cell bank that represents a genetically diverse pool of stem cell lines (Faden et al . 2003, Lott & Savulescu 2007). This kind of stem cell bank would require the creation of embryos from gamete donors who share the same HLA-types (i.e., similar versions of the genes that mediate immune recognition and rejection).
Each of these enterprises has its own set of ethical issues. In the case of research cloning, some raise concerns, for example, that the perfection of cloning techniques for research purposes will enable the pursuit of reproductive cloning, and that efforts to obtain the thousands of eggs required for the production of cloned embryos will result in the exploitation of women who provide the eggs (President’s Council on Bioethics 2002, Norsigian 2005). With respect to stem cell banks, it is not practically possible to create a bank of HESCs that will provide a close immunological match for all recipients. This gives rise to the challenge of determining who will have biological access to stem cell therapies. We might construct the bank so that it provides matches for the greatest number of people in the population, gives everyone an equal chance of finding a match, or ensures that all ancestral/ethnic groups are fairly represented in the bank (Faden et al . 2003, Bok, Schill, & Faden 2004, Greene 2006).
There are, however, more general challenges to the creation of embryos for research and therapeutic purposes. Some argue that the creation of embryos for non-reproductive ends is morally problematic, regardless of whether they are created through cloning or in vitro fertilization. There are two related arguments that have been advanced to morally distinguish the creation of embryos for reproductive purposes from the creation of embryos for research and therapeutic purposes. First, each embryo created for procreative purposes is originally viewed as a potential child in the sense that each is a candidate for implantation and development into a mature human. In contrast, embryos created for research or therapies are viewed as mere tools from the outset (Annas, Caplan & Elias 1996, President’s Council on Bioethics 2002). Second, while embryos created for research and therapy are produced with the intent to destroy them, the destruction of embryos created for reproduction is a foreseeable but unintended consequence of their creation (FitzPatrick 2003).
One response to the first argument has been to suggest that we could, under certain conditions, view all research embryos as potential children in the relevant sense. If all research embryos were included in a lottery in which some of them were donated to individuals for reproductive purposes, all research embryos would have a chance at developing into mature humans (Devander 2005). Since those who oppose creating embryos for research would likely maintain their opposition in the research embryo lottery case, it is arguably irrelevant whether embryos are viewed as potential children when they are created. Of course, research embryos in the lottery case would be viewed as both potential children and potential research tools. But this is also true in the case of embryos created for reproductive purposes where patients are open to donating spare embryos to research.
As to the second argument, the distinction between intending and merely foreseeing harms is one to which many people attach moral significance, and it is central to the Doctrine of Double Effect. But even if one holds that this is a morally significant distinction, it is not clear that it is felicitous to characterize the destruction of spare embryos as an unintended but foreseeable side-effect of creating embryos for fertility treatment. Fertility clinics do not merely foresee that some embryos will be destroyed, as they choose to offer patients the option of discarding embryos and carry out the disposal of embryos when patients request it. Patients who elect that their embryos be discarded also do not merely foresee the embryos’ destruction; their election of that option manifests their intention that the embryos be destroyed. There is thus reason to doubt that there is a moral distinction between creating embryos for research and creating them for reproductive purposes, at least given current fertility clinic practices.
Recent scientific work suggests it is possible to derive gametes from human pluripotent stem cells. Researchers have generated sperm and eggs from mouse ESCs and iPSCs and have used these stem cell-derived gametes to produce offspring (Hayashi 2011; Hayashi 2012). While it may take several years before researchers succeed in deriving gametes from human stem cells, the research holds much promise for basic science and clinical application. For example, the research could provide important insights into the fundamental processes of gamete biology, assist in the understanding of genetic disorders, and provide otherwise infertile individuals a means of creating genetically related children. The ability to derive gametes from human stem cells could also reduce or eliminate the need for egg donors and thus help overcome concerns about exploitation of donors and the risks involved in egg retrieval. Nonetheless, the research gives rise to some controversial issues related to embryos, genetics, and assisted reproductive technologies (D. Mathews et al . 2009).
One issue arises from the fact that some research on stem cell-derived gametes requires the creation of embryos, regardless of whether one is using ESCs or iPSCs. To establish that a particular technique for deriving human gametes from stem cells produces functional sperm and eggs, it is necessary to demonstrate that the cells can produce an embryo. This entails the creation of embryos through in vitro fertilization. Since it would not be safe to implant embryos created during the early stages of the research, the likely disposition of the embryos is that they would be destroyed. In such instances, the research would implicate all of the moral issues surrounding the creation and destruction of embryos for research. However, the creation of embryos for research in this situation would not necessitate the destruction of the embryos, as it does when embryos are created to derive stem cell lines. One could in principle store them indefinitely rather than destroy them. This would still leave one subject to the objection that life is being created for instrumental purposes. But the force of the objection is questionable since it is not clear that this instrumental use is any more objectionable than the routine and widely accepted practice of creating excess IVF embryos in the reproductive context to increase the probability of generating a sufficient number of viable ones to produce a pregnancy.
Further issues emerge with the prospect of being able to produce large quantities of eggs from stem cells. As the capacity to identify disease and non-disease related alleles through preimplantation genetic diagnosis (PGD) expands, the ability to create large numbers of embryos would substantially increase the chances of finding an embryo that possesses most or all of the traits one wishes to select. This would be beneficial in preventing the birth of children with genetic diseases. But matters would become morally contentious if it were possible to select for non-disease characteristics, such as sexual orientation, height, superior intelligence, memory, and musical ability. One common argument against using PGD in this way is that it could devalue the lives of those who do not exhibit the chosen characteristics. Another concern is that employing PGD to select for non-disease traits would fail to acknowledge the “giftedness of life” by treating children as “objects of our design or products of our will or instruments of our ambition” rather accepting them as they are given to us (Sandel 2004, 56). There is additionally a concern about advances in genetics heightening inequalities where certain traits confer social and economic advantages and only the well-off have the resources to access the technology (Buchanan 1995). Of course, one can question whether the selection of non-disease traits would in fact lead to devaluing other characteristics, whether it would alter the nature of parental love, or whether it is distinct enough from currently permitted methods of gaining social and economic advantage to justify regulating the practice. Nonetheless, the capacity to produce human stem cell-derived gametes would make these issues more pressing.
There have been a number of recent scientific studies in which stem cells have, under certain in vitro culture conditions, self-organized into three-dimensional structures that resemble and recapitulate some of the functions of human organs (Lancaster & Knoblich 2014; Clevers 2016). These “organoids” have been established with human stem cells for a variety of organs, including, among others, the kidney, liver, gut, pancreas, retina, and brain. In addition to organoids, stem cells have been shown to self-organize into embryo-like structures in vitro . Human embryonic stem cells have formed structures – referred to as “gastruloids” – that bear some resemblance to embryos during gastrulation, which is the stage several days after implantation where the body plan and some tissues tissue types, including the central nervous system, start to develop (Warmflash et al. 2014; Deglincerti et al . 2016; Shahbazi 2016). Researchers have also combined mouse embryonic stem cells and trophoblast stem cells to create “synthetic embryos,” which have a structure akin to pre-implantation embryos (Rivron et al . 2018). Synthetic embryos have been shown to implant into the mouse uterus, though their potential to develop to term has not been demonstrated.
While these scientific advances offer promising avenues for better understanding human development and disease, they also raise some novel and challenging ethical issues. In the case of organoids, cerebral organoids raise the most vexing issues. Researchers have produced cerebral organoids with a degree of development similar to that of a few-months-old embryo, and have already used them to study how the Zika virus causes microcephaly in fetuses (Garcez et al . 2016). At present, there is some evidence that cerebral organoids may be able to receive afferent stimulations that produce simple sensations (Quadrato et al . 2017). However, they currently lack the kind of mature neural networks and sensory inputs and outputs essential to the development of cognition. If, through bioengineering, human cerebral organoids were to develop the capacity for cognition, that would provide grounds for ascribing an elevated moral status to them, and it would raise concomitant issues about our moral obligations towards them. In the nearer term, it is more likely that cerebral organoids will develop some degree of consciousness Assuming we have a shared understanding of consciousness (e.g., phenomenal consciousness), one challenge is to identify means of measuring the presence of consciousness, since a cerebral organoid cannot communicate its internal states (Lavazza & Massimini 2018). But even if we can verify that an organoid is conscious, there remains the question of the moral significance of consciousness (Shepherd 2018). There is debate over whether consciousness has intrinsic value (Lee 2018), and whether in some cases it is better for a conscious being to not possess it (Kahane & Savulescu 2009). Those who reject the intrinsic value and moral significance of consciousness might find the case of a conscious entity that has led a solely disembodied existence, emerges and persists in the absence of any social or cultural nexus, and lacks beliefs and desires, to be a paradigmatic case where the value of consciousness is doubtful.
With respect to gastruloids and synthetic embryos (if the latter are successfully produced with human stem cells), the central question is whether these entities are sufficiently like human embryos in their structure and functions to give rise to moral concerns about their use in research. Gastruloids do not possess all the characteristics of an embryo, as they do not form all of the embryonic tissues (e.g., they do not have the trophectoderm, which mediates the attachment to the uterus). At the same time, gastruloids may, with extra-embryonic tissues, achieve a developmental stage in which they manifest a whole body plan. Recall that one argument (discussed in Section 1.1 above) for rejecting that human embryos are human beings is that the cells that comprise the early embryo do not function in a coordinated way to regulate and preserve a single organism. Gastruloids can in principle operate with this higher level of coordination. While one may still reject that this characteristic of gastruloids confers human rights on them, their more advanced stage of development might ground reasonable claims for according them greater respect than embryos at an earlier stage. In the case of both gastruloids and human synthetic embryos, the possibility that they ultimately lack the potential to develop into mature human beings may be of significance in morally distinguishing them from normal human embryos. As noted previously (in section 1.2 above), one argument for ascribing a high moral status to human embryos and for distinguishing the potential of human embryos from the potential of somatic cells and embryonic stem cells is that embryos have an “active disposition” and “intrinsic power” to develop into mature humans on their own. If synthetic embryos and gastruloids do not possess this disposition and power, then those who oppose some forms of human embryo research might not object to the creation and use of human gastruloids and synthetic embryos for research.
- Annas, G., Caplan, A., and Elias, S., 1996, “The Politics of Human-Embryo Research—Avoiding Ethical Gridlock,” New England Journal of Medicine , 334: 1329–32.
- Benn, S.I., 1973, “Abortion, Infanticide, and Respect for Persons,” in The Problem of Abortion , Joel Feinberg (ed.), Belmont, CA: Wadsworth: 92–103.
- Bok H., Schill K.E., and Faden R.R., 2004, “Justice, Ethnicity, and Stem-Cell Banks,” Lancet , 364(9429): 118–21.
- Buchanan, A., 1995, “Equal Opportunity and Genetic Intervention,” Social Philosophy and Policy , 12(2): 105–35.
- Burtchaell, J.T., 1989, “The Use of Aborted Fetal Tissue in Research: A Rebuttal,” IRB: A Review of Human Subjects Research , 11(2): 9–12.
- Curzer, H., 2004, “The Ethics of Embryonic Stem Cell Research,” Journal of Medicine and Philosophy , 29(5): 533–562.
- Damschen, G., Gomez-Lobo, A., and Schonecker, D., 2006, “Sixteen Days? A reply to B. Smith and B. Brogaard on the Beginning of Human Individuals,” Journal of Medicine and Philosophy , 31: 165–175.
- Clevers, H., 2016, “Modeling Development and Disease with Organoids,” Cell , 165: 1586–1597.
- Deglincerti, A., et al ., 2016, “Self-Organization of the Attached In Vitro Human Embryo,” Nature , 533: 251–54.
- Devolder, K., 2005, “Human Embryonic Stem Cell Research: Why the Discarded-Created Distinction Cannot Be Based on the Potentiality Argument,” Bioethics , 19(2): 167–86.
- Devolder, K., and Harris, J., 2007, “The Ambiguity of the Embryo: Ethical Inconsistency in the Human Embryonic Stem Cell Debate,” Metaphilosophy , 38(2–3): 153–169.
- Dworkin, R., 1992, Life’s Dominion , New York: Vintage.
- Faden, R.R., et al ., 2003, Public Stem Cell Banks: Considerations of Justice in Stem Cell Therapy, Hastings Center Report , 33: 13–27.
- Feinberg, J., 1986, “Abortion,” in Matters of Life and Death , T. Regan (ed.), New York: Random House.
- FitzPatrick, W., 2003, “Surplus Embryos, Nonreproductive Cloning, and the Intend/Foresee Distinction,” Hastings Center Report , 33: 29–36.
- Garzes, P.P., et al ., 2016, “Zika Virus Impairs Growth in Human Neurospheres and Brain Organoids,” Science , 352: 816–18.
- George, R.P., and Gomez-Lobo, A., 2002, “Statement of Professor George (Joined by Dr. Gomez-Lobo),” in Human Cloning and Human Dignity: An Ethical Inquiry , report by the President’s Council on Bioethics: 258–266.
- Green, R., 2002, “Benefiting from ‘Evil’; An Incipient Moral Problem in Human Stem Cell Research,” Bioethics , 16(6): 544–556.
- Greene, M., 2006, “To Restore Faith and Trust: Justice and Biological Access to Cellular Therapies,” Hastings Center Report , 36(1): 57–63.
- Hayashi, K., et al ., 2011, “Reconstitution of the Mouse Germ Cell Specification Pathway in Culture by Pluripotent Stem Cells,” Cell , 146(4): 519–532.
- Hayashi, K., et al ., 2012, “Offspring from Oocytes Derived from In Vitro Primordial Germ Cell-Like Cells in Mice,” Science , 338(6109): 971–975.
- Holm, S., 2003, “The Ethical Case Against Stem Cell Research,” Cambridge Quarterly of Healthcare Ethics , 12: 372–83.
- Kahane, G. and Savulescu, J., 2009, “Brain Damage and the Moral Significance of Consciousness,” Journal of Medicine and Philosophy , 34: 6–26.
- Kuhse, H., and Singer, P., 1992, “Individuals, Human, and Persons: The Issue of Moral Status,” in Embryo Experimentation: Ethical, Legal, and Social Issues , P. Singer, et al. (eds.), Cambridge: Cambridge University Press, 65–75
- Lancaster, M.A., and Knoblich, J.A., 2014, “Organogenesis in a Dish: Modeling Development and Disease Using Organoid Technologies,” Science , 345(6194): 1247125.
- Lavazza, A. and Massimini, M., 2018, “Cerebral Organoids: Ethical Issues and Consciousness Assessment,” Journal of Medical Ethics , 44: 606–10.
- Lee, A.Y., 2018, “Is Consciousness Intrinsically Valuable,” Philosophical Studies , 20: 1–17.
- Lee, P., and George R., 2006, “Human-Embryo Liberation: A Reply to Peter Singer,” National Review Online (25 January). [ Available online ]
- Lott, J.P., and Savulescu, J., 2007, “Towards a Global Human Embryonic Stem Cell Bank,” American Journal of Bioethics , 7(8): 37–44.
- Marquis, D., 2002, “Stem Cell Research: The Failure of Bioethics,” Free Inquiry , 23(1): 40–44.
- Mathews, D., et al ., 2009, “Pluripotent Stem Cell-Derived Gametes: Truth and (Potential) Consequences,” Cell: Stem Cell , 5: 11–14.
- May, L., 1992, Sharing Responsibility , Chicago: University of Chicago Press.
- McMahan, J., 2002, The Ethics of Killing: Problems at the Margins of Life , New York: Oxford University Press.
- McMahan, J., 2007a, “Killing Embryos for Stem Cell Research,” Metaphilosophy , 38(2–3): 170–189.
- –––, 2007b, “Infanticide,” Utilitas , 19: 131–159.
- Norsigian, J., 2005, “Risks to Women in Embryo Cloning,” Boston Globe , February 25.
- Parfit, D., 1984, Reasons and Persons , Oxford: Clarendon Press.
- Quadrato, G., et al ., 2017, “Cell Diversity and Network Dynamics in Photosensitive Human Brain Organoids,” Nature , 545: 48–53.
- Rivron, N.C., et al ., 2018, “Blastocyst-Like Structures Generated Solely from Stem Cells,” Nature , 557: 106–11.
- Robertson, J., 1988, “Fetal Tissue Transplant Research is Ethical,” IRB: A Review of Human Subjects Research , 6(10): 5–8.
- –––, 1999, “Ethics and Policy In Embryonic Stem Cell Research,” Kennedy Institute of Ethics Journal , 2(9): 109–36.
- Sagan, A., and Singer, P., 2007, “The Moral Status of Stem Cells,” Metaphilosophy , 38(2–3): 264–284
- Sandel, M., 2004, “The Case Against Human Perfection,” Atlantic Monthly , 293(3): 51–62.
- Savulescu, J., 1999, “Should We Clone Human Beings?” Journal of Medical Ethics , 25(2): 87–98.
- Shahbazi, M.N., et al ., 2016, “Self-Organization of the Human Embryo in the Absence of Maternal Tissues,” Nature Cell Biology , 18: 700–08.
- Shepherd, J., 2018, “Ethical (and Epistemological) Issues Regarding Consciousness in Cerebral Organoids,” Journal of Medical Ethics , 44: 611–12.
- Smith, B., and Brogaard, B., 2003, “Sixteen Days,” Journal of Medicine and Philosophy , 28: 45–78.
- Siegel, A., 2003, “Locating Convergence: Ethics, Public Policy, and Human Stem Cell Research,” in The Stem Cell Controversy , M. Ruse and C. Pynes (eds.), Amherst, NY: Prometheus Books.
- –––, 2004, “Temporal Restrictions and the Impasse on Human Embryonic Stem Cell Research,” The Lancet , 364(9429): 215–18.
- Strong, C., 1997, “The Moral Status of Preembryos, Embryos, Fetuses, and Infants,” Journal of Medicine and Philosophy , 22(5): 457–78
- Takahashi, K., et al ., 2007, “Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors,” Cell , 131: 861–872.
- Thomson, J.A., et al ., 1998, “Embryonic Stem Cell Lines Derived from Human Blastocysts,” Science , 282: 1145–47.
- Tooley, M., 1983, Abortion and Infanticide , New York: Oxford University Press.
- Warmflash, A., et al ., 2014, “A Method to Recapitulate Early Embryonic Spatial Patterning in Human Embryonic Stem Cells,” Nature Methods , 11: 847–854.
- Warren, M.A., 1973, “On the Moral and Legal Status of Abortion,” Monist , 57: 43–61.
- Yu, J., et al ., 2007, “Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells,” Science , 318: 1917–1920.
How to cite this entry . Preview the PDF version of this entry at the Friends of the SEP Society . Look up topics and thinkers related to this entry at the Internet Philosophy Ontology Project (InPhO). Enhanced bibliography for this entry at PhilPapers , with links to its database.
Other Internet Resources
- President’s Council on Bioethics, 2002, Human Cloning and Human Dignity: An Ethical Inquiry
- U.S. Conference of Catholic Bishops, 2001, Fact Sheet: President Bush’s Stem Cell Decision
- International Society for Stem Cell Research
- Stem Cell Resources from the American Association for the Advancement of Science
- Stem Cell Research and Applications , recommendations and findings from the AAAS and the Institute for Civil Society.
- Medline Plus: Stem Cells
- The Pew Forum on Religion and Public Life: Bioethics
- The Hinxton Group: An International Consortium on Stem Cell, Ethics, and Law
-->abortion, ethics of --> | cloning | double effect, doctrine of | ethics, biomedical: chimeras, human/non-human | parenthood and procreation | systems and synthetic biology, philosophy of
Copyright © 2018 by Andrew Siegel < asiegel @ jhu . edu >
- Accessibility
Support SEP
Mirror sites.
View this site from another server:
- Info about mirror sites
The Stanford Encyclopedia of Philosophy is copyright © 2023 by The Metaphysics Research Lab , Department of Philosophy, Stanford University
Library of Congress Catalog Data: ISSN 1095-5054
- Published: 07 July 2014
Ethical issues in stem cell research and therapy
- Nancy MP King 1 &
- Jacob Perrin 2
Stem Cell Research & Therapy volume 5 , Article number: 85 ( 2014 ) Cite this article
156k Accesses
140 Citations
25 Altmetric
Metrics details
Rapid progress in biotechnology has introduced a host of pressing ethical and policy issues pertaining to stem cell research. In this review, we provide an overview of the most significant issues with which the stem cell research community should be familiar. We draw on a sample of the bioethics and scientific literatures to address issues that are specific to stem cell research and therapy, as well as issues that are important for stem cell research and therapy but also for translational research in related fields, and issues that apply to all clinical research and therapy. Although debate about the moral status of the embryo in human embryonic stem cell research continues to have relevance, the discovery of other highly multipotent stem cell types and alternative methods of isolating and creating highly multipotent stem cells has raised new questions and concerns. Induced pluripotent stem cells hold great promise, but care is needed to ensure their safety in translational clinical trials, despite the temptation to move quickly from bench to bedside. A variety of highly multipotent stem cells - such as mesenchymal stem/stromal cells and stem cells derived from amniotic fluid, umbilical cord blood, adipose tissue, or urine - present the opportunity for widespread biobanking and increased access. With these increased opportunities, however, come pressing policy issues of consent, control, and justice. The imperatives to minimize risks of harm, obtain informed consent, reduce the likelihood of the therapeutic misconception, and facilitate sound translation from bench to bedside are not unique to stem cell research; their application to stem cell research and therapy nonetheless merits particular attention. Because stem cell research is both scientifically promising and ethically challenging, both the application of existing ethical frameworks and careful consideration of new ethical implications are necessary as this broad and diverse field moves forward.
Introduction
As every reader of this journal knows, ‘stem cell research’ is a category of enormous breadth and complexity. Current and potential therapeutic applications for stem cells are numerous. Stem cell researchers may be engaged in many different endeavors, including but not limited to seeking new sources of highly multipotent stem cells and methods of perpetuating them; creating induced pluripotent stem cell (iPSC) lines to study genetic disorders or explore pharmacogenomics; conducting animal or early-phase human studies of experimental stem cell interventions; or working with stem cells and biomaterials to develop organoids and other products for use in regenerative medicine, to name only a few possibilities.
In this review of selected major ethical issues in stem cell research and therapy, we briefly describe and discuss the most significant ethical implications of this wide-ranging and fast-moving field. Our discussion addresses research oversight in the historical context of human embryonic stem cell (hESC) research; clinical translation and uncertainty; the profound tension between the desire for clinical progress and the need for scientific caution; and issues of consent, control, commercialization, and justice arising from stem cell banking, disease modeling, and drug discovery. We seek to make stem cell scientists more aware of the need for clarity of discussion and to improve professional and public understanding of the ethical and policy issues affecting this important but early research. A review this brief is necessarily general; our hope is that researchers can use this discussion as a starting point for more in-depth identification and analysis of issues pertinent to specific translational research projects [ 1 – 3 ].
Stem cell research: oversight and clinical translation
The basic system of regulation and review of research involving humans and animals as subjects [ 4 , 5 ] is familiar to investigators. Recently, however, the term ‘translational’ has come to describe a line of research inquiry intended to stretch from bench to bedside and beyond. This has helped to emphasize that thinking about ethical issues should begin at the earliest stages of preclinical research. Ethics in both research and clinical settings is most effective when it is preventive.
In this respect, stem cell research is not unique; stem cell researchers should ask themselves the same questions about the trajectory of their translational research as would any other biomedical researcher [ 6 ]. Oversight of cell-based interventions does, however, include additional features that, while adding complexity to the regulatory process, also make it easier to take a long view, by requiring attention to the use of stem cells at all research stages. Increasing pressures for the rapid clinical translation and commercialization of stem cell products underscore the value of this long view [ 7 – 14 ].
The ethical issues that all researchers face during clinical translation begin with the need to ask a meaningful question, the answer to which has both scientific and social value and can be reached by the study as designed when properly conducted [ 6 , 15 ]. The risks of harm and the potential benefits to society from the development of generalizable knowledge (and, sometimes, potential direct benefit to patient-subjects) must be weighed and balanced at each stage of the research. Sound justification is necessary to support moving from the laboratory into animal studies, and from animals into human subjects, as well as through relevant phases of research with humans [ 15 – 18 ]. Minimizing the risks of harm, selecting and recruiting appropriate patient-subjects, facilitating informed decision making through the consent form and process, and avoiding the ‘therapeutic misconception’, whereby unduly high expectations affect all interested parties to a clinical trial, are all significant research ethics considerations, especially in first-in-human and other early-phase studies [ 19 – 26 ]. To many researchers, these considerations are simply requirements of sound and responsible study design, as exemplified, for example, in US Food and Drug Administration (FDA) guidance documents and investigational new drug requirements [ 27 ]. It should come as no surprise, however, that research design and research ethics are closely intertwined [ 1 , 6 , 15 ].
Stem cell research may give rise to heightened concern in several of these areas. One such concern is clarity of language. The term ‘stem cell’ by itself is broad and non-specific enough to be confusing; it can refer to hESCs, to iPSCs, to other types of multipotent and highly multipotent stem cells (including but not limited to stem cells derived from amniotic fluid, umbilical cord blood, adipose tissue, or urine), or to determined or adult stem cells like hematopoietic stem cells (HSCs), which have long been used in standard therapies. Patients, science reporters, and the public, on hearing the term ‘stem cell’, may thus find it difficult to distinguish between experimental stem cell interventions and proven stem cell therapies of long standing, such as treatments involving autologous or allogeneic HSC transplantation. The commercial availability worldwide of unproven ‘stem cell therapies’ that have not been studied in translational research adds to this confusion [ 12 , 14 , 24 – 26 , 28 , 29 ].
Human embryonic stem cells and embryonic stem cell research oversight committees
Hopes that the ethical controversy surrounding hESCs would become irrelevant when new sources of highly multipotent stem cells became available have proven somewhat premature. hESCs remain scientifically promising and continue to have important uses, even as research with iPSCs and other highly multipotent stem cells gains momentum [ 30 – 32 ]. A brief discussion thus seems warranted.
The first hESC line was derived in 1998, ushering in one of the most public, spirited, and intractable debates in research ethics: the moral status of the embryo from which hESCs are derived. To harvest hESCs, it is first necessary to destroy the 5-day-old preimplantation embryo. Opponents of hESC research argue that because the embryo is capable of developing into a human being, it has significant moral standing; therefore, its destruction is unethical. Some proponents of hESC research deny that the embryo has any moral status; others grant it limited moral status but argue that the value of this limited status is far outweighed by the potential benefits that can result from hESC research [ 24 , 33 ].
The ethical implications of hESC research in the US have been reflected in federal funding policy and in research oversight. In 2003, the National Academy of Sciences (NAS) established a committee to develop guidelines for institutions and investigators conducting hESC research [ 9 ]. The NAS Guidelines for Human Embryonic Stem Cell Research , most recently amended in 2010 [ 34 ], comprehensively address permissible and impermissible categories of hESC research and recommend the establishment of embryonic stem cell research oversight committees (ESCROs) to assist in research review. They also incorporate National Institutes of Health guidelines promulgated after a 2009 federal funding expansion, recommend oversight of research with human pluripotent stem cells, and address questions of consent from all donors of biomaterials, creation and use of embryos for research purposes, and animal-human chimeras.
Many research institutions have created ESCROs or ‘SCROs’ to review hESC and iPSC research; others rely on their institutional review boards or their animal care and use committees or both. As stem cell research diversifies, its ethical oversight also becomes more diverse, and questions have been raised regarding the ongoing need for specialized committees like ESCROs and SCROs [ 9 , 10 ]. The NAS Guidelines are nonetheless likely to continue providing guidance for a variety of oversight bodies reviewing stem cell research [ 9 , 10 , 32 ].
Induced pluripotent stem cells on the translational pathway
Controversy about the derivation and use of hESCs led investigators to seek less ethically fraught but maximally useful types of stem cells [ 31 ]. The history of iPSCs is one of seeking efficient ways to induce pluripotency that minimize the risk of teratoma development [ 35 ]. Although the rapidly developing science has reduced risks of harm and has increased the efficiency of pluripotent cell line creation to some extent, safety and efficacy concerns remain [ 36 ]. Indeed, the most recent advance in inducing pluripotency - stimulus-triggered acquisition of pluripotency, or STAP [ 37 ] - was widely heralded [ 38 ] but has since been called into question [ 39 ]. Obokata and colleagues [ 37 ] presented data suggesting that subjecting somatic cells to various stresses could quickly and safely produce iPSCs, but their results have not proven reproducible.
In research with iPSCs as well as with other types of stem cells, it is essential that preclinical studies in animal models and other media be sufficient to justify the progression to clinical trials. Toxicity and the risk of tumorigenicity must be assessed for all stem cell-based products, especially when genetically modified, in order to minimize the risks of harm as far as feasible before moving to humans [ 11 , 12 , 16 , 17 , 26 , 40 ].
Concern about the research use of animals - especially non-human primates - in preclinical research, including iPSC research, is growing and must be addressed; at the same time, researchers are increasingly aware that good animal models are often unavailable or inadequate to predict effects in humans. Thus, considerable uncertainty continues to surround first-in-human trials and other early-phase studies using stem cells, even as the rapid pace and apparently improving safety of iPSC creation tempt the field to move rapidly into clinical research and even therapeutic applications [ 5 , 25 , 41 ].
Clinical trials: uncertainty and human subjects
Clinical trials of iPSCs and other highly pluripotent stem cell interventions generally enroll patients as subjects at all trial stages, as using healthy volunteers may raise safety concerns or compromise the value of the data. All clinical trials must, of course, be carefully designed, rigorously justified, and properly conducted in order to protect the rights, interests, and welfare of trial subjects and contribute to generalizable knowledge [ 11 , 12 , 15 – 17 , 25 , 26 , 35 , 40 ]. Stem cell researchers can and should benefit from the lessons learned by gene transfer researchers: rapid transition to clinical applications without sufficient understanding of the mechanisms of effect is both inefficient and unwise [ 11 , 12 , 25 , 42 ].
The Geron trial provides just one instructive example. In late January 2009, the FDA approved the first clinical trial of an hESC-based experimental intervention for spinal cord injury. The product, oligodendrocyte progenitor cells (OPCs), is thought to remyelinate spinal cord axons. The trial was to enroll a small number of patient-subjects with recent serious spinal cord lesions. It was placed on hold once by the FDA to ensure the purity and safety of the OPCs and ultimately was halted by the sponsor, Geron Corporation (Menlo Park, CA, USA), for reasons of cost, after only four patient-subjects had received the intervention.
Both the trial’s design and its ultimate discontinuation were controversial. Its design caused controversy because the subjects were enrolled very soon after a serious injury, making understanding and consent challenging in this first-in-human trial and in addition making it potentially difficult to distinguish between spontaneous recovery of function and remyelination attributable to the intervention. Patients with older lesions, though very probably in a better position to make decisions about trial participation, have scar tissue that makes remyelination unlikely or impossible. The sponsor’s premature discontinuation of the trial was problematic because data insufficiency renders worthless not only its own investment but also those made by patient-subjects and investigators. The outcome had the potential to discourage pioneering stem cell research in the future [ 25 , 43 , 44 ]. Nonetheless, identifying the optimal time for post-injury intervention, both to maximize the potential for assessing effects on remyelination and to promote an optimal decision-making process by patient-subjects, is of ongoing concern to spinal cord injury researchers studying cell-based interventions [ 45 ]. More recently, discussions of ethical and design issues in particular stem cell trials (for example, macular degeneration [ 2 ] and cardiovascular disease [ 3 ]) highlight the difficult balance between the imperatives of caution and progress for first-in-human trials in high-profile areas like stem cell intervention research.
Disclosure and discussion of uncertainty with potential subjects in stem cell trials are essential in order to reduce the incidence of therapeutic misconception, whereby research subjects and also investigators and oversight bodies view research as a treatment modality or significantly overestimate the likelihood of direct benefit or both [ 19 – 22 , 41 ]. This information transparency also helps protect the integrity of the research process and the safety of patients in the face of increasing global availability of unapproved and unproven stem cell ‘treatments’ [ 11 , 12 , 16 , 24 – 26 ].
Many types of multipotent and highly multipotent stem cells have been identified as potentially suitable for clinical applications. Some of the most significant challenges faced in clinical application include how quickly to move forward in the face of great promise, great uncertainty, and great clinical need; how to regard research with investigational interventions that are difficult to standardize and impossible to undo; and how to define and describe these uncertainties in the consent process. A growing number of prestigious academics from both science and bioethics are calling attention to these challenges [ 2 , 24 , 26 , 42 ].
One prominent scientist commentator compares the current state of stem cell research with the histories of HSC transplantation and gene transfer research, citing several principles: risks of harm should be commensurate with the severity of the condition under study, preclinical animal models remain critically important, and gaining insight into therapeutic mechanisms is essential to the success of a line of clinical research. He advocates ‘a conservative approach to clinical translation of stem cell therapies’ at present, not because of risks of harm, ‘but rather because our understanding of the mechanisms by which stem cells might prove useful, and in which diseases, remains primitive’ [ 25 ]. Similarly, in an international survey of stem cell scientists and scholars of ethical issues in stem cell research, a prolific bioethics research group has identified increasing concerns arising from pressures for clinical translation, commercialization, and oversight of new stem cell technologies [ 14 ].
Highly multipotent stem cells: biobanking, disease modeling, and drug discovery
Some applications of stem cell research, such as disease modeling, drug discovery and testing, cell line banking, and commercialization of stem cell therapies, also give rise to ethical considerations specific to the field [ 11 , 12 , 14 , 16 , 24 – 26 , 28 , 29 ]. iPSCs and other highly multipotent stem cells have many additional important uses outside the typical clinical research trajectory. The creation and use of disease-specific iPSC lines, both alone and in combination with regenerative medicine products (for example, to produce ex vivo organoids), are essential components of disease modeling and drug discovery. ‘Body-on-a-chip’ types of three-dimensional organoid arrays hold great promise for improving drug development, disease modeling, and pharmacogenomic research, by lowering costs, speeding results, and increasing the safety and potential efficacy signaling of first-in-human trials, and considerable research is under way [ 46 ]. That promise is as yet unrealized, but questions of consent and control arise even at the bench. Because iPSC lines are derived from the somatic cells of identifiable individuals, disclosing to those individuals the planned and envisioned uses of iPSCs derived from the cells they have donated and obtaining consent from them are critical for the creation and sharing of cell line research libraries and the future uses of biomaterials derived from previously donated biospecimens [ 24 , 26 , 47 – 50 ].
As potentially therapeutic applications proliferate for different highly multipotent stem cell types and as technical barriers to the collection and perpetuation of cell lines continue to fall, proposed research and treatment uses abound for both autologous and allogeneic stem cells. In particular, the development of public and other broadly accessible biobanking models for stem cells derived from umbilical cord blood, amniotic fluid and placental tissue, urine, and adipose tissue holds promise for easy collection of good allograft matches for a large percentage of the population but also requires attention to ethical and policy issues [ 26 , 51 ].
