Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List

Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
Striking findings from 2023
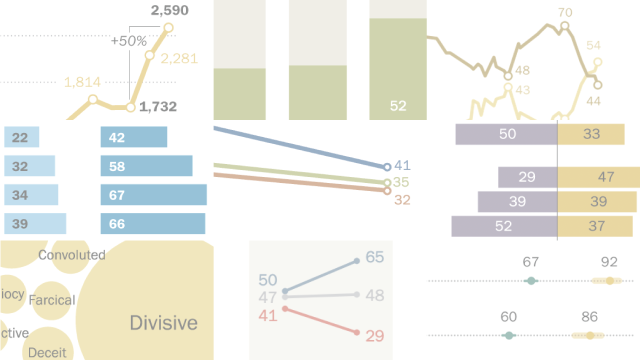
Pew Research Center has gathered data around some of this year’s defining news stories, from the rise of artificial intelligence to the debate over affirmative action in college admissions . Here’s a look back at 2023 through some of our most striking research findings.
These findings only scratch the surface of the Center’s research from this past year .
A record-high share of 40-year-olds in the U.S. have never been married, according to a Center analysis of the most recent U.S. Census Bureau data . As of 2021, a quarter of 40-year-olds had never been married – up from 6% in 1980.
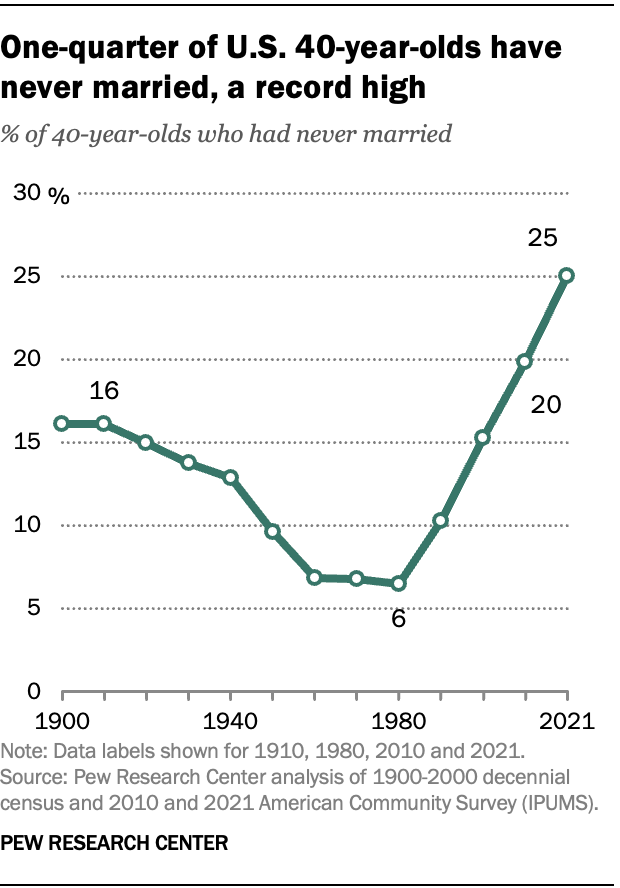
In 2021, the demographic groups most likely not to have ever been married by age 40 include men, Black Americans and those without a four-year college degree.
A Center survey conducted in April found that relatively few Americans see marriage as essential for people to live a fulfilling life compared with factors like job satisfaction and friendship. While majorities say that having a job or career they enjoy (71%) and having close friends (61%) are extremely or very important for living a fulfilling life, far fewer say this about having children (26%) or being married (23%). Larger shares, in fact, say having children (42%) or being married (44%) are not too or not at all important.
About half of Americans say the increased use of artificial intelligence in daily life makes them feel more concerned than excited – up 14 percentage points from last year, according to an August survey . Overall, 52% of Americans say they feel this way, an increase from 38% in December 2022.
Just 10% of adults say they are more excited than concerned about the increased use of AI, while 36% say they feel an equal mix of these emotions.
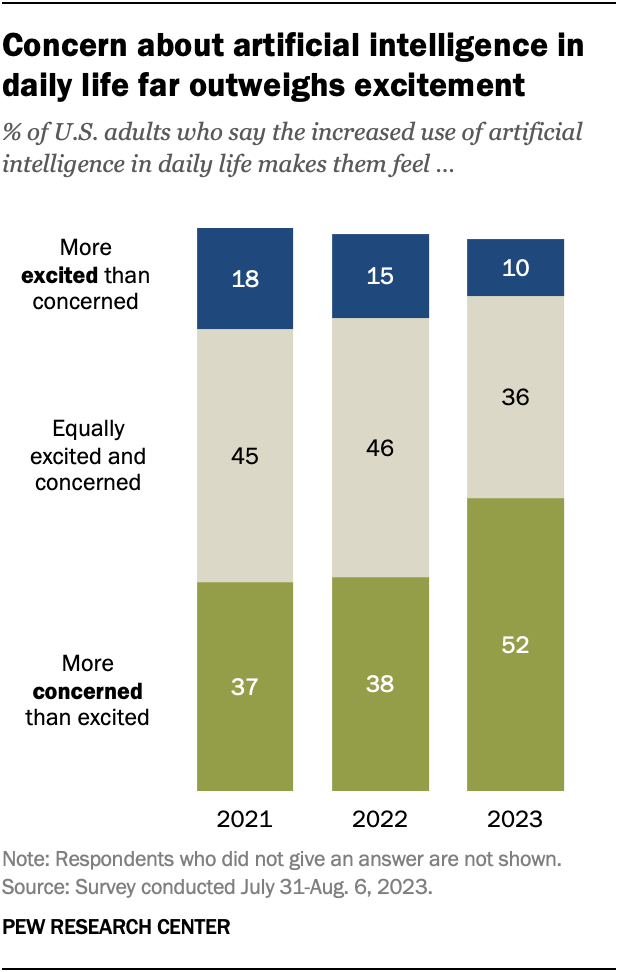
The rise in concern about AI has taken place alongside growing public awareness of the technology. Nine-in-ten adults say they have heard either a lot (33%) or a little (56%) about artificial intelligence. The share of those who have heard a lot is up 7 points since December 2022.
For the first time in over 30 years of public opinion polling, Americans’ views of the U.S. Supreme Court are more negative than positive, a July survey found . A narrow majority (54%) have an unfavorable view of the high court, while fewer than half (44%) express a favorable one.
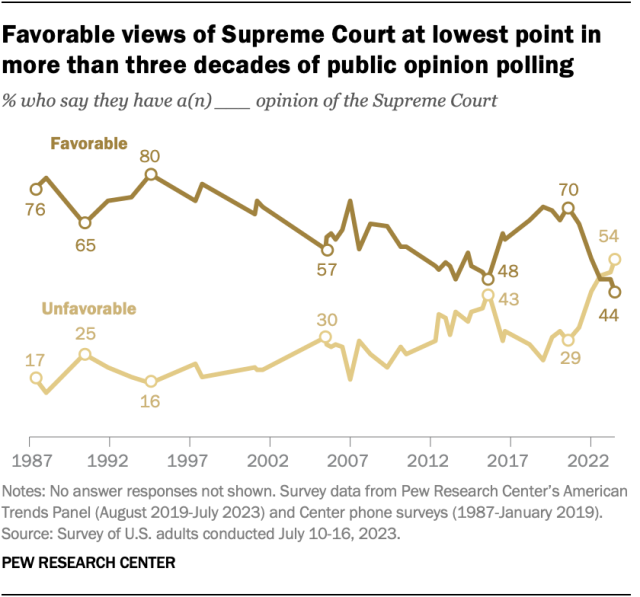
The court’s favorable rating has declined 26 percentage points since 2020, following a series of high-profile rulings on issues including affirmative action in college admissions, LGBTQ+ rights and student loans. The drop in favorability is primarily due to a decline among Democrats and Democratic-leaning independents, just 24% of whom express a favorable opinion of the court.
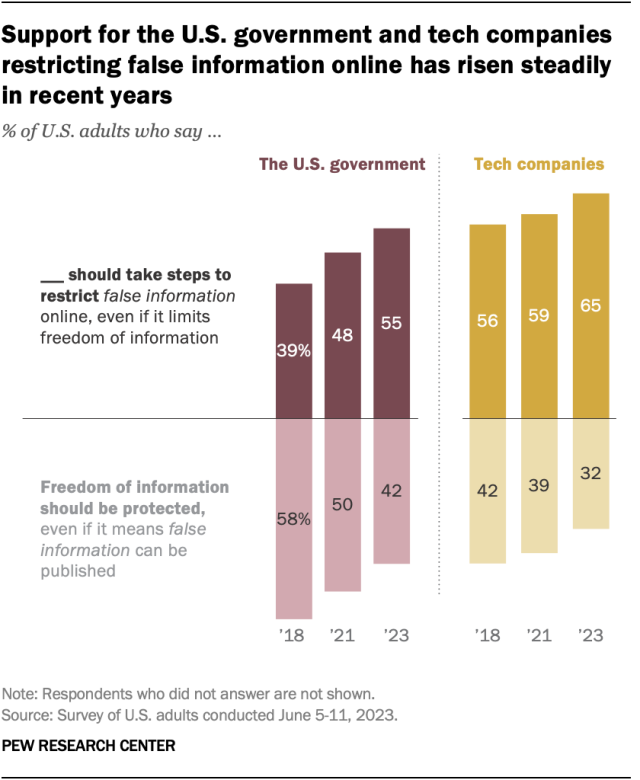
A growing share of U.S. adults say the federal government should take steps to restrict false information online, even if it limits freedom of information, a June survey found . The share of U.S. adults with this view has risen from 39% in 2018 to 55% in 2023.
In the most recent survey, 42% of adults took the opposite view, saying the government should protect freedom of information, even if it means false information can be published.
Still, Americans remain more likely to say that tech companies – rather than the U.S. government – should be responsible for restricting false information online. About two-thirds (65%) said this in June.
The number of U.S. children and teens killed by gunfire rose 50% in just two years, according to a 2023 analysis of data from the Centers for Disease Control and Prevention (CDC). In 2019, there were 1,732 gun deaths among U.S. children and teens under 18. By 2021, that figure had increased to 2,590.
The gun death rate among children and teens – a measure that adjusts for changes in the nation’s population – rose 46% during that span.
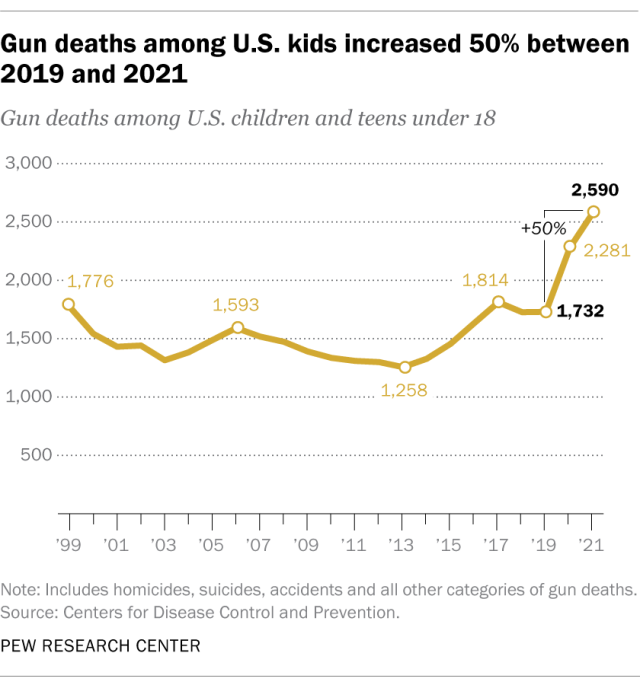
Both the number and rate of children and teens killed by gunfire in 2021 were the highest since at least 1999, the earliest year for which this information is available in the CDC’s mortality database.
Most Asian Americans view their ancestral homelands favorably – but not Chinese Americans, according to a multilingual, nationally representative survey of Asian American adults .
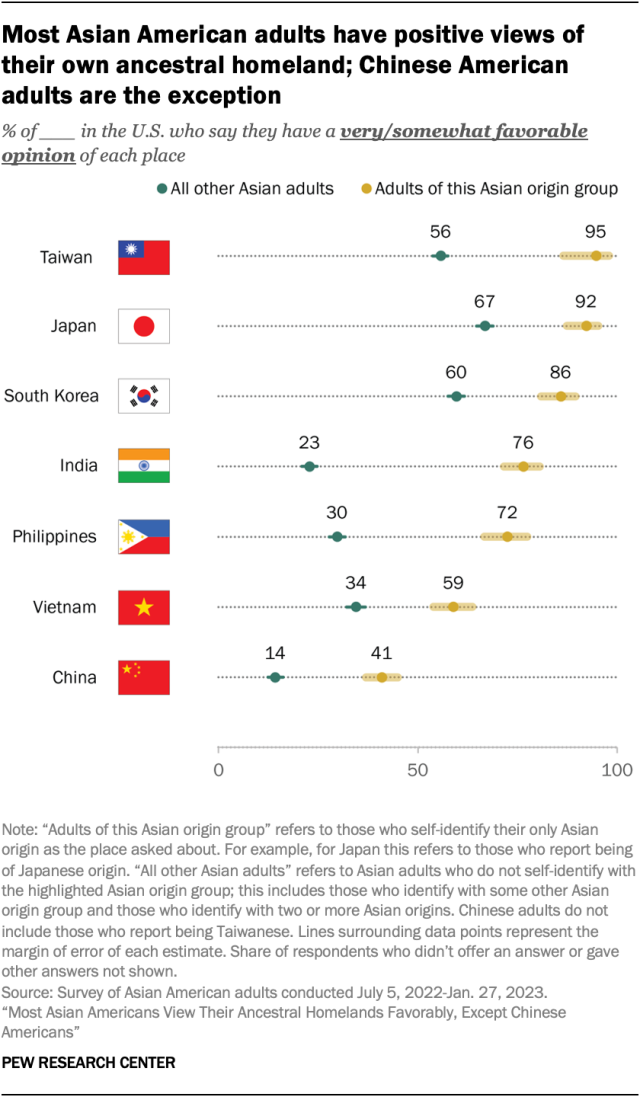
Only about four-in-ten Chinese Americans (41%) have a favorable opinion of China, while 35% have an unfavorable one. Another 22% say they have a neither favorable nor unfavorable view. This stands in contrast to how other Asian Americans view their ancestral homelands. For instance, about nine-in-ten Taiwanese and Japanese Americans have a very or somewhat favorable opinion of their place of origin, as do large majorities of Korean, Indian and Filipino Americans.
While Chinese Americans’ views of China are more mixed, they still have a more favorable opinion of the country than other Asian adults do. Just 14% of other Asian Americans view China favorably.
Even before the Israel-Hamas war, Israelis had grown more skeptical of a two-state solution. In a survey conducted in March and April , prior to the war, just 35% of Israelis thought “a way can be found for Israel and an independent Palestinian state to coexist peacefully.” This share had declined by 9 percentage points since 2017 and 15 points since 2013.
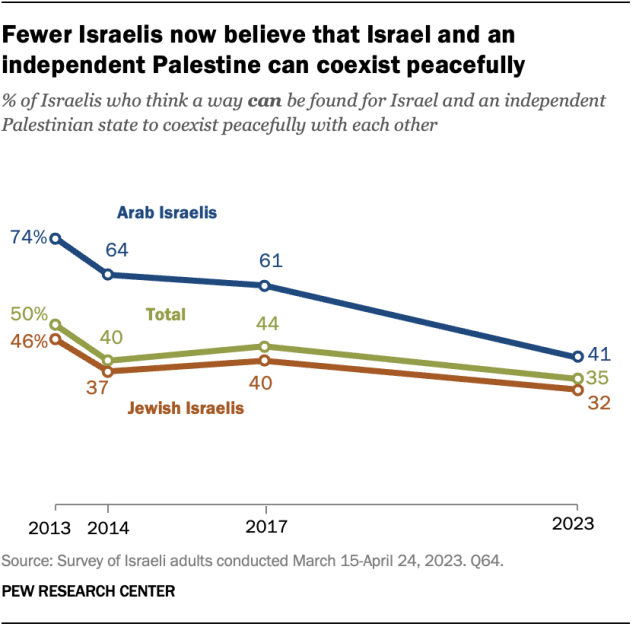
Among both Arabs and Jews living in Israel, there have been declines over the past decade in the share of people who believe that a peaceful coexistence between Israel and an independent Palestinian state is possible.
A majority of Americans say they would tip 15% or less for an average restaurant dining experience, including 2% who wouldn’t leave a tip at all, an August survey shows . The survey presented respondents with a hypothetical scenario in which they went to a sit-down restaurant and had average – but not exceptional – food and service. About six-in-ten (57%) say they would leave a tip of 15% or less in this situation. Another 12% say they would leave a tip of 18%, and a quarter of people say they’d tip 20% or more.
Adults in lower-income households and those ages 65 and older are more likely than their counterparts to say they would tip 15% or less in a situation like this.
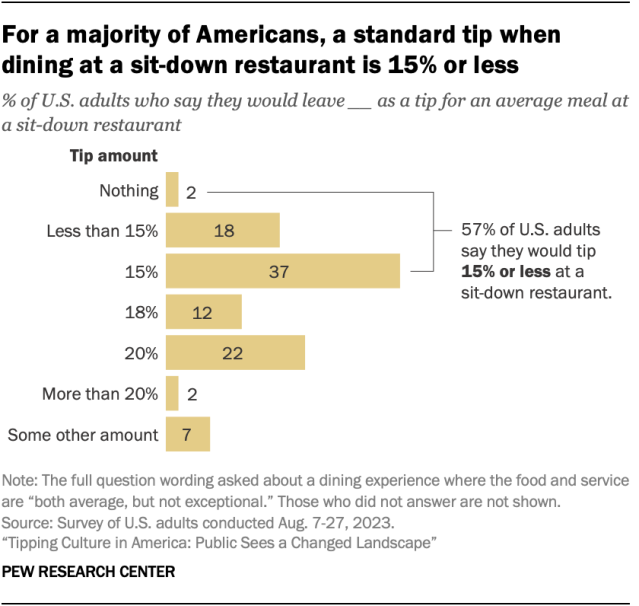
Partisan views of Twitter – the social media platform now called X – have shifted over the last two years, with Republican users’ views of the site growing more positive and those of Democratic users becoming more negative, according to a March survey . The share of Republican and GOP-leaning users who said the site is mostly bad for American democracy fell from 60% in 2021 to 21% earlier this year. At the same time, the share of Republican users who said the site is mostly good for democracy rose from 17% to 43% during the same span.
Democrats’ views moved in the opposite direction during that time frame. The percentage of Democratic and Democratic-leaning Twitter users who said the platform is good for American democracy decreased from 47% to 24%, while the share who said it is bad for democracy increased – though more modestly – from 28% to 35%.
These changes in views follow Elon Musk’s takeover of the platform in fall 2022.
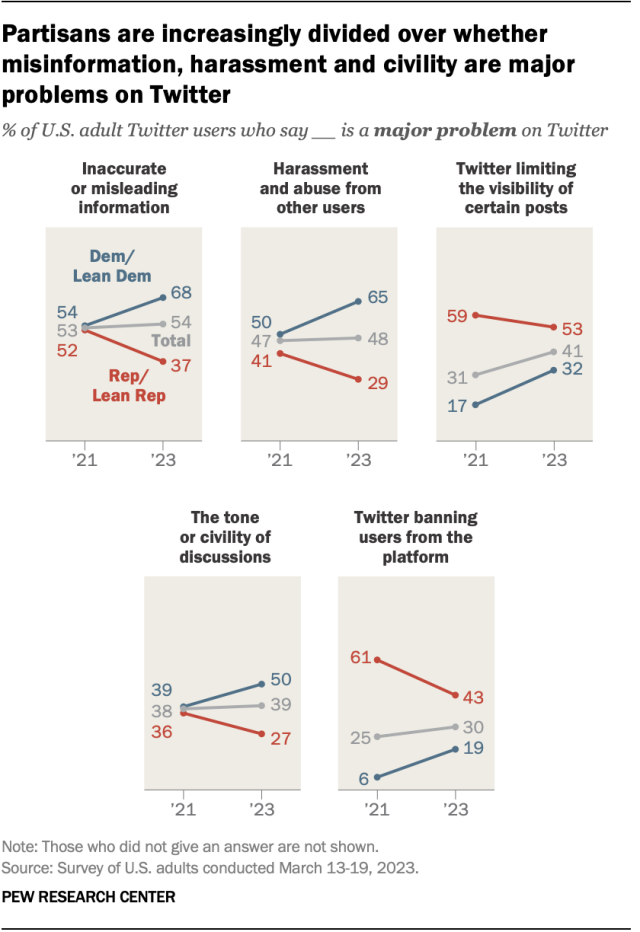
Nearly half of U.S. workers who get paid time off don’t take all the time off their employer offers, according to a February survey of employed Americans . Among those who say their employer offers paid time off for vacation, doctors’ appointments or to deal with minor illnesses, 46% say they take less time off than they are allowed. A similar share (48%) say they typically take all the time off they are offered.
Among those who don’t take all their paid time off, the most common reasons cited are not feeling the need to take more time off (52% say this), worrying they might fall behind at work (49%), and feeling badly about their co-workers taking on additional work (43%).
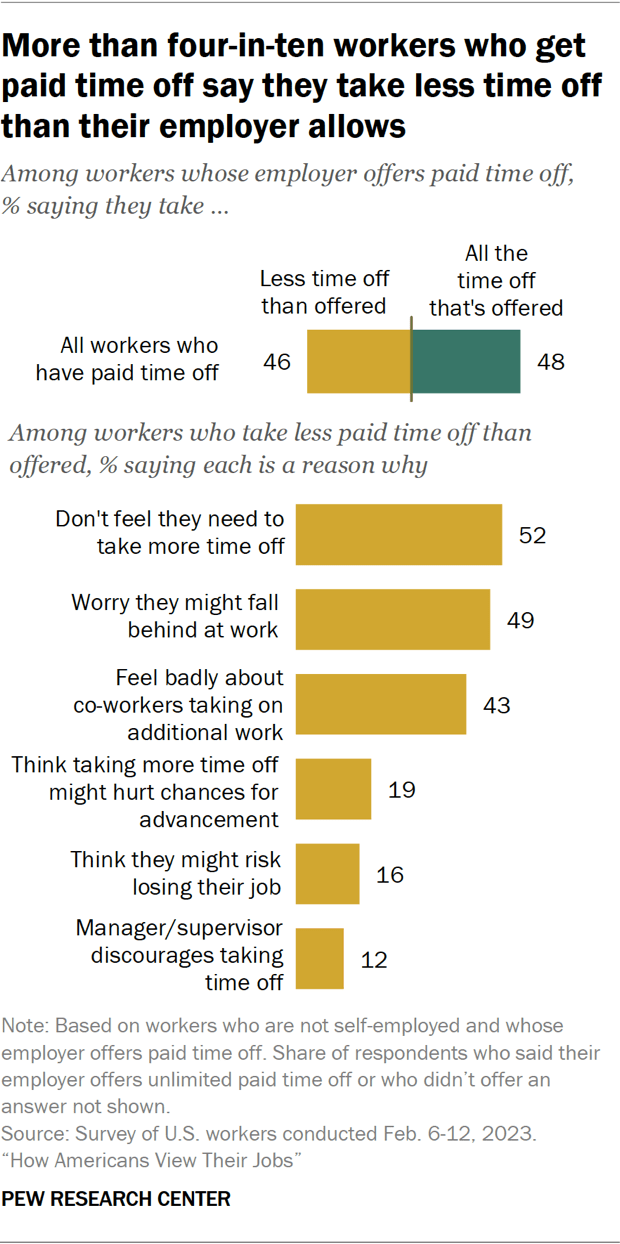
Smaller shares cite other concerns, including the feeling that taking more time off might hurt their chances for job advancement (19%) or that they might risk losing their job (16%). Some 12% say their manager or supervisor discourages them from taking time off.
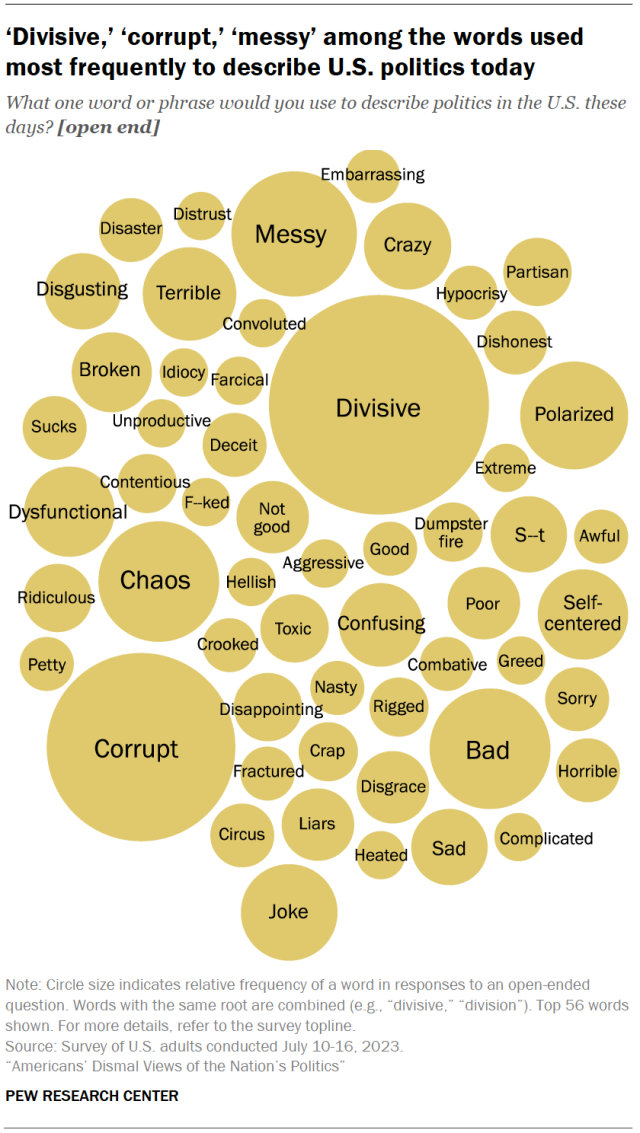
An overwhelming majority of Americans (79%) express a negative sentiment when asked to describe politics in the United States these days, a July survey found . Just 2% offer a positive word or phrase, while 10% say something neutral.
Among those who volunteered an answer, 8% use the word “ divisive” or variations of it, while 2% cite the related term “polarized.” “Corrupt” is the second-most frequent answer, given by 6% of respondents.
The top 15 most cited words also include “messy,” “chaos,” “broken” and “dysfunctional.” Many respondents are even more negative in their views: “terrible,” “disgusting,” “disgrace” and the phrase “dumpster fire” are each offered by at least 1% of respondents.
Around half of Americans (53%) say they have ever been visited by a dead family member in a dream or in another form, according to a spring survey . Overall, 46% of Americans report that they’ve been visited by a dead family member in a dream, while 31% report having been visited by dead relatives in some other form.
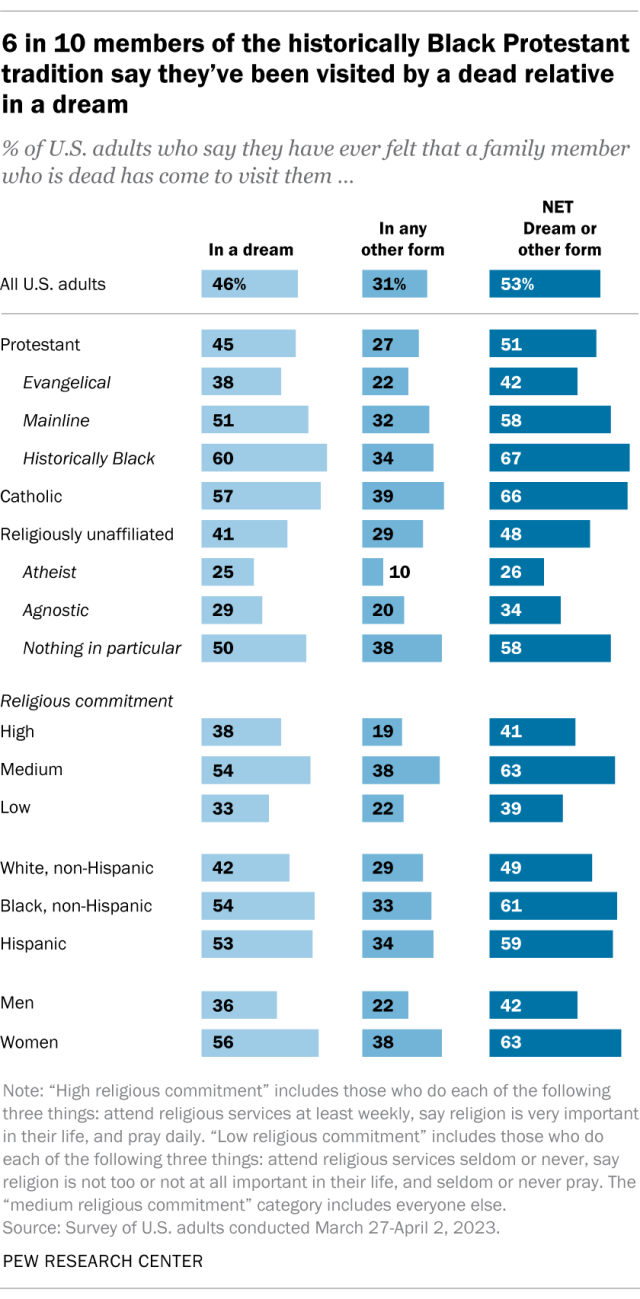
Women are more likely than men to report these experiences.
While the survey asked whether people have had interactions with dead relatives, it did not ask for explanations. So, we don’t know whether people view these experiences as mysterious or supernatural, whether they see them as having natural or scientific causes, or some of both.
For example, the survey did not ask what respondents meant when they said they had been visited in a dream by a dead relative. Some might have meant that relatives were trying to send them messages or information from beyond the grave. Others might have had something more commonplace in mind, such as dreaming about a favorite memory of a family member.
More Americans disapprove than approve of selective colleges and universities taking race and ethnicity into account when making admissions decisions, according to another spring survey , fielded before the Supreme Court ruled on the practice in June. Half of U.S. adults disapprove of colleges considering race and ethnicity to increase diversity at the schools, while a third approve and 16% are not sure.
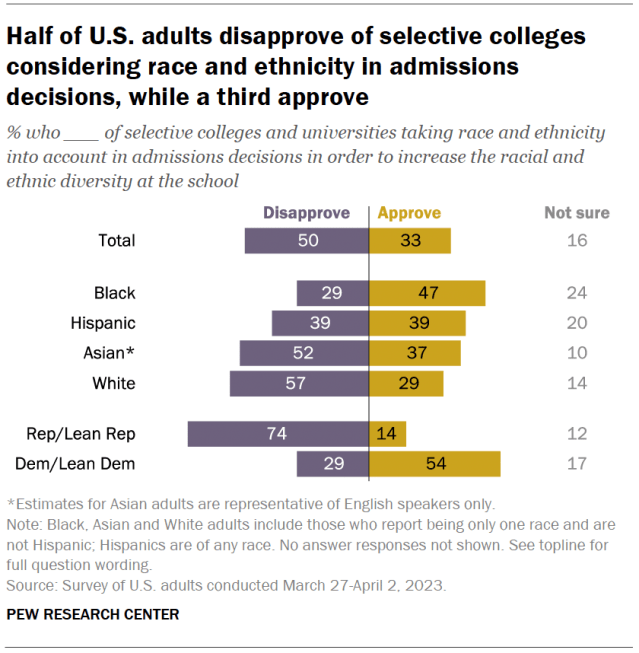
Views differ widely by party, as well as by race and ethnicity. Around three-quarters of Republicans and Republican leaners (74%) disapprove of the practice, while 54% of Democrats and Democratic leaners approve of it.
Nearly half of Black Americans (47%) say they approve of colleges and universities considering race and ethnicity in admissions, while smaller shares of Hispanic (39%), Asian (37%) and White (29%) Americans say the same.
The share of Americans who say science has had a mostly positive effect on society has declined since 2019, before the coronavirus outbreak, a fall survey shows : 57% say science has had a mostly positive effect on society, down from 73% in 2019.
About a third of adults (34%) now say the impact of science on society has been equally positive and negative. And 8% say science has had a mostly negative impact on society.
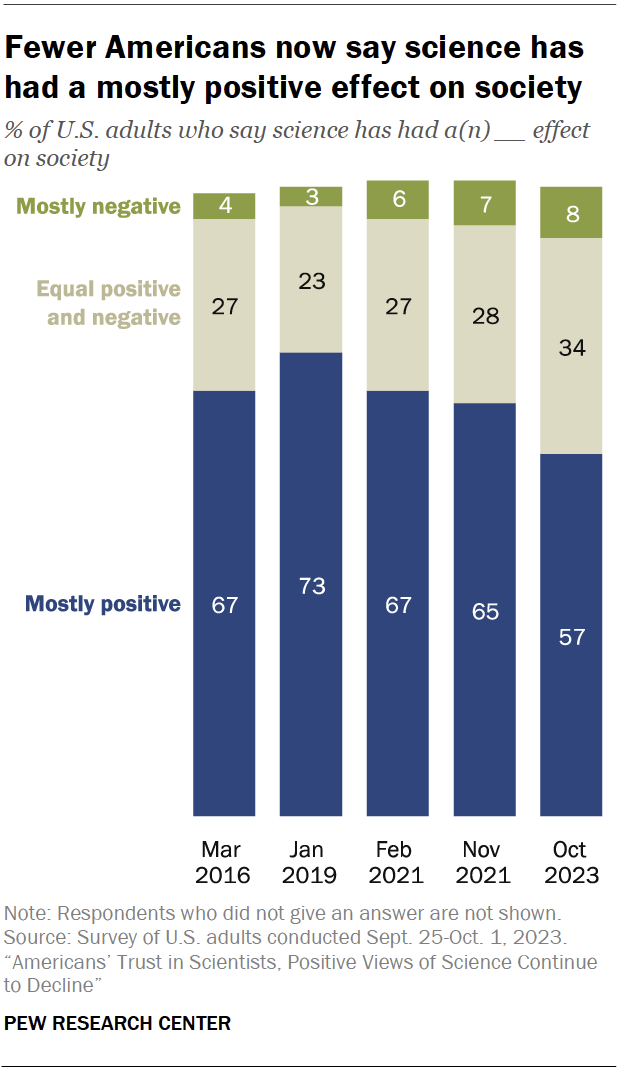
Democrats have become much more likely than Republicans to say science has had a mostly positive impact on society (69% vs. 47%). This gap is the result of steeper declines in positive ratings among Republicans than among Democrats since 2019 (down 23 points and 8 points, respectively).
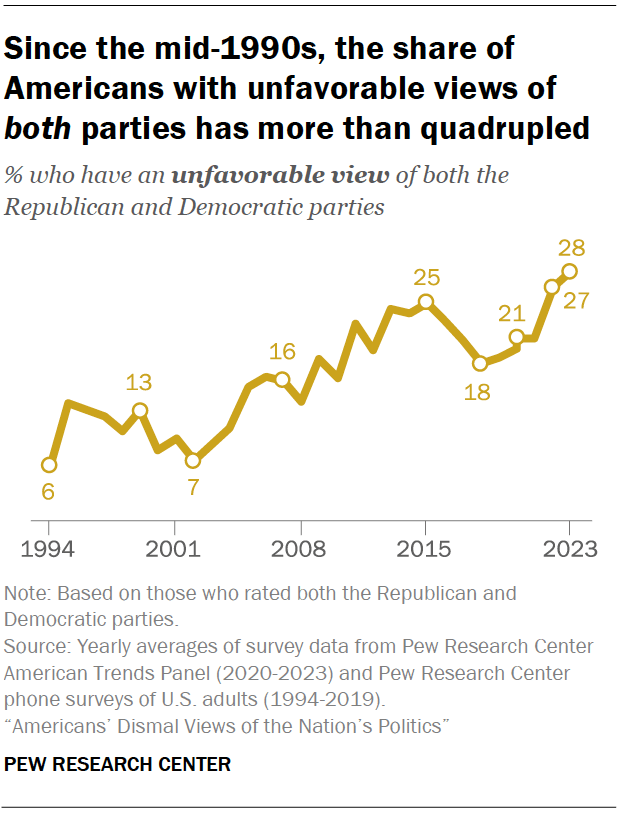
Nearly three-in-ten Americans express an unfavorable opinion of both major political parties – the highest share in at least three decades, according to a July survey . Overall, 28% of Americans have an unfavorable opinion of both the Republican and Democratic parties. This is more than quadruple the share in 1994, when just 6% of Americans viewed both parties negatively.
A majority of Americans say TikTok is a threat to national security, according to a survey conducted in May . About six-in-ten adults (59%) see the social media platform as a major or minor threat to national security in the United States. Just 17% say it is not a threat to national security and another 23% aren’t sure.
Views vary by partisanship and age. Seven-in-ten Republicans and GOP leaners say TikTok is at least a minor threat to national security, compared with 53% of Democrats and Democratic leaners. Conservative Republicans are more likely than moderate or liberal Republicans – or Democrats of any ideology – to say the view the app as a major threat.
Nearly half of those ages 65 and older (46%) see TikTok as a major threat to national security, compared with a much smaller share (13%) of adults ages 18 to 29.
Read the other posts in our striking findings series:
- Striking findings from 2022
- Striking findings from 2021
- 20 striking findings from 2020
- 19 striking findings from 2019
- 18 striking findings from 2018
- 17 striking findings from 2017
- 16 striking findings from 2016
- 15 striking findings from 2015
- 14 striking findings from 2014
- Affirmative Action
- Artificial Intelligence
- Asian Americans
- Business & Workplace
- Death & Dying
- Defense & National Security
- Family & Relationships
- Misinformation Online
- Other Topics
- Politics & Policy
- Social Media
- Supreme Court
- Trust in Science
- Twitter (X)
- Unmarried Adults
- War & International Conflict

Katherine Schaeffer is a research analyst at Pew Research Center .
Private, selective colleges are most likely to use race, ethnicity as a factor in admissions decisions
Americans and affirmative action: how the public sees the consideration of race in college admissions, hiring, asian americans hold mixed views around affirmative action, more americans disapprove than approve of colleges considering race, ethnicity in admissions decisions, hispanic enrollment reaches new high at four-year colleges in the u.s., but affordability remains an obstacle, most popular.
901 E St. NW, Suite 300 Washington, DC 20004 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
© 2024 Pew Research Center
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih research matters.
December 21, 2023
2023 NIH Research Highlights - Promising Medical Findings
Results with potential for enhancing human health.
With NIH support, scientists across the United States and around the world conduct wide-ranging research to discover ways to enhance health, lengthen life and reduce illness and disability. Groundbreaking NIH-funded research often receives top scientific honors. In 2023, these honors included two NIH-supported scientists who received Nobel Prizes . Here’s just a small sample of the NIH-supported research accomplishments in 2023. Also see this year's Human Health Advances and Basic Research Insights .
Printer-friendly version of full 2023 NIH Research Highlights

Immune and hormonal features of Long COVID
About one in eight people who survive an acute SARS-CoV-2 infection go on to have persistent symptoms. The processes that give rise to this syndrome, known as Long COVID, remain unclear. Researchers found several immune and hormonal differences between people with Long COVID and those without. Another study found that infection with a common cold virus may predispose some people to develop Long COVID . This year, researchers also discovered how COVID-19 may damage cells’ energy production and potentially cause some symptoms of Long COVID.
20230911-me-cfs.jpg
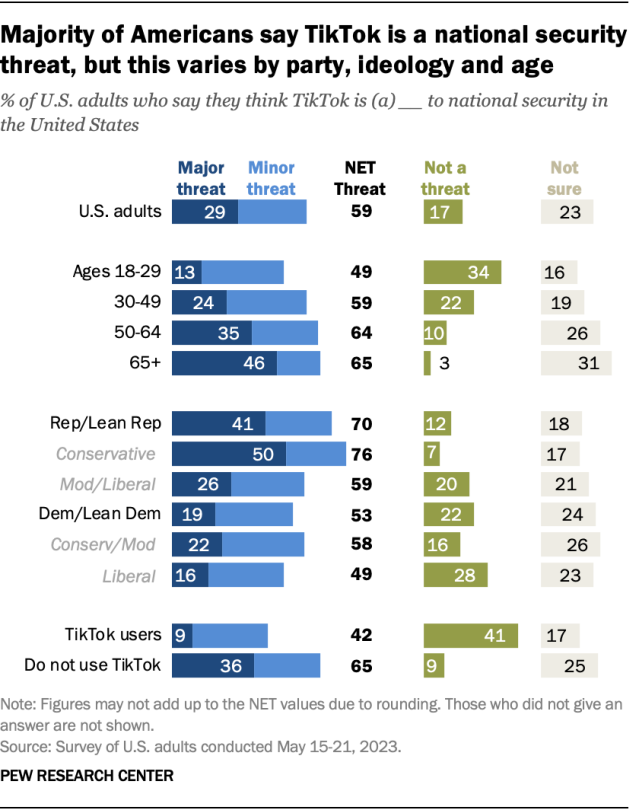
Protein may be linked to exercise intolerance in ME/CFS
People with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) live with debilitating symptoms. These including exhaustion, exercise intolerance, cognitive problems and worsening of symptoms after even mild exertion. A study suggested that high levels of a protein called WASF3 may reduce energy production in the muscle cells of people with ME/CFS. Blocking this protein in cells in the laboratory restored energy production, suggesting a potential new strategy for treating the condition.
20230214-bodyparts.png
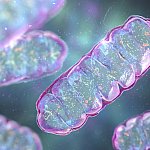
Engineering skin grafts for complex body parts
Advances in bioengineering have allowed researchers to grow new patches of skin in the lab. But these skin patches have been small and limited in shape. Using new techniques, scientists grew strong skin in the shape of a full human hand. This technology has the potential to help heal burns and other damage to complex body parts with less trauma and scarring.
20220110-alz.jpg

Blood test for early Alzheimer’s detection
One of the first stages of Alzheimer’s disease involves the formation of toxic aggregates of a protein called amyloid beta (Aβ). The ability to detect these early would let scientists test new treatments before irreparable brain damage occurs. Researchers developed a blood test that could detect the toxic Aβ aggregates before Alzheimer’s symptoms appeared. This is one of several promising approaches to early diagnosis of Alzheimer’s and other dementias.
20230314-diet.jpg

Erythritol and cardiovascular events
Artificial sweeteners can help people reduce their sugar and calorie intake. But little is known about the long-term health consequences. Researchers found that elevated blood levels of the artificial sweetener erythritol were associated with increased risk of heart attack and stroke. When used as a sweetener, erythritol is typically added at levels more than 1,000-fold higher than those found naturally in foods. The results highlight the need to further study erythritol’s long-term effects on cardiovascular health.
Read more 2023 NIH Research Highlights: Basic Research Insights
Connect with Us
- More Social Media from NIH
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Research Highlights in 2023

A tale of Silk Road violence as told by a lake’s mud
Soot and other remnants of fires record ancient warfare along the storied trade route.

Hybrid workers scurry to the office when the bosses appear
Key-card use by more than 40,000 technology-company employees hints at who’s coming to the office and why.

Where ‘green ghost’ lightning gets its emerald hue
A range of elements in planetary dust provide the colour for high-altitude natural fireworks.
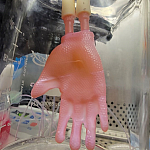
Weird waves in water emulate those in quantum matter
Unusually large wave patterns made by vibrating a container of water resemble those seen in Bose–Einstein condensates.

The Tree of Life, emoji version
What about the worms? Analysis of the 112 official emojis that represent organisms shows that there is a bias against invertebrates.

Dirty air is linked to smaller babies across huge swathes of Asia and Africa
Exposure to high levels of ozone causes mothers in low- and middle-income countries to give birth to infants with a low birth weight.

Wee VR googles give mice a true immersive experience
Headset could make it easier to study reactions in the animals’ brains to simulated situations.

Earth is warming but Mount Everest is getting chillier
Winds triggered by climate change sweep cold air down from the summit of Mount Everest and other Himalayan peaks, leading to a cooling trend.

Powerful X-ray reveals the inner life of an electric-vehicle battery
Researchers get an unprecedented glimpse of how ions behave during a drive.
Mini fat particles help to turn platelets into protein factories
Genetically modified blood cells could churn out therapeutic proteins for treating inflammation and other conditions.

Holiday side dish: a big helping of indoor air pollution
Particulate-matter levels in US homes peak during mealtime — and especially on Thanksgiving and Christmas.

Why coffee particles clump and make a mess during grinding
Scientists studying the electrical charge on coffee particles stumble on a secret to a better cup of joe.

This bird escaped extinction — but its genes hint at an ominous future
The extravagantly feathered Seychelles paradise flycatcher lacks genetic diversity, which might hamper its resilience to climate change and other threats.

How immense mountains create one of the rainiest places on Earth
The western coast of Colombia can get more than 26 metres of rain a year, thanks to the influence of air jets hitting the Andes range.

Earliest known fossil mosquito is a blood-sucking surprise
Insects trapped in amber reveal that male mosquitoes, too, could once extract blood.
A low-cost electron microscope maps proteins at speed
Bespoke cryo-electron microscope reveals 3D details of cellular structures — and is an order of magnitude cheaper than its rivals.
Tinkering with immune cells gives cancer treatment a boost
Tumours respond more readily to radiation and other therapies in mice without a specific protein in their dendritic cells.

The hunt for dark-matter particles ventures into the wild
Sensors deployed at magnetically quiet rural sites looked for axions and ‘hidden photons’ — with no luck yet.

Dolphins have a feel for electric fields
The bottlenose dolphin’s keen ‘electroreception’ sense might help it to locate buried prey and navigate the seas.

