Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- Ethical Considerations in Research | Types & Examples

Ethical Considerations in Research | Types & Examples
Published on October 18, 2021 by Pritha Bhandari . Revised on May 9, 2024.
Ethical considerations in research are a set of principles that guide your research designs and practices. Scientists and researchers must always adhere to a certain code of conduct when collecting data from people.
The goals of human research often include understanding real-life phenomena, studying effective treatments, investigating behaviors, and improving lives in other ways. What you decide to research and how you conduct that research involve key ethical considerations.
These considerations work to
- protect the rights of research participants
- enhance research validity
- maintain scientific or academic integrity
Table of contents
Why do research ethics matter, getting ethical approval for your study, types of ethical issues, voluntary participation, informed consent, confidentiality, potential for harm, results communication, examples of ethical failures, other interesting articles, frequently asked questions about research ethics.
Research ethics matter for scientific integrity, human rights and dignity, and collaboration between science and society. These principles make sure that participation in studies is voluntary, informed, and safe for research subjects.
You’ll balance pursuing important research objectives with using ethical research methods and procedures. It’s always necessary to prevent permanent or excessive harm to participants, whether inadvertent or not.
Defying research ethics will also lower the credibility of your research because it’s hard for others to trust your data if your methods are morally questionable.
Even if a research idea is valuable to society, it doesn’t justify violating the human rights or dignity of your study participants.
Here's why students love Scribbr's proofreading services
Discover proofreading & editing
Before you start any study involving data collection with people, you’ll submit your research proposal to an institutional review board (IRB) .
An IRB is a committee that checks whether your research aims and research design are ethically acceptable and follow your institution’s code of conduct. They check that your research materials and procedures are up to code.
If successful, you’ll receive IRB approval, and you can begin collecting data according to the approved procedures. If you want to make any changes to your procedures or materials, you’ll need to submit a modification application to the IRB for approval.
If unsuccessful, you may be asked to re-submit with modifications or your research proposal may receive a rejection. To get IRB approval, it’s important to explicitly note how you’ll tackle each of the ethical issues that may arise in your study.
There are several ethical issues you should always pay attention to in your research design, and these issues can overlap with each other.
You’ll usually outline ways you’ll deal with each issue in your research proposal if you plan to collect data from participants.
| Voluntary participation | Your participants are free to opt in or out of the study at any point in time. |
|---|---|
| Informed consent | Participants know the purpose, benefits, risks, and funding behind the study before they agree or decline to join. |
| Anonymity | You don’t know the identities of the participants. Personally identifiable data is not collected. |
| Confidentiality | You know who the participants are but you keep that information hidden from everyone else. You anonymize personally identifiable data so that it can’t be linked to other data by anyone else. |
| Potential for harm | Physical, social, psychological and all other types of harm are kept to an absolute minimum. |
| Results communication | You ensure your work is free of or research misconduct, and you accurately represent your results. |
Voluntary participation means that all research subjects are free to choose to participate without any pressure or coercion.
All participants are able to withdraw from, or leave, the study at any point without feeling an obligation to continue. Your participants don’t need to provide a reason for leaving the study.
It’s important to make it clear to participants that there are no negative consequences or repercussions to their refusal to participate. After all, they’re taking the time to help you in the research process , so you should respect their decisions without trying to change their minds.
Voluntary participation is an ethical principle protected by international law and many scientific codes of conduct.
Take special care to ensure there’s no pressure on participants when you’re working with vulnerable groups of people who may find it hard to stop the study even when they want to.
Informed consent refers to a situation in which all potential participants receive and understand all the information they need to decide whether they want to participate. This includes information about the study’s benefits, risks, funding, and institutional approval.
You make sure to provide all potential participants with all the relevant information about
- what the study is about
- the risks and benefits of taking part
- how long the study will take
- your supervisor’s contact information and the institution’s approval number
Usually, you’ll provide participants with a text for them to read and ask them if they have any questions. If they agree to participate, they can sign or initial the consent form. Note that this may not be sufficient for informed consent when you work with particularly vulnerable groups of people.
If you’re collecting data from people with low literacy, make sure to verbally explain the consent form to them before they agree to participate.
For participants with very limited English proficiency, you should always translate the study materials or work with an interpreter so they have all the information in their first language.
In research with children, you’ll often need informed permission for their participation from their parents or guardians. Although children cannot give informed consent, it’s best to also ask for their assent (agreement) to participate, depending on their age and maturity level.
Anonymity means that you don’t know who the participants are and you can’t link any individual participant to their data.
You can only guarantee anonymity by not collecting any personally identifying information—for example, names, phone numbers, email addresses, IP addresses, physical characteristics, photos, and videos.
In many cases, it may be impossible to truly anonymize data collection . For example, data collected in person or by phone cannot be considered fully anonymous because some personal identifiers (demographic information or phone numbers) are impossible to hide.
You’ll also need to collect some identifying information if you give your participants the option to withdraw their data at a later stage.
Data pseudonymization is an alternative method where you replace identifying information about participants with pseudonymous, or fake, identifiers. The data can still be linked to participants but it’s harder to do so because you separate personal information from the study data.
Confidentiality means that you know who the participants are, but you remove all identifying information from your report.
All participants have a right to privacy, so you should protect their personal data for as long as you store or use it. Even when you can’t collect data anonymously, you should secure confidentiality whenever you can.
Some research designs aren’t conducive to confidentiality, but it’s important to make all attempts and inform participants of the risks involved.
As a researcher, you have to consider all possible sources of harm to participants. Harm can come in many different forms.
- Psychological harm: Sensitive questions or tasks may trigger negative emotions such as shame or anxiety.
- Social harm: Participation can involve social risks, public embarrassment, or stigma.
- Physical harm: Pain or injury can result from the study procedures.
- Legal harm: Reporting sensitive data could lead to legal risks or a breach of privacy.
It’s best to consider every possible source of harm in your study as well as concrete ways to mitigate them. Involve your supervisor to discuss steps for harm reduction.
Make sure to disclose all possible risks of harm to participants before the study to get informed consent. If there is a risk of harm, prepare to provide participants with resources or counseling or medical services if needed.
Some of these questions may bring up negative emotions, so you inform participants about the sensitive nature of the survey and assure them that their responses will be confidential.
The way you communicate your research results can sometimes involve ethical issues. Good science communication is honest, reliable, and credible. It’s best to make your results as transparent as possible.
Take steps to actively avoid plagiarism and research misconduct wherever possible.
Plagiarism means submitting others’ works as your own. Although it can be unintentional, copying someone else’s work without proper credit amounts to stealing. It’s an ethical problem in research communication because you may benefit by harming other researchers.
Self-plagiarism is when you republish or re-submit parts of your own papers or reports without properly citing your original work.
This is problematic because you may benefit from presenting your ideas as new and original even though they’ve already been published elsewhere in the past. You may also be infringing on your previous publisher’s copyright, violating an ethical code, or wasting time and resources by doing so.
In extreme cases of self-plagiarism, entire datasets or papers are sometimes duplicated. These are major ethical violations because they can skew research findings if taken as original data.
You notice that two published studies have similar characteristics even though they are from different years. Their sample sizes, locations, treatments, and results are highly similar, and the studies share one author in common.
Research misconduct
Research misconduct means making up or falsifying data, manipulating data analyses, or misrepresenting results in research reports. It’s a form of academic fraud.
These actions are committed intentionally and can have serious consequences; research misconduct is not a simple mistake or a point of disagreement about data analyses.
Research misconduct is a serious ethical issue because it can undermine academic integrity and institutional credibility. It leads to a waste of funding and resources that could have been used for alternative research.
Later investigations revealed that they fabricated and manipulated their data to show a nonexistent link between vaccines and autism. Wakefield also neglected to disclose important conflicts of interest, and his medical license was taken away.
This fraudulent work sparked vaccine hesitancy among parents and caregivers. The rate of MMR vaccinations in children fell sharply, and measles outbreaks became more common due to a lack of herd immunity.
Research scandals with ethical failures are littered throughout history, but some took place not that long ago.
Some scientists in positions of power have historically mistreated or even abused research participants to investigate research problems at any cost. These participants were prisoners, under their care, or otherwise trusted them to treat them with dignity.
To demonstrate the importance of research ethics, we’ll briefly review two research studies that violated human rights in modern history.
These experiments were inhumane and resulted in trauma, permanent disabilities, or death in many cases.
After some Nazi doctors were put on trial for their crimes, the Nuremberg Code of research ethics for human experimentation was developed in 1947 to establish a new standard for human experimentation in medical research.
In reality, the actual goal was to study the effects of the disease when left untreated, and the researchers never informed participants about their diagnoses or the research aims.
Although participants experienced severe health problems, including blindness and other complications, the researchers only pretended to provide medical care.
When treatment became possible in 1943, 11 years after the study began, none of the participants were offered it, despite their health conditions and high risk of death.
Ethical failures like these resulted in severe harm to participants, wasted resources, and lower trust in science and scientists. This is why all research institutions have strict ethical guidelines for performing research.
If you want to know more about statistics , methodology , or research bias , make sure to check out some of our other articles with explanations and examples.
- Normal distribution
- Measures of central tendency
- Chi square tests
- Confidence interval
- Quartiles & Quantiles
- Cluster sampling
- Stratified sampling
- Thematic analysis
- Cohort study
- Peer review
- Ethnography
Research bias
- Implicit bias
- Cognitive bias
- Conformity bias
- Hawthorne effect
- Availability heuristic
- Attrition bias
- Social desirability bias
Ethical considerations in research are a set of principles that guide your research designs and practices. These principles include voluntary participation, informed consent, anonymity, confidentiality, potential for harm, and results communication.
Scientists and researchers must always adhere to a certain code of conduct when collecting data from others .
These considerations protect the rights of research participants, enhance research validity , and maintain scientific integrity.
Research ethics matter for scientific integrity, human rights and dignity, and collaboration between science and society. These principles make sure that participation in studies is voluntary, informed, and safe.
Anonymity means you don’t know who the participants are, while confidentiality means you know who they are but remove identifying information from your research report. Both are important ethical considerations .
You can only guarantee anonymity by not collecting any personally identifying information—for example, names, phone numbers, email addresses, IP addresses, physical characteristics, photos, or videos.
You can keep data confidential by using aggregate information in your research report, so that you only refer to groups of participants rather than individuals.
These actions are committed intentionally and can have serious consequences; research misconduct is not a simple mistake or a point of disagreement but a serious ethical failure.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
Bhandari, P. (2024, May 09). Ethical Considerations in Research | Types & Examples. Scribbr. Retrieved September 13, 2024, from https://www.scribbr.com/methodology/research-ethics/
Is this article helpful?

Pritha Bhandari
Other students also liked, data collection | definition, methods & examples, what is self-plagiarism | definition & how to avoid it, how to avoid plagiarism | tips on citing sources, get unlimited documents corrected.
✔ Free APA citation check included ✔ Unlimited document corrections ✔ Specialized in correcting academic texts
- Privacy Policy

Home » Ethical Considerations – Types, Examples and Writing Guide
Ethical Considerations – Types, Examples and Writing Guide
Table of Contents

Ethical Considerations
Ethical considerations in research refer to the principles and guidelines that researchers must follow to ensure that their studies are conducted in an ethical and responsible manner. These considerations are designed to protect the rights, safety, and well-being of research participants, as well as the integrity and credibility of the research itself
Some of the key ethical considerations in research include:
- Informed consent: Researchers must obtain informed consent from study participants, which means they must inform participants about the study’s purpose, procedures, risks, benefits, and their right to withdraw at any time.
- Privacy and confidentiality : Researchers must ensure that participants’ privacy and confidentiality are protected. This means that personal information should be kept confidential and not shared without the participant’s consent.
- Harm reduction : Researchers must ensure that the study does not harm the participants physically or psychologically. They must take steps to minimize the risks associated with the study.
- Fairness and equity : Researchers must ensure that the study does not discriminate against any particular group or individual. They should treat all participants equally and fairly.
- Use of deception: Researchers must use deception only if it is necessary to achieve the study’s objectives. They must inform participants of the deception as soon as possible.
- Use of vulnerable populations : Researchers must be especially cautious when working with vulnerable populations, such as children, pregnant women, prisoners, and individuals with cognitive or intellectual disabilities.
- Conflict of interest : Researchers must disclose any potential conflicts of interest that may affect the study’s integrity. This includes financial or personal relationships that could influence the study’s results.
- Data manipulation: Researchers must not manipulate data to support a particular hypothesis or agenda. They should report the results of the study objectively, even if the findings are not consistent with their expectations.
- Intellectual property: Researchers must respect intellectual property rights and give credit to previous studies and research.
- Cultural sensitivity : Researchers must be sensitive to the cultural norms and beliefs of the participants. They should avoid imposing their values and beliefs on the participants and should be respectful of their cultural practices.
Types of Ethical Considerations
Types of Ethical Considerations are as follows:
Research Ethics:
This includes ethical principles and guidelines that govern research involving human or animal subjects, ensuring that the research is conducted in an ethical and responsible manner.
Business Ethics :
This refers to ethical principles and standards that guide business practices and decision-making, such as transparency, honesty, fairness, and social responsibility.
Medical Ethics :
This refers to ethical principles and standards that govern the practice of medicine, including the duty to protect patient autonomy, informed consent, confidentiality, and non-maleficence.
Environmental Ethics :
This involves ethical principles and values that guide our interactions with the natural world, including the obligation to protect the environment, minimize harm, and promote sustainability.
Legal Ethics
This involves ethical principles and standards that guide the conduct of legal professionals, including issues such as confidentiality, conflicts of interest, and professional competence.
Social Ethics
This involves ethical principles and values that guide our interactions with other individuals and society as a whole, including issues such as justice, fairness, and human rights.
Information Ethics
This involves ethical principles and values that govern the use and dissemination of information, including issues such as privacy, accuracy, and intellectual property.
Cultural Ethics
This involves ethical principles and values that govern the relationship between different cultures and communities, including issues such as respect for diversity, cultural sensitivity, and inclusivity.
Technological Ethics
This refers to ethical principles and guidelines that govern the development, use, and impact of technology, including issues such as privacy, security, and social responsibility.
Journalism Ethics
This involves ethical principles and standards that guide the practice of journalism, including issues such as accuracy, fairness, and the public interest.
Educational Ethics
This refers to ethical principles and standards that guide the practice of education, including issues such as academic integrity, fairness, and respect for diversity.
Political Ethics
This involves ethical principles and values that guide political decision-making and behavior, including issues such as accountability, transparency, and the protection of civil liberties.
Professional Ethics
This refers to ethical principles and standards that guide the conduct of professionals in various fields, including issues such as honesty, integrity, and competence.
Personal Ethics
This involves ethical principles and values that guide individual behavior and decision-making, including issues such as personal responsibility, honesty, and respect for others.
Global Ethics
This involves ethical principles and values that guide our interactions with other nations and the global community, including issues such as human rights, environmental protection, and social justice.
Applications of Ethical Considerations
Ethical considerations are important in many areas of society, including medicine, business, law, and technology. Here are some specific applications of ethical considerations:
- Medical research : Ethical considerations are crucial in medical research, particularly when human subjects are involved. Researchers must ensure that their studies are conducted in a way that does not harm participants and that participants give informed consent before participating.
- Business practices: Ethical considerations are also important in business, where companies must make decisions that are socially responsible and avoid activities that are harmful to society. For example, companies must ensure that their products are safe for consumers and that they do not engage in exploitative labor practices.
- Environmental protection: Ethical considerations play a crucial role in environmental protection, as companies and governments must weigh the benefits of economic development against the potential harm to the environment. Decisions about land use, resource allocation, and pollution must be made in an ethical manner that takes into account the long-term consequences for the planet and future generations.
- Technology development : As technology continues to advance rapidly, ethical considerations become increasingly important in areas such as artificial intelligence, robotics, and genetic engineering. Developers must ensure that their creations do not harm humans or the environment and that they are developed in a way that is fair and equitable.
- Legal system : The legal system relies on ethical considerations to ensure that justice is served and that individuals are treated fairly. Lawyers and judges must abide by ethical standards to maintain the integrity of the legal system and to protect the rights of all individuals involved.
Examples of Ethical Considerations
Here are a few examples of ethical considerations in different contexts:
- In healthcare : A doctor must ensure that they provide the best possible care to their patients and avoid causing them harm. They must respect the autonomy of their patients, and obtain informed consent before administering any treatment or procedure. They must also ensure that they maintain patient confidentiality and avoid any conflicts of interest.
- In the workplace: An employer must ensure that they treat their employees fairly and with respect, provide them with a safe working environment, and pay them a fair wage. They must also avoid any discrimination based on race, gender, religion, or any other characteristic protected by law.
- In the media : Journalists must ensure that they report the news accurately and without bias. They must respect the privacy of individuals and avoid causing harm or distress. They must also be transparent about their sources and avoid any conflicts of interest.
- In research: Researchers must ensure that they conduct their studies ethically and with integrity. They must obtain informed consent from participants, protect their privacy, and avoid any harm or discomfort. They must also ensure that their findings are reported accurately and without bias.
- In personal relationships : People must ensure that they treat others with respect and kindness, and avoid causing harm or distress. They must respect the autonomy of others and avoid any actions that would be considered unethical, such as lying or cheating. They must also respect the confidentiality of others and maintain their privacy.
How to Write Ethical Considerations
When writing about research involving human subjects or animals, it is essential to include ethical considerations to ensure that the study is conducted in a manner that is morally responsible and in accordance with professional standards. Here are some steps to help you write ethical considerations:
- Describe the ethical principles: Start by explaining the ethical principles that will guide the research. These could include principles such as respect for persons, beneficence, and justice.
- Discuss informed consent : Informed consent is a critical ethical consideration when conducting research. Explain how you will obtain informed consent from participants, including how you will explain the purpose of the study, potential risks and benefits, and how you will protect their privacy.
- Address confidentiality : Describe how you will protect the confidentiality of the participants’ personal information and data, including any measures you will take to ensure that the data is kept secure and confidential.
- Consider potential risks and benefits : Describe any potential risks or harms to participants that could result from the study and how you will minimize those risks. Also, discuss the potential benefits of the study, both to the participants and to society.
- Discuss the use of animals : If the research involves the use of animals, address the ethical considerations related to animal welfare. Explain how you will minimize any potential harm to the animals and ensure that they are treated ethically.
- Mention the ethical approval : Finally, it’s essential to acknowledge that the research has received ethical approval from the relevant institutional review board or ethics committee. State the name of the committee, the date of approval, and any specific conditions or requirements that were imposed.
When to Write Ethical Considerations
Ethical considerations should be written whenever research involves human subjects or has the potential to impact human beings, animals, or the environment in some way. Ethical considerations are also important when research involves sensitive topics, such as mental health, sexuality, or religion.
In general, ethical considerations should be an integral part of any research project, regardless of the field or subject matter. This means that they should be considered at every stage of the research process, from the initial planning and design phase to data collection, analysis, and dissemination.
Ethical considerations should also be written in accordance with the guidelines and standards set by the relevant regulatory bodies and professional associations. These guidelines may vary depending on the discipline, so it is important to be familiar with the specific requirements of your field.
Purpose of Ethical Considerations
Ethical considerations are an essential aspect of many areas of life, including business, healthcare, research, and social interactions. The primary purposes of ethical considerations are:
- Protection of human rights: Ethical considerations help ensure that people’s rights are respected and protected. This includes respecting their autonomy, ensuring their privacy is respected, and ensuring that they are not subjected to harm or exploitation.
- Promoting fairness and justice: Ethical considerations help ensure that people are treated fairly and justly, without discrimination or bias. This includes ensuring that everyone has equal access to resources and opportunities, and that decisions are made based on merit rather than personal biases or prejudices.
- Promoting honesty and transparency : Ethical considerations help ensure that people are truthful and transparent in their actions and decisions. This includes being open and honest about conflicts of interest, disclosing potential risks, and communicating clearly with others.
- Maintaining public trust: Ethical considerations help maintain public trust in institutions and individuals. This is important for building and maintaining relationships with customers, patients, colleagues, and other stakeholders.
- Ensuring responsible conduct: Ethical considerations help ensure that people act responsibly and are accountable for their actions. This includes adhering to professional standards and codes of conduct, following laws and regulations, and avoiding behaviors that could harm others or damage the environment.
Advantages of Ethical Considerations
Here are some of the advantages of ethical considerations:
- Builds Trust : When individuals or organizations follow ethical considerations, it creates a sense of trust among stakeholders, including customers, clients, and employees. This trust can lead to stronger relationships and long-term loyalty.
- Reputation and Brand Image : Ethical considerations are often linked to a company’s brand image and reputation. By following ethical practices, a company can establish a positive image and reputation that can enhance its brand value.
- Avoids Legal Issues: Ethical considerations can help individuals and organizations avoid legal issues and penalties. By adhering to ethical principles, companies can reduce the risk of facing lawsuits, regulatory investigations, and fines.
- Increases Employee Retention and Motivation: Employees tend to be more satisfied and motivated when they work for an organization that values ethics. Companies that prioritize ethical considerations tend to have higher employee retention rates, leading to lower recruitment costs.
- Enhances Decision-making: Ethical considerations help individuals and organizations make better decisions. By considering the ethical implications of their actions, decision-makers can evaluate the potential consequences and choose the best course of action.
- Positive Impact on Society: Ethical considerations have a positive impact on society as a whole. By following ethical practices, companies can contribute to social and environmental causes, leading to a more sustainable and equitable society.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Appendix in Research Paper – Examples and...

Thesis Statement – Examples, Writing Guide

Research Methods – Types, Examples and Guide

Research Project – Definition, Writing Guide and...

Assignment – Types, Examples and Writing Guide

Research Methodology – Types, Examples and...
Ethical considerations in research: Best practices and examples

To conduct responsible research, you’ve got to think about ethics. They protect participants’ rights and their well-being - and they ensure your findings are valid and reliable. This isn’t just a box for you to tick. It’s a crucial consideration that can make all the difference to the outcome of your research.
In this article, we'll explore the meaning and importance of research ethics in today's research landscape. You'll learn best practices to conduct ethical and impactful research.
Examples of ethical considerations in research
As a researcher, you're responsible for ethical research alongside your organization. Fulfilling ethical guidelines is critical. Organizations must ensure employees follow best practices to protect participants' rights and well-being.
Keep these things in mind when it comes to ethical considerations in research:
Voluntary participation
Voluntary participation is key. Nobody should feel like they're being forced to participate or pressured into doing anything they don't want to. That means giving people a choice and the ability to opt out at any time, even if they've already agreed to take part in the study.
Informed consent
Informed consent isn't just an ethical consideration. It's a legal requirement as well. Participants must fully understand what they're agreeing to, including potential risks and benefits.
The best way to go about this is by using a consent form. Make sure you include:
- A brief description of the study and research methods.
- The potential benefits and risks of participating.
- The length of the study.
- Contact information for the researcher and/or sponsor.
- Reiteration of the participant’s right to withdraw from the research project at any time without penalty.
Anonymity means that participants aren't identifiable in any way. This includes:
- Email address
- Photographs
- Video footage
You need a way to anonymize research data so that it can't be traced back to individual participants. This may involve creating a new digital ID for participants that can’t be linked back to their original identity using numerical codes.
Confidentiality
Information gathered during a study must be kept confidential. Confidentiality helps to protect the privacy of research participants. It also ensures that their information isn't disclosed to unauthorized individuals.
Some ways to ensure confidentiality include:
- Using a secure server to store data.
- Removing identifying information from databases that contain sensitive data.
- Using a third-party company to process and manage research participant data.
- Not keeping participant records for longer than necessary.
- Avoiding discussion of research findings in public forums.
Potential for harm
The potential for harm is a crucial factor in deciding whether a research study should proceed. It can manifest in various forms, such as:
- Psychological harm
- Social harm
- Physical harm
Conduct an ethical review to identify possible harms. Be prepared to explain how you’ll minimize these harms and what support is available in case they do happen.
Fair payment
One of the most crucial aspects of setting up a research study is deciding on fair compensation for your participants. Underpayment is a common ethical issue that shouldn't be overlooked. Properly rewarding participants' time is critical for boosting engagement and obtaining high-quality data. While Prolific requires a minimum payment of £6.00 / $8.00 per hour, there are other factors you need to consider when deciding on a fair payment.
First, check your institution's reimbursement guidelines to see if they already have a minimum or maximum hourly rate. You can also use the national minimum wage as a reference point.
Next, think about the amount of work you're asking participants to do. The level of effort required for a task, such as producing a video recording versus a short survey, should correspond with the reward offered.
You also need to consider the population you're targeting. To attract research subjects with specific characteristics or high-paying jobs, you may need to offer more as an incentive.
We recommend a minimum payment of £9.00 / $12.00 per hour, but we understand that payment rates can vary depending on a range of factors. Whatever payment you choose should reflect the amount of effort participants are required to put in and be fair to everyone involved.
Ethical research made easy with Prolific
At Prolific, we believe in making ethical research easy and accessible. The findings from the Fairwork Cloudwork report speak for themselves. Prolific was given the top score out of all competitors for minimum standards of fair work.
With over 25,000 researchers in our community, we're leading the way in revolutionizing the research industry. If you're interested in learning more about how we can support your research journey, sign up to get started now.
You might also like

High-quality human data to deliver world-leading research and AIs.