Justice in stem cell research and treatment
Justice is a necessary but neglected consideration in all scientific research. Like many novel biotechnologies, gene- and cell-based and regenerative medicine interventions and products can be extraordinarily costly and time- and labor-intensive to develop and use. Justice thus requires attention to the costs of developing stem cell therapies and making them available, with the goal of reducing unfair disparities in access. Cost is a standard distributive justice concern. Less commonly discussed is the effect of research funding decisions on health disparities - both priority-setting within research and priority between research funding and funding for medical care, public health, and other public goods [ 19 – 21 ].
Justice considerations are addressed in stem cell research and therapy in several ways. The first is biobanking policy and practice. The rationale for public stem cell banking is to provide a resource for transplantation of blood-forming HSCs to virtually anyone. Ideally, large-scale banking efforts could store enough different lines of broadly multipotent and pluripotent stem cells, suitable for use in regenerative medicine applications, to provide good matches for nearly the entire population of the US. Comprehensive systems for the collection, storage, and use of stem cells of different types are, however, still in the early stages of technological and policy development. Scientific, practical, and ethical challenges include ensuring broad availability of matches for those in need, determining access for both research and therapy, refining consent forms and processes, and protecting confidentiality in labeling and information linkage [ 11 , 12 , 16 , 26 , 51 , 52 ]. Thus, large-scale biobanking of stem cell lines holds the potential to greatly increase access to stem cell therapies and reduce costs, but because available allogeneic matches may not be perfect, balancing the harms and benefits of biobanking remains critical.
The second justice-promoting feature of stem cell research and therapy has some similarities. Attempts to standardize and streamline production are more prevalent in stem cell-related research than elsewhere - especially in development of cell-based products and in regenerative medicine. In other new technologies like gene transfer, standardization, the development of platform technologies, and attempts at large-scale, cost-reducing production are in their infancy. This production perspective is an important step in reducing time, labor, and costs and thus increasing access, but it could also have interesting ethical implications. Autologous or individually ‘compounded’ cell-based interventions will certainly be more costly and less readily available - and will take more time to produce - than allogeneic and other ‘mass-produced’ cell-based interventions, which may provide a less-than-perfect match or fit for a given patient. Such differences could have efficacy implications that must be monitored and balanced against cost savings and access gains [ 51 ].
A final justice consideration that is heightened in the stem cell context is the simple reality that important work dedicated to improving the health of the public takes place in a market system with its attendant pressures of competition and commercialization. The attempt to ensure that hope does not become hype and that hype does not become fraud is a matter of justice. Thus, sound practice in clinical translation, careful discussion in the media, and even seeking balance between scientific transparency and data-sharing and the intellectual property interests of industry all have important justice implications [ 13 , 16 , 24 – 29 , 42 , 53 ]. As research funding shrinks and competitive pressures grow, it may become increasingly difficult to move deliberately toward clinical translation and to allocate research resources wisely. This is especially likely as more is learned about how to reduce the risks of harm from the creation and use of iPSCs and as the costs of careful progress continue to increase. The fewer resources we have, the more important it is to allocate funds to maximize the likelihood of knowledge development in areas of greatest promise and clinical need [ 14 , 21 ].
Individual researchers may at first regard justice considerations as somewhat removed from their daily work at bench or bedside. The goals of advancing knowledge and, ultimately, improving human health are nonetheless social goals, not merely scientific goals. Researchers make vital contributions to societal views about the value of - and best directions for - scientific progress. For this reason alone, it is worthwhile for researchers to keep in mind the population-level applications of stem cell research as well as the effects of stem cell therapy on individual health.
Summary and conclusions
As our discussion has shown, many of the ethical and policy issues that are most significant for stem cell research and therapy are similar to those arising in other novel biotechnologies. Consideration of these issues in both scientific and bioethics literatures addresses many common themes: the minimization of risks of harm; the importance of information disclosure and informed consent; the potential for overpromising, overexpectations, and the therapeutic misconception; and the pressure from disease constituencies and commercial entities to move quickly into the clinic, too often at the expense of understanding basic mechanisms. In the realm of clinical translation, trial-specific examinations of ethical issues continue to provide important guidance, not only with regard to the trials specifically considered but also as models for investigators starting down new translational pathways.
Although the creation and use of hESCs have long been the unique focus of stem cell ethics, more current controversies include the creation, for research use, of human embryos, human-animal chimeras, and gametes. Yet these marquee controversies are, in the long run, less important for the field as a whole than are more mundane, justice-oriented concerns like the creation and use of stem cell banks for research and therapy, facilitation of ‘off-the-shelf’ stem cell applications that could be less costly though perhaps less than perfect, and questions of consent, provenance, and policy. Finally, moving forward with the right blend of creativity and caution is essential, in the interest of both science and patients. In all areas of stem cell research and therapy, nuanced consideration and discussion of the best translational pathways, as viewed by ethics as well as science, will play a vital role in balancing hope and hype now and in the future, as the field continues its rapid progress.
Abbreviations
embryonic stem cell research oversight (committee)
US Food and Drug Administration
human embryonic stem cell
hematopoietic stem cell
induced pluripotent stem cell
National Academy of Sciences
oligodendrocyte progenitor cell
stem cell research oversight (committee).
Lo B, Parham L: Resolving ethical issues in stem cell clinical trials: the example of Parkinson disease. J Law Med Ethics. 2010, 38: 257-266. 10.1111/j.1748-720X.2010.00486.x.
Article PubMed Google Scholar
Habets MG, van Delden JJ, Bredenoord AL: The inherent ethical challenge of first-in-human pluripotent stem cell trials. Regen Med. 2014, 9: 1-3. 10.2217/rme.13.83.
Article CAS PubMed Google Scholar
Niemansburg SL, Teraa M, Hesam H, van Delden JJ, Verhaar MC, Bredenoord AL: Stem cell trials for cardiovascular medicine: ethical rationale. Tiss Eng Part A. 2013, [Epub ahead of print]
Google Scholar
Levine R: Ethics and Regulation of Clinical Research. 1988, New York: Yale University Press
Gilbert S, Kaebnick GE, Murray TH: Special Report: Animal research ethics: evolving views and practices. Hastings Center Rep. 2012, 42: S1-S39.
Article Google Scholar
Joffe S, Miller FG: Bench to bedside: mapping the moral terrain of clinical research. Hastings Center Rep. 2008, 38: 30-42.
Arcidiacono JA, Blair JW, Benton KA: US Food and Drug Administration international collaborations for cellular therapy product regulation. Stem Cell Res Ther. 2012, 3: 38-42. 10.1186/scrt129.
Article PubMed Central PubMed Google Scholar
Caulfield T, Zarzeczny A, McCormick J, Bubela T, Critchley C, Einsiedel E, Galipeau J, Harmon S, Huynh M, Hyun I, Illes J, Isasi R, Joly Y, Laurie G, Lomax G, Longstaff H, McDonald M, Murdoch C, Ogbogu U, Owen-Smith J, Pattinson S, Premji S, von Tigerstrom B, Winickoff DE: The stem cell research environment: a patchwork of patchworks. Stem Cell Rev. 2009, 5: 82-88. 10.1007/s12015-009-9071-3.
Greely H: Assessing ESCROs: yesterday and tomorrow. Am J Bioeth. 2013, 13: 44-52.
Lomax GP, Peckman SR: Stem cell policy exceptionalism: proceed with caution. Stem Cell Rev Rep. 2012, 8: 299-304. 10.1007/s12015-011-9305-z.
Hyun I, Lindvall O, Ahrlund-Richter L, Cattaneo E, Cavazzana-Calvo M, Cossu G, De Luca M, Fox IJ, Gerstle C, Goldstein RA, Hermeren G, High KA, Kim HO, Lee HP, Levy-Lahad E, Li L, Lo B, Marshak DR, McNab A, Munsie M, Nakauchi H, Rao M, Rooke HM, Valles CS, Srivastava A, Sugarman J, Taylor PL, Veiga A, Wong AL, Zoloth L, Daley GQ: New ISSCR guidelines underscore major principles for responsible translational stem cell research. Cell Stem Cell. 2008, 3: 607-609. 10.1016/j.stem.2008.11.009.
International Society for Stem Cell Research: Guidelines for the clinical translation of stem cells. [ http://www.isscr.org/docs/guidelines/isscrglclinicaltrans.pdf ]
Caulfield T: Stem cell research and economic promises. J Law Med Ethics. 2010, 38: 303-313. 10.1111/j.1748-720X.2010.00490.x.
Caulfield T, Rachul C, Zarzeczny A: The evolution of policy issues in stem cell research: an international survey. Stem Cell Rev Rep. 2012, 8: 1037-1042. 10.1007/s12015-012-9404-5.
Emanuel EJ, Wendler D, Grady C: What makes clinical research ethical?. JAMA. 2000, 283: 2701-2711. 10.1001/jama.283.20.2701.
Kato K, Kimmelman J, Robert J, Sipp D, Sugarman J: Ethical and policy issues in the clinical translation of stem cells: report of a focus session at the ISSCR annual meeting. Cell Stem Cell. 2012, 11: 765-767. 10.1016/j.stem.2012.11.004.
London AJ, Kimmelman J, Emborg ME: Beyond access vs. protection in trials of innovative therapies. Science. 2010, 328: 829-830. 10.1126/science.1189369.
Article PubMed Central CAS PubMed Google Scholar
King NM, Cohen-Haguenauer O: En route to ethical recommendations for gene transfer clinical trials. Mol Ther. 2008, 16: 432-438. 10.1038/mt.2008.13.
Dresser R: First-in-human trial participants: not a vulnerable population, but vulnerable nonetheless. J Law Med Ethics. 2009, 37: 38-50.
Dresser R: Stem cell research as innovation: expanding the ethical and policy conversation. J Law Med Ethics. 2010, 38: 332-341. 10.1111/j.1748-720X.2010.00492.x.
Dresser R: Alive and well: the research imperative. J Law Med Ethics. 2012, 40: 915-921.
Dresser R: The ubiquity and utility of the therapeutic misconception. Soc Philos Policy. 2002, 19: 271-294. 10.1017/S0265052502192119.
King NM, Henderson GE, Churchill LR, Davis AM, Hull SC, Nelson DK, Parham-Vetter PC, Rothschild BB, Easter MM, Wilfond BS: Consent forms and the therapeutic misconception: the example of gene transfer research. IRB. 2005, 27: 1-
Hyun I: The bioethics of stem cell research and therapy. J Clin Invest. 2010, 120: 71-75. 10.1172/JCI40435.
Daley GQ: The promise and perils of stem cell therapeutics. Cell Stem Cell. 2012, 10: 740-749. 10.1016/j.stem.2012.05.010.
Sugarman J: Human stem cell ethics: beyond the embryo. Cell Stem Cell. 2008, 2: 529-533. 10.1016/j.stem.2008.05.005.
Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research: Guidance for industry: considerations for the design and conduct of early-phase clinical trials of cellular and gene therapy products (DRAFT). 2013, [ http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/UCM359073.pdf ]
Sipp D: Direct-to-consumer stem cell marketing and regulatory responses. Stem Cells Translational Med. 2013, 2: 638-640. 10.5966/sctm.2013-0040.
Weissman I: Stem cell therapies could change medicine if they get the chance. Cell Stem Cell. 2012, 10: 663-665. 10.1016/j.stem.2012.05.014.
Hyun I, Hochedlinger K, Jaenish R, Yamanaka S: New advances in iPS cell research do not obviate the need for human embryonic stem cells. Cell Stem Cell. 2007, 4: 367-368.
King NM, Coughlin CN, Atala A: Pluripotent stem cells: the search for the ‘perfect’ source. Minn J Law Sci Technol. 2011, 12: 715-730.
Ishii T, Pera RA, Greely HT: Ethical and legal issues arising in research on inducing human germ cells from pluripotent stem cells. Cell Stem Cell. 2013, 13: 145-148. 10.1016/j.stem.2013.07.005.
Cohen CB: Renewing the Stuff of Life: Stem Cells, Ethics, and Public Policy. 2007, New York: Oxford University Press
Human Embryonic Stem Cell Research Advisory Committee, The National Academies: Final Report and 2010 Amendments to the National Academies’ Guidelines for Human Embryonic Stem Cell Research. 2010, Washington, DC: National Academies Press
Yamanaka S: Induced pluripotent stem cells: past, present, and future. Cell Stem Cell. 2012, 10: 678-684. 10.1016/j.stem.2012.05.005.
Pera MF: Stem cells: the dark side of induced pluripotency. Nature. 2011, 471: 46-47. 10.1038/471046a.
Obokata H, Wakayama T, Sasai Y, Kojima K, Vacanti MP, Niwa H, Yamato M, Vacanti CA: Stimulus-triggered fate conversion of somatic cells into pluripotency. Nature. 2014, 505: 641-647. 10.1038/nature12968.
Cyranoski D: Acid bath offers easy path to stem cells. Nature. 2014, 505: 596-10.1038/505596a.
Cyranoski D: Acid-bath stem-cell study under investigation. Nature. 2014, [ http://www.nature.com/news/acid-bath-stem-cell-study-under-investigation-1.14738 ]
Kimmelman J, Baylis F, Glass KG: Stem cell trials: lessons from gene transfer research. Hastings Cent Rep. 2006, 36: 23-26.
Hyun I: Allowing innovative stem cell based therapies outside of clinical trials: ethical and policy challenges. J Law Med Ethics. 2010, 38: 277-285. 10.1111/j.1748-720X.2010.00488.x.
Wilson JM: A history lesson for stem cells. Science. 2009, 324: 727-728. 10.1126/science.1174935.
Bretzner F, Gilbert F, Baylis F, Brownstone RM: Target populations for first-in-human embryonic stem cell research in spinal cords. Cell Stem Cell. 2011, 8: 468-475. 10.1016/j.stem.2011.04.012.
Lukovic D, Stojkovic M, Moreno-Manzano V, Bhattacharya SS, Erceg S: Perspectives and future directions of human pluripotent stem cell-based therapies: lessons from Geron’s clinical trial for spinal cord injury. Stem Cells Dev. 2014, 23: 1-4. 10.1089/scd.2013.0266.
Illes J, Reimer C, Kwon BK: Stem cell clinical trials for spinal cord injury: readiness, reluctance, redefinition. Stem Cell Rev. 2011, 7: 997-1005. 10.1007/s12015-011-9259-1.
Esch MB, King TL, Shuler ML: The role of body-on-a-chip devices in drug and toxicity studies. Ann Rev Biomed Eng. 2011, 13: 55-72. 10.1146/annurev-bioeng-071910-124629.
Article CAS Google Scholar
Lowenthal J, Lipnick S, Rao M, Hull SC: Specimen collection for induced pluripotent stem cell research: harmonizing the approach to informed consent. Stem Cells Translational Med. 2012, 1: 409-421. 10.5966/sctm.2012-0029.
Lomax GP, Hull SC, Lowenthal J, Rao M, Isasi R: The DISCUSS project: induced pluripotent stem cell lines from previously collected research biospecimens and informed consent: points to consider. Stem Cells Translational Med. 2013, 2: 727-730. 10.5966/sctm.2013-0099.
Lomax GP, Shepard KA: Return of results in translational iPS cell research: considerations for donor informed consent. Stem Cell Res Ther. 2013, 4: 6-7. 10.1186/scrt154.
Hyun I: The bioethics of iPS cell based drug discovery. Clin Pharmacol Ther. 2011, 89: 646-647. 10.1038/clpt.2010.308.
King NM, Coughlin CN, Furth M: Ethical issues in regenerative medicine. Wake Forest Intellectual Property Law J. 2009, 9: 216-238.
Faden RR, Dawson L, Bateman-House AS, Agnew DM, Bok H, Brock DW, Chakravarti A, Gao XJ, Greene M, Hansen JA, King PA, O’Brien SJ, Sachs DH, Schill KE, Siegel A, Solter D, Suter SM, Verfaillie CM, Walters LB, Gearhart JD: Public stem cell banks: considerations of justice in stem cell research and therapy. Hastings Center Rep. 2003, 33: 13-27.
Caulfield T, Rachul C: Science spin: iPSC research in the news. Clin Pharmacol Ther. 2011, 89: 644-646. 10.1038/clpt.2010.309.
Download references
Author information
Authors and affiliations.
Department of Social Sciences and Health Policy, Wake Forest School of Medicine, Medical Center Blvd, Winston-Salem, NC, 27157, USA
Nancy MP King
Center for Bioethics, Health, and Society, Wake Forest University, 1834 Wake Forest Rd, Winston-Salem, NC, 27106, USA
Jacob Perrin
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Nancy MP King .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
King, N.M., Perrin, J. Ethical issues in stem cell research and therapy. Stem Cell Res Ther 5 , 85 (2014). https://doi.org/10.1186/scrt474
Download citation
Published : 07 July 2014
DOI : https://doi.org/10.1186/scrt474
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Pluripotent Stem Cell
- Clinical Translation
- Stem Cell Therapy
- Stem Cell Research
Stem Cell Research & Therapy
ISSN: 1757-6512
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Utility Menu
GA4 tracking code

Examining the ethics of embryonic stem cell research

Following the recent passage by both houses of Congress of the Stem Cell Research Enhancement Act of 2007, which would permit federal funding of research using donated surplus embryonic stem cells from fertility clinics, the president has once again threatened a veto.
Because neither the House nor the Senate had sufficient votes to override a presidential veto, it appears unlikely this new bill will be enacted into law, further stalling the pace of this research. “This bill crosses a moral line that I and others find troubling,” stated Bush, following the Senate’s vote.
SCL : What are th e main arguments for and against embryonic stem cell research? MS : Proponents argue that embryonic stem cell research holds great promise for understanding and curing diabetes, Parkinson’s disease, spinal cord injury, and other debilitating conditions. Opponents argue that the research is unethical, because deriving the stem cells destroys the blastocyst, an unimplanted human embryo at the sixth to eighth day of development. As Bush declared when he vetoed last year’s stem cell bill, the federal government should not support “the taking of innocent human life.”
It is surprising that, despite the extensive public debate—in Congress, during the 2004 and 2006 election campaigns, and on the Sunday morning talk shows—relatively little attention has been paid to the moral issue at the heart of the controversy: Are the opponents of stem cell research correct in their claim that the unimplanted human embryo is already a human being, morally equivalent to a person?

“It is important to be clear about the embryo from which stem cells are extracted. It is not implanted and growing in a woman’s uterus. It is not a fetus. It has no recognizable human features or form. It is, rather, a blastocyst, a cluster of 180 to 200 cells, growing in a petri dish, barely visible to the naked eye.”
SCL : What are the contradictions in Bush’s stance? MS : Before we address that, it is important to be clear about the embryo from which stem cells are extracted. It is not implanted and growing in a woman’s uterus. It is not a fetus. It has no recognizable human features or form.
It is, rather, a blastocyst, a cluster of 180 to 200 cells, growing in a petri dish, barely visible to the naked eye. Such blastocysts are either cloned in the lab or created in fertility clinics. The bill recently passed by Congress would fund stem cell research only on excess blastocysts left over from infertility treatments.
The blastocyst represents such an early stage of embryonic development that the cells it contains have not yet differentiated, or taken on the properties of particular organs or tissues—kidneys, muscles, spinal cord, and so on. This is why the stem cells that are extracted from the blastocyst hold the promise of developing, with proper coaxing in the lab, into any kind of cell the researcher wants to study or repair.
The moral and political controversy arises from the fact that extracting the stem cells destroys the blastocyst. It is important to grasp the full force of the claim that the embryo is morally equivalent to a person, a fully developed human being.
For those who hold this view, extracting stem cells from a blastocyst is as morally abhorrent as harvesting organs from a baby to save other people’s lives. This is the position of Senator Sam Brownback, Republican of Kansas, a leading advocate of the right-to-life position. In Brownback’s view, “a human embryo . . . is a human being just like you and me; and it deserves the same respect that our laws give to us all.
If Brownback is right, then embryonic stem cell research is immoral because it amounts to killing a person to treat other people’s diseases.
SCL : What is the basis for the belief that personhood begins at conception? MS : Some base this belief on the religious conviction that the soul enters the body at the moment of conception. Others defend it without recourse to religion, by the following line of reasoning: Human beings are not things. Their lives must not be sacrificed against their will, even for the sake of good ends, like saving other people’s lives. The reason human beings must not be treated as things is that they are inviolable. At what point do humans acquire this inviolability? The answer cannot depend on the age or developmental stage of a particular human life. Infants are inviolable, and few people would countenance harvesting organs for transplantation even from a fetus.
Every human being—each one of us—began life as an embryo. Unless we can point to a definitive moment in the passage from conception to birth that marks the emergence of the human person, we must regard embryos as possessing the same inviolability as fully developed human beings.
SCL : By this line of reasoning, human embryos are inviolable and should not be used for research, even if that research might save many lives. MS : Yes, but this argument can be challenged on a number of grounds. First, it is undeniable that a human embryo is “human life” in the biological sense that it is living rather than dead, and human rather than, say, bovine.
But this biological fact does not establish that the blastocyst is a human being, or a person. Any living human cell (a skin cell, for example) is “human life” in the sense of being human rather than bovine and living rather than dead. But no one would consider a skin cell a person, or deem it inviolable. Showing that a blastocyst is a human being, or a person, requires further argument.
Some try to base such an argument on the fact that human beings develop from embryo to fetus to child. Every person was once an embryo, the argument goes, and there is no clear, non-arbitrary line between conception and adulthood that can tell us when personhood begins. Given the lack of such a line, we should regard the blastocyst as a person, as morally equivalent to a fully developed human being.
SCL : What is the flaw in this argument? MS : Consider an analogy: although every oak tree was once an acorn, it does not follow that acorns are oak trees, or that I should treat the loss of an acorn eaten by a squirrel in my front yard as the same kind of loss as the death of an oak tree felled by a storm. Despite their developmental continuity, acorns and oak trees differ. So do human embryos and human beings, and in the same way. Just as acorns are potential oaks, human embryos are potential human beings.
The distinction between a potential person and an actual one makes a moral difference. Sentient creatures make claims on us that nonsentient ones do not; beings capable of experience and consciousness make higher claims still. Human life develops by degrees.
SCL : Yet there are people who disagree that life develops by degrees, and believe that a blastocyst is a person and, therefore, morally equivalent to a fully developed human being. MS : Certainly some people hold this belief. But a reason to be skeptical of the notion that blastocysts are persons is to notice that many who invoke it do not embrace its full implications.
President Bush is a case in point. In 2001, he announced a policy that restricted federal funding to already existing stem cell lines, so that no taxpayer funds would encourage or support the destruction of embryos. And in 2006, he vetoed a bill that would have funded new embryonic stem cell research, saying that he did not want to support “the taking of innocent human life.”
“The distinction between a potential person and an actual one makes a moral difference. Sentient creatures make claims on us that nonsentient ones do not; beings capable of experience and consciousness make higher claims still. Human life develops by degrees.”
But it is a striking feature of the president’s position that, while restricting the funding of embryonic stem cell research, he has made no effort to ban it. To adapt a slogan from the Clinton administration, the Bush policy might be summarized as “don’t fund, don’t ban.” But this policy is at odds with the notion that embryos are human beings.
SCL : If Bush’s policy were consistent with his stated beliefs, how, in your opinion, would it differ from his current “don’t fund, don’t ban” policy? MS : If harvesting stem cells from a blastocyst were truly on a par with harvesting organs from a baby, then the morally responsible policy would be to ban it, not merely deny it federal funding.
If some doctors made a practice of killing children to get organs for transplantation, no one would take the position that the infanticide should be ineligible for federal funding but allowed to continue in the private sector. In fact, if we were persuaded that embryonic stem cell research were tantamount to infanticide, we would not only ban it but treat it as a grisly form of murder and subject scientists who performed it to criminal punishment.
SCL : Couldn’t it be argued, in defense of the president’s policy, that Congress would be unlikely to enact an outright ban on embryonic stem cell research? MS : Perhaps. But this does not explain why, if the president really considers embryos to be human beings, he has not at least called for such a ban, nor even called upon scientists to stop doing stem cell research that involves the destruction of embryos. In fact, Bush has cited the fact that “there is no ban on embryonic stem cell research” in touting the virtues of his “balanced approach.”
The moral oddness of the Bush “don’t fund, don’t ban” position confused even his spokesman, Tony Snow. Last year, Snow told the White House press corps that the president vetoed the stem cell bill because he considered embryonic stem cell research to be “murder,” something the federal government should not support. When the comment drew a flurry of critical press attention, the White House retreated. No, the president did not believe that destroying an embryo was murder. The press secretary retracted his statement, and apologized for having “overstated the president’s position.”
How exactly the spokesman had overstated the president’s position is unclear. If embryonic stem cell research does constitute the deliberate taking of innocent human life, it is hard to see how it differs from murder. The chastened press secretary made no attempt to parse the distinction. His errant statement that the president considered embryo destruction to be “murder” simply followed the moral logic of the notion that embryos are human beings. It was a gaffe only because the Bush policy does not follow that logic.
SCL : You have stated that the president’s refusal to ban privately funded embryonic stem cell research is not the only way in which his policies betray the principle that embryos are persons. How so? MS : In the course of treating infertility, American fertility clinics routinely discard thousands of human embryos. The bill that recently passed in the Senate would fund stem cell research only on these excess embryos, which are already bound for destruction. (This is also the position taken by former governor Mitt Romney, who supports stem cell research on embryos left over from fertility clinics.) Although Bush would ban the use of such embryos in federally funded research, he has not called for legislation to ban the creation and destruction of embryos by fertility clinics.
SCL : If embryos are morally equivalent to fully developed human beings, doesn’t it then follow that allowing fertility clinics to discard thousands of embryos is condoning mass murder? MS : It does. If embryos are human beings, to allow fertility clinics to discard them is to countenance, in effect, the widespread creation and destruction of surplus children. Those who believe that a blastocyst is morally equivalent to a baby must believe that the 400,000 excess embryos languishing in freezers in U.S. fertility clinics are like newborns left to die by exposure on a mountainside. But those who view embryos in this way should not only be opposing embryonic stem cell research; they should also be leading a campaign to shut down what they must regard as rampant infanticide in fertility clinics.
Some principled right-to-life opponents of stem cell research meet this test of moral consistency. Bush’s “don’t fund, don’t ban” policy does not. Those who fail to take seriously the belief that embryos are persons miss this point. Rather than simply complain that the president’s stem cell policy allows religion to trump science, critics should ask why the president does not pursue the full implications of the principle he invokes.
If he does not want to ban embryonic stem cell research, or prosecute stem cell scientists for murder, or ban fertility clinics from creating and discarding excess embryos, this must mean that he does not really consider human embryos as morally equivalent to fully developed human beings after all.
But if he doesn’t believe that embryos are persons, then why ban federally funded embryonic stem cell research that holds promise for curing diseases and saving lives?

The Ethics of Embryonic Stem Cell Research
Devolder, K., (2015), ' The Ethics of Embryonic Stem Cell Research ', (Oxford: Oxford University Press)

Oxford University Press
Embryonic stem cell research holds unique promise for developing therapies for currently incurable diseases and conditions, and for important biomedical research. However, the process through which embryonic stem cells are obtained involves the destruction of early human embryos. Katrien Devolder focuses on the tension between the popular view that an embryo should never be deliberately harmed or destroyed, and the view that embryonic stem cell research, because of its enormous promise, must go forward. She provides an in-depth ethical analysis of the major philosophical and political attempts to resolve this tension. One such attempt involves the development of a middle ground position, which accepts only types or aspects of embryonic stem cell research deemed compatible with the view that the embryo has a significant moral status. An example is the position that it can be permissible to derive stem cells from embryos left over from in vitro fertilisation but not from embryos created for research. Others have advocated a technical solution. Several techniques have been proposed for deriving embryonic stem cells, or their functional equivalents, without harming embryos. An example is the induced pluripotent stem cell technique. Through highlighting inconsistencies in the arguments for these positions, Devolder argues that the central tension in the embryonic stem cell debate remains unresolved. This conclusion has important implications for the stem cell debate, as well as for policies inspired by this debate.
"As an academic bioethicist with experience in the clinical setting, it is important to me that context and morality are married. Devolder's book accomplishes this task nicely, beginning in the introduction with a consideration of the potential use of embryonic stem cell (if not the embryo as a whole) for the alleviation of pain and disease. She convincingly directs us towards our moral obligation to allieviate suffering, underscoring that embryonic stem cell research is thus a moral enterprise." - Ayesha Ahmad, London School of Economics, Times Higher Education
"In her small but well written and insightful monograph Katrien Devolder is focusing on these "middle-ground positions" together with technical solutions to the dilemma. The author has been working on reproductive ethics in general and on embryo and stem cell research ethics in particular for more than ten years. Her book is based on several previously published articles, but it is far more than a mere collection or a re-use of essays." - Marco Stier, Ethical Theory and Moral Practice
"Devolders study is a tour de force, exhibiting real skill and imagination in the use of analogies to test our moral intuitions... The Ethics of Embryonic Stem Cell Research is a solid contribution to our stem cell debates. Neither partisan nor committed to advocacy for any side, it displays epistemic honesty and exhibits the value of philosophical analysis at its best." - Ronald M. Green, Monash Bioethics Review
- Research article
- Open access
- Published: 12 May 2020
Ethical challenges regarding the use of stem cells: interviews with researchers from Saudi Arabia
- Ghiath Alahmad ORCID: orcid.org/0000-0002-3331-4378 1 ,
- Sarah Aljohani 1 &
- Muath Fahmi Najjar 1
BMC Medical Ethics volume 21 , Article number: 35 ( 2020 ) Cite this article
18k Accesses
10 Citations
Metrics details
With the huge number of patients who suffer from chronic and incurable diseases, medical scientists continue to search for new curative methods for patients in dire need of treatment. Interest in stem cells is growing, generating high expectations in terms of the possible benefits that could be derived from stem cell research and therapy. However, regardless of the hope of stem cells changing and improving lives, there are many ethical, religious, and political challenges and controversies that affect the research, and mandated to establish ethical guidelines and regulations. In Saudi Arabia, key stakeholders play an active role in discussing the ethics of stem cell research and therapy. The focus of the study was to explore professionals’ perceptions related to the ethical challenges of using stem cells in research and treatment in Saudi Arabia.
A qualitative research study was conducted to explore and describe the perceptions of 25 professionals employed at different tertiary hospitals in the various regions of Saudi. A thematic analysis was performed to search for and identify the most significant perceptions shared by the participants. Four themes were generated based on the ethical challenges of four areas related to stem cell use, including (1) forbidden and permitted sources of stem cells, (2) informed consent, (3) beneficence, and (4) ethical regulations and guidelines.
The study identified that there is a growing need to advance the knowledge, education, and awareness related to stem cell research and treatment in Saudi Arabia.
Peer Review reports
Literature highlights the significance of understanding stem cell therapy and research to support the development of regenerative medicine [ 1 , 2 , 3 ]. McLaren reported that there are millions of individuals suffering from and succumbing to “incurable degenerative diseases of the nervous system, heart, liver, pancreas, and other organs” annually [ 3 ]. Similarly, Lovell-Badge discussed the fact that stem cells offer great hope for patients with enervating illnesses such as “diabetes, Parkinson’s, and Huntington’s diseases” [ 4 ]. In this context, medical practitioners consider stem cells as the hope and light for many patients who are suffering and in dire need of a cure [ 1 ]. However, as indicated by several authors, the use of stem cells, present many ethical, political, and even religious challenges, either related to resources, use, or rights of donors [ 5 , 6 ]. Bharadwaj mentioned the increasing movements of social and government concerns regarding stem cell research and clinical medication in countries such as the United States, United Kingdom, and Japan. More recently, emerging countries also incorporated stem cell therapies in their practices [ 7 ].
Lo and Parham provided a classification of the different ethical issues based on the four phases of stem cell research. The first phase is the “donation of biological material,” highlighting the problem of “informed and voluntary consent” [ 2 ]. The second phase, research with human embryonic stem cells, creates several ethical issues. These issues include the “destruction of embryos and the creation of embryos for research purposes” as well as financial compensation to oocyte donors, medical hazards related to the retrieval of the oocyte, and the need to protect the reproductive interests of women undergoing infertility treatment [ 2 ]. The third phase of the research is using stem cell lines obtained from other institutions leading to the issue of adverse legal and ethical principles [ 2 ]. The fourth and last step is the use of stem cells in clinical trials, encompassing both the advantages and disadvantages of the trial and informed consent [ 2 ].
Stem cell research in Saudi Arabia
Many Arabic countries conduct research with stem cells, as evidenced by the hundreds of scientific papers published in this field. Saudi Arabia is ahead in stem cell research as many universities, such as King Saud University and King Faisal Specialized Hospital and Research Center, started stem cell research more than 20 years ago [ 8 ]. In addition, many other research institutions, established later, play a leading role in this field such as King Abdullah International Medical Research Center with a specialized stem cell research department, including a stem cell registry containing more than 10,000 donors and the Cord Blood Bank [ 9 ].
From an ethical perspective, Saudi Arabia was the first country in the region to have ethical regulations related to the use of and research with stem cells. The Research Ethics Law promulgated in 2010 and its implementing regulation in 2012 includes all ethical guidelines to control stem cell research [ 10 ]. This was followed in 2014 by Jordan where a specific law about stem cell is announced [ 11 ]. In addition to these national laws, many research centers have institutional guidelines, for example King Faisal Specialized Hospital and Research Center and King Abdullah International Medical Research Center.
Though there is an abundance of stem cell research, the ethical component has not been researched in depth. There is no literature related to the views of physicians and researchers about the ethical challenges of using stem cells. It is important to explore this important issue to enhance the ethical component and maintaining the progress of stem cell research through finding appropriate solutions of all ethical challenges and obstacles.
Research design
A qualitative research design was used to explore and describe the perceptions and experiences of participants regarding the ethical challenges of using stem cells in a “subjective and reflexive manner” [ 12 ]. The aim was to gather, explore, analyze, and extract the most meaningful perceptions of the sample using interviews. The qualitative approach was deemed appropriate for the study to emphasize the content of the data to explore a specific phenomenon.
Data collection
We collected data from professionals employed at tertiary hospitals from various regions in Saudi Arabia where stem cell research have been conducted or are being conducted. The target population included physicians from any medical specialty or non-physician researchers doing stem cell research. We visited the potential hospitals and presented the objectives and purpose of the study. We collected contact information of potential participants with the permission from the gatekeepers of the hospitals and other pertinent representatives. We used a snowball sampling technique, defined by Clark and Creswell as the sampling of individuals based on the recommendations and suggestions of others [ 12 ]. Once a participant agreed to participate, we actively inquired from the participant to identify other possible candidates for the study. The technique allowed us to recruit 25 participants. The demographic characteristics of the sample are displayed in Table 1 .
We conducted individual semi-structured interviews with open-ended questions and audiotaped the interviews. Before conducting the interviews, written informed consent was obtained. The consent form highlighted voluntary participation, no monetary reward or promise. The privacy and confidentiality clauses were explained thoroughly. The interviews were held in a private room at the preferred time and date of the participants. Each interview lasted between 40 and 60 min.
