Pharmaceutical Medicine

Working as a Clinical Research Physician: A Gateway into the Pharmaceutical Industry
Ever wondered what it is like working as a clinical research physician? Check out our career guide where we hear first hand from Dr Fahimi, what it is like being a clinical research physician and how to get involved!

A day in the life of someone working as a clinical research physician
What is a clinical research physician.
To understand the role of a clinical research physician, one needs to have a basic understanding about drug development.
Let’s say a pharma company called ‘ABC Therapeutics Ltd’ owns a molecule that they want to develop as a potential new treatment for hypertension.
After appropriate laboratory and animal testing, they then decide to put the molecule through a series of clinical trials to show that the molecule is safe for humans and able to lower the blood pressure in hypertensive subjects.
These clinical trials need to be run by a third party. This is when independent clinical trial companies come in, running the clinical trials to support drug development in order to prove or disprove the above.
A clinical research physician acts as a Principal Investigator or Sub-Investigator for a particular site.
Let’s say ABC Therapeutic Ltd appoints 20 different clinical trial companies or sites to help them to recruit 300 trial participants. At each site you will need at least one clinical research physician to run the clinical trial and monitor participants.
When working as a clinical research physician, you need to read and understand the protocol, which outlines the inclusion and exclusion criteria, as well as procedures and data needed during the trial. When a participant comes to the site for a visit, you’ll be the face of the company and the trial.
You need to determine if a participant is suitable for the study or not, provide them with information and trial medication and follow them up until they finish the study. This could range from 1 day to a few years, depending on the protocol.

What are the working hours?
Usually office hours. In my case, 8:30 am to 4:30 pm and no regular night shifts, long day shifts or weekend shifts. Once in a while you may need to come outside the regular office hours, depending on participant availability and protocol. However, this is rarely the case.
What does a typical day look like for you? Do you work in a team?
My main task is to see trial participants in the clinic. The first visit is called the screening visit, depending on the protocol there might be follow ups. I will meet with them at each visit up until their participation in the trial is completed.
During the screening visit, I check participants’ health status, review current medications, perform a physical examination and complete rating scales as outlined by the study protocol. I also spend a significant amount of time answering queries from trial participants about any issue they may face during the trial and queries from the sponsor about a particular participant.
As part of preparations for the new studies, I also attend meetings, spend time reading and understanding study protocols, attend protocol training and provide training for medical and non medical colleagues. As a medic you are expected to lead the team at your site, which may include a site manager, study coordinators, clinical trial assistants, data coordinators and nurses.
What are some pros and cons of working as a clinical research physician?

1. Greater earning potential
2. Nine-to-five job, with no weekends and no night shifts
3. Less stressful than a purely clinical job
4. Great work life balance
5. I still have a lot of patient contact
1. Lower pension contributions than the NHS
2. Lower total daily steps on my Fitbit!
3. Multiple, simultaneous project deadlines
How does one get into working as a clinical research physician?
You don’t need any specific experience to apply for a clinical research physician job. However, you can do the Good Clinical Practice online training for free on the National Institute for Health website , which will look good on your CV.
If you simply search ‘clinical research physician’ on LinkedIn or Indeed, you’ll find that there are ample of job listings out there. If you can’t find suitable jobs near you, set the new job alert on these websites or feel free to contact me . I’m also more than happy to guide any aspiring research physician out there when preparing for interviews.
Or check out the Medic Footprints Jobs page to see any current clinical research opportunities!
When is the right time to apply?
Ideally you should have completed any of the Royal Colleges Memberships when you apply. You can apply if you don’t have any of these memberships, but in the long term, the membership will help you when you are further down your career.
Strictly speaking, the ideal time to apply is either between ST3 and ST4 level or after CCT for GP. You can apply before, just after FY2 or after ST5 and onward and after specialty CCT.

Is there a visa sponsorship available for those on a Tier 2 visa?
For the last three years, I have never seen any job listings with a visa sponsorship. So the answer here is ‘no’. You need to have either a British passport, ‘indefinite leave to remain’ or a dependent visa to apply for a clinical research physician job.
Possibilities in Clinical Research
What does the career progression look like.
There are various subspecialties in the pharmaceutical industry : clinical research and development, medical affairs , drug safety, regulatory, pharmacovigilance etc. In clinical research, you can progress to senior clinical research physician, associate medical director, medical director and chief medical officer.
Or you can branch out to other subspecialties like a safety physician or pharmacovigilance physician. The idea is to step a foot in the industry as a clinical research physician and build up from there.
How about working as a clinical research physician in non-commercial settings?
You can work as a clinical research physician in the NHS, for example with the National Institute for Health Research (NIHR) , which actually does both commercial and non-commercial research.
Is it possible to do both clinical research and clinical practice at the same time?
Yes, it is possible, especially if you are a GP. It’s about how you negotiate this with your employer. Obviously, some employers may not allow this. I have seen a few GP colleagues who are still doing some GP sessions per week, while also working as a research physician.
I heard there is a specific training programme. Is it true?
Yes, it’s called Pharmaceutical Medicine Specialty Training (PMST) under the Faculty of Pharmaceutical Medicine . This is a 4-year programme and at the end of completion, CCT is awarded. One of the criteria for PMST is 4-years of clinical practice after medical school. Which is why I encourage foundation doctors to practice for a few more years before applying for a clinical research physician job.
Last but not least – Dr Fahimi, how did you get into clinical research?
It was spring 2017 in Beverley, just outside Hull. I had just been on the phone, talking to a recruiter about a job as a clinical research physician in London. In order to cope with the confusion and mixed feelings of the thought of leaving the NHS and working for a private company, I first had to sit down.
I still vividly remember I was next to Beverley Minster on a nice British spring, when all this happened.
The truth is, I had been searching for an alternative career for quite a while at that time. But realising that it was going to happen sooner rather than later was a scary thought.
I finally accepted a job offer from a clinical trial company where my journey of working as a clinical research physician began.
Any final advice?
As with any other specialty, pharmaceutical medicine may or may not be right for you. I have seen a few colleagues who joined the industry as a research physician, but then leave the job after a few months due to one reason or another.
Speaking for myself, I have to say that I enjoy every single minute working in clinical research and I am happy to have made the career change when I did!
My hope is that this Q&A will have given you a small insight into the world of pharmaceutical medicine and the life of a research physician.

Did we spark your interest?
Maybe you are wondering what else is out there in terms of alternative careers for doctors? Check out our many Career Guides or get in touch with questions!
- Latest Posts
Nubli Fahimi
Latest posts by nubli fahimi ( see all ).
- Working as a Clinical Research Physician: A Gateway into the Pharmaceutical Industry - 24th December 2020
Also in Pharmaceutical Medicine
Career Support
How to Nail any Question in a Non-Clinical Interview
How to get into a career in Pharma

The Real Doctors of Pharma

5 points to consider for a career in pharma
Start your journey here.
Browse hundreds of articles and guides to discover more!

Clinical Research Overview and Roles
Clinical Research Professionals (CRPs) at Penn are integral members of the research team. The goal is to have highly trained, competent and motivated individuals in these roles.
We continue to look for research stories to highlight. If you have a research difference-maker story, research highlight or breakthrough story, please share it with us . These will be reflected in the Featured Highlights section of the Penn Medicine Clinical Research website.
CRP job functions include:
- Data Collection
- Analysis, or Monitoring
- Case Management Of Protocol Participants
- Recruitment And Enrollment Of Human Subjects
- Protection of Subjects And Subjects’ Rights
- Development of Informed Consent Documents
- Preparation of Adverse Event Experience Reports
- Construction or Monitoring Of Case Report Forms
- Maintenance of Drug Accountability Records
- Development of Grants And Budgets
- Preparation of Reports
- Educating Other Healthcare Professionals, Patients or Families About Clinical Trials
- Protocol Development
- Program Administration
- Auditing Research Program
Training of CRPs is largely competency based using the CTSA framework followed by ACRP. The ACRP modules, depicted below, are supplemented with Penn specific content.
Clinical Research Operations
OVERVIEW: This role includes clinical research assistant, clinical research coordinator
TRAINING: for more information see Required Training section under Clinical Research Professionals .
Clinical Research Nursing
OVERVIEW: The specialized practice of professional nursing focused on maintaining equilibrium between care of the research participant and fidelity to the research protocol. This specialty practice incorporates human subject protection; care coordination and continuity; contribution to clinical science; clinical practice; and study management throughout a variety of professional roles, practice settings, and clinical specialties*
Clinical Research Monitoring/ Quality
OVERVIEW: Monitoring is the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded and reported in accordance with the protocol, SOPs, GCP and applicable regulatory requirements. The clinical research monitor is the person with direct access to, and oversight, of the site research activities. They conduct monitoring visits in accordance with a monitoring plan and provide feedback and training to sites as necessary.
Clinical Research Regulatory Affairs
OVERVIEW: A Regulatory Affairs specialist is responsible for knowing and operating within the legal framework established for clinical research and product development of drugs and devices. This includes obtaining necessary approvals from local authorities such as the IRB and federal authorities such as the FDA to conduct research in humans. Regulatory Affairs also ensures essential documents are maintained and always inspection ready. Skills of a Regulatory Affairs Specialists include a basic understanding of research, project management, preparing submissions, and effective communication.
Clinical Research Data Management
OVERVIEW: This role manages research data, creation of datasets for investigators, performing data quality assurance checks, creation of new summary variables for analysis, and creation of data tables for reports and papers. This role provides general administrative support for research data, completion of data collection forms, extracting and entering required clinical data from medical records and patient research charts to Case Report Forms (eCRFs/CRFs).
Clinical Research Project Management
OVERVIEW: This role oversees the operation and execution of all aspects of a clinical research study. Includes recruitment of participants and implementation of objectives for each study
*Reference: International Association of Clinical Research Nurses. (2012) "Enhancing Clinical Research Quality and Safety Through Specialized Nursing Practice". Scope and Standards of Practice Committee Report
Highlight your clinical research story: We continue to look for stories to highlight. If you have a research difference-maker story, research highlight or breakthrough story, please share it with us . These will be reflected in the Featured Highlights section of the Penn Medicine Clinical Research website.
Clinical Researcher
Navigating a Career as a Clinical Research Professional: Where to Begin?
Clinical Researcher June 9, 2020