Follow us on
All Rights Reserved Prolific 2024

Research Ethics & Ethical Considerations
A Plain-Language Explainer With Examples
By: Derek Jansen (MBA) | Reviewers: Dr Eunice Rautenbach | May 2024
Research ethics are one of those “ unsexy but essential ” subjects that you need to fully understand (and apply) to conquer your dissertation, thesis or research paper. In this post, we’ll unpack research ethics using plain language and loads of examples .
Overview: Research Ethics 101
- What are research ethics?
- Why should you care?
- Research ethics principles
- Respect for persons
- Beneficence
- Objectivity
- Key takeaways
What (exactly) are research ethics?
At the simplest level, research ethics are a set of principles that ensure that your study is conducted responsibly, safely, and with integrity. More specifically, research ethics help protect the rights and welfare of your research participants, while also ensuring the credibility of your research findings.
Research ethics are critically important for a number of reasons:
Firstly, they’re a complete non-negotiable when it comes to getting your research proposal approved. Pretty much all universities will have a set of ethical criteria that student projects need to adhere to – and these are typically very strictly enforced. So, if your proposed study doesn’t tick the necessary ethical boxes, it won’t be approved .
Beyond the practical aspect of approval, research ethics are essential as they ensure that your study’s participants (whether human or animal) are properly protected . In turn, this fosters trust between you and your participants – as well as trust between researchers and the public more generally. As you can probably imagine, it wouldn’t be good if the general public had a negative perception of researchers!
Last but not least, research ethics help ensure that your study’s results are valid and reliable . In other words, that you measured the thing you intended to measure – and that other researchers can repeat your study. If you’re not familiar with the concepts of reliability and validity , we’ve got a straightforward explainer video covering that below.
The Core Principles
In practical terms, each university or institution will have its own ethics policy – so, what exactly constitutes “ethical research” will vary somewhat between institutions and countries. Nevertheless, there are a handful of core principles that shape ethics policies. These principles include:
Let’s unpack each of these to make them a little more tangible.
Ethics Principle 1: Respect for persons
As the name suggests, this principle is all about ensuring that your participants are treated fairly and respectfully . In practical terms, this means informed consent – in other words, participants should be fully informed about the nature of the research, as well as any potential risks. Additionally, they should be able to withdraw from the study at any time. This is especially important when you’re dealing with vulnerable populations – for example, children, the elderly or people with cognitive disabilities.
Another dimension of the “respect for persons” principle is confidentiality and data protection . In other words, your participants’ personal information should be kept strictly confidential and secure at all times. Depending on the specifics of your project, this might also involve anonymising or masking people’s identities. As mentioned earlier, the exact requirements will vary between universities, so be sure to thoroughly review your institution’s ethics policy before you start designing your project.
Need a helping hand?
Ethics Principle 2: Beneficence
This principle is a little more opaque, but in simple terms beneficence means that you, as the researcher, should aim to maximise the benefits of your work, while minimising any potential harm to your participants.
In practical terms, benefits could include advancing knowledge, improving health outcomes, or providing educational value. Conversely, potential harms could include:
- Physical harm from accidents or injuries
- Psychological harm, such as stress or embarrassment
- Social harm, such as stigmatisation or loss of reputation
- Economic harm – in other words, financial costs or lost income
Simply put, the beneficence principle means that researchers must always try to identify potential risks and take suitable measures to reduce or eliminate them.

Ethics Principle 3: Objectivity
As you can probably guess, this principle is all about attempting to minimise research bias to the greatest degree possible. In other words, you’ll need to reduce subjectivity and increase objectivity wherever possible.
In practical terms, this principle has the largest impact on the methodology of your study – specifically the data collection and data analysis aspects. For example, you’ll need to ensure that the selection of your participants (in other words, your sampling strategy ) is aligned with your research aims – and that your sample isn’t skewed in a way that supports your presuppositions.
If you’re keen to learn more about research bias and the various ways in which you could unintentionally skew your results, check out the video below.
Ethics Principle 4: Integrity
Again, no surprises here; this principle is all about producing “honest work” . It goes without saying that researchers should always conduct their work honestly and transparently, report their findings accurately, and disclose any potential conflicts of interest upfront.
This is all pretty obvious, but another aspect of the integrity principle that’s sometimes overlooked is respect for intellectual property . In practical terms, this means you need to honour any patents, copyrights, or other forms of intellectual property that you utilise while undertaking your research. Along the same vein, you shouldn’t use any unpublished data, methods, or results without explicit, written permission from the respective owner.
Linked to all of this is the broader issue of plagiarism . Needless to say, if you’re drawing on someone else’s published work, be sure to cite your sources, in the correct format. To make life easier, use a reference manager such as Mendeley or Zotero to ensure that your citations and reference list are perfectly polished.
FAQs: Research Ethics
Research ethics & ethical considertation, what is informed consent.
Informed consent simply means providing your potential participants with all necessary information about the study. This should include information regarding the study’s purpose, procedures, risks, and benefits. This information allows your potential participants to make a voluntary and informed decision about whether to participate.
How should I obtain consent from non-English speaking participants?
What about animals.
When conducting research with animals, ensure you adhere to ethical guidelines for the humane treatment of animals. Again, the exact requirements here will vary between institutions, but typically include minimising pain and distress, using alternatives where possible, and obtaining approval from an animal care and use committee.
What is the role of the ERB or IRB?
An ethics review board (ERB) or institutional review board (IRB) evaluates research proposals to ensure they meet ethical standards. The board reviews study designs, consent forms, and data handling procedures, to protect participants’ welfare and rights.
How can I obtain ethical approval for my project?
This varies between universities, but you will typically need to submit a detailed research proposal to your institution’s ethics committee. This proposal should include your research objectives, methods, and how you plan to address ethical considerations like informed consent, confidentiality, and risk minimisation. You can learn more about how to write a proposal here .
How do I ensure ethical collaboration when working with colleagues?
Collaborative research should be conducted with mutual respect and clear agreements on roles, contributions, and publication credits. Open communication is key to preventing conflicts and misunderstandings. Also, be sure to check whether your university has any specific requirements with regards to collaborative efforts and division of labour.
How should I address ethical concerns relating to my funding source?
Key takeaways: research ethics 101.
Here’s a quick recap of the key points we’ve covered:
- Research ethics are a set of principles that ensure that your study is conducted responsibly.
- It’s essential that you design your study around these principles, or it simply won’t get approved.
- The four ethics principles we looked at are: respect for persons, beneficence, objectivity and integrity
As mentioned, the exact requirements will vary slightly depending on the institution and country, so be sure to thoroughly review your university’s research ethics policy before you start developing your study.

Psst... there’s more!
This post was based on one of our popular Research Bootcamps . If you're working on a research project, you'll definitely want to check this out ...
Great piece!!!
Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Print Friendly

What Are the Ethical Considerations in Research Design?
When I began my work on the thesis I was always focused on my research. However, once I began to make my way through research, I realized that research ethics is a core aspect of the research work and the foundation of research design.
Research ethics play a crucial role in ensuring the responsible conduct of research. Here are some key reasons why research ethics matter:

Let us look into some of the major ethical considerations in research design.
Ethical Issues in Research
There are many organizations, like the Committee on Publication Ethics , dedicated to promoting ethics in scientific research. These organizations agree that ethics is not an afterthought or side note to the research study. It is an integral aspect of research that needs to remain at the forefront of our work.
The research design must address specific research questions. Hence, the conclusions of the study must correlate to the questions posed and the results. Also, research ethics demands that the methods used must relate specifically to the research questions.
Voluntary Participation and Consent
An individual should at no point feel any coercion to participate in a study. This includes any type of persuasion or deception in attempting to gain an individual’s trust.
Informed consent states that an individual must give their explicit consent to participate in the study. You can think of consent form as an agreement of trust between the researcher and the participants.
Sampling is the first step in research design . You will need to explain why you want a particular group of participants. You will have to explain why you left out certain people or groups. In addition, if your sample includes children or special needs individuals, you will have additional requirements to address like parental permission.
Confidentiality
The third ethics principle of the Economic and Social Research Council (ESRC) states that: “The confidentiality of the information supplied by research subjects and the anonymity of respondents must be respected.” However, sometimes confidentiality is limited. For example, if a participant is at risk of harm, we must protect them. This might require releasing confidential information.
Risk of Harm
We should do everything in our power to protect study participants. For this, we should focus on the risk to benefit ratio. If possible risks outweigh the benefits, then we should abandon or redesign the study. Risk of harm also requires us to measure the risk to benefit ratio as the study progresses.
Research Methods
We know there are numerous research methods. However, when it comes to ethical considerations, some key questions can help us find the right approach for our studies.
i. Which methods most effectively fit the aims of your research?
ii. What are the strengths and restrictions of a particular method?
iii. Are there potential risks when using a particular research method?
For more guidance, you can refer to the ESRC Framework for Research Ethics .
Ethical issues in research can arise at various stages of the research process and involve different aspects of the study. Here are some common examples of ethical issues in research:
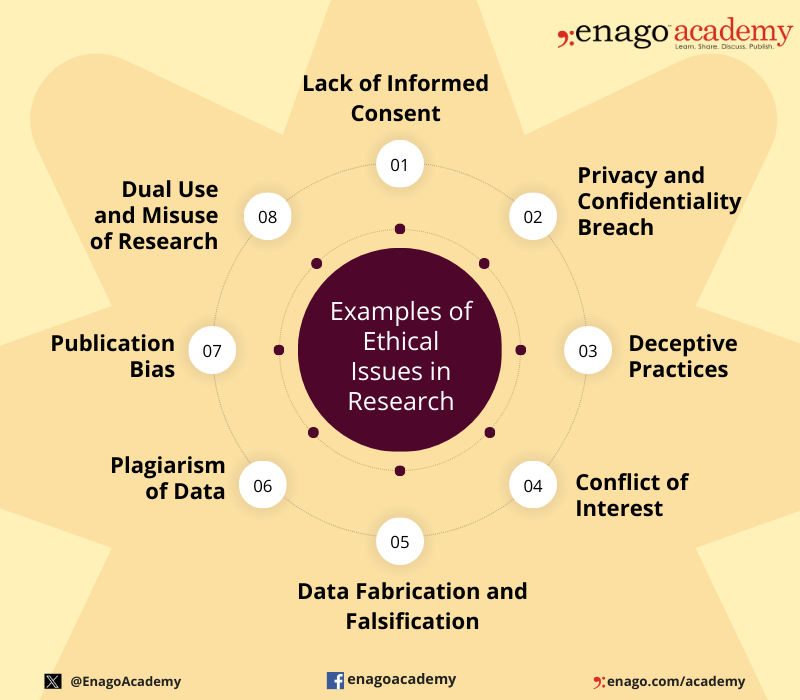
Institutional Review Boards
The importance of ethics in research cannot be understated. Following ethical guidelines will ensure your study’s validity and promote its contribution to scientific study. On a personal level, you will strengthen your research and increase your opportunities to gain funding.
To address the need for ethical considerations, most institutions have their own Institutional Review Board (IRB). An IRB secures the safety of human participants and prevents violation of human rights. It reviews the research aims and methodologies to ensure ethical practices are followed. If a research design does not follow the set ethical guidelines, then the researcher will have to amend their study.
Applying for Ethical Approval
Applications for ethical approval will differ across institutions. Regardless, they focus on the benefits of your research and the risk to benefit ratio concerning participants. Therefore, you need to effectively address both in order to get ethical clearence.
Participants
It is vital that you make it clear that individuals are provided with sufficient information in order to make an informed decision on their participation. In addition, you need to demonstrate that the ethical issues of consent, risk of harm, and confidentiality are clearly defined.
Benefits of the Study
You need to prove to the panel that your work is essential and will yield results that contribute to the scientific community. For this, you should demonstrate the following:
i. The conduct of research guarantees the quality and integrity of results.
ii. The research will be properly distributed.
iii. The aims of the research are clear and the methodology is appropriate.
Integrity and transparency are vital in the research. Ethics committees expect you to share any actual or potential conflicts of interest that could affect your work. In addition, you have to be honest and transparent throughout the approval process and the research process.
The Dangers of Unethical Practices
There is a reason to follow ethical guidelines. Without these guidelines, our research will suffer. Moreover, more importantly, people could suffer.
The following are just two examples of infamous cases of unethical research practices that demonstrate the importance of adhering to ethical standards:
- The Stanford Prison Experiment (1971) aimed to investigate the psychological effects of power using the relationship between prisoners and prison officers. Those assigned the role of “prison officers” embraced measures that exposed “prisoners” to psychological and physical harm. In this case, there was voluntary participation. However, there was disregard for welfare of the participants.
- Recently, Chinese scientist He Jiankui announced his work on genetically edited babies . Over 100 Chinese scientists denounced this research, calling it “crazy” and “shocking and unacceptable.” This research shows a troubling attitude of “do first, debate later” and a disregard for the ethical concerns of manipulating the human body Wang Yuedan, a professor of immunology at Peking University, calls this “an ethics disaster for the world” and demands strict punishments for this type of ethics violation.
What are your experiences with research ethics? How have you developed an ethical approach to research design? Please share your thoughts with us in the comments section below.
I love the articulation of reasoning and practical examples of unethical research
Rate this article Cancel Reply
Your email address will not be published.

Enago Academy's Most Popular Articles

- AI in Academia
- Trending Now
6 Leading AI Detection Tools for Academic Writing — A comparative analysis
The advent of AI content generators, exemplified by advanced models like ChatGPT, Claude AI, and…

- Reporting Research
Choosing the Right Analytical Approach: Thematic analysis vs. content analysis for data interpretation
In research, choosing the right approach to understand data is crucial for deriving meaningful insights.…

- Industry News
China’s Ministry of Education Spearheads Efforts to Uphold Academic Integrity
In response to the increase in retractions of research papers submitted by Chinese scholars to…

Comparing Cross Sectional and Longitudinal Studies: 5 steps for choosing the right approach
The process of choosing the right research design can put ourselves at the crossroads of…

- Publishing Research
- Understanding Ethics
Understanding the Difference Between Research Ethics and Compliance
Ethics refers to the principles, values, and moral guidelines that guide individual or group behavior…
Unlocking the Power of Networking in Academic Conferences
Avoiding the AI Trap: Pitfalls of relying on ChatGPT for PhD applications
10 Ways to Help Students Restore Focus on Learning
Switching Your Major As a Researcher: Things to Consider Before Making the Decision

Sign-up to read more
Subscribe for free to get unrestricted access to all our resources on research writing and academic publishing including:
- 2000+ blog articles
- 50+ Webinars
- 10+ Expert podcasts
- 50+ Infographics
- 10+ Checklists
- Research Guides
We hate spam too. We promise to protect your privacy and never spam you.
- Promoting Research
- Career Corner
- Diversity and Inclusion
- Infographics
- Expert Video Library
- Other Resources
- Enago Learn
- Upcoming & On-Demand Webinars
- Peer Review Week 2024
- Open Access Week 2023
- Conference Videos
- Enago Report
- Journal Finder
- Enago Plagiarism & AI Grammar Check
- Editing Services
- Publication Support Services
- Research Impact
- Translation Services
- Publication solutions
- AI-Based Solutions
- Thought Leadership
- Call for Articles
- Call for Speakers
- Author Training
- Edit Profile
I am looking for Editing/ Proofreading services for my manuscript Tentative date of next journal submission:

Which among these features would you prefer the most in a peer review assistant?
Instant insights, infinite possibilities
A guide to ethical considerations in research
Last updated
12 March 2023
Reviewed by
Miroslav Damyanov
Whether you are conducting a survey, running focus groups , doing field research, or holding interviews, the chances are participants will be a part of the process.
Taking ethical considerations into account and following all obligations are essential when people are involved in your research. Upholding academic integrity is another crucial ethical concern in all research types.
So, how can you protect your participants and ensure that your research is ethical? Let’s take a closer look at the ethical considerations in research and the best practices to follow.
Make research less tedious
Dovetail streamlines research to help you uncover and share actionable insights
- The importance of ethical research
Research ethics are integral to all forms of research. They help protect participants’ rights, ensure that the research is valid and accurate, and help minimize any risk of harm during the process.
When people are involved in your research, it’s particularly important to consider whether your planned research method follows ethical practices.
You might ask questions such as:
Will our participants be protected?
Is there a risk of any harm?
Are we doing all we can to protect the personal data and information we collect?
Does our study include any bias?
How can we ensure that the results will be accurate and valid?
Will our research impact public safety?
Is there a more ethical way to complete the research?
Conducting research unethically and not protecting participants’ rights can have serious consequences. It can discredit the entire study. Human rights, dignity, and research integrity should all be front of mind when you are conducting research.
- How to conduct ethical research
Before kicking off any project, the entire team must be familiar with ethical best practices. These include the considerations below.
Voluntary participation
In an ethical study, all participants have chosen to be part of the research. They must have voluntarily opted in without any pressure or coercion to do so. They must be aware that they are part of a research study. Their information must not be used against their will.
To ensure voluntary participation, make it clear at the outset that the person is opting into the process.
While participants may agree to be part of a study for a certain duration, they are allowed to change their minds. Participants must be free to leave or withdraw from the study at any time. They don’t need to give a reason.
Informed consent
Before kicking off any research, it’s also important to gain consent from all participants. This ensures participants are clear that they are part of a research study and understand all of the information related to it.
Gaining informed consent usually involves a written consent form—physical or digital—that participants can sign.
Best practice informed consent generally includes the following:
An explanation of what the study is
The duration of the study
The expectations of participants
Any potential risks
An explanation that participants are free to withdraw at any time
Contact information for the research supervisor
When obtaining informed consent, you should ensure that all parties truly understand what they are signing and their obligations as a participant. There should never be any coercion to sign.
Anonymity is key to ensuring that participants cannot be identified through their data. Personal information includes things like participants’ names, addresses, emails, phone numbers, characteristics, and photos.
However, making information truly anonymous can be challenging, especially if personal information is a necessary part of the research.
To maintain a degree of anonymity, avoid gathering any information you don’t need. This will minimize the risk of participants being identified.
Another useful tool is data pseudonymization, which makes it harder to directly link information to a real person. Data pseudonymization means giving participants fake names or mock information to protect their identity. You could, for example, replace participants’ names with codes.
Confidentiality
Keeping data confidential is a critical aspect of all forms of research. You should communicate to all participants that their information will be protected and then take active steps to ensure that happens.
Data protection has become a serious topic in recent years and should be taken seriously. The more information you gather, the more important it is to heavily protect that data.
There are many ways to protect data, including the following:
Restricted access: Information should only be accessible to the researchers involved in the project to limit the risk of breaches.
Password protection : Information should not be accessible without access via a password that complies with secure password guidelines.
Encrypted data: In this day and age, password protection isn’t usually sufficient. Encrypting the data can help ensure its security.
Data retention: All organizations should uphold a data retention policy whereby data gathered should only be held for a certain period of time. This minimizes the risk of breaches further down the line.
In research where participants are grouped together (such as in focus groups), ask participants not to pass on what has been discussed. This helps maintain the group’s privacy.
Data falsification
Regardless of what your study is about or whether it involves humans, it’s always unethical to falsify data or information. That means editing or changing any data that has been gathered or gathering data in ways that skew the results.
Bias in research is highly problematic and can significantly impact research integrity. Data falsification or misrepresentation can have serious consequences.
Take the case of Korean researcher Hwang Woo-suk, for example. Woo-suk, once considered a scientific leader in stem-cell research, was found guilty of fabricating experiments in the field and making ethical violations. Once discovered, he was fired from his role and sentenced to two years in prison.
All conflicts of interest should be declared at the outset to avoid any bias or risk of fabrication in the research process. Data must be collected and recorded accurately, and analysis must be completed impartially.
If conflicts do arise during the study, researchers may need to step back to maintain the study’s integrity. Outsourcing research to neutral third parties is necessary in some cases.
Potential for harm
Another consideration is the potential for harm. When completing research, it’s important to ensure that your participants will be safe throughout the study’s duration.
Harm during research could occur in many forms.
Physical harm may occur if your participants are asked to perform a physical activity, or if they are involved in a medical study.
Psychological harm can occur if questions or activities involve triggering or sensitive topics, or if participants are asked to complete potentially embarrassing tasks.
Harm can be caused through a data breach or privacy concern.
A study can cause harm if the participants don’t feel comfortable with the study expectations or their supervisors.
Maintaining the physical and mental well-being of all participants throughout studies is an essential aspect of ethical research.
- Gaining ethical approval
Gaining ethical approval may be necessary before conducting some types of research.
The US Department of Health and Human Services (HHS) and the US Food and Drug Administration (FDA) advise that approval is likely required for studies involving people.
To gain approval, it’s necessary to submit a proposal to an Institutional Review Board (IRB). The board will check the proposal and ensure that the research aligns with ethical practices. It will allow the project to proceed if it meets requirements.
Not gaining appropriate approval could invalidate your study, so it’s essential to pay attention to all local guidelines and laws.
- The dangers of unethical practices
Not maintaining ethical standards in research isn’t just questionable—it can be dangerous too. Many historical cases show just how widespread the ramifications can be.
The case of Korean researcher Hwang Woo-suk shows just how critical it is to obtain information ethically and accurately represent findings.
A case in 1998, which involved fraudulent data reporting, further proves this point.
The study, now debunked, was completed by Andrew Wakefield. It suggested there may be a link between the measles, mumps, and rubella (MMR) vaccine and autism in children. It was later found that the data was manipulated to show a causal link when there wasn’t one. Wakefield’s medical license was removed as a result, but the fraudulent study was still widely cited and continues to cause vaccine hesitancy among many parents.
Large organizational bodies have also been a part of unethical research. The alcohol industry, for example, was found to be highly influential in a major public health study in an attempt to prove that moderate alcohol consumption had health benefits. Five major alcohol companies pledged approximately $66 million to fund the study.
However, the World Health Organization (WHO) is clear that research shows there is no safe level of alcohol consumption. After pressure from many organizations, the study was eventually pulled due to biasing by the alcohol industry. Despite this, the idea that moderate alcohol consumption is better than abstaining may still appear in public discourse.
In more extreme cases, unethical research has led to medical studies being completed on people without their knowledge and against their will. The atrocities committed in Nazi Germany during World War II are an example.
Unethical practices in research are not just problematic or in conflict with academic integrity; they can seriously harm public health and safety.
- The ethical way to research
Considering ethical concerns and adopting best practices throughout studies is essential when conducting research.
When people are involved in studies, it’s important to consider their rights. They must not be coerced into participating, and they should be protected throughout the process.
Accurate reporting, unbiased results, and a genuine interest in answering questions rather than confirming assumptions are all essential aspects of ethical research.
Ethical research ultimately means producing true and valuable results for the benefit of everyone impacted by your study.
What are ethical considerations in research?
Ethical research involves a series of guidelines and considerations to ensure that the information gathered is valid and reliable. These guidelines ensure that:
People are not harmed during research
Participants have data protection and anonymity
Academic integrity is upheld
Not maintaining ethics in research can have serious consequences for those involved in the studies, the broader public, and policymakers.
What are the most common ethical considerations?
To maintain integrity and validity in research, all biases must be removed, data should be reported accurately, and studies must be clearly represented.
Some of the most common ethical guidelines when it comes to humans in research include avoiding harm, data protection, anonymity, informed consent, and confidentiality.
What are the ethical issues in secondary research?
Using secondary data is generally considered an ethical practice. That’s because the use of secondary data minimizes the impact on participants, reduces the need for additional funding, and maximizes the value of the data collection.
However, secondary research still has risks. For example, the risk of data breaches increases as more parties gain access to the information.
To minimize the risk, researchers should consider anonymity or data pseudonymization before the data is passed on. Furthermore, using the data should not cause any harm or distress to participants.
Should you be using a customer insights hub?
Do you want to discover previous research faster?
Do you share your research findings with others?
Do you analyze research data?
Start for free today, add your research, and get to key insights faster
Editor’s picks
Last updated: 18 April 2023
Last updated: 27 February 2023
Last updated: 22 August 2024
Last updated: 5 February 2023
Last updated: 16 August 2024
Last updated: 9 March 2023
Last updated: 30 April 2024
Last updated: 12 December 2023
Last updated: 11 March 2024
Last updated: 4 July 2024
Last updated: 6 March 2024
Last updated: 5 March 2024
Last updated: 13 May 2024
Latest articles
Related topics, .css-je19u9{-webkit-align-items:flex-end;-webkit-box-align:flex-end;-ms-flex-align:flex-end;align-items:flex-end;display:-webkit-box;display:-webkit-flex;display:-ms-flexbox;display:flex;-webkit-flex-direction:row;-ms-flex-direction:row;flex-direction:row;-webkit-box-flex-wrap:wrap;-webkit-flex-wrap:wrap;-ms-flex-wrap:wrap;flex-wrap:wrap;-webkit-box-pack:center;-ms-flex-pack:center;-webkit-justify-content:center;justify-content:center;row-gap:0;text-align:center;max-width:671px;}@media (max-width: 1079px){.css-je19u9{max-width:400px;}.css-je19u9>span{white-space:pre;}}@media (max-width: 799px){.css-je19u9{max-width:400px;}.css-je19u9>span{white-space:pre;}} decide what to .css-1kiodld{max-height:56px;display:-webkit-box;display:-webkit-flex;display:-ms-flexbox;display:flex;-webkit-align-items:center;-webkit-box-align:center;-ms-flex-align:center;align-items:center;}@media (max-width: 1079px){.css-1kiodld{display:none;}} build next, decide what to build next, log in or sign up.
Get started for free
- The Open University
- Accessibility hub
- Guest user / Sign out
- Study with The Open University