‘Early dark energy’ fails to solve mystery of cosmic expansion
The extra ingredient would explain why the Universe is expanding so fast now — but conflicts with data from ancient quasars.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Here’s how you know
- U.S. Department of Health and Human Services
- National Institutes of Health
Selected Research Results
Research spotlights of selected studies are shown below. For a full list of published NCCIH Research to-date, see PubMed .
Spotlights for 2023

Adding Mindfulness-Oriented Recovery Enhancement (MORE) to Methadone Treatment Provides Therapeutic Benefits for People With Opioid Use Disorder and Chronic Pain According to a recent study, adding a remote group therapy mindfulness program to standard methadone treatment leads to therapeutic benefits in people with opioid use disorder and chronic pain. The study, conducted by researchers at the Robert Wood Johnson Medical School, Rutgers School of Public Health, and University of Utah, was funded by the National Center for Complementary and Integrative Health (NCCIH) and published in the journal JAMA Psychiatry .
December 2023

New Machine Learning Strategy for Optimizing Interventions in Causal Model Design Researchers developed a new active learning—or machine learning—strategy that outperformed existing approaches for identifying optimal interventions when designing causal models. The new approach, which was developed by researchers from Massachusetts Institute of Technology and Harvard University, was recently described in a paper in Nature Machine Intelligence . The research was partially funded by the National Center for Complementary and Integrative Health.
October 2023
The Mechanoreceptive Ion Channel PIEZO2 Plays a Critical Role in Sexual Function Uncovering the biomechanical processes underlying human touch and sensation is critical to understanding this essential human function and key to discovering potential new approaches to treating pain, a key National Center for Complementary and Integrative Health (NCCIH) priority. NCCIH’s research is contributing to a growing understanding of the mechanoreceptive ion channel PIEZO2 and its essential role in discriminative touch in both mice and humans, in different parts of the body.
August 2023
PIEZO2 Ion Channel Plays a Key Role in Gastrointestinal Motility and Bowel Sensation New research has identified mechanisms involved in sensing the presence of food in the gastrointestinal (GI) tract and controlling the transit of GI contents. The findings demonstrate a key role for the protein PIEZO2 in controlling GI motility, a process critical for proper digestion, nutrient absorption, and waste removal. This research, conducted jointly by the National Center for Complementary and Integrative Health, the National Institute of Neurological Disorders and Stroke, the Scripps Research Institute, and other collaborating institutions, was published in a recent issue of the journal Cell.

U.S. National Survey Data Show High Rates of New Cases and Persistence of Chronic Pain New cases of chronic pain occur more often among U.S. adults than new cases of several other common conditions, including diabetes, depression, and high blood pressure. Among people who have chronic pain, almost two-thirds will still have it the following year. These findings come from a new analysis of National Health Interview Survey (NHIS) data by investigators from the National Center for Complementary and Integrative Health, Seattle Children’s Research Institute, and University of Washington, published in JAMA Network Open .

The Prevalence of Pain Among Sexual Minority Adults Is Higher Than Among Straight Adults, National Survey Data Show Pain prevalence is significantly higher among sexual minority adults than straight adults, with the highest levels among those who identify as bisexual or “something else,” followed by those who identify as gay or lesbian, according to a new analysis of 2013–2018 data from the National Health Interview Survey (NHIS). This analysis, published in the journal Pain, was conducted by researchers from the University of Western Ontario; University at Buffalo, State University of New York; Michigan State University; Ohio State University; and National Center for Complementary and Integrative Health.

When Taken Orally, Δ9-Tetrahydrocannabinol With Cannabidiol Can Result in Stronger Drug Effects Than Δ9-Tetrahydrocannabinol Alone New research contradicts prior claims and clarifies the interactions between the two main cannabinoids—Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD)—when they are ingested orally as part of an edible cannabis product. The study’s findings suggest that CBD can inhibit the metabolism of Δ9-THC when the two are consumed together, leading to increased effects than when the same dose of Δ9-THC is consumed without CBD. The study, recently published in the journal JAMA Network Open , was led by researchers at Johns Hopkins University School of Medicine and partially funded by the National Center for Complementary and Integrative Health.
February 2023
Research Results by Year
More Published Research
Cochrane Collaboration Complementary Medicine Reviews—plain-language summaries and abstracts of research on complementary health approaches.
NCCIH-funded studies in PubMed—a pre-designated search of all published, NCCIH-funded research to date.

Suggested Searches
- Climate Change
- Expedition 64
- Mars perseverance
- SpaceX Crew-2
- International Space Station
- View All Topics A-Z
Humans in Space
Earth & climate, the solar system, the universe, aeronautics, learning resources, news & events.
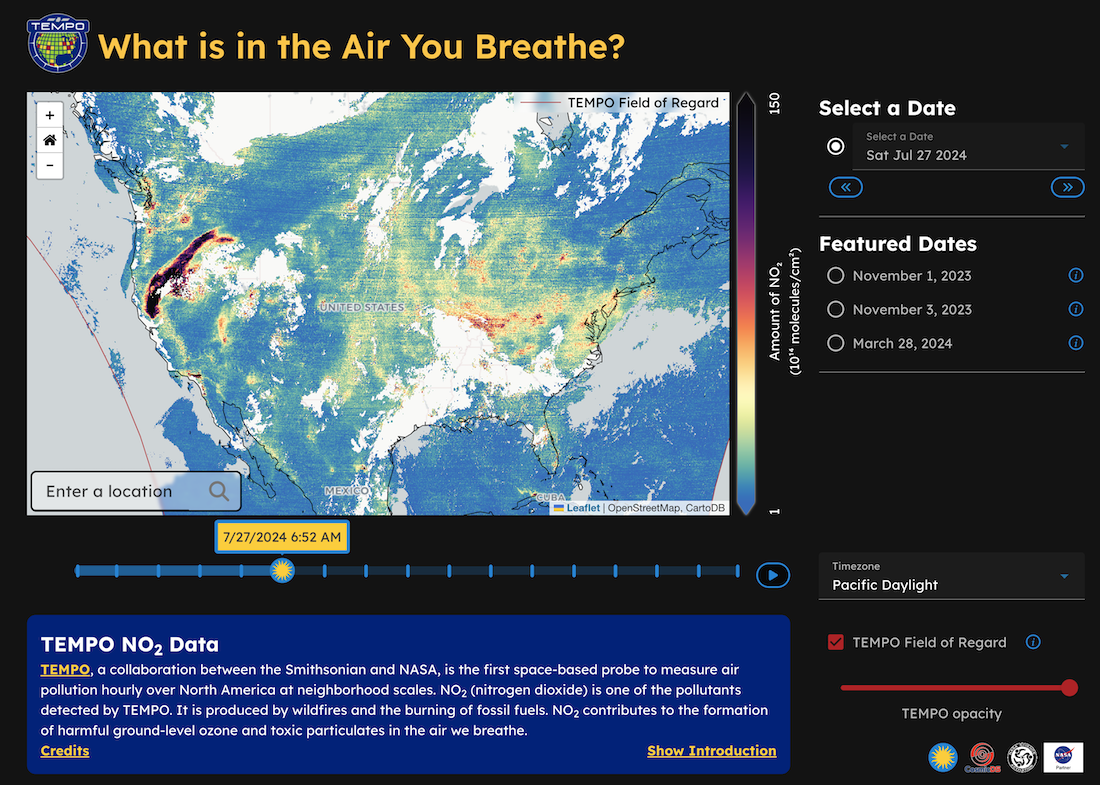
New TEMPO Cosmic Data Story Makes Air Quality Data Publicly Available

NASA’s X-59 Progresses Through Tests on the Path to Flight

NASA Demonstrates ‘Ultra-Cool’ Quantum Sensor for First Time in Space
- Search All NASA Missions
- A to Z List of Missions
- Upcoming Launches and Landings
- Spaceships and Rockets
- Communicating with Missions
- James Webb Space Telescope
- Hubble Space Telescope
- Why Go to Space
- Commercial Space
- Destinations
- Living in Space
- Explore Earth Science
- Earth, Our Planet
- Earth Science in Action
- Earth Multimedia
- Earth Science Researchers
- Pluto & Dwarf Planets
- Asteroids, Comets & Meteors
- The Kuiper Belt
- The Oort Cloud
- Skywatching
- The Search for Life in the Universe
- Black Holes
- The Big Bang
- Dark Energy & Dark Matter
- Earth Science
- Planetary Science
- Astrophysics & Space Science
- The Sun & Heliophysics
- Biological & Physical Sciences
- Lunar Science
- Citizen Science
- Astromaterials
- Aeronautics Research
- Human Space Travel Research
- Science in the Air
- NASA Aircraft
- Flight Innovation
- Supersonic Flight
- Air Traffic Solutions
- Green Aviation Tech
- Drones & You
- Technology Transfer & Spinoffs
- Space Travel Technology
- Technology Living in Space
- Manufacturing and Materials
- Science Instruments
- For Kids and Students
- For Educators
- For Colleges and Universities
- For Professionals
- Science for Everyone
- Requests for Exhibits, Artifacts, or Speakers
- STEM Engagement at NASA
- NASA's Impacts
- Centers and Facilities
- Directorates
- Organizations
- People of NASA
- Internships
- Our History
- Doing Business with NASA
- Get Involved
NASA en Español
- Aeronáutica
- Ciencias Terrestres
- Sistema Solar
- All NASA News
- Video Series on NASA+
- Newsletters
- Social Media
- Media Resources
- Upcoming Launches & Landings
- Virtual Events
- Sounds and Ringtones
- Interactives
- STEM Multimedia

Primary Instrument for Roman Space Telescope Arrives at NASA Goddard

Artemis Emergency Egress System Emphasizes Crew Safety

What’s New With the Artemis II Crew

Food in Space
Airborne Surface, Cryosphere, Ecosystem, and Nearshore Topography

Amendment 42: A.30 Understanding Changes in High Mountain Asia Deferred to ROSES-25

Citizen Science Earth Projects
The Summer Triangle’s Hidden Treasures

Solar Eclipse Data Story Helps the Public Visualize the April 2024 Total Eclipse

NASA’s Perseverance Rover to Begin Long Climb Up Martian Crater Rim

NASA Selects 5 New Roman Technology Fellows in Astrophysics
NASA Citizen Scientists Spot Object Moving 1 Million Miles Per Hour

Perseverance Pays Off for Student Challenge Winners

NASA Aircraft Gathers 150 Hours of Data to Better Understand Earth

Collegiate Teams to Focus on Aviation Solutions for Agriculture in 2025 Gateways to Blue Skies Competition

Amendment 41: DRAFT F.13 Lunar Terrain Vehicle Instruments Program Released for Community Comment.

NASA Tests Deployment of Roman Space Telescope’s ‘Visor’

How Do I Navigate NASA Learning Resources and Opportunities?

NASA Challenge Seeks ‘Cooler’ Solutions for Deep Space Exploration
NASA Explores Industry, Partner Interest in Using VIPER Moon Rover

How NASA Citizen Science Fuels Future Exoplanet Research

There Are No Imaginary Boundaries for Dr. Ariadna Farrés-Basiana

Astronauta de la NASA Frank Rubio

Diez maneras en que los estudiantes pueden prepararse para ser astronautas
Groundbreaking results from space station science in 2023, melissa l. gaskill, a new spin on pulsars, learning from lightning, regenerating tissue in space, mighty muscles in microgravity, better ultrasound images, this is your brain in space, improving solar materials, understanding bubbles in foams, answering burning questions, the robot hop.
Lee esta historia en español aquí .
The International Space Station is a microgravity research lab hosting groundbreaking technology demonstrations and scientific investigations. More than 3,700 investigations conducted to date have generated more than 4,000 research articles published in scientific journals. In 2023, the orbiting lab hosted approximately 500 investigations.
See more space station research achievements and findings in the Annual Highlights of Results publication , and read highlights of results published between October 2022 and October 2023 below:
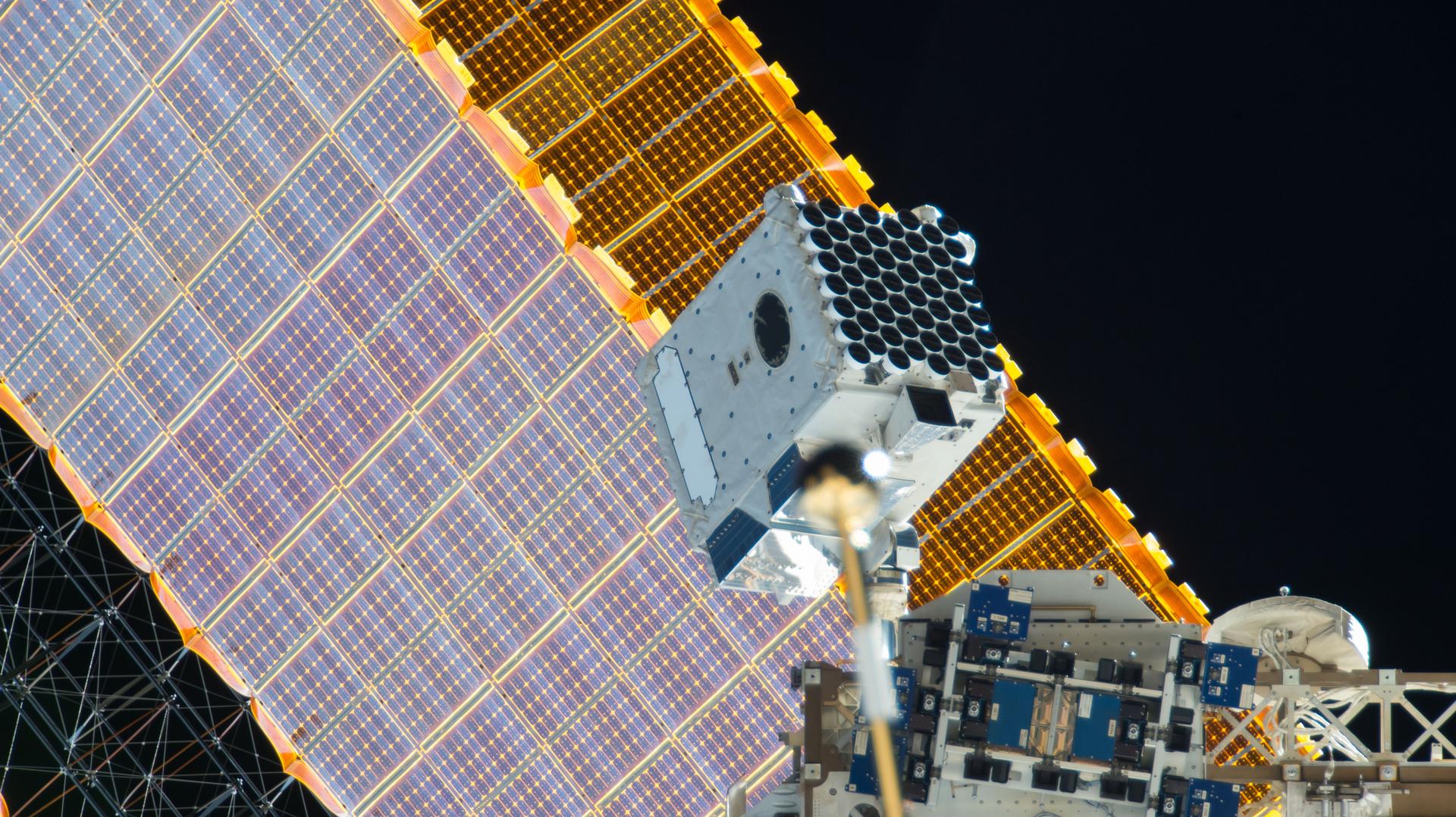
Neutron stars, ultra-dense matter left behind when massive stars explode as supernovas, are also called pulsars because they spin and emit X-ray radiation in beams that sweep the sky like lighthouses. The Neutron star Interior Composition Explorer ( NICER ) collects this radiation to study the structure, dynamics, and energetics of pulsars. Researchers used NICER data to calculate rotations of six pulsars and update mathematical models of their spin properties. Precise measurements enhance the understanding of pulsars, including their production of gravitational waves, and help address fundamental questions about matter and gravity.

Atmosphere-Space Interactions Monitor ( ASIM ) studies how upper-atmospheric electrical discharges generated by severe thunderstorms affect Earth’s atmosphere and climate. These events occur well above the altitudes of normal lightning and storm clouds. Using ASIM data, researchers reported the first detailed observations of development of a of negative leader, or initiation of a flash, from in-cloud lightning. Understanding how thunderstorms disturb the high-altitude atmosphere could improve atmospheric models and climate and weather predictions.
Tissue Regeneration-Bone Defect ( Rodent Research-4 (CASIS)), sponsored by the ISS National Lab, examined wound healing mechanisms in microgravity. Researchers found that microgravity affected the fibrous and cellular components of skin tissue. Fibrous structures in connective tissue provide structure and protection for the body’s organs. This finding is an initial step to use connective tissue regeneration to treat disease and injuries for future space explorers.
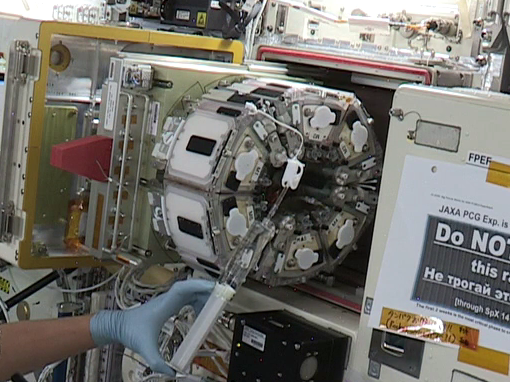
JAXA (Japan Aerospace Exploration Agency) developed the Multiple Artificial-gravity Research System (MARS), which generates artificial gravity in space. Three JAXA investigations, MHU-1 , MHU-4 , and MHU-5 , used the artificial-gravity system to examine the effect on skeletal muscles from different gravitation loads – microgravity, lunar gravity (1/6 g ), and Earth gravity (1 g ). Results show that lunar gravity protects against loss of some muscle fibers but not others. Different gravitational levels may be needed to support muscle adaptation on future missions.

Vascular Echo , an investigation from CSA (Canadian Space Agency), examined changes in blood vessels and the heart during and after spaceflight using ultrasound and other measures. Researchers compared 2D ultrasound technology with a motorized 3D ultrasound and found that 3D is more accurate. Better measurements could help maintain crew health in space and quality of life for people on Earth.

The Brain-DTI investigation from ESA (European Space Agency) tested whether the brain adapts to weightlessness by using previously untapped connections between neurons. MRI scans of crew members before and after spaceflight demonstrate functional changes in specific brain regions, confirming the adaptability and plasticity of the brain under extreme conditions. This insight supports the development of ways to monitor brain adaptations and countermeasures to promote healthy brain function in space and for those with brain-related disorders on Earth.
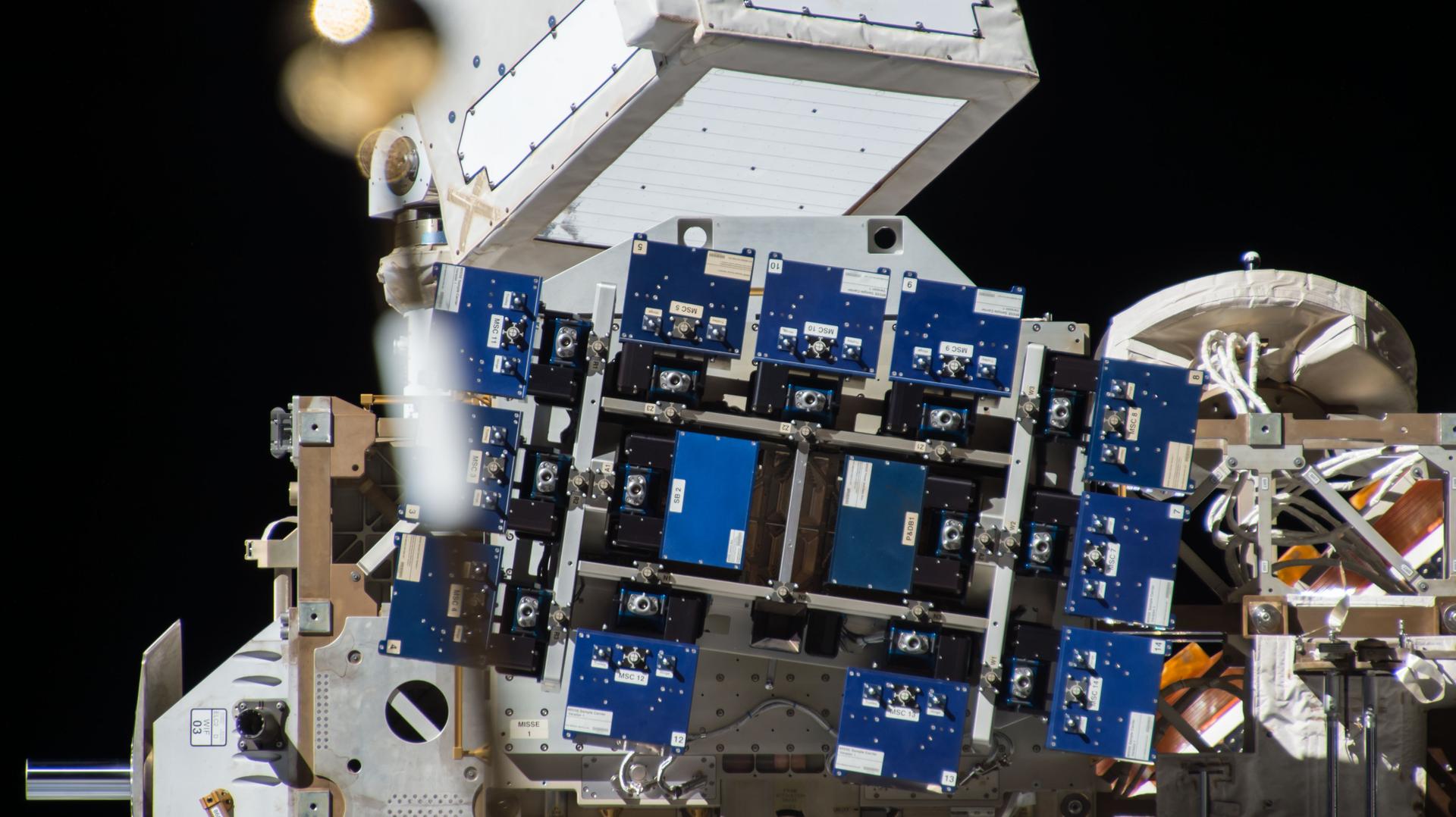
Metal halide perovskite (MHP) materials convert sunlight into electrical energy and show promise for use in thin-film solar cells in space due to low cost, high performance, suitability for in-space manufacturing, and defect and radiation tolerance. For Materials International Space Station Experiment-13-NASA ( MISSE-13-NASA ), which continues a series investigating how space affects various materials, researchers exposed perovskite thin films to space for ten months. Results confirmed their durability and stability in this environment. This finding could lead to improvements in MHP materials and devices for space applications such as solar panels.

Wet foams are dispersions of gas bubbles in a liquid matrix. An ESA investigation, FSL Soft Matter Dynamics or FOAM , examines coarsening, a thermodynamic process where large bubbles grow at the expense of smaller ones. Researchers determined the coarsening rates for various types of foams and found close agreement with theoretical predictions. A better understanding of foam properties could help scientists improve these substances for a variety of uses, including firefighting and water treatment in space and making detergents, food, and medicine on Earth.
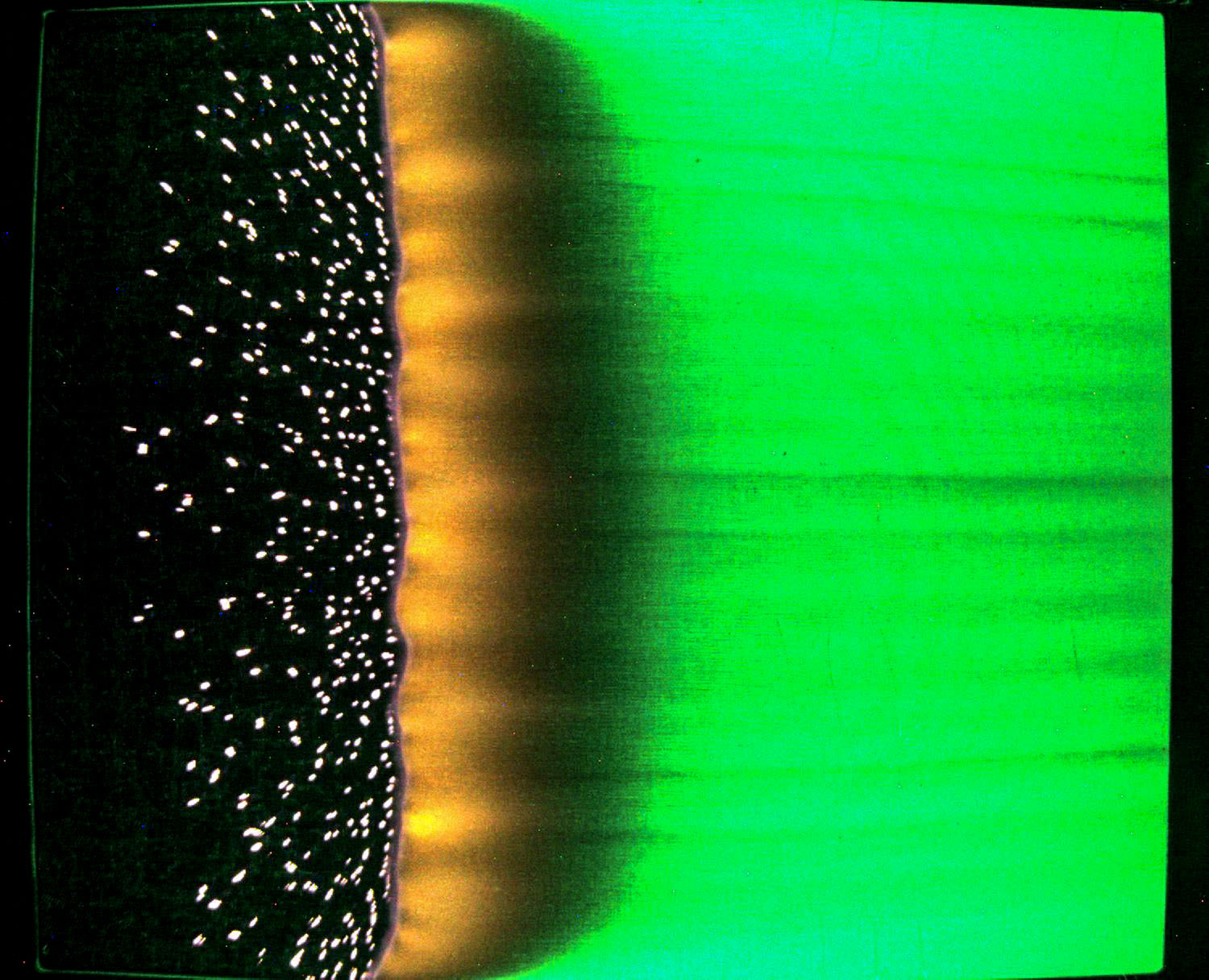
Fire is a constant concern in space. The Saffire series of experiments studies flame conditions in microgravity using empty Cygnus resupply spacecraft that have undocked from the space station. Saffire-IV examined fire growth with different materials and conditions and showed that a technique called color pyrometry can determine the temperature of a spreading flame. The finding helps validate numerical models of flame properties in microgravity and provides insight into fire safety on future missions.
Astrobatics tests robotic movement using hopping or self-toss maneuvers by the station’s Astrobee robots. In low gravity, robots could move faster, use less fuel, and cover otherwise impassable terrain with these maneuvers, expanding their orbital and planetary capabilities. Results verified the viability of the locomotion method and showed that it provides a greater range of distance. The work is a step toward autonomous robotic helpers in space and on other celestial bodies, potentially reducing the need to expose astronauts to risky environments.
Melissa Gaskill International Space Station Research Communications Team Johnson Space Center
Search this database of scientific experiments to learn more about those mentioned above.
Discover More Topics
Space Station Research Results

Latest News from Space Station Research

Opportunities and Information for Researchers

ISS National Laboratory


- Adolescent and Young Adult Cancer
- Bile Duct Cancer
- Bladder Cancer
- Brain Cancer
- Breast Cancer
- Cervical Cancer
- Childhood Cancer
- Colorectal Cancer
- Endometrial Cancer
- Esophageal Cancer
- Head and Neck Cancer
- Kidney Cancer
- Liver Cancer
- Lung Cancer
- Mouth Cancer
- Mesothelioma
- Multiple Myeloma
- Neuroendocrine Tumors
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Skin Cancer/Melanoma
- Stomach Cancer
- Testicular Cancer
- Throat Cancer
- Thyroid Cancer
- Prevention and Screening
- Diagnosis and Treatment
- Research and Clinical Trials
- Survivorship

Request an appointment at Mayo Clinic

Cancer research highlights from 2023
Share this:.
By Mayo Clinic staff
Researchers at Mayo Clinic Comprehensive Cancer Center spent 2023 studying the biology of cancer and new ways to predict, prevent, diagnose and treat the disease. Their discoveries are creating hope and transforming the quality of life for people with cancer today and in the future. Here are some highlights from their research over the past year:
Mayo Clinic researchers link ovarian cancer to bacteria colonization in the microbiome.
A specific colonization of microbes in the reproductive tract is commonly found in people with ovarian cancer, according to a study from the Mayo Clinic Center for Individualized Medicine . Published in Scientific Reports and led by Marina Walther-Antonio, Ph.D. , a Mayo Clinic researcher, and Abigail Asangba, Ph.D., the discovery strengthens the evidence that the bacterial component of the microbiome — a community of microorganisms that also consists of viruses, yeasts and fungi — is an important indicator for early detection, diagnosis and prognosis of ovarian cancer . The study also suggests that a higher accumulation of pathogenic microbes plays a role in treatment outcomes and could be a potential indicator for predicting a patient's prognosis and response to therapy. Read more .
Artificial intelligence is forging a new future for colorectal cancer and other digestive system diseases.
Colonoscopy remains the gold standard in detecting and preventing colorectal cancer , but the procedure has limitations. Some studies suggest that more than half of post-colonoscopy colon cancer cases arise from lesions missed at patients' previous colonoscopies. In 2022, Michael Wallace, M.D. , a Mayo Clinic gastroenterologist, published the results of an international, multicenter study testing the impact of adding artificial intelligence (AI) to routine colonoscopies. His team, including James East, M.D. , a Mayo Clinic gastroenterologist, and other researchers from the U.S., the U.K., Italy, Germany and Ireland, found that incorporating AI into colonoscopies reduced the risk of missing polyps by 50%. Read more .
A big step forward: Bringing DNA sequencing data to routine patient care.
The Tapestry study , an extensive genomic sequencing clinical research study, aims to complete exome sequencing (sequencing the protein-coding regions of a genome) for 100,000 Mayo Clinic patients. The results will be integrated into patients’ electronic health records for three hereditary conditions, and the amassed data will contribute to a research dataset stored within the Mayo Clinic Cloud on the Omics Data Platform. The overall hope of Tapestry is to accelerate discoveries in individualized medicine to tailor prevention, diagnosis and treatment to a patient's unique genetic makeup. It is poised to advance evidence that exome sequencing, when applied to a diverse and comprehensive general population, can proficiently identify carriers of genetic variants that put them at higher risk for a disease, allowing them to take preventive measures. Read more .
Patients with multiple tumors in one breast may not need a mastectomy.
Patients who have multiple tumors in one breast may be able to avoid a mastectomy if surgeons can remove the tumors while leaving enough breast tissue, according to research led by the Alliance in Clinical Trials in Oncology and Mayo Clinic Comprehensive Cancer Center . Patients would receive breast-conserving therapy — a lumpectomy followed by whole-breast radiation therapy — rather than mastectomy . The study is published in the Journal of Clinical Oncology . Historically, women with multiple tumors in one breast have been advised to have a mastectomy. Now, patients can be offered a less invasive option with faster recovery, resulting in better patient satisfaction and cosmetic outcomes, says Judy Boughey, M.D. , lead author, Mayo Clinic breast surgical oncologist and the W.H. Odell Professor of Individualized Medicine. Read more .
Staging pancreatic cancer early with minimally invasive surgery shows positive results in patient prognosis.
A study published in the Journal of the American College of Surgeons reveals that performing a minor surgical procedure on patients newly diagnosed with pancreatic cancer helps to identify cancer spread early and determine the stage of cancer. The researchers add that the surgery ideally should be performed before the patient begins chemotherapy. "This is an important study because it supports that staging laparoscopy may help determine a patient's prognosis and better inform treatment so that patients avoid unhelpful or potentially harmful surgical therapy," says Mark Truty, M.D. , a Mayo Clinic surgical oncologist who led the research. Read more .
Mayo Clinic study reveals proton beam therapy may shorten breast cancer treatment.
In a trial published in The Lancet Oncology , Mayo Clinic Comprehensive Cancer Center researchers uncovered evidence supporting a shorter treatment time for people with breast cancer . The study compared two separate dosing schedules of pencil-beam scanning proton therapy , known for its precision in targeting cancer cells while preserving healthy tissue to reduce the risk of side effects. The investigators found that both 25-day and 15-day proton therapy schedules resulted in excellent cancer control while sparing surrounding non-cancerous tissue. Further, complication rates were comparable between the two study groups. "We can now consider the option of 15 days of therapy for patients based on the similar treatment outcomes observed," says Robert Mutter, M.D. , a Mayo Clinic radiation oncologist and physician-scientist. Read more .
Harnessing the immune system to fight ovarian cancer.
Mayo Clinic research is biomanufacturing an experimental, cell-based ovarian cancer vaccine and combining it with immunotherapy to study a "one-two punch" approach to halting ovarian cancer progression. This research begins with a blood draw from people with advanced ovarian cancer whose tumors have returned after standard surgery and chemotherapy. White blood cells are extracted from the blood, biomanufactured to become dendritic cells and returned to the patient. Dendritic cells act as crusaders that march through the body, triggering the immune system to recognize and fight cancer. "We're building on an earlier phase 1 clinical trial that showed promising results in terms of survival after the dendritic cell-based vaccine," says Matthew Block, M.D., Ph.D. , co-principal investigator and Mayo Clinic medical oncologist. "Of the 18 evaluable patients in the phase 1 study, 11 had cancer return, but seven of them — 40% — have been cancer-free for almost 10 years. We typically expect 90% of patients in this condition to have the cancer return." Read more .
New gene markers detect Lynch syndrome-associated colorectal cancer.
Researchers from Mayo Clinic Comprehensive Cancer Center and Mayo Clinic Center for Individualized Medicine have discovered new genetic markers to identify Lynch syndrome-associated colorectal cancer with high accuracy. Studies are underway to determine if these genetic markers are in stool samples and, if so, how this could lead to a non-invasive screening option for people with Lynch syndrome . The research was published in Cancer Prevention Research , a journal of the American Association for Cancer Research. "This is an exciting finding that brings us closer to the reality that clinicians may soon be able to offer a non-invasive cancer screening option to patients with the highest risk of getting cancer," says Jewel Samadder, M.D. , co-lead author of the paper and a Mayo Clinic gastroenterologist. Read more .
Mayo Clinic prepares to biomanufacture a new CAR-T cell therapy for B-cell blood cancers.
Mayo Clinic research has developed a new type of chimeric antigen receptor-T cell therapy (CAR-T cell therapy) aimed at killing B-cell blood cancers that have returned and are no longer responding to treatment. This pioneering technology, designed and developed in the lab of Hong Qin, M.D., Ph.D. , a Mayo Clinic cancer researcher, killed B-cell tumors grown in the laboratory and tumors implanted in mouse models. The preclinical findings are published in Cancer Immunology, Immunotherapy . "This study shows our experimental CAR-T cell therapy targets several blood cancers, specifically chronic lymphocytic leukemia," says Dr. Qin. "Currently, there are six different CAR-T cell therapies approved for treatment of relapsed blood cancers. While the results are impressive, not everyone responds to this treatment. Our goal is to provide novel cell therapies shaped to each patient's individual need." Read more .
Related Posts

Research shows screening protocols fail to catch a notable number of people carrying genetic mutations associated with increased cancer risk.

Marty Kedian successfully underwent a groundbreaking surgery at Mayo Clinic that restored his voice and his ability to swallow and breathe on his own.