Clinical Researcher—June 2020 (Volume 34, Issue 6)
PEER REVIEWED
Bridget Kesling, MACPR; Carolynn Jones, DNP, MSPH, RN, FAAN; Jessica Fritter, MACPR; Marjorie V. Neidecker, PhD, MEng, RN, CCRP
Those seeking an initial career in clinical research often ask how they can “get a start” in the field. Some clinical research professionals may not have heard about clinical research careers until they landed that first job. Individuals sometimes report that they have entered the field “accidentally” and were not previously prepared. Those trying to enter the clinical research field lament that it is hard to “get your foot in the door,” even for entry-level jobs and even if you have clinical research education. An understanding of how individuals enter the field can be beneficial to newcomers who are targeting clinical research as a future career path, including those novices who are in an academic program for clinical research professionals.
We designed a survey to solicit information from students and alumni of an online academic clinical research graduate program offered by a large public university. The purpose of the survey was to gain information about how individuals have entered the field of clinical research; to identify facilitators and barriers of entering the field, including advice from seasoned practitioners; and to share the collected data with individuals who wanted to better understand employment prospects in clinical research.
Core competencies established and adopted for clinical research professionals in recent years have informed their training and education curricula and serve as a basis for evaluating and progressing in the major roles associated with the clinical research enterprise.{1,2} Further, entire academic programs have emerged to provide degree options for clinical research,{3,4} and academic research sites are focusing on standardized job descriptions.
For instance, Duke University re-structured its multiple clinical research job descriptions to streamline job titles and progression pathways using a competency-based, tiered approach. This led to advancement pathways and impacted institutional turnover rates in relevant research-related positions.{5,6} Other large clinical research sites or contract research organizations (CROs) have structured their onboarding and training according to clinical research core competencies. Indeed, major professional organizations and U.S. National Institutes of Health initiatives have adopted the Joint Task Force for Clinical Trial Competency as the gold standard approach to organizing training and certification.{7,8}
Recent research has revealed that academic medical centers, which employ a large number of clinical research professionals, are suffering from high staff turnover rates in this arena, with issues such as uncertainty of the job, dissatisfaction with training, and unclear professional development and role progression pathways being reported as culprits in this turnover.{9} Further, CROs report a significant shortage of clinical research associate (CRA) personnel.{10} Therefore, addressing factors that would help novices gain initial jobs would address an important workforce gap.
This mixed-methods survey study was initiated by a student of a clinical research graduate program at a large Midwest university who wanted to know how to find her first job in clinical research. Current students and alumni of the graduate program were invited to participate in an internet-based survey in the fall semester of 2018 via e-mails sent through the program listservs of current and graduated students from the program’s lead faculty. After the initial e-mail, two reminders were sent to prospective participants.
The survey specifically targeted students or alumni who had worked in clinical research. We purposefully avoided those students with no previous clinical research work experience, since they would not be able to discuss their pathway into the field. We collected basic demographic information, student’s enrollment status, information about their first clinical research position (including how it was attained), and narrative information to describe their professional progression in clinical research. Additional information was solicited about professional organization membership and certification, and about the impact of graduate education on the acquisition of clinical research jobs and/or role progression.
The survey was designed so that all data gathered (from both objective responses and open-ended responses) were anonymous. The survey was designed using the internet survey instrument Research Electronic Data Capture (REDCap), which is a secure, web-based application designed to support data capture for research studies. REDCap provides an intuitive interface for validated data entry; audit trails for tracking data manipulation and export procedures; automated export procedures for seamless data downloads to common statistical packages; and procedures for importing data from external sources.{11}
Data were exported to Excel files and summary data were used to describe results. Three questions solicited open-ended responses about how individuals learned about clinical research career options, how they obtained their first job, and their advice to novices seeking their first job in clinical research. Qualitative methods were used to identify themes from text responses. The project was submitted to the university’s institutional review board and was classified as exempt from requiring board oversight.
A total of 215 survey invitations were sent out to 90 current students and 125 graduates. Five surveys were returned as undeliverable. A total of 48 surveys (22.9%) were completed. Because the survey was designed to collect information from those who were working or have worked in clinical research, those individuals (n=5) who reported (in the first question) that they had never worked in clinical research were eliminated. After those adjustments, the total number completed surveys was 43 (a 20.5% completion rate).
The median age of the participants was 27 (range 22 to 59). The majority of respondents (89%) reported being currently employed as clinical research professionals and 80% were working in clinical research at the time of graduate program entry. The remaining respondents had worked in clinical research in the past. Collectively, participants’ clinical research experience ranged from less than one to 27 years.
Research assistant (20.9%) and clinical research coordinator (16.3%) were the most common first clinical research roles reported. However, a wide range of job titles were also reported. When comparing entry-level job titles of participants to their current job title, 28 (74%) respondents reported a higher level job title currently, compared to 10 (26%) who still had the same job title.
Twenty-four (65%) respondents were currently working at an academic medical center, with the remaining working with community medical centers or private practices (n=3); site management organizations or CROs (n=2); pharmaceutical or device companies (n=4); or the federal government (n=1).
Three respondents (8%) indicated that their employer used individualized development plans to aid in planning for professional advancement. We also asked if their current employer provided opportunities for professional growth and advancement. Among academic medical center respondents, 16 (67%) indicated in the affirmative. Respondents also affirmed growth opportunities in other employment settings, with the exception of one respondent working in government and one respondent working in a community medical center.
Twenty-five respondents indicated membership to a professional association, and of those, 60% reported being certified by either the Association of Clinical Research Professionals (ACRP) or the Society of Clinical Research Associates (SoCRA).
Open-Ended Responses
We asked three open-ended questions to gain personal perspectives of respondents about how they chose clinical research as a career, how they entered the field, and their advice for novices entering the profession. Participants typed narrative responses.
“Why did you decide to pursue a career in clinical research?”
This question was asked to find out how individuals made the decision to initially consider clinical research as a career. Only one person in the survey had exposure to clinical research as a career option in high school, and three learned about such career options as college undergraduates. One participant worked in clinical research as a transition to medical school, two as a transition to a doctoral degree program, and two with the desire to move from a bench (basic science) career to a clinical research career.
After college, individuals either happened across clinical research as a career “by accident” or through people they met. Some participants expressed that they found clinical research careers interesting (n=6) and provided an opportunity to contribute to patients or improvements in healthcare (n=7).
“How did you find out about your first job in clinical research?”
Qualitative responses were solicited to obtain information on how participants found their first jobs in clinical research. The major themes that were revealed are sorted in Figure 1.
Figure 1: How First Jobs in Clinical Research Were Found
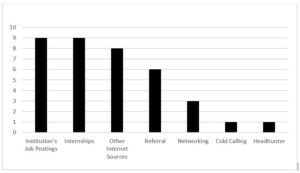
Some reported finding their initial job through an institution’s job posting.
“I worked in the hospital in the clinical lab. I heard of the opening after I earned my bachelor’s and applied.”
Others reported finding about their clinical research position through the internet. Several did not know about clinical research roles before exploring a job posting.
“In reviewing jobs online, I noticed my BS degree fit the criteria to apply for a job in clinical research. I knew nothing about the field.”
“My friend recommended I look into jobs with a CRO because I wanted to transition out of a production laboratory.”
“I responded to an ad. I didn’t really know that research could be a profession though. I didn’t know anything about the field, principles, or daily activities.”
Some of the respondents reported moving into a permanent position after a role as an intern.
“My first clinical job came from an internship I did in my undergrad in basic sleep research. I thought I wanted to get into patient therapies, so I was able to transfer to addiction clinical trials from a basic science lab. And the clinical data management I did as an undergrad turned into a job after a few months.”
“I obtained a job directly from my graduate school practicum.”
“My research assistant internship [as an] undergrad provided some patient enrollment and consenting experience and led to a CRO position.”
Networking and referrals were other themes that respondents indicated had a direct impact on them finding initial employment in clinical research.
“I received a job opportunity (notice of an opening) through my e-mail from the graduate program.”
“I was a medical secretary for a physician who did research and he needed a full-time coordinator for a new study.”
“I was recommended by my manager at the time.”
“A friend had a similar position at the time. I was interested in learning more about the clinical research coordinator position.”
“What advice do you have for students and new graduates trying to enter their first role in clinical research?”
We found respondents (n=30) sorted into four distinct categories: 1) a general attitude/approach to job searching, 2) acquisition of knowledge/experience, 3) actions taken to get a position, and 4) personal attributes as a clinical research professional in their first job.
Respondents stressed the importance of flexibility and persistence (general attitude/approach) when seeking jobs. Moreover, 16 respondents stressed the importance of learning as much as they could about clinical research and gaining as much experience as they could in their jobs, encouraging them to ask a lot of questions. They also stressed a broader understanding of the clinical research enterprise, the impact that clinical research professional roles have on study participants and future patients, and the global nature of the enterprise.
“Apply for all research positions that sound interesting to you. Even if you don’t meet all the requirements, still apply.”
“Be persistent and flexible. Be willing to learn new skills and take on new responsibilities. This will help develop your own niche within a group/organization while creating opportunities for advancement.”
“Be flexible with salary requirements earlier in your career and push yourself to learn more [about the industry’s] standards [on] a global scale.”
“Be ever ready to adapt and change along with your projects, science, and policy. Never forget the journey the patients are on and that we are here to advance and support it.”
“Learning the big picture, how everything intertwines and works together, will really help you progress in the field.”
In addition to learning as much as one can about roles, skills, and the enterprise as a whole, advice was given to shadow or intern whenever possible—formally or through networking—and to be willing to start with a smaller company or with a lower position. The respondents stressed that novices entering the field will advance in their careers as they continue to gain knowledge and experience, and as they broaden their network of colleagues.
“Take the best opportunity available to you and work your way up, regardless [if it is] at clinical trial site or in industry.”
“Getting as much experience as possible is important; and learning about different career paths is important (i.e., not everyone wants or needs to be a coordinator, not everyone goes to graduate school to get a PhD, etc.).”
“(A graduate) program is beneficial as it provides an opportunity to learn the basics that would otherwise accompany a few years of entry-level work experience.”
“Never let an opportunity pass you up. Reach out directly to decision-makers via e-mail or telephone—don’t just rely on a job application website. Be willing to start at the bottom. Absolutely, and I cannot stress this enough, [you should] get experience at the site level, even if it’s just an internship or [as a] volunteer. I honestly feel that you need the site perspective to have success at the CRO or pharma level.”
Several personal behaviors were also stressed by respondents, such as knowing how to set boundaries, understanding how to demonstrate what they know, and ability to advocate for their progression. Themes such as doing a good job, communicating well, being a good team player, and sharing your passion also emerged.
“Be a team player, ask questions, and have a good attitude.”
“Be eager to share your passion and drive. Although you may lack clinical research experience, your knowledge and ambition can impress potential employers.”
“[A] HUGE thing is learning to sell yourself. Many people I work with at my current CRO have such excellent experience, and they are in low-level positions because they didn’t know how to negotiate/advocate for themselves as an employee.”
This mixed-methods study used purposeful sampling of students in an academic clinical research program to gain an understanding of how novices to the field find their initial jobs in the clinical research enterprise; how to transition to a clinical research career; and how to find opportunities for career advancement. There are multiple clinical research careers and employers (see Figure 2) available to individuals working in the clinical research enterprise.
Figure 2: Employers and Sample Careers
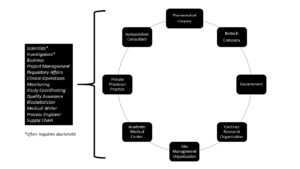
Despite the need for employees in the broad field of clinical research, finding a pathway to enter the field can be difficult for novices. The lack of knowledge about clinical research as a career option at the high school and college level points to an opportunity for broader inclusion of these careers in high school and undergraduate curricula, or as an option for guidance counselors to be aware of and share with students.
Because most clinical research jobs appear to require previous experience in order to gain entry, novices are often put into a “Catch-22” situation. However, once hired, upward mobility does exist, and was demonstrated in this survey. Mobility in clinical research careers (moving up and general turnover) may occur for a variety of reasons—usually to achieve a higher salary, to benefit from an improved work environment, or to thwart a perceived lack of progression opportunity.{9}
During COVID-19, there may be hiring freezes or furloughs of clinical research staff, but those personnel issues are predicted to be temporary. Burnout has also been reported as an issue among study coordinators, due to research study complexity and workload issues.{12} Moreover, the lack of individualized development planning revealed by our sample may indicate a unique workforce development need across roles of clinical research professionals.
This survey study is limited in that it is a small sample taken specifically from a narrow cohort of individuals who had obtained or were seeking a graduate degree in clinical research at a single institution. The study only surveyed those currently working in or who have a work history in clinical research. Moreover, the majority of respondents were employed at an academic medical center, which may not fully reflect the general population of clinical research professionals.
It was heartening to see the positive advancement in job titles for those individuals who had been employed in clinical research at program entry, compared to when they responded to the survey. However, the sample was too small to draw reliable correlations about job seeking or progression.
Although finding one’s first job in clinical research can be a lengthy and discouraging process, it is important to know that the opportunities are endless. Search in employment sites such as Indeed.com, but also search within job postings for targeted companies or research sites such as biopharmguy.com (see Table 1). Created a LinkedIn account and join groups and make connections. Participants in this study offered sound advice and tips for success in landing a job (see Figure 3).
Table 1: Sample Details from an Indeed.Com Job Search
| Clinical Research Patient Recruiter | PPD | Bachelor’s degree and related experience |
| Clinical Research Assistant | Duke University | Associate degree |
| Clinical Trials Assistant | Guardian Research Network | Bachelor’s degree and knowledge of clinical trials |
| Clinical Trials Coordinator | Advarra Health Analytics | Bachelor’s degree |
| Clinical Research Specialist | Castle Branch | Bachelor’s degree and six months in a similar role |
| Clinical Research Technician | Rose Research Center, LLC | Knowledge of Good Clinical Practice and experience working with patients |
| Clinical Research Lab Coordinator | Coastal Carolina Research Center | One year of phlebotomy experience |
| Project Specialist | WCG | Bachelor’s degree and six months of related experience |
| Data Coder | WCG | Bachelor’s degree or currently enrolled in an undergraduate program |
Note: WCG = WIRB Copernicus Group
Figure 3: Twelve Tips for Finding Your First Job
- Seek out internships and volunteer opportunities
- Network, network, network
- Be flexible and persistent
- Learn as much as possible about clinical research
- Consider a degree in clinical research
- Ask a lot of questions of professionals working in the field
- Apply for all research positions that interest you, even if you think you are not qualified
- Be willing to learn new skills and take on new responsibilities
- Take the best opportunity available to you and work your way up
- Learn to sell yourself
- Sharpen communication (written and oral) and other soft skills
- Create an ePortfolio or LinkedIn account
Being willing to start at the ground level and working upwards was described as a positive approach because moving up does happen, and sometimes quickly. Also, learning soft skills in communication and networking were other suggested strategies. Gaining education in clinical research is one way to begin to acquire knowledge and applied skills and opportunities to network with experienced classmates who are currently working in the field.
Most individuals entering an academic program have found success in obtaining an initial job in clinical research, often before graduation. In fact, the student initiating the survey found a position in a CRO before graduation.
- Sonstein S, Seltzer J, Li R, Jones C, Silva H, Daemen E. 2014. Moving from compliance to competency: a harmonized core competency framework for the clinical research professional. Clinical Researcher 28(3):17–23. doi:10.14524/CR-14-00002R1.1. https://acrpnet.org/crjune2014/
- Sonstein S, Brouwer RN, Gluck W, et al. 2018. Leveling the joint task force core competencies for clinical research professionals. Therap Innov Reg Sci .
- Jones CT, Benner J, Jelinek K, et al. 2016. Academic preparation in clinical research: experience from the field. Clinical Researcher 30(6):32–7. doi:10.14524/CR-16-0020. https://acrpnet.org/2016/12/01/academic-preparation-in-clinical-research-experience-from-the-field/
- Jones CT, Gladson B, Butler J. 2015. Academic programs that produce clinical research professionals. DIA Global Forum 7:16–9.
- Brouwer RN, Deeter C, Hannah D, et al. 2017. Using competencies to transform clinical research job classifications. J Res Admin 48:11–25.
- Stroo M, Ashfaw K, Deeter C, et al. 2020. Impact of implementing a competency-based job framework for clinical research professionals on employee turnover. J Clin Transl Sci.
- Calvin-Naylor N, Jones C, Wartak M, et al. 2017. Education and training of clinical and translational study investigators and research coordinators: a competency-based approach. J Clin Transl Sci 1:16–25. doi:10.1017/cts.2016.2
- Development, Implementation and Assessment of Novel Training in Domain-based Competencies (DIAMOND). Center for Leading Innovation and Collaboration (CLIC). 2019. https://clic-ctsa.org/diamond
- Clinical Trials Talent Survey Report. 2018. http://www.appliedclinicaltrialsonline.com/node/351341/done?sid=15167
- Causey M. 2020. CRO workforce turnover hits new high. ACRP Blog . https://acrpnet.org/2020/01/08/cro-workforce-turnover-hits-new-high/
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–81.
- Gwede CK, Johnson DJ, Roberts C, Cantor AB. 2005. Burnout in clinical research coordinators in the United States. Oncol Nursing Forum 32:1123–30.
A portion of this work was supported by the OSU CCTS, CTSA Grant #UL01TT002733.
Bridget Kesling, MACPR, ( [email protected] ) is a Project Management Analyst with IQVIA in Durham, N.C.
Carolynn Jones, DNP, MSPH, RN, FAAN, ( [email protected] ) is an Associate Professor of Clinical Nursing at The Ohio State University College of Nursing, Co-Director of Workforce Development for the university’s Center for Clinical and Translational Science, and Director of the university’s Master of Clinical Research program.
Jessica Fritter, MACPR, ( [email protected] ) is a Clinical Research Administration Manager at Nationwide Children’s Hospital and an Instructor for the Master of Clinical Research program at The Ohio State University.
Marjorie V. Neidecker, PhD, MEng, RN, CCRP, ( [email protected] ) is an Assistant Professor of Clinical Nursing at The Ohio State University Colleges of Nursing and Pharmacy.
Sorry, we couldn't find any jobs that match your criteria.