Data analysis
After completing the 25 interview transcripts, data analysis commenced. The analysis involved identifying, analyzing, and reporting the most frequent and meaningful patterns or themes [ 12 ]. The analysis followed Braun and Clarke’s six-step process [ 13 ]. First, we familiarized ourselves with the interviews and actively read and reread the transcripts and generated initial codes as the second step. In the third step, we searched for themes across the data and the initial codes were categorized in the themes. We were mindful of the three objectives and purposes of the study to identify the most important points and concepts. The fourth step entailed the constant review of the themes, with the original data or the interviews. In the fifth step, the themes were named. Finally, the last step is the creation of the report as presented in the next section. We used the NVivo12 by QSR software to assist in the management and systematic tabulation of the themes.
A thematic analysis was performed to search for the most significant and meaningful responses from the sample. The thematic analysis resulted in four themes to address the three key objectives of the study. From the participant perspective, some sources are forbidden due to ethical issues. In addition, researchers and professionals must obtain an informed consent at all times (following the IRB) and follow the international regulations regarding stem cell research. The participants expressed the need to clarify the purpose of the research and storage procedures. Table 2 displays the themes in response to the study objectives.
Theme I: an exploration of the sample’s views regarding forbidden and permitted sources of stem cells
The participants’ position regarding the sources of stem cells can be classified in two categories: permitted and forbidden resources. The majority felt that some sources of stem cells should be forbidden because they may lead to serious religious issues, but some sources were considered safe and acceptable.
Adult stem cells as a source was considered safe providing the extraction is done within the prescribed processes and guidelines. An interviewee said, “S ources which are like skin liver heart these are allowed… Adult stem cells- allowed.” A second participant added, “Adult stem cells [are] approved for clinical use.”
Pluripotent stem cells are becoming an acceptable resource of stem cells, due to their positive and safe characteristics. An interviewee shared that the use of pluripotent stem cells is continuously advancing with the hope of curing different diseases, saying: “ Why not? It’s a new science, and the people are trying to use pluripotent stem cells in another type of... a different kind of disease, and there is a lot of clinical intervention a lot of clinical trials still under investigation there is no clear answer.” Another participant indicated that pluripotent stem cells are similar to adult stem cells and can easily be replicated, saying: “Pluripotent stem cells it’s actually an adult cell you reprogramed the genetic and you move it back so you can do anything with it.”
According to our interviewees, the umbilical cord is a safe and promising stem cell source. A participant commented, “umbilical cord is one of the best sources of stem cells.”
The participants also indicated that the placenta is a permitted source, and they experienced no issues as the placenta was used previously to extract stem cells. A participant stated that using the placenta is not harmful, saying, “We used to collect stem cells from the placenta. It is not invasive.”
Obtaining stem cells from fetuses were perceived differently. One group clearly and completely forbids any use of stem cells from any fetus, either intentionally aborted or accidently miscarried, regardless of the age of fetus. One of the interviewees responded that the use is strictly prohibited, stating, “This is forbidden” and a second indicated clearly and strongly, “Miscarried fetuses before reaching [120 days] the same, the same, forbidden.” This group of professionals stated that the aborted fetus is rejected as the institutions and stakeholders are aware that the use of such a source may lead to ethical dilemmas. As one of the participants expressed, “there may be ethical issues that go along with the use of an aborted fetus.” In addition, the use of embryonic stem cells may lead to more serious and critical religious issues. A participant stated, “That they must adhere to the religious teaching that one must not touch or alter the fetus.”
However, some of the participant accepted fetuses aborted for therapeutic reasons, but forbid stem cells obtained from fetuses aborted for non-therapeutic reasons. An interviewee said, “However, these source of stem cells that we’re using is probably less chaotic, and hence should be utilized or the regulations should be applied like any other biological materials.” Spontaneously miscarried fetuses can be accepted as a resource of stem cells, if they are less than 120 days of age. One interviewee said, “If the fetus is less than 120 days old, it is not considered a human, and we can use its stem cells.”
Theme II: an exploration of professionals’ opinions regarding the ethical challenges of securing informed consent, with IRB approval
The second thematic category explored the sample’s perceptions concerning the challenges related to obtaining informed consent for stem cell research. The participants emphasized the importance of informed consent to guarantee the voluntary participation of donors. One of the interviewees said, “We do have consent actually, we never collect stem cells without taking a consent from the patient. Sure, sure yeah, we take the permission before we start collecting the cells.” However, for umbilical cord blood, consent is obtained from parents, usually during the routine visits to clinics during the pregnancy, as expressed by one interviewee.
According to the participants, the consent should explain and clearly describe the purpose of the research. A participant said: “I think that the donor should be informed what exactly we are doing with tissue that he has that we take it from him.” Another said, “The scope of the research should be properly presented. Such practice will protect both parties, the researcher and donor, from future issues.”
All donor rights should be mentioned in the informed consent. One of the interviewees said, “Donors’ rights musts be clarified and explained to them, including, but not limited to, withdrawal right.”
The explanation should be in understandable, clear language and the terms and conditions of the forms should be simplified. The communication must be sufficient to ensure the donor understand fully. A participant narrated, “The researchers must take the time to orient and explain the content of the informed consent to the volunteers.” The informed consent documents should have been reviewed and approved by an ethics committee. A participant discussed the process of procuring the form, as follows: We submit the consent to the research office as a part of the submitted research proposal and then you will get IRB approval for all proposals including the informed consent.”
Theme III: an exploration of the professionals’ perceptions regarding the ethical challenges related to the benefit resulting from the stem cell research
The participants described the potential value of the sources of stem cells in the field of medicine and research. According to the majority, any use of stem cells should be beneficial, either to the donors or to the public. A participant said, “Even if there is no direct benefit to the donors themselves, but at least stem cells research should have some potential benefits to others.” A second opinion was “There are different applications and uses of the umbilical cord and it can save many patients today.” When using stem cells in treatment, the approved procedures should be followed meticulously, as explained by one of the interviewees, “Not following approved methods may lead to serious consequences.”
The sample emphasized the responsibility of being transparent when informing and communicating with the patient about any potential benefit. “It is very important not give the patients false hope about treatment by stem cells,” as expressed by one of the interviewees. A second participant was concerned about false hopes based on wrong assumptions, “I am very sad to see hopeless people spend all their earnings and energy in trying something that can never be a success.”
Theme IV: an exploration of stem cell research regulations
Four subthemes were developed related to the regulations related to stem cell research theme, including the importance of following international regulations, the need to use international guidelines based on Islamic laws (fatwas) and beliefs, no national law related to stem cells, and the need to increase the researchers’ knowledge about the ethical guidelines of stem cells.
The majority of the sample considered following regulations and guidelines consistently every time stem cells are used as an important issue. A participant explained, “ We have to follow the set procedures by regulators and the law because it involves safety of patients.” Our findings indicate that our researchers are using international regulations in their current practice and research with stem cells. One researcher said, “ Actually we are already use international guidelines. We used to follow that when I was getting my training in the west, and we here continue do the same.” Another researcher justified why international regulations should be followed, “Following the international regulations on stem cell research is needed for two reasons: the ethical principles are the same, and we are in many cases part of international multicenter research.” However, when applying these international regulations, Islamic law and fatwas should be taken in account. One of the participants explained, “Nothing that contradicts Sariah is acceptable, and this is true when it comes to stem cells, especially when it comes to the permitted or forbidden resources.”
The participants also indicated a lack of standardization in the local setting. A participant narrated that currently, they follow only the international regulations for their clinical trials and research. The participant described the increasing need for a targeted local policy related to stem cells, saying: “There is no national standardization as far as I know.” According to a second participant, the main issue in Saudi is the actual lack of a national law related to stem cell research and therapy, he commented, “Ethics must be a priority in Saudi to be able to create laws that would be in line with the local religious or spiritual beliefs.” Another participant also expressed the need to create local guidelines, which should match the international guidelines and not contradict the Islamic law. A last perspective was as follows: “Definitely, it’s good to have a supporting in fatwa for our patients satisfaction. This is because patients and the community rely on the fatwa more than the IRB. They don’t know about the IRB. So, I think that’s why the need the fatwa. I think we would reassure our patients about that.”
Many participants admitted that researchers lack adequate knowledge and information regarding ethical considerations and guidelines about stem cells research. One of the participants said, “The researchers themselves need to be trained or oriented in a formal setting to become aware about the ethical guidelines of using stem cells. ”
Though researchers are doing research in the stem cell field, they realize the ethical challenges they are facing in their research. Having spent a significant part of their scientific life in Western countries, they are aware of ethical issues; however at the same time, their cultural and religious background plays a role in their perceptions regarding the ethical challenges and how to deal with them.
The first point to manage appropriately is the source of stem cells, classified in permitted and forbidden sources. While the sample accepts adult stem cells in general, they have a different point of view regarding embryonic stem cells. These stem cells are affected by ethical, legal, and religious considerations, especially regarding the method of obtaining the cells and more specifically, when it results in destroying embryos who may have a degree of dignity and humanity, similar to other researchers, societies and universities in the west [ 14 ]. A particular concern is if the fetus is more than 120 days old, the time of soul installment according to Islamic law [ 15 ], which is in the middle between the two opposing opinions: the first sees embryos less than complete and conscious persons, while the second sees them equal to all human beings and should not be treated differently. The sensitivity related to using stem cells from embryonic sources resulted in a significant increase in the interest of adult stem cells in medical research, even though the lesser importance they have.
The researchers’ points of view about permitted and forbidden sources, as stated in the Saudi law of ethics of research on living creatures and its implementing regulations [ 10 ], match almost completely except for limiting the use of pluripotent stem cells to laboratories only. The researchers did not mention extra fetuses (extra fertilized eggs) which, as prescribed in the Saudi law, are not permitted as a source of stem cells. The reason may be because it is neither a common practice nor legal to use this source. Researchers where satisfied with the available sources, namely cord blood and imported cell lines.
The source of stem cells was not the only point discussed by the sample. They highlighted several factors to be considered to ensure stem cell research is ethical. The points are a component of the general rules related to conducting ethical medical research, locally and internationally [ 16 ]. It was expected that the sample would mention obtaining informed consent prior to any stem cell donation, adult or embryonic, before use. Informed consent should also be obtained from donors of adult stem cells. However, for embryonic stem cells, consent should be obtained from the parents. It is noteworthy that the researchers highlighted the importance of clarifying the purpose of stem cell donation to the potential donors to avoid any possibility of employing practices that may invalidate the consent. Mandating review and approval of an ethics committee of the informed consent form protect the donors who may miss understanding some points in the informed consent documents. Although there is no direct benefit to the donors from stem cell research, the altruism principle is an important motivation to donate stem cells, which is supported by studies in other regions [ 17 ]. The participants mentioned the importance that research should not be futile but have a direct or indirect benefit. The researchers recognize that stem cells have the potential of future success and many people, especially patients with chronic diseases and difficult to treat diseases, have placed their hope on stem cells. So, it is understandable that the sample mentioned repeatedly that patients should be warned against false hope and they emphasized the importance of transparency in stem cell treatment or research; the idea that is highlighted by other researchers [ 18 ].
The awareness of researchers about the importance of respecting and complying to international guidelines can be understood in the context of receiving tertiary education abroad where they internalized the international guidelines and conducted research according to these guidelines. Frequently, the current research in Saudi Arabia is a continuation of their previous research during their training. The second reason which explains the importance of following international guidelines is that the majority of research is multi-center international studies and following the same principles is essential for success.
The harmonization of ethical and legal rules related to stem cell research with the Islamic point of view is important due to two reasons. Firstly, the acceptance or willing participation of potential donors will significantly increase if they are informed that the research is in line with Islamic law, and secondly, the Research Ethics Law in Saudi Arabia mandates that all practices in stem cell research should be in line with Islamic rules to be allowed and legitimate. However, the sample where not sufficiently aware of the regulation related to stem cell research mentioned in the Saudi Law of Research Ethics [ 10 ]. This caveat reflects a lack of responsibility about keeping themselves updated, as the law is readily available on the website of the National Committee of Bioethics www.kacst.edu.sa . From another perspective, the offices responsible in the National Committee of Bioethics should promote the law efficiently to raise awareness in researchers and donors.
In conclusion, the participants of the study indicate various ethical challenges regarding the use of stem cells in research. For the majority of the participants, specific stem cell sources are forbidden in Saudi. Particularly, embryonic stem cells as the use may result in serious religious issues. The participants also reject aborted or (some) miscarried fetuses as a source. In response to the second objective, the ethical principles and challenges related to stem cell research were identified. The sample emphasized the importance of always securing IRB approval of the informed consent documents. Informed consent should include an explanation of the scope of the research and the participants’ rights, in simple understandable language to ensure complete understanding.
The majority of the participants reported that they already follow the international regulations related to stem cell research, which they had been exposed to during their studies and training, mostly in Western countries. However, surprisingly, they are not necessarily aware of existing national local laws, which reflects a critical need of research ethics education in general and in stem cell ethics in particular, through courses, conferences, and university programs-which are currently lacking in Saudi. Also, conducting analytic and comparative studies about stem cells in Saudi research ethics law may help to increase awareness among researchers. Additional in-depth research to include different categories with different levels will be very important at the next stage.
Availability of data and materials
The datasets generated during the study are available from the corresponding author on reasonable request.
Bongso A, Richards M. History and perspective of stem cell research. Best Pract Res Clin Obstet Gynaecol. 2004;18(6):827–42.
Article Google Scholar
Lo B, Parham L. Ethical issues in stem cell research. Endocr Rev. 2009;30(3):204–13.
McLaren A. Ethical and social considerations of stem cell research. Nature. 2001;414(6859):129–31.
Lovell-Badge R. The future for stem cell research. Nature. 2001;414(6859):88–91.
Aksoy S. Making regulations and drawing up legislation in Islamic countries under conditions of uncertainty, with special reference to embryonic stem cell research. J Med Ethics. 2005;31(7):399–403.
Kimmelman J, Hyun I, Benvenisty N, Caulfield T, Heslop HE, Murry CE, Sipp D, Studer L, Sugarman J, Daley GQ. Policy: global standards for stem-cell research. Nature. 2016;533(7603):311–3.
Bharadwaj A. Stem cell intersections: perspectives and experiences. In: Global perspectives on stem cell technologies. Cham: Palgrave Macmillan; 2018. p. 1–24.
Chapter Google Scholar
Al Douri M, Wahdan M, Al Hilali A, Jeha MT, Zwaan F, Van Dijken P, Batniji F, Qasim M, Al Anazi K, Al Saghair F, Shafi T. The experience of bone marrow transplantation at Riyadh armed forces hospital. Saudi J Kidney Dis Transpl. 1996;7(2):199.
Google Scholar
Fakhoury HA, Alaskar AS, Hajeer AH. A novel HLA-DQ allele, HLA-DQB1* 05: 48, found in the Saudi stem cells donor registry. Tissue Antigens. 2015;86(3):218.
Doe J. Title of subordinate document. In: The dictionary of substances and their effects: Royal Society of Chemistry; 1999. http://www.rsc.org/dose/title/of/subordinate/document . Accessed 15 Jan 1999.
Regulations of the Law of Ethics of Research on Living Creatures. National Committee of BioEthics. 2016. https://prod.kau.edu.sa/Med/ali/files/Publications/Guide/National_Committe_of_BioEthics- Regulations_of_the_Law_of_Ethics_of_Research_on_Living_Creatures.pdf . Accessed 14 Mar 2020.
Jordanian Stem cell law. 2014. https://site.eastlaws.com/GeneralSearch/Home/ArticlesTDetails?MasterID=1759114&MasterID=1759114 . Accessed 16 Mar 2020.
Clark VL, Creswell JW. Understanding research: a consumer's guide. Pearson Higher Ed; 2014.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
Klimanskaya I, Chung Y, Becker S, Lu SJ, Lanza R. Human embryonic stem cell lines derived from single blastomeres. Nature. 2006;444(7118):481–5.
Salihu HM. Genetic counselling among Muslims: questions remain unanswered. Lancet. 1997;350(9083):1035–6.
World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ. 2001;79(4):373.
Bergstrom TC, Garratt RJ, Sheehan-Connor D. One chance in a million: altruism and the bone marrow registry. Am Econ Rev. 2009;99(4):1309–34.
Download references
Acknowledgements
We would like to thank the experts involved in stem cell research who agreed to participate in our study.
This study was funded by King Abdullah International Medical Research Center. There was no role of the funding body in the study design, collection, analysis, interpretation of data and in the manuscript writing.
Author information
Authors and affiliations.
King Abdullah International Medical Research Center (KAIMRC), King Saud Bin Abdulaziz University for Health Sciences, King Abdulaziz Medical City, Ministry of National Guard - Health Affairs, P.O. Box 22490, Mail Code 1515, Riyadh, 11426, Saudi Arabia
Ghiath Alahmad, Sarah Aljohani & Muath Fahmi Najjar
You can also search for this author in PubMed Google Scholar
Contributions
(GA) designed, directed, analyzed and interpreted the study interviewee data and he was the major contributor in writing the manuscript. (MN) carried out the interviews with study subjects and he helped in drafting the manuscript. (SA) helped in interviewing, drafting, and reviewing the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Ghiath Alahmad .
Ethics declarations
Ethics approval and consent to participate.
The study has been approved by the Institutional Review Board (IRB) at king Abdulaziz medical city (KAMC) under the grant number: RC16/188/R. The written informed consent has been obtained from the interviewed professionals before conducting the interviews.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Alahmad, G., Aljohani, S. & Najjar, M.F. Ethical challenges regarding the use of stem cells: interviews with researchers from Saudi Arabia. BMC Med Ethics 21 , 35 (2020). https://doi.org/10.1186/s12910-020-00482-6
Download citation
Received : 24 March 2020
Accepted : 05 May 2020
Published : 12 May 2020
DOI : https://doi.org/10.1186/s12910-020-00482-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Qualitative
- Informed consent
BMC Medical Ethics
ISSN: 1472-6939
- General enquiries: [email protected]
- Search Menu
- Sign in through your institution
- Advance articles
- Mini-reviews
- ESHRE Pages
- Editor's Choice
- Supplements
- Author Guidelines
- Submission Site
- Reasons to Publish
- Open Access
- Advertising and Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- Journals Career Network
- About Human Reproduction
- About the European Society of Human Reproduction and Embryology
- Editorial Board
- Self-Archiving Policy
- Dispatch Dates
- Contact ESHRE
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Introduction, what are (embryonic) stem cells, potential applications of hes cells and state‐of‐the‐art, ethical exploration, the status of hes cells, instrumental use of embryos, ethics of using surplus ivf embryos as a source of hes cells, therapeutic cloning, conclusions and recommendations, acknowledgements.
- < Previous
Human embryonic stem cells: research, ethics and policy
- Article contents
- Figures & tables
- Supplementary Data
Guido de Wert, Christine Mummery, Human embryonic stem cells: research, ethics and policy, Human Reproduction , Volume 18, Issue 4, April 2003, Pages 672–682, https://doi.org/10.1093/humrep/deg143
- Permissions Icon Permissions
The use of human embryos for research on embryonic stem (ES) cells is currently high on the ethical and political agenda in many countries. Despite the potential benefit of using human ES cells in the treatment of disease, their use remains controversial because of their derivation from early embryos. Here, we address some of the ethical issues surrounding the use of human embryos and human ES cells in the context of state‐of‐the‐art research on the development of stem cell based transplantation therapy.
Human embryonic stem cells (hES cells) are currently discussed not only by the biologists by whom they were discovered but also by the medical profession, media, ethicists, governments and politicians. There are several reasons for this. On the one hand, these ‘super cells’ have a major clinical potential in tissue repair, with their proponents believing that they represent the future relief or cure of a wide range of common disabilities; replacement of defective cells in a patient by transplantation of hES cell‐derived equivalents would restore normal function. On the other hand, the use of hES cells is highly controversial because they are derived from human pre‐implantation embryos. To date, most embryos used for the establishment of hES cell lines have been spare embryos from IVF, but the creation of embryos specifically for deriving hES cells is also under discussion. The most controversial variant of this is the transfer of a somatic cell‐nucleus from a patient to an enucleated oocyte (unfertilized egg) in order to produce hES cells genetically identical to that patient for ‘autologous’ transplantation (so‐called ‘therapeutic’ cloning); this may prevent tissue rejection.
The question ‘Can these cells be isolated and used and, if so, under what conditions and restrictions’ is presently high on the political and ethical agenda, with policies and legislation being formulated in many countries to regulate their derivation. The UK has been the first to pass a law governing the use of human embryos for stem cell research. The European Science Foundation has established a committee to make an inventory of the positions taken by governments of countries within Europe on this issue ( European Science Foundation, 2001 ).
In order to discuss the moral aspects of the isolation and use of hES cells, which is the aim of the present article, it is first essential to understand exactly what these cells are, where they come from, their intended applications and to define the ethical questions to be addressed.
‘Stem cells’ are primitive cells with the capacity to divide and give rise to more identical stem cells or to specialize and form specific cells of somatic tissues. Broadly speaking, two types of stem cell can be distinguished: embryonic stem (ES) cells which can only be derived from pre‐implantation embryos and have a proven ability to form cells of all tissues of the adult organism (termed ‘pluripotent’), and ‘adult’ stem cells, which are found in a variety of tissues in the fetus and after birth and are, under normal conditions, more specialized (‘multipotent’) with an important function in tissue replacement and repair.
hES cells are derived from the so‐called ‘inner cell mass’ of blastocyst stage embryos that develop in culture within 5 days of fertilization of the oocyte ( Thomson et al ., 1998 ; Reubinoff et al ., 2000 ). Although hES cells can form all somatic tissues, they cannot form all of the other ‘extraembryonic’ tissues necessary for complete development, such as the placenta and membranes, so that they cannot give rise to a complete new individual. They are therefore distinct from the ‘totipotent’ fertilized oocyte and blastomere cells deriving from the first cleavage divisions. hES cells are also immortal, expressing high levels of a gene called telomerase, the protein product of which ensures that the telomere ends of the chromosomes are retained at each cell division and the cells do not undergo senescence. The only other cells with proven pluripotency similar to that of ES cells are embryonic germ (EG) cells, which as their name implies, have been derived from ‘primordial germ cells’ that would ultimately form the gametes if the fetus had not been aborted. In humans, hEG cells were first established in culture in 1998, shortly after the first hES cells, from tissue derived from an aborted fetus ( Shamblott et al ., 1998 ). Biologically, hEG cells have many properties in common with hES cells ( Shamblott et al ., 2001 ).
In the adult individual, a variety of tissues have also been found to harbour stem cell populations. Examples include the brain, skeletal muscle, bone marrow and umbilical cord blood, although the heart, by contrast, contains no stem cells after birth (reviewed in McKay 1997 ; Fuchs and Segre, 2000 ; Watt and Hogan, 2000 ; Weissman et al ., 2000 ; Blau et al ., 2001 ; Spradling et al ., 2001 ). These adult stem cells have generally been regarded as having the capacity to form only the cell types of the organ in which they are found, but recently they have been shown to exhibit an unexpected versatility ( Ferrari et al ., 1998 ; Bjornson et al ., 1999 ; Petersen et al ., 1999 ; Pittenger et al ., 1999 ; Brazelton et al ., 2000 ; Clarke et al ., 2000 ; Galli et al ., 2000 ; Lagasse et al ., 2000 ; Mezey et al ., 2000 ; Sanchez‐Ramos et al ., 2000 ; Anderson et al ., 2001 ; Jackson et al ., 2001 ; Orlic et al ., 2001 ). Evidence is strongest in animal experiments, but is increasing in humans, that adult stem cells originating in one germ layer can form a variety of other derivatives of the same germ layer (e.g. bone marrow‐to‐muscle within the mesodermal lineage), as well as transdifferentiate to derivatives of other germ layers (e.g. bone marrow‐to‐brain between the mesodermal and ectodermal lineages). To what extent transdifferentiated cells are immortal or acquire appropriate function in host tissue remains largely to be established but advances in this area are rapid, particularly for multipotent adult progenitor cells (MAPCs) of bone marrow ( Reyes and Verfaillie, 2001 ). Answers to these questions with respect to MAPCs, in particular whether they represent biological equivalents to hES and can likewise be expanded indefinitely whilst retaining their differentiation potential, are currently being addressed ( Jiang et al . 2002 ; Schwartz et al ., 2002 ; Verfaillie, 2002 ; Zhao et al ., 2002 ). For other adult stem cell types, such as those from brain, skin or intestine ( Fuchs and Segre, 2000 ), this may remain unclear for the immediate future. Although the discussion here concerns hES cells and the use of embryos, the scientific state‐of‐the‐art on other types of stem cell is important in the context of the ‘subsidiarity principle’ (see below).
In theory, hES cells could be used for many different purposes ( Keller and Snodgrass, 1999 ). Examples in fundamental research on early human development are the causes of early pregnancy loss, aspects of embryonic ageing and the failure of pregnancy in older women (where genetic defects in the oocyte appear to be important). A second category might be toxicology, more specifically research on possible toxic effects of new drugs on early embryonic cells which are often more sensitive than adult cells (drug screening). The most important potential use of hES cells is, however, clinically in transplantation medicine, where they could be used to develop cell replacement therapies. This, according to most researchers in the field represents the real ‘home run’ and it is the ethics of using embryos in this aspect of medicine that will be discussed here. Examples of diseases caused by the loss, or loss of function, of only one or a limited number of cell types and which could benefit from hES cell‐based therapies include diabetes, Parkinson’s disease, stroke, arthritis, multiple sclerosis, heart failure and spinal cord lesions. Although it is known that hES cells are capable of generating neural, cardiac, skeletal muscle, pancreas and liver cells in teratocarcinomas in vivo in immunodeficient mice as well as in tissue culture, it would be an illusion to consider that cell‐therapies will have widespread application in the short term (i.e. within a couple of years). It is unfortunate that sensational treatment in the media, which implied the generation of whole organs from hES cells, initially left this impression so that the more realistic view emerging is already a disappointment to some patient groups. Nonetheless, a proper scientific evaluation of the therapeutic potential is being carried out in countries that allow the isolation and/or use of existing hES cells. The ethical questions here then also include whether the establishment of new hES cell lines can be justified, in the realisation that eventual therapies, based on either hES or adult stem cells are long‐term perspectives.
There are, at least in theory, various sources of hES cells. In most cases to date, these have been spare IVF embryos, although IVF embryos have been specifically created for the purpose of stem cell isolation ( Lanzendorf et al ., 2001 ). In one variant of ‘embryo creation’, it has even been reported that normally organized blastocysts develop from chimeras of two morphologically non‐viable embryos ( Alikani and Willadsen, 2002 ). The most revolutionary option would be the creation of embryos specifically for the purpose of isolating stem cells via ‘nuclear transfer’ (‘therapeutic cloning’). This option is purported to be the optimal medical use of hES technology since the nuclear DNA of the cells is derived from a somatic cell of a patient to receive the transplant, reducing the chances of tissue rejection (see Barrientos et al ., 1998 ; 2000). It is of note that the oocyte in this case is not fertilized, but receives maternal and paternal genomes from the donor cell nucleus. Since by some definitions an embryo is the result of fertilization of an oocyte by sperm, there is no absolute consensus that nuclear transfer gives rise to an embryo (see below).
The establishment of embryonic cell lines is becoming increasingly efficient, with up to 50% of spare IVF embryos that develop into blastocysts after thawing at the 8‐cell stage reported to yield cell lines. There are reports of efficiencies much lower than 50%, however, the quality of the donated embryos being an important determinant of success. Growth of the cell lines over extended periods and in some cases under defined conditions ( Xu et al ., 2001 ) has also been reported, but the controlled expansion and differentiation to specific cell types is an area where considerable research will be required before cell transplantation becomes clinical practice (for review, see Passier and Mummery, 2003 ). In addition, research will be required on how to deliver cells to the appropriate site in the patient to ensure that they survive, integrate in the host tissue and adopt appropriate function. These are the current scientific challenges that will have to be overcome before cell therapy becomes clinical practice; the problems are common to both hES and adult stem cells. The efficiency of establishing embryonic stem cell lines from nuclear transfer embryos is currently unknown, but expected to be lower than from IVF embryos.
In the following section, the status of hES cells is first considered. The questions of whether it is acceptable to use pre‐implantation embryos as a source of ES cells for research on cell transplantation therapy and if so, whether embryo use should be limited to spare embryos or may also include the creation of embryos via nuclear transfer (‘therapeutic cloning’), are then addressed.
What is the ontological status of hES cells? Should they be considered equivalent to embryos or not? Let us first consider the status of the ‘naked’, isolated inner cell mass (ICM; the source for deriving hES cell lines). The ICM is as it were the ‘essence’ of the pre‐implantation embryo, the precursor of the ‘embryo proper’. The isolated ICM, however, no longer has the potential to develop into a fetus and child, as trophoblast cells, necessary for implantation and nourishment of the embryo, and extra‐embryonic endoderm, are absent. It does not necessarily follow, though, that the isolated ICM is no longer an embryo—we suggest that the whole, isolated ICM could best be qualified as a disabled, ‘non‐viable’ embryo (even though it might, at least in theory, be ‘rescued’ by enveloping the ICM with sufficient trophoblast cells).
What, then, is the status of the individual cells from the ICM once isolated, and the embryonic stem cell lines derived from them? Should we consider these cells/cell lines to be non‐viable embryos too? We would argue that when the cells of the ICM begin to spread and grow in culture, the ICM disintegrates and the non‐viable embryo perishes. Some might argue that hES cells are embryos, because, although hES cells in themselves cannot develop into a human being, they might if they were ‘built into’ a cellular background able to make extra‐embryonic tissues necessary for implantation and nutrition of the embryo. At present this is only possible by ‘embryo reconstruction’ in which the ICM of an existing embryo is replaced by ES cells ( Nagy et al ., 1993 ). Commentators who, against this background, regard hES cells as equivalent to embryos, apparently take recourse to the opinion that any cell from which a human being could in principle be created, even when high technology (micromanipulation) would be required to achieve this, should be regarded as an embryo. An absurd implication of this ‘inclusive’ definition of an embryo is that one should then also regard all somatic cells as equivalent to embryos—after all, a somatic nucleus may become an embryo after nuclear transplantation in an enucleated oocyte. It is therefore unreasonable to regard hES cells as equivalent to embryos.
Research into the development of cell‐replacement therapy requires the instrumental use of pre‐implantation embryos from which hES cells are derived since current technology requires lysis of the trophectoderm and culture of the ICM; the embryo disintegrates and is thus destroyed. As has already been discussed extensively in the embryo‐research debate, considerable differences of opinion exist with regard to the ontological and moral status of the pre‐implantation embryo ( Hursthouse, 1987 ). On one side of the spectrum are the ‘conceptionalist’ view (‘the embryo is a person’) and the ‘strong’ version of the potentiality‐argument (‘because of the potential of the embryo to develop into a person, it ought to be considered as a person’). On the other side of the spectrum we find the view that the embryo (and even the fetus) as a ‘non‐person’ ought not to be attributed any moral status at all. Between these extremes are various intermediates. Here, there is a kind of ‘overlapping consensus’: the embryo has a real, but relatively low moral value. The most important arguments are the moderate version of the potentiality argument (‘the embryo deserves some protection because of its potential to become a person’) and the argument concerning the symbolic value of the embryo (the embryo deserves to be treated with respect because it represents the beginning of human life). Differences of opinion exist on the weight of these arguments (how much protection does the embryo deserve?) and their extent (do they apply to pre‐implantation embryos?). In view of the fact that up to 14 days of development, before the primitive streak develops and three germ layers appear, embryos can split and give rise to twins or two embryos may fuse into one, it may reasonably be argued that at these early stages there is in principle no ontological individuality; this limits the moral value of an embryo.
Pre‐implantation embryos are generally regarded from the ethical point of view as representing a single class, whereas in fact ∼50–60% of these embryos are aneuploid and mostly non‐viable. For non‐viable embryos, the argument of potentiality does not of course apply. Their moral status is thus only based on their symbolic value, which is already low in ‘pre‐individualized’ pre‐implantation embryos. The precise implications of this moral difference for the regulation of the instrumental use of embryos is, however, beyond the scope of the present article.
The view that research with pre‐implantation embryos should be categorically forbidden is based on shaky premises and would be difficult to reconcile with the wide social acceptance of contraceptive intrauterine devices. The dominant view in ethics is that the instrumental use of pre‐implantation embryos, in the light of their relative moral value, can be justified under certain conditions. The international debate focuses on defining these conditions.
Possible objections are connected to the principle of proportionality, the slippery slope argument, and the principle of subsidiarity.
Proportionality
It is generally agreed that research involving embryos should be related to an important goal, sometimes formulated as ‘an important health interest’ (the principle of proportionality). Opinions differ on how this should be interpreted and made operational. In a number of countries, research on pre‐implantation embryos is permitted provided it is related to human reproduction. Internationally, however, such a limitation is being increasingly regarded as too restrictive ( De Wert et al ., 2002 ). The isolation of hES cells for research into cell‐replacement therapies operates as a catalyst for this discussion. It is difficult to argue that research into hES cells is disproportional. If embryos may be used for research into the causes or treatment of infertility, then it is inconsistent to reject research into the possible treatment of serious invalidating diseases as being not sufficiently important. The British Nuffield Council on Bioethics ( Nuffield Council on Bioethics, 2000 ) also saw no reason for making a moral distinction between research into diagnostic methods or reproduction and research into potential cell therapies.
Even if one argued that there is a difference between the two types of research, research on cell therapy would, if anything, be more defensible than research on reproduction. One (in our opinion somewhat dubious) argument is to be found in McGee and Caplan (1999 ); here the suggestion is made that in using embryos for cell therapy, no embryos are actually sacrificed: ‘In the case of embryos already slated to be discarded after IVF, the use of stem cells may actually lend permanence to the embryo. Our point here is that the sacrifice of an early human embryo, whether it involves a human person or not, is not the same as the sacrifice of an adult because life of a 100‐cell embryo is contained in its cells nuclear DNA.’ In other words, the unique characteristic of an embryo is its DNA; by transplanting cells containing this DNA to a new individual, the DNA is preserved and the embryo therefore not sacrificed—a ‘win–win’ situation for both the embryo and cell transplant recipient. The implication is thus that the use of embryos for cell transplantation purposes is ethically preferable to disposing of them or using them in other (‘truly destructive’) types of research. This extreme genetic ‘reductionism’ is highly disputable and not convincing: the fact that embryos are actually sacrificed in research into cell therapy is masked. A second, more convincing, argument, that the instrumental use of embryos is in principle easier to justify for isolation of hES cells than, for example, research directed towards improving IVF, is that it has potentially far wider clinical implications. It therefore, unquestionably meets the proportionality requirement.