Study results show a new surgical platform enables real-time diagnoses and tailored surgical treatment in the operating room.
Investor Relations
News Details
Johnson & johnson reports q4 and full-year 2023 results.
- 2023 Fourth-Quarter reported sales growth of 7.3% to $21.4 Billion with operational growth of 7.2%* and adjusted operational growth of 5.7%*. Operational growth excluding COVID-19 Vaccine of 10.9%*
- 2023 Fourth-Quarter Earnings per share (EPS) of $1.70 increasing 39.3% and adjusted EPS of $2.29 increasing by 11.7%*
- 2023 Full-Year reported sales growth of 6.5% to $85.2 Billion with operational growth of 7.4%* and adjusted operational growth of 5.9%*. Operational growth excluding COVID-19 Vaccine of 9.0%*
- 2023 Full-Year EPS of $5.20 decreasing 15.3% due to a special one-time charge in the First-Quarter, and adjusted EPS of $9.92 increasing by 11.1%*
- Company re-confirms guidance for 2024 with operational sales 5 growth of 5.0% - 6.0%* and adjusted operational EPS of $10.55 - $10.75, reflecting growth of 7.4%* at the mid-point
NEW BRUNSWICK, N.J.--(BUSINESS WIRE)-- Johnson & Johnson (NYSE: JNJ) today announced results for fourth-quarter and full year 2023. “Johnson & Johnson’s full year 2023 results reflect the breadth and competitiveness of our business and our relentless focus on delivering for patients,” said Joaquin Duato, Chairman and Chief Executive Officer. “We have entered 2024 from a position of strength, and I am confident in our ability to lead the next wave of health innovation.”
Unless otherwise noted, the financial results and earnings guidance included below reflect the continuing operations of Johnson & Johnson.
Overall Financial Results
|
|
|
| |||||
|
|
|
|
|
|
|
|
|
| Reported Sales | $21,395 | $19,939 | 7.3% |
| $85,159 | $79,990 | 6.5% |
| Net Earnings | $4,132 | $3,227 | 28.0% |
| $13,326 | $16,370 | (18.6)% |
| EPS (diluted) | $1.70 | $1.22 | 39.3% |
| $5.20 | $6.14 | (15.3)% |
|
|
|
|
|
|
| ||
|
|
|
| |||||
|
|
|
|
|
|
|
|
|
| Operational Sales |
|
| 7.2% |
|
|
| 7.4% |
| Adjusted Operational Sales |
|
| 5.7% |
|
|
| 5.9% |
| Adjusted Net Earnings | $5,562 | $5,432 | 2.4% |
| $25,409 | $23,796 | 6.8% |
| Adjusted EPS (diluted) | $2.29 | $2.05 | 11.7% |
| $9.92 | $8.93 | 11.1% |
| Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures included in accompanying schedules | ||
| Excludes the impact of translational currency | ||
| Excludes the net impact of acquisitions and divestitures and translational currency | ||
| Excludes intangible amortization expense and special items | ||
| Excludes COVID-19 Vaccine | ||
| Note: Values may have been rounded | ||
Regional Sales Results
|
|
| |||||
|
|
|
|
|
|
|
|
| U.S. | $12,009 | $10,820 | 11.0% | 11.0 | - | 8.8 |
| International | 9,386 | 9,119 | 2.9 | 2.7 | 0.2 | 2.1 |
| Worldwide | $21,395 | $19,939 | 7.3% | 7.2 | 0.1 | 5.7 |
|
|
|
| ||||
|
|
|
|
|
|
|
|
| U.S. | $46,444 | $41,981 | 10.6% | 10.6 | - | 8.2 |
| International | 38,715 | 38,009 | 1.9 | 3.8 | (1.9) | 3.4 |
| Worldwide | $85,159 | $79,990 | 6.5% | 7.4 | (0.9) | 5.9 |
| Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures included in accompanying schedules | ||
| Excludes the impact of translational currency | ||
| Excludes the net impact of acquisitions and divestitures and translational currency | ||
| Note: Values may have been rounded | ||
Segment Sales Results
|
|
| ||||||
|
|
|
|
|
|
|
| |
| Innovative Medicine | $13,722 | $13,163 | 4.2% | 4.0 | 0.2 | 4.0 | |
| MedTech | 7,673 | 6,776 | 13.3 | 13.4 | (0.1) | 9.1 | |
| Worldwide | $21,395 | $19,939 | 7.3% | 7.2 | 0.1 | 5.7 | |
|
|
| ||||||
|
|
|
|
|
|
|
| |
| Innovative Medicine | $54,759 | $52,563 | 4.2% | 4.8 | (0.6) | 4.9 | |
| MedTech | 30,400 | 27,427 | 10.8 | 12.4 | (1.6) | 7.8 | |
| Worldwide | $85,159 | $79,990 | 6.5% | 7.4 | (0.9) | 5.9 | |
| Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures included in accompanying schedules | ||
| Excludes the impact of translational currency | ||
| Excludes the net impact of acquisitions and divestitures and translational currency | ||
| Note: The Innovative Medicine segment was previously referred to as the Pharmaceutical segment | ||
| Values may have been rounded | ||
Full Year 2023 Segment Commentary:
Operational sales* reflected below excludes the impact of translational currency. Adjusted operational sales* reflected below excludes the net impact of acquisitions and divestitures and translational currency.
Innovative Medicine
Innovative Medicine worldwide operational sales, excluding the COVID-19 Vaccine, grew 7.2%*. Growth was driven by DARZALEX (daratumumab), ERLEADA (apalutamide), TECVAYLI (teclistamab-cqyv) in Other Oncology, and CARVYKTI (ciltacabtagene autoleucel) in Oncology, STELARA (ustekinumab) and TREMFYA (guselkumab) in Immunology, and SPRAVATO (esketamine) in Neuroscience. Growth was partially offset by ZYTIGA (abiraterone acetate) and IMBRUVICA (ibrutinib) in Oncology, and REMICADE (infliximab) in Immunology. Including the COVID-19 Vaccine, Innovative Medicine worldwide operational sales grew 4.8%*.
MedTech worldwide operational sales grew 12.4%*, with the acquisition of Abiomed contributing 4.7%. MedTech worldwide adjusted operational sales grew 7.8%*, driven primarily by electrophysiology products in Interventional Solutions, contact lenses in Vision, wound closure products in General Surgery, and biosurgery in Advanced Surgery.
Notable New Announcements in the Quarter:
The information contained in this section should be read together with Johnson & Johnson’s other disclosures filed with the Securities and Exchange Commission, including its Current Reports on Form 8-K, Quarterly Reports on Form 10-Q and Annual Reports on Form 10-K. Copies of these filings are available online at www.sec.gov , www.jnj.com or on request from Johnson & Johnson. The reader is also encouraged to review all other news releases and information available in the Investor Relations section of the company’s website at News Releases , as well as Innovative Medicine , MedTech , www.factsabouttalc.com , www.factsaboutourprescriptionopioids.com , and www.LLTManagementInformation.com.
| Regulatory | U.S. Food and Drug Administration Grants Full Approval for BALVERSA to Treat Locally Advanced or Metastatic Bladder Cancer with Select Genetic Alterations |
|
| Biosense Webster Announces Regulatory Approval of VARIPULSE Pulsed Field Ablation (PFA) Platform in Japan |
| |
| Janssen Submits Marketing Authorisation Application to the European Medicines Agency Seeking Approval of Lazertinib, in combination with RYBREVANT (amivantamab), for the First-Line Treatment of Patients with EGFR-Mutated Non-Small Cell Lung Cancer |
| |
| Johnson & Johnson Submits Supplemental Biologics License Application and New Drug Application to U.S. FDA Seeking Approval of RYBREVANT (amivantamab-vmjw) Plus Lazertinib for the Treatment of Patients with EGFR-Mutated Non-Small Cell Lung Cancer (NSCLC) |
| |
| Johnson & Johnson’s Investigational TAR-200 Granted U.S. FDA Breakthrough Therapy Designation for the Treatment of High-Risk Non-Muscle-Invasive Bladder Cancer |
| |
| Janssen Submits Application to the European Medicines Agency for RYBREVANT (amivantamab) in Combination with Chemotherapy for the Treatment of Adult Patients with Advanced EGFR-Mutated Non-Small Cell Lung Cancer After Failure of Prior Therapy |
| |
| Janssen Submits Supplemental Biologics License Application to U.S. FDA Seeking Approval of RYBREVANT (amivantamab-vmjw) Plus Chemotherapy for the Treatment of Patients with EGFR-Mutated Non-Small Cell Lung Cancer Who Progressed on or after Osimertinib |
| |
| MONARCH Platform for Bronchoscopy Receives Regulatory License for China |
| |
| Data Release | Johnson & Johnson highlights its preeminent leadership in hematology through differentiated blood cancer portfolio and pipeline with new clinical and real-world data at ASH |
|
| New Real-World Data Show TREMFYA (guselkumab) Was Associated With Clinically Meaningful Improvements in Patient-Reported Outcomes for Adults Living With Active Psoriatic Arthritis |
| |
| Phase 2 Nipocalimab Data Establish Proof of Mechanism in Adults Living with Moderate to Severe Rheumatoid Arthritis, Supporting its Progression into a Combination Study |
| |
| New Biosense Webster QDOT MICRO Catheter Data Demonstrate Very High-Power, Short-Duration Ablations Improved Quality of Life and Reduced Healthcare Utilization for AFib Patients |
| |
| New Phase 3 TREMFYA (guselkumab) Results in Ulcerative Colitis Show a 77 Percent Overall Clinical Response Rate and Early Symptom Improvement |
| |
| Janssen Aims to Define New Standards of Care in the Treatment of Solid Tumor Cancers with Transformative Data Planned for Presentation at ESMO |
| |
| Product Launch | Ethicon Introduces ETHIZIA Hemostatic Sealing Patch, Clinically Proven to Stop Disruptive Bleeding |
|
| Other | Johnson & Johnson to Acquire Ambrx, Advancing Next Generation Antibody Drug Conjugates to Transform the Treatment of Cancer |
|
| Johnson & Johnson Announces Key Drivers for Long-Term Competitive Growth at Enterprise Business Review |
| |
| Johnson & Johnson Names Eugene A. Woods, Chief Executive Officer of Advocate Health, to its Board of Directors |
| |
| Johnson & Johnson MedTech Acquires Laminar, Inc. |
| |
| Johnson & Johnson MedTech Provides Details and Timeline for General Surgery Robot |
| |
| Johnson & Johnson Announces Departure of Ashley McEvoy, Tim Schmid Named Executive Vice President, Worldwide Chairman of MedTech |
| |
| Subsequent to the quarter | ||
Full-Year 2024 Guidance:
Johnson & Johnson does not provide GAAP financial measures on a forward-looking basis because the company is unable to predict with reasonable certainty the ultimate outcome of legal proceedings, unusual gains and losses, acquisition-related expenses, and purchase accounting fair value adjustments without unreasonable effort. These items are uncertain, depend on various factors, and could be material to Johnson & Johnson's results computed in accordance with GAAP.
|
|
|
|
| Adjusted Operational Sales Change vs. Prior Year / Mid-point | 5.0% – 6.0% / 5.5% |
|
| Operational Sales / Mid-point Change vs. Prior Year / Mid-point | $88.2B – $89.0B / $88.6B 5.0% – 6.0% / 5.5% | 5.0% – 6.0% / 5.5% |
| Estimated Reported Sales / Mid-point Change vs. Prior Year / Mid-point | $87.8B – $88.6B / $88.2B 4.5% – 5.5% / 5.0% |
|
|
|
|
|
| Adjusted Operational EPS (Diluted) / Mid-point Change vs. Prior Year / Mid-point | $10.55 – $10.75 / $10.65 6.4% – 8.4% / 7.4% | $10.55 – $10.75 / $10.65 7.3% Mid-point |
| Adjusted EPS (Diluted) / Mid-point Change vs. Prior Year / Mid-point | $10.55 – $10.75 / $10.65 6.4% – 8.4% / 7.4% |
|
| Average Shares Outstanding (Diluted) | ~2,435 million |
|
| Non-GAAP financial measure; excludes the net impact of acquisitions and divestitures | ||
| Non-GAAP financial measure; excludes the impact of translational currency | ||
| Calculated using Euro Average Rate: January 2024 = $1.09 and December 2023 = $1.09 (Illustrative purposes only) | ||
| Non-GAAP financial measure; excludes intangible amortization expense and special items | ||
| Excludes COVID-19 Vaccine | ||
| Full Year 2024 Projected Average Shares Outstanding (Diluted) reflects impact from the Kenvue exchange offer | ||
| Note: Percentages may have been rounded | ||
Other modeling considerations will be provided on the webcast.
Webcast Information:
Johnson & Johnson will conduct a conference call with investors to discuss this earnings release today at 8:30 a.m., Eastern Time. A simultaneous webcast of the call for investors and other interested parties may be accessed by visiting the Johnson & Johnson website . A replay and podcast will be available approximately two hours after the live webcast in the Investor Relations section of the company's website at events-and-presentations .
About Johnson & Johnson:
At Johnson & Johnson, we believe health is everything. Our strength in healthcare innovation empowers us to build a world where complex diseases are prevented, treated, and cured, where treatments are smarter and less invasive, and solutions are personal. Through our expertise in Innovative Medicine and MedTech, we are uniquely positioned to innovate across the full spectrum of healthcare solutions today to deliver the breakthroughs of tomorrow, and profoundly impact health for humanity. Learn more at https://www.jnj.com/ .
Non-GAAP Financial Measures:
* “Operational sales growth” excluding the impact of translational currency, “adjusted operational sales growth” excluding the net impact of acquisitions and divestitures and translational currency, as well as “adjusted net earnings”, “adjusted diluted earnings per share” and “adjusted operational diluted earnings per share” excluding after-tax intangible amortization expense and special items, are non-GAAP financial measures and should not be considered replacements for, and should be read together with, the most comparable GAAP financial measures. Except for guidance measures, reconciliations of these non-GAAP financial measures to the most directly comparable GAAP financial measures can be found in the accompanying financial schedules of the earnings release and the Investor Relations section of the company's website at quarterly results .
Copies of the financial schedules accompanying this earnings release are available on the company’s website at quarterly results . These schedules include supplementary sales data, a condensed consolidated statement of earnings, reconciliations of non-GAAP financial measures, and sales of key products/franchises. Additional information on Johnson & Johnson, including adjusted income before tax by segment, an Innovative Medicine pipeline of selected compounds in late stage development and a copy of today’s earnings call presentation can also be found in the Investor Relations section of the company's website at quarterly results .
Note to Investors Concerning Forward-Looking Statements:
This press release contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things: future operating and financial performance, product development, and market position and business strategy. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Johnson & Johnson. Risks and uncertainties include, but are not limited to: economic factors, such as interest rate and currency exchange rate fluctuations; competition, including technological advances, new products and patents attained by competitors; challenges inherent in new product research and development, including uncertainty of clinical success and obtaining regulatory approvals; uncertainty of commercial success for new and existing products; challenges to patents; the impact of patent expirations; the ability of the Company to successfully execute strategic plans, including restructuring plans; the impact of business combinations and divestitures; manufacturing difficulties or delays, internally or within the supply chain; product efficacy or safety concerns resulting in product recalls or regulatory action; significant adverse litigation or government action, including related to product liability claims; changes to applicable laws and regulations, including tax laws and global health care reforms; trends toward health care cost containment; changes in behavior and spending patterns of purchasers of health care products and services; financial instability of international economies and legal systems and sovereign risk; increased scrutiny of the health care industry by government agencies; the Company’s ability to realize the anticipated benefits from the separation of the Company’s Consumer Health business; and the New Consumer Health Company’s ability to succeed as a standalone publicly traded company. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson’s Annual Report on Form 10-K for the fiscal year ended January 1, 2023, including in the sections captioned “Cautionary Note Regarding Forward-Looking Statements” and “Item 1A. Risk Factors,” and in Johnson & Johnson’s subsequent Quarterly Reports on Form 10-Q and other filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov , www.jnj.com or on request from Johnson & Johnson. Any forward-looking statement made in this release speaks only as of the date of this release. Johnson & Johnson does not undertake to update any forward-looking statement as a result of new information or future events or developments.
| (Unaudited; Dollars in Millions) | |||||||||||||||||||||||||||
| Percent Change | Percent Change | ||||||||||||||||||||||||||
|
|
|
|
| Total |
| Operations |
| Currency |
|
|
|
|
| Total |
| Operations |
| Currency | |||||||||
| Innovative Medicine | |||||||||||||||||||||||||||
| U.S. |
|
|
| 9.5 |
| 9.5 |
| - |
|
|
|
| 9.0 |
| 9.0 |
| - |
| |||||||||
| International |
|
|
| (2.5 | ) | (3.1 | ) | 0.6 |
|
|
|
| (1.5 | ) | (0.2 | ) | (1.3 | ) | |||||||||
|
|
|
| 4.2 |
| 4.0 |
| 0.2 |
|
|
|
| 4.2 |
| 4.8 |
| (0.6 | ) | ||||||||||
| Innovative Medicine excluding COVID-19 Vaccine | |||||||||||||||||||||||||||
| U.S. |
|
|
| 9.5 |
| 9.5 |
| - |
|
|
|
| 9.4 |
| 9.4 |
| - |
| |||||||||
| International |
|
|
| 9.8 |
| 9.4 |
| 0.4 |
|
|
|
| 2.6 |
| 4.3 |
| (1.7 | ) | |||||||||
|
|
|
| 9.7 |
| 9.5 |
| 0.2 |
|
|
|
| 6.5 |
| 7.2 |
| (0.7 | ) | ||||||||||
| MedTech | |||||||||||||||||||||||||||
| U.S. |
|
|
| 14.1 |
| 14.1 |
| - |
|
|
|
| 14.2 |
| 14.2 |
| - |
| |||||||||
| International |
|
|
| 12.4 |
| 12.8 |
| (0.4 | ) |
|
|
| 7.7 |
| 10.6 |
| (2.9 | ) | |||||||||
|
|
|
| 13.3 |
| 13.4 |
| (0.1 | ) |
|
|
| 10.8 |
| 12.4 |
| (1.6 | ) | ||||||||||
| U.S. |
|
|
| 11.0 |
| 11.0 |
| - |
|
|
|
| 10.6 |
| 10.6 |
| - |
| |||||||||
| International |
|
|
| 2.9 |
| 2.7 |
| 0.2 |
|
|
|
| 1.9 |
| 3.8 |
| (1.9 | ) | |||||||||
| Worldwide |
|
|
| 7.3 |
| 7.2 |
| 0.1 |
|
|
|
| 6.5 |
| 7.4 |
| (0.9 | ) | |||||||||
| U.S. |
|
|
| 11.0 |
| 11.0 |
| - |
|
|
|
| 10.9 |
| 10.9 |
| - |
| |||||||||
| International |
|
|
| 10.8 |
| 10.7 |
| 0.1 |
|
|
|
| 4.6 |
| 6.7 |
| (2.1 | ) | |||||||||
| Worldwide excluding COVID-19 Vaccine |
|
|
| 10.9 |
| % | 10.9 |
| 0.0 |
|
|
|
| 8.0 |
| % | 9.0 |
| (1.0 | ) | |||||||
| Percentages have been calculated using actual, non-rounded figures and, therefore, may not recalculate precisely. | |||||||||
| Refer to supplemental sales reconciliation schedule | |||||||||
| Previously referred to as Pharmaceutical | |||||||||
| (Unaudited; Dollars in Millions) | |||||||||||||||||||||||||||
| Percent Change | Percent Change | ||||||||||||||||||||||||||
|
|
| Total | Operations | Currency |
|
| Total | Operations | Currency | ||||||||||||||||||
| U.S. |
|
|
| 11.0 |
| % | 11.0 |
| - |
|
|
|
| 10.6 |
| % | 10.6 |
| - |
| |||||||
| Europe |
|
|
| (3.2 | ) | (5.8 | ) | 2.6 |
|
|
|
| (1.2 | ) | (2.2 | ) | 1.0 |
| |||||||||
| Western Hemisphere excluding U.S. |
|
|
| 14.0 |
| 18.1 |
| (4.1 | ) |
|
|
| 10.7 |
| 15.8 |
| (5.1 | ) | |||||||||
| Asia-Pacific, Africa |
|
|
| 9.7 |
| 12.1 |
| (2.4 | ) |
|
|
| 3.9 |
| 9.5 |
| (5.6 | ) | |||||||||
| International |
|
|
| 2.9 |
| 2.7 |
| 0.2 |
|
|
|
| 1.9 |
| 3.8 |
| (1.9 | ) | |||||||||
| Worldwide |
|
|
| 7.3 |
| % | 7.2 |
| 0.1 |
|
|
|
| 6.5 |
| % | 7.4 |
| (0.9 | ) | |||||||
| : Percentages have been calculated using actual, non-rounded figures and, therefore, may not recalculate precisely. | |||||||||||||||||||||||||||
|
|
|
| Percent | ||||||||||
|
|
|
|
|
|
|
| Increase | ||||||
|
|
|
|
|
|
|
| (Decrease) | ||||||
|
|
|
|
|
|
|
|
| 7.3 | |||||
|
|
|
|
|
|
|
|
| 11.7 | |||||
|
|
|
|
|
|
|
|
| 5.4 | |||||
|
|
|
|
|
|
|
|
| 8.8 | |||||
|
|
|
|
|
|
|
|
| 20.8 | |||||
|
|
|
|
|
|
|
|
| ||||||
|
|
|
|
|
|
|
|
| ||||||
|
|
|
|
|
|
|
|
| ||||||
|
|
|
|
|
|
|
|
| ||||||
|
|
|
|
|
|
|
|
| 25.7 | |||||
|
|
|
|
|
|
|
|
| 13.2 | |||||
|
|
|
|
|
|
|
|
| 28.0 | |||||
|
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
| 39.3 | |||||||
|
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
|
|
| ||||||
|
|
|
|
|
|
|
|
| (3.8) | |||||
|
|
|
|
|
|
|
|
| 2.4 | |||||
|
|
|
|
|
|
| 11.7 | |||||||
|
|
|
|
|
|
|
|
| ||||||
| * Basic shares of 2,407.2 are used to calculate loss per share in the fourth quarter of 2023 as use of diluted shares when in a loss position would be anti-dilutive. | |||||||||||||
| See Reconciliation of Non-GAAP Financial Measures. | |||||||||||||
|
|
|
|
| Percent | |||||||
|
|
|
|
|
|
|
|
| Increase | |||
|
|
|
|
|
|
|
|
| (Decrease) | |||
|
|
|
|
|
|
| 6.5 | |||||
|
|
|
|
|
|
| 8.0 | |||||
|
|
|
|
|
|
| 5.8 | |||||
|
|
|
|
|
|
| 6.3 | |||||
|
|
|
|
|
|
| 6.7 | |||||
|
|
|
|
|
|
| ||||||
|
|
|
|
|
|
| ||||||
|
|
|
|
|
|
| ||||||
|
|
|
|
|
|
| ||||||
|
|
|
|
|
|
| (22.2) | |||||
|
|
|
|
|
|
| (41.9) | |||||
|
|
|
|
|
|
| (18.6) | |||||
|
|
|
|
| ||||||||
|
|
|
|
| ||||||||
|
|
|
|
| (15.3) | |||||||
|
|
|
|
| ||||||||
|
|
|
|
| ||||||||
|
|
|
|
|
|
| ||||||
|
|
|
|
|
|
| 6.6 | |||||
|
|
|
|
|
|
| 6.8 | |||||
|
|
|
|
| 11.1 | |||||||
|
|
|
|
|
|
| ||||||
| See Reconciliation of Non-GAAP Financial Measures. | |||||||||||
| (Dollars in Millions Except Per Share Data) |
|
|
|
|
|
|
| |
| Net Earnings from Continuing Operations, after tax- as reported |
|
|
|
| ||||
| Litigation related | 166 | 262 | 7,152 | 866 | ||||
| Intangible Asset Amortization expense | 1,148 | 977 | 4,532 | 3,944 | ||||
| COVID-19 Vaccine related costs | 10 | 821 | 663 | 1,474 | ||||
| Restructuring related | 139 | 119 | 798 | 372 | ||||
| Medical Device Regulation | 88 | 88 | 311 | 296 | ||||
| Acquisition, integration and divestiture related | 237 | 196 | 339 | 196 | ||||
| (Gains)/losses on securities | (435) | 6 | 641 | 690 | ||||
| IPR&D impairments | 58 | 173 | 313 | 783 | ||||
| Other | - | - | - | (7) | ||||
| Tax impact on special item adjustments | 75 | (394) | (2,694) | (1,294) | ||||
| Tax legislation and other tax related | (56) | (43) | 28 | 106 | ||||
| Adjusted Net Earnings from Continuing Operations, after tax |
|
|
|
| ||||
| Average shares outstanding (Diluted) | 2,430.7 | 2,650.1 | 2,560.4 | 2,663.9 | ||||
| Adjusted net earnings per share from Continuing Operations (Diluted) |
|
|
|
| ||||
| Operational adjusted net earnings per share from Continuing Operations (Diluted) |
|
| ||||||
|
| ||
| COVID-19 Vaccine related costs include remaining commitments and obligations, including external manufacturing network exit costs and required clinical trial expenses, associated with the Company's completion of its COVID-19 vaccine contractual commitments. | ||
| In fiscal 2023, the company completed a prioritization of its research and development (R&D) investment within the Innovative Medicine segment to focus on the most promising medicines with the greatest benefit to patients. This resulted in the exit of certain programs within therapeutic areas. The R&D program exits are primarily in infectious diseases and vaccines including the discontinuation of its respiratory syncytial virus (RSV) adult vaccine program, hepatitis and HIV development. The restructuring expenses of $55 million in the quarter ($479 million Q4 YTD) include the termination of partnered and non-partnered program costs and asset impairments. | ||
| In fiscal 2023, the company initiated a restructuring program of its Orthopaedics franchise within the MedTech segment to streamline operations by exiting certain markets, product lines and distribution network arrangements. The restructuring expenses of $84 million in the quarter ($319 million Q4 YTD) primarily includes inventory and instrument reserves related to the market and product exits. | ||
| European Medical Device Regulation (MDR) costs represent one-time compliance costs for the Company’s previously registered products. MDR is a replacement of the existing European Medical Devices Directive regulatory framework, and manufacturers of currently marketed medical devices were required to comply with EU MDR beginning in May 2021. The Company considers the adoption of EU MDR to be a significant one-time regulatory change and is not indicative of on-going operations. The Company has excluded only external third-party regulatory and consulting costs from its MedTech operating segments' measures of profit and loss used for making operating decisions and assessing performance which is expected to be completed during 2024. | ||
| The tax impact related to special item adjustments reflects the current and deferred income taxes associated with the above pre-tax special items in arriving at adjusted earnings. | ||
|
|
|
|
|
| ||
| U.S. | 9.5% |
| 14.1% |
| 11.0% | |
| International | (2.5)% |
| 12.4% |
| 2.9% | |
|
|
|
|
|
| ||
|
|
|
|
|
| ||
| U.S. |
|
|
|
|
| |
| International | 0.6 |
| (0.4) |
| 0.2 | |
|
|
|
|
|
| ||
|
|
|
|
|
| ||
| U.S. | 9.5% |
| 14.1% |
| 11.0% | |
| International | (3.1)% |
| 12.8% |
| 2.7% | |
|
|
|
|
|
| ||
|
|
|
|
|
| ||
| U.S. |
|
| (7.2) |
| (2.3) | |
| International |
|
| (1.9) |
| (0.7) | |
|
|
|
|
|
| ||
|
|
|
|
|
| ||
| U.S. | 0.0 |
| 0.3 |
| 0.1 | |
| International | 0.1 |
| 0.1 |
| 0.1 | |
|
|
|
|
|
| ||
|
|
|
|
|
| ||
| U.S. | 9.5% |
| 7.2% |
| 8.8% | |
| International | (3.0)% |
| 11.0% |
| 2.1% | |
| Percentages are based on actual, non-rounded figures and may not sum | ||||||
|
|
|
|
|
| ||
|
|
|
|
|
| ||
|
|
|
|
|
| ||
| U.S. | 9.0% |
| 14.2% |
| 10.6% | |
| International | (1.5)% |
| 7.7% |
| 1.9% | |
|
|
|
|
|
| ||
|
|
|
|
|
| ||
| U.S. |
|
|
|
|
| |
| International | (1.3) |
| (2.9) |
| (1.9) | |
|
|
|
|
|
| ||
|
|
|
|
|
| ||
| U.S. | 9.0% |
| 14.2% |
| 10.6% | |
| International | (0.2)% |
| 10.6% |
| 3.8% | |
|
|
|
|
|
| ||
|
|
|
|
|
| ||
| U.S. |
|
| (7.7) |
| (2.5) | |
| International |
|
| (1.7) |
| (0.6) | |
|
|
|
|
|
| ||
|
|
|
|
|
| ||
| U.S. | 0.0 |
| 0.1 |
| 0.1 | |
| International | 0.2 |
| 0.1 |
| 0.2 | |
|
|
|
|
|
| ||
|
|
|
|
|
| ||
| U.S. | 9.0% |
| 6.6% |
| 8.2% | |
| International | 0.0% |
| 9.0% |
| 3.4% | |
| Percentages are based on actual, non-rounded figures and may not sum | ||||||
|
|
| ||||||||||||||||||
|
|
| ||||||||||||||||||
|
|
|
|
| ||||||||||||||||
| US |
|
| 8.1% | 8.1% | - |
|
|
| 4.6% | 4.6% | - | ||||||||
| Intl |
|
| 19.0% | 17.7% | 1.3% |
|
|
| 10.4% | 12.0% | -1.6% | ||||||||
| WW |
|
| 11.6% | 11.2% | 0.4% |
|
|
| 6.6% | 7.1% | -0.5% | ||||||||
| REMICADE | |||||||||||||||||||
| US |
|
| -7.4% | -7.4% | - |
|
|
| -19.3% | -19.3% | - | ||||||||
| US Exports |
|
| -15.1% | -15.1% | - |
|
|
| -28.0% | -28.0% | - | ||||||||
| Intl |
|
| -13.4% | -12.4% | -1.0% |
|
|
| -23.9% | -21.3% | -2.6% | ||||||||
| WW |
|
| -9.6% | -9.4% | -0.2% |
|
|
| -21.5% | -20.7% | -0.8% | ||||||||
| SIMPONI / SIMPONI ARIA | |||||||||||||||||||
| US |
|
| -7.8% | -7.8% | - |
|
|
| -3.6% | -3.6% | - | ||||||||
| Intl |
|
| 10.1% | 11.8% | -1.7% |
|
|
| 5.4% | 9.3% | -3.9% | ||||||||
| WW |
|
| 0.1% | 0.8% | -0.7% |
|
|
| 0.6% | 2.4% | -1.8% | ||||||||
| STELARA | |||||||||||||||||||
| US |
|
| 10.1% | 10.1% | - |
|
|
| 9.0% | 9.0% | - | ||||||||
| Intl |
|
| 26.4% | 23.9% | 2.5% |
|
|
| 16.7% | 17.4% | -0.7% | ||||||||
| WW |
|
| 15.3% | 14.5% | 0.8% |
|
|
| 11.7% | 11.9% | -0.2% | ||||||||
| TREMFYA | |||||||||||||||||||
| US |
|
| 21.6% | 21.6% | - |
|
|
| 16.5% | 16.5% | - | ||||||||
| Intl |
|
| 19.4% | 17.8% | 1.6% |
|
|
| 21.2% | 22.4% | -1.2% | ||||||||
| WW |
|
| 21.0% | 20.5% | 0.5% |
|
|
| 17.9% | 18.3% | -0.4% | ||||||||
| OTHER IMMUNOLOGY | |||||||||||||||||||
| US |
|
| -22.8% | -22.8% | - |
|
|
| -33.8% | -33.8% | - | ||||||||
| Intl |
|
| - | - | - |
|
|
| - | - | - | ||||||||
| WW |
|
| -22.8% | -22.8% | - |
|
|
| -33.8% | -33.8% | - | ||||||||
| US |
|
| -14.6% | -14.6% | - |
|
|
| -10.7% | -10.7% | - | ||||||||
| Intl |
|
| -55.8% | -58.2% | 2.4% |
|
|
| -22.6% | -23.9% | 1.3% | ||||||||
| WW |
|
| -44.7% | -46.5% | 1.8% |
|
|
| -18.9% | -19.8% | 0.9% | ||||||||
| COVID-19 VACCINE | |||||||||||||||||||
| US |
|
| - | - | - |
|
|
| * | * | - | ||||||||
| Intl |
|
| -93.7% | -95.2% | 1.5% |
|
|
| -45.8% | -47.2% | 1.4% | ||||||||
| WW |
|
| -93.7% | -95.2% | 1.5% |
|
|
| -48.8% | -50.1% | 1.3% | ||||||||
| EDURANT / rilpivirine | |||||||||||||||||||
| US |
|
| -12.4% | -12.4% | - |
|
|
| -3.7% | -3.7% | - | ||||||||
| Intl |
|
| 6.4% | 1.5% | 4.9% |
|
|
| 14.8% | 12.1% | 2.7% | ||||||||
| WW |
|
| 5.8% | 1.0% | 4.8% |
|
|
| 14.1% | 11.5% | 2.6% | ||||||||
| PREZISTA / PREZCOBIX / REZOLSTA / SYMTUZA | |||||||||||||||||||
| US |
|
| -14.4% | -14.4% | - |
|
|
| -3.2% | -3.2% | - | ||||||||
| Intl |
|
| 3.1% | -0.8% | 3.9% |
|
|
| -9.2% | -10.4% | 1.2% | ||||||||
| WW |
|
| -11.0% | -11.8% | 0.8% |
|
|
| -4.6% | -4.9% | 0.3% | ||||||||
| OTHER INFECTIOUS DISEASES | |||||||||||||||||||
| US |
|
| -30.4% | -30.4% | - |
|
|
| -34.5% | -34.5% | - | ||||||||
| Intl |
|
| -5.6% | -4.9% | -0.7% |
|
|
| -3.8% | -0.4% | -3.4% | ||||||||
| WW |
|
| -7.8% | -7.1% | -0.7% |
|
|
| -6.7% | -3.6% | -3.1% | ||||||||
|
|
| ||||||||||||||||||
|
|
| ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
| US |
|
| 12.1% | 12.1% | - |
|
|
| 13.9% | 13.9% | - | ||||||||
| Intl |
|
| -5.5% | -2.0% | -3.5% |
|
|
| -7.5% | -3.7% | -3.8% | ||||||||
| WW |
|
| 3.7% | 5.4% | -1.7% |
|
|
| 3.6% | 5.4% | -1.8% | ||||||||
| CONCERTA / Methylphenidate | |||||||||||||||||||
| US |
|
| 5.7% | 5.7% | - |
|
|
| 52.5% | 52.5% | - | ||||||||
| Intl |
|
| 7.9% | 9.2% | -1.3% |
|
|
| 12.2% | 16.4% | -4.2% | ||||||||
| WW |
|
| 7.4% | 8.5% | -1.1% |
|
|
| 21.6% | 24.9% | -3.3% | ||||||||
| INVEGA SUSTENNA / XEPLION / INVEGA TRINZA / TREVICTA | |||||||||||||||||||
| US |
|
| 8.0% | 8.0% | - |
|
|
| 6.7% | 6.7% | - | ||||||||
| Intl |
|
| -15.5% | -15.7% | 0.2% |
|
|
| -14.6% | -12.8% | -1.8% | ||||||||
| WW |
|
| 0.3% | 0.3% | 0.0% |
|
|
| -0.6% | 0.0% | -0.6% | ||||||||
| SPRAVATO | |||||||||||||||||||
| US |
|
| 72.3% | 72.3% | - |
|
|
| 79.7% | 79.7% | - | ||||||||
| Intl |
|
| 87.1% | 83.4% | 3.7% |
|
|
| * | * | * | ||||||||
| WW |
|
| 74.1% | 73.6% | 0.5% |
|
|
| 84.1% | 84.0% | 0.1% | ||||||||
| OTHER NEUROSCIENCE | |||||||||||||||||||
| US |
|
| -23.0% | -23.0% | - |
|
|
| -7.3% | -7.3% | - | ||||||||
| Intl |
|
| -5.0% | 3.2% | -8.2% |
|
|
| -11.3% | -5.4% | -5.9% | ||||||||
| WW |
|
| -8.7% | -2.3% | -6.4% |
|
|
| -10.4% | -5.9% | -4.5% | ||||||||
| US |
|
| 23.0% | 23.0% | - |
|
|
| 22.1% | 22.1% | - | ||||||||
| Intl |
|
| 12.8% | 12.0% | 0.8% |
|
|
| 1.6% | 2.9% | -1.3% | ||||||||
| WW |
|
| 17.6% | 17.2% | 0.4% |
|
|
| 10.5% | 11.2% | -0.7% | ||||||||
| CARVYKTI | |||||||||||||||||||
| US |
|
| * | * | - |
|
|
| * | * | - | ||||||||
| Intl |
|
| * | * | - |
|
|
| * | * | - | ||||||||
| WW |
|
| * | * | * |
|
|
| * | * | * | ||||||||
| DARZALEX | |||||||||||||||||||
| US |
|
| 22.5% | 22.5% | - |
|
|
| 25.4% | 25.4% | - | ||||||||
| Intl |
|
| 22.3% | 21.8% | 0.5% |
|
|
| 18.6% | 20.2% | -1.6% | ||||||||
| WW |
|
| 22.4% | 22.2% | 0.2% |
|
|
| 22.2% | 22.9% | -0.7% | ||||||||
| ERLEADA | |||||||||||||||||||
| US |
|
| 4.4% | 4.4% | - |
|
|
| 10.0% | 10.0% | - | ||||||||
| Intl |
|
| 35.6% | 34.0% | 1.6% |
|
|
| 44.8% | 46.0% | -1.2% | ||||||||
| WW |
|
| 19.8% | 19.0% | 0.8% |
|
|
| 26.9% | 27.5% | -0.6% | ||||||||
| IMBRUVICA | |||||||||||||||||||
| US |
|
| -19.8% | -19.8% | - |
|
|
| -24.4% | -24.4% | - | ||||||||
| Intl |
|
| -2.6% | -3.8% | 1.2% |
|
|
| -7.5% | -6.7% | -0.8% | ||||||||
| WW |
|
| -8.9% | -9.7% | 0.8% |
|
|
| -13.7% | -13.2% | -0.5% | ||||||||
| ZYTIGA / abiraterone acetate | |||||||||||||||||||
| US |
|
| -52.0% | -52.0% | - |
|
|
| -32.1% | -32.1% | - | ||||||||
| Intl |
|
| -23.6% | -23.2% | -0.4% |
|
|
| -50.7% | -49.1% | -1.6% | ||||||||
| WW |
|
| -25.6% | -25.3% | -0.3% |
|
|
| -49.9% | -48.4% | -1.5% | ||||||||
| OTHER ONCOLOGY | |||||||||||||||||||
| US |
|
| * | * | - |
|
|
| * | * | - | ||||||||
| Intl |
|
| 32.2% | 28.4% | 3.8% |
|
|
| 16.9% | 17.0% | -0.1% | ||||||||
| WW |
|
| * | * | * |
|
|
| * | * | * | ||||||||
|
| |||||||||||||||||||
|
|
| ||||||||||||||||||
|
|
|
|
|
| |||||||||||||||
| US |
|
| 20.2% | 20.2% | - |
|
|
| 15.0% | 15.0% | - | ||||||||
| Intl |
|
| 8.6% | 10.9% | -2.3% |
|
|
| 4.3% | 8.3% | -4.0% | ||||||||
| WW |
|
| 16.7% | 17.4% | -0.7% |
|
|
| 11.6% | 12.9% | -1.3% | ||||||||
| OPSUMIT | |||||||||||||||||||
| US |
|
| 20.5% | 20.5% | - |
|
|
| 14.1% | 14.1% | - | ||||||||
| Intl |
|
| 7.9% | 8.3% | -0.4% |
|
|
| 4.6% | 7.2% | -2.6% | ||||||||
| WW |
|
| 16.2% | 16.3% | -0.1% |
|
|
| 10.6% | 11.6% | -1.0% | ||||||||
| UPTRAVI | |||||||||||||||||||
| US |
|
| 24.3% | 24.3% | - |
|
|
| 20.1% | 20.1% | - | ||||||||
| Intl |
|
| 26.4% | 31.4% | -5.0% |
|
|
| 17.3% | 21.8% | -4.5% | ||||||||
| WW |
|
| 24.6% | 25.4% | -0.8% |
|
|
| 19.7% | 20.4% | -0.7% | ||||||||
| OTHER PULMONARY HYPERTENSION | |||||||||||||||||||
| US |
|
| -29.4% | -29.4% | - |
|
|
| -28.6% | -28.6% | - | ||||||||
| Intl |
|
| -9.7% | -3.9% | -5.8% |
|
|
| -10.3% | -2.9% | -7.4% | ||||||||
| WW |
|
| -16.2% | -12.4% | -3.8% |
|
|
| -16.7% | -12.0% | -4.7% | ||||||||
| US |
|
| -15.9% | -15.9% | - |
|
|
| -4.5% | -4.5% | - | ||||||||
| Intl |
|
| -4.7% | -6.7% | 2.0% |
|
|
| -9.4% | -9.1% | -0.3% | ||||||||
| WW |
|
| -13.7% | -14.1% | 0.4% |
|
|
| -5.5% | -5.5% | 0.0% | ||||||||
| XARELTO | |||||||||||||||||||
| US |
|
| -21.2% | -21.2% | - |
|
|
| -4.4% | -4.4% | - | ||||||||
| Intl |
|
| - | - | - |
|
|
| - | - | - | ||||||||
| WW |
|
| -21.2% | -21.2% | - |
|
|
| -4.4% | -4.4% | - | ||||||||
| OTHER | |||||||||||||||||||
| US |
|
| 15.9% | 15.9% | - |
|
|
| -5.0% | -5.0% | - | ||||||||
| Intl |
|
| -4.7% | -6.7% | 2.0% |
|
|
| -9.4% | -9.1% | -0.3% | ||||||||
| WW |
|
| 2.8% | 1.5% | 1.3% |
|
|
| -7.6% | -7.4% | -0.2% | ||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||
| See footnotes at end of schedule | |||||||||||||||||||
|
|
| ||||||||||||||||||
|
|
| ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
| US |
|
| 61.0% | 61.0% | - |
|
|
| 67.5% | 67.5% | - | ||||||||
| Intl |
|
| 40.8% | 41.7% | -0.9% |
|
|
| 27.5% | 31.7% | -4.2% | ||||||||
| WW |
|
| 51.9% | 52.3% | -0.4% |
|
|
| 47.7% | 49.8% | -2.1% | ||||||||
| ELECTROPHYSIOLOGY | |||||||||||||||||||
| US |
|
| 22.0% | 22.0% | - |
|
|
| 20.7% | 20.7% | - | ||||||||
| Intl |
|
| 28.0% | 29.0% | -1.0% |
|
|
| 17.3% | 21.5% | -4.2% | ||||||||
| WW |
|
| 24.7% | 25.2% | -0.5% |
|
|
| 19.1% | 21.1% | -2.0% | ||||||||
| ABIOMED | |||||||||||||||||||
| US |
|
| * | * | - |
|
|
| * | * | - | ||||||||
| Intl |
|
| * | * | - |
|
|
| * | * | - | ||||||||
| WW |
|
| * | * | - |
|
|
| * | * | - | ||||||||
| OTHER INTERVENTIONAL SOLUTIONS | |||||||||||||||||||
| US |
|
| 8.8% | 8.8% | - |
|
|
| 6.7% | 6.7% | - | ||||||||
| Intl |
|
| 26.5% | 27.1% | -0.6% |
|
|
| 7.3% | 11.4% | -4.1% | ||||||||
| WW |
|
| 20.4% | 20.7% | -0.3% |
|
|
| 7.1% | 9.9% | -2.8% | ||||||||
| US |
|
| 2.9% | 2.9% | - |
|
|
| 3.8% | 3.8% | - | ||||||||
| Intl |
|
| 10.5% | 8.8% | 1.7% |
|
|
| 4.6% | 5.8% | -1.2% | ||||||||
| WW |
|
| 5.6% | 5.0% | 0.6% |
|
|
| 4.1% | 4.6% | -0.5% | ||||||||
| HIPS | |||||||||||||||||||
| US |
|
| 6.3% | 6.3% | - |
|
|
| 5.6% | 5.6% | - | ||||||||
| Intl |
|
| -2.0% | -3.9% | 1.9% |
|
|
| -1.2% | -0.1% | -1.1% | ||||||||
| WW |
|
| 3.4% | 2.7% | 0.7% |
|
|
| 3.0% | 3.5% | -0.5% | ||||||||
| KNEES | |||||||||||||||||||
| US |
|
| 4.5% | 4.5% | - |
|
|
| 5.3% | 5.3% | - | ||||||||
| Intl |
|
| 18.1% | 15.6% | 2.5% |
|
|
| 10.2% | 11.1% | -0.9% | ||||||||
| WW |
|
| 9.2% | 8.4% | 0.8% |
|
|
| 7.1% | 7.5% | -0.4% | ||||||||
| TRAUMA | |||||||||||||||||||
| US |
|
| 3.6% | 3.6% | - |
|
|
| 3.6% | 3.6% | - | ||||||||
| Intl |
|
| 5.9% | 4.1% | 1.8% |
|
|
| 4.1% | 4.8% | -0.7% | ||||||||
| WW |
|
| 4.4% | 3.8% | 0.6% |
|
|
| 3.8% | 4.0% | -0.2% | ||||||||
| SPINE, SPORTS & OTHER | |||||||||||||||||||
| US |
|
| -0.7% | -0.7% | - |
|
|
| 2.4% | 2.4% | - | ||||||||
| Intl |
|
| 17.5% | 16.5% | 1.0% |
|
|
| 5.4% | 7.3% | -1.9% | ||||||||
| WW |
|
| 6.2% | 5.8% | 0.4% |
|
|
| 3.7% | 4.5% | -0.8% | ||||||||
|
| |||||||||||||||||||
|
|
| ||||||||||||||||||
|
|
|
|
|
| |||||||||||||||
| US |
|
| 4.6% | 4.6% | - |
|
|
| 3.4% | 3.4% | - | ||||||||
| Intl |
|
| 7.2% | 7.8% | -0.6% |
|
|
| 3.7% | 7.0% | -3.3% | ||||||||
| WW |
|
| 6.1% | 6.4% | -0.3% |
|
|
| 3.6% | 5.5% | -1.9% | ||||||||
| ADVANCED | |||||||||||||||||||
| US |
|
| 2.5% | 2.5% | - |
|
|
| 2.8% | 2.8% | - | ||||||||
| Intl |
|
| 7.0% | 7.2% | -0.2% |
|
|
| 1.9% | 5.1% | -3.2% | ||||||||
| WW |
|
| 5.1% | 5.2% | -0.1% |
|
|
| 2.2% | 4.2% | -2.0% | ||||||||
| GENERAL | |||||||||||||||||||
| US |
|
| 6.4% | 6.4% | - |
|
|
| 4.0% | 4.0% | - | ||||||||
| Intl |
|
| 7.4% | 8.3% | -0.9% |
|
|
| 5.3% | 8.7% | -3.4% | ||||||||
| WW |
|
| 7.0% | 7.5% | -0.5% |
|
|
| 4.8% | 6.8% | -2.0% | ||||||||
| US |
|
| 6.8% | 6.8% | - |
|
|
| 4.8% | 4.8% | - | ||||||||
| Intl |
|
| 4.6% | 6.4% | -1.8% |
|
|
| 4.5% | 7.9% | -3.4% | ||||||||
| WW |
|
| 5.5% | 6.6% | -1.1% |
|
|
| 4.6% | 6.6% | -2.0% | ||||||||
| CONTACT LENSES / OTHER | |||||||||||||||||||
| US |
|
| 8.9% | 8.9% | - |
|
|
| 6.8% | 6.8% | - | ||||||||
| Intl |
|
| 4.2% | 7.2% | -3.0% |
|
|
| 2.7% | 7.0% | -4.3% | ||||||||
| WW |
|
| 6.1% | 7.9% | -1.8% |
|
|
| 4.5% | 6.9% | -2.4% | ||||||||
| SURGICAL | |||||||||||||||||||
| US |
|
| 0.4% | 0.4% | - |
|
|
| -1.8% | -1.8% | - | ||||||||
| Intl |
|
| 5.6% | 4.7% | 0.9% |
|
|
| 8.6% | 10.0% | -1.4% | ||||||||
| WW |
|
| 3.7% | 3.1% | 0.6% |
|
|
| 4.9% | 5.8% | -0.9% | ||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||
| Columns and rows within tables may not add due to rounding. Percentages have been calculated using actual, non-rounded figures and, therefore, may not recalculate precisely |
| * Percentage greater than 100% or not meaningful |
| (1) Operational growth excludes the effect of translational currency |
| (2) Unaudited |
| (3) Certain prior year amounts have been reclassified to conform to current year product disclosures |
| (4) Previously referred to as Pharmaceutical |
| (5) Reported as U.S. sales |
| | ||||||||||||||||||||||||
| (Dollars in Millions) | ||||||||||||||||||||||||
| Percent Change | Percent Change | |||||||||||||||||||||||
|
|
|
|
| Total |
| Operations |
| Currency |
|
|
| Total | Operations | Currency | ||||||||||
| Innovative Medicine | ||||||||||||||||||||||||
| U.S. |
|
|
| 9.5 |
| 9.5 | - |
|
|
| 9.0 |
| 9.0 | - | ||||||||||
| International |
|
|
| (2.5) | (3.1) | 0.6 |
|
|
| (1.5) | (0.2) | (1.3) | ||||||||||||
| Worldwide |
|
|
| 4.2 | 4.0 | 0.2 |
|
|
| 4.2 | 4.8 | (0.6) | ||||||||||||
| COVID-19 Vaccine | ||||||||||||||||||||||||
| U.S. |
|
|
| - | - | - |
|
|
| * | * | - | ||||||||||||
| International |
|
|
| (93.7) | (95.2) | 1.5 |
|
|
| (45.8) | (47.2) | 1.4 | ||||||||||||
| Worldwide |
|
|
| (93.7) | (95.2) | 1.5 |
|
|
| (48.8) | (50.1) | 1.3 | ||||||||||||
| Innovative Medicine excluding COVID-19 Vaccine | ||||||||||||||||||||||||
| U.S. |
|
|
| 9.5 | 9.5 | - |
|
|
| 9.4 | 9.4 | - | ||||||||||||
| International |
|
|
| 9.8 | 9.4 | 0.4 |
|
|
| 2.6 | 4.3 | (1.7) | ||||||||||||
| Worldwide |
|
|
| 9.7 | 9.5 | 0.2 |
|
|
| 6.5 | 7.2 | (0.7) | ||||||||||||
| Worldwide | ||||||||||||||||||||||||
| U.S. |
|
|
| 11.0 | 11.0 | - |
|
|
| 10.6 | 10.6 | - | ||||||||||||
| International |
|
|
| 2.9 | 2.7 | 0.2 |
|
|
| 1.9 | 3.8 | (1.9) | ||||||||||||
| Worldwide |
|
|
| 7.3 | 7.2 | 0.1 |
|
|
| 6.5 | 7.4 | (0.9) | ||||||||||||
| COVID-19 Vaccine | ||||||||||||||||||||||||
| U.S. |
|
|
| - | - | - |
|
|
| * | * | - | ||||||||||||
| International |
|
|
| (93.7) | (95.2) | 1.5 |
|
|
| (45.8) | (47.2) | 1.4 | ||||||||||||
| Worldwide |
|
|
| (93.7) | (95.2) | 1.5 |
|
|
| (48.8) | (50.1) | 1.3 | ||||||||||||
| Worldwide | ||||||||||||||||||||||||
| U.S. |
|
|
| 11.0 | 11.0 | - |
|
|
| 10.9 | 10.9 | - | ||||||||||||
| International |
|
|
| 10.8 | 10.7 | 0.1 |
|
|
| 4.6 | 6.7 | (2.1) | ||||||||||||
| Worldwide excluding COVID-19 Vaccine |
|
|
| 10.9 |
| 10.9 | - |
|
|
| 8.0 |
| 9.0 | (1.0) | ||||||||||
| : Columns and rows within tables may not add due to rounding | ||||||||||||||||||||||||
| * Percentage greater than 100% or not meaningful | ||||||||||||||||||||||||
| Johnson & Johnson and Subsidiaries | |||||||||||||||||||
| Reconciliation of Non-GAAP Financial Measures | |||||||||||||||||||
| Q4 QTD - Income Before Tax* and Research & Development Expense by Segment | |||||||||||||||||||
| Dollars in Millions | |||||||||||||||||||
| Innovative Medicine | MedTech | Unallocated | Worldwide Total | ||||||||||||||||
| 2023 | 2022 | 2023 | 2022 |
| 2023 | 2022 |
|
| 2023 | 2022 | |||||||||
| Reported Income Before Tax by Segment From Continuing Operations | $ | 4,238 | 3,223 | 404 | 806 | 184 | (189) | 4,826 | 3,840 | ||||||||||
|
|
|
|
|
|
|
|
| ||||||||||||
| Intangible asset amortization expense | 747 | 717 | 401 | 260 | - | - | 1,148 | 977 | |||||||||||
| In-process research and development impairments | - | 173 | 58 | - | - | - | 58 | 173 | |||||||||||
| Litigation related | 17 | 76 | 149 | 136 | - | 50 | 166 | 262 | |||||||||||
| Loss/(gain) on securities | (112) | 23 | (59) | (17) | (264) | - | (435) | 6 | |||||||||||
| Restructuring related | 55 | 31 | 84 | 88 | - | - | 139 | 119 | |||||||||||
| Acquisition, integration and divestiture related | 175 | (104) | 62 | 300 | - | - | 237 | 196 | |||||||||||
| Medical Device Regulation | - | - | 88 | 88 | - | - | 88 | 88 | |||||||||||
| COVID-19 Vaccine related costs | 10 | 821 | - | - | - | - | 10 | 821 | |||||||||||
| Adjusted Income Before Tax by Segment From Continuing Operations | $ | 5,130 | 4,960 | 1,187 | 1,661 | (80) | (139) | 6,237 | 6,482 | ||||||||||
|
|
|
|
|
|
|
|
| ||||||||||||
| *Estimated as of 1/23/2024 | |||||||||||||||||||
| As Reported Research and Development Expense | $ | 3,357 | 3,070 | 1,123 | 640 | 4,480 | 3,710 | ||||||||||||
|
|
|
|
|
|
| ||||||||||||||
| Johnson & Johnson and Subsidiaries | |||||||||||||||||||
| Reconciliation of Non-GAAP Financial Measures | |||||||||||||||||||
| Q4 YTD - Income Before Tax* and Research & Development Expense by Segment | |||||||||||||||||||
| Dollars in Millions | |||||||||||||||||||
| Innovative Medicine | MedTech | Unallocated | Worldwide Total | ||||||||||||||||
| 2023 | 2022 | 2023 | 2022 |
| 2023 | 2022 |
|
| 2023 | 2022 | |||||||||
| Reported Income Before Tax by Segment From Continuing Operations | $ | 18,246 | 15,647 | 4,669 | 4,447 | (7,853) | (735) | 15,062 | 19,359 | ||||||||||
|
|
|
|
|
|
|
|
| ||||||||||||
| Intangible asset amortization expense | 2,983 | 2,911 | 1,549 | 1,033 | - | - | 4,532 | 3,944 | |||||||||||
| In-process research and development impairments | 206 | 783 | 107 | - | - | - | 313 | 783 | |||||||||||
| Litigation related | (108) | 104 | 190 | 612 | 7,070 | 150 | 7,152 | 866 | |||||||||||
| Loss/(gain) on securities | 362 | 696 | (102) | (6) | 381 | - | 641 | 690 | |||||||||||
| Restructuring related | 479 | 63 | 319 | 309 | - | - | 798 | 372 | |||||||||||
| Acquisition, integration and divestiture related | 175 | (104) | 164 | 300 | - | - | 339 | 196 | |||||||||||
| Medical Device Regulation | - | - | 311 | 296 | - | - | 311 | 296 | |||||||||||
| COVID-19 Vaccine related costs | 663 | 1,474 | - | - | - | - | 663 | 1,474 | |||||||||||
| Other | - | - | - | - | - | (7) | - | (7) | |||||||||||
| Adjusted Income Before Tax by Segment From Continuing Operations | $ | 23,006 | 21,574 | 7,207 | 6,991 | (402) | (592) | 29,811 | 27,973 | ||||||||||
|
|
|
|
|
|
|
|
| ||||||||||||
| *Estimated as of 1/23/2024 | |||||||||||||||||||
| As Reported Research and Development Expense | $ | 11,963 | 11,642 | 3,122 | 2,493 | 15,085 | 14,135 | ||||||||||||
|
|
|
|
|
|
| ||||||||||||||
| Cost of products sold | $ | 6,798 | (1,131) | (83) | (42) | (12) | - | - | 5,530 | ||||||||||||||||
| Selling, marketing and admin expenses |
| 5,810 | (8) | 5,802 | |||||||||||||||||||||
| Research and development expense |
| 4,480 | (16) | (38) | (1) | 4,425 | |||||||||||||||||||
| Other (Income) / Expense |
| (421) | (17) | (166) | - | (221) | 435 | 3 | - | (387) | |||||||||||||||
| In-process research and development impairments |
| 58 | (58) | - | |||||||||||||||||||||
| Restructuring |
| 56 | (56) | - | |||||||||||||||||||||
| Provision for taxes on income |
| 694 | 175 | (134) | 13 | 16 | 30 | (191) | 16 | - | 56 | - | 675 | ||||||||||||
| Net Earnings from Continuing Operations |
| 4,132 | 973 | 300 | 45 | 123 | 207 | (244) | 72 | 10 | (56) | - | 5,562 | ||||||||||||
| Cost of products sold | $ | 6,084 | (977) | (25) | (33) | (160) | - | - | 4,889 | ||||||||||||||||
| Selling, marketing and admin expenses |
| 5,339 | (9) | 5,330 | |||||||||||||||||||||
| Research and development expense |
| 3,710 | - | (46) | (114) | 3,550 | |||||||||||||||||||
| Other (Income) / Expense |
| 795 | - | (262) | (19) | (196) | (6) | (547) | - | (235) | |||||||||||||||
| In-process research and development impairments |
| 173 | (173) | - | |||||||||||||||||||||
| Restructuring |
| 75 | (75) | - | |||||||||||||||||||||
| Provision for taxes on income |
| 613 | 148 | (36) | 40 | 19 | 5 | 2 | 17 | 199 | 43 | - | 1,050 | ||||||||||||
| Net Earnings from Continuing Operations |
| 3,227 | 829 | 298 | 133 | 100 | 191 | 4 | 71 | 622 | (43) | - | 5,432 | ||||||||||||
| Cost of products sold | $ | 26,553 | (4,511) | (309) | (133) | (189) | 21,411 | ||||||||||||||||||
| Selling, marketing and admin expenses |
| 21,512 | (29) | - | 21,483 | ||||||||||||||||||||
| Research and development expense |
| 15,085 | (32) | (149) | (99) | 14,805 | |||||||||||||||||||
| Other (Income) / Expense |
| 6,634 | (21) | (7,152) | - | (307) | (641) | (375) | - | (1,862) | |||||||||||||||
| In-process research and development impairments |
| 313 | (313) | - | - | - | - | - | |||||||||||||||||
| Restructuring |
| 489 | (489) | - | |||||||||||||||||||||
| Provision for taxes on income |
| 1,736 | 707 | 1,505 | 70 | 157 | 52 | (9) | 57 | 155 | (28) | - | 4,402 | ||||||||||||
| Net Earnings from Continuing Operations |
| 13,326 | 3,825 | 5,647 | 243 | 641 | 287 | 650 | 254 | 508 | 28 | - | 25,409 | ||||||||||||
| Cost of products sold | $ | 24,596 | (3,944) | (62) | (109) | (456) | 20,025 | ||||||||||||||||||
| Selling, marketing and admin expenses |
| 20,246 | (28) | 20,218 | |||||||||||||||||||||
| Research and development expense |
| 14,135 | - | (159) | (304) | 13,672 | |||||||||||||||||||
| Other (Income) / Expense |
| 810 | - | (866) | (35) | (196) | (690) | (714) | 7 | (1,684) | |||||||||||||||
| In-process research and development impairments |
| 783 | (783) | - | - | ||||||||||||||||||||
| Restructuring |
| 275 | (275) | - | |||||||||||||||||||||
| Provision for taxes on income |
| 2,989 | 590 | (125) | 178 | 66 | 5 | 166 | 56 | 360 | (106) | (2) | 4,177 | ||||||||||||
| Net Earnings from Continuing Operations |
| 16,370 | 3,354 | 991 | 605 | 306 | 191 | 524 | 240 | 1,114 | 106 | (5) | 23,796 | ||||||||||||
Media contact: Tesia Williams [email protected] Investor contact: Jessica Moore [email protected]
Source: Johnson & Johnson
Multimedia Files:
Contact Investor Relations
Questions please contact us:.