The Industry Shift Toward Decentralized Clinical Trials: Impacts on Quality Management, Participant Outcomes, and Data Management

Insights into the Clinical Research Associate Career Pathway

The Future of Multi-Omics in Cancer Clinical Trials
Clinical Research Physician
Description, responsibilities.
- Lead and oversee clinical research projects and trials.
- Design and implement clinical studies.
- Ensure compliance with regulatory requirements and ethical standards.
- Analyze clinical data and interpret results.
- Collaborate with cross-functional teams, including scientists, statisticians, and regulatory affairs specialists.
- Write and review clinical study reports.
- Present research findings at conferences and meetings.
- Publish results in peer-reviewed journals.
- Stay up-to-date with the latest advancements in medical research.
- Contribute to the development of new treatment protocols.
- Monitor patient safety and manage adverse event reporting.
- Develop and maintain study documentation and records.
- Provide medical expertise and guidance to research teams.
- Participate in the selection and training of research staff.
- Ensure the integrity and quality of clinical data.
- Manage budgets and resources for clinical research projects.
- Communicate effectively with stakeholders, including sponsors and regulatory authorities.
- Develop and implement strategies for patient recruitment and retention.
- Conduct literature reviews and stay informed about relevant scientific literature.
- Mentor and train junior researchers and medical staff.
Requirements
- Medical degree (MD or equivalent) with a valid medical license.
- Board certification in a relevant medical specialty.
- Minimum of 5 years of experience in clinical research.
- Strong knowledge of clinical trial design and methodology.
- Familiarity with regulatory requirements and ethical standards in clinical research.
- Excellent analytical and problem-solving skills.
- Ability to interpret and analyze complex clinical data.
- Strong written and verbal communication skills.
- Experience in writing and reviewing clinical study reports.
- Proven track record of publishing research findings in peer-reviewed journals.
- Ability to present research findings at conferences and meetings.
- Strong organizational and project management skills.
- Ability to work collaboratively with cross-functional teams.
- Attention to detail and commitment to quality.
- Proficiency in using clinical data management and statistical software.
- Ability to manage multiple projects and meet deadlines.
- Strong leadership and mentoring skills.
- Commitment to continuous learning and professional development.
- Experience in patient recruitment and retention strategies.
- Knowledge of Good Clinical Practice (GCP) guidelines.
Potential interview questions
- Can you describe your experience with clinical trial design and implementation?
- How do you ensure compliance with regulatory requirements and ethical standards in your research?
- Can you provide an example of a clinical study you have led and the outcomes achieved?
- How do you approach data analysis and interpretation in clinical research?
- What strategies do you use to communicate complex scientific information to non-experts?
- Can you discuss a time when you had to manage an adverse event during a clinical trial?
- How do you stay current with the latest advancements in medical research?
- What is your experience with writing and reviewing clinical study reports?
- How do you handle conflicts or disagreements within a research team?
- Can you describe your experience with patient recruitment and retention in clinical trials?
- What role do you believe mentorship plays in clinical research?
- How do you ensure the integrity and quality of clinical data?
- Can you discuss your experience with presenting research findings at conferences?
- What are your strategies for managing budgets and resources for clinical research projects?
- How do you prioritize and manage multiple research projects simultaneously?
- Can you provide an example of a challenging clinical research project and how you overcame the challenges?
- What is your experience with using clinical data management and statistical software?
- How do you contribute to the development of new treatment protocols?
- Can you discuss your experience with publishing research findings in peer-reviewed journals?
- What motivates you to work in clinical research?
Needed Skills
Related job descriptions.
CLINICAL RESEARCH PHYSICIAN JOB DESCRIPTION
Find detail information about clinical research physician job description, duty and skills required for clinical research physician position.
What do clinical research physician do?
A Clinical Research Physician (CRP) is responsible for overseeing clinical trials, performing safety medical reviews, and building relationships with professionals in the pharmaceutical field. CRPs also play a critical role in developing regulatory documents for the pharmaceutical industry.
Does clinical research pay well?
Clinical research associate salaries can range from entry-level jobs that pay $68,986 a year to mid-career salaries of $82,198 a year. These salaries are significantly higher than the average salary in the United States, and they are especially important for people who want to become medical professionals. Clinical research associate careers can be very rewarding, and they offer a lot of opportunity for advancement.
What is a career in clinical research?
Clinical research often involves conducting studies in order to try and find new treatments for patients. This can involve working with your employer in order to conduct trials which can help improve the safety and effectiveness of treatments. Clinical research is a rewarding career, and if you have qualifications or experience within life sciences, you may be able to find a career in this field.
What is the highest paying job in clinical research?
There are a number of jobs in clinical research that offer competitive salaries and amazing opportunity for growth. These jobs may include Clinical Research Physician, Clinical Laboratory Director, Clinical Trial Manager, Regional Clinical Research Associate, and Clinical Project Leader. Each position has its own unique set of responsibilities and demands. The paychecks for these jobs vary greatly, but they are all excellent places to work if you are looking to start or grow your career in clinical research.
How do you become a physician researcher?
There are four pathways to become a physician scientist: Complete MD training and then conduct extended research through fellowship training. Complete MD training and then return to graduate school to earn a PhD degree. Complete PhD training and then enter medical school to earn an MD degree. The first two pathways are the most common, and require only a completed MD degree. The third pathway is more complex, but may lead to a successful career as a physician scientist if you have experience in research. The fourth pathway may be the best for you if you are interested in becoming a doctor with a science background and want to pursue a career in medicine that involves practicing medicine as well as conducting research.
Is clinical research a medical field?
Medical research is a branch of medical science which deals with research in order to assess the potential treatment methods for humans. This includes medications, devices, and diagnostic products. Medical research is important because it allows doctors to find new ways to help people.
Is clinical research a good career?
Clinical research is one of the most important and challenging fields of study in medicine. It involves performing experiments on animals in order to learn more about diseases and treatments. Clinicians can earn a salary of around $50,000 per year when they work in this field. However, the level of preparation required for this profession is high. In order to be a successful clinician, you will need to have a degree in medical science or an equivalent field. Moreover, you will also need to pass an entrance exam and undergo a rigorous training program.
How do I get started in clinical research?
Clinical Research Associates (CRA) are individuals who have completed undergraduate or graduate study in a healthcare field and are now working in the clinic, hospital, or research setting. CRA experience can be found working as interns at Fortune 500 companies, as site managers at clinical research organizations (CROs), or as researchers at government agencies.
Is CRA a good career?
The demand for clinical research professionals has increased 4000 percent since 2000. Clinical research is a vital component of many businesses, and with the increasing number of diseases and treatments that need to be studied, the demand for these professionals has only grown. This is a great opportunity for those who are interested in working in this field, as the pay is typically quite good and there are many opportunities for advancement.
Which job is best in clinical research?
The field of clinical research is full of opportunities for career growth. Clinical research data specialists are able to collect and analyze data to make decisions that impact patients. Clinical investigator or clinical researcher have the ability to conduct groundbreaking research and improve patient care. Research pharmacist work with Pharma companies to develop and test new products. Clinical laboratory technologist or technician are responsible for many tasks in a laboratory, including testing drugs and vaccines, measuring blood sugar levels, and analyzing protein specimens. Medical laboratory scientist work with doctors and other medical professionals to conduct tests that help diagnose diseases or track progress in patients. Marketing and communications professionals are responsible for creating public relations campaigns that promote the good work of clinical research institutions.
Is clinical research stressful?
Clinical research can be a daunting task. It can be full of unpredictable pain, long hours, and meager entry-level pay. However, for those who are willing to put in the hard work, it can be a rewarding experience. clinical research can be filled with intense pain, long hours, and little hope of advancement. However, for those who are determined to succeed, it can be a rewarding experience.
What can I do after clinical research?
The medical field is full of opportunity for those with an exciting and innovative mind. With the right skills, you can become a Principal Investigator, Medical Advisor, Drug Developer, or Regulatory Affairs Manager. In this highly competitive field, it is essential to have strong writing and communication abilities as well as the ability to work with others in a team environment. If you are interested in pursuing a career in medicine, please submit your resume today.
Is a masters in clinical research worth it?
Most clinical trials are used to test new treatments and vaccines in humans in order to determine if they are safe and effective. This research can also help to develop new products and therapies. The global clinical trials market is expected to grow at a CAGR of 4.5% over the next five years, according to a recent study. This is due to the increasing demand for new treatments and vaccines as well as the growing interest in Forskolin supplements.
What is a clinical research associate salary?
It is difficult to find a job in India that is not full of stress and tension. Clinical research associate salaries are very low, and many workers are forced to work long hours without any possibility of rest or vacation. Entry-level positions start at peanuts, while most experienced researchers make a significant amount of money.
Is clinical research coordinator a good job?
As a clinical research coordinator, you will work with investigators in order to collect data and improve the research project. This can be a challenging and rewarding job, as you will often have to work with highly skilled professionals in order to achieve results. The job market is constantly changing, so it is important to keep up with the latest trends in order to maintain a competitive edge.
Do physician-scientists see patients?
Physician-scientists are experts in the study of health, disease, and delivery of patient care. They use their knowledge to improve the understanding of how the body works and how to provide good care for patients.
Can doctors do research without a PhD?
"I can do research as a physician. I have a PhD in medical science, but I don't need one to do research. I can use my creativity to come up with ideas for treatments and treatments that may be helpful for my patients." - source.
Are clinical scientists doctors?
Clinical scientists work with a variety of different tools and techniques to help them understand the behavior and health of patients. They use these tools to diagnose and treat diseases. Clinical scientists also work with other healthcare professionals to create new treatments and cures for diseases.
Is Neet required for clinical research?
NEET is not a mandatory requirement for many careers in the medical field, but it can provide valuable experience and qualifications for many careers in the biotechnology and pharmaceutical industries. Many students who complete the program can pursue a career in both these industries.
Who is eligible for clinical research?
Doctoral degree in Clinical Research is an essential step for any medical professional. A doctoral degree in Clinical Research gives you the knowledge and skills necessary to conduct studies in the field of medicine. Clients and scientists always look for someone with a strong clinical background when choosing researchers. A doctoral degree in Clinical Research can help you develop your research skills, which will then help you become a better doctor.
What is the difference between medical research and clinical research?
Medical research is conducted in order to learn about potential treatments that could help patients in the future. Clinical research is conducted to test new medications on a small group of people in order to see if they are effective.
Where do clinical researchers work?
clinical researchers gather data and monitor side effects and patient wellbeing in the laboratory. They investigate medication efficacy, potency, dosing, side effects, as well as drug impact on study volunteers. Their work helps to improve patient care and may lead to new treatments for diseases.
Should I study clinical research?
Clinical research is one of the most demanding and rewarding aspects of scientific work. It helps scientists find new ways to help people, and it can be very rewarding when a new treatment is found to be safe and effective.
Why do I want to work in clinical research?
When it comes to clinical research, there is no lack of opportunities. Clinical trials are a means by which scientists can test new treatments and vaccines against disease. This research can help lead to new discoveries that could then be used to prevent or treat illnesses. There is a growing demand for this type of research, as new therapies and vaccines become available more frequently. This is echoed by the clinical trials industry itself, which is expected to grow at a rate of 20 percent annually through 2024. In addition, this sector is highly lucrative- earning an estimated $46.8 billion in 2019 alone. This success comes with a number of challenges, however. First and foremost, it can be difficult to get funding for these trials from traditional sources. As a result, many companies have turned to the private sector for assistance. This has had positive effects, as the private sector typically invests in longer-term projects that are less likely to be affected by political instability or economic volatility. Despite these challenges, there are still opportunities for the clinical research industry in 2019. The field value is expected to grow at an alarming rate, so it?s important that companies stay ahead of the curve and invest in novel technologies and applications.
Can you do clinical research with a Phd?
Most science PhDs may transition into other career positions after completing their degree, such as a clinical trials project manager or medical science liaison. After completing their degree, science PhDs are highly skilled in critical thinking and problem solving, which makes them well-suited for a variety of positions in the scientific community.
How do you become a CRA?
In order to become a clinical research associate (CRA), you will need to have an undergraduate degree in life sciences, nursing, biotech or medical sciences and a diploma or certification in clinical research from a reputed institute. As a crafter of healthcare products and treatments, you will be able to contribute to the development of new therapies and therapies for patients. With experience in both patient care and scientific research, you will be able to develop innovative methods for testing new treatments and therapies.
Is clinical research a good career in India?
Clinical research is an attractive industry for researchers in India because of the potential for great growth and job opportunities. Not only are medical, pharmaceutical and paramedical professionals needed, but also project management staff, regulatory authorities, government and the society at large. Clinical research offers researchers a great opportunity to study new treatments and cures for diseases and helps develop new products.
How do I get a CRA job with no experience?
If you are not experienced in monitoring your company's financial performance, you will likely increase your chances of being a successful CRA if you take some courses. For example, if you have spent a few weeks or months learning the GCP software, you will be able to meet the basic requirements for training as a CRA.
Which country is best for clinical research?
The United States leads the pack when it comes to clinical studies, with almost 150,000 reports dating back to 2008. France is second in terms of research activity, with just over 30,000 studies on record. Five of the top 10 countries are located in Europe.
What is the highest paying job in clinical research in India?
In India, the highest salary for a Clinical Research Associate is ?7.1 Lakhs per year (?59.2k per month). This salary is much higher than the average salary in India. A Clinical Research Associate in India can make a lot of money, and this is an excellent career for someone who wants to work in a field that requires a lot of hard work and dedication.
How stressful is CRA job?
As a CRA, you may feel stressed most of the time. even when you're on vacation or sick, you can't help but think about all the work that still needs to be done. Your body is feeling the stress.
What makes a good clinical research associate?
A good CRA must be self-confident, flexible and adaptable to the changing environment. He/she should be able to operate through relationships that are built on trust, respect, loyalty and an ability to relate to people. A good CRA is able to identify and develop opportunities that can provide value for his or her clients.
How do I prepare for a CRA interview?
1. Why are you interested in clinical research? 2. What experience do you have in this field? 3. What challenges have you faced while conducting clinical research? 4. How would you go about solving these challenges?
What comes after MSc clinical research?
The MSc Clinical Research provides the students with a wealth of skills and knowledge which can be used in the field of medicine science. They can work at high-level posts like Clinical Research Analyst, Biostatistician, Clinical Research Project Coordinator, and Clinical Research Project Manager. These posts provide opportunities for the students to work on exciting and challenging projects which will help them to develop their scientific abilities.
How do you become a clinical research director?
Clinical research directors are responsible for performing clinical trials, designing and conducting research, and monitoring and analyzing the results. They need a Bachelor's or Master's degree in medical sciences or a related field, as well as a graduate level certification in clinical social work or registered nurse. Some jobs may require you to be a Licensed Clinical Social Worker (LCSW) or a Registered Nurse (RN).
How can I become a medical researcher after 12th?
If you're interested in becoming a medical researcher, you need to first have a good level of critical thinking skills and research skills. You'll also need to be able to observe and collect data.
How much do research monitors get paid?
Clinical Research Monitors are vital members of any research team. They are responsible for monitoring the safety and effectiveness of treatments and products, as well as ensuring that team members are up to date on scientific advances. In a busy clinical setting, Clinical Research Monitors can play an important role in keeping both patients and researchers safe.
What is the difference between a CRA and CTA?
The CRA is a responsible and important role in the process of learning and clinical trials. They are responsible for preparing, maintaining, tracking and archiving study documentation, as well as processing data collected throughout the duration of the trial. The CTA is an important office-based position that involves a great deal of work.
What is the difference between CRA and CRC?
The CRA is a system that requires individuals to have a college degree or equivalent in order to participate in it. CRCs, on the other hand, can work with only a high school diploma. This difference can make a big difference when it comes to finding employment or studying for exams.