Slippery slope
The slippery slope argument can be considered as having two variants, one empirical and the other logical. The empirical version involves a prediction of the future: ‘Acceptance of practice X will inevitably lead to acceptance of (undesirable) practice Y. To prevent Y, X must be banned’. The logical version concerns the presumed logical implications resulting from the moral justification of X: ‘Justification of X automatically implies acceptance of (undesirable) practice Y’. In this context the problem often lies in the lack of precise definition of X: ‘The difficulty in making a conceptual distinction between X and Y that is sharp enough to justify X without at the same time justifying Y, is a reason to disallow X.’ Both versions of the argument play a role in the debate about the isolation of hES cells for research into cell replacement therapy. An example of the logical version is that acceptance of hES cells for the development of stem cell therapy for the treatment of serious disease automatically means there is no argument against acceptance of use, for example, for cosmetic rejuvenation (Nuffield Council on Bioethics, 2000). The main difficulty is, according to these critics, the ‘grey area’ between these two extremes. One answer to this objection is to consider each case individually rather than reject all cases out of hand. One could use the same objection for example against surgery, which can equally be used for serious as well as trivial treatments.
An example of the empirical version of the slippery slope argument is that the use of hES cells for the development of cell therapy would inevitably lead to applications in germ‐line gene therapy and in therapeutic cloning, then ultimately reproductive cloning. This version of the argument is unconvincing too; even if germ line gene therapy and therapeutic cloning would be categorically unacceptable, which is not self‐evident, it does not necessarily follow from this that the use of hES cells for cell‐therapy is unacceptable. The presumed automatism in the empirical version of the slippery slope argument is disputable.
Subsidiarity
A further condition for the instrumental use of embryos is that no suitable alternatives exist that may serve the same goals of the research. This is termed ‘the principle of subsidiarity’. Critics of the use of hES cells claim that at least three such alternatives exist, which have in common that they do not require the instrumental use of embryos: (i) xenotransplantation; (ii) human embryonic germ cells (hEG cells), and (iii) adult stem cells.
The question is not whether these possible alternatives require further research (this is, at least for the latter two, largely undisputed), but whether only these alternatives should be the subject of research. Is a moratorium for isolating hES cells required, or is it preferable to carry out research on the different options, including the use of hES cells, in parallel?
The answer to this question depends on how the principle of subsidiarity ought to be applied. Although the principle of subsidiarity is meant to express concern for the (albeit limited) moral value of the embryo, it is a sign of ethical one‐dimensionality to present every alternative, which does not use embryos, as a priori superior. For the comparative ethical analysis of hES cells from pre‐implantation embryos on the one hand, and the possible alternatives mentioned on the other, a number of relevant aspects should be taken into account. These include: the burdens and/or risks of the different options for the patient and his or her environment; the chance that the alternative options have the same (probably broad) applicability as hES cells from pre‐implantation embryos; and the time‐scale in which clinically useful applications are to be expected.
A basis for initiating a comparative ethical analysis is set out below:
(i) Xenotransplantation is viewed at present as carrying a risk, albeit limited, of cross‐species infections and an accompanying threat to public health. This risk is, at least for the time being, an ethical and safety threshold for clinical trials. Apart from that, the question may be raised from a perspective of animal ethics whether it is reasonable to breed and kill animals in order to produce transplants, when at the same time spare human embryos are available which would otherwise be discarded;
(ii) In principle, the use of hEG cells from primordial germ cells of dead fetuses seems from a moral perspective to be more acceptable than the instrumental use of living pre‐implantation embryos, provided that the decision to abort was not motivated by the use of fetal material for transplantation purposes. To date, however, hEG cells have been difficult to isolate and culture, with only one research group reporting success ( Shamblott et al ., 1998 ; 2001). In addition, research in mice suggests abnormal reprogramming of these cells in culture: chimeric mice generated between mouse (m)EG cells and pre‐implantation embryos develop abnormally while chimeras using mouse (m)ES cells develop as normally as non‐chimeric mice ( Steghaus‐Kovac, 1999 ; Surani, 2001 ). This makes the outcome of eventual clinical application of these cells difficult to predict in terms of health risks for the recipient.
(iii) Analysis of the developmental potential of adult stem cells is a rapidly evolving field of research, particularly in animal model systems. Experiments carried out within the last two years have demonstrated, for example, that bone marrow cells can give rise to nerve cells in mouse brain ( Mezey et al ., 2000 ), neural cells from mouse brain can turn into blood and muscle ( Bjornson et al ., 1999 ; Galli et al ., 2000 ), and even participate in the development of chimeric mouse embryos up to mid‐gestation ( Clarke et al ., 2000 ). Although apparently spectacular in demonstrating that neural stem cells from mice can form most cell types under the appropriate conditions, it is still unclear whether true plasticity in terms of function has been demonstrated or whether the cells simply ‘piggy‐back’ with normal cells during development. Published evidence of ‘plasticity’ in adult human stem cells is more limited, but recent evidence suggests that the MAPCs from bone marrow may represent a breakthrough ( Jiang et al ., 2002 ; Schwartz et al ., 2002 ;). They are accessible. Collection is relatively non‐destructive for surrounding tissue compared, for example, with the collection of neural stem cells from adult brain, although their numbers are low: 1 in 10 8 of these cells exhibit the ability to form populations of nerve, muscle and a number of other cell types and they only become evident after several months of careful culture. Clonal analysis has provided rigorous proof of plasticity: a single haematopoietic stem cell can populate a variety of tissues when injected into lethally irradiated mice ( Krause et al ., 2001 ) or into blastocyst stage embryos to generate chimeric embryos ( Jiang et al ., 2002 ). Nonetheless, there are potential hazards to using cells that have been cultured for long periods for transplantation and although MAPCs seem to have normal chromosomes, it is important to establish that the pathways governing cell proliferation are unperturbed. This is also true for hES cells. However, the powerful performance of mES cells in restoring function in a rat model for Parkinson’s disease ( Kim et al ., 2002 ), has not yet been matched by MAPCs. Bone marrow stem cells have been shown very recently to restore function to some extent in a mouse heart damaged by coronary ligation, an experiment that mimics the conditions of the human heart soon after infarction ( Orlic et al ., 2001 ). Although clinical restoration of function in a damaged organ is usually sought rather longer after the original injury than in these experiments, which were performed before scar tissue had formed, this approach will certainly be worth pursuing. An alternative, non‐invasive, haematopoietic stem cell source is umbilical cord blood. This is used clinically for transplantation as an alternative to bone marrow in patients for whom no bone marrow match is available. Cord blood contains precursors of a number of lineages but its pluripotency, or even multipotency, is far from proven. Nevertheless, the prospect of autologous transplantation of haematopoietic stem cells of bone marrow in the long term makes this an important research area in terms of alternatives to therapeutic cloning (see below).
Although studies with adult stem cells so far have been encouraging, Galli (2000 ), author of the first adult neural stem studies and much cited by advocates of the view that adult stem cells have a proven developmental potency equal to that of ES cells, himself disagrees entirely with this viewpoint (see Editorial, 2000 ). It has even been suggested that the results from adult stem cell research are being misinterpreted for political motives and ‘hints of the versatility of the adult cells have been over interpreted, overplayed and over hyped’ ( Vastag, 2001 ). Opponents of ES cell research are now heralding Verfaillie’s adult stem cells as proof that work on hES cells is no longer needed. However the stem cell research community and Verfaillie herself ( Vastag, 2002 ) have called for more research on both adult and embryonic stem cells. ES cells that can perform as powerfully as those described by Kim et al . (2002 ) in the rat Parkinson model make it far too early in the game for them to be discounted ( Editorial, 2002 ).
The question remains, however, should a moratorium be imposed on isolating hES cells for research in cell therapy in the light of the indisputably promising results from adult stem cell research? The lack of consensus arises largely from disagreement on interpretation of the subsidiarity principle. Against the restrictive viewpoint that research on hES cells may only take place if there is proof that adult stem cells are not optimally useful, there is the more permissive viewpoint that hES cell research may, and indeed should, take place so long it is unclear whether adult stem cells are complete or even partial alternatives.
On the basis of the following arguments, a less restrictive interpretation of the subsidiarity principle is morally justified. ( Stem Cell Research, 2000 ) To begin with, the most optimistic expectation is that only in the long run will adult stem cells prove to have equal plasticity and developmental potential as hES cells (and be as broadly applicable in the clinic), and there is a reasonable chance that this will never turn out to be the case. If hES cells from pre‐implantation embryos have more potential clinical applications in the short term, then the risk of a moratorium is that patients will be deprived of benefit. This in itself is a reason to forgo a moratorium—assuming that the health interests of patients overrule the relative moral value of pre‐implantation embryos. Secondly, the simultaneous development of different research strategies is preferable, considering that research on hES cells will probably contribute to speeding up and optimising clinical applications of adult stem cells. In particular, the stimuli to drive cells in particular directions of differentiation may be common to both cell types, while methods of delivery to damaged tissue are as likely to be common as complementary. A moratorium on hES cell research would remove the driving force behind adult stem cell research.
A final variant on adult stem cell sources concerns the use of embryonal carcinoma (EC) cells, a stem cell population found in tumours (teratocarcinomas) of young adult patients. These cells have properties very similar to hES cells. The results of a phase I (safety) trial using these cells in 11 stroke victims in the USA have recently been published and permission granted by the Food and Drug Administration (FDA) for a phase II trial (effectivity) ( Kondziolka et al ., 2000 ). The patients received neural cells derived from retinoic acid (vitamin A) treatment of teratocarcinoma stem cells. Although the scientific and ethical consensus is that these trials were premature in terms of potential risk of teratocarcinoma development at the transplant site, all patients survived with no obvious detrimental effects, no tumour formation and in two cases a small improvement in symptoms. After two years, the transplanted cells were still detectable by scanning ( Kondziolka et al ., 2000 ). Despite its controversial nature, this trial has nevertheless probably set a precedent for similar trials using neural derivatives of hES, the best controlled differentiation pathway of hES cells at the present time ( Reubinoff et al ., 2001 ; Zhang et al ., 2001 ). Proponents believe that such trials would be feasible even in the short term ( McKay, 1997 ). Neural differentiation of hEC cells is fairly easy to induce reproducibly but most other forms of differentiation are not; even if ultimately regarded as ‘safe’, hEC cells will not replace hES cells in terms of developmental potential and are therefore not regarded as an alternative.
In view of both the only relative moral value of pre‐implantation embryos and the uncertainties and risks of the potential alternative sources for the development of cell therapy, a moratorium for isolating human embryonic stem cells is unjustified.
Before discussing the ethical issues around ‘therapeutic cloning’, the term itself requires consideration. To avoid confusion, it has been proposed that the term ‘cloning’ be reserved for reproductive cloning and that ‘Nuclear transplantation to produce stem cells’ would be better terminology for therapeutic cloning ( NAS report, 2002 ; Vogelstein et al ., 2002 ). Others have pointed out the disadvantage of this alternative term, namely that it masks the fact that an embryo is created for instrumental use. More important in our opinion however, is that the use of the adverb ‘therapeutic’ suggests that hES cell therapy is already a reality: strictu sensu there can only be a question of therapeutic applications once clinical trials have started. In the phase before clinical trials, it is only reasonable to refer to research on nuclear transfer as ‘research cloning’ or ‘nuclear transplantation for fundamental scientific research’, aimed at future applications of therapeutic cloning.
Some consider this technology to be ethically neutral; they claim that the ‘construct’ produced is not a (pre‐implantation) embryo. Qualifications suggested for these constructs include: activated oocyte, ovasome, transnuclear oocyte cell, etc. ( Kiessling, 2001 ; Hansen, 2002 ) However, to restrict the definition of ‘embryo’ to the product of fertilization in the post‐Dolly era is a misleading anachronism. Although the purpose of therapeutic cloning is not the creation of a new individual and it is unlikely that the viability of the constructed product is equivalent to that of an embryo derived from sexual reproduction, it is not correct to say that an embryo has not been created.
The core of the problem is that here human embryos are created solely for instrumental use. Whether or not this can be morally justified—and if so, under what conditions—has already been an issue of debate for years in the context of the development of ‘assisted reproductive technologies’ (ART). Is it acceptable to create embryos for research, and if so, is therapeutic cloning morally acceptable too?
A preliminary question: is it justified to create embryos for research?
Article 18 of the European Convention on Human Rights and Biomedicine forbids the creation of embryos for all research purposes ( Council of Europe, 1996 ). However, this does not close the ethical and political debates in individual EU member states.
In the ‘classical’ normative debate on embryo research, two perspectives can be distinguished: a ‘fetalist’ perspective (focusing on the moral value of the embryo), and a ‘feminist’ perspective (with the interests of women, particularly candidate oocyte donors, playing a central role) ( Raymond, 1987 ). Both perspectives have a different outlook on the question of whether or not there is a decisive moral distinction between research with spare IVF embryos on the one hand, and creating embryos for research on the other. In other words: is the difference between these practices such that the former can be acceptable under specific conditions, and the latter absolutely not?
Fetalist perspective
Instrumentalization of the embryo is sometimes regarded as far greater and fundamentally different when it involves the creation of embryos for research purposes rather than the use of spare embryos. This difference, however, is just gradual. Not only is the embryo used completely instrumentally in both cases, the moral status is also identical. The difference is in the intention at fertilization, which, although a real difference, is relative. It is a misconception to think that in the context of regular IVF treatment every embryo is created as a ‘goal in itself’: the goal is the solution of involuntary childlessness and the loss of some embryos is a calculated risk beforehand.

Feminist perspective
From a feminist perspective, the creation of embryos for research should be evaluated critically in as far as it may require hormone treatment of a woman to obtain oocytes for research purposes: can this be morally justified when it requires unpleasant treatment of the donor with no benefit at all, or even a detrimental outcome, for her own state of health? A first objection is that women themselves become objects of instrumental use. Here, however, an analogy can be made with recruiting healthy research subjects. Relevant considerations concern whether or not the research serves an important goal, whether the burdens and risks to the subjects are proportional, and whether valid informed consent of the research subject/donor is given. The second objection is that the health risks to the women themselves are too high and the degree of discomfort disproportional. Difference of opinion exists, however, also among women, about the disproportionality of hormone treatment. There are, furthermore, several potential alternatives that do not require hormone treatment of healthy women. One involves the in‐vitro maturation (IVM) of immature oocytes after their isolation from dead donors or donors having ovaries removed for other reasons. IVM is successful in cattle and sheep (efficiency ∼40%), although it is, for the moment, much lower in humans.
In conclusion, from both a fetalist and a feminist perspective there is no overriding categorical objection against bringing pre‐implantation embryos into existence for instrumental use. If the research cannot be conducted using spare embryos and its importance for human health is beyond doubt, we believe the creation of embryos specifically for research is morally justified subject to the required oocytes being obtained in a morally sound way.
Ethics of therapeutic cloning
Can therapeutic cloning be morally acceptable? The principle of proportionality, the slippery slope, and the principle of subsidiarity enter the debate again, but in a slightly different way.
It is doubtful whether the principle of proportionality provides a convincing a‐priori objection against therapeutic cloning. If it is considered acceptable to create embryos for research aimed at improving ART (freezing of oocytes; IVM of oocytes, etc…), then it is inconsistent to reject therapeutic cloning beforehand as being disproportional. Maybe even some opponents of creating embryos for the improvement of ART can conditionally accept therapeutic cloning because of the important health interests of patients.
Slippery‐slope
A consequentialist objection (fashioned as a ‘slippery‐slope’ argument) is that therapeutic cloning will inevitably lead to reproductive cloning. This objection is not convincing; if reproductive cloning is categorically unacceptable (the debate on this issue is still ongoing), it is reasonable to prohibit this specific technology, and not to ban other, non‐reproductive, applications of cloning. A second objection that could be raised in this context is that the creation of embryos through cloning for the isolation of stem cells could in the long term be used to justify the initiation of pregnancy from these embryos and their use simply as a vehicle for generating sufficient cells of the required type for transplantation; the pregnancy would be interrupted the moment the appropriate developmental stage was reached ( Lanza et al ., 2002 ). Relevant questions here are: is this a realistic scenario in the human (or just science fiction), would it be unacceptable, and is it unavoidable?
In terms of being a realistic means of generating genetically identical (fetal) tissue for transplantation, it could theoretically be an option, but whether it would actually be useful would depend on the alternatives available at the time transplantation techniques themselves have been perfected to clinical applicability (see below).
In terms of moral acceptability, most people would consider pregnancy‐and‐abortion‐for‐transplantation to be far more difficult to justify than the creation of pre‐implantation embryos for instrumental use in vitro , firstly because of the higher moral status/symbolic value of the fetus, and secondly because of the significantly greater burden of pregnancy‐and‐abortion‐for‐transplantation for women. ( De Wert et al ., 2002 ) Even though many countries do forbid pregnancy‐for‐transplantation, it has been argued that it could be morally justified as a last resort, on the basis that sacrificing a fetus (a potential person) may be justified in order to rescue the life of a person.
Finally, in scrutinising the slippery slope argument, it is important to assess whether instrumental use of pre‐implantation embryos makes pregnancy‐for‐abortion unavoidable. Again, the apparent automatism is disputable: if we reject pregnancy‐for‐abortion as being unacceptable, we can continue its prohibition.
Taking these points for and against together, the slippery slope argument does not provide a convincing basis for banning therapeutic cloning.
Therapeutic cloning can only be morally acceptable if there are no good alternatives. It is important to note that therapeutic cloning strictu sensu is not likely to be short‐term prospect. Apart from unsolved technical difficulties with nuclear transfer itself in human oocytes ( Cibelli et al ., 2002 ), much basic research is still needed to determine whether the differentiation of hES cells can be controlled and sufficient cell numbers generated to be a useful therapy. This research can be done with spare IVF embryos. In this light, creation of embryos for therapeutic cloning is, in our opinion, premature. Although critics of this point of view could use our own argument that delay in the development of research cloning could, just as a moratorium on hES cell isolation and research, have negative consequences for patients, the evidence suggests that further optimization of the technology as such could take place in animals. We believe that the duration of any ‘delay’ in offering therapy to patients would not then be of real significance.
At the same time, research on potential alternatives for therapeutic cloning, which likewise avoid (or at least reduce) the problem of rejection but which do not involve the creation of human embryos for instrumental use, should be stimulated. For the comparative ethical analysis, it is again important to avoid the pitfall of one‐dimensionality. Possible alternative options include: (i) the use of adult cells, both stem cells and differentiated cells; (ii) making optimal use of spare embryos: embryo‐banks and immuno‐tolerance and (iii) the use of entities with an undetermined status: ‘hybrids’ and ‘parthenotes’.
Adult cells
Adult tissue is a potential source of two alternatives: stem cells, which may be induced to transdifferentiate by extracellular signals, and somatic cells (nuclei) which require direct reprogramming signals, for example from an oocyte after nuclear transfer, to adopt a new fate. Both sources will, however, require substantial research to become realistic alternatives. Until it has been shown that adult stem cells at some point re‐express ES cell markers we will never know if transdifferentiation or direct reprogramming are the same or not.
For direct reprogramming of somatic nuclei, new methods may be developed which do not require nuclear transfer to oocyte cytoplasm. Examples of current work in this area include the study of cellular hybrids derived from the fusion of (embryonic) stem cells with somatic or adult stem cells ( Surani, 2001 ; Terada et al ., 2002; Ying et al ., 2002 ). An understanding of the basic mechanisms underlying reprogramming is already being undertaken in mice, cattle and sheep and indeed, the creation of ‘Dolly’ re‐initiated a wave of research in nuclear reprogramming in mammals. The ultimate aim of this research in the context of cell transplantation therapy would be chemically‐induced nuclear re‐programming in the test‐tube to derive the required cell type, obviating the necessity for therapeutic cloning altogether. First evidence that this might be feasible demonstrated direct reprogramming of fibroblasts to neural cells and T‐cells in culture by temporary permeabilization of the fibroblasts to allow them to take up extracts of neural and T‐cells, respectively ( Hakelien et al ., 2002 ). In this sense, therapeutic cloning may be regarded, perhaps, as a temporary option; in the long term it will be replaced by a direct reprogramming alternative.
Research on direct reprogramming of adult somatic nuclei may ultimately require the creation of human embryos for instrumental use. In view of the importance of this research, both in terms of the contribution to the development of cell therapy and the potential ultimately to reduce the instrumental use of human embryos by developing an alternative for therapeutic cloning, this research would no doubt also meet the principle of proportionality.
Optimal use of spare embryos
Various strategies should be considered. Firstly, the generation of a bank of hES cell lines from a wide spectrum of genotypes is required to be able to offer a reasonable tissue match for every patient requiring a cellular transplant. Estimates of the number of independent cell lines that would actually be required for this vary greatly, from a few hundred to several thousand. Such a bank is already being discussed in the UK but could ultimately be established as a European resource. However, even very good tissue matches between donor and recipient require some degree of immunosuppressive therapy, which has long term negative side‐effects for patients, including increased risk of tumorigenesis
Secondly, there should be further development and application of ‘immunotolerance’ methodology. This may be particularly useful in combination with matching from an hES cell bank. The observation that patients receiving bone marrow transplants are more immunotolerant to other tissue transplantation from the same donor have led to the suggestion that immunotolerance may also be induced by initial injection of hES‐derived haematopoietic cells followed by the cell type of interest derived from the same hES cell line ( Kaufman et al ., 2001 ). The transplant may then be tolerated without being genetically identical, and lower doses or no immunosuppressives required. The combination of ‘near match’ with immunotolerance is probably a promising option.
For certain genetically based diseases, autologous transplantation may not always be appropriate since the transplanted tissue will bear the same genetic defect. Immunotolerance hES cell strategies may then be a particularly attractive or the only option. Should the success rates be very high, then attempts to create genetically identical transplantable tissue may become superfluous, not only for these, but for all patients. If, however, it works imperfectly or only for some patients, then therapeutic cloning may well remain an important option for the majority of all other patients.
Creating entities with an undefined status
Various alternative options raise classification problems, as the entities created to obtain cells have an undefined status. Firstly, transplanting the somatic nucleus of a patient into an enucleated animal oocyte. The logic behind this variant of therapeutic cloning is twofold: one, assuming that the ‘units’ thus created are not human embryos because only their nuclear but not mitochondrial DNA is human, advocates of this strategy argue that it circumvents the controversial issue of the instrumental use of human embryos. Two, a technical advantage of this approach would be that plenty of animal oocytes would be available; the feminist objection to creating human embryos for research would, of course, not apply.
It is not yet known whether this is a scientifically realistic option (whether hES cells can be effectively obtained following this approach). Animal research has so far been limited and not generally successful ( Barrientos et al ., 1998 ; 2001); polymorphic interspecies differences in mitochondrial DNA are thought to make such reconstructed zygotes non‐viable or prone to major developmental abnormalities. There are however, unvalidated reports of successful applications of the technique in China. The Donaldson Committee advocated a ban on this approach, but without any argumentation (Stem Cell Research, 2000). However, if this were a realistic option scientifically, then we believe that the issues involved deserve further ethical discussion. The major questions that should be addressed include: is the risk acceptable? As for xenotransplantation, there is also here the risk of cross‐species infection, although this may be extremely small, because the nuclear DNA of the animal, which may harbour viruses, is removed from the oocyte. Is it reasonable to argue that this ‘artificial combination’ should not be considered equivalent to a human embryo? Since the entire nuclear DNA is human, the reconstructed combination should, we think, be regarded as a human embryo. The procedure should thus not be presented as an ‘embryo saving’ variant of therapeutic cloning. However, only further in‐utero research with reconstructed animal embryos, for example embryos created by transplanting the somatic nucleus of a rat into an enucleated mouse oocyte, will provide a more definitive answer. Finally: in‐vitro research may well show that embryos obtained by transplanting a human somatic nucleus into an enucleated animal oocyte are non‐viable (like parthenotes, see below). The moral status of non‐viable pre‐implantation embryos, and more particularly, the question as to whether the conditions for research using non‐viable embryos may be more permissive than the conditions for using viable embryos, needs further debate (see earlier).
A second option may be the generation of parthenogenetic embryos for the isolation of hES cell lines. Here, an unfertilized (haploid) oocyte is treated chemically such that it becomes diploid, with two identical sets of the maternal chromosomes. These uniparental embryos are by definition gynogenetic and never result in viable offspring, because they fail to generate extra‐embryonic tissues. Nevertheless, in mice (see Boediono et al ., 1999 ) and in apes ( Cibelli et al ., 2002 ), parthenotes have been shown to develop to the blastocyst stage and yield cell lines with properties not distinguishable from ES cells derived from fertilized oocytes. However, in view of the fact that some genes are genomically imprinted, such that they are expressed only if inherited via the male germ line, ES cells derived from parthenotes may well be abnormal. First attempts at parthenogenesis in humans have not yielded hES cell lines ( Cibelli et al ., 2002 ). It is important to realise that such hES cell lines, if developed in humans, would only provide a tissue match for the oocyte donor, i.e. women of reproductive age. Although it has been speculated that two sets of male chromosomes could also be used in parthenotes, there is no evidence that this is a real option.
Cibelli and colleagues have referred to parthenogenesis as cloning. Whether this is correct depends on the timing of parthenogenesis: if initiated before the first complete meiotic division, then the procedure amounts to cloning (the same genotype as the female); if after the first meiotic division (ie recombination and loss of half) then it is not cloning. In this light, the experiments of Cibelli et al . (2002) would not qualify as cloning in the strict sense.
Some will certainly argue that the parthenote is not an embryo; parthenogenesis would then be classified as an ‘embryo‐saving’ strategy. As the parthenote undergoes the first divisions normally and is at these stages not distinguishable from embryos derived by normal fertilization, we would argue that it should be regarded as a non‐viable embryo. In the light of its non‐viability, the potentiality argument is not applicable. The moral status of parthenotes may therefore be regarded as very low, lower even than that of normal viable embryos at the same stage (see earlier). Thus, although not an ‘embryo‐saving alternative’, all other things being equal, parthenogenesis may be regarded as ethically preferable to the generation of viable embryos by fertilization or nuclear transfer (for instrumental use). In addressing the question of whether this research is premature given the current lack of proof that human ES cells are clinically useful as a source of transplantable cells, the lower moral status of parthenotes should be taken into account.
Regarding moral judgements as a ‘quasi stable equilibrium’ is particularly appropriate when applied to the ethics of isolating hES cells for research into cell replacement therapy. Stem cell research is highly dynamic, with many questions and ‘unknowns’. New insights into the effectiveness, risks and usefulness of the various alternatives may have immediate consequences for the ethical evaluation of the isolation of hES cells.
The status of the pre‐implantation embryo is the most sensitive and disputed point in the debate on isolation of hES cells for research. The dominant view in ethics, however, is that the moral status of the pre‐implantation embryo is relatively low and that the instrumental use of these embryos can be morally justified under some conditions.
The moral status of non‐viable pre‐implantation embryos is lower than the moral status of viable pre‐implantation embryos. The precise implications of this difference in moral status for the regulation of the instrumental use of embryos need further ethical scrutiny.
Both the principle of proportionality and a permissive interpretation of the principle of subsidiarity, make a moratorium on the isolation of hES cells unjustified.
Parallel research on alternatives is important and requires major support. Research on hES cells can provide an important impetus in this context.
The moral difference between research on surplus embryos and the creation of embryos for research is only gradual. A complete ban on creating embryos for instrumental use in research is morally unjustified.
A categorical ban on research on human therapeutic cloning is not justified, although the creation of embryos by cloning for the isolation of hES cells is, at the present time, premature. The necessary research can currently be carried out using animal embryos and surplus human IVF embryos.
Research into potential alternatives for therapeutic cloning, which does not require human embryos or which requires only the use of spare embryos, should be stimulated.
Banning the transplantation of a human somatic nucleus to an animal oocyte (as a variant of therapeutic cloning) is premature and morally unjustified.
The question whether therapeutic cloning should be allowed, becomes acute if research with spare embryos suggests that usable transplants can be obtained in vitro from hES cells and if the possible alternatives for therapeutic cloning are less promising or need more time for development than is currently expected. In that case, therapeutic cloning can be morally justified on the basis of both the principle of proportionality and the principle of subsidiarity.
We are grateful to Drs K.Lawson and J.Geraedts for comments on the manuscript.
Alikani, M. and Willadsen, S.M. ( 2002 ) Human blastocysts from aggregated mono‐nucleated cells of two or more non‐viable zygote‐derived embryos. Reprod. Biomed. Online , 5 , 56 –58.
Anderson, D.J., Gage, F.H. and Weissman, I.L. ( 2001 ) Can stem cells cross lineage boundaries? Nat. Med. , 7 , 393 –395.
Barrientos, A., Kenyon, L. and Moraes, C.T. ( 1998 ) Human xeno mitochondrial cybrids. Cellular models of mitochondrial complex I deficiency. J. Biol. Chem. , 273 , 14210 –14217.
Barrientos, A., Muller, S., Dey, R., Weinberg, J. and Moraes, C.T. ( 2000 ) Cytochrome c oxidase assembly in primates is sensitive to small evolutionary variations in amino acid sequence. Mol. Biol. Evol. , 17 , 1508 –1519.
Boediono, A., Suzuki, T., Li, L.Y. and Godke, RA. ( 1999 ). Offspring born from chimeras reconstructed from parthenogenetic and in vitro fertilized bovine embryos. Mol. Reprod. Dev. , 53 , 159 –170.
Bjornson, C.R., Rietze, R.L., Reynolds, B.A., Magli, M.C. and Vescovi, A.L. ( 1999 ) Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science , 283 , 534 –537.
Blau, H.M., Brazelton, T.R. and Weimann, J.M. ( 2001 ) The evolving concept of a stem cell. Entity of function? Cell , 105 , 829 –841.
Brazelton, T.R., Rossi, F.M., Keshet, G.I. and Blau, H.M. ( 2000 ) From marrow to brain: expression of neuronal phenotypes in adult mice. Science , 290 , 1775 –1779.
Cibelli, J.B., Grant, K.A., Chapman, K.B., Cunniff, K., Worst, T., Green, H.L., Walker, S.J., Gutin, P.H., Vilner, L., Tabar, V. et al . ( 2002 ) Parthenogenetic stem cells in nonhuman primates. Science , 295 , 819 .
Clarke, D.L., Johansson, C.B., Wilbertz, J., Veress, B., Nilsson, E., Karlstrom, H., Lendahl, U. and Frisen, J. ( 2000 ) Generalized potential of adult neural stem cells. Science , 288 , 1559 –1561.
Council of Europe ( 1996 ) Convention on Human Rights and Biomedicine. Strasburg.
De Wert, G., Berghmans, R., Boer, G.J., Andersen, S., Brambati, B., Carvalho, A.S., Dierickx, K.M., Elliston, S., Nunez, P., Osswald, W. et al . ( 2002 ) Ethical guidance on human embryonic and fetal tissue transplantation: A European overview. Med., Health Care and Philosophy , 5 , 79 –90.
Editorial ( 2002 ) Nature .
European Science Foundation ( 2001 ) Human stem cell research: scientific uncertainties and ethical dilemmas. European Science Foundation Policy Briefing , 14.
Ferrari, G., Cusella‐De Angelis, G., Coletta, M., Paolucci, E., Stornaiuolo, A., Cossu, G. and Mavilio, F. ( 1998 ) Muscle regeneration by bone marrow‐derived myogenic progenitors. Science , 279 , 1528 –1530.
Fuchs, E. and Segre, J.A. ( 2000 ) Stem cells: a new lease on life. Cell , 100 , 143 –155.
Galli, R., Borello, U., Gritti, A., Minasi, M.G., Bjornson, C., Coletta, M., Mora, M., De Angelis, M.G., Fiocco, R., Cossu, G. et al . ( 2000 ) Skeletal myogenic potential of human and mouse neural stem cells. Nat. Neurosci. , 3 , 986 –991.
Hakelien, A.M., Landsverk, H.B., Robl, J.M., Skallhegg, B.S. and Collas, P. ( 2002 ) Reprogramming fibroblasts to express T‐cell functions using cell extracts. Nat. Biotechnol. , 20 , 460 –466.
Hansen, J‐E.S. ( 2002 ) Embryonic stem cell production through therapeutic cloning has fewer ethical problems than stem cell harvest from surplus IVF embryos. J. Med. Ethics , 28 , 86 –88.
Human Fertilisation and Embryology Act ( 1990 ) HFEA, London
Hursthouse, R. ( 1987 ) Beginning lives . Blackwell, Oxford.
Jackson, K.A., Majka, S.M., Wang, H., Pocius, J., Hartley, C.J., Majesky, M.W., Entman, M.L., Michael, L.H., Hirschi, K.K. and Goodell, M.A. ( 2001 ) Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J. Clin. Invest. , 107 , 1395 –1402.
Jiang, Y., Jahagirdar, B.N., Reinhardt, R.L., Schwartz, R.E., Keene, C.D., Ortiz‐Gonzalez, X.R., Reyes, M., Lenvik, T., Lund, T., Blackstad, M. et al . ( 2002 ) Pluripotency of mesenchymal stem cells derived from adult bone marrow. Nature , 418 , 41 –49.
Kaufman, D.S., Hanson, E.T., Lewis, R.L., Auerbach, R. and Thomson, J.A. ( 2001 ) Hematopoietic colony forming cells derived human embryonic stem cells. Proc. Natl Acad. Sci. USA , 98 , 10716 –10721.
Keller, G. and Snodgrass, H.R. ( 1999 ) Human embryonic stem cells: The future is now. Nat. Med. , 5 , 151 –152.
Kiessling, A. ( 2001 ) In the stem‐cell debate, new concepts need new words. Nature , 413 , 453.
Kim, J‐H., Auerbach,J.M., Rodriguez‐Gomez, J.A., Velasco, I.Gavin, D., Lumelsky,N., Lee, S‐H., Nguyen,J., Sanchez‐Pernaute, R., Bankiewicz, K. and McKay, R. ( 2002 ) Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson′s disease. Nature , 418 , 50 ‐56
Kondziolka, D., Wechsler, L., Goldstein, S., Meltzer, C., Thulborn, K.R., Gebel, J., Jannetta, P., DeCesare, S., Elder, E.M., McGrogan, M. et al . ( 2000 ). Transplantation of cultured human neuronal cells for patients with stroke. Neurology , 55 , 565 –569.
Krause, D.S., Theise, N.D., Collector, M.I., Henegariu, O., Hwang, S., Gardner, R., Neutzel, S. and Sharkis, S.J.( 2001 ) Multi‐organ, multi‐lineage engraftment by a single bone marrow‐derived stem cell. Cell , 105 , 369 –377.
Lagasse, E., Connors, H., Al‐Dhalimy, M., Reitsma, M., Dohse, M., Osborne, L., Wang, X., Finegold, M., Weissman, I.L. and Grompe, M. ( 2000 ) Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Medicine , 6 , 1229 –1234.
Lanza, RP., Chung, H.Y., Yoo, J.J., Wettstein, P.J., Blackwell, C., Borson, N., Hofmeister, E., Schuch, G., Soker, S., Moraes, C.T. et al .( 2002 ) Generation of histocompatible tissues using nuclear transplantation. Nature Biotech ., advance online publication DOI: 10.1038/nbt703.