Online Help
Our 24/7 cancer helpline provides information and answers for people dealing with cancer. We can connect you with trained cancer information specialists who will answer questions about a cancer diagnosis and provide guidance and a compassionate ear.
Chat live online
Select the Live Chat button at the bottom of the page
Call us at 1-800-227-2345
Available any time of day or night
Our highly trained specialists are available 24/7 via phone and on weekdays can assist through online chat. We connect patients, caregivers, and family members with essential services and resources at every step of their cancer journey. Ask us how you can get involved and support the fight against cancer. Some of the topics we can assist with include:
- Referrals to patient-related programs or resources
- Donations, website, or event-related assistance
- Tobacco-related topics
- Volunteer opportunities
- Cancer Information
For medical questions, we encourage you to review our information with your doctor.
Cancer Facts and Statistics
- 2024 Cancer Facts & Figures
- 2023 Cancer Facts & Figures
- 2022 Cancer Facts & Figures
- 2021 Cancer Facts & Figures
- 2020 Cancer Facts & Figures
- 2019 Cancer Facts & Figures
- 2018 Cancer Facts & Figures
- 2017 Cancer Facts & Figures
- 2016 Cancer Facts & Figures
- 2015 Cancer Facts & Figures
- 2014 Cancer Facts & Figures
- 2013 Cancer Facts & Figures
- 2012 Cancer Facts & Figures
- 2011 Cancer Facts & Figures
- 2010 Cancer Facts & Figures
- 2009 Cancer Facts & Figures
- 2008 Cancer Facts & Figures
- 2007 Cancer Facts & Figures
- Colorectal Cancer Facts & Figures
- Breast Cancer Facts & Figures
- Cancer Facts & Figures for Hispanic and Latino People
- Cancer Facts & Figures for African American/Black People
- Cancer Facts & Figures AANHPI
- Cancer Prevention & Early Detection Facts & Figures
- Cancer Treatment & Survivorship Facts & Figures
- Global Cancer Facts & Figures
- The Global Burden of Cancer in Women
Cancer Facts & Figures 2023

Cancer Facts & Figures 2023 is an educational companion for Cancer Statistics 2023, a scientific paper published in the American Cancer Society journal, CA: A Cancer Journal for Clinicians . These annual reports provide:
- Estimated numbers of new cancer cases and deaths in 2023 by cancer site and US state
- Current cancer incidence, mortality, and survival statistics
- Information on cancer symptoms, risk factors, early detection, and treatment
Also see this news story: Incidence Rate Drops for Cervical Cancer But Rises for Prostate Cancer
2023 Special Section: Lung Cancer
This special section provides an overview of lung cancer occurrence in the united states, including information about risk factors, prevention, and early detection, as well as what the american cancer society is doing to reduce the burden..

Download the PDF
Cancer Facts & Figures Report 2023
Special Section Lung Cancer

Download PowerPoint Presentation
Slide Set: Annual Cancer Facts & Figures 2023
Present up-to-date information and statistics about cancer like an expert with this PowerPoint slide set of graphics and tables, complete with explanatory text.
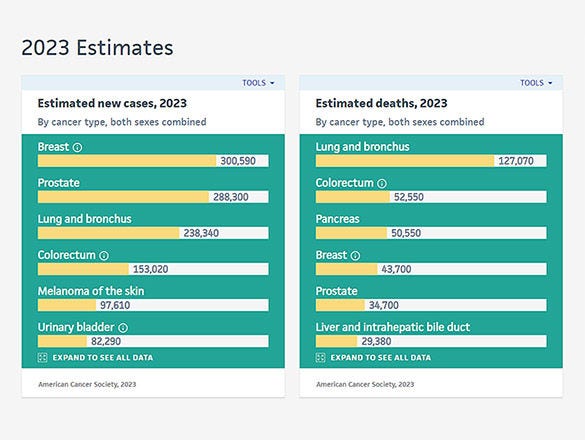
All Cancer I ACS Statistics Center
The 2023 annual report provides an estimated numbers of new cancer cases, deaths, survivors and information on prevention, early detection & treatment.

Most requested Tables & Figures
Trends in Age-adjusted Cancer Death Rates by Site, US, 1930-2020
- For Males (PDF)
- For Females (PDF)
Estimated Number of New Cancer Cases and Deaths, US, 2023
- By Sex (PDF)
Estimated Number for Selected Cancers by State, US, 2023 (PDF)
- Of New Cases (PDF)
- Of Deaths (PDF)
Leading Sites of New Cancer Cases and Deaths - 2023 Estimates (PDF)
Probability of Developing Invasive Cancer During Selected Age Intervals by Sex, US, 2017-2019 (PDF)
Incidence and Mortality Rates for Selected Cancers by Race and Ethnicity, US, 2015-2020 (PDF)
2023 Supplemental Data
Estimated Number for the 4 Major Cancers by Sex & Age Group, 2023
- Of New Cases (PDF)
- Of Deaths (PDF)
Estimated Number of New Cases & Deaths by State for 21 Cancer Sites, 2023 (PDF)
Lifetime Probability of Developing & Dying from Cancer for 23 Sites, 2017-2019 (PDF)
See Highlights of More ACS Cancer Research
Find links to research from ACS staff and grantees about the different cancer types, cancer disparities, and healthy eating and activity.
Glossary for Nonscientists
Featured term: cancer incidence (also called cancer occurrence ).
The number of new diagnoses of cancer, or new cases, in a group. Incidence counts can give information about a specific group but can’t be used to compare groups because the numbers alone don’t account for the size of the group or age ranges of the people in it. Comparing groups requires cancer incidence rates.
Citations, Credits, & Permissions
Suggested citation: Cancer Facts & Figures 2023 . Atlanta: American Cancer Society, Inc. 2022.
Please note that any reproduction or re-use of this publication or portions of it should credit the appropriate edition of the American Cancer Society Annual Cancer Facts & Figures publication. See the PDF contents page for more copyright info and permissions for use.
Help us end cancer as we know it, for everyone.

- Skip to Content
- Skip to Main Navigation
- Skip to Search

NSSE NSSE NSSE

- Conceptual Framework
- Positions and Policies
- Topical Modules
- Engagement Indicators
- High-Impact Practices
- Campus Contacts
- Administration Checklist
- Population File
- Customizing NSSE
- Recruitment Method
- Encouraging Participation
- Terms of Participation
- Accessibility
- Data Security
- NSSE Administration Overview
- Sample Report
- Institutional Report Guide
- Report Builder
- Data Summaries & Interactive Displays
- NSSE Data User’s Guide
- Data Codebooks
- Response Rates
- FSSE Portal Log-in
- FSSE Scales
- Disciplinary Areas
- Consortium Questions
- Admin Preparation
- Confidentiality
- Administration Customization
- Institutional Review Board (IRB)
- Locating Your Data & Results
- Administration Summary
- FSSE Respondent Profile
- FSSE Topical Module Report
- FSSE Disciplinary Area Report
- FSSE-NSSE Combined Report
- FSSE Frequency Report
- FSSE Snapshot Report
- FSSE Overview
- Content Summaries
- Data Visualizations
- Data Use Examples
- Analysis Resources
- Data User's Guide
- Psychometric Portfolio
- About BCSSE
- BCSSE Scales
- New Student Check-In
- Registration & Pricing
- Administration Protocol and Procedures
- BCSSE Contacts
- Demonstration Dashboard Portal
- Institution Participation Agreement
- Data Security and Accessibility
- BCSSE Overview
- Accessing BCSSE Data
- Summary Tables
- Additional Resources
- Dashboard Log-in
- NSSE Institute
- Institution Examples
- NSSE Data Use in Brief
- Displaying Results
- Data Use Teams
- Search for examples
- Participating Institution Search
- NSSE Channel
- Tips for More Inclusive Data Sharing and Analysis
- Accreditation Toolkits
- Campuswide Mapping
- Sharing and Disseminating NSSE Results
- Effective Educational Practice Documented
- Contextualizing NSSE Effect Sizes
- Custom Analysis
Annual Results 2023
- 1. Rebounding Engagement
- 2. Digging Deeper Into HIP Quality
- 3. Hot Topics in Higher Ed
- Past Annual Results
- Foundational Publications
- Featured Publications
- Recent Presentations
- Lessons from the Field
- DEEP Practice Briefs
- Special Projects
- NSSE Essentials
- Search Posts
- Institution Login
Our Research: Projects, Publications, and More
- Annual Results
Engagement Insights: Survey Findings on the Quality of Undergraduate Education
Story features, a note from our director.
- Complex Identity Demographics & Relationship-Building Aspects of Engagement
Some LGBTQ+ Students More Likely to Participate in Undergraduate Research
- Institution Story (CSULB): Using NSSE’s Expanded Student Identity Categories
Welcome to the 202 3 edition of Engagement Insights , NSSE’s annual dissemination of selected research findings and institutional stories that have broad relevance to the improvement of undergraduate education.
This year’s Annual Results focuses on the theme: Engaging Students in Relationship-Rich Education. Below, you will find the main story , some related follow-up analyses, and an example of institutional use. We also plan to roll out new “Special Reports” that dive deeper into other content throughout the course of 2024 , so stay tuned for more NSSE findings!
I am excited to offer my first introduction as NSSE director, to our 2023 Annual Results. As an undergraduate student, I was the beneficiary of deliberate engagement efforts and had the fortune of participating in High-Impact Practices that enriched my educational experiences. As a practitioner, I spent years facilitating HIPs and working to ensure they were enacted in quality ways, and their benefits were widely accessible. As a scholar, I work to interrogate, refine, and amplify the impact of quality educational experiences like HIPs by empowering the people who deliver them. For these reasons I am excited and well-positioned to chart the future of NSSE, working alongside my NSSE colleagues to provide campuses across North America with sound assessment, quality data, and useful insight into students’ academic and co-curricular experiences.

This year, we are especially focused on the role of relationships in student undergraduate experiences, and the importance of those relationships in their overall learning, development, and success.
Meaningful connections are central to our humanity, of which interpersonal relationships are paramount. This year, we are especially focused on the role of relationships in student undergraduate experiences, and the importance of those relationships in their overall learning, development, and success. Felten and Lambert talk about this as Relationship-Rich Education , which offers a label to capture what staff, administrators, faculty members and scholars across the postsecondary landscape have known to be true from the beginning – relationships matter.
Key, but perhaps understated, in the concept of engagement are students’ relationships; relationships to the experiences they have in college, and to the people that support them along the way. NSSE offers insight into the impact of those relationships, underscoring the importance of interpersonal relationships (whether with peers or staff) to students’ academic success. The following spotlights from our 2023 Annual Results are some of many examples of NSSE’s commitment to providing campus with high quality data, our commitment to support equitable student experiences and outcomes, and the ways we are charting new paths forward as we think about survey design that seeks to reflect, rather than enforce, students’ identities and expressions. ♦
Using Complex Identity Demographics to Explore Relationship-Building Aspects of Engagement

For this year’s Annual Results, we would like to expand on Felten and Lambert’s (2020) conception of “ Relationship-Rich Education ” to think about how identities can interact and influence the various relationships students form during their higher education experience.
As student populations are incredibly diverse even within institutions, examining educational quality and student experiences means we must look at subgroups in the data we collect. This work is critical to our understanding of student engagement, allowing us to learn about growing student subpopulations, find areas of inequity, close gaps in achievement, and redefine our conceptualizations of student success. Some aspects of the respondent experience can be relatively easy to capture in a survey question, but others, particularly questions about identity, can be a challenge. Some of these challenges include students' ongoing identity development and exploration, evolving conception and language related to identity, and the potential political and material consequences for certain identity groups. Socioeconomic status, for example, is a broad concept of one’s access to financial, social, cultural, and human capital resources, but it is frequently simplified to questions about first-generation status, family wealth, or eligibility for federal grants.
NSSE staff have invested in continuous improvements to the survey instrument to allow for more inclusive explorations of engagement data. Some examples of complex identity items that have been recently updated are racial/ethnic identification, gender identity, sexual orientation, and reported disabilities or conditions that impact learning, working, or living activities. To be sensitive to the fluid and contextual nature of identity, NSSE respondents can choose multiple components of an aspect of identity. Prioritizing this benefit to respondents, however, can create difficulties for analysis and understanding of the data. These difficulties are a function, and opportunity to push the limits, of traditional strategies for analysis and representation of quantitative data.
The following vignettes highlight what we can learn from taking advantage of the complexity of this data and digging deeper into traditionally aggregated groups of students. We will first look at what we can learn about relationship-building aspects of engagement for students commonly grouped as bi- or multi-racial when we disaggregate by common combinations of selected racial/ethnic heritage. Next, we will look at sense of belonging for students with differing conditions or disabilities that impact their learning, working, or living activities. Finally, we will combine what we learned in these explorations to further examine belonging for students with intersections of these aspects of identity.
Complicating Bi-/Multi-Racial Categories
In the 2023 NSSE administration , 18,621 (10%) first-year and senior students in the U.S. identified with more than one racial/ethnic identification. One might be tempted to report and explore experiences for a combined category of students who selected more than one identification, particularly if institutions have a relatively small number of such students, but we can learn so much more about this diverse group of students with a closer look.
Just a simple examination of common pairs or trios of identity (Table 1) reveals how diverse this group of students really is.
|
| Subgroups of Students Who Chose More Than One Racial/Ethnic Identification |
| Latine + White | 5994 | 35.3 |
| Asian + White | 2703 | 15.9 |
| Black + White | 1746 | 10.3 |
| Indigenous + White | 1198 | 7.0 |
| Black + Latine | 1064 | 6.3 |
| MENA + White | 661 | 3.9 |
| White + another | 413 | 2.4 |
| Indigenous + Latine | 411 | 2.4 |
| Asian + Latine | 351 | 2.1 |
| NHPI + White | 340 | 2.0 |
| Indigenous + Latine + White | 295 | 1.7 |
| Asian + NHPI | 270 | 1.6 |
| Asian + Black | 267 | 1.6 |
| Black + Indigenous | 247 | 1.5 |
| Black + Latine + White | 224 | 1.3 |
| Asian + NHPI + White | 213 | 1.3 |
| Black + Indigenous + White | 177 | 1.0 |
| Black + another | 172 | 1.0 |
| Asian + Latine + White | 146 | 0.9 |
| Asian + another | 104 | 0.6 |
| Note: MENA = Middle Eastern or North African, NHPI = Native Hawaiian or Pacific Islander |
, , , , , and are all examples of relationship-building aspects of engagement. Positive relationships with peers, faculty, and staff are of benefit to students academically, socioemotionally, and professionally. Traditionally marginalized students can benefit the most from supportive relationships with others providing campuses that center relationship-based learning with a mechanism for fostering more equitable student experiences. |
By dividing select relationship-building aspects of engagement into terciles, we can see how these subgroups of students distribute across low, moderate, or high amounts of engagement. Examining these relationships with ꭓ 2 ( p < .001) tests and adjusted standardized residuals (ASR ≥ 2) as an effect size criterion allows us to see notable patterns in how these students experience relationship-building aspects of engagement. (See Harris et al., 2018 for another example of how this could be done.)
In Table 2 we can see subgroups of these students who experience disproportionately high select relationship-building aspects of engagement and some that experience disproportionately less. For example, we see that students who identify with Asian + White, NHPI + White, Asian + NHPI, or Asian + NHPI + White engage in a disproportionately high amount of Collaborative Learning . More simply, although students with these identities may engage in low or moderate amounts of Collaborative Learning , more often than not, they tend to do noticeably more. Instead of trying to understand the experiences of students who select more than one racial/ethnic identification as a single aggregate group, we can see that experiences within vary greatly and more inclusive aggregations could be made by looking for patterns in engagement or other outcomes of interest.
Reflective & Integrative Learning | Asian + White, Asian + Latine | MENA + White, White + another, Indigenous + Latine |
| Collaborative Learning | Black + White, Indigenous + White, Indigenous + Latine, Black + Indigenous + White, Black + another | Asian + White, NHPI + White, Asian + NHPI, Asian + NHPI + White |
| Student-Faculty Interaction | Latine + White, Asian + White, | Black + Latine, Black + Indigenous, Black + Indigenous + White, Black + another |
| Quality of Interactions | Black + Latine, MENA + White, White + another, Black + another | Latine + White, Indigenous + Latine + White |
| Supportive Environment | White + another, Indigenous + Latine | Latine + White |
| Sense of Belonging | Black + Latine, White + another, Indigenous + Latine + White, Black + another | Latine + White |
| Note: MENA = Middle Eastern or North African, NHPI = Native Hawaiian or Pacific Islander |
Another Layer of Complexity
To understand the experiences of a whole student, when possible, we need to consider student identity beyond a single demographic. This can be an incredibly daunting task! But taking even small steps to integrate other information about student experiences can help to form a clearer picture of complex situations. In 2023 , 38,619 (22%) of the first-year and senior students in the U.S. who responded to NSSE have a disability or condition that impacts their learning, working, or living activities.
response is yes, respondents receive the following:
Sensory disability Physical disability Mental health or developmental disability Another disability or condition |
The context of the disability or condition , however, can impact students in vastly diverse ways . Additionally, nearly t hree out of four (74%) of these s tudents have multiple disabilities or conditions that affect their experiences , further complicating matters . Looking at students with disabilities in the aggregate will likely hide important differences in experience . Expansive disaggregation would be idea l , but looking within general subcategories, such as sensory or phy sical disabilities, can be a good start.
Focusing on one of our relationship-building aspects of engagement, Sense of Belonging , students with sensory disabilities, physical disabilities , and disabilities or conditions such as chronic medical conditions or learning disabilities feel disproportionately greater sense of belonging ( ꭓ 2 ( 8 ; 37,964) = 234.3, p < .001 ) but students with mental health o r developmental disabilities or multiple disabilities or conditions across these broad categories feel disproportionately lower sense of belonging. These findings could signal that institution s are more equipped or motivated to create inclusive spaces for students with sensory or physical disabilities and chronic medical conditions. It could also indicate inclusion efforts focus on more obvious disabilities as opposed to disabilities that may be less apparent such as developmental disabilities and those related to mental health .

We previously found that students who identified as Black + Latine , White + another, Indigenous + Latine + White, or Black + another feel disproportionately less belonging at their institution and that students with mental health or developmental disabilities feel similarly . Combining these aspects of identity, we see that students with these select racial/ethnic identifications and mental health or developmental disabilities feel even less belonging than their peers (Figure 1 ).
These are certainly not the only ways to explore data through disaggregation or intersecting identity lenses , but they are a simple way to start. Understanding engagement experiences through identity can be complex and intimidating , but small steps are better than none . W e can often learn more about unique student experiences when we question the norms of our data practice s , whether that involves the ways we aggregate or disaggregate data or how we often ask questions of our data using one demographic at a time.
Questions to Consider
Often the most compelling findings result in more questions. Here we offer a series of questions to consider with your own institutional data:
- How inclusive is the student demographic data you collect?
- Do findings on your campus mirror those found here?
- What other aspects of student identity can you use to add layers of complexity to your findings?
- Are counseling services and disability services prepared to support students with complex racial/ethnic identities?
- Are racial/ethnic cultural centers prepared to support students with mental health or other disability concerns?
- How can efforts to increase sense of belonging or other relationship-building engagement, such as those embedded in the outcomes we feature here, incorporate understandings of multiple aspects of student identity? ♦
Back to Top

Participation with faculty in undergraduate r esearch (U G R) is invaluable for academic development, fostering critical thinking, and providing mentorship opportunities. However, for LGBTQ+ students, these collaborations can be both empowering and challenging due to the social dynamics and inherent biases present in academic environments.
For example, Woodford and Kulick (2015) observed that the perceptions and experiences of student s identifying as lesbian, gay, bisexual, or transgender affected their academic and social integration within the campus. However, Kilgo et al . ( 2019 ) found that among all high-impact practices, only undergraduate research positively predicted LGBTQ+ students’ academic development . They further concluded that it was students’ relationship s with the instructors that mediated the difference.
NSSE's Undergraduate Research Question
- Done or in progress
- Plan to do
- Do not plan to do
- Have not decided
A few years ago , NSSE found that —percentagewise—many more LGBT Q + seniors ( about a third) participated in UG R , compared to a quarter of their straight peers. Similarly, around a third of seniors who d id not identify with the man/woman gender binary participated in UG R , compared to around a quarter of seniors identifying as men or women (Kinzie & BrckaLorenz, 2021).
Now i n 2023, w ith NSSE’s recently expanded and more inclusive gender identity and sexual orientation options , a detailed revisiting of these results is possible , and the same overall pattern s emerge . For example, a third of seniors who identified as “ genderqueer, non-binary, or gender nonconforming ” and “ trans / transgender ” participated in UGR , compared to a quarter of those who identified as “ woman ” or “ man ” (Figure 2 ) . Participation by sexual orientation reveals even larger gaps. F ully 37% of “ q ueer” senior s participated in research with faculty, and “demisexual,” “bisexual,” and others were also substantially more likely to participate than their “straight or heterosexual” peers (23%) (Figure 3 ).

Note: N is the number of seniors within the category who responded to the undergraduate research question. Students could select more than one category.

On the face of it , t hese results are encouraging for students who often find campus environments to be challenging (Woodford & Kulick, 2015) . V iewed alon g side the findings of Kilgo et al . (2019) , they offer support to NSSE’s assertion that the quality of high-impact practices depends on how well they are designed and facilitated . Yet, questions remain as to the reasons for these positive results. Do LGBTQ+ students find more welcoming spaces with faculty? Are they shut out of opportunities in other areas , leading them to pursue majors that offer more research experiences ? Are students who identify as genderqueer, trans, etc. more likely to be “research-oriented” with the inquisitiveness , scholarly approach, and inclination to study unanswered questions ?
D espite these ongoing questions , engaging in UGR can nevertheless provide opportunities for empowerment , advocacy , and academic development through more sustained and engaging student-instructor relationships, where as , according to Kilgo et al. (2019) , other high-impact practices may not . For instance, supportive and inclusive mentors can serve as allies, creating a better climate for these students to express their identities and contribute meaningfully to research projects. More work sh ould be done to explore how other high-impact practices are implemented and their potential to support LGBTQ+ populations. ♦
Institution Story (CSULB): Using NSSE’s Expanded Student Identity Categories for More Tailored Results

Though college students' mental health and wellness has been a concern for many years, it has recently emerged as a widespread problem that can seriously impact academic success. To inform the enhancement of institutional support for student’s mental health, California State University, Long Beach (CSULB) is reviewing a range of data to gain a more nuanced sense of students’ access to campus resources and impressions of services. One of their data sources is NSSE’s Mental Health & Well-Being Topical Module . Gary Coyne, Associate Director for Assessment and Evaluation in Student Affairs, has been disaggregating the CSULB data by standard demographic items including gender, first-generation status, and major, and plans to use NSSE’s expanded student identity items to further explore their data through disaggregated ranking. Specifically, results regarding who students identify as most supportive of their mental health and well-being and their awareness of a range of sources of help on campus will be ranked within subgroups. Knowing more about sources of support by identity groups can lend insights into fostering more productive relationships and tailored support offerings. The use of NSSE’s more expansive student identity categories, coupled with analytic approaches that preserve small sub-groups, can help reveal salient variation among student populations and inform the development of customized support.
Let us know how your institution plans to use the updated student identity items!
Email NSSE
Felton, P. & Lambert, L. M. (2020). Relationship-rich education: How human connections drive success in college . John Hopkins University Press.
Harris, J. C., BrckaLorenz, A., & Nelson Laird, T. (2018). Engaging in the margins: Exploring differences in biracial students’ engagement by racial heritage. Journal of Student Affairs Research and Practice , 58 (2), 137-154. https://doi.org/10.1080/19496591.2018.1406364
Kilgo, C. A., Linley, J. L., Renn, K. A., & Woodford, M. R. (2019). High-impact for whom? The influence of environment and identity on lesbian, gay, bisexual, and queer college students' participation in high-impact practices. Journal of College Student Development , 60 (4), 421-436. https://doi.org/10.1353/csd.2019.0038
Kinzie, J., & BrckaLorenz, A. (2021). Expectations for and Quality Experiences in Undergraduate Research Over Time: Perspectives of Students and Faculty. Journal of the Scholarship of Teaching and Learning , 21 (1). https://doi.org/10.14434/josotl.v21i1.30842
Woodford, M. R., & Kulick, A. (2015). Academic and social integration on campus among sexual minority students: The impacts of psychological and experiential campus climate. American Journal of Community Psychology, 55, 13-24. https://doi.org/10.1007/s10464-014-9683-x
Banner Image: Saint Paul University
Background images: St. Johns' University New York, Southeast Missouri State University, Cedar Crest College
Evidence-Based Improvement in Higher Education resources and social media channels
Evidence-Based Improvement in Higher Education
Center for Postsecondary Research Indiana University School of Education 201 N. Rose Avenue Bloomington, IN 47405-1006 Phone: 812.856.5824 Email: [email protected]
FinancialResearch.gov