More jobs related with Clinical

- CONNECTED Networking
- APPLIED Learning
- PUBLICATIONS
- NEWS & HIGHLIGHTS
What is a Clinical Researcher ?
Clinical Researchers are responsible for designing and conducting clinical trials to test the safety and efficacy of new drugs, medical devices, and treatments. They work with teams of scientists and medical professionals to develop protocols, recruit participants, and collect and analyze data. They also play a critical role in the development of new medical treatments and the advancement of medical knowledge. Clinical Researchers may work for pharmaceutical companies, medical device manufacturers, academic medical centers, or research organizations.
Get Clinical Researcher Jobs Emailed to You
By signing up, you agree to the terms of use and privacy policy
Clinical Researcher Job Description Template
Job overview.
We are seeking a highly motivated and experienced Clinical Researcher to join our team and contribute to advancing the field of medical research. As a Clinical Researcher, you will play a critical role in conducting studies and trials to determine the safety and effectiveness of new treatments and medications. The successful candidate will be a detail-oriented individual with excellent communication and organizational skills, and a passion for making a difference in people's lives.
Clinical Researcher Responsibilities & Duties
- Design, plan, and implement clinical trials and research studies.
- Collect and analyze data from clinical trials and research studies.
- Prepare reports, publications, and presentations to share results and findings with colleagues, stakeholders, and the general public.
- Maintain accurate and detailed records of all research activities.
- Ensure that all research activities are conducted in compliance with ethical, legal, and regulatory requirements.
- Collaborate with cross-functional teams, including physicians, scientists, and regulatory specialists.
- Stay up-to-date with current trends and advancements in the field of clinical research.
Clinical Researcher Qualifications & Skills
- Previous experience in a leadership role in clinical research.
- Publication record in peer-reviewed journals.
- Knowledge of current trends and advancements in the field of clinical research.
- Strong network of professional contacts in the field of clinical research.
- Master's or PhD degree in a relevant field, such as medicine, biology, or public health.
- 3-5 years of experience in clinical research or a related field.
- Excellent written and verbal communication skills.
- Ability to manage multiple projects and deadlines effectively.
- Strong attention to detail and accuracy in record-keeping.
- Experience with data analysis and statistical methods.
- Familiarity with regulatory requirements for clinical research.
Ready to post a job using this template?
Related job descriptions.
See job descriptions similar to a Clinical Researcher
Analytical Research Chemist
Biochemistry scientist, biological chemist, biophysicist, biophysics researcher, clinical biochemist, clinical laboratory scientist, formulation scientist, pharmaceutical scientist, physical biochemist, protein biochemist, ready to start hiring.
Looking to hire the best talent for your company? Post your job to reach qualified candidates in just minutes. With our user-friendly platform, you can create a compelling job description and start receiving applications right away.
Powered by Web Scribble Solutions , Inc.
SLAS Terms of Use | Privacy Notice (non-EU / UK residents) | Privacy Notice (EU / UK residents)
© 2024 Society for Laboratory Automation and Screening

The Job Description of a Clinical Research Associate
Clinical research associates are also referred to as CRAs. They are responsible for organizing and administering clinical trials of a current or new drug to assess and determine the risks and the benefits associated with using them. Clinical research associates are often employed by clinical contract agencies and pharmaceutical companies. There are many things that a clinical research associate needs to handle, and we will discuss them in this article.
Their responsibilities
The key responsibilities that a clinical research associate has to carry out in their course of duty include:
Writing reports
Progress monitoring during the trial duration
Collection and authentication of forms that are used for data collection
Giving the clinicians instructions about how trials are to be conducted
Designing different trial materials as well as providing different study centers that have sufficient quantities
Setting up the trial centers and then disbanding them
Identifying the clinicians and briefing them
Writing down the procedures of the drug trials
The employers
Typical employers include:
The academic departments in hospitals
Clinical contract houses and agencies (CROs)
Pharmaceutical companies
Available vacancies are usually advertised in the national newspapers, online, specialist agencies, and very specific journals. This field is very competitive, therefore it can be useful for applicants to have the relevant analytical and scientific techniques. In addition, pharmaceutical research, medical sales, nursing, or laboratory work knowledge can be very useful in clinical research associate positions.
The process of recruitment involves an interview, which is very technical. You will need to be trained to get the kind of qualifications that are necessary to excel in this area. To achieve this, you need to be qualified in life sciences or medical sciences. To learn more about clinical research professional courses, visit CCRPS .
Unveiling the Role of Clinical Research Associates
Clinical Research Coordinator : Dive into the responsibilities and skills required for effective clinical research coordination.
Pharmacovigilance Certification : Explore the essential aspects of pharmacovigilance to ensure drug safety and regulatory compliance.
CRA (Clinical Research Associate) : Delve into the core responsibilities and duties of a Clinical Research Associate in managing clinical trials.
ICH-GCP : Understand the principles and guidelines outlined by the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) Good Clinical Practice (GCP).
Clinical Trials Assistant Training : Acquire the foundational knowledge and skills necessary to support clinical trial operations effectively.
Advanced Clinical Research Project Manager Certification : Elevate your expertise in clinical research project management with advanced certification training.
Advanced Principal Investigator Physician Certification : Gain advanced certification tailored for Principal Investigator Physicians involved in clinical research studies.
Medical Monitor Certification : Explore the critical role of medical monitoring in ensuring the safety and integrity of clinical trials.

Different Categories of Clinical Research Jobs Available as Careers
Understanding the scope of clinical research and what it entails.