My OpenLearn Profile
Personalise your OpenLearn profile, save your favourite content and get recognition for your learning

Addressing ethical issues in your research proposal
This article explores the ethical issues that may arise in your proposed study during your doctoral research degree.
What ethical principles apply when planning and conducting research?
Research ethics are the moral principles that govern how researchers conduct their studies (Wellcome Trust, 2014). As there are elements of uncertainty and risk involved in any study, every researcher has to consider how they can uphold these ethical principles and conduct the research in a way that protects the interests and welfare of participants and other stakeholders (such as organisations).
You will need to consider the ethical issues that might arise in your proposed study. Consideration of the fundamental ethical principles that underpin all research will help you to identify the key issues and how these could be addressed. As you are probably a practitioner who wants to undertake research within your workplace, consider how your role as an ‘insider’ influences how you will conduct your study. Think about the ethical issues that might arise when you become an insider researcher (for example, relating to trust, confidentiality and anonymity).
What key ethical principles do you think will be important when planning or conducting your research, particularly as an insider? Principles that come to mind might include autonomy, respect, dignity, privacy, informed consent and confidentiality. You may also have identified principles such as competence, integrity, wellbeing, justice and non-discrimination.
Key ethical issues that you will address as an insider researcher include:
- Gaining trust
- Avoiding coercion when recruiting colleagues or other participants (such as students or service users)
- Practical challenges relating to ensuring the confidentiality and anonymity of organisations and staff or other participants.
(Heslop et al, 2018)
A fuller discussion of ethical principles is available from the British Psychological Society’s Code of Human Research Ethics (BPS, 2021).
You can also refer to guidance from the British Educational Research Association and the British Association for Applied Linguistics .

Ethical principles are essential for protecting the interests of research participants, including maximising the benefits and minimising any risks associated with taking part in a study. These principles describe ethical conduct which reflects the integrity of the researcher, promotes the wellbeing of participants and ensures high-quality research is conducted (Health Research Authority, 2022).
Research ethics is therefore not simply about gaining ethical approval for your study to be conducted. Research ethics relates to your moral conduct as a doctoral researcher and will apply throughout your study from design to dissemination (British Psychological Society, 2021). When you apply to undertake a doctorate, you will need to clearly indicate in your proposal that you understand these ethical principles and are committed to upholding them.
Where can I find ethical guidance and resources?
Professional bodies, learned societies, health and social care authorities, academic publications, Research Ethics Committees and research organisations provide a range of ethical guidance and resources. International codes such as the Universal Declaration of Human Rights underpin ethical frameworks (United Nations, 1948).
You may be aware of key legislation in your own country or the country where you plan to undertake the research, including laws relating to consent, data protection and decision-making capacity, for example, the Data Protection Act, 2018 (UK). If you want to find out more about becoming an ethical researcher, check out this Open University short course: Becoming an ethical researcher: Introduction and guidance: What is a badged course? - OpenLearn - Open University
You should be able to justify the research decisions you make. Utilising these resources will guide your ethical judgements when writing your proposal and ultimately when designing and conducting your research study. The Ethical Guidelines for Educational Research (British Educational Research Association, 2018) identifies the key responsibilities you will have when you conduct your research, including the range of stakeholders that you will have responsibilities to, as follows:
- to your participants (e.g. to appropriately inform them, facilitate their participation and support them)
- clients, stakeholders and sponsors
- the community of educational or health and social care researchers
- for publication and dissemination
- your wellbeing and development
The National Institute for Health and Care Research (no date) has emphasised the need to promote equality, diversity and inclusion when undertaking research, particularly to address long-standing social and health inequalities. Research should be informed by the diversity of people’s experiences and insights, so that it will lead to the development of practice that addresses genuine need. A commitment to equality, diversity and inclusion aims to eradicate prejudice and discrimination on the basis of an individual or group of individuals' protected characteristics such as sex (gender), disability, race, sexual orientation, in line with the Equality Act 2010.
The NIHR has produced guidance for enhancing the inclusion of ‘under-served groups’ when designing a research study (2020). Although the guidance refers to clinical research it is relevant to research more broadly.
You should consider how you will promote equality and diversity in your planned study, including through aspects such as your research topic or question, the methodology you will use, the participants you plan to recruit and how you will analyse and interpret your data.
What ethical issues do I need to consider when writing my research proposal?

You might be planning to undertake research in a health, social care, educational or other setting, including observations and interviews. The following prompts should help you to identify key ethical issues that you need to bear in mind when undertaking research in such settings.
1. Imagine you are a potential participant. Think about the questions and concerns that you might have:
- How would you feel if a researcher sat in your space and took notes, completed a checklist, or made an audio or film recording?
- What harm might a researcher cause by observing or interviewing you and others?
- What would you want to know about the researcher and ask them about the study before giving consent?
- When imagining you are the participant, how could the researcher make you feel more comfortable to be observed or interviewed?
2. Having considered the perspective of your potential participant, how would you take account of concerns such as privacy, consent, wellbeing and power in your research proposal?
[Adapted from OpenLearn course: Becoming an ethical researcher, Week 2 Activity 3: Becoming an ethical researcher - OpenLearn - Open University ]
The ethical issues to be considered will vary depending on your organisational context/role, the types of participants you plan to recruit (for example, children, adults with mental health problems), the research methods you will use, and the types of data you will collect. You will need to decide how to recruit your participants so you do not inappropriately exclude anyone. Consider what methods may be necessary to facilitate their voice and how you can obtain their consent to taking part or ensure that consent is obtained from someone else as necessary, for example, a parent in the case of a child.
You should also think about how to avoid imposing an unnecessary burden or costs on your participants. For example, by minimising the length of time they will have to commit to the study and by providing travel or other expenses. Identify the measures that you will take to store your participants’ data safely and maintain their confidentiality and anonymity when you report your findings. You could do this by storing interview and video recordings in a secure server and anonymising their names and those of their organisations using pseudonyms.
Professional codes such as the Code of Human Research Ethics (BPS, 2021) provide guidance on undertaking research with children. Being an ‘insider’ researching within your own organisation has advantages. However, you should also consider how this might impact on your research, such as power dynamics, consent, potential bias and any conflict of interest between your professional and researcher roles (Sapiro and Matthews, 2020).
How have other researchers addressed any ethical challenges?
The literature provides researchers’ accounts explaining how they addressed ethical challenges when undertaking studies. For example, Turcotte-Tremblay and McSween-Cadieux (2018) discuss strategies for protecting participants’ confidentiality when disseminating findings locally, such as undertaking fieldwork in multiple sites and providing findings in a generalised form. In addition, professional guidance includes case studies illustrating how ethical issues can be addressed, including when researching online forums (British Sociological Association, no date).
Watch the videos below and consider what insights the postgraduate researcher and supervisor provide regarding issues such as being an ‘insider researcher’, power relations, avoiding intrusion, maintaining participant anonymity and complying with research ethics and professional standards. How might their experiences inform the design and conduct of your own study?
Postgraduate researcher and supervisor talk about ethical considerations
Your thoughtful consideration of the ethical issues that might arise and how you would address these should enable you to propose an ethically informed study and conduct it in a responsible, fair and sensitive manner.
British Educational Research Association (2018) Ethical Guidelines for Educational Research. Available at: https://www.bera.ac.uk/publication/ethical-guidelines-for-educational-research-2018 (Accessed: 9 June 2023).
British Psychological Society (2021) Code of Human Research Ethics . Available at: https://cms.bps.org.uk/sites/default/files/2022-06/BPS%20Code%20of%20Human%20Research%20Ethics%20%281%29.pdf (Accessed: 9 June 2023).
British Sociological Association (2016) Researching online forums . Available at: https://www.britsoc.co.uk/media/24834/j000208_researching_online_forums_-cs1-_v3.pdf (Accessed: 9 June 2023).
Health Research Authority (2022) UK Policy Framework for Health and Social Care Research . Available at: https://www.hra.nhs.uk/planning-and-improving-research/policies-standards-legislation/uk-policy-framework-health-social-care-research/uk-policy-framework-health-and-social-care-research/#chiefinvestigators (Accessed: 9 June 2023).
Heslop, C., Burns, S., Lobo, R. (2018) ‘Managing qualitative research as insider-research in small rural communities’, Rural and Remote Health , 18: pp. 4576.
Equality Act 2010, c. 15. Available at: https://www.legislation.gov.uk/ukpga/2010/15/introduction (Accessed: 9 June 2023).
National Institute for Health and Care Research (no date) Equality, Diversity and Inclusion (EDI) . Available at: https://arc-kss.nihr.ac.uk/public-and-community-involvement/pcie-guide/how-to-do-pcie/equality-diversity-and-inclusion-edi (Accessed: 9 June 2023).
National Institute for Health and Care Research (2020) Improving inclusion of under-served groups in clinical research: Guidance from INCLUDE project. Available at: https://www.nihr.ac.uk/documents/improving-inclusion-of-under-served-groups-in-clinical-research-guidance-from-include-project/25435 (Accessed: 9 June 2023).
Sapiro, B. and Matthews, E. (2020) ‘Both Insider and Outsider. On Conducting Social Work Research in Mental Health Settings’, Advances in Social Work , 20(3). Available at: https://doi.org/10.18060/23926
Turcotte-Tremblay, A. and McSween-Cadieux, E. (2018) ‘A reflection on the challenge of protecting confidentiality of participants when disseminating research results locally’, BMC Medical Ethics, 19(supplement 1), no. 45. Available at: https://bmcmedethics.biomedcentral.com/articles/10.1186/s12910-018-0279-0
United Nations General Assembly (1948) The Universal Declaration of Human Rights . Resolution A/RES/217/A. Available at: https://www.un.org/en/about-us/universal-declaration-of-human-rights#:~:text=Drafted%20by%20representatives%20with%20different,all%20peoples%20and%20all%20nations . (Accessed: 9 June 2023).
Wellcome Trust (2014) Ensuring your research is ethical: A guide for Extended Project Qualification students . Available at: https://wellcome.org/sites/default/files/wtp057673_0.pdf (Accessed: 9 June 2023).
More articles from the research proposal collection

Writing your research proposal
A doctoral research degree is the highest academic qualification that a student can achieve. The guidance provided in these articles will help you apply for one of the two main types of research degree offered by The Open University.
Level: 1 Introductory
Defining your research methodology
Your research methodology is the approach you will take to guide your research process and explain why you use particular methods. This article explains more.

Writing your proposal and preparing for your interview
The final article looks at writing your research proposal - from the introduction through to citations and referencing - as well as preparing for your interview.
Free courses on postgraduate study

Are you ready for postgraduate study?
This free course, Are you ready for postgraduate study, will help you to become familiar with the requirements and demands of postgraduate study and ensure you are ready to develop the skills and confidence to pursue your learning further.

Succeeding in postgraduate study
This free course, Succeeding in postgraduate study, will help you to become familiar with the requirements and demands of postgraduate study and to develop the skills and confidence to pursue your learning further.

Applying to study for a PhD in psychology
This free OpenLearn course is for psychology students and graduates who are interested in PhD study at some future point. Even if you have met PhD students and heard about their projects, it is likely that you have only a vague idea of what PhD study entails. This course is intended to give you more information.
Become an OU student
Ratings & comments, share this free course, copyright information, publication details.
- Originally published: Tuesday, 27 June 2023
- Body text - Creative Commons BY-NC-SA 4.0 : The Open University
- Image 'Pebbles balance on a stone see-saw' - Copyright: Photo 51106733 / Balance © Anatoli Styf | Dreamstime.com
- Image 'Camera equipment set up filming a man talking' - Copyright: Photo 42631221 © Gabriel Robledo | Dreamstime.com
- Image 'Writing your proposal and preparing for your interview' - Copyright: Photo 133038259 / Black Student © Fizkes | Dreamstime.com
- Image 'Writing your research proposal' - Copyright free
- Image 'Addressing ethical issues in your research proposal' - Copyright: Photo 50384175 / Children Playing © Lenutaidi | Dreamstime.com
- Image 'Defining your research methodology' - Copyright free
- Image 'Succeeding in postgraduate study' - Copyright: © Everste/Getty Images
- Image 'Applying to study for a PhD in psychology' - Copyright free
- Image 'Are you ready for postgraduate study?' - Copyright free
Rate and Review
Rate this article, review this article.
Log into OpenLearn to leave reviews and join in the conversation.
Article reviews
For further information, take a look at our frequently asked questions which may give you the support you need.
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, automatically generate references for free.
- Knowledge Base
- Methodology
- Ethical Considerations in Research | Types & Examples
Ethical Considerations in Research | Types & Examples
Published on 7 May 2022 by Pritha Bhandari . Revised on 6 July 2024.
Ethical considerations in research are a set of principles that guide your research designs and practices. Scientists and researchers must always adhere to a certain code of conduct when collecting data from people.
The goals of human research often include understanding real-life phenomena, studying effective treatments, investigating behaviours, and improving lives in other ways. What you decide to research and how you conduct that research involve key ethical considerations.
These considerations work to:
- Protect the rights of research participants
- Enhance research validity
- Maintain scientific integrity
Table of contents
Why do research ethics matter, getting ethical approval for your study, types of ethical issues, voluntary participation, informed consent, confidentiality, potential for harm, results communication, examples of ethical failures, frequently asked questions about research ethics.
Research ethics matter for scientific integrity, human rights and dignity, and collaboration between science and society. These principles make sure that participation in studies is voluntary, informed, and safe for research subjects.
You’ll balance pursuing important research aims with using ethical research methods and procedures. It’s always necessary to prevent permanent or excessive harm to participants, whether inadvertent or not.
Defying research ethics will also lower the credibility of your research because it’s hard for others to trust your data if your methods are morally questionable.
Even if a research idea is valuable to society, it doesn’t justify violating the human rights or dignity of your study participants.
Prevent plagiarism, run a free check.
Before you start any study involving data collection with people, you’ll submit your research proposal to an institutional review board (IRB) .
An IRB is a committee that checks whether your research aims and research design are ethically acceptable and follow your institution’s code of conduct. They check that your research materials and procedures are up to code.
If successful, you’ll receive IRB approval, and you can begin collecting data according to the approved procedures. If you want to make any changes to your procedures or materials, you’ll need to submit a modification application to the IRB for approval.
If unsuccessful, you may be asked to re-submit with modifications or your research proposal may receive a rejection. To get IRB approval, it’s important to explicitly note how you’ll tackle each of the ethical issues that may arise in your study.
There are several ethical issues you should always pay attention to in your research design, and these issues can overlap with each other.
You’ll usually outline ways you’ll deal with each issue in your research proposal if you plan to collect data from participants.
| Voluntary participation | Your participants are free to opt in or out of the study at any point in time. |
|---|---|
| Informed consent | Participants know the purpose, benefits, risks, and funding behind the study before they agree or decline to join. |
| Anonymity | You don’t know the identities of the participants. Personally identifiable data is not collected. |
| Confidentiality | You know who the participants are but keep that information hidden from everyone else. You anonymise personally identifiable data so that it can’t be linked to other data by anyone else. |
| Potential for harm | Physical, social, psychological, and all other types of harm are kept to an absolute minimum. |
| Results communication | You ensure your work is free of plagiarism or research misconduct, and you accurately represent your results. |
Voluntary participation means that all research subjects are free to choose to participate without any pressure or coercion.
All participants are able to withdraw from, or leave, the study at any point without feeling an obligation to continue. Your participants don’t need to provide a reason for leaving the study.
It’s important to make it clear to participants that there are no negative consequences or repercussions to their refusal to participate. After all, they’re taking the time to help you in the research process, so you should respect their decisions without trying to change their minds.
Voluntary participation is an ethical principle protected by international law and many scientific codes of conduct.
Take special care to ensure there’s no pressure on participants when you’re working with vulnerable groups of people who may find it hard to stop the study even when they want to.
Informed consent refers to a situation in which all potential participants receive and understand all the information they need to decide whether they want to participate. This includes information about the study’s benefits, risks, funding, and institutional approval.
- What the study is about
- The risks and benefits of taking part
- How long the study will take
- Your supervisor’s contact information and the institution’s approval number
Usually, you’ll provide participants with a text for them to read and ask them if they have any questions. If they agree to participate, they can sign or initial the consent form. Note that this may not be sufficient for informed consent when you work with particularly vulnerable groups of people.
If you’re collecting data from people with low literacy, make sure to verbally explain the consent form to them before they agree to participate.
For participants with very limited English proficiency, you should always translate the study materials or work with an interpreter so they have all the information in their first language.
In research with children, you’ll often need informed permission for their participation from their parents or guardians. Although children cannot give informed consent, it’s best to also ask for their assent (agreement) to participate, depending on their age and maturity level.
Anonymity means that you don’t know who the participants are and you can’t link any individual participant to their data.
You can only guarantee anonymity by not collecting any personally identifying information – for example, names, phone numbers, email addresses, IP addresses, physical characteristics, photos, and videos.
In many cases, it may be impossible to truly anonymise data collection. For example, data collected in person or by phone cannot be considered fully anonymous because some personal identifiers (demographic information or phone numbers) are impossible to hide.
You’ll also need to collect some identifying information if you give your participants the option to withdraw their data at a later stage.
Data pseudonymisation is an alternative method where you replace identifying information about participants with pseudonymous, or fake, identifiers. The data can still be linked to participants, but it’s harder to do so because you separate personal information from the study data.
Confidentiality means that you know who the participants are, but you remove all identifying information from your report.
All participants have a right to privacy, so you should protect their personal data for as long as you store or use it. Even when you can’t collect data anonymously, you should secure confidentiality whenever you can.
Some research designs aren’t conducive to confidentiality, but it’s important to make all attempts and inform participants of the risks involved.
As a researcher, you have to consider all possible sources of harm to participants. Harm can come in many different forms.
- Psychological harm: Sensitive questions or tasks may trigger negative emotions such as shame or anxiety.
- Social harm: Participation can involve social risks, public embarrassment, or stigma.
- Physical harm: Pain or injury can result from the study procedures.
- Legal harm: Reporting sensitive data could lead to legal risks or a breach of privacy.
It’s best to consider every possible source of harm in your study, as well as concrete ways to mitigate them. Involve your supervisor to discuss steps for harm reduction.
Make sure to disclose all possible risks of harm to participants before the study to get informed consent. If there is a risk of harm, prepare to provide participants with resources, counselling, or medical services if needed.
Some of these questions may bring up negative emotions, so you inform participants about the sensitive nature of the survey and assure them that their responses will be confidential.
The way you communicate your research results can sometimes involve ethical issues. Good science communication is honest, reliable, and credible. It’s best to make your results as transparent as possible.
Take steps to actively avoid plagiarism and research misconduct wherever possible.
Plagiarism means submitting others’ works as your own. Although it can be unintentional, copying someone else’s work without proper credit amounts to stealing. It’s an ethical problem in research communication because you may benefit by harming other researchers.
Self-plagiarism is when you republish or re-submit parts of your own papers or reports without properly citing your original work.
This is problematic because you may benefit from presenting your ideas as new and original even though they’ve already been published elsewhere in the past. You may also be infringing on your previous publisher’s copyright, violating an ethical code, or wasting time and resources by doing so.
In extreme cases of self-plagiarism, entire datasets or papers are sometimes duplicated. These are major ethical violations because they can skew research findings if taken as original data.
You notice that two published studies have similar characteristics even though they are from different years. Their sample sizes, locations, treatments, and results are highly similar, and the studies share one author in common.
Research misconduct
Research misconduct means making up or falsifying data, manipulating data analyses, or misrepresenting results in research reports. It’s a form of academic fraud.
These actions are committed intentionally and can have serious consequences; research misconduct is not a simple mistake or a point of disagreement about data analyses.
Research misconduct is a serious ethical issue because it can undermine scientific integrity and institutional credibility. It leads to a waste of funding and resources that could have been used for alternative research.
Later investigations revealed that they fabricated and manipulated their data to show a nonexistent link between vaccines and autism. Wakefield also neglected to disclose important conflicts of interest, and his medical license was taken away.
This fraudulent work sparked vaccine hesitancy among parents and caregivers. The rate of MMR vaccinations in children fell sharply, and measles outbreaks became more common due to a lack of herd immunity.
Research scandals with ethical failures are littered throughout history, but some took place not that long ago.
Some scientists in positions of power have historically mistreated or even abused research participants to investigate research problems at any cost. These participants were prisoners, under their care, or otherwise trusted them to treat them with dignity.
To demonstrate the importance of research ethics, we’ll briefly review two research studies that violated human rights in modern history.
These experiments were inhumane and resulted in trauma, permanent disabilities, or death in many cases.
After some Nazi doctors were put on trial for their crimes, the Nuremberg Code of research ethics for human experimentation was developed in 1947 to establish a new standard for human experimentation in medical research.
In reality, the actual goal was to study the effects of the disease when left untreated, and the researchers never informed participants about their diagnoses or the research aims.
Although participants experienced severe health problems, including blindness and other complications, the researchers only pretended to provide medical care.
When treatment became possible in 1943, 11 years after the study began, none of the participants were offered it, despite their health conditions and high risk of death.
Ethical failures like these resulted in severe harm to participants, wasted resources, and lower trust in science and scientists. This is why all research institutions have strict ethical guidelines for performing research.
Ethical considerations in research are a set of principles that guide your research designs and practices. These principles include voluntary participation, informed consent, anonymity, confidentiality, potential for harm, and results communication.
Scientists and researchers must always adhere to a certain code of conduct when collecting data from others .
These considerations protect the rights of research participants, enhance research validity , and maintain scientific integrity.
Research ethics matter for scientific integrity, human rights and dignity, and collaboration between science and society. These principles make sure that participation in studies is voluntary, informed, and safe.
Anonymity means you don’t know who the participants are, while confidentiality means you know who they are but remove identifying information from your research report. Both are important ethical considerations .
You can only guarantee anonymity by not collecting any personally identifying information – for example, names, phone numbers, email addresses, IP addresses, physical characteristics, photos, or videos.
You can keep data confidential by using aggregate information in your research report, so that you only refer to groups of participants rather than individuals.
These actions are committed intentionally and can have serious consequences; research misconduct is not a simple mistake or a point of disagreement but a serious ethical failure.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the ‘Cite this Scribbr article’ button to automatically add the citation to our free Reference Generator.
Bhandari, P. (2024, July 05). Ethical Considerations in Research | Types & Examples. Scribbr. Retrieved 9 September 2024, from https://www.scribbr.co.uk/research-methods/ethical-considerations/
Is this article helpful?