2023 Annual Report to Congress
The 2023 OFR Annual Report discusses our assessment of risks associated with the U.S. financial system and the performance of the OFR.
Financial stability risks have increased since last year's report and remain elevated in 2023. Multiple indicators signal an upcoming economic slowdown—potentially magnified by persistent inflation, ongoing geopolitical risks, and global conflicts.
- Download Full Report
- Summary of Risks
- Press Release
- Status of the OFR
From the Office of the Director
It is my pleasure to deliver the Office of Financial Research's 2023 Annual Report to Congress. Approaching my second year as Acting Director of the OFR, I continue to lead the talented and dedicated OFR staff with a principal focus on supporting the Financial Stability Oversight Council (Council) and its member agencies.
As noted in this year's report, the information we cover describes our research and analysis as of September 30, 2023, the end of the fiscal year (FY). In an ever-changing environment, however, we recognize that much has evolved since that time. The OFR will continue to monitor and analyze risks to financial stability, remaining agile to identify and examine emerging threats as they arise now and in the future...
Read the Director's letter
Report Highlights
Economic indicators.
- To manage core inflation, the Federal Reserve and other central banks are intent on keeping policy rates higher for longer. This policy posture has the effect of increasing borrowing costs for both companies and households, potentially dampening economic growth.
- Credit risks have built up in the commercial real estate (CRE) sector as borrowing rates have increased, pushing valuations significantly lower. Of particular concern is the decline in valuations of office space, as vacancy rates have increased following the rise of the work-from-home (WFH) trend.
- As labor markets remain tight, consumer spending and liquidity remain resilient, but consumer debt has risen while household savings have declined. This is particularly true for households with weaker credit. Delinquencies for certain segments have reverted to prepandemic levels, though they remain within historically low ranges overall.
Financial Institutions
- Several regional banking institutions failed or self-liquidated in the first half of 2023—largely due to an influx of deposits during the pandemic, followed by the banks’ failure to manage interest rate risks as financial conditions reversed.
- The property insurance sector is facing unprecedented stress that is expected to continue for an extended period. While P&C insurers have benefited from increased investment income from rising interest rates, this benefit has often been offset by rapidly rising claims costs, especially in property-exposed lines such as homeowners' insurance. While insurers may have been able to pass some of their increased costs on to consumers, some insurers have instead opted to exit certain states more prone to natural catastrophes.
- The level of Treasury market implied volatility exceeded those seen in March 2020—when a flight to cash led to the unwinding of positions to meet margin payments, which put more downward pressure on Treasury prices, thus increasing Treasury yields.
Financial Markets
- Higher rates and the Federal Reserve’s quantitative tightening have been accompanied by volatility in the bond and equity markets.
- Banks experienced a large-scale outflow of deposits, with much of the funds going into MMFs and other passive investment vehicles.
- Banks also provide substantial lending to small and medium-sized companies, and tighter credit conditions as banks curtail lending can potentially destabilize such companies with weaker balance sheets. Similar trends exist in the leveraged loan markets, where borrowing costs have risen sharply during a period of weaker earnings growth.
Digital Assets & Cybersecurity
- Over the past year, turmoil in the digital assets markets has exposed and even increased the high level of interconnectedness between digital asset firms and traditional markets, highlighting the impact of digital assets on financial institutions.
- The percentage of organizations affected by ransomware has risen from 79% to 87% in 2023. This surge in ransomware attacks has resulted in the highest proportion of data breaches in the financial services industry since 2018.
You are now leaving the OFR’s website.
You will be redirected to:
You are now leaving the OFR Website. The website associated with the link you have selected is located on another server and is not subject to Federal information quality, privacy, security, and related guidelines. To remain on the OFR Website, click 'Cancel'. To continue to the other website you selected, click 'Proceed'. The OFR does not endorse this other website, its sponsor, or any of the views, activities, products, or services offered on the website or by any advertiser on the website.
Thank you for visiting www.financialresearch.gov.
What can we help you find?
Merck announces fourth-quarter and full-year 2023 financial results.
February 1, 2024 6:30 am ET
- Fourth-Quarter and Full-Year Sales Reflect Sustained Growth Across Oncology and Vaccines
- Fourth-Quarter Worldwide Sales Were $14.6 Billion, an Increase of 6% From Fourth Quarter 2022; Excluding LAGEVRIO, Growth Was 11%; Excluding LAGEVRIO and the Impact of Foreign Exchange, Growth Was 13%
- Fourth-Quarter GAAP Loss per Share Was $0.48; Non-GAAP EPS Was $0.03; GAAP Loss per Share and Non-GAAP EPS Include a Charge of $1.69 per Share for a Collaboration With Daiichi Sankyo
- KEYTRUDA Sales Grew 19% to $25.0 Billion; Excluding the Impact of Foreign Exchange, Sales Grew 21%
- GARDASIL/GARDASIL 9 Sales Grew 29% to $8.9 Billion; Excluding the Impact of Foreign Exchange, Sales Grew 33%
- LAGEVRIO Sales Declined 75% to $1.4 Billion; Excluding the Impact of Foreign Exchange, Sales Declined 74%
- Full-Year 2023 GAAP EPS Was $0.14; Non-GAAP EPS Was $1.51; GAAP and Non-GAAP EPS Include Charges of $6.21 per Share for Certain Business Development Transactions
- Obtained FDA Priority Review of Biologics License Applications for V116, an Investigational Pneumococcal Conjugate Vaccine, as Well as Merck and Daiichi Sankyo’s Patritumab Deruxtecan, in the Fourth Quarter
- Received Multiple FDA Approvals Across Oncology Portfolio in 2023
- Initiated More Than 20 Phase 3 Study Starts, Including the Progression of Eight Novel Assets Into Phase 3 in 2023
- Augmented Pipeline Through Acquisitions of Prometheus and Imago, and Collaboration Agreements With Daiichi Sankyo and Kelun-Biotech in 2023
- Anticipates Worldwide Sales To Be Between $62.7 Billion and $64.2 Billion
- Expects Non-GAAP EPS To Be Between $8.44 and $8.59
RAHWAY, N.J.--(BUSINESS WIRE)-- Merck (NYSE: MRK), known as MSD outside the United States and Canada, today announced financial results for the fourth quarter and full year of 2023.
“2023 was another very strong year for Merck. I am extremely pleased by the progress we’ve made to develop and deliver transformative therapies and vaccines that will help save and improve lives around the world. We reached more than 500 million people with our medicines last year alone, over half of which were donations, including through our program to treat river blindness,” said Robert M. Davis, chairman and chief executive officer, Merck. “We also made investments of approximately $30 billion in research and development in our ongoing effort to discover, develop and collaborate to propel the next generation of impactful innovations. As we move forward, I’m confident that our strong momentum will continue, underpinned by the unwavering dedication of our talented global team.”
Financial Summary
| $ in millions, except EPS amounts |
|
| ||||||
|
|
|
|
|
|
|
|
| |
| Sales | $14,630 | $13,830 | 6% | 7% | $60,115 | $59,283 | 1% | 4% |
| GAAP net (loss) income | (1,226) | 3,017 | N/M | N/M | 365 | 14,519 | -97% | -95% |
| Non-GAAP net income that excludes certain items | 66 | 4,129 | -98% | N/M | 3,837 | 19,005 | -80% | -75% |
| GAAP EPS | (0.48) | 1.18 | N/M | N/M | 0.14 | 5.71 | -98% | -95% |
| Non-GAAP EPS that excludes certain items | 0.03 | 1.62 | -98% | N/M | 1.51 | 7.48 | -80% | -75% |
| *Refer to table on page 9. N/M - Not meaningful | ||||||||
Generally Accepted Accounting Principles (GAAP) loss/earnings per share (EPS) assuming dilution was a loss per share of $0.48 for the fourth quarter and EPS of $0.14 for the full year of 2023. Non-GAAP EPS was $0.03 for the fourth quarter and $1.51 for the full year of 2023. GAAP loss per share and non-GAAP EPS in the fourth quarter of 2023 include a charge of $1.69 per share related to the collaboration with Daiichi Sankyo. GAAP and non-GAAP EPS for the full years of 2023 and 2022 include charges of $6.21 and $0.22 per share, respectively, related to certain collaborations, licensing agreements and asset acquisitions.
Non-GAAP EPS excludes acquisition- and divestiture-related costs, including pretax intangible asset impairment research and development (R&D) charges of $779 million in the fourth quarter and full year of 2023 related to gefapixant, and $780 million and $1.7 billion in the fourth quarter and full year of 2022, respectively, primarily related to nemtabrutinib. Non-GAAP EPS also excludes restructuring costs, including costs for the recently approved 2024 Restructuring Program, as well as income and losses from investments in equity securities.
Fourth-Quarter Sales Performance
The following table reflects sales of the company’s top products and significant performance drivers.
|
|
| ||||
| $ in millions |
|
|
|
|
|
| Total Sales | $14,630 | $13,830 | 6% | 7% |
|
| Pharmaceutical | 13,141 | 12,180 | 8% | 8% | Increase driven by growth in oncology, vaccines and hospital acute care, partially offset by a decline in virology, due to LAGEVRIO, and diabetes. Excluding LAGEVRIO and impact of foreign exchange, growth of 14%. |
| KEYTRUDA | 6,608 | 5,450 | 21% | 22% | Growth driven by increased global uptake in earlier-stage indications, including triple-negative breast cancer and renal cell carcinoma (RCC), and continued strong global demand from metastatic indications. |
| GARDASIL/GARDASIL 9 | 1,871 | 1,470 | 27% | 27% | Growth due to strong global demand, particularly in China, and public-sector buying patterns in the U.S. |
| JANUVIA/JANUMET | 787 | 913 | -14% | -13% | Decline primarily due to generic competition in several international markets, particularly in Europe, and lower demand in the U.S. |
| PROQUAD, M-M-R II and VARIVAX | 545 | 526 | 4% | 3% | Growth largely due to higher pricing in the U.S. |
| BRIDION | 429 | 441 | -3% | -3% | Decline primarily due to generic competition in certain ex-U.S. markets, particularly in Europe, partially offset by higher demand in the U.S. |
| Lynparza* | 315 | 292 | 8% | 8% | Growth driven primarily by higher pricing in the U.S. |
| Lenvima* | 226 | 216 | 5% | 5% | Growth primarily due to higher demand in the U.S., partially offset by timing of shipments in China. |
| LAGEVRIO | 193 | 825 | -77% | -76% | Decline due to nonrecurrence of sales in the U.K. and lower demand in Japan and Australia. |
| ROTATEQ | 185 | 139 | 34% | 33% | Growth primarily due to public-sector buying patterns in the U.S. and timing of shipments in China. |
| VAXNEUVANCE | 176 | 138 | 28% | 26% | Growth largely driven by launches in Europe and continued uptake for the pediatric indication in the U.S. Prior-year quarter benefited from inventory stocking in the U.S. in preparation for pediatric launch. |
| Animal Health | 1,278 | 1,230 | 4% | 4% | Growth primarily driven by higher demand for Companion Animal products. |
| Livestock | 808 | 814 | -1% | 0% | Decline primarily due to timing of shipments for ruminant products, largely offset by higher pricing across the product portfolio and higher demand for swine products. |
| Companion Animal | 470 | 416 | 13% | 12% | Growth primarily due to higher demand and timing of shipments for BRAVECTO line of products, as well as higher pricing. Sales of BRAVECTO were $197 million and $168 million in the current and prior-year quarters, respectively, which represented growth of 18%, or 19% excluding the impact of foreign exchange. |
| Other Revenues** | 211 | 420 | -50% | -1% | Decline primarily due to impact of revenue hedging activities. |
| *Alliance revenue for this product represents Merck’s share of profits, which are product sales net of cost of sales and commercialization costs. **Other revenues are comprised primarily of revenues from third-party manufacturing arrangements and miscellaneous corporate revenues, including revenue-hedging activities. | |||||
Full-Year Revenue Performance
The following table reflects sales of the company’s top pharmaceutical products, as well as sales of Animal Health products.
|
|
| |||
| $ in millions |
|
|
|
|
| Total Sales | $60,115 | $59,283 | 1% | 4% |
| Pharmaceutical | 53,583 | 52,005 | 3% | 5% |
| KEYTRUDA | 25,011 | 20,937 | 19% | 21% |
| GARDASIL/GARDASIL 9 | 8,886 | 6,897 | 29% | 33% |
| JANUVIA/JANUMET | 3,366 | 4,513 | -25% | -23% |
| PROQUAD, M-M-R II and VARIVAX | 2,368 | 2,241 | 6% | 6% |
| BRIDION | 1,842 | 1,685 | 9% | 11% |
| LAGEVRIO | 1,428 | 5,684 | -75% | -74% |
| Lynparza* | 1,199 | 1,116 | 7% | 9% |
| Lenvima* | 960 | 876 | 10% | 11% |
| ROTATEQ | 769 | 783 | -2% | -1% |
| VAXNEUVANCE | 665 | 170 | N/M | N/M |
| Animal Health | 5,625 | 5,550 | 1% | 3% |
| Livestock | 3,337 | 3,300 | 1% | 4% |
| Companion Animal | 2,288 | 2,250 | 2% | 3% |
| Other Revenues** | 907 | 1,728 | -48% | -15% |
| *Alliance revenue for this product represents Merck’s share of profits, which are product sales net of cost of sales and commercialization costs. **Other revenues are comprised primarily of revenues from third-party manufacturing arrangements and miscellaneous corporate revenues, including revenue-hedging activities. N/M - Not meaningful | ||||
Full-year 2023 pharmaceutical sales grew 3% to $53.6 billion. Pharmaceutical sales growth was primarily driven by higher sales in oncology, particularly KEYTRUDA, higher sales of vaccines, reflecting strong growth of combined sales of GARDASIL/GARDASIL 9 and VAXNEUVANCE, as well as growth in hospital acute care products, including PREVYMIS and BRIDION. Pharmaceutical sales growth in 2023 was partially offset by lower sales of the COVID-19 medication LAGEVRIO, as well as lower sales of JANUVIA and JANUMET, primarily reflecting generic competition in many ex-U.S. markets and lower demand in the U.S., and lower sales of PNEUMOVAX 23 as the market continues to shift toward newer adult pneumococcal conjugate vaccines. Pharmaceutical sales growth for the full year of 2023 was 14% excluding LAGEVRIO and the unfavorable impact of foreign exchange.
Full-year 2023 Animal Health sales grew 1% to $5.6 billion. Excluding the unfavorable impact of foreign exchange, Animal Health sales grew 3%, primarily due to higher pricing. Full-year sales growth was also driven by higher demand for livestock products, led by poultry and swine products, partially offset by lower demand for ruminant products. Sales of BRAVECTO were $1.1 billion in 2023, which represented growth of 4%, or 5% excluding the impact of foreign exchange, primarily reflecting higher pricing.
Fourth-Quarter and Full-Year Expense, EPS and Related Information
The table below presents selected expense information.
| $ in millions |
|
|
|
|
|
|
| |||||
| Cost of sales | $3,911 | $454 | $117 | $- | $3,340 |
| Selling, general and administrative | 2,804 | 24 | 29 | - | 2,751 |
| Research and development | 9,628 | 790 | - | - | 8,838 |
| Restructuring costs | 255 | - | 255 | - | - |
| Other (income) expense, net | 78 | (35) | - | (61) | 174 |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Cost of sales | $3,881 | $482 | $38 | $- | $3,361 |
| Selling, general and administrative | 2,687 | 39 | 20 | - | 2,628 |
| Research and development | 3,775 | 740 | - | - | 3,035 |
| Restructuring costs | 49 | - | 49 | - | - |
| Other (income) expense, net | (75) | (69) | - | 80 | (86) |
| $ in millions |
|
|
|
|
|
|
|
|
| |||||
| Cost of sales | $16,126 | $2,018 | $211 | $- | $- | $13,897 |
| Selling, general and administrative |
10,504 |
86 |
122 | - | - |
10,296 |
| Research and development | 30,531 | 819 | 1 | - | - | 29,711 |
| Restructuring costs | 599 | - | 599 | - | - | - |
| Other (income) expense, net | 466 | (47) | - | (279) | 573 | 219 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Cost of sales | $17,411 | $2,059 | $205 | $- | $- | $15,147 |
| Selling, general and administrative | 10,042 | 176 | 94 | - | - | 9,772 |
| Research and development | 13,548 | 1,676 | 30 | - | - | 11,842 |
| Restructuring costs | 337 | - | 337 | - | - | - |
| Other (income) expense, net | 1,501 | (207) | - | 1,348 | - | 360 |
GAAP Expense, EPS and Related Information
Gross margin was 73.3% for the fourth quarter of 2023 compared with 71.9% for the fourth quarter of 2022. The increase was primarily due to the favorable impacts of lower LAGEVRIO sales, which have a low gross margin, lower manufacturing facilities costs and product mix, partially offset by the unfavorable impacts of foreign exchange and higher restructuring costs. Gross margin was 73.2% for the full year of 2023 compared with 70.6% for the full year of 2022. The increase was primarily due to the favorable impacts of lower LAGEVRIO sales, product mix, lower manufacturing facilities costs, and lower revenue from third-party manufacturing arrangements, partially offset by the unfavorable impact of foreign exchange.
Selling, general and administrative (SG&A) expenses were $2.8 billion in the fourth quarter of 2023, an increase of 4% compared with the fourth quarter of 2022. The increase was primarily due to higher administrative costs, including higher compensation and benefit costs, partially offset by lower promotional spending. Full-year 2023 SG&A expenses were $10.5 billion, an increase of 5% compared with the full year of 2022. The increase was primarily due to higher administrative costs, including higher compensation and benefit costs, and higher promotional spending, partially offset by the favorable impact of foreign exchange and lower acquisition- and divestiture-related costs.
R&D expenses were $9.6 billion in the fourth quarter of 2023 compared with $3.8 billion in the fourth quarter of 2022. R&D expenses were $30.5 billion for the full year of 2023 compared with $13.5 billion for the full year of 2022. The increase in the fourth quarter and full year of 2023 reflects a $5.5 billion charge for the collaboration with Daiichi Sankyo, and higher development costs due to spending on clinical programs, including newly acquired programs, as well as higher compensation and benefit costs (reflecting in part increased headcount). The increase in R&D expenses for the full year was also due to charges of $11.4 billion in the aggregate for the acquisitions of Prometheus Biosciences, Inc. (Prometheus) and Imago BioSciences, Inc. (Imago). The increase in R&D expenses for the full year was partially offset by lower intangible asset impairment charges in 2023 and charges of $690 million in the aggregate in 2022 for collaboration and licensing agreements with Moderna, Inc. (Moderna), Orna Therapeutics (Orna) and Orion Corporation (Orion).
Other (income) expense, net, was $78 million of expense in the fourth quarter of 2023 compared with $75 million of income in the fourth quarter of 2022, primarily due to higher exchange losses and higher net interest expense, partially offset by net gains from investments in equity securities for the fourth quarter of 2023 compared with net losses from investments in equity securities for the fourth quarter of 2022, and lower pension settlement costs. Other (income) expense, net, was $466 million of expense in the full year of 2023 compared with $1.5 billion of expense in the full year of 2022, primarily due to net gains from investments in equity securities in 2023 compared with net losses from investments in equity securities in 2022, and lower pension settlement costs, partially offset by a $572.5 million charge in 2023 related to settlements with certain plaintiffs in the Zetia antitrust litigation.
The effective tax rate was 40.1% for the fourth quarter of 2023 compared with 14.1% in the fourth quarter of 2022. The effective tax rate for the fourth quarter of 2023 includes a 29.2 percentage point impact resulting from the charge for the Daiichi Sankyo collaboration. The effective tax rate was 80.0% for the full year of 2023 compared with 11.7% for the full year of 2022. The full-year 2023 effective tax rate reflects an aggregate 65.6 percentage point unfavorable impact, resulting from charges for asset acquisitions (for which no tax benefits were recognized) as well as the charge for the Daiichi Sankyo collaboration.
GAAP loss per share was $0.48 for the fourth quarter of 2023 compared with EPS of $1.18 for the fourth quarter of 2022, primarily driven by the charge in 2023 related to the collaboration with Daiichi Sankyo, the unfavorable impact of foreign exchange and higher restructuring costs, partially offset by a beneficial impact from the tax rate and operational strength in the business. GAAP EPS was $0.14 for the full year of 2023 compared with EPS of $5.71 for the full year of 2022. The EPS decline in 2023 was primarily due to higher charges for certain business development transactions, the unfavorable impact of foreign exchange and the tax rate, as well as a charge related to settlements with certain plaintiffs in the Zetia antitrust litigation, partially offset by the beneficial impacts of operational strength in the business, better performance from equity investments and lower intangible asset impairment charges.
Non-GAAP Expense, EPS and Related Information
Non-GAAP gross margin was 77.2% for the fourth quarter of 2023 compared with 75.7% for the fourth quarter of 2022. Non-GAAP gross margin was 76.9% for the full year of 2023 compared with 74.4% for the full year of 2022. The non-GAAP gross margin improvement in the fourth quarter and full year of 2023 was primarily due to the favorable impacts of lower LAGEVRIO sales, which have a low gross margin, product mix, and lower manufacturing facilities costs. The increase in non-GAAP gross margin for the full year was also due to lower revenue from third-party manufacturing arrangements. The non-GAAP gross margin improvement in the fourth quarter and full year of 2023 was partially offset by the unfavorable impact of foreign exchange.
Non-GAAP SG&A expenses were $2.8 billion for the fourth quarter of 2023 compared with $2.6 billion for the fourth quarter of 2022. The increase was primarily due to higher administrative costs, including higher compensation and benefit costs, partially offset by lower promotional spending. Full-year 2023 non-GAAP SG&A expenses were $10.3 billion, an increase of 5% compared with the full year of 2022. The increase was primarily due to higher administrative costs, including higher compensation and benefit costs, and higher promotional spending, partially offset by the favorable impact of foreign exchange.
Non-GAAP R&D expenses were $8.8 billion in the fourth quarter of 2023 compared with $3.0 billion in the fourth quarter of 2022. Non-GAAP R&D expenses were $29.7 billion for the full year of 2023 compared with $11.8 billion for the full year of 2022. The increase in the fourth quarter and full year of 2023 reflects a $5.5 billion charge for the collaboration with Daiichi Sankyo, and higher development costs due to spending on clinical programs, including newly acquired programs, as well as higher compensation and benefit costs (reflecting in part increased headcount). The increase in non-GAAP R&D expenses for the full year was also due to charges of $11.4 billion in the aggregate for the acquisitions of Prometheus and Imago. The increase in R&D expenses for the full year was partially offset by charges of $690 million in the aggregate in 2022 for collaboration and licensing agreements with Moderna, Orna and Orion.
Non-GAAP other (income) expense, net, was $174 million of expense in the fourth quarter of 2023 compared with $86 million of income in the fourth quarter of 2022, primarily due to higher exchange losses and higher net interest expense, partially offset by lower pension settlement costs. Non-GAAP other (income) expense, net, was $219 million of expense in the full year of 2023 compared with $360 million of expense in the full year of 2022, primarily due to lower pension settlement costs.
The non-GAAP effective tax rate was 114.2% for the fourth quarter of 2023 compared with 15.6% in the fourth quarter of 2022. The non-GAAP effective tax rate for the fourth quarter of 2023 includes a 101.1 percentage point unfavorable impact resulting from the charge for the Daiichi Sankyo collaboration. The non-GAAP effective tax rate was 35.8% for the full year of 2023 compared with 14.2% for the full year of 2022. The full-year 2023 non-GAAP effective tax rate reflects an aggregate 21.2 percentage point unfavorable impact, resulting from charges for asset acquisitions (for which no benefits were recognized) as well as the charge for the Daiichi Sankyo collaboration.
Non-GAAP EPS was $0.03 for the fourth quarter of 2023 compared with $1.62 for the fourth quarter of 2022. The non-GAAP EPS decline in the fourth quarter was primarily due to the charge in 2023 related to the collaboration with Daiichi Sankyo and the unfavorable impact of foreign exchange, partially offset by a beneficial impact from the tax rate and operational strength in the business. Non-GAAP EPS was $1.51 for the full year of 2023 compared with $7.48 for the full year of 2022. The non-GAAP EPS decline for the full year was primarily due to higher charges for certain business development transactions, the unfavorable impact of foreign exchange and the tax rate, partially offset by operational strength in the business.
A reconciliation of GAAP to non-GAAP net (loss) income and (loss) earnings per share is provided in the table that follows.
|
|
| |||
| $ in millions, except EPS amounts |
|
|
|
|
|
|
|
|
|
|
| GAAP EPS | $(0.48) | $1.18 | $0.14 | $5.71 |
| Difference | 0.51 | 0.44 | 1.37 | 1.77 |
| Non-GAAP EPS that excludes items listed below | $0.03 | $1.62 | $1.51 | $7.48 |
|
|
|
|
|
|
|
|
|
|
|
|
| GAAP net (loss) income | $(1,226) | $3,017 | $365 | $14,519 |
| Difference | 1,292 | 1,112 | 3,472 | 4,486 |
| Non-GAAP net income that excludes items listed below | $66 | $4,129 | $3,837 | $19,005 |
|
|
|
|
|
|
|
|
|
|
|
|
| Acquisition- and divestiture-related costs | $1,233 | $1,192 | $2,876 | $3,704 |
| Restructuring costs | 401 | 107 | 933 | 666 |
| (Income) loss from investments in equity securities | (61) | 80 | (279) | 1,348 |
| Charge for Zetia antitrust litigation settlements | - | - | 573 | - |
| Increase to net loss/decrease to net income before taxes | 1,573 | 1,379 | 4,103 | 5,718 |
| Estimated income tax (benefit) expense | (281) | (267) | (631) | (1,232) |
| Increase to net loss/decrease to net income | $1,292 | $1,112 | $3,472 | $4,486 |
2024 Restructuring Program
Merck recently approved a new restructuring program (2024 Restructuring Program) intended to continue the optimization of the company’s Human Health global manufacturing network as the future pipeline shifts to new modalities, and also to optimize the Animal Health global manufacturing network to improve supply reliability and increase efficiency. The company recorded charges in its GAAP results of $190 million related to the 2024 Restructuring Program for the fourth quarter and full year of 2023.
Pipeline and Portfolio Highlights
In the fourth quarter, Merck continued to make significant progress advancing its broad portfolio and pipeline across key therapeutic areas, representing continued momentum toward addressing patient needs.
In oncology, Merck received multiple U.S. Food and Drug Administration (FDA) approvals, including KEYTRUDA plus Padcev for the first-line treatment of adult patients with locally advanced or metastatic urothelial cancer and WELIREG for the treatment of certain patients with previously treated advanced RCC, among other approvals. The FDA also accepted and granted Priority Review to Merck and Daiichi Sankyo’s Biologics License Application (BLA) for patritumab deruxtecan for the treatment of certain patients with previously treated locally advanced or metastatic EGFR-mutated non-small cell lung cancer (NSCLC). The FDA set a Prescription Drug User Fee Act (PDUFA), or target action, date of June 26, 2024. In addition, Merck showed meaningful progress in its robust oncology pipeline, initiating Phase 3 trials for four investigational medicines, including bomedemstat (LSD1 inhibitor), nemtabrutinib (BTK inhibitor), MK-2870 (anti-TROP2 antibody-drug conjugate) and MK-5684 (CYP11A1 inhibitor).
In vaccines, Merck received Priority Review from the FDA for a BLA for V116, the company’s investigational, 21-valent pneumococcal conjugate vaccine specifically designed to protect adults, based on results from multiple Phase 3 trials. The FDA set a PDUFA date of June 17, 2024. If approved, V116 would be the first pneumococcal conjugate vaccine to include serotypes responsible for approximately 83 percent of adult invasive pneumococcal disease in individuals 65 and older, according to U.S. Centers for Disease Control and Prevention data from 2018-2021.
In hospital acute care, the European Commission (EC) approved PREVYMIS for prevention of cytomegalovirus (CMV) disease in high-risk adult kidney transplant recipients and extended 200-day dosing in adult hematopoietic stem cell transplant (HSCT) recipients who are at high risk for late CMV infection and disease.
Merck continued to augment its pipeline through business development, and in January 2024, entered into a definitive agreement to acquire Harpoon Therapeutics, Inc. (Harpoon), for an approximate total equity value of $680 million, further diversifying its oncology pipeline.
Notable recent news releases on Merck’s pipeline and portfolio are provided in the table that follows.
|
| FDA Approved Expanded Indication for KEYTRUDA Plus Padcev as First-Line Treatment for Adult Patients With Locally Advanced or Metastatic Urothelial Cancer, Based on Results From Phase 3 KEYNOTE-A39 Trial |
|
| FDA Approved Merck’s WELIREG as Treatment for Patients With Advanced RCC Following a PD-1 or PD-L1 Inhibitor and a VEGF-TKI, Based on Results From LITESPARK-005 Trial |
| |
| FDA Approved Merck’s KEYTRUDA Plus Chemoradiotherapy as Treatment for Patients With FIGO 2014 Stage III-IVA Cervical Cancer, Based on Results From Phase 3 KEYNOTE-A18 Trial |
| |
| FDA Approved Merck’s KEYTRUDA Plus Chemotherapy as First-Line Treatment for Locally Advanced Unresectable or Metastatic HER2-Negative Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma, Based on Results From Phase 3 KEYNOTE-859 Trial |
| |
| FDA Approved Merck’s KEYTRUDA Plus Gemcitabine and Cisplatin as Treatment for Patients With Locally Advanced Unresectable or Metastatic Biliary Tract Cancer, Based on Results From Phase 3 KEYNOTE-966 Trial |
| |
| EC Approved KEYTRUDA Plus Chemotherapy for New First-Line Indications in Advanced HER2-Negative Gastric or GEJ Adenocarcinoma in Tumors Expressing PD-L1 (CPS ≥1) and Advanced Biliary Tract Cancer, Based on Results From Phase 3 KEYNOTE-859 and KEYNOTE-966 Trials |
| |
| FDA Granted Priority Review to Merck and Daiichi Sankyo’s BLA for Patritumab Deruxtecan for the Treatment of Certain Patients With Previously Treated Locally Advanced or Metastatic EGFR-Mutated NSCLC, Based on Results From Phase 2 HERTHENA-Lung01 Trial; FDA Set PDUFA Date of June 26, 2024 |
| |
| Merck Announced Phase 3 Trial Initiations for Bomedemstat, Nemtabrutinib, MK-2870 and MK-5684, Four Investigational Candidates From Promising Hematology and Oncology Pipeline |
| |
| Merck and Moderna Initiated INTerpath-002, a Phase 3 Study Evaluating V940 (mRNA-4157) in Combination With KEYTRUDA for Adjuvant Treatment of Patients With Certain Types of Resected NSCLC |
| |
| KEYTRUDA Reduced the Risk of Death by 38% Versus Placebo as Adjuvant Therapy for Patients With RCC at an Increased Risk of Recurrence Following Nephrectomy, Based on Results From Phase 3 KEYNOTE-564 Trial |
| |
| KEYTRUDA Significantly Improved Disease-Free Survival as Adjuvant Therapy Versus Observation in High-Risk Patients With Localized Muscle-Invasive and Locally Advanced Urothelial Carcinoma After Surgery, Based on Results From Phase 3 AMBASSADOR/KEYNOTE-123 Trial |
| |
| Moderna and Merck Announced V940 (mRNA-4157) in Combination With KEYTRUDA Demonstrated Continued Improvement in Recurrence-Free Survival and Distant Metastasis-Free Survival in Patients With High-Risk Stage III/IV Melanoma Following Complete Resection Versus KEYTRUDA at Three Years, Based on Results From Phase 2b Randomized KEYNOTE-942/mRNA-4157-P201 Study |
| |
|
| FDA Granted Priority Review to Merck’s New BLA for V116, an Investigational, 21-valent Pneumococcal Conjugate Vaccine Specifically Designed to Protect Adults, Based on Results From Multiple Phase 3 Trials; FDA Set PDUFA Date of June 17, 2024 |
|
| Merck’s V116, an Investigational, 21-valent Pneumococcal Conjugate Vaccine Specifically Designed to Protect Adults, Demonstrated Superior Immunogenicity for 10 of 11 Unique Serotypes Compared to PCV20 in Adults 50 Years of Age and Older, Based on Results From Phase 3 STRIDE-3 Trial |
|
Full-Year 2024 Financial Outlook
The following table summarizes the company’s full-year financial outlook.
|
|
|
| Sales | $62.7 to $64.2 billion |
| Non-GAAP gross margin | Approximately 80.5% |
| Non-GAAP operating expenses | $25.1 to $26.1 billion |
| Non-GAAP other (income) expense, net | Approximately $200 million expense |
| Non-GAAP effective tax rate | 14.5% to 15.5% |
| Non-GAAP EPS | $8.44 to $8.59 |
| Share count (assuming dilution) | Approximately 2.54 billion |
| *The company does not have any non-GAAP adjustments to sales. **Includes approximately $650 million of R&D expense related to the recently announced Harpoon acquisition, which is expected to close in the first half of 2024. Outlook does not assume any additional significant potential business development transactions. ***Includes a one-time charge of approximately $0.26 per share related to the Harpoon acquisition. | |
Merck has not provided a reconciliation of forward-looking non-GAAP gross margin, non-GAAP operating expenses, non-GAAP other (income) expense, net, non-GAAP effective tax rate and non-GAAP EPS to the most directly comparable GAAP measures, given it cannot predict with reasonable certainty the amounts necessary for such a reconciliation, including intangible asset impairment charges, legal settlements, and income and losses from investments in equity securities either owned directly or through ownership interests in investment funds, without unreasonable effort. These items are inherently difficult to forecast and could have a significant impact on the company’s future GAAP results.
Merck anticipates full-year 2024 sales to be between $62.7 billion and $64.2 billion, including a negative impact of foreign exchange of approximately 2% at mid-January 2024 exchange rates. The negative impact is primarily due to the devaluation of the Argentine peso, which the company expects will largely be offset by inflation-related price increases, consistent with market practice.
The outlook for operating expenses reflects incremental R&D spending expected to be incurred to advance the development of promising programs related to the acquisitions of Prometheus, Imago and Harpoon, as well as the collaborations with Daiichi Sankyo and Kelun-Biotech.
Merck’s full-year non-GAAP effective income tax rate is expected to be between 14.5% and 15.5%.
Merck expects full-year 2024 non-GAAP EPS to be between $8.44 and $8.59, including a negative impact of foreign exchange of approximately $0.25 per share. In 2023, non-GAAP EPS of $1.51 was negatively impacted by charges of $6.21 per share related to certain acquisitions and collaboration agreements.
In early January 2024, Merck announced the acquisition of Harpoon, which is expected to close in the first half of 2024, and result in a non-tax deductible charge of approximately $650 million of R&D expense included in non-GAAP results. The impact of the transaction on expected full-year non-GAAP EPS is approximately $0.26 per share, which is included in the 2024 outlook.
Consistent with past practice, the financial outlook does not assume additional significant potential business development transactions.
Earnings Conference Call
Investors, journalists and the general public may access a live audio webcast of the earnings conference call on Thursday, Feb. 1, at 9 a.m. ET via this weblink . A replay of the webcast, along with the sales and earnings news release, supplemental financial disclosures, prepared remarks and slides highlighting the results, will be available at www.merck.com .
All participants may join the call by dialing (800) 779-6561 (U.S. and Canada Toll-Free) or (773) 756-4619 and using the access code 5958465.
About Merck
At Merck, known as MSD outside of the United States and Canada, we are unified around our purpose: We use the power of leading-edge science to save and improve lives around the world. For more than 130 years, we have brought hope to humanity through the development of important medicines and vaccines. We aspire to be the premier research-intensive biopharmaceutical company in the world – and today, we are at the forefront of research to deliver innovative health solutions that advance the prevention and treatment of diseases in people and animals. We foster a diverse and inclusive global workforce and operate responsibly every day to enable a safe, sustainable and healthy future for all people and communities. For more information, visit www.merck.com and connect with us on X (formerly Twitter ) , Facebook , Instagram , YouTube and LinkedIn .
Forward-Looking Statement of Merck & Co., Inc., Rahway, N.J., USA
This news release of Merck & Co., Inc., Rahway, N.J., USA (the “company”) includes “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. These statements are based upon the current beliefs and expectations of the company’s management and are subject to significant risks and uncertainties. There can be no guarantees with respect to pipeline candidates that the candidates will receive the necessary regulatory approvals or that they will prove to be commercially successful. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements.
Risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of the global outbreak of novel coronavirus disease (COVID-19); the impact of pharmaceutical industry regulation and health care legislation in the United States and internationally; global trends toward health care cost containment; technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; the company’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of the company’s patents and other protections for innovative products; and the exposure to litigation, including patent litigation, and/or regulatory actions.
The company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise. Additional factors that could cause results to differ materially from those described in the forward-looking statements can be found in the company’s Annual Report on Form 10-K for the year ended December 31, 2022, and the company’s other filings with the Securities and Exchange Commission (SEC) available at the SEC’s Internet site ( www.sec.gov ).
Generic product names are provided below.
Pharmaceutical BRIDION (sugammadex) GARDASIL ( Human Papillomavirus Quadrivalent [Types 6, 11, 16 and 18] Vaccine, Recombinant ) GARDASIL 9 (Human Papillomavirus 9-valent Vaccine, Recombinant) JANUMET (sitagliptin and metformin HCl) JANUVIA (sitagliptin) KEYTRUDA (pembrolizumab) LAGEVRIO (molnupiravir) Lenvima (lenvatinib) Lynparza ( olaparib) M-M-R II (Measles, Mumps and Rubella Virus Vaccine Live) PNEUMOVAX 23 (Pneumococcal Vaccine Polyvalent) PREVYMIS ( letermovir ) PROQUAD (Measles, Mumps, Rubella and Varicella Virus Vaccine Live) ROTATEQ (Rotavirus Vaccine, Live, Oral, Pentavalent) VARIVAX (Varicella Virus Vaccine Live) VAXNEUVANCE ( Pneumococcal 15-valent Conjugate Vaccine ) WELIREG ( belzutifan )
Animal Health BRAVECTO (fluralaner)
| ________________________________ |
| Net (loss) income attributable to Merck & Co., Inc. |
| Merck is providing certain 2023 and 2022 non-GAAP information that excludes certain items because of the nature of these items and the impact they have on the analysis of underlying business performance and trends. Management believes that providing this information enhances investors’ understanding of the company’s results because management uses non-GAAP results to assess performance. Management uses non-GAAP measures internally for planning and forecasting purposes and to measure the performance of the company along with other metrics. In addition, senior management’s annual compensation is derived in part using a non-GAAP pre-tax income metric. This information should be considered in addition to, but not as a substitute for or superior to, information prepared in accordance with GAAP. For a description of the non-GAAP adjustments, see Table 2a attached to this release. |
| Includes expenses for the amortization of intangible assets and purchase accounting adjustments to inventories recognized as a result of acquisitions of businesses, intangible asset impairment charges and expense or income related to changes in the estimated fair value measurement of liabilities for contingent consideration. R&D expenses include intangible asset impairment charges of $779 million in both the fourth quarter and full year of 2023 related to gefapixant and $780 million and $1.7 billion in the fourth quarter and full year of 2022, respectively, largely related to nemtabrutinib. Also includes integration, transaction and certain other costs associated with acquisitions and divestitures, as well as amortization of intangible assets related to collaborations and licensing arrangements. |
|
|
|
|
|
|
| |||||||||||||||
| Sales | $ | 14,630 |
| $ | 13,830 |
| 6 | % | $ | 60,115 |
| $ | 59,283 |
| 1 | % | ||||
| Costs, Expenses and Other | ||||||||||||||||||||
| Cost of sales |
| 3,911 |
|
| 3,881 |
| 1 | % |
| 16,126 |
|
| 17,411 |
| -7 | % | ||||
| Selling, general and administrative |
| 2,804 |
|
| 2,687 |
| 4 | % |
| 10,504 |
|
| 10,042 |
| 5 | % | ||||
| Research and development |
| 9,628 |
|
| 3,775 |
| * |
| 30,531 |
|
| 13,548 |
| * | ||||||
| Restructuring costs |
| 255 |
|
| 49 |
| * |
| 599 |
|
| 337 |
| 78 | % | |||||
| Other (income) expense, net |
| 78 |
|
| (75 | ) | * |
| 466 |
|
| 1,501 |
| -69 | % | |||||
| (Loss) Income Before Taxes |
| (2,046 | ) |
| 3,513 |
| * |
| 1,889 |
|
| 16,444 |
| -89 | % | |||||
| Income Tax (Benefit) Provision |
| (821 | ) |
| 495 |
|
| 1,512 |
|
| 1,918 |
| ||||||||
| Net (Loss) Income |
| (1,225 | ) |
| 3,018 |
| * |
| 377 |
|
| 14,526 |
| -97 | % | |||||
| Less: Net Income Attributable to Noncontrolling Interests |
| 1 |
|
| 1 |
|
| 12 |
|
| 7 |
| ||||||||
| Net (Loss) Income Attributable to Merck & Co., Inc. | $ | (1,226 | ) | $ | 3,017 |
| * | $ | 365 |
| $ | 14,519 |
| -97 | % | |||||
| (Loss) Earnings per Common Share Assuming Dilution | $ | (0.48 | ) | $ | 1.18 |
| * | $ | 0.14 |
| $ | 5.71 |
| -98 | % | |||||
| Average Shares Outstanding Assuming Dilution |
| 2,533 |
|
| 2,548 |
|
| 2,547 |
|
| 2,542 |
| ||||||||
| Tax Rate |
| 40.1 | % |
| 14.1 | % |
| 80.0 | % |
| 11.7 | % | ||||||||
| * 100% or greater | ||||||||||||||||||||
| Because the company recorded a net loss in the fourth quarter of 2023, no potential dilutive common shares were used in the computation of loss per common share assuming dilution as the effect would have been anti-dilutive. | ||||||||||||||||||||
| Cost of sales |
|
|
| 454 |
| 117 |
| 571 |
| $ | 3,340 |
| |||||||||||||||
| Selling, general and administrative |
|
|
| 24 |
| 29 |
| 53 |
|
| 2,751 |
| |||||||||||||||
| Research and development |
|
|
| 790 |
| 790 |
|
| 8,838 |
| |||||||||||||||||
| Restructuring costs |
|
|
| 255 |
| 255 |
|
| – |
| |||||||||||||||||
| Other (income) expense, net |
|
|
| (35 | ) | (61 | ) | (96 | ) |
| 174 |
| |||||||||||||||
| Loss Before Taxes |
|
|
| (1,233 | ) | (401 | ) | 61 |
| (1,573 | ) |
| (473 | ) | |||||||||||||
| Income Tax Provision (Benefit) |
|
|
| (227 | ) |
|
| (67 | ) |
|
| 13 |
|
|
| (281 | ) |
| (540 | ) | |||||||
| Net (Loss) Income |
|
|
| (1,006 | ) | (334 | ) | 48 |
| (1,292 | ) |
| 67 |
| |||||||||||||
| Net (Loss) Income Attributable to Merck & Co., Inc. |
|
|
| (1,006 | ) | (334 | ) | 48 |
| (1,292 | ) |
| 66 |
| |||||||||||||
| (Loss) Earnings per Common Share Assuming Dilution |
|
|
| (0.40 | ) | (0.13 | ) | 0.02 |
| (0.51 | ) | $ | 0.03 |
| |||||||||||||
| Tax Rate |
|
|
|
| 114.2 | % | |||||||||||||||||||||
| Cost of sales |
|
|
| 2,018 |
| 211 |
| 2,229 |
| $ | 13,897 |
| |||||||||||||||
| Selling, general and administrative |
|
|
| 86 |
| 122 |
| 208 |
|
| 10,296 |
| |||||||||||||||
| Research and development |
|
|
| 819 |
| 1 |
| 820 |
|
| 29,711 |
| |||||||||||||||
| Restructuring costs |
|
|
| 599 |
| 599 |
|
| – |
| |||||||||||||||||
| Other (income) expense, net |
|
|
| (47 | ) | (279 | ) | 573 |
|
|
| 247 |
|
| 219 |
| |||||||||||
| Income Before Taxes |
|
|
| (2,876 | ) | (933 | ) | 279 |
| (573 | ) | (4,103 | ) |
| 5,992 |
| |||||||||||
| Income Tax Provision (Benefit) |
|
|
| (476 | ) |
|
| (155 | ) |
|
| 60 |
|
|
| (60 | ) |
|
| (631 | ) |
| 2,143 |
| |||
| Net Income |
|
|
| (2,400 | ) | (778 | ) | 219 |
| (513 | ) | (3,472 | ) |
| 3,849 |
| |||||||||||
| Net Income Attributable to Merck & Co., Inc. |
|
|
| (2,400 | ) | (778 | ) | 219 |
| (513 | ) | (3,472 | ) |
| 3,837 |
| |||||||||||
| Earnings per Common Share Assuming Dilution |
|
|
| (0.94 | ) | (0.31 | ) | 0.08 |
| (0.20 | ) | (1.37 | ) | $ | 1.51 |
| |||||||||||
| Tax Rate |
|
|
|
| 35.8 | % | |||||||||||||||||||||
| Only the line items that are affected by non-GAAP adjustments are shown. | |||||||||||||||||||||||||||
| Merck is providing certain non-GAAP information that excludes certain items because of the nature of these items and the impact they have on the analysis of underlying business performance and trends. Management believes that providing non-GAAP information enhances investors’ understanding of the company’s results because management uses non-GAAP measures to assess performance. Management uses non-GAAP measures internally for planning and forecasting purposes and to measure the performance of the company along with other metrics. In addition, senior management’s annual compensation is derived in part using a non-GAAP pretax income metric. The non-GAAP information presented should be considered in addition to, but not as a substitute for or superior to, information prepared in accordance with GAAP. | |||||||||||||||||||||||||||
| Amounts included in cost of sales primarily reflect expenses for the amortization of intangible assets. Amounts included in selling, general and administrative expenses reflect integration, transaction and certain other costs related to acquisitions and divestitures. Amounts included in research and development expenses primarily reflect a $779 million in-process research and development (IPR&D) impairment charge related to gefapixant, which was obtained as part of the 2016 Afferent Pharmaceuticals acquisition, and expenses for the amortization of intangible assets. Amounts included in other (income) expense, net, primarily reflect royalty income related to the prior termination of the Sanofi-Pasteur MSD joint venture. Additionally, other (income) expense, net, for the full year includes a $37 million loss on the sale of a business. | |||||||||||||||||||||||||||
| Amounts primarily include employee separation costs and accelerated depreciation associated with facilities to be closed or divested related to activities under the company's formal restructuring programs. | |||||||||||||||||||||||||||
| Reflects a charge related to settlements with certain plaintiffs in the Zetia antitrust litigation. | |||||||||||||||||||||||||||
| Represents the estimated tax impacts on the reconciling items based on applying the statutory rate of the originating territory of the non-GAAP adjustments. | |||||||||||||||||||||||||||
| Because the company recorded a net loss in the fourth quarter of 2023, no potential dilutive common shares were used in the computation of loss per common share assuming dilution as the effect would have been anti-dilutive. | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||
| Keytruda | 5,795 | 6,271 | 6,338 | 6,608 | 25,011 | 4,809 | 5,252 | 5,426 | 5,450 | 20,937 | 21 | 22 | 19 | 21 | |||
| Alliance Revenue – Lynparza | 275 | 310 | 299 | 315 | 1,199 | 266 | 275 | 284 | 292 | 1,116 | 8 | 8 | 7 | 9 | |||
| Alliance Revenue – Lenvima | 232 | 242 | 260 | 226 | 960 | 227 | 231 | 202 | 216 | 876 | 5 | 5 | 10 | 11 | |||
| Welireg | 42 | 50 | 54 | 72 | 218 | 18 | 27 | 38 | 40 | 123 | 79 | 78 | 77 | 77 | |||
| Alliance Revenue – Reblozyl | 43 | 47 | 52 | 70 | 212 | 52 | 33 | 39 | 41 | 166 | 69 | 69 | 28 | 28 | |||
| Gardasil / Gardasil 9 | 1,972 | 2,458 | 2,585 | 1,871 | 8,886 | 1,460 | 1,674 | 2,294 | 1,470 | 6,897 | 27 | 27 | 29 | 33 | |||
| ProQuad / M-M-R II / Varivax | 528 | 582 | 713 | 545 | 2,368 | 470 | 578 | 668 | 526 | 2,241 | 4 | 3 | 6 | 6 | |||
| RotaTeq | 297 | 131 | 156 | 185 | 769 | 216 | 173 | 256 | 139 | 783 | 34 | 33 | -2 | -1 | |||
| Vaxneuvance | 106 | 168 | 214 | 176 | 665 | 5 | 12 | 16 | 138 | 170 | 28 | 26 | * | * | |||
| Pneumovax 23 | 96 | 92 | 140 | 85 | 412 | 173 | 153 | 131 | 145 | 602 | -42 | -43 | -32 | -31 | |||
| Vaqta | 40 | 42 | 69 | 29 | 180 | 36 | 35 | 64 | 39 | 173 | -24 | -25 | 4 | 4 | |||
| Bridion | 487 | 502 | 424 | 429 | 1,842 | 395 | 426 | 423 | 441 | 1,685 | -3 | -3 | 9 | 11 | |||
| Prevymis | 129 | 143 | 157 | 175 | 605 | 94 | 103 | 114 | 118 | 428 | 49 | 49 | 41 | 43 | |||
| Dificid | 65 | 76 | 74 | 87 | 302 | 52 | 66 | 77 | 67 | 263 | 30 | 30 | 15 | 15 | |||
| Zerbaxa | 50 | 54 | 53 | 61 | 218 | 30 | 46 | 43 | 49 | 169 | 25 | 23 | 29 | 30 | |||
| Noxafil | 60 | 55 | 51 | 46 | 213 | 57 | 60 | 62 | 58 | 238 | -21 | -16 | -11 | -4 | |||
| Primaxin | 80 | 53 | 41 | 39 | 213 | 58 | 64 | 63 | 54 | 239 | -28 | -28 | -11 | -6 | |||
| Alliance Revenue - Adempas/Verquvo | 99 | 68 | 92 | 108 | 367 | 72 | 98 | 88 | 82 | 341 | 31 | 31 | 8 | 8 | |||
| Adempas | 59 | 65 | 65 | 66 | 255 | 61 | 63 | 57 | 57 | 238 | 15 | 11 | 7 | 8 | |||
| Lagevrio | 392 | 203 | 640 | 193 | 1,428 | 3,247 | 1,177 | 436 | 825 | 5,684 | -77 | -76 | -75 | -74 | |||
| Isentress / Isentress HD | 123 | 136 | 119 | 105 | 483 | 158 | 147 | 161 | 167 | 633 | -37 | -37 | -24 | -23 | |||
| Belsomra | 56 | 63 | 58 | 54 | 231 | 69 | 69 | 62 | 59 | 258 | -8 | -6 | -11 | -6 | |||
| Simponi | 180 | 180 | 179 | 171 | 710 | 186 | 181 | 173 | 166 | 706 | 3 | -2 | 1 | - | |||
| Remicade | 51 | 48 | 45 | 43 | 187 | 61 | 53 | 49 | 44 | 207 | -1 | -2 | -9 | -8 | |||
| Januvia | 551 | 511 | 581 | 547 | 2,189 | 779 | 756 | 717 | 561 | 2,813 | -2 | -2 | -22 | -20 | |||
| Janumet | 329 | 354 | 255 | 240 | 1,177 | 454 | 476 | 417 | 353 | 1,700 | -32 | -32 | -31 | -29 | |||
| 584 | 553 | 549 | 595 | 2,283 | 602 | 528 | 603 | 583 | 2,319 | 2 | 2 | -2 | - | ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
| Livestock | 849 | 807 | 874 | 808 | 3,337 | 832 | 826 | 829 | 814 | 3,300 | -1 | - | 1 | 4 | |||
| Companion Animal | 642 | 649 | 526 | 470 | 2,288 | 650 | 641 | 542 | 416 | 2,250 | 13 | 12 | 2 | 3 | |||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
| *200% or greater | |||||||||||||||||
| Sum of quarterly amounts may not equal year-to-date amounts due to rounding. | |||||||||||||||||
| Only select products are shown. | |||||||||||||||||
| Alliance Revenue represents Merck’s share of profits, which are product sales net of cost of sales and commercialization costs. | |||||||||||||||||
| Alliance Revenue represents royalties and a milestone payment of $20 million received in the first quarter of 2022. | |||||||||||||||||
| Total Vaccines sales were $3,133 million, $3,557 million, $4,002 million and $2,962 million in the first, second, third and fourth quarter of 2023, respectively, and $2,481 million, $2,709 million, $3,552 million and $2,554 million in the first, second, third and fourth quarter of 2022, respectively. | |||||||||||||||||
| Alliance Revenue represents Merck's share of profits from sales in Bayer's marketing territories, which are product sales net of cost of sales and commercialization costs. | |||||||||||||||||
| Net product sales in Merck's marketing territories. | |||||||||||||||||
| Total Diabetes sales were $950 million, $951 million, $924 million and $876 million in the first, second, third and fourth quarter of 2023, respectively, and $1,305 million, $1,300 million, $1,231 million and $1,012 million in the first, second, third and fourth quarter of 2022, respectively. | |||||||||||||||||
| Includes Pharmaceutical products not individually shown above. | |||||||||||||||||
| Other Revenues are comprised primarily of revenues from third-party manufacturing arrangements and miscellaneous corporate revenues, including revenue-hedging activities. Other Revenues related to the receipt of upfront and milestone payments for out-licensed products were $51 million, $3 million and $65 million in the first, second and third quarter of 2023, respectively, and $114 million, $32 million, $10 million and $10 million in the first, second, third and fourth quarter of 2022, respectively. | |||||||||||||||||
Media Contacts: Robert Josephson (203) 914-2372 [email protected] Michael Levey (215) 872-1462 [email protected] Investor Contacts: Peter Dannenbaum (732) 594-1579 [email protected] Steven Graziano (732) 594-1583 [email protected]
Merck Logo Horizontal Teal Grey RGB (211 KB)
Financial Highlights (1.06 MB)

Sign up for email alerts
Unsubscribe from email alerts
Related links

Company Statements
Read our latest company statements.

Media library
Access videos, logos, photos, and infographics.