THE LARGEST COLLECTION OF CLINICAL RESEARCH JOBS ON EARTH
- Search Clinical Research Jobs
- Browse Clinical Research Jobs
- Post Clinical Research Jobs
- Refer A Friend
- Browse Sales Jobs
- Clinical Research Physician Jobs
- Job Description
- Article Search
Supports PDF, DOC, DOCX, TXT, XLS, WPD, HTM, HTML files up to 5 MB
On ClinicalResearchCrossing
Clinical Research Physician Jobs description
| "); } |
Testimonial of the WeekOnly ClinicalResearchCrossing consolidates every job it can find in the domain and puts all of the job listings it locates in one place.
 Clinical Research Coordinator Associate🔍 school of medicine, stanford, california, united states. The Division of Cardiovascular Medicine, within Stanford University Department of Medicine, is a dynamic and innovative center dedicated to excellence in research, medical education, and clinical care. Our Division is driven by over 90 faculty members and a cadre of staff who are the pillar of strength in the Division's ongoing efforts into the prevention and treatment of cardiovascular disease. We are seeking a Clinical Research Coordinator Associate (CRCA) who is passionate about clinical research and wants to deliver results. The CRCA will have the opportunity to work with a robust clinical research team, hand in hand with the Principal Investigators, Clinical Research Manager and other associates in support of the Interventional Affinity Group and the Structural Heart Research Program. The successful applicant will collaborate with external vendors, and enjoy working in a dynamic work environment. Exceptional diplomacy, interpersonal and communication skills are essential, as is a high degree of integrity. Attention to detail and the ability to manage multiple responsibilities simultaneously are also critical attributes. If you are interested in supporting translational medicine and contributing to Stanford Medicine’s mission, we invite you to join our team. Duties include:
DESIRED QUALIFICATIONS:
EDUCATION & EXPERIENCE (REQUIRED):
KNOWLEDGE, SKILLS AND ABILITIES (REQUIRED):
CERTIFICATIONS & LICENSES:
PHYSICAL REQUIREMENTS:
WORKING CONDITIONS:
WORKING STANDARDS:
The expected pay range for this position is $31.73 - $36.54 hourly. Stanford University provides pay ranges representing its good faith estimate of what the university reasonably expects to pay for a position. The pay offered to a selected candidate will be determined based on factors such as (but not limited to) the scope and responsibilities of the position, the qualifications of the selected candidate, departmental budget availability, internal equity, geographic location and external market pay for comparable jobs. At Stanford University, base pay represents only one aspect of the comprehensive rewards package. The Cardinal at Work website provides detailed information on Stanford’s extensive range of benefits and rewards offered to employees. Specifics about the rewards package for this position may be discussed during the hiring process. Why Stanford is for You Imagine a world without search engines or social platforms. Consider lives saved through first-ever organ transplants and research to cure illnesses. Stanford University has revolutionized the way we live and enrich the world. Supporting this mission is our diverse and dedicated 17,000 staff. We seek talent driven to impact the future of our legacy. Our culture and unique perks empower you with:
The job duties listed are typical examples of work performed by positions in this job classification and are not designed to contain or be interpreted as a comprehensive inventory of all duties, tasks, and responsibilities. Specific duties and responsibilities may vary depending on department or program needs without changing the general nature and scope of the job or level of responsibility. Employees may also perform other duties as assigned. Consistent with its obligations under the law, the University will provide reasonable accommodations to applicants and employees with disabilities. Applicants requiring a reasonable accommodation for any part of the application or hiring process should contact Stanford University Human Resources at [email protected]. For all other inquiries, please submit a contact form . Stanford is an equal employment opportunity and affirmative action employer. All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, disability, protected veteran status, or any other characteristic protected by law.
My SubmissionsTrack your opportunities. Similar Listings School of Medicine, Stanford, California, United States 📁 Research Post Date: Jan 29, 2024 Post Date: Aug 05, 2022 Post Date: Aug 16, 2024 Global Impact We believe in having a global impactClimate and sustainability. Stanford's deep commitment to sustainability practices has earned us a Platinum rating and inspired a new school aimed at tackling climate change. Medical InnovationsStanford's Innovative Medicines Accelerator is currently focused entirely on helping faculty generate and test new medicines that can slow the spread of COVID-19. From Google and PayPal to Netflix and Snapchat, Stanford has housed some of the most celebrated innovations in Silicon Valley. Advancing EducationThrough rigorous research, model training programs and partnerships with educators worldwide, Stanford is pursuing equitable, accessible and effective learning for all. Working Here We believe you matter as much as the work I love that Stanford is supportive of learning, and as an education institution, that pursuit of knowledge extends to staff members through professional development, wellness, financial planning and staff affinity groups. School of Engineering  I get to apply my real-world experiences in a setting that welcomes diversity in thinking and offers support in applying new methods. In my short time at Stanford, I've been able to streamline processes that provide better and faster information to our students. Phillip Cheng Office of the Vice Provost for Student Affairs  Besides its contributions to science, health, and medicine, Stanford is also the home of pioneers across disciplines. Joining Stanford has been a great way to contribute to our society by supporting emerging leaders. Denisha Clark School of Medicine  I like working in a place where ideas matter. Working at Stanford means being part of a vibrant, international culture in addition to getting to do meaningful work. Office of the President and Provost Getting Started We believe that you can love your jobJoin Stanford in shaping a better tomorrow for your community, humanity and the planet we call home.
View All Jobs  Clinical Research Regulatory Specialist I – II, Center for Clinical Research (Hybrid)Position # : 2400071Q Position : Clinical Research Regulatory Specialist I – II, Center for Clinical Research (Hybrid) UH Facility : Cleveland Medical Center Shift : Days Status : Full-time Work Days of Week : M-F City : Cleveland Click Here if you ARE a Current Employee Click Here if you are NOT a Current Employee Next you will be prompted to log in. To Heal. To Teach. To Discover. These words are more than just our mission, they embody the opportunities available to the world-class doctors, nurses, health care professionals and support staff who choose to join University Hospitals and Be the Difference. From entry to executive level, every position and every professional at University Hospitals has a direct impact on our ability to offer excellent care to our patients, their families and our community. Join Us and Be the difference in Advancing the Science of Health and the Art of Compassion. Equal Opportunity Employer – minorities/females/veterans/individuals with disabilities/sexual orientation/gender identity. Sign up for job alerts Share This Job
JOB DESCRIPTION & QUALIFICATIONS Description What You Will Do
Additional Responsibilities
Work Experience
Knowledge, Skills, & Abilities
Licenses and Certifications
Physical Demands
Travel Requirements  UH Cleveland Medical CenterUH Cleveland Medical Center is University Hospitals’ flagship academic medical center. With more than 1,000 registered beds, UH Cleveland Medical Center provides primary, specialty and subspecialty medical and surgical care in the heart of Cleveland’s University Circle.  Working at University HospitalsCareers at University Hospitals offers opportunities to provide care locally in the Cleveland area with the power to impact healthcare nationwide. As a leader in our industry, our staff share a commitment to Be the Difference in the lives of our patients and our communities, from Northeast Ohio and beyond. Take a look at this video to discover how we’re building an organization for the future, today. Note: video content was obtained prior to the COVID-19 pandemic. ANEESAH DAVIS: You have a choice to be here or not be here. The choice for me to just actually just stay here is just, I love it. I love it here to be honest and the opportunities that it allowed me to have and grow. That's basically why I choose to stay here. SONORA HOWSON: My favorite part is probably the people I work with. I mean I'm interested in the procedures and I feel like we do a lot of good. But like the thing that has me excited to come back every day is just that I work with such an incredible team. Really talented people and it feels like a family. I know that's like kind of trite to say but it does feel like that. ALVIN MANIGAULT: One of the things I was really impressed with my time being at UH, just from a managerial and leadership perspective, I think they do a phenomenal job. My leadership specifically in terms of just being involved and engaged and also listening as well to matters, issues or concerns and then working to establish some type of implementing change. I think subsequently for me as an employee, it really makes me feel as if I'm heard, my voice is heard and that also my perspective can have an impact on the overall overarching picture as well. Similar JobsSee All Jobs  To contact University Hospitals Human Resources department, or to request a reasonable accommodation, please call 216-767-8900 or send us an email at [email protected] Equal Employment Opportunity StatementIt is the policy of University Hospitals to provide equal opportunity to all of our employees and applicants for employment. Decisions concerning employment, transfers and promotions or other conditions of employment are all made upon the basis of the best qualified candidate without regard to race, color, national origin, age, religion, veteran status, disability, gender, sexual orientation, gender identity, or marital status in accordance with Federal and Ohio law. A qualified individual with a disability will be considered for employment on the same basis as non- disabled applicants if he/she can perform the essential functions of the job sought, with or without a reasonable accommodation, and without imposing a direct threat to the health or safety of others or him/herself. EEO is the Law Join Our Talent CommunityTo learn more about our employment opportunities and how UH is advancing care through the science of health and the art of compassion, sign up for our job alerts. Stay Connected
Copyright © 2020 University Hospitals  Clinical Research Coordinator
Job Summary:The Department of Radiology, University of Wisconsin - Madison, School of Medicine & Public Health is seeking a Clinical Research Coordinator (CRC) to help advance exciting medical imaging and disease-focused projects! The CRC works as part of a team, which includes physicians, PhD researchers, imaging staff, and other research support staff, to support and advance a portfolio of interesting research projects. Radiology projects focus on anything from the development of novel imaging techniques, to work towards understanding and curing disease. The role of a CRC is quite varied - an average day could include responsibilities ranging from recruiting and enrolling subjects into a study, to coordinating and conducting actual subject visits, to collaborations with the study team on a wide variety of topics. Responsibilities:Institutional statement on diversity:. Diversity is a source of strength, creativity, and innovation for UW-Madison. We value the contributions of each person and respect the profound ways their identity, culture, background, experience, status, abilities, and opinion enrich the university community. We commit ourselves to the pursuit of excellence in teaching, research, outreach, and diversity as inextricably linked goals. The University of Wisconsin-Madison fulfills its public mission by creating a welcoming and inclusive community for people from every background - people who as students, faculty, and staff serve Wisconsin and the world. For more information on diversity and inclusion on campus, please visit: Diversity and Inclusion Preferred Bachelor's Degree Qualifications:Required - Research experience. Preferred - At least one year experience with clinical research studies. - Experience working within a higher education setting. - Prior experience working within a healthcare setting. The ideal candidate will have strong attention to detail and ability to think critically and/or strategically to solve problems. Full or Part Time: 80% - 100% This position may require some work to be performed in-person, onsite, at a designated campus work location. Some work may be performed remotely, at an offsite, non-campus work location. The incumbent must have schedule flexibility to occasionally work outside of standard 8:00AM- 5:00PM weekday hours. The position occasionally requires travel to other, local UW locations. Appointment Type, Duration:Ongoing/Renewable Minimum $48,000 ANNUAL (12 months) Depending on Qualifications The starting full time salary for the position is $48,000 but commensurate with experience and qualifications. Actual pay is determined at hire and is based on experience and qualifications. Employees in this position can expect to receive benefits such as generous vacation, holidays, and sick leave; competitive insurances and savings accounts; retirement benefits. Benefits information can be found at ( https://hr.wisc.edu/benefits/ ). Additional Information:Applicants for this position will be considered for the titles listed in this posting. The title is determined by the experience and qualifications of the finalist. This position has been identified as a position of trust with access to vulnerable populations. The selected candidate will be required to pass an initial caregiver check to be eligible for employment under the Wisconsin Caregiver Law and every four years. University sponsorship is not available for this position, including transfers of sponsorship. The selected applicant will be responsible for ensuring their continuous eligibility to work in the United States (i.e. a citizen or national of the United States, a lawful permanent resident, a foreign national authorized to work in the United States without the need of an employer sponsorship) on or before the effective date of appointment. This position is an ongoing position that will require continuous work eligibility. UW-Madison is not an E-Verify employer, and therefore, is not eligible to employ F1-OPT STEM Extension participants. If you are selected for this position you must provide proof of work authorization and eligibility to work. How to Apply:To apply for this position, please click on the "Apply Now" button. You will be asked to upload a current resume/CV and a cover letter briefly describing your qualifications and experience. Amanda Joncas [email protected] 608-262-2716 Relay Access (WTRS): 7-1-1. See RELAY_SERVICE for further information. Official Title:Clin Res Coord I(RE015) or Clin Res Coord II(RE016) Department(s):A53-MEDICAL SCHOOL/RADIOLOGY/RADIOLOGY Employment Class:Academic Staff-Renewable Job Number:The university of wisconsin-madison is an equal opportunity and affirmative action employer.. You will be redirected to the application to launch your career momentarily. Thank you! Frequently Asked Questions Applicant Tutorial Disability Accommodations Pay Transparency Policy Statement Refer a FriendYou've sent this job to a friend! Website feedback, questions or accessibility issues: [email protected] . Learn more about accessibility at UW–Madison . © 2016–2024 Board of Regents of the University of Wisconsin System • Privacy Statement Before You Go..Would you like to sign-up for job alerts. Thank you for subscribing to UW–Madison job alerts! Resume Builder
Director, Clinical Research Job DescriptionDirector, clinical research duties & responsibilities. To write an effective director, clinical research job description, begin by listing detailed duties, responsibilities and expectations. We have included director, clinical research job description templates that you can modify and use. Sample responsibilities for this position include: Director, Clinical Research QualificationsQualifications for a job description may include education, certification, and experience. Licensing or Certifications for Director, Clinical ResearchList any licenses or certifications required by the position: FEMA, ACRP, OCN, PMP, CPSM, ASCP, CCRP, CRC, ACP Education for Director, Clinical ResearchTypically a job would require a certain level of education. Employers hiring for the director, clinical research job most commonly would prefer for their future employee to have a relevant degree such as Bachelor's and Master's Degree in Medical, Education, Nursing, Business, Medicine, Management, Health, Healthcare, Oncology, Science Skills for Director, Clinical ResearchDesired skills for director, clinical research include: Desired experience for director, clinical research includes: Director, Clinical Research Examples