Pritha Bhandari
Other students also liked, a quick guide to experimental design | 5 steps & examples, data collection methods | step-by-step guide & examples, how to avoid plagiarism | tips on citing sources.

Ethical Considerations
Ethical Considerations can be specified as one of the most important parts of the research. Dissertations may even be doomed to failure if this part is missing.
According to Bryman and Bell (2007) [1] the following ten points represent the most important principles related to ethical considerations in dissertations:
- Research participants should not be subjected to harm in any ways whatsoever.
- Respect for the dignity of research participants should be prioritised.
- Full consent should be obtained from the participants prior to the study.
- The protection of the privacy of research participants has to be ensured.
- Adequate level of confidentiality of the research data should be ensured.
- Anonymity of individuals and organisations participating in the research has to be ensured.
- Any deception or exaggeration about the aims and objectives of the research must be avoided.
- Affiliations in any forms, sources of funding, as well as any possible conflicts of interests have to be declared.
- Any type of communication in relation to the research should be done with honesty and transparency.
- Any type of misleading information, as well as representation of primary data findings in a biased way must be avoided.
In order to address ethical considerations aspect of your dissertation in an effective manner, you will need to expand discussions of each of the following points to at least one paragraph:
1. Voluntary participation of respondents in the research is important. Moreover, participants have rights to withdraw from the study at any stage if they wish to do so.
2. Respondents should participate on the basis of informed consent. The principle of informed consent involves researchers providing sufficient information and assurances about taking part to allow individuals to understand the implications of participation and to reach a fully informed, considered and freely given decision about whether or not to do so, without the exercise of any pressure or coercion. [2]
3. The use of offensive, discriminatory, or other unacceptable language needs to be avoided in the formulation of Questionnaire/Interview/Focus group questions.
4. Privacy and anonymity or respondents is of a paramount importance.
5. Acknowledgement of works of other authors used in any part of the dissertation with the use of Harvard/APA/Vancouver referencing system according to the Dissertation Handbook
6. Maintenance of the highest level of objectivity in discussions and analyses throughout the research
7. Adherence to Data Protection Act (1998) if you are studying in the UK
In studies that do not involve primary data collection, on the other hand, ethical issues are going to be limited to the points d) and e) above.
Most universities have their own Code of Ethical Practice. It is critically important for you to thoroughly adhere to this code in every aspect of your research and declare your adherence in ethical considerations part of your dissertation.
My e-book, The Ultimate Guide to Writing a Dissertation in Business Studies: a step by step assistance offers practical assistance to complete a dissertation with minimum or no stress. The e-book covers all stages of writing a dissertation starting from the selection to the research area to submitting the completed version of the work within the deadline. John Dudovskiy

[1] Bryman, A. & Bell, E. (2007) “Business Research Methods”, 2nd edition. Oxford University Press.
[2] Saunders, M., Lewis, P. & Thornhill, A. (2012) “Research Methods for Business Students” 6th edition, Pearson Education Limited.

Top 5 Ethical Considerations in Research

While research is based on the pillars of innovation, trust, and transparency, it also requires scientists and academics to abide by certain ethical considerations in research. The world is increasingly dependent on the scientific community to come up with solutions to global problems. When inaccurate or plagiarised results are published, only to be retracted later, it leads not only to wasted time and resources, but also threatens the precious trust that people have in the scientific process and scholarly publishing. Therefore, it is important for researchers to understand and abide by these ethical considerations in research not only when conducting experiments but also when publishing the results.
The National Research Council of the National Academies defines ethics and integrity in research as a series of good practices, which include among other aspects, intellectual honesty in performing and reporting research, fairness in peer reviews, transparency in communication, collegiality in scientific interactions, and protection and care of human and animal subjects during research. 1 To put it simply, ethical considerations in research refers to a code of conduct that must be followed when planning, conducting, and reporting research.
Despite these ethical considerations in research, many researchers are left grappling with ethical dilemmas and many face journal rejection, retractions, loss of employment, and other penalties because they lack or did not completely adhere to the mandated guidelines. To make it simpler for you, we’ve put together key ethical considerations in research every author should know about.
Table of Contents
- Steer clear of accidental or self-plagiarism with Paperpal’s plagiarism checker. Try it now!
Fabrication and falsification of data or results
- Check your paper’s submission readiness with Paperpal for Manuscript. Try it now!
Ethical approvals, informed consent, privacy, and confidentiality
Duplicate submissions and salami slicing.
- Go from the first draft to submission readiness in half the time. Try Paperpal now!
Frequently Asked Questions (FAQs)
Plagiarism and duplication of others’ work.
Avoiding plagiarism is one of the basic ethical considerations in research. Plagiarism is presenting someone else’s work as your own without acknowledging them, which is unacceptable in scientific research. This unauthorised use is akin to stealing ideas, thoughts, or words and using them for your benefit.
However, all research is built on previously published work and many researchers end up relying too much on the work of others. Violations of this ethical consideration in research may happen due to a lack of experience or skill, forgetting to correctly cite a source or not understanding what constitutes plagiarism. Regardless of the reason, plagiarism is considered a serious ethical violation making it imperative for researchers to understand this key ethical consideration in research and take care to avoid unintentional or self-plagiarism in their work. You can do this by acknowledging people who have contributed to your research, properly citing all sources used in the research paper, and avoiding a direct copy-paste of text from other sources.
Steer clear of accidental or self-plagiarism with Paperpal’s plagiarism checker. Try it now!
Conducting and reporting research methods, data, and results honestly is at the very top of the list of ethical considerations in research. Fabrication is making up data or results, while falsification is manipulating or altering data or results, both of which are seen as major ethical violations. As a researcher, you need to steer clear of the temptation to make up data, exaggerate findings, and mislead readers with vague or contradictory explanations.
It is always better to be honest and state all aspects of your research and its results accurately instead of over-exaggerating findings and being found out. If found guilty of violating this ethical consideration in research, you risk being heavily penalized, being suspended or expelled, face rejection, or be forced to retract your paper, all of which will impact your credibility as a researcher. Take time to review your work carefully so that you can identify and eliminate even inadvertent errors in data presentation. It may be a good idea to keep a full record of your research, so that you can go back to check on certain sections if so required.
Conflicts of interest and potential for bias
Conflicts of interest occur when competing financial obligations, personal values and stands, or professional interests compromise a researcher’s ability to be objective. While conflicts of interest are not a major ethical consideration in research and scholarly publishing, not recognizing or declaring them is seen as unethical. It is critical for researchers to identify and disclose any and all potential conflicts of interest when submitting their manuscript for publication.
Ethical considerations in research require researchers to keep aside personal biases and conduct research in an objective manner without letting your own views or cultural perspectives seep into the research study. Stay vigilant and avoid any kind of discrimination and focus instead on the scientific competence and integrity of people involved in research. It may help to discuss your study with peers or have your supervisor review your research plan and data to see if they can identify any possible bias.
Check your paper’s submission readiness with Paperpal for Manuscript. Try it now!
Before you start any study involving people or animals, make sure you meet the ethical considerations in research methodology along with the requisite approvals from respective review boards. It is important to respect the rights of the subjects with regards to informed consent, privacy, and confidentiality. Create detailed and well-designed research plans, taking help from your mentor or supervisor if needed, that reduce harm to the subjects and maximize benefits both for the participants and those conducting the research. Similarly, researchers working with animals must get the necessary permissions and make sure they are properly cared for.
One of the key ethical considerations in research is ensuring the manuscripts you submit to journals are original and have not been published before or submitted elsewhere. Researchers who intentionally submit a paper to multiple journals are seen as breaching the basic standards and ethical considerations in research. In fact, authors are required to disclose details of related or similar papers at the time manuscript submission. This holds true even of the paper is in another language or has been published only in a particular region.
Similarly, researchers must avoid “slicing” their manuscript into segments for publication in different journals to boost their publication output as it is considered unethical. As a rule, if the “slices” of study share the same aim, methodology, and study group it must be submitted as a single manuscript and should never be broken down or published separately.
Understanding and following these ethical considerations in research goes a long way in ensuring that your work earns the trust and support of your peers, supervisors, and the wider community. However, this is far easier said than done. Fortunately, today there are trusted AI writing tools like Paperpal that can make your journey a smoother one.
Make your manuscript submission ready with Paperpal
Manuscript submission is one of the most important steps in the publication journey, and you need to make sure you’ve covered all the set ethical guidelines. Here, it’s not unrealistic to be tired or even frustrated with the many small but essential checks required when preparing your research manuscript for journal submission. This is where Paperpal comes in, with a full suite of language and technical checks to help move your manuscript closer to journal acceptance.
Paperpal is a comprehensive AI academic writing tool that is tailored to support students and researchers worldwide achieve high-quality content in half the time. Based on over 21 years of STM expertise and trained on millions of language corrections by leading academic editors across subject areas, Paperpal can quickly grasp academic context and provide accurate suggestions to improve your writing quality.
The Paperpal Prime subscription is the most effective AI solution for academics, offering unlimited language checks, paraphrasing support, consistency checks, as well as a trusted online plagiarism checker and a suite of 30+ checks to ensure your manuscript is submission ready. Supercharge your writing and effortlessly deliver high-quality writing that has the best change of publication success.
Go from the first draft to submission readiness in half the time. Try Paperpal now!
Ethical considerations in research are important to ensure the protection of participants’ rights, well-being, and dignity. Ethical guidelines help researchers maintain integrity, promote fairness, and minimize harm. By following ethical principles, such as obtaining informed consent, ensuring participant confidentiality, and conducting research with integrity, researchers uphold the ethical standards necessary for trustworthy and responsible research. Ethical considerations also contribute to the credibility and reliability of research outcomes and help build public trust in the scientific community.
Researchers can protect participant confidentiality by implementing various measures. These include obtaining informed consent regarding data confidentiality, using anonymization techniques to remove personal identifiers, securely storing and transmitting data, and restricting access to sensitive information. Researchers must adhere to legal and ethical obligations to safeguard participants’ privacy and confidentiality. Additionally, when reporting research findings, researchers should use aggregated data or pseudonyms to further protect participant identities. By prioritizing participant confidentiality, researchers demonstrate respect for individuals’ privacy rights and foster trust in the research process.
Researchers obtain informed consent by providing participants with clear and comprehensive information about the research study. This includes explaining the purpose, procedures, potential risks, benefits, and any alternatives available. Participants must have the opportunity to ask questions and fully understand the implications of their participation before providing voluntary and informed consent. Researchers should document the consent process through written consent forms or other appropriate means, ensuring that participants have freely given their consent and have the option to withdraw at any time without repercussions.
- Integrity in Scientific Research: Creating an Environment That Promotes Responsible Conduct , Institute of Medicine National Research Council of the National Academics, 2002. https://nap.nationalacademies.org/catalog/10430/integrity-in-scientific-research-creating-an-environment-that-promotes-responsible
Paperpal is a comprehensive AI writing toolkit that helps students and researchers achieve 2x the writing in half the time. It leverages 21+ years of STM experience and insights from millions of research articles to provide in-depth academic writing, language editing, and submission readiness support to help you write better, faster.
Get accurate academic translations, rewriting support, grammar checks, vocabulary suggestions, and generative AI assistance that delivers human precision at machine speed. Try for free or upgrade to Paperpal Prime starting at US$19 a month to access premium features, including consistency, plagiarism, and 30+ submission readiness checks to help you succeed.
Experience the future of academic writing – Sign up to Paperpal and start writing for free!
Related Reads:
- Self-Plagiarism in Research: What it is and How to Avoid It
- 3 Easy Ways for Researchers to Improve Their Academic Vocabulary
- Good Writing Habits: 7 Ways to Improve Your Academic Writing
- Ethical Research Practices For Research with Human Subjects
9 Steps to Writing a Good Research Paper
Research manuscript structure: understanding different parts of a manuscript, you may also like, how to choose a dissertation topic, how to write an abstract in research papers..., how to write dissertation acknowledgements, how to write a high-quality conference paper, measuring academic success: definition & strategies for excellence, phd qualifying exam: tips for success , ai in education: it’s time to change the..., is it ethical to use ai-generated abstracts without..., what are journal guidelines on using generative ai..., should you use ai tools like chatgpt for....
Ethical Considerations in Research
- First Online: 22 October 2021
Cite this chapter

- Jan Recker ORCID: orcid.org/0000-0002-2072-5792 2
Part of the book series: Progress in IS ((PROIS))
3691 Accesses
1 Citations
This chapter draws attention to ethical considerations as they pertain to research in information systems (IS). Ethics define the principles of right and wrong conduct in the community of IS scholars. This chapter discusses the role of ethics in IS research, the difficulty of acting ethically in research, and presents guidelines for ethical conduct in performing research and publishing research.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Allen, G. N., Ball, N. L., & Smith, H. J. (2011). Information Systems Research Behaviors: What Are the Normative Standards? MIS Quarterly, 35 (3), 533–551.
Article Google Scholar
Bedeian, A. G., Taylor, S. G., & Miller, A. N. (2010). Management Science on the Credibility Bubble: Cardinal Sins and Various Misdemeanors. Academy of Management Learning & Education, 9 (4), 715–725.
Google Scholar
Bettis, R. A., Ethiraj, S., Gambardella, A., Helfat, C., & Mitchell, W. (2016). Creating Repeatable Cumulative Knowledge in Strategic Management. Strategic Management Journal, 37 (2), 257–261.
Bhattacharjee, Y. (2013). The Mind of a Con Man. The New York Times Magazine . Retrieved February 8, from https://www.nytimes.com/2013/04/28/magazine/diederik-stapels-audacious-academic-fraud.html
Carver, J. D., Dellva, B., Emmanuel, P. J., & Parchure, R. (2011). Ethical Considerations in Scientific Writing. Indian Journal of Sexually Transmitted Diseases and AIDS, 32 (2), 124–128.
CITI Program. (2010). The Trusted Standard in Research, Ethics, and Compliance Training . CITI Program. Retrieved February 8, 2021 from https://about.citiprogram.org/en/homepage/
Clarke, R. (2006). Plagiarism by Academics: More Complex Than It Seems. Journal of the Association for Information Systems, 7 (5), 91–121.
Dennis, A. R., Brown, S. A., Wells, T. M., & Rai, A. (2020). Editor’s Comments: Replication Crisis or Replication Reassurance: Results of the IS Replication Project. MIS Quarterly, 44 (3), iii–vii.
Gray, P. (2009). Journal Self-Citation I: Overview of the Journal Self-Citation Papers—The Wisdom of the IS Crowd. Communications of the Association for Information Systems, 25 (1), 1–10.
Kerr, N. L. (1998). HARKing: Hypothesizing After the Results are Known. Personality and Social Psychology Review, 2 (3), 196–217.
Kock, N. (2001). A Case of Academic Plagiarism. Communications of the ACM, 42 (7), 96–104.
Kock, N., & Davison, R. (2003). Dealing with Plagiarism in the Information Systems Research Community: A Look at Factors that Drive Plagiarism and Ways to Address Them. MIS Quarterly, 27 (4), 511–532.
Makri, A. (2021). What do Journalists say About Covering Science During the COVID-19 Pandemic? Nature Medicine, 27 , 17–20. https://doi.org/10.1038/s41591-020-01207-3
McNutt, M. (2016). Taking Up TOP. Science, 352 (6290), 1147.
Mertens, W., & Recker, J. (2020). New Guidelines for Null Hypothesis Significance Testing in Hypothetico-Deductive IS Research. Journal of the Association for Information Systems, 21 (4), 1072–1102. https://doi.org/10.17705/1jais.00629
Molloy, J. C. (2011). The Open Knowledge Foundation: Open Data Means Better Science. PLoS Biology, 9 (12), e1001195.
Nosek, B. A., Alter, G., Banks, G. C., Borsboom, D., Bowman, S. D., Breckler, S. J., Buck, S., Chambers, C. D., Chin, G., Christensen, G., Contestabile, M., Dafoe, A., Eich, E., Freese, J., Glennerster, R., Goroff, D., Green, D. P., Hesse, B., Humphreys, M., et al. (2015). Promoting an Open Research Culture. Science, 348 (6242), 1422–1425.
O’Boyle, E. H., Jr., Banks, G. C., & Gonzalez-Mulé, E. (2017). The Chrysalis Effect: How Ugly Initial Results Metamorphosize Into Beautiful Articles. Journal of Management, 43 (2), 376–399.
Recker, J., & Lekse, D. (2016). A Field Study of Spatial Preferences in Enterprise Microblogging. Journal of Information Technology, 31 (2), 115–129.
Recker, J., & Mendling, J. (2016). The State-of-the-Art of Business Process Management Research as Published in the BPM Conference: Recommendations for Progressing the Field. Business & Information Systems Engineering, 58 (1), 55–72.
Recker, J., Safrudin, N., & Rosemann, M. (2012). How Novices Design Business Processes. Information Systems, 37 (6), 557–573.
Resnik, D. B. (2016). Ethics in Science. In P. Humphreys (Ed.), The Oxford Handbook of Philosophy of Science (pp. 252–273). Oxford University Press.
Resnik, D. B. (2020). What Is Ethics in Research & Why Is It Important? National Institute of Environmental Health Sciences. Retrieved February 3, 2021 from https://www.niehs.nih.gov/research/resources/bioethics/whatis/index.cfm
Resnik, D. B., & Dinse, G. E. (2012). Do U.S. Research Institutions Meet or Exceed Federal Mandates for Instruction in Responsible Conduct of Research? A National Survey. Academic Medicine, 87 (9), 1237–1242. https://doi.org/10.1097/ACM.0b013e318260fe5c
Starbuck, W. H. (2016). 60th Anniversary Essay: How Journals Could Improve Research Practices in Social Science. Administrative Science Quarterly, 61 (2), 165–183.
Warren, M. (2018). First Analysis of ‘Pre-registered’ Studies Shows Sharp Rise in Null Findings. Nature , October 24. d41586-41018-07118-41581.
Wasserstein, R. L., & Lazar, N. A. (2016). The ASA’s Statement on P-values: Context, Process, and Purpose. The American Statistician, 70 (2), 129–133.
Wasserstein, R. L., Schirm, A. L., & Lazar, N. A. (2019). Moving to a World Beyond “p < 0.05”. The American Statistician, 73 (Suppl 1), 1–19.
Zelt, S., Recker, J., Schmiedel, T., & vom Brocke, J. (2018). Development and Validation of an Instrument to Measure and Manage Organizational Process Variety. Plos ONE, 13 (10), e0206198.
Download references
Author information
Authors and affiliations.
University of Hamburg, Hamburg, Germany
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Recker, J. (2021). Ethical Considerations in Research. In: Scientific Research in Information Systems. Progress in IS. Springer, Cham. https://doi.org/10.1007/978-3-030-85436-2_7
Download citation
DOI : https://doi.org/10.1007/978-3-030-85436-2_7
Published : 22 October 2021
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-85435-5
Online ISBN : 978-3-030-85436-2
eBook Packages : Business and Management Business and Management (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Springer Nature - PMC COVID-19 Collection