We are committed to providing leading innovations for today and the future that save and improve lives around the world.
Forward-looking statement of Merck & Co., Inc., Rahway, N.J., USA
This website of Merck & Co., Inc., Rahway, N.J., USA (the “company”) includes “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. These statements are based upon the current beliefs and expectations of the company’s management and are subject to significant risks and uncertainties. There can be no guarantees with respect to pipeline candidates that the candidates will receive the necessary regulatory approvals or that they will prove to be commercially successful. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements. Risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of pharmaceutical industry regulation and health care legislation in the United States and internationally; global trends toward health care cost containment; technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; the company’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of the company’s patents and other protections for innovative products; and the exposure to litigation, including patent litigation, and/or regulatory actions. The company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise. Additional factors that could cause results to differ materially from those described in the forward-looking statements can be found in the company’s Annual Report on Form 10-K for the year ended December 31, 2023 and the company’s other filings with the Securities and Exchange Commission (SEC) available at the SEC’s Internet site (www.sec.gov). No Duty to Update The information contained in this website was current as of the date presented. The company assumes no duty to update the information to reflect subsequent developments. Consequently, the company will not update the information contained in the website and investors should not rely upon the information as current or accurate after the presentation date.
You are leaving Merck.com
Welcome to merck.com.
By continuing, you will be directed to a site intended only for residents of the United States and Canada. We are called MSD everywhere, except in the United States and Canada where we are known as Merck & Co Inc, Rahway, NJ USA.
- Our company
- Sustainability
- Social impact
years of refreshing the world
The Coca‑Cola Company has been refreshing the world and making a difference for over 137 years. Explore our Purpose & Vision, History and more.
- Purpose & Company Vision
- The Coca‑Cola System
- Our Board of Directors
- COCA-COLA HISTORY
- Our Origins
- Our First Bottle
- Sustainability History
- Advertising History
brands worldwide
We've established a portfolio of drinks that are best positioned to grow in an ever-changing marketplace.
From trademark Coca‑Cola to Sports, Juice & Dairy Drinks, Alcohol Ready-to-Drink Beverages and more, discover some of our most popular brands in North America and from around the world.
- Coca‑Cola
- + View More
- COFFEE & TEA
- Costa Coffee
- Gold Peak Tea
- JUICES & DAIRY
- Minute Maid
- Fresca Mixed
- Jack Daniel's & Coca‑Cola
- Simply Spiked
- Topo Chico Hard Seltzer
OUR PLANET MATTERS
Our purpose is to refresh the world and make a difference. See how our company and system employees make this possible every day and learn more about our areas of focus in sustainability.
- Water Stewardship
- 2030 Water Strategy Key Goals
- Sustainable Agriculture
- Principles for Sustainable Agriculture (PSAs)
- Sustainable Packaging
- Collection Strategy
- Packaging Design
- Partnership
- In Our Products
- Sugar Reduction
- 2022 Business & Sustainability Report
- Sustainability & Governance Resource Center
PEOPLE MATTER
We aim to improve people's lives, from our employees to those who touch our business to the many communities we call home.
- Diversity, Equity and Inclusion
- Leadership Council
- Employee Groups
- People & Communities
- Women Empowerment
- Project Last Mile
- HUMAN RIGHTS
- Human Rights Governance
- Stories of IMPACT
- Sports & Entertainment
- Paris 2024 Hub
- Coca‑Cola Foundation
- Partnerships
- Supplier Diversity
We believe working at The Coca‑Cola Company is an opportunity to build a meaningful career while helping us make a real difference on a global scale.
- LIFE AT COCA-COLA
- Career Development
- Work With Us
- CAREER AREAS
- Early Career
- Experienced Professionals
- Accessible Workplace
- HIRING PROCESS
- Application Process
- Coca‑Cola Company Jobs
- Coca‑Cola System Jobs
GET THE LATEST
Catch up on the latest Coca‑Cola news from around the globe - from exciting brand innovation to the latest sustainability projects.
- WHAT OTHERS ARE READING
- Taste the Transformation: Coca‑Cola and Grammy-Award Winning Artist Rosalía Break Boundaries With Limited-Edition Coke Creation
- Coca‑Cola Brings Together Iconic Andy Warhol Painting with Illustrious Roster of Master Classics and Contemporary Works in New Global 'Masterpiece' Campaign
- A Deeper Look at Coca‑Cola's Emerging Business in Alcohol
- LATEST ARTICLES
- Coca‑Cola Zero Sugar Invites Fans to #TakeATaste
- Simply Mixology Raises the Bar of the At-Home Mocktail and Cocktail Experience
- Sprite, Fresca and Seagram's Tap Mark Ronson and Madlib to Create a 'Clear' Connection
- View all news
Coca‑Cola Reports Fourth Quarter and Full-Year 2023 Results
Global Unit Case Volume Grew 2% for the Quarter and 2% for the Full Year
Net Revenues Grew 7% for the Quarter and 6% for the Full Year; Organic Revenues (Non-GAAP) Grew 12% for the Quarter and 12% for the Full Year
Operating Income Grew 10% for the Quarter and 4% for the Full Year; Comparable Currency Neutral Operating Income (Non-GAAP) Grew 20% for the Quarter and 16% for the Full Year
Fourth Quarter EPS Declined 2% to $0.46; Comparable EPS (Non-GAAP) Grew 10% to $0.49; Full Year EPS Grew 13% to $2.47; Comparable EPS (Non-GAAP) Grew 8% to $2.69
Cash Flow from Operations Was $11.6 Billion for the Full Year, Up 5%; Full-Year Free Cash Flow (Non-GAAP) Was $9.7 Billion for the Full Year, Up 2%
Company Provides 2024 Financial Outlook
ATLANTA, Feb. 13, 2024 – The Coca‑Cola Company today reported fourth quarter and full-year 2023 results. “During the year, our people and partners rose to meet new challenges, allowing us to excel globally and deliver in a dynamic world,” said James Quincey, Chairman and CEO of The Coca‑Cola Company. “As we begin a new year, we’re confident that our all-weather strategy, powerful portfolio and harmonized system will continue to create value for our stakeholders in 2024 and for the long term.”
Quarterly / Full-Year Performance
- Revenues : For the quarter, net revenues grew 7% to $10.8 billion, and organic revenues (non-GAAP) grew 12%, driven by 9% growth in price/mix and 3% growth in concentrate sales. The quarter included one additional day, which resulted in a 1-point tailwind to revenue growth. For the full year, net revenues grew 6% to $45.8 billion, and organic revenues (non-GAAP) grew 12%, driven by 10% growth in price/mix and 2% growth in concentrate sales. For both the quarter and the full year, organic revenue (non-GAAP) performance was strong across all operating segments.
- Operating margin : For the quarter, operating margin was 21.0% versus 20.5% in the prior year, while comparable operating margin (non-GAAP) was 23.1% versus 22.7% in the prior year. For the full year, operating margin was 24.7% versus 25.4% in the prior year, while comparable operating margin (non-GAAP) was 29.1% versus 28.7% in the prior year. Operating margin performance included items impacting comparability and currency headwinds. For both the quarter and full year, comparable operating margin (non-GAAP) expansion was primarily driven by strong topline growth, partially offset by an increase in marketing investments versus the prior year, as well as currency headwinds.
- Earnings per share : For the quarter, EPS declined 2% to $0.46, while comparable EPS (non-GAAP) grew 10% to $0.49. EPS performance included the impact of a 14-point currency headwind, while comparable EPS (non-GAAP) performance included the impact of a 13-point currency headwind. For the full year, EPS grew 13% to $2.47, and comparable EPS (non-GAAP) grew 8% to $2.69. EPS performance included the impact of an 8-point currency headwind, while comparable EPS (non-GAAP) performance included the impact of a 7-point currency headwind.
- Market share : For both the quarter and the full year, the company gained value share in total nonalcoholic ready-to-drink (NARTD) beverages.
- Cash flow : Cash flow from operations was $11.6 billion for the full year, an increase of $581 million versus the prior year, driven by strong business performance and working capital initiatives, partially offset by a transition tax payment and currency headwinds. Free cash flow (non-GAAP) was $9.7 billion for the full year, an increase of $213 million versus the prior year.
Company Updates
- Connecting with consumers through experiences and digital engagement: The company continues to leverage its marketing transformation to build globally scaled marketing platforms tailored to local consumers. In the fourth quarter, “The World Needs More Santas” campaign was executed in over 80 markets, continuing the company’s rich history of celebrating the holidays. The company leveraged the success of its first AI-based platform, “Create Real Magic”, by inviting consumers to create sharable, digital greeting cards featuring iconic brand assets such as cherished depictions of Santa Claus and the Coca‑Cola polar bear. Local initiatives generated buzz and excitement, such as the Coca‑Cola Caravan Truck Tour, which travelled throughout nearly 60 countries around the world making over a thousand stops and meeting over 16 million consumers to share in the magic. In total, the holiday campaign experiences garnered approximately 9 billion impressions on social media. By combining the company’s global scale with local relevancy, the holiday activation contributed to Trademark Coca‑Cola® volume and value share gains as well as unit case volume and transactions growth for both the quarter and for the full year.
- Building a system that is increasingly positioned for sustainable long-term growth: Since the start of 2023, the company completed the refranchising of company-owned bottling operations in Vietnam to a subsidiary of Swire Pacific Limited (Swire), completed the sale of its stake in the bottler in Pakistan to Coca‑Cola İçecek and completed the sale of its stake in the bottler in Indonesia to Coca‑Cola Europacific Partners (CCEP). More recently, the company completed the refranchising of a portion of its company-owned bottling operations in India to existing franchise bottlers and received regulatory approval to sell its bottling operations in the Philippines to CCEP and Aboitiz Equity Ventures, which is expected to close towards the end of February 2024. Additionally, the company recently completed the sale of its stake in the largest bottler in Thailand to Swire and existing shareholders of the bottler. Our franchise business model has enabled the company to develop a strong global footprint with a local touch in markets around the world. The company continuously works to optimize the system with trusted, capable and motivated bottling partners allowing it to focus on building and growing consumer-loved brands.
- Delivering on our purpose by empowering people to drive growth: The company’s strategy is centered around people, starting with the employees who are critical in bringing this strategy to life. In November, the company was ranked #1 on the 2023 American Opportunity Index, which assessed how effectively companies enable employees to progress in their careers in the United States. Around the world, the company provides thousands of jobs and is invested in the long-term success of its employees. Over the year, the company enhanced its learning and related technologies, making strides towards nurturing a more innovative and informed workforce. The company’s 2023 Culture & Engagement Survey results underscore the strong levels of employee pride and growth opportunities with a strong number of respondents saying they are proud to work at The Coca‑Cola Company and see good opportunities to learn and grow in their roles.
Operating Review – Three Months Ended December 31, 2023
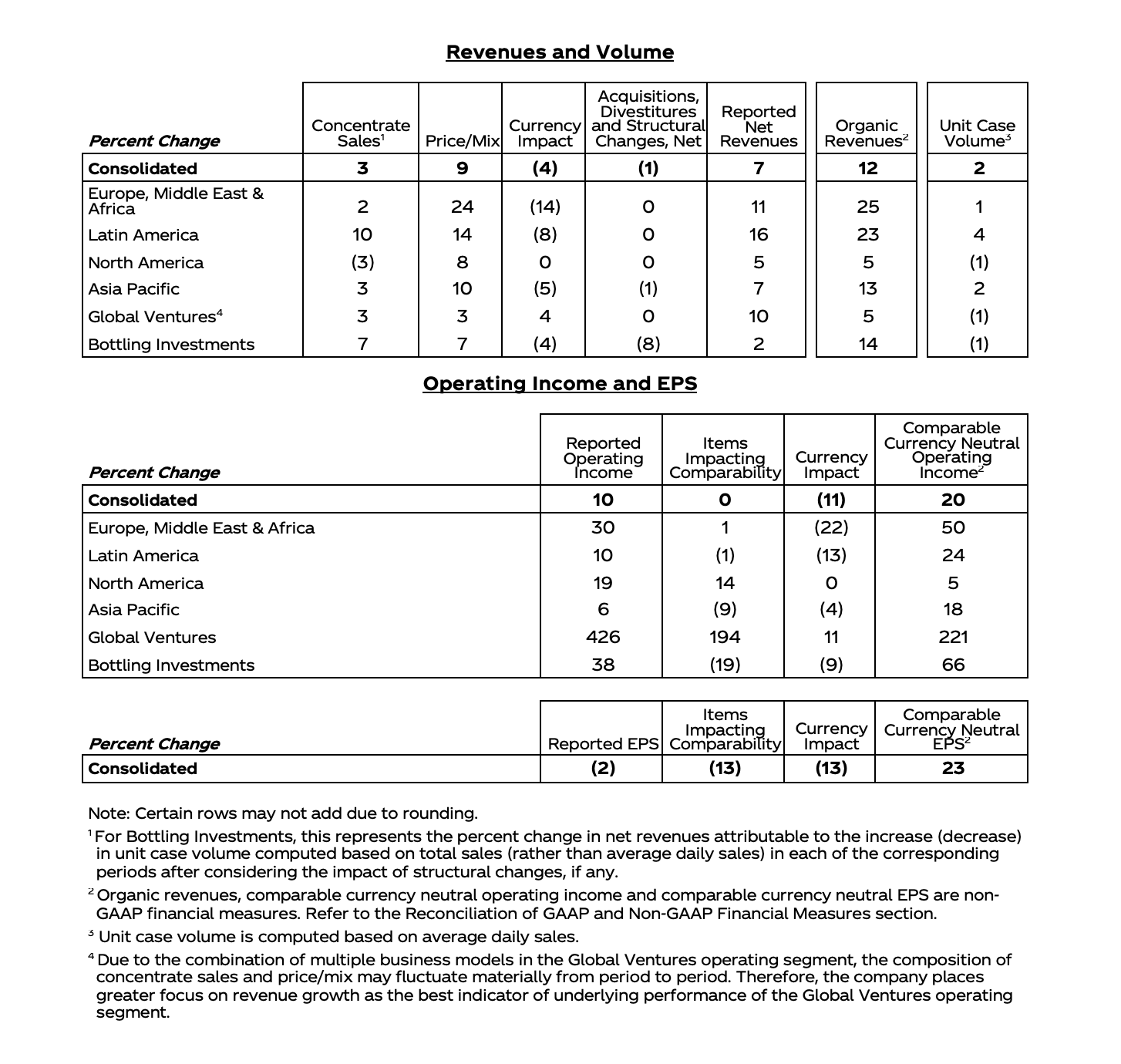
Operating Review – Year Ended December 31, 2023
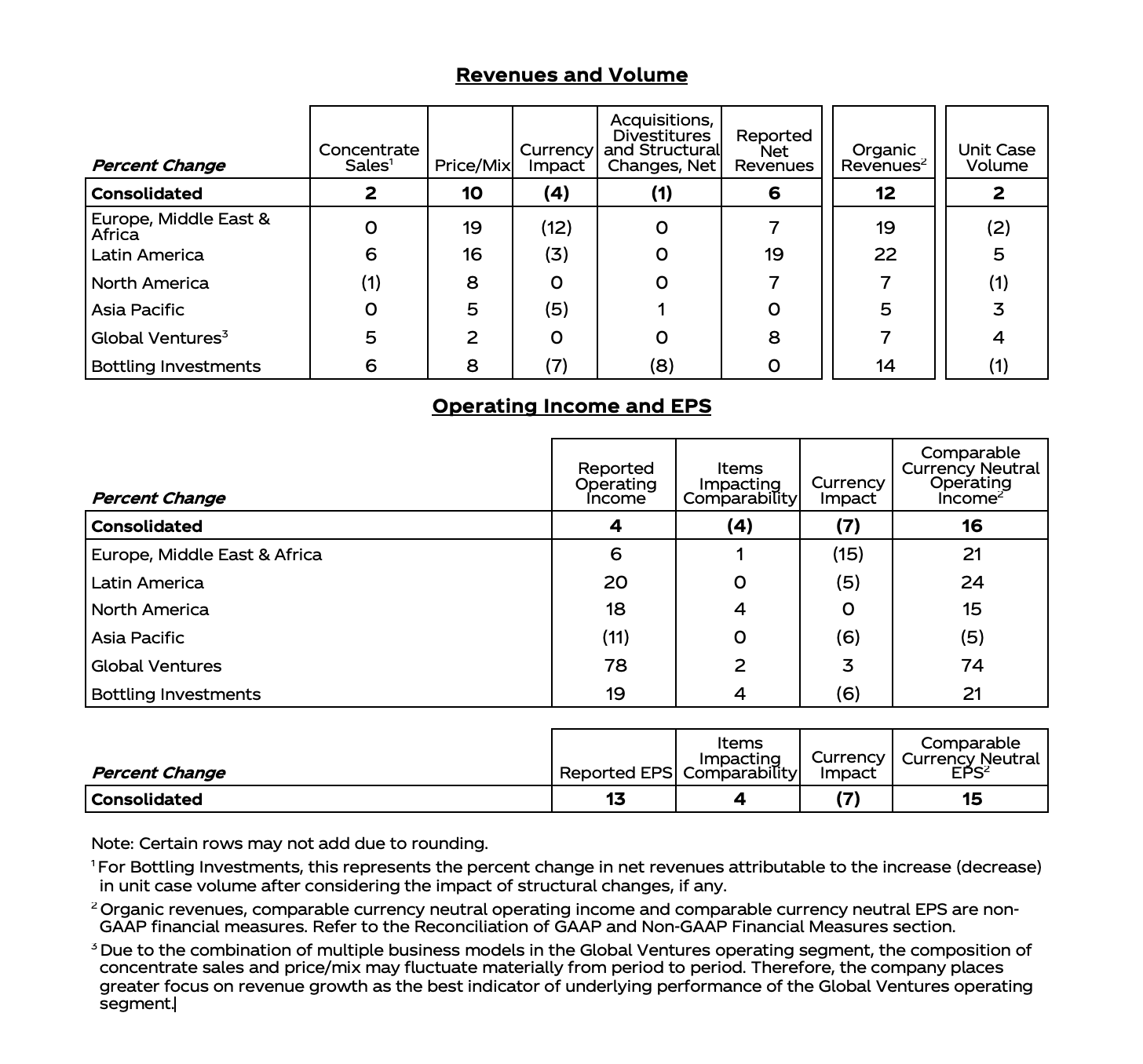
In addition to the data in the preceding tables, operating results included the following:
Consolidated
- Unit case volume grew 2% for the quarter. Developed markets were even as growth in Mexico and Germany was offset by declines in the United States and Chile. Developing and emerging markets grew 4%, driven by growth in Brazil and India. For the full year, unit case volume grew 2%. Developed markets grew 1%, driven by growth in Mexico and Germany. Developing and emerging markets grew 2%, driven by growth in India and Brazil, partially offset by the suspension of business in Russia in 2022.
Unit case volume performance included the following:
- Sparkling soft drinks grew 2% for both the quarter and the full year, primarily driven by growth in Latin America and Asia Pacific. Trademark Coca‑Cola® grew 2% for both the quarter and the full year, primarily driven by growth in Latin America and Asia Pacific. Coca‑Cola Zero Sugar grew 4% for the quarter and 5% for the full year, driven by growth in Latin America and North America. Sparkling flavors grew 1% for both the quarter and the full year, primarily driven by growth in Asia Pacific.
- Juice, value-added dairy and plant-based beverages grew 6% for the quarter and 2% for the full year. This performance benefited from growth in Minute Maid® Pulpy in China, Mazoe® in Africa and fairlife® in the United States.
- Water, sports, coffee and tea was even for the quarter and grew 1% for the full year. Water grew 1% for the quarter and 2% for the full year, primarily driven by growth in Latin America. Sports drinks declined 1% for the quarter and were even for the year. Full year performance was benefited by growth in Powerade® in Latin America, offset by a decline in BODYARMOR®. Coffee declined 7% for the quarter and grew 3% for the year. Full year performance was benefited by strong performance of Costa® coffee in the United Kingdom and China. Tea was even for the quarter and declined 1% for the full year, as growth in Latin America and Europe, Middle East and Africa was more than offset by declines in North America and doğadan® in Türkiye.
- Price/mix grew 9% for the quarter and 10% for the full year, primarily driven by pricing actions in the marketplace, including the continued impact of hyperinflationary markets, and favorable mix. For the quarter, concentrate sales were 1 point ahead of unit case volume, primarily due to one additional day.
- Operating income grew 10% for the quarter and 4% for the full year, which included items impacting comparability and currency headwinds. Comparable currency neutral operating income (non-GAAP) grew 20% for the quarter and 16% for the year. For both the quarter and the full year, performance was driven by organic revenue (non-GAAP) growth across all operating segments, partially offset by an increase in marketing investments.
Europe, Middle East & Africa
- Unit case volume grew 1% for the quarter, driven by growth in water, sports, coffee and tea as well as juice, value-added dairy and plant-based beverages. Growth was led by Germany and Nigeria.
- Price/mix grew 24% for the quarter, approximately one-third of which was driven by the continued impact of pricing in hyperinflationary markets and the remaining driven primarily by pricing actions across operating units. For the quarter, concentrate sales were 1 point ahead of unit case volume, primarily due to one additional day.
- Operating income grew 30% for the quarter, which included a 22-point currency headwind. Comparable currency neutral operating income (non-GAAP) grew 50% for the quarter, primarily driven by organic revenue (non-GAAP) growth across all operating units.
- For the year, the company gained value share in total NARTD beverages, led by share gains in Türkiye, Nigeria and Germany.
Latin America
- Unit case volume grew 4% for the quarter, driven by growth in Trademark Coca‑Cola and water, sports, coffee and tea. Growth was led by Brazil and Mexico.
- Price/mix grew 14% for the quarter, primarily driven by the continued impact of inflationary pricing in Argentina and pricing actions, partially offset by incremental investments in the marketplace. For the quarter, concentrate sales were 6 points ahead of unit case volume, primarily due to one additional day and the timing of concentrate shipments.
- Operating income grew 10% for the quarter, which included a 15-point currency headwind and items impacting comparability. Comparable currency neutral operating income (non-GAAP) grew 24% for the quarter, primarily driven by strong organic revenue (non-GAAP) growth, partially offset by an increase in marketing investments.
- For the year, the company gained value share in total NARTD beverages, led by share gains in Brazil, Colombia and Mexico.
North America
- Unit case volume declined 1% for the quarter, as growth in juice, value-added dairy and plant-based beverages and Trademark Coca‑Cola was more than offset by a decline in water, sports, coffee and tea.
- Price/mix grew 8% for the quarter, primarily driven by pricing actions already in the marketplace, timing related adjustments and favorable category mix. For the quarter, concentrate sales were 2 points behind unit case volume, primarily due to the timing of concentrate shipments, partially offset by one additional day.
- Operating income grew 19% for the quarter, which included items impacting comparability. Comparable currency neutral operating income (non-GAAP) grew 5% for the quarter, primarily driven by organic revenue (non-GAAP) growth.
- For the year, the company gained value share in total NARTD beverages, driven by sparkling soft drinks and value-added dairy beverages.
Asia Pacific
- Unit case volume grew 2% for the quarter, primarily driven by growth in juice, value-added dairy and plant-based beverages and sparkling flavors. Growth was led by India and China.
- Price/mix grew 10% for the quarter, primarily driven by pricing actions in the marketplace and favorable category mix. For the quarter, concentrate sales were 1 point ahead of unit case volume, primarily due to one additional day.
- Operating income grew 6% for the quarter, which included items impacting comparability and a 13-point currency headwind. Comparable currency neutral operating income (non-GAAP) grew 18% for the quarter, driven by organic revenue (non-GAAP) growth across all operating units and lower operating costs, partially offset by an increase in marketing investments.
- For the year, the company gained value share in total NARTD beverages, led by share gains in India, the Philippines, South Korea and Japan.
Global Ventures
- Net revenues grew 10%, and organic revenues (non-GAAP) grew 5% for the quarter, primarily driven by the strong performance of Costa® coffee in the United Kingdom and China.
- Operating income and comparable currency neutral operating income (non-GAAP) both had robust growth for the quarter, driven by organic revenue (non-GAAP) growth and lower costs.
Bottling Investments
- Unit case volume declined 1% for the quarter, as growth in India and the Philippines was more than offset by the impact of refranchising bottling operations.
- Price/mix grew 7% for the quarter, driven by pricing actions across most markets.
- Operating income grew 38% for the quarter, which included items impacting comparability and an 8-point currency headwind. Comparable currency neutral operating income (non-GAAP) grew 66% for the quarter, driven by organic revenue (non-GAAP) growth, partially offset by higher operating costs.
Capital Allocation Update
- Reinvesting in the business: The company continued to invest in its various lines of business and spent $1.9 billion on capital expenditures in 2023, an increase of 25% versus the prior year.
- Continuing to grow the dividend: The company paid dividends totaling $8.0 billion during 2023. The company has increased its dividend in each of the last 61 years.
- M&A initiatives: In 2023, the company did not make any significant acquisitions. The company continues to evaluate inorganic growth opportunities through brands and capabilities. In 2023, with respect to divestitures, the company made progress towards refranchising company-owned bottling operations.
- Share repurchases: In 2023, the company issued $0.5 billion of shares in connection with the exercise of stock options by employees and purchased $2.3 billion of shares. Consequently, net share repurchases (non-GAAP) were $1.7 billion. The company’s remaining share repurchase authorization is approximately $6 billion.
The 2024 outlook information provided below includes forward-looking non-GAAP financial measures, which management uses in measuring performance. The company is not able to reconcile full-year 2024 projected organic revenues (non-GAAP) to full-year 2024 projected reported net revenues, full-year 2024 projected comparable net revenues (non-GAAP) to full-year 2024 projected reported net revenues, full-year 2024 projected underlying effective tax rate (non-GAAP) to full-year 2024 projected reported effective tax rate, full-year 2024 projected comparable currency neutral EPS (non-GAAP) to full-year 2024 projected reported EPS, or full-year 2024 projected comparable EPS (non-GAAP) to full-year 2024 projected reported EPS without unreasonable efforts because it is not possible to predict with a reasonable degree of certainty the exact timing and exact impact of acquisitions, divestitures and structural changes throughout 2024; the exact timing and exact amount of items impacting comparability throughout 2024; and the exact impact of fluctuations in foreign currency exchange rates throughout 2024. The unavailable information could have a significant impact on the company’s full-year 2024 reported financial results.
Full Year 2024
The company expects to deliver organic revenue (non-GAAP) growth of 6% to 7%.
For comparable net revenues (non-GAAP), the company expects a 2% to 3% currency headwind based on the current rates and including the impact of hedged positions, in addition to a 4% to 5% headwind from acquisitions, divestitures and structural changes.
The company’s underlying effective tax rate (non-GAAP) is estimated to be 19.2%. This does not include the impact of ongoing tax litigation with the IRS, if the company were not to prevail.
Given the above considerations, the company expects to deliver comparable currency neutral EPS (non-GAAP) growth of 8% to 10% and comparable EPS (non-GAAP) growth of 4% to 5%, versus $2.69 in 2023.
Comparable EPS (non-GAAP) percentage growth is expected to include a 4% to 5% currency headwind based on the current rates and including the impact of hedged positions, in addition to an approximate 2% headwind from acquisitions, divestitures and structural changes.
The company expects to generate free cash flow (non-GAAP) of approximately $9.2 billion through cash flow from operations of approximately $11.4 billion, less capital expenditures of approximately $2.2 billion. This does not include any potential payments related to ongoing tax litigation with the IRS.
First Quarter 2024 Considerations
Comparable net revenues (non-GAAP) are expected to include an approximate 4% currency headwind based on the current rates and including the impact of hedged positions, in addition to an approximate 2% headwind from acquisitions, divestitures and structural changes.
Comparable EPS (non-GAAP) percentage growth is expected to include an approximate 8% currency headwind based on the current rates and including the impact of hedged positions, in addition to an approximate 1% headwind from acquisitions, divestitures and structural changes.
The first quarter has one less day compared to the first quarter of 2023.
- All references to growth rate percentages and share compare the results of the period to those of the prior year comparable period, unless otherwise noted.
- All references to volume and volume percentage changes indicate unit case volume, unless otherwise noted. All volume percentage changes are computed based on average daily sales in the fourth quarter, unless otherwise noted, and are computed on a reported basis for the full year. “Unit case” means a unit of measurement equal to 192 U.S. fluid ounces of finished beverage (24 eight-ounce servings), with the exception of unit case equivalents for Costa non-ready-to-drink beverage products which are primarily measured in number of transactions. “Unit case volume” means the number of unit cases (or unit case equivalents) of company beverages directly or indirectly sold by the company and its bottling partners to customers or consumers.
- “Concentrate sales” represents the amount of concentrates, syrups, beverage bases, source waters and powders/minerals (in all instances expressed in unit case equivalents) sold by, or used in finished beverages sold by, the company to its bottling partners or other customers. For Costa non-ready-to-drink beverage products, “concentrate sales” represents the amount of beverages, primarily measured in number of transactions (in all instances expressed in unit case equivalents) sold by the company to customers or consumers. In the reconciliation of reported net revenues, “concentrate sales” represents the percent change in net revenues attributable to the increase (decrease) in concentrate sales volume for the geographic operating segments and the Global Ventures operating segment after considering the impact of structural changes, if any. For the Bottling Investments operating segment for the fourth quarter, this represents the percent change in net revenues attributable to the increase (decrease) in unit case volume computed based on total sales (rather than average daily sales) in each of the corresponding periods after considering the impact of structural changes, if any. For the Bottling Investments operating segment for the full year, this represents the percent change in net revenues attributable to the increase (decrease) in unit case volume after considering the impact of structural changes, if any. The Bottling Investments operating segment reflects unit case volume growth for consolidated bottlers only.
- “Price/mix” represents the change in net operating revenues caused by factors such as price changes, the mix of products and packages sold, and the mix of channels and geographic territories where the sales occurred.
- First quarter 2023 financial results were impacted by one less day as compared to first quarter 2022, and fourth quarter 2023 financial results were impacted by one additional day as compared to fourth quarter 2022. Unit case volume results for the quarters are not impacted by the variances in days due to the average daily sales computation referenced above.
Conference Call
The company is hosting a conference call with investors and analysts to discuss fourth quarter and full-year 2023 operating results today, Feb. 13, 2024, at 8:30 a.m. ET. The company invites participants to listen to a live webcast of the conference call on the company’s website, http://www.coca-colacompany.com, in the “Investors” section. An audio replay in downloadable digital format and a transcript of the call will be available on the website within 24 hours following the call. Further, the “Investors” section of the website includes certain supplemental information and a reconciliation of non-GAAP financial measures to the company’s results as reported under GAAP, which may be used during the call when discussing financial results.
Forward-Looking Statements
This press release may contain statements, estimates or projections that constitute “forward-looking statements” as defined under U.S. federal securities laws. Generally, the words “believe,” “expect,” “intend,” “estimate,” “anticipate,” “project,” “will” and similar expressions identify forward-looking statements, which generally are not historical in nature. Forward-looking statements are subject to certain risks and uncertainties that could cause The Coca‑Cola Company’s actual results to differ materially from its historical experience and our present expectations or projections. These risks include, but are not limited to, unfavorable economic and geopolitical conditions, including the direct or indirect negative impacts of the conflict between Russia and Ukraine and conflicts in the Middle East; increased competition; an inability to be successful in our innovation activities; changes in the retail landscape or the loss of key retail or foodservice customers; an inability to expand our business in emerging and developing markets; an inability to successfully manage the potential negative consequences of our productivity initiatives; an inability to attract or retain a highly skilled and diverse workforce; disruption of our supply chain, including increased commodity, raw material, packaging, energy, transportation and other input costs; an inability to successfully integrate and manage our acquired businesses, brands or bottling operations or an inability to realize a significant portion of the anticipated benefits of our joint ventures or strategic relationships; failure by our third-party service providers and business partners to satisfactorily fulfill their commitments and responsibilities; an inability to renew collective bargaining agreements on satisfactory terms, or we or our bottling partners experience strikes, work stoppages, labor shortages or labor unrest; obesity and other health-related concerns; evolving consumer product and shopping preferences; product safety and quality concerns; perceived negative health consequences of certain ingredients, such as non-nutritive sweeteners and biotechnology-derived substances, and of other substances present in our beverage products or packaging materials; failure to digitalize the Coca‑Cola system; damage to our brand image, corporate reputation and social license to operate from negative publicity, whether or not warranted, concerning product safety or quality, workplace and human rights, obesity or other issues; an inability to successfully manage new product launches; an inability to maintain good relationships with our bottling partners; deterioration in our bottling partners’ financial condition; an inability to successfully manage our refranchising activities; increases in income tax rates, changes in income tax laws or the unfavorable resolution of tax matters, including the outcome of our ongoing tax dispute or any related disputes with the U.S. Internal Revenue Service (“IRS”); the possibility that the assumptions used to calculate our estimated aggregate incremental tax and interest liability related to the potential unfavorable outcome of the ongoing tax dispute with the IRS could significantly change; increased or new indirect taxes; changes in laws and regulations relating to beverage containers and packaging; significant additional labeling or warning requirements or limitations on the marketing or sale of our products; litigation or legal proceedings; conducting business in markets with high-risk legal compliance environments; failure to adequately protect, or disputes relating to, trademarks, formulas and other intellectual property rights; changes in, or failure to comply with, the laws and regulations applicable to our products or our business operations; fluctuations in foreign currency exchange rates; interest rate increases; an inability to achieve our overall long-term growth objectives; default by or failure of one or more of our counterparty financial institutions; impairment charges; an inability to protect our information systems against service interruption, misappropriation of data or cybersecurity incidents; failure to comply with privacy and data protection laws; evolving sustainability regulatory requirements and expectations; increasing concerns about the environmental impact of plastic bottles and other packaging materials; water scarcity and poor quality; increased demand for food products, decreased agricultural productivity and increased regulation of ingredient sourcing due diligence; climate change and legal or regulatory responses thereto; adverse weather conditions; and other risks discussed in our filings with the Securities and Exchange Commission (“SEC”), including our Annual Report on Form 10-K for the year ended December 31, 2022, and our subsequently filed Quarterly Reports on Form 10-Q, which filings are available from the SEC. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. We undertake no obligation to publicly update or revise any forward-looking statements.
Download Q4 and Full-Year 2023 Earnings Results
Related Content
Coca‑Cola Collaborates with Tech Partners to Create Bottle Prototype Made from 100% Plant-Based Sources

Coca‑Cola Launches ‘Real Magic’ Brand Platform, Including Refreshed Visual Identity and Global Campaign

Iteration, for Good: How Project Last Mile is Supporting COVID-19 Vaccine Distribution in Africa and Beyond