Related Job DescriptionsCreate a Resume in Minutes with Professional Resume Templates I am an EmployerI am a candidate. RECRUITMENT FRAUD ALERTIt has been brought to our attention that there has been fraudulent activity by scammers attempting to represent themselves as Parexel employees or recruiters. These individuals are attempting to reach potential job seekers through online chat interviews and sending false offer letters, representing Parexel without our consent. If you’re concerned that you’ve been contacted by an unauthorized Parexel recruiter or employee, please notify [email protected] . You may also report suspicious fraudulent activity to your local law enforcement agency. Thank you. 
My research opens up new medical possibilities. And I do it Search Jobs Radius: Enter distance 5 15 25 35 50 Clinical Research Associate (CRA II/Senior) - US homebasedAs a Clinical Research Associate (CRA) at Parexel, you act as an integral part to get treatments to patients sooner. Our CRAs' priority is the safety and well-being of the patients. As you travel to investigator sites and perform your monitoring duties, we encourage you to be inquisitive, take accountability, build relationships, and act with integrity. Join a team with a wide variety of experiences and knowledge, and work on global projects within a broad scope of therapeutic areas. We’re looking for people who want to grow personally and professionally and support their colleagues globally and cross-functionally. Success ProfileDo you have these soft skills and interpersonal traits to succeed at Parexel?
About This RoleWorking with heart At the end of the day we’re all patients. Because of this, the Parexel team takes our work personally and does it with empathy and heart. When our values align, there’s no limit to what we can achieve. Our clinical operating model drives effectiveness, reduces handoffs and increases employee, client and site satisfaction. As a Clinical Research Associate at Parexel, you will be joining a team with a wide variety of experiences and knowledge. We hire talent that takes initiative to do things better, smarter and faster- people who want to grow personally and professionally. Your time here At Parexel, the CRA role offers the opportunity to go beyond the role of a typical clinical monitor. CRA’s are accountable for using their expertise to build and maintain the site relationship and ensure they are set up for success. As the clinical sites’ sole point of contact, this includes addressing and resolving site issues and questions. You will also manage site quality and delivery from site identification through to close-out. As a CRA, Parexel will offer you world class technology and training catered to your individual experience. You can anticipate less travel and a lower protocol load in comparison to industry peers. Your hard work may be rewarded through a bonus incentive program, the opportunity to work within multiple therapeutic areas and an opportunity to advance your career in clinical research. If impact, flexibility, and career development appeal to you, Parexel may be your next home. What you’ll do
A little about us Parexel is proud to be a leading Clinical Research Organization with team members across the globe. As a member of our team, you will get to know your colleagues on a personal level. Have a question? A clinical research leader, project team member, technology “super user” or colleague are a phone call away. Our clinical research teams meet regularly to have discussions in an open environment, allowing our team members to share their expertise and promote learning within the team. Management supports and encourages career growth through consistent performance discussions and evaluations. Whether your interest is to progress into a more senior level CRA role, into line management, Quality, Project Leadership or a variety of other positions, Parexel prides itself on career opportunities for our employees. More about you On your first day we’ll expect you to have:
EEO Disclaimer Parexel is an equal opportunity employer. Qualified applicants will receive consideration for employment without regard to legally protected status, which in the United States includes race, color, religion, sex, sexual orientation, gender identity, national origin, disability, or protected veteran status. Share this job Explore this locationCheck It Out Potential Career PathParexel offers various career paths and internal development programs for CRAs to advance to the next level. This could include enhancing your technical position, moving into management, or shifting to other areas of the business.
Employee Insights Nick on a day in the life of a CRANick Burger | Clinical Research Associate II Watch Video  Liliana on CRA tasks and our recognition programLiliana Belmares Flores | Senior Clinical Research Associate  Marlayna on why Parexel is a great place for CRAsMarlayna Fitts | APEX CRA Field Coach TEAM IMPRESSIONS Why Clinical Research Associates work at ParexelPatient-focused in everything we doWe push the boundaries of what is possible to create clinical trials that are inclusive, innovative, and patient-focused Supportive and inclusive environmentWe foster collaboration, teamwork, respect, and inclusivity, to work together to achieve common goals. Career growth and developmentWe develop your skills through training, mentorship, and career advancement programs. Flexible work arrangementsWe focus on outputs and results, not where and when you work. Diverse therapeutic areas and project exposureWe continuously learn from a broad exposure from early to late phase clinical trials and our cros-functional global teams. Advanced TechnologyWe recognize the importance of first-time quality to bring treatments to patients faster. Learn About Our Culture Neuroscience CareersNeuroscience diseases affect the very core of one’s being and experience. We work diligently to lighten the burden for patients and families alike. Explore opportunities to join our team.   Careers in Inflammation & ImmunologyEach day, we’re working toward building a deeper connection and understanding with those who count on us – the patients.  Cell and Gene CareersCell and gene therapies (CAGT) have rapidly emerged as among the fastest-growing spaces in all of biopharma R&D, and Parexel was an early entrant into this space.  Our work cultureLearn about our culture, perks, learning opportunities, and our corporate responsibility approach. Careers in OncologyOur dedicated people, innovative approaches, and culture of caring all work together to ease the journey for patients and investigators.  Careers in Rare DiseasesIn rare disease, every single patient is precious. Discover where your skills can create a life-changing achievement for every person involved.  Emerging Talent ProgramsBegin your career journey at Parexel with our emerging talent programs or our internships, placements and apprenticeships. Advance science by keeping the patients at the heart.  About our hiring processIt’s all about finding the right fit, for you and for us. Our recruiters work with you and our hiring managers to bring together a team and culture where everyone can grow and be successful.  Hear about Xoli's role as Sr. Director of Patient InclusionDiscover Xoli's passion for fostering diversity within the clinical trial industry. Learn about her new role, the importance of patient engagement, and Parexel's commitment to creating an inclusive workplace.  Aida Sabo, Sr. VP DEI, on Diversity, Equity & Inclusion at ParexelDiscover how Aida drives DEI change at Parexel. Learn why it's crucial, goes beyond diversity dimensions, and embraces the advantage of valuing differences.  Follow us on Social MediaParexel is present on several social media channels where we post our latest updates. Follow, interact, and rate us!  Medical Writing OpportunitiesJoin Parexel's global team of 700+ medical writers. Deliver impactful, high-quality content and advance your career in medical writing.  Flexible Work ArrangementIn-office, home-based or a mix? What’s your preference? We value the work-life balance of our employees, and as such Parexel is offering maximum flexibility to our employees wherever possible.  Clinical Operations OpportunitiesAre you passionate about making a difference in the fight against cancer and beyond? At Parexel's Global Clinical Operations, we are dedicated to putting patients at the heart of every clinical trial. With a global footprint and 5000 Clinical Operations team members worldwide, we are impacting clinical research.  A day in a life of a Project LeaderChristine and Ewa share what a Project Leader does, why to become a Project Leader, and what mindset is needed. Explore how a Project Leader resembles a cheerleader or a coxswain of a rowing boat!  Meet Lola: Senior Clinical Research AssociateLola shares about her position as a Senior Clinical Research Associate (Sr. CRA), what skills are needed for her role, and what she enjoys outside of work!  Roles within our Functional Service Provider devisionWithin our outsourcing model, you are deployed as an experienced colleague for our customers, benefiting from exposure to both the clinical research organization (CRO) and sponsor experience.  Roles within Data OperationsImpact patients with a role in Biostatistics, Statistical Programming and Data Management.  Meet Amrita: Manager, Statistical ProgrammerAmrita tells us about how Parexel has helped her grow within her career with working flexibility and opportunities to learn.  Meet Janice: Principal Statistical Programmer FSPJanice You shares why she choose Parexel FSP. Find out which skills are needed in her role, what she enjoys the most, as well as what challenges her.  Meet Neha: Senior BiostatisticianNeha describes her role as a Senior Biostatistician and why she chose Parexel.  Meet Rahul: Senior Health Economics AssociateRahul describes his role as a Senior Health Economics Associate in the Health Economics and Outcomes Research team  Meet Joanna: Senior Medical WriterJoanna provides insights about her role as a Senior Medical Writer. She describes what excites her about the role and what it is like working with highly educated, motivated, and professional colleagues.  Meet Mary: Principal Medical WriterMary provides insights about her role as a Principal Medical Writer. She shares what it is like working with her fellow colleagues, how Parexel has supported her career development, and what excites her most about the work she does.  Meet Chanakarn: Clinical Research Associate IICharnakarn talks about what it is like to be a CRA, and the support she has been given to achieve her career goals.  Rebuilding Careers: How Ashwini rediscovered her confidence at ParexelMeet Ashwini Somayaji, Senior Manager for Medical Writing Services. Ashwini's career took an unexpected turn with a five-year break. However, her determination and passion led her to Parexel, where she reignited her professional aspirations. With the support and guidance of her colleagues, Ashwini's journey became one of rebuilding confidence and career growth.  Returning with Passion: What Marlayna experienced coming back to ParexelAfter a brief departure, Marlayna joined as a Senior Clinical Research Associate and transitioned later into being a field coach and mentor for new CRAs. When returning Marlayna discovered a company more committed than ever to employee well-being and belonging.  Meet Sheryl: Principal Regulatory Affairs Consultant, Regulatory StrategySheryl, Principal Regulatory Affairs Consultant, talks about why she joined Parexel and how she keeps the patient first.  Meet Kanika: Manager in Project ManagerKanika describes her role within finance operations and why she chose to join Parexel  Meet Cheri & Tarryn: Project Specialist IICheri and Tarryn are sisters and Project Specialists at Parexel South Africa. Learn about their lives, roles and the culture of the Project Planning & Support (PPS) department.  Meet Jamie: Diversity & InclusionJamie offers her perspective on Parexel’s inclusivity to LGBTQ+ colleagues, how she feels about being "out" at work, and more.  Meet Kirill: Executive Director, FSP BiometricsKirill describes what excites him about his role and how he tries to challenge and encourage his staff.  Meet Santino: Clinical Operations LeaderSantino's career at Parexel, from a Project Specialist to a Clinical Operations Leader, showcases a diverse journey through Clinical Operations, fueled by a passion for the pharmaceutical industry and a commitment to impacting patient lives. His experience highlights Parexel's dedication to professional growth and the embodiment of the "We Care" promise in every facet of their work.  Meet Siddhika: Clinical Data Analyst IIIMeet Siddhika, Clinical Data Analyst III as she explains why she decided to apply to Parexel after a six year career break  Meet Jitender: Director, Health EconomicsJitender describes his role as a Director, Health Economics, supporting our clients with strategic recommendations and delivering the value story of new treatments.  Meet Jagan: Director, India Assistant Compliance OfficerJagan describes his role as a Director within the compliance team based in India  Catalyst Award Winner 2022Catalyst is advancing workplaces that work for Women - Parexel was recognized for "Leveraging Gender Partnership to Advance Women in Leadership."  Meet Jennifer: Associate Director, Scientific Services, MedComJennifer discusses her role as an Associate Director, Scientific Services in Medical Communications, and the challenges she enjoys.  Meet Simona: Principal Consultant, Regulatory & AccessSimona shares how Parexel has supported her career development, the day-to-day activities of being a Principal Consultant at R&A, and much more!  UK Career Webinar — Accelerate your career in Clinical Project LeadershipView this career webinar to hear from our Clinical Project Leadership team in the UK about their opportunities for growth and the team's culture at Parexel.  Meet Joy: Senior Director, Statistical ProgrammingJoy joined Parexel in 2006 and has since then built an incredible career and lasting relationships with her colleagues. She is passionate about programming and finds fulfillment in supporting clinical trials. Outside of work, she enjoys hiking and spending time with loved ones.  Meet Celine: Director for Integrated Solutions StrategyCeline talks about her silver award in The PharmaTimes Clinical Researcher of the Year (Americas) competition, which she received while working as a Senior Project Leader at Parexel.  Meet Tina: Manager, Project Finance ExcellenceMeet Tina Huang, Project Finance Excellence Manager, as she discusses hAssociate Director, Scientific Services, MedComer role and what it's like to work at Parexel Taipei  Meet Amelia: Senior Manager, Medical Writing ServicesMeet Amelia Young, Senior Manager, Medical Writing Services, as she discusses her role at Parexel  Meet Andrea: Manager, FSPAndrea discusses why she returned to Parexel, what excites her about being an FSP Manager, and the best career advice she ever received!  Meet Catherine: Associate Project DirectorPeople are Catherine's passion. She enjoys showing a project team how their work fits into the bigger picture, sharing knowledge and celebrating accomplishments. Explore her career advice, newly established behaviors, and more!  Meet Jahanara: Vice President, FSP Biometrics, IndiaJahanara is proud of growing within Parexel and our Women in Leadership program.  Meet Nayoung: VP & APAC Head of Enterprise Account LeadershipNayoung participated in our Women in Leadership program and an MBA program and believes the advice from Parexel colleagues to BE BOLD encouraged her to take the risk to move forward.  Meet Chalermporn: Senior Clinical Research AssociateChalermporn talks about what it is like to be a CRA at Parexel, and the support she has been given to pursue her career.  Meet Dorothy: Senior Document Quality Reviewer Medical Writing ServicesDorothy shares her story about returning to work after a career break, and how she arrived at Parexel having previously worked for another CRO.  Watch Replay: Why Biotech Matters More Than EverInsights on working with Biotech clients to rapidly take new science from the bench through registration.  Career Blog - Should I consider a mentor  Meet Laurias: Manager, Clinical OperationsLaurias shares how Parexel has supported his career development from CRA to Clin Ops Manager and flexibility within the workplace.  Meet Jens: Senior Director, Medical Writing ServicesMeet Jens Zurrahn, Senior Director for Medical Writing Services, as he discusses his role at Parexel.  Meet Vivek: Senior Manager, Statistical Programming FSPVivek describes his role as a Senior Manager within the Statistical Programming Functional Service Provider (FSP) team.  Watch Replay: Online Seminar Italy Clinical Research Associates Putting the Patients FirstView this career seminar to hear from our Clinical Operations colleagues and Clinical Research Associates about the role of a CRA and working in Clinical Operations at Parexel.  Watch Replay: Online Seminar UK Clinical Research Associates Putting the Patient FirstView this webinar to hear from our Clinical Operations colleagues and Clinical Research Associates about the role of a CRA working in Clinical Operations at Parexel  Watch Replay: Online Seminar EMEA Your Skill Set Could Save Lives Working as a Stats ProgrammerView this webinar to hear from our Data Operations colleagues and about the role of Statistical Programming in the Clinical Research Industry  Meet Wipawee: Clinical Research Associate I (CRA I)Wipawee shares about her role as a Clinical Research Associate (CRA) at the Parexel Thailand office, including the training and CRA job responsibilities. Learn about what she considers to be the most attractive part of working as a CRA in Parexel.  