Ethical Issues in Research: Perceptions of Researchers, Research Ethics Board Members and Research Ethics Experts
Marie-josée drolet.
1 Department of Occupational Therapy (OT), Université du Québec à Trois-Rivières (UQTR), Trois-Rivières (Québec), Canada
Eugénie Rose-Derouin
2 Bachelor OT program, Université du Québec à Trois-Rivières (UQTR), Trois-Rivières (Québec), Canada
Julie-Claude Leblanc
Mélanie ruest, bryn williams-jones.
3 Department of Social and Preventive Medicine, School of Public Health, Université de Montréal, Montréal (Québec), Canada
In the context of academic research, a diversity of ethical issues, conditioned by the different roles of members within these institutions, arise. Previous studies on this topic addressed mainly the perceptions of researchers. However, to our knowledge, no studies have explored the transversal ethical issues from a wider spectrum, including other members of academic institutions as the research ethics board (REB) members, and the research ethics experts. The present study used a descriptive phenomenological approach to document the ethical issues experienced by a heterogeneous group of Canadian researchers, REB members, and research ethics experts. Data collection involved socio-demographic questionnaires and individual semi-structured interviews. Following the triangulation of different perspectives (researchers, REB members and ethics experts), emerging ethical issues were synthesized in ten units of meaning: (1) research integrity, (2) conflicts of interest, (3) respect for research participants, (4) lack of supervision and power imbalances, (5) individualism and performance, (6) inadequate ethical guidance, (7) social injustices, (8) distributive injustices, (9) epistemic injustices, and (10) ethical distress. This study highlighted several problematic elements that can support the identification of future solutions to resolve transversal ethical issues in research that affect the heterogeneous members of the academic community.
Introduction
Research includes a set of activities in which researchers use various structured methods to contribute to the development of knowledge, whether this knowledge is theoretical, fundamental, or applied (Drolet & Ruest, accepted ). University research is carried out in a highly competitive environment that is characterized by ever-increasing demands (i.e., on time, productivity), insufficient access to research funds, and within a market economy that values productivity and speed often to the detriment of quality or rigour – this research context creates a perfect recipe for breaches in research ethics, like research misbehaviour or misconduct (i.e., conduct that is ethically questionable or unacceptable because it contravenes the accepted norms of responsible conduct of research or compromises the respect of core ethical values that are widely held by the research community) (Drolet & Girard, 2020 ; Sieber, 2004 ). Problematic ethics and integrity issues – e.g., conflicts of interest, falsification of data, non-respect of participants’ rights, and plagiarism, to name but a few – have the potential to both undermine the credibility of research and lead to negative consequences for many stakeholders, including researchers, research assistants and personnel, research participants, academic institutions, and society as a whole (Drolet & Girard, 2020 ). It is thus evident that the academic community should be able to identify these different ethical issues in order to evaluate the nature of the risks that they pose (and for whom), and then work towards their prevention or management (i.e., education, enhanced policies and procedures, risk mitigation strategies).
In this article, we define an “ethical issue” as any situation that may compromise, in whole or in part, the respect of at least one moral value (Swisher et al., 2005 ) that is considered socially legitimate and should thus be respected. In general, ethical issues occur at three key moments or stages of the research process: (1) research design (i.e., conception, project planning), (2) research conduct (i.e., data collection, data analysis) and (3) knowledge translation or communication (e.g., publications of results, conferences, press releases) (Drolet & Ruest, accepted ). According to Sieber ( 2004 ), ethical issues in research can be classified into five categories, related to: (a) communication with participants and the community, (b) acquisition and use of research data, (c) external influence on research, (d) risks and benefits of the research, and (e) selection and use of research theories and methods. Many of these issues are related to breaches of research ethics norms, misbehaviour or research misconduct. Bruhn et al., ( 2002 ) developed a typology of misbehaviour and misconduct in academia that can be used to judge the seriousness of different cases. This typology takes into consideration two axes of reflection: (a) the origin of the situation (i.e., is it the researcher’s own fault or due to the organizational context?), and (b) the scope and severity (i.e., is this the first instance or a recurrent behaviour? What is the nature of the situation? What are the consequences, for whom, for how many people, and for which organizations?).
A previous detailed review of the international literature on ethical issues in research revealed several interesting findings (Beauchemin et al., 2021 ). Indeed, the current literature is dominated by descriptive ethics, i.e., the sharing by researchers from various disciplines of the ethical issues they have personally experienced. While such anecdotal documentation is relevant, it is insufficient because it does not provide a global view of the situation. Among the reviewed literature, empirical studies were in the minority (Table 1 ) – only about one fifth of the sample (n = 19) presented empirical research findings on ethical issues in research. The first of these studies was conducted almost 50 years ago (Hunt et al., 1984 ), with the remainder conducted in the 1990s. Eight studies were conducted in the United States (n = 8), five in Canada (n = 5), three in England (n = 3), two in Sweden (n = 2) and one in Ghana (n = 1).
Summary of Empirical Studies on Ethical Issues in Research by the year of publication
| References | Country | Types of research participants | Study design |
|---|---|---|---|
| Hunt et al., ( ) | USA | marketing researchers | mixed-methods |
| Pope & Vetter ( ) | USA | members of the American psychological association | quantitative |
| Swazey et al., ( ) | USA | doctoral candidates and faculty members | quantitative |
| Balk ( ) | USA | study participants | mixed-methods |
| Sigmon ( ) | USA | psychopathology researchers | quantitative |
| Fraser ( ) | UK | education researchers | qualitative |
| Lynöe et al., ( ) | Sweden | research ethics board members, researchers, healthcare politicians and district nurses | quantitative |
| Bouffard ( ) | Canada | researchers, health professionals and patients | qualitative |
| Davison ( ) | UK | social work researchers | qualitative |
| Miyazaki & Taylor ( ) | USA | non-traditional undergraduate students | quantitative |
| Mondain & Bologo ( ) | Ghana | researcher participants and other stakeholders | qualitative |
| Wiegand & Funk ( ) | Canada | nurses | quantitative |
| McGinn ( ) | USA | nanotechnology researchers | quantitative |
| Colnerud ( ) | Sweden | researchers | qualitative |
| Lierville et al., ( ) | Canada | Managers, Researchers, Unit Leaders and Practitioners | Qualitative |
| Giorgini et al., ( ) | USA | researchers | mixed-methods |
| Birchley et al., ( ) | UK | smart-home researchers | qualitative |
| Jarvis ( ) | Canada | research participants (women and their family members), health care providers and key stakeholders | qualitative |
| Drolet & Girard ( ) | Canada | occupational therapist researchers | qualitative |
Further, the majority of studies in our sample (n = 12) collected the perceptions of a homogeneous group of participants, usually researchers (n = 14) and sometimes health professionals (n = 6). A minority of studies (n = 7) triangulated the perceptions of diverse research stakeholders (i.e., researchers and research participants, or students). To our knowledge, only one study has examined perceptions of ethical issues in research by research ethics board members (REB; Institutional Review Boards [IRB] in the USA), and none to date have documented the perceptions of research ethics experts. Finally, nine studies (n = 9) adopted a qualitative design, seven studies (n = 7) a quantitative design, and three (n = 3) a mixed-methods design.
More studies using empirical research methods are needed to better identify broader trends, to enrich discussions on the values that should govern responsible conduct of research in the academic community, and to evaluate the means by which these values can be supported in practice (Bahn, 2012 ; Beauchemin et al., 2021 ; Bruhn et al., 2002 ; Henderson et al., 2013 ; Resnik & Elliot, 2016; Sieber 2004 ). To this end, we conducted an empirical qualitative study to document the perceptions and experiences of a heterogeneous group of Canadian researchers, REB members, and research ethics experts, to answer the following broad question: What are the ethical issues in research?
Research Methods
Research design.
A qualitative research approach involving individual semi-structured interviews was used to systematically document ethical issues (De Poy & Gitlin, 2010 ; Hammell et al., 2000 ). Specifically, a descriptive phenomenological approach inspired by the philosophy of Husserl was used (Husserl, 1970 , 1999 ), as it is recommended for documenting the perceptions of ethical issues raised by various practices (Hunt & Carnavale, 2011 ).
Ethical considerations
The principal investigator obtained ethics approval for this project from the Research Ethics Board of the Université du Québec à Trois-Rivières (UQTR). All members of the research team signed a confidentiality agreement, and research participants signed the consent form after reading an information letter explaining the nature of the research project.
Sampling and recruitment
As indicated above, three types of participants were sought: (1) researchers from different academic disciplines conducting research (i.e., theoretical, fundamental or empirical) in Canadian universities; (2) REB members working in Canadian organizations responsible for the ethical review, oversight or regulation of research; and (3) research ethics experts, i.e., academics or ethicists who teach research ethics, conduct research in research ethics, or are scholars who have acquired a specialization in research ethics. To be included in the study, participants had to work in Canada, speak and understand English or French, and be willing to participate in the study. Following Thomas and Polio’s (2002) recommendation to recruit between six and twelve participants (for a homogeneous sample) to ensure data saturation, for our heterogeneous sample, we aimed to recruit approximately twelve participants in order to obtain data saturation. Having used this method several times in related projects in professional ethics, data saturation is usually achieved with 10 to 15 participants (Drolet & Goulet, 2018 ; Drolet & Girard, 2020 ; Drolet et al., 2020 ). From experience, larger samples only serve to increase the degree of data saturation, especially in heterogeneous samples (Drolet et al., 2017 , 2019 ; Drolet & Maclure, 2016 ).
Purposive sampling facilitated the identification of participants relevant to documenting the phenomenon in question (Fortin, 2010 ). To ensure a rich and most complete representation of perceptions, we sought participants with varied and complementary characteristics with regards to the social roles they occupy in research practice (Drolet & Girard, 2020 ). A triangulation of sources was used for the recruitment (Bogdan & Biklen, 2006 ). The websites of Canadian universities and Canadian health institution REBs, as well as those of major Canadian granting agencies (i.e., the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, and the Social Sciences and Humanities Research Council of Canada, Fonds de recherche du Quebec), were searched to identify individuals who might be interested in participating in the study. Further, people known by the research team for their knowledge and sensitivity to ethical issues in research were asked to participate. Research participants were also asked to suggest other individuals who met the study criteria.
Data Collection
Two tools were used for data collecton: (a) a socio-demographic questionnaire, and (b) a semi-structured individual interview guide. English and French versions of these two documents were used and made available, depending on participant preferences. In addition, although the interview guide contained the same questions, they were adapted to participants’ specific roles (i.e., researcher, REB member, research ethics expert). When contacted by email by the research assistant, participants were asked to confirm under which role they wished to participate (because some participants might have multiple, overlapping responsibilities) and they were sent the appropriate interview guide.
The interview guides each had two parts: an introduction and a section on ethical issues. The introduction consisted of general questions to put the participant at ease (i.e., “Tell me what a typical day at work is like for you”). The section on ethical issues was designed to capture the participant’s perceptions through questions such as: “Tell me three stories you have experienced at work that involve an ethical issue?” and “Do you feel that your organization is doing enough to address, manage, and resolve ethical issues in your work?”. Although some interviews were conducted in person, the majority were conducted by videoconference to promote accessibility and because of the COVID-19 pandemic. Interviews were digitally recorded so that the verbatim could be transcribed in full, and varied between 40 and 120 min in duration, with an average of 90 min. Research assistants conducted the interviews and transcribed the verbatim.
Data Analysis
The socio-demographic questionnaires were subjected to simple descriptive statistical analyses (i.e., means and totals), and the semi-structured interviews were subjected to qualitative analysis. The steps proposed by Giorgi ( 1997 ) for a Husserlian phenomenological reduction of the data were used. After collecting, recording, and transcribing the interviews, all verbatim were analyzed by at least two analysts: a research assistant (2nd author of this article) and the principal investigator (1st author) or a postdoctoral fellow (3rd author). The repeated reading of the verbatim allowed the first analyst to write a synopsis, i.e., an initial extraction of units of meaning. The second analyst then read the synopses, which were commented and improved if necessary. Agreement between analysts allowed the final drafting of the interview synopses, which were then analyzed by three analysts to generate and organize the units of meaning that emerged from the qualitative data.
Participants
Sixteen individuals (n = 16) participated in the study, of whom nine (9) identified as female and seven (7) as male (Table 2 ). Participants ranged in age from 22 to 72 years, with a mean age of 47.5 years. Participants had between one (1) and 26 years of experience in the research setting, with an average of 14.3 years of experience. Participants held a variety of roles, including: REB members (n = 11), researchers (n = 10), research ethics experts (n = 4), and research assistant (n = 1). As mentioned previously, seven (7) participants held more than one role, i.e., REB member, research ethics expert, and researcher. The majority (87.5%) of participants were working in Quebec, with the remaining working in other Canadian provinces. Although all participants considered themselves to be francophone, one quarter (n = 4) identified themselves as belonging to a cultural minority group.
Description of Participants
| Participant number | Gender | Age | Year(s) of experience | Participant’s role(s) |
|---|---|---|---|---|
| P1 | F | 20–25 | 1–5 | REB member, and research assistant |
| P2 | F | 45–50 | 10–15 | REB member |
| P3 | F | 35–40 | 20–25 | Researcher |
| P4 | H | 55–60 | 20–25 | REB member, research ethics expert, and researcher |
| P5 | H | 70–75 | 20–25 | REB member and researcher |
| P6 | H | 45–50 | 5–10 | REB member |
| P7 | H | 40–45 | 5–10 | REB member, research ethics expert, and researcher |
| P8 | H | 45–50 | 15–20 | REB member, research ethics expert, and researcher |
| P9 | F | 35–40 | 5–10 | REB member |
| P10 | F | 65–70 | 25–30 | Researcher and research ethics expert |
| P11 | F | 60–65 | 20–25 | REB member |
| P12 | F | 45 − 40 | 20–25 | Researcher |
| P13 | F | 40–45 | 5–10 | REB member |
| P14 | H | 30–35 | 1–15 | Researcher |
| P15 | F | 40–45 | 5–10 | REB member and researcher |
| P16 | H | 50–55 | 20–25 | Researcher |
With respect to their academic background, most participants (n = 9) had a PhD, three (3) had a post-doctorate, two (2) had a master’s degree, and two (2) had a bachelor’s degree. Participants came from a variety of disciplines: nine (9) had a specialty in the humanities or social sciences, four (4) in the health sciences and three (3) in the natural sciences. In terms of their knowledge of ethics, five (5) participants reported having taken one university course entirely dedicated to ethics, four (4) reported having taken several university courses entirely dedicated to ethics, three (3) had a university degree dedicated to ethics, while two (2) only had a few hours or days of training in ethics and two (2) reported having no knowledge of ethics.
Ethical issues
As Fig. 1 illustrates, ten units of meaning emerge from the data analysis, namely: (1) research integrity, (2) conflicts of interest, (3) respect for research participants, (4) lack of supervision and power imbalances, (5) individualism and performance, (6) inadequate ethical guidance, (7) social injustices, (8) distributive injustices, (9) epistemic injustices, and (10) ethical distress. To illustrate the results, excerpts from verbatim interviews are presented in the following sub-sections. Most of the excerpts have been translated into English as the majority of interviews were conducted with French-speaking participants.