Lanzendorf, S.E., Boyd, C.A., Wright, D.L., Muasher, S., Oehninger, S. and Hodgen, G.D. ( 2001 ) Use of human gametes obtained from anonymous donors for the production of human embryonic stem cell lines. Fertil. Steril. , 76 , 132 –137.
McGee, G. and Caplan, A. ( 1999 ) The ethics and politics of small sacrifices in stem cell research. Kennedy Institute of Ethics J. , 9 , 151 –158.
McKay, R. ( 1997 ) Stem cells in the central nervous system. Science , 276 , 66 –71.
Mezey, E., Chandross, K.J., Harta, G., Maki, R.A. and McKercher, S.R. ( 2000 ) Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science , 290 , 1779 –1782.
Nagy, A., Rossant, J., Nagy, R., Abramow‐Newerly, W. and Roder, J.C. ( 1993 ) Derivation of completely cell culture‐derived mice from early‐passage embryonic stem cells. Proc. Natl Acad. Sci. USA , 90 , 8424 –8428.
NAS (2002) Board on Life Sci., National Research Council and Board on Neuroscience and Behavioural Health, Institute of Medicine. Stem cells and future of regenerative medicine. National Academy Press. www.nap.edu/books/0309076307/html .
Nuffield Council on Bioethics ( 2000 ). Stem Cell Therapy: the ethical issues. A discussion paper. April, 2000.
Orlic, D., Kajstura, J., Chimenti, S., Jakoniuk, I., Anderson, S.M., Li, B., Pickel, J., McKay, R., Nadal‐Ginard, B., Bodine, D.M. et al . ( 2001 ) Bone marrow cells regenerate infarcted myocardium. Nature , 401 , 701 –705.
Passier, R. and Mummery, C.L ( 2003 ) The origin and use of embryonic and adult stem cells in differentiation and tissue repair: review in Spotlight Issue on Cell Transplantation and Stem cells in the Cardiovascular system. Cardiovasc. Res. , in press.
Petersen, B.E., Bowen, W.C., Patrene, K.D., Mars ,W.M., Sullivan, A.K., Murase, N., Boggs, S.S., Greenberger, J.S. and Goff, J.P. ( 1999 ) Bone marrow as a potential source of hepatic oval cells. Science , 284 , 1168 –1170.
Pittenger, M.F., Mackay, A.M., Beck, S.C., Jaiswal, R.K., Douglas, R., Mosca, J.D., Moorman, M.A., Simonetti, D.W., Craig, S. and Marshak, D.R. ( 1999 ) Multilineage potential of adult human mesenchymal stem cells. Science , 284 , 143 –147.
Raymond, J.G. ( 1987 ) Fetalists and feminists: they are not the same. In Spallone, P. and Steinberg D.L. (eds), Made to Order: The Myth of Reproductive and Genetic Progress. Pergamon Press, Oxford, pp. 58 –65.
Reubinoff, B.E., Pera, M.F., Fong, C.Y., Trounson, A. and Bongso, A. ( 2000 ) Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nature Biotechnol. , 18 , 399 –404.
Reubinoff, B.E., Itsykson, P., Turetsky, T., Pera, M.F., Reinhartz, E., Itzik, A. and Ben‐Hur, T. ( 2001 ) Neural progenitors from human embryonic stem cells. Nature Biotechnol. , 19 , 1134 –1140.
Reyes, M. and Verfaillie, C.M. ( 2001 ) Characterization of multipotent adult progenitor cells, a subpopulation of mesenchymal stem cells. Ann. N.Y. Acad. Sci. , 938 , 231 –233.
Sanchez‐Ramos, J., Song, S., Cardozo‐Pelaez, F., Hazzi, C., Stedeford, T., Willing, A., Freeman, T.B., Saporta, S., Janssen, W., Patel, N. et al . ( 2000 ) Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp. Neurol. , 164 , 247 –256.
Schwartz, R.E., Reyes, M., Koodie, L., Blacksrad, M., Lund, T., Lenvik, T., Johnson, S., Hu, W‐H. and Verfaillie, C. ( 2002 ) Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte‐like cells. J. Clin. Invest. , 109 , 1291 –302.
Shamblott, M.J., Axelman, J., Wang, S., Bugg, E.M., Littlefield, J.W., Donovan, P.J., Blumenthal, P.D., Huggins, G.R. and Gearhart, J.D. ( 1998 ) Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc. Natl Acad. Sci. USA , 95 , 13726 –13731.
Shamblott, M.J., Axelman, J., Littlefield, J.W., Blumenthal, P.D., Huggins, G.R., Cui Y., Cheng, L. and Gearhart, J.D. ( 2001 ) Human embryonic germ cell derivatives express a broad range of developmentally distinct markers and proliferate extensively in vitro. Proc. Natl Acad. Sci. USA , 98 , 113 –118.
Spradling, A., Drummond‐Barbosa, D. and Kai, T. ( 2001 ) Stem cells find their niche. Nature , 414 , 98 –104.
Steghaus‐Kovac, S. ( 1999 ) Ethical loophole closing up for stem cell researchers. Science , 286 , 31.
Stem Cell Research ( 2000 ) Medical progress with responsibility. A report from the chief medical officer’s expert group reviewing the potential of developments in stem cell research and cell nuclear replacement to benefit human health . Department of Health, UK.
Stem cells show their muscle ( 2000 ). Editorial. Nature Neurosci ., 3 , 961 .
Surani, M.A. ( 2001 ) Re‐programming of genome function through epigenetic inheritance. Nature , 414 , 122 –128.
Terada, N., Hamazaki, T., Oka, M., Hoki, M., Masterlerz, D.M., Nakano, Y., Meyer, E.M., Morel, L., Petersen, B.E. and Scott, E.W. ( 2002 ) Bone marrow cell adopt the phenotype of other cells by spontaneous cell fusion. Nature , 416 , 542 –545.
Thomson, J.A., Itskovitz‐Eldor, J., Shapiro, S.S., Waknitz, M.A., Swiergiel, J.J., Marshall, V.S. and Jones, J.M. ( 1998 ) Embryonic stem cell lines derived from human blastocysts. Science , 282 , 1145 –1147.
Vastag, B. ( 2001 ) Many say adult stem cell reports overplayed. JAMA , 286 , 293 .
Vastag, B. ( 2002 ) New bioethics council offers no recommendations. JAMA , 287 , 2934 –2935.
Verfaillie, CM. ( 2002 ) Hematopoietic stem cells for transplantation. J. Nat. Immunol. , 3 , 314 –317.
Vogelstein, B., Alberts, B. and Shine, K. ( 2002 ) Please don’t call it cloning! Science , 295 , 1237.
Watt, F.M. and Hogan, B.L. ( 2000 ) Out of Eden: stem cells and their niches. Science , 287 , 1427 –1430.
Weissman, I.L. ( 2000 ) Stem cells: units of development, units of regeneration, and units in evolution. Cell , 100 , 157 –168.
Xu, C., Inokuma, M.S., Denham, J., Golds, K., Kundu ,P., Gold, J.D. and Carpenter, M.K. ( 2001 ) Feeder‐free growth of undifferentiated human embryonic stem cells. Nature Biotechnol. , 19 , 971 –974.
Ying, Q.L., Nichols, J., Evans, E.P. and Smith, A.G. ( 2002 ) Changing potency by spontaneous fusion. Nature , 416 , 545 –548.
Zhao, L.R., Duan, W.M., Reyes, M., Keene, C.D., Verfaillie, C.M. and Low, W.C. ( 2002 ). Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp. Neurol. , 174 , 11 –20.
Zhang, S.C., Wernig, M., Duncan, I.D., Brustle, O. and Thomson, J.A.( 2001 ) In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. , 19 , 1129 –1133.
| Month: | Total Views: |
|---|---|
| December 2016 | 1 |
| January 2017 | 171 |
| February 2017 | 1,500 |
| March 2017 | 1,170 |
| April 2017 | 1,084 |
| May 2017 | 750 |
| June 2017 | 467 |
| July 2017 | 597 |
| August 2017 | 598 |
| September 2017 | 1,139 |
| October 2017 | 1,645 |
| November 2017 | 2,083 |
| December 2017 | 8,778 |
| January 2018 | 8,533 |
| February 2018 | 8,951 |
| March 2018 | 10,734 |
| April 2018 | 9,553 |
| May 2018 | 8,886 |
| June 2018 | 6,825 |
| July 2018 | 5,720 |
| August 2018 | 7,373 |
| September 2018 | 8,448 |
| October 2018 | 9,047 |
| November 2018 | 10,957 |
| December 2018 | 8,946 |
| January 2019 | 7,194 |
| February 2019 | 8,164 |
| March 2019 | 9,951 |
| April 2019 | 8,540 |
| May 2019 | 7,917 |
| June 2019 | 6,011 |
| July 2019 | 6,438 |
| August 2019 | 6,926 |
| September 2019 | 6,759 |
| October 2019 | 4,764 |
| November 2019 | 3,270 |
| December 2019 | 2,229 |
| January 2020 | 1,943 |
| February 2020 | 2,477 |
| March 2020 | 2,435 |
| April 2020 | 2,431 |
| May 2020 | 1,215 |
| June 2020 | 1,334 |
| July 2020 | 979 |
| August 2020 | 1,056 |
| September 2020 | 1,558 |
| October 2020 | 1,901 |
| November 2020 | 1,846 |
| December 2020 | 975 |
| January 2021 | 1,028 |
| February 2021 | 1,051 |
| March 2021 | 1,450 |
| April 2021 | 1,301 |
| May 2021 | 1,014 |
| June 2021 | 816 |
| July 2021 | 423 |
| August 2021 | 633 |
| September 2021 | 1,097 |
| October 2021 | 1,237 |
| November 2021 | 1,358 |
| December 2021 | 917 |
| January 2022 | 854 |
| February 2022 | 904 |
| March 2022 | 1,247 |
| April 2022 | 1,141 |
| May 2022 | 902 |
| June 2022 | 567 |
| July 2022 | 372 |
| August 2022 | 462 |
| September 2022 | 823 |
| October 2022 | 884 |
| November 2022 | 1,000 |
| December 2022 | 550 |
| January 2023 | 589 |
| February 2023 | 630 |
| March 2023 | 766 |
| April 2023 | 519 |
| May 2023 | 467 |
| June 2023 | 245 |
| July 2023 | 218 |
| August 2023 | 284 |
| September 2023 | 471 |
| October 2023 | 632 |
| November 2023 | 510 |
| December 2023 | 485 |
| January 2024 | 496 |
| February 2024 | 504 |
| March 2024 | 937 |
| April 2024 | 565 |
| May 2024 | 505 |
| June 2024 | 336 |
| July 2024 | 216 |
| August 2024 | 271 |
| September 2024 | 58 |
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1460-2350
- Copyright © 2024 European Society of Human Reproduction and Embryology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Stem cell research ethics: consensus statement on emerging issues
Affiliation.
- 1 Health Law Institute, Faculty of Law, School of Public Health, University of Alberta, Edmonton AB.
- PMID: 17915069
- DOI: 10.1016/s1701-2163(16)32632-9
- J Obstet Gynaecol Can. 2007 Dec;29(12):971. Grenier, Glenn [corrected to Griener, Glenn]
This article is a consensus statement by an international interdisciplinary group of academic experts and Canadian policy-makers on emerging ethical, legal and social issues in human embryonic stem cells (hESC) research in Canada. The process of researching consensus included consultations with key stakeholders in hESC research (regulations, stem cell researchers, and research ethics experts), preparation and distribution of background papers, and an international workshop held in Montreal in February 2007 to discuss the papers and debate recommendations. The recommendations provided in the consensus statement focus on issues of immediate relevance to Canadian policy-makers, including informed consent to hESC research, the use of fresh embryos in research, management of conflicts of interest, and the relevance of public opinion research to policy-making.
PubMed Disclaimer
Similar articles
- An investigation of embryo donation, informed consent, and research oversight in Canadian human embryonic stem cell research. Nelson E, Ogbogu U, Caulfield T. Nelson E, et al. J Obstet Gynaecol Can. 2007 Dec;29(12):997-1002. doi: 10.1016/S1701-2163(16)32692-5. J Obstet Gynaecol Can. 2007. PMID: 18053386
- Administrative and research policies required to bring cellular therapies from the research laboratory to the patient's bedside. Yim R. Yim R. Transfusion. 2005 Oct;45(4 Suppl):144S-58S. doi: 10.1111/j.1537-2995.2005.00616.x. Transfusion. 2005. PMID: 16181400 Review.
- Human stem cell ethics: beyond the embryo. Sugarman J. Sugarman J. Cell Stem Cell. 2008 Jun 5;2(6):529-33. doi: 10.1016/j.stem.2008.05.005. Cell Stem Cell. 2008. PMID: 18522846
- Ethics and stem cell therapeutics for cardiovascular disease. Sugarman J. Sugarman J. Prog Cardiovasc Dis. 2007 Jul-Aug;50(1):1-6. doi: 10.1016/j.pcad.2007.02.003. Prog Cardiovasc Dis. 2007. PMID: 17631433 Review.
- Constructing an ethical framework for embryo donation to research: is it time for a restricted consent policy? Ehrich K, Farsides B, Williams C, Scott R. Ehrich K, et al. Hum Fertil (Camb). 2011 Jun;14(2):115-21. doi: 10.3109/14647273.2011.557762. Epub 2011 Apr 5. Hum Fertil (Camb). 2011. PMID: 21463226
- Human pluripotent stem cell registry: Operations, role and current directions. Kurtz A, Mah N, Chen Y, Fuhr A, Kobold S, Seltmann S, Müller SC. Kurtz A, et al. Cell Prolif. 2022 Aug;55(8):e13238. doi: 10.1111/cpr.13238. Epub 2022 May 6. Cell Prolif. 2022. PMID: 35522426 Free PMC article. Review.
- Policy recommendations for addressing privacy challenges associated with cell-based research and interventions. Ogbogu U, Burningham S, Ollenberger A, Calder K, Du L, El Emam K, Hyde-Lay R, Isasi R, Joly Y, Kerr I, Malin B, McDonald M, Penney S, Piat G, Roy DC, Sugarman J, Vercauteren S, Verhenneman G, West L, Caulfield T. Ogbogu U, et al. BMC Med Ethics. 2014 Feb 3;15:7. doi: 10.1186/1472-6939-15-7. BMC Med Ethics. 2014. PMID: 24485220 Free PMC article.
- Sino-Canadian collaborations in stem cell research: a scientometric analysis. Ali-Khan SE, Ray M, McMahon DS, Thorsteinsdóttir H. Ali-Khan SE, et al. PLoS One. 2013;8(2):e57176. doi: 10.1371/journal.pone.0057176. Epub 2013 Feb 28. PLoS One. 2013. PMID: 23468927 Free PMC article.
- Criteria for authorship in bioethics. Resnik DB, Master Z. Resnik DB, et al. Am J Bioeth. 2011 Oct;11(10):17-21. doi: 10.1080/15265161.2011.603795. Am J Bioeth. 2011. PMID: 21943265 Free PMC article.
- Comparison of participant information and informed consent forms of five European studies in genetic isolated populations. Mascalzoni D, Janssens AC, Stewart A, Pramstaller P, Gyllensten U, Rudan I, van Duijn CM, Wilson JF, Campbell H, Quillan RM; EUROSPAN consortium. Mascalzoni D, et al. Eur J Hum Genet. 2010 Mar;18(3):296-302. doi: 10.1038/ejhg.2009.155. Epub 2009 Oct 14. Eur J Hum Genet. 2010. PMID: 19826451 Free PMC article.
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- Elsevier Science
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
University of Notre Dame
Fresh Writing
A publication of the University Writing Program
- Home ›
- Essays ›
The Ethics of Embryonic Stem Cell Research
By Belin Mirabile
Published: July 31, 2016
What if I told you that researchers could cure diseases such as Parkinson's disease and multiple sclerosis? Odds are, you would be in favor of ending the suffering of the thousands of people who currently battle such diseases. These cures and many more are the potential results of embryonic stem cell research. Embryonic stem cells are stem cells isolated from embryos during a specific stage of development known as the blastocyst stage. These stem cells can renew themselves and reproduce to form all cell types of the body. Research utilizing these stem cells requires the destruction of an embryo, making the practice a point of moral, scientific, religious, and political controversy. Many argue that the destruction of embryos for research purposes is unethical based on the belief that embryos qualify as forms of life that deserve respect. Those in favor of embryonic stem cell research deem such a loss acceptable for the future benefits that this research could have on thousands of lives. While various arguments surround this debate, the main point of controversy is the source of stem cells used and the method with which they are obtained. In this paper, I will establish what stem cells are and the difference between embryonic and adult stem cells; then I will evaluate the two main arguments in the embryonic stem cell research debate; and finally, I will analyze the ethics of these arguments to come to the conclusion that embryonic stem cell research is ethical under certain circumstances.
Overview of Stem Cell Research
As defined by "The Human Embryonic Stem Cell Debate: Science, Ethics, and Public Policy," human embryonic stem cells are "a self-renewing cell line that gives rise to all cells and tissues of the body" (Holland 3). Most stem cells are only able to differentiate into a single form of offspring cells, otherwise known as progeny cells. For example, hematopoietic stem cells are a type of stem cells that can only form blood cells and skin stem cells can similarly only produce skin cells. These types of stem cells are referred to as adult stem cells or somatic stem cells because they are gathered from patients after birth (Devolder 5). Meanwhile, embryonic stem cells are pluripotent, meaning they have the capacity to produce all cells and tissues of the body (Holland 5). Embryonic stem cells, however, only have this pluripotent potential for the particular five-to-seven-day stage of embryonic development known as the blastocyst stage, after which they can only reproduce a single cell type ("The Ethics of Embryonic Stem Cell Research" 123).
Stem cells, in general, hold great promise for the future of medicine. Thus far, stem cell-based therapies have been developed to treat illnesses that previously had no cure. One example is bone marrow transplantation to treat leukemia and other blood disorders. The hematopoietic stem cells in bone marrow are injected into a patient who has severely reduced blood cell levels and these stem cells generate new blood cells, restoring the patient's immune system (Devolder 5). Therapies such as this will continue to be discovered with the support of stem cell research.
In addition to the development of revolutionary therapies, stem cell research also provides valuable information about mechanisms regulating cell growth, migration, and differentiation. Scientists can learn about these processes by studying stem cells that have been stimulated to differentiate into different types of body cells. The discovery of new information about these concepts will allow scientists to better understand early human development and how tissues are maintained throughout life (8).
Embryonic stem cells are particularly valuable not only because of their pluripotent qualities, but also because of their ability to renew themselves. This is done by "divid[ing] asynchronously – at different times – into one differentiated daughter cell 1 and one stem cell-like daughter cell." This unique self-renewing quality of embryonic stem cells allows them to continuously grow even in laboratory conditions. Other types of stem cells eventually lose the ability to divide, making them less valuable for research purposes. Embryonic stem cells' ability to be produced in large quantities allows researchers to make progress in regenerative medicine, using these cells to develop new functional cells, tissues, and organs. The healthy cells are implanted into the patient, serving as treatment to permanently repair failing organs (Holland 5). The otherwise lack of treatment for loss of organ function displays the valuable potential of embryonic stem cells.
The sources of embryonic stem cells are a main point of controversy in the debate regarding embryonic stem cell research. Some possible sources for these stem cells include embryos created via in vitro fertilization (for either research or reproduction); five-to-nine-week old embryos or fetuses obtained through elective abortion; and embryos created through cloning or what is known as somatic cell nuclear transfer (Liu 1). Somatic cell nuclear transfer is the laboratory creation of a viable embryo by implanting a donor nucleus from a body cell into an egg cell. The ethics of obtaining embryonic stem cells via these sources can be questionable and have led to disputes that I will later address.
Research utilizing human embryonic stem cell lines has focused on the potential to generate replacement tissues for malfunctioning cells or organs (Liu 1). A specific technique has been isolated to utilize stem cells in order to repair a damaged tissue or organ:
"If a damaged tissue or organ cannot repair itself, stem cells could be obtained from these different stem cell sources [organs and tissues from individuals after birth; gametes, tissues, and organs from aborted fetuses; inner cell mass of early embryos]. Scientists could then culture these stem cells by creating conditions that enable them to replicate many times in a petri dish without differentiating. Such a population of proliferating stem cells originating from a single parent group of stem cells is a stem cell line. Stem cells from this stem cell line could then be coaxed to differentiate in to the desired cell type, and be transferred into the patient so that they can repair the damaged tissue or organ" (Devolder 6).
Other examples of research efforts include treatment of spinal cord injury, multiple sclerosis, Parkinson's disease, Alzheimer's disease, and diabetes. Researchers also hope to use specialized cells to replace dysfunctional cells in the brain, spinal cord, pancreas, and other organs (2).
Federal funding of embryonic research has been strictly regulated since 1994 when President Clinton declared such research would not be funded by the government. Following this executive order, Congress passed the Dickey Amendment in 1996, prohibiting "federally appropriated funds from being used for either the creation of human embryos for research purposes or for research in which a human embryo or embryos are destroyed, discarded, or knowingly subjected to risk of injury or death" (Liu 2). Embryonic research has continued nonetheless by means of alternative funding. In 2001, President Bush declared that federal funding would be granted to human embryonic research on a restricted basis. However, these funds were only to be awarded for research on already existing stem cell lines. No funding was to be granted for "the use of stem cell lines derived from newly destroyed embryos, the creation of any human embryos for research purposes, or cloning of human embryos for any purposes" (3-4).
The debate over funding for embryonic stem cell research depends heavily on the ethical status of the research. There are two main arguments surrounding the ethics of embryonic stem cell research: the research is ethical because of the unique potential that embryonic stem cells have to cure currently untreatable diseases; and the research is unethical because it requires the destruction of life in the form of an embryo or fetus. Ultimately, the possible benefits and controversial status of life that an embryo embodies qualify embryonic stem cell research as ethical, as long as the stem cells are obtained in an ethical manner.
Arguments for Embryonic Stem Cell Research
In the realm of stem cell research, embryonic and adult stem cells are often compared. The controversial use of embryonic stem cells is supported on the basis of the many advantages that they have over adult stem cells. Embryonic stem cells are easier to obtain; they have a greater cell growth, otherwise known as proliferation, capacity; and they are more versatile. Embryonic stem cells are isolated from embryos in the blastocyst stage and the process damages the structure of the embryo to a point from which the embryo can no longer develop. Because these stem cells are obtained at a point when the inner cell mass is concentrated in the embryo, they are more easily obtained than adult stem cells, which are limited in quantity. Another valuable benefit of embryonic stem cells is their ability to multiply readily and proliferate indefinitely when cultured in the proper conditions (Devolder 9). Lastly, embryonic stem cells' pluripotent quality is the main factor that distinguishes them from adult stem cells (10). The ability to differentiate into any cell type creates greater possibilities for the application of embryonic stem cells.
Supporters of embryonic stem cell research argue that the research is justified, though it requires the destruction of an embryo, because of the potential for developing cures and preventing unavoidable suffering. These backers often disagree with the belief that "a blastocyst – even one that is not implanted in a woman's uterus – has the same ethical status as a further-developed human" (Clemmitt 702). Arthur Caplan, professor of medical ethics at the University of Pennsylvania, asserts that "an embryo in a dish is more like a set of instructions or blueprint for a house. It can't build the house. For the cells to develop into a human being requires an interactive process in the uterus between the embryo and the mother" (Clemmitt 702).
Others in favor of the research, such as Heron, a biotechnology company, claim that "not to develop the technology would do great harm to over 100 million patients in the United States alone who are affected by diseases potentially treatable by the many medical applications of hES [human Embryonic Stem] cells" (Holland 11-12). One example is the previously stated method of using embryonic stem cells to repair damaged tissue or organs. The only way to restore cellular function in an organ is to literally replace the lost cells and embryonic stem cells provide the best option for producing these cells (3).
Embryonic stem cells do also have some disadvantages that should be considered when making the argument for further support of embryonic stem cell research. Unlike adult stem cells, embryonic stem cells have a higher risk of causing tumor formation in the patient's body after the stem cells are implanted. This is due to their higher capacities for proliferation and differentiation (Devolder 11). Embryonic stem cell-based therapies also possess the risk of immunorejection – rejection of the stem cells by the patient's immune system. Because embryonic stem cells are derived from embryos donated for research after in vitro fertilization treatment, the marker molecules on the surfaces of the cells may not be recognized by the patient's body, and therefore may be destroyed as the result of a defense mechanism by the body (Holland 11). This is a problem that will require a solution if embryonic stem cell research is to be the basis for future therapeutic medicine.
Arguments against Embryonic Stem Cell Research
Currently, the isolation of embryonic stem cells requires the destruction of an early embryo. Many people hold the belief that a human embryo has significant moral status, and therefore should not be used merely as a means for research. One position that opponents of embryonic stem cell research assert is what "The Ethics of Embryonic Stem Cell Research" calls the full moral status view (14). This view holds that "the early embryo has the same moral status, that is, the same basic moral rights, claims, or interests as an ordinary adult human being." This moral status is believed to be acquired at the point of fertilization or an equivalent event such as the completion of somatic cell nuclear transfer. Therefore, with full moral status as a human being, an embryo should not be deliberately destroyed for research purposes simply because it is human (Devolder 15). The Roman Catholic Church is a strong supporter of this view, opposing stem cell research on the grounds that it is a form of abortion. Several other groups, including American evangelicals and Orthodox ethicists, consider "blastocysts to have the same status as fully developed human beings" and therefore oppose embryonic stem cell research for this reason. Beliefs regarding the moral status of an embryo are subjective, and also their own controversial issue, which complicates the task of creating a universal law for the use of embryonic stem cells for research.
Others in opposition, such as Kevin T. Fitzgerald, a Jesuit priest who is a bioethicist and professor of oncology at Georgetown University Medical School, do not consider the moral status of an embryo, but rather assert that Embryos should be protected because they are "that which we all once were" (Clemmitt 701). This view is very similar to moral philosopher and professor of philosophy as the University of California at Irvine Philip Nickel's "Loss of Future Life Problem" in regards to embryonic stem cell research. The Loss of Future Life Problem holds that it is unethical to take the lives of future humans by destroying embryos for research (Tobis 64). This stance stresses the potential of those future lives that will never have the chance to reach fulfillment if destroyed for research. In a retroactive sense, this can cause us to question "what if the embryo that developed into Albert Einstein was destroyed for embryonic stem cell research?" It is impossible for one to know the value that is lost in each embryo taken for research purposes, if that embryo is created with the plan of developing into an adult human being.
The response to this problem is that the particular blastocysts that are harvested for embryonic stem cell research are taken from (1) embryos that are frozen during in vitro fertilization procedures and never implanted, (2) donated egg cells, and (3) embryos created specifically for the purpose of generating new stem cell lines. In each of these cases, the embryo at hand does not have a future life in plan and therefore, nothing is lost by using such embryonic stem cells for research. For embryos created via in vitro fertilization, the researchers using the embryos are not making a decision that results in the loss of a future life. The future life of said embryo is lost when the decision is made to not implant it. Therefore, the Loss of Future Life Problem is not a valid objection to research using embryonic stem cells from frozen IVF embryos that are never implanted. Donated egg cells can be fertilized in a lab or through somatic cell nuclear transfer, a process described earlier in this paper. Embryos created specifically for the purpose of contributing to stem cell research have no actual future life to be lost from the moment of conception. In both of these cases, the intent of fertilization is not to create a future adult human being, and so the Loss of Future Life Problem does not apply to these sources of embryonic stem cells.
"In terms of the Loss of Future Life Problem, the key question is again whether the embryo is being deprived of future life, and again the answer depends on whether the embryo is removed from a woman's reproductive system, in which case it is likely that it is being deprived of future life that it would otherwise go on to have. If fertilization takes place outside a woman's body, by contrast, then the embryo is not already on its way toward a future life, so destroying it does not deprive it of that particular future" (Tobis 66-67).
As shown by the various arguments in this essay, the debate over embryonic stem cell research is a multifaceted scientific, moral, ethical, and political issue. Embryonic stem cells, with their pluripotent potential and self-renewing quality, hold great value for scientific researchers in search of cures for untreatable diseases, progress in regenerative medicine, or a better understanding of early human development. However, the ethical question still arises, "do the ends justify the means?"
Varying views regarding the ethical status of an embryo answer this question in different ways, though it is commonly accepted that if the means of obtaining the embryonic stem cells are ethical, then the resulting research of those stem cells is also ethical. For example, if a donated egg is fertilized in a lab with the intention of being used for future research purposes, the resulting research is therefore morally justified.
This is not to be said that the life of an early-stage embryo is to be taken lightly. More so that our moral perception of these embryos is different than that of a later-stage fetus, an infant, or an adult human being. Phillip Nickel asserts this subconscious difference, claiming that,
"while it's well known that many embryos are shed naturally, in very early abortions and miscarriages, no one makes an effort to save or grieve for them, as frequently happens with later-stage fetuses. This shows that people do view embryos as somewhat different from people, even though they may not realize it" (Clemmitt 702).
Thus, the moral distinction between a blastocyst and a developed fetus weakens the moral arguments in opposition to embryonic stem cell research. After all, if this research can reduce suffering for thousands of people, are we not morally obligated to pursue it?
Scientists in support of embryonic stem cell research are currently restricted by the limited amounts of federal funding and embryonic stem cell lines available for research. Many argue that these restrictions are preventing further scientific development and weakening the United States' position as a leading nation in biomedical research. Some scientists worry that if strict regulations of stem cell research continue, private companies may bypass the standards put in place by the National Institute of Health and conduct unregulated research (Clemmitt 700). If the United States wishes to remain a premiere country in biomedical research and maintain order and control of embryonic research being performed, action must be taken to address this issue.
Overall, though the destruction of a life is typically held to be unethical, the moral status of an embryo in the blastocyst stage is unclear and therefore cannot be equated to the moral status of an adult human being. Also, ethical sources of embryonic stem cells exist that do not take the life of future beings (i.e. unwanted frozen embryos produced via in vitro fertilization, donated egg cells fertilized in a laboratory). For these reasons, in combination with the possibility of reducing suffering for future beings, embryonic stem cell research is ethical under certain circumstances. As long as the stem cells are isolated in a manner that does not harm an embryo with the plan of developing into an adult human, the subsequent research is ethically justified. With this in mind, embryonic stem cell research should receive greater government funding so that continued progress can be made.
1 In cell division, a parent cell divides into two or more daughter cells.
Works Cited
Clemmitt, Marcia. "Stem Cell Research." CQ Researcher 1 Sept. 2006: 697-720. Web. 25 Nov. 2015.
Devolder, Katrien. The Ethics of Embryonic Stem Cell Research . First ed. 2015. Issues in Biomedical Ethics. Print.
Holland, Suzanne, Lebacqz, Karen, and Zoloth, Laurie. The Human Embryonic Stem Cell Debate: Science, Ethics, and Public Policy . Cambridge, Mass.: MIT, 2001. Basic Bioethics. Web. 17 Nov. 2015.
Liu, Edward Chan-Young. Background and Legal Issues Related to Human Embryonic Stem Cell Research . American Law Division, 2008. Print.
"The Ethics of Embryonic Stem Cell Research." Embryo Politics . Ithaca; London: Cornell UP, 2011. 120. Print.
Tobis, Jerome S., Ronald Baker Miller, and Kristen R. Monroe. Fundamentals Of The Stem Cell Debate : The Scientific, Religious, Ethical, And Political Issues . Berkeley: University of California Press, 2008. eBook Collection (EBSCOhost) . Web. 17 Nov. 2015.

Belin Mirabile
Belin Mirabile was born and raised in Phoenixville, Pennsylvania, a suburb of Philadelphia. She is currently majoring in Mechanical Engineering at Notre Dame with a minor in Catholic Social Tradition. When tasked with the assignment of writing a rhetorical essay that evaluates a point of ethical controversy, Belin wanted to choose a topic that relates to her interest in Bioengineering. Embryonic stem cell research stood out as a current issue that would be interesting to evaluate in the form of a researched essay. After her four years at Notre Dame, Belin plans to pursue a career related to Bioengineering that contributes in some fashion to the betterment of human health. Belin would like to thank her Writing and Rhetoric professor, John Duffy, for transforming her opinion of writing and giving her every tool to be a successful writer .

The Ethics of Human Cloning and Stem Cell Research
- Markkula Center for Applied Ethics
- Focus Areas
- Bioethics Resources
Report from a conference on state regulation of cloning and stem cell research.
"California Cloning: A Dialogue on State Regulation" was convened October 12, 2001, by the Markkula Center for Applied Ethics at Santa Clara University. Its purpose was to bring together experts from the fields of science, religion, ethics, and law to discuss how the state of California should proceed in regulating human cloning and stem cell research.
A framework for discussing the issue was provided by Center Director of Biotechnology and Health Care Ethics Margaret McLean, who also serves on the California State Advisory Committee on Human Cloning. In 1997, the California legislature declared a "five year moratorium on cloning of an entire human being" and requested that "a panel of representatives from the fields of medicine, religion, biotechnology, genetics, law, bioethics and the general public" be established to evaluate the "medical, ethical and social implications" of human cloning (SB 1344). This 12-member Advisory Committee on Human Cloning convened five public meetings, each focusing on a particular aspect of human cloning: e.g., reproductive cloning, and cloning technology and stem cells. The committee is drafting a report to the legislature that is due on December 31, 2001. The report will discuss the science of cloning, and the ethical and legal considerations of applications of cloning technology. It will also set out recommendations to the legislature regarding regulation of human cloning. The legislature plans to take up this discussion after January. The moratorium expires the end of 2002.
What should the state do at that point? More than 80 invited guests came to SCU for "California Cloning" to engage in a dialogue on that question. These included scientists, theologians, businesspeople from the biotechnology industry, bioethicists, legal scholars, representatives of non-profits, and SCU faculty. Keynote Speaker Ursula Goodenough, professor of biology at Washington University and author of Genetics , set the issues in context with her talk, "A Religious Naturalist Thinks About Bioethics." Four panels addressed the specific scientific, religious, ethical, and legal implications of human reproductive cloning and stem cell research. This document gives a brief summary of the issues as they were raised by the four panels.
Science and Biotechnology Perspectives
Thomas Okarma, CEO of Geron Corp., launched this panel with an overview of regenerative medicine and distinguished between reproductive cloning and human embryonic stem cell research. He helped the audience understand the science behind the medical potential of embryonic stem cell research, with an explanation of the procedures for creating stem cell lines and the relationship of this field to telomere biology and genetics. No brief summary could do justice to the science. The reader is referred to the report of the National Bioethics Advisory Committee (http://bioethics.georgetown.edu/nbac/stemcell.pdf) for a good introduction.
Responding to Okarma, were J. William Langston, president of the Parkinson’s Institute, and Phyllis Gardner, associate professor of medicine and former dean for medical education at Stanford University. Both discussed the implications of the president’s recent restrictions on stem cell research for the non-profit sector. Langston compared the current regulatory environment to the Reagan era ban on fetal cell research, which he believed was a serious setback for Parkinson’s research. He also pointed out that stem cell research was only being proposed using the thousands of embryos that were already being created in the process of fertility treatments. These would ultimately be disposed of in any event, he said, arguing that it would be better to allow them to serve some function rather than be destroyed. President Bush has confined federally-funded research to the 64 existing stem cell lines, far too few in Langston’s view. In addition, Langston opposed bans on government funding for stem cell research because of the opportunities for public review afforded by the process of securing government grants.