Location: Quakertown DepotCome work with a supportive team of 30 colleagues in Quakertown to provide packaging, labeling, and global distribution of clinical trial materials.  Meet Ben: Senior Regulatory Affairs ConsultantBen provides and insight into his role as a regulatory affairs consultant. He also talks openly about being part of the LGBTQ+ community and how Parexel's flexible work arrangements help him as a single father.  Meet iCRA Twins Marina Palumbo and Anna KorelisDouble the passion and double the commitment to working With Heart™. Read on to learn more about these colleagues whose family and work lives are uniquely intertwined at Parexel!  Meet Rebecca: Senior Data Management LeadRebecca talks about her day-to-day activities as a Senior Data Management Lead and why Parexel's core value of Empowerment and Accountability stand out to her.  Meet Sanjay: India Country Head & Head CTS&LSanjay discusses his dual role, Parexel’s strong collaborative and cohesive working environment, and how our patient-centric culture makes him feel connected.  Location: GermanyAt Parexel Germany are 750+ employees, we have an Office, Early Phase Clinical Unit and Logistics Depot, and 40+ nationalities.  Meet Yogeeta: Senior Document Quality ReviewerYogeeta talks about returning to work after a 3-year career break, and the support she received from her manager at Parexel India.  Watch Replay: Online Seminar Spain Clinical Research Associates Putting the Patients FirstView this career seminar to hear from our Clinical Operations colleagues and Clinical Research Associates about the role of a CRA working in Clinical Operations at Parexel.  Meet Virginia: Project Quality & Risk ManagementVirginia shares what it is like working at Parexel Argentina and how it has given her the opportunity to meet with a wide range of creative-minded people and this is what keeps her on her toes.  Meet Nadia: Senior Data Management LeadNadia shares details about her role as a Senior Data Management Lead and what she finds rewarding in her job.  Meet Tom: Medical ITTom talks about the knowledge and experience he has gained at Parexel; along with what is different upon him rejoining.  Meet Barbara, a Senior Clinical Operations Leader who generates excitementBarbara is living her passion for Physics by tutoring kids in her neighborhood and sparking their joy in the subject. Her interest in sparking excitement is coming in handy for her role as a Senior Clinical Operations Leader as well. Do you like to excite your team?  Meet Julia: CVP, Head of Medical Writing and GMBA IrelandJulia provides leadership insights, inspiration and advice from her 20+ years at Parexel.  Recognition ProgramAn interview about the value of the Recognition Program and the high engagement of Parexel's employees.  Meet Bob: Biostatistician II FSPBob shares about his roles as a Biostatistician II within Parexel FSP, his direct involvement with the client's team and goals, and what is needed to be successful in his role.  Meet Urvashi: Medical Writer IUrvashi tells us why she chose Parexel and what she enjoys being a Medical Writer I  Meet Reyad: Senior Clinical Research AssociateReyad, Senior Clinical Research Associate, shares how Parexel supports flexibility within the workplace and what he finds to make a great leader.  Meet Margaret: Clinical Research NurseFind out why Margaret enjoys working as a Nurse in Clinical Research and working at Parexel, also how Parexel supports her in a way she hasn't experienced anywhere else.  Meet Penny: Senior Clinical Research AssociatePenny, Senior Clinical Research Associate, shares the reason why she kept coming back to Parexel, the responsibilities of her role and how she keeps the patient at the heart of everything she does.  Women at ParexelParexel employee base is 70 % female, and we are proud to say 60 % of managers+ and 46 % of VP-level+ are female. Yet we are committed to improving these numbers with several leadership programs for female and male colleagues!  Meet Adriane, a Clinical Operations Leader sharing insights and tipsDiscover Adriane's role as a Clinical Operations Leader. She shares insights into her daily responsibilities, the skills crucial for success, and the rewarding challenges she faces. Find out how Parexel supported her career development and get inspired by her advice for professional growth.  Roles within Medical CommunicationsMake a difference with a role in Medical Communications  Meet Seeba: Regional Director for Project Planning and SupportSeeba describes her role within Project Planning and Support.  Meet Blessy: Data Management Lead IBlessy describes her role, what excites her about it, and how she came to work in Clinical Data.  Blog: Should I consider engaging with a mentorLets review what mentoring really means, and how it can benefit you  Meet Robbin: Associate Manager, Statistical ProgrammingRobbin joined Parexel for her Placement year in 2014 and has since progressed into a Associate Manager, Statistical Programming. Dedication and communication are her driving skills.  Meet Ekaterina: Senior Statistical ProgrammerEkaterina loves challenges and new tasks! She feels her contribution to clinical trials really helps people and that inspires her.  Great Place to Work - IndiaIn February 2023, Parexel India has been certified as a Great Place to Work®, for the second time in three years — on average, scores for Parexel India increased in all categories by 10 to 15 points.  Meet Swarnalatha: Senior Principal Statistical Programmer FSPSwarnalatha has been working for over 14 years in the pharma industry as a Statistical Programmer, and has been impressed with the work flexibility and empowerment of female colleagues at Parexel.  Meet Mati: Medical Writer IIMati provides an insight into his role as a Medical Writer II in the Taipei office in Taiwan. He also openly talks about being part of the LGBTQ+ community at Parexel and how welcoming the Taipei office is!  Meet Angeli: Senior Project SpecialistAngeli shares what it is like to work as a Project Specialist and with her colleagues within the Project Planning & Support department.  Meet Agnieszka: Senior Clinical Operations LeaderAgnieszka shares about her role as a Senior Clinical Operations Leader, the skills needed, the challenges and teamwork. She is looking back on a 17-year career path at Parexel.  Meet Lillie: Clinical Research Associate ILillie shares what her role as a CRA I looks like and how she got started at Parexel.  Coming back to Parexel: Looking forward to strong collaborationAfter a brief departure, Ira Mills (Senior Scientific Specialist) found himself being drawn back to Parexel. He missed the strong working and personal collaboration with his colleagues and the broad institutional support. Parexel not only cares deeply about patients but also about its employees.  Location: ArgentinaWork where you will find flexible working options, a supportive atmosphere, constant learning, and more.  Location: IndiaJoin one of our 5 locations in India. Parexel India employs ~5770 employees, which represents 25% of our global population. We offer a supportive and fun work culture, flexibility, career growth, and learning opportunities.  Re-excel: Return to workDo you want to return to work after an extended period of time away from the workplace? Parexel has many opportunities for those interesting in re-establishing a meaningful career with heart. Now is the time to re-excel at Parexel!  Roles within Scientific Data OrganizationBe at the core of what we do at Parexel with a role in our Scientific Data Organization  Meet Emmanuel: Senior Clinical Research AssociateEmmanuel shares about his day to day duties and how he emphasizes the patients' wellbeing, by running smooth trials. Due to his great work, he was recently awarded for Extraordinary Monitoring Efforts.  Meet Andrea: Initiation Clinical Research Associate IIAndrea talks about what it is like to be an iCRA and the opportunities she has been given to progress her career.  Meet Jayashree: Senior Clinical Data AnalystJayashree details what it's like to be a CDA and what skills you need to be successful in the role. Being able to contribute to a good cause through clinical trials is a genuine reason as to why she enjoys her job.  Roles within AI LabsDiscover how Parexel AI Labs is leveraging technology and AI to improve clinical trials, advance patient safety, and transform our everyday work.  Meet Donata, a Senior Project Leader focusing on quality and growthDonata's advancement from an entry-level position to Senior Project Leader at Parexel showcases her dedication to quality and determination to exceed client expectations. Her journey reflects the supportive and growth-oriented environment at Parexel, where passion and hard work pave the way for making a meaningful impact.  Meet Madalina: Clinical Operations LeaderAs a Clinical Operations Leader, Madalina invites people to join the wonderful and life-changing experience of working With Heart and passion for the future of medicine.  Meet Carolina, a Senior Project Leader with a stellar growth storyCarolina's remarkable growth story, achieving 5 promotions within 10 years highlights her supportive team and the importance of personal growth. She progressed from Project Specialist to Senior Project Leader and moved from Argentina to the US.  Meet Kathryn: Clinical Research Associate IIKathryn, Clinical Research Associate II, tells us why she chose Parexel and how she keeps the patient at the heart of everything she does.  Learning and developmentWe believe that investing in your professional and personal development is an investment in Parexel, and we want to help you realize your full potential and career. Ensuring we have a fully trained and capable workforce is a key part of delivering quality work and patient safety.  Benefits & SupportAt Parexel, we prioritize putting people first, allowing you to achieve your best work. Explore the Flexible Work Program, our "Bravo" Recognition Program, and how we build our well-being in our supportive culture. Diversity, Equity & Inclusion at ParexelLearn how Parexel embraces diversity and strives for equity and inclusion in its workforce, clinical trials, and supplier partnerships.  Join our APEX Program and become a CRAJoin Parexel's Accelerated Program of Education, Exposure, and Experience (APEX) and embark on a 6-month journey to become a remarkable Clinical Research Associate (CRA). With hands-on experience and expert guidance, we'll help you excel in the field.  Meet Mwango: SVP & Global Head of Regulatory StrategyMwango shares how she came to Parexel after spending 16 years at the FDA to experience the process and considerations from the drug developer’s perspective. Read about her role as a VP-Technical and what valuable advice she offers to those looking to work With Heart™.  Meet Mark, a Corporate Real Estate & Services Manager, proud to be part of Parexel's global missionMark shares about his background in the U.S. Navy and how he has been able to transition his skillset to leading a team as a Corporate Real Estate & Services Manager at Parexel.  What is a Clinical Research Organization?A Clinical Research Organization (CRO) is a company contracted by a pharmaceutical, biological or medical device manufacturer to manage clinical research studies and other services to support product development. Learn about the four phases and the key functions of clinical trials.  Meet Catia, Director of Clinical Operations in the Americas at Parexel BiotechCatia discusses her impressive 15-year career progression at Parexel. Learn from her experiences and insights into people management, the importance of support, and advice for Clinical Operations Leaders.  Parexel's NewsroomRead our corporate news, press releases, as well as our ESG report.  Medical Writing Careers Webinar ReplayOur expert medical writers share their extensive industry experience. Whether you're a seasoned medical writer or just starting, this webinar caters to all skill levels, offering valuable insight into the life of a Medical Writer and what it is like to work at Parexel.  Why leaders work at ParexelLearn more about Parexel's exceptional team dedicated to efficient, high-quality clinical development services. With a culture of care, collaboration, and ownership, we put patients first.  Meet Cheng Cai: Director, Clinical Pharmacology Modeling and Simulation (CPMS)Cheng is passionate about advancing clinical research and improving patient outcomes. He enjoys collaborating with his talented team and building mathematical models in his role. Outside of work, he leads an active lifestyle and values quality time with his family through various activities.  Parexel Military Talent CommunityWe know ‘serving’ is a core value of many of our military community. At Parexel, you can continue on your mission to serve, by joining an organization dedicated to improving the lives of patients worldwide. The skills and values you have developed in your military career or as a military spouse are transferrable to meaningful careers here at Parexel.  Video on Working With Heart™ - Christina's Clinical Operations Manager PerspectiveView how Christina reflects on her work experience, her impact on patients and her management style of respect and growth. Christina is leading a team of Clinical Operations Leaders who manage groundbreaking trials in the biotech space.  Parexel's Careers BlogLearn more about our teams, our company culture and benefits, hear from our employees, find hiring resources and more.  Blog: Do you take your career seriouslyTaking the time to review your career path is an important, but sometimes overwhelming, task that many of us gloss over.  Meet Nadia: Principal BiostatisticianNadia Seniavina talks about her role as a Principal Biostatistician and what excites her most working in Parexel  Meet Steve, Sr. Director Cloud & Infrastructure SolutionsDiscover how his passion for resilient technology solutions is making a difference in global clinical trials and transforming patients' lives worldwide. Steve started as an intern and now leads the team that supports the technology tools he helped build during his early years at Parexel.  A day in a life of a Clinical Operations Leader (COL)Viviana and Jani share what a Clinical Operations Leader does, what it takes to be in their role, and why to work in Clinical Operations. Joining Parexel as a COL means taking on significant responsibilities, being open-minded and making a meaningful impact in clinical research.  Meet Chrishni, a Senior Project Leader with an exciting global journeyDiscover Chrishni's inspiring journey at Parexel, where her love for France led her to relocate from Australia and thrive in her career based in Lyon. She enjoys collaborating with her team and finding a harmonious work-life balance to indulge in her passions.  Meet Doreen, a Project Leader focusing on patient-centric researchExplore Doreen's journey as a Project Leader at Parexel, where she embraces her role in improving patient materials and ensuring patient-friendly and inclusive studies. Additionally, learn about Doreen's onboarding process, her Line Manager's support for work-life balance, and how she enjoys adventures with her children.  Meet Xin Ni, a Clinical Data Analyst I at Parexel (Malaysia)Xin Ni joined Parexel Malaysia as Clinical Data Analyst Intern since 2022, and was shortlisted and hired as a permanent Clinical Data Analyst I (CDAI). Let’s visit her story in the data management team and get more insight of Gen Z workers, The Future Innovators.  Meet Marije, a Clinical Operations Manager in Parexel, is most proud of becoming a people leaderMarije, a former nurse and now Clinical Operations Manager in Parexel, is dedicated to enhancing patients' lives through her involvement in clinical research. Marije's exceptional leadership skills and impressive career growth further highlight her as a motivating individual.  Meet Adrian, a Site Care Partner navigating the complex landscape of clinical trials and prioritizing patient careAdrian works as an outsourced Site Care Partner (SCP) for a client within the clinical research and pharmaceutical industry. Learn more about Adrian's experience and insights on how he contributes to the success of clinical trials and prioritizing patient care.  Meet Rachel Smith: Global Head of Rare Disease, CoERachel, Global Head of Rare Disease, CoE, advocates for rare disease patients and drives innovation in clinical trials at Parexel. Her own diagnosis gives her a unique perspective on patient experiences and outcomes. Discover Rachel's inspiring projects and her dedication to advancing rare disease research.  Meet Jessica, an Associate Project Director guiding trials through the complex regulatory landscapeJessica works as an Associate Project Director within our Regulatory and Access Global Project Leadership team. Learn more about Jessica's experience and how she contributes to the success of clinical trials.  Meet Anthony, a Sr. Principal Medical Writer contributing to lifesaving treatmentsExplore his career reflections, motivations, and proud moments in contributing to medical advancements through his work.  Why Clinical Operations Leaders work at ParexelListen to Elizabeth Edwards, SVP Clinical Operations at Parexel, as she delves into why she works With Heart™, the impact of Clinical Operations Leaders, how Parexel supports their success, and what makes Parexel's culture unique.  A Day in the Life: Discover Sinan's Impactful Journey as a VP Technical of Regulatory Strategy at ParexelSinan, VP Technical of Regulatory Strategy, is dedicated to putting patients first by helping to navigate the complex landscapes of clinical trials. He has great satisfaction in guiding clients to innovative products and trial designs,  Meet Julie, a Senior Clinical Assistant, proud of her contributionJulie welcomes volunteers at the Early Phase Clinic reception in Berlin, manages payments, and caters to the volunteers in the kitchen.  