- China Mainland
- Saudi Arabia
- Türkiye
- United Arab Emirates
- South Africa
- Australia and New Zealand
- Belgium and Luxembourg
- Czech Republic & Slovakia
- Netherlands
- Poland & The Baltics
- Switzerland
- United Kingdom
Press Releases
February 06, 2024
Gilead Sciences Announces Fourth Quarter and Full Year 2023 Financial Results
Product Sales Excluding Veklury Increased Year-Over-Year by 7% for Full Year 2023
Biktarvy Sales Increased Year-Over-Year by 14% for Full Year 2023
Oncology Sales Increased Year-Over-Year by 37% for Full Year 2023
FOSTER CITY, Calif.--(BUSINESS WIRE)-- Gilead Sciences, Inc. (Nasdaq: GILD) announced today its results of operations for the fourth quarter and full year 2023.
“This was another strong year of revenue growth for Gilead’s base business, driven by both HIV and Oncology,” said Daniel O’Day, Gilead’s Chairman and Chief Executive Officer. “The strength of the business provides a solid foundation as we enter a new catalyst-rich phase for the company. We are expecting several milestones in 2024, including updates on long-acting HIV prevention and treatment, Cell Therapy and Trodelvy.”
Fourth Quarter 2023 Financial Results
- Total fourth quarter 2023 revenue decreased 4% to $7.1 billion compared to the same period in 2022, primarily due to lower Veklury® (remdesivir) and HIV sales, partially offset by higher Oncology sales.
- Diluted Earnings Per Share (“EPS”) decreased to $1.14 in the fourth quarter 2023 compared to $1.30 in the same period in 2022, primarily due to higher total costs and expenses, and lower Veklury revenues, partially offset by unrealized gains on equity investments in 2023 compared to losses in 2022, and lower tax expense.
- Non-GAAP diluted EPS increased to $1.72 in the fourth quarter 2023 compared to $1.67 in the same period in 2022, primarily due to lower total costs and expenses, partially offset by lower Veklury revenues.
- As of December 31, 2023, Gilead had $8.4 billion of cash, cash equivalents and marketable debt securities compared to $7.6 billion as of December 31, 2022.
- During the fourth quarter 2023, Gilead generated $2.2 billion in operating cash flow.
- During the fourth quarter 2023, Gilead paid cash dividends of $943 million and utilized $150 million to repurchase common stock.
Fourth Quarter 2023 Product Sales
Total fourth quarter 2023 product sales decreased 4% to $7.1 billion compared to the same period in 2022. Total product sales, excluding Veklury, of $6.3 billion were flat compared to the same period in 2022, with higher Oncology sales partially offset by lower HIV sales.
HIV product sales decreased 2% to $4.7 billion in the fourth quarter 2023 compared to the same period in 2022, primarily driven by lower average realized price due to channel mix, partially offset by higher demand and channel inventory dynamics.
- Biktarvy® (bictegravir 50mg/emtricitabine (“FTC”) 200mg/tenofovir alafenamide (“TAF”) 25mg) sales increased 7% to $3.1 billion in the fourth quarter 2023 compared to the same period in 2022, primarily reflecting higher demand, partially offset by lower average realized price due to channel mix.
- Descovy® (FTC 200mg/TAF 25mg) sales decreased 5% to $509 million in the fourth quarter 2023 compared to the same period in 2022, primarily driven by unfavorable pricing dynamics in the United States, partially offset by higher demand and channel inventory dynamics.
The Liver Disease portfolio sales were $691 million in the fourth quarter 2023 and remained flat compared to the same period in 2022. Sales were impacted by unfavorable pricing dynamics, offset by higher demand across chronic hepatitis C virus (“HCV”) and chronic hepatitis delta virus (“HDV”) products.
Cell Therapy product sales increased 11% to $466 million in the fourth quarter 2023 compared to the same period in 2022.
- Yescarta® (axicabtagene ciloleucel) sales increased 9% to $368 million in the fourth quarter 2023 compared to the same period in 2022, primarily driven by strong demand in relapsed or refractory (“R/R”) large B-cell lymphoma (“LBCL”) outside the United States.
- Tecartus® (brexucabtagene autoleucel) sales increased 19% to $98 million in the fourth quarter 2023 compared to the same period in 2022, driven by increased demand in R/R adult acute lymphoblastic leukemia (“ALL”) and in R/R mantle cell lymphoma (“MCL”).
Trodelvy ® (sacituzumab govitecan-hziy) sales increased 53% to $299 million in the fourth quarter 2023 compared to the same period in 2022, reflecting higher demand in both the United States and Europe.
Veklury sales decreased 28% to $720 million in the fourth quarter 2023 compared to the same period in 2022, primarily driven by lower rates of COVID-19 related hospitalizations. Veklury sales generally reflect COVID-19 related rates and severity of infections and hospitalizations, as well as the availability, uptake and effectiveness of vaccinations and alternative treatments for COVID-19.
Fourth Quarter 2023 Product Gross Margin, Operating Expenses and Tax
- Product gross margin was 70.4% in the fourth quarter 2023 compared to 81.0% in the same period in 2022, primarily driven by restructuring expenses related to changes in our manufacturing strategy, intangible asset amortization expenses related to Trodelvy following approval for pretreated HR+/HER2- metastatic breast cancer in February 2023, and product mix. Non-GAAP product gross margin was 86.1% in the fourth quarter 2023 compared to 86.8% in the same period in 2022, primarily driven by product mix.
- Research and development (“R&D”) expenses were $1.4 billion in the fourth quarter 2023 compared to $1.5 billion in the same period in 2022, driven by the timing of clinical activities and valuation adjustments to the MYR-related contingent consideration, partially offset by increased investments in Oncology. Non-GAAP R&D expenses were $1.5 billion in the fourth quarter 2023 and in the same period in 2022. Decreases due to the timing of clinical activities were offset by increased investments in Oncology.
- Acquired in-process research and development (“IPR&D”) expenses were $347 million in the fourth quarter 2023, primarily driven by payments related to collaborations with Arcellx, Inc. (“Arcellx”), Assembly Biosciences, Inc. (“Assembly”), and Compugen Ltd. (“Compugen”), as well as a milestone payment related to the acquisition of XinThera, Inc. (“XinThera”).
- Selling, general and administrative (“SG&A”) and non-GAAP SG&A expenses were $1.6 billion in the fourth quarter 2023 compared to $2.0 billion in the same period in 2022. The decrease was primarily driven by a 2022 charge related to the termination of the Trodelvy collaboration agreement with Everest Medicines (“Everest”) that did not repeat.
- The effective tax rate (“ETR”) was 14.3% in the fourth quarter 2023 compared to 19.6% in the same period in 2022, primarily due to remeasurement of certain deferred tax liabilities and non-taxable unrealized gains on equity investments. Non-GAAP ETR was 17.1% in the fourth quarter 2023 compared to 16.8% in the same period in 2022.
Full Year 2023 Financial Results
- Total full year 2023 revenue decreased 1% to $27.1 billion compared to 2022, driven by a reduction of $1.7 billion in Veklury sales, largely offset by higher HIV and Oncology sales.
- Diluted EPS increased to $4.50 in the full year 2023 compared to $3.64 in 2022, primarily due to lower IPR&D impairment expenses, lower unrealized losses on equity investments, and higher interest income, partially offset by higher cost of goods sold and operating expenses, and lower Veklury sales.
- Non-GAAP diluted EPS decreased to $6.72 in the full year 2023 compared to $7.26 in 2022. This was primarily driven by higher total costs and expenses, as well as lower Veklury sales, partially offset by a decrease in tax reserves as a result of reaching an agreement with a tax authority, and higher interest income.
Full Year 2023 Product Sales
Total full year 2023 product sales of $26.9 billion were relatively flat compared to the same period in 2022, with lower Veklury sales largely offset by higher HIV and Oncology sales. Total product sales, excluding Veklury, increased 7% to $24.7 billion in the full year 2023 compared to 2022, primarily driven by higher HIV and Oncology sales.
HIV product sales increased 6% to $18.2 billion in the full year 2023 compared to 2022, primarily reflecting higher demand across treatment and prevention, in addition to higher average realized price and favorable channel inventory dynamics.
- Biktarvy sales increased 14% to $11.8 billion in the full year 2023 compared to 2022, primarily reflecting higher demand as well as higher average realized price.
- Descovy sales increased 6% to $2.0 billion in the full year 2023 compared to 2022, primarily driven by favorable channel inventory dynamics and higher demand.
The Liver Disease portfolio sales decreased 1% to $2.8 billion in the full year 2023 compared to 2022. The decrease was primarily driven by unfavorable HCV pricing dynamics and foreign exchange rates, partially offset by higher demand across HCV, HDV and chronic hepatitis B virus (“HBV”) products.
Cell Therapy product sales increased 28% to $1.9 billion in the full year 2023 compared to 2022.
- Yescarta sales increased 29% to $1.5 billion in the full year 2023 compared to 2022, primarily driven by increased demand in R/R LBCL.
- Tecartus sales increased 24% to $370 million in the full year 2023 compared to 2022, primarily driven by increased demand in in R/R ALL and R/R MCL.
Trodelvy sales increased 56% to $1.1 billion in the full year 2023 compared to 2022, reflecting strong demand in new and existing geographies.
Veklury sales decreased 44% to $2.2 billion in the full year 2023 compared to 2022, primarily driven by lower rates of COVID-19 related hospitalizations in all regions.
Full Year 2023 Product Gross Margin, Operating Expenses and Tax
- Product gross margin was 75.9% in the full year 2023 compared to 79.0% in 2022, primarily driven by restructuring expenses related to the aforementioned changes in our manufacturing strategy, increased amortization expenses, and product mix. Non-GAAP product gross margin was 86.3% in the full year 2023 compared to 86.6% in 2022, primarily driven by product mix.
- R&D and non-GAAP R&D expenses were $5.7 billion in the full year 2023 compared to $5.0 billion in 2022. This primarily reflects increased clinical activities across our Oncology and Virology programs.
- Acquired IPR&D expenses were $1.2 billion in the full year 2023, primarily driven by payments related to the collaboration with Arcellx, as well as the acquisition of XinThera and Tmunity Therapeutics Inc. (“Tmunity”).
- SG&A expenses were $6.1 billion in the full year 2023 compared to $5.7 billion in 2022. Non-GAAP SG&A expenses were $6.1 billion in the full year 2023 compared to $5.6 billion in 2022. The increases in SG&A and non-GAAP SG&A expenses are primarily due to a $525 million litigation settlement with certain plaintiffs in the HIV antitrust litigation and increased commercial activities in Oncology and HIV, partially offset by the 2022 charge related to the termination of the Trodelvy collaboration agreement with Everest.
- The ETR and non-GAAP ETR were 18.2% and 15.2%, respectively, in the full year 2023 compared to 21.5% and 19.3%, respectively, in 2022. Lower ETR is primarily due to a decrease in tax reserves as a result of reaching agreement with a tax authority on certain tax positions.
Guidance and Outlook
Gilead is providing full-year 2024 guidance below:
- Total product sales between $27.1 billion and $27.5 billion.
- Total product sales, excluding Veklury, between $25.8 billion and $26.2 billion.
- Total Veklury sales of approximately $1.3 billion.
- Diluted EPS between $5.15 and $5.55.
- Non-GAAP diluted EPS between $6.85 and $7.25.
Additional information and a reconciliation between GAAP and non-GAAP financial information for the 2024 guidance is provided in the accompanying tables. The financial guidance is subject to a number of risks and uncertainties. See the Forward-Looking Statements section below.
Key Updates Since Our Last Quarterly Release
- Announced that the Phase 3 OAKTREE trial of obeldesivir in non-hospitalized participants without risk factors for developing severe COVID-19 did not meet its primary endpoint of improvement in time to symptom alleviation. Obeldesivir was well-tolerated in this large study population.
- Announced that the Phase 3 EVOKE-01 study of Trodelvy versus docetaxel in previously treated metastatic non-small cell lung cancer did not meet its primary endpoint of overall survival. While not statistically powered, we observed an encouraging trend in a subgroup of patients non-responsive to prior anti-PD-(L)1 immunotherapy, that we may potentially explore further.
- Presented new data at the San Antonio Breast Cancer Symposium 2023, including a post-hoc, subgroup analysis of clinical outcomes by age from the Phase 3 TROPiCS-02 study of Trodelvy in HR+/HER2- metastatic breast cancer.
- Presented new data at the American Society of Hematology 2023 Annual Meeting (“ASH”) including long-term follow-up across Yescarta trials in R/R LBCL, first-line high-risk LBCL, and R/R follicular lymphoma, as well as real-world data for Tecartus in R/R MCL and B-cell ALL.
- Received U.S. Food and Drug Administration (“FDA”) approval of Yescarta’s label update to include overall survival (“OS”) data from the Phase 3 ZUMA-7 study, which showed a statistically significant OS improvement for Yescarta in second-line R/R LBCL versus standard of care.
- Received FDA approval of a manufacturing process change resulting in reduced median turnaround time for Yescarta in the U.S. to an anticipated 14 days (from 16 days previously).
- Our partner Arcellx presented updated data at ASH from the Phase 1 trial evaluating anitocabtagene autoleucel (“anito-cel”) in R/R multiple myeloma. With 26.5 months of median follow-up, the data demonstrated continued deep and durable responses, including in patients with extramedullary disease, with median progression-free survival not reached. In addition, no cases of delayed neurotoxicity events or parkinsonian symptoms were observed.
- Announced expansion of the Arcellx collaboration to include exercising an option for the ARC-SparX ACLX-001 program in multiple myeloma, expanding the scope of the existing anito-cel collaboration to include lymphomas, and a further equity investment of $200 million.
- Announced an amended collaboration agreement with Arcus Biosciences, Inc. (“Arcus”), including an update to the domvanalimab development collaboration program, and an additional $320 million equity investment.
- Announced an exclusive license agreement with Compugen for later-stage development and commercialization of novel pre-clinical anti-IL18 binding protein antibodies, including COM503, that have the potential to treat various tumor types.
- Announced that Ted Love has joined Gilead’s Board of Directors.
- Named to Dow Jones Sustainability World Index for third consecutive year and added to the North America Index for the first year, reflecting Gilead’s ongoing commitment to corporate responsibility and sustainability.
- The company’s Board of Directors declared a quarterly dividend of $0.77 per share of common stock for the first quarter of 2024. The dividend is payable on March 28, 2024, to stockholders of record at the close of business on March 15, 2024. Future dividends will be subject to Board approval.
Certain amounts and percentages in this press release may not sum or recalculate due to rounding.
Conference Call
At 2:00 p.m. Pacific Time today, Gilead will host a conference call to discuss Gilead’s results. A live webcast will be available on http://investors.gilead.com and will be archived on www.gilead.com for one year.
Non-GAAP Financial Information
The information presented in this document has been prepared in accordance with U.S. generally accepted accounting principles (“GAAP”), unless otherwise noted as non-GAAP. Management believes non-GAAP information is useful for investors, when considered in conjunction with Gilead’s GAAP financial information, because management uses such information internally for its operating, budgeting and financial planning purposes. Non-GAAP information is not prepared under a comprehensive set of accounting rules and should only be used to supplement an understanding of Gilead’s operating results as reported under GAAP. Non-GAAP financial information generally excludes acquisition-related expenses including amortization of acquired intangible assets and inventory step-up charges, and other items that are considered unusual or not representative of underlying trends of Gilead’s business, fair value adjustments of equity securities and discrete and related tax charges or benefits associated with changes in tax related laws and guidelines. Although Gilead consistently excludes the amortization of acquired intangible assets from the non-GAAP financial information, management believes that it is important for investors to understand that such intangible assets were recorded as part of acquisitions and contribute to ongoing revenue generation. Non-GAAP measures may be defined and calculated differently by other companies in the same industry. Reconciliations of the non-GAAP financial measures to the most directly comparable GAAP financial measures are provided in the accompanying tables.
About Gilead Sciences
Gilead Sciences, Inc. is a biopharmaceutical company that has pursued and achieved breakthroughs in medicine for more than three decades, with the goal of creating a healthier world for all people. The company is committed to advancing innovative medicines to prevent and treat life-threatening diseases, including HIV, viral hepatitis, COVID-19 and cancer. Gilead operates in more than 35 countries worldwide, with headquarters in Foster City, California.
Forward-Looking Statements
Statements included in this press release that are not historical in nature are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Gilead cautions readers that forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially. These risks and uncertainties include those relating to: Gilead’s ability to achieve its anticipated full year 2024 financial results, including as a result of the uncertainty of the amount and timing of Veklury revenues; Gilead’s ability to make progress on any of its long-term ambitions or strategic priorities laid out in its corporate strategy; Gilead’s ability to accelerate or sustain revenues for its virology, oncology and other programs; Gilead’s ability to realize the potential benefits of acquisitions, collaborations or licensing arrangements, including the arrangements with Arcellx, Arcus, Assembly, Compugen, Galapagos NV, MYR, Tmunity, and XinThera; patent protection and estimated loss of exclusivity for our products and product candidates; Gilead’s ability to initiate, progress or complete clinical trials within currently anticipated timeframes or at all, the possibility of unfavorable results from ongoing and additional clinical trials, including those involving Tecartus, Trodelvy (including EVOKE-01), Yescarta, anito-cel and domvanalimab, and the risk that safety and efficacy data from clinical trials may not warrant further development of Gilead’s product candidates or the product candidates of Gilead’s strategic partners; Gilead’s ability to submit new drug applications for new product candidates or expanded indications in the currently anticipated timelines; Gilead’s ability to receive regulatory approvals in a timely manner or at all, and the risk that any such approvals, if granted, may be subject to significant limitations on use; Gilead’s ability to successfully commercialize its products; the risk of potential disruptions to the manufacturing and supply chain of Gilead’s products; pricing and reimbursement pressures from government agencies and other third parties, including required rebates and other discounts; a larger than anticipated shift in payer mix to more highly discounted payer segments; market share and price erosion caused by the introduction of generic versions of Gilead products; the risk that physicians and patients may not see advantages of these products over other therapies and may therefore be reluctant to prescribe the products, including Yescarta; and other risks identified from time to time in Gilead’s reports filed with the SEC, including annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K. In addition, Gilead makes estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses and related disclosures. Gilead bases its estimates on historical experience and on various other market specific and other relevant assumptions that it believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. There may be other factors of which Gilead is not currently aware that may affect matters discussed in the forward-looking statements and may also cause actual results to differ significantly from these estimates. Further, results for the quarter ended December 31, 2023 are not necessarily indicative of operating results for any future periods. Gilead directs readers to its press releases, annual reports on Form 10-K, quarterly reports on Form 10-Q and other subsequent disclosure documents filed with the SEC. Gilead claims the protection of the Safe Harbor contained in the Private Securities Litigation Reform Act of 1995 for forward-looking statements.
The reader is cautioned that forward-looking statements are not guarantees of future performance and is cautioned not to place undue reliance on these forward-looking statements. All forward-looking statements are based on information currently available to Gilead and Gilead assumes no obligation to update or supplement any such forward-looking statements other than as required by law. Any forward-looking statements speak only as of the date hereof or as of the dates indicated in the statements.
Gilead owns or has rights to various trademarks, copyrights and trade names used in its business, including the following: GILEAD®, GILEAD SCIENCES®, KITE™, AMBISOME®, ATRIPLA®, BIKTARVY®, CAYSTON®, COMPLERA®, DESCOVY®, DESCOVY FOR PREP®, EMTRIVA®, EPCLUSA®, EVIPLERA®, GENVOYA®, HARVONI®, HEPCLUDEX®, HEPSERA®, JYSELECA®, LETAIRIS®, ODEFSEY®, SOVALDI®, STRIBILD®, SUNLENCA® , TECARTUS®, TRODELVY®, TRUVADA®, TRUVADA FOR PREP®, TYBOST®, VEKLURY®, VEMLIDY®, VIREAD®, VOSEVI®, YESCARTA® and ZYDELIG®. KEYTRUDA® is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, United States. Other trademarks are the property of their respective owners.
For more information on Gilead Sciences, Inc., please visit www.gilead.com or call the Gilead Public Affairs Department at 1-800-GILEAD-5 (1-800-445-3235).
|
| ||||||||||||||||
|
|
|
|
|
| ||||||||||||
|
|
|
|
|
| ||||||||||||
|
|
|
|
|
|
|
|
|
| ||||||||
| Revenues: |
|
|
|
|
|
|
|
| ||||||||
| Product sales |
| $ | 7,070 |
|
| $ | 7,333 |
|
| $ | 26,934 |
|
| $ | 26,982 |
|
| Royalty, contract and other revenues |
|
| 45 |
|
|
| 56 |
|
|
| 182 |
|
|
| 299 |
|
| Total revenues |
|
| 7,115 |
|
|
| 7,389 |
|
|
| 27,116 |
|
|
| 27,281 |
|
| Costs and expenses: |
|
|
|
|
|
|
|
| ||||||||
| Cost of goods sold |
|
| 2,090 |
|
|
| 1,396 |
|
|
| 6,498 |
|
|
| 5,657 |
|
| Research and development expenses |
|
| 1,408 |
|
|
| 1,548 |
|
|
| 5,718 |
|
|
| 4,977 |
|
| Acquired in-process research and development expenses |
|
| 347 |
|
|
| 158 |
|
|
| 1,155 |
|
|
| 944 |
|
| In-process research and development impairment |
|
| 50 |
|
|
| — |
|
|
| 50 |
|
|
| 2,700 |
|
| Selling, general and administrative expenses |
|
| 1,608 |
|
|
| 2,020 |
|
|
| 6,090 |
|
|
| 5,673 |
|
| Total costs and expenses |
|
| 5,503 |
|
|
| 5,122 |
|
|
| 19,511 |
|
|
| 19,951 |
|
| Operating income |
|
| 1,612 |
|
|
| 2,267 |
|
|
| 7,605 |
|
|
| 7,330 |
|
| Interest expense |
|
| (252 | ) |
|
| (227 | ) |
|
| (944 | ) |
|
| (935 | ) |
| Other income (expense), net |
|
| 293 |
|
|
| (9 | ) |
|
| 198 |
|
|
| (581 | ) |
| Income before income taxes |
|
| 1,653 |
|
|
| 2,031 |
|
|
| 6,859 |
|
|
| 5,814 |
|
| Income tax expense |
|
| (236 | ) |
|
| (398 | ) |
|
| (1,247 | ) |
|
| (1,248 | ) |
| Net income |
|
| 1,417 |
|
|
| 1,633 |
|
|
| 5,613 |
|
|
| 4,566 |
|
| Net loss attributable to noncontrolling interest |
|
| 12 |
|
|
| 7 |
|
|
| 52 |
|
|
| 26 |
|
| Net income attributable to Gilead |
| $ | 1,429 |
|
| $ | 1,640 |
|
| $ | 5,665 |
|
| $ | 4,592 |
|
| Basic earnings per share attributable to Gilead |
| $ | 1.15 |
|
| $ | 1.31 |
|
| $ | 4.54 |
|
| $ | 3.66 |
|
| Shares used in basic earnings per share attributable to Gilead calculation |
|
| 1,248 |
|
|
| 1,252 |
|
|
| 1,248 |
|
|
| 1,255 |
|
| Diluted earnings per share attributable to Gilead |
| $ | 1.14 |
|
| $ | 1.30 |
|
| $ | 4.50 |
|
| $ | 3.64 |
|
| Shares used in diluted earnings per share attributable to Gilead calculation |
|
| 1,256 |
|
|
| 1,264 |
|
|
| 1,258 |
|
|
| 1,262 |
|
| Cash dividends declared per share |
| $ | 0.75 |
|
| $ | 0.73 |
|
| $ | 3.00 |
|
| $ | 2.92 |
|
|
|
|
|
|
|
|
|
|
| ||||||||
| Research and development expenses as a % of revenues |
|
| 19.8 | % |
|
| 20.9 | % |
|
| 21.1 | % |
|
| 18.2 | % |
| Selling, general and administrative expenses as a % of revenues |
|
| 22.6 | % |
|
| 27.3 | % |
|
| 22.5 | % |
|
| 20.8 | % |
|
| ||||||||||||||||||
|
|
|
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
| Product sales: |
|
|
|
|
|
|
|
|
|
|
|
| ||||||
| HIV |
| $ | 4,693 |
| $ | 4,772 |
|
|
|
| $ | 18,175 |
| $ | 17,194 |
|
|
|
| Oncology |
|
| 765 |
|
| 614 |
|
|
|
|
| 2,932 |
|
| 2,139 |
|
|
|
| Liver Disease |
|
| 691 |
|
| 694 |
|
|
|
|
| 2,784 |
|
| 2,798 |
|
|
|
| Other |
|
| 201 |
|
| 252 |
|
|
|
|
| 859 |
|
| 946 |
|
|
|
| Total product sales excluding Veklury |
|
| 6,350 |
|
| 6,333 |
|
|
|
|
| 24,750 |
|
| 23,077 |
|
|
|
| Veklury |
|
| 720 |
|
| 1,000 |
|
|
|
|
| 2,184 |
|
| 3,905 |
|
|
|
| Total product sales |
|
| 7,070 |
|
| 7,333 |
|
|
|
|
| 26,934 |
|
| 26,982 |
|
|
|
| Royalty, contract and other revenues |
|
| 45 |
|
| 56 |
|
|
|
|
| 182 |
|
| 299 |
|
|
|
| Total revenues |
| $ | 7,115 |
| $ | 7,389 |
|
|
|
| $ | 27,116 |
| $ | 27,281 |
|
|
|
|
| ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
| ||||||||||||||
|
|
|
|
|
|
|
|
|
| ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||
| Non-GAAP: |
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||
| Cost of goods sold |
| $ | 980 |
|
| $ | 968 |
|
|
|
|
| $ | 3,697 |
|
| $ | 3,602 |
|
|
|
|
| Research and development expenses |
| $ | 1,452 |
|
| $ | 1,544 |
|
|
|
|
| $ | 5,720 |
|
| $ | 4,968 |
|
|
|
|
| Acquired IPR&D expenses |
| $ | 347 |
|
| $ | 158 |
|
|
|
|
| $ | 1,155 |
|
| $ | 944 |
|
|
|
|
| Selling, general and administrative expenses |
| $ | 1,597 |
|
| $ | 2,020 |
|
|
|
|
| $ | 6,060 |
|
| $ | 5,587 |
|
|
|
|
| Other income (expense), net |
| $ | 104 |
|
| $ | 52 |
|
|
|
|
| $ | 365 |
|
| $ | 77 |
|
|
|
|
| Diluted EPS |
| $ | 1.72 |
|
| $ | 1.67 |
|
|
|
|
| $ | 6.72 |
|
| $ | 7.26 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||
| Product gross margin |
|
| 86.1 | % |
|
| 86.8 | % |
|
|
|
| 86.3 | % |
|
| 86.6 | % |
|
| ||
| Research and development expenses as a % of revenues |
|
| 20.4 | % |
|
| 20.9 | % |
|
|
|
| 21.1 | % |
|
| 18.2 | % |
|
| ||
| Selling, general and administrative expenses as a % of revenues |
|
| 22.4 | % |
|
| 27.3 | % |
|
|
|
| 22.3 | % |
|
| 20.5 | % |
|
| ||
| Operating margin |
|
| 38.5 | % |
|
| 36.5 | % |
|
|
|
| 38.7 | % |
|
| 44.6 | % |
|
| ||
| Effective tax rate |
|
| 17.1 | % |
|
| 16.8 | % |
|
|
|
| 15.2 | % |
|
| 19.3 | % |
|
| ||
| ________________________________ NM - Not Meaningful | ||
|
|
| Refer to Non-GAAP Financial Information section above for further disclosures on non-GAAP financial measures. A reconciliation between GAAP and non-GAAP financial information is provided in the tables below. |
|
|
| Equal to GAAP financial information. |
|
| ||||||||||||||||
|
|
|
|
|
| ||||||||||||
|
|
|
|
|
| ||||||||||||
|
|
|
|
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
|
|
|
| ||||||||
| GAAP cost of goods sold |
| $ | 2,090 |
|
| $ | 1,396 |
|
| $ | 6,498 |
|
| $ | 5,657 |
|
| Acquisition-related – amortization |
|
| (580 | ) |
|
| (428 | ) |
|
| (2,271 | ) |
|
| (2,013 | ) |
| Restructuring | . |
| (479 | ) |
|
| — |
|
|
| (479 | ) |
|
| (42 | ) |
| Other |
|
| (51 | ) |
|
| — |
|
|
| (51 | ) |
|
| — |
|
| Non-GAAP cost of goods sold |
| $ | 980 |
|
| $ | 968 |
|
| $ | 3,697 |
|
| $ | 3,602 |
|
|
|
|
|
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
|
|
|
| ||||||||
| GAAP product gross margin |
|
| 70.4 | % |
|
| 81.0 | % |
|
| 75.9 | % |
|
| 79.0 | % |
| Acquisition-related – amortization |
|
| 8.2 | % |
|
| 5.8 | % |
|
| 8.4 | % |
|
| 7.5 | % |
| Restructuring | . |
| 6.8 | % |
|
| — | % |
|
| 1.8 | % |
|
| 0.2 | % |
| Other |
|
| 0.7 | % |
|
| — | % |
|
| 0.2 | % |
|
| — | % |
| Non-GAAP product gross margin |
|
| 86.1 | % |
|
| 86.8 | % |
|
| 86.3 | % |
|
| 86.6 | % |
|
|
|
|
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
|
|
|
| ||||||||
| GAAP research and development expenses |
| $ | 1,408 |
|
| $ | 1,548 |
|
| $ | 5,718 |
|
| $ | 4,977 |
|
| Acquisition-related – other costs |
|
| 59 |
|
|
| (1 | ) |
|
| 22 |
|
|
| 13 |
|
| Restructuring | . |
| (15 | ) | . |
| (4 | ) |
|
| (20 | ) |
|
| (22 | ) |
| Non-GAAP research and development expenses |
| $ | 1,452 |
|
| $ | 1,544 |
|
| $ | 5,720 |
|
| $ | 4,968 |
|
|
|
|
|
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
|
|
|
| ||||||||
| GAAP IPR&D impairment |
| $ | 50 |
|
| $ | — |
|
| $ | 50 |
|
| $ | 2,700 |
|
| IPR&D impairment |
|
| (50 | ) |
|
| — |
|
|
| (50 | ) |
|
| (2,700 | ) |
| Non-GAAP IPR&D impairment |
| $ | — |
|
| $ | — |
|
| $ | — |
|
| $ | — |
|
|
|
|
|
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
|
|
|
| ||||||||
| GAAP selling, general and administrative expenses |
| $ | 1,608 |
|
| $ | 2,020 |
|
| $ | 6,090 |
|
| $ | 5,673 |
|
| Acquisition-related – other costs |
|
| — |
|
|
| (1 | ) |
|
| (2 | ) |
|
| (3 | ) |
| Restructuring |
|
| (11 | ) | . |
| 1 |
|
|
| (28 | ) |
|
| 2 |
|
| Other |
|
| — |
|
|
| — |
|
|
| — |
|
|
| (85 | ) |
| Non-GAAP selling, general and administrative expenses |
| $ | 1,597 |
|
| $ | 2,020 |
|
| $ | 6,060 |
|
| $ | 5,587 |
|
|
|
|
|
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
|
|
|
| ||||||||
| GAAP operating income |
| $ | 1,612 |
|
| $ | 2,267 |
|
| $ | 7,605 |
|
| $ | 7,330 |
|
| Acquisition-related – amortization |
|
| 580 |
|
|
| 428 |
|
|
| 2,271 |
|
|
| 2,013 |
|
| Acquisition-related – other costs |
|
| (59 | ) | . |
| 2 |
|
|
| (20 | ) |
|
| (10 | ) |
| Restructuring |
|
| 505 |
| . |
| 2 |
|
|
| 527 |
|
|
| 62 |
|
| IPR&D impairment |
|
| 50 |
| . |
| — |
|
|
| 50 |
|
|
| 2,700 |
|
| Other |
|
| 51 |
|
|
| — |
|
|
| 51 |
|
|
| 85 |
|
| Non-GAAP operating income |
| $ | 2,739 |
|
| $ | 2,699 |
|
| $ | 10,484 |
|
| $ | 12,180 |
|
|
|
|
|
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
|
|
|
| ||||||||
| GAAP operating margin |
|
| 22.7 | % |
|
| 30.7 | % |
|
| 28.0 | % |
|
| 26.9 | % |
| Acquisition-related – amortization |
|
| 8.1 | % |
|
| 5.8 | % |
|
| 8.4 | % |
|
| 7.4 | % |
| Acquisition-related – other costs |
|
| (0.8 | ) % |
|
| — | % |
|
| (0.1 | ) % |
|
| — | % |
| Restructuring | . |
| 7.1 | % |
|
| — | % |
|
| 1.9 | % |
|
| 0.2 | % |
| IPR&D impairment |
|
| 0.7 | % |
|
| — | % |
|
| 0.2 | % |
|
| 9.9 | % |
| Other |
|
| 0.7 | % |
|
| — | % |
|
| 0.2 | % |
|
| 0.3 | % |
| Non-GAAP operating margin |
|
| 38.5 | % |
|
| 36.5 | % |
|
| 38.7 | % |
|
| 44.6 | % |
|
|
|
|
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
|
|
|
| ||||||||
| GAAP other income (expense), net |
| $ | 293 |
|
| $ | (9 | ) |
| $ | 198 |
|
| $ | (581 | ) |
| (Gain) loss from equity securities, net |
|
| (189 | ) |
|
| 61 |
|
|
| 167 |
|
|
| 657 |
|
| Non-GAAP other income (expense), net |
| $ | 104 |
|
| $ | 52 |
|
| $ | 365 |
|
| $ | 77 |
|
|
| ||||||||||||||||
|
|
|
|
|
| ||||||||||||
|
|
|
|
|
| ||||||||||||
|
|
|
|
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
|
|
|
| ||||||||
| GAAP effective tax rate |
|
| 14.3 | % |
|
| 19.6 | % |
|
| 18.2 | % |
|
| 21.5 | % |
| Income tax effect of above non-GAAP adjustments and discrete and related tax adjustments |
|
| 2.8 | % |
|
| (2.8 | ) % |
|
| (3.0 | ) % |
|
| (2.1 | ) % |
| Non-GAAP effective tax rate |
|
| 17.1 | % |
|
| 16.8 | % |
|
| 15.2 | % |
|
| 19.3 | % |
|
|
|
|
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
|
|
|
| ||||||||
| GAAP net income attributable to Gilead |
| $ | 1,429 |
|
| $ | 1,640 |
|
| $ | 5,665 |
|
| $ | 4,592 |
|
| Acquisition-related – amortization |
|
| 460 |
|
|
| 346 |
|
|
| 1,805 |
|
|
| 1,610 |
|
| Acquisition-related – other costs |
|
| (59 | ) |
|
| 1 |
|
|
| (29 | ) |
|
| (12 | ) |
| Restructuring |
|
| 414 |
| . |
| 2 |
|
|
| 431 |
|
|
| 47 |
|
| IPR&D impairment |
|
| 35 |
|
|
| — |
|
|
| 35 |
|
|
| 2,057 |
|
| (Gain) loss from equity securities, net |
|
| (171 | ) |
|
| 60 |
|
|
| 180 |
|
|
| 630 |
|
| Discrete and related tax charges |
|
| 12 |
|
|
| 57 |
|
|
| 326 |
|
|
| 175 |
|
| Other |
|
| 40 |
|
|
| — |
|
|
| 40 |
|
|
| 59 |
|
| Non-GAAP net income attributable to Gilead |
| $ | 2,161 |
|
| $ | 2,106 |
|
| $ | 8,454 |
|
| $ | 9,158 |
|
|
|
|
|
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
|
|
|
| ||||||||
| GAAP diluted earnings per share |
| $ | 1.14 |
|
| $ | 1.30 |
|
| $ | 4.50 |
|
| $ | 3.64 |
|
| Acquisition-related – amortization |
|
| 0.37 |
|
|
| 0.27 |
|
|
| 1.43 |
|
|
| 1.28 |
|
| Acquisition-related – other costs |
|
| (0.05 | ) |
|
| — |
|
|
| (0.02 | ) |
|
| (0.01 | ) |
| Restructuring | . |
| 0.33 |
|
|
| — |
|
|
| 0.34 |
|
|
| 0.04 |
|
| IPR&D impairment |
|
| 0.03 |
|
|
| — |
|
|
| 0.03 |
|
|
| 1.63 |
|
| (Gain) loss from equity securities, net |
|
| (0.14 | ) |
|
| 0.05 |
|
|
| 0.14 |
|
|
| 0.50 |
|
| Discrete and related tax charges |
|
| 0.01 |
|
|
| 0.05 |
|
|
| 0.26 |
|
|
| 0.14 |
|
| Other |
|
| 0.03 |
|
|
| — |
|
|
| 0.03 |
|
|
| 0.05 |
|
| Non-GAAP diluted earnings per share |
| $ | 1.72 |
|
| $ | 1.67 |
|
| $ | 6.72 |
|
| $ | 7.26 |
|
|
|
|
|
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
|
|
|
| ||||||||
| Cost of goods sold adjustments |
| $ | 1,110 |
|
| $ | 428 |
|
| $ | 2,801 |
|
| $ | 2,055 |
|
| Research and development expenses adjustments |
|
| (44 | ) |
|
| 4 |
|
|
| (2 | ) |
|
| 9 |
|
| IPR&D impairment adjustments |
|
| 50 |
|
|
| — |
|
|
| 50 |
|
|
| 2,700 |
|
| Selling, general and administrative expenses adjustments |
|
| 11 |
|
|
| — |
|
|
| 30 |
|
|
| 86 |
|
| Total non-GAAP adjustments to costs and expenses |
|
| 1,127 |
|
|
| 432 |
|
|
| 2,879 |
|
|
| 4,850 |
|
| Other income (expense), net, adjustments |
|
| (189 | ) |
|
| 61 |
|
|
| 167 |
|
|
| 657 |
|
| Total non-GAAP adjustments before income taxes |
|
| 938 |
|
|
| 493 |
|
|
| 3,046 |
|
|
| 5,507 |
|
| Income tax effect of non-GAAP adjustments above |
|
| (218 | ) |
|
| (84 | ) |
|
| (583 | ) |
|
| (1,116 | ) |
| Discrete and related tax charges |
|
| 12 |
|
|
| 57 |
|
|
| 326 |
|
|
| 175 |
|
| Total non-GAAP adjustments to net income attributable to Gilead |
| $ | 732 |
|
| $ | 466 |
|
| $ | 2,789 |
|
| $ | 4,566 |
|
| ______________________________ | ||
|
|
| Relates to amortization of acquired intangibles and inventory step-up charges. |
|
|
| Relates to a write-off of an intangible asset related to the restructuring of our collaboration with Galapagos NV during the fourth quarter of 2023. |
|
|
| Relates to donations to the Gilead Foundation, a California nonprofit organization, during the second quarter of 2022. |
|
|
| Adjustments include integration expenses, contingent consideration fair value adjustments and other expenses associated with Gilead’s acquisitions of MYR GmbH, MiroBio, Ltd., Tmunity Therapeutics, Inc. and XinThera, Inc. |
|
|
| Represents discrete and related deferred tax charges or benefits primarily associated with acquired intangible assets and transfers of intangible assets from a foreign subsidiary to Ireland and the United States. |
|
| ||
|
|
|
|
|
|
|
|
| GAAP projected product gross margin |
| 76.0% - 77.0% |
| Acquisition-related expenses and other |
| ~ 9.0% |
| Non-GAAP projected product gross margin |
| 85.0% - 86.0% |
|
|
|
|
|
|
|
|
| GAAP projected operating income |
| $8,700 - $9,200 |
| Acquisition-related expenses and other |
| ~ 2,500 |
| Non-GAAP projected operating income |
| $11,200 - $11,700 |
|
|
|
|
|
|
|
|
| GAAP projected effective tax rate |
| ~ 21% |
| Income tax effect of above non-GAAP adjustments, and discrete and related tax adjustments |
| (~ 2%) |
| Non-GAAP projected effective tax rate |
| ~ 19% |
|
|
|
|
|
|
|
|
| GAAP projected diluted EPS |
| $5.15 - $5.55 |
| Acquisition-related expenses and other, and discrete and related tax adjustments |
| ~ 1.70 |
| Non-GAAP projected diluted EPS |
| $6.85 - $7.25 |
| ________________________________ | ||
|
|
| Our full-year guidance excludes the potential impact of any (i) acquisitions or business development transactions that have not been executed, (ii) future fair value adjustments of equity securities and (iii) discrete tax charges or benefits associated with changes in tax related laws and guidelines that have not been enacted, as Gilead is unable to project such amounts. The non-GAAP full-year guidance includes non-GAAP adjustments to actual current period results as well as adjustments for the known future impact associated with events that have already occurred, such as future amortization of our intangible assets and the future impact of discrete and related deferred tax charges or benefits primarily associated with acquired intangible assets and transfers of intangible assets from a foreign subsidiary to Ireland and the United States. |
|
| ||||||
|
|
|
| ||||
|
|
|
|
|
| ||
|
|
|
|
|
| ||
| Cash, cash equivalents and marketable debt securities |
| $ | 8,428 |
| $ | 7,630 |
| Accounts receivable, net |
|
| 4,660 |
|
| 4,777 |
| Inventories |
|
| 3,366 |
|
| 2,820 |
| Property, plant and equipment, net |
|
| 5,317 |
|
| 5,475 |
| Intangible assets, net |
|
| 26,454 |
|
| 28,894 |
| Goodwill |
|
| 8,314 |
|
| 8,314 |
| Other assets |
|
| 5,586 |
|
| 5,262 |
| Total assets |
| $ | 62,125 |
| $ | 63,171 |
|
|
|
|
|
| ||
| Current liabilities |
| $ | 11,280 |
| $ | 11,237 |
| Long-term liabilities |
|
| 28,096 |
|
| 30,725 |
| Stockholders’ equity |
|
| 22,749 |
|
| 21,209 |
| Total liabilities and stockholders’ equity |
| $ | 62,125 |
| $ | 63,171 |
| ________________________________ | ||
|
|
| As of December 31, 2023 and 2022, there were 1,246 and 1,247 shares of common stock issued and outstanding, respectively. |
|
| ||||||||||||||||
|
|
|
|
|
| ||||||||||||
|
|
|
|
|
| ||||||||||||
|
|
|
|
|
|
|
|
|
| ||||||||
| Net cash provided by operating activities |
| $ | 2,168 |
|
| $ | 2,566 |
|
| $ | 8,006 |
|
| $ | 9,072 |
|
| Net cash used in investing activities |
|
| (726 | ) |
|
| (374 | ) |
|
| (2,265 | ) |
|
| (2,466 | ) |
| Net cash used in financing activities |
|
| (1,100 | ) |
|
| (1,554 | ) |
|
| (5,125 | ) |
|
| (6,469 | ) |
| Effect of exchange rate changes on cash and cash equivalents |
|
| 37 |
|
|
| 75 |
|
|
| 57 |
|
|
| (63 | ) |
| Net change in cash and cash equivalents |
|
| 380 |
|
|
| 713 |
|
|
| 673 |
|
|
| 74 |
|
| Cash and cash equivalents at beginning of period |
|
| 5,705 |
|
|
| 4,699 |
|
|
| 5,412 |
|
|
| 5,338 |
|
| Cash and cash equivalents at end of period |
| $ | 6,085 |
|
| $ | 5,412 |
|
| $ | 6,085 |
|
| $ | 5,412 |
|
|
|
|
|
|
| ||||||||||||
|
|
|
|
|
| ||||||||||||
|
|
|
|
|
|
|
|
|
| ||||||||
| Net cash provided by operating activities |
| $ | 2,168 |
|
| $ | 2,566 |
|
| $ | 8,006 |
|
| $ | 9,072 |
|
| Capital expenditures |
|
| (214 | ) |
|
| (181 | ) |
|
| (585 | ) |
|
| (728 | ) |
| Free cash flow |
| $ | 1,954 |
|
| $ | 2,386 |
|
| $ | 7,421 |
|
| $ | 8,344 |
|
| ________________________________ | ||
|
|
| Free cash flow is a non-GAAP liquidity measure. Please refer to our disclosures in the Non-GAAP Financial Information section above. |
|
| ||||||||||||
|
|
|
|
|
| ||||||||
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
|
|
|
| ||||
|
|
|
|
|
|
|
|
|
| ||||
| Biktarvy – U.S. |
| $ | 2,588 |
| $ | 2,423 |
| $ | 9,692 |
| $ | 8,510 |
| Biktarvy – Europe |
|
| 333 |
|
| 295 |
|
| 1,253 |
|
| 1,103 |
| Biktarvy – Other International |
|
| 188 |
|
| 200 |
|
| 905 |
|
| 777 |
|
|
|
| 3,109 |
|
| 2,918 |
|
| 11,850 |
|
| 10,390 |
|
|
|
|
|
|
|
|
|
| ||||
| Complera / Eviplera – U.S. |
|
| 6 |
|
| 17 |
|
| 47 |
|
| 74 |
| Complera / Eviplera – Europe |
|
| 15 |
|
| 37 |
|
| 70 |
|
| 113 |
| Complera / Eviplera – Other International |
|
| 3 |
|
| 3 |
|
| 12 |
|
| 13 |
|
|
|
| 24 |
|
| 58 |
|
| 129 |
|
| 200 |
|
|
|
|
|
|
|
|
|
| ||||
| Descovy – U.S. |
|
| 457 |
|
| 479 |
|
| 1,771 |
|
| 1,631 |
| Descovy – Europe |
|
| 25 |
|
| 26 |
|
| 100 |
|
| 118 |
| Descovy – Other International |
|
| 28 |
|
| 31 |
|
| 114 |
|
| 123 |
|
|
|
| 509 |
|
| 537 |
|
| 1,985 |
|
| 1,872 |
|
|
|
|
|
|
|
|
|
| ||||
| Genvoya – U.S. |
|
| 447 |
|
| 543 |
|
| 1,752 |
|
| 1,983 |
| Genvoya – Europe |
|
| 48 |
|
| 64 |
|
| 205 |
|
| 284 |
| Genvoya – Other International |
|
| 22 |
|
| 33 |
|
| 103 |
|
| 136 |
|
|
|
| 517 |
|
| 640 |
|
| 2,060 |
|
| 2,404 |
|
|
|
|
|
|
|
|
|
| ||||
| Odefsey – U.S. |
|
| 258 |
|
| 295 |
|
| 1,012 |
|
| 1,058 |
| Odefsey – Europe |
|
| 71 |
|
| 85 |
|
| 294 |
|
| 364 |
| Odefsey – Other International |
|
| 11 |
|
| 11 |
|
| 44 |
|
| 47 |
|
|
|
| 340 |
|
| 392 |
|
| 1,350 |
|
| 1,469 |
|
|
|
|
|
|
|
|
|
| ||||
| Stribild – U.S. |
|
| 15 |
|
| 20 |
|
| 72 |
|
| 88 |
| Stribild – Europe |
|
| 5 |
|
| 7 |
|
| 21 |
|
| 29 |
| Stribild – Other International |
|
| 2 |
|
| 3 |
|
| 8 |
|
| 10 |
|
|
|
| 22 |
|
| 29 |
|
| 101 |
|
| 127 |
|
|
|
|
|
|
|
|
|
| ||||
| Truvada – U.S. |
|
| 12 |
|
| 37 |
|
| 82 |
|
| 113 |
| Truvada – Europe |
|
| 3 |
|
| 3 |
|
| 13 |
|
| 15 |
| Truvada – Other International |
|
| 3 |
|
| 5 |
|
| 19 |
|
| 18 |
|
|
|
| 18 |
|
| 45 |
|
| 114 |
|
| 147 |
|
|
|
|
|
|
|
|
|
| ||||
| Revenue share – Symtuza – U.S. |
|
| 104 |
|
| 97 |
|
| 382 |
|
| 348 |
| Revenue share – Symtuza – Europe |
|
| 32 |
|
| 42 |
|
| 133 |
|
| 168 |
| Revenue share – Symtuza – Other International |
|
| 3 |
|
| 3 |
|
| 13 |
|
| 14 |
|
|
|
| 139 |
|
| 142 |
|
| 529 |
|
| 530 |
|
|
|
|
|
|
|
|
|
| ||||
| Other HIV – U.S. |
|
| 13 |
|
| 4 |
|
| 37 |
|
| 15 |
| Other HIV – Europe |
|
| 1 |
|
| 5 |
|
| 12 |
|
| 24 |
| Other HIV – Other International |
|
| 1 |
|
| 3 |
|
| 7 |
|
| 17 |
|
|
|
| 15 |
|
| 12 |
|
| 56 |
|
| 57 |
|
|
|
|
|
|
|
|
|
| ||||
| Total HIV – U.S. |
|
| 3,899 |
|
| 3,914 |
|
| 14,848 |
|
| 13,820 |
| Total HIV – Europe |
|
| 533 |
|
| 566 |
|
| 2,102 |
|
| 2,219 |
| Total HIV – Other International |
|
| 261 |
|
| 293 |
|
| 1,226 |
|
| 1,155 |
|
|
| $ | 4,693 |
| $ | 4,772 |
| $ | 18,175 |
| $ | 17,194 |
|
| ||||||||||||
|
|
|
|
|
| ||||||||
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
|
|
|
| ||||
|
|
|
|
|
|
|
|
|
| ||||
|
|
|
|
|
|
|
|
|
| ||||
| Tecartus – U.S. |
| $ | 66 |
| $ | 61 |
| $ | 245 |
| $ | 221 |
| Tecartus – Europe |
|
| 27 |
|
| 19 |
|
| 110 |
|
| 75 |
| Tecartus – Other International |
|
| 5 |
|
| 1 |
|
| 15 |
|
| 3 |
|
|
|
| 98 |
|
| 82 |
|
| 370 |
|
| 299 |
|
|
|
|
|
|
|
|
|
| ||||
| Yescarta – U.S. |
|
| 187 |
|
| 219 |
|
| 811 |
|
| 747 |
| Yescarta – Europe |
|
| 140 |
|
| 103 |
|
| 547 |
|
| 355 |
| Yescarta – Other International |
|
| 42 |
|
| 15 |
|
| 140 |
|
| 57 |
|
|
|
| 368 |
|
| 337 |
|
| 1,498 |
|
| 1,160 |
|
|
|
|
|
|
|
|
|
| ||||
| Total Cell Therapy – U.S. |
|
| 253 |
|
| 281 |
|
| 1,055 |
|
| 968 |
| Total Cell Therapy – Europe |
|
| 167 |
|
| 122 |
|
| 658 |
|
| 430 |
| Total Cell Therapy – Other International |
|
| 46 |
|
| 17 |
|
| 156 |
|
| 60 |
|
|
|
| 466 |
|
| 419 |
|
| 1,869 |
|
| 1,459 |
|
|
|
|
|
|
|
|
|
| ||||
|
|
|
|
|
|
|
|
|
| ||||
| Trodelvy – U.S. |
|
| 226 |
|
| 146 |
|
| 777 |
|
| 525 |
| Trodelvy– Europe |
|
| 48 |
|
| 44 |
|
| 217 |
|
| 143 |
| Trodelvy – Other International |
|
| 24 |
|
| 4 |
|
| 68 |
|
| 12 |
|
|
|
| 299 |
|
| 195 |
|
| 1,063 |
|
| 680 |
|
|
|
|
|
|
|
|
|
| ||||
| Total Oncology – U.S. |
|
| 479 |
|
| 427 |
|
| 1,833 |
|
| 1,494 |
| Total Oncology – Europe |
|
| 216 |
|
| 166 |
|
| 875 |
|
| 573 |
| Total Oncology – Other International |
|
| 70 |
|
| 21 |
|
| 224 |
|
| 73 |
|
|
|
| 765 |
|
| 614 |
|
| 2,932 |
|
| 2,139 |
|
|
|
|
|
|
|
|
|
| ||||
|
|
|
|
|
|
|
|
|
| ||||
| Ledipasvir / Sofosbuvir – U.S. |
|
| 11 |
|
| 19 |
|
| 39 |
|
| 46 |
| Ledipasvir / Sofosbuvir – Europe |
|
| 1 |
|
| 4 |
|
| 12 |
|
| 17 |
| Ledipasvir / Sofosbuvir – Other International |
|
| 5 |
|
| 8 |
|
| 19 |
|
| 51 |
|
|
|
| 17 |
|
| 31 |
|
| 70 |
|
| 115 |
|
|
|
|
|
|
|
|
|
| ||||
| Sofosbuvir / Velpatasvir – U.S. |
|
| 216 |
|
| 214 |
|
| 859 |
|
| 844 |
| Sofosbuvir / Velpatasvir – Europe |
|
| 74 |
|
| 67 |
|
| 323 |
|
| 355 |
| Sofosbuvir / Velpatasvir – Other International |
|
| 89 |
|
| 87 |
|
| 355 |
|
| 331 |
|
|
|
| 378 |
|
| 369 |
|
| 1,537 |
|
| 1,530 |
|
|
|
|
|
|
|
|
|
| ||||
| Other HCV – U.S. |
|
| 25 |
|
| 27 |
|
| 104 |
|
| 115 |
| Other HCV – Europe |
|
| 10 |
|
| 9 |
|
| 43 |
|
| 40 |
| Other HCV – Other International |
|
| 3 |
|
| 3 |
|
| 12 |
|
| 10 |
|
|
|
| 37 |
|
| 39 |
|
| 160 |
|
| 166 |
|
|
|
|
|
|
|
|
|
| ||||
| Total HCV – U.S. |
|
| 251 |
|
| 260 |
|
| 1,002 |
|
| 1,005 |
| Total HCV – Europe |
|
| 84 |
|
| 80 |
|
| 378 |
|
| 413 |
| Total HCV – Other International |
|
| 97 |
|
| 98 |
|
| 386 |
|
| 392 |
|
|
| $ | 432 |
| $ | 439 |
| $ | 1,767 |
| $ | 1,810 |
|
| |||||||||||||
|
|
|
|
|
| |||||||||
|
|
|
|
|
| |||||||||
|
|
|
|
|
|
|
|
|
| |||||
|
|
|
|
|
|
|
|
|
| |||||
| Vemlidy – U.S. |
| $ | 115 |
| $ | 123 |
|
| $ | 410 |
| $ | 429 |
| Vemlidy – Europe |
|
| 10 |
|
| 8 |
|
|
| 38 |
|
| 35 |
| Vemlidy – Other International |
|
| 92 |
|
| 89 |
|
|
| 414 |
|
| 379 |
|
|
|
| 217 |
|
| 220 |
|
|
| 862 |
|
| 842 |
|
|
|
|
|
|
|
|
|
| |||||
| Viread – U.S. |
|
| 4 |
|
| 2 |
|
|
| 8 |
|
| 6 |
| Viread – Europe |
|
| 6 |
|
| 6 |
|
|
| 22 |
|
| 23 |
| Viread – Other International |
|
| 12 |
|
| 14 |
|
|
| 52 |
|
| 62 |
|
|
|
| 21 |
|
| 22 |
|
|
| 83 |
|
| 91 |
|
|
|
|
|
|
|
|
|
| |||||
| Other HBV/HDV – U.S. |
|
| — |
|
| (1 | ) |
|
| — |
|
| — |
| Other HBV/HDV – Europe |
|
| 22 |
|
| 14 |
|
|
| 72 |
|
| 55 |
|
|
|
| 22 |
|
| 13 |
|
|
| 72 |
|
| 55 |
|
|
|
|
|
|
|
|
|
| |||||
| Total HBV/HDV – U.S. |
|
| 119 |
|
| 124 |
|
|
| 418 |
|
| 435 |
| Total HBV/HDV – Europe |
|
| 37 |
|
| 28 |
|
|
| 133 |
|
| 112 |
| Total HBV/HDV – Other International |
|
| 103 |
|
| 103 |
|
|
| 466 |
|
| 441 |
|
|
|
| 259 |
|
| 255 |
|
|
| 1,017 |
|
| 988 |
|
|
|
|
|
|
|
|
|
| |||||
| Total Liver Disease – U.S. |
|
| 370 |
|
| 384 |
|
|
| 1,421 |
|
| 1,440 |
| Total Liver Disease – Europe |
|
| 121 |
|
| 108 |
|
|
| 511 |
|
| 525 |
| Total Liver Disease – Other International |
|
| 200 |
|
| 202 |
|
|
| 852 |
|
| 833 |
|
|
|
| 691 |
|
| 694 |
|
|
| 2,784 |
|
| 2,798 |
|
|
|
|
|
|
|
|
|
| |||||
| Veklury – U.S. |
|
| 364 |
|
| 395 |
|
|
| 972 |
|
| 1,575 |
| Veklury – Europe |
|
| 181 |
|
| 142 |
|
|
| 408 |
|
| 702 |
| Veklury – Other International |
|
| 175 |
|
| 462 |
|
|
| 805 |
|
| 1,628 |
|
|
|
| 720 |
|
| 1,000 |
|
|
| 2,184 |
|
| 3,905 |
|
|
|
|
|
|
|
|
|
| |||||
| AmBisome – U.S. |
|
| 4 |
|
| 9 |
|
|
| 43 |
|
| 57 |
| AmBisome – Europe |
|
| 68 |
|
| 66 |
|
|
| 260 |
|
| 258 |
| AmBisome – Other International |
|
| 39 |
|
| 42 |
|
|
| 189 |
|
| 182 |
|
|
|
| 111 |
|
| 117 |
|
|
| 492 |
|
| 497 |
|
|
|
|
|
|
|
|
|
| |||||
| Letairis – U.S. |
|
| 36 |
|
| 60 |
|
|
| 142 |
|
| 196 |
|
|
|
|
|
|
|
|
|
| |||||
| Other – U.S. |
|
| 28 |
|
| 44 |
|
|
| 118 |
|
| 135 |
| Other – Europe |
|
| 9 |
|
| 13 |
|
|
| 40 |
|
| 65 |
| Other – Other International |
|
| 17 |
|
| 18 |
|
|
| 66 |
|
| 53 |
|
|
|
| 54 |
|
| 75 |
|
|
| 225 |
|
| 253 |
|
|
|
|
|
|
|
|
|
| |||||
| Total Other – U.S. |
|
| 68 |
|
| 113 |
|
|
| 304 |
|
| 388 |
| Total Other – Europe |
|
| 77 |
|
| 79 |
|
|
| 301 |
|
| 323 |
| Total Other – Other International |
|
| 56 |
|
| 61 |
|
|
| 255 |
|
| 235 |
|
|
|
| 201 |
|
| 252 |
|
|
| 859 |
|
| 946 |
|
|
|
|
|
|
|
|
|
| |||||
| Total product sales – U.S. |
|
| 5,180 |
|
| 5,234 |
|
|
| 19,377 |
|
| 18,716 |
| Total product sales – Europe |
|
| 1,128 |
|
| 1,061 |
|
|
| 4,197 |
|
| 4,342 |
| Total product sales – Other International |
|
| 762 |
|
| 1,037 |
|
|
| 3,361 |
|
| 3,924 |
|
|
| $ | 7,070 |
| $ | 7,333 |
|
| $ | 26,934 |
| $ | 26,982 |
| _______________________________ | ||
|
|
| Represents Gilead’s revenue from cobicistat (“C”), FTC and TAF in Symtuza (darunavir/C/FTC/TAF), a fixed dose combination product commercialized by Janssen Sciences Ireland Unlimited Company. |
|
|
| Includes Atripla, Emtriva, Sunlenca and Tybost. |
|
|
| Amounts consist of sales of Harvoni and the authorized generic version of Harvoni sold by Gilead’s separate subsidiary, Asegua Therapeutics LLC. |
|
|
| Amounts consist of sales of Epclusa and the authorized generic version of Epclusa sold by Gilead’s separate subsidiary, Asegua Therapeutics LLC. |
|
|
| Includes Vosevi and Sovaldi. |
|
|
| Includes Hepcludex and Hepsera. |
|
|
| Includes Cayston, Jyseleca, Ranexa and Zydelig. |
Investors: Jacquie Ross, CFA [email protected]
Media: Ashleigh Koss [email protected]
Intersititial body copy
Some content on this site is not intended for people outside the United States.
- Article Information
Data Sharing Statement
- As Ozempic’s Popularity Soars, Here’s What to Know About Semaglutide and Weight Loss JAMA Medical News & Perspectives May 16, 2023 This Medical News article discusses chronic weight management with semaglutide, sold under the brand names Ozempic and Wegovy. Melissa Suran, PhD, MSJ
- Patents and Regulatory Exclusivities on GLP-1 Receptor Agonists JAMA Special Communication August 15, 2023 This Special Communication used data from the US Food and Drug Administration to analyze how manufacturers of brand-name glucagon-like peptide 1 (GLP-1) receptor agonists have used patent and regulatory systems to extend periods of market exclusivity. Rasha Alhiary, PharmD; Aaron S. Kesselheim, MD, JD, MPH; Sarah Gabriele, LLM, MBE; Reed F. Beall, PhD; S. Sean Tu, JD, PhD; William B. Feldman, MD, DPhil, MPH
- What to Know About Wegovy’s Rare but Serious Adverse Effects JAMA Medical News & Perspectives December 12, 2023 This Medical News article discusses Wegovy, Ozempic, and other GLP-1 receptor agonists used for weight management and type 2 diabetes. Kate Ruder, MSJ
- GLP-1 Receptor Agonists and Gastrointestinal Adverse Events—Reply JAMA Comment & Response March 12, 2024 Ramin Rezaeianzadeh, BSc; Mohit Sodhi, MSc; Mahyar Etminan, PharmD, MSc
- GLP-1 Receptor Agonists and Gastrointestinal Adverse Events JAMA Comment & Response March 12, 2024 Karine Suissa, PhD; Sara J. Cromer, MD; Elisabetta Patorno, MD, DrPH
- GLP-1 Receptor Agonist Use and Risk of Postoperative Complications JAMA Research Letter May 21, 2024 This cohort study evaluates the risk of postoperative respiratory complications among patients with diabetes undergoing surgery who had vs those who had not a prescription fill for glucagon-like peptide 1 receptor agonists. Anjali A. Dixit, MD, MPH; Brian T. Bateman, MD, MS; Mary T. Hawn, MD, MPH; Michelle C. Odden, PhD; Eric C. Sun, MD, PhD
- Glucagon-Like Peptide-1 Receptor Agonist Use and Risk of Gallbladder and Biliary Diseases JAMA Internal Medicine Original Investigation May 1, 2022 This systematic review and meta-analysis of 76 randomized clinical trials examines the effects of glucagon-like peptide-1 receptor agonist use on the risk of gallbladder and biliary diseases. Liyun He, MM; Jialu Wang, MM; Fan Ping, MD; Na Yang, MM; Jingyue Huang, MM; Yuxiu Li, MD; Lingling Xu, MD; Wei Li, MD; Huabing Zhang, MD
- Cholecystitis Associated With the Use of Glucagon-Like Peptide-1 Receptor Agonists JAMA Internal Medicine Research Letter October 1, 2022 This case series identifies cases reported in the US Food and Drug Administration Adverse Event Reporting System of acute cholecystitis associated with use of glucagon-like peptide-1 receptor agonists that did not have gallbladder disease warnings in their labeling. Daniel Woronow, MD; Christine Chamberlain, PharmD; Ali Niak, MD; Mark Avigan, MDCM; Monika Houstoun, PharmD, MPH; Cindy Kortepeter, PharmD
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Sodhi M , Rezaeianzadeh R , Kezouh A , Etminan M. Risk of Gastrointestinal Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss. JAMA. 2023;330(18):1795–1797. doi:10.1001/jama.2023.19574
Manage citations:
© 2024
- Permissions
Risk of Gastrointestinal Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss
- 1 Faculty of Medicine, University of British Columbia, Vancouver, British Columbia, Canada
- 2 StatExpert Ltd, Laval, Quebec, Canada
- 3 Department of Ophthalmology and Visual Sciences and Medicine, University of British Columbia, Vancouver, Canada
- Medical News & Perspectives As Ozempic’s Popularity Soars, Here’s What to Know About Semaglutide and Weight Loss Melissa Suran, PhD, MSJ JAMA
- Special Communication Patents and Regulatory Exclusivities on GLP-1 Receptor Agonists Rasha Alhiary, PharmD; Aaron S. Kesselheim, MD, JD, MPH; Sarah Gabriele, LLM, MBE; Reed F. Beall, PhD; S. Sean Tu, JD, PhD; William B. Feldman, MD, DPhil, MPH JAMA
- Medical News & Perspectives What to Know About Wegovy’s Rare but Serious Adverse Effects Kate Ruder, MSJ JAMA
- Comment & Response GLP-1 Receptor Agonists and Gastrointestinal Adverse Events—Reply Ramin Rezaeianzadeh, BSc; Mohit Sodhi, MSc; Mahyar Etminan, PharmD, MSc JAMA
- Comment & Response GLP-1 Receptor Agonists and Gastrointestinal Adverse Events Karine Suissa, PhD; Sara J. Cromer, MD; Elisabetta Patorno, MD, DrPH JAMA
- Research Letter GLP-1 Receptor Agonist Use and Risk of Postoperative Complications Anjali A. Dixit, MD, MPH; Brian T. Bateman, MD, MS; Mary T. Hawn, MD, MPH; Michelle C. Odden, PhD; Eric C. Sun, MD, PhD JAMA
- Original Investigation Glucagon-Like Peptide-1 Receptor Agonist Use and Risk of Gallbladder and Biliary Diseases Liyun He, MM; Jialu Wang, MM; Fan Ping, MD; Na Yang, MM; Jingyue Huang, MM; Yuxiu Li, MD; Lingling Xu, MD; Wei Li, MD; Huabing Zhang, MD JAMA Internal Medicine
- Research Letter Cholecystitis Associated With the Use of Glucagon-Like Peptide-1 Receptor Agonists Daniel Woronow, MD; Christine Chamberlain, PharmD; Ali Niak, MD; Mark Avigan, MDCM; Monika Houstoun, PharmD, MPH; Cindy Kortepeter, PharmD JAMA Internal Medicine
Glucagon-like peptide 1 (GLP-1) agonists are medications approved for treatment of diabetes that recently have also been used off label for weight loss. 1 Studies have found increased risks of gastrointestinal adverse events (biliary disease, 2 pancreatitis, 3 bowel obstruction, 4 and gastroparesis 5 ) in patients with diabetes. 2 - 5 Because such patients have higher baseline risk for gastrointestinal adverse events, risk in patients taking these drugs for other indications may differ. Randomized trials examining efficacy of GLP-1 agonists for weight loss were not designed to capture these events 2 due to small sample sizes and short follow-up. We examined gastrointestinal adverse events associated with GLP-1 agonists used for weight loss in a clinical setting.
We used a random sample of 16 million patients (2006-2020) from the PharMetrics Plus for Academics database (IQVIA), a large health claims database that captures 93% of all outpatient prescriptions and physician diagnoses in the US through the International Classification of Diseases, Ninth Revision (ICD-9) or ICD-10. In our cohort study, we included new users of semaglutide or liraglutide, 2 main GLP-1 agonists, and the active comparator bupropion-naltrexone, a weight loss agent unrelated to GLP-1 agonists. Because semaglutide was marketed for weight loss after the study period (2021), we ensured all GLP-1 agonist and bupropion-naltrexone users had an obesity code in the 90 days prior or up to 30 days after cohort entry, excluding those with a diabetes or antidiabetic drug code.
Patients were observed from first prescription of a study drug to first mutually exclusive incidence (defined as first ICD-9 or ICD-10 code) of biliary disease (including cholecystitis, cholelithiasis, and choledocholithiasis), pancreatitis (including gallstone pancreatitis), bowel obstruction, or gastroparesis (defined as use of a code or a promotility agent). They were followed up to the end of the study period (June 2020) or censored during a switch. Hazard ratios (HRs) from a Cox model were adjusted for age, sex, alcohol use, smoking, hyperlipidemia, abdominal surgery in the previous 30 days, and geographic location, which were identified as common cause variables or risk factors. 6 Two sensitivity analyses were undertaken, one excluding hyperlipidemia (because more semaglutide users had hyperlipidemia) and another including patients without diabetes regardless of having an obesity code. Due to absence of data on body mass index (BMI), the E-value was used to examine how strong unmeasured confounding would need to be to negate observed results, with E-value HRs of at least 2 indicating BMI is unlikely to change study results. Statistical significance was defined as 2-sided 95% CI that did not cross 1. Analyses were performed using SAS version 9.4. Ethics approval was obtained by the University of British Columbia’s clinical research ethics board with a waiver of informed consent.
Our cohort included 4144 liraglutide, 613 semaglutide, and 654 bupropion-naltrexone users. Incidence rates for the 4 outcomes were elevated among GLP-1 agonists compared with bupropion-naltrexone users ( Table 1 ). For example, incidence of biliary disease (per 1000 person-years) was 11.7 for semaglutide, 18.6 for liraglutide, and 12.6 for bupropion-naltrexone and 4.6, 7.9, and 1.0, respectively, for pancreatitis.
Use of GLP-1 agonists compared with bupropion-naltrexone was associated with increased risk of pancreatitis (adjusted HR, 9.09 [95% CI, 1.25-66.00]), bowel obstruction (HR, 4.22 [95% CI, 1.02-17.40]), and gastroparesis (HR, 3.67 [95% CI, 1.15-11.90) but not biliary disease (HR, 1.50 [95% CI, 0.89-2.53]). Exclusion of hyperlipidemia from the analysis did not change the results ( Table 2 ). Inclusion of GLP-1 agonists regardless of history of obesity reduced HRs and narrowed CIs but did not change the significance of the results ( Table 2 ). E-value HRs did not suggest potential confounding by BMI.
This study found that use of GLP-1 agonists for weight loss compared with use of bupropion-naltrexone was associated with increased risk of pancreatitis, gastroparesis, and bowel obstruction but not biliary disease.
Given the wide use of these drugs, these adverse events, although rare, must be considered by patients who are contemplating using the drugs for weight loss because the risk-benefit calculus for this group might differ from that of those who use them for diabetes. Limitations include that although all GLP-1 agonist users had a record for obesity without diabetes, whether GLP-1 agonists were all used for weight loss is uncertain.
Accepted for Publication: September 11, 2023.
Published Online: October 5, 2023. doi:10.1001/jama.2023.19574
Correction: This article was corrected on December 21, 2023, to update the full name of the database used.
Corresponding Author: Mahyar Etminan, PharmD, MSc, Faculty of Medicine, Departments of Ophthalmology and Visual Sciences and Medicine, The Eye Care Center, University of British Columbia, 2550 Willow St, Room 323, Vancouver, BC V5Z 3N9, Canada ( [email protected] ).
Author Contributions: Dr Etminan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Sodhi, Rezaeianzadeh, Etminan.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Sodhi, Rezaeianzadeh, Etminan.
Critical review of the manuscript for important intellectual content: All authors.
Statistical analysis: Kezouh.
Obtained funding: Etminan.
Administrative, technical, or material support: Sodhi.
Supervision: Etminan.
Conflict of Interest Disclosures: None reported.
Funding/Support: This study was funded by internal research funds from the Department of Ophthalmology and Visual Sciences, University of British Columbia.
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Advanced search