Gardner talked about the differences between academic and commercial research, suggesting that both were important for the advancement of science and its application. Since most of the current stem cell lines are in the commercial sector and the president has banned the creation of new lines, she worried that universities would not continue to be centers of research in this important area. That, she argued, would cut out the more serendipitous and sometimes more altruistic approaches of academic research. Also, it might lead to more of the brain drain represented by the recent move of prominent UCSF stem cell researcher Roger Pedersen to Britain. Gardner expressed a hope that the United States would continue to be the "flagship" in stem cell research. Her concerns were echoed later by moderator Allen Hammond, SCU law professor, who urged the state, which has been at the forefront of stem cell research to consider the economic impact of banning such activity. All three panelists commended the decision of the state advisory committee to deal separately with the issues of human cloning and stem cell research.
Religious Perspectives
Two religion panelists, Suzanne Holland and Laurie Zoloth, are co editors of The Human Embryonic Stem Cell Debate: Science, Ethics and Public Policy (MIT Press, 2001). Holland, assistant professor of Religious and Social Ethics at the University of Puget Sound, began the panel with a discussion of Protestant ideas about the sin of pride and respect for persons and how these apply to human reproductive cloning. Given current safety concerns about cloning, she was in favor of a continuing ban. But ultimately, she argued, cloning should be regulated rather than banned outright. In fact, she suggested, the entire fertility industry requires more regulation. As a basis for such regulation, she proposed assessing the motivation of those who want to use the technology. Those whose motives arise from benevolence--for example, those who want to raise a child but have no other means of bearing a genetically related baby--should be allowed to undergo a cloning procedure. Those whose motives arise more from narcissistic considerations -- people who want immortality or novelty -- should be prohibited from using the technology. She proposed mandatory counseling and a waiting period as a means of assessing motivation.
Zoloth reached a different conclusion about reproductive cloning based on her reading of Jewish sources. She argued that the availability of such technology would make human life too easily commodified, putting the emphasis more on achieving a copy of the self than on the crucial parental act of creating "a stranger to whom you would give your life." She put the cloning issue in the context of a system where foster children cannot find homes and where universal health care is not available for babies who have already been born. While Zoloth reported that Jewish ethicists vary considerably in their views about reproductive cloning, there is fairly broad agreement that stem cell research is justified. Among the Jewish traditions she cited were:
The embryo does not have the status of a human person.
There is a commandment to heal.
Great latitude is permitted for learning.
The world is uncompleted and requires human participation to become whole.
Catholic bioethicist Albert Jonsen, one of the deans of the field, gave a historical perspective on the cloning debate, citing a paper by Joshua Lederburg in the 1960s, which challenged his colleagues to look at the implications of the then-remote possibility. He also traced the development of Catholic views on other new medical technologies. When organ transplantation was first introduced, it was opposed as a violation of the principal, "First, do no harm" and as a mutilation of the human body. Later, the issue was reconceived in terms of charity and concern for others. One of the key questions, Jonsen suggested, is What can we, as a society that promotes religious pluralism, do when we must make public policy on issues where religious traditions may disagree. He argued that beneath the particular teachings of each religion are certain broad themes they share, which might provide a framework for the debate. These include human finitude, human fallibility, human dignity, and compassion.
Ethics Perspectives
Lawrence Nelson, adjunct associate professor of philosophy at SCU, opened the ethics panel with a discussion of the moral status of the human embryo. Confining his remarks to viable, extracorporeal embryos (embryos created for fertility treatments that were never implanted), Nelson argued that these beings do have some moral status--albeit it weak--because they are alive and because they are valued to varying degrees by other moral agents. This status does entitle the embryo to some protection. In Nelson’s view, the gamete sources whose egg and sperm created these embryos have a unique connection to them and should have exclusive control over their disposition. If the gamete sources agree, Nelson believes the embryos can be used for research if they are treated respectfully. Some manifestations of respect might be:
They are used only if the goal of the research cannot be obtained by other methods.
The embryos have not reached gastrulation (prior to 14 to 18 days of development).
Those who use them avoid considering or treating them as property.
Their destruction is accompanied by some sense of loss or sorrow.
Philosophy Professor Barbara MacKinnon (University of San Francisco), editor of Human Cloning: Science, Ethics, and Public Policy , began by discussing the distinction between reproductive and therapeutic cloning and the slippery slope argument. She distinguished three different forms of this argument and showed that for each, pursuing stem cell research will not inevitably lead to human reproductive cloning. MacKinnon favored a continuing ban on the latter, citing safety concerns. Regarding therapeutic cloning and stem cell research, she criticized consequentialist views such as that anything can be done to reduce human suffering and that certain embryos would perish anyway. However, she noted that non-consequentialist concerns must also be addressed for therapeutic cloning, among them the question of the moral status of the early embryo. She also made a distinction between morality and the law, arguing that not everything that is immoral ought to be prohibited by law, and showed how this position relates to human cloning.
Paul Billings, co-founder of GeneSage, has been involved in crafting an international treaty to ban human reproductive cloning and germ-line genetic engineering. As arguments against human cloning he cited:
There is no right to have a genetically related child.
Cloning is not safe.
Cloning is not medically necessary.
Cloning could not be delivered in an equitable manner.
Billings also believes that the benefits of stem cell therapies have been "wildly oversold." Currently, he argues, there are no effective treatments coming from this research. He is also concerned about how developing abilities in nuclear transfer technology may have applications in germ-line genetic engineering that we do not want to encourage. As a result, he favors the current go-slow approach of banning the creation of new cell lines until some therapies have been proven effective. At the same time, he believes we must work to better the situation of the poor and marginalized so their access to all therapies is improved.
Legal Perspectives
Member of the State Advisory Committee on Human Cloning Henry "Hank" Greely addressed some of the difficulties in creating a workable regulatory system for human reproductive cloning. First he addressed safety, which, considering the 5 to 10 times greater likelihood of spontaneous abortion in cloned sheep, he argued clearly justifies regulation. The FDA has currently claimed jurisdiction over this technology, but Greely doubted whether the courts would uphold this claim. Given these facts, Greely saw three alternatives for the state of California:
Do nothing; let the federal government take care of it.
Create an FDA equivalent to regulate the safety of the process, an alternative he pointed out for which the state has no experience.
Continue the current ban on the grounds of safety until such time as the procedure is adjudged safe. Next Greely responded to suggestions that the state might regulate by distinguishing between prospective cloners on the basis of their motivation, for example, denying a request to clone a person to provide heart tissue for another person but okaying a request if cloning were the only opportunity a couple might have to conceive a child. Greely found the idea of the state deciding on such basis deeply troubling because it would necessitate "peering into someone’s soul" in a manner that government is not adept at doing.
The impact of regulation on universities was the focus of Debra Zumwalt’s presentation. As Stanford University general counsel, Zumwalt talked about the necessity of creating regulations that are clear and simple. Currently, federal regulations on stem cells are unclear, she argued, making it difficult for universities and other institutions to tell if they are in compliance. She believes that regulations should be based on science and good public policy rather than on politics. As a result, she favored overall policy being set by the legislature but details being worked out at the administrative level by regulatory agencies with expertise. Whatever regulations California develops should not be more restrictive than the federal regulations, she warned, or research would be driven out of the state. Like several other speakers, Zumwalt was concerned about federal regulations restricting stem cell research to existing cell lines. That, she feared, would drive all research into private hands. "We must continue to have a public knowledge base," she said. Also, she praised the inherent safeguards in academic research including peer review, ethics panels, and institutional review boards.
SCU Presidential Professor of Ethics and the Common Good June Carbone looked at the role of California cloning decisions in contributing to the governance of biotechnology. California, she suggested, cannot address these issues alone, and thus might make the most useful contribution by helping to forge a new international moral consensus through public debate. Taking a lesson from U.S. response to recent terrorist attacks, she argued for international consensus based on the alliance of principle and self-interest. Such consensus would need to be enforced both by carrot and stick and should, she said, include a public-private partnership to deal with ethical issues. Applying these ideas to reproductive cloning, she suggested that we think about which alliances would be necessary to prevent or limit the practice. Preventing routine use might be accomplished by establishing a clear ethical and professional line prohibiting reproductive cloning. Preventing exceptional use (a determined person with sufficient money to find a willing doctor) might not be possible. As far as stem cell research is concerned, Carbone argued that the larger the investment in such research, the bigger the carrot--the more the funder would be able to regulate the process. That, she suggested, argues for a government role in the funding. If the professional community does not respect the ethical line drawn by politicians, and alternative funding is available from either public sources abroad or private sources at home, the U.S. political debate runs the risk of becoming irrelevant.
"California Cloning" was organized by the Markkula Center for Applied Ethics and co-sponsored by the Bannan Center for Jesuit Education and Christian Values; the Center for Science, Technology, and Society; the SCU School of Law; the High Tech Law Institute; the Howard Hughes Medical Institute Community of Science Scholars Initiative; and the law firm of Latham & Watkins.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 08 September 2024
Peering into the mind: unraveling schizophrenia’s secrets using models
- João V. Nani ORCID: orcid.org/0000-0002-4336-1709 1 , 2 ,
- Alysson R. Muotri 3 &
- Mirian A. F. Hayashi ORCID: orcid.org/0000-0001-6437-4923 1 , 2
Molecular Psychiatry ( 2024 ) Cite this article
Metrics details
- Biological techniques
- Neuroscience
Schizophrenia (SCZ) is a complex mental disorder characterized by a range of symptoms, including positive and negative symptoms, as well as cognitive impairments. Despite the extensive research, the underlying neurobiology of SCZ remain elusive. To overcome this challenge, the use of diverse laboratory modeling techniques, encompassing cellular and animal models, and innovative approaches like induced pluripotent stem cell (iPSC)-derived neuronal cultures or brain organoids and genetically engineered animal models, has been crucial. Immortalized cellular models provide controlled environments for investigating the molecular and neurochemical pathways involved in neuronal function, while iPSCs and brain organoids, derived from patient-specific sources, offer significant advantage in translational research by facilitating direct comparisons of cellular phenotypes between patient-derived neurons and healthy-control neurons. Animal models can recapitulate the different psychopathological aspects that should be modeled, offering valuable insights into the neurobiology of SCZ. In addition, invertebrates’ models are genetically tractable and offer a powerful approach to dissect the core genetic underpinnings of SCZ, while vertebrate models, especially mammals, with their more complex nervous systems and behavioral repertoire, provide a closer approximation of the human condition to study SCZ-related traits. This narrative review provides a comprehensive overview of the diverse modeling approaches, critically evaluating their strengths and limitations. By synthesizing knowledge from these models, this review offers a valuable source for researchers, clinicians, and stakeholders alike. Integrating findings across these different models may allow us to build a more holistic picture of SCZ pathophysiology, facilitating the exploration of new research avenues and informed decision-making for interventions.
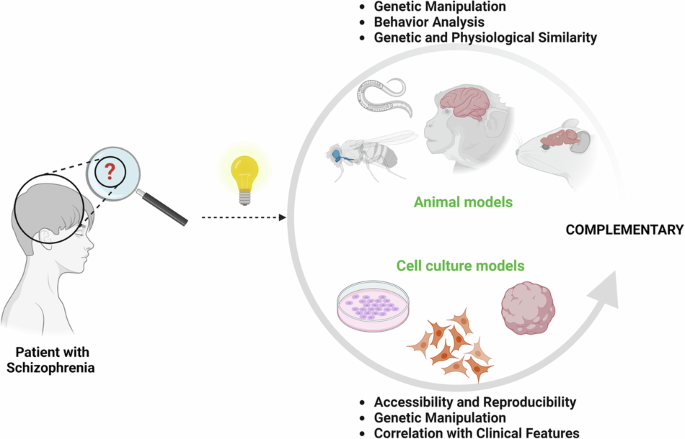
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
251,40 € per year
only 20,95 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
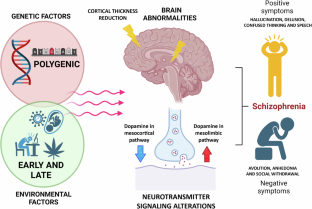
Similar content being viewed by others
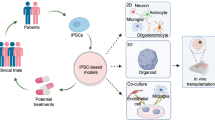
Opportunities and limitations for studying neuropsychiatric disorders using patient-derived induced pluripotent stem cells
Advancing preclinical models of psychiatric disorders with human brain organoid cultures
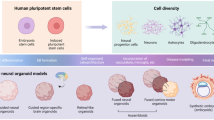
Human pluripotent stem cell (hPSC) and organoid models of autism: opportunities and limitations
Reynolds EH, Kinnier Wilson JV. Psychoses of epilepsy in Babylon: the oldest account of the disorder. Epilepsia. 2008;49:1488–90. https://doi.org/10.1111/j.1528-1167.2008.01614.x .
Article PubMed Google Scholar
Evans K, McGrath J, Milns R. Searching for schizophrenia in ancient Greek and Roman literature: a systematic review. Acta Psychiatr Scand. 2003;107:323–30. https://doi.org/10.1034/j.1600-0447.2003.00053.x .
Article CAS PubMed Google Scholar
Falkai P, Rossner MJ, Schulze TG, Hasan A, Brzózka MM, Malchow B, et al. Kraepelin revisited: schizophrenia from degeneration to failed regeneration. Mol Psychiatry. 2015;20:671–6. https://doi.org/10.1038/mp.2015.35 .
Jablensky A. The diagnostic concept of schizophrenia: its history, evolution, and future prospects. Dialogues Clin Neurosci. 2010;12:271–87. https://doi.org/10.31887/DCNS.2010.12.3/ajablensky .
Article PubMed PubMed Central Google Scholar
Rössler W, Riecher-Rössler A, Meise U. Wilhelm Griesinger and the concept of community care in 19th-century Germany. Hosp Community Psychiatry. 1994;45:818–22. https://doi.org/10.1176/ps.45.8.818 .
Glucksman ML. Freud’s “Project”: The Mind-Brain Connection Revisited. Psychodyn Psychiatry. 2016;44:69–90. https://doi.org/10.1521/pdps.2016.44.1.69 .
Tran The J. Freud, Griesinger and Foville: the influence of the nineteenth-century psychiatric tradition in the Freudian concept of delusion as an ‘attempt at recovery. Hist Psychiatry. 2021;32:323–34. https://doi.org/10.1177/0957154X211013726 .
Kane JM, Correll CU. Past and present progress in the pharmacologic treatment of schizophrenia. J Clin Psychiatry. 2010;71:1115–24. https://doi.org/10.4088/JCP.10r06264yel .
Kesby JP, Eyles DW, McGrath JJ, Scott JG. Dopamine, psychosis and schizophrenia: the widening gap between basic and clinical neuroscience. Transl Psychiatry. 2018;8:30 https://doi.org/10.1038/s41398-017-0071-9 .
Article CAS PubMed PubMed Central Google Scholar
Jauhar S, Johnstone M, McKenna PJ. Schizophrenia. Lancet. 2022;399:473–86. https://doi.org/10.1016/S0140-6736(21)01730-X .
Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39:1129–38. https://doi.org/10.1093/schbul/sbs118 .
Ban TA. Neuropsychopharmacology and the genetics of schizophrenia: a history of the diagnosis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:753–62. https://doi.org/10.1016/j.pnpbp.2004.05.021 .
Janoutová J, Janácková P, Serý O, Zeman T, Ambroz P, Kovalová M, et al. Epidemiology and risk factors of schizophrenia. Neuro Endocrinol Lett. 2016;37:1–8.
PubMed Google Scholar
Radua J, Ramella-Cravaro V, Ioannidis JPA, Reichenberg A, Phiphopthatsanee N, Amir T, et al. What causes psychosis? An umbrella review of risk and protective factors. World Psychiatry. 2018;17:49–66. https://doi.org/10.1002/wps.20490 .
Tandon R, Gaebel W, Barch DM, Bustillo J, Gur RE, Heckers S, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150:3–10. https://doi.org/10.1016/j.schres.2013.05.028 .
Hjorthøj C, Stürup AE, McGrath JJ, Nordentoft M. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry. 2017;4:295–301. https://doi.org/10.1016/S2215-0366(17)30078-0 .
Correll CU, Schooler NR. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat. 2020;16:519–34. https://doi.org/10.2147/NDT.S225643 .
Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–62. https://doi.org/10.1016/S0140-6736(13)60733-3 .
Maric NP, Jovicic MJ, Mihaljevic M, Miljevic C. Improving current treatments for schizophrenia. Drug Dev Res. 2016;77:357–67. https://doi.org/10.1002/ddr.21337 .
Nucifora FC Jr, Woznica E, Lee BJ, Cascella N, Sawa A. Treatment resistant schizophrenia: clinical, biological, and therapeutic perspectives. Neurobiol Dis. 2019;131:104257. https://doi.org/10.1016/j.nbd.2018.08.016 .
Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–96. https://doi.org/10.1001/archpsyc.1988.01800330013001 .
Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373:31–41. https://doi.org/10.1016/S0140-6736(08)61764-X .
Fonseca M, Carmo F, Martel F. Metabolic effects of atypical antipsychotics: molecular targets. J Neuroendocrinol. 2023;35:e13347. https://doi.org/10.1111/jne.13347 .
Ribeiro ELA, de Mendonça Lima T, Vieira MEB, Storpirtis S, Aguiar PM. Efficacy and safety of aripiprazole for the treatment of schizophrenia: an overview of systematic reviews. Eur J Clin Pharmacol. 2018;74:1215–33. https://doi.org/10.1007/s00228-018-2498-1 .
Gaitonde SA, Avet C, de la Fuente Revenga M, Blondel-Tepaz E, Shahraki A, Pastor AM, et al. Pharmacological fingerprint of antipsychotic drugs at the serotonin 5-HT2A receptor. Mol Psychiatry. 2024. https://doi.org/10.1038/s41380-024-02531-7 .
Korth C, Fangerau H. Blood tests to diagnose schizophrenia: self-imposed limits in psychiatry. Lancet Psychiatry. 2020;7:911–4. https://doi.org/10.1016/S2215-0366(20)30058-4 .
Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388:86–97. https://doi.org/10.1016/S0140-6736(15)01121-6 .
Ferrer I, Martinez A, Boluda S, Parchi P, Barrachina M. Brain banks: benefits, limitations and cautions concerning the use of post-mortem brain tissue for molecular studies. Cell Tissue Bank. 2008;9:181–94. https://doi.org/10.1007/s10561-008-9077-0 .
Abashkin DA, Kurishev AO, Karpov DS, Golimbet VE. Cellular models in schizophrenia research. Int J Mol Sci. 2021;22:8518. https://doi.org/10.3390/ijms22168518 .
Strickland JC. Guide to research techniques in neuroscience. J Undergrad Neurosci Educ. 2014;13:R1–2.
PubMed Central Google Scholar
Maqsood MI, Matin MM, Bahrami AR, Ghasroldasht MM. Immortality of cell lines: challenges and advantages of establishment. Cell Biol Int. 2013;37:1038–45. https://doi.org/10.1002/cbin.10137 .
Wiatrak B, Kubis-Kubiak A, Piwowar A, Barg E. PC12 cell line: cell types, coating of culture vessels, differentiation and other culture conditions. Cells. 2020;9:958. https://doi.org/10.3390/cells9040958 .
Westerink RH, Ewing AG. The PC12 cell as model for neurosecretion. Acta Physiol. 2008;192:273–85. https://doi.org/10.1111/j.1748-1716.2007.01805.x .
Article CAS Google Scholar
Wang L, Chen Q, Ma R, Zhang B, Yang P, Cao T, et al. Insight into mitochondrial dysfunction mediated by clozapine-induced inhibition of PGRMC1 in PC12 cells. Toxicology. 2023;491:153515. https://doi.org/10.1016/j.tox.2023.153515 .
Jankowska U, Skupien-Rabian B, Swiderska B, Prus G, Dziedzicka-Wasylewska M, Kedracka-Krok S. Proteome analysis of PC12 cells reveals alterations in translation regulation and actin signaling induced by clozapine. Neurochem Res. 2021;46:2097–111. https://doi.org/10.1007/s11064-021-03348-4 .
Zhuo C, Xu Y, Hou W, Chen J, Li Q, Liu Z, et al. Mechanistic/mammalian target of rapamycin and side effects of antipsychotics: insights into mechanisms and implications for therapy. Transl Psychiatry. 2022;12:13. https://doi.org/10.1038/s41398-021-01778-w .
Xiong YJ, Song YZ, Zhu Y, Zuo WQ, Zhao YF, Shen X, et al. Neuroprotective effects of olanzapine against rotenone-induced toxicity in PC12 cells. Acta Pharmacol Sin. 2020;41:508–15. https://doi.org/10.1038/s41401-020-0378-6 .
Wei Z, Bai O, Richardson JS, Mousseau DD, Li XM. Olanzapine protects PC12 cells from oxidative stress induced by hydrogen peroxide. J Neurosci Res. 2003;73:364–8. https://doi.org/10.1002/jnr.10668 .
Magliaro BC, Saldanha CJ. Clozapine protects PC-12 cells from death due to oxidative stress induced by hydrogen peroxide via a cell-type specific mechanism involving inhibition of extracellular signal-regulated kinase phosphorylation. Brain Res. 2009;1283:14–24. https://doi.org/10.1016/j.brainres.2009.05.063 .
Wang H, Xu H, Dyck LE, Li XM. Olanzapine and quetiapine protect PC12 cells from beta-amyloid peptide(25-35)-induced oxidative stress and the ensuing apoptosis. J Neurosci Res. 2005;81:572–80. https://doi.org/10.1002/jnr.20570 .
Ishizuka K, Paek M, Kamiya A, Sawa A. A review of disrupted-in-schizophrenia-1 (DISC1): neurodevelopment, cognition, and mental conditions. Biol Psychiatry. 2006;59:1189–97. https://doi.org/10.1016/j.biopsych.2006.03.065 .
Dahoun T, Trossbach SV, Brandon NJ, Korth C, Howes OD. The impact of disrupted-in-schizophrenia 1 (DISC1) on the dopaminergic system: a systematic review. Transl Psychiatry. 2017;7:e1015. https://doi.org/10.1038/tp.2016.282 .
Kamiya A, Tomoda T, Chang J, Takaki M, Zhan C, Morita M, et al. DISC1-NDEL1/NUDEL protein interaction, an essential component for neurite outgrowth, is modulated by genetic variations of DISC1. Hum Mol Genet. 2006;15:3313–23. https://doi.org/10.1093/hmg/ddl407 .
Taya S, Shinoda T, Tsuboi D, Asaki J, Nagai K, Hikita T, et al. DISC1 regulates the transport of the NUDEL/LIS1/14-3-3epsilon complex through kinesin-1. J Neurosci. 2007;27:15–26. https://doi.org/10.1523/JNEUROSCI.3826-06.2006 .
Pletnikov MV, Xu Y, Ovanesov MV, Kamiya A, Sawa A, Ross CA. PC12 cell model of inducible expression of mutant DISC1: new evidence for a dominant-negative mechanism of abnormal neuronal differentiation. Neurosci Res. 2007;8:234–44. https://doi.org/10.1016/j.neures.2007.03.003 .
Pezzini F, Bettinetti L, Di Leva F, Bianchi M, Zoratti E, Carrozzo R, et al. Transcriptomic profiling discloses molecular and cellular events related to neuronal differentiation in SH-SY5Y neuroblastoma cells. Cell Mol Neurobiol. 2017;37:665–82. https://doi.org/10.1007/s10571-016-0403-y .
Kovalevich J, Langford D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol Biol. 2013;1078:9–21. https://doi.org/10.1007/978-1-62703-640-5_2 .
Shin JH, Park SJ, Kim ES, Jo YK, Hong J, Cho DH. Sertindole, a potent antagonist at dopamine D 2 receptors, induces autophagy by increasing reactive oxygen species in SH-SY5Y neuroblastoma cells. Biol Pharm Bull. 2012;35:1069–75. https://doi.org/10.1248/bpb.b12-00009 .
Lee JG, Cho HY, Park SW, Seo MK, Kim YH. Effects of olanzapine on brain-derived neurotrophic factor gene promoter activity in SH-SY5Y neuroblastoma cells. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1001–6. https://doi.org/10.1016/j.pnpbp.2010.05.013 .
Goldie BJ, Barnett MM, Cairns MJ. BDNF and the maturation of posttranscriptional regulatory networks in human SH-SY5Y neuroblast differentiation. Front Cell Neurosci. 2014;8:325. https://doi.org/10.3389/fncel.2014.00325 .
Svoboda J, Stankova A, Entlerova M, Stuchlik A. Acute administration of MK-801 in an animal model of psychosis in rats interferes with cognitively demanding forms of behavioral flexibility on a rotating arena. Front Behav Neurosci. 2015;9:75. https://doi.org/10.3389/fnbeh.2015.00075 .
Zhu D, Zhang J, Wu J, Li G, Yao W, Hao J, et al. Paliperidone protects SH-SY5Y cells against MK-801-induced neuronal damage through inhibition of Ca(2+) influx and regulation of SIRT1/miR-134 signal pathway. Mol Neurobiol. 2016;53:2498–509. https://doi.org/10.1007/s12035-015-9217-z .
Unal G, Dokumaci AH, Ozkartal CS, Yerer MB, Aricioglu F. Famotidine has a neuroprotective effect on MK-801 induced toxicity via the Akt/GSK-3β/β-catenin signaling pathway in the SH-SY5Y cell line. Chem Biol Interact. 2019;314:108823. https://doi.org/10.1016/j.cbi.2019.108823 .
Mellios N, Sur M. The emerging role of microRNAs in schizophrenia and autism spectrum disorders. Front Psychiatry. 2012;3:39. https://doi.org/10.3389/fpsyt.2012.00039 .
Ryskalin L, Limanaqi F, Frati A, Busceti CL, Fornai F. mTOR-related brain dysfunctions in neuropsychiatric disorders. Int J Mol Sci. 2018;19:2226. https://doi.org/10.3390/ijms19082226 .
Kruse AO, Bustillo JR. Glutamatergic dysfunction in schizophrenia. Transl Psychiatry. 2022;12:500. https://doi.org/10.1038/s41398-022-02253-w .
Eastwood SL, Walker M, Hyde TM, Kleinman JE, Harrison PJ. The DISC1 Ser704Cys substitution affects centrosomal localization of its binding partner PCM1 in glia in human brain. Hum Mol Genet. 2010;19:2487–96. https://doi.org/10.1093/hmg/ddq130 .
Piñero-Martos E, Ortega-Vila B, Pol-Fuster J, Cisneros-Barroso E, Ruiz-Guerra L, Medina-Dols A, et al. Disrupted in schizophrenia 1 (DISC1) is a constituent of the mammalian mitochondrial contact site and cristae organizing system (MICOS) complex, and is essential for oxidative phosphorylation. Hum Mol Genet. 2016;25:4157–69. https://doi.org/10.1093/hmg/ddw250 .
Ramos A, Rodríguez-Seoane C, Rosa I, Gorroño-Etxebarria I, Alonso J, Veiga S, et al. Proteomic studies reveal disrupted in schizophrenia 1 as a player in both neurodevelopment and synaptic function. Int J Mol Sci. 2018;20:119. https://doi.org/10.3390/ijms20010119 .
Trossbach SV, Bader V, Hecher L, Pum ME, Masoud ST, Prikulis I, et al. Misassembly of full-length Disrupted-in-Schizophrenia 1 protein is linked to altered dopamine homeostasis and behavioral deficits. Mol Psychiatry. 2016;21:1561–72. https://doi.org/10.1038/mp.2015.194 .
Pertile RA, Cui X, Eyles DW. Vitamin D signaling and the differentiation of developing dopamine systems. Neuroscience. 2016;333:193–203. https://doi.org/10.1016/j.neuroscience.2016.07.020 .
Sei Y, Li Z, Song J, Ren-Patterson R, Tunbridge EM, Iizuka Y, et al. Epistatic and functional interactions of catechol-o-methyltransferase (COMT) and AKT1 on neuregulin1-ErbB signaling in cell models. PLoS ONE. 2010;5:e10789. https://doi.org/10.1371/journal.pone.0010789 .
Wu S, Wang P, Tao R, Yang P, Yu X, Li Y, et al. Schizophrenia associated microRNA 148b 3p regulates COMT and PRSS16 expression by targeting the ZNF804A gene in human neuroblastoma cells. Mol Med Rep. 2020;22:1429–39. https://doi.org/10.3892/mmr.2020.11230 .
Khavari B, Mahmoudi E, Geaghan MP, Cairns MJ. Oxidative stress impact on the transcriptome of differentiating neuroblastoma cells: implication for psychiatric disorders. Int J Mol Sci. 2020;21:9182. https://doi.org/10.3390/ijms21239182 .
Larouche A, Berube P, Sarret P, Grignon S. Subacute H2O2, but not poly(IC), upregulates dopamine D2 receptors in retinoic acid differentiated SH-SY5Y neuroblastoma. Synapse. 2008;62:70–3. https://doi.org/10.1002/syn.20458 .
Deslauriers J, Lefrançois M, Larouche A, Sarret P, Grignon S. Antipsychotic-induced DRD2 upregulation and its prevention by α-lipoic acid in SH-SY5Y neuroblastoma cells. Synapse. 2011;65:321–31. https://doi.org/10.1002/syn.20851 .
Sato T, Okumura F, Kano S, Kondo T, Ariga T, Hatakeyama S. TRIM32 promotes neural differentiation through retinoic acid receptor-mediated transcription. J Cell Sci. 2011;124:3492–502. https://doi.org/10.1242/jcs.088799 .
Yap MY, Lo YL, Talbot K, Ong WY. Oxidative stress reduces levels of dysbindin-1A via its PEST domain. Neurochem Int. 2014;79:65–9. https://doi.org/10.1016/j.neuint.2014.10.001 .
Jäkel S, Dimou L. Glial cells and their function in the adult brain: a journey through the history of their ablation. Front Cell Neurosci. 2017;11:24. https://doi.org/10.3389/fncel.2017.00024 .
Silver J, Schwab ME, Popovich PG. Central nervous system regenerative failure: role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harb Perspect Biol. 2014;7:a020602. https://doi.org/10.1101/cshperspect.a020602 .
Almeida PGC, Nani JV, Oses JP, Brietzke E, Hayashi MAF. Neuroinflammation and glial cell activation in mental disorders. Brain Behav Immun Health. 2019;2:100034. https://doi.org/10.1016/j.bbih.2019.100034 .
Nani JV, Almeida PGC, Noto C, Bressan RA, Brietzke E, Hayashi MAF. Unraveiling the correlation among neurodevelopmental and inflammatory biomarkers in patients with chronic schizophrenia. Nord J Psychiatry. 2022;76:559–64. https://doi.org/10.1080/08039488.2021.2023217 .
Gadelha A, Yonamine CM, Nering M, Rizzo LB, Noto C, Cogo-Moreira H, et al. Angiotensin converting enzyme activity is positively associated with IL-17a levels in patients with schizophrenia. Psychiatry Res. 2015;229:702–7. https://doi.org/10.1016/j.psychres.2015.08.018 .
Hartmann SM, Heider J, Wüst R, Fallgatter AJ, Volkmer H. Microglia-neuron interactions in schizophrenia. Front Cell Neurosci. 2024;18:1345349. https://doi.org/10.3389/fncel.2024.1345349 .
Goldsmith DR, Rapaport MH. Inflammation and negative symptoms of schizophrenia: implications for reward processing and motivational deficits. Front Psychiatry. 2020;11:46. https://doi.org/10.3389/fpsyt.2020.00046 .
De Vries GH, Boullerne AI. Glial cell lines: an overview. Neurochem Res. 2010;35:1978–2000. https://doi.org/10.1007/s11064-010-0318-9 .
Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27:229–37. https://doi.org/10.1016/0165-5728(90)90073-v .
Horvath RJ, Nutile-McMenemy N, Alkaitis MS, Deleo JA. Differential migration, LPS-induced cytokine, chemokine, and NO expression in immortalized BV-2 and HAPI cell lines and primary microglial cultures. J Neurochem. 2008;107:557–69. https://doi.org/10.1111/j.1471-4159.2008.05633.x .
Sarapultsev A, Gusev E, Komelkova M, Utepova I, Luo S, Hu D. JAK-STAT signaling in inflammation and stress-related diseases: implications for therapeutic interventions. Mol Biomed. 2023;4:40. https://doi.org/10.1186/s43556-023-00151-1 .
Long Y, Wang Y, Shen Y, Huang J, Li Y, Wu R, et al. Minocycline and antipsychotics inhibit inflammatory responses in BV-2 microglia activated by LPS via regulating the MAPKs/ JAK-STAT signaling pathway. BMC Psychiatry. 2023;23:514. https://doi.org/10.1186/s12888-023-05014-1 .
Zheng LT, Hwang J, Ock J, Lee MG, Lee WH, Suk K. The antipsychotic spiperone attenuates inflammatory response in cultured microglia via the reduction of proinflammatory cytokine expression and nitric oxide production. J Neurochem. 2008;107:1225–35. https://doi.org/10.1111/j.1471-4159.2008.05675.x .
Racki V, Marcelic M, Stimac I, Petric D, Kucic N. Effects of haloperidol, risperidone, and aripiprazole on the immunometabolic properties of BV-2 microglial cells. Int J Mol Sci. 2021;22:4399. https://doi.org/10.3390/ijms22094399 .
Ng BG, Xu G, Chandy N, Steyermark J, Shinde DN, Radtke K, et al. Biallelic mutations in FUT8 cause a congenital disorder of glycosylation with defective fucosylation. Am J Hum Genet. 2018;102:188–95. https://doi.org/10.1016/j.ajhg.2017.12.009 .
Fukuda T, Hashimoto H, Okayasu N, Kameyama A, Onogi H, Nakagawasai O, et al. Alpha1,6-fucosyltransferase-deficient mice exhibit multiple behavioral abnormalities associated with a schizophrenia-like phenotype: importance of the balance between the dopamine and serotonin systems. J Biol Chem. 2011;286:18434–43. https://doi.org/10.1074/jbc.M110.172536 .
Lu X, Zhang D, Shoji H, Duan C, Zhang G, Isaji T, et al. Deficiency of α1,6-fucosyltransferase promotes neuroinflammation by increasing the sensitivity of glial cells to inflammatory mediators. Biochim Biophys Acta Gen Subj. 2019;1863:598–608. https://doi.org/10.1016/j.bbagen.2018.12.008 .
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. https://doi.org/10.1016/j.cell.2006.07.024 .
McKinney CE. Using induced pluripotent stem cells derived neurons to model brain diseases. Neural Regen Res. 2017;12:1062–7. https://doi.org/10.4103/1673-5374.211180 .
Heider J, Vogel S, Volkmer H, Breitmeyer R. Human iPSC-derived glia as a tool for neuropsychiatric research and drug development. Int J Mol Sci. 2021;22:10254. https://doi.org/10.3390/ijms221910254 .