Meet Steve, a VP Technical of Regulatory Strategy responsible for steering clients through the complexities of regulatory requirementsSteve works as a VP Technical within Regulatory Strategy, where he is working to guide clients through the regulatory journey from initial clinical trials to achieving market success. Read more about what a day in the life of a VP Technical at Parexel looks like and how Steve is able to utilize his FDA background and apply his skills to his current position.  Meet Inhye, a Senior Clinical Research Nurse contributing to societyInhye finds pride in her role in contributing to society. She emphasizes putting patients first and ensuring volunteer safety and comfort during clinical trials. Learn more insights into her journey with Parexel, her reasons for choosing the organization, and the supportive work environment.  Working as a Nurse in Clinical Research at ParexelAntje and Katharina share insights into working as a Nurse in Clinical Research, where innovative medications are developed through early-phase and first-in-human studies.  Project Leadership OpportunitiesLearn how Project Leaders partner with pharmaceutical and biotech clients, manage with determination and With Heart, and our unwavering commitment to patient-centricity.  Meet Rebecca, a Project Leader who is passionate about empathyDiscover Rebecca's insights on effective leadership, collaboration, and patient-centric approaches.  Meet Theodora, a Site Contract LeaderTheodora Chung, Site Contract Leader, offers us an insight into her role, which is to oversee the whole clinical trial budget and contract planning, drafting and negotiations till its execution. She is passionate about working in the team.  Location: MexicoLearn about Parexel Mexico, where you can be part of a rapidly growing team dedicated to improving patient health. Enjoy flexible work options, a supportive and collaborative culture, and continuous professional growth. Discover how you can make a global impact with us.  New Medicines, Novel InsightsParexel's insight-generation engine, our people, share perspectives on patient-guided clinical research.  New Medicines, Novel Insights: Advancing Precision OncologyThis report examines some obstacles drug developers encounter and describes strategies to help bring precision cancer medicines more quickly and certainly to market—to benefit an ever-increasing proportion of patients.  Meet David, a champion for disability inclusionHis leadership fosters workplace and clinical research inclusivity. Explore David's inspiring journey from combat medic to Parexel VP and Disability Steering Committee co-lead. Clinical Research Coordinator A (Department of Radiology)
Job Description
Qualifications
Job Location - City, State Philadelphia, Pennsylvania Department / School Perelman School of Medicine Pay Range $43,888.00 - $44,947.00 Annual Rate Salary offers are made based on the candidate's qualifications, experience, skills, and education as they directly relate to the requirements of the position, as well as internal and market factors and grade profile. Affirmative Action Statement Penn adheres to a policy that prohibits discrimination on the basis of race, color, sex, sexual orientation, gender identity, religion, creed, national or ethnic origin, citizenship status, age, disability, veteran status, or any other legally protected class. Special Requirements Background check required after a conditional job offer is made. Consideration of the background check will be tailored to the requirements of the job. University Benefits
Application Details (to apply use the Apply Now button below.)How to Apply: Please click the "Apply Now" button below or visit: https://wd1.myworkdaysite.com/recruiting/upenn/careers-at-penn Jobs at the University of Pennsylvania. When inquiring or applying for positions within the University of Pennsylvania, please also reference AcademicCareers.com Applicants with dual-career considerations can find university jobs such as professor jobs, dean jobs, chair / department head jobs, and other faculty jobs and professional and administrative staff employment opportunities at the University of Pennsylvania and at other institutions of higher education in the region on www.AcademicCareers.com To receive email alerts when new jobs at the University of Pennsylvania are posted, job seekers can sign up at new job openings at the University of Pennsylvania.  A Great Place to Work Penn's innovative schools, centers, and divisions offer a vast array of positions in a broad range of fields. Penn is the largest private employer in Philadelphia. Here you can find new opportunities as your career develops—from your first job out of school to your first leadership role, and all points in between. You can discover new work settings as your goals evolve, and even change professions. We’re famous for research and education, but did you know Penn also offers positions in areas such as financial management, hospitality, transportation, real estate development, investments, public safety, fundraising, marketing, communications, and information technology (IT)? Penn is a leading employer in key fields. We are regularly ranked as one of the best places to work in IT, and our Office of Development and Alumni Relations is one of the premier organizations in the industry. If you have a passion for excellence in education, research, or service to the community, Penn is the right choice for any point in your professional journey. University Overview The University of Pennsylvania, the largest private employer in Philadelphia, is a world-renowned leader in education, research, and innovation. This historic, Ivy League school consistently ranks among the top 10 universities in the annual U.S. News & World Report survey. Penn has 12 highly-regarded schools that provide opportunities for undergraduate, graduate and continuing education, all influenced by Penn's distinctive interdisciplinary approach to scholarship and learning. Penn offers a unique working environment within the city of Philadelphia. The University is situated on a beautiful urban campus, with easy access to a range of educational, cultural, and recreational activities. With its historical significance and landmarks, lively cultural offerings, and wide variety of atmospheres, Philadelphia is the perfect place to call home for work and play. The University offers a competitive benefits package that includes excellent healthcare and tuition benefits for employees and their families, generous retirement benefits, a wide variety of professional development opportunities, supportive work and family benefits, a wealth of health and wellness programs and resources, and much more. When inquiring or applying for positions within the University of Pennsylvania, please also reference AcademicCareers.com  COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK  Senior Financial Analyst
Position Summary The Senior Financial Analyst is responsible for clinical practice payroll accounting/budgeting and endowment management for multiple clinical departments. Reporting to the Manager of Budget and Finance, responsibilities include the following: Responsibilities
Minimum Qualifications Requires a Bachelor's degree or equivalent in education and experience, plus three (3) years of related experience. Preferred Qualifications
Other Requirements
Equal Opportunity Employer / Disability / Veteran Columbia University is committed to the hiring of qualified local residents. Commitment to DiversityColumbia university is dedicated to increasing diversity in its workforce, its student body, and its educational programs. achieving continued academic excellence and creating a vibrant university community require nothing less. in fulfilling its mission to advance diversity at the university, columbia seeks to hire, retain, and promote exceptionally talented individuals from diverse backgrounds. , share this job. Thank you - we'll send an email shortly. Other Recently Posted Jobs Heavy Cleaner (Floater)Heavy cleaner. Refer someone to this job 
Wait! Before you go, are you interested in a career at Columbia University? Sign up here! Thank you, for sharing your information. A member of our team will reach out to you soon!  This website uses cookies as well as similar tools and technologies to understand visitors' experiences. By continuing to use this website, you consent to Columbia University's usage of cookies and similar technologies, in accordance with the Columbia University Website Cookie Notice .  |
IMAGES
VIDEO
COMMENTS
Design and conduct early clinical Phase 1 and Proof-Of-Concept development trials, assess clinical safety and efficacy data, and write clinical study reports, clinical sections of INDs, Investigator Brochures. This individual will also work closely with biomarker, discovery and translational research scientists. Ensures optimal study conduct.
Medical Doctor/Physician for Clinical Research. Elite Clinical Network. Chandler, AZ 85224. $100 - $140 an hour. Part-time + 1. Monday to Friday + 1. Easily apply. $100-$140/hr DOE for working on research and/or ownership share if you want to open a private practice on top of doing research with us. Active 5 days ago ·.
To understand the role of a clinical research physician, one needs to have a basic understanding about drug development. Let's say a pharma company called 'ABC Therapeutics Ltd' owns a molecule that they want to develop as a potential new treatment for hypertension.. After appropriate laboratory and animal testing, they then decide to put the molecule through a series of clinical trials ...
What does a Clinical Research Physician do? Clinical Research Associate (CRAs) are responsible for coordinating and overseeing the execution of studies and clinical trials. They have a hand in everything from recruiting study participants to creating study documentation, collecting patient data, and performing quality assurance audits to ensure ...
A clinical research physician (CRP) conducts research and performs clinical studies to develop improved care options for local physicians and their patients. As a CRP, your job duties include overseeing clinical trials, performing safety medical reviews, and building relationships with professionals in the pharmaceutical field.
Clinical Research Monitoring/ Quality. OVERVIEW: Monitoring is the act of overseeing the progress of a clinical trial, and of ensuring that it is conducted, recorded and reported in accordance with the protocol, SOPs, GCP and applicable regulatory requirements.The clinical research monitor is the person with direct access to, and oversight, of the site research activities.
Clinical research is an important part of the healthcare industry, an industry that is expanding faster than any other (in the U.S.) Combine the overall growth of healthcare sector, with rapid growth of new technologies and innovations, clinical research presents a huge employment opportunity which should see continued growth for a long time.
As a leading global contract research organization (CRO) with a passion for scientific rigor and decades of clinical development experience, Fortrea provides pharmaceutical, biotechnology, and medical device customers a wide range of clinical development, patient access and technology solutions across more than 20 therapeutic areas.
Job Description - Clinical Research Physician Job Description: Clincal Research Physician / v.1 / Effective Date: 01-Dec-2017 Template ID: CCR_TMPL -QM_001 / Version: v.3 / Effective Date: 12-Oct-2017 Page 1 of 2 JOB FUNCTION / RESPO NSIBILITIES setting up, documenting and communicating project specific medical scientific
Job Types: Part-time, Contract. Schedule: License/Certification: Ability to Commute: Ability to Relocate: Work Location: In person. Search Clinical research physician jobs. Get the right Clinical research physician job with company ratings & salaries. 108 open jobs for Clinical research physician.
We have included clinical research job description templates that you can modify and use. Sample responsibilities for this position include: Hire, orient, train, and conduct performance reviews for staff handling research administration activities associated with the conducting of clinical trials. Monitor staffing levels, and identify adequate ...
Recent research has revealed that academic medical centers, which employ a large number of clinical research professionals, are suffering from high staff turnover rates in this arena, with issues such as uncertainty of the job, dissatisfaction with training, and unclear professional development and role progression pathways being reported as ...
The Clinical Research Physician will also be responsible for writing and reviewing clinical study reports, presenting findings at conferences, and publishing results in peer-reviewed journals. Additionally, the role requires staying up-to-date with the latest advancements in medical research and contributing to the development of new treatment ...
Clinical Researcher: Duties, Skills and How To Become One
A Clinical Research Physician (CRP) is responsible for overseeing clinical trials, performing safety medical reviews, and building relationships with professionals in the pharmaceutical field. CRPs also play a critical role in developing regulatory documents for the pharmaceutical industry.
Clinical Researchers are responsible for designing and conducting clinical trials to test the safety and efficacy of new drugs, medical devices, and treatments. They work with teams of scientists and medical professionals to develop protocols, recruit participants, and collect and analyze data. They also play a critical role in the development ...
Clinical Research Associate Job Description
The key responsibilities that a clinical research associate has to carry out in their course of duty include: Writing reports. Progress monitoring during the trial duration. Collection and authentication of forms that are used for data collection. Giving the clinicians instructions about how trials are to be conducted.
Job Description. 4.5. 166 votes for Clinical Research Specialist. Clinical research specialist provides financial, administrative, and organizational support for assigned clinical trials and sites in accordance with Good Clinical Practice (GCP), guidelines, standards, federal regulations, and SOPs.
Clinical Research Physician Jobs description on ClinicalResearchCrossing.com, clinical research physician jobs, career description & job details page.
The Division of Cardiovascular Medicine, within Stanford University Department of Medicine, is a dynamic and innovative center dedicated to excellence in research, medical education, and clinical care. Our Division is driven by over 90 faculty members and a cadre of staff who are the pillar of strength in the Division's ongoing efforts into the prevention and treatment of cardiovascular disease.
Coordinate and manage the regulatory operations for clinical research studies conducted by principal investigator(s) at University Hospitals Case Medical Center. (20%) Complete regulatory documentation required to conduct clinical research with the IRB, pharmaceutical sponsor, and other internal/external agencies or committees. (20%)
As a company with over 70,000+ dedicated employees, Fresenius Medical Care AG is quite aware of the many scams that job seekers may face. If you receive any type of communication on behalf of Fresenius Medical Care AG that seems inappropriate or suspicious, we urge you to report this activity to:
Clinical Research Associate Job Description Template
Job Summary: The Department of Radiology, University of Wisconsin - Madison, School of Medicine & Public Health is seeking a Clinical Research Coordinator (CRC) to help advance exciting medical imaging and disease-focused projects! The CRC works as part of a team, which includes physicians, PhD researchers, imaging staff, and other research support staff, to support and advance a portfolio of ...
The Director of Clinical Research, Oncology, will be the key physician contact for sites and investigators during site start-up and study execution. The Director will act as a liaison between the company and clinical investigators. 2. Director, Clinical Research Job Description. Job Description Example.
Summer Undergraduate Research Fellowship Program (SURF) UT Dallas Green Fellows; Supporting a Culture of Clinical Trials. Our research teams help plan, conduct, fund, administer, and report on clinical trials across the broad spectrum of health conditions and diseases. More than 1,000 trials are currently underway, including these areas of ...
Learn more about applying for Clinical Research Associate (CRA II/Senior) - US ... Remote - United States of America Job ID R0000026880 Category Clinical Trials Date Posted 08/28/2024. Apply Now Save Job. Jump To: ... biological or medical device manufacturer to manage clinical research studies and other services to support product development. ...
Clinical Research Coordinator A Job Description Summary ... resolving data queries, review of medical records, scheduling study-related tests in EPIC/Pennchart, Radiology, and other research systems; preparing and processing regulatory documents for submission to the IRB, and all regulatory committees from study start-up to completion ...
Job Type: Officer of Administration Bargaining Unit: Regular/Temporary: Regular End Date if Temporary: Hours Per Week: 35 Standard Work Schedule: Building: Salary Range: 75,700-87,700 The salary of the finalist selected for this role will be set based on a variety of factors, including but not limited to departmental budgets, qualifications, experience, education, licenses, specialty, and ...