Ethical issues in research according to the participants
Research Integrity
The research environment is highly competitive and performance-based. Several participants, in particular researchers and research ethics experts, felt that this environment can lead both researchers and research teams to engage in unethical behaviour that reflects a lack of research integrity. For example, as some participants indicated, competition for grants and scientific publications is sometimes so intense that researchers falsify research results or plagiarize from colleagues to achieve their goals.
Some people will lie or exaggerate their research findings in order to get funding. Then, you see it afterwards, you realize: “ah well, it didn’t work, but they exaggerated what they found and what they did” (participant 14). Another problem in research is the identification of authors when there is a publication. Very often, there are authors who don’t even know what the publication is about and that their name is on it. (…) The time that it surprised me the most was just a few months ago when I saw someone I knew who applied for a teaching position. He got it I was super happy for him. Then I looked at his publications and … there was one that caught my attention much more than the others, because I was in it and I didn’t know what that publication was. I was the second author of a publication that I had never read (participant 14). I saw a colleague who had plagiarized another colleague. [When the colleague] found out about it, he complained. So, plagiarism is a serious [ethical breach]. I would also say that there is a certain amount of competition in the university faculties, especially for grants (…). There are people who want to win at all costs or get as much as possible. They are not necessarily going to consider their colleagues. They don’t have much of a collegial spirit (participant 10).
These examples of research misbehaviour or misconduct are sometimes due to or associated with situations of conflicts of interest, which may be poorly managed by certain researchers or research teams, as noted by many participants.
Conflict of interest
The actors and institutions involved in research have diverse interests, like all humans and institutions. As noted in Chap. 7 of the Canadian Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS2, 2018),
“researchers and research students hold trust relationships, either directly or indirectly, with participants, research sponsors, institutions, their professional bodies and society. These trust relationships can be put at risk by conflicts of interest that may compromise independence, objectivity or ethical duties of loyalty. Although the potential for such conflicts has always existed, pressures on researchers (i.e., to delay or withhold dissemination of research outcomes or to use inappropriate recruitment strategies) heighten concerns that conflicts of interest may affect ethical behaviour” (p. 92).
The sources of these conflicts are varied and can include interpersonal conflicts, financial partnerships, third-party pressures, academic or economic interests, a researcher holding multiple roles within an institution, or any other incentive that may compromise a researcher’s independence, integrity, and neutrality (TCPS2, 2018). While it is not possible to eliminate all conflicts of interest, it is important to manage them properly and to avoid temptations to behave unethically.
Ethical temptations correspond to situations in which people are tempted to prioritize their own interests to the detriment of the ethical goods that should, in their own context, govern their actions (Swisher et al., 2005 ). In the case of researchers, this refers to situations that undermine independence, integrity, neutrality, or even the set of principles that govern research ethics (TCPS2, 2018) or the responsible conduct of research. According to study participants, these types of ethical issues frequently occur in research. Many participants, especially researchers and REB members, reported that conflicts of interest can arise when members of an organization make decisions to obtain large financial rewards or to increase their academic profile, often at the expense of the interests of members of their research team, research participants, or even the populations affected by their research.
A company that puts money into making its drug work wants its drug to work. So, homeopathy is a good example, because there are not really any consequences of homeopathy, there are not very many side effects, because there are no effects at all. So, it’s not dangerous, but it’s not a good treatment either. But some people will want to make it work. And that’s a big issue when you’re sitting at a table and there are eight researchers, and there are two or three who are like that, and then there are four others who are neutral, and I say to myself, this is not science. I think that this is a very big ethical issue (participant 14). There are also times in some research where there will be more links with pharmaceutical companies. Obviously, there are then large amounts of money that will be very interesting for the health-care institutions because they still receive money for clinical trials. They’re still getting some compensation because its time consuming for the people involved and all that. The pharmaceutical companies have money, so they will compensate, and that is sometimes interesting for the institutions, and since we are a bit caught up in this, in the sense that we have no choice but to accept it. (…) It may not be the best research in the world, there may be a lot of side effects due to the drugs, but it’s good to accept it, we’re going to be part of the clinical trial (participant 3). It is integrity, what we believe should be done or said. Often by the pressure of the environment, integrity is in tension with the pressures of the environment, so it takes resistance, it takes courage in research. (…) There were all the debates there about the problems of research that was funded and then the companies kept control over what was written. That was really troubling for a lot of researchers (participant 5).
Further, these situations sometimes have negative consequences for research participants as reported by some participants.
Respect for research participants
Many research projects, whether they are psychosocial or biomedical in nature, involve human participants. Relationships between the members of research teams and their research participants raise ethical issues that can be complex. Research projects must always be designed to respect the rights and interests of research participants, and not just those of researchers. However, participants in our study – i.e., REB members, researchers, and research ethics experts – noted that some research teams seem to put their own interests ahead of those of research participants. They also emphasized the importance of ensuring the respect, well-being, and safety of research participants. The ethical issues related to this unit of meaning are: respect for free, informed and ongoing consent of research participants; respect for and the well-being of participants; data protection and confidentiality; over-solicitation of participants; ownership of the data collected on participants; the sometimes high cost of scientific innovations and their accessibility; balance between the social benefits of research and the risks to participants (particularly in terms of safety); balance between collective well-being (development of knowledge) and the individual rights of participants; exploitation of participants; paternalism when working with populations in vulnerable situations; and the social acceptability of certain types of research. The following excerpts present some of these issues.
Where it disturbs me ethically is in the medical field – because it’s more in the medical field that we’re going to see this – when consent forms are presented to patients to solicit them as participants, and then [these forms] have an average of 40 pages. That annoys me. When they say that it has to be easy to understand and all that, adapted to the language, and then the hyper-technical language plus there are 40 pages to read, I don’t understand how you’re going to get informed consent after reading 40 pages. (…) For me, it doesn’t work. I read them to evaluate them and I have a certain level of education and experience in ethics, and there are times when I don’t understand anything (participant 2). There is a lot of pressure from researchers who want to recruit research participants (…). The idea that when you enter a health care institution, you become a potential research participant, when you say “yes to a research, you check yes to all research”, then everyone can ask you. I think that researchers really have this fantasy of saying to themselves: “as soon as people walk through the door of our institution, they become potential participants with whom we can communicate and get them involved in all projects”. There’s a kind of idea that, yes, it can be done, but it has to be somewhat supervised to avoid over-solicitation (…). Researchers are very interested in facilitating recruitment and making it more fluid, but perhaps to the detriment of confidentiality, privacy, and respect; sometimes that’s what it is, to think about what type of data you’re going to have in your bank of potential participants? Is it just name and phone number or are you getting into more sensitive information? (participant 9).
In addition, one participant reported that their university does not provide the resources required to respect the confidentiality of research participants.
The issue is as follows: researchers, of course, commit to protecting data with passwords and all that, but we realize that in practice, it is more difficult. It is not always as protected as one might think, because professor-researchers will run out of space. Will the universities make rooms available to researchers, places where they can store these things, especially when they have paper documentation, and is there indeed a guarantee of confidentiality? Some researchers have told me: “Listen; there are even filing cabinets in the corridors”. So, that certainly poses a concrete challenge. How do we go about challenging the administrative authorities? Tell them it’s all very well to have an ethics committee, but you have to help us, you also have to make sure that the necessary infrastructures are in place so that what we are proposing is really put into practice (participant 4).
If the relationships with research participants are likely to raise ethical issues, so too are the relationships with students, notably research assistants. On this topic, several participants discussed the lack of supervision or recognition offered to research assistants by researchers as well as the power imbalances between members of the research team.
Lack of Supervision and Power Imbalances
Many research teams are composed not only of researchers, but also of students who work as research assistants. The relationship between research assistants and other members of research teams can sometimes be problematic and raise ethical issues, particularly because of the inevitable power asymmetries. In the context of this study, several participants – including a research assistant, REB members, and researchers – discussed the lack of supervision or recognition of the work carried out by students, psychological pressure, and the more or less well-founded promises that are sometimes made to students. Participants also mentioned the exploitation of students by certain research teams, which manifest when students are inadequately paid, i.e., not reflective of the number of hours actually worked, not a fair wage, or even a wage at all.
[As a research assistant], it was more of a feeling of distress that I felt then because I didn’t know what to do. (…) I was supposed to get coaching or be supported, but I didn’t get anything in the end. It was like, “fix it by yourself”. (…) All research assistants were supposed to be supervised, but in practice they were not (participant 1). Very often, we have a master’s or doctoral student that we put on a subject and we consider that the project will be well done, while the student is learning. So, it happens that the student will do a lot of work and then we realize that the work is poorly done, and it is not necessarily the student’s fault. He wasn’t necessarily well supervised. There are directors who have 25 students, and they just don’t supervise them (participant 14). I think it’s really the power relationship. I thought to myself, how I saw my doctorate, the beginning of my research career, I really wanted to be in that laboratory, but they are the ones who are going to accept me or not, so what do I do to be accepted? I finally accept their conditions [which was to work for free]. If these are the conditions that are required to enter this lab, I want to go there. So, what do I do, well I accepted. It doesn’t make sense, but I tell myself that I’m still privileged, because I don’t have so many financial worries, one more reason to work for free, even though it doesn’t make sense (participant 1). In research, we have research assistants. (…). The fact of using people… so that’s it, you have to take into account where they are, respect them, but at the same time they have to show that they are there for the research. In English, we say “carry” or take care of people. With research assistants, this is often a problem that I have observed: for grant machines, the person is the last to be found there. Researchers, who will take, use student data, without giving them the recognition for it (participant 5). The problem at our university is that they reserve funding for Canadian students. The doctoral clientele in my field is mostly foreign students. So, our students are poorly funded. I saw one student end up in the shelter, in a situation of poverty. It ended very badly for him because he lacked financial resources. Once you get into that dynamic, it’s very hard to get out. I was made aware of it because the director at the time had taken him under her wing and wanted to try to find a way to get him out of it. So, most of my students didn’t get funded (participant 16). There I wrote “manipulation”, but it’s kind of all promises all the time. I, for example, was promised a lot of advancement, like when I got into the lab as a graduate student, it was said that I had an interest in [this particular area of research]. I think there are a lot of graduate students who must have gone through that, but it is like, “Well, your CV has to be really good, if you want to do a lot of things and big things. If you do this, if you do this research contract, the next year you could be the coordinator of this part of the lab and supervise this person, get more contracts, be paid more. Let’s say: you’ll be invited to go to this conference, this big event”. They were always dangling something, but you have to do that first to get there. But now, when you’ve done that, you have to do this business. It’s like a bit of manipulation, I think. That was very hard to know who is telling the truth and who is not (participant 1).
These ethical issues have significant negative consequences for students. Indeed, they sometimes find themselves at the mercy of researchers, for whom they work, struggling to be recognized and included as authors of an article, for example, or to receive the salary that they are due. For their part, researchers also sometimes find themselves trapped in research structures that can negatively affect their well-being. As many participants reported, researchers work in organizations that set very high productivity standards and in highly competitive contexts, all within a general culture characterized by individualism.
Individualism and performance
Participants, especially researchers, discussed the culture of individualism and performance that characterizes the academic environment. In glorifying excellence, some universities value performance and productivity, often at the expense of psychological well-being and work-life balance (i.e., work overload and burnout). Participants noted that there are ethical silences in their organizations on this issue, and that the culture of individualism and performance is not challenged for fear of retribution or simply to survive, i.e., to perform as expected. Participants felt that this culture can have a significant negative impact on the quality of the research conducted, as research teams try to maximize the quantity of their work (instead of quality) in a highly competitive context, which is then exacerbated by a lack of resources and support, and where everything must be done too quickly.
The work-life balance with the professional ethics related to work in a context where you have too much and you have to do a lot, it is difficult to balance all that and there is a lot of pressure to perform. If you don’t produce enough, that’s it; after that, you can’t get any more funds, so that puts pressure on you to do more and more and more (participant 3). There is a culture, I don’t know where it comes from, and that is extremely bureaucratic. If you dare to raise something, you’re going to have many, many problems. They’re going to make you understand it. So, I don’t talk. It is better: your life will be easier. I think there are times when you have to talk (…) because there are going to be irreparable consequences. (…) I’m not talking about a climate of terror, because that’s exaggerated, it’s not true, people are not afraid. But people close their office door and say nothing because it’s going to make their work impossible and they’re not going to lose their job, they’re not going to lose money, but researchers need time to be focused, so they close their office door and say nothing (participant 16).
Researchers must produce more and more, and they feel little support in terms of how to do such production, ethically, and how much exactly they are expected to produce. As this participant reports, the expectation is an unspoken rule: more is always better.
It’s sometimes the lack of a clear line on what the expectations are as a researcher, like, “ah, we don’t have any specific expectations, but produce, produce, produce, produce.” So, in that context, it’s hard to be able to put the line precisely: “have I done enough for my work?” (participant 3).
Inadequate ethical Guidance
While the productivity expectation is not clear, some participants – including researchers, research ethics experts, and REB members – also felt that the ethical expectations of some REBs were unclear. The issue of the inadequate ethical guidance of research includes the administrative mechanisms to ensure that research projects respect the principles of research ethics. According to those participants, the forms required for both researchers and REB members are increasingly long and numerous, and one participant noted that the standards to be met are sometimes outdated and disconnected from the reality of the field. Multicentre ethics review (by several REBs) was also critiqued by a participant as an inefficient method that encumbers the processes for reviewing research projects. Bureaucratization imposes an ever-increasing number of forms and ethics guidelines that actually hinder researchers’ ethical reflection on the issues at stake, leading the ethics review process to be perceived as purely bureaucratic in nature.
The ethical dimension and the ethical review of projects have become increasingly bureaucratized. (…) When I first started working (…) it was less bureaucratic, less strict then. I would say [there are now] tons of forms to fill out. Of course, we can’t do without it, it’s one of the ways of marking out ethics and ensuring that there are ethical considerations in research, but I wonder if it hasn’t become too bureaucratized, so that it’s become a kind of technical reflex to fill out these forms, and I don’t know if people really do ethical reflection as such anymore (participant 10). The fundamental structural issue, I would say, is the mismatch between the normative requirements and the real risks posed by the research, i.e., we have many, many requirements to meet; we have very long forms to fill out but the research projects we evaluate often pose few risks (participant 8). People [in vulnerable situations] were previously unable to participate because of overly strict research ethics rules that were to protect them, but in the end [these rules] did not protect them. There was a perverse effect, because in the end there was very little research done with these people and that’s why we have very few results, very little evidence [to support practices with these populations] so it didn’t improve the quality of services. (…) We all understand that we have to be careful with that, but when the research is not too risky, we say to ourselves that it would be good because for once a researcher who is interested in that population, because it is not a very popular population, it would be interesting to have results, but often we are blocked by the norms, and then we can’t accept [the project] (participant 2).
Moreover, as one participant noted, accessing ethics training can be a challenge.
There is no course on research ethics. […] Then, I find that it’s boring because you go through university and you come to do your research and you know how to do quantitative and qualitative research, but all the research ethics, where do you get this? I don’t really know (participant 13).
Yet, such training could provide relevant tools to resolve, to some extent, the ethical issues that commonly arise in research. That said, and as noted by many participants, many ethical issues in research are related to social injustices over which research actors have little influence.
Social Injustices
For many participants, notably researchers, the issues that concern social injustices are those related to power asymmetries, stigma, or issues of equity, diversity, and inclusion, i.e., social injustices related to people’s identities (Blais & Drolet, 2022 ). Participants reported experiencing or witnessing discrimination from peers, administration, or lab managers. Such oppression is sometimes cross-sectional and related to a person’s age, cultural background, gender or social status.
I have my African colleague who was quite successful when he arrived but had a backlash from colleagues in the department. I think it’s unconscious, nobody is overtly racist. But I have a young person right now who is the same, who has the same success, who got exactly the same early career award and I don’t see the same backlash. He’s just as happy with what he’s doing. It’s normal, they’re young and they have a lot of success starting out. So, I think there is discrimination. Is it because he is African? Is it because he is black? I think it’s on a subconscious level (participant 16).
Social injustices were experienced or reported by many participants, and included issues related to difficulties in obtaining grants or disseminating research results in one’s native language (i.e., even when there is official bilingualism) or being considered credible and fundable in research when one researcher is a woman.
If you do international research, there are things you can’t talk about (…). It is really a barrier to research to not be able to (…) address this question [i.e. the question of inequalities between men and women]. Women’s inequality is going to be addressed [but not within the country where the research takes place as if this inequality exists elsewhere but not here]. There are a lot of women working on inequality issues, doing work and it’s funny because I was talking to a young woman who works at Cairo University and she said to me: “Listen, I saw what you had written, you’re right. I’m willing to work on this but guarantee me a position at your university with a ticket to go”. So yes, there are still many barriers [for women in research] (participant 16).
Because of the varied contextual characteristics that intervene in their occurrence, these social injustices are also related to distributive injustices, as discussed by many participants.
Distributive Injustices
Although there are several views of distributive justice, a classical definition such as that of Aristotle ( 2012 ), describes distributive justice as consisting in distributing honours, wealth, and other social resources or benefits among the members of a community in proportion to their alleged merit. Justice, then, is about determining an equitable distribution of common goods. Contemporary theories of distributive justice are numerous and varied. Indeed, many authors (e.g., Fraser 2011 ; Mills, 2017 ; Sen, 2011 ; Young, 2011 ) have, since Rawls ( 1971 ), proposed different visions of how social burdens and benefits should be shared within a community to ensure equal respect, fairness, and distribution. In our study, what emerges from participants’ narratives is a definite concern for this type of justice. Women researchers, francophone researchers, early career researchers or researchers belonging to racialized groups all discussed inequities in the distribution of research grants and awards, and the extra work they need to do to somehow prove their worth. These inequities are related to how granting agencies determine which projects will be funded.
These situations make me work 2–3 times harder to prove myself and to show people in power that I have a place as a woman in research (participant 12). Number one: it’s conservative thinking. The older ones control what comes in. So, the younger people have to adapt or they don’t get funded (participant 14).
Whether it is discrimination against stigmatized or marginalized populations or interest in certain hot topics, granting agencies judge research projects according to criteria that are sometimes questionable, according to those participants. Faced with difficulties in obtaining funding for their projects, several strategies – some of which are unethical – are used by researchers in order to cope with these situations.
Sometimes there are subjects that everyone goes to, such as nanotechnology (…), artificial intelligence or (…) the therapeutic use of cannabis, which are very fashionable, and this is sometimes to the detriment of other research that is just as relevant, but which is (…), less sexy, less in the spirit of the time. (…) Sometimes this can lead to inequities in the funding of certain research sectors (participant 9). When we use our funds, we get them given to us, we pretty much say what we think we’re going to do with them, but things change… So, when these things change, sometimes it’s an ethical decision, but by force of circumstances I’m obliged to change the project a little bit (…). Is it ethical to make these changes or should I just let the money go because I couldn’t use it the way I said I would? (participant 3).
Moreover, these distributional injustices are not only linked to social injustices, but also epistemic injustices. Indeed, the way in which research honours and grants are distributed within the academic community depends on the epistemic authority of the researchers, which seems to vary notably according to their language of use, their age or their gender, but also to the research design used (inductive versus deductive), their decision to use (or not use) animals in research, or to conduct activist research.
Epistemic injustices
The philosopher Fricker ( 2007 ) conceptualized the notions of epistemic justice and injustice. Epistemic injustice refers to a form of social inequality that manifests itself in the access, recognition, and production of knowledge as well as the various forms of ignorance that arise (Godrie & Dos Santos, 2017 ). Addressing epistemic injustice necessitates acknowledging the iniquitous wrongs suffered by certain groups of socially stigmatized individuals who have been excluded from knowledge, thus limiting their abilities to interpret, understand, or be heard and account for their experiences. In this study, epistemic injustices were experienced or reported by some participants, notably those related to difficulties in obtaining grants or disseminating research results in one’s native language (i.e., even when there is official bilingualism) or being considered credible and fundable in research when a researcher is a woman or an early career researcher.
I have never sent a grant application to the federal government in English. I have always done it in French, even though I know that when you receive the review, you can see that reviewers didn’t understand anything because they are English-speaking. I didn’t want to get in the boat. It’s not my job to translate, because let’s be honest, I’m not as good in English as I am in French. So, I do them in my first language, which is the language I’m most used to. Then, technically at the administrative level, they are supposed to be able to do it, but they are not good in French. (…) Then, it’s a very big Canadian ethical issue, because basically there are technically two official languages, but Canada is not a bilingual country, it’s a country with two languages, either one or the other. (…) So I was not funded (participant 14).
Researchers who use inductive (or qualitative) methods observed that their projects are sometimes less well reviewed or understood, while research that adopts a hypothetical-deductive (or quantitative) or mixed methods design is better perceived, considered more credible and therefore more easily funded. Of course, regardless of whether a research project adopts an inductive, deductive or mixed-methods scientific design, or whether it deals with qualitative or quantitative data, it must respect a set of scientific criteria. A research project should achieve its objectives by using proven methods that, in the case of inductive research, are credible, reliable, and transferable or, in the case of deductive research, generalizable, objective, representative, and valid (Drolet & Ruest, accepted ). Participants discussing these issues noted that researchers who adopt a qualitative design or those who question the relevance of animal experimentation or are not militant have sometimes been unfairly devalued in their epistemic authority.
There is a mini war between quantitative versus qualitative methods, which I think is silly because science is a method. If you apply the method well, it doesn’t matter what the field is, it’s done well and it’s perfect ” (participant 14). There is also the issue of the place of animals in our lives, because for me, ethics is human ethics, but also animal ethics. Then, there is a great evolution in society on the role of the animal… with the new law that came out in Quebec on the fact that animals are sensitive beings. Then, with the rise of the vegan movement, [we must ask ourselves]: “Do animals still have a place in research?” That’s a big question and it also means that there are practices that need to evolve, but sometimes there’s a disconnection between what’s expected by research ethics boards versus what’s expected in the field (participant 15). In research today, we have more and more research that is militant from an ideological point of view. And so, we have researchers, because they defend values that seem important to them, we’ll talk for example about the fight for equality and social justice. They have pressure to defend a form of moral truth and have the impression that everyone thinks like them or should do so, because they are defending a moral truth. This is something that we see more and more, namely the lack of distance between ideology and science (participant 8).
The combination or intersectionality of these inequities, which seems to be characterized by a lack of ethical support and guidance, is experienced in the highly competitive and individualistic context of research; it provides therefore the perfect recipe for researchers to experience ethical distress.
Ethical distress
The concept of “ethical distress” refers to situations in which people know what they should do to act ethically, but encounter barriers, generally of an organizational or systemic nature, limiting their power to act according to their moral or ethical values (Drolet & Ruest, 2021 ; Jameton, 1984 ; Swisher et al., 2005 ). People then run the risk of finding themselves in a situation where they do not act as their ethical conscience dictates, which in the long term has the potential for exhaustion and distress. The examples reported by participants in this study point to the fact that researchers in particular may be experiencing significant ethical distress. This distress takes place in a context of extreme competition, constant injunctions to perform, and where administrative demands are increasingly numerous and complex to complete, while paradoxically, they lack the time to accomplish all their tasks and responsibilities. Added to these demands are a lack of resources (human, ethical, and financial), a lack of support and recognition, and interpersonal conflicts.
We are in an environment, an elite one, you are part of it, you know what it is: “publish or perish” is the motto. Grants, there is a high level of performance required, to do a lot, to publish, to supervise students, to supervise them well, so yes, it is clear that we are in an environment that is conducive to distress. (…). Overwork, definitely, can lead to distress and eventually to exhaustion. When you know that you should take the time to read the projects before sharing them, but you don’t have the time to do that because you have eight that came in the same day, and then you have others waiting… Then someone rings a bell and says: “ah but there, the protocol is a bit incomplete”. Oh yes, look at that, you’re right. You make up for it, but at the same time it’s a bit because we’re in a hurry, we don’t necessarily have the resources or are able to take the time to do things well from the start, we have to make up for it later. So yes, it can cause distress (participant 9). My organization wanted me to apply in English, and I said no, and everyone in the administration wanted me to apply in English, and I always said no. Some people said: “Listen, I give you the choice”, then some people said: “Listen, I agree with you, but if you’re not [submitting] in English, you won’t be funded”. Then the fact that I am young too, because very often they will look at the CV, they will not look at the project: “ah, his CV is not impressive, we will not finance him”. This is complete nonsense. The person is capable of doing the project, the project is fabulous: we fund the project. So, that happened, organizational barriers: that happened a lot. I was not eligible for Quebec research funds (…). I had big organizational barriers unfortunately (participant 14). At the time of my promotion, some colleagues were not happy with the type of research I was conducting. I learned – you learn this over time when you become friends with people after you enter the university – that someone was against me. He had another candidate in mind, and he was angry about the selection. I was under pressure for the first three years until my contract was renewed. I almost quit at one point, but another colleague told me, “No, stay, nothing will happen”. Nothing happened, but these issues kept me awake at night (participant 16).
This difficult context for many researchers affects not only the conduct of their own research, but also their participation in research. We faced this problem in our study, despite the use of multiple recruitment methods, including more than 200 emails – of which 191 were individual solicitations – sent to potential participants by the two research assistants. REB members and organizations overseeing or supporting research (n = 17) were also approached to see if some of their employees would consider participating. While it was relatively easy to recruit REB members and research ethics experts, our team received a high number of non-responses to emails (n = 175) and some refusals (n = 5), especially by researchers. The reasons given by those who replied were threefold: (a) fear of being easily identified should they take part in the research, (b) being overloaded and lacking time, and (c) the intrusive aspect of certain questions (i.e., “Have you experienced a burnout episode? If so, have you been followed up medically or psychologically?”). In light of these difficulties and concerns, some questions in the socio-demographic questionnaire were removed or modified. Talking about burnout in research remains a taboo for many researchers, which paradoxically can only contribute to the unresolved problem of unhealthy research environments.
Returning to the research question and objective
The question that prompted this research was: What are the ethical issues in research? The purpose of the study was to describe these issues from the perspective of researchers (from different disciplines), research ethics board (REB) members, and research ethics experts. The previous section provided a detailed portrait of the ethical issues experienced by different research stakeholders: these issues are numerous, diverse and were recounted by a range of stakeholders.
The results of the study are generally consistent with the literature. For example, as in our study, the literature discusses the lack of research integrity on the part of some researchers (Al-Hidabi et al., 2018 ; Swazey et al., 1993 ), the numerous conflicts of interest experienced in research (Williams-Jones et al., 2013 ), the issues of recruiting and obtaining the free and informed consent of research participants (Provencher et al., 2014 ; Keogh & Daly, 2009 ), the sometimes difficult relations between researchers and REBs (Drolet & Girard, 2020 ), the epistemological issues experienced in research (Drolet & Ruest, accepted; Sieber 2004 ), as well as the harmful academic context in which researchers evolve, insofar as this is linked to a culture of performance, an overload of work in a context of accountability (Berg & Seeber, 2016 ; FQPPU; 2019 ) that is conducive to ethical distress and even burnout.
If the results of the study are generally in line with those of previous publications on the subject, our findings also bring new elements to the discussion while complementing those already documented. In particular, our results highlight the role of systemic injustices – be they social, distributive or epistemic – within the environments in which research is carried out, at least in Canada. To summarize, the results of our study point to the fact that the relationships between researchers and research participants are likely still to raise worrying ethical issues, despite widely accepted research ethics norms and institutionalized review processes. Further, the context in which research is carried out is not only conducive to breaches of ethical norms and instances of misbehaviour or misconduct, but also likely to be significantly detrimental to the health and well-being of researchers, as well as research assistants. Another element that our research also highlighted is the instrumentalization and even exploitation of students and research assistants, which is another important and worrying social injustice given the inevitable power imbalances between students and researchers.
Moreover, in a context in which ethical issues are often discussed from a micro perspective, our study helps shed light on both the micro- and macro-level ethical dimensions of research (Bronfenbrenner, 1979 ; Glaser 1994 ). However, given that ethical issues in research are not only diverse, but also and above all complex, a broader perspective that encompasses the interplay between the micro and macro dimensions can enable a better understanding of these issues and thereby support the identification of the multiple factors that may be at their origin. Triangulating the perspectives of researchers with those of REB members and research ethics experts enabled us to bring these elements to light, and thus to step back from and critique the way that research is currently conducted. To this end, attention to socio-political elements such as the performance culture in academia or how research funds are distributed, and according to what explicit and implicit criteria, can contribute to identifying the sources of the ethical issues described above.
Contemporary culture characterized by the social acceleration
The German sociologist and philosopher Rosa (2010) argues that late modernity – that is, the period between the 1980s and today – is characterized by a phenomenon of social acceleration that causes various forms of alienation in our relationship to time, space, actions, things, others and ourselves. Rosa distinguishes three types of acceleration: technical acceleration , the acceleration of social changes and the acceleration of the rhythm of life . According to Rosa, social acceleration is the main problem of late modernity, in that the invisible social norm of doing more and faster to supposedly save time operates unchallenged at all levels of individual and collective life, as well as organizational and social life. Although we all, researchers and non-researchers alike, perceive this unspoken pressure to be ever more productive, the process of social acceleration as a new invisible social norm is our blind spot, a kind of tyrant over which we have little control. This conceptualization of the contemporary culture can help us to understand the context in which research is conducted (like other professional practices). To this end, Berg & Seeber ( 2016 ) invite faculty researchers to slow down in order to better reflect and, in the process, take care of their health and their relationships with their colleagues and students. Many women professors encourage their fellow researchers, especially young women researchers, to learn to “say No” in order to protect their mental and physical health and to remain in their academic careers (Allaire & Descheneux, 2022 ). These authors also remind us of the relevance of Kahneman’s ( 2012 ) work which demonstrates that it takes time to think analytically, thoroughly, and logically. Conversely, thinking quickly exposes humans to cognitive and implicit biases that then lead to errors in thinking (e.g., in the analysis of one’s own research data or in the evaluation of grant applications or student curriculum vitae). The phenomenon of social acceleration, which pushes the researcher to think faster and faster, is likely to lead to unethical bad science that can potentially harm humankind. In sum, Rosa’s invitation to contemporary critical theorists to seriously consider the problem of social acceleration is particularly insightful to better understand the ethical issues of research. It provides a lens through which to view the toxic context in which research is conducted today, and one that was shared by the participants in our study.
Clark & Sousa ( 2022 ) note, it is important that other criteria than the volume of researchers’ contributions be valued in research, notably quality. Ultimately, it is the value of the knowledge produced and its influence on the concrete lives of humans and other living beings that matters, not the quantity of publications. An interesting articulation of this view in research governance is seen in a change in practice by Australia’s national health research funder: they now restrict researchers to listing on their curriculum vitae only the top ten publications from the past ten years (rather than all of their publications), in order to evaluate the quality of contributions rather than their quantity. To create environments conducive to the development of quality research, it is important to challenge the phenomenon of social acceleration, which insidiously imposes a quantitative normativity that is both alienating and detrimental to the quality and ethical conduct of research. Based on our experience, we observe that the social norm of acceleration actively disfavours the conduct of empirical research on ethics in research. The fact is that researchers are so busy that it is almost impossible for them to find time to participate in such studies. Further, operating in highly competitive environments, while trying to respect the values and ethical principles of research, creates ethical paradoxes for members of the research community. According to Malherbe ( 1999 ), an ethical paradox is a situation where an individual is confronted by contradictory injunctions (i.e., do more, faster, and better). And eventually, ethical paradoxes lead individuals to situations of distress and burnout, or even to ethical failures (i.e., misbehaviour or misconduct) in the face of the impossibility of responding to contradictory injunctions.
Strengths and Limitations of the study
The triangulation of perceptions and experiences of different actors involved in research is a strength of our study. While there are many studies on the experiences of researchers, rarely are members of REBs and experts in research ethics given the space to discuss their views of what are ethical issues. Giving each of these stakeholders a voice and comparing their different points of view helped shed a different and complementary light on the ethical issues that occur in research. That said, it would have been helpful to also give more space to issues experienced by students or research assistants, as the relationships between researchers and research assistants are at times very worrying, as noted by a participant, and much work still needs to be done to eliminate the exploitative situations that seem to prevail in certain research settings. In addition, no Indigenous or gender diverse researchers participated in the study. Given the ethical issues and systemic injustices that many people from these groups face in Canada (Drolet & Goulet, 2018 ; Nicole & Drolet, in press ), research that gives voice to these researchers would be relevant and contribute to knowledge development, and hopefully also to change in research culture.
Further, although most of the ethical issues discussed in this article may be transferable to the realities experienced by researchers in other countries, the epistemic injustice reported by Francophone researchers who persist in doing research in French in Canada – which is an officially bilingual country but in practice is predominantly English – is likely specific to the Canadian reality. In addition, and as mentioned above, recruitment proved exceedingly difficult, particularly amongst researchers. Despite this difficulty, we obtained data saturation for all but two themes – i.e., exploitation of students and ethical issues of research that uses animals. It follows that further empirical research is needed to improve our understanding of these specific issues, as they may diverge to some extent from those documented here and will likely vary across countries and academic research contexts.
Conclusions
This study, which gave voice to researchers, REB members, and ethics experts, reveals that the ethical issues in research are related to several problematic elements as power imbalances and authority relations. Researchers and research assistants are subject to external pressures that give rise to integrity issues, among others ethical issues. Moreover, the current context of social acceleration influences the definition of the performance indicators valued in academic institutions and has led their members to face several ethical issues, including social, distributive, and epistemic injustices, at different steps of the research process. In this study, ten categories of ethical issues were identified, described and illustrated: (1) research integrity, (2) conflicts of interest, (3) respect for research participants, (4) lack of supervision and power imbalances, (5) individualism and performance, (6) inadequate ethical guidance, (7) social injustices, (8) distributive injustices, (9) epistemic injustices, and (10) ethical distress. The triangulation of the perspectives of different members (i.e., researchers from different disciplines, REB members, research ethics experts, and one research assistant) involved in the research process made it possible to lift the veil on some of these ethical issues. Further, it enabled the identification of additional ethical issues, especially systemic injustices experienced in research. To our knowledge, this is the first time that these injustices (social, distributive, and epistemic injustices) have been clearly identified.