Page SC, Sripathy SR, Farinelli F, Ye Z, Wang Y, Hiler DJ, et al. Electrophysiological measures from human iPSC-derived neurons are associated with schizophrenia clinical status and predict individual cognitive performance. Proc Natl Acad Sci USA. 2022;119:e2109395119. https://doi.org/10.1073/pnas.2109395119 .
Hribkova H, Svoboda O, Bartecku E, Zelinkova J, Horinkova J, Lacinova L, et al. Clozapine reverses dysfunction of glutamatergic neurons derived from clozapine-responsive schizophrenia patients. Front Cell Neurosci. 2022;16:830757. https://doi.org/10.3389/fncel.2022.830757 .
Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–5. https://doi.org/10.1038/nature09915 .
Hook V, Brennand KJ, Kim Y, Toneff T, Funkelstein L, Lee KC. Human iPSC neurons display activity-dependent neurotransmitter secretion: aberrant catecholamine levels in schizophrenia neurons. Stem Cell Rep. 2014;3:531–8. https://doi.org/10.1016/j.stemcr.2014.08.001 .
Zuccoli GS, Nascimento JM, Moraes-Vieira PM, Rehen SK, Martins-de-Souza D. Mitochondrial, cell cycle control and neuritogenesis alterations in an iPSC-based neurodevelopmental model for schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2023. https://doi.org/10.1007/s00406-023-01605-x .
Tay SH, Winanto, Khong ZJ, Koh YH, Ng SY. Generation of cortical, dopaminergic, motor, and sensory neurons from human pluripotent stem cells. Methods Mol Biol. 2022;2549:359–77. https://doi.org/10.1007/7651_2021_399 .
Ni P, Noh H, Park GH, Shao Z, Guan Y, Park JM, et al. iPSC-derived homogeneous populations of developing schizophrenia cortical interneurons have compromised mitochondrial function. Mol Psychiatry. 2020;25:3103–4. https://doi.org/10.1038/s41380-019-0423-3 .
Kathuria A, Lopez-Lengowski K, Watmuff B, McPhie D, Cohen BM, Karmacharya R. Synaptic deficits in iPSC-derived cortical interneurons in schizophrenia are mediated by NLGN2 and rescued by N-acetylcysteine. Transl Psychiatry. 2019;9:321. https://doi.org/10.1038/s41398-019-0660-x .
Park JM, Liu D, Park GH, Noh H, Ni P, Yin C, et al. Migratory cortical interneuron-specific transcriptome abnormalities in schizophrenia. J Psychiatr Res. 2021;137:111–6. https://doi.org/10.1016/j.jpsychires.2021.02.054 .
Shao Z, Noh H, Bin Kim W, Ni P, Nguyen C, Cote SE, et al. Dysregulated protocadherin-pathway activity as an intrinsic defect in induced pluripotent stem cell-derived cortical interneurons from subjects with schizophrenia. Nat Neurosci. 2019;22:229–42. https://doi.org/10.1038/s41593-018-0313-z .
Farrelly LA, Zheng S, Schrode N, Topol A, Bhanu NV, Bastle RM, et al. Chromatin profiling in human neurons reveals aberrant roles for histone acetylation and BET family proteins in schizophrenia. Nat Commun. 2022;13:2195. https://doi.org/10.1038/s41467-022-29922-0 .
Nascimento JM, Saia-Cereda VM, Zuccoli GS, Reis-de-Oliveira G, Carregari VC, Smith BJ, et al. Proteomic signatures of schizophrenia-sourced iPSC-derived neural cells and brain organoids are similar to patients’ postmortem brains. Cell Biosci. 2022;12:189. https://doi.org/10.1186/s13578-022-00928-x .
Hoffman GE, Hartley BJ, Flaherty E, Ladran I, Gochman P, Ruderfer DM, et al. Transcriptional signatures of schizophrenia in hiPSC-derived NPCs and neurons are concordant with post-mortem adult brains. Nat Commun. 2017;8:2225. https://doi.org/10.1038/s41467-017-02330-5 .
Lin M, Pedrosa E, Hrabovsky A, Chen J, Puliafito BR, Gilbert SR, et al. Integrative transcriptome network analysis of iPSC-derived neurons from schizophrenia and schizoaffective disorder patients with 22q11.2 deletion. BMC Syst Biol. 2016;10:105. https://doi.org/10.1186/s12918-016-0366-0 .
Li J, Ryan SK, Deboer E, Cook K, Fitzgerald S, Lachman HM, et al. Mitochondrial deficits in human iPSC-derived neurons from patients with 22q11.2 deletion syndrome and schizophrenia. Transl Psychiatry. 2019;9:302. https://doi.org/10.1038/s41398-019-0643-y .
Kim NS, Wen Z, Liu J, Zhou Y, Guo Z, Xu C, et al. Pharmacological rescue in patient iPSC and mouse models with a rare DISC1 mutation. Nat Commun. 2021;12:1398. https://doi.org/10.1038/s41467-021-21713-3 .
Kikuchi M, Nakazawa T, Kinoshita M, Yamamori H, Yasuda Y, Fujimoto M, et al. Methylation analysis in monozygotic twins with treatment-resistant schizophrenia and discordant responses to clozapine. Front Psychiatry. 2021;12:734606. https://doi.org/10.3389/fpsyt.2021.734606 .
Kathuria A, Lopez-Lengowski K, Roffman JL, Karmacharya R. Distinct effects of interleukin-6 and interferon-γ on differentiating human cortical neurons. Brain Behav Immun. 2022;103:97–108. https://doi.org/10.1016/j.bbi.2022.04.007 .
Guennewig B, Bitar M, Obiorah I, Hanks J, O’Brien EA, Kaczorowski DC, et al. THC exposure of human iPSC neurons impacts genes associated with neuropsychiatric disorders. Transl Psychiatry. 2018;8:89. https://doi.org/10.1038/s41398-018-0137-3 .
Trindade P, Nascimento JM, Casas BS, Monteverde T, Gasparotto J, Ribeiro CT, et al. Induced pluripotent stem cell-derived astrocytes from patients with schizophrenia exhibit an inflammatory phenotype that affects vascularization. Mol Psychiatry. 2023;28:871–82. https://doi.org/10.1038/s41380-022-01830-1 .
Koskuvi M, Lehtonen Š, Trontti K, Keuters M, Wu YC, Koivisto H, et al. Contribution of astrocytes to familial risk and clinical manifestation of schizophrenia. Glia. 2022;70:650–60. https://doi.org/10.1002/glia.24131 .
Couch ACM, Solomon S, Duarte RRR, Marrocu A, Sun Y, Sichlinger L, et al. Acute IL-6 exposure triggers canonical IL6Ra signaling in hiPSC microglia, but not neural progenitor cells. Brain Behav Immun. 2023;110:43–59. https://doi.org/10.1016/j.bbi.2023.02.007 .
Qian X, Song H, Ming GL. Brain organoids: advances, applications and challenges. Development. 2019;146:dev166074. https://doi.org/10.1242/dev.166074 .
Trujillo CA, Muotri AR. Brain organoids and the study of neurodevelopment. Trends Mol Med. 2018;24:982–90. https://doi.org/10.1016/j.molmed.2018.09.005 .
Trujillo CA, Gao R, Negraes PD, Gu J, Buchanan J, Preissl S, et al. Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell. 2019;25:558–69.e7. https://doi.org/10.1016/j.stem.2019.08.002 .
Tan HY, Cho H, Lee LP. Human mini-brain models. Nat Biomed Eng. 2021;5:11–25. https://doi.org/10.1038/s41551-020-00643-3 .
Bagley JA, Reumann D, Bian S, Lévi-Strauss J, Knoblich JA. Fused cerebral organoids model interactions between brain regions. Nat Methods. 2017;14:743–51. https://doi.org/10.1038/nmeth.4304 .
Kathuria A, Lopez-Lengowski K, Jagtap SS, McPhie D, Perlis RH, Cohen BM, et al. Transcriptomic landscape and functional characterization of induced pluripotent stem cell-derived cerebral organoids in schizophrenia. JAMA Psychiatry. 2020;77:745–54. https://doi.org/10.1001/jamapsychiatry.2020.0196 .
Benson CA, Powell HR, Liput M, Dinham S, Freedman DA, Ignatowski TA, et al. Immune factor, TNFα, disrupts human brain organoid development similar to schizophrenia-schizophrenia increases developmental vulnerability to TNFα. Front Cell Neurosci. 2020;14:233. https://doi.org/10.3389/fncel.2020.00233 .
Notaras M, Lodhi A, Dündar F, Collier P, Sayles NM, Tilgner H, et al. Schizophrenia is defined by cell-specific neuropathology and multiple neurodevelopmental mechanisms in patient-derived cerebral organoids. Mol Psychiatry. 2022;27:1416–34. https://doi.org/10.1038/s41380-021-01316-6 .
Notaras M, Lodhi A, Fang H, Greening D, Colak D. The proteomic architecture of schizophrenia iPSC-derived cerebral organoids reveals alterations in GWAS and neuronal development factors. Transl Psychiatry. 2021;11:541. https://doi.org/10.1038/s41398-021-01664-5 .
Bradshaw NJ, Hayashi MA. NDE1 and NDEL1 from genes to (mal)functions: parallel but distinct roles impacting on neurodevelopmental disorders and psychiatric illness. Cell Mol Life Sci. 2017;74:1191–210. https://doi.org/10.1007/s00018-016-2395-7 .
Dal Mas C, Nani JV, Noto C, Yonamine CM, da Cunha GR, Mansur RB, et al. Ndel1NDEL1 oligopeptidase activity as a potential biomarker of early stages of schizophrenia. Schizophr Res. 2019;208:202–8. https://doi.org/10.1016/j.schres.2019.02.021 .
Gadelha A, Machado MF, Yonamine CM, Sato JR, Juliano MA, Oliveira V, et al. Plasma Ndel1NDEL1 enzyme activity is reduced in patients with schizophrenia—a potential biomarker? J Psychiatr Res. 2013;47:657–63. https://doi.org/10.1016/j.jpsychires.2013.01.009 .
Ye F, Kang E, Yu C, Qian X, Jacob F, Yu C, et al. DISC1 regulates neurogenesis via modulating kinetochore attachment of Ndel1NDEL1/Nde1 during mitosis. Neuron. 2017;96:1041–-54.e5. https://doi.org/10.1016/j.neuron.2017.10.010 .
Papes F, Camargo AP, de Souza JS, Carvalho VMA, Szeto RA, LaMontagne E, et al. Transcription Factor 4 loss-of-function is associated with deficits in progenitor proliferation and cortical neuron content. Nat Commun. 2022;13:2387. https://doi.org/10.1038/s41467-022-29942-w .
Goo BS, Mun DJ, Kim S, Nhung TTM, Lee SB, Woo Y, et al. Schizophrenia-associated mitotic arrest deficient-1 (MAD1) regulates the polarity of migrating neurons in the developing neocortex. Mol Psychiatry. 2023;28:856–70. https://doi.org/10.1038/s41380-022-01856-5 .
Robinson NB, Krieger K, Khan FM, Huffman W, Chang M, Naik A, et al. The current state of animal models in research: a review. Int J Surg. 2019;72:9–13. https://doi.org/10.1016/j.ijsu.2019.10.015 .
Suo S, Ishiura S, Van Tol HH. Dopamine receptors in C. elegans . Eur J Pharmacol. 2004;500:159–66. https://doi.org/10.1016/j.ejphar.2004.07.021 .
Furukubo-Tokunaga K. Modeling schizophrenia in flies. Prog Brain Res. 2009;179:107–15. https://doi.org/10.1016/S0079-6123(09)17912-8 .
Beauchamp A, Yee Y, Darwin BC, Raznahan A, Mars RB, Lerch JP. Whole-brain comparison of rodent and human brains using spatial transcriptomics. Elife. 2022;11:e79418. https://doi.org/10.7554/eLife.79418 .
Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106-107:1–16. https://doi.org/10.1016/j.pneurobio.2013.04.001 .
Nani JV, Rodríguez B, Cruz FC, Hayashi MAF. Animal models in psychiatric disorder studies. Animal models in medicine and biology. IntechOpen. 2020. https://doi.org/10.5772/intechopen.89034 .
Nagy S, Maurer GW, Hentze JL, Rose M, Werge TM, Rewitz K. AMPK signaling linked to the schizophrenia-associated 1q21.1 deletion is required for neuronal and sleep maintenance. PLoS Genet. 2018;14:e1007623. https://doi.org/10.1371/journal.pgen.1007623 .
Mullin AP, Sadanandappa MK, Ma W, Dickman DK, VijayRaghavan K, Ramaswami M, et al. Gene dosage in the dysbindin schizophrenia susceptibility network differentially affect synaptic function and plasticity. J Neurosci. 2015;35:325–38. https://doi.org/10.1523/JNEUROSCI.3542-14.2015 .
Hidalgo S, Castro C, Zárate RV, Valderrama BP, Hodge JJL, Campusano JM. The behavioral and neurochemical characterization of a Drosophila dysbindin mutant supports the contribution of serotonin to schizophrenia negative symptoms. Neurochem Int. 2020;138:104753. https://doi.org/10.1016/j.neuint.2020.104753 .
Lu B, Shao L, Feng S, Wang T, Zhong Y. The β-alanyl-monoamine synthase ebony is regulated by schizophrenia susceptibility gene dysbindin in Drosophila. Sci China Life Sci. 2014;57:46–51. https://doi.org/10.1007/s11427-013-4595-9 .
Sawamura N, Ando T, Maruyama Y, Fujimuro M, Mochizuki H, Honjo K, et al. Nuclear DISC1 regulates CRE-mediated gene transcription and sleep homeostasis in the fruit fly. Mol Psychiatry. 2008;13:1138–48. https://doi.org/10.1038/mp.2008.101 .
Shao L, Lu B, Wen Z, Teng S, Wang L, Zhao Y, et al. Disrupted-in-Schizophrenia-1 (DISC1) protein disturbs neural function in multiple disease-risk pathways. Hum Mol Genet. 2017;26:2634–48. https://doi.org/10.1093/hmg/ddx147 .
Ferguson L, Petty A, Rohrscheib C, Troup M, Kirszenblat L, Eyles DW, et al. Transient dysregulation of dopamine signaling in a developing drosophila arousal circuit permanently impairs behavioral responsiveness in adults. Front Psychiatry. 2017;8:22. https://doi.org/10.3389/fpsyt.2017.00022 .
Moulin TC, Stojanovic T, Rajesh RP, Pareek T, Donzelli L, Williams MJ, et al. Effects of transient administration of the NMDA receptor antagonist MK-801 in drosophila melanogaster activity, sleep, and negative geotaxis. Biomedicines. 2023;11:192. https://doi.org/10.3390/biomedicines11010192 .
Navrotskaya V, Wnorowski A, Turski W, Oxenkrug G. Effect of kynurenic acid on pupae viability of drosophila melanogaster cinnabar and cardinal eye color mutants with altered tryptophan-kynurenine metabolism. Neurotox Res. 2018;34:324–31. https://doi.org/10.1007/s12640-018-9891-5 .
Christensen EL, Beasley A, Radchuk J, Mielko ZE, Preston E, Stuckett S, et al. ngn-1/neurogenin activates transcription of multiple terminal selector transcription factors in the Caenorhabditis elegans nervous system. G3. 2020;10:1949–62. https://doi.org/10.1534/g3.120.401126 .
Chen SY, Huang PH, Cheng HJ. Disrupted-in-Schizophrenia 1-mediated axon guidance involves TRIO-RAC-PAK small GTPase pathway signaling. Proc Natl Acad Sci USA. 2011;108:5861–6. https://doi.org/10.1073/pnas.1018128108 .
Monte GG, Nani JV, de Almeida Campos MR, Dal Mas C, Marins LAN, Martins LG, et al. Impact of nuclear distribution element genes in the typical and atypical antipsychotics effects on nematode Caenorhabditis elegans : Putative animal model for studying the pathways correlated to schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:19–30. https://doi.org/10.1016/j.pnpbp.2018.12.010 .
Pedrini M, Cao B, Nani JVS, Cerqueira RO, Mansur RB, Tasic L, et al. Advances and challenges in development of precision psychiatry through clinical metabolomics on mood and psychotic disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2019;93:182–8. https://doi.org/10.1016/j.pnpbp.2019.03.010 .
Molina JD, Avila S, Rubio G, López-Muñoz F. Metabolomic connections between schizophrenia, antipsychotic drugs and metabolic syndrome: a variety of players. Curr Pharm Des. 2021;27:4049–61. https://doi.org/10.2174/1381612827666210804110139 .
Rodríguez B, Nani JV, Almeida PGC, Brietzke E, Lee RS, Hayashi MAF. Neuropeptides and oligopeptidases in schizophrenia. Neurosci Biobehav Rev. 2020;108:679–93. https://doi.org/10.1016/j.neubiorev.2019.11.024 .
Campeiro JD, Nani JV, Monte GG, Almeida PGC, Mori MA, Hayashi MAF. Regulation of monoamine levels by typical and atypical antipsychotics in Caenorhabditis elegans mutant for nuclear distribution element genes. Neurochem Int. 2021;147:105047. https://doi.org/10.1016/j.neuint.2021.105047 .
Karmacharya R, Lynn SK, Demarco S, Ortiz A, Wang X, Lundy MY, et al. Behavioral effects of clozapine: involvement of trace amine pathways in C. elegans and M. musculus . Brain Res. 2011;1393:91–9. https://doi.org/10.1016/j.brainres.2011.04.010 .
Osuna-Luque J, Rodríguez-Ramos Á, Gámez-Del-Estal MDM, Ruiz-Rubio M. Behavioral mechanisms that depend on dopamine and serotonin in Caenorhabditis elegans interact with the antipsychotics risperidone and aripiprazole. J Exp Neurosci. 2018;12:1179069518798628. https://doi.org/10.1177/1179069518798628 .
Refai O, Blakely RD. Blockade and reversal of swimming-induced paralysis in C. elegans by the antipsychotic and D2-type dopamine receptor antagonist azaperone. Neurochem Int. 2019;123:59–68. https://doi.org/10.1016/j.neuint.2018.05.013 .
Maior RS, Nishijo H, Caixeta FV. Editorial: Non-human primate models of psychiatric disorders. Front Behav Neurosci. 2021;15:774064. https://doi.org/10.3389/fnbeh.2021.774064 .
Fedigan LM. Ethical issues faced by field primatologists: asking the relevant questions. Am J Primatol. 2010;72:754–71. https://doi.org/10.1002/ajp.20814 .
Campbell PD, Granato M. Zebrafish as a tool to study schizophrenia-associated copy number variants. Dis Model Mech. 2020;13:dmm043877. https://doi.org/10.1242/dmm.043877 .
Carlsson A, Lindqvist M. Effect of chlorpromazine or haloperidol on formation of 3methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol Toxicol. 1963;20:140–4. https://doi.org/10.1111/j.1600-0773.1963.tb01730.x .
McCutcheon RA, Abi-Dargham A, Howes OD. Schizophrenia, dopamine and the striatum: from biology to symptoms. Trends Neurosci. 2019;42:205–20. https://doi.org/10.1016/j.tins.2018.12.004 .
Canetta S, Kellendonk C. Can we use mice to study schizophrenia? Philos Trans R Soc Lond B Biol Sci. 2018;373:20170032. https://doi.org/10.1098/rstb.2017.0032 .
Wang P, Li M, Zhao A, Ma J. Application of animal experimental models in the research of schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2021;186:209–27. https://doi.org/10.1002/ajmg.b.32863 .
Winship IR, Dursun SM, Baker GB, Balista PA, Kandratavicius L, Maia-de-Oliveira JP, et al. An overview of animal models related to schizophrenia. Can J Psychiatry. 2019;64:5–17. https://doi.org/10.1177/0706743718773728 .
Sato K. Why is prepulse inhibition disrupted in schizophrenia? Med Hypotheses. 2020;143:109901. https://doi.org/10.1016/j.mehy.2020.109901 .
Nikiforuk A. Assessment of cognitive functions in animal models of schizophrenia. Pharmacol Rep. 2018;70:639–49. https://doi.org/10.1016/j.pharep.2018.01.009 .
Jones CA, Watson DJ, Fone KC. Animal models of schizophrenia. Br J Pharmacol. 2011;164:1162–94. https://doi.org/10.1111/j.1476-5381.2011.01386.x .
van den Buuse M. Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects. Schizophr Bull. 2010;36:246–70. https://doi.org/10.1093/schbul/sbp132 .
Blot K, Bai J, Otani S. The effect of non-competitive NMDA receptor antagonist MK-801 on neuronal activity in rodent prefrontal cortex: an animal model for cognitive symptoms of schizophrenia. J Physiol Paris. 2013;107:448–51. https://doi.org/10.1016/j.jphysparis.2013.04.003 .
Cadinu D, Grayson B, Podda G, Harte MK, Doostdar N, Neill JC. NMDA receptor antagonist rodent models for cognition in schizophrenia and identification of novel drug treatments, an update. Neuropharmacology. 2018;142:41–62. https://doi.org/10.1016/j.neuropharm.2017.11.045 .
Featherstone RE, Kapur S, Fletcher PJ. The amphetamine-induced sensitized state as a model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1556–71. https://doi.org/10.1016/j.pnpbp.2007.08.025 .
Weidenauer A, Bauer M, Sauerzopf U, Bartova L, Praschak-Rieder N, Sitte HH, et al. Making sense of: sensitization in schizophrenia. Int J Neuropsychopharmacol. 2016;20:1–10. https://doi.org/10.1093/ijnp/pyw081 .
Peleg-Raibstein D, Knuesel I, Feldon J. Amphetamine sensitization in rats as an animal model of schizophrenia. Behav Brain Res. 2008;191:190–201. https://doi.org/10.1016/j.bbr.2008.03.037 .
Wearne TA, Mirzaei M, Franklin JL, Goodchild AK, Haynes PA, Cornish JL. Methamphetamine-induced sensitization is associated with alterations to the proteome of the prefrontal cortex: implications for the maintenance of psychotic disorders. J Proteome Res. 2015;14:397–410. https://doi.org/10.1021/pr500719f .
Abekawa T, Ito K, Nakagawa S, Nakato Y, Koyama T. Effects of aripiprazole and haloperidol on progression to schizophrenia-like behavioural abnormalities and apoptosis in rodents. Schizophr Res. 2011;125:77–87. https://doi.org/10.1016/j.schres.2010.08.011 .
Herrera AS, Casanova JP, Gatica RI, Escobar F, Fuentealba JA. Clozapine pre-treatment has a protracted hypolocomotor effect on the induction and expression of amphetamine sensitization. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47:1–6. https://doi.org/10.1016/j.pnpbp.2013.07.023 .
Almey A, Arena L, Oliel J, Shams WM, Hafez N, Mancinelli C, et al. Interactions between estradiol and haloperidol on perseveration and reversal learning in amphetamine-sensitized female rats. Horm Behav. 2017;89:113–20. https://doi.org/10.1016/j.yhbeh.2016.12.010 .
Jaaro-Peled H. Gene models of schizophrenia: DISC1 mouse models. Prog Brain Res. 2009;179:75–86. https://doi.org/10.1016/S0079-6123(09)17909-8 .
Nani JV, Fonseca MC, Engi SA, Perillo MG, Dias CS, Gazarini ML, et al. Decreased nuclear distribution nudE-like 1 enzyme activity in an animal model with dysfunctional disrupted-in-schizophrenia 1 signaling featuring aberrant neurodevelopment and amphetamine-supersensitivity. J Psychopharmacol. 2020;34:467–77. https://doi.org/10.1177/0269881119897562 .
Nason MW Jr, Adhikari A, Bozinoski M, Gordon JA, Role LW. Disrupted activity in the hippocampal-accumbens circuit of type III neuregulin 1 mutant mice. Neuropsychopharmacology. 2011;36:488–96. https://doi.org/10.1038/npp.2010.180 .
Schneider S, Götz K, Birchmeier C, Schwegler H, Roskoden T. Neuregulin-1 mutant mice indicate motor and sensory deficits, indeed few references for schizophrenia endophenotype model. Behav Brain Res. 2017;322:177–85. https://doi.org/10.1016/j.bbr.2017.01.022 .
Tomiyama K, O’Tuathaigh CM, O’Sullivan GJ, Kinsella A, Lai D, Harvey RP, et al. Phenotype of spontaneous orofacial dyskinesia in neuregulin-1 ‘knockout’ mice. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:330–3. https://doi.org/10.1016/j.pnpbp.2008.12.010 .
Benedetti A, Molent C, Barcik W, Papaleo F. Social behavior in 16p11.2 and 22q11.2 copy number variations: insights from mice and humans. Genes Brain Behav. 2022;21:e12787. https://doi.org/10.1111/gbb.12787 .
Stark KL, Xu B, Bagchi A, Lai WS, Liu H, Hsu R, et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008;40:751–60. https://doi.org/10.1038/ng.138 .
Horev G, Ellegood J, Lerch JP, Son YE, Muthuswamy L, Vogel H, et al. Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. Proc Natl Acad Sci USA. 2011;108:17076–81. https://doi.org/10.1073/pnas.1114042108 .
Kimber WL, Hsieh P, Hirotsune S, Yuva-Paylor L, Sutherland HF, Chen A, et al. Deletion of 150 kb in the minimal DiGeorge/velocardiofacial syndrome critical region in mouse. Hum Mol Genet. 1999;8:2229–37. https://doi.org/10.1093/hmg/8.12.2229 .
Estes ML, McAllister AK. Maternal immune activation: implications for neuropsychiatric disorders. Science. 2016;353:772–7. https://doi.org/10.1126/science.aag3194 .
Haddad FL, Patel SV, Schmid S. Maternal immune activation by poly I:C as a preclinical model for neurodevelopmental disorders: a focus on autism and schizophrenia. Neurosci Biobehav Rev. 2020;113:546–67. https://doi.org/10.1016/j.neubiorev.2020.04.012 .
Guerrin CGJ, Doorduin J, Sommer IE, de Vries EFJ. The dual hit hypothesis of schizophrenia: evidence from animal models. Neurosci Biobehav Rev. 2021;131:1150–68. https://doi.org/10.1016/j.neubiorev.2021.10.025 .
Uzuneser TC, Speidel J, Kogias G, Wang AL, de Souza Silva MA, Huston JP, et al. Disrupted-in-schizophrenia 1 (DISC1) overexpression and juvenile immune activation cause sex-specific schizophrenia-related psychopathology in rats. Front Psychiatry. 2019;10:222. https://doi.org/10.3389/fpsyt.2019.00222 .
Feigenson KA, Kusnecov AW, Silverstein SM. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev. 2014;38:72–93. https://doi.org/10.1016/j.neubiorev.2013.11.006 .
Giovanoli S, Engler H, Engler A, Richetto J, Feldon J, Riva MA, et al. Preventive effects of minocycline in a neurodevelopmental two-hit model with relevance to schizophrenia. Transl Psychiatry. 2016;6:e772. https://doi.org/10.1038/tp.2016.38 .
Liu R, Meng X, Yu X, Wang G, Dong Z, Zhou Z, et al. From 2D to 3D co-culture systems: a review of co-culture models to study the neural cells interaction. Int J Mol Sci. 2022;23:13116. https://doi.org/10.3390/ijms232113116 .
Dixon TA, Muotri AR. Advancing preclinical models of psychiatric disorders with human brain organoid cultures. Mol Psychiatry. 2023;28:83–95. https://doi.org/10.1038/s41380-022-01708-2 .
Mateos-Aparicio P, Bello SA, Rodríguez-Moreno A. Challenges in physiological phenotyping of hiPSC-derived neurons: from 2D cultures to 3D brain organoids. Front Cell Dev Biol. 2020;8:797. https://doi.org/10.3389/fcell.2020.00797 .
Ardhanareeswaran K, Mariani J, Coppola G, Abyzov A, Vaccarino FM. Human induced pluripotent stem cells for modelling neurodevelopmental disorders. Nat Rev Neurol. 2017;13:265–78. https://doi.org/10.1038/nrneurol.2017.45 .
Volpato V, Webber C. Addressing variability in iPSC-derived models of human disease: guidelines to promote reproducibility. Dis Model Mech. 2020;13:dmm042317. https://doi.org/10.1242/dmm.042317 .
Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, et al. Assembly of functionally integrated human forebrain spheroids. Nature. 2017;545:54–9. https://doi.org/10.1038/nature22330 .
Pham MT, Pollock KM, Rose MD, Cary WA, Stewart HR, Zhou P, et al. Generation of human vascularized brain organoids. Neuroreport. 2018;29:588–93. https://doi.org/10.1097/WNR.0000000000001014 .
Sloan SA, Andersen J, Pașca AM, Birey F, Pașca SP. Generation and assembly of human brain region-specific three-dimensional cultures. Nat Protoc. 2018;13:2062–85. https://doi.org/10.1038/s41596-018-0032-7 .
Jeziorski J, Brandt R, Evans JH, Campana W, Kalichman M, Thompson E, et al. Brain organoids, consciousness, ethics and moral status. Semin Cell Dev Biol. 2023;144:97–102. https://doi.org/10.1016/j.semcdb.2022.03.020 .
Dwyer DS. Crossing the worm-brain barrier by using Caenorhabditis elegans to explore fundamentals of human psychiatric illness. Mol Neuropsychiatry. 2018;3:170–9. https://doi.org/10.1159/000485423 .
van Alphen B, van Swinderen B. Drosophila strategies to study psychiatric disorders. Brain Res Bull. 2013;92:1–11. https://doi.org/10.1016/j.brainresbull.2011.09.007 .
Cannarozzi G, Schneider A, Gonnet G. A phylogenomic study of human, dog, and mouse. PLoS Comput Biol. 2007;3:e2. https://doi.org/10.1371/journal.pcbi.0030002 .
Forrest AD, Coto CA, Siegel SJ. Animal models of psychosis: current state and future directions. Curr Behav Neurosci Rep. 2014;1:100–16. https://doi.org/10.1007/s40473-014-0013-2 .
Białoń M, Wąsik A. Advantages and limitations of animal schizophrenia models. Int J Mol Sci. 2022;23:5968. https://doi.org/10.3390/ijms23115968 .
Liang SG, Greenwood TA. The impact of clinical heterogeneity in schizophrenia on genomic analyses. Schizophr Res. 2015;161:490–5. https://doi.org/10.1016/j.schres.2014.11.019 .
Chandra A, Miller BJ, Goldsmith DR. Predictors of successful anti-inflammatory drug trials in patients with schizophrenia: a meta-regression and critical commentary. Brain Behav Immun. 2023;114:154–62. https://doi.org/10.1016/j.bbi.2023.08.001 .
Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry. 2015;2:258–70. https://doi.org/10.1016/S2215-0366(14)00122-9 .
Borbély E, Scheich B, Helyes Z. Neuropeptides in learning and memory. Neuropeptides. 2013;47:439–50. https://doi.org/10.1016/j.npep.2013.10.012 .
Perez-Bonilla P, Santiago-Colon K, Leinninger GM. Lateral hypothalamic area neuropeptides modulate ventral tegmental area dopamine neurons and feeding. Physiol Behav. 2020;223:112986. https://doi.org/10.1016/j.physbeh.2020.112986 .
Griebel G. Neuropeptide receptor ligands for the treatment of schizophrenia: focus on neurotensin and tachykinins. Curr Pharm Des. 2015;21:3807–12. https://doi.org/10.2174/1381612821666150605105859 .
LaCrosse AL, Olive MF. Neuropeptide systems and schizophrenia. CNS Neurol Disord Drug Targets. 2013;12:619–32. https://doi.org/10.2174/1871527311312050010 .
Harrow M, Jobe TH, Tong L. Long-term effectiveness of antipsychotics. Psychol Med. 2023;53:1129–33. https://doi.org/10.1017/S0033291721001732 .
Telias M. Neural differentiation protocols: how to choose the correct approach. Neural Regen Res. 2023;18:1273–4. https://doi.org/10.4103/1673-5374.360171 .
Martino M, Magioncalda P. A three-dimensional model of neural activity and phenomenal-behavioral patterns. Mol Psychiatry. 2024. https://doi.org/10.1038/s41380-023-02356-w .
Howes OD, Onwordi EC. The synaptic hypothesis of schizophrenia version III: a master mechanism. Mol Psychiatry. 2023;28:1843–56. https://doi.org/10.1038/s41380-023-02043-w .
Ye H, Fussenegger M. Optogenetic Medicine: Synthetic Therapeutic Solutions Precision-Guided by Light. Cold Spring Harb Perspect Med. 2019;9:a034371. https://doi.org/10.1101/cshperspect.a034371 .
Qiao C, Li D, Guo Y, Liu C, Jiang T, Dai Q, et al. Evaluation and development of deep neural networks for image super-resolution in optical microscopy. Nat Methods. 2021;18:194–202. https://doi.org/10.1038/s41592-020-01048-5 .
Lopez-Lengowski K, Kathuria A, Gerlovin K, Karmacharya R. Co-culturing microglia and cortical neurons differentiated from human induced pluripotent stem cells. J Vis Exp. 2021. https://doi.org/10.3791/62480 .
Tan SY, Feng X, Cheng LKW, Wu AR. Vascularized human brain organoid on-chip. Lab Chip. 2023;23:2693–709. https://doi.org/10.1039/d2lc01109c .
Cheng C, Chen W, Jin H, Chen X. A Review of Single-Cell RNA-Seq Annotation, Integration, and Cell-Cell Communication. Cells. 2023;12:1970. https://doi.org/10.3390/cells12151970 .
Wu Y, Zhang CY, Wang L, Li Y, Xiao X. Genetic insights of schizophrenia via single cell RNA-sequencing analyses. Schizophr Bull. 2023;49:914–22. https://doi.org/10.1093/schbul/sbad002 .
Chilla GS, Yeow LY, Chew QH, Sim K, Prakash KNB. Machine learning classification of schizophrenia patients and healthy controls using diverse neuroanatomical markers and Ensemble methods. Sci Rep. 2022;12:2755. https://doi.org/10.1038/s41598-022-06651-4 .
Santos Febles E, Ontivero Ortega M, Valdés Sosa M, Sahli H. Machine Learning Techniques for the Diagnosis of Schizophrenia Based on Event-Related Potentials. Front Neuroinform. 2022;16:893788. https://doi.org/10.3389/fninf.2022.893788 .
Montazeri M, Montazeri M, Bahaadinbeigy K, Montazeri M, Afraz A. Application of machine learning methods in predicting schizophrenia and bipolar disorders: a systematic review. Health Sci Rep. 2022;6:e962. https://doi.org/10.1002/hsr2.962 .
Hauser TU, Skvortsova V, De Choudhury M, Koutsouleris N. The promise of a model-based psychiatry: building computational models of mental ill health. Lancet Digit Health. 2022;4:e816–28. https://doi.org/10.1016/S2589-7500(22)00152-2 .