Advanced Search
First contact physiotherapy: an evaluation of clinical effectiveness and costs
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- ORCID record for Nicola E Walsh
- ORCID record for Serena Halls
- ORCID record for Rachel Thomas
- ORCID record for Alice Berry
- ORCID record for Cathy Liddiard
- ORCID record for Margaret E Cupples
- ORCID record for Heather Gage
- ORCID record for Daniel Jackson
- ORCID record for Fiona Cramp
- ORCID record for Hannah Stott
- ORCID record for Paula Kersten
- ORCID record for Justin Jagosh
- ORCID record for Peter Williams
- Figures & Data
Background First contact physiotherapy practitioners (FCPPs) are embedded within general practice, providing expert assessment, diagnosis, and management plans for patients with musculoskeletal disorders (MSKDs), without the prior need for GP consultation.
Aim To determine the clinical effectiveness and costs of FCPP models compared with GP-led models of care.
Design and setting Multiple site case-study design of general practices in the UK.
Method General practice sites were recruited representing the following three models: 1) GP-led care; 2) FCPPs who could not prescribe or inject (FCPPs-standard [St]); and 3) FCPPs who could prescribe and/or inject (FCPPs-additional qualifications [AQ]). Patient participants from each site completed outcome data at baseline, 3 months, and 6 months. The primary outcome was the SF-36 Physical Component Summary (PCS) score. Healthcare usage was collected for 6 months.
Results In total, 426 adults were recruited from 46 practices across the UK. Non-inferiority analysis showed no significant difference in physical function (SF-36 PCS) across all three arms at 6 months ( P = 0.667). At 3 months, a significant difference in numbers improving was seen between arms: 54.7% ( n = 47) GP consultees, 72.4% ( n = 71) FCPP-St, and 66.4% ( n = 101) FCPP-AQ ( P = 0.037). No safety issues were identified. Following initial consultation, a greater proportion of patients received medication (including opioids) in the GP-led arm (44.7%, n = 42), compared with FCPP-St (18.4%, n = 21) and FCPP-AQ (24.7%, n = 40) ( P <0.001). NHS costs (initial consultation and over 6-month follow-up) were significantly higher in the GP-led model (median £105.5 per patient) versus FCPP-St (£41.0 per patient) and FCPP-AQ (£44.0 per patient) ( P <0.001).
Conclusion FCPP-led models of care provide safe, clinically effective patient management, with cost-benefits and reduced opioid use in this cohort.
- general practice
- physiotherapy
- delivery of health care
- musculoskeletal diseases
- Introduction
General practice is experiencing unprecedented demand for appointments at a time when the number of fully qualified GPs is falling, part-time working is increasing, and average patient caseload is rising. 1 The Additional Roles Reimbursement Scheme was introduced in 2019 with the intention of growing the capacity of the primary care workforce. 2 First contact physiotherapy practitioners (FCPPs) were one of five professional roles initially identified for expedited implementation, 2 in recognition of the growing demands musculoskeletal disorders (MSKDs) place on general practice, which account for up to 30% of consultations. 3 FCPPs have an extended appointment time (normally 20 minutes) to assess, diagnose, and determine the most appropriate interventions and manage onward referral for patients without the prior need for GP consultation. 4 Some FCPPs also have the capability to provide injection therapy, and following legislation change in 2013, licensed physiotherapists can independently prescribe, including, since 2015, some controlled drugs. 5 By 2024, all adults in England consulting with a suspected MSKD should be offered a consultation with a FCPP within their local practice. 6
Since its inception, local service evaluations indicate that FCPPs reduce the need for GP consultation, referral to secondary care services, and prescribed medications, while improving patient and staff satisfaction. 7 The only large-scale evaluation of FCPP was conducted as part of an NHS England national pilot of the initiative and reported against pre-determined criteria including the following: re-consultation rates with the GP; improvements in patient symptoms at 3 months; provision of self-management and/or exercise advice for the condition; and impact on ability to work. 8 Pre-determined criteria were largely successfully met, apart from limited information on presenteeism and the ability to work. While this evaluation provided important data on the potential of FCPP, there was no insight regarding longer-term clinical outcomes, use of healthcare resources, or differences in outcomes compared with traditional GP-led models of care.
The current study aimed to determine the impact of FCPP on clinical outcomes and healthcare resource use for 6 months post-consultation compared with GP-led models of care.
| Introducing first contact physiotherapy practitioners (FCPPs) into general practice provides access to expert skills in musculoskeletal disorders (MSKDs) and helps manage patient demand for appointments; MSKD consultations account for up to one-third of GP workload. This study found that FCPPs provide a safe, clinically effective, and cost-beneficial alternative to GP-led consultations. FCPPs also positively impact on medication use (including opioids) and patients improve quicker than those who have not initially consulted with GPs. Embedding FCPP as a standard model in general practice will provide benefits for patients and savings for the healthcare system while reducing the number of patients consulting GPs with MSKDs. |
How this fits in
Setting and practice recruitment
General practices across the UK were invited to participate either via expressions of interest in response to a previous survey regarding FCPP provision, 9 or through advertisement via Clinical Research Networks. The aim was to recruit across all four nations, from a range of urban and rural areas, and differing levels of deprivation; deprivation index was based on practice report and confirmed by nationally available data. 10 – 13
Description of services
General practice study sites were categorised into the following three study arms, according to their existing service provision:
no FCPP service: MSKD management with GP-led consultation (‘GP’);
standard FCPP with no additional competencies for prescribing and/or injecting (‘FCPP-St’); and
FCPP with additional qualifications to prescribe and/or inject (‘FCPP-AQ’).
Participant recruitment
Patients who attended appointments for MSKDs in the study sites were given recruitment materials by the clinician or an allocated practice staff member. They were invited to contact the study team for further information, or to express their willingness to participate. Volunteers were screened for eligibility.
The inclusion criteria were as follows: 1) patients consulting with a suspected MSKD episode, defined as any acute or chronic disorder related to the spinal or peripheral musculoskeletal (MSK) system; 2) patients not consulted for the same problem in preceding 3 months; and 3) patients aged ≥18 years. The exclusion criteria were as follows: 1) receiving palliative care; and 2) non-English speaking and unwilling to provide informed consent and communicate through an interpreter.
Eligible participants provided written, informed consent. Recruitment started in December 2019, slowed in January 2020, owing to the emerging COVID-19 pandemic, and paused in March 2020. Recruitment re-started under COVID-19 restrictions in July 2020 and ended in April 2022. Final assessments were completed in October 2022.
Data collection
Information on age, gender, reason for consultation, MSK risk (using STarT MSK), education, and employment were collected by telephone at baseline (post-consultation). Participants were also asked about their consultation experience and any safety concerns (to be reported elsewhere). There were no notable differences across groups.
Questionnaires regarding Patient Reported Outcome Measures (PROMs) were posted to participants following initial consultation (baseline) and at 3 months and 6 months post-consultation. The questionnaires were self-completed and returned by post. The primary outcome measure was the change from baseline to 6 months in the SF-36 Physical Component Summary (PCS) score. 14 Secondary clinical outcomes were SF-36 Mental Component Summary score; Musculoskeletal Health Questionnaire (MSK-HQ, total and physical); perceived safety of health care, using the healthcare experience in general practice survey, short form (Patient Reported Experiences and Outcomes of Safety in Primary Care; PREOS-PC Q5), on a 10-point scale: completely unsafe (0) to completely safe (10); and Roland–Morris Disability Questionnaire (for patients with low back pain). EQ-5D-5L, a generic measure of health-related quality of life, was gathered for use in the economic evaluation. 15
Sample size
The total participants required per arm was 181 across 39 sites. This was based on a non-inferiority margin of 2 units in SF-36 PCS scale, 14 a minimal clinically important difference of 4 points 16 and standard deviation (SD) 6.5, 17 a one-sided P = 0.05 non-inferior hypothesis test, with 80% power, a design effect of 1.09 for a cluster size of 14 and an intraclass correlation coefficient (ICC) of 0.0075, 18 and 20% attrition. COVID-19 impacted recruitment, so figures were revisited. Actual attrition rates were used (5%) and number of sites were increased ( n = 46), which required a total sample size of n = 462 ( n = 154 per arm).
Data analyses
The primary outcome was the change in SF-36 PCS score from baseline to 6 months compared between arms, using a one-way analysis of variance; in case of difference, a post-hoc unpaired t -test was performed. Further comparisons were undertaken in the context of stepwise linear regression modelling, incorporating demographic and clinical data, including baseline SF-36 PCS score. Outcomes from baseline to 3 months are also reported.
Economic analysis
The base case economic analysis adopted an NHS and social care perspective. Information on service use related to the MSK condition was gathered retrospectively by telephone interview at 3 months and 6 months, using a tailored version of the Client Service Receipt Inventory (CSRI). 19 This included: NHS and private healthcare services (primary, community, accident and emergency [A&E], outpatient referrals, and inpatient stays) and social care. Unit costs 20 , 21 were applied to service use and summed (months 1–6) at the participant level, including the cost of the index consultation (see Supplementary Information S1). Group costs were inspected and compared. Owing to the skewed nature of the total costs data, stepwise logistic regression was used to model the presence or absence of additional costs over and above the cost of the initial presentation, with service model as a dummy variable and baseline demographic and clinical factors as covariates. A societal perspective was included through consideration of self-reported days off work and inability to perform usual activities, and the private perspective through out-of-pocket expenditures.
Analyses were carried out using IBM SPSS Statistics (version 27). Database access can be requested via: http://researchdata.uwe.ac.uk/703 .
A total of 426 participants were recruited from 46 general practices across the UK, with a range of deprivation indices and rural or urban locations. Of the 426 participants, there were 110 (25.8%) from GP-led care, 124 (29.1%) from FCPP-St, and 192 (45.1%) from FCPP-AQ. A total of 46 GP practices were involved: 13 GP-led care practices (with 1, 2, 2, 5, 6, 6, 7, 10, 11, 14, 14, 15, and 17 participants), 15 FCPP-St practices (with 1, 3, 3, 3, 4, 4, 5, 7, 7, 9, 9, 14, 15, 17, and 23 participants), and 18 FCPP-AQ practices (with 1, 1, 4, 4, 6, 8, 8, 9, 11, 12, 14, 15, 15, 16, 16, 16, 17, and 19 participants). The study completion rates in each arm for PROMs and CSRIs, along with attrition patterns, can be seen in Supplementary Table S1.
Mean age was 63 years (SD 13.2); 34.1% ( n = 145) were male and 97.8% ( n = 408) reported White ethnicity. There were no statistically significant differences in individual baseline demographics between arms. There was some discrepancy in practice-level deprivation across arms, with a higher representation of low deprived practices in the FCPP-St arm ( Table 1 ). Data were returned at all three time points by 377 (88.5%) participants, including 320 (75.1%) who provided completed PROM and CSRI data. Details of attrition from the study are given in Supplementary Table S1.
- View inline
Baseline demographics: summary statistics with comparison of the three service models
Clinical data revealed no statistically significant differences between arms at baseline, except for the EQ-5D-5L (visual analogue scale [VAS]; better state of health reported in FCPP-St model) and for MSK-HQ total (a more desirable MSK status was indicated in FCPP-St model). Participants reported a range of peripheral and spinal diagnoses (up to two pain sites); given the previously reported high incidence of low back pain in primary care, 18 a 24.9% ( n = 106/426) prevalence was noted ( Table 2 ).
Baseline clinical summary for each of the three service models
Outcomes analysis
The primary outcome variable was the change in SF-36 PCS score from baseline to 6 months; in an unadjusted analysis, no statistically significant difference was found between arms ( Table 3 ). This was confirmed under linear regression, with a final model ( R 2 = 0.138, n = 332) predicting change = 15.074–0.333x (SF-36 PCS score at baseline) + 2.377 (if university educated) + 2.402 (if in full-time employment). Service model along with age at baseline, gender (male: yes/no), ethnic origin (White: yes/no), whether MSKD area at baseline included back (yes/no), whether MSKD area at baseline included knee or leg or hip or foot or ankle (yes/ no), and whether the presented MSK condition had affected employment or ability to perform usual activities (yes/ no) were not significant (see Supplementary Table S2).
Primary and secondary outcome changes from baseline to 3 months and from baseline to 6 months (positive changes indicate improvement)
However, when each of these change outcomes was simplified from the change in continuous score to an improved or worsened/stayed the same scenario, a statistically significant difference between arms was seen in two instances. At 3 months, the FCPP-St and FCPP-AQ service models delivered a statistically significant greater improvement rate for the primary outcome variable SF-36 PCS score compared with the GP-led service model ( P = 0.037). At 6 months, the FCPP-St and FCPP-AQ service models delivered a statistically significant greater improvement rate for the secondary outcome MSK-HQ physical compared with the GP-led service model ( P = 0.016; Table 3 ). No other statistically significant differences in outcomes were found between arms. No safety issues were identified.
Healthcare utilisation and costs
The initial consultation was assumed to be face-to-face with a GP, FCPP-St, or FCPP-AQ. CSRI data were available for 370/426 (86.9%) of participants at 3 months, 348 (81.7%) at 6 months (see Supplementary Table S1). Health service use after the initial consultation was low in all arms, most being within general practice; few participants reported hospital use. Key health service usage (GP and physiotherapist) and prescribing outcomes are shown in Table 4 . In the 3 months following initial consultation, a greater proportion of patients received medication (including opioids) in the GP-led arm (44.7%; n = 42) compared with FCPP-St (18.4%; n = 21) and FCPP-AQ (24.7%; n = 40) (χ 2 P <0.001) . A full breakdown of NHS service use, including medication prescribing, at 3 months and 6 months, is shown in Supplementary Tables S3 and S4. There was scattered use of the private sector while use of over-the-counter medications was commonplace (see Supplementary Tables S5 and S6).
Key self-reported NHS service usages associated with the presenting musculoskeletal condition, not including initial presentation, at 3 months and 6 months
Group mean total costs (health services, excluding medications) over 6-month follow-up for the three service models are shown in Table 5 . Comparisons were performed both excluding and including inpatient (planned MSK surgery) events, and assuming the FCPP-St and FCPP-AQ were both working at salary level band 7; a sensitivity analysis was performed with the FCPP-AQ costed at the higher band 8a. In each comparison, there is a statistically significant difference in costs between the three models ( P <0.001) with the GP model the more costly (median £105.5 per patient versus £41.0 for FCPP-St and £44.0 for FCPP-AQ in the band 7 calculation), and no statistically significant difference between the FCPP-St and FCPP-AQ. In the band 8a comparison, the FCPP-AQ was significantly more costly than the FCPP-St. Regarding days lost through inability to work or perform usual activities, the FCPP-St model showed greater reductions in days lost compared with GP-led care and FCPP-AQ, but there was no statistically significant difference between GP-led care and FCPP-AQ ( Table 6 ). Only eight participants had absences covered by sick notes in the first 3 months and three during the second period (two of which were new).
Total costs (£) summary statistics, months 0–6
Changes in days lost (unable to work or perform usual activities), with comparisons of the three service models
Backwards stepwise logistic regression to model the presence or absence of additional health service costs in months 0–6 over and above the initial presentation (excluding inpatient), with re-running of the final model to include additional participants for whom data were missing only for non-significant predictors, led to the model in Supplementary Table S2 (with Nagelkerke R 2 = 0.072, n = 334). The model demonstrates a significantly (2.181 times) higher likelihood of incurring additional costs after the initial consultation with a GP-led service model compared with a FCPP-St or FCPP-AQ service model. Higher scores in baseline SF-36 PCS score are also significantly associated with a lower likelihood of incurring additional cost (adjusted odds ratio of 0.966 implies that a participant with a baseline SF-36 PCS score, which is 10 points higher than another participant, is 0.966 10 = 0.708 times less likely to incur additional cost). No other predictors were statistically significant.
The analysis demonstrated that neither FCPP model was inferior in relation to clinical outcome at 6-month post-consultation compared with the GP-led model, but both were significantly less costly; P <0.001. There were no significant differences in quality-of-life changes (based on EQ-5D-5L) between the models at 3 months or 6 months, so given the cost differentials, no formal cost-effectiveness analysis was undertaken ( Tables 3 and 5 ).
Analysis demonstrated no statistically significant difference in clinical outcomes between different service models after 6 months. However, the GP-led model of care was approximately 2.5 times costlier than the FCPP-St and FCPP-AQ models. Furthermore, at 3 months, a greater proportion of patients who consulted with FCPPs had improved, compared with those who had consulted with GPs, and time off work or unable to perform usual activities was reduced in the FCPP-St consultees.
Strengths and limitations
To the authors’ knowledge, this is the first study that has compared GP-and FCPP-led models of care for MSKDs and included data from all four UK nations. It provides a robust overview of the service innovation to support decision making, and a qualitative analysis, which was conducted concurrently, will allow further interpretation of findings.
Recruitment was severely hampered by the COVID-19 pandemic, yet this study still provides the most extensive dataset of FCPPs to date. There was uneven recruitment across study arms and sites because the drive for FCPP recruitment, resulting from the Additional Roles Reimbursement Scheme, made the identification of GP-led sites challenging; and recruitment within some individual sites was lower than anticipated. At site level, there was some variation in deprivation across arms: the FCPP-St consisted of relatively more practices with lower levels of deprivation compared with the other arms, which may explain the higher levels of quality of life (EQ-5D-5L [VAS] and MSK-HQ) reported at baseline within this arm. However, while these differences were of statistical significance, neither was of clinical significance, based on previously reported levels of minimum clinical important difference 23 , 24 and, importantly, there was no difference in the primary outcome measure at baseline across arms. All sites that expressed an interest in participation were recruited, so this variation did not result from selective recruitment. Furthermore, at the level of individual participants, no significant differences were found between groups regarding levels of education or employment.
The sample was almost exclusively White and not representative of practice cohorts despite efforts for diverse recruitment at practice and patient level. Only 12/46 (26.1%) sites returned requested data regarding numbers invited to participate in the study, so how representative the study sample is of those eligible is unable to be reported. Much of the recruitment was undertaken under COVID-19 restrictions, which disproportionately impacted people of ethnic minority heritage, which may have influenced decision to participate, although in consultation with recruitment sites, it was identified that fewer people from ethnic minority communities consult FCPP staff. There was potential recruitment bias as not all eligible participants consented to join the study.
Comparison with existing literature
To the authors’ knowledge, this is the first study to show a comparison between GP and FCPP clinical outcomes and resource use, confirming the proposed benefits of the new model of care. While at 6 months there were no differences in patient improvement across the models studied, at 3 months a significantly greater proportion of patients who consulted with FCPPs had improved compared with GP consultees, with positive impact on ability to work or perform usual activities in FCPP-St ( P = 0.005). Previous work highlighted GP propensity for pharmacological management rather than guideline-based self-management and rehabilitation strategies, which may account for these differences; 25 – 27 indeed, a greater proportion of patients under GP-led care were prescribed medication, including opioid derivatives. The authors are unable to identify any factors in the study design that would account for this finding and believe this is a result of clinical decision making. Other work has shown that FCPPs with a licence to prescribe are still reluctant to use this intervention, instead choosing to use their capability to deprescribe where possible and intervene with non-pharmacological measures. 28
From an onward resource use perspective, data showed minimal reliance on other services within each model and therefore relatively low costs. For services that were used, there was a greater number of referrals onto outpatient physiotherapy by GPs, as would be expected; other work has suggested GP overuse of magnetic resonance imaging (MRI), but this was not found. 29 These data were obtained through self-report so may have been subject to recall bias. It is noted, however, that other studies report the similarities in self-report versus medical record review, and in some cases note greater accuracy with patient recall. 30
A previous evaluation in England reported that GP workload was positively impacted by FCPPs. It found most patients did not consult their GP with the same problem within 3 months of seeing the FCPP. 8 This concurs with the present study’s findings that only 23/276 (8.3%) of patients consulted the GP for the same problem having seen the FCPP, whereas many more (30.9%) initial GP consultees re-consulted the GP for the same problem within the study period ( Table 4 ).
A predominant aim of introducing FCPPs is to make better use of resources in general practice. The present study shows clear cost benefits to implementing FCPP models compared with GP-led care given the extent of MSKD consultations in primary care. 3
Implications for research and practice
This research supports continued implementation of FCPP in general practice as a safe, clinically effective, and cost-beneficial approach to managing people with MSKDs. Given FCPPs’ low reliance on prescription medications, it may also assist in reducing opioid prescriptions in primary care. Further research is required to understand why there appears to be disproportionate consultations from people of ethnic minority heritage to ensure appropriate access for all.
- Acknowledgments
The FRONTIER team would like to thank all participants for their time and valuable contribution to the study. The authors would also like to thank Gemma Artz, Pete Young, Jude Hancock, Alison Diaper, the Study Steering Committee, and the research team at Bristol, North Somerset and South Gloucestershire Integrated Care Board for their expertise and support.
This study was funded by the National Institute for Health and Care Research (NIHR) Health and Social Care Delivery Research Programme (reference: 16/116/03). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Ethical approval
Granted on 18 June 2019 (Integrated Research Application System ID: 261530; Research Ethics Committee reference: 19/NI/0108). Health Research Authority approval was granted on 25 June 2019.
University of the West of England Database Repository Database access can be requested via: http://researchdata.uwe.ac.uk/703 .
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article:
bjgp.org/letters
- Received October 25, 2023.
- Revision requested November 28, 2023.
- Accepted January 22, 2024.
- © The Authors
This article is Open Access: CC BY 4.0 licence ( http://creativecommons.org/licences/by/4.0/ ).
- British Medical Association
- NHS England, NHS Improvement
- NHS England
- Chartered Society of Physiotherapy
- Home Office
- Jordan KP ,
- 10. ↵ Ministry of Housing, Communities & Local Government . The English Indices of Deprivation 2019 Frequently asked questions (FAQs) , https://assets.publishing.service.gov.uk/media/5dfb3d7ce5274a3432700cf3/IoD2019_FAQ_v4.pdf (accessed 29 Jul 2024).
- Scottish Government
- Northern Ireland Statistics and Research Agency
- Kosinski M ,
- Bjorner JB ,
- EuroQol Research Foundation
- Aeschlimann A ,
- Ogollah RO ,
- Salisbury C ,
- Montgomery AA ,
- Hollinghurst S ,
- Personal Social Service Research Unit (PSSRU)
- Devlin NJ ,
- Reneman MF ,
- Speijer BLGN ,
- Lancaster G ,
- Hayward R ,
- Wallis JA ,
- Ackerman IN ,
- Brusco NK ,
- Smithson J ,
- Parkunan A ,
- Wallace E ,
- Moriarty F ,
- McGarrigle C ,
Online First
Thank you for recommending British Journal of General Practice.
NOTE: We only request your email address so that the person to whom you are recommending the page knows that you wanted them to see it, and that it is not junk mail. We do not capture any email address.
Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager

- Tweet Widget
- Facebook Like
- Google Plus One
Jump to section
More in this toc section.
- The first 100 days after childbirth: cross-sectional study of maternal clinical events and health needs from primary care
- Media depictions of primary care teleconsultation safety: a thematic analysis of UK newspapers
Related Articles
Cited by....

British Journal of General Practice

- Academic Departments
- Centers, Institutes & Labs
- Faculty Directory
- Offices & Programs
- Patient Care
- Student Resources
- Faculty Resources
Can't find what you're looking for?
- Centers, Institutes & Labs
- Offices & Programs
This is a site-wide search. if you already know what you're looking for, try visiting a section of the site first to see A-Z listings.
Results for " "

Clinical and Research Faculty Positions
Happenings at mu school of medicine, stories that inspire.

Rajiv R. Mohan, PhD, named 2023 AAAS Fellow

Leary honored with 2023-24 Shared Governance Award
Wan Elected to American Institute for Medical and Biological Engineering College of Fellows

A 40-year career: LeFevre’s legacy
See All News
Columbia, MO 65212 Contact
Emergency Information
Sign up for our monthly newsletter
Copyright © 2024 — Curators of the University of Missouri . All rights reserved. DMCA and other copyright information . Equal Opportunity/Access/Affirmative Action/Pro Disabled & Veteran Employer . For website issues, contact MU Health Care Communications . Contact the MU School of Medicine .

How to cite ChatGPT

Use discount code STYLEBLOG15 for 15% off APA Style print products with free shipping in the United States.
We, the APA Style team, are not robots. We can all pass a CAPTCHA test , and we know our roles in a Turing test . And, like so many nonrobot human beings this year, we’ve spent a fair amount of time reading, learning, and thinking about issues related to large language models, artificial intelligence (AI), AI-generated text, and specifically ChatGPT . We’ve also been gathering opinions and feedback about the use and citation of ChatGPT. Thank you to everyone who has contributed and shared ideas, opinions, research, and feedback.
In this post, I discuss situations where students and researchers use ChatGPT to create text and to facilitate their research, not to write the full text of their paper or manuscript. We know instructors have differing opinions about how or even whether students should use ChatGPT, and we’ll be continuing to collect feedback about instructor and student questions. As always, defer to instructor guidelines when writing student papers. For more about guidelines and policies about student and author use of ChatGPT, see the last section of this post.
Quoting or reproducing the text created by ChatGPT in your paper
If you’ve used ChatGPT or other AI tools in your research, describe how you used the tool in your Method section or in a comparable section of your paper. For literature reviews or other types of essays or response or reaction papers, you might describe how you used the tool in your introduction. In your text, provide the prompt you used and then any portion of the relevant text that was generated in response.
Unfortunately, the results of a ChatGPT “chat” are not retrievable by other readers, and although nonretrievable data or quotations in APA Style papers are usually cited as personal communications , with ChatGPT-generated text there is no person communicating. Quoting ChatGPT’s text from a chat session is therefore more like sharing an algorithm’s output; thus, credit the author of the algorithm with a reference list entry and the corresponding in-text citation.
When prompted with “Is the left brain right brain divide real or a metaphor?” the ChatGPT-generated text indicated that although the two brain hemispheres are somewhat specialized, “the notation that people can be characterized as ‘left-brained’ or ‘right-brained’ is considered to be an oversimplification and a popular myth” (OpenAI, 2023).
OpenAI. (2023). ChatGPT (Mar 14 version) [Large language model]. https://chat.openai.com/chat
You may also put the full text of long responses from ChatGPT in an appendix of your paper or in online supplemental materials, so readers have access to the exact text that was generated. It is particularly important to document the exact text created because ChatGPT will generate a unique response in each chat session, even if given the same prompt. If you create appendices or supplemental materials, remember that each should be called out at least once in the body of your APA Style paper.
When given a follow-up prompt of “What is a more accurate representation?” the ChatGPT-generated text indicated that “different brain regions work together to support various cognitive processes” and “the functional specialization of different regions can change in response to experience and environmental factors” (OpenAI, 2023; see Appendix A for the full transcript).
Creating a reference to ChatGPT or other AI models and software
The in-text citations and references above are adapted from the reference template for software in Section 10.10 of the Publication Manual (American Psychological Association, 2020, Chapter 10). Although here we focus on ChatGPT, because these guidelines are based on the software template, they can be adapted to note the use of other large language models (e.g., Bard), algorithms, and similar software.
The reference and in-text citations for ChatGPT are formatted as follows:
- Parenthetical citation: (OpenAI, 2023)
- Narrative citation: OpenAI (2023)
Let’s break that reference down and look at the four elements (author, date, title, and source):
Author: The author of the model is OpenAI.
Date: The date is the year of the version you used. Following the template in Section 10.10, you need to include only the year, not the exact date. The version number provides the specific date information a reader might need.
Title: The name of the model is “ChatGPT,” so that serves as the title and is italicized in your reference, as shown in the template. Although OpenAI labels unique iterations (i.e., ChatGPT-3, ChatGPT-4), they are using “ChatGPT” as the general name of the model, with updates identified with version numbers.
The version number is included after the title in parentheses. The format for the version number in ChatGPT references includes the date because that is how OpenAI is labeling the versions. Different large language models or software might use different version numbering; use the version number in the format the author or publisher provides, which may be a numbering system (e.g., Version 2.0) or other methods.
Bracketed text is used in references for additional descriptions when they are needed to help a reader understand what’s being cited. References for a number of common sources, such as journal articles and books, do not include bracketed descriptions, but things outside of the typical peer-reviewed system often do. In the case of a reference for ChatGPT, provide the descriptor “Large language model” in square brackets. OpenAI describes ChatGPT-4 as a “large multimodal model,” so that description may be provided instead if you are using ChatGPT-4. Later versions and software or models from other companies may need different descriptions, based on how the publishers describe the model. The goal of the bracketed text is to briefly describe the kind of model to your reader.
Source: When the publisher name and the author name are the same, do not repeat the publisher name in the source element of the reference, and move directly to the URL. This is the case for ChatGPT. The URL for ChatGPT is https://chat.openai.com/chat . For other models or products for which you may create a reference, use the URL that links as directly as possible to the source (i.e., the page where you can access the model, not the publisher’s homepage).
Other questions about citing ChatGPT
You may have noticed the confidence with which ChatGPT described the ideas of brain lateralization and how the brain operates, without citing any sources. I asked for a list of sources to support those claims and ChatGPT provided five references—four of which I was able to find online. The fifth does not seem to be a real article; the digital object identifier given for that reference belongs to a different article, and I was not able to find any article with the authors, date, title, and source details that ChatGPT provided. Authors using ChatGPT or similar AI tools for research should consider making this scrutiny of the primary sources a standard process. If the sources are real, accurate, and relevant, it may be better to read those original sources to learn from that research and paraphrase or quote from those articles, as applicable, than to use the model’s interpretation of them.
We’ve also received a number of other questions about ChatGPT. Should students be allowed to use it? What guidelines should instructors create for students using AI? Does using AI-generated text constitute plagiarism? Should authors who use ChatGPT credit ChatGPT or OpenAI in their byline? What are the copyright implications ?
On these questions, researchers, editors, instructors, and others are actively debating and creating parameters and guidelines. Many of you have sent us feedback, and we encourage you to continue to do so in the comments below. We will also study the policies and procedures being established by instructors, publishers, and academic institutions, with a goal of creating guidelines that reflect the many real-world applications of AI-generated text.
For questions about manuscript byline credit, plagiarism, and related ChatGPT and AI topics, the APA Style team is seeking the recommendations of APA Journals editors. APA Style guidelines based on those recommendations will be posted on this blog and on the APA Style site later this year.
Update: APA Journals has published policies on the use of generative AI in scholarly materials .
We, the APA Style team humans, appreciate your patience as we navigate these unique challenges and new ways of thinking about how authors, researchers, and students learn, write, and work with new technologies.
American Psychological Association. (2020). Publication manual of the American Psychological Association (7th ed.). https://doi.org/10.1037/0000165-000
Related and recent
Comments are disabled due to your privacy settings. To re-enable, please adjust your cookie preferences.
APA Style Monthly
Subscribe to the APA Style Monthly newsletter to get tips, updates, and resources delivered directly to your inbox.
Welcome! Thank you for subscribing.
APA Style Guidelines
Browse APA Style writing guidelines by category
- Abbreviations
- Bias-Free Language
- Capitalization
- In-Text Citations
- Italics and Quotation Marks
- Paper Format
- Punctuation
- Research and Publication
- Spelling and Hyphenation
- Tables and Figures
Full index of topics

IMAGES
COMMENTS
Here's a look back at 2023 through some of our most striking research findings. These findings only scratch the surface of the Center's research from this past year. A record-high share of 40-year-olds in the U.S. have never been married, according to a Center analysis of the most recent U.S. Census Bureau data. As of 2021, a quarter of 40 ...
The results highlight the need to further study erythritol's long-term effects on cardiovascular health. Read more 2023 NIH Research Highlights: Basic Research Insights. NIH findings with potential for enhancing human health include insights into Long COVID and ME/CFS, and a promising blood test for early Alzheimer's detection.
The Science staff named glucagon-like peptide-1 therapies as the 2023 Breakthrough of the Year, but there were many other research advances that caught our attention last year. All of our runners-up to the Breakthrough of the Year had an impact on both science and society last year. Read more about the major science breakthroughs of 2023.
We may soon be seeing the interim results in 2023 of a multi-center sickle-cell disease trial of gene editing sponsored by CRISPR Therapeutics and Vertex Pharmaceuticals. This is a single-arm ...
Research Highlight 04 Dec 2023 A low-cost electron microscope maps proteins at speed Bespoke cryo-electron microscope reveals 3D details of cellular structures — and is an order of magnitude ...
March 2023. When Taken Orally, Δ9-Tetrahydrocannabinol With Cannabidiol Can Result in Stronger Drug Effects Than Δ9-Tetrahydrocannabinol Alone. New research contradicts prior claims and clarifies the interactions between the two main cannabinoids—Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD)—when they are ingested orally as ...
As we make progress on these priorities, we delivered strong results in fiscal year 2023, including a record $211 billion in revenue and over $88 billion in operating income. ... Fiscal Year 2023 Compared with Fiscal Year 2022. Research and development expenses increased $2.7 billion or 11% driven by investments in cloud engineering and LinkedIn.
Between Oct. 1, 2022, and Sept. 30, 2023, we identified a total of 330 articles associated with station research. Of these 330 articles, 268 appeared in peer-reviewed journals, 59 in conference proceedings, and 3 in gray literature such as books, magazines, technical reports, or patents.
The International Space Station is a microgravity research lab hosting groundbreaking technology demonstrations and scientific investigations. More than 3,700 investigations conducted to date have generated more than 4,000 research articles published in scientific journals. In 2023, the orbiting lab hosted approximately 500 investigations.
December 27, 2023. Estimated reading time: 6 minutes. By Mayo Clinic staff. Researchers at Mayo Clinic Comprehensive Cancer Center spent 2023 studying the biology of cancer and new ways to predict, prevent, diagnose and treat the disease. Their discoveries are creating hope and transforming the quality of life for people with cancer today and ...
NEW BRUNSWICK, N.J.--(BUSINESS WIRE)-- Johnson & Johnson (NYSE: JNJ) today announced results for fourth-quarter and full year 2023. "Johnson & Johnson's full year 2023 results reflect the breadth and competitiveness of our business and our relentless focus on delivering for patients," said Joaquin Duato, Chairman and Chief Executive Officer.
Full-year 2023 revenues totaled $58.5 billion, a decrease of $41.8 billion, or 42%, compared to full-year 2022, reflecting an operational decline of $40.8 billion, or 41%, and an unfavorable ...
Cancer Facts & Figures 2023 is an educational companion for Cancer Statistics 2023, a scientific paper published in the American Cancer Society journal, CA: A Cancer Journal for Clinicians. These annual reports provide: Estimated numbers of new cancer cases and deaths in 2023 by cancer site and US state. Current cancer incidence, mortality, and ...
Welcome to the 202 3 edition of Engagement Insights, NSSE's annual dissemination of selected research findings and institutional stories that have broad relevance to the improvement of undergraduate education.. This year's Annual Results focuses on the theme: Engaging Students in Relationship-Rich Education. Below, you will find the main story, some related follow-up analyses, and an ...
The 2023 OFR Annual Report discusses our assessment of risks associated with the U.S. financial system and the performance of the OFR. Financial stability risks have increased since last year's report and remain elevated in 2023. Multiple indicators signal an upcoming economic slowdown—potentially magnified by persistent inflation, ongoing ...
Non-GAAP EPS excludes acquisition- and divestiture-related costs, including pretax intangible asset impairment research and development (R&D) charges of $779 million in the fourth quarter and full year of 2023 related to gefapixant, and $780 million and $1.7 billion in the fourth quarter and full year of 2022, respectively, primarily related to ...
Unit case volume results for the quarters are not impacted by the variances in days due to the average daily sales computation referenced above. Conference Call. The company is hosting a conference call with investors and analysts to discuss fourth quarter and full-year 2023 operating results today, Feb. 13, 2024, at 8:30 a.m. ET.
CAMBRIDGE, Mass.--(BUSINESS WIRE)--Feb. 8, 2024-- Forrester Research, Inc. (Nasdaq: FORR) today announced fourth-quarter and full-year financial results for 2023, with contract value (CV) down by 4%, at $332.1 million, compared with the prior year. Commenting on the results, George F. Colony, Forrester's CEO and chairman, stated, "2023 was a challenging year, with revenue down 11% and CV ...
FOSTER CITY, Calif.-- (BUSINESS WIRE)-- Gilead Sciences, Inc. (Nasdaq: GILD) announced today its results of operations for the fourth quarter and full year 2023. "This was another strong year of revenue growth for Gilead's base business, driven by both HIV and Oncology," said Daniel O'Day, Gilead's Chairman and Chief Executive Officer.
Pfizer reported its results for the fourth quarter and full year of 2023. Full-year 2023 revenues were $58.5 billion, reflecting a 41% operational decline due primarily to the expected decline in COVID-19 revenues, while fourth-quarter revenues were $14.2 billion, reflecting a 42% operational decline. Excluding contributions from its COVID portfolio, Pfizer achieved 7% operational revenue […]
How Organizations Can Rebound in 2023. The good news is this: While only 32% of U.S. employees overall were engaged in 2022, there are organizations that have more than doubled this percentage ...
Fourth-Quarter & Full-Year 2023 Highlights. Good 2023 performance met or exceeded outlooks for organic revenue, adjusted EBITDA margin and FCF. Q4 organic revenue growth of 7% and FY organic revenue growth of 6%. "Big 3" Business Segments (Legal, Corporates and Tax & Accounting) revenues grew 8% organically in Q4 and 7% for the full year.
operational revenue growth of Pfizer's non-COVID products in the fourth quarter of 2023, achieving our full-year. 2023 non-COVID operational revenue growth target of 6% to 8%. In addition, we are on track to deliver at least. $4.0 billion in annual net cost savings by the end of 2024 from our cost realignment program.
Glucagon-like peptide 1 (GLP-1) agonists are medications approved for treatment of diabetes that recently have also been used off label for weight loss. 1 Studies have found increased risks of gastrointestinal adverse events (biliary disease, 2 pancreatitis, 3 bowel obstruction, 4 and gastroparesis 5) in patients with diabetes. 2-5 Because such patients have higher baseline risk for ...
2023 5INTERNET CRIME REPORT THE IC3'S ROLE IN COMBATTING CYBER CRIME1 1 Accessibility description: Image lists the I 3's primary functions including partnering with private sector and with local, state, federal, and international agencies: hosting a reporting portal at www.ic3.gov; providing a central hub to
It turns out that an amino acid that our body produces may play a huge role in longevity. Here's what scientists say about taurine.
These results are consistent with an additive complex genetic risk architecture of ASD involving rare and common variation and further suggest that language delay is a core biological feature of ASD. ... Research Article July 24, 2023. Analytical study of the Lorenz system: Existence of infinitely many periodic orbits and their topological ...
Results In total, 426 adults were recruited from 46 practices across the UK. Non-inferiority analysis showed no significant difference in physical function (SF-36 PCS) across all three arms at 6 months (P = 0.667).At 3 months, a significant difference in numbers improving was seen between arms: 54.7% (n = 47) GP consultees, 72.4% (n = 71) FCPP-St, and 66.4% (n = 101) FCPP-AQ (P = 0.037).
Results for "" Pages. No Results. View All Pages. Faculty. No Results ... About / Faculty and Physician Careers / Clinical and Research Faculty Positions. Happenings at MU School of Medicine Stories That Inspire. Rajiv R. Mohan, PhD, named 2023 AAAS Fellow ... PhD, named 2023 AAAS Fellow. Leary honored with 2023-24 Shared Governance Award. Wan ...
When prompted with "Is the left brain right brain divide real or a metaphor?" the ChatGPT-generated text indicated that although the two brain hemispheres are somewhat specialized, "the notation that people can be characterized as 'left-brained' or 'right-brained' is considered to be an oversimplification and a popular myth" (OpenAI, 2023).