Finally, this study brought to the fore several problematic elements that are important to address if the research community is to develop and implement the solutions needed to resolve the diverse and transversal ethical issues that arise in research institutions. A good starting point is the rejection of the corollary norms of “publish or perish” and “do more, faster, and better” and their replacement with “publish quality instead of quantity”, which necessarily entails “do less, slower, and better”. It is also important to pay more attention to the systemic injustices within which researchers work, because these have the potential to significantly harm the academic careers of many researchers, including women researchers, early career researchers, and those belonging to racialized groups as well as the health, well-being, and respect of students and research participants.
Acknowledgements
The team warmly thanks the participants who took part in the research and who made this study possible. Marie-Josée Drolet thanks the five research assistants who participated in the data collection and analysis: Julie-Claude Leblanc, Élie Beauchemin, Pénéloppe Bernier, Louis-Pierre Côté, and Eugénie Rose-Derouin, all students at the Université du Québec à Trois-Rivières (UQTR), two of whom were active in the writing of this article. MJ Drolet and Bryn Williams-Jones also acknowledge the financial contribution of the Social Sciences and Humanities Research Council of Canada (SSHRC), which supported this research through a grant. We would also like to thank the reviewers of this article who helped us improve it, especially by clarifying and refining our ideas.
Competing Interests and Funding
As noted in the Acknowledgements, this research was supported financially by the Social Sciences and Humanities Research Council of Canada (SSHRC).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- Al-Hidabi, Abdulmalek, M. D., & The, P. L. (2018). Multiple Publications: The Main Reason for the Retraction of Papers in Computer Science. In K. Arai, S. Kapoor, & R. Bhatia (eds), Future of Information and Communication Conference (FICC): Advances in Information and Communication, Advances in Intelligent Systems and Computing (AISC), Springer, vol. 886, pp. 511–526
- Allaire, S., & Deschenaux, F. (2022). Récits de professeurs d’université à mi-carrière. Si c’était à refaire… . Presses de l’Université du Québec
- Aristotle . Aristotle’s Nicomachean Ethics. Chicago: The University of Chicago Press; 2012. [ Google Scholar ]
- Bahn S. Keeping Academic Field Researchers Safe: Ethical Safeguards. Journal of Academic Ethics. 2012; 10 :83–91. doi: 10.1007/s10805-012-9159-2. [ CrossRef ] [ Google Scholar ]
- Balk DE. Bereavement Research Using Control Groups: Ethical Obligations and Questions. Death Studies. 1995; 19 :123–138. doi: 10.1080/07481189508252720. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Beauchemin, É., Côté, L. P., Drolet, M. J., & Williams-Jones, B. (2021). Conceptualizing Ethical Issues in the Conduct of Research: Results from a Critical and Systematic Literature Review. Journal of Academic Ethics , Early Online. 10.1007/s10805-021-09411-7
- Berg, M., & Seeber, B. K. (2016). The Slow Professor . University of Toronto Press
- Birchley G, Huxtable R, Murtagh M, Meulen RT, Flach P, Gooberman-Hill R. Smart homes, private homes? An empirical study of technology researchers’ perceptions of ethical issues in developing smart-home health technologies. BMC Medical Ethics. 2017; 18 (23):1–13. doi: 10.1186/s12910-017-0183-z. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Blais, J., & Drolet, M. J. (2022). Les injustices sociales vécues en camp de réfugiés: les comprendre pour mieux intervenir auprès de personnes ayant séjourné dans un camp de réfugiés. Recueil annuel belge d’ergothérapie , 14, 37–48
- Bogdan, R. C., & Biklen, S. K. (2006). Qualitative research in education: An introduction to theory and methods . Allyn & Bacon
- Bouffard C. Le développement des pratiques de la génétique médicale et la construction des normes bioéthiques. Anthropologie et Sociétés. 2000; 24 (2):73–90. doi: 10.7202/015650ar. [ CrossRef ] [ Google Scholar ]
- Bronfenbrenner, U. (1979). The Ecology of Human development. Experiments by nature and design . Harvard University Press
- Bruhn JG, Zajac G, Al-Kazemi AA, Prescott LD. Moral positions and academic conduct: Parameters of tolerance for ethics failure. Journal of Higher Education. 2002; 73 (4):461–493. doi: 10.1353/jhe.2002.0033. [ CrossRef ] [ Google Scholar ]
- Clark, A., & Sousa (2022). It’s time to end Canada’s obsession with research quantity. University Affairs/Affaires universitaires , February 14th. https://www.universityaffairs.ca/career-advice/effective-successfull-happy-academic/its-time-to-end-canadas-obsession-with-research-quantity/?utm_source=University+Affairs+e-newsletter&utm_campaign=276a847f 70-EMAIL_CAMPAIGN_2022_02_16&utm_medium=email&utm_term=0_314bc2ee29-276a847f70-425259989
- Colnerud G. Ethical dilemmas in research in relation to ethical review: An empirical study. Research Ethics. 2015; 10 (4):238–253. doi: 10.1177/1747016114552339. [ CrossRef ] [ Google Scholar ]
- Davison J. Dilemmas in Research: Issues of Vulnerability and Disempowerment for the Social Workers/Researcher. Journal of Social Work Practice. 2004; 18 (3):379–393. doi: 10.1080/0265053042000314447. [ CrossRef ] [ Google Scholar ]
- DePoy E, Gitlin LN. Introduction to Research. St. Louis: Elsevier Mosby; 2010. [ Google Scholar ]
- Drolet, M. J., & Goulet, M. (2018). Travailler avec des patients autochtones du Canada ? Perceptions d’ergothérapeutes du Québec des enjeux éthiques de cette pratique. Recueil annuel belge francophone d’ergothérapie , 10 , 25–56
- Drolet MJ, Girard K. Les enjeux éthiques de la recherche en ergothérapie: un portrait préoccupant. Revue canadienne de bioéthique. 2020; 3 (3):21–40. doi: 10.7202/1073779ar. [ CrossRef ] [ Google Scholar ]
- Drolet MJ, Girard K, Gaudet R. Les enjeux éthiques de l’enseignement en ergothérapie: des injustices au sein des départements universitaires. Revue canadienne de bioéthique. 2020; 3 (1):22–36. [ Google Scholar ]
- Drolet MJ, Maclure J. Les enjeux éthiques de la pratique de l’ergothérapie: perceptions d’ergothérapeutes. Revue Approches inductives. 2016; 3 (2):166–196. doi: 10.7202/1037918ar. [ CrossRef ] [ Google Scholar ]
- Drolet MJ, Pinard C, Gaudet R. Les enjeux éthiques de la pratique privée: des ergothérapeutes du Québec lancent un cri d’alarme. Ethica – Revue interdisciplinaire de recherche en éthique. 2017; 21 (2):173–209. [ Google Scholar ]
- Drolet MJ, Ruest M. De l’éthique à l’ergothérapie: un cadre théorique et une méthode pour soutenir la pratique professionnelle. Québec: Presses de l’Université du Québec; 2021. [ Google Scholar ]
- Drolet, M. J., & Ruest, M. (accepted). Quels sont les enjeux éthiques soulevés par la recherche scientifique? In M. Lalancette & J. Luckerhoff (dir). Initiation au travail intellectuel et à la recherche . Québec: Presses de l’Université du Québec, 18 p
- Drolet MJ, Sauvageau A, Baril N, Gaudet R. Les enjeux éthiques de la formation clinique en ergothérapie. Revue Approches inductives. 2019; 6 (1):148–179. doi: 10.7202/1060048ar. [ CrossRef ] [ Google Scholar ]
- Fédération québécoise des professeures et des professeurs d’université (FQPPU) Enquête nationale sur la surcharge administrative du corps professoral universitaire québécois. Principaux résultats et pistes d’action. Montréal: FQPPU; 2019. [ Google Scholar ]
- Fortin MH. Fondements et étapes du processus de recherche. Méthodes quantitatives et qualitatives. Montréal, QC: Chenelière éducation; 2010. [ Google Scholar ]
- Fraser DM. Ethical dilemmas and practical problems for the practitioner researcher. Educational Action Research. 1997; 5 (1):161–171. doi: 10.1080/09650799700200014. [ CrossRef ] [ Google Scholar ]
- Fraser, N. (2011). Qu’est-ce que la justice sociale? Reconnaissance et redistribution . La Découverte
- Fricker, M. (2007). Epistemic Injustice: Power and the Ethics of Knowing . Oxford University Press
- Giorgi A, et al. De la méthode phénoménologique utilisée comme mode de recherche qualitative en sciences humaines: théories, pratique et évaluation. In: Poupart J, Groulx LH, Deslauriers JP, et al., editors. La recherche qualitative: enjeux épistémologiques et méthodologiques. Boucherville, QC: Gaëtan Morin; 1997. pp. 341–364. [ Google Scholar ]
- Giorgini V, Mecca JT, Gibson C, Medeiros K, Mumford MD, Connelly S, Devenport LD. Researcher Perceptions of Ethical Guidelines and Codes of Conduct. Accountability in Research. 2016; 22 (3):123–138. doi: 10.1080/08989621.2014.955607. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Glaser, J. W. (1994). Three realms of ethics: Individual, institutional, societal. Theoretical model and case studies . Kansas Cuty, Sheed & Ward
- Godrie B, Dos Santos M. Présentation: inégalités sociales, production des savoirs et de l’ignorance. Sociologie et sociétés. 2017; 49 (1):7. doi: 10.7202/1042804ar. [ CrossRef ] [ Google Scholar ]
- Hammell KW, Carpenter C, Dyck I. Using Qualitative Research: A Practical Introduction for Occupational and Physical Therapists. Edinburgh: Churchill Livingstone; 2000. [ Google Scholar ]
- Henderson M, Johnson NF, Auld G. Silences of ethical practice: dilemmas for researchers using social media. Educational Research and Evaluation. 2013; 19 (6):546–560. doi: 10.1080/13803611.2013.805656. [ CrossRef ] [ Google Scholar ]
- Husserl E. The crisis of European sciences and transcendental phenomenology. Evanston, IL: Northwestern University Press; 1970. [ Google Scholar ]
- Husserl E. The train of thoughts in the lectures. In: Polifroni EC, Welch M, editors. Perspectives on Philosophy of Science in Nursing. Philadelphia, PA: Lippincott; 1999. [ Google Scholar ]
- Hunt SD, Chonko LB, Wilcox JB. Ethical problems of marketing researchers. Journal of Marketing Research. 1984; 21 :309–324. doi: 10.1177/002224378402100308. [ CrossRef ] [ Google Scholar ]
- Hunt MR, Carnevale FA. Moral experience: A framework for bioethics research. Journal of Medical Ethics. 2011; 37 (11):658–662. doi: 10.1136/jme.2010.039008. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jameton, A. (1984). Nursing practice: The ethical issues . Englewood Cliffs, Prentice-Hall
- Jarvis K. Dilemmas in International Research and the Value of Practical Wisdom. Developing World Bioethics. 2017; 17 (1):50–58. doi: 10.1111/dewb.12121. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kahneman D. Système 1, système 2: les deux vitesses de la pensée. Paris: Flammarion; 2012. [ Google Scholar ]
- Keogh B, Daly L. The ethics of conducting research with mental health service users. British Journal of Nursing. 2009; 18 (5):277–281. doi: 10.12968/bjon.2009.18.5.40539. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lierville AL, Grou C, Pelletier JF. Enjeux éthiques potentiels liés aux partenariats patients en psychiatrie: État de situation à l’Institut universitaire en santé mentale de Montréal. Santé mentale au Québec. 2015; 40 (1):119–134. doi: 10.7202/1032386ar. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lynöe N, Sandlund M, Jacobsson L. Research ethics committees: A comparative study of assessment of ethical dilemmas. Scandinavian Journal of Public Health. 1999; 27 (2):152–159. doi: 10.1177/14034948990270020401. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Malherbe JF. Compromis, dilemmes et paradoxes en éthique clinique. Anjou: Éditions Fides; 1999. [ Google Scholar ]
- McGinn R. Discernment and denial: Nanotechnology researchers’ recognition of ethical responsibilities related to their work. NanoEthics. 2013; 7 :93–105. doi: 10.1007/s11569-013-0174-6. [ CrossRef ] [ Google Scholar ]
- Mills, C. W. (2017). Black Rights / White rongs. The Critique of Racial Liberalism . Oxford University Press
- Miyazaki AD, Taylor KA. Researcher interaction biases and business ethics research: Respondent reactions to researcher characteristics. Journal of Business Ethics. 2008; 81 (4):779–795. doi: 10.1007/s10551-007-9547-5. [ CrossRef ] [ Google Scholar ]
- Mondain N, Bologo E. L’intentionnalité du chercheur dans ses pratiques de production des connaissances: les enjeux soulevés par la construction des données en démographie et santé en Afrique. Cahiers de recherche sociologique. 2009; 48 :175–204. doi: 10.7202/039772ar. [ CrossRef ] [ Google Scholar ]
- Nicole, M., & Drolet, M. J. (in press). Fitting transphobia and cisgenderism in occupational therapy, Occupational Therapy Now
- Pope KS, Vetter VA. Ethical dilemmas encountered by members of the American Psychological Association: A national survey. The American Psychologist. 1992; 47 (3):397–411. doi: 10.1037/0003-066X.47.3.397. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Provencher V, Mortenson WB, Tanguay-Garneau L, Bélanger K, Dagenais M. Challenges and strategies pertaining to recruitment and retention of frail elderly in research studies: A systematic review. Archives of Gerontology and Geriatrics. 2014; 59 (1):18–24. doi: 10.1016/j.archger.2014.03.006. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rawls, J. (1971). A Theory of Justice . Harvard University Press
- Resnik DB, Elliott KC. The Ethical Challenges of Socially Responsible Science. Accountability in Research. 2016; 23 (1):31–46. doi: 10.1080/08989621.2014.1002608. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rosa, H. (2010). Accélération et aliénation. Vers une théorie critique de la modernité tardive . Paris, Découverte
- Sen, A. K. (2011). The Idea of Justice . The Belknap Press of Harvard University Press
- Sen, A. K. (1995). Inegality Reexaminated . Oxford University Press
- Sieber JE. Empirical Research on Research Ethics. Ethics & Behavior. 2004; 14 (4):397–412. doi: 10.1207/s15327019eb1404_9. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sigmon ST. Ethical practices and beliefs of psychopathology researchers. Ethics & Behavior. 1995; 5 (4):295–309. doi: 10.1207/s15327019eb0504_1. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Swazey JP, Anderson MS, Lewis KS. Ethical Problems in Academic Research. American Scientist. 1993; 81 (6):542–553. [ Google Scholar ]
- Swisher LL, Arsalanian LE, Davis CM. The realm-individual-process-situation (RIPS) model of ethical decision-making. HPA Resource. 2005; 5 (3):3–8. [ Google Scholar ]
- Tri-Council Policy Statement (TCPS2) (2018). Ethical Conduct for Research Involving Humans . Government of Canada, Secretariat on Responsible Conduct of Research. https://ethics.gc.ca/eng/documents/tcps2-2018-en-interactive-final.pdf
- Thomas SP, Pollio HR. Listening to Patients: A Phenomenological Approach to Nursing Research and Practice. New York: Springer Publishing Company; 2002. [ Google Scholar ]
- Wiegand DL, Funk M. Consequences of clinical situations that cause critical care nurses to experience moral distress. Nursing Ethics. 2012; 19 (4):479–487. doi: 10.1177/0969733011429342. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Williams-Jones B, Potvin MJ, Mathieu G, Smith E. Barriers to research on research ethics review and conflicts of interest. IRB: Ethics & Human Research. 2013; 35 (5):14–20. [ PubMed ] [ Google Scholar ]
- Young, I. M. (2011). Justice and the Politics of difference . Princeton University Press
- Introduction
- Conclusions
- Article Information
eAppendix. Interview Guide
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Youssef A , Nichol AA , Martinez-Martin N, et al. Ethical Considerations in the Design and Conduct of Clinical Trials of Artificial Intelligence. JAMA Netw Open. 2024;7(9):e2432482. doi:10.1001/jamanetworkopen.2024.32482
Manage citations:
© 2024
- Permissions
Ethical Considerations in the Design and Conduct of Clinical Trials of Artificial Intelligence
- 1 Departments of Radiology, Stanford University School of Medicine, Stanford, California
- 2 Center for Biomedical Ethics, Stanford University School of Medicine, Stanford, California
- 3 Department of Psychiatry, Stanford University School of Medicine, Stanford, California
- 4 Department of Ophthalmology and Visual Sciences, University of Iowa Hospital and Clinics, Iowa City
- 5 Electrical and Computer Engineering, University of Iowa, Iowa City
- 6 Division of Endocrinology, Department of Pediatrics, The Johns Hopkins School of Medicine, Baltimore, Maryland
- 7 Department of Anesthesiology, Division of Pediatric Cardiac Anesthesia, Stanford, California
Question How generalizable are current National Institutes of Health (NIH) ethical principles for conduct of clinical trials to clinical trials of artificial intelligence (AI), and what unique ethical considerations arise in trials of AI?
Findings In this qualitative study, interviews with 11 investigators involved in clinical trials of AI for diabetic retinopathy screening confirmed the applicability of current ethical principles but also identified unique challenges, including assessing social value, ensuring scientific validity, fair participant selection, evaluation of risk-to-benefit ratio in underrepresented groups, and navigating complex consent processes.
Meaning These results suggest ethical challenges unique to clinical trials of AI, which may provide important guidance for empirical and normative ethical efforts to enhance the conduct of AI clinical trials.
Importance Safe integration of artificial intelligence (AI) into clinical settings often requires randomized clinical trials (RCT) to compare AI efficacy with conventional care. Diabetic retinopathy (DR) screening is at the forefront of clinical AI applications, marked by the first US Food and Drug Administration (FDA) De Novo authorization for an autonomous AI for such use.
Objective To determine the generalizability of the 7 ethical research principles for clinical trials endorsed by the National Institute of Health (NIH), and identify ethical concerns unique to clinical trials of AI.
Design, Setting, and Participants This qualitative study included semistructured interviews conducted with 11 investigators engaged in the design and implementation of clinical trials of AI for DR screening from November 11, 2022, to February 20, 2023. The study was a collaboration with the ACCESS (AI for Children’s Diabetic Eye Exams) trial, the first clinical trial of autonomous AI in pediatrics. Participant recruitment initially utilized purposeful sampling, and later expanded with snowball sampling. Study methodology for analysis combined a deductive approach to explore investigators’ perspectives of the 7 ethical principles for clinical research endorsed by the NIH and an inductive approach to uncover the broader ethical considerations implementing clinical trials of AI within care delivery.
Results A total of 11 participants (mean [SD] age, 47.5 [12.0] years; 7 male [64%], 4 female [36%]; 3 Asian [27%], 8 White [73%]) were included, with diverse expertise in ethics, ophthalmology, translational medicine, biostatistics, and AI development. Key themes revealed several ethical challenges unique to clinical trials of AI. These themes included difficulties in measuring social value, establishing scientific validity, ensuring fair participant selection, evaluating risk-benefit ratios across various patient subgroups, and addressing the complexities inherent in the data use terms of informed consent.
Conclusions and Relevance This qualitative study identified practical ethical challenges that investigators need to consider and negotiate when conducting AI clinical trials, exemplified by the DR screening use-case. These considerations call for further guidance on where to focus empirical and normative ethical efforts to best support conduct clinical trials of AI and minimize unintended harm to trial participants.
The integration of artificial intelligence (AI) into health care promises to address long-standing challenges, offering innovative solutions to improve patient outcomes, health equity, clinician productivity, and system efficiency. 1 , 2 As the deployment of AI interventions expands, clinical evidence becomes increasingly crucial in validating their efficacy and safety. 3 - 7 However, there exists a notable gap between the extensive theoretical research on ethical concerns in AI applications in health care and the practical challenges encountered by clinical investigators in clinical settings. 8 This empirical study aims to bridge this gap by examining the practical ethical considerations in the design and implementation of clinical trials involving AI.
Early detection of diabetic retinopathy (DR) is a vanguard area in clinical AI; the first US Food and Drug Administration (FDA) De Novo–authorized autonomous AI was for diabetic eye examinations. 9 We collaborated with investigators from the first National Institutes of Health (NIH)-funded randomized clinical trial (RCT) of autonomous AI, the AI for Children’s Diabetic Eye Exams Study (ACCESS), which was designed to determine the efficacy of autonomous AI screening for DR in a diverse population of youth with diabetes. 10
Ethical frameworks for clinical research, shaped by landmark documents such as the Nuremberg Code, Declaration of Helsinki, Belmont Report, CIOMS guidelines, and the US Common Rule, form the bedrock of research ethics. 11 - 13 Emanuel et al 12 have further delineated 7 core principles for clinical trial ethics, endorsed by the NIH: social and clinical value, scientific validity, fair participant selection, favorable risk-benefit ratio, independent review, informed consent, and respect for human participants. 14 However, the complexities inherent to AI, such as clinical efficacy, algorithmic fairness, and reproducibility of results, pose unique challenges. 15 - 17 Systematic reviews have highlighted significant limitations in AI clinical trials, such as the absence of clinically relevant endpoints and a high risk of bias, raising questions about the suitability of traditional ethical frameworks in AI contexts. 4 , 18 - 21 While there is a consensus on the necessity for increased transparency of randomized clinical trials of AI (AI-RCTs), current guidelines primarily focus on standardized reporting and fall short from addressing ethical considerations in the design of these clinical trials. 22 , 23
This qualitative study aimed to address 2 primary research questions: (1) To what extent are the 7 NIH ethical principles 14 created by Emanuel and Grady 12 generalizable to clinical trials of AI? and (2) What are the ethical considerations that may be unique to clinical trials of AI?
This qualitative study was approved by the Johns Hopkins Medicine institutional review board. All study participants were informed about the waiver of written consent and provided verbal consent to participate voluntarily, without financial compensation. We followed the Consolidated Criteria for Reporting Qualitative Research ( COREQ ) reporting guidelines.
We employed both a deductive and an inductive approach to data collection. We used a deductive approach described to test the applicability of the NIH’s 7 core ethical principles—clinical and social value, scientific validity, fair participant selection, favorable risk-benefit ratio, independent review, informed consent, and respect for human participants. 24 We also utilized a modified grounded theory approach for the discovery of novel themes. 25
Participants in this study included clinical investigators, institutional review ethicists, and clinical trialists involved in autonomous AI trials for diabetic retinopathy screening. The selection criteria were aligned with the study’s aim of examining ethical challenges in the design and conduct of AI-based clinical trials. Initially, purposive, nonprobabilistic sampling was used to recruit 6 participants from the ACCESS study ( NCT05131451 ). 10 Two authors (R.W. and D.C.) invited investigators from the ACCESS study to participate in this study. To enhance the generalizability of our findings, we employed snowball sampling method to identify participants involved in concurrent RCTs of AI for diabetic retinopathy screening in low-income countries, resulting in the inclusion of 3 participants from a nonprofit organization and 2 from the private sector.
Data collection occurred from November 2022 to February 2023. Interviews were conducted in English via video call (Zoom) by a qualitative research scientist (A.Y.) with over 7 years of experience in qualitative research. Interviews ranged from 30 to 60 minutes and were guided by a set of questions addressing demographic questions, the 7 ethical principles endorsed by the NIH—clinical and social value, scientific validity, fair participant selection, favorable risk-benefit ratio, independent review, informed consent, and respect for human participants—and open-ended questions to explore additional ethical considerations (eAppendix in Supplement 1 ). All interviews were transcribed by a professional service, and MAXQDA version 2022.2 software (VERBI GmbH) was used for managing and analyzing the data.
Following initial transcript analysis, a study coauthor (A.Y.) developed a codebook for systematic independent coding. Two authors (A.Y. and A.N.) independently coded all interviews, achieving a Cohen κ score greater than 0.8 for interrater reliability. Discrepancies were resolved through consensus coding with 3 coauthors (R.W., N.M., and D.C.). Theoretical saturation was achieved after the tenth interview, as no new insights emerged from the eleventh interview. To maintain reflexivity, we kept a detailed audit trail (A.Y.). This trail included reflections on both the interviewees’ and interviewer’s perceptions. These reflections were critically examined in weekly research meetings with all authors, challenging emerging hypotheses to reduce confirmation bias and ensure theme credibility. 26
We conducted interviews with 11 investigators with experience conducting AI-RCT for DR screening, with a mean (SD) age of 47.5 (12.0) years (7 male [64%], 4 female [36%]; 3 Asian [27%], 8 White [73%]) ( Table 1 ). Participants came from academia, the nonprofit sector, and industry, bringing diverse expertise in ethics, ophthalmology, translational medicine, biostatistics, AI development and deployment, and policy.
While recognizing the importance of the 7 ethical principles in AI clinical trials, participants identified unique ethical challenges specific to AI trials. These challenges demand a nuanced understanding of how to appropriately apply these principles in the context of AI clinical trials. Table 2 outlines participants’ perspectives on the 7 principles within the context of AI clinical trials. Table 3 presents novel ethical considerations that emerged from the inductive analysis, highlighting specific challenges faced during the implementation of these trials.
When applied to clinical trials of AI in clinical settings, participants identified several unique applications of the 7 ethical principles. Common themes across principles included the added difficulty in accounting for equitable access to care and the need for transparency with patients.
Participants recognized AI’s potential to improve clinical outcomes, comparable with the potential outcomes of non-AI based RCTs (ie, drugs or medical devices). Specifically, in this RCT focused on DR, they perceived AI’s potential to reduce health disparities as a clear metric for social value. However, they expressed uncertainty defining and quantifying the social value of an AI intervention compared with its clinical benefits.
Participants recognized RCTs as a criterion standard for demonstrating clinical efficacy of the AI intervention. However, they identified unique challenges specific to AI RCTs. One participant questioned the appropriateness of prioritizing individualized outcome parameters—such as patient outcome—rather than outcomes for groups or populations. They noted the difficulty of comparing AI interventions with the variable criterion standard of usual care, which can differ significantly across clinical settings.
Fair participant selection in AI clinical trials emerged as a significant topic, particularly regarding the accurate representation of the patient population of focus. Study participants highlighted the challenges in evaluating the efficacy of the AI intervention across patient subgroups, who are often affected by limited access to care and can be underrepresented in clinical trials. One participant pointed out the complexities of ensuring equitable access studying the impact of AI screening on patient groups that may access less regular diabetes screening and care.
Participants recognized the complexity of balancing the risks and benefits of AI interventions across diverse patient groups. They noted the difficulty in estimating the harm-to-benefit ratio of AI interventions relative to the known risks of standard care, a challenge exacerbated by limited representation of patient groups facing health inequities in retrospective standalone studies of algorithm performance.
Participants identified key ethical concerns presented in AI clinical trials, emphasizing the need for transparent communication about the risk and benefits of an AI intervention tailored to patients with varying levels of health literacy. They questioned whether patients fully understand the extent toward which their data might be used beyond the trial itself. Additionally, concerns were raised about the adequacy of current informed consent processes and institutional ethical review readiness to assess the risks and benefits of AI interventions.
Participants highlighted additional ethical challenges beyond the established 7 ethical principles ( Table 3 ). These included: (1) Whose values prevail in AI systems design and implementation? (2) Can AI integration enhance clinical workflows without compromising patient safety? (3) How to balance the economic incentives with the ethical obligations to adopt effective AI intervention that can improve patients’ outcomes? (4) What are the ethical implications of expanding DR screening without enhancing treatment access?
Participants critiqued the broadly defined term “value,” noting its varied interpretations across different stakeholders including health systems, clinicians, and patients. They questioned whose values are prioritized during the design and implementation of AI systems. Additionally, concerns were raised about AI’s adaptability to individual patient needs. For instance, while clinicians can adjust treatments to ensure affordability and effectiveness, AI systems may lack this flexibility due to their predefined operational parameters and potential downstream effects.
Said a participant who specializes in informatics, “So patient value can take all what people really, really, really care about, which includes spiritual and religious issues which do not go into health services research considerations, don’t go into societal considerations…so the very word value I would bet is not well-defined.” A participant with a research focus in ophthalmology pointed out that cost of care varied by community, “Somebody who had very limited resources couldn’t afford to pay $60 a month for drops.…So, the decision that we make about how much quality we can afford needs to be made with respect to local economic scales.”
From the participants’ perspective, integrating AI tools into clinical workflows introduces a significant tension. There is a drive for AI to enhance clinical workflows, but this drive carries the inherent risk of complicating an already complex workflow, especially when the definitive clinical benefits of AI are still unclear. This tension is further amplified when contemplating potential risks to research participants during clinical trials. “I think part of it, to not be able to do an RCT with an AI tool, would come down to just clinical workflow,” said a participant working in optometry. “If you’re trying to interject something into a workflow that’s already overloaded and pretty strapped.…I think that’s a limitation when it comes to implementing these because you have to set up, you know, both arms. You have to then set up multiple workflows and you’re already…you’re already trying to interject a new workflow where it hadn’t been before, which can be complicated.”
“I think the clinical value is that there’s quite a bit of hype about AI and we know that for sure AI can do certain things better than humans in many different contexts, but just because AI is better than humans that do certain things doesn’t mean if we incorporate AI will it necessarily improve the outcomes or the metrics that we’re interested in,” said a respondent specializing in ophthalmology and machine learning. “So I think it’s very important to, in clinical trial[s] involving AI, to show that it improves outcomes. That’s a completely open question by now that we don’t know for the most part whether incorporating AI in clinical workflow improves outcome, so I think this is a very important question to answer. And you could only answer that using [a] randomized clinical trial.”
Participants highlighted a critical tension in developing and validating AI tools in health care, balancing economic pressures with ethical imperatives. The ethical mandate to make these tools universally accessible clashes with the high costs associated with conducting RCTs, deemed the criterion standard for validation, particularly in the US and Europe. This economic challenge has prompted AI developers to shift RCTs to developing countries, raising concerns about potentially deepening health care inequities.
One participant who is both an AI developer and clinician-scientist expressed the dilemma facing AI developers and health systems: “If you don’t get creators and people like me and investors excited about the potential return, it will stop. That’s just the way it is. I was struggling as a developer, what is the balance between making…so if you see that more access and better outcomes is good and you expect people to pay for that, how do you put a charge so that you don’t make a charge too high?” The same participant also added insight on payment models for AI, suggesting a focus on health equity. “How should we be paying for AI?” he said. “If as a taxpayer or society you’re paying for something, then health equity should be the main guiding star.”
One participant in ophthalmology criticized the inherently high costs associated with RCTs: “Randomized control trials…there’s sort of an inherent assumption that they have to cost 40 million dollars. I just think that’s unethical. We have to come up with a way of delivering the kind of evidence, the high-quality evidence that can provide in a way that’s affordable for lower middle-income countries.”
Finally, the practical implications of AI were discussed. “If you’re improving the screening, then that in and of itself is sufficient to understand this is something that benefits patients,” said a participant working in AI and machine learning in health care. “Now whether a system is willing to pay for such an [AI solution], that’s a different question.”
A stated goal of AI is to broaden the reach of ophthalmology screening to populations currently underserved, thereby reducing their risk of blindness from DR. However, participants highlighted a complex array of clinical and ethical questions associated with this proposed use of AI. One concern is whether merely expanding access to DR screening without simultaneously improving access to treatment might create ethical dilemmas downstream.
“If someone’s not accessing services for diabetes in general regularly and not coming in for their well visits,…they’re not controlling their condition in the first place, they’re probably coming in less, they’re less likely to get the AI screening even if it’s available to them in their clinic and they’re more at risk for diabetic retinopathy because they’re not [accessing care],” said a researcher specializing in biostatistics and clinical trials. “If we’re trying to target and reduce those disparities, I think it would be important then to see where they’re falling off and why they’re not getting screened, or are they getting screened and not going for follow-ups.”
One participant emphasized the potential benefits of AI for the “bottom billion,” a uniquely underserved population, highlighting the slower arrival of such technologies to these groups. Another noted the generalization issues that can arise if AI models are trained on data from a narrow demographic. “I’ve been pleasantly surprised at how well the generalization has shown so far,” he said. “But I also think that if you only train for people from one small part of the world, then you would have a generalization problem.”
Finally, the cost-effectiveness of AI diagnostics was questioned, particularly in contexts where the financial burden might outweigh the clinical benefits. “If the AI is efficacious, it’s an accurate diagnostic, but is this cost-effective if it costs a million dollars to run?” asked one participant. “Whether a system is willing to pay for such a product is another question.”
This study is, to our knowledge, the first to explore the practical ethical considerations involved in designing and executing AI clinical trials. We draw on the experiences of investigators conducting the first NIH-funded RCT of an autonomous AI for DR screening, along with related trials. While we found consensus among stakeholders regarding generalizability of the NIH’s 7 ethical principles to clinical trials of AI, we identified important areas of uncertainty regarding social value, scientific validity, fair participant selection, favorable benefit-risk ratio, and informed consent ( Table 2 ). Thematic analysis of participants’ experiences in DR screening trials across various settings also highlighted novel ethical considerations specific to AI clinical trials, independent of the 7 principles.
When discussing the 7 ethical principles, defining and measuring the social value of AI in clinical trials proved to be complex. Perspectives ranged from prioritizing patient views to measurable reduction in health care inequities. Moreover, participants struggled to generalize social value across trials due to its context-dependent nature and the lack of defined metrics to measure the social impact of AI interventions. While the clinical value of AI in improving patient outcomes is similar to that of traditional drug or device trials, evaluating AI introduces additional complexities as it functions as both a cognitive tool and a workflow intervention. Establishing a control, typically defined as the standard of care, can vary significantly across different clinical environments. This variability may limit the generalizability of AI interventions, as an AI system validated in one setting might not perform effectively in another setting.
In addition, the goal of using an AI to expand access for populations with limited access presents an ethical tension between the desirable social value of reducing access inequity with the limitations of an AI with biased training data (at least until better access for all groups is delivered) while outcomes are still being evaluated. For future trials, it will be crucial for researchers to clearly define the desired social value and establish specific, measurable outcomes that demonstrate the AI intervention efficacy achieving this value.
Participants also expressed nuanced concerns about ensuring a favorable benefit-to-risk ratio and obtaining true informed consent for AI interventions. While uncertainty is inherent in clinical research, balancing the unknown risks of AI screening against the known risks of untreated DR presented unique challenges. Interviewees noted that different population subgroups likely had different risk-benefit ratios when AI risks are compared with current screening methods or the absence of screening. Moreover, challenges in ensuring fair patient selection were noted, particularly in scenarios where patients facing health disparities are less likely to access care. Ensuring informed consent emerged as a significant challenge, particularly in communicating the benefits, risks, and data use terms for AI interventions to participants with varying levels of health literacy.
The exploration of novel ethical considerations in the context of AI clinical trials revealed several critical issues ( Table 3 ). Participants expressed concerns about whose values are prioritized in the design and implementation of AI systems, emphasizing that current definitions fail to capture the diverse needs and priorities of all stakeholders, including patients, clinicians, and health systems. An ethical challenge is emerging in trials of AI around value capture; if a desired outcome of an AI tool is “value” (cost, labor, or access savings), who decides what outcome value is prioritized and how value savings are redistributed within a health care system or community is unknown. 27 This uncertainty presents a crucial ethical knowledge gap in ensuring that AI trials are responsive to clinical contexts.
The integration of AI into clinical workflows also generated tension. RCT investigators aimed to evaluate AI effectiveness without compromising patient safety or disrupting established workflows with proven efficacy. Clinicians expressed concern that modifying care workflows to accommodate AI could unintentionally affect patient care or increase staff workload. Therefore, it is crucial to carefully assess the impact of AI on clinical workflows before implementation.
Participants also raised ethical concerns about using AI to improve access and equity without a clear financial incentive. They questioned what would incentivize a health system to invest in such technologies. Participants expressed concern around using AI to improve screening access in resource-constrained settings. For example, in the context of an AI clinical trial for DR screening, the AI system may be tested in a well-resourced clinical setting where patient can immediately receive follow-up treatment from an ophthalmologist if diagnosed with DR. However, if this AI system is later to be deployed in an underresourced setting, even if it accurately identifies patients needing treatment, the lack of access to follow-up care might prevent these patients from receiving the necessary interventions. This raises an ethical question: Is it appropriate to evaluate the AI’s efficacy in a controlled environment with follow-up care, knowing that in its clincial application, such care might be inaccessible?
Findings from this study suggest that the concept of equipoise—the ethical balance necessary in clinical trials—is more complex in AI interventions. AI, as a systems intervention, not only affects individual patient care but also integrates with and transforms health care workflows and system operations, complicating the evaluation of its effectiveness in clinical trials.
This study had several limitations. The participant pool was relatively small, comprising 11 individuals from 4 US academic institutions, and was restricted to investigators involved in AI clinical trials. While the deductive approach allowed us to systematically apply the 7 ethical principles to our data, it may inherently have limited the scope of conclusions by focusing on predefined frameworks. However, the inductive components of our study enabled us to explore novel insights and themes that emerged directly from the data, thereby enriching our understanding and identification of ethical issues beyond the initial framework. Although the theoretical concepts uncovered may be applicable to AI clinical trials in other areas, the scope of this study was limited to clinical trials of AI in diabetic retinopathy screening. Thus, the generalizability of these findings requires further validation in future studies. Furthermore, it is important to note that the 7 principles centered in this article have been criticized as being parochial, or at least Western-centric. 28 , 29 The potential vulnerability of the 7 principles raised by this criticism is magnified as AI development for health care is occurring globally.
This study addresses an important gap in practical understanding of how clinical investigators actually navigate ethical considerations arising with the design and conduct of AI clinical trials. It reveals a general consensus on the utility of NIH’s 7 ethical principles for clinical trials of AI but also important areas of uncertainty in social value, scientific validity, fair participant selection, favorable risk-benefit ratio, and informed consent. These findings highlight important considerations that should be addressed in future iterations of ethical guidance for AI trials. As Emanuel and Grady 12 aptly noted, “Like a constitution, these requirements can be reinterpreted, refined, and revised.…Yet these requirements must all be considered and met to ensure that clinical research, wherever practiced, is ethical.”
Accepted for Publication: July 15, 2024.
Published: September 6, 2024. doi:10.1001/jamanetworkopen.2024.32482
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Youssef A et al. JAMA Network Open .
Corresponding Author: Alaa Youssef, PhD, Department of Radiology, Stanford University School of Medicine, Stanford Center for Artificial Intelligence and Medical Imaging, 1701 Page Mill Rd, Mail code 5467, Stanford, CA 94304 ( [email protected] ).
Author Contributions : Drs Youssef and Char had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Youssef, Abramoff, Wolf, Char.
Acquisition, analysis, or interpretation of data: Youssef, Nichol, Martinez-Martin, Larson, Wolf, Char.
Drafting of the manuscript: Youssef, Nichol, Abramoff, Wolf, Char.
Critical review of the manuscript for important intellectual content: Youssef, Martinez-Martin, Larson, Wolf, Char.
Statistical analysis: Youssef, Nichol, Abramoff.
Obtained funding: Wolf, Char.
Administrative, technical, or material support: Nichol, Wolf, Char.
Supervision: Larson, Abramoff, Wolf, Char.
Conflict of Interest Disclosures: Dr Larson reported holding shares in Bunker Hill Health Shareholder outside the submitted work; he reported receiving research support from Siemens Healthineers and the Gordon and Betty Moore Foundation outside the submitted work. Dr Abramoff reported work as director and consultant with Digital Diagnostics Inc outside the submitted work, where he also holds equity and has patent application assigned; he reported chairing the Healthcare AI Coalition Foundational Principles of AI Collaborative Community for Ophtalmic Imgaing and serving as committee member of the American Academy of Ophthalmology AI Committee, AI Workgroup Digital Medicine Payment Advisory Group, and the Collaborative Community for Ophthalmic Imaging. Dr Wolf reported grants from Novo Nordisk as primary investigator for a clinical research site outside the submitted work. No other disclosures were reported.
Funding/Support: This study was funded by the National Eye Institute (R01EY033233-01).
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: We extend our sincere gratitude to the ACCESS study team and all participating individuals. Their invaluable contributions, dedicated time, and willingness to share their insights have been fundamental to the success of this research. We are deeply grateful for their openness and the rich perspectives they have provided.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