Lally J, Watkins R, Nash S, Shetty H, Gardner-Sood P, Smith S, et al. The representativeness of participants with severe mental illness in a psychosocial clinical trial. Front Psychiatry. 2018;9:654. https://doi.org/10.3389/fpsyt.2018.00654 .
Huxley P, Krayer A, Poole R, Prendergast L, Aryal S, Warner R. Schizophrenia outcomes in the 21st century: a systematic review. Brain Behav. 2021;11:e02172. https://doi.org/10.1002/brb3.2172 .
Levy N. The use of animal as models: ethical considerations. Int J Stroke. 2012;7:440–2. https://doi.org/10.1111/j.1747-4949.2012.00772.x .
Qureshi MNI, Oh J, Cho D, Jo HJ, Lee B. Multimodal discrimination of schizophrenia using hybrid weighted feature concatenation of brain functional connectivity and anatomical features with an extreme learning machine. Front Neuroinform. 2017;11:59. https://doi.org/10.3389/fninf.2017.00059 .
Download references
Acknowledgements
All figures were created with BioRender.com. We would like to thank the anonymous reviewers for their constructive and helpful comments, which have significantly improved the quality and clarity of our manuscript.
This work was supported by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) [Nos. 2022/00527-8; 2020/01107-7; 2019/13112-8; 2019/08287-3; 2017/02413-1; 2014/50891-1 (INCT 2014 - TRANSLATIONAL MEDICINE)], CAPES and CNPq. MAFH is also a recipient of a fellowship from CNPq [39337/2016-0]. JVN is recipient of a Fellowship from FAPESP Nos. 2022/03297-3 and 2019/09207-3).
Author information
Authors and affiliations.
Department of Pharmacology, Escola Paulista de Medicina (EPM), Universidade Federal de São Paulo (UNIFESP), São Paulo, SP, Brazil
João V. Nani & Mirian A. F. Hayashi
National Institute for Translational Medicine (INCT-TM, CNPq/FAPESP/CAPES), Ribeirão Preto, Brazil
Department of Pediatrics and Department of Molecular and Cellular Medicine, University of California, San Diego, La Jolla, CA, USA
Alysson R. Muotri
You can also search for this author in PubMed Google Scholar
Contributions
JVN was responsible for the literature review, writing, and figure preparation. JVN, MAFH, and ARM contributed equally to the conceptualization, editing, and revision of the paper.
Corresponding authors
Correspondence to João V. Nani or Mirian A. F. Hayashi .
Ethics declarations
Competing interests.
ARM is a co-founder and has an equity interest in TISMOO, a company dedicated to genetic analysis and human brain organogenesis, focusing on therapeutic applications customized for autism spectrum disorders and other neurological disorders origin genetics. The terms of this arrangement have been reviewed and approved by the University of California, San Diego, following its conflict-of-interest policies.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Nani, J.V., Muotri, A.R. & Hayashi, M.A.F. Peering into the mind: unraveling schizophrenia’s secrets using models. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-024-02728-w
Download citation
Received : 23 August 2023
Revised : 21 August 2024
Accepted : 27 August 2024
Published : 08 September 2024
DOI : https://doi.org/10.1038/s41380-024-02728-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Med Sci
- v.15(1); 2018
Ethical and Safety Issues of Stem Cell-Based Therapy
Vladislav volarevic.
1 University of Kragujevac, Serbia, Faculty of Medical Sciences, Department of Microbiology and Immunology, Center for Molecular Medicine and Stem Cell Research;
Bojana Simovic Markovic
Marina gazdic.
2 University of Kragujevac, Serbia, Faculty of Medical Sciences, Department of Genetics;
Ana Volarevic
Nemanja jovicic.
3 University of Kragujevac, Serbia, Faculty of Medical Sciences, Department of Histology and Embryology;
Nebojsa Arsenijevic
Lyle armstrong.
4 Institute of Genetic Medicine, Newcastle University, UK;
Valentin Djonov
5 Institute of Anatomy, University of Bern, Bern, Switzerland.
Majlinda Lako
Miodrag stojkovic.
Results obtained from completed and on-going clinical studies indicate huge therapeutic potential of stem cell-based therapy in the treatment of degenerative, autoimmune and genetic disorders. However, clinical application of stem cells raises numerous ethical and safety concerns.
In this review, we provide an overview of the most important ethical issues in stem cell therapy, as a contribution to the controversial debate about their clinical usage in regenerative and transplantation medicine.
We describe ethical challenges regarding human embryonic stem cell (hESC) research, emphasizing that ethical dilemma involving the destruction of a human embryo is a major factor that may have limited the development of hESC-based clinical therapies. With previous derivation of induced pluripotent stem cells (iPSCs) this problem has been overcome, however current perspectives regarding clinical translation of iPSCs still remain. Unlimited differentiation potential of iPSCs which can be used in human reproductive cloning, as a risk for generation of genetically engineered human embryos and human-animal chimeras, is major ethical issue, while undesired differentiation and malignant transformation are major safety issues.
Although clinical application of mesenchymal stem cells (MSCs) has shown beneficial effects in the therapy of autoimmune and chronic inflammatory diseases, the ability to promote tumor growth and metastasis and overestimated therapeutic potential of MSCs still provide concerns for the field of regenerative medicine.
This review offers stem cell scientists, clinicians and patient's useful information and could be used as a starting point for more in-depth analysis of ethical and safety issues related to clinical application of stem cells.
Introduction
Stem cells have raised tremendous expectations among the medical doctors, researchers, patients, and the general public due to their capacity to differentiate into a broad range of cell types. Stem cell researchers are engaged in different endeavors, including treating genetic disorders and generating new stem cell-derived human tissues and biomaterials for use in pharmacy genomics and regenerative medicine. Results obtained from completed and on-going clinical studies indicate huge therapeutic potential of stem cell-based therapy in the treatment of degenerative, autoimmune and genetic disorders 1 , 2 .
However, clinical application of stem cells raises some ethical and safety concerns. In this review we provide an overview of the most important ethical issues in stem cell research and therapy, as a contribution to the debate about their clinical use in regenerative and transplantation medicine. We describe and discuss ethical challenges regarding human embryonic stem cell (hESC) research, therapeutic potential and clinical translation of induced pluripotent stem cell (iPSC) and safety issues of mesenchymal stem cell (MSC)-based therapy.
Our hope is that stem cell scientists and clinicians will use the information presented herein as a starting point for more in-depth analysis of ethical and safety issues related to clinical translation of stem cells since controversial regulation and application of stem cell therapy has been falsely celebrated not only in countries with lax medical regulations but also in many developed countries. For instance, in 2016, 351 US businesses engaged in frequently unproven and direct-to-consumer marketing of different stem cell interventions was offered at 570 clinics 3 .
Ethical and safety concerns regarding hESC-based therapy
hESCs are stem cells derived from the pluripotent inner cell mass of the pre-implantation embryos 4 , 5 . hESCs express typical pluripotent stem cell markers such as octamer-binding transcription factor 3/4 ( OCT3/4 ), stage specific embryonic antigens 3 and 4 (SSEA-3 and SSEA-4), TRA-1-60, TRA-1-81 and alkaline phosphatase, possess high levels of telomerase activity and show normal karyotype 6 , 7 . hESCs have capacity to differentiate into cell types of all three germ layers [endoderm, mesoderm, and ectoderm] under in vitro and in vivo conditions 6 , 7 . Consequently, hESCs hold great promise in understanding of early human embryology and for developing the cell replacement strategies for the treatment of human diseases (Figure (Figure1 1 ).

Schematic diagram describing characteristics of ESCs. Embryonic stem cells (ESCs) are harvested from a blastocyst. Embryonic stem (ES) cells are derived from the inner cell mass of the pre-implantation embryo. Fully characterized hESCs express typical pluripotent stem cell markers such as octamer-binding transcription factor 3/4 (OCT3/4), stage specific embryonic antigens 3 and 4 (SSEA-3 and SSEA-4), TRA-1-60, and TRA-1-81.These cells are pluripotent, meaning they can differentiate into cells from all three germ layers (ectoderm, mesoderm and endoderm). Main ethical issues (labeled with question marks): isolation of ESCs involves the destruction of a human embryo; transplantation of undifferentiated ESCs may result with a formation of teratomas, tumors that contain all three germ layers.
Nevertheless, the ethical dilemma involving the destruction of a human embryo was and remains a major factor that has slowed down the development of hESC-based clinical therapies.
The fundamental question is: Whether it is morally acceptable to pursue novel therapies for curing illnesses at the expense of destroying an early human embryo? This debate brings out individual opinions so deeply rooted in basic moral beliefs that developing a definitive policy acceptable to everyone seems unlikely. This ethical dilemma is portrayed in different legislation that exists throughout the world regulating hESCs research 8 , 9 . For example, in many countries including United Kingdom, it is illegal to perform nuclear transfer (NT) for reproductive or therapeutic purposes, while use of hESCs for research is allowed. Other countries retain more extreme stances, as is the case of Italy where there is a prohibition on all hESC-based research. On contrary, it is legal to use supernumerary in vitro fertilization (IVF)-derived embryos for derivation of new hESCs lines and to perform NT for the generation of patient-specific stem cells in the United Kingdom 10 - 12 . United States banned production of any hESCs line that requires the destruction of an embryo and research using hESCs lines is limited on usage of lines created prior to August 9, 2001. Present restrictions have additionally slowed the progress of hESCs technology and provide a significant barrier to the development of cell based clinical therapies. Additionally, the ethical debate surrounding the harvest of hESCs has made research on this topic controversial, and as a result, the majority of studies were focused on animal models 13 .
It is important to highlight that beside ethical concerns, safety issues regarding hESC-based therapy are the main problem for their clinical use. The pluripotency of hESCs is a double-edged sword; the same plasticity that permits hESCs to generate hundreds of different cell types also makes them difficult to control after in vivo transplantation 14 . When undifferentiated hESCs are transplanted, teratomas, tumors that contain all three germ layers, could develop [Figure [Figure1] 1 ] 15 . Studies have revealed that appearance of teratoma is between 33-100% in hESC-transplanted immunodeficient mice, depending on the implantation site, cell maturation, purity, and implantation techniques 16 , 17 .
Currently, the only way to ensure that teratoma will not develop after hESC transplantation is to differentiate them in desired and mature cell type before injection and screen them for the presence of undifferentiated cells. When such procedures were rigorously followed, teratomas were not observed in over 200 animals transplanted with hESC-derived cardiomyocytes 18 . However, unwanted and uncontrolled differentiation of hESCs was still noticed despite following up of this procedure. Primitive population of nestin+ neuroepithelial cells, that continued to proliferate in the striatum, was noticed in rats with Parkinson disease, 70 days after transplantation of hESC-derived dopamine neurons 19 . This raises a cautionary flag and suggests that even committed progenitors can proliferate excessively after transplantation, a problem that may be solved by improving purification methods.
However, despite these safety concerns, recently published data 20 suggest that under controlled conditions, hESC-derived cells could serve as a potentially safe new source in regenerative medicine.
Clinical trial that investigates potential of hESC-based therapy for the treatment of diabetes mellitus is opened and recruitment of patients has begun in 2014 21 . The goal of this study is to evaluate the safety and efficacy of VC-01, an implant containing hESCs derived pancreatic progenitor cells encapsulated by an immune protecting device, which would allow the cells to proliferate and differentiate into mature β-cells in vivo without the possibility of immune rejection 22 .
Recently, Song and co-workers 20 , reported that subretinal transplantation of hESC-derived retinal pigment epithelial cells (hESC-RPE) in four Asian patients: two with dry age-related macular degeneration and two with Stargardt macular dystrophy was safe and well tolerated procedure. Visual acuity improved 9-19 letters in three patients and remained stable [+1 letter] in one patient. During one year follow-up period, serious safety issues related to the transplanted cells such as: adverse proliferation, tumorigenicity, ectopic tissue formation, was not observed. Based on these encouraging results, during the past few years, several clinical trials are investigating therapeutic potential of hESC-RPE in patients with Stargardt macular dystrophy and advanced dry age related macular degeneration (Table (Table1, 1 , left panel) and promising results are expecting in next year.
Clinical trials using hESC-RPE and iPSC-derived cells
| hESC-RPE cellular therapy | iPSC-derived cells in clinical trials | ||
|---|---|---|---|
| Condition | ClinicalTrials.gov Identifier number/ Phase/ Status | Condition | ClinicalTrials.gov Identifier number/ Source/ Status |
| NCT02903576/ I/II/ study is currently recruiting participants | | NCT02564484/ blood/ study is currently recruiting participants | |
| NCT01344993/ I/II/ study has been completed | NCT02246491/ blood, skin/ study is currently recruiting participants | ||
| NCT01345006/ I/II/ study has been completed | | NCT02926963/ hair, skin/ study is currently recruiting participants | |
| NCT03046407/ early 1/ study is currently recruiting participants | | NCT02193724/ skin, blood/ study is currently recruiting participants | |
| NCT01469832/ I/II/ study has been completed | NCT02720939/ blood/ study is currently recruiting participants | ||
| | NCT02590692/ I/II/ study is currently recruiting participants | | NCT02896387/ skin, cornea/ study is currently recruiting participants |
| NCT02755428/ early 1/ study is currently recruiting participants | |||
| | NCT02749734/ I/ study is currently recruiting participants | NCT02980302/ skin/ study is currently recruiting participants | |
| NCT02286089/ I/II/ study is currently recruiting participants | |||
| NCT03102138/ I/ study is currently recruiting participants | |||
Advances and challenges of iPSC technology
iPSC are very similar to hESCs in terms of karyotype, phenotype, telomerase activity and capacity for differentiation. However, iPSCs are considered morally superior to hESCs since their generation does not require destruction of embryos 23 . Takahashi and Yamanaka demonstrated the first direct reprogramming of mammalian somatic cells 24 . Up-regulation of “Yamanaka factors”: sex determining region Y box-containing gene 2 [ SOX2 ], OCT3/4 , tumor suppressor Krüppel-like factor 4 [ KLF4 ], and proto-oncogene c-MYC managed to reprogram differentiated somatic cells in the pluripotent state 24 .
Since then, iPSCs technology provides a historic opportunity to move away from embryo destruction and opened a new era of personalized medicine. Patient-specific iPSCs may be helpful in drug screening, generating in vitro models of human diseases, and novel reproductive techniques (Figure (Figure2). 2 ). In vitro , patient-specific iPSCs can differentiate to specific cell types which enable testing of new drugs in patient-specific conditions. Since iPSC-derived cells are generated from somatic cells previously obtained from a patient, there is no risk of immune rejection after their transplantation 25 . The development of reproductive technology enables generation of gametes (sperm and eggs) from human iPSCs 26 . This technique could be helpful for treating infertility, however, the use of iPSC-derived gametes raises set of ethical concerns related to the potential exploitation of created embryos, human NT, and risk of change natural reproduction including the possibility to derive gametes for same-sex reproduction, as well as in the asexual reproduction 26 .

Potential applications of human induced pluripotent stem cells (iPSCs). iPSC technology can be potentially utilized in disease modeling, drug discovery, gene therapy, and cell replacement therapy. Genetic mutations can be corrected by gene targeting approaches before or after reprogramming. iPSCs are considered morally superior then ESCs since their generation do not require destruction of embryos. Introduction of the four transcription factors-“Yamanaka factors“ (Oct-4, Sox-2, Klf-4, and c-Myc) leads to reprogramming of a somatic cell to an iPSC which can further differentiate into different types of cells. Two types of methods for the delivery of reprogramming factors into the somatic cells can be used: integrating viral vector systems and non-integrating methods. The main safety issue regarding iPSC-based therapy (labeled with question marks) is the risk of teratoma formation which might happen if patient receive iPSC-derived cells that contain undifferentiated iPSC and dilemma whether retroviral and lentiviral-free iPSC are safe for clinical application.
As for hESCs the main safety issue regarding iPSC-based therapy is the risk of teratoma formation which can happened if patient receive iPSC-derived cells that contain undifferentiated iPSC (Figure (Figure2). 2 ). Uncontrolled proliferation and differentiation of transplanted undifferentiated iPSCs may result in generation of tumors and/or undesired differentiation of iPSCs in broad range of somatic cells 27 . Thus, development of more effective methods for generation of purified populations of autologous iPSC-derived differentiated cells remains a challenge for personalized and regenerative medicine 28 .
It is important to highlight here that due to the genomic instability of iPSCs 29 , even improved protocols for their differentiation, does not guarantee safe clinical application and underlines several differences compared to hESCs 30 - 32 .
Transformation of iPSCs into tumor cells could be a consequence of oncogenic properties of the reprogramming cocktail (use of c-MYC) 33 , or insertional mutagenesis induced by the reprogramming with integrating retroviral or lentiviral vectors which disrupts endogenous genes 34 . Recently, clinical trial that investigated potential of autologous iPSC-RPE for the treatment of advanced neovascular age-related macular degeneration has been stopped 35 . Although transplantation of iPSC-RPE in the first enrolled patient was well tolerated after one year follow-up, study was stopped when it moved on to a possible second patient. Since iPSC, derived from second patient contained mutation, they did not pass a genomic validation step and the team led by Takahashi decided to at least temporarily suspend the trial. However, what remains unclear at this time and what should be explored is whether the mutation in the second patient's iPSC was pre-existing in the patient's fibroblasts or it occurred during the reprogramming process itself.
In order to make the transition of iPSC-based therapy from lab to clinic, recently conducted research studies are focusing on identifying new molecular strategies that can increase cell reprogramming efficiency without causing genetic and epigenetic abnormalities in the iPSCs 36 . Several types of non-integrating methods have been developed [use of non-integrating adenoviral vectors, repeated transfection of plasmids, Cre-loxP- mediated recombination , PiggyBac- transposition] 37 - 41 .
Unfortunately, there is still insufficient data to argue that these retroviral and lentiviral-free iPSC are safe for clinical application (Figure (Figure2). 2 ). Accordingly, further in vitro and in vivo , animal, studies are necessary to develop optimized growth and differentiation protocols and reliable safety assays to evaluate the potential of iPSCs and iPSC-derived differentiated cells for clinical application in patients.
Several clinical trials that are going to explore clinical potential of iPSC-derived cells are currently recruiting patients (Table (Table1, 1 , right panel) and scientific and public community curiously expects these results.
Mesenchymal stem cells: key players in the cell-based therapy of immune-mediated diseases
Mesenchymal stem cells are adult, fibroblast-like, multipotent cells, most frequently isolated from bone marrow (BM), adipose tissue (AT) and umbilical cord blood (UCB) 42 . The International Society for Cellular Therapy formulated minimal criteria for uniform characterization of MSCs such as plastic adherence, potential for differentiation in osteogenic, chondrogenic, and adipogenic lineage, cell surface expression of CD105, CD73, CD90 and the absence of hematopoietic markers CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR (Figure (Figure3) 3 ) 43 .

Differentiation ability and immune-modulatory characteristics of MSCs. MSCs are adult, fibroblast-like, multipotent cells, most frequently isolated from bone marrow (BM), adipose tissue (AT) and umbilical cord blood (UCB). Minimal criteria for characterization of MSCs are: cell surface expression of CD105, CD73, CD90 and the absence of hematopoietic markers CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR. MSCs have been applied clinically in patients with inflammatory bowel diseases (IBD), liver disorders and cardiac diseases with very encouraging results. MSCs possess broad spectrum of immuno-modulatory capacities. Serious adverse events noticed in some of MSC-treated patients could be explained by the fact that MSCs either suppress or promote inflammation in dependence of inflammatory environment to which they are exposed to. The primary concerns for clinical application of MSCs (labeled with question marks) are unwanted differentiation of the transplanted MSCs and their potential to suppress anti-tumor immune response and generate new blood vessels that may promote tumor growth and metastasis.
These cells can differentiate into a variety of cell types of mesodermal origin and due to their plasticity, some studies 44 - 46 claim that MSCs can differentiate towards cells of neuro-ectodermal (neurons, astrocytes, and oligodendrocytes) or endodermal (hepatocytes) origin 47 . In addition to their differentiation potential, MSCs possess broad spectrum of immuno-modulatory capacities 48 . MSCs 'primed' by pro-inflammatory cytokines (interferon gamma and tumor necrosis factor alpha) adopt immunosuppressive phenotype, and through cell-to-cell contact (engagement of the inhibitory molecule programmed death 1 with its ligands) or through the production of soluble factors (transforming growth factor-β (TGF-β), interleukin (IL)-10, hepatocyte growth factor (HGF), prostaglandin E2, nitric oxide, indoleamine 2,3 dioxygenase and heme-oxygenase-1) modulate the adaptive and innate immune response 42 , 49 . In addition, MSCs lack the expression of membrane bound molecules involved in immune rejection which enable their allogenic transplantation 50 .
Accordingly, the past decade has witnessed an outstanding scientific production focused towards the possible clinical applications of MSCs in the therapy of autoimmune and chronic inflammatory diseases including inflammatory bowel diseases (IBD), liver disorders and cardiac diseases with very encouraging results (Figure (Figure3) 3 ) 51 - 70 .
MSCs in IBD therapy
Instantly, there are two routes for the administration of MSCs in IBDs patients: intravenous administration for the systemic control of intestinal inflammation in the therapy of luminal Chron's disease (CD) and ulcerative colitis (UC), and the local administration as a therapeutic approach for patients with perianal fistulazing CD 51 - 58 . Administration of autologous or allogeneic MSCs derived from BM and AT achieved significant clinical efficacy in patients with fistulazing CD by attenuating local immune response and by promoting tissue repair 51 - 58 .
Results obtained in huge number of clinical trials 51 - 55 indicate that local application of autologous and allogeneic BM-MSCs and AT-MSCs are simple, safe, and beneficial therapy for the treatment of perianal fistulas in CD patients with no adverse effects. On contrary, adverse effects have been reported in three of nine improved clinical trials 56 that investigated therapeutic potential of intravenously injected MSCs.
Study conducted by Duijvestein and coworkers 56 documented that 6 weeks after MSCs treatment, three patients required surgery due to disease worsening. Similar results were noticed in another clinical trial 57 . In this study, autologous MSCs, derived from marrow aspirate and propagated for 2-3 weeks with fibrinogen depleted human platelet lysate, were administered to IBD patients. Twelve patients received single MSCs intravenous infusion of 2, 5 or 10 million cells/kg and serious adverse events were seen in seven patients. Aggravation of disease was noticed in five patients while adverse events in other two patients were possibly related to the infusion of MSCs 57 .
Moreover, serious side effects were seen in patients with moderate to severe UC that received Multistem (stem cells derived from adult BM and non-embryonic tissue sources) as potentially new therapeutic agent for the treatment of UC 58 .
Serious adverse events noticed in some of MSC-treated patients could be explained by the fact that MSCs either suppress or promote inflammation in dependence of inflammatory environment to which they are exposed to 59 . When MSCs are transplanted in the tissue with high levels of pro-inflammatory cytokines (IFN-γ, TNF-α, IL-12, IL-6, IL-17 and IL-23), MSCs adopt an immuno-suppressive phenotype and modify maturation of DCs, promote conversion of macrophages in anti-inflammatory M2 phenotype and suppress proliferation and activation of T lymphocytes, NK and NKT cells. In the presence of low levels of inflammatory cytokines, MSCs adopt a pro-inflammatory phenotype and produce inflammatory cytokines that promote neutrophil and T cell activation and enhance immune response and inflammation 59 .
MSC-based therapy of liver diseases
Over the past few years, several clinical trials used MSCs to treat patients with liver diseases 60 - 65 . Obtained results demonstrated that MSCs treatment improved liver function in safe and well tolerated manner 60 - 65 . Amer and colleagues demonstrated the safety and short-term therapeutic effect of autologous transplantation of bone marrow MSCs-derived hepatocyte-like cells in patients with end-stage liver failure 61 . In patients with liver failure caused by hepatitis B virus infection, autologous transplantation of BM-MSCs provided short-term efficacy in respect to several clinical and biochemical parameters, but long-term outcomes were not markedly improved 62 . Recent studies reported that infusion of umbilical cord-derived MSCs was well tolerated in patients with decompensated cirrhosis, and in patients suffering from acute on chronic liver failure, resulting in significant improvement of liver function and increased survival rates 64 , 65 .
MSCs as a promising tool in the therapy of cardiac diseases
Several studies have examined therapeutic potential of autologous and allogeneic MSCs in the treatment of acute myocardial infarction (MI) 66 - 70 . In a phase I clinical study 66 , 53 patients were randomized to receive either allogeneic MSCs or placebo, 7 to 10 days after MI. An improvement of overall clinical status was noticed 6 months after intravenous infusion of MSCs. Chen and colleagues 67 administered autologous MSCs intra-coronary in patients with subacute MI and observed decreased perfusion defect, improved left ventricular ejection fraction, and left ventricular remodeling 3 months after therapy.
Currently, there are several published or ongoing clinical trials that demonstrated beneficent effects of MSC-based therapy in the treatment of chronic ischemic cardiomyopathy. Injection of MSCs attenuated fibrosis, induced neo-angiogenesis, enhanced contractility, and improved the quality of life of patients with chronic ischemic cardiomyopathy 66 - 70 . Additionally, it was reported that intracoronary transplantation of autologous MSCs reduced episodes of tachycardia in patients with chronic ischemic cardiomyopathy and implanted cardioverter defibrillator 69 . Haack-Sørensen and co-workers performed demonstrated that intra-myocardial injections of autologous MSC significantly improve quality of life, physical limitation and angina stability of patients with chronic coronary artery disease and refractory angina 70 .
The other side of the coin: safety issues regarding MSCs-based therapy
Despite these promising results, safety issues regarding MSCs-based therapy are still a matter of debate, especially in the long-term follow up. The primary concern is unwanted differentiation of the transplanted MSCs and their potential to suppress anti-tumor immune response and generate new blood vessels that may promote tumor growth and metastasis.
MSCs have a potential to differentiate into undesired tissues, including bone and cartilage. Encapsulated structures were found in the infarcted areas of myocardium after transplantation of MSCs. The structures contained calcifications or ossifications 71 . Study conducted by Yoon et colleagues showed that transplantation of unfractionated BM-derived cells into acutely infarcted myocardium may induce development of intra-myocardial calcification 72 .
It was recently reported that three women suffering from macular degeneration, within a week of undergoing “adipose tissue stem cell”-based therapy developed complications including vision loss, detached retinas and bleeding and are now totally blind and unlikely to recover 73 . The treatment involved combining fat tissue removed from the patients' abdomens with enzymes to obtain “adipose-derived” stem cells. These were mixed with blood plasma containing large numbers of platelets and injected into the women's eyes. Although, usually experimental eye procedures are tested on one eye first so that if something goes wrong the patient is still able to see with the other eye, in this trial both eyes were treated at once which, at the end, resulted with complete blindness in these patients.
These results suggest that local microenvironment in which MSCs engraft contains factors that induce unwanted differentiation of transplanted MSCs in vivo. Therefore, new research studies should be focused in definition of factors and signaling pathways that are responsible for the fate of MSCs after their in vivo administration.
In addition to unwanted differentiation, MSCs may bridge the gap between anti-tumor immune response and neo-angiogenesis in malignant diseases, thus promoting tumor growth and metastasis. After injection, MSCs migrate towards primary tumors 74 where due to their immuno-modulatory characteristics; suppress anti-tumor immune response resulting with an increased tumor growth 75 , 76 . We showed that injection of human MSCs promotes tumor growth and metastasis in tumor bearing mice, which was accompanied by lower cytotoxic activity of NK and CD8+ T cells and increased presence of immuno-suppressive IL-10 producing T lymphocytes and CD4+Foxp3+ T regulatory cells 77 . MSCs promote polarization of immune response towards anti-inflammatory Th2 pathway creating an immunosuppressive environment which enables progression of tumor growth and metastasis 77 .
Additionally, MSCs promote metastasis by enhancing generation of new blood vessels. MSCs have the capacity to differentiate into endothelial cells and to create a capillary network 78 , 79 . Injected MSCs migrate to the metastatic sites 74 and produce pro-angiogenic factors: vascular endothelial growth factor, basic fibroblast growth factor, TGF-β, platelet-derived growth factor, angiopoietin-1, placental growth factor, IL-6, monocyte chemotactic protein-1, HGF, resulting with neo-vascularization 80 .
Conclusions
The creation and clinical use of hESCs have long been the unique focus of stem cell ethics. Current ethical controversies regarding stem cell-based therapy are focused on the unlimited differentiation potential of iPSCs which can be used in human cloning, as a risk for generation of human embryos and human-animal chimeras.
Since undesired differentiation and malignant transformation are major safety issues regarding transplantation of iPSCs and iPSC-derived cells, protocols for differentiation of iPSCs should be optimized in order to ensure the purity of iPSC-derived populations of differentiated cells before their clinical use. Considering the fact that MSCs are frequently and worldwide offered as universal human remedy but may promote tumor growth and metastasis, studies which utilize MSCs should be focused in continuous monitoring and long-term follow-up of MSC-treated animal models in order to determine possible pro-tumorigenic and other detrimental effects of MSC-based therapy.
Acknowledgments
This study was supported by “Start Up for Science” grant funded by Phillip Morris and Center for Leadership Development, Swiss National Science Foundation project (SCOPES IZ73Z0_152454/1), Serbian Ministry of Science (ON175069 and ON175103) and Faculty of Medical Sciences University of Kragujevac (MP01/14 and MP01/12). Lako holds an ERC fellowship (614620).
Abbreviations
| hESC | human embryonic stem cell |
| iPSCs | induced pluripotent stem cells |
| MSCs | mesenchymal stem cells |
| OCT3/4 | octamer-binding transcription factor 3/4 |
| SSEA-3 and SSEA-4 | stage specific embryonic antigens 3 and 4 |
| NT | nuclear transfer |
| IVF | in vitro fertilization |
| hESC-RPE | retinal pigment epithelial cells |
| BM | bone marrow |
| AT | adipose tissue |
| UCB | umbilical cord blood |
| TGF-β | transforming growth factor-β |
| IL | interleukin |
| HGF | hepatocyte growth factor |
| IBD | inflammatory bowel diseases |
| CD | Chron's disease |
| UC | ulcerative colitis |
| MI | myocardial infarction. |

COMMENTS
Recognizing the ethical implications of stem cell research
hESC Research •Local oversight:Each institution should establish an oversight committee to review and monitor all proposals to conduct hESC research •ESCRO/SCRO committeesshould include representatives of the public and persons with expertise in developmental biology, stem cell research, molecular biology, assisted reproduction, and
Ethics of Stem Cell Research. First published Fri Apr 25, 2008; substantive revision Wed Dec 19, 2018. Human embryonic stem cell (HESC) research offers much hope for alleviating the human suffering brought on by the ravages of disease and injury. HESCs are characterized by their capacity for self-renewal and their ability to differentiate into ...
In this article, I place stem cell research in a broader ethics and policy context by describing three considerations that merit more attention in the debate. These include the following: (1) truth-telling and scientific integrity; (2) priorities in resource allocation for research and health care; and (3) responsibilities in civic discourse ...
The essays address the ethics of stem cell research from a variety of viewpoints. The first essay by Katrien Devolder and John Harris argues that our view of embryos is ethically inconsistent. This inconsistency is evident in the example of identical twins, which result from splitting of the early embryo.
Ethical issues in stem cell research and therapy
Nevertheless, the fact that an outcome was going to happen anyway does often mitigate causing or ensuring that outcome. A second way in which the argument is. 3 See my footnote 7 for a discussion of how the ethics of the situation would be different if the cells could be extracted without destroying the embryo.
Insoo Hyun1,2,*. Stem cells are increasingly being used to model human develop-ment and disease in the form of self-organizing embryo models, brain organoids, and neurological chimeras. These new research di-rections are resurrecting old embryo debates around moral status and personhood. Hyun considers how these old questions are tackled in ...
The International Stem Cell Forum (ISCF) supports research on all stem cell types, including adult stem cells, embryonic stem cells, and stem cells from umbilical cord blood (1). The Ethics Working Party (EWP) of the ISCF has 19 participants from 17 (2) countries. In 2004, the EWP defined its mission as (i) clarification of ethical issues and, where possible, the international harmonization of ...
Examining the ethics of embryonic stem cell research
The ethical implications of stem cell research are often discussed in terms of risks, side effects, and safety, which are examples of hard impacts. In this article, Assen and colleagues argue that to understand the broader spectrum of ethical implications of stem cell research on science and society, it is important to recognize the so-called soft impacts.
Summary of possible uses of stem cells: To provide lab-grown human or animal tissue for identifying new treatments for disease, including new drugs and other substances, rather than using animals. To produce new human tissue and organs to replace those lost in injury or disease. See box, Organ regeneration.
The author has been working on reproductive ethics in general and on embryo and stem cell research ethics in particular for more than ten years. Her book is based on several previously published articles, but it is far more than a mere collection or a re-use of essays." - Marco Stier, Ethical Theory and Moral Practice
Abstract. Stem cells are increasingly being used to model human development and disease in the form of self-organizing embryo models, brain organoids, and neurological chimeras. These new research directions are resurrecting old embryo debates around moral status and personhood. Hyun considers how these old questions are tackled in these new ...
Research design. A qualitative research design was used to explore and describe the perceptions and experiences of participants regarding the ethical challenges of using stem cells in a "subjective and reflexive manner" [].The aim was to gather, explore, analyze, and extract the most meaningful perceptions of the sample using interviews.
Ethical Issues in Stem Cell Research - PMC - NCBI
Human embryonic stem cells: research, ethics and policy
The process of researching consensus included consultations with key stakeholders in hESC research (regulations, stem cell researchers, and research ethics experts), preparation and distribution of background papers, and an international workshop held in Montreal in February 2007 to discuss the papers and debate recommendations.
One position that opponents of embryonic stem cell research assert is what "The Ethics of Embryonic Stem Cell Research" calls the full moral status view (14). This view holds that "the early embryo has the same moral status, that is, the same basic moral rights, claims, or interests as an ordinary adult human being."
Human embryonic stem (hES) cells are unique in their demonstrated potential to differentiate into all cell lineages. Reports by T. Wakayama et al. ("Differentiation of embryonic stem cell lines generated from adult somatic cells by nuclear transfer," 27 Apr., p. 740) and N. Lumelsky et al. ("Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic ...
GERMANY. The Central Ethics Committee for Stem Cell Research (2002) Reviews and evaluates applications for import and use of hESCs as required by the Stem Cell Act. Issues a written opinion on each application to the licensing authority—the Robert Koch Institute. 2002 Act ensuring the protection of embryos in connection with the importation ...
The Ethics of Human Cloning and Stem Cell Research
Supported by evidence from preclinical models, GWAS, stem cell research, and MRI/PET (magnetic resonance imaging/positron-emission tomography) Imaging, the proposed third version of the synaptic ...
Ethical and Safety Issues of Stem Cell-Based Therapy - PMC
Metrum Research Group, Tariffville, Connecticut, USA. Search for more papers by this author ... Search for more papers by this author. Timothy Waterhouse, Timothy Waterhouse ... of vedolizumab for acute graft-versus-host disease prophylaxis in adults undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT) and assess potential ...