IMAGES
VIDEO
COMMENTS
Ethical considerations in research are a set of principles that guide your research designs and practices. Scientists and researchers must always adhere to a certain code of conduct when collecting data from people. The goals of human research often include understanding real-life phenomena, studying effective treatments, investigating ...
These considerations are designed to protect the rights, safety, and well-being of research participants, as well as the integrity and credibility of the research itself. Some of the key ethical considerations in research include: Informed consent: Researchers must obtain informed consent from study participants, which means they must inform ...
At Prolific, we believe in making ethical research easy and accessible. The findings from the Fairwork Cloudwork report speak for themselves. Prolific was given the top score out of all competitors for minimum standards of fair work. With over 25,000 researchers in our community, we're leading the way in revolutionizing the research industry.
Ethics Principle 1: Respect for persons. As the name suggests, this principle is all about ensuring that your participants are treated fairly and respectfully. In practical terms, this means informed consent - in other words, participants should be fully informed about the nature of the research, as well as any potential risks. Additionally ...
The following are just two examples of infamous cases of unethical research practices that demonstrate the importance of adhering to ethical standards: The Stanford Prison Experiment (1971) aimed to investigate the psychological effects of power using the relationship between prisoners and prison officers. Those assigned the role of "prison ...
a paper can be considered for publication. if you cannot attest to this, most reputable journals will not publish your paper . A Scientist's Responsibilities That said, ethical considerations do not stop when the last subject leaves. However, the focus of ethical consideration does change. Now you must
3. Justice. IRB indicates institutional review board. framework for evaluating research is outlined by Emanuel et al.7 Steps suggested in the process of eval-uating ethical research include: 1.Value in terms of the knowledge extracted and applied from the research. 2.Scientific validity reflecting the methodology.
Research is a critical aspect of any design journey, but ensuring that the research is accurate, unbiased, and ethical is just as important.. Whether you are conducting a survey, running focus groups, doing field research, or holding interviews, the chances are participants will be a part of the process.. Taking ethical considerations into account and following all obligations are essential ...
In the conduct of research, several key principles and actions must be observed and preserved. These include freedom from harm, right to self-determination, right to privacy, and right to anonymity and confidentiality (See Figure 2). Freedom from physical or mental harm or discomfort should be of utmost concern to the researcher.
Ethical Statement Templates The following statements provide templates for the different types of ethical statements required for journal articles. Authors may use these as a guide when drafting their manuscripts. Please note there are many different types of statements and situations, so several examples are provided, but may not cover all cases.
of power and authority are all 'ethical considerations inherent in and raised. by ESL research' (p. . 1) . Koulouriotis further reiterates the point that a great. proportion of research in ESL ...
Principles that come to mind might include autonomy, respect, dignity, privacy, informed consent and confidentiality. You may also have identified principles such as competence, integrity, wellbeing, justice and non-discrimination. Key ethical issues that you will address as an insider researcher include: Gaining trust.
organizations are also examined. Specifically, within this paper the importance of ethical conduct while engaging with research, especially WIL research using human participants, is discussed, including the need to obtain ethical approval and consideration of issues around informed consent, conflict of interest, risk of harm and confidentiality.
Revised on 6 July 2024. Ethical considerations in research are a set of principles that guide your research designs and practices. Scientists and researchers must always adhere to a certain code of conduct when collecting data from people. The goals of human research often include understanding real-life phenomena, studying effective treatments ...
In order to address ethical considerations aspect of your dissertation in an effective manner, you will need to expand discussions of each of the following points to at least one paragraph: 1. Voluntary participation of respondents in the research is important. Moreover, participants have rights to withdraw from the study at any stage if they ...
Ethical Considerations in Research. Research is an important component of expanding knowledge and understanding the. world, and the people that inhabit the it. As Fiske (2018) notes, how people ...
Fabrication and falsification of data or results. Conducting and reporting research methods, data, and results honestly is at the very top of the list of ethical considerations in research. Fabrication is making up data or results, while falsification is manipulating or altering data or results, both of which are seen as major ethical violations.
The study of research-related ethical dilemmas is what "ethics" is all about, say Mirza et al. (2023). Meyer (2008) highlighted that ethical considerations in research stem from ancient Greek ...
Ethical considerations related to conducting research also involve standards for the storage and analysis of research data so cases like the Schön scandal can be reviewed expeditiously. Generally speaking, all research data, including primary materials and raw data, such as survey questionnaires, measurements, recordings, and computer results, must be stored in secure and durable storage ...
In this paper, we discuss core ethical and methodological considerations in the design and implementation of qualitative research in the COVID-19 era, and in pivoting to virtual methods—online interviews and focus groups; internet-based archival research and netnography, including social media; participatory video methods, including photo ...
Introduction. Research includes a set of activities in which researchers use various structured methods to contribute to the development of knowledge, whether this knowledge is theoretical, fundamental, or applied (Drolet & Ruest, accepted).University research is carried out in a highly competitive environment that is characterized by ever-increasing demands (i.e., on time, productivity ...
According to Arifin (2018), ethical considerations in a qualitative study have a particular resonance due to the in-depth nature of the study process, which is central to protecting human subjects ...
Ethical frameworks for clinical research, shaped by landmark documents such as the Nuremberg Code, Declaration of Helsinki, Belmont Report, CIOMS guidelines, and the US Common Rule, form the bedrock of research ethics. 11-13 Emanuel et al 12 have further delineated 7 core principles for clinical trial ethics, endorsed by the NIH: social and ...
Email: [email protected]. This is the third of four papers to be published in Research Ethics Review in 2009, that address methodological issues of relevance to research ethics committees. It focuses on three issues: the representativeness of study participants, the size of the study and